Abstract
Lutetium-177 [T½ = 6.76 d; Eβ (max) = 0.497 MeV; maximum tissue range ~2.5 mm; 208 keV γ-ray] is one of the most important theranostic radioisotope used for the management of various oncological and non-oncological disorders. The present review chronicles the advancement in the last decade in 177Lu-radiopharmacy with a focus on 177Lu produced via direct 176Lu (n, γ) 177Lu nuclear reaction in medium flux research reactors. The specific nuances of 177Lu production by various routes are described and their pros and cons are discussed. Lutetium, is the last element in the lanthanide series. Its chemistry plays a vital role in the preparation of a wide variety of radiopharmaceuticals which demonstrate appreciable in vivo stability. Traditional bifunctional chelators (BFCs) that are used for 177Lu-labeling are discussed and the upcoming ones are highlighted. Research efforts that resulted in the growth of various 177Lu-based radiopharmaceuticals in preclinical and clinical settings are provided. This review also summarizes the results of clinical studies with potent 177Lu-based radiopharmaceuticals that have been prepared using medium specific activity 177Lu produced by direct neutron activation route in research reactors. Overall, the review amply demonstrates the practicality of the medium specific activity 177Lu towards formulation of various clinically useful radiopharmaceuticals, especially for the benefit of millions of cancer patients in developing countries with limited reactor facilities.
Keywords: 177Lu, direct neutron activation, DOTATATE, intrinsically radiolabeled nanoparticles, medium flux research reactors, specific activity, PSMA-617, targeted therapy, TENIS
Introduction
Over the last several decades, the production and application of radiometals have matured from largely ambiguous and experimental technologies to indispensable components of routine practices in nuclear medicine. This transition has been driven by the mutually essential advances in radiochemical processing, biomedical sciences, synthetic organic and inorganic chemistry, nuclear imaging technology and cancer biology. Radiometals which are γ-emitters or positron emitters are generally used for diagnostic applications utilizing single photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging modalities. Contrastingly, particulate emitting radiometals (α, β-, conversion and/or Auger electrons) are in high demand for internal radiotherapy. Dosimetry is the major challenge with internal radiotherapy due to uncertainty in measurement of radiation dose associated with particulate radiation from outside the patient body. To circumvent this limitation, presently efforts are emerging towards combining the diagnosis (molecular imaging) and the internal radiotherapy (molecular targeted therapy) and this concept is known as “theranostics” [1-5].
The fundamental requirement of theranostics is that the same or similar chemical compound should be labelled with a diagnostic and a therapeutic radionuclide. For the diagnostic part of the investigation, SPECT and PET imaging techniques are used. To meet the requirement of theranostics, the two radionuclides chosen are preferably of the same chemical element, even though chemically similar elements are also utilized occasionally. A more preferable option in this regard is to use intrinsically theranostic radiometals which by virtue of their suitable γ or β+ emission can be used for diagnostic imaging and due to suitable particulate emission can be used for targeted therapy. The use of the same radiometal would give an accurate idea regarding the pharmacokinetics of the radiopharmaceutical administered for dosimetric estimation.
Several intrinsically theranostic radiometals such as 47Sc, 64Cu, 67Cu, 153Sm, 166Ho, 177Lu, 186Re, etc., with fascinating nuclear decay characteristics (as summarized in Table 1) have been studied for preparation of radiopharmaceuticals in preclinical and clinical settings [6-10]. Although nuclear decay characteristics are of paramount importance for selection of an intrinsically theranostic radiometal, its success in real clinical context also depends on the practicality of large-scale production with acceptable purity and the supply logistics. In this regard, 177Lu has numerous advantages in comparison to other intrinsically theranostic radiometals [11,12]. The nuclear decay properties of 177Lu such as its relatively long half-life, the energy of β- particles and the energy and abundance of the γ-photons make it suitable for use in preparation of theranostic radiopharmaceuticals for targeting small-sized primary tumors and their metastatic sites [12].
Table 1.
Nuclear decay characteristics of some intrinsically theranostic radiometals
| Radiometal | Decay mode | T½ (h) | Emax (β), MeV (%) | Principal γ-energy, keV (%) | Mean tissue range (mm) |
|---|---|---|---|---|---|
| 47Sc | β-, γ | 80.4 | 0.60 (100) | 159.4 (68) | 0.20 |
| 64Cu | β+, β-, γ (EC) | 12.7 | β+: 0.65 (19) | 511 (38.6) | 0.19 |
| β-: 0.58 (40) | |||||
| 67Cu | β-, γ | 61.9 | 0.58 (100) | 184.6 (46.7) | 0.19 |
| 93.3 (16.6) | |||||
| 91.3 (7.3) | |||||
| 153Sm | β-, γ | 46.3 | 0.81 (100) | 103 (28.3) | 0.30 |
| 161Tb | β-, γ | 165.8 | 0.59 (100) | 48.9 (17.0) | 0.20 |
| 74.6 (10.2) | |||||
| 166Ho | β-, γ | 26.8 | 1.86 (100) | 80.6 (6.2) | 0.84 |
| 177Lu | β-, γ | 161.0 | 0.50 (100) | 208 (11) | 0.16 |
| 113 (6.6) | |||||
| 186Re | β-, γ | 89.2 | 1.10 (92.5) | 137 (9) | 0.43 |
| 188Re | β-, γ | 16.9 | 2.10 (100) | 155 (15) | 0.98 |
Clinical efficacies of several 177Lu-based radiopharmaceuticals have been established in different parts of the world [12]. A quick “Pubmed” search shows that more than 1500 papers have been published in the last decade on 177Lu-based radiopharmaceuticals and their utilization in preclinical and clinical settings. In this review, our objective to provide an apt and wide-ranging overview of the 177Lu-based radiopharmaceuticals developed over the last one decade, with focus on utilization of 177Lu produced via direct neutron activation route. The choice of BFCs which are used for 177Lu-labeling to the different carrier molecules are discussed. The development of various ligands and nanoconstructs for site- and event-specific targeting are summarized, and the likelihood and fascinating opportunities for future development which might help in translating this exciting research area towards routine clinical use are discussed.
Production of 177Lu
Lutetium-177 is among the very few radioisotopes that can be produced both in a cyclotron as well as in a nuclear reactor. The schematic illustrating the production of 177Lu in a cyclotron and nuclear reactor is shown in Figure 1. The cyclotron production route is relatively less explored because of the low batch yields of the process which is unsuitable to meet the clinical demands. Nevertheless, it is feasible approach to produce 177Lu of equivalent quality without depending on nuclear reactors to fulfil the requirements of research and development laboratories and academic institutions. The details of production of 177Lu by various production methodologies in nuclear reactor or cyclotron are dealt with in the following sections.
Figure 1.
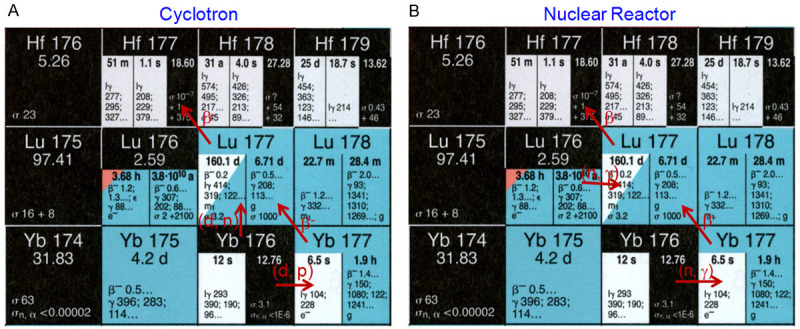
Production of 177Lu in (A) cyclotron via 176Yb (d, n) 177Lu and 176Yb (d, p) 177Yb→177Lu reactions and (B) nuclear reactor via 176Lu (n, γ) 177Lu and 176Yb (n, γ) 177Yb→177Lu reactions.
Cyclotron production route
The production of 177Lu in a cyclotron has been explored more from an academic perspective. For the first time, Hermanne et al. studied the deuteron induced nuclear reactions for production of no-carrier-added (NCA) Lu radioisotopes and reported the excitation functions for their formation [13]. Similar results were also reported by Maneti et al. and Khandaker et al. [14,15]. In these studies, stacked foil activation technique on natural Yb targets at incident deuteron energies up to 20 MeV was explored. The authors inferred from these studies that two different nuclear reactions, viz., 176Yb (d, n) 177Lu and 176Yb (d, p) 177Yb→177Lu, contributed to the generation of 177Lu by deutron bombardment on Yb target. By analysis of the excitation functions, it was revealed that the principal route for the production of 177Lu was the indirect nuclear reaction 176Yb (d, p) 177Yb→177Lu, where co-production of 177mLu impurity (T½ = 160.5 d) could be minimized [16,17]. Kambali calculated that the end of bombardment (EOB) yields for the direct i.e. 176Yb (d, n) 177Lu reaction and indirect i.e. 176Yb (d, p) 177Yb reaction were 0.519 and 181.1 MBq/µA h, respectively [17]. In order to enhance the overall yield of 177Lu and minimize the radionuclidic impurities due to extraneous Lu radioisotopes, highly enriched 176Yb target must be used. Use of enriched 176Yb could yield 177Lu free from other coproduced 177Lu radionuclides such as 170Lu, 171Lu, 172Lu, 173Lu and 174Lu [12].
In another approach, Medvedev et al. investigated the feasibility of production of 177Lu in a proton accelerator by irradiation of Ta and Hf targets [18]. The authors reported both theoretical and experimental activation cross-sections of proton induced reactions at 100 and 200 MeV proton beam energy. The largest cross-section of ~20 mb was found for the Hf target at 195 MeV proton energy. In addition to the small reaction cross-section, a major shortcoming of this approach is the co-production of extraneous Lu isotopes reducing the specific activity and radionuclidic purity required for the practical applications.
Reactor production route
Two independent routes are adopted for production of 177Lu in nuclear reactors and these are the most commonly used methods for large-scale production of 177Lu for therapeutic purposes world over [11,19]. The first is the “direct” production of 177Lu via 176Lu (n, γ) 177Lu reaction in medium to high flux research reactors. The other approach involves “indirect” production of 177Lu via 176Yb (n, γ) 177Yb→177Lu by irradiating enriched 176Yb target in high flux research reactors. In the direct route, the post irradiation radiochemical processing involves simple dissolution of the irradiated target, whereas, the indirect route involves radiochemical separation of 177Lu from the bulk irradiated Yb target. The major advantage of the indirect route is that it yields NCA 177Lu and the product is free from the long-lived radionuclidic impurity, 177mLu. However, the success of this method depends on availability of a suitable method for radiochemical separation of miniscule quantities (in µg level) of NCA 177Lu from bulk irradiated Yb target (in g quantities). Since, Lu and Yb are adjacent members of the lanthanide series, their chemical properties are similar which makes the separation process quite challenging. Over the last several years numerous methods of radiochemical separation based on solvent extraction, ion exchange, electrochemical technique, either alone or in combination have been reported for separation of NCA 177Lu from the irradiated target [20-28]. All these methods involve laboratory scale separation and the details of large-scale separation in industrial settings to meet the surging demand of the nuclear medicine departments have not yet been reported. As a result, the technology for production of NCA 177Lu is not available to most of the countries and the radioisotope is supplied by few commercial manufacturers, which restricts it widespread clinical utility.
Generally, highly enriched (>98%) 176Yb target is required for the production of NCA 177Lu by the indirect route. This is because the natural abundance of 176Yb is only 12.6%. Irradiation of natural Yb target will not only result in lower production yield but also lead to production of significantly higher activity level of 175Yb, which will increase the radiation dose to working personnel during target processing and radiochemical separation. Moreover, on irradiation of natural Yb target, some stable isotopes of Lu will be produced as decay products which will significantly lower the specific activity of 177Lu obtained after radiochemical separation. Use of highly enriched target would lead to the production of NCA 177Lu with specific activity approaching the theoretical value of ~110 Ci (4.1 TBq)/g [12,20]. Considering the contribution due to thermal neutron capture only, the activity of NCA 177Lu produced by indirect route can be calculated using the following equations (Equations 1-5):
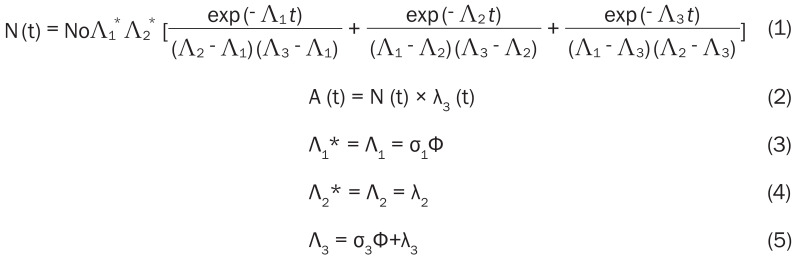 |
where, No is the number of 176Yb atoms used as target (at t = 0), A (t) is the activity of 177Lu produced after irradiation for time ‘t’, σ1 is the thermal neutron capture cross-section of 175Yb, σ3 is the thermal neutron capture cross-section of 177Lu, λ2 is the decay constant of 177Yb, λ3 is the decay constant of 177Lu, Φ is the flux of the reactor.
Contrarily, while calculating the 177Lu activity produced via direct 176Lu (n, γ) 177Lu reaction, two noteworthy effects have to be considered [11]. First, significant target burn up is caused due to the high cross section of 176Lu and therefore the number of target atoms does not remain constant during the course of irradiation. Second, the reaction cross section, which is a function of neutron velocity [σ (vn)], exhibits a strong resonance at 0.1413 eV and therefore it deviates from the 1/vn law. For such situations, the Westcott convention has to be adopted for calculating the activity produced [29] (Equation 6):
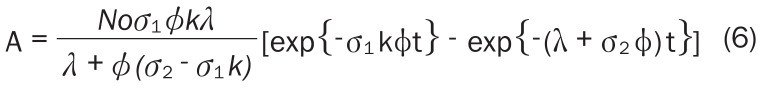 |
where, No is the number of 176Lu atoms used as target (at t = 0), λ the decay constant of 177Lu (in s-1), σ1 the thermal neutron capture cross section of 176Lu at the neutron velocity of 2,200 ms-1 (2,090 b), σ2 is the thermal neutron capture cross section of 177Lu at the neutron velocity of 2,200 ms-1 (1,000 b), Φ the thermal neutron flux of the reactor (in cm-2s-1), t is the time of irradiation and ‘k’ is so called k-factor which can be expressed as (Equation 7):
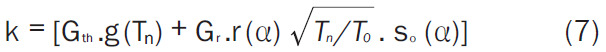 |
Where, Gth is the thermal neutron self-shielding factor, Gr is the epithermal neutron self-shielding factor, g (Tn) is the Westcott factor with thermal neutron temperature Tn, r (α) √(Tn/T0 ). is the Westcott spectral index, so (α) is the so (Er)-α, where so and Er are constants. The k-factor depends upon several parameters such as, thermal energy cross-section at a reference temperature, resonance integral, epithermal index, epithermal flux shape factor, Westcott g-factor, temperature, Cadmium cut-off energy, effective resonance energy and flux attenuation factor for thermal and epithermal neutrons [29]. Generally, the value of ‘k’ is reported to be between 1.5 and 2.5 [30].
Since, the natural abundance of 176Lu is only 2.6%, enriched lutetium target (>70% enriched in 176Lu) is required for production of 177Lu with adequate specific activity by the direct route. Owing to the large cross-section, the consumption of the target in a typical batch is very low. Generally, 25-100 µg of 176Lu target is required for production of 37 GBq (1 Ci) of 177Lu depending on the flux of the reactor. The yields of 177Lu produced by the direct and indirect route on irradiation of 1 mg each of Lu2O3 (80% enriched in 176Lu) and Yb2O3 (99.9% enriched in 176Yb) targets, respectively, under different flux conditions were calculated using Equations 1 and 6 and plotted in Figure 2. For simplicity, only the thermal neutron contribution was considered in both the cases and the k-factor was assumed as 1.75. It can be seen from the figures that production of 177Lu by the direct route requires cautious optimization of the time of irradiation, which diverges between 5-20 days depending on the flux of the reactor. Under the optimal conditions, the specific activity of >740 GBq (20 Ci)/mg can easily be achieved in the direct production route on irradiation in medium flux research reactor (Φ >1014 n.cm-2.s-1). In sharp contrast to the direct production route, the yield of 177Lu in the indirect route is drastically lower based on the target mass at all flux conditions. This significantly increases the cost of production of 177Lu by the indirect route, as the process involves the use of highly enriched target and the reaction cross-section is low (2.85 b). Additionally, the process involves intricate radiochemical separation which further escalates the cost. Utilization of enriched targets for radioisotope production involving low activation cross-section reaction is not a viable proposition, as considerable portion of the target does not capture neutrons. As seen from Figure 2, it is advisable to produce 177Lu by the indirect route only if there is access to high flux research reactor (Φ >5 × 1014 n.cm-2.s-1) for radioisotope production. Even then, the process would be practical only if there is provision to efficiently recover the unused enriched target after the radiochemical separation. According the International Atomic Energy Agency database, majority of the operational research reactors for isotope production are medium flux research reactors [31]. For its widespread utilization, 177Lu can be cost-effectively produced by the direct route in these medium flux research reactors. Nevertheless, NCA 177Lu produced in large-scale in high flux research reactors is available commercially and is preferred by certain groups.
Figure 2.
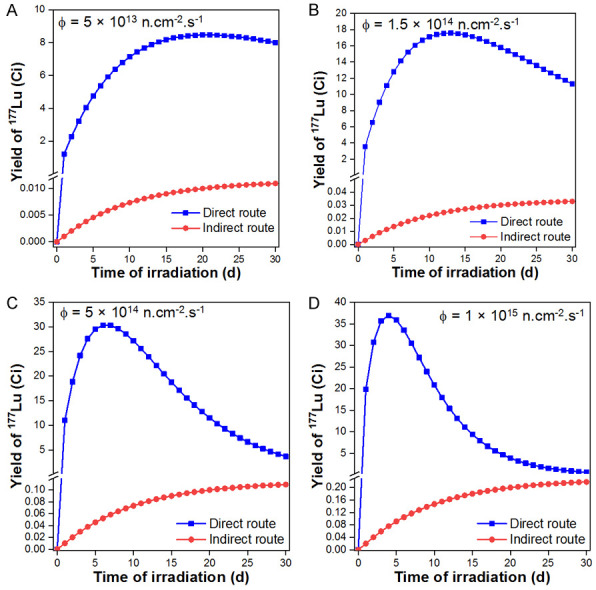
Comparative evaluation of the yield of 177Lu produced via direct 176Lu (n, γ) 177Lu and indirect 176Yb (n, γ) 177Yb→177Lu routes when irradiated at flux of (A) 5 × 1013 n.cm-2.s-1, (B) 1.5 × 1014 n.cm-2.s-1, (C) 5 × 1014 n.cm-2.s-1 and (D) 1 × 1015 n.cm-2.s-1 for different irradiation times.
A major concern with 177Lu produced by the direct route is the co-production of small amount of long-lived 177mLu as the radionuclidic impurity [11]. Owing to the low cross-section (~7 b) of the 176Lu (n, γ) 177mLu reaction and the long half-life of the radioisotope, the yield of 177mLu is relatively low under most irradiation conditions. Figure 3 shows the activity of 177mLu co-produced at different thermal neutron flux values when irradiated for different time periods. Especially, under medium flux conditions the level of 177mLu in 177Lu is <0.02% and is therefore not a significant issue [12]. Lutetium-177m, being a radionuclidic impurity, is taken up in the same tissues where the uptake of 177Lu-based radiopharmaceutical occurs. However, its duration of retention in the tissues will be much more prolonged compared to 177Lu because of its much longer half-life. Moreover, if metabolization of the targeting agent occurs, free 177mLu can be excreted or skeletal uptake may occur. This is not a cause of much concern as the radiation dose due to 177mLu will be low. However, from waste management point of view, the presence of 177mLu in 177Lu is problematic especially in certain countries with stringent waste disposal protocols [12]. Assuming that a few μCi of 177mLu would be present in a curie of 177Lu used in a nuclear medicine clinic, the containment and post-decay release of the radioactive waste generated has to be carefully managed by design of appropriate delay tanks. Overall, it can be inferred that direct production of 177Lu is the most practical route in countries which have access to medium flux research reactors for radioisotope production.
Figure 3.
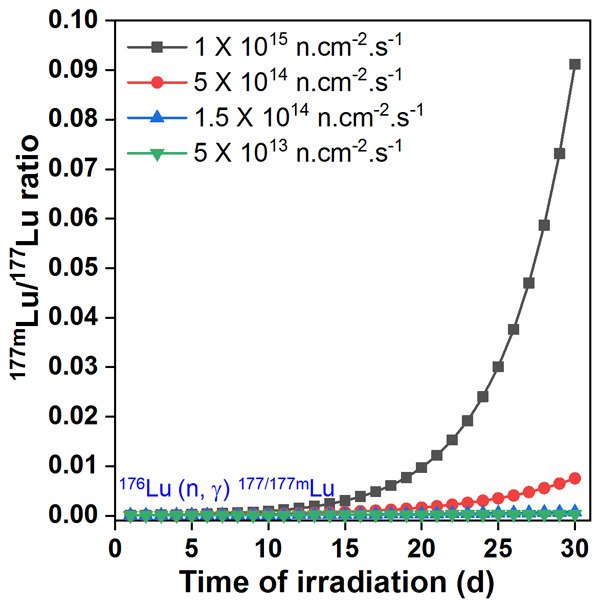
Calculated 177mLu/177Lu ratios during production of 177Lu via direct 176Lu (n, γ) 177Lu route when irradiated at different fluxes for different irradiation times in nuclear reactor.
Complexes of 177Lu with various bifunctional chelators
Selection of an efficient BFC is essential for preparation of 177Lu-based radiopharmaceutical. This section describes the coordination chemistry of lutetium and the design concept of BFCs for preparation of 177Lu-based radiopharmaceuticals. Being the last member of the lanthanide series Lu with 71 electrons has [Xe]4f145d16s2 configuration. As a result, it loses two outermost 6s electron and the lone 5d electron to generate +3 metal ion species during chemical reaction. As Lu3+ species, it has empty s, p, d orbitals and fully filled f orbitals. Since 4f electrons are tightly bound by high effective nuclear charge, they have hardly any contribution to bond formation. Accordingly, coordination between Lu3+ ion and the chelator are predominantly electrostatic and governed by the hard and soft acid base (HSAB) principle. Being a hard Lewis acid, Lu3+ forms strong complexes with chelators which have high Lewis bases such as carboxylates. Due to lanthanide contraction, Lu3+ has the smallest ionic radius (86.1 pm) among the lanthanides. Therefore, because of the high charge density and narrow coordination field, limited number of ligands can be placed around Lu3+. Mostly, reciprocal number dictate the coordination number between the ligands without any pertinent effect ascribable to the (s, p, and d) orbitals involved in bond formation.
Chelators for Lu3+ are designed based on the following explicit requirements: (i) Labeling efficiency: The process of radiolabeling is performed using a very low-concentration of 177Lu[Lu3+], wherein a high radiolabeling yield is required. Therefore, it is expected that Lu-chelator complexation should be kinetically favored. (ii) In vivo stability: The metal-chelator complex should be insusceptible to hydrolysis under physiological conditions. Additionally, compared to competing proteins, such as human serum albumin (HSA) and transferrin, the chelator should have a higher affinity for Lu [12,32]. (iii) Metal selectivity: The chelator should have sufficient selectivity towards 177Lu[Lu3+], such that 177Lu[Lu3+] is not replaceable by another metal ions such as Na+, Mg2+, K+, Ca2+, Fe2+, Fe3+, Co2+, or Zn2+ which co-exist under physiological conditions.
A wide variety of structures have been explored to develop an ideal BFC to satisfy these requirements [8,12,33-41]. In fact, Lu3+ forms stable complexes with several BFCs and coordination numbers of 6, 7, 8, and 9 have been reported. The common BFCs used for Lu3+ are shown in Figure 4 and their stability constants are summarized in Table 2. Among them, DOTA and its derivatives are the most widely used BFCs for preparation of 177Lu-based radiopharmaceuticals. The DOTA moiety forms the Na[Lu(DOTA)(H2O)]4H2O nine-coordinated complex with high thermodynamic stability and kinetic inertness. The reported Na[Lu(DOTA)(H2O)]4H2O complex has capped square antiprism geometry where the basal plane is occupied by four amine nitrogens of the macrocycle, the capped plane is occupied by four carboxylate oxygens of the carboxylic residues, and the capping position is occupied by a water molecule. In addition to DOTA-based agents, direct radiolabeling of phosphonate analogs of aminocarboxylates, such as ethylenediamine tetra (methylene phosphonic acid) (EDTMP) and 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetramethylene phosphonic acid (DOTMP) have been used for preparation of radiopharmaceuticals for bone pain palliation [42-46]. It is interesting to note that the Lu-aminophosphonate complex (such as Lu-DOTMP) is generally more stable than the Lu-aminocarboxylate complex (such as Lu-DOTA) [12].
Figure 4.
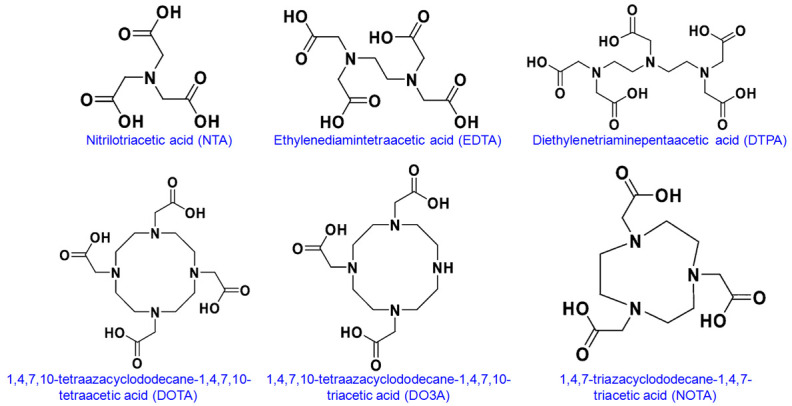
Structures of the common bifunctional chelator used for radiolabeling with 177Lu.
Table 2.
Commonly used BFCs for Lu3+ and log stability constants [12]
| BFC | log stability constant |
|---|---|
| DTPA | 12.5 |
| EDTA | 19.8 |
| NTA | 22.4 |
| DOTA | 25.4 |
| DO3A | 23.0 |
| NOTA | 15.3 |
For preparation of target specific radiopharmaceuticals, the BFC needs to be conjugated with targeting ligands such as peptides or antibodies. Numerous methods of conjugation are known, among which, the carboxylate or isothiocyanate group in the BFC structure conjugated with the amino group of the targeting ligand, is the approach most widely used [12]. In certain cases, a linker can also be unified between the chelator and the targeting ligand to affect the pharmacokinetics of the radiopharmaceutical [47-49]. These linker groups, such as hydrocarbon chains (CH2)n, polyethylene glycol (PEG), or polypeptide linkers, can adjust the pharmacokinetics and biodistribution by altering the overall charge and hydrophilicity of the radiopharmaceutical. In all radiolabeling procedures, pH of the reaction mixture plays a crucial role in determining the complexation yield. For DOTA-based chelators, the rate of complex formation increases with increase in pH of the medium. However, at pH >6, Lu3+ forms insoluble Lu(OH)3. Therefore, the optimum pH for radiolabeling is between 5 and 6, and is generally maintained using sodium acetate or ammonium acetate buffers. Interestingly, radiolabeling for DOTMP and EDTMP is done at pH >8, maintained using bicarbonate buffer. This is possible because several folds higher excess of ligand is used compared to Lu3+ ions [12]. Only a small fraction of these ligands is radiolabeled and the large excess of the ligands prevent formation of lutetium-hydroxo species through rapid formation of intermediary complexes.
A significant disadvantage of macrocyclic DOTA-based BFCs for preparation of radiopharmaceuticals is the slow rate of complexation with Lu3+ ions [12]. Accordingly, heating becomes essential in most radiolabeling processes to increase the kinetics of the chelation process. Generally, heating is performed at 90-95°C for 25-30 min to obtain near quantitative radiolabeling yield [50-52]. This especially becomes a serious challenge when DOTA is conjugated with temperature sensitive biomolecules such as monoclonal antibodies or their engineered fragments. From this perspective, diethylene triamine penta acetic acid (DTPA) based BFCs have been proposed for room temperature radiolabeling with 177Lu [53-57]. However, some DTPA based complexes are found to dissociate under physiological conditions and release free Lu3+ ions in vivo [12]. To circumvent this limitation, recently, AAZTA (1,4-bis (carboxymethyl)-6-[bis (carboxymethyl)]amino-6-methylperhydro-1,4-diazepine) based chelators were developed which demonstrated quantitative labeling (>95%) with 177Lu at room temperature within 5 minutes (Figure 5) [58-61]. The complexes demonstrated excellent radiochemical stability and therefore this class of chelators holds promise for development of new generation of 177Lu-based radiopharmaceuticals.
Figure 5.
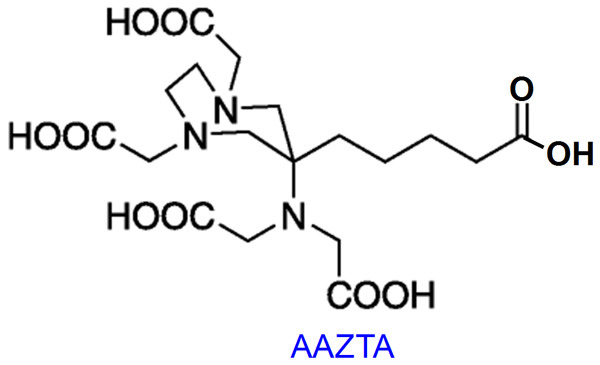
Structure of AAZTA chelator.
Potential 177Lu-based radiopharmaceuticals in preclinical and clinical settings
A number of 177Lu-based agents have been prepared using 177Lu produced by direct neutron activation route in medium flux research reactors, some of which have been translated to the clinic. Targeting ligands such as peptides, monoclonal antibodies and their engineered fragments, phosphonates, small molecules and nanoparticles have been evaluated as potential agents for targeted radiotherapy. An outline of the research efforts that have predicted and established the practicality of 177Lu produced by direct neutron activation route for potential applications in targeted therapy is given below.
Somatostatin receptor targeting agents
Somatostatin (sst) receptors are typically overexpressed in a variety of tumors of neuroendocrine origin in the pancreas, lung, intestine and thyroid [62,63]. Over the last two decades, there has been concerted research in the field of sst targeting in oncology for both diagnostic and therapeutic indications [64,65]. Currently, five subtypes (sst1-sst5) have been identified and cloned, out of which sst2 receptors are predominantly expressed in neuroendocrine tumors (NETs) [66-68]. The presence of sst2 receptors in NETs has opened up the avenue for peptide receptor radionuclide therapy (PRRT). In this regard, 177Lu labeled sst analog, 1,4,7,10-tetraazacyclododecane-NI,NII,NIII,NIIII-tetraacetic acid(D)Tyr3-octreotate (DOTATATE) is an established agent in the management of patients with inoperable or metastatic NETs [69-79]. This radiopharmaceutical is able to irradiate tumors and their metastases via internalization through sst2 receptors, generally overexpressed on the cell membrane. This radiopharmaceutical has evolved mainly during the years 2001-2010 and till recently, was the most widely used 177Lu-labeled agent in clinical context. Nevertheless, significant volume of clinical studies with 177Lu-DOTATATE have been performed during the last decade using medium specific activity 177Lu produced by direct neutron activation route, which are summarized below.
The protocol for formulation of clinical dose of 177Lu-DOTATATE using medium specific activity 177Lu was optimized by Das et al. [80,81]. A peptide/metal ratio of ~2 was found to be optimal for complexation wherein radiolabeling yields >95% could be obtained. Subsequently, Mathur et al. performed systematic studies towards bulk scale formulation of “ready-to-use” 177Lu-DOTATATE in a centralized radiopharmacy [82]. The authors observed that 740 MBq/mL of 177Lu-DOTATATE formulation with gentisic acid (1.5% w/v) was safe for human use (>98% radiochemical purity) for more than 1 week from the date of production when stored at -70°C. Preliminary clinical studies carried out in NET patients with several batches of 177Lu-DOTATATE formulation exhibited desired uptake in the tumor and its metastatic sites to obtain the expected therapeutic outcome. This strategy would aid towards widespread deployment of this radiopharmaceutical from centralized radiopharmacy to distant nuclear medicine centers for the maximum benefit of the cancer patients. It is known that the kidneys are the critical organs in PRRT, as the radiopharmaceutical gets cleared from the biological system through the renal route [83,84]. Consequently, renal function is known to worsen after PRRT. From this perspective, Gupta et al. analyzed the glomerular filtration rate (GFR), increase in serum creatinine (SCr), and changes in hemogram parameters between pretherapy and at least 6 months after last cycle post-therapy with 177Lu-DOTATATE [84]. It was observed that 177Lu-DOTATATE therapy led to worsening of renal function revealed by a decline in GFR and increase in SCr. Hematologic toxicity was relatively rare and could be managed as encountered. In this study, the authors analyzed GFR and SCr levels 6 weeks after completion of therapy. However, a long follow up would be required to conclude about the final outcome and toxicity of 177Lu-DOTATATE therapy. The same group of authors further performed dosimetric analyses of kidneys, liver, spleen, pituitary gland and neuroendocrine tumors of 61 patients treated with 177Lu-DOTATATE [85]. Favorable biodistribution was observed with 177Lu-DOTATATE with high affinity to tumors overexpressing sst receptor. The results indicated that highest doses were delivered to the kidneys and spleen with some doses to the liver and pituitary gland. However, there were no serious clinical consequences. It could be concluded that 177Lu-DOTATATE therapy resulted in high tumor doses with likelihood of injection of up to 40 GBq of cumulative activity in fractions.
In an interesting study, Jois et al. evaluated the feasibility of 177Lu-DOTATATE therapy in non-131I avid metastatic differentiated thyroid carcinoma patients [86]. However, the authors could not arrive at a definite conclusion on the therapeutic efficacy of 177Lu-DOTATATE in the studied group of patients and further studies would be required. Basu et al. demonstrated that metastatic Merkel cell carcinoma patients responded favorably to targeted therapy with 177Lu-DOTATATE [87]. The authors inferred that considering the relative well tolerability, minimal side-effects and specificity of the treatment, 177Lu-DOTATATE therapy could evolve as the first line therapy in patients of metastatic Merkel cell carcinoma. Nevertheless, further examination in a greater number of patients would be required in the future. From study in a large number of patients over a period of 5 years, Danthala et al. showed that 177Lu-DOTATATE therapy could be considered irrespective of prior chemotherapy, surgery or extent of disease [88]. The dose delivered was primarily responsible for treatment response and progression free survival and the best results were obtained when more than two cycles of 177Lu-DOTATATE therapy were given, with cautious monitoring of the likely side effects. The follow up imaging in a typical responding patient after four cycles of 177Lu-DOTATATE therapy is shown in Figure 6. In another similar study, Ballal et al. evaluated the outcome, toxicity, survival, and quality of life after concomitant 177Lu-DOTATATE and Capecitabine therapy in large number of patients with advanced neuroendocrine tumor [89]. The authors observed that concomitant 177Lu-DOTATATE and Capecitabine therapy was highly effective, delayed the time to progression, and improved the overall survival in patients with metastatic neuroendocrine tumors. Life-threatening toxicity was not reported in any of the patients. Overall, this combined therapeutic modality can be applicable in selective patients with aggressive disease where the primary goal is not to achieve complete resolution of the diseases, but rather to arrest disease progression. Similar results were also reported by Yadav et al. in patients with malignant paragangliomas [90]. In the recent times, numerous clinical studies have been reported which demonstrated the efficacy of 177Lu-DOTATATE therapy in metastatic neuroendocrine tumors [91-102]. In future, multi-institutional randomized clinical trials would be desirable to further establish the safety and clinical efficacy of this treatment modality in a large number of patients.
Figure 6.
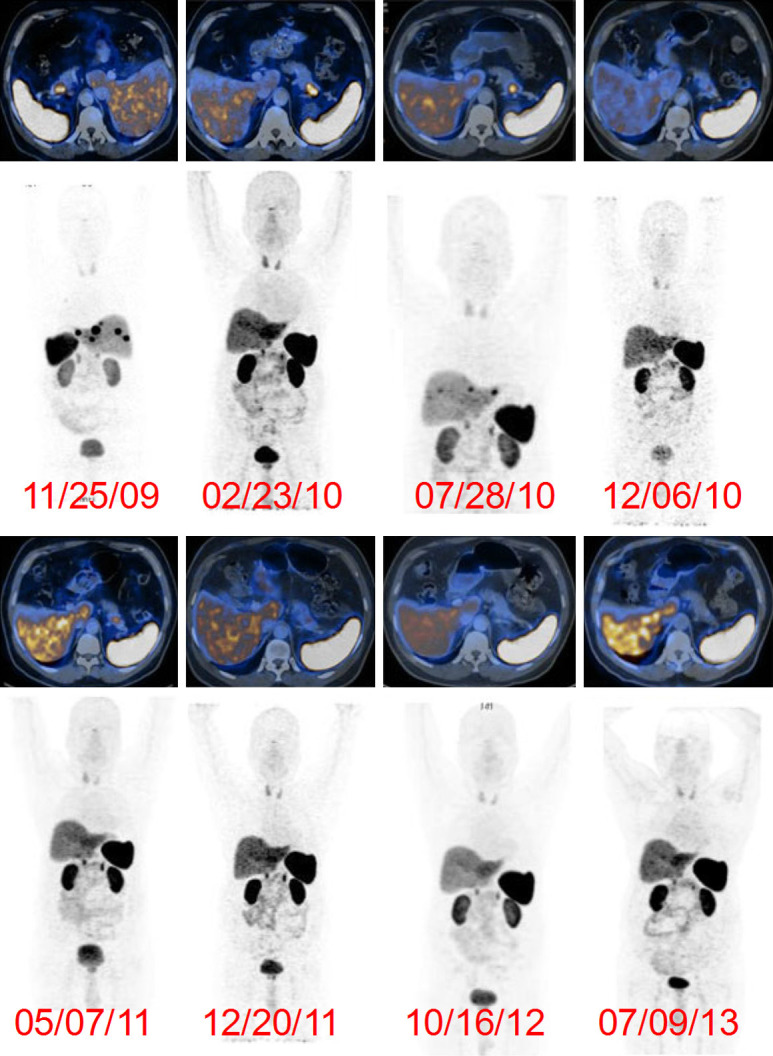
Post-therapy, 68Ga-DOTA-NOC PET scans in a 57-year-old man with pancreatic neuroendocrine tumor with extensive liver metastases. The patient was treated with four cycles of 177Lu-DOTATATE and on regular follow-up showed significant decreases in both the primary tumor in the pancreas and hepatic metastases. Adapted from Ref [88].
Prostate specific membrane antigen inhibitor
Prostate cancer is among the most common malignancies in men globally, with 10-20% of the cases progressing to metastatic castration resistant prostate cancer (mCRPC) [103,104]. Approximately, 90% of mCRPC patients present with bone metastases causing severe pain, stress fractures, poor quality of life and morbidity [104]. A variety of therapeutic procedures such as chemotherapy, androgen deprivation therapy and immunotherapy have conventionally been proposed in the treatment of mCRPC. However, very few therapeutic options are available for the patients who have the disease that progresses with steadily increasing serum prostate specific antigen (PSA) level. Especially for such patients, prostate specific membrane antigen (PSMA), a type II transmembrane glycoprotein overexpressed in prostate cancer, is an excellent target in the imaging and therapy of mCRPC [105,106]. Several urea based peptidomimmetic agents have been reported for targeting PSMA, out of which the commercially available PSMA-617 has received widespread attention for radiolabeling with 177Lu [105,106]. The structure of PSMA-617 is shown in Figure 7. In fact, 177Lu-PSMA-617 therapy which evolved within a very short span of time in the last decade has revolutionized the radionuclide therapy of mCRPC [107-116]. The growth of this radiopharmaceutical has been so rapid and huge that it has overtaken 177Lu-DOTATATE as the most widely used 177Lu-based radiopharmaceutical in clinical context.
Figure 7.
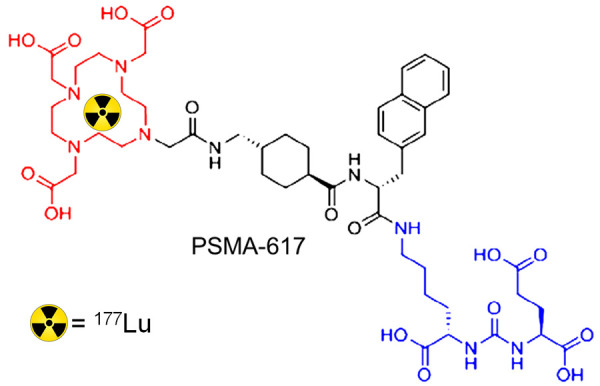
Structure of PSMA-617.
Utilizing medium specific activity 177Lu produced by direct neutron activation route in medium flux research reactor, our group was among the first to report the protocol for formulation of therapeutic dose (7.4 GBq) of 177Lu-PSMA-617 in a hospital radiopharmacy [117]. Preliminary clinical studies were performed in five patients with biopsy proven mCRPC. In vivo SPECT imaging post administration of therapeutic dose showed specific targeting of the radiopharmaceutical in the lesion sites and similar biodistribution pattern as in diagnostic 68Ga-PSMA-11 PET scans performed earlier. Clinical investigations conducted after therapy did not show any adversative side effects in any of the five patients and also there was significant reduction in PSA levels in the five patients demonstrating the preliminary efficacy of the therapeutic procedure. This work was further extended to scale-up the procedure for formulation of 177Lu-PSMA-617 in a centralized radiopharmacy [118]. The protocol was optimized for multidose formulation (>40 GBq) of ready-to-use 177Lu-PSMA-617 which met the requirements for clinical use and could be shipped to nuclear medicine centers for administration up to 4 days from the date of formulation. These procedures would aid toward widespread utilization of medium specific activity 177Lu in prostate cancer therapy especially in the developing countries.
To determine the safe activity of 177Lu-PSMA-617 that can be administered to prevent hematological, liver and renal toxicity, Yadav et al. studied the pharmacokinetics and dosimetry of the radiopharmaceuticals in 26 patients with mCRPC [119]. The mean administered activity in the overall population was 2.52±1.3 GBq. Normal physiological uptakes were observed in the lacrimal glands, salivary glands (parotid glands and submandibular glands), liver, spleen, kidneys, intestines and urinary bladder in all the patients. It was observed that the organs with the highest absorbed doses were the salivary glands, followed by the kidneys, receiving 1.24±0.26 and 0.99±0.31 mGy/MBq, respectively. The mean absorbed doses to the liver, urinary bladder and red marrow were 0.36±0.10, 0.243±0.09 and 0.048±0.05 mGy/MBq, respectively. The mean whole-body dose was 0.016±0.003 mGy/MBq. From the point of view of radiation dosimetry, it could be concluded that 177Lu-PSMA-617 is a safe option for treatment in mCRPC patients. The same group of authors further studied the safety and efficacy of 177Lu-PSMA-617 in 31 mCRPC patients (age range: 38-81 years) [120]. All the patients underwent 68Ga-PSMA-11 PET/CT scan prior to inclusion for therapy and were administered with 5069±1845 MBq dose of 177Lu-PSMA-617 ranging from one to four cycles. Standard physiological uptake of 177Lu-PSMA-617 occurred in the lacrimal glands, salivary glands, liver, intestines, spleen, kidneys, and bladder (Figure 8). In typical patients with complete remission, the mean SUVmax of the tumor lesions reduced from 32.67 to 0.38 after 177Lu-PSMA-617 therapy (Figure 8). Further, the authors observed biochemical response in 70.9% (22/31) and clinical response in 67.7% (21/31) patients. Serious life-threatening toxicity was not observed in any of the 31 patients. It was a concluded that 177Lu-PSMA-617 therapy is a simple and effective procedure with no significant adverse effects and improves the quality of life of end stage mCRPC patients. Similar clinical outcomes were observed in another study reported by Suman et al. [121]. However, these studies need to be performed in larger number of patients who need to be followed up for an extended period of time to arrive at a definitive conclusion. Recently, Adnan and Basu studied the imaging characteristics, peculiarities and response to 177Lu-PSMA-617 therapy in patients with urinary bladder metastasis by prostate cancer in accordance with Gleason score and dual tracer (68Ga-PSMA-11 and 18F-FDG) PET study [122]. The authors inferred that 18F-FDG PET/CT along with 68Ga-PSMA-11 PET/CT, preferably at the baseline, can promote prognostic and predictive implications in therapy of mCRPC using 177Lu-PSMA-617. The major limitation of this study was that it was conducted on just 3 patients and therefore, future studies in larger number of patients would be a worthwhile exercise.
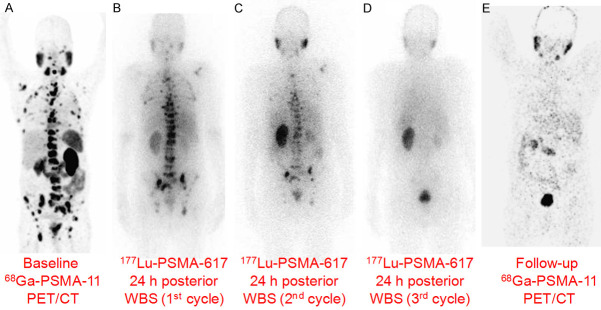
Figure 8.
177Lu-PSMA-617 therapy in a 65-year-old man with mCRPC. (A) The baseline pre-therapy diagnostic 68Ga-PSMA-11 PET/CT showed PSMA-avid extensive skeletal metastases, (B) Posterior whole body scintigraphy (WBS) 24 h after administration of first cycle of 177Lu-PSMA-617, (C) Posterior WBS 24 h after administration of second cycle of 177Lu-PSMA-617 showed remarkable reduction in uptake, (D) Posterior WBS 24 h after administration of third cycle of 177Lu-PSMA-617 did not show any abnormal uptake, (E) After three cycles of therapy, the follow-up diagnostic 68Ga-PSMA-11 PET/CT scan showed near complete metabolic response with resolution of the PSMA-avid metastases. Adapted from Ref [120].
Bone pain palliation agents
Bone is one of the common sites of metastases from various types of cancers, which include that of prostate, breast, lung, kidney, bladder, thyroid, lymphomas and sarcomas [123-128]. Of these, primary cancers of prostate, breast and lung account for >80% cases of bone metastases. These metastatic lesions on bone surface have huge impact in the quality of life of those patients, causing complications such as severe bone pain, pathologic fractures, hypercalcemia, and spinal cord compression [129]. The management of bone pain involves a multidisciplinary approach involving systemic and nonsystemic treatments. However, many treatment options have limitations in their efficacy and duration. Some of them manifest significant adverse effects that seriously compromise the quality of life of the cancer patients. Over the last few decades, radiopharmaceuticals are being used widely as an alternate method for palliative care of metastatic bone pain [43,129-135]. In this regard, EDTMP and DOTMP (Figure 9A and 9B) labeled with 177Lu have been clinically established as effective radiopharmaceuticals for bone pain palliation [42,45]. Though these radiopharmaceuticals were developed at the beginning of this century, significant progress took place in the clinical evolution of these radiopharmaceuticals over the last decade and hence dealt in here. Few other bone-targeting 177Lu-based radiopharmaceuticals have been developed and used in clinical settings over the last decade, a summary of which is provided in Table 3.
Figure 9.
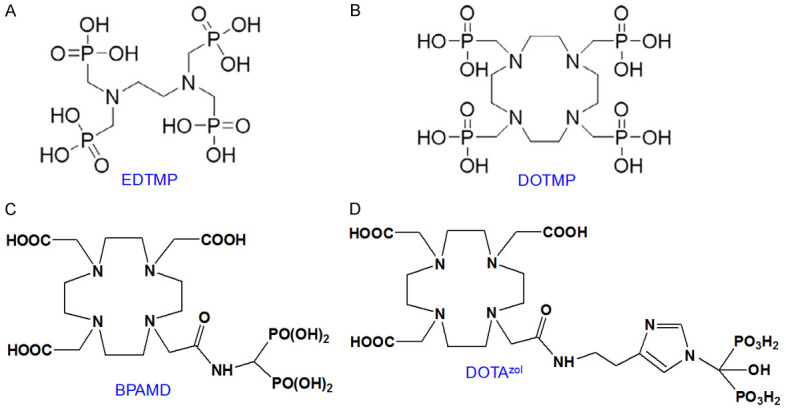
Structures of (A) EDTMP, (B) DOTMP, (C) BPAMD and (D) DOTAzol.
Table 3.
Bone targeting 177Lu-based radiopharmaceuticals used in clinical context
| Formulation | Type of study | Total population | Clinical outcome | Ref. |
|---|---|---|---|---|
| 177Lu-EDTMP | Phase 0 | 6 patients with metastatic prostate cancer | 177Lu-EDTMP has excellent pharmacokinetic and dosimetric properties, besides being safe and effective. | [136] |
| Phase I | 21 patients with metastatic prostate cancer | |||
| Phase II | 44 patients with documented breast carcinoma (12 patients) or castration-resistant prostate carcinoma (32 patients) and skeletal metastases | 177Lu-EDTMP was found to be a safe and effective radiopharmaceutical for bone pain palliation in patients with metastatic prostate and breast carcinoma. There were no differences in efficacy or toxicity between patients receiving low-dose and high-dose 177Lu-EDTMP. | [137] | |
| 177Lu-DOTMP | Preliminary clinical investigation | 5 male patients with metastatic prostate cancer or lung cancer | Desired human pharmacokinetic features, namely, preferential localization of 177Lu-DOTMP in skeletal lesion sites and almost no uptake in soft tissue or any other major nontarget organ was observed. Also, reduction of pain and almost no adverse side effects in patients was exhibited. | [44] |
| Systematic clinical investigation in larger cohort of patients | 27 male patients with histologically proven carcinoma and painful widespread skeletal metastases | 177Lu-DOTMP is an effective treatment option for immediate bone pain palliation with improvement in quality of life. The notable adverse effects were transient mild-moderate hematotoxicity and was well-tolerated by all the patients. | [141] | |
| 177Lu-BPAMD | Preliminary clinical investigation | 2 patients with metastatic prostate cancer | Significant reduction in pain after 1 week without any adverse effect. | [149] |
| 177Lu-DOTAzol | Systematic clinical investigation | 40 patients with metastatic prostate, breast or lung cancer | According to the visual analogue score (VAS) criteria, complete, partial, and minimal responses were observed in 11 (27.5%), 20 (50%), and 5 patients (12.5%), respectively with an overall response rate of 90% with 27.5% of complete response and 50% of partial response. Overall, 177Lu-DOTAzol is an ideal, safe and effective agent in the treatment of metastatic bone pain. | [157] |
Using 177Lu produced via direct neutron activation route, the results of Phase 0/I study dealing with the pharmacokinetics, dosimetry and toxicity analysis of 177Lu-EDTMP in patients was first reported by Bal et al. [136]. In this study, the authors assessed the biokinetics of skeletal and non-skeletal uptake of 177Lu-EDTMP (172.7-206.9 MBq) in 6 different patients with metastatic prostate cancer. The authors obtained the data of whole skeletal uptake, blood and fractionated urine samples and performed dosimetric calculations. Prolonged retention of activity in bone was observed in all the patients. It was observed that excretion took place mainly through renal route and blood clearance was rapid and biphasic. The mean estimated dose to the red marrow was 0.8±0.15 mGy/MBq while the mean total body dose was 0.16±0.04 mGy/MBq. A maximum tolerated dose (MTD) of 2000-3250 MBq for 177Lu-EDTMP was calculated. For the phase I study, therapeutic dose (692-5550 MBq) of 177Lu-EDTMP was administered in patients with metastatic prostate cancer. The toxicity of the dose was evaluated by assessment of hemoglobin levels, platelet and leukocyte counts over 12 weeks. Out of the 21 patients, transient toxicity was observed in 14 patients of whom 6 had baseline toxicity. Grade 3-4 toxicity was manifested in significantly higher number of patients beyond the MTD. Visual analog scale (VAS) score was used for analyzing the pain relief and it was found that 86% of the patients got relief within the period of 7 weeks. Based on these results, the authors concluded that 177Lu-EDTMP not only has excellent pharmacokinetic and dosimetric characteristics but is also safe and effective. The study was extended by the same group of authors, wherein they conducted Phase II study with 177Lu-EDTMP in patients with breast and prostate cancer [137]. In this study also, the radiopharmaceutical was found to be safe and efficacious. There was no difference in toxicity or efficacy between patients receiving low and high doses of 177Lu-EDTMP.
In another interesting study, Thapa et al. compared 177Lu-EDTMP with United States Food and Drug Administration (US FDA) approved agent, 153Sm-EDTMP in patients with skeletal metastases due to primary cancer [138]. The patients were divided into two groups: one group was administered with 177Lu-EDTMP and the other group was administered with 153Sm-EDTMP. Doses of both the radiopharmaceuticals were maintained at 37 MBq/kg of body weight. Analgesic, pain, quality of life scores and bone proliferation marker were used to examine efficacy. From this study, the authors could conclude that 177Lu-EDTMP has pain response efficacy similar to that of 153Sm-EDTMP, coupled with minimal side effects and improved quality of life. Overall, 177Lu-EDTMP is a safe alternative to 153Sm-EDTMP and in view of relatively longer half-life of 177Lu, 177Lu-EDTMP is especially advantageous for centers having no nearby access to 153Sm-EDTMP. A similar clinical study was later reported by Sharma et al., where the authors highlighted that both 177Lu-EDTMP and 153Sm-EDTMP offered competitive efficacy for palliative care of metastatic bone pain and can be interchangeably used as per the availability [139].
Comparative studies of 177Lu-EDTMP with its macrocyclic analog 177Lu-DOTMP in preclinical settings established that 177Lu-EDTMP has marginally higher skeletal accumulation in comparison to that of 177Lu-DOTMP, while the latter showed slightly faster blood clearance along with lower retention in liver and kidneys [140]. Also, 177Lu-DOTMP demonstrates superior kinetic robustness compared to 177Lu-EDTMP. Our group has developed a robust and easily adaptable protocol for formulation of clinically relevant dose of 177Lu-DOTMP in a hospital radiopharmacy [44]. The radiolabeled formulation demonstrated excellent in vitro stability and desired pharmacokinetics in preclinical settings. Preliminary clinical investigations were carried out in limited number of patients which showed specific skeletal accumulation with preferential localization in the metastatic lesion sites and almost no uptake in soft tissue or any other major non-target organ (Figure 10). Pain reduction was observed in all the patients 1 week after therapy. Recently, investigation in a larger cohort of patients was carried out by us to infer on the clinical efficacy and safety of the radiopharmaceutical [141]. In this study, 27 patients with painful skeletal metastases were administered with 37 MBq/kg dose of 177Lu-DOTMP. Overall response was seen in 77.8% of patients with significant improvement in median VAS and mean analgesic score (AS) at 2 months post-therapy. Best response was seen in patients with breast cancer (100%) followed by prostate cancer (81%) and lung cancer (28%). Improvement in quality of life was noted in 40% of patients. However, grade 2/3 anaemia, grade 1/2 leukopenia and grade 1/3 thrombocytopenia were seen in 37%, 11.1%, and 18.5% patients, respectively in the follow-up. It could be inferred that 177Lu-DOTMP was an effective treatment option for bone pain palliation and led to improvement in quality of life. The radiopharmaceutical appeared to be safe with mild to moderate hematotoxicity which was well tolerated by the patients. Nevertheless, controlled studies with larger sample size are warranted for better evaluation of the overall survival of the patients.
Figure 10.
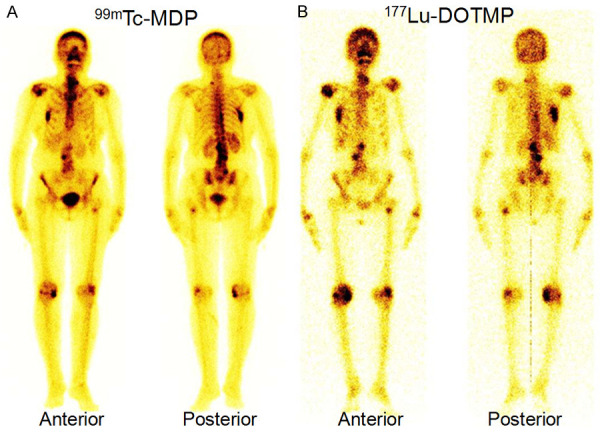
Anterior and posterior images from (A) 99mTc-MDP WBS of a 55-year-old woman with breast cancer showing widespread skeletal metastases, (B) 177Lu-DOTMP post-therapy images showing uptake pattern similar to that of 99mTc-MDP bone scan.
A strategy which has recently evolved is to form well-defined and highly stable complex by keeping the bisphosphonate moiety out of the radiometal chelating framework and using the conventional bifunctional chelating approach for complexation with 177Lu [142-148]. In this regard, a new macrocyclic bisphosphonate [4-{[bis-(phosphonomethyl))carbamoyl]methyl}-7,10-bis(car-boxymethyl)-1,4,7,10-tetraazacyclododec-1-yl)acetic acid)] (BPAMD) has been developed which guarantees 177Lu-complexation with high thermodynamic stability and appreciable kinetic rigidity in vivo (Figure 9C) [145,146]. Consequently, BPAMD concentration in the radiopharmaceutical could be significantly lowered which aided towards targeting micrometastatic sites, resulting in enhanced therapeutic efficacy. Our group has developed a protocol for formulation of therapeutically relevant dose of 177Lu-BPAMD using medium specific activity 177Lu produced by direct 176Lu (n,γ) 177Lu reaction [149]. After establishing the efficacy of the product in preclinical settings, preliminary clinical investigations were carried out in limited number of patients with metastatic bone pain. Intense uptake of the radiopharmaceutical was observed in the metastatic skeletal lesions with insignificant uptake in any other major non-targeted organs. In all the patients, considerable reduction in pain was observed after one week without any adverse effects.
Despite promising clinical results obtained in palliative radiotherapy using 177Lu-BPAMD, there is still much potential for improvement with regard to further enhancement of accumulation of the radiolabeled agent in bone metastases and minimizing the uptake in non-targeted organs. In this regard, the side chains on the central carbon atom of the bisphosphonate moiety play a significant role in the bisphosphonate’s activity, i.e., in terms of affinity to hydroxyapatite (the constituent of bone). Higher bone accumulation can be observed using a hydroxybisphosphonate because of increased affinity for hydroxyapatite [150]. Furthermore, an aromatic nitrogen atom in the side chain could cause building of another hydrogen bond and thereby also raise bone accumulation [146,151]. One such bisphosphosphonate is zoledronic acid, which has also been found to influence biochemical processes. These molecules possess an inhibiting effect on the farnesyl diphosphate synthase (FPPS) and inhibition of this enzyme causes an increased apoptosis rate [146,151]. Taking these factors into consideration, a DOTA-conjugated zoledronate (DOTAzol, Figure 9D) was synthesized and labeled with 68Ga and 177Lu and examined in in vitro and ex vivo biodistribution studies, as well as small animal PET and SPECT studies [152-156]. Recently, Yadav et al. evaluated the safety and efficacy of 177Lu-DOTAzol as bone pain palliation agent in 40 patients suffering from bone metastasis due to variety of cancers [157]. The patients were treated with either 1 or 2 cycles of 177Lu-DOTAzol. The biodistribution and uptake of 177Lu-DOTAzol in a patient with skeletal metastases from prostate cancer is shown in Figure 11. According to the VAS response assessment criteria, complete, partial, and minimal responses were observed in 11 (27.5%), 20 (50%), and 5 patients (12.5%), respectively with an overall response rate of 90%. Though this was a non-randomized clinical study, the promising results obtained herein amply demonstrated that 177Lu-DOTAzol is an ideal, safe and effective agent in the treatment of metastatic bone pain.
Figure 11.
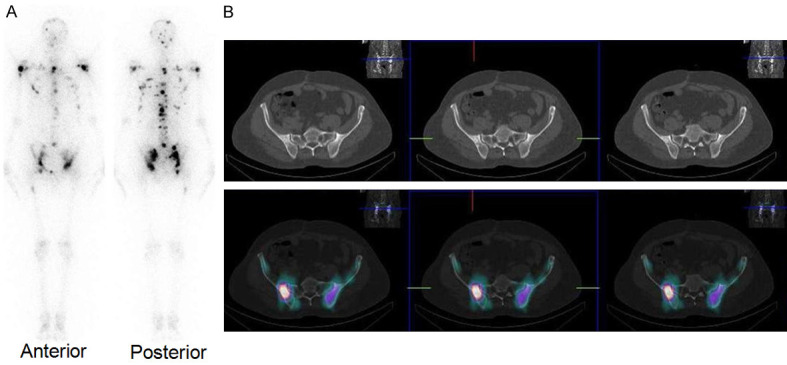
A 55-year-old male diagnosed with prostatic adenocarcinoma was administered with 177Lu-DOTAzol. (A) Post-therapy WBS 24 h post-administration showed uptake in multiple skeletal sites, (B) Post-therapy SPECT/CT showed uptake in the pelvic bone metastases. Adapted from Ref [157].
Integrin αvβ3 targeting agents
Integrin αvβ3, which is a receptor for the extracellular matrix proteins with the exposed arginine(R)-glycine(G)-aspartic acid(D) tripeptide sequence, plays an important role in angiogenesis during tumor growth and metastasis. For both early detection as well as treatment of rapidly growing solid tumors, the over-expression of integrin αvβ3 presents an interesting molecular target The preparation and biological evaluation of 177Lu-labeled cyclic RGD peptide dimer E[c(RGDfK)]2 (E = glutamic acid) coupled with DOTA (1,4,7,10-tetraazacyclododecane-1,4,710-tetraacetic acid) [177Lu-DOTA-E[c(RGDfK)]2] in BALB/c bearing human glioblastoma (U87MG) xenograft was reported by Shi et al. [158] and Luna-Gutierrez et al. [159]. The radiotracer demonstrated specific uptake in the tumor. Realizing the scope of this agent for future clinical investigation, our group optimized the protocol for formulation of clinically relevant doses of 177Lu-DOTA-E[c(RGDfK)]2 using medium specific activity 177Lu produced via direct neutron activation route [160]. In this study, a therapeutic dose (7.4 GBq) of the radiolabeled agent was formulated in ~63 GBq/μM specific activity with high yield (98.2±0.7%) and appreciable in vitro stability. Biodistribution studies carried out in C57BL/6 mice bearing melanoma tumors revealed specific accumulation of the radiolabeled conjugate in tumor (3.80±0.55% ID/g at 30 min p.i.) with high tumor-to-blood and tumor-to-muscle ratios. This reported protocol could be followed to prepare clinical dose of the radiolabeled agent in a hospital radiopharmacy.
In a latest development, Parihar et al. demonstrated the utility of 177Lu-DOTA-E[c(RGDfK)]2 in treatment of patients with differentiated thyroid carcinoma and presenting with thyroglobulin elevation with negative 131I scintigraphy (TENIS) [161]. In fact, TENIS in thyroid cancer patients causes a serious management challenge due to limited treatment modalities. Recently, RGD peptides have been identified as effective agents for this purpose due to overexpression of integrin αvβ3 in differentiated thyroid cancer [161]. The authors reported the case of a 54-year-old female patient with papillary thyroid cancer who developed TENIS syndrome after receiving 500 GBq of 131I in cumulative doses. The patient experienced considerable adverse effects with hardly any clinical improvement on sorafenib therapy for 1 year. She also presented with severe pain and a palpable hard mass in the pre-sternal region. After 68Ga-DOTA-E[c(RGDfK)]2 PET/CT scan for evaluating the extent of the disease and pre-therapy assessment, she was administered with 5.5 GBq of 177Lu-DOTA-E[c(RGDfK)]2 (Figure 12). Post-therapy follow-up showed significant pain relief and reduced pre-sternal swelling, suggesting clinical benefit. At 4 months post-therapy, 68Ga-DOTA-E[c(RGDfK)]2 PET/CT scan showed reduced uptake in the cancerous lesions indicating clinical benefit (Figure 12). Though the results of this preliminary clinical study are quite promising, randomized clinical trials in large cohort of patients are warranted to understand the real benefits of this approach.
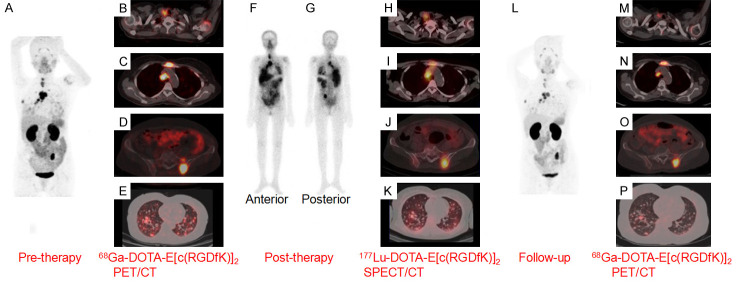
Figure 12.
A 54-year-old woman with papillary thyroid carcinoma who developed TENIS syndrome after receiving 500 GBq of 131I in cumulative doses, was administered with 177Lu-DOTA-E[(cRGDfK)2] therapy. 68Ga-DOTA-E[(cRGDfK)2] PET/CT was performed to evaluate disease extent and for pre-therapy assessment. (A) Pre-therapy, the maximum intensity projection (MIP) image with 68Ga-DOTA-E[(cRGDfK)2] PET scan, transaxial fused PET/CT images showed increased tracer uptake in the (B) thyroid remnant, (C) cervical lymph nodes (D) mediastinal lymph node, lytic skeletal lesions with soft tissue component in the sternum and left iliac bone, (E) multiple lung nodules. Post-therapy WBS in (F) anterior and (G) posterior views revealing the overall distribution of 177Lu-DOTA-E[(cRGDfK)2] and transaxial fused SPECT/CT images (H-K) showing tracer uptake at sites corresponding to 68Ga-DOTA- E[(cRGDfK)2] -avid lesions. (L) Post-therapy follow-up 68Ga-DOTA- E[(cRGDfK)2] PET/CT MIP image and transaxial fused PET/CT images showed tracer uptake in the (M) thyroid remnant with cervical lymph nodes, (N) mediastinal lymph node, lytic skeletal lesions with significant reduction in soft tissue component in the sternum, (O) left iliac bone and (P) multiple lung nodules, suggesting response to therapy. Adapted from Ref [161].
Monoclonal antibodies
Over the last several years, radiolabeled monoclonal antibodies are being increasingly used for personalized management of various types of cancers [54,162-166]. Lutetium-177 is an important radioisotope in this regard as its relatively long half-life matches the pharmacokinetics of the antibodies. Our group had reported the formulation of clinically relevant doses of 177Lu-labeled cetuximab using low specific activity 177Lu and demonstrated its efficacy in preclinical settings [167]. Subsequently, in order to improve the pharmacokinetics, Fab fragments of cetuximab were generated by papain digestion and used for radiolabeling with 177Lu for formulation of therapeutically relevant doses [168]. The promising results obtained in these studies might expedite the process of formulation of large doses of 177Lu-labeled immunoconjugates in clinical settings for the benefit of the cancer patients. In a recent preclinical study, a CD38 targeting monoclonal antibody (daratumumab) was radiolabeled with 89Zr and 177Lu for potential theranostic applications [169]. The results suggested that CD38 is a lymphoma specific marker, meriting further exploration in clinical context. The authors reported high and persistent tumor uptake and significant tumor inhibition with radiolabeled daratumumab for the theranostic of CD38-positive cancer in mice model. Histological and hematological analyses established negligible toxicity of 177Lu-labeled daratumumab in mice model. It was inferred that further advancement of this strategy would aid significantly in lymphoma patient stratification and management.
The first clinical study with 177Lu-labeled monoclonal antibody using low specific activity 177Lu was reported by Yadav et al., wherein the authors studied the dosimetry of 177Lu-DOTA-rituximab in 10 patients with relapsed/refractory non-Hodgkin’s lymphoma [170]. In this study, rituximab (375 mg/m2), followed by 1.85 GBq of 177Lu-DOTA-rituximab was administered as a slow intravenous infusion and SPECT images were acquired and internal dose estimation was performed using OLINDA software. The authors determined that the effective half-life of 177Lu-DOTA-rituximab was 100±28 h and the critical organ in their study was the red marrow. The average total body dose, effective dose, and effective dose equivalent calculated in all 10 patients were 0.13±0.02, 0.15±0.03, and 0.22±0.04 mGy/MBq, respectively. A major limitation of this study was the lack of generalization or extrapolation of doses in the clinical setting. Hence, patient specific dosimetry is warranted in a larger cohort of patients to get rid of the variations and reduce the possibility of dose-limiting toxicity. In another study, Bhusari et al. formulated clinically relevant doses of 177Lu-DOTA-trastuzumab and performed feasibility of radioimmunotherapy assessment in breast cancer patients [171]. The patient studies showed the localization of 177Lu-DOTA-trastuzumab at primary as well as metastatic sites (HER2 positive) in the planar and SPECT/CT images (Figure 13). No tracer uptake was observed in HER2 negative patients that indicated the specificity of the radioimmunoconjugate (Figure 13). Despite promising results, more clinical studies need to be performed with escalating trastuzumab doses and dosimetric evaluation to determine the therapeutic index of 177Lu-DOTA-trastuzumab.
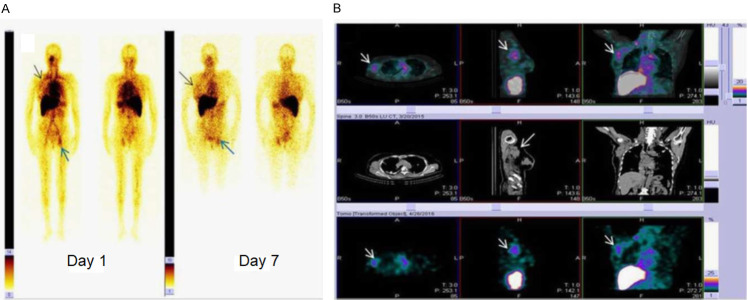
Figure 13.
A 60-year-old breast cancer patient (HER2 +ve) was administered with 177Lu-DOTA-trastuzumab therapy. A. WBS at day 1 and day 7 post administration of 177Lu-DOTA-trastuzumab. Tracer uptake can be observed in primary breast tumor (black arrow head). The bone metastasis in the acetabulum region was visualized at day 1 and day 7 (blue arrow heads). B. SPECT/CT, CT, and SPECT images showing lymph node metastases which could not be localized on WBS (white arrow heads). Adapted from Ref [171].
Radiation synovectomy agents
In radiation synovectomy, beta-emitting radionuclides in colloidal or particulate form (1-10 μm size range) are intraarticularly injected into the affected synovial joints. This is an effective treatment modality in patients suffering from inflammatory-rheumatoid and degenerative joint diseases [172-180]. In this treatment modality, the uptake of radionuclides occurs in the synovial lining cells, phagocytized by the outermost cellular layer of the synovial membrane and deliver radiation dose to the synovium without excessive irradiation of surrounding tissue. Owing to its favorable nuclear decay characteristics, cost-effective availability and ease of production, medium specific activity 177Lu is an appealing radioisotope for preparation of radiation synovectomy agents. For the first time, our group reported a kit-based strategy for expedient formulation of ~400 MBq dose of 177Lu-labeled hydroxyapatite (177Lu-HA) particles at hospital radiopharmacy and its clinical investigations in patients with rheumatoid arthritis of knee joints [181,182]. Significant improvement in disease condition was observed in all the patients within 15 days post-therapy. No significant onset of pain was observed within 30 days post-therapy. Similar results were obtained on administration of 177Lu-HA in patients with rheumatoid arthritis of wrist joints (Figure 14). Despite encouraging results, clinical studies on larger number of patients and pain response over a longer period of time are warranted to understand the efficacy of this treatment modality.
Figure 14.
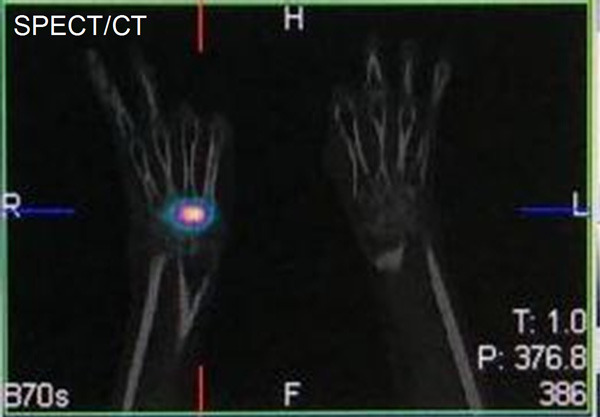
A 56-year-old male patient with rheumatoid arthritis of wrist joint. SPECT/CT image 24 h post-administration of 222 MBq of 177Lu-HA.
In a preliminary clinical study, Arora et al. synthesized 177Lu-labeled tin colloid (mean size: 241±47 nm) and injected into the infected knee joint of a patient [183]. SPECT scan revealed homogenous distribution of the product in the intra-articular space. No leakage of activity was observed outside the knee joint even 7 days after injection, indicating good binding of the radiolabeled agent and its in vivo stability. As an extension of this work, the same group of authors evaluated the efficacy of 177Lu-labeled tin colloid in 29 patients with chronic synovitis caused by various inflammatory knee joint diseases which were refractory to conventional therapy [184]. Out of the 29 patients, 21 were responders and 8 were non-responders at 3 months after radiation synovectomy. No serious adverse effects were observed in any of the patients. The authors concluded that 177Lu-labeled tin colloid is a useful treatment modality and is beneficial for the patients with shorter duration of disease and with normal or minor radiographic findings. In a recent study, the same group of authors further compared the clinical efficacy of 177Lu-labeled tin colloid with 188Re-labeled tin chloride for radiation synovectomy to reduce pain in patients with chronic inflammatory arthritis of knee [185]. The authors administered 188Re-labeled tin chloride in 27 patients and 177Lu-labeled tin colloid in 30 patients. Out of 30, 20 patients responded to radiosynovectomy with 177Lu-labeled tin colloid and 21 patients out of 27 responded to the same with 188Re-labeled tin colloid. Overall, the authors concluded that 177Lu-labeled tin colloid is an effective alternative to 188Re-labeled tin colloid for radiation synovectomy especially in places which do not have access to 188W/188Re generators.
Radiolabeled nanoparticles
Over the last decade, a large variety of radiolabeled nanoparticles have been synthesized and assessed for their possible use in cancer imaging and therapy [186-198]. The advantages of using radiolabeled nanoparticles for this purpose are manifold [194]. Firstly, compared to conventional radiotracers, radiolabeled nanoparticles act as signal amplifiers leading to increased contrast indices and enhanced sensitivity. Secondly, nanoparticles can be conjugated with variety of targeting ligands such as peptides, antibodies, aptamers for enhanced uptake and retention by active targeting in addition to enhanced permeability and retention (EPR) effect. Thirdly, nanoparticles offer the scope of multimodality imaging which provides synergistic advantages over any single imaging modality. Fourthly, both diagnostic and therapeutic capabilities can be integrated in the same nanoplatform, thereby giving the benefits of theranostics. Finally, different therapeutic modalities (chemotherapy, radionuclide therapy, etc.) can be incorporated in the same nanoplatforms giving rise to enhanced therapeutic benefits.
For the first time, Arora et al. reported the synthesis of poly(D,L-Lactide-co-Glycolide) (PLGA) nanospheres coated with polyethylene glycol (PEG) [199]. The nanoparticles were spherical in shape with mean particle diameter of ~300 nm. 177Lu-DOTATATE was encapsulated with >75% efficiency in the synthesized nanoparticles by incubating the nanoparticles with the radiolabeled agent at 37°C, overnight. The authors performed in vivo SPECT imaging and biodistribution studies in normal rats after intravenous administration of the radiolabeled nanoparticles and observed reduced renal retention of 177Lu-DOTATATE. These results suggest the potential of this strategy in achieving reduction in nephrotoxicity and unnecessary radiation dose to the normal tissue, which might have implications in targeted therapy of neuroendocrine tumors. Subsequently, in another study, the same group of authors evaluated the in vitro toxicity of the radiolabeled nanoparticles in U87MG (human glioblastoma) cell line [200]. The highest cytotoxicity that could be achieved on radioresistant U87MG cells was 35.8%. In vivo SPECT imaging and biodistribution studies in tumor bearing Wistar rats showed the highest tumor uptake of ~4.5% ID/g at 24 h post-injection (p.i.). The main limitations of these studies are the very slow method of radiolabeling and the lack of data to prove the therapeutic potential of the radiolabeled nanoparticles.
Recently, attempts have been made towards synthesis of intrinsically radiolabeled nanoparticles which represents a new paradigm in nanomedicine research [201-210]. The intrinsically radiolabeled nanoparticles offer several advantages over the conventional chelator-based radiolabeling techniques: this approach offers highly efficient, radiochemically more stable, faster and robust radiolabeling possibilities without changing the innate pharmacokinetics of the nanoparticles. This strategy is especially advantageous for low specific activity radioisotopes such as 177Lu produced by direct neutron activation route using natural targets for development of effective therapeutic agents. In this direction, our group has reported the synthesis of intrinsically radiolabeled [177Lu]Lu2O3 nanoparticles entrapped in human serum albumin scaffold, as schematically illustrated in Figure 15 [211]. The particle size of the nanoparticles was in the range of 2-6 nm. In vitro cell binding and toxicity studies in murine melanoma (B16F10) cells demonstrated the suitability of the radiolabeled nanoparticles as a therapeutic agent. Ex vivo biodistribution studies in melanoma tumor bearing mice demonstrated fast and high accumulation of the radiolabeled nanoparticles in the tumor (11.7±2.1% ID/g at 4 h p.i.) with significant retention up to 7 days. The therapeutic efficacy of the intrinsically radiolabeled nanoparticles was demonstrated by tumor regression studies performed over a period of 21 days (Figure 16). The promising results obtained in this study demonstrate the potential of this class of radiolabeled nanoparticles for potential clinical translation.
Figure 15.
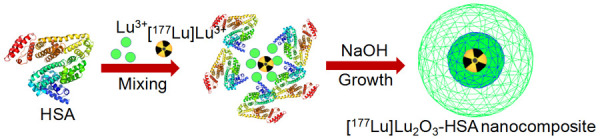
Schematic illustration of the synthesis of intrinsically radiolabeled [177Lu]Lu2O3-HSA nanocomposite. Adapted from Ref [211].
Figure 16.
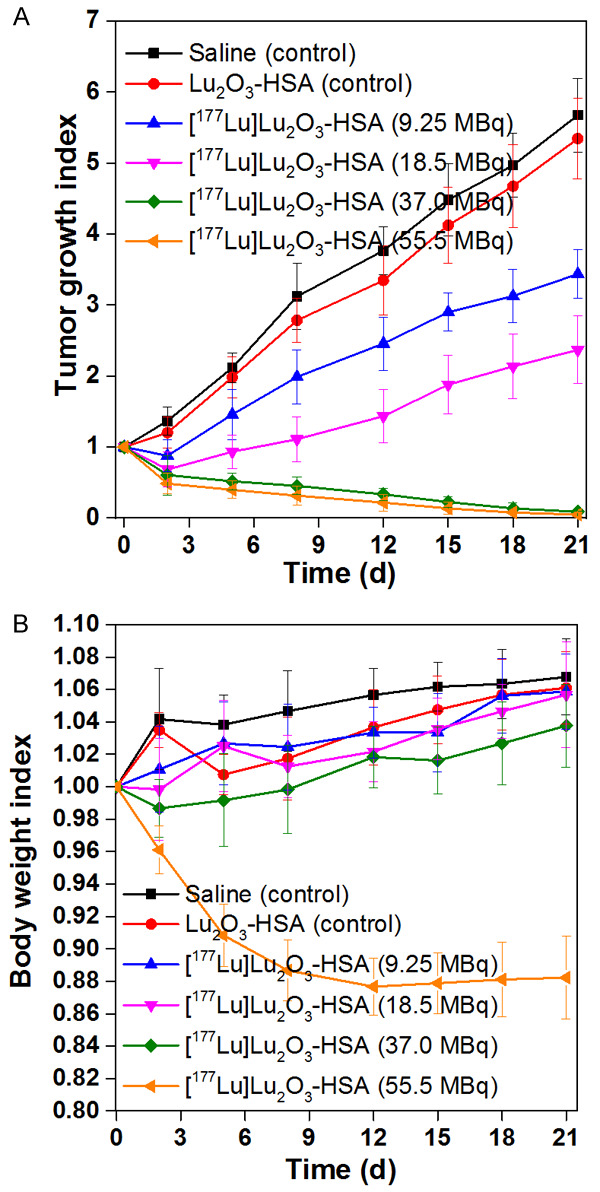
Tumor regression studies with [177Lu]Lu2O3-HSA nanocomposite. (A) Tumor growth index and (B) body weight index curves of C57BL/6 mice bearing melanoma tumor after intravenous injection of saline (control), Lu2O3-HSA (control), 9.25 MBq of [177Lu]Lu2O3-HSA nanocomposite, 18.5 MBq [177Lu]Lu2O3-HSA nanocomposite, 37 MBq [177Lu]Lu2O3-HSA nanocomposite and 55.5 MBq [177Lu]Lu2O3-HSA nanocomposite. Adapted from Ref [211].
Conclusions
In this treatise, the multifaceted aspects of 177Lu-radiopharmacy were discussed emphasizing on the utility of 177Lu produced via direct neutron activation route over the last decade in clinical context. Surely, 177Lu has emerged as the most widely used therapeutic radioisotope after 131I and the clinical efficacies of several 177Lu-based radiopharmaceuticals have amply been demonstrated. The favorable nuclear decay characteristics and the widespread and affordable availability of the radioisotope are the main reasons behind its widespread clinical use. The relatively long half-life of 177Lu provides distinct logistic advantages especially in countries with limited reactor facilities for radioisotope production. The feasibility of large-scale production with adequate specific activity in medium flux research reactors via facile direct neutron activation route is another desirable feature for widespread utilization of this radioisotope. According to the IAEA research reactor database [31], there are >250 operational nuclear reactors worldwide, out of which 50 reactors have thermal neutron flux over 1 × 1014 n.cm-2.s-1 which is acceptable for production of 177Lu by the direct route for preparation of target specific radiopharmaceuticals. These reactors have good geographical distribution and hence their utilization would ensure cost-effective and widespread utilization of 177Lu for clinical use.
Though, some groups especially in the developed countries have preference for NCA 177Lu in view of its higher specific activity, the production of the radioisotope by the indirect route is still a costly proposition and requires access to high flux research reactors, very few of which are presently available in the world. In the indirect route, only a very small fraction of the enriched 176Yb target is transformed into 177Lu due to the low cross section of 176Yb. The 177Lu recovery from the target requires the employment of effective Lu/Yb separation methods, whose characteristics are not only crucial for the practicality of the indirect production route but also increases the overall cost. In this review, the practical and unclear issues regarding production of 177Lu by both the routes in a nuclear reactor were discussed to provide the readers an in-depth holistic overview regarding the production aspects.
The chemistry of 177Lu in the form of Lu3+ allows for facile radiolabeling either directly with different biomolecules such as EDTMP and DOTMP or through the use of BFCs. Among the various BFCs studied, DOTA is undoubtedly the ‘gold standard’ in preparation of 177Lu-based radiopharmaceuticals. Complexes of 177Lu with DOTA and its derivatives are formed with high radiochemical yield and they exhibit excellent in vitro and in vivo stability. However, the kinetics of the complexation process with macrocyclic DOTA chelator is slow and requires heating at ~90°C for achieving near quantitative yield. This is in fact undesirable for radiolabeling temperature sensitive biomolecules such as monoclonal antibodies. From this perspective, the new AAZTA chelator which manifests rapid room temperature radiolabeling with high stability is a valuable proposition and would especially be advantageous towards 177Lu-based radioimmunotherapy. The use of AAZTA chelator would also simplify the radiopharmacy practices for other 177Lu-based theranostic agents.
Till recently, 177Lu-DOTATATE held the distinction of being the most widely used 177Lu-based radiopharmaceutical, which has now been overtaken by 177Lu-PSMA-617 because of the much larger number of prostate cancer cases compared to neuroendocrine tumors. Together, 177Lu-PSMA-617 and 177Lu-DOTATATE encompass for use of >90% of the 177Lu produced worldwide. It has been amply demonstrated from the large number of clinical studies summarized in this review that even while using medium specific activity 177Lu produced by the direct neutron activation route, 177Lu based radiopharmaceuticals can be prepared with adequate radiochemical purity and specific activity for effective and efficient targeted cancer therapy. The concept of intrinsically radiolabeled nanoparticles is an emerging concept in personalized cancer therapy which would aid toward development of agents which show enhanced tumor targeting even while using low specific activity 177Lu produced by inexpensive natural Lu target. However, for clinical translation of this emerging therapeutic modality, synthesis of biocompatible and biodegradable nanoplatforms which demonstrate renal clearable properties to minimize toxicity concerns is desirable [212-216]. Also, there is an ever-increasing possibility of identifying new molecular targeting vectors such as new peptides, monoclonal antibodies and their engineered fragments, nanobodies, aptamers, etc. for synthesis of new generation of 177Lu based radiopharmaceuticals for personalized cancer treatment. The present advances are just the tip of the iceberg and more exciting avenues are yet to open up. To explore the ‘gold mine’ of 177Lu-radiopharmacy to its fullest potential, increased interdisciplinary collaboration and knowledge exchange among various stakeholders in this field, which include, funding agencies, regulatory authorities, clinicians, and scientists should be encouraged for development and clinical deployment of newer 177Lu-based agents for the benefit of the millions of cancer patients worldwide.
Disclosure of conflict of interest
None.
References
- 1.Turner JH. Recent advances in theranostics and challenges for the future. Br J Radiol. 2018;91:20170893. doi: 10.1259/bjr.20170893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choudhury P, Gupta M. Personalized & precision medicine in cancer: a theranostic approach. Curr Radiopharm. 2017;10:166–170. doi: 10.2174/1874471010666170728094008. [DOI] [PubMed] [Google Scholar]
- 3.Farolfi A, Lima GM, Oyen W, Fanti S. Molecular imaging and theranostics-a multidisciplinary approach. Semin Nucl Med. 2019;49:247–254. doi: 10.1053/j.semnuclmed.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Filippi L, Chiaravalloti A, Schillaci O, Cianni R, Bagni O. Theranostic approaches in nuclear medicine: current status and future prospects. Expert Rev Med Devices. 2020;17:331–343. doi: 10.1080/17434440.2020.1741348. [DOI] [PubMed] [Google Scholar]
- 5.Jeelani S, Reddy RC, Maheswaran T, Asokan GS, Dany A, Anand B. Theranostics: a treasured tailor for tomorrow. J Pharm Bioallied Sci. 2014;6:S6–8. doi: 10.4103/0975-7406.137249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boros E, Packard AB. Radioactive transition metals for imaging and therapy. Chem Rev. 2019;119:870–901. doi: 10.1021/acs.chemrev.8b00281. [DOI] [PubMed] [Google Scholar]
- 7.Brandt M, Cardinale J, Aulsebrook ML, Gasser G, Mindt TL. An overview of PET radiochemistry, part 2: radiometals. J Nucl Med. 2018;59:1500–1506. doi: 10.2967/jnumed.117.190801. [DOI] [PubMed] [Google Scholar]
- 8.Kostelnik TI, Orvig C. Radioactive main group and rare Earth metals for imaging and therapy. Chem Rev. 2019;119:902–956. doi: 10.1021/acs.chemrev.8b00294. [DOI] [PubMed] [Google Scholar]
- 9.Mikolajczak R, van der Meulen NP, Lapi SE. Radiometals for imaging and theranostics, current production, and future perspectives. J Labelled Comp Radiopharm. 2019;62:615–634. doi: 10.1002/jlcr.3770. [DOI] [PubMed] [Google Scholar]
- 10.Notni J, Wester HJ. Re-thinking the role of radiometal isotopes: towards a future concept for theranostic radiopharmaceuticals. J Labelled Comp Radiopharm. 2018;61:141–153. doi: 10.1002/jlcr.3582. [DOI] [PubMed] [Google Scholar]
- 11.Dash A, Pillai MR, Knapp FF Jr. Production of 177Lu for targeted radionuclide therapy: available options. Nucl Med Mol Imaging. 2015;49:85–107. doi: 10.1007/s13139-014-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee S, Pillai MR, Knapp FF. Lutetium-177 therapeutic radiopharmaceuticals: linking chemistry, radiochemistry, and practical applications. Chem Rev. 2015;115:2934–2974. doi: 10.1021/cr500171e. [DOI] [PubMed] [Google Scholar]
- 13.Hermanne A, Takacs S, Goldberg MB, Lavie E, Shubin YN, Kovalev S. Deuteron-induced reactions on Yb: measured cross sections and rationale for production pathways of carrier-free, medically relevant radionuclides. Nucl Instrum Methods Phys Res B. 2006;247:223–231. [Google Scholar]
- 14.Manenti S, Groppi F, Gandini A, Gini L, Abbas K, Holzwarth U, Simonelli F, Bonardi M. Excitation function for deuteron induced nuclear reactions on natural ytterbium for production of high specific activity 177gLu in no-carrier-added form for metabolic radiotherapy. Appl Radiat Isot. 2011;69:37–45. doi: 10.1016/j.apradiso.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Khandaker MU, Haba H, Kassim HA. Production of 177Lu, a potential radionuclide for diagnostic and therapeutic applications. AIP Conf Proc. 2015;1657:120003. [Google Scholar]
- 16.Manenti S, Bonardi ML, Gini L, Groppi F. Physical optimization of production by deuteron irradiation of high specific activity 177gLu suitable for radioimmunotherapy. Nucl Med Biol. 2014;41:407–409. doi: 10.1016/j.nucmedbio.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Kambali I. Production of Lu-177 radionuclide using deuteron beams: comparison between (d,n) and (d,p) nuclear reactions. J Phys Conf Ser. 2018;1120:012011. [Google Scholar]
- 18.Medvedev DG, Mausner LF, Greene GA, Hanson AL. Activation of natural Hf and Ta in relation to the production of 177Lu. Appl Radiat Isot. 2008;66:1300–1306. doi: 10.1016/j.apradiso.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 19.Kuznetsov RA, Bobrovskaya KS, Svetukhin VV, Fomin AN, Zhukov AV. Production of lutetium-177: process aspects. Radiochemistry. 2019;61:381–395. [Google Scholar]
- 20.Dash A, Chakravarty R, Knapp FF Jr, Pillai AM. Indirect production of no carrier added (NCA) 177Lu from irradiation of enriched 176Yb: options for ytterbium/lutetium separation. Curr Radiopharm. 2015;8:107–118. doi: 10.2174/1874471008666150312161942. [DOI] [PubMed] [Google Scholar]
- 21.Vosoughi S, Salek N, Arani SS, Samani AB, Maragheh MG. Investigation of radiolabeling efficacy by enhancement of the chemical form of no carrier added 177Lu isolated by electro amalgamation process. Curr Radiopharm. 2021 doi: 10.2174/1874471014666210122150134. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Chakravarty R, Das T, Dash A, Venkatesh M. An electro-amalgamation approach to isolate no-carrier-added 177Lu from neutron irradiated Yb for biomedical applications. Nucl Med Biol. 2010;37:811–820. doi: 10.1016/j.nucmedbio.2010.04.082. [DOI] [PubMed] [Google Scholar]
- 23.Mikolajczak R, Parus JL, Pawlak D, Zakrzewska E, Michalak W, Sasinowska I. Reactor produced 177Lu of specific activity and purity suitable for medical applications. J Radioanal Nucl Chem. 2003;257:53–57. [Google Scholar]
- 24.Van So L, Morcos N, Zaw M, Pellegrini P, Greguric I. Alternative chromatographic processes for no-carrier added 177Lu radioisotope separation. J Radioanal Nucl Chem. 2008;277:663. [Google Scholar]
- 25.Horwitz EP, McAlister DR, Bond AH, Barrans RE, Williamson JM. A process for the separation of 177Lu from neutron irradiated 176Yb targets. Appl Radiat Isot. 2005;63:23–36. doi: 10.1016/j.apradiso.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto K, Matsuoka H, Uchida S. Production of no-carrier-added 177Lu via the 176Yb(n,γ)177Yb→177Lu process. J Radioanal Nucl Chem. 2003;255:575–579. [Google Scholar]
- 27.Lebedev NA, Novgorodov AF, Misiak R, Brockmann J, Rösch F. Radiochemical separation of no-carrier-added 177Lu as produced via the 176Yb(n,gamma)177Yb→177Lu process. Appl Radiat Isot. 2000;53:421–425. doi: 10.1016/s0969-8043(99)00284-5. [DOI] [PubMed] [Google Scholar]
- 28.Lahiri S, Nayak D, Nandy M, Das NR. Separation of carrier free lutetium produced in proton activated ytterbium with HDEHP. Appl Radiat Isot. 1998;49:911–913. [Google Scholar]
- 29.Dvorakova Z. Production and chemical processing of 177Lu for nuclear medicine at the Munich research reactor FRM-II. Ph.D. Thesis. (Available online at: https://mediatum.ub.tum.de/doc/625564/document.pdf 2007; Accessed on September 1, 2021)
- 30.Chakraborty S, Vimalnath KV, Lohar SP, Shetty P, Dash A. On the practical aspects of large-scale production of 177Lu for peptide receptor radionuclide therapy using direct neutron activation of 176Lu in a medium flux research reactor: the Indian experience. J Radioanal Nucl Chem. 2014;302:233–243. [Google Scholar]
- 31.IAEA Research Reactor Database. (https://nucleus.iaea.org/RRDB/RR/ReactorSearch.aspx) Accessed on September 1, 2021.
- 32.Liu S. Bifunctional coupling agents for radiolabeling of biomolecules and target-specific delivery of metallic radionuclides. Adv Drug Deliv Rev. 2008;60:1347–1370. doi: 10.1016/j.addr.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bailey GA, Price EW, Zeglis BM, Ferreira CL, Boros E, Lacasse MJ, Patrick BO, Lewis JS, Adam MJ, Orvig C. H2azapa: a versatile acyclic multifunctional chelator for 67Ga, 64Cu, 111In, and 177Lu. Inorg Chem. 2012;51:12575–12589. doi: 10.1021/ic302225z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brechbiel MW. Bifunctional chelates for metal nuclides. Q J Nucl Med Mol Imaging. 2008;52:166–173. [PMC free article] [PubMed] [Google Scholar]
- 35.Parus JL, Pawlak D, Mikolajczak R, Duatti A. Chemistry and bifunctional chelating agents for binding 177Lu. Curr Radiopharm. 2015;8:86–94. doi: 10.2174/1874471008666150312160440. [DOI] [PubMed] [Google Scholar]
- 36.Boros E, Holland JP. Chemical aspects of metal ion chelation in the synthesis and application antibody-based radiotracers. J Labelled Comp Radiopharm. 2018;61:652–671. doi: 10.1002/jlcr.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng H, Wang H, Li Z. Matching chelators to radiometals for positron emission tomography imaging-guided targeted drug delivery. Curr Drug Targets. 2015;16:610–624. doi: 10.2174/1389450116666150707100702. [DOI] [PubMed] [Google Scholar]
- 38.Park JA, Kim JY. Recent advances in radiopharmaceutical application of matched-pair radiometals. Curr Top Med Chem. 2013;13:458–469. doi: 10.2174/1568026611313040006. [DOI] [PubMed] [Google Scholar]
- 39.Price EW, Orvig C. Matching chelators to radiometals for radiopharmaceuticals. Chem Soc Rev. 2014;43:260–290. doi: 10.1039/c3cs60304k. [DOI] [PubMed] [Google Scholar]
- 40.Price EW, Zeglis BM, Cawthray JF, Lewis JS, Adam MJ, Orvig C. What a difference a carbon makes: H4octapa vs H4C3octapa, ligands for In-111 and Lu-177 radiochemistry. Inorg Chem. 2014;53:10412–10431. doi: 10.1021/ic501466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramogida CF, Orvig C. Tumour targeting with radiometals for diagnosis and therapy. Chem Commun (Camb) 2013;49:4720–4739. doi: 10.1039/c3cc41554f. [DOI] [PubMed] [Google Scholar]
- 42.Askari E, Harsini S, Vahidfar N, Divband G, Sadeghi R. 177Lu-EDTMP for metastatic bone pain palliation: a systematic review and meta-analysis. Cancer Biother Radiopharm. 2021;36:383–390. doi: 10.1089/cbr.2020.4323. [DOI] [PubMed] [Google Scholar]
- 43.Zakaly HMH, Mostafa MYA, Deryabina D, Zhukovsky M. Comparative studies on the potential use of 177Lu-based radiopharmaceuticals for the palliative therapy of bone metastases. Int J Radiat Biol. 2020;96:779–789. doi: 10.1080/09553002.2020.1729441. [DOI] [PubMed] [Google Scholar]
- 44.Chakraborty S, Vimalnath KV, Rajeswari A, Chakravarty R, Sarma HD, Radhakrishnan E, Kamaleshwaran K, Shinto AS, Dash A. A “mix-and-use” approach for formulation of human clinical doses of 177Lu-DOTMP at hospital radiopharmacy for management of pain arising from skeletal metastases. J Labelled Comp Radiopharm. 2017;60:410–419. doi: 10.1002/jlcr.3517. [DOI] [PubMed] [Google Scholar]
- 45.Das T, Shinto A, Karuppuswamy Kamaleshwaran K, Banerjee S. Theranostic treatment of metastatic bone pain with 177Lu-DOTMP. Clin Nucl Med. 2016;41:966–967. doi: 10.1097/RLU.0000000000001409. [DOI] [PubMed] [Google Scholar]
- 46.Kumar C, Sharma R, Das T, Korde A, Sarma H, Banerjee S, Dash A. 177Lu-DOTMP induces G2/M cell cycle arrest and apoptosis in MG63 cell line. J Labelled Comp Radiopharm. 2018;61:837–846. doi: 10.1002/jlcr.3651. [DOI] [PubMed] [Google Scholar]
- 47.De León-Rodríguez LM, Kovacs Z. The synthesis and chelation chemistry of DOTA-peptide conjugates. Bioconjug Chem. 2008;19:391–402. doi: 10.1021/bc700328s. [DOI] [PubMed] [Google Scholar]
- 48.Parry JJ, Kelly TS, Andrews R, Rogers BE. In vitro and in vivo evaluation of 64Cu-labeled DOTA-linker-bombesin(7-14) analogues containing different amino acid linker moieties. Bioconjug Chem. 2007;18:1110–1117. doi: 10.1021/bc0603788. [DOI] [PubMed] [Google Scholar]
- 49.Hernandez R, Czerwinski A, Chakravarty R, Graves SA, Yang Y, England CG, Nickles RJ, Valenzuela F, Cai W. Evaluation of two novel 64Cu-labeled RGD peptide radiotracers for enhanced PET imaging of tumor integrin αvβ3 . Eur J Nucl Med Mol Imaging. 2015;42:1859–1868. doi: 10.1007/s00259-015-3085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guzik P, Siwowska K, Fang HY, Cohrs S, Bernhardt P, Schibli R, Müller C. Promising potential of [177Lu] Lu-DOTA-folate to enhance tumor response to immunotherapy-a preclinical study using a syngeneic breast cancer model. Eur J Nucl Med Mol Imaging. 2021;48:984–994. doi: 10.1007/s00259-020-05054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hänscheid H, Hartrampf PE, Schirbel A, Buck AK, Lapa C. Intraindividual comparison of [177Lu] Lu-DOTA-EB-TATE and [177Lu] Lu-DOTA-TOC. Eur J Nucl Med Mol Imaging. 2021;48:2566–2572. doi: 10.1007/s00259-020-05177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miranda ACC, Durante ACR, Fuscaldi LL, Barbezan AB, de Lima CR, Perini E, de Araújo EB. Anti-HER2 monoclonal antibody based-radioimmunoconjugates: assessment of the chelating agent influence. Bioorg Med Chem. 2021;33:115996. doi: 10.1016/j.bmc.2021.115996. [DOI] [PubMed] [Google Scholar]
- 53.Akbar MU, Bokhari TH, Ahmad MR, Zia KM, Roohi S, Ayub N, Sohaib M. Radiosynthesis, radiochemical, and biological characterization of 177Lu-labeled diethylenetriamine penta-acetic acid. J Cell Biochem. 2019;120:14510–14517. doi: 10.1002/jcb.28712. [DOI] [PubMed] [Google Scholar]
- 54.Ehlerding EB, Lacognata S, Jiang D, Ferreira CA, Goel S, Hernandez R, Jeffery JJ, Theuer CP, Cai W. Targeting angiogenesis for radioimmunotherapy with a 177Lu-labeled antibody. Eur J Nucl Med Mol Imaging. 2018;45:123–131. doi: 10.1007/s00259-017-3793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi J, Liu Z, Jia B, Yu Z, Zhao H, Wang F. Potential therapeutic radiotracers: preparation, biodistribution and metabolic characteristics of 177Lu-labeled cyclic RGDfK dimer. Amino Acids. 2010;39:111–120. doi: 10.1007/s00726-009-0382-0. [DOI] [PubMed] [Google Scholar]
- 56.Tsai WK, Zettlitz KA, Dahlbom M, Reiter RE, Wu AM. Evaluation of [131I] I- and [177Lu] Lu-DTPA-A11 minibody for radioimmunotherapy in a preclinical model of PSCA-expressing prostate cancer. Mol Imaging Biol. 2020;22:1380–1391. doi: 10.1007/s11307-020-01518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou L, Chen L, Yang L, Cai L, Liu L, Zhao Y, Feng Y, Liu N, Zhao Y, Xia Y, Wei H, Chen Y. Preliminary studies of 177Lu-diethylenetriamine penta-acetic acid-deoxyglucose in hepatic tumor-bearing mice. Cancer Biother Radiopharm. 2020;35:33–40. doi: 10.1089/cbr.2019.2903. [DOI] [PubMed] [Google Scholar]
- 58.Greifenstein L, Grus T, Nagel J, Sinnes JP, Rösch F. Synthesis and labeling of a squaric acid containing PSMA-inhibitor coupled to AAZTA(5) for versatile labeling with 44Sc, 64Cu, 68Ga and 177Lu. Appl Radiat Isot. 2020;156:108867. doi: 10.1016/j.apradiso.2019.108867. [DOI] [PubMed] [Google Scholar]
- 59.Pfister J, Summer D, Rangger C, Petrik M, von Guggenberg E, Minazzi P, Giovenzana GB, Aloj L, Decristoforo C. Influence of a novel, versatile bifunctional chelator on theranostic properties of a minigastrin analogue. EJNMMI Res. 2015;5:74. doi: 10.1186/s13550-015-0154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sinnes JP, Bauder-Wüst U, Schäfer M, Moon ES, Kopka K, Rösch F. 68Ga, 44Sc and 177Lu-labeled AAZTA(5)-PSMA-617: synthesis, radiolabeling, stability and cell binding compared to DOTA-PSMA-617 analogues. EJNMMI Radiopharm Chem. 2020;5:28. doi: 10.1186/s41181-020-00107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinnes JP, Nagel J, Rösch F. AAZTA(5)/AAZTA(5)-TOC: synthesis and radiochemical evaluation with 68Ga, 44Sc and 177Lu. EJNMMI Radiopharm Chem. 2019;4:18. doi: 10.1186/s41181-019-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ambrosini V, Kunikowska J, Baudin E, Bodei L, Bouvier C, Capdevila J, Cremonesi M, de Herder WW, Dromain C, Falconi M, Fani M, Fanti S, Hicks RJ, Kabasakal L, Kaltsas G, Lewington V, Minozzi S, Cinquini M, Öberg K, Oyen WJG, O’Toole D, Pavel M, Ruszniewski P, Scarpa A, Strosberg J, Sundin A, Taïeb D, Virgolini I, Wild D, Herrmann K, Yao J. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur J Cancer. 2021;146:56–73. doi: 10.1016/j.ejca.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amini A, Dehdar F, Jafari E, Gholamrezanezhad A, Assadi M. Somatostatin receptor scintigraphy in a patient with myocarditis. Mol Imaging Radionucl Ther. 2021;30:50–53. doi: 10.4274/mirt.galenos.2019.03164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu C, Zhang H. Somatostatin receptor based imaging and radionuclide therapy. Biomed Res Int. 2015;2015:917968. doi: 10.1155/2015/917968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwekkeboom DJ, de Herder WW, Krenning EP. Somatostatin receptor-targeted radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:173–185. ix. doi: 10.1016/j.ecl.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Patel YC, Greenwood MT, Panetta R, Demchyshyn L, Niznik H, Srikant CB. The somatostatin receptor family. Life Sci. 1995;57:1249–1265. doi: 10.1016/0024-3205(95)02082-t. [DOI] [PubMed] [Google Scholar]
- 67.Bruns C, Weckbecker G, Raulf F, Lübbert H, Hoyer D. Characterization of somatostatin receptor subtypes. Ciba Found Symp. 1995;190:89–101. doi: 10.1002/9780470514733.ch6. discussion 101-10. [DOI] [PubMed] [Google Scholar]
- 68.Schonbrunn A, Gu YZ, Brown PJ, Loose-Mitchell D. Function and regulation of somatostatin receptor subtypes. Ciba Found Symp. 1995;190:204–217. doi: 10.1002/9780470514733.ch13. [DOI] [PubMed] [Google Scholar]
- 69.Brabander T, Nonnekens J, Hofland J. The next generation of peptide receptor radionuclide therapy. Endocr Relat Cancer. 2019;26:C7–C11. doi: 10.1530/ERC-19-0186. [DOI] [PubMed] [Google Scholar]
- 70.Cordova C, Kurz SC. Advances in molecular classification and therapeutic opportunities in meningiomas. Curr Oncol Rep. 2020;22:84. doi: 10.1007/s11912-020-00937-4. [DOI] [PubMed] [Google Scholar]
- 71.Das S, Al-Toubah T, El-Haddad G, Strosberg J. 177Lu-DOTATATE for the treatment of gastroenteropancreatic neuroendocrine tumors. Expert Rev Gastroenterol Hepatol. 2019;13:1023–1031. doi: 10.1080/17474124.2019.1685381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kendi AT, Halfdanarson TR, Packard A, Dundar A, Subramaniam RM. Therapy With (177)Lu-DOTATATE: clinical implementation and impact on care of patients with neuroendocrine tumors. AJR Am J Roentgenol. 2019;213:309–317. doi: 10.2214/AJR.19.21123. [DOI] [PubMed] [Google Scholar]
- 73.Mittra ES. Neuroendocrine tumor therapy: 177Lu-DOTATATE. AJR Am J Roentgenol. 2018;211:278–285. doi: 10.2214/AJR.18.19953. [DOI] [PubMed] [Google Scholar]
- 74.Ramage J, Naraev BG, Halfdanarson TR. Peptide receptor radionuclide therapy for patients with advanced pancreatic neuroendocrine tumors. Semin Oncol. 2018;45:236–248. doi: 10.1053/j.seminoncol.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 75.Satapathy S, Mittal BR. 177Lu-DOTATATE peptide receptor radionuclide therapy versus everolimus in advanced pancreatic neuroendocrine tumors: a systematic review and meta-analysis. Nucl Med Commun. 2019;40:1195–1203. doi: 10.1097/MNM.0000000000001103. [DOI] [PubMed] [Google Scholar]
- 76.Satapathy S, Mittal BR, Bhansali A. ‘Peptide receptor radionuclide therapy in the management of advanced pheochromocytoma and paraganglioma: a systematic review and meta-analysis’. Clin Endocrinol (Oxf) 2019;91:718–727. doi: 10.1111/cen.14106. [DOI] [PubMed] [Google Scholar]
- 77.Wang LF, Lin L, Wang MJ, Li Y. The therapeutic efficacy of 177Lu-DOTATATE/DOTATOC in advanced neuroendocrine tumors: a meta-analysis. Medicine (Baltimore) 2020;99:e19304. doi: 10.1097/MD.0000000000019304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J, Song Q, Cai L, Xie Y, Chen Y. The efficacy of 177Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) in patients with metastatic neuroendocrine tumours: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2020;146:1533–1543. doi: 10.1007/s00432-020-03181-2. [DOI] [PubMed] [Google Scholar]
- 79.Basu S, Chakraborty S, Parghane RV, Kamaldeep , Ranade R, Thapa P, Asopa RV, Sonawane G, Nabar S, Shimpi H, Chandak A, Vimalnath KV, Ostwal V, Ramaswamy A, Bhandare M, Chaudhari V, Shrikhande SV, Sirohi B, Dash A, Banerjee S. One decade of ‘Bench-to-Bedside’ peptide receptor radionuclide therapy with indigenous [177Lu]Lu-DOTATATE obtained through ‘Direct’ neutron activation route: lessons learnt including practice evolution in an Indian setting. Am J Nucl Med Mol Imaging. 2020;10:178–211. [PMC free article] [PubMed] [Google Scholar]
- 80.Das T, Chakraborty S, Banerjee S, Venkatesh M. On the preparation of a therapeutic dose of 177Lu-labeled DOTA-TATE using indigenously produced 177Lu in medium flux reactor. Appl Radiat Isot. 2007;65:301–308. doi: 10.1016/j.apradiso.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 81.Das T, Chakraborty S, Kallur KG, Venkatesh M, Banerjee S. Preparation of patient doses of 177Lu-DOTA-TATE using indigenously produced 177Lu: the Indian experience. Cancer Biother Radiopharm. 2011;26:395–400. doi: 10.1089/cbr.2010.0881. [DOI] [PubMed] [Google Scholar]
- 82.Mathur A, Prashant V, Sakhare N, Chakraborty S, Vimalnath KV, Mohan RK, Arjun C, Karkhanis B, Seshan R, Basu S, Korde A, Banerjee S, Dash A, Sachdev SS. Bulk scale formulation of therapeutic doses of clinical grade ready-to-use 177Lu-DOTA-TATE: the intricate radiochemistry aspects. Cancer Biother Radiopharm. 2017;32:266–273. [PubMed] [Google Scholar]
- 83.Dash A, Chakraborty S, Pillai MR, Knapp FF Jr. Peptide receptor radionuclide therapy: an overview. Cancer Biother Radiopharm. 2015;30:47–71. doi: 10.1089/cbr.2014.1741. [DOI] [PubMed] [Google Scholar]
- 84.Gupta SK, Singla S, Bal C. Renal and hematological toxicity in patients of neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-DOTATATE. Cancer Biother Radiopharm. 2012;27:593–599. doi: 10.1089/cbr.2012.1195. [DOI] [PubMed] [Google Scholar]
- 85.Gupta SK, Singla S, Thakral P, Bal CS. Dosimetric analyses of kidneys, liver, spleen, pituitary gland, and neuroendocrine tumors of patients treated with 177Lu-DOTATATE. Clin Nucl Med. 2013;38:188–194. doi: 10.1097/RLU.0b013e3182814ac1. [DOI] [PubMed] [Google Scholar]
- 86.Jois B, Asopa R, Basu S. Somatostatin receptor imaging in non-131I-avid metastatic differentiated thyroid carcinoma for determining the feasibility of peptide receptor radionuclide therapy with 177Lu-DOTATATE: low fraction of patients suitable for peptide receptor radionuclide therapy and evidence of chromogranin A level-positive neuroendocrine differentiation. Clin Nucl Med. 2014;39:505–510. doi: 10.1097/RLU.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 87.Basu S, Ranade R. Favorable response of metastatic merkel cell carcinoma to targeted 177Lu-DOTATATE therapy: will PRRT evolve to become an important approach in receptor-positive cases? J Nucl Med Technol. 2016;44:85–87. doi: 10.2967/jnmt.115.163527. [DOI] [PubMed] [Google Scholar]
- 88.Danthala M, Kallur KG, Prashant GR, Rajkumar K, Raghavendra Rao M. 177Lu-DOTATATE therapy in patients with neuroendocrine tumours: 5 years’ experience from a tertiary cancer care centre in India. Eur J Nucl Med Mol Imaging. 2014;41:1319–1326. doi: 10.1007/s00259-014-2710-1. [DOI] [PubMed] [Google Scholar]
- 89.Ballal S, Yadav MP, Damle NA, Sahoo RK, Bal C. Concomitant 177Lu-DOTATATE and capecitabine therapy in patients with advanced neuroendocrine tumors: a long-term-outcome, toxicity, survival, and quality-of-life study. Clin Nucl Med. 2017;42:e457–e466. doi: 10.1097/RLU.0000000000001816. [DOI] [PubMed] [Google Scholar]
- 90.Yadav MP, Ballal S, Bal C. Concomitant 177Lu-DOTATATE and capecitabine therapy in malignant paragangliomas. EJNMMI Res. 2019;9:13. doi: 10.1186/s13550-019-0484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Basu S, Parghane RV, Banerjee S. Availability of both [177Lu] Lu-DOTA-TATE and [90Y] Y-DOTATATE as PRRT agents for neuroendocrine tumors: can we evolve a rational sequential duo-PRRT protocol for large volume resistant tumors? Eur J Nucl Med Mol Imaging. 2020;47:756–758. doi: 10.1007/s00259-019-04546-7. [DOI] [PubMed] [Google Scholar]
- 92.Parghane RV, Naik C, Talole S, Desmukh A, Chaukar D, Banerjee S, Basu S. Clinical utility of 177Lu-DOTATATE PRRT in somatostatin receptor-positive metastatic medullary carcinoma of thyroid patients with assessment of efficacy, survival analysis, prognostic variables, and toxicity. Head Neck. 2020;42:401–416. doi: 10.1002/hed.26024. [DOI] [PubMed] [Google Scholar]
- 93.Parghane RV, Talole S, Basu S. Prevalence of hitherto unknown brain meningioma detected on 68Ga-DOTATATE positron-emission tomography/computed tomography in patients with metastatic neuroendocrine tumor and exploring potential of 177Lu-DOTATATE peptide receptor radionuclide therapy as single-shot treatment approach targeting both tumors. World J Nucl Med. 2019;18:160–170. doi: 10.4103/wjnm.WJNM_39_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parghane RV, Mitra A, Upadhye T, Rakshit S, Banerjee S, Basu S. Sequential duo-peptide receptor radionuclide therapy with indigenous 90Y-DOTATATE and 177Lu-DOTATATE in large-volume neuroendocrine tumors: posttherapy bremsstrahlung and pet/ct imaging following 90Y-DOTATATE treatment. Clin Nucl Med. 2020;45:714–715. doi: 10.1097/RLU.0000000000003182. [DOI] [PubMed] [Google Scholar]
- 95.Parghane RV, Ostwal V, Ramaswamy A, Bhandare M, Chaudhari V, Talole S, Shrikhande SV, Basu S. Long-term outcome of “Sandwich” chemo-PRRT: a novel treatment strategy for metastatic neuroendocrine tumors with both FDG- and SSTR-avid aggressive disease. Eur J Nucl Med Mol Imaging. 2021;48:913–923. doi: 10.1007/s00259-020-05004-5. [DOI] [PubMed] [Google Scholar]
- 96.Sitani K, Parghane RV, Talole S, Basu S. Long-term outcome of indigenous 177Lu-DOTATATE PRRT in patients with metastatic advanced neuroendocrine tumours: a single institutional observation in a large tertiary care setting. Br J Radiol. 2021;94:20201041. doi: 10.1259/bjr.20201041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parghane RV, Talole S, Basu S. 131I-MIBG negative progressive symptomatic metastatic paraganglioma: response and outcome with 177Lu-DOTATATE peptide receptor radionuclide therapy. Ann Nucl Med. 2021;35:92–101. doi: 10.1007/s12149-020-01541-z. [DOI] [PubMed] [Google Scholar]
- 98.Jaiswal SK, Sarathi V, Memon SS, Garg R, Malhotra G, Verma P, Shah R, Sehemby MK, Patil VA, Jadhav S, Lila AR, Shah NS, Bandgar TR. 177Lu-DOTATATE therapy in metastatic/inoperable pheochromocytoma-paraganglioma. Endocr Connect. 2020;9:864–873. doi: 10.1530/EC-20-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Adnan A, Kudachi S, Ramesh S, Prabhash K, Basu S. Metastatic or locally advanced mediastinal neuroendocrine tumours: outcome with 177Lu-DOTATATE-based peptide receptor radionuclide therapy and assessment of prognostic factors. Nucl Med Commun. 2019;40:947–957. doi: 10.1097/MNM.0000000000001054. [DOI] [PubMed] [Google Scholar]
- 100.Adnan A, Basu S. Combined 177Lu-DOTATATE peptide receptor radionuclide therapy and platinum-based chemotherapy in recurrent, metastatic sinonasal neuroendocrine carcinoma: a promising therapeutic option. J Nucl Med Technol. 2020;48:292–294. doi: 10.2967/jnmt.119.237354. [DOI] [PubMed] [Google Scholar]
- 101.Kalshetty A, Ramaswamy A, Ostwal V, Basu S. Resistant functioning and/or progressive symptomatic metastatic gastroenteropancreatic neuroendocrine tumors: efficacy of 177Lu-DOTATATE peptide receptor radionuclide therapy in this setting. Nucl Med Commun. 2018;39:1143–1149. doi: 10.1097/MNM.0000000000000926. [DOI] [PubMed] [Google Scholar]
- 102.Ramesh S, Kudachi S, Basu S. Peptide receptor radionuclide therapy with 177Lu-DOTATATE in carcinoid heart disease: a contraindication or a promising treatment approach bettering chances for corrective surgery? J Nucl Med Technol. 2018;46:292–294. doi: 10.2967/jnmt.118.210179. [DOI] [PubMed] [Google Scholar]
- 103.Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: a review. JAMA. 2017;317:2532–2542. doi: 10.1001/jama.2017.7248. [DOI] [PubMed] [Google Scholar]
- 104.Sumanasuriya S, De Bono J. Treatment of advanced prostate cancer-a review of current therapies and future promise. Cold Spring Harb Perspect Med. 2018;8:a030635. doi: 10.1101/cshperspect.a030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pillai MRA, Nanabala R, Joy A, Sasikumar A, Russ Knapp FF. Radiolabeled enzyme inhibitors and binding agents targeting PSMA: effective theranostic tools for imaging and therapy of prostate cancer. Nucl Med Biol. 2016;43:692–720. doi: 10.1016/j.nucmedbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 106.Chakravarty R, Siamof CM, Dash A, Cai W. Targeted α-therapy of prostate cancer using radiolabeled PSMA inhibitors: a game changer in nuclear medicine. Am J Nucl Med Mol Imaging. 2018;8:247–267. [PMC free article] [PubMed] [Google Scholar]
- 107.Bögemann M, Herrmann K, Radtke JP, Rahbar K. PSMA radioligand therapy in patients with advanced prostate cancer. Urologe A. 2020;59:680–686. doi: 10.1007/s00120-020-01205-w. [DOI] [PubMed] [Google Scholar]
- 108.Gupta M, Karthikeyan G, Choudhury PS, Babu Koyyala VP, Sharma M, Jain P, Talwar V, Singh A, Rawal S. A walk with Lu-177 PSMA: how close we have reached from bench to bedside? Cancer Invest. 2020;38:486–492. doi: 10.1080/07357907.2020.1811301. [DOI] [PubMed] [Google Scholar]
- 109.Iravani A, Violet J, Azad A, Hofman MS. Lutetium-177 prostate-specific membrane antigen (PSMA) theranostics: practical nuances and intricacies. Prostate Cancer Prostatic Dis. 2020;23:38–52. doi: 10.1038/s41391-019-0174-x. [DOI] [PubMed] [Google Scholar]
- 110.Satapathy S, Mittal BR, Sood A. Visceral metastases as predictors of response and survival outcomes in patients of castration-resistant prostate cancer treated with 177Lu-labeled prostate-specific membrane antigen radioligand therapy: a systematic review and meta-analysis. Clin Nucl Med. 2020;45:935–942. doi: 10.1097/RLU.0000000000003307. [DOI] [PubMed] [Google Scholar]
- 111.Sun M, Niaz MO, Nelson A, Skafida M, Niaz MJ. Review of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Cureus. 2020;12:e8921. doi: 10.7759/cureus.8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.von Eyben FE, Bauman G, von Eyben R, Rahbar K, Soydal C, Haug AR, Virgolini I, Kulkarni H, Baum R, Paganelli G. Optimizing PSMa radioligand therapy for patients with metastatic castration-resistant prostate cancer. a systematic review and meta-analysis. Int J Mol Sci. 2020;21:9054. doi: 10.3390/ijms21239054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weber M, Hadaschik B, Ferdinandus J, Rahbar K, Bögemann M, Herrmann K, Fendler WP, Kesch C. Prostate-specific membrane antigen-based imaging of castration-resistant prostate cancer. Eur Urol Focus. 2021;7:279–287. doi: 10.1016/j.euf.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 114.Wester HJ, Schottelius M. PSMA-targeted radiopharmaceuticals for imaging and therapy. Semin Nucl Med. 2019;49:302–312. doi: 10.1053/j.semnuclmed.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 115.Zang J, Liu Q, Sui H, Wang R, Jacobson O, Fan X, Zhu Z, Chen X. 177Lu-EB-PSMA radioligand therapy with escalating doses in patients with metastatic castration-resistant prostate cancer. J Nucl Med. 2020;61:1772–1778. doi: 10.2967/jnumed.120.242263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.von Eyben FE, Roviello G, Kiljunen T, Uprimny C, Virgolini I, Kairemo K, Joensuu T. Third-line treatment and 177Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: a systematic review. Eur J Nucl Med Mol Imaging. 2018;45:496–508. doi: 10.1007/s00259-017-3895-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chakraborty S, Chakravarty R, Shetty P, Vimalnath KV, Sen IB, Dash A. Prospects of medium specific activity 177Lu in targeted therapy of prostate cancer using 177Lu-labeled PSMA inhibitor. J Labelled Comp Radiopharm. 2016;59:364–371. doi: 10.1002/jlcr.3414. [DOI] [PubMed] [Google Scholar]
- 118.Chakraborty S, Vimalnath KV, Chakravarty R, Sarma HD, Dash A. Multidose formulation of ready-to-use 177Lu-PSMA-617 in a centralized radiopharmacy set-up. Appl Radiat Isot. 2018;139:91–97. doi: 10.1016/j.apradiso.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 119.Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A, Bal C. Post-therapeutic dosimetry of 177Lu-DKFZ-PSMA-617 in the treatment of patients with metastatic castration-resistant prostate cancer. Nucl Med Commun. 2017;38:91–98. doi: 10.1097/MNM.0000000000000606. [DOI] [PubMed] [Google Scholar]
- 120.Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A, Bal C. 177Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2017;44:81–91. doi: 10.1007/s00259-016-3481-7. [DOI] [PubMed] [Google Scholar]
- 121.Suman S, Parghane RV, Joshi A, Prabhash K, Bakshi G, Talole S, Banerjee S, Basu S. Therapeutic efficacy, prognostic variables and clinical outcome of 177Lu-PSMA-617 PRLT in progressive mCRPC following multiple lines of treatment: prognostic implications of high FDG uptake on dual tracer PET-CT vis-à-vis Gleason score in such cohort. Br J Radiol. 2019;92:20190380. doi: 10.1259/bjr.20190380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Adnan A, Basu S. Comparison of dual-tracer PET and CT features to conventional risk categories in assessing response to 177Lu-PSMA-617 therapy for metastatic prostate adenocarcinoma with urinary bladder involvement. J Nucl Med Technol. 2020;48:148–153. doi: 10.2967/jnmt.119.235960. [DOI] [PubMed] [Google Scholar]
- 123.Kattepur AK, Gulia A, Jones RL, Rastogi S. Extraskeletal osteosarcomas: current update. Future Oncol. 2021;17:825–835. doi: 10.2217/fon-2020-0802. [DOI] [PubMed] [Google Scholar]
- 124.Liu F, Ke J, Song Y. Application of biomarkers for the prediction and diagnosis of bone metastasis in breast cancer. J Breast Cancer. 2020;23:588–598. doi: 10.4048/jbc.2020.23.e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marques C, Roberts C, Matos VMJ, Buikstra JE. Cancers as rare diseases: terminological, theoretical, and methodological biases. Int J Paleopathol. 2021;32:111–122. doi: 10.1016/j.ijpp.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 126.Mollica V, Rizzo A, Rosellini M, Marchetti A, Ricci AD, Cimadamore A, Scarpelli M, Bonucci C, Andrini E, Errani C, Santoni M, Montironi R, Massari F. Bone targeting agents in patients with metastatic prostate cancer: state of the art. Cancers (Basel) 2021;13:546. doi: 10.3390/cancers13030546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peters C, Vandewiele J, Lievens Y, van Eijkeren M, Fonteyne V, Boterberg T, Deseyne P, Veldeman L, De Neve W, Monten C, Braems S, Duprez F, Vandecasteele K, Ost P. Adoption of single fraction radiotherapy for uncomplicated bone metastases in a tertiary centre. Clin Transl Radiat Oncol. 2021;27:64–69. doi: 10.1016/j.ctro.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Soni S, Torvund M, Mandal CC. Molecular insights into the interplay between adiposity, breast cancer and bone metastasis. Clin Exp Metastasis. 2021;38:119–138. doi: 10.1007/s10585-021-10076-0. [DOI] [PubMed] [Google Scholar]
- 129.Dash A, Das T, Knapp FFR. Targeted radionuclide therapy of painful bone metastases: past developments, current status, recent advances and future directions. Curr Med Chem. 2020;27:3187–3249. doi: 10.2174/0929867326666190201142814. [DOI] [PubMed] [Google Scholar]
- 130.Bouman-Wammes EW, de Klerk JMH, Bloemendal HJ, Van Dodewaard-de Jong JM, Lange R, Ter Heine R, Verheul HMW, Van den Eertwegh AJM. Bone-targeting radiopharmaceuticals as monotherapy or combined with chemotherapy in patients with castration-resistant prostate cancer metastatic to bone. Clin Genitourin Cancer. 2019;17:e281–e292. doi: 10.1016/j.clgc.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 131.Manafi-Farid R, Masoumi F, Divband G, Saidi B, Ataeinia B, Hertel F, Schweighofer-Zwink G, Morgenroth A, Beheshti M. Targeted palliative radionuclide therapy for metastatic bone pain. J Clin Med. 2020;9:2622. doi: 10.3390/jcm9082622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miranda J, Viñal D, Pinto Á. Radium 223 for the treatment of metastatic castration-resistant prostate cancer. Arch Esp Urol. 2019;72:500–507. [PubMed] [Google Scholar]
- 133.Murray I, Du Y. Systemic radiotherapy of bone metastases with radionuclides. Clin Oncol (R Coll Radiol) 2021;33:98–105. doi: 10.1016/j.clon.2020.11.028. [DOI] [PubMed] [Google Scholar]
- 134.Sadremomtaz A, Masoumi M. An assessment of bone-seeking radionuclides for palliation of metastatic bone pain in a vertebral model. Ann Nucl Med. 2019;33:252–264. doi: 10.1007/s12149-019-01329-w. [DOI] [PubMed] [Google Scholar]
- 135.Wada Y, Anbai A, Kumagai S, Okuyama E, Hatakeyama K, Takagi N, Hashimoto M. Effect of the types of pretreatment imaging modalities on the treatment response to palliative radiation for painful bone metastases from solid cancer: a single-center retrospective analysis. Radiat Oncol. 2019;14:98. doi: 10.1186/s13014-019-1310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bal C, Arora G, Kumar P, Damle N, Das T, Chakraborty S, Banerjee S, Venkatesh M, Zaknun JJ, Pillai MR. Pharmacokinetic, dosimetry and toxicity study of 177Lu-EDTMP in patients: phase 0/I study. Curr Radiopharm. 2016;9:71–84. doi: 10.2174/1874471008666150313105000. [DOI] [PubMed] [Google Scholar]
- 137.Agarwal KK, Singla S, Arora G, Bal C. 177Lu-EDTMP for palliation of pain from bone metastases in patients with prostate and breast cancer: a phase II study. Eur J Nucl Med Mol Imaging. 2015;42:79–88. doi: 10.1007/s00259-014-2862-z. [DOI] [PubMed] [Google Scholar]
- 138.Thapa P, Nikam D, Das T, Sonawane G, Agarwal JP, Basu S. Clinical efficacy and safety comparison of 177Lu-EDTMP with 153Sm-EDTMP on an equidose basis in patients with painful skeletal metastases. J Nucl Med. 2015;56:1513–1519. doi: 10.2967/jnumed.115.155762. [DOI] [PubMed] [Google Scholar]
- 139.Sharma S, Singh B, Koul A, Mittal BR. Comparative therapeutic efficacy of 153Sm-EDTMP and 177Lu-EDTMP for bone pain palliation in patients with skeletal metastases: patients’ pain score analysis and personalized dosimetry. Front Med (Lausanne) 2017;4:46. doi: 10.3389/fmed.2017.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chakraborty S, Das T, Sarma HD, Venkatesh M, Banerjee S. Comparative studies of 177Lu-EDTMP and 177Lu-DOTMP as potential agents for palliative radiotherapy of bone metastasis. Appl Radiat Isot. 2008;66:1196–1205. doi: 10.1016/j.apradiso.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 141.Bollampally N, Shukla J, Mittal BR, Sood A, Mohanty M, Kapoor R, Vatsa R, Satapathy S, Chakravarty R, Chakraborty S, Dash AK. Efficacy and safety of 177Lu-DOTMP in palliative treatment of symptomatic skeletal metastases: a prospective study. Nucl Med Commun. 2021;42:964–971. doi: 10.1097/MNM.0000000000001425. [DOI] [PubMed] [Google Scholar]
- 142.Keeling GP, Sherin B, Kim J, San Juan B, Grus T, Eykyn TR, Rösch F, Smith GE, Blower PJ, Terry SYA, T M de Rosales R. [68Ga] Ga-THP-Pam: a bisphosphonate PET tracer with facile radiolabeling and broad calcium mineral affinity. Bioconjug Chem. 2020;32:1276–1289. doi: 10.1021/acs.bioconjchem.0c00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meckel M, Fellner M, Thieme N, Bergmann R, Kubicek V, Rösch F. In vivo comparison of DOTA based 68Ga-labelled bisphosphonates for bone imaging in non-tumour models. Nucl Med Biol. 2013;40:823–830. doi: 10.1016/j.nucmedbio.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 144.Meckel M, Kubíček V, Hermann P, Miederer M, Rösch F. A DOTA based bisphosphonate with an albumin binding moiety for delayed body clearance for bone targeting. Nucl Med Biol. 2016;43:670–678. doi: 10.1016/j.nucmedbio.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 145.Meckel M, Nauth A, Timpe J, Zhernosekov K, Puranik AD, Baum RP, Rösch F. Development of a [177Lu] BPAMD labeling kit and an automated synthesis module for routine bone targeted endoradiotherapy. Cancer Biother Radiopharm. 2015;30:94–99. doi: 10.1089/cbr.2014.1720. [DOI] [PubMed] [Google Scholar]
- 146.Pfannkuchen N, Meckel M, Bergmann R, Bachmann M, Bal C, Sathekge M, Mohnike W, Baum RP, Rösch F. Novel radiolabeled bisphosphonates for PET diagnosis and endoradiotherapy of bone metastases. Pharmaceuticals (Basel) 2017;10:45. doi: 10.3390/ph10020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rabie A, Enayati R, Yousefnia H, Jalilian AR, Shamsaei M, Zolghadri S, Bahrami-Samani A, Hosntalab M. Preparation, quality control and biodistribution assessment of 153Sm-BPAMD as a novel agent for bone pain palliation therapy. Ann Nucl Med. 2015;29:870–876. doi: 10.1007/s12149-015-1014-2. [DOI] [PubMed] [Google Scholar]
- 148.Wu Z, Zha Z, Choi SR, Plössl K, Zhu L, Kung HF. New 68Ga-PhenA bisphosphonates as potential bone imaging agents. Nucl Med Biol. 2016;43:360–371. doi: 10.1016/j.nucmedbio.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 149.Chakraborty S, Shetty P, Chakravarty R, Vimalnath KV, Kumar C, Sarma HD, Vatsa R, Shukla J, Mittal BR, Dash A. Formulation of ‘ready-to-use’ human clinical doses of 177Lu-labeled bisphosphonate amide of DOTA using moderate specific activity 177Lu and its preliminary evaluation in human patient. Radiochim Acta. 2020;108:661–672. [Google Scholar]
- 150.Palma E, Correia JD, Campello MP, Santos I. Bisphosphonates as radionuclide carriers for imaging or systemic therapy. Mol Biosyst. 2011;7:2950–2966. doi: 10.1039/c1mb05242j. [DOI] [PubMed] [Google Scholar]
- 151.Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 152.Fernandez R, Eppard E, Lehnert W, Jimenez-Franco LD, Soza-Ried C, Ceballos M, Ribbeck J, Kluge A, Roesch F, Meckel M, Zhernosekov K, Kramer V, Amaral H. Evaluation of safety and dosimetry of 177Lu DOTA-ZOL for therapy of bone metastases. J Nucl Med. 2021;62:1126–1132. doi: 10.2967/jnumed.120.255851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Khawar A, Eppard E, Roesch F, Ahmadzadehfar H, Kürpig S, Meisenheimer M, Gaertner FC, Essler M, Bundschuh RA. Biodistribution and post-therapy dosimetric analysis of [177Lu] Lu-DOTA(ZOL) in patients with osteoblastic metastases: first results. EJNMMI Res. 2019;9:102. doi: 10.1186/s13550-019-0566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Khawar A, Eppard E, Roesch F, Ahmadzadehfar H, Kürpig S, Meisenheimer M, Gaertner FC, Essler M, Bundschuh RA. Preliminary results of biodistribution and dosimetric analysis of [68Ga] Ga-DOTA(ZOL): a new zoledronate-based bisphosphonate for PET/CT diagnosis of bone diseases. Ann Nucl Med. 2019;33:404–413. doi: 10.1007/s12149-019-01348-7. [DOI] [PubMed] [Google Scholar]
- 155.Meckel M, Bergmann R, Miederer M, Roesch F. Bone targeting compounds for radiotherapy and imaging: *Me(III)-DOTA conjugates of bisphosphonic acid, pamidronic acid and zoledronic acid. EJNMMI Radiopharm Chem. 2017;1:14. doi: 10.1186/s41181-016-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Meisenheimer M, Kürpig S, Essler M, Eppard E. DOTA-ZOL: a promising tool in diagnosis and palliative therapy of bone metastasis-challenges and critical points in implementation into clinical routine. Molecules. 2020;25:2988. doi: 10.3390/molecules25132988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yadav MP, Ballal S, Meckel M, Roesch F, Bal C. [177Lu] Lu-DOTA-ZOL bone pain palliation in patients with skeletal metastases from various cancers: efficacy and safety results. EJNMMI Res. 2020;10:130. doi: 10.1186/s13550-020-00709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shi J, Fan D, Dong C, Liu H, Jia B, Zhao H, Jin X, Liu Z, Li F, Wang F. Anti-tumor effect of integrin targeted 177Lu-3PRGD2 and combined therapy with endostar. Theranostics. 2014;4:256–266. doi: 10.7150/thno.7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Luna-Gutiérrez M, Ferro-Flores G, Ocampo-García B, Jiménez-Mancilla N, Morales-Avila E, De León-Rodríguez L, Isaac-Olivé K. 177Lu-labeled monomeric, dimeric and multimeric RGD peptides for the therapy of tumors expressing αvβ3 integrins. J Labelled Comp Radiopharm. 2012;55:140–148. [Google Scholar]
- 160.Chakraborty S, Sarma HD, Vimalnath KV, Pillai MR. Tracer level radiochemistry to clinical dose preparation of 177Lu-labeled cyclic RGD peptide dimer. Nucl Med Biol. 2013;40:946–954. doi: 10.1016/j.nucmedbio.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 161.Parihar AS, Sood A, Kumar R, Bhusari P, Shukla J, Mittal BR. Novel use of 177Lu-DOTA-RGD2 in treatment of 68Ga-DOTA-RGD2-avid lesions in papillary thyroid cancer with TENIS. Eur J Nucl Med Mol Imaging. 2018;45:1836–1837. doi: 10.1007/s00259-018-4036-x. [DOI] [PubMed] [Google Scholar]
- 162.Camacho X, Calzada V, Fernandez M, Alonso O, Chammas R, Riva E, Gambini JP, Cabral P. 177Lu-DOTA-bevacizumab: radioimmunotherapy agent for melanoma. Curr Radiopharm. 2017;10:21–28. doi: 10.2174/1874471009666161010155246. [DOI] [PubMed] [Google Scholar]
- 163.Deshayes E, Ladjohounlou R, Le Fur P, Pichard A, Lozza C, Boudousq V, Sevestre S, Jarlier M, Kashani R, Koch J, Sosabowski J, Foster J, Chouin N, Bruchertseifer F, Morgenstern A, Kotzki PO, Navarro-Teulon I, Pouget JP. Radiolabeled antibodies against müllerian-inhibiting substance receptor, type II: new tools for a theranostic approach in ovarian cancer. J Nucl Med. 2018;59:1234–1242. doi: 10.2967/jnumed.118.208611. [DOI] [PubMed] [Google Scholar]
- 164.Minnix M, Adhikarla V, Caserta E, Poku E, Rockne R, Shively JE, Pichiorri F. Comparison of CD38 targeted alpha- vs beta-radionuclide therapy of disseminated multiple myeloma in an animal model. J Nucl Med. 2020;62:795–801. doi: 10.2967/jnumed.120.251983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mortensen AC, Spiegelberg D, Haylock AK, Lundqvist H, Nestor M. Preclinical evaluation of a novel engineered recombinant human anti-CD44v6 antibody for potential use in radio-immunotherapy. Int J Oncol. 2018;52:1875–1885. doi: 10.3892/ijo.2018.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Timmermand OV, Elgqvist J, Beattie KA, Örbom A, Larsson E, Eriksson SE, Thorek DLJ, Beattie BJ, Tran TA, Ulmert D, Strand SE. Preclinical efficacy of hK2 targeted [(177)Lu] hu11B6 for prostate cancer theranostics. Theranostics. 2019;9:2129–2142. doi: 10.7150/thno.31179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chakravarty R, Chakraborty S, Sarma HD, Nair KV, Rajeswari A, Dash A. 90Y/177Lu-labelled Cetuximab immunoconjugates: radiochemistry optimization to clinical dose formulation. J Labelled Comp Radiopharm. 2016;59:354–363. doi: 10.1002/jlcr.3413. [DOI] [PubMed] [Google Scholar]
- 168.Chakravarty R, Rohra N, Chakraborty S, Jain R, Sarma HD, Dash A. Clinically relevant radioactive dose formulation of 177Lu-labeled cetuximab-fab fragment for potential use in cancer theranostics. Chem Select. 2018;3:242–248. [Google Scholar]
- 169.Kang L, Li C, Rosenkrans ZT, Huo N, Chen Z, Ehlerding EB, Huo Y, Ferreira CA, Barnhart TE, Engle JW, Wang R, Jiang D, Xu X, Cai W. CD38-targeted theranostics of lymphoma with 89Zr/177Lu-labeled daratumumab. Adv Sci (Weinh) 2021;8:2001879. doi: 10.1002/advs.202001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yadav MP, Singla S, Thakral P, Ballal S, Bal C. Dosimetric analysis of 177Lu-DOTA-rituximab in patients with relapsed/refractory non-Hodgkin’s lymphoma. Nucl Med Commun. 2016;37:735–742. doi: 10.1097/MNM.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 171.Bhusari P, Vatsa R, Singh G, Parmar M, Bal A, Dhawan DK, Mittal BR, Shukla J. Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients. Int J Cancer. 2017;140:938–947. doi: 10.1002/ijc.30500. [DOI] [PubMed] [Google Scholar]
- 172.Ahmad I, Nisar H. Dosimetry perspectives in radiation synovectomy. Phys Med. 2018;47:64–72. doi: 10.1016/j.ejmp.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 173.García-Colmenero L, Martin-Ezquerra G, Monfort J, Pujol RM. Persistent cutaneous ulcers after Yttrium-90 synovectomy, an unusual complication: two case reports and a review of the literature. Int Wound J. 2017;14:508–511. doi: 10.1111/iwj.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kitonyi GW, Kitonyi JM. Radiation synovectomy: treatment option for haemophilia patients with chronic haemarthrosis: a review. East Afr Med J. 2009;86(Suppl):S71–75. doi: 10.4314/eamj.v86i12.62907. [DOI] [PubMed] [Google Scholar]
- 175.Knut L. Radiosynovectomy in the therapeutic management of arthritis. World J Nucl Med. 2015;14:10–15. doi: 10.4103/1450-1147.150509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lopez-Olivo MA, Kakpovbia-Eshareturi V, des Bordes JK, Barbo A, Christensen R, Suarez-Almazor ME. Treating early undifferentiated arthritis: a systematic review and meta-analysis of direct and indirect trial evidence. Arthritis Care Res (Hoboken) 2018;70:1355–1365. doi: 10.1002/acr.23474. [DOI] [PubMed] [Google Scholar]
- 177.Mollon B, Lee A, Busse JW, Griffin AM, Ferguson PC, Wunder JS, Theodoropoulos J. The effect of surgical synovectomy and radiotherapy on the rate of recurrence of pigmented villonodular synovitis of the knee: an individual patient meta-analysis. Bone Joint J. 2015;97-B:550–557. doi: 10.1302/0301-620X.97B4.34907. [DOI] [PubMed] [Google Scholar]
- 178.Rodriguez-Merchan EC. Radiosynovectomy in haemophilia. Blood Rev. 2019;35:1–6. doi: 10.1016/j.blre.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 179.Teyssler P, Kolostova K, Bobek V. Radionuclide synovectomy in haemophilic joints. Nucl Med Commun. 2013;34:291–297. doi: 10.1097/MNM.0b013e32835ed50c. [DOI] [PubMed] [Google Scholar]
- 180.van der Zant FM, Boer RO, Moolenburgh JD, Jahangier ZN, Bijlsma JW, Jacobs JW. Radiation synovectomy with (90)Yttrium, (186)Rhenium and (169)Erbium: a systematic literature review with meta-analyses. Clin Exp Rheumatol. 2009;27:130–139. [PubMed] [Google Scholar]
- 181.Chakraborty S, Vimalnath KV, Rajeswari A, Shinto A, Sarma HD, Kamaleshwaran K, Thirumalaisamy P, Dash A. Preparation, evaluation, and first clinical use of 177Lu-labeled hydroxyapatite (HA) particles in the treatment of rheumatoid arthritis: utility of cold kits for convenient dose formulation at hospital radiopharmacy. J Labelled Comp Radiopharm. 2014;57:453–462. doi: 10.1002/jlcr.3202. [DOI] [PubMed] [Google Scholar]
- 182.Shinto AS, Kamaleshwaran KK, Chakraborty S, Vyshakh K, Thirumalaisamy SG, Karthik S, Nagaprabhu VN, Vimalnath KV, Das T, Banerjee S. Radiosynovectomy of painful synovitis of knee joints due to rheumatoid arthritis by intra-articular administration of 177Lu-labeled hydroxyapatite particulates: first human study and initial indian experience. World J Nucl Med. 2015;14:81–88. doi: 10.4103/1450-1147.153908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Arora G, Singh M, Jha P, Tripathy S, Bal C, Mukherjee A, Shamim SA. Formulation and characterization of lutetium-177-labeled stannous (tin) colloid for radiosynovectomy. Nucl Med Commun. 2017;38:587–592. doi: 10.1097/MNM.0000000000000684. [DOI] [PubMed] [Google Scholar]
- 184.Jha P, Arora G, Shamim SA, Mukherjee A, Gautam D, Ballal S, Kumar U, Ansari TM, Bal C. Lutetium-177 tin colloid radiosynovectomy in patients with inflammatory knee joint conditions intractable to prevailing therapy. Nucl Med Commun. 2018;39:803–808. doi: 10.1097/MNM.0000000000000871. [DOI] [PubMed] [Google Scholar]
- 185.Shamim SA, Arora G, Jha P, Gupta P, Behera A, Mukherjee A, Prabhu M, Ansari MT, Vyas S, Bal C. Comparison of Lutetium-177 tin colloid and Rhenium-188 tin colloid radiosynovectomy in chronic knee arthritis. Nucl Med Commun. 2020;41:721–726. doi: 10.1097/MNM.0000000000001210. [DOI] [PubMed] [Google Scholar]
- 186.Andreou C, Pal S, Rotter L, Yang J, Kircher MF. Molecular imaging in nanotechnology and theranostics. Mol Imaging Biol. 2017;19:363–372. doi: 10.1007/s11307-017-1056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Aranda-Lara L, Morales-Avila E, Luna-Gutiérrez MA, Olivé-Alvarez E, Isaac-Olivé K. Radiolabeled liposomes and lipoproteins as lipidic nanoparticles for imaging and therapy. Chem Phys Lipids. 2020;230:104934. doi: 10.1016/j.chemphyslip.2020.104934. [DOI] [PubMed] [Google Scholar]
- 188.Datta P, Ray S. Nanoparticulate formulations of radiopharmaceuticals: strategy to improve targeting and biodistribution properties. J Labelled Comp Radiopharm. 2020 doi: 10.1002/jlcr.3839. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 189.Forte E, Fiorenza D, Torino E, Costagliola di Polidoro A, Cavaliere C, Netti PA, Salvatore M, Aiello M. Radiolabeled PET/MRI nanoparticles for tumor imaging. J Clin Med. 2019;9:89. doi: 10.3390/jcm9010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Frantellizzi V, Conte M, Pontico M, Pani A, Pani R, De Vincentis G. New frontiers in molecular imaging with superparamagnetic iron oxide nanoparticles (SPIONs): efficacy, toxicity, and future applications. Nucl Med Mol Imaging. 2020;54:65–80. doi: 10.1007/s13139-020-00635-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Holzwarth U, Ojea Jimenez I, Calzolai L. A random walk approach to estimate the confinement of α-particle emitters in nanoparticles for targeted radionuclide therapy. EJNMMI Radiopharm Chem. 2018;3:9. doi: 10.1186/s41181-018-0042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jeon J. Review of therapeutic applications of radiolabeled functional nanomaterials. Int J Mol Sci. 2019;20:2323. doi: 10.3390/ijms20092323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ni D, Jiang D, Ehlerding EB, Huang P, Cai W. Radiolabeling silica-based nanoparticles via coordination chemistry: basic principles, strategies, and applications. Acc Chem Res. 2018;51:778–788. doi: 10.1021/acs.accounts.7b00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pellico J, Gawne PJ, T M de Rosales R. Radiolabelling of nanomaterials for medical imaging and therapy. Chem Soc Rev. 2021;50:3355–3423. doi: 10.1039/d0cs00384k. [DOI] [PubMed] [Google Scholar]
- 195.Peltek OO, Muslimov AR, Zyuzin MV, Timin AS. Current outlook on radionuclide delivery systems: from design consideration to translation into clinics. J Nanobiotechnology. 2019;17:90. doi: 10.1186/s12951-019-0524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Polyak A, Ross TL. Nanoparticles for SPECT and PET imaging: towards personalized medicine and theranostics. Curr Med Chem. 2018;25:4328–4353. doi: 10.2174/0929867324666170830095553. [DOI] [PubMed] [Google Scholar]
- 197.Ranjbar Bahadori S, Mulgaonkar A, Hart R, Wu CY, Zhang D, Pillai A, Hao Y, Sun X. Radiolabeling strategies and pharmacokinetic studies for metal based nanotheranostics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13:e1671. doi: 10.1002/wnan.1671. [DOI] [PubMed] [Google Scholar]
- 198.Silva F, Cabral Campello MP, Paulo A. Radiolabeled gold nanoparticles for imaging and therapy of cancer. Materials (Basel) 2020;14:4. doi: 10.3390/ma14010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Arora G, Shukla J, Ghosh S, Maulik SK, Malhotra A, Bandopadhyaya G. PLGA nanoparticles for peptide receptor radionuclide therapy of neuroendocrine tumors: a novel approach towards reduction of renal radiation dose. PLoS One. 2012;7:e34019. doi: 10.1371/journal.pone.0034019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Arora G, Dubey P, Shukla J, Ghosh S, Bandopadhyaya G. Evaluation of cytotoxic and tumor targeting capability of 177Lu-DOTATATE-nanoparticles: a trailblazing strategy in peptide receptor radionuclide therapy. Ann Nucl Med. 2016;30:334–345. doi: 10.1007/s12149-016-1067-x. [DOI] [PubMed] [Google Scholar]
- 201.Chakravarty R, Chakraborty S, Guleria A, Kumar C, Kunwar A, Nair KVV, Sarma HD, Dash A. Clinical scale synthesis of intrinsically radiolabeled and cyclic RGD peptide functionalized 198Au nanoparticles for targeted cancer therapy. Nucl Med Biol. 2019;72-73:1–10. doi: 10.1016/j.nucmedbio.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 202.Chen D, Yang D, Dougherty CA, Lu W, Wu H, He X, Cai T, Van Dort ME, Ross BD, Hong H. In vivo targeting and positron emission tomography imaging of tumor with intrinsically radioactive metal-organic frameworks nanomaterials. ACS Nano. 2017;11:4315–4327. doi: 10.1021/acsnano.7b01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen F, Valdovinos HF, Hernandez R, Goel S, Barnhart TE, Cai W. Intrinsic radiolabeling of Titanium-45 using mesoporous silica nanoparticles. Acta Pharmacol Sin. 2017;38:907–913. doi: 10.1038/aps.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Heo GS, Zhao Y, Sultan D, Zhang X, Detering L, Luehmann HP, Zhang X, Li R, Choksi A, Sharp S, Levingston S, Primeau T, Reichert DE, Sun G, Razani B, Li S, Weilbaecher KN, Dehdashti F, Wooley KL, Liu Y. Assessment of copper nanoclusters for accurate in vivo tumor imaging and potential for translation. ACS Appl Mater Interfaces. 2019;11:19669–19678. doi: 10.1021/acsami.8b22752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lamb J, Holland JP. Advanced methods for radiolabeling multimodality nanomedicines for SPECT/MRI and PET/MRI. J Nucl Med. 2018;59:382–389. doi: 10.2967/jnumed.116.187419. [DOI] [PubMed] [Google Scholar]
- 206.Wall MA, Shaffer TM, Harmsen S, Tschaharganeh DF, Huang CH, Lowe SW, Drain CM, Kircher MF. Chelator-free radiolabeling of SERRS nanoparticles for whole-body PET and intraoperative raman imaging. Theranostics. 2017;7:3068–3077. doi: 10.7150/thno.18019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhan Y, Ehlerding EB, Shi S, Graves SA, Goel S, Engle JW, Liang J, Cai W. Intrinsically zirconium-89-labeled manganese oxide nanoparticles for in vivo dual-modality positron emission tomography and magnetic resonance imaging. J Biomed Nanotechnol. 2018;14:900–909. doi: 10.1166/jbn.2018.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chakravarty R, Chakraborty S, Ningthoujam RS, Vimalnath Nair KV, Sharma KS, Ballal A, Guleria A, Kunwar A, Sarma HD, Vatsa RK, Dash A. Industrial-scale synthesis of intrinsically radiolabeled 64CuS nanoparticles for use in positron emission tomography (PET) imaging of cancer. Ind Eng Chem Res. 2016;55:12407–12419. [Google Scholar]
- 209.Chakravarty R, Chakraborty S, Guleria A, Kunwar A, Sarma HD, Dash A. Facile one-pot synthesis of intrinsically radiolabeled 64Cu-human serum albumin nanocomposite for cancer targeting. Chem Select. 2017;2:8043–8051. [Google Scholar]
- 210.Chakravarty R, Chakraborty S, Guleria A, Shukla R, Kumar C, Vimalnath Nair KV, Sarma HD, Tyagi AK, Dash A. Facile one-pot synthesis of intrinsically radiolabeled and cyclic RGD conjugated 199Au nanoparticles for potential use in nanoscale brachytherapy. Ind Eng Chem Res. 2018;57:14337–14346. [Google Scholar]
- 211.Chakravarty R, Guleria A, Jadhav S, Kumar C, Debnath AK, Sarma HD, Chakraborty S. Bioinspired synthesis of intrinsically 177Lu-labeled hybrid nanoparticles for potential cancer therapy. Ind Eng Chem Res. 2020;59:22492–22500. [Google Scholar]
- 212.Zhang X, Chen F, Turker MZ, Ma K, Zanzonico P, Gallazzi F, Shah MA, Prater AR, Wiesner U, Bradbury MS, McDevitt MR, Quinn TP. Targeted melanoma radiotherapy using ultrasmall 177Lu-labeled α-melanocyte stimulating hormone-functionalized core-shell silica nanoparticles. Biomaterials. 2020;241:119858. doi: 10.1016/j.biomaterials.2020.119858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chen F, Goel S, Hernandez R, Graves SA, Shi S, Nickles RJ, Cai W. Dynamic positron emission tomography imaging of renal clearable gold nanoparticles. Small. 2016;12:2775–2782. doi: 10.1002/smll.201600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cheng L, Jiang D, Kamkaew A, Valdovinos HF, Im HJ, Feng L, England CG, Goel S, Barnhart TE, Liu Z, Cai W. Renal-clearable PEGylated porphyrin nanoparticles for image-guided photodynamic cancer therapy. Adv Funct Mater. 2017;27:1702928. doi: 10.1002/adfm.201702928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ehlerding EB, Chen F, Cai W. Biodegradable and renal clearable inorganic nanoparticles. Adv Sci (Weinh) 2016;3:1500223. doi: 10.1002/advs.201500223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Shen S, Jiang D, Cheng L, Chao Y, Nie K, Dong Z, Kutyreff CJ, Engle JW, Huang P, Cai W, Liu Z. Renal-clearable ultrasmall coordination polymer nanodots for chelator-free 64Cu-labeling and imaging-guided enhanced radiotherapy of cancer. ACS Nano. 2017;11:9103–9111. doi: 10.1021/acsnano.7b03857. [DOI] [PMC free article] [PubMed] [Google Scholar]