Abstract
Background
The availability of linear accelerators (linac) for research purposes is often limited and therefore alternative radiation sources are needed to conduct radiobiological research. The National Centre for Radiation Research in Poland recently developed an intraoperative mobile linac that enables electron irradiation at energies ranging from 4 to 12 MeV and dose rates of 5 or 10 Gy/min. The present study was conducted to evaluate the electron beam parameters of this intraoperative linac and to verify the set-up to evaluate out-of-field doses in a water phantom, which were determined through dosimetric and biological response measurements.
Materials and methods
The distribution of radiation doses along and across the radiation beam were measured in a water phantom using a semiconductor detector and absolute doses using an ionisation chamber. Two luminal breast cancer cell lines (T-47D and HER2 positive SK-BR-3) were placed in the phantom to study radiation response at doses ranging from 2 to 10 Gy. Cell response was measured by clonogenic assays.
Results and Conclusion
The electron beam properties, including depth doses and profiles, were within expected range for the stated energies. These results confirm the viability of this device and set-up as a source of megavoltage electrons to evaluate the radiobiological response of tumour cells.
Keywords: electron accelerator, intraoperative radiotherapy, breast cancer, clonogenic assay
Introduction
Due to technical advances in photon beam radiotherapy, which allow for highly conformal dose painting, the clinical use of electron beam radiotherapy has decreased over time. Nevertheless, electrons continue to play an important role in specific applications such as intraoperative radiotherapy (e.g., breast cancer) and the emerging technique known as flash radiotherapy [1–3].
Intraoperative radiotherapy is performed immediately after surgical removal of the tumour to eradicate any remaining cancer cells located in or around the tumour bed [4, 5]. The electrons are produced by a mobile accelerator unit. However, since demand for intraoperative radiotherapy is relatively limited due to strict eligibility criteria for this treatment, these units often have greater availability for research purposes than conventional linear accelerators (linacs), thus representing an excellent source of electrons for research purposes [6, 7]. However, few studies have been performed to verify the viability of using intraoperative linacs to perform radiobiological tests [8]. Moreover, very few studies have been conducted to evaluate out-of-field doses produced by electron beams, which are dependent on the applicators used during electron irradiation [9–14] and intraoperative electron radiation therapy [15–17]. Importantly, the advent of FLASH radiotherapy may increase research interest in out-of-field doses from electron therapy [2, 18].
Given the need for more research in verifying the beam parameters in electron radiotherapy, we also wanted to confirm the potential of a novel intraoperative linac developed for clinical treatment at the National Centre for Radiation Research in Poland for radiobiological studies.
In this context, the present study had two main aims: 1) to evaluate the electron beam properties (4–12 MeV) from this intraoperative linac, and 2) to assess the potential to use this system set-up to verify out-of-field doses in a water phantom using dosimetric and biological response measurements.
Materials and methods
Source of electrons
For this study, we used an intraoperative accelerator being developed under another project conducted by the National Centre for Radiation Research in Poland. This device allows for irradiation with a time stable beam of electrons at energies ranging from 4 to 12 MeV.
Measurements of beam parameters
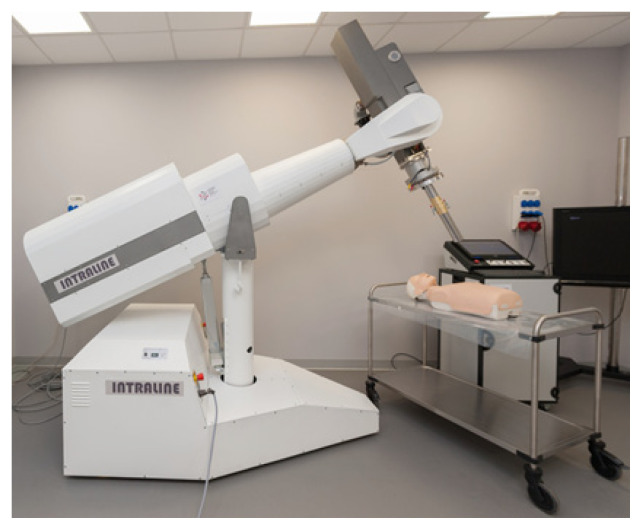
The accelerator (Fig. 1) was built specifically for intraoperative radiotherapy. It can generate electrons with energies ranging from 4 to 12 MeV to deliver a radiation dose rate of 5 or 10 Gy/min at each energy.
Figure 1.
General view of the mobile intraoperative linear accelerator which being developed by the National Centre for Radiation Research
The electron source of this accelerator is a triode gun controlled by a triode modulator. The cathode is polarized with a voltage of −15 kV and heated with a current of 1.44 A at each energy output. The number of emitted electrons and thus control of the dose rate is regulated by setting the appropriate polarity of the grid voltage (range: −80 to 100 V) [19]. The microwave power source is a magnetron with a maximum power of 3.2 MW. The magnetron is powered by a modulator that produces a heating source for the cathode and a proper pulse anode voltage, which is dependent on the required energy [20]. The magnetron works in a constant magnetic field generated by an electromagnet. The electron beam energy value is set using the appropriate anode voltage level of the magnetron. After the electron beam leaves the area where it is accelerated through the microwave power generated by the magnetron, it is shaped by an array of scattering and equalizing films and the final beam is formed by a circular applicator. In this study, a steel applicator with a circular cross section of Ø 100 mm was used.
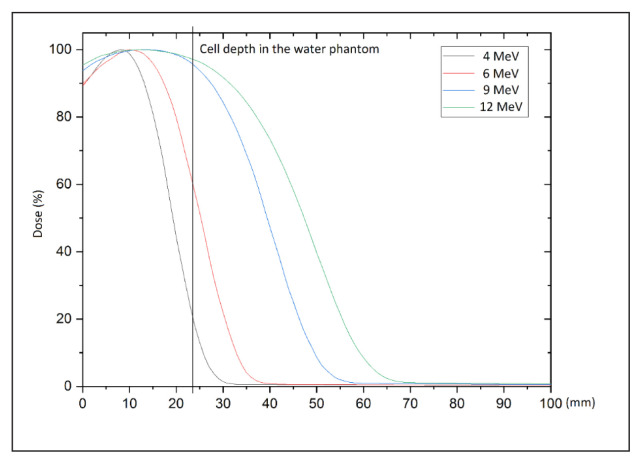
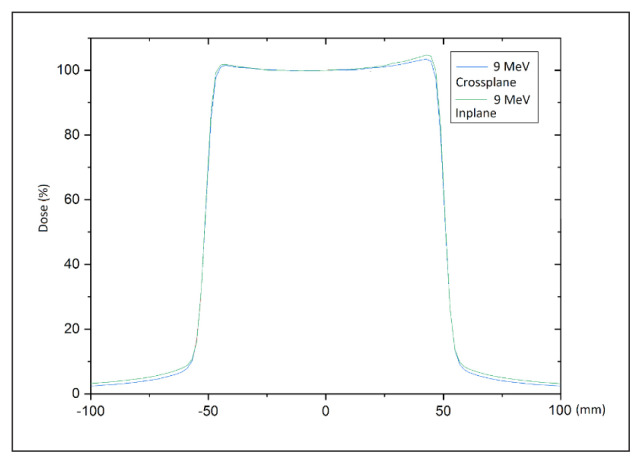
To determine the dose distribution along and across the radiation beam, dosimetric characteristics were measured in a water phantom using a semiconductor detector (60012 diode; PTW, Freiburg, Germany), which was specifically selected because the measurements are carried out under non-reference conditions due to the characteristics of mobile accelerators (smaller than the standard source to surface distance [SSD] used, round shaped field, high dose rate and dose per pulse) [21, 22]. The percentage depth dose curves for the available radiation energies (4–12 MeV) were determined and used to obtain the following parameters: depth of the maximum dose; depth of 80% and 50% of maximum dose (R80 and R50, respectively); practical range; percentage of dose on the surface; and the level of contamination with photon radiation. A function profile was determined perpendicular to the central axis at the depth of the maximum dose to check the symmetry and flatness of the radiation beam.
Based on the measured dose distributions, we selected the optimal parameters for the irradiation of biological material and the design of the flask holder. The final measurements included the following parameters: absolute dose; repeatability; and dose linearity from the number of monitor units to ensure appropriate conditions for the irradiation of cell lines and to determine survival curves. A Marcus type chamber and a PTW Unidos electrometer were placed in the phantom at the depth at which the cancer cells would be located.
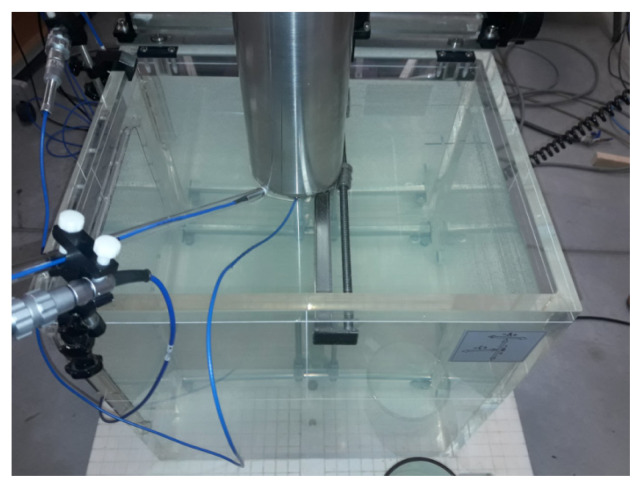
All accelerator parameters were set and measured using a 600 × 500 × 408 mm3 PTW MP3 water phantom (Fig. 2). Depth curves showing the energy measurements (Fig. 3) and profiles showing beam homogeneity (Fig. 4) were measured using a PTW 60012 diode detector. Figure 2 shows the assembled phantom.
Figure 2.
A measuring set-up consisting of a water phantom and a set of detectors used to obtain dose distributions for the diameter of the applicator (10 cm)
Figure 3.
Depth dose curves for 4, 6, 8 and 12 MeV. The position of the cell tubes at the depth is marked with a vertical line
Figure 4.
Beam profiles measured at depth of the maximum dose for 9 or 12 MeV
Preparation of set-up and irradiation of tumour cells
Cell lines and culture conditions
Experiments were conducted on two luminal breast cancer cell lines—T-47D (ATCC HTB-133, Rockville, MD) and HER2 positive SK-BR-3 (ATCC HTB-30, Rockville, MD) —both of which are classified as adherent cells with epithelial morphology. Tumour cells were grown under standard conditions (temperature 37°C, atmosphere 5% CO2, humidity 95%). The T-47D line was cultured in RPMI 1640 medium (Biowest; Nuaillé, France) supplemented with 10% heat inactivated fetal bovine serum (FBS), 1% penicillin/streptomycin (Sigma, Aldrich, Merck KGaA; Darmstadt, Germany) and insulin at a concentration of 0.2 UL/ml (Bioton, Poland). The SK-BR-3 cells were cultured in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 supplemented with 10% FBS and 1% penicillin/streptomycin solution (all from Sigma, Aldrich, Merck KGaA; Darmstadt, Germany). Cells were passaged a mean of once weekly with a 0.25% trypsin solution in EDTA (Sigma-Aldrich, Merck KGaA; Darmstadt, Germany).
Irradiation conditions
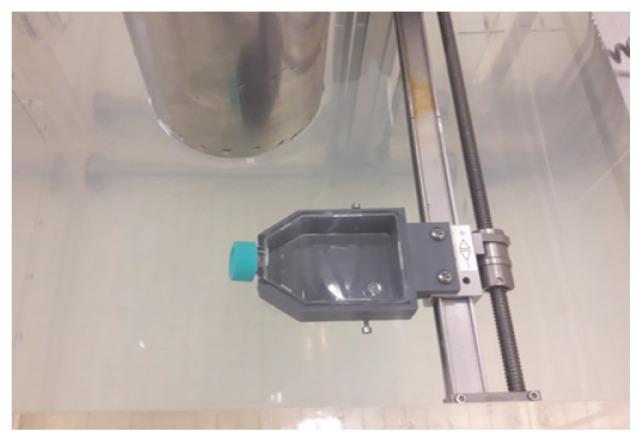
A three-dimensional (3D) printer was used to print a purpose-built holder (Fig. 5) to ensure precise, repeatable placement of the bottles containing the cancer cells. This holder was then installed in the water phantom in place of the original measuring probe (Fig. 6).
Figure 5.
T-25 cell culture bottle placed in the holder. The holder was specially designed and printed in the 3D technique
Figure 6.
Bottle holder fixed in the water phantom. This system enables precise positioning of the sample in the desired position
Cells were seeded in T-25 bottles with a cap but no filter (Nest, China). After the culture medium was removed, the T-25 bottles were filled with a sterile PBS solution (Sigma-Aldrich, Germany) to create water conditions similar to those found in the human body. The cells were transported to the accelerator in a portable Mini-Cell NB203M CO2 incubator (N-Biotek, Korea). Unirradiated control cells (0 Gy) were handled in the same way as the irradiated cells. The cells were irradiated at the following doses: 2, 4, 6, 8 and 10 Gy. For each cell line, we repeated the radiobiological tests three times.
Clonogenic assay
A clonogenic assay was performed as described elsewhere [24, 25]. Briefly, after irradiation, the cells were counted using a Luna II automated cell counter (Logos Biosystems) plated on 6-well plates and incubated for 10–14 days. The clonogenic assay was closed when control colonies contained ≥ 50 cells. Following the incubation period, the plates were washed with PBS solution, fixed with 70% ethanol (POCH S.A.) and stained with Coomassie Brilliant Blue buffer (Sigma-Aldrich). Colonies were counted manually. Plating efficiency (PE) was calculated as a ratio of counted colonies to seeded cells multiplied by 100%. The surviving fraction (SF) was calculated as the ratio of PE irradiated cells to PE control cells.
The optimal range of electrons for a 24-mm deep T-25 cell culture bottle is obtained with an energy of either 9 or 12 MeV. The dashed line on the depth profile graph (Fig. 3) shows the depth of the cells in the bottle mounted in the water phantom. Before the test, the water in the phantom was heated to 37°C to ensure that the water temperature did not influence cell survival.
Results
Measured beam properties, including depth doses and flatness/symmetry of the profiles for energies 4–12 MeV are shown in Figures 3 and 4.
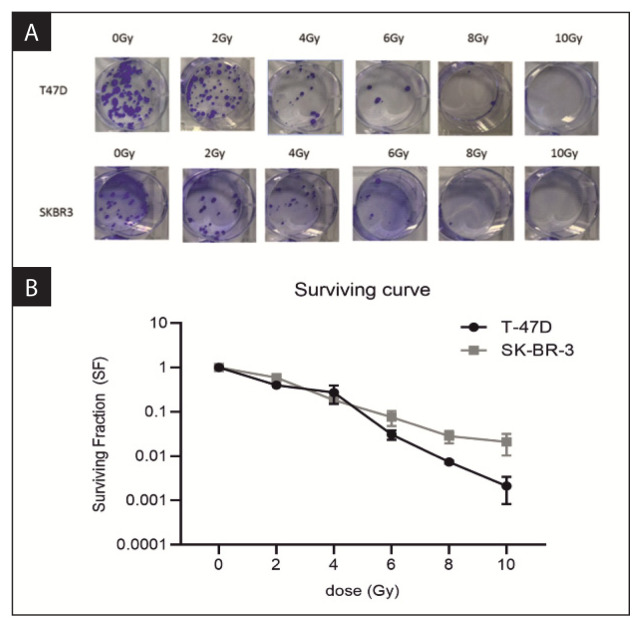
The clonogenic assay was performed to measure the SF of cells irradiated with 2, 4, 6, 8 and 10 Gy (Fig. 7A). Based on the results of this experiment, survival curves were generated (Fig. 7B), which showed that cell survival decreased in line with higher radiation doses.
Figure 7.
Radiobiological response of breast cancer cells to ionising radiation. A. Results of the clonogenic assays in T-47D and SK-BR-3 cell lines treated with 2, 4, 6,8 and 10 Gy of irradiation. B. Survival curves of the T-47D and SK-BR-3 cells based on the results of the clonogenic assays
As expected, increases in the radiation dose led to a decrease in the fraction of surviving cells. The SF for the SK-BR-3 line was much higher than for the T-47D line, indicating a greater radiation resistance for the SK-BR-3 line.
Discussion
The present study was performed to evaluate the properties of electron beams with 4 to 12 MeV from a novel intraoperative linac. Our findings show that the electron beam properties, including depth doses and profiles (flatness and symmetry), were within the expected range for stated energies from 4 to 12 MeV [26, 27].
We also evaluated the potential of this new linac to perform out-of-field dose verification and biological cell response. Two luminal breast cancer cell lines were evaluated to study radiation response (clonogenic assay) at doses ranging from 2 to 10 Gy. These analyses show that this intraoperative linac and set-up appears to be a suitable source of mega-voltage electrons and can thus be used to evaluate the radiobiological response of tumour cells. The intraoperative accelerator produces megavoltage electrons ranging from 4 to 12 MeV with a dose rate of 5 or 10 Gy/min for each energy. The dosimetric measurements and biological response of the breast cancer cells to irradiation confirmed the assumed properties of the beam.
In this study, we have developed a procedure to perform repeatable, accurate testing with controlled cell irradiation, thereby allowing us to assess the effects of radiation on cell survival. The results confirmed both the accuracy of the biological procedures used and the physical parameters of the accelerator. Cells were irradiated at different doses to determine and calculate survival curves. The maximum dose (10 Gy) killed 98.5% of SK-BR-3 cells and 99.5% of T-47D cells. As expected, higher radiation doses resulting in greater decreases in the fraction of surviving cells.
Our data demonstrate that this intraoperative accelerator is a suitable source of megavoltage electrons, which can be used to evaluate the radiobiological response of tumour cells. This set-up in a water phantom can also be used to determine the properties of out-of-field beams to evaluate low dose radiation (0.05–1 Gy) caused by scattered radiation.
This validated technology can be used for all radiobiological tests to analyse cell survival curves for various cell lines. In this case, the observed decrease in surviving cells was as expected, confirming the dose measurement accuracy of the dosimeter.
Conclusions
This study demonstrates that the electron beam properties from this novel intraoperative linear accelerator, including depth doses and profiles, were within expected range for stated energies from 4 to 12 MeV at dose rates modes of 5 and 10 Gy/min. These findings support the use of this linac as a suitable source of megavoltage electrons to test the radiobiological response of tumour cells.
The set-up, which was specifically designed and constructed for in vitro testing, can be used for dose measurements and biological response for locations along and outside the central axis and the radiation field.
Footnotes
Conflicts of interest
None declared.
Funding
The study was supported by the National Science Centre of Poland, grant no. 2015/19/B/NZ7/03811.
References
- 1.Ronga MG, Cavallone M, Patriarca A, et al. Back to the Future: Very High-Energy Electrons (VHEs) and Their Potential Application in Radiation Therapy. Cancers (Basel) 2021;13(19) doi: 10.3390/cancers13194942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moeckli R, Gonçalves Jorge P, Grilj V, et al. Commissioning of an ultra-high dose rate pulsed electron beam medical LINAC for FLASH RT preclinical animal experiments and future clinical human protocols. Med Phys. 2021;48(6):3134–3142. doi: 10.1002/mp.14885. [DOI] [PubMed] [Google Scholar]
- 3.Fastner G, Gaisberger C, Kaiser J, et al. ESTRO IORT Task Force/ACR OP recommendations for intraoperative radiation therapy with electrons (IOERT) in breast cancer. Radiother Oncol. 2020;149:150–157. doi: 10.1016/j.radonc.2020.04.059. [DOI] [PubMed] [Google Scholar]
- 4.Piotrowski I, Kulcenty K, Murawa D, et al. Biologiczne aspekty śródoperacyjnej radioterapii i roli płynów pooperacyjnych w terapii raka piersi. Lett Oncol Sci. 2016;13(2):30–37. doi: 10.21641/los.13.2.13. [DOI] [Google Scholar]
- 5.Piotrowski I, Kulcenty K, Wichtowski M, et al. Intraoperative Radiotherapy of Breast Cancer and Its Biological Effects. Breast Care (Basel) 2017;12(2):109–113. doi: 10.1159/000454673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minafra L, Bravatà V, Russo G, et al. Gene Expression Profiling of MCF10A Breast Epithelial Cells Exposed to IOERT. Anticancer Res. 2015;35(6):3223–3234. [PubMed] [Google Scholar]
- 7.Bravatà V, Minafra L, Russo G, et al. High-dose Ionizing Radiation Regulates Gene Expression Changes in the MCF7 Breast Cancer Cell Line. Anticancer Res. 2015;35(5):2577–2591. [PubMed] [Google Scholar]
- 8.Piotrowski I, Kulcenty K, Murawa D, et al. Surgical wound fluids from patients treated with intraoperative radiotherapy induce radiobiological response in breast cancer cells. Med Oncol. 2018;36(2):14. doi: 10.1007/s12032-018-1243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iktueren B, Bilge H, Karacam S, et al. The peripheral dose outside the applicator in electron beams of Oncor linear accelerator. Radiat Prot Dosimetry. 2012;150(2):192–197. doi: 10.1093/rpd/ncr392. [DOI] [PubMed] [Google Scholar]
- 10.Haghparast A, Amiri F, Yarahmadi M, et al. The peripheral dose outside the applicator in electron beams of an Elekta linear accelerator. Australas Phys Eng Sci Med. 2018;41(3):647–655. doi: 10.1007/s13246-018-0660-9. [DOI] [PubMed] [Google Scholar]
- 11.Chow JCL, Grigorov GN. Peripheral dose outside applicators in electron beams. Phys Med Biol. 2006;51(12):N231–N240. doi: 10.1088/0031-9155/51/12/N01. [DOI] [PubMed] [Google Scholar]
- 12.Jabbari N, Hashemi-Malayeri B, Farajollahi AR, et al. Monte Carlo calculation of scattered radiation from applicators in low energy clinical electron beams. Nukleonika. 2007;52:97–103. [Google Scholar]
- 13.Perec A, Kubo H. Radiation leakage through electron applicators on Clinac-1800 accelerators. Med Phys. 1990;17(4):715–719. doi: 10.1118/1.596472. [DOI] [PubMed] [Google Scholar]
- 14.Alabdoaburas MM, Mege JP, Chavaudra J, et al. Experimental assessment of out-of-field dose components in high energy electron beams used in external beam radiotherapy. J Appl Clin Med Phys. 2015;16(6):435–448. doi: 10.1120/jacmp.v16i6.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adrich P. Technical Note: Monte Carlo study on the reduction in x-ray contamination of therapeutic electron beams for Intraoperative Radiation Therapy by means of improvements in the design of scattering foils. Med Phys. 2019;46(8):3378–3384. doi: 10.1002/mp.13647. [DOI] [PubMed] [Google Scholar]
- 16.García-Cases F, Perez-Calatayud J, Ballester F, et al. Peripheral dose around a mobile linac for intraoperative radiotherapy: radiation protection aspects. J Radiol Prot. 2018;38(4):1393–1411. doi: 10.1088/1361-6498/aae5a0. [DOI] [PubMed] [Google Scholar]
- 17.Mahdavi SR, Tutuni M, Farhood B, et al. Measurement of peripheral dose to the pelvic region and the associated risk for cancer development after breast intraoperative electron radiation therapy. J Radiol Prot. 2019;39(1):278–291. doi: 10.1088/1361-6498/aafdc8. [DOI] [PubMed] [Google Scholar]
- 18.Matuszak N, Suchorska WM, Milecki P, et al. FLASH Radiotherapy: an emerging approach in radiation therapy. Rep Pract Oncol Radiother. :2021. doi: 10.5603/RPOR.a2022.0038. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scharf W. Akceleratory biomedyczne. Wydawnictwo Naukowe PWN, Warszawa. 1994 [Google Scholar]
- 20.Pracz J, Syntfeld-Każuch A. Akcelerator IntraLine–IORT do radioterapii śródoperacyjnej. Post Tech Jądr. 2017;60(3):21–28. [Google Scholar]
- 21.Scalchi P, Ciccotelli A, Felici G, et al. Use of parallel-plate ionization chambers in reference dosimetry of NOVAC and LIAC mobile electron linear accelerators for intraoperative radiotherapy: a multi-center survey. Med Phys. 2017;44(1):321–332. doi: 10.1002/mp.12020. [DOI] [PubMed] [Google Scholar]
- 22.Laitano RF, Guerra AS, Pimpinella M, et al. Charge collection efficiency in ionization chambers exposed to electron beams with high dose per pulse. Phys Med Biol. 2006;51(24):6419–6436. doi: 10.1088/0031-9155/51/24/009. [DOI] [PubMed] [Google Scholar]
- 23.Gunderson LL, Willett CG, Harrison LB, Calvo FA. Intraoperative Irradiation: Techniques and Results. Humana Press; Totowa: p. 1999. [Google Scholar]
- 24.Musielak M. The assessment of the effect of ionizing radiation dose and dose rate for breast cancer cells. Lett Oncol Sci. 2018;19(4):117–125. doi: 10.21641/los.15.4.82. [DOI] [Google Scholar]
- 25.Joiner M. Quantifing cell kill and cell survival. In: Joiner M, Van der Kogel A, editors. Basic Clinical Radiobiology. Hodder Arnold; London: 2009. pp. 41–55. [Google Scholar]
- 26.Medyczne urządzenia elektryczne. Część 2-1: Szczegółowe wymagania bezpieczeństwa akceleratorów elektronów w zakresie od 1 MeV do 50 MeV. PKN. Warszawa: 2005. [Google Scholar]
- 27.Medical electrical equipment — Medical electron accelerators — Functional performance characteristics. Geneva, IEC 60976: [Google Scholar]