Abstract
Regeneration plays an instrumental role in biological development and damage repair by constructing and replacing cells, tissues, and organs. Since regenerative capacity declines with age, promoting regeneration is heralded as a potential strategy for delaying aging. On this premise, mechanisms that regulate regeneration have been extensively studied across species and in different tissues. However, an open and comprehensive database collecting and standardizing the abundant data generated in regeneration research, such as high-throughput sequencing data, remains to be developed. In this work, we constructed Regeneration Roadmap to systematically and comprehensively collect such information over 2.38 million data entries across 11 species and 36 tissues, including regeneration-related genes, bulk and single-cell transcriptomics, epigenomics, and pharmacogenomics data. In this database, users can explore regulatory and expression changes of regeneration-associated genes in different species and tissues. Regeneration Roadmap provides the research community with a long-awaited and valuable data resource featuring convenient computing and visualizing tools, which is publicly available at https://ngdc.cncb.ac.cn/regeneration/index.
INTRODUCTION
Regenerative processes renew and reconstruct damaged cells, tissues, organs and even entire body parts in all living organisms. In both acute injury and continuous environmental stress settings, regeneration ensures organismal survival and homeostasis (1–5). However, with age increasing, regenerative capacity declines, and a gradual loss of function attributes to the well-documented reduced regenerative capacity of the stem/progenitor cell pool (6–9). Consequently, enhancing stem cell function is viewed as a potential mechanism for driving regeneration and delaying aging (10). More broadly, translation of the fundamental principles governing regeneration holds significant promise for treating complex human diseases, especially degenerative diseases related to aging (11,12).
The application of high-throughput omics technologies (including RNA-seq, scRNA-seq, ChIP-seq and ATAC-seq) has accumulated multidimensional data at an unprecedented scale and depth in the field of regeneration (13–16). To date, omics technologies have been leveraged to study stem cell self-renewal, fate determination and organ regeneration, enabling pioneering large-scale analyses of regeneration-related molecular programs and regulatory mechanisms across species (14,17–22). However, such regeneration-related data and information are presently scattered across project-specific data depositions, preventing a full realization of their collective impact. As regeneration multi-omics studies from a broad spectrum of regeneration models are increasingly emerging, aggregating such data into a database has become an urgent need for the field.
Current regeneration-related databases, including REGene (23), LimbformDB (24), NvERTx (25) and Myo-REG (26), are primarily knowledge databases that consolidate the published literature on regeneration-associated genes or signal pathways. Although databases with experimental data have been published, no database exists that allows users to upload regeneration multi-omics data, perform an interactive query, joint analysis and visualize such shared data. To propel the regenerative biology field and fully utilize the accumulating high-throughput sequencing data, we built Regeneration Roadmap, a comprehensive regeneration database for storing, managing, and integrating multi-omics sequencing data across multiple species.
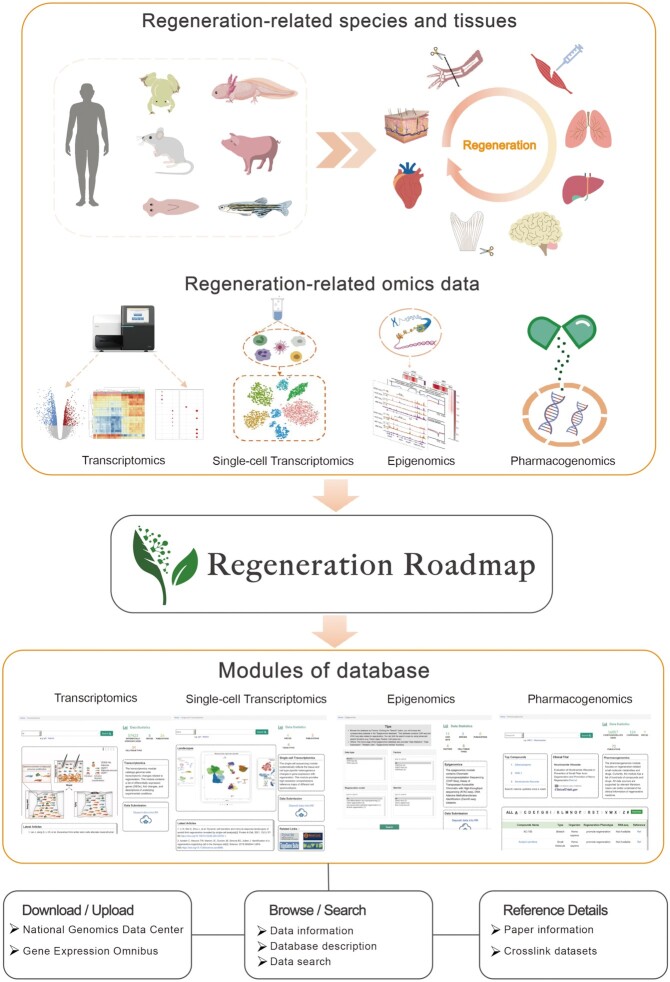
The scope of Regeneration Roadmap is to enable an understanding of regeneration processes across species and tissues. The database encompasses data sets collected from the published literature and retrieved from existing databases on wide-ranging regeneration models under different physiological or pathological conditions. In aggregate, Regeneration Roadmap now comprises a spectrum of regeneration-related omics data, including transcriptomics, single-cell transcriptomics, epigenomics, and pharmacogenomics. In addition, we provide search, visualization, and download functions for regeneration gene sets named Genes Archived in Regeneration Roadmap (GARR), which are manually collected from the literature of regeneration. Hence, Regeneration Roadmap provides users with interactive and user-friendly functionalities that enable exploration of specific gene expression changes and the construction of regulatory networks associated with regeneration.
DATABASE CONTENT
The current implementation of Regeneration Roadmap includes four modules: transcriptomics, single-cell transcriptomics, epigenomics, and pharmacogenomics (Table 1 and Figure 1). Furthermore, we have manually curated a collection of regeneration-related genes, hereafter referred to as GARR. The GARR section presently includes 553 regeneration-related genes from 7 species across 24 different tissues. The gene sets span typical regeneration processes, including planarian regeneration (27), zebrafish lateral regeneration (28), newt limb and heart regeneration (29), human pancreatic islet (30) and liver regeneration (31). Each gene in GARR has been attributed to these regeneration processes. Therefore, GARR provides a benchmark section for Regeneration Roadmap to help our users better interpret the biological implications of regeneration-related genes.
Table 1.
The URL links for each module in the Regeneration Roadmap database
| Module | Website |
|---|---|
| Homepage | https://ngdc.cncb.ac.cn/regeneration/index |
| Regeneration-related Genes | https://ngdc.cncb.ac.cn/regeneration/regeneration_related_genes |
| Transcriptomics | https://ngdc.cncb.ac.cn/regeneration/rna-seq |
| Single-cell Transcriptomics | https://ngdc.cncb.ac.cn/regeneration/single-cell |
| Epigenomics | https://ngdc.cncb.ac.cn/regeneration/epigenomics |
| Pharmacogenomics | https://ngdc.cncb.ac.cn/regeneration/regeneration-drugs |
Figure 1.
Overview of the Regeneration Roadmap database. The current implementation of Regeneration Roadmap includes four modules: transcriptomics, single-cell transcriptomics, epigenomics, and pharmacogenomics. The Genes Archived in Regeneration Roadmap (GARR), a collection of regeneration-related genes, has been manually curated.
Transcriptomics module
The transcriptomics module currently contains more than 57 400 differentially expressed genes (DEGs) totally which were identified during regeneration or regeneration intervention across eight species. Users can find the genes they are interested in through the convenient keyword search function. For example, lepb is highly induced in both regenerating hearts and fins of zebrafish as revealed by the transcriptome analysis, and it is easy to explore the expression changes of this gene in other species or tissues across our database (2). In addition, users can also click on one picture of the corresponding data set to view and download all DEGs in this article, as well as basic gene descriptions from RefSeq (32) and gene ontology obtained from AmiGO (33). Over time, we will continue to collect more high-quality regeneration research data sets as these become available, and users are encouraged to upload their data to the database as well. Thus, in this module, we have integrated a large amount of RNA sequencing data from published regeneration research articles, making it possible to cross-check the expression changes of any gene during the regeneration process.
Single-cell transcriptomics module
This module encompasses single-cell resolution gene expression changes during axolotl limb regeneration (20,34), xenopus tail regeneration (13), planarian whole-body regeneration (35), and mouse and human prostate regeneration (36). It contains more than 689 000 cells during regeneration across 6 species. For example, single-cell transcriptomics analysis of axolotl limb regeneration recently revealed gene expression changes and could be explored easily in our database (20). In this module, users can view the newly published regeneration-related single-cell data sets in the ‘Landscapes’ scroll bar. When users click on an atlas, they will go to the sub-page of the data set. From that point on, users can easily explore the gene expression patterns of specific cell types, visualize these in an interactive interface and download information about DEGs. Alternatively, users can search for genes of interest in the search column, and quickly find the relevant information at single-cell resolution by selecting species and tissues. Moreover, we also provide a single-cell data upload portal to facilitate users to upload their single-cell sequencing data to our website. Altogether, by collecting datasets from multi-organ single-cell transcriptomes, this module provides a valuable resource for the regeneration research community for assessing common and specific regeneration mechanisms in different organs across species.
Epigenomics module
This module allows users to query epigenomic information during regeneration or regeneration intervention across six tissues from 11 datasets. It currently contains largely Chromatin Immunoprecipitation Sequencing (ChIP-Seq) data that identifies how specific regeneration-related loci are regulated by histone modifications and transcription factors (37–39). It also contains Assay for Transposase-Accessible Chromatin Sequencing (ATAC-seq) data, one of the most powerful approaches for genome-wide chromatin accessibility profiling (40). These data can visualize enrichment changes of a transcription factor or chromatin modification at a specific gene locus during regeneration. For example, ChIP-seq of the histone modifications H3K27Ac, H3K27me3 and H3K4me3 recently revealed the importance of epigenomics during adult zebrafish muscle regeneration in vivo (38). Furthermore, this module will be expanded to include a broader range of epigenetic data, including DNA methylation, DamID-seq (DNA adenine methyltransferase identification) and R-loop mapping. All data included in this module originate from published high-quality datasets on regeneration research, and datasets will be updated continuously. We manually annotated the dataset in the dataset display page, including the data types, regeneration modes, species, factors, and literature information involved, and supported advanced search functions for further screening. Users can easily visualize these data with WashU Epigenome Browser on our website (41). Users can also input specific gene coordinates simultaneously to show target enrichment data at the specified genomic loci. In conclusion, this module aims to provide a systematic platform to support further research on epigenomic regulation of regenerative processes.
Pharmacogenomics module
The pharmacogenomics module allows users to query compounds related to regeneration, providing summary data of regeneration-related small molecule compounds, targets, pathways, and their roles in the regeneration process. The module currently lists 124 compounds, including dibenzazepine (42) and nicotinamide riboside (43), as well as RNA-seq datasets from drug therapy analyses, revealing gene expression changes caused by specific regeneration-promoting drugs. The data is primarily curated from the published literature and the DrugBank database (44). On the pharmacogenomics module homepage, users can browse through the ‘Top small molecules’ function, which lists compounds with high search volumes; and ‘Clinical Trials’, which presents clinical trial data for compounds related to regeneration. Importantly, users can easily search by the name of the drugs and related genes to access detailed information about specific compounds. If a corresponding RNA-seq dataset is available, a link will be provided in the gene expression tables. As the first database containing information about regeneration-related compounds, we provide a comprehensive list of identified regeneration-promoting compounds to date and include information on gene regulation and expression changes caused by the drugs.
CONCLUDING REMARKS
As a process relying on stem cell self-renewal, differentiation and tissue reconstruction in all living organisms, regeneration is central to physiological processes such as development and tissue damage repair. Since the decline of stem cell regenerative function is a hallmark of the aging process, boosting stem cell self-renewal capacity to drive regeneration can thereby potentially delay aging and/or mitigate aging phenotypes. With the application of high-throughput omics technology (including RNA-seq, scRNA-seq, ChIP-seq and ATAC-seq) in regeneration studies, researchers stand to discover unknown biological events that occur during regeneration processes and that can be targeted therapeutically. To pave the way towards these goals, we constructed Regeneration Roadmap—a multi-species and multi-omics database for regenerative biology that spans (i) currently over 2.38 million data entries across 11 species and 36 tissues, which will be updated continually to display high-quality regeneration omics data and improved functionalities, including four modules on transcriptomics, single-cell transcriptomics, epigenomics and pharmacogenomics; (ii) a collection of 533 regeneration-related genes, GARR, that characterize typical regeneration processes from seven species and 24 different tissues, where each gene has been attributed to corresponding regeneration processes and (iii) a user-friendly website through which to explore and share data and resources, and users can quickly search for information related to regeneration across species or tissues. With the combined efforts of our users and our team, the database will be updated continuously to add high-quality omics data from the ever-growing regeneration research and will stay open and freely available to the public. It also provides the functions for the interactive query, joint analysis, and visualization of such shared data. In the future, we will continue to add more bioinformatic tools. Regeneration Roadmap is bound to become an important resource for the regeneration field but also for the broader life science community.
DATA AVAILABILITY
All data in Regeneration Roadmap is available to the researchers (https://ngdc.cncb.ac.cn/regeneration/index). Users can directly download the search results in the corresponding module without registration or login (Table 1).
ACKNOWLEDGEMENTS
The authors thank Lei Bai, Qun Chu, Jing Lu, Ying Yang, Ruijun Bai, Jing Chen and Luyang Tian for administrative assistance.
Contributor Information
Wang Kang, State Key Laboratory of Membrane Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China; China National Center for Bioinformation, Beijing 100101, China; CAS Key Laboratory of Genomic and Precision Medicine, Beijing Institute of Genomics, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Tong Jin, China National Center for Bioinformation, Beijing 100101, China; National Genomics Data Center & CAS Key Laboratory of Genome Sciences and Information, Beijing Institute of Genomics, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Tao Zhang, China National Center for Bioinformation, Beijing 100101, China; National Genomics Data Center & CAS Key Laboratory of Genome Sciences and Information, Beijing Institute of Genomics, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Shuai Ma, State Key Laboratory of Membrane Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China; Institute for Stem cell and Regeneration, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China; Beijing Institute for Stem Cell and Regenerative Medicine, Beijing 100101, China.
Haoteng Yan, Advanced Innovation Center for Human Brain Protection, and National Clinical Research Center for Geriatric Disorders, Xuanwu Hospital Capital Medical University, Beijing 100053, China; Aging Translational Medicine Center, Xuanwu Hospital, Capital Medical University, Beijing 100053, China.
Zunpeng Liu, Institute for Stem cell and Regeneration, Chinese Academy of Sciences, Beijing 100101, China; State Key Laboratory of Stem Cell and Reproductive Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China; Beijing Institute for Stem Cell and Regenerative Medicine, Beijing 100101, China.
Zhejun Ji, Institute for Stem cell and Regeneration, Chinese Academy of Sciences, Beijing 100101, China; State Key Laboratory of Stem Cell and Reproductive Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China; Beijing Institute for Stem Cell and Regenerative Medicine, Beijing 100101, China.
Yusheng Cai, State Key Laboratory of Membrane Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China; Institute for Stem cell and Regeneration, Chinese Academy of Sciences, Beijing 100101, China; Beijing Institute for Stem Cell and Regenerative Medicine, Beijing 100101, China.
Si Wang, Advanced Innovation Center for Human Brain Protection, and National Clinical Research Center for Geriatric Disorders, Xuanwu Hospital Capital Medical University, Beijing 100053, China; Aging Translational Medicine Center, Xuanwu Hospital, Capital Medical University, Beijing 100053, China.
Moshi Song, State Key Laboratory of Membrane Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China; Institute for Stem cell and Regeneration, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China; Beijing Institute for Stem Cell and Regenerative Medicine, Beijing 100101, China.
Jie Ren, China National Center for Bioinformation, Beijing 100101, China; Institute for Stem cell and Regeneration, Chinese Academy of Sciences, Beijing 100101, China; CAS Key Laboratory of Genomic and Precision Medicine, Beijing Institute of Genomics, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Baoyang Hu, Institute for Stem cell and Regeneration, Chinese Academy of Sciences, Beijing 100101, China; State Key Laboratory of Stem Cell and Reproductive Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China; Beijing Institute for Stem Cell and Regenerative Medicine, Beijing 100101, China.
Qi Zhou, Institute for Stem cell and Regeneration, Chinese Academy of Sciences, Beijing 100101, China; State Key Laboratory of Stem Cell and Reproductive Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China; Beijing Institute for Stem Cell and Regenerative Medicine, Beijing 100101, China.
Weiqi Zhang, China National Center for Bioinformation, Beijing 100101, China; Institute for Stem cell and Regeneration, Chinese Academy of Sciences, Beijing 100101, China; CAS Key Laboratory of Genomic and Precision Medicine, Beijing Institute of Genomics, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Jing Qu, Institute for Stem cell and Regeneration, Chinese Academy of Sciences, Beijing 100101, China; State Key Laboratory of Stem Cell and Reproductive Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China; Beijing Institute for Stem Cell and Regenerative Medicine, Beijing 100101, China.
Yiming Bao, China National Center for Bioinformation, Beijing 100101, China; National Genomics Data Center & CAS Key Laboratory of Genome Sciences and Information, Beijing Institute of Genomics, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Guang-Hui Liu, State Key Laboratory of Membrane Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China; Institute for Stem cell and Regeneration, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China; Advanced Innovation Center for Human Brain Protection, and National Clinical Research Center for Geriatric Disorders, Xuanwu Hospital Capital Medical University, Beijing 100053, China; Aging Translational Medicine Center, Xuanwu Hospital, Capital Medical University, Beijing 100053, China; Beijing Institute for Stem Cell and Regenerative Medicine, Beijing 100101, China.
FUNDING
National Key Research and Development Program of China [2020YFA0804000]; Strategic Priority Research Program of the Chinese Academy of Sciences [XDA16010000]; National Key Research and Development Program of China [2018YFC2000100, 2020YFA0112201, 2017YFA0103304, 2017YFA0102802, 2018YFA0107203, 2020YFA0113400]; National Natural Science Foundation of China [81921006, 81625009, 91749202, 81861168034, 91949209, 92049304, 81822018, 82071588, 92049116, 32000500, 81922027, 81870228, 82125011, 82122024]; Key Research Program of the Chinese Academy of Sciences [KFZD-SW-221]; Program of Beijing Municipal Science and Technology Commission [Z191100001519005]; Program of the Beijing Natural Science Foundation [Z190019, JQ20031]; K. C. Wong Education Foundation [GJTD-2019-06, GJTD-2019-08]; Youth Innovation Promotion Association of CAS [2021078, E1CAZW0401]; State Key Laboratory of Stem Cell and Reproductive Biology; State Key Laboratory of Membrane Biology; Milky Way Research Foundation (MWRF); Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences [2020-JKCS-011]; Genomics Data Center Construction of Chinese Academy of Sciences [WX145XQ07-04]; Professional Association of the Alliance of International Science Organizations [ANSO-PA-2020-07]; Open Biodiversity and Health Big Data Programme of IUBS. Funding for open access charge: National Key Research and Development Program of China [2020YFA0804000].
Conflict of interest statement. None declared.
REFERENCES
- 1. Maruyama T., Jeong J., Sheu T.J., Hsu W.. Stem cells of the suture mesenchyme in craniofacial bone development, repair and regeneration. Nat. Commun. 2016; 7:10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kang J., Hu J., Karra R., Dickson A.L., Tornini V.A., Nachtrab G., Gemberling M., Goldman J.A., Black B.L., Poss K.D.. Modulation of tissue repair by regeneration enhancer elements. Nature. 2016; 532:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quaife-Ryan G.A., Sim C.B., Ziemann M., Kaspi A., Rafehi H., Ramialison M., El-Osta A., Hudson J.E., Porrello E.R.. Multicellular transcriptional analysis of mammalian heart regeneration. Circulation. 2017; 136:1123–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ye L., D’Agostino G., Loo S.J., Wang C.X., Su L.P., Tan S.H., Tee G.Z., Pua C.J., Pena E.M., Cheng R.B.et al.. Early regenerative capacity in the porcine heart. Circulation. 2018; 138:2798–2808. [DOI] [PubMed] [Google Scholar]
- 5. Hutson T.H., Kathe C., Palmisano I., Bartholdi K., Hervera A., De Virgiliis F., McLachlan E., Zhou L., Kong G., Barraud Q.et al.. Cbp-dependent histone acetylation mediates axon regeneration induced by environmental enrichment in rodent spinal cord injury models. Sci. Transl. Med. 2019; 11:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim J.Y., Ohn J., Yoon J.S., Kang B.M., Park M., Kim S., Lee W., Hwang S., Kim J.I., Kim K.H.et al.. Priming mobilization of hair follicle stem cells triggers permanent loss of regeneration after alkylating chemotherapy. Nat. Commun. 2019; 10:3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shook B.A., Wasko R.R., Rivera-Gonzalez G.C., Salazar-Gatzimas E., Lopez-Giraldez F., Dash B.C., Munoz-Rojas A.R., Aultman K.D., Zwick R.K., Lei V.et al.. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science. 2018; 362:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shcherbina A., Larouche J., Fraczek P., Yang B.A., Brown L.A., Markworth J.F., Chung C.H., Khaliq M., de Silva K., Choi J.J.et al.. Dissecting murine muscle stem cell aging through regeneration using integrative genomic analysis. Cell Rep. 2020; 32:107964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aging Atlas C. Aging Atlas: a multi-omics database for aging biology. Nucleic Acids Res. 2021; 49:D825–D830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yan P., Li Q., Wang L., Lu P., Suzuki K., Liu Z., Lei J., Li W., He X., Wang S.et al.. FOXO3-Engineered Human ESC-Derived vascular cells promote vascular protection and regeneration. Cell Stem Cell. 2019; 24:447–461. [DOI] [PubMed] [Google Scholar]
- 11. Ambrosi T.H., Scialdone A., Graja A., Gohlke S., Jank A.M., Bocian C., Woelk L., Fan H., Logan D.W., Schurmann A.et al.. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017; 20:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murphy M.P., Koepke L.S., Lopez M.T., Tong X., Ambrosi T.H., Gulati G.S., Marecic O., Wang Y., Ransom R.C., Hoover M.Y.et al.. Articular cartilage regeneration by activated skeletal stem cells. Nat. Med. 2020; 26:1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aztekin C., Hiscock T.W., Marioni J.C., Gurdon J.B., Simons B.D., Jullien J.. Identification of a regeneration-organizing cell in the Xenopus tail. Science. 2019; 364:653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ben-Yair R., Butty V.L., Busby M., Qiu Y., Levine S.S., Goren A., Boyer L.A., Burns C.G., Burns C.E.. H3K27me3-mediated silencing of structural genes is required for zebrafish heart regeneration. Development. 2019; 146:178632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y., Tatakis D.N.. Human gingiva transcriptome during wound healing. J. Clin. Periodontol. 2017; 44:394–402. [DOI] [PubMed] [Google Scholar]
- 16. Yan P., Liu Z., Song M., Wu Z., Xu W., Li K., Ji Q., Wang S., Liu X., Yan K.et al.. Genome-wide R-loop landscapes during cell differentiation and reprogramming. Cell Rep. 2020; 32:107870. [DOI] [PubMed] [Google Scholar]
- 17. Spina E.J., Guzman E., Zhou H., Kosik K.S., Smith W.C.. A microRNA-mRNA expression network during oral siphon regeneration in Ciona. Development. 2017; 144:1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bai H., Guo J., Chang C., Guo X., Xu C., Jin W.. Comprehensive analysis of lncRNA-miRNA-mRNA during proliferative phase of rat liver regeneration. J. Cell. Physiol. 2019; 234:18897–18905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qu M., Xiong L., Lyu Y., Zhang X., Shen J., Guan J., Chai P., Lin Z., Nie B., Li C.et al.. Establishment of intestinal organoid cultures modeling injury-associated epithelial regeneration. Cell Res. 2021; 31:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li H., Wei X., Zhou L., Zhang W., Wang C., Guo Y., Li D., Chen J., Liu T., Zhang Y.et al.. Dynamic cell transition and immune response landscapes of axolotl limb regeneration revealed by single-cell analysis. Protein Cell. 2021; 12:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Llorens-Bobadilla E., Zhao S., Baser A., Saiz-Castro G., Zwadlo K., Martin-Villalba A.. Single-Cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain injury. Cell Stem Cell. 2015; 17:329–340. [DOI] [PubMed] [Google Scholar]
- 22. Lei J., Jiang X., Li W., Ren J., Wang D., Ji Z., Wu Z., Cheng F., Cai Y., Yu Z.R.et al.. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein Cell. 2021; https://doi.org/10.1007/s13238-021-00860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao M., Rotgans B., Wang T., Cummins S.F.. REGene: a literature-based knowledgebase of animal regeneration that bridge tissue regeneration and cancer. Sci. Rep. 2016; 6:23167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lobo D., Feldman E.B., Shah M., Malone T.J., Levin M.. Limbform: a functional ontology-based database of limb regeneration experiments. Bioinformatics. 2014; 30:3598–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Warner J.F., Guerlais V., Amiel A.R., Johnston H., Nedoncelle K., Rottinger E.. NvERTx: a gene expression database to compare embryogenesis and regeneration in the sea anemone Nematostella vectensis. Development. 2018; 145:162867. [DOI] [PubMed] [Google Scholar]
- 26. Palma A., Cerquone Perpetuini A., Ferrentino F., Fuoco C., Gargioli C., Giuliani G., Iannuccelli M., Licata L., Micarelli E., Paoluzi S.et al.. Myo-REG: A portal for signaling interactions in muscle regeneration. Front. Physiol. 2019; 10:1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reddien P.W. The cellular and molecular basis for planarian regeneration. Cell. 2018; 175:327–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thomas E.D., Raible D.W.. Distinct progenitor populations mediate regeneration in the zebrafish lateral line. Elife. 2019; 8:e43736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Middleton R.C., Rogers R.G., De Couto G., Tseliou E., Luther K., Holewinski R., Soetkamp D., Van Eyk J.E., Antes T.J., Marban E.. Newt cells secrete extracellular vesicles with therapeutic bioactivity in mammalian cardiomyocytes. J. Extracell. Vesicles. 2018; 7:1456888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watanabe H., Saito H., Rychahou P.G., Uchida T., Evers B.M.. Aging is associated with decreased pancreatic acinar cell regeneration and phosphatidylinositol 3-kinase/Akt activation. Gastroenterology. 2005; 128:1391–1404. [DOI] [PubMed] [Google Scholar]
- 31. Hu J., Srivastava K., Wieland M., Runge A., Mogler C., Besemfelder E., Terhardt D., Vogel M.J., Cao L., Korn C.et al.. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science. 2014; 343:416–419. [DOI] [PubMed] [Google Scholar]
- 32. Haft D.H., DiCuccio M., Badretdin A., Brover V., Chetvernin V., O’Neill K., Li W., Chitsaz F., Derbyshire M.K., Gonzales N.R.et al.. RefSeq: an update on prokaryotic genome annotation and curation. Nucleic Acids Res. 2018; 46:D851–D860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carbon S., Ireland A., Mungall C.J., Shu S., Marshall B., Lewis S., Ami G.O.H., Web Presence Working G.. AmiGO: online access to ontology and annotation data. Bioinformatics. 2009; 25:288–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leigh N.D., Dunlap G.S., Johnson K., Mariano R., Oshiro R., Wong A.Y., Bryant D.M., Miller B.M., Ratner A., Chen A.et al.. Transcriptomic landscape of the blastema niche in regenerating adult axolotl limbs at single-cell resolution. Nat. Commun. 2018; 9:5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benham-Pyle B.W., Brewster C.E., Kent A.M., Mann F.G. Jr, Chen S., Scott A.R., Box A.C., Sanchez Alvarado A.. Identification of rare, transient post-mitotic cell states that are induced by injury and required for whole-body regeneration in Schmidtea mediterranea. Nat. Cell Biol. 2021; 23:939–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karthaus W.R., Hofree M., Choi D., Linton E.L., Turkekul M., Bejnood A., Carver B., Gopalan A., Abida W., Laudone V.et al.. Regenerative potential of prostate luminal cells revealed by single-cell analysis. Science. 2020; 368:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee H.J., Hou Y., Chen Y., Dailey Z.Z., Riddihough A., Jang H.S., Wang T., Johnson S.L.. Regenerating zebrafish fin epigenome is characterized by stable lineage-specific DNA methylation and dynamic chromatin accessibility. Genome Biol. 2020; 21:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tingle C.F., Magnuson B., Zhao Y., Heisel C.J., Kish P.E., Kahana A.. Paradoxical changes underscore epigenetic reprogramming during adult zebrafish extraocular muscle regeneration. Invest. Ophthalmol. Vis. Sci. 2019; 60:4991–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yeola A., Subramanian S., Oliver R.A., Lucas C.A., Thoms J.A.I., Yan F., Olivier J., Chacon D., Tursky M.L., Srivastava P.et al.. Induction of muscle-regenerative multipotent stem cells from human adipocytes by PDGF-AB and 5-azacytidine. Sci. Adv. 2021; 7:1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thompson J.D., Ou J., Lee N., Shin K., Cigliola V., Song L., Crawford G.E., Kang J., Poss K.D.. Identification and requirements of enhancers that direct gene expression during zebrafish fin regeneration. Development. 2020; 147:191262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li D., Hsu S., Purushotham D., Sears R.L., Wang T.. WashU Epigenome Browser update 2019. Nucleic Acids Res. 2019; 47:W158–W165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu J., Dong X., Li W., Zhao L., Zhou L., Sun S., Li H.. Dibenzazepine promotes cochlear supporting cell proliferation and hair cell regeneration in neonatal mice. Cell Prolif. 2020; 53:e12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mukherjee S., Chellappa K., Moffitt A., Ndungu J., Dellinger R.W., Davis J.G., Agarwal B., Baur J.A.. Nicotinamide adenine dinucleotide biosynthesis promotes liver regeneration. Hepatology. 2017; 65:616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wishart D.S., Feunang Y.D., Guo A.C., Lo E.J., Marcu A., Grant J.R., Sajed T., Johnson D., Li C., Sayeeda Z.et al.. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018; 46:D1074–D1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data in Regeneration Roadmap is available to the researchers (https://ngdc.cncb.ac.cn/regeneration/index). Users can directly download the search results in the corresponding module without registration or login (Table 1).