Abstract
Auxin signaling regulates growth and developmental processes in plants. The core of nuclear auxin signaling relies on just three components: TIR1/AFBs, Aux/IAAs, and ARFs. Each component is itself made up of several domains, all of which contribute to the regulation of auxin signaling. Studies of the structural aspects of these three core signaling components have deepened our understanding of auxin signaling dynamics and regulation. In addition to the structured domains of these components, intrinsically disordered regions within the proteins also impact auxin signaling outcomes. New research is beginning to uncover the role intrinsic disorder plays in auxin-regulated degradation and subcellular localization. Structured and intrinsically disordered domains affect auxin perception, protein degradation dynamics, and DNA binding. Taken together, subtle differences within the domains and motifs of each class of auxin signaling component affect signaling outcomes and specificity.
Over the past three decades, scientists have elucidated the molecular and genetic mechanisms that underlie major aspects of nuclear auxin signaling. The auxin molecule has many functions, but the most well studied is its role in regulating transcription. Initial genetic studies of auxin-resistant plants uncovered the major players involved in auxin transcriptional control. In all members of the plant lineage, three protein families make up this nuclear auxin signaling pathway that affects the transcription of up to a third of all genes in the angiosperm Arabidopsis thaliana (Mutte et al. 2018; Powers et al. 2019). The F-box protein TRANSPORT INHIBITOR RESPONSE 1 (TIR1) and its AUXIN SIGNALING F-BOX (AFB) paralogs are auxin receptors and are responsible for regulating the abundance of the Auxin/INDOLE-3-ACETIC-ACID proteins (Aux/IAAs). The Aux/IAAs are a family of transcriptional corepressors that interact with the third family, the AUXIN RESPONSE FACTORS (ARFs). The ARFs are transcription factors with diverse functions (Fig. 1).
Figure 1.
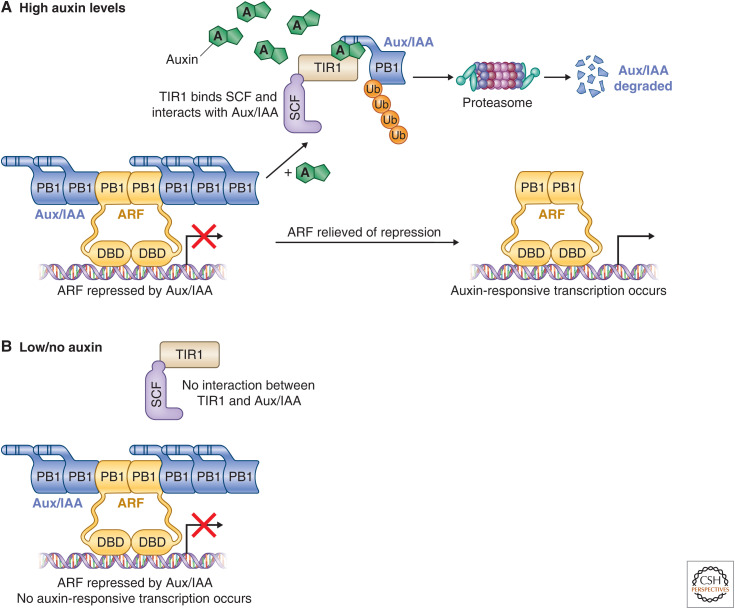
The canonical nuclear auxin signaling pathway. The core nuclear auxin signaling pathway is made up of three components: TIR1/AFBs, AUX/IAAs, and ARFs. (A) High auxin levels; (B) Low/no auxin. Under low auxin conditions, the AUX/IAAs interact with ARFs via the carboxy-terminal PB1 domain found in both protein families and prevent transcription of auxin-responsive genes. As auxin levels increase, the F-box protein and auxin-receptor TIR1 binds the molecule, which stabilizes interactions with its target, the AUX/IAAs. The AUX/IAAs are polyubiquitinated and degraded by the 26S proteasome, relieving the ARFs of repression and allowing auxin-responsive transcription to occur. (DBD) DNA-binding domain.
These three components form the core of the nuclear auxin signaling pathway. Their interactions with one another, themselves, and other proteins influence nearly every aspect of plant growth and development. Genetic studies of each family have yielded insights into the mechanisms of nuclear auxin signaling; however, genetic redundancy has prevented a complete understanding of the function of each member. Recent work on the biochemical and structural aspects of auxin signaling has resulted in an increased understanding of the complexities of this signaling module. Here, we will discuss what is known about the structured and unstructured domains found in each family, and how these domains contribute to auxin signaling outcomes. We will also discuss new questions raised by our structural understanding of the nuclear auxin signaling pathway.
TIR1/AFBs
The Auxin Signaling F-Box Protein
The TIR1 gene was originally identified in a mutant screen for plants with altered growth in the presence of the auxin transport inhibitor NPA (Ruegger et al. 1997, 1998). Unlike other genes identified in the initial screen, tir1 mutants were also resistant to exogenous auxin (Ruegger et al. 1998). Genetic studies placed TIR1 in the same pathway as other auxin signaling components, such as AXR1 and AXR6, an upstream regulator and component of the ubiquitin ligase complexes, respectively (Lincoln et al. 1990; Leyser et al. 1993; del Pozo et al. 2002; Hellmann et al. 2003). Consistent with the function of AXR1 and AXR6, sequence analysis of TIR1 suggested that it is an F-box protein that links ubiquitin conjugation directly to auxin signaling (Ruegger et al. 1998; Gray et al. 1999). Like other F-box proteins, TIR1 interacts with the Arabidopsis SKP1 orthologs ASK1 and ASK2, via its amino-terminal F-box domain (Fig. 2A,B; Ruegger et al. 1998; Gray et al. 1999; Dharmasiri et al. 2005a,b; Kepinski and Leyser 2005; Tan et al. 2007). In addition to its F-box domain, TIR1 has several leucine-rich repeats (LRRs) (Ruegger et al. 1998; Gray et al. 1999; Tan et al. 2007). TIR1 is a member of a small sub-clade of F-box proteins that includes COI1, involved in jasmonic acid (JA) signaling, as well as the AFBs (Dharmasiri et al. 2005b; Mutte et al. 2018). Increasingly higher-order knockout mutants of the TIR1/AFB family display increasing severity of developmental defects, suggesting that TIR1 and the AFBs act redundantly to regulate auxin signaling (Dharmasiri et al. 2005b; Parry et al. 2009; Prigge et al. 2020).
Figure 2.
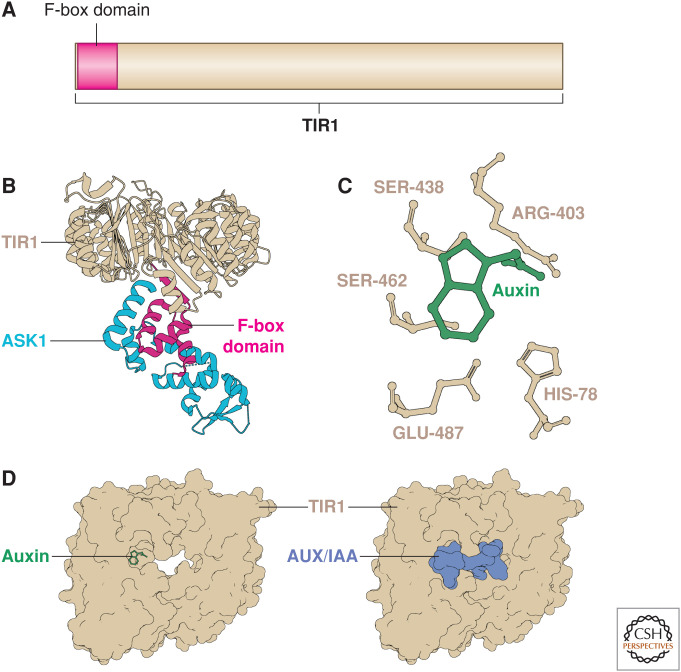
TIR1 is the auxin receptor. (A) The TIR1 protein encodes a 48-amino-acid F-box domain at its amino-terminus that is required for interactions with the SCF complex. (B) The TIR1 crystal structure (tan) in complex with the SCF adaptor component ASK1 (cyan) was resolved by Tan et al. (2007). The F-box domain (magenta) contacts the helices of ASK1 at several points and disturbing this interaction results in a dominant-negative form of TIR1. (C) Critical residues in the auxin-binding pocket of TIR1 are required for its function. This region was targeted to generate the synthetic ccvTIR1 form. (D) Upon auxin (green) binding, TIR1 (tan) can strongly interact with the degron (DII) of the AUX/IAA (pale blue) that seals in the auxin molecule.
Initial protein stability studies suggested that TIR1 directly regulates Aux/IAA levels (discussed below) by polyubiquitinating the Aux/IAAs in the presence of increased auxin. However, the mechanism of auxin perception was still unknown (Gray et al. 2001; Zenser et al. 2001, 2003; Tian et al. 2003; Kepinski and Leyser 2004; Yang et al. 2004). Studies of other F-box proteins raised the possibility that modification of either TIR1 or the substrate Aux/IAA in the presence of auxin promoted their direction interaction. However, subsequent biochemical and structural studies of TIR1 and the Aux/IAAs showed that TIR1 is the auxin receptor itself (Fig. 2C). Two studies published simultaneously showed that radiolabeled auxin interacts directly with both TIR1 and an Aux/IAA (Dharmasiri et al. 2005a; Kepinski and Leyser 2005). In addition, TIR1–Aux/IAA interactions occur in a cell-free system in the presence of auxin, suggesting that no further modification of either TIR1 or Aux/IAA is required for auxin-induced interaction (Dharmasiri et al. 2003, 2005a; Tan et al. 2007). Taken together, this work resulted in a model whereby auxin acts as a “molecular glue” directly interacting with both the E3 complex and its substrate (Fig. 2D; Tan et al. 2007).
Under low auxin conditions, TIR1/AFBs display low affinity for the Aux/IAA corepressors (Fig. 1; Kepinski and Leyser 2004, 2005; Yang et al. 2004; Dharmasiri et al. 2005a,b; Dreher et al. 2006; Tan et al. 2007; Maraschin Fdos et al. 2009; Calderón Villalobos et al. 2012). As auxin levels increase, the molecule binds in a pocket of the TIR1/AFB (Fig. 2C; Tan et al. 2007; Lee et al. 2014). When TIR1/AFBs bind the auxin molecule, the affinity for the Aux/IAAs increases, stabilizing the interaction between the F-box and the corepressor that results in polyubiquitination of the Aux/IAAs and their degradation (Tian et al. 2003; Kepinski and Leyser 2004, 2005; Yang et al. 2004; Dharmasiri et al. 2005a,b; Dreher et al. 2006; Tan et al. 2007; Maraschin Fdos et al. 2009; Calderón Villalobos et al. 2012). Auxin contacts both the TIR1-binding pocket and the Aux/IAA degron (DII, see below) to generate a coreceptor complex (Fig. 2D; Kepinski and Leyser 2004, 2005; Yang et al. 2004; Dharmasiri et al. 2005a,b; Dreher et al. 2006; Tan et al. 2007; Maraschin Fdos et al. 2009; Calderón Villalobos et al. 2012). Changes to either the auxin-binding surface, or the interaction surfaces between TIR1 and the Aux/IAA, result in stabilization of the Aux/IAA (Rouse et al. 1998; Nagpal et al. 2000; Worley et al. 2000; Gray et al. 2001; Tiwari et al. 2001; Dharmasiri et al. 2003, 2005a,b; Zenser et al. 2003; Kepinski and Leyser 2004, 2005; Tan et al. 2007; Maraschin Fdos et al. 2009; Calderón Villalobos et al. 2012; Uzunova et al. 2016). This coreceptor complex is the core of the nuclear auxin response pathway. The dynamics of auxin perception by TIR1 necessarily dictate the steady state of Aux/IAAs in the system, which in turn modulates auxin responses.
Consistent with other components of auxin signaling, the TIR1/AFB family is larger in angiosperms (six in Arabidopsis) than in bryophytes (one in Marchantia polymorpha and four in Physcomitrella patens) (Dharmasiri et al. 2005b; Parry et al. 2009; Flores-Sandoval et al. 2015; Mutte et al. 2018). The expansion of TIR1/AFBs in the flowering plants suggests subfunctionalization (Parry et al. 2009). Indeed, recent studies indicate that TIR1/AFBs from both within and between species have distinct auxin-binding affinities and different affinities for the Aux/IAAs (Calderón Villalobos et al. 2012; Havens et al. 2012; Pierre-Jerome et al. 2013; Lee et al. 2014; Moss et al. 2015; Uzunova et al. 2016; Báez et al. 2020). These small differences act as points of tunability in the auxin response. Mutating residues in the auxin-binding pocket or the interaction surface of TIR1/AFBs impairs the ability of the F-box to interact with Aux/IAAs without affecting the ability of TIR1/AFBs to interact with the SCF complex. Initial observations show that TIR1 binds full-length Aux/IAA with a higher affinity than a peptide encompassing the degron (see below) (Worley et al. 2000; Gray et al. 2001; Ramos et al. 2001; Kepinski and Leyser 2004, 2005; Yang et al. 2004; Dharmasiri et al. 2005a; Tan et al. 2007; Calderón Villalobos et al. 2012). Similar results were obtained using purified proteins and also in the yeast synthetic auxin signaling system (Havens et al. 2012; Pierre-Jerome et al. 2014; Moss et al. 2015). Maize TIR1/AFBs show differing degradation dynamics depending on the Aux/IAA tested (Báez et al. 2020). Binding pocket size and electrostatic potential affect TIR1/AFB-binding affinities and specificities for different active auxins (Lee et al. 2014; Uzunova et al. 2016). Further studies are required to see how different auxins impact the ability of low- and high-affinity TIR1/AFB interactions to proceed in vivo.
Mutations in either the F-box domain or the auxin-binding pocket affect TIR1 function. However, the relative contributions of each domain to TIR1/AFB function result in differing phenotypes. If the F-box domain is perturbed, a dominant-negative form of TIR1 or AFBs is generated (Yu et al. 2015). It is hypothesized that signal transduction fails to occur because the Aux/IAAs are not polyubiquitinated and degraded (Yu et al. 2015). Consistent with that hypothesis, these mutations result in phenotypes similar to dominant gain-of-function Aux/IAA mutations (Rouse et al. 1998; Nagpal et al. 2000; Worley et al. 2000; Tiwari et al. 2001; Fukaki et al. 2002; Yu et al. 2015). The TIR1-auxin-Aux/IAA coreceptor is stabilized and protected from endogenous, functioning TIR1 and AFBs. Mutations in the ligand-binding pocket only affect the ability of TIR1 to interact with Aux/IAAs (Yu et al. 2013).
The vast majority of TIR1/AFBs are broadly expressed and show overlapping biological functions (Dharmasiri et al. 2005b; Parry et al. 2009; Lavy et al. 2016; Prigge et al. 2020). Consistent with its role in regulating nuclear auxin signaling, the TIR1/AFBs are nuclear localized proteins with the exception of AFB1, which is found primarily in the cytoplasm (Prigge et al. 2020). Like the other TIR1/AFBs, AFB1 can interact with Aux/IAAs in an auxin-dependent manner; however, it has lower affinity for the SCF complex than other members of the family. This suggests that AFB1 regulates auxin responses in a distinct manner. The biochemical basis for nuclear or cytoplasmic localization of the TIR1/AFB family is not understood.
Synthetic Auxin Signaling
Studies of the TIR1-auxin-Aux/IAA coreceptor module have resulted in synthetic biology approaches in several different organisms. The yeast synthetic auxin signaling system has led to new insights into auxin signaling kinetics (Pierre-Jerome et al. 2014; Báez et al. 2020). In addition, the TIR1-auxin-Aux/IAA coreceptor module has been used to modulate protein stability in other organisms. The auxin-induced degron (AID) system relies on an Aux/IAA degron fused to a protein of interest and coexpressed with TIR1 (Nishimura et al. 2009). Upon auxin application, the target protein is degraded by TIR1 resulting in controlled protein degradation and depletion. The AID system has been used in a number of different study systems from yeast to mammalian tissue culture (Kanke et al. 2011; Holland et al. 2012; Trost et al. 2016).
Synthetic approaches have also been used to generate orthogonal auxin signaling systems in Arabidopsis. Knowledge of the structural basis for auxin perception and signaling in the nuclear auxin-response pathway was used to develop a novel TIR1 variant (ccvTIR1) and synthetic auxin (cvxIAA) pair (Uchida et al. 2018). The auxin-binding pocket in ccvTIR1 has been enlarged and binds endogenous auxin molecules poorly, but still perceives the synthetic auxin analog, cvxIAA. This molecule has a large aromatic ring and has no bioactivity in the absence of ccvTIR1. When cvxIAA is applied, ccvTIR1 functions normally (Uchida et al. 2018; Mazur et al. 2020). Early studies relying on this system have elucidated new aspects of auxin signaling by increasing the control researchers have over their study system and allowing for examination of TIR1-mediated auxin responses. For example, a role for TIR1 in PIN repolarization was interrogated using this synthetic auxin signaling system (Mazur et al. 2020). One could imagine higher order combinations of ccvTIR1 and ccvAFBs to better identify the contributions of different TIR1/AFBs to specific aspects of auxin signaling.
AUX/IAAs
The Aux/IAA family (34 members in Arabidopsis) is the other major class of transcription factor involved in auxin signaling. Originally identified in screens for auxin-resistant mutants, these highly structured proteins, along with the class A ARFs, provide much of our understanding of auxin-responsive transcription (Fig. 1; for review, see Powers and Strader 2020). The Aux/IAAs are smaller than the ARFs and lack a DNA-binding domain (DBD) (Fig. 3A). Aux/IAA proteins have three major domains, DI that encodes the repressive domain, DII that encodes the Aux/IAA degron, and a type I/II Phox and Bem1 (PB1) domain that was previously termed DIII/DIV (Fig. 3A; Han et al. 2014; Korasick et al. 2014; Nanao et al. 2014).
Figure 3.
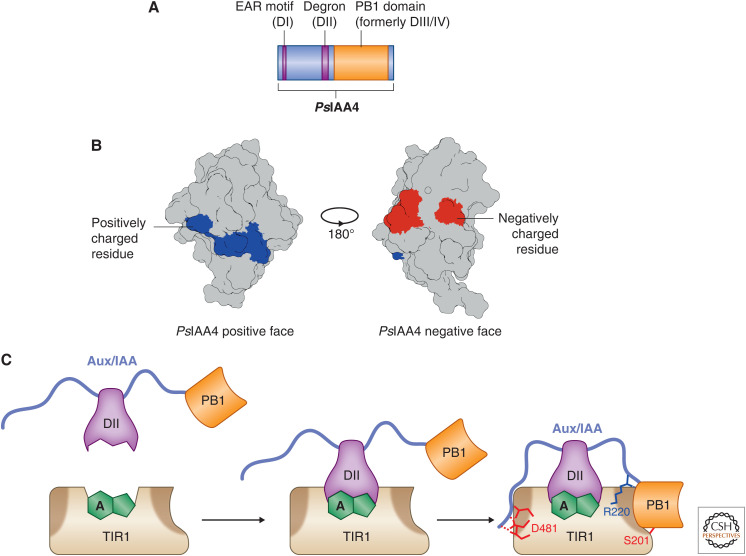
The AUX/IAAs are modular proteins with only a single structured domain. (A) An idealized motif and domain structure based on the Pisum sativum IAA4 protein. The only globular domain is the carboxy-terminal PB1 domain that mediates interactions with other AUX/IAAs and the ARFs. Both the degron (DII) and the EAR motif (DI) are short linear motifs that mediate interactions with TIR1 and the TPL corepressor family, respectively. (B) The atomic structure of the PsIAA4 PB1 domain here, resolved by Dinesh et al. (2015), shows that each face of the PB1 domain is enriched for either positively (blue) or negatively (red) charged residues that facilitate head-to-tail interactions with one another. (C) The current model for TIR1–AUX/IAA interactions now incorporates the intrinsically disordered regions that flank the degron. Key residues on both TIR1 and the AUX/IAA help stabilize the degron-mediated interaction and ensure downstream degradation and auxin signaling as shown in Niemeyer et al. (2020).
The Aux/IAA PB1 domain mediates interactions between Aux/IAAs and between Aux/IAAs and the ARFs (Fig. 1). Under low auxin conditions, this interaction results in repression of auxin-responsive transcription via an amino-terminal ethylene-responsive element-binding factor-associated amphiphilic repression (EAR) motif (DI) (reviewed in Kagale and Rozwadowski 2011 and Powers and Strader 2020).
Domain I
The amino-terminal DI region encodes a canonical EAR motif in the Aux/IAAs (Fig. 3; Ulmasov et al. 1997b; Tiwari et al. 2004). This motif is necessary to repress transcription of auxin-responsive genes in the absence of auxin (Szemenyei et al. 2008). Like other canonical EAR motifs, the Aux/IAA EAR domain recruits TOPLESS (TPL) and TPL-RELATED (TPR) corepressors to loci. TPL and TPR in turn recruit histone deacetylases, which remove marks of active transcription from histones (Long et al. 2006; Szemenyei et al. 2008). Dominant mutants of Aux/IAAs were suppressed in the absence of functional TPL and TPR proteins (Szemenyei et al. 2008). The Aux/IAAs repress transcription of auxin-responsive genes via their interactions with the ARFs (see below).
Domain II
Of the 34 Aux/IAAs found in Arabidopsis, 29 have a conserved degron in the DII region of the protein. DII is the most well-studied motif in the Aux/IAAs. The discovery and characterization of DII not only helped push the field of auxin signaling forward by explaining one of the core mechanisms (Worley et al. 2000; Gray et al. 2001; Ramos et al. 2001; Tiwari et al. 2001; Zenser et al. 2001), but it is also used to generate reporters that measure auxin abundance and signaling activity (Brunoud et al. 2012).
In the presence of auxin, DII interacts with both the auxin molecule and the TIR1/AFB surface, and is ultimately polyubiquitinated and degraded via the ubiquitin-proteasome pathway (Fig. 2D; Dreher et al. 2006; Tan et al. 2007; Calderón Villalobos et al. 2012). The Aux/IAAs are traditionally considered to be rapidly degrading proteins even in the absence of auxin (Abel et al. 1994). The interaction between different Aux/IAA DII motifs and members of the TIR1/AFB family affects the rate of Aux/IAA degradation and therefore auxin sensitivity in different cell types (Ouellet et al. 2001; Calderón Villalobos et al. 2012; Havens et al. 2012; Pierre-Jerome et al. 2014; Moss et al. 2015; Báez et al. 2020; Niemeyer et al. 2020).
Initial characterization of the Aux/IAAs was primarily through dominant mutations in the DII domain, stabilizing the Aux/IAAs and resulting in an auxin-resistant phenotype (Worley et al. 2000; Gray et al. 2001; Tiwari et al. 2001; Zenser et al. 2001). Since those initial observations, DII mutations have been used to investigate differences between Aux/IAA function and circumvent genetic redundancy.
The PB1 Domain
Like ARFs, Aux/IAA proteins have a carboxy-terminal type-I/II PB1 domain (Fig. 3A,B). Originally, this region was classified as two distinct conserved domains, termed domains III and IV (DIII/IV). Recent structural analyses show DIII/DIV assumes a single globular domain that shares many of the hallmarks of a type-I/II PB1 domain, including a negative and positive face with conserved lysine and aspartic acid residues, respectively (Han et al. 2014; Dinesh et al. 2015), as originally proposed by Guilfoyle and Hagen (2012) (Fig. 3B).
Amino acid substitutions in the positive face of the IAA16 PB1 domain that affect its ability to multimerize can suppress dominant DII mutations, suggesting that these interactions are necessary for Aux/IAA function in vivo (Korasick et al. 2014). In addition, mutations that disrupt IAA17 or IAA19 PB1 domain oligomerization increase the magnitude of auxin response in protoplast experiments, suggesting they are less effective repressors (Nanao et al. 2014). Conversely, mutations that disrupt IAA14 PB1 domain oligomerization fail to suppress the dominant DII mutation in vivo (Pierre-Jerome et al. 2016), suggesting that oligomerization is not required for IAA14 function. Taken together, these data suggest that some Aux/IAAs, like IAA16, may require oligomerization for full repressive activity. Alternatively, IAA16 may interact with its target ARFs along a single PB1 face, and, thus, disrupting this face of the PB1 domain abrogates its ability to bind targets in addition to disrupting oligomerization. Other Aux/IAAs, like IAA14, may interact with target ARFs along either face, which would be consistent with no single-face mutation resulting in decreased IAA14 repressive activity. Further work is necessary to determine whether Aux/IAA oligomerization is necessary for its function in planta. Further, these results raise the possibility that auxin signaling outcomes are regulated in part by Aux/IAA affinity for ARFs in a PB1 domain face-specific manner.
PB1 domain interactions between ARFs and Aux/IAAs display higher affinity than either ARF–ARF or Aux/IAA–Aux/IAA interactions (Han et al. 2014; Nanao et al. 2014). Thus, low levels of Aux/IAA proteins may efficiently bind members of the ARF family and repress transcription, whereas Aux/IAA–Aux/IAA interactions are less stable. Rapid turnover of the Aux/IAAs and the weak self-interactions via the PB1 domain may act to tune the thresholds required for specific auxin signaling events to occur. These weaker self-interactions may also prevent complete repression of ARF targets, including the Aux/IAAs themselves, likely maintaining auxin sensitivity in a physiologically relevant range and ensuring appropriate negative feedback regulation on the auxin signaling module.
Not all Aux/IAA–ARF interactions are equal. For example, ARF PB1 domains are not interchangeable in the Marchantia auxin signaling pathway. Additional structural modeling data suggests some ARF PB1 domains prevent Aux/IAA PB1 domain oligomerization, stopping both strong Aux/IAA transcriptional repression and sequestration of otherwise functional Aux/IAAs (Kato et al. 2020). In this model, the negatively charged face of the ARF PB1 domain interacts with the positively charged lysine face of the Aux/IAA PB1 domain in a manner that obscures the surface of the OPCA face of the Aux/IAA PB1 domain, preventing further interactions. Biochemical and structural analysis of TPL and TPR function suggests that tetramers are the active form of the TPL corepressor complex (Ke et al. 2015). The Aux/IAA PB1 domain, in addition to mediating interactions with the ARFs, may also provide a scaffold for higher order TPL and TPR interactions to occur through Aux/IAA multimerization. Indeed, Aux/IAA with mutated PB1 domains are less effective repressors (Korasick et al. 2014; Nanao et al. 2014; Dinesh et al. 2015). Further work is required to determine the actual interaction affinities and oligomeric state of different Aux/IAA and ARF PB1 domains for one another and each other.
The Aux/IAA PB1 domain regulates a number of biological functions either directly via protein–protein interactions, or indirectly via functional complex formation. Further studies that attempt to disentangle the contribution of Aux/IAA oligomerization from Aux/IAA–ARF interaction, as well as studies investigating interaction specificity, will be critical to understanding how the large Aux/IAA family contributes to so many distinct auxin signaling outcomes. The contribution of each face of the PB1 domain to any or all of these functions also remains a mystery.
Disorder in the Aux/IAAs
In addition to their ordered regions, the Aux/IAAs also have regions of intrinsic disorder (Niemeyer et al. 2020). These intrinsically disordered regions (IDRs) contribute to Aux/IAA–auxin–TIR1 interaction specificity and stability. The role of intrinsic disorder in protein and biological function in auxin signaling is only just beginning to be understood (see ARF intrinsic disorder below). Unlike ordered protein regions, disordered regions adopt a broad range of possible conformations. Ordered protein regions maintain a stable secondary and tertiary structure, whereas proteins with regions of intrinsic disorder lack a stable secondary structure. Protein conformation studies can present an ensemble of possible conformations, and the lack of strong predictive modeling due to the complex multifaceted nature of disordered regions makes hypothesis generation difficult (for review, see Holehouse 2019 and Uversky 2019). IDR analysis relies more on general amino acid composition and spacing than specific amino acid sequence. However, the biological and molecular functions of disordered regions can be studied via traditional genetic means.
Many Aux/IAA proteins in Arabidopsis have IDRs that span the region between the EAR motif (DI) and the PB1 domain (Niemeyer et al. 2020). Initial studies of the Aux/IAA degron (DII) suggested that regions flanking the conserved domain contribute to auxin signal transduction, but the mechanism was not understood (Calderón Villalobos et al. 2012; Moss et al. 2015). These flanking regions are intrinsically disordered and they contribute, along with the PB1 domain, to the interaction surface between Aux/IAAs and TIR1/AFBs (Fig. 3C; Niemeyer et al. 2020).
A model has emerged where the two IDR flanking DII contribute to the stability of the Aux/IAA–auxin–TIR1 interaction (Fig. 3C; Niemeyer et al. 2020). The regions act to clamp DII into the binding pocket on TIR1 and ensure the interaction lasts long enough to transfer ubiquitin to Aux/IAA, marking it for degradation. Interestingly, the DII flanking regions appear to be evolutionarily constrained; however, not all Aux/IAAs in Arabidopsis show similar amino acid compositions.
AUXIN-RESPONSE FACTORS
The ARF transcription factors are represented by large gene families in many plant species (Mutte et al. 2018). Mutant plants lacking functional ARFs have diverse developmental and growth phenotypes consistent with their roles in auxin signaling. However, in many cases, mutation of a single ARF does not result in a mutant phenotype (Sessions et al. 1997; Harper et al. 2000; Hardtke et al. 2004; Li et al. 2004; Tian et al. 2004; Ellis et al. 2005; Okushima et al. 2005; Wilmoth et al. 2005), suggesting overlapping functions. The ARFs fall into three ancient clades and have a conserved domain structure (Fig. 4A; Mutte et al. 2018). At the amino terminus lies a B3 DBD flanked by dimerization domains (Fig. 4B). A carboxy-terminal type-I/II PB1 domain promotes interactions between ARFs and the Aux/IAAs as well as between the ARFs themselves (Fig. 4C,D). The region that lies in between the amino-terminal DBD and the carboxy-terminal PB1 domain is known as the middle region (Fig. 4A). Class A ARFs, originally called activator ARFs, are defined by glutamine-rich middle regions, whereas class B and C ARFs, classically defined as repressor ARFs, are recognized by serine-rich middle regions (Ulmasov et al. 1997a, 1999a,b; Mutte et al. 2018). The middle regions of all ARFs contain varying degrees of intrinsic disorder and are implicated in transcriptional activity, subcellular localization, and protein condensation.
Figure 4.
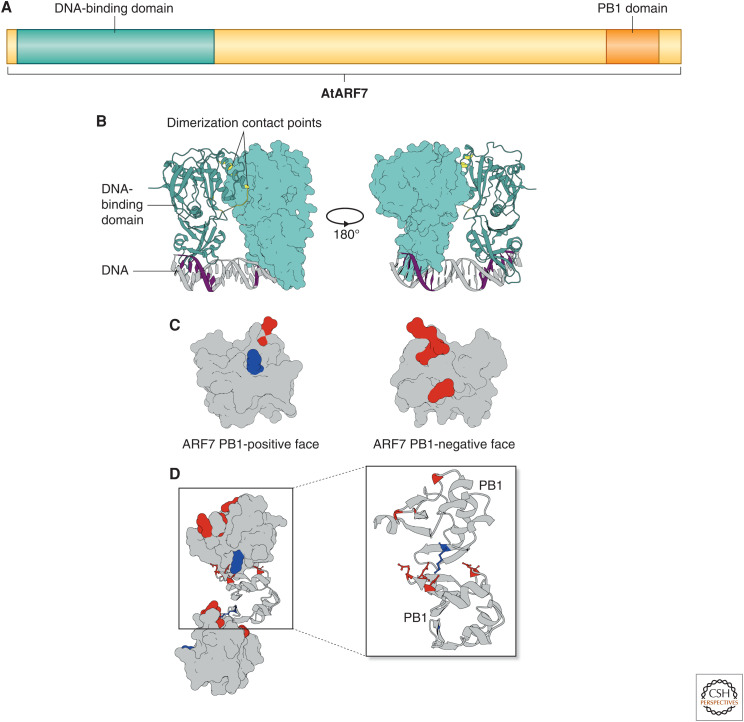
The ARFs are defined by three major domains. (A) The ARFs are defined by two globular domains, the amino-terminal DNA-binding domain (DBD) and the carboxy-terminal PB1 domain, and an intrinsically disordered middle region that lies between them, based on the Arabidopsis ARF7 protein model. (B) The crystal structure of the class B ARF1 DBD binding an ER7 AuxRE, resolved by Boer et al. (2014), shows dimerization contact points (yellow) that stabilize the interactions with TGTCTC consensus sequence (purple) in an antiparallel manner. (C) The ARF7 PB1 domain crystal structure resolved by Korasick et al. (2014), like the AUX/IAA PB1 domain, has both a positively and negatively charged face. (D) These faces interact in a head-to-tail fashion to generate strings of PB1 domains in the crystal structure. The lysine residue on the positive face interacts with the aspartic and glutamic acid residues on the negative face similar to a bar magnet. Perturbing these residues results in the loss of PB1 domain interactions along that face.
The ARF DNA-Binding Domain
The ARF DBD is made up of a canonical B3 DBD (Ulmasov et al. 1997a, 1999b; Boer et al. 2014; Mutte et al. 2018). Flanking the B3 DBD lie two regions that make up a dimerization domain, which contributes to ARF homodimerization and stabilizes DNA binding (Fig. 4B; Boer et al. 2014). Conserved amino acid residues along the dimerization surface and DNA-binding surface are necessary for ARF function (Boer et al. 2014; Pierre-Jerome et al. 2016; Lanctot et al. 2020). These results suggest that dimerization of ARF DBDs may dictate some aspects of ARF signaling specificity.
All studied ARFs bind to the same consensus sequence, the auxin response element (AuxRE) (Ulmasov et al. 1995, 1997a,b, 1999a,b; Boer et al. 2014; Pierre-Jerome et al. 2016; Lanctot et al. 2020). Synthetic AuxREs, like DR5, have served as auxin signaling reporters for over two decades (Ulmasov et al. 1997b). Naturally occurring AuxREs share the same core sequence TGTC followed by two variable positions. DNA affinity purification sequencing (DAP-seq) experiments have confirmed these findings for multiple Arabidopsis and maize ARFs (O'Malley et al. 2016; Galli et al. 2018). The initial biochemical characterization of the ARF DBD resulted in a caliper model in which ARF dimers bound DNA with differing AuxRE topologies or grammar, thus driving ARF DNA-binding specificity (Boer et al. 2014). ARF1 shows preferences for everted repeats with seven or eight base pair spacing, whereas ARF5 can bind everted repeats with five to nine base pair spacing. Similar conclusions were drawn from global binding patterns of maize and Arabidopsis ARFs by DAP experiments, and the yeast synthetic auxin signaling system (Boer et al. 2014; Pierre-Jerome et al. 2016; Lanctot et al. 2020). In all published studies, class A and B ARFs show distinct preferences for AuxRE grammar. These observations raise the possibility that the ARF DBD dictates signaling specificity by reducing binding affinity for loci with suboptimal AuxRE grammar. Further studies of class A and B ARF DNA binding suggests that inverted AuxRE repeats with eight-base-pair spacing (IR8) can recruit both classes of ARFs, whereas DR5-binding sites only recruit the class A ARFs (Freire-Rios et al. 2020). Consistent with this finding, meta-analysis of auxin transcriptional response data sets shows that IR8 motifs are found upstream of genes that show both repression and induction upon auxin signaling and DR5 motifs are only found upstream of genes with auxin-induced expression (Freire-Rios et al. 2020). Taken together, this suggests that some auxin-responsive targets can be bound by both class A and class B ARFs and others can only be bound by class A ARFs. ARF DBD swap experiments in the nonvascular plants Physcomitrella and Marchantia also suggest that ARFs compete for similar genomic targets (Lavy et al. 2016; Kato et al. 2020).
Marchantia has a single member from each ancestral class of ARF (Flores-Sandoval et al. 2015; Kato et al. 2020). Domain swap experiments showed that for MpARF1 and MpARF2, the class A and class B ARFs, respectively, the DBDs were interchangeable. Thus, replacement of the MpARF1 DBD with the same domain from MpARF2 resulted in a functional protein and could rescue the Mparf1 loss of function mutation (Kato et al. 2020). Replacing the MpARF1 DBD with the MpARF3, the class C ARF, DBD was unable to rescue Mparf1. These data suggest a model in which class A and class B ARFs compete for genomic targets and class C ARFs participate in separate processes.
Differences in AuxRE-binding affinities present an appealing model for ARF DNA-binding specificity. However, experiments in the yeast synthetic auxin signaling system suggest that some ARFs may activate transcription on a single AuxRE, but dimerization between the ARFs is necessary for transcription to occur (Lanctot et al. 2020). Consistent with the yeast synthetic signaling system, enrichment for single AuxREs upstream of auxin-responsive genes is also detectable (Freire-Rios et al. 2020). The biochemical basis for differences in DNA-binding specificity to single AuxRE binding sites is not yet known.
The ARF PB1 Domain
The DBD is not the only ARF interaction domain, however. The carboxy-terminus of most ARFs contain a type-I/II PB1 domain (Mutte et al. 2018). This class of PB1 domain has two faces: a positively charged, basic face and a negatively charged, acidic face (Fig. 4C). The ARF PB1 domain, previously referred to as domains III and IV, is shared with Aux/IAAs (Guilfoyle and Hagen 2012). Like other type-I/II PB1 domains, the ARF PB1 domains allow for head-to-tail oligomerization where the positive face of a single PB1 domain will interact with the negative face of another PB1 domain (Fig. 4D; Korasick et al. 2014, 2015; Nanao et al. 2014). Oligomerization was observed both in size exclusion chromatography and protein crystallization experiments in the ARFs (Han et al. 2014; Korasick et al. 2014; Nanao et al. 2014).
The positive face is characterized by an invariant lysine residue that interacts with an array of conserved aspartic and glutamic acids (Fig. 4C). Mutations in the lysine residue disrupt interactions with the negative face preventing oligomerization (Han et al. 2014; Korasick et al. 2014; Nanao et al. 2014; Powers et al. 2019). The PB1 domain likely contributes to ARF function in a number of ways, including interactions between ARFs and Aux/IAAs, which is required for appropriate auxin signal transduction (Vernoux et al. 2011; Han et al. 2014; Nanao et al. 2014). In Arabidopsis, a transgenic copy of ARF19 with a mutation on the positive face that ablates oligomerization showed increases in transcription of both auxin-responsive genes and novel targets in the absence of auxin (Powers et al. 2019). This result suggests that the ARF19 PB1 mutant is acting as a constitutive auxin signaling factor likely due to its lack of interaction with its transcriptional corepressor, the Aux/IAAs. Interestingly, in vivo oligomerization data suggests that the ARF19 PB1 mutant fails to dimerize in the nucleus (Powers et al. 2019). This observation raises the possibility that the ARF PB1 domain, and not the DBD, primarily contributes to ARF homodimerization. Indeed, PB1 mutants showed reduced transcriptional activity in the yeast-synthetic auxin signaling system compared to wild-type ARFs (Pierre-Jerome et al. 2016; Lanctot et al. 2020).
Unlike the DBD, class A and class B PB1 domains seem to have differing functions in Marchantia. Domain swap experiments show that only the MpARF1 PB1 domain can rescue the Mparf1 mutant (Kato et al. 2020). When the MpARF1 PB1 domain was replaced with either the MpARF2 or MpARF3 PB1 domain, the plant showed severe developmental defects and a loss of auxin responsiveness. Differences in charge distribution along the PB1 interaction surfaces may explain the differences in function; however, this has not been tested empirically. This data raises the possibility that PB1-mediated interactions, both between different ARFs and between ARFs and Aux/IAAs, drive signaling specificity by tuning auxin responsiveness.
In addition to differential Aux/IAA ARF interactions, the ARF PB1 is necessary for transcriptional control. In the synthetic yeast auxin signaling system, mutations impairing PB1 domain interactions negatively affected some, but not all, of the reporters tested (Pierre-Jerome et al. 2016; Lanctot et al. 2020). ARF19 with a mutant PB1 domain that prevents dimerization failed to activate transcription on single AuxRE targets, but is able to function on paired AuxRE targets. Unlike the PB1 domain, DBD dimerization was necessary in all cases. This data raises the possibility that the ARF PB1 domain modulates the ability of the ARFs to activate transcription on some AuxREs and serves to stabilize ARF dimerization under less ideal AuxRE grammar. All experiments testing the contribution of the ARF PB1 domain to transcriptional activity have relied on ideal AuxRE grammar with short spacing (DR5). The multimeric nature of the ARF PB1 domain also raises the possibility that AuxREs located thousands of base pairs apart from one another can be spanned by multimeric complexes of the ARFs. Further work is necessary to determine whether ARFs can span large genomic distances, and whether those multimeric complexes are biologically relevant.
Taken together, a more complex model of ARF-mediated auxin responsiveness emerges (Fig. 5B; Lavy et al. 2016; Kato et al. 2020). In tissues where class A ARF nuclear abundance is high, and therefore ARF–Aux/IAA interactions are prevalent, auxin responsiveness is high. In tissues where class B ARF abundance is high, and therefore lacking ARF–Aux/IAA interactions, auxin responsiveness is inhibited. This gradient of auxin responsiveness requires that class A and B ARFs share transcriptional targets and that Aux/IAA interact only with class A ARFs. Many of the observations of ARF function and activity are consistent between angiosperms and nonvascular plants. However, it remains to be seen whether plants with larger ARF gene families are governed by the same general principles seen in Physcomitrella and Marchantia (Lavy et al. 2016).
Figure 5.
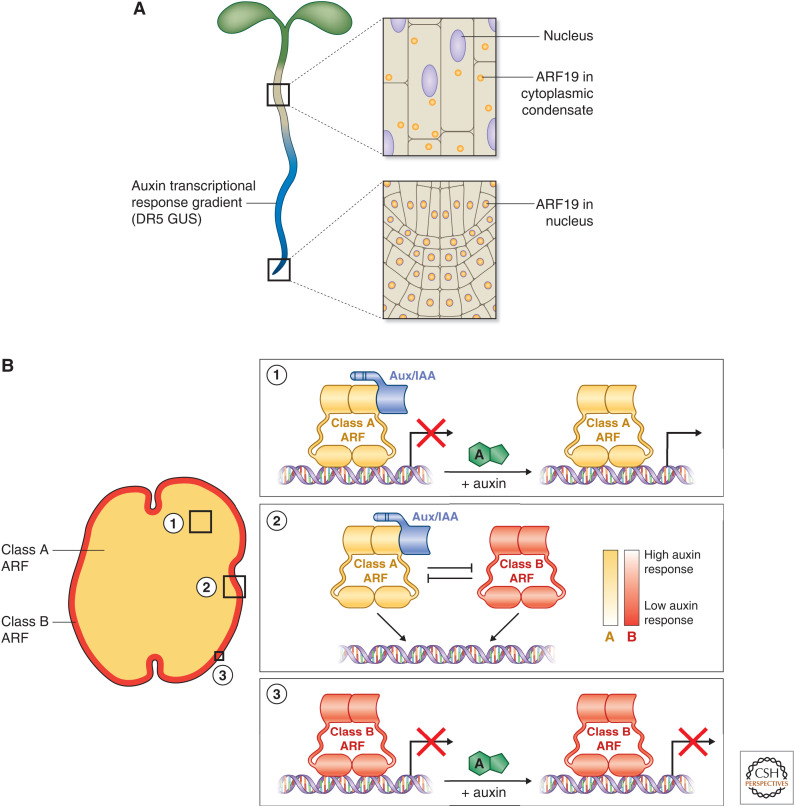
Auxin signaling relies on abundant, nuclear class A ARFs. (A) Powers et al. (2019) show that auxin signaling is absent in tissues in which ARF19 has moved out of the nucleus into cytoplasmic condensates. This effect can be eliminated if ARF19 remains nuclear. (B) As data in Kato et al. (2020) show, auxin signaling is present in the tissues that express class A ARF1 in Marchantia and absent in the tissues that express class B ARF2. Taken together, these two studies present a model in which high abundance of nuclear localized class A ARFs define auxin-responsive tissues, whereas tissues lacking class A ARFs entirely or from the nucleus are generally auxin insensitive.
The ARF Middle Region
Whereas structural domains in the ARFs have been well characterized, the unstructured middle region is less understood. Initial reports on ARF function identified the middle region, making up approximately one-half of the total protein length in most ARFs, as containing activation and repressive domains in carrot protoplast experiments (Ulmasov et al. 1999a; Tiwari et al. 2003). Class A ARFs act generally as auxin-inducible activators, whereas the class B ARFs act as repressors. Interestingly, activation and repressive activity can be decoupled from auxin induction by expressing the middle region alone in a synthetic transcription factor assay in carrot protoplasts (Tiwari et al. 2003).
A conserved TPL-binding motif (RLFGV) in Arabidopsis class B ARFs acts as a repressor domain in vivo (Choi et al. 2018). Interestingly, some class B ARFs also encode a canonical EAR motif in addition to the RLFGV motif. In the case of ARF2, for example, both the conserved RLFGV motif and the additional EAR motif are required for ARF2 to function as a transcriptional corepressor; however, only the RLFGV motif is required for TPL interactions in yeast two-hybrid experiments (Choi et al. 2018). Taken together with data from Marchantia, it suggests that class B ARFs act as auxin-insensitive negative regulators of auxin-responsive genes (Fig. 5B).
Unlike ARF repressor domains, the ARF activation domain remains unknown. This is likely due in part to the intrinsic disorder found in the class A ARF middle region. Most activation domains are defined not by sequence similarity or identity, but by sequence features such as hydrophobicity and negative charge (Ravarani et al. 2018; Staller et al. 2018; Erijman et al. 2020). Further, whether class B ARFs can act as transcriptional activators at certain loci or in the presence of other unknown cofactors remains unknown.
In addition to direct activation of transcription, class A ARFs may induce transcription indirectly by recruiting the SWITCH/SUCROSE NONFERMENTING (SWI/SNF) chromatin-remodeling complex (reviewed in Clapier and Cairns 2009). The ARF5 middle region can recruit the SWI/SWF complex through interactions with BRAHMA and SPLAYED (Wu et al. 2015). These interactions are improved in the presence of auxin and increase chromatin accessibility at ARF5-binding sites (Wu et al. 2015). These results suggest a model in which ARF5, and possibly additional class A ARFs, alter nucleosome positioning to make more transcription factor-binding sites accessible (Wu et al. 2015; Weijers and Wagner 2016).
The intrinsic disorder found in the middle region of class A ARFs, and to a lesser degree in class B and C ARFs, does not dictate transcription potential alone. The middle regions of two class A ARFs that regulate lateral root development, ARF7 and ARF19, are necessary for subcellular localization (Fig. 5A; Powers et al. 2019). ARF19 displays differential cellular localization based on tissue type, with ARF19 localized to the nucleus in young, dividing root tissue and in the cytoplasm in mature root tissue. Mutating the PB1 domain alters this localization pattern, driving more ARF19 into the nucleus in mature roots compared to wild-type ARF19. The PB1 domain is necessary for this behavior but insufficient. The ARF middle region, cooperatively with the PB1 domain, drives differences in localization through protein condensation (see below). The ARF19 middle region and PB1 domain are required for protein condensation and cytoplasmic localization of ARF19 in the mature regions of the Arabidopsis root. If either the PB1 domain or middle region is perturbed, ARF19 localizes to the nucleus in these tissues. Interestingly, the mature zone of the root showed reduced or absent auxin-induced expression of the DR5 GUS reporter (Fig. 5A). However, plants expressing ARF19 with a mutated PB1 domain show expansion of DR5 GUS expression into these mature tissues, consistent with increased nuclear localization of ARF19. This data is supported by increased transcript abundance of both novel and known auxin-responsive genes (Powers et al. 2019). The PB1 domain likely increases the local concentration of ARF19 and that the intrinsic disorder of the middle region contributes to phase separation and protein condensation (for review, see Holehouse 2019 and Powers and Strader 2020). The relationship between ARF localization and transcriptional activity provides another possible route of regulation for the auxin signaling cascade (for review, see Lanctot and Nemhauser 2020). Understanding the mechanisms that drive ARF condensation, and the degree to which other ARFs participate in similar processes, will be crucial for our understanding of auxin signaling specificity.
Posttranslational modifications (for review, see Powers and Strader 2020) and alternative splicing of the ARFs (for review, see Lanctot and Nemhauser 2020) also contribute to ARF function. Understanding the contribution of different ARF posttranscriptional and posttranslational modifications to ARF function may further explain how a single, albeit large, transcription factor family can impact so many distinct processes.
CONCLUDING REMARKS
The structural aspects of nuclear auxin signaling have shed light on one of the main questions in auxin biology. The roles of protein–protein and protein–DNA interaction specificity, protein degradation dynamics, and subcellular localization all provide insights into how a module made up of only three components can result in such diverse signaling outcomes. Furthermore, the disordered regions of the ARFs and Aux/IAAs are opening new avenues for understanding how these proteins function in vivo. More work is required to fully understand how the ensemble of structures, modifications, and interactions in these disordered regions contribute to auxin signaling specificity and outcomes. In addition to intrinsic disorder, there is an emerging role for protein subcellular localization in our understanding of auxin signaling potential. The ARFs appear to potentiate activity in different cell types based on nuclear and cytoplasmic localization patterns and are regulated by both posttranscriptional and posttranslational mechanisms. Uncovering the mechanisms that regulate ARF localization patterns may provide new insights into hormone cross talk as well as how plants integrate environmental and developmental signals.
ACKNOWLEDGMENTS
The authors thank members of the Strader laboratory for their critical reading of this manuscript, as well as support from the National Science Foundation Postdoctoral Research Program (IOS-1907098 to N.M.), the National Institutes of Health (R35 GM136338 to L.C.S.), and the National Science Foundation (IOS-1453750 to L.C.S.). Artwork by Debbie Maizels, Zoobotanicals Scientific Illustration.
Footnotes
Editors: Dolf Weijers, Karin Ljung, Mark Estelle, and Ottoline Leyser
Additional Perspectives on Auxin Signaling available at www.cshperspectives.org
REFERENCES
- Abel S, Oeller PW, Theologis A. 1994. Early auxin-induced genes encode short-lived nuclear proteins. Proc Natl Acad Sci 91: 326–330. 10.1073/pnas.91.1.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez RR, Buckley Y, Yu H, Chen Z, Gallavotti A, Nemhauser J, Moss BL. 2020. A synthetic approach allows rapid characterization of the maize nuclear auxin response circuit. Plant Physiol 182: 1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer DR, Freire-Rios A, van den Berg WA, Saaki T, Manfield IW, Kepinski S, López-Vidrieo I, Franco-Zorrilla JM, de Vries SC, Solano R, et al. 2014. Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell 156: 577–589. 10.1016/j.cell.2013.12.027 [DOI] [PubMed] [Google Scholar]
- Brunoud G, Wells DM, Oliva M, Larrieu A, Mirabet V, Burrow AH, Beeckman T, Kepinski S, Traas J, Bennett MJ, et al. 2012. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 482: 103–106. 10.1038/nature10791 [DOI] [PubMed] [Google Scholar]
- Calderón Villalobos LI, Lee S, De Oliveira C, Ivetac A, Brandt W, Armitage L, Sheard LB, Tan X, Parry G, Mao H, et al. 2012. A combinatorial TIR1/AFB-Aux/IAA co-receptor system for differential sensing of auxin. Nat Chem Biol 8: 477–485. 10.1038/nchembio.926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HS, Seo M, Cho HT. 2018. Two TPL-binding motifs of ARF2 are involved in repression of auxin responses. Front Plant Sci 9: 372. 10.3389/fpls.2018.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapier CR, Cairns BR. 2009. The biology of chromatin remodeling complexes. Annu Rev Biochem 78: 273–304. 10.1146/annurev.biochem.77.062706.153223 [DOI] [PubMed] [Google Scholar]
- del Pozo JC, Dharmasiri S, Hellmann H, Walker L, Gray WM, Estelle M. 2002. AXR1-ECR1-dependent conjugation of RUB1 to the Arabidopsis cullin AtCUL1 is required for auxin response. Plant Cell 14: 421–433. 10.1105/tpc.010282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Jones AM, Estelle M. 2003. Auxin action in a cell-free system. Curr Biol 13: 1418–1422. 10.1016/S0960-9822(03)00536-0 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. 2005a. The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445. 10.1038/nature03543 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M. 2005b. Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9: 109–119. 10.1016/j.devcel.2005.05.014 [DOI] [PubMed] [Google Scholar]
- Dinesh DC, Kovermann M, Gopalswamy M, Hellmuth A, Calderón Villalobos LI, Lilie H, Balbach J, Abel S. 2015. Solution structure of the PsIAA4 oligomerization domain reveals interaction modes for transcription factors in early auxin response. Proc Natl Acad Sci 112: 6230–6235. 10.1073/pnas.1424077112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher KA, Brown J, Saw RE, Callis J. 2006. The Arabidopsis Aux/IAA protein family has diversified in degradation and auxin responsiveness. Plant Cell 18: 699–714. 10.1105/tpc.105.039172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis CM, Nagpal P, Young JC, Hagen G, Guilfoyle TJ, Reed JW. 2005. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 132: 4563–4574. 10.1242/dev.02012 [DOI] [PubMed] [Google Scholar]
- Erijman A, Kozlowski L, Sohrabi-Jahromi S, Fishburn J, Warfield L, Schreiber J, Noble WS, Söding J, Hahn S. 2020. A high-throughput screen for transcription activation domains reveals their sequence features and permits prediction by deep learning. Mol Cell 78: 890. 10.1016/j.molcel.2020.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Sandoval E, Eklund DM, Bowman JL. 2015. A simple auxin transcriptional response system regulates multiple morphogenetic processes in the liverwort Marchantia polymorpha. PLoS Genet 11: e1005207. 10.1371/journal.pgen.1005207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire-Rios A, Tanaka K, Crespo I, Wijk Evd, Sizentsova Y, Levitsky V, Lindhoud S, Fontana M, Hohlbein J, Boer DR, et al. 2020. Architecture of DNA elements mediating ARF transcription factor binding and auxin-responsive gene expression in Arabidopsis. Proc Nat Acad Sci 117: 24557–24566. 10.1073/pnas.2009554117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H, Tameda S, Masuda H, Tasaka M. 2002. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J 29: 153–168. 10.1046/j.0960-7412.2001.01201.x [DOI] [PubMed] [Google Scholar]
- Galli M, Khakhar A, Lu Z, Chen Z, Sen S, Joshi T, Nemhauser JL, Schmitz RJ, Gallavotti A. 2018. The DNA binding landscape of the maize AUXIN RESPONSE FACTOR family. Nat Commun 9: 4526. 10.1038/s41467-018-06977-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, del Pozo JC, Walker L, Hobbie L, Risseeuw E, Banks T, Crosby WL, Yang M, Ma H, Estelle M. 1999. Identification of an SCF ubiquitin-ligase complex required for auxin response in Arabidopsis thaliana. Genes Dev 13: 1678–1691. 10.1101/gad.13.13.1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M. 2001. Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414: 271–276. 10.1038/35104500 [DOI] [PubMed] [Google Scholar]
- Guilfoyle TJ, Hagen G. 2012. Getting a grasp on domain III/IV responsible for auxin response factor-IAA protein interactions. Plant Sci 190: 82–88. 10.1016/j.plantsci.2012.04.003 [DOI] [PubMed] [Google Scholar]
- Han M, Park Y, Kim I, Kim EH, Yu TK, Rhee S, Suh JY. 2014. Structural basis for the auxin-induced transcriptional regulation by Aux/IAA17. Proc Natl Acad Sci 111: 18613–18618. 10.1073/pnas.1419525112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Ckurshumova W, Vidaurre DP, Singh SA, Stamatiou G, Tiwari SB, Hagen G, Guilfoyle TJ, Berleth T. 2004. Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL 4. Development 131: 1089–1100. 10.1242/dev.00925 [DOI] [PubMed] [Google Scholar]
- Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E. 2000. The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12: 757–770. 10.1105/tpc.12.5.757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens KA, Guseman JM, Jang SS, Pierre-Jerome E, Bolten N, Klavins E, Nemhauser JL. 2012. A synthetic approach reveals extensive tunability of auxin signaling. Plant Physiol 160: 135–142. 10.1104/pp.112.202184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann H, Hobbie L, Chapman A, Dharmasiri S, Dharmasiri N, del Pozo C, Reinhardt D, Estelle M. 2003. Arabidopsis AXR6 encodes CUL1 implicating SCF E3 ligases in auxin regulation of embryogenesis. EMBO J 22: 3314–3325. 10.1093/emboj/cdg335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holehouse AS. 2019. IDPs and IDRs in biomolecular condensates. In Intrinsically disordered proteins (ed. Salvi N), pp. 209–255. Academic, Cambridge, MA. [Google Scholar]
- Holland AJ, Fachinetti D, Han JS, Cleveland DW. 2012. Inducible, reversible system for the rapid and complete degradation of proteins in mammalian cells. Proc Natl Acad Sci 109: E3350–E3357. 10.1073/pnas.1216880109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagale S, Rozwadowski K. 2011. EAR motif-mediated transcriptional repression in plants: an underlying mechanism for epigenetic regulation of gene expression. Epigenetics 6: 141–146. 10.4161/epi.6.2.13627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanke M, Nishimura K, Kanemaki M, Kakimoto T, Takahashi TS, Nakagawa T, Masukata H. 2011. Auxin-inducible protein depletion system in fission yeast. BMC Cell Biol 12: 8. 10.1186/1471-2121-12-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Mutte SK, Suzuki H, Crespo I, Das S, Radoeva T, Fontana M, Yoshitake Y, Hainiwa E, van den Berg W, et al. 2020. Design principles of a minimal auxin response system. Nat Plants 6: 473–482. 10.1038/s41477-020-0662-y [DOI] [PubMed] [Google Scholar]
- Ke J, Ma H, Gu X, Thelen A, Brunzelle JS, Li J, Xu HE, Melcher K. 2015. Structural basis for recognition of diverse transcriptional repressors by the TOPLESS family of corepressors. Sci Adv 1: e1500107. 10.1126/sciadv.1500107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S, Leyser O. 2004. Auxin-induced SCFTIR1-Aux/IAA interaction involves stable modification of the SCFTIR1 complex. Proc Natl Acad Sci 101: 12381–12386. 10.1073/pnas.0402868101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S, Leyser O. 2005. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435: 446–451. 10.1038/nature03542 [DOI] [PubMed] [Google Scholar]
- Korasick DA, Westfall CS, Lee SG, Nanao MH, Dumas R, Hagen G, Guilfoyle TJ, Jez JM, Strader LC. 2014. Molecular basis for AUXIN RESPONSE FACTOR protein interaction and the control of auxin response repression. Proc Natl Acad Sci 111: 5427–5432. 10.1073/pnas.1400074111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korasick DA, Chatterjee S, Tonelli M, Dashti H, Lee SG, Westfall CS, Fulton DB, Andreotti AH, Amarasinghe GK, Strader LC, et al. 2015. Defining a two-pronged structural model for PB1 (Phox/Bem1p) domain interaction in plant auxin responses. J Biol Chem 290: 12868–12878. 10.1074/jbc.M115.648253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctot A, Nemhauser JL. 2020. It's Morphin’ time: how multiple signals converge on ARF transcription factors to direct development. Curr Opin Plant Biol 57: 1–7. 10.1016/j.pbi.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctot A, Taylor-Teeples M, Oki EA, Nemhauser J. 2020. Specificity in auxin responses is not explained by the promoter preferences of activator ARFs. Plant Physiol 182: 1533–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy M, Prigge MJ, Tao S, Shain S, Kuo A, Kirchsteiger K, Estelle M. 2016. Constitutive auxin response in Physcomitrella reveals complex interactions between Aux/IAA and ARF proteins. eLife 5: e13325. 10.7554/eLife.13325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Sundaram S, Armitage L, Evans JP, Hawkes T, Kepinski S, Ferro N, Napier RM. 2014. Defining binding efficiency and specificity of auxins for SCFTIR1/AFB-Aux/IAA co-receptor complex formation. Acs Chem Biol 9: 673–682. 10.1021/cb400618m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser HM, Lincoln CA, Timpte C, Lammer D, Turner J, Estelle M. 1993. Arabidopsis auxin-resistance gene AXR1 encodes a protein related to ubiquitin-activating enzyme E1. Nature 364: 161–164. 10.1038/364161a0 [DOI] [PubMed] [Google Scholar]
- Li H, Johnson P, Stepanova A, Alonso JM, Ecker JR. 2004. Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev Cell 7: 193–204. 10.1016/j.devcel.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M. 1990. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2: 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA, Ohno C, Smith ZR, Meyerowitz EM. 2006. TOPLESS regulates apical embryonic fate in Arabidopsis. Science 312: 1520–1523. 10.1126/science.1123841 [DOI] [PubMed] [Google Scholar]
- Maraschin Fdos S, Memelink J, Offringa R. 2009. Auxin-induced, SCFTIR1-mediated poly-ubiquitination marks AUX/IAA proteins for degradation. Plant J 59: 100–109. 10.1111/j.1365-313X.2009.03854.x [DOI] [PubMed] [Google Scholar]
- Mazur E, Kulik I, Hajný J, Friml J. 2020. Auxin canalization and vascular tissue formation by TIR1/AFB-mediated auxin signaling in Arabidopsis. New Phytol 226: 1375–1383. 10.1111/nph.16446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss BL, Mao H, Guseman JM, Hinds TR, Hellmuth A, Kovenock M, Noorassa A, Lanctot A, Villalobos LI, Zheng N, et al. 2015. Rate motifs tune auxin/indole-3-acetic acid degradation dynamics. Plant Physiol 169: 803–813. 10.1104/pp.15.00587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutte SK, Kato H, Rothfels C, Melkonian M, Wong GK, Weijers D. 2018. Origin and evolution of the nuclear auxin response system. eLife 7: e33399. 10.7554/eLife.33399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal P, Walker LM, Young JC, Sonawala A, Timpte C, Estelle M, Reed JW. 2000. AXR2 encodes a member of the Aux/IAA protein family. Plant Physiol 123: 563–574. 10.1104/pp.123.2.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanao MH, Vinos-Poyo T, Brunoud G, Thévenon E, Mazzoleni M, Mast D, Lainé S, Wang S, Hagen G, Li H, et al. 2014. Structural basis for oligomerization of auxin transcriptional regulators. Nat Commun 5: 3617. 10.1038/ncomms4617 [DOI] [PubMed] [Google Scholar]
- Niemeyer M, Moreno Castillo E, Ihling CH, Iacobucci C, Wilde V, Hellmuth A, Hoehenwarter W, Samodelov SL, Zurbriggen MD, Kastritis PL, et al. 2020. Flexibility of intrinsically disordered degrons in AUX/IAA proteins reinforces auxin co-receptor assemblies. Nat Commun 11: 2277. 10.1038/s41467-020-16147-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Fukagawa T, Takisawa H, Kakimoto T, Kanemaki M. 2009. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat Methods 6: 917–922. 10.1038/nmeth.1401 [DOI] [PubMed] [Google Scholar]
- Okushima Y, Mitina I, Quach HL, Theologis A. 2005. AUXIN RESPONSE FACTOR 2 (ARF2): a pleiotropic developmental regulator. Plant J 43: 29–46. 10.1111/j.1365-313X.2005.02426.x [DOI] [PubMed] [Google Scholar]
- O'Malley RC, Huang SSC, Song L, Lewsey MG, Bartlett A, Nery JR, Galli M, Gallavotti A, Ecker JR. 2016. Cistrome and epicistrome features shape the regulatory DNA landscape. Cell 165: 1280–1292. 10.1016/j.cell.2016.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet F, Overvoorde PJ, Theologis A. 2001. IAA17/AXR3: biochemical insight into an auxin mutant phenotype. Plant Cell 13: 829–841. 10.1105/tpc.13.4.829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Calderon-Villalobos LI, Prigge M, Peret B, Dharmasiri S, Itoh H, Lechner E, Gray WM, Bennett M, Estelle M. 2009. Complex regulation of the TIR1/AFB family of auxin receptors. Proc Natl Acad Sci 106: 22540–22545. 10.1073/pnas.0911967106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre-Jerome E, Moss BL, Nemhauser JL. 2013. Tuning the auxin transcriptional response. J Exp Bot 64: 2557–2563. 10.1093/jxb/ert100 [DOI] [PubMed] [Google Scholar]
- Pierre-Jerome E, Jang SS, Havens KA, Nemhauser JL, Klavins E. 2014. Recapitulation of the forward nuclear auxin response pathway in yeast. Proc Natl Acad Sci 111: 9407–9412. 10.1073/pnas.1324147111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre-Jerome E, Moss BL, Lanctot A, Hageman A, Nemhauser JL. 2016. Functional analysis of molecular interactions in synthetic auxin response circuits. Proc Natl Acad Sci 113: 11354–11359. 10.1073/pnas.1604379113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Strader LC. 2020. Regulation of auxin transcriptional responses. Dev Dyn 249: 483–495. 10.1002/dvdy.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Holehouse AS, Korasick DA, Schreiber KH, Clark NM, Jing H, Emenecker R, Han S, Tycksen E, Hwang I, et al. 2019. Nucleo-cytoplasmic partitioning of ARF proteins controls auxin responses in Arabidopsis thaliana. Mol Cell 76: 177–190.e5. 10.1016/j.molcel.2019.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Platre M, Kadakia N, Zhang Y, Greenham K, Szutu W, Pandey BK, Bhosale RA, Bennett MJ, Busch W, et al. 2020. Genetic analysis of the Arabidopsis TIR1/AFB auxin receptors reveals both overlapping and specialized functions. eLife 9: e54740. 10.7554/eLife.54740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JA, Zenser N, Leyser O, Callis J. 2001. Rapid degradation of auxin/indoleacetic acid proteins requires conserved amino acids of domain II and is proteasome dependent. Plant Cell 13: 2349–2360. 10.1105/tpc.010244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravarani CN, Erkina TY, De Baets G, Dudman DC, Erkine AM, Babu MM. 2018. High-throughput discovery of functional disordered regions: investigation of transactivation domains. Mol Syst Biol 14: e8190. 10.15252/msb.20188190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse D, Mackay P, Stirnberg P, Estelle M, Leyser O. 1998. Changes in auxin response from mutations in an AUX/IAA gene. Science 279: 1371–1373. 10.1126/science.279.5355.1371 [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M. 1997. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 9: 745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M. 1998. The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast Grr1p. Genes Dev 12: 198–207. 10.1101/gad.12.2.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A, Nemhauser JL, McColl A, Roe JL, Feldmann KA, Zambryski PC. 1997. ETTIN patterns the Arabidopsis floral meristem and reproductive organs. Development 124: 4481–4491. [DOI] [PubMed] [Google Scholar]
- Staller MV, Holehouse AS, Swain-Lenz D, Das RK, Pappu RV, Cohen BA. 2018. A high-throughput mutational scan of an intrinsically disordered acidic transcriptional activation domain. Cell Syst 6: 444–455.e6. 10.1016/j.cels.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szemenyei H, Hannon M, Long JA. 2008. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319: 1384–1386. 10.1126/science.1151461 [DOI] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. 2007. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446: 640–645. 10.1038/nature05731 [DOI] [PubMed] [Google Scholar]
- Tian Q, Nagpal P, Reed JW. 2003. Regulation of Arabidopsis SHY2/IAA3 protein turnover. Plant J 36: 643–651. 10.1046/j.1365-313X.2003.01909.x [DOI] [PubMed] [Google Scholar]
- Tian CE, Muto H, Higuchi K, Matamura T, Tatematsu K, Koshiba T, Yamamoto KT. 2004. Disruption and overexpression of auxin response factor 8 gene of Arabidopsis affect hypocotyl elongation and root growth habit, indicating its possible involvement in auxin homeostasis in light condition. Plant J 40: 333–343. 10.1111/j.1365-313X.2004.02220.x [DOI] [PubMed] [Google Scholar]
- Tiwari SB, Wang XJ, Hagen G, Guilfoyle TJ. 2001. AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 13: 2809–2822. 10.1105/tpc.010289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Hagen G, Guilfoyle T. 2003. The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell 15: 533–543. 10.1105/tpc.008417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Hagen G, Guilfoyle TJ. 2004. Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 16: 533–543. 10.1105/tpc.017384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost M, Blattner AC, Lehner CF. 2016. Regulated protein depletion by the auxin-inducible degradation system in Drosophila melanogaster. Fly (Austin) 10: 35–46. 10.1080/19336934.2016.1168552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Takahashi K, Iwasaki R, Yamada R, Yoshimura M, Endo TA, Kimura S, Zhang H, Nomoto M, Tada Y, et al. 2018. Chemical hijacking of auxin signaling with an engineered auxin-TIR1 pair. Nat Chem Biol 14: 299–305. 10.1038/nchembio.2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Liu ZB, Hagen G, Guilfoyle TJ. 1995. Composite structure of auxin response elements. Plant Cell 7: 1611–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. 1997a. ARF1, a transcription factor that binds to auxin response elements. Science 276: 1865–1868. 10.1126/science.276.5320.1865 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. 1997b. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. 1999a. Activation and repression of transcription by auxin-response factors. Proc Natl Acad Sci 96: 5844–5849. 10.1073/pnas.96.10.5844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. 1999b. Dimerization and DNA binding of auxin response factors. Plant J 19: 309–319. 10.1046/j.1365-313X.1999.00538.x [DOI] [PubMed] [Google Scholar]
- Uversky VN. 2019. Intrinsically disordered proteins and their “mysterious” (meta)physics. Front Phys 10.3389/fphy.2019.00010 [DOI] [Google Scholar]
- Uzunova VV, Quareshy M, Del Genio CI, Napier RM. 2016. Tomographic docking suggests the mechanism of auxin receptor TIR1 selectivity. Open Biol 6: 160139. 10.1098/rsob.160139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Brunoud G, Farcot E, Morin V, Van den Daele H, Legrand J, Oliva M, Das P, Larrieu A, Wells D, et al. 2011. The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol Syst Biol 7: 508. 10.1038/msb.2011.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D, Wagner D. 2016. Transcriptional responses to the auxin hormone. Annu Rev Plant Biol 67: 539–574. 10.1146/annurev-arplant-043015-112122 [DOI] [PubMed] [Google Scholar]
- Wilmoth JC, Wang S, Tiwari SB, Joshi AD, Hagen G, Guilfoyle TJ, Alonso JM, Ecker JR, Reed JW. 2005. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J 43: 118–130. 10.1111/j.1365-313X.2005.02432.x [DOI] [PubMed] [Google Scholar]
- Worley CK, Zenser N, Ramos J, Rouse D, Leyser O, Theologis A, Callis J. 2000. Degradation of Aux/IAA proteins is essential for normal auxin signalling. Plant J 21: 553–562. 10.1046/j.1365-313x.2000.00703.x [DOI] [PubMed] [Google Scholar]
- Wu MF, Yamaguchi N, Xiao J, Bargmann B, Estelle M, Sang Y, Wagner D. 2015. Auxin-regulated chromatin switch directs acquisition of flower primordium founder fate. eLife 4: e09269. 10.7554/eLife.09269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Lee S, So JH, Dharmasiri S, Dharmasiri N, Ge L, Jensen C, Hangarter R, Hobbie L, Estelle M. 2004. The IAA1 protein is encoded by AXR5 and is a substrate of SCFTIR1. Plant J 40: 772–782. 10.1111/j.1365-313X.2004.02254.x [DOI] [PubMed] [Google Scholar]
- Yu H, Moss BL, Jang SS, Prigge M, Klavins E, Nemhauser JL, Estelle M. 2013. Mutations in the TIR1 auxin receptor that increase affinity for auxin/indole-3-acetic acid proteins result in auxin hypersensitivity. Plant Physiol 162: 295–303. 10.1104/pp.113.215582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Zhang Y, Moss BL, Bargmann BO, Wang R, Prigge M, Nemhauser JL, Estelle M. 2015. Untethering the TIR1 auxin receptor from the SCF complex increases its stability and inhibits auxin response. Nat Plants 1: 14030. 10.1038/nplants.2014.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenser N, Ellsmore A, Leasure C, Callis J. 2001. Auxin modulates the degradation rate of Aux/IAA proteins. Proc Natl Acad Sci 98: 11795–11800. 10.1073/pnas.211312798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenser N, Dreher KA, Edwards SR, Callis J. 2003. Acceleration of Aux/IAA proteolysis is specific for auxin and independent of AXR1. Plant J 35: 285–294. 10.1046/j.1365-313X.2003.01801.x [DOI] [PubMed] [Google Scholar]