Abstract
With rising global demand for food proteins and significant environmental impact associated with conventional animal agriculture, it is important to develop sustainable alternatives to supplement existing meat production. Since fat is an important contributor to meat flavor, recapitulating this component in meat alternatives such as plant based and cell cultured meats is important. Here, we discuss the topic of cell cultured or tissue engineered fat, growing adipocytes in vitro that could imbue meat alternatives with the complex flavor and aromas of animal meat. We outline potential paths for the large scale production of in vitro cultured fat, including adipogenic precursors during cell proliferation, methods to adipogenically differentiate cells at scale, as well as strategies for converting differentiated adipocytes into 3D cultured fat tissues. We showcase the maturation of knowledge and technology behind cell sourcing and scaled proliferation, while also highlighting that adipogenic differentiation and 3D adipose tissue formation at scale need further research. We also provide some potential solutions for achieving adipose cell differentiation and tissue formation at scale based on contemporary research and the state of the field.
Keywords: Adipose, cell culture, tissue engineering, food, cellular agriculture, cultured fat, cultured meat, cultivated fat, cultivated meat, in vitro meat, in vitro fat, livestock, pig, pork, porcine, cow, beef, bovine, chicken, avian, galline, duck, buffalo, livestock, RNA delivery, omega 3, macroscale, scale up
Introduction
Conventional livestock production has been calculated by the United Nation’s Food and Agriculture Organization to be a significant contributor to human impact on the environment, comprising an estimated 15–18% of anthropogenic greenhouse gas (GHG) emissions [1,2]. For comparison, this is higher than the 14% attributed to GHG emissions from global transportation, although the actual value of livestock GHGs is disputed [3–5]. Currently, animal farming uses at least one-third of arable land and fresh water [1,6,7]. Conventional animal agriculture also generates wastes such as manure, which contributes to groundwater and local ecosystem contamination as well as eutrophication [1,8–11]. Meat consumption is the largest driver of livestock production, and concerns over it extend to human health impacts, playing a role in the growth of antimicrobial resistance and the emergence of pathogenic diseases. The emergence of antimicrobial resistance has been associated with antibiotic use in animal agriculture systems, causing significant health and food safety complications through human contact with contaminated food, water or manure and the horizontal gene transfer of antibiotic resistance genes amongst microbes [12–15]. The emergence and spread of zoonotic diseases have been connected to livestock farming practices, with over half of all human pathogens classified as zoonotic and 73% of emergent pathogens identified as zoonotic [16,17].
As meat production is predicted to increase 1.8-fold by 2050 from 2005/2007 levels, the impact of conventionally produced meats on areas such as disease and the environment are at risk of further increasing [18]. Due to this, the emergence of alternative meats and proteins would be beneficial for supplementing existing production to meet rising demand for food proteins. Alternative proteins should be comparable in taste, texture, and sensory properties in order to satisfy the same consumer demand that drives meat consumption. Ideally, such alternatives should be made with minimal impact to the environment to promote food system sustainability. Plant based meats utilize plant or other non-animal components to mimic animal meat, bypassing the low efficiency feed to food conversion ratios encountered when raising livestock for meat (3% of calories and protein for beef) [19–21]. As an example, Beyond Meat produces plant based meat alternatives on a large scale, including a burger patty calculated to involve 90% less GHG emissions and land use, while requiring less than half the energy and over 99% less water when compared to traditional livestock-derived beef [22,23]. Similarly, the Impossible Burger claims to require 87% less water, 96% less land, 89% less GHGs and 92% less aquatic pollutants than its beef equivalent [24].
Cultured meat (also called in vitro, cultivated, lab grown, cell-based meat) is another alternative to traditional animal agriculture that aims to produce the skeletal muscle and adipose tissues that normally comprise animal meats, except using in vitro tissue and biological engineering techniques [25,26]. By directly growing meat in vitro, energy and nutrients can be more efficiently focused on the outcome – muscle and adipose as opposed to unused components such as the lungs and nerves [25,27,28]. While processes to produce cultured meat are still being scaled up, estimates based on current industry values and projections involve the emission of fewer GHGs and the use of much less land when compared to ruminant meat like beef and lamb. Estimations of energy requirements vary more widely, ranging far below and above that of conventional beef production [29–33]. Compared to the weight of livestock increasing linearly over time, the timeframe to generate cultured meat tissues in vitro is thought to be faster due to the exponential nature of in vitro cell proliferation (cell numbers doubling during each round of proliferation) [34–39]. Cultured meat is estimated to only require several weeks of growth, as opposed to months or years for pork and beef [34,40–42]. The direct use of animal muscle and fat cells in cultured meat might also provide a greater resemblance in taste and nutrition to conventional meat when compared to plant based meats [23,29,43–45]. Moreover, control over cell biology during tissue cultivation, as well as the overall production process, allows for the fine tuning of nutritional properties to improve human health, where muscle and fat cells can be engineered to produce vital nutrients such as anti-oxidative carotenoids that would otherwise not be found (or only at low concentrations) in conventional meat [46]. Taken together, these factors suggest that cultured meat production systems may be able to offer healthier, more efficient, and more environmentally compatible options to traditional animal sourced meats. Furthermore, opportunities to improve food safety at all levels of need also derive from the cultured meat production process.
To date, plant based and cultured meat alternatives have focused on mimicking the muscle component of meat [26,47,48]. However, fat is also a crucial component, contributing to sensory and textural attributes [49]. For example, an increase in fat content improved the juiciness and tenderness ratings of Japanese black steer samples, with maximum evaluation scores attributed to samples containing 36% crude fat [50]. Beef samples with an intramuscular fat content over 10% had significantly higher amounts of lipid-derived flavor volatiles, implying that the adipose content of meat improves flavor as well as texture [51]. The lipid component of meat is also responsible for the complex, undefined, species-specific flavor that is present in meat from different animals, which is not provided by conventional ingredients used in plant based meats or fats. This means that the inclusion of adipocytes may be important for plant based and cultured meats that aim to fully capture the sensory profile of conventional meat [52,53]. For plant based meats, this could mean directly supplementing cultured adipocytes to generate a hybrid plant based meat containing the aforementioned species-specific flavors (e.g., pork flavors). In addition to species specific flavors, the addition of adipocytes to plant meats could generally aid in incorporating an animal “essence”, or general meat specific flavor [52]. Hundreds of volatile compounds comprise the aroma of cooked animal meat, with the majority of them originating from the degradation of lipids upon heating [54,55].
Since fat plays a crucial role in the consumption of animal meat, cultured adipocytes are hypothesized to be key to sensory qualities in cultured and plant based meats. This review contains a guide to the biology and engineering surrounding larger scale adipocyte cell cultures, with consideration for culturing fat for food applications. We provide an evaluation of the cell sources that may be used to produce cultured fat, followed by techniques for proliferating these cells at scale. We also discuss efficient induction of adipogenesis of the cells after large scale proliferation, as well as how cultured adipocytes might ultimately be converted from individual cells into bulk, macroscale fat tissue.
Cell Sources for Scaling Adipocyte Culture
Interest in large scale adipocyte culture has centered on the potential use of human cells in personalized medicine [56]. With the recent emergence of cultured meat products, attention has expanded to include adipocytes from non-human animal sources that can be used to augment cell-based meats. Various cell sources (adipogenic precursor cells) can be utilized for cultured fat, related to proliferative capacity and lipid accumulation. Senescence is an issue that also needs to be considered in the process. Some common cell types used in adipose tissue engineering applications, including pluripotent stem cells (PSCs), can be passaged indefinitely while maintaining some proliferative and adipogenic capacities. Both natural and engineered cells are reviewed here as sources for large scale production of adipose tissue for food. Here, we designate dedifferentiated fat (DFAT), PSCs, mesenchymal stem cells (MSCs), adipose derived stem cells (ADSCs), fibroadipogenic progenitors (FAPs), and preadipocytes as non-engineered cells and highlight their potential as cell sources for large scale adipocyte generation. In the following section, we discuss cells, such as MSCs, that eventually senesce and potentially lose adipogenic capacity. However, primary cells, including MSCs, ADSCs, and preadipocytes (i.e., stromal vascular fraction-isolated cells) often show improved lipid accumulation and differentiation potential compared to most PSCs. We focus on some of the most promising livestock PSC sources and discuss future steps to improve their proliferative and adipogenic capacities. These cells are generally free from genetic manipulation or mutagenesis, but they can also be bioengineered to increase proliferative capacity or prevent senescence (i.e., immortalization) [57]. Bioengineered cells, including induced pluripotent stem cells (iPSCs), transdifferentiated cells, and genetically engineered cells are also considered as potential sources for food outcomes. In this analysis we include non-livestock species that may not be directly applicable to large scale adipocyte culture for food, however that their inclusion remains relevant as a guide for researchers. Non-livestock cells provide validated models that are important tools in developing new approaches relevant for cultured meat and fat. For example, readers may refer to adipogenic genes targeted in murine studies to enhance their own work with livestock adipocytes. Though not typically considered livestock, murine cells ultimately may find use as food for cats, with at least one group currently working to scale up mouse cell production for pet food [58]. A summary of non-engineered cells reviewed in this section is provided in Table 1, while engineered cells are summarized in Table 2.
Table 1.
Summary of non-engineered cell sources for large scale adipose production.
| Cell Type | Species | Main outcome(s) | Reference(s) |
|---|---|---|---|
| DFAT | Porcine | Cells expressed stemness markers CD34, Sca1, CD90, and CD45; cells also expressed mature adipocyte markers Oct3/4, Sox2, c-Myc, and Klf4 | [63] |
| Porcine | Cells remained viable over at least 37 passages | [69] | |
| Porcine | Cells viable over 60 days in culture; expressed adipocyte genes PPARγ, aP2, LPL, and adiponectin | [70] | |
| Bovine | Improved cell growth in plates versus flasks; Wagyu-IM DFAT cells used | [73] | |
| Bovine | Improved adipogenesis with appropriate media formulation, but adipogenesis not as efficient as with porcine cells | [71] | |
| Avian (Chicken) | Incorporation of differential plating techniques (two hour “pre-plate”) helped improve intramuscular chicken DFAT purity | [95] | |
| MSCs/ADSCs/Preadipocytes | Porcine | ADSCs successfully isolated, cultured over 28 passages; positively expressed the stem cell surface markers CD29, CD44, CD71, CD73, CD90, CD105, and CD166, as well as vimentin | [86] |
| Porcine | Bone marrow MSCs experienced decreased proliferation at later passages (>P15), but also exhibited increased adipogenesis | [87] | |
| Porcine | Clonal pig intramuscular preadipocytes were cultured up to 35 passages | [88] | |
| Bovine, Buffalo | Bovine and Buffalo ASCs lasted 15 and 25 passages in culture respectively. | [89] | |
| Bovine | Cells successfully isolated, cultured over 25 passages; expressed high levels of β-integrin, CD44, and CD73 | [90] | |
| Bovine | Clonal cow intramuscular preadipocytes were cultured for over 60 passages | [103] | |
| Bovine | Adipogenic differentiation was effective when media was supplemented with insulin, dexamethasone, isobutylmethylxanthine, octanoate, and 2% Intralipid | [115] | |
| Bovine | MSCs experienced the highest degree of lipid accumulation in serum free media supplemented with ascorbic acid | [92] | |
| Bovine | Bovine preadipocytes co-cultured with mature adipocytes resulted in improved adipogenesis | [93] | |
| Bovine | Isolation methods for bovine preadipocytes exist; additional methods are necessary for future applications | [82] | |
| Avian (Chicken) | ADSCs successfully isolated, cultured over 37 passages; cells expressed CD29, CD31, CD44, CD71 and CD73 | [116] | |
| Avian (Chicken) | Cells isolated from the stromal vascular fraction of excised adipose tissue readily accumulated lipid when treated with adipogenic culture media | [95] | |
| Avian (Duck) | Preadipocytes isolated from different duck breeds exhibited varying rates of lipid accumulation | [96] | |
| Murine | GSH and melatonin prevent senescence, maintain differentiation potential of cells over at least 9 passages | [104] | |
| Human | 3D aggregate culture improved adipogenesis compared to suspension culture; some proliferative capacity maintained | [117] | |
| Human | Immortalization may not be necessary to maintain proliferation and achieve large scale production of certain ADSCs | [118] | |
| Human | ADSCs successfully expanded in animal serum-free media | [119] | |
| Human | Cells expressed CD34 and CD90; large scale in vitro adipogenesis demonstrated; cells secreted adipogenic ECM after 60 days in culture | [85] | |
| Human | Osteogenic genes (RUNX2, SP7, ATF4, BGLAP) more likely to be expressed in DFAT than ADSCs | [120] | |
| Human | Preadipocytes have good adipogenic potential; potential varies based on harvest area | [121] | |
| PSCs | Porcine | Using culture medium containing FGF2 and LIF, and inhibitors of MAPK14, MAPK8, TGFB1, MAP2K1, GSK3A and BMP results in lentiviral-reprogrammed PSCs becoming naïve-state stem cells; cells were viable >45 passages, expressed pPOU5F1 and pSOX2 | [112] |
| Porcine | Transgene insertion-free generation of porcine iPSCs | [122] | |
| Porcine | Serum-free derivation and propagation of pig ESCs | [123] | |
| Bovine | The first report of successful bovine ESC generation and propagation | [124] | |
| Bovine | Feeder free bovine ESCs via replacement using Matrigel or recombinant vitronectin | [110] | |
| Avian (Chicken) | Long term in vitro ESC proliferation (>25 passages) with supporting DF-1 chicken fibroblast feeder cells | [125] | |
| Human | PPARγ2 transfection greatly improved the adipogenic differentiation of iPSCs and ESCs. Cells were first differentiated to MSCs and then to adipocytes | [113] |
Table 2.
Engineered cell sources for large scale adipose production.
| Cell Type | Species | Main outcome(s) | Refs. |
|---|---|---|---|
| Immortalized cells with adipogenic capacity | Avian (chicken) preadipocytes | Expression of telomerase reverse transcriptase (TERT) and/or telomerase RNA enable >100 doublings of preadipocytes, while maintaining adipogenicity. | [126] |
| Murine preadipocytes | Conditional expression of the SV40 T antigen imparts immortality during proliferation, and cessation of SV40 expression allows adipogenic differentiation. | [127] | |
| Human preadipoctyes | Combined expression of TERT with the papillomavirus E7 oncoprotein immortalized preadipocytes while maintaining adipogenicity. | [153] | |
| Avian (chicken) fibroblasts | Adipogenesis with treatment with fatty acids and insulin or all-trans retinoic acid. | [128] | |
| Porcine fibroblasts | Immortalized fibroblasts from peripheral blood. Adipogenesis in media supplemented with dexamethasone, 3-isobutyl-1-methylxanthine, indomethacin, and insulin. | [129] | |
| Bovine fibroblasts | Immortalized bovine embryonic fibroblasts. Ectopic expression of PPARγ2 induces adipogenesis in adipogenic media. | [130] | |
| Murine fibroblasts | Adipogenesis with increased serum concentration in the media. | [133] | |
| Reprogrammed cells from food-relevant species* | Porcine iPSCs | Doxycycline-inducible expression of human Oct4, Sox2, Klf4, c-Myc, Nanog and Lin28 in primary ear fibroblasts result in reprogramming and the ability to differentiate into all three germ layers. At time of publication (2009) showed stability over 41 passages. | [154] |
| Bovine iPSCs | Retroviral insertion of bovine Oct4, Sox2, Klf4, c-Myc, Lin28, and NANOG into embryonic fibroblasts resulted in the ability to differentiate into all three germ layers. At time of publication (2011) showed stability over 16 passages. | [155] | |
| Leporine (Rabbit) | Lentiviral insertion of human Oct3/4, Sox2, Klf4 and c-Myc into liver cells resulted in the ability to differentiate into all three germ layers. At the time of publication (2010) showed stability over 50 passages. | [156] | |
| Ovine | Lentiviral insertion of doxycycline-inducible mouse Oct4, Sox2, Klf4, and c-Myc into fetal fibroblasts resulted in the ability to differentiate into all three germ layers, though this capacity was unstable over more than two passages. | [157] | |
| Avian (Chicken) | Lentiviral insertion of mammalian Oct4, Sox2, Klf4, and c-Myc into embryonic fibroblasts resulted in the ability to differentiate into all three germ layers. At the time of publication (2013), showed stability over 20 passages. | [158] | |
| Piscine (Zebrafish) | Lentiviral insertion of mammalian Oct4, Sox2, Klf4, and c-Myc into embryonic fibroblasts resulted in the ability to differentiate into all three germ layers. Stability over >5 passages was not described. | [158] |
First publication of iPSCs from these species listed here. For more in-depth review, the reader is referred to [159].
Non-Engineered Cells (Primary Cells)
Dedifferentiated fat (DFAT) cells
DFAT cells have emerged as a cell source for engineered fat applications [59–61]. DFAT cells are derived from lipid-containing (mature) adipocytes, which possess the ability to symmetrically or asymmetrically proliferate, replicate, and redifferentiate or transdifferentiate. Though adipocytes were previously thought to be terminally differentiated, their ability to proliferate into large numbers of daughter cells was initially documented using cells from the anterior abdominal walls of human infants and children [62]. The origins and characteristics of DFAT cells have been reviewed elsewhere [59], and more recent studies have elucidated some of the mechanisms by which DFAT cells maintain both proliferation and differentiation capacities [63]. Briefly, DFAT cells are distinguishable from other adipocytes via the expression of the stem cell markers cluster of differentiation (CD)34, Stem cells antigen (Sca)1, CD90, and CD45, as well as octamer binding transcription factor (Oct)3/4, sex-determining region y-box 2 (Sox2), myc proto-oncogene (c-Myc), and Krüppel-like factor 4 (Klf4). Under specific culture conditions, DFAT cells are multipotent; forming adipocytes, chondrocytes [64], osteoblasts [65], skeletal myocytes [66], cardiomyocytes [67], and smooth muscle cells [68].
Porcine DFAT cells maintain proliferation and adipogenicity over at least 37 passages [69]. DFAT cells accumulated more lipid than stromal vascular-isolated preadipocytes, MSCs, and ADSCs, highlighting their enhanced potential for use in large scale cultured fat production [69]. Porcine DFAT cells also grew and accumulated lipids over 60 days in culture, and consistently expressed adipocyte genes peroxisome proliferator-activated receptor gamma (PPARγ), adipocyte protein 2 (aP2), lipoprotein lipase (LPL), and adiponectin [70]. Additionally, these cells maintained the capacity for myogenic differentiation when the culture medium was changed to include galectin-1. Interestingly, flow cytometric analysis showed that the porcine DFAT cells displayed similar cell-surface antigen profiles to MSCs, including markers CD44, CD29, and CD90, suggesting that DFAT cells maintain their high proliferative capacity during long-term culture at least in part by reverting to a stem-like state. The mechanisms by which this reversion takes place are yet to be elucidated, but improving understanding of these cellular processes will be a key factor in scaling up the cell-based production of adipose tissue. Overall, DFAT cells are promising for large scale engineered fat production in terms of both proliferative capacity and adipogenic potential.
Bovine DFAT cells have also been explored as a potential cell source for engineered adipose tissue. Bovine DFAT cells were not as adipogenic or proliferative as porcine cells, possibly due to the culture parameters evaluated [71]. Prior work has optimized and improved adipogenesis conditions for human, murine, and porcine cells, which share commonalities in terms of their biology. There remains a need to develop culture parameters for adipogenesis from bovine cells, and some recent work has focused on addressing this gap. Wagyu-intramuscular fat (IMF) DFAT cells cultured in flat plates versus flasks enabled more facile removal of contaminating cell types, allowing more subpopulations of cells to be adipogenic compared to cells cultured in standard tissue culture flasks [71]. Recent work highlighted new techniques for robust lipid accumulation in bovine DFAT cells through the use of low fetal bovine serum (FBS) media during adipogenic differentiation [72]. Despite the limitations compared to porcine DFAT cells, bovine DFAT cells can be returned to a proliferative state, and recent work has demonstrated simple and optimized culture conditions for improving this process [73]. Taken together, existing work illustrates that improved culture conditions and media formulations should improve the proliferative and adipogenic capacity of bovine DFAT cells to improve yields of both proliferative and differentiating DFAT for scaling adipocyte generation.
Mesenchymal Stem cells (MSCs), Adipose Derived Stem Cells (ADSCs), Fibroadipogenic Progenitors (FAPs) and Preadipocytes
Other cell sources such as MSCs, ADSCs, FAPs and preadipocytes merit continued investigation with similar proliferative and adipogenic capacity to DFAT cells [74]. MSCs are multipotent cells derived from various tissues such as bone marrow, adipose, cord blood, muscle and neural tissue, while ADSCs specifically refer to MSCs derived from adipose [75–77]. FAPs typically refer to intramuscular MSCs, while sometimes also including the general medley of cells (fibroblasts, endothelial cells) within the interstitial space of skeletal muscle [78–80]. Preadipocytes refer to adipose progenitor cells (derived from any cell type) that are committed to adipogenesis, but are still able to proliferate [81]. In this review, we broadly consider preadipocytes, FAPs and MSCs from all tissues as one group, due to similarities in terms of proliferative ability, culture media requirements and most importantly, their ability to differentiate into adipocytes. ADSCs and general preadipocytes also typically share similar isolation protocols, where adipose tissue is broken down mechanically and enzymatically, then subsequently centrifuged to capture its stromal vascular cell fraction [82–84]. Human ADSCs have been shown to generate a complete adipose tissue in vitro, expressing adiponectin and PPARγ while fibroblasts present in the cell population secreted an extracellular matrix (ECM). After 60 days cultured tissues were positive for type I collagen and fibronectin, eventually becoming comparable with adult human adipose tissue after forming vessels and collagenic fibers during long-term culture (120 days) [85].
Various livestock preadipocytes and MSCs have been reported in the literature, with longevity for in vitro culture typically lasting between at least 15 to 37 passages for pig, cow, buffalo, chicken and duck cells [82,86–96]. The maintenance of adipogenicity throughout an isolated cell population’s lifespan is a separate variable though, with reports of adipogenicity waning before the cessation of proliferation [83,97]. Waning adipogenicity of MSCs in particular has been accompanied by an increase in osteogenic differentiation, which could point to factors such as substrate stiffness influencing MSC differentiation [98–101]. Hard surfaces such as tissue culture flasks, well plates and petri dishes have been shown to promote osteogenesis in MSCs [102]. At the same time, there are concurrent reports of an increase in adipogenicity during extended MSC culture, so the exact changes to cell adipogenicity during long term culture may depend on numerous factors such as the cell type, culture conditions and perhaps even batch-to-batch variation [87].
One solution to cell longevity and adipogenicity may be to generate clonal cell lines from isolated MSCs and preadipocytes, where particularly proliferative and adipogenic cells are selected from the bulk population to screen for the best fat precursor cells. Indeed, the longest lasting MSCs or preadipocytes reported in the literature have undergone clonal selection, with porcine intramuscular preadipocyte clones in one study maintaining proliferative and adipogenic ability for at least 35 passages [88]. For bovine cells, a clonal intramuscular preadipocyte line has been allegedly passaged over 60 times while retaining the ability to adipogenically differentiate [103]. While there is a report of uncloned chicken ADSCs lasting for 37 passages, the study reported a subculture ratio of 1:2, which is much lower than what other groups have published (e.g., ~1:7 for the clonal porcine cells). Despite this, it is likely true that the variability in in vitro cell proliferation observed across the range of reported passage numbers could reflect how protocol optimization could play a role in maximizing the longevity of cultured MSCs and preadipocytes. For example, switching from a standard DMEM+10% FBS culture media to a custom formulation (with 5% FBS) extended the proliferation of porcine ADSCs from 34.8 population doublings to 54.9, with no signs of stopping at the end of the 110-day experiment [97]. The addition of glutathione (GSH) and melatonin as antioxidants during ADSC cell culture has also been shown to combat cell senescence while preserving stemness and multidirectional differentiation potential [104]. Recently characterized transcriptional profiles and single cell RNA-seq data may also offer clues for maintaining the proliferative and adipogenic potential of ADSCs [105]. Ultimately, combining multiple approaches for improved MSC and preadipocyte growth (clonal selection, culture media optimization, substrate stiffness) might yield the most favorable results, giving rise to MSCs and preadipocytes that maintain high levels of proliferative capacity and adipogenicity over extended in vitro culture.
Pluripotent Stem Cells (PSCs)
Mouse embryonic stem cells are considered the “original” PSC, and have helped illuminate many of the conditions favorable to adipogenesis [56]. While their role in deciphering potential adipogenic mechanisms has been invaluable, mouse cells are largely not appropriate for uses in regenerative medicine and cultured meat. Instead, several livestock sources of ESCs have been developed in the last decade, and are reviewed comprehensively elsewhere [106].
Promising recent work has demonstrated that bovine stem cell generation is becoming more feasible at larger scales [106–108]. Bovine pluripotent stem cells hold the potential to substantially advance cultured meat by providing a livestock source of cells that can be differentiated into all the necessary components of meat (muscle and fat). Bovine expanded potential stem cells (bEPSCs) were recently established from preimplantation embryos of both wild-type and somatic cell nuclear transfer (SCNT) bovine embryos [109]. bEPSCs expressed high levels of pluripotency genes and were able to differentiate towards adipogenic lineages in vitro, and cells remained viable in their undifferentiated state for at least 50 passages [109]. Additionally, stable bovine embryonic stem cells (bESCs) were recently reported [110]. Combined with recent advances in simplified, effective, culture conditions that no longer rely on mouse embryonic fibroblast feeder cells, bESCs may soon be a reliable cell source for cultured adipose production. Recently, bESC lines were cultured and derived using a base medium consisting of commercially available supplements and only 1% bovine serum albumin (BSA) [110]. The newly derived bESC lines were easy to establish, simple to propagate and stable after long-term culture. Additionally, the bESCs expressed pluripotency markers and remained viable for over 35 passages. bESCs maintained normal karyotype and the ability to differentiate into all 3 germ lineages, suggesting they can be used for adipocyte generation. In addition to media and culture conditions, recently developed differentiation methods, such as forward programming in established livestock PSCs to guide differentiation, can also be integrated into bovine and porcine approaches to establish cell lines for generation of adipose tissue [107]. As more bovine stem cell lines are established, novel methodologies will be adopted and refined to generate cell lines useful for cultured meat.
Porcine stem cells are another promising cell source for cultured meat applications. Though much research has focused on the potential uses of porcine DFAT cells for cultured adipose tissue, several studies have attempted to generate and characterize porcine PSCs. This is partially due to the high applicability of porcine cell and tissue models in human medicine, as well as the constant search for improved gamete generation and breeding schemes of farmed animals [111]. One study found that using culture medium containing basic fibroblast growth factor (bFGF), leukemia inhibitory factor (LIF), inhibitors of mitogen activated protein kinase (MAPK)14, MAPK8, transforming growth factor beta (TGFβ)1, MAP2K1, glycogen synthase kinase (GSK)3A and bone morphogenetic protein (BMP), lentiviral-reprogrammed porcine PSCs were transformed into naïve-state stem cells [112]. The cells were viable for over 45 passages and expressed the pluripotency-related endogenous genes pPOU5F1 and pSOX2. RNA-sequencing (RNAseq) data suggested that the key mechanism was blocking TGFβ1 and BMP signaling. Many compounds can block these pathways, making this finding an important future direction to explore in the generation of adipocytes for cultured fat. It is worth noting that a significant limitation of PSCs is that the cells must be first be differentiated into MSCs, and then adipogenically differentiated into fat [113]. These complex processes take time and result in some off-target effects, such as osteogenic or chondrogenic differentiation [114]. Direct conversion of PSCs to adipocytes or to readily accumulate lipid (i.e., become adipocyte-like cells), would make this process a more viable option. Despite this concern, PSCs remain a promising cell source for large scale cultured fat production.
Conclusions
Ultimately, highly proliferative and adipogenic cells are crucial for large scale cultured fat production. Studies highlighted in this section demonstrate that there are cells capable of proliferating for dozens of passages while remaining adipogenic. Of the various cell types listed, it appears that DFAT cells may be the most promising due to strong adipogenic capacity coupled with the ability to remain proliferative over extended in vitro culture. Clonal MSC-type cells are another good option, and selecting for promising clones from a bulk population of isolated cells brings their proliferative and adipogenic capacity close to that of DFAT cells. It is important to note that strategies such as clonal selection are also applicable and potentially beneficial to DFAT cells [72]. DFAT cells and MSC-type cells are also abundantly available in adipose tissue, minimizing primary cell isolation requirements. While PSCs are known to be very proliferative, their path to adipogenic differentiation is less characterized and more difficult. One limitation in the field is that most cell lines presented here have been characterized in “proof of concept” studies, with few cells proliferated and adipogenically differentiated at scale. While industrial and commercial proliferation of cells for cultured fat can rely on scaling frameworks established for other cells, as the demand for large scale adipose tissue engineering increases, novel approaches will be needed for generating sufficient cell biomass for cultured fat and meat.
Engineered cells for robust expansion and adipogenesis
Along with primary or native cell populations, engineered and adapted cell lines offer significant opportunities for scaling cultured fat production. For instance, immortalized somatic cell lines offer advantages in terms of proliferative capacity and control over cell fate and could therefore improve the dependability and scalability of cultured fat production. Precedent exists for this in food-relevant species, such as chicken, where primary preadipocytes have been immortalized through overexpression of Telomerase Reverse Transcriptase (TERT) alone or in concert with telomerase RNA (TR) to generate immortalized chicken preadipocytes (ICP1) [126]. This work showed that ectopic TERT alone could overcome the population doubling limits of primary preadipocytes, and that co-expression with TR could drastically improve proliferation rates (a ~30% rate increase compared to TERT alone). These preadipocytes maintained adipogenic potential and underwent robust lipid accumulation following long-term expansion. Alternatively, genetic immortalization of murine preadipocytes has been achieved through the conditional expression of the simian virus 40 (SV40) T-antigen during proliferation [127]. Application of these systems to various livestock species could offer promising options for cultured fat production. In addition to immortalized preadipocytes, the immortalization of alternate cell types with subsequent induction of adipogenesis could offer scalable solutions for cultured fat. For instance, adipogenesis in immortalized DF-1 chicken fibroblasts was demonstrated by treatment with fatty acids, insulin, and retinoic acid [128]. Immortalization of bovine and porcine fibroblasts was demonstrated with overexpression of TERT, cyclin-dependent kinase 4 (CDK4) and cyclin D1 [129,130]. Taken together, these results indicate that the immortalization of numerous cell types could help provide scalable solutions for cultured fat production.
In addition to engineering cells to generate immortalized somatic cell lines, spontaneous immortalization of cells can be pursued through routine subculturing or treatment with mutagenic agents such as radiation or chemical mutagens [131]. Under these conditions, some percentage of random mutations will induce an escape from cellular senescence, thereby immortalizing the cells. Indeed, spontaneous immortalization through subculture was the method through which the DF-1 chicken fibroblast cell line was established, as well as the commonly-used mammalian adipogenic fibroblast line—murine 3T3-L1’s [132,133]. While these spontaneously generated cell lines may not be considered engineered, per se, neither are they unaltered, due to the selection for cells that have undergone specific mutations. However, it is possible that spontaneous immortalization could offer regulatory advantages compared with genetically engineered cells, particularly in jurisdictions that disallow any genetic engineering in food production [134]. Similarly, various methods for transiently inhibiting cellular senescence could offer non-genetically modified organism (GMO) opportunities for “immortalizing” cells. For instance, human fibroblasts treated with mRNA encoding TERT showed a 1012-fold increase in total cell number compared with untreated cells, without requiring modifications to the genome [135]. Such transient genetic strategies could overcome regulatory issues facing GMOs, while offering the bioprocess benefits of genetically immortalized cells. Other non-genetic methods can be explored as well. For instance, small molecule inhibition of the PAPD5 polymerase—which halts endogenous TERT activity by triggering degradation of the telomerase RNA component—can recover TERT activity in stem cells, extending proliferative capacity [136]. Additionally, antioxidant treatment has been shown to reduce senescence in adipose-derived stem cells while maintaining adipogenic potential [104].
Along with immortalizing somatic cells, adult cells can be reprogrammed to a stem cell state by the overexpression of Yamanaka factors (Oct3/4, Sox2, Klf4, and c-Myc), which are naturally expressed in high levels in embryonic stem cells [137]. These cells—called induced pluripotent stem cells (iPSCs)—are capable of indefinite expansion and differentiation into a range of cell types, including lipid accumulating adipocytes [138]. iPSCs offer a range of potential advantages for cultured fat production: They are capable of expansion in suspension bioreactors with rapid growth rates (~20 hour doubling times for porcine iPSCs in suspension culture) [139]; they can be obtained through entirely non-invasive means, such as via keratinocytes from hair follicles [140]; they can be generated through transient mRNA delivery, thus potentially avoiding regulatory hurdles facing fully engineered GMOs [141]; and they have been successfully generated for many livestock species, including pigs, cattle, chicken, sheep, and goats, as reviewed extensively by others [106,142–145]. Despite these advantages, iPSCs offer their own challenges for cultured fat production. For instance, unless produced through transient mRNA methods—and potentially even still—iPSCs could face regulatory hurdles as genetically controlled cells, as the specific regulatory status of transgenic, gene-edited, and transiently altered cells for cultured meat is yet to be determined. Additionally, iPSCs often require challenging or complex differentiation processes. For instance, differentiation into lipid accumulating adipocytes can be less robust in iPSCs than ADSCs, and often involves a transition through an MSC state, which can complicate the differentiation process [113,138]. Lastly, iPSC generation from livestock species have to-date offered some unique challenges compared with human or murine iPSCs, such as requiring additional genes (e.g., TERT) to be expressed or relying on continued Yamanaka factor expression, potentially indicating incomplete reprogramming [142,144,146,147]. Despite these challenges, the stated advantages of iPSCs warrant extensive exploration of their use for cultured fat and could allow for highly scalable production systems.
Lastly, along with engineering efforts that are directly related to the immortalization or reprogramming of cells, genetic strategies can be used to engineer cell lines towards optimal properties for large scale fat production. For instance, engineering the previously mentioned immortalized chicken preadipocyte (ICP1) cells to overexpress BMP4 increased cell proliferation, which could improve process dynamics and economics [148]. Additionally, ICP1 cells engineered to overexpress perilipin 1 (PLIN1) or retinoid X receptor α (RXRα) showed improved lipid accumulation and adipogenic differentiation [149,150]. In both iPSCs and fibroblasts, overexpression of PPARγ can be used to improve adipogenic differentiation and lipid accumulation [113,151]. In fibroblasts, commensurate overexpression of the CCAAT/enhancer-binding protein alpha (CEBPα) can further improve adipogenesis [151,152]. Taken together, these results demonstrate how immortalization or reprogramming and subsequent genetic engineering strategies can generate cell lines that are capable of both robust proliferation and differentiation to enable the large scale production of high-quality cultured fat. A summary of engineered cell sources for large scale adipose production is provided in Table 2.
In all these possible cell engineering efforts, consumer and regulatory considerations play an important role in affecting the eventual application of permanently or transiently engineered cells. This could be particularly true for the above approaches, as the development of high-tech foods—which genetically modified cultured meat would certainly be categorized as—can be met with skepticism from consumers [160]. Ultimately, further research is required to determine the impact that such genetic strategies would have on consumer acceptance. From a regulatory standpoint, the impact of cell engineering will likely vary significantly by country. In the United States, two genetically modified animals have to-date been approved for human consumption, and therefore point towards a potential path for regulatory approval of genetically engineered cultured meat products [161,162].
Large scale Cell Proliferation Techniques for Cultured Fat
After an appropriate adipogenic cell source is selected, methods of proliferating the cells to produce sufficient biomass for food applications will be required. Many scalable cell production systems exist from the biotechnology and pharmaceutical spaces and should be applicable to cultured fat cell production for food purposes. However, there may be unique challenges associated with adipocyte culture due to cell buoyancy during lipid accumulation as well as costs associated with process controls and growth factors. Scalable cell proliferation techniques are grouped here into two approaches: Suspension and adherent culture-based strategies.
Suspension Culture
One promising avenue for large scale adipose culture is via suspension culture. Suspension culture is the process of growing single cells or cell aggregates in growth medium with agitation, allowing for proliferation without attachment to a culture flask. Adaptation of adherent cells to suspension culture has been widely explored in the literature, and prior to inoculation in a suspension system cells are generally adapted to serum-free medium [163,164]. Although adherent culture can achieve high cell density (discussed further below), suspension culture is widely used in various biotechnology applications and large scale infrastructure already exists that could be easily transferred to fat-relevant cell types [165–167]. Suspension culture is also favored for scale-up compared with adherent culture because cell growth is limited by cell concentration (or aggregate size) as opposed to surface area. Furthermore, suspension cultures are less labor-intensive, provide a more homogenous culture environment, and require fewer resources (culture flasks, enzymes to passage adherent cultures, et cetera.) [168].
Suspension bioreactor types
Many different bioreactor types have been explored to facilitate suspension cell culture (Figure 1A). The most common suspension bioreactor is a stirred suspension bioreactor (SSB), comprised of a cylindrical culture vessel and mechanism to stir the media to prevent excessive cell aggregation [169]. SSBs are typically characterized by the media replenishment mechanism involved in the process: batch (no replenishment, proliferation until cell harvest), fed-batch (media replacement in batches), or continuous (constant media replacement). Other relevant suspension bioreactors are wave (disposable flexible systems on an undulating surface to gently mix), shake-flask (flasks that are agitated on shaking incubators), and airlift (gas is injected from the bottom of the culture vessel) [170–172]. Cell types relevant to fat culture have been grown in SSBs (bovine ADSCs, avian fibroblasts, porcine iPSCs) and wave bioreactors (avian fibroblasts) [139,169,173]. Computational fluid dynamic models suggest that airlift bioreactors may be the most realistic and efficient production method for large scale cultured meat production (although specific cell types/species were not directly modeled) [174]. While these studies present promising evidence for the production of cultured fat-relevant cell types in suspension, future work will be necessary to optimize bioreactor type, as well as media and culture conditions to ensure efficient, cost-effective, fat culture scale-up depending on the cell type used. Despite the limited literature on livestock adipose suspension culture, a large volume of work has been published using suspension culture to scale-up human and rodent fat-relevant cell types and can serve as a base for the generation of large scale fat production for food applications. Human MSCs, ESCs, and iPSCs have been expanded in SSBs and maintain proliferative potential [175–179]. Notably, human iPSCs and ADSCs retained their ability to differentiate while still in suspension. This could be a promising route to large scale fat production where livestock stem cells are expanded in suspension and adipogenic differentiation is induced after sufficient cell mass has been produced [180]. 3D adipose spheroids generated using preadipocytes and hADSCs in suspension using low attachment tissue culture plates show increased differentiation efficiency and more accurately represent the 3D microenvironment [180,181]. One potential explanation for this is that cell shape has been shown to regulate stem cell fate, and cells that are allowed to remain circular (such as in suspension as opposed to flattening during adherent culture) will favor adipogenesis over osteogenesis [182].
Figure 1.
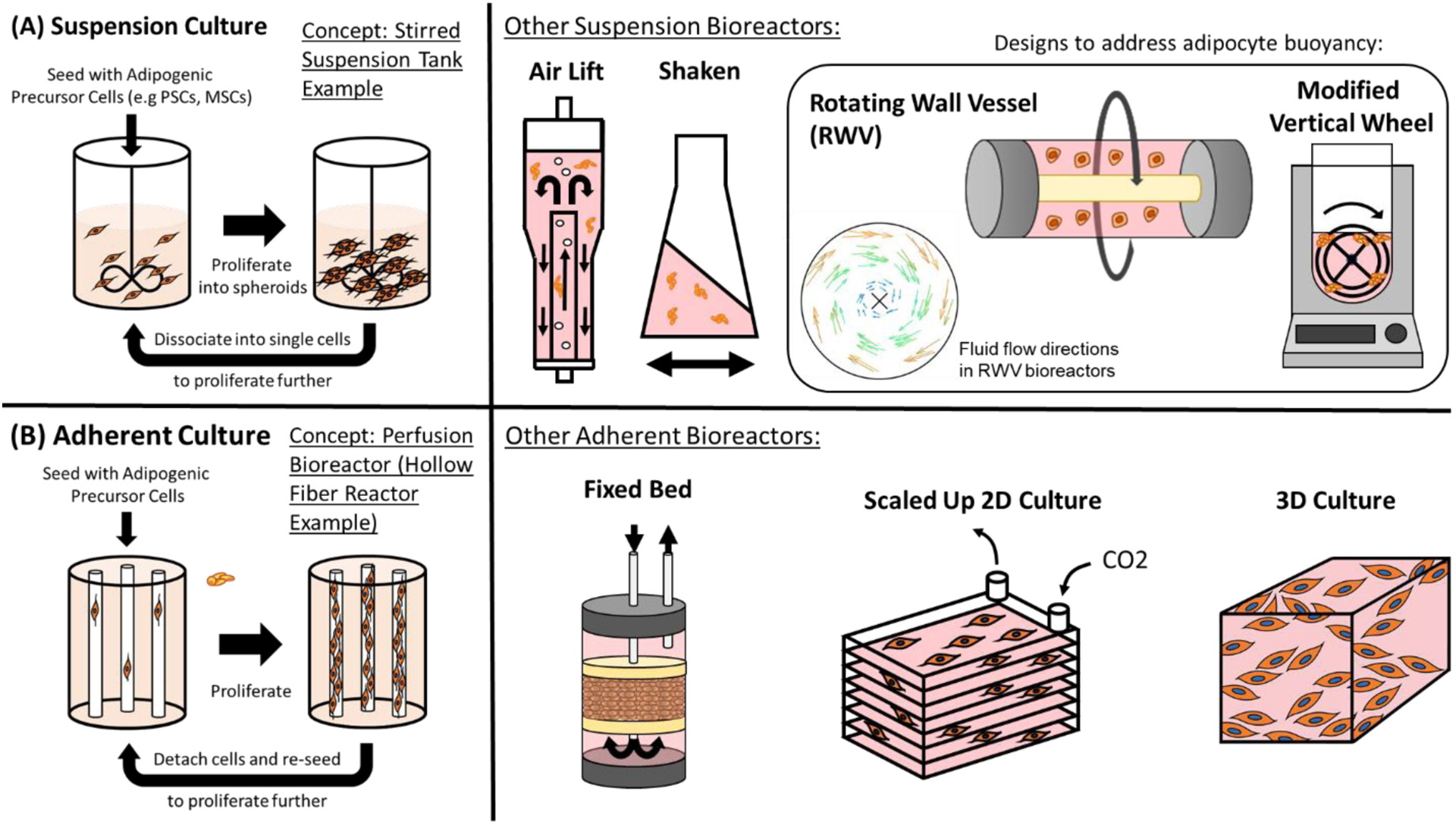
Overview of (A) suspension and (B) adherent culture-based bioreactors for the large scale proliferation of adipogenic precursor cells in order to obtain large numbers of cells for the downstream generation of adipocytes. The fluid velocity tracking diagram (short arrows) for rotating wall vessel bioreactors is reproduced from [197].
Many cell types self-organize into aggregates that proliferate, retain differentiation potential, and more accurately recapitulate a natural 3D environment than single cell suspensions [177]. Culturing cell aggregates in suspension eliminates the complexity of designing edible microcarriers (or removing inedible materials at the end of cell culture), while producing higher cell numbers versus microcarriers since the entire aggregate is comprised of cells. Cell aggregate-based suspension culture can theoretically be applied to many different cell types (iPSCs, MSCs, hADSCs, preadipocytes, etc.) [176,180,181,183]. One important consideration in the suspension culture of aggregates is the formation of a necrotic core when cell density and aggregate size exceeds the limit where nutrients can reach the central cells [184]. While this is essential to consider when designing systems, engineering controls can be enacted to either harvest or passage spheroids into single cell suspensions before they become necrotic. Unfortunately, when considering scaling-up cultured fat production, increased passaging may result in increased process complexity and would likely have to be optimized for specific cell types used. The necrotic core challenge could also be addressed by co-culturing fat-relevant cell types with endothelial cells to transport nutrients throughout the aggregate [185]. For example, a previous study generated vascularized human subcutaneous white adipose tissue spheroids that retained the ability to differentiate into unilocular adipocytes [186].
Overcoming Adipocyte Buoyancy in Suspension
While aggregate cell culture could be a promising route for simple and efficient fat scale-up, the inherent nature of lipid-producing cells will likely present unique challenges. As lipid accumulation increases, cells become buoyant and float to the surface of culture systems. This interferes with nutrient/oxygen flow in the bioreactor and decreases yield [185]. One option would be to simply harvest cells once they become buoyant and allow proliferation of non-lipid loaded cells to continue below, thus allowing for “self-selection” of differentiated fat cells on the culture surface. Another option is to incorporate methods that allow continued culture of buoyant adipocytes in suspension. For example, it may be necessary to use dense microcarriers to counteract adipocyte buoyancy, although this would lower yield compared to cell aggregate-based systems as mentioned.
Perhaps the most promising avenue to overcome buoyancy issues would be to adopt specialty suspension bioreactors for fat accumulation. Rotating wall vessel bioreactors (RWVBs) initially developed by NASA to simulate a microgravity environment produce rotational forces that draw cells towards the center of the bioreactor chamber, and may thus work for cells that sink downwards and float upwards (see “Rotating Wall Vessel” in Figure 1A) [187]. RWVBs comprise a rotating cylindrical culture vessel surrounded by a membrane to allow oxygenation are favored for cell types that are sensitive to shear stress [188,189]. In contrast to SSBs, RWVBs do not require an internal mixing device and instead are completely filled with culture medium, while cells and nutrients are mixed by gentle rotation of the entire vessel. Two types of RWVBs exist: High Aspect Ratio Vessels (HARV, developed by NASA to stimulate a microgravity environment) and Slow-Turning Lateral Vessels (STLVs) [190]. HARV bioreactors have been used to culture lipid-containing 3T3-L1 aggregates and lead to increased adipogenesis in MSCs [191,192]. Hollow fiber membranes can also be added to RWVBs to enhance efficiency/cell health [193]. Alternatively, a system designed to draw floating adipose cells back into the media (e.g., a modified Vertical Wheel Bioreactor) could enable efficient culture of buoyant cells [183,194]. Another potentially relevant bioreactor type is the random positioning machine (RPM) bioreactor, which rotates a culture vessel over multiple axes to ultimately create a microgravity environment. RPMs require a large footprint relative to RWVBs however, and may be more difficult to scale up [195,196].
Adherent Culture
Using adherent cell culture strategies is another approach towards upscaling adipocyte cultures (Figure 1B). While suspension culture offers some advantages by foregoing the need to fabricate and utilize scaffolds, there are potential advantages to scaling the growth of adipocytes using adherent cell culture strategies. Given that ADSCs and adipocytes are sensitive to mechanical cues, one major advantage of utilizing adherent culture strategies is the potential to increase culture efficiency by stimulating cells using mechanical cues. For example, adult ADSCs undergo adipogenesis by simply tuning the substrate stiffness to physiologically relevant levels of adipose tissue (~2 kPa) [198]. In addition, adipocytes differentiated on more compliant substrates exhibited higher levels of lipid accumulation when compared to fat cells grown on less deformable substrates [199]. There is also potential to utilize cell adherence onto substrates to apply mechanical cues to stimulate adipogenesis in stem cells. Thus, the use of adherent cell culture strategies would allow for the tuning of mechanical environments, which could be harnessed to improve the growth of adipocyte precursors and lipid accumulation of differentiated adipocytes.
Additionally, while the occurrence of cell buoyancy upon adipocyte differentiation presents challenges during suspension culture upscale, one benefit of growing adipocytes in adherent bioreactors would be to prevent this occurrence by either having cells attached to an immobile 2D surface. Culture surfaces could be treated further to maintain cell attachment, for example with the use of elastin-like peptides [200]. On the other hand, cell buoyancy could be harnessed as a potential advantage in both suspension culture and adherent culture to improve the efficiency of cell harvesting by selecting for differentiated adipocytes which lift off, thus freeing up surface area for proliferating cells.
Next, we will discuss current technological approaches towards upscaling adherent cell cultures with specific examples highlighted towards progress with adipocyte-relevant cell types. For a more extensive review on general approaches towards upscaling adherent cell cultures, we refer the reader to a recent review [201].
Adherent Bioreactor Types
Given that cell culture techniques are best characterized and established in 2D, one approach is to iterate and improve upon the efficiency of conventional 2D culture techniques for adipocyte culture upscale. For example, 2D multilayered flasks offer a scale up approach for the growth of adherent cells. While the use of multilayered flasks is not feasible with static media at a larger scale, one study found that the incorporation of active gas ventilation resulted in >95% cell viability of hiPSCs cultured within 10 layers with a total surface area of 6320 cm2 [202]. Additionally, a 44% increase in cell proliferation of hiPSCs was observed when compared to cells grown in static media [202]. Using the same culture system, gas transport could be further enhanced with oxygen-permeable layers, similar to culture vessels which are commercially available such as Corning®’s HYPERflask or HYPERstack. While there are some improvements towards upscaling 2D culture methods, the approach remains limiting for upscale and additional innovations would be necessary to make 2D culturing a viable approach. Specifically, it will be important to address the technical challenge of increasing the amount of surface area for cell attachment while achieving proper nutrient and oxygen mixing as culture vessels increase in volume.
The utilization of microcarriers offers the advantages of adherent cell culture techniques with the benefit of growing cells in suspension to increase scalability, providing a promising approach towards upscaling adipocyte cell culture [203]. Microcarrier beads are widely used to increase surface area for adherent cell attachment and to increase yield efficiency in cells that are unable to form spheroids or aggregates in suspension [204]. It is important to note that the generation of microcarrier scaffolds adds an additional processing step which may add to overall production costs. However, if mechanical cues could substitute for expensive growth factors, and similarly stimulate important cell growth and differentiation pathways, the added cost of scaffold fabrication could potentially eliminate other production costs. Additionally, the choice of scaffold material will be important to consider for not only manufacturing costs, but also production logistics and the resulting adipose tissue properties. For example, additional complexities to the cell harvesting process can occur if synthetic carriers are used given that production facilities must separate cells from the carriers. Alternatively, microcarriers could be edible and engineered to match the mechanical properties of adipose tissue to increase cell differentiation efficiency [199]. However, it will be important to consider the effects of integrating scaffolds on the taste, texture, and nutritional profiles of the final adipose tissue product.
Another approach towards upscaling adherent culture of adipocytes is through the use of perfusion bioreactors, where media is passed through adhered cell cultures, such as hollow fiber bioreactors (HFBs). HFBs can be designed to have multiple oxygen and nutrient permeable tubing running in parallel, which provides a substrate for adherent cells to grow on. Using HFBs, researchers have demonstrated its ability to support scalable culture with well-characterized proliferation and differentiation of MSCs, ADSCs, and iPSCs [35,205–208]. Using a similar perfusion approach, fixed-bed bioreactors (FBBs) can be utilized. Here, cells are immobilized within macroporous carriers, packed within a cylindrical bioreactor, and perfused with media. The approach provides a higher surface area to volume ratio, and thus, less cell passaging is required [209,210]. It also provides a low shear stress environment for mammalian cells. Importantly, its ability to support the proliferation of adipocyte precursors, such as MSCs, has been demonstrated at laboratory scale [211]. Further innovations among perfusion-type bioreactors are underway, for example, with the recent development of an interchangeable production line system which incorporates feeder cells for the supplementation of growth factors and allows for adjustments in media circulation flow. Thus, media can flow to deliver essential growth factors to adherent cells and be reversed for other essential processes such as for media recycling. Though the process is currently geared towards muscle cell proliferation, the approach could be tuned towards adipocyte cell culture provided that the feeder cells are producing the appropriate growth factors needed [212].
Although there is strong potential to scale-up adipocyte cultures using scaffold suspensions or perfusion bioreactors, nutrient and oxygen mixing is key and becomes more challenging as these systems are scaled [209]. Additionally, there are concerns over the use of expensive enzymes for cell passaging and harvesting when growing cells under adherent culture conditions. To address this challenge, innovative approaches are being developed which are designed to have cells proliferate and self-detach via cleavable peptides [213]. This approach would allow for additional cells to grow on newly unoccupied substrates as well as increase the ease of cell harvesting, while reducing costs. It is important to note that this approach is not limited to 2D culture and could be applied to 3D culture techniques as well. Lastly, the potential effects on adipocyte precursor proliferation and differentiation when culturing in 2D must be considered given that studies have shown differences in cell behavior and gene expression when compared to 3D cultures [200,214,215]. For example, primary mouse adipocyte exhibit phenotypes similar to those seen in vivo—such as the presence of unilocular lipid droplets—when grown in 3D compared to 2D [216]. Thus, methods for upscaling adipocytes in 3D adherent cultures should be considered.
To replicate 3D environments similar to native adipose tissue, one approach to consider is the encapsulation of adipocytes within hydrogels. Though there has been extensive research regarding hydrogel encapsulation for tissue engineering applications, more recent innovations in biomaterial development have improved culturing efficiency. For example, the culture of cells using the thermoreversible Mebiol Gel which has been shown to support the growth of high cell densities (~20 million cells/mL using hMSCs), provides increased ease in cell harvesting [217]. Importantly, encapsulation of adipocyte precursors within hydrogels would protect cultures from high shear stress in the presence of tank mixing or fluid flow in a perfusion bioreactor.
Another option is the culture of adipocytes within porous scaffolds in a bioreactor [218]. However, similar to cell aggregates in suspension, nutrient and oxygen diffusion limitations exist using a top-down approach in 3D tissue engineering. Diffusion rates are dependent on multiple factors such as scaffold porosity, nutrient molecule size, and media viscosity, which can limit 3D cultures to a range of tissue thickness from ~100 μm − 1 mm when cultured in static media and in the absence of vasculature [219–221]. As a result, there is potential to use a modular, or bottom-up approach in which smaller microtissues can be cultured as building blocks for larger 3D structures [222]. For example, this can be achieved using biodegradable microcarriers which assists in the formation of adipocyte cell aggregates in suspension and does not remain in the final tissue culture product. However, in this scenario, it will be important to use food-grade scaffold materials for cultured meat applications [223]. Another approach towards engineering adherent adipocyte spheroids is through surface chemistry treatments on 2D substrates. For example, the introduction of surface charge induces spheroid formation while surface conjugation with appropriate peptides results in consistent cell attachment to the substrate [200]. Using this approach provides the benefits of growing adipocyte precursors in 3D, while avoiding issues of adipocytes lifting off during differentiation.
Overall, upscaling adipose tissue using adherent culture methods offers potential to overcome current hurdles with adipocyte cell culture such as increasing the efficiency of preadipocyte proliferation and adipocyte differentiation via mechanical stimuli, reducing shear stress on cells, and providing methods towards engineering 3D adipose tissue for structured cuts of meat. However, suspension culture certainly presents advantages towards adipocyte cell culture upscale. For example, foregoing the need to produce scaffolds could significantly reduce production costs. Importantly, the most efficient method will likely vary according to cell type and target product type. Therefore, it will be important to continue research to determine which culture method is most efficient for adipocyte culture upscale according to each cell type and application.
Culture Media Considerations
Since one goal of plant based and cultured meat is to supplement conventional animal agriculture, there is intense effort in developing culture media formulations that do not depend on animal products such as serum from blood. For the cultivation of cultured fat cells, three types of culture media formulations are usually considered (Figure 2A): A proliferation medium that supports and promotes the expansion of adipogenic precursor cells to achieve a desired amount of biomass, followed by an adipogenic induction medium (also called differentiation medium) that initiates adipogenesis within precursor cells. Adipogenic differentiation is typically accompanied by cell cycle arrest, where cell division no longer takes place [224]. Once the adipogenic program has been sufficiently activated, cultured cells are often switched to a pared down lipid accumulation medium (also called maintenance medium) containing fewer adipogenic compounds at concentrations sufficient to maintain adipogenic gene expression [225].
Figure 2.
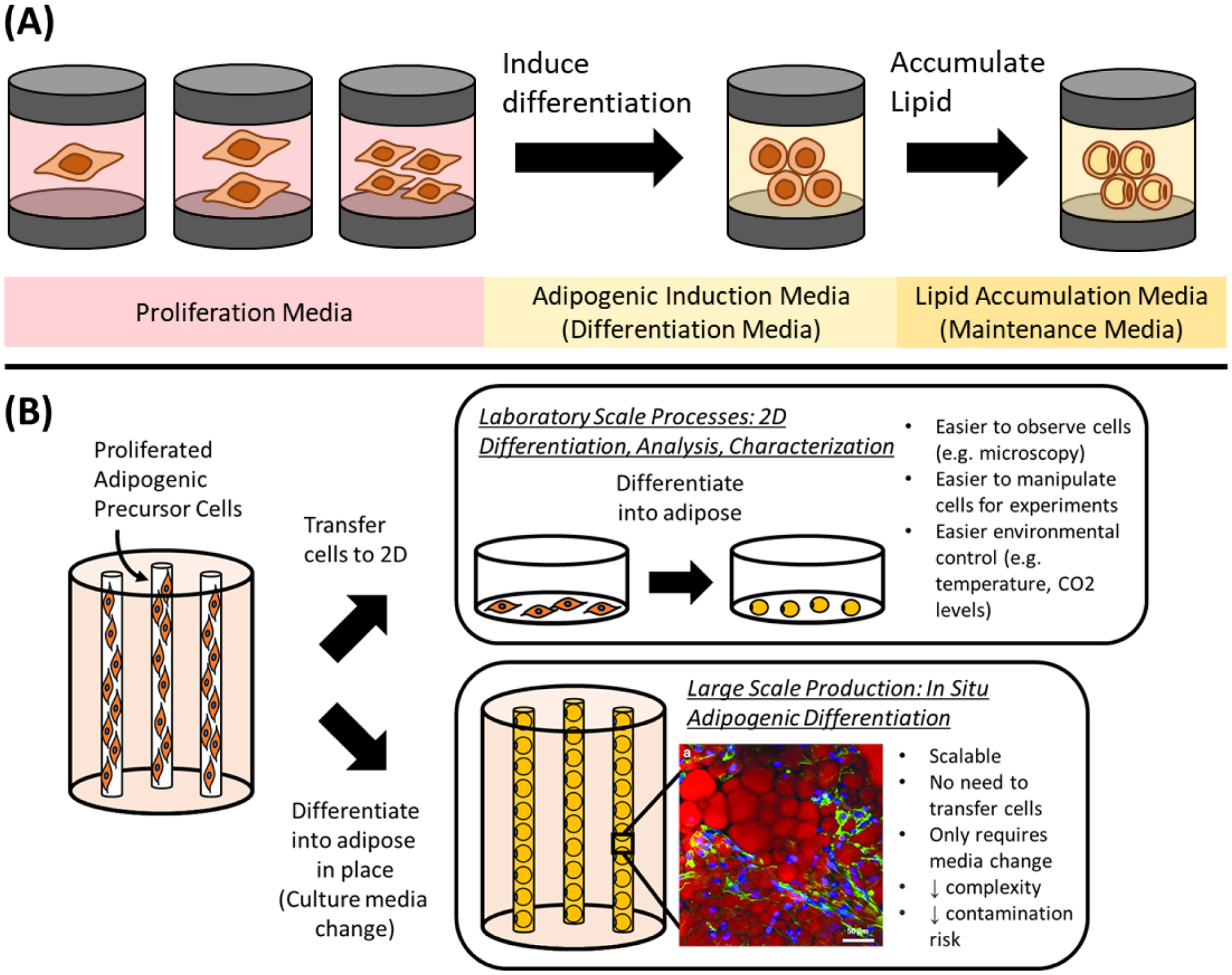
(A) Biological sequence of events for adipogenic differentiation, where proliferated adipose precursor cells are differentiated via an adipogenic induction medium (differentiation medium), followed by a switch to a medium designed to foster subsequent lipid accumulation (maintenance medium) after the cultured cells become primed for adipogenesis. (B) In situ adipogenic differentiation using a hollow fiber bioreactor as an example - leveraging the same proliferation bioreactor environment to enable the differentiation of adipogenic precursor cells by merely changing the culture media. This is juxtaposed with the conventional practice of taking cells out of the proliferation bioreactor and differentiating them on 2D cell culture substrates. Inlay of hollow fiber differentiated adipose from [259] stained with AdipoRed, scale bar is 50 μm.
For proliferation media, numerous serum free formulations have been devised for the adipogenesis-competent cells that we have covered in this review, albeit often still comprising of animal components such as growth factors, extracellular matrix components and other signaling molecules [173,175,176,226–232]. Individual animal components are often produced recombinantly, but the question then shifts to cost and commercial viability. Low cost recombinant protein production may be feasible when performed at scale though; and simple serum free formulations only requiring a few recombinant factors exist for certain cell types such as MSCs [227,233–235]. Other approaches that may avoid the use of recombinant growth factors exist in the form of purported serum replacements such as algae, yeast, mushroom, numerous plant extracts, as well as various plant protein hydrolysates [236–240]. Synthetic peptides also show promise as a way of mimicking full growth factors such as FGF2 at a lower cost [241]. If desired, even animal products such as pork plasma could be marshalled to supplement culture media as a way to valorize waste and by-products from the meat industry [242].
For adipogenic differentiation and maintenance media, there have been numerous reports across species of serum free adipogenesis, as well as some cases documenting how the exclusion of serum can even lead to improved adipocyte lipid accumulation and gene expression [72,91,92,231,232,243,244]. As was the case for proliferation media, there have been reports of plant based culture media components promoting adipogenic differentiation in cultured cells such as MSCs [238]. It is important to note though, that adipogenesis media formulations may not be readily interchangeable across species due to differences in adipose biology between livestock species [71,245–247]. For example, porcine and avian adipocytes use glucose as the primary building block for fatty acid synthesis, while bovine and ovine adipocytes use acetate [247]. These differences may relate to how optimal adipogenic differentiation media for adipocytes of varying species can differ considerably: Ovine cells have been shown to require a prolonged period of adipogenic induction (via the inclusion of a PPARγ agonist in their differentiation culture media) in order to accumulate intracellular lipid, whereas murine cells show no such requirement and begin accumulating lipids much more readily with fewer adipogenic media factors [248,249]. For other cell types, there has been success with the adipogenic differentiation of human, mouse and porcine DFAT cells, but similar differentiation media formulations do not as robustly induce adipogenesis in bovine DFAT cells [71]. The optimization of adipogenesis in less elucidated species such as cows is taking place over time though, with insights emerging on alternative adipogenic factors that are able to induce lipid accumulation. For example, addition of the growth factor BMP4 during the proliferation stage of bovine skeletal muscle MSCs has been shown to improve adipogenesis, while small molecules such as certain retinoids and carotenoids have been shown to perform better than rosiglitazone (PPARγ agonist) in bovine preadipocytes [76,250]. Very recently, robust adipogenesis has been demonstrated in bovine DFAT cells using adipogenic media containing 0.25% and 2.5% FBS, as opposed to the standard 10% FBS [72]. The same DFAT cells treated with was also found to exhibit maximal glycerol-3-phosphate dehydrogenase (GPDH) activity when treated with 1 mM acetate.
During the large scale production of cultured meat, culture media is one of the largest contributors to cost [42,251]. As such, media recycling may be an important consideration for boosting efficiency and extending culture media utility. The buildup of metabolic waste products is often the limiting factor that requires culture media changes in conventional cell culture, often limiting cell growth as other nutrients and growth factors remain present at sufficient amounts. In a smaller scale (100 ml working volume) suspension bioreactor that refined culture media via dialysis, media recycling with only 1 media change permitted a culture of iPSCs to proliferate at the same rate as a conventional culture with 5 media changes [252]. In this system, saving unused growth factors and nutrients led to a 2 to 3-fold reduction of the amount of TGF-β1, insulin, transferrin, ascorbic acid and selenium required during cell proliferation. It is important to note though, that while this example of media recycling offers great cost reduction potential (because growth factors are the largest component of culture media cost), dialysis is known to be water intensive. In this example, the 2 to 3-fold reduction of certain media components required a 4 to 5-fold increase in water usage due to the need for dialysis media [251]. Alternative methods of recycling culture media or extending its use are thus worth investigating. For example, extending culture media has also been explored through the co-culture of mammalian cells with algae, with decreased lactic acid and ammonia levels observed in co-culture groups when compared to mono-culture controls. It was hypothesized that algae-produced oxygen promoted aerobic respiration, while ammonia was metabolized by co-cultured algae to produce amino acids [253,254].
Metabolic and genetic engineering approaches are additional options for reducing culture media costs. Glutamine synthetase overexpression is an option for reducing glutamine requirements in culture media, while also providing a method of reducing NH3 levels as it is an substrate of the enzyme [255]. Chinese hamster ovary (CHO) cells engineered to overexpress enzymes of the phenylalanine-to-tyrosine biosynthesis pathway gained the ability to grow in culture media devoid of tyrosine, while also producing fewer growth inhibitory compounds associated with bottlenecks during tyrosine synthesis [256]. In the same study, improved proliferation was observed in CHO cells treated with CRISPR to knock out the enzyme branched chain aminotransferase 1, which halted the production of various compounds that were inhibitory to cell growth. For more information and examples of cell optimizations for improved growth and viability, we refer the reader to [257].
Several key challenges lay in the way of effective culture media development for cultured fat. However, various paths exist that may help overcome these obstacles. The inclusion of recombinant proteins in serum free media formulations for adipocyte precursor cell growth represents a pain point in cost, but solutions may exist with the use of small molecules or peptides that can mimic full growth factor proteins. The emergence of alternate adipogenic differentiation media formulations for difficult cases such as bovine adipogenic precursor cells bodes well for achieving robust lipid accumulation in the future. Culture media requirements may also be fundamentally lowered through the rational engineering of cultured cells to limit the production of inhibitory metabolites and reduce exogenous growth factor requirements. Media recycling may also be an important component in extending a single batch of culture media, because expended media often contain unused nutrients and growth factors.
Methods to Induce Adipogenic Differentiation and Lipid Accumulation at Scale
In previous sections we have outlined studies on the scaled proliferation of adipogenic precursor cells such as MSCs and PSCs. Many groups have demonstrated the feasibility of using stirred suspension tanks or hollow fiber bioreactors for large scale cell production. However, in these studies cells are often adipogenically differentiated using traditional 2D culture techniques [139,176,177,258]. During large scale processes, this would lead to a bottleneck in production as transferring large numbers of cells to well plates and petri dishes for differentiation would not be tenable. Thus, scalable methods of differentiating proliferated adipose precursor cells are needed.
In situ Adipogenesis
One method to induce adipogenesis at scale would be to perform an in situ differentiation, where cells proliferated in a bioreactor are differentiated in place via a change in culture media or through the addition of adipogenesis-inducing factors (Figure 2B). This has the advantage of eliminating cell transfer from proliferation bioreactors to a separate bioreactor or apparatus for differentiation, enabling reduced complexity, cost and risk of contamination. For hollow fiber bioreactors, such an in situ adipogenesis approach has been reported using polyethersulfone (PES) fibers, where cells were expanded for 2–3 weeks and then differentiated via a change from proliferation to adipogenic media [259,260]. After 2 weeks of differentiation, cultured MSCs on the hollow fibers had differentiated better than 2D cultured adipocytes. For food applications, it may be possible to detach PES hollow fiber-grown adipocytes (e.g., proteolytically with enzymes such as TrypLE, Accutase and TrypZean) for further downstream processing [206,261–263]. Alternatively, edible hollow fibers could be used for process simplicity and to avoid obstacles such as hollow fiber clogging during cell detachment, while also preserving adipocyte extracellular matrix (ECM) components that would typically be degraded when using proteolytic enzyme-based cell detachment approaches [264,265].
For suspension cultures, in situ adipogenic differentiation was demonstrated using stirred suspension tank and rotating wall vessel bioreactors, where adipogenically differentiated MSC spheroids (via an addition of adipogenic factors to the media) accumulated more lipid when compared to 2D controls [192]. ESC spheroids have also been adipogenically differentiated, but only in static suspension cultures [266]. While promising, these studies on MSCs and ESCs were performed using preformed spheroids, which in terms of cell expansion is less efficient and more complicated to scale up when compared to suspension cultures that are inoculated with single cells [194]. Nonetheless, the fact that other studies have demonstrated MSC and ESC spheroid formation from single cell inoculations suggests that an efficient expansion of MSCs and ESCs – followed by their in situ differentiation into adipocytes – should be possible [175,258]. This has been demonstrated beyond MSCs and ESCs, where single cell inoculated 3T3-L1s in a rotating wall vessel bioreactor formed spheroids and accumulated lipid [191]. 3T3-L1 spheroids were also differentiated into fat upon deliberate induction with adipogenic media, which is important for preventing cells from spontaneously differentiating during proliferation and exiting the cell cycle [185].
Potential Food-Safe Strategies to Induce and Enhance Adipogenic Differentiation
While the in situ adipogenic differentiation of cells within proliferation bioreactors has been outlined as an attractive option for large scale cultured fat production, the formulations of differentiation media typically used to induce adipogenesis often contain ingredients that may be questionable for a food production process. Conventional adipogenic differentiation media commonly contains ingredients that are not approved for food use (e.g., 3-isobutyl-1-methylxanthine), or compounds (indomethacin, rosiglitazone, dexamethasone) that are used as medical drugs [267]. Although the use of such compounds may only be required transiently during the initial induction of adipogenesis – and may thus be diluted to negligible levels by media exchanges during extended cell culture – here we cover alternative options for inducing adipogenesis that may be relevant to large scale food production scenarios (Figure 2B).
Free Fatty Acids to Induce Adipogenesis
Certain free fatty acids (FFAs) have been characterized as agonists of PPARγ and have thus been investigated as adipogenesis-inducing compounds [268]. For livestock cells, bovine stromal vascular cells from adipose tissue have been induced to differentiate using a cocktail of monounsaturated and branched chain fatty acids [82]. In experiments of FFAs added to conventional differentiation media for bovine stromal vascular cells, oleic and linoleic acid were found to be the most efficacious for promoting lipid accumulation [269]. For bovine muscle satellite cells, oleic acid was found to promote adipogenic gene expression and lipid accumulation [270]. FFA-based adipogenesis has been demonstrated with porcine preadipocytes using oleic acid, linoleic acid and to a lesser extent the omega 3 FFA α-linolenic acid [271]. Oleic acid alone has been shown to be sufficient for inducing lipid accumulation in chicken preadipocytes [272,273]. Preadipocyte cell lines such as 3T3-L1s have also been differentiated with FFAs [274].
FFA-induced adipogenesis appears to have not been reported for PSCs, however the differentiation path of PSCs to adipocytes typically routes through an MSC or MSC-like cell phase, followed by adipogenic induction via a conventional differentiation media designed for MSC adipogenesis with cow, pig and chicken cells [113,114,275]. The fact that the final stage of PSC to adipocyte differentiation is the same as MSC adipogenesis suggests that it may be possible to substitute conventional differentiation media formulations with an FFA-based protocol once PSCs are induced to become MSC-like.
While FFAs have been shown to have PPARγ activity and FFA differentiation has been shown to be sufficient in many cases, experiments have shown that FFAs coupled with conventional PPARγ agonists (e.g., rosiglitazone, ciglitazone) can yield superior results versus either treatment alone [88,270,273]. Thus, the most optimal food-safe strategies for adipogenic differentiation will likely require pairing FFA treatment with other adipogenic factors. Relevant supplements that may enhance adipogenesis include beta carotene, biotin, pantothenate, as well as conventional growth factors such as BMP4 [76,225,250,267,276]. Numerous phytochemicals reported to promote adipogenesis such as green propolis extract could also be used to induce lipid accumulation alongside FFAs, although whether these various compounds are food-safe needs to be determined [277–281]. Glucose levels also influence adipogenesis [282]. Nonetheless, the discovery of oleic acid and linoleic acid as effective FFAs in the induction of livestock cell adipogenesis presents a favorable opportunity for large scale cultured fat production, as these FFAs are present at high concentrations in plant based oils such as olive and soybean oil [283,284]. The direct use and efficacy of soybean oil in promoting adipogenesis has been explored through its use in Intralipid, a soybean oil emulsion with clinical applications that has found use in adipose tissue engineering [285–287]. With a demonstrated adipogenic effect in livestock cells and the ready availability of plant based sources, FFAs may be an appealing, food safe and cost-effective option for inducing adipogenesis during large scale cultured fat production.
As an additional note, it is important to keep in mind that FFA administration can have both beneficial and deleterious effects on adipogenic differentiation, depending on the particular FFA, the treated cell type or species, and likely other unelucidated variables. Combined octanoate and oleate administration has been shown to synergistically promote lipid accumulation in porcine preadipocytes [88]. However, octanoate has also been reported to inhibit lipid accumulation in 3T3-L1s and human adipocytes [288]. Similar effects have been reported with omega 3 fatty acids, with reports of its administration both promoting and inhibiting adipogenesis [289]. One reason for this discrepancy could be the omega 3 source, with one group reporting negative effects when using FFAs from one supplier, which was not observed with FFAs from another supplier [290]. Outcomes also differ based on the specific omega 3 FFA tested [291,292]. It may also be possible that omega 3 supplementation is beneficial to lipid accumulation media only when compared to non-adipogenically differentiating culture media, as there is a pattern in the literature where positive results for omega 3 are seen when they are compared to media without other adipogenic factors, while negative results are reported when the control group involves a conventional adipogenic differentiation regime [292–295]. Interestingly, a study by Li et al. (2017) shows data for omega 3 supplementation to culture media with and without conventional adipogenic differentiation factors [296]. All omega 3 FFAs tested – alpha linoleic acid (ALA), stearidonic acid (SDA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) – induced considerable lipid accumulation relative to the undifferentiated sample, but FFA treated cells also accumulated less lipid than plain un-supplemented differentiation media. Another investigation by Murali et al. (2014) tested EPA and DHA with undifferentiated and conventionally differentiated cells, although curiously in this case the EPA and DHA groups (FFAs added to conventional differentiation media) surpassed the conventional differentiation media in terms of lipid accumulation [297]. Nonetheless, it is likely that omega 3 FFAs carry some ability to induce adipogenesis when used in isolation with undifferentiated cells, but whether they are synergistic or antagonistic with other adipogenic factors is unclear. Omega 3 administration appears to at least enrich cultured adipocytes with itself though – EPA or DHA treatment leads to cell enrichment of the administered FFA, even when they result in diminished total lipid accumulation versus plain adipogenic differentiation media [296]. Lastly, one limitation with research on in vitro omega 3 supplementation is that the vast majority of studies take place with 3T3-L1 cells. Some studies on omega 3s and other in vitro adipocytes exist, especially for fish, but more research would better inform upon the role of omega 3 FFAs in the context of large scale cultured fat (e.g., improved nutritional profile of the cultured cells, but potentially diminished lipid accumulation) [271,291,298–302].
Genetic Engineering: Inducible Expression of Adipogenic Genes
When using engineered cell lines, it may be possible to include genes known to initiate adipocyte differentiation and pair them with inducible promoters to selectively activate them when differentiation is desired during the cultured fat production process. A selection of inducible promoters (also called conditional promoters) has been published elsewhere [303] and a list of adipogenic gene targets is provided below (Table 3).
Table 3.
List of genes that have been ectopically expressed to induce adipogenesis in various adipogenic precursor cells.
| Adipogenic Gene | Species | Cell Types | Reference(s) |
|---|---|---|---|
| Peroxisome proliferator-activated receptor gamma (PPARγ) | Human, Mouse, Chicken, Cow, Goat, Pig | PSCs/MSCs, Myoblasts, Fibroblasts | [113,151,304–310] |
| CCAAT/enhancer-binding protein alpha and beta (C/EBPα, β) | Mouse, Chicken, Goat | Myoblasts, Fibroblasts, Preadipocytes | [304,306,308,311–313] |
| Sterol regulatory element-binding protein-1 (SREBP-1) | Mouse, Chicken | Fibroblasts | [306,314] |
| Fatty acid binding protein 4 (FABP4) | Cow | MSCs | [315] |
| Zinc finger protein 423 (Zfp423) | Cow | MSCs | [316,317] |
| Zinc finger and BTB domain containing 16 (ZBTB16) | Cow | DFAT cells | [318] |
| Perilipin 1 (PLIN1) | Cow | Preadipocytes | [319] |
| Kruppel-like factor 13 (KLF13) | Pig | MSCs | [320] |
| Retinoid X receptor α (RXRα) | Chicken | Preadipocytes | [150] |
| Phosphoenolpyruvate carboxykinase 1 (PCK1) | Buffalo | Preadipocytes | [321] |
| Early B-cell factor (Ebf) 1–3 | Mouse | Fibroblasts | [322] |
| Runt-related transcription factor 1 (RUNX1) | Mouse | MSCs | [323] |
| Cyclin-dependent kinase 4 (CDK4) | Mouse | Fibroblasts | [324] |
RNA Delivery: GMO-Free Alternatives for Adipogenesis
DNA-based genetic engineering is a powerful tool in modulating protein and gene expression to direct cell differentiation of large scale proliferated cells. However, it is also viewed unfavorably by certain consumers and governing bodies [325,326]. RNA-based technologies such as mRNA delivery present an alternative approach to modulating adipogenic protein expression without modifications to the cell genome. Here, mRNA of an adipogenic gene is delivered to a cultured cell, where it is then translated into a protein that ultimately promotes adipogenesis [327]. The delivered mRNA does not interact with the cell’s genome, so no genetic modification takes place. Delivered RNA is also transient and eventually degraded by the cell, which reduces the risk of exposure when consuming the final food product. There is also a low risk of human exposure to RNA during food consumption, as biologically there are multitudinous barriers to RNA exposure, such as the presence of RNAse in saliva and the GI tract, as well as the low pH of the stomach [328]. For a commercial cultured fat process, the close to 100% transfection efficiency of RNA delivery also means that a majority of proliferated cells could be converted into adipocytes, which is advantageous for yield and cost [329–332]. There have been no reports of using mRNA to express adipogenic genes and generate adipocytes from precursor cells. However, there is precedent for the use of mRNA in modifying cell types, as the approach has found wide usage in the high efficiency generation of iPSCs from cells such as fibroblasts, as well as in the differentiation of iPSCs into other cell types such as neurons and muscle cells [141,333–336]. mRNA could also be used to generate dCas9 proteins within cells to facilitate CRISPR activation (CRISPRa), which epigenetically induces transcription of endogenous genes without actual changes to the DNA code. CRISPRa has been used to induce adipogenesis in murine C3H 10T1/2 MSCs through the upregulation of PPARγ2, and it has generally been shown to yield stronger and longer lasting gene expression when compared to the direct translation of target proteins during conventional mRNA delivery [337,338]. Even with CRISPRa approaches, the effects of RNA-based gene expression is temporary. However, this may be fitting for adipogenic differentiation, as it has been reported that a transient expression of PPARγ and C/EBPα (via DNA transfection) is sufficient for adipogenic differentiation in MSCs [339].
RNA-based approaches also enable adipogenesis through the repression of anti-adipogenic genes. Numerous microRNAs (miRNAs) in human, rodent and livestock species have been characterized for their ability to repress anti-adipogenic genes and could be delivered exogenously to cultured cells [340,340–347]. Epigenetic approaches have also been explored with CRISPR inhibition (CRISPRi), where muscle satellite cells were transdifferentiated into adipocytes through the epigenetic repression of MyoD [348].
With recent developments in RNA vaccine production, RNA production has been shown to be scalable and low cost [349,350]. Moreover, the use of technologies such as self-amplifying mRNAs (saRNAs) stand to further reduce cost by reducing the number of RNA molecules required during transfection by orders of magnitude, while also extending their duration of efficacy [351–353]. The breadth of RNA-based techniques allows for many different approaches to promoting adipogenesis – for example, numerous long noncoding RNAs (lncRNAs) that promote adipogenesis have been characterized and could also be used to induce adipogenic differentiation [354,355]. Ultimately, with RNA delivery one can modulate the same adipogenic genes targeted during direct DNA-based genetic engineering, but without affecting the host genome and potentially avoiding a GMO designation.
3D Culture to Enhance Adipogenesis
To promote more robust adipogenesis in cultured cells, growth of cells in 3D culture environments is a promising approach. In 2D culture, bovine stromal vascular cells only undergo limited lipid accumulation when differentiated using a conventional adipogenic induction medium (insulin, dexamethasone, IBMX. However, cultivation of the same cells under 3D conditions in spheroids caused a significant increase in lipid accumulation and adipogenic gene expression, rescuing adipogenesis [356]. 3D spheroid environments have also been shown to improve adipogenesis in human MSCs in multiple reports [192,200]. During 3D spheroid culture, adipose stromal vascular cells have even been reported to robustly accumulate lipid while also self-organizing to form a network of capillary vessels [186]. Other forms of 3D culture (sponge scaffolds, hydrogel encapsulation) have also been shown to promote robust adipogenesis in cultured cells [214,357–359]. 3D cultures of 3T3-L1s embedded in agarose gels enabled their adipogenic differentiation without the need for an adipogenic induction medium, which might imply that a 3D cell environment is inherently favorable for adipogenesis [360].
Strategies to Form Macroscale Cultured Fat Tissue
To fully mimic the mouthfeel experienced when eating native fat tissue from an animal, macroscale adipose tissue needs to be generated from adipocytes. After proliferating cells at scale and subsequently differentiating them to induce lipid accumulation, a cohesive structure on the tissue-scale is required to prevent fat cells from falling apart. Here, we discuss two major strategies to forming macroscale cultured fat tissue: 1) Growing adipose tissue using 3D culture techniques and sustaining the tissue using vascularization or perfusion techniques, or 2) Growing small adipocyte tissue constructs, adipocyte aggregates or individual adipocytes and combining them at the end of culture to mimic macroscale 3D adipose tissue. Either case may involve the use of a scaffolding or binder material to hold adipocytes together, made of biomaterials with similar texture profiles as native adipose, while being animal-free and unobtrusive in terms of flavor.
Macroscale 3D Culture: The Need to Address Nutrient Diffusion
One method of generating tangible, macroscale fat tissues is through bulk 3D tissue culture, where 3D constructs are formed instead of the cell monolayers that are produced from 2D culture (Figure 3D) [361]. However, large macroscale tissues require a method of delivering oxygen and nutrients to the tissue interior [362,363]. In the body this is addressed by the circulatory system, where blood vessels interspersed throughout our tissues replenish cell nutrients while removing metabolic waste products. For macroscale tissue engineering, the limitations of mass transport can be solved by manually incorporating perfusion channels into cultured tissues to permit culture media flow inside the tissue. This has been explored in numerous ways, such as via the 3D printing of sacrificial channel templates, incorporation of porous tubes, laser ablation of 3D tissues, as well as casting cell-laden hydrogels around a wire which is taken out after gel polymerization [285,364–366]. However, the close channel spacing required to limit nutrient diffusion distances to several hundred microns necessitates a very dense packing of perfusion channels, increasing the cost and complexity of macroscale 3D culture. Each channel also needs to be small, as otherwise a large volume of the cultured tissue will ultimately consist of empty channels. Due to these limitations, there are few examples of perfusion culture systems scaled up to generate large amounts of 3D tissue. In one example, nutrient diffusion limits led to only 21% (by volume) of a hollow fiber bioreactor growing skeletal muscle actually consisting of cultured tissue, with the majority of space being taken up by its perfusion channels [367]. For channel-perfused 3D cultures, more work is required to develop scalable methods of incorporating small, dense arrays of channels in cultured tissues in order for this approach to be viable.
Figure 3.
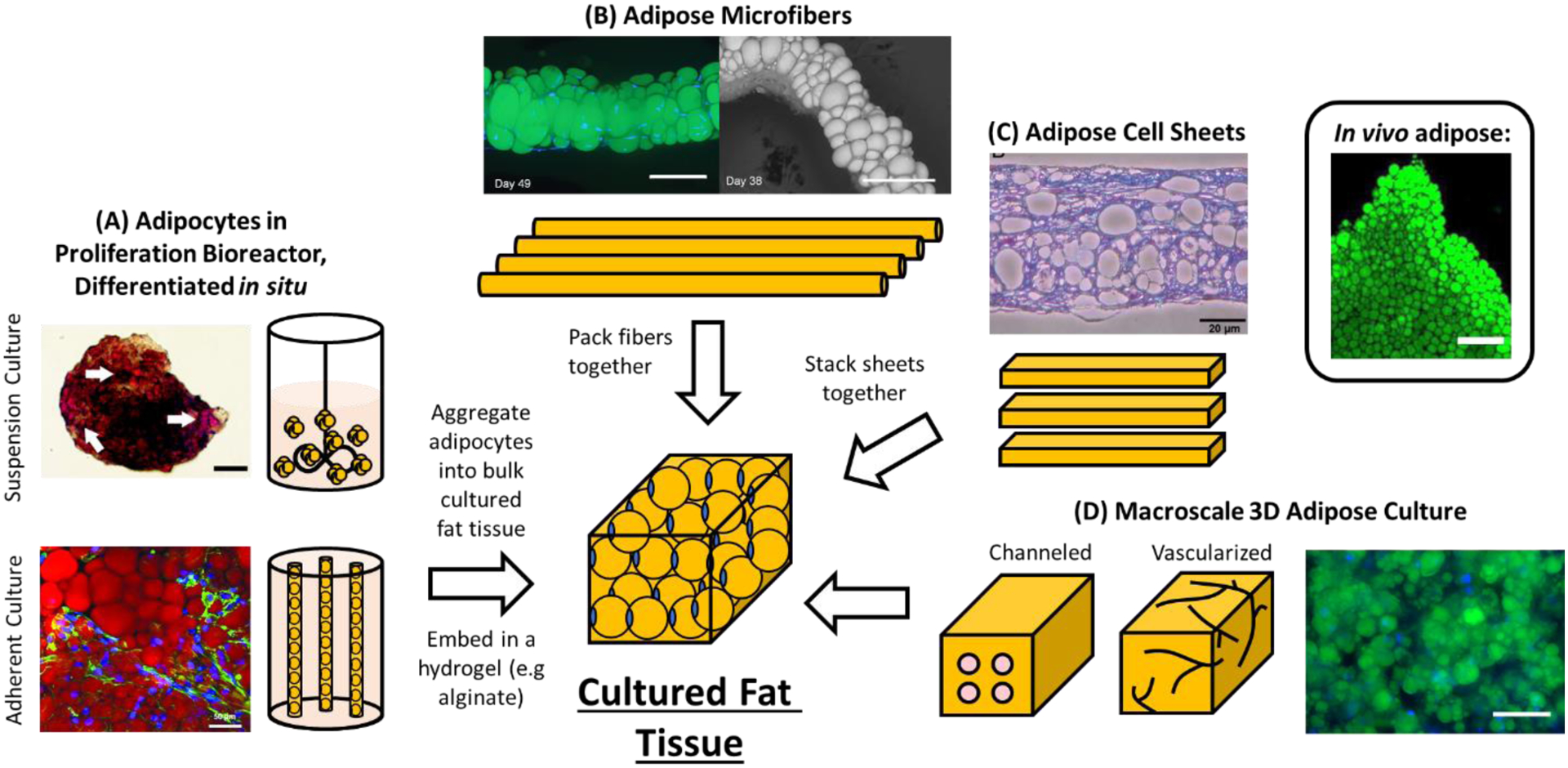
Strategies for producing cultured fat, either via adipose aggregation or macroscale 3D culture. (A) Cultured fat produced by proliferating, followed by differentiating adipocytes in the same bioreactor, then aggregating the cells and binding them together with a food-safe binder to form a cultured fat tissue construct. The Oil Red O stained adipose spheroid is from [192]. Scale bar 100 μm. AdipoRed stained hollow fiber adipocytes are from [259]. Scale bar 50 μm. (B) Unilocular adipocytes in alginate-fibrin-collagen microfibers after extended cell culture [377]. Microfibers can be packed together to form macroscale cultured fat tissues. Scale bars 100 μm. (C) Adipose cell sheets prepared from differentiated MSCs [378]. Stacked sheets spontaneously fuse to form larger tissues. Scale bar 50 μm. (D) Macroscale 3D tissue culture of adipocytes could be performed to form bulk cultured fat constructs. These 3D tissues would require channels to ensure culture media access to the cells on the interior to avoid necrosis. The DAPI (nuclei, blue) and BODIPY (lipids, green) stained image is of 3T3-L1 MBX (5 million cells/ml) cultured under 3D conditions in a 3 mg/ml fibrin hydrogel for 20 days (unpublished data). Scale bar 100 μm. The image of in vivo adipose from newborn mouse also stained with BODIPY is from [379] (scale bar 200 μm).
Another solution to nutrient diffusion limitations may be to vascularize tissues via co-culture with endothelial cells. In 3D culture, endothelial cells spontaneously form perfusable capillary-like blood vessels that resemble in vivo microvasculature [368]. Cell to cell communication from endothelial co-culture has also been reported to be beneficial for adipogenic differentiation, and there have been many reports of vascularized adipose tissue formation in the literature [186,357,369–373]. However, vasculature from cultured endothelial cells is not formed immediately at the beginning of culture, possibly leaving cells deprived of nutrition for too long before culture media perfusion is established [374]. Additionally, the limited volume of media perfusable through capillary sized vessels may not deliver a sufficient amount of nutrition to sustain a high number of cells residing in very large tissue constructs [375,376]. Interestingly, the limitations of manually created perfusion channels and self-assembled endothelial capillaries appear to be antithetical – capillaries form small and dense channels that are difficult to achieve using engineered channels, while larger engineered channels enable sufficient nutrient flow and mass transport into macroscale tissue constructs. It is possible that a hybrid system consisting of both engineered perfusion channels and endothelial capillaries might be advantageous in addressing nutrient diffusion during large macroscale 3D culture. However, to date no perfusion systems readily applicable to the cost effective scale-up of bulk 3D culture in general are available.
Adipose Tissue From Cellular Aggregates (Aggregated Adipose)
Unlike the muscle component of meat, which comprises an elaborate structural hierarchy of muscle fibrils bundled to form muscle fibers, native white adipose tissue is largely a dense aggregation of adipocyte spheres, with the fat cells occupying a majority of the tissue volume held together by a sparse ECM protein network [379,380]. Thus, it may be possible for a simple aggregation of individually grown adipocytes, small adipose spheroids, or miniature 3D tissue constructs to produce an macroscale tissue that is organoleptically comparable to native fat. Growing cells or small tissues sidesteps the obstacle of delivering nutrients to the interior of large in vitro tissues and allows nutrients and waste to diffuse across shorter distances of dense tissue, which is important for enabling strong cell growth and for obtaining highly differentiated and lipid-laden adipocytes [362,363,381]. One method in which this has been approached has been through the culture of 3D adipose microfibers. In this system, MSCs were suspended in two-phase microfibers comprising of a protective alginate shell with a fibrin-collagen core, after which the fibers were cultured in adipogenic induction and maintenance media to produce 3D microfiber tissues containing large unilocular adipocytes that resembled in vivo fat tissue. After adipogenic differentiation, alginate shells of the microfibers were degraded and the remaining adipose fibers were packed in molds overnight, where the fibers fused into a single tissue (Figure 3B) [377]. Alginate-based microfibers have also been successfully differentiated into adipose using FFAs [82]. Aggregated adipose would also pair well with a cell sheet engineering approach, which has been successfully used with MSCs to form sheets containing lipid-laden adipocytes that spontaneously fuse with each other when pressed together [382–385]. During cultured fat production, individual sheets of adipocytes and their secreted ECM could be stacked together to build large tissues (Figure 3C).
Another approach to achieve aggregated adipose tissue would be to build upon the concept of in situ adipose differentiation discussed previously. After proliferating and differentiating large numbers of adipocytes in scalable bioreactors such as a hollow fiber bioreactor, adipocyte clusters could be removed from the reactor and packed together to recapitulate the dense adipocyte arrangement observed in native adipose (Figure 3A). While simple and scalable, aggregating separately grown adipocytes with this approach may result in cultured fat containing a less developed ECM component than native adipose. However, the major contributions of fat tissue to meat flavor (e.g., species specific flavors) come from the lipid fraction of the tissue, rather than the ECM [52,53]. Additionally, cultured fat formed from aggregated adipocytes would not be completely devoid of any ECM proteins, as some ECM is autologously deposited during in vitro culture, especially when using techniques such as spheroid-based cultures where cell aggregates are held together by secreted ECM [192,386–388]. It may also be possible to deliberately bolster the ECM component of cultured adipose by upregulating ECM deposition during in vitro cell culture, which has been shown through the supplementation of ECM building blocks (e.g., hydroxyproline) in the culture media, as well as through co-culture strategies (e.g., adding fibroblasts) [389].
In lieu of fully formed adipose ECM, a hydrogel scaffold or binder with characteristics similar to fat could be employed to encapsulate aggregated adipocytes. Scaffold requirements for aggregated cultured fat would be different from typical tissue engineering as it only needs to hold cells together - aggregation already produces the final cultured fat product and no further cell culture is required. Ideally, a scaffold used in aggregated adipose would be unobtrusive in terms of flavor, similar to adipose tissue in terms of texture and animal-free. Numerous gelling ingredients such as alginate and carrageenan have been used in the food industry as fat replacers and may thus be good options for binding adipose aggregates (Table 4). Another option could be to bind fat cells together using transglutaminase and protein from a source such as soy, where adipocyte aggregates become crosslinked within a proteinaceous network to form a bulk tissue construct [390].
Table 4.
Potential gelling and binding ingredients to use with “aggregated adipose” forms of cultured fat.
| Gelling or Binding Ingredient | Ingredient Source/Type | References |
|---|---|---|
| Alginate | Seaweed | [391–393] |
| Carrageenan | Seaweed | [391,392] |
| Cellulose | Plant Fiber | [214,394] |
| Corn Zein | Plant Protein | [395] |
| Guar Gum | Plant Fiber | [396,397] |
| Hyaluronic Acid | Animal ECM; Microbially Produced | [214] |
| Inulin | Plant Fiber | [398,399] |
| Konjac | Plant Fiber | [400] |
| Oat Bran | Plant Fiber | [393] |
| Pea Proteins | Plant Protein | [395] |
| Pectin | Plant Fiber | [399,401] |
| Soy Protein Isolate | Plant Protein | [390,395] |
| Starches | Plant | [402] |
| Wheat Gluten | Plant Protein | [395] |
| Xanthan Gum | Microbially Produced | [397,403] |
Characterization of Sensory Properties and Nutrition in Cultured Fat
While it may be possible to produce 3D cultured fat tissues, to date no nutrition nor sensory evaluation data on cultured fat has been published. After safety studies are conducted, nutritional properties should be characterized and sensory evaluation panels should be explored to elucidate cultured fat properties such as taste and aroma. Cultured fat texture can also be evaluated through mechanical assessments such as uniaxial compression testing and rheology, as well as shear force measurements for bite force related data [390,404–407]. In addition to conventional methods such as sensory panels and mechanical testing, mass spectrometry (MS)-based analysis is one of the fastest growing and most widely used analytical methods in food science today [408]. For cultured fat, MS-based analyses could be used to enable comparisons between animal-derived and in vitro-grown fats through the screening of compounds present in tested samples. Lipidomics can be performed using liquid or gas chromatography-MS (LC-MS and GC-MS) to obtain fatty acid composition data and thus inform on its sensory and nutritional qualities [409]. For example, analysis of fatty acid compositions using GC-MS revealed that linoleic acid supplementation in cultured adipocytes enabled a corresponding increase of the fatty acid in the cells, increasing the unsaturated to saturated fat ratio when compared to un-supplemented adipocytes [410]. The change in lipid composition also demonstrated the tunability of cultured fat, analogous to how different feeds in cattle can impact their fatty acid make-up [411–413]. In the same study, profiles of phospholipid content in the cultured adipocytes were also obtained, which could be juxtaposed with native adipose to potentially shed light on how their flavor may differ, as phospholipids are thought to be a major contributor to flavor within the lipid fraction of meat [414,415]. Generally, the ability to tune fatty acid profiles in cultured fat holds promise for enhancing its nutritional properties. For example, adipocytes could be cultured to have reduced levels of omega 6 (n-6) polyunsaturated fatty acids, which are reported to have pro-inflammatory properties associated with risk of cardiovascular disease and rheumatoid arthritis [416,417]. In addition to evaluating the fatty acid profile of cultured adipose cells, GC-MS based based headspace analyses of volatile organic compounds (VOCs) released by cultured and native fat tissues upon heating would enable the characterization of aromas that are released during cooking [418–420]. Ultimately, in depth omics characterizations empower producers to fine tune in vitro fats for improved sensory, taste and nutritional properties, or to accurately recapitulate native adipose tissue.
Conclusions and Future Perspectives
With the impact of conventional livestock production on the environment and its increasing rate of consumption, developing sustainable alternatives to supplement livestock-derived meat are of growing significance. Since fat is an important component of flavor, aroma and texture in meat, reproducing these features without animal agriculture using cultured adipocytes should greatly contribute to the sensory and textural experience of consuming meat alternatives such as plant based and cell cultured meats. In this review we outline various considerations and potential paths for the large scale production of in vitro cultured fat, including adipogenic precursors during cell proliferation, methods to proliferate cells at scale, methods to differentiate cells at scale and strategies for converting differentiated adipocytes into macroscale 3D cultured fat tissues. For large scale cultured fat production, various cell source options with proliferative and adipogenic capacities exist, with and without the use of genetic modifications. Adipose biology surrounding certain livestock species may need to be further elucidated to enable more efficient large scale adipose production. Methods, infrastructure and bioreactor systems exist for the large scale proliferation of adipogenic precursor cells, owing to previous developments in the biopharmaceutical and biotechnology fields. However, specific considerations (cell buoyancy) for adipocyte cell culture may require adaptations to existing systems. Current solutions for adipogenic differentiation involve compounds not approved for use in humans, while FFAs, RNAs, genetic engineering and other strategies offer potential methods of differentiation that are likely to be scalable and food safe. While much research has been done on scalable cell proliferation, and promising options exist for adipogenic differentiation, research on the conversion of adipocytes into fat tissue is scarce. 3D cell culture approaches promote adipogenesis and could inherently produce macroscale tissues, but tissue perfusion systems for large 3D tissues remains to be further developed before this approach can be used to efficiently create bulk adipose tissues. An alternative strategy to forming macroscale adipose tissue is to aggregate smaller, separately grown adipose tissue constructs, which could circumvent the need for nutrient perfusion. Lastly, while data on the nutrition and taste of cell cultured fat are not yet available, numerous analytical tools exist to characterize the lipid and volatile fractions of adipose tissue samples once production is scaled up.
Acknowledgments
We thank the NIH (P41EB027062), New Harvest, the Good Food Institute, ARPA-E (DE-AR0001233) and many colleagues for the support of the work reviewed here.
List of Abbreviations
- ADSC
adipose derived stem cell
- ALA
alpha-linoleic acid
- aP2
adipocyte protein 2
- BM-MSCs
bone marrow mesenchymal stem cells
- BMP
bone morphogenetic protein
- C/EBPα, β
CCAAT/enhancer-binding protein alpha and beta
- CD
clusters of differentiation
- CDK4
cyclin-dependent kinase 4
- CHO
chinese hamster ovary
- c-Myc
myc proto-oncogene
- COVID
coronavirus disease 2019
- DFAT
dedifferentiated fat
- DHA
docosahexaenoic acid
- DMEM
Dulbecco’s modified Eagle medium
- Ebf
early b-cell factor
- ECM
extracellular matrix
- ESC
embryonic stem cell
- EPA
eicosapentaenoic acid
- F12
Nutrient Mixture F-12
- FABP4
fatty acid binding protein 4
- FAP
fibroadipogenic progenitor
- FBB
fixed-bed bioreactor
- FBS
fetal bovine serum
- FFA
free fatty acid
- FGF
fibroblast growth factor
- GC-MS
gas chromatography-mass spectrometry
- GHG
greenhouse gas
- GSK
glycogen synthase kinase
- HARV
high aspect ratio vessel
- HFB
hollow fiber bioreactor
- HS
horse serum
- IBMX
isobutylmethylxanthine
- ICP1
immortalized chicken preadipocytes
- iPSC
induced pluripotent stem cell
- Klf
Krüppel-like factor
- LC-MS
liquid chromatography-mass spectrometry
- LIF
leukemia inhibitory factor
- LPL
lipoprotein lipase
- MAPK
mitogen activated protein kinase
- MS
mass spectrometry
- MSC
mesenchymal stem cell
- Oct
octamer binding transcription factor
- PCK1
phosphoenolpyruvate carboxykinase 1
- PLIN1
perilipin 1
- PPARγ
peroxisome proliferator-activated receptor gamma
- PSC
pluripotent stem cell
- RXRα
retinoid X receptor α
- RNAseq
RNA-sequencing
- RUNX1
runt-related transcription factor 1
- Sca
stem cells antigen
- Sox2
sex-determining region y-box 2
- SDA
stearidonic acid
- SREBP-1
sterol regulatory element-binding protein-1
- STLV
slow-turning lateral vessel
- SV40
simian virus 40
- TERT
telomerase reverse transcriptase
- TGFβ
transforming growth factor beta
- TR
telomerase RNA
- VOC
volatile organic compound
- ZF
Zinc finger
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Steinfeld H, Gerber P, Wassenaar TD, Castel V, Rosales MM, de Haan C. Livestock’s long shadow: environmental issues and options. Rome: Food and Agriculture Organization of the United Nations; 2006. ISBN: 978-92-5-105571-7 [Google Scholar]
- [2].Gerber PJ, Food and Agriculture Organization of the United Nations, editors. Tackling climate change through livestock: a global assessment of emissions and mitigation opportunities. Rome: Food and Agriculture Organization of the United Nations; 2013. ISBN: 978-92-5-107920-1 [Google Scholar]
- [3].Glatzle A Questioning key conclusions of FAO publications ‘Livestock’s Long Shadow’ (2006) appearing again in ‘Tackling Climate Change Through Livestock’ (2013). Pastoralism 2014;4:1. 10.1186/2041-7136-4-1 [DOI] [Google Scholar]
- [4].Goodland R, Anhang J. Livestock and climate change. 2009
- [5].IPCC. Climate change 2014: mitigation of climate change: Working Group III contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. New York, NY: Cambridge University Press; 2014. ISBN: 978-1-107-05821-7 [Google Scholar]
- [6].Herrero M, Henderson B, Havlík P, Thornton PK, Conant RT, Smith P, et al. Greenhouse gas mitigation potentials in the livestock sector. Nat Clim Change 2016;6:452–61. 10.1038/nclimate2925 [DOI] [Google Scholar]
- [7].Eshel G, Shepon A, Makov T, Milo R. Land, irrigation water, greenhouse gas, and reactive nitrogen burdens of meat, eggs, and dairy production in the United States. Proc Natl Acad Sci 2014;111:11996–2001. 10.1073/pnas.1402183111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Me L Reviving Dead Zones. Sci Am 2006;295:78–85 [DOI] [PubMed] [Google Scholar]
- [9].Leip A, Billen G, Garnier J, Grizzetti B, Lassaletta L, Reis S, et al. Impacts of European livestock production: nitrogen, sulphur, phosphorus and greenhouse gas emissions, land-use, water eutrophication and biodiversity. Environ Res Lett 2015;10:115004. 10.1088/1748-9326/10/11/115004 [DOI] [Google Scholar]
- [10].Zonderland-Thomassen MA, Lieffering M, Ledgard SF. Water footprint of beef cattle and sheep produced in New Zealand: water scarcity and eutrophication impacts. J Clean Prod 2014;73:253–62. 10.1016/j.jclepro.2013.12.025 [DOI] [Google Scholar]
- [11].Wu H, Yuan Z, Zhang L, Bi J. Eutrophication mitigation strategies: perspectives from the quantification of phosphorus flows in socioeconomic system of Feixi, Central China. J Clean Prod 2012;23:122–37. 10.1016/j.jclepro.2011.10.019 [DOI] [Google Scholar]
- [12].Abdalla SE, Abia ALK, Amoako DG, Perrett K, Bester LA, Essack SY. From Farm-to-Fork: E. Coli from an Intensive Pig Production System in South Africa Shows High Resistance to Critically Important Antibiotics for Human and Animal Use. Antibiotics 2021;10:178. 10.3390/antibiotics10020178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tiseo K, Huber L, Gilbert M, Robinson TP, Van Boeckel TP. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020;9. 10.3390/antibiotics9120918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Boeckel TPV, Glennon EE, Chen D, Gilbert M, Robinson TP, Grenfell BT, et al. Reducing antimicrobial use in food animals. Science 2017;357:1350–2. 10.1126/science.aao1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ma F, Xu S, Tang Z, Li Z, Zhang L. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosaf Health 2021;3:32–8. 10.1016/j.bsheal.2020.09.004 [DOI] [Google Scholar]
- [16].Woolhouse MEJ, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg Infect Dis 2005;11:1842–7. 10.3201/eid1112.050997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jones BA, Grace D, Kock R, Alonso S, Rushton J, Said MY, et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc Natl Acad Sci U S A 2013;110:8399–404. 10.1073/pnas.1208059110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Alexandratos N, Bruinsma J. World Agriculture towards 2030/2050: the 2012 revision. Food and Agriculture Organization of the United Nations; 2012. http://www.fao.org/3/ap106e/ap106e.pdf [Google Scholar]
- [19].Shepon A, Eshel G, Noor E, Milo R. Energy and protein feed-to-food conversion efficiencies in the US and potential food security gains from dietary changes. Environ Res Lett 2016;11:105002. 10.1088/1748-9326/11/10/105002 [DOI] [Google Scholar]
- [20].Detzel A, Krüger M, Busch M, Blanco-Gutiérrez I, Varela C, Manners R, et al. Life cycle assessment of animal-based foods and plant-based protein-rich alternatives: an environmental perspective. J Sci Food Agric 2021;n/a. 10.1002/jsfa.11417 [DOI] [PubMed] [Google Scholar]
- [21].Saerens W, Smetana S, Van Campenhout L, Lammers V, Heinz V. Life cycle assessment of burger patties produced with extruded meat substitutes. J Clean Prod 2021;306:127177. 10.1016/j.jclepro.2021.127177 [DOI] [Google Scholar]
- [22].Heller MC, Keoleian GA. Beyond Meat’s Beyond Burger Life Cycle Assessment: A detailed comparison between a plant-based and an animal-based protein source. University of Michigan; 2018. http://css.umich.edu/publication/beyond-meats-beyond-burger-life-cycle-assessment-detailed-comparison-between-plant-based (accessed May 9, 2021) [Google Scholar]
- [23].Smetana S, Profeta A, Voigt R, Kircher C, Heinz V. Meat substitution in burgers: nutritional scoring, sensorial testing, and Life Cycle Assessment. Future Foods 2021;4:100042. 10.1016/j.fufo.2021.100042 [DOI] [Google Scholar]
- [24].Khan S, Loyola C, Dettling J, Hester J. COMPARATIVE ENVIRONMENTAL LCA OF THE IMPOSSIBLE BURGER WITH CONVENTIONAL GROUND BEEF BURGER. Quantis; 2019. https://assets.ctfassets.net/hhv516v5f7sj/4exF7Ex74UoYku640WSF3t/cc213b148ee80fa2d8062e430012ec56/Impossible_foods_comparative_LCA.pdf (accessed November 15, 2021) [Google Scholar]
- [25].Datar I, Betti M. Possibilities for an in vitro meat production system. Innov Food Sci Emerg Technol 2010;11:13–22. 10.1016/j.ifset.2009.10.007 [DOI] [Google Scholar]
- [26].Post MJ. Cultured meat from stem cells: Challenges and prospects. Meat Sci 2012;92:297–301. 10.1016/j.meatsci.2012.04.008 [DOI] [PubMed] [Google Scholar]
- [27].Jayathilakan K, Sultana K, Radhakrishna K, Bawa AS. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: a review. J Food Sci Technol 2012;49:278–93. 10.1007/s13197-011-0290-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bhat ZF, Fayaz H. Prospectus of cultured meat—advancing meat alternatives. J Food Sci Technol 2011;48:125–40. 10.1007/s13197-010-0198-7 [DOI] [Google Scholar]
- [29].Odegard I, Sinke P. LCA of cultivated meat. Future projections for different scenarios. CE Delft; 2021. https://cedelft.eu/publications/rapport-lca-of-cultivated-meat-future-projections-for-different-scenarios/ (accessed September 5, 2021) [Google Scholar]
- [30].Tuomisto HL, Teixeira de Mattos MJ. Environmental Impacts of Cultured Meat Production. Environ Sci Technol 2011;45:6117–23. 10.1021/es200130u [DOI] [PubMed] [Google Scholar]
- [31].Tuomisto HL. Environmental impacts of cultured meat: alternative production scenarios. EU Sci. Hub - Eur. Comm, 2015 [Google Scholar]
- [32].Sun Z, Yu Q, Han L. The environmental prospects of cultured meat in China. J Integr Agric 2015;14:234–40. 10.1016/S2095-3119(14)60891-1 [DOI] [Google Scholar]
- [33].Mattick CS, Landis AE, Allenby BR, Genovese NJ. Anticipatory Life Cycle Analysis of In Vitro Biomass Cultivation for Cultured Meat Production in the United States. Environ Sci Technol 2015;49:11941–9. 10.1021/acs.est.5b01614 [DOI] [PubMed] [Google Scholar]
- [34].Allan SJ, De Bank PA, Ellis MJ. Bioprocess Design Considerations for Cultured Meat Production With a Focus on the Expansion Bioreactor. Front Sustain Food Syst 2019;3. 10.3389/fsufs.2019.00044 [DOI] [Google Scholar]
- [35].Hanley PJ, Mei Z, Durett AG, Cabreira-Harrison M da G, Klis M, Li W, et al. Efficient Manufacturing of Therapeutic Mesenchymal Stromal Cells Using the Quantum Cell Expansion System. Cytotherapy 2014;16:1048–58. 10.1016/j.jcyt.2014.01.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Orden E, Ban-Tokuda T, Barrio A, Lapitan R, Delavaud C, Chilliard Y, et al. Effects of species and sex on plasma hormone and metabolite concentrations in crossbred Brahman cattle and crossbred water buffalo. Asian Australas J Anim Sci 2007 [Google Scholar]
- [37].Manoj M, Gandhi RS, Raja T, Verma A, Singh A, Sachdeva GK. Growth Rates and Growth Curve in Sahiwal Cattle 2012. 10.5146/IJDS.V65I4.25867.G11917 [DOI]
- [38].Tamzil MH, Ichsan M, Jaya N, Taqiuddin M. Growth Rate, Carcass Weight and Percentage Weight of Carcass Parts of Laying Type Cockerels, Kampong Chicken and Arabic Chicken in Different Ages. Undefined 2015 [Google Scholar]
- [39].Rauw WM, de Mercado de la Peña E, Gomez-Raya L, García Cortés LA, Ciruelos JJ, Gómez Izquierdo E. Impact of environmental temperature on production traits in pigs. Sci Rep 2020;10:2106. 10.1038/s41598-020-58981-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Madden L, Juhas M, Kraus WE, Truskey GA, Bursac N. Bioengineered human myobundles mimic clinical responses of skeletal muscle to drugs. ELife 2015;4:e04885. 10.7554/eLife.04885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Food and Agriculture Organization of the United Nations. Guidelines for slaughtering, meat cutting and further processing. Rome: 1991. ISBN: 92-5-102921-0 [Google Scholar]
- [42].Vergeer R, Ingrid Odegard, Pelle Sinke. TEA of cultivated meat. Future projections for different scenarios. CE Delft; 2021. https://cedelft.eu/publications/tea-of-cultivated-meat/ (accessed May 25, 2021) [Google Scholar]
- [43].van Vliet S, Bain JR, Muehlbauer MJ, Provenza FD, Kronberg SL, Pieper CF, et al. A metabolomics comparison of plant-based meat and grass-fed meat indicates large nutritional differences despite comparable Nutrition Facts panels. Sci Rep 2021;11:13828. 10.1038/s41598-021-93100-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ben-Arye T, Shandalov Y, Ben-Shaul S, Landau S, Zagury Y, Ianovici I, et al. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat Food 2020:1–11. 10.1038/s43016-020-0046-532395719 [DOI] [Google Scholar]
- [45].World’s first lab-grown burger is eaten in London. BBC News; 2013 [Google Scholar]
- [46].Stout AJ, Mirliani AB, Soule-Albridge EL, Cohen JM, Kaplan DL. Engineering carotenoid production in mammalian cells for nutritionally enhanced cell-cultured foods. Metab Eng 2020;62:126–37. 10.1016/j.ymben.2020.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].He J, Evans NM, Liu H, Shao S. A review of research on plant-based meat alternatives: Driving forces, history, manufacturing, and consumer attitudes. Compr Rev Food Sci Food Saf 2020;19:2639–56. 10.1111/1541-4337.12610 [DOI] [PubMed] [Google Scholar]
- [48].Moritz MSM, Verbruggen SEL, Post MJ. Alternatives for large-scale production of cultured beef: A review. J Integr Agric 2015;14:208–16. 10.1016/S2095-3119(14)60889-3 [DOI] [Google Scholar]
- [49].Frank D, Joo S-T, Warner R. Consumer Acceptability of Intramuscular Fat. Korean J Food Sci Anim Resour 2016;36:699–708. 10.5851/kosfa.2016.36.6.699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Iida F, Saitou K, Kawamura T, Yamaguchi S, Nishimura T. Effect of fat content on sensory characteristics of marbled beef from Japanese Black steers. Anim Sci J Nihon Chikusan Gakkaiho 2015;86:707–15. 10.1111/asj.12342 [DOI] [PubMed] [Google Scholar]
- [51].Frank D, Kaczmarska K, Paterson J, Piyasiri U, Warner R. Effect of marbling on volatile generation, oral breakdown and in mouth flavor release of grilled beef. Meat Sci 2017;133:61–8. 10.1016/j.meatsci.2017.06.006 [DOI] [PubMed] [Google Scholar]
- [52].Hornstein I, Crowe PF. Meat Flavor Chemistry, Flavor Studies on Beef and Pork. J Agric Food Chem 1960;8:494–8. 10.1021/jf60112a022 [DOI] [Google Scholar]
- [53].Mottram DS. Flavour formation in meat and meat products: a review. Food Chem 1998;62:415–24. 10.1016/S0308-8146(98)00076-4 [DOI] [Google Scholar]
- [54].Ba HV, Hwang I, Jeong D, Touseef A. Principle of Meat Aroma Flavors and Future Prospect. IntechOpen; 2012. ISBN: 978-953-51-0868-9. 10.5772/51110 [DOI] [Google Scholar]
- [55].Mottram DS, Edwards RA, Macfie JHH. A comparison of the flavour volatiles from cooked beef and pork meat systems. J Sci Food Agric 1982;33:934–44. 10.1002/jsfa.2740330917 [DOI] [Google Scholar]
- [56].Bahmad HF, Daouk R, Azar J, Sapudom J, Teo JCM, Abou-Kheir W, et al. Modeling Adipogenesis: Current and Future Perspective. Cells 2020;9:2326. 10.3390/cells9102326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Romito A, Cobellis G. Pluripotent Stem Cells: Current Understanding and Future Directions. Stem Cells Int 2016;2016:1–20. 10.1155/2016/9451492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kolodny L Meet the company making mouse meat cat treats without harming animals. CNBC 2021. https://www.cnbc.com/2021/08/15/meet-the-company-making-mouse-meat-cat-treats-without-harming-animals.html (accessed November 16, 2021) [Google Scholar]
- [59].Wei S, Zan L, Hausman GJ, Rasmussen TP, Bergen WG, Dodson MV. Dedifferentiated adipocyte-derived progeny cells (DFAT cells): Potential stem cells of adipose tissue. Adipocyte 2013;2:122–7. 10.4161/adip.23784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sugihara H, Yonemitsu N, Miyabara S, Yun K. Primary cultures of unilocular fat cells: Characteristics of growth in vitro and changes in differentiation properties. Differentiation 1986;31:42–9. 10.1111/j.1432-0436.1986.tb00381.x [DOI] [PubMed] [Google Scholar]
- [61].Zhang HH, Kumar S, Barnett AH, Eggo MC. Ceiling culture of mature human adipocytes: use in studies of adipocyte functions. J Endocrinol 2000;164:119–28. 10.1677/joe.0.1640119 [DOI] [PubMed] [Google Scholar]
- [62].Adebonojo F Studies on Human Adipose Cells in Culture: Relation of Cell Size and Cell Multiplication to Donor Age. Yale J Biol Med 1975;48:9–16 [PMC free article] [PubMed] [Google Scholar]
- [63].Wei S, Duarte MS, Zan L, Du M, Jiang Z, Guan L, et al. Cellular and Molecular Implications of Mature Adipocyte Dedifferentiation. J Genomics 2013;1:5–12. 10.7150/jgen.3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol 2008;215:210–22. 10.1002/jcp.21304 [DOI] [PubMed] [Google Scholar]
- [65].Oki Y, Watanabe S, Endo T, Kano K. Mature Adipocyte-Derived Dedifferentiated Fat Cells Can Trans-Differentiate into Osteoblasts In Vitro and In Vivo only by All-Trans Retinoic Acid. Cell Struct Funct 2008;33:211–22. 10.1247/csf.08038 [DOI] [PubMed] [Google Scholar]
- [66].Kazama T, Fujie M, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can transdifferentiate into skeletal myocytes in vitro. Biochem Biophys Res Commun 2008;377:780–5. 10.1016/j.bbrc.2008.10.046 [DOI] [PubMed] [Google Scholar]
- [67].Jumabay M, Matsumoto T, Yokoyama S, Kano K, Kusumi Y, Masuko T, et al. Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. J Mol Cell Cardiol 2009;47:565–75. 10.1016/j.yjmcc.2009.08.004 [DOI] [PubMed] [Google Scholar]
- [68].Sakuma T, Matsumoto T, Kano K, Fukuda N, Obinata D, Yamaguchi K, et al. Mature, Adipocyte Derived, Dedifferentiated Fat Cells Can Differentiate Into Smooth Muscle-Like Cells and Contribute to Bladder Tissue Regeneration. J Urol 2009;182:355–65. 10.1016/j.juro.2009.02.103 [DOI] [PubMed] [Google Scholar]
- [69].Nobusue H, Kano K. Establishment and characteristics of porcine preadipocyte cell lines derived from mature adipocytes. J Cell Biochem 2009:n/a–n/a. 10.1002/jcb.22431 [DOI] [PubMed] [Google Scholar]
- [70].Peng X, Song T, Hu X, Zhou Y, Wei H, Peng J, et al. Phenotypic and Functional Properties of Porcine Dedifferentiated Fat Cells during the Long-Term Culture In Vitro. BioMed Res Int 2015;2015:1–10. 10.1155/2015/673651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wei S, Du M, Jiang Z, Duarte MS, Fernyhough-Culver M, Albrecht E, et al. Bovine dedifferentiated adipose tissue (DFAT) cells. Adipocyte 2013;2:148–59. 10.4161/adip.24589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Oki Y, Hagiwara R, Matsumaru T, Kano K. Effect of volatile fatty acids on adipocyte differentiation in bovine dedifferentiated fat (DFAT) cells in vitro. Genes Cells 2021;n/a. 10.1111/gtc.12903 [DOI] [PubMed] [Google Scholar]
- [73].Wei S, Duarte MS, Du M, Paulino PVR, Jiang Z, Albrecht E, et al. Bovine mature adipocytes readily return to a proliferative state. Tissue Cell 2012;44:385–90. 10.1016/j.tice.2012.08.001 [DOI] [PubMed] [Google Scholar]
- [74].O’Neill EN, Cosenza ZA, Baar K, Block DE. Considerations for the development of cost-effective cell culture media for cultivated meat production. Compr Rev Food Sci Food Saf 2021;20:686–709. 10.1111/1541-4337.12678 [DOI] [PubMed] [Google Scholar]
- [75].Parekkadan B, Milwid JM. Mesenchymal Stem Cells as Therapeutics. Annu Rev Biomed Eng 2010;12:87–117. 10.1146/annurev-bioeng-070909-105309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wang Y, Ren L, Xing Y, Hu X, Li Q, Xu L, et al. BMP4 and Rosiglitazone Improves Adipogenesis of Bovine Fetal Muscle Derived Progenitor Cells. Pak J Zool 2020;53. 10.17582/journal.pjz/20190718150746 [DOI] [Google Scholar]
- [77].Zhang J, Liu Y, Chen Y, Yuan L, Liu H, Wang J, et al. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int 2020;2020:e8810813. 10.1155/2020/8810813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Li X, Fu X, Yang G, Du M. Review: Enhancing intramuscular fat development via targeting fibro-adipogenic progenitor cells in meat animals. Animal 2020;14:312–21. 10.1017/S175173111900209X [DOI] [PubMed] [Google Scholar]
- [79].Biferali B, Proietti D, Mozzetta C, Madaro L. Fibro–Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Front Physiol 2019;10:1074. 10.3389/fphys.2019.01074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Judson RN, Low M, Eisner C, Rossi FM. Isolation, Culture, and Differentiation of Fibro/Adipogenic Progenitors (FAPs) from Skeletal Muscle. Methods Mol Biol Clifton NJ 2017;1668:93–103. 10.1007/978-1-4939-7283-8_7 [DOI] [PubMed] [Google Scholar]
- [81].Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res 2012;53:227–46. 10.1194/jlr.R021089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mehta F, Theunissen R, Post MJ. Adipogenesis from Bovine Precursors. In: Rønning SB, editor. Myogenesis Methods Protoc, New York, NY: Springer; 2019, p. 111–25. 10.1007/978-1-4939-8897-6_8 [DOI] [PubMed] [Google Scholar]
- [83].Zhao Y, Waldman SD, Flynn LE. The effect of serial passaging on the proliferation and differentiation of bovine adipose-derived stem cells. Cells Tissues Organs 2012;195:414–27. 10.1159/000329254 [DOI] [PubMed] [Google Scholar]
- [84].Chen Y-J, Liu H-Y, Chang Y-T, Cheng Y-H, Mersmann HJ, Kuo W-H, et al. Isolation and Differentiation of Adipose-Derived Stem Cells from Porcine Subcutaneous Adipose Tissues. JoVE J Vis Exp 2016:e53886. 10.3791/53886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].D’Andrea F, De Francesco F, Ferraro GA, Desiderio V, Tirino V, De Rosa A, et al. Large-Scale Production of Human Adipose Tissue from Stem Cells: A New Tool for Regenerative Medicine and Tissue Banking. Tissue Eng Part C Methods 2008;14:233–42. 10.1089/ten.tec.2008.0108 [DOI] [PubMed] [Google Scholar]
- [86].Zhang S, Bai C, Zheng D, Gao Y, Fan Y, Li L, et al. Identification and characterization of pig adipose-derived progenitor cells. Can J Vet Res 2016;80:309–17 [PMC free article] [PubMed] [Google Scholar]
- [87].Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol 2005;205:194–201. 10.1002/jcp.20376 [DOI] [PubMed] [Google Scholar]
- [88].Sanosaka M, Minashima T, Suzuki K, Watanabe K, Ohwada S, Hagino A, et al. A combination of octanoate and oleate promotes in vitro differentiation of porcine intramuscular adipocytes. Comp Biochem Physiol B Biochem Mol Biol 2008;149:285–92. 10.1016/j.cbpb.2007.09.019 [DOI] [PubMed] [Google Scholar]
- [89].Sampaio RV, Chiaratti MR, Santos DCN, Bressan FF, Sangalli JR, Sá ALA, et al. Generation of bovine (Bos indicus) and buffalo (Bubalus bubalis) adipose tissue derived stem cells: isolation, characterization, and multipotentiality. Genet Mol Res 2015;14:53–62. 10.4238/2015.January.15.7 [DOI] [PubMed] [Google Scholar]
- [90].Lu T, Xiong H, Wang K, Wang S, Ma Y, Guan W. Isolation and Characterization of Adipose-derived Mesenchymal Stem Cells (ADSCs) from Cattle. Appl Biochem Biotechnol 2014;174:719–28. 10.1007/s12010-014-1128-3 [DOI] [PubMed] [Google Scholar]
- [91].Lengi AJ, Corl BA. Factors influencing the differentiation of bovine preadipocytes in vitro. J Anim Sci 2010;88:1999–2008. 10.2527/jas.2009-2439 [DOI] [PubMed] [Google Scholar]
- [92].Jurek S, Sandhu MA, Trappe S, Bermúdez-Peña MC, Kolisek M, Sponder G, et al. Optimizing adipogenic transdifferentiation of bovine mesenchymal stem cells: a prominent role of ascorbic acid in FABP4 induction. Adipocyte 2020;9:35–50. 10.1080/21623945.2020.1720480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Strieder-Barboza C, Thompson E, Thelen K, Contreras GA. Technical note: Bovine adipocyte and preadipocyte co-culture as an efficient adipogenic model. J Dairy Sci 2019;102:3622–9. 10.3168/jds.2018-15626 [DOI] [PubMed] [Google Scholar]
- [94].Lu T, Pei W, Wang K, Zhang S, Chen F, Wu Y, et al. In vitro culture and biological properties of broiler adipose-derived stem cells. Exp Ther Med 2018;16:2399–407. 10.3892/etm.2018.6445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Cui HX, Guo LP, Zhao GP, Liu RR, Li QH, Zheng MQ, et al. Method using a co-culture system with high-purity intramuscular preadipocytes and satellite cells from chicken pectoralis major muscle. Poult Sci 2018;97:3691–7. 10.3382/ps/pey023 [DOI] [PubMed] [Google Scholar]
- [96].Wang S, Zhang Y, Xu Q, Yuan X, Dai W, Shen X, et al. The differentiation of preadipocytes and gene expression related to adipogenesis in ducks (Anas platyrhynchos). PloS One 2018;13:e0196371. 10.1371/journal.pone.0196371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wang K-H, Kao A-P, Wangchen H, Wang F-Y, Chang C-H, Chang C-C, et al. Optimizing proliferation and characterization of multipotent stem cells from porcine adipose tissue. Biotechnol Appl Biochem 2008;51:159–66. 10.1042/BA20070201 [DOI] [PubMed] [Google Scholar]
- [98].Hadden WJ, Young JL, Holle AW, McFetridge ML, Kim DY, Wijesinghe P, et al. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc Natl Acad Sci 2017;114:5647–52. 10.1073/pnas.1618239114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol 2017;18:728–42. 10.1038/nrm.2017.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006;126:677–89. 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- [101].Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 2010;9:518–26. 10.1038/nmat2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Yang Y, Wang K, Gu X, Leong KW. Biophysical Regulation of Cell Behavior-Cross Talk between Substrate Stiffness and Nanotopography. Eng Beijing China 2017;3:36–54. 10.1016/J.ENG.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Aso H, Abe H, Nakajima I, Ozutsumi K, Yamaguchi T, Takamori Y, et al. A Preadipocyte Clonal Line from Bovine Intramuscular Adipose Tissue: Nonexpression of GLUT-4 Protein during Adipocyte Differentiation. Biochem Biophys Res Commun 1995;213:369–75. 10.1006/bbrc.1995.2141 [DOI] [PubMed] [Google Scholar]
- [104].Liao N, Shi Y, Zhang C, Zheng Y, Wang Y, Zhao B, et al. Antioxidants inhibit cell senescence and preserve stemness of adipose tissue-derived stem cells by reducing ROS generation during long-term in vitro expansion. Stem Cell Res Ther 2019;10:306. 10.1186/s13287-019-1404-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Liu X, Xiang Q, Xu F, Huang J, Yu N, Zhang Q, et al. Single-cell RNA-seq of cultured human adipose-derived mesenchymal stem cells. Sci Data 2019;6:190031. 10.1038/sdata.2019.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Navarro M, Soto DA, Pinzon CA, Wu J, Ross PJ, Navarro M, et al. Livestock pluripotency is finally captured in vitro. Reprod Fertil Dev 2020;32:11–39. 10.1071/RD19272 [DOI] [PubMed] [Google Scholar]
- [107].Pawlowski M, Ortmann D, Bertero A, Tavares JM, Pedersen RA, Vallier L, et al. Inducible and Deterministic Forward Programming of Human Pluripotent Stem Cells into Neurons, Skeletal Myocytes, and Oligodendrocytes. Stem Cell Rep 2017;8:803–12. 10.1016/j.stemcr.2017.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Yu X, Fang X, Gao M, Mi J, Zhang X, Xia L, et al. Isolation and Identification of Bovine Preadipocytes and Screening of MicroRNAs Associated with Adipogenesis. Animals 2020;10:818. 10.3390/ani10050818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zhao L, Gao X, Zheng Y, Wang Z, Zhao G, Ren J, et al. Establishment of bovine expanded potential stem cells. Proc Natl Acad Sci 2021;118:e2018505118. 10.1073/pnas.2018505118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Soto DA, Navarro M, Zheng C, Halstead MM, Zhou C, Guiltinan C, et al. Simplification of culture conditions and feeder-free expansion of bovine embryonic stem cells. Sci Rep 2021;11:11045. 10.1038/s41598-021-90422-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Brevini TAL, Antonini S, Cillo F, Crestan M, Gandolfi F. Porcine embryonic stem cells: Facts, challenges and hopes. Theriogenology 2007;68:S206–13. 10.1016/j.theriogenology.2007.05.043 [DOI] [PubMed] [Google Scholar]
- [112].Yuan Y, Park J, Tian Y, Choi J, Pasquariello R, Alexenko AP, et al. A six-inhibitor culture medium for improving naïve-type pluripotency of porcine pluripotent stem cells. Cell Death Discov 2019;5:104. 10.1038/s41420-019-0184-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ahfeldt T, Schinzel RT, Lee Y-K, Hendrickson D, Kaplan A, Lum DH, et al. Programming human pluripotent stem cells into white and brown adipocytes. Nat Cell Biol 2012;14:209–19. 10.1038/ncb2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Mohsen-Kanson T, Hafner A-L, Wdziekonski B, Takashima Y, Villageois P, Carrière A, et al. Differentiation of Human Induced Pluripotent Stem Cells into Brown and White Adipocytes: Role of Pax3. STEM CELLS 2014;32:1459–67. 10.1002/stem.1607 [DOI] [PubMed] [Google Scholar]
- [115].Lengi AJ, Corl BA. Factors influencing the differentiation of bovine preadipocytes in vitro1. J Anim Sci 2010;88:1999–2008. 10.2527/jas.2009-2439 [DOI] [PubMed] [Google Scholar]
- [116].Lu T, Pei W, Wang K, Zhang S, Chen F, Wu Y, et al. In vitro culture and biological properties of broiler adipose-derived stem cells. Exp Ther Med 2018. 10.3892/etm.2018.6445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Welter JF, Penick KJ, Solchaga LA. Assessing Adipogenic Potential of Mesenchymal Stem Cells: A Rapid Three-Dimensional Culture Screening Technique. Stem Cells Int 2013;2013:1–8. 10.1155/2013/806525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Schneider S, Unger M, van Griensven M, Balmayor ER. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur J Med Res 2017;22:17. 10.1186/s40001-017-0258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Czapla J, Matuszczak S, Kulik K, Wiśniewska E, Pilny E, Jarosz-Biej M, et al. The effect of culture media on large-scale expansion and characteristic of adipose tissue-derived mesenchymal stromal cells. Stem Cell Res Ther 2019;10:235. 10.1186/s13287-019-1331-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Sasahara Y, Kubota Y, Kosaka K, Adachi N, Yamaji Y, Nagano H, et al. Adipose-Derived Stem Cells and Ceiling Culture-Derived Preadipocytes Cultured from Subcutaneous Fat Tissue Differ in Their Epigenetic Characteristics and Osteogenic Potential: Plast Reconstr Surg 2019;144:644–55. 10.1097/PRS.0000000000005913 [DOI] [PubMed] [Google Scholar]
- [121].Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA, et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol-Regul Integr Comp Physiol 2002;282:R1286–96. 10.1152/ajpregu.00653.2001 [DOI] [PubMed] [Google Scholar]
- [122].Li D, Secher J, Hyttel P, Ivask M, Kolko M, Hall VJ, et al. Generation of transgene-free porcine intermediate type induced pluripotent stem cells. Cell Cycle 2018;17:2547–63. 10.1080/15384101.2018.1548790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Choi K-H, Lee D-K, Kim SW, Woo S-H, Kim D-Y, Lee C-K. Chemically Defined Media Can Maintain Pig Pluripotency Network In Vitro. Stem Cell Rep 2019;13:221–34. 10.1016/j.stemcr.2019.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Bogliotti YS, Wu J, Vilarino M, Okamura D, Soto DA, Zhong C, et al. Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc Natl Acad Sci U S A 2018;115:2090–5. 10.1073/pnas.1716161115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zhang L, Wu Y, Li X, Wei S, Xing Y, Lian Z, et al. An Alternative Method for Long-Term Culture of Chicken Embryonic Stem Cell In Vitro. Stem Cells Int 2018;2018:e2157451. 10.1155/2018/2157451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wang W, Zhang T, Wu C, Wang S, Wang Y, Li H, et al. Immortalization of chicken preadipocytes by retroviral transduction of chicken TERT and TR. PLoS ONE 2017;12. 10.1371/journal.pone.0177348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Morganstein DL, Christian M, Turner JJO, Parker MG, White R. Conditionally immortalized white preadipocytes: a novel adipocyte models. J Lipid Res 2008;49:679–85. 10.1194/jlr.D700029-JLR200 [DOI] [PubMed] [Google Scholar]
- [128].Lee J, Kim D-H, Suh Y, Lee K. Research Note: Potential usage of DF-1 cell line as a new cell model for avian adipogenesis. Poult Sci 2021;100:101057. 10.1016/j.psj.2021.101057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Wang X, Moutsoglou D. Osteogenic and adipogenic differentiation potential of an immortalized fibroblast-like cell line derived from porcine peripheral blood. Vitro Cell Dev Biol - Anim 2009;45:584–91. 10.1007/s11626-009-9231-4 [DOI] [PubMed] [Google Scholar]
- [130].Yin J, Jin X, Beck S, Kang DH, Hong Z, Li Z, et al. In vitro myogenic and adipogenic differentiation model of genetically engineered bovine embryonic fibroblast cell lines. Biotechnol Lett 2010;32:195–202. 10.1007/s10529-009-0142-y [DOI] [PubMed] [Google Scholar]
- [131].Trott DA, Cuthbert AP, Overell RW, Russo I, Newbold RF. Mechanisms involved in the immortalization of mammalian cells by ionizing radiation and chemical carcinogens. Carcinogenesis 1995;16:193–204. 10.1093/carcin/16.2.193 [DOI] [PubMed] [Google Scholar]
- [132].Foster DN, Foster LK. Immortalized cell lines for virus growth. US5672485A, 1996
- [133].Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell 1974;3:127–33. 10.1016/0092-8674(74)90116-0 [DOI] [PubMed] [Google Scholar]
- [134].Schmidt SM, Belisle M, Frommer WB. The evolving landscape around genome editing in agriculture. EMBO Rep 2020;21:e50680. 10.15252/embr.202050680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Ramunas J, Yakubov E, Brady JJ, Corbel SY, Holbrook C, Brandt M, et al. Transient delivery of modified mRNA encoding TERT rapidly extends telomeres in human cells. FASEB J 2015;29:1930–9. 10.1096/fj.14-259531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Nagpal N, Wang J, Zeng J, Lo E, Moon DH, Luk K, et al. Small-Molecule PAPD5 Inhibitors Restore Telomerase Activity in Patient Stem Cells. Cell Stem Cell 2020;26:896–909.e8. 10.1016/j.stem.2020.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature 2010;465:704–12. 10.1038/nature09229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Taura D, Noguchi M, Sone M, Hosoda K, Mori E, Okada Y, et al. Adipogenic differentiation of human induced pluripotent stem cells: Comparison with that of human embryonic stem cells. FEBS Lett 2009;583:1029–33. 10.1016/j.febslet.2009.02.031 [DOI] [PubMed] [Google Scholar]
- [139].Burrell K, Dardari R, Goldsmith T, Toms D, Villagomez DAF, King WA, et al. Stirred Suspension Bioreactor Culture of Porcine Induced Pluripotent Stem Cells. Stem Cells Dev 2019;28:1264–75. 10.1089/scd.2019.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Re S, Dogan AA, Ben-Shachar D, Berger G, Werling AM, Walitza S, et al. Improved Generation of Induced Pluripotent Stem Cells From Hair Derived Keratinocytes – A Tool to Study Neurodevelopmental Disorders as ADHD. Front Cell Neurosci 2018;12. 10.3389/fncel.2018.00321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Steinle H, Weber M, Behring A, Mau-Holzmann U, Schlensak C, Wendel HP, et al. Generation of iPSCs by Nonintegrative RNA-Based Reprogramming Techniques: Benefits of Self-Replicating RNA versus Synthetic mRNA. Stem Cells Int 2019;2019:e7641767. 10.1155/2019/7641767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Soto DA, Ross PJ. Pluripotent stem cells and livestock genetic engineering. Transgenic Res 2016;25:289–306. 10.1007/s11248-016-9929-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Pessôa LV de F, Bressan FF, Freude KK. Induced pluripotent stem cells throughout the animal kingdom: Availability and applications. World J Stem Cells 2019;11:491–505. 10.4252/wjsc.v11.i8.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Ogorevc J, Orehek S, Dovč P. Cellular reprogramming in farm animals: an overview of iPSC generation in the mammalian farm animal species. J Anim Sci Biotechnol 2016;7:10. 10.1186/s40104-016-0070-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Fuet A, Pain B. Chicken Induced Pluripotent Stem Cells: Establishment and Characterization. In: Sheng G, editor. Avian Reptil. Dev. Biol. Methods Protoc, New York, NY: Springer; 2017, p. 211–28. 10.1007/978-1-4939-7216-6_14 [DOI] [PubMed] [Google Scholar]
- [146].Bao L, He L, Chen J, Wu Z, Liao J, Rao L, et al. Reprogramming of ovine adult fibroblasts to pluripotency via drug-inducible expression of defined factors. Cell Res 2011;21:600–8. 10.1038/cr.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Hall VJ, Kristensen M, Rasmussen MA, Ujhelly O, Dinnyés A, Hyttel P. Temporal Repression of Endogenous Pluripotency Genes during Reprogramming of Porcine Induced Pluripotent Stem Cells. Cell Reprogramming 2012;14:204–16. 10.1089/cell.2011.0089 [DOI] [PubMed] [Google Scholar]
- [148].Chen H, Liu C, Chen C, Su Z, Shu J, Zhang M, et al. Bone morphogenetic protein 4 regulates immortalized chicken preadipocyte proliferation by promoting G1/S cell cycle progression. FEBS Open Bio 2019;9:1109–18. 10.1002/2211-5463.12640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Wn Z, Yx W, H L. [Effect of perilipin 1 on chicken preadipocyte lipid accumulation]. Bao Xi Fen Yu Mian Zi Xue Yi Zhi Za Chin J Cell Mol Immunol 2012;28:944–7 [PubMed] [Google Scholar]
- [150].Sun Y, Zhai G, Li R, Zhou W, Li Y, Cao Z, et al. RXRα Positively Regulates Expression of the Chicken PLIN1 Gene in a PPARγ-Independent Manner and Promotes Adipogenesis. Front Cell Dev Biol 2020;8. 10.3389/fcell.2020.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 1994;79:1147–56. 10.1016/00928674(94)90006-X [DOI] [PubMed] [Google Scholar]
- [152].Liu W, Meridew JA, Aravamudhan A, Ligresti G, Tschumperlin DJ, Tan Q. Targeted regulation of fibroblast state by CRISPR-mediated CEBPA expression. Respir Res 2019;20:281. 10.1186/s12931-019-1253-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Darimont C, Zbinden I, Avanti O, Leone-Vautravers P, Giusti V, Burckhardt P, et al. Reconstitution of telomerase activity combined with HPV-E7 expression allow human preadipocytes to preserve their differentiation capacity after immortalization. Cell Death Differ 2003;10:1025–31. 10.1038/sj.cdd.4401273 [DOI] [PubMed] [Google Scholar]
- [154].Wu Z, Chen J, Ren J, Bao L, Liao J, Cui C, et al. Generation of Pig Induced Pluripotent Stem Cells with a Drug-Inducible System. J Mol Cell Biol 2009;1:46–54. 10.1093/jmcb/mjp003 [DOI] [PubMed] [Google Scholar]
- [155].Han X, Han J, Ding F, Cao S, Lim SS, Dai Y, et al. Generation of induced pluripotent stem cells from bovine embryonic fibroblast cells. Cell Res 2011;21:1509–12. 10.1038/cr.2011.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Honda A, Hirose M, Hatori M, Matoba S, Miyoshi H, Inoue K, et al. Generation of Induced Pluripotent Stem Cells in Rabbits. J Biol Chem 2010;285:31362–9. 10.1074/jbc.M110.150540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Li Y, Cang M, Lee AS, Zhang K, Liu D. Reprogramming of Sheep Fibroblasts into Pluripotency under a Drug-Inducible Expression of Mouse-Derived Defined Factors. PLOS ONE 2011;6:e15947. 10.1371/journal.pone.0015947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Rosselló RA, Chen C-C, Dai R, Howard JT, Hochgeschwender U, Jarvis ED. Mammalian genes induce partially reprogrammed pluripotent stem cells in non-mammalian vertebrate and invertebrate species. ELife 2013;2:e00036. 10.7554/eLife.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Su Y, Zhu J, Salman S, Tang Y. Induced pluripotent stem cells from farm animals. J Anim Sci 2020;98. 10.1093/jas/skaa343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Tomiyama AJ, Kawecki NS, Rosenfeld DL, Jay JA, Rajagopal D, Rowat AC. Bridging the gap between the science of cultured meat and public perceptions. Trends Food Sci Technol 2020;104:144–52. 10.1016/j.tifs.2020.07.019 [DOI] [Google Scholar]
- [161].AquAdvantage Salmon Approval Letter and Appendix. US Food Drug Adm; 2020. https://www.fda.gov/animal-veterinary/animals-intentional-genomic-alterations/aquadvantagesalmon-approval-letter-and-appendix (accessed November 16, 2021) [Google Scholar]
- [162].FDA Approves First-of-its-Kind Intentional Genomic Alteration in Line of Domestic Pigs for Both Human Food, Potential Therapeutic Uses. US Food Drug Adm; 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-its-kind-intentional-genomic-alteration-line-domestic-pigs-both-human-food (accessed November 16, 2021) [Google Scholar]
- [163].Tsao YS, Condon R, Schaefer E, Lio P, Liu Z. Development and improvement of a serum-free suspension process for the production of recombinant adenoviral vectors using HEK293 cells. Cytotechnology 2001;37:189–98. 10.1023/A:1020555310558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Malm M, Saghaleyni R, Lundqvist M, Giudici M, Chotteau V, Field R, et al. Evolution from adherent to suspension: systems biology of HEK293 cell line development. Sci Rep 2020;10:18996. 10.1038/s41598-020-76137-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Taticek RA, Choi C, Phan S-E, Palomares LA, Shuler ML. Comparison of Growth and Recombinant Protein Expression in Two Different Insect Cell Lines in Attached and Suspension Culture. Biotechnol Prog 2001;17:676–84. 10.1021/bp010061g [DOI] [PubMed] [Google Scholar]
- [166].Chen VC, Couture LA. The suspension culture of undifferentiated human pluripotent stem cells using spinner flasks. Methods Mol Biol Clifton NJ 2015;1283:13–21. 10.1007/7651_2014_118 [DOI] [PubMed] [Google Scholar]
- [167].Backer MP, Metzger LS, Slaber PL, Nevitt KL, Boder GB. Large-scale production of monoclonal antibodies in suspension culture. Biotechnol Bioeng 1988;32:993–1000. 10.1002/bit.260320807 [DOI] [PubMed] [Google Scholar]
- [168].Pörtner R, Nagel-Heyer S, Goepfert C, Adamietz P, Meenen NM. Bioreactor design for tissue engineering. J Biosci Bioeng 2005;100:235–45. 10.1263/jbb.100.235 [DOI] [PubMed] [Google Scholar]
- [169].Hanga MP, Ali J, Moutsatsou P, Raga FA de la, Hewitt CJ, Nienow A, et al. Bioprocess development for scalable production of cultivated meat. Biotechnol Bioeng 2020;117:3029–39. 10.1002/bit.27469 [DOI] [PubMed] [Google Scholar]
- [170].Rodrigues ME, Costa AR, Henriques M, Azeredo J, Oliveira R. Wave characterization for mammalian cell culture: residence time distribution. New Biotechnol 2012;29:402–8. 10.1016/j.nbt.2011.10.006 [DOI] [PubMed] [Google Scholar]
- [171].Singh V Disposable bioreactor for cell culture using wave-induced agitation. Cytotechnology 1999;30:149–58. 10.1023/A:1008025016272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Varley J, Birch J. Reactor design for large scale suspension animal cell culture. Cytotechnology 1999;29:177. 10.1023/A:1008008021481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Mullen N, Park N, Jones C, Bowman T, Bignone P, SANTO VE, et al. In vitro avian food product. US20200392461A1, 2020
- [174].Li X, Zhang G, Zhao X, Zhou J, Du G, Chen J. A conceptual air-lift reactor design for large scale animal cell cultivation in the context of in vitro meat production. Chem Eng Sci 2020;211:115269. 10.1016/j.ces.2019.115269 [DOI] [Google Scholar]
- [175].Allen LM, Matyas J, Ungrin M, Hart DA, Sen A. Serum-Free Culture of Human Mesenchymal Stem Cell Aggregates in Suspension Bioreactors for Tissue Engineering Applications. Stem Cells Int 2019;2019:e4607461. 10.1155/2019/4607461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Alimperti S, Lei P, Wen Y, Tian J, Campbell AM, Andreadis ST. Serum-free spheroid suspension culture maintains mesenchymal stem cell proliferation and differentiation potential. Biotechnol Prog 2014;30:974–83. 10.1002/btpr.1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Steiner D, Khaner H, Cohen M, Even-Ram S, Gil Y, Itsykson P, et al. Derivation, propagation and controlled differentiation of human embryonic stem cells in suspension. Nat Biotechnol 2010;28:361–4. 10.1038/nbt.1616 [DOI] [PubMed] [Google Scholar]
- [178].Nampe D, Joshi R, Keller K, Nieden NI zur, Tsutsui H. Impact of fluidic agitation on human pluripotent stem cells in stirred suspension culture. Biotechnol Bioeng 2017;114:2109–20. 10.1002/bit.26334 [DOI] [PubMed] [Google Scholar]
- [179].Yabe SG, Fukuda S, Nishida J, Takeda F, Nashiro K, Okochi H. Induction of functional islet-like cells from human iPS cells by suspension culture. Regen Ther 2019;10:69–76. 10.1016/j.reth.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Wang Y-H, Wu J-Y, Chou P-J, Chen C-H, Wang C-Z, Ho M-L, et al. Characterization and evaluation of the differentiation ability of human adipose-derived stem cells growing in scaffold-free suspension culture. Cytotherapy 2014;16:485–95. 10.1016/j.jcyt.2013.07.015 [DOI] [PubMed] [Google Scholar]
- [181].Klingelhutz AJ, Gourronc FA, Chaly A, Wadkins DA, Burand AJ, Markan KR, et al. Scaffold-free generation of uniform adipose spheroids for metabolism research and drug discovery. Sci Rep 2018;8:1–12. 10.1038/s41598-017-19024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Dev Cell 2004;6:483–95. 10.1016/S1534-5807(04)00075-9 [DOI] [PubMed] [Google Scholar]
- [183].Nogueira DES, Rodrigues CAV, Carvalho MS, Miranda CC, Hashimura Y, Jung S, et al. Strategies for the expansion of human induced pluripotent stem cells as aggregates in single-use Vertical-Wheel™ bioreactors. J Biol Eng 2019;13:74. 10.1186/s13036-019-0204-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Sutherland R, Carlsson J, Durand R, Yuhas J. Spheroids in Cancer Research. Cancer Res 1981;41:2980–4 [Google Scholar]
- [185].Daquinag AC, Souza GR, Kolonin MG. Adipose Tissue Engineering in Three-Dimensional Levitation Tissue Culture System Based on Magnetic Nanoparticles. Tissue Eng Part C Methods 2013;19:336–44. 10.1089/ten.tec.2012.0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Muller S, Ader I, Creff J, Leménager H, Achard P, Casteilla L, et al. Human adipose stromal-vascular fraction self-organizes to form vascularized adipose tissue in 3D cultures. Sci Rep 2019;9:1–10. 10.1038/s41598-019-43624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Ingram M, Techy GB, Saroufeem R, Yazan O, Narayan KS, Goodwin TJ, et al. Three-dimensional growth patterns of various human tumor cell lines in simulated microgravity of a NASA bioreactor. Vitro Cell Dev Biol - Anim 1997;33:459–66. 10.1007/s11626-997-0064-8 [DOI] [PubMed] [Google Scholar]
- [188].Hammond TG, Hammond JM. Optimized suspension culture: the rotating-wall vessel. Am J Physiol-Ren Physiol 2001;281:F12–25. 10.1152/ajprenal.2001.281.1.F12 [DOI] [PubMed] [Google Scholar]
- [189].Liu Y, Liu T, Fan X, Ma X, Cui Z. Ex vivo expansion of hematopoietic stem cells derived from umbilical cord blood in rotating wall vessel. J Biotechnol 2006;124:592–601. 10.1016/j.jbiotec.2006.01.020 [DOI] [PubMed] [Google Scholar]
- [190].Gerecht-Nir S, Cohen S, Itskovitz-Eldor J. Bioreactor cultivation enhances the efficiency of human embryoid body (hEB) formation and differentiation. Biotechnol Bioeng 2004;86:493–502. 10.1002/bit.20045 [DOI] [PubMed] [Google Scholar]
- [191].Frye CA, Patrick CW. Three-dimensional adipose tissue model using low shear bioreactors. In Vitro Cell Dev Biol Anim 2006;42:109–14. 10.1290/0509055.1 [DOI] [PubMed] [Google Scholar]
- [192].Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods 2010;16:735–49. 10.1089/ten.TEC.2009.0432 [DOI] [PubMed] [Google Scholar]
- [193].Song K, Yan X, Zhang Y, Song F, Lim M, Fang M, et al. Numberical simulation of fluid flow and three-dimensional expansion of tissue engineering seed cells in large scale inside a novel rotating wall hollow fiber membrane bioreactor. Bioprocess Biosyst Eng 2015;38:1527–40. 10.1007/s00449-015-1395-6 [DOI] [PubMed] [Google Scholar]
- [194].Borys BS, Dang T, So T, Rohani L, Revay T, Walsh T, et al. Overcoming bioprocess bottlenecks in the large-scale expansion of high-quality hiPSC aggregates in vertical-wheel stirred suspension bioreactors. Stem Cell Res Ther 2021;12:55. 10.1186/s13287-020-02109-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Borst AG, van Loon JJWA. Technology and Developments for the Random Positioning Machine, RPM. Microgravity Sci Technol 2009;21:287–92. 10.1007/s12217-008-9043-2 [DOI] [Google Scholar]
- [196].van Loon JJWA. Some history and use of the random positioning machine, RPM, in gravity related research. Adv Space Res 2007;39:1161–5. 10.1016/j.asr.2007.02.016 [DOI] [Google Scholar]
- [197].Varley MC, Markaki AE, Brooks RA. Effect of Rotation on Scaffold Motion and Cell Growth in Rotating Bioreactors. Tissue Eng Part A 2017;23:522–34. 10.1089/ten.tea.2016.0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Young DA, Choi YS, Engler AJ, Christman KL. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials 2013;34:8581–8. 10.1016/j.biomaterials.2013.07.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Chandler EM, Berglund CM, Lee JS, Polacheck WJ, Gleghorn JP, Kirby BJ, et al. Stiffness of photocrosslinked RGD-alginate gels regulates adipose progenitor cell behavior. Biotechnol Bioeng 2011;108:1683–92. 10.1002/bit.23079 [DOI] [PubMed] [Google Scholar]
- [200].Turner PA, Gurumurthy B, Bailey JL, Elks CM, Janorkar AV. Adipogenic differentiation of human adipose-derived stem cells grown as spheroids. Process Biochem 2017;59:312–20. 10.1016/j.procbio.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Bellani CF, Ajeian J, Duffy L, Miotto M, Groenewegen L, Connon CJ. Scale-Up Technologies for the Manufacture of Adherent Cells. Front Nutr 2020;7. 10.3389/fnut.2020.575146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Tohyama S, Fujita J, Fujita C, Yamaguchi M, Kanaami S, Ohno R, et al. Efficient Large-Scale 2D Culture System for Human Induced Pluripotent Stem Cells and Differentiated Cardiomyocytes. Stem Cell Rep 2017;9:1406–14. 10.1016/j.stemcr.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Bodiou V, Moutsatsou P, Post MJ. Microcarriers for Upscaling Cultured Meat Production. Front Nutr 2020;7. 10.3389/fnut.2020.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Martin Y, Eldardiri M, Lawrence-Watt DJ, Sharpe JR. Microcarriers and their potential in tissue regeneration. Tissue Eng Part B Rev 2011;17:71–80. 10.1089/ten.TEB.2010.0559 [DOI] [PubMed] [Google Scholar]
- [205].Nold P, Brendel C, Neubauer A, Bein G, Hackstein H. Good manufacturing practice-compliant animal-free expansion of human bone marrow derived mesenchymal stroma cells in a closed hollow-fiber-based bioreactor. Biochem Biophys Res Commun 2013;430:325–30. 10.1016/j.bbrc.2012.11.001 [DOI] [PubMed] [Google Scholar]
- [206].Barckhausen C, Rice B, Baila S, Sensebé L, Schrezenmeier H, Nold P, et al. GMP-Compliant Expansion of Clinical-Grade Human Mesenchymal Stromal/Stem Cells Using a Closed Hollow Fiber Bioreactor. In: Gnecchi M, editor. Mesenchymal Stem Cells Methods Protoc, New York, NY: Springer; 2016, p. 389–412. 10.1007/978-1-4939-3584-0_23 [DOI] [PubMed] [Google Scholar]
- [207].Paccola Mesquita FC, Hochman-Mendez C, Morrissey J, Sampaio LC, Taylor DA. Laminin as a Potent Substrate for Large-Scale Expansion of Human Induced Pluripotent Stem Cells in a Closed Cell Expansion System. Stem Cells Int 2019;2019:e9704945. 10.1155/2019/9704945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Schive SW, Fjukstad R, Josefsen D, Katavić M, Abadpour S, Gullestad H, et al. Automated isolation and expansion of human adipose tissue-derived stem cells for a seamless translation into clinical trials. CellR4 2018;6:e2519 [Google Scholar]
- [209].Kumar A, Starly B. Large scale industrialized cell expansion: producing the critical raw material for biofabrication processes. Biofabrication 2015;7:044103. 10.1088/1758-5090/7/4/044103 [DOI] [PubMed] [Google Scholar]
- [210].Pörtner R, Platas Barradas OBJ. Cultivation of Mammalian Cells in Fixed-Bed Reactors. In: Pörtner R, editor. Anim. Cell Biotechnol. Methods Protoc, Totowa, NJ: Humana Press; 2007, p. 353–69. 10.1007/978-1-59745-399-8_17 [DOI] [Google Scholar]
- [211].Mizukami A, Orellana MD, Caruso SR, de Lima Prata K, Covas DT, Swiech K. Efficient expansion of mesenchymal stromal cells in a disposable fixed bed culture system. Biotechnol Prog 2013;29:568–72. 10.1002/btpr.1707 [DOI] [PubMed] [Google Scholar]
- [212].羽生 雄毅, 川 島一公. Growth induction system, growth induction control apparatus, growth induction control method, and growth induction control program. WO2017191691A1, 2017 [Google Scholar]
- [213].Miotto M, Gouveia R, Abidin FZ, Figueiredo F, Connon CJ. Developing a Continuous Bioprocessing Approach to Stromal Cell Manufacture. ACS Appl Mater Interfaces 2017;9:41131–42. 10.1021/acsami.7b09809 [DOI] [PubMed] [Google Scholar]
- [214].Henriksson I, Gatenholm P, Hägg DA. Increased lipid accumulation and adipogenic gene expression of adipocytes in 3D bioprinted nanocellulose scaffolds. Biofabrication 2017;9:015022. 10.1088/1758-5090/aa5c1c [DOI] [PubMed] [Google Scholar]
- [215].Turner PA, Tang Y, Weiss SJ, Janorkar AV. Three-Dimensional Spheroid Cell Model of In Vitro Adipocyte Inflammation. Tissue Eng Part A 2015;21:1837–47. 10.1089/ten.tea.2014.0531 [DOI] [PubMed] [Google Scholar]
- [216].Louis F, Kitano S, Mano JF, Matsusaki M. 3D collagen microfibers stimulate the functionality of preadipocytes and maintain the phenotype of mature adipocytes for long term cultures. Acta Biomater 2019;84:194–207. 10.1016/j.actbio.2018.11.048 [DOI] [PubMed] [Google Scholar]
- [217].Lei Y, Jeong D, Xiao J, Schaffer DV. Developing Defined and Scalable 3D Culture Systems for Culturing Human Pluripotent Stem Cells at High Densities. Cell Mol Bioeng 2014;7:172–83. 10.1007/s12195-014-0333-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Choi JS, Yang H-J, Kim BS, Kim JD, Lee SH, Lee EK, et al. Fabrication of porous extracellular matrix scaffolds from human adipose tissue. Tissue Eng Part C Methods 2010;16:387–96. 10.1089/ten.TEC.2009.0276 [DOI] [PubMed] [Google Scholar]
- [219].Place TL, Domann FE, Case AJ. Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research. Free Radic Biol Med 2017;113:311–22. 10.1016/j.freeradbiomed.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Coyle R, Yao J, Richards D, Mei Y. The Effects of Metabolic Substrate Availability on Human Adipose-Derived Stem Cell Spheroid Survival. Tissue Eng Part A 2019;25:620–31. 10.1089/ten.tea.2018.0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Murphy KC, Hung BP, Browne-Bourne S, Zhou D, Yeung J, Genetos DC, et al. Measurement of oxygen tension within mesenchymal stem cell spheroids. J R Soc Interface 2017;14:20160851. 10.1098/rsif.2016.0851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Tiruvannamalai-Annamalai R, Armant DR, Matthew HWT. A Glycosaminoglycan Based, Modular Tissue Scaffold System for Rapid Assembly of Perfusable, High Cell Density, Engineered Tissues. PLOS ONE 2014;9:e84287. 10.1371/journal.pone.0084287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Chung HJ, Park TG. Injectable Cellular Aggregates Prepared from Biodegradable Porous Microspheres for Adipose Tissue Engineering. Tissue Eng Part A 2008;15:1391–400. 10.1089/ten.tea.2008.0344 [DOI] [PubMed] [Google Scholar]
- [224].Fajas L Adipogenesis: a cross-talk between cell proliferation and cell differentiation. Ann Med 2003;35:79–85. 10.1080/07853890310009999 [DOI] [PubMed] [Google Scholar]
- [225].Lee M-J, Fried SK. Optimal Protocol for the Differentiation and Metabolic Analysis of Human Adipose Stromal Cells. Methods Enzymol 2014;538:49–65. 10.1016/B978-0-12-800280-3.00004-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Li Y, Powell S, Brunette E, Lebkowski J, Mandalam R. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol Bioeng 2005;91:688–98. 10.1002/bit.20536 [DOI] [PubMed] [Google Scholar]
- [227].Liu C-H, Wu M-L, Hwang S-M. Optimization of serum free medium for cord blood mesenchymal stem cells. Biochem Eng J 2007;33:1–9. 10.1016/j.bej.2006.08.005 [DOI] [Google Scholar]
- [228].Kolkmann AM, Post MJ, Rutjens MAM, van Essen ALM, Moutsatsou P. Serum-free media for the growth of primary bovine myoblasts. Cytotechnology 2020;72:111–20. 10.1007/s10616-019-00361-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Zhang X, Xue B, Li Y, Wei R, Yu Z, Jin J, et al. A novel chemically defined serum- and feeder-free medium for undifferentiated growth of porcine pluripotent stem cells. J Cell Physiol 2019. 10.1002/jcp.28185 [DOI] [PubMed] [Google Scholar]
- [230].Ma Y, Yu T, Cai Y, Wang H. Preserving self-renewal of porcine pluripotent stem cells in serum-free 3i culture condition and independent of LIF and b-FGF cytokines. Cell Death Discov 2018;4:1–14. 10.1038/s41420-017-0015-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Boone C, Grégoire F, Remacle C. Culture of porcine stromal-vascular cells in serum-free medium: differential action of various hormonal agents on adipose conversion. J Anim Sci 2000;78:885–95. 10.2527/2000.784885x [DOI] [PubMed] [Google Scholar]
- [232].Broad TE, Ham RG. Growth and adipose differentiation of sheep preadipocyte fibroblasts in serum-free medium. Eur J Biochem 1983;135:33–9. 10.1111/j.1432-1033.1983.tb07614.x [DOI] [PubMed] [Google Scholar]
- [233].Bell AA, Hansen PJ, De Vries A. Profitability of bovine somatotropin administration to increase first insemination conception rate in seasonal dairy herds with heat stress. Livest Sci 2009;126:38–45. 10.1016/j.livsci.2009.05.015 [DOI] [Google Scholar]
- [234].Marsh WE, Galligan DT, Chalupa W. Economics of Recombinant Bovine Somatotropin Use in Individual Dairy Herds. J Dairy Sci 1988;71:2944–58. 10.3168/jds.S0022-0302(88)79892-6 [DOI] [Google Scholar]
- [235].Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: Influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng 2006;93:1152–63. 10.1002/bit.20828 [DOI] [PubMed] [Google Scholar]
- [236].Ho YY, Lu HK, Lim ZFS, Lim HW, Ho YS, Ng SK. Applications and analysis of hydrolysates in animal cell culture. Bioresour Bioprocess 2021;8:93. 10.1186/s40643-021-00443-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Ng JY, Chua ML, Zhang C, Hong S, Kumar Y, Gokhale R, et al. Chlorella vulgaris Extract as a Serum Replacement That Enhances Mammalian Cell Growth and Protein Expression. Front Bioeng Biotechnol 2020;8:1068. 10.3389/fbioe.2020.564667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Saud B, Malla R, Shrestha K. A Review on the Effect of Plant Extract on Mesenchymal Stem Cell Proliferation and Differentiation. Stem Cells Int 2019;2019:e7513404. 10.1155/2019/7513404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Benjaminson MA, Gilchriest JA, Lorenz M. In vitro edible muscle protein production system (MPPS): stage 1, fish. Acta Astronaut 2002;51:879–89 [DOI] [PubMed] [Google Scholar]
- [240].George F, Kerschen D, Nuffel AV, Rees JF, Donnay I, George F, et al. Plant protein hydrolysates (plant peptones) as substitutes for animal proteins in embryo culture medium. Reprod Fertil Dev 2009;21:587–98. 10.1071/RD08147 [DOI] [PubMed] [Google Scholar]
- [241].Rubert Pérez CM, Álvarez Z, Chen F, Aytun T, Stupp SI. Mimicking the Bioactivity of Fibroblast Growth Factor-2 Using Supramolecular Nanoribbons. ACS Biomater Sci Eng 2017;3:2166–75. 10.1021/acsbiomaterials.7b00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Andreassen RC, Pedersen ME, Kristoffersen KA, Rønning SB. Screening of by-products from the food industry as growth promoting agents in serum-free media for skeletal muscle cell culture. Food Funct 2020;11:2477–88. 10.1039/C9FO02690H [DOI] [PubMed] [Google Scholar]
- [243].Lee M-J, Wu Y, Fried SK. A modified protocol to maximize differentiation of human preadipocytes and improve metabolic phenotypes. Obes Silver Spring Md 2012;20:2334–40. 10.1038/oby.2012.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Matsubara Y, Endo T, Kano K. Fatty acids but not dexamethasone are essential inducers for chick adipocyte differentiation in vitro. Comp Biochem Physiol A Mol Integr Physiol 2008;151:511–8. 10.1016/j.cbpa.2008.07.002 [DOI] [PubMed] [Google Scholar]
- [245].Mellouk N, Ramé C, Barbe A, Grandhaye J, Froment P, Dupont J. Chicken Is a Useful Model to Investigate the Role of Adipokines in Metabolic and Reproductive Diseases. Int J Endocrinol 2018;2018. 10.1155/2018/4579734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Dodson MV, Hausman GJ, Guan L, Du M, Rasmussen TP, Poulos SP, et al. Lipid metabolism, adipocyte depot physiology and utilization of meat animals as experimental models for metabolic research. Int J Biol Sci 2010;6:691–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Bergen WG, Mersmann HJ. Comparative Aspects of Lipid Metabolism: Impact on Contemporary Research and Use of Animal Models. J Nutr 2005;135:2499–502. 10.1093/jn/135.11.2499 [DOI] [PubMed] [Google Scholar]
- [248].Pu Y, Veiga-Lopez A. PPARγ agonist through the terminal differentiation phase is essential for adipogenic differentiation of fetal ovine preadipocytes. Cell Mol Biol Lett 2017;22:6. 10.1186/s11658-017-0037-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Arimochi H, Sasaki Y, Kitamura A, Yasutomo K. Differentiation of preadipocytes and mature adipocytes requires PSMB8. Sci Rep 2016;6:26791. 10.1038/srep26791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].García-Rojas P, Antaramian A, González-Dávalos L, Villarroya F, Shimada A, Varela-Echavarría A, et al. Induction of peroxisomal proliferator-activated receptor and peroxisomal proliferator-activated receptor coactivator 1 by unsaturated fatty acids, retinoic acid, and carotenoids in preadipocytes obtained from bovine white adipose tissue1,2. J Anim Sci 2010;88:1801–8. 10.2527/jas.2009-2579 [DOI] [PubMed] [Google Scholar]
- [251].Risner D, Li F, Fell JS, Pace SA, Siegel JB, Tagkopoulos I, et al. Preliminary Techno-Economic Assessment of Animal Cell-Based Meat. Foods 2021;10:3. 10.3390/foods10010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Nath SC, Nagamori E, Horie M, Kino-Oka M. Culture medium refinement by dialysis for the expansion of human induced pluripotent stem cells in suspension culture. Bioprocess Biosyst Eng 2017;40:123–31. 10.1007/s00449-016-1680-z [DOI] [PubMed] [Google Scholar]
- [253].Haraguchi Y, Kagawa Y, Sakaguchi K, Matsuura K, Shimizu T, Okano T. Thicker three-dimensional tissue from a “symbiotic recycling system” combining mammalian cells and algae. Sci Rep 2017;7:41594. 10.1038/srep41594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Haraguchi Y, Shimizu T. Three-dimensional tissue fabrication system by co-culture of microalgae and animal cells for production of thicker and healthy cultured food. Biotechnol Lett 2021. 10.1007/s10529-021-03106-0 [DOI] [PubMed] [Google Scholar]
- [255].Genovese NJ, SCHULZE EN, DESMET DN. Compositions and methods for increasing the efficiency of cell cultures used for food production. WO2019014652A1, 2019 [Google Scholar]
- [256].Mulukutla BC, Mitchell J, Geoffroy P, Harrington C, Krishnan M, Kalomeris T, et al. Metabolic engineering of Chinese hamster ovary cells towards reduced biosynthesis and accumulation of novel growth inhibitors in fed-batch cultures. Metab Eng 2019;54:54–68. 10.1016/j.ymben.2019.03.001 [DOI] [PubMed] [Google Scholar]
- [257].Fischer S, Handrick R, Otte K. The art of CHO cell engineering: A comprehensive retrospect and future perspectives. Biotechnol Adv 2015;33:1878–96. 10.1016/j.biotechadv.2015.10.015 [DOI] [PubMed] [Google Scholar]
- [258].Li X, Ma R, Gu Q, Liang L, Wang L, Zhang Y, et al. A fully defined static suspension culture system for large-scale human embryonic stem cell production. Cell Death Dis 2018;9:1–9. 10.1038/s41419-018-0863-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Gerlach JC, Lin Y-C, Brayfield CA, Minteer DM, Li H, Rubin JP, et al. Adipogenesis of Human Adipose-Derived Stem Cells Within Three-Dimensional Hollow Fiber-Based Bioreactors. Tissue Eng Part C Methods 2012;18:54–61. 10.1089/ten.tec.2011.0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Minteer DM, Young MT, Lin Y-C, Over PJ, Rubin JP, Gerlach JC, et al. Analysis of type II diabetes mellitus adipose-derived stem cells for tissue engineering applications. J Tissue Eng 2015;6:2041731415579215. 10.1177/2041731415579215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Krishnan A, Woodard SL. TrypZean™: An Animal-Free Alternative to Bovine Trypsin. In: Howard JA, Hood EE, editors. Commer. Plant-Prod. Recomb. Protein Prod. Case Stud, Berlin, Heidelberg: Springer; 2014, p. 43–63. 10.1007/978-3-662-43836-7_4 [DOI] [Google Scholar]
- [262].Bajpai R, Lesperance J, Kim M, Terskikh AV. Efficient propagation of single cells accutase-dissociated human embryonic stem cells. Mol Reprod Dev 2008;75:818–27. 10.1002/mrd.20809 [DOI] [PubMed] [Google Scholar]
- [263].Rourou S, Riahi N, Kallel H. A Protocol for Cell Detachment of Vero Cells Grown Under Fully Animal Component Free Conditions and on Cytodex 1 Microcarriers. In: Jenkins N, Barron N, Alves P, editors. Proc. 21st Annu. Meet. Eur. Soc. Anim. Cell Technol. ESACT Dublin Irel June 7–10 2009, Dordrecht: Springer Netherlands; 2012, p. 383–6. 10.1007/978-94-007-0884-6_62 [DOI] [Google Scholar]
- [264].Mirmohseni A, Seyed Dorraji MS, Figoli A, Tasselli F. Chitosan hollow fibers as effective biosorbent toward dye: Preparation and modeling. Bioresour Technol 2012;121:212–20. 10.1016/j.biortech.2012.06.067 [DOI] [PubMed] [Google Scholar]
- [265].Modrzejewska Z, Eckstein W. Chitosan hollow fiber membranes. Biopolymers 2004;73:61–8. 10.1002/bip.10510 [DOI] [PubMed] [Google Scholar]
- [266].Yan L, Jiang B, Li E, Wang X, Ling Q, Zheng D, et al. Scalable Generation of Mesenchymal Stem Cells from Human Embryonic Stem Cells in 3D. Int J Biol Sci 2018;14:1196–210. 10.7150/ijbs.25023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Scott MA, Nguyen VT, Levi B, James AW. Current Methods of Adipogenic Differentiation of Mesenchymal Stem Cells. Stem Cells Dev 2011;20:1793–804. 10.1089/scd.2011.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta BBA - Mol Basis Dis 2011;1812:1007–22. 10.1016/j.bbadis.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Yanting C, Yang QY, Ma GL, Du M, Harrison JH, Block E. Dose- and type-dependent effects of long-chain fatty acids on adipogenesis and lipogenesis of bovine adipocytes. J Dairy Sci 2018;101:1601–15. 10.3168/jds.2017-13312 [DOI] [PubMed] [Google Scholar]
- [270].Li XZ, Yan Y, Zhang JF, Sun JF, Sun B, Yan CG, et al. Oleic acid in the absence of a PPARγ agonist increases adipogenic gene expression in bovine muscle satellite cells1. J Anim Sci 2019;97:4114–23. 10.1093/jas/skz269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Ding S-T, Wang J-C, Mersmann HJ. Effect of unsaturated fatty acids on porcine adipocyte differentiation. Nutr Res 2003;23:1059–69. 10.1016/S0271-5317(03)00081-2 [DOI] [Google Scholar]
- [272].Shang Z, Guo L, Wang N, Shi H, Wang Y, Li H. Oleate promotes differentiation of chicken primary preadipocytes in vitro. Biosci Rep 2014;34. 10.1042/BSR20130120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Cheng B, Wu M, Xu S, Zhang X, Wang Y, Wang N, et al. Cocktail supplement with rosiglitazone: a novel inducer for chicken preadipocyte differentiation in vitro. Biosci Rep 2016;36. 10.1042/BSR20160049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Sprenger S, Woldemariam T, Chaturvedi LS. Induction of Adipogenic Genes by Novel Serum-Free Conditions From Pre-adipocyte 3T3-L1 and ST2 Cells. Cureus 2021;13. 10.7759/cureus.13831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Yuan C, Chakraborty S, Chitta KK, Subramanian S, Lim TE, Han W, et al. Fast Adipogenesis Tracking System (FATS)—a robust, high-throughput, automation-ready adipogenesis quantification technique. Stem Cell Res Ther 2019;10. 10.1186/s13287-019-1141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Huang H, Song T-J, Li X, Hu L, He Q, Liu M, et al. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci 2009;106:12670–5. 10.1073/pnas.0906266106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Chen Y-W, Chien Y-H, Yu Y-H. Taiwanese green propolis ethanol extract promotes adipocyte differentiation and alleviates TNF-α-mediated downregulation of adiponectin expression. J Funct Foods 2020;73:104135. 10.1016/j.jff.2020.104135 [DOI] [Google Scholar]
- [278].Boudreau A, Poulev A, Ribnicky DM, Raskin I, Rathinasabapathy T, Richard AJ, et al. Distinct Fractions of an Artemisia scoparia Extract Contain Compounds With Novel Adipogenic Bioactivity. Front Nutr 2019;6:18. 10.3389/fnut.2019.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Ahn S, Lee M, An S, Hyun S, Hwang J, Lee J, et al. 2-Formyl-komarovicine promotes adiponectin production in human mesenchymal stem cells through PPARγ partial agonism. Bioorg Med Chem 2018;26. 10.1016/j.bmc.2018.01.019 [DOI] [PubMed] [Google Scholar]
- [280].Kim S, Han Y, Ahn S, An S, Shin J, Choi H, et al. Kojyl cinnamate esters are peroxisome proliferator-activated receptor α/γ dual agonists. Bioorg Med Chem 2018;26. 10.1016/j.bmc.2018.10.010 [DOI] [PubMed] [Google Scholar]
- [281].Rho HS, Hong SH, Park J, Jung H-I, Park Y-H, Lee JH, et al. Kojyl cinnamate ester derivatives promote adiponectin production during adipogenesis in human adipose tissue-derived mesenchymal stem cells. Bioorg Med Chem Lett 2014;24:2141–5. 10.1016/j.bmcl.2014.03.034 [DOI] [PubMed] [Google Scholar]
- [282].Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, Bianchi K, et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci 2008;105:1226–31. 10.1073/pnas.0711402105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Casado-Díaz A, Dorado G, Quesada-Gómez JM. Influence of olive oil and its components on mesenchymal stem cell biology. World J Stem Cells 2019;11:1045–64. 10.4252/wjsc.v11.i12.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Matthaus B, Özcan MM. Fatty acid and tocopherol contents of several soybean oils. Nat Prod Res 2014;28:589–92. 10.1080/14786419.2014.883396 [DOI] [PubMed] [Google Scholar]
- [285].Li X, Xia J, Nicolescu CT, Massidda MW, Ryan TJ, Tien J. Engineering of microscale vascularized fat that responds to perfusion with lipoactive hormones. Biofabrication 2018;11:014101. 10.1088/1758-5090/aae5fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Hadri KE, Glorian M, Monsempes C, Dieudonné M-N, Pecquery R, Giudicelli Y, et al. In Vitro Suppression of the Lipogenic Pathway by the Nonnucleoside Reverse Transcriptase Inhibitor Efavirenz in 3T3 and Human Preadipocytes or Adipocytes. J Biol Chem 2004;279:15130–41. 10.1074/jbc.M312875200 [DOI] [PubMed] [Google Scholar]
- [287].Wu P, Sato K, Suzuta F, Hikasa Y, Kagota K. Effects of Lipid-Related Factors on Adipocyte Differentiation of Bovine Stromal-Vascular Cells in Primary Culture. J Vet Med Sci 2000;62:933–9. 10.1292/jvms.62.933 [DOI] [PubMed] [Google Scholar]
- [288].Han J, Farmer S, Kirkland J, Corkey B, Yoon R, Pirtskhalava T, et al. Octanoate Attenuates Adipogenesis in 3T3-L1 Preadipocytes. J Nutr 2002;132:904–10. 10.1093/jn/132.5.904 [DOI] [PubMed] [Google Scholar]
- [289].Todorčević M, Hodson L. The Effect of Marine Derived n-3 Fatty Acids on Adipose Tissue Metabolism and Function. J Clin Med 2015;5. 10.3390/jcm5010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Wójcik C, Lohe K, Kuang C, Xiao Y, Jouni Z, Poels E. Modulation of adipocyte differentiation by omega-3 polyunsaturated fatty acids involves the ubiquitin-proteasome system. J Cell Mol Med 2014;18:590–9. 10.1111/jcmm.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Ghnaimawi S, Rebello L, Baum J, Huang Y. DHA but not EPA induces the trans-differentiation of C2C12 cells into white-like adipocytes phenotype. PLOS ONE 2021;16:e0249438. 10.1371/journal.pone.0249438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Song J, Li C, Lv Y, Zhang Y, Amakye WK, Mao L. DHA increases adiponectin expression more effectively than EPA at relative low concentrations by regulating PPARγ and its phosphorylation at Ser273 in 3T3-L1 adipocytes. Nutr Metab 2017;14:52. 10.1186/s12986-017-0209-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Kim H-K, Della-Fera M, Lin J, Baile CA. Docosahexaenoic Acid Inhibits Adipocyte Differentiation and Induces Apoptosis in 3T3-L1 Preadipocytes. J Nutr 2006;136:2965–9. 10.1093/jn/136.12.2965 [DOI] [PubMed] [Google Scholar]
- [294].Manickam E, Sinclair AJ, Cameron-Smith D. Suppressive actions of eicosapentaenoic acid on lipid droplet formation in 3T3-L1 adipocytes. Lipids Health Dis 2010;9:57. 10.1186/1476-511X-9-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Barber E, Sinclair AJ, Cameron-Smith D. Comparative actions of omega-3 fatty acids on in-vitro lipid droplet formation. Prostaglandins Leukot Essent Fatty Acids 2013;89:359–66. 10.1016/j.plefa.2013.07.006 [DOI] [PubMed] [Google Scholar]
- [296].Li Y, Rong Y, Bao L, Nie B, Ren G, Zheng C, et al. Suppression of adipocyte differentiation and lipid accumulation by stearidonic acid (SDA) in 3T3-L1 cells. Lipids Health Dis 2017;16:181. 10.1186/s12944-017-0574-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Murali G, Desouza CV, Clevenger ME, Ramalingam R, Saraswathi V. Differential effects of eicosapentaenoic acid and docosahexaenoic acid in promoting the differentiation of 3T3-L1 preadipocytes. Prostaglandins Leukot Essent Fatty Acids 2014;90:13–21. 10.1016/j.plefa.2013.10.002 [DOI] [PubMed] [Google Scholar]
- [298].Todorcević M, Vegusdal A, Gjøen T, Sundvold H, Torstensen BE, Kjaer MA, et al. Changes in fatty acids metabolism during differentiation of Atlantic salmon preadipocytes; effects of n-3 and n-9 fatty acids. Biochim Biophys Acta 2008;1781:326–35. 10.1016/j.bbalip.2008.04.014 [DOI] [PubMed] [Google Scholar]
- [299].Zhao M, Chen X. Eicosapentaenoic acid promotes thermogenic and fatty acid storage capacity in mouse subcutaneous adipocytes. Biochem Biophys Res Commun 2014;450:1446–51. 10.1016/j.bbrc.2014.07.010 [DOI] [PubMed] [Google Scholar]
- [300].Riera-Heredia N, Lutfi E, Gutiérrez J, Navarro I, Capilla E. Fatty acids from fish or vegetable oils promote the adipogenic fate of mesenchymal stem cells derived from gilthead sea bream bone potentially through different pathways. PLoS ONE 2019;14:e0215926. 10.1371/journal.pone.0215926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Scholefield AM, Tocher DR, Schuller KA. Dynamics of fatty acid metabolism in a cell line from southern bluefin tuna (Thunnus maccoyii). Aquaculture 2015;449:58–68. 10.1016/j.aquaculture.2015.02.017 [DOI] [PubMed] [Google Scholar]
- [302].Scholefield AM, Schuller KA. Cell proliferation and long chain polyunsaturated fatty acid metabolism in a cell line from southern bluefin tuna (Thunnus maccoyii). Lipids 2014;49:703–14. 10.1007/s11745-014-3910-y [DOI] [PubMed] [Google Scholar]
- [303].Kallunki T, Barisic M, Jäättelä M, Liu B. How to Choose the Right Inducible Gene Expression System for Mammalian Studies? Cells 2019;8. 10.3390/cells8080796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Hu E, Tontonoz P, Spiegelman BM. Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci U S A 1995;92:9856–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Yu Y, Liu B-H, Mersmann H, Ding S. Porcine peroxisome proliferator-activated receptor induces transdifferentiation of myocytes into adipocytes. J Anim Sci 2006;84:2655–65. 10.2527/jas.2005-645 [DOI] [PubMed] [Google Scholar]
- [306].Liu S, Wang Y, Wang L, Wang N, Li Y, Li H. Transdifferentiation of fibroblasts into adipocyte-like cells by chicken adipogenic transcription factors. Comp Biochem Physiol A Mol Integr Physiol 2010;156:502–8. 10.1016/j.cbpa.2010.04.003 [DOI] [PubMed] [Google Scholar]
- [307].Ge L, Kang J, Dong X, Luan D, Su G, Li G, et al. Myostatin site-directed mutation and simultaneous PPARγ site-directed knockin in bovine genome. J Cell Physiol 2021;236:2592–605. 10.1002/jcp.30017 [DOI] [PubMed] [Google Scholar]
- [308].Yamanouchi K, Ban A, Shibata S, Hosoyama T, Murakami Y, Nishihara M. Both PPARgamma and C/EBPalpha are sufficient to induce transdifferentiation of goat fetal myoblasts into adipocytes. J Reprod Dev 2007;53:563–72. 10.1262/jrd.18169 [DOI] [PubMed] [Google Scholar]
- [309].Gu H, Zhou Y, Yang J, Li J, Peng Y, Zhang X, et al. Targeted overexpression of PPARγ in skeletal muscle by random insertion and CRISPR/Cas9 transgenic pig cloning enhances oxidative fiber formation and intramuscular fat deposition. FASEB J Off Publ Fed Am Soc Exp Biol 2021;35:e21308. 10.1096/fj.202001812RR [DOI] [PubMed] [Google Scholar]
- [310].Ren D, Collingwood TN, Rebar EJ, Wolffe AP, Camp HS. PPARγ knockdown by engineered transcription factors: exogenous PPARγ2 but not PPARγ1 reactivates adipogenesis. Genes Dev 2002;16:27–32. 10.1101/gad.953802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Freytag SO, Paielli DL, Gilbert JD. Ectopic expression of the CCAAT/enhancer-binding protein alpha promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev 1994;8:1654–63. 10.1101/gad.8.14.1654 [DOI] [PubMed] [Google Scholar]
- [312].Lin FT, Lane MD. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc Natl Acad Sci 1994;91:8757–61. 10.1073/pnas.91.19.8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Wu Z, Xie Y, Bucher NL, Farmer SR. Conditional ectopic expression of C/EBP beta in NIH-3T3 cells induces PPAR gamma and stimulates adipogenesis. Genes Dev 1995;9:2350–63. 10.1101/gad.9.19.2350 [DOI] [PubMed] [Google Scholar]
- [314].Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev 1996;10:1096–107. 10.1101/gad.10.9.1096 [DOI] [PubMed] [Google Scholar]
- [315].Zhang L, Zhao Y, Ning Y, Wang H, Zan L. Ectopical expression of FABP4 gene can induce bovine muscle-derived stem cells adipogenesis. Biochem Biophys Res Commun 2017;482:352–8. 10.1016/j.bbrc.2016.11.067 [DOI] [PubMed] [Google Scholar]
- [316].Huang Y, Das AK, Yang Q-Y, Zhu M-J, Du M. Zfp423 Promotes Adipogenic Differentiation of Bovine Stromal Vascular Cells. PLOS ONE 2012;7:e47496. 10.1371/journal.pone.0047496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Guan L, Hu X, Liu L, Xing Y, Zhou Z, Liang X, et al. bta-miR-23a involves in adipogenesis of progenitor cells derived from fetal bovine skeletal muscle. Sci Rep 2017;7:43716. 10.1038/srep43716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Wei S, Zhang M, Zheng Y, Yan P. ZBTB16 Overexpression Enhances White Adipogenesis and Induces Brown-Like Adipocyte Formation of Bovine White Intramuscular Preadipocytes. Cell Physiol Biochem 2018;48:2528–38. 10.1159/000492697 [DOI] [PubMed] [Google Scholar]
- [319].Li S, Raza SHA, Zhao C, Cheng G, Zan L. Overexpression of PLIN1 Promotes Lipid Metabolism in Bovine Adipocytes. Anim Open Access J MDPI 2020;10. 10.3390/ani10111944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Jiang S, Wei H, Song T, Yang Y, Zhang F, Zhou Y, et al. KLF13 promotes porcine adipocyte differentiation through PPARγ activation. Cell Biosci 2015;5:28. 10.1186/s13578-015-0016-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Huang J, Feng X, Zhu R, Guo D, Wei Y, Cao X, et al. Comparative transcriptome analysis reveals that PCK1 is a potential gene affecting IMF deposition in buffalo. BMC Genomics 2020;21:710. 10.1186/s12864-020-07120-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Jimenez MA, Åkerblad P, Sigvardsson M, Rosen ED. Critical Role for Ebf1 and Ebf2 in the Adipogenic Transcriptional Cascade. Mol Cell Biol 2007;27:743–57. 10.1128/MCB.01557-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Wosczyna MN, Perez Carbajal EE, Wagner MW, Paredes S, Konishi CT, Liu L, et al. Targeting microRNA-mediated gene repression limits adipogenic conversion of skeletal muscle mesenchymal stromal cells. Cell Stem Cell 2021. 10.1016/j.stem.2021.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Abella A, Dubus P, Malumbres M, Rane SG, Kiyokawa H, Sicard A, et al. Cdk4 promotes adipogenesis through PPARγ activation. Cell Metab 2005;2:239–49. 10.1016/j.cmet.2005.09.003 [DOI] [PubMed] [Google Scholar]
- [325].Mohorčich J, Reese J. Cell-cultured meat: Lessons from GMO adoption and resistance. Appetite 2019;143:104408. 10.1016/j.appet.2019.104408 [DOI] [PubMed] [Google Scholar]
- [326].Singh P, Borthakur A, Singh AA, Kumar A, Singh KK. Policy Issues in Genetically Modified Crops: A Global Perspective. Academic Press; 2020. ISBN: 978-0-12-820945-5 [Google Scholar]
- [327].Tavernier G, Andries O, Demeester J, Sanders NN, De Smedt SC, Rejman J. mRNA as gene therapeutic: How to control protein expression. J Controlled Release 2011;150:238–47. 10.1016/j.jconrel.2010.10.020 [DOI] [PubMed] [Google Scholar]
- [328].Petrick JS, Brower-Toland B, Jackson AL, Kier LD. Safety assessment of food and feed from biotechnology-derived crops employing RNA-mediated gene regulation to achieve desired traits: A scientific review. Regul Toxicol Pharmacol 2013;66:167–76. 10.1016/j.yrtph.2013.03.008 [DOI] [PubMed] [Google Scholar]
- [329].Moradian H, Lendlein A, Gossen M. Strategies for simultaneous and successive delivery of RNA. J Mol Med 2020;98:1767–79. 10.1007/s00109-020-01956-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Plews J, Li J, Jones M, Moore H, Mason C, Andrews P, et al. Activation of Pluripotency Genes in Human Fibroblast Cells by a Novel mRNA Based Approach. PloS One 2010;5:e14397. 10.1371/journal.pone.0014397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Matsui A, Uchida S, Ishii T, Itaka K, Kataoka K. Messenger RNA-based therapeutics for the treatment of apoptosis-associated diseases. Sci Rep 2015;5:15810. 10.1038/srep15810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Zou S, Scarfo K, Nantz MH, Hecker JG. Lipid-mediated delivery of RNA is more efficient than delivery of DNA in non-dividing cells. Int J Pharm 2010;389:232–43. 10.1016/j.ijpharm.2010.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Xue Y, Zhan X, Sun S, Karuppagounder SS, Xia S, Dawson VL, et al. Synthetic mRNAs Drive Highly Efficient iPS Cell Differentiation to Dopaminergic Neurons. Stem Cells Transl Med 2018;8:112–23. 10.1002/sctm.18-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Warren L, Lin C. mRNA-Based Genetic Reprogramming. Mol Ther 2019;27:729–34. 10.1016/j.ymthe.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Goparaju SK, Kohda K, Ibata K, Soma A, Nakatake Y, Akiyama T, et al. Rapid differentiation of human pluripotent stem cells into functional neurons by mRNAs encoding transcription factors. Sci Rep 2017;7. 10.1038/srep42367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Akiyama T, Sato S, Chikazawa-Nohtomi N, Soma A, Kimura H, Wakabayashi S, et al. Efficient differentiation of human pluripotent stem cells into skeletal muscle cells by combining RNA-based MYOD1-expression and POU5F1-silencing. Sci Rep 2018;8:1189. 10.1038/s41598-017-19114-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Kwon JB, Vankara A, Ettyreddy AR, Bohning JD, Gersbach CA. Myogenic Progenitor Cell Lineage Specification by CRISPR/Cas9-Based Transcriptional Activators. Stem Cell Rep 2020. 10.1016/j.stemcr.2020.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Lundh M, Pluciñska K, Isidor MS, Petersen PSS, Emanuelli B. Bidirectional manipulation of gene expression in adipocytes using CRISPRa and siRNA. Mol Metab 2017;6:1313–20. 10.1016/j.molmet.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Sheyn D, Pelled G, Tawackoli W, Su S, Ben-David S, Gazit D, et al. Transient overexpression of Pparγ2 and C/ebpα in mesenchymal stem cells induces brown adipose tissue formation. Regen Med 2013;8:295–308. 10.2217/rme.13.25 [DOI] [PubMed] [Google Scholar]
- [340].Chen K, He H, Xie Y, Zhao L, Zhao S, Wan X, et al. miR-125a-3p and miR-483–5p promote adipogenesis via suppressing the RhoA/ROCK1/ERK1/2 pathway in multiple symmetric lipomatosis. Sci Rep 2015;5:11909. 10.1038/srep11909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Gerin I, Bommer GT, McCoin CS, Sousa KM, Krishnan V, MacDougald OA. Roles for miRNA-378/378* in adipocyte gene expression and lipogenesis. Am J Physiol-Endocrinol Metab 2010;299:E198–206. 10.1152/ajpendo.00179.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Wang L, Zhang S, Zhang W, Cheng G, Khan R, Junjvlieke Z, et al. miR-424 Promotes Bovine Adipogenesis Through an Unconventional Post-Transcriptional Regulation of STK11. Front Genet 2020;11. 10.3389/fgene.2020.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [343].Yu X, Fang X, Gao M, Mi J, Zhang X, Xia L, et al. Isolation and Identification of Bovine Preadipocytes and Screening of MicroRNAs Associated with Adipogenesis. Anim Open Access J MDPI 2020;10. 10.3390/ani10050818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344].Romao JM, Jin W, He M, McAllister T, Guan LL. MicroRNAs in bovine adipogenesis: genomic context, expression and function. BMC Genomics 2014;15:137. 10.1186/1471-2164-15-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Dong P, Mai Y, Zhang Z, Mi L, Wu G, Chu G, et al. MiR-15a/b promote adipogenesis in porcine pre-adipocyte via repressing FoxO1. Acta Biochim Biophys Sin 2014;46:565–71. 10.1093/abbs/gmu043 [DOI] [PubMed] [Google Scholar]
- [346].Shi X-E, Li Y-F, Jia L, Ji H-L, Song Z-Y, Cheng J, et al. MicroRNA-199a-5p Affects Porcine Preadipocyte Proliferation and Differentiation. Int J Mol Sci 2014;15:8526. 10.3390/ijms15058526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Ma X, Sun J, Zhu S, Du Z, Li D, Li W, et al. MiRNAs and mRNAs Analysis during Abdominal Preadipocyte Differentiation in Chickens. Animals 2020;10:468. 10.3390/ani10030468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Chen J, Wang C, Kuang S. Transdifferentiation of Muscle Satellite Cells to Adipose Cells Using CRISPR/Cas9-Mediated Targeting of MyoD. Methods Mol Biol Clifton NJ 2019;1889:25–41. 10.1007/978-1-4939-8897-6_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [349].Kis Z, Kontoravdi C, Dey AK, Shattock R, Shah N. Rapid development and deployment of high-volume vaccines for pandemic response. J Adv Manuf Process 2020;2:e10060. 10.1002/amp2.10060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Pascolo S MESSENGER RNA: THE INEXPENSIVE BIOPHARMACEUTICAL. J Multidiscip Eng Sci Technol 2017;4:6937 [Google Scholar]
- [351].Blakney AK, Ip S, Geall AJ. An Update on Self-Amplifying mRNA Vaccine Development. Vaccines 2021;9:97. 10.3390/vaccines9020097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Bloom K, van den Berg F, Arbuthnot P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther 2020:1–13. 10.1038/s41434-020-00204-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Blakney AK, McKay PF, Hu K, Samnuan K, Jain N, Brown A, et al. Polymeric and lipid nanoparticles for delivery of self-amplifying RNA vaccines. J Controlled Release 2021;338:201–10. 10.1016/j.jconrel.2021.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Squillaro T, Peluso G, Galderisi U, Di Bernardo G. Long non-coding RNAs in regulation of adipogenesis and adipose tissue function. ELife 2020;9:e59053. 10.7554/eLife.59053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [355].Xiao T, Liu L, Li H, Sun Y, Luo H, Li T, et al. Long Noncoding RNA ADINR Regulates Adipogenesis by Transcriptionally Activating C/EBPα. Stem Cell Rep 2015;5:856–65. 10.1016/j.stemcr.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [356].Ma YN, Wang B, Wang ZX, Gomez NA, Zhu MJ, Du M. Three-dimensional spheroid culture of adipose stromal vascular cells for studying adipogenesis in beef cattle. Animal 2018;12:2123–9. 10.1017/S1751731118000150 [DOI] [PubMed] [Google Scholar]
- [357].Kang JH, Gimble JM, Kaplan DL. In Vitro 3D Model for Human Vascularized Adipose Tissue. Tissue Eng Part A 2009;15:2227–36. 10.1089/ten.tea.2008.0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [358].Kang S-W, Cha B-H, Park H, Park K-S, Lee KY, Lee S-H. The Effect of Conjugating RGD into 3D Alginate Hydrogels on Adipogenic Differentiation of Human Adipose-Derived Stromal Cells. Macromol Biosci 2011;11:673–9. 10.1002/mabi.201000479 [DOI] [PubMed] [Google Scholar]
- [359].Abbott RD, Wang RY, Reagan MR, Chen Y, Borowsky FE, Zieba A, et al. The use of silk as a scaffold for mature, sustainable unilocular adipose 3D tissue engineered systems. Adv Healthc Mater 2016;5:1667–77. 10.1002/adhm.201600211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [360].Aulthouse AL, Freeh E, Newstead S, Stockert AL. Part 1: A Novel Model for Three-Dimensional Culture of 3T3-L1 Preadipocytes Stimulates Spontaneous Cell Differentiation Independent of Chemical Induction Typically Required in Monolayer. Nutr Metab Insights 2019;12. 10.1177/1178638819841399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [361].Yang F, Carmona A, Stojkova K, Huitron EIG, Goddi A, Bhushan A, et al. A 3D human adipose tissue model within a microfluidic device. Lab Chip 2021;21:435–46. 10.1039/D0LC00981D [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat Med 1997;3:177–82. 10.1038/nm0297-177 [DOI] [PubMed] [Google Scholar]
- [363].Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol 2005;23:821–3. 10.1038/nbt0705-821 [DOI] [PubMed] [Google Scholar]
- [364].Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci 2016;113:3179–84. 10.1073/pnas.1521342113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [365].Sarig-Nadir O, Livnat N, Zajdman R, Shoham S, Seliktar D. Laser Photoablation of Guidance Microchannels into Hydrogels Directs Cell Growth in Three Dimensions. Biophys J 2009;96:4743–52. 10.1016/j.bpj.2009.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366].Lovett M, Cannizzaro C, Daheron L, Messmer B, Vunjak-Novakovic G, Kaplan DL. Silk fibroin microtubes for blood vessel engineering. Biomaterials 2007;28:5271–9. 10.1016/j.biomaterials.2007.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [367].Yamamoto Y, Ito A, Jitsunobu H, Yamaguchi K, Kawabe Y, Mizumoto H, et al. Hollow Fiber Bioreactor Perfusion Culture System for Magnetic Force-Based Skeletal Muscle Tissue Engineering. J Chem Eng Jpn 2012;45:348–54. 10.1252/jcej.11we237 [DOI] [Google Scholar]
- [368].Andrée B, Ichanti H, Kalies S, Heisterkamp A, Strauß S, Vogt P-M, et al. Formation of three-dimensional tubular endothelial cell networks under defined serum-free cell culture conditions in human collagen hydrogels. Sci Rep 2019;9:1–11. 10.1038/s41598-019-41985-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [369].Lai N, Jayaraman A, Lee K. Enhanced Proliferation of Human Umbilical Vein Endothelial Cells and Differentiation of 3T3-L1 Adipocytes in Coculture. Tissue Eng Part A 2009;15:1053–61. 10.1089/ten.tea.2008.0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [370].Louis F, Piantino M, Liu H, Kang D-H, Sowa Y, Kitano S, et al. Bioprinted Vascularized Mature Adipose Tissue with Collagen Microfibers for Soft Tissue Regeneration. Cyborg Bionic Syst 2021;2021:1–15. 10.34133/2021/1412542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Volz A-C, Hack L, Kluger PJ. A cellulose-based material for vascularized adipose tissue engineering. J Biomed Mater Res B Appl Biomater n.d;0. 10.1002/jbm.b.34235 [DOI] [PubMed] [Google Scholar]
- [372].Wittmann K, Dietl S, Ludwig N, Berberich O, Hoefner C, Storck K, et al. Engineering Vascularized Adipose Tissue Using the Stromal-Vascular Fraction and Fibrin Hydrogels. Tissue Eng Part A 2015;21:1343–53. 10.1089/ten.tea.2014.0299 [DOI] [PubMed] [Google Scholar]
- [373].Yang F, Cohen RN, Brey EM. Optimization of Co-Culture Conditions for a Human Vascularized Adipose Tissue Model. Bioengineering 2020;7:114. 10.3390/bioengineering7030114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [374].Unal AZ, Jeffs SE, West JL. 3D Co-Culture with Vascular Cells Supports Long-Term Hepatocyte Phenotype and Function In Vitro. Regen Eng Transl Med 2018;4:21–34. 10.1007/s40883-018-0046-2 [DOI] [Google Scholar]
- [375].Tetzlaff F, Fischer A. Human Endothelial Cell Spheroid-based Sprouting Angiogenesis Assay in Collagen. BIO-Protoc 2018;8. 10.21769/BioProtoc.2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [376].Moya ML, Hsu Y-H, Lee AP, Hughes CCW, George SC. In Vitro Perfused Human Capillary Networks. Tissue Eng Part C Methods 2013;19:730–7. 10.1089/ten.tec.2012.0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [377].Hsiao AY, Okitsu T, Teramae H, Takeuchi S. 3D Tissue Formation of Unilocular Adipocytes in Hydrogel Microfibers. Adv Healthc Mater 2016;5:548–56. 10.1002/adhm.201500673 [DOI] [PubMed] [Google Scholar]
- [378].Aubin K, Safoine M, Proulx M, Audet-Casgrain M-A, Côté J-F, Têtu F-A, et al. Characterization of In Vitro Engineered Human Adipose Tissues: Relevant Adipokine Secretion and Impact of TNF-α. PLOS ONE 2015;10:e0137612. 10.1371/journal.pone.0137612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [379].Han J, Lee J-E, Jin J, Lim JS, Oh N, Kim K, et al. The spatiotemporal development of adipose tissue. Development 2011;138:5027–37. 10.1242/dev.067686 [DOI] [PubMed] [Google Scholar]
- [380].Berry DC, Stenesen D, Zeve D, Graff JM. The developmental origins of adipose tissue. Dev Camb Engl 2013;140:3939–49. 10.1242/dev.080549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [381].Sheng X, Tucci J, Malvar J, Mittelman SD. Adipocyte differentiation is affected by media height above the cell layer. Int J Obes 2005 2014;38:315–20. 10.1038/ijo.2013.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [382].Imashiro C, Shimizu T. Fundamental Technologies and Recent Advances of Cell-Sheet-Based Tissue Engineering. Int J Mol Sci 2021;22. 10.3390/ijms22010425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [383].Shahin-Shamsabadi A, Selvaganapathy PR. π-SACS: pH Induced Self-Assembled Cell Sheets Without the Need for Modified Surfaces. ACS Biomater Sci Eng 2020;6:5346–56. 10.1021/acsbiomaterials.0c01073 [DOI] [PubMed] [Google Scholar]
- [384].Labbé B, Marceau-Fortier G, Fradette J. Cell Sheet Technology for Tissue Engineering: The Self-Assembly Approach Using Adipose-Derived Stromal Cells. In: Gimble JM, Bunnell BA, editors. Adipose-Deriv. Stem Cells Methods Protoc, Totowa, NJ: Humana Press; 2011, p. 429–41. 10.1007/978-1-61737-960-4_31 [DOI] [PubMed] [Google Scholar]
- [385].Scahill SD, Hunt M, Rogers CL, Lau FH. A Microphysiologic Platform for Human Fat: Sandwiched White Adipose Tissue. J Vis Exp JoVE 2018. 10.3791/57909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [386].Nederman T, Norling B, Glimelius B, Carlsson J, Brunk U. Demonstration of an extracellular matrix in multicellular tumor spheroids. Cancer Res 1984;44:3090–7 [PubMed] [Google Scholar]
- [387].Bauman E, Feijão T, Carvalho DTO, Granja PL, Barrias CC. Xeno-free pre-vascularized spheroids for therapeutic applications. Sci Rep 2018;8:230. 10.1038/s41598-017-18431-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Kumar P, Satyam A, Fan X, Collin E, Rochev Y, Rodriguez BJ, et al. Macromolecularly crowded in vitro microenvironments accelerate the production of extracellular matrix-rich supramolecular assemblies. Sci Rep 2015;5:8729. 10.1038/srep08729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [389].Thorrez L, DiSano K, Shansky J, Vandenburgh H. Engineering of Human Skeletal Muscle With an Autologous Deposited Extracellular Matrix. Front Physiol 2018;9. 10.3389/fphys.2018.01076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [390].Dreher J, Blach C, Terjung N, Gibis M, Weiss J. Formation and characterization of plant-based emulsified and crosslinked fat crystal networks to mimic animal fat tissue. J Food Sci 2020;85:421–31. 10.1111/1750-3841.14993 [DOI] [PubMed] [Google Scholar]
- [391].Kumbhar V, Chatli MK, Wagh R, Kumar P, Malav O, Mehta N. Efficacy of Composite Fat Replacer Mixture of Sodium Alginate and Carrageenan for Development of Low Fat Pork Patties. Int J Livest Res 2018;8:1. 10.5455/ijlr.20180421070155 [DOI] [Google Scholar]
- [392].Lin K-W, Keeton JT. Textural and Physicochemical Properties of Low-Fat, Precooked Ground Beef Patties Containing Carrageenan and Sodium Alginate. J Food Sci 1998;63:571–4. 10.1111/j.1365-2621.1998.tb15787.x [DOI] [Google Scholar]
- [393].Pintado T, Herrero AM, Jiménez-Colmenero F, Ruiz-Capillas C. Emulsion gels as potential fat replacers delivering β-glucan and healthy lipid content for food applications. J Food Sci Technol 2016;53:4336–47. 10.1007/s13197-016-2432-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [394].Production of Low-Fat Emulsified Cooked Sausages Using Amorphous Cellulose Gel n.d. [DOI]
- [395].Yashini M, Sunil CK, Sahana S, Hemanth SD, Chidanand DV, Rawson A. Protein-based Fat Replacers – A Review of Recent Advances. Food Rev Int 2019;37:197–223. 10.1080/87559129.2019.1701007 [DOI] [Google Scholar]
- [396].Rather SA, Masoodi FA, Akhter R, Rather JA, Amin F. Effects of guar gum as a fat substitute in low fat meat emulsions. J Food Process Preserv 2017;41:e13249. 10.1111/jfpp.13249 [DOI] [Google Scholar]
- [397].Rather SA, Masoodi FA, Akhter R, Rather JA, Gani A, Wani SM, et al. Application of guar–xanthan gum mixture as a partial fat replacer in meat emulsions. J Food Sci Technol 2016;53:2876–86. 10.1007/s13197-016-2270-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [398].Glisic M, Baltic M, Glisic M, Trbovic D, Jokanovic M, Parunovic N, et al. Inulin-based emulsion-filled gel as a fat replacer in prebiotic- and PUFA-enriched dry fermented sausages. Int J Food Sci Technol 2019;54:787–97. 10.1111/ijfs.13996 [DOI] [Google Scholar]
- [399].Silva-Vazquez R, Flores-Giron E, Quintero-Ramos A, Hume ME, Mendez-Zamora G. Effect of inulin and pectin on physicochemical characteristics and emulsion stability of meat batters. CyTA - J Food 2018;16:306–10. 10.1080/19476337.2017.1403490 [DOI] [Google Scholar]
- [400].Kim DH, Shin DM, Seo HG, Han SG. Effects of konjac gel with vegetable powders as fat replacers in frankfurter-type sausage. Asian-Australas J Anim Sci 2019;32:1195–204. 10.5713/ajas.18.0781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [401].Wongkaew M, Sommano SR, Tangpao T, Rachtanapun P, Jantanasakulwong K. Mango Peel Pectin by Microwave-Assisted Extraction and Its Use as Fat Replacement in Dried Chinese Sausage. Foods 2020;9:450. 10.3390/foods9040450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [402].Verma AK, Chatli MK, Kumar D, Kumar P, Mehta N. Efficacy of Sweet Potato Powder and Added Water as Fat Replacer on the Quality Attributes of Low-fat Pork Patties. Asian-Australas J Anim Sci 2015;28:252–9. 10.5713/ajas.14.0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [403].Rather SA, Masoodi FA, Akhter R, Gani A, Wani SM, Malik AH. Xanthan gum as a fat replacer in goshtaba-a traditional meat product of India: effects on quality and oxidative stability. J Food Sci Technol 2015;52:8104–12. 10.1007/s13197-015-1960-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [404].Schreuders FKG, Schlangen M, Kyriakopoulou K, Boom RM, van der Goot AJ. Texture methods for evaluating meat and meat analogue structures: A review. Food Control 2021;127:108103. 10.1016/j.foodcont.2021.108103 [DOI] [Google Scholar]
- [405].Nishimura T The role of intramuscular connective tissue in meat texture. Anim Sci J 2010;81:21–7. 10.1111/j.1740-0929.2009.00696.x [DOI] [PubMed] [Google Scholar]
- [406].Therkildsen M, Greenwood PL, Starkey CP, McPhee M, Walmsley B, Siddell J, et al. Collagen, intramuscular fat and proteolysis affect Warner-Bratzler shear-force of muscles from Bos taurus breed types differently at weaning, after backgrounding on pasture, and after feedlotting. Anim Prod Sci 2020;61:432–43. 10.1071/AN20349 [DOI] [Google Scholar]
- [407].Martínez-Álvaroi M, Penalba V, Blasco A, Hernández P. Effect of divergent selection for intramuscular fat on sensory traits and instrumental texture in rabbit meat. J Anim Sci 2016;94:5137–43. 10.2527/jas.2016-0850 [DOI] [PubMed] [Google Scholar]
- [408].Andjelković U, Josić D. Mass spectrometry based proteomics as foodomics tool in research and assurance of food quality and safety. Trends Food Sci Technol 2018;77:100–19. 10.1016/j.tifs.2018.04.008 [DOI] [Google Scholar]
- [409].Breitkopf SB, Ricoult SJH, Yuan M, Xu Y, Peake DA, Manning BD, et al. A relative quantitative positive/negative ion switching method for untargeted lipidomics via high resolution LC-MS/MS from any biological source. Metabolomics Off J Metabolomic Soc 2017;13. 10.1007/s11306-016-1157-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [410].Ouellette M-È, Bérubé J-C, Bourget J-M, Vallée M, Bossé Y, Fradette J. Linoleic acid supplementation of cell culture media influences the phospholipid and lipid profiles of human reconstructed adipose tissue. PLOS ONE 2019;14:e0224228. 10.1371/journal.pone.0224228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [411].Urrutia O, Mendizabal JA, Alfonso L, Soret B, Insausti K, Arana A. Adipose Tissue Modification through Feeding Strategies and Their Implication on Adipogenesis and Adipose Tissue Metabolism in Ruminants. Int J Mol Sci 2020;21:3183. 10.3390/ijms21093183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [412].Bermingham EN, Agnew M, Reis MG, Taukiri K, Jonker A, Cameron-Smith D, et al. Assessment of atherogenic index, long-chain omega-3 fatty acid and phospholipid content of prime beef: a survey of commercially sourced New Zealand Wagyu and Angus beef cattle. Anim Prod Sci 2020;61:179–90. 10.1071/AN19427 [DOI] [Google Scholar]
- [413].Scollan ND, Choi N-J, Kurt E, Fisher AV, Enser M, Wood JD. Manipulating the fatty acid composition of muscle and adipose tissue in beef cattle. Br J Nutr 2001;85:115–24. 10.1079/BJN2000223 [DOI] [PubMed] [Google Scholar]
- [414].Huang Y-C, Li H-J, He Z-F, Wang T, Qin G. Study on the flavor contribution of phospholipids and triglycerides to pork. Food Sci Biotechnol 2010;19:1267–76. 10.1007/s10068-010-0181-0 [DOI] [Google Scholar]
- [415].Mottram DS, Edwards RA. The role of triglycerides and phospholipids in the aroma of cooked beef. J Sci Food Agric 1983;34:517–22. 10.1002/jsfa.2740340513 [DOI] [Google Scholar]
- [416].Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J Nutr Metab 2012;2012:539426. 10.1155/2012/539426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [417].Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA 2002;288:2569–78. 10.1001/jama.288.20.2569 [DOI] [PubMed] [Google Scholar]
- [418].Watanabe A, Ueda Y, Higuchi M, Shiba N. Analysis of volatile compounds in beef fat by dynamic-headspace solid-phase microextraction combined with gas chromatography-mass spectrometry. J Food Sci 2008;73:C420–425. 10.1111/j.1750-3841.2008.00764.x [DOI] [PubMed] [Google Scholar]
- [419].Wang X, Zhu L, Han Y, Xu L, Jin J, Cai Y, et al. Analysis of volatile compounds between raw and cooked beef by HS-SPME–GC–MS. J Food Process Preserv 2018;42:e13503. 10.1111/jfpp.13503 [DOI] [Google Scholar]
- [420].O’Sullivan MG, Byrne DV, Jensen MT, Andersen HJ, Vestergaard J. A comparison of warmed-over flavour in pork by sensory analysis, GC/MS and the electronic nose. Meat Sci 2003;65:1125–38. 10.1016/S0309-1740(02)00342-X [DOI] [PubMed] [Google Scholar]


