Significance Statement
Relatively little is known about the relative safety and efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors in patients with advanced (stage 4) CKD. The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial enrolled patients with CKD with or without type 2 diabetes (mean eGFR 43 ± 12 ml/min per 1.73m2), finding that patients receiving the drug had lower risks of major kidney and cardiovascular events and an attenuation of progressive eGFR loss compared with patients receiving placebo. In this analysis within a subgroup of patients with stage 4 CKD and albuminuria, the authors found that the benefits of the SGLT2 inhibitor dapagliflozin in patients with baseline eGFR<30 ml/min per 1.73m2 were consistent with those observed in the DAPA-CKD trial overall, with no evidence of increased risks.
Keywords: dapagliflozin, SGLT2 inhibitor, chronic kidney disease, stage 4 CKD
Abstract
Background
In the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) randomized, placebo-controlled trial, the sodium-glucose cotransporter 2 inhibitor dapagliflozin significantly reduced risk of kidney failure and prolonged survival in patients with CKD with or without type 2 diabetes.
Methods
Adults with eGFR of 25–75 ml/min per 1.73 m2 and urinary albumin-to-creatinine ratio of 200–5000 mg/g had been randomized to receive dapagliflozin 10 mg/d or placebo. Here, we conducted a prespecified analysis of dapagliflozin’s effects in patients with stage 4 CKD (eGFR,30 ml/min per 1.73 m2) at baseline. The primary end point was a composite of time to ≥50% sustained decline in eGFR, ESKD, or kidney or cardiovascular death. Secondary end points were a kidney composite (same as the primary end point but without cardiovascular death), a composite of cardiovascular death or heart failure hospitalization, and all-cause death.
Results
A total of 293 participants with stage 4 CKD received dapagliflozin and 331 received placebo. Patients with stage 4 CKD randomized to dapagliflozin experienced a 27% (95% confidence interval [95% CI]: −2 to 47%) reduction in the primary composite endpoint, and 29% (−2 to 51%), 17% (−53 to 55%), and 32% (−21 to 61%) reductions in the kidney, cardiovascular and mortality endpoints, respectively, relative to placebo. Interaction P-values were 0.22, 0.13, 0.63, and 0.95, respectively, comparing CKD stages 4 versus 2/3. The eGFR slope declined by 2.15 and 3.38 ml/min per 1.73 m2 per year in the dapagliflozin and placebo groups, respectively (P=0.005). Patients treated with dapagliflozin or placebo had similar rates of serious adverse events and adverse events of interest.
Conclusions
Among patients with stage 4 CKD and albuminuria, the effects of dapagliflozin were consistent with those observed in the DAPA-CKD trial overall, with no evidence of increased risks.
Relative to patients with normal or near normal kidney function, patients with CKD experience higher rates of death, cardiovascular events, and hospitalization,1,2 and experience poorer health status, including impaired physical function, cognitive function, and health-related quality of life.3–6 Patients with advanced (stage 4) CKD are particularly vulnerable to cardiovascular events and other complications, including progression to kidney failure.
Randomized clinical trials conducted more than two decades ago established the benefits of inhibitors of the renin-angiotensin-aldosterone system (RAAS) in attenuating progression of CKD associated with type 2 diabetes and other forms of proteinuric CKD. Thereafter, RAAS inhibitors became widely recommended, and not only yielded benefits on kidney disease-related composite end points (e.g., death, the need for dialysis or kidney transplantation, or doubling of serum creatinine), but also enhanced control of hypertension and reduced complications of heart failure, both of which frequently accompanied CKD.7–10 In practice, however, the persistence of RAAS inhibitor prescription has been limited by transient increases in serum creatinine and/or hyperkalemia, often prompting drug discontinuation, particularly among patients with advanced CKD.11
Several sodium-glucose cotransporter 2 (SGLT2) inhibitors have been shown to reduce rates of death and cardiovascular events among patients with type 2 diabetes.12–14 Initially, SGLT2 inhibitors were not recommended for use in patients with impaired kidney function, due to diminished efficacy vis-à-vis glycemic control.15,16 The Canagliflozin and Renal Events in Diabetes and Established Nephropathy Clinical Evaluation (CREDENCE) trial was the first of the SGLT2 inhibitor trials with a primary cardiorenal composite end point, conducted in a population with substantial albuminuria and/or impaired kidney function. CREDENCE enrolled patients exclusively with type 2 diabetes, urine albumin-to-creatinine ratio (UACR) >300 to 5000 mg/g, and eGFR 30–90 ml/min per 1.73 m2 at screening, and demonstrated significant reductions in the risk of kidney and cardiovascular events.17 In contrast, the Dapagliflozin And Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial enrolled patients with and without type 2 diabetes, with UACR 200–5000 mg/g, and with eGFR 25–75 ml/min per 1.73 m2 at screening.18,19 In DAPA-CKD, 624 of 4304 (14%) randomized patients had stage 4 CKD at baseline, allowing for a robust assessment of safety in this subpopulation, and a detailed assessment of the effects of dapagliflozin on primary and key secondary efficacy end points.
Methods
DAPA-CKD was a randomized, double-blind, placebo-controlled, multicenter clinical trial; manuscripts describing trial design, baseline characteristics, primary results, and results stratified by diabetes status and history of cardiovascular disease have been previously published.18–22 The trial was sponsored by AstraZeneca and conducted at 386 sites in 21 countries from February of 2017 through June of 2020 and registered at ClinicalTrials.gov (NCT03036150). All participants provided written, informed consent before any study-specific procedure commenced. The safety of patients in the trial was overseen by an independent data and safety monitoring committee.
Participants
Adults with or without type 2 diabetes, with eGFR 25–75 ml/min per 1.73 m2, and with UACR 200–5000 mg/g were eligible for participation. We required patients to be treated with a stable dose of RAAS inhibitor for ≥4 weeks unless medically contraindicated. Key exclusion criteria included a documented diagnosis of type 1 diabetes, polycystic kidney disease, lupus nephritis, or ANCA-associated vasculitis. A complete list of inclusion and exclusion criteria and the trial protocol have been previously published.18,19
Procedures
Participants were randomly assigned to dapagliflozin 10 mg once daily or matching placebo, in accordance with the sequestered, fixed-randomization schedule, with the use of balanced blocks to ensure an approximate 1:1 ratio of the two regimens. Randomization was stratified by diabetes status and UACR (≤ or >1000 mg/g). We calculated eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation and incorporated results from the equation as originally defined, including a term for self-reported race (Black versus non-Black). Recruitment of participants with eGFR 60–75 ml/min per 1.73 m2 was limited to no more than 10% of trial participants. Whereas participants with eGFR<25 ml/min per 1.73 m2 at screening were not enrolled, participants only discontinued study drug (dapagliflozin or placebo) if they developed diabetic ketoacidosis, became pregnant, or developed an adverse event (AE) that in the opinion of the investigator was a contraindication to ongoing treatment. The protocol did not mandate discontinuation of study drug when participants reached a certain eGFR threshold. Participants and all study personnel (except the Independent Data Monitoring Committee) were masked to treatment allocation.
After randomization, in-person study visits were performed after 2 weeks; after 2, 4, and 8 months; and at 4-month intervals thereafter. At each follow-up visit, study personnel recorded vital signs, obtained blood and urine samples, and recorded information on potential study end points, AEs, concomitant therapies, and study drug adherence.
End Points
The primary composite end point was time to ≥50% sustained decline in eGFR (confirmed by a second serum creatinine measurement after at least 28 days), onset of ESKD (defined as maintenance dialysis for at least 28 days, kidney transplantation, or eGFR<15 ml/min per 1.73 m2 confirmed by a second measurement after at least 28 days), or death from kidney or cardiovascular cause. Key secondary end points were time to: (1) a composite kidney end point of ≥50% sustained decline in eGFR, kidney failure, or death from kidney disease; (2) a composite cardiovascular end point defined as hospitalization for heart failure or cardiovascular death; and (3) all-cause death. Additional prespecified and post hoc exploratory end points included a composite of dialysis, kidney transplantation, or kidney death; a composite of cardiovascular death, myocardial infarction or stroke, time to first hospitalization for heart failure, ESKD or all-cause death, ESKD, or heart failure hospitalization; a composite of ESKD, myocardial infarction, stroke, hospitalization for heart failure, or cardiovascular death; and a composite of ESKD, hospitalization for heart failure, or all-cause death. We also considered change in eGFR slope as an exploratory efficacy end point.
All efficacy end points were adjudicated by a masked, independent Clinical Events Committee, except for the quantitative assessments of eGFR which were obtained from our central laboratory.
Safety
Given extensive prior experience with dapagliflozin, we limited our ascertainment of AEs to serious adverse events (SAEs), AEs resulting in the discontinuation of study drug, and AEs of special interest (symptoms of volume depletion, kidney-related events, major hypoglycemia, bone fractures, amputations, and potential diabetic ketoacidosis). Potential diabetic ketoacidosis events were adjudicated by an independent adjudication committee.
Statistical Analyses
The overall analytic approach, power calculation, and prespecified statistical analysis plan have been previously published.18,19 All analyses presented here followed the intention-to-treat principle. Briefly, we conducted time-to-event analyses using a proportional hazards (Cox) regression stratified by randomization factors (diabetes status and UACR), adjusting for baseline eGFR, yielding hazard ratios (HRs) and 95% confidence intervals (95% CIs) from model parameter coefficients and standard errors. For the purpose of the current prespecified analysis, we evaluated the primary, key secondary, and other prespecified efficacy end points in patients within the subcohort of patients with baseline stage 4 CKD (eGFR<30 ml/min per 1.73 m2), and compared results with the larger subpopulation (86%) of patients with stages 2/3 CKD (eGFR≥30 ml/min per 1.73 m2), including a multiplicative interaction term between randomized treatment group and stage. For time-to-event analyses, we assessed for nonuniformity of HRs with the Akaike’s information criterion. We considered P values <0.05 to be statistically significant.
We conducted two post hoc exploratory analyses within the stage 4 CKD subgroup, comparing results for the primary composite and kidney composite end points by diabetes status and by higher versus lower levels of albuminuria.
We analyzed the effects of dapagliflozin on the mean on-treatment eGFR slope by fitting a two-slope mixed effects linear spline model (with a knot at week 2) with a random intercept and random slopes for treatment. The model included fixed effects for treatment, CKD stage, and stratification factors (diabetes status and UACR), and a continuous, fixed covariate for time-to-visit. To determine eGFR slopes for the CKD stage subgroups (4 versus 2/3), we added to the model all possible interaction terms for treatment effect, CKD stage, and time-to-visit, assuming an unstructured variance-covariance matrix. We computed the mean total slope as a weighted combination of the acute and chronic slopes to reflect the mean rate of eGFR change until the last on-treatment visit. We also presented the pattern of change in mean eGFR using a restricted maximum likelihood repeated measures approach. This latter analysis included fixed effects of treatment, visit, treatment-by-visit interaction, and treatment–by–CKD stage interaction. We added interaction terms between CKD stage, visit, and treatment assignment to assess the change in eGFR for the CKD stage subgroups (4 versus 2/3).
We performed all analyses with SAS version 9.4 (SAS Institute) or R version 4.0.2 (R Foundation).
Results
Six hundred and twenty-four (14%) of 4304 randomized patients had stage 4 CKD at baseline. Figure 1 shows the Consolidated Standards of Reporting Trials (CONSORT) diagram for patients enrolled in DAPA-CKD and those randomized by baseline CKD stage (4 versus 2/3). Supplemental Figure 1 shows the distribution of baseline eGFR<30 ml/min per 1.73 m2 in the dapagliflozin (n=293) and placebo (n=331) groups.
Figure 1.
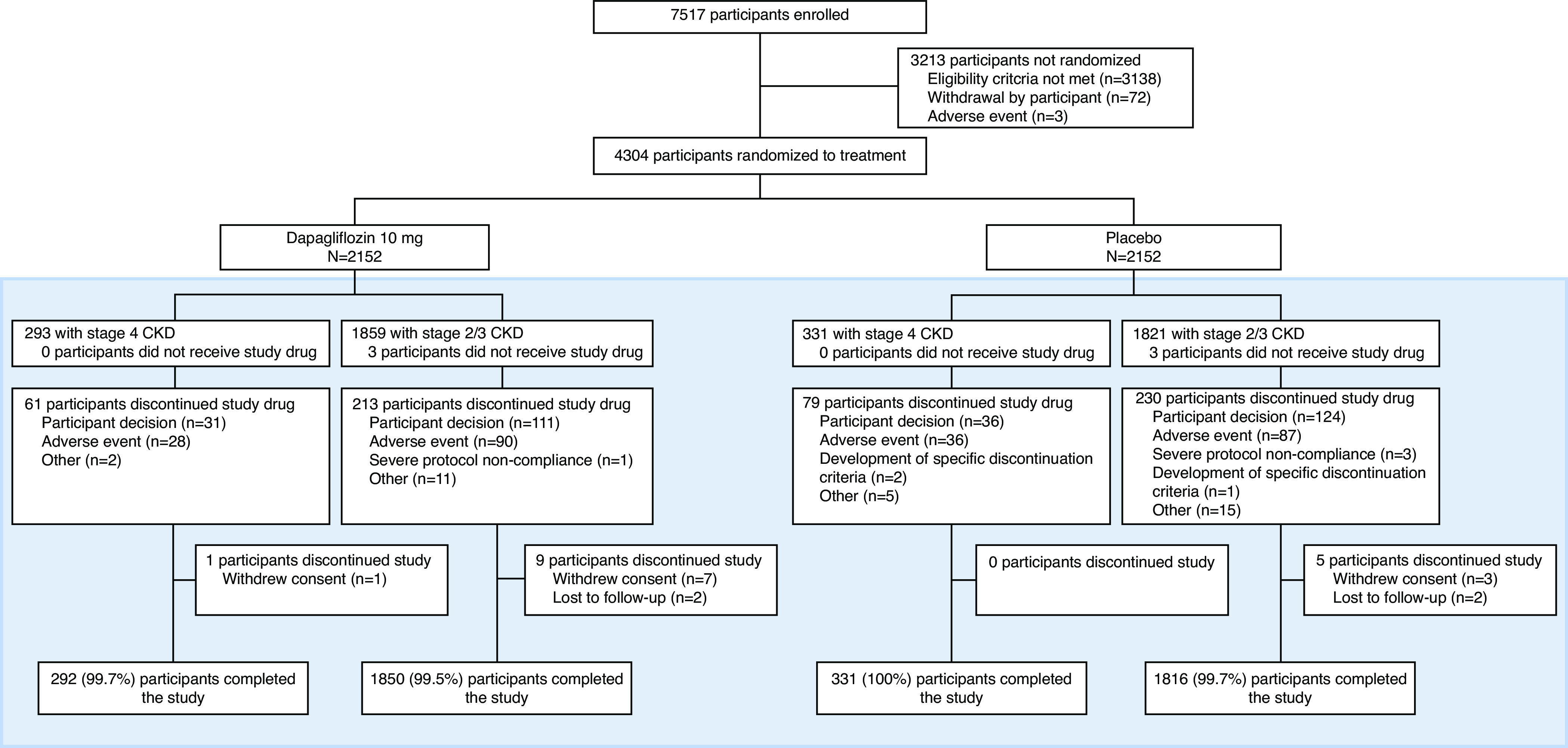
Participant flow chart by CKD stage. Box indicates subgroups in the current prespecified analysis; stage 4 CKD, eGFR<30 ml/min per 1.73 m2; stage 2/3 CKD, eGFR≥30 ml/min per 1.73 m2.
Table 1 shows baseline characteristics of randomized patients stratified by CKD stage (4 versus 2/3) and randomized treatment group. The mean age, proportion female, and distribution by race were similar across CKD stages. Randomized patients with stage 4 CKD were less likely to have type 2 diabetes, had higher UACR, were less likely to be treated with RAAS inhibitors, and were more likely to be treated with diuretics.
Table 1.
Characteristics of the patients at baseline according to CKD stage and randomized treatment assignment
| Characteristic | Baseline Stage 4 CKD (n=624) |
Baseline Stages 2/3 CKD (n=3680) |
||
|---|---|---|---|---|
| Dapagliflozin (n=293) |
Placebo (n=331) |
Dapagliflozin (n=1859) |
Placebo (n=1821) |
|
| Age (years) | 61.9 (11.8) | 62.6 (12.4) | 61.8 (12.1) | 61.8 (12.1) |
| Female sex, n (%) | 103 (35.2) | 122 (36.9) | 606 (32.6) | 594 (32.6) |
| Race, n (%) | ||||
| White | 155 (52.9) | 180 (54.4) | 969 (52.1) | 986 (54.2) |
| Black | 12 (4.1) | 11 (3.3) | 92 (5.0) | 76 (4.2) |
| Asian | 96 (32.8) | 113 (34.1) | 653 (35.1) | 605 (33.2) |
| Other | 39 (10.2) | 33 (8.2) | 145 (7.8) | 154 (8.5) |
| Body mass index (kg/m2) | 29.6 (6.7) | 29.0 (6.2) | 29.4 (5.9) | 29.8 (6.3) |
| Current smoker, n (%) | 43 (14.7) | 44 (13.3) | 240 (12.9) | 257 (14.1) |
| Blood pressure (mmHg) | ||||
| Systolic | 139.4 (19.1) | 137.1 (18.4) | 136.3 (17.2) | 137.5 (17.1) |
| Diastolic | 78.0 (10.9) | 76.4 (10.9) | 77.4 (10.6) | 77.7 (10.1) |
| eGFR (ml/min per 1.73 m2) | 26.8 (1.8) | 26.8 (1.8) | 45.8 (11.2) | 45.9 (11.2) |
| Hemoglobin (g/L) | 120.8 (16.7) | 120.0 (16.2) | 129.8 (18.0) | 129.3 (18.0) |
| Serum potassium (mEq/L) | 4.8 (0.6) | 4.8 (0.6) | 4.6 (0.5) | 4.6 (0.5) |
| Median UACR (Q1–Q3) | 1279 (642–2470) | 1212 (577–2289) | 920 (448–1841) | 911 (472–1791) |
| UACR>1000 mg/g, n (%) | 168 (57.3) | 183 (55.3) | 880 (47.3) | 848 (46.6) |
| Type 2 diabetes diagnosis, n (%) | 190 (64.9) | 211 (63.8) | 1265 (68.1) | 1240 (68.1) |
| Cardiovascular disease, n (%) | 98 (33.5) | 133 (40.2) | 715 (38.5) | 664 (36.5) |
| Baseline medication, n (%) | ||||
| ACE inhibitor/ARB | 278 (94.9) | 310 (93.7) | 1816 (97.7) | 1770 (97.2) |
| Diuretic | 154 (52.6) | 176 (53.2) | 774 (41.6) | 778 (42.7) |
| Statin | 180 (61.4) | 214 (64.7) | 1215 (65.4) | 1185 (65.1) |
Data are shown as mean (SD) unless otherwise stated. Stage 4 CKD, eGFR<30 ml/min per 1.73 m2; stages 2/3 CKD, eGFR≥30 ml/min per 1.73 m2. Q1, 25th percentile; Q3, 75th percentile; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker.
Effects of Dapagliflozin on Discrete Events
Fifty-nine of 293 (20%) patients with stage 4 CKD at baseline randomized to dapagliflozin experienced a primary composite end point, compared with 87 of 331 (26%) randomized to placebo. Figure 2A shows the cumulative incidence of the primary composite end point in patients with stage 4 CKD at baseline in both groups (HR 0.73; 95% CI, 0.53 to 1.02). Figure 2, B, C, and D, shows cumulative incidence curves for the key secondary end points: ≥50% sustained decline in eGFR, kidney failure, or death from kidney disease (HR 0.71; 95% CI, 0.49 to 1.02); hospitalization for heart failure or cardiovascular death (HR 0.83; 95% CI, 0.45 to 1.53); and all-cause death (HR 0.68; 95% CI, 0.39 to 1.21). Forty-nine, 18, and 19 patients with stage 4 CKD at baseline randomized to dapagliflozin and 73, 24, and 31 patients with stage 4 CKD at baseline randomized to placebo experienced the key secondary end points, respectively.
Figure 2.
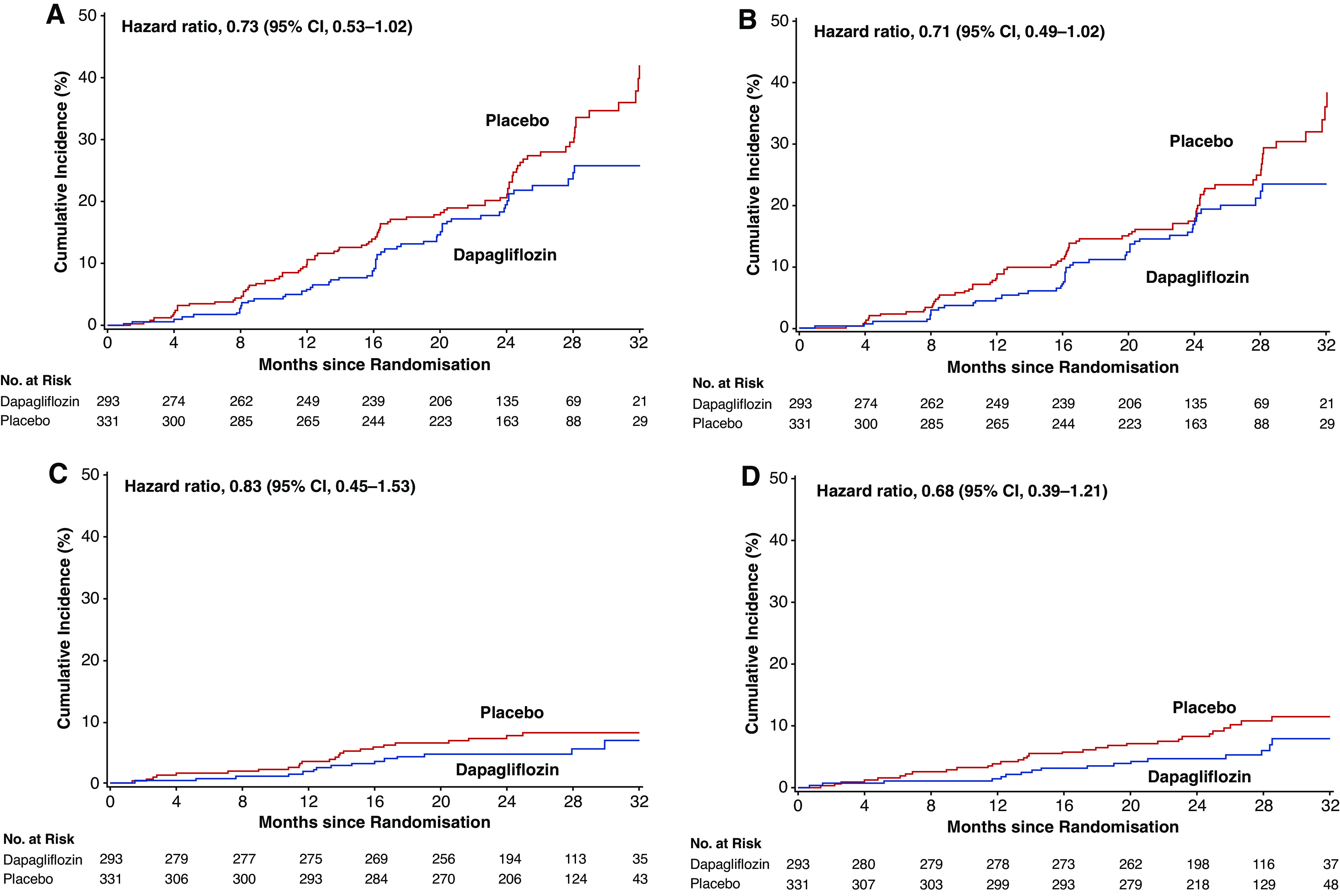
Kaplan–Meier curves for the cumulative incidence of (A) the primary composite end points, (B) the kidney composite end point, (C) hospitalization for heart failure or cardiovascular death, and (D) all-cause death in patients with stage 4 CKD at baseline. Primary composite end point, sustained eGFR decline ≥50%, ESKD, or kidney or cardiovascular death; secondary kidney composite end point, sustained eGFR decline ≥50%, ESKD, or kidney death.
The trial was not powered to detect a statistically significant difference in the primary and key secondary end points in modest-sized subgroups. However, when comparing treatment effects in patients with stage 4 CKD at baseline with those in patients with stages 2/3 CKD, there were no significant differences (interaction P values for treatment assignment by CKD stage 0.22, 0.13, 0.63, and 0.95, respectively, for the primary composite end point and the three key secondary end points in sequence shown above). Figure 3 shows Forest plots for the primary and secondary outcomes by baseline CKD stage. Supplemental Figures 2 and 3 show Forest plots for prespecified and post hoc exploratory end points, respectively. For all end points, HRs for dapagliflozin were below 1.0, and there were no significant interactions (effect modification) by CKD stage.
Figure 3.
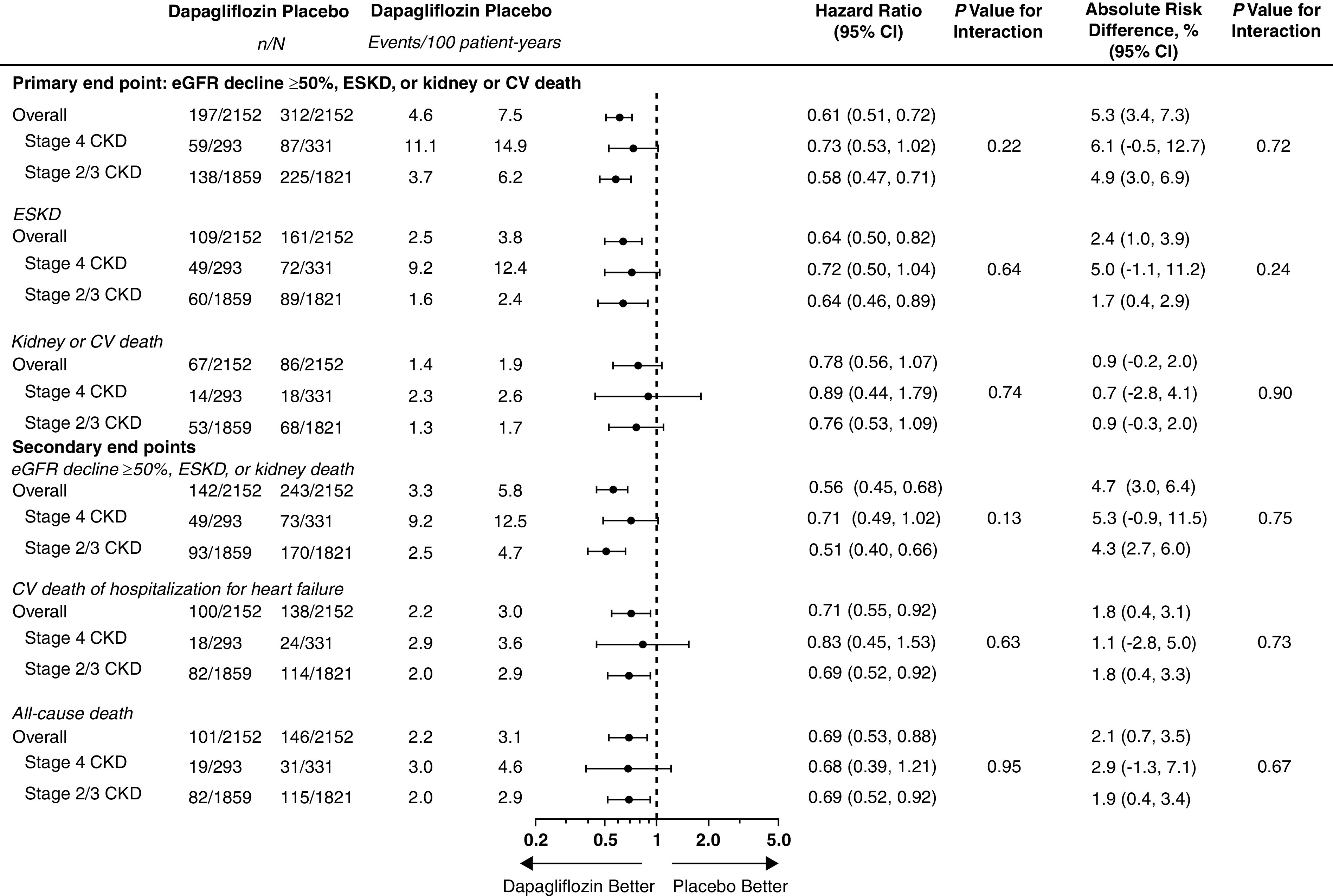
Forest plots for the primary and secondary end points by baseline CKD stage. Stage 4 CKD, eGFR<30 ml/min per 1.73 m2; stages 2/3 CKD, eGFR≥30 ml/min per 1.73 m2. CV, cardiovascular; n, number with events; N, total number.
Post Hoc Exploratory Sub-Subgroup Analyses
Within the stage 4 CKD subgroup, there was no detectable heterogeneity of effect of dapagliflozin on the primary composite or kidney composite end points by diabetes status or degree of albuminuria. For the primary composite end point, HRs were 0.84 (95% CI, 0.57 to 1.23) and 0.52 (95% CI, 0.27 to 1.02) in patients with and without diabetes, respectively (interaction P=0.23), and 0.79 (0.37 to 1.69) and 0.72 (0.50 to 1.05) in patients with UACR≤1000 mg/g and >1000 mg/g, respectively (interaction P=0.94). Corresponding HRs for the kidney composite end point were 0.92 (95% CI, 0.60 to 1.42) and 0.48 (0.24 to 0.96), respectively (interaction P=0.11), and 0.63 (0.24 to 1.69) and 0.73 (0.49 to 1.08), respectively (interaction P=0.71).
Effects of Dapagliflozin on eGFR Slopes
Figure 4 shows least squares mean change in eGFR slope (±SEM) in the stage 4 CKD dapagliflozin and placebo groups: total slope −2.15 (0.32) and −3.38 (0.31) ml/min per 1.73 m2 per year, corresponding to a between-groups difference of 1.23 ml/min per 1.73 m2 per year (95% CI, 0.36 to 2.09; P=0.005). The acute decline in eGFR (baseline to 2 weeks) was more pronounced in patients treated with dapagliflozin compared with placebo: acute decline −2.10 (0.37) and −0.68 (0.35) ml/min per 1.73 m2 per 2 weeks (P=0.005), whereas the chronic slopes (week 2 and beyond) were −1.33 (0.32) and −3.16 (0.31) ml/min per 1.73 m2 per year, corresponding to a between-groups difference of 1.82 ml/min per 1.73 m2 per year (95% CI, 0.96 to 2.68; P<0.0001).
Figure 4.
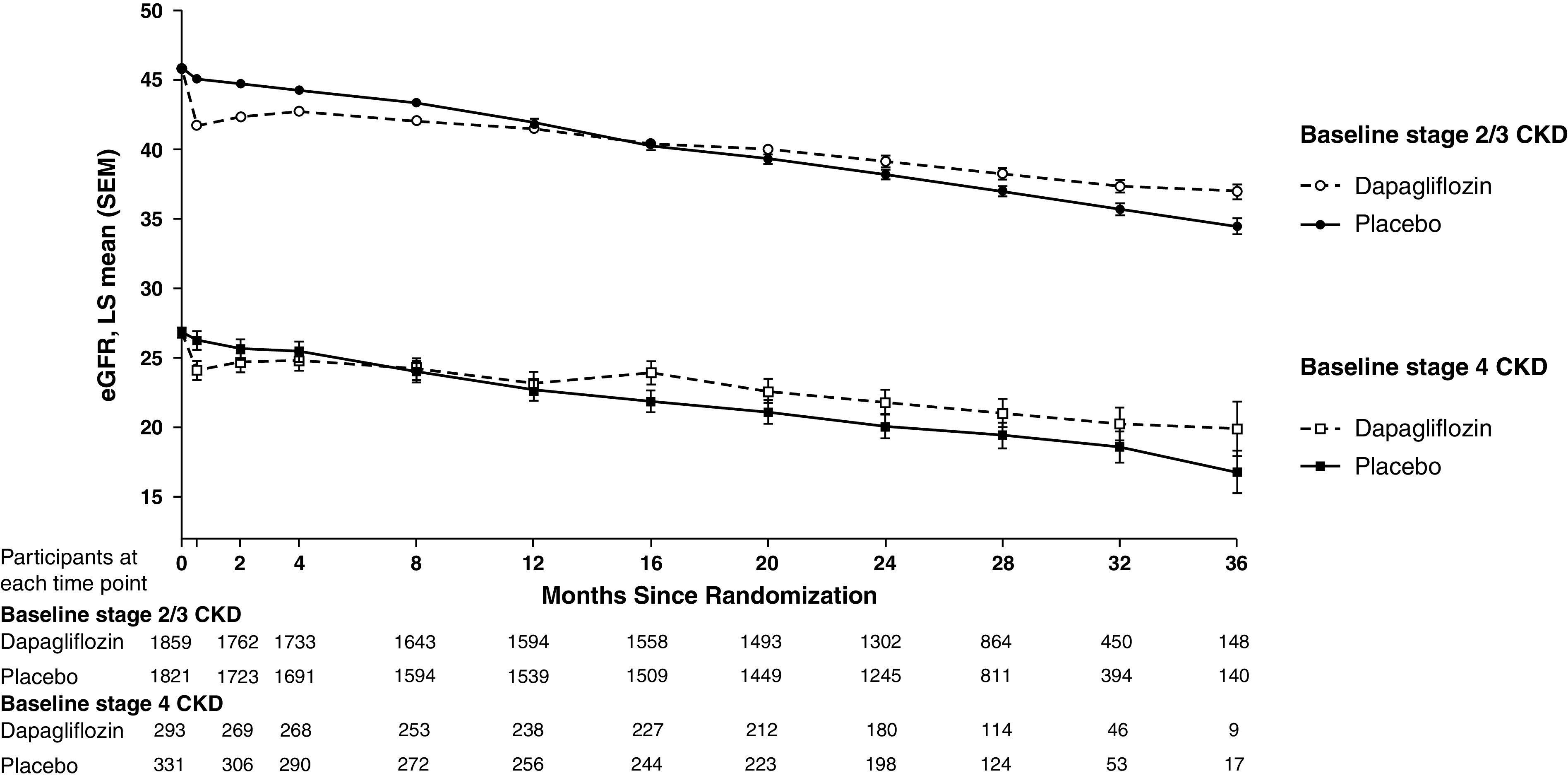
LS mean change in eGFR over the study in those with baseline stage 4 or stages 2/3 CKD. On the basis of the two-slope model. Total slopes (SEM): stage 4 CKD, dapagliflozin −2.15 (0.32), placebo −3.38 (0.31); stage 2/3 CKD, dapagliflozin −2.98 (0.12), placebo −3.87 (0.12) ml/min per 1.73 m2 per year. Chronic slope (SEM): stage 4 CKD, dapagliflozin −1.33 (0.32), placebo −3.68 (0.12); stage 2/3 CKD, dapagliflozin −1.73 (0.12), placebo −3.68 (0.12) ml/min per 1.73 m2 per year. Acute slope (SEM): stage 4 CKD, dapagliflozin −2.10 (0.37), placebo −0.68 (0.35); stage 2/3 CKD, dapagliflozin −3.19 (0.15), placebo −0.64 (0.15) ml/min per 1.73 m2 per two weeks. Stage 4 CKD, eGFR<30 ml/min per 1.73 m2; stages 2/3 CKD, eGFR 30 ml/min per 1.73 m2. Error bars indicate SEM. LS, least squares; SEM, standard error of the mean.
The magnitude of benefit of dapagliflozin on the total eGFR slope was similar in patients with stage 4 CKD at baseline compared with patients with stages 2/3 CKD at baseline (difference [±SEM]: 1.23 [0.44] versus 0.89 [0.17] ml/min per 1.73 m2 per year; interaction P value=0.48).
SAEs
Table 2 shows a summary of SAEs and AEs of special interest observed in patients stratified by treatment group and CKD stage. As expected, patients with stage 4 CKD at baseline were more likely to experience one or more SAEs or AEs resulting in discontinuation of study drug. Overall incidence of SAEs was numerically lower in patients treated with dapagliflozin versus placebo across both CKD stage strata. Although kidney-related AEs were observed more frequently in patients with stage 4 CKD at baseline, neither the proportion of patients (15% versus 13%) nor the relative odds of experiencing kidney-related AEs (1.12; 95% CI, 0.71 to 1.77) were significantly increased in patients randomized to dapagliflozin.
Table 2.
Safety outcomes by baseline CKD stage and treatment assignment
| Outcome, n/N (%) | Dapagliflozin | Placebo | Odds Ratio (95% CI) |
P Value Interaction |
|---|---|---|---|---|
| Discontinuation due to AE | 0.61 | |||
| Baseline stage 4 CKD | 28 of 293 (9.6) | 36 of 331 (10.9) | 0.87 (0.51 to 1.45) | |
| Baseline stages 2/3 CKD | 90 of 1856 (4.8) | 87 of 1818 (4.8) | 1.01 (0.75 to 1.37) | |
| Any SAEa | 0.49 | |||
| Baseline stage 4 CKD | 101 of 293 (34.5) | 138 of 331 (41.7) | 0.74 (0.53 to 1.02) | |
| Baseline stages 2/3 CKD | 532 of 1856 (28.7) | 591 of 1818 (32.5) | 0.83 (0.72 to 0.96) | |
| AEs of interest | ||||
| Amputationb | 0.95 | |||
| Baseline stage 4 CKD | 3 of 293 (1.0) | 4 of 331 (1.2) | 0.85 (0.17 to 3.87) | |
| Baseline stages 2/3 CKD | 32 of 1856 (1.7) | 35 of 1818 (1.9) | 0.89 (0.55 to 1.45) | |
| Any definite or probable diabetic ketoacidosis | NC | |||
| Baseline stage 4 CKD | 0 | 1 of 331 (0.3) | NC | |
| Baseline stages 2/3 CKD | 0 | 1 of 1818 (0.1) | NC | |
| Fracturec | 0.26 | |||
| Baseline stage 4 CKD | 11 of 293 (3.8) | 15 of 331 (4.5) | 0.82 (0.36 to 1.81) | |
| Baseline stages 2/3 CKD | 74 of 1856 (4.0) | 54 of 1818 (3.0) | 1.36 (0.95 to 1.95) | |
| Renal-related AEc | 0.13 | |||
| Baseline stage 4 CKD | 43 of 293 (14.7) | 44 of 331 (13.3) | 1.12 (0.71 to 1.77) | |
| Baseline stages 2/3 CKD | 112 of 1856 (6.0) | 144 of 1818 (7.9) | 0.75 (0.58 to 0.96) | |
| Major hypoglycemiad | 0.37 | |||
| Baseline stage 4 CKD | 2 of 293 (0.7) | 8 of 331 (2.4) | 0.28 (0.04 to 1.12) | |
| Baseline stages 2/3 CKD | 12 of 1856 (0.6) | 20 of 1818 (1.1) | 0.59 (0.28 to 1.18) | |
| Volume depletionc | 0.39 | |||
| Baseline stage 4 CKD | 14 of 293 (4.8) | 15 of 331 (4.5) | 1.06 (0.50 to 2.24) | |
| Baseline stages 2/3 CKD | 113 of 1856 (6.1) | 75 of 1818 (4.1) | 1.51 (1.12 to 2.04) |
Stage 4 CKD, eGFR<30 ml/min per 1.73 m2; stages 2/3 CKD, eGFR≥30 ml/min per 1.73 m2. NC, not calculable; n, number with events; N, total number.
Includes death.
Surgical or spontaneous/nonsurgical amputation, excluding amputation due to trauma.
On the basis of predefined list of preferred terms.
AE with the following criteria confirmed by the investigator: (1) symptoms of severe impairment in consciousness or behavior; (2) need of external assistance; (3) intervention to treat hypoglycemia; (4) prompt recovery of acute symptoms after the intervention.
Discussion
In this prespecified subgroup analysis of the DAPA-CKD trial, we show beneficial effects of dapagliflozin on kidney and cardiovascular end points in patients with stage 4 CKD at baseline, similar in magnitude to the larger group of patients with stages 2/3 CKD, and significant attenuation of loss of kidney function as reflected by the eGFR slopes over time.
The first series of randomized cardiovascular outcome trials using SGLT2 inhibitors were primarily designed to meet regulatory requirements for ensuring cardiovascular safety in patients with type 2 diabetes according to the 2008 Guidance for Industry.12–14 These trials included relatively few patients with CKD at baseline, and very few participants developed kidney failure requiring dialysis or kidney transplantation, or died from kidney disease.23,24 Moreover, these studies were not primarily designed to assess the effects of SGLT2 inhibitors on kidney disease–related end points.
CREDENCE was the first SGLT2 inhibitor trial restricted to patients with CKD and with a primary end point that was kidney disease–related. The trial showed a 30% lower relative risk of the primary composite end point of kidney failure (defined as the provision of dialysis, kidney transplantation, or a sustained eGFR<15 ml/min per 1.73 m2), doubling of serum creatinine, or death from kidney or cardiovascular causes in patients randomized to canagliflozin.17 The mean eGFR in CREDENCE was 56.2 ml/min per 1.73 m2; 174 of 4401 (4%) randomized patients had stage 4 CKD at baseline. CREDENCE investigators recently reported effects of canagliflozin on eGFR slope in patients with stage 4 CKD.25 The reported placebo-adjusted difference in eGFR slope was 1.91 ml/min per 1.73 m2 per year (95% CI, 0.18 to 3.64), similar in magnitude to what we observed in DAPA-CKD.
Results presented here from DAPA-CKD extend the findings from CREDENCE by demonstrating safety and efficacy of dapagliflozin in a cohort of patients with stage 4 CKD more than 3.5-fold larger, and which included patients with CKD without type 2 diabetes. Moreover, we demonstrate numerically lower composite event rates, and a 32% lower rate of death from any cause, with benefits similar to those observed among patients with mild-to-moderate (stages 2/3) CKD, and no safety signals.
These results build upon findings reported by Dekkers et al. who demonstrated benefits of dapagliflozin relative to placebo on surrogate kidney and cardiovascular outcomes (including UACR and eGFR) in a pooled analysis of patients from 11 placebo-controlled trials with baseline eGFR 12 to <45 ml/min per 1.73 m2 (n=220).26 Among 136 (62%) patients with baseline UACR>30 mg/g, dapagliflozin 5 mg and 10 mg resulted in relative reductions in UACR of 47% (21% to 65%) and 38% (10% to 58%), respectively. Change in eGFR did not differ among groups. In addition, in the EMPagliflozin outcomE tRial in patients with chrOnic heaRt failure with reduced ejection fraction (EMPEROR-Reduced) trial, empagliflozin reduced the risks of cardiovascular death or heart failure hospitalization (the primary composite end point) and kidney failure or a sustained ≥40% decline in eGFR in 3730 patients with heart failure and reduced ejection fraction with and without CKD and type 2 diabetes.27 Two hundred and four patients had stage 4 CKD at baseline; patients with baseline eGFR as low as 20 ml/min per 1.73 m2 were enrolled. The effects of empagliflozin on cardiovascular and kidney end points did not differ significantly across five eGFR categories: >90, 60 to <90, 45 to <60, 30 to <45, and <30 ml/min per 1.73 m2.
That dapagliflozin and other SGLT2 inhibitors can be safely used in patients with advanced CKD offers clinicians a strategy to provide additional benefit in patients on RAAS inhibitors. Indeed, we found that the acute effects (baseline to 2 weeks) of dapagliflozin on eGFR were attenuated in patients with stage 4 CKD relative to those with stages 2/3 CKD (1.42 versus 2.56 ml/min per 1.73 m2 per 2 weeks). Although patients with more advanced (stage 5) CKD at baseline were not enrolled in DAPA-CKD, it is noteworthy that neither dapagliflozin nor placebo was discontinued when eGFR declined to <15 ml/min per 1.73 m2. Moreover, there was no increase in SAEs or AEs of special interest in patients with stage 4 CKD at baseline randomized to dapagliflozin, 30 (10%) of whom developed kidney failure during the trial.
There are several strengths to this analysis. Data were derived from a randomized trial and major kidney and cardiovascular events were adjudicated by an independent panel. Trial participants were diverse by age, sex, country of origin, and primary cause of kidney disease. Background therapy was excellent, with nearly all patients treated with RAAS inhibitors and other agents proven to reduce rates of cardiovascular disease. There are also several limitations. First, the trial was stopped early following a recommendation from the Independent Data Monitoring Committee. As a result, the trial accrued fewer than 75% of its anticipated number of events, and the precision of our estimated treatment effects in the stage 4 CKD subgroup was diminished. We did not collect eGFR after the completion of the trial, which might have increased the observed difference in eGFR slope between groups if a fraction of the initial decline observed in treated patients were reversible. Finally, the proportion of Black patients in DAPA-CKD was relatively low, although, in aggregate, the number and proportion of Black patients treated with dapagliflozin across multiple trials with type 2 diabetes, CKD, and heart failure is substantial,12,19,28 and a favorable benefit risk profile has been demonstrated across racial groups.
In summary, among patients with stage 4 CKD and albuminuria, with and without type 2 diabetes, the effects of dapagliflozin on reducing the risks of major kidney and cardiovascular events and attenuating progressive loss of eGFR are consistent with those observed in the trial overall, with no evidence of increased risks. Dapagliflozin should be considered part of the therapeutic armamentarium for patients with stage 4 CKD and albuminuria.
Disclosures
G.M. Chertow has received fees from AstraZeneca for the Dapagliflozin And Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial steering committee, research grants from Amgen, the National Institute of Diabetes and Digestive and Kidney Diseases, and National Institute of Allergy and Infectious Disease; he is on the board of directors for Satellite Healthcare, is Co-Editor of Brenner & Rector's The Kidney (Elsevier); has received fees for advisory boards for Ardelyx, Baxter, CloudCath, Cricket, DiaMedica, Durect, DxNow, Outset, and Reata; and holds stock options for Ardelyx, CloudCath, Durect, DxNow, Eliaz Therapeutics, Outset, Physiowave, and PuraCath; has received fees from Akebia, Amgen, Ardelyx, Baxter, Cricket, DiaMedica, Gilead, Miromatrix, Reata, Sanifit, Unicycive, and Vertex for trial steering committees; and has received fees for DSMB service from Angion, Bayer, and ReCor. R. Correa-Rotter has received fees from AstraZeneca for the DAPA-CKD trial steering committee; speaker fees from Amgen, Astra Zeneca, Boehringer Ingelheim, Janssen, and Takeda; research support from GlaxoSmithKline and Novo Nordisk; honoraria for advisory boards from Boehringer Ingelheim, Medtronic, and Novo Nordisk; Consultancy Agreements from Astra Zeneca, Boehringer Ingelheim, GSK, and Novo Nordisk; reports Scientific Advisor or Membership via National Leader Anemia Studies in Chronic Kidney Disease: Erythropoiesis Via a Novel Prolyl Hydroxylase Inhibitor Daprodustat study with GSK, and National Leader Effect of semaglutide versus placebo on the progression of renal impairment in subjects with T2DM and CKD (FLOW) study with Novo Nordisk; Editorial Board Current Opinion in Nephrology and Hypertension, Nefrologia Latinoamericana, and Revista de Investigación Clinica, and Associate Editor of Blood Purification; and Other Interests/Relationships as Member of the International Society of Nephrology, Member of the National Kidney Foundation, Member of the Mexican Institute for Research in Nephrology, Member of the Latin American Society of Nephrology and Hypertension, and Member of the European Dialysis and Transplant Association-European Renal Association. J.L. Gorriz has received honoraria for lectures from AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Janssen, MSD, Mundipharma, Novartis, and Novo Nordisk; and for advisory boards from AstraZeneca, Boehringer Ingelheim, Janssen-Mundipharma, and MSD. J. Gorriz also reports Consultancy Agreements with AstraZeneca, Boehringer, MSD, Mundipharma, and Novo Nordisk; and Research Funding from AstraZeneca. H.J.L. Heerspink has received support from AstraZeneca to his institution for the DAPA-CKD trial; fees to his institution for his participation in advisory boards for Janssen, Merck, Mitsubishi Tanabe, and Mundipharma; as a consultant for AbbVie, Bayer, Boehringer Ingelheim, Chinook, CSL Pharma, Dimerix Fresenius, Gilead, Janssen, Merck, Mitsubishi Tanabe, Mundi Pharma, Novo Nordisk, Retrophin, and Travere Pharmaceuticals; for participation in steering committees for Bayer, Chinook, CSL Pharma, Gilead, and Janssen; research support from AbbVie, AstraZeneca, Boehringer Ingelheim, and Janssen; and for Speakers Bureau from AstraZeneca. F.F. Hou has received honoraria from AstraZeneca as an executive member of the DAPA-CKD study; and has received honoraria from AbbVie for participation in a steering committee. F. Hou also reports Consultancy Agreements with AbbVie and AstraZeneca; Honoraria from AbbVie; and Member on the editorial board of Kidney International, Kidney Medicine, Current Opinion in Nephrology and Hypertension, and Kidney Disease (Basel). N. Jongs has no conflicts of interest to declare. J.J.V. McMurray has received support to his institution, Glasgow University, for work on clinical trials, consulting, and other activities: AbbVie, Alnylam, Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Cardurion, Cyclerion, Cytokinetics, DalCor, GSK, Ionis, KBP Biosciences, Kidney Research UK, Merck, Novartis, Pfizer, Servier, Theracos, and Vifor-Fresenius. He has received personal lecture fees: Abbott, Alkem Metabolics, Eris Lifesciences, Hickman, Lupin, Medscape/Heart.Org, ProAdWise Communications, Radcliffe Cardiology, Servier, Sun Pharmaceuticals, and The Corpus; Other Interests/Relationships as Director of Global Clinical TrialPartners Ltd (GCTP). P. Rossing has received fees to his institution for research support from AstraZeneca and Novo Nordisk; for steering group participation from AstraZeneca, Bayer, Gilead, and Novo Nordisk; for lectures from Bayer, Eli Lilly, and Novo Nordisk; for advisory boards from Boehringer Ingelheim and Sanofi; and reports Scientific Advisor or Membership with Bayer Astellas, MSD, and Mundipharma, with all honoraria to institution. C.D. Sjöström, B.V. Stefánsson, and A.M. Langkilde are employees and stockholders of AstraZeneca. R.D. Toto reports consultancy agreements with Amgen, Boehringer-Ingelheim, Relypsa, Bayer, AstraZeneca, Reata Pharmaceuticals, Otsuka, Medscape, ACI, and Vifor Pharma; honoraria from Relypsa, Akebia, ACI, Amgen, Bayer, Boehringer-Ingelheim, AstraZeneca, Reata Pharmaceuticals, Otsuka, Medscape, and Vifor; and scientific advisor or membership with Akebia, Amgen, Boehringer-Ingelheim, Relypsa, Bayer, AstraZeneca, Reata Pharmaceuticals, Otsuka, Medscape, ACI, and Vifor. P. Vart reports Scientific Advisor or Membership as Associate Editor for BMC Public Health. D.C. Wheeler provides ongoing consultancy services to AstraZeneca and has received honoraria and/or consultancy fees from Amgen, Astellas, Bayer, Boehringer Ingelheim, Gilead, GlaxoSmithKline, Janssen, Merck Sharp and Dohme, Mundipharma, Napp, Pharmacosmos, Reata, Tricida, Vifor Fresenius, and Zydus; Speakers Bureau from Amgen, Astellas, AstraZeneca, Janssen, Merck Sharp and Dohme, Mundipharma, Napp, and Vifor Fresenius; and Other Interests/Relationships as Honorary Professorial Fellow, George Institute for Global Health.
Funding
This work was supported by AstraZeneca.
Supplementary Material
Acknowledgments
The authors thank all investigators, trial teams, and patients for their participation in the trial. The authors would also like to acknowledge Parita Sheth and Nicola Truss, inScience Communications, London, UK, for assistance in editing and preparation of figures.
Glenn M. Chertow was involved in the study design, data collection, and analysis or interpretation of the data, and wrote the first draft of the manuscript. Hiddo J.L. Heerspink, David C. Wheeler, John J.V. McMurray, Fan Fan Hou, Ricardo Correa-Rotter, Peter Rossing, and Robert D. Toto are members of the study’s executive committee and were involved in the study design, data collection, and analysis or interpretation of the data. Niels Jongs and Priya Vart performed the data analyses. C. David Sjöström, Anna Maria Langkilde, and Bergur V. Stefánsson were involved in the study design, conduct of the study, and interpretation of data. Jose Luis Gorriz was involved in interpretation of the data. All authors reviewed the manuscript drafts, provided approval of the final version for submission, and take responsibility for the accuracy and integrity of the data.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021020167/-/DCSupplemental.
Supplemental Figure 1. Distribution of eGFR values at baseline in those with eGFR <30 ml/min per 1.73 m2.
Supplemental Figure 2. Forest plots for the prespecified and exploratory end points by baseline CKD stage.
Supplemental Figure 3. Forest plots for the post hoc exploratory end points by baseline CKD stage.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, et al. : Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Kurella M, Chertow GM, Luan J, Yaffe K: Cognitive impairment in chronic kidney disease. J Am Geriatr Soc 52: 1863–1869, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Madan P, Kalra OP, Agarwal S, Tandon OP: Cognitive impairment in chronic kidney disease. Nephrol Dial Transplant 22: 440–444, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Padilla J, Krasnoff J, Da Silva M, Hsu CY, Frassetto L, Johansen KL, et al. : Physical functioning in patients with chronic kidney disease. J Nephrol 21: 550–559, 2008 [PubMed] [Google Scholar]
- 6.Mujais SK, Story K, Brouillette J, Takano T, Soroka S, Franek C, et al. : Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol 4: 1293–1301, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, et al. : Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med 354: 131–140, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, et al. ; The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group: Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med 334: 939–945, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. ; RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, et al. : Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 354: 359–364, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Palmer BF: Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 351: 585–592, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. ; DECLARE–TIMI 58 Investigators: Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380: 347–357, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. ; CANVAS Program Collaborative Group: Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. ; EMPA-REG OUTCOME Investigators: Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Petrykiv S, Sjöström CD, Greasley PJ, Xu J, Persson F, Heerspink HJL: Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol 12: 751–759, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheen AJ: Pharmacokinetics, pharmacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease. Clin Pharmacokinet 54: 691–708, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. ; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Heerspink HJL, Stefansson BV, Chertow GM, Correa-Rotter R, Greene T, Hou FF, et al. ; DAPA-CKD Investigators: Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 35: 274–282, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. ; DAPA-CKD Trial Committees and Investigators: Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Wheeler DC, Stefansson BV, Batiushin M, Bilchenko O, Cherney DZI, Chertow GM, et al. : The Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) trial: baseline characteristics. Nephrol Dial Transplant 35: 1700–1711, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheeler DC, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, et al. ; DAPA-CKD Trial Committees and Investigators: Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 9: 22–31, 2021 [DOI] [PubMed] [Google Scholar]
- 22.McMurray JJV, Wheeler DC, Stefánsson BV, Jongs N, Postmus D, Correa-Rotter R, et al. ; DAPA-CKD Trial Committees and Investigators: Effect of dapagliflozin on clinical outcomes in patients with chronic kidney disease, with and without cardiovascular disease. Circulation 143: 438–448, 2021 [DOI] [PubMed] [Google Scholar]
- 23.Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. : Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 7: 606–617, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. ; EMPA-REG OUTCOME Investigators: Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323–334, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Bakris G, Oshima M, Mahaffey KW, Agarwal R, Cannon CP, Capuano G, et al. : Effects of canagliflozin in patients with baseline eGFR <30 ml/min per 1.73 m2: subgroup analysis of the randomized CREDENCE trial. Clin J Am Soc Nephrol 15: 1705–1714, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dekkers CCJ, Petrykiv S, Laverman GD, Cherney DZ, Gansevoort RT, Heerspink HJL: Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes Metab 20: 1988–1993, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. ; EMPEROR-Reduced Trial Investigators: Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383: 1413–1424, 2020 [DOI] [PubMed] [Google Scholar]
- 28.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. ; DAPA-HF Trial Committees and Investigators: Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381: 1995–2008, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


