Abstract
Weak hypomorph mutations in the enhancer of yellow genes, e(y)1 and e(y)2, of Drosophila melanogaster were discovered during the search for genes involved in the organization of interaction between enhancers and promoters. Previously, the e(y)1 gene was cloned and found to encode TAFII40 protein. Here we cloned the e(y)2 gene and demonstrated that it encoded a new ubiquitous evolutionarily conserved transcription factor. The e(y)2 gene is located at 10C3 (36.67) region and is expressed at all stages of Drosophila development. It encodes a 101-amino-acid protein, e(y)2. Vertebrates, insects, protozoa, and plants have proteins which demonstrate a high degree of homology to e(y)2. The e(y)2 protein is localized exclusively to the nuclei and is associated with numerous sites along the entire length of the salivary gland polytene chromosomes. Both genetic and biochemical experiments demonstrate an interaction between e(y)2 and TAFII40, while immunoprecipitation studies demonstrate that the major complex, including both proteins, appears to be distinct from TFIID. Furthermore, we provide genetic evidence suggesting that the carboxy terminus of dTAFII40 is important for mediating this interaction. Finally, using an in vitro transcription system, we demonstrate that recombinant e(y)2 is able to enhance transactivation by GAL4-VP16 on chromatin but not on naked DNA templates, suggesting that this novel protein is involved in the regulation of transcription.
Despite the enormous progress made in unraveling the complexities of regulated gene transcription during the past few years (9, 21, 30), novel regulatory factors are still being discovered. We are interested in factors that are involved in the organization of interaction between enhancers and promoters, a key process in transcription control. Previously, during the search for such factors, we identified mutations in three genes named enhancers of yellow [e(y)1, e(y)2, and e(y)3], as they influenced yellow expression in the bristles that was activated by a tissue-specific enhancer (15). In combination with the zeste null allele, mutations in these genes strongly inhibited enhancer-dependent white expression (14). The zeste protein recognizes DNA sequences located in the enhancer and promoter regions of certain genes (e.g., the white gene) and is able to mediate protein-protein interactions to generate multimeric zeste complexes (4, 29). In spite of the fact that some mutations of zeste changing the specificity of zeste protein-protein interaction may strongly inhibit the target gene transcription depending on enhancer activity, the null allele of zeste induces only a weak effect on gene expression. The synergistic effects of the zeste null mutation and mutations in the e(y) genes on inhibition of enhancer-dependent white expression suggests that these genes share similar functions.
The e(y)1 gene was recently cloned and shown to encode Drosophila melanogaster (d) TAFII40 protein (also called dTAFII42) (37). TAFIIs or TATA-binding protein-associated factors are components of TFIID, a basal RNA polymerase II transcription factor. TAFIIs are highly conserved from yeast to mammals (for reviews, see references 39 and 40) and are considered to perform important functions in transcription initiation, core promoter recognition, and transcription activation as coactivators that mediate signals from enhancer-bound regulatory proteins (7, 9, 10, 18, 19, 23, 25, 42). Both the human (h) and the yeast (y) homologues of dTAFII40 (hTAFII31 and yTAFII17) are subunits not only of TFIID but also of the recently identified TBP-free TAFII-containing multiprotein complexes (including hTFTC, hPCAF, hSTAGA, and ySAGA [3, 6, 8]).
The second isolated e(y) gene mutation, e(y)21, has diverse weak effects on fly morphology: short stocky body, separated wings, eyes with altered facets, and low fertility (15). It also influences the phenotype of weak mutations in the yellow, white, cut, and scute genes (13, 26). Thus, the genetic data suggest that the e(y)2 protein influences the expression of many different genes.
Here we report the identification of the e(y)2 gene and demonstrate that it encodes a novel, ubiquitous, evolutionarily conserved chromatin-associated protein that does not contain any known structural domains. The e(y)2 protein is present in all tissues and at all stages of Drosophila development. It enhances transcription activation in an in vitro transcription system on chromatin but not on naked DNA templates. The e(y)2 protein coimmunoprecipitates with TAFII40 and some other components of the Drosophila TFIID complex. Genetic data also demonstrate e(y)2-TAFII40 interaction. Thus, genetic and biochemical data together suggest that e(y)2 participates in transcription regulation.
MATERIALS AND METHODS
Genetic crosses.
Cultivation of flies, the mutations and constructs used in this work were described elsewhere (14, 24, 37).
P{w+, e(y)2+} construction and P element-mediated transformation.
P{w+, e(y)2+} was obtained by the insertion of fragment shown on Fig. 1C (the 5′-XhoI restriction site, introduced by PCR with the primer atCTCGAGtaagacgtcgccgaggtgt, and the 3′-BamHI genomic site were used) into pCaSpeR 3 vector. The P{w+, e(y)2+} construct and p25.7wc (22) were injected into C(1)RM, yf/y2w e(y)21/Y preblastoderm embryos as described previously (31, 38). Chromosomal insertions of P{w+, e(y)2+} were tested by the reversion of the “w” phenotype, and the number of inserted copies was determined by Southern blot analysis using P element sequence as a probe.
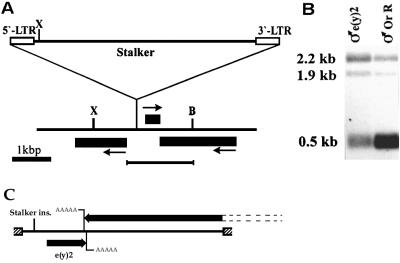
FIG. 1.
Cloning of the e(y)2 gene. (A) Map of the obtained clone showing site of the Stalker insertion. Black boxes indicate the regions of localization of corresponding transcripts. Arrows show the direction of transcription. B, BamHI; X, XhoI. The underlined fragment was used for the rescue of the wild phenotype. (B) Northern blot hybridization with poly(A)+ RNA from males of Oregon R and e(y)11 strains. XhoI-BamHI fragment was used as a probe. (C) Schematic presentation of the construct used for phenotype rescue. Shaded boxes indicate polylinker pCaSpeR 3 vector, and transcripts are indicated by black arrows showing the direction of the transcription. The inserted genomic region includes the whole e(y)2 transcript (0.5 kb) with the adjacent 5′ sequences and the 3′ portion of the 2.2-kb neighboring transcript. The two 3′ ends of the transcripts slightly overlap.
Construction of libraries.
Construction of cDNA and genomic libraries, RNA isolation, and Northern blot analysis were performed as described previously (37).
Preparation of extracts.
Nuclear extracts from Drosophila embryos (TRAX), which efficiently worked in in vitro transcription reactions, were used for the immunoprecipitation experiments. Extracts were obtained as described previously (34) by the lysis of nuclei from 0- to 6-h embryos with 0.4 M ammonium sulfate. The final extract contained 15 to 20 mg of protein per ml in HEMG 100 buffer (25 mM HEPES, pH 7.6; 100 mM KCl; 12.5 mM MgCl2; 0.1 mM EDTA, pH 8.0; 1 mM dithiothreitol [DTT]; 0.2 mM phenylmethylsulfonyl fluoride [PMSF]; 10% glycerol). Cytoplasmic extracts used for chromatin assembly were obtained as described previously (2, 5). Dechorionated, 0- to 90-min embryos of Drosophila were washed with EW (0.7% NaCl; 0.05% Triton X-100), 0.7% NaCl, and EX-10 buffer (10 mM HEPES-KOH, pH 7.6; 10 mM KCl; 6.5 mM MgCl2; 0.5 mM EGTA; 10% glycerol; 1 mM DTT; 0.2 mM PMSF) and then homogenized in Potter-Elvehjem homogenizer in EX-10 buffer. Turbid cytoplasmic extracts obtained after centrifugation for 5 min at 17,000 × g were further centrifuged for 2 h at 190,000 × g. Cytoplasmic extracts from Drosophila cell culture (Schneider) were obtained by lysis of cells washed in buffer (15 mM potassium phosphate, pH 7.0; 80 mM KCl; 16 mM NaCl; 5 mM MgCl2; 1% PEG 6000). Cells were homogenized in small glass-Teflon homogenizer, and nuclei were pelleted by centrifugation for 5 min at 17,000 × g.
Immunoprecipitation Superose-6 chromatography, Western blot analysis, and immunodetection experiments.
The recombinant His-tagged e(y)2 protein was expressed using the pQE-30 expression vector (Qiagen). To generate e(y)2 antibodies, the affinity-purified His-tagged e(y)2 protein was injected into rabbits. Rabbit polyclonal antibodies raised against His-tagged e(y)2 protein were affinity purified and used in Western blot analysis, immunodetection, and immunoprecipitation experiments.
In immunoprecipitation experiments, 150 μg of nuclear extract in 400 to 500 μl of immunoprecipitation buffer (IP buffer; 25 mM Tris-HCl, pH 7.9; 10% [vol/vol] glycerol; 0.1% NP-40; 0.5 mM DTT; 5 mM MgCl2) containing 100 mM KCl was immunoprecipitated with 40 μl of protein A-Sepharose (Pharmacia) and approximately 2 μg of antibody. Antibody-protein A-Sepharose-bound complexes were washed three times with IP buffer containing 0.5 M KCl and two times with IP buffer containing 0.1 M KCl. In the experiment shown in Fig. 4D, antibody-protein A-Sepharose-bound complexes were washed with IP buffer containing 1 M KCl. After being washed 10 μl of beads was boiled in sample buffer, and proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For chromatography, 200 μl of nuclear extract (TRAX) was loaded on a Superose-6 10/30 column and equilibrated with buffer (20 mM HEPES-KOH, pH 7.6; 400 mM KCl; 1 mM MgCl2; 0.5 mM EGTA; 1 mM DTT; 20% glycerol) at a flow rate of 0.1 ml/min. Fractions of 0.5 ml were collected. The antibodies used were described previously (17). Immunostaining of polytene chromosomes and tissue sections was performed as described previously (37).
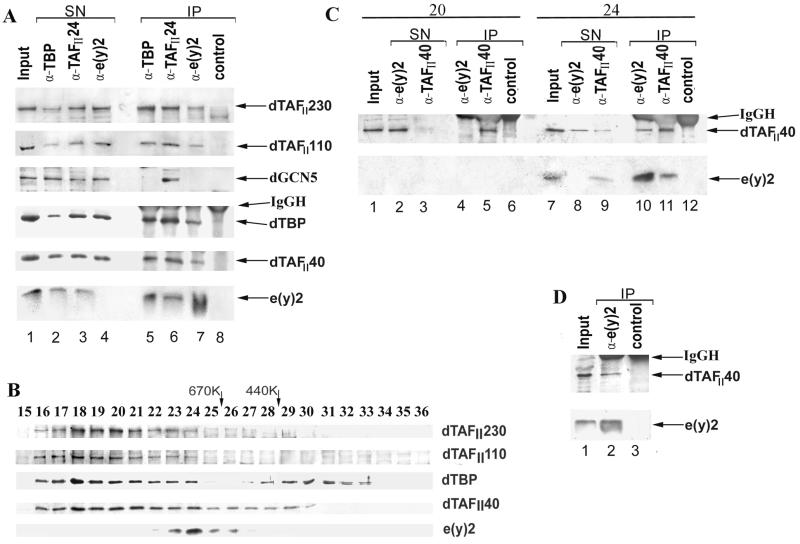
FIG. 4.
e(y)2 and TAFII40 are stably associated within a high-molecular-mass complex. (A) The nuclear extract was immunoprecipitated with polyclonal antibodies (α) raised against dTBP, e(y)2, dTAFII24, or a rabbit preimmune serum (control). The input nuclear fraction (Input), supernatant of the immunoprecipitations (SN), and the protein A-Sepharose-bound proteins (IP), washed with 500 mM KCl containing IP buffer, were resolved by SDS-PAGE on a 10 or 15% (lower panel) polyacrylamide gel. Blots were probed with antibodies raised against dTAFII230, dTAFII110, hGCN5, dTBP, dTAFII40, and e(y)2, respectively. Note that the aliquots for the IP lanes (A, C, and D) are two to three times larger than those for the Input and SN lanes (approximately 1/6 and 1/15 of the material, respectively), except in the panel for TAFII40, where approximately equal aliquots were taken. (B) Western blot analysis of fractions from Superose-6 gel filtration column with the antibodies against e(y)2 and different components of TFIID. The column was calibrated with thyroglobulin (670K) and ferritin (440K) size standards (Pharmacia). Protein fractions eluted from the column were separated by SDS-PAGE on a 10 or 15% (lower panel) polyacrylamide gel. After transfer, the blots were probed with antibodies against dTAFII230, dTAFII110, dTBP, dTAFII40, and e(y)2, respectively. (C) Immunoprecipitation with antibodies against e(y)2, TAFII40, or preimmune serum (control) using fractions 20 and 24 of the Superose-6 column. Blots were probed with antibodies against dTAFII40 and e(y)2. The indications are the same as in panel A. (D) Immunoprecipitation of nuclear extract with the antibodies against e(y)2 and a control preimmune serum. Protein A-Sepharose-bound proteins were washed three times with IP buffer containing 1 M KCl. Blots were probed with antibodies against dTAFII40 and e(y)2. The indications are the same as in panel A.
Chromatin assembly and incubation in transcription extract.
A 7.75-kb plasmid containing hsp26 minigene (33) was used as a template. The chromatin reconstitution on DNA templates immobilized on Dynabeads M280 (Dynal) and the monitoring of chromatin assembly were performed as described earlier (5, 27, 32, 34). Naked or chromatin DNA templates (1.5 μg) immobilized on beads were incubated in a scaled-up transcription reaction (34) containing 30 μl of transcription extract (TRAX) per 200 μl of total volume in the presence of 2.5 mM ATP for 30 min at 26°C. Beads were washed three times with 400 μl of HEMG 100 and resuspended in SDS gel loading buffer.
In vitro transcription experiments.
The in vitro transcription system was as previously described (11, 12). Chromatin was assembled using Drosophila embryonic extract (28) on supercoiled circular DNA and tested by micrococcal nuclease digestion as described earlier (2, 12). His-tagged e(y)2 and/or GAL4-VP16 were added to the template and incubated for 30 min at 27°C prior to transcription initiation. Transcription was quantitated by S1 nuclease analysis (35, 41) by using the 32P-labeled probe that hybridized with the transcripts from the (17M)5β2G and pG1 sites to yield fragments of 179 and 60 nucleotides, respectively. Transcription was quantitated using a PhosphorImager.
Search for e(y)2 homologues and analysis of amino acid sequences.
Searches were performed using the BLAST (National Center for Biotechnology Information) computer program (1). UniGene clusters Hs.56002, Rn.3365, and Mm.10219 correspond to human, rat, and mouse expressed sequence tags (EST). The final nucleotide sequences of human, rat, and mouse cDNAs were obtained as a result of alignment of all EST sequences. To confirm the sequences, we cloned and sequenced e(y)2 cDNA using reverse transcription-PCR. The multiple sequence alignment of proteins was done with the PIMA 1.4 program (36); the pairwise sequence alignment was done with the BLAST 2 program (1). Sequences obtained in this work were submitted to GenBank under the following accession numbers: genomic DNA in region of localization of Drosophila e(y)2 gene (AF173294); cDNA of the e(y)2 gene from D. melanogaster (AF173295), mouse (AF173297), and human (AF173296).
RESULTS
Cloning of the e(y)2 gene and structure of the e(y)2 gene and protein.
The e(y)21 mutation was shown to be induced by insertion of the Stalker mobile element (16) into a site localized to the 10C2-C4 region of the X chromosome according to deletion mapping and in situ hybridization with a Stalker probe. The e(y)2 gene was cloned by chromosomal walk from the gene encoding the largest subunit of RNA polymerase II located in the same region (20, 43). Clones containing sequences homologous to Stalker were found on the first step of the walk. The adjacent genomic sequence was used as a probe for the isolation of wild-type clone from the Oregon R strain. Three transcripts were mapped close to the place of Stalker insertion (Fig. 1A). However, only the smallest of them (0.5 kb) was under-represented in the mutant e(y)21 strain compared to the wild-type one (Fig. 1B).
To prove that the 0.5-kb transcript corresponds to the e(y)2 gene, the wild-type genomic region encoding this transcript (see Fig. 1C) was inserted into the pCaSpeR3 vector and microinjected in embryos of the y2 w e(y)21 strain. A complete reconstitution of the wild-type phenotype, e(y)2+, took place in four independent transgenic y2 w e(y)21 P{w+, e(y)2+} lines.
Thus, the 0.5-kb transcript does indeed correspond to the e(y)2 gene. Sequencing of the obtained genomic and cDNA clones showed the absence of introns in the e(y)2 gene. The deduced amino acid sequence revealed a small protein, 101 amino acids long, without any homology to known proteins.
The e(y)21 mutation is caused by the insertion of the Stalker mobile element 167 bp upstream of the 5′ end of the largest cDNA clone. The size and structure of the e(y)2 transcript were not changed by this mutation. The only molecular effect of Stalker insertion was a reduced level of e(y)2 transcription (ca. three- to fourfold decrease of the mRNA content in adult males). Thus, e(y)21 represents a weak hypomorph mutation.
Presence of the homologous genes in other species.
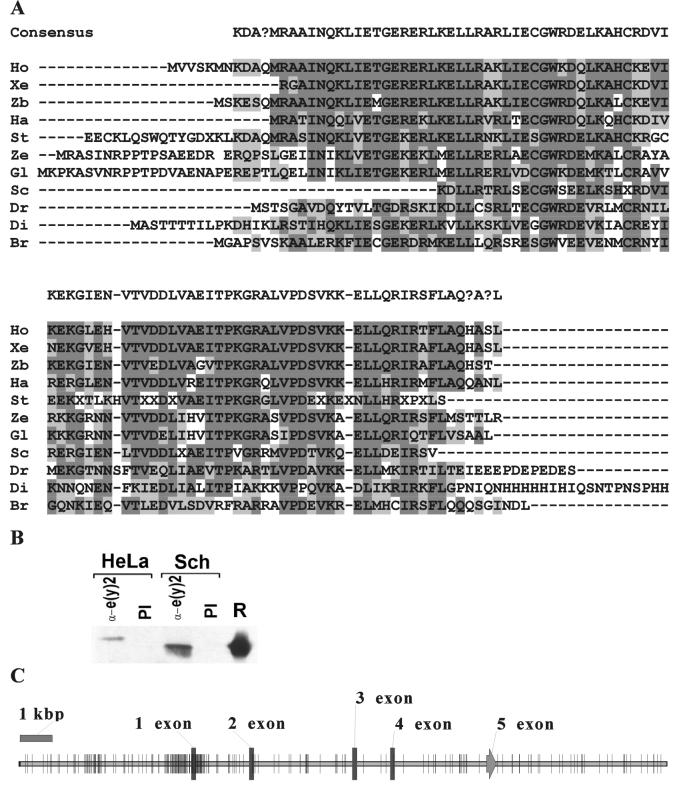
No homologues of the e(y)2 gene were detected by BLAST search among already-known genes. On the other hand, a search in EST data bank has revealed cDNA's encoding homologous proteins in a wide range of species from mammals and protozoa to plants (Fig. 2A). The e(y)2 homologues were found among EST clones obtained from different human (including bone, brain, heart, and kidney) and mouse (including unfertilized egg, embryo, kidney, liver, and muscle) tissues. The 5′ upstream region of human homologue is CpG-rich (80 CpG for 1 kbp of promoter region), a characteristic feature of housekeeping genes (Fig. 2C).
FIG. 2.
e(y)2 homologues from different species. (A) Result of sequence alignment of e(y)2 from different species. Identical amino acids are represented in dark boxes; similar ones are represented by light boxes. Ho, Homo sapiens; Xe, Xenopus laevis; Zb, Danio rerio (zebrafish); Ha, Halocynthia roretzi; St, Strongylocentrotus purpuratus; Ze, Zea mays; Gl, Glycine max; Sc, Schistosoma mansoni; Dr, Drosophila melanogaster; Di, Dictyostelium discoideum; Br, Brugia malayi. (B) Antibodies against Drosophila e(y)2 recognize human homologue. The immunoprecipitation with the antibodies against e(y)2 and preimmune serum (PI) of nuclear extracts of HeLa and Schneider (Sch) cells are shown. We used SDS–15% PAGE for resolution of the proteins. A Western blot was probed with the antibodies against Drosophila e(y)2. (C) Map of the human e(y)2 gene obtained from the sequence of chromosome 8 (accession number AC021237). Short segments indicate the positions of individual CpG sites.
All homologous proteins are of similar size, and the region of homology is spread over the entire length of different e(y)2 proteins (Fig. 2A). The amino acid sequences of human, rabbit, rat, and mouse proteins are identical. Human and Drosophila proteins contain 48% identical and 27% similar amino acids. Still the human protein is recognized by polyclonal antibodies directed against recombinant Drosophila e(y)2 (Fig. 2B).
The e(y)2 protein has a nuclear localization and is present in all tissues of D. melanogaster.
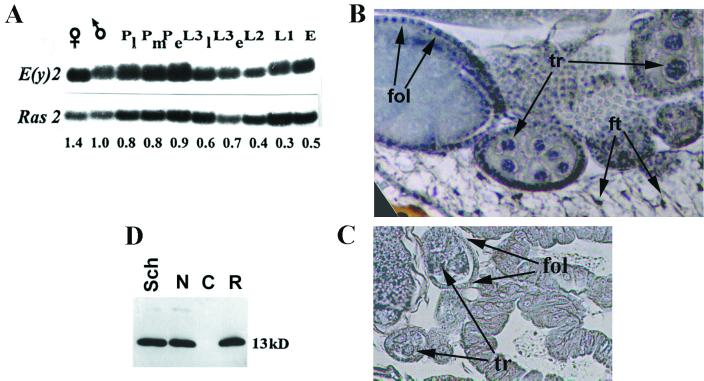
The e(y)2 gene is actively transcribed at all stages of development of D. melanogaster (Fig. 3A). The e(y)2 protein is present in the nuclei of all tissues of adult flies (Fig. 3B) and was detected in the nuclei from the earliest stages of embryonic development (data not shown). Separation of Drosophila Schneider cell homogenate into nuclear and cytoplasmic fractions also demonstrated e(y)2 protein to be limited to the nuclear fraction (Fig. 3D). The content of e(y)2 protein per nucleus in cell culture was roughly estimated on the basis of Western blot analysis. It is equal to ca. 1.2 × 104 molecules per nucleus, i.e., ca. 1 e(y)2 molecule per 50 nucleosomes (data not shown).
FIG. 3.
Pattern of expression of the e(y)2 gene. (A) Content of e(y)2 mRNA at different stages of development of D. melanogaster (Oregon R): adult females (♀) and males (♂); late (Pl), middle (Pm), and early (Pe) pupae; late-third (L3l), early-third (L3e)-, second (L2)-, and first (L1)-instar larvae; and embryos (E). Signals on Northern blot were normalized according to the results of Ras2 hybridization (26). The relative level of mRNA content is indicated below. The content of e(y)2 mRNA in males was taken for 1. (B) Immunostaining of frontal tissue section of female abdomen with antibodies to e(y)2 protein. Arrows indicate the nuclei of follicular cells (fol), trophocytes (tr), and fat cells (ft). The places of e(y)2 localization are blue. Secondary horseradish peroxidase-conjugated antibodies and Sigma Fast DAB were used for visualization. (C) Control staining with preimmune serum. (D) Western blot of whole-cell (Sch.), cytoplasmic (C), and nuclear (N) extracts from Schneider cells, probed with polyclonal antibodies raised against e(y)2. R, recombinant protein.
Genetic evidence for interaction between e(y)2 and TAFII40.
Genetic analysis of the e(y)21 mutation permitted us to further examine the molecular function of e(y)2. Previously, we described the e(y)11 mutation of the e(y)1/TAFII40 gene (37). It is noteworthy that although the viability of both e(y)11 and e(y)21 strains of flies is not severely compromised, the combination of the e(y)11 and e(y)21 mutations is lethal at the late larval and early pupal stages of development (Table 1). Thus, the e(y)11 mutation strongly enhances the effect of the weak e(y)21 mutation and vice versa. Note, that both the e(y)21 (Fig. 1B) and the e(y)11 mutations (37) individually decrease expression of the e(y)2 or the TAFII40 genes, respectively, at the transcription level. The viability of e(y)21 e(y)11 flies is rescued in strains carrying a single copy of either the P{w+, e(y)1+} or the P{w+, e(y)2+} constructs which express the wild-type e(y)1 and e(y)2 genes, respectively (Table 1).
TABLE 1.
Genetic interaction between mutations in the e(y)1 and e(y)2 genesa
| Genotype | % Survival |
|---|---|
| y2 e(y)11 | 100 |
| y2 e(y)21 | 90 |
| y2 w e(y)21 e(y)11 | 2 |
| y2 w e(y)21 e(y)11; P{e(y)2+}/+ | 60 |
| y2 w e(y)21 e(y)11; P{e(y)1+}-21/+ | 40 |
| y2 w e(y)21 e(y)11; P{e(y)1+}-22/+ | 80 |
| y2 w e(y)21 e(y)11; P{Δe(y)1}-2/+ | 3 |
| y2 w e(y)21 e(y)11; P{Δe(y)1}-3/+ | 4 |
| y2 w e(y)21 e(y)11; P{Δe(y)1}-2/P{Δe(y)1}-3 | 5 |
Abbreviations: P{e(y)1+}-21 and P{e(y)1+}-22 are different single insertions of P{e(y)1+} in the second chromosome; P{Δe(y)1}-2 is a single insertion in the second chromosome; P{Δe(y)1}-3 is a single insertion in the third chromosome. The level of viability was calculated as a ratio of males carrying e(y) mutations to FM4 males. For each combination of mutations no less than 200 FM4 males were scored.
In contrast, when the e(y)21 e(y)11 flies are complemented with one or two copies of the P{w+, Δe(y)1} transposon, expressing a C-terminally truncated version of e(y)1/TAFII40 (the last 25 amino acids of TAFII40 are replaced with 17 amino acids encoded by the Stalker sequences [see reference 37]), this transposon is not able to reverse the lethal phenotype of the e(y)21 e(y)11 double mutant, in spite of the fact that the same P{w+, Δe(y)1} transposon restores the defects of the e(y)11 mutant. Thus, in the presence of abnormally low e(y)2 protein concentration, truncated TAFII40 protein cannot function properly, suggesting an important role for the C-terminal domain of TAFII40 in the lethal phenotype of the e(y)21 e(y)11 flies. Together, these genetic experiments suggest the existence of an interaction between e(y)2 and TAFII40. Since dTAFII40 is a subunit of TFIID and possibly of other Drosophila TAFII-containing complexes (17), e(y)2 may also interact with these complexes.
Biochemical experiments to examine the interaction between e(y)2 and dTAFII40.
To further study the genetically identified interaction between e(y)2 and TAFII40, we analyzed the proteins that coimmunoprecipitated together with either e(y)2 or different subunits of the distinct TAFII-containing complexes from Drosophila embryo nuclear extract. TFIID and other TAFII-containing complexes were immunoprecipitated using antibodies directed against either Drosophila TATA-binding protein (dTBP) or dTAFII24, one of two recently discovered Drosophila homologues of human TAFII30 (17), while e(y)2-associated proteins were immunoprecipitated with a polyclonal sera raised against recombinant e(y)2. The proteins were then analyzed by Western blotting (Fig. 4A).
In a good agreement with the genetic data, antibodies to e(y)2 coimmunoprecipitated dTAFII40 and also other bona fide Drosophila TAFIIs (such as dTAFII230, dTAFII110, and dTAFII24) and TBP (Fig. 4A, lane 7, and data not shown). The antibodies raised against either TBP (lane 5) or dTAFII24 (lane 6) also coimmunoprecipitated e(y)2. A control immunoprecipitation with preimmune serum and probing the Western blots with several different antibodies against dTAFII230, dTAFII110, dTAFII40, dTAFII24, TBP, and GCN5 confirmed the specificity of the immunoprecipitations (compare lanes 5 to 7 to lane 8).
Thus, the 13-kDa e(y)2 protein interacts with either TBP- or different TAFII-containing complexes. The immunoprecipitation with antibodies to e(y)2 depleted more than 90% of the e(y)2 protein from the input nuclear extract but reduced only slightly the amounts of TBP and of the different TAFIIs (Fig. 4A). Vice versa, the amount of e(y)2 coprecipitating with TBP and TAFII24 did not seem to be stoichiometric, suggesting that only a minor fraction of e(y)2 may be associated with the TFIID complex.
To further study the association of e(y)2 with TAFII-containing multiprotein complexes, we carried out gel filtration experiments. Drosophila embryo nuclear extract was fractionated on a Superose-6 column. Western blot analysis of the Superose-6 fractions (Fig. 4B) revealed that e(y)2 eluted as a single peak and was present in fractions with apparent relative molecular masses of between 600 and 900 kDa, indicating that e(y)2 is a component of a large protein complex. dTAFIIs and TBP eluted in fractions with apparent relative molecular masses of more than 800 kDa (Fig. 4B, fractions 16 to 24). The single e(y)2 elution peak only slightly overlaps with TFIID-containing fractions (Fig. 4B, fractions 23 and 24). Interestingly, the TAFII40 elution peak is much larger (Fig. 4B, fractions 16 to 30) than that of the other tested dTAFIIs and TBP, and thus the overlap is more prominent in the case of TAFII40 and e(y)2.
To control the specificity of e(y)2 and TAFII40 complex formation, we performed an immunoprecipitation with fraction 24 containing the maximal amount of e(y)2 and fraction 20 containing almost no e(y)2 (Fig. 4C). The e(y)2 antibodies precipitated neither e(y)2 nor TAFII40 from fraction 20 (Fig. 4C, lanes 4 and 5), proving the absence of nonspecific precipitation. Antibodies raised against TAFII40 precipitated almost all TAFII40 and a very significant amount of e(y)2 in the fraction 24 (lane 11) and, vice versa, antibodies to e(y)2 precipitated almost all e(y)2 and about a half of the TAFII40 in the fraction 24 (lane 10). Thus, e(y)2 and TAFII40 are stably associated in fraction 24. In contrast, when the anti-e(y)2 immunoprecipitation from fraction 24 was tested for the presence of other TFIID components by Western blot, antibodies raised against TBP, TAFII110 and TAFII230 gave negative results (data not shown), suggesting that after gel filtration the other TAFII-containing complexes and the e(y)2-TAFII40-containing complex are separated. Nevertheless, the e(y)2-TAFII40 interaction seems to be relatively stable since TAFII40 was still detected in anti-e(y)2 immunoprecipitations from the nuclear extract after the resin-bound proteins were washed in more stringent conditions (with IP buffer containing 1 M KCl) (Fig. 4D).
The e(y)2 protein is associated with chromatin.
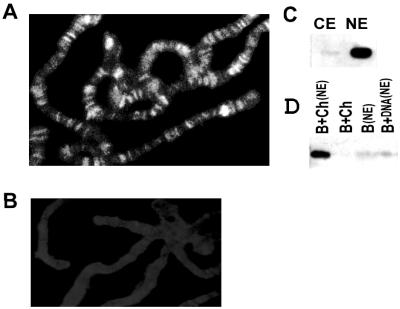
Considering the above-mentioned properties of e(y)2 protein, one might expect e(y)2 to be associated with chromosomes (37, 17). Antibody staining of Drosophila polytene chromosomes shows e(y)2 to be located in a large number of loci. Approximately 200 strong e(y)2-binding sites were detected on polytene chromosomes (Fig. 5A). The e(y)2 protein does not contain any known DNA-binding domain, suggesting that it binds DNA through the interaction with other proteins or multiprotein complexes.
FIG. 5.
e(y)2 is associated with chromatin. (A) Immunostaining of polytene chromosomes from Oregon R larvae with antibodies to e(y)2 and Cy3-conjugated secondary antibodies. Magnification, ×1,000. (B) Control immunostaining with preimmune serum and Cy3-conjugated secondary antibodies. (C) Western blot of cytoplasmic (CE) and nuclear (NE) extracts from Drosophila embryos probed with antibodies to e(y)2. (D) Binding of e(y)2 to chromatin immobilized on paramagnetic beads. Incubation of beads (B), chromatin (B+Ch), or naked DNA (B+DNA) with nuclear extract (NE), is as indicated above each lane.
To test whether e(y)2 can associate with chromatin, we compared its ability to bind chromatin or naked DNA. Chromatin was assembled by incubating DNA template containing the RNA polymerase II promoter of hsp26 minigene that was immobilized on paramagnetic beads for 6 h with a cytoplasmic chromatin assembly extract from 0- to 90-min preblastoderm embryos (32). The chromatin template did not contain e(y)2 since it was not detected in cytoplasmic extract used for nucleosome assembly (Fig. 5C). The purified immobilized chromatin was incubated for 30 min in nuclear in vitro transcription extract from 0- to 6-h embryos (34) and washed with 100 mM KCl. Following the incubation of the chromatin template with the nuclear extract, the e(y)2 protein was efficiently bound to chromatin (Fig. 5D). Note that the e(y)2 protein or the e(y)2-containing protein complexes had only a very low affinity for the naked DNA after incubation in nuclear extract [Fig. 5D, B+DNA(NE) and B(NE) as a control]. Thus, the e(y)2 protein or e(y)2-containing protein complexes are able to bind to chromatin templates in vitro.
The influence of e(y)2 on transcription in vitro.
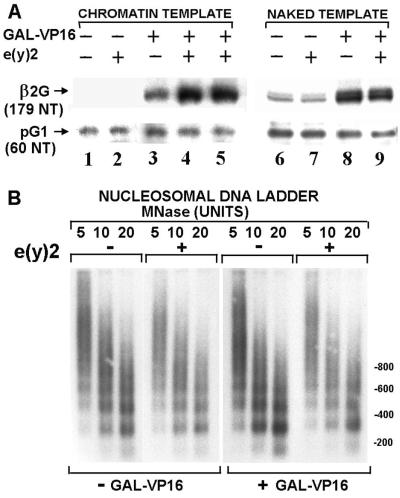
To investigate the function of e(y)2 at the molecular level, we studied the influence of the recombinant Drosophila protein on the GAL4-VP16 activated transcription in a cell-free system using chromatin templates. Chromatin was assembled on supercoiled (17M)5β2G template, containing five GAL4-binding sites upstream of the mouse retinoic acid receptor β2 core promoter linked to −9 to +1516 chicken β-globin gene sequences (12). The naked pG1 (35) template containing −109 to +1516 β-globin gene sequences was used as an internal control of basal transcription. Micrococcal nuclease digestion of (17M)5β2G chromatin templates demonstrates that the total chromatin structure (ethidium bromide staining [data not shown]) and nucleosome repeat length within the proximal promoter (−40 to +5) (Fig. 6B) were not affected by the presence of e(y)2. The e(y)2 protein had no effect on transcription in the absence of activator (Fig. 6A) or on a chromatin template lacking GAL4-binding sites (data not shown). However, we observed in the presence of GAL4-VP16 a moderate (four- to fivefold) but reproducible activation of transcription by e(y)2 protein on chromatin but not on naked cognate DNA templates. This result suggests that e(y)2 can potentiate transcriptional activation from chromatin templates.
FIG. 6.
e(y)2 activates transcription on chromatin template. (A) Transcription was performed on (17M)5β2G (β2G) chromatin or naked templates (200 pM) using a HeLa cell nuclear extract (100 μg) in the presence or absence of GAL4-VP16 (1 nM) and e(y)2 (5 nM [lanes 2, 4, 7, and 9] or 1 nM [lane 5]) in a final reaction volume of 50 μl. The ratio of added recombinant e(y)2 to endogenous e(y)2 was 10 in lanes 2, 4, 7, and 9 or 2 in lane 5. S1 nuclease analysis was performed after deproteinization (see Materials and Methods). (B) Analysis of the structure of chromatin reconstituted in the presence or absence of e(y)2 (5 nM) and GAL4-VP16 (1 nM). The template was digested with various concentrations of micrococcal nuclease in a final volume of 80 μl, separated on a 1.5% gel, and Southern blotted using a 32P-labeled probe corresponding to the region from positions −40 to +5 of the β2G proximal promoter. Hybridization with the probes corresponding to a region 5′ of the GAL4 binding sites gave a similar result (data not shown).
DISCUSSION
By combining biochemical and classical genetic approaches, we have characterized a new ubiquitously expressed, evolutionary conserved transcription factor, e(y)2. The e(y)2 gene was identified in a genetic screen designed to identify factors facilitating communication between enhancers and promoters (15). Genetic data have shown that it influences the expression of a wide range of genes, suggesting that the e(y)2 gene plays a important role in transcription (13–15, 26).
The e(y)2 mRNA is present at all stages of development. Furthermore, e(y)2 protein is present in all tissues and is associated with numerous sites along the entire length of the salivary gland polytene chromosomes, as could be expected for a factor playing role in transcription of vast spectrum of genes. Interestingly, approximately three times more sites were detected on polytene chromosomes with the anti-e(y)2 antibodies than with antibodies raised against dTAFII16 and dTAFII24 (17).
Homologues of the e(y)2 protein were detected in many higher eukaryotes from mammals to plants. The high degree of evolutionary conservation of the protein (100% conservation among mammals) suggests an important role for e(y)2 protein in cell metabolism. As was the case for Drosophila, the e(y)2 mRNA was detected in many different tissues and at different stages of development in humans, rats, and mice. Thus, e(y)2 homologues also appear to be ubiquitous proteins. Database searches did not reveal any known functional domains in e(y)2. While e(y)2 does not contain any sequence similarity to the proteins of HMG family, it does share several features, including small size and chromatin binding.
What is the function of e(y)2 protein? The genetic data obtained previously revealed the interaction between e(y)1/TAFII40 and e(y)2 genes. The e(y)21 and e(y)11 mutations have the same effect on white and yellow expression, and the combination of these mutations is lethal (14, 15). Interestingly, the lethality induced by combination of the e(y)21 and e(y)11 mutations cannot be suppressed by the high level of synthesis of the TAFII40 protein lacking its carboxy terminus. All other effects of e(y)11 mutation are suppressed by the latter. This suggests that the function of TAFII40 determined by its carboxy-terminal amino acids has a special relationship to the function of e(y)2 protein. The data obtained here and in a previous study (37) are the first indication for a functional role for the TAFII40 carboxy terminus.
Importantly, the genetic interaction data is confirmed by biochemical experiments, since e(y)2 and TAFII40 were found to interact in several distinct immunoprecipitation experiments either from a crude nuclear extract or from more purified fractions. Using gel filtration followed by immunoprecipitation, we showed that e(y)2 and TAFII40 proteins are associated and cofractionate as an entity with a large molecular mass (600 to 900 kDa). The e(y)2 and TAFII40 interaction in such an entity is relatively stable, surviving 1 M KCl treatment. Considering the size of the e(y)2-TAFII40-containing fractions (600 to 900 kDa), it is highly possible that e(y)2 and TAFII40 are associated with other proteins. However, e(y)2 and TAFII40 seem not to be associated with TFIID, since we could not coimmunoprecipitate with e(y)2 TBP and some TFIID-associated TAFIIs from the corresponding fraction after gel filtration. It should be pointed out that dTAFIIs are components of not only TFIID but also the recently described Drosophila TAFII-HAT (histone-acetyltransferase) complex (17). Thus, our experiments suggest that TAFII40 is a component of an unknown complex of 600 to 900 kDa, which also contains e(y)2.
If the anti-e(y)2 immunoprecipitation experiments are carried out with nonfractionated extracts, some TFIID components (i.e., TBP, dTAFII230, and dTAFII110) coimmunoprecipitate with e(y)2. This is in contrast to the absence of significant overlapping of the complexes containing e(y)2 and those of TBP and the above-mentioned TAFIIs upon gel filtration (Fig. 4B). A possible explanation for this is that, while the complex containing e(y)2 and TAFII40 is stable, the binding of e(y)2 to TFIID is loose and is destroyed during fractionation. If the filter shown in Fig. 4B is overexposed, the traces of e(y)2 are visible in many more fractions, some overlapping with TFIID (not shown). Thus, a continuous dissociation of the complex could occur during migration through the column. A putative loose association of e(y)2 with TFIID may explain why e(y)2 has not been noticed before and why the addition of recombinant e(y)2 enhances the activity of the extract containing endogenous e(y)2 (Fig. 6).
We observed that, in vitro, transcriptional activation by GAL4-VP16 was potentiated by e(y)2 on chromatin but not on naked DNA templates. Interestingly, there is no e(y)2 homologue in yeast, where regulation of transcription by high-order chromatin structure has not been well established. Perhaps e(y)2 regulates transcription directly through the action of the detected complex containing e(y)2 and TAFII40 or through a putative loose interaction of e(y)2 with TFIID. Alternatively, it is conceivable that the complex containing e(y)2 may play a role in chromatin remodeling (3, 6, 8).
ACKNOWLEDGMENTS
We are greatly indebted to T. Belenkaya for help with genetic analysis; to A. L. Greenleaf for the clone of the large subunit of RNA polymerase II; to R. Tjian, Y. Nakatani, K. Nightingale, and M. Brand for the gift of reagents and valuable advice; and to A. Kashirskii for making the colored photographs.
This work was supported by an International Research Scholar's award from the Howard Hughes Medical Institute to P.G., by EMBO fellowship ALTF517-1999 to A.S., and by INSERM, CNRS, the Hôpital Universitaire de Strasbourg, ARC, FRM, the Ligue Nationale contre le Cancer, and HFSP to L.T. The work of E.N. and S.G. was supported by a grant from the Centre for Medical Studies, University of Oslo, and by the grant 00-04-22000 from the Russian Foundation for Basic Research.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker P B, Tsukiyama T, Wu C. Chromatin assembly extracts from Drosophila embryos. Methods Cell Biol. 1994;44:207–223. doi: 10.1016/s0091-679x(08)60915-2. [DOI] [PubMed] [Google Scholar]
- 3.Bell B, Tora L. Regulation of gene expression by multiple forms of TFIID and other novel TAFII-containing complexes. Exp Cell Res. 1999;246:11–19. doi: 10.1006/excr.1998.4294. [DOI] [PubMed] [Google Scholar]
- 4.Bickel S, Pirrotta V. Self-association of the Drosophila zeste protein is responsible for transvection effects. EMBO J. 1990;9:2959–2967. doi: 10.1002/j.1460-2075.1990.tb07488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blank T A, Sandaltzopoulos R, Becker P B. Biochemical analysis of chromatin structure and function using Drosophila embryo extracts. Methods. 1997;12:28–35. doi: 10.1006/meth.1997.0444. [DOI] [PubMed] [Google Scholar]
- 6.Brand M, Yamamoto K, Staub A, Tora L. Identification of TATA-binding protein-free TAFII-containing complex subunits suggests a role in nucleosome acetylation and signal transduction. J Biol Chem. 1999;274:18285–18289. doi: 10.1074/jbc.274.26.18285. [DOI] [PubMed] [Google Scholar]
- 7.Brou C, Wu J, Ali S, Scheer E, Lang C, Davidson I, Chambon P, Tora L. Different TBP-associated factors are required for mediating the stimulation of transcription in vitro by the acidic transactivator GAL-VP16 and the two nonacidic activation functions of the estrogen receptor. Nucleic Acids Res. 1993;21:5–12. doi: 10.1093/nar/21.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown C E, Lechner I, Howe I, Workman J L. The many HATs of transcription coactivators. Trends Biochem Sci. 2000;25:15–19. doi: 10.1016/s0968-0004(99)01516-9. [DOI] [PubMed] [Google Scholar]
- 9.Burke T W, Kadonaga J T. Drosophila TFIID binds to a conserved downstream basal promoter element that is present in many TATA-box-deficient promoters. Genes Dev. 1996;10:711–724. doi: 10.1101/gad.10.6.711. [DOI] [PubMed] [Google Scholar]
- 10.Burley S K, Roeder R G. Biochemistry and structural biology of transcription factor IID (TFIID) Annu Rev Biochem. 1996;65:769–799. doi: 10.1146/annurev.bi.65.070196.004005. [DOI] [PubMed] [Google Scholar]
- 11.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dilworth F J, Fromental-Ramain C, Remboutsika E, Benecke A, Chambon P. Ligand-dependent activation of transcription in vitro by retinoic acid receptor alpha/retinoid X receptor alpha heterodimers that mimics transactivation by retinoids in vivo. Proc Natl Acad Sci USA. 1999;96:1995–2000. doi: 10.1073/pnas.96.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gause M, Georgieva S, Georgiev P. Phenotypic reversion of the gypsy-induced mutation scD1 of Drosophila melanogaster by replicative transposition of a sc enhancer to the yellow gene and by mutations in the enhancer of yellow and zeste loci. Mol Gen Genet. 1996;253:370–376. doi: 10.1007/pl00008603. [DOI] [PubMed] [Google Scholar]
- 14.Georgiev P G. Identification of mutations in three genes that interact with zeste in the control of white gene expression in Drosophila melanogaster. Genetics. 1994;138:733–739. doi: 10.1093/genetics/138.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgiev P G, Gerasimova T I. Novel genes influencing the expression of the yellow locus and mdg4 (gypsy) in Drosophila melanogaster. Mol Gen Genet. 1989;220:121–126. doi: 10.1007/BF00260865. [DOI] [PubMed] [Google Scholar]
- 16.Georgiev P G, Kiselev S L, Simonova O B, Gerasimova T I. A novel transposition system in Drosophila melanogaster depending on the Stalker mobile genetic element. EMBO J. 1990;9:2037–2044. doi: 10.1002/j.1460-2075.1990.tb07370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgieva S G, Kirschner D B, Jagla T, Nabirochkina E, Hanke S, Schenkel H, de Lorenzo C, Sinha P, Jagla K, Mechler B, Tora L. Two novel Drosophila TAFIIs have homology with human TAFII30 and are differentially regulated during development. Mol Cell Biol. 2000;20:1639–1648. doi: 10.1128/mcb.20.5.1639-1648.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodrich J A, Hoey T, Thut C J, Admon A, Tjian R. Drosophila TAFII40 interacts with both a VP16 activation domain and the basal transcription factor TFIIB. Cell. 1993;75:519–530. doi: 10.1016/0092-8674(93)90386-5. [DOI] [PubMed] [Google Scholar]
- 19.Jacq X, Brou C, Lutz Y, Davidson I, Chambon P, Tora L. Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell. 1994;79:107–117. doi: 10.1016/0092-8674(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 20.Jokerst R S, Weeks J R, Zehring W A, Greenleaf A L. Analysis of the gene encoding the largest subunit of RNA polymerase II in Drosophila. Mol Gen Genet. 1989;215:266–275. doi: 10.1007/BF00339727. [DOI] [PubMed] [Google Scholar]
- 21.Kadonaga J T. Eukaryotic transcription: an interlaced network of transcription factors and chromatin-modifying machines. Cell. 1998;92:307–313. doi: 10.1016/s0092-8674(00)80924-1. [DOI] [PubMed] [Google Scholar]
- 22.Karess R E, Rubin G M. Analysis of P transposable element functions in Drosophila. Cell. 1984;38:135–46. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- 23.Klemm R D, Goodrich J A, Zhou S, Tjian R. Molecular cloning and expression of the 32-kDa subunit of human TFIID reveals interactions with VP16 and TFIIB that mediate transcriptional activation. Proc Natl Acad Sci USA. 1995;92:5788–5792. doi: 10.1073/pnas.92.13.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsley D L, Zimm G G. The genome of Drosophila melanogaster. New York, N.Y: Academic Press; 1992. [Google Scholar]
- 25.Lu H, Levine A J. Human TAFII31 protein is a transcriptional coactivator of the p53 protein. Proc Natl Acad Sci USA. 1995;92:5154–5158. doi: 10.1073/pnas.92.11.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melnikova L, Kulikov A, Georgiev P. Interactions between cut wing mutations and mutations in zeste, and the enhancer of yellow and Polycomb group genes of Drosophila melanogaster. Mol Gen Genet. 1996;252:230–236. doi: 10.1007/BF02173768. [DOI] [PubMed] [Google Scholar]
- 27.Nightingale K P, Wellinger R E, Sogo J M, Becker P B. Histone acetylation facilitates RNA polymerase II transcription of the Drosophila hsp26 gene in chromatin. EMBO J. 1998;17:2865–2876. doi: 10.1093/emboj/17.10.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pazin M J, Kamakaka R T, Kadonaga J T. ATP-dependent nucleosome reconfiguration and transcriptional activation from preassembled chromatin templates. Science. 1994;266:2007–2011. doi: 10.1126/science.7801129. [DOI] [PubMed] [Google Scholar]
- 29.Pirrotta V, Manet E, Hardon E, Bickel S, Benson M. Structure and sequence of the Drosophila zeste gene. EMBO J. 1987;6:791–799. doi: 10.1002/j.1460-2075.1987.tb04821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roeder R G. The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem Sci. 1996;21:327–335. [PubMed] [Google Scholar]
- 31.Rubin G M, Spradling A C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 32.Sandaltzopoulos R, Blank T, Becker P B. Transcriptional repression by nucleosomes but not H1 in reconstituted preblastoderm Drosophila chromatin. EMBO J. 1994;13:373–379. doi: 10.1002/j.1460-2075.1994.tb06271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandaltzopoulos R, Mitchelmore C, Bonte E, Wall G, Becker P B. Dual regulation of the Drosophila hsp26 promoter in vitro. Nucleic Acids Res. 1995;23:2479–2487. doi: 10.1093/nar/23.13.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandaltzopoulos R, Quivy J P, Becker P B. Analysis of protein/DNA interactions by solid-phase footprinting. Methods Mol Cell Biol. 1995;5:176–181. [Google Scholar]
- 35.Sassone-Corsi P, Duboule D, Chambon P. Viral enhancer activity in teratocarcinoma cells. Cold Spring Harbor Symp Quant Biol. 1985;50:747–752. doi: 10.1101/sqb.1985.050.01.092. [DOI] [PubMed] [Google Scholar]
- 36.Smith R F, Smith T F. Pattern-induced multi-sequence alignment (PIMA) algorithm employing secondary structure-dependent gap penalties for use in comparative protein modelling. Protein Eng. 1992;5:35–41. doi: 10.1093/protein/5.1.35. [DOI] [PubMed] [Google Scholar]
- 37.Soldatov A, Nabirochkina E, Georgieva S, Belenkaja T, Georgiev P. TAFII40 protein is encoded by the e(y)1 gene: biological consequences of mutations. Mol Cell Biol. 1999;19:3769–3778. doi: 10.1128/mcb.19.5.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spradling A C, Rubin G M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- 39.Tansey W P, Herr W. TAFs: guilt by association? Cell. 1997;88:729–732. doi: 10.1016/s0092-8674(00)81916-9. [DOI] [PubMed] [Google Scholar]
- 40.Tjian R, Maniatis T. Transcriptional activation: a complex puzzle with few easy pieces. Cell. 1994;77:5–8. doi: 10.1016/0092-8674(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 41.Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989;59:477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- 42.Verrijzer C P, Tjian R. TAFs mediate transcriptional activation and promoter selectivity. Trends Biochem Sci. 1996;21:338–342. [PubMed] [Google Scholar]
- 43.Voelker R A, Wisely G B, Huang S M, Gyurkovics H. Genetic and molecular variation in the RpII215 region of Drosophila melanogaster. Mol Gen Genet. 1985;201:437–445. [Google Scholar]