Abstract
Objective: This meta-analysis aims to assess whether elevated De Ritis ratio is associated with poor prognosis in patients with coronavirus 2019 (COVID-19).
Methods: A systematic literature search was performed using PubMed, Embase, and EuropePMC databases up until September 17, 2021. De Ritis ratio is also known as Aspartate aminotransferase/alanine transaminase (AST/ALT) ratio. The main outcome was poor prognosis, a composite of mortality, severity, the need for ICU care, and intubation. The effect measure was odds ratios (ORs) and mean differences. We generated sensitivity and specificity, negative and positive likelihood ratio (NLR and PLR), diagnostic odds ratio (DOR), and area under curve (AUC).
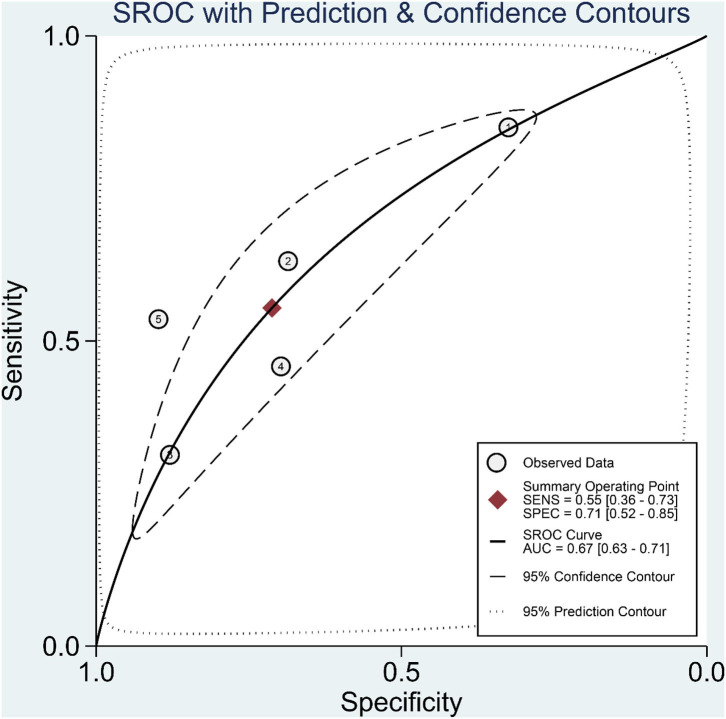
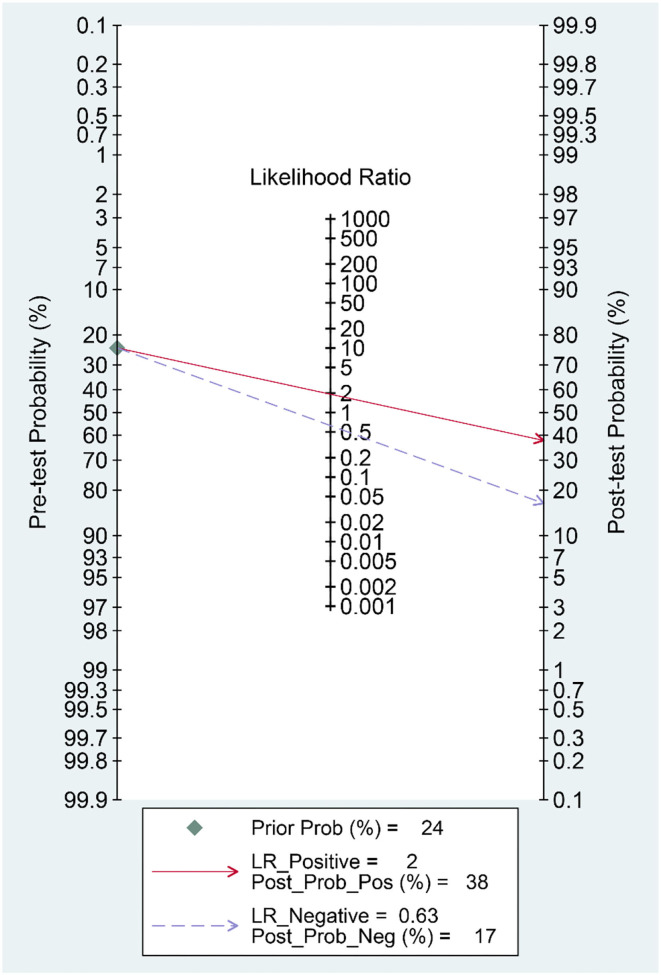
Results: There were eight studies with 4,606 patients. De Ritis ratio was elevated in 44% of the patients. Patients with poor prognosis have higher De Ritis ratio [mean difference 0.41 (0.31, 0.50), p < 0.001; I2: 81.0%] and subgroup analysis showed that non-survivors also have higher De Ritis Ratio [mean difference 0.47 (0.46, 0.48), p < 0.001; I2: 0%]. Elevated De Ritis ratio was associated with poor prognosis [OR 3.28 (2.39, 4.52), p < 0.001; I2: 35.8%]. It has a sensitivity of 55% (36–73), specificity of 71% (52–85), PLR 1.9, NLR.63, DOR of 3 (2–4), and AUC of.67 (0.63–0.71). The posterior probability of poor prognosis was 38% if De Ritis is elevated, while 17% if De Ritis is not elevated.
Conclusion: Elevated De Ritis ratio is associated with poor prognosis in patients with COVID-19.
Systematic Review Registration: PROSPERO ID: CRD42020216634.
Keywords: coronavirus—COVID-19, liver enzyme, transaminase, SARS-CoV-2, De Ritis ratio
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread rapidly and causes a considerable number of deaths worldwide (1). Although most patients with coronavirus 2019 disease (COVID-19) have mild-to-moderate symptoms, they may develop severe COVID-19 with multi-organ dysfunction, cardiorespiratory collapse, coagulopathy and thrombosis, sepsis, and even death (2, 3). Common symptoms include fever, cough and dyspnea, and minor symptoms are dysgeusia, anosmia, gastrointestinal symptoms, cutaneous manifestation, and headache (4–6). Although the virus primarily affects the lungs, it may invade and damage other organs, such as the heart and vasculature, coagulation system, liver, kidneys, intestine, and central nervous system (7–12).
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been reported to cause a varying degree of liver injury (13). Liver injury is more frequently found in patients with severe COVID-19 and is associated with an increased risk of poor outcomes (14). The ratio between the two most routinely requested liver function panel, the aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio or more commonly known as the De Ritis ratio, was recently reported as a possible biomarker for prognostication in patients with COVID-19 (15). Therefore, we conducted a systematic review and meta-analysis to evaluate the association between De Ritis ratio and composite poor outcomes in COVID-19.
Materials and Methods
The study was registered in the PROSPERO database (CRD42020216634) and was conducted per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Eligibility Criteria
Research articles (both prospective and retrospective cohorts) that contain information on De Ritis ratio and mortality, severity, intensive care unit (ICU) care admission or need for intubation were included in the study. We excluded preprints, review articles, editorial, commentaries, conference abstracts, letters, and case reports/series.
Search Strategy and Study Selection
We performed a systematic literature search from PubMed database, Embase database, and EuropePMC database with the search terms “COVID-19” OR “2019-nCoV” OR “SARS-CoV-2” AND “De Ritis Ratio” OR “AST ALT Ratio.” The search was finalized on September 17, 2021. The PubMed search strategy was [(COVID-19) OR (2019-nCoV) OR (SARS-CoV-2)] AND [(De Ritis Ratio) OR (AST ALT Ratio)]. Two independent authors performed the initial search and duplicate removal. The inclusion and exclusion criteria served as the basis for article exclusion during the title or abstract screening and evaluation of full-text articles.
Data Collection
Data extraction from the eligible studies was conducted by two authors who are independently using pre-built forms containing the author, study design, origin, AST, ALT, cut-off for elevated De Ritis ratio, sample size, age, gender, obesity, diabetes, elevated liver enzymes, and outcome of interests.
The main outcome was poor prognosis, a composite of mortality, severity, need for ICU care, and need for intubation. Mortality was defined as non-survivor or death.
Severity was defined according to the studies inclusion parameters, need for ICU care, and intubation. The effect measure was the odds ratios (ORs) and mean differences. Diagnostic meta-analysis was performed to generate diagnostic values, which consisted of sensitivity, specificity, negative and positive likelihood ratio (NLR and PLR), diagnostic odds ratio (DOR), and area under curve (AUC).
Risk of Bias Assessment
The risk of bias assessment was performed independently by two authors with the help of Newcastle-Ottawa Scale (NOS). Discrepancies were resolved by discussion. The Egger's test and Deek's funnel plot asymmetry test was used to assess the presence of small-study effects and publication bias, respectively.
Statistical Analysis
STATA 16 (College Station, TX) was used to perform statistical analysis. Meta-analysis of proportions was performed to pool the incidence of elevated De Ritis Ratio. DerSimonian and Laird method random-effects models were used to pool ORs and mean differences, notwithstanding heterogeneity. p < 0.05 were considered statistically significant. Inter-study heterogeneity was evaluated using the I-squared (I2) and Cochrane Q test, an I2 > 50% or p < 0.10 indicates substantial heterogeneity. We performed pooling of sensitivity and specificity and generated a summary receiver operating characteristic (SROC) curve. Relationship between prior probability and posterior probability was evaluated using Fagan's nomogram. Subgroup analysis was performed for mortality outcome.
Results
Baseline Characteristics
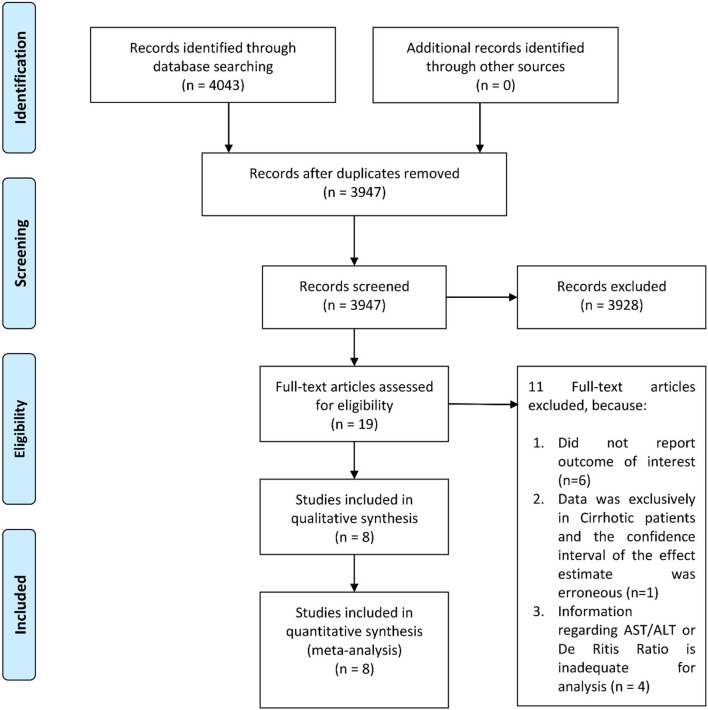
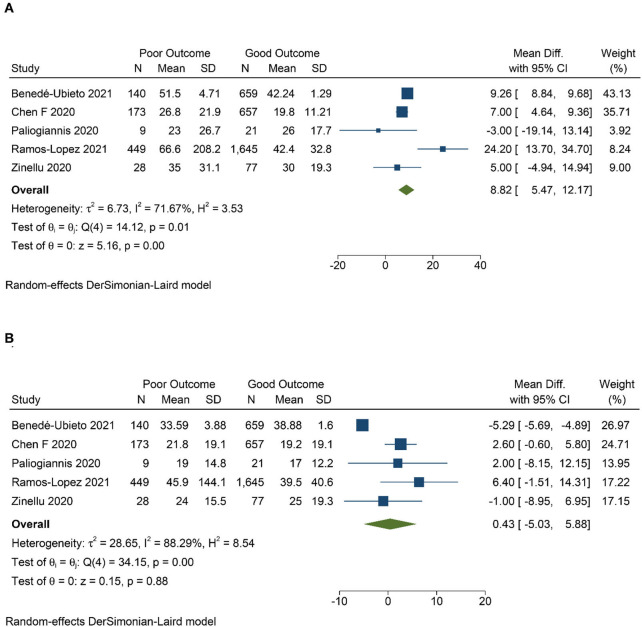
There were eight studies with 4,606 patients in this meta-analysis (Figure 1) (7, 16–19). The mean age of patients in this study was 64.3 years, whereas 46.3% of the patients were male. The characteristics of the studies are presented in Table 1. Patients with poor prognosis have higher AST levels [mean difference 8.82 (5.47, 12.17), p < 0.001; I2: 71.7%, p = 0.007] (Figure 2A), but not ALT levels [mean difference 0.43 (−5.03, 5.88), p = 0.878; I2: 88.3%, p < 0.001] (Figure 2B). De Ritis ratio was elevated in 24% of the patients. Poor prognosis occurs in 26% of the patients.
Figure 1.
PRISMA flowchart.
Table 1.
Characteristics of the included studies.
| Authors | Study design | Study origin | Cut-off for elevated De Ritis | Samples | Age (mean) | Male (%) | Obesity | Diabetes (%) | Elevated liver enzymes (%) | Outcome | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Benedé-Ubieto (20) | RC | Spain | NA | 799 | Stratified | 54.7 | NA | NA | NA | Mortality (17.5%) | 6 |
| Chen (18) | RC | China | >1 | 227 | 51 | 45.5 | NA | 9 | 4.5 | Mortality (11.8%) | 5 |
| Medetalibeyoglu (17) | RC | Turkey | >1 | 554 | 66.2 | 58.7 | BMI (29.39) | 22.7 | 153/554 (27.6) | Severity (13.9%), Mortality (7.2%) | 7 |
| Paliogiannis (19) | RC | Italy | NA | 60 | 71.5 | 60 | NA | 25 | NA | Mortality (30.0%) | 6 |
| Qin (16) | RC | China | >1.38 | 567 | 55 | 43.6 | NA | 15 | 103/567 (18.2) | Mortality (11.5%) | 8 |
| Ramos-Lopez (21) | RC | Spain | 1.29 | 2,094 | 66.9 | 39.4 | NA | NA | NA | Mortality + ICU (21.4%) | 8 |
| Yadlapati (22) | RC | United States | 1.2 | 200 | 66.5 | 45.5 | 50 | NA | 110/200 (55) | Mortality (26%) | 6 |
| Zinellu (7) | RC | Italy | >1.63 | 105 | 72 | 66.7 | 21.9 | 21 | 51/105 (48.6) | Mortality (26.7%) | 9 |
BMI, body mass index; NOS, newcastle-ottawa scale; RC, retrospective cohort.
Figure 2.
Mean difference in aspartate aminotransferase (AST) (A) and alanine transaminase (ALT) (B) level between poor and good prognosis.
Elevated De Ritis Ratio and Poor Prognosis
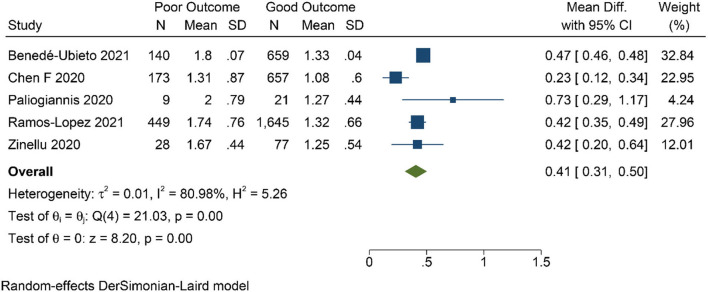
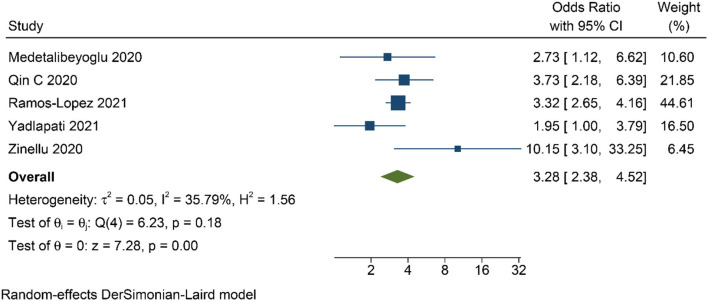
Patients with poor prognosis have higher De Ritis ratio [mean difference 0.41 (0.31, 0.50), p < 0.001; I2: 81.0%, p < 0.001] (Figure 3) and subgroup analysis showed that non-survivors also have higher De Ritis Ratio [mean difference 0.47 (0.46, 0.48), p < 0.001; I2: 0%, p = 0.463]. Elevated De Ritis ratio was associated with poor prognosis [OR 3.28 (2.39, 4.52), p < 0.001; I2: 35.8%, p = 0.182] (Figure 4) and subgroup analysis also showed that elevated De Ritis ratio was associated with mortality [OR 3.36 (1.93, 5.85), p < 0.001; I2: 51.7%, p = 0.102]. It has a sensitivity of 55% (36–73), specificity of 71% (52–85), PLR 1.9, NLR 0.63, DOR of 3 (2–4), and AUC of 0.67 (0.63–0.71) (Figure 5). The posterior probability of poor prognosis was 38% if De Ritis was elevated, while 17% if De Ritis was not elevated (Figure 6).
Figure 3.
Mean difference in De Ritis ratio between poor and good prognosis.
Figure 4.
Odds ratio for elevated De Ritis ratio and poor prognosis.
Figure 5.
Summary receiver operating characteristic (SROC) curve.
Figure 6.
Fagan's nomogram.
Risk of Bias Assessment
Newcastle-Ottawa Scale (NOS) indicates a low-moderate risk of bias (Table 1). There is no indication of small-study effects in the relationship between elevated De Ritis ratio and poor prognosis (p = 0.488). Deek's funnel plot asymmetry test was non-significant (p = 0.81).
Discussion
Early identification of patients at risk for developing severe COVID-19 is crucial during the pandemic. Previous studies highlighted that individuals with advanced age, high body mass index, and physical inactivity had greater morbidity and mortality from COVID-19, along with the presence of various comorbidities, such as cardiovascular disease, diabetes, chronic obstructive pulmonary disease, hypertension, and chronic kidney disease (23–31). Several inflammatory parameters, comprising C-reactive protein, D-dimer, procalcitonin, interleukin-6, and ferritin, are often higher in patients with severe and critically ill with COVID-19 (8). An increase in liver-related biomarkers, particularly AST, ALT, total bilirubin concentrations, and gamma-glutamyl transferase in patients with COVID-19 have been reported (32, 33).
Although hepatic damage is not commonly seen as a major characteristic of COVID-19, liver injury is an emerging concern because it may indicate a severe disease course (2). The mechanism for liver involvement in COVID-19 remains obscure. Previous liver pathology reports showed the presence of moderate microvesicular steatosis along with mild inflammation in several areas (34). These patterns are also observed in drug-induced liver injury and sepsis, although these findings are not unique, they might provide insight into the mechanism involved in liver injury induced by COVID-19 (35). The SARS-CoV-2 may invade the liver directly through the angiotensin receptor enzyme 2 (ACE2) receptor, which serves as the novel coronavirus' entry point. It has been found that bile duct epithelial cells (cholangiocytes) express a high amount of ACE2 receptors (36). Liver dysfunction may also be caused by drug-induced liver injury or an overactive inflammatory response, including cytokine storm and pneumonia-associated hypoxia (2, 7). Antivirals used in the treatment of COVID-19 are postulated to cause drug-induced liver injury (37).
Serum concentrations of ALT and AST, without exception, are the most frequently ordered liver panel for evaluating liver injury in all laboratories. ALT is present in the cytosol of hepatocytes, while AST is present in the cytoplasm and mitochondria of the hepatocyte (38). ALT activity in the liver is ~10-fold higher than that of the heart and skeletal muscles, which emphasizes its function to indicate parenchymal liver disease or injury. Meanwhile, AST has the greatest activity in the liver, cardiac, and skeletal muscle, but also exhibits in other tissues including kidneys, pulmonary, brain, pancreas, red blood cells, and white blood cells. Therefore, ALT is a more specific biomarker for liver damage compared to AST, indicating liver-biliary disease, myocardial injury, and rhabdomyolysis (7, 15). AST and ALT are found in the liver with a 2.5:1 ratio but with different turnaround time, resulting in a relatively similar level of serum of AST and ALT in healthy populations (38).
The De Ritis ratio or the AST/ALT ratio is a promising biochemical parameter for prognostication in COVID-19. In the present study, elevated De Ritis ratio was associated with 3-fold increased risk for poor prognosis in patients with COVID-19. Although the cut-off values for elevated De Ritis ratio are different from these five studies (Table 1), the result of this meta-analysis has low heterogeneity (I2: 35.8%). Nonetheless, the difference in the cut-off value used between those studies caused a highly varied diagnostic value (Figure 3) with an overall sensitivity of 55%, specificity of 71%, and AUC of 0.67. These variations further translate into the uncertainty of the optimal cut-off value for De Ritis Ratio as a prognostic factor in COVID-19 and merit further investigations.
Interestingly, Qin et al. indicated that De Ritis ratio of ≥1.38 was independently associated with poor prognosis irrespective of AST elevation (≤40 and >40 U/L) (16). They showed that AST/ALT ratio elevation was associated with a more severely computed tomography scan findings, higher severity, and positive linear association with other prognostic markers (e.g., c-reactive protein, procalcitonin, interleukin-6, D-Dimer, lactate, LDH, and creatine Kinase-MB). Additionally, Chen et al. showed the association of AST/ALT ratio with liver injury and severity of COVID-19. However, the number of outcomes or risk estimates (e.g., OR) of this study interest was not available (18).
There were two studies on the association of De Ritis ratio with other specific biochemical parameters (e.g., creatinine kinase and serum ALT), but were excluded from the analysis due to its irrelevance with our outcome of interest (15, 39).
The limitations of the current study were primarily caused by the small quantity of the included studies. Moreover, the retrospective-observational nature and the small sample size of the included studies should be taken into account in extrapolating the results of this meta-analysis, where selection bias and confounding factors may be evident. We also could not dismiss the possibility of publication bias due to the small number of studies. Despite our limitations, this meta-analysis has brought early evidence of using the De Ritis ratio for prognostication in COVID-19.
Implication for Clinical Practice
Although this “traditional” ratio was initially found in 1957 as a diagnostic test for viral hepatitis (40), it is still commonly used and proves to be a valuable indicator of liver disease (38). It is a promising, straightforward, and readily available parameter for poor prognosis in COVID-19. This meta-analysis showed that AST, but not ALT, was significantly associated with poor prognosis in COVID-19. This supports the use of De Ritis ratio in addition to AST and ALT levels. However, we suggest, including this parameter and other accessible hematological markers, to improve the prognostic performance of the model for COVID-19. De Ritis ratio would be better for this marker to be a part of a prognostic model rather than a stand-alone examination. A predictive model comprising of readily available tools may be of value, especially in rural areas where sophisticated prognostic biomarkers are often not available.
In conclusion, elevated De Ritis ratio is associated with poor prognosis in patients with COVID-19.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
RP: conceptualization, methodology, formal analysis, investigation, and writing—original draft. IH: data curation, investigation, writing—original draft, and project administration. ML: data curation, investigation, and writing—original draft. EY and RV: investigation and writing—original draft. AL, SN, BS, and RK: investigation and writing—review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
Abbreviations
- ACE2
Angiotensin receptor enzyme 2
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- AUC
Area under curve
- COVID-19
Coronavirus disease 2019
- DOR
Diagnostic odds ratio
- OR
Odds ratio
- PLR
Positive likelihood ratio
- NLR
Negative likelihood ratio
- SARS-CoV-2
Severe cute respiratory syndrome coronavirus 2.
References
- 1.Livingston E, Bucher K, Rekito A. Coronavirus Disease 2019 and Influenza 2019-2020. JAMA. (2020) 323:1122. 10.1001/jama.2020.2633 [DOI] [PubMed] [Google Scholar]
- 2.Lim MA, Pranata R, Huang I, Yonas E, Soeroto AY, Supriyadi R. multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Heal Dis. (2020) 7:2054358120938573. 10.1177/2054358120938573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wijaya I, Andhika R, Huang I. The use of therapeutic-dose anticoagulation and its effect on mortality in patients with COVID-19: a systematic review. Clin Appl Thromb. (2020) 26:1076029620960797. 10.1177/1076029620960797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck. (2020) 42:1252–8. 10.1002/hed.26204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Vito A, Geremia N, Fiore V, Princic E, Babudieri S, Madeddu G. Clinical features, laboratory findings and predictors of death in hospitalized patients with COVID-19 in Sardinia, Italy. Eur Rev Med Pharmacol Sci. (2020) 24:7861–8. 10.26355/eurrev_202007_22291 [DOI] [PubMed] [Google Scholar]
- 6.De Vito A, Fiore V, Princic E, Geremia N, Napodano CMP, Muredda AA, et al. Predictors of infection, symptoms development, and mortality in people with SARS-CoV-2 living in retirement nursing homes. PLoS ONE. (2021) 16:e0248009. 10.1371/journal.pone.0248009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zinellu A, Arru F, De Vito A, Sassu A, Valdes G, Scano V, et al. The De Ritis ratio as prognostic biomarker of in-hospital mortality in COVID-19 patients. Eur J Clin Invest. (2021) 51:1–8. 10.1111/eci.13427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. (2020) 14:175346662093717. 10.1177/1753466620937175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wibowo A, Pranata R, Lim MA, Akbar MR, Martha JW. Endotheliopathy marked by high von Willebrand Factor (vWF) antigen in COVID-19 is associated with poor outcome: a systematic review and meta-analysis. Int J Infect Dis. (2021). 10.1016/j.ijid.2021.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wibowo A, Pranata R, Astuti A, Tiksnadi BB, Martanto E, Martha JW, et al. Left and right ventricular longitudinal strains are associated with poor outcome in COVID-19: a systematic review and meta-analysis. J Intensive Care. (2021) 9:9. 10.1186/s40560-020-00519-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martha JW, Pranata R, Wibowo A, Lim MA. Tricuspid annular plane systolic excursion (TAPSE) measured by echocardiography and mortality in COVID-19: a systematic review and meta-analysis. Int J Infect Dis. (2021) 105:351–6. 10.1016/j.ijid.2021.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wibowo A, Pranata R, Akbar MR, Purnomowati A, Martha JW. Prognostic performance of troponin in COVID-19: a diagnostic meta-analysis and meta-regression. Int J Infect Dis. (2021) 105:312–8. 10.1016/j.ijid.2021.02.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kukla M, Skonieczna-Zydecka K, Kotfis K, Maciejewska D, Łoniewski I, Lara LF, et al. COVID-19, MERS and SARS with concomitant liver injury—systematic review of the existing literature. J Clin Med. (2020) 9:1420. 10.3390/jcm9051420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pranata R, Yonas E, Huang I, Lim MA, Nasution SA, Kuswardhani RAT. Fibrosis-4 index and mortality in coronavirus disease 2019. Eur J Gastroenterol Hepatol. (2021). 10.1097/MEG.0000000000002091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yazar H, Kayacan Y, Ozdin M. De Ritis ratio and biochemical parameters in COVID-19 patients. Arch Physiol Biochem. (2020) 1–5. 10.1080/13813455.2020.1788604 [DOI] [PubMed] [Google Scholar]
- 16.Qin C, Wei Y, Lyu X, Zhao B, Feng Y, Li T, et al. High aspartate aminotransferase to alanine aminotransferase ratio on admission as risk factor for poor prognosis in COVID-19 patients. Sci Rep. (2020) 10:16496. 10.1038/s41598-020-73575-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medetalibeyoglu A, Catma Y, Senkal N, Ormeci A, Cavus B, Kose M, et al. The effect of liver test abnormalities on the prognosis of COVID-19. Ann Hepatol. (2020) 19:614–21. 10.1016/j.aohep.2020.08.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen F, Chen W, Chen J, Xu D, Xie W, Wang X, et al. Clinical features and risk factors of COVID-19-associated liver injury and function: a retrospective analysis of 830 cases. Ann Hepatol. (2021) 21:100267. 10.1016/j.aohep.2020.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paliogiannis P, Zinellu A, Scano V, Mulas G, de Riu G, Pascale RM, et al. Laboratory test alterations in patients with COVID-19 and non COVID-19 interstitial pneumonia: a preliminary report. J Infect Dev Ctries. (2020) 14:685–90. 10.3855/jidc.12879 [DOI] [PubMed] [Google Scholar]
- 20.Benedé-Ubieto R, Estévez-Vázquez O, Flores-Perojo V, et al. Abnormal liver function test in patients infected with coronavirus (SARS-CoV-2): a retrospective single-center study from Spain. J Clin Med. (2021) 10:1039. 10.3390/jcm10051039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramos-Lopez O, San-Cristobal R, Martinez-Urbistondo D, et al. Proinflammatory and hepatic features related to morbidity and fatal outcomes in COVID-19 patients. J Clin Med. (2021) 10:3112. 10.3390/jcm10143112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yadlapati S. Prevailing patterns of liver enzymes in patients with COVID-19 infection and association with clinical outcomes. Ann Gastroenterol. (2021). 10.20524/aog.2021.0573. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andhika R, Huang I, Wijaya I. Severity of COVID-19 in end-stage kidney disease patients on chronic dialysis. Ther Apher Dial. (2020) 25:706–9. 10.1111/1744-9987.13597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression: diabetes and COVID-19. Diabetes Metab Syndr Clin Res Rev. (2020) 14:395–403. 10.1016/j.dsx.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin-Angiotensin-Aldosterone Syst. (2020) 21:147032032092689. 10.1177/1470320320926899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pranata R, Huang I, Lim MA, Wahjoepramono EJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19–systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. (2020) 29:104949. 10.1016/j.jstrokecerebrovasdis.2020.104949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pranata R, Soeroto AY, Huang I, Lim MA, Santoso P, Permana H, et al. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tuberc Lung Dis. (2020) 24:838–43. 10.5588/ijtld.20.0278 [DOI] [PubMed] [Google Scholar]
- 28.Pranata R, Lim MA, Yonas E, Vania R, Lukito AA, Siswanto BB, et al. Body mass index and outcome in patients with COVID-19: a dose–response meta-analysis. Diabetes Metab. (2021) 47:101178. 10.1016/j.diabet.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yonas E, Alwi I, Pranata R, Huang I, Lim MA, Gutierrez EJ, et al. Effect of heart failure on the outcome of COVID-19 — a meta analysis and systematic review. Am J Emerg Med. (2020) 46:204–11. 10.1016/j.ajem.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pranata R, Supriyadi R, Huang I, Permana H, Lim MA, Yonas E, et al. The association between chronic kidney disease and new onset renal replacement therapy on the outcome of COVID-19 patients: a meta-analysis. Clin Med Insights Circ Respir Pulm Med. (2020) 14:1179548420959165. 10.1177/1179548420959165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pranata R, Henrina J, Raffaello WM, Lawrensia S, Huang I. Diabetes and COVID-19: the past, the present, and the future. Metabolism. (2021) 121:154814. 10.1016/j.metabol.2021.154814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paliogiannis P, Zinellu A. Bilirubin levels in patients with mild and severe Covid-19: a pooled analysis. Liver Int. (2020) 40:1787–8. 10.1111/liv.14477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. (2020) 8:420–2. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: the current evidence. United Eur Gastroenterol J. (2020) 8:509–19. 10.1177/2050640620924157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. (2020). 10.1101/2020.02.03.931766 [DOI] [Google Scholar]
- 37.Ma C, Cong Y, Zhang H. COVID-19 and the digestive system. Am J Gastroenterol. (2020) 115:1003–6. 10.14309/ajg.0000000000000691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. (2013) 34:117–30. [PMC free article] [PubMed] [Google Scholar]
- 39.Safari R, Gholizadeh P, Marofi P, Zeinalzadeh E, Pagliano P, Ganbarov K, et al. Alteration of liver biomarkers in patients with SARS-CoV-2 (COVID-19). J Inflamm Res. (2020) 13:285–92. 10.2147/JIR.S257078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Ritis F, Coltorti M, Giusti G. An enzymic test for the diagnosis of viral hepatitis: the transaminase serum activities. Clin Chim Acta. (1957) 2:70–4. 10.1016/0009-8981(57)90027-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.