Highlights
-
•
Standard procedures and appropriate assessment of exercise are proposed for the commonly used animal models related to chronic exercise (e.g., treadmill running, voluntary wheel running, swimming exercise, and resistance exercise) in cardiovascular research.
-
•
Optimal design of animal exercise studies in cardiovascular research should consider the choice of exercise models, control of exercise protocols, exercise at different stages of disease, and other factors, such as age, sex, and genetic background.
-
•
An optimal design for studying exercise-induced physiological cardiac growth and its related beneficial effects against cardiovascular diseases is presented.
Keywords: Animal studies, Cardiovascular disease, Cardiovascular research, Exercise, Exercise models
Abstract
Growing evidence has demonstrated exercise as an effective way to promote cardiovascular health and protect against cardiovascular diseases However, the underlying mechanisms of the beneficial effects of exercise have yet to be elucidated. Animal exercise studies are widely used to investigate the key mechanisms of exercise-induced cardiovascular protection. However, standardized procedures and well-established evaluation indicators for animal exercise models are needed to guide researchers in carrying out effective, high-quality animal studies using exercise to prevent and treat cardiovascular diseases. In our review, we present the commonly used animal exercise models in cardiovascular research and propose a set of standard procedures for exercise training, emphasizing the appropriate measurements and analysis in these chronic exercise models. We also provide recommendations for optimal design of animal exercise studies in cardiovascular research, including the choice of exercise models, control of exercise protocols, exercise at different stages of disease, and other considerations, such as age, sex, and genetic background. We hope that this position paper will promote basic research on exercise-induced cardiovascular protection and pave the way for successful translation of exercise studies from bench to bedside in the prevention and treatment of cardiovascular diseases.
Graphical abstract
1. Introduction
Exercise has long been proved to be an effective way to promote cardiovascular health and protect against cardiovascular diseases (CVDs). Regular exercise reduces the risk of CVDs and improves cardiac function in patients.1 Exercise capacity and cardiorespiratory fitness are also powerful predictors of CVD events and all-cause mortality in patients and in healthy populations.2 There is solid evidence demonstrating the beneficial effects of exercise on hypertension, coronary heart diseases, and heart failure.3, 4, 5 Properly designed exercise training has become a well-established intervention for patients with heart failure.6,7 Indeed, there has been increasing interest in and investigations focusing on the vital mechanisms of exercise in protecting the cardiovascular system.
The benefits of exercise are multifaceted.8 Exercise have direct effects on the cardiovascular system, leading to physiological cardiac growth (cardiomyocyte hypertrophy and proliferation) and adaptive angiogenesis.9, 10, 11 Exercise can also regulate cardiac excitation/contraction coupling and mitochondrial energy metabolism and can attenuate cardiac myocyte death and fibrotic process in response to pathological stimulus.12,13 Besides the cardiovascular system, the benefits of exercise are also associated with neurohumoral regulation and systemic organ communications.14, 15, 16 These investigations not only improve our understanding of the mechanisms of exercise but also help to identify novel therapies that can mimic the benefits of exercise for patients with CVDs.17
2. Purpose and current status of animal exercise studies
2.1. Purpose of animal exercise studies
Clinical exercise prescription aims to realize an objective scientific exercise-training program for patients.18 These programs may vary in exercise modality, intensity, frequency, and duration according to the differing physical conditions and disease states of patients. Despite increasing evidence demonstrating the benefits of regular and chronic exercise in the prevention and treatment of CVDs, the underlying mechanisms have yet to be elucidated.8
Preclinical animal studies are useful and practical for establishing programmed exercise training and for examining the effects of exercise interventions in various experimental cardiac-injury models. Aerobic exercise training (e.g., treadmill running, voluntary wheel running, and swimming) and resistance training (e.g., squat-training, ladder climbing) have most frequently been adopted in rodent studies19 and have demonstrated that exercise training is capable of protecting the heart from a variety of cardiac injuries, such as myocardial infarction, cardiac ischemia–reperfusion injury, diabetic cardiomyopathy, atherosclerosis, cardiac fibrosis, and heart failure.20, 21, 22, 23, 24, 25 These positive findings have encouraged more and more scientists to focus their research on this field.
2.2. Current status of animal exercise studies
Exercise can be generally categorized as dynamic exercise or static exercise. Dynamic exercise (e.g., treadmill running, voluntary wheel running, and swimming), commonly applied in experimental animal studies, has multiple advantages, such as enhancing the capacity of oxygen transport in the cardiovascular system and muscles, improving hemodynamics and exercise capacity, and regulating autonomic functions. Indeed, dynamic exercise is a useful strategy for reducing cardiovascular risk factors and adverse events.26 Static exercise (e.g., resistance training) helps to increase muscle mass, enhance muscle strength and contractility, and improve basal metabolic rate.25 Thus, different animal exercise models lead to different systemic and cardiac adaptations and should be carefully chosen according to the targeted organs and functions studied in CVDs.
Different exercise intensity, frequency, and duration can substantially influence the systemic effects of exercise as well as its benefits to the heart.19,27 Long-term moderate-intensity aerobic exercise or interval high-intensity aerobic exercise are the most commonly used exercise modes for studying the cellular and molecular mechanisms of exercise-induced cardiac protection.28,29 Short-term high-intensity or strenuous exercise is a good way to assess cardiopulmonary fitness, exercise capacity, and rapid metabolic changes in cardiac and skeletal muscles derived from exercise.30 However, to achieve an effective exercise protocol, exercise validity and efficacy need to be proved. One example of exercise validity would be assessing physiological cardiac growth when studying exercise-induced cardiac hypertrophy.21 For exercise efficacy, it is important to both unveil its functional protection and to identify its underlying cellular and molecular mechanisms in CVDs.8 Comparisons of different modes of exercise in preventing and treating CVDs also help translate results from basic research to clinical exercise prescriptions.31,32
Because researchers from different groups may use their own conventional exercise protocols and evaluation standards in their animal exercise studies, it is sometimes difficult to reproduce and compare the results from different groups. For example, in addition to running speed, running time, running frequency, and running duration, the inclination of treadmill lanes is also a critical parameter that influences animal-exercise performance and muscle strength after treadmill running.33 However, measurements of maximal oxygen uptake (VO2max), time to exhaustion from exercise (endurance capacity), and grip strength help to compare equitably the results obtained from various laboratories.34,35
Under these circumstances, it is necessary to obtain expert opinions and make scientific statements about the standardization and optimization of animal exercise studies in cardiovascular research. Guidelines for animal exercise and training protocols for cardiovascular studies, published in the American Journal of Physiology—Heart and Circulatory Physiology, presents the most commonly used animal exercise models, ranging from small animals (rats and mice) to large animals (horses, pigs, dogs, and rabbits).36 The guidelines provide excellent and very useful steps that researchers can take to perform and control the quality of their animal exercise studies, especially when standardizing procedures and applying rigorous quality controls to animal-exercise models with the intention of analyzing kinetic parameters and reproducing tests of physiological functions affected by exercise.36 In our statement, we provide rules for best practices and for optimal selection, design, and control of animal-exercise studies, especially insofar as these studies relate to chronic exercise used for exercise-induced cardioprotection. Our statement also summarizes and identifies useful evaluation indicators for cardiovascular responses to different types of exercise. Standardized procedures and well-established evaluation indicators for animal exercise models are greatly needed to help scientific researchers to carry out effective and efficient animal exercise studies that can be used for developing prevention and treatment techniques for CVDs.
3. Animal-exercise models used in cardiovascular research
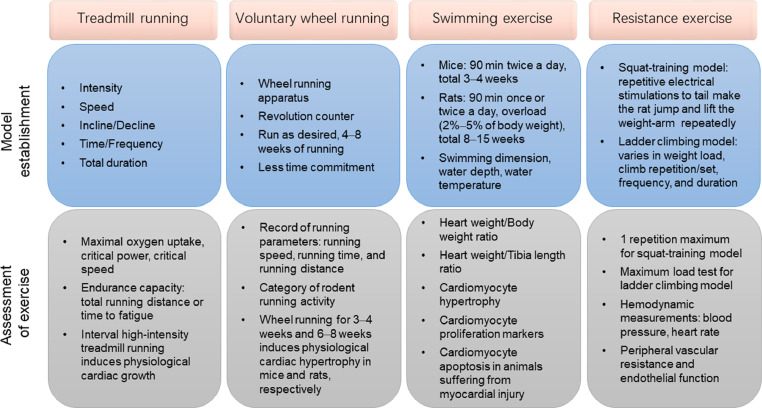
Rodent exercise training models are the most commonly used animal models in studying the effects of exercise on CVDs. However, large-animal exercise models are more practical for providing translational research of exercise interventions because the cardiovascular systems of large animals have more similarities to the human cardiovascular system. Below, we propose standard procedures for exercise training and identify appropriate evaluation measurements and methods of analysis in commonly used animal models employing chronic exercise, including treadmill running, voluntary wheel running, swimming exercise, and resistance exercise, in cardiovascular research (Fig. 1).
Fig. 1.
Animal exercise models commonly used in cardiovascular research. Model establishment and assessment of exercise for treadmill running, voluntary wheel running, swimming exercise, and resistance exercise.
3.1. Treadmill running
3.1.1. Model establishment
Treadmill running is among the most commonly used exercise modalities used in rodents for studying the effects of endurance running on skeletal muscles and the cardiovascular system.37,38 Although treadmill-running protocols vary among investigation groups, they can be categorized basically as either moderate-intensity continuous running or interval high-intensity running.39,40 The categorization of exercise intensity is generally dependent on the VO2max, which represents the greatest possible amount of oxygen supply during exercise.35 Generally, exercise in which 50%−70% of VO2max is reached is defined as moderate-intensity exercise, and exercise in which 85%−90% of VO2max is reached is defined as high-intensity exercise.40, 41, 42 However, for those individuals or animals who do not attain a VO2 plateau during exercise, the highest VO2 attained is termed peak oxygen uptake (VO2peak). Increasing evidence has demonstrated that critical power (CP) and critical speed (CS) are more sensitive functional parameters of exercise performance than VO2max.43 The hyperbolic power/speed−duration curve is constructed by the subject performing exercise at a constant power or speed to the point of exhaustion.44,45 The power asymptote is known as CP (or CS when exercise intensity is measured by speed), which is a “fatigue threshold” that distinguishes exercise performance with a sustainable physiological response to exercise (<CP) from the development of fatigue during exercise (>CP).45 For example, rats that run below CS have sustained exercise performance, while those that run above CS have significantly disproportionate blood flow, especially in highly glycolytic muscle fibers.46 CS can also be determined in mouse treadmill running, which is estimated to be ∼75% of VO2max.47
The optimal protocols for measuring VO2max of rats and mice during treadmill running in a metabolic chamber can be applied by warm-up running for 15 min at 40%–50% of VO2max before the VO2max protocol, which is then increased by 0.03 m/s every 2 min in settled slopes.41,42 Intensity-controlled treadmill running in rats and mice indicates that the inclination of the treadmill significantly affects VO2max. For example, an incline of 25° yields the highest VO2max in rats and the best cardiovascular load in mice.41,42 Researchers should determine the inclination of treadmill in association with running speed. For moderate-intensity continuous treadmill running, rodents should run at an incline of 0°−30°.38,48, 49, 50, 51, 52 For interval high-intensity treadmill running, an incline of 25° has proved to be efficient for inducing cardiovascular responses.41,42
Moderate-intensity continuous treadmill running (e.g., for adaptive training of mice) should begin with 5 m/min and 10 min of running on the first day and increase progressively by 2 m/min and 10 min/day until the running speed reaches 10−20 m/min with a duration of 30−120 min/day.38,52,53 The daily running duration (30−120 min), running frequency (once or twice per day), and total running duration (weeks to months) vary depending on disease models, functions investigated, and scientific questions posed. Along with an increase in animal exercise capacity, running speed should be progressively elevated weekly.54,55 Although various treadmill-running protocols have been reported in the literature, investigators should always make the running behaviors of mice or rats consistent by using bottle brushes, puffs of air, or electrical shocks. Animals that fail to learn proper running protocols on the treadmill should be excluded from the study.
Compared to moderate-intensity continuous treadmill running, interval high-intensity treadmill running requires the rodents to have adaptive training to ensure that they can run at 50%−60% of VO2max for at least 30 min. After their adaptation to this running period, mice or rats should have a daily warm-up training session for 10−20 min at 50%−60% of VO2max, followed by 5−8 intervals alternating between 4−8 min of high-intensity running (at 85%−90% of VO2max) and 2−3 min of recovery running (at 50%−60% of VO2max).40, 41, 42 Functionally, aerobic interval high-intensity treadmill running has been shown to increase cardiomyocyte contractility and calcium sensitivity.40,56 Interval high-intensity treadmill running post myocardial infarction is protective for attenuating cardiac remodeling and improving cardiac function.57
3.1.2. Assessment of exercise
Intensity-controlled treadmill running provides reliable and well-controlled endurance training when a VO2max measurement is used.41,42 With treadmill running, VO2max progressively increases when measured at the start of every week. Indeed, the workload (usually adjusted by running speed) is increased accordingly throughout the running period. It has been reported that at low to moderate exercise intensity, there is a close correlation between running speed and VO2max.58 However, this correlation is not accurate enough to control the exercise intensity strictly because the correlation is not present within a range of 85%−90% of VO2max.58 Thus, accurate control of exercise intensity should be ensured by adjusting the running speed on the basis of serial measurements of VO2max.41,42
Endurance exercise can significantly increase VO2max as well as endurance capacity.59 Endurance capacity in treadmill training is determined by the total running distance or the time to fatigue when mice or rats are exhausted and cannot run any farther or fail to keep pace with the treadmill.60 For mice or rats finishing a total duration of moderate-intensity continuous running training and after adapting to the running environment, they should begin running at the same speed as usual (e.g., 15 m/min), which should be increased progressively (e.g., by 1 m/min every 4 min). After the mice or rats are exhausted and cannot keep running despite being pushed, the total running distance or exercise time is determined in order to evaluate endurance capacity.61 The exhaustion of rats can be confirmed by placing the rats on their backs and determining that the rats cannot turn over immediately.60 The exhaustion of mice can be determined when a mouse fails to get back on the treadmill after 3 consecutive attempts to continue running.62
Because mice and rats are nocturnal animals, running training during their dark cycle is more effective. Measurements of VO2max and endurance capacity should also be taken at a fixed time to ensure the reproducibility and reliability of data. For example, rat endurance capacity can be influenced by liver and skeletal muscle glycogen concentrations that may fluctuate during a day.63,64 Moreover, the running environment should not be noisy, and the room temperature should be kept between 20°C and 22°C.
The reported effects of continuous treadmill running on physiological cardiac hypertrophy have been controversial,39,51,65,66 and they may be related to different strains of rodents used as well as the exercise intensity employed in exercise protocols.48,67,68 For example, C57BL/6J mice showed no significant increase in exercise capacity or cardiac growth after moderate-intensity treadmill running, whereas FVB/NJ mice developed obvious cardiac growth using this protocol.49 However, interval high-intensity treadmill running in rats and mice controlled by VO2max measurements has been shown to be effective in increasing ventricular weight.41,42 In addition to promoting peripheral vascular effects and systemic metabolic regulation, treadmill running also brings about improvements in cardiac contractility and myofilament calcium sensitivity,69,70 reduces mitochondrial reactive oxygen species production and mitochondrial permeability transition pore opening,71, 72, 73 attenuates endoplasmic reticulum stress,74,75 and inhibits cellular apoptosis and aging signaling in the heart.76
3.2. Voluntary wheel running
3.2.1. Model establishment
The wheel-running model is a voluntary running exercise applicable to both rats and mice.77, 78, 79 The wheel-running apparatus for each animal is set in a single cage that is connected to a transducer and revolution counter for calculation and record of running parameters. Because animals are allowed to run at the speed, duration, and frequency they desire, voluntary wheel running requires less investigator time commitment, which is particularly applicable for long-term studies, including those on aging.80 As nocturnal animals, rodents usually run during their dark cycle. Indeed, wheel running is good for rodents in that they run voluntarily during their active times each day, and it is important for investigators to facilitate the light–dark cycle of rodents. An unexpected light exposure will alter their voluntary running performance. The investigator should also check the record of running activity and ensure that all running wheels and revolution counters work well. The hygiene of animals using running wheels and the health status of these animals, including paw or toenail injuries, should be carefully checked.
3.2.2. Assessment of exercise
It is essential to record the running parameters of animals every day, including running speed, running time, and running distance.81 Rodents should be adapted to the running wheel for 1−2 days before initiating a record of running parameters. Increasing evidence has demonstrated that the running distance usually peaks at ∼4 weeks of voluntary wheel running and will remain stable or slightly decline thereafter.81 The record of running parameters also helps to categorize rodents’ running activity. Variations have been found in the voluntary wheel running of adult rats; these variations have been categorized as low activity (2−5 km/day), medium activity (>5–≤11 km/day), and high activity (>11 km/day).82 It has been reported that adult male C57/BL6 mice run an average distance of 6.8 km/day (3.1–16.2 km/day).83 Animals that are not willing to run on a voluntary wheel (e.g., C57/BL6 mice that run <3.1 km/day) could be excluded from the study. Although the frequency of exclusions of rodents from studies has not yet been fully determined, increasing the number of animals used for voluntary wheel running in a study helps to reduce the impact of exclusions.84
Although it is not practical to measure exercise capacity in voluntary wheel-running rodents, growing evidence has shown that voluntary wheel running can significantly increase VO2max in these rodents, although to a much less extent than treadmill running.81 Unlike the intensity-dependent effects of treadmill running on physiological cardiac hypertrophy, voluntary wheel running for 3−4 weeks in mice83,85,86 and 6−8 weeks in rats87, 88, 89, 90 can induce physiological cardiac hypertrophy. Voluntary wheel running-induced physiological cardiac hypertrophy shows different transcriptomic and metabolomic profiles compared to pathological hypertrophy.91 Voluntary wheel running for 4−8 weeks can also reduce myocardial infarction and cardiac ischemia/reperfusion injury, which have been found to be related to endothelial nitric oxide synthase expression and β3-adrenergic receptor activation.78,79
3.3. Swimming exercise
3.3.1. Model establishment
Swimming training of rodents is a commonly used exercise model for studying the physiological adaptations of skeletal muscles and the cardiovascular system to exercise, including physiological cardiac growth.92,93 Mice that received a programmed swimming-exercise protocol starting with 10-min sessions twice a day and increasing to 90-min sessions twice a day, and then maintaining 90-min sessions twice a day, 5−7 days/week for 3−4 weeks, had increased heart weight and developed physiological cardiac hypertrophy.94,95 It is important that investigators respect a standard protocol in order to observe significant physiological cardiac growth after swimming exercise.
Although the swimming apparatus is inexpensive, it is important to ensure that the swimming dimensions, water depth, and water temperature remain constant.96 For a swimming tank or bucket with a diameter of 65–80 cm, a total of 8–10 mice should swim together because mice tend to swim more vigorously when grouped together. However, it is crucial to avoid “gang swimming”, which causes animals to push one another under the water. The water depth should be set to >10–12 cm, and the water temperature should be set to 30°C−32°C. This helps to ensure an efficient swimming exercise and prevents mice from diving to the bottom or changing swimming speed because of lower temperatures.97 A thermometer should be kept in the water during the whole course of swimming, and warm water should be added carefully into the bucket to maintain the water temperature at 30°C−32°C. Additionally, the swim buckets can be placed on large heat pads to help maintain a constant temperature. Mice should have a 5-min swimming adaptation session on the day before the formal exercise program begins. A protocol that begins with 10 min of swimming twice a day and increases by 10 min each day until two 90-min sessions each day are reached is important for training mice to be proficient swimmers. Although various mouse strains have been used in swimming-exercise models,98, 99, 100 the ages and the initial body weights of mice should be randomized so that they are identical in the sedentary and swimming groups. C57BL/6 mice that are 8−10 weeks old and weigh 24−25 g are suitable for beginning a swimming-exercise program.95 When the mice are swimming, investigators should be vigilant regarding their swimming behavior and use a bottle brush when it is necessary to keep them swimming. Some mice may also need a little rest to avoid any adverse animal events. They can be picked up for 30 s or so and rest on the palm of the researcher's hand before being placed back in the water. Keeping notes on mice that continually float or need rest is also important because cardiac hypertrophy at the endpoint may not be expected for those mice. After their swimming exercise, mice need to be dried with a towel and ventilated in a warm place. Any environment that is either too hot or too cold will cause unexpected illness in the animals. Mice should rest at least 4 h between the 2 daily swimming sessions. Mice that are subjected to an exhaustive swimming exercise program should swim with an overload of 5% of body weight. Exhaustion can be confirmed when a mouse fails to swim to the surface of the water within 7 s.101
Compared to mice, rats used in swimming exercises produce and accumulate more air bubbles in their fur, which results in more floating or bobbing behaviors instead of actual swimming. Thus, rats usually need an overload (2%−5% of body weight) that is effected by attaching a balance weight to the chest or about 5 cm from the end of the tail during swimming.102 Exhaustion can be defined as rats’ being unable to swim to the surface for breathing within 10 s.102, 103, 104 The swimming dimension should be 1000−1500 cm2 per rat, and the water depth should be greater than the length of the rat from nose to tail.105,106 A swimming tank with a diameter of 65−80 cm is suitable only for 2 rats swimming at the same time. It is also important that rats have a 5-min swimming adaptation session on the day before the formal exercise program begins. Then rats begin with 10-min swimming on the first day of the formal exercise program, and an overload will be added on the third day of swimming. Swimming duration should increase by 10 min/day until a total of 60−90 min is attained (once or twice a day). The total swimming duration can vary from 8 to 15 weeks, which is much longer than the mouse swimming protocol.107, 108, 109, 110
3.3.2. Assessment of exercise
After the swimming exercise, the heart weight, heart weight body weight ratio (HW/BW), and heart weight to tibia length ratio (HW/TL) should be determined.111 Both the HW/BW ratio and HW/TL ratio are commonly used to determine exercise-induced cardiac growth, although the increase in the HW/BW ratio may be overestimated due to the change in body weight after exercise. For swimming-trained mice and rats, an increase of about 12%−15% or more in HW/BW ratio (or HW/TL ratio) can be considered to be effective physiological cardiac growth due to exercise.21,98,112,113 According to the literature, there is no significant difference in the the degree of cardiac hypertrophy after swimming exercise in rats and mice.92,98,108–110,113, 114, 115, 116, 117 Heart rate can be reduced or remain unchanged, but blood pressure is not altered in swimming-exercised rodents.98,114, 115, 116, 117 It is also recommended that citrate synthase activity in mixed skeletal muscles be measured, including the tibialis anterior and gastrocnemius.98,112 A similar increase in citrate synthase activity indicates that there is no difference in exercise capacity between differently exercised groups.98,112,118
Multiple factors, such as genetic modification of animals, disease conditions, and exercise at differing stages of disease, can influence the effects of exercise, so it is not practical to set uniform, predetermined exclusion criteria for exercise studies. However, animals that exhibit certain exercise behaviors indicating that they cannot adapt to the swimming environment, including drowning, continually floating, or losing more than 15% of their body weight, can be excluded from the analysis.
Histological analysis of the cross-sectional area of the cardiomyocytes should be conducted to confirm the hypertrophic growth of the heart.95 The pathological hypertrophy-marker genes, atrial natriuretic peptide and brain natriuretic peptide, do not increase with exercise training.21 In addition to physiological hypertrophy, swimming training can promote cardiomyocyte proliferation and renewal.119 Cardiac cell proliferation and renewal have been reported to be necessary to mediate the protective effect of swimming training in the murine model of cardiac ischemia-reperfusion injury.120 Interestingly, some of the best-characterized pathways in physiological cardiac growth (e.g., insulin-like growth factor 1/phosphoinositide 3-kinase/protein kinase B (IGF1/PI3K/AKT), downregulated CCAAT/enhancer binding protein β (C/EBPβ) and upregulated Cbp/p300-interacting transactivator with ED-rich carboxy-terminal domain-4 (CITED4), and non-coding RNAs, such as microRNA-222 (miR-222) and miR-17-3p), also show beneficial effects against pathological cardiac remodeling.21,92,95,119 The mechanisms underlying the cardioprotective effects of swimming exercise could be related to the improved survival (pro-proliferative and anti-apoptotic effects) of cardiomyocytes, cardiac electrical and contraction functions, myocardial glycolipid metabolism, and mitochondrial adaptations.8
3.4. Resistance exercise
3.4.1. Model establishment
Compared with aerobic exercise, resistance exercise has been much less studied in cardiovascular adaptations and diseases. The most commonly used methods of resistance training in animal models include squat-training and ladder climbing. Squat-training requires a specially designed apparatus first described by Tamaki et al.121 Rats wear a canvas jacket that is connected to a wooden bar with a loading weight on it. When the rat tail is stressed by an electrical stimulation (20 V, 0.3-s duration, at 3-s intervals), the rat jumps and lifts the weight-arm of the apparatus repeatedly.122 Rats should be preconditioned to this apparatus for about 2 weeks. The squat-training requires 12 repetitive stimulations per set, with 90 s of rest after each set. The sets should be administered 4 times/day and last for 4 weeks (5 days/week).122 The weight loads should be regulated to 65%−75% of 1 repetition maximum (1 RM; for details, see “3.4.2 Assessment of exercise”).122 This model was first used to analyze skeletal muscle adaptations and metabolic changes in response to resistance training123,124 and was later used to analyze physiological cardiac hypertrophy and cardiac diseases.25,55,125
Compared with the special apparatus required for squat-training, the equipment used for ladder climbing or grille climbing is less complicated. For ladder-climbing exercise, the ladder is about 1 m in length with an 80°−85° incline, and the interval between steps is generally 0.5−1.0 cm. The animals need to be adapted to the climb for 1 week, with gradually increasing loads (usually beginning with 10%−20% of body weight). However, climb repetitions (8−15 repetitions per set), number of climb sets (e.g., 4 sets/day), frequency of climbs (3−5 days/week), and duration of climbs (from weeks to months) can vary depending on the type of research being conducted. Likewise, the weight load attached to the tail can be maintained at 40%−60% of the maximum load55 or can be gradually increased to 90%−100% of the maximum load after each climb set.126 Because of the differing parameters used in ladder climbing, the data reported by different groups are difficult to compare.
3.4.2. Assessment of exercise
The weight loads added to the animals should be regularly controlled and adjusted for both squat-training and ladder climbing. For squat-training, 1 RM is defined as the maximum weight lifted when the animal is able to jump in response to an electronic stimulation.121 The 1 RM should be measured every 2 weeks; this serves as a basis for controlling the weight loads (usually set at 65%−75% of 1 RM) and represents an important index for characterizing training load.122 For ladder climbing, the maximum load test needs to be conducted at the start, during (every 45 days), and after the completion of resistance training.127 After adapting to the climbing activity for 5 consecutive days, rats or mice should climb the ladder with an initial load of 75% of body weight, which is increased by 15% of body weight after finishing each climb.128 The weight load when the animal cannot climb the entire ladder after 3 successive stimulations to the tail is defined as the maximum load.55
Hemodynamic measurements after resistance exercise training should include the arterial systolic and diastolic blood pressure and heart rate.129 Resistance training can reduce blood pressure (diastolic pressure and mean arterial pressure) and peripheral vascular resistance and improve endothelial function in hypertensive and diabetic rats.129, 130, 131 The squat-training (load of 65%–75% of 1 RM, for 4 weeks) can induce a slight increase in the left ventricle weight.122 However, the effect of climbing-exercise training on cardiac hypertrophy has been much less studied.132 Nevertheless, resistance exercise has been found to be beneficial in preventing and treating hypertension, myocardial infarction, ischemia reperfusion injury, heart failure, and diabetic cardiomyopathy.72,133,134
4. Optimal design of animal-exercise studies in cardiovascular research
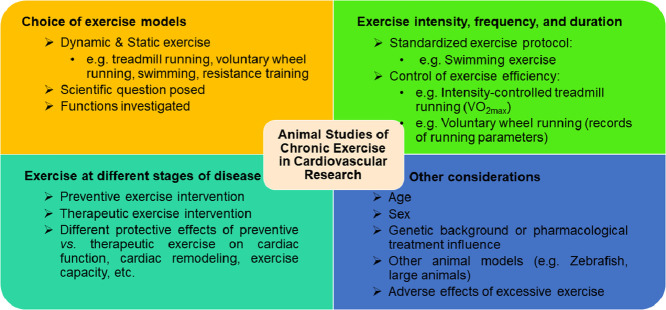
Various exercise models have been used in studies of cardiovascular physiology and CVDs. For optimal design of animal-exercise studies in cardiovascular research, we provide consensus recommendations concerning the choice of exercise models, the control of exercise protocols, exercise at different stages of disease, and other considerations that should be marked. An instructive outline of the optimal design of animal-exercise studies in cardiovascular research, with an example of studying exercise-induced physiological cardiac growth and its related beneficial effects against CVDs, is presented in Fig. 2.
Fig. 2.
Optimal design of animal studies in cardiovascular research. CVD = cardiovascular disease; I/R = ischemia-reperfusion; VO2max = maximal oxygen uptake.
4.1. Choice of exercise models
Dynamic exercise, especially treadmill running, is the most commonly used exercise model in the study of CVDs. Treadmill running, conducted either before or after the onset of CVDs, has been shown to have protective effects in various CVDs, such as myocardial infarction, ischemia–reperfusion injury, atherosclerosis, hypertension, diabetic cardiomyopathy, and heart failure.19 In addition, swimming training protects mice from pathological remodeling and signs of heart failure in response to aortic-banding, myocardial infarction, and ischemia–reperfusion injury.21,112,120 Numerous studies have also reported the beneficial effects of exercise in CVDs using other exercise models, such as voluntary wheel running and resistance training.122,135
A primary issue in current exercise studies in cardiovascular research involves choosing an exercise model. The choice of exercise models can be associated with multiple important factors, including the scientific question posed and the functions investigated. One representative example of this is exercise-induced physiological cardiac growth, which is characterized by a physiological process without cardiac fibrosis and cardiac dysfunction.98,136 Understanding whether and how exercise-induced physiological cardiac growth can protect the heart may provide new opportunities for identifying novel therapeutic targets for CVDs.137 Using swimming training and voluntary wheel-running exercise models, scientists have proved that such aerobic dynamic training can induce both cardiomyocyte hypertrophy and proliferation.21,119 Although interval high-intensity treadmill running has been shown to induce physiological cardiac growth,41,42 moderate-intensity continuous treadmill running or resistance training are not very efficient exercise models for inducing cardiac growth and cardiomyocyte proliferation.39,65,66 Protocols and effects of different types of exercise on cardiac physiological hypertrophy and other cardiovascular responses are listed in Supplementary Tables 1 and 2.
4.2. Exercise intensity, frequency, and duration
Exercise intensity, frequency, and duration are essential parameters that should be seriously considered and decided upon before animal studies start. Some exercise models require strict control of exercise parameters. For example, mice that swim for 90 min twice a day, 5−7 days/week, for 3−4 weeks can develop obvious physiological cardiac growth.21,92,95 Because evidence has shown that the voluntary running distance usually peaks at ∼4 weeks, the commonly used running duration is 4−8 weeks for the voluntary wheel-running model.81 It is quite important to adhere to a standardized exercise protocol in order to achieve the obvious exercise-associated phenotypes.
Exercise parameters can vary among research groups. In all research reports, it is quite important that the exercise parameters be described in detail in Methods section. For example, a large number of studies using treadmill running do not or cannot measure VO2max but describe only the running speed instead. This absence of parameters, especially for interval high-intensity treadmill running (which requires regular measurements of VO2max), is insufficient for controlling exercise intensity during the study.58 As for voluntary wheel running, the running distance can vary substantially, even in a single group of animals.82 However, experimental data (e.g., running speed, running time, and running distance) about voluntary wheel running are not always provided by the investigators. A clear presentation of these exercise parameters is necessary for promoting the standardization of exercise models and facilitating data comparisons among the various research groups.
Spontaneous or modified animal models of CVDs are widely used in cardiovascular research. Low- to moderate-intensity aerobic exercise attenuates cardiac remodeling in spontaneously hypertensive rats, and the settings of running speed, duration, and frequency are not significantly different from those of healthy wild-type animals.138, 139, 140 The beneficial effects of aerobic exercise have been shown to exist in other spontaneous disease models, such as reduced obesity in fatty Zucker rats (by improving insulin resistance), increased functional vasodilation, and reduced heart glycogen content and mitochondrial apoptotic signaling.141, 142, 143 However, compared to lean Zucker rats, obese Zucker rats have lower VO2max.141 Although VO2max can be increased by treadmill running in both lean and obese Zucker rats, VO2max still remains lower in trained obese Zucker rats than in trained lean ones.141 In addition to VO2max, determinations of the maximal lactate steady state and the speed at maximum exercise test help to prescribe and evaluate exercise in obese rat models.144, 145, 146, 147 Indeed, some disease models may influence animal exercise capacity. In these circumstances, measurements of exercise capacity are necessary to ensure accurate control of exercise intensity and proper presentation of results and conclusions.148
4.3. Exercise at various stages of disease
Although a growing number of CVD studies have shown the benefits of exercise in animal models, the clinical translational potential must be more carefully examined. For example, exercise carried out at differing stages of a disease is an important issue that should be considered in designing the study. Exercise before and after the onset of heart failure is categorized as primary and secondary prevention of heart failure, respectively.18 Despite the fact that preventive exercise interventions have been widely proved to attenuate cardiovascular injury and preserve cardiac function, exercise interventions occurring after the onset of CVDs may have differing effects.55 Aerobic and resistance exercise conducted in rats 3 months after myocardial infarction improved exercise capacity and maximum load carrying, respectively.55 However, the onset of exercise 3 months after myocardial infarction failed to reduce infarct size or attenuate cardiac dysfunction in rats.55 Indeed, exercise training as a therapy carried out after cardiac injury deserves further investigation. The mechanisms related to the therapeutic effects of exercise post injury need to be explained and differentiated from the effects of preventive exercise interventions.9,57,126
4.4. Other considerations
4.4.1. Age
Most exercise studies use young or adult animals in cardiovascular research. Because aging is an independent risk factor for CVDs, it is important to use aging animals in studying the effects of exercise.149 Young and middle-aged mice (up to 20 months) may perform treadmill running at the same running speed. However, older mice (24 months of age) have reduced exercise capacity, and their running speed should be regulated according to VO2max measurements.150 Old mice (24−30 months of age) that run 45 min at 10 m/min at a 10° incline, 5 days/week for 8 weeks, have improved exercise capacity, diastolic function, and contractile reserves as well as increased capillary density and reduced pulmonary congestion.76 Old animals can also be used in studies involving voluntary wheel running.76 Thus, the effects of exercise need to be explored through the use of aging animals in CVD studies.
4.4.2. Sex
Sex-related differences in cardiac structures and functions, as well as in cardiovascular pathophysiology, have been studied increasingly.151, 152, 153 Males and females can have different exercise capacities as well as systemic and cardiac responses when involved in exercise training.134 Although some early studies showed that running exercise induced similar skeletal muscle adaptations in male and female rats, only the isolated hearts from male rats showed higher stroke work, stroke volume, coronary flow, and myocardial oxygen consumption.154 In exercise models, female mice showed better exercise performance than males after treadmill running and voluntary wheel running, and female mice had greater physiological cardiac hypertrophy than male mice after voluntary wheel running.155 Studies of sex differences in response to exercise, as well as the separate underlying mechanisms, are strongly warranted.
4.4.3. Genetic background or pharmacological treatment influence
Genetic manipulations in rodents and even in large animals can be used to identify the the mechanisms related to exercise-associated effects in CVD studies. However, genetic backgrounds as well as pharmacological interventions in animals may influence their exercise capacities. One study found that endothelial nitric oxide synthase or β3-adrenergic receptor-deficient mice exercised to a lesser degree than wild mice during a 4-week voluntary wheel-running exercise.78 Measurements of running parameters (e.g., running distance) and VO2max are necessary to assess the impact of genetic background on exercise capacity in animal-exercise models. Indeed, myocardial-specific genetic manipulations are a better strategy for avoiding unexpected influences on other cell types, organs, or systems, which may also have less impact on exercise capacity.98,111
4.4.4. Other animal models
Although rodents are the most commonly used species in animal-exercise studies, other species, such as zebrafish, rabbits, dogs, swine, and horses, can also be used for exercise training.156, 157, 158, 159 More details about large-animal exercise models can be found in the Guidelines for animal exercise and training protocols for cardiovascular studies, published in the American Journal of Physiology—Heart and Circulatory Physiology.36 Using large-animal exercise models has obvious advantages over small-animal models because large animals have cardiovascular structures and functional responses to exercise that are more similar to those of humans. However, the laboratory facilities, breeding, and maintenance of large animals, the exercise protocols used, and the measurements to be taken are all factors that influence researchers’ abilities to carry out large-animal studies. Nevertheless, large-animal exercise models are valuable in promoting both an understanding of the mechanisms at work and the translational value of CVD studies related to exercise.
The zebrafish genome has a close relationship to the human genome.160 Zebrafish exercise models have been increasingly used in studies of exercise physiology, CVDs, and toxicology.161, 162, 163, 164, 165 A swimming protocol for zebrafish larvae, in which they swam at 5 body lengths/s (BL/s), 18 h/day, for 7 days for 9-day-old larvae and 12 days for 21-day-old larvae, did not influence cardiac activity such as heart rate, systolic and diastolic ventricular volume, and cardiac output.166 Instead, this swimming protocol improved the oxygen-carrying capacity of blood and increased the capillarization and mitochondrial density in muscle tissues, as measured by red blood cell count, cast of the vascular bed, and electron microscopy.166 In another study, zebrafish that were 14 days old began with 3 BL/s swimming for the first week and then maintained swimming at 5 BL/s after the second week, for 6 h/day for 10 weeks.167 The result was that they had upregulation of the muscle growth factor myogenin and proliferating cell nuclear antigen in both the heart and axial muscle.167 This swimming protocol enhanced slow aerobic-muscle development in the axial musculature, while the heart muscle showed a shift toward a faster phenotype but did not become more aerobic.167 In yet another study, adult zebrafish swimming at 13 BL/s, 6 h/day, 5 days/week for 4 weeks, had increased proliferation of cardiomyocytes under normal condition.163 Zebrafish swimming using this protocol for 13 experimental days also displayed increased cardiomyocyte proliferation and improved cardiac recovery and function after cryoinjury.163 Indeed, zebrafish swimming is emerging as an interesting model for studying the effects and mechanisms of physical exercise on the heart and CVDs.
4.4.5. Adverse effects of excessive exercise
Although most studies are designed to study the beneficial effects of exercise, it is also important to highlight the adverse effects caused by excessive exercise. In 1 study, high-intensity running exercise (e.g., 28 m/min for 60 min, 5 days/week for 16 weeks) increased atrial fibrillation susceptibility in rats, which has been associated with autonomic changes, atrial dilation, and atrial fibrosis.168 Likewise, mice swimming in a steady water current (90 min per session, daily for 6 weeks) or running on a treadmill with a 30° incline (at 21 m/min for 120 min, daily for 6 weeks) increased atrial arrhythmia susceptibility.169
In models relying on forced exercise (e.g., the exhaustive exercise test), experienced laboratory personnel should observe the animals’ exercise behaviors closely. The animals should not be forced to engage in exercise when exhaustion occurs. In addition, responses to improper exercise such as cessation of swimming, constant floating, increased defecation, hyperalgesia, or biomarkers of peripheral muscle fatigue, should be assessed because improper exercise may cause psychological distress in animals.170, 171, 172 Although the exact impact of psychological distress on experimental results has not yet been fully determined, it has been reported that forced treadmill running, but not voluntary wheel running, effectively induces neuroprotection in stroke.173 Certainly, it is important to choose proper exercise protocols in order to reduce pain and distress in animal-exercise experiments.
5. Functional experiments to determine targets in response to exercise
Because of the rapid development of omics technology, such as genomics, proteomics, and metabonomics, as well as microarray profiling and in silico analysis, more and more potential targets in response to exercise are able to be revealed.21,95,119 First, it is critical to confirm the expressions of predicted targets in the hearts of exercised animals using real-time quantitative polymerase chain reactions and Western blot analysis. Specific biochemical validation experiments are needed for noncoding RNAs, such as circular RNAs and long non-coding RNAs, that are identified in the hearts of exercised animals.174,175 Next, it is essential to conduct functional experiments in order to determine the roles of these targets in the hearts of exercised animals. Increasing evidence indicates that targets identified in exercised animal hearts have the potential to mitigate cardiac injury in multiple ways.176 Molecules that are regulated in exercised animal hearts may promote physiological hypertrophy of cardiomyocytes and may have the potential to enhance the expression of proliferation markers of cardiomyocytes and prevent cardiomyocyte apoptosis as well.9
For studies of exercise-induced physiological cardiac hypertrophy, the molecules that were screened out need to be further investigated based on functional experiments. In in vitro experiments, primary cultured neonatal rat or mouse cardiomyocytes are essential for determining the functional roles of exercise-responsive molecules in regulating the physiological hypertrophy and the proliferation markers in cardiomyocytes.119,175 The oxygen glucose deprivation/reperfusion-induced apoptosis model can be used to examine whether these molecules have antiapoptosis effects in cultured neonatal cardiomyocytes.21,95 It is noteworthy that cardiomyocytes derived from human embryonic stem cells or human-induced pluripotent stem cells are valuable in confirming these functions in human cardiomyocytes.177,178 In in vivo experiments, exercise-associated cardiac phenotypes, such as physiological cardiac hypertrophy (without dysregulation of pathological hypertrophy markers, e.g., atrial natriuretic peptide and brain natriuretic peptide) and increased expression of proliferation markers (e.g., 5-ethynyl-2’-deoxyuridine, Ki-67, phosphohistone H3, and Aurora B) in the myocardium deserve to be validated in exercised animal models such as murine models of swimming exercise by using genetically engineered or modified animals.92,98,175,179 A deep understanding of the functions and mechanisms of exercise-responsive molecules paves the way for identifying novel myocardial protective or therapeutic targets for CVDs.8,137,176
6. Translational values and conclusions
Animal studies using exercise interventions have greatly enlarged our understanding of the beneficial effects of exercise in preventing and treating CVDs. It is noteworthy that the involved cellular and molecular mechanisms contributing to exercise-induced cardiovascular adaptations are potential interventional targets that can be used to provide cardiovascular protective effects.17,180 The molecule-based therapies regulating the PI3K/Akt signaling pathway or calcium handling and the gene therapies or RNA-based therapies targeting the key molecules identified in animal exercise models, such as miR-222, miR-17-3p, and sarco(endo)plasmic reticulum Ca(2+)– ATPase 2a, may mimic, to at least some degree, the effects of exercise.21,95,112 The molecule mechanisms identified in the control of physiological cardiac growth have the potential to mitigate detrimental hypertrophy and heart failure.137 Additionally, exercise-induced regulation of circulating components such as cytokines, myokines, microRNAs, and other non-coding RNAs, may serve as biomarkers for risk stratification, treatment, and prognosis of CVDs.181, 182, 183 Indeed, animal studies using exercise interventions have great translational value for identifying novel targets and developing promising clinical approaches to mitigate CVDs.
Despite the intensive interest in studying the beneficial effects and mechanisms of exercise on CVDs, the successful translation of basic research into clinical applications is still limited. Multiple factors contribute to this, including the inappropriate use of animal exercise models, which may influence the evaluation of exercise-induced effects on CVDs and limit the comparison of results from different studies. We hope that this scientific statement will help scientists to design and perform high-quality animal studies better by using exercise interventions that will help in the treatment of CVDs. Rigorous respect for the use of standardized procedures in exercise training and good adherence to exercise protocols during the study are warranted. Collectively, a well-designed, well-performed, and quality-controlled animal exercise study and a deep understanding of the mechanisms of exercise-associated cardioprotection will pave the way for successful translation of exercise studies from bench to bedside in the prevention and treatment of CVDs.
Acknowledgments
This work was supported by grants from the National Key Research and Development Project (2020YFA0803800 to YB), National Natural Science Foundation of China (82020108002 and 81911540486 to JX, 81772444 to LW, 81772466 to RD), Innovation Program of Shanghai Municipal Education Commission (2017-01-07-00-09-E00042 to JX), Science and Technology Commission of Shanghai Municipality (18410722200 and 17010500100 to JX), and “Dawn” Program of the Shanghai Education Commission (19SG34 to JX). We thank all members of the Committee on Cardiac Rehabilitation, Chinese Medical Doctors’ Association.
Authors’ contributions
JX and LG designed the concept and structure of the position paper and provided substantial revisions to the draft manuscript; YB and LW designed the concept and structure of the position paper. All authors were actively involved in writing and composing subsection of the position paper. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2021.08.002.
Contributor Information
Lan Guo, Email: guolan1993@126.com.
Junjie Xiao, Email: junjiexiao@shu.edu.cn.
Appendix. Supplementary materials
References
- 1.Lee IM, Sesso HD, Oguma Y, Paffenbarger RS., Jr Relative intensity of physical activity and risk of coronary heart disease. Circulation. 2003;107:1110–1116. doi: 10.1161/01.cir.0000052626.63602.58. [DOI] [PubMed] [Google Scholar]
- 2.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen BK, Saltin B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(Suppl. 3):S1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 4.Dun Y, Thomas RJ, Smith JR, et al. High-intensity interval training improves metabolic syndrome and body composition in outpatient cardiac rehabilitation patients with myocardial infarction. Cardiovasc Diabetol. 2019;18:104. doi: 10.1186/s12933-019-0907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luan X, Tian X, Zhang H, et al. Exercise as a prescription for patients with various diseases. J Sport Health Sci. 2019;8:422–441. doi: 10.1016/j.jshs.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Rev Esp Cardiol (Engl Ed) 2016;69:1167. doi: 10.1016/j.rec.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Forman DE, Sanderson BK, Josephson RA, et al. Heart failure as a newly approved diagnosis for cardiac rehabilitation: Challenges and opportunities. J Am Coll Cardiol. 2015;65:2652–2659. doi: 10.1016/j.jacc.2015.04.052. [DOI] [PubMed] [Google Scholar]
- 8.Bernardo BC, Ooi JYY, Weeks KL, et al. Understanding key mechanisms of exercise-induced cardiac protection to mitigate disease: Current knowledge and emerging concepts. Physiol Rev. 2018;98:419–475. doi: 10.1152/physrev.00043.2016. [DOI] [PubMed] [Google Scholar]
- 9.Vujic A, Lerchenmüller C, Wu TD, et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat Commun. 2018;9:1659. doi: 10.1038/s41467-018-04083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo J, McMullen JR, Sobkiw CL, et al. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol. 2005;25:9491–9502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinonen I, Kudomi N, Kemppainen J, et al. Myocardial blood flow and its transit time, oxygen utilization, and efficiency of highly endurance-trained human heart. Basic Res Cardiol. 2014;109:413. doi: 10.1007/s00395-014-0413-1. [DOI] [PubMed] [Google Scholar]
- 12.Lai CH, Ho TJ, Kuo WW, et al. Exercise training enhanced SIRT1 longevity signaling replaces the IGF1 survival pathway to attenuate aging-induced rat heart apoptosis. Age (Dordr) 2014;36:9706. doi: 10.1007/s11357-014-9706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werner C, Hanhoun M, Widmann T, et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52:470–482. doi: 10.1016/j.jacc.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 14.Safdar A, Saleem A, Tarnopolsky MA. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol. 2016;12:504–517. doi: 10.1038/nrendo.2016.76. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Lv Y, Li G, Xiao J. MicroRNAs in heart and circulation during physical exercise. J Sport Health Sci. 2018;7:433–441. doi: 10.1016/j.jshs.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q, Chen L, Liang X, et al. Exercise attenuates angiotensin-induced muscle atrophy by targeting PPARγ/miR-29b. J Sport Health Sci. 2021 doi: 10.1016/j.jshs.2021.06.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bei Y, Zhou Q, Sun Q, Xiao J. Exercise as a platform for pharmacotherapy development in cardiac diseases. Curr Pharm Des. 2015;21:4409–4416. doi: 10.2174/1381612821666150803150008. [DOI] [PubMed] [Google Scholar]
- 18.Cattadori G, Segurini C, Picozzi A, Padeletti L, Anzà C. Exercise and heart failure: An update. ESC Heart Fail. 2018;5:222–232. doi: 10.1002/ehf2.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng R, Wang L, Li Z, et al. A systematic comparison of exercise training protocols on animal models of cardiovascular capacity. Life Sci. 2019;217:128–140. doi: 10.1016/j.lfs.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang H, Jia D, Zhang B, et al. Exercise improves cardiac function and glucose metabolism in mice with experimental myocardial infarction through inhibiting HDAC4 and upregulating GLUT1 expression. Basic Res Cardiol. 2020;115:28. doi: 10.1007/s00395-020-0787-1. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Xiao J, Zhu H, et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21:584–595. doi: 10.1016/j.cmet.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMullen JR, Amirahmadi F, Woodcock EA, et al. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci U S A. 2007;104:612–617. doi: 10.1073/pnas.0606663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veeranki S, Givvimani S, Kundu S, Metreveli N, Pushpakumar S, Tyagi SC. Moderate intensity exercise prevents diabetic cardiomyopathy associated contractile dysfunction through restoration of mitochondrial function and connexin 43 levels in db/db mice. J Mol Cell Cardiol. 2016;92:163–173. doi: 10.1016/j.yjmcc.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J, Jang HJ, Schellingerhout D, et al. Effects of exercise training and detraining on atheromatous matrix metalloproteinase activity in mice. Atherosclerosis. 2020;299:15–23. doi: 10.1016/j.atherosclerosis.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Melo SFS, da Silva Júnior ND, Barauna VG, et al. Cardiovascular adaptations induced by resistance training in animal models. Int J Med Sci. 2018;15:403–410. doi: 10.7150/ijms.23150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: Potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dun Y, Thomas RJ, Medina-Inojosa JR, et al. High-intensity interval training in cardiac rehabilitation: Impact on fat mass in patients with myocardial infarction. Mayo Clin Proc. 2019;94:1718–1730. doi: 10.1016/j.mayocp.2019.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powers SK, Demirel HA, Vincent HK, et al. Exercise training improves myocardial tolerance to in vivo ischemia–reperfusion in the rat. Am J Physiol. 1998;275:R1468–R1477. doi: 10.1152/ajpregu.1998.275.5.R1468. [DOI] [PubMed] [Google Scholar]
- 29.Rolim N, Skårdal K, Høydal M, et al. Aerobic interval training reduces inducible ventricular arrhythmias in diabetic mice after myocardial infarction. Basic Res Cardiol. 2015;110:44. doi: 10.1007/s00395-015-0502-9. [DOI] [PubMed] [Google Scholar]
- 30.He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouyang A, Olver TD, Emter CA, et al. Chronic exercise training prevents coronary artery stiffening in aortic-banded miniswine: Role of perivascular adipose-derived advanced glycation end products. J Appl Physiol (1985) 2019;127:816–827. doi: 10.1152/japplphysiol.00146.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer P, Gayda M, Juneau M, Nigam A. High-intensity aerobic interval exercise in chronic heart failure. Curr Heart Fail Rep. 2013;10:130–138. doi: 10.1007/s11897-013-0130-3. [DOI] [PubMed] [Google Scholar]
- 33.Hirai DM, Copp SW, Holdsworth CT, et al. Skeletal muscle microvascular oxygenation dynamics in heart failure: Exercise training and nitric oxide-mediated function. Am J Physiol Heart Circ Physiol. 2014;306:H690–H698. doi: 10.1152/ajpheart.00901.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musch TI, Moore RL, Hilty MR. Effects of dynamic exercise training on the metabolic and cardiocirculatory responses to exercise in the rat model of myocardial infarction and heart failure. Am J Cardiol. 1988;62:20E–24E. doi: 10.1016/s0002-9149(88)80005-5. [DOI] [PubMed] [Google Scholar]
- 35.Musch TI, Bruno A, Bradford GE, Vayonis A, Moore RL. Measurements of metabolic rate in rats: A comparison of techniques. J Appl Physiol (1985) 1988;65:964–970. doi: 10.1152/jappl.1988.65.2.964. [DOI] [PubMed] [Google Scholar]
- 36.Poole DC, Copp SW, Colburn TD, et al. Guidelines for animal exercise and training protocols for cardiovascular studies. Am J Physiol Heart Circ Physiol. 2020;318:H1100–H1138. doi: 10.1152/ajpheart.00697.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janice Sánchez B, Tremblay AK, Leduc-Gaudet JP, et al. Depletion of HuR in murine skeletal muscle enhances exercise endurance and prevents cancer-induced muscle atrophy. Nat Commun. 2019;10:4171. doi: 10.1038/s41467-019-12186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fenning A, Harrison G, Dwyer D, Rose'Meyer R, Brown L. Cardiac adaptation to endurance exercise in rats. Mol Cell Biochem. 2003;251:51–59. doi: 10.1007/978-1-4419-9238-3_8. [DOI] [PubMed] [Google Scholar]
- 39.Fewell JG, Osinska H, Klevitsky R, et al. A treadmill exercise regimen for identifying cardiovascular phenotypes in transgenic mice. Am J Physiol. 1997;273:H1595–H1605. doi: 10.1152/ajpheart.1997.273.3.H1595. [DOI] [PubMed] [Google Scholar]
- 40.Kemi OJ, Haram PM, Loennechen JP, et al. Moderate vs. high exercise intensity: Differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res. 2005;67:161–172. doi: 10.1016/j.cardiores.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Wisløff U, Helgerud J, Kemi OJ, Ellingsen O. Intensity-controlled treadmill running in rats: VO(2max) and cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2001;280:H1301–H1310. doi: 10.1152/ajpheart.2001.280.3.H1301. [DOI] [PubMed] [Google Scholar]
- 42.Kemi OJ, Loennechen JP, Wisløff U, Ellingsen Ø. Intensity-controlled treadmill running in mice: Cardiac and skeletal muscle hypertrophy. J Appl Physiol (1985) 2002;93:1301–1309. doi: 10.1152/japplphysiol.00231.2002. [DOI] [PubMed] [Google Scholar]
- 43.Whipp BJ, Ward SA. Quantifying intervention-related improvements in exercise tolerance. Eur Respir J. 2009;33:1254–1260. doi: 10.1183/09031936.00110108. [DOI] [PubMed] [Google Scholar]
- 44.Jones AM, Vanhatalo A, Burnley M, et al. Critical power: Implications for determination of VO2max and exercise tolerance. Med Sci Sports Exerc. 2010;42:1876–1890. doi: 10.1249/MSS.0b013e3181d9cf7f. [DOI] [PubMed] [Google Scholar]
- 45.Poole DC, Burnley M, Vanhatalo A, Rossiter HB, Jones AM. Critical power: An important fatigue threshold in exercise physiology. Med Sci Sports Exerc. 2016;48:2320–2334. doi: 10.1249/MSS.0000000000000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Copp SW, Hirai DM, Musch TI, Poole DC. Critical speed in the rat: Implications for hindlimb muscle blood flow distribution and fibre recruitment. J Physiol. 2010;588:5077–5087. doi: 10.1113/jphysiol.2010.198382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Billat VL, Mouisel E, Roblot N, Melki J. Inter- and intrastrain variation in mouse critical running speed. J Appl Physiol (1985) 2005;98:1258–1263. doi: 10.1152/japplphysiol.00991.2004. [DOI] [PubMed] [Google Scholar]
- 48.Yalçin F, Kucukler N, Cingolani O, et al. Evolution of ventricular hypertrophy and myocardial mechanics in physiological and pathological hypertrophy. J Appl Physiol (1985) 2019;126:354–362. doi: 10.1152/japplphysiol.00199.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibb AA, McNally LA, Riggs DW, Conklin DJ, Bhatnagar A, Hill BG. FVB/NJ mice are a useful model for examining cardiac adaptations to treadmill exercise. Front Physiol. 2016;7:636. doi: 10.3389/fphys.2016.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gibb AA, Epstein PN, Uchida S, et al. Exercise-induced changes in glucose metabolism promote physiological cardiac growth. Circulation. 2017;136:2144–2157. doi: 10.1161/CIRCULATIONAHA.117.028274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore RL, Musch TI, Yelamarty RV, et al. Chronic exercise alters contractility and morphology of isolated rat cardiac myocytes. Am J Physiol. 1993;264:C1180–C1189. doi: 10.1152/ajpcell.1993.264.5.C1180. [DOI] [PubMed] [Google Scholar]
- 52.Al-Asoom LI, BA Al-Shaikh, Bamosa AO. El-Bahai MN Comparison of Nigella sativa- and exercise-induced models of cardiac hypertrophy: Structural and electrophysiological features. Cardiovasc Toxicol. 2014;14:208–213. doi: 10.1007/s12012-014-9244-4. [DOI] [PubMed] [Google Scholar]
- 53.Diffee GM, Nagle DF. Regional differences in effects of exercise training on contractile and biochemical properties of rat cardiac myocytes. J Appl Physiol (1985) 2003;95:35–42. doi: 10.1152/japplphysiol.00951.2002. [DOI] [PubMed] [Google Scholar]
- 54.Kim HJ, Lee WJ. Low-intensity aerobic exercise training: Inhibition of skeletal muscle atrophy in high-fat-diet-induced ovariectomized rats. J Exerc Nutrition Biochem. 2017;21:19–25. doi: 10.20463/jenb.2017.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomes MJ, Pagan LU, Lima ARR, et al. Effects of aerobic and resistance exercise on cardiac remodelling and skeletal muscle oxidative stress of infarcted rats. J Cell Mol Med. 2020;24:5352–5362. doi: 10.1111/jcmm.15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wisløff U, Loennechen JP, Falck G, et al. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovasc Res. 2001;50:495–508. doi: 10.1016/s0008-6363(01)00210-3. [DOI] [PubMed] [Google Scholar]
- 57.Kraljevic J, Marinovic J, Pravdic D, et al. Aerobic interval training attenuates remodelling and mitochondrial dysfunction in the post-infarction failing rat heart. Cardiovasc Res. 2013;99:55–64. doi: 10.1093/cvr/cvt080. [DOI] [PubMed] [Google Scholar]
- 58.Høydal MA, Wisløff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: Practical implications for exercise training. Eur J Cardiovasc Prev Rehabil. 2007;14:753–760. doi: 10.1097/HJR.0b013e3281eacef1. [DOI] [PubMed] [Google Scholar]
- 59.Lambert MI, Noakes TD. Dissociation of changes in VO2max, muscle QO2, and performance with training in rats. J Appl Physiol (1985) 1989;66:1620–1625. doi: 10.1152/jappl.1989.66.4.1620. [DOI] [PubMed] [Google Scholar]
- 60.Helwig B, Schreurs KM, Hansen J, et al. Training-induced changes in skeletal muscle Na+-K+ pump number and isoform expression in rats with chronic heart failure. J Appl Physiol (1985) 2003;94:2225–2236. doi: 10.1152/japplphysiol.00279.2002. [DOI] [PubMed] [Google Scholar]
- 61.Rodrigues LO, Oliveira A, Lima NR, Machado-Moreira CA. Heat storage rate and acute fatigue in rats. Braz J Med Biol Res. 2003;36:131–135. doi: 10.1590/s0100-879x2003000100018. [DOI] [PubMed] [Google Scholar]
- 62.Scheiman J, Luber JM, Chavkin TA, et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. 2019;25:1104–1109. doi: 10.1038/s41591-019-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Conlee RK. Muscle glycogen and exercise endurance: A twenty-year perspective. Exerc Sport Sci Rev. 1987;15:1–28. [PubMed] [Google Scholar]
- 64.Moore RL, Hilty MR, Musch TI. Effect of aortic arterial catheterization on tissue glycogen content. J Appl Physiol (1985) 1988;65:2752–2756. doi: 10.1152/jappl.1988.65.6.2752. [DOI] [PubMed] [Google Scholar]
- 65.Morán M, Saborido A, Megías A. Ca2+ regulatory systems in rat myocardium are altered by 24 weeks treadmill training. Pflugers Arch. 2003;446:161–168. doi: 10.1007/s00424-003-1019-x. [DOI] [PubMed] [Google Scholar]
- 66.Bellafiore M, Sivverini G, Palumbo D, et al. Increased cx43 and angiogenesis in exercised mouse hearts. Int J Sports Med. 2007;28:749–755. doi: 10.1055/s-2007-964899. [DOI] [PubMed] [Google Scholar]
- 67.Gunadi JW, Tarawan VM, Setiawan I, Lesmana R, Wahyudianingsih R, Supratman U. Cardiac hypertrophy is stimulated by altered trainingintensity and correlates with autophagy modulation in male Wistar rats. BMC Sports Sci Med Rehabil. 2019;11:9. doi: 10.1186/s13102-019-0121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gholipour M, Tabrizi A. The role of Hippo signaling pathway in physiological cardiac hypertrophy. Bioimpacts. 2020;10:251–257. doi: 10.34172/bi.2020.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnsen AB, Høydal M, Røsbjørgen R, Stølen T, Wisløff U. Aerobic interval training partly reverse contractile dysfunction and impaired Ca2+ handling in atrial myocytes from rats with post infarction heart failure. PLoS One. 2013;8:e66288. doi: 10.1371/journal.pone.0066288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kemi OJ, Ellingsen O, Ceci M, et al. Aerobic interval training enhances cardiomyocyte contractility and Ca2+ cycling by phosphorylation of CaMKII and Thr-17 of phospholamban. J Mol Cell Cardiol. 2007;43:354–361. doi: 10.1016/j.yjmcc.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Magalhães J, Gonçalves IO, Lumini-Oliveira J, et al. Modulation of cardiac mitochondrial permeability transition and apoptotic signaling by endurance training and intermittent hypobaric hypoxia. Int J Cardiol. 2014;173:40–45. doi: 10.1016/j.ijcard.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 72.Alleman RJ, Tsang AM, Ryan TE, et al. Exercise-induced protection against reperfusion arrhythmia involves stabilization of mitochondrial energetics. Am J Physiol Heart Circ Physiol. 2016;310:H1360–H1370. doi: 10.1152/ajpheart.00858.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ascensão A, Lumini-Oliveira J, Machado NG, et al. Acute exercise protects against calcium-induced cardiac mitochondrial permeability transition pore opening in doxorubicin-treated rats. Clin Sci (Lond) 2011;120:37–49. doi: 10.1042/CS20100254. [DOI] [PubMed] [Google Scholar]
- 74.Bozi LH, Jannig PR, Rolim N, et al. Aerobic exercise training rescues cardiac protein quality control and blunts endoplasmic reticulum stress in heart failure rats. J Cell Mol Med. 2016;20:2208–2212. doi: 10.1111/jcmm.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bourdier G, Flore P, Sanchez H, Pepin JL, Belaidi E, Arnaud C. High-intensity training reduces intermittent hypoxia-induced ER stress and myocardial infarct size. Am J Physiol Heart Circ Physiol. 2016;310:H279–H289. doi: 10.1152/ajpheart.00448.2015. [DOI] [PubMed] [Google Scholar]
- 76.Roh JD, Houstis N, Yu A, et al. Exercise training reverses cardiac aging phenotypes associated with heart failure with preserved ejection fraction in male mice. Aging Cell. 2020;19:e13159. doi: 10.1111/acel.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Noble EG, Moraska A, Mazzeo RS, et al. Differential expression of stress proteins in rat myocardium after free wheel or treadmill run training. J Appl Physiol (1985) 1999;86:1696–1701. doi: 10.1152/jappl.1999.86.5.1696. [DOI] [PubMed] [Google Scholar]
- 78.Calvert JW, Condit ME, Aragón JP, et al. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of β(3)-adrenergic receptors and increased nitric oxide signaling: Role of nitrite and nitrosothiols. Circ Res. 2011;108:1448–1458. doi: 10.1161/CIRCRESAHA.111.241117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Waard MC, van Haperen R, Soullié T, Tempel D, de Crom R, Duncker DJ. Beneficial effects of exercise training after myocardial infarction require full eNOS expression. J Mol Cell Cardiol. 2010;48:1041–1049. doi: 10.1016/j.yjmcc.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 80.Holloszy JO. Exercise increases average longevity of female rats despite increased food intake and no growth retardation. J Gerontol. 1993;48:B97–100. doi: 10.1093/geronj/48.3.b97. [DOI] [PubMed] [Google Scholar]
- 81.Overton JM, Tipton CM, Matthes RD, Leininger JR. Voluntary exercise and its effects on young SHR and stroke-prone hypertensive rats. J Appl Physiol (1985) 1986;61:318–324. doi: 10.1152/jappl.1986.61.1.318. [DOI] [PubMed] [Google Scholar]
- 82.Rodnick KJ, Reaven GM, Haskell WL, Sims CR, Mondon CE. Variations in running activity and enzymatic adaptations in voluntary running rats. J Appl Physiol (1985) 1989;66:1250–1257. doi: 10.1152/jappl.1989.66.3.1250. [DOI] [PubMed] [Google Scholar]
- 83.Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol (1985) 2001;90:1900–1908. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- 84.Goh J, Ladiges W. Voluntary wheel running in mice. Curr Protoc Mouse Biol. 2015;5:283–290. doi: 10.1002/9780470942390.mo140295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luckey SW, Haines CD, Konhilas JP, Luczak ED, Messmer-Kratzsch A, Leinwand LA. Cyclin D2 is a critical mediator of exercise-induced cardiac hypertrophy. Exp Biol Med (Maywood) 2017;242:1820–1830. doi: 10.1177/1535370217731503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buitrago M, Lorenz K, Maass AH, et al. The transcriptional repressor Nab1 is a specific regulator of pathological cardiac hypertrophy. Nat Med. 2005;11:837–844. doi: 10.1038/nm1272. [DOI] [PubMed] [Google Scholar]
- 87.Gulve EA, Rodnick KJ, Henriksen EJ, Holloszy JO. Effects of wheel running on glucose transporter (GLUT4) concentration in skeletal muscle of young adult and old rats. Mech Ageing Dev. 1993;67:187–200. doi: 10.1016/0047-6374(93)90122-8. [DOI] [PubMed] [Google Scholar]
- 88.Stones R, Natali A, Billeter R, Harrison S, White E. Voluntary exercise-induced changes in beta2-adrenoceptor signalling in rat ventricular myocytes. Exp Physiol. 2008;93:1065–1075. doi: 10.1113/expphysiol.2008.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Natali AJ, Wilson LA, Peckham M, Turner DL, Harrison SM, White E. Different regional effects of voluntary exercise on the mechanical and electrical properties of rat ventricular myocytes. J Physiol. 2002;541:863–875. doi: 10.1113/jphysiol.2001.013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sakamoto M, Minamino T, Toko H, et al. Upregulation of heat shock transcription factor 1 plays a critical role in adaptive cardiac hypertrophy. Circ Res. 2006;99:1411–1418. doi: 10.1161/01.RES.0000252345.80198.97. [DOI] [PubMed] [Google Scholar]
- 91.Lai L, Leone TC, Keller MP, et al. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: A multisystems approach. Circ Heart Fail. 2014;7:1022–1031. doi: 10.1161/CIRCHEARTFAILURE.114.001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim J, Wende AR, Sena S, et al. Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Mol Endocrinol. 2008;22:2531–2543. doi: 10.1210/me.2008-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dun Y, Liu S, Zhang W, Xie M, Qiu L. Exercise combined with rhodiola sacra supplementation improves exercise capacity and ameliorates exhaustive exercise-induced muscle damage through enhancement of mitochondrial quality control. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/8024857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McMullen JR, Shioi T, Huang WY, et al. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. J Biol Chem. 2004;279:4782–4793. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- 95.Shi J, Bei Y, Kong X, et al. miR-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia-rerfusion injury. Theranostics. 2017;7:664–676. doi: 10.7150/thno.15162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Evangelista FS, Brum PC, Krieger JE. Duration-controlled swimming exercise training induces cardiac hypertrophy in mice. Braz J Med Biol Res. 2003;36:1751–1759. doi: 10.1590/s0100-879x2003001200018. [DOI] [PubMed] [Google Scholar]
- 97.Iivonen H, Nurminen L, Harri M, Tanila H, Puoliväli J. Hypothermia in mice tested in Morris water maze. Behav Brain Res. 2003;141:207–213. doi: 10.1016/s0166-4328(02)00369-8. [DOI] [PubMed] [Google Scholar]
- 98.McMullen JR, Shioi T, Zhang L, et al. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Taniike M, Yamaguchi O, Tsujimoto I, et al. Apoptosis signal-regulating kinase 1/p38 signaling pathway negatively regulates physiological hypertrophy. Circulation. 2008;117:545–552. doi: 10.1161/CIRCULATIONAHA.107.710434. [DOI] [PubMed] [Google Scholar]
- 100.Soares DDS, Pinto GH, Lopes A, et al. Cardiac hypertrophy in mice submitted to a swimming protocol: Influence of training volume and intensity on myocardial renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2019;316:R776–R782. doi: 10.1152/ajpregu.00205.2018. [DOI] [PubMed] [Google Scholar]
- 101.Tung YT, Hsu YJ, Liao CC, Ho ST, Huang CC, Huang WC. Physiological and biochemical effects of intrinsically high and low exercise capacities through multiomics approaches. Front Physiol. 2019;10:1201. doi: 10.3389/fphys.2019.01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McArdle WD, Montoye HJ. Reliability of exhaustive swimming in the laboratory rat. J Appl Physiol. 1966;21:1431–1434. doi: 10.1152/jappl.1966.21.4.1431. [DOI] [PubMed] [Google Scholar]
- 103.Tanaka M, Nakamura F, Mizokawa S, Matsumura A, Nozaki S, Watanabe Y. Establishment and assessment of a rat model of fatigue. Neurosci Lett. 2003;352:159–162. doi: 10.1016/j.neulet.2003.08.051. [DOI] [PubMed] [Google Scholar]
- 104.Shui L, Yi RN, Wu YJ, et al. Effects of mongolian warm acupuncture on iNOS/NO and inflammatory cytokines in the hippocampus of chronic fatigue rats. Front Integr Neurosci. 2019;13:78. doi: 10.3389/fnint.2019.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sturek ML, Bedford TG, Tipton CM, Newcomer L. Acute cardiorespiratory responses of hypertensive rats to swimming and treadmill exercise. J Appl Physiol Respir Environ Exerc Physiol. 1984;57:1328–1332. doi: 10.1152/jappl.1984.57.5.1328. [DOI] [PubMed] [Google Scholar]
- 106.Laughlin MH, Mohrman SJ, Armstrong RB. Muscular blood flow distribution patterns in the hindlimb of swimming rats. Am J Physiol. 1984;246:H398–H403. doi: 10.1152/ajpheart.1984.246.3.H398. [DOI] [PubMed] [Google Scholar]
- 107.Iemitsu M, Miyauchi T, Maeda S, et al. Cardiac hypertrophy by hypertension and exercise training exhibits different gene expression of enzymes in energy metabolism. Hypertens Res. 2003;26:829–837. doi: 10.1291/hypres.26.829. [DOI] [PubMed] [Google Scholar]
- 108.Medeiros A, Oliveira EM, Gianolla R, Casarini DE, Negrão CE, Brum PC. Swimming training increases cardiac vagal activity and induces cardiac hypertrophy in rats. Braz J Med Biol Res. 2004;37:1909–1917. doi: 10.1590/s0100-879x2004001200018. [DOI] [PubMed] [Google Scholar]
- 109.Palabiyik O, Tastekin E, Doganlar ZB, Tayfur P, Dogan A, Vardar SA. Alteration in cardiac PI3K/Akt/mTOR and ERK signaling pathways with the use of growth hormone and swimming, and the roles of miR21 and miR133. Biomed Rep. 2019;10:97–106. doi: 10.3892/br.2018.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yeves AM, Villa-Abrille MC, Pérez NG, Medina AJ, Escudero EM, Ennis IL. Physiological cardiac hypertrophy: Critical role of AKT in the prevention of NHE-1 hyperactivity. J Mol Cell Cardiol. 2014;76:186–195. doi: 10.1016/j.yjmcc.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 111.Riehle C, Wende AR, Zhu Y, et al. Insulin receptor substrates are essential for the bioenergetic and hypertrophic response of the heart to exercise training. Mol Cell Biol. 2014;34:3450–3460. doi: 10.1128/MCB.00426-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weeks KL, Gao X, Du XJ, et al. Phosphoinositide 3-kinase p110α is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ Heart Fail. 2012;5:523–534. doi: 10.1161/CIRCHEARTFAILURE.112.966622. [DOI] [PubMed] [Google Scholar]
- 113.Ikeda H, Shiojima I, Ozasa Y, et al. Interaction of myocardial insulin receptor and IGF receptor signaling in exercise-induced cardiac hypertrophy. J Mol Cell Cardiol. 2009;47:664–675. doi: 10.1016/j.yjmcc.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaplan ML, Cheslow Y, Vikstrom K, et al. Cardiac adaptations to chronic exercise in mice. Am J Physiol. 1994;267:H1167–H1173. doi: 10.1152/ajpheart.1994.267.3.H1167. [DOI] [PubMed] [Google Scholar]
- 115.Iemitsu M, Miyauchi T, Maeda S, et al. Physiological and pathological cardiac hypertrophy induce different molecular phenotypes in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R2029–R2036. doi: 10.1152/ajpregu.2001.281.6.R2029. [DOI] [PubMed] [Google Scholar]
- 116.Ma Z, Qi J, Meng S, Wen B, Zhang J. Swimming exercise training-induced left ventricular hypertrophy involves microRNAs and synergistic regulation of the PI3K/AKT/mTOR signaling pathway. Eur J Appl Physiol. 2013;113:2473–2486. doi: 10.1007/s00421-013-2685-9. [DOI] [PubMed] [Google Scholar]
- 117.Noh J, Wende AR, Olsen CD, et al. Phosphoinositide dependent protein kinase 1 is required for exercise-induced cardiac hypertrophy but not the associated mitochondrial adaptations. J Mol Cell Cardiol. 2015;89:297–305. doi: 10.1016/j.yjmcc.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harrison BC, Bell ML, Allen DL, Byrnes WC, Leinwand LA. Skeletal muscle adaptations in response to voluntary wheel running in myosin heavy chain null mice. J Appl Physiol (1985) 2002;92:313–322. doi: 10.1152/japplphysiol.00832.2001. [DOI] [PubMed] [Google Scholar]
- 119.Boström P, Mann N, Wu J, et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bei Y, Fu S, Chen X, et al. Cardiac cell proliferation is not necessary for exercise-induced cardiac growth but required for its protection against ischaemia/reperfusion injury. J Cell Mol Med. 2017;21:1648–1655. doi: 10.1111/jcmm.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tamaki T, Uchiyama S, Nakano S. A weight-lifting exercise model for inducing hypertrophy in the hindlimb muscles of rats. Med Sci Sports Exerc. 1992;24:881–886. [PubMed] [Google Scholar]
- 122.Barauna VG, Batista ML, Jr, Costa Rosa LF, et al. Cardiovascular adaptations in rats submitted to a resistance-training model. Clin Exp Pharmacol Physiol. 2005;32:249–254. doi: 10.1111/j.1440-1681.2005.04180.x. [DOI] [PubMed] [Google Scholar]
- 123.Yaspelkis BB, 3rd, Singh MK, Trevino B, Krisan AD, Collins DE. Resistance training increases glucose uptake and transport in rat skeletal muscle. Acta Physiol Scand. 2002;175:315–323. doi: 10.1046/j.1365-201X.2002.00998.x. [DOI] [PubMed] [Google Scholar]
- 124.Notomi T, Lee SJ, Okimoto N, et al. Effects of resistance exercise training on mass, strength, and turnover of bone in growing rats. Eur J Appl Physiol. 2000;82:268–274. doi: 10.1007/s004210000195. [DOI] [PubMed] [Google Scholar]
- 125.Barauna VG, Rosa KT, Irigoyen MC, de Oliveira EM. Effects of resistance training on ventricular function and hypertrophy in a rat model. Clin Med Res. 2007;5:114–120. doi: 10.3121/cmr.2007.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grans CF, Feriani DJ, Abssamra ME, et al. Resistance training after myocardial infarction in rats: its role on cardiac and autonomic function. Arq Bras Cardiol. 2014;103:60–68. doi: 10.5935/abc.20140093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hornberger TA, Jr, Farrar RP. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol. 2004;29:16–31. doi: 10.1139/h04-002. [DOI] [PubMed] [Google Scholar]
- 128.Sanches IC, Conti FF, Sartori M, Irigoyen MC, De Angelis K. Standardization of resistance exercise training: Effects in diabetic ovariectomized rats. Int J Sports Med. 2014;35:323–329. doi: 10.1055/s-0033-1351254. [DOI] [PubMed] [Google Scholar]
- 129.Araujo AJ, Santos AC, Souza Kdos S, et al. Resistance training controls arterial blood pressure in rats with L-NAME-induced hypertension. Arq Bras Cardiol. 2013;100:339–346. [PubMed] [Google Scholar]
- 130.Mota MM, Silva TL, Fontes MT, et al. Resistance exercise restores endothelial function and reduces blood pressure in type 1 diabetic rats. Arq Bras Cardiol. 2014;103:25–32. doi: 10.5935/abc.20140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Macedo FN, Mesquita TR, Melo VU, et al. Increased nitric oxide bioavailability and decreased sympathetic modulation are involved in vascular adjustments induced by low-intensity resistance training. Front Physiol. 2016;7:265. doi: 10.3389/fphys.2016.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Souza MR, Pimenta L, Pithon-Curi TC, Bucci M, Fontinele RG, De Souza RR. Effects of aerobic training, resistance training, or combined resistance-aerobic training on the left ventricular myocardium in a rat model. Microsc Res Tech. 2014;77:727–734. doi: 10.1002/jemt.22394. [DOI] [PubMed] [Google Scholar]
- 133.Ramkumar V, Barrington WW, Jacobson KA, Stiles GL. Demonstration of both A1 and A2 adenosine receptors in DDT1 MF-2 smooth muscle cells. Mol Pharmacol. 1990;37:149–156. [PMC free article] [PubMed] [Google Scholar]
- 134.Fernandes AA, Faria Tde O, Ribeiro Júnior RF, et al. A single resistance exercise session improves myocardial contractility in spontaneously hypertensive rats. Braz J Med Biol Res. 2015;48:813–821. doi: 10.1590/1414-431X20154355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Laufs U, Wassmann S, Czech T, et al. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:809–814. doi: 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- 136.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: Experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 137.Hill JA. Braking bad hypertrophy. N Engl J Med. 2015;372:2160–2162. doi: 10.1056/NEJMcibr1504187. [DOI] [PubMed] [Google Scholar]
- 138.Pagan LU, Damatto RL, Gomes MJ, et al. Low-intensity aerobic exercise improves cardiac remodelling of adult spontaneously hypertensive rats. J Cell Mol Med. 2019;23:6504–6507. doi: 10.1111/jcmm.14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pagan LU, Damatto RL, Cezar MD, et al. Long-term low intensity physical exercise attenuates heart failure development in aging spontaneously hypertensive rats. Cell Physiol Biochem. 2015;36:61–74. doi: 10.1159/000374053. [DOI] [PubMed] [Google Scholar]
- 140.Libonati JR, Sabri A, Xiao C, Macdonnell SM, Renna BF. Exercise training improves systolic function in hypertensive myocardium. J Appl Physiol (1985) 2011;111:1637–1643. doi: 10.1152/japplphysiol.00292.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sebai M, Lu S, Xiang L, Hester RL. Improved functional vasodilation in obese Zucker rats following exercise training. Am J Physiol Heart Circ Physiol. 2011;301:H1090–H1096. doi: 10.1152/ajpheart.00233.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lajoie C, Calderone A, Trudeau F, et al. Exercise training attenuated the PKB and GSK-3 dephosphorylation in the myocardium of ZDF rats. J Appl Physiol (1985) 2004;96:1606–1612. doi: 10.1152/japplphysiol.00853.2003. [DOI] [PubMed] [Google Scholar]
- 143.Peterson JM, Bryner RW, Sindler A, Frisbee JC, Alway SE. Mitochondrial apoptotic signaling is elevated in cardiac but not skeletal muscle in the obese Zucker rat and is reduced with aerobic exercise. J Appl Physiol (1985) 2008;105:1934–1943. doi: 10.1152/japplphysiol.00037.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Almeida JA, Petriz AB, Gomes CP, Araujo RC, Pereira RW, Franco OL. Exercise training at MLSS decreases weight gain and increases aerobic capacity in obese Zucker rats. Int J Sports Med. 2014;35:199–202. doi: 10.1055/s-0033-1349872. [DOI] [PubMed] [Google Scholar]
- 145.Almeida JA, Petriz BA, Gomes CP, Rocha LA, Pereira RW, Franco OL. Determination of the maximal lactate steady state in obese Zucker rats. Int J Sports Med. 2013;34:214–217. doi: 10.1055/s-0032-1316360. [DOI] [PubMed] [Google Scholar]
- 146.Stoyell-Conti FF, Irigoyen MC, Sartori M, et al. Aerobic training is better than resistance training on cardiac function and autonomic modulation in female ob/ob mice. Front Physiol. 2019;10:1464. doi: 10.3389/fphys.2019.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rodrigues B, Figueroa DM, Mostarda CT, Heeren MV, Irigoyen MC, De Angelis K. Maximal exercise test is a useful method for physical capacity and oxygen consumption determination in streptozotocin-diabetic rats. Cardiovasc Diabetol. 2007;6:38. doi: 10.1186/1475-2840-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Campos HO, Drummond LR, Rodrigues QT, Lima PM, Prímola-Gomes TN, Coimbra CC. Exercise capacity in different stages of hypertension in spontaneously hypertensive rats. J Sports Med Phys Fitness. 2020;60:800–805. doi: 10.23736/S0022-4707.20.10369-4. [DOI] [PubMed] [Google Scholar]
- 149.Horowitz AM, Fan X, Bieri G, et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science. 2020;369:167–173. doi: 10.1126/science.aaw2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Reynolds JC, Lee C. Mouse fitness as determined through treadmill running and walking. Methods Mol Biol. 2020;2144:57–65. doi: 10.1007/978-1-0716-0592-9_5. [DOI] [PubMed] [Google Scholar]
- 151.Zhou Q, Bei Y. Gender differences in cardiovascular diseases. J Cardiovasc Transl Res. 2020;13:1–2. doi: 10.1007/s12265-020-09956-9. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Y, Liu B, Zhao R, Zhang S, Yu XY, Li Y. The influence of sex on cardiac physiology and cardiovascular diseases. J Cardiovasc Transl Res. 2020;13:3–13. doi: 10.1007/s12265-019-09898-x. [DOI] [PubMed] [Google Scholar]
- 153.Stein GY, Ben-Gal T, Kremer A, et al. Gender-related differences in hospitalized heart failure patients. Eur J Heart Fail. 2013;15:734–741. doi: 10.1093/eurjhf/hft024. [DOI] [PubMed] [Google Scholar]
- 154.Schaible TF, Penpargkul S, Scheuer J. Cardiac responses to exercise training in male and female rats. J Appl Physiol Respir Environ Exerc Physiol. 1981;50:112–117. doi: 10.1152/jappl.1981.50.1.112. [DOI] [PubMed] [Google Scholar]
- 155.Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA. Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol. 2004;287:H2768–H2776. doi: 10.1152/ajpheart.00292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Langsetmo I, Poole DC. V(O2) recovery kinetics in the horse following moderate, heavy, and severe exercise. J Appl Physiol (1985) 1999;86:1170–1177. doi: 10.1152/jappl.1999.86.4.1170. [DOI] [PubMed] [Google Scholar]
- 157.Duncker DJ, Zhang J, Crampton MJ, Bache RJ. Alpha 1-adrenergic tone does not influence the transmural distribution of myocardial blood flow during exercise in dogs with pressure overload left ventricular hypertrophy. Basic Res Cardiol. 1995;90:73–83. doi: 10.1007/BF00795126. [DOI] [PubMed] [Google Scholar]
- 158.Merkus D, Sorop O, Houweling B, Hoogteijling BA, Duncker DJ. KCa+ channels contribute to exercise-induced coronary vasodilation in swine. Am J Physiol Heart Circ Physiol. 2006;291:H2090–H2097. doi: 10.1152/ajpheart.00315.2006. [DOI] [PubMed] [Google Scholar]
- 159.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: A role for angiotensin II. Circulation. 2000;102:1854–1862. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- 160.Howe K, Clark MD, Torroja CF, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bagatto B, Pelster B, Burggren WW. Growth and metabolism of larval zebrafish: Effects of swim training. J Exp Biol. 2001;204:4335–4343. doi: 10.1242/jeb.204.24.4335. [DOI] [PubMed] [Google Scholar]
- 162.Marit JS, Weber LP. Persistent effects on adult swim performance and energetics in zebrafish developmentally exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Aquat Toxicol. 2012;106-7:131–139. doi: 10.1016/j.aquatox.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 163.Rovira M, Borràs DM, Marques IJ, Puig C, Planas JV. Physiological responses to swimming-induced exercise in the adult zebrafish regenerating heart. Front Physiol. 2018;9:1362. doi: 10.3389/fphys.2018.01362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Palstra AP, Rovira M, Rizo-Roca D, Torrella JR, Spaink HP, Planas JV. Swimming-induced exercise promotes hypertrophy and vascularization of fast skeletal muscle fibres and activation of myogenic and angiogenic transcriptional programs in adult zebrafish. BMC Genomics. 2014;15:1136. doi: 10.1186/1471-2164-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.LeMoine CM, Craig PM, Dhekney K, Kim JJ, McClelland GB. Temporal and spatial patterns of gene expression in skeletal muscles in response to swim training in adult zebrafish (Danio rerio) J Comp Physiol B. 2010;180:151–160. doi: 10.1007/s00360-009-0398-5. [DOI] [PubMed] [Google Scholar]
- 166.Pelster B, Sänger AM, Siegele M, Schwerte T. Influence of swim training on cardiac activity, tissue capillarization, and mitochondrial density in muscle tissue of zebrafish larvae. Am J Physiol Regul Integr Comp Physiol. 2003;285:R339–R347. doi: 10.1152/ajpregu.00110.2003. [DOI] [PubMed] [Google Scholar]
- 167.van der Meulen T, Schipper H, van den Boogaart JG, Huising MO, Kranenbarg S, van Leeuwen JL. Endurance exercise differentially stimulates heart and axial muscle development in zebrafish (Danio rerio) Am J Physiol Regul Integr Comp Physiol. 2006;291:R1040–R1048. doi: 10.1152/ajpregu.00116.2006. [DOI] [PubMed] [Google Scholar]
- 168.Guasch E, Benito B, Qi X, et al. Atrial fibrillation promotion by endurance exercise: Demonstration and mechanistic exploration in an animal model. J Am Coll Cardiol. 2013;62:68–77. doi: 10.1016/j.jacc.2013.01.091. [DOI] [PubMed] [Google Scholar]
- 169.Aschar-Sobbi R, Izaddoustdar F, Korogyi AS, et al. Increased atrial arrhythmia susceptibility induced by intense endurance exercise in mice requires TNFα. Nat Commun. 2015;6:6018. doi: 10.1038/ncomms7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Abdelhamid RE, Kovacs KJ, Pasley JD, Nunez MG, Larson AA. Forced swim-induced musculoskeletal hyperalgesia is mediated by CRF2 receptors but not by TRPV1 receptors. Neuropharmacology. 2013;72:29–37. doi: 10.1016/j.neuropharm.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.National Research Council of The National Academies . The National Academies Press; Washington, DC: 2003. Guidelines for the care and use of mammals in neuroscience and behavioral research. [PubMed] [Google Scholar]
- 172.Finsterer J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet Disord. 2012;13:218. doi: 10.1186/1471-2474-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hayes K, Sprague S, Guo M, et al. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathol. 2008;115:289–296. doi: 10.1007/s00401-008-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Das S, Shah R, Dimmeler S, et al. Noncoding RNAs in cardiovascular disease: Current knowledge, tools and technologies for investigation, and future directions: A scientific statement from the American Heart Association. Circ Genom Precis Med. 2020;13 doi: 10.1161/HCG.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 175.Gao R, Wang L, Bei Y, et al. LncRNA CPhar induces cardiac physiological hypertrophy and promotes functional recovery after myocardial ischemia-reperfusion injury. Circulation. 2021;144:303–317. doi: 10.1161/CIRCULATIONAHA.120.050446. [DOI] [PubMed] [Google Scholar]
- 176.Wang H, Xie Y, Guan L, Elkin K, Xiao J. Targets identified from exercised heart: Killing multiple birds with one stone. NPJ Regen Med. 2021;6:23. doi: 10.1038/s41536-021-00128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Madonna R, Van Laake LW, Botker HE, et al. ESC working group on cellular biology of the heart: position paper for cardiovascular research: Tissue engineering strategies combined with cell therapies for cardiac repair in ischaemic heart disease and heart failure. Cardiovasc Res. 2019;115:488–500. doi: 10.1093/cvr/cvz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Viereck J, Bührke A, Foinquinos A, et al. Targeting muscle-enriched long non-coding RNA H19 reverses pathological cardiac hypertrophy. Eur Heart J. 2020;41:3462–3474. doi: 10.1093/eurheartj/ehaa519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lerchenmuller C, Rabolli CP, Yeri A, et al. CITED4 protects against adverse remodeling in response to physiological and pathological stress. Circ Res. 2020;127:631–646. doi: 10.1161/CIRCRESAHA.119.315881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang L, Wang J, Li G, Xiao J. Non-coding RNAs in physiological cardiac hypertrophy. Adv Exp Med Biol. 2020;1229:149–161. doi: 10.1007/978-981-15-1671-9_8. [DOI] [PubMed] [Google Scholar]
- 181.Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Meinecke A, Mitzka S, Just A, et al. Cardiac endurance training alters plasma profiles of circular RNA MBOAT2. Am J Physiol Heart Circ Physiol. 2020;319:H13–H21. doi: 10.1152/ajpheart.00067.2020. [DOI] [PubMed] [Google Scholar]
- 183.Mooren FC, Viereck J, Krüger K, Thum T. Circulating microRNAs as potential biomarkers of aerobic exercise capacity. Am J Physiol Heart Circ Physiol. 2014;306:H557–H563. doi: 10.1152/ajpheart.00711.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.