Abstract
Purpose
Hyposmia is a common feature of COVID-19 and Parkinson’s disease (PD). As parkinsonism has been reported after COVID-19, a link has been hypothesized between SARS-CoV2 infection and PD. We aimed to evaluate brain metabolic correlates of isolated persistent hyposmia after mild-to-moderate COVID-19 and to compare them with metabolic signature of hyposmia in drug-naïve PD patients.
Methods
Forty-four patients who experienced hyposmia after SARS-COV2 infection underwent brain [18F]-FDG PET in the first 6 months after recovery. Olfaction was assessed by means of the 16-item “Sniffin’ Sticks” test and patients were classified as with or without persistent hyposmia (COVID-hyposmia and COVID-no-hyposmia respectively). Brain [18F]-FDG PET of post-COVID subgroups were compared in SPM12. COVID-hyposmia patients were also compared with eighty-two drug-naïve PD patients with hyposmia. Multiple regression analysis was used to identify correlations between olfactory test scores and brain metabolism in patients’ subgroups.
Results
COVID-hyposmia patients (n = 21) exhibited significant hypometabolism in the bilateral gyrus rectus and orbitofrontal cortex with respect to COVID-non-hyposmia (n = 23) (p < 0.002) and in middle and superior temporal gyri, medial/middle frontal gyri, and right insula with respect to PD-hyposmia (p < 0.012). With respect to COVID-hyposmia, PD-hyposmia patients showed hypometabolism in inferior/middle occipital gyri and cuneus bilaterally. Olfactory test scores were directly correlated with metabolism in bilateral rectus and medial frontal gyri and in the right middle temporal and anterior cingulate gyri in COVID-hyposmia patients (p < 0.006) and with bilateral cuneus/precuneus and left lateral occipital cortex in PD-hyposmia patients (p < 0.004).
Conclusion
Metabolic signature of persistent hyposmia after COVID-19 encompasses cortical regions involved in olfactory perception and does not overlap metabolic correlates of hyposmia in PD.
Keywords: COVID-19, Brain PET, Parkinson’s disease, Anosmia, [18F]FDG
Introduction
COVID-19 due to SARS-CoV-2 infection has been associated with a number of neurological manifestations and complications [1]. The severity, occurrence, and duration of these symptoms during the disease course has been demonstrated to be largely variable [1]. In this framework, loss or reduction of smell (anosmia/hyposmia) and taste (hypogeusia/ageusia) can occur in absence of any other clinical feature covering the entire spectrum of disease severity and time course of COVID-19 [2, 3]. In particular, olfactory dysfunction has been repeatedly reported as a frequent persistent symptom both in the subacute stage and several weeks or months after infection [3]. A potential link has been investigated between hyposmia and an increased risk to develop other neurological mid- and long-term sequelae also in patients who experienced mild or moderate COVID-19 [4–6]. An increasing body of evidence suggests that hyposmia and other long-lasting symptoms after SARS-CoV-2 infection might be associated with cortical hypometabolism as assessed by [18F]-fluorodeoxyglucose (FDG) PET [7–15]. The majority of available FDG PET studies during or after COVID-19 has been carried out in patients who experienced hyposmia after severe disease and/or in association with other neurological sequelae [7–15]. Conversely, from an epidemiological and functional perspective, it is relevant to quantify the burden of persistent symptoms especially in the vast majority of patients who, actually, suffered from mild or moderate disease to assess the impact of these sequelae on the healthcare system in the future. In fact, considering the high spreading of SARS-CoV-2 infection worldwide, COVID-19 is expected to significantly contribute to the overall burden of hyposmia in the next years [16]. A deeper exploration of the pathophysiological and prognostic relevance of persistent hyposmia after COVID-19 is also relevant considering the strong association between olfactory dysfunction and neurodegenerative diseases, including Parkinson’s disease (PD), dementia with Lewy bodies, and Alzheimer’s disease [17–19]. Interestingly, while in PD olfactory dysfunction is independent of cognitive impairment at baseline, PD patients who exhibit decreased olfactory ability have a significantly increased risk of cognitive decline which is associated with a more prominent hypometabolism on FDG PET in posterior cortical regions since baseline [17, 20]. Accordingly, FDG PET also provides prognostic information in PD patients [20, 21]. Of note, the occurrence of parkinsonism following a viral infection is a well-known phenomenon, in which both causal and coincidental links are theoretically possible (i.e., unmasking of underlying, pre-symptomatic, neurologic disorder) [22]. Potential links between the SARS-Cov-2 virus and PD and the risk of a “wave” of new diagnosis of PD after the pandemic emergency have been advocated since the first months after the beginning of the pandemic emergence [23]. However, only few cases have been reported documenting the presence of parkinsonism during or after COVID-19 [24–27]. Whether COVID-19 could unmask or precipitate an underlying neurodegenerative condition or even trigger such a neurodegenerative cascade is still unknown. Therefore, it would be of interest to compare the functional imaging correlates of hyposmia after COVID-19 and in a neurodegenerative disease typically preceded by hyposmia, such as PD, to further disclose the potential pathophysiological link between COVID-19 and neurodegeneration and to further investigate the prognostic value of persistent hyposmia after COVID-19. In this framework, FDG PET may represent a suitable tool to capture the potential common metabolic signature of hyposmia after COVID-19 and in PD patients. This kind of group analysis might be more informative and epidemiologically relevant in patients who experienced mild or moderate COVID-19 without structural lesions. Given these premises, we aimed to evaluate the brain metabolic correlates of isolated persistent hyposmia after COVID-19 in a group of patients who experienced mild-to-moderate disease and to compare these findings with the same analysis in patients who showed a complete resolution of hyposmia after COVID-19 as well as in patients with de novo PD patients with hyposmia.
Materials and methods
Patients’ recruitment and selection
Post-COVID patients
Patients were recruited among subjects who underwent whole-body and dedicated brain [18F]-FDG PET for other clinical reasons more than 4 weeks after SARS-CoV-2 infection in our institution between May 2020 and May 2021. Preliminary inclusion criteria were previous SARS-CoV-2 infection, confirmed by polymerase chain reaction (PCR) at the time of initial symptoms, presence of hyposmia at the time of COVID-19 and PET examinations performed between 1 and 6 months after recovery from infection. The recovery phase was defined with symptom recovery and at least one negative swab test after infection. Severity of COVID-19 was established according to the international guidelines [28]. Briefly, mild disease has been defined as the presence of signs and symptoms of COVID-19 (e.g., fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste, and smell) in absence of shortness of breath, dyspnea, or abnormal chest imaging while moderate illness is associated to the evidence of lower respiratory tract disease during clinical assessment or imaging in presence of oxygen saturation (SpO2) ≥ 94% without an additional oxygen supply [28]. Patients that previously required mechanical ventilation or showed severe respiratory distress syndrome due to SARS-CoV-2 infection were thus excluded, given the potential independent effect of these clinical scenarios on brain metabolism. By contrast, lymphopenia and elevations of C-reactive protein, d-dimer, lactate dehydrogenase, and ferritin were not exclusion criteria [28]. Hospitalization was not considered an exclusion criterion as especially in the first wave of the pandemic emergence criteria for hospitalization were less clearly established.
Exclusion criteria were demonstration of brain lesions on CT/MRI, previous diagnosis of encephalopathy/encephalitis, or cerebrovascular disorders due to or concomitant with the SARS-CoV-2 infection, or any other previous, or current neurological or psychiatric symptoms or disease. Patients with a history of anosmia before SARS-CoV-2 infection, as well as those treated with chemotherapy or previous radiotherapy in the head and neck district for oncological reasons, were also excluded. Overall, sixty-five consecutive patients in the recovery phase after mild or moderate COVID-19 underwent brain [18F]-FDG PET in the selected period. Twenty-one patients did not meet inclusion criteria (mainly as previously they received chemotherapy and/or for previous or current neurological diseases); thus, forty-four patients were enrolled and underwent the olfactory test. In fact, while self-reported newly onset loss of smell is important from an infection control perspective, self-reporting may result in misdiagnosis. Patients’ characteristics are reported in Table 1. The study was approved by the regional ethical committee (CER Liguria code 671/2021); all procedures and informed consent collection were in accordance with the ethical standards of the 1964 Helsinki declaration.
Table 1.
Patients’ characteristics
| Characteristics | Post-COVID patients with hyposmia (n = 21) | Post-COVID patients without hyposmia (n = 23) | PD patients with hyposmia (either 8 or 16 items tests) (n = 82) | PD patients with hyposmia (16 items test) (n = 16) |
|---|---|---|---|---|
| Age (years) | 62.1 ± 11.6 (range 51–85) | 59.9 ± 12.9 (range 51–82) | 71.8 ± 7.4 (range 50–84) | 73.1 ± 7.2 (range 58–84) |
| Sex | ||||
| Male | 9/21 | 9/23 | 48/82 | 7/16 |
| Female | 12/21 | 14/23 | 34/82 | 9/16 |
| Time since diagnosis of SARS-CoV-2 infection (weeks) | 13.7 ± 7 (range 4–25) | 16.3 ± 9.1 (range 4–25) | n.a.* | n.a.* |
| MDS-UPDRS-III | n.a | n.a | 23.1 ± 7.2 | 27.0 ± 2.1 |
| MMSE | n.a | n.a | 28.1 ± 2.0 | 28.7 ± 3.3 |
| Olfactory test (number of correct answers) | ||||
| < 4/16 | 2 | 0 | -** | 8 |
| 5–7/16 | 8 | 0 | - | 3 |
| 8–11/16 | 11 | 0 | - | 5 |
| 12–14/16 | 0 | 20 | - | 0 |
| 15–16/16 | 0 | 2 | - | 0 |
Values are shown as mean ± standard deviation (range)
MDS-UPDRS-III, Movement Disorders Unified Parkinson’s Disease Rating Scale, motor section; MMSE, Mini-Mental State Examination; n.a., not available
*No previous SARS-CoV2 infection was previously documented in de novo PD group
**This wider subgroup of de novo PD patients with hyposmia was selected based on two different olfactory tests (either 16 or 8 items tests with hyposmia defined as the number of correct answers ≤ 11 or ≤ 2 respectively). For this reason, the number of correct answers is only summarized for patients who underwent the 16-item sniffing tests
De novo drug-naïve PD patients
Between January 2015 and May 2021, we examined 123 consecutive drug-naïve PD patients who underwent a full neuropsychological assessment and [123I]FP-CIT-SPECT imaging [29]. Inclusion criteria for the present study were availability of a brain [18F]-FDG PET within 3 months following clinical diagnosis (mainly due to their inclusion in previous prospective studies in our center) and presence of hyposmia as documented by means of olfaction test (see below). The exclusion criteria were the use of dopaminergic or serotonergic medication, the presence of medical comorbidities, and dementia or other neuropsychiatric diseases.
Olfactory test
In post-CO4D patients, olfaction was assessed by means of the 16-item “Sniffin’ Sticks” identification test [30, 31] on the day of PET examination. The “Sniffin’ Sticks” battery is used in daily clinical practice as well as in research. Briefly, odors were delivered using felt-tip pens (“Sniffin’ Sticks”) of approximately 14 cm in length and an inner diameter of 1.3 cm. These pens carry a tampon soaked with 4 ml of liquid odorant. For odor presentation, the cap is removed from the pen for approximately 10 s, and the pen’s tip was brought in front of the subject’s nose and carefully moved from left to right nostril and backward. Odor identification comprises common and familiar odorants. Subjects were presented with single pens and asked to identify and label the smell, using four alternative descriptors for each pen. Between-pen intervals were approximately 20 s. The total score was the sum of correctly identified pens; thus, subjects could score between 0 and 16 points. Based on the references reported in the “Sniffin’ Sticks” instructions, hyposmia was defined as a score ≤ 11 points (correct answers) [32]. The number of correct answers was recorded and patients were classified as patients with or without hyposmia. Based on the results, twenty-one post-COVID patients were still showing hyposmia (COVID-hyposmia) while twenty-three showed normal olfactory test (COVID-no-hyposmia) results. Characteristics of these 44 patients are detailed in Table 1.
The 16-item “Sniffin’ Sticks” test was not available for all de novo PD patients as in the past the 8-item smell diskettes olfactory test was used in the outpatient clinic in our institution [33]. The 8-item smell diskettes olfactory test is based on reusable diskettes as applicators of 8 different odorants. Using a questionnaire with illustrations, the test is designed as a triple forced multiple-choice test resulting in a score of 0 to 8 correct answers. For the 8-item diskettes olfactory test, hyposmia is defined as making at least 2 mistakes on the questionnaire. Accordingly, to identify de novo PD patients with hyposmia, we initially considered both patients who underwent the 16-item Sniffin olfactory test and those who underwent the 8-item smell diskettes olfactory test.
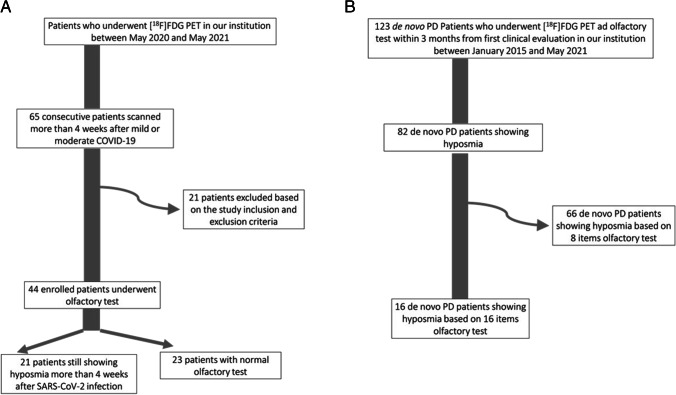
Among the one hundred and twenty-three included patients, eighty-two drug-naïve de novo PD patients showed hyposmia and were initially included in the group analyses (PD-hyposmia). Accordingly, the FDG PET images of these patients were used for the within groups comparisons in SPM (see below). However, for a proper comparison of results with the metabolic correlates of hyposmia in COVID-19, for multiple regression analysis between brain metabolism and olfaction we only selected the sixteen out of 82 PD patients having the 16-item Sniffin olfactory test available (see below). De novo PD patients’ characteristics are reported in Table 1. A schematic representation of the steps that narrowed post-COVID and de novo PD subgroups to the final number of patients is presented in Fig. 1.
Fig. 1.
A, B Schematic representation of the steps that narrowed post-COVID and de novo PD subgroups to the final number of patients
FDG brain PET acquisition and image processing
Brain FDG PET acquisition was performed in all patients’ groups according to the European Association of Nuclear Medicine (EANM) guidelines on Siemens Biograph 16 PET/CT system (EARL certified scanner)[34]. Images preprocessing was conducted using Statistical Parametric Mapping software version 12 (SPM12; Wellcome Trust Center for Neuroimaging, London, UK) [35]. Brain FDG PET images were spatially normalized using the Montreal Neurological Institute (MNI) atlas. All the default choices of SPM were followed with the exception of spatial normalization. For this study, the H215O SPM-default template was replaced by a brain FDG PET as detailed elsewhere [36]. The spatially normalized set of images was then smoothed with an 8-mm isotropic Gaussian filter to blur individual variations in gyral anatomy and to increase the signal-to-noise ratio. For all SPM analyses described below, a FWE-corrected height threshold < 0.05 both at peak and cluster levels was considered significant. Then a height threshold (peak-level significance) of p < 0.001, with a correction for multiple comparison at cluster level using the family-wise error (FWE) rate for a p-cluster < 0.05 was also considered to minimize type II errors and for a wider exploration of metabolic correlates of olfactory function in all patients’ subgroups. Only clusters with size k ≥ 100 voxels were considered.
Correlation between olfactory function and brain metabolism
After preprocessing, multiple regression analysis in SPM12 was used to identify the correlation between olfactory test scores and brain metabolism in post-COVID patients with and without persistent hyposmia as well as in the subgroup of PD patients with hyposmia who underwent the 16-item sniffing test. Age and gender were included as nuisance variables. To avoid influence of disease severity, in the correlation analysis involving de novo PD patients, the Movement Disorders Unified Parkinson’s Disease Rating Scale, motor section (MDS-UPDRS-III) score, and MMSE score were also included as nuisances.
Brain FDG PET comparisons
Metabolic correlates of hyposmia in post-COVID and de novo PD patients were also investigated by comparing the three groups (COVID-hyposmia, COVID-non-hyposmia, and PD-hyposmia) in the same ANOVA statistical model in SPM, with age and sex as nuisance variables. Then, to further characterize brain metabolism in COVID-hyposmia patients, a post hoc analysis (two-sample t-test) was performed to directly compare brain metabolism in these patients with respect to COVID-non-hyposmia and PD-hyposmia groups (both 1, − 1 and − 1, 1 contrasts were explored; again, age and sex were included as nuisance variables).
Results
Metabolic correlates of hyposmia after mild or moderate COVID-19 and in de novo PD
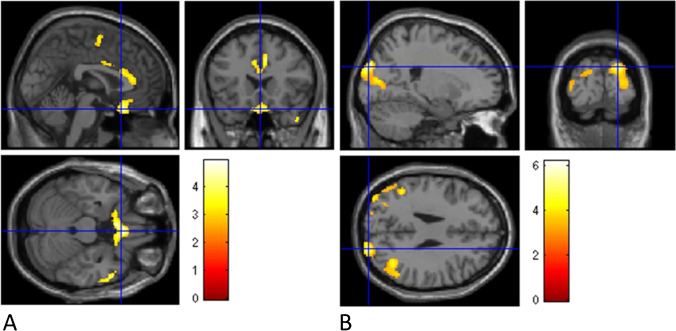
In COVID-hyposmia patients, olfactory test scores were directly correlated with metabolism in the rectus, medial frontal gyri of both hemispheres, and in right middle temporal and anterior cingulate gyri (p < 0.006, Fig. 2). By contrast, in PD-hyposmia patients, olfactory test scores were directly correlated with metabolic levels of cuneus/precuneus in both hemispheres and of the left lateral occipital cortex (p < 0.004, Fig. 2). No significant correlations were highlighted between brain metabolism and olfactory test score in post-COVID patients without hyposmia. (Details on coordinates and z-score are reported in Table 2.)
Fig. 2.
Correlation between Sniffin-test scores and whole-brain metabolism in COVID-hyposmia (A) and PD-hyposmia patients (B). A significant direct correlation was highlighted between olfaction and brain metabolism in rectus, middle temporal, and medial frontal gyri on both hemispheres and in the right precentral gyrus and anterior cingulate in COVID-hyposmia patients and in cuneus/precuneus on both hemispheres and in the lateral occipital cortex in the left hemisphere in PD-hyposmia patients. Clusters with significant hypometabolism are shown superimposed on an MRI template. The color bars indicate the level of z-scores for significant voxels. (See Table 2 for details on coordinates and z-scores.)
Table 2.
Metabolic correlates of hyposmia in post-COVID (A) and de novo PD patients (B)
| Cluster extent | Cluster level | Cortical region | Peak level | Talairach coordinates | Cortical region | BA | ||
|---|---|---|---|---|---|---|---|---|
| Corrected p value | Maximum z-score | |||||||
| A | ||||||||
| 420 | 0.006 | |||||||
| L-frontal | 4.7 | − 6 | 16 | − 24 | Rectal gyrus | 11 | ||
| R-frontal | 4.5 | 2 | 24 | − 18 | Medial frontal gyrus | 25 | ||
| R-limbic | 4.09 | 10 | 6 | 36 | Cingulate gyrus | 24 | ||
| R-limbic | 3.75 | 8 | 18 | 36 | Cingulate gyrus | 32 | ||
| R-temporal | 3.65 | 58 | 8 | − 20 | Middle temporal gyrus | 21 | ||
| B | ||||||||
| 1964 | 0.001 | |||||||
| R-occipital | 6.20 | 22 | − 92 | 28 | Cuneus | 19 | ||
| R-parietal | 5.30 | 42 | − 60 | 32 | Angular gyrus | 39 | ||
| L-parietal | 4.90 | − 42 | − 78 | 40 | Precuneus | 19 | ||
| 155 | 0.034 | L-occipital | 4.44 | − 40 | − 84 | 28 | Superior occipital gyrus | 19 |
| L-occipital | 4.27 | − 20 | − 84 | 16 | Middle occipital gyrus | 18 | ||
Uncorrected p < 0.001 at peak level and p < 0.05 FWE-corrected at cluster level were accepted as statistically significant. In the “cluster level” section on the left, the corrected p value and the brain lobe with hypometabolism are reported. In the “peak level” section on the right, the z-score and peak coordinates, the corresponding cortical region, and Brodmann area (BA) are reported
L, left; R, right
Groups’ comparisons
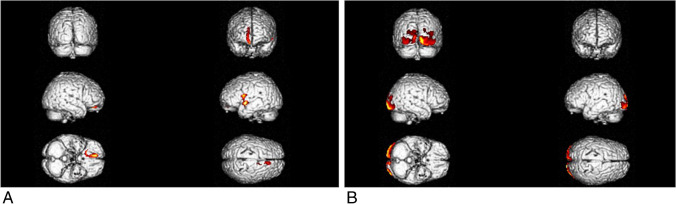
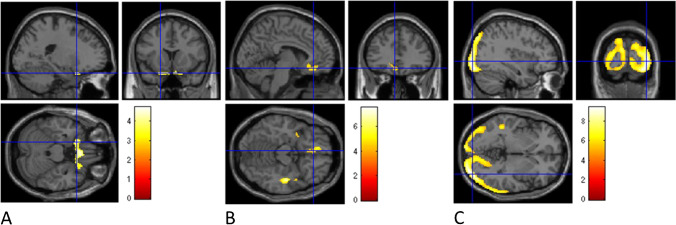
Metabolism in the three subgroups differed in the right rectus gyrus and in bilateral parahippocampal gyri and anterior cingulate and in the occipital lateral cortex on both hemispheres (p < 0.001 Fig. 3A, B). This pathophysiological difference was further investigated in a post hoc analysis aiming to directly compare COVID-hyposmia patients with the other patients’ groups. In this analysis, COVID-hyposmia patients exhibited significant hypometabolism with respect to COVID-non-hyposmia in a bilateral and symmetric cluster encompassing gyrus rectus and inferior, middle, and superior frontal gyri (p < 0.002, Fig. 4A) while (changing the direction of the contrast) no regions of significant hypometabolism were identified in COVID-non-hyposmia. (Details on coordinates and z-score are reported in Table 3.)
Fig. 3.
A, B Results of the ANOVA statistical model comparing the three groups of patients (COVID-hyposmia, COVID-non-hyposmia, and PD-hyposmia) with age and sex as nuisance variables. Clusters with significant hypometabolism are shown superimposed on a 3D brain rendering. Direct comparison between patients subgroups was explored in the post hoc analyses (Fig. 4A–C)
Fig. 4.
Comparisons between COVID-hyposmia on one side and COVID-non-hyposmia and PD-hyposmia respectively. COVID-hyposmia patients showed significant hypometabolism with respect to COVID-non-hyposmia in a bilateral and symmetric cluster encompassing gyrus rectus and inferior, middle, and superior frontal gyrus (A) while (changing the direction of the contrast) no regions of significant hypometabolism were identified in COVID-non-hyposmia. With respect to PD-hyposmia patients COVID-hyposmia patients showed hypometabolism in middle and superior temporal gyri, medial, and middle frontal gyri on both hemispheres and right insula (B). PD-hyposmia patients showed relative hypometabolism with respect to COVID-hyposmia in wide cortical regions mainly involving inferior and middle occipital gyri and cuneus in both hemispheres (C). Clusters with significant hypometabolism are shown superimposed on an MRI template. The color bars indicate the level of z-scores for significant voxels. (See Tables 3 and 4 for details on coordinates and z-scores.)
Table 3.
Comparisons between post-COVID patients with and without hyposmia
| Cluster extent | Cluster level | Cortical region | Peak level | Talairach coordinates | Cortical region | BA | ||
|---|---|---|---|---|---|---|---|---|
| Corrected p value | Maximum z-score | |||||||
| 874 | 0.002 | |||||||
| L-frontal | 4.7 | − 20 | 14 | − 24 | Inferior frontal gyrus | 47 | ||
| R-frontal | 4.5 | 4 | 22 | − 24 | Rectal gyrus | 11 | ||
| L-frontal | 4.5 | 0 | 22 | − 26 | Rectal gyrus | 11 | ||
| R-frontal | 4.1 | 40 | 16 | − 10 | Inferior frontal gyrus | 47 | ||
| R-frontal | 4.09 | 34 | 34 | − 14 | Middle frontal gyrus | 11 |
p < 0.05
FWE-corrected both at peak and at cluster level were accepted as statistically significant. In the “cluster level” section on the left, the corrected p value and the brain lobe with hypometabolism are reported. In the “peak level” section on the right, the z-score and peak coordinates, the corresponding cortical region, and Brodmann area (BA) are reported
L, left; R, right
Post-COVID patients with persistent hyposmia showed relative hypometabolism with respect to de novo PD patients in three small clusters located within middle and superior temporal gyri, medial, and middle frontal gyri on both hemispheres and right insula (p < 0.012, Fig. 4B). In turn, with respect to COVID-hyposmia, PD-hyposmia patients showed relative hypometabolism in wide cortical regions mainly involving inferior and middle occipital gyri and cuneus in both hemispheres (p < 0.002, Fig. 4C). (Details on coordinates and z-score are reported in Table 4.)
Table 4.
Comparisons between post-COVID and de novo PD patients with hyposmia
| Cluster extent | Cluster level | Cortical region | Peak level | Talairach coordinates | cortical region | BA | ||
|---|---|---|---|---|---|---|---|---|
| Corrected p value | Maximum z-score | |||||||
| A Relative hypometabolism in post-COVID patients | ||||||||
| 781 | 0.012 | |||||||
| R-frontal | 6.5 | 32 | 2 | 36 | Middle frontal gyrus | 6 | ||
| R-temporal | 5.73 | 44 | 6 | − 32 | Medial temporal gyrus | 21 | ||
| R-temporal | 5.68 | 44 | − 24 | − 8 | Superior temporal gyrus | 22 | ||
| R-frontal | 5.61 | 32 | 10 | 20 | Insula | 13 | ||
| L-temporal | 5.26 | − 36 | − 4 | − 32 | Middle temporal gyrus | 21 | ||
| L-temporal | 5.22 | − 34 | 4 | − 20 | Superior temporal gyrus | 38 | ||
| L-frontal | 5.11 | − 10 | 26 | − 8 | Middle frontal gyrus | 11 | ||
| B Relative hypometabolism in de novo PD patients | ||||||||
| 1964 | 0.002 | |||||||
| R-occipital | 8.4 | 38 | − 92 | − 2 | Inferior occipital gyrus | 18 | ||
| R-occipital | 8.33 | 8 | − 102 | 10 | Cuneus | 18 | ||
| L-occipital | 8.25 | − 10 | − 90 | 36 | Cuneus | 19 | ||
| L-occipital | 8.22 | − 34 | − 86 | 6 | Middles occipital gyrus | 19 | ||
p < 0.05
FWE-corrected both at peak and cluster level were accepted as statistically significant. In the “cluster level” section on the left, the corrected p value and the brain lobe with hypometabolism are reported. In the “peak level” section on the right, the z-score and peak coordinates, the corresponding cortical region, and Brodmann area (BA) are reported
L, left; R, right
Discussion
The present study highlights the capability of FDG PET to capture functional correlates of persistent hyposmia even in patients who suffered from mild or moderate COVID-19. Namely, isolated persistent hyposmia was directly correlated with metabolism in the bilateral rectus, middle temporal, medial frontal gyri, and in the right anterior cingulate cortex. Of note, this metabolic signature was absent in patients who experienced a complete resolution of hyposmia after recovery from infection. Moreover, post-COVID patients with hyposmia showed relative hypometabolism in the orbitofrontal cortex with respect to patients who fully recovered after infection and even with respect to de novo PD patients with hyposmia. Conversely, when compared with post-COVID patients with or without hyposmia, hyposmic de novo PD patients demonstrated hypometabolism in a large and bilateral cluster mainly involving medial and lateral occipital cortex. The total lack of any topographic overlap between metabolic correlates of hyposmia in PD and post-COVID patients was further testified by the hereby identified hyposmia-related metabolic signature of PD. In fact, in hyposmic de novo PD patients, we demonstrated an opposite pattern with respect to post-COVID patients with a direct correlation between olfactory test and metabolism in posterior regions, namely cuneus/precuneus in both hemispheres and in the left lateral occipital cortex.
Previous studies have highlighted the presence of cortical regions of relative hypometabolism in patients still presenting a wide range of neurological symptoms in the subacute stage of COVID-19 as well as in the so-called long COVID patients [7–15]. In particular, evidence was provided about neocortical (frontoparietal) dysfunction associated with cognitive decline in patients with subacute COVID-19 initially requiring inpatient treatment [15]. Of note, impairment sense of smell was documented with an olfactory test in 25/29 of those patients (although, for the study inclusion criteria, all patients were still presenting at least two neurological symptoms). Interestingly, a relative hypometabolism of the olfactory gyrus and connected limbic/paralimbic regions, with a further extension to the brainstem and the cerebellum, was also documented in a group of long COVID patients including 10 patients with hypo/anosmia [10]. Case reports providing FDG PET data in patients with isolated hyposmia after COVID-19 have been made available since the first months after the beginning of the pandemic and again these findings pointed to a role of the orbitofrontal cortex [7]. Niesen and colleagues reported that around 2 weeks after infection, sudden loss of smell in COVID patients is associated with subtle cerebral metabolic changes in core olfactory and high-order cortical areas [14]. We previously reported relative hypometabolism in a small group of patients with isolated hyposmia between 1 and 3 months after infection in bilateral parahippocampal and fusiform gyri and in left insula [9]. While confirming that long-lasting neurological symptoms of COVID-19 tend to be associated with cortical hypometabolism at FDG PET, the present findings extend the evidence of cortical hypometabolism to patients who recovered from mild-to-moderate disease and who experienced persistent hyposmia in absence of any other cognitive and neurological complaint.
Indeed, during the last year, the scientific community has paid great attention to the risk and predictors of neurological sequelae of COVID-19 [1, 37]. As hyposmia is also e feature a of prodromal PD and of other neurodegenerative diseases and given the fact that that parkinsonism has been reported in few patients following COVID-19, a hypothetical link between SARS-CoV-2 infection and PD has been previously proposed [23].
The occurrence of transient or permanent parkinsonism following a viral infection is a well-known phenomenon. At least in theory, after a viral infection, parkinsonism may occur for different reasons and underlying mechanisms [22]. In fact, in the course of viral infections with neurotropism such as SARS-Cov2, structural and functional damage may involve the substantia nigra and/or nigrostriatal dopaminergic projections. Indeed, SPECT and PET presynaptic dopaminergic imaging showed abnormal results in few published cases with the onset of parkinsonism during or just after severe COVID-19 infection [23, 24]. It should be noted, however, that impairment of presynaptic dopaminergic imaging implies either substantia nigra or nigrostriatal involvement, but it is not per se diagnostic of PD as this pathway can be damaged by other pathological processes, including vascular damage that can occur during COVID-19 [38, 39]. A second pathophysiological hypothesis to explain a diagnosis of PD after COVID-19 is related to the potential failure of reserve and compensations mechanisms during or just after infection thus unmasking a pre-symptomatic PD [40, 41]. Finally, as a more speculative scenario, the hypothetical possibility exists that a viral infection triggers a series of processes that result in the development of PD over the long term in susceptible individuals [26]. Indeed, in patients with sporadic PD, it has been demonstrated that intracellular aggregates of alpha-synuclein also involve the olfactory bulb and anterior olfactory nucleus [42]. Similarly, dysfunction or atrophy of olfactory bulb has been repeatedly reported in subjects with COVID-19 [43].
Despite these hypothetical mechanisms, the causal association of SARS-CoV-2 infection with the development of PD is presently not supported by robust evidence and it is well evident that our results do not support a common functional/cortical substrate for hyposmia in PD and COVID-19.
Data are already available on the pathophysiological differences between patients with PD and otherwise olfactory dysfunctional individuals supporting specific central origin of hyposmia in PD patients [44]. From the structural/microstructural point of view, previous resting-state and diffuse tensor imaging MRI studies have demonstrated that PD is associated with disruption of olfactory areas situated in the temporal lobes and in the orbitofrontal cortex [45]. However, PET imaging with different tracers provided insight into the underlying neurotransmission pathway. Bohnen and colleagues demonstrated by means of acetylcholinesterase (AChE) PET that cholinergic denervation is a more robust determinant of hyposmia than nigrostriatal dopaminergic denervation in PD [46]. The typical posterior PD hypometabolic pattern that we have here identified is in line with these findings as, in PD patients, hypometabolism in these regions has been previously linked to cholinergic dysfunction as well as to the risk of developing clinically significant cognitive impairment [29]. Accordingly, the present findings are in keeping with the hypothesis that olfactory judgement/interpretation and, not only olfactory perception, is altered in PD patients. Similarly, although a further investigation of these aspects is outside the aims of the present study, it is possible that both olfactory and cognitive dysfunctions are markers of a PD subtype characterized by a more “diffuse” degenerative process [47].
By contrast, in the present group of post-COVID patients with hyposmia, regions of relative hypometabolism were demonstrated to be confined to the core olfactory cortical area and in their major connections with prominent involvement of the orbitofrontal regions [48]. While the PET-only nature of the present study prevented the exploration of structural networks underlying hyposmia in post-COVID patients, the topography of relative hypometabolism seems to support a more perception-related hypometabolism.
Indeed, significantly reduced glucose metabolism in bilateral rectus gyrus, bilateral superior and medial orbitofrontal cortex, bilateral thalamus, left hippocampus and parahippocampus, and superior temporal pole was previously demonstrated in patients with post-traumatic anosmia (possibly simply due to sinonasal tract disruption or direct stretching of olfactory nerve fibers at the cribriform plate thus with a cortical deafferentation effect) [49]. Similarly, these regions were also identified in the few published studies that previously explored brain metabolism during olfactory stimulation by means of FDG PET [50]. The results were in keeping with the clusters hereby highlighted thus potentially strengthening the present results and conclusions. As a matter of fact, mechanisms previously advocated to explain the presence of anosmia in patients with COVID-19 also included peripheral damage and olfactory cleft syndrome [2]. Of note, FDG PET data have been used in the past also to investigate pathophysiology of other sense disturbances such as blindness and deafness and it has been demonstrated that, also in subacute stages, glucose hypometabolism is usually observed in related cortical central area mainly as a result of diaschisis (and not necessary to direct damage) [51, 52].
Notably, the present study also included a group of patients who were complaining of hyposmia at the time of infection and who experienced complete resolution of olfactory function during their recovery. No correlation between brain metabolism and the olfactory test was highlighted in this group of patients. This finding is in line with the capability of FDG PET/CT to capture and closely correlate with ongoing clinical symptoms. Interestingly, it was previously shown that long COVID patients show a common pattern of hypometabolism when grouped according to persistent functional complaints (including olfactory impairment) while not when grouped based on more prominent neurological symptoms at the time of infection [12]. Of note, the only available study providing follow-up FDG PET data in post-COVID patients demonstrated a significant recovery of regional neuronal function as well as of cognition at 6 months follow-up in a group of patients showing cognitive impairment after COVID-19 [13]. The lack of correlation between brain metabolism and olfactory test scores in patients who suffered from hyposmia during infection but who fully recovered might reflect lack of long-term disease-related substrates in these patients. This finding is in line with the notion that isolated hyposmia in absence of a severe disease course is not predictive of long-term functional sequelae [53].
The present study has some limitations, mainly related to its naturalistic observational nature and to the small groups of patients being submitted to FDG PET for other clinical reasons, including the suspect or follow-up of oncological diseases. Brain lesions were radiologically excluded in all patients, and to reduce the potential confounding effect of comorbidities, we also excluded patients submitted to chemotherapy and/or to radiotherapy in the head and neck district. Indeed, despite the small number of post-COVID patients, the present study represents the first FDG study including patients who suffered from mild or moderate COVID-19 both with and without isolated persistent hyposmia. In fact, at present, there is still a lack of studies directly comparing COVID patients with or without neurological sequelae. The demonstration of hyposmia by means of an olfactory test (that was not possible in larger epidemiologic studies) and the exclusion of patients who suffered from COVID-related pneumonia and who requested COVID-oriented treatment or mechanical ventilation are a further strength of the present study [54].
Conclusions
In conclusion, the present study suggests that persistent hyposmia after mild or moderate COVID-19 is associated with dysfunction in cortical regions directly involved in olfactory perception or with regions densely interconnected with the primary olfactory cortex. This metabolic signature is absent in patients who experienced a full resolution of the olfactory disturbances present at the time of infection. Finally, a metabolic functional substrate of hyposmia in PD and COVID-19 did not overlap. A more marked impairment in olfactory judgement might underlie hyposmia in PD patients while a more restricted perception deficit seems to explain hyposmia in COVID-19. The potential long-term neurological sequelae of COVID-19 are of interest from the clinical and even economical points of view. Accordingly, further pathophysiological studies targeting symptoms common to COVID-19 and chronic neurological diseases with the aim of exploring potential common pathways are of interest also to avoid unjustified claims about a future high incidence of neurodegenerative diseases secondary to the SARS-CoV-2 pandemic.
Acknowledgements
The authors are grateful to radiologic technologists, nurses, and administrative personal of the Nuclear Medicine Unit of the IRCCS Policlinico San Martino for their contribution to the acquisition of brain PET data of the patients included in this study.
Funding
This work was supported by a grant from the Italian Ministry of Health—Rete Italiana di Neuroscienze.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the regional ethical committee (CER Liguria code 671/2020).
Consent to participate
Informed consent was obtained from all individual participants included in the study. The results of all the performed exams have been provided to the patients.
Conflict of interest
Silvia Morbelli received speaking honoraria from GE Healthcare and AAA. Flavio Nobili received fees from BIAL for consultation, from GE Healthcare for teaching talks, and from Roche for board participation. Dario Arnaldi received fees from Fidia for lectures and board participation. Matteo Pardini receives research support from Novartis and Nutricia and fees from Novartis, Merck, and Biogen. All other authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Neurology
Silvia Chiola and Maria Isabella Donegani are contributed equally to this work
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boscolo-Rizzo P, Polesel J, Spinato G, Menegaldo A, Fabbris C, Calvanese L, Borsetto D, Hopkins C. Predominance of an altered sense of smell or taste among long-lasting symptoms in patients with mildly symptomatic COVID-19. Rhinology. 2020;58:524–525. doi: 10.4193/Rhin20.263. [DOI] [PubMed] [Google Scholar]
- 4.Estiri H, Strasser ZH, Brat GA, Semenov YR, Patel CJ, Murphy SN. Evolving phenotypes of non-hospitalized patients that indicate long COVID 2021. 10.1101/2021.04.25.21255923 [DOI] [PMC free article] [PubMed]
- 5.Almeria M, Cejudo JC, Sotoca J, Deus J, Krupinski J. Cognitive profile following COVID-19 infection: clinical predictors leading to neuropsychological impairment. Brain Behav Immun Health. 2020;9:100163. doi: 10.1016/j.bbih.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guedj E, Lazarini F, Morbelli S, Ceccaldi M, Hautefort C, Kas A, Radulesco T, Salmon-Ceron D, Eldin C. Long COVID and the brain network of Proust’s madeleine: targeting the olfactory pathway. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morbelli S, Ekmekcioglu O, Barthel H, Albert NL, Boellaard R, Cecchin D, Guedj E, Lammertsma AA, Law I, Penuelas I, Semah F, Traub-Weidinger T, van de Giessen E, Varrone A, Garibotto V, EANM Neuroimaging Committee COVID-19 and the brain: impact on nuclear medicine in neurology. Eur J Nucl Med Mol Imaging. 2020;47:2487–2492. doi: 10.1007/s00259-020-04965-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guedj E, Million M, Dudouet P, Tissot-Dupont H, Bregeon F, Cammilleri S, Raoult D. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Eur J Nucl Med Mol Imaging. 2021;48:592–595. doi: 10.1007/s00259-020-04973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niesen M, Trotta N, Noel A, Coolen T, Fayad G, Leurkin-Sterk G, Delpierre I, Henrard S, Sadeghi N, Goffard JC, Goldman S, De Tiège X. Structural and metabolic brain abnormalities in COVID-19 patients with sudden loss of smell. Eur J Nucl Med Mol Imaging. 2021;48:1890–1901. doi: 10.1007/s00259-020-05154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guedj E, Campion JY, Dudouet P, Kaphan E, Bregeon F, Tissot-Dupont H, Guis S, Barthelemy F, Habert P, Ceccaldi M, Million M, Raoult D, Cammilleri S, Eldin C. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48:2823–2833. doi: 10.1007/s00259-021-05215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yousefi-Koma A, Haseli S, Bakhshayeshkaram M, Raad N, Karimi-Galougahi M. Multimodality imaging with PET/CT and MRI reveals hypometabolism in tertiary olfactory cortex in parosmia of COVID-19. Acad Radiol. 2021;28:749–751. doi: 10.1016/j.acra.2021.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sollini M, Morbelli S, Ciccarelli M, Cecconi M, Aghemo A, Morelli P, Chiola S, Gelardi F, Chiti A. Long COVID hallmarks on [18F]FDG-PET/CT: a case-control study. Eur J Nucl Med Mol Imaging. 2021;7:1–11. doi: 10.1007/s00259-021-05294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blazhenets G, Schroeter N, Bormann T, Thurow J, Wagner D, Frings L, Weiller C, Meyer PT, Dressing A, Hosp JA. Slow but evident recovery from neocortical dysfunction and cognitive impairment in a series of chronic COVID-19 patients. J Nucl Med. 2021;62:910–915. doi: 10.2967/jnumed.121.262128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donegani MI, Miceli A, Pardini M, Bauckneht M, Chiola S, Pennone M, Marini C, Massa F, Raffa S, Ferrarazzo G, Arnaldi D, Sambuceti G, Nobili F, Morbelli S. Brain metabolic correlates of persistent olfactory dysfunction after SARS-Cov2 infection. Biomedicines. 2021;9:287. doi: 10.3390/biomedicines9030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosp JA, Dressing A, Blazhenets G, Bormann T, Rau A, Schwabenland M, Thurow J, Wagner D, Waller C, Niesen WD, Frings L, Urbach H, Prinz M, Weiller C, Schroeter N, Meyer PT. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain. 202;144:1263–1276. [DOI] [PMC free article] [PubMed]
- 16.Varghese J, Sandmann S, Ochs K, Schrempf IM, Frömmel C, Dugas M, Schmidt HH, Vollenberg R, Tepasse PR. Persistent symptoms and lab abnormalities in patients who recovered from COVID-19. Sci Rep. 2021;11:12775. doi: 10.1038/s41598-021-91270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Arch Neurol. 1998;55:84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- 18.Utsumi K, Fukatsu R, Yamada R, Takamaru Y, Hara Y, Yasumura S. Characteristics of initial symptoms and symptoms at diagnosis in probable dementia with Lewy body disease: incidence of symptoms and gender differences. Psychogeriatrics. 2020;20(5):737–745. doi: 10.1111/psyg.12586. [DOI] [PubMed] [Google Scholar]
- 19.Walker IM, Fullard ME, Morley JF, Duda JE. Olfaction as an early marker of Parkinson’s disease and Alzheimer’s disease. Handb Clin Neurol. 2021;182:317–329. doi: 10.1016/B978-0-12-819973-2.00030-7. [DOI] [PubMed] [Google Scholar]
- 20.Nobili F, Morbelli S, Arnaldi D, Ferrara M, Campus C, Brugnolo A, Mazzei D, Mehrdad N, Sambuceti G, Rodriguez G. Radionuclide brain imaging correlates of cognitive impairment in Parkinson’s disease (PD) J Neurol Sci. 2011;310:31–35. doi: 10.1016/j.jns.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 21.Meles SK, Renken RJ, Pagani M, Teune LK, Arnaldi D, Morbelli S, Nobili F, van Laar T, Obeso JA, Rodríguez-Oroz MC, Leenders KL. Abnormal pattern of brain glucose metabolism in Parkinson’s disease: replication in three European cohorts. Eur J Nucl Med Mol Imaging. 2020;47:437–450. doi: 10.1007/s00259-019-04570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rebholz H, Braun RJ, Ladage D, Knoll W, Kleber C, Hassel AW. Loss of olfactory function-early indicator for COVID-19, other viral infections and neurodegenerative disorders. Front Neurol. 2020;11:569333. doi: 10.3389/fneur.2020.569333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merello M, Bhatia KP, Obeso JA. SARS-CoV-2 and the risk of Parkinson’s disease: facts and fantasy. Lancet Neurol. 2021;20:94–95. doi: 10.1016/S1474-4422(20)30442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morassi M, Palmerini F, Nici S, Magni E, Savelli G, Guerra UP, Chieregato M, Morbelli S, Vogrig A. SARS-CoV-2-related encephalitis with prominent parkinsonism: clinical and FDG-PET correlates in two patients. J Neurol. 2021;21:1–8. doi: 10.1007/s00415-021-10560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makhoul K, Jankovic J. Parkinson’s disease after COVID-19. J Neurol Sci. 2021;422:117331. doi: 10.1016/j.jns.2021.117331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen ME, Eichel R, Steiner-Birmanns B, et al. A case of probable Parkinson’s disease after SARS-CoV-2 infection. Lancet Neurol. 2020;19:804–805. doi: 10.1016/S1474-4422(20)30305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faber I, Brandão PRP, Menegatti F, et al. Coronavirus disease 2019 and parkinsonism: a non-post-encephalitic case. Mov Disord. 2020;35:1721–1722. doi: 10.1002/mds.28277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandhi RT, Lynch JB, del Rio C. Mild or moderate COVID-19. NEJM. 2020;383(18):1757e66. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 29.Orso B, Arnaldi D, Girtler N, Brugnolo A, Doglione E, Mattioli P, Biassoni E, Fancellu R, Massa F, Bauckneht M, Chiola S, Morbelli S, Nobili F, Pardini M. Dopaminergic and serotonergic degeneration and cortical [18F]fluorodeoxyglucose positron emission tomography in de novo Parkinson’s disease. Mov Disord. 2021 doi: 10.1002/mds.28654. [DOI] [PubMed] [Google Scholar]
- 30.Kobal G, Hummel T, Sekinger B, et al. “Sniff Sticks”: screening of olfactory performance. Rhinology. 1996;34:222–226. [PubMed] [Google Scholar]
- 31.Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol. 2019;276:719–728. doi: 10.1007/s00405-018-5248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briner HR, Simmen D. Smell diskettes as screening test of olfaction. Rhinology. 1999;37:145–148. [PubMed] [Google Scholar]
- 33.Arnaldi D, Morbelli S, Brugnolo A, Girtler N, Picco A, Ferrara M, Accardo J, Buschiazzo A, de Carli F, Pagani M, Nobili F. Functional neuroimaging and clinical features of drug naive patients with de novo Parkinson’s disease and probable RBD. Parkinsonism Relat Disord. 2016;29:47–53. doi: 10.1016/j.parkreldis.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 34.Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Någren K, Darcourt J, Kapucu OL, Tatsch K, Bartenstein P, et al. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2103–2110. doi: 10.1007/s00259-009-1264-0. [DOI] [PubMed] [Google Scholar]
- 35.Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2:189–210. doi: 10.1002/hbm.460020402. [DOI] [Google Scholar]
- 36.Morbelli S, Piccardo A, Villavecchia G, Dessi B, Brugnolo A, Piccini A, Caroli A, Frisoni G, Rodriguez G, Nobili F. Mapping brain morphological and functional conversion patterns in amnestic MCI: a voxel-based MRI and FDG-PET study. Eur J Nucl Med Mol Imaging. 2010;37:36–45. doi: 10.1007/s00259-009-1218-6. [DOI] [PubMed] [Google Scholar]
- 37.Toniolo S, Scarioni M, Di Lorenzo F, Hort J, Georges J, Tomic S, Nobili F, Frederiksen KS, Management Group of the EAN Dementia and Cognitive Disorders Scientific Panel Dementia and COVID-19, a bidirectional liaison: risk factors, biomarkers, and optimal health care. J Alzheimers Dis. 2021 doi: 10.3233/JAD-210335. [DOI] [PubMed] [Google Scholar]
- 38.Tiraboschi P, Corso A, Guerra UP, Nobili F, Piccardo A, Calcagni ML, Volterrani D, Cecchin D, Tettamanti M, Antelmi L, Vidale S, Sacco L, Merello M, Stefanini S, Micheli A, Vai P, Capitanio S, Gabanelli SV, Riva R, Pinto P, Biffi AM, Muscio C, SCILLA Working Group (123) I-2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl) nortropane single photon emission computed tomography and (123) I-metaiodobenzylguanidine myocardial scintigraphy in differentiating dementia with Lewy bodies from other dementias: A comparative study. Ann Neurol. 2016;80:368–78. doi: 10.1002/ana.24717. [DOI] [PubMed] [Google Scholar]
- 39.Morbelli S, Esposito G, Arbizu J, Barthel H, Boellaard R, Bohnen NI, Brooks DJ, Darcourt J, Dickson JC, Douglas D, Drzezga A, Dubroff J, Ekmekcioglu O, Garibotto V, Herscovitch P, Kuo P, Lammertsma A, Pappata S, Peñuelas I, Seibyl J, Semah F, Tossici-Bolt L, Van de Giessen E, Van Laere K, Varrone A, Wanner M, Zubal G, Law I. EANM practice guideline/SNMMI procedure standard for dopaminergic imaging in Parkinsonian syndromes 1.0. Eur J Nucl Med Mol Imaging. 2020;47:1885–1912. doi: 10.1007/s00259-020-04817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helmich RC, Bloem BR. The impact of the COVID-19 pandemic on Parkinson’s disease: hidden sorrows and emerging opportunities. J Park Dis. 2020;10:351–354. doi: 10.3233/JPD-202038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hainque E, Grabli D. Rapid worsening in Parkinson’s disease may hide COVID-19 infection. Parkinsonism Relat Disord. 2020;S1353–8020:30117–30126. doi: 10.1016/j.parkreldis.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paschen L, Schmidt N, Wolff S, Cnyrim C, van Eimeren T, Zeuner KE, et al. The olfactory bulb volume in patients with idiopathic Parkinson’s disease. Eur J Neurol. 2015;22:1068–1073. doi: 10.1111/ene.12709. [DOI] [PubMed] [Google Scholar]
- 43.Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol. 2020;77:1028–1029. doi: 10.1001/jamaneurol.2020.2125. [DOI] [PubMed] [Google Scholar]
- 44.Crespo Cuevas AM, Ispierto L, Vilas D, Planas A, Planas A, Isern I, Sanchez J, De Haro J, Alvarez R. Distinctive olfactory pattern in Parkinson’s disease and non-neurodegenerative causes of hyposmia. Neurodegener Dis. 2018;18:143–149. doi: 10.1159/000488680. [DOI] [PubMed] [Google Scholar]
- 45.Haghshomar M, Dolatshahi M, Ghazi Sherbaf F, Sanjari Moghaddam H, Shirin Shandiz M, Aarabi MH. Disruption of inferior longitudinal fasciculus microstructure in Parkinson’s disease: a systematic review of diffusion tensor imaging studies. Front Neurol. 2018;9:598. doi: 10.3389/fneur.2018.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bohnen NI, Müller ML, Kotagal V, Koeppe RA, Kilbourn MA, Albin RL, Frey KA. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson’s disease. Brain. 2010;133:1747–1754. doi: 10.1093/brain/awq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Postuma R, Gagnon JF. Cognition and olfaction in Parkinson’s disease. Brain. 2010;133:e160. doi: 10.1093/brain/awq225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richard L. Doty, Olfaction in Richard L. Doty, Olfaction, Editor(s). Encyclopedia of the human brain, Academic Press, 2002; 717–727, ISBN 9780122272103,
- 49.Gao X, Wu D, Li X, Su B, Sun Z, Nie B, Zhang X, Wei Y. Altered glucose metabolism of the olfactory-related cortices in anosmia patients with traumatic brain injury. Eur Arch Otorhinolaryngol. 2021 doi: 10.1007/s00405-021-06754-0. [DOI] [PubMed] [Google Scholar]
- 50.Chiaravalloti A, Pagani M, Micarelli A, Di Pietro B, Genovesi G, Alessandrini M, Schillaci O. Cortical activity during olfactory stimulation in multiple chemical sensitivity: a (18)F-FDG PET/CT study. Eur J Nucl Med Mol Imaging. 2015;42:733–740. doi: 10.1007/s00259-014-2969-2. [DOI] [PubMed] [Google Scholar]
- 51.Chu WJ, Mason GF, Pan JW, Hetherington HP, Liu HG, San Pedro EC, Mountz JM. Regional cerebral blood flow and magnetic resonance spectroscopic imaging findings in diaschisis from stroke. Stroke. 2002;33:1243–1248. doi: 10.1161/01.STR.0000015240.75199.BE. [DOI] [PubMed] [Google Scholar]
- 52.Dietemann S, Noblet V, Imperiale A, Blondet C, Namer IJ. FDG PET findings of the brain in sudden blindness caused by bilateral central retinal artery occlusion revealing giant cell arteritis. Clin Nucl Med. 2015;40:45–46. doi: 10.1097/RLU.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 53.Salamanna F, Veronesi F, Martini L, Landini MP, Fini M. Post-COVID-19 syndrome: the persistent symptoms at the post-viral stage of the disease. A systematic review of the current data. Front Med (Lausanne). 2021;8:653516. doi: 10.3389/fmed.2021.653516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antczak J, Popp R, Hajak G, Zulley J, Marienhagen J, Geisler P. Positron emission tomography findings in obstructive sleep apnea patients with residual sleepiness treated with continuous positive airway pressure. J Physiol Pharmacol. 2007;5(Suppl. 5):25–35. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.