Abstract
Delirium is a serious and complex problem facing critically ill patients, their families, and the health care system. When delirium develops, it is associated with prolonged hospital stays, increased costs, and long-term cognitive impairment in many patients. This article uses a clinical case to discuss our approach to delirium prevention and treatment in the ICU. We believe that an effective strategy to combat delirium requires implementation and adherence to a pain and sedation protocol as part of bundled care, use of a validated tool to detect delirium when present, and a focus on nonpharmacologic care strategies, including reorientation, early mobility, and incorporating family into care when possible. At present, the evidence does not support the routine administration of medications to prevent or treat delirium. A pharmacologic approach may be needed for agitated delirium, and we discuss our evaluation of the evidence for and against particular medications. Although delirium can be a distressing problem, there is evidence that it can be addressed through careful attention to prevention, detection, and minimizing the long-term impact on patients and their families.
Key Words: critical care, delirium, implementation
Abbreviations: CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; RCT, randomized controlled trial
Delirium, a form of acute brain dysfunction, is a common and complex problem in the ICU. It is characterized by an acute change or fluctuation in mentation, inattention, and either disorganized thinking or an altered level of consciousness.1 Cohort studies have found that delirium is present in 60% to 80% of patients who are mechanically ventilated and 20% to 50% of nonventilated patients.2, 3, 4, 5, 6 It is independently associated with increased mortality, prolonged duration of mechanical ventilation, and longer ICU and hospital stays.3,7,8 Each additional day with delirium is associated with a 20% increased risk of prolonged hospitalization, with an annual cost estimated to be more than US $164 billion.9 The impact is not limited to the hospital stay, for delirium is associated with a cascade of long-term cognitive impairment, functional decline, and caregiver burden.
Although ICU delirium has been a growing focus of research and quality improvement efforts over the past 30 years, it remains a significant burden for patients, their families, and the health care system. The pathophysiology is poorly understood, and its detection and diagnosis require sustained efforts at training bedside nurses and physicians. Effective prevention and treatment strategies are available to target delirium in the ICU; they require dedicated attention from the ICU leadership team and a multipronged approach. Let us consider the following case example.
Case Example
A 72-year-old woman is admitted to the ICU overnight with sepsis and moderate ARDS secondary to bacterial pneumonia. She is intubated and receiving lung-protective ventilation as well as broad-spectrum antimicrobial therapy. Her oxygenation has continued to improve over the course of the night, and she remains off vasopressor medications in the morning. She is receiving a continuous infusion of fentanyl. On your examination, she opens her eyes briefly to voice but does not make eye contact. Her daughter is in the room, and she informs you that her mother has a history of mild cognitive impairment and diabetes.
Given the patient’s age, comorbid conditions, and critical illness, you are concerned that she is at risk for developing delirium during her ICU stay. During multidisciplinary rounds, you discuss with your team targeting light sedation (Richmond Agitation-Sedation Scale score of –1 to +1) and performing a spontaneous awakening and breathing trial that morning. The bedside nurse discusses a goal for early mobilization with the multidisciplinary team. You are hopeful the patient may be able to sit on the edge of the bed today. You ask her daughter (present on rounds) to stay for the day and discuss with her what delirium may look like in her mother.
Components to a Delirium Strategy
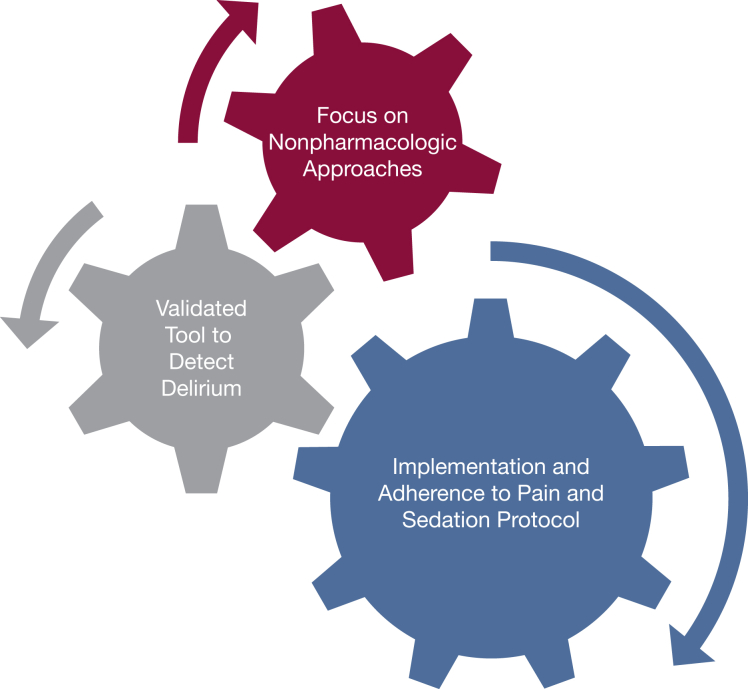
Developing an effective prevention and treatment strategy targeting delirium requires a multipronged approach at both the ICU and individual patient level. We believe the following components are critical to any strategy: (1) implementing and adhering to a pain and sedation protocol in your ICU; (2) using a validated tool to detect delirium; and (3) and focusing on nonpharmacologic interventions first (Fig 1). At present, the evidence does not support starting medications (eg, antipsychotic agents) to prevent delirium or to treat hypoactive delirium when present. We do acknowledge, however, that a pharmacologic strategy may be needed to treat agitated delirium when symptoms are distressing to the patient or are affecting care.
Figure 1.
Components of an effective delirium prevention and management strategy.
Implementation and Adherence to an Interdisciplinary Pain, Agitation, and Sedation Protocol
The backbone of an effective delirium prevention and treatment strategy is an ICU culture of adherence to pain and sedation protocols and an emphasis on nonpharmacologic strategies targeting delirium.
In our experience, implementation and adherence to a pain, agitation, and sedation protocol is the first and most important step in both preventing and treating ICU delirium. The key ingredients are: (1) routine assessment of pain and agitation; (2) setting a target for sedation using a validated scoring system; and (3) involving the multidisciplinary team. An example of this approach is the ICU Liberation Bundle (also referred to as the ABCDE and, more recently, the ABCDEF bundle), which includes assessing pain regularly using standardized tools, performing both spontaneous awakening and breathing trials, targeting a depth of sedation and choosing the right medication, assessing delirium, focusing on early mobility, and involving the family in patient care.10,11 Both analyses that established the value of coordinating awakening and breathing trials and early mobilization in routine ICU care included routine daily delirium assessments.12,13 In fact, early mobility in the ICU is associated with a significant reduction in delirium duration when it is combined with a daily interruption of sedatives.12 Implementation of the ABCDEF bundle is associated with less delirium as well as significant and meaningful improvements in likelihood of surviving, having less coma and physical restraint use, being liberated from mechanical ventilation, and being discharged home.14,15 A systematic review by Trogrlić et al16 concluded that delirium screening embedded in a larger bundled intervention is more likely to positively affect clinical outcomes, stating “it is the circumstances leading to or sustaining brain dysfunction that should be dealt with in the first place.” Despite the known benefits of bundle implementation, evidence suggests that these may not be routinely used in clinical care.17 In an 18-month interprofessional study that implemented the ABCDE bundle into everyday care in multiple ICUs, the following factors were identified as facilitators to successful bundle implementation: daily interdisciplinary rounds, the use of standardized delirium and screening instruments, and intense and sustained education.11
Although it may not be possible to distill the distinct component(s) of the ABCDEF bundle that are most beneficial regarding delirium prevention, the relationship between sedation and delirium deserves particular attention. Evidence that targeting light sedation reduces the incidence of delirium is conflicting; however, light sedation is associated with reduced time to extubation and a decreased rate of tracheostomy.18 The Society of Critical Care Medicine’s Clinical Practice Guidelines for the Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU (published most recently in 2018) recommend targeting “light sedation,” which, in most studies, was defined as a Richmond Agitation-Sedation Scale score of –2 to +1 or its equivalent using other scales. We agree that light sedation should be the default target in the ICU, not the exception; some restlessness can be tolerated as long as the patient is not overtly agitated, uncomfortable, or unsafe. In those patients with ARDS, as with our case example, managing the respiratory drive can be considered first before increasing the depth of sedation. This may involve optimizing mechanical ventilator strategies to target patient ventilator synchrony or treating fever or acidosis if present.19 In other patients, such as postoperative patients, a strategy of immediate interruption of sedation may be the most appropriate.20
Use a Validated Tool to Detect Delirium
Delirium remains a clinical diagnosis, and one that can be easily overlooked by the health care team.21,22 Validated tools exist to detect delirium in ICU patients. The Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) and the Intensive Care Delirium Screening Checklist are the most widely studied and used.23,24 Implementing screening with a validated tool is crucial to any delirium prevention and management strategy. Given the high incidence of delirium in critically ill patients, it is important to address with the ICU team why specific recognition of this diagnosis is important for patient care. Is delirium just expected for ICU patients? Furthermore, without a specific medication to treat delirium, why does diagnosing it matter?
First, delirium may be the first presentation of a new, treatable ICU complication such as an infection, electrolyte abnormality, or medication side effect. The presence of new delirium should prompt an investigation for a precipitating cause. Second, screening for delirium is a key component of the ABCDEF care bundle, and implementing routine delirium assessments with spontaneous awakening trials and early mobility efforts has been shown to increase the mobility achieved for patients compared with performing just awakening trials and early mobility without delirium screening. As noted by Miller et al,25 “With respect to the ABCDE bundle, the whole truly is greater than the sum of its individual parts.” Finally, knowing the prevalence of delirium in an ICU is important for changing the local culture to focus on its prevention.
Focus on Nonpharmacologic Approaches
A combination of risk factors likely interact to increase the vulnerability to delirium, and reducing the number or severity of these factors may help prevent delirium or mitigate its effects when present.26,27 There is moderate to strong evidence that multicomponent interventions targeting several delirium risk factors concurrently are effective in preventing delirium in hospitalized, non-ICU patients.28 A Cochrane systematic review found that multicomponent, nonpharmacologic interventions reduced the incidence of delirium compared with usual care (relative risk, 0.69; 95% CI, 0.59-0.81; seven studies, moderate-quality evidence).26 Nonpharmacologic interventions targeting one or more of these risk factors are most effective when implemented together.28 Examples of the interventions studied included activities such as: providing clocks, introducing cognitively stimulating exercises such as reminiscing, encouraging early mobilization, providing vision and hearing aids if needed, preserving uninterrupted sleep by minimizing nighttime nursing interventions and laboratory draws, and carefully reviewing medications.26,28 The American Geriatrics Society and the American College of Surgeons released clinical practice guidelines for the prevention and treatment of postoperative delirium in 2014. They recommend that multicomponent, nonpharmacologic delirium interventions be delivered to older at-risk adults, including mobility and walking, avoiding physical restraints, orienting to surroundings, sleep hygiene, and nutrition.29 These interventions, implemented and monitored by an interdisciplinary team, reduced the incidence of delirium by 30% to 40% in hospitalized older adults.27,30, 31, 32 Although the evidence is weaker for the management of delirium, a majority of studies suggested a benefit.29
The impact of multicomponent, nonpharmacologic interventions in preventing and treating delirium in the ICU is less clear. A 2018 Cochrane review of interventions for preventing ICU delirium in adults found the quality of evidence to be moderate to very low.23 The conclusion of the review was that more research is needed to explore the benefits and harms of nonpharmacologic interventions to prevent delirium (eg, changes in the ICU environment and nursing care tailored to prevent delirium). A systematic review by Rivosecchi et al33 also concluded that the evidence was in favor of a nonpharmacologic, multicomponent delirium prevention and treatment strategy. The 2018 Society of Critical Care Medicine’s Pain, Agitation, Delirium, Immobility, and Sleep guidelines do recommend using a multicomponent, nonpharmacologic intervention that is focused on reducing modifiable risk factors of delirium, improving cognition, and optimizing sleep, mobility, hearing, and vision (conditional recommendation, low quality of evidence).18 However, a more recent (2019) systematic review of 15 trials concluded that the current evidence does not support the use of nonpharmacologic interventions.34 The nonpharmacologic interventions included in this meta-analysis were diverse and not all multicomponent (eg, interventions included earplugs or bright light therapy), which may in part explain the negative results.
Overall, there are very few rigorous studies to date evaluating preventative nurse care and environmental interventions to prevent ICU delirium. Moon and Lee35 tested an ICU delirium-preventative nursing care protocol (including orientation, aiding visual and hearing impairments, prioritizing sleep and reducing pain, and reducing noise and bright lights) vs standard nursing care and found no effect on the event rate of ICU delirium, mortality, or length of stay; the study was at risk for cross-contamination that may have diluted the effect of the intervention. Kamdar et al36 conducted a pre-post observational study of 300 medical ICU patients. The nonpharmacologic interventions included environmental measures to promote sleep such as providing earplugs and eye masks and minimizing overhead paging practices combined with mobilization; the authors reported a 20% reduction in incidence of delirium or coma and 5% increase in delirium-free or coma-free days. Several clinical trials are ongoing in this area that will provide additional evidence on the benefits or harms of multicomponent, nonpharmacologic interventions in ICU patients.37,38,39
Despite limited evidence, in a well-resourced ICU with a strong existing culture of adherence to spontaneous awakening and breathing trials, delirium screening, and early mobilization, it is reasonable to include additional nonpharmacologic interventions targeting prevention and treatment. There is insufficient evidence to recommend specific components to include in such a bundle. One approach may be the one taken by Rivosecchi et al33 in a quality improvement project at the University of Pittsburgh Medical Center, Presbyterian Hospital. In this study, the authors evaluated the current components already in place in their ICU (eg, noise reduction, avoiding restraints, clock in room, mobilization) and identified additional components included in other bundles that may be feasible, such as opening of blinds, providing music and a calendar, cognitive stimulation, and a delirium prevention kit that included earplugs. The authors emphasized the multidisciplinary approach to both the creation of the intervention and in carrying out its components as key ingredients to their success. We agree that implementation of a multicomponent delirium care bundle on top of the ABCDEF bundle may help foster a culture of multidisciplinary collaboration and additional attention and focus to delirium. Attention must be paid that resources are not diverted away from other evidence-based practices shown to have an impact (eg, awakening and breathing trials, early mobilization).12,13
Return to Case Example
Let us return to the 72-year-old woman with moderate ARDS. During the spontaneous awakening and breathing trial that morning, the bedside nurse administers the CAM-ICU. Your patient displays a fluctuating course of mental status, agitation, and inattention. She is CAM-ICU positive. The results of this positive CAM-ICU prompt your team to take several actions. First, you add a diagnosis of delirium to her problem list. The acknowledgment of delirium by the treating team of clinicians is important, for it reinforces to the multidisciplinary team the importance of delirium screening and detection in your ICU. It prompts a discussion about inciting causes and modifiable risk factors. You review laboratory test results to ensure there are no new electrolyte disturbances or signs of new infection. Finally, the acknowledgment results in additional focus on nonpharmacologic approaches to the prevention and treatment of delirium. The blinds are opened during the day, and noise is reduced at night; the patient has her glasses on, and a clock and calendar are visible from her bed. Restraints are minimized, and the television is turned off. She is prioritized for early mobility by physical therapy. You ask her daughter to reorient her throughout the day and discuss things familiar to the patient, including recent events and family stories. She remains CAM-ICU positive throughout the day but now calm. During the evening shift, the patient has periods of extreme agitation. You consider extubation at this point but she now requires increased oxygen support. Her agitation persists, and you are asked about pharmacologic interventions to treat her delirium.
Suggested Approach to Pharmacologic Management for Agitated Delirium
No pharmacologic intervention has been consistently shown to prevent delirium or reduce the duration of delirium when present.9,23 Despite conflicting evidence and studies in noncritically ill patients identifying significant adverse effects, antipsychotic agents remain the most common treatment for ICU delirium.9,40 At present, results of randomized controlled trials (RCTs) do not support the routine use of antipsychotic agents for the prevention or treatment of delirium in the ICU. Page et al41 tested early treatment with IV haloperidol 2.5 mg every 8 hours initiated within 72 h of ICU admission in 142 participants (Haloperidol Effectiveness in ICU Delirum [HOPE-ICU]) and found no difference in number of days alive without delirium in the haloperidol group compared with the placebo group. van den Boogaard et al42 evaluated prophylactic haloperidol three times daily intravenously in a three-arm, parallel RCT (Prophylactic Haloperidol Use for Delirium in ICU Patients with a High Risk for Delirium [REDUCE]). In this study, the use of prophylactic haloperidol compared with placebo did not improve survival at 28 days (primary outcome) or the secondary outcome of delirium incidence. Both studies failed to show a difference in ventilator-free days.41,42 A double-blind, placebo-controlled trial of haloperidol, ziprasidone, or placebo in 566 ICU patients with respiratory failure or shock did not alter the duration of delirium in the Modifying the Impact of ICU-Induced Neurological Dysfunction-USA (MIND-USA) study.43 It is important to note, however, that the majority of patients (89%) in the MIND-USA study developed hypoactive delirium, and the results may not be generalizable to the subset of patients with hyperactive delirium. However, there was no evidence of reduction in median number of days of hyperactive delirium in groups randomized to receive either haloperidol or ziprasidone compared with placebo (in all three groups: median days, 0; interquartile range, 0-1 day).
Burry et al9 synthesized the results of RCTs, including quasi-RCTs, of pharmacologic treatments of delirium in critically ill adults for the Cochrane Review Library. Their review included 14 trials that examined the following: antipsychotic agents (n = 10), alpha2-agonists (n = 3 [all dexmedetomidine], statins (n = 2), and opioids, serotonin agonists, and cholinesterase inhibitors (all, n = 1). There was no evidence of a difference between placebo and any drug in terms of delirium-free days, days with coma, physical restraint use, length of stay, long-term cognitive outcomes, or mortality. The authors concluded that dexmedetomidine (a selective alpha2-adrenoreceptor agonist with sedative, anxiolytic, and analgesic properties) may shorten delirium duration, although this small effect was based on a single study of 71 participants. The study was a double-blind, parallel-group, placebo-controlled RCT of dexmedetomidine vs placebo in intubated patients with agitated delirium.44 The results showed that addition of dexmedetomidine to standard care resulted in more ventilator-free hours at 7 days. Of note, this study screened > 21,000 admissions to enroll 74 participants and may not be generalizable to the majority of ICU patients with delirium.
The 2018 Pain, Agitation, Delirium, Immobility, and Sleep Guidelines from the Society of Critical Care Medicine recommend against using haloperidol, an atypical antipsychotic, a statin, or ketamine to prevent delirium in all critically ill adults (conditional recommendation, very low to low quality of evidence).18 Single, randomized studies have shown a reduction in delirium incidence in surgical populations with these therapies,45, 46, 47 but given the absence of a significant impact on other important outcomes and the relative low severity of illness in the groups studied, the panel did not believe that the potential risks and costs of exposing a large proportion of critically ill patients to these medications outweighed the benefits. The panel similarly recommended against routine use of haloperidol, an atypical antipsychotic, or a statin as treatment for delirium when symptoms developed.18 The authors did acknowledge that patients experiencing significant distress secondary to delirium symptoms (eg, anxiety, hallucinations, delusions) or at risk of physically harming themselves or others may benefit from short-term use of an antipsychotic. The guidelines issue a conditional recommendation for the use of dexmedetomidine to treat delirium when agitation precludes weaning or extubation.
Four semi-systematic reviews (defined as not fulfilling two or more of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria)48 and a subgroup analysis (including two trials and a total of 415 patients) in a semi-systematic review found evidence of a beneficial effect of dexmedetomidine compared with alternate sedatives.49, 50, 51, 52, 53 Conversely, a systematic review assessing the effect of prophylactic use of alpha2-agonists compared with alternative sedatives on the subsequent risk of delirium found no evidence of an effect.54 Barbateskovic et al55 published a systematic overview of reviews and meta-analyses addressing pharmacologic interventions for both the prevention and treatment of delirium in ICUs. After evaluating 378 reviews, the authors concluded that the evidence for the use of pharmacologic interventions for the prevention or treatment of delirium is “poor to sparse.”
At present, we cannot make a strong recommendation in support of any pharmacologic treatment targeting the prevention or treatment of delirium. As ICU physicians, we recognize there is a subset of patients that develop significant symptoms of distress despite adequate pain control such as severe agitation, anxiety, hallucinations, or delusions. In those patients, we are often pressed to use a pharmacologic intervention. Based on the current evidence, we think it is reasonable to trial dexmedetomidine in intubated patients. Although we cannot make a strong argument in support of any particular agent, we can recommend against the routine use of benzodiazepines. Benzodiazepine administration, particularly in the form of a continuous infusion, has been shown to increase the risk of delirium in critically ill patients.56, 57, 58, 59 We also recommend that if a medication is started to treat delirium, it should be discontinued when no longer needed and prior to discharge in all patients.
Return to Case Example
You assess your patient with agitated delirium at the bedside. You adjust the ventilator to target patient ventilator synchrony, with some modest improvement in her oxygenation. You determine it is now her agitation that is precluding her extubation. You start her on dexmedetomidine, and she is calm and compliant with the ventilator. The next morning, you are able to extubate her successfully on dexmedetomidine and discontinue it later that morning. She continues to struggle with delirium over the next 48 h, and you continue to emphasize nonpharmacologic interventions to the team. The patient does improve and is stable for transfer to the floor. Prior to transfer, you discuss with the patient’s daughter that she may benefit from close follow-up and cognitive screening following discharge.
Conclusions
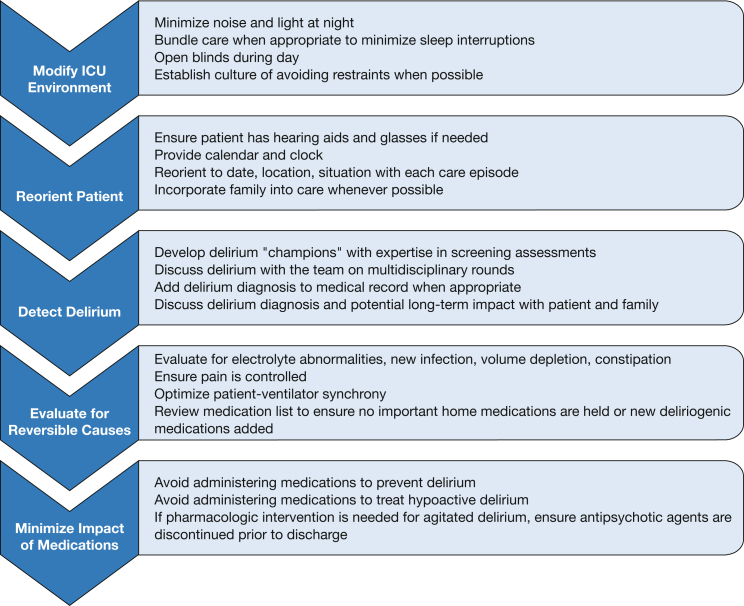
Delirium remains a distressing problem for critically ill patients, their families, and the ICU team. As treating clinicians, we understand the frustration that delirium can cause for all members of the team. Detecting it requires routine screening and sustained efforts at education and training. Despite decades of research and numerous RCTs, we do not yet have an effective pharmacologic therapy to prevent delirium or shorten its duration for those who develop it. Despite these challenges, we remain optimistic. We do have effective strategies to reduce the burden of delirium, including adequate treatment of pain, avoidance of oversedation, and early mobilization (Fig 2). We can ensure that patients have their glasses and hearing aids, their blinds open, and their families present when possible. We can reorient them each time we enter the room and ask them about their past. We can foster a culture of multidisciplinary collaboration critical to preventing, detecting, and treating delirium and its complications.
Figure 2.
Recommendations to reduce the burden of ICU delirium.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: Dr Hough is supported by National Institutes of Health grant [K24HL141526, PI].
References
- 1.American Psychiatric Association . American Psychiatric Society; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. [Google Scholar]
- 2.Ely E.W., Inouye S.K., Bernard G.R., et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 3.Ely E.W., Gautam S., Margolin R., et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois M.J., Bergeron N., Dumont M., Dial S., Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27:1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 5.Rudolph J.L., Jones R.N., Levkoff S.E., et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119:229–236. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Rompaey B., Elseviers M.M., Schuurmans M.J., Shortridge-Baggett L.M., Truijen S., Bossaert L. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care. 2009;13:R77. doi: 10.1186/cc7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ely E.W., Shintani A., Truman B., et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 8.Pisani M.A., Kong S.Y., Kasl S.V., Murphy T.E., Araujo K.L., Van Ness P.H. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burry L., Hutton B., Williamson D.R., et al. Pharmacological interventions for the treatment of delirium in critically ill adults. Cochrane Database Syst Rev. 2019;9:CD011749. doi: 10.1002/14651858.CD011749.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandharipande P., Banerjee A., McGrane S., Ely E.W. Liberation and animation for ventilated ICU patients: the ABCDE bundle for the back-end of critical care. Crit Care. 2010;14:157. doi: 10.1186/cc8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balas M.C., Vasilevskis E.E., Burke W.J., et al. Critical care nurses' role in implementing the "ABCDE bundle" into practice. Crit Care Nurse. 2012;32:35–38. doi: 10.4037/ccn2012229. 40-47; quiz 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweickert W.D., Pohlman M.C., Pohlman A.S., et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girard T.D., Kress J.P., Fuchs B.D., et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 14.Barnes-Daly M.A., Phillips G., Ely E.W. Improving hospital survival and reducing brain dysfunction at seven California community hospitals: implementing PAD guidelines via the ABCDEF bundle in 6,064 patients. Crit Care Med. 2017;45:171–178. doi: 10.1097/CCM.0000000000002149. [DOI] [PubMed] [Google Scholar]
- 15.Pun B.T., Balas M.C., Barnes-Daly M.A., et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med. 2019;47:3–14. doi: 10.1097/CCM.0000000000003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trogrlić Z., van der Jagt M., Bakker J., et al. A systematic review of implementation strategies for assessment, prevention, and management of ICU delirium and their effect on clinical outcomes. Crit Care. 2015;19:157. doi: 10.1186/s13054-015-0886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luetz A., Balzer F., Radtke F.M., et al. Delirium, sedation and analgesia in the intensive care unit: a multinational, two-part survey among intensivists. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devlin J.W., Skrobik Y., Gélinas C., et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 19.Chanques G., Constantin J.M., Devlin J.W., et al. Analgesia and sedation in patients with ARDS. Intensive Care Med. 2020;46:2342–2356. doi: 10.1007/s00134-020-06307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chanques G., Conseil M., Roger C., et al. Immediate interruption of sedation compared with usual sedation care in critically ill postoperative patients (SOS-Ventilation): a randomised, parallel-group clinical trial. Lancet Respir Med. 2017;5:795–805. doi: 10.1016/S2213-2600(17)30304-1. [DOI] [PubMed] [Google Scholar]
- 21.Inouye S.K., Westendorp R.G., Saczynski J.S. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spronk P.E., Riekerk B., Hofhuis J., Rommes J.H. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35:1276–1280. doi: 10.1007/s00134-009-1466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herling S.F., Greve I.E., Vasilevskis E.E., et al. Interventions for preventing intensive care unit delirium in adults. Cochrane Database Syst Rev. 2018;11 doi: 10.1002/14651858.CD009783.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergeron N., Dubois M.J., Dumont M., Dial S., Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 25.Miller M.A., Govindan S., Watson S.R., Hyzy R.C., Iwashyna T.J. ABCDE, but in that order? A cross-sectional survey of Michigan intensive care unit sedation, delirium, and early mobility practices. Ann Am Thorac Soc. 2015;12:1066–1071. doi: 10.1513/AnnalsATS.201501-066OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siddiqi N., Harrison J.K., Clegg A., et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. doi: 10.1002/14651858.CD005563.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inouye S.K., Bogardus S.T., Jr., Charpentier P.A., et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 28.Oh E.S., Fong T.G., Hshieh T.T., Inouye S.K. Delirium in older persons: advances in diagnosis and treatment. JAMA. 2017;318:1161–1174. doi: 10.1001/jama.2017.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inouye S., Robinson T., Blaum C., et al. American Geriatrics Society Abstracted Clinical Practice Guideline for Postoperative Delirium in Older Adults. J Am Geriatr Soc. 2015;63:142–150. doi: 10.1111/jgs.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inouye S.K., Bogardus S.T., Jr., Baker D.I., Leo-Summers L., Cooney L.M., Jr. The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J Am Geriatr Soc. 2000;48:1697–1706. doi: 10.1111/j.1532-5415.2000.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 31.Rubin F.H., Williams J.T., Lescisin D.A., Mook W.J., Hassan S., Inouye S.K. Replicating the Hospital Elder Life Program in a community hospital and demonstrating effectiveness using quality improvement methodology. J Am Geriatr Soc. 2006;54:969–974. doi: 10.1111/j.1532-5415.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 32.Holt R., Young J., Heseltine D. Effectiveness of a multi-component intervention to reduce delirium incidence in elderly care wards. Age Ageing. 2013;42:721–727. doi: 10.1093/ageing/aft120. [DOI] [PubMed] [Google Scholar]
- 33.Rivosecchi R.M., Smithburger P.L., Svec S., Campbell S., Kane-Gill S.L. Nonpharmacological interventions to prevent delirium: an evidence-based systematic review. Crit Care Nurse. 2015;35:39–49. doi: 10.4037/ccn2015423. [DOI] [PubMed] [Google Scholar]
- 34.Bannon L., McGaughey J., Verghis R., Clarke M., McAuley D.F., Blackwood B. The effectiveness of non-pharmacological interventions in reducing the incidence and duration of delirium in critically ill patients: a systematic review and meta-analysis. Intensive Care Med. 2019;45:1–12. doi: 10.1007/s00134-018-5452-x. [DOI] [PubMed] [Google Scholar]
- 35.Moon K.J., Lee S.M. The effects of a tailored intensive care unit delirium prevention protocol: a randomized controlled trial. Int J Nurs Stud. 2015;52:1423–1432. doi: 10.1016/j.ijnurstu.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 36.Kamdar B.B., Yang J., King L.M., et al. Developing, implementing, and evaluating a multifaceted quality improvement intervention to promote sleep in an ICU. Am J Med Qual. 2014;29:546–554. doi: 10.1177/1062860613509684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institutes of Health Clinical Center. Impact of non-pharmacological prevention measures on the incidence of delirium in adult intensive care units. NCT03125252. ClinicalTrials.gov. National Institutes of Health; 2017. Updated November 12, 2020. http://clinicaltrials.gov/ct2/show/ NCT03125252
- 38.National Institutes of Health Clinical Center. Impact of Nursing Delirium Preventive Interventions in the Intensive Care Unit (UNDERPIN-ICU). NCT03002701. ClinicalTrials.gov. National Institutes of Health; 2016. Updated October 9, 2020. http://clinicaltrials.gov/ct2/show/ NCT03002701
- 39.National Institutes of Health Clinical Center. The effects of a model-based sensory stimulation intervention on preventing delirium among intensive care unit patients: a randomised controlled trial. NCT04306016. ClinicalTrials.gov. National Institutes of Health; 2020. Updated March 17, 2021. http://clinicaltrials.gov/ct2/show/NCT04306016
- 40.Barr J., Fraser G.L., Puntillo K., et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 41.Page V.J., Ely E.W., Gates S., et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2013;1:515–523. doi: 10.1016/S2213-2600(13)70166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Boogaard M., Slooter A.J.C., Bruggemann R.J.M., et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA. 2018;319:680–690. doi: 10.1001/jama.2018.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girard T.D., Exline M.C., Carson S.S., et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379:2506–2516. doi: 10.1056/NEJMoa1808217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reade M.C., Eastwood G.M., Bellomo R., et al. Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA. 2016;315:1460–1468. doi: 10.1001/jama.2016.2707. [DOI] [PubMed] [Google Scholar]
- 45.Wang W., Li H.L., Wang D.X., et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial. Critical Care Med. 2012;40:731–739. doi: 10.1097/CCM.0b013e3182376e4f. [DOI] [PubMed] [Google Scholar]
- 46.Prakanrattana U., Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesthesia Intensive Care. 2007;35:714–719. doi: 10.1177/0310057X0703500509. [DOI] [PubMed] [Google Scholar]
- 47.Su X., Meng Z.T., Wu X.H., et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388:1893–1902. doi: 10.1016/S0140-6736(16)30580-3. [DOI] [PubMed] [Google Scholar]
- 48.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia Z.Q., Chen S.Q., Yao X., Xie C.B., Wen S.H., Liu K.X. Clinical benefits of dexmedetomidine versus propofol in adult intensive care unit patients: a meta-analysis of randomized clinical trials. J Surg Res. 2013;185:833–843. doi: 10.1016/j.jss.2013.06.062. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H., Lu Y., Liu M., et al. Strategies for prevention of postoperative delirium: a systematic review and meta-analysis of randomized trials. Crit Care. 2013;17:R47. doi: 10.1186/cc12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X., Xie G., Zhang K., et al. Dexmedetomidine vs propofol sedation reduces delirium in patients after cardiac surgery: a meta-analysis with trial sequential analysis of randomized controlled trials. J Crit Care. 2017;38:190–196. doi: 10.1016/j.jcrc.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 52.Lin Y.Y., He B., Chen J., Wang Z.N. Can dexmedetomidine be a safe and efficacious sedative agent in post-cardiac surgery patients? A meta-analysis. Crit Care. 2012;16:R169. doi: 10.1186/cc11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasin L., Landoni G., Nardelli P., et al. Dexmedetomidine reduces the risk of delirium, agitation and confusion in critically Ill patients: a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. 2014;28:1459–1466. doi: 10.1053/j.jvca.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 54.Chen K., Lu Z., Xin Y.C., Cai Y., Chen Y., Pan S.M. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst Rev. 2015;1:CD010269. doi: 10.1002/14651858.CD010269.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barbateskovic M., Krauss S.R., Collet M.O., et al. Pharmacological interventions for prevention and management of delirium in intensive care patients: a systematic overview of reviews and meta-analyses. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-024562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaal I.J., Devlin J.W., Hazelbag M., et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015;41:2130–2137. doi: 10.1007/s00134-015-4063-z. [DOI] [PubMed] [Google Scholar]
- 57.Pandharipande P., Shintani A., Peterson J., et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Pandharipande P.P., Pun B.T., Herr D.L., et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 59.Carson S.S., Kress J.P., Rodgers J.E., et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med. 2006;34:1326–1332. doi: 10.1097/01.CCM.0000215513.63207.7F. [DOI] [PubMed] [Google Scholar]