Abstract
Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is widely known as a tumor suppressor gene. It is located on chromosome 10q23 with 200 kb, and has dual activity of both protein and lipid phosphatase. In addition, as a targeted gene in multiple pathways, PTEN has a variety of physiological activities, such as those regulating the cell cycle, inducing cell apoptosis, and inhibiting cell invasion, etc. The PTEN gene have been identified in many kinds of cancers due to its mutations, deletions and inactivation, such as lung cancer, liver cancer, and breast cancer, and they are closely connected with the genesis and progression of cancers. To a large extent, the tumor suppressive function of PTEN is realized through its inhibition of the PI3K/AKT signaling pathway which controls cells apoptosis and development. In addition, PTEN loss has been associated with the prognosis of many cancers, such as lung cancer, liver cancer, and breast cancer. PTEN gene is related to many cancers and their pathological development. On the basis of a large number of related studies, this study describes in detail the structure, regulation, function and classical signal pathways of PTEN, as well as the relationship between various tumors related to PTEN. In addition, some drug studies targeting PTEN/PI3K/AKT/mTOR are also introduced in order to provide some directions for experimental research and clinical treatment of tumors.
Keywords: PTEN, structure, function, pathway, cancer
Introduction
Phosphatase and tensin homolog deleted on chromosome ten (PTEN) are essential for normal cells and are widely concerned and studied tumor suppressor genes [1-3]. PTEN was first discovered in 1997, when the mutation at the 10q23 site on chromosome 10 was studied [4,5]. In early reports, PTEN was considered as a protein located only in the cytoplasm. Nevertheless, it is now clear that it can exist in the nucleus or cytoplasm [6-9]. In the cytoplasm, PTEN interacts with its cytoplasmic targets to regulate cell growth, proliferation, apoptosis, adhesion, migration and invasion. In the nucleus, PTEN can maintain chromosome stability and DNA double strand break repair, so protecting the completeness of the genome [6,7,10]. Because PTEN is very important to many cellular processes, the expression of PTEN is strictly regulated by many cellular mechanisms, which exert effect on the transcriptional, post-transcriptional and post-translational levels [11-13]. Since then, many studies have confirmed that the decrease of PTEN level or activity induces the accumulation of PIP3, and is related to the activation of proto-oncogene AKT, so establishing an important link between PTEN and phosphatidylinositol 3-kinase (PI3K) pathway [14-16]. PI3K/AKT pathway is extremely significant for the growth, proliferation and survival of tumor cells. Many researches have shown that PTEN can regulate PI3K/AKT signal pathway through the dephosphorylation of D3 phosphatidylinositol 3, 4, 5-trisphosphate (PIP3) [17,18].
It is reported that PTEN inhibits tumorigenesis through different mechanisms. In recent years, it has been found that the mutation, deletion and expression of PTEN gene are closely associated with the development of cancers [19-22]. According to reports, PTEN absence leads to phosphorylation mediated by AKT and the activation of nuclear factor kappa-B (NF-κB) activity, promoting P53 degradation. P53 degradation reduces the apoptotic ability of cells and induces cell cycle progression [23-25]. Inactivation of PTEN also results in MAPK stimulation and activation of mammalian target of rapamycin (mTOR) kinase complex 1 (mTORC1). Apart from the inherent tumor inhibitory function of PTEN, PTEN can also affect the occurrence and development of tumor cells by regulating some information molecules such as focal adhesion kinase (FAK) [26-28], mitogen activated protein kinase (MAPK) [29,30], hypoxia inducible factor-1 (HIF-1) [31] and vascular endothelial growth factor (VEGF) [32]. In addition, some studies have also emphasized the key function of PTEN in tumor microenvironment, which plays important role in tumor cells, stroma and immune response at different levels, so as to control the occurrence, development and metastasis of the disease [33-35].
As PTEN gene is involved in many kinds of cancers and the pathological process of cancers. In this study, we describe the details of PTEN structure and function and the association between various cancers associated with PTEN on the basis of a myriad of pertinent studies. The aim is to provide a clearer understanding of future revelations.
Information related to PTEN
The structure of PTEN
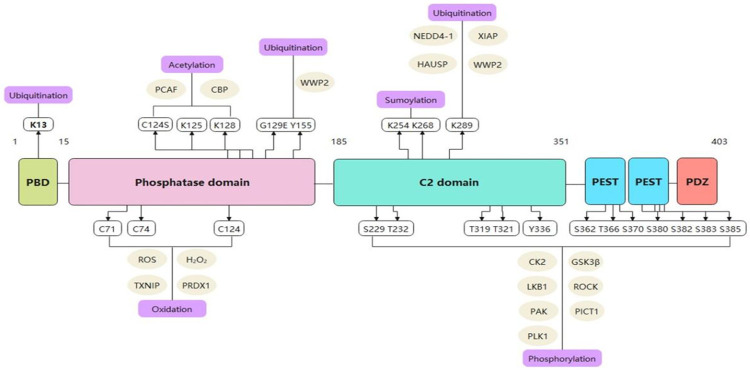
PTEN is located on chromosome 10q23.3 with a 200 kb and consists of 9 exons and 8 introns, encoding a protein 403 amino acids long with a relative molecular mass of approximately 47 kDa [36]. The amino acid (N) terminus of the protein structure can remove phosphorylate groups from phosphotyrosine, phosphoserine and phosphothreonine on highly acidic substrates, but its catalytic activity is weak [36,37] (Figure 1). In addition, the PTEN structure contains a phosphatase domain, like to protein phosphatases. However, it has an expanded active site that is indispensable to regulating phosphoinositol substrates [38,39]. PTEN also has a C2 domain that is connected to the phospholipid membrane in vitro. Furthermore, the phosphatase and C2 domains participate in a broad interface, which shows that the C2 domain may be has important impact on in positioning the catalytic domain on the membrane [40].
Figure 1.
The structure of PTEN gene and occurrence of mutations in exons. Abbreviation: PBD: a p-hosphatidylinositol-4,5-bisphosphate (Ptdlns (4,5) P2)-bind-ing domain).
On the other hand, PTEN contains a characteristic motif of protein tyrosine phosphatases and bispecific protein phosphatases, which indicates that it is both a protein and lipid phosphatase [5]. The crystal structure of PTEN indicates a separation of the two significant domains, an N-terminal phosphatase domain (residues 7-185) and a C-terminal C2 domain (residues 186-351) [6,36]. Each of these two domains contains five central β-sheets with one α-helix on one side and four α-helices on another side, similar to the domains conferring specificity to other phosphatases [3,36]. A short N-terminal (PIP2)-binding domain carries the conserved phosphatase motif HCSSGSSR, which is similar to the catalytic domain of tyrosine phosphatases and serine/threonine phosphatases, with the function to dephosphorylate tyrosine and serine/threonine residues and facilitate PTEN to resist to cancer development [38]. An N-terminal phosphatase domain promotes phospholipid hydrolysis. A C2 domain constituted by two antiparallel β-sheets with two small α-helix strands exerts significant impact on mediating the binding of signalrelated proteins to cell membranes [12,19]. Furthermore, the phosphatase terminus plays indispensable roles in the interaction between a ligand and phosphate head. Nevertheless, three loops, a P loop (H123CKAGKGR130), a WPD loop (residues 88-98), and a TI loop (residues 160-171) include residues participating in catalysis which can control the PIP2 and C2 domains and their interactions [4,36,41]. In addition, the C-terminal tail contains a domain with a PEST sequence comprising proline, glutamic acid, serine, and threonine and various phosphorylation sites and a PDZ interaction motif that can bind to lipids [36,42]. Two natural mutations in the phosphatase domain disrupt the tumor-inhibiting ability of PTEN. The C124S mutation causes dysfunction of PTEN protein and lipid phosphatase activities, while the G129E mutation disrupts only the PTEN lipid phosphatase activity [43-45]. Although the N-terminal phosphatase domain mainly participates in PTEN the physiological activity, most tumor-related PTEN mutations are associated with the C2 domain and C-terminus, indicating that the C-terminal sequence is very important to protect the function of PTEN [46] (Figure 2). Papa et al. proved that PTEN homodimerization enhances its lipid phosphatase function stably through the C-terminal tail [47].
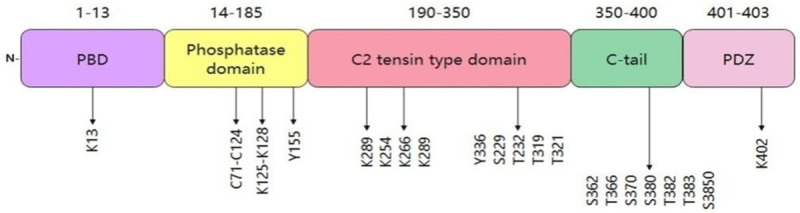
Figure 2.
Posttranslational regulation of PTEN at specific sites. Abbreviation: PBD: a phosphatidyli-nositol-4,5-bisphosphate (Ptdlns (4,5) P2)-bind-ing domain).
The regulation of PTEN
The expression control of PTEN
The expression and function of PTEN are strictly regulated at transcription, post-transcription and post-translation levels [3,11]. Apart from genetic deletions or somatic mutations in human cancers, these regulatory molecules, which control the expression and function of PTEN, can lead to changes in the level of PTEN, thereby promoting the occurrence and development of tumors in different ways [19,22,47]. Epigenetic and transcriptional silencing, as well as disorders in microRNAs (miRNAs) and competitive endogenous RNA (Cerna) systems have been shown to inhibit PTEN expression [15,16]. Recently, the processing pseudogenes (PTENP1) of PTEN plus pseudogenes are very interested in the regulation of PTEN, and the increased regulation is attracting great interest because it increases the complexity of regulating PTEN expression.
The transcriptional regulation of PTEN
Many molecules directly interact with PTEN promoter and promote or inhibit PTEN transcription. These molecules include early growth response transcription factor 1 (EGR1), peroxisome proliferation activated receptor gamma (PPARγ), activating transcription factor 2 (ATF2) and tumor suppressor p53 [48,49]. It is reported that P53 upregulates PTEN transcription through functional p53 binding element upstream of PTEN promoter [50,51]. The transcription of PTEN is suppressed through zinc finger proteins Snail and Slug [52]. Other transcription factors such as Cbf-1 (C-kinetin promoter binding factor-1) and c-Jun, antisense transcripts of NF-ΚB and PTEN pseudogenes (PTENP1) also interact with PTEN promoter and reduce the transcription of PTEN [53-56]. In addition, it has been reported that several kinds of miRNA, including miR-205, miR-122, and miR-21, bind to the 3’ untranslated region of PTEN mRNA, leading to the decrease of PTEN mRNA [11,15,57-60].
Post-translational regulation of PTEN
Many post-translational mechanisms can exert effect on the activity and stability of PTEN, including phosphorylation, oxidation, acetylation, ubiquitin, and SUMOylation [1,61,62]. The catalytic activity of PTEN can be regulated by phosphorylation at specific points on the C2 and C-Tail domains. Under the help of casein kinase 2 and glycogen synthase kinase 3 (GSK3), the specific serine and threonine residues (Ser380, Thr382, Thr383 and Ser385) at the end of PTEN C are phosphorylated, resulting in a decrease in phosphatase activity [63].
The oxidation of PTEN by hydrogen peroxide promoted the formation of disulfide bonds between Cys124 and Cys71 residues, leading to conformational changes, and then changed the binding sites of PTEN substrates, which bring about the loss of PTEN phosphatase activity [64,65].
Under the stimulation of growth factors, PCAF acetylates PTEN on lysine residues 125, 128 located in the catalytic site of PTEN, leading to inactivating PTEN and activating PI3K signal pathway. It is reported that CREB can promote the acetylation of PTEN through the Lys402 in the PTENPDZ binding region [66-68].
Ubiquitin affects the degradation of PTEN. Neural progenitor cells expressing NEDD4-1 can promote the ubiquitination of PTEN [69,70]. The polyubiquitination of PTEN leads to the decrease of protein stability and the degradation of PTEN through proteasome-mediated decay, while the ubiquitination of PTEN on Lys13 and Lys289 promotes PTEN transport in the nucleus [69,71].
The connection of ubiquitin-associated modifiers to the protein is also one of the post-translational regulatory mechanisms of PTEN [72]. The SUMOylation of PTEN on Lys266 contributes to PTEN aggregation on the plasma membrane, while the SUMO of PTEN on Lys254 participates in promoting the nuclear localization of PTEN [73,74].
Typical signal pathways related to PTEN
Under physiological conditions, G protein-coupled receptors (GPCRs) or receptor tyrosine kinase (RTK) (including IGFR, PDGFR, EGFR and c-Met) are stimulated through many molecules, including growth factors, hormones and extracellular matrix (ECM) components to activate PI3K, and then activated PI3K catalyzes the phosphorylation of phosphatidylinositol (4, 5)-diphosphate (PIP2) to PIP3 [75-77]. Subsequently, the production of PIP3 causes proteins containing the PH domain to be attracted to the cell membrane, including AKT and PDK1 [78,79]. On the cell membrane, PDK1 phosphorylates and activates AKT on Thr308, which in turn activates multiple effect targets, including GSK3, forkhead box O (FoxO) protein and mTORC1 target, thereby regulating various cell processes including apoptosis, proliferation, and metabolism [80-83]. Interestingly, the largest AKT activation needs the other one phosphorylation event on Ser473, which is catalyzed by MTORC2, and mTORC2 also be regulated through PIP3 [84-86]. However, among a variety of substrates, PTEN mainly targets and dephosphorylates PIP3, thus becoming the main passive regulator of PI3K/AKT signal through decreasing the level of PIP3 and restraining the recruitment of subsequent information molecules and AKT activation [14,87].
The functions of PTEN
The mechanisms by which PTEN controls cellular proliferation, migration, apoptosis, adhesion, and genetic stability impact various cell signal transmission pathways and molecules, forming a complex system.
Regulation of the cell cycle and induction of apoptosis
The PTEN gene exerts important impact on cell migration and cell apoptosis, which suppresses tumorigenicity and cell growth [88,89]. PTEN expression inhibits SCC-4 cell apoptosis by inducing the PI3K/AKT signaling pathway and increasing the level of the Bcl-2-interacting mediator of cell death [90]. Through its lipid phosphatase activity, PTEN dephosphorylates the 3-phosphoinositide products of PI3K. Moreover, many vital survival kinases, such as PDK1 and AKT, and other proteins that are not kinases can be activated by 3-phosphoinositides. Therefore, PTEN negatively regulates the AKT pathway, and the role of AKT in apoptosis prevention has been well documented [91,92]. Furthermore, by hydrolyzing PIP3, PTEN antagonizes the activity of PI3K to generate PIP2, which inhibits the activation of downstream signaling molecules and ultimately inhibits cell proliferation, growth and survival [33,93,94]. In summary, PTEN can regulate the cell cycle and induce cell death through various signaling pathways.
Inhibition of cell invasion
It has been widely shown that the protein phosphatase activity of PTEN has important effect on its ability to inhibit cell invasion [91,92,95,96]. The epithelial-mesenchymal transition (EMT) is considered to be one of the key factors of cell invasion and metastasis. Downregulation of PTEN can activate the PI3K/AKT pathway, thus promoting the invasion ability of cancer cells and facilitating the EMT [96]. Upregulation of PTEN can inhibit the EMT and tumor cell invasion. This effect may be realized by the downregulation of the Hedgehog (Hh) signaling pathway. Ecadherin and β-catenin can enhance cell-cell adhesion, and their decreased expression is connected to cancer cells invasion and metastasis [97]. The overexpression of PTEN is positively related to the expression of B-catenin cells and negatively correlated with the expression of cadherin and vimentin, indicating that B-catenin is related to the EMT and may be involved in the assembly of adhesion connections during the EMT [98]. Therefore, PTEN can suppress the EMT by downregulating the Hh signaling pathway, thus inhibiting cell invasion.
Regulation of tumor drug resistance
PTEN suppresses tumors by inhibiting tumor proliferation induced by P13K/AKT pathway activation [99]. Moreover, evidence has shown that PI3K inhibitors can enhance the sensitivity of NSCLC cells with high levels of phosphorylated AKT to medically induced cellular apoptosis [100]. Recent evidence suggests that activated AKT/PKB causes cell resistance to drug-induced apoptosis by phosphorylating downstream targets [101]. Furthermore, PTEN also regulates both the antitumor effect of the anaphase-promoting complex (APC) and its regulatory factor Ecadherin in the nucleus independent of its lipid phosphatase activity [91]. Through the aforementioned mechanisms, PTEN can regulate tumor drug resistance.
Others functions
Many other vital functions of PTEN have been verified. For example, lipid phosphatase activity of PTEN on cell membranes has been established, but PTEN also exhibits nuclear functions. Centrosome stabilization requires PTEN binding to centromeric protein C1 (CENP-C1), while DNA repair protein RAD51-mediated DNA double-strand break (DSB) repair requires PTEN nuclear localization [91,102]. Additionally, PTEN can regulate cellular migration, adhesion, and stretching through regulating FAK activity by dephosphorylation and can modulate membrane channels [93]. In addition, studies have demonstrated that PTEN deficiency can also increase cell activity. In summary, PTEN has a nuclear function, controls cell migration, adhesion and stretching, and regulates cell activity.
The roles of PTEN in some cancers
As mentioned above, PTEN is a vital gene in cell growth, development, mobility, apoptosis, signal transduction and other cellular processes, processes that contribute greatly to its tumor suppressor function. The detailed mechanism and effect of PTEN in cancers are presented in Table 1. PTEN is able to control cell apoptosis and survival by restraining the PI3K/AKT pathway because of its lipid phosphatase activity [103,104]. PTEN is a phosphatase for phosphoinositol lipids, which are regulated to be critically involved in cellular adhesion and tumor metastasis [105]. PTEN has been demonstrated that dephosphorylize the FAK regulating cell migration. The function of PTEN disorders and its FAK substrate are significantly associated with multiple cancers [33,106,107]. Furthermore, as a dual protein and lipid phosphatase, PTEN interrupts downstream AKT activation by dephosphorylating the secondary messenger produced by PI3K, thus affecting tumorigenesis [108,109].
Table 1.
The mechanisms and effects of PTEN in different cancers
| Disease | Mechanism | Effect | References |
|---|---|---|---|
| Lung Cancer | PI3K/AKT signaling pathway↓ | regulating the proliferation and apoptosis of tumor cells, leading to cell malignant transformation, tumor cell migration and adhesion, angiogenesis and extracellular matrix degradation | [100,114] |
| HDAC inhibitors ↓ | regulating a variety of genes and pathways in tumor cells and enhancing the anti-tumor effects of other anti-tumor drugs and radiotherapy | [116-119] | |
| SHCBP1↓ | a key role in the apoptosis of lung cancer cells | [115] | |
| Ovarian Cancer | miRNA-200a↓ | Inhibiting proliferation and invasion of ovarian cancer cells | [123] |
| miRNA-205↑ | Promoting proliferation and invasion of OC cells and inhibiting angiogenesis | [122] | |
| miRNA-552↓ | Inhibiting the proliferation and metastasis of OC | [59] | |
| PI3K pathway↓ | enhanced apoptosis and radiation sensitivity | [125] | |
| Epithelial Ovarian Cancer | miR-21↑ | EOC tumor development and poor prognosis | [124] |
| Liver cancer | PRL-3↓→AKT pathway↓ | Inhibiting the aggressive progression of HCC | [134,135] |
| miR-21↑ | Triggering cell death in liver cancer cells | [136,137] | |
| Colon Cancer | PI3K/AKT/NF-κB pathway↓ | inhibiting colon cancer progression | [106] |
| AR↓ | inhibiting the proliferation of colon cancer cells | [108] | |
| microRNA-26b↓ | Inhibiting the invasiveness, migration and stem cell-like phenotype of colorectal cancer | [139] | |
| Breast Cancer | AKT, NF-κB↑→P53 degradation↑ | reducing the apoptotic ability of cells and induces cell cycle progression | [141] |
| MiR-142-5p↑→PTEN↓ | Inhibiting the invasion of breast cancer cells | [148] | |
| Gastric Cancer | miR-718↓ | Inhibiting the proliferation and invasion of gastric cancer cells | [153] |
| PI3K signaling pathway↑ | inducing drug resistance by inducing the expression of multi-drug resistance protein-1 | [154-156] | |
| phosphorylation and activation of SRC kinase↑ | resistance to chemotherapy drugs such as trastuzumab | [160] | |
| Prostate Cancer | PLce, miR-20b→PTEN↓ | inhibiting tumor cell proliferation | [164-168] |
| Pancreatic Cancer | JARID1B↓ | Inhibiting cancer cell proliferation and tumor growth | [169] |
| PI3K/AKT signaling pathway | Inhibiting tumor cell proliferation | [172-174] | |
| Esophageal Cancer | the phosphorylation of AKT↓ | Affecting the development of cancer cells | [175] |
| JARID1B↓ | Inhibiting the proliferation of esophageal carcinoma cells and tumor growth | [169,176] | |
| miR-93-5p↓ | Inhibiting the proliferation of receptor cancer cells | [177] | |
| Endometrial Carcinoma | miR-205→PTEN/AKT↓ | Inhibiting tumor cell apoptosis | [178] |
Abbreviations: HDAC: histone deacetylase inhibitors; SHCBP1: SH2-binding protein 1; OC: Ovarian cancer; EOC: epithelial ovarian cancer; HCC: hepatocellular carcinoma; PRL-3: regenerating liver-3; AR: aldose reductase; PLCe: Phospholipase Ce; JARID1B: Jumonji AT-rich interactive domain 1B; PI3K: phosphatidylinositol 3-kinase.
In addition, PTEN gene mutations have been widely demonstrated is related to cancers; specifically, loss of post-translational expression results in abnormal cells proliferation, apoptosis, movement, and adhesion. The details of these effects are listed in Table 2. Different parts of PTEN are associated with the development of cancers. For example, the P-loop (residues 123-130) contains four mutated residues, His123, Lys125, Gly127, and Lys128, which are important for identifying changes in the loop [110] and reducing protein activity by approximately 50-60%. The TI loop has four conserved residues: Val166, Thr167, Ile168 and Gln171. These amino acids are related to the C2 domain and the phosphatase domain. Thr167 and Gln171 are frequently mutated residues in the TI loop, and these mutations lead to 60-75% dysfunction. Mutation to the His93 residue in the WPD loop has the same effect on protein activity, reducing PTEN function by approximately 75%. In addition, frequent mutations at the D5 site may lead to the occurrence of cancer [36]. Chromosome 10q heterozygosity was reported in cases of endometrial cancer [111,112]. It has also been indicated that PTEN is much more likely to be mutated than other genes, including Kras and p53 [113]. Different mutant amino acid residues are mutated in each loop, with each being critical for reducing protein activity.
Table 2.
The effect of genetic mutations on cancers
| Disease | Part | Effect | References |
|---|---|---|---|
| Cancer | Entire loss | Abnormal cell proliferation, apoptosis, migration, and adhesion | [6,36] |
| P-loop | identifying changes in the loop and reduce protein activity | [110] | |
| Ti ring | functional loss and occurrence of cancer | [36] | |
| Lung cancer | Entire loss | poor prognosis and resistance to EGFR and TKIs | [116] |
| Ovarian cancer | Entire loss | inducing tubal cancer and subsequently to involve the ovaries; producing serous borderline tumors of FTE and endometriosis carcinoma | [125] |
| Liver Cancer | Entire loss | high malignant potential/poor prognosis | [134] |
| poor cell differentiation | [133] | ||
| Breast Cancer | Entire loss | overgrowth, proliferation, survival, and metabolism of tumor cells | [3,141-147] |
| Gastric Cancer | Entire loss | tumor resistance | [150-152] |
| Prostate Cancer | Entire loss | changes in a variety of genes and pathways that affect the progression of cancer | [167,168] |
| Esophageal Cancer | Gene mutation | Stability decline, leading to the development of endometrial cancer | [113] |
Abbreviations: EGFR: epidermal growth factor receptor; TKIs: tyrosine kinase inhibitors; FTE: fallopian tube epithelium; PI3K: phosphatidylinositol 3-kinase.
PTEN mutations, or partial deletions, are common in all types of tumors. Abnormalities in cell proliferation, adhesion, migration, and apoptosis resulting from loss of PTEN posttranslational regulation are usually associated with cancer occurrence, development, and metastasis. Therefore, PTEN and its functionally related proteins are promising new anticancer drugs, and the potential of PTEN use in gene therapy and other therapeutics should be fully explored.
PTEN and lung cancer
As a tumor suppressor, PTEN inhibitory effects are largely realized through its lipid phosphatase activity that inhibits PI3K/AKT activation, as the PI3K/AKT signaling pathway regulates proliferation and migration of tumor cells. However, PTEN is often mutated in cancer, and this change to PTEN leads to malignant transformation, tumor cells migration and adhesion, extracellular matrix degradation and angiogenesis [100,114]. Furthermore, PTEN expression is regulated by SH2-binding protein 1 (SHCBP1) downregulation, as silencing SHCBP1 can lead to significant increase in PTEN expression. This suggests that SHCBP1 may be upregulated in lung cancer and may have an important role in the apoptosis of tumor cells; this role is related to the expression of PTEN [115]. In addition, histone deacetylase (HDAC) inhibitors can regulate a variety of genes and pathways in tumor cells and enhance the antitumor effects of other antitumor drugs and radiotherapy; therefore, HDAC inhibitors have shown strong anticancer effects [116]. More importantly, the target of HDACs is PTEN, and HDAC inhibition upregulates the expression of PTEN [117-119]. The expression of PTEN can affect the expression of HDACs and SHCBPI, and then affect lung cancer cells, suggesting that PTEN may be an effective therapeutic target for lung cancer. In lung cancer, PTEN deficiency is related to poor prognosis and resistance to EGFR and other tyrosine kinase inhibitors (TKIs), such as erlotinib. However, suberoylanilide hydroxamic acid (SAHA) can upregulate PTEN expression and increase tumor cell apoptosis [116], thus alleviating erlotinib resistance. However, the specific molecular mechanism remains to be determined.
PTEN and ovarian cancer (OC)
It is reported the absence of PTEN is linked to ovarian cancer. The loss of PTEN in tubal epithelial cells is sufficient to induce tubal cancer and subsequently involves the ovaries. Furthermore, homozygous PTEN deletion produces borderline serous tumors of the fallopian tube epithelium (FTE) and endometriosis-associated carcinoma, which are similar to human precursor lesions [120].
PTEN is regulated by various microRNAs, which play vital roles in OC. Studies have suggested that PTEN serves as a target gene for miRNA-200a, miR-205, and miR-552 [120-122]. MIR-205 participates in the positive feedback of cell proliferation and invasion, and contributes to cell proliferation and invasion through inhibiting PTEN expression [121]. Furthermore, exosomal miR-205 can inhibit angiogenesis through silencing PTEN, thereby activating the downstream AKT pathway, indicating a new mechanism by which exosomal miR-205 is related to OC metastasis [122]. miR-552 can also directly activate PTEN expression by interacting with the 3’-UTR of its mRNA, promoting the proliferation and metastasis of OC. More importantly, PTEN siRNA disrupted the apparent ability of miR-552 to induce the growth and metastasize between OC cells, compared to its effect on control cells. Moreover, miR-552 may be a good prognostic biomarker for patients with OC [59]. The miR-200a can directly bind to PTEN and negatively regulate the mRNA expression of PTEN in SKOV3 or OVCAR3 cells. By inhibiting PTEN expression, miRNA-200a contributes to the proliferation and invasion of OC cells [123]. In human epithelial ovarian cancer (EOC), there may be an intercommunication between miR-21 and PTEN [124]. On the one hand, miR-21 was overexpressed in clinical EOC tumors and EOC cell lines. On the other hand, PTEN gene expression was significantly decreased. These findings indicate that the overexpression of miR-21 and the downregulation of PTEN can regulate EOC cells. Furthermore, downregulation of PTEN may contribute to miR-21 expression [124].
In terms of drug resistance and radiotherapy, studies have found that PTEN, which inhibits the function of PI3K at the molecular level, is upregulated by paeonol and inhibits the activation of the PI3K pathway [125]. This inhibition may be the cause of an increased apoptosis rate and enhanced radiation sensitivity, which can support the development of barriers to radiotherapy resistance in OC [125].
Overall, PTEN is regulated by various microRNAs, which play vital roles in OC, and the absence of PTEN is associated with the occurrence of OC. PTEN can also lead to apoptosis and increased radiotherapy sensitivity, contributing to the formation of a barrier to radiotherapy resistance in OC. As suggested, PTEN is a very important regulatory gene in OC.
PTEN and liver cancer
As a negative regulator of the EGFR/PI3K/AKT signaling pathway, loss and mutation of PTEN often occur in liver cancer [126]. A progenitor cell mechanism may be associated with the PTEN mutations observed in human liver cancer and high malignant potential/poor prognosis [126]. Twenty-nine percent of HCC tissues lost cytoplasmic PTEN, and 25% of HCC tissues lost all expression of PTEN. In HCC tissues, PTEN expression was significantly reducing than that in adjacent nonneoplastic tissues [127,128], and its downregulation was associated with poor differentiation [129,130]. High levels of reactive oxygen species (ROS) are associated with tumorigenesis in PTEN-deficient mouse models [131]. Moreover, the deletion and downregulation of PTEN were significantly associated with the overexpression of fatty acid synthase (FAS) and histological grade of HCC. In addition, PTEN deficiency is related to poor prognosis in patients with advanced HCC. When FAS is overexpressed, the situation worsens [132].
In liver cancer cells, both the expression and the tumor suppressive ability of PTEN are significant [132]. Notably, the expression density of PTEN is connected with the development of liver cancer. This phenomenon may protect of the body itself in the case where tumor cells further express PTEN in adjacent cancer tissues under high-pressure conditions [133]. PTEN can act as a negative switch of the AKT pathway, thereby promoting the aggressive progression of hepatocellular carcinoma (HCC) by activating the AKT pathway [134]. On the other hand, the level and activity of PTEN in liver cancer are changed by various complex mechanisms. For instance, the expression of PI3K is negatively connected to the expression of PTEN. PI3K overexpression may be closely correlated with the formation of tumors. The anticancer effect of PTEN depends on the extent of its negative regulation of PI3K signaling [135]. In addition, phosphatase in regenerating liver-3 (PRL-3) can enhance the phosphorylation level of PTEN to reduce the PTEN level. Through this negative regulation of PTEN expression, PRL-3 may activate the PI3K/AKT signaling pathway, which promotes HCC progression [134]. PTEN serves as the downstream target of miR-21, and ectopic miR-21-mediated downregulation of PTEN and highly upregulated miR-21 expression were evident in hepatocellular carcinoma cell lines. PTEN is involved with miR-21 triggering of liver cancer cell death [136,137].
In summary, the expression of PTEN is closely connected with the occurrence and development of liver cancer, and its mechanism is related to various other mechanisms, such as those associated with the AKT pathway, PI3K, PRL-3, and miR-21. Deletions and mutations are frequent in liver cancer. PTEN is associated with the prognosis and drug resistance of liver cancer, making it a potential diagnostic and prognostic marker.
PTEN and colon cancer
In terms of the association between PTEN variations and cancers, a considerable proportion of patients with colon cancer (34.3%) showed PTEN expression deficiency [138]. In terms of mechanisms, it has been revealed that PTEN inhibits colon cancer progression through restraining paxillin expression downstream of the PI3K/AKT/NF-κB pathway [106]. In addition, PTEN expression can be regulated by aldose reductase (AR). Studies have shown that AR inhibition can inhibit PTEN phosphorylation induced by growth factors, thereby activating PTEN and increasing the expression of this protein in tumor cells, thus inhibiting colon cancer cells proliferation [108]. Furthermore, inhibition of PTEN increases the expression of microRNA-26b and contributes to the invasiveness, migration and stem cell-like phenotype of colorectal cancer (CRC) [139]. In summary, PTEN deficiency is closely associated with the occurrence of colon cancer, and this gene can affect PI3K/AKT/NF-κB, AR and miR-26b to inhibit colon cancer, and therefore, PTEN may be a new prognostic biomarker or therapeutic target.
PTEN and breast cancer
Breast cancer is the most frequent cancer in women and PTEN gene is strongly linked to it [140]. First, PTEN deficiency leads to the overgrowth, survival, proliferation, and metabolism of tumor cells [3]. It causes AKT-mediated phosphorylation and increased NF-κB activity, thereby promoting P53 degradation. Then, P53 degradation reduces the apoptotic ability of cells and induces cell cycle progression [141]. Heterozygosity disorders in chromosome 10q23 are evident in advanced sporadic tumors, including breast cancer [58,142-144].
In addtion, a recent meta-analysis demonstrated that hypermethylation of the PTEN promoter is considered to be among the most important mechanisms for inactivating PTEN in ductal carcinoma in situ (DCIS) and invasive ductal carcinoma of the breast, suggesting that the inactivation of PTEN is involved in the early stage of breast neoplasia [145,146]. In terms of PTEN and cancer prognosis, it has also been previously reported that tumor cells with loss of PTEN function lead to poor prognoses [147]. The inactivation of PTEN was significantly associated with a decrease in 5-year overall survival and disease-free survival rates in breast tumor patients. PTEN reduction was significantly correlated with tumor volume increases, estrogen progesterone receptor (ER)/progesterone receptor (PR) negativity, axillary lymph node metastasis positivity, and advanced stage and local recurrence of breast cancer, indicating a worsening prognosis [145].
Some researches have also demonstrated that the level of PTEN mRNA in breast cancer tissue is significantly decreased. The expression level of mIR-142-5p was positively and negatively correlated with PTEN, and the PTEN level was related to tumor size and metastasis [148].
PTEN and gastric cancer (GC)
Scientists found that the expression of PTEN decreased gradually as GC progressed [93]. The expression of PTEN in primary tumors was obviously lower than that in adjacent non-tumor tissues [149]. Therefore, the decrease or loss of PTEN expression is a dynamic process in gastric cancer progression and the level of PTEN can be considered as an indicator for the diagnosis of GC pathological status [150-152].
In terms of prognosis, PTEN and miR-718 have been identified as prognostic factors for GC. MiR-718 can promote the proliferation and invasion of GC cells through targeting PTEN mRNA [153]. Thus, PTEN is a prognostic risk factor for poor prognosis of GC. These findings are helpful for studying the progress and treatment of GC. In addition, tumor resistance is mainly caused by the inactivation of PTEN and subsequent activation of the AKT pathway [154-156]. To date, many mechanisms have been proven for the specific role of PTEN in endowing tumor cells with chemotherapy drug resistance. First, through inducing the expression of multidrug resistance protein 1 (MRP1), the PI3K signaling pathway is activated, especially PI3K3a and PAKT, which induces PTEN-induced drug resistance [157-159]. In addition, reduced expression of PTEN in cancer cells can lead to increased phosphorylation and activation of SRC kinase, leading to resistance to chemotherapy drugs such as trastuzumab [160].
In conclusion, the dysfunction of PTEN in GC leads to multiple processes. PTEN level not only can be used as a diagnostic indicator of GC pathological status but also as a risk factor for the poor prognosis in patients with GC. PTEN also has an inseparable relationship with drug resistance and is a promising potential therapeutic factor for cancers. However, further research is needed to study how PTEN regulates the interactions between these processes, interaction dynamics, and homeostasis under pathological conditions.
PTEN and prostate cancer
PTEN mutations can cause alterations in various genes and pathways that affect the development of prostate cancer, which may be significant to the individualized treatment of prostate cancer. Thus, drugs directed at lipid metabolism pathways may be targeted to PTEN-mutant prostate cancer in the development of new treatments for patients. As patients with PTEN mutations may be more sensitive to docetaxel and because these patients need early intervention to prolong their survival, docetaxel chemotherapy may be the most effective treatment [161]. In addition, advanced disease and poor prognosis are associated with PTEN mutations, and known mechanisms of ectopic PTEN effects include PTEN deletion, dysregulated transcription, and epigenetic modification [162,163]. In general, the disease progression of various cancer types is related to low PTEN expression levels [164-166], indicating the importance of PTEN mutation in disease progression. Furthermore, PTEN is associated with two oncogenes: phospholipase Ce (PLCe) and miR-20b in prostate cancer. PLCe expression downregulates PTEN expression in cancer cell lines and inhibits tumor cell proliferation by the PTEN/AKT signaling pathway [167], while miR-20b can restrain PTEN expression through directly combine with the 3’-UTR of PTEN mRNA [168].
PTEN has a strong link with oncogenes, but the mechanisms remain unclear. Reduced expression of PTEN is often related to the progression of many types of cancers and is one of the important potential mechanisms by which PTEN mutations are associated with cancer progression. However, the determination of the mechanisms and verification of gene mutations in cancer needs further molecular biological and clinical experimental research, and the characterization of the relationship between PTEN mutations and specific events in prostate cancer requires data from larger samples to produce the most accurate results.
PTEN and pancreatic cancer
PTEN is an essential factor in regulating the development of pancreatic cancer cells. PTEN plays a vital role in Jumonji AT-rich interactive domain 1B (JARID1B)-promoted cell and tumor proliferation. JARID1B may affect the activation of PTEN by regulating the methylation of lysine 4 on histone H3 (H3K4), thus promoting PC cell proliferation and tumor growth [169]. Moreover, studies have indicated that miR-486 can facilitate the proliferation of CAPAN-2 human pancreatic cancer cells through targeting PTEN. The tumor suppressor gene PTEN is the regulatory target gene of miR-486 [170]. At the protein level, miR-486 can negatively regulate the expression of PTEN. Moreover, PTEN gene overexpression disrupts the proliferation of miR-486 mimics because miR-486 is functionally targeted by PTEN in CAPAN-2 cells [171]. Furthermore, PTEN can be downregulated to target NF-κB and cMyc in pancreatic cancer cell lines through the activation of the PI3K/AKT signaling pathway, thereby playing an inhibitory role in pancreatic cancer [172]. Furthermore, PTEN is related to the regulation of pancreatic cancer cell angiogenesis, which may be related to chemotherapy resistance and tumor recurrence [173,174]. In conclusion, there is still a lack of effective diagnostic markers, drug targets, and treatment strategies to successfully treat PC. PTEN acts mainly on JARID1B and miR-486 in pancreatic cancer, both of which are potential therapeutic targets.
PTEN and esophageal cancer (EC)
Studies on EC have suggested that the downregulation of PTEN in EC is due to the hypermethylation of its promoter region [175]. The downregulation of PTEN can inhibit the phosphorylation of AKT in EC cells, while long intergenic nonprotein-coding RNA 184 (LINC00184) can activate AKT phosphorylation, thus positively regulating PTEN gene methylation [175]. Furthermore, other studies have shown other factors that affect PTEN and thus the development of cancer cells. For example, JARID1B can promote the proliferation of esophageal carcinoma cells and tumor growth after activating PTEN [169,176]. miR-93-5p may affect the expression of p21 and Cyclin D1, downstream proteins of PTEN in the PTEN/PI3K/AKT pathway, so contributed to cancer cells proliferation [177].
In conclusion, the downregulation of PTEN in EC is due to the hypermethylation at its promoter region. However, the relationship between the regulation of glucose metabolism by LINC00184 and EC cell tumors remains unclear and requires further study. On the other hand, both JARID1B and miR-93-5p can promote the proliferation of cancer cells by affecting PTEN, making them potential therapeutic targets to cure EC.
PTEN and endometrial carcinoma
Deficiency of the phosphatase and catalytic activities of the PTEN protein has been associated with various types of cancer, including endometrial cancer (EDC). Mutant PTEN is less stable than normal PTEN. Substrate binding sites in PTEN are abrogated when PTEN is mutated, leading to the development of endometrial cancer [113]. Furthermore, the miRNAs associated with PTEN are often dysregulated in human cancers. Among these miRNAs, miR-205 can directly regulate the expression of PTEN in endometrial tumor cells and result in cell apoptosis inhibition. As an oncogene, miR-205 restrains apoptosis through targeting the PTEN/AKT pathway. Thus, the increasing expression of miR-205 in cancer cells may have essential effect on EDC progression [178].
In terms of prognosis, it has been shown that PTEN is associated with clinicopathological factors and prognosis in EDC patients. The decrease in PTEN expression is associated with poor prognosis. By contrast, EDC patients with high level of PTEN had low malignant tumor levels, diminished proliferative activity and a better prognosis [179]. In summary, the dysregulation and loss of PTEN expression are related to endometrial cancer and prognosis, which is a potential factor for cancer treatment.
Drugs of targeting the PTEN/PI3K/AKT/mTOR axis
The overall active role of PI3K/AKT/mTOR signaling pathway in cell growth and development makes it possible for small molecule inhibitors of PI3K, AKT or mTOR to target the treatment of PTNE deficient cancer [180-183]. As the most common abnormal regulatory pathway in tumors, this pathway has attracted more and more attention because of its potential in targeted therapy of many kinds of malignant tumors. In this context, a variety of inhibitors for this pathway are mainly targeted at PI3Ks, AKT and mTOR [181,184-186]. PI3K inhibitors include LY294002 [187], wortmannin [188], curcumin [189], BLY719 [190], BKM120 [191], idelalisib [192], copanlisib [193], etc. AKT inhibitors include perifosine [194], celecoxib [195], GSK690693 [196], deguelin [197], MK-2206 [198], etc. mTOR inhibitors include RAD-001 (everolimus) [199], CCI-779 (temsirolimus) [200], AP23573 (deforolimus) [201] and so on.
The mTOR-based inhibitors temsirolimus and everolimus, as well as PI3K-based inhibitors idelalisib and copanlisib, have been approved by the Food and Drug Administration for clinical anticancer treatment [192,193,199,200,202]. Curcumin (NCT03211104, NCT03980509), perifosine (NCT01048580, NCT01224730), celecoxib (NCT03896113, NCT02429427), GSK690693 (NCT00666081), MK-2206 (NCT01147211, NCT01240928), AP23573 (NCT00704054, NCT00122343) have entered the clinical experimental research in the treatment of tumors. However, LY294002, Wortmannin [186] and deguelin are still in the stage of experimental research [187,203].
Although the most effective anti-tumor effect of PTEN is the passive regulation of PI3K/mTOR/AKT carcinogenic signal pathway, but further tumor inhibition functions have been reported, such as chromosome integrity and DNA repair [10,74]. At present, some small molecular inhibitors have been experimentally explored as a potential treatment by pharmacological inhibition of PTEN. For example, bpv (phen), bpv (pic), bpv (HOpic), bpv (pis), Vo-OH-pic, the effect of this inhibitor on PTEN can be reversed by reductant, just like the inhibition of PTEN by ROS [204-206]. SF1670, an inhibitor targeting PTEN, has been found that SF1670 restrains cells apoptosis and inflammation by inhibiting PTEN and activating AKT, thus preventing intervertebral disc degeneration [207]. It has also been found that SF1670 protects PC12 cells from cell death induced by oxygen-glucose deprivation by restraining PTEN [208]. However, there are few studies on the mechanism of SF1670 inhibiting PTEN in tumor.
On the PI3K/AKT/mTOR axis, PTEN plays important tumor inhibitory role through regulating transcription, translation, cell cycle progression, inducing cell death, stimulating angiogenesis and stem cell self-renewal [17,34,94,164,209]. From this point of view, it can be considered that it is of great benefit to strengthen the research and development of PTEN activators in the future. However, PTEN inhibition is considered as a potential treatment. Most pathological conditions depend on the direct negative regulation of PIP3 phosphatase activity on the signal of PI3K/AKT/mTOR pathway. The evidence shows that PTEN protein phosphatase activity and non-catalytic PTEN activity exert significant function on physiological and pathological processes. Whether it is feasible to selectively inhibit the activity of small molecular PTEN lipoprotein phosphatase or protein phosphatase remains to be discussed.
Perspectives and future directions
PTEN has been extensively studied by scholars as a tumor factor. PTEN inhibits tumorigenesis by various mechanisms, including phosphatase-dependent and independent activities, subcellular localization and protein-protein interactions, affecting many physiological and pathological processes, including growth, development, survival, DNA repair and cells movement [3,11,61,210]. To date, considerable progress has been made in the study of PTEN mutation and deficiency in cancers, and knowledge of anticancer mechanisms, prognoses and drug resistance in different cancer types has advanced. In terms of the mechanism of cancer inhibition, the PI3K/AKT pathway has been widely and frequently mentioned. However, there are still a few cancers for which PTEN has been rarely studied, and more extensive research is needed. As a vital tumor suppressor gene, the main function of PTEN is to control apoptosis and regulate the cycle of cancer cells. Apart from the inherent tumor inhibitory function of PTEN, some researches have also emphasized the key function of PTEN in regulating tumor microenvironment. It acts on cancer cells, stroma and immune response at different levels, thereby promoting the occurrence, development and metastasis of the diseases [1,35,211-215]. In view of the fact that PTEN is an important target with a variety of biological functions in tumors, the future drug research on PTEN will be of great significance to the treatment and prognostic diagnosis of tumors. Although, in the PTEN/PI3K/AKT/mTOR axis, targeted PI3K, AKT and mTOR inhibitors have appeared or even entered clinical trials, there are still few studies on drugs related to PTEN. However, whether to develop an activator or an inhibitor of PTEN still needs follow-up experimental studies to provide more evidence.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81873076), the Hundred Talents Program from Shanghai University of Traditional Chinese Medicine and Innovation Project for Undergraduates of Shanghai University of Traditional Chinese Medicine (202110268228).
Disclosure of conflict of interest
None.
References
- 1.Chen CY, Chen J, He L, Stiles BL. PTEN: tumor suppressor and metabolic regulator. Front Endocrinol (Lausanne) 2018;9:338. doi: 10.3389/fendo.2018.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Álvarez-Garcia V, Tawil Y, Wise HM, Leslie NR. Mechanisms of PTEN loss in cancer: it’s all about diversity. Semin Cancer Biol. 2019;59:66–79. doi: 10.1016/j.semcancer.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Milella M, Falcone I, Conciatori F, Cesta Incani U, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S, Cognetti F, Ciuffreda L. PTEN: multiple functions in human malignant tumors. Front Oncol. 2015;5:24. doi: 10.3389/fonc.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahia PL. PTEN, a unique tumor suppressor gene. Endocr Relat Cancer. 2000;7:115–129. doi: 10.1677/erc.0.0070115. [DOI] [PubMed] [Google Scholar]
- 5.Simpson L, Parsons R. PTEN: life as a tumor suppressor. Exp Cell Res. 2001;264:29–41. doi: 10.1006/excr.2000.5130. [DOI] [PubMed] [Google Scholar]
- 6.Thies KA, Lefler JE, Leone G, Ostrowski MC. PTEN in the stroma. Cold Spring Harb Perspect Med. 2019;9:a036111. doi: 10.1101/cshperspect.a036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho J, Cruise ES, Dowling RJO, Stambolic V. PTEN nuclear functions. Cold Spring Harb Perspect Med. 2020;10:a036079. doi: 10.1101/cshperspect.a036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Yin Y. PTEN in chromatin remodeling. Cold Spring Harb Perspect Med. 2020;10:a036160. doi: 10.1101/cshperspect.a036160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bononi A, Pinton P. Study of PTEN subcellular localization. Methods. 2015;77-78:92–103. doi: 10.1016/j.ymeth.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou SQ, Ouyang M, Brandmaier A, Hao H, Shen WH. PTEN in the maintenance of genome integrity: from DNA replication to chromosome segregation. Bioessays. 2017;39 doi: 10.1002/bies.201700082. 10.1002/bies.201700082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Jiang X. Post-translational regulation of PTEN. Oncogene. 2008;27:5454–5463. doi: 10.1038/onc.2008.242. [DOI] [PubMed] [Google Scholar]
- 12.De Melo J, He L, Tang D. The protein-protein interaction-mediated inactivation of PTEN. Curr Mol Med. 2014;14:22–33. doi: 10.2174/1566524013666131118100542. [DOI] [PubMed] [Google Scholar]
- 13.Ho J, Bassi C, Stambolic V. Characterization of nuclear PTEN and its post translational modifications. Methods. 2015;77-78:104–111. doi: 10.1016/j.ymeth.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–198. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- 15.Liu HY, Zhang YY, Zhu BL, Feng FZ, Yan H, Zhang HY, Zhou B. miR-21 regulates the proliferation and apoptosis of ovarian cancer cells through PTEN/PI3K/AKT. Eur Rev Med Pharmacol Sci. 2019;23:4149–4155. doi: 10.26355/eurrev_201905_17917. [DOI] [PubMed] [Google Scholar]
- 16.Cao HL, Gu MQ, Sun Z, Chen ZJ. miR-144-3p contributes to the development of thyroid tumors through the PTEN/PI3K/AKT pathway. Cancer Manag Res. 2020;12:9845–9855. doi: 10.2147/CMAR.S265196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuoka S, Ueda M. Mutual inhibition between PTEN and PIP3 generates bistability for polarity in motile cells. Nat Commun. 2018;9:4481. doi: 10.1038/s41467-018-06856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 20.Teng DH, Hu R, Lin H, Davis T, Iliev D, Frye C, Swedlund B, Hansen KL, Vinson VL, Gumpper KL, Ellis L, El-Naggar A, Frazier M, Jasser S, Langford LA, Lee J, Mills GB, Pershouse MA, Pollack RE, Tornos C, Troncoso P, Yung WK, Fujii G, Berson A, Steck PA, et al. MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res. 1997;57:5221–5225. [PubMed] [Google Scholar]
- 21.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 22.Al Bashir S, Alzoubi A, Alfaqih MA, Kheirallah K, Smairat A, Haddad H, Al-Dwairy A, Fawwaz BAB, Alzoubi M, Trpkov K. PTEN loss in a prostate cancer cohort from jordan. Appl Immunohistochem Mol Morphol. 2020;28:389–394. doi: 10.1097/PAI.0000000000000732. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abrão F, Modotti WP, Spadoto-Dias D, Bueloni-Dias FN, Leite NJ, Peres GF, Elias LV, Domingues MAC, Dias R. Concomitant p53 and PTEN immunoexpression to predict the risk of malignancy in endometrial polyps. Medicine (Baltimore) 2018;97:e12304. doi: 10.1097/MD.0000000000012304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasparyan M, Lo MC, Jiang H, Lin CC, Sun D. Combined p53- and PTEN-deficiency activates expression of mesenchyme homeobox 1 (MEOX1) required for growth of triple-negative breast cancer. J Biol Chem. 2020;295:12188–12202. doi: 10.1074/jbc.RA119.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C, Yang R, Yue D, Zhang Z. Expression of FAK and PTEN in bronchioloalveolar carcinoma and lung adenocarcinoma. Lung. 2009;187:104–109. doi: 10.1007/s00408-008-9130-6. [DOI] [PubMed] [Google Scholar]
- 27.Hu C, Zhou H, Liu Y, Huang J, Liu W, Zhang Q, Tang Q, Sheng F, Li G, Zhang R. ROCK1 promotes migration and invasion of non-small-cell lung cancer cells through the PTEN/PI3K/FAK pathway. Int J Oncol. 2019;55:833–844. doi: 10.3892/ijo.2019.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Yu Q, He J, Zha X. Study of the PTEN gene expression and FAK phosphorylation in human hepatocarcinoma tissues and cell lines. Mol Cell Biochem. 2004;262:25–33. doi: 10.1023/b:mcbi.0000038212.78008.7f. [DOI] [PubMed] [Google Scholar]
- 29.Li ZH, Li L, Kang LP, Wang Y. MicroRNA-92a promotes tumor growth and suppresses immune function through activation of MAPK/ERK signaling pathway by inhibiting PTEN in mice bearing U14 cervical cancer. Cancer Med. 2018;7:3118–3131. doi: 10.1002/cam4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Pan H, Lu LY, Wang XQ, Li BX, Kelly K, Lin HS. Gambogic acid induces cell apoptosis and inhibits MAPK pathway in PTEN(-/-)/p53(-/-) prostate cancer cells in vitro and ex vivo. Chin J Integr Med. 2018;24:109–116. doi: 10.1007/s11655-017-2410-3. [DOI] [PubMed] [Google Scholar]
- 31.Chang RM, Xu JF, Fang F, Yang H, Yang LY. MicroRNA-130b promotes proliferation and EMT-induced metastasis via PTEN/p-AKT/HIF-1α signaling. Tumour Biol. 2016;37:10609–10619. doi: 10.1007/s13277-016-4919-z. [DOI] [PubMed] [Google Scholar]
- 32.Shen W, Li HL, Liu L, Cheng JX. Expression levels of PTEN, HIF-1α, and VEGF as prognostic factors in ovarian cancer. Eur Rev Med Pharmacol Sci. 2017;21:2596–2603. [PubMed] [Google Scholar]
- 33.Xu W, Yang Z, Zhou SF, Lu N. Posttranslational regulation of phosphatase and tensin homolog (PTEN) and its functional impact on cancer behaviors. Drug Des Devel Ther. 2014;8:1745–1751. doi: 10.2147/DDDT.S71061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, Williams LJ, Deng W, Chen G, Mbofung R, Lazar AJ, Torres-Cabala CA, Cooper ZA, Chen PL, Tieu TN, Spranger S, Yu X, Bernatchez C, Forget MA, Haymaker C, Amaria R, McQuade JL, Glitza IC, Cascone T, Li HS, Kwong LN, Heffernan TP, Hu J, Bassett RL Jr, Bosenberg MW, Woodman SE, Overwijk WW, Lizée G, Roszik J, Gajewski TF, Wargo JA, Gershenwald JE, Radvanyi L, Davies MA, Hwu P. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016;6:202–216. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conciatori F, Bazzichetto C, Falcone I, Ciuffreda L, Ferretti G, Vari S, Ferraresi V, Cognetti F, Milella M. PTEN function at the interface between cancer and tumor microenvironment: implications for response to immunotherapy. Int J Mol Sci. 2020;21:5337. doi: 10.3390/ijms21155337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 37.Gericke A, Munson M, Ross AH. Regulation of the PTEN phosphatase. Gene. 2006;374:1–9. doi: 10.1016/j.gene.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 38.Koul D, Jasser SA, Lu Y, Davies MA, Shen R, Shi Y, Mills GB, Yung WK. Motif analysis of the tumor suppressor gene MMAC/PTEN identifies tyrosines critical for tumor suppression and lipid phosphatase activity. Oncogene. 2002;21:2357–2364. doi: 10.1038/sj.onc.1205296. [DOI] [PubMed] [Google Scholar]
- 39.Sim CH, Gabriel K, Mills RD, Culvenor JG, Cheng HC. Analysis of the regulatory and catalytic domains of PTEN-induced kinase-1 (PINK1) Hum Mutat. 2012;33:1408–1422. doi: 10.1002/humu.22127. [DOI] [PubMed] [Google Scholar]
- 40.Redfern RE, Redfern D, Furgason ML, Munson M, Ross AH, Gericke A. PTEN phosphatase selectively binds phosphoinositides and undergoes structural changes. Biochemistry. 2008;47:2162–2171. doi: 10.1021/bi702114w. [DOI] [PubMed] [Google Scholar]
- 41.Maier D, Jones G, Li X, Schönthal AH, Gratzl O, Van Meir EG, Merlo A. The PTEN lipid phosphatase domain is not required to inhibit invasion of glioma cells. Cancer Res. 1999;59:5479–5482. [PubMed] [Google Scholar]
- 42.Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem. 2001;70:247–279. doi: 10.1146/annurev.biochem.70.1.247. [DOI] [PubMed] [Google Scholar]
- 43.Steelman LS, Navolanic PM, Sokolosky ML, Taylor JR, Lehmann BD, Chappell WH, Abrams SL, Wong EW, Stadelman KM, Terrian DM, Leslie NR, Martelli AM, Stivala F, Libra M, Franklin RA, McCubrey JA. Suppression of PTEN function increases breast cancer chemotherapeutic drug resistance while conferring sensitivity to mTOR inhibitors. Oncogene. 2008;27:4086–4095. doi: 10.1038/onc.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fragoso R, Barata JT. Kinases, tails and more: regulation of PTEN function by phosphorylation. Methods. 2015;77-78:75–81. doi: 10.1016/j.ymeth.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Kotelevets L, Trifault B, Chastre E, Scott MGH. Posttranslational regulation and conformational plasticity of PTEN. Cold Spring Harb Perspect Med. 2020;10:a036095. doi: 10.1101/cshperspect.a036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shenoy SS, Nanda H, Lösche M. Membrane association of the PTEN tumor suppressor: electrostatic interaction with phosphatidylserine-containing bilayers and regulatory role of the C-terminal tail. J Struct Biol. 2012;180:394–408. doi: 10.1016/j.jsb.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papa A, Wan L, Bonora M, Salmena L, Song MS, Hobbs RM, Lunardi A, Webster K, Ng C, Newton RH, Knoblauch N, Guarnerio J, Ito K, Turka LA, Beck AH, Pinton P, Bronson RT, Wei W, Pandolfi PP. Cancer-associated PTEN mutants act in a dominant-negative manner to suppress PTEN protein function. Cell. 2014;157:595–610. doi: 10.1016/j.cell.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LoRusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J. Clin. Oncol. 2016;34:3803–3815. doi: 10.1200/JCO.2014.59.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noorolyai S, Shajari N, Baghbani E, Sadreddini S, Baradaran B. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–128. doi: 10.1016/j.gene.2019.02.076. [DOI] [PubMed] [Google Scholar]
- 50.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J. Clin. Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadeghi N, Gerber DE. Targeting the PI3K pathway for cancer therapy. Future Med Chem. 2012;4:1153–1169. doi: 10.4155/fmc.12.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perumal E, So Youn K, Sun S, Seung-Hyun J, Suji M, Jieying L, Yeun-Jun C. PTEN inactivation induces epithelial-mesenchymal transition and metastasis by intranuclear translocation of β-catenin and snail/slug in non-small cell lung carcinoma cells. Lung Cancer. 2019;130:25–34. doi: 10.1016/j.lungcan.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 53.Man X, Piao C, Lin X, Kong C, Cui X, Jiang Y. USP13 functions as a tumor suppressor by blocking the NF-kB-mediated PTEN downregulation in human bladder cancer. J Exp Clin Cancer Res. 2019;38:259. doi: 10.1186/s13046-019-1262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haddadi N, Lin Y, Travis G, Simpson AM, Nassif NT, McGowan EM. PTEN/PTENP1: ‘regulating the regulator of RTK-dependent PI3K/Akt signalling’, new targets for cancer therapy. Mol Cancer. 2018;17:37. doi: 10.1186/s12943-018-0803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao LQ, Yang XW, Chen YB, Zhang DW, Jiang XF, Xue P. Exosomal miR-21 regulates the TETs/PTENp1/PTEN pathway to promote hepatocellular carcinoma growth. Mol Cancer. 2019;18:148. doi: 10.1186/s12943-019-1075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao X, Qin T, Mao J, Zhang J, Fan S, Lu Y, Sun Z, Zhang Q, Song B, Li L. PTENP1/miR-20a/PTEN axis contributes to breast cancer progression by regulating PTEN via PI3K/AKT pathway. J Exp Clin Cancer Res. 2019;38:256. doi: 10.1186/s13046-019-1260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, Hu K, Gong G, Zhu D, Wang Y, Liu H, Wu X. Upregulation of MiR-205 transcriptionally suppresses SMAD4 and PTEN and contributes to human ovarian cancer progression. Sci Rep. 2017;7:41330. doi: 10.1038/srep41330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li N, Miao Y, Shan Y, Liu B, Li Y, Zhao L, Jia L. MiR-106b and miR-93 regulate cell progression by suppression of PTEN via PI3K/Akt pathway in breast cancer. Cell Death Dis. 2017;8:e2796. doi: 10.1038/cddis.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao W, Han T, Li B, Ma Q, Yang P, Li H. miR-552 promotes ovarian cancer progression by regulating PTEN pathway. J Ovarian Res. 2019;12:121. doi: 10.1186/s13048-019-0589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang X, Liu C, Li H, Guo L. Effects of miR-21 on proliferation and apoptosis of WT cells via PTEN/Akt pathway. Exp Ther Med. 2020;19:2155–2160. doi: 10.3892/etm.2019.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leslie NR, Kriplani N, Hermida MA, Alvarez-Garcia V, Wise HM. The PTEN protein: cellular localization and post-translational regulation. Biochem Soc Trans. 2016;44:273–278. doi: 10.1042/BST20150224. [DOI] [PubMed] [Google Scholar]
- 62.Chen L, Liu S, Tao Y. Regulating tumor suppressor genes: post-translational modifications. Signal Transduct Target Ther. 2020;5:90. doi: 10.1038/s41392-020-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nussinov R, Zhang M, Tsai CJ, Jang H. Phosphorylation and driver mutations in PI3Kα and PTEN autoinhibition. Mol Cancer Res. 2021;19:543–548. doi: 10.1158/1541-7786.MCR-20-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Panera N, Gnani D, Piermarini E, Petrini S, Bertini E, Nobili V, Pastore A, Piemonte F, Alisi A. High concentrations of H2O2 trigger hypertrophic cascade and phosphatase and tensin homologue (PTEN) glutathionylation in H9c2 cardiomyocytes. Exp Mol Pathol. 2016;100:199–206. doi: 10.1016/j.yexmp.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Verrastro I, Tveen-Jensen K, Woscholski R, Spickett CM, Pitt AR. Reversible oxidation of phosphatase and tensin homolog (PTEN) alters its interactions with signaling and regulatory proteins. Free Radic Biol Med. 2016;90:24–34. doi: 10.1016/j.freeradbiomed.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Okumura K, Mendoza M, Bachoo RM, DePinho RA, Cavenee WK, Furnari FB. PCAF modulates PTEN activity. J Biol Chem. 2006;281:26562–26568. doi: 10.1074/jbc.M605391200. [DOI] [PubMed] [Google Scholar]
- 67.Kim JE, Lee DS, Park H, Kang TC. Src/CK2/PTEN-mediated GluN2B and CREB dephosphorylations regulate the responsiveness to AMPA receptor antagonists in chronic epilepsy rats. Int J Mol Sci. 2020;21:9633. doi: 10.3390/ijms21249633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li P, Wang J, Zhao X, Ru J, Tian T, An Y, Tang L, Bai Y. PTEN inhibition attenuates endothelial cell apoptosis in coronary heart disease via modulating the AMPK-CREB-Mfn2-mitophagy signaling pathway. J Cell Physiol. 2020;235:4878–4889. doi: 10.1002/jcp.29366. [DOI] [PubMed] [Google Scholar]
- 69.Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo C, Erdjument-Bromage H, Tempst P, Chi SG, Kim HJ, Misteli T, Jiang X, Pandolfi PP. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shao C, Li Z, Ahmad N, Liu X. Regulation of PTEN degradation and NEDD4-1 E3 ligase activity by numb. Cell Cycle. 2017;16:957–967. doi: 10.1080/15384101.2017.1310351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang JM, Schiapparelli P, Nguyen HN, Igarashi A, Zhang Q, Abbadi S, Amzel LM, Sesaki H, Quiñones-Hinojosa A, Iijima M. Characterization of PTEN mutations in brain cancer reveals that pten mono-ubiquitination promotes protein stability and nuclear localization. Oncogene. 2017;36:3673–3685. doi: 10.1038/onc.2016.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.González-Santamaría J, Campagna M, Ortega-Molina A, Marcos-Villar L, de la Cruz-Herrera CF, González D, Gallego P, Lopitz-Otsoa F, Esteban M, Rodríguez MS, Serrano M, Rivas C. Regulation of the tumor suppressor PTEN by SUMO. Cell Death Dis. 2012;3:e393. doi: 10.1038/cddis.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang J, Yan J, Zhang J, Zhu S, Wang Y, Shi T, Zhu C, Chen C, Liu X, Cheng J, Mustelin T, Feng GS, Chen G, Yu J. SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane. Nat Commun. 2012;3:911. doi: 10.1038/ncomms1919. [DOI] [PubMed] [Google Scholar]
- 74.Bassi C, Ho J, Srikumar T, Dowling RJ, Gorrini C, Miller SJ, Mak TW, Neel BG, Raught B, Stambolic V. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science. 2013;341:395–399. doi: 10.1126/science.1236188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horn D, Hess J, Freier K, Hoffmann J, Freudlsperger C. Targeting EGFR-PI3K-AKT-mTOR signaling enhances radiosensitivity in head and neck squamous cell carcinoma. Expert Opin Ther Targets. 2015;19:795–805. doi: 10.1517/14728222.2015.1012157. [DOI] [PubMed] [Google Scholar]
- 76.Zhang HX, Yang JJ, Zhang SA, Zhang SM, Wang JX, Xu ZY, Lin RY. HIF-1α promotes inflammatory response of chronic obstructive pulmonary disease by activating EGFR/PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2018;22:6077–6084. doi: 10.26355/eurrev_201809_15946. [DOI] [PubMed] [Google Scholar]
- 77.Han J, Yu J, Dai Y, Li J, Guo M, Song J, Zhou X. Overexpression of miR-361-5p in triple-negative breast cancer (TNBC) inhibits migration and invasion by targeting RQCD1 and inhibiting the EGFR/PI3K/Akt pathway. Bosn J Basic Med Sci. 2019;19:52–59. doi: 10.17305/bjbms.2018.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 79.Bamodu OA, Chang HL, Ong JR, Lee WH, Yeh CT, Tsai JT. Elevated PDK1 expression drives PI3K/AKT/MTOR signaling promotes radiation-resistant and dedifferentiated phenotype of hepatocellular carcinoma. Cells. 2020;9:746. doi: 10.3390/cells9030746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mora A, Sakamoto K, McManus EJ, Alessi DR. Role of the PDK1-PKB-GSK3 pathway in regulating glycogen synthase and glucose uptake in the heart. FEBS Lett. 2005;579:3632–3638. doi: 10.1016/j.febslet.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 81.Zheng X, Xie L, Qin J, Shen H, Chen Z, Jin Y. Effects of wortmannin on phosphorylation of PDK1, GSK3-beta, PTEN and expression of Skp2 mRNA after ischemia/reperfusion injury in the mouse kidney. Int Urol Nephrol. 2008;40:185–192. doi: 10.1007/s11255-007-9215-9. [DOI] [PubMed] [Google Scholar]
- 82.Boreddy SR, Pramanik KC, Srivastava SK. Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clin Cancer Res. 2011;17:1784–1795. doi: 10.1158/1078-0432.CCR-10-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park SG, Long M, Kang JA, Kim WS, Lee CR, Im SH, Strickland I, Schulze-Luehrmann J, Hayden MS, Ghosh S. The kinase PDK1 is essential for B-cell receptor mediated survival signaling. PLoS One. 2013;8:e55378. doi: 10.1371/journal.pone.0055378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yan Y, Huang H. Interplay among PI3K/AKT, PTEN/FOXO and AR signaling in prostate cancer. Adv Exp Med Biol. 2019;1210:319–331. doi: 10.1007/978-3-030-32656-2_14. [DOI] [PubMed] [Google Scholar]
- 85.Jhanwar-Uniyal M, Wainwright JV, Mohan AL, Tobias ME, Murali R, Gandhi CD, Schmidt MH. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv Biol Regul. 2019;72:51–62. doi: 10.1016/j.jbior.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 86.Kim HK, Bhattarai KR, Junjappa RP, Ahn JH, Pagire SH, Yoo HJ, Han J, Lee D, Kim KW, Kim HR, Chae HJ. TMBIM6/BI-1 contributes to cancer progression through assembly with mTORC2 and AKT activation. Nat Commun. 2020;11:4012. doi: 10.1038/s41467-020-17802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Papa A, Pandolfi PP. The PTEN-PI3K axis in cancer. Biomolecules. 2019;9:153. doi: 10.3390/biom9040153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chai C, Song LJ, Han SY, Li XQ, Li M. MicroRNA-21 promotes glioma cell proliferation and inhibits senescence and apoptosis by targeting SPRY1 via the PTEN/PI3K/AKT signaling pathway. CNS Neurosci Ther. 2018;24:369–380. doi: 10.1111/cns.12785. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Han JB, Huang ML, Li F, Yang R, Chen SM, Tao ZZ. MiR-214 mediates cell proliferation and apoptosis of nasopharyngeal carcinoma through targeting both WWOX and PTEN. Cancer Biother Radiopharm. 2020;35:615–625. doi: 10.1089/cbr.2019.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao Q, Zhang L, Zhang B, Wang QY, Sun CF, Dong XT, Ying J. Phosphatase and tensin homolog overexpression decreases proliferation and invasion and increases apoptosis in oral squamous cell carcinoma cells. Oncol Lett. 2014;8:1058–1064. doi: 10.3892/ol.2014.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11:289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weng LP, Brown JL, Eng C. PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum Mol Genet. 2001;10:599–604. doi: 10.1093/hmg/10.6.599. [DOI] [PubMed] [Google Scholar]
- 93.Xu WT, Yang Z, Lu NH. Roles of PTEN (phosphatase and tensin homolog) in gastric cancer development and progression. Asian Pac J Cancer Prev. 2014;15:17–24. doi: 10.7314/apjcp.2014.15.1.17. [DOI] [PubMed] [Google Scholar]
- 94.Ye B, Jiang LL, Xu HT, Zhou DW, Li ZS. Expression of PI3K/AKT pathway in gastric cancer and its blockade suppresses tumor growth and metastasis. Int J Immunopathol Pharmacol. 2012;25:627–636. doi: 10.1177/039463201202500309. [DOI] [PubMed] [Google Scholar]
- 95.Hlobilkova A, Guldberg P, Thullberg M, Zeuthen J, Lukas J, Bartek J. Cell cycle arrest by the PTEN tumor suppressor is target cell specific and may require protein phosphatase activity. Exp Cell Res. 2000;256:571–577. doi: 10.1006/excr.2000.4867. [DOI] [PubMed] [Google Scholar]
- 96.Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, Gleave M, Wu H. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012;72:1878–1889. doi: 10.1158/0008-5472.CAN-11-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tanaka N, Odajima T, Ogi K, Ikeda T, Satoh M. Expression of E-cadherin, alpha-catenin, and beta-catenin in the process of lymph node metastasis in oral squamous cell carcinoma. Br J Cancer. 2003;89:557–563. doi: 10.1038/sj.bjc.6601124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie S, Lu Z, Lin Y, Shen L, Yin C. Upregulation of PTEN suppresses invasion in Tca8113 tongue cancer cells through repression of epithelial-mesenchymal transition (EMT) Tumour Biol. 2016;37:6681–6689. doi: 10.1007/s13277-015-4486-8. [DOI] [PubMed] [Google Scholar]
- 99.Li M, Peng Z, Ren W, Wang Z. Small activating ribonucleic acid reverses tyrosine kinase inhibitor resistance in epidermal growth factor receptor-mutant lung cancer by increasing the expression of phosphatase and tensin homolog. Thorac Cancer. 2016;7:481–485. doi: 10.1111/1759-7714.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun H, Ma H, Wang J, Xia L, Zhu G, Wang Z, Sun J, Chen Z. Phosphatase and tensin homolog deleted on chromosome 10 degradation induced by NEDD4 promotes acquired erlotinib resistance in non-small-cell lung cancer. Tumour Biol. 2017;39:1010428317709639. doi: 10.1177/1010428317709639. [DOI] [PubMed] [Google Scholar]
- 101.Poh TW, Pervaiz S. LY294002 and LY303511 sensitize tumor cells to drug-induced apoptosis via intracellular hydrogen peroxide production independent of the phosphoinositide 3-kinase-Akt pathway. Cancer Res. 2005;65:6264–6274. doi: 10.1158/0008-5472.CAN-05-0152. [DOI] [PubMed] [Google Scholar]
- 102.Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, Yin Y. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 103.Kim RH, Mak TW. Tumours and tremors: how PTEN regulation underlies both. Br J Cancer. 2006;94:620–624. doi: 10.1038/sj.bjc.6602994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kotelevets L, van Hengel J, Bruyneel E, Mareel M, van Roy F, Chastre E. The lipid phosphatase activity of PTEN is critical for stabilizing intercellular junctions and reverting invasiveness. J Cell Biol. 2001;155:1129–1135. doi: 10.1083/jcb.200105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang S, Yu D. PI(3)king apart PTEN’s role in cancer. Clin Cancer Res. 2010;16:4325–4330. doi: 10.1158/1078-0432.CCR-09-2990. [DOI] [PubMed] [Google Scholar]
- 106.Zhang LL, Mu GG, Ding QS, Li YX, Shi YB, Dai JF, Yu HG. Phosphatase and tensin homolog (PTEN) represses colon cancer progression through inhibiting paxillin transcription via PI3K/AKT/NF-κB pathway. J Biol Chem. 2015;290:15018–15029. doi: 10.1074/jbc.M115.641407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chatzizacharias NA, Kouraklis GP, Theocharis SE. Focal adhesion kinase: a promising target for anticancer therapy. Expert Opin Ther Targets. 2007;11:1315–1328. doi: 10.1517/14728222.11.10.1315. [DOI] [PubMed] [Google Scholar]
- 108.Saxena A, Tammali R, Ramana KV, Srivastava SK. Aldose reductase inhibition prevents colon cancer growth by restoring phosphatase and tensin homolog through modulation of miR-21 and FOXO3a. Antioxid Redox Signal. 2013;18:1249–1262. doi: 10.1089/ars.2012.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 110.Barford D, Flint AJ, Tonks NK. Crystal structure of human protein tyrosine phosphatase 1B. Science. 1994;263:1397–1404. [PubMed] [Google Scholar]
- 111.Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 112.Zhang H, Zhao X, Liu S, Li J, Wen Z, Li M. 17betaE2 promotes cell proliferation in endometriosis by decreasing PTEN via NFkappaB-dependent pathway. Mol Cell Endocrinol. 2010;317:31–43. doi: 10.1016/j.mce.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 113.Mondal SK, Sen MK. Loss of phosphatase activity in PTEN (phosphatase and tensin homolog deleted on chromosome ten) results in endometrial carcinoma in humans: an in-silico study. Heliyon. 2020;6:e03106. doi: 10.1016/j.heliyon.2019.e03106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li XB, Yang Y, Zhang HQ, Yue WT, Zhang TM, Lu BH, Li J, Liu Z, Wang QH, Gao Y, Hu AM, Zhang HM, Shi HL, Hu FB, Li BL. High levels of phosphatase and tensin homolog expression predict favorable prognosis in patients with non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2015;19:2231–2239. [PubMed] [Google Scholar]
- 115.Wang F, Li Y, Zhang Z, Wang J, Wang J. SHCBP1 regulates apoptosis in lung cancer cells through phosphatase and tensin homolog. Oncol Lett. 2019;18:1888–1894. doi: 10.3892/ol.2019.10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu PF, Gao WW, Sun CL, Ma T, Hao JQ. Suberoylanilide hydroxamic acid overcomes erlotinib-acquired resistance via phosphatase and tensin homolog deleted on chromosome 10-mediated apoptosis in non-small cell lung cancer. Chin Med J (Engl) 2020;133:1304–1311. doi: 10.1097/CM9.0000000000000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Min A, Im SA, Kim DK, Song SH, Kim HJ, Lee KH, Kim TY, Han SW, Oh DY, Kim TY, O’Connor MJ, Bang YJ. Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA), enhances anti-tumor effects of the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib in triple-negative breast cancer cells. Breast Cancer Res. 2015;17:33. doi: 10.1186/s13058-015-0534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pan L, Lu J, Wang X, Han L, Zhang Y, Han S, Huang B. Histone deacetylase inhibitor trichostatin a potentiates doxorubicin-induced apoptosis by up-regulating PTEN expression. Cancer. 2007;109:1676–1688. doi: 10.1002/cncr.22585. [DOI] [PubMed] [Google Scholar]
- 119.Gan YH, Zhang S. PTEN/AKT pathway involved in histone deacetylases inhibitor induced cell growth inhibition and apoptosis of oral squamous cell carcinoma cells. Oral Oncol. 2009;45:e150–154. doi: 10.1016/j.oraloncology.2009.05.563. [DOI] [PubMed] [Google Scholar]
- 120.Russo A, Czarnecki AA, Dean M, Modi DA, Lantvit DD, Hardy L, Baligod S, Davis DA, Wei JJ, Burdette JE. PTEN loss in the fallopian tube induces hyperplasia and ovarian tumor formation. Oncogene. 2018;37:1976–1990. doi: 10.1038/s41388-017-0097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chu P, Liang A, Jiang A, Zong L. miR-205 regulates the proliferation and invasion of ovarian cancer cells via suppressing PTEN/SMAD4 expression. Oncol Lett. 2018;15:7571–7578. doi: 10.3892/ol.2018.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang J, Wu X. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9:8206–8220. doi: 10.7150/thno.37455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jiang JH, Lv QY, Yi YX, Liao J, Wang XW, Zhang W. MicroRNA-200a promotes proliferation and invasion of ovarian cancer cells by targeting PTEN. Eur Rev Med Pharmacol Sci. 2018;22:6260–6267. doi: 10.26355/eurrev_201810_16033. [DOI] [PubMed] [Google Scholar]
- 124.Hao B, Zhang J. miRNA-21 inhibition suppresses the human epithelial ovarian cancer by targeting PTEN signal pathway. Saudi J Biol Sci. 2019;26:2026–2029. doi: 10.1016/j.sjbs.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou HM, Sun QX, Cheng Y. Paeonol enhances the sensitivity of human ovarian cancer cells to radiotherapy-induced apoptosis due to downregulation of the phosphatidylinositol-3-kinase/Akt/phosphatase and tensin homolog pathway and inhibition of vascular endothelial growth factor. Exp Ther Med. 2017;14:3213–3220. doi: 10.3892/etm.2017.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rountree CB, Ding W, He L, Stiles B. Expansion of CD133-expressing liver cancer stem cells in liver-specific phosphatase and tensin homolog deleted on chromosome 10-deleted mice. Stem Cells. 2009;27:290–299. doi: 10.1634/stemcells.2008-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sze KM, Wong KL, Chu GK, Lee JM, Yau TO, Ng IO. Loss of phosphatase and tensin homolog enhances cell invasion and migration through AKT/Sp-1 transcription factor/matrix metalloproteinase 2 activation in hepatocellular carcinoma and has clinicopathologic significance. Hepatology. 2011;53:1558–1569. doi: 10.1002/hep.24232. [DOI] [PubMed] [Google Scholar]
- 128.Chen JS, Wang Q, Fu XH, Huang XH, Chen XL, Cao LQ, Chen LZ, Tan HX, Li W, Bi J, Zhang LJ. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: association with MMP-9. Hepatol Res. 2009;39:177–186. doi: 10.1111/j.1872-034X.2008.00449.x. [DOI] [PubMed] [Google Scholar]
- 129.Hu TH, Huang CC, Lin PR, Chang HW, Ger LP, Lin YW, Changchien CS, Lee CM, Tai MH. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer. 2003;97:1929–1940. doi: 10.1002/cncr.11266. [DOI] [PubMed] [Google Scholar]
- 130.Wu SK, Wang BJ, Yang Y, Feng XH, Zhao XP, Yang DL. Expression of PTEN, PPM1A and P-Smad2 in hepatocellular carcinomas and adjacent liver tissues. World J Gastroenterol. 2007;13:4554–4559. doi: 10.3748/wjg.v13.i34.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Y, He L, Zeng N, Sahu D, Cadenas E, Shearn C, Li W, Stiles BL. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) signaling regulates mitochondrial biogenesis and respiration via estrogen-related receptor α (ERRα) J Biol Chem. 2013;288:25007–25024. doi: 10.1074/jbc.M113.450353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu X, Qin X, Fei M, Hou W, Greshock J, Bachman KE, Wooster R, Kang J, Qin CY. Combined phosphatase and tensin homolog (PTEN) loss and fatty acid synthase (FAS) overexpression worsens the prognosis of Chinese patients with hepatocellular carcinoma. Int J Mol Sci. 2012;13:9980–9991. doi: 10.3390/ijms13089980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yin LR, Chen ZX, Zhang SJ, Sun BG, Liu YD, Huang HZ. Expression of phosphatase and tensin homolog deleted on chromosome ten in liver of athymic mice with hepatocellular carcinoma and the effect of Fuzheng Jiedu Decoction. World J Gastroenterol. 2008;14:108–113. doi: 10.3748/wjg.14.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li BH, Wang Y, Wang CY, Zhao MJ, Deng T, Ren XQ. Up-regulation of phosphatase in regenerating liver-3 (PRL-3) contributes to malignant progression of hepatocellular carcinoma by activating phosphatase and tensin homolog deleted on chromosome ten (PTEN)/phosphoinositide 3-kinase (PI3K)/AKT signaling pathway. Med Sci Monit. 2018;24:8105–8114. doi: 10.12659/MSM.913307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sun B, Meng J, Xiang T, Chen Z, Li Y, Lu L, Zhang S, Chen X. Jianpijiedu fang improves survival of hepatocarcinoma mice by affecting phosphatase and tensin homolog, phosphoinositide 3-kinase, and focal adhesion kinase. J Tradit Chin Med. 2013;33:479–485. doi: 10.1016/s0254-6272(13)60152-1. [DOI] [PubMed] [Google Scholar]
- 136.Liu H, Cheng L, Cao D, Zhang H. Suppression of miR-21 expression inhibits cell proliferation and migration of liver cancer cells by targeting phosphatase and tensin homolog (PTEN) Med Sci Monit. 2018;24:3571–3577. doi: 10.12659/MSM.907038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang X, He H, Lu Y, Ren W, Teng KY, Chiang CL, Yang Z, Yu B, Hsu S, Jacob ST, Ghoshal K, Lee LJ. Indole-3-carbinol inhibits tumorigenicity of hepatocellular carcinoma cells via suppression of microRNA-21 and upregulation of phosphatase and tensin homolog. Biochim Biophys Acta. 2015;1853:244–253. doi: 10.1016/j.bbamcr.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lin PC, Lin JK, Lin HH, Lan YT, Lin CC, Yang SH, Chen WS, Liang WY, Jiang JK, Chang SC. A comprehensive analysis of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) loss in colorectal cancer. World J Surg Oncol. 2015;13:186. doi: 10.1186/s12957-015-0601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fan D, Lin X, Zhang F, Zhong W, Hu J, Chen Y, Cai Z, Zou Y, He X, Chen X, Lan P, Wu X. MicroRNA 26b promotes colorectal cancer metastasis by downregulating phosphatase and tensin homolog and wingless-type MMTV integration site family member 5A. Cancer Sci. 2018;109:354–362. doi: 10.1111/cas.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Csolle MP, Ooms LM, Papa A, Mitchell CA. PTEN and other PtdIns(3,4,5)P(3) lipid phosphatases in breast cancer. Int J Mol Sci. 2020;21:9189. doi: 10.3390/ijms21239189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang X, Simpson ER, Brown KA. p53: protection against tumor growth beyond effects on cell cycle and apoptosis. Cancer Res. 2015;75:5001–5007. doi: 10.1158/0008-5472.CAN-15-0563. [DOI] [PubMed] [Google Scholar]
- 142.Ngeow J, Sesock K, Eng C. Breast cancer risk and clinical implications for germline PTEN mutation carriers. Breast Cancer Res Treat. 2017;165:1–8. doi: 10.1007/s10549-015-3665-z. [DOI] [PubMed] [Google Scholar]
- 143.Costa C, Wang Y, Ly A, Hosono Y, Murchie E, Walmsley CS, Huynh T, Healy C, Peterson R, Yanase S, Jakubik CT, Henderson LE, Damon LJ, Timonina D, Sanidas I, Pinto CJ, Mino-Kenudson M, Stone JR, Dyson NJ, Ellisen LW, Bardia A, Ebi H, Benes CH, Engelman JA, Juric D. PTEN loss mediates clinical cross-resistance to CDK4/6 and PI3Kα inhibitors in breast cancer. Cancer Discov. 2020;10:72–85. doi: 10.1158/2159-8290.CD-18-0830. [DOI] [PubMed] [Google Scholar]
- 144.Adamczyk A, Niemiec J, Janecka A, Harazin-Lechowska A, Ambicka A, Grela-Wojewoda A, Domagała-Haduch M, Cedrych I, Majchrzyk K, Kruczak A, Ryś J, Jakubowicz J. Prognostic value of PIK3CA mutation status, PTEN and androgen receptor expression for metastasis-free survival in HER2-positive breast cancer patients treated with trastuzumab in adjuvant setting. Pol J Pathol. 2015;66:133–141. doi: 10.5114/pjp.2015.53009. [DOI] [PubMed] [Google Scholar]
- 145.Xu F, Zhang C, Cui J, Liu J, Li J, Jiang H. The prognostic value and potential drug target of phosphatase and tensin homolog in breast cancer patients: a meta-analysis. Medicine (Baltimore) 2017;96:e8000. doi: 10.1097/MD.0000000000008000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Luo S, Chen J, Mo X. The association of PTEN hypermethylation and breast cancer: a meta-analysis. Onco Targets Ther. 2016;9:5643–5650. doi: 10.2147/OTT.S111684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Windarti I, Harahap WA, Nindrea RD, Yerizel E, Rustamadji P. The prognostic significance of phosphatase and tensin homolog loss in breast cancer. Open Access Maced J Med Sci. 2019;7:3716–3720. doi: 10.3889/oamjms.2019.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu W, Wang W. MicroRNA-142-5p modulates breast cancer cell proliferation and apoptosis by targeting phosphatase and tensin homolog. Mol Med Rep. 2018;17:7529–7536. doi: 10.3892/mmr.2018.8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang Z, Yuan XG, Chen J, Lu NH. Is NEDD4-1 a negative regulator of phosphatase and tensin homolog in gastric carcinogenesis? World J Gastroenterol. 2012;18:6345–6348. doi: 10.3748/wjg.v18.i43.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li M, Sun H, Song L, Gao X, Chang W, Qin X. Immunohistochemical expression of mTOR negatively correlates with PTEN expression in gastric carcinoma. Oncol Lett. 2012;4:1213–1218. doi: 10.3892/ol.2012.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bai ZG, Ye YJ, Shen DH, Lu YY, Zhang ZT, Wang S. PTEN expression and suppression of proliferation are associated with Cdx2 overexpression in gastric cancer cells. Int J Oncol. 2013;42:1682–1691. doi: 10.3892/ijo.2013.1875. [DOI] [PubMed] [Google Scholar]
- 152.Li Y, Cui J, Zhang CH, Yang DJ, Chen JH, Zan WH, Li B, Li Z, He YL. High-expression of DJ-1 and loss of PTEN associated with tumor metastasis and correlated with poor prognosis of gastric carcinoma. Int J Med Sci. 2013;10:1689–1697. doi: 10.7150/ijms.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu S, Tian Y, Zhu C, Yang X, Sun Q. High miR-718 suppresses phosphatase and tensin homolog (PTEN) expression and correlates to unfavorable prognosis in gastric cancer. Med Sci Monit. 2018;24:5840–5850. doi: 10.12659/MSM.909527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Oki E, Baba H, Tokunaga E, Nakamura T, Ueda N, Futatsugi M, Mashino K, Yamamoto M, Ikebe M, Kakeji Y, Maehara Y. Akt phosphorylation associates with LOH of PTEN and leads to chemoresistance for gastric cancer. Int J Cancer. 2005;117:376–380. doi: 10.1002/ijc.21170. [DOI] [PubMed] [Google Scholar]
- 155.Hwang PH, Kim SY, Lee JC, Kim SJ, Yi HK, Lee DY. PTEN/MMAC1 enhances the growth inhibition by anticancer drugs with downregulation of IGF-II expression in gastric cancer cells. Exp Mol Med. 2005;37:391–398. doi: 10.1038/emm.2005.49. [DOI] [PubMed] [Google Scholar]
- 156.Yu HG, Ai YW, Yu LL, Zhou XD, Liu J, Li JH, Xu XM, Liu S, Chen J, Liu F, Qi YL, Deng Q, Cao J, Liu SQ, Luo HS, Yu JP. Phosphoinositide 3-kinase/Akt pathway plays an important role in chemoresistance of gastric cancer cells against etoposide and doxorubicin induced cell death. Int J Cancer. 2008;122:433–443. doi: 10.1002/ijc.23049. [DOI] [PubMed] [Google Scholar]
- 157.Lee JT Jr, Steelman LS, McCubrey JA. Phosphatidylinositol 3’-kinase activation leads to multidrug resistance protein-1 expression and subsequent chemoresistance in advanced prostate cancer cells. Cancer Res. 2004;64:8397–8404. doi: 10.1158/0008-5472.CAN-04-1612. [DOI] [PubMed] [Google Scholar]
- 158.Esteva FJ, Guo H, Zhang S, Santa-Maria C, Stone S, Lanchbury JS, Sahin AA, Hortobagyi GN, Yu D. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177:1647–1656. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu JL, Wang ZW, Hu LM, Yin ZQ, Huang MD, Hu ZB, Shen HB, Shu YQ. Genetic variants in the PI3K/PTEN/AKT/mTOR pathway predict platinum-based chemotherapy response of advanced non-small cell lung cancers in a Chinese population. Asian Pac J Cancer Prev. 2012;13:2157–2162. doi: 10.7314/apjcp.2012.13.5.2157. [DOI] [PubMed] [Google Scholar]
- 160.Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW, Xiong Y, Tseng LM, Li SH, Ding Z, Sahin AA, Esteva FJ, Hortobagyi GN, Yu D. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17:461–469. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sun J, Li S, Wang F, Fan C, Wang J. Identification of key pathways and genes in PTEN mutation prostate cancer by bioinformatics analysis. BMC Med Genet. 2019;20:191. doi: 10.1186/s12881-019-0923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 163.Steelman LS, Bertrand FE, McCubrey JA. The complexity of PTEN: mutation, marker and potential target for therapeutic intervention. Expert Opin Ther Targets. 2004;8:537–550. doi: 10.1517/14728222.8.6.537. [DOI] [PubMed] [Google Scholar]
- 164.Wang Y, Romigh T, He X, Tan MH, Orloff MS, Silverman RH, Heston WD, Eng C. Differential regulation of PTEN expression by androgen receptor in prostate and breast cancers. Oncogene. 2011;30:4327–4338. doi: 10.1038/onc.2011.144. [DOI] [PubMed] [Google Scholar]
- 165.Seront E, Pinto A, Bouzin C, Bertrand L, Machiels JP, Feron O. PTEN deficiency is associated with reduced sensitivity to mTOR inhibitor in human bladder cancer through the unhampered feedback loop driving PI3K/Akt activation. Br J Cancer. 2013;109:1586–1592. doi: 10.1038/bjc.2013.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bruni P, Boccia A, Baldassarre G, Trapasso F, Santoro M, Chiappetta G, Fusco A, Viglietto G. PTEN expression is reduced in a subset of sporadic thyroid carcinomas: evidence that PTEN-growth suppressing activity in thyroid cancer cells mediated by p27kip1. Oncogene. 2000;19:3146–3155. doi: 10.1038/sj.onc.1203633. [DOI] [PubMed] [Google Scholar]
- 167.Wang X, Fan Y, Du Z, Fan J, Hao Y, Wang J, Wu X, Luo C. Knockdown of phospholipase Cε (PLCε) inhibits cell proliferation via phosphatase and tensin homolog deleted on chromosome 10 (PTEN)/AKT signaling pathway in human prostate cancer. Med Sci Monit. 2018;24:254–263. doi: 10.12659/MSM.908109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Guo J, Xiao Z, Yu X, Cao R. miR-20b promotes cellular proliferation and migration by directly regulating phosphatase and tensin homolog in prostate cancer. Oncol Lett. 2017;14:6895–6900. doi: 10.3892/ol.2017.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shen X, Cheng G, Xu L, Wu W, Chen Z, Du P. Jumonji AT-rich interactive domain 1B promotes the growth of pancreatic tumors via the phosphatase and tensin homolog/protein kinase B signaling pathway. Oncol Lett. 2018;16:267–275. doi: 10.3892/ol.2018.8618. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 170.Gao ZJ, Yuan WD, Yuan JQ, Yuan K, Wang Y. miR-486-5p functions as an oncogene by targeting PTEN in non-small cell lung cancer. Pathol Res Pract. 2018;214:700–705. doi: 10.1016/j.prp.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 171.Xia L, Song M, Sun M, Chen W, Yang C. miR-486 promotes Capan-2 pancreatic cancer cell proliferation by targeting phosphatase and tensin homolog deleted on chromosome 10 (PTEN) Front Genet. 2019;10:541. doi: 10.3389/fgene.2019.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ying H, Elpek KG, Vinjamoori A, Zimmerman SM, Chu GC, Yan H, Fletcher-Sananikone E, Zhang H, Liu Y, Wang W, Ren X, Zheng H, Kimmelman AC, Paik JH, Lim C, Perry SR, Jiang S, Malinn B, Protopopov A, Colla S, Xiao Y, Hezel AF, Bardeesy N, Turley SJ, Wang YA, Chin L, Thayer SP, DePinho RA. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-κB-cytokine network. Cancer Discov. 2011;1:158–169. doi: 10.1158/2159-8290.CD-11-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gu J, Wang D, Zhang J, Zhu Y, Li Y, Chen H, Shi M, Wang X, Shen B, Deng X, Zhan Q, Wei G, Peng C. GFRα2 prompts cell growth and chemoresistance through down-regulating tumor suppressor gene PTEN via Mir-17-5p in pancreatic cancer. Cancer Lett. 2016;380:434–441. doi: 10.1016/j.canlet.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 174.Wang L, Lv Y, Li G, Xiao J. MicroRNAs in heart and circulation during physical exercise. J Sport Health Sci. 2018;7:433–441. doi: 10.1016/j.jshs.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li W, Huang K, Wen F, Cui G, Guo H, He Z, Zhao S. LINC00184 silencing inhibits glycolysis and restores mitochondrial oxidative phosphorylation in esophageal cancer through demethylation of PTEN. EBioMedicine. 2019;44:298–310. doi: 10.1016/j.ebiom.2019.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 176.Kano Y, Konno M, Ohta K, Haraguchi N, Nishikawa S, Kagawa Y, Hamabe A, Hasegawa S, Ogawa H, Fukusumi T, Noguchi Y, Ozaki M, Kudo T, Sakai D, Satoh T, Ishii M, Mizohata E, Inoue T, Mori M, Doki Y, Ishii H. Jumonji/Arid1b (Jarid1b) protein modulates human esophageal cancer cell growth. Mol Clin Oncol. 2013;1:753–757. doi: 10.3892/mco.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu MX, Liao J, Xie M, Gao ZK, Wang XH, Zhang Y, Shang MH, Yin LH, Pu YP, Liu R. miR-93-5p transferred by exosomes promotes the proliferation of esophageal cancer cells via intercellular communication by targeting PTEN. Biomed Environ Sci. 2018;31:171–185. doi: 10.3967/bes2018.023. [DOI] [PubMed] [Google Scholar]
- 178.Zhang G, Hou X, Li Y, Zhao M. MiR-205 inhibits cell apoptosis by targeting phosphatase and tensin homolog deleted on chromosome ten in endometrial cancer Ishikawa cells. BMC Cancer. 2014;14:440. doi: 10.1186/1471-2407-14-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liang Y, Lin B, Ye Z, Chen S, Yu H, Chen C, Zhang X, Zhou K, Zeng J. Triple-high expression of phosphatase and tensin homolog (PTEN), estrogen receptor (ER) and progesterone receptor (PR) may predict favorable prognosis for patients with type I endometrial carcinoma. J Cancer. 2020;11:1436–1445. doi: 10.7150/jca.33720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ebrahimi S, Hosseini M, Shahidsales S, Maftouh M, Ferns GA, Ghayour-Mobarhan M, Hassanian SM, Avan A. Targeting the Akt/PI3K signaling pathway as a potential therapeutic strategy for the treatment of pancreatic cancer. Curr Med Chem. 2017;24:1321–1331. doi: 10.2174/0929867324666170206142658. [DOI] [PubMed] [Google Scholar]
- 181.Lee D, Yu EJ, Ham IH, Hur H, Kim YS. AKT inhibition is an effective treatment strategy in ARID1A-deficient gastric cancer cells. Onco Targets Ther. 2017;10:4153–4159. doi: 10.2147/OTT.S139664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Juric D, Rodon J, Tabernero J, Janku F, Burris HA, Schellens JHM, Middleton MR, Berlin J, Schuler M, Gil-Martin M, Rugo HS, Seggewiss-Bernhardt R, Huang A, Bootle D, Demanse D, Blumenstein L, Coughlin C, Quadt C, Baselga J. Phosphatidylinositol 3-kinase α-selective inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors: results from the first-in-human study. J. Clin. Oncol. 2018;36:1291–1299. doi: 10.1200/JCO.2017.72.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Peng X, Zhou J, Li B, Zhang T, Zuo Y, Gu X. Notch1 and PI3K/Akt signaling blockers DAPT and LY294002 coordinately inhibit metastasis of gastric cancer through mutual enhancement. Cancer Chemother Pharmacol. 2020;85:309–320. doi: 10.1007/s00280-019-03990-4. [DOI] [PubMed] [Google Scholar]
- 184.Roncolato F, Lindemann K, Willson ML, Martyn J, Mileshkin L. PI3K/AKT/mTOR inhibitors for advanced or recurrent endometrial cancer. Cochrane Database Syst Rev. 2019;10:CD012160. doi: 10.1002/14651858.CD012160.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Uko NE, Güner OF, Matesic DF, Bowen JP. Akt pathway inhibitors. Curr Top Med Chem. 2020;20:883–900. doi: 10.2174/1568026620666200224101808. [DOI] [PubMed] [Google Scholar]
- 186.Ihara M, Shichijo K, Takeshita S, Kudo T. Wortmannin, a specific inhibitor of phosphatidylinositol-3-kinase, induces accumulation of DNA double-strand breaks. J Radiat Res. 2020;61:171–176. doi: 10.1093/jrr/rrz102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang Y, Kuramitsu Y, Baron B, Kitagawa T, Tokuda K, Akada J, Maehara SI, Maehara Y, Nakamura K. PI3K inhibitor LY294002, as opposed to wortmannin, enhances AKT phosphorylation in gemcitabine-resistant pancreatic cancer cells. Int J Oncol. 2017;50:606–612. doi: 10.3892/ijo.2016.3804. [DOI] [PubMed] [Google Scholar]
- 188.Gomes AM, Pinto TS, da Costa Fernandes CJ, da Silva RA, Zambuzzi WF. Wortmannin targeting phosphatidylinositol 3-kinase suppresses angiogenic factors in shear-stressed endothelial cells. J Cell Physiol. 2020;235:5256–5269. doi: 10.1002/jcp.29412. [DOI] [PubMed] [Google Scholar]
- 189.Zhao S, Pi C, Ye Y, Zhao L, Wei Y. Recent advances of analogues of curcumin for treatment of cancer. Eur J Med Chem. 2019;180:524–535. doi: 10.1016/j.ejmech.2019.07.034. [DOI] [PubMed] [Google Scholar]
- 190.Yang C, Sheng Y, Shi X, Liu Y, He Y, Du Y, Zhang G, Gao F. CD44/HA signaling mediates acquired resistance to a PI3Kα inhibitor. Cell Death Dis. 2020;11:831. doi: 10.1038/s41419-020-03037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Garrido-Castro AC, Saura C, Barroso-Sousa R, Guo H, Ciruelos E, Bermejo B, Gavilá J, Serra V, Prat A, Paré L, Céliz P, Villagrasa P, Li Y, Savoie J, Xu Z, Arteaga CL, Krop IE, Solit DB, Mills GB, Cantley LC, Winer EP, Lin NU, Rodon J. Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2020;22:120. doi: 10.1186/s13058-020-01354-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zirlik K, Veelken H. Idelalisib. Recent Results Cancer Res. 2018;212:243–264. doi: 10.1007/978-3-319-91439-8_12. [DOI] [PubMed] [Google Scholar]
- 193.Krause G, Hassenrück F, Hallek M. Copanlisib for treatment of B-cell malignancies: the development of a PI3K inhibitor with considerable differences to idelalisib. Drug Des Devel Ther. 2018;12:2577–2590. doi: 10.2147/DDDT.S142406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Karagul MI, Aktas S, Yilmaz SN, Yetkin D, Celikcan HD, Cevik OS. Perifosine and vitamin D combination induces apoptotic and non-apoptotic cell death in endometrial cancer cells. EXCLI J. 2020;19:532–546. doi: 10.17179/excli2019-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tołoczko-Iwaniuk N, Dziemiańczyk-Pakieła D, Nowaszewska BK, Celińska-Janowicz K, Miltyk W. Celecoxib in cancer therapy and prevention-review. Curr Drug Targets. 2019;20:302–315. doi: 10.2174/1389450119666180803121737. [DOI] [PubMed] [Google Scholar]
- 196.Altomare DA, Zhang L, Deng J, Di Cristofano A, Klein-Szanto AJ, Kumar R, Testa JR. GSK690693 delays tumor onset and progression in genetically defined mouse models expressing activated Akt. Clin Cancer Res. 2010;16:486–496. doi: 10.1158/1078-0432.CCR-09-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang Y, Ma W, Zheng W. Deguelin, a novel anti-tumorigenic agent targeting apoptosis, cell cycle arrest and anti-angiogenesis for cancer chemoprevention. Mol Clin Oncol. 2013;1:215–219. doi: 10.3892/mco.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xing Y, Lin NU, Maurer MA, Chen H, Mahvash A, Sahin A, Akcakanat A, Li Y, Abramson V, Litton J, Chavez-MacGregor M, Valero V, Piha-Paul SA, Hong D, Do KA, Tarco E, Riall D, Eterovic AK, Wulf GM, Cantley LC, Mills GB, Doyle LA, Winer E, Hortobagyi GN, Gonzalez-Angulo AM, Meric-Bernstam F. Phase II trial of AKT inhibitor MK-2206 in patients with advanced breast cancer who have tumors with PIK3CA or AKT mutations, and/or PTEN loss/PTEN mutation. Breast Cancer Res. 2019;21:78. doi: 10.1186/s13058-019-1154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Babiker HM, Karass M, Recio-Boiles A, Chandana SR, McBride A, Mahadevan D. Everolimus for the treatment of advanced pancreatic ductal adenocarcinoma (PDAC) Expert Opin Investig Drugs. 2019;28:583–592. doi: 10.1080/13543784.2019.1632289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chen Z, Yang H, Li Z, Xia Q, Nie Y. Temsirolimus as a dual inhibitor of retinoblastoma and angiogenesis via targeting mTOR signalling. Biochem Biophys Res Commun. 2019;516:726–732. doi: 10.1016/j.bbrc.2019.06.127. [DOI] [PubMed] [Google Scholar]
- 201.Mita M, Sankhala K, Abdel-Karim I, Mita A, Giles F. Deforolimus (AP23573) a novel mTOR inhibitor in clinical development. Expert Opin Investig Drugs. 2008;17:1947–1954. doi: 10.1517/13543780802556485. [DOI] [PubMed] [Google Scholar]
- 202.Bethesda (MD): National Library of Medicine (US); 2006. Copanlisib. Drugs and lactation database (LactMed) [Google Scholar]
- 203.Moschetta MG, Leonel C, Maschio-Signorini LB, Borin TF, Gelaleti GB, Jardim-Perassi BV, Ferreira LC, Sonehara NM, Carvalho LGS, Hellmén E, de Campos Zuccari DAP. Evaluation of angiogenesis process after metformin and LY294002 treatment in mammary tumor. Anticancer Agents Med Chem. 2019;19:655–666. doi: 10.2174/1871520619666181218164050. [DOI] [PubMed] [Google Scholar]
- 204.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 205.Lee CU, Hahne G, Hanske J, Bange T, Bier D, Rademacher C, Hennig S, Grossmann TN. Redox modulation of PTEN phosphatase activity by hydrogen peroxide and bisperoxidovanadium complexes. Angew Chem Int Ed Engl. 2015;54:13796–13800. doi: 10.1002/anie.201506338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pulido R. PTEN inhibition in human disease therapy. Molecules. 2018;23:285. doi: 10.3390/molecules23020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fu HY, Shen L, Gao XS, Cui DX, Cui ZY. SF1670 inhibits apoptosis and inflammation via the PTEN/Akt pathway and thus protects intervertebral disc degeneration. Eur Rev Med Pharmacol Sci. 2020;24:8694–8702. doi: 10.26355/eurrev_202009_22806. [DOI] [PubMed] [Google Scholar]
- 208.Farajdokht F, Mohaddes G, Karimi-Sales E, Kafshdooz T, Mahmoudi J, Aberoumandi SM, Karimi P. Inhibition of PTEN protects PC12 cells against oxygen-glucose deprivation induced cell death through mitoprotection. Brain Res. 2018;1692:100–109. doi: 10.1016/j.brainres.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 209.Li A, Qiu M, Zhou H, Wang T, Guo W. PTEN, insulin resistance and cancer. Curr Pharm Des. 2017;23:3667–3676. doi: 10.2174/1381612823666170704124611. [DOI] [PubMed] [Google Scholar]
- 210.Heinrich F, Chakravarthy S, Nanda H, Papa A, Pandolfi PP, Ross AH, Harishchandra RK, Gericke A, Lösche M. The PTEN tumor suppressor forms homodimers in solution. Structure. 2015;23:1952–1957. doi: 10.1016/j.str.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Malaney P, Uversky VN, Davé V. PTEN proteoforms in biology and disease. Cell Mol Life Sci. 2017;74:2783–2794. doi: 10.1007/s00018-017-2500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.VanderLaan PA, Rangachari D, Mockus SM, Spotlow V, Reddi HV, Malcolm J, Huberman MS, Joseph LJ, Kobayashi SS, Costa DB. Mutations in TP53, PIK3CA, PTEN and other genes in EGFR mutated lung cancers: correlation with clinical outcomes. Lung Cancer. 2017;106:17–21. doi: 10.1016/j.lungcan.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kong FR, Lv YH, Yao HM, Zhang HY, Zhou Y, Liu SE. LncRNA PCAT6 promotes occurrence and development of ovarian cancer by inhibiting PTEN. Eur Rev Med Pharmacol Sci. 2019;23:8230–8238. doi: 10.26355/eurrev_201910_19132. [DOI] [PubMed] [Google Scholar]
- 214.Li W, Jiang Y, Wu X, Yang F. Targeted regulation of miR-26a on PTEN to affect proliferation and apoptosis of prostate cancer cells. Cancer Biother Radiopharm. 2019;34:480–485. doi: 10.1089/cbr.2018.2664. [DOI] [PubMed] [Google Scholar]
- 215.Zhang B, Zhang X, Jin M, Hu L, Zang M, Qiu W, Wang S, Liu B, Liu S, Guo D. CagA increases DNA methylation and decreases PTEN expression in human gastric cancer. Mol Med Rep. 2019;19:309–319. doi: 10.3892/mmr.2018.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]