Abstract
Type 1 diabetes is thought to occur as a result of the loss of insulin-producing pancreatic β cells by an environmentally triggered autoimmune reaction. In rodent models of diabetes, streptozotocin (STZ), a genotoxic methylating agent that is targeted to the β cells, is used to trigger the initial cell death. High single doses of STZ cause extensive β-cell necrosis, while multiple low doses induce limited apoptosis, which elicits an autoimmune reaction that eliminates the remaining cells. We now show that in mice lacking the DNA repair enzyme alkylpurine-DNA-N-glycosylase (APNG), β-cell necrosis was markedly attenuated after a single dose of STZ. This is most probably due to the reduction in the frequency of base excision repair-induced strand breaks and the consequent activation of poly(ADP-ribose) polymerase (PARP), which results in catastrophic ATP depletion and cell necrosis. Indeed, PARP activity was not induced in APNG−/− islet cells following treatment with STZ in vitro. However, 48 h after STZ treatment, there was a peak of apoptosis in the β cells of APNG−/− mice. Apoptosis was not observed in PARP-inhibited APNG+/+ mice, suggesting that apoptotic pathways are activated in the absence of significant numbers of DNA strand breaks. Interestingly, STZ-treated APNG−/− mice succumbed to diabetes 8 months after treatment, in contrast to previous work with PARP inhibitors, where a high incidence of β-cell tumors was observed. In the multiple-low-dose model, STZ induced diabetes in both APNG−/− and APNG+/+ mice; however, the initial peak of apoptosis was 2.5-fold greater in the APNG−/− mice. We conclude that APNG substrates are diabetogenic but by different mechanisms according to the status of APNG activity.
Type 1 diabetes occurs in a genetically susceptible human population as a result of the loss of the insulin-producing pancreatic β cells. The disease is thought to be triggered by an environmental agent(s) that initiates processes leading to an eventual β-cell-destructive autoimmune response (6, 12, 14). In animal models of the disease, the first observable abnormality is an initial low level of β-cell death (27, 28) that primes antigen-presenting cells such as dendritic cells and macrophages (19). This leads to the proliferation of autoreactive lymphocytes and the ensuing selective elimination of the remaining β cells.
Much of what is known about the cellular mechanisms leading to type 1 diabetes has come from the study of both the nonobese diabetic (NOD) mouse and the use of the methylating agent streptozotocin [2-deoxy-2-(3-methyl-3-nitrosourea)-1-d-glucopyranose] (STZ) as the environmental trigger for the disease. STZ is actively transported into pancreatic β cells via the Glut-2 glucose transporter. It reacts at many sites in DNA but in particular at the ring nitrogen and exocyclic oxygen atoms of the DNA bases, predominantly producing 7-methylguanine, 3-methyladenine (3-meA), and O6-methylguanine adducts (1, 37). 3-meA and 7-methylguanine are removed by the action of alkylpurine-DNA-N-glycosylase (APNG) (also referred to as 3-methyladenine DNA glycosylase), leaving an apurinic/apyrimidinic (AP) site that is acted upon by an AP endonuclease. The resulting DNA strand breaks activate poly(ADP-ribose) polymerase (PARP), which synthesizes polymers of ADP-ribose from NAD+, modifying acceptor proteins at the site of DNA damage (38). PARP is thus part of a protein complex that includes XRCC1, DNA polymerase β, and DNA ligase III and is required for the efficient resynthesis and ligation steps of base excision repair (5).
Therefore, a single high dose of STZ will produce a large number of DNA strand breaks, leading to the overactivation of PARP. This results in a catastrophic fall in cellular NAD+ levels and thus to nonphysiological concentrations of ATP, loss of membrane integrity, and necrotic β-cell death (30). In agreement with this, mice deficient in PARP or treated with PARP inhibitors are protected from STZ-induced β-cell necrosis and do not develop hyperglycemia (2, 25, 33). However, although the initial destruction of the β cells can be avoided by the use of PARP inhibitors, the resulting long-term biological consequence of this is the development of a high incidence of pancreatic β-cell tumors (36, 40). Conversely, a regimen of five daily subdiabetogenic doses of STZ (the multiple-low-dose STZ model, MLDS) induces a peak of apoptotic β-cell death after 5 days of STZ treatment (27), while a second peak of apoptosis is seen at 11 days, when lymphocytic infiltration of the islet occurs. The preimmune stage of β-cell apoptosis has also been reported to occur in the NOD mouse model (28). However, the molecular mechanisms leading to the initial peak of STZ-induced β-cell apoptosis in the MLDS model are unclear, and it is also unknown whether the initial induction of β-cell apoptosis is important in triggering the resulting autoimmune reaction.
Thus, while the role of PARP in STZ-induced β-cell death has been studied extensively, we were interested in determining the role of APNG in modulating both the cytotoxic effects of a single high dose of STZ and its effect in the MLDS model of diabetes. Using a recently described APNG-deficient mouse strain (8), our results showed that although APNG−/− mice were substantially resistant to single, high-dose STZ-induced β-cell necrosis and the initial onset of diabetes, a smaller peak of β-cell apoptosis was observed 48 h after treatment. However, analogous to the onset of autoimmune diabetes in the NOD mouse model but in contrast to that reported for PARP-inhibited animals, the STZ-treated APNG−/− mice eventually succumbed to diabetes after several months due to a marked autoimmune reaction. Additionally, in the MLDS model of diabetes, the APNG−/− mice also exhibited an initial increased sensitivity to β-cell apoptosis.
MATERIALS AND METHODS
Mice.
APNG−/− mice were generated as previously described (8). They were backcrossed onto a C57BL/6J background for nine generations, and the APNG+/− mice thus generated were crossed to yield the male mice used in these experiments. All animal experiments were carried out under the Animals (Scientific Procedures) Act 1986, in the United Kingdom.
Animal dosing.
STZ (Sigma) was dissolved in sodium citrate buffer (pH 4.5) and injected intraperitoneally, either as a single high dose of 140 mg/kg or, for the MLDS experiments, as five daily doses of 40 mg/kg. In some experiments, 30 min before STZ treatment, 3-aminobenzamide (3-ab; Sigma) prepared in 0.9% saline was administered by intravenous injection at a dose of 340 mg/kg of body weight. Control animals were similarly injected with vehicle only.
Determination of pancreatic insulin and glucose levels.
Pancreata were dissected; either they were placed entirely in 10 ml of acidified ethanol, or they were divided longitudinally and one half was placed in acidified ethanol while the other was prepared for histological examination. For insulin extraction, the organ was cut into small pieces and then sonicated twice for 30 s at 216 μm (peak-to-peak amplitude; Heat Systems). The sonicated material was kept at 4°C for 16 h to extract the insulin and then aliquoted for storage at −20°C. The insulin concentration was measured by radioimmunoassay, using rat insulin as the standard as previously described (15). The blood glucose level was determined by reflectance photometry (Glucotrend blood glucose meter; Boehringer Mannheim) in conjunction with oxidoreductase reaction strips (Glucotrend glucose strips; Roche).
Histological examination of pancreata.
Mice were sacrificed at 8, 12, 24, 48, and 96 h after STZ treatment. At autopsy, the pancreata were fixed in 4% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.2), processed for paraffin embedding, sectioned (3 μm), and stained with hematoxylin-eosin. The percentage of morphologically abnormal β cells was calculated by scoring 500 to 1,000 islet cells per animal and was used as an indication of β-cell death. Scoring β-cell apoptosis using β-cell-specific stains was not appropriate since the cytoplasmic and nuclear morphology was not good enough when these methods were used. Since necrotic and apoptotic nuclei are smaller than normal nuclei, the morphological scoring can be regarded as only semiquantitative. Therefore, apoptosis and necrosis was confirmed by transmission electron microscopy. Mice were perfused with 4% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.2), and 1- by 3-mm slices of pancreas were transferred to 3% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for 3 h, resin embedded, and stained for electron microscopy as previously described (20).
Immunohistochemistry. (i) Insulin.
Formalin-fixed pancreata were embedded in paraffin, and 3-μm-thick sections were cut and mounted onto 3-aminopropyltriethoxysilane-coated slides. After being dewaxed through xylene and absolute ethanol, the slides were rehydrated through decreasing concentrations of ethanol (100, 90, 70, and 40%) and rinsed in distilled water. The slides were washed thoroughly in Tris-buffered saline (pH 7.5) (TBS), and endogenous peroxidase was blocked by incubation with 3% H2O2 in TBS for 20 min. The slides were again washed in water and TBS and incubated with 5% normal rabbit serum for 20 min, before being exposed to the primary antibody, a polyclonal guinea pig anti-swine insulin (1:4 dilution; Dako) at 4°C overnight. After being washed in TBS, the slides were incubated with the secondary antibody, biotinylated rabbit anti-guinea pig immunoglobulins (1:200 dilution; Dako) for 30 min at room temperature, washed in TBS, and incubated with an avidin-biotin-horseradish peroxidase complex (Dako) for 40 min at room temperature. The antibody-antigen complexes were visualized by 3,3-diaminobenzidine (Sigma) staining for 5 min. Finally, the slides were counterstained with hematoxylin, dehydrated, and mounted.
(ii) T-cell markers CD4 and CD8.
Pancreata were snap frozen in liquid nitrogen, and 4- to 5-μm sections were prepared. The sections were then fixed in cold acetone for 10 min, air dried, and washed in TBS. Endogenous peroxidase was blocked as above. The sections were incubated with 5% normal rabbit serum for 20 min and then exposed overnight to one of the primary antibodies, either rat anti-mouse CD4+, or rat anti-mouse CD8+ (BD PharMingen), at 2 μg/ml. After being washed in TBS, the sections were incubated with biotinylated rabbit anti-rat immunoglobulins (1:300 dilution; Dako) for 30 min at room temperature. Treatment with avidin-biotin-horseradish peroxidase and then 3,3-diaminobenzidine was as used for the detection of insulin.
Isolation of pancreatic islets and treatment with STZ.
Islets were isolated using the method of Lake et al. (22). Using this method, 150 to 200 islets could be reliably obtained. Groups of 150 freshly isolated islets (one animal) were incubated for 30 min in 2.2 mM STZ in Ham's F-10 medium (Life Technologies) or in medium only.
Isolation of islet nuclei and estimation of PARP activity.
PARP activity in isolated islets was measured essentially as previously described (3). Briefly, following STZ treatment, the islets were washed in Ham's F-10 medium and resuspended in a solution containing 250 mM sucrose, 10 mM HEPES (pH 7.4), 2.5 mM EDTA, 2 mM cysteine, and 0.02% bovine serum albumin. The islets were left on ice for 5 min to lyse and then dispersed by rapid pipetting. The nuclei were pelleted by centrifugation at 1,000 × g for 3 min and resuspended in 100 μl of buffer (50 mM Tris-HCl [pH 7.5], 30% glycerol, 1 mM EDTA, 0.5 mM EGTA) and added to an equal volume of 100 mM Tris-HCl (pH 8.0)–20 mM 2-mercaptoethanol–10 mM MgCl2 containing 5 μCi of [2,5,8-3H]NAD (Amersham Pharmacia Biotech). The mixture was vortexed briefly and incubated for 30 min at 37°C; then 1 ml of ice-cold stop solution (10% trichloroacetic acid, 2% sodium pyrophosphate decahydrate) was added. The samples were left on ice for 45 min and then centrifuged at 12,000 × g for 10 min. The supernatant was aspirated, and the pellet was washed three more times with 1 ml of stop solution and then once in 0.6 M perchloric acid. The pellet resuspended and left overnight in 200 μl of 0.04 N NaOH. A portion (150 μl) of the solution was added to a scintillant, and the amount of radioactivity present was measured in a scintillation counter. The remainder of the solution was used to measure DNA content as previously described (21). Results are expressed as femtomoles of NAD+ incorporated per minute per microgram of DNA.
Statistical analysis.
The results obtained with groups from each study were first analyzed using analysis of variance. Groups that showed differences were further analyzed by Student's t test.
RESULTS
Effect of APNG deletion on β-cell survival following a single high dose of STZ.
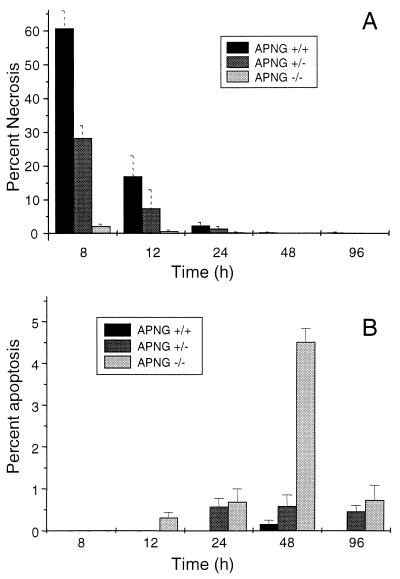
To test our hypothesis that APNG-deleted mice should be resistant to STZ-induced necrosis of pancreatic β cells, normal mice (APNG+/+) and mice heterozygous (APNG+/−) or homozygous (APNG−/−) for the APNG null mutation were injected with a single dose of STZ (140 mg/kg) and scored for islet cell morphology by light microscopy. Under light microscopy, necrotic islet cells could be easily identified by their pycnotic nuclei and fragmented cytoplasm (Fig. 1A). For the three mouse strains, the difference in the number of necrotic islet cells after treatment was striking (Fig. 2A). At 8 h after the STZ dose, 60% of the cells from normal mice showed nuclear pycnosis whereas fewer than 5% of the cells from APNG−/− mice did so. In normal mice, the number of islet cells containing pycnotic nuclei was reduced to background levels over the next 2 days. No delayed increase in nuclear pycnosis was observed in the islets from APNG−/− mice (Fig. 2A). Interestingly, there was a gene dosage effect, with islet cells from APNG+/− mice exhibiting approximately half the normal level of nuclear pycnosis at 8, 12, and 24 h postdose, although this was statistically significant only for the 8- and 12-h values.
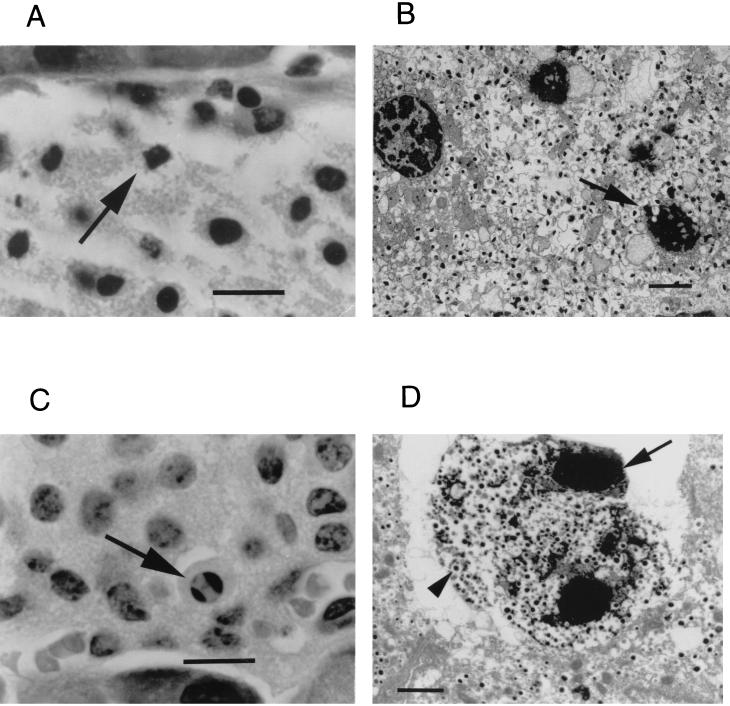
FIG. 1.
Light and electron microscopy of sections of pancreatic islets at 8 and 48 h after the STZ dose (140 mg/kg). (A and B) Light microscopy of islet cells from APNG+/+ mice reveals extensive necrosis with fragmented nuclei (arrow) at 8 h postdose (A), while electron microscopy shows nuclei clumped with ill-defined edges (arrow) and karyolysis, indicative of necrosis (B). (C) In contrast, light microscopy of islets from APNG−/− mice 48 h postdose showed increased numbers of apoptotic islet cells with shrunken cytoplasm and intact nuclear and cytoplasmic membranes. Nuclei were clumped into well-defined masses marginated against the nuclear membrane (arrow). (D) Electron microscopy of the APNG−/− STZ-treated islets showed cells with condensed nuclear bodies (arrow), intact membranes, and condensed cytoplasm containing insulin granules (arrowhead), indicative of an apoptotic β cell. Magnification, ×400 (A and C); bar, 10 μm; ×2,800 (B and D); bar, 2 μm.
FIG. 2.
Proportion of islet cells showing nuclear pycnosis consistent with necrosis (A) and islet cell apoptosis (B) in APNG+/+, APNG+/−, and APNG−/− mice 8 to 96 h after a single injection of STZ (140 mg/kg). Each data point represents the mean and standard error of the mean for 500 islet cells from four animals.
The primary mode of cell death for islet cells from STZ-treated APNG+/+ mice was confirmed to be necrosis by transmission electron microscopy (Fig. 1B). The necrotic β cells displayed clumping of the chromatin with ill-defined edges and karyolysis, while the mitochondria were swollen. At 24 h postdose, most of the β cells were dead, leaving an area of necrosis at the center of the islets. In contrast, the islets of APNG−/− mice at 8 h postdose contained only an occasional necrotic β cell. However, in APNG−/− mice and to a lesser extent in APNG+/− mice, significant numbers of apoptotic islet cells were seen from 24 h, with a peak at 48 h postdose (Fig. 2B). Under light microscopy, hematoxylin-and-eosin-stained apoptotic β cells could be identified by their chromatin morphology, size, and cytoplasmic staining (Fig. 1C). Apoptotic nuclei were either clumped and marginated or fragmented into regularly shaped membrane-bound bodies. Apoptotic cells appeared shrunken and more densely eosinophilic. Apoptosis was confirmed by transmission electron microscopy; apoptotic cells appeared shrunken but retained intact nuclear and cell membranes (Fig. 1D). Nuclear chromatin was clumped into well-defined masses marginated against the nuclear membrane or in separate membrane-bound bodies. Apoptotic islet cells stained positive for insulin, confirming that they were β cells (data not shown).
Effect of APNG deletion on PARP activation.
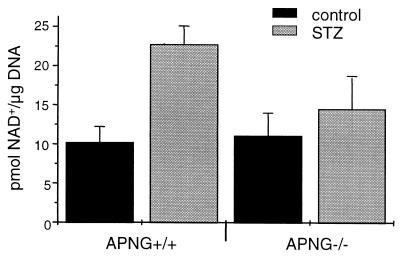
To confirm that APNG deletion attenuated the activation of PARP, thereby maintaining cellular ATP levels and preventing the initial catastrophic necrosis, pancreatic islets were isolated from APNG+/+ and APNG−/− mice and treated with STZ in vitro. Measurement of PARP activity made by the incorporation of [3H]NAD+ into poly(ADP-ribose) polymers showed that APNG−/− islets had significantly less PARP activity after STZ treatment than did APNG+/+ islets (P < 0.01) and were not significantly different from untreated controls (P = 0.19) (Fig. 3). Therefore, since DNA strand breaks are required for PARP activation, this result indicates that the bulk of the STZ-induced DNA base adducts were not repaired by base excision in the absence of APNG. It is reasonable to suggest that the persistence of at least a subset of these adducts could be responsible for the apoptotic response observed in the APNG−/− mice.
FIG. 3.
PARP activity in islets isolated from APNG+/+ and APNG−/− mice. PARP activity was measured in islet cells following incubation for 30 min in 2.2 mM STZ or medium alone. While PARP activity was significantly increased in STZ-treated islets from APNG+/+ mice compared to untreated controls (P < 0.001), there was no significant difference in PARP activity between STZ-treated and control islets from APNG−/− mice. Error bars indicated the mean ± standard deviation.
Effect of APNG deletion on high-dose-STZ-induced diabetes.
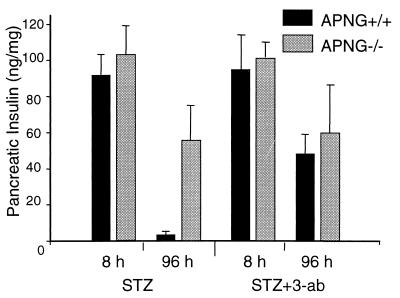
The pancreatic insulin content was measured at 8 and 96 h to give an indication of the extent of pancreatic β-cell destruction after a single high dose of STZ. Figure 4 shows that at 8 h, pancreatic insulin levels in both APNG+/+ and APNG−/− mice were similar. By 96 h, the pancreatic insulin content in APNG+/+ mice had fallen by 90% to around 4 ng of insulin per mg of pancreas whereas the levels in STZ-treated APNG−/− mice were reduced by only 50% of the control level (Fig. 4). These results corroborate the observed histological findings and confirm that the insulin-secreting β cells are the apoptotic cells of the APNG−/− islets. Since previous reports had shown that PARP-deficient mice were also resistant to STZ-induced diabetes (2, 25, 33), we were interested in knowing the effect of the PARP inhibitor 3-ab on diabetes induction in-the APNG−/− mice. Pretreatment of normal mice with 3-ab (340 mg/kg) substantially protected them against a fall in pancreatic insulin levels at 96 h (Fig. 4), in agreement with the previous reports. However, pretreatment with 3-ab had no effect on the insulin levels of STZ-treated APNG−/− mice at 96 h (Fig. 4). Indeed, there was no significant difference between the pancreatic insulin levels in 3-ab-pretreated APNG+/+ and APNG−/− mice and APNG−/− mice treated with STZ alone. Since PARP acts at the DNA strand breaks arising from the action of APNG and AP-endonuclease, these results indicate that the APNG-deleted cells survive because of the absence of DNA strand scission and that any inhibition of a later step in the pathway has no effect on cell survival.
FIG. 4.
Effect of pretreatment with 3-ab on pancreatic insulin levels of APNG+/+ and APNG−/− mice, 8 and 96 h after treatment with a single dose of STZ (140 mg/kg). Error bars indicate the mean ± the standard error of the mean.
To further compare the mechanisms by which the lack of APNG or PARP leads to cell survival in this system, we investigated the effect of 3-ab on islet cell morphology at 8 and 48 h after STZ treatment in APNG+/+ and APNG−/− mice. As expected, histological analysis at 8 h showed that 3-ab-pretreated APNG+/+ mice displayed considerably less β-cell necrosis than did those treated with STZ alone (P < 0.001) (Table 1). Similarly, PARP inhibition in APNG−/− mice resulted in a small but significant reduction in islet cell necrosis (P < 0.05) (Table 1). This most probably reflects a low level of strand break induction by nonenzymatic depurination and glycosylase-catalyzed removal of STZ-induced oxidative base damage to the DNA in the APNG−/− mice. For apoptosis at 48 h, it is clear from Table 1 that 3-ab treatment of APNG+/+ mice did not produce a peak of apoptosis similar to that found in APNG−/− mice. The inhibition of PARP had no significant effect on the peak of apoptosis observed in APNG−/− islets (P = 0.15) (Table 1). These results suggest that it is the persistence of DNA adducts that act as a signal for the cell to undergo apoptosis.
TABLE 1.
Effect of 3-ab on high-dose-STZ-induced β-cell necrosis at 8 h and apoptosis at 48 h.
| Mouse strain | 3-ab | % Necrosis at 8 ha (no. of mice) | % Apoptosis at 48 ha (no. of mice) |
|---|---|---|---|
| APNG+/+ | − | 73 ± 3.0 (5) | 0.25 ± 0.16 (4) |
| + | 0.5 ± 0.3 (5) | 0.87 ± 0.26 (4) | |
| APNG−/− | − | 2.1 ± 0.7 (5) | 4.3 ± 1.0 (4) |
| + | 0.2 ± 0.1 (5) | 2.63 ± 0.56 (5) |
For apoptosis values, P < 0.01 for both comparisons between APNG+/+ and APNG−/− mice. For necrosis and apoptosis values, data are expressed as the mean ± the standard error of the mean.
Induction of diabetes in APNG−/− mice after a single high dose of STZ.
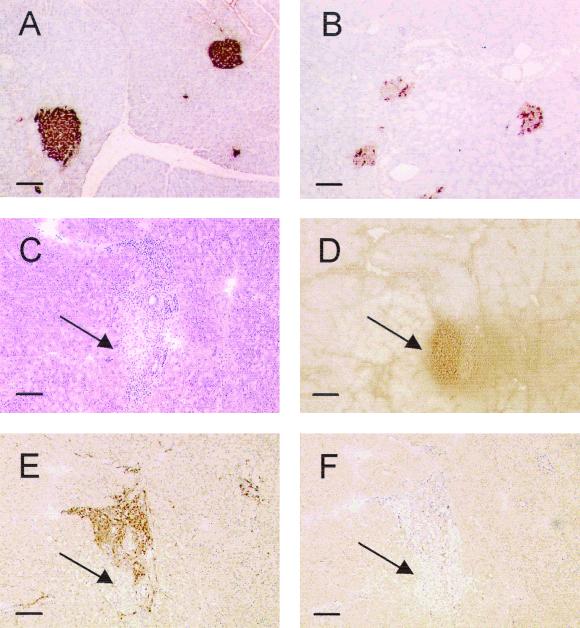
To assess the long-term effects of high-dose STZ treatment in APNG−/− mice, STZ-treated animals were monitored for general well-being over several months. At approximately 8 months postdose, all the mice were diabetic, with fasting blood glucose levels averaging above 10 mM and significantly reduced pancreatic insulin levels compared to equivalently aged untreated controls (Table 2). Several mice also exhibited marked lipolysis, with greatly diminished fat pads. Immumohistological examination of the pancreata showed a marked decrease in islet insulin content and marked CD4+ lymphocytic proliferation, but not CD8+ proliferation, both around and within the islets (Fig. 5), which was not seen in the controls (data not shown). Although no similar study has been reported for PARP-deficient mice, rodents pretreated with 3-ab developed β-cell specific insulomas 1 year after receiving a single high dose of STZ (36, 40). Thus, the method of cell death reported here, resulting from the persistence of DNA adducts or the lack of DNA strand breaks, may have implications for the generation of secondary tumors following treatment with chemotherapeutic alkylating agents.
TABLE 2.
Long-term effects of a single high dose of STZ on blood glucose and pancreatic insulin levels in APNG−/− mice
| Treatment | Blood glucose level (mM)a (no. of mice) | Pancreatic insulin level (ng/mg)a (no. of mice) |
|---|---|---|
| None (controls) | 5.48 ± 0.89 (7) | 82.3 ± 28 (6) |
| STZ (140 mg/kg) | 13.1 ± 3.3 (11)b | 7.97 ± 5.19 (7) |
P < 0.001 for comparisons between controls and STZ-treated mice. Values are expressed as the mean ± standard devation.
Values do not include one animal, where the glucose level was >44 mM.
FIG. 5.
Immunohistochemical determination of autoimmune diabetes in APNG−/− mice 8 months after treatment with STZ. (A and B) Formalin-fixed sections from untreated (A) and STZ-treated (B) APNG−/− mice were stained for insulin as described in Materials and Methods. (C to F) CD4+ and CD8+ immunostaining was carried out on frozen sections. Hematoxylin-eosin (C) and insulin (D) staining indicate the position of the islet in the section, while staining for the specific lymphocyte markers shows evidence of CD4+ (E) but not CD8+ (F) lymphocytic invasion in the islet. The more porous nature of the frozen sections compared to the formalin-fixed sections made them unsuitable for quantitative assessment of insulin content by this method. Arrows indicate the position of the islet in the section. Bar, 100 μm.
Effect of APNG deletion on MLDS-induced β-cell apoptosis.
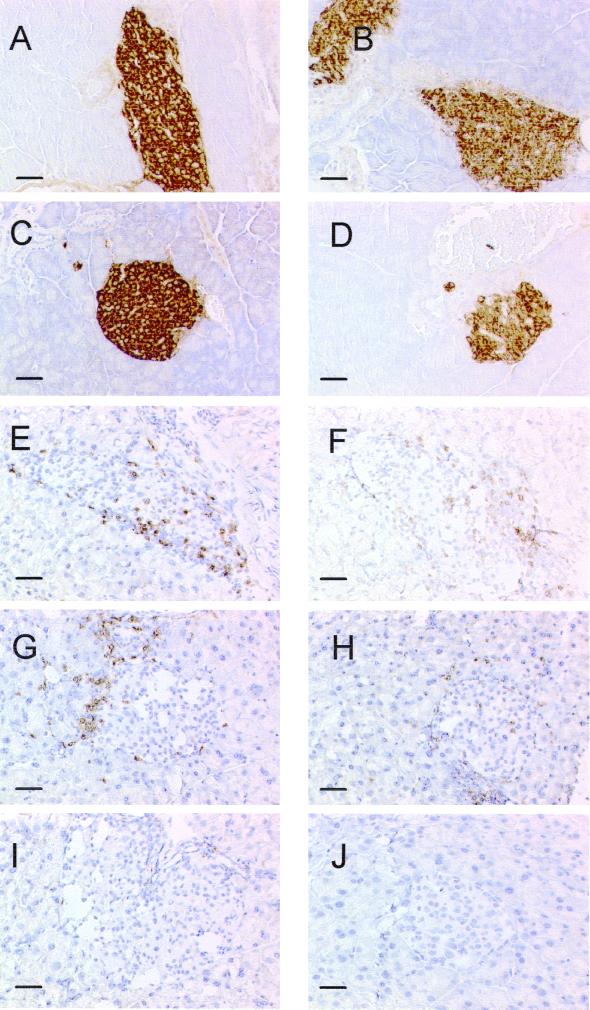
Since MLDS is a commonly used rodent model of type 1 diabetes, APNG+/+ and APNG−/− mice were treated with STZ (40 mg/kg) for 5 days, and 6 h after the last dose their pancreata were dissected for histological examination. Pancreata were also removed on day 11 to determine the effect of this regimen on pancreatic insulin levels in these mouse strains. On day 5, islets from both strains showed evidence of apoptosis, with APNG−/− mice showing a 2.5-fold increase in β-cell apoptosis compared with their normal littermates (Table 3). By day 11, however, this situation had been reversed (Table 3), with less apoptosis being present in APNG−/− mice. At this later time, pancreatic insulin levels were approximately 30% of control values in both mouse strains (data not shown), and this was borne out by immunohistochemical staining for insulin, which showed a decrease in staining intensity for both STZ-treated APNG-deficient and normal mice (Fig. 6A to D). Islets from both strains showed evidence of a low-grade CD4+ and CD8+ lymphocytic infiltration at this time (Fig. 6E to J). Therefore, under this STZ regimen, both mouse strains succumbed similarly to diabetes. However, evidence from the cell morphology studies suggests that the initial signaling events leading to apoptosis are different and occur more rapidly in the absence of APNG.
TABLE 3.
Islet cell apoptosis following MLDS treatment of APNG+/+ and APNG−/− mice
| Mouse strain | Day | % Apoptosis (no. of mice)a |
|---|---|---|
| APNG+/+ | 5 | 0.98 ± 0.1b (5) |
| 11 | 1.56 ± 0.12c (11) | |
| APNG−/− | 5 | 2.42 ± 0.21b (5) |
| 11 | 1.03 ± 0.18c (11) |
Values are expressed as the mean ± the standard error of the mean.
Day 5 values (P < 0.001).
Day 11 values (P < 0.05).
FIG. 6.
Immunohistochemical determination of MLDS-induced autoimmune diabetes in APNG+/+ and APNG−/− mice on day 11. (A to D) Formalin-fixed pancreata stained for insulin. (E to J) Frozen sections of pancreata stained for CD4+ (E, G, and I) or CD8+ (F, H, and J). (A and B) APNG+/+ control and MLDS, respectively; (C and D) APNG−/− control and MLDS, respectively; (E and F) APNG+/+; (G and H) APNG−/−; (I and J) control sections from untreated APNG−/− mice. Bar, 50 μm.
DISCUSSION
This study demonstrates that APNG−/− mice are essentially resistant to the immediate cytotoxic effects of a single high dose of STZ, analogous to that described for PARP-deficient mice (2, 25, 33). However, significant differences in the extent and timing of islet cell apoptosis were observed in both this and the MLDS model of type 1 diabetes, suggesting that at least a subset of the unrepaired DNA adducts can act as signals for apoptosis. The finding that both APNG−/− and PARP-deficient mice are resistant to single-high-STZ-dose-induced β-cell necrosis provides further evidence that the necrosis is caused by DNA adduct removal, the subsequent induction of DNA strand breaks by AP-endonuclease, and the activation of PARP. This is also supported by our observation that in contrast to APNG+/+ islets, APNG−/− islets treated in vitro do not show significant PARP activation. Since APNG−/− and APNG+/+ mice have the same basal levels of PARP activity, the finding that APNG−/− mice lack a significant STZ-induced activation of PARP indicates that the majority of STZ-induced DNA strand breaks are due to the action of APNG on DNA adducts that are substrates for this enzyme.
The presence of significant amounts of STZ-induced β-cell apoptosis in APNG−/− mice is a novel finding and supports previous reports that PARP inhibitors protect cultured β cells against STZ-induced necrosis but not against cytokine-induced apoptosis (16, 18). To our knowledge, this is the first in vivo evidence of increased apoptosis resulting from a reduced repair of DNA adducts and supports a previous report of the induction of apoptosis in cell lines lacking APNG treated in vitro with MeOSO2(CH2)2-lexitropsin, which almost exclusively forms 3-meA adducts (10). 3-meA is known to block DNA replication by inhibiting the action of DNA polymerases (23), and thus it is likely that stalled replication-transcription forks at 3-meA adducts are the ultimate apoptotic signaling lesions in STZ-treated APNG−/− cells. Since 3-meA in DNA has a half-life of only 24 h under physiological conditions in vitro (24), its potential toxicity in cells undergoing replication decreases with time, and this probably explains the timing of the peak of apoptosis observed in islets from APNG−/− mice after STZ treatment. However, this could also be due in part to the formation of DNA strand breaks resulting from the spontaneous depurination of 3-meA over this period.
Evidence that 3-meA depurination was not the major apoptotic signaling event was obtained from studies of islet cell morphology following STZ treatment: 3-ab-pretreated APNG+/+ mice showed significantly less β-cell apoptosis than did APNG−/− mice, consistent with the hypothesis that the unrepaired DNA adducts, or a fraction thereof, act as the apoptotic signal. However, the degree of cell death, as measured by pancreatic insulin levels, did not differ between APNG−/− and PARP-inhibited APNG+/+ mice. Considering the kinetics of apoptotic cell death over time, the degree of apoptosis seen in APNG−/− mice would adequately explain the fall in pancreatic insulin levels in these mice. Since the mode of STZ-induced β-cell death in 3-ab-pretreated APNG+/+ mice is unclear, it is possible that there may be another mechanism of delayed β-cell death. STZ is known to damage mitochondria, inhibiting ATP production (7), and this would lead to loss of membrane integrity and necrosis. Necrosis by this mechanism, therefore, may not involve nuclear DNA damage detectable as pycnotic nuclei 8 h postdose and may be difficult to detect by light microscopy, especially if it is a minor pathway.
Apoptosis is the mode of β-cell death in the NOD mouse model (28). DNA adduct formation and APNG may also play a role in the NOD mouse model and in immune-mediated β-cell apoptosis. In addition to the N-methylpurines, which are an integral part of methylation damage, APNG releases 1,N6-ethenoadenine and deaminated adenine, both of which are generated endogenously by a number of processes, including macrophage-induced NO synthesis and lipid peroxidation (9, 11, 26). Cytokine-generated reactive oxygen species cause DNA strand breaks and β-cell apoptosis in vitro and are thought to play a role in the induction of the immune-mediated β-cell apoptosis seen in NOD mice (35). Thus, it is possible that APNG may also protect against NO and some immune-mediated apoptosis. Consistent with the idea that low levels of APNG may contribute to susceptibility to autoimmunity in NOD mice is the finding that NOD mice are very sensitive to multiple low doses of STZ but relatively resistant to a single high dose of STZ (32). The present study suggests that strain differences in sensitivity to STZ could be explained by differences in APNG activity and thus supports a recent article reporting differences in DNA strand break induction and PARP activation in two mouse strains (4).
The finding that APNG−/− mice treated with a single high dose of STZ develop a delayed lymphocytic proliferation and diabetes contrasts with earlier reports on the induction of β-cell tumors in rats given combined treatments of STZ and PARP inhibitors (36, 40). These are important results since they indicate that (i) inhibition of PARP may contribute to carcinogenesis and (ii) the persistence of DNA adducts can lead to an autoimmune reaction. It is possible that other adducts such as O6-methylguanine or DNA lesions induced by oxidative damage are responsible for the tumors seen in PARP-inhibited mice. Alternatively, the fidelity of DNA repair synthesis may be compromised in PARP-inhibited β cells since strand rejoining is known to occur more slowly in PARP-treated cells (39). On the other hand, while the deletion of APNG greatly reduces the ability of the cells to carry out base excision repair of these adducts, perhaps crucially, the signaling apparatus for other types of DNA damage, such as that caused by reactive oxygen species, remains in place. Relevant to this is the recent finding that PARP can promote inflammation through its interaction with the redox-regulated transcription factor NF-κB (31). In response to many agents, including genotoxins and oxidative stress, this family of transcription factors is involved both in the up-regulation of expression of inducible nitric oxide synthase and several proinflammatory cytokines and in the prevention of apoptosis initiation (13). Indeed, the inappropriate expression of NF-κB and the resulting autoimmune and inflammatory response has been proposed as the critical event in the development of type 1 diabetes (17). Thus, in APNG−/− mice the biological consequence of an active NF-κB signaling system is susceptibility to STZ-induced autoimmune diabetes. We are currently assessing the biological response of PARP−/− and APNG-PARP double-null mice in this system.
The lack of both β-cell apoptosis and any reported autoimmunity in PARP-inhibited mice treated with a single high dose of STZ suggests that β-cell apoptosis and not necrosis may be necessary for the induction of the autoimmune reaction. These findings are in keeping with recent studies by O'Brien et al., who showed that PARP-inhibited NOD mice are protected from both β-cell apoptosis and the ensuing autoimmune reaction (29). The observation that the immune reaction is CD4 positive suggests a role for antigen-presenting cells in the activation of the immune system. It is possible, then, that the difference between apoptotic and necrotic stimulation of the immune system may lie in the extent to which antigen-presenting cells are activated after taking up the remains of the β cell.
In the MLDS model, the timing and extent of apoptosis in APNG−/− mice is consistent with a role for unrepaired 3-meA adducts in this pathway. Although a proportion of the DNA adducts can be removed in normal mice, daily treatment of STZ would lead to an increase in the residual number of unrepaired adducts. Thus, APNG−/− mice may show more apoptosis than APNG+/+ mice by virtue of their inability to remove STZ-induced DNA adducts. This is further supported by the observation that APNG−/− mice treated with a single high dose of STZ also show β-cell apoptosis. For MLDS, the cumulative effect of the STZ treatment resulted in the same biological outcome, irrespective of APNG status. A major unresolved issue is why the autoimmune reaction is delayed 8 months after a single high dose of STZ in APNG−/− mice but occurs at day 11 in the MLDS model. One explanation may be that a single high dose of STZ may not produce the same T-lymphocyte imbalance that the MLDS regimen is thought to produce. Additionally, as C57BL/6J mice age, they develop immune dysregulation, again involving NF-κB signaling (34). Thus, autoimmunity may require both apoptotic β-cell priming of antigen-presenting cells and dysregulation of the lymphocyte subsets. Further studies are required to determine the relationship between the persistence of DNA adducts, apoptosis, and the immune response.
In conclusion, this study has revealed three important findings: (i) APNG deficiency leads to resistance to STZ-induced necrosis, (ii) DNA adduct persistence can lead to cellular apoptosis in vivo, and (iii) the inhibition of base excision repair before the induction of DNA strand breaks can radically alter the biological outcome, preventing the onset of tumorigenesis and promoting autoimmunity.
ACKNOWLEDGMENTS
At the Paterson Institute, we thank M. A. Willington for his excellent technical support during part of this work and G. Forster for carrying out the immunohistochemistry. We also thank Peter O'Connor for his critical comments on the manuscript. Electron microscopy was performed by C. Winterford of the University of Queensland Medical School.
J.W.C. acknowledges support by the Princess Alexandra Hospital Research and Development foundation and a Princess Alexandra Hospital Private Practice Scholarship. This work was supported by the Cancer Research Campaign UK (CRC).
REFERENCES
- 1.Bennett R A, Pegg A E. Alkylation of DNA in rat tissues following administration of streptozotocin. Cancer Res. 1981;41:2786–2790. [PubMed] [Google Scholar]
- 2.Burkart V, Wang Z-Q, Radons J, Heller B, Herceg Z, Stingl L, Wagner E F, Kolb H. Mice lacking the poly(ADP-ribose)polymerase gene are resistant to pancreatic beta-cell destruction and diabetes development induced by streptozotocin. Nat Med. 1999;5:314–319. doi: 10.1038/6535. [DOI] [PubMed] [Google Scholar]
- 3.Cardinal J W, Allan D J, Cameron D P. Poly(ADP-ribose)polymerase activation determines strain sensitivity to streptozotocin-induced β cell death in inbred mice. J Mol Endocrinol. 1998;22:65–70. doi: 10.1677/jme.0.0220065. [DOI] [PubMed] [Google Scholar]
- 4.Cardinal J W, Allan D J, Cameron D P. Differential metabolite accumulation may be the cause of strain differences in sensitivity to streptozotocin-induced β-cell death in inbred mice. Endocrinology. 1998;139:2885–2891. doi: 10.1210/endo.139.6.6048. [DOI] [PubMed] [Google Scholar]
- 5.Dantzer F, de la Rubia G, Ménissier-de Murcia J, Hostomsky Z, de Murcia G, Schreiber V. Base excision repair is impaired in mammalian cells lacking poly(ADP-ribose) polymerase-1. Biochemistry. 2000;39:7559–7569. doi: 10.1021/bi0003442. [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Epidemiology Research International. Preventing insulin dependent diabetes mellitus: the environmental challenge. Br Med J. 1987;295:479–481. doi: 10.1136/bmj.295.6596.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eizirik D L, Sandler S, Sener A, Malaisse W J. Defective catabolism of d-glucose and l-glutamine in mouse pancreatic islets maintained in culture after streptozotocin exposure. Endocrinology. 1988;123:1001–1007. doi: 10.1210/endo-123-2-1001. [DOI] [PubMed] [Google Scholar]
- 8.Elder R H, Jansen J G, Weeks R J, Willington M A, Deans B, Watson A J, Mynett K J, Bailey J A, Cooper D P, Rafferty J A, Heeran M C, Wijnhoven S W, van Zeeland A A, Margison G P. Alkylpurine-DNA-N-glycosylase knockout mice show increased susceptibility to induction of mutations by methyl methanesulfonate Mol. Cell Biol. 1998;18:5828–5837. doi: 10.1128/mcb.18.10.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Ghissassi F, Barbin A, Nair J, Bartsch H. Formation of 1,N6-ethenoadenine and 3,N4-ethenocytosine by lipid peroxidation products and nucleic acid bases. Chem Res Toxicol. 1995;8:278–283. doi: 10.1021/tx00044a013. [DOI] [PubMed] [Google Scholar]
- 10.Engelward B P, Allan J M, Dreslin A J, Kelly J D, Wu M M, Samson L D. A chemical and genetic approach together define the biological consequences of 3-methyladenine lesions in the mammalian genome. J Biol Chem. 1998;273:5412–5418. doi: 10.1074/jbc.273.9.5412. [DOI] [PubMed] [Google Scholar]
- 11.Felley-Bosco E. Role of nitric oxide in genotoxicity: implications for carcinogenesis. Cancer Metastasis Rev. 1998;17:25–37. doi: 10.1023/a:1005948420548. [DOI] [PubMed] [Google Scholar]
- 12.Feutren G, Papoz L, Assan R, Vialettes B, Karsenty G, Vexiau P, Du Rostu H, Rodier M, Sirmai J, Lallemand A, et al. Cyclosporin increases the rate and length of remisions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet. 1986;ii:119–124. doi: 10.1016/s0140-6736(86)91943-4. [DOI] [PubMed] [Google Scholar]
- 13.Foo S Y, Nolan G P. NF-κB to the rescue. Trends Genet. 1999;15:229–235. doi: 10.1016/s0168-9525(99)01719-9. [DOI] [PubMed] [Google Scholar]
- 14.Gepts W, Lecompte G. The pancreatic islets in diabetes. Am J Med. 1993;70:105–115. doi: 10.1016/0002-9343(81)90417-4. [DOI] [PubMed] [Google Scholar]
- 15.Gordon C, Yates A P, Davies D. Evidence for a direct action of exogenous insulin on the pancreatic islets of diabetic mice: islet response to insulin preincubation. Diabetologia. 1985;28:291–294. doi: 10.1007/BF00271688. [DOI] [PubMed] [Google Scholar]
- 16.Ha H C, Snyder S H. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci USA. 1999;96:13978–13982. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho E, Bray T M. Antioxidants, NF-κB activation, and diabetogenesis. Proc Soc Exp Biol Med. 1999;222:205–213. doi: 10.1046/j.1525-1373.1999.d01-137.x. [DOI] [PubMed] [Google Scholar]
- 18.Hoorens A, Pipeleers D. Nicotinamide protects human beta cells against chemically-induced necrosis, but not against cytokine-induced apoptosis. Diabetologia. 1999;42:55–59. doi: 10.1007/s001250051113. [DOI] [PubMed] [Google Scholar]
- 19.Jansen A, van Hagen M, Drexhage H A. Defective maturation and function of antigen presenting cells in type 1 diabetes. Lancet. 1995;345:491–492. doi: 10.1016/s0140-6736(95)90586-3. [DOI] [PubMed] [Google Scholar]
- 20.Kerr J F R, Gobe G C, Winterford C M, Harmon B V. Cell death, anatomical methods in cell death. Methods Cell Biol. 1995;46:1–26. doi: 10.1016/s0091-679x(08)61921-4. [DOI] [PubMed] [Google Scholar]
- 21.Kissane J M, Robins E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958;233:184–188. [PubMed] [Google Scholar]
- 22.Lake S P, Anderson J, Chamberlain J, Gardner S J, Bell P R F, James R F L. Bovine serum albumin density gradient isolation of rat pancreatic islets. Transplantation. 1987;43:805–808. [PubMed] [Google Scholar]
- 23.Larson K, Sahm J, Shenkar R, Strauss B. Methylation-induced blocks to in vitro DNA replication. Mutat Res. 1985;150:77–84. doi: 10.1016/0027-5107(85)90103-4. [DOI] [PubMed] [Google Scholar]
- 24.Margison G P, O'Connor P J. Biological implications of the instability of the N-glycosidic bond of 3-methyldeoxyadenosine in DNA. Biochim Biophys Acta. 1973;331:349–356. doi: 10.1016/0005-2787(73)90021-x. [DOI] [PubMed] [Google Scholar]
- 25.Masutani M, Suzuki H, Kamada N, Watanabe M, Ueda O, Nozaki T, Jishage K, Watanabe T, Sugimoto T, Nakagama H, Ochiya T, Sugimura T. Poly(ADP-ribose) polymerase gene disruption conferred mice resistant to streptozotocin-induced diabetes. Proc Natl Acad Sci USA. 1999;96:2301–2304. doi: 10.1073/pnas.96.5.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair J, Gal A, Tamir S, Tannenbaum S R, Wogan G N, Bartsch H. Etheno adducts in spleen DNA of SJL mice stimulated to overproduce nitric oxide. Carcinogenesis. 1998;19:2081–2084. doi: 10.1093/carcin/19.12.2081. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien B A, Harmon B V, Cameron D P, Allan D J. Beta-cell apoptosis is responsible for the development of IDDM in the multiple low-dose streptozotocin model. J Pathol. 1996;178:176–181. doi: 10.1002/(SICI)1096-9896(199602)178:2<176::AID-PATH433>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien B A, Harmon B V, Cameron D P, Allan D J. Apoptosis is the mode of β-cell death responsible for the development of IDDM in the nonobese diabetic (NOD) mouse. Diabetes. 1997;46:1–8. doi: 10.2337/diab.46.5.750. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien B A, Harmon B V, Cameron D P, Allan D J. Nicotinamide prevents the development of diabetes in the cyclophosphamide-induced NOD mouse model by reducing beta-cell apoptosis. J Pathol. 2000;191:86–92. doi: 10.1002/(SICI)1096-9896(200005)191:1<86::AID-PATH573>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto H. Molecular basis of experimental diabetes: degeneration, oncogenesis and regeneration of pancreatic B-cells of islets of langerhans. Bioessays. 1985;2:15–21. [Google Scholar]
- 31.Oliver F J, Ménissier-de Murcia J, Nacci C, Decker P, Andriantsitohaina R, Muller S, de Murcia G. Resistance to endotoxic shock as a consequence of defective NF-κB activation in poly (ADP-ribose) polymerase-1 deficient mice. EMBO J. 1999;18:4446–4454. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orlow S, Yasunami R, Boitard C, Bach J F. Early induction of diabetes in NOD mice by streptozotocin. C R Acad Sci Sec III. 1987;304:77–78. [PubMed] [Google Scholar]
- 33.Pieper A A, Brat D J, Krug D K, Watkins C C, Gupta A, Blackshaw S, Verma A, Wang Z-Q, Snyder S H. Poly(ADP-ribose) polymerase-deficient mice are protected from streptozotocin-induced diabetes. Proc Natl Acad Sci USA. 1999;96:3059–3064. doi: 10.1073/pnas.96.6.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poynter M E, Daynes R A. Peroxisome proliferator-activated receptor α activation modulates cellular redox status, represses nuclear factor-κB signaling and reduces inflammatory cytokine production in aging. J Biol Chem. 1998;273:32833–32841. doi: 10.1074/jbc.273.49.32833. [DOI] [PubMed] [Google Scholar]
- 35.Rabinovitch A, Suarez-Pinzon W L. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol. 1998;55:1139–1149. doi: 10.1016/s0006-2952(97)00492-9. [DOI] [PubMed] [Google Scholar]
- 36.Rakieten N, Gordon B S, Beaty A, Cooney D A, Davis R D, Schein P S. Pancreatic islet cell tumors produced by the combined action of streptozotocin and nicotinamide. Proc Soc Exp Biol Med. 1971;137:280–283. doi: 10.3181/00379727-137-35561. [DOI] [PubMed] [Google Scholar]
- 37.Saffhill R, Margison G P, O'Connor P J. Mechanisms of carcinogenesis induced by alkylating agents. Biochim Biophys Acta. 1985;823:111–145. doi: 10.1016/0304-419x(85)90009-5. [DOI] [PubMed] [Google Scholar]
- 38.Shall S, de Murcia G. Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat Res. 2000;460:1–15. doi: 10.1016/s0921-8777(00)00016-1. [DOI] [PubMed] [Google Scholar]
- 39.Trucco C, Oliver F J, de Murcia G, Ménissier-de Murcia J. DNA repair defect in poly(ADP-ribose)polymerase-deficient cell lines. Nucleic Acids Res. 1998;26:2644–2649. doi: 10.1093/nar/26.11.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamagami T, Miwa A, Takasawa S, Yamamoto H, Okamoto H. Induction of rat pancreatic B-cell tumors by the combined administration of streptozotocin or alloxan and poly(adenosine diphosphate ribose) synthetase inhibitors. Cancer Res. 1985;45:1845–1849. [PubMed] [Google Scholar]