Abstract
Background:
Early, chronic, low-level fluoride exposure has been linked to attention-deficit hyperactivity disorder (ADHD) and learning deficits in children. Rodent studies suggest a link between fluoride exposure and internalizing behaviors. No human studies have examined the impact of fluoride on internalizing behaviors during adolescence.
Objective:
To evaluate the relationship between urinary fluoride and early adolescent internalizing symptoms in the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS).
Methods:
Participants in CCAAPS provided non-fasting spot urine samples at age 12 years (n=286). Urine samples were analyzed using a microdiffusion method to determine childhood urinary fluoride (CUF) concentrations and were log transformed for analyses. Caregivers of CCAAPS participants completed the Behavior Assessment System for Children-2 (BASC-2) at the age 12 study visit to assess internalizing symptoms (e.g., anxiety, depression, somatization), and a composite score of the three domains; T-scores ≥ 60 were used to identify adolescents in a clinically “at-risk” range. Race, age of the adolescent, household income, maternal age at birth, caregiver depression, caregiver-child relationships, and age 12-year serum cotinine concentrations were considered covariates in regression models. Sex-specific effects of fluoride exposures were investigated through the inclusion of interaction terms.
Results:
Higher CUF concentrations were significantly associated with increased somatization (β = 3.64, 95% CI 0.49, 6.81) and internalizing composite T-scores in a clinically “at-risk” range (OR=2.9, 95% CI 1.24, 6.9). Compared to females, males with higher CUF concentrations had more internalizing (pinteraction=0.04) and somatization symptoms (pinteraction=0.02) and were nearly seven times more likely to exhibit “at-risk” internalizing symptomology. CUF concentrations were not significantly associated with depression or anxiety symptoms.
Conclusions:
This is the first study to link fluoride exposure and internalizing symptoms, specifically somatization. Somatization represents an interface of physical and psychological health. Continued follow-up will help shed light on the sex-specific relationship between fluoride and mental health and the role of somatization.
Keywords: Fluoride, adolescents, mental health, somatization
Introduction
Internalizing disorders, such as anxiety and depression, are the most common mental health conditions affecting children and adolescents1 and rank in the top 10 for disability-adjusted life years (DALYs) for adolescents.2 These disorders are associated with developmental impairment, increased risk of developing substance abuse disorders, increased suicide attempts, suicides, educational underachievement, and later adult economic disadvantage.3 During pre- and early adolescence major neurostructural and neurofunctional changes occur, and these changes affect brain regions that subserve affective and inhibitory processing and have been linked to internalizing disorders.4 Additionally, neurodevelopmental changes in these regions and networks that underlie internalizing disorders and may increase adolescents’ vulnerability to neurotoxicants.5,6
Fluoride is a mineral that occurs naturally in many foods and water making exposure nearly inexorable. To prevent tooth decay, water fluoridation has been supplied to nearly 75% of United States residents through community water systems7 and is considered one of the top 10 public health achievements. Research suggests that children retain up to 80% of ingested fluoride, while the remaining fluoride is renally excreted. In comparison, adults typically excrete 50% of ingested fluoride.8,9
While there is agreement that water fluoridation has reduced dental caries, there is much debate about the potential neurotoxic effects of fluoride. Fluoride exposure has been associated with impaired cognition,10 attention-deficit hyperactivity disorder (ADHD),11,12 and decreased intelligence (IQ)13–16 among Canadian, Chinese, and Mexican children. However, many of these studies have been criticized for their inability to accurately measure fluoride concentrations, developmental outcomes, and/or control for crucial confounders.17,18 Very little is known about fluoride’s impact on internalizing disorders in humans. However, animal studies suggest fluoride induces anxiety- and depression-like behaviors in mice.19–21
Both animal and human studies suggest fluoride might impact neurodevelopmental outcomes differently in males and females. Male rats exposed to fluoride exhibit more task avoidance, learning deficits, and anxiety-like behaviors compared to their female counterparts.22,23 Similar observations have been reported in human studies with prenatal and childhood exposure to fluoride associated with lower full-scale IQ among males, with one study reporting a 4.49 point reduction in males compared to females.14,24The same sex-specific association (i.e., vulnerability among males) was also observed among children in Mexico where a 0.5 mg/day increase in dietary fluoride was associated with a 3.5 point reduction in IQ among males, but not females.25 It is unclear whether similar sex-specific effects would be observed in studies examining the effects of fluoride on internalizing behaviors.
With these considerations in mind, we sought to examine the associations and sex differences between urinary fluoride and internalizing symptoms (i.e., anxiety, depression, and somatization) among adolescents in Greater Cincinnati. While our analysis is cross-sectional, to our knowledge, it is the first study in the United States and the first to focus on the relationship between fluoride and internalizing symptoms during a vulnerable neurodevelopmental period.
Methods
Study Population
Participants were enrolled in the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS), a previously described prospective birth cohort of children born between 2001 and 2003 in the Greater Cincinnati Region with enrollment eligibility based on the location of the adolescent’s residence from a major roadway.26,27 All participants live in communities supplied by fluorinated water. Enrolled children and caregivers completed demographic and clinical evaluations at ages 1, 2, 3, 4, 7, and 12 years to obtain information regarding the participant’s health and general wellbeing, housing characteristics, residential history, and secondhand smoke (SHS) exposure. The study was approved by the Institutional Review Boards of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center. Participants and parents provided informed assent and consent, respectively.
Urinary Fluoride Measurements
Spot urine samples were collected under normal (non-fasting) conditions at age 12 years during a clinic visit. Samples were stored at −20 C before analysis. Childhood urinary fluoride (CUF) concentrations were analyzed at the Indiana University School of Dentistry. Briefly, urine samples (5 mL) were thawed, and ionic fluoride was diffused from the sample using the hexamethyldisiloxane (HDMS)-diffusion method.28 To correct for dilution in spot urine samples, urinary specific gravity (SG) was measured using a zero-setting calibrated ATAGO® Pen Refractometer under darkened conditions against a standard check (using a fluoride standard traceable to the National Institute of Standards and Technology (NIST)). To account for variations in urine dilution, we adjusted CUF concentrations for SG using the following equation: CUFSG = CUFi × (SGM-1)/(SGi −1), where CUFSG is the SG-adjusted fluoride concentration (in micrograms of fluoride per milliliter), CUFi is the observed fluoride concentration, SGi is the SG of the individual urine sample, and SGM is the median SG for the cohort.29,30 Dilution-corrected CUF was used in analyses.
Internalizing Behaviors Assessment
At the 12-year study visit, parents completed the Behavior Assessment System for Children-2 (BASC-2).31 The BASC-2 is a parent-reported survey used to gain a parent’s perspective on their adolescent’s behavior. Both adaptive and clinical behavior are assessed, and this assessment can be combined with other measures to assess the risk of behavioral problems. Specifically, BASC-2 scores consist of composite and subscale T-scores with a mean (± SD) of 50 ± 10 and were calculated using combined gender norms. All subscale scores were calculated, but anxiety, depression, and somatization subscales along with the internalizing symptoms composite were selected for this analysis a priori because these symptoms have implications for the development of internalizing psychopathology. Higher T-scores represent more reported internalizing symptoms; T-scores ≥ 60 represent potentially clinically relevant symptoms. All assessments were conducted in English; no participants requested assessments in alternate languages.
Statistical Analyses
Descriptive statistics were used to examine the distribution of adolescent, maternal, and household characteristics at the age 12-year visit and potential outliers across exposure and outcome measures. Sex differences were compared using χ2 and T-tests, as appropriate. Means and standard deviations are reported for the continuous variables; frequencies are reported for categorical variables. Outliers (CUF >3 IQR) were removed (n = 5, CUF >4.0 mg/L); CUF values were right-skewed and thus were natural log-transformed to obtain a normal distribution.
Separate unadjusted linear regression models were used to assess the relationship between CUF concentrations and each BASC-2 internalizing outcome (continuous T-scores); adjusted linear regression models were subsequently developed. Covariates included in all models were selected based on prior literature demonstrating their relationship with internalizing outcomes or their potential role as a confounder in the relationship between fluoride and neurobehavior. Covariates included the adolescents’ race (Black/non-Black), age at the 12-year visit, household income at the age 12-year visit, caregiver depression assessed by the Beck Depression Inventory second edition (BDI-2),32 relational frustration of the parent-child relationship (assessed by the Parenting Relationship Questionnaire),33 and age 12-year serum cotinine concentrations.32–39 Given that internalizing symptoms are common in children independent of psychopathology/disease, we conducted secondary logistic regression analyses using a T-score cutoff of ≥ 60 to represent potentially clinically meaningful symptomology. In both primary and secondary analyses, effect modification of CUF concentrations by sex was examined by including an interaction term. Covariates included in the final adjusted models were either significantly (p < 0.05) associated with the outcome, or their inclusion resulted in a greater than 10% change in the CUF parameter estimate. Statistical analyses were performed using SAS® version 9.4.
Results
Characteristics of Participants
Comparisons of demographics, exposure, and other characteristics between CCAAPS participants completing the age 12-year visit (n = 344) and the analytic sample (n = 286) are broken down by sex are provided in Table 1.
Table 1.
Comparison of characteristics in the CCAAPS cohort at the 12-year visit, analytic sample, and by the adolescent’s sex.
| Year 12 Study Visit (n = 344)a |
Analytic Sample (n = 286)a |
Males (n = 161) |
Females (n = 125) |
|
|---|---|---|---|---|
| Characteristic (s) | n (%) or Mean (SD) | n (%) or Mean (SD) | n (%) or Mean (SD) | n (%) or Mean (SD) |
| Sex | ||||
| Male | 191 (55%) | 161 (56%) | 161 (100%) | -- |
| Female | 153 (45%) | 125 (44%) | -- | 125 (100%) |
| Race/Ethnicity b | ||||
| White | 261 (76%) | 219 (77%) | 121 (75%) | 98 (78%) |
| Black/More than One Race | 83 (24%) | 67 (23%) | 40 (25%) | 27 (22%) |
| Age at 12 y visit (in years) | 12.2 (0.79) | 12.1 (0.76) | 12 (0.70) | 12.2 (0.82) |
| Serum Cotinine (age 12 yr.) in ng/ml | 0.32 (1.33) | 0.27 (1.10) | 0.32 (1.3) | 0.20 (0.57) |
| Parent-Reported BASC-2 Outcomes | ||||
| Internalizing Composite | 50.6 (11.1) | 50.1 (10.8) | 49.6 (11.6) | 50.7 (9.6) |
| T-score ≥ 60 (n, %) | 67 (20%) | 56 (19%) | 31 (19%) | 23 (18%) |
| Depression Subscale | 49.9 (10.2) | 49.2 (9.9) | 49 (10.3) | 49.5 (9.5) |
| T-score ≥ 60 (n, %) | 48 (14%) | 37 (13%) | 23 (14%) | 12 (10%) |
| Anxiety Subscale | 52.1 (12.0) | 51.7 (11.6) | 50.1 (11.7) | 53.6 (11.2) |
| T-score ≥ 60 (n, %) | 84 (25%) | 68 (24%) | 30 (19%) | 36 (29%) |
| Somatization Subscale | 49.4 (11.4) | 49.3 (10.0) | 49.9 (12.8) | 48.6 (9.2) |
| T-score ≥ 60 (n, %) | 56 (17%) | 50 (17%) | 34 (21%) | 15 (12%) |
| Caregiver Depression using BDI-2 | 6.4 (4.0) | 6.3 (7.3) | 6.1 (6.6) | 6.6 (8.2) |
| Age at Enrollment in Years | 30.7 (5.9) | 30.2 (5.9) | 30.2 (6.1) | 30.6 (5.6) |
| Relational Frustration T-Score | 48.2 (9.5) | 48.02 (9.2) | 48.1 (8.7) | 47.9 (9.8) |
| Yr 12 Household Income c | ||||
| < $20,000 | 39 (11%) | 30 (10%) | 16 (10%) | 14 (11%) |
| $20,000 to < $40,000 | 41 (12%) | 34 (12%) | 21 (13%) | 13 (10%) |
| $40,000 to < $70,000 | 63 (13%) | 52 (18%) | 29 (18%) | 23 (18%) |
| $70,000 to < $90,000 | 43 (12%) | 39 (14%) | 25 (15%) | 14 (11%) |
| > $90,000 | 140 (41%) | 121 (42%) | 64 (40%) | 57 (46%) |
| CUF at Age 12 y Visit in mg/L (n = 334) | 0.88 (0.36) | 0.88 (0.37) | 0.88 (0.32) | 0.88 (0.39) |
Includes participants with complete exposure, outcome, and covariate data
Reported at study enrollment
Percentages do not add to 100 as some individuals refused to provide income data (n = 16); Differences in means and proportions were tested using T-tests and Chi-square statistics, respectively. The only observed statistical difference was on average females tend to exhibit elevated anxiety T-scores (Chi-square p=0.04); males were more likely to exhibit elevated somatization T-scores (Chi-square p=0.05). Abbreviations: Behavioral Assessment System for Children, second edition (BASC-2), Beck Depression Inventory second edition (BDI-2), childhood urinary fluoride (CUF), standard deviation (SD).
At the 12-year visit, 344 participants were assessed, with 55% (n = 191) being male and 44.5% (n = 153) being female. Participants were on average 12.2 ± 0.79 years old; mothers were on average age 30 years at delivery (30.7 ± 5.9 years). The adolescents were 75.9% (n = 261) white and 24.1% (n = 83) Black or more than one race. There was a diverse range of household income concentrations, with 11.4% (n = 39) earning less than $20,000, 12.0% (n = 41) earning between $20,000 and $40,000, 14.4% (n = 63) earning between $40,000 and $70,000, 12.6% (n = 43) earning between $70,000 and $90,000, and 40.9% (n = 140) earning greater than $90,000.
Of the 286 participants with urine samples and cotinine measurements who were included in the analytic sample, the population was 56.3% male (n = 161) and 43.7% female (n = 125) and had an average age of 12.1 ± 0.76 years old. The average maternal age at delivery was 30.2 ± 5.9 years. This sample was 76.6% (n = 219) white and 23.4% (n = 67) black or biracial. The household income ranged from 10.5% (n = 30) earning less than $20,000, 11.9% (n = 34) earning between $20,000 and $40,000, 18.2% (n = 52) earning between $40,000 and $70,000, 13.6% (n = 39) earning between $70,000 and $90,000, and 42.3% (n = 121) earning greater than $90,000. Descriptive characteristics of the study population did not vary between the adolescents completing the age 12-year visit and those included in the analysis. Further, we did not observe differences by sex with respect to race, age, income, caregiver depressive symptoms (i.e., BDI-2 score), relational frustration scores, or serum cotinine.
CUF concentrations and Internalizing Behaviors
The average CUF concentration was 0.88 ± 0.37 mg/L with CUF concentrations ranging from 0.28 to 2.46 mg/L. Similar concentrations were observed in the analytic sample and did not vary by sex. Average BASC scores were also similar among the full and analytic samples with average internalizing composite scores and subscale scores near 50; females, however, were more likely to have elevated and potentially clinically relevant anxiety T-scores (T-score ≥ 60, p = 0.04) while their male counterparts were more likely to have elevated and potentially clinically relevant somatization T-scores (p = 0.05).
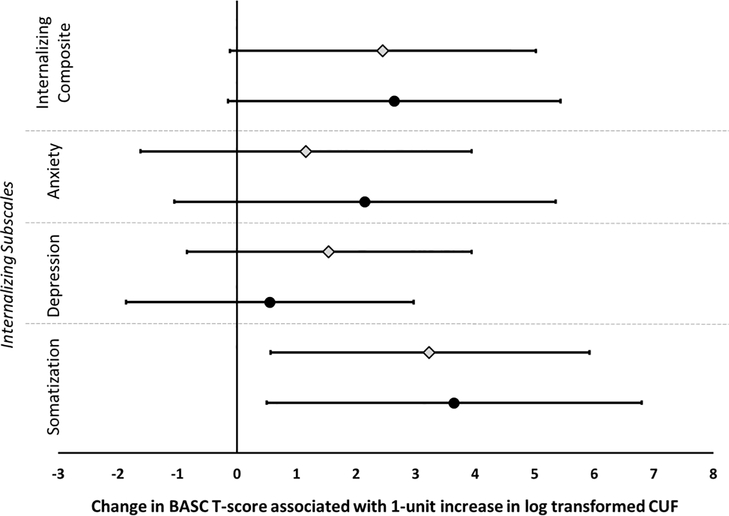
In the primary unadjusted analyses, CUF concentration was significantly associated with parent-reported internalizing symptoms, particularly somatization (Figure 1). After adjustment for covariates, a positive but not statistically significant association was observed between CUF concentrations and increased internalizing behaviors (β = 2.64, 95% CI −0.15 to 5.44, p = 0.06). However, the relationship between CUF and internalizing symptoms was specifically driven by the strong and significant associations between CUF concentrations and increased somatization T-scores (β = 3.64, 95% CI 0.49 to 6.81, p = 0.02) and, to a lesser extent, anxiety (β = 2.15, 95% CI −1.05 to 5.35, p = 0.15); CUF was not associated with depression behaviors (β = 0.55, 95% CI −1.86 to 2.97, p = 0.65). Secondary analyses suggested that adolescents with higher urinary fluoride are more likely to have an internalizing composite T-score in a clinically “at-risk” range (OR 2.9, 95% CI 1.24 to 6.9, p = 0.01) but are not more likely to have elevated “at-risk” subscale scores (p-values range 0.19 to 0.47).
Figure 1. Relationship between CUF concentrations and parent-reported internalizing outcomes.
Analysis is adjusted for adolescents’ race, sex, age, total family income at the age 12-year visit, maternal depression, serum cotinine (yr. 12), and PRQ relational frustration. Unadjusted (diamonds) and adjusted (circles) are provided along with the 95% confidence intervals.
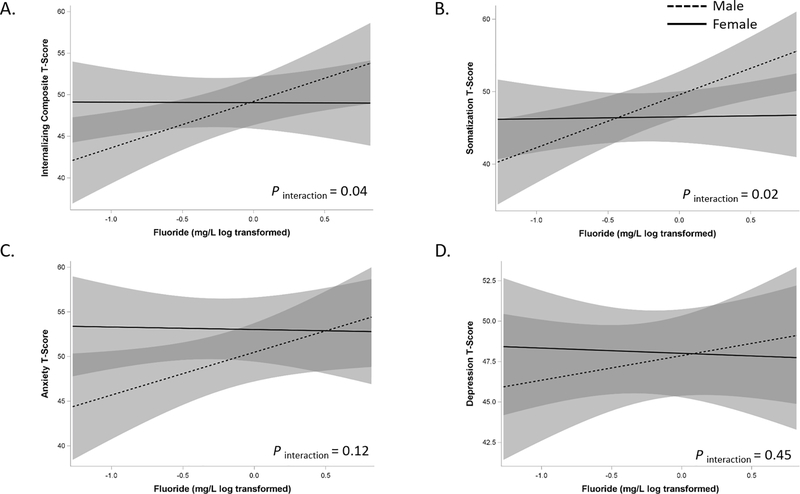
Sex-Specific Effects of CUF on Internalizing Behaviors
Primary analyses utilizing continuous BASC-2 T-scores suggested sex-specific effects. Specifically, significant sex differences were observed in the relationship between CUF and internalizing (Figure 2A, pinteraction = 0.04) and somatization behaviors (Figure 2B, pinteraction = 0.02). Compared to female participants, male participants with higher CUF concentrations exhibited more internalizing and somatization behaviors; similar trends were observed for anxiety and depression subscales, but the associations were not significant (Figure 2C and 2D). Secondary analyses suggested males with higher fluoride have nearly seven times greater odds of having an internalizing composite T-score in a clinically “at-risk” range (OR= 7.9, 95% CI: 2.15 to 29.0) compared to females (OR= 1.13, 95% CI: 0.34 to 3.76) with similar increased odds for elevated “at-risk” somatization T-scores [males (OR=4.18, 95%, CI: 1.27 to 13.69); females (OR= 0.52, 95% CI: 0.13 to 2.06). We did not observe significant sex-specific effects of CUF on “at-risk” scores for anxiety (pinteraction = 0.19) or depression (pinteraction = 0.15), although trends were similar with males being more “at-risk”.
Figure 2. Sex-specific associations between CUF and internalizing (A), somatization (B), anxiety (C), and depression (D) outcomes:
Models are adjusted for the adolescents’ race, age, total family income at the age 12-year visit, maternal depression, serum cotinine (yr. 12), and PRQ relational frustration. Male (dashed line) and female (solid) effects are provided along with the 95% confidence intervals.
Discussion
To date, this is the first epidemiologic study to examine the association between internalizing behavioral and child/adolescent fluoride exposure. Fluoride exposure is widespread across the United States and concerns have been raised about its neurotoxicity. Many studies of fluoride-related neurotoxicity, which have emerged from Canadian and Mexican cohorts, suggest that fluoride is related to decreased cognition,10 (7) ADHD,11 and decreased full-scale IQ.13,14,40 Unfortunately, little is known about the role of fluoride in the development of internalizing pathology. Our findings suggest the effects of fluoride extend beyond adverse cognitive effects and the risk of externalizing symptoms. In our sample, youth with higher childhood urinary fluoride concentrations experienced more somatization and were more likely to have “at-risk” scores on the BASC-2 internalizing composite, particularly among males.
Our findings are consistent with several rodent studies linking fluoride and internalizing-like behaviors. In adult mice given 68 mg/L NaF in water, 120 days of exposure produced depressive and anxiety-like behaviors compared to control mice. Tests conducted to assess internalizing behaviors included an elevated zero maze test, emergence test, light/dark exploration test, novel object recognition test, forced swimming test, and tail suspension test. Additionally, hippocampal messenger ribonucleic acid (mRNA) concentrations were significantly decreased.19 Another study found that 3-week-old female mice given 50 mg/L NaF in water for 39 weeks spent longer periods in the open arms of an elevated plus-maze compared to controls when tested after 23 weeks of treatment. Concentrations of fluoride in the brain were twice as high in treated mice as in control mice; treated mice had significantly higher concentrations of serum serotonin as well.20 Moreover, 4-week-old rats given 5 mg/L and 10 mg/L NaF in water for four weeks had longer latency times in a Morris water maze and longer immobility times in a tail suspension test suggesting impaired spatial memory and increased depression-like behaviors. Rats that were given 10 mg/L NaF in water spent significantly more time in the open arms of an elevated plus-maze test and had longer immobility times in a forced swimming test. These results show altered emotional cognition, indicating increased anxiety and depression behaviors.21
Our findings underscore the need to examine developmental periods beyond those that are typically studied (e.g., prenatal periods) to include adolescence and beyond. During pre- and early adolescence, major neurostructural and neurofunctional changes occur, particularly in regions and structures involved in affective and regulatory brain regions that underlie internalizing pathology.5 The brain is uniquely susceptible to environmental insults during this critical developmental window. The effects of fluoride exposure in adulthood have also been shown in clinical and pre-clinical studies. Multiple animal studies have implicated fluoride in the formation of reactive oxygen species and inflammation. Male adult rats subjected to 10 ppm NaF in water for thirty days showed altered concentrations of stress biomarkers. Rats in the treatment group were found to have elevated malondialdehyde concentrations and reduced superoxide dismutase concentrations in the brain, which signified an increase in oxidative stress. Interestingly, these changes did not occur in rats exposed to 2.1 ppm NaF in water, which matches the guidelines put forth by the World Health Organization regarding appropriate levels in drinking water.41 Another rat study found that chronic fluorosis, induced by 50 ppm NaF in water over six months, significantly increased N-methyl-d-aspartate receptor (GluN1 and GluN2B) expression compared to controls. These receptors are thought to play a role in brain plasticity and synaptic development.42,43 Increased calcium ion concentration in neurons and higher rates of apoptosis in the brain were also noted in rats experiencing fluorosis.44 Increased neuronal apoptosis has been observed in other studies as well, one of which utilized adult rats receiving 60–120 ppm NaF in water for 10 weeks. This study also found microglial activation and increased brain inflammation.45 Researchers have also investigated fluoride-based effects in the uptake of [3H] glucose in the brain. Adult rats given 50 ppm NaF in water have been shown to have increased [3H]glucose uptake in the brain, suggesting increased neuronal activity.46 In adults, fluoride exposure has been associated with adverse outcomes such as increased dementia risk.47,48
We found that the associations between fluoride, somatization, and internalizing symptoms were particularly strong in males, suggesting a sex-specific effect. Interestingly, accumulating data (reviewed by Green et al.49) suggest that fluoride produces sex-specific neurodevelopmental effects. The review concluded that male offspring appeared to be more susceptible to adverse outcomes following prenatal, but not postnatal, fluoride exposure. However, only two of the studies included in the review examined behaviors related to internalizing pathology (i.e., anxiety- and depression-like behaviors), both in rats.23,50 One rat study focusing on learning and memory reported no change in emotional state based on latencies in escape responses. The timing of postnatal exposure ranged from birth to postnatal day 21 (e.g., ~ 6 human years).22 No such studies were conducted in human children, meaning that children and adolescents over ten years old are woefully underrepresented in this field of study. Fluoride exposure during adolescence could represent another critical window given the changing patterns of psychopathology around puberty and a noticeable shift in the prevalence of internalizing disorders. The most notable illustration of this is the emergence of sex differences in depression; while rates of depression for both sexes are similar during adolescence, from adolescence into adulthood women are twice as likely to develop depressive and anxiety disorders.51–53 Given the puberty-related biological changes during adolescence in conjunction with our findings, these data suggest that the adolescent period likely represents a second critical window of fluoride exposure. It should also be noted that by the age of 12 years, the prevalence of physician diagnosed behavioral, anxiety, or learning problems was 28% in the CCAAPS population. Thus, another possibility is early-life exposure to fluoride might increase the risk of other neurodevelopmental disorders that in turn contribute to internalizing symptoms.
There are two major strengths of our study. First, our study focuses on fluoride-related internalizing symptoms in young adolescents, which might represent a vulnerable population; studies to date have examined fluoride-related cognitive and externalizing outcomes primarily focusing on prenatal exposure. Fluoride is both absorbed and eliminated quickly; the half-life of fluoride in the gastrointestinal track and plasma is approximately thirty minutes and 6–10 hours, respectively. Fluoride elimination rates are also thought to be dependent on the dose received and the concentrations of other minerals in the body such as calcium resulting in children retaining higher proportions of absorbed fluoride compared to adults.54 Second, many of the published studies have not controlled for important confounders for which the CCAAPS cohort has comprehensive assessments of including socioeconomic status, parental psychological functioning, as well as assessments of the parent-child relationship. Despite these strengths, some limitations warrant consideration. First, we collected only one spot urine sample in a cross-sectional study design, and thus cannot address temporal associations. Second, the internalizing symptoms represent parent/caregiver reports which may differ from those reported by the adolescent or assessed by a clinician. Third, whether these symptoms represent psychiatric disorders (e.g., major depressive disorder, generalized anxiety disorder) cannot be determined in this sample.
Further, fluoride could contribute directly to somatic symptoms rather than to somatization proper. High-dose fluoride supplementation (30 mg/day) in adults with osteoporosis has been associated with abdominal pain, nausea, and vomiting in addition to atrophic gastritis and gastric epithelial desquamation.55 Additionally, in pediatric patients, ingestions of fluoride produce gastrointestinal symptoms (nausea, vomiting, diarrhea, abdominal pain) in nearly one-third of patients.56 Fourth, this study did not estimate the total fluoride intake of participants which would include assessing sources of fluoride exposure and how the source of exposure relates to internalizing symptomology; urinary fluoride concentrations were the only measurement used to assess fluoride exposure. Lastly, we were unable to examine the effects of prenatal fluoride exposure on internalizing behaviors, which is an established critical window for cognitive effects. Thus, prenatal exposure could also play a role in later life internalizing symptomology.
Conclusions
This is the first study to be conducted on youths living in the United States and the results suggest that childhood urinary fluoride concentrations are significantly associated with increased internalizing symptoms and, in particular, somatization. Despite males and females having comparable urinary fluoride concentrations, males may be at greater risk for adverse effects of fluoride exposure as the association between fluoride concentrations and internalizing symptoms was more robust among males. Due to the ubiquitous exposure to fluoride and the lack of research on the potential neuropsychiatric effects in developing adolescents, more research is needed. These studies should consider sources of fluoride exposure across all developmental windows (prenatal and childhood) as well as explore mechanisms of action. Such studies may inform clinical screening for psychopathology in the most vulnerable individuals and help to determine the best methods of exposure reduction during critical developmental timeframes.
Highlights:
Adolescents with elevated urinary fluoride concentrations exhibit more somatization symptoms.
Males may represent an at-risk population for fluoride-related internalizing behaviors.
While somatization is typically comorbid with anxiety and depression, fluoride concentrations were not associated with increased depressive or anxiety symptoms.
Acknowledgments:
We thank the CCAAPS study participants for their time and contribution to this research.
Funding Source:
Funding for this project was provided by the National Institutes of Environmental Health Sciences (NIEHS) P30 ES006096, R00 ES024116, R01 ES019890, R01 ES11170, and R01 ES027224 and the National Center for Advancing Translational Sciences (NCATS) UL1 TR001425. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations:
- ADHD
attention-deficit hyperactivity disorder
- BASC-2
Behavior Assessment System for Children-2
- BDI-2
Beck Depression Inventory second edition
- CCAAPS
Cincinnati Childhood Allergy and Air Pollution Study
- CDC
Centers for Disease Control and Prevention
- CUF
childhood urinary fluoride
- DALYs
disability-adjusted life years
- EPA
Environmental Protection Agency
- HDMS
hexamethyldisiloxane
- IQ
intelligence quotient
- IQR
interquartile range
- mRNA
messenger ribonucleic acid
- NIH
National Institutes of Health
- NIST
National Institute of Standards and Technology
- PRQ
Parenting Relationship Questionnaire
- SD
standard deviation
- SG
specific gravity
- SHS
secondhand smoke
Footnotes
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Merikangas K, He J, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/J.JAAC.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Husky DMM, Olfson DM, He MJ, Nock DMK, Swanson MSA, Merikangas DKR. Twelve-Month Suicidal Symptoms and Use of Services Among Adolescents: Results From the National Comorbidity Survey. Psychiatr Serv. 2012;63(10):989. doi: 10.1176/APPI.PS.201200058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strawn J, Dominick K, Patino L, Doyle C, Picard L, Phan K. Neurobiology of Pediatric Anxiety Disorders. Curr Behav Neurosci reports. 2014;1(3):154–160. doi: 10.1007/S40473-014-0014-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arain M, Haque M, Johal L, et al. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. 2013;9:449. doi: 10.2147/NDT.S39776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci. 2004;101(21):8174–8179. doi: 10.1073/PNAS.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Data & Statistics | Community Water Fluoridation | Division of Oral Health | CDC. Accessed July 30, 2021. https://www.cdc.gov/fluoridation/statistics/index.htm [Google Scholar]
- 8.Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Published online September 17, 1997. doi: 10.17226/5776 [DOI] [PubMed]
- 9.Council NR. Fluoride in Drinking Water: A Scientific Review of EPA’s Standards. Fluoride Drink Water. Published online March 22, 2006. doi: 10.17226/11571 [DOI] [Google Scholar]
- 10.Bashash M, Thomas D, Hu H, et al. Prenatal fluoride exposure and cognitive outcomes in children at 4 and 6–12 years of age in Mexico. Environ Health Perspect. 2017;125(9). doi: 10.1289/EHP655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malin AJ, Till C. Exposure to fluoridated water and attention deficit hyperactivity disorder prevalence among children and adolescents in the United States: An ecological association Children’s Environmental Health. Environ Heal A Glob Access Sci Source. 2015;14(1):1–10. doi: 10.1186/s12940-015-0003-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bashash M, Marchand M, Hu H, et al. Prenatal fluoride exposure and attention deficit hyperactivity disorder (ADHD) symptoms in children at 6–12 years of age in Mexico City. Environ Int. 2018;121:658–666. doi: 10.1016/j.envint.2018.09.017 [DOI] [PubMed] [Google Scholar]
- 13.Das K, Mondal NK. Dental fluorosis and urinary fluoride concentration as a reflection of fluoride exposure and its impact on IQ level and BMI of children of Laxmisagar, Simlapal Block of Bankura District, W.B., India. Environ Monit Assess 2016 1884. 2016;188(4):1–14. doi: 10.1007/S10661-016-5219-1 [DOI] [PubMed] [Google Scholar]
- 14.Green R, Lanphear B, Hornung R, et al. Association between Maternal Fluoride Exposure during Pregnancy and IQ Scores in Offspring in Canada. JAMA Pediatr. 2019;173(10):940–948. doi: 10.1001/jamapediatrics.2019.1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu X, Chen J, Li Y, et al. Threshold effects of moderately excessive fluoride exposure on children’s health: A potential association between dental fluorosis and loss of excellent intelligence. Environ Int. 2018;118:116–124. doi: 10.1016/j.envint.2018.05.042 [DOI] [PubMed] [Google Scholar]
- 16.Till C, Green R, Flora D, et al. Fluoride exposure from infant formula and child IQ in a Canadian birth cohort. Environ Int. 2020;134. doi: 10.1016/J.ENVINT.2019.105315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabour S, Ghorbani Z. Developmental fluoride neurotoxicity: Clinical importance versus statistical significance. Environ Health Perspect. 2013;121(3):1362–1368. doi: 10.1289/ehp.1206192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett JR. Low prenatal exposures to fluoride: Are there neurotoxic risks for children? Environ Health Perspect. 2017;125(10). doi: 10.1289/EHP2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Zhang J, Niu R, Manthari RK, Yang K, Wang J. Effect of fluoride exposure on anxiety- and depression-like behavior in mouse. Chemosphere. 2019;215:454–460. doi: 10.1016/j.chemosphere.2018.10.070 [DOI] [PubMed] [Google Scholar]
- 20.Lu F, Zhang Y, Trivedi A, et al. Fluoride related changes in behavioral outcomes may relate to increased serotonin. Physiol Behav. 2019;206:76–83. doi: 10.1016/j.physbeh.2019.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu F, Ma J, Zhang H, et al. Fluoride exposure during development affects both cognition and emotion in mice. Physiol Behav. 2014;124:1–7. doi: 10.1016/j.physbeh.2013.10.027 [DOI] [PubMed] [Google Scholar]
- 22.Bera I, Sabatini R, Auteri P, et al. (PDF) Neurofunctional effects of developmental sodium fluoride exposure in rats. Published 2007. Accessed June 2, 2021. https://www.researchgate.net/publication/5965976_Neurofunctional_effects_of_developmental_sodium_fluoride_exposure_in_rats [PubMed]
- 23.Bartos M, Gumilar F, Bras C, et al. Neurobehavioural effects of exposure to fluoride in the earliest stages of rat development. Physiol Behav. 2015;147:205–212. doi: 10.1016/j.physbeh.2015.04.044 [DOI] [PubMed] [Google Scholar]
- 24.Saeed M, Malik RN, Kamal A. Fluorosis and cognitive development among children (6–14 years of age) in the endemic areas of the world: a review and critical analysis. Environ Sci Pollut Res. 2020;27(3):2566–2579. doi: 10.1007/s11356-019-06938-6 [DOI] [PubMed] [Google Scholar]
- 25.Cantoral A, Téllez-Rojo MM, Malin AJ, et al. Dietary fluoride intake during pregnancy and neurodevelopment in toddlers: A prospective study in the progress cohort [published online ahead of print, 2021 Aug 31]. Neurotoxicology. 2021;87:86–93. doi: 10.1016/j.neuro.2021.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeMasters GK, Wilson K, Levin L, et al. HIGH PREVALENCE OF AEROALLERGEN SENSITIZATION AMONG INFANTS OF ATOPIC PARENTS. J Pediatr. 2006;149(4):505. doi: 10.1016/J.JPEDS.2006.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan PH, LeMasters G, Biagini J, et al. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol. 2005;116(2):279–284. doi: 10.1016/J.JACI.2005.05.014 [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Mier EA, Cury JA, Heilman JR, et al. Development of gold standard ion-selective electrode-based methods for fluoride analysis. Caries Res. 2011;45(1):3–12. doi: 10.1159/000321657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas DB, Basu N, Martinez-Mier EA, et al. Urinary and plasma fluoride levels in pregnant women from Mexico City. Environ Res. 2016;150:489–495. doi: 10.1016/j.envres.2016.06.046 [DOI] [PubMed] [Google Scholar]
- 30.Till C, Green R, Grundy JG, et al. Community water fluoridation and urinary fluoride concentrations in a national sample of pregnant women in Canada. Environ Health Perspect. 2018;126(10). doi: 10.1289/EHP3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds CR, Kamphaus RW, Vannest KJ. Behavior Assessment System for Children (BASC). In: Encyclopedia of Clinical Neuropsychology. Springer; New York; 2011:366–371. doi: 10.1007/978-0-387-79948-3_1524 [DOI] [Google Scholar]
- 32.Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck depression inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- 33.Kamphaus R, Reynolds C. Parenting Relationship Questionnaire. San Antonio, TX: Pearson Clin Assess. Published online 2008. [Google Scholar]
- 34.Ashford J, van Lier P, Timmermans M, P C, HM K. Prenatal smoking and internalizing and externalizing problems in children studied from childhood to late adolescence. J Am Acad Child Adolesc Psychiatry. 2008;47(7):779–787. doi: 10.1097/CHI.0B013E318172EEFB [DOI] [PubMed] [Google Scholar]
- 35.Bandiera FC, Arheart KL, Caban-Martinez AJ, et al. Secondhand Smoke Exposure and Depressive Symptoms. Psychosom Med. 2010;72(1):68. doi: 10.1097/PSY.0B013E3181C6C8B5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouchard M, Bellinger DC, Weuve J, et al. Blood lead levels and major depressive disorder, panic disorder, and generalized anxiety disorder in U.S. young adults. Arch Gen Psychiatry. 2009;66(12):1313. doi: 10.1001/ARCHGENPSYCHIATRY.2009.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood J, McLeod B, Sigman M, WC H, BC C. Parenting and childhood anxiety: theory, empirical findings, and future directions. J Child Psychol Psychiatry. 2003;44(1):134–151. doi: 10.1111/1469-7610.00106 [DOI] [PubMed] [Google Scholar]
- 38.Möller E, Nikolić M, Majdandžić M, SM B. Associations between maternal and paternal parenting behaviors, anxiety and its precursors in early childhood: A meta-analysis. Clin Psychol Rev. 2016;45:17–33. doi: 10.1016/J.CPR.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 39.Wen D, Poh J, Ni S, et al. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl Psychiatry. 2017;7(4). doi: 10.1038/TP.2017.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farmus L, Till C, Green R, et al. Critical windows of fluoride neurotoxicity in Canadian children. Environ Res. 2021;200:111315. doi: 10.1016/J.ENVRES.2021.111315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akinrinade ID, Memudu AE, Ogundele OM, Ajetunmobi OI. Interplay of glia activation and oxidative stress formation in fluoride and aluminium exposure. Pathophysiology. 2015;22(1):39–48. doi: 10.1016/J.PATHOPHYS.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 42.Qiu S, Li XY, Zhuo M. Post-translational modification of NMDA receptor GluN2B subunit and its roles in chronic pain and memory. Semin Cell Dev Biol. 2011;22(5):521–529. doi: 10.1016/J.SEMCDB.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 43.Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140(1–2):1–47. doi: 10.1016/S0166-4328(02)00272-3 [DOI] [PubMed] [Google Scholar]
- 44.Wei N, Dong YT, Deng J, et al. Changed expressions of N-methyl-d-aspartate receptors in the brains of rats and primary neurons exposed to high level of fluoride. J Trace Elem Med Biol. 2018;45:31–40. doi: 10.1016/J.JTEMB.2017.09.020 [DOI] [PubMed] [Google Scholar]
- 45.Yan N, Liu Y, Liu S, et al. Fluoride-Induced Neuron Apoptosis and Expressions of Inflammatory Factors by Activating Microglia in Rat Brain. Mol Neurobiol. 2016;53(7):4449–4460. doi: 10.1007/s12035-015-9380-2 [DOI] [PubMed] [Google Scholar]
- 46.Rogalska A, Kuter K, Żelazko A, Głogowska-Gruszka A, Świętochowska E, Nowak P. Fluoride Alteration of [3H]Glucose Uptake in Wistar Rat Brain and Peripheral Tissues. Neurotox Res. 2017;31(3):436. doi: 10.1007/S12640-017-9709-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russ TC, Killin LOJ, Hannah J, Batty GD, Deary IJ, Starr JM. Aluminium and fluoride in drinking water in relation to later dementia risk. Br J Psychiatry. 2020;216(1):29–34. doi: 10.1192/BJP.2018.287 [DOI] [PubMed] [Google Scholar]
- 48.Goschorska M, Baranowska-Bosiacka I, Gutowska I, Metryka E, Skórka-Majewicz M, Chlubek D. Potential Role of Fluoride in the Etiopathogenesis of Alzheimer’s Disease. Int J Mol Sci. 2018;19(12). doi: 10.3390/IJMS19123965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green R, Rubenstein J, Popoli R, R C, C T. Sex-specific neurotoxic effects of early-life exposure to fluoride: A review of the epidemiologic and animal literature. Curr Epidemiol reports. 2020;7(4):263–273. doi: 10.1007/S40471-020-00246-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flace P, Benagiano V, Vermesan D, et al. Effects of developmental fluoride exposure on rat ultrasonic vocalization, acoustic startle reflex and pre-pulse inhibition (European Review for Medical and Pharmacological Sciences (2010) 14, 6 (507–512)). Eur Rev Med Pharmacol Sci. 2010;14(12):1074. [PubMed] [Google Scholar]
- 51.Mendle J, Ferrero J. Detrimental psychological outcomes associated with pubertal timing in adolescent boys. Dev Rev. 2012;32(1):49–66. doi: 10.1016/J.DR.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mendle J, Turkheimer E, Emery R. Detrimental Psychological Outcomes Associated with Early Pubertal Timing in Adolescent Girls. Dev Rev. 2007;27(2):151–171. doi: 10.1016/J.DR.2006.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mendle J Why Puberty Matters for Psychopathology. Published online 2014. doi: 10.1111/cdep.12092 [DOI] [Google Scholar]
- 54.Washington, DC: The National Academies Press. 2006. 10.17226/11571 [DOI] [Google Scholar]
- 55.Das TK, Susheela AK, Gupta IP, Dasarathy S, Tandon RK. Toxic effects of chronic fluoride ingestion on the upper gastrointestinal tract. J Clin Gastroenterol. 1994;18(3):194–199. doi: 10.1097/00004836-199404000-00004 [DOI] [PubMed] [Google Scholar]
- 56.Augenstein WL, Spoerke DG, Kulig KW, et al. Fluoride ingestion in children: a review of 87 cases. Pediatrics. 1991;88(5):907–912. [PubMed] [Google Scholar]