Abstract
Over the past few years, nanotechnology has been attracting considerable research attention because of their outstanding mechanical, electromagnetic and optical properties. Nanotechnology is an interdisciplinary field comprising nanomaterials, nanoelectronics, and nanobiotechnology, as three areas which extensively overlap. The application of metal nanoparticles (MNPs) has drawn much attention offering significant advances, especially in the field of medicine by increasing the therapeutic index of drugs through site specificity preventing multidrug resistance and delivering therapeutic agents efficiently. Apart from drug delivery, some other applications of MNPs in medicine are also well known such as in vivo and in vitro diagnostics and production of enhanced biocompatible materials and nutraceuticals. The use of metallic nanoparticles for drug delivery systems has significant advantages, such as increased stability and half-life of drug carrier in circulation, required biodistribution, and passive or active targeting into the required target site. Green synthesis of MNPs is an emerging area in the field of bionanotechnology and provides economic and environmental benefits as an alternative to chemical and physical methods. Therefore, this review aims to provide up-to-date insights on the current challenges and perspectives of MNPs in drug delivery systems. The present review was mainly focused on the greener methods of metallic nanocarrier preparations and its surface modifications, applications of different MNPs like silver, gold, platinum, palladium, copper, zinc oxide, metal sulfide and nanometal organic frameworks in drug delivery systems.
Keywords: Nanocarriers, Metallic nanoparticles, Drug delivery, Drug carriers
Introduction
Nanotechnology is a science that deals with the preparation of nanosize particles ranging from 1 to 100 nm employing diverse synthetic strategies, particle structure and size modification [1]. The technological leap of controlling materials at nanoscale provides for a big revolution in medical and healthcare treatments and therapies. The use of nanoparticles in different fields like molecular biology, physics, organic and inorganic chemistry, medicine, and material science is unexpectedly augmented nowadays [2, 3]. It is reported that moving from bulk materials into nanosize will change their physicochemical properties, which can be utilized in diverse biomedical applications. Nanoparticles are extremely attractive for several biomedical applications mainly due to its high surface to volume ratio with the capability to interact with the molecular or cellular process and the possibility to influence their functions [4]. Drug delivery systems (DDS) are one of the greatest promising applications of human health care and represent an eternally progressing field for medical sciences. In spite of vast development in the field of DDS, still it is a crucial challenge for formulation scientists to develop an appropriate carrier that is efficient for drug delivery to the body with maximum benefit to risk ratio [5]. The development of drug-delivery systems has been an active field of multidisciplinary research for more than 20 years and has led to successful improvement of treatments for different pathologies [6, 7]. Nanoscale drug delivery systems are an important route to reduce the side effects and improve the treatment efficacy of chemotherapy drugs. The use of nanostructured materials provides incomparable liberty to customize the intrinsic properties of the drug in DDS such as drug release characteristics, dissolution, solubility, bioavailability, t1/2, and immunogenicity [8]. Another important feature of nanostructured materials (NSM) is its comparable size of the cell organelles in the human cell and this property makes them a potential candidate for DDS as the variety of physiological processes take place at nanoscales (Fig. 1). Formation of stable interactions with ligands, variability in size and shape, high carrier capacity and convenience of binding of both hydrophilic and hydrophobic substances make nanoparticles favorable platforms for the target-specific and controlled delivery of micro and macromolecules in disease therapy [9]. The side effects and poor bioavailability associated with existing drugs make NSM as an effective drug delivery system. It means that the delivery of therapeutic agent directly to the tumor cells is a critical challenge. Some conventional chemotherapy drugs used to treat cancer can increase the risk of heart problems, which can also occur with radiation therapy which includes myocardial infarction, heart attack, stroke and blood clot [10]. By using metal nanoparticles, drug delivery system can minimize these types of side effects because they directly target the affected organ and thereby reduces the side effects.
Fig. 1.
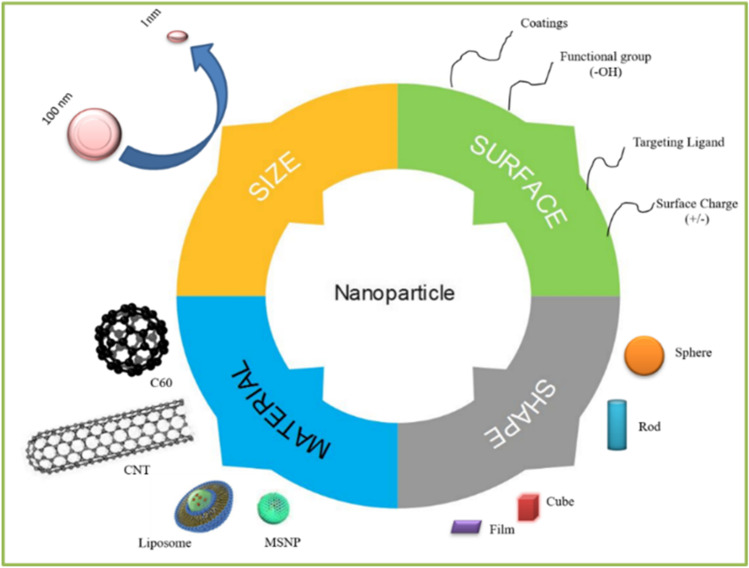
The size, shape, material, and surface of the nanoparticles
In the field of nanobiotechnology, synthesis of MNPs is considered as a progressive area attracting scientific research with significant importance on imaging and drug delivery [11, 12]. MNPs are of great interest because of its optical properties such as surface plasmon resonance (SPR) with the ability to control optical field which makes them potential candidates for biomedical applications. The small size of MNPs facilitates them to infiltrate through the biological or physiological membrane that is usually impermeable to other macromolecules [13, 14]. To alter the pharmacokinetics properties, the surface of the MNPs can be tuned accordingly. For example the circulation time of the MNPs within the body can be enhanced by reducing the non-specific uptake by mononuclear phagocyte system through coating the surface of MNPs with polyethylene glycol (PEG). Over the past years, there is a rapid improvement in the utilization of MNPs as drug delivery carriers for the treatment of tumor cells [15]. Applying radiation or removing tumor surgically is the most common treatment for cancer. Utilizing therapeutic agents is non-invasive process and produce promising results when compared with other treatments, still they have some problem in practice: the selectivity of the treatments cannot reach the target site, tumor cells cannot be killed completely and the side effects cannot be controlled [16, 17]. To overcome these problems, the MNPs are selected to synthesize smart drug delivery systems to make the agents attack directly to the target site [18, 19]. To synthesize such type of drug delivery systems, we need to investigate the tumor and select materials for responding tumoral signal and thereby releasing drug to the target site. The growth of tumor tissues is completely different from normal healthy tissues, in addition to these, they have some biochemical differences and pathological differences; it provides chances for drug delivery system to attack the tumor selectively [20, 21]. Conjugation of targeting ligands such as antibodies, peptides or sequence of nucleic acid to target tissue or specific disease organ is mainly associated with surface chemistry of MNPs [22, 23]. Functionalized MNPs deliver the therapeutic drugs effectively by substantially increasing drug payload to the site of action into specific cells with minimal adverse effects, thereby diagnosis and treatment occur at the cellular level [24, 25]. By considering the effective advantages of MNPs due to their large surface area, tunable hole size, high pore volume and informal surface variation, this review explores the current challenges and perspectives of various metal-based nanomaterials for their potential applications in drug delivery systems especially in the treatment of cancer, diabetes, inflammation and in anti-viral therapy.
Methods of synthesizing MNPs
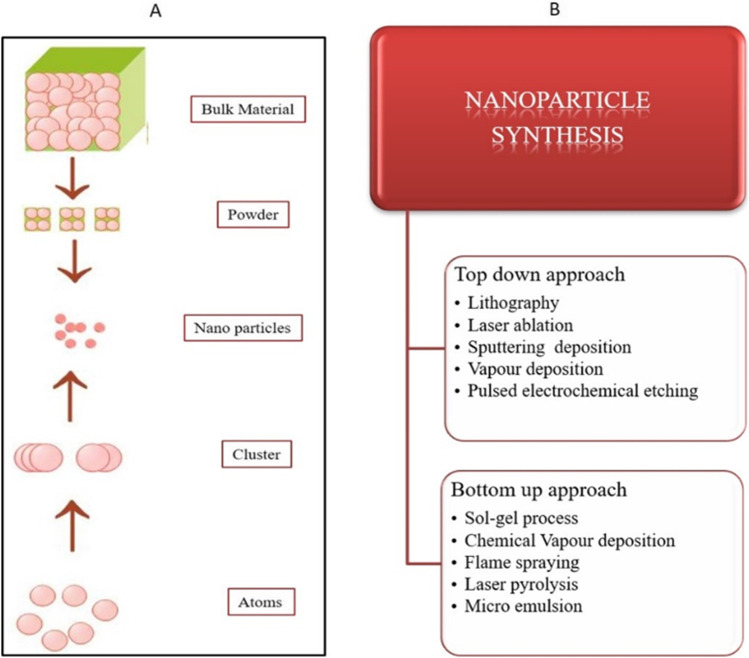
The synthesis of MNPs majorly involves two different approaches named as top-down approach or dispersion method and bottom-up approach or condensation method [26]. In top-down approach, the nanoparticles are formed by size reduction method where bulk materials are broken down into small materials. This can be accomplished with the use of ultrasonic generators of greater intensity operating at frequencies around 1,200,000 rpm [27]. Another dispersion method includes the generation of an electric arc within the liquid. Metal is dispersed as vapor from the electrode due to the intense heat produced from the electric arc which condensed further to form MNPs [28]. In the bottom-up assembly, nanostructures are fabricated atom by atom or particle by particle to build up nanostructure. This can be attained by a high degree of supersaturation followed by nuclei growth [29, 30]. By considering these two approaches, various scientists have reported a variety of chemical and physical methods for production of MNPs, for instance, chemical reduction [31], microemulsion [32, 33], thermal decomposition [34, 35], sonochemical [36, 37], polyol method [38, 39], microwave-assisted method [40, 41], laser ablation [42], sputtering deposition [43], lithography [44], pulsed electrochemical etching [45], vapor deposition [46] and sol–gel [47] (Fig. 2). In chemical methods, the harsh chemical additives are added to prepare the MNPs. For example, dimethyl formamide, hydrazine, and sodium borohydride are used as reducing agents and capping agents. Moreover, this method needs to be maintained under some physical conditions like high temperature and vacuum and also these methods need to face some environmental issues because of using toxic chemicals and the waste byproducts are discharged into soil or rivers which will affect the microorganisms, plants and human health [48]. So the researchers aim to minimize the hazards by synthesizing nanomaterials via greener methods by using plant parts (root, fruit, leaves, stem and flowers), and this method was considered as ecofriendly, simple, fast, and stable method.
Fig. 2.
A Schematic representation of top-down and bottom-up approaches. B Various methods involved in the synthesis of nanoparticles
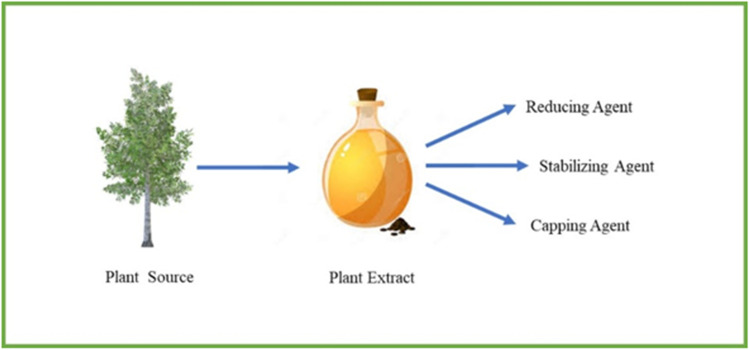
When the synthesis of MNPs was carried out by the green chemistry principle, the produced particle has more compatibility and also the greener synthesis of nanoparticles protects the environment from toxic effects [49–51]. In phytonanotechnology, the NP synthesis was carried out by using water as a solvent and this technique was non-toxic and the process was completed in a single step [52, 53]. The plant extract contains proteins, amino acids, vitamins as well as secondary metabolites which act as reducing agent, capping agent, and stabilizing agent during metal NP synthesis. By using this greener approach, hazards can be minimized compared to chemical synthesis. The reference guide for the researchers, scientists and the chemists are the 12 principles of Green Chemistry to develop the low toxic nanomaterials. The greener way of synthesizing nanoparticles, mainly used in the field of nanomedicine and nanodrug delivery systems. In ancient time, humans widely used these plant-based products as medicine for several diseases. Now nearly 25% of medicines are derived from natural resources [54]. The plant-based natural compounds are basis for the invention of novel drugs [55] and they have outstanding characteristics like chemical diversity, biological property and very less hazards. Natural compounds are proved to be effective for the treatment of several diseases, in addition they have many advantages like low cost, lower side effect and less toxicity [56–58].
Metallic nanoparticles in drug delivery
In the field of nanotechnology, metallic nanoparticles have shown the number of properties, and it has unlocked many new pathways in nanotechnology especially in targeted drug delivery systems (Fig. 3) [59]. MNPs are extensively utilized as drug delivery carriers for various therapeutic agents (antibodies, nucleic acids, chemotherapeutic drugs, peptides, etc.). Most of the MNPs like silver, gold, palladium, titanium, zinc, and copper nanoparticles possess enhanced tunable optical properties. Moreover, their surface can be easily functionalized to conjugate targeting agents and active biomolecules through H-bonding, covalent bonding and electrostatic interactions. In addition to this, multiple drugs can be easily loaded to achieve higher therapeutic efficacy [60]. MNPs are attained from their capability to increase the aqueous solubility of hydrophobic drug compound, enhanced circulation time of drugs in the blood and repress or eliminate fast renal drug excretion. Multifunctional nanoparticles have greater ability than conventional nanoparticles, to perform several goals synergistically such as co-delivery of multiple bioactive(s) with imaging agents, target-specific delivery through surface ligand decoration and simultaneously attainment of cancer therapeutics as well as diagnostics (Fig. 4) [61–63]. In drug delivery, there are three main goals: targeting therapeutic agent to the site of action, minimizing adverse effects of the drug to the healthy tissues or organs and controlling release of drug to avoid the classical overdosing/underdosing cycle [64]. MNPs provided a model to achieve these goals. Hence, the coating of MNP surface has been optimized to control the drug loading, drug delivery, and drug release in the target area [65]. Surface coating aim, in addition to improve biocompatibility and reduce side effects, is endowing MNPs with functional groups to be more suitable for drug combination. Efficient drug delivery through MNPs depends on two important factors: (i) design of MNPs for slow and sustained release of drug and (ii) ability of MNPs to distribute the therapeutic drugs to the target areas, without disturbing the other normal cells [66, 67]. By using active and passive targeting, these factors could be achieved easily. Passive targeting is possible due to the exclusive changes in the cancer vasculature. Owing to the fast growth of tumors, blood vessels and junctions are not shaped appropriately and can be leaky and lose. Due to the unique size of MNPs, they are able to travel through these loose junctions resulting in better accumulation at the tumor site and over time is primarily utilized for cancer therapeutics [68]. On the other side, in active targeting strategy, MNPs are conjugated with various active ligands that bind with specific cell surface receptors and ultimately lead to take the load to the desired site and release the drug [69].
Fig. 3.
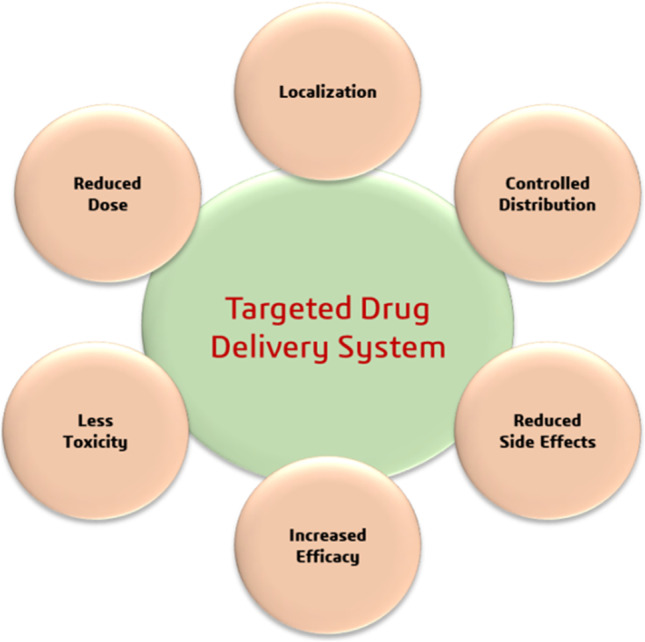
Advantages of targeted drug delivery system
Fig. 4.
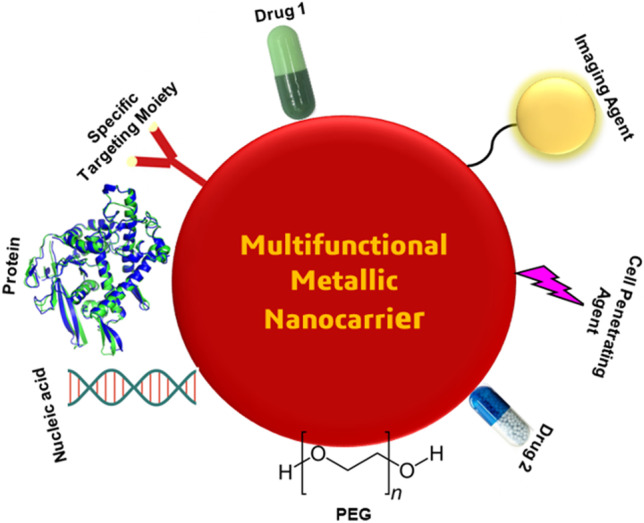
Multifunctional MNP-based delivery systems for targeting, delivery, and imaging
MNPs are explored in early and clinical studies for detection, diagnosis and treatment of several diseases. MNPs have received widespread attention based on their unique material and size-dependent physicochemical property which are impossible with organic NPs [70, 71]. FDA-approved metal nanoparticle–based nanomedicines in clinical use have been shown to enhance the bioavailability and efficacy of drug delivery systems, while at the same time reducing side effects due to their properties like improved targeted delivery to active cellular uptake. By adjusting their sizes and shapes, surface chemistry and doping techniques, the designed MNPs can decompose rapidly under specific physiological conditions and are thereby easily absorbed by various metabolic pathways without affecting the healthy tissues [72]. For example, MNPs approved for cancer therapy by FDA or EMA and clinical trials are given in Table. 1.
Table 1.
Metal nanoparticles for cancer therapy approved by FDA or EMA and clinical trials
| Nanomaterial | Sponsor/company name | Indication/application | Clinical trial identifier |
|---|---|---|---|
| Iron oxide |
Iron-based inorganic nanoparticles Magnablate I (University College London) |
Prostate cancer | NCT02033447 (Ph 0) |
| Magnetic iron oxide NPs | MagProbeTM (University of New Mexico) | Detection of leukemia | NCT01411904 |
| Gold nanoparticle with iron oxide silica cell | NANOM (Ural Medical University) | Plasmonic photothermal and stem cell therapy of atherosclerosis | NCT01270139 (not applicable), NCT01436123 (Ph I) |
| Spherical gold nanoparticle | NU-0129 (Northwestern University) | Recurrent glioblastoma or gliosarcoma undergoing surgery | NCT03020017 (Ph 0) |
| Silver nanoparticle gel | SilvaSorb (Madigan Army Medical Center) | Anti-bacterial | NCT00659204 (Ph III) |
| Nanocrystalline silver | Acticoat | Pemphigus; pemphigoid | NCT02365675 (not applicable) |
| Adhesive doped with Zn oxide + Cu nanoparticles in a (5%/0.2% concentration) | Cu/Zn nanoparticles (University of Chile) | Dental varies | NCT03635138 (not applicable) |
| Zinc oxide nanoparticles | Zinc oxide nanoparticles | Foot dermatoses; dental caries | NCT04000386 (not applicable), NCT03478150 (not applicable) |
| Titanium dioxide nanoparticles | Cairo University | Denture stomatitis | NCT02950584 (Ph I) |
| Mixture of gold and silver nanoparticles | NanoCare Gold | Caries class II | NCT03669224 (not applicable) |
| Hafnium oxide nanoparticle | Nanobiotix | Prostate adenocarcinoma | NCT02805894 |
| Silver nanoparticle/calcium hydroxide | Cairo University | Postoperative pain | NCT03692286 (Ph IV), NCT04213716 (Ph II) |
Characteristic properties of MNPs
Metallic nanoparticles generally possess large surface energies which is one of the most important quantities in understanding the thermodynamics of particles. The increase in surface area to volume ratio leads to an increase in dominance behavior of the atoms at the surface rather than the interior of the particle which subsequently results in an increase in overall surface energy [73]. By using various approaches such as molecular dynamics simulations, ab initio calculations and classical thermodynamic calculations, the surface energy of the metallic nanoparticle can be determined [74]. The high surface area of MNPs provides more possible reactive sites with high surface energy which makes them ideal candidates in drug delivery systems [75, 76].
Surface modification of MNPs
Surface modification is a rapidly growing area of focus in the fields of nanoscience and technology, along with the design and development of nanomaterials [77]. Surface modification is necessary to stabilize a nanoparticle and prevent agglomeration. It is also essential to improve the properties of MNPs in terms of biocompatibility, wettability, adhesion and toxicity up to sufficient extent prior to their practical application in drug delivery systems [78]. The surface modification of noble metals is typically carried out by adherence, mainly with a thiol group, disulfide ligands, amines, nitriles, carboxylic acids, and phosphines. The development and the utilization of organosulphur in the surface modification was mainly attributed to its chemical feasibility in the formation of strong covalent bond with noble metals such as Ag, Au, Cu, Pt, Hg, and Fe [79–81]. The crucial factor that enables the strong coordinate bond between the metal surface organo-sulfur is the higher affinity of sulfur to metal surfaces, thus making the organo-sulfur compounds readily absorbable. Surface modification of nanoparticles with long-chain polymers such as polyethylene glycol (PEG) was shown to minimize non-specific protein absorption onto the nanoparticle surface. Due to its intrinsic physicochemical properties, PEG is a favorable polymer for therapeutic nanoparticles, which decreases their phagocytic uptake and reduces their accumulation in non-target organs [82].
Greener approach in the synthesis of MNPs
Green chemistry for sustainable development has been universally studied extensively for the past 15 years [83]. The three important conditions for the synthesis of nanomaterials are the selection of environment-friendly solvent, a good reducing agent and a good stabilizing agent. Generally, the chemical methods used are too costly and incorporate the use of hazardous and toxic chemicals responsible for many risks to the environment [4], but the biosynthetic way is considered to be a safe and environmental-friendly green approach to synthesize nanoparticles for biomedical applications. The researchers from throughout the world is continuously involved in developing eco-friendly methods to produce products that are environmentally friendly and non-toxic, also highly effective, via the implementation of green nanotechnology [84, 85]. In biology and medicine field, the used nanoparticles should have the ability of less toxicity or absence of toxicity, high compatibility and biodegradability [86]. Metal nanoparticles have different target organs and reaction response mechanisms and they have the ability to penetrate easily to the body through the skin and overcome the biological barriers, bind to nucleic acids and proteins, are embedded in cell membranes, penetrate into cell nucleus and alter their functions, and express more biological activity due to their smaller size and large surface area [87, 88]. In spite of tremendous advantages of greener methods over other chemical methods in nanoparticle synthesis, these greener approaches also associated some minor disadvantages. The plant extracts cannot be manipulated by optimizing synthesis as a choice of nanoparticles through genetic engineering. In order to increase the productivity as a future approach, researchers are involved in the genetic modification of plants with improved metal tolerance and accumulation capacities.
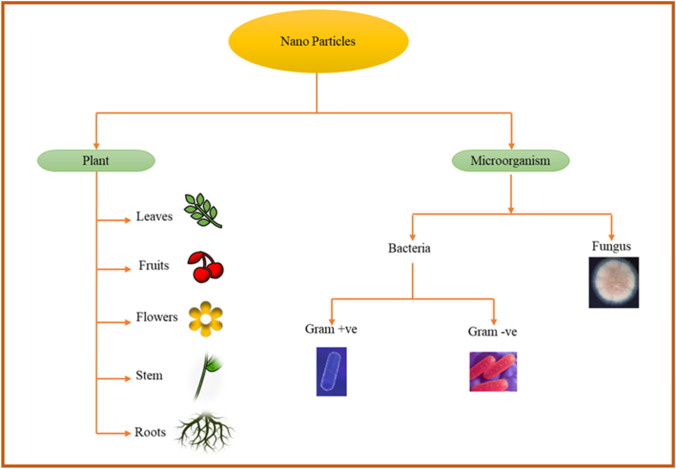
Synthesis of MNPs from biological source
The bio-mediated synthesis using microbes (Table 2) and plants (Table 3) has grown as a favorable substitute to traditional methods of nanoparticle synthesis [89]. Microorganisms and plants provide potential nanofactories for low cost and eco-friendly synthesis of various metallic nanoparticles such as silver, gold, palladium, copper, and metal oxides (Fig. 5).
Table 2.
Microbe-mediated nanoparticles
| Species | Type of microorganism | Mode | Metal | Size (nm) | Reference |
|---|---|---|---|---|---|
| Candida albicans | Fungi | Extracellular |
Ag Au |
60–80 20–40 |
[115] [116] |
| Fusarium oxysporum | Fungi | Extracellular |
Au CdS Zr |
20–40 5–20 3–11 |
[117] [118] [119] |
| Rhodopseudomonas capsulata | Bacteria | Extracellular | Au | 10–20 | [120] |
| Vibrio alginolyticus | Bacteria | Intra cellular and Extracellular | Ag | 50–100 | [121] |
| Pseudomonas stutzeri AG259 | Bacteria | Extracellular | Ag | 200 | [95] |
| Pseudomonas aeruginosa | Bacteria | Intracellualar | Pd, Ag, Rh, Ni, Fe, Co, Pt, Li | - | [96] |
| Shewanella loihica PV-4 | Bacteria | Extracellular | Pd, Pt | 2–7 | [97] |
| actinobacteria Rhodococcus NCIM 2891 | Actinomycetes | Extracellular | Ag | 10 | [101] |
| actinobacteria, Streptacidiphilus durhamensis | Actinomycetes | Extracellular | Ag | 8–48 | [102] |
| Streptomyces griseoruber, Streptomyces capillispiralis Ca-1 | Actinomycetes | Extracellular |
Cu Au |
- |
[103] [104] |
| Schizophyllum radiatum, | Fungi | Extracellular | Ag | - | [105] |
| Rhizopus oryzae | Fungi | Extracellular | Au | - | [109] |
| Candida utilis NCIM 3469 | Yeast | Extracellular | Ag | 20–80 | [111] |
| Candida lusitaniae | Yeast | Extracellular | Ag | 2–10 | [112] |
Table 3.
Plant-mediated greener nanoparticles
| Plant | Origin | Mode | Metal | Size (nm) | Reference |
|---|---|---|---|---|---|
| Andrographis paniculata | Leaves | Extracellular | Ag | 40–60 | [132] |
| Brassica oleracea | Arial parts | Extracellular | Ag | 36 | [133] |
| Camellia sinensis | Leaves | Extracellular | ZnO | 9–17.5 | [134] |
| Sapium sebiferum | Leaves | Extracellular | Pd | 2–14 | [135] |
| Spinacia oleracea | Leaves | Extracellular | ZnO | 40.9 | [136] |
| Alternanthera dentate | Leaves | Extracellular | Ag | 50–100 | [131] |
| Euphorbia nivulia | Plant | Extracellular | CuO | - | [130] |
Fig. 5.
Biosynthesis of MNPs
From bacteria and actinomycetes
Single bacteria have the ability of converting toxic metal ions into non-toxic NPs [90]. It is a promising source of synthesizing NPs because of its utilizing low energy and process controllability [91]. For example, the silver nanoparticles were synthesized from the source of Streptacidiphilus durhamensis HGG16n and it had a size range of 8–48 nm [92]; similarly Bacillus endophyticus [93] and Deinococcus radiodurans [94] are capable of producing silver nanoparticles with different shapes and sizes. Bacteria have the ability to reduce metallic ions into nanoparticles and are one of the most suitable applicants for nanoparticle synthesis because of their ease of handling. Silver nanoparticles (AgNP) synthesized by using the strain of Pseudomonas stutzeri AG259 with the size range of less than 200 nm by using NADH-dependant reductase enzyme that supplies electrons and itself oxidises to NAD+ [95]. The transfer of electrons from NADH shows the bio reduction of silver ions into silver nanoparticles. In 2012, Srivastava et al. reported that Pseudomonas aeruginosa has the ability to synthesize different nanoparticles intracellularly for example Pd, Ag, Rh, Ni, Fe, Co, Pt. and Li nanoparticles [96].
Nowadays, researchers are moving towards the synthesis and development of various nanoparticles such as palladium, platinum, and tellurium. For example, Ahmed et al. reported synthesis of ultra-small palladium and platinum nanoparticles by Shewanella loihica PV-4 within the size range of 2–7 nm [97]. Srinath et al. reported the synthesis of AgNPs using Bacillus subtilis [98] extracted from gold mine have high resistance to gold ions toxicity and can synthesize silver nanoparticle (AuNPs) efficiently. Sintubin et al. suggested that the cell wall of the bacteria may act as a capping agent for the production of NPs, stabilizing them by preventing aggregation. These author also shows the reduction process and the formation of nanoparticle may stimulate when there is an increase in the pH of the culture medium [99]. Generally, actinomycetes are used for the synthesis of extracellular enzymes and secondary metabolites [100] as well as they have ability for the biosynthesis of nanoparticles as they have unparalleled ability for the production of different bioactive compounds and contain high protein content. In 2012, Otari et al. prepared the silver nanoparticles by using Actinobacteria Rhodococcus NCIM 2891 [101] with the size range of 10 nm and spherical shape. Buszewski et al. prepared stable spherical shaped silver nanoparticle with the size range of 8 to 48 nm by using acidophilic actinobacteria, Streptacidiphilus durhamensis [102]. The recent reports described the synthesis of copper and gold nanoparticles using actinomycetes of Streptomyces griseoruber and Streptomyces capillispiralis Ca-1, respectively [103, 104].
From fungi and yeast
Fungal biosynthesis of nanoparticles is another easy and uncomplicated approach which has been promoted extensively for the production of nanoparticles. For the nanoparticle synthesis, fungi have higher productivity and higher tolerance to metals when compared with bacteria [105] as well as have the ability of higher bioaccumulation towards metal ions that leads to the production of nanoparticle that is efficient as well as low cost. Trichoderma viride reported AuNP biosynthesis within 10 min at 30 °C and have a property of catalyst (biocatalyst) and strong anti-microbial agents. Metuku et al. have reported white rot fungus and Schizophyllum radiatum, which have the ability of producing well-dispersed stable silver nanoparticles [106]. Candida albicans extract was used to prepare the Au and AgNPs [107] and the resulting nanoparticles are monodisperse and highly crystalline; the selenium nanoparticles (SeNPs) with the diameter range of 500 nm were prepared by using marine fungus Aspergillus terreus [108]. Kitching et al. reported the in vitro production of gold nanoparticles by using Rhizopus oryzae (cell surface proteins) as it has biomedical and bio catalytic applications [109]. Yeast has the ability to absorb and collect high concentrations of toxic metal ions from their surroundings [110]. Waghmare et al. reported the extracellular biosynthesis of AgNP using Candida utilis NCIM 3469. The obtained nanoparticles possess circular shape with a size range between 20 and 80 nm and they have anti-bacterial activity against some pathogenic strains (Staphylococcus aureus) [111]. In 2016, Eugenio et al. extract a yeast strain, Candida lusitaniae from the gut of a termite and show the production of silver nanoparticles with a diameter in the range of 2–10 nm [112].
From virus
Viruses can be employed for the preparation of nanoconjugates and nanocomposites with metal nanoparticles which are important materials in drug delivery and cancer therapy. The structural and biochemical stability of plant viruses provides a safe nanotechnology application as well as its ease of cultivation and non-toxicity in animals and humans. Cao et al. used red clover necrotic mosaic virus (RCNMV) for the synthesis of nanoparticles for the controlled delivery of doxorubicin drug for cancer therapy [113] as well as Le et al. investigated the ability of potato virus X nanoparticles for the delivery of doxorubicin drug for cancer treatment [114].
From plants
Plants are rich in secondary metabolites (alkaloids, flavonoids, and phenolic acids). Some of the studies have shown that the metabolites act as reducing, capping and stabilizing agents and inhibit the aggregation and agglomeration of the MNPs (Fig. 6) [122–124]. The bio-reduction of metal NPs using plant sources involved into three main phases. The first one is activation step, another one is thermodynamic stability and the last one is termination phase [125]. The concentration of the plant extracts influence the arrangement of the formed nanoparticles as well as the temperature and pH of the plant extract control the size and growth of the nanoparticles [126]. El-Kemary et al. investigated the biosynthesis of AgNPs using a leaf extract of Ambrosia maritime, which are rich in secondary metabolites and have strongly influenced the NP yield [127].
Fig. 6.
Role of secondary metabolites in the formation of MNPs
The use of plants for the production of metallic nanoparticles has become more focused in recent years because of its rapid, environmentally friendly, pathogenic and economical protocol providing a single-step mechanism for biological process [128]. Relatively high levels of steroids, saponins, carbohydrates and flavonoids act as capping-reducing agents and phyto-constituents that provide stability to silver nanoparticles [129]. Copper oxide nanoparticles were synthesized extracellularly by using a medicinal plant extract of Euphorbia nivulia and the particles are stabilized by the terpenoids and peptides present within the latex [130]. The aqueous extract of Alternanthera dentate was used for the synthesis of silver nanoparticles with the size range of 50–100 nm. The extracellular silver nanoparticles were synthesized within 10 min [131].
The mechanism for the plant based biosynthesis of n0 as zero valent metal atoms by extract polyphenols APOH when reacting with a metal halogen precursor is as follows:
Factors influencing the greener synthesis of metallic nanoparticle
Light: The rate of biosynthesis processes can affect when it has exposure to sunlight; for example, generally, the biosynthesis of AgNps was achieved at 12 h but exposure to sunlight reduced the reaction time up to 12 h to 5 min. It was observed by Raut et al. [137].
Temperature and heating rate: The temperature can straightly affect the size, shape and rate of the formation of biosynthetic NPs [138]. For example, the size-selective synthesis of CuNPs could be achieved by adjusting the reaction temperature; the resulting nanoparticles have the diameters ranging from 5 to 25 nm as well as the shape of the CuNPs also change with the reaction temperature, enabling the formation of rod-shaped and cubical shape products [139].
pH: pH shows important effects on the shapes and sizes of NPs. For example, at pH 7, the gold nanoparticle was synthesized by using Rhodopseudomonas capsulate; it gives spherical shaped AuNPs but changing the pH to 4, it gives nanoplates. So changing of the pH value gives the different morphology and it has been proved by He et al. [120].
Time: Reaction time is also affecting the size and morphology of NPs. In increasing the reaction rate, the size and breadth of the synthesized nanoparticles can be reduced [140, 141].
Toxicity of green nanoparticle
The green nanoparticles can cause damage to the cell membrane, oxidative DNA damage and inhibit the electron transport chain as these have the ability to enter into cells and cause destruction when present above a threshold limit. However, the exact inhibition mechanism has not been demonstrated because there is limited available data on the biological activity of the green nanoparticles [142].
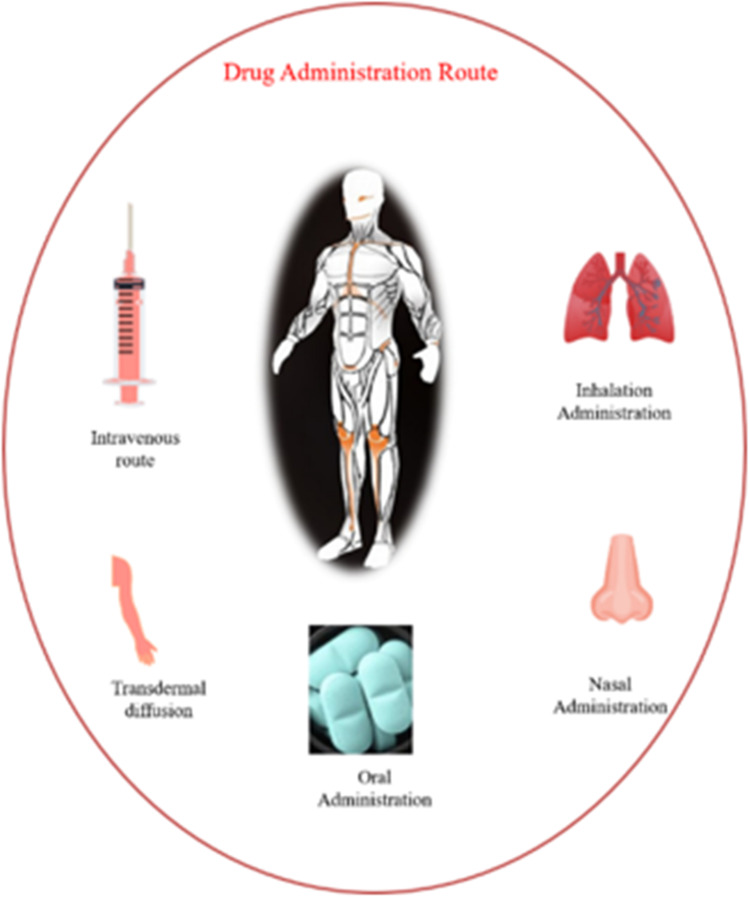
Administration routes of drug delivery
The choice of delivery route is driven by patient acceptance, drug properties (such as its solubility), access to a disease location, or efficacy in dealing with a specific disease (Fig. 7). There are several reports of drug delivery based on metal nanoparticles via transdermal drug delivery system (TDDS) and intravenous. Intravenous (IV) route is an invasive technique, although it has the advantage of avoiding drug dysfunction during the first pass metabolism and rapidly increasing drug concentration in a proper cycle. Compared to IV, TDDS offers many benefits, e.g., (1) the skin is easily accessible for absorption and it is harmless and patient-friendly and (2) TDDS drugs can be more systematically released into the system (146).
Fig. 7.
Schematic representation of drug administrative route using MNPs
Transdermal route
In the skin, the penetration of drugs occurs by the process of spreading throughout the skin layers filled with byproducts. Basic properties of drugs manage molecular flow through these routes. The skin generally acts as a shield for efficient drug delivery, but there are other ways to achieve proper absorption. Various infiltration pathways are recognized in TDDS: (1) intercellular, (2) intra/trans cellular and (3) trans appendageal. Transdermal drug delivery avoids problems such as gastrointestinal irritation, metabolism, variations in delivery rates and disruption caused by food. It is also acceptable for patients under anesthesia. This technique is normally non-invasive and aesthetically acceptable and can be used to deliver local delivery for several days. Suksaeree et al. have reported silver nanoparticle (AgNP)-loaded anti-microbial wound dressing patch using ethyl cellulose as a matrix membrane and diethyl phthalate as a plasticizer. The anti-microbial wound dressing patch did not exhibit any interaction between the matrix membrane and AgNPs. The AgNPs were evenly dispersed in the patch. The patch could control the release of silver at 102.98 ± 4.11% over 12 h [143].
Intravenous route
MNPs can be administered using different routes namely intravenous, intraperitoneal injection, pulmonary inhalation and oral administration. The intravenous route delivers almost instantaneous response and permits systematic control of the rate of drug contribution into the body. It is also suitable for drugs which cannot be absorbed by the gastrointestinal tract or which cannot be injected into muscles or other tissues; equally important, it overcomes the problem of first-pass metabolism. Expensive drugs such as peptides and proteins are delivered efficiently by intravenous route. Intravenous administration overcomes the degradation by proteolytic enzymes. The main advantage of intravenous drug delivery is the rapid onset of action and complete bioavailability of drugs even with low doses. [144].
Various metallic nanocarriers in drug delivery systems
Silver nanoparticles
Silver is the most profit-oriented precious metal used in the preparation of NPs and nanomaterials. These are known because of their anti-bacterial, anti-viral, anti-fungal, antioxidant and unusually enhanced physicochemical properties compared to the bulk material such as optical, thermal, electrical and catalytic properties [145]. About 500 t of silver nanoparticles (AgNPs), used in various industries and everyday life, is produced per year. Rising demand for silver nanomaterials requires the development of eco-friendly synthesis methods. AgNPs interrelate with microbes and discharge the silver ion in the de-activation of cellular-based enzymes, delayed membrane penetrability. Silver nanoparticles have been found to induce cytotoxicity via apoptosis and necrosis toward a range of different cell types. Moreover, they exhibit results against secondary effects of current therapies as well, such as deoxyribonucleic acid (DNA) damage, generation of reactive oxygen species (ROS), increasing leakage of lactate dehydrogenase (LDH) and inhibiting stem cell differentiation.
AgNPs in cancer therapy
Recent advancements in the field of oncology and therapeutic research of nanodiagnostic and nanotherapeutic agents have gave important improvements in the use of nanoscale metal particles as a solid carrier for the site-specific delivery of the drug release process [146]. Cancer is one of the leading causes of mortality in the modern world, with more than 10 million new cases every year. Anti-cancer activities of silver nanoparticles (AgNPs) are also being widely studied (Table 4). AgNPs have the ability to stimulate the production of reactive oxygen species (ROS) and thus destroying the mitochondrial respiratory chain of cancer cells. The (AgNPs) have proven promising anti-cancer effects [147]. Some researchers have reported that AgNPs induced a cytotoxic effect against leukemic cells. Guo et al. studied the PVP-coated AgNPs could effectively reduce the activity of acute myeloid leukemia (AML) cells and stimulate DNA damage and apoptosis through the generation of reactive oxygen species (ROS) and the release of silver ions. Sahu et al. reported the significant concentration-dependent cytotoxicity of AgNPs in human liver (HepG2) cells in the concentration range of 1 to 20 μg/mL [148]. El-Deeb et al. reported the simple, low cost and eco-friendly synthesis of AgNPs by using honey bees. The prepared nanocarrier was used for the treatment of colon carcinoma Caco-2 cells. They noted that the obtained AgNPs could be safely used with concentrations up to 39 μg/mL with 60% inhibition of Caco-2 cell proliferation [149]. AgNPs have exhibited cytotoxic effect to MCF-7 breast cancer cells at 20 µg/mL for 48 h [150] and also its suppressed lung cancer cells, H1299 in which 50% of cells were killed at 5 µg/mL [151] In colon cancer cells, AgNPs have shown effective killings of 5 to 28 g/mL in cell lines such as HCT116 [152], Caco-2 [153] and HT-29 [154] as well as AGNPs have been shown to have great potential to act as a nanocarrier to deliver anti-cancer drugs to cancer cells [155, 156]. This evidence strongly supports the potential use of AGNPs for cancer chemotherapy [157]. K. X. Lee et al. prepared AgPCA (PCA: protocatechuic acid) by using Garcinia mangostana fruit peel extract. They reported AgNPs loaded with PCA (AgPCA) resulted in 80% of inhibition at 15.6 µg/mL as compared to AgNPs which only killed 5% of HCT116 colorectal cells at the same concentration [158].
Table 4.
AgNPs in anti-cancer therapy
| S. no | Material used | Activity/cell line | Reference |
|---|---|---|---|
| 1 | PVP-coated AgNPs | Acute myeloid leukemia (AML) cells | [149] |
| 2 | AgNPs | Human liver (HepG2) cells | [148] |
| 3 | AgNPs | Colon carcinoma Caco-2 cells | [149] |
| 4 | AgNPs | Cytotoxic effect to MCF-7 breast cancer | [150] |
| 5 | AgNPs | Colon cancer cells/ HCT116, Caco-2 and HT-29 | [152–155] |
| 6 | AgPCA | HCT116 colorectal cells | [159] |
| 7 | AgNPs-MTX | Lung cancer cell line (A-549) | [160] |
| 8 | (MTX-GO/AgNPs) | Anti-cancer pharmacological activity | [161] |
| 9 | Ag-NGO-DOX | HepG2 cancer cell line and HEK293 cell line | [163] |
| 10 | DOX-AgNPs | Inhibition of the proliferation of cancer cells B16F10 | [164] |
| 11 | IMAB-AgNPs | Cytotoxicity in MCF-7 cells | [165] |
| 12 | AgNPs | Cytotoxicity and apoptosis in A2780 cells | [166] |
Rozalen et al. [159] synthesized AgNPs (size of 11.13 ± 2.3 nm), then it is coupled with methotrexate (AgNPs-MTX) and it shows anti-cancer activity, and the lower dose of AgNPs-MTX was needed compared to free methotrexate, as well as the authors reported that the anti-cancer activity was more noticeable in the colon cancer cell line (HTC-116) than in the lung cancer cell line (A-549). Thapa et al. prepared the nanocomposite of AgNPs embedded in graphene oxide (GO) and coupled with methotrexate (MTX-GO/AgNPs). The nanocarrier combines the combined effect of AgNPs, which increase the production of reactive oxygen species that cause DNA damage, leading to improved cellular apoptosis. The combinational therapy system MTX-GO/AgNPs potentially evaluated for effective folate receptor-targeted treatment of cancers [160]. Palai et al. [161] synthesized AgNPs coupled with doxorubicin by utilizing a biosynthesis approach. The drug release by the nanosystem was studied at two different pHs of 7.4 and 5.4. From the result, at acidic pH higher rate of drug release was observed as well as at both pH, it was possible to note a controlled release of doxorubicin up to 120 h. The authors reported the synthesized nanocarriers had a less damaging effect on cancer cells of the HeLa cell line compared to free doxorubicin; it showed less cytotoxicity in the normal HaCaT keratinocyte cell line and the nanosystem shows less harmful effect on normal cells compared to free doxorubicin.
Zeng et al. (2018) [162] prepared AgNPs coated with graphene oxide (Ag-NGO) as doxorubicin nanocarriers. The Ag-NGO-DOX nanocarrier has the potential to deliver the drug and released it at a targeted location, due to the intracellular acidic pH responsive release of DOX, making viable the drug to reach the nucleus of cancer cells (HepG2 cancer cell line and HEK293 cell line). In 2015, Patra et al. [163] synthesized AgNPs by using Butea monosperma leaf extract, then the AgNPs were coupled with doxorubicin. The DOX-AgNPs nanosystem produced greater inhibition of the proliferation of cancer cells B16F10 of murine melanoma, and MCF-7 than free doxorubicin. In 2017, Shandiz et al. [164] synthesized AgNPs coupled with imatinib (IMAB) through biosynthesis (Eucalyptus procera extract) method. The authors finalize that the IMAB release could be split into two stages. In the first stage, till ~ 40 h, there was a burst release of the drug and in the second stage; the drug release was gradual till a release of 86.56 ± 2.04% at 80 h of contact time in a releasing inducing medium of phosphate buffer. The IMAB-AgNPs show greater cytotoxicity in MCF-7 cells than the free drug of imatinib.
Yu-Guo Yuan and collaborators have shown that the synergism between AgNPs and gemcitabine generated a higher cytotoxicity and apoptosis in A2780 cells, compared with the use of the therapeutic agent without nanoparticles [165]. Moreover, it was demonstrated that AgNPs can improve the responsiveness to gemcitabine or salinomycin in ovarian cancer cells, leading to an increased level of different proapoptotic genes, such as tumor protein p53, p21, Bax, Bak, and activation of caspases 3 and 9. The synergistic effect between silver nanoparticles and camptothecin in human cervical cancer cells (HeLa) was inferred from their ability to activate caspases 9, 6, and 3. Moreover, increased levels of p53, p21, cyt C, Bid, Bax, Bak and modulated expressions of Akt1, RAF, MEK, Erk1/2, JNK, P38, NF-κB and Cyclin D which are known as molecules involved in cell survival were observed in HeLa cells after using AgNPs combined with camptothecin [166]. AgNPs are also active significant therapeutics for HIV infection treatment. AgNPs induce anti-viral properties through the binding process to gp120 with the subsequent inhibition of CD4-reliant.
AgNPs as anti-viral agents
The development of resistance by various viral pathogens against anti-viral agents signifies another main cause of death, which is a major concern of the pharmaceutical, medical and biotechnological systems [166]. In recent years, there is a tremendous growth in the emerging applications of AgNPs as anti-viral agents, due to their inhibitory efficacy against numerous viruses, including certain strains of hepatitis, coronavirus, influenza, herpes, recombinant respiratory syncytial virus and human immunodeficiency virus [167]. It is widely accepted that AgNPs contribute towards the inhibition of virus because of effective interactions with sulfhydra, amino, carboxyl, phosphate and imidazole groups. A delivery system was designed at nanoscale with AgNPs for zanamivir medication which is commonly used to treat and prevent influenza virus. Similarly surface enrichment of the AgNPs with amantadine was developed to avoid the resistance shown by H1N1 virus [168]. Compared with single drug treatments, self-assembly of amantadine and zanamivir over the surface of AgNPs produces better inhibitory potential of neuraminidase and hemagglutinin activity.
Gold nanoparticles
Gold nanoparticles (AuNPs) are effective radiosensitizers in medical applications such as drug delivery and cancer therapy [169]. Au NPs can deliver multiple drug molecules, recombinant proteins, vaccines or nucleotides into their targets and can control drug release via biological stimuli (internal) or light activation (external). Au NPs are known to be an effective nanocarrier for various drugs such as peptides, plasmid deoxynucleic acids (pDNAs), proteins, small interfering ribonucleic acids (siRNAs) and chemo-therapeutic agents. AuNP-based drug delivery has gained considerable attention because of its prominent performance [170]. Gold particles that are modified with nuclear localization signal from simian virus (SV40) have been widely used for drug carrier to nucleus. Surface functionalization is one of the most favorable properties of Au NPs in the biomedical domain [171].
AuNPs in cancer therapy
For gold nanoparticles to be effective as a pharmaceutical, it is essential to have a firm understanding of their biodistribution/accumulation in living systems. To achieve this, it is necessary to have proper characterization of the nanomaterials and a good animal model with an appropriate sample size and robust statistical analyses. The use of gold nanoparticles (AuNPs) reduces the risk of side effects and controls the damage to healthy cells [172]. AuNPs are a novel factor in cancer therapy and show aggregation [173] and size-dependent cytotoxic activity against various cancer cells [174, 175]. The mechanism beyond the anti-cancer activity of AuNP is quite difficult and not well understood. AuNPs have positive charges, while cancer/normal cell membranes contain negatively charged substances such as lipids having counter-charges is responsible for taking over and internalizing AuNPs [176]. Another way for gold nanoparticles to enter cells is by endocytosis as shown in the study, endocytosis causes small AuNPs to accumulate within HeLa cells [177]. The surface of AuNPs can be functionalized with various biomolecules such as DNA, peptides and antibodies. For example, 13-nm colloidal Au has been combined to methotrexate which is an analog of folic acid and has the ability to restrain the growth and reproduction of cancer cell and has been usually used as an anti-cancer drug. Gold nanoparticles containing cedoximab and gemcitabine (anti-cancer drugs) were developed for TDDS and were able to inhibit pancreatic tumor cells in vitro and tumor model [178]. Eghtedari et al. have fabricated the AuNPs for in vivo targeting to breast cancer cells. Herceptin was used to functionalize the AuNPs by molecular recognition of breast cancer cells along with PEG [179].
AuNPs in the treatment of bacterial infections
Due to the development and extent of drug resistance by bacterial pathogens, it resulted in the decrease in effectiveness of antibiotics over period of time. The so-called “antibiotic resistance crisis” caused by drug-resistant bacteria results in additional medical costs of up to billions of dollars annually [180]. AuNPs exhibit a combination of physical, chemical, optical, and electronic properties unique from other biomedical nanotechnologies and provide a highly multifunctional platform in drug delivery systems as joint anti-bacterials [181]. Gentamicin sulfate is an aminoglycoside and has a broad range of anti-bacterial activities. However, gentamicin sulfate has some serious side effects such as ototoxicity and nephrotoxicity, which restrict its usage. It also does not make cell membrane efficiently and is highly soluble to water. Gentamicin can be used for the treatment of serious microbial infection when it is conjugated to Au NPs [182]. Ampicillin functionalized AuNP prepared by Brown et al. was an effective broad-spectrum fungicide that can resist Gram-negative and Gram-positive bacteria. This functionalization method mainly utilizes ampicillin’s ability to permeate the outer membrane of bacteria. Nanoparticles enter the bacteria to achieve anti-bacterial effects. This combined anti-bacterial effect can destroy ampicillin-resistant bacteria [183].
AuNPs in the treatment of diabetes and inflammation
Govindaraju et al. synthesized guavanoic acid functionalized gold nanoparticles and the strong in vitro anti-diabetic activity of guavanoic acid functionalized gold nanoparticles was studied by using L6 rat skeletal muscle cell lines. The active nanoparticles of guanonic acid have been found to enhance the function of insulin-dependent glucose uptake [184]. Yujie Zhang et al. prepared the two types of glucose-responsive high-drug loading AuNCs (gold nanocluster) which were produced for insulin release. The first type of AuNCs was synthesized based on CR9 peptide and it was coupled with 4-carboxy-3-fluorophenylboronic acid (FPBA–COOH) and insulin molecules to obtain AuNC nanocomplex drug namely, (CR9–AuNC–FPBA–Ins) and the second type of AuNCs was synthesized based on bovine serum albumin (BSA), the carboxyl-enriched BSA–AuNCs were coupled with 4-aminophenylboronic acid (NH2–PBA) molecules and insulin, labeled as BSA–AuNC–PBA–Ins and then the micro needle (MN) patch was loaded with the above AuNC nanocomplex drugs. The authors have successfully reported the soluble and glucose-responsive insulin-releasing MN patch system for the treatment of type 1 diabetes. MNs are fused with high-drug loading AuNC nanocarrier drugs, where gold nanomaterials enhance the mechanical strength of MNs to effectively penetrate the skins of mice [185]. Similarly they developed a gold nanocluster (GNCs) based glucose-responsive insulin releasing system for glucose control in diabetes. The GNCs prepared with one-step reaction and the GNCs are coupled with the following materials like 4-carboxyphenylboronic acid (PBA) and 4-carboxy-3-fluorophenylboronic acid (FPBA). Complex prepared for glucose-responsive insulin release and glucose regulation in diabetes. This nanocomplex has the ability to release insulin quickly into the hyperglycemic state and effectively maintains blood glucose levels in the type 1 diabetic mice at the normoglycemic level for up to 48 h [186].
Nanoparticles have the ability to penetrate epithelial cells and inflammatory cells resulting in better efficacy and better durability in the treatment. They also have the best selection of target sites such as inflammatory cells or tissues [187]. Kim et al. prepared methotrexate (MTX)-loaded Au/Fe/Au plasmonic nanoparticles coupled with arginylglycylaspartic acid (RGD) for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis. Under near-infrared (NIR) irradiation, the Au half-shell produces heat at the inflammation area and speeds up the release of MTX. Fe half-shell can deliver nanoparticles to swollen joints under the action of an external magnetic field and increase their residence time in the joints. The study combined the use of low-infrared radiation and external magnetic field so that a low-dose MTX can achieve the best therapeutic effect for arthritis [188].
Palladium nanoparticles
To overcome the inherent limitations of conventional drug therapies with many limitations, such as low selectively, rapid excretion and severe toxicity, the controlled drug delivery system has been gaining attention. Pd is a very precious metal with extraordinary catalytic, powerful mechanical and electroanalytical properties [189]. Pd nano-based structures have been developed as self-therapeutics, with proven anti-bacterial and cytotoxic pharmacological activity [190]. The high porosity of PdNPs was the genius property for loading anti-cancer drugs. The therapeutic drug can be indirectly conjugated to the PdNPs through a linking molecule. Shanthi et al. have developed pH responsive carrier of palladium NPs with DOX through acid labile linkage and exhibited pH-mediated release of doxorubicin in endosomal compartment of drug-resistant cervical cancer cells [191]. For example, Dox, which was glued to PEGylated PdNPs through a hydrazine bond, showed a pH-responsive releasing profile in human cervical cancer cells (HeLa) and strong anti-tumor efficacy against HeLa tumor xenograft models in vivo. As mentioned earlier PdNPs possess anti-cancer activity on various cancer cell lines resulting in potential toxicity, which may affect the results of drug-delivery studies. The effect of cancer drug on targeted cells/tumors need to be carefully examined by correlating the cytotoxicity of mono-modal PdNPs as self-therapeutics and that of dual-modal cancer drug-loaded PdNPs. The in vitro experiment revealed that 20 µg/mL of PdNPs which was synthesized by chaga mushroom extract was able to kill approximately 20% of HeLa cells; meanwhile, 30% of HeLa cells were killed by the Dox-loaded PdNPs (20 µg/mL of Pd).
Platinum nanoparticles
Platinum nanoparticles (PtNPs) are noteworthy scientific tools that are being explored in various biotechnological, nanomedicinal and pharmacological fields. They are unique because of their large surface area and their numerous biomedical applications as anti-microbial, antioxidant and anti-cancer properties [192]. However, recent reports have demonstrated the toxicity of PtNPs, limiting the use of PtNPs in healthcare and medicine. As a consequence, it is an ultimate challenge to design and develop biocompatible PtNPs for cancer therapy [193]. Another key challenge in drug delivery is low circulation and retention of nanoparticles and nanodrug conjugates inside tumors limiting the efficacy of the nanomedicine [194]. Generally, these nanoconjugates are often cleared from the body in a short time following their recognition by the immune system. In this context, pegylation (PEG is FDA approved) of nanoparticles has become a popular technique; which increases the blood circulation and retention time and decreases rapid clearance and non-specific interactions with serum proteins. Mukherjee et al. have reported improved delivery of doxorubicin using rationally designed PEGylated platinum nanoparticles for the treatment of melanoma. Intraperitoneal (IP) administration of PtNPs-DOX shows substantial reduction of tumor growth in subcutaneous murine melanoma tumor model compared to control group with free drug. Upregulation of tumor suppressor protein p53 and downregulation of SOX2 and Ki-67 proliferation markers in melanoma tumor tissue indicates probable molecular mechanism for the anti-cancer activity of PtNPs-DOX [195].
Copper nanoparticles
Copper nanoparticles (CuNPs) have gradually become an active area of research due to their unique physical, chemical, electrical and optical properties, low cost, availability and exhibit good anti-bacterial properties [196, 197]. The prime advantage of CuNPs is their low cost and its availability compared to gold and silver nanoparticles, resulting in the sample synthesis and various applications of CuNPs [198, 199]. Copper is a readily available metal and one of the essential trace elements in most living organisms. Experimental studies on the development of targeted drug delivery and bioimaging molecules resulted in the formation of transferrin (Tf) templated copper nanoclusters (Tf-CuNCs) with enhanced luminescence. The corresponding nanomolecules were further formulated into spherical transferrin copper nanocluster-doxorubicin (Dox) NPs (Tf-CuNC-Dox-NPs) of spherical shape, based on the electrostatic interactions with doxorubicin. The newly synthesized nanomaterials were further assessed in vivo on TfR (transferrin receptor) positive DLA (Dalton’s lymphoma ascites) bearing mice revealing enhanced inhibition of tumor growth and prolongation of the survival of the animals [200]. Kamble et al. have reported curcumin-capped CuNPs as possible inhibitors of human breast cancer cells and angiogenesis: a comparative study with native curcumin [201]. Vikram et al. mupirocin coupled copper nanoparticles were synthesized to overwhelm drug resistance in Staphylococcus aureus, responsible for dermal skin infections. In vitro release study of CuNPs shows 96.5% release of drug and show effective anti-bacterial activity against Staphylococcus aureus [202].
Zinc oxide nanoparticles
Zinc oxide (ZnO) NPs have promising approach in drug delivery system in cancer, diabetes, and inflammation therapy. Zhang et al. have synthesized ZnO NPs as a drug carrier for the anti-cancer drug daunorubicin (DNR) by using simple one-step process at room temperature in the presence of air. The investigation discloses that the combination of ZnO-NPs-DNR has stimulated a significant reduced cytotoxicity of anti-cancer drug and considerable increase in the cancer cell targeting mediated by ROS in human hepatocarcinoma cells (SMMC-7721 cells) [203]. J. Hussein et al. synthesized green ZnO NPs using the solid state approach in the dry state capped by gum arabic (GA) exhibit as an excellent anti-diabetic agent with high cytocompatibility as a nanodelivery system. Following this, hydrophobic docosahexaenoic acid (DHA) was loaded on ZnO NPs to investigate the anti-diabetic efficacy of free DHA compared with loaded DHA NPs. The blood glucose, insulin resistance, oxidant and antioxidants, cholesterol, triglycerides, fatty acid parameters and phosphoinositide 3-kinase (PI3K) levels are demonstrated in the preparation of diabetes in experimental rats. It confirms that the biocompatibility and physicochemical properties of zinc oxide nanoparticles played a significant role in the treatment of diabetes [204]. P. Chauhan et al. have investigated the changes assisted by the treatment of streptozotocin influenced diabetic rats with nanocurcumin-zinc oxide (ZnO-NC) nanoparticles, encapsulated in chitosan (CS-ZnO-NC) on different biomarkers associated to the metabolic and oxidative accelerations were observed in this experimental model (streptozotocin induced diabetic Wistar rats) of diabetes mellitus. The CS-ZnO-NC is useful for the treatment of diabetes mellitus and has no toxicity [205]. Ekta Yadav et al. have prepared ZnO NPs by using Trianthema portulacastrum Linn. (TP) plant extract via co-precipitation method. These greener nanoparticles showed strong in vitro anti-inflammatory activity. Topical treatment with ZnOTP on injured skin tissue led to rapid closure and wound healing due to reduced collagen fibers, tissue granulation, antioxidant pressure, and inflammatory reactions [206].
Titanium dioxide nanoparticles
Titanium dioxide (TiO2) or titania is a semiconductor metal oxide with potential applications in drug delivery systems especially in cancer because of its chemical stability as well as low-toxicity and low cost [207, 208]. Titanium dioxide exists in two crystalline forms namely rutile and anatase which is chemically more active. Rutile form of TiO2 nanoparticles can also be called as titanium dioxide fine particles (TiO2 FPs). With the increase in crystalline nature of anatase form, there is an increase in the generation of reactive oxygen species which substantially makes the anatase phase more toxic to healthy cells when compared to rutile phase. The rutile titanium dioxide is considered as chemically inert but when the particles become smaller, the surface area will increase and therefore the rutile titanium dioxide particles can become harmful, according to the studies. Also, the chemical changes carried out on the surface of nanoparticles cause alterations in the overall activity of TiO2 NPs [209]. Various nanostructures of TiO2 like TiO2NPs, TiO2 nanotubes, TiO2 matrices, as well as TiO2 capsules and TiO2 whiskers (TiO2 Ws) have emerging applications as drug delivery systems for different anti-cancer drugs, such as gambogic acid (GA), cisplatin, valproic acid, doxorubicin (DOX), temozolomide (TMZ) and daunorubicin (DNR). For example, one-dimensional TiO2 Ws clearly enhanced the cytotoxic effects of DNR by increasing its dose inside human SMMC-7721 hepatocarcinoma cells [210]. Similarly the anti-tumor efficacy of TMZ, a therapeutic drug for brain gliomas, could be improved by coupling the TMZ with TiO2 nanostructures [211]. In addition to this encapsulation of valproic acid into TiO2 matrices led to gradual but long lasting release of valproic acid in numerous diseases [212, 213]. Moreover, GA and DNR from GA-TiO2 and DNR-TiO2 nanocomposites represented more potential anti-tumor efficiency in human leukemia K562 cells, when compared with its individual drugs [214, 215].
Metal sulfide nanoparticles
Metal sulfide nanomaterials (MeSNPs) are a novel class of metal-containing nanomaterials composed of metal ions and sulfur compounds [216, 217]. During the past decade, scientists found that the MeSNPs engineered by specific approaches not only had high biocompatibility but also exhibited unique physicochemical properties for cancer therapy. Yang et al. have reported engineered polydopamine (PDA)-coated hollow mesoporous nickel sulfide (NiS) NPs (hm-NiS) for the delivery of doxorubicin (DOX) [218]. The encapsulation efficiency and loading capacity of DOX were respectively estimated as 66.9 and 7.1%. The high efficiency of drug encapsulation not only resulted from the internal cavity and hollow mesoporous structural framework of hm-NiS but also from the strong interaction between DOX and PDA. Li et al. designed a kind of mesoporous hollow CuS nanoparticles (H-CuS NPs) for delivering chlorine6 (Ce6, a kind of photosensitizer) and DOX to tumor sites [219]. The thermo-responsive degradation feature of H-CuS NPs could trap drugs interiorly in the cavity of H-CuS nanovehicles, thus functioning as a removable plug and thereby attained the controlled release of drugs by light-induced thermal stimuli. The attribute was highly useful for targeted delivery of the drug, which only caused a minimal release of drug non-specifically in the circulation, thereby enhancing the drug bioavailability in tumor tissues via improved permeability and retention effects. Hou et al. have reported copper sulfide nanoparticle-based localized drug delivery system as an effective cancer synergistic treatment and theranostic platform [220]. They introduced diffusion molecular retention (DMR) tumor targeting effect, a new strategy that employed transferrin (Tf)-modified hollow mesoporous CuS nanoparticles (HMCuS NPs) to undergo extensive diffuse through the interstitium and tumor retention after a peritumoral (PT) injection. The results exhibited that TfHMCuS NPs prolonged the local accumulation and retention together with slow vascular uptake and extensive interstitial diffusion, which was consistent with the biodistribution studies of AS/Tf-HMCuS NPs. Xie et al. engineered a kind of two-dimensional tin sulfide nanosheets (SnS NSs) with a high loading rate of DOX (up to about 200% in weight) through electrostatic absorption between the negative potential carriers and positively charged DOX [221], which was larger than that of mesoporous MeSNs (about 7%).
Nanoscale metal organic frameworks
Metal organic frameworks (MOFs) are considered as a potential class of nanocarriers for drug delivery owing to well-defined structure, ultrahigh surface area and porosity, tunable pore size, and easy chemical functionalization [222]. Recently, these materials have been scaled down to nanometer sizes, and this results in the production of nanoscale metal–organic frameworks (NMOFs) for biomedical applications [223]. The unique properties of NMOFs such as large pore volume, highly ordered structure and large surface area enable them to adsorb functional molecules on their external surface or open channels, as well as trap these molecules inside the framework [224]. NMOFs possess several potential advantages over conventional nanomedicines such as their structural and chemical diversity, their high loading capacity, and their intrinsic biodegradability [225]. To increase the biological functionality and biocompatibility functionalization of MOFs with biomolecules such as nucleobases, saccharides, peptides, and amino acids have been employed as the organic ligands to prepare bio-MOFs. Zirconium-based MOF was prepared as a nanocarrier for the controlled release of ibuprofen in an acidic phosphate buffer solution [226]. Horcajada and collaborators reported the use of a series of non-toxic porous iron (III)-based nanoMOFs [227], i.e., MIL-53, MIL-88A, MIL-88Bt, MIL-89, MIL-100 and MIL-101-NH2, as carriers of antineoplastic and retroviral drugs, including busulfan, azidothymidine triphosphate, doxorubicin or cidofovir, ibuprofen, etc. ZIF-8 (Zeolitic Imidazolate Framework) is an outstanding representative for drug delivery, which is constructed by zinc ions and 2-methylimidazole and possesses unique merits such as high porosity and stability, good biosecurity, and pH-induced degradability. Recently, Willner and co-workers encapsulated two drugs (e.g., insulin and anti-vascular endothelial growth factor aptamer (VEGF aptamer) and glucose oxidase (GOx) into ZIF-8 nanoparticles to build a glucose-responsive vehicle for drug controlled release [228]. Zhuang et al. have reported optimized metal organic framework nanospheres for drug delivery. Camptothecin encapsulated ZIF-8 particles show enhanced cell death, indicative of internalization and intracellular release of the drug [229].
Conclusion
The interdisciplinary multifunctional nature of nanotechnology and nanodrugs enabled diversification and advancements to improve the quality of life. However at present, the basic theory of nanotechnology applied in medicine and the preparation of nanodrugs are not fully explored. Therefore, extensive research has to be carried out in the field of nanomedicine especially in drug delivery systems. Nanomedicine is constantly looking for new and improved treatments for diseases, which need to have a high efficacy and be cost-effective, creating a large demand on scientific research to discover such new treatments. One important aspect of any treatment is the ability to be able to target only the illness and not cause harm to another healthy part of the body. Nowadays, there is a tremendous improvement in the utilization of metal nanoparticles as targeted drug delivery systems because of their availability, biocompatibility, and stability. These behaviors allow the drugs to be encapsulated and delivered straightly to the targeted sites thereby produces higher biological effect. The development of metallic nanoparticles is rapid and multidirectional and the improved practical potential of metallic nanoparticle highlights their potency as new tools for future drug delivery therapeutic modalities especially in the cancer, inflammation, diabetes and anti-viral therapy.
Acknowledgements
The authors sincerely thank the management of Kalasalingam Academy of Research and Education for their constant encouragement and support and providing all the necessary facilities for carrying out this research work.
Declarations
Conflict of interest
The authors declare no competing interests.
References
- 1.Ibrahim K, Khalid S, Idrees K. Nanoparticles: properties, applications and toxicities. Arab. J. Chem. 2019;12:908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- 2.Salata OV. Applications of nanoparticles in biology and medicine. J. Nanobiotechnology. 2004;2:1–6. doi: 10.1186/1477-3155-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang EC, Wang AZ. Nanoparticles and their applications in cell and molecular biology. Integr Biol (Camb). 2014;6:9–26. doi: 10.1039/c3ib40165k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nath D, Banerjee P. Green nanotechnology-a new hope for medical biology. Environ Toxicol Pharmacol. 2013;36:997–1014. doi: 10.1016/j.etap.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Felice B, Prabhakaran MP, Rodríguez AP, Ramakrishna S. Drug delivery vehicles on a nano-engineering perspective. Mater. Sci. Eng. C. 2014;41:178–195. doi: 10.1016/j.msec.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 6.Choudhury SR, Ordaz J, Lo CL, Damayanti NP, Zhou F, Irudayaraj J. Zinc oxide nanoparticles-induced reactive oxygen species promotes multimodal cyto- and epigenetic toxicity. Toxicol. Sci. 2017;156:261–274. doi: 10.1093/toxsci/kfw252. [DOI] [PubMed] [Google Scholar]
- 7.Qadri S, Haik Y, Mensah-Brown E, Bashir G, Fernandez-Cabezudo MJ, al-Ramadi BK. Metallic nanoparticles to eradicate bacterial bone infection. Nanomed. Nanotechnol. 2017;13:2241–50. doi: 10.1016/j.nano.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Ramadi KB, Mohamed YA, Al-Sbiei A, Almarzooqi S, Bashir G, Al Dhanhani A. Acute systemic exposure to silver-based nanoparticles induces hepatotoxicity and NLRP3-dependent inflammation. Nanotoxicology. 2016;10:1061–74. doi: 10.3109/17435390.2016.1163743. [DOI] [PubMed] [Google Scholar]
- 9.Martinho N, Damge C, Reis CP. Recent advances in drug delivery systems. J. Biomater. Nanobiotechnol. 2011;2:510–526. doi: 10.4236/jbnb.2011.225062. [DOI] [Google Scholar]
- 10.Jahangirian H, Lemraski EG, Webster TJ, Rafiee-Moghaddam R, Abdollahi Y. A review of drug delivery systems based on nanotechnology and green chemistry: green nanomedicine. Int. J. Nanomed. 2017;12:2957–2978. doi: 10.2147/IJN.S127683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jinjun S, Alexander VR, Omid FC, Robert L. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10:3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qadri S, Abdulrehman T, Azzi J, Mansour S, Haik Y. AgCuB nanoparticle eradicates intracellular S. aureus infection in bone cells: in vitro. Emergent. Mater. 2019;2:219–31. doi: 10.1007/s42247-019-00035-7. [DOI] [Google Scholar]
- 13.Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. 2012;8:147–166. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Noruzi M, Zare D, Khoshnevisan K, Davoodi D. Rapid green synthesis of gold nanoparticles using Rosa hybrida petal extract at room temperature. Spectrochim. Acta A: Mol. Biomol Spectrosc. 2011;79:1461–1465. doi: 10.1016/j.saa.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Al Tamimi S, Ashraf S, Abdulrehman T, Parray A, Mansour SA, Haik Y. Synthesis and analysis of silver–copper alloy nanoparticles of different ratios manifest anticancer activity in breast cancer cells. Cancer Nanotechnol. 2020;11:1–16. doi: 10.1186/s12645-020-00069-1. [DOI] [Google Scholar]
- 16.Narayanan KB, Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010;156:1–13. doi: 10.1016/j.cis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Mirza AZ, Siddiqui FA. Nanomedicine and drug delivery: a mini review. Int. Nano Lett. 2014;4:94. doi: 10.1007/s40089-014-0094-7. [DOI] [Google Scholar]
- 18.Miele E, Spinelli GP, Miele E, Di Fabrizio E, Ferretti E, Tomao S, Gulino A. Nanoparticle-based delivery of small interfering RNA: challenges for cancer therapy. Int. J. Nanomed. 2012;7:3637. doi: 10.2147/IJN.S23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quan Q, Zhang Y. Lab-ona-Tip (LOT): where nanotechnology can revolutionize fibre optics. Nanobiomedicine. 2015;2(2008):5–10. doi: 10.5772/60518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bamrungsap S, Zhao Z, Chen T, Wang L, Li C, Fu T, Tan W. Nanotechnology in therapeutics a focus on nanoparticles as a drug delivery system. Nanomedicine. 2012;7:1253–1271. doi: 10.2217/nnm.12.87. [DOI] [PubMed] [Google Scholar]
- 21.Sahoo SK, Parveen S, Panda JJ. The present and future of nanotechnology in human health care. Nanomed.: Nanotechnol. Biol. Med. 2007;3:20–31. doi: 10.1016/j.nano.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Sengani M, Grumezescu AM, Rajeswari VD. Recent trends and methodologies in gold nanoparticle synthesis – a prospective review on drug delivery aspect. OpenNano. 2017;2:37–46. doi: 10.1016/j.onano.2017.07.001. [DOI] [Google Scholar]
- 23.Liyanage PY, Hettiarachchi SD, Zhou Y, Ouhtit A, Seven ES, Oztan CY. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta Rev. Cancer. 2019;1871(2):419–433. doi: 10.1016/j.bbcan.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad MZ, Akhter S, Jain GK, Rahman M, Pathan SA, Ahmad FJ. Metallic nanoparticles: technology overview and drug delivery applications in oncology. Expert Opin. Drug Deliv. 2010;7(8):927–942. doi: 10.1517/17425247.2010.498473. [DOI] [PubMed] [Google Scholar]
- 25.Alalaiwe A. The clinical pharmacokinetics impact of medical nanometals on drug delivery system. Nanomed. Nanotechnol. Biol. Med. 2019;17:47–61. doi: 10.1016/j.nano.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Isaacoff B, Brown K. Progress in top-down control of bottom-up assembly. Nano Lett. 2017;17:6508–6510. doi: 10.1021/acs.nanolett.7b04479. [DOI] [PubMed] [Google Scholar]
- 27.Sreekanth TVM, Nagajyothi PC, Muthuraman P, Enkhtaivan G, Vattikuti SVP, Tettey CO. Ultra-sonication-assisted silver nanoparticles using Panax ginseng root extract and their anti-cancer and antiviral activities. J. Photochem. Photobiol. B Biol. 2018;188:6–11. doi: 10.1016/j.jphotobiol.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Rampino LD, Nord FF. Preparation of palladium and platinum synthetic high polymer catalysts and the relationship between particle size and rate of hydrogenation. J. Am. Chem. Soc. 1941;63(10):2745–2749. doi: 10.1021/ja01855a070. [DOI] [Google Scholar]
- 29.Brown KR, Walter D, Natan MJ. Seeding of colloidal Au nanoparticle solutions. 2. Improved control of particle size and shape. Chem. Mater. 2000;12(2):306–313. doi: 10.1021/cm980065p. [DOI] [Google Scholar]
- 30.Bastus NG, Comenge J, Puntes V. Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: size focusing versus Ostwald ripening. Langmuir. 2011;27(17):11098–11105. doi: 10.1021/la201938u. [DOI] [PubMed] [Google Scholar]
- 31.Khan JA, Kudgus RA, Szabolcs A, Dutta S, Wang E, Cao S, et al. Designing nanoconjugates to effectively target pancreatic cancer cells in vitro and in vivo. PLoS One. 2011;6(6):e20347. doi: 10.1371/journal.pone.0020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu L, An X. Silver nanoparticles synthesis using H2 as reducing agent in toluene-supercritical CO2 microemulsion. J. Supercrit. Fluids. 2015;99:29–37. doi: 10.1016/j.supflu.2014.12.024. [DOI] [Google Scholar]
- 33.Yıldırım OA, Durucan C. Synthesis of zinc oxide nanoparticles elaborated by microemulsion method. J. Alloys Compd. 2010;506(2):944–949. doi: 10.1016/j.jallcom.2010.07.125. [DOI] [Google Scholar]
- 34.Khalil MI, Al-Qunaibit MM, Al-Zahem AM, Labis JP. Synthesis and characterization of ZnO nanoparticles by thermal decomposition of a curcumin zinc complex. Arab. J. Chem. 2014;6(6):1178–1184. doi: 10.1016/j.arabjc.2013.10.025. [DOI] [Google Scholar]
- 35.Chin S, Park E, Kim M, Jurng J. Photocatalytic degradation of methylene blue with TiO2 nanoparticles prepared by a thermal decomposition process. Powder Technol. 2010;201(2):171–176. doi: 10.1016/j.powtec.2010.03.034. [DOI] [Google Scholar]
- 36.Abulizi A, Yang GH, Okitsu K, Zhu J-J. Synthesis of MnO2 nanoparticles from sonochemical reduction of MnO4− in water under different pH conditions. Ultrason. Sonochem. 2014;21(5):1629–1634. doi: 10.1016/j.ultsonch.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 37.Zhu J, Liu S, Palchik O, Koltypin Y, Gedanken A. Shape-controlled synthesis of silver nanoparticles by pulse sonoelectrochemical methods. Langmuir. 2000;16(16):6396–6399. doi: 10.1021/la991507u. [DOI] [Google Scholar]
- 38.Dhas NA, Raj CP, Gedanken A. Synthesis, characterization, and properties of metallic copper nanoparticles. Chem. Mater. 1998;10(5):1446–1452. doi: 10.1021/cm9708269. [DOI] [Google Scholar]
- 39.Lu L, An X. Silver nanoparticles synthesis using H2 as reducing agent in toluene–supercritical CO2 microemulsion. J. Supercrit. Fluids. 2015;99:29–37. doi: 10.1016/j.supflu.2014.12.024. [DOI] [Google Scholar]
- 40.Komarneni S, Katsuki H. Nanophase materials by a novel microwave-hydrothermal process. Pure Appl. Chem. 2002;74(9):1537–1543. doi: 10.1351/pac200274091537. [DOI] [Google Scholar]
- 41.Sharma D, Sharma S, Kaith BS, Rajput J, Kaur M. Synthesis of ZnO nanoparticles using surfactant free in-air and microwave method. Appl. Surf. Sci. 2011;257(22):9661–9672. doi: 10.1016/j.apsusc.2011.06.094. [DOI] [Google Scholar]
- 42.Barcikowski S, Devesa F, Moldenhauer K. Impact and structure of literature on nanoparticle generation by laser ablation in liquids. J. Nanopart Res. 2009;11:1883–1893. doi: 10.1007/s11051-009-9765-0. [DOI] [Google Scholar]
- 43.Bell J, Chen Z, Olofinjana A. Synthesis of amorphous carbon nitride using reactive ion beam sputtering deposition with grazing bombardment. Diam. Relat. Mater. 2001;10:2184–2189. doi: 10.1016/S0925-9635(01)00505-2. [DOI] [Google Scholar]
- 44.Tapaszto L, Dobrik G, Lambin P, Biro LP. Tailoring the atomic structure of graphene nanoribbons by scanning tunneling microscope lithography. Nat. Nanotechnol. 2008;3:397–401. doi: 10.1038/nnano.2008.149. [DOI] [PubMed] [Google Scholar]
- 45.Nissinen T, Ikonen T, Lama M, Riikonen J, Lehto VP. Improved production efficiency of mesoporous silicon nanoparticles by pulsed electrochemical etching. Powder Technol. 2016;288:360–365. doi: 10.1016/j.powtec.2015.11.015. [DOI] [Google Scholar]
- 46.Manawi YM, Ihsanullah, Samara A, Al-Ansari T, Atieh MA. A review of carbon nanomaterials’ synthesis via the chemical vapor deposition (CVD) method. Materials. 2018;11:822. doi: 10.3390/ma11050822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yi G, Wu Z, Sayer M. Preparation of Pb(Zr, Ti)O3 thin films by sol gel processing: electrical, optical, and electro-optic properties. J. Appl. Phys. 1988;64:2717–2724. doi: 10.1063/1.341613. [DOI] [Google Scholar]
- 48.S. Rana, P.T. Kalaichelvan, Ecotoxicity of nanoparticles. ISRN Toxicology, 574648 (2013) [DOI] [PMC free article] [PubMed]
- 49.Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J. Nanopart Resvol. 2008;10:507–517. doi: 10.1007/s11051-007-9275-x. [DOI] [Google Scholar]
- 50.Tiwari DK, Behari J, Sen P. Time and dose-dependent antimicrobial potential of Ag nanoparticles synthesized by top-down approach. Curr. Sci. 2008;95:647–655. [Google Scholar]
- 51.Luechinger NA, Grass RN, Athanassiou EK, Stark WJ. Bottom-up fabrication of metal/metal nanocomposites from nanoparticles of immiscible metals. Chem. Mater. 2010;22:155–160. doi: 10.1021/cm902527n. [DOI] [Google Scholar]
- 52.Singh P, Kim YJ, Yang DC. A strategic approach for rapid synthesis of gold and silver nanoparticles by Panax ginseng leaves. Artif. Cells Nanomed. Biotechnol. 2016;44:1949–1957. doi: 10.3109/21691401.2015.1115410. [DOI] [PubMed] [Google Scholar]
- 53.Singh P, Kim YJ, Wang C, Mathiyalagan R, El-Agamy Farh M, Yang DC. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif. Cells Nanomed. Biotechnol. 2016;44:811–816. doi: 10.3109/21691401.2015.1008514. [DOI] [PubMed] [Google Scholar]
- 54.Swamy MK, Sinniah UR. Patchouli (Pogostemon cablin Benth.): botany, grotechnology and biotechnological aspects. Ind. Crops Prod. 2016;87:161–176. doi: 10.1016/j.indcrop.2016.04.032. [DOI] [Google Scholar]
- 55.Mohanty SK, Swamy MK, Sinniah UR, Anuradha M. Leptadenia reticulata (Retz.) Wight & Arn. (Jivanti): botanical, agronomical, phytochemical, pharmacological, and biotechnological aspects. Molecules. 2017;22:1019. doi: 10.3390/molecules22061019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodrigues T, Reker D, Schneider P, Schneider G. Counting on natural products for drug design. Nat. Chem. 2016;8:531. doi: 10.1038/nchem.2479. [DOI] [PubMed] [Google Scholar]
- 57.Thilakarathna SH, Rupasinghe H. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 2013;5:3367–3387. doi: 10.3390/nu5093367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watkins R, Wu L, Zhang C, Davis RM, Xu B. Natural product-based nanomedicine: recent advances and issues. Int. J. Nanomed. 2015;10:6055. doi: 10.2147/IJN.S92162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cooper ER. Nanoparticles: a personal experience for formulating poorly water soluble drugs. J. Control. Release. 2010;141(3):300–302. doi: 10.1016/j.jconrel.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 60.Thanki K, Gangwal RP, Sangamwar AT, Jain S. Oral delivery of anticancer drugs: challenges and opportunities. J. Control Release. 2013;170(1):15–40. doi: 10.1016/j.jconrel.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 61.Gao L, Liu G, Ma J, Wang X, Zhou L, Li X. Drug nanocrystals: in vivo performances. J. Control Release. 2012;160(3):418–430. doi: 10.1016/j.jconrel.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 62.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303(5665):1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 63.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263(5153):1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 64.Mandal D, Maran A, Yaszemski MJ, Bolander ME, Sarkar G. Cellular uptake of gold nanoparticles directly cross-linked with carrier peptides by osteosarcoma cells. J. Mater. Sci. Mater. Med. 2009;20(1):347–350. doi: 10.1007/s10856-008-3588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan G, Onur MA, Sagam N. Utilization of gold nanostructures in biomedical applications. Turk. J. Biol. 2012;36(6):607–621. [Google Scholar]
- 66.Langer R. Drug delivery and targeting. Nature. 1998;392(Suppl. 6679):5–10. [PubMed] [Google Scholar]
- 67.D. Delcassian, A.K. Patel, A.B. Cortinas, R. Langer, Drug delivery across length scales. J. Drug Target. 29(3), 229–243 (2019) [DOI] [PubMed]
- 68.Yih TC, Al-Fandi M. Engineered nanoparticles as precise drug delivery systems. J. Cell. Biochem. 2006;97(6):1184–1190. doi: 10.1002/jcb.20796. [DOI] [PubMed] [Google Scholar]
- 69.Bhattacharyya S, Kudgus RA, Bhattacharya R, Mukherjee P. Inorganic nanoparticles in cancer therapy. Pharm. Res. 2011;28(2):237–259. doi: 10.1007/s11095-010-0318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nishiyama N. Nanomedicine: nanocarriers shape up for long life. Nat. Nanotechnol. 2007;2(4):203–204. doi: 10.1038/nnano.2007.88. [DOI] [PubMed] [Google Scholar]
- 71.Conde J, Doria G, Baptista P. Noble metal nanoparticles applications in cancer. J. Drug Deliv. 2012;2012:751075. doi: 10.1155/2012/751075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010;62(11):1064–1079. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sau TK, Rogach AL, Jackel F, Klar TA, Feldmann J. Properties and applications of colloidal non spherical noble metal nanoparticles. Adv. Mater. 2010;22:1805–1825. doi: 10.1002/adma.200902557. [DOI] [PubMed] [Google Scholar]
- 74.Sperling RA, Gil PR, Zhang F, Zanella M, Parak WJ. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008;37:1896–1908. doi: 10.1039/b712170a. [DOI] [PubMed] [Google Scholar]
- 75.Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology and medicine. Acc. Chem. Res. 2008;41:1578–1586. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 76.Lee KS, El-Sayed MA. Gold and silver nanoparticles in sensing and imaging: sensitivity of plasmon response to size, shape, and metal composition. J. Phys. Chem. B. 2006;110:19220–19225. doi: 10.1021/jp062536y. [DOI] [PubMed] [Google Scholar]
- 77.Schmitt CP, Genix AC, Alauzun JG, Sztucki M, Oberdisse J, Mutin PH. Surface modification of alumina-coated silica nanoparticles in aqueous sols with phosphonic acids and impact on nanoparticle interactions. Phys. Chem. Chem. Phys. 2015;17:19173–19182. doi: 10.1039/C5CP01925G. [DOI] [PubMed] [Google Scholar]
- 78.Vert M, Doi Y, Hellwich KH, Hess M, Hodge P, Kubisa P, Rinaudo M, Schue F. Terminology for biorelated polymers and applications (IUPAC recommendations 2012) Pure Appl. Chem. 2012;84:377–410. doi: 10.1351/PAC-REC-10-12-04. [DOI] [Google Scholar]
- 79.Arzt E. Size effects in materials due to microstructural and dimensional constraints: a comparative review. Acta Mater. 1998;46:5611–5626. doi: 10.1016/S1359-6454(98)00231-6. [DOI] [Google Scholar]
- 80.Patzke GR, Zhou Y, Kontic R, Conrad F. Oxide nanomaterials: synthetic developments, mechanistic studies, and technological innovations. Angew. Chem. Int. Ed. 2011;50:826–859. doi: 10.1002/anie.201000235. [DOI] [PubMed] [Google Scholar]
- 81.Rodriguez JA, Fernandez-Garcia M. Synthesis, properties, and applications of oxide nanomaterials. Hoboken: Wiley; 2007. [Google Scholar]
- 82.Jung Soo S, Qingguo X, Namho K, Justin H, Laura EM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clark JH, Macquarrie DJ. Handbook of green chemistry and technology. Hoboken: Wiley; 2008. [Google Scholar]
- 84.Bhainsa KC, DSouza SF. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B: Biointerfaces. 2006;47:160–174. doi: 10.1016/j.colsurfb.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 85.Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine. 2007;3:168–171. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 86.Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, Taliansky ME, Kalinina NO. Green nanotechnologies: synthesis of metal nanoparticles using plants. Acta Nat. 2014;6:35–44. doi: 10.32607/20758251-2014-6-1-35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ritter SK. EPA analysis suggests green success. Chem. Eng. News. 2015;93:32–43. doi: 10.1021/cen-09305-scitech1. [DOI] [Google Scholar]
- 88.Nasrollahzadeh M, Issaabadi Z, Sajadi SM. Green synthesis of a Cu/MgO nanocomposite by Cassytha filiformis L. extract and investigation of its catalytic activity in the reduction of methylene blue, congo red and nitro compounds in aqueous media. RSC Adv. 2018;8:3723–3735. doi: 10.1039/C7RA13491F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh P, Kim YJ, Zhang D, Yang DC. Trends Biotechnol Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016;34:588–599. doi: 10.1016/j.tibtech.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 90.Garole DJ, Choudhary BC, Paul D, Borse AU. Sorption and recovery of platinum from simulated spent catalyst solution and refinery wastewater using chemically modified biomass as a novel sorbent. Environ. Sci. Pollut. Res. 2018;25:10911–10925. doi: 10.1007/s11356-018-1351-5. [DOI] [PubMed] [Google Scholar]
- 91.Fang X, Wang Y, Wang Z, Jiang Z, Dong M. Microorganism assisted synthesized nanoparticles for catalytic applications. Energies. 2019;12:190. doi: 10.3390/en12010190. [DOI] [Google Scholar]
- 92.Buszewski B, Railean-Plugaru V, Pomastowski P, Rafińska K, Szultka-Mlynska M, Golinska P, Wypij M, Laskowski D, Dahm H. Antimicrobial activity of biosilver nanoparticles produced by a novel Streptacidiphilus durhamensis strain. J. Microbiol. Immunol. Infect. 2018;51:45–54. doi: 10.1016/j.jmii.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 93.Gan L, Zhang S, Zhang Y, He S, Tian Y. Biosynthesis, characterization and antimicrobial activity of silver nanoparticles by a halotolerant Bacillus endophyticus SCU-L. Prep. Biochem. Biotechnol. 2018;48:582–588. doi: 10.1080/10826068.2018.1476880. [DOI] [PubMed] [Google Scholar]
- 94.Li J, Tian B, Li T, Dai S, Weng Y, Lu J, Xu X, Jin Y, Pang R, Hua Y. Biosynthesis of Au, Ag and Au–Ag bimetallic nanoparticles using protein extracts of Deinococcus radiodurans and evaluation of their cytotoxicity. Int. J. Nanomed. 2018;13:1411. doi: 10.2147/IJN.S149079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klaus-Joerger T, Joerger R, Olsson E, Granqvist C-G. Bacteria as workers in the living factory: metal-accumulating bacteria and their potential for materials science. Trends Biotechnol. 2001;19:15–20. doi: 10.1016/S0167-7799(00)01514-6. [DOI] [PubMed] [Google Scholar]
- 96.Srivastava SK, Constanti M. Room temperature biogenic synthesis of multiple nanoparticles (Ag, Pd, Fe, Rh, Ni, Ru, Pt Co, and Li) by Pseudomonas aeruginosa SM1. J. Nanopart. Res. 2012;14:831. doi: 10.1007/s11051-012-0831-7. [DOI] [Google Scholar]
- 97.Ahmed E, Kalathil S, Shi L, Alharbi O, Wang P. Synthesis of ultra-small platinum, palladium and gold nanoparticles by Shewanella loihica PV-4 electrochemically active biofilms and their enhanced catalytic activities. J. Saudi Chem. Soc. 2018;22:919–929. doi: 10.1016/j.jscs.2018.02.002. [DOI] [Google Scholar]
- 98.Srinath B, Namratha K, Byrappa K. Eco-friendly synthesis of gold nanoparticles by Bacillus subtilis and their environmental applications. Adv. Sci. Lett. 2018;24:5942–5946. doi: 10.1166/asl.2018.12224. [DOI] [Google Scholar]
- 99.Sintubin L, De Windt W, Dick J, Mast J, van der Ha D, Verstraete W, Boon N. Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl. Microbiol. Biotechnol. 2009;84:741–749. doi: 10.1007/s00253-009-2032-6. [DOI] [PubMed] [Google Scholar]
- 100.Manimaran M, Kannabiran K. Lett. Appl. Microbiol. 2017;64:40–408. doi: 10.1111/lam.12730. [DOI] [PubMed] [Google Scholar]
- 101.Otari S, Patil R, Nadaf N, Ghosh S, Pawar S. Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus sp. Mater. Lett. 2012;72:92–94. doi: 10.1016/j.matlet.2011.12.109. [DOI] [Google Scholar]
- 102.Buszewski V, Railean-Plugaru P, Pomastowski P, Rafinska K, Szultka-Mlynska M, Golinska P, Wypij M, Laskowski D, Dahm H. Antimicrobial activity of biosilver nanoparticles produced by a novel Streptacidiphilus durhamensis strain. J. Microbiol. Immunol. Infect. 2016;20:1–10. doi: 10.1016/j.jmii.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 103.Ranjitha V, Rai VR. Actinomycetes mediated synthesis of gold nanoparticles from the culture supernatant of Streptomyces griseoruber with special reference to catalytic activity. Biotech. 2017;7:299. doi: 10.1007/s13205-017-0930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hassan SE, Salem SS, Fouda A, Awad MA, El-Gamal MS, Abdo AM. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J. Radiat. Res. Appl. Sci. 2018;30:1–9. [Google Scholar]
- 105.Singh P, Kim YJ, Zhang D, Yang DC. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016;34:588–599. doi: 10.1016/j.tibtech.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 106.Metuku RP, Pabba S, Burra S, Gudikandula K, Charya MS. Biosynthesis of silver nanoparticles from Schizophyllum radiatum HE 863742.1: their characterization and antimicrobial activity. Biotech. 2014;4:227–234. doi: 10.1007/s13205-013-0138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ahmad T, Wani IA, Manzoor N, Ahmed J, Asiri AM. Biosynthesis, structural characterization and antimicrobial activity of gold and silver nanoparticles. Colloids Surf. B. 2013;107:227. doi: 10.1016/j.colsurfb.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 108.Yin Y, Yang X, Hu L, Tan Z, Zhao L, Zhang Z, Liu J, Jiang G. Superoxide-mediated extracellular biosynthesis of silver nanoparticles by the fungus Fusarium oxysporum. Environ. Sci. Technol. Lett. 2016;3:160–165. doi: 10.1021/acs.estlett.6b00066. [DOI] [Google Scholar]
- 109.Kitching M, Choudhary P, Inguva S, Guo Y, Ramani M, Das SK, Marsili E. Fungal surface protein mediated one-pot synthesis of stable and hemocompatible gold nanoparticles. Enzyme Microb. Technol. 2016;95:76–84. doi: 10.1016/j.enzmictec.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 110.Shah M, Fawcett D, Sharma S, Tripathy S, Poinern G. Green synthesis of metallic nanoparticles via biological entities. Materials. 2015;8:7278–7308. doi: 10.3390/ma8115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Waghmare SR, Mulla MN, Marathe SR, Sonawane KD. Ecofriendly production of silver nanoparticles using Candida utilis and its mechanistic action against pathogenic microorganisms. Biotech. 2015;5:33–38. doi: 10.1007/s13205-014-0196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eugenio M, Muller N, Frases S, Almeida-Paes R, Lima LMT, Lemgruber L, Farina M, de Souza W, SantAnna C. Yeast-derived biosynthesis of silver/silver chloride nanoparticles and their antiproliferative activity against bacteria. RSC Adv. 2016;6:9893–9904. doi: 10.1039/C5RA22727E. [DOI] [Google Scholar]
- 113.Cao J, Guenther RH, Sit TL, Opperman CH, Lommel SA, Willoughby JA. Loading and release mechanism of red clover necrotic mosaic virus derived plant viral nanoparticles for drug delivery of doxorubicin. Small. 2014;10:5126–5136. doi: 10.1002/smll.201400558. [DOI] [PubMed] [Google Scholar]
- 114.Le DH, Lee KL, Shukla S, Commandeur U, Steinmetz NF. Potato virus X, a filamentous plant viral nanoparticle for doxorubicin delivery in cancer therapy. Nanoscale. 2017;9:2348–2357. doi: 10.1039/C6NR09099K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chauhan A, Zubair S, Tufail S, Sherwani A, Sajid M, Raman SC, Azam A, Owais M. Fungus-mediated biological synthesis of gold nanoparticles: potential in detection of liver cancer. Int. J. Nanomed. 2011;6:2305. doi: 10.2147/IJN.S23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ahmad T, Wani IA, Manzoor N, Ahmed J, Asiri AM. Biosynthesis, structural characterization and antimicrobial activity of gold and silver nanoparticles. Colloids Surf. B. 2013;107:227. doi: 10.1016/j.colsurfb.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 117.Abhilash KBD. Pandey, Microbial synthesis of iron-based nanomaterials-a review. Bull. Mater. Sci. 2011;34:191–198. doi: 10.1007/s12034-011-0076-6. [DOI] [Google Scholar]
- 118.Ahmad A, Mukherjee P, Mandal D, Senapati S, Khan MI, Kumar R, Sastry M. Enzyme mediated extracellular synthesis of CdS nanoparticles by the fungus, Fusarium oxysporum. J. Am. Chem. Soc. 2002;124:12108–12119. doi: 10.1021/ja027296o. [DOI] [PubMed] [Google Scholar]
- 119.Bansal V, Rautaray D, Ahmada A, Sastry M. Biosynthesis of zirconiananoparticles using the fungus Fusarium oxysporum. J. Mater. Chem. 2004;14:3303–3305. doi: 10.1039/b407904c. [DOI] [Google Scholar]
- 120.He S, Guo Z, Zhang Y, Zhang S, Wang J, Gu N. Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulate. Mater. Lett. 2007;61:3984. doi: 10.1016/j.matlet.2007.01.018. [DOI] [Google Scholar]
- 121.D. Venkataraman, K. Kalimuthu, R.K.P. Sureshbabu, G. Sangiliyandi, Metal Nanoparticles in Microbiology, ed. M. Rai and N. Duran, Springer, XI 17–35 (2011)
- 122.Kuppusamy P, Yusoff MM, Maniam GP, Govindan N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications–an updated report. Saudi Pharm. J. 2016;24:473. doi: 10.1016/j.jsps.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638. doi: 10.1039/c1gc15386b. [DOI] [Google Scholar]
- 124.Kharissova OV, Dias HVR, Kharisov BI, Perez BO, Victor M, Perez J. The greener synthesis of nanoparticles. Trends Biotechnol. 2013;31:240. doi: 10.1016/j.tibtech.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 125.Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, Taliansky ME, Kalinina NO. “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Nat. 2014;6:35–44. doi: 10.32607/20758251-2014-6-1-35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.R. Sankar, P. Manikandan, V. Malarvizhi, T. Fathima, K.S. Shivashangari, V. Ravikumar, Spectrochim. Acta Part A. 121, 746–750 (2014) [DOI] [PubMed]
- 127.El-Kemary M, Zahran M, Khalifa SAM, El-Seedi HR. Spectral characterisation of the silver nanoparticles biosynthesised using Ambrosia maritima plant. Micro Nano Lett. 2016;11:311. doi: 10.1049/mnl.2015.0572. [DOI] [Google Scholar]
- 128.Li S, Shen Y, Xie A, Yu X, Zhang X, Yang L, Li C. Rapid, room-temperature synthesis of amorphous selenium/protein composites using Capsicum annuum L extract. Nanotechnol. Nanotechnol. 2007;18:405101. doi: 10.1088/0957-4484/18/40/405101. [DOI] [Google Scholar]
- 129.Kumar CG, Mamidyala SK. Extracellular synthesis of silver nanoparticles using culture supernatant of Pseudomonas aeruginosa. Colloids Surf. B. 2011;84:462–466. doi: 10.1016/j.colsurfb.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 130.Srikar SK, Giri DD, Pal DB, Mishra P, Upadhyay S. Light induced green synthesis of silver nanoparticles using aqueous extract of Prunus amygdalus. Green Sustain. Chem. 2016;6:26. doi: 10.4236/gsc.2016.61003. [DOI] [Google Scholar]
- 131.Selvakannan PR, Swami A, Srisathiyanarayanan D, Shirude PS, Pasricha R, Mandale AB, Sastry M. Synthesis of aqueous Au core− Ag shell nanoparticles using tyrosine as a pH-dependent reducing agent and assembling phase-transferred silver nanoparticles at the air-water interface. Langmuir. 2004;20:7825. doi: 10.1021/la049258j. [DOI] [PubMed] [Google Scholar]
- 132.Sinha SN, Paul D. Phytosynthesis of silver nanoparticles using andrographis paniculata leaf extract and evaluation of their antibacterial activities. Spectrosc. Lett. 2015;48:600. doi: 10.1080/00387010.2014.938756. [DOI] [Google Scholar]
- 133.Tamileswari R, Haniff Nisha M, Jesurani SS. Green synthesis of silver nanoparticles using Brassica oleracea (cauliflower) and Brassica oleracea Capitata (cabbage) and the analysis of antimicrobial activity. Int. J. Eng. Res. Technol. 2015;4:1071. [Google Scholar]
- 134.Zahran M, El-Kemary M, Khalifa S, El-Seedi H. Spectral studies of silver nanoparticles biosynthesized by Origanum majorana. Green Process. Synth. 2018;7:100–105. doi: 10.1515/gps-2016-0183. [DOI] [Google Scholar]
- 135.Tahir K, Nazir S, Li B, Ahmad A, Nasir T, Khan AU, Shah SAA, Khan ZUH, Yasin G, Hameed MU. Sapium sebiferum leaf extract mediated synthesis of palladium nanoparticles and in vitro investigation of their bacterial and photocatalytic activities. J. Photochem. Photobiol. B. 2016;164:164–173. doi: 10.1016/j.jphotobiol.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 136.Lakshmi SJ, Bai RR, Sharanagouda H, Ramachandra CT, Nadagouda S, Doddagoudar SR. Biosynthesis and characterization of ZnO nanoparticles from spinach (Spinacia oleracea) leaves and its effect on seed quality parameters of greengram (Vigna radiata) Int. J. Curr. Microbiol. Appl. Sci. 2017;6:3376–3384. doi: 10.20546/ijcmas.2017.609.416. [DOI] [Google Scholar]
- 137.Raut RW, Mendhulkar VD, Kashid SB. Synthesis of silver nanoparticles using Withania somnifera leaf powder and silver nitrate. J. Photochem. Photobiol. B. 2014;132:45. doi: 10.1016/j.jphotobiol.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 138.Fouad RR, Aljohani HA, Shoueir KR. Biocompatible poly(vinyl alcohol) nanoparticle-based binary blends for oil spill control. Mar. Pollut. Bull. 2016;112:46. doi: 10.1016/j.marpolbul.2016.08.046. [DOI] [PubMed] [Google Scholar]
- 139.Mott D, Galkowski J, Wang L, Luo J, Zhong CJ. Synthesis of size-controlled and shaped copper nanoparticles. Langmuir. 2007;23:5740. doi: 10.1021/la0635092. [DOI] [PubMed] [Google Scholar]
- 140.Punjabi K, Choudhary P, Samant L, Mukhejee S, Vaidya S, Chowdhary A. Biosynthesis of nanoparticles: a review. Int. J. Pharm. Sci. Rev. Res. 2015;30:219. [Google Scholar]
- 141.Darroudi M, Ahmad MB, Zamiri R, Zak AK, Abdullah AH, Ibrahim NA. Time-dependent effect in green synthesis of silver nanoparticles. Int. J. Nanomed. 2011;6:677. doi: 10.2147/IJN.S17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gopinath V, Priyadarshini S, Loke MF, Arunkumar J, Marsili E, Mubarak Ali D, Velusamy P, Vadivelu J. Biogenic synthesis, characterization of antibacterial silver nanoparticles and its cell cytotoxicity. Arab. J. Chem. 2017;10:1107–1117. doi: 10.1016/j.arabjc.2015.11.011. [DOI] [Google Scholar]
- 143.Suksaeree J, Thuengernthong A, Pongpichayasiri K, Maneewattanapinyo P, Settharaksa S, Pichayakorn W. Formulation and evaluation of matrix type transdermal patch containing silver nanoparticles. J. Polym. Environ. 2018;26:4369–4375. doi: 10.1007/s10924-018-1305-5. [DOI] [Google Scholar]
- 144.M. Sharma, Transdermal and intravenous nano drug delivery systems, applications of targeted nano drugs and delivery systems, Elsevier Inc.; 499–550 (2019)
- 145.Le B, Stellacci OF. Antibacterial activity of silver nanoparticles: a surface science insight. Nano Today. 2015;10:339–354. doi: 10.1016/j.nantod.2015.04.002. [DOI] [Google Scholar]
- 146.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 147.Balmain A, Gray J, Ponder B. The genetics and genomics of cancer. Nat. Genet. 2003;33:238–244. doi: 10.1038/ng1107. [DOI] [PubMed] [Google Scholar]
- 148.Sahu SC, Zheng J, Graham L, Chen L, Ihrie J, Yourick JJ, Sprando RL. Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture. J. Appl. Toxicol. 2014;34:1155–1166. doi: 10.1002/jat.2994. [DOI] [PubMed] [Google Scholar]
- 149.El-Deeb NM, El-Sherbiny IM, El-Aassara MR, Hafez EE. Novel trend in colon cancer therapy using silver nanoparticles synthesized by honey bee. J. Nanomed. Nanotechnol. 2015;6:265. [Google Scholar]
- 150.Chung IM, Park I, Seung-Hyun K, Thiruvengadam M, Rajakumar G. Plant-mediated synthesis of silver nanoparticles: their characteristic properties and therapeutic applications. Nanoscale Res. Lett. 2016;11:40. doi: 10.1186/s11671-016-1257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.He Y, Du Z, Ma S, Liu Y, Li D, Huang H, Jiang S, Cheng S, Wu W, Zhang K. Effects of green-synthesized silver nanoparticles on lung cancer cells in vitro and grown as xenograft tumors in vivo. Int. J. Nanomed. 2016;11:1879. doi: 10.2147/IJN.S103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gurunathan S, Qasim M, Park C, Yoo H, Kim JH, Hong K. Cytotoxic potential and molecular pathway analysis of silver nanoparticles in human colon cancer cells HCT116. Int. J. Mol. Sci. 2018;19:2269. doi: 10.3390/ijms19082269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bohmert L, Niemann B, Lichtenstein D, Juling S, Lampen A. Molecular mechanism of silver nanoparticles in human intestinal cells. Nanotoxicology. 2015;9:852–860. doi: 10.3109/17435390.2014.980760. [DOI] [PubMed] [Google Scholar]
- 154.Mani S, Balasubramanian MG, Ponnusamy P, Vijayan P. Antineoplastic effect of PAC capped silver nanoparticles promote apoptosis in HT-29 human colon cancer cells. J. Clust. Sci. 2019;30:483–493. doi: 10.1007/s10876-019-01510-1. [DOI] [Google Scholar]
- 155.Chugh H, Sood D, Chandra I, Tomar V, Dhawan G, Chandra R. Role of gold and silver nanoparticles in cancer nano-medicine. Artif. Cells Nanomed. Biotechnol. 2018;46:1210–1220. doi: 10.1080/21691401.2018.1449118. [DOI] [PubMed] [Google Scholar]
- 156.Mathur P, Jha S, Ramteke S, Jain N. Pharmaceutical aspects of silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018;46:115–126. doi: 10.1080/21691401.2017.1414825. [DOI] [PubMed] [Google Scholar]
- 157.Hsin YH, Chen CF, Huang S, Shih TS, Lai PS, Chueh PJ. The apoptotic effect of nanosilver is mediated by a ROS-and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol. Lett. 2008;179:130–139. doi: 10.1016/j.toxlet.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 158.Lee KX, Shameli K, Mohamad SE, Yew YP, Isa EDM, Yap HY, Lim WL, Teow SY. Bio-mediated synthesis and characterisation of silver nanocarrier, and its potent anticancer action. Nanomaterials. 2019;9:1–19. doi: 10.3390/nano9101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rozalen M, Sánchez-Polo M, Fernandez-Perales M, Widmann TJ, Rivera-Utrilla J. Synthesis of controlled-size silver nanoparticles for the administration of methotrexate drug and its activity in colon and lung cancer cells. RSC Adv. 2020;10:10646–10660. doi: 10.1039/C9RA08657A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Thapa RK, Kim JH, Jeong JH, Shin BS, Choi HG, Yong CS, Kim JO. Silver nanoparticle-embedded grapheme oxide-methotrexate for targeted cancer treatment. Colloids Surf. B Biointerfaces. 2017;153:95–103. doi: 10.1016/j.colsurfb.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 161.Palai PK, Mondal A, Chakraborti CK, Banerjee I, Pal K. Green synthesized amino-PEGylated silver decorated grapheme nanoplatform as a tumor-targeted controlled drug delivery system. SN Appl. Sci. 2019;1:269. doi: 10.1007/s42452-019-0287-9. [DOI] [Google Scholar]
- 162.Zeng F, Xu D, Zhan C, Liang C, Zhao W, Zhang J, Feng H, Ma X. Surfactant-free synthesis of graphene oxide coated silver nanoparticles for SERS biosensing and intracellular drug delivery. ACS Appl. Nano Mater. 2018;1:2748–2753. doi: 10.1021/acsanm.8b00444. [DOI] [Google Scholar]
- 163.Patra S, Mukherjee S, Barui AK, Ganguly A, Sreedhar B, Patra CR. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;53:298–309. doi: 10.1016/j.msec.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 164.Shandiz SAS, Ardestani MS, Shahbazzadeh D, Assadi A, Cohan RA, Asgary V, Salehi S. Novel imatinib-loaded silver nanoparticles for enhanced apoptosis of human breast cancer MCF-7 cells. Artif. Cells Nanomed. Biotechnol. 2017;45:1–10. doi: 10.1080/21691401.2016.1202257. [DOI] [PubMed] [Google Scholar]
- 165.Yuan YG, Peng QL, Gurunathan S. Silver nanoparticles enhance the apoptotic potential of gemcitabine in human ovarian cancer cells: combination therapy for effective cancer treatment. Int. J. Nanomed. 2017;12:6487–6502. doi: 10.2147/IJN.S135482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gurunathan S, Qasim M, Choi Y, Tae Do J, Park C, Hong K, Kim JH, Song H. Antiviral potential of nanoparticles-can nanoparticles fight against coronaviruses? Nanomaterials. 2020;10:1645. doi: 10.3390/nano10091645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Y.G. Yuan, S. Zhang, J.Y. Hwang, I.K. Kong, Silver nanoparticles potentiates cytotoxicity and apoptotic potential of camptothecin in human cervical cancer cells. Oxid. Med. Cell Longev. 6121328 (2018) [DOI] [PMC free article] [PubMed]
- 168.Li Y, Lin Z, Zhao M, Guo M, Xu T, Wang C, Xia H, Zhu B. Reversal of H1N1 influenza virus-induced apoptosis by silver nanoparticles functionalized with amantadine. RSC Adv. 2016;6:89679–89686. doi: 10.1039/C6RA18493F. [DOI] [Google Scholar]
- 169.Jain S, Hirst DG, O’Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012;85:101–113. doi: 10.1259/bjr/59448833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chow EK, Ho D. Cancer nanomedicine: from drug delivery to imaging. Sci. Transl. Med. 2013;5:216rv4. doi: 10.1126/scitranslmed.3005872. [DOI] [PubMed] [Google Scholar]
- 171.Alkilany A, Murphy C. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J. Nanopart. Res. 2010;12:2313–2333. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tiloke C, Phulukdaree A, Anand K, Gengan RM, Chuturgoon AA. Moringa oleifera gold nanoparticles modulate oncogenes, tumor suppressor genes, and caspase-9 splice variants in A549 cells. J. Cell Biochem. 2016;117:2302–2314. doi: 10.1002/jcb.25528. [DOI] [PubMed] [Google Scholar]
- 173.Cui W, Li J, Zhang Y, Rong H, Lu W, Jiang L. Effects of aggregation and the surface properties of gold nanoparticles on cytotoxicity and cell growth. Nanomedicine: NBM. 2012;8:46–53. doi: 10.1016/j.nano.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 174.Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, Schmid G, Brandau W, Jahnen-Dechent W. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 175.Gong N, Chen S, Jin S, Zhang J, Wang PC, Liang XJ. Effect of the physicochemical properties of gold nanostructures on cellular internalization. Regen. Biomater. 2015;2:273–280. doi: 10.1093/rb/rbv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Albanese A, Tang PS, Chan WCW. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 177.Muhammad UF, Novosad V, Rozhkova AE, Wali H, Ali A, Fateh AA, Neogi PB, Neogi A, Wang Z. Gold nanoparticles-enabled efficient dual delivery of anticancer therapeutics to HeLa cells. Sci. Rep. 2018;8:2907. doi: 10.1038/s41598-018-21331-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 178.Patra CR, Bhattacharya R, Mukhopadhyay D, Mukherjee P. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv. Drug Deliv. Rev. 2010;8:346–361. doi: 10.1016/j.addr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Eghtedari M, Liopo AV, Copland JA, Oraevsky AA, Motamed M. Engineering of hetero-functional gold nanorods for the in vivo molecular targeting of breast cancer cells. Nano Lett. 2009;9:287–291. doi: 10.1021/nl802915q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Golkar Z, Bagazra O, Pace DG. Bacteriophage therapy: a potential solution for the antibiotic resistance crisis. J. Infect. Dev. Ctries. 2014;8:129–136. doi: 10.3855/jidc.3573. [DOI] [PubMed] [Google Scholar]
- 181.Gould IM, Bal AM. New antibiotic agents in the pipeline and how they can overcome microbial resistance. Virulence. 2013;4:185–191. doi: 10.4161/viru.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.C. Su, K. Huang, L. Hao-Hong, L. You-Guang, Z. Da-Li, Antibacterial properties of functionalized gold nanoparticles and their application in oral biology. J. Nanomater. 5616379 (2020)
- 183.Brown AN, Smith K, Samuels TA, Lu J, Obare SO, Scott ME. Nanoparticles functionalized with ampicillin destroy multiple-antibiotic-resistant isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and methicillin-resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012;78:2768–2774. doi: 10.1128/AEM.06513-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Govindaraju K, Suganya KSU. In vitro anti-diabetic assessment of guavanoic acid functionalized gold nanoparticles in regulating glucose transport using L6 rat skeletal muscle cells. RSC Med. Chem. 2020;11:814–822. doi: 10.1039/D0MD00125B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang Y, Wu M, Tan D, Liu Q, Xia R, Chen M, Liu Y, Xue L, Lei Y. A dissolving and glucose-responsive insulin-releasing microneedle patch for type 1 diabetes therapy. J. Mater. Chem. B. 2021;9:648–657. doi: 10.1039/D0TB02133D. [DOI] [PubMed] [Google Scholar]
- 186.Zhang Y, Wu M, Dai W, Li Y, Wang X, Tan D, Yang Z, Liu S, Xue L, Lei Y. Gold nanoclusters for controlled insulin release and glucose regulation in diabetes. Nanoscale. 2019;11:6471–6479. doi: 10.1039/C9NR00668K. [DOI] [PubMed] [Google Scholar]
- 187.Agarwal H, Nakara A, Shanmugam VK. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: a review. Biomed. Pharmacother. 2019;109:2561–2572. doi: 10.1016/j.biopha.2018.11.116. [DOI] [PubMed] [Google Scholar]
- 188.Kim HJ, Lee SM, Park KH, Mun CH, Park YB, Yoo KH. Drug-loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis. Biomaterials. 2015;61:95–102. doi: 10.1016/j.biomaterials.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 189.Yaqoob SB, Adnan R, Khan RMR, Rashid M. Gold, silver, and palladium nanoparticles: a chemical tool for biomedical applications. Front. Chem. 2020;8:376. doi: 10.3389/fchem.2020.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Leso V, Iavicoli I. Palladium nanoparticles: toxicological effects and potential implications for occupational risk assessment. Int. J. Mol. Sci. 2018;19:503. doi: 10.3390/ijms19020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shanthi K, Vimala K, Gopi D, Kannan S. Fabrication of a pH responsive DOXconjugated PEGylated palladium nanoparticle mediated drug delivery system: an in vitro and in vivo evaluation. RSC Adv. 2015;5:44998–45014. doi: 10.1039/C5RA05803A. [DOI] [Google Scholar]
- 192.Johnstone TC, Suntharalingam K, Lippard SJ. The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery and Pt (IV) prodrugs. Chem. Rev. 2016;116:3436–3486. doi: 10.1021/acs.chemrev.5b00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang Z, Chen L, Huang C, Huang Y, Jia N. Albumin-mediated platinum nanocrystals for in vivo enhanced computed tomography imaging. J. Mater. Chem. B. 2017;5:3498–3510. doi: 10.1039/C7TB00561J. [DOI] [PubMed] [Google Scholar]
- 194.Doherty RE, Sazanovich IV, McKenzie LK, Stasheuski AS, Coyle R, Baggaley E, Bottomley S, Weinstein JA, Bryant HE. Photodynamic killing of cancer cells by a platinum(II) complex with cyclometallating ligand. Sci. Rep. 2016;6:22668. doi: 10.1038/srep22668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mukherjee S, Kotcherlakota R, Haqueac S, Bhattacharyaa D, Mahesh Kumar J, Chakravarty S, Patraac CR. Improved delivery of doxorubicin using rationally designed PEGylated platinum nanoparticles for the treatment of melanoma. Mater. Sci. Eng. C. 2020;108:110375. doi: 10.1016/j.msec.2019.110375. [DOI] [PubMed] [Google Scholar]
- 196.Gawande MB, Goswami A, Felpin FX, Asefa T, Huang X, Silva R, Zou X, Zboril R, Rajender S, Varma S. Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem. Rev. 2016;116:3722–3811. doi: 10.1021/acs.chemrev.5b00482. [DOI] [PubMed] [Google Scholar]
- 197.Ranu BC, Dey R, Chatterjee T, Ahammed S. Copper nanoparticle-catalyzed carbon-carbon and carbon-heteroatom bond formation with a greener perspective. Chemsuschem. 2012;5:22–44. doi: 10.1002/cssc.201100348. [DOI] [PubMed] [Google Scholar]
- 198.Ahn Y, Jeong Y, Lee D, Lee Y. Copper nanowire–graphene core–shell nanostructure for highly stable transparent conducting electrodes. ACS Nano. 2015;9:3125–3133. doi: 10.1021/acsnano.5b00053. [DOI] [PubMed] [Google Scholar]
- 199.S. Bhanushali, P. Ghosh, A. Ganesh, W. Cheng, 1D copper nanostructures: progress, challenges and opportunities. Small. 11, 1232–1252 (2015) [DOI] [PubMed]
- 200.Goswami U, Dutta A, Raza A, Kandimalla R, Kalita S, Sankar Ghosh S, Chattopadhyay A. Transferrin-copper nanocluster-doxorubicin nanoparticles as targeted theranostic cancer nanodrug. ACS Appl. Mater Interfaces. 2018;10:3282–3294. doi: 10.1021/acsami.7b15165. [DOI] [PubMed] [Google Scholar]
- 201.Kamble S, Utage B, Mogle P, Kamble R, Hese S, Dawane B, Gacche R. Evaluation of curcumin capped copper nanoparticles as possible inhibitors of human breast cancer cells and angiogenesis: a comparative study with native curcumin. AAPS PharmSciTech. 2016;17:1030–1041. doi: 10.1208/s12249-015-0435-5. [DOI] [PubMed] [Google Scholar]
- 202.Verma V, Kaushik D. Mupirocin Mounted copper nanoparticle offered augmented drug delivery against resistant bacteria. Indian J. Pharm. Educ. Res. 2020;54:637–646. doi: 10.5530/ijper.54.3.113. [DOI] [Google Scholar]
- 203.Zhang H, Chen B, Jiang H. A strategy for ZnO nanorod mediated multi-mode cancer treatment. Biomaterials. 2011;32:1906–1914. doi: 10.1016/j.biomaterials.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 204.Hussein J, Attia MF, El M, El-daly SM, Mohamed N, El-khayat Z, El-naggar ME. Solid state synthesis of docosahexaenoic acid-loaded zinc oxide nanoparticles as a potential antidiabetic agent in rats. Int. J. Biol. Macromol. 2019;140:1305–1314. doi: 10.1016/j.ijbiomac.2019.08.201. [DOI] [PubMed] [Google Scholar]
- 205.Chauhan P, Mahajan S, Prasad GBKS. Preparation and characterization of CS-ZnO-NC nanoparticles for imparting anti-diabetic activities in experimental diabetes. J. Drug Deliv. Sci. Technol. 2019;52:738–747. doi: 10.1016/j.jddst.2019.05.020. [DOI] [Google Scholar]
- 206.Yadav E, Singh D, Yadav P, Verma A. Ameliorative effect of biofabricated ZnO nanoparticles of Trianthema portulacastrum Linn and inflammation. RSC Adv. 2018;8:21621–21635. doi: 10.1039/C8RA03500H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ziental D, Goslinska BC, Mlynarczyk DT, Sobotta AG, Stanisz B, Goslinski T, Sobotta L. Titanium dioxide nanoparticles: prospects and applications in medicine. Nanomaterials. 2020;10:387. doi: 10.3390/nano10020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xu J, Sun Y, Huang J, Chen C, Liu G, Jiang Y, Zhao Y, Jiang Y. Photokilling cancer cells using highly cell-specific antibody–TiO2 bioconjugates and electroporation. Bioelectrochemistry. 2007;71:217–222. doi: 10.1016/j.bioelechem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 209.Ni W, Li M, Cui J, Xing Z, Li Z, Wu X, Song E, Gong M, Zhou W. 808 nm light triggered black TiO2 nanoparticles for killing of bladder cancer cells. Mater. Sci. Eng. C. 2017;81:252–260. doi: 10.1016/j.msec.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 210.Chen Y, Wan Y, Wang Y, Zhang H, Jiao Z. Anticancer efficacy enhancement and attenuation of side effects of doxorubicin with titanium dioxide nanoparticles. Int. J. Nanomed. 2011;6:2321–2326. doi: 10.2147/IJN.S25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rehman FU, Rauf MA, Ullah S, Shaikh S, Qambrani A, Muhammad P, Hanif S. Ultrasound-activated nano-TiO2 loaded with temozolomide paves the way for resection of chemoresistant glioblastoma multiforme. Cancer Nanotechnol. 2021;12:17. doi: 10.1186/s12645-021-00088-6. [DOI] [Google Scholar]
- 212.Lopez T, Sotelo J, Navarrete J, Ascencio JA. Synthesis of TiO2 nanostructured reservoir with temozolomide: structural evolution of the occluded drug. Opt. Mater. 2006;29:88–94. doi: 10.1016/j.optmat.2006.03.033. [DOI] [Google Scholar]
- 213.Uddin M, Mondal D, Morris CA, Lopez T, Diebold U, Gonzalez RD. An in vitro controlled release study of valproic acid encapsulated in a Titania ceramic matrix. App. Surf Sci. 2011;257:7920–7927. doi: 10.1016/j.apsusc.2011.03.079. [DOI] [Google Scholar]
- 214.Xu P, Wang R, Ouyang J, Chen B. A new strategy for TiO2 whiskers mediated multi-mode cancer treatment. Nanoscale Res. Let. 2015;10:1–11. doi: 10.1186/s11671-015-0796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang H, Wang C, Chen B, Wang X. Daunorubicin-TiO2 nanocomposites as a “smart” pH-responsive drug delivery system. Int. J. Nanomed. 2012;7:235–242. doi: 10.2147/IJN.S27722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Li N, Sun Q, Yu Z, Gao X, Pan W, Wan X, Tang B. Nuclear-targeted photothermal therapy prevents cancer recurrence with near-infrared triggered copper sulfide nanoparticles. ACS Nano. 2018;12:5197–5206. doi: 10.1021/acsnano.7b06870. [DOI] [PubMed] [Google Scholar]
- 217.Yi X, Chen L, Chen J, Maiti D, Chai ZF, Liu Z, Yang K. Biomimetic copper sulfide for chemo-radiotherapy: enhanced uptake and reduced efflux of nanoparticles for tumor cells under ionizing radiation. Adv. Func. Mater. 2018;28:11. doi: 10.1002/adfm.201705161. [DOI] [Google Scholar]
- 218.Yang R, Li R, Zhang L, Xu Z, Kang Y, Xue P. Facile synthesis of hollow mesoporous nickel sulphide nanoparticles for highly efficient combinatorial photothermal–chemotherapy of cancer. J. Mater. Chem. B. 2020;8:7766. doi: 10.1039/D0TB01448F. [DOI] [PubMed] [Google Scholar]
- 219.Li Q, Sun L, Hou M, Chen Q, Yang R, Zhang L, Xu Z, Kang Y, Xue P. Phase-change material packaged within hollow copper sulfide nanoparticles carrying doxorubicin and chlorine 6 for fluorescence-guided trimodal therapy of cancer. ACS Appl. Mater. Interfaces. 2019;11:417–429. doi: 10.1021/acsami.8b19667. [DOI] [PubMed] [Google Scholar]
- 220.Hou L, Shan X, Hao L, Feng Q, Zhang Z. Copper sulfide nanoparticle-based localized drug delivery system as an effective cancer synergistic treatment and theranostic platform. Acta Biomater. 2017;54:307–320. doi: 10.1016/j.actbio.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 221.Xie Z, Wang D, Fan T, Xing C, Li Z, Tao W, Liu L, Bao S, Fana D, Zhang H. Black phosphorus analogue tin sulfide nanosheets: synthesis and application as near-infrared photothermal agents and drug delivery platforms for cancer therapy. J. Mater. Chem. B. 2018;6:4747. doi: 10.1039/C8TB00729B. [DOI] [PubMed] [Google Scholar]
- 222.Sun Y, Zheng L, Yang Y, Qian X, Fu T, Li X, Yang Z, Yan H, Cui C, Tan W. Metal–organic framework nanocarriers for drug delivery in biomedical applications. Nano-Micro Lett. 2020;12:103. doi: 10.1007/s40820-020-00423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sun CY, Qin C, Wang XL, Su ZM. Metal–organic frameworks as potential drug delivery systems. Expert Opin. Drug Deliv. 2013;10:89–101. doi: 10.1517/17425247.2013.741583. [DOI] [PubMed] [Google Scholar]
- 224.Gimenez-Marques M, Hidalgo T, Serre C, Horcajada P. Nanostructured metal–organic frameworks and their bio-related applications. Coord. Chem. Rev. 2016;307:342–360. doi: 10.1016/j.ccr.2015.08.008. [DOI] [Google Scholar]
- 225.Lakshmi BA, Kim S. Current and emerging applications of nanostructured metal–organic frameworks in cancer-targeted theranostics. Mater. Sci. Eng. C. 2019;105:110091. doi: 10.1016/j.msec.2019.110091. [DOI] [PubMed] [Google Scholar]
- 226.Wang HL, Yeh H, Li BH, Lin CH, Hsiao TC, Tsai DH. Zirconium-based metal–organic framework nanocarrier for the controlled release of ibuprofen. ACS Appl. Nano Mater. 2019;2:3329–3334. doi: 10.1021/acsanm.9b00834. [DOI] [Google Scholar]
- 227.Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T, Eubank J.F, Heurtaux D, Clayette P, Kreuz C, Chang J.S, Hwang Y.K, Marsaud V, Bories P.N, Cynober L, Gil S, Ferey G, Couvreur P, Grefln R. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nature Materials. 2010;9:172. doi: 10.1038/nmat2608. [DOI] [PubMed] [Google Scholar]
- 228.Chen WH, Yu X, Liao WC, Sohn YS, Cecconello A, Kozell A, Nechushtai R, Willner I. ATP-responsive aptamer-based metal–organic framework nanoparticles (NMOFs) for the controlled release of loads and drugs. Adv. Funct. Mater. 2017;27:1702102. doi: 10.1002/adfm.201702102. [DOI] [Google Scholar]
- 229.Zhuang J, Kuo CH, Chou LY, Liu DY, Weerapana E, Tsung CK. Optimized metal-organic-framework nanospheres for drug delivery: evaluation of small-molecule encapsulation. ACS Nano. 2014;8:2812–2819. doi: 10.1021/nn406590q. [DOI] [PubMed] [Google Scholar]