Highlights
-
•
Agriculture plays an important role in a country's economy. In modern intensive agricultural practices, chemical fertilizers and pesticides are applied on large scale to increase crop production in order to meet the nutritional requirements of the ever-increasing world population. However, rapid urbanization with shrinking agricultural lands, dramatic change in climatic conditions and extensive use of agrochemicals in agricultural practices has been found to cause environmental disturbances and public health hazards affecting food security and sustainability in agriculture. Besides this, agriculture soils are continuously losing their quality and physical properties as well as their chemical (imbalance of nutrients) and biological health due to indiscriminate use of agrochemicals. Plant-associated microbes with their plant growth- promoting traits have enormous potential to solve these challenges and play a crucial role in enhancing plant biomass and crop yield under greenhouse and field conditions. The beneficial mechanisms of plant growth improvement include enhanced availability of nutrients (i.e., N, P, K, Zn and S), phytohormone modulation, biocontrol of phytopathogens and amelioration of biotic and abiotic stresses. This plant-microbe interplay is indispensable for sustainable agriculture and these microbes may perform essential role as an ecological engineer to reduce the use of chemical fertilizers. Various steps involved for production of solid-based or liquid biofertilizer formulation include inoculum preparation, addition of cell protectants such as glycerol, lactose, starch, a good carrier material, proper packaging and best delivery methods. In addition, recent developments of formulation include entrapment/microencapsulation, nano-immobilization of microbial bioinoculants and biofilm-based biofertilizers. Thus, inoculation with beneficial microbes has emerged as an innovative eco-friendly technology to feed global population with available resources. This review critically examines the current state-of-art on use of microbial strains as biofertilizers in different crop systems for sustainable agriculture and in maintaining soil fertility and enhancing crop productivity. It is believed that acquisition of advanced knowledge of plant-PGPR interactions, bioengineering of microbial communities to improve the performance of biofertilizers under field conditions, will help in devising strategies for sustainable, environment-friendly and climate smart agricultural technologies to deliver short and long terms solutions for improving crop productivity to feed the world in a more sustainable manner.
Keywords: Beneficial microorganisms, Rhizosphere, Biofertilizers, Soil fertility, Crop production, Sustainable agriculture
Abbreviations: PGP, Plant growth-promoting; PSB, Phosphate-solubilizing bacteria; PGPR, Plant growth-promoting rhizobacteria; DRB, Deleterious rhizospheric bacteria; IAA, Indole acetic acid; GA, Gibberellic acid; HCN, Hydrogen cyanide; ACC, 1-aminocyclopropane-1-carboxylic acid; KMB, Potassium mobilizing bacteria; CAT, Catalase; POD, Peroxidase; APX, Ascorbate peroxidase; ABA, Abscisic acid; GPX, Glutathione/thioredoxin peroxidase; IAR, Intrinsic antibiotic resistance; DAPG, 2, 4-diacetyl phloroglucinol; PCA, Phenazine-1-carboxylic acid; ISR, Induced systemic resistance; SAR, Systemic acquired resistance; PAMPs, Pathogen associated molecular patterns; MAMPs, Microbes associated molecular patterns; AM, Arbuscular mycorrhiza; BNF, Biological nitrogen fixation; BGA, Blue green algae; KSMs, Potassium-solubilizing microbes; SOB, Sulphur oxidizing bacteria
Abstract
Modern intensive agricultural practices face numerous challenges that pose major threats to global food security. In order to address the nutritional requirements of the ever-increasing world population, chemical fertilizers and pesticides are applied on large scale to increase crop production. However, the injudicious use of agrochemicals has resulted in environmental pollution leading to public health hazards. Moreover, agriculture soils are continuously losing their quality and physical properties as well as their chemical (imbalance of nutrients) and biological health. Plant-associated microbes with their plant growth- promoting traits have enormous potential to solve these challenges and play a crucial role in enhancing plant biomass and crop yield. The beneficial mechanisms of plant growth improvement include enhanced nutrient availability, phytohormone modulation, biocontrol of phytopathogens and amelioration of biotic and abiotic stresses. Solid-based or liquid bioinoculant formulation comprises inoculum preparation, addition of cell protectants such as glycerol, lactose, starch, a good carrier material, proper packaging and best delivery methods. Recent developments of formulation include entrapment/microencapsulation, nano-immobilization of microbial bioinoculants and biofilm-based biofertilizers. This review critically examines the current state-of-art on use of microbial strains as biofertilizers and the important roles performed by these beneficial microbes in maintaining soil fertility and enhancing crop productivity.
Graphical abstract
1. Introduction
Agriculture sector contributes towards one third share in global gross domestic products. However, with the increasing trend in human population, world's population has been estimated to rise upto 9.5 billion by 2050, leading to high food demand (Green et al., 2005; Gerland et al., 2014). Besides availability of limited fertile land, urbanisation, unexpected weather events connected to climate change, abiotic and biotic stresses are the major constraints for the production of several crops (Glaser and Lehr, 2019). Furthermore, soil quality, availability of nutrients, environmental conditions as well as the biological health of the soil are other important criteria for improving crop yield per unit area for achieving the targeted goal of food security (Tilman et al., 2011). During recent high input farming systems and technologies, chemical fertilizers (consisting of N, P or K) are applied excessively to provide the plant nutrient requirement for increasing the agriculture productivity worldwide. However, only a limited amount (30–40%) of these nutrients is absorbed by the plants due to low fertilizer-use efficiency and rest is lost to soil causing environmental pollution. In addition, heavy metals and radionuclides are present in chemical fertilizers, which are difficult to degrade, thus making them persistent pollutants in nature. Another major issue related to application of excessive chemical fertilizers is eutrophication of water sources. These pollution problems leading to public health hazards necessitated the development of technologies that are sustainable and eco-friendly, which could reduce the application of synthetic fertilizers (Santoyo et al., 2012; Zhang et al., 2021). Therefore, application of beneficial microbiomes as biofertilizers in sustainable agriculture practices has emerged as innovative and environment-friendly technology for improving soil fertility and plant growth (Adesemoye et al., 2009; Bertola et al., 2019; Ullah et al., 2019a; Murgese et al., 2020; Fasusi et al. 2021).
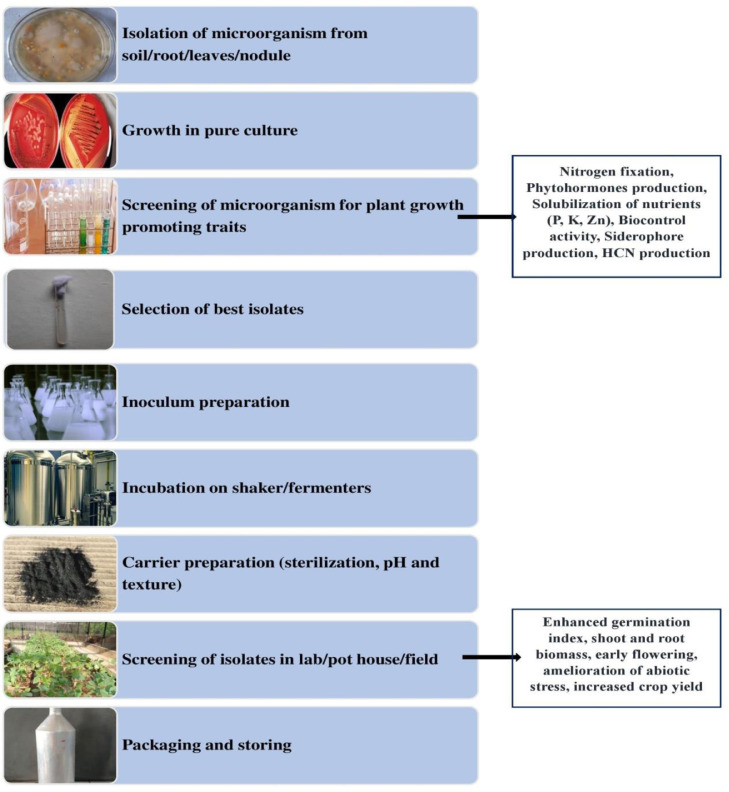
All terrestrial plants are colonized by diverse, complex and interactive communities of microorganisms (Sasse et al., 2018; Xiong et al., 2021). The colonization of microbes on the plants can be either epiphytic, endophytic or rhizospheric (Rossmann et al., 2017). The study of microbial communities inhabiting the diverse habitats and their collective contribution to plant growth development and protection has received intense interest over the last two decades. Various microorganisms present within the plant rhizosphere include procaryotic organisms i.e., bacteria and archaea, unicellular protozoa, multicellular eucaryotic nematodes and fungi, and viruses (Glick and Gamalaro, 2021). Many of these microorganisms within the plant's microbiome play many vital roles in promoting the growth and development of agriculturally important crop plants (Bakker et al., 2013; Bulgarelli et al., 2013; Fasusi et al., 2021). For screening of efficient beneficial microbial strains for use as biofertilizer, microorganisms isolated from the rhizosphere are screened for plant growth-promoting (PGP) traits along with effective colonization ability (Andreozzi et al., 2019; Pandey et al., 2019). Various beneficial properties of PGP microbes for utilization as biofertilizer include decomposition of organic matter, enhancing nutrient availability, production of phytohormones and contribution towards mitigation of abiotic and biotic stresses (Lalitha, 2017; Sehrawat and Sindhu, 2019; Zhang et al., 2021). Biofertilizers comprise of living or latent cells, which are applied either to soil, seed or seedlings for improving nutrients availability and uptake from soil (Fasusi et al., 2021). Use of biofertilizers has currently emerged as cost effective and ecofriendly alternative than chemical-based fertilizers. Substantial progress has been achieved recently in development of effective biofertilizers for different crops.
2. Rhizosphere biology and microbial diversity
Plants release a significant proportion (varying from 2% to even 50%) of their photosynthates as rhizo-deposits or root littre into the rhizosphere (Kuzyakov and Domanski, 2000; Jones et al., 2004; Huang et al., 2014). The rhizo-deposits, including sugars, organic acids, amino acids, fatty acids, phenolics, nucleotides, sterols and vitamins, provide critical carbon sources for rhizobacteria and plant pathogens (Sasse et al., 2018). In a stride to evaluate the impact of root exudates on proliferation of the microorganisms in the rhizosphere, Kawasaki et al. (2021) altered the expression of transporters in rice (Oryza sativa L.) and wheat (Triticum aestivum L.), which affected the release of substrates (simple organic anions, including malate, citrate, and γ-amino butyric acid) from root apices. These altered level of root exudates, either separately or in combination, encouraged the proliferation of specific beneficial root microbiomes from the soil. However, the root type (seminal or nodal), position along the roots (apex or base) and soil type also had a greater influence on microbiome structure. Thus, rhizosphere harbours a great abundance of varied microorganisms, several of which support the plants in nutrient procurement from soil and in suppressing pathogenic invasion (Bulgarelli et al., 2013; Leach et al., 2017). Plant-associated microbiome includes nitrogen fixers, phosphate-solubilizing bacteria (PSB), mycorrhizal fungi, biocontrol agents, bioremediation agents, plant growth promoting rhizobacteria (PGPR) and pathogenic microbes (Sehrawat and Sindhu, 2019; Singh et al., 2021).
Applications of recent technologies to explore taxonomic and functional components of diverse microbiomes have resulted in selection and manipulation of particular microbial community from rhizosphere for sustainable crop production (Brewin et al., 1990; Mohanram and Kumar, 2019; Gupta et al., 2021). The information regarding microbial community composition, species relative abundance in a niche and signaling between microbes and plants in the rhizosphere contributes towards establishing a relationship between crop plants, environmental factors and ecosystem functions (Torsvik et al., 1996; Garbeva et al., 2008; Sehrawat et al., 2020). Recent developments in next generation sequencing techniques allowed significant improvement in interpreting the functioning of microbiomes specifically inhabiting the crop rhizosphere (Gupta et al., 2021). Also, the plant genetic diversity alongwith soil properties tend to influence the composition and diversity of rhizospheric community (Garbeva et al., 2008; Vorholt et al., 2017; Jiang, 2017; Xu, 2018; Matthews et al., 2019) and these communities may share similarities within different agroecosystems.
The methodological progress made so far has provided the information about composition of microbiome inhabiting a specific crop (Busby, 2017; de Vries et al., 2020) and the selection basis of a particular beneficial microbial species from soil rhizosphere. It is estimated that bulk soil in general contains 106 to 109 bacterial cells per gram of soil and there is remarkable ten-fold enrichment of bacterial numbers in the rhizosphere zone (Tkacz et al., 2020; Wang et al., 2020; Glick and Gamalaro, 2021). Similarly, population of other organisms including fungi, actinomycetes, algae, protozoa and nematodes get stimulated in the rhizosphere. For understanding the structure, diversity and functions of rhizosphere microbiome and their interactions with different environmental factors, traditional approaches are complemented with modern omics-based approach based on next-generation sequencing (NGS) technologies (Gupta et al., 2021; Raghu et al., 2021). Bioprospecting the emerging field of ecosystems’ engineering and plant–microbe interactions in the rhizosphere marks a promising opportunity to confer tolerance towards abiotic and biotic stresses to host plant and support the nutrition of host plants for developing sustainable solutions to improve crop productivity under current and future climatic conditions (Singh et al., 2021).
Current unravelling of the complex microbial communities using molecular tools showed that fertile soil contains both beneficial as well as detrimental organisms, which act as facilitators of plant processes (Zipfel and Oldroyd, 2017; Thoms et. al., 2021). For example, nitrogen-fixing, phosphate-solubilizing, plant growth-promoting rhizobacteria and AM fungi provide nutrients to the plants leading to stimulation of plant growth (Hurek et al., 2002; Dobbelaere et al., 2003; Mohanty et al., 2021). Some microbes secrete phytohormones such as indole acetic acid (IAA), gibberellins (GA) and cytokinins that change root architecture (Jangu and Sindhu, 2011; Duca et al., 2014; Khan et al., 2020). On the other hand, particular microorganisms isolated from the soil or rhizosphere have been found to inhibit the growth and activity of phytopathogens, and may also alter plant immune responses and community ecology (Sahu and Sindhu, 2011; Sehrawat and Sindhu, 2019; Wang et al., 2022). Secretion of specific metabolites such as flavonoids, acetosyringone, strigolactones and MBOA (break-down product of root exuded benzoxazinoid) may act as attractant for beneficial microbes and as a signal to trigger plant immunity (Torres-Vera et al., 2014; Hu et al., 2018; Phour et al., 2020). On the contrary, detrimental organisms adversely affect the growth and development of plants, and are termed as deleterious rhizospheric bacteria (DRB) (Barazani and Friedman, 2001). However, the proliferation, population and distribution of beneficial as well as pathogenic microbes varies with soil pH, temperature, moisture and nutrient availability (Dumbrell et al., 2010; Laceg and Wilson, 2001). Therefore, understanding of rhizosphere biology in context to climate change and abiotic stresses is the urgent need to harness beneficial microbial interactions as a low-input technology for agricultural sustainability (Dubey et al., 2016).
3. Mechanisms of action of beneficial microbes
Microbes being phylogenetically diverse and multifaceted, interact with plants in different ways including symbiosis, parasitism, commensalism, amensalism and neutralism (Glick and Gamalaro, 2021). The growth of these microbes is dependent on plant photosynthesis and reciprocate by influencing plant growth, thereby collectively termed as plant microbiome (Wang et al., 2008; Lebeis et al., 2012; Klaus and Bulgarelli, 2015; Mu¨ller et al., 2016; Zhang et al., 2021). Recently, beneficial plant-microbiome associations are being exploited for improving crop production. Plant beneficial microbes improve soil properties, increase availability of soil nutrients, enhance resistance towards pathogens and also produce plant growth-stimulating hormones (Chaparro et al., 2012; Wasai and Minamisawa, 2018; Kour et al., 2020b; Yadav et al., 2020a). Though, soil microbiome is constituted of bacteria, fungi, algae, protozoa, archaea and viruses, but beneficial bacterial communities make important contributions towards improving crop productivity for the sustainable agriculture (Mueller and Sachs, 2015; Adesemoye and Kloepper, 2009; Haney et al., 2015; Berg et al., 2016; Mohanty et al., 2021).
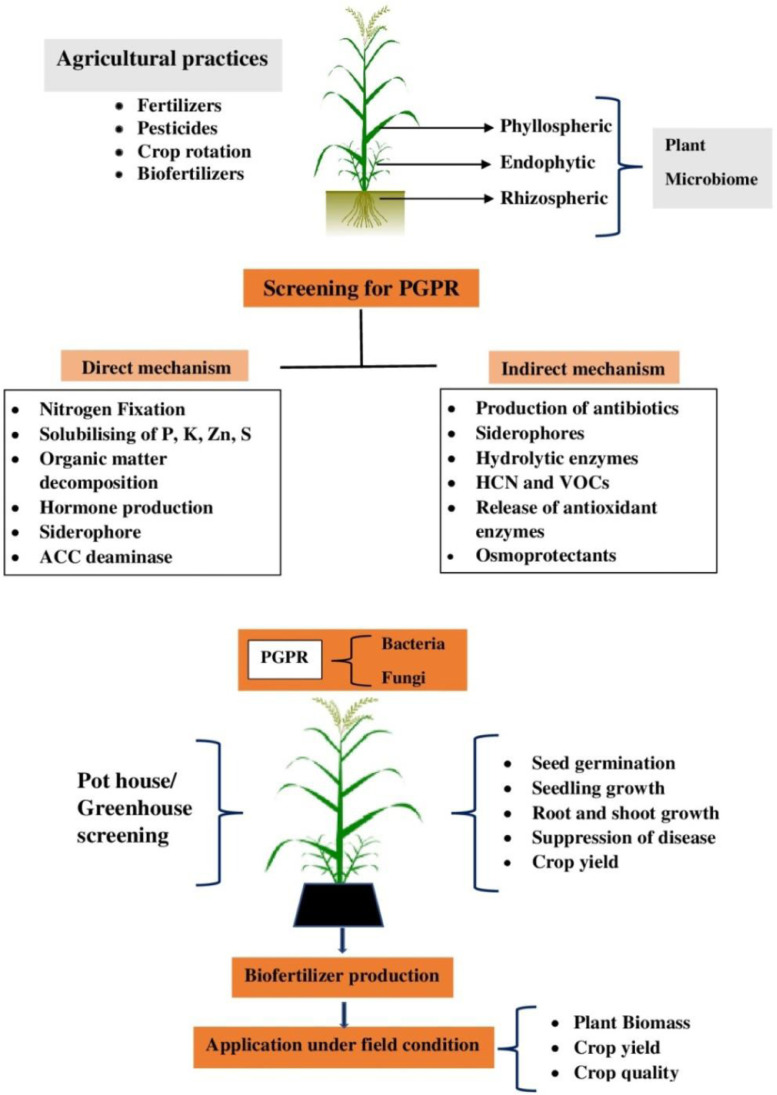
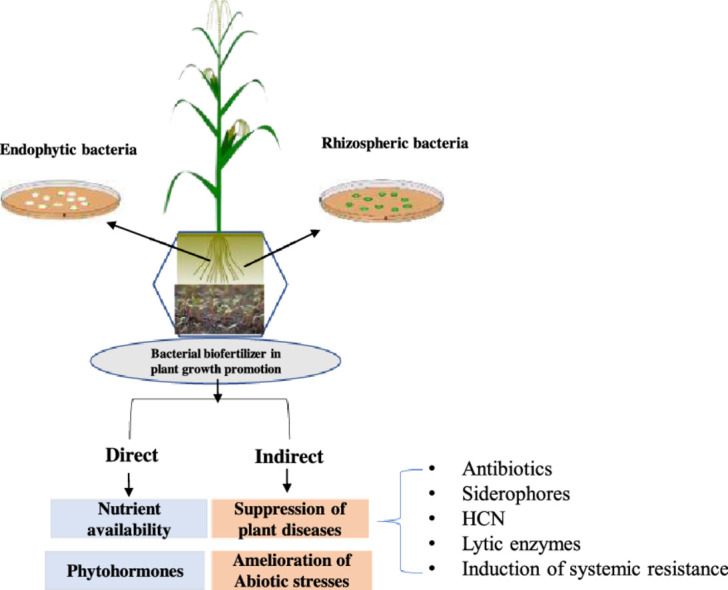
Rhizosphere-inhabiting microorganisms contribute towards plant growth promotion either through direct mechanism or indirect mechanisms. Enhanced availability of nutrients and phytohormone production are directly involved in plant growth promotion (Malik and Sindhu, 2011; Santoyo et al., 2021b), whereas suppression of diseases by biocontrol agents, amelioration of abiotic stresses and bioremediation of pollutants and contaminants are the indirect mechanisms that contribute towards improved plant health and crop productivity (Glick and Gamalero, 2021; Sehrawat et al., 2021; Zhang et al., 2021) (Fig. 1).
Fig. 1.
A schematic view of screening the rhizosphere bacteria for beneficial traits, their pot house evaluation and subsequent selection for use in biofertilizer production and application.
3.1. Direct mechanisms involved in plant growth promotion
Beneficial bacterial inoculants provide nitrogen, phosphorous, potassium and other plant nutrients to the crop without any chemical input to soil leading to improvement of plant growth and increase in crop yield (Singh and Gupta, 2018; Tiwari et al., 2018; Vimal et al., 2018; Basu et al., 2021). Moreover, production and excretion of different phytohormones i.e., IAA, gibberellins (GA) and cytokinins have been reported to increase the root surface area for more adsorption of plant nutrients from the soil (Jangu and Sindhu, 2011; Duca et al., 2014; Khan et al., 2016; 2020).
3.1.1. Enhanced nutrient availability
About 16 micro- and macro-nutrients are essentially required for the growth of plants and deficiency of any of these nutrients could lead to malfunctioning and imbalanced growth. Nutrient availability is affected by different parameters of soil, climate and type of crop plant. Soil microbes maintains the optimum concentration of soil nutrients, hence providing better plant growth and crop yield (Richardson et al., 2009; Hirel et al., 2011; Kumar et al., 2021) (Table 1). Rhizosphere management, through use of beneficial microbes helps to enhance nutrient availability in soil for the better plant growth via solubilization of zinc, potassium and phosphate, nitrogen fixation and phytohormones production (Sehrawat and Sindhu, 2019; Sharma et al., 2019). Mycorrhizal fungi and PGPR are considered to play crucial role in nutrient availability in soil along with amelioration of stresses (Santoyo et al., 2021a) and thus, these microorganisms have become important components towards the effective functioning of soil ecosystem.
Table 1.
Inoculation responses of beneficial rhizobacteria on plant growth.
| Types of biofertilizer | Bacterial strain used | Plant growth promoting activity | Effect on plant productivity parameters | References |
| Nitrogen- fixing bacteria | Azotobacter sp. strain Avi2 (MCC 3432) | Nitrogen fixation, production of IAA, siderophore | improved vegetative and reproductive growth in rice | Banik and Dangar, 2019 |
| Azotobacter chroococcum, A. vinelandii | Nitrogen fixation, P solubilization, production of NH3, HCN, IAA | Increased shoot and root length, leaf and root number, chlorophyll content of maize | Jain et al., 2021 | |
| Herbaspirillum seropedicae (strain ZAE94) | Nitrogen fixation, production of siderophore, IAA | Enhanced mineral uptake, increased diameter, weight, length of ear, number of grains/rows, cob weight and grain yield | Ávila et al., 2020 | |
| Enterobacter cloacae (PGLO9) | Nitrogen fixation, phosphorous solubilization, siderophore production, ACC deaminase | Enhanced potato growth and yield, significant increase in vegetative growth parameters including root and shoot length, root as well as shoot biomass | Verma and Agrawal, 2018 | |
| Bradyrhizobium sp. | Nitrogen fixation, siderophore and IAA production, P solubilisation | Increased vegetative parameters and seed yield in mung bean | Alkurtany et al., 2018 | |
| Rhizobium meliloti | Nitrogen fixation, production of siderophore and chitinase | Increased growth and yield, pods quality and better use of nitrogen in peanut | Mondal et al., 2020 | |
| Phosphate-solubilizing bacteria (PSB) | Pseudomonas stutzeri (CSP03), Bacillus subtilis (TTP02), Pseudomonas putida (PHP03) | Solubilization of P and zinc, IAA, N2 fixation, siderophore, NH3 production | Enhanced plant growth in arid soils | El-Sayed et al., 2014 |
| Alcaligenes faecalis sub sp. faecalis str S8 | P solubilization, production of IAA, hydrogen cyanide, chitinolytic, proteolytic and pectinolytic enzymes | Better growth, increased root weight and length, plant height and aerial plant part weight | Abdallah et al., 2016 | |
| Bacillus subtilis LK14 | P solubilization, production of IAA | Enhanced host plant's nutrient uptake and amelioration of stress | Khan et al., 2016 | |
| R. leguminosarum Pseudomonas moraviensis, Bacillus halotolerans, Enterobacter hormaechei and Pseudomonas frederiksbergensis |
P solubilisation P solubilization, production of IAA, Siderophores, cellulase, ammonia and metal tolerance |
Improved growth and yield, and better resistance against drought in soybean improved wheat seed germination, plant growth, nitrogen and potassium uptake, and Zn absorption | Igiehon et al., 2019; Fahsi et al., 2021 | |
| Potassium-solubilizing bacteria (KSB) | Bacillus edaphicus strain NBT | Potassium solubilisation | Increased root and shoot Growth, and K content in cotton and rape | Sheng, 2005 |
| Bacillus muciloginosus | Potassium solubilisation | Increased the plant biomass, yield and uptake of K in Sudan grass | Basak and Biswas, 2008 | |
| Acidothiobacillus ferooxidans Bacillus cereus | Potassium solubilization Potassium solubilization | Increased growth and yield, improved oil composition in pumpkinIncreased plant height, branches number, shoot dry weight, K uptake and total yield of potato | Ansari et al., 2017; Ali et al., 2021 | |
| Coinoculation of beneficial bacteria | Azotobacter, phosphate solubilising bacteria and potash mobilizing bacteria | Nitrogen fixation, P solubilization, K mobilization, IAA production | Increased yield components and yield of wheat, improved soil nutrients balance, increased microbial activity in the rhizosphere | Game et al., 2020 |
| Azospirillum lipoferum or Azotobacter chroococcum Bacillus megaterium, Arthrobacter chlorophenolicus, Enterobacter sp., Pseudomonas aeruginosa | Nitrogen fixation, IAA production Production of IAA, HCN, siderophore, P-solubilization and N-fixation |
Enhanced growth parameters, pigments, K+, osmolytes, K+/ Na+ ratio and the activity of CAT, POD and APX of the salt-affected maize plants Increased plant height, grain yield, straw yield and nutrients acquisition |
Latef et al., 2020; Kumar et al., 2021 |
IAA: Indole acetic acid; HCN: Hydrogen cyanide; ACC: 1-aminocyclopropane-1-carboxylic acid; PSB: Phosphate solubizing bacteria; KSB: Potassium solubilizing bacteria; CAT: Catalase; POD: Peroxidase; APX: Ascorbate peroxidase.
The application of beneficial microorganisms as biofertilizers helps in increasing nutrients levels either by; (i) influencing metabolism of plant, thus altering composition of root exudates, (ii) influencing the solubility and availability of nutrients, (iii) increasing the interactions with other soil microbes (Sindhu and Suneja, 1997; Adesemoye and Kloepper, 2009; Fitter et al., 2011; Miransari, 2011a; Miransari, 2011b). Microbes mineralize nutrients by acidolysis, oxidoreduction, chelation or by secreting compounds like oxalate, gluconate, citrate, catechol, lactate and pseudobactin (Marschner and Rengel, 2007; Uroz et al., 2009; Parmar and Sindhu, 2019). Arbuscular mycorrhizal fungi tend to associate symbiotically with terrestrial plants and increase availability and uptake of water and minerals, in return of consuming carbon from plant (Javaid, 2009; Kumar et al., 2021). Host plant provides a suitable environment or habitat for the germination of fungal spores into fungal hyphae, thus creating a mycorrhizosphere. Thus, there are huge numbers of microorganisms in the rhizospheric region, which play important role in the release of phosphorus, potassium and zinc from different insoluble compounds in soil (Sindhu et al., 2014, 2016, 2019b; Wallenstein, 2017; Zhang et al., 2021). The application of nutrient-mobilizing microbial inoculants has been found to stimulate the root and shoot growth, enhanced nutrient uptake and increased seed yield of different crops under pot house as well as in field under different agro-environmental conditions (Etesami et al., 2021; Jia et al., 2021; Patel et al., 2021; Santoyo et al., 2021b) (Table 1).
In various studies conducted to establish the role of PGPR in increasing nutrient availability and plant growth promotion, inoculation of Azotobacter chroococcum was found to increase the the contents of total nitrogen (N) and total phosphorus (P) in maize plants relative to the uninoculated control treatment (Song et al. (2021). A. chroococcum treatments changed the contents of soil ammonium-N (NH4+-N) at the seedling stage (+17.78%) and heading stage (+34.48%), as well as soil nitrate-N (NOx-N; −23.94%) and soil available P (Olsen-P; −15.38%) at the heading stage. The average grain weight over 4 years was higher (+17.07%) in A. chroococcum inoculation treatment than that in the control treatment. In similar studies, coinoculation of sugarcane with different PSB strains including Bacillus sp. BACBR04, Bacillus sp. BACBR06 and Rhizobium sp. RIZBR01, along with use of compost as a P source, resulted in increased P content in shoot compared with the uninoculated treatments, which received only compost or triple superphosphate (i.e. soluble P) (Estrada-Bonilla et al., 2021). This treatment also displayed an increase of nitrogen and potassium content in plant tissue. Filipini et al. (2021) showed that application of Azospirillum brasilense, on seed or by foliar spraying, and seed inoculation of Rhizobium tropici, had an synergistic effect and increased plant biomass, accumulated nitrogen, thousand-grain weight and grain yield of common bean (Phaseolus vulgaris L.). in a field experiment.
3.1.2. Phytohormone production
Plants as well as bacteria synthesize certain phytohormones or plant growth regulators in very low concentrations, which influence the root and shoot growth, shape, flowering, senescence and seed growth along with various physiological processes including cell division, development, gene expression and stress responses (Jangu and Sindhu, 2011; Malik and Sindhu, 2011; Khan et al., 2020). Phytohormones increase root hair length and surface area of roots, and thus nutrient and water uptake ability of the plant roots are improved (Tsegaye et al., 2017). Enhanced metabolic activity due to production of the phytohormones helps in defence, normal functioning of cell and abiotic stress management (Khan et al., 2020). Plant growth-promoting bacteria either secretes hormones or alters concentration of hormones within the plant during biotic and abiotic stresses.
Phytohormones are grouped into five classes; auxin, cytokinins, gibberllins, ethylene and abscisic acid (Cassán et al., 2014). Apart from these, some other classes have also been identified like jasmonates, brassinosteroids and strigolactones, which act as targets for the metabolic engineering to construct crop plants which can withstand abiotic stress. Most of the PGPRs are known to produce cytokinins, auxins and ethylene, but only limited microbes are known to secrete gibberllins (van Loon, 2007; Egamberdieva et al., 2017; Abd Allah et al., 2018). Pseudomonas has been reported as an excellent IAA producer genus; however, P. putida is better than P. fluorescens in terms of IAA production (Bharucha et al., 2013; Kumar et al., 2015a). Batista et al. (2021) demonstrated that the genome of the Bacillus thuringiensis strain RZ2MS9 harbours the complete set of genes required for indole acetic acid production. Inoculation of tomato with B. thuringiensis strain RZ2MS9 caused 24% increase in the shoot dry weight of the Micro-Tom (MT). The application also modified the root architecture of tomato, with an increase of 26% in the average lateral root length and inhibition of the axial root. Moreover, RZ2MS9-treated MT plants also presented elongated root cortical cells with intensified mitotic activity at the cellular level. On the other hand, no growth alteration was detected in the auxin-insensitive diageotropic (dgt) plants either with or without the RZ2MS9 inoculation.
The production of cytokinins and gibberellins have been reported in various microbial genera and these bioactive hormones affect seed germination, stem elongation, root hair development, flowering, fruit setting and other developmental processes (Maheshwari et al., 2015; Kang et al., 2019). Phytohormone strigolactones production by plants and microbial species has been associated with root and shoot system's regulation, leaf senescence and nutrient stress (Torres-Vera et al., 2014; Visentin et al., 2016; Xie et al., 2019). The inoculation of ACC deaminase-producing PGPR strains, which lowers the stress hormone ethylene concentration in plant roots, protect plants against abiotic stress (Gamalero and Glick, 2015) and increases plant growth (Khandelwal and Sindhu, 2012). Salomon et al. (2014) isolated Bacillus licheniformis Rt4M10 and Pseudomonas fluorescens Rt6M10 from rhizospheric regions of grape vine, which produced ABA, IAA and gibberellins. Ghosh et al. (2019) depicted alleviated water stress due to the modulation in endogenic accumulation and relocation of ABA, gibberellic acid and cytokinin in both shoot and roots of plant by Pseudomonas putida strain GAP-P45.
3.2. Indirect mechanisms contributing towards plant growth stimulation
Plant pathogens including harmful bacteria, fungi and viruses cause various diseases on different crop plants. These diseases caused by plant pathogens adversely affect global crop productivity and account for 20–40% yield losses annually in various cereal and legume crops (Oerke, 2006). Injudicious application of pesticides for disease control causes environmental pollution leading to public health hazards and therefore, efforts are being made to characterize antagonistic microorganisms for use as biopesticides for increasing agricultural crop production (Santoyo et al., 2012; Anand et al., 2020; Jiao et al., 2021; Wang et al., 2022). The mechanisms to control the diseases include the synthesis and secretion of siderophores, hydrolytic enzymes, antibiotics, volatile organic compounds, hydrogen cyanide and induction of systemic resistance (Santoyo et al., 2012; Sehrawat and Sindhu, 2019, Sharma et al., 2019; Khanna et al., 2021). Memenza-Zegarra and Zúñiga-Dávila, (2021) isolated 26 strains from the rhizosphere of common bean (Phaseolus vulgaris) plants and most of the strains inhibited the growth of pathogenic fungi i.e. Sclerotinia, Fusarium and Rhizoctonia due to the production of both volatile and non-volatile organic compounds, hydrolytic enzymes, siderophores and antifungal lipopeptide. Bacillus IcBac2.1 strain showed significant inhibition of the majority of phytopathogens due to production of antifungal lipopeptides. Similarly, Alcaligenes TvPs2.4 and Pseudomonas TvPs1.6 strains showed the highest growth inhibition against the tested phytopathogens. SPME/GC–MS analysis of culture filterates showed that each strain produced 21 volatile organic compounds and the highest concentration of dimethyl disulfide and D-limonene compounds were obtained.
3.2.1. Siderophore production
Iron is one of the vital elements involved in plant metabolism and its deficiency could lead to abnormal respiration and photosynthesis (Zuo and Zhang, 2011). Iron is present as Fe3+ in aerobic environments, which is a major resource in soil. Fe3+ readily forms hydroxides and oxyhydroxides, which becomes unavailable to microbes and plants as they consume iron as Fe+2 form (Pahari and Mishra, 2017; Ghazy and El-Nahrawy, 2021). Ferrous is obtained via siderophore secretion and these siderophores are the chelating compounds with low-molecular-weight. Siderophore forms a complex with Fe3+, after which or Fe3+ form is reduced to Fe2+, which is released into the cell (Kashyap et al., 2017). This easily absorbable Fe2+ is either directly taken up as iron-siderophore complex or the iron is exchanged via a ligand (Rasouli-Sadaghiani et al., 2014; Novo et al., 2018). Siderophores are composed of electron-rich atoms like oxygen and nitrogen, which binds up with cations (Chu et al., 2010; Ghavami et al., 2017). Apart from iron mobilization, siderophores are also involved in uptake of molybdenum and vanadium (nitrogenase co-factors) for Azotobacter vinelandii (McRose et al., 2017). However, when Mo was limited, then production of catechol type siderophore was increased (McRose et al., 2017).
Siderophores are produced by Pseudomonas, Aeromonas, Azotobacter, Bacillus, Rhizobium, Azadirachta, Streptomyces, Burkholderia and Serratia (Sahu and Sindhu, 2011; Sabet and Mortazaeinezhad, 2018; Sultana et al., 2021). Siderophore-producing bacteria were found to play crucial role in growth promotion and biocontrol activity. Iron nutrition in graminaceous and dicot plants was enhanced by siderophore-producing fluorescent Pseudomonas species (Shirley et al., 2011). Inoculation of siderophore-producing Bacillus subtilis in pepper showed significant suppression of Fusarium wilt disease caused by Fusarium oxysporum (Yu et al., 2011). Similarly, the production of siderophores by fungal species i.e. Penicillium citrinum, Aspergillus niger and Trichoderma harzianum also contributed towards biocontrol activity and their inoculation resulted in increased growth of chickpea (Cicer arietinum) (Yadav et al., 2011). Siderophore-producing Pseudomonas sp. were found to control plant disease in green gram (Vigna radiata) and resulted in promotion of plant growth (Sahu and Sindhu, 2011). Similarly, inoculation of siderophore-producing Pseudomonas aeruginosa caused suppression of the disease in chilli and paddy as well (Sasirekha and Srividya, 2016; Kumar et al., 2017b). The inoculation of groundnut with siderophore-producing Bacillus species caused 82% release of iron on day 32 of plant growth (Sarwar et al., 2020). Ghazy and El-Nahrawy (2021) reported siderophore production and antagonistic activities in B. subtilis MF497446 and Pseudomonas koreensis MG209738 strains. Inoculation of maize with B. subtilis and P. koreensis suppressed the pre- and post-emergence damping off disease caused by Cephalosporium maydis under greenhouse experiment. In field experiment, coinoculation of a mixture of B. subtilis and P. koreensis showed significant increases in catalase (CAT), peroxidase (POX) and polyphenol oxidase (PPO) activities, as well as total chlorophyll and carotenoids than control treatments during the two growing seasons. Similarly, the highest effect in reducing infection and increasing the thickness of the sclerenchymatous sheath layer surrounding the vascular bundles in maize stem was observed reflecting the increase in yield and yield parameters.
3.2.2. Enzyme production
Metabolic activity of any organism is regulated by the activity of various enzymes. Extracellular enzymes secreted by bacteria, archaea and fungi in the soil causes depolymerization and mineralization of structurally complex biomolecules in soil. The activities and synthesis of these enzymes could be manipulated to facilitate carbon sequestration, bioremediation and for plant growth promotion (Burns et al., 2013). PGPRs synthesize diversity of enzymes, for instance Pseudomonas, Bacillus, Xanthomonas and Agrobacterium sp. produces proteases and lipases (Ghodsalavi et al., 2013). Under abiotic stress conditions, various enzymes i.e., ascorbate peroxidase (APX), catalase (CAT), glutathione/thioredoxin peroxidase (GPX) and glutathione S-transferase help in amelioration of stress (Willekens et al., 1995; Wagner et al., 2002; Mittler et al., 2004; Nivetha et al., 2021). Hydrogen peroxidase enzyme is also known to function as a signal molecule in biotic and abiotic stress, photosynthesis and cell cycle (Sofo et al., 2015).
Salinity stress tolerance was enhanced in wheat by inoculation of PGPRs due to the alleviated concentration and activity of numerous antioxidant enzymes including manganese-dependent superoxide dismutase (MnSOD), peroxidase (POD), catalase, glutathione reductase (GR) and ascorbate peroxidase (Bharti et al., 2016). Yasmin et al. (2016) studied the inoculation effect of Pseudomonas sp. Rh323 and Pseudomonas sp. in rice and observed a strong activity of polyphenol oxidase in leaves, while a maximum activity of phenylalanine ammonia-lyase and peroxidase was observed for the plants inoculated with Pseudomonas sp. in contrast to control. Further, production of lytic enzymes for instance, chitinases, lipases, proteases, cellulase and β-1,3 glucanases by beneficial microbes was found to inhibit the growth of pathogenic fungi including Botrytis, Rhizoctonia, Sclerotium, Phytophthora, Pythium and Fusarium (Hayat et al., 2010). Five Pseudomonas strains were found to produce chitinase and cellulases, and these bacteria showed growth inhibition of Pythium aphanidermatum and Rhizoctonia solani on medium plates (Sindhu and Dadarwal, 2001). Coinoculation of these antagonistic Pseudomonas strains with Mesorhizobium sp. Cicer strain resulted in significant increase in nodule biomass under sterilized conditions. PGPRs inoculation was found to prevent diseases like rhizome rot disease and leaf blight disease of turmeric (Vinayarani and Prakash, 2018), collar rot disease in peanut (Gajera and Vakharia, 2012) and early blight disease in tomato (Babu et al., 2015).
Haroon et al. (2021) demonstrated that inoculation of ACC deaminase- and exopolysaccharides (EPS)-secreting strains of Bacillus megaterium, B. tequilensis and Pseudomonas putida positively invigorated growth attributes such as relative water content and photosynthetic pigments of wheat seedlings under saline conditions. Plants inoculated with PGPR also showed decreased concentration of malondialdehyde (MDA) and hydrogen peroxide (H2O2). Besides this, inoculation of PGPR also reduced electrolytic leakage and enhanced enzymatic activity for the scavenging of reactive oxygen species (ROS) along with increased production of proline and total soluble sugar. Higher expression of Salt Overly Sensitive (SOS1 and SOS4) genes was observed by qPCR expression analysis of selected genes, predicting their potential role to tolerate salinity stress in wheat plants.
Chitinase has been reported as another prominent plant protecting enzyme against pathogens (Maksimov et al., 2011) and can be extracted from Trichoderma, Pseudomonas and Bacillus (Babu et al., 2015). Sixty-three bacterial isolates obtained from termite mounds showed termite killing ability under Petri plate conditions and the production of chitinase, lipase and protease was correlated with termiticidal activity (Rakshiya et al., 2016). Recently, many biocontrol agents have been found to suppress various plant diseases along with stimulation of the growth and yield of different crops under pot house and field conditions (Sharma et al., 2018). Of the 90 endophytic and rhizospheric isolates obtained from field-grown common bean plants, 12 bacterial strains consisting of Bacillus amyloliquefaciens, B. halotolerans, B. velezensis, Agrobacterium fabrum and Pseudomonas lini, exhibited up to 71% inhibition of Fusarium sp., Macrophomina sp. and Alternaria sp. on common bean cv. Coco blanc (Sendi et al., 2020). Biochemical analysis of the antagonistic and plant growth-promoting activities of these biocontrol strains revealed the production of xylanases, chitinases, siderophores, HCN, phosphate-solubilizing activity and indole-3-acetic acid. However, the complex interactions between the plant, environment and biocontrol agents, are the reasons behind inconsistency observed in disease suppression and plant growth stimulation.
3.2.3. Antibiotic production
Soil serves as microbial pool for the growth and maintenance of diversity of organisms, including commensals, pathogens and symbionts (Mendes et al., 2013). With increasing population of microbes, competition for food and space also increases, which leads to adaptation of different strategies by different microbial species for their survival and establishment in particular niche (Song et al., 2005; Demanèche et al., 2008; Philippot et al., 2010; Arora et al., 2013a). The most popular strategy adopted for survival during microbial competition is the antibiotic production (Sehrawat and Sindhu, 2019; Jiao et al., 2021). Antibiotics are low-molecular-weight heterogenous compounds, which are toxic against competing microbial strains (Duffy, 2003). Antibiotics can be either volatile (aldehydes, ketones, alcohols and sulphides) or non-volatile (phenylpyrrole, cyclic lipopeptide amino polyols and heterocyclic nitrogenous compounds) (Gouda et al., 2017; Fernando et al., 2018). Antibiotics may possess antimicrobial, antiviral, antioxidant, antitumor, anti-helminthic, phytotoxic and/or cytotoxic activities, and may also act as plant growth-promoting compounds at low concentration (Kim, 2012). In response, microbes develop IAR (intrinsic antibiotic resistance) against the antibiotics, therefore antibiotic-producing strain and the competing strain with IAR provides survival strategies (Nesme and Simonet, 2015). Further, antibiotics produced by PGPR are kind of antagonistic agents produced against phytopathogens (Glick et al., 2007; van Loon, 2007). Antibiotics inhibit the growth of detrimental organisms due to distortion of cell membrane, inhibition of translation, arrest at ribosomal RNA formation stage and inhibition of cell wall synthesis (Maksimov et al., 2011).
PGPR strains have been found to produce various types of antibiotics such as 2,4-diacetyl phloroglucinol (DAPG), phenazine-1-carboxylic acid (PCA), phenazine-1-carboxamide, pyroluteorin, pyrrolnitrin, oomycin A, viscosinamide, butyroaminectone, kyanoaminectone, zymicrolactone, zymicrolactone A, rhamnolipids, cepacyamide A, ecomycins, pseudomonic acid, azomycin and cepafungins (Sindhu et al., 2010; Sindhu et al., 2017; Santoyo et al., 2019). Besides this, various Bacillus strains have been reported to produce antibiotics like mycosubtilin, bacillomycin D, iturins, fengycin, surfactin and zwittermicin A, while on the other hand fluorescent Pseudomonas produces pyoluteorin, phenazines, oomycin A, 2,4-diacetyl phloroglucinol, viscosin and massetolide A. Various antibiotics like surfactins, fengycin, phenazine and DAPG phenazine were retrieved from the rhizosphere of wheat maize, potato and rice, which showed antagonism against pathogenic fungi including Fusarium moniliforme, Fusarrium oxysporum, Aspergillus niger, Aspergillus flavus and Collectotrichum falcatum (Ali et al., 2020). Secretion of phenazine-1-carboxylic acid and 2,4-DAPG antibiotics by the Pseudomonas sp. caused suppression of Rhizoctonia solani growth (Mendes et al., 2011). Similarly, the production of DAPG antibiotic by Pseudomonas fluorescens suppressed the infestation of Meloidogyne incognita (nematode) and Fusarium oxysporum (Meyer et al., 2016). Sundaramoorthy and Balabaskar (2013) showed that combined application of Bacillus subtilis and P. fluorescens caused maximum reduction of Fusarium wilt disease under greenhouse conditions and a significant stimulatory effect on plant height and dry weight of tomato plants (upto 27% increase) was observed compared to non-bacterized control.
3.2.4. Induced systemic resistance
Plant responds to any pathogenic attack with a defence mechanism, which includes induced systemic resistance (ISR) and systemic acquired resistance (SAR). The attack of pathogens on plant is counteracted by two strong responses, which includes jasmonate pathway and ethylene pathway, named on the basis of the signaling molecule involved (Pangesti et al., 2016). In case of ISR, flagellar proteins, O-antigen side chain, chitin, pyoverdine, lipopeptide surfactants and salicylic acid act as signaling molecules (Doornbos et al., 2012). Different routes are adopted by biocontrol agents to ensure ISR in plants, which include phytohormones secretion, PAMPs (pathogen associated molecular patterns), MAMPs (microbes associated molecular patterns) and production of elicitors molecules, which could be volatile organic compounds, siderophores, phytases and miRNAs (Rodriguez et al., 2019; Abdul Malik et al., 2020).
Some of the strains of Bacillus, Pseudomonas, Serratia and Rhizobium are not host specific and are able to provide resistance to a variety of hosts (Choudhary et al., 2007). About 80% disease incidence reduction in spotted wilt virus in tomato was observed by Beris et al., (2018) after inoculation with Bacillus amyloliquefaciens and the disease control was correlated with salicylic acid signaling pathway. Kousar et al. (2020) depicted the resistance against the insect Spodoptera litura in tomato by the application of Bacillus endophyticus and Pseudomonas aeruginosa via the secretion of compounds like abscisic acid, salicylic acid, phenolics and IAA. Serratia marcescens strain 90–166 produced catechol-type siderophore, which induced resistance in cucumber to various pathogens i.e. Fusarium oxysporum, Colletotrichum orbiculare, Pseudomonas syringae, Erwinia tracheiphila and cucumber mosaic virus (Press et al., 2001).
Induced systemic resistance against pathogens resulted in deposition of callose, lignin and phenolics in epidermal and cortical cell walls, boosted expression of stress genes and overproduction of enzymes including peroxidases, phenylalanine ammona lyase, chitinase and polyphenol oxidase along with increase production of phytoalexin (Heil and Bostock, 2002; Yi et al., 2013). In poplar plant, Salicylic acid activated the biosynthesis of catechin and pro-anthocyanidins, which decreased proliferation of the foliar root fungus Melamspora larcipopulina (Ullah et al., 2019b). The platelet-activating factor acetyl hydrolase produced by Trichoderma harzianum induced the resistance in maize via jasmonic acid signaling pathway regulation against the foliar pathogen Curvularia lunata (Yu et al., 2015). Strain RS11 acted as a positive regulator for genes involved in ethylene and jasmonic acid biosynthesis and regulated defence against the necrotrophic pathogens Botrytic cinerea and Alternaria alternata (Singh et al., 2019).
In a study conducted to establish the role of beneficial microbes in strengthening plant immune system through ISR, single and consortium of two selected Streptomyces strains (Streptomyces shenzhenensis TKSC3 and Streptomyces sp. SS8) was applied in rice and the treatments suppressed ISR-mediated bacterial leaf streak (BLS) disease caused by infection of Xanthomonas oryzae pv. oryzicola (Xoc) (Hata et al., 2021). Streptomyces treatments (both single and consortium) increased peroxidase (POX), polyphenol oxidase, phenylalanine ammonia-lyase, and β,1–3 glucanase (GLU) accumulation compared to untreated plant. Consortium treatment TKSC3 + SS8 showed the highest disease suppression efficiency (81.02%) and the lowest area under the disease progress curve value (95.79), making it the best to control BLS disease. In addition, consortium treatment TKSC3 + SS8 induced the highest POX and GLU enzyme activities at 114.32 μmol/min/mg protein and 260.32 abs/min/mg protein, respectively. Moreover, seed bacterization of rice by Streptomyces strains improved seed germination and vigor relative to the untreated seed. Seed bacterization with consortium treatment of two selected Streptomyces strains also increased seed germination, root length, and dry weight by 20%, 23% and 33%, respectively under greenhouse conditions. In another study, transcriptomic analysis of defense-related genes using the markers of the salicylic acid (SA) signaling pathway (PR-1A and GLUA) or jasmonic acid/ethylene (JA/ET) signaling pathway (CHI3, LOXD, and PAL), showed increased transcription patterns in tomato plants treated with Bacillus subtilis MBI600 (Bs MBI600) or Fusarium oxysporum f. sp. radicis-lycopersici – Forl (Samaras et al., 2021). Besides this, transcriptional activation of two auxin-related genes (SiPin6 and SiLax4) was also observed. The application of Bs MBI600 on pathogens-inoculated tomato plants revealed satisfactory disease control efficacy compared to chemical treatment and also caused significant increases in shoot and root lengths. Similarly, Taha et al. (2021) showed that compound 6-pentyl-α-pyrone (6PP) obtained from endophytic fungal strain Trichoderma koningii CTX1172 (AUMC 11,520) induced systemic resistance in tobacco (Nicotiana tabacum cv. White Burley) plant against tobacco mosaic virus (TMV) and exhibited 10–60% symptoms inhibition at low concentrations (10–30 μg mL−1) achieving 100% biocontrol efficacy at high concentrations (40 and 50 μg mL−1) compared with control. Application of 6PP not only increased the accumulation of proline, but also increased the activities of pathogenesis-related enzymes (superoxide dismutase, peroxidase and polyphenol oxidase), indicating that 6PP acts as elicitor for induction of resistance in tobacco against TMV. On the molecular level, plants treated with 6PP also showed augmented and rapid expression of defense-related genes including PR-a, PR-b and PR-10, implying the potential of a pyrone compound in biocontrol of plant viral disease.
3.2.5. Production of hydrogen cyanide and ammonia
Another significant trait for biocontrol activity by the PGPRs is the production of hydrogen cyanide (HCN) and ammonia. Some of the rhizobacterial strains are well known for the simultaneous synthesis of both HCN and ammonia giving a synergistic effect on growth of plants (Kumar et al., 2016b). HCN has been reported to chelate metal ions, makes phosphorous available in soil and also contributes as highly toxic metabolite against growth of phytopathogens (Rijavec and Lapanje, 2016). Production of hydrogen cyanide ensures the use of PGPR strains as biocontrol agent in agriculture (Rijavec and Lapanje, 2016; Sehrawat et al., 2022). Cyanogenic strain of Pseudomonas fluorescencs were found to cause enhancement in germination rate, length of root and shoot of wild barley, rye and wheat (Heydari et al., 2008). HCN producing PGPB strains are used as biofertilizer as they influence growth and yield of various crops (Rijavec and Lapanje, 2016; Kumar et al., 2017b, Kumar et al., 2016a). Apart from HCN, ammonia produced by rhizobacterial strains provides nitrogen to the plant, thus promoting biomass and elongation of root and shoot (Marques et al., 2010).
Bacillus sp. isolated from Phaseolus vulgaris were found to produce HCN, which can inhibit phytopathogens including Rhizoctonia sclerotinia, Fusarium oxysporum, F. solani, Sclerotinia sclerotiorum, Macrophomina phaseolina and Colletotricum sp. (Kumar et al., 2012b). Zain et al. (2019) characterized bacteria that showed antagonism against phytopathogenic Fusarium spp. in the cotton and sugarcane rhizosphere. It was demonstrated that production of lytic enzymes, IAA, HCN and phosphate solubilization in these isolated rhizobacteria contributed towards the control of pathogen's growth and caused the promotion of plant growth. Sendi et al. (2020) showed that twelve bacterial strains (endophytic and rhizospheric isolates) obtained from field-grown common bean plants exhibited up to 71% of inhibition of the three pathogenic strains belonging to Fusarium sp., Macrophomina sp. and Alternaria sp. Biochemical analysis of the antagonistic and plant growth-promoting activities revealed the phosphate-solubilizing activity and production of xylanases, chitinases, siderophore, HCN and indole-3-acetic-acid. In another study, Anand et al. (2020) reported that HCN is a dominant inhibitor when mycelial growth inhibition was carried out using in vitro volatile compounds of Phytophthora infestans (causal agent of late blight of potato). HCN-negative mutants (Dhcn) were obtained from two cyanogenic Pseudomonas strains, P. putida R32 and P. chlororaphis R47. Further in vitro studies of volatile-mediated interactions demonstrated that HCN played a major role in growth inhibition of mycelium (57% in R47 and 80% in R32). But when combined interaction study of volatile and diffusible compound was carried out, a low inhibition was observed by HCN.
Pathak et al. (2021) screened 39 Bacillus isolates for plant growth promoting traits in vitro and found that 48.7% isolates were IAA producers, 38.4% of the isolates showed the ability to solubilize the phosphate and 71.8% isolates were able to produce ammonia. All the isolates showed the ability to produce hydrogen cyanide and protease. In another study, thirteen bacteria were isolated from salt-polluted soil (Sharma et al., 2021). Isolates HB6P2 and HB6J2 showed maximum tolerance to salts at 10% followed by HB4A1, HB4N3 and HB8P1. All the salt-tolerant bacterial isolates showed HCN production with maximum production by HB6J2 isolate and ammonia production was maximum in HB6P2 (12.3) and least in HB8P1 (6.2). Three potent isolates HB6J2, HB8P1 and HB4N3 were identified as Bacillus paramycoides, Bacillus amyloliquefaciens and Bacillus pumilus, respectively using 16S rDNA sequencing. These bacteria may play an important role in the recycling of plant nutrients through phytostimulation and phytoremediation.
4. Types of biofertilizers
Biofertilizers are the formulation of living or latent cells of microbes, which provides additional advantage in nutrient uptake and plant performance in rhizosphere. The biofertilizer formulation technique is simple with low installation cost and the former can be composed of single or a mix of two or more diverse microbial strains including Acetobacter, Azotobacter, Bacillus, Pseudomonas, Rhizobium, PGPB or plant growth promoting bacteria and AM or arbuscular mycorrhiza (Basu et al., 2021; Fausi et al., 2021; Mohanty et al., 2021). Biofertilizers are subdivided into different groups (Fig. 2), which are as follows:
Fig. 2.
Categories of different biofertilizers along with microbial species involved.
4.1. Nitrogen-fixing microbes
The process of converting atmospheric nitrogen into ammonia by the diazotrophic microbes is known as biological nitrogen fixation (BNF). BNF allows the replenishment of total nitrogen content and the fixed nitrogen regulates the crop growth and yield. Chemical fertilizers cause increased nitrogen oxide emission, water eutrophication and soil acidification. Whereas, biologically fixed nitrogen is sustainable and is less available for leaching and volatilization. Nitrogen fixation is more or less limited to bacteria and archaea, which forms a large portion of diazotrophic organisms. Nitrogen-fixing groups include green sulphur bacteria, firmibacteria, actinomycetes, cyanobacteria and all subdivisions of the proteobacteria. However, only methanogens are able to fix nitrogen among archaea. Different bacterial strains are able to carry out nitrogen fixation with different physiologies including: aerobic (for example, Azotobacter), anaerobic (Clostridium), facultatively anaerobic (Klebsiella) or heterotrophs; anoxygenic (Rhodobacter) or oxygenic (Anabaena) phototrophs; and chemolithotrophs (Leptospirillum ferrooxidans). Diazotrophs are inhabitants of varied habitats of soil and water, and contribution of different diazotrophic bacteria varies from 20 kg-300 kg N/ha/year (Table 2). Diazotrophic bacteria can develop association with grasses, symbiotic relationship with termites, cyanobacterial symbioses, actinorhizal association with woody plants and symbiosis with legumes leading to root nodule formation.
Table 2.
Amount of nitrogen fixed by different microbial strains.
| Category | Biofertilizers | Amount/ha/year | References |
|---|---|---|---|
| Free-living | Azotobacter | 20–40 Kg N | Thomas and Singh, 2019 |
| Blue green algae | 20–40 Kg N | Singh et al., 2016 | |
| Symbiotic | Rhizobium | 50–300 Kg N | Brahmaprakash and Sahu, 2012 |
| Azolla-Anabaena | 30–60 Kg N | Kollah et al., 2016 | |
| Frankia | 89.7 Kg N | Brahmaprakash and Sahu, 2012 | |
| Associative | Azospirillum | 20–160 Kg N | Okumura et al., 2013; Pathak et al., 2017 |
| Acetobacter diazotrophicus | 20- 150 Kg N | Boddey et al., 1995 |
Nitrogenase is the key enzyme, which carries out conversion of dinitrogen into ammonia during the process of nitrogen fixation. It is a complex metalloenzyme having conserved mechanistic and structural properties (Rees and Howard, 2000; Lawson and Smith, 2002). Nitrogenase is constituted of two components; a dimeric Fe (iron) protein (dinitrogenase reductase) and a heterodimeric MoFe protein (dinitrogenase). The Fe protein is an ATP dependent electron donor, while the MoFe protein is the catalytic site possessor. Though diazotrophs have molybdenum-iron nitrogenase enzyme but under the scarcity of molybdenum, some of the microbes have alternative nitrogenase having vanadium-iron or iron-iron cofactors as observed in Azotobacter vinelandii and Rhodobacter capsulatus (Eady, 1996). Nitrogenase is an oxygen sensitive enzyme and for Fe protein it is deliberated by the [4Fe-4S] cluster, which is exposed on surface and functions as a bridge between the subunits of dimer. There are two different kinds of metal centres within MoFe protein: P cluster (8Fe-7S), FeMo cofactor (MoFe7S9 homocitrate), substrate reduction site (Einsle et al., 2002; Seefeldt et al., 2004). Dinitrogen reduction can be expressed by the equation as follows:
| N2 + 8 e– + 8 H+ + 16 Mg ATP → 2 NH3 + H2 + 16 Mg ADP + 16 Pi |
Nitrogenase is encoded by at least 20 nitrogen fixation (nif) genes in aerobic diazotrophic bacteria. In model organism Klebsiella pneumoniae, nif genes are organized in seven operons known as nif cluster (spanning over 24 kb DNA located either on plasmids or chromosome), which comprises various nif genes including structural, regulatory and supplementary genes. Gene nifH encodes Fe protein, while Mo-Fe protein is encoded by nifD and nifK, collectively these genes are the structural nif genes. However, some additional genes are also involved in nif gene regulation, maturation process of electron transport, Fe-Mo cofactor biosynthesis and assembly, which includes nifE, nifN, nifX, nifQ, nifW, nifV, nifA, nifB, nifZ and nifS (Masepohl et al., 2002; Lee et al., 2000). In addition, four operons containing fixABCX, fixGHIS, fixLK and fixNOQP genes have been identified in Rhizobium meliloti (Kallas et al., 1985; Earl et al., 1987), which were involved in electron transfer, regulatory and other accessory functions to carry out nitrogen fixation by nitrogenase (Fischer, 1994; Edgren and Nordlund, 2004; Wongdee et al., 2018).
4.1.1. Symbiotic nitrogen-fixing microbes
Symbiotic association with roots of legumes is formed by species of Mesorhizobium, Azorhizobium, Allorhizobium, Rhizobium and Sinorhizobium (collectively termed as Rhizobium) (Sindhu and Dadarwal, 1997). Various Rhizobium strains form nodules on specific leguminous plants contributing to the enhanced growth, increased nutrition of plant and improvement in soil fertility (Sindhu et al., 2019b). Another important aspect of nitrogen fixation is leghemoglobin formation in nodules, which helps in maintaining a low concentration of oxygen necessary for the activity of oxygen-sensitive nitrogenase (Marchal, and Vanderleyden, 2000). The process of nitrogen fixation carried out by Rhizobium enables the legumes to be less dependent on chemical fertilizers as compared to the non-leguminous plants (Goyal et al., 2021).
Inoculation of effective strains of rhizobia in various legumes produced a significant increase in plant biomass and grain yield of various legume crops (Sindhu et al., 1992; Thies et al., 1991; Goel et al., 2001). However, numerous failures and inconsistencies have been reported in achieving yield increases following inoculation with rhizobial strains under field conditions (Miller and May, 1991). Moreover, the left-over nitrogen in the field after harvesting of legumes was found often equivalent to the application of 30–80 Kg of fertilizer nitrogen per hactare (Sindhu et al. 1992). Mfilinge et al. (2014) revealed that inoculation of Rhizobium strains in soybean altogether enhanced growth and yield constituents such as number of branches bearing pod per plant, total number of pods per plant and seed number per pod. So also, inoculation of Rhizobium leguminosarum strains onto seeds of pea and lentil brought about enhancement of nodulation, shoot/root weight and yield of pea seed (Bourion et al., 2017). The effect of variations in the rhizosphere microbial communities and their interactions with bradyrhizobia was found to affect the symbiotic efficiency in soybean (Han et al., 2020).
In exception, Acetobacter strains showed the potential of nitrogen fixation under aerobic conditions and in symbiotic relationship with sugarcane. Acetobacter produced gibberellic acid and indole acetic acid essential for the proliferation of rootlets, thus increasing the surface area for efficient nutrients and water uptake, and enhancing phosphate solubilization leading to promotion of the growth and sugar recovery in sugarcane. Another symbiont Azolla has been reported to associate with nitrogen-fixing blue green algae (BGA), Anabaena azollae, which is a highly efficient biofertilizer strain. Anabaena is frequently observed in the rice fields and fixes approximately 40–60 Kg N/ha of rice crop (Kannaiyan, 1993).
Sometimes, dual inoculation strategies have shown more stimulation of plant growth and increase in crop yield than single inoculation (Sivaramaiah et al., 2007; Chaudhary and Sindhu, 2016). For example, combined inoculation of Bradyrhizobium spp. and Azospirillum brasilense significantly increased the soybean yield in comparison to single inoculation of Bradyrhizobium spp. in a field experiment (Hungria et al. 2015). Similarly, the application of rhizobia with cyanobacterium Anabaena laxa and Trichoderma sp. showed promotive effects on nodulation, nitrogen fixation and crop yield of pea, chickpea and lentil (Babu et al., 2015). In another study, 20% increase in wood yield of non-nodulating legume Schizolobium parahyba var. amazonicum was observed by coinoculation of AM fungi and PGPRs (Cely et al., 2016). Kavadia et al. (2021) evaluated the effect of tripartite symbiotic associations on cowpea plants grown under limited N supply, with or without a symbiotic nitrogen-fixing bacterium, Sinorhizobium meliloti and combinations of three different arbuscular mycorrhizal fungal species namely Dominikia disticha, Claroideoglomus etunicatum and Rhizophagus irregularis in a pot gnotobiotic trial. Inoculation with both AMF and S. meliloti increased above ground biomass production compared to inoculation with AMF only, but the positive stimulation effect depended on the specific AMF partners used. Single inoculation with AMF showed a highly positive impact on the growth and P uptake of cowpea, but the nitrogen-fixing bacteria (NFB) inoculation was needed to address N deficiency in planta. The AMF composition in plant roots was also altered in the presence of the S. meliloti. Plant nitrogen content of cowpea plants significantly increased under the presence of both symbionts compared to AMF alone, while phosphorus content was hardly affected by dual inoculations. However, the efficiency of synergism depends on the specific AMF partners used and it is not related to their colonization levels.
4.1.2. Free-living nitrogen fixing bacteria
Azotobacter is one of the prime members among free-living diazotrophic bacteria (Aasfar et al., 2021). Different Azotobacter strains have been isolated from neutral to alkaline soil and usually found in the rhizosphere of various non-legume crops including cotton, wheat, rice and vegetables (Sindhu and Lakshminaryana, 1982; Jain et al., 2021). Arable cultivated soil is chiefly inhabited by Azotobacter chroococcum along with Azotobacter insignis, A. beijerinckii, A. macrocytogens and A. vinelandii, which potentially fixes up to 2–18 mg N/g of carbon used in culture medium (Moraditochaee et al., 2014; Smercina et al., 2019). Some of the Azotobacter strains have been found to act as potential biocontrol agent and also reported to excrete bioactive compounds like phytohormones, which promotes mineral uptake by enhancing the root growth (Mahanty et al., 2016; Noar and BrunoBa´rcena, 2018). Azotobacter vinelendii secretes azotobactin siderophore under iron deficiency (Noar and BrunoBa´rcena, 2018). Wang et al. (2018) conducted an experiment with Azotobacter chroococcum to evaluate its effect on nitrogen fixation. An alteration was observed in structural configuration of nitrogenase, which uplifted the nitrogen fixation by 158%, when carbon source was applied at 4 μg/mL concentration.
Pandey and Kumar (1989) concluded the findings of various experiments and stated that Azotobacter inoculation could significantly enhance the yields of different crops including maize, rice, sorghum, pearl millet and wheat by 0–72% as compared to the uinoculated controls without any amendments. The addition of farm yard manure and fertilizers caused 8–43% increase in the yields of wheat over the control treatment. Lakshminarayana et al. (2000) inoculated wheat (WH291) with Azotobacter chroococcum strain A103 and observed a 16.3% increase in grain yield, while inoculation of same wheat variety with analogue-resistant mutants of same strain of Azotobacter increased grain yield by 10–30% under field conditions. Sangwan et al. (2012) showed that seed treatment of wheat variety WH711 with Bacillus strain SYB101 caused 32.6% increase in seed yield, whereas this strain caused 23.1% increase in seed yield of another wheat variety Raj3765 in comparison to uninoculated control treatment. On the other hand, Azotobacter chroococcum inoculation resulted in only 7.4% increase in case of Raj3765 variety.
Chaudhary et al. (2013) reported that inoculation with salinity tolerant Azotobacter strains caused significant increase in total nitrogen, biomass and grain yield of wheat variety WH157 in earthen pots containing saline soil under pot house conditions. Maximum increase in plant growth parameters was obtained after inoculation with Azotobacter strain ST24 at fertilization dose of 120 Kg N/ha. Yousefi and Barzegar (2014) observed the outcome of collective inoculation of Azotobacter and Pseudomonas in comparison to control and reported a rise in grain yield, harvest index, biomass and protein content of wheat by 34.3, 7.7, 12.5 and 13.6%, respectively. Around 10–12% increase in crop productivity has been reported after the inoculation with Azotobacter strains under different agroclimatic conditions. Different species of Azotobacter fix 20–40 Kg/ha/year of nitrogen and inoculation of selected strains enhanced germination and vigour of young plants along with grain yield in wheat (Kader et al., 2002). Jain et al. (2021) found that all the isolated 24 Azotobacter strains showed IAA, siderophore, HCN, and ammonia production, whereas seven Azotobacter strains showed phosphate solubilization. Significant diversity was revealed among all the isolates by Amplified Ribosomal DNA Restriction Analysis (ARDRA) and the dendrogram differentiated twenty-four of the strains into two major clusters at a similarity coefficient of 0.64. The amounts of acetylene reduced (N2 fixation) by Azotobacter strains varied in the range of 1.31 to 846.56 nmol C2H4 mg protein−1 h−1. Inoculation of Azotobacter strains significantly increased the various plant growth parameters of maize plantlets under pot studies.
Another important free-living nitrogen-fixing group is cyanobacteria, which are anaerobic, photosynthetic blue green algae and are inhabitants of alkaline moist soil. Free-living photosynthetic cyanobacteria (Anabaena, Nostoc, Aulosira, Calothrix etc.), or symbiotic cyanobacteria (Azolla-Anabaena system) or blue green algae (BGA), which are commonly found in lakes, ponds, springs, wetlands, streams and rivers, has been reported to fix about 4–6 billion kilograms of N2 annually (Song et al. 2005; Singh et al. 2016). The prominent genera Nostoc and Anabaena were described to fix up to 20–25 Kg N/ha. These are used as biofertilizer for rice crop during rainy season. Azolla sp. is generally used for wetland and livestock feed. The latter synthesises micronutrients such as proteins, amino acids and lipids. Cyanobacteria have also been reported for increasing water holding capacity (Saadatnia and Riahi, 2009), soil fertility and crop yield apart from the synthesis of phytohormones, vitamins and amino acids (Rodríguez et al., 2006). It further decreases the growth of weeds and soil salinity, while increases the phosphorous levels in the soil (Wilson, 2006; Bhuvaneshwari and Singh, 2015). Cyanobacterial strains are known to increase the growth and yield of chilli, cotton, barley, oats, maize, tomato and radish (Thajuddin and Subramanian, 2005). Species of Tolypothrix, Calothrix, Nostoc linkia, Aulosira fertilisima, Anabaena variabilis and Scytonema are applied for the cultivation of rice under lowland and upland conditions (Prasad and Prasad, 2001).
4.1.3. Associative nitrogen-fixing microbes
Azospirillum is usually applied as biofertilizer on wetlands in many countries including Italy, Mexico, Belgium, Africa, USA, Pakistan, France, Germany, Uruguay, Australia, Argentina and Brazil (Okon and Labandera-Gonzalez, 1994; Bashan and De-Bashan, 2010; Hungria et al., 2010; Glick, 2014; Mehnaz, 2015; Pereg et al., 2016). Azospirillum species associates with plant roots and synthesize various compounds involved in plant growth promotion, for instance IAA, gibberellins and cytokinin. At present, around 17 diverse species of Azospirillum have been characterized, although Azospirillum brasilense and Azospirillum lipoferum are the most studied ones (Rodrigues et al., 2015). Azospirillum alone fixes nitrogen upto 20–40 Kg/ha/year in non-legumes. Azospirillum species change the root morphology for the increased plant nutrient efficiency (Fibach-Paldi et al., 2011) and it furthers supports plants under stress conditions by modulating osmosis and elasticity of cell wall (Richardson et al., 2009; Groppa et al., 2012). Bacilio et al. (2004) showed that inoculation of A. lipoferum strain JA4 showed improved plant growth (higher height and dry weight of root as well as shoots) under continous irrigation with 160 mM NaCl when contrasted with uninoculated control plants. Double inoculation of Rhizobium species with Azospirillum and/or other PGPR strains significantly improved nodule number, nitrogen fixation, plant biomass and total nitrogen contents of several legumes in contrast with single inoculation with Rhizobium alone or uninoculated plants (Molla et al., 2001; Remans et al., 2008).
Alen'kina and Nikitina, (2021) evaluated the effect of lectins from two strains of Azospirillum brasilense Sp7 (epiphyte) and Azospirillum brasilense Sp245 (endophyte) on germination and growth characteristics of the host wheat plant under abiotic stresses. The lectins of A. brasilense Sp7 and Sp245 neutralized the negative effects of simulated abiotic stresses, heavy metals (СuSO4, CoSO4, ZnSO4, Pb(CH3COO)2), hypo- and hyperthermic stress, salinization and drought with different efficiency, causing a decrease in seed germination of wheat. For both Azospirillum strains, the most pronounced effect on germination was observed in the case of exposure to heavy metals. Thus, stimulating effect of lectins was demonstrated on the length and number of roots of wheat seedlings. The lectin of the endophytic strain showed a higher efficiency as compared to the lectin of the epiphytic strain.
4.2. Phosphate-solubilizing/mobilizing microbes
Phosphorous is a vital macronutrient required for the growth and development of a plant (Bamagoos et al., 2021). Soil harbours a fair volume of phosphorous amounting to a range of 400–1200 mg/Kg of the soil. But the concentration of soluble or inorganic available phosphorus i.e., orthophosphate is very low, hence low availability of phosphorous in the soil results in reduction of the crop yield (Miller et al., 2010; Wang et al., 2017). Usually, phosphorous exists in the form of tricalcium, dicalcium phosphate and minerals. The process of solubilization and mineralization in soil i.e., conversion of organic form of phosphate into inorganic form is carried out by phosphate-solubilizing bacteria (Oteino et al., 2015; Tandon et al., 2020). PSB secretes organic acids like citric acid and gluconic acids, which solubilizes the organic reservoirs of phosphates. Also, PSB secretes phytases and nucleases enzymes for mineralizing the organic reservoirs of phosphates (Novo et al., 2018; Ku et al., 2018). PSBs are also well known for producing secondary metabolites such as IAA and siderophores, which causes plant growth promotion (Hariprasad and Niranjana, 2009). Interestingly, ability to produce indole acetic acid was associated with improved phosphate solubilizing activity of rhizobacteria (Alemneh et al., 2021) and addition of L-tryptophan to growth media was found to increase the P-solubilizing activity of PSB that were able to produce IAA greater than 20 µg mL−1.
Numerous microbes including Escherichia freundii, Bacillus, Pseudomonas, Achromobacter, Brevibacterium, Erwinia sp., Flavobacterium sp., Micrococcus sp., Corynebacterium, Xanthomonas sp., Nostoc, Rhodococcus sp., Serratia phosphaticum, Acytonema, Calothrix brauna and Tolypothrix ceylonica, Bacillus, Pseudomonas, Achromobacter, Brevibacterium, Burkholderia, Sarcina sp. and Scytonema have been reported to solubilize phosphorous in soil (Oteino et al., 2015; Santoyo et al., 2021b). Similarly, many fungi including Fusarium sp., Rhodotrula minuta, Saccharomyces cerevisiae, Torula thermophila, Paeciliomyces, Penicillium, Sclerotium rolfisi, Cephalosporium sp., Aspergillus sp., Cylindrocladium sp., and Alternaria sp., were reported to show phosphate solubilization (Sindhu et al., 2014). The phosphorus solubilizing microbial strains like Aspergillus, Bacillus, Escherichia, Arthrobacter and Pseudomonas have been reported to solubilize upto 30–35 kg P2O5/ha (Gaur et al., 2004).
Inoculation with three strains of phosphorus-solubilizing bacteria i.e., Pseudomonas fluorescens strains (CB501, CD511 and CE509) was carried out in Zea mays under greenhouse conditions (Henri et al., 2008). Obvious results of enhanced growth, yield and phosphorous uptake were recorded. P. fluorescens strain CB501 showed maximum growth promotion of maize plants with a global effect of +37%, followed by CE509 strain (+21.2%) and CD511 strain (+16.7%). Yousefi et al. (2011) conducted an experiment with four types of soil including loam, sandy loam, clay and clay loam soil types, and three phosphorus fertilizers were taken at the dose of 0, 20 and 40 mg/kg soil along with four levels of PSM or phosphate solubilizing microorganisms. They observed the maximum shoot dry matter in clay loam soil (21.5 g/pot). Further when PSB and AMF were inoculated as a combination than shoot dry matter, spike number and grain yield were increased by 52, 19 and 26%, respectively as compared to control. Qureshi et al. (2012) studied the effect of Bacillus sp. inoculation on cotton. An increased seed yield (1630 kg ha−1) was observed as compared to control (1511 Kg ha−1) at pH 8.3 for clay loam soil under field conditions. Further, a positive effect was recorded on plant parameters like plant height, number of bolls and soil available phosphorous on inoculation with Bacillus sp.
Ditta et al. (2018) showed that inoculation of PSB in chickpea caused an increase of 23%, 13%, 17% and 15% in number of nodules per plant, shoot length, number of pods per plant and grain yield, respectively in chickpea. Further, there was a rise in soil aggregate stability (37%) and 2.35 times more phosphorous was released from rock phosphate. Also, nitrogen, phosphorous and protein content was enhanced in straw and chickpea i.e. 11%, 42% and 16%, respectively. Wang et al. (2021) reported that inoculation of peanut with purple non-sulfur bacteria Rhodopseudomonas palutris, PSB Burkholderia cepacia ISOP5 and a coinoculation of these two bacteria could enhance the yield by 12.5%, 8.1% and 19.5% after 5 years of inoculation. Further, these treatments also influenced nitrogen absorption and protein content in peanut seeds. Inoculation of these bacteria also caused an increased composition of genes functioning in organic phosphorous mineralization and inorganic phosphorus solubilization. However, inoculum did not affect bacterial community diversity and richness.
Mycorrhiza also play crucial role in phosphorus mobilization, nutrient cycling and enhancement of microbial biomass. Generally, indigenous arbuscular mycorrhizae (AM) are found in soil, which colonizes the plant roots and stimulate plant growth. Inoculation of low phosphorous soil with mycorrhiza causes a sudden increase in availability of phosphorous. Arbuscular mycorrhizal fungi are symbiotic in nature and readily associate with cereals and horticulture plants (Dalpe and Monreal, 2004), and are best known for improving phosphorus bioavailability. Fungal hyphae are long enough to penetrate those far-off soils where plant roots fail to reach and thus plants with mycorrhizal association surpass the non-mycorrhizal association ones in terms of exploring soil nutrients (Pandey et al., 2019). Genera like Scutellospora, Glomus, Acaulospora and Gigaspora are frequently utilized as biofertilizers.
Toro et al. (1997) described that coinoculation of G. intraradices and Bacillus subtilis in onion results in improved accumulation of N and P along with increased plant biomass. The inoculated rhizobacteria released Pi from the added rock phosphate (RP) and at least 75% of the P in dually inoculated plants was derived from the added RP. Yao et al. (2005) studied the growth-promoting effect of Glomus intraradices and Gigaspora margarita on Litchi chinensis. Glomus mosseae is widely studied and reported to increase the weight and length of shoot and root in wheat (Bhale et al., 2018). Mycorrhiza also positively influences the soil structure (Bhat et al., 2017). The positive effects of mycorrhizal inoculation include improvement in plant health, soil productivity, soil aggregate stability and increase in crop yield (Begum et al., 2019). Therefore, three-way interactions among bacteria, fungi, and plant result in biogeochemical phosphorous cycling and sustainable nutrient avaialability to plants.
Etesami et al. (2021) reviewed the contributions of silicon addition along with inoculation of phosphate–solubilizing bacteria and arbuscular mycorrhizal fungi (AMF) in improving the P availability. The combined strategy of using Si along with AMF and PSB was found highly useful in improving the P availability and its uptake by plants compared to using either of them alone. In similar studies, Nacoon et al. (2021) evaluated the effects of coinoculation between an arbuscular mycorrhizal fungus and PSB to promote the growth and production of sunchoke under field conditions. The results showed the presence of PSB and AMF colonization at the harvest stage in both years. PSB was found to positively affect AMF spore density and colonization rate. Inoculation with both AMF and PSB was positively correlated with growth and production of sunchoke. Coinoculation of AMF and PSB was found to enhance various plant parameters, suggesting that AMF and PSB could effectively promote growth and production of sunchoke under field conditions.
4.3. Potassium-solubilizing microbes
Potassium is ranked at third position as crucial plant nutrient after nitrogen and phosphorous (Ding et al., 2021; Patel et al., 2021). Potassium is available in plentiful amount in the soil but only a small fraction (1–2%) of it is available to plants. Hence, a system of continuous replenishment of potassium in soil solution is needed for its adequate availability to crop plants (Park et al., 2009; Meena et al., 2014; Parmar and Sindhu, 2019). Like other nutrients, potassium also influences growth and development of plants, and if it is not supplied in required amount, plant growth will be slow with poorly developed roots and low yield (Williams and Pittman, 2010). Potassium also affects important physiological processes such as starch production, root growth and stomatal movement (Gallegos-Cedillo et al., 2016). In deficiency of potassium, root growth becomes slow and gets poorly developed, seeds will be of small size and disease susceptibility will be more leading to reduction in crop yield (Troufflard et al., 2010).
PGPRs present in the soil and rhizosphere convert the potassium present in insoluble form into soluble form. Some of the potassium-solubilizing microbes (KSMs) are Acidothiobacillus, Enterobacter hormaechei, Paenibacillus sp., Aminobacter, Pseudomonas, Paenibacillus glucanolyticus, Sphingomonas, Aminobacter, Bacillus circulans, Burkholderia, and Acidothiobacillus ferrooxidans (Uroz et al., 2007; Parmar and Sindhu, 2019). Organic acid secreted by microorganisms’ leads to dissolution of potassium, therefore KSMs application may be a promising strategy for improving crop productivity (Sindhu et al., 2016). Inoculation studies of potassium-solubilizing bacteria showed increased germination percentage, seedling vigour, uptake of nutrients, growth and yield of plant on the application of KSMs (Basak and Biswas, 2009; Singh et al., 2010; Sindhu et al., 2016). Similar effects of improved NPK content and growth of root as well as shoot was observed by Sheng and He (2006), when wheat was inoculated with Bacillus edaphicus strain in soil with little potassium content. Singh et al. (2010) evaluated the inoculation effect of consortia comprising of Azotobacter chroococcum, Rhizobium sp. and Bacillus mucilaginosus in maize and wheat. Results showed significant assimilation of potassium, which resulted in enhanced crop productivity. Plant growth-promoting effects have been demonstrated by inoculation of potassium-solubilizing microbes in various crops including pepper, cucumber (Han and Lee, 2006), wheat (Sheng and He, 2006), cotton, rape (Sheng, 2005) and Sudan grass (Basak and Biswas, 2009).
A compilation of 20 greenhouse experiments and 12 field trials showed that inoculation with KSM improved the crop yield by an average of 17% in different crops (Basak et al. 2020). Ashfaq et al. (2020) showed that inoculation of the five best halotolerant potassium solubilizing bacterial strains i.e. Acinetobacter pitti strain L1/4, A. pitti strain L3/3, Rhizobium pusense strain L3/4, Caprivadus oxalaticus strain L4/12 and Ochrobactrum ciceri strain L5/1 significantly improved the shoot length, fresh weight, dry weight and chlorophyll contents of paddy plants grown under saline conditions. Similarly, inoculation of Paenibacillus mucilaginosus caused improved growth of apple seedlings (Chen et al., 2020). Bakhshandeh et al. (2020) reported that four plant growth-promoting microorganisms i.e., Bacillus cereus (B1), Bacillus megaterium (B2), Trichoderma longibrachiatum (F1) and Trichoderma simmonsii (F2) promoted soybean seed germination and seedling growth under laboratory and pot experiment conditions. Combinations of these PGPM and single inoculations of B2, F1, F2 were considered to be the best treatments for improving seed germination, seedling growth and potassium uptake of soybean plants in both experiments. Ali et al. (2021) reported that inoculation with the potassium solubilizing bacterium Bacillus cereus significantly increased the plant height, branch number and shoot dry weight of potato by about 15, 27 and 26%, respectively. The biofertilization of potato with B. cereus significantly increased the total yield of potato by 21% in comparison to uninoculated plants. A total potato tuber yield of 40 ton per hectare was achieved by application of K-feldspar at a rate of 240 kg K2O per hactare along with inoculation of B. cereus.
Ding et al. (2021) applied 50 and 100% of K recommended dose with or without potassium solubilizing bacteria (PSB) and 40 kg of humic acid (HA) ha−1 to faba bean (Vicia faba L., cv. Giza 843) plants grown in sandy loam soils. Maximum potassium use efficiency (KUE) (40%) was obtained in the soil treated with HA and PSB. Maximum growth and yield of faba bean plants was observed by application of humic acid and PSB to the plants fertilized with 50% of the recommended dose. Chlorophyll and carbohydrates in the leaves were increased by 36 and 50%, respectively, above the control, as results of HA and PSB application. Adding half of K requirements for faba bean in a mineral form with 40 kg of HA and PSB led to 14% and 19% increases in the seed and straw yield compared to the full mineral fertilization without bacterial inoculation. In another study, Raji and Thangavelu (2021) isolated fifteen culturable saxicolous (rock-dwelling) bacterial isolates with varied K solubilizing ability from two sites. Of these, four potential K solubilizer isolates were identified as Bacillus subtilis, Bacillus cereus, Bacillus licheniformis and Burkholderia cenocepacia by 16S rRNA gene sequencing. Potassium solubilization differed among the bacterial isolates and was significantly influenced by K sources. Isolated KSB produced different organic acids, indole acetic acid and siderophore under in vitro conditions. Inoculation of KSB improved the tomato plant growth parameters like plant height, leaf area, total root length, root/shoot ratio, and tissue K content in sterilized and unsterilized soils under greenhouse conditions. Higher residual K content was also observed in the KSB inoculated post-harvest soils. Simiarly, an experiment was conducted in wheat (Triticum aestivum L.), which comprised twelve-treatment combinations (Patel et al., 2021). The treatments consisted of two levels of farm yard manure (FYM) viz., 0 t ha−1 (F0) and 10 t ha−1 (F1) and two levels of potassium mobilizing bacteria (KMB) viz., without KMB (KMB0) and with KMB (KMB1) and three levels of potassium viz., 0 kg K2O ha−1 (K0), 20 kg K2O ha−1 (K1) and 40 kg K2O ha−1 (K2). The application of FYM, potassium mobilizing bacteria and potassium showed significant increase in root biomass, dry matter, spike length, total number of tillers and grain yield of wheat. Treatment combination KMB1K2 (KMB along with potassium @ 40 kg ha−1) recorded significantly the highest spike length (10.54 cm), whereas treatment combination F1KMB1K2 (FYM @ 10 t ha−1 along with KMB and potassium @ 40 kg ha−1) recorded significantly higher grain yield (5640 kg ha−1) of wheat.
4.4. Zinc solubilizing microbes
Among micronutrients, zinc deficiency is the most widespread nutrient deficiency (Hafeez et al., 2013). Deficiency of zinc (Zn) imparts negative effects not only to plants but also to human health. Deficiency of zinc is ranked at 5th position in terms of human-related death in under developed countries. Zinc is involved in the synthesis of chlorophyll, enzymes, proteins and metabolic reactions (Ali et al., 2008). Plants suffering from zinc deficiency produce symptoms like chlorosis, low membrane integrity and leaf size, retarded shoot growth, reduced grain yield, pollen formation, root development, water uptake and transport and increased vulnerability to heat, light and fungal infections (Tavallali et al., 2010; Kamran et al., 2017). In wheat, Zn deficiency causes stunted growth and yellowing of leaves. Hence, it becomes utmost important to address zinc deficiency as a top priority concern among other micronutrients (Hussain et al., 2018; Kumar et al., 2019).
Although the zinc requirement of a plant can be met by the chemical fertilizers (Reyes and Brinkman, 1989), but these agrochemicals pose a threat to the environment. The alternative technology for providing zinc to the plant is to inoculate the crop with the zinc-solubilizing microorganisms. A major portion of zinc available to plant is provided by the microbial activity (Sindhu et al., 2019a). Microbes produce organic acids, which cause decline in pH and these organic acids act on zinc complexes in soil, thus cause sequestering the zinc cation. Zinc solubilization may involve production of chelated ligands, siderophore (Saravanan et al., 2011) and redox system present on cell membrane (Wakatsuki, 1995; Chang et al., 2005). Prominent zinc-solubilizing microbes are Pseudomonas sp., Rhizobium spp., Bacillus aryabhattai, Thiobacillus thioxidans and Azospirillum sp. (Ijaz et al., 2019). Naseer et al. (2020) characterized bacterial isolates having the ability to solubilize indigenous zinc oxide along with multifarious plant growth promoting traits. Based on their sequencing, bacterial isolates were identified as Bacillus megaterium strain AN24, Bacillus aryabhattai strain AN30, Bacillus megaterium strain AN31 and Bacillus megaterium strain AN35.
Inoculation with zinc solubilizing bacteria Bacillus aryabhattai showed growth-promoting effect in maize (Mumtaz et al., 2017). Jha (2019) demonstrated that inoculation with zinc solubilizing Pseudomonas pseudoalcaligenes and Bacillus pumilus enhanced the plant height and dry weight of rice. Bacillus strains provided a strong base for developing biofertilizer. Zaheer et al. (2019) studied the inoculatiom effect of two phosphorus and Zn-solubilizing bacterial strains i.e. Bacillus sp. strain AZ17 and Pseudomonas sp. strain AZ5 on the growth of chickpea plant. Bacterial strains AZ5 and AZ17 increased the grain yield by 17.47% and 17.34% as compared to control, while strain AZ5 was better than the latter and enhanced the Zn uptake (26.12%), P uptake (26.12%), dry weight of nodules (22.53%), number of nodules (26.32%), straw weight (16.04%) and grain yield (17.47%). Bhatt, (2020) isolated zinc solubilizing bacteria from Capsicum annuum L. and isolate CDK25 was found to be the most potent owing to its maximum zinc solubilization ability. Isolate CDK25 was also endowed with multiple PGP attributes viz., phosphate solubilization, phytase production, indole acetic acid and siderophore production. This isolate was identifed as Bacillus megaterium based on 16S rRNA gene sequencing. Hussain et al. (2020) detected the effect of zinc solubilising Bacillus spp. AZ6 on maize and an overall increase in biomass, growth, chlorophyll content (90%) and yield was reported. Vaid et al. (2020) studied the effective Zn mobilization to rice grains using rhizobacterial consortium and the grain yield of rice during two years was increased by 19.7–27.9 and 17.1–20.4% over control.
Kang et al. (2021) demonstrated that Leclercia adecarboxylata MO1 produced siderophores that could solubilize Zn and silicate, and it showed a tolerance to Zn supplementation (2 and 5 mM) in growth medium. This bacterium also produced significant amounts of IAA. Bacterization of cucumber plants with L. adecarboxylata MO1 strain decreased hydrogen peroxide (H2O2) and Zn uptake in both roots and shoots, and also improved antioxidant systems (e.g. catalase (CAT), peroxidase (POD), polyphenol peroxidase (PPO), superoxide anion (SOA), lipid peroxidation (MDA), and glutathione (GSH)). Inoculation with L. adecarboxylata also reduced stress-responsive endogenous abscisic acid (ABA) and salicylic acid (SA) in plants grown under Zn toxicity of 2 and 5 mM as compared with non-inoculated plants under control conditions. In another study, Bashir et al. (2021) evaluated the effect of zinc-lysine chelate alone (0.1, 0.5, 1.0 and 1.5%) as seed priming and in combination with Zn-solubilizing bacteria (PMEL-1, PMEL-48, PMEL-57 and PMEL-71)) on grain biofortification of autumn maize. Results indicated that Zn accumulation was 18.5% higher in the seeds primed with 1.5% solution of Zn-lysine chelate and inoculation of ZSB strains compared to control treatments. Seed priming with 1.5% Zn-lysine chelate in combination with ZSB inoculation significantly improved cob diameter and cob length by 16.75% and 42% during 2016 and by 11.36% and 34.35% during 2017. The Zn contents were increased by 15.3%, 15.6%, 49.1%, and 33.0% in grain, cob-pith, stem and roots, respectively compared from control. Thus, the combined application of 1.5% Zn-lysine chelates along with ZSB inoculation could be used for combating malnutrition.
Batool et al. (2021) isolated 50 bacterial strains from chickpea rhizosphere and were screened for in vitro Zn solubilizing efficiency by culturing on tris-minimal agar medium supplemented with insoluble Zn compounds (ZnO and ZnCO3). Six potential zinc solubilizing bacteria (ZnSB) (ZnSB7, Paenibacillus polymyxa; ZnSB11, Ochrobactrum intermedium; ZnSB13, Bacillus cereus; ZnSB21, Streptomyces; ZnSB24, Stenotrophomonas maltophilia; and ZnSB25, Arthrobacter globiformi) were selected based on Zn solubilization efficiency. Seed inoculation of chickpea with ZnSB13 exhibited maximum phosphatase, dehydrogenase and microbial activities in plant rhizosphere in pot experiment, and caused a maximum availability of soil Zn. Inoculation with ZnSB13 strain maintained higher net photosynthetic rate, transpiration rate and stomatal conductance and water use efficiency that caused considerable increase in dry biomass, nodulation and yield of chickpea. Furthermore, inoculation of ZnSB13 exhibited maximum increase in grain N, grain P and Zn contents in root, shoot and grains of chickpea, suggesting improved Zn biofortification in chickpea.
4.5. Sulphur oxidizing microbes
Macronutrient sulphur is needed in high amount by plants as it is a constituent of macromolecules like amino acids (cysteine, cystine and methionine) and also involved in regulation of various enzymes like superoxide dismutase, ascorbate peroxidase, monodehydro-ascorbate reductase, dehydro-ascorbate reductase and glutathione reductase. Sulphur deficiency causes chlorosis and low lipid content along with lower plant growth and yield (Saha et al., 2018). Soil is composed of organic as well as inorganic sulphur and the process of conversion of organic sulphur into plant utilizable inorganic sulphur (i.e., SO42−) form is carried out by sulphur-oxidizing bacteria (SOB) including Xanthobacter, Alcaligenes, Bacillus, Pseudomonas, Thiobacillus sp., Thiobacillus thioparous and T. thioxidans (Kertesz and Mirleau, 2004; Riaz et al., 2020). Sulphur-oxidizing microorganisms also exhibited other plant growth-promoting activities.
Hoda and Gomaa (2005) reported that inoculation of SOB and specific root nodule bacteria (Okadin) in cowpea plants along with application of municipal refuse compost, cabronite and elemental sulphur considerably increased the dry weights and number of seeds of cowpea along with different minerals (S, K, N, P, Fe, Mn, Zn and Cu) of the used soil. In another experiment, the application of municipal refuse compost was found to maximize the role of sulphur and SOB. Chemoautotrophic (Thiobacillus ferrooxidans) and heterotrophic sulphur oxidizing bacteria (SOB; M2 and A12 strains) were inoculated along with pyrite as sulphur sources into canola seeds (Brassica campestris var toria) cv. Bhavani. SOBs were found to increase the grain and straw yields, and up to fourfold increase in nitrogen uptake was observed by bacterization in S-deficient alkaline soil (Anne et al., 2014). Besides nitrogen, uptake of other nutrients like Fe, Cu and Mn was also increased due to nutrient use efficiency of these microbes. Maximum S-content up to 8.34 mg/g of plant biomass was recorded in treatment having inoculation of A12 strain along with pyrite. Increased plant height, nitrogen uptake and yield were observed for maize when it was inoculated with Thiobacillus sp. (Pourbabaee et al., 2020). Similarly, inoculation of sulphur-oxidizing bacteria increased bulb weight and diameter in garlic (Pourbabaee et al., 2020). Thus, sulphur oxidizing microbes can be exploited for use as potential biofertilizers under alkaline soil conditions for onion, oats, ginger, grape, garlic and cauliflower (da Silva Júnior et al., 2018; Macik et al., 2020).
Boroujeni et al. (2021) isolated salt-tolerant sulfur-oxidizing bacteria of genus Halothiobacillus from saline and sulfidic habitats of Iran. Three species (eight strains) of Halothiobacillus genus were identified, which belonged to H. neapolitanus, H. hydrothermalis and H. halophilus. Salinity (0, 0.5, 1, 2 and 4 M NaCl) caused a significant impact on bacterial biomass and sulfate production during the oxidation of thiosulfate and elemental sulfur. The highest amount of biomass and sulfate was produced by H. neapolitanus strain I19 at 0.5 and 1 M NaCl concentration, suggesting that the application of these bacteria to increase sulfate storage of saline soils and for crop production. Amin and Mihoub (2021) examined the impacts of applying a mixture of bone-wood chips biochar and sulfur at different rates with sulfur-oxidizing bacteria (Thiobacillus spp.) in calcareous sandy soil. The results revealed that applying biochar amendment improved significantly labile P and P associated with calcium fractions compared with control. In a similar study, Gilani et al. (2021) conducted two-location field experiment to investigate the effect of Thiobacillus and different levels of sulfur fertilizer on growth and physiological indices in the replacement intercropping of sesame and mung bean. Sulfur fertilizer was used at three levels: control level (S0), 50% of recommended amount (S1), 100% of recommended amount (S2). Thiobacillus bacteria were used at two levels: T0 and T1. The results showed that growth indices chlorophyll a, chlorophyll b, total chlorophyll, biological yield, and grain yield of sesame and mung bean were significantly affected by the studied treatments. Also, sulfur fertilizer, 100% of the recommended amount, increased all studied indices in both plants except for chlorophyll a in sesame. Interaction effects of cropping ratio and location on growth indices, grain biological yield, chlorophyll a of sesame and mung bean were significant.
4.6. Plant growth promoting rhizobacteria
PGPR includes bacteria, which are free living in nature and obtained from the rhizosphere having the capability to produce and secrete metabolites, which promote plant growth after colonizing their roots (Beneduzi et al., 2012). Upon inoculation, PGPR help the plant to withstand drought stress (Timmusk et al., 2014; Niu et al., 2018; Ilyas et al., 2020), salinity (Mayak et al., 2004; Bharti et al., 2013) and biotic stress (de Vasconcellos and Cardoso, 2009; Verma et al., 2016). Inoculation of PGPRs has been reported to enhance seed germination, soil fertility and plant growth via the production of auxins, ethylene, gibberellins etc. (Jang et al., 2017; Tahir et al., 2017). Members from various genera like Agrobacterium, Arthrobacter, Alcaligenes, Azotobacter, Acinetobacter, Actinoplanes, Bacillus, Frankia, Pseudomonas, Rhizobium, Micrococcus, Streptomyces, Xanthomonas, Enterobacter, Cellulomonas, Serratia, Flavobacterium, Thiobacillus etc. are included in PGPR (Glick and Gamalaro, 2021; Kumar et al., 2021; Santoyo et al., 2021b).
Many PGPRs have been reported to promote plant growth and crop yield by increasing nutrient availability and uptake, producing plant hormones and suppressing of soil-borne diseases (Santoyo et al., 2021b). Valetti et al. (2018) observed that inoculation of rapeseed with six phosphorus solubilizing strains (including three strains of Bacillus and one each of Serratia, Arthrobacter and Pantoea) enhanced final crop yield by 21–44% even without P application under field conditions. Some of the PGPR strains possessed multiple beneficial activities and their inoculation may cause synergistic effect leading to enhanced crop production. For example, Bacillus strain M-3, Burkholderia strain OSU-7 and Pseudomonas strain BA-8 showed the capability of phosphorus solubilization along with production of IAA and cytokinin (Aslantas et al., 2007). The improved nutrient status and production of plant hormones caused by inoculation of these PGPR strains increased the fruit yield by 73.7, 88.2 and 137.5%, respectively in young apple trees. In another experiment, inoculation of P. fluorescens strain N21.4, having the capability to produce siderophores and chitinases, to blackberry roots resulted in enhanced plant growth and number of fruits alongwith increase in promotion of total phenolics, flavonols and epicatechins/catechins metabolites (Garcia-Seco et al., 2015). Coinoculation of PSB and PGPR strains i.e. Bacillus polymyxa, Rhizobium and Pseudomonas fluorescens showed significant enhancement in nutrient uptake over the single inoculation of PGPR (Rhizobium or Pseudomonas fluorescens), PSB (Bacillus polymyxa) or control uninoculated plants in wheat (Singh and Singh, 2012; Jaybhay et al., 2017).
Pellegrini et al. (2021) evaluated the effects of a four bacterial strain consortium comprising of Azospirillum brasilense, Gluconacetobacter diazotrophicus, Herbaspirillum seropedicae and Burkholderia ambifaria on growth of Allium cepa L. and on soil health. The results showed a positive influence of bacterial application on plant growth, with increased plant height (+18%), total chlorophylls (+42%), crop yields (+13%), and bulb dry matter (+3%) with respect to the control under field conditions. Differences were also observed between control and treatments in the bulb extracts in terms of total phenolic contents (+25%) and antioxidant activities (+20%). Bacterial consortium also caused an increase in total organic carbon, organic matter and available phosphorus, as well as higher concentrations of nutrients in soil than the control at harvest. Kumar et al. (2021) characterized most effective Bacillus pumilus strain JPVS11 out of 36 plant growth-promoting bacteria (ST-PGPB) isolated from sodic soil of eastern Uttar Pradesh, India. This strain showed production of IAA, ACC deaminase activity, P-solubilization, proline accumulation and exopolysaccharides (EPS) production at different concentrations of NaCl (0 –1200 mM). Pot experiment was conducted on rice (Oryza sativa L.) variety CSR46 at different NaCl concentrations (0, 50, 100, 200, and 300 mM) with and without inoculation of Bacillus pumilus strain JPVS11. Inoculation of Bacillus pumilus strain JPVS11 improved the growth performance of rice as compared to non-inoculated and a significant enhancement of plant height (12.90–26.48%), root length (9.55–23.09%), chlorophyll content (10.13–27.24%), carotenoids (8.38–25.44%), plant fresh weight (12.33–25.59%), and dry weight (8.66–30.89%) were recorded from 50 to 300 mM NaCl concentration in inoculated plants as compared to non-inoculated. Moreover, the plants inoculated with Bacillus pumilus strain JPVS11 showed improvement in antioxidant enzyme activities of catalase and superoxide dismutase. Besides, the significant improvement in soil enzyme activities, such as alkaline phosphatase, acid phosphatase, urease and β-glucosidase were recorded in inoculated pots as compared to non-inoculated plants.
Kubi et al. (2021) identified the salt-tolerant rhizobacterium Pseudomonas psychrotolerans CS51, which produced several biochemicals like indole-3-acetic acid (33 ± 1.8 ng/mL) and gibberellic acid (GA3; 38 ± 1.3 and GA4; 23 ± 1.2 ng/mL) in Luria-Bertani (LB) media. The sole inoculation of P. psychrotolerans isolate CS51, silicon (3 mM) and combined CS51 + Si significantly enhanced maize biomass and chlorophyll content under normal and salinity stress (200 mM). Phytohormonal results showed that salinity stress increased abscisic acid (ABA; three folds) and jasmonic acid (JA; 49.20%). However, the sole and combined isolate CS51 + Si application markedly reduced ABA (1.5 folds) and JA content (14.89%). Besides, the sole and isolate CS51 + Si co-application strengthened the antioxidant system, such as flavonoid (97%) and polyphenol (19.64%), and lowered the proline content (57.69%) under NaCl stress. Similarly, the CS51 and Si inoculation (solely or combined) significantly enhanced the Si uptake (4 folds) and reduced the Na+ uptake (42.30%) in maize plants under NaCl stress. The results suggested that combining of isolate CS51 with Si can be used against salinity stress in maize plants and may be commercialized as a biofertilizer. Thus, applications of diverse microbial inoculants may also enhance the agri-produce quality and promote the synthesis of functional secondary metabolites along with improvement in the productivity of agricultural crops.
5. Commercial biofertilizers: Preparation and application
For any product to produce desired effect on crop, it must be perfectly formulated and applied. With current scenario, efficient microbial inoculants have to be characterized, which could be used as inoculant for wide range of soil conditions (Etesami et al., 2021; Patel et al., 2021; Santoyo et al., 2021b). However, the bioinoculants available in global market are either of low quality or contain contamination of other undesired microbes (Herrmann and Lesueur, 2013; Yadav and Chandra, 2014). Thus, inconsistency is observed in performance of bioinoculants when applied under field conditions. Also, some of the inoculants are not able to produce the same stimulatory effect in the field, which can be due to inappropriate formulation (Vassilev et al., 2015; Biradar and Santhosh, 2018). Hence, inoculant formulation is a critical aspect and should be prepared in a way that allows high survivability of PGPR from storage period to application (Soumare et al., 2019; Amenaghawon et al., 2021). Bioinoculant formulation comprises inoculum preparation, addition of additives, opting for a good carrier, sterilization of carrier material, up scaling, quality control measures and proper packaging with the best delivery methods (Fig. 3). Formulation can be solid-based or liquid, the former further can be either dry or wet as per the requirement (Berninger et al., 2017; Oliveira et al., 2017; Berger et al., 2018).
Fig. 3.
Flow chart for selection of beneficial microbial strains and different steps involved in preparation of biofertilizer.
5.1. Liquid formulation
Combination or mixture of microbial cultures and different liquids like water, oil and polymers constitute the liquid inoculants and the latter is mixed with culture so to increase stability, adhesion and dispersion capacity (Lee et al., 2016). Liquid inoculants are dominating the market as they are easy to prepare and cheap over solid formulation (Lee et al., 2016; Dey, 2021). Once the growth of bacterial cultures is harvested/prepared, some protective agents like natural polymers (e.g., xanthan gum, carrageenan, arabic gum, alginate, gelatin, etc.), synthetic polymers [e.g., polyvinyl pyrrolidone (PVP) and polyvinyl alcohol], glycerol, horticultural oil, monosaccharides (glucose) or disaccharides (e.g., lactose) are added in the liquid medium to prolong the viability of microbial culture through storage (Lee et al., 2016; Valetti et al., 2016; Bernabeu et al., 2018). The added polymers have been reported to create a microenvironment that is having high water activity, which limits heat transfer, thus maintaining conditions necessary for bacterial survival (Mugnier and Jung, 1985). However, microbes are vulnerable to abiotic stresses mainly due to nutrient limitation and thermal shock, which lowers the population of viable cells (He et al., 2015; Berger et al., 2018; Bernabeu et al., 2018). The minimum number of viable cell in a formulation must be 107 cfu/mL during cold storage and application (He et al., 2015; Valetti et al., 2016).
Riddech et al. (2021) used PGPR strains Bacillus licheniformis BDS31 and Pseudomonas azotoformans C2–114 for the production of biofertilizer in the form of granules and powder. The bacterial suspension of 1010 cfu/ml was immobilized on different substrates including the binder polyvinylpyrolidone, starch and sodium alginate. The survival of bacterial cells was found highest in the granule biofertilizer supplemented with mannitol (Granule + M3: 59% mannitol, 10% PVP, 6% sodium alginate, 10% starch, 5% bacterial cells and 10% water). The seedling growth of Dipterocarpus alatus was enhanced by application of biofertilizer and it also promoted plant height and the number of leaves at 30 days after cultivation of plant seedlings. Such efficiency was comparable to that obtained from chemical and commercial biofertilizer.
During storage, protective agents regulate or maintain nutrients, physical characteristics and impart osmo-protection (Lee et al., 2016; Berninger et al., 2017). Some of the oils from horticulture crops are safe and a cheap option of additional nutrition for bacteria like Rhodopseudomonas palustris PS3 (Lee et al., 2016). Pero-dexin, which is an industrial waste by-product of coconut water and starch can cut down the cost of formulation along with the additional advantage of nutrient source for bacteria (Abbas et al., 2014; Anith et al., 2016). Some of the cell protectants such as glycerol shields the cells from tension and helps in balancing transmembrane traffic and osmotic pressure (Li et al., 2009). Further it has high water activity, thus prevent the cell from desiccation and due to this fluidity, the formulated inoculant will be easily applied to seeds (Singleton et al., 2002). Another cell protectent is lactose that possesses high water binding capacity and it prevents the formation of ice crystal during cold storage. Moreover, it stabilizes the cell membrane and maintains the protein integrity and functions (Leslie et al., 1995). Lactose contains eight hydroxyl groups, which protects the bacterial cell, during storage, from free radicals (Zárate et al., 2005). Another fascinating technique, which is proved to be efficient is the biofilm-based biofertilizers (Das et al., 2017). Currently, PGPRs are immobilised on a fungal matrix for biofilm formation. In biofilms, bacterial cell shows enhanced survival and PGPR activity as compared to free cell (Rabin et al., 2015).
For evaluating the role of organic fertilizer in improving nutrient acquisition and promotion of plant growth, Li et al. (2021) applied liquid organic fertilizer in sunflower cropland and investigated its response on rhizosphere microbial community structure and co-occurrence patterns. Compared with the untreated soils, organic fertilizer treatment increased soil nutrient concentrations by 13.8–137.1% while reducing soil pH and salinity by 5.6% and 54.7%, respectively. Organic fertilizer treatment also improved sunflower yield, plant number, and plant height by 28.6–67.3%. Following organic fertilizer treatment, the relative abundances of some halotolerant microbes and phytopathogenic fungi were reduced in organic fertilizer-treated soils, in contrast to increases in the relative abundances of plant growth-promoting microbes and organic matter decomposers, such as Nocardioides, Rhizophagus and Stachybotrys. More keystone taxa (e.g., Amycolatopsis, Variovorax and Gemmatimonas) were positively correlated with soil nutrient concentrations and crop yield-related traits in organic fertilizer-treated soils. Overall, liquid organic fertilizer amendment ameliorated the adverse effects of salinity-alkalinity stress on sunflower yield by improving soil quality and optimizing rhizosphere microbial community structure and co-occurrence patterns.
5.2. Solid formulation
Solid formulations contain the microbial culture preparations either in the form of granules or powder and contain organic or inorganic carrier molecules. Solid bioinoculant formulation can be bifurcated based upon particle size and applications (Lee et al., 2016). Solid formulation containing protectants, additives and carriers can be either wet or dry, but the composition more or less remains same. However, in wet formulation, there is no drying process due to which the water content remains high throughout storage and even during application as well. Further the wet formulation is alginate-based (Joe et al., 2014; Liffourrena and Lucchesi, 2018), peat (Oliveira et al., 2017; Zhou et al., 2017), clay (Schoebitz et al., 2014), biogas sludge mixed with enriched soil (Mukhtar et al., 2017) and biochar (Tripti et al., 2017). Carriers must be selected on the basis of cost, toxicity, chemical stability and suitable for management by farmers (Malusá et al., 2012; Bashan et al., 2014).
In solid wet formulation, PGPR immobilization prevents the effect of harsh conditions and is carried out either by biofilm formation or adhesion on a support or curbing over alginate beads (Rabin et al., 2015). Marcelino et al. (2016) entrapped Azospirillum brasilense Ab-V5 on biodegradable foam via biofilm formation and the foam was made from conventional compounds, which were further mixed with by products from industries like e.g., sugarcane bagasse, glycerol and cassava starch. Liffourrena and Lucchesi (2018) carried out entrapment of Pseudomonas putida A (ATCC 12,633) over alginate beads, which was supplemented with a high resistant inorganic perlite. It additionally provided the mechanical stability to the beads and enhanced survival of bacteria. Another recent technique includes nano-immobilization of microbial bioinoculants by electro-spinning (De Gregorio et al., 2017). Dry formulations are deprived of high-water content, which provides extended survival of bioinoculant (Melin et al., 2006).
Dry inoculants are dried by using freeze drying, air drying, dessication and spray drying. Spray drying and shade drying are low-cost methods while the methods like lyophilization as well as spray drying are equipment-based methods and are energy intensive due to which they are expensive (Ruíz-Valdiviezo et al., 2015; Berninger et al., 2017; Basheer et al., 2018). Freeze drying is a soft dehydration method having a cell protector mixed with the bacterial cells (Wessman et al., 2013; Berninger et al., 2016; Tamreihao et al., 2016).
Among powder inoculation, talc is the most basic natural carrier, which is used for long term storage due its reduced moisture absorption andrelative hydrophobicity, which averts hydrate bridge formation (Martínez-Álvarez et al., 2016). On the other hand, dry systems of PGPR immobilized by entrapment/microencapsulation in alginate or zeolite have been successfully developed (Campos et al., 2014; Berninger et al., 2017). Along with the carrier material, some of the adhesive or protective substances are added for enhanced survival (Schoebitz et al., 2012). These substances include gelatin (Berninger et al., 2017), carboxymethyl cellulose (Martínez-Álvarez et al., 2016; Basheer et al., 2018), arabic gum (Berninger et al., 2016), disaccharides such as lactose and sucrose (Cabrefiga et al., 2014; Molina-Romero et al., 2017), maltodextrin (Campos et al., 2014) and milk-derived compounds (Berninger et al., 2017). The efficacy of cell protectants totally depends upon the microbial species involved and the former concentration needs to be optimized according to species (Morgan et al., 2006). Sodium glutamate makes the cell membrane more fluid by stabilizing the headgroups and it influences the membrane conservation and also increases the bacterial resistance to drying (Martos et al., 2007). Further, amino groups of milk proteins react with the amino groups of carrier molecules, thus stabilizes the protein structure (Sharma et al., 2014). Like glycerol and maize bran residue, whey is also a cheap compound, which serves as additional nutrient as well as protectant (Vassilev et al., 2017; Zhang et al., 2018).
6. Mode of application of formulated biofertilizers
For any biofertilizer strain to become effective, it must possess the competitive ability for its survival, persistence and establishment under the provided environmental conditions (Sindhu and Dadarwal, 2000). Once the PGPR strain is established, its population starts rising and provides desired growth-promoting effects. The performance results of biofertilizer inoculation are slower in comparison to chemical fertilizers. But their effect is long lasting, which results in improvement of soil fertility. There are several methods for the application of biofertilizers i.e., roots dipping, soil application and seed inoculation with the application of either liquid or dry formulation (Mahanty et al., 2017). Though, precautions must be taken before the application of biofertilizer. For instance, one must avoid storing the prepared solution of microbes’ overnight and direct exposure to sunlight. Further, for storage of biofertilizer optimum range is between 0°C to 35°C.
For the application on seeds, roots or soil, the suspension of the dry bioinoculant is prepared by rehydrating dry bioinoculant (Malusá et al., 2012; Berninger et al., 2017). On the other hand, liquid formulations are used as such in their native form and require no rehydration. For seed application, carrier biofertilizer is diluted with water or jaggery forming slurry, to which sterilized seeds are added and mixed so to provide a uniform coating. After that the coated seeds are air dried before sowing in the field (Lawal and Babalola, 2014). Root dipping method is opted for transplanted crops and in this biofertilizer is mixed with water. Roots are dipped into the diluted biofertilizer suspension and kept for a while after that they are transplanted. Soil application is done when farmer is about to sow the seeds. Before sowing, biofertilizer is sprayed or spread on the soil (Lawal and Babalola, 2014).
7. Limitations in biofertilizer production
Several constraints are involved in commercial production of biofertilizers at a large scale, which include poor quality of soil, competition to inoculated strain, abiotic stress, contamination, lack of effective strain, skills, carrier material, awareness, storage, and even lack of regulations and safety standards (Bharti and Suryavanshi, 2021). The credibility of the biofertilizers depends on labelling the product with expiry date, name of microbes and action of microbes (Naveed et al., 2015; Timmusk et al., 2017). Manufacturers must select the robust exotic species, which must be further tested under different environment conditions like different crops and soil. Quality control should be compulsory at every level of biofertilizer production. The product must possess 2–3 months shelf life, which can be further extended by supplementing with additives and nutrients along with a carrier. Further, emphasis must be given for amendment with organic matter like vermicompost and farmyard manure (Mamnabi et al., 2020) so to cut the application of chemical fertilizer and to maintain soil quality and PGPR efficacy without affecting growth and yield of crop. Coinoculation or microbial consortium could provide better result of plant development and increased nutrition uptake as well (Zeffa et al., 2020; Santoyo et al., 2021) but there must be proper experimentation to study the synergistic effect of microbes on different crops. There must be set regulations for development, maintaince and promotion of biofertilizers. Moreover, subsidies and incentives must be given by the government to farmers to promote organic farming by use of PGPR as biofertilizers. The encouragement of farmers regarding use of biofertilizers may help in restoration of soil fertility with additional advantage of sustainable crop production.
8. Potential of biofertilizer in agricultural market
History of biofertilizers initiated around 120 years ago with the registration of Nitragin, which is Rhizobium inoculation for legume plant. For almost 100 years, rhizobial strains are available in the market as bioinoculants (O'Callaghan, 2016). According to Verma et al. (2019) biofertilizers constitute around 5% of total fertilizers available in the market. Among biofertilizers, approximately 150 products are microbial strains which are registered for farming (Table 3). Consumers are recently highly concerned about the food safety, environment and rising pesticide residues in food, which has forced them to prefer chemical-free products. Recently, organic retail sales have increased in the following countries: USA, Germany, India, China, Switzerland and Denmark. At present, the worth of biofertilizer market is USD 2.3 billion, which is likely to increaseup to USD 3.9 billion by 2025 recording a CAGR of 11.6% during the forecast period.
Table 3.
Different microbial biofertilizers available in market.
| Types of biofertilizer | Bacterial strain | Product name | Application for crops | References |
|---|---|---|---|---|
| Nitrogen-fixing biofertilizer | Azotobacter chroococcum | Bioazoto, Bhoomi Rakshak, Azonik | Wheat, sorghum, maize, mustard, cotton, vegetables, horticulture crops, flowers, orchids, plantation crops, ornamental and forest plants | Singh et al., 2014; National fertilizer limited, 2018 |
| Azotobacter chroococcum | Dimargon | Uribe et al., 2010 | ||
| Azotobacter chroococcum | Azotovit | Mishra and Arora, 2016 | ||
| Azotobacter vinelandii | Rhizosum N | Mehnaz, 2016 | ||
| Azospirillum lipoferum | Biospirillum, Green Plus | For normal, acidic and dry soil, paddy and other crops | National fertilizer limited, 2018 | |
| Azospirillum brasilense | Azo-S | Singh et al., 2014 | ||
| Azospirillum brasilense | Bio N | Mishra and Arora, 2016; Chakdar and Pabbi, 2020 | ||
| Azospirillum brasilense | Rhizosum Aqua | Klimek-kopyra et al., 2018 | ||
| Azospirillum brasilense | Azobacterin | Garcia-Fraila et al., 2017 | ||
| Gluconoacetobacterdiazotrophicus | Sugar-Plus | For sugarcane | International Panaacea Limited, 2018 | |
| Pulses (grams, peas, lentils, moong, urd, cowpea and arhar), fodder legumes (barseem and lucerne) and forest tree legumes (subabul, shisam and shinsh) | ||||
| Rhizobium sp. | Rizotorphin | Mikhailouskaya and Bogdevitch, 2009 | ||
| Bradyrhizobium japonicum | Bio Agro 10 | Uribe et al., 2010 | ||
| Rhizobium leguminosarum | Nodulator XL | Garcia-Fraile et al., 2017 | ||
| Phosphate-solubilizing/mobilizing biofertilizer | Pseudomonas striata, B. polymyxa and B. megaterium | P sol B | For all crops | Mehnaz, 2016 |
| Bacillus megaterium | BioPhos | Dash et al., 2017 | ||
| Bacillus mucilaginous and B. subtilis | CBF | Celador-Lera et al., 2018 | ||
| Potassium-solubilizing biofertilizer | Bacillus mucilaginous | Bio-NPK, BioPotash | For all crops | Singh et al. 2014 |
| Bacillus circulans | Kaliplant | Mikhailouskaya and Bogdevitch, 2009 | ||
| Frateuria aurantia | K sol B | Mehnaz, 2016 | ||
| Zinc-solubilizing biofertilizer | Thiobacillus thiooxidans | Zn sol B | Wheat, paddy, pulses, citrus, ginger etc. | Mehnaz, 2016 |
| Thiobacillus thiooxidans | Biozinc | National fertilizers limited, 2018 | ||
| Sulphur-oxidizing biofertilizer | Thiobacillus thiooxidans | S sol B | For cereals, oilseeds, fiber crops, plantation crops, medicinal crops, vegetables, flowers, orchards,forage crops, and ornamentals | Mehnaz, 2016 |
| Delftia acidovorans and Bradyrhizobium japonicum | BioBoost | Adesemoye et al., 2017 | ||
| Plant growth- promoting biofertilizer | Pseudomonas chlorapsis | Cedomon | For all crops | Mehnaz, 2016 |
| Azotobacter chroococcum and Pseudomonas fluorescens | Bio Gold | Mehnaz, 2016; Minaxi Saxena et al., 2013 |
Among biofertilizer market, the share of rhizobia meets about 79% of world demand, while PSB possess 15% share. There are numbers of manufacturing companies, which are ruling global market. Some of the companies of North America and Asia are T Stanes & Company Ltd. (India), Novozymes (Denmark), International Panaacea Limited (India), SOM Phytopharma Ltd. (India), Symborg (Spain), Madras Fertilizers Limited (India), Kan Biosys (India), Kiwa Biotech (China), Gujarat State Fertilizers and Chemicals Ltd. (India), Mapleton Agribiotech (Australia), National Fertilizers Limited (India), Lallemand Inc. (Canada), Rashtriya Chemicals & Fertilizers Ltd, (India) and Rizobacter Argentina S.A (Argentina). Biofertilizers can be easily developed by a small company for the use at small field. For instance, in America, Azospirillum strain is selected from the field after robust testing and after that suitable formulation are prepared, which is made available in the market. Currently, over 100 of Azospirillum strains are commercially accessible, which intended to boost crop yield mainly in wheat, maize, and soybean in South America (Cassán and Diaz-Zorita, 2016). In India and China, around one lakh hactares and 167 million hactares of area is under organic farming, respectively (Sekhar et al., 2016).
9. Conclusion
Biofertilizer inoculation is a promising strategy to improve crop yields and reduce the use of chemical fertilizers, thereby creating environment-friendly sustainable agriculture (Basu et al., 2021; Chakraborty and Akhtar, 2021; Mohanty et al, 2021). Various plant growth-promoting microbes have been characterized for beneficial traits, which play a significant role in improving the accessibility of nutrients such as N, P, K, Zn and S, in modulation of phytohormones, suppression of plant diseases and alleviation of abiotic stresses (Wasai and Minamisawa, 2018; Glick and Gamalaro, 2021). The inoculations of individual or consortium of beneficial microorganisms have been found to improve plant biomass and crop yield under greenhouse and field conditions (Santoyo et al., 2021b). However, several constraints have been found to limit crop growth under field conditions under diverse agricultural ecosystems in some cases and the inoculation of multifunctional PGPR strains does not improve plant growth, yield of crop and quality of agri-produce. Because the growth conditions of microbes are generally different under laboratory and greenhouse conditions, which affect the survival and functioning of inoculated microorganisms under field conditions (Sindhu and Dadarwal, 2000). Currently, the amendment of these beneficial biofertilizer strains is made with organic materials, cell protectants and nanoparticles to increase their survival and efficacy leading to improvement in crop production. Moreover, sequencing of a large number of microbial genomes and identification of specific genes provided a powerful tool to enhance the synthesis and release of PGP metabolites by the beneficial microbes (Bakker et al., 2012; Köberl et al., 2015). Further, technological developments such as the advent of next-generation sequencing, gene editing and bioengineering of microbial communities, in silico modeling of proteins and synthetic biology (Berg et al., 2014; Kaul et al. 2021; Ke et al., 2021) may allow the manipulation of plants and microbes to deliver short and long terms solutions for improving crop productivity to feed the world in a more sustainable manner.
Funding
Being a review article, no funding was involved in compilation of the information in this review chapter.
Authors' contributions
All the authors have substantial (equal) contribution in compilation of this review chapter. All authors have read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
Authors thank the faculty members of the Microbiology department, CCS Haryana Agricultural University, Hisar for their critical reading of the manuscript and suggestions for improving the quality of the manuscript.
References
- Aasfar A., Bargaz A., Yaakoubi K., Hilali A., Bennis I., Zeroual Y., Meftah Kadmiri I. Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.628379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas M.T., Hamza M.A., Youssef H.H., Youssef G.H., Fayez M., Monib M., Hegazi N.A. Bio-preparate support the productivity of potato plants grown under desert farming conditions of north Sinai: five years of field trials. J. Adv. Res. 2014;5:41–48. doi: 10.1016/j.jare.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd_Allah E.F., Alqarawi A.A., Hashem A., Radhakrishnan R., Al-Huqail A.A., Al-Otibi F.O.N. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J. Plant Interact. 2018;13(1):37–44. [Google Scholar]
- Abdallah R.A.B., Trabelsi B.M., Nefzi A., Khiareddine H.J., Remadi M.D. Isolation of endophytic bacteria from Withania somnifera and assessment of their ability to suppress Fusarium wilt disease in tomato and to promote plant growth. J. Plant Pathol. Microbiol. 2016;7:352. [Google Scholar]
- Adesemoye A., Kloepper J. Plant–microbes interactions in enhanced fertilizer-use efficiency. Appl. Microbiol. Biotechnol. 2009;85:1–12. doi: 10.1007/s00253-009-2196-0. [DOI] [PubMed] [Google Scholar]
- Adesemoye A.O., Torbert H.A., Kloepper J.W. Plant growth promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb. Ecol. 2009;58:921–929. doi: 10.1007/s00248-009-9531-y. [DOI] [PubMed] [Google Scholar]
- Adesemoye, A.O., Yuen, G., Watts, D.B., 2017. Microbial inoculants for optimized plant nutrient use in integrated pest and input management systems. In: Kumar, V. (Ed.), Probiotics and Plant Health. Springer Nature Singapore Pte Ltd, pp. 21–40.
- Alemneh A.A., Cawthray G.R., Zhou Y., Ryder M.H., Denton M.D. Ability to produce indole acetic acid is associated with improved phosphate solubilizing activity of rhizobacteria. Arch. Microbiol. 2021;203:3825–3837. doi: 10.1007/s00203-021-02364-w. [DOI] [PubMed] [Google Scholar]
- Alen'kina S.A., Nikitina V.E. Stimulating effect from lectins of associative bacteria of the genus Azospirillum on the germination and morphometric characteristics of spring wheat sprouts in simulated abiotic stress. Russ J Plant Physiol. 2021;68:315–321. doi: 10.1134/S1021443721010027. [DOI] [Google Scholar]
- Ali A.A., Awad M.Y.M., Hegab S.A., Abd El Gawad A.M., Eissa M.A. Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J. Plant. Nutr. 2021;44(3):411–420. doi: 10.1080/01904167.2020.1822399. [DOI] [Google Scholar]
- Ali S., Riaz A.K., Ghazal M., Arif M., Fida M., Saiqa B. Assessment of different crop nutrient management practices for yield improvement. Aust. J. Crop Sci. 2008;2:150–157. [Google Scholar]
- Alkurtany A., Ali S., Mahdi W. The efficiency of prepared biofertilizer from local isolate of Bradyrhizobium sp. on growth and yield of mungbean plant. Iraqi J. Agric. Sci. 2018:49. [Google Scholar]
- Amenaghawon A.N., Anyalewechi C.L., Kusuma H.S. Fabrication approaches for biofertilizers. Biofertilizers. 2021:491–515. doi: 10.1002/9781119724995.ch16. [DOI] [Google Scholar]
- Amin A.E.A.Z., Mihoub A. Effect of sulfur-enriched biochar in combination with sulfur-oxidizing bacterium (Thiobacillus spp.) on release and distribution of phosphorus in high calcareous P-fixing soils. J. Soil Sci. Plant Nutr. 2021;21:2041–2047. doi: 10.1007/s42729-021-00500-5. [DOI] [Google Scholar]
- Anand, A., Chinchilla, D., Tan, C., Mene-Sarane, L., L'Haridon, F., Weisskopf, L., 2020. Contribution of hydrogen cyanide to the antagonistic activity of Pseudomonas strains against Phytophthora infestans. Microorganisms 8, 1144. doi:10.3390/microorganisms8081144. [DOI] [PMC free article] [PubMed]
- Anith K.N., Vaishakhi A.S., Viswanathan A., Varkey S., Aswini S. Population dynamics and efficiency of coconut water based liquid formulation of Pseudomonas fluorescens AMB-8. J. Trop. Agric. 2016;54:184–189. [Google Scholar]
- Anne R., Joseph Suresh, K Kavimandan, Kolluru V.B.R., Tilak Lata Nain. Response of canola and wheat to amendment of pyrite and sulphur-oxidizing bacteria in soil. Arch. Agron. Soil Sci. 2014;60(3):367–375. doi: 10.1080/03650340.2013.799275. [DOI] [Google Scholar]
- Ansari M.H., Hashemabadi D., Kaviani B. Effect of cattle manure and sulphur on yield and oil composition of pumpkin (Cucurbita pepo var. Styriaca) inoculated with Thiobacillus thiooxidans in calcareous soil. Comm. Soil Sci. Plant Anal. 2017;48:2103–2118. [Google Scholar]
- Arora N.K., Tewari S., Singh R. Plant-microbe symbiosis: fundamentals and advances. Springer; New Delhi: 2013. Multifaceted plant-associated microbes and their mechanisms diminish the concept of direct and indirect PGPRs; pp. 411–449. [Google Scholar]
- Ashfaq M., Hassan H.M., Ghazali A.H., Ahmad M. Halotolerant potassium solubilizing plant growth promoting rhizobacteria may improve potassium availability under saline conditions. Environ. Monit. Assess. 2020;192:697. doi: 10.1007/s10661-020-08655-x. [DOI] [PubMed] [Google Scholar]
- Aslantas R., Cakmakc R., Sahin F. Effect of plant growth promoting rhizobacteria on young apple tree growth and fruit yield under orchard conditions. Sci. Hortic. 2007;111(4):371–377. [Google Scholar]
- Ávila J.S., Ferreira J.S., Santos J.S., Rocha P.A.D., Baldani V.L. Green manure, seed inoculation with Herbaspirillum seropedicae and nitrogen fertilization on maize yield. Rev. Bras. Eng. Agrícola Ambient. 2020;24:590–595. [Google Scholar]
- Babu A.N., Jogaiah S., Ito S.I., Nagaraj A.K., Tran L-S.P. Improvement of growth, fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase. Plant Sci. 2015;231:62–73. doi: 10.1016/j.plantsci.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Bacilio M., Roderiguez H., Moreno M. Mitigation of salt stress in wheat seedlings by gfp-tagged Azospirillum lipoferum. Biol. Fertil. Soils. 2004;40:188–193. [Google Scholar]
- Bakhshandeh E., Gholamhosseini M., Yaghoubian Y., Pirdashti H. Plant growth promoting microorganisms can improve germination, seedling growth and potassium uptake of soybean under drought and salt stress. Plant Growth Regul. 2020;90(1):123–136. [Google Scholar]
- Bakker M., Manter D., Sheflin A., Weir T., Vivanco J. Harnessing the rhizosphere microbiome through plant breeding and agricultural management. Plant Soil. 2012;360:1–13. [Google Scholar]
- Bakker P.A.H.M., Berendsen R.L., Doombos R.F., Wintermans P.C.A., Pieterse C.M.J. The rhizosphere revisited: root microbiomics. Front. Plant Sci. 2013;4:165. doi: 10.3389/fpls.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamagoos A.A., Alharby H.F., Belal E.E., Khalaf A.E.A., Abdelfattah M.A., Rady M.M., Ali E.F., Mersal G.A.M. Phosphate-solubilizing bacteria as a panacea to alleviate stress effects of high soil CaCO3 content in Phaseolus vulgaris with special reference to P-releasing enzymes. Sustainability. 2021;13(13):7063. doi: 10.3390/su13137063. [DOI] [Google Scholar]
- Banik A., Dangar T.K. Application of rice (Oryza sativa L.) root endophytic diazotrophic Azotobacter sp. strain AviZ (MCC 3432) can increase rice yield under green house and field condition. Microbiol. Res. 2019;219:56–65. doi: 10.1016/j.micres.2018.11.004. [DOI] [PubMed] [Google Scholar]
- Barazani O., Friedman J. Allelopathic bacteria and their impact on higher plants. Crit. Rev. Microbiol. 2001;27:41–55. doi: 10.1080/20014091096693. [DOI] [PubMed] [Google Scholar]
- Basak B.B., Biswas D.R. Influence of potassium solubilizing microorganism (Bacillus mucilogenous) and waste mica on potassium uptake dynamics by Sudan grass (Sorghum vulgare Pers) grown under two alfisols. Plant Soil. 2008;317:235–255. [Google Scholar]
- Basak B.B., Maity A., Ray P., Biswas D.R., Roy S. Potassium supply in agriculture through biological potassium fertilizer: A promising and sustainable option for developing countries. Arch. Agron. Soil Sci. 2020 doi: 10.1080/03650340.2020.1821191. [DOI] [Google Scholar]
- Bashan Y., de- Bashan L.E., Prabhu S.R., Hernandez J.P. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013) Plant Soil. 2014;378:1–33. [Google Scholar]
- Bashan Y., de-Bashan L.E. Chapter two-how the plant growth-promoting bacterium Azospirillum promotes plant growth-a critical assessment. Adv. Agron. 2010;108:77–136. [Google Scholar]
- Basheer J., Ravi A., Mathew J., Krishnankutty R.E. Assessment of plant-probiotic performance of novel endophytic Bacillus sp. in talc-based formulation. Probiotics Antimicrob. Proteins. 2018;11:256–263. doi: 10.1007/s12602-018-9386-y. [DOI] [PubMed] [Google Scholar]
- Bashir S., Basit A., Abbas R.N., Naeem S., Naeem S., Bashir S., Ahmed N., Ahmed M.S., Ilyas M.Z., Aslam Z., Alotaibi S.S., El-Shehawi A.M., Li Y. Combined application of zinc-lysine chelate and zinc-solubilizing bacteria improves yield and grain biofortification of maize (Zea mays L.) PLOS One. 2021;16(7) doi: 10.1371/journal.pone.0254647. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Basu A., Prasad P., Das S.N., Kalam S., Sayyed R.Z., Reddy M.S., El Enshasy H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability. 2021;13:1140. doi: 10.3390/su13031140. [DOI] [Google Scholar]
- Batista B.D., Dourado M.N., Figueredo E.F., Hortencio R.O., Marques J.P.R., Piotto F.A., Bonatelli M.L., Settles M.L., Azevedo J.L., Quecine M.C. The auxin-producing Bacillus thuringiensis RZ2MS9 promotes the growth and modifies the root architecture of tomato (Solanum lycopersicum cv. Micro-Tom) Arch. Microbiol. 2021;203:3869–3882. doi: 10.1007/s00203-021-02361-z. [DOI] [PubMed] [Google Scholar]
- Batool S., Asghar H.N., Shehzad M.A., Yasin S., Sohaib M., Nawaz F., Akhtar G., Mubeen K., Zahir Z.A., Uzair M. Zinc-solubilizing bacteria-mediated enzymatic and physiological regulations confer zinc biofortification in chickpea (Cicer arietinum L.) J. Soil Sci. Plant Nutr. 2021;21:2456–2471. doi: 10.1007/s42729-021-00537-6. [DOI] [Google Scholar]
- Begum N., Qin C., Ahanger M.A., Raza S., Khan M.I., Ahmed N., Ashraf M., Zhang L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019;10:1068. doi: 10.3389/fpls.2019.01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneduzi A., Ambrosini A., Passaglia L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012;35:1044–1051. doi: 10.1590/s1415-47572012000600020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg G., Grube M., Schloter M., Smalla K. Unraveling the plant microbiome: looking back and future perspectives. Front. Microbiol. 2014;5:148. doi: 10.3389/fmicb.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg G., Rybakova D., Grube M. The plant microbiome explored: implications for experimental botany. J. Exp. Bot. 2016;67:995–1002. doi: 10.1093/jxb/erv466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B., Patz S., Ruppel S., Dietel K., Faetke S., Junge H., Becker M. Successful formulation and application of plant-growth promoting Kosakoniar adicincitans in maize cultivation. Biomed. Res. Int. 2018 doi: 10.1155/2018/6439481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beris D., Theologidis I., Skandalis N. Bacillus amyloliquefaciens strain MBI600 induces salicylic acid dependent resistance in tomato plants against tomato spotted wilt virus and potato virus Y. Sci. Rep. 2018;8:10320. doi: 10.1038/s41598-018-28677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabeu P.R., García S.S., López A.C., Vio S.A., Carrasco N., Boiardi J.L., Luna M.F. Assessment of bacterial inoculant formulated with Paraburkholderia tropica to enhance wheat productivity. World J. Microbiol. Biotechnol. 2018;34:81. doi: 10.1007/s11274-018-2461-4. [DOI] [PubMed] [Google Scholar]
- Berninger T., Mitter B., Preininger C. The smaller, the better? The size effect of alginate beads carrying plant growth-promoting bacteria for seed coating. J. Microencapsul. 2016;33:127–136. doi: 10.3109/02652048.2015.1134690. [DOI] [PubMed] [Google Scholar]
- Berninger T., Mitter B., Preininger C. Zeolite-based, dry formulations for conservation and practical application of Paraburkholderia phytofirmans PsJN. J. Appl. Microbiol. 2017;122:974–986. doi: 10.1111/jam.13360. [DOI] [PubMed] [Google Scholar]
- Bertola M., Mattarozzi M., Sanangelantoni A.M., Careri M., Visioli G. PGPB colonizing three-year biochar-amended soil: towards biochar-mediated biofertilization. J. Soil Sci. Plant Nutr. 2019;19:841–850. [Google Scholar]
- Bhale U., Bansode S., Singh S. fungi and their role in sustainable development: Current Perspectives. Springer; Berlin/Heidelberg, Germany: 2018. Multifactorial role of arbuscular mycorrhizae in agroecosystem; pp. 205–220. [Google Scholar]
- Bharti, N. and Suryavanshi, M., 2021. Quality control and regulations of biofertilizers: Current scenario and future prospects. In: Rakshit, A., Meena, V.S., Parihar, M., Singh, H.B., Singh, A.K. (Eds.), Biofertilizers, Woodhead Publishing, pp. 133–141. 10.1016/B978-0-12-821667-5.00018-X.
- Bharti N., Pandey S.S., Barnawal D., Patel V.K., Kalra A. Plant-growth promoting rhizobacteria Dietzianatrono limnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016;6:34768. doi: 10.1038/srep34768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti N., Yadav D., Barnawal D., Maji D., Kalra A. Exiguobacterium oxidotolerans, a halotolerant plant-growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L.) Pennell under primary and secondary salt stress. J. World Microbiol. Biotechnol. 2013;29:379–387. doi: 10.1007/s11274-012-1192-1. [DOI] [PubMed] [Google Scholar]
- Bharucha U., Kamlesh P., Ujjval B., Trivedi Optimization of indole acetic acid production by Pseudomonas putida UB1 and its effect as plant growth-promoting rhizobacteria on mustard (Brassica nigra) Agric. Res. 2013;2(3):215–221. [Google Scholar]
- Bhat R.A., Dervash M.A., Mehmood M.A., Skinder B.M., Rashid A., Bhat J.I.A., Singh D.V., Lone R. Mycorrhiza-Nutrient Uptake, Biocontrol, Ecorestoration. Springer; Berlin/Heidelberg, Germany: 2017. Mycorrhizae: A sustainable industry for plant and soil environment; pp. 473–502. [Google Scholar]
- Bhatt K., Maheshwari D.K. Zinc solubilizing bacteria (Bacillus megaterium) with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. 3 Biotech. 2020;10:36. doi: 10.1007/s13205-019-2033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneshwari K., Singh P.K. Response of nitrogen-fixing water fern Azolla biofertilization to rice crop. 3 Biotech. 2015;5:523–529. doi: 10.1007/s13205-014-0251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biradar B.P., Santhosh G.P. Cell protectants, adjuvants, surfactant and preservative and their role in increasing the shelf life of liquid inoculant formulations of Pseudomonas fluorescens. Int. J. Pure Appl. Biosci. 2018;6:116–122. [Google Scholar]
- Boddey R.M., de Oliveira O.C., Urquiaga S., Reis V.M., Olivares F.L., Baldani V.L.D., Dobereiner J. Biological nitrogen fixation associated with sugar cane and rice: contributions and prospects for improvement. Plant Soil. 1995;174:195–209. [Google Scholar]
- Boroujeni S.M., Kalbasi M., Asgharzadeh A., Baharlouei J. Evaluating the potential of Halothiobacillus bacteria for sulfur oxidation and biomass production under saline soil. Geomicrobiol. J. 2021;38:57–65. doi: 10.1080/01490451.2020.1809571. [DOI] [Google Scholar]
- Bourion V., Heulin-Gotty K., Aubert V., Tisseyre P., Chabert-Martinello M., Pervent M., Delaitre C., Vile D., Siol M., Duc G., Brunel B. Coinoculation of pea core-collection with diverse rhizobial strains shows competitiveness for nodulation and efficiency of nitrogen fixation are distinct traits in interaction. Front. Plant Sci. 2017;8:2249–2263. doi: 10.3389/fpls.2017.02249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmaprakash G., Sahu P.K. Biofertilizers for sustainability. J. Indian Inst. Sci. 2012;92:37–62. [Google Scholar]
- Brewin, N.J., Rae, A.L., Perotto, S., Knox, P., Roberts, K., Le Gal, M.F., Sindhu, S.S., Wood, E.A. and Kannenberg, E.L., 1990. Immunological dissection of the plant-microbe interface in pea nodules. In: Gresshoff P.M., Roth J., Stacey G. and Newton W.E. (Eds.), Nitrogen Fixation: Achievements and Objectives. Chapman and Hall, New York, London. pp. 227–234.
- Bulgarelli D., Schlaeppi K., Spaepen S., van Themaat E.V.L., Schulze-Lefert P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013;64:807–838. doi: 10.1146/annurev-arplant-050312-120106. [DOI] [PubMed] [Google Scholar]
- Burns R.G., de Forest J.L., Marxsen J., Sinsabaugh R.L., Stromberger M.E., Wallenstein M.D., Weintraub M.N., Zoppini A. Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol. Biochem. 2013;58:216–234. [Google Scholar]
- Busby P.E. Research priorities for harnessing plant microbiomes in sustainable agriculture. Plos Biol. 2017;15 doi: 10.1371/journal.pbio.2001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrefiga J., Francés J., Montesinos E., Bonaterra A. Improvement of a dry formulation of Pseudomonas fluorescens EPS62e for fire blight disease biocontrol by combination of culture osmoadaptation with a freeze-drying lyoprotectant. J. Appl. Microbiol. 2014;117:1122–1131. doi: 10.1111/jam.12582. [DOI] [PubMed] [Google Scholar]
- Campos D.C., Acevedo F., Morales E., Aravena J., Amiard V., Jorquera M.A., Inostroza N.G., Rubilar M. Microencapsulation by spray drying of nitrogen fixing bacteria associated with lupin nodules. World J. Microbiol. Biotechnol. 2014;30:2371–2378. doi: 10.1007/s11274-014-1662-8. [DOI] [PubMed] [Google Scholar]
- Cassán F., Diaz-Zorita M. Azospirillum sp. in current agriculture: From the laboratory to the field. Soil Biol. Biochem. 2016;103:117–130. [Google Scholar]
- Cassán F., Vanderleyden J., Spaepen S. Physiological and agronomical aspects of phytohormone production by model plant-growth-promoting rhizobacteria (PGPR) belonging to the genus Azospirillum. J. Plant Growth Regul. 2014;33:440–459. [Google Scholar]
- Celador-Lera, L., Jimenez-Go´mez, A., Menendez, E., Rivas, R., 2018. Biofertilizers based on bacterial endophytes isolated from cereals: potential solution to enhance these crops. In: Meena, V.S. (Ed.), Role of Rhizospheric Microbes in Soil Volume 1 Stress Management and Agricultural Sustainability. Springer Nature, Singapore Pte Ltd, pp. 175–203. 10.1007/978-981-10-8402-7.
- Cely M.V., Siviero M.A., Emiliano J., Spago F.R., Freitas V.F., Barazetti A.R., Goya E.T., Lamberti G.D.S., dos Santos I.M., De Oliveira A.G., Andrade G. Inoculation of Schizolobium parahyba with mycorrhizal fungi and plant growth-promoting rhizobacteria increases wood yield under field conditions. Front. Plant Sci. 2016;7:1708. doi: 10.3389/fpls.2016.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakdar, H., Pabbi, S., 2020. Microbial bioinoculants for sustainable agriculture: trends, constraints and future perspectives. In: Chandra, R., Sobti, R.C. (Eds.), Microbes for Sustainable Development and Bioremediation. CRC Press, pp. 343–358.
- Chakraborty T., Akhtar N. Biofertilizers: Prospects and challenges for future. Biofertilizers. 2021:575–590. doi: 10.1002/9781119724995.ch20. [DOI] [Google Scholar]
- Chang H.B., Lin C.W., Huang H.J. Zinc induced cell death in rice (Oryza sativa L.) roots. Plant Growth Regul. 2005;46:261–266. [Google Scholar]
- Chaparro J.M., Sheflin A.M., Manter D.K., Vivanco J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils. 2012;48(5):489–499. [Google Scholar]
- Chaudhary D., Narula N., Sindhu S.S., Behl R.K. Plant growth stimulation of wheat (Triticum aestivum L.) by inoculation of salinity tolerant Azotobacter strains. Physiol. Mol. Biol. Plants. 2013;19:515–519. doi: 10.1007/s12298-013-0178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary S.R., Sindhu S.S. Growth stimulation of clusterbean (Cyamopsis tetragonoloba) by coinoculation with rhizosphere bacteria and Rhizobium. Legume Res. 2016;39(6):1003–1012. [Google Scholar]
- Chen Y-H., Yang X-Z., Li Z., An X-H., Ma R-P., Li Y-Q., Cheng C-G. Efficiency of potassium-solubilizing Paenibacillus mucilaginosus for the growth of apple seedling. J. Integr. Agric. 2020;19(10):2458–2469. [Google Scholar]
- Choudhary D.K., Prakash A., Johri B.N. Induced systemic resistance (ISR) in plants: mechanism of action. Indian J. Microbiol. 2007;47(4):289–297. doi: 10.1007/s12088-007-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B.C., Garcia-Herrero A., Johanson T.H., Krewulak K.D., Lau C.K., Peacock R.S. Siderophore uptake in bacteria and the battle for iron with the host; a bird's eye view. Biometals. 2010;23(4):601–611. doi: 10.1007/s10534-010-9361-x. [DOI] [PubMed] [Google Scholar]
- da Silva Júnior S., Stamford N.P., Oliveira W.S., Silva E.V.N., de Rosalia E., Silva Santos C.E., de Freitas A.D.S., da Silva V.S.G. Microbial biofertilizer increases nutrient uptake on grape (Vitis labrusca L.) grown in an alkaline soil reclaimed by sulphur and Acidithiobacillus. Aust. J. Crop Sci. 2018;12:1695. [Google Scholar]
- Dalpe Y., Monreal M. Arbuscular mycorrhiza inoculum to support sustainable cropping systems. Crop Manag. 2004;3 [Google Scholar]
- Das K., Rajawat M.V.S., Saxena A.K., Prasanna R. Development of Mesorhizobium ciceri-based biofilms and analyses of their antifungal and plant growth promoting activity in chickpea challenged by Fusarium wilt. Indian J. Microbiol. 2017;57:48–59. doi: 10.1007/s12088-016-0610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash, N., Pahari, A., Dangar, T.K., 2017. Functionalities of phosphate- solubilizing bacteria of rice rhizosphere: techniques and perspectives. In: Shukla, P. (Ed.), Recent Advances in Applied Microbiology. Springer Nature, Singapore, Pte Ltd, pp. 151–163.
- De Gregorio P.R., Michavila G., Ricciardi Muller L., de Souza Borges C., Pomares M.F., Saccol de Sá E.L., Pereira C., Vincent P.A. Beneficial rhizobacteria immobilized in nanofibers for potential application as soybean seed bioinoculants. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vasconcellos R.L.F., Cardoso E.J.B.N. Rhizospheric Streptomycetes as potential biocontrol agents of Fusarium and Armillaria pine rot and as PGPR for Pinus taeda. J. Biocontr. 2009;54:807–816. [Google Scholar]
- de Vries F.T., Griffiths R.I., Knight C.G., Nicolitch O., Williams A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science. 2020;368:270. doi: 10.1126/science.aaz5192. [DOI] [PubMed] [Google Scholar]
- Demanèche S., Sanguin H., Poté J., Navarro E., Bernillon D., Mavingui P. Antibiotic resistant soil bacteria in transgenic plant fields. Proc. Natl. Acad. Sci. U.S.A. 2008;105(10):3957–3962. doi: 10.1073/pnas.0800072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A. Liquid biofertilizers and their applications: An overview. Environmental and Agricultural Microbiology. 2021:275–292. doi: 10.1002/9781119525899.ch13. [DOI] [Google Scholar]
- Ding Z., Ali E.F., Almaroai Y.A., Eissa M.A., Abeed A.H.A. Effect of potassium solubilizing bacteria and humic acid on faba bean (Vicia faba L.) plants grown on sandy loam soils. J. Soil Sci. Plant Nutr. 2021;21:791–800. doi: 10.1007/s42729-020-00401-z. [DOI] [Google Scholar]
- Ditta A., Imtiaz M., Mehmood S., Rizwan M.S., Mubeen F., Aziz O., Qian Z., Ijaz R., Tu S. Rock phosphate-enriched organic fertilizer with phosphate-solubilizing microorganisms improves nodulation, growth, and yield of legumes. Commun. Soil Sci. Plant Anal. 2018;49(21):2715–2725. [Google Scholar]
- Dobbelaere S., Vanderleyden J., Okon Y. Plant growth-promoting effects of diazotrophs in the rhizosphere. Crit. Rev. Plant Sci. 2003;22:107–149. [Google Scholar]
- Doornbos R.F., van Loon L.C., Bakker P.A. Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agron. Sustain. Dev. 2012;32(1):227–243. [Google Scholar]
- Dubey R.K., Tripathi V., Dubey P.K., Singh H.B., Abhilash P.C. Exploring rhizospheric interactions for agricultural sustainability: the need for integrative research on multi-trophic interactions. J. Clean Prod. 2016;115:362–365. [Google Scholar]
- Duca D., Lorv J., Patten C.L., Rose D., Glick B.R. Indole-3-acetic acid in plant-microbe interactions. Antonie Van Leeuwenh. 2014;106:85–125. doi: 10.1007/s10482-013-0095-y. [DOI] [PubMed] [Google Scholar]
- Duffy B. Pathogen self-defense: Mechanisms to counteract microbial antagonism. Annu. Rev. Phytopathol. 2003;41:501–538. doi: 10.1146/annurev.phyto.41.052002.095606. [DOI] [PubMed] [Google Scholar]
- Dumbrel A.J., Nelson M., Helgason T., Dytham C., Fitter A.H. Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J. 2010;4:337–345. doi: 10.1038/ismej.2009.122. [DOI] [PubMed] [Google Scholar]
- Eady R.R. Structure–function relationships of alternative nitrogenases. Chem. Rev. 1996;96:3013–3030. doi: 10.1021/cr950057h. [DOI] [PubMed] [Google Scholar]
- Earl C., Ronson C., Ausubel F. Genetic and structural analysis of the Rhizobium meliloti fixA, fixB, fixC, and fixX genes. J. Bacteriol. 1987;169:1127–1136. doi: 10.1128/jb.169.3.1127-1136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgren T., Nordlund S. The fixABCX genes in Rhodospirillum rubrum encode a putative membrane complex participating in electron transfer to nitrogenase. J. Bacteriol. 2004;186:2052–2060. doi: 10.1128/JB.186.7.2052-2060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egamberdieva D., Wirth S.J., Alqarawi A.A., Abd-Allah E.F., Hashem A. Phytohormones and beneficial microbes: essential components for plants to balance stress and fitness. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einsle O., Akif-Tezcan F., Susana L.A., Andrade Nitrogenase MoFe-protein at 1.16 Å resolution: a central ligand in the FeMo-cofactor. Science. 2002;297:1696–1700. doi: 10.1126/science.1073877. [DOI] [PubMed] [Google Scholar]
- El-Sayed W.S., Akhkha A., El-Nagga M., Elbadry M. In vitro antagonistic activity, plant growth promoting traits and phylogenetic affiliation of rhizobacteria associated with wild plants grown in arid soil. Front. Microbiol. 2014;5:651. doi: 10.3389/fmicb.2014.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Bonilla G.A., Durrer A., Cardoso E.J.B.N. Use of compost and phosphate-solubilizing bacteria affect sugarcane mineral nutrition, phosphorus availability and the soil bacterial community. Appl. Soil Ecol. 2021;157 doi: 10.1016/j.apsoil.2020.103760. [DOI] [Google Scholar]
- Etesami H., Jeong B.R., Glick B.R. Contribution of arbuscular mycorrhizal fungi, phosphate–solubilizing bacteria and silicon to P uptake by plant. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.699618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahsi N., Mahdi I., Mesfioui A., Biskri L., Allaoui A. Plant growth-promoting rhizobacteria isolated from the Jujube (Ziziphus lotus) plant enhance wheat growth, Zn uptake and heavy metal tolerance. Agriculture. 2021;11:316. doi: 10.3390/agriculture11040316. [DOI] [Google Scholar]
- Fasusi O.A., Cruz C., Babalola O.O. Agricultural sustainability: Microbial biofertilizers in rhizosphere management. Agriculture. 2021;11:163. doi: 10.3390/agriculture11020163. [DOI] [Google Scholar]
- Fernando W., Nakkeeran S., Zhang Y., Savchuk S. Biological control of Sclerotinia sclerotiorum (lib.) de Bary by Pseudomonas and Bacillus species on canola petals. Crop Prot. 2018;26:100–107. [Google Scholar]
- Fibach-Paldi S., Burdman S., Okon Y. Key physiological properties contributing to rhizosphere adaptation and plant growth promotion abilities of Azospirillum brasilense. FEMS Microbiol. Lett. 2011;326:99–108. doi: 10.1111/j.1574-6968.2011.02407.x. [DOI] [PubMed] [Google Scholar]
- Filipini L.D., Pilatti F.K., Meyer E., Ventura B.S., Lourenzi C.R., Lovato P.E. Application of Azospirillum on seeds and leaves, associated with Rhizobium inoculation, increases growth and yield of common bean. Arch. Microbiol. 2021;203:1033–1038. doi: 10.1007/s00203-020-02092-7. [DOI] [PubMed] [Google Scholar]
- Fischer H.M. Genetic regulation of nitrogen fixation in rhizobia. Microbiol. Rev. 1994;58:352–386. doi: 10.1128/mr.58.3.352-386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitter A.H., Helgason T., Hodge A. Nutritional exchanges in the arbuscular mycorrhizal symbiosis: implications for sustainable agriculture. Fungal Biol. Rev. 2011;25:1–5. [Google Scholar]
- Gajera H.P., Vakharia D.N. Production of lytic enzymes by Trichoderma isolates during in vitro antagonism with Aspergillus niger, the causal agent of collar rot of peanut. Braz. J. Microbiol. 2012;43(1):43–52. doi: 10.1590/S1517-83822012000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos-Cedillo V.M., Urrestarazu M., Álvaro J.E. Influence of salinity on transport of nitrates and potassium by means of the xylem sap content between roots and shoots in young tomato plants. J. Soil Sci. Plant Nutr. 2016;16(4):991–998. [Google Scholar]
- Gamalero E., Glick B.R. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015;169:13–22. doi: 10.1104/pp.15.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Game B.C., Ilhe B.M., Pawar V.S., Khandagale P.P. Effect of Azotobacter, phosphate solubilizing bacteria and potash mobilizing bacteria inoculants on productivity of wheat (Triticum aestivum L.) Intern. J. Curr. Microbiol. Appl. Sci. 2020;9(3):2800–2807. [Google Scholar]
- Garbeva P., van Elsas J.D., van Veen J.A. Rhizosphere microbial community and its response to plant species and soil history. Plant Soil. 2008;302:19–32. [Google Scholar]
- Garcı´a-Fraile, P., Menendez, E., Lera, L.C., Dı´ez-Mendez, A., Jimenez-Go´mez, A., Marcos-Garcı´a, M., Cruz-Gonza´lez, X.A., Martı´nez-Hidalgo, P., Mateos, P.F., Rivas, R., 2017. Bacterial probiotics: a truly green revolution. In: Kumar, V. (Ed.), Probiotics and Plant Health. Springer Nature Singapore, Pte Ltd, pp. 131–162.
- Garcia-Seco D., Zhang Y., Gutierrez-Man˜ero F.J., Martin C., Ramos-Solano B. Application of Pseudomonas fluorescens to blackberry under field conditions improves fruit quality by modifying flavonoid metabolism. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0142639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur R., Noam S., Johri B., Rossi P., Aragno M. Diacetylphloroglucinol-producing pseudomonads do not influence AM fungi in wheat rhizosphere. Curr. Sci. 2004;88:453–457. [Google Scholar]
- Ghavami N., Alikhani H.A., Pourbabaei A.A., Besharati H. Effects of two new siderophore-producing rhizobacteria on growth and iron content of maize and canola plants. J. Plant Nutr. 2017;40(5):736–746. [Google Scholar]
- Ghazy N., El-Nahrawy S. Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant. Arch. Microbiol. 2021;203:1195–1209. doi: 10.1007/s00203-020-02113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghodsalavi B., Ahmadzadeh M., Soleimani M., Madloo P.B., Taghizad-Farid R. Isolation and characterization of rhizobacteria and their effects on root extracts of Valeriana officinalis. Aust. J. Crop Sci. 2013;7(3):338–344. [Google Scholar]
- Ghosh D., Gupta A., Mohapatra S. Dynamics of endogenous hormone regulation in plants by phytohormone secreting rhizobacteria under water-stress. Symbiosis. 2019;77:265–278. [Google Scholar]
- Gilani A., Abbasdokht H., Gholami A. Effects of Thiobacillus and different levels of sulfur fertilizer on growth and physiological indices in intercropping of sesame (L.) and mung bean (Vigna radiata L.) Gesunde Pflanzen. 2021;73:317–333. doi: 10.1007/s10343-021-00554-6. [DOI] [Google Scholar]
- Glaser B., Lehr V.I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2019;9:9338. doi: 10.1038/s41598-019-45693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014;169:30–39. doi: 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Glick B.R., Cheng Z., Czarny J., Duan J. Springer; New York: 2007. New perspectives and approaches in plant growth-promoting rhizobacteria research. In: Promotion of plant growth by ACC deaminase-producing soil bacteria; pp. 329–339. [Google Scholar]
- Glick B.R., Gamalaro E. Recent developments in the study of plant microbiomes. Microorganisms. 2021;9:1533. doi: 10.3390/microorganisms9071533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A.K., Sindhu S.S., Dadarwal K.R. Symbiotic effectiveness of bacteriocin producing and non-producing strains of Rhizobium in green gram (Vigna radiata) Indian J. Expt. Biol. 2001;39:821–823. [PubMed] [Google Scholar]
- Gouda S., Kerry R.G., Das G., Paramithiotis S., Shin H.S., Patra J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2017;206:131–140. doi: 10.1016/j.micres.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Goyal R.K., Mattoo A.K., Schmidt M.A. Rhizobial–host interactions and symbiotic nitrogen fixation in legume crops toward agriculture sustainability. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.669404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R.E., Cornell S.J., Scharlemann J.P.W., Balmford A. Farming and the fate of wild nature. Science. 2005;307:550–555. doi: 10.1126/science.1106049. [DOI] [PubMed] [Google Scholar]
- Groppa M.D., Benavides M.P., Zawoznik M.S. Root hydraulic conductance, aquaporins and plant growth promoting microorganisms: a revision. Appl. Soil Ecol. 2012;61:247–254. [Google Scholar]
- Gupta, T., Chakraborty, D., Sarkar, A., 2021. Structural and functional rhizospheric microbial diversity analysis by cutting-edge biotechnological tools. In: Pudake, R.N., Sahu, B.B., Kumari, M., Sharma A.K. (Eds.), Omics science for rhizosphere biology. Springer Nature, Singapore.. 10.1007/978-981-16-0889-6_9.
- Hafeez B., Khanif Y.M., Saleem M. Role of zinc in plant nutrition—a review. Am. J. Expt. Agric. 2013;3(2):374–391. [Google Scholar]
- Han H.S., Lee K. Effect of co-inoculation with phosphate and potassium solubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant Soil Environ. 2006;52:130. [Google Scholar]
- Han Q., Ma Q., Chen Y., Tian B., Xu L., Bai Y., Chen W., Li X. Variation in rhizosphere microbial communities and its association with the symbiotic efficiency of rhizobia in soybean. ISME J. 2020;14:1915–1928. doi: 10.1038/s41396-020-0648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney C.H., Samuel B.S., Bush J., Ausubel F.M. Associations with rhizosphere bacteria can confer and adaptive advantage to plants. Nat. Plants. 2015;1:15051. doi: 10.1038/nplants.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariprasad P., Niranjana S. Isolation and characterization of phosphate solubilizing rhizobacteria to improve plant health of tomato. Plant Soil. 2009;316:13–24. [Google Scholar]
- Haroon U., Khizar M., Liaquat F., Ali M., Akbar M., Tahir K., Batool S.S., Kamal A., Chaudhary H.J., Munis M.F.H. Halotolerant plant growth-promoting rhizobacteria induce salinity tolerance in wheat by enhancing the expression of SOS genes. J. Plant Growth Regul. 2021 doi: 10.1007/s00344-021-10457-5. [DOI] [Google Scholar]
- Hata E.M., Yusof M.T., Zulperi D. Induction of systemic resistance against bacterial leaf streak disease and growth promotion in rice plant by Streptomyces shenzhenesis TKSC3 and Streptomyces sp. SS8. Plant Pathol. J. 2021;37(2):173–181. doi: 10.5423/PPJ.OA.05.2020.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat R., Ali S., Amara U., Khalid R., Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann. Microbiol. 2010;60(4):579–598. [Google Scholar]
- He Y.H., Peng Y.J., Wu Z.S., Han Y., Dang Y. Survivability of Pseudomonas putida RS-198 in liquid formulations and evaluation its growth-promoting abilities on cotton. J. Animal Plant Sci. 2015;3:180–189. [Google Scholar]
- Heil M., Bostock R.M. Induced systemic resistance (ISR) against pathogens in the context of induced plant defences. Ann. Bot. 2002;89(5):503–512. doi: 10.1093/aob/mcf076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henri F., Laurette N.N., Annette D., John Q., Wolfgang M., François-Xavier E., Dieudonné N. Solubilization of inorganic phosphates and plant growth promotion by strains of Pseudomonas fluorescens isolated from acidic soils of Cameroon. Afric. J. Microbiol. Res. 2008;2:171–178. [Google Scholar]
- Herrmann L., Lesueur D. Challenges of formulation and quality of biofertilizers for successful inoculation. Appl. Microbiol. Biotechnol. 2013;97:8859–8873. doi: 10.1007/s00253-013-5228-8. [DOI] [PubMed] [Google Scholar]
- Heydari S., Moghadam P.R., Kennedy Arab S.M. Proceedings “Competition for Resources in a Changing World: New Drive for Rural Development”, 7-9 October 2008, Tropentag, Hohenheim. 2008. Hydrogen cyanide production ability by Pseudomonas fluorescens bacteria and their inhibition potential on weed germination. [Google Scholar]
- Hirel B., Te´tu T., Lea P., Dubois F. Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability. 2011;3:1452–1485. [Google Scholar]
- Hoda A.M., Gomma A.M. Faba bean growth and green yield and its quality as influenced by the application of bio-organic farming system. J. Appl. Sci. Res. 2005;1(5):380–385. [Google Scholar]
- Hu L., Robert C.A.M., Cadat S., Zhang X., Ye M., Li B., Manzo D., Chervet N., Steinger T., van der Heijden M.G.A., Schlaeppi K., Erb M. Root exudate metabolites derive plant-soil feed backs on growth and defense by shaping the rhizosphere microbiota. Nature Commun. 2018;9:2738. doi: 10.1038/s41467-018-05122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Chaparro J.M., Reardon K.F., Zhang R., Shen Q., Vivanco J.M. Rhizosphere interactions: root exudates, microbes, and microbial communities. Botany. 2014;92:267–275. doi: 10.1139/cjb-2013-0225. [DOI] [Google Scholar]
- Hungria M., Campo R.J., Souza E.M., Pedrosa F.O. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil. 2010;331:413–425. [Google Scholar]
- Hungria M., Nogueira M.A., Araujo R.S. Soybean seed coinoculation with Bradyrhizobium spp. and Azospirillum brasilense: a new biotechnological tool to improve yield and sustainability. AJPS. 2015;6(6):8–11. [Google Scholar]
- Hurek T., Handley L.L., Reinhold-Hurek B., Pich´e Y. Azoarcus grass endophytes contribute fixed nitrogen to the plant in an unculturable state. Mol. Plant Microb. Interact. 2002;15:233–242. doi: 10.1094/MPMI.2002.15.3.233. [DOI] [PubMed] [Google Scholar]
- Hussain A., Zahir Z.A., Asghar H.N., Ahmad M., Jamil M., Naveed M., Akhtar M.F.U.Z. Role of Rhizospheric Microbes in Soil. Springer; Berlin/Heidelberg, Germany: 2018. Zinc solubilizing bacteria for zinc biofortification in cereals: A step toward sustainable nutritional security; pp. 203–227. [Google Scholar]
- Hussain A., Zahir Z.A., Ditta A., Tahir M.U., Ahmad M., Mumtaz M.Z., Hayat K., Hussain S. Production and implication of bio-activated organic fertilizer enriched with zinc-solubilizing bacteria to boost up maize (Zea mays L.) production and biofortification under two cropping seasons. Agronomy. 2020;10:39. [Google Scholar]
- Igiehon N.O., Babalola O.O., Aremu B.R. Genomic insights into plant growth promoting rhizobia capable of enhancing soybean germination under drought stress. BMC Microbiol. 2019;19:159. doi: 10.1186/s12866-019-1536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz M., Ali Q., Ashraf S., Kamran M., Rehman A. Microbiome in Plant Health and Disease. Springer; Berlin/Heidelberg, Germany: 2019. Development of future bioformulations for sustainable agriculture; pp. 421–446. [Google Scholar]
- Ilyas N., Mumtaz K., Akhtar N., Yasmin H., Sayyed R., Khan W., Enshasy H.A.E., Dailin D.J., Elsayed E.A., Ali Z. Exopolysaccharides producing bacteria for the amelioration of drought stress in wheat. J. Sustain. 2020;12:8876. [Google Scholar]
- International Panaacea Limited, 2018. https://www.iplbiologicals.com.
- Jain D., Sharma J., Kaur G., Bhojiya A.A., Chauhan S., Sharma V., Suman A., Mohanty S.R., Maharjan E. Phenetic and molecular diversity of nitrogen-fixing plant growth-promoting Azotobacter isolated from semiarid regions of India. Hindawi BioMed. Res. Intern. 2021;2021 doi: 10.1155/2021/6686283. 9 pages. [DOI] [Google Scholar]
- Jang J.H., Woo S.Y., Kim S.H., Khaine I., Kwak M.J., Lee H.K., Lee T.Y., Lee W.Y. Effects of increased soil fertility and plant growth-promoting rhizobacteria inoculation on biomass yield, energy value, and physiological response of poplar in short-rotation coppices in a reclaimed tideland: A case study in the Saemangeum area of. Korea. J. Biomass. 2017;107:29–38. [Google Scholar]
- Jangu O.P., Sindhu S.S. Differential response of inoculation with indole acetic acid producing Pseudomonas sp. in green gram (Vigna radiata L.) and black gram (vigna mungo L.) Microbiol. J. 2011;1:159–173. [Google Scholar]
- Javaid A. Arbuscular mycorrhizal mediated nutrition in plants. J. Plant Nutr. 2009;32:1595–1618. [Google Scholar]
- Jaybhay S.A., Taware S.P., Varghese P. Microbial inoculation of Rhizobium and phosphate solubilizing bacteria along with inorganic fertilizers for sustainable yield of soybean [Glycine max (L.) Merrill] J. Plant Nutr. 2017;40(15):2209–2216. [Google Scholar]
- Jha Y. The importance of zinc-mobilizing rhizosphere bacteria to the enhancement of physiology and growth parameters for paddy under salt-stress conditions. Jordan J. Biol. Sci. 2019;12(2):167–173. [Google Scholar]
- Jiang Y.J. Plant cultivars imprint the rhizosphere bacterial community composition and association networks. Soil Biol. Biochem. 2017;109:145–155. [Google Scholar]
- Jiao X., Takishita Y., Zhou G., Smith D.L. Plant associated rhizobacteria for biocontrol and plant growth enhancement. Front. Plant Sci. 2021;17 doi: 10.3389/fpls.2021.634796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe M.M., Saravanan V.S., Islam M.R., Sa T. Development of alginate-based aggregate inoculants of Methylobacterium sp. and Azospirillum brasilense tested under in vitro conditions to promote plant growth. J. Appl. Microbiol. 2014;116:408–423. doi: 10.1111/jam.12384. [DOI] [PubMed] [Google Scholar]
- Jones D.L., Hodge A., Kuzyakov Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004;164:459–480. doi: 10.1111/j.1469-8137.2004.01130.x. [DOI] [PubMed] [Google Scholar]
- Kader M., Mian M., Hoque M. Effects of Azotobacter inoculant on the yield and nitrogen uptake by wheat. J. Biol. Sci. 2002;2:259–261. [Google Scholar]
- Kallas T., Coursin T., Rippka R. Different organization of nif genes in non-heterocystous and heterocystous cyanobacteria. Plant Mol. Biol. 1985;5:321–329. doi: 10.1007/BF00020630. [DOI] [PubMed] [Google Scholar]
- Kamran S., Shahid I., Baig D.N., Rizwan M., Malik K.A., Mehnaz S. Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Front. Microbiol. 2017;8:2593. doi: 10.3389/fmicb.2017.02593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.M., Adhikari A., Lee K.E., Park Y.G., Shahzad R., Lee I.J. Gibberellin producing rhizobacteria Pseudomonas korensis mu2 enhance growth of lettuce (Lactuca sativa) and Chinese cabbage (Brassica rapa chinensis) J. Microbiol. Biotechnol. Food Sci. 2019;9(2):166–170. [Google Scholar]
- Kang S-M., Shahzad R., Khan M.A., Hasnain Z., Lee K-E., Park H-S., Kim L-R., Lee I-J. Ameliorative effect of indole-3-acetic acid- and siderophore-producing Leclercia adecarboxylata MO1 on cucumber plants under zinc stress. J. Plant Inter. 2021;16:30–41. doi: 10.1080/17429145.2020.1864039. [DOI] [Google Scholar]
- Kannaiyan S. Nitrogen contribution by Azolla to rice crop. Energy. 1993;400:1–67. [Google Scholar]
- Kashyap, A.S., Pandey, V.K., Manzar, N., Kannojia, P., Singh, U.B., Sharma, P.K., 2017. Role of plant growth-promoting rhizobacteria for improving crop productivity in sustainable agriculture. In: Singh, D., Singh, H., Prabha, R. (Eds.), Plant-Microbe Interactions in Agro-Ecological Perspectives. Springer, Singapore, pp. 673–693.
- Kaul S., Choudhary M., Gupta S., Dhar M.K. Engineering host microbiome for crop improvement and sustainable agriculture. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.635917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavadia A., Omirou M., Fasoula D.A., Louka F., Ehaliotis C., Ioannides I.M. Co-inoculations with rhizobia and arbuscular mycorrhizal fungi alters mycorrhizal composition and lead to synergistic growth effects in cowpea that are fungal combination-dependent. Appl. Soil Ecol. 2021;167 doi: 10.1016/j.apsoil.2021.104013. [DOI] [Google Scholar]
- Kawasaki A., Dennis P.G., Forstner C., Raghavendra A.H.H., Mathesius U., Richardson A.E., Delhaize E., Gilliham M., Watt M., Ryan P.R. Manipulating exudate composition from root apices shapes the microbiome throughout the root system. Plant Physiol. 2021;2021:1–17. doi: 10.1093/plphys/kiab337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke J., Wang B., Yoshikuni Y. Microbiome engineering: Synthetic biology of plant-associated microbiomes in sustainable agriculture. Trends Biotechnol. 2021;39:244–261. doi: 10.1016/j.tibtech.2020.07.008. [DOI] [PubMed] [Google Scholar]
- Kertesz M.A., Mirleau P. The role of microbes in plant sulphur nutrition. J. Exp. Bot. 2004;55:1939–1945. doi: 10.1093/jxb/erh176. [DOI] [PubMed] [Google Scholar]
- Khan A.L., Halo B.A., Elyassi A., Ali S., Al-Hosni K., Hussain J. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Electron. J. Biotechnol. 2016;21:58–64. [Google Scholar]
- Khan N., Bano A., Ali S., Babar Md.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020;90:189–203. [Google Scholar]
- Khandelwal A., Sindhu S.S. Expression of 1-aminocyclopropane-1-carboxylate deaminase in rhizobia promotes nodulation and plant growth of cluster bean (Cyamopsis tetragonoloba L.) Res. J. Microbiol. 2012;7:158–170. [Google Scholar]
- Khanna, K., Kohli, S.K., Sharma, P., Kour, J., Singh, A.D., Sharma, N., Ohri, P., Bhardwaj, R., 2021. Antioxidant potential of plant growth-promoting rhizobacteria (PGPR) in agricultural crops infected with root-knot nematodes. In: Singh H.B., Vaishnav A., Sayyed R. (Eds.), Antioxidants in plant-microbe interaction. Springer Nature, Singapore. 10.1007/978-981-16-1350-0_16.
- Kim S.D. Colonizing ability of Pseudomonas fluorescens 2112, among collections of 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens spp. in pea rhizosphere. J. Microbiol. Biotechnol. 2012;22:763–770. doi: 10.4014/jmb.1112.12039. [DOI] [PubMed] [Google Scholar]
- Klaus S., Bulgarelli D. The plant microbiome at work. Mol. Plant Microbe Interact. 2015;28(3):212–217. doi: 10.1094/MPMI-10-14-0334-FI. [DOI] [PubMed] [Google Scholar]
- Klimek-Kopyra A., Zajac T., Oleksy A., Kulig B., Slizowska A. The value of different vegetative indices (NDVI, GAI) for the assessment of yield potential of pea (Pisum sativum L.) at different growth stages and under varying management practices. Acta. Agrobot. 2018;71:1–12. [Google Scholar]
- Köberl M., White R.A., Erschen S., El-Arabi T.F., Jansson J.K., Berg G. Draft genome sequence of Streptomyces sp. strain Wb2n-11, a desert isolate with broad-spectrum antagonism against soilborne phytopathogens. Genome Announc. 2015;3(4) doi: 10.1128/genomeA.00860-15. e00860-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollah B., Patra A.K., Mohanty S.R. Aquatic microphylla Azolla: A perspective paradigm for sustainable agriculture, environment, and global climate change. Environ. Sci. Pollut. Res. 2016;23:4358–4369. doi: 10.1007/s11356-015-5857-9. [DOI] [PubMed] [Google Scholar]
- Ku Y., Xu G., Tian X., Xie H., Yang X., Cao C. Root colonization and growth promotion of soybean, wheat and Chinese cabbage by Bacillus cereus YL6. PloS One. 2018;13(11) doi: 10.1371/journal.pone.0200181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubi H.A.A., Khan M.A., Adhikari A., Imran M., Kang S-M., Hamayun M., Lee I-J. Silicon and plant growth-promoting rhizobacteria Pseudomonas psychrotolerans CS51 mitigates salt stress in Zea mays L. Agriculture. 2021;11(3):272. doi: 10.3390/agriculture11030272. [DOI] [Google Scholar]
- Kumar A., Dewangan S., Lawate P., Bahadur I., Prajapati S. Plant Growth Promoting Rhizobacteria for Sustainable Stress Management. Springer; Berlin/Heidelberg, Germany: 2019. Zinc-solubilizing bacteria: A boon for sustainable agriculture; pp. 139–155. [Google Scholar]
- Kumar A., Maurya B.M., Raghuwanshi R. The microbial consortium of indigenous rhizobacteria improving plant health, yield and nutrient content in wheat (Triticum aestivum) J. Plant Nutr. 2021;44:1942–1956. doi: 10.1080/01904167.2021.1884706. [DOI] [Google Scholar]
- Kumar A., Singh A.K., Kaushik M.S., Mishra S.K., Raj P., Singh P.K. Interaction of turmeric (Curcuma longa L.) with beneficial microbes: a review. 3 Biotech. 2017;7(6):357. doi: 10.1007/s13205-017-0971-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Singh R., Yadav A., Giri D.D., Singh P.K., Pandey K.D. Isolation and characterization of bacterial endophytes of Curcuma longa (L.) 3 Biotech. 2016;6:60. doi: 10.1007/s13205-016-0393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Singh V., Singh M., Singh P.P., Singh S.K., Singh P.K. Isolation of plant growth promoting rhizobacteria and their impact on growth and curcumin content in Curcuma longa L. Biocat. Agric. Biotechnol. 2016;8:1–7. [Google Scholar]
- Kumar P., Dubey R.C., Maheshwari D.K. Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol. Res. 2012;167(8):493–499. doi: 10.1016/j.micres.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Kumar V., Kumar A., Pandey K.D., Roy B.K. Isolation and characterization of bacterial endophytes from the roots of Cassia tora L. Ann. Microbiol. 2015;65:1391–1399. [Google Scholar]
- Kuzyakov Y., Domanski G. Carbon input by plants into the soil. J. Plant Nutr. Soil Sci. 2000;163:421–431. [Google Scholar]
- Laceg M.J., Wilson C.R. Relationship of common seab incidence of potatoes grown in Tasmanian ferrosol soils with pH, exchangeable cations and other chemical properties of those soils. J. Phytopathol. 2001;149:679–683. [Google Scholar]
- Lakshminarayana K., Shukla Bela, Sindhu S.S., Kumari Parveen, Narula N., Sheoran R.K. Analogue-resistant mutants of Azotobacter chrococcum derepressed for nitrogenase activity and early ammonia excretion having potential as inoculants for cereal crops. Indian J. Expt. Biol. 2000;38:373–378. [PubMed] [Google Scholar]
- Latef H.A., Alhmad M.F.A., Zakir A. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum reinforces maize growth by improving physiological activities under saline conditions. J. Plant Growth Regul. 2020;39:1293–1308. [Google Scholar]
- Lawal T.E., Babalola O.O. Relevance of biofertilizers to agriculture. J. Hum. Ecol. 2014;47:35–43. [Google Scholar]
- Lawson, D.M., Smith, B.E., 2002. In: Sigel, A., Sigel, H. (Eds.), Metal Ions in Biological Systems Vol. 39 Marcel Dekker, New York, pp. 75–119. [PubMed]
- Leach J.E., Triplett L.R., Argueso C.T., Trivedi P. Communication in the phytobiome. Cell. 2017;169:587–596. doi: 10.1016/j.cell.2017.04.025. [DOI] [PubMed] [Google Scholar]
- Lebeis S.L., Rott M., Dangl J.L., Schulze-Lefert P. Culturing a plant microbiome community at the cross-rhodes. New Phytol. 2012;196(2):341–344. doi: 10.1111/j.1469-8137.2012.04336.x. [DOI] [PubMed] [Google Scholar]
- Lee S., Reth A., Meletzus D., Sevilla M., Kennedy C. Characterization of a major cluster of nif, fix, and associated genes in a sugarcane endophyte, Acetobacter diazotrophicus. J. Bacteriol. 2000;182:7088–7091. doi: 10.1128/jb.182.24.7088-7091.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.K., Lur H.S., Lo K.J., Cheng K.C., Chuang C.C., Tang S.J., Yang Z.W., Liu C.T. Evaluation of the effects of different liquid inoculant formulations on the survival and plant-growth-promoting efficiency of Rhodopseudomonas palustris strain PS3. Appl. Microbiol. Biotechnol. 2016;100:7977–7987. doi: 10.1007/s00253-016-7582-9. [DOI] [PubMed] [Google Scholar]
- Leslie S.B., Israeli E., Lighthart B., Crowe J.H., Crowe L.M. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol. 1995;61:3592–3597. doi: 10.1128/aem.61.10.3592-3597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Luo N., Ji C., Li J., Zhang L., Xiao L., She X., Liu Z., Li Y., Liu C., Guo Q., Lai H. Liquid organic fertilizer amendment alters rhizosphere microbial community structure and co-occurrence patterns and improves sunflower yield under salinity-alkalinity stress. Microb. Ecol. 2021 doi: 10.1007/s00248-021-01870-0. [DOI] [PubMed] [Google Scholar]
- Li L., Ye Y., Pan L., Zhu Y., Zheng S., Lin Y. The induction of trehalose and glycerol in Saccharomyces cerevisiae in response to various stresses. Biochem. Biophys. Res. Commun. 2009;387:778–783. doi: 10.1016/j.bbrc.2009.07.113. [DOI] [PubMed] [Google Scholar]
- Liffourrena A.S., Lucchesi G.I. Alginate-perlite encapsulated Pseudomonas putida A (ATCC 12633) cells: preparation, characterization and potential use as plant inoculants. J. Biotechnol. 2018;278:28–33. doi: 10.1016/j.jbiotec.2018.04.019. [DOI] [PubMed] [Google Scholar]
- Macik M., Gryta A., Frac M. Biofertilizers in agriculture: An overview on concepts, strategies, and effects on soil microorganisms. Adv. Agron. 2020;160:31. [Google Scholar]
- Mahanty T., Bhattacharjee S., Goswami M., Bhattacharyya P., Das B., Ghosh A., Tribedi P. Biofertilizers: a potential approach for sustainable agriculture development. Environ. Sci. Poll. Res. 2016;23:1–21. doi: 10.1007/s11356-016-8104-0. [DOI] [PubMed] [Google Scholar]
- Mahanty T., Bhattacharjee S., Goswami M., Bhattacharyya P., Das B., Ghosh A., Tribedi P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Poll. Res. 2017;24:3315–3335. doi: 10.1007/s11356-016-8104-0. [DOI] [PubMed] [Google Scholar]
- Maheshwari D.K., Dheeman S., Agarwal M. Bacterial metabolites in sustainable agroecosystems. Springer; Cham: 2015. Phytohormone-producing PGPR for sustainable agriculture; pp. 159–182. [Google Scholar]
- Maksimov I.V., Abizgil'Dina R.R., Pusenkova L.I. Plant growth promoting rhizobacteria as alternative to chemical crop protectors from pathogens. Appl. Biochem. Microbiol. 2011;47(4):333–345. [PubMed] [Google Scholar]
- Malik D.K., Sindhu S.S. Phytostimulatory effect of IAA-producing Pseudomonas strains on nodulation and plant growth of chickpea (Cicer arietinum) Physiol. Mol. Biol. Plants. 2011;17:25–32. doi: 10.1007/s12298-010-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malusá E., Sas-Paszt L., Ciesielska J. Technologies for beneficial microorganisms inocula used as biofertilizers. Sci. World J. 2012 doi: 10.1100/2012/491206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamnabi S., Nasrollahzadeh S., Ghassemi-Golezani K., Raei Y. Improving yield related physiological characteristics of spring rapeseed by integrated fertilizer management under water deficit conditions. Saudi J. Biol. Sci. 2020;27(3):797–804. doi: 10.1016/j.sjbs.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelino P.R.F., Milani K.M.L., Mali S., Dos Santos O.J.A.P., de Oliveira A.L.M. Formulations of polymeric biodegradable low-cost foam by melt extrusion to deliver plant growth-promoting bacteria in agricultural systems. Appl. Microbiol. Biotechnol. 2016;100:7323–7338. doi: 10.1007/s00253-016-7566-9. [DOI] [PubMed] [Google Scholar]
- Marchal K., Vanderleyden J. Oxygen paradox” of dinitrogen-fixing bacteria. Biol. Fertil. Soils. 2000;30:363–373. [Google Scholar]
- Marques A.P.G.C., Pires C., Moreira H., Rangel A.O.S.S., Castro P.M.L. Assessment of the plant growth promotion abilities of six bacterial isolates using Zeamays as indicator plant. Soil Biol. Biochem. 2010;42:1229–1235. [Google Scholar]
- Marschner, P., Rengel, Z., 2007. Contributions of rhizosphere interactions to soil. In: Abbott, L.K., Murphy, D.V., (Eds.) Soil biological fertility—a key to sustainable land use in agriculture. Kluwer Academic Publishers, Dordrecht, pp. 81–98.
- Martínez-Álvarez J.C., Castro-Martínez C., Sánchez-Peña P., Gutiérrez-Dorado R., Maldonado-Mendoza I.E. Development of a powder formulation based on Bacillus cereus sensulato strain B25 spores for biological control of Fusarium verticillioides in maize plants. World J. Microbiol. Biotechnol. 2016;32:75. doi: 10.1007/s11274-015-2000-5. [DOI] [PubMed] [Google Scholar]
- Martos G.I., Minahk C.J., Font de Valdez G., Morero R. Effects of protective agents on membrane fluidity of freeze-dried Lactobacillus delbrueckii sp. bulgaricus. Lett. Appl. Microbiol. 2007;45:282–288. doi: 10.1111/j.1472-765X.2007.02188.x. [DOI] [PubMed] [Google Scholar]
- Masepohl B., Drepper T., Paschen A., Gross S., Pawlowski A. Regulation of nitrogen fixation in the phototrophic purple bacterium Rhodobacter capsulatus. J. Mol. Microbiol. Biotechnol. 2002;4:243–248. [PubMed] [Google Scholar]
- Matthews A., Pierce S., Hipperson H., Raymond B. Rhizobacterial community assembly patterns vary between crop species. Front. Microbiol. 2019;10:581. doi: 10.3389/fmicb.2019.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayak S., Tirosh T., Glick B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004;42:565–572. doi: 10.1016/j.plaphy.2004.05.009. [DOI] [PubMed] [Google Scholar]
- McRose D.L., Baars O., Morel F.M.M., Kraepiel A.M.L. Siderophore production in Azotobacter vinelandii in response to Fe-, Mo- and V-limitation. Environ. Microbiol. 2017;19(9):3595–3605. doi: 10.1111/1462-2920.13857. [DOI] [PubMed] [Google Scholar]
- Meena V.S., Maurya B., Verma J.P. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol. Res. 2014;169:337–347. doi: 10.1016/j.micres.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Mehnaz S. In: Plant Microbes Symbiosis: Applied Facets. Arora N.K., editor. Springer; India, New Delhi: 2015. Azospirillum: A biofertilizer for every crop; pp. 297–314. [Google Scholar]
- Mehnaz S. In: Bioformulations: For Sustainable Agriculture. Arora N.K., editor. Springer; India: 2016. An overview of globally available bioformulations; pp. 267–281. [Google Scholar]
- Melin P., Håkansson S., Eberhard T.H., Schnürer J. Survival of the biocontrol yeast Pichia anomala after long-term storage in liquid formulations at different temperatures, assessed by flow cytometry. J. Appl. Microbiol. 2006;100:264–271. doi: 10.1111/j.1365-2672.2005.02778.x. [DOI] [PubMed] [Google Scholar]
- Memenza-Zegarra M., Zúñiga-Dávila D. Bioprospection of native antagonistic rhizobacteria from the Peruvian coastal ecosystems associated with Phaseolus vulgaris. Curr. Microbiol. 2021;78:1418–1431. doi: 10.1007/s00284-021-02388-x. [DOI] [PubMed] [Google Scholar]
- Mendes R., Garbeva P., Raaijmakers J.M. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013;37(5):634–663. doi: 10.1111/1574-6976.12028. [DOI] [PubMed] [Google Scholar]
- Mendes R., Kruijt M., de Bruijn I., Dekkers E., van der Voort M., Schneider J.H., Piceno Y.M., De Santis T.Z., Andersen G.L., Bakker P.A., Raaijmakers J.M. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science. 2011;332:1097–1100. doi: 10.1126/science.1203980. [DOI] [PubMed] [Google Scholar]
- Meyer S.L.F., Everts K.L., McSpadden Gardener B., Marler E.P., Abdelnabby H.M.E., Skantar A.M. Assessment of DAPG-producing Pseudomonas fluorescens for management of Meloidogyne incognita and Fusarium oxysporum on watermelon. J. Nematol. 2016;48(1):43–53. [PMC free article] [PubMed] [Google Scholar]
- Mfilinge A., Mtei K., Ndakidemi P. Effect of Rhizobium inoculation and supplementation with phosphorus and potassium on growth and total leaf chlorophyll (Chl) content of bush bean Phaseolus vulgaris L. Agricul. Sci. 2014;5:1413–1419. [Google Scholar]
- Mikhailouskaya N., Bogdevitch I. Effect of biofertilizers on yield and quality of long-fibred flax and cereal grains. Agron. Res. 2009;7:412–418. [Google Scholar]
- Miller, R.H., May, S., 1991. Legume inoculation: successes and failures. In: rhizosphere and plant growth. Keister, D.L., Cregan, P.B. (Eds.). Kluwer, Dordrecht. pp. 123–134.
- Miller S.H., Browne P., Prigent-Cambaret C., Combes-Meynet E., Morrissey J.P., O'Gara F. Biochemical and genomic comparison of inorganic phosphate solubilization in Pseudomonas species. Environ. Microbiol. Rep. 2010;2:403–411. doi: 10.1111/j.1758-2229.2009.00105.x. [DOI] [PubMed] [Google Scholar]
- Minaxi Saxena J., Chandra S., Nain L. Synergistic effect of phosphate solubilizing rhizobacteria and arbuscular mycorrhiza on growth and yield of wheat plants. J. Soil Sci. Plant Nutr. 2013;13:511–525. [Google Scholar]
- Miransari M. Arbuscular mycorrhizal fungi and nitrogen uptake. Arch. Microbiol. 2011;193:77–81. doi: 10.1007/s00203-010-0657-6. [DOI] [PubMed] [Google Scholar]
- Miransari M. Interactions between arbuscular mycorrhizal fungi and soil bacteria. Appl. Microbiol. Biotechnol. 2011;89:917–930. doi: 10.1007/s00253-010-3004-6. [DOI] [PubMed] [Google Scholar]
- Mishra, J., Arora, N.K., 2016. Bioformulations for plant growth promotion and combating phytopathogens: a sustainable approach. In: Arora, N.K., Balestrini, R., Mehnaz, S. (Eds.), Bioformulations: For Sustainable Agriculture. Springer India, pp. 3–33. 10.1007/978-81-322-2779-3.
- Mittler R., Vanderauwera S., Gollery M., Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9(10):490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Mohanram S., Kumar P. Rhizosphere microbiome: revisiting the synergy of plant-microbe interactions. Ann. Microbiol. 2019;69:307–320. [Google Scholar]
- Mohanty P., Singh P.K., Chakraborty D., Mishra S., Pattnaik R. Insight into the role of PGPR in sustainable agriculture and environment. Front. Sustain. Food Syst. 2021;5 doi: 10.3389/sufs.2021.667150. [DOI] [Google Scholar]
- Molina-Romero D., Baez A., Quintero-Hernández V., Castañeda-Lucio M., Fuentes-Ramírez L.E., del Rocío Bustillos-Cristales M., Rodríguez-Andrade O., Morales-García Y.E., Munive A., Muñoz-Rojas J. Compatible bacterial mixture, tolerant to desiccation, improves maize plant growth. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla A.H., Shamsuddin Z.H., Halimi M.S., Morziah M., Puteh A.B. Potential for enhancement of root growth and nodulation of soybean co-inoculated with Azospirillum and Bradyrhizobium in laboratory systems. Soil Biol. Biochem. 2001;33:457–463. [Google Scholar]
- Mondal M., Skalicky M., Garai S., Hossain A., Sarkar S., Banerjee H., Kundu R., Brestic M., Barutcular C., Erman M. Supplementing nitrogen in combination with Rhizobium inoculation and soil mulch in peanut (Arachis hypogaea L.) production system: Part II. Effect on phenology, growth, yield attributes, pod quality, profitability and nitrogen use efficiency. Agronomy. 2020;10:1513. [Google Scholar]
- Moraditochaee M., Azarpour E., Bozorgi H.R. Study effects of bio-fertilizers, nitrogen fertilizer and farmyard manure on yield and physiochemical properties of soil in lentil farming. Int. J. Biosci. 2014;4:41–48. [Google Scholar]
- Morgan C.A., Herman N., White P.A., Vesey G. Preservation of micro-organisms by drying; a review. J. Microbiol. Methods. 2006;66:183–193. doi: 10.1016/j.mimet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Mueller U.G., Sachs J.L. Engineering microbiomes to improve plant and animal health. Trends Microbiol. 2015;23:606–617. doi: 10.1016/j.tim.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Mugnier J., Jung G. Survival of bacteria and fungi in relation to water activity and the solvent properties of water in biopolymer. Appl. Environ. Microbiol. 1985;50:108–114. doi: 10.1128/aem.50.1.108-114.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D.B., Vogel C., Bai Y., Vorholt J.A. The plant microbiota: System-level insights and perspectives. Annu. Rev. Genet. 2016;50:211–234. doi: 10.1146/annurev-genet-120215-034952. [DOI] [PubMed] [Google Scholar]
- Mumtaz M.Z., Ahmad M., Jamil M., Hussain T. Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol. Res. 2017;202:51–60. doi: 10.1016/j.micres.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Murgese P., Santamaria P., Leoni B., Crecchio C. Ameliorative effects of PGPB on yield, physiological parameters, and nutrient transporter genes expression in Barattiere (Cucumis melo L.) J. Soil Sci. Plant Nutr. 2020;20:784–793. [Google Scholar]
- Nacoon S., Jogloy S., Riddech N., Mongkolthanaruk W., Ekprasert J., Cooper J., Boonlue S. Combination of arbuscular mycorrhizal fungi and phosphate solubilizing bacteria on growth and production of Helianthus tuberosus under field condition. Sci. Rep. 2021;11:650. doi: 10.1038/s41598-021-86042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseer I., Ahmad M., Hussain A., Jamil M. Potential of zinc solubilizing Bacillus strains to improve rice growth under axenic conditions. Pakistan J. Agric. Sci. 2020;57(4):1057–1071. [Google Scholar]
- National fertilizers limited., 2018. https://www.nationalfertilizers.com/index.php⁇. option=com_content&view=article&id=140&Itemid=156&Lang=en.
- Naveed M., Mehboob I., Shaker M.A., Hussain M.B., Farooq M. Biofertilizers in Pakistan: Initiatives and limitations. Intern. J. Agric. Biol. 2015;17:411–420. [Google Scholar]
- Nesme J., Simonet P. The soil resistome: a critical review of antibiotic resistance origins, ecology, and dissemination potential in telluric bacteria. Environ. Microbiol. 2015;17(4):913–930. doi: 10.1111/1462-2920.12631. [DOI] [PubMed] [Google Scholar]
- Niu X., Song L., Xiao Y., Ge W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. J. Front. Microbiol. 2018;8:2580. doi: 10.3389/fmicb.2017.02580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivetha, N., Lavanya, A.K., Vikram, K.V., Asha, A.D., Sruthi, K.S., Bandeppa, S., Annapurna, K., Paul, S., 2021. PGPR-mediated regulation of antioxidants: Prospects for abiotic stress management in plants. In: Singh, H.B., Vaishnav, A., Sayyed, R. (eds), Antioxidants in plant-microbe interaction. Springer Nature, Singapore. 10.1007/978-981-16-1350-0_23.
- Noar J.D., Bruno-Barcena J.M. Azotobacter vinelandii: the source of 100 years of discoveries and many more to come. Nat. Microbiol. 2018;164:421–436. doi: 10.1099/mic.0.000643. [DOI] [PubMed] [Google Scholar]
- Novo, L.A., Castro, P.M., Alvarenga, P., da Silva, E.F., 2018. Plant growth-promoting rhizobacteria-assisted phytoremediation of mine soils. In: Prasad, M.N.V., de Campos Favas, P.J., Maiti, S.K., (Eds.), Bio-Geotechnologies for Mine Site Rehabilitation. Elsevier Inc., Amsterdam, pp. 281–295.
- O'Callaghan M. Microbial inoculation of seed for improved crop performance: issues and opportunities. Appl. Microbiol. Biotechnol. 2016;100:5729–5746. doi: 10.1007/s00253-016-7590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerke E.C. Crop losses to pests. J. Agric. Sci. 2006;44(1):31–43. doi: 10.1017/S0021859605005708. [DOI] [Google Scholar]
- Okon Y., Labandera-Gonzalez C.A. Agronomic applications of Azospirillum: evaluation of 20 years worldwide field inoculation. Soil Biol Biochem. 1994;26:1591–1601. [Google Scholar]
- Okumura R.S., Mariano D.D.C., Dallacort R., Nogueira de Albuquerque A., Lobato A.D.S., Guedes E.S., Neto C., Oliveira da Conceicao H.E., Alves G.R. Azospirillum: A new and efficient alternative to biological nitrogen fixation in grasses. J. Food Agric. Environ. 2013;2:1142–1146. [Google Scholar]
- Oliveira A.L., Santos O.J., Marcelino P.R., Milani K.M., Zuluaga M.Y., Zucareli C., Gonçalves L.S. Maize inoculation with Azospirillum brasilense Ab-V5 cells enriched with exopolysaccharides and polyhydroxybutyrate results in high productivity under low N fertilizer input. Front. Microbiol. 2017;8:1873. doi: 10.3389/fmicb.2017.01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteino N., Lally R.D., Kiwanuka S., Lloyd A., Ryan D., Germaine K.J. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015;6:745. doi: 10.3389/fmicb.2015.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahari A., Mishra B.B. Characterization of siderophore producing rhizobacteria and its effect on growth performance of different vegetables. Intern. J. Curr. Microbiol. App. Sci. 2017;6:1398–1405. [Google Scholar]
- Pandey A., Kumar S. Potential of Azotobacters and Azospirilla as biofertilizers for upland agriculture: A review. J. Sci. Ind. Res. 1989;48:134–144. [Google Scholar]
- Pandey D., Kehri H.K., Zoomi I., Akhtar O., Singh A.K. Recent Advancement in White Biotechnology through Fungi. Springer; Berlin/Heidelberg, Germany: 2019. Mycorrhizal fungi: Biodiversity, ecological significance, and industrial applications; pp. 181–199. [Google Scholar]
- Pangesti N., Reichelt M., van de Mortel J.E. Jasmonic acid and ethylene signaling pathways regulate glucosinolate levels in plants during rhizobacteria-induced systemic resistance against a leaf-chewing herbivore. J. Chem. Ecol. 2016;42:1212–1225. doi: 10.1007/s10886-016-0787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.H., Lee C.Y., Son H.J. Mechanism of insoluble phosphate solubilization by Pseudomonas fluorescens RAF15 isolated from ginseng rhizosphere and its plant growth-promoting activities. Lett. Appl. Microbiol. 2009;49:222–228. doi: 10.1111/j.1472-765X.2009.02642.x. [DOI] [PubMed] [Google Scholar]
- Parmar P., Sindhu S.S. The novel and efficient method for isolating potassium solubilizing bacteria from rhizosphere soil. Geomicrobiol. J. 2019;36(2):130–136. [Google Scholar]
- Patel S.H., Viradiya M.B., Prajapati B.J. Effect of potassium and potassium mobilizing bacteria (KMB) with and without FYM on yield of wheat (Triticum aestivum L.) J. Pharmac. Phytochem. 2021;10(1):1615–1620. [Google Scholar]
- Pathak D., Kumar M., Rani K. Microorganisms for Green Revolution. Springer; Berlin/Heidelberg, Germany: 2017. Biofertilizer application in horticultural crops; pp. 215–227. [Google Scholar]
- Pathak E., Sanjyal A., Regmi C.R., Paudel S., Shrestha A. Screening of potential plant growth promoting properties of Bacillus species isolated from different regions of Nepal. Nepal J. Biotechnol. 2021;9(1):79–84. doi: 10.3126/njb.v9i1.38672. [DOI] [Google Scholar]
- Pellegrini M., Spera D.M., Ercole C., Del Gallo M. Allium cepa L. inoculation with a consortium of plant growth-promoting bacteria: Effects on plants, soil and the autochthonous microbial community. Microorganisms. 2021;9(3):639. doi: 10.3390/microorganisms9030639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereg L., Luz E., Bashan Y. Assessment of affinity and specificity of Azospirillum for plants. Plant Soil. 2016;399:389–414. [Google Scholar]
- Philippot L., Andersson S.G.E., Battin T.J., Prosser J.I., Schimel J.P., Whitman W.B., Hallin S. The ecological coherence of high bacterial taxonomic ranks. Nat. Rev. Microbiol. 2010;8:523–529. doi: 10.1038/nrmicro2367. [DOI] [PubMed] [Google Scholar]
- Phour M., Sehrawat A., Sindhu S.S., Glick B.R. Interkingdom signaling in plant-rhizomicrobiome interactions for sustainable agriculture. Microbiol. Res. 2020;241 doi: 10.1016/j.micres.2020.126589. 19 pp. [DOI] [PubMed] [Google Scholar]
- Pourbabaee A.A., Koohbori Dinekaboodi S., Seyed Hosseini H.M., Alikhani H.A., Emami S. Potential application of selected sulfur-oxidizing bacteria and different sources of sulfur in plant growth promotion under different moisture conditions. Commun. Soil Sci. Plant Anal. 2020;51:735–745. [Google Scholar]
- Prasad R., Prasad B. Cyanobacteria as a source biofertilizer for sustainable agriculture. Nepal J. Plant Sci. Bot. Orientalis. 2001;8:127–133. [Google Scholar]
- Press C.M., Loper J.E., Kleopper J.W. Role of iron in rhizobacteria-mediated induced systemic resistance of cucumber. Phytopathology. 2001;91:593–598. doi: 10.1094/PHYTO.2001.91.6.593. [DOI] [PubMed] [Google Scholar]
- Qureshi M.A., Ahmad Z.A., Akhtar N., Iqbal A., Mujeeb F., Shakir M.A. Role of phosphate solubilizing bacteria (PSB) in enhancing P availability and promoting cotton growth. J. Anim. Plant Sci. 2012;22:204–210. [Google Scholar]
- Rabin N., Zheng Y., Opoku-Temeng C., Du Y., Bonsu E., Sintim H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015;7:493–512. doi: 10.4155/fmc.15.6. [DOI] [PubMed] [Google Scholar]
- Raghu, S., Kumar, S., Suyal, D.C., Sahu, B., Kumar, V., Soni, R., 2021. Molecular tools to explore rhizosphere microbiome. In: Nath, M., Bhatt, D., Bhargava, P., Choudhary, D.K. (Eds.), Microbial metatranscriptomics belowground. Springer Nature, Singapore. 10.1007/978-981-15-9758-9_2.
- Raji M., Thangavelu M. Isolation and screening of potassium solubilizing bacteria from saxicolous habitat and their impact on tomato growth in different soil types. Arch. Microbiol. 2021;203:3147–3161. doi: 10.1007/s00203-021-02284-9. [DOI] [PubMed] [Google Scholar]
- Rakshiya Y.S., Verma M.K., Sindhu S.S. Efficacy of antagonistic soil bacteria in management of subterranean termites (Isoptera) Res. Environ. Life Sci. 2016;9:949–955. [Google Scholar]
- Rasouli-Sadaghiani, M., Malakouti, M.J., Khavazi, K., Miransari, M., 2014. Siderophore efficacy of fluorescent pseudomonads affecting labeled iron (59Fe) uptake by wheat (Triticum aestivum L.) genotypes differing in Fe efficiency. In: Miransari, M. (Ed.), Use of Microbes for the Alleviation of Soil Stresses. Springer, New York, pp. 121–132.
- Rees D.C., Howard J.B. Nitrogenase: standing at the crossroads. Curr.Opin. Chem. Biol. 2000;4:559–566. doi: 10.1016/s1367-5931(00)00132-0. [DOI] [PubMed] [Google Scholar]
- Remans R., Ramaekers L., Shelkens S., Hernandez G., Garcia A., Reyes G.L., Mendez N., Toscano V., Mullin M., Galvez L., Vanderleyden J. Effect of Rhizobium-Azospirillum co-inoculation on nitrogen fixation and yield of two contrasting Phaseolus vulgaris L. genotypes cultivated across different conditions in Cuba. Plant Soil. 2008;312:25–37. [Google Scholar]
- Riaz U., Mehdi S.M., Iqbal S., Khalid H.I., Qadir A.A., Anum W., Ahmad M., Murtaza G. Bioremediation and Biotechnology. Springer; Berlin/Heidelberg, Germany: 2020. Bio-fertilizers: Eco-Friendly approach for plant and soil environment; pp. 189–213. [Google Scholar]
- Richardson A., Barea J.M., McNeill A., Prigent-Combaret C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil. 2009;321:305–339. [Google Scholar]
- Riddech N., Phibunwatthanawong T., Sarin P. Suitable formulation of microbial inoculants as a bio-fertilizer for promoting growth of hairy-leafed apitong (Dipterocarpus alatus) Waste Biomass, Valor. 2021 doi: 10.1007/s12649-021-01526-7. [DOI] [Google Scholar]
- Rijavec T., Lapanje A. Hydrogen cyanide in the rhizosphere: not suppressing plant pathogens, but rather regulating availability of phosphate. Front. Microbiol. 2016;7:1785. doi: 10.3389/fmicb.2016.01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, A.C., Bonifacio, A., de Araujo, F.F., Junior, M.A.L., Figueiredo, Md.V.B., 2015. Azospirillum sp. as a challenge for agriculture. In: Maheshwari, D.K. (Ed.) Bacterial Metabolites in Sustainable Agroecosystem. Springer International Publishing, Cham, pp. 29–51. doi:10.1007/978-3-319-24654-3_2.
- Rodríguez A., Stella A., Storni M., Zulpa G., Zaccaro M. Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Syst. 2006;2:1. doi: 10.1186/1746-1448-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann, M., Sarango-Flores, S.W., Chiaramonte, J.B., Kmit, M.C.P., Mendes, R., 2017. Plant microbiome: composition and functions in plant compartments. In: Pylro, V., Roesch, L. (Eds.) The Brazilian microbiome. Springer, Cham, pp. 7–20.
- Ruíz-Valdiviezo V.M., Canseco L.M.C.V., Suárez L.A.C., Gutiérrez-Miceli F.A., Dendooven L., Rincón-Rosales R. Symbiotic potential and survival of native rhizobia kept on different carriers. Braz. J. Microbiol. 2015;46:735–742. doi: 10.1590/S1517-838246320140541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadatnia H., Riahi H. Cyanobacteria from paddy fields in Iran as a biofertilizer in rice plants. Plant Soil Environ. 2009;55:207–212. [Google Scholar]
- Sabet H., Mortazaeinezhad F. Yield, growth and Fe uptake of cumin (Cuminum cyminum L.) affected by Fe-nano, Fe-chelated and Fe-siderophore fertilization in the calcareous soils. J. Trace Elements Med. Biol. 2018;50:154–160. doi: 10.1016/j.jtemb.2018.06.020. [DOI] [PubMed] [Google Scholar]
- Saha B., Saha S., Roy P.D., Padhan D., Pati S., Hazra G.C. Role of Rhizospheric Microbes in Soil. Springer; Berlin/Heidelberg, Germany: 2018. Microbial transformation of sulphur: An approach to combat the sulphur deficiencies in agricultural soils; pp. 77–97. [Google Scholar]
- Sahu G.K., Sindhu S.S. Disease control and plant growth promotion of green gram by siderophore-producing Pseudomonas sp. Res. J. Microbiol. 2011;6:735–749. [Google Scholar]
- Salomon M.V., Bottini R., de Souza Filho G.A., Cohen A.C., Moreno D., Gil M. Bacteria isolated from roots and rhizosphere of Vitis vinifera retard water losses, abscisic acid accumulation and synthesis of defence-related terpenes in in vitro cultured grapevine. Physiol. Plant. 2014;151(4):359–374. doi: 10.1111/ppl.12117. [DOI] [PubMed] [Google Scholar]
- Samaras A., Roumeliotis E., Ntasiou P., Karaoglanidis G. Bacillus subtilis MBI600 promotes growth of tomato plants and induces systemic resistance contributing to the control of soilborne pathogens. Plants. 2021;10(6):1113. doi: 10.3390/plants10061113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan V.P., Sindhu S.S., Dahiya O.S., Kharb R.P.S. Improvement of wheat (Triticum aestivum L.) yield inder field conditions by inoculation of microbial strains. Microbiol. J. 2012;2(3):86–95. [Google Scholar]
- Santoyo G., Gamalero E., Glick B.R. Mycorrhizal-bacterial amelioration of plant abiotic and biotic stress. Front. Sustain. Food System. 2021;5 [Google Scholar]
- Santoyo G., Guzman-Guzman P., Parra-Cota F.I., de los Santos-Villalobos S., Orozco-Mosqueda M.C., Glick B.R. Plant growth stimulation by microbial consortia. Agronomy. 2021;11:219. doi: 10.3390/qgronomy11020219. [DOI] [Google Scholar]
- Santoyo G., Orozco-Mosqueda M.C., Govindappa M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: a review. Biocontr. Sci. Technol. 2012;22:855–872. [Google Scholar]
- Saravanan, V.S., Kumar, M.R., Sa, T.M., 2011. Microbial zinc solubilization and their role on plants, in Bacteria in Agrobiology: Plant Nutrient Management, Maheshwari, D.K. (Ed.). Berlin: Springer, pp. 47–63.
- Sarwar S., Khaliq A., Yousra M., Sultan T., Ahmad N., Khan M.Z. Screening of siderophore-producing PGPRs isolated from groundnut (Arachis hypogaea L.) rhizosphere and their influence on iron release in soil. Commun. Soil Sci. Plant Anal. 2020;51(12):1680–1692. [Google Scholar]
- Sasirekha B., Srividya S. Siderophore production by Pseudomonas aeruginosa FP6, a biocontrol strain for Rhizoctonia solani and Colletotrichum gloeosporioides causing diseases in chilli. Agric. Nat. Resour. 2016;50:250–256. [Google Scholar]
- Sasse J., Martinoia E., Northen T. Feed your friends: do plant exudates shape the root microbiome? Trends Plant Sci. 2018;23(1):25–41. doi: 10.1016/j.tplants.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Schoebitz M., Mengual C., Roldán A. Combined effects of clay immobilized Azospirillum brasilense and Pantoea dispersa and organic olive residue on plant performance and soil properties in the revegetation of a semiarid area. Sci. Total Environ. 2014;466:67–73. doi: 10.1016/j.scitotenv.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Schoebitz M., Simonin H., Poncelet D. Starch filler and osmoprotectants improve the survival of rhizobacteria in dried alginate beads. J. Microencapsul. 2012;29:532–538. doi: 10.3109/02652048.2012.665090. [DOI] [PubMed] [Google Scholar]
- Seefeldt L.C., Dance I.G., Dean D.R. Substrate interactions with nitrogenase: Fe versus Mo. Biochemistry. 2004;43:1401–1409. doi: 10.1021/bi036038g. [DOI] [PubMed] [Google Scholar]
- Sehrawat A., Sindhu S.S. Potential of biocontrol agents in plants disease control for improving food safety. Def. Life Sci. J. 2019;4(4):220–225. [Google Scholar]
- Sehrawat A., Sindhu S.S., Glick B.R. Hydrogen cyanide production by soil bacteria: Biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere. 2022;32(1):15–38. (in press) [Google Scholar]
- Sekhar M., Riotte J., Ruiz L., Jouquet P., Braun J.J. Influences of climate and agriculture on water and biogeochemical cycles: Kabini critical zone observatory. Proc. Indian Natl. Sci. Acad. 2016;82:833–846. [Google Scholar]
- Sendi Y., Pfeiffer T., Koch E., Mhadhbi H., Mrabet M. Potential of common bean (Phaseolus vulgaris L.) root microbiome in the biocontrol of root rot disease and traits of performance. J. Plant Dis. Protec. 2020;127:453–462. [Google Scholar]
- Sharma A., Dev K., Sourirajan A., Choudhary M. Isolation and characterization of salt-tolerant bacteria with plant growth-promoting activities from saline agricultural fields of Haryana, India. J. Genet. Eng. Biotechnol. 2021;19:99. doi: 10.1186/s43141-021-00186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Dahiya A., Sindhu S.S. Harnessing proficient rhizobacteria to minimize the use of agrochemicals. Intern. J. Curr. Microbiol. Appl. Sci. 2019;7(10):3186–3197. [Google Scholar]
- Sharma R., Sanodiya B.S., Thakur G.S., Jaiswal P., Sharma A., Bisen P.S. Standardization of lyophilization medium for Streptococcus thermophilus subjected to viability escalation on freeze drying. Microbiol. Res. 2014;5:5402. [Google Scholar]
- Sharma R., Sindhu S., Sindhu S.S. Suppression of Alternaria blight disease and plant growth promotion of mustard (Brassica juncea L.) by antagonistic rhizosphere bacteria. Appl. Soil Ecol. 2018;129:145–150. [Google Scholar]
- Sheng X. Growth promotion and increased potassium uptake of cotton and rape by a potassium releasing strain of Bacillus edaphicus. Soil Biol. Biochem. 2005;37:1918–1922. [Google Scholar]
- Sheng X.F., He L.Y. Solubilization of potassium-bearing minerals by a wild-type strain of Bacillus edaphicus and its mutants and increased potassium uptake by wheat. Can. J. Microbiol. 2006;52:66–72. doi: 10.1139/w05-117. [DOI] [PubMed] [Google Scholar]
- Shirley M., Avoscan L., Bernaud E., Vansuyt G., Lamanceau P. Comparison of iron acquisition from Fe-pyoverdine by strategy I and strategy II plants. Botany. 2011;89(10):731–735. [Google Scholar]
- Sindhu, S.S., Dadarwal, K.R., 1997. Molecular aspects of host specificity in Rhizobium-legume symbiosis and possibilities of inducing nodule in non-leguminous crops. In: Dadarwal, K.R. (ed.), Biotechnological Approaches in Soil Microorganisms for Sustainable Crop Production. Scientific Publishers, Jodhpur. pp. 39–69.
- Sindhu S.S., Dadarwal K.R. Competition for nodulation among rhizobia in legume-Rhizobium symbiosis. Indian J. Microbiol. 2000;40(4):211–246. [Google Scholar]
- Sindhu S.S., Dadarwal K.R. Chitinolytic and cellulolytic Pseudomonas sp. antagonistic to fungal pathogens enhances nodulation by Mesorhizobium sp. Cicer in chickpea (Cicer arietinum) Microbiol. Res. 2001;156:353–358. doi: 10.1078/0944-5013-00120. [DOI] [PubMed] [Google Scholar]
- Sindhu S.S., Dadarwal K.R., Davis T.M. Non-nodulating chickpea breeding line for the study of symbiotic nitrogen fixation potential. Indian J. Microbiol. 1992;32:175–180. [Google Scholar]
- Sindhu S.S., Lakshminaryana K. Survival and competitive ability of ammonia excreting and non-ammonia excreting Azotobacter chroococcum strains in sterile soil. Plant Soil. 1982;69:79–84. [Google Scholar]
- Sindhu, S.S., Parmar, P., Phour, M., Sehrawat, A., 2016. Potassium solubilizing microorganisms (KSMs) and its effect on plant growth improvement. In: Meena, V.S., Verma, J.P., Maurya, B.R., Meena, R.S. (Eds.), Potassium Solubilizing Microbes (KSMs). Springer-verlag, Berlin, Heidelberg, pp. 171–185.
- Sindhu, S.S., Phour, M., Choudhary, S.R., Choudhary, D., 2014. Phosphorus cycling: Prospects of using rhizosphere microorganisms for improving phosphorus nutrition of plants. In: Parmar, N., Singh, A. (Eds.), Geomicrobiology and Biogeochemistry. Springer-Verlag, Berlin, Heidelberg, pp. 199–237.
- Sindhu, S.S., Sharma, R., Sindhu, S., Phour, M., 2019a. Plant nutrient management through inoculation of zinc solubilizing bacteria for sustainable agriculture. In: Giri, B., Prasad, R., Wu, Q.S., Verma, A. (Eds.), Biofertilizers for Sustainable Agriculture and Environment, Springer Nature Springer, Pte Ltd. pp. 173–201.
- Sindhu, S.S., Sharma, R., Sindhu, S., Sehawat, A., 2019b. Soil fertility improvement by symbiotic rhizobia for sustainable agriculture. In: Panpatte, D.G., Jhala, V.K. (Eds.), Soil fertility management for sustainable development. Springer Nature, Singapore, Pte Ltd. pp. 101–166.
- Sindhu, S.S., Suneja, S., Dadarwal, K.R., 1997. Plant growth promoting rhizobacteria and their role in crop productivity. In: Dadarwal, K.R. (ed.), Biotechnological Approaches in Soil Microorganisms for Sustainable Crop Production. Scientific Publishers, Jodhpur. pp. 149–191.
- Singh G., Biswas D., Marwaha T. Mobilization of potassium from waste mica by plant growth promoting rhizobacteria and its assimilation by maize (Zea mays) and wheat (Triticum aestivum L.): a hydroponics study under phytotron growth chamber. J. Plant Nutr. 2010;33:1236–1251. [Google Scholar]
- Singh J.S., Gupta V.K. Soil microbial biomass: a key soil driver in management of ecosystem functioning. Sci. Total. Environ. 2018;634:497–500. doi: 10.1016/j.scitotenv.2018.03.373. [DOI] [PubMed] [Google Scholar]
- Singh J.S., Kumar A., Rai A.N., Singh D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016;7:529. doi: 10.3389/fmicb.2016.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S., Kumar, V., Dhanjal, D.S., Dhaka, S.V., Thotapalli, S., Singh, J., Anil, L.K.T., Aguilar-Marcelino, L., 2021. Rhizosphere biology: A key to agricultural sustainability. In: Yadav, A.N., Singh, J., Singh, C., Yadav, N., (Eds.), Current Trends in Microbial Biotechnology for Sustainable Agriculture. Environmental and microbial biotechnology. Springer Nature, Singapore, 10.1007/978-981-15-6949-4_7.
- Singh S., Singh B.K., Yadav S.M., Gupta A.K. Potential of biofertilizers in crop production in Indian agriculture. Am. J. Plant Nutr. Fertil. Technol. 2014;4:33–40. [Google Scholar]
- Singh S., Singh J.P. Effect of organic and inorganic nutrient sources on some soil properties and wheat yield. J. Indian Soc. Soil Sci. 2012;60(3):237–240. [Google Scholar]
- Singh V., Singh D., Gautam J.K., Nandi A.K. RS11/FLD is a positive regulator for defence against necrotrophic pathogens. Physiol. Mol. Plant Pathol. 2019;107:40–45. [Google Scholar]
- Singleton P., Keyser H., Sande E. In: Inoculants and Nitrogen Fixation of Legumes in Vietnam. ACIAR Proceedings. Herridge D., editor. 2002. Development and evaluation of liquid inoculants; pp. 52–66. [Google Scholar]
- Sivaramaiah N., Malik D.K., Sindhu S.S. Improvement in symbiotic efficiency of chickpea (Cicer arietinum) by coinoculation of Bacillus strains with Mesorhizobium sp. Cicer. Indian J. Microbiol. 2007;47(1):51–56. doi: 10.1007/s12088-007-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smercina D.N., Evans S.E., Friesen M.L., Tiemann L.K. To fix or not to fix: Controls on free-living nitrogen fixation in the rhizosphere. Appl. Environ. Microbiol. 2019;85(6) doi: 10.1128/AEM.02546-18. e02546-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofo A., Scopa A., Nuzzaci M., Vitti A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Intern. J. Mol. Sci. 2015;16:1356–1357. doi: 10.3390/ijms160613561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.S., Jeon J.H., Lee J.H., Jeong S.H., Jeong B.C. Molecular characterization of TEM-type beta-lactamases identified in cold-seep sediments of Edison Seamount (south of Lihir Island, Papua New Guinea) J. Microbiol. 2005;43:172–178. [PubMed] [Google Scholar]
- Song Y., Li Z., Liu J., Zou Y., Lv C., Chen F. Evaluating the impacts of Azotobacter chroococcum inoculation on soil stability and plant property of maize crop. J. Soil Sci. Plant Nutr. 2021;21:824–831. doi: 10.1007/s42729-020-00404-w. [DOI] [Google Scholar]
- Soumare A., Boubekri K., Lyamlouli K., Hafidi M., Ouhdouch Y., Kouisni L. From isolation of phosphate solubilizing microbes to their formulation and use as biofertilizers: Status and needs. Front. Bioengin. Biotechnol. 2019;7:425. doi: 10.3389/fbioe.2019.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana S., Alam S., Karim M.M. Screening of siderophore-producing salt-tolerant rhizobacteria suitable for supporting plant growth in saline soils with iron limitation. J. Agric. Food Res. 2021;4 [Google Scholar]
- Sundaramoorthy S., Balabaskar P. Evaluation of combined efficacy of Pseudomonas fluorescens and Bacillus subtilis in managing tomato wilt caused by Fusarium oxysporum f. sp. lycopersici (FOL) Plant Pathol. J. 2013;12(4):154–161. [Google Scholar]
- Taha M.A., Ismaiel A.A., Ahmed R.M. 6-pentyl-α-pyrone from Trichoderma koningii induces systemic resistance in tobacco against tobacco mosaic virus. Eur. J. Plant Pathol. 2021;159:81–93. doi: 10.1007/s10658-020-02142-2. [DOI] [Google Scholar]
- Tahir H.A., Gu Q., Wu H., Raza W., Hanif A., Wu L., Colman M.V., Gao X. Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. J. Front. Microbiol. 2017;8:171. doi: 10.3389/fmicb.2017.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamreihao K., Ningthoujam D.S., Nimaichand S., Singh E.S., Reena P., Singh S.H., Nongthomba U. Biocontrol and plant growth promoting activities of a Streptomyces corchorusii strain UCR3-16 and preparation of powder formulation for application as biofertilizer agents for rice plant. Microbiol. Res. 2016;192:260–270. doi: 10.1016/j.micres.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Tandon A., Fatima T., Anshu Shukla D., Tripathi P., Srivastava S., Singh P.C. Phosphate solubilization by Trichoderma koningiopsis (NBRI-PR5) under abiotic stress conditions. JKSUS. 2020;32(1):791–798. [Google Scholar]
- Tavallali V., Rahemi M., Eshghi S., Kholdebarin B., Ramezanian A. Zinc alleviates salt stress and increases antioxidant enzyme activity in the leaves of pistachio (Pistacia vera L. ‘Badami’) seedlings. Turk. J. Agric. 2010;34:349–359. [Google Scholar]
- Thajuddin N., Subramanian G. Cyanobacterial biodiversity and potential applications in biotechnology. Curr. Sci. 2005;89:47–57. [Google Scholar]
- Thies J.E., Singleton P.W., Bohlool B.B. Influence of size of indigenous rhizobial populations on establishment and symbiotic performance of introduced rhizobia on field-grown legumes. Appl. Environ. Microbiol. 1991;57(1):19–28. doi: 10.1128/aem.57.1.19-28.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L., Singh I. Biofertilizers for Sustainable Agriculture and Environment. Springer; Berlin/Heidelberg, Germany: 2019. Microbial biofertilizers: Types and applications; pp. 1–19. [Google Scholar]
- Thoms D., Liang Y., Haney C.H. Maintaining symbiotic homoestasis: How do plant engage with beneficial microorganisms while at the same time restricting pathogens? Mol. Plant Microbe Interact. 2021 doi: 10.1094/MPMI-11-20-0318-FI. [DOI] [PubMed] [Google Scholar]
- Tilman D., Balzer C., Hill J., Befort B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA. 2011;108:20260–20264. doi: 10.1073/pnas.1116437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk S., Abd El-Daim I.A., Copolovici L., Tanilas T., Kännaste A., Behers L., Nevo E., Seisenbaeva G., Stenström E., Niinemets Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS One. 2014;9:e96086. doi: 10.1371/journal.pone.0096086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk S., Behers L., Muthoni J., Muraya A., Aronsson A.C. Perspectives and challenges of microbial application for crop improvement. Front. Plant Sci. 2017;8:49. doi: 10.3389/fpls.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S., Singh C., Singh J.S. Land use changes: a key ecological driver regulating methanotrophs abundance in upland soils. Energy Ecol. Environ. 2018;3:355–371. [Google Scholar]
- Tkacz A., Pini F., Turner T.R., Bestion E., Simmonds J., Greenland A., Cheema J., Emms D.M., Uany C., Poole P.S.P. Agricultural selection of wheat has been shaped by plant-microbe interctions. Front. Microbiol. 2020;11:132. doi: 10.3389/fmicb.2020.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro M., Azcon R., Barea J.M. Improvement of arbuscular mycorrhiza development by inoculation of soil with phosphate solubilizing rhizobacteria to improve rock phosphate bioavailability (32P) and nutrient cycling. Appl. Environ. Microbiol. 1997;63:4408–4412. doi: 10.1128/aem.63.11.4408-4412.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Vera R., Garcia J.M., Pozo M.J., Lopez-Raez J.A. Do strigolactones contribute to plant defence? Mol. Plant Pathol. 2014;15(2):211–216. doi: 10.1111/mpp.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsvik V., Sorheim R., Goksoyr J. Total bacterial diversity in soil and sediment communities – A review. J. Ind. Microbiol. 1996;17:170–178. [Google Scholar]
- Tripti K., Adarsh U., Zeba K., Vipin A. Biochar and flyash inoculated with plant growth promoting rhizobacteria act as potential biofertilizer for luxuriant growth and yield of tomato plant. J. Environ. Manage. 2017;190:20–27. doi: 10.1016/j.jenvman.2016.11.060. [DOI] [PubMed] [Google Scholar]
- Troufflard S., Mullen W., Larson T.R., Graham I.A., Crozier A., Amtmann A., Armengaud P. Potassium deficiency induces the biosynthesis of oxylipins and glucosinolates in Arabidopsis thaliana. BMC Plant Biol. 2010;10:172. doi: 10.1186/1471-2229-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsegaye Z., Assefa F., Beyene D. Properties and application of plant growth promoting rhizobacteria. Intern. J. Curr. Trend Pharmacobiol. Med. Sci. 2017;2(1):30–43. [Google Scholar]
- Ullah C., Tsai C.J., Unsicker S.B., Xue L., Reichelt M., Gershenzon J., Hammerbacher A. Salicyclic acid activates poplar defence against the biotrophic rust fungus Melamspora larcipopulinavia increased biosynthesis of catechin and pro-anthocyanidins. New Phytol. 2019;221(2):960–975. doi: 10.1111/nph.15396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah N., Ditta A., Khalid A., Mehmood S., Rizwan M.S., Ashraf M., Mubeen F., Imtiaz M., Iqbal M.M. Integrated effect of algal biochar and plant growth promoting rhizobacteria on physiology and growth of maize under deficit irrigations. J. Soil Sci. Plant Nutr. 2019;20:346–356. doi: 10.1007/s42729-019-00112-0. [DOI] [Google Scholar]
- Uribe, D., Sanchez-Nieves, J., Vanegas, J., 2010. Role of microbial biofertilizers in the development of a sustainable agriculture in the tropics. In: Dion, P. (Ed.), Soil Biology and Agriculture in the Tropics. Springer-Verlag, Berlin Heidelberg, pp. 235–250. 10.1007/978-3-642-05076-3.
- Uroz S., Calvaruso C., Turpault M.P., Frey-klett P. Mineral weathering by bacteria: ecology, actors and mechanisms. Trends Microbiol. 2009;17:378–387. doi: 10.1016/j.tim.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Uroz S., Calvaruso C., Turpault M.P., Pierrat J.C., Mustin C., Frey-Klett P. Effect of the mycorrhizosphere on the genotypic and metabolic diversity of the bacterial communities involved in mineral weathering in a forest soil. Appl. Environ. Microbiol. 2007;73:3019–3027. doi: 10.1128/AEM.00121-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaid S.K., Srivastava P.C., Pachauri S.P., Sharma A., Rawat D., Shankhadhar S.C., Shukla A.K. Effective zinc mobilization to rice grains using rhizobacterial consortium. Israel J. Plant Sci. 2020;67(3-4):145–157. [Google Scholar]
- Valetti L., Angelini J.G., Taurian T., Ibáñez F.J., Muñoz V.L., Anzuay M.S., Ludueña L.M., Fabra A. Development and field evaluation of liquid inoculants with native Bradyrhizobial strains for peanut production. Afr. Crop Sci. J. 2016;24:1–13. doi: 10.4314/acsj.v24i1.1. [DOI] [Google Scholar]
- Valetti L., Iriarte L., Fabra A. Growth promotion of rapeseed (Brassica napus) associated with the inoculation of phosphate solubilizing bacteria. Appl. Soil Ecol. 2018;132:1–10. [Google Scholar]
- van Loon L.C. Plant responses to plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 2007;119:243–254. [Google Scholar]
- Vassilev N., Malusa E., Requena A.R., Martos V., López A., Maksimovic I., Vassileva M. Potential application of glycerol in the production of plant beneficial microorganisms. J. Ind. Microbiol. Biotechnol. 2017;44:735–743. doi: 10.1007/s10295-016-1810-2. [DOI] [PubMed] [Google Scholar]
- Vassilev N., Vassileva M., Lopez A., Martos V., Reyes A., Maksimovic I., Eichler-Löbermann B., Malusa E. Unexploited potential of some biotechnological techniques for biofertilizer production and formulation. Appl. Microbiol. Biotechnol. 2015;99:4983–4996. doi: 10.1007/s00253-015-6656-4. [DOI] [PubMed] [Google Scholar]
- Verma, M., Mishra, J., Arora, N.K., 2019. Plant growth-promoting rhizobacteria: diversity and applications. In: Sobti, R.C. (Ed.), Environmental Biotechnology: For Sustainable Future. Springer Nature Singapore, Pte Ltd, pp. 129–173.
- Verma P., Agrawal N., Shahi S.K. Effect of rhizobacterial strain Enterobacter cloacae strain pglo9 on potato plant growth and yield. Plant Arch. 2018;18:2528–2532. [Google Scholar]
- Verma P., Yadav A.N., Khannam K.S., Kumar S., Saxena A.K., Suman A. Molecular diversity and multifarious plant growth promoting attributes of Bacilli associated with wheat (Triticum aestivum L.) rhizosphere from six diverse agro-ecological zones of India. J. Basic Microbiol. 2016;56:44–58. doi: 10.1002/jobm.201500459. [DOI] [PubMed] [Google Scholar]
- Vimal S.R., Patel V.K., Singh J.S. Plant growth promoting Curtobacterium albidum strain SRV4: an agriculturally important microbe to alleviate salinity stress in paddy plants. Ecol. Indic. 2018;105:553–562. [Google Scholar]
- Vinayarani G., Prakash H.S. Growth promoting rhizospheric and endophytic bacteria from Curcuma longa L. as biocontrol agents against rhizome rot and leaf blight diseases. Plant Pathol. J. 2018;34(3):218–235. doi: 10.5423/PPJ.OA.11.2017.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visentin I., Vitali M., Ferrero M., Zhang Y., Ruyter-Spira C., Novak D., Sternad M., Lovisulo C., Schubert A., Cardinale F. Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. New Phytol. 2016;212(4):954–963. doi: 10.1111/nph.14190. [DOI] [PubMed] [Google Scholar]
- Vorholt J.A., Vogel C., Carlstrom C.I., Muller D.B. Establishing causality: Opportunities of synthetic communities for plant microbiome research. Cell Host Microbe. 2017;22:142–155. doi: 10.1016/j.chom.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Wagner E., Luche S., Penna L., Chevallet M., van Dorsselaer A., Leize-Wagner E. A method for detection of overoxidation of cysteines: peroxiredoxins areoxidized in vivo at the active-site cysteine during oxidative stress. Biochem. J. 2002;366(3):777–785. doi: 10.1042/BJ20020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatsuki T. Metal oxidoreduction by microbial cells. J. Ind. Microbiol. 1995;14:169–177. doi: 10.1007/BF01569900. [DOI] [PubMed] [Google Scholar]
- Wallenstein M.D. Managing and manipulating the rhizosphere microbiome for plant health: A systems approach. Rhizosphere. 2017;3:230–232. [Google Scholar]
- Wang D., Lv S., Jiang P., Li Y. Roles, regulation, and agricultural application of plant phosphate transporters. Front. Plant Sci. 2017;8:817. doi: 10.3389/fpls.2017.00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Li H., Zhang M., Song Y., Huang J., Huang H. Carbon dots enhance the nitrogen fixation activity of Azotobacter chroococcum. ACS Appl. Mater. Interf. 2018;10:16308–16314. doi: 10.1021/acsami.8b03758. [DOI] [PubMed] [Google Scholar]
- Wang H.X., Geng Z.L., Zeng Y., Shen Y.M. Enriching plant microbiota for a metagenomic library construction. Environ. Microbiol. 2008;10(10):2684–2691. doi: 10.1111/j.1462-2920.2008.01689.x. [DOI] [PubMed] [Google Scholar]
- Wang X., Wang M., Xie X., Guo S., Zhou Y., Zhang X., Yu N., Wang E. An amplification selection model for quantified rhizosphere microbiota assembly. Sci. Bullet. 2020;65:983–986. doi: 10.1016/j.scib.2020.03.005. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu H., Shen Z., Miao Y., Wang J., Jiang X., Shen Q., Li R. Richness and antagonistic effects co-affect plant growth promotion by synthetic microbial consortia. Appl. Soil Ecol. 2022;170 doi: 10.1016/j.apsoil.2021.104300. [DOI] [Google Scholar]
- Wang Y., Peng S., Hua Q., Qiu C., Wu P., Liu X., Lin X. The long-term effects of using phosphate-solubilizing bacteria and photosynthetic bacteria as biofertilizers on peanut yield and soil bacteria community. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.693535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasai S., Minamisawa K. Plant-associated microbes: From rhizobia to plant microbiomes. Microbes Environ. 2018;33:1–3. doi: 10.1264/jsme2.ME3301rh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessman P., Håkansson S., Leifer K., Rubino S. Formulations for freeze-drying of bacteria and their influence on cell survival. J. Vis. Exp. 2013;78:4058. doi: 10.3791/4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willekens H., Inze´ D., van Montagu M., van Camp W. Catalases in plants. Mol. Breed. 1995;1(3):207–228. [Google Scholar]
- Williams L., Pittman J. Springer; Berlin, Germany: 2010. Cell Biology of Metals and Nutrients, Plant Cell Monographs; pp. 95–117. [Google Scholar]
- Wilson L. Cyanobacteria: a potential nitrogen source in rice fields. Texas Rice. 2006;6:9–10. [Google Scholar]
- Wongdee J., Boonkerd N., Teaumroong N., Tittabutr P., Giraud E. Regulation of nitrogen fixation in Bradyrhizobium sp. strain DOA9 involves two distinct NifA regulatory proteins that are functionally redundant during symbiosis but not during free-living growth. Front. Microbiol. 2018;9:1644. doi: 10.3389/fmicb.2018.01644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Mori N., Yoneyama K., Nomura T., Uchida K., Yoneyama K., Akiyama K. Lotus lactone, a non-canonical strigolactone from Lotus japonicus. Phytochemistry. 2019;157:200–205. doi: 10.1016/j.phytochem.2018.10.034. [DOI] [PubMed] [Google Scholar]
- Xiong J., Lu J., Li X., Qui Q., Chen J., Yan C. Effect of rice (Oryza sativa L.) genotype on yield: Evidence from recruiting spatially consistent rhizosphere microbiome. Soil Biol. Biochem. 2021;161 [Google Scholar]
- Xu J. The structure and function of the global citrus rhizosphere microbiome. Nat. Commun. 2018;9:4894. doi: 10.1038/s41467-018-07343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A.K., Chandra K. Mass production and quality control of microbial inoculants. Proc. Indian Natl. Sci. Acad. 2014;80:483–489. [Google Scholar]
- Yadav J., Verma J.P., Tiwari K.N. Plant growth promoting activities of fungi and their effect on chickpea plant growth. Asian J. Biol. Sci. 2011;4(3):291–299. [Google Scholar]
- Yao Q., Zhu H., Chen J. Growth responses and endogenous IAA and iPAs changes of litchi (Litchi chinensis Sonn.) seedlings induced by arbuscular mycorrhizal fungal inoculation. Sci. Hortic. 2005;105:145–151. [Google Scholar]
- Yasmin S., Zaka A., Imran A., Zahid M.A., Yousaf S., Rasul G. Plant growth promotion and suppression of bacterial leaf blight in rice by inoculated bacteria. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0160688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H.S., Yang J.W., Ryu C.M. ISR meets SAR outside: additive action of the endophyte Bacillus pumilus INR7 and the chemical inducer, benzothiadiazole, on induced resistance against bacterial spot in field-grown pepper. Front. Plant Sci. 2013;4:122. doi: 10.3389/fpls.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi A.A., Barzegar A.R. Effect of Azotobacter and Pseudomonas bacteria inoculation on wheat yield under field condition. Intern. J. Agric. Crop Sci. 2014;7:616. [Google Scholar]
- Yousefi A.A., Khavazi K., Moezi A.A., Rejali F., Nadian H.A. Phosphate solubilizing bacteria and arbuscular mycorrhizal fungi impacts on inorganic phosphorus fractions and wheat growth. World Appl. Sci. J. 2011;15:1310–1318. [Google Scholar]
- Yu C., Fan L., Gao J., Wang M., Wu Q., Tang J., Li Y., Chan J. The platelet-activating factor acetyl hydrolase gene derived from Trichoderma harzianum induces maize resistance to Curvularia lunata through the jasmonic acid signaling pathway. J. Environ. Sci. Health B. 2015;50(10):708–717. doi: 10.1080/03601234.2015.1048104. [DOI] [PubMed] [Google Scholar]
- Zaheer A., Malik A., Sher A., Qaisrani M.M., Mehmood A., Khan S.U., Ashraf M., Mirza Z., Karim S., Rasool M. Isolation, characterization, and effect of phosphate-zinc-solubilizing bacterial strains on chickpea (Cicer arietinum L.) growth. Saudi J. Biol. Sci. 2019;26(5):1061–1067. doi: 10.1016/j.sjbs.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain M., Yasmin S., Hafeez F. Isolation and characterization of plant growth promoting antagonistic bacteria from cotton and sugarcane plants for suppression of phytopathogenic Fusarium species. Iran J. Biotechnol. 2019;17(2):61–70. doi: 10.21859/ijb.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zárate G., Juárez Tomás M.S., Nader-Macias M.E. Effect of some pharmaceutical excipients on the survival of probiotic vaginal lactobacilli. Can. J. Microbiol. 2005;51:483–489. doi: 10.1139/w05-031. [DOI] [PubMed] [Google Scholar]
- Zeffa D.M., Fantin L.H., Koltun A., de Oliveira A., Nunes M., Canteri M.G., Gonçalves L. Effects of plant growth-promoting rhizobacteria on co-inoculation with Bradyrhizobium in soybean crop: a meta-analysis of studies from 1987 to 2018. Peer J. 2020;8:e7905. doi: 10.7717/peerj.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Liu Y., Wang G. Integrated use of maize bran residue for one-step phosphate bio-fertilizer production. Appl. Biochem. Biotechnol. 2018;187:1475–1487. doi: 10.1007/s12010-018-2874-4. [DOI] [PubMed] [Google Scholar]
- Zhang J., Cook J., Nearing J.T., Zhang J., Raudonis R., Glick B.R., Langille M.G.I., Cheng Z. Harnessing the plant microbiome to promote the growth of agricultural crops. Microbiol. Res. 2021;245 doi: 10.1016/j.micres.2020.126690. pp 1–14. [DOI] [PubMed] [Google Scholar]
- Zhou J., Deng B., Zhang Y., Cobb A.B., Zhang Z. Molybdate in rhizobial seed-coat formulations improves the production and nodulation of alfalfa. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C., Oldroyd G.E.D. Plant signaling in symbiosis and immunity. Nature. 2017;543:328–336. doi: 10.1038/nature22009. [DOI] [PubMed] [Google Scholar]
- Zuo Y., Zhang F. Soil and crop management strategies to prevent iron deficiency in crops. Plant Soil. 2011;339(1-2):83–95. [Google Scholar]