Abstract
Advances in our understanding of uremic retention solutes, and improvements in hemodialysis membranes and other techniques designed to remove uremic retention solutes, offer opportunities to readdress the definition and classification of uremic toxins. A consensus conference was held to develop recommendations for an updated definition and classification scheme on the basis of a holistic approach that incorporates physicochemical characteristics and dialytic removal patterns of uremic retention solutes and their linkage to clinical symptoms and outcomes. The major focus is on the removal of uremic retention solutes by hemodialysis. The identification of representative biomarkers for different classes of uremic retention solutes and their correlation to clinical symptoms and outcomes may facilitate personalized and targeted dialysis prescriptions to improve quality of life, morbidity, and mortality. Recommendations for areas of future research were also formulated, aimed at improving understanding of uremic solutes and improving outcomes in patients with CKD.
Keywords: uremia, dialysis, middle molecule, definition, classification
Background
Uremia is a broad term that has been variably used to describe the buildup of metabolic waste products, such as urea, that occurs with diminished kidney function. Along with the retention of metabolic waste products, patients with advanced kidney disease typically experience a constellation of symptoms that may include nausea, vomiting, fatigue, anorexia, muscle cramps, pruritus, mental status changes, and others, which lead to a reduced quality of life and excess morbidity and mortality. Given the retention of metabolic waste products with advanced kidney disease, there has been much interest in using dialysis techniques to remove these substances with the hope that symptoms and outcomes would also improve. However, this goal has only been partially achieved, and outcomes for patients with kidney disease remain suboptimal. Although our knowledge of solutes that build up with uremia has increased, there is a growing recognition that dialysis prescriptions (both hemodialysis and peritoneal) may not be effective in their removal. Furthermore, technological advances, such as the development of new hemodialysis membranes and the ability to perform high efficiency hemodiafiltration, enable the removal of molecules from the body up to a mass of approximately 50 kDa (1). Besides, other new technologies are being developed to remove toxins that build up with abnormal kidney function (2).
In light of these developments, an expert conference was convened to identify limitations in the definition and classification of uremic retention solutes and toxins. Experts in the field were tasked with a comprehensive review of the current definition and classification of uremic retention solutes, and posed several critical questions and recommendations to define these toxins better and map future studies for improving outcomes.
Materials and Methods
A diverse panel of clinicians and researchers representing experts in the field of uremia and uremic toxins were identified and invited by the conference chair (C.R.) to participate. In addition, a few individuals were chosen on the basis of experience in managing consensus processes. The conference was held virtually, over 3 days from November 30 to December 2, 2020, with additional small group sessions over the subsequent weeks. This consensus meeting used a modified Delphi method to achieve consensus, as previously described (3).
The consensus conference began with a preconference comprehensive literature search and appraisal of scientific evidence to identify key themes that are central to uremia and uremic toxins. Conference participants were divided into three workgroups (Supplemental Table 1) and were tasked with addressing the following themes: critical appraisal of limitations in the current definition/classification of uremic retention solutes; rationale for updating definition and classification of uremic retention solutes and molecules of interest in the field of maintenance hemodialysis; and proposal of a new classification of solutes of interest in uremia and hemodialysis. Literature searches were performed using the National Institutes of Health PubMed platform. Individual workgroups presented their output to conference participants during the three online plenary sessions for debate, discussion, and suggested revisions. In addition, recommendations for research were formulated for all key areas. The final product was then assessed and aggregated in a videoconference session attended by all attendees, who approved the consensus recommendations. A detailed description of the methodology is provided in the Supplemental Methods.
Redefining Uremic Toxins
Rationale
In 2003, the European Uremic Toxin (EUTox) Work Group proposed five criteria for an organic solute to be classified as a uremic toxin (Figure 1, left column) (4). Inorganic solutes (e.g., water, potassium, sodium, magnesium, phosphate) were excluded in these criteria given the available literature on these solutes and their divergent intradialytic removal patterns from other solutes of interest.
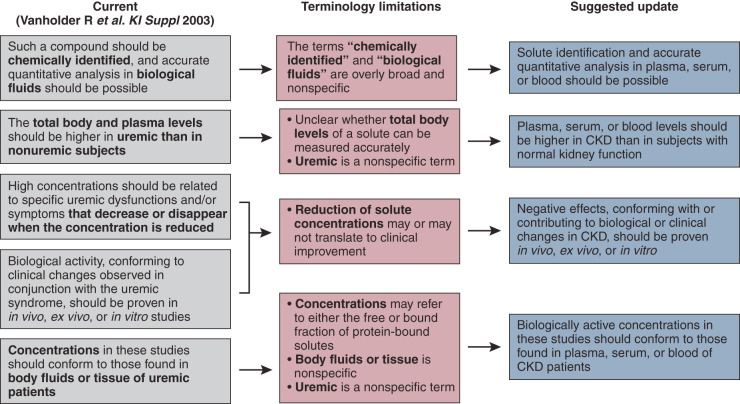
Figure 1.
Definition of uremic toxins. The left panel represents the current definition of uremic toxins, with the bold text indicating terminology that we identified as needing an update. The middle panel elaborates on the limitations associated with the identified terms. The right panel shows the newly proposed definition of uremic toxins.
The current view of uremic toxicity incorporates many solutes that are retained during kidney failure and have different physiochemical characteristics and diverse adverse effects on biologic systems (5). Moreover, there are differences in toxicity of solutes, depending on whether a solute is studied alone or in conjunction with other solutes that may interact in complex ways (6,7). Besides, protein-bound solutes exhibit a large variation in their binding affinities to various plasma proteins (8,9), and toxicity may be exerted by the free fraction or the total concentration of these solutes (10). Undisputable proof of the toxicity of a specific solute can in principle only be obtained if selective removal is linked with improved outcomes and amelioration of symptoms, but such studies have been conducted only for a few uremic toxins (10); in those patients, proof of toxicity is seldom unequivocal, likely because the effect of specific toxins may be superseded by that of other solutes with overlapping biologic effects and which may interact in various ways (11).
By 2012, EUTox listed 146 uremic retention solutes (4,8). New technologies enable expansion of the list, creating a more comprehensive picture of uremic toxicity than was initially appreciated (6,11,12). In this context, the question was raised of whether the current definition of a uremic toxin can be maintained or requires revision. We concluded that modifications are necessary to accommodate new advances in the field, especially with the development of newer hemodialysis techniques. Figure 1 summarizes the current definition, the terminological limitations of that definition, and the proposed update.
Recommendations
-
1.
We suggest the current definition of uremic toxins should be adapted in terminology to account for the growth in knowledge in the field (Figure 1).
-
2.
We suggest the scope of the definition should remain limited to organic solutes.
Physicochemical Classification of Uremic Toxins
Rationale
In 2003, EUTox categorized uremic toxins according to their physicochemical characteristics that affect clearance during hemodialysis. This classification was essentially inspired by the need to simplify and organize uremic toxicity concepts within a framework of therapeutic removal approaches, mainly by hemodialysis. These classes include small water-soluble compounds with low molecular mass (<500 Da), protein-bound solutes, and so-called middle molecules (≥500 Da) (13). Of note, the term middle molecule is a misnomer as it refers to peptides with a low molecular weight. The term was likely partially inspired by the removal pattern of hemodialysis membranes used at the time of formulation of the middle molecule hypothesis (14).
Statement 1. The Current Physicochemical Classification of Uremic Toxins Does Not Adequately Address or Reflect How Current/Modern Hemodialysis Technologies (Mechanisms of Adsorption, Convection, and Diffusion) Remove Toxins
The current physiochemical subdivision can be considered artificial because there is a continuum in the molecular weight of uremic solutes, and any cutoff on the basis of molecular weight is arbitrary (10). Besides, the degree of protein binding for these uremic solutes is variable and complicates any schema based solely on molecular weight. Nevertheless, because hemodialysis remains the most frequently applied therapeutic strategy to reduce the concentration of uremic toxins in advanced CKD, the most practical classification approach is on the basis of the principles of removal patterns by hemodialysis, noting it only applies to conventional hemodialysis, and not to peritoneal dialysis or hemodialysis time frames deviating from typical 4-hour thrice-weekly sessions (15,16). Also of note, the original classification does not account for the compartmental partitioning behavior of solutes within the body (17) or alternative strategies to decrease uremic toxin concentration (e.g., preservation of residual kidney function [18,19], adsorptive techniques [20], or strategies aimed at decreasing solute generation [21–23]). Finally, it should be acknowledged that any classification on the basis of dialysis strategies does not take into account that uremic signs and symptoms in advanced kidney disease may be present before the initiation of dialysis.
The mechanism of adsorption to hemodialysis membranes plays a role in removing uremic toxins, although membranes with truly enhanced adsorptive properties are still in the pipeline (20,24–29). Concerning the clinically available membranes, a marked reduction in the sieving coefficient for solutes with molecular mass >12 kDa demonstrates the adsorptive phenomenon of membrane caking derived from the deposition of plasma proteins (albumin-bound or soluble uremic toxins included) obstructing some pores, causing a time-dependent loss of efficiency during the hemodialysis session (30).
Newer hemodialysis membranes are likely to change the ability to remove higher molecular weight solutes that may be toxic. The ability to remove larger uremic toxins relies largely on convection. The high-flux dialyzer, when applied in the hemodialysis modality, has a molecular mass cutoff of 25 kDa (31), being boosted up to 30 kDa when in hemodiafiltration mode (32). A new class of membranes is the medium cutoff membrane, with a cutoff of 56 kDa, a mean pore radius of 5 nm, and a fiber inner diameter of 180 µm (33). As a comparison, the high-flux membrane has a mean pore radius of 3.9 nm and an inner diameter of approximately 200 µm (1,31,33,34). Clearance is more efficient for larger molecules (25−58 kDa) with medium cutoff membranes than it is for high-flux membranes. Clinical trials have consistently demonstrated increased clearance of larger molecular weight molecules, such as complement factor D, free κ light chains, TNF-α, and β2-microglobulin (35,36). We believe the classification of middle molecules should include the effect of different hemodialysis membranes on their clearance, ultimately allowing the personalization of therapies. We recognize this approach is limited in that it is focused solely on hemodialysis versus other forms of KRT, such as peritoneal dialysis and transplantation.
Recommendation
-
1.
We suggest the definition of uremic toxins should be on the basis of hemodialysis strategies, membranes, and removal patterns, acknowledging that any classification on the basis of cutoff values and/or molecular spatial configuration or charge would be arbitrary and likely will need to be changed as technological development changes solute removal patterns.
Classification on the Basis of Toxicity
Rationale
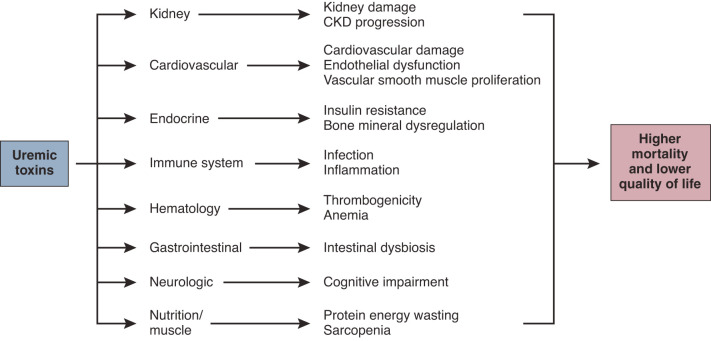
Uremic toxicity negatively affects multiple organ systems and metabolic pathways (Figure 2); cardiovascular damage (37), increased susceptibility to infection (38), and neurologic manifestations (39) are major factors affecting mortality and quality of life of patients with CKD. However, the current physicochemical classification of uremic toxins provides no insight into where benefit may come from increased clearance of a class of uremic toxins, or where problems may lie by inadequate clearance of a class of uremic toxins.
Figure 2.
Uremic toxins and related systemic disorders. The pathophysiologic effect of uremic toxins on organ systems and associated disorders linked with outcomes. Many organ systems influence each other and contribute to kidney damage and cardiovascular morbidity.
Statement 2. The Current Physicochemical Classification of Uremic Toxins Does Not Adequately Reflect the Biologic Consequences of the Toxins and Is Not Able to Identify which Toxins Possess the Most Clinical Relevance
Wolley and colleagues (40) reviewed the breadth of effect for one group of uremic toxins, a subgroup of middle molecules with molecular masses >15 kDa. The authors demonstrated how these molecules are involved in chronic inflammation, cardiovascular disease, secondary immunodeficiency, and symptom burden. Their review emphasizes that a physicochemical classification of uremic toxins does not aid clinicians in addressing a specific complication of kidney failure. For example, in a patient at high risk of cardiovascular diseases, there will be involvement of uremic toxins from small water-soluble, middle-molecule, and protein-bound groups. Likewise, for the clinicians trying to improve the outcomes for a patient with recurrent infections, they will have to target uremic toxins from all three groups (water soluble, middle molecules, and protein bound). There may therefore be a logic to looking at a reclassification of uremic toxins on the basis of clinical consequences.
In 2018, a scoring system for uremic retention solutes was developed to classify solutes according to the experimental and clinical evidence of their toxicity (10). This unique classification was on the basis of objective and reproducible criteria and considered most uremic solutes then known (Table 1) despite limitations (e.g., it is a scoring system on the basis of the number of conclusive studies). Thus, solutes that are studied most frequently have a higher likelihood of reaching a high score; the classification lacks systematic literature analysis, it provides a framework for defining target molecules for future uremic toxicity analyses and removal studies. The expert group considered other classification systems, but felt this was the most evidence-based approach available.
Table 1.
Uremic toxins with the highest toxicity evidence score
| Highest Evidence Score (4) | Second Highest Evidence Score (3) |
|---|---|
| p-cresyl sulfate | Advanced glycation end products |
| β2-microglobulin | Indoxyl sulfate |
| Asymmetric dimethyl arginine | Uric acid |
| Kynurenines | Ghrelin |
| Carbamylated compounds | Indole acetic acid |
| Fibroblast growth factor-23 | Parathyroid hormone |
| IL-6 | Phenyl acetic acid |
| TNF-α | Trimethyl methylamine-N-oxide |
| Symmetric dimethyl arginine | Retinol binding protein |
| Endothelin | |
| Immunoglobulin light chains | |
| IL-1β | |
| IL-8 | |
| Neuropeptide Y | |
| Lipids and lipoproteina |
Adapted from Vanholder et al. (10). The ranking was on the basis of the number of experimental and clinical studies showing toxicity with a downgrade if 25% of the retrieved studies showed no effect or a benefit. A score between 4 and 0 was possible, with only the toxins scoring 4 or 3 displayed in this table. Per score the toxins are ranked top to bottom according to the proven number of affected organ systems.
Post-transcriptional modifications.
Recommendations
-
1.
We suggest using the 2018 classification system (10) that reflects the degree of known toxicity on the basis of published peer-reviewed literature to define the pathophysiologic effect of each uremic retention solute. Periodic updates will be required as new evidence of the toxicity of solutes becomes available, and new solutes are identified.
-
2.
We suggest the pathophysiologic effect of each uremic toxins (e.g., inflammatory, cardiovascular) and solute origin (e.g., intestinal generation, post-translational modification) should be stated wherever available.
-
3.
We suggest focusing on a limited number of key body system effects that are the most prominent in uremia, such as cardiovascular damage, susceptibility to infection, and neurologic manifestations for pathophysiologic classification.
Classification on the Basis of Patient Outcomes
Rationale
In addition to the high morbidity and mortality associated with kidney failure, patients have a high symptom burden. Studies have demonstrated that reducing the symptom burden is as, if not more, important to many patients than an extended survival. Therefore, there has been much interest in recent years in developing robust, reproducible methods (41–44) to measure the patient experience. Additionally, there are now coordinated international research programs (45) targeting methods for improving what patients with kidney failure experience. However, the current classification of uremic toxins does not include patient experience or outcomes. The current uremic toxins classification does not help clinicians prescribe a dialysis regime for a patient with restless leg syndrome, fatigue, or prolonged recovery time after a dialysis session. Therefore, it would now be logical to look at the classification of uremic toxins in light of the symptoms and patient outcomes they cause. A classification such as this could then allow dialysis prescriptions to be specific to individual patient complaints, such as pruritus or restless leg syndrome.
Statement 3. The Current Physicochemical Classification of Uremic Toxins Does Not Adequately Address Patient Experience or Outcomes and Does Not Reflect Personal Patient Characteristics by which the Dialysis Prescription Should Be Made (e.g., Targeting the Prevention of Cardiovascular Disease, Loss of Residual Kidney Function, Deterioration of Vascular Access, or Quality of Life)
Since the original classification of uremic retention solutes, significant advances have been made to understand their clinical effect in uremia. For example, urea, once considered biologically inert, has been associated with insulin release (46), free radical production (47), apoptosis (48), and disruption of the intestinal barrier (49). Similarly, molecules such as β2-microglobulin, complement factor D, immunoglobulin free light chains, endothelin, and fibroblast growth factor-23 have been shown to have significant effects on the cardiovascular system, inflammation, and fibrosis (13,50–53). In contrast, studies demonstrating adverse effects of molecules, such as adiponectin, IL-10, leptin, resistin, or visfatin are lacking (54). The HEMO (55) and the MPO (56) studies suggested high-flux hemodialysis membranes compared with low-flux hemodialysis membranes are associated with lower risk of mortality in certain subgroups of patients with long dialysis vintage, diabetes, and serum albumin of ≤4 g/dl. Although not conclusive, these results indicate a potential advantage of increasing the spectrum of hemodialytic removal of uremic toxins to include larger molecules. It should be noted that the retention of inorganic solutes, which, per the definition, are not considered uremic toxins, and may offset or supersede any beneficial effects derived from the removal of organic solutes given their undisputed link to cardiovascular morbidity and mortality. The classification of uremic solutes does not express their clinical relevance, nor does it identify candidate molecules whose dialysis clearance and blood levels may be monitored to assure dialysis adequacy and improvement in clinical outcomes (10,13). Therefore, future classification attempts must aim to map patient profile or phenotype to a single or panel of biomarkers and suggest reduction or removal techniques that can be best utilized to decrease levels.
Recommendations
-
1.
Future studies should focus on correlating solute concentrations or the effect of interventions on solute concentrations with clinically relevant outcomes and outcomes of importance to patients.
-
2.
Ideally, dialysis prescriptions would be tailored to improve these symptoms and quality of life on the basis of removal patterns of uremic solutes linked to symptoms and outcomes.
Assessment of Toxin Measurement and Removal Capacity
Rationale
A marker of solute removal should be linked to its toxicity (and improvement of symptoms with removal) and be representative of other toxins with comparable characteristics. Given the unpredictable effect of kinetics on removing various uremic toxins in intermittent dialysis strategies such as maintenance hemodialysis (16,57,58), we suggest that (prehemodialysis) concentration after a sufficiently long equilibration is a better measure of toxin removal than clearance or pre- to postremoval ratio calculations. Depending on the efficiency of removal, multicompartmental solutes will need different equilibration times (Figure 3). An equilibration time of 4 weeks allows most solutes (except those with very low dialytic concentration reduction ratios, which are observed when the volume of distribution is large relative to the dialytic clearance) to reach equilibrium while minimizing the occurrence of confounders (e.g., loss of residual kidney function, need for antibiotics, changes in dialytic prescription, changes in dietary intake).
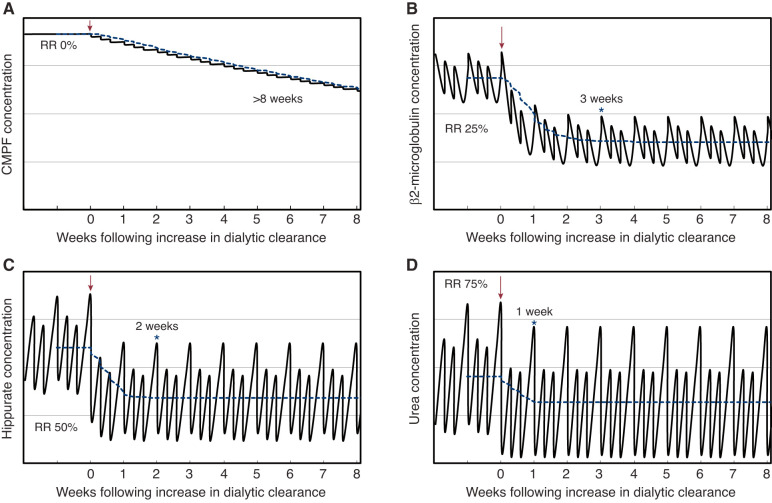
Figure 3.
The modeled effect of increasing dialytic clearance on time required to reach solute concentration equilibrium. The modeled effect demonstrates that solutes may be classified according to time to reach steady state. Each panel illustrates the time required for solute concentration to reach equilibrium after an increase in dialytic clearance with 4-hour thrice-weekly treatment. Modeling was performed for four hypothetical solutes with varying dialytic RRs (0% for CMPF [A], 25% for β2-microglobulin [B], 50% for hippurate [C], and 75% for urea [D], respectively). Dialytic clearance was increased two-fold for solutes with RR 25%, 50%, and 75%, and was increased from 0 ml/min to 1 ml/min for the solute with RR 0%. Intercompartmental clearances were assumed to be higher than the dialytic clearance such that the accessible compartment refills rapidly from nonaccessible compartments during dialysis. The RR can therefore refer to blood, plasma, or serum concentrations. Constant generation and absence of nondialytic clearance of each solute were also assumed. Solute concentrations are presented without any unit on the y axis, with the weeks after increase in dialytic clearance on the x axis. The arrow indicates the time at which dialytic clearance is increased. The asterisk (*) indicates the time at which concentrations are within 1% of equilibrium for each solute during each week of dialysis from then on. The dashed blue line represents the average solute concentration over each week. CMPF, 3-Carboxy-4-methyl-5-propyl-2-furanpropionic acid; RR, reduction ratio.
Recommendations
-
1.
For assessment of toxin removal by extracorporeal treatment, we recommend measuring the prehemodialysis concentration of a toxin after a period of equilibration (≥4 weeks).
-
2.
For comparability reasons, we suggest using the same equilibration time (4 weeks) to study any other strategy than extracorporeal removal to decrease toxin concentration (e.g., medication, dietary intervention, xenobiotics, and others).
Proposal for a New Classification System of Uremic Solutes
Rationale
It should be emphasized that decreased uremic toxin clearance due to low GFR is not the sole reason for toxin accumulation in kidney failure. For example, excessive production of cytokines and soluble receptors due to local tissue inflammation is a major contributor to middle-molecule accumulation (54). Besides, gut dysbiosis generates a broad spectrum of uremic toxins (57). Thus, a broader view of uremic solutes that goes beyond simply retention with poor GFR is needed. Recent data regarding the origin of uremic toxins, and the new development of hemodialysis methods and new membranes with the ability to clear uremic toxins with specific characteristics, or by using drugs/molecules to facilitate the shift from bound fraction to free fraction (58), lead us to propose a new classification beyond the classic physicochemical classification.
Statement 4. New Measurement Tools for Uremic Toxins Are Needed in Each Class that Go Beyond Physicochemical Classification
Because the available tests (limited to a few relevant molecules, such as phosphate, urea, serum creatinine) are not sufficient for clinical needs, new validated biomarkers are needed. For example, the accumulation of toxins in the uremic milieu nurtures an intermediate inflamed phenotype related to oxidative stress, fibrosis, senescence, mitochondrial dysfunction, and tissue hypoxia that promote premature aging (59) by vascular calcification, left ventricular hypertrophy, osteoporosis, sarcopenia, frailty, and cognitive dysfunction. Thus, to better target the intermediate inflammatory phenotype, we suggest considering the kinetics of a wide range of uremic toxins in addition to the urea kinetics. The ideal biomarker should be inexpensive, easy to measure, globally available, correlate with severity of disease, and be sensitive to early subclinical disease, recovery, and response to therapy. We believe the new classification is clinically more relevant.
Recommendation
-
1.
The new classification schema must link uremic solutes to traditional clinical outcomes and quality of life measures, including pruritus, restless legs syndrome, and recovery time after dialysis (60,61).
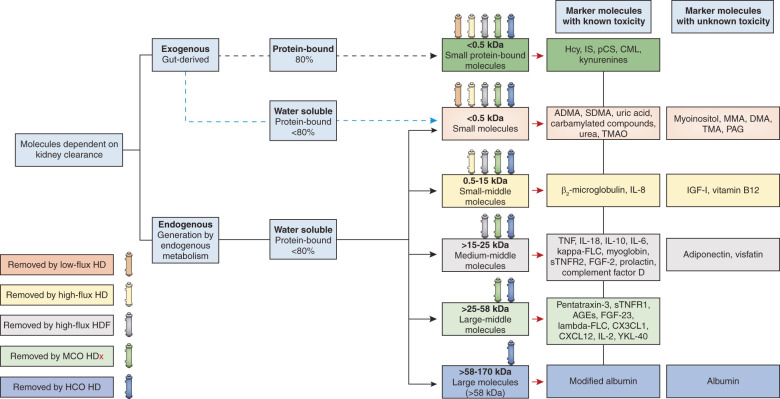
We propose a panel of biomarkers representing each uremic toxin class (Figure 4). Small (<500 Da) water-soluble molecules and urea (60 Da) correspond to the criteria mentioned above and could be included in the biomarker panel. Creatinine (113 Da) could also be considered a biomarker of small water-soluble toxins, but only if factors that are known to confound its concentration, such as age, muscle mass, Kt/V, and normalized protein catabolic rate are accounted for (62). However, it should be noted there is little evidence linking creatinine directly with uremic symptoms or outcomes. For evaluation of small-middle molecular mass (0.5−15 kDa) clearance, we recommend using parathyroid hormone (9.5 kDa) and β2-microglobulin (11.8 kDa). For estimation of medium-middle (>15−25 kDa) and large-middle (>25−58 kDa) molecular mass clearance, we recommend analyses of κ (22.5 kDa) and λ (45 kDa) free light chains, respectively. Until validation of a more widely available estimate of protein-bound solutes, clearance of protein-bound solutes is best estimated by analyses of indoxyl sulfate and paracresyl sulfate. It should be noted that residual kidney function can significantly contribute to the removal of solutes for which protein binding limits clearance by hemodialysis. Finally, it is important to recognize that the evidence base for use of some biomarkers is immature and requires additional study. Importantly, studies linking removal of these biomarkers to clinical outcomes are required.
Figure 4.
New definition and classification of uremic toxins. The third column from right to left subdivides molecules according to their protein affinity and is followed by a column that describes their molecular weight. On the top of each box of the molecular weight column, each colored dialyzer represents a dialysis modality and its expected capacity to remove the substances with molecular mass within the range represented in the box underneath. Although all dialyzer types remove small water-soluble compounds and protein-bound compounds, removal of protein-bound compounds is less pronounced. The black broken line indicates that many compounds with protein binding ≥80% are intestinally generated; the blue broken line indicates that some small water-soluble compounds may be intestinally generated. ADMA, asymmetric dimethylarginine; AGEs, advanced glycosylation end products; CML, carboxymethyl lysine; CXCL12, C-X-C motif chemokine 12; CX3CL1, chemokine (C-X3-C motif) ligand 1; DMA, dimethylamine; FGF, fibroblast growth factor; FLC, free light chain; HCO, high cutoff; Hcy, homocysteine; HD, hemodialysis; HDF, hemodiafiltration; HDx, expanded hemodialysis; IGF-1, insulin-like growth factor-1; IL, interleukin; IS, indoxyl sulfate; MCO, medium cutoff; MMA, monomethylamine; PAG, phenylacetylglutamine; pCS, para-cresyl sulfate; SMDA, symmetric dimethylarginine; sTNFR, soluble tumor necrosis factor receptor; TMA, trimethylamine; TMAO, trimethylamine-N-oxide; YKL-40, chitinase-3-like protein 1.
Recommendation
-
1.
Candidate biomarkers representing different types of uremic retention solutes should be identified and used as proxies to study various dialytic and nondialytic removal strategies.
Statement 5. Available and Newer Dialysis Technology (Including Membranes) Must Be Measured for Its Effective Removal of Uremic Toxins in Each Class
In recent years, the clearance profiles of the latest generation of hemodialyzer membranes have improved remarkably. Several characteristics should be considered for the evaluation of new membranes. These include new permeability indices, the hydrophilic or hydrophobic nature of membranes, adsorption capacity, and electrical potential (63). Furthermore, molecular weight retention onset, molecular weight cutoff, and the mass transfer area coefficient should be measured (64). Some studies support the choice of high volume postdilution hemodiafiltration over the current dialysis techniques (65,66). Beyond diffusion and convection, the removal pattern of the uremic toxins by hemodialysis methods could be enhanced by adsorption techniques (58), or by using drugs or molecules to facilitate the shift from bound fraction to free fraction (67). Consideration of uremic toxin characteristics has an effect on treatment choice. Therefore, clinicians should consider molecular radius, electrical charges, protein binding solute characteristics, high versus low molecular weight, hydrophilic versus hydrophobic, endogenous versus exogenous, secretion by kidney tubules, and different volumes of distribution (68).
Statement 6. Prototype Uremic Biomarkers Should Be Validated as New Measurement Tools of Uremic Toxicity
Identifying prototype biomarkers that could be used to optimize the management of kidney failure is essential. Current methodologies for the evaluation of the adequacy of dialysis, such as Kt/V, should not be abandoned until high-quality clinical studies support the use of novel biomarkers. These biomarkers need to be linked to improving clinical outcomes, that is, they are directly or indirectly linked to uremic toxicity processes in vivo. These biomarkers need to predict uremic manifestations, provide information about mechanisms and prognosis, improve the safety of interventions to address uremia, or be used as a surrogate marker of a uremic toxin or clinical outcome. The relationship between the accumulation of uremic toxins, intervention, and outcome should be considered. Although the role of various uremic toxins in pathophysiological processes that drive morbidity has been widely studied, the extent of the effect after intervention is less clear. Moreover, the effect between a change in biomarkers and aspects of quality of life is virtually unexplored. Novel treatments should establish whether a change in uremic toxin biomarkers affects traditional clinical outcomes and whether it improves quality of life. In addition, the cost of using novel biomarkers must be assessed and be sensitive to resource-constrained environments to ensure widespread uptake.
Biomarkers need to be sensitive to subclinical toxicities and respond to extracorporeal or enhanced endogenous toxin removal. A multidimensional understanding of disease biology using omics technologies (genomics, transcriptomics, proteomics, cytometric, and metabolomics) and “big data” methodologies is necessary for understanding the complex pathophysiology of uremia (Supplemental Figure 1). After the relationship between uremia pathophysiology and target biomarkers is understood, a biomarker discovery phase should follow, by testing candidate biomarkers in patients with CKD to identify biomarkers with the highest performance. The highest performers should be validated in a larger, diverse group of patients. In the next phase, studies need to assess the effect of biomarker-guided protocols on clinical outcomes. Finally, test platform development with rapid turnaround time, low cost, and high accuracy should be completed before implementation in clinical practice.
Research Recommendations
Given the many unknowns in the field of uremic toxins, the consensus group felt strongly that continued research was critical. A research agenda was identified and listed in Supplemental Table 2. This agenda links with the above statements and enhances the move away from the classification of uremic solutes based solely on physiochemistry and removal patterns on the basis of prior dialytic techniques and membranes. Adherence to the research agenda is likely to yield substantial increases in our knowledge base regarding the uremic syndrome and ultimately improve patients’ outcomes.
Summary
Advances in our understanding of uremic toxins and the availability of new hemodialysis membranes and techniques have led to a reappraisal of the definition and classification of uremic toxins. We recommend a more holistic classification that includes physicochemical characteristics and correlation to clinical symptoms and outcomes. Besides, the identification of representative biomarkers that correlate with removal patterns and are clinically relevant in terms of toxicity may lead to more personalized and targeted dialysis prescriptions and facilitate the search for nondialysis strategies that have the opportunity of improving the quality of life and outcomes for patients with advanced kidney disease. Validation of the novel classification will require big data methodologies, validation in external cohorts, and experimental evidence of toxicity. Of note, new data on uremic toxins and removal techniques are continuously being published and these recommendations may therefore require modifications as new results become available.
Disclosures
C. Hutchison reports consultancy agreements with, receiving research funding from, and receiving honoraria from Baxter. C. Ronco reports consultancy agreements with Asahi, Astute, Baxter, B. Braun, Biomerieux, Bioporto, Cytosorbents, General Electric (GE), Jafron, Medtronic, OCD, and Toray; receiving honoraria from Astute, Baxter, B. Braun, Estor, Fresenius, GE, Jafron, Medtronic, and Toray; and reports serving as the Editor-in-Chief of Blood Purification and Contributions to Nephrology and Cardiorenal Medicine and as an Associate Editor of Nephrology Dialysis and Transplantation. H. Kawanishi reports receiving honoraria from Kyowa-Kirin Co. Ltd.; reports serving as a scientific advisor or member of PDOPPS Steering Committee; reports serving on the Editorial Boards of Blood Purification, Peritoneal Dialysis International, and The Journal of Vascular Access; and reports serving as president of International Society of Blood Purification. K. Kashani reports consultancy agreements with AM PHARMA; reports receiving research funding from La Jolla Inc.; and reports serving as a scientific advisor or member of GE, La Jolla Inc., and MediBeacon Inc. L. Juillard reports consultancy agreements with Amgen, Astellas, Baxter, Fresenius, Hemotech, Leo, Novartis, Otsuka, Sanofi, and Vifor; reports receiving research funding from Amgen, Baxter, and Sanofi; reports receiving honoraria from Amgen, Astellas, Baxter, Fresenius, Hemotech, Leo, Novartis, Otsuka, Sanofi, and Vifor; and serving as a scientific advisor or member of Amgen, Astellas, Baxter, Fresenius, Hemotech, Leo, Novartis, and Vifor. M. Cozzolino reports receiving research funding from Abbvie, Baxter, Keryx, and Shire; reports receiving honoraria from Abbvie, Amgen, Baxter, Shire, and Vifor-Fresenius; reports serving as a scientific advisor or member of and reports speakers bureau for Abbvie, Amgen, Keryx, Shire, and Vifor. M.H. Rosner reports consultancy agreements with Baxter; reports receiving research funding from Kadmon and National Institutes of Health; reports receiving honoraria from the American Society of Nephrology and Baxter; reports serving as an Editor-at-Large of CJASN; and reports serving as a scientific advisor or member of American Society of Nephrology and on the Data Safety Monitoring Boards of clinical trials sponsored by AstraZeneca, Reata, and Travere. M. Kaushik reports receiving honoraria from and speakers bureau for Baxter Healthcare and Fresenius Medical Care; and serving as a member of ARA-EDTA, European Society of Intensive Care Medicine, International Society of Nephrology, NKF, Singapore Society of Nephrology, and Society of Transplantation Singapore. P.J. Blankestijn reports receiving consulting fees and receiving honoraria, fees paid to the institution, from Baxter and Medtronic; reports receiving research funding from Ablative Solutions, the European Commission, and Recor; and reports serving on the Editorial Board of Nephrology Dialysis Transplantation. P. Stenvinkel reports receiving consultancy fees, research grants, and speaker's honoraria from Amgen, Astellas, AstraZeneca, Baxter Healthcare, Bayer, Fresenius Medical Care, Pfizer, Reata, and Vifor. R. Vanholder reports consultancy agreements with Baxter Healthcare, BBraun, Fresenius Medical Care, Jafron, Kibow, and Nextkidney Project; has received travel support and honoraria from Baxter Healthcare and B. Braun Avitum; reports serving as an advisor to B. Braun Avitum, Baxter Healthcare, Debiotech, Fresenius Medical Care, Jafron, Kibow, and the Dutch Kidney Foundation; and reports serving as a scientific advisor or member of European Kidney Health Alliance, International Scientific Advisory Board Dutch Kidney Foundation, JASN, Nature Reviews Nephrology, and Nephrology Dialysis Transplantation. T. Reis reports employment with Clinica de Doencas Renais de Brasilia; consultancy agreements with AstraZeneca, Baxter, Contatti Medical (CytoSorbents), and Eurofarma; and reports receiving honoraria and speakers bureau from AstraZeneca, Baxter, B. Braun, Contatti Medical (CytoSorbents), Eurofarma, and Jafron. Z. Massy reports receiving research funding from Amgen, Baxter, the French government, Fresenius Medical Care, Genzyme-Sanofi, GlaxoSmithKline, Lilly, Merck Sharp and Dohme-Chibret, and Otsuka and government support for CKD REIN project and experimental projects; reports receiving honoraria on consultation fees to charities or for travel from AstraZeneca, Baxter, and Genzyme-Sanofi; and serving as a scientific advisor or member of Journal of Nephrology, Journal of Renal Nutrition, Kidney International, Nephrology Dialysis Transplantation, and Toxins. All remaining authors have nothing to disclose.
Funding
Support for the consensus conference was from Baxter Healthcare through an unrestricted educational grant to C. Ronco.
Supplementary Material
Acknowledgments
Baxter Healthcare did not participate in the meeting or have any role in the preparation of the consensus statements or manuscript. Because Dr. Mitchell H. Rosner is an Editor-at-Large of CJASN, he was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript.
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02660221/-/DCSupplemental.
Supplemental Table 1. Information regarding workgroups and work product.
Supplemental Table 2. Research recommendations for improving our understanding of uremic solutes, their dialytic removal, and their effect on clinical outcomes.
Supplemental Figure 1. Big data–driven discovery and validation of candidate uremic retention solutes.
References
- 1.Boschetti-de-Fierro A, Voigt M, Storr M, Krause B: MCO membranes: Enhanced selectivity in high-flux class. Sci Rep 5: 18448, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanholder RC, Eloot S, Glorieux GLRL: Future avenues to decrease uremic toxin concentration. Am J Kidney Dis 67: 664–676, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Kellum JA, Bellomo R, Ronco C: Acute Dialysis Quality Initiative (ADQI): Methodology. Int J Artif Organs 31: 90–93, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Vanholder R, Glorieux G, De Smet R, Lameire N; European Uremic Toxin Work Group : New insights in uremic toxins. Kidney Int Suppl 63: S6–S10, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Vanholder R, Baurmeister U, Brunet P, Cohen G, Glorieux G, Jankowski J; European Uremic Toxin Work Group : A bench to bedside view of uremic toxins. J Am Soc Nephrol 19: 863–870, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Meert N, Schepers E, Glorieux G, Van Landschoot M, Goeman JL, Waterloos M-A, Dhondt A, Van der Eycken J, Vanholder R: Novel method for simultaneous determination of p-cresylsulphate and p-cresylglucuronide: Clinical data and pathophysiological implications. Nephrol Dial Transplant 27: 2388–2396, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Pletinck A, Glorieux G, Schepers E, Cohen G, Gondouin B, Van Landschoot M, Eloot S, Rops A, Van de Voorde J, De Vriese A, van der Vlag J, Brunet P, Van Biesen W, Vanholder R: Protein-bound uremic toxins stimulate crosstalk between leukocytes and vessel wall. J Am Soc Nephrol 24: 1981–1994, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A; European Uremic Toxin Work Group : Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23: 1258–1270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, Berland Y, Brunet P: The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int 65: 442–451, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Vanholder R, Pletinck A, Schepers E, Glorieux G: Biochemical and clinical impact of organic uremic retention solutes: A comprehensive update. Toxins (Basel) 10: 33, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanholder R, Boelaert J, Glorieux G, Eloot S: New methods and technologies for measuring uremic toxins and quantifying dialysis adequacy. Semin Dial 28: 114–124, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Rhee EP, Souza A, Farrell L, Pollak MR, Lewis GD, Steele DJR, Thadhani R, Clish CB, Greka A, Gerszten RE: Metabolite profiling identifies markers of uremia. J Am Soc Nephrol 21: 1041–1051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W; European Uremic Toxin Work Group (EUTox) : Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int 63: 1934–1943, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Babb AL, Ahmad S, Bergström J, Scribner BH: The middle molecule hypothesis in perspective. Am J Kidney Dis 1: 46–50, 1981 [DOI] [PubMed] [Google Scholar]
- 15.Hai X, Landeras V, Dobre MA, DeOreo P, Meyer TW, Hostetter TH: Mechanism of prominent trimethylamine oxide (TMAO) accumulation in hemodialysis patients. PLoS One 10: e0143731, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eloot S, van Biesen W, Dhondt A, de Smet R, Marescau B, De Deyn PP, Verdonck P, Vanholder R: Impact of increasing haemodialysis frequency versus haemodialysis duration on removal of urea and guanidino compounds: A kinetic analysis. Nephrol Dial Transplant 24: 2225–2232, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Ward RA, Greene T, Hartmann B, Samtleben W: Resistance to intercompartmental mass transfer limits β2-microglobulin removal by post-dilution hemodiafiltration. Kidney Int 69: 1431–1437, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Snauwaert E, Holvoet E, Van Biesen W, Raes A, Glorieux G, Vande Walle J, Roels S, Vanholder R, Askiti V, Azukaitis K, Bayazit A, Canpolat N, Fischbach M, Godefroid N, Krid S, Litwin M, Obrycki L, Paglialonga F, Ranchin B, Samaille C, Schaefer F, Schmitt CP, Spasojevic B, Stefanidis CJ, Van Dyck M, Van Hoeck K, Collard L, Eloot S, Shroff R: Uremic toxin concentrations are related to residual kidney function in the pediatric hemodialysis population. Toxins (Basel) 11: 235, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marquez IO, Tambra S, Luo FY, Li Y, Plummer NS, Hostetter TH, Meyer TW: Contribution of residual function to removal of protein-bound solutes in hemodialysis. Clin J Am Soc Nephrol 6: 290–296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tijink MSL, Wester M, Glorieux G, Gerritsen KGF, Sun J, Swart PC, Borneman Z, Wessling M, Vanholder R, Joles JA, Stamatialis D: Mixed matrix hollow fiber membranes for removal of protein-bound toxins from human plasma. Biomaterials 34: 7819–7828, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Mair RD, Sirich TL, Plummer NS, Meyer TW: Characteristics of colon-derived uremic solutes. Clin J Am Soc Nephrol 13: 1398–1404, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM, Szeto C-C, McWhinney BC, Ungerer JPJ, Campbell KL: Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A randomized trial. Clin J Am Soc Nephrol 11: 223–231, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stinghen AEM, Massy ZA, Vlassara H, Striker GE, Boullier A: Uremic toxicity of advanced glycation end products in CKD. J Am Soc Nephrol 27: 354–370, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harm S, Falkenhagen D, Hartmann J: Pore size: A key property for selective toxin removal in blood purification. Int J Artif Organs 37: 668–678, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Oshihara W, Fujieda H, Ueno Y: A new poly(methyl methacrylate) membrane dialyzer, NF, with adsorptive and antithrombotic properties. In Scientific Aspects of Dialysis Therapy: JSDT/ISBP Anniversary Edition. Contrib Nephrol, edited by Kawanishi H, Takemoto Y., Basel, Karger, 2017, pp 230-236. Available at: https://www.karger.com/Article/FullText/450806. Accessed January 23, 2021 [DOI] [PubMed]
- 26.Yamamoto S, Ito T, Sato M, Goto S, Kazama JJ, Gejyo F, Narita I: Adsorption of protein-bound uremic toxins using activated carbon through direct hemoperfusion in vitro. Blood Purif 48: 215–222, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Rocchetti MT, Cosola C, di Bari I, Magnani S, Galleggiante V, Scandiffio L, Dalfino G, Netti GS, Atti M, Corciulo R, Gesualdo L: Efficacy of divinylbenzenic resin in removing indoxyl sulfate and p-cresol sulfate in hemodialysis patients: Results from an in vitro study and an in vivo pilot trial (xuanro4-Nature 3.2). Toxins (Basel) 12: 170, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geremia I, Stamatialis D: Innovations in dialysis membranes for improved kidney replacement therapy. Nat Rev Nephrol 16: 550–551, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Pavlenko D, Giasafaki D, Charalambopoulou G, van Geffen E, Gerritsen KGF, Steriotis T, Stamatialis D: Carbon adsorbents with dual porosity for efficient removal of uremic toxins and cytokines from human plasma. Sci Rep 7: 14914, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hulko M, Haug U, Gauss J, Boschetti-de-Fierro A, Beck W, Krause B: Requirements and pitfalls of dialyzer sieving coefficients comparisons. Artif Organs 42: 1164–1173, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boschetti-de-Fierro A, Voigt M, Storr M, Krause B: Extended characterization of a new class of membranes for blood purification: The high cut-off membranes. Int J Artif Organs 36: 455–463, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Maduell F, Rodas L, Broseta JJ, Gomez M, Xipell M, Guillen E, Montagud-Marrahi E, Arias-Guillén M, Fontseré N, Vera M, Rico N: Medium cut-off dialyzer versus eight hemodiafiltration dialyzers: Comparison using a global removal score. Blood Purif 48: 167–174, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Hulko M, Dietrich V, Koch I, Gekeler A, Gebert M, Beck W, Krause B: Pyrogen retention: Comparison of the novel medium cut-off (MCO) membrane with other dialyser membranes. Sci Rep 9: 6791, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronco C, Brendolan A, Lupi A, Metry G, Levin NW: Effects of a reduced inner diameter of hollow fibers in hemodialyzers. Kidney Int 58: 809–817, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Weiner DE, Falzon L, Skoufos L, Bernardo A, Beck W, Xiao M, Tran H: Efficacy and Safety of Expanded hemodialysis with the theranova 400 dialyzer: A randomized controlled trial. Clin J Am Soc Nephrol 15: 1310–1319, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnasamy R, Hawley CM, Jardine MJ, Roberts MA, Cho Y, Wong M, Heath A, Nelson CL, Sen S, Mount PF, Pascoe EM, Vergara LA, Paul-Brent P-A, Toussaint ND, Johnson DW, Hutchison CA: A tRial Evaluating Mid cut-Off Value membrane clearance of Albumin and Light chains in HemoDialysis patients: A safety device study. Blood Purif 49: 468–478, 2020 [DOI] [PubMed] [Google Scholar]
- 37.Van Biesen W, De Bacquer D, Verbeke F, Delanghe J, Lameire N, Vanholder R: The glomerular filtration rate in an apparently healthy population and its relation with cardiovascular mortality during 10 years. Eur Heart J 28: 478–483, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Wang HE, Gamboa C, Warnock DG, Muntner P: Chronic kidney disease and risk of death from infection. Am J Nephrol 34: 330–336, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zoccali C, Vanholder R, Massy ZA, Ortiz A, Sarafidis P, Dekker FW, Fliser D, Fouque D, Heine GH, Jager KJ, Kanbay M, Mallamaci F, Parati G, Rossignol P, Wiecek A, London G; European Renal and Cardiovascular Medicine (EURECA-m) Working Group of the European Renal Association – European Dialysis Transplantation Association (ERA-EDTA) : The systemic nature of CKD. Nat Rev Nephrol 13: 344–358, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Wolley M, Jardine M, Hutchison CA: Exploring the clinical relevance of providing increased removal of large middle molecules. Clin J Am Soc Nephrol 13: 805–814, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pittman ZCL, John SG, McIntyre CW: Collection of daily patient reported outcomes is feasible and demonstrates differential patient experience in chronic kidney disease. Hemodial Int 21: 265–273, 2017 [DOI] [PubMed] [Google Scholar]
- 42.Nair D, Wilson FP: Patient-reported outcome measures for adults with kidney disease: Current measures, ongoing initiatives, and future opportunities for incorporation into patient-centered kidney care. Am J Kidney Dis 74: 791–802, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Willik EM, Meuleman Y, Prantl K, van Rijn G, Bos WJW, van Ittersum FJ, Bart HAJ, Hemmelder MH, Dekker FW: Patient-reported outcome measures: Selection of a valid questionnaire for routine symptom assessment in patients with advanced chronic kidney disease: A four-phase mixed methods study. BMC Nephrol 20: 344, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Penny J, Salerno FR, Hur L, McIntyre C: Expanded dialysis (HDx): Is there an impact on patient reported symptom? Nephrol Dial Transplant 35: 2020 [Google Scholar]
- 45.Tong A, Manns B, Hemmelgarn B, Wheeler DC, Evangelidis N, Tugwell P, Crowe S, Van Biesen W, Winkelmayer WC, O’Donoghue D, Tam-Tham H, Shen JI, Pinter J, Larkins N, Youssouf S, Mandayam S, Ju A, Craig JC; SONG-HD Investigators : Establishing core outcome domains in hemodialysis: Report of the Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) consensus workshop. Am J Kidney Dis 69: 97–107, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koppe L, Nyam E, Vivot K, Manning Fox JE, Dai X-Q, Nguyen BN, Trudel D, Attané C, Moullé VS, MacDonald PE, Ghislain J, Poitout V: Urea impairs β cell glycolysis and insulin secretion in chronic kidney disease. J Clin Invest 126: 3598–3612, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Apolito M, Du X, Pisanelli D, Pettoello-Mantovani M, Campanozzi A, Giacco F, Maffione AB, Colia AL, Brownlee M, Giardino I: Urea-induced ROS cause endothelial dysfunction in chronic renal failure. Atherosclerosis 239: 393–400, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trécherel E, Godin C, Louandre C, Benchitrit J, Poirot S, Mazière J-C, Massy ZA, Galmiche A: Upregulation of BAD, a pro-apoptotic protein of the BCL2 family, in vascular smooth muscle cells exposed to uremic conditions. Biochem Biophys Res Commun 417: 479–483, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Vaziri ND, Yuan J, Norris K: Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol 37: 1–6, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuragano T, Kida A, Furuta M, Nanami M, Otaki Y, Hasuike Y, Nonoguchi H, Nakanishi T: The impact of β2-microglobulin clearance on the risk factors of cardiovascular disease in hemodialysis patients. ASAIO J 56: 326–332, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Nakano T, Matsui M, Inoue I, Awata T, Katayama S, Murakoshi T: Free immunoglobulin light chain: Its biology and implications in diseases. Clin Chim Acta 412: 843–849, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Kohan DE, Inscho EW, Wesson D, Pollock DM: Physiology of endothelin and the kidney. In: Comprehensive Physiology, edited by Terjung R, American Physiological Society, 2011, p c100039. Available at: http://doi.wiley.com/10.1002/cphy.c100039. Accessed January 23, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuczera P, Adamczak M, Wiecek A: Fibroblast Growth Factor-23: A potential uremic toxin. Toxins (Basel) 8: 369, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castillo-Rodríguez E, Pizarro-Sánchez S, Sanz AB, Ramos AM, Sanchez-Niño MD, Martin-Cleary C, Fernandez-Fernandez B, Ortiz A: Inflammatory cytokines as uremic toxins: “Ni son todos los que estan, ni estan todos los que son.” Toxins (Basel) 9: 114, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rocco MV, Dwyer JT, Larive B, Greene T, Cockram DB, Chumlea WC, Kusek JW, Leung J, Burrowes JD, McLeroy SL, Poole D, Uhlin L; HEMO Study Group : The effect of dialysis dose and membrane flux on nutritional parameters in hemodialysis patients: Results of the HEMO Study. Kidney Int 65: 2321–2334, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Locatelli F, Martin-Malo A, Hannedouche T, Loureiro A, Papadimitriou M, Wizemann V, Jacobson SH, Czekalski S, Ronco C, Vanholder R; Membrane Permeability Outcome (MPO) Study Group : Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol 20: 645–654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Graboski AL, Redinbo MR: Gut-derived protein-bound uremic toxins. Toxins (Basel) 12: 590, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perego AF: Adsorption techniques: Dialysis sorbents and membranes. Blood Purif 35: 48–51, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Kooman JP, Kotanko P, Schols AMWJ, Shiels PG, Stenvinkel P: Chronic kidney disease and premature ageing. Nat Rev Nephrol 10: 732–742, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Legrand K, Speyer E, Stengel B, Frimat L, Ngueyon Sime W, Massy ZA, Fouque D, Laville M, Combe C, Jacquelinet C, Durand AC, Edet S, Gentile S, Briançon S, Ayav C: Perceived health and quality of life in patients with CKD, including those with kidney failure: Findings from national surveys in France. Am J Kidney Dis 75: 868–878, 2020 [DOI] [PubMed] [Google Scholar]
- 61.Anderson NE, Calvert M, Cockwell P, Dutton M, Kyte D: The use of patient-reported outcomes in patients treated with maintenance hemodialysis: A perspective. Am J Kidney Dis 74: 399–406, 2019 [DOI] [PubMed] [Google Scholar]
- 62.Vernaglione L, Marangi AL, Cristofano C, Giordano R, Chimienti S, Basile C: Predictors of serum creatinine in haemodialysis patients: A cross-sectional analysis. Nephrol Dial Transplant 18: 1209–1213, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Ronco C, Clark WR: Haemodialysis membranes. Nat Rev Nephrol 14: 394–410, 2018 [DOI] [PubMed] [Google Scholar]
- 64.Ronco C, Neri M, Lorenzin A, Garzotto F, Clark WR: Multidimensional classification of dialysis membranes. In: Contributions to Nephrology, edited by Ronco C, Karger Press, 2017, pp 115–126. Available at: https://www.karger.com/Article/FullText/479260. Accessed January 23, 2021 [DOI] [PubMed] [Google Scholar]
- 65.Basile C, Davenport A, Blankestijn PJ: Why choose high volume online post-dilution hemodiafiltration? J Nephrol 30: 181–186, 2017 [DOI] [PubMed] [Google Scholar]
- 66.Canaud B, Blankestijn PJ, Davenport A, Bots ML: Reconciling and closing the loop between evidence-based and practice-based medicine: The case for hemodiafiltration. Am J Kidney Dis 68: 176–179, 2016 [DOI] [PubMed] [Google Scholar]
- 67.Scholze A, Rinder C, Beige J, Riezler R, Zidek W, Tepel M: Acetylcysteine reduces plasma homocysteine concentration and improves pulse pressure and endothelial function in patients with end-stage renal failure. Circulation 109: 369–374, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Cozzolino M, Ronco C: Medium cut-off membranes: Incremental or quantum leap innovation in haemodialysis? [published online ahead of print December 22, 2020]. Blood Purif 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.