Abstract
Polo-like kinase I (PLK1), a cell cycle regulating kinase, has been shown to have oncogenic function in several cancers. Although PLK1 inhibitors, such as BI2536, BI6727 (volasertib) and NMS-1286937 (onvansertib) are generally well-tolerated with a favorable pharmacokinetic profile, clinical successes are limited due to partial responses in cancer patients, especially those in advanced stages. Recently, combination therapies targeting multiple pathways are being tested for cancer management. In this review, we first discuss structure and function of PLK1, role of PLK1 in cancers, PLK1 specific inhibitors, and advantages of using combination therapy versus monotherapy followed by a critical account on PLK1-based combination therapies in cancer treatments, especially highlighting recent advancements and challenges. PLK1 inhibitors in combination with chemotherapy drugs and targeted small molecules have shown superior effects against cancer both in vitro and in vivo. PLK1-based combination therapies have shown increased apoptosis, disrupted cell cycle, and potential to overcome resistance in cancer cells/tissues over monotherapies. Further, with successes in preclinical experiments, researchers are validating such approaches in clinical trials. Although PLK1-based combination therapies have achieved initial success in clinical studies, there are examples where they have failed to improve patient survival. Therefore, further research is needed to identify and validate novel biologically informed co-targets for PLK1-based combinatorial therapies. Employing a network-based analysis, we identified potential PLK1 co-targets that could be examined further. In addition, understanding the mechanisms of synergism between PLK1 inhibitors and other agents may lead to a better approach on which agents to pair with PLK1 inhibition for optimum cancer treatment.
Keywords: PLK1, Cancer, Combination therapies, Targeted therapy, Immunotherapy
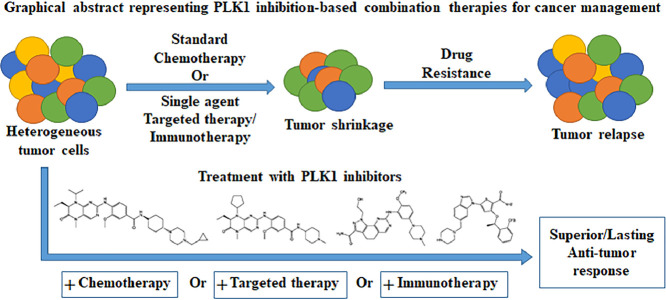
Graphical abstract
Introduction
Cancer is a major health problem globally, ranking among the top two causes of death before the age of 70 years in majority of countries [1]. In 2021, it is estimated there will be 1,898,160 people diagnosed with cancer and 608,570 cancer-related deaths in the United States alone [1]. Although several cancers can be surgically removed, this may not always be an option due to location, severity, and invasiveness of the disease. Alternatively, many cancers can be treated with non-surgical approaches such as chemotherapy, targeted therapy, immunotherapy, and radiotherapy [2]. However, even with this array of available approaches, cancer management continues to be an enormous challenge for clinicians and researchers. A majority of available therapies for cancer management are associated with drug resistance and recurrence, prompting the need for novel therapeutic options, including combination therapies that target more than one signaling pathway [3]. Studies have shown that targeted combination therapies often result in superior efficacies against cancer in both preclinical and clinical settings. An emerging druggable target for cancer therapeutic is polo-like kinase 1 (PLK1), which could be used in conjunction with other therapeutic approaches such as chemotherapy, other targeted therapies, and immunotherapy.
Mammalian polo‐like kinases (PLKs) consist of a family of five serine/threonine kinases (PLKs1-5) with distinct functions and expression patterns in mammalian cells. Like PLK1, most PLKs have C‐terminal polo‐box domains (PBDs) that recognize substrates, along with N‐terminal kinase domains that phosphorylate the substrates (Fig. 1A) [4]. Among PLKs, PLK1 is the most extensively studied family member, with critical functions in cell cycle progression, especially in G2-M checkpoint, mitosis, and cytokinesis [5]. PLK1 activity is tightly regulated during cell cycle progression via binding to other phosphorylated scaffold proteins. Recently, Li et al. have shown that the PLK1 activity may be controlled by a balanced methylation and phosphorylation switch. This study suggested that the methyltransferase G9a methylates PLK1 at Lys209 regulates PLK1 activity by antagonizing phosphorylation of T210 [6]. Further, it has been shown that PLK1 is overexpressed in several cancer types, and higher expression of PLK1 is associated with poor overall and disease-free survival [7]. Based on considerable amounts of research, PLK1 is being considered as a promising target for cancer therapy. Interestingly, in certain cancers, PLK1 has been shown to serve as a tumor suppressor (reviewed in [8]). The exact reason for this dichotomy is not known at this time, although with the importance of PLK1 in chromosome segregation it may be due to its effects on chromosomal instability, which is known to have the same dichotomous relationship with cancer promotion. This was shown by de Carcer et al., who found that PLK1 suppressed the development of Kras-induced and Her2-induced mammary gland tumors due to increased rates of chromosomal instability [9]. For clarity and brevity, in this review, we will focus only on cancer types in which PLK1 acts as a tumor promoter and which are sensitive to PLK1 inhibitors. The observed overexpression of PLK1 and its oncogenic role in several cancers has led to development of PLK1-specific inhibitors, which have been studied in preclinical and clinical settings (reviewed in [10]).
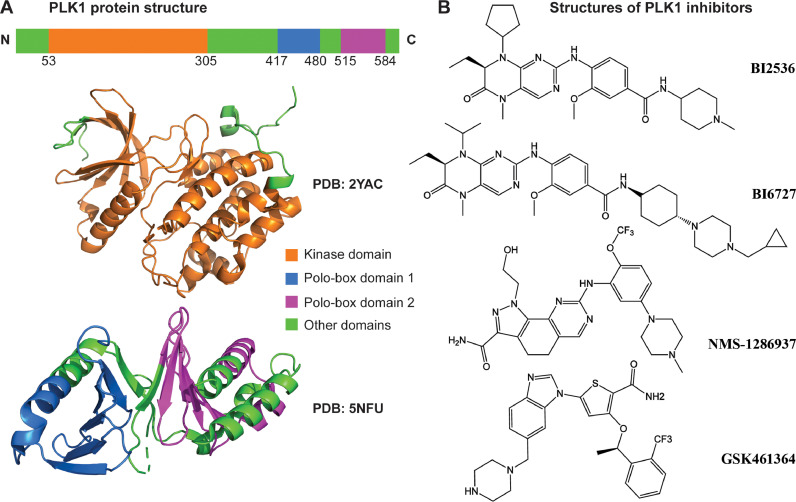
Fig. 1.
PLK1 structure and inhibitors. (A) PLK1 protein structure. The protein comprises a Kinase domain (orange) and two Polo-box domains (blue and purple). The protein 3D structures are acquired from Protein Data Bank (PDB) and visualized in PyMol. (B) PLK1 small molecule inhibitors. Four PLK1 inhibitors that have been used in clinical trials are displayed: BI2536, BI6727, NMS-1286937, and GSK461364 (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
It is interesting to note that one of the unique molecular features of PLK1 is that it offers two independent moieties for drug targeting: a kinase domain (KD) at the N-terminus and a polo-box domain (PBD) at the C-terminus. Most available PLK1 inhibitors are designed to bind to either the ATP binding pocket of the KD (ATP-competitive inhibitors) or the phosphopeptide binding site of the PBD (non-ATP-competitive inhibitors) [11]. ATP-competitive PLK1 inhibitors have certain limitations when used as a therapeutic agent, including (i) weak target selectivity due to the high similarity between PLK family members, (ii) high risk of toxicity due to the complex roles of PLK1 in various diseases, (iii) emergence of drug resistance due to frequent mutations at the ATP-binding region. It may be helpful for future research to focus on enhancing drug selectivity by targeting unique or less conserved residues of the PLK1 KD. Similarly, non-ATP-competitive inhibitors could be explored to overcome these limitations by modulating interactions between PLK1 PBD and its corresponding phosphopeptide motif to obtain superior efficacy [12].
Currently, there are more than 10 commercially available PLK1-specific inhibitors, of which at least four (BI2536, BI6727 (volasertib), GSK461364, and NMS-1286937(onvansertib)) have been evaluated in clinical trials (Fig. 1B) [10,13]. All four of these PLK1 inhibitors are ATP-competitive inhibitors and share a similar profile of action. Among these, GSK461364, BI2536, and BI6727 interact with Cys133 [14], while NMS-1286937 interacts with Glu131 in addition to Cys133 [15]. Although PLK1 inhibition-based monotherapy has shown promise in clinical trials, it was not able to achieve complete response for all patients. To address this problem, researchers have started combining PLK1 inhibitors with other agents [3]. The rationale for using combination therapy in cancer treatment is mostly based on tumor characteristics of plasticity and heterogeneity [16]. There are three main scenarios where combination therapy show advantages over monotherapy: (i) due to the genetic/epigenetic variance among patients, a drug effective on certain patients may not be effective on other patients; (ii) within tumors, only a fraction of tumor cells may be sensitive to a drug, resulting in incomplete destruction of tumor; (iii) the selection pressure from one drug may press the rising of drug resistance cells de novo [17]. To avoid these setbacks, combination therapy provides opportunities for (1) targeting more than two pathways so it may eliminate cancer cells with various genetic backgrounds, and (2) imparting superior or synergistic anticancer effects resulting in a rapid elimination before the development of drug resistance [18]. Detailed information on PLK1 expression, gene and protein structures, functional role in different diseases, as well as the design and development of PLK1 inhibitors has been discussed earlier [11]. In this review we focus on PLK1-based combination therapies for cancer management, including combinations with chemotherapies, targeted therapies, and immunotherapies.
PLK1 inhibitors combined with chemotherapy
Different from targeted drugs, chemotherapy drugs usually affect broad range of pathways rather than a specific one and frequently become ineffective due to drug resistance developed by cancer cells [19]. To overcome the resistance to chemotherapy, an alternative or additional treatment is needed, such as PLK1 inhibition combined with chemotherapy agents. Among PLK1 inhibitors, BI2536, BI6727, and NMS-1286937 are mostly used as backbone of such combination therapy (see Fig. 1B for the structure). Here, we discuss combinatorial therapies based on PLK1 inhibitors and chemotherapy agents.
BI2536 in combination with chemotherapy
BI2536, an ATP-competitive PLK1-specific inhibitor, has been explored as a cytotoxic drug in various cancers [10]. However, in clinical trials, BI2536 was shown to be associated with limited anti-tumor activity and dose-limiting side effects [20,21]. Therefore, to reduce side effects by lowering dose, BI2536 has been explored further in combination with chemotherapy drugs in several preclinical studies (Fig. 2A, Table 1). Stehle et al. demonstrated that co-treatment of eribulin, a novel microtubule-interfering drug, with BI2536 was significantly more effective than monotherapy in reducing cell viability and colony formation capability of rhabdomyosarcoma (RMS) cells. Moreover, eribulin and BI2536 combination impeded tumor growth in an in vivo model of RMS, whereas monotherapy with either agent failed to exhibit antitumor activity [22]. Another recent study tested the combination of BI2536 and nocodazole, a microtubule poison that arrests cells at M phase, in DU145 advanced prostate cancer (PCa) cells. This combination treatment synergistically induced apoptosis and inhibited viability of PCa cells, but not normal prostate epithelial cells [23].
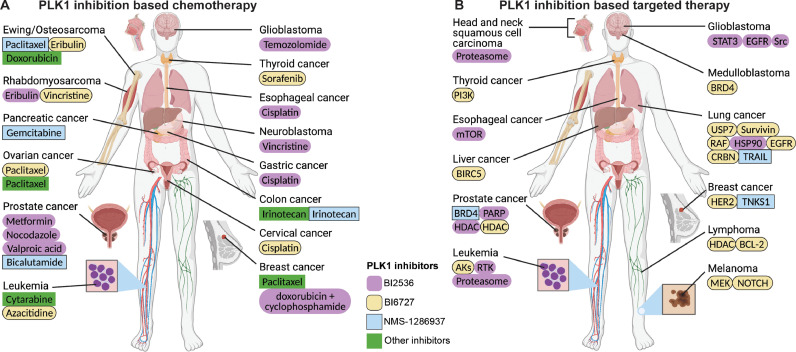
Fig. 2.
Summary of PLK1 inhibition-based chemotherapy and targeted therapy. (A) PLK1 inhibition-based chemotherapy. The cancers treated by a PLK1 inhibitor and a chemotherapy drug are displayed in the human figure. Under the cancer names are the chemotherapy drugs, highlighted by 4 different colors as PLK1 inhibitors. (B) PLK1 inhibition-based targeted therapy. The cancers are treated by targeting PLK1 and the other pathway. Under the cancer names are the targeted pathways, highlighted by PLK1 inhibitors in 4 different colors. The purple, orange, blue, and green backgrounds represent BI2536, BI6727, NMS-1286937, and other PLK1 inhibitors, respectively. (Created with Biorender.com and edited with Adobe Illustrator) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Table 1.
PLK1 inhibitors combined with chemotherapy.
| Plk1 Inhibitor | Cancer Type | Chemotherapy drug | Affected process | Affected pathways | Ref. |
|---|---|---|---|---|---|
| BI2536 | Rhabdomyosarcoma | Eribulin | Cell viability, DNA fragmentation, colony formation, cell cycle | Caspase-3/8/9 | [22] |
| BI2536 | Prostate cancer | Nocodazole | Cell viability, cell apoptosis | PARP | [23] |
| BI2536 | Triple-negative breast cancer | Doxorubicin + Cyclophosphamide | Tumor growth | NA | [24] |
| BI2536 | IDH1 mutant gliomas | Temozolomide | Colony formation, tumor growth | CHK1 | [25] |
| BI2536 | Gastric cancer cells | Cisplatin | Cell viability, cell invasion, G2/M arrest, cell apoptosis | Cdc25C, Cdc2, CCNB1 | [26] |
| BI2536 | Oesophageal squamous cell carcinoma | Cisplatin | Cell apoptosis, cell cycle, colony formation | BAX, Caspase-3, GSDME, H2AX | [27] |
| BI2536 | Neuroblastoma | Vincristine | DNA fragmentation, colony formation, cell cycle | BAX/BAK, BCL2, MCL1, Caspase-9 | [28] |
| BI2536 | Squamous cell carcinoma | Cpt11 | Cell apoptosis, tumor growth | PARP, Caspase-3 | [29] |
| BI6727 | Cervical cancer | Cisplatin | Cell proliferation, tumor growth | NA | [30] |
| BI6727 | Rhabdomyosarcoma | Vincristine | Cell viability | NA | [31] |
| BI6727 | Thyroid cancer | Sorafenib | Tumor growth | NA | [32] |
| BI6727 | Ovarian cancer | Paclitaxel | Cell apoptosis, cell cycle, colony formation | BCL2, Caspase-3, BAK | [33] |
| BI6727 | Acute myeloid leukemia | Azacitidine | Cell viability | NA | [34] |
| BI6727 | Ewing sarcoma | Eribulin | DNA fragmentation, cell viability, cell cycle | BCL2, BAX, MCL1 | [35] |
| NMS-1286937 | Triple-negative breast cancer | Paclitaxel | Cell proliferation | NA | [36] |
| NMS-1286937 | Mucinous ovarian carcinoma | Paclitaxel | Cell viability, cell cycle, tumor growth | Caspase-3, H2AX | [37] |
| NMS-1286937 | Leukemia, colon cancer | Cytarabine/irinotecan | Tumor growth | NA | [38] |
| NMS-1286937 | Osteosarcoma | Doxorubicin | Cell cycle | NA | [39] |
| TAK-960 | Colorectal cancer | Irinotecan | Tumor growth | NA | [40] |
| GSK461364 | Pancreatic cancer | Gemcitabine | Tumor growth | Ki-67 | [41] |
| GSK461364 | Osteosarcoma | Paclitaxel | Cell viability | NA | [42] |
| Genistein | Paclitaxel-resistant prostate cancer | Bicalutamide | Cell viability | NA | [43] |
Another interesting study showed that the combination of doxorubicin and cyclophosphamide with BI2536 impaired tumor relapse in HBCx-10 model, a human triple-negative breast cancer (TNBC)-derived xenograft model suggesting that BI2536 in combination with conventional chemotherapy is more efficient to achieve complete response and inhibit tumor relapse in vivo [24]. Further, BI2536 significantly improved the efficacy of DNA alkylating agent temozolomide (TMZ) in vitro as well as in a xenograft mouse model using isocitrate dehydrogenase 1 (IDH1) mutant astrocytes. This is important because up to 90% of grades II-III gliomas contain a single mutant IDH1 allele that may be associated with TMZ resistance through G2 checkpoint adaptation facilitated by PLK1 activation [25].
Lian et al. evaluated the efficacy of BI2536 in combination with cisplatin, a commonly used chemotherapy agent, in gastric cancer. The results from this study showed that BI2536 enhanced the cisplatin-induced inhibitory effects on cell viability and invasion of cisplatin-resistant gastric cancer cells (SGC-7901/DDP) by inducing G2/M arrest and decreasing the expression of p-Cdc25C as well as increasing the expression of p-Cdc2 and cyclin B1. Moreover, several other pathways associated with cellular functions including Wnt/β-catenin and MEK/ERK/RSK1 pathways were affected by BI2536 in SGC-7901/DDP cells [26]. Another study demonstrated that low doses of BI2536 when combined with cisplatin increased chemosensitivity by inducing pyroptosis in esophageal squamous cell carcinoma (ESCC) in vivo and in vitro, and these effects were associated with BAX/Caspase-3/GSDME (gasdermin family protein) pathway [27]. Czaplinski et al. showed that BI2536 synergizes with vinca alkaloids to trigger apoptosis in neuroblastoma (NB) cells. Further, BI2536 in combination with the chemotherapeutic drug vincristine suppressed long-term clonogenic survival of NB cells as well as inhibited tumor growth in vivo in the chorioallantoic membrane (CAM) model of NB in which cells were seeded on the CAM of chicken embryos and allowed to form tumors. Mechanistically, this study demonstrated the involvement of mitochondrial pathway of apoptosis BAX/BAK, BCL2 and MCL1 in NB cells [28]. Zuco et al. investigated the therapeutic potential of camptothecins (CPTs) in combination with BI2536 in cervical squamous cell carcinoma (CSCC). The combination of CPT11 and BI2536 was well-tolerated and resulted in improved anti-tumor effects against SCC xenografts when compared to single-agent treatments. These effects were associated with increased apoptosis as shown by PARP activation [29].
BI6727 in combination with chemotherapeutic drugs
BI6727 (volasertib) is a potent and selective small-molecule inhibitor of PLK1 with a half-maximal inhibitory concentration (IC50) of 0.87 nM as determined using a cell-free in vitro assay. BI6727 has been shown to have good tissue penetration, long terminal half-life, and potent efficacy in inhibiting tumor growth with encouraging safety profiles in animal models (reviewed in [10]. As a strong candidate with its apoptotic and anti-proliferative effects in conjunction with its encouraging safety profile, BI6727 has also been found to show synergistic anti-proliferative effects with a wide variety of other anticancer agents (Fig. 2A, Table 1).
Combining BI6727 and cisplatin was found to be more effective at inhibiting cervical cancer cell growth than either agent alone. Further, the combination showed synergistic tumor growth inhibition in nude mice compared to cisplatin and BI6727 treatments individually [30]. BI6727 has also been shown to be synergistically cytotoxic against pediatric cancer cell lines in vitro in combination with vincristine [31]. Another study showed that BI6727 dose-dependently inhibited thyroid cancer cell survival in four cell lines from different genetic backgrounds and when combined with chemotherapeutic sorafenib, and was more effective at inhibiting tumor growth in vitro and in vivo [32]. In ovarian cancer cells, BI6727 in combination with paclitaxel demonstrated enhanced G2/M phase cell cycle arrest and synergistically sensitized ovarian cancer cells to paclitaxel, thereby reducing paclitaxel-induced neurotoxicity [33].
When used in combination with BI6727, chemotherapeutic drug azacitidine was found to be required in lower concentrations for similar anti-proliferative effects in leukemia cell lines. The study also found BI6727 in conjunction with PI3K inhibitor LY294002 to have a synergistic relationship in primary AML cell lines, with pAKT level as a predictive marker in testing [34]. BI6727 has been found to show synergistic anti-proliferative response, when used in combination with eribulin against Ewing sarcoma cell lines [35]. The combination was found to significantly enhance DNA fragmentation and induced mitotic arrest at G2/M phase followed by cell death (via caspase-3, 8, and 9 activations) and suppressed colony formation. Furthermore, this co-treatment triggered the activation of pro-apoptotic protein BAX as well as inactivation of anti-apoptotic BCL-2 protein, further supporting their use in anticancer treatment [35]. Overall, the effect of BI6727 is mostly synergistic with a wide variety of other anticancer therapeutics, and therefore should be further evaluated as a potential co-treatment option for cancer management.
NMS-1286937 in combination with chemotherapy
NMS-1286937 (NMS-P937) is a third generation orally available PLK1 inhibitor that has also been assessed in combination with certain chemotherapy drugs (Fig. 2A, Table 1). Recently, Giordano et al. tested the combination of NMS-1286937 and paclitaxel in SUM159 xenograft model of TNBC, which is intrinsically resistant to chemotherapy. The authors found that the combination of NMS-1286937 and paclitaxel was significantly superior in exerting anti-tumor effects when compared to single-agent treatment [36]. Similarly, combination of NMS-1286937 and paclitaxel acted synergistically both in vitro and in vivo in nude mice transplanted with mucinous ovarian cancer cell line (MCAS cells), with more sustained tumor regression and tumor growth inhibition, as well as longer survival time [37]. Another study evaluated NMS-1286937 in combination with DNA-damaging agent cytarabine (CPT11) in two different xenograft models. The combination in a disseminated leukemia model was well tolerated and clearly showed increased mouse survival in HT29 human colon adenocarcinoma–bearing mice, with strikingly synergistic effects with regression of all tumors [38]. In a study, Sero et al. used human osteosarcoma cell lines to evaluate the in vitro efficacy of NMS-1286937 either as a single agent or combined with traditional drugs used as chemotherapy for osteosarcoma. The combined treatment of NMS-1286937 with doxorubicin was found to have synergistic anti-proliferative effects against doxorubicin-resistant cells. Interestingly, sequential drug exposure was more effective in overcoming the antagonistic drug effects, especially when NMS-1286937 was administered as the first agent before other chemotherapeutic agents [39].
Other PLK1 inhibitors in combination with chemotherapy
There are several other PLK1 inhibitors that have shown anti-proliferative effects against various cancer cell lines (Fig. 2A, Table 1), alone or in combination with other agents. In a study, colorectal cancer (CRC) cell lines were treated with an ATP-competitive PLK1 inhibitor, TAK-960, which induced G2/M-phase mitotic arrest and inhibited proliferation in CRC cells. Additionally, when paired with irinotecan and cetuximab, TAK-960 had additive antitumor benefits [40]. Interestingly, PLK1 inhibitor GSK461364A appears to have even greater antitumor benefits. In a recent study, when combined with gemcitabine, a standard-of-care chemotherapy drug for pancreatic adenocarcinoma, PLK1 inhibition significantly increased cellular apoptosis in cells and reduced tumor growth in an orthotopic pancreatic cancer xenograft tumor model [41]. Additionally, in osteoscarcoma cells, GSK461364A showed synergistic cytotoxic effects when combined with paclitaxel [42]. The PLK1-paclitaxel synergy was also found in paclitaxel-resistant PCa cells, which have higher levels of PLK1 and p-PLK1 compared to parental cell lines. When paclitaxel-resistant PCa cell lines were exposed to PLK1 inhibitor genistein, it down-regulated the expression of MDR1 and MRP1, which are key components of chemo-resistance and cells went through apoptotic cell death [43]. Overall, PLK1 inhibition has been found to reduce the ability of cancer cells to divide and evidence suggest that combination with chemotherapeutic drugs may lead to an effective therapeutic approach for individuals battling cancer, even potentially after developing resistance to other chemotherapies. However, further research is needed to compare different PLK1 inhibitors to determine which agent may be best for each scenario.
PLK1 inhibitors in combination with targeted therapy
Another approach to combination therapy is the use of PLK1 inhibitors with other targeted inhibitors. Generally, this comes with less side effects and allows for better tumor response, when treating two or more pathways at the same time. Here we focus on combination therapies with two most studied PLK1 inhibitors, BI2536 and BI6727, as well as discuss on other less commonly used PLK1 inhibitors that have been used with targeted therapies.
BI2536 in combination with targeted therapy
BI2536 has been administered in combination with multiple targeted inhibitors for cancer treatment (Fig. 2B, Table 2). Although the FDA has recently approved sotorasib, a RAS GTPase family inhibitor, for adult patients with KRAS G12C‑mutated metastatic non-small cell lung cancer (NSCLC) [44], treatment of KRAS mutant cancers is pharmacologically challenging because of the limited clinically available KRAS-specific inhibitors [45]. Therefore, much effort has been put forth to target alternative pathways in KRAS mutant cancers, such as dual inhibition of PLK1 and RhoA/Rho kinase (ROCK). In a recent study, BI2536 and fasudil (a ROCK inhibitor), were tested in KRAS-mutant and wild-type cancer cell lines, as well as normal human cells [46]. It was found that the combined inhibition of PLK1 and ROCK led to much-reduced cell viability in KRAS-mutant, but not wild-type cancer cells and normal cells. In the same study, a set of experiments using a transgenic lox-STOP-lox (LSL)-KRASG12D mouse model confirmed that the superior anti-growth effects in combination treatment over single drug and vehicle control groups translated to in vivo situations. A closely related signaling pathway, mammalian target of rapamycin (mTOR), was also targeted in another study to treat ESCC. In ESCC, PLK1 depletion markedly inhibited phosphorylation levels of mTOR and its downstream effector ribosomal protein S6 [47]. The combination of BI2536 and mTOR inhibitor rapamycin synergistically reduced growth and colony formation capacity of multiple ESCC cells in vitro.
Table 2.
PLK1 inhibitors combined with targeted therapy.
| PLK1 inhibitor | Cancer Type | Interacting Protein | Interacting protein inhibition | Affected process | Affected pathways | Ref |
|---|---|---|---|---|---|---|
| BI2536 | KRAS-mutant cancers | ROCK | fasudil | Apoptosis, cell cycle arrest | p21 | [46] |
| BI2536 | Esophageal squamous cell carcinoma | mTOR | Rapamycin | Colony formation | S6, 4E-BP1, AKT | [47] |
| BI2536 | Glioblastoma | STAT3 | Stattic | Apoptosis | MYC | [48] |
| BI2536 | Glioblastomas | EGFR | Gefitinib and temozolomide | Colony formation, tumor growth | NA | [49] |
| BI2536 | EGFRvIII positive glioma | Src | Saracatinib | Neurosphere formation, cell apoptosis | NOTCH1, SOX2 | [49] |
| BI2536 | Castration-resistant prostate cancer | HDAC | Metformin | Colony formation, cell metabolism, tumor growth | TP53 | [50] |
| BI2536 | Castration-Resistant Prostate Cancer | PARP | Olaparib | G2/M arrest, colony formation | Ki67, Caspase-3 | [51],[52] |
| BI2536, BI6727 | Prostate cancer | HDAC | Valproic acid, Vorinostat | Cell viability, colony formation | NA | [53] |
| BI2536 | Chronic myeloid leukemia | Bcr-Abl fusion protein | Imatinib | Cell cycle, apoptosis | NA | [54] |
| BI2536 | Bcr-Abl-positive leukemia | Proteasome | Bortezomib | Cell viability | STAT5 | [55] |
| BI2536 | Squamous cell carcinoma of the head and neck | Proteasome | Bortezomib | Cell proliferation | NA | [56] |
| BI6727 | Melanoma | MEK | JTP-74017 | Apoptosis, cell cycle arrest | TP53 | [58] |
| BI6727 | Melanoma | NOTCH | MK0752 | Apoptosis, colony formation | MAPK, PI3K, RAS, Apobec3G, BTK, FCER1G | [59] |
| BI6727 | Non-small cell lung cancer | Survivin | Sepantronium | Apoptosis, colony formation | NA | [60] |
| BI6727 | Non-small cell lung cancer | EGFR | Erlotinib | G2/M arrest and apoptosis | PARP | [61],[62] |
| BI6727 | Paclitaxel-resistant lung cancer | USP7 | P22077 | Apoptosis | MDR1/ABCB1 | [63] |
| BI6727 | NRAS-mutant non-small cell lung cancer | RAF | LXH254 | G2/M phase arrest, colony formation | NA | [65] |
| BI6727 | Non-small cell lung cancer | CRBN | CC-885 | Cell death, cell cycle | PLK1, TCTP, MYTL | [66] |
| BI6727 | Double-hit lymphoma | BCL-2 | ABT-199 | Tumorigenicity | NA | [68] |
| BI6727 | Double-hit lymphoma | HDAC | Vorinostat | Cell apoptosis | NA | [69] |
| BI6727 | Non-Hodgkin's lymphoma | HDAC | Belinostat | Cell apoptosis, cell viability, cell cycle, tumor growth | H2AX, Caspase-3, PARP | [70] |
| BI6727 | Anaplastic thyroid cancer | PI3K | BKM120 | Mitotic slippage, endoreduplication | AKT, HH3, H2AX, PARP | [71] |
| BI6727 | Medulloblastoma | BRD4 | MK-8628 | Cell viability | BRD4, CCND1 | [72] |
| BI6727 | TP53-mutated hepatocellular carcinoma | BIRC5 | YM155 | Cell viability, apoptosis, tumor growth | NA | [73] |
| SBE13 | Multiple cancer cells | PKCβ | Enzastaurin | Cell proliferation, cell cycle | PLK1, GSK3B | [74] |
| GSK461364 | Castration-resistant prostate cancer | BRD4 | JQ1 | Colony formation, tumor growth | AR/Ki67, Caspase-3 | [75] |
| GW843682X | Triple-negative breast cancer | TNKS1 | XAV939 | Cell invasion and migration, apoptosis | PARP cleavage | [76] |
| RO3280 | Non-small cell lung cancer | TRAIL receptors | rhTRAIL | Cell apoptosis, cell cycle, tumor growth | MCL-1, PARP | [77] |
Combined treatment of BI2536 and stattic (an inhibitor of signal transducer and activator of transcription 3; STAT3), was shown to have superior antiproliferative effects on glioblastoma (GBM) cells in vitro, as well as in vivo xenografts [48]. Another study evaluated PLK1-based combination therapy in GBM with EGFRvIII mutation and found BI2536 and gefitinib (an EFGRvIII inhibitor) could significantly abridge tumor growth in vivo, although drug resistance eventually emerged after a period of treatment. To overcome drug resistance, a third chemotherapy drug, temozolomide, was added to the combination of BI2536 and gefitinib, followed by a complete reduction of tumor growth with this “multi-orthogonal” approach [49]. This study also looked at GBM stem cells, a subpopulation of GBM similar to neural stem cells (NSCs) that have recently evolved as a new therapeutic target for GBM treatment. In GBM stem cells bearing EGFRvIII mutant, the combined inhibition of PLK1 with BI2536 and Src (a downstream target of EGFRvIII) with Saracatinib resulted in diminished cell self-renewal in vitro and prolonged disease-free survival of tumor-bearing mice in vivo.
BI2536-involved targeted therapy has also been studied in treating prostate cancer. For castration-resistant prostate cancer (CRPC) with wild-type but suppressed TP53, researchers have explored reactivation of TP53 pathway as a potential treatment option. A study by Chen et al. found that co-administration of SIRT1 and SIRT2 inhibitor tenovin-1 and BI2536 increased TP53 protein levels and cell death in LNCaP and C4-2 PCa cells (both wild-type TP53), but not in TP53-null PC-3 cells. Further, both BI2536 and Tennovin-1 increased the anti-tumor activity of metformin, an inhibitor of oxidative phosphorylation, in a p53 dependent manner [50]. BI2536 was also tested in the treatment of BRCA-mutant CRPC together with olaparib, an FDA-approved PARP inhibitor. CRPCs with BRCA mutants have defects in repairing DNA double-strand breaks (DSBs). The DSBs are significantly increased by PARP inhibitors olaparib, eventually leading to chromatin break and cell death [51]. PLK1 inhibition by BI2536 significantly enhances the efficacy of olaparib in 22Rν1 cells (BRAC2 mutant), showing increased PARP cleavage, apoptosis and DSBs [52]. Additionally, Wissing et al. used BI2536 combination with histone deacetylase (HDAC) inhibitors valproic acid or vorinostat in PCa and showed synergistic effect depending on type and concentration of the inhibitor as well as PCa cell line, especially for lower doses of BI2536 and HDAC rather than high doses [53].
Interestingly, BI2536 was found to synergize with imatinib and nilotinib in inhibiting the growth of imatinib-resistant chronic myelogenous leukemia (CML) cells. Synergistic effects of this combination were observed on cells with Bcr-Abl mutants, including T315I [54]. In another study, Bucur et al. demonstrated that bortezomib, a selective FDA-approved proteasome inhibitor for treatment of mantle cell lymphoma and multiple myeloma, in combination with BI2536, appears to be an effective strategy for targeting both tyrosine kinase inhibitor (TKIs) -resistant and -sensitive Bcr-Abl-positive leukemic cells. This combination induced a marked downregulation of total and phosphorylated Bcr-Abl protein levels, induced caspase activation, PARP cleavage and cell death compared with single treatments, in K562 and K562-R cells [55]. However, the exact mechanism of synergy of this combination needs to be further explored. Bortezomib has also been studied in combination with BI2536 against head and neck squamous cell carcinoma (SCCHN) cell lines and showed significantly higher anti-proliferative activity compared with Bortezomib alone [56]. These findings indicated that the anti-proliferative effect of Bortezomib was significantly enhanced by adding BI2536 and may be able to be applied to multiple cancer types.
BI6727 in combination with targeted therapy
As a structurally optimized version of BI2536, BI6727 has been tested in more cancer types than BI2536 (Fig. 2B, Table 2). NRAS mutant melanoma accounts for ∼20% of total melanoma cases, and is difficult to treat due to the lack of inhibitors specific to NRAS mutant [57]. An alternative approach is to target pathways other than NRAS. In a collaborative study, we found that combined inhibition of MEK (using JTP-74017) and PLK1 (using BI6727) showed superior antitumor effects against NRAS mutant melanoma both in vitro and in vivo [58]. Further, in a recent study, we demonstrated that the combined inhibition of PLK1 and NOTCH may be suitable for the treatment of melanoma with various genetic backgrounds, including those with BRAF or NRAS mutants. We found that the expression of PLK1 and NOTCH1 were positively correlated in melanoma patients, and combined treatment of BI6727 and NOTCH inhibitor MK0752 resulted in a synergistic antiproliferative response in A375 (BRAFmt), SK-MEL-2 (BRAFmt/TP53mt), and SK-MEL-28 (NRASmt) human melanoma cells. Using RNA-sequencing analysis, we identified the modulations of several key genes relevant to melanoma progression/metastasis, including MAPK, PI3K, and RAS, as well as some new genes such as Apobec3G, BTK, and FCER1G, which have not been well studied in melanoma [59].
Additionally, there are several PLK1-based combinatorial strategies that have been tested for lung cancer treatment. BI6727 coupled with sepantronium, an inhibitor of survivin, showed potent growth inhibition of various NSCLC cell lines [60]. Erlotinib, a small molecule to treat EGFRmt NSCLCs, suffers from drug resistance due to the development of subsequent T790M mutation in EGFR [61]. Interestingly, PLK1 was shown to be overexpressed in T790Mmt NSCLCs, and the inhibition of PLK1 by BI6727 and EGFR by Erlotinib was synergistic and caused apoptosis in vitro and delayed tumor growth in vivo using T790Mmt NSCLC cells [62]. PLK1 inhibition was also found to serve as a potential approach to overcome the resistance of NSCLCs to a chemotherapy drug paclitaxel. It was found that USP7 (ubiquitin-specific-processing protease 7) was highly expressed in paclitaxel-resistant NSCLCs, and combination treatment with USP7 inhibitor P22077 and BI6727 showed decreased cell viability in Paclitaxel-resistant NSCLCs [63]. This is an exciting way to combine targeted therapy with traditional chemotherapy agents. Similar to NRAS-mutant melanoma, although NRASmt NSCLCs account for only ∼1% of NSCLC cases, its treatment remains challenging due to lack of NRAS-specific inhibitors [64]. An alternative approach that has been researched is to simultaneously inhibit PLK1 and RAF, where the administration of RAF inhibitor LXH254 and BI6727 caused enhanced G2/M-phase arrest and reduced colony formation in NRAS-mutant NSCLCs [65]. Likewise, coupling of BI6727 with CC-885, a modulator of E3 ubiquitin ligase complex CUL4-CRBN, showed inhibitory effects in both PLK1wt and PLK1mt NSCLCs [66]. CC-885 modulates CUL4-CRBN activity allowing it to ubiquitinate PLK1 that leads to PLK1 degradation. The CC-885 facilitated PLK1 degradation synchronized with BI6727, resulting in a superior inhibition of PLK1 pathway in NSCLC cells with either mutant or wild-type PLK1, such as degradation of PLK1, reduced phosphorylation of PLK1 substrate and increased cell apoptosis.
The BI6727-based combination therapies have also been tested in the treatment of blood cancer and lymphomas. As a subtype of blood cancer, acute lymphoblastic leukemia (ALL) is derived from lymphoid progenitor cells and has two subtypes B-cell ALL (B-ALL) and T-cell ALL (T-ALL) [67]. PLK1-based inhibition was also evaluated in double-hit lymphoma (DHL), a type of B cell lymphomas, where the combined PLK1 and BCL2 inhibition showed therapeutic advances in DHL treatment. A combination treatment of BI6727 and ABT-199 (BCL2 inhibitor) was found to result in reduced DHL cell growth and clonogenic survival, as well as growth of PDX tumors [68]. Another study focusing on DHL treatment has found that BI6727 showed synergistically antiproliferative effects with JQ1 (bromodomain inhibitor) or with vorinostat (HDACs inhibitor) at lower concentrations than when independently used [69]. BI6727 also interacts synergistically with belinostat (HDACs inhibitor) in various non-Hodgkin's lymphoma cell lines and double-hit DLBCL cells, yielding a marked increase in cell death via caspase activation, DNA damage, mitotic arrest at M and G2/M phases, and mitotic errors compared to individual treatments [70]. Only when combined with BI6727, belinostat treatment showed marked downregulation in c-Myc protein and mRNA expression, suggesting cell-lethality of the agents. While mouse experiments using independent treatment in vivo showed modest results, this combination therapy yielded sharp tumor growth reductions with minimal toxicity [70].
Lastly, there have been studies on BI6727 and other agents against prostate cancer, thyroid cancer, hepatic cancer, and medulloblastoma. Additionally, the combination therapy of BI6727 and BKM120 (PI3K inhibitor) has shown antiproliferative effects against anaplastic thyroid cancer both in vitro and in vivo [71]; similar synergism was also found in the treatment of medulloblastoma using BET inhibitor MK-8628 with BI6727 [72], and the treatment of TP53-mutated hepatocellular carcinoma using BIRC5 inhibitor YM155 with BI6727 [73].
Other PLK1 inhibitor-based targeted therapy
Additional PLK1 inhibitors, such as SBE13, GSK461364, GW843682X, and RO3280, have been employed in PLK1 inhibition-based targeted therapy (Fig. 2B, Table 2). SBE13 was administered with enzastaurin, a PKCβ inhibitor, to target cancer cells with various TP53 statuses. The study found that cancers with wild-type TP53 are more resistant to the combination therapy than p53-deficient cells [74]. Another study demonstrated that the combination of PLK1 inhibitor GSK461364A and BRD4 inhibitor JQ1 showed a synergistic effect against CRPC in both cell lines and xenograft mouse model, likely due to enhanced inhibition of c-Myc and AR (androgen receptor) pathways [75]. As the most aggressive form of breast cancer, TNBC is difficult to treat because it does not respond to hormonal therapy or inhibition of HER2 protein receptors. A novel approach found that PLK1 inhibitor GW843682X in combination with tankyrase-1 (TNKS1) inhibitor XVA939 significantly reduced the invasion and migration, and enhanced apoptosis in MDA-MB231 TNBC cells [76].
In another study, Noor et al. examined the efficacy of PLK1 inhibitor RO3280 in combination with TNF-related apoptosis-inducing ligand (TRAIL) receptor agonist. TRAIL, also known as Apo-2 ligand, is a member of the TNF cytokine family and has been shown to play an important role in innate and adaptive immunity and its activity is considered as a natural path to eliminate cancer cells. This study demonstrated that the combination of rhTRAIL (a TRAIL receptor agonist), and RO3280 synergistically reduced cell viability, and increased apoptosis in NSCLC cells, in vitro. Further, this combination significantly reduced tumor growth in vivo in NSCLC xenograft model. Mechanistically, this study found that G2/M cell cycle arrest and downregulation of Mcl-1 and STAT3 activity following treatment with RO3280 may contribute to the sensitization of TRAIL-induced apoptosis in NSCLC [77]. Although these PLK1 inhibitors show promising preliminary research, additional research is needed to determine their efficacy when used with other targeted therapies in other cancers as well as in clinical studies.
PLK1 inhibitors in combination with immunotherapy
In the armamentarium of cancer therapeutics, for certain cancers, some of the most effective agents are the ones that target body's immune systems. These agents mimic or spur the body into expanding its own immune system against cancers that are present already. In a recent study, Li et al. investigated the associations of PLK1 expression with tumor immunity in several cancer types and analyzed the drug sensitivities of PLK1 inhibitors with tumor immunity in cancer cell lines [78]. This study showed that high PLK1 expression was significantly correlated with low antitumor immunity, and PLK1 inhibition promoted tumor antigen presentation in vitro. This suggests that combining PLK1 inhibitors may boost the anti-tumor response of the immunotherapy, leading to a synergistic anti-tumor response. Unfortunately, at this time more studies are needed to determine if these results can be replicated and expanded into a potent anticancer therapy regimen.
Clinical trials focusing on PLK1 inhibition-based combination therapy
As the use of PLK1 inhibitors alone in clinical trials has been reviewed by us earlier [10], here we focus on PLK1 inhibitors in combination with other agents in clinical trials (Table 3). Due to its success in vitro and in vivo, second-generation PLK1 inhibitor, BI6727, was employed in phase I/II combination clinical trial against AML. In phase I, 31 patients with relapsed or refractory AML were treated with BI6727 and low-dose cytarabine (LDAC), a chemotherapy drug for AML treatment [79]. As a result, 5 patients achieved a complete response (CR), 2 patients achieved partial response (PR), and 6 patients achieved stable disease (SD). The phase I trial was soon advanced to phase II, where 87 patients were enrolled and treated with BI6727+LDAC or LDAC only [80]. The group treated with BI6727+LDAC showed a favorable response rate of 31.0% (13 of 42 patients) as compared to LDAC monotherapy 13.3% (6 of 45 patients), as well as an improved OS vs LDAC only (median OS 8.0 vs 5.2 months). Continuing from these exciting findings, the phase III clinical trial has been launched and is expected to complete in 2021 (NCT01721876) [81]. Next, population pharmacokinetics (PK) of BI6727 and LDAC were evaluated in patients enrolled in phase II and III trials [82]. It was found that variability in total clearance of BI6727 was low-to-mild among patients, clearance of LDAC was faster in those with high body surface area, and there was no evidence showing that BI6727 and LDAC influence PK of each other. In another phase I clinical trial, BI6727 and decitabine, an AML chemotherapy drug for adult patients, were delivered to 13 AML patients, among which 2 patients achieved CR and one received PR [83]. To follow this success with PLK1 inhibitors, NMS-1286937, a third generation, oral PLK1 inhibitor, was recently used in a phase 1b AML clinical trial, where the efficacy of NMS-1286937+LDAC and NMS-1286937+decitabine was evaluated in 40 patients [13]. The result showed that NMS-1286937+decitabine combination was well tolerated and achieved 24% CR rate (5 out of 21 evaluable patients).
Table 3.
Clinical trials focusing on PLK1 inhibition based combination therapy.
| Disease | Phase | Patient's condition | Number of patients enrolled | Plk1 inhibitor | The other inhibitor | Cycle (days) | Efficacy | Ref | NCT # | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Dose | Administration | Name | Dose | Administration | ||||||||
| Acute myeloid leukemia | II | Ineligible for intensive induction therapy | 87 | BI6727 | 350 mg BI6727 on days 1 and 15) | IV | LDAC | 20 mg BID on days 1–10 | SC | 28 | PFS: 2.3 month with LDAC; 5.6 months with combination | [79,80] | NCT01721876 |
| Acute myeloid leukemia | I | Aged above 65, ineligible for intensive therapy | 13 | BI6727 | 300–400 mg on days 1 and 15 | IV | Decitabine | 20 mg/m2 on days 1–5 | IV | 28 | 2CR, 1PR | [83] | NCT02003573 |
| Acute myeloid leukemia | I | ECOG Performance Status 0–2 | 23 | NMS-1286937 | 12–60 mg/m2 on days 1–5 | Oral | Decitabine | 20 mg/m2 on days 1–5 | IV | 28 | 5CR | [13] | NCT03303339 |
| Solid tumors | I | Advanced non-resectable and/or metastatic disease, failure of conventional treatment | 57 | BI6727 | 150–300 mg on day 1 | IV | Afatinib | Schedule A: 30–50 mg on days 2–21 Schedule B: 50–90 mg on days 2–6 | Oral | 21 | 2PR, 8SD | [84] | NCT01206816 |
| Solid tumors | I | Advanced, nonresectable and/or metastatic solid tumors | 28 | BI6727 | 300 mg on day 1 | IV | Itraconazole | 200 mg on days 1–18 | Oral | 21 | 25SD | [85] | NCT01772563 |
| Solid tumors | I | Advanced metastatic solid tumors, failure of conventional treatment | 30 | BI6727 | 100–300 mg on day 8 | IV | Nintedanib | 200 mg BID days 1–7 and days 9–28 | Oral | 28 | 1 CR, 1 PR, 16 SD | [86] | NCT01022853 |
| Non-small cell lung cancer | II | Recurrent, advanced, or metastatic NSCLC, progressed after platinum-based chemotherapy regimen | 131 | BI6727 | 250–300 mg on day 1 | IV | Pemetrexed | 500 mg/m2 | IV | 21 | PFS: 5.3 months with pemetrexed; 1.4 months with BI6727; 3.3 months with combination | [87] | NCT00824408 |
In addition to AML, BI6727-based combination therapy was also evaluated in solid tumors. However, these clinical studies either stopped at phase I or failed to achieve the primary goal in phase II. To study the dose tolerance and PK of BI6727 plus afatinib (an EGFR inhibitor), a phase I clinical trial was conducted with 57 patients with advanced, unresectable, and/or metastatic solid tumors [84]. These patients either have no available effective therapy or have failed conventional therapy so were administered various doses of BI6727 and afatinib as part of the trial. Two patients achieved PR, 8 patients achieved SD, and PK profiles of both drugs were independent of each other; maximum tolerated dose (MTD) of BI6727 and afatinib was 300 and 70 mg/day, respectively. In another phase I clinical trial, the researchers studied whether the co-administration of itraconazole, a Hedgehog pathway inhibitor, could affect the PK profile of BI6727 [85]. A total of 28 patients with advanced, recurrent or metastasized solid tumors were enrolled in the study. These patients were sequentially administered with BI6727 plus itraconazole for cycle 1, and BI6727 monotherapy for cycle 2. It was shown that there was no PK interaction between BI6727 and itraconazole, and SD was observed in 25 of 28 patients although no CR or PR was observed.
In a recent phase I dose-escalation clinical study by de Braud et al., BI6727 was used in combination with nintedanib, a potent and orally bioavailable triple angiokinase inhibitor of PDGF, VEGF, and bFGF receptors in patients with advanced metastatic solid tumors who failed on conventional treatment. This study has shown that BI6727 could be combined with fixed-dose nintedanib at the recommended single-agent dose. This combination had a manageable safety profile without unexpected or overlapping adverse events, and showed antitumor activity with significant disease stabilization [86].
Another BI6727-based phase II clinical trial in solid tumors was conducted in patients with advanced NSCLCs refractory to platinum-based chemotherapy [87]. The patients were randomized to receive BI6727, pemetrexed, and combined BI6727 with pemetrexed. Unexpectedly, patients treated by pemetrexed alone received better PFS than the combined treatment of BI6727 and pemetrexed, indicating an antagonistic effect between the two drugs. Although these studies appear to show a lack of response for PLK1-based combination therapies, one catch is that the enrolled patients were mostly diagnosed with advanced tumors or tumors resistant to previous treatment. This may have limited the apparent efficacies of the combination therapies. Therefore, future clinical trials should be planned to enroll patients with various disease stages for solid tumors.
Future perspectives
In PLK1-overexpressing cancers, combining PLK1 inhibitory drugs with chemotherapies and targeted therapies has shown success in numerous in vitro and in vivo studies. The superior or synergistic anticancer effects appear to be widely observed and can be seen in various cancers with different genetic landscapes. In general, combination treatments showed superior anti-proliferative effects on cell lines tested, including increased apoptosis, reduced colony formation, and cell cycle arrest. Although immunotherapies have been becoming more prevalent in cancer therapies, there is a marked lack of studies testing the combination of PLK1 inhibitors and immunotherapies. However, the preliminary studies we found in this avenue of research show great potential and suggest that it should be explored much more in the future.
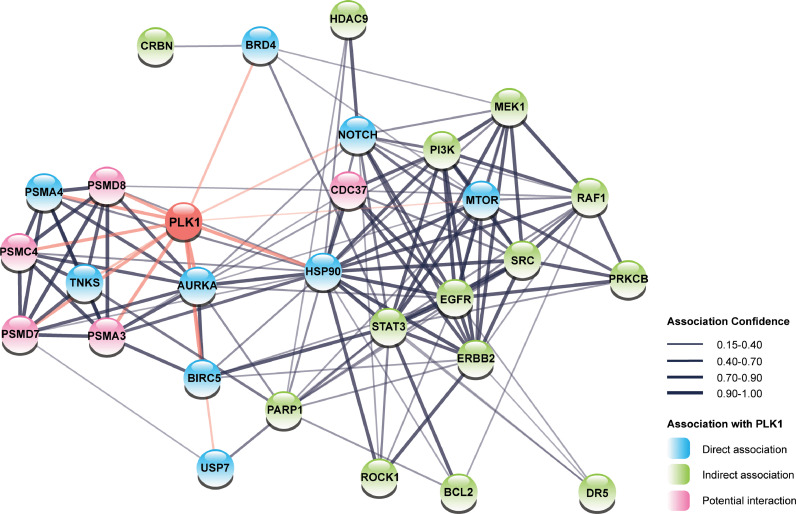
One area of research, we think will be particularly fruitful, is that of PLK1 inhibitors combined with biologically informed co-targets, with an aim to achieve synergistic responses. Employing a network analysis using the STRING database, we plotted PLK1 along with the signaling molecules identified in PLK1-based combined targeted therapies section and found that many co-targets are either directly or indirectly related to PLK1 (Fig. 3). We also identified 5 additional targets that are closely related to the network: PSMD7, PSMD8, PSMC4, PSMA3, and CDC37. These targets could be examined further as novel PLK1 inhibition-based targeted therapies. Among those novel potential targets, CDC37, known as cell division cycle 37, forms a complex with HSP90 as an important molecular chaperone that regulates the activity and stability of multiple proteins [88]. Disruption of such a complex destabilizes PLK1 and leads to its degradation, making CDC37 inhibitors promising agents for co-inhibition with PLK1 [89]. PSMD7, PSMC4, and PSMD8 are all subunits of the 19S regulatory particle of the 26S proteasome, and PSMA3 is a subunit of the 20S core particle of the 26S proteasome. Although the development of inhibitors for such subunits has been elusive, the chemotherapeutic inhibition of 26S proteasome has been found to impair cancer cell viability, as discussed in the previous section [90].
Fig. 3.
Network analysis on PLK1 and its co-targets for targeted therapy. This network shows how PLK1 interacts with its co-targets, as well as potential association of new targets with the existing targets. The thickness of the edge represents the confidence of the association between two nodes. The blue, green, and pink nodes represent a direct association, indirect association, and potential interaction with PLK1 (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
As a second-generation PLK1 inhibitor, BI6727 has largely replaced BI2536 for both preclinical and clinical usage. More recently and in the future, it is likely that NMS-1286937 will see an increasing use. The advantage of this third generation PLK1 inhibitor is that it is orally delivered, while BI6727 is an intravenously injected drug. Although BI6727-based combination therapy has been explored in phase I to phase III clinical trials, such clinical trials are more successful on leukemia rather than solid tumors, where most patients enrolled are in advanced stage or nonresponsive to previous treatments. At the same time, clinical trials using NMS-1286937-based combination therapy are remarkably limited, calling for the planning of more NMS-1286937-based clinical trials.
Conclusions
Overall, PLK1 is a promising drug target for cancer treatment, and the administration of PLK1 inhibitors with other agents have achieved success in clinical and preclinical settings. BI2536, BI6727 and NMS-1286937 are the three most frequently used inhibitors for PLK1 inhibition-based combination therapy, and these three inhibitors have been evaluated in clinical trials alone or with other agents. We believe that PLK1 inhibition-based combination therapeutic strategies with chemotherapy and targeted therapy could be superior to existing approaches for the treatment of various cancers. However, additional research is still needed to reveal the potentials of such combinatorial strategies, especially with immunotherapies.
CRediT authorship contribution statement
Shengqin Su: Conceptualization, Writing – review & editing. Gagan Chhabra: Writing – review & editing. Chandra K. Singh: Writing – review & editing. Mary A. Ndiaye: Writing – review & editing. Nihal Ahmad: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
The authors have stated that they have no conflicts of interest.
Acknowledgments
Acknowledgments
This work was partially supported by funding from the NIH (R01 AR059130 and P30 CA014520) and the Department of Veterans Affairs (VA Merit Review Awards I01 CX001441 and I01 BX004221; and a Research Career Scientist Award IK6 BX003780).
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Miller K.D., Nogueira L., Mariotto A.B., Rowland J.H., Yabroff K.R., Alfano C.M., et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019;69(5):363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 3.Bayat Mokhtari R., Homayouni T.S., Baluch N., Morgatskaya E., Kumar S., Das B., et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022–38043. doi: 10.18632/oncotarget.16723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Combes G., Alharbi I., Braga L.G., Elowe S. Playing polo during mitosis: PLK1 takes the lead. Oncogene. 2017;36(34):4819–4827. doi: 10.1038/onc.2017.113. [DOI] [PubMed] [Google Scholar]
- 5.Zitouni S., Nabais C., Jana S.C., Guerrero A., Bettencourt-Dias M. Polo-like kinases: structural variations lead to multiple functions. Nat. Rev. Mol. Cell Biol. 2014;15(7):433–452. doi: 10.1038/nrm3819. [DOI] [PubMed] [Google Scholar]
- 6.Li W., Wang H.Y., Zhao X., Duan H., Cheng B., Liu Y., et al. A methylation-phosphorylation switch determines Plk1 kinase activity and function in DNA damage repair. Sci. Adv. 2019;5(3):eaau7566. doi: 10.1126/sciadv.aau7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z., Sun Q., Wang X. PLK1, a potential target for cancer therapy. Transl. Oncol. 2017;10(1):22–32. doi: 10.1016/j.tranon.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Carcer G. The mitotic cancer target polo-like kinase 1: oncogene or tumor suppressor? Genes (Basel) 2019;10(3):208. doi: 10.3390/genes10030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Carcer G., Venkateswaran S.V., Salgueiro L., El Bakkali A., Somogyi K., Rowald K., et al. Plk1 overexpression induces chromosomal instability and suppresses tumor development. Nat. Commun. 2018;9(1):3012. doi: 10.1038/s41467-018-05429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutteridge R.E., Ndiaye M.A., Liu X., Ahmad N. Plk1 inhibitors in cancer therapy: from laboratory to clinics. Mol. Cancer Ther. 2016;15(7):1427–1435. doi: 10.1158/1535-7163.MCT-15-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shakeel I., Basheer N., Hasan G.M., Afzal M., Hassan M.I. Polo-like Kinase 1 as an emerging drug target: structure, function and therapeutic implications. J. Drug Target. 2021;29(2):168–184. doi: 10.1080/1061186X.2020.1818760. [DOI] [PubMed] [Google Scholar]
- 12.Yun T., Qin T., Liu Y., Lai L. Discovery of non-ATP-competitive inhibitors of polo-like kinase 1. ChemMedChem. 2016;11(7):713–717. doi: 10.1002/cmdc.201600051. [DOI] [PubMed] [Google Scholar]
- 13.Zeidan A.M., Ridinger M., Lin T.L., Becker P.S., Schiller G.J., Patel P.A., et al. A phase Ib study of onvansertib, a novel oral PLK1 inhibitor, in combination therapy for patients with relapsed or refractory acute myeloid leukemia. Clin. Cancer Res. 2020;26(23):6132–6140. doi: 10.1158/1078-0432.CCR-20-2586. [DOI] [PubMed] [Google Scholar]
- 14.Palmisiano N.D., Kasner M.T. Polo-like kinase and its inhibitors: ready for the match to start? Am. J. Hematol. 2015;90(11):1071–1076. doi: 10.1002/ajh.24177. [DOI] [PubMed] [Google Scholar]
- 15.Beria I., Ballinari D., Bertrand J.A., Borghi D., Bossi R.T., Brasca M.G., et al. Identification of 4,5-dihydro-1H-pyrazolo[4,3-h]quinazoline derivatives as a new class of orally and selective Polo-like kinase 1 inhibitors. J. Med. Chem. 2010;53(9):3532–3551. doi: 10.1021/jm901713n. [DOI] [PubMed] [Google Scholar]
- 16.Boshuizen J., Peeper D.S. Rational cancer treatment combinations: an urgent clinical need. Mol. Cell. 2020;78(6):1002–1018. doi: 10.1016/j.molcel.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Marusyk A., Janiszewska M., Polyak K. Intratumor heterogeneity: the rosetta stone of therapy resistance. Cancer Cell. 2020;37(4):471–484. doi: 10.1016/j.ccell.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer A.C., Sorger P.K. Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell. 2017;171(7):1678–1691. doi: 10.1016/j.cell.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; e13
- 19.Alfarouk K.O., Stock C.M., Taylor S., Walsh M., Muddathir A.K., Verduzco D., et al. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71. doi: 10.1186/s12935-015-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Awad M.M., Chu Q.S., Gandhi L., Stephenson J.J., Govindan R., Bradford D.S., et al. An open-label, phase II study of the polo-like kinase-1 (Plk-1) inhibitor, BI 2536, in patients with relapsed small cell lung cancer (SCLC) Lung Cancer. 2017;104:126–130. doi: 10.1016/j.lungcan.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Sebastian M., Reck M., Waller C.F., Kortsik C., Frickhofen N., Schuler M., et al. The efficacy and safety of BI 2536, a novel Plk-1 inhibitor, in patients with stage IIIB/IV non-small cell lung cancer who had relapsed after, or failed, chemotherapy: results from an open-label, randomized phase II clinical trial. J. Thorac. Oncol. 2010;5(7):1060–1067. doi: 10.1097/JTO.0b013e3181d95dd4. [DOI] [PubMed] [Google Scholar]
- 22.Stehle A., Hugle M., Fulda S. Eribulin synergizes with Polo-like kinase 1 inhibitors to induce apoptosis in rhabdomyosarcoma. Cancer Lett. 2015;365(1):37–46. doi: 10.1016/j.canlet.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Gheghiani L., Shang S., Fu Z. Targeting the PLK1-FOXO1 pathway as a novel therapeutic approach for treating advanced prostate cancer. Sci. Rep. 2020;10(1):12327. doi: 10.1038/s41598-020-69338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maire V., Nemati F., Richardson M., Vincent-Salomon A., Tesson B., Rigaill G., et al. Polo-like kinase 1: a potential therapeutic option in combination with conventional chemotherapy for the management of patients with triple-negative breast cancer. Cancer Res. 2013;73(2):813–823. doi: 10.1158/0008-5472.CAN-12-2633. [DOI] [PubMed] [Google Scholar]
- 25.Koncar R.F., Chu Z., Romick-Rosendale L.E., Wells S.I., Chan T.A., Qi X., et al. PLK1 inhibition enhances temozolomide efficacy in IDH1 mutant gliomas. Oncotarget. 2017;8(9):15827–15837. doi: 10.18632/oncotarget.15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lian G., Li L., Shi Y., Jing C., Liu J., Guo X., et al. BI2536, a potent and selective inhibitor of polo-like kinase 1, in combination with cisplatin exerts synergistic effects on gastric cancer cells. Int. J. Oncol. 2018;52(3):804–814. doi: 10.3892/ijo.2018.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu M., Wang Y., Yang D., Gong Y., Rao F., Liu R., et al. A PLK1 kinase inhibitor enhances the chemosensitivity of cisplatin by inducing pyroptosis in oesophageal squamous cell carcinoma. EBioMedicine. 2019;41:244–255. doi: 10.1016/j.ebiom.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czaplinski S., Hugle M., Stiehl V., Fulda S. Polo-like kinase 1 inhibition sensitizes neuroblastoma cells for vinca alkaloid-induced apoptosis. Oncotarget. 2016;7(8):8700–8711. doi: 10.18632/oncotarget.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuco V., De Cesare M., Zaffaroni N., Lanzi C., Cassinelli G. PLK1 is a critical determinant of tumor cell sensitivity to CPT11 and its inhibition enhances the drug antitumor efficacy in squamous cell carcinoma models sensitive and resistant to camptothecins. Oncotarget. 2015;6(11):8736–8749. doi: 10.18632/oncotarget.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie F.F., Pan S.S., Ou R.Y., Zheng Z.Z., Huang X.X., Jian M.T., et al. Volasertib suppresses tumor growth and potentiates the activity of cisplatin in cervical cancer. Am. J. Cancer Res. 2015;5(12):3548–3559. [PMC free article] [PubMed] [Google Scholar]
- 31.Abbou S., Lanvers-Kaminsky C., Daudigeos-Dubus E., Dret L.L.E, Laplace-Builhe C., Molenaar J., et al. Polo-like kinase inhibitor volasertib exhibits antitumor activity and synergy with vincristine in pediatric malignancies. Anticancer Res. 2016;36(2):599–609. [PubMed] [Google Scholar]
- 32.Lin S.F., Lin J.D., Yeh C.N., Huang Y.T., Chou T.C., Wong R.J. Targeting PLKs as a therapeutic approach to well-differentiated thyroid cancer. Endocr. Relat. Cancer. 2019;26(8):727–738. doi: 10.1530/ERC-18-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noack S., Raab M., Matthess Y., Sanhaji M., Kramer A., Gyorffy B., et al. Synthetic lethality in CCNE1-amplified high grade serous ovarian cancer through combined inhibition of Polo-like kinase 1 and microtubule dynamics. Oncotarget. 2018;9(40):25842–25859. doi: 10.18632/oncotarget.25386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adachi Y., Ishikawa Y., Kiyoi H. Identification of volasertib-resistant mechanism and evaluation of combination effects with volasertib and other agents on acute myeloid leukemia. Oncotarget. 2017;8(45):78452–78465. doi: 10.18632/oncotarget.19632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WeiDelta L.M., Hugle M., Fulda S. Eribulin alone or in combination with the PLK1 inhibitor BI 6727 triggers intrinsic apoptosis in Ewing sarcoma cell lines. Oncotarget. 2017;8(32):52445–52456. doi: 10.18632/oncotarget.17190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giordano A., Liu Y., Armeson K., Park Y., Ridinger M., Erlander M., et al. Polo-like kinase 1 (Plk1) inhibition synergizes with taxanes in triple negative breast cancer. PLoS One. 2019;14(11) doi: 10.1371/journal.pone.0224420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Affatato R., Carrassa L., Chila R., Lupi M., Restelli V., Damia G. Identification of PLK1 as a new therapeutic target in mucinous ovarian carcinoma. Cancers (Basel) 2020;12(3):672. doi: 10.3390/cancers12030672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valsasina B., Beria I., Alli C., Alzani R., Avanzi N., Ballinari D., et al. NMS-P937, an orally available, specific small-molecule polo-like kinase 1 inhibitor with antitumor activity in solid and hematologic malignancies. Mol. Cancer Ther. 2012;11(4):1006–1016. doi: 10.1158/1535-7163.MCT-11-0765. [DOI] [PubMed] [Google Scholar]
- 39.Sero V., Tavanti E., Vella S., Hattinger C.M., Fanelli M., Michelacci F., et al. Targeting polo-like kinase 1 by NMS-P937 in osteosarcoma cell lines inhibits tumor cell growth and partially overcomes drug resistance. Investig. New Drugs. 2014;32(6):1167–1180. doi: 10.1007/s10637-014-0158-6. [DOI] [PubMed] [Google Scholar]
- 40.Klauck P.J., Bagby S.M., Capasso A., Bradshaw-Pierce E.L., Selby H.M., Spreafico A., et al. Antitumor activity of the polo-like kinase inhibitor, TAK-960, against preclinical models of colorectal cancer. BMC Cancer. 2018;18(1):136. doi: 10.1186/s12885-018-4036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J., Wang R., Schweickert P.G., Karki A., Yang Y., Kong Y., et al. Plk1 inhibition enhances the efficacy of gemcitabine in human pancreatic cancer. Cell Cycle. 2016;15(5):711–719. doi: 10.1080/15384101.2016.1148838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chou Y.S., Yen C.C., Chen W.M., Lin Y.C., Wen Y.S., Ke W.T., et al. Cytotoxic mechanism of PLK1 inhibitor GSK461364 against osteosarcoma: mitotic arrest, apoptosis, cellular senescence, and synergistic effect with paclitaxel. Int. J. Oncol. 2016;48(3):1187–1194. doi: 10.3892/ijo.2016.3352. [DOI] [PubMed] [Google Scholar]
- 43.Shin S.B., Woo S.U., Yim H. Cotargeting Plk1 and androgen receptor enhances the therapeutic sensitivity of paclitaxel-resistant prostate cancer. Ther. Adv. Med. Oncol. 2019;11 doi: 10.1177/1758835919846375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blair H.A. Sotorasib: first Approval. Drugs. 2021;81(13):1573–1579. doi: 10.1007/s40265-021-01574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bar-Sagi D., Knelson E.H. Sequist LV. A bright future for KRAS inhibitors. Nat. Cancer. 2020;1(1):25–27. doi: 10.1038/s43018-019-0016-8. [DOI] [PubMed] [Google Scholar]
- 46.Wang J., Hu K., Guo J., Cheng F., Lv J., Jiang W., et al. Suppression of KRas-mutant cancer through the combined inhibition of KRAS with PLK1 and ROCK. Nat. Commun. 2016;7:11363. doi: 10.1038/ncomms11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu T.T., Yang K.X., Yu J., Cao Y.Y., Ren J.S., Hao J.J., et al. Co-targeting PLK1 and mTOR induces synergistic inhibitory effects against esophageal squamous cell carcinoma. J. Mol. Med. 2018;96(8):807–817. doi: 10.1007/s00109-018-1663-4. (Berl) [DOI] [PubMed] [Google Scholar]
- 48.Wang H., Tao Z., Feng M., Li X., Deng Z., Zhao G., et al. Dual PLK1 and STAT3 inhibition promotes glioblastoma cells apoptosis through MYC. Biochem. Biophys. Res. Commun. 2020;533(3):368–375. doi: 10.1016/j.bbrc.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Li X., Tao Z., Wang H., Deng Z., Zhou Y., Du Z. Dual inhibition of Src and PLK1 regulate stemness and induce apoptosis through Notch1-SOX2 signaling in EGFRvIII positive glioma stem cells (GSCs) Exp. Cell. Res. 2020;396(1) doi: 10.1016/j.yexcr.2020.112261. [DOI] [PubMed] [Google Scholar]
- 50.Chen L., Ahmad N., Liu X. Combining p53 stabilizers with metformin induces synergistic apoptosis through regulation of energy metabolism in castration-resistant prostate cancer. Cell Cycle. 2016;15(6):840–849. doi: 10.1080/15384101.2016.1151582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deeks E.D. Olaparib: first global approval. Drugs. 2015;75(2):231–240. doi: 10.1007/s40265-015-0345-6. [DOI] [PubMed] [Google Scholar]
- 52.Li J., Wang R., Kong Y., Broman M.M., Carlock C., Chen L., et al. Targeting Plk1 to enhance efficacy of olaparib in castration-resistant prostate cancer. Mol. Cancer Ther. 2017;16(3):469–479. doi: 10.1158/1535-7163.MCT-16-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wissing M.D., Mendonca J., Kortenhorst M.S., Kaelber N.S., Gonzalez M., Kim E., et al. Targeting prostate cancer cell lines with polo-like kinase 1 inhibitors as a single agent and in combination with histone deacetylase inhibitors. FASEB J. 2013;27(10):4279–4293. doi: 10.1096/fj.12-222893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gleixner K.V., Ferenc V., Peter B., Gruze A., Meyer R.A., Hadzijusufovic E., et al. Polo-like kinase 1 (Plk1) as a novel drug target in chronic myeloid leukemia: overriding imatinib resistance with the Plk1 inhibitor BI 2536. Cancer Res. 2010;70(4):1513–1523. doi: 10.1158/0008-5472.CAN-09-2181. [DOI] [PubMed] [Google Scholar]
- 55.Bucur O., Stancu A.L., Goganau I., Petrescu S.M., Pennarun B., Bertomeu T., et al. Combination of bortezomib and mitotic inhibitors down-modulate Bcr-Abl and efficiently eliminates tyrosine-kinase inhibitor sensitive and resistant Bcr-Abl-positive leukemic cells. PLoS One. 2013;8(10):e77390. doi: 10.1371/journal.pone.0077390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leinung M., Hirth D., Tahtali A., Diensthuber M., Stover T., Wagenblast J. Fighting cancer from different signalling pathways: effects of the proteasome inhibitor Bortezomib in combination with the polo-like-kinase-1-inhibitor BI2536 in SCCHN. Oncol. Lett. 2012;4(6):1305–1308. doi: 10.3892/ol.2012.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munoz-Couselo E., Adelantado E.Z., Ortiz C., Garcia J.S., Perez-Garcia J. NRAS-mutant melanoma: current challenges and future prospect. Onco Targets Ther. 2017;10:3941–3947. doi: 10.2147/OTT.S117121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Posch C., Cholewa B.D., Vujic I., Sanlorenzo M., Ma J., Kim S.T., et al. Combined inhibition of MEK and Plk1 has synergistic antitumor activity in NRAS mutant melanoma. J. Invest. Dermatol. 2015;135(10):2475–2483. doi: 10.1038/jid.2015.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Su S., Chhabra G., Ndiaye M.A., Singh C.K., Ye T., Huang W., et al. PLK1 and NOTCH positively correlate in melanoma and their combined inhibition results in synergistic modulations of key melanoma pathways. Mol. Cancer Ther. 2021;20(1):161–172. doi: 10.1158/1535-7163.MCT-20-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong M., Ren M., Silva J., Kennedy T., Choi J., Cowell J.K., et al. Sepantronium is a DNA damaging agent that synergizes with PLK1 inhibitor volasertib. Am. J. Cancer Res. 2014;4(2):135–147. [PMC free article] [PubMed] [Google Scholar]
- 61.Yun C.H., Mengwasser K.E., Toms A.V., Woo M.S., Greulich H., Wong K.K., et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl. Acad. Sci. U. S. A. 2008;105(6):2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y., Singh R., Wang L., Nilsson M., Goonatilake R., Tong P., et al. Polo-like kinase 1 inhibition diminishes acquired resistance to epidermal growth factor receptor inhibition in non-small cell lung cancer with T790M mutations. Oncotarget. 2016;7(30):47998–48010. doi: 10.18632/oncotarget.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin S.B., Kim C.H., Jang H.R., Yim H. Combination of Inhibitors of USP7 and PLK1 has a Strong Synergism against Paclitaxel Resistance. Int. J. Mol. Sci. 2020;21(22):8629. doi: 10.3390/ijms21228629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jordan E.J., Kim H.R., Arcila M.E., Barron D., Chakravarty D., Gao J., et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov. 2017;7(6):596–609. doi: 10.1158/2159-8290.CD-16-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park S., Kim T.M., Cho S.Y., Kim S., Oh Y., Kim M., et al. Combined blockade of polo-like kinase and pan-RAF is effective against NRAS-mutant non-small cell lung cancer cells. Cancer Lett. 2020;495:135–144. doi: 10.1016/j.canlet.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 66.Li L., Xue W., Shen Z., Liu J., Hu M., Cheng Z., et al. A cereblon modulator CC-885 induces CRBN- and p97-dependent plk1 degradation and synergizes with volasertib to suppress lung cancer. Mol. Ther. Oncolytics. 2020;18:215–225. doi: 10.1016/j.omto.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Terwilliger T., Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6):e577. doi: 10.1038/bcj.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ren Y., Bi C., Zhao X., Lwin T., Wang C., Yuan J., et al. PLK1 stabilizes a MYC-dependent kinase network in aggressive B cell lymphomas. J. Clin. Investig. 2018;128(12):5517–5530. doi: 10.1172/JCI122533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kikuchi H., Higuchi T., Hashida Y., Taniguchi A., Kamioka M., Taguchi T., et al. Generation and characteristics of a novel "double-hit" high grade B-cell lymphoma cell line DH-My6 with MYC/IGH and BCL6/IGH gene arrangements and potential molecular targeted therapies. Oncotarget. 2018;9(71):33482–33499. doi: 10.18632/oncotarget.26060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen T., Parker R., Hawkins E., Holkova B., Yazbeck V., Kolluri A., et al. Synergistic interactions between PLK1 and HDAC inhibitors in non-Hodgkin's lymphoma cells occur in vitro and in vivo and proceed through multiple mechanisms. Oncotarget. 2017;8(19):31478–31493. doi: 10.18632/oncotarget.15649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Martino D., Yilmaz E., Orlacchio A., Ranieri M., Zhao K., Di Cristofano A. PI3K blockage synergizes with PLK1 inhibition preventing endoreduplication and enhancing apoptosis in anaplastic thyroid cancer. Cancer Lett. 2018;439:56–65. doi: 10.1016/j.canlet.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han Y., Lindner S., Bei Y., Garcia H.D., Timme N., Althoff K., et al. Synergistic activity of BET inhibitor MK-8628 and PLK inhibitor Volasertib in preclinical models of medulloblastoma. Cancer Lett. 2019;445:24–33. doi: 10.1016/j.canlet.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 73.Li Y., Zhao Z.G., Luo Y., Cui H., Wang H.Y., Jia Y.F., et al. Dual targeting of Polo-like kinase 1 and baculoviral inhibitor of apoptosis repeat-containing 5 in TP53-mutated hepatocellular carcinoma. World J. Gastroenterol. 2020;26(32):4786–4801. doi: 10.3748/wjg.v26.i32.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lange L., Keppner-Witter S., Grigat J., Spankuch B. Combinatorial inhibition of Plk1 and PKCbeta in cancer cells with different p53 status. Oncotarget. 2014;5(8):2263–2275. doi: 10.18632/oncotarget.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mao F., Li J., Luo Q., Wang R., Kong Y., Carlock C., et al. Plk1 inhibition enhances the efficacy of BET epigenetic reader blockade in castration-resistant prostate cancer. Mol. Cancer Ther. 2018;17(7):1554–1565. doi: 10.1158/1535-7163.MCT-17-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ha G.H., Kim D.Y., Breuer E.K., Kim C.K. Combination treatment of polo-like kinase 1 and tankyrase-1 inhibitors enhances anticancer effect in triple-negative breast cancer cells. Anticancer Res. 2018;38(3):1303–1310. doi: 10.21873/anticanres.12352. [DOI] [PubMed] [Google Scholar]
- 77.Noor A., Umelo I.A., Kronenberger P., Giron P., De Vlieghere E., De Wever O., et al. Targeting polo-like kinase 1 and TRAIL enhances apoptosis in non-small cell lung cancer. Oncotarget. 2018;9(47):28731–28744. doi: 10.18632/oncotarget.25618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li M., Liu Z., Wang X. Exploration of the combination of PLK1 inhibition with immunotherapy in cancer treatment. J. Oncol. 2018;2018 doi: 10.1155/2018/3979527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bug G., Schlenk R.F., Müller-Tidow C., Lübbert M., Krämer A., Fleischer F., et al. Phase I/II study of BI 6727 (volasertib), an intravenous polo-like kinase-1 (Plk1) inhibitor, in patients with acute myeloid leukemia (AML): results of the dose finding for BI 6727 in combination with low-dose cytarabine. Blood. 2010;116(21):3316. [Google Scholar]
- 80.Dohner H., Lubbert M., Fiedler W., Fouillard L., Haaland A., Brandwein J.M., et al. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood. 2014;124(9):1426–1433. doi: 10.1182/blood-2014-03-560557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeAngelo D.J., Sekeres M.A., Ottmann O.G., Sanz M.A., Naoe T., Taube T., et al. Phase III randomized trial of volasertib combined with low-dose cytarabine (LDAC) versus placebo plus LDAC in patients aged ≥65 years with previously untreated, acute myeloid leukemia (AML) ineligible for intensive remission induction therapy. Clin. Lymphoma Myeloma Leuk. 2015;15:S194. [Google Scholar]
- 82.Solans B.P., Fleury A., Freiwald M., Fritsch H., Haug K., Troconiz I.F. Population pharmacokinetics of volasertib administered in patients with acute myeloid leukaemia as a single agent or in combination with cytarabine. Clin. Pharmacokinet. 2018;57(3):379–392. doi: 10.1007/s40262-017-0566-9. [DOI] [PubMed] [Google Scholar]
- 83.Cortes J., Podoltsev N., Kantarjian H., Borthakur G., Zeidan A.M., Stahl M., et al. Phase 1 dose escalation trial of volasertib in combination with decitabine in patients with acute myeloid leukemia. Int. J. Hematol. 2021;113(1):92–99. doi: 10.1007/s12185-020-02994-8. [DOI] [PubMed] [Google Scholar]
- 84.Machiels J.P., Peeters M., Herremans C., Surmont V., Specenier P., De Smet M., et al. A phase I study of volasertib combined with afatinib, in advanced solid tumors. Cancer Chemother. Pharmacol. 2015;76(4):843–851. doi: 10.1007/s00280-015-2860-2. [DOI] [PubMed] [Google Scholar]
- 85.Lang I., Liu D., Fritsch H., Taube T., Chizhikov E., Liptai B. Potential drug-drug interactions with combination volasertib + itraconazole: a phase I, fixed-sequence study in patients with solid tumors. Clin. Ther. 2020;42(11):2214–2224. doi: 10.1016/j.clinthera.2020.09.015. [DOI] [PubMed] [Google Scholar]
- 86.de Braud F., Cascinu S., Spitaleri G., Pilz K., Clementi L., Liu D., et al. A phase I, dose-escalation study of volasertib combined with nintedanib in advanced solid tumors. Ann. Oncol. 2015;26(11):2341–2346. doi: 10.1093/annonc/mdv354. [DOI] [PubMed] [Google Scholar]
- 87.Ellis P.M., Leighl N.B., Hirsh V., Reaume M.N., Blais N., Wierzbicki R., et al. A randomized, open-label phase II trial of volasertib as monotherapy and in combination with standard-dose pemetrexed compared with pemetrexed monotherapy in second-line treatment for non-small-cell lung cancer. Clin. Lung Cancer. 2015;16(6):457–465. doi: 10.1016/j.cllc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 88.Verba K.A., Agard D.A. How Hsp90 and Cdc37 lubricate kinase molecular switches. Trends Biochem. Sci. 2017;42(10):799–811. doi: 10.1016/j.tibs.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.D’Annessa I., Hurwitz N., Pirota V., Beretta G.L., Tinelli S., Woodford M., et al. Design of disruptors of the Hsp90-Cdc37 interface. Molecules. 2020;25(2):360. doi: 10.3390/molecules25020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsvetkov P., Adler J., Myers N., Biran A., Reuven N., Shaul Y. Oncogenic addiction to high 26S proteasome level. Cell Death. Dis. 2018;9(7):773. doi: 10.1038/s41419-018-0806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]