SUMMARY
The human body is full of an extensive number of commensal microbes, consisting of bacteria, viruses, and fungi, collectively termed the human microbiome. The initial acquisition of microbiota occurs from both the external and maternal environments, and the vast majority of them colonize the gastrointestinal tract (GIT). These microbial communities play a central role in the maturation and development of the immune system, the central nervous system, and the GIT system and are also responsible for essential metabolic pathways. Various factors, including host genetic predisposition, environmental factors, lifestyle, diet, antibiotic or nonantibiotic drug use, etc., affect the composition of the gut microbiota. Recent publications have highlighted that an imbalance in the gut microflora, known as dysbiosis, is associated with the onset and progression of neurological disorders. Moreover, characterization of the microbiome-host cross talk pathways provides insight into novel therapeutic strategies. Novel preclinical and clinical research on interventions related to the gut microbiome for treating neurological conditions, including autism spectrum disorders, Parkinson’s disease, schizophrenia, multiple sclerosis, Alzheimer’s disease, epilepsy, and stroke, hold significant promise. This review aims to present a comprehensive overview of the potential involvement of the human gut microbiome in the pathogenesis of neurological disorders, with a particular emphasis on the potential of microbe-based therapies and/or diagnostic microbial biomarkers. This review also discusses the potential health benefits of the administration of probiotics, prebiotics, postbiotics, and synbiotics and fecal microbiota transplantation in neurological disorders.
KEYWORDS: fecal-microbiota transplantation, gut microbiota, neurodegenerative disorders, neuropsychiatric disorders, probiotic
INTRODUCTION
Microorganisms have inhabited the Earth for billions of years, and these organisms can be found in almost every habitat in nature. Abundant and diverse microbial communities also coexist in the bodies of host organisms, including humans. Bacteria and their hosts constantly exchange genes and metabolites. Under the microscopic world of host-microbe interactions, microbes harbor many metabolic pathways, exchange signals, cross talk with the host, mediate different complicated pathways, and are significant drivers of host evolution (1–3). There is no exception for the human body to be occupied by ample and different microbial inhabitants. Most of the microbes inhabiting humans, including bacteria, viruses, fungi, and archaea, reside in the human gastrointestinal tract (GIT) and are termed “gut microbiota” (4). Microbiome investigations have accelerated at a remarkable pace in the last 2 decades, demonstrating the significant number of ways that these symbiotic microflorae influence the daily life of human beings. It has been found that the gut microbiome has implications in the host physiology, both in healthy and in diseased conditions, and can be considered a central regulator of host pathology (5). Advancements in DNA sequencing and microbiome bioinformatics have led to a less expensive but more sophisticated structural and functional microbiota analysis. Intriguingly, 99% of the genetic information of human body are microbial, numbering over 100 million genes. Considering the human-microbiota coevolution, the microbiome plays a pivotal part in the development and programming of all body systems (6, 7). Despite our parental genome, which is consistent in the duration of the host lifetime, the microbiota is dynamic, diverse, and capable of external input, highlighting its capacity as a novel target for clinical/therapeutic intervention (8–10). The gut microbiome plays an essential role in several physiological processes, such as maintenance of homeostasis, immunomodulation, and regulation of the central system (CNS) and enteric nervous system (ENS) (11–14). Furthermore, studies in mouse models have also indicated the microbiota’s pivotal role in central neuroinflammation, neurodevelopment, mood, and behavior (15). Dysbiosis of the gut microbial communities is particularly associated with a variety of central nervous system (CNS) disorders. Increasing evidence suggests that there is a bidirectional gut-brain communication system between CNS, microflora, and the gut: i.e., the microbiota-gut-brain axis (MGBA) (16, 17), Alzheimer’s disease (AD) (18), multiple sclerosis (MS) (19), Parkinson’s disease (PD) (20, 21), and schizophrenia (SCZ) (22). In particular, reducing the number of bifidobacteria was reported in individuals with AD (23). In addition, anti-inflammatory bacteria, such as the bacterial genera Coprococcus, Roseburia, and Blautia spp., were substantially less abundant in stool samples from subjects with PD (24). According to another study on children suffering from autism spectrum disorder (ASD), the relative numbers of bacteria and overall diversity are significantly reduced (25). In the present review, we discuss the association of the gut microbiome and the CNS functions relating to neurological disorders (Fig. 1).
FIG 1.
Association of gut microbiome and CNS functions in neurological diseases.
MICROBIOME-GUT-BRAIN AXIS
Microbiome Development
The initial colonization of microbes in the human body occurs at birth, when fetal exposure to the maternal microbiota happens in the uterus. Several studies indicate that vaginal or C-section delivery influences the seeding process of the neonatal microbiome profiles, causing definite differences in their microbiome (26). Other factors in the first stages of life, including the maternal-to-fetal exchange of microbes during delivery, prematurity, breastfeeding, host genetics, environment, maternal infection, obesity, and stress, as well as antibiotic and nonantibiotic exposure, can significantly disturb the microbiome profiles of the newborns (27). The composition of microbes inhabiting the GIT is relatively stable throughout life and is unique to every individual.
Several investigations on the metabolic activity of GIT microflora and the dynamic cross-talks of microbe-host interactions revealed the necessity of these microbial communities in maintaining the host homeostasis and health (28). In other words, the complex biochemical signaling between the GIT and the brain, known as the gut-brain axis (GBA), is mediated by the CNS, ENS, and gastrointestinal (GI) microbiota. Recent studies on GBA have shown the importance of intestinal microbiota involvement in these bidirectional interactions, namely, within the brain to GI microbiota and vice versa, via neural, immune, humoral, and endocrine links (29). Research on the GBA has gained remarkable consideration over the past several years, since the imbalances in the GI microflora can impact the brain’s physiology, cognition, and also behavior (30–34). The human GI microbiota contains more than 100 bacterial species (35, 36), including two primary bacterial phylotypes, i.e., Firmicutes and Bacteroidetes, and fewer Actinomyces, Fusobacterium, Proteobacteria, and Verrucomicrobia (37). Various elements such as aging, infection, drugs, diseases, and nutrition may influence the microbiota and human health (38, 39).
Neuronal Pathways for Gut-Brain Axis Interactions
There are two neuroanatomical pathways by which the gut communicates with the brain. First, the brain and gut communicate directly via the vagus nerve (VN) and the autonomic nervous system (ANS) in the spinal cord. Second, the bicommunication ENS of the GIT, which, in addition to ANS and VN, leads to the bilateral interaction within the gut and brain (40). Bacteria build a direct neural connection between the brain and GI microflora through the VN and stimulation of ENS afferent neurons (41). Moreover, vagal activation represents anti-inflammatory effects and many positive impacts on the gut microbiota, as well as the probiotic stem, from the vagal activity (40). Several preclinical investigations have revealed that the pathophysiology and pathogenesis of intestinal disorders, including inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS), in addition to neurological diseases and psychiatric conditions, including anxiety, depression, ASD, AD, MS, and PD, have implications with imbalance of the gut microbial communities or “dysbiosis” in the gut microbiota (Fig. 2) (42–49).
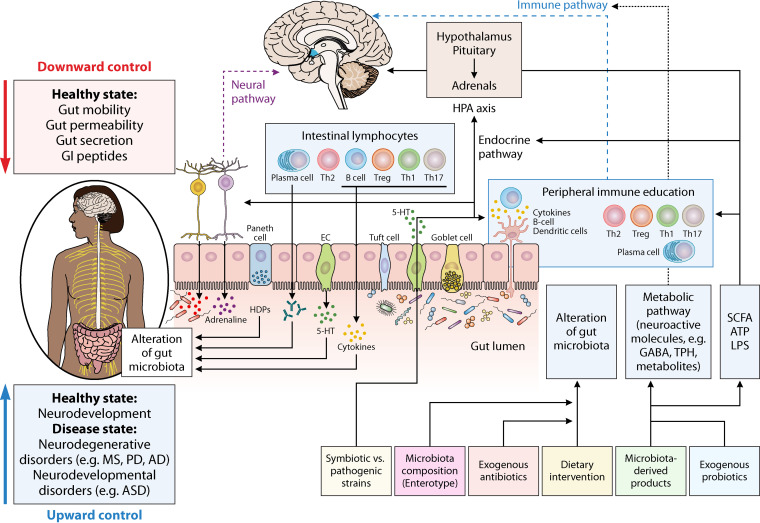
FIG 2.
Molecular communication pathways among the microbiota and the brain via the gut-brain axis (GBA). Several direct including, vagus nerve, and indirect pathways, such as cytokines, SCFA, and essential dietary amino acids (e.g., tyrosine, histidine, and tryptophan), have roles in modulation of the gut-brain axis by gut microbiota. The gut-brain axis is comprised of the immune pathway (including cytokines); microbial metabolites; the neuroactive pathway, such as neuroactive metabolites and neurotransmitters; and the neural pathway (spinal nerves, enteric nervous system, and vagus nerve); the endocrine pathway; and the hypothalamic-pituitary-adrenal axis. Microbes residing in gastrointestinal tract are capable of neurotransmitters synthesis, including GABA, serotonin, dopamine, and noradrenaline, locally playing an essential part of the cross talk between the host and the microbiome. Bacterial neuroactive metabolites and dietary molecules can alter the brain and behavior in several ways that are still being discovered, such as influencing epithelial cells to affect the function of the epithelial barrier, hormone release from enteroendocrine cells, and modulation of microglial and immune cells functions through dendritic cells. Abbreviations: AD, Alzheimer’s disease; ASD, autism spectrum disorder; MS, multiple sclerosis; PD, Parkinson’s disease; HDP, host defense proteins; EC, enterochromaffin cells; 5-HT, 5-hydroxytryptamine (serotonin); SCFAs, short-chain fatty acids; GABA, γ-aminobutyric acid; LPS, lipopolysaccharide; TPH, tryptophan hydroxylase.
Chemical Signaling between the Gut and the Brain
Since there are several interactions within the microbiome-gut-brain axis (MGBA) via multiple mechanisms (38, 50–52), the GI microbiome communicates with the CNS is predominantly through the immune-related, neural, endocrine, and metabolic signaling pathways (53). Using neurotransmitters such as dopamine, γ-aminobutyric acid (GABA), serotonin or 5-hydroxytryptamine (5-HT), neuropeptides, hormones (such as secretion of corticotrophin-releasing hormone in the hypothalamic-pituitary-adrenal [HPA] axis), and short-chain fatty acids (SCFAs), the brain and the residing microbes in the gut communicate with each other (Fig. 2) (50, 54–56). Moreover, the gut microbiota has an impact on the regulation of neurotransmitter secretion, such as serotonin. For instance, Bifidobacterium infantis affects central serotonin transmission by increasing plasma tryptophan, a precursor to serotonin levels (57). It has also been reported that several neurotransmitters, including acetylcholine, dopamine, noradrenaline, and serotonin are synthesized by different bacterial species (58, 59). Microbiota-derived metabolites, including vitamins, neuroactive metabolites (e.g., SCFAs), and neurotransmitters, mediate bidirectional MGBA interactions to modulate host neurophysiology and immunity (42, 60, 61). How these microbial products influence brain function still needs to be elucidated, since blood-brain barrier (BBB) and several feedback loops inhibit direct access to the brain. Accordingly, they exert influence directly following their transportation across the BBB or indirectly via neuroendocrine, immune, or vagal pathways (61–64).
MGBA in the Context of Psychiatry
As previously described, the intestinal bacterial species can interact reciprocally with the CNS via several mechanisms, such as alteration of the microbial population (65), stimulation of the immune system (66), and through neural pathways (67) and tryptophan metabolic pathways (68, 69) and via microbial metabolites (70). The GI microflora can affect the CNS by synthesizing or mimicking different neuroactive compounds, such as serotonin, melatonin, histamine, GABA, acetylcholine, and catecholamines (71). Moreover, the SCFA products of microbial carbohydrate fermentation can also influence the CNS by entering the systemic circulation (72). Furthermore, the GI microbiota produces several proteins and neurotrophins, such as postsynaptic density protein 95 (PSD-95), synaptophysin, and brain-derived neurotrophic factor (BDNF) (67). It has been reported that several other factors can affect the MGBA, including the intestinal microbiota, permeable intestine, and hypersensitivity to food antigens (73). The VN can transfer endocrine, neuronal, and microbial alterations in the GIT to the brain (74). The gut epithelial lining includes ENS neuron endings, which can sense and respond directly to the luminal contents or indirectly to the neurochemicals synthesized by the GI microflora or enteroendocrine cells, in addition to their communication through the VN. Antibiotics and nonantibiotic drugs, GI neuromodulators/neurotransmitters, infectious or noninfectious agents, essential metabolites, and sensory vagal neurons altogether transfer the required information about the intestinal state to the CNS (75). Thus, various afferent and efferent pathways are involved in the functioning of the GBA. Through the bidirectional connection in the MGBA, the HPA, the CNS regulatory areas of satiety, and the neuropeptides synthesized by sensory neurons can influence GI microfloral composition. These interactions can affect the pathogenesis of several diseases where inflammation is mainly involved, such as ASD and ADHD, as well as mood and anxiety disorders (76, 77). Chrobak et al. (78) reported that chronic inflammation might play a substantial role in the etiopathology of major depressive disorder (MDD), and the dysregulation of homeostasis of intestinal bacterial species may give rise to such inflammation, suggesting a central role of the GI microflora in affecting brain development, mood, and behavior. These authors concluded that physiological and emotional stress can affect gut microbiome composition (79). Jacka et al. also reported that dietary interventions hold significant promise as a potential target for improving psychiatric disorders through modulation of the intestinal microbiome (80, 81). Several neuropsychiatric and behavioral disorders are correlated with responsive stress conditions, including chronic stress, dysregulated HPA axis stress response, and individual coping skills and strategies and resilience to stress, which are now found to be potentially modifiable targets through modulation of the gut microbiome. Through bidirectional interactions between the GI microflora and other environmental risk factors, including stress and diet, it has been proposed that targeting the gut microbiome may impact the prevention and treatment of mental and behavioral disorders. However, this subject still needs further investigation.
Gut Microbiome and Neurodevelopment
Brain development is an intricate process that generally begins in the third gestational week and continues through late adolescence (82). Various factors can affect brain development, among which the role of GI microflora has been recently discovered. Recently, the impact of the gut microbiome in behavioral modulation has been demonstrated in both rodents and humans (43). In addition, there have been many studies on the correlation between a healthy microbiome and appropriate development of neural systems and neural circuits (83). Over 100 trillion microbes inhabit the GIT (84). Different factors, such as modes of delivery, the type of feeding, the mother’s diet, and antibiotic and nonantibiotic drug administrations, have a significant influence on the infant gut microbiome composition and its maturation (85). The first 3 years of life are considered the central period for the formation of GI microbiota and brain synaptogenesis (86). Moreover, investigations on germfree (GF) mouse models have suggested a correlation between GI microflora, behavioral performance, and brain functions. Stress responses were much more intense than the specific pathogen-free (SPF) mice (87). Moreover, it has been reported that the reduced levels of synaptogenesis markers in the GF-mouse models, including synaptophysin (88, 89) and PSD95, which are responsible for synaptic maturation, respectively (90, 91), highlight the importance of commensal bacteria in the development of the brain. Based on these results, the gut microflora appears to have a central role in forming the neural networks during brain development. As previously stated, there is an association between CNS functions and the GIT, known as the cross talk between the brain and the gut, interacting with the VN, the ENS, the immune system, and the circulation (85). The GI microbiome can influence the nervous system, whether by direct input transmissions into the brain via the VN or by indirectly activating the entire GI tract’s ENS (92). However, the molecular mechanisms underlying the behavioral traits influenced by probiotic administration throughout the VN have not been fully elucidated, and no operational alteration in the neuronal pathways of these particular regions was considered. Remarkably, administration of Lactobacillus rhamnosus (JB-1) resulted in decreased anxiety and corticosterone levels, as well as upregulation of GABA receptors in the brains of BALB/c mouse models. In addition, the absence of similar outcomes in vagotomized animal models supports the involvement of vagal nerve in MGBA that can be affected by certain probiotics (93). Indeed, investigations on healthy adults with the minor allele rs16944 as a risk factor increasing the interleukin-1β (IL-1β) production revealed that the treatment with probiotics could alleviate anxiety symptoms, suggesting the application of psychobiotics in personalized anxiety disorder therapy (94). It should be noted that some of the behavioral phenotypes influenced by gut-residing commensals are independent of the ANS and the VN. Accordingly, it was concluded that the gut flora induces central levels of neurotrophic factors of the brain, as well as affecting behavior, in mice, independent of the ANS and GI neurotransmitters (95). Gut microorganisms can exchange sensory information with the host via the production of a large number of metabolites, including the neurotransmitters, GABA, serotonin, dopamine, and noradrenaline, as well as several vitamins and SCFAs, in the gut lumen (96). Subsequently, some of these molecules can pass through the BBB, get to the brain, and influence the neuronal circuits. Among these metabolites, SCFA, the main metabolite produced from dietary fiber (DF) fermentation by colonic bacteria, performs critical roles in regulating neuroimmunoendocrine, metabolic homeostatic, infectious, and inflammatory functions (68, 97). SCFA is exploited as an essential energy source by neurons and glial cells in the CNS, contributing to brain development. Studies indicate that butyrate and propionate act as histone deacetylase (HDAC) inhibitors and ligands for a subset of G protein-coupled receptors affecting gene expression and host epigenome (98). Moreover, symbionts residing in the GI tract play a primary role in host immune system development. Microbe-derived metabolites and the microbe-associated molecular patterns (MAMPs) of the GI tract can stimulate immune cell activation and regulate brain behavior and functions (99). Investigations revealed that the GI microbiome and their products substantially affect microglia, the major resident macrophages in the brain, before birth until adulthood and can regulate their inflammatory responses in the CNS (100). Microglia play a significant role in regulating the correctness of neuronal network wiring in the CNS through synaptic pruning during brain development (101). Consequently, altered GI microflora correlates with several microglia-associated neurological disorders in humans (102). Furthermore, SCFA treatment, which contributes to the complete recovery of innate immune response impairments in GF mice, suggests that GI microflora signals play a pivotal role in maintaining microglia functions (102). New clinical evidence has implied correlations between GI tract perturbations and neurological diseases. Several studies reported that a subset of autistic individuals shows symptoms of chronic constipation, higher intestinal permeability, abdominal pain, and disturbed intestinal microbiota, thus providing a possible connection between dysbiosis and neurodevelopmental disorders (103). Microbial transfer from the mother to the fetus, the mode of delivery, antibiotic exposure, and the dietary regimen can change infant microbiota colonization and maturation. These environmental factors in the composition of intestinal commensals and functions can contribute to the long-lasting impact on human host health and may result in the development of illnesses later in life (104). In addition, mouse studies have shown that supplementation of antibiotics during pregnancy leads to the disruption of maternal and neonatal intestinal microflora, subsequent reduction in locomotor activities, and alterations in neonate behavior (105). Accordingly, clinical evidence supports the association of antibiotic-induced dysbiosis with the development of several neurodevelopmental disorders, including schizophrenia, depression, and bipolar disorders. Furthermore, imbalanced development of the host microbiota during premature birth is associated with a much higher risk of developing psychiatric disorders, such as depression and schizophrenia (106). Although more studies are needed to clarify the molecular interconnection between the above-mentioned factors and neurodevelopmental disorders, manipulating early-life microbiota can be considered a profitable approach for preventing ASD and other neurological disorders (106).
Gut Microbiota-Brain Signaling through the Immune System
The immune system and the CNS are both complex and organized systems that control and regulate numerous functions throughout the body, sharing common characteristics in operational modes and developmental processes. It has been acknowledged that molecules involved in innate immunity, such as Toll-like receptors (TLRs), cytokines, the complement family, and adaptive immunity-related molecules such as antibody receptor and the major histocompatibility complex (MHC), are also produced in the brain and play critical modulatory roles in brain development. Moreover, despite previous beliefs about the brain as an immune-privileged organ, it contains meningeal lymphatic vessels. The presence of lymphatic vessels in the meninges provides insight into a possible link between the CNS peripheral immune system, influencing autoimmunity (107). Moreover, lymphocyte and microglia can regulate cognition and are also essential for the correct wiring of neuronal circuits (108). The vasculature of most areas of the brain develops tissue-specific properties of selective BBB, allowing the passage of required molecules to the brain and limiting the penetration of potentially toxic substances or cells. However, circumventricular organs, including the median eminence, pineal gland, area postrema, and subfornical organ, contain highly permeable capillaries (109). The vasculature of this specific area, located adjacent to the third and fourth ventricles, is characterized by fenestrated capillaries essential for the functions of these nuclei, which detect either the solute concentration of blood or the secretion of molecules into the bloodstream (110).
Microglia are macrophages that constitute up to 10% of all neural cells (111). They are responsible for the fundamental role of active immune defense in the CNS (112). Moreover, the immune cells have the ability to infiltrate the brain. Either the infiltrating immune cells or the microglia are capable of interacting efficiently with the CNS and affect brain function and pathology as well (113, 114). Microglia emerge from the embryonic progenitor cells and can undergo the process of renewing themselves in the CNS. They are involved not only in the typical immune functions, such as phagocytosis and antigen presentation, but also in some brain physiological activities (115). Immune cells such as neutrophils, macrophages, T cells, and natural killer (NK) cells enter from the brain’s peripheral circulating blood. Microglia have a tremendous impact on behavior and on some neurological disorders, such as neurodegenerative diseases (116, 117). Currently, it has been indicated that the GI microbiome plays a central role in developing the immunity of the brain. The GI microbiome affects microglial cell maturation in the uterus from the initial levels of development. The existence of gut microflora is also essential for the microglia function in adulthood (118). To illustrate further, a study in GF mice exhibited weakened microglial maturation. The gut microbiota may influence the microglia maturation by producing SCFAs (102).
Based on these findings, there is no doubt that the GI microflora plays a substantial part in the onset of several autoimmune disorders and that brain-infiltrating immune cells are vital in CNS autoimmunity. Recent investigations on brain autoimmunity diseases, including MS and experimental autoimmune encephalomyelitis (EAE), highlight the importance of microbial communities residing in the gut (119, 120). Remarkably, microbiome transplantation from individuals with MS to GF mice triggered an increased risk of EAE compared to healthy donors as negative controls. Moreover, the modulation of T lymphocytes by the gut microbiota and their derived metabolites, such as SCFAs, leads to reduced EAE-associated axon damage (120–122). Other microbial metabolites, such as tryptophan, regulate the inflammation of CNS in the EAE models and mediate elevated astrocyte activation (123). Generally, adult brain neurogenesis is influenced by the gut microbiota of the host. Antibiotic treatments of adult mice affect both the diversity of gut microbial communities and the neurogenesis of the hippocampus (124). Interestingly, the administration of mice with normal SPF flora versus probiotics in the neurogenesis-deficient mice revealed that only probiotics have the capability to reconstitute the gut microbiota. Moreover, supplementation of reconstituted microbial flora SPF mice with probiotics demonstrated an increased number of infiltrating Ly6C high monocytes and showed improved neurogenesis (125).
Under normal circumstances, the immune system and microbiota collaborate, leading to the appropriate immune responses. However, disorders such as autoimmune and inflammatory diseases are rooted in the failure of immune responses (126). Many recent studies demonstrate a connection between the gut microbiome and the brain and between the gut microbiome and immune system modulation. Studies on GF and control rodents revealed that the absence of an intestinal microbiome intensifies anxiety-like behavior. Moreover, this behavior could entirely be cured, if the gut microbiota was recovered in early life. Microbial metabolites can be transferred to the brain through blood circulation and affect the VN or immune system and inflammatory responses, indicating that the disturbance of microbial metabolism due to dysbiosis can have a tremendous impact on anxiety-related disorders (127). In addition, GI microbiome-derived products, including LPS and amyloids, have been shown to be important contributors to particular signaling pathways and proinflammatory cytokines involved in neurodegenerative inflammation (128). Hence, dysbiosis of GI microbes in AD patients and “leaky gut syndrome” can be considered central pathophysiological links in the transport of microbiome-derived neurotoxic products across the BBB, leading to the progression of AD (129).
Gut microbial communities modulate immune responses in the gut, either directly or indirectly, by recruiting different immune cells under various immunological conditions. Next-generation sequencing (NGS) and metagenome analysis have revealed that there are five main phyla in the mammalian gut microbiome: including Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, and Verrucomicrobia (130). Although there is a unique gut microbiome fingerprint between individuals, there is a balance in its microbial composition, which would negatively affect the host (131). Dynamic and heterogeneous features of the GIT are fundamental in the homeostasis of the host. It has been reported that imbalanced immune system development, characterized by a reduced level of GI lymphocytes, reduced the numbers of immunoglobins A (IgAs), antimicrobial peptides (AMPs), and immature gut-associated lymphoid tissues (GALT) in GF mice compared to wild-type mice, highlighting the essential significant role of the GI microbiome in the formation of host immunity (132). Moreover, the enteric immune system is vital in distinguishing between commensals and pathogens and in determining what leads to an immunological tolerance to them. Consequently, intestinal flora can modulate immune system development and function, shaping the gut microbial communities and regulating the pathogens on mucosal surfaces of the intestinal tract. For instance, it has been reported that a decreased number of T helper 1 (Th1) and Th17 cells, together with a decrease in IL-22 and IL-17 in GF mice, contributes to a lower number of lamina propria (LP)-associated CD4+ lymphocytes (133, 134). Specific bacterial species perform a fundamental role in the formation of the immunological functions of different immune cells. Th17 cells are capable of the antipathogen host defense, and multiple reports also indicate their importance in the pathogenesis of autoimmune diseases. A reduction in these types of cells indicates a correlation between decreasing or the complete absence of segmented filamentous bacteria (SFB) in the GI microflora, indicating their potential in the differentiation of Th17 cells and the induction of IL-22 and IL-17 (135, 136).
In addition, the GI microbiome influences the development of intestine-resident B lymphocytes at the LP, since lower counts of B cells at the LP have been reported in GF mice (137). Moreover, this type of cell is also able to produce IgA as strong regulators of the microbial compositions. This indicates prominence in contributing an immune tolerance to the commensal microbes and reaching a broad diversification for IgA at the LP (138). Accordingly, these investigations concluded that the gut microbiome influences the induction and the development of intestinal T and B cell responses. The gut microbiome plays an essential role in the activation of CD8+ T lymphocytes in the gut. Recent investigations highlight the importance of the functional metabolic patterns induced by the microbial communities within the GIT milieu rather than the presence of a single or a microbial consortium leading to the modulation of mucosal immunity. The microbial communities residing in the gut are an important source of various metabolites, and the interactions between these microbial metabolites and the gut-mucosal immune cells are important for T-reg cell differentiation or T-eff cell properties (139). SCFAs, such as butyrate, produced from microbial DF fermentation are mainly produced by Firmicutes, despite acetate and propionate being primarily fermented by Bacteroidetes (140). Investigations of SCFAs demonstrated that, in addition to the enhancement of the function and the number of regulatory T lymphocytes in the enteric system, they can also promote anti-inflammatory effects and gut-barrier function based on their inhibitory effects on the transcription factor NF-κB and HDAC activity (141–144). Tryptophan derivatives produced by microbial gut flora binding to the aryl hydrocarbon receptor (AhR) affect the function of the intestinal immune system. Moreover, the production of indole-3-aldehyde and tryptophan catabolism by a commensal gut bacterium, e.g., Lactobacillus reuteri, ultimately lead to binding to the AhR and trigger the IL-22 pathway (145, 146). Furthermore, the intestinal microflora can produce arginine derivatives including, diamine, spermine, spermidine, and polyamines, causing the modulation of immune responses by enhancing the homeostasis of both resident immune cells and the intestinal mucosa (147, 148).
GUT MICROBIOTA AND AGING
Recent advancements in NGS and metagenomic technologies have made it possible for scientists to investigate compositional changes in the gut microbiota of the elderly (149). Based on recent research, increases in Bacteroidetes and Proteobacteria, especially Gammaproteobacteria, and remarkable reductions in Firmicutes and Bifidobacterium have been observed with aging (150, 151). These age-associated variations can be linked to many external factors, such as diet and a decrease in fibrous foods (152), a large amount of antibiotic consumption, and general alteration of living conditions (153). The ENS is one of the most intricate and substantial parts of the peripheral nervous system, which is made up of small ganglia and neurons. These neurons are distributed throughout the GIT membrane (154, 155). The ENS has a significant role in intestinal cell activities, nutrient absorption, and gut hormonal secretion (154, 156, 157).
The enteric nervous system (ENS) goes through a dramatic developmental change during the host life, while maintaining its flexibility in terms of its pathophysiological functions (156, 158). Therefore, it seems quite logical that the ENS starts to debilitate, along with the host microbiota, immune system, and physiology, with aging (Fig. 3). Various studies have focused on this topic, but the mechanisms remain ambiguous since the results are not consistent (159). Some investigations have observed a decrease in the amount and function of the myenteric neurons with aging (160, 161), while other studies have reported no such consequences (162, 163). Hence, whether the ENS undergoes changes with aging is still being seriously debated. Investigations on ENS alterations with aging have indicated changes in the morphology of enteric ganglia (164, 165), the identification of degenerating nerve fibers (163, 166), and α-synuclein (α-syn) and lipofuscin accumulation (167, 168). These reports indicate a correlation between aging and ENS degeneration, although some mechanisms and pathways remain to be elucidated. Hence, it is reasonable to hypothesize that the ENS undergoes some degenerative changes in line with the changes in host physiology, metabolism, microbiota, and the immune system associated with aging (Fig. 4).
FIG 3.
Schematic presentation of molecular pathways by which the changes related to age in gastrointestinal tract (GIT) microflora and the neuro-entero-endocrine system might influence the brain’s health through dysfunction of the gut-brain signaling pathway. In healthy adult individuals, the balanced gut microflora and gut barrier integrity contribute to maintaining balanced microbial communities and their metabolites, including SCFAs. In addition, the appropriate production of neurotransmitters in the gastrointestinal tract aids in the maintenance of controlled enteric- inflammatory and immune systems via the balanced proliferation of macrophages and dendritic cells, which finally leads to controlled gut-brain communication and appropriate functioning of the CNS. However, in the senescent host, an alteration in the diversity of gastrointestinal microbial communities and disruption of gut barrier integrity contributes to the perturbation of the biochemical and microbial microenvironment of the epithelial cell lining of the GI tract through unbalanced levels of SCFA, LPS, 5-HT, histamine, secretory immunoglobin (sIgA), etc. Consequently, inducing an overactivated inflammatory environment in the intestinal environment leads to the disruption of healthy gut-brain communication. Abbreviations: SCFA, short-chain fatty acids; LPS, lipopolysaccharide; 5-HT, 5-hydroxytryptamine; TNF-α, tumor necrosis factor α; IFN-δ, interferon δ; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein 1; CNS, central nervous system.
FIG 4.
Organizational structure of the main steps implicated in the bioinformatic analysis of the gastrointestinal microbiome. The general overview of the bioinformatic analysis pipelines is divided into two branches based on the type of sequencing, including 16S rRNA gene microbial profiling and shotgun metagenomics. After microbial DNA extraction and sequencing, the pipeline determines the taxonomic profiling of the gut microbiota and the genomes’ reconstruction, in addition to a functional analysis of the genes. This schematic presentation depicts the major steps and may be modified according to the analysis’ ultimate objective. ASV, amplicon sequence variant; OTU, operational taxonomic unit.
Considering the distinct functional and morphological characteristics of ENS cells, different cell types play a major role in the susceptibility of age-related diseases. The burden of oxidative DNA damage and reactive oxygen species (ROS) production in metabolically active nerve cells might be another underlying element of ENS aging, both directly or indirectly (169). According to studies highlighting the importance of reducing calcium-binding protein expression in geriatric animal models, calcium dysregulation can also be considered to be involved in ENS senescence (170, 171). It has also been reported that during the aging process, sodium channel gene expression in the enteric nervous cells is significantly altered (172). Further studies on the magnitude and features of these age-related alterations are needed. Moreover, additional research on the age-related impacts on the ENS are essential and may contribute to a new understanding of these complex aging and gastrointestinal tract associations, and they may also introduce novel opportunities to discover new therapeutic approaches for treating various age-related neurological disorders, as well as for improving the quality of life among geriatric populations. Likewise, considering the microbes colonizing in the vicinity of enteric neurons in the gastrointestinal tract, it appears that the ENS is highly correlated with or may be affected by the intestinal microflora. Several studies have indicated that ENS development in newborn infants is shaped by early exposure to gut-resident microbes (173–175). Furthermore, the intestinal microbiota can regulate the initial colonization of LP by glial cells, as well as homeostasis (176).
Similarly to the substantial changes of the gut microbiota during the aging process (161), enteric neurons show more susceptibility to age-related impairments in adults (168). The altered equilibrium of the gut microbiota, including the enhancement of opportunistic pathobionts and the reduction of beneficial or commensal microbes, can contribute to the different microbial metabolite profiles of the gastrointestinal tract (177, 178). Consequently, the elevated levels of intestinal inflammation induced by the age-related alterations of the gut microflora influence the ENS and cause impairments or loss of different physiological and neurochemical functions of the enteric neurons, contributing to the onset of age-associated diseases (157, 178).
Remarkably, it has also been concluded that the ENS modulates the gut microflora’s community composition, maintaining and promoting intestinal health. Moreover, the absence of gut microbial communities can lead to abnormal and altered functions of the ENS (179). It has also been shown that the development of gut dysbiosis and intestinal pathology is correlated with perturbed gastrointestinal motility (178–180), indicating the essential role of the ENS in gut microbial population maintenance and prevention of the overgrowth of pathobionts that can drive host diseases. Although the exact mechanisms still need to be clarified, the complex cross talk and interactions among gut microbiota, the mucosal immune system, enteric neurons, and intestinal epithelial cells in the view of the GBA and neuropathies of elderly populations highlight the importance of research in this area and the need for further research (157, 181, 182).
Several studies demonstrated that the aged microbiome itself is sufficient to cause cognitive impairment. Investigations on microbiota transplantation have indicated that an aged gut microbiome can cause morbidity in young recipients. For example, it was reported that gut microbiota transplantation from old donor mice to GF recipient mice contributed to increased intestinal inflammation and permeability, which showed a correlation with higher levels of Proteobacteria and TM7 bacteria (183). A recent study showed that fecal microbiota transplantation (FMT) from aged donor mice contributed to impairments in spatial learning and memory in young recipient mice, followed by a significant reduction of SCFA-producing bacteria, including Faecalibaculum, Lachnospiraceae, and Ruminococcaceae (184). Furthermore, FMT from aged mouse models to GF mice led to a reduction in fecal SCFA production, the promotion of depressive-like behavior, and short-term memory impairments, indicating that the aged gut microbiome is able to reduce SCFA levels of the host and subsequent cognitive decline (185). In addition, Li et al. reported that FMT from aging donors to young recipient rats led to higher cognitive behavior impairment, synaptic structural alterations, elevated levels of glycation end products, and increased inflammatory and oxidative stress in recipient young rats (186).
GUT MICROBIOTA IN NEURODEVELOPMENTAL DISORDERS
Autism Spectrum Disorder
Autism spectrum disorder (ASD) comprises a complex range of neurodevelopmental symptoms, including the impairment of social interactions and communication, together with restrictive and repetitive patterns of behaviors (187). Recent epidemiological studies from the CDC Autism and Developmental Disabilities Monitoring (ADDM) Network revealed that an incidence of 1 in 54 children had been identified with ASD. Moreover, male individuals with ASD outnumbered females by 4.3:1 in 2016 (188). The exact etiology of ASD is still undetermined. However, there is considerable clinical evidence indicating that genetic and environmental factors play crucial roles in the onset of the disease. More than 100 genes and genomic regions that impact the development of CNS have been identified, which may be associated with the development of ASD (189). Environmental factors, such as malnutrition, viruses, and developmental errors during infancy (190) and, specifically, maternal autoantibody against seven proteins in the developing brain (191) have also been associated with ASD. These environmental factors have now been shown to have a much more crucial contribution in ASD than was previously thought (192). In recent decades, it has been discovered that gut microbiota and brain interactions play a critical role in neuropsychiatric diseases such as autism. Moreover, about 40% of individuals with ASD experience more gastrointestinal dysfunction (193, 194), including alteration of bowel function and abdominal cramps (pain), diarrhea, reflux, and vomiting (195). Accordingly, the correlation of the GI symptoms and severity of ASD shows the importance of the connection between the gut microbiota and the brain (196).
The composition of gut microbiota is related to age. Alterations in the normal composition of the gut microbial communities would increase the numbers of pathogenic microbes and thereby subsequent infections (197, 198). Moreover, recent data suggest that GI disturbances and CNS symptoms in autistic patients may be related to inflammatory states induced by gut dysbiosis (52, 187). According to the latest studies, autistic children have shown significant gut microbiota composition changes, and the GI symptoms may represent the inflammatory processes (199). Inflammation is correlated with increased permeability of the intestinal mucosal barrier to the bacterial neurotoxic peptides such as lipopolysaccharide (LPS) and the production of inflammatory cytokines (195). Bacterial metabolites play a vitally important contribution in the GBA; therefore, disruption in gut-brain signaling may be involved in neuropsychiatric disorders such as ASD and PD (200). It has been demonstrated that a high-fat diet that influences fetal microbiota in pregnant women may also be related to ASD (201). Moreover, breastfeeding for 6 months reduced the chances of ASD manifestation, whereas formula-feeding was associated with an increase in the abundance of Clostridium difficile in the gut (202). Since probiotics can play an anti-inflammatory role and reduce the gastrointestinal symptoms of IBD subjects (203–206), it has been reported that microbial interventions, such as probiotics, can contribute to the reduction of social behavioral symptoms and the level of inflammation in individuals with ASD (207). Recent studies on the potential of microbial intervention in preventing and treating ASD have been summarized in Table 1. The gut microbiota in autistic children and even adults is thoroughly different from healthy controls (200). Investigations on fecal samples of autistic children indicated a decrease of Bacteroidetes/Firmicutes ratio due to a reduction in numbers of Bacteroidetes (200) Furthermore, an increased level of Lactobacillus, Clostridium, Desulfovibrio, Caloramator, Alistipes, Sarcina, Akkermansia, Sutterellaceae, and Enterobacteriaceae has been seen in children with ASD (200, 208, 209). Many researchers have evaluated the abundance of Clostridium and its role as a risk factor. Weekly treatment with vancomycin on autistic children resulted in a significant improvement of neurobehavioral and gastrointestinal symptoms (210). Apart from the role of gut microbiota in the immune system development, Clostridium sends signals to the brain via ENS or afferent fibers of the VN (211) and controls gut permeability (212, 213). It has been reported that the gut microbes can play a significant role in gut permeability by producing metabolites such as phenols, SCFAs, and free amino acids (214). Autistic children have high rates of propionic acid and acetic acid but a low rate of butyric acid (200). The final products of nondigested carbohydrates, which may be relevant in ASD pathogenesis (215), are SCFAs such as propionic acid, butyric acid, and acetic acid (216). A comparison of fecal and plasma metabolomes between ASD and typically developing children showed mitochondrial dysfunction; different levels of phenolic microbial metabolites, lipids, and amino acids and xenobiotic metabolism in the ASD children can potentially be used as molecular biomarkers for ASD (217). After precise evaluation of plasma metabolites in children with ASD in another study, the levels of plasma metabolites, including nicotinamide riboside, IMP, iminodiacetate, methylsuccinate, galactonate, valylglycine, sarcosine, and leucylglycine, were significantly low. However, after microbiota transfer therapy (MTT), substantial changes were seen in these metabolites, making some of them similar to those seen in typically developing children (218). Overall, alterations in the composition of microbial communities of the gut have been confirmed in subjects with ASD. However, considering the heterogeneity of the participating patients and several conflicting results, it is hard to develop a unique profile for ASD. Considering the inconsistency in the association of bowel dysfunction and the severity of social behavior impairments in the ASD patients, these data suggest that we should consider two different subtypes for the ASD with different grades of inflammation associated with gastrointestinal comorbidities. Among various therapeutic ASD approaches, several studies have investigated the potential of using probiotics to alleviate autistic symptoms. Despite the promising results of probiotic treatments, assessments of tolerability and safety should be considered. Regarding the limitations in microbiota analysis methods, further use of randomized, placebo-controlled clinical trials is necessary in order to validate the efficiency of probiotics in the treatment of ASD (218).
TABLE 1.
Microbial intervention in autism spectrum disorder
| Source | Study design | Sample size (n) | Study population | Microbial intervention | Study duration | Key finding(s) |
|---|---|---|---|---|---|---|
| Fecal microbiota transplantation | ||||||
| Kang et al., 2017 and 2019 (16, 435) | An open-label, double-blind, randomized placebo-controlled study | 38 participants (18 ASD, 20 controls) | 7- to 16-yr-old autistic children with mild to severe gastrointestinal symptoms; control group comprised of 20 age-neurotypical gender-matched children without gut disorders. | FMT from standardized healthy donors of human gut microbiome | 18 wks | Alleviation of ASD and GI symptoms 2 yrs after the MTT termination. |
| Significant correlation between ASD and GI symptoms improvements. | ||||||
| A considerable increase in bacterial diversity of autistic fecal samples 2 yrs after the MTT termination. | ||||||
| Zhao et al., 2019 (436) | Open-label, randomized waitlist-controlled study | 48 participants; FMT group (n = 24); waitlist group (n = 24) | FMT group in which patients received twice FMT through gastroscopy and colonoscopy under anaesthesia; waitlist group in which patients received only rehabilitation training. | The fresh fecal suspension was isolated from one anonymous healthy donor. | 18 mo | FMT treatment was safe and well tolerated. |
| ASD symptoms ameliorated. | ||||||
| Changes in GI microbiome of ASD individuals to a healthy state. | ||||||
| Urbonas and Cervinskiene, 2018 (437) | - | 5 participants | Boys (5 to 8 yrs); FMT per formation was done every mo, three times for every individual with mild GI and ASD symptoms. | The donor feces from healthy individuals were infused into the cecum. | 2 mo | FMT treatment was well tolerated. |
| Positive effect on GI and ASD symptoms. | ||||||
| Sharon et al., 2019 (17) | Preclinical study–animal study | 14 to 121 per group per analysis | Mouse model: offspring of mice with FMT from human ASD patients. Relevant groups (all GF WT mice) FMT: (i) offspring human mild ASD-FMT; (ii) offspring human ASD-FMT; and (iii) offspring human ND-FMT. | Gut microbiome from human donors with ASD or typically developing controls. | - | Mice transplanted with human ASD microbiome, but not typically developing (TD), demonstrating ASD-like behaviors. |
| TD and ASD microbiota provide distinct metabolome profiles in mice. | ||||||
| Improvement of social and repetitive behaviors in BTBR mice treated with 5AV or taurine. | ||||||
| Probiotics | ||||||
| Liu et al., 2019 (438) | A randomized, double-blind, placebo-controlled trial | 80 participants | Boys with ASD aged 7 to 15 in Taiwan | Lactobacillus plantarum PS128 (PS128) | 4 wks | Improvements in some autism symptoms, primarily those linked to rule-breaking behaviors such as hyperactivity/impulsivity. |
| PS128 intervention is age related, with more considerable results noticed in younger cases, suggesting the importance of early interventions. | ||||||
| Shaaban, 2018 (439) | Prospective, open-label study, observational clinical cohort | 30 participants | 5- to 9-yr-old children with ASD, 63% male, Egypt | Lactobacillus acidophilus, L. rhamnosus, Bifidobacterium longum | 3 mo | Elevation in levels of Bifidobacteria in fecal samples. |
| Improvement of behavior. | ||||||
| Ameliorated GI symptoms. | ||||||
| Slykerman, 2018 (440) | Two-center, randomized, double-blind, placebo-controlled study | 474 participants | Children monitored from birth to 11 yrs, New Zealand | Lactobacillus rhamnosus, Bifidobacterium animalis, B. lactis HN019 | 2 yrs | Behavior exacerbated with probiotics. |
| Prebiotics | ||||||
| Sanctuary, 2019 (397) | Randomized, double-blind, controlled trial | 8 participants | 2- to 11-yr-old children with ASD, 87.5% male | Bifidobacterium longum subsp. infantis (UCD272); bovine colostrum product (BCP) | 12 wks | Reduction in the amt of certain GI symptoms BCP only versus BCP+B. infantis-treated groups. |
| The group that received only BCP showed remarkable improvement in stereotypy and irritability scores. | ||||||
| Reduction in TNF-α and IL-13 production might be an improvement factor. | ||||||
| Grimaldi, 2018 (441) | Randomized, double-blind, placebo-controlled study | 41 participants | 4- to 11-yr-old children with ASD, 31 male and 10 females | Prebiotic B-GOS mixture and GLUCIDEX maltodextrin as placebo | 6 wks | B-GOS intervention resulted in subsequent metabolic shifts in urine spectrum profile and fecal samples. |
| Decreased gastrointestinal discomfort but no considerable difference in sleep or GI symptoms. |
Schizophrenia
Schizophrenia (SCZ) is a serious mental illness associated with some combination of auditory hallucinations, delusions, and disordered thought and behavior, impairing daily function and social interaction (219). The physiopathology of SCZ is not yet explained, but recent investigations have shown that environmental factors increase the risk of developing SCZ in individuals who may already have a genetic predisposition to the disease. Dysfunction of neurotransmitter systems in multiple systems has been widely investigated, particularly highlighting the importance of abnormalities in signaling, including dopamine, serotonin, glutamate, and GABA (220–222). Increased evidence implies that SCZ can be considered a system disorder with both neuropsychiatric and psychotic conditions (223, 224). Moreover, the significance of inflammation and the possible role of gastrointestinal systems in the etiology of SCZ is under consideration (225). The role of the GI tract microflora is crucial in the neurogenerative pathways and gut microbiome, and their microbial metabolite perturbations have been shown to influence mood and behavior (16, 226, 227). Gut microbiome alterations have been correlated with several neurodevelopmental (213, 228) and neurological (229) disorders. It has been recently shown that FMT from subjects with SCZ can induce SCZ-related behavioral symptoms in GF recipient mice. This is associated with the altered levels of GABA, glutamine, and glutamate in the hippocampus. It indicates that the microbiomes of schizophrenic patients can have an impact on the neurochemistry, which may be related to these human conditions (230). There are still no reports identifying the functionality of the specific bacteria that foster alterations in the behavior of the examined recipient mouse models (231, 232). Based on various reports, Actinobacteria, Proteobacteria, Bacteroidetes, and Firmicutes show the largest differences between the two cohorts in individuals with SCZ (230). Interestingly, antibiotic supplementation reduced engulfment of synapses by the microglial cells in an in vitro study. Microglial cells reduce the density of CNS synapses, and this is considered to be an essential step in the development of SCZ (233). After the examination of electronic health records of a cohort of adolescents, the administration of minocycline was linked to a slight reduction in the incidence rate of SCZ, implying that more studies are needed to investigate the association of the microbiota in SCZ. In accordance with the severe nature and intricacy of SCZ, no research has yet confirmed any behavioral symptom alleviation through the possibility for probiotic supplementation in SCZ patients. However, several investigations have considered that probiotic administrations may alleviate at least the digestive disorder associated with SCZ (Table 2). Furthermore, severe GI problems were reduced in one human clinical trial, without any alteration in the psychiatric symptoms in subjects with SCZ (234). Another human trial demonstrated a correlation between Candida albicans and GI problems, where improvement in psychiatric symptoms was seen in males administered a specific probiotic supplementation (comprising L. rhamnosus GG and B. animalis subsp. lactis Bb12) and who were seronegative for C. albicans (235). A full array of novel, possibly therapeutic interventions for severe psychotic disorders have been discovered, including considering bowel comfort. More research is required to improve our understanding of the GI microbiome’s involvement in SCZ using longitudinal data analysis and larger sample sizes. Functional and taxonomic classification of the GI microbes is essential for a comprehensive understanding of the GI microbiota. Metagenomic shotgun DNA sequencing in association with bioinformatic analysis tools provides better characterization of the gut-residing microbes. These provide a more precise perspective of the biological properties of the bacteria and their capacity to affect host physiology. Considering that most human intestinal microbiota are unculturable, the development of culture-independent methods, i.e., metagenomics, metatranscriptomics, and metabolomics, is essential to identify the activities, metabolism, and physiological roles of the unculturable members of the intestinal microbiota (Fig. 4).
TABLE 2.
Microbial intervention in schizophrenia
| Source | Study design | Sample size (n) | Study population | Microbial strains | Treatment duration | Key finding(s) |
|---|---|---|---|---|---|---|
| Fecal microbiota transplantation | ||||||
| Xie et al., 2019 (442) | Case report, pre and postintervention assessment | 1 participant | 1 male MDD patient with GI symptoms and alopecia, 86 yrs old | Six-time FMT via colonoscopy, 22-yr-old healthy male donor | 18 mo | Improvement in symptoms of depression. |
| Increased BMI, improvement of appetite, no abdominal pain or distension. | ||||||
| Cai et al. 2019 (443) | Case report, pre- and postintervention assessment | 1 participant | 1 female MDD patient, 79 yrs old | 6-yr-old grandson, single-time FMT via gastroscope | 6 mo | Optimization of the intestinal microflora of patients with depression through the use of FMT. |
| Substantial increase and reduction in Firmicutes and Bacteroides count, respectively. | ||||||
| Alleviate depression-related symptoms by restoring or reconstructing the constitution of the intestinal microflora. | ||||||
| Liu et al. 2020 (473) | Preclinical, animal study | 18 participants | 18 8-wk-old GF rats | Microbiota transplantation from healthy or depressed humans between ages 18 to 60 into GF mice | 4 wks | Depression microbiota-treated rats demonstrated depression-like behavior. |
| The decrease of hippocampal neurotransmitter levels through depression microbiota transplantation. | ||||||
| Depression microbiota transplantation induced abnormalities in the HPA axis, an inflammatory reaction, and mitochondrial dysfunction. | ||||||
| Probiotics | ||||||
| Severance et al., 2017 (235) | Randomized, placebo-controlled, longitudinal pilot study | 56. participants | Aged between 18–65 yrs, patients with at least moderately severe psychotic symptoms | Combined B. animalis subsp. lactis strain Bb12 and L. rhamnosus strain GG | 14 wks | In SCZ, probiotic supplementation contributes to the reduction of elevated Candida yeast antibody levels. |
| Relieved yeast-related bowel discomfort over compared to placebo. | ||||||
| Candida albicans seropositivity was associated with worse psychiatric symptoms. | ||||||
| Okubo et al., 2019 (444) | open-label single-arm study | 29. participants | Patients aged 20 yrs or more; not admitted to hospital within at least 6 mo after last discharge | Bifidobacterium breve A-1 | 8 wks | Probable enervation of depressive and anxiety symptoms in SCZ by enhancement in the function of gastrointestinal epithelial barrier. |
| Dickerson, 2014 (234) | A randomized, placebo-controlled, double-blind trial | 58 participants | Aged between 18 to 65 yrs, patients with at least moderately severe psychotic symptoms. | Combined B. animalis subsp. lactis strain Bb12 and L. rhamnosus strain GG | 14 wks | Probiotic administration might reduce the development of severe bowel difficulties in SCZ patients. |
| Tomasik et al. 2015 (445) | A randomized, Placebo-Controlled Trial | 57. participants | 65. outpatients diagnosed with schizophrenia or schizoaffective disorder | B. animalis subsp. lactis strain Bb12, Lactobacillus rhamnosus strain GG | 14 wks | Probiotic administration may ameliorate gastrointestinal leakage control in SCZ. |
| Synbiotics | ||||||
| Haghighat et al., 2019 (446) | Three-arm parallel design, placebo-controlled, double-blind, randomized controlled trial | 75 participants | Clinically stable hemodialytic patients with MDD, aged 30 to 65 yrs | Synbiotic (15 g of prebiotics, 5 g of probiotic containing Lactobacillus acidophilus T16, Bifidobacterium bifidum BIA-6, B. lactis BIA-7, and B. longum BIA-8) probiotics (5 g probiotics as in synbiotic group with 15 g of maltodextrin as placebo) or placebo (20 g of maltodextrin) | 12 wks | Improvement in serum BDNF level and depression symptoms through synbiotic supplementation in comparison to the probiotic administration in subjects with HD, particularly in depression symptoms. |
GUT MICROBIOTA AND NEURODEGENERATIVE DISORDERS
Multiple Sclerosis
Multiple sclerosis (MS) is an immune-mediated, chronic neurological disease of the CNS, involving damaged axons and demyelination and affecting approximately 2.3 million people worldwide. Its incidence is higher in females (236–238). The pathogenic hallmark of MS is the development of inflammatory focal demyelinated plaques in the CNS, including either gray or white matter of the spinal cord and brain and triggering a neuroinflammatory response contributing to the demyelination of specialized cells, including oligodendrocytes, and consequent neurodegeneration. Due to the abnormal permeability of the BBB, various cells of the immune system infiltrate into CNS neuronal cells, leading to the onset of demyelination. Myelin antigen-specific T cells (CD8+ and CD4+ T cells) cross this barrier, contributing to a series of events leading to the formation of demyelinating lesions (239). Recent studies on the MS mouse models, including the EAE model, indicate the primary role of CD4+ T lymphocytes in the etiopathogenesis of MS (240). Particularly, CD4+ Th17 and Th1 lymphocytes have the most prominent responsibilities in the onset of MS. Th1 contributes to the secretion of delta interferon (IFN-δ), fostering the production of macrophage enzymes following their activation. Moreover, IFN-δ stimulates reactive nitrogen and oxygen species production, leading to the nitrosative and oxidative damage of cellular structures, respectively. Th1 cells are also capable of IL-12 production, which induces the secretion of the tumor necrosis factor (TNF-α) and IFN-δ, leading to the chronic inflammatory response and further tissue damage. In addition, the production of particular cytokines, including IL-22, IL-21, and IL-17, mediated by Th17 cells, leads to chronic inflammatory progression. CD4+ T lymphocytes that recognize CNS self-antigens, such as Th1 and Th17, are involved in the pathophysiology of MS (241). In addition to CD8+ and CD4+ cells, other immune cells are implicated in MS pathogenesis, including NK cells, microglial cells, and macrophages (242). The molecular interactions between these cells and their cytokines maintain the inflammatory cascades within the CNS. MS has been divided into several clinical variants, including relapsing-remitting MS, which is the most prevalent, and progressive relapsing MS, as well as primary progressive MS and secondary progressive MS (SPMS). Genetic predisposition and environmental factors both have a fundamental role in the etiology of MS (243, 244). Recent investigations stated that the gut commensal microbial communities are also responsible for several immune-mediated disorders such as MS and can be considered a novel environmental risk factor. In other terms, the gut microbiota is responsible for immunomodulation, altering BBB integrity and functionality, stimulation of the autoimmune demyelinating process, and interplay directly with various cell types existing in the CNS (245). Rather than the wide-ranging differences in α- or β-diversity of the gut microbiome, the cross-sectional investigations have mainly revealed that distinct taxonomic alterations are observed in children with MS compared to healthy individuals (19). Several investigations have assessed the efficacy of microbial transplantation from MS patients into two distinct EAE models; these studies highlight the significance of CD+ T cells producing IL-10 in GI-microflora-mediated immunomodulation (Table 3) (120, 123). In addition, the presence of the SFB in the GI tract, which may act in Th17 cell activation, significantly influences the MS-like symptoms in EAE mice (120). According to the definition of MS as a demyelinating disease, preclinical antibacterial studies have shown, after converging the data from the GF mouse models, that GI tract microbiota can regulate the production of myelin in the prefrontal cortex in a mouse model (123, 246). Furthermore, regarding the fundamental role of gut microflora in the regulation of the BBB, GF-mouse studies have shown that there might be an association between the microbiome and the loss of integrity of BBB as a major hallmark of MS (247). It has also been demonstrated that dietary supplementation with SCFA, or bacterial producers of SCFA, can reverse the loss of BBB integrity. Moreover, diet-induced change in the structure of gut-residing microbial communities has also been involved in the manifestation of EAE (Table 3) (248). Evidence indicates that the gut microbiota can regulate plenty of neuroinflammatory pathways. However, complementary studies are essential to understand the exact contributory mechanisms in the etiopathology of MS (123, 183, 249, 250). Animal and human studies imply that gut microflora may be responsible for many aspects of MS physiopathology. The question is still open as to how the gut microbiome can be effectively manipulated as an intervention to hinder relapse and alleviate symptoms at maximum levels. In a pilot experiment, supplementation of a particular probiotic formulation (containing Bifidobacterium, Lactobacillus, and Streptococcus species) could reverse microbiota alterations and modulated the inflammatory responses, indicating that this kind of microbiota-targeted therapy is promising (Table 3) (250), although further investigations are necessary for confirmation of these results.
TABLE 3.
Microbial intervention in multiple sclerosis
| Source | Study design | Sample size (n) | Study population | Microbial strains | Study duration | Key finding(s) |
|---|---|---|---|---|---|---|
| Fecal microbiota transplantation | ||||||
| Makkawi et al., 2018 (447) | Human (SPMS) Case report |
1 participant | A 61-yr-old woman with MS | Not mentioned. | >10 yrs | Amelioration of the Expanded Disability Status Scale (EDSS). |
| Cekanaviciute et al., 2017 (123) | Preclinical animal study | 142 participants | 71 MS patients not undergoing treatment and 71 healthy controls | Human MS or healthy fecal microbiota transplantation | 70 days | Immunoregulatory effects in MS via healthy human gut microbiota transplantation. |
| Berer et al., 2017 (120) | Preclinical animal study | 38 participants | 20 humanized transgenic 6-wk-old, germ-free RR mice MS-FMT; 18 humanized transgenic 6-wk-old, germ-free RR mice HT-FMT | The transplantation of fecal samples was from selected healthy and MS twin pairs. | 12 wks | MS-FMT contributed or spontaneous autoimmune encephalomyelitis in mouse models. |
| Probiotics | ||||||
| Salehipour et al., 2017 (448) | Randomized, placebo-controlled, double-blind trial | 24 participants | MOG-induced EAE in female C57BL/6 mice, 8 to 10 wks old, n = 8 per group | Treatment (T) groups: T1, L. plantarum; T2, B. animalis; T3, both probiotics; 109 CFU | 22 days | T1, T2, and remarkably in T3. |
| Mitigation of EAE in mice through motivating polarization of CD4+ T cells toward T-reg by induction of anti-inflammatory cytokines and transcription factors. | ||||||
| Inhibition of proinflammatory cytokines, resulting in the suppression of leukocyte infiltration, proliferation of autoreactive T cells into CNS. | ||||||
| Salami et al., 2019 (449) | Randomized, placebo-controlled, double-blind trial | 58 participants | 20 to 60 yrs, n = 24 per group | Probiotic capsules containing Bifidobacterium infantis, B. lactis, Lactobacillus reuteri, L. casei, L. fermentum, L. plantarum, and 2 × 109 CFU. | 16 wks | Decrease in several inflammatory markers, such as hs-CRP levels and IL-6. |
| Enhancement in levels of nitric oxide and IL-10. | ||||||
| Secher et al., 2017 (450) | Preclinical animal study | 60 to 80 participants | MOG-induced EAE in male C57/BL6Jmice, 8 to 12 wks old, n = 30–40 per group | The archetypal K-12 E. coli strain MG1655 and the probiotic E. coli Nissle 1917 (ECN) | 30 days | EAE progression and severity is correlated with variation in the intestinal barrier function is associated with the impact of probiotic treatment on improving the intestinal permeability may be beneficial in controlling CNS pathogenesis and neuroinflammation. |
Parkinson’s Disease
Parkinson’s disease (PD) is a progressive and multicentric neurodegenerative disease caused by the deposition of α-synuclein (α-syn) in the dopaminergic nerve cells in part of the center of the brain, the substantia nigra. These processes foster the gradual aggregation of round lamellated eosinophilic cytoplasmic inclusions, known as Lewy bodies. However, the exact mechanism of the PD pathogenesis is still vague, and it is probably a multifactorial disease, and various theories have been introduced in that respect (251). Aging is a substantial risk factor for PD development and progression, affecting several cellular pathways leading to the impairment of these processes and causing neurodegeneration. Conceivably, the same molecular perturbations that can be tolerated by young neurons show some catastrophic consequences in an aged one (252). The onset of the clinical symptoms of PD is primarily revealed with impaired motor symptoms, including muscular rigidity, resting tremor, akinesia, and postural instability (253). PD is rare before the age of 50, but the occurrence raises 5- to 10-fold with aging. It occurs primarily in men and involves 5 to >35 new cases per 100,000 individuals annually (254, 255). Dopaminergic neurons degenerate progressively, and there is a strong interrelationship between the nonmotor and motor symptoms such as depression (256), dementia (257), and GI problems, including constipation, abnormal salivation, defecatory dysfunction, nausea, and dysphagia. PD symptoms vary among individuals (258, 259). Several investigations have proposed that GI abnormalities in PD subjects are linked to intestinal dysbiosis and α-synuclein deposits in the ENS (260, 261). Due to the initial GI involvement in PD and the high potential of physiological interactions between host microbiomes, it has been suggested that the GI microflora may influence PD (49). Abnormal gastrointestinal functioning, especially constipation, affects up to 80% of PD-affected individuals and may happen years before the motor symptoms (262, 263). Idiopathic constipation is a major associated factor in PD and is related to neurodegenerative alteration in the ENS (49). α-syn neurodegeneration in the ENS may be one of the premotor clinical signs of PD (253). It is related to chronic constipation and physiological alterations in the GI wall. The gut microbiome probably affects the enteric neurons involved in α-syn secretion (264). These changes have been observed at the beginning of PD before the onset of motor symptoms, which can be considered a premotor biomarker (253). Different investigations have been carried out on the correlation between the gut microbiome and PD. One study observed a considerable reduction in Prevotellaceae species rather than in the relative counts of Enterobacteriaceae in the fecal samples of subjects with PD (49).
Members of the Prevotellaceae family are known as gut commensals, which take the lead in the production of SCFAs through DF fermentation and mucin in the gut (84). Enhancement of systemic exposure of bacterial endotoxins and gut permeability induced by the reduction in the Prevotellaceae population can trigger the uncontrolled expression and misfold the α-syn colon (265, 266). Furthermore, the severity of gait difficulty and postural instability is positively correlated with the relative abundance of the Enterobacteriaceae population in the gut (49). Overgrowth of Enterobacteriaceae in the gut results in enhancing LPS titration as a part of the Gram-negative bacterial cell wall in the serum (267). Consequently, it has been shown that due to the increased absorption of LPS in blood samples of subjects with PD, the systemic concentrations of LPS binding protein are surprisingly high (268, 269). According to one recent study on this matter, the number of opportunistic pathogens was increased significantly in patients with PD, which was acknowledged for the first time. Also, the decreased level of SCFA-producing bacteria and the growth in probiotics was confirmed (270). In addition, pyrosequencing of the variable regions, including V1-V3 of the bacterial 16S rRNA to study the fecal microbiota, showed a considerable decrease (77.6%) in the abundance of Prevotellaceae compared to the reference group. This enterotype is responsible for the biosynthesis of thiamine, folate, and neuroactive SCFAs. As such, the supplementation of these vitamins and SCFAs may be beneficial as therapy for PD (49). Moreover, the relevant results have shown an increased abundance of Lactobacillaceae, which, with Prevotellaceae, is related to the gut hormone ghrelin. It has also been reported that ghrelin secretion in patients with PD is reduced (271). Overall, the findings of the study revealed a connection between the GI microflora, PD, and the microbiome’s role as a potential biological marker. Further microbiome analysis may increase the accuracy and clarify the relationships, as well as mechanisms (49). Keshavarzian et al. observed an immense change in mucosa-associated and feces microbiota in the subjects with PD compared to healthy controls. The dysbiotic imbalance observed in PD patients could influence inflammation given that dysbiosis can impair intestinal barrier function and trigger immune activation and systemic inflammatory response (272–275). The persistence of PD affects the microbial community, and gut bacteria may have a role in some abdominal symptoms such as constipation and colonial inflammation (24). Briefly, LPS and other bacterial neurotoxins enter the bloodstream after crossing the intestinal wall, contributing to the disruption of the intestine’s epithelial barrier of the intestine (276). The presence of bacterial LPS in the bloodstream results in the production of inflammatory cytokines via nuclear factor-κB (NF-κB) and TLR4, resulting in systemic inflammation (268, 276). BBB disruption induced by bacterial LPS and inflammatory cytokines, including TNF-α, IL-1β, and IL-6, triggers the α-syn accumulation (277–281). Dopaminergic neuronal loss located in the substantia nigra can occur as a result of BBB breakdown (282). Since the intestinal barrier disruption in PD leads to the elevation of microbial translocation and higher proinflammatory gene profiles, colonic biopsy specimens show enhancement in the expression of TLR4 or bacterial endotoxin-specific ligand, CD3+ T cells, and other cytokines. Moreover, a decrease in SCFA-producing bacteria during intestinal dysbiosis has been reported in PD. Despite the TLR4-KO mice bearing dysbiotic microbiota, investigations of TLR4-KO mice treated with rotenone caused a reduction in neurodegeneration, neuroinflammation, intestinal and motor dysfunction, and intestinal inflammation compared to wild-type animals that were administered rotenone, suggesting the vital role of TLR4-mediated inflammation in the brain or intestinal inflammation, which might be one of the essential drivers causing PD neurodegeneration (283). Therefore, the relative enhancement of LPS after enhancing the Enterobacteriaceae population in the gut has been correlated with PD development (258, 284). Occlusion and other tight-junction proteins are vital for intestinal barrier structure (285). Dysbiosis of the gut, which degrades the occludins, results in the reinforcement of intestinal permeability (266, 286). Another study on PD patients investigated the increase of Ralstonia, Enterococcus, and Proteobacteria concentrations in their mucosa, leading to the elevation of proinflammatory cytokines. A substantial reduction in the population of butyrate-producing bacterial species considered anti-inflammatory, such as Blautia, Coprococcus, Faecalibacterium, and Roseburia, have been reported in the fecal samples of the subjects with PD. Moreover, it has been reported that LPS biosynthetic gene expression was increased in the microbiota of stool samples of the PD patients (24). Interestingly, it has been reported that the infections with Helicobacter pylori can be considered a significant triggering factor in the pathogenesis of PD (287). The results demonstrated that overgrowth of small intestinal bacterial communication, which is known as small intestinal bacterial overgrowth (SIBO), has been associated with motor dysfunction, especially in PD patients (288, 289).
Adams et al. investigated the presence of gingipain R1 (RgpA) using a fluorescent antibody in a PD population. These authors concluded that gingipain protease and LPS of Porphyromonas gingivalis lead to abnormal blood clots in their PD samples. Also, their finding that gingipain antibody signal was observed in only clots of PD samples confirmed the potential of this bacterium in the pathology of PD. They further suggested that infection of P. gingivalis might play an essential role in the etiology and/or a risk factor for PD (290). It has been reported that peripheral inflammation induced by P. gingivalis resulted in the imbalance of gut microbiota, a decrease of dopaminergic neurons in the substantia nigra, increased intestinal permeability, and enhanced microglial activation in the pathophysiology of leucine-rich repeat kinase 2 (LRRK2)-associated PD (291).
According to the definition by FAO/WHO, probiotics are determined as viable microorganisms, which when ingested/administered in appropriate amounts, confer a health benefit on the host. Probiotics, including lactobacilli and bifidobacteria, have been shown to alleviate PD-like conditions (Table 4) (292). Bacillus spp. as a probiotic bacterium has the ability to convert l-tyrosine to L-DOPA, which is an essential precursor molecule of dopamine, and its conversion to dopamine is carried out via DOPA decarboxylase (293). It has been reported that the regular administration of fermented milk beverages containing Lactobacillus casei shirota results in an enhancement of bowel movements through a reduction in the number of fecal staphylococci in individuals with PD (294). Microbial communities in the gut actively produce polyphenols from dietary flavanols, interfering with α-synuclein misfolding and toxicity, a fundamental pathological mechanism of the PD and other α-synucleinopathies. Investigations on heterogenic humanized gnotobiotic mice with oral administration of flavanol-rich preparation (FRP) revealed particular distinctions in the production of FRP-derived metabolites, affecting α-synuclein misfolding or inflammation. Studies on Drosophila models of α-synucleinopathy revealed its effect on motor dysfunctions, which leads to modulation of its onset and progression. In vitro investigations revealed that in bacterial fermentation, particular bacteria can produce these bioactive phenolic acids. Altogether, it has been concluded that varying the dietary flavanols induced by heterogenous gut microbiota among individuals demonstrates the potential of probiotic-, prebiotic-, and symbiotic-based strategies in modulating the progression of PD and other synucleopathies (Table 4) (295). However, most of the information about the connection between the microbiome and PD is based on studies of the colon mucus and feces microbiome. There are still no studies on other gastrointestinal disorders in PD, such as stomach infection by Helicobacter pylori and excessive growth of small intestine bacteria (24). Currently, there is no unanimity among results obtained from different studies on the correlation between the microbiome and PD. However, studies in this area are ongoing, and microbiome results produce valuable information. According to the reviews, there is a connection between the medication used by PD patients and the gut-residing microbes, which is not surprising due to the gut microbiota’s role in the metabolism of prescribed medicines and even the consequences of drugs on the microbial composition (296, 297). Therefore, study of the gastric microbiome can provide information about the toxicity of PD drugs. Further investigation is needed on the impact of Levodopa and Carbidopa and other medications on patients (298). It may be possible to predict, delay, or prevent the disease through analysis of metagenomes of bacterium-derived extracellular vesicles using human body samples (299). Also, it has been shown that a vegetarian diet, including SCFAs, will boost the level of UDPRS III and decrease the effective daily dosage of levodopa-equivalent. Thus, there may be an effective nonpharmacologic therapy for PD patients (300). A complete perception of the interaction of the GI microbiome and the GBA exists and the brain may clarify PD’s etiological and progression factors to provide new therapeutic approaches. For instance, FMT and evaluation of the gut microbiome as a novel biomarker for PD clinical diagnosis may reveal an alternative treatment to traditional therapeutic methods (Table 4) (301). Gut complaints can occur during the initial stages of PD; this could assist in the early diagnosis of the disorder, prior to the emergence of motor symptoms such as tremor and rigidity. There is no doubt that microbiome studies can provide helpful information on PD, but for now, we cannot rely on them as biomarkers (302).
TABLE 4.
Microbial intervention in Parkinson’s disease
| Source | Study design | Sample size (n) | Study population | Microbial strains | Study duration | Key finding(s) |
|---|---|---|---|---|---|---|
| Fecal microbiota transplantation | ||||||
| Huang et al., 2019 (451) | Human case report | 1 participant | A 71-yr-old male patient presented with 7 yrs of PD and intractable constipation | FMT was obtained a 26-yr-old male | 12 wks | Probable therapeutic effects of gut microbiome reconstruction on PD patients, especially those with gastrointestinal symptoms. |
| Sun et al. 2018 (452) | Preclinical animal study | 10 to 15 per group | 8-wk-old male C57BL/6 mice: (i) normal control without any treatment (n = 15); (ii) MPTP+PBS group (n = 15); and (iii) MPTP+FMT group (n = 15) | Collection of fresh fecal pellets from PD mice or healthy control mice | 1 wk | FMT ameliorates motor symptoms of PD mice. |
| Improvement of serotonin content of PD mice and striatal dopamine resulted by FMT. | ||||||
| Reducing the glial-mediated neuroinflammation through normal SCFAs improvement by FMT. | ||||||
| Reducing the gut inflammation and neuroinflammation probably by suppressing TLR4/TBK1/NF-jB/TNF-α signaling pathway. | ||||||
| Zhou et al., 2019 (453) | Preclinical animal study | 8 per group | 6-wk-old male C57BL/6J mice separated into four groups randomly: (i) NS-AL group (normal saline injection + fed ad libitum); (ii) NSFMD group (normal saline injection + fasting diet); (ii) MPTP-AL group (MPTP + fed ad libitum); and (iv) MPTP-FMD group (MPTP + fasting diet) | Feces from fed ad libitum, fasting-mimicking diet, and normal mice treated with saline by intraperitoneal injection | 4 wks | Recovering the motor function via FMD. |
| Improvement in the degeneration of dopaminergic neurons in the substantia nigra. | ||||||
| Increasing the levels of 5-HT and DA in the striatum of PD mice, possibly by reshaping gut microflora to modulate microbial dysbiosis, that contributes to the alterations in SCFAs. | ||||||
| Probiotics | ||||||
| Borazabadi et al., 2018 (454) | Randomized, double-blind, placebo-controlled trial | 50 participants | 50 individuals were randomly divided into two groups to take either placebo (n = 25 each group, one capsule daily) or 8 × 109 CFU/day probiotic supplements | Probiotic capsule comprised of Lactobacillus acidophilus L. fermentum, L. reuteri, and Bifidobacterium bifidum | 12 wks | Enhancement of TNF-α, TGF-β, IL-1, IL-8, and PPAR-γ gene expression in subjects with PD via probiotic supplementation. |
| No impact on VEGF and LDLR gene expression, oxidative stress, and inflammation biological markers. | ||||||
| Tamtaji et al, 2018 (455) | Randomized, double-blind, placebo-controlled trial | 60 participants | 60 individuals with PD (aged 50-90 yrs), randomly distributed into two groups in order to take either probiotic or placebo (n = 30 each group) | Probiotic comprised of Bifidobacterium bifidum, Lactobacillus acidophilus, L. reuteri, and L. fermentum | 12 wks | Improvement of the Movement Disorder Society-Unified Parkinson’s Disease rating scale, and insulin metabolism. |
| Georgescu et al, 2016 (456) | Randomized, placebo-controlled, double-blind trial | 40 participants | 40 PD patients (17 males, 23 females; mean age 76.05 ± 2.09 yrs) randomly divided into two groups in order to take either probiotics or trimebutine 200 mg | Lactobacillus acidophilus and Bifidobacterium infantis | 3 mo | Ameliorated the symptoms of bloating and abdominal pain. |
| Synbiotics | ||||||
| Barichella et al, 2016 (396) | Tertiary setting, single center, double-blind, randomized, placebo-controlled trial | 80 participants | Subjects with PD randomly divided into two classes, supplemented with fermented milk, including multiple probiotic strains and prebiotic fiber or placebo (a pasteurized, fermented, fiber-free milk) | Lactobacillus rhamnosus GG, L. acidophilus, L. plantarum, L. paracasei, L. delbrueckii subsp. bulgaricus, and Bifidobacterium (fermented milk), Streptococcus salivarius subsp. thermophilus, Enterococcus faecium | 4 wks | Consumption of fermented milk was better than placebo in alleviating constipation in PD patients. |
Alzheimer’s Disease
Alzheimer’s disease (AD) is a chronic irreversible brain disorder, in which progressive degeneration of brain cells leads to memory impairments, cognitive decline. It is the most common type of dementia in the elderly (303). Patients with AD demonstrate serious impairments in learning, behavior, and memory, severe enough to influence daily activities. AD is characterized by neuronal cell death in the brain and progressive synaptic failure, along with amyloid-β (Aβ) deposition around or outside neurons, accompanied by an aggregation of abnormal-phosphorylation of the microtubule-associated protein tau (or τ proteins) in dendrites and axons of cortical neurons (304–306). Aβ accumulation and aggregation of τ proteins contribute to a decrease in the stabilization of microtubules, synaptic failure, and perturbation of Ca2+ homeostasis in neurons, ultimately leading to neuronal apoptosis (307, 308). Although numerous investigations have been performed on AD etiopathology, the underlying mechanisms of AD are incompletely defined, and current Aβ therapies confer limited reduction in symptoms (309). It has been reported that amyloid might act as an AMP in the brain (310). Recent studies have discovered that the pathogenesis of AD is correlated with neuroinflammation in the CNS induced by peripheral infections (311, 312). Common characteristics of tau and Aβ deposition in AD subjects are seen in mice infected with herpes simplex virus 1 (HSV-1). The high intracellular levels of cholesterol 25-hydroxylase (CH25H) induced by viral infection are essential for modulating both the Aβ production and AD susceptibility (313, 314). Furthermore, previous studies have demonstrated the potential connections between AD and other microbial infections, including fungal, Chlamydia pneumonia, and spirochaete infections (315–317). Over the past several decades, there has been no evident treatment for AD, but there have been some glimmers of hope with the discovery that the gut microbiota may have a possible significant role in the pathogenesis of AD. Although it is not considered a new concept, some investigations have provided evidence that AD may have a microbial origin (318). Determination of microbe-derived metabolites from the gut microflora in the cerebrospinal fluid of individuals with AD is correlated with AD biomarkers, such as phosphorylated tau and tau/Aβ42, suggest the implication of the gut microbiota in the onset of AD (319). A study reported that, according to the bacterial 16S rRNA sequence analysis from Aβ precursor protein transgenic mouse (APP) fecal samples, compared to wild-type mouse model controls, significant differences had been revealed in the gut microbial compositions (320). It has also been shown transgenic mouse models with AD phenotypes have various gut microbes. The effect of the microbial intervention on AD has been summarized (Table 5) (320–322). Moreover, studies on GF mice demonstrated that no symptoms of amyloid plaque and neuroinflammation could be seen in the absence of microbes (320). Furthermore, based on the results of cross-sectional studies, the abundance of two bacterial taxa Escherichia and Shigella, which are involved in inflammatory responses, increased dramatically in fecal samples of patients with AD compared to healthy controls (323). Two main conditions, which may be related to a peripheral inflammatory state in patients who suffer from cognitive impairment and brain amyloidosis, include an increased frequency of proinflammatory Escherichia and Shigella and a decrease in the frequency of anti-inflammatory Eubacterium rectale. The idea proposed is that there is a link between gut microbiota dysbiosis and systemic inflammation that can be a contributing factor in the neurodegeneration that occurs in the brain of patients with AD (23). These observations are based on small-scale studies, and more studies with larger statistical groups are required to evaluate the gut microbiota involvement in AD progression. Some scientists have stated that the infective agents that are found in the brain of patients with AD can be involved in developing this disease, but robust evidence is essential in this regard (324). The use of synthesized neurotoxic inhibitors was reported as beneficial in a recent study for AD treatment. In this study, a pathogen called Porphyromonas gingivalis, which is involved in chronic periodontitis, was recognized in the brains of patients with AD (325). The colonization of these bacteria in the brain resulted in increased production of Aβ1-42. Moreover, the neurotoxic gingipains have damaging impacts on the tau protein Aβ1-42. Moreover, the neurotoxic gingipains have a damaging impact on the tau protein (191). Shukla et al. reported a potential association between gut microbiota dysbiosis and AD-associated neuroinflammation. They discovered a positive correlation between the increase in abnormal expression of gut NLRP3 and activation of the peripheral inflammasome, which enhances neuroinflammation with AD progression. Hence, they observed considerable alterations in the gut microbiota composition in young and aged 5xFAD mouse models compared to age-matched control mice. Also, com 5xFAD mice displayed impaired gut barrier function according to the loss of adherens and tight-junction proteins compared to nontransgenics. Moreover, it has been demonstrated that higher expression of gut microbial inflammasome proteins might be an essential leading factor in the activation of downstream cytotoxic and inflammatory mediators. Accordingly, NLRP3 inflammasome-mediated neuroinflammation may be promoted via gastrointestinal NLRP3. Therefore, gut microbiota modulation may be a possible strategy for treating AD-associated neurological disorders in genetically susceptible individuals (326). Honarpisheh et al. (327) also investigated the gastrointestinal Aβ load prior to the brain and their involvement in GBA interaction and AD. Comparing Tg2576 mouse models of AD, including presymptomatic and symptomatic transgenics to wild types, these researchers observed that vascular Aβ peptide deposition in the intestinal epithelial barrier (IEB) disrupted the IEB and that dysregulation of absorption occurs before its cerebral aggregation. These researchers also concluded that alterations on the GBA are correlated with higher levels of inflammatory plasma cytokines, such as IL-9, IP-10, and VEGF. Considering the gut dysfunction in AD patients, future therapeutic strategies for AD treatment might involve the early manipulation of gut microbiota (327). According to the involvement of gut microbiota in the development of Aβ pathology in AD, Wang et al. developed a novel framework to determine AD underlying mechanism via GBA and translate manipulation of gut microbiota to reach clinical practice. Using APPSWE/PS1ΔE9 mice receiving the fecal donor transplantation from aged (16 months) APPSWE/PS1ΔE9 mice following short-term antibiotic cocktail treatment, Wang et al. collected fecal pellets for further analysis. FMT reconstitution in pre-antibiotic-treated mice was attributable primarily to the donor sources such as Clostridia and Coriobacteriaceae, contributing to higher deposition of Aβ plaques. Intriguingly, after microbiota engraftment, activation of astrocytes surrounding Aβ plaques was inhibited rather than microglia (328).
TABLE 5.
Microbial intervention in Alzheimer’s disease
| Source | Study design | Sample size (n) | Study population | Microbial strains | Study duration | Key finding(s) |
|---|---|---|---|---|---|---|
| Fecal microbiota transplantation | ||||||
| Dodiya et al., 2019 (322) | Preclinical animal study | 8 to 9 per group per analysis | APPPS1-21 transgenic mouse models. Relevant groups (all AB-treated APPPS1-21 male mice): (i) FMT, APPPS1-21-FMT; (ii) no FMT, vehicle | FMT from age-matched APPPS1-21 male donor mice | 24 days | ABX-mediated perturbations of the microbiota have sex-specific, selective influence on microglial homeostasis and brain Aβ amyloidosis. |
| Fujii et al., 2019 (457) | Preclinical animal study | 7 per group | Mice receiving feces from a human AD patient. Relevant groups (FMT (GF [WT mice]): (i) human-HC-FMT and (ii) human-AD-FMT | Subjects with AD and age-matched HC | 71 wks | Alteration of fecal metabolome and behavior in recipient mice via FMT from Alzheimer’s disease patients. |
| Zhan et al., 2018 (458) | Preclinical animal study | 7 to 8 per group per analysis | Mice receiving feces from SAMP8 mice. Relevant groups: FMT, (i) SAMP8-FMT and (ii) SAMR1-FMT; no FMT, (iii) control and (iv) vehicle (pseudo-GF). | Feces from SAMP8 or SAMR1 mice | 21 days from first FMT | Cognitive dysfunction in SAMP8 mice due to abnormal microbial communities of the gut microbiota. |
| Probiotics | ||||||
| Leblhuber et al., 2018 (459) | Explorative intervention study | 20 participants | 11 males, 9 females, aged 76.7 ± 9.6 yrs of patients with AD | Lactobacillus casei W56, L. lactis W19, L. acidophilus W22, L. paracasei W20, L. plantarum W62, L. salivarius W24, Bifidobacterium lactis W51, B. bifidum W23, and B. lactis W52 | 28 days | Influencing the tryptophan metabolism and the composition of gastrointestinal bacteria. |
| Association between neopterin concentrations and Kyn/Trp contributing to the activation of dendritic cells and/or macrophages. | ||||||
| Den et al., 2020 (460) | Meta-analysis of randomized controlled trials | – | Five investigation comprised of 154 and 143 cases in the probiotics and control group, respectively | – | – | Enhancement of cognition in MCI or AD patients or through probiotic administration, potentially reducing the levels of oxidative and inflammatory biomarkers. |
| Tamtaji et al., 2018 (461) | Randomized, double-blind, controlled trial | 79 participants | 27 patients receiving selenium and probiotic supplementation and 26 patients receiving only selenium as Placebo | Selenium (200 μg/day) in addition to probiotic containing Bifidobacterium bifidum, B. longum, and Lactobacillus acidophilus | 12 wks | Ameliorated function of cognition and some improvement in metabolic profiles through cosupplementation of probiotic and selenium to subjects with AD. |
| Hwang et al., 2019 (462) | Multicenter, randomized, double-blind, controlled trial | 100 participants | 100 individuals with Mild cognitive impairment (MCI) were determined to take placebo (800 mg/day, n = 50) or DW2009 randomly (800 mg/day, n = 50) | Lactobacillus plantarum C29 | 12 wks | Safe administration of DW2009 to improve cognitive function in individuals with MCI. |
| Kobayashi et al., 2019 (463) | Randomized, double-blind, controlled trial | 121 participants | Bifidobacterium breve A1 = 61; placebo = 60 | Sole (Bifidobacterium breve A1) | 12 wks | Considerable difference between placebo groups and B. breve A1 and terms of MMSE total score in the MCI patients. |
| Agahi et al., 2018 (464) | Randomized, double-blind, controlled trial | 48 participants | 23 control group receiving placebo capsules (500 mg maltodextrin); 25 probiotic group receiving capsules containing a mixture of probiotic bacteria | Bifidobacterium bifidum, B. longum, B. lactis, Lactobacillus fermentum, L. plantarum, and L. acidophilus | 12 wks | No significant impact of probiotic administration in subjects with severe AD. |
The prolonged administration of broad-spectrum antibiotics could also decrease Aβ accumulation and regulate the innate immunological responses influencing Aβ amyloidosis in the mouse models with AD (329). Moreover, a reduction in microglia and astrocyte aggregation around amyloid plaques in the hippocampus, as well as insoluble Aβ plaques, was reported through periodic treatment with antibiotic cocktails in transgenic mice (322, 330). It has also been reported that by comparing fecal SCFAs and microbial composition between wild-type and AD mouse models at different ages, a considerable reduction of Butyricicoccus and Ruminococcus, and elevations in Proteobacteria and Verrucomicrobia in mice with AD phenotypes were observed, providing the altered microbial composition and diversity. The decline in the level of SCFAs suggests perturbation in at least 30 metabolic pathways (321). A prior study has also suggested that microglial activation inhibits the Aβ clearance and degradation and that further Aβ accumulation leads to the pathology of AD. Moreover, elevated levels of Aβ deposits contribute to the release of several proinflammatory mediators within microglia, such as ROS, iNOS, NF-κB, and COX2, thereby promoting neuroinflammation in AD patients (331).
These investigations indicate that certain species of the gut microbiota can activate Aβ signaling pathways and contribute to the pathogenesis of AD and also play a crucial role in the molecular modulation of AD. Probiotic supplementation and nutritional interventions may become a promising therapeutic approach to hinder AD progression.
Epilepsy
Epilepsy is a debilitating neurological disorder that affects about 65 million people worldwide (332). Although there have been many new advances in medical sciences, the exact etiopathology still needs to be fully elucidated (333). Accordingly, in about one-half of patients with epilepsy, the cause of the disease is unknown. It has been reported that epileptic patients spend 13 times more on medical expenses than do normal subjects (334). The remarkable socioeconomic impact of epilepsy is due to a higher mortality and disability rate than for the normal population (335, 336). Consequently, these data indicate that much effort is needed to find more effective strategies toward prevention, curing, and treatment. Today, only 70% of patients affected by epilepsy will achieve full seizure control, despite being on drug therapy with anti-epileptic drugs (AEDs) (332). Therefore, about one-third of patients with epilepsy will have refractory seizures that affect their daily living activities. It has been found that both environmental and genetic factors determine predisposition to epilepsy. Also, several studies suggest an association between intestinal bacterial species and the pathophysiology of epilepsy. These findings imply the gut microbiota’s role in treating epilepsy (337). Gut microbiome dysbiosis is associated with the development of neuropsychiatric diseases such as epilepsy (332). It has been reported that the healthy gut microflora consists of microbial communities, which indicates both anti- and proinflammatory effects. As a result, there is a correlation between a balanced gut microbiota and a healthy brain and the immune system (338). Recent studies have suggested that chronic inflammation plays a significant role in the onset and progression of epilepsy (339). It has also been demonstrated that gut microflora can regulate immune and inflammatory responses. Consequently, manipulating the gut microbiome has potential as a therapeutic strategy for epilepsy. Alternative treatment strategies for uncontrolled epilepsy include VN stimulation and a ketogenic diet (KD) (332). Hence, manipulating the diversity of the gut microbiota can be considered a potential therapeutic approach. It has been found in several studies that there is a variation of gut microbial profiles between cases with various types of therapeutic approaches of uncontrolled epilepsy compared to the healthy population. All these studies indicated an increased Firmicutes/Bacteroidetes ratio in uncontrolled epilepsy (340–342). Some bacteria belonging to the phylum Firmicutes are capable of regulating neurotransmitter levels (341). Further analysis of the gut microbiota, including α-diversity, indicates remarkable diverging outcomes. In another study, Peng et al. reported the increased abundance of Firmicutes compared to Bacteroidetes. Also, measurements of α-diversity in the drug-resistant patients compared to drug-responsive patients demonstrated similarity to the healthy subjects. Substantially, higher levels of α-diversity were linked to an unusually increased number of rare intestinal bacterial species. Also, at the genus level, significant differences were reported. According to these outcomes, it can be assumed that bacteria have a role in the effective treatment of epilepsy. Interestingly, the intestinal gut microbiota can modulate zonisamide metabolism, which is an antiepileptic drug (343). Moreover, increased numbers of Lactobacillus and Bifidobacterium revealed a correlation with fewer seizures per year (341). It has been reported that a KD in epileptic patients reduces the rate of seizures and is also linked to an alteration of intestinal microflora composition and function (344). Sewal et al. reported that the intraperitoneal injection of LPS induced a higher predisposition to seizure in rats, while increasing the permeability of the BBB and higher levels of proinflammatory cytokines in the brain. (345). Moreover, controversial findings have been reported on whether antibiotic administration induces or protect against seizures in preclinical and clinical studies. Significantly, possible pro-epileptic effects of the underlying infectious disease during treatment or direct antibiotic-induced neurotoxic side effects might rather be involved. In addition, several investigations have indicated a positive effect of supplementation of probiotics in epilepsy (346, 347). Medel-Matus et al. reported that FMT from chronically stressed rat donors accelerated kindling and also the duration of kindled seizures in sham-stressed rats (348). Another study by Olson et al. indicated that a ketogenic diet mediates the antiseizure effects in a GF mouse model for temporal lobe epilepsy. Indeed, these researchers found that the seizure threshold in SPF mice elevated after transplantation with KD microbiota or long-term treatment of bacterial species, including Akkermansia muciniphila, Parabacteroides distasonis, and Parabacteroides merdae (349).
Stroke and Brain Injury
Globally, stroke and brain injury are important causes of morbidity and mortality. Commensal bacteria of the gut microflora could be associated with the development of stroke through the modulation of several risk factors of cerebrovascular disease, including atherosclerosis, diabetes, dyslipidemia, and arterial hypertension. Diet can also be considered a significant risk factor. In addition, linking atherosclerosis and dysbiosis can directly influence microbiome composition and diversity (350, 351). However, mounting evidence suggests that the gut microbiome might play a more direct role in cerebrovascular diseases and stroke. Trimethylamine n-oxide (TMAO) as a microbiota-derived metabolite can be synthesized from dietary choline, which can be detected in body fluids and tissues. Recent investigations indicate that TMAO is associated with increased risk of cerebrovascular and cardiovascular diseases, suggesting the probable modulation of gut microflora through the therapeutic potential of this metabolite (352, 353). Moreover, cross-sectional investigations implied an imbalance in the composition of the gut microbiota in individuals with stroke compared to healthy controls. The elevated levels of TMAO in plasma samples were associated with a higher risk of stroke and cardiovascular events in a dose-dependent manner in a longitudinal study of more than 4,000 cases. The reduction of TMAO levels induced by antibiotic supplementation highlighted the importance of intestinal bacterial species in the synthesis of this compound (354). Meanwhile, patients with stroke and transient ischemic attack showed relatively lower levels of TMAO compared to the cases with asymptomatic atherosclerosis (354). Preclinical studies indicate that administration of phosphatidylcholine metabolites such as choline and TMAO can upregulate the expression of macrophage scavenger receptors involved in atherosclerosis, which probably occurs due to the presence of bacterial species residing in the gut. Moreover, research on GF mice demonstrated that the administration of choline is not correlated with a higher rate of atherosclerosis and contributes to a decrease in the aortic plaque volume (355, 356). However, regarding the effect of diet on both TMAO and choline and also the detrimental and protective impact of the gut microbiota in the onset and progression of atherosclerosis, it is important not to overinterpret the results of preclinical studies (355). A healthy microbiome plays a substantial part in the recovery of atherosclerotic lesions. Supplementation of broad-spectrum antibiotics following middle-cerebral-artery occlusion is associated with reduced survival in mice (357). Antibiotic-induced alterations in GI microflora also led to downregulation of IL-17-associated chemokine expression and a decrease in the migration of proinflammatory IL-17 γδ T cells. Therefore, the intestinal bacterial species emerge to modulate the neuroinflammation after a stroke by regulating intestinal T-cell infiltration to the brain (358). Moreover, post-stroke FMT containing SCFA-producers, including Lactobacillus fermentum, Bifidobacterium longum, Faecalibacterium prausnitzii, and Clostridium symbiosum, relieved cognitive impairments and inflammation after stroke and also increase plasma, gut, and brain SCFA concentrations, promoting post-stroke recovery in aged models (359). It has also been reported that transferring FMT from individuals with stroke into antibiotic-administered mice and also from a stroke model into GF mice increased the size of ischemic cerebral lesions and associated functional impairments. In addition, a reduction in the number and diversity of Bacteroidetes has been reported subsequent to the stroke (350). Recent research on the potential of microbial intervention in the prevention and treatment of epilepsy has been summarized in Table 6. Another study implied that patients with transient ischemic attack and stroke show a higher number of opportunistic pathogens, including Desulfovibrio, Enterobacter, Megasphaera, and Osicillibacter, and a decreased number of the beneficial or commensal genera, such as Bacteroides, Faecalibacterium, and Prevotella (354). Moreover, the increased abundance of Prevotellaceae and Peptococcaceae shows a correlation with stroke severity (351). Accordingly, treatment with a particular bacterial strain, i.e., C. butyricum, improved cognitive function in a mouse model with ischemia/reperfusion and decreased neuronal injury (360). According to these explanations, the role of the gut microbiota in the onset and progression of stroke and brain injury is not yet fully understood. Although preclinical and clinical studies have provided intriguing results (Table 7), further studies are required. Supplementing a diet with psychobiotics has been suggested to reduce psychiatric outcomes and comorbidity following traumatic brain injury (361). However, more clinical investigations are needed to elucidate the potential of such microbial therapeutic interventions.
TABLE 6.
Microbial intervention in epilepsy
| Source | Study design | Sample size (n) | Study population | Microbial intervention | Study duration | Key finding(s) |
|---|---|---|---|---|---|---|
| Fecal microbiota transplantation | ||||||
| He et al., 2017 (465) | Human case report of generalized epilepsy | 1 participant | A 22-yr-old girl, with a 17-yr history of epilepsy | FMT was obtained from a primary school girl by gastroscopy under anaesthesia | 20 mo | Seizure-free without antiepileptic drugs. |
| Decreasing the Crohn’s disease activity index or CDAI to 104 points after 12-mo regulation of menstrual cycle. | ||||||
| Medel-Matus et al., 2018 (348) | Preclinical study–animal study | 6 per group | The study included the following groups: (i) sham stress, no FMT; (ii) stress, no FMT; (iii) sham‐stressed recipients, FMT from sham‐stressed donors; (iv) stressed recipients, FMT from sham‐stressed donors; (v) sham‐stressed recipients, FMT from stressed donors; and (vi) stressed recipients, FMT from stressed donors. | FMT was obtained by one anonymous healthy donor | 18 mo | Perturbations in the GI microflora induced by chronic stress led to increased susceptibility to epilepsy. |
| Probiotics | ||||||
| Yeom et al., 2018 (347) | Prospective observational study | 228 participants | Neonates, rotavirus positive (n = 78); age, 6.2 (±4.1) days | Probiotic supplementation containing Saccharomyces boulardii and only one with Lactobacillus casei | 4 wks | Immediate supplementation of probiotics after birth may reduce neonatal seizures associated with rotavirus about 10-fold. |
| Gómez-Eguílaz et al. 2018 (346) | Interventional, Prospective and Open label study | 45 participants | Patients with drug resistant epilepsy (n = 45); mean age, 44 (±13.5) yrs | Probiotic supplementation containing Bacteroides, Lactobacillus, and Streptococcus spp. | 4 mo | Seizure no. reduction in 28.9% of patients (≥50%). |
| Improvement in Quality of Life score. | ||||||
| Bagheri et al. 2019 (466) | Preclinical study–animal model | 8 per group (n = 40) | 2-mo-old pentylenetetrazol-induced epilepsy in male Wistar rats | Probiotic supplement with Bifidobacterium infantis, Lactobacillus reuteri, and L. rhamnosus | 3 wks | Improvement in epileptic condition and cognitive behavior, ↓MDA, and NO. |
TABLE 7.
Microbial intervention in stroke and brain injury
| Source | Study design | Sample size (n) | Study population | Microbial intervention | Study duration | Key finding(s) |
|---|---|---|---|---|---|---|
| Fecal microbiota transplantation | ||||||
| Spychala et al., 2018 (467) | Preclinical study–animal study | 2 to 16 per group per analysis | C57BL/6 male mice (18–20 mo old) with MCAO (middle cerebral artery occlusion)-induced transient focal cerebral ischemia | FMT was obtained from young male C57BL/6 male mice (8–12 wks) | 2 mo | 9‐Fold decrease in Firmicutes/Bacteroidetes ratio. |
| Improved recovery and increased survival following MCAO. | ||||||
| Xia et al., 2019 (468) | Preclinical study–animal study | 10 per group, 4 to 9 per group per analysis | 6-wk-old male C57BL/6 mice with MCAO-induced transient focal cerebral ischemia | FMT was obtained from three individuals of lower stroke dysbiosis index (SDI) or higher SDI | 17 days | FMT obtained from high-SDI patients developed severe brain injury, and also increased IL-17+ γδ T cells in the gut rather than FMT from low-SDI patient. |
| Probiotics | ||||||
| Akhoundzad et al., 2018 (469) | Preclinical study–animal model | 5 per group (n = 30) | Focal cerebral ischemia in BLC57 mice | Bifidobacterium breve, Lactobacillus acidophilus, L. bulgaricus, L. casei, | 2 wks | Improvement in stroke conditions or brain ischemic damage. |
| Higher levels of malondialdehyde. | ||||||
| Reduction of TNF-α. | ||||||
| Gong et al., 2021 (470) | Prospective observational study | 40 participants | Premature infants born by C-section at 28 to 37 wks gestational age | Bifidobacterium longum, Enterococcus faecalis, and Lactobacillus acidophilus | 4 mo | Restoration of the normal gut microbiota associated with the decreased brain injury. |
| Rahmati et al., 2019 (471) | Preclinical study–animal model | 10 per group (n = 50) | Male Swiss albino mice (2 to 4 mo old) | Bifidobacterium breve ZTBbr.22, B. longum ZT-Lca.106, Lactobacillus acidophilus ZTLac.51, L. bulgaricus ZT-Lbu.90, L. casei ZT-Lca.106, L. rhamnosus ZT Lrh.54, and Streptococcus thermophilus ZT-Sth | 3 wks | Reduced damage of hippocampus. |
| Prevention of the memory deficit and spatial learning by apoptotic suppression. | ||||||
| Synbiotics | ||||||
| Jahangiri et al., 2017 (472) | Randomized clinical trial | 65 participants | Ischemic and hemorrhagic patients (between the ages of 20 and 80) | Synbiotic supplement including Lactobacillus, Bifidobacterium, Streptococcus thermophilus, and fructooligosaccharides | 1 wk | Increasing the frequency of defecation in patients. |
ROLE OF MICROBIAL INTERVENTION IN NEUROLOGICAL DISORDERS
Fecal Microbiota Transplantation
Based on the recent findings revealing the potential of the microbial interventions in the regulation of intestinal dysbiosis-driven neurological disorders, fecal microbiota transplantation (FMT) appears to be a promising therapeutic strategy (Fig. 5). This relatively new treatment method comprises the transfer from a healthy stool sample donor, along with its microbes and metabolites, to a recipient (297). This method is currently being used to treat Clostridium difficile infections, considered a hospital infection, and is used along with antibiotic treatment (297). Through FMT, the healthy microbiota replaces itself via reproduction and produces bioactive metabolites. FMT is done by using endoscopies, enemas, and freeze-dried material oral feeding. The potential of this method has been examined for the treatment of neurological disorders such as PD, ASD, and MS (18, 299–301). One of the advantages of this method is that no significant side effect has been reported, and it is considered safe, even in high-risk patients (302, 303). In a recent study on ASD mice, the effect of an in vitro cultured gut microbial transplant (GMT) was evaluated, which considerably alleviated the anxiety-like behavior in mice (304). In another study by Zhao et al., groups with ASD who received FMT were examined via colonoscopy, and the results proved that ASD-related symptoms improved drastically and that their gut microbiota changed to a healthy state. However, more studies are needed for further clarification of the impact of FMT on ASD patients (305). Numerous studies have been conducted on AD in animal models (290, 292, 306), although no specific research have been conducted on human patients. The results of a study on mouse models of AD demonstrated that cognitive dysfunction was related to a change in gut microbiota composition; therefore, modifying this microbiota through FMT proved to be effective in alleviating the cognitive dysfunction in AD (307). Many studies have been done on FMT on various neurological diseases, and many trials are ongoing. Thus, a bulk of evidence will be available shortly.
FIG 5.
Modulation of gut microbiota by therapeutic microbial interventions. Microbial interventions, including probiotics, prebiotics postbiotics, synbiotics, and fecal microbiota transplant (FMT), improve the microbiota-gut-brain axis through modification of the microbial communities. Modulation of the microbial composition, regulation of their essential metabolites, or both can improve neurological complications by increasing neurochemicals and SCFAs and reducing intestinal permeability, regulation of neural, metabolic, and immune pathways. Each approach can be improved by personalized medicine for more effective management of a patient’s pathophysiology.
Probiotic
Variations in the gut microbiota composition and function among healthy individuals and patients have been identified for various neurological disorders. It has been acknowledged that diet can affect the microbiome composition, modifying the function of the GBA. Multiple therapeutic interventions have been applied to treat gut microbiome dysbiosis, bringing back the intestinal microflora balance and improving clinical outcomes in neurological disorders, including the use of probiotics (362). The word “probiotic” was first coined in 1974 and is defined by the World Health Organization (WHO) as live microorganisms with beneficial impacts on host health if taken in appropriate amounts (363). The use of probiotics in common foods and also as pills is gaining popularity (364). Probiotics are mainly comprised of Bifidobacterium and lactic acid-producing bacteria, e.g., Lactobacillus. Mounting evidence suggests that metabolites synthesized by probiotics are essential mediators of diet-induced host-microbe interactions. Also, it has been reported that several gut-residing bacterial species, such as Bacteroides, Clostridium, Bifidobacterium, Peptostreptococcus, Lactobacillus, and Ruminococcus, can produce multiple tryptophan catabolites, including indole, 3-methylindole, indoleacetic acid (IAA), tryptamine, etc. (365–370). Emerging data indicate that microbiome-derived tryptophan catabolites affect host health. It has been demonstrated that these metabolites can bind to the AhR, leading to the activation of the immune system, improving the intestinal barrier function, stimulating gastric motor activity (as well as the secretion of GI hormones), exerting systemic or local antioxidant, anti-inflammatory effects, and possibly modulating the gut microbiome and metabolome (64, 371). It has been reported that tryptophan catabolites synthesized by commensal microbiota induce microglial AhR activation, inhibiting the activation of NF-κB signaling, VEGF-B, and TGF-α production (372). Furthermore, AhR is highly expressed in dendritic cells, controlling differentiation and function (373). The production of retinoic acid, kynurenine, and AhR-driven cytokine in dendritic cells enhances the differentiation of T-reg cells, suppressing the development of EAE as an animal model of MS (374). In addition, astrocytes play pivotal roles in inflammation-mediated neurodegeneration by exerting neurotoxic effects and activating and recruiting other cells implicated in the pathogenesis of CNS (375). It has also been reported that transcriptional profiles of astrocytes demonstrated upregulated AhR expression in terms of EAE and animal models of MS (376). Recent studies supported by clinical evidence related to several neurological disorders, including ASD and PD, have demonstrated increasing attention on using pro- and prebiotics to modulate the GI microbiota. Altogether, these findings have led researchers to examine probiotics in various neurological dysfunctions (377). Several investigations using mouse models have revealed that the administration of probiotics can be beneficial for several neurological disorders (e.g., ASD, epilepsy, and AD), causing improvement in cognitive outcomes. However, there is still little clinical evidence on the effectiveness of probiotic administration in neurological dysfunction in humans (Fig. 5) (378). An investigation of several children with ASD aged from 3 to 12 years, with anxiety and GI symptoms, found that the administration of a particular formulation termed VISBIOME that included eight different probiotic strains, mainly Lactobacillus, was safe, and resulted in health improvements in the ASD and GI symptoms of the patients who retained Lactobacillus (379). Moreover, promising results have been found when using probiotics to treat human neurodegenerative disorders, including AD. First, it has been reported that Lactobacillus plantarum was able to improve cognition and increase the levels of acetylcholine esterase in the brain in a mouse model of AD (380). Similar results have been found in other studies due to Aβ injection with Lactobacillus acidophilus, Lactobacillus fermentum, Bifidobacterium lactis, and Bifidobacterium longum in rodent sporadic models with AD (381–383). Another randomized clinical study identified that probiotic administration with Lactobacillus rhamnosus GG (ATCC 53103) could potentially reduce ADHD development in 75 infants with ASD and could reduce the development of neuropsychiatric disorders (384). Evidence also demonstrates the impact of probiotics in PD patients (385). A recent study showed that long-term administration of probiotics consisting of six bacterial strains alleviated the motor impairments and had neuroprotective effects on dopaminergic neurons in a genetic mouse model of PD (386).
Prebiotic
According to the International Scientific Association for Probiotics and Prebiotics (ISAPP), “a prebiotic is termed for its nonviable food components that are specifically utilized by the host-microbial populations and has health benefits.” As an alternative to probiotic supplementation, prebiotics can be used to regulate the gut microbial flora (387). This group of compounds is identified by their ability to affect the health of the GI tract and consists of nondigestible oligosaccharides (NDOs), human milk oligosaccharides (HMOs), and soluble, fermentable fibers (Fig. 5) (387). Despite the potential of prebiotic therapies in enhancing beneficial bacteria such as bifidobacteria and lactobacilli, only a small number of studies have examined the beneficial impacts of these compounds on gut microflora in both humans and animals. An investigation of the effect of galacto- and fructo-oligosaccharides or their combination in male mice showed that these compounds have antidepressant, anxiolytic impacts and reverse the effects of chronic stress (388). In a placebo-controlled clinical trial, the administration of N-acetylcysteine for 8 weeks conferred a decline in irritability and repetitive behavior in infants with ASD (389). Moreover, supplementation with of B GOS (Bimuno), a commercial prebiotic drug, and a restrictive diet led to the amelioration of the behavior of the ASD children, probably due to higher levels of Lactobacillus and Bifidobacterium abundance (390). A recent study has also shown that prebiotic lactulose can improve a cognitive deficit in AD mouse models through autophagy and anti-inflammation pathways (391). Consequently, these findings appear to be show that probiotics and prebiotics can be effective treatment options for neurological disorders. However, additional investigations are required to understand the underlying mechanisms in detail, considering that mere correlation does not necessarily indicate causation.
Synbiotic
Synbiotics are defined as a mixture of prebiotics and probiotics in which the prebiotics favor the probiotic microorganisms in terms of their growth and metabolism and improve their viability and benefits, influencing the host by increasing the abundance of beneficial microbes in the GIT. The combination used in synbiotics must be appropriate so that the probiotic microorganism’s survival in the GIT is supported (392). Studies have demonstrated that the use of synbiotics is more effective than the use of probiotics or prebiotics alone (393, 394). The results have shown that a synbiotic agent consists of GOS and a multistrain probiotic, including Lactobacillus helveticus and Bifidobacterium longum, resulted in a reduction of the symptoms of depression and improved the tryptophan signaling in MDD (395). The results of the treatment with a synbiotic containing multistrain probiotics and a prebiotic in a randomized control trial led to an improvement in functional GI symptoms in a PD cohort (Fig. 5) (396). The administration of Bifidobacterium infantis with oligosaccharide as a synbiotic proved effective in alleviating the gut-related disorders in ASD (397). However, more studies are needed on the effects of synbiotics on the MGBA (398).
Postbiotic
Postbiotics, also known as metabiotics, biogenics, or CFSs (cell-free supernatants), consist of bacterial fermentation metabolites and soluble factors obtained from live bacteria or released after bacterial cell lysis (363, 399), such as SCFAs, enzymes, AMPs, teichoic acids, endo- and exopolysaccharides, cell surface proteins, vitamins, plasmalogens, and organic acids (400). Paraprobiotics are defined as nonviable or inactivated microbial cells, while some researchers include them as a subgroup of postbiotic (331, 401, 402). Paraprobiotics are structural components that may trigger biological activity of the hosts if administered in proper amounts. Inactivation can be achieved via various methods such as physical (heat-killed probiotics, UV irradiation, or sonication) or chemical methods (325). The two terms—postbiotics and paraprobiotics—were coined in recent years to clarify that probiotics are not the only viable compounds to positively impact human health (403). The bioactive compounds, such as gut peptides, which are the result of bacterial interactions with the host, are considered postbiotics. Few investigations have been conducted on the impact of bioactive compounds on brain health, although recent investigations have shed light on the general effect of the gut microbiota and SCFAs on specific diseases (404). Regarding brain health, research on mice suffering from psychosocial stress treated with a SCFA combination (acetate, propionate, and butyrate) demonstrated anxiolytic effects (405). Rats treated with propionic acid showed a phenotype similar to autism (406). It has been shown that the gut microbiota can affect behavior, stress, anxiety, and depression (407). Although the gut peptides cannot directly be used as an intervention with the GBA signaling, a lucrative psychobiotics therapy is aimed at specific microbiota to modulate specific gut peptides (Fig. 5).
There are some studies on paraprobiotic efficacy on the MGBA in humans. Treatment of medical students under university exam stress with a heat-killed washed paraprobiotic CP2305 for 12 weeks reduced their stress levels and ameliorated their sleeping patterns and basal cortisol output (408). A number of studies have shown the benefits of several heat-killed probiotics to alleviate anxiety, depression, and chronic stress in preclinical models. Wei et al. reported that heat-killed L. paracasei PS23 alleviates the corticosterone-induced anxiogenic-like phenotype, improving dopamine levels in the hippocampus and prefrontal cortex. Heat-killed paraprobiotics would benefit from probiotics due to an increased shelf life and safety profile, showing an essential advantage in marketing (409). However, heat-killed preparations are not always effective on all probiotic strains. In an investigation done by Liu et al., it has been reported that heat-killed L. plantarum PS128 showed no statistical difference compared to the GF group (409).
Psychotropics and the Microbiome
Mounting evidence suggests that there has been an increased insight into the importance of the gut microbiome in mediating both the efficacy and the adverse effects of different medications such as psychotropics. Undoubtedly, antibiotics demonstrate the most effective and direct way of influencing the GI microflora (410, 411). Meanwhile, emerging evidence suggests that in addition to the modulation of drug pharmacokinetics, nonantibiotic drugs can alter gut microbiome structure, with potential impacts on mood and behavior (412). On the other hand, there is growing emphasis on the interactions between the gut microbiome and drugs, supporting the notion that gut microflora can affect drug metabolism and absorption. In a large-scale cohort investigation, Falony et al. reported that medical interventions, including antibiotics, antidepressants, benzodiazepines, etc., can alter the composition of the gut microbiome (296). Moreover, deep sequencing of gut microbiomes from 1,135 individuals demonstrated an association between the gut microbiota and various groups of drugs (297). Predictably, antibiotics were substantially correlated with alterations in the GI microbiome. Remarkably, the authors provided evidence for effects on the GI microbiome by several other therapeutic drugs, such as metformin, laxatives, statins, and proton pump inhibitors (PPIs). Polypharmacy, which refers to the concurrent use of several medications for the treatment of a patient, has also been linked with alteration of the gut microbiota. One investigation revealed that there was a notable negative correlation between the number of administered medicines and microbial diversity (413). Particularly, antidepressants, PPIs, and antipsychotics had the greatest association with taxon abundance. Furthermore, Tomizawa et al. investigated the effect of psychotropic medications on the GI microflora of 40 patients with anxiety and/or MDD. In their cohort study, the researchers concluded that antipsychotics reduced GI microbiome α-diversity. These researchers determined that the dosage of antipsychotics was negatively associated with α-diversity in these patients (414). Multiple in vitro studies have been performed to assess the antimicrobial activities of nonantibiotic drugs (415–418), all of which have been shown to have antimicrobial activity, possibly affecting CNS function by interacting with a special molecular target. Other reports revealed the antimicrobial activity of antidepressant selective serotonin reuptake inhibitors (SSRIs), fluoxetine, sertraline, citalopram, and paroxetine on Bacillus, Clostridium, Enterococcus, Pseudomonas, and Staphylococcus strains (412, 419, 420). In a recent study by Chait et al., the authors tested the antimicrobial activity of different classes of antidepressants on 12 commensal bacterial strains of the GI microflora. Most of the antidepressants examined possessed a considerable concentration-dependent inhibition on the growth of examined bacterial strains (421). Moreover, in an in vivo investigation on male BALB/c mice gut microbiota, the authors found that desipramine could increase in β-diversity and reduced richness compared to controls (422). These authors also found that the number of Adlercreutzia, Ruminococcus, and unclassified Alphaproteobacteria was reduced in the mouse models supplemented with desipramine. Tricyclic antidepressants, including amitriptyline, have also been shown to exert in vitro antimicrobial activity on pathogenic bacterial strains, e.g., Bacillus spp., Staphylococcus spp., and Vibrio cholerae (423), and imipramine exhibited growth inhibition on Yersinia enterocolitica and E. coli, respectively (424). The effects of psychotropic drugs on the gut microbiota composition were examined in a cohort study of elderly hospitalized patients. Within the tested medicines, antipsychotics showed the highest negative correlation with microbial community alpha diversity compared to PPIs and antidepressants. In another investigation on subjects with bipolar disease, Flowers et al. reported that the treatment with atypical antipsychotics (APP) is correlated with decreased microbial diversity in females, but not in APP-treated male patients (425). Patients in this cohort administered APPs exhibited a considerable increase and decrease in the number of Lachnospiraceae and Akkermansia, respectively. In a large-scale, in vitro screening study of more than 1,000 drugs against 40 representatives of gut commensals, it was reported that 24% of the tested drugs showed growth inhibition on at least one bacterial strain (412). These drugs exerted antimicrobial activity against a considerably similar species pattern, suggesting that direct antibacterial activity might be a part of their pharmacological effects, which should not be considered a side effect (426). Hence, there is an urgent need to assess the potential impacts of psychotropic medications on GI microflora.
CONCLUSION AND FUTURE AIMS
Awareness of neurological diseases is increasing around the world, and it is predicted that by 2030, the number of individuals with neurological disorders will increase by 13%. Due to the complex etiopathology of neurological diseases, it is important to identify more reliable biomarkers and practical therapeutic approaches. An enormous number of microbes, such as archaea, bacteria, protists, and fungi, generally termed the microbiome, reside in and on our bodies. Numerous investigations have concluded that an imbalance in the composition of GI microflora is correlated with particular abnormal physiological conditions, emphasizing the importance of the MGBA in an individual’s health status. The presence of a biological link between the gastrointestinal microbial communities, the CNS, and immune signaling implies that both immunological and neurological activities in the brain could be impacted either directly by microbiota-derived products or indirectly by systemic microbial signals. Meanwhile, more studies are essential to achieve a comprehensive perspective on the GBA. Numerous investigations indicate that the gut microbiome is crucial for the proper development and function of the brain. Several preclinical and clinical studies concluded that the GIT microbiome has implications in neurological dysfunctions, including AD, MS, PD, and ASD. However, many complementary studies need to be conducted, and overinterpretation of data should be avoided. Hence, to elucidate the complex interactions between the MGBA and neurological dysfunction, more appropriate designs and controlled investigations are necessary. The application of interventional approaches, such as probiotics, prebiotics, and fecal transplantation therapies, can aid researchers in identifying more comprehensive causes and the effects of underlying pathways. Application of microbial interventions, such as FMT, supplementation of probiotics, and prebiotics, etc., in the prevention and treatment of neurological disorders as a routine therapeutic strategy raises some questions and challenges. Microbial interventions may theoretically be responsible for several side effects, including sepsis, adverse metabolic activities, gene transfer, and excessive immune response activation in susceptible individuals (427). Another extremely important challenge involves the safety and tolerability of microbial therapeutic interventions in high-risk groups such as children, immunocompromised patients, and elderly individuals. Therefore, according to the greater risk of any microbial therapeutic intervention in high-risk groups, more caution should be considered to avoid possible side effects. Despite several studies reporting the safety of probiotics, some authors have recently performed more investigation with more clinical trials to detect possible side effects (428). Consequently, more studies on the effect of microbial therapeutic interventions possible interactions with other therapies, the appropriate size of the sample, and longer follow-up studies should be considered. Since the beneficial effects on neurologic disorders are different and dependent on the therapeutic bacterial strain, it is essential to conduct more extensive studies to identify the most beneficial single or microbial formulation for each particular neurologic disease (429). In addition, it is crucial to consider the adequate amounts of probiotics and other microbial interventions when administered. The optimal dose and duration of treatment have not been completely determined (430). In neurologic disorders, the time of administration, the formulation, and the quantity of probiotic and other microbial interventions are widely different in preclinical and clinical trials. More investigations are needed to determine a target population of microbial therapeutic intervention, such as the optimal phase of the disease and the age of the patient (431). Furthermore, unlike the high diversity of microbiota, probiotic formulations are restricted to only a few diversities of beneficial bacteria. In particular, obligate anaerobes are usually underrepresented in commercial products. However, the death of a premature infant due to probiotic supplementation contamination raises doubts about the safety of some microbial therapeutic products (432). The ability of each strain to remain viable and effective at a given target should also be taken into account. Indeed, other studies have demonstrated that once the dysbiosis after colonization of pathogenic microbial communities sets in, probiotics and prebiotics have not been effective, and other therapeutic strategies, such as engineered bacteria, could be administered (433). According to the general safety of FMT, a possible adverse effect has been reported to be linked with its preparation under a protocol that lacks screening for multidrug-resistant organisms. Consequently, careful testing and donor screening should be considered to minimize the safety concern of FMT, particularly during the COVID-19 pandemic (434).
At present, defining a healthy microbial flora is perhaps one of the most challenging topics because of the significant interindividual differences in the GI microbiome. However, microbiota-targeted therapies can also be beneficial since they might lead to a step toward precision medicine approaches. Indeed, further research is required to understand the effects of external and internal factors that impede the gut microflora from being altered by nutritional or other interventions. It is also essential to further explain the microbial structure down to the strain level, utilizing metagenome analysis and multi-omics methods rather than 16S rRNA gene sequencing. Meanwhile, expansion beyond the bacteriome is required to fully appreciate the importance of neuroregulatory mechanisms, particularly in the virome and bacteriophage fields. The application of systems biology approaches will be vital in multi-omics data integration. Furthermore, the understanding the molecular mechanisms underlying the bidirectional microbiota-gut-brain cross talk and pinpointing the functions of the microbial products and their possible host interactions will be essential. The influence of both microbe-derived metabolites and dietary compounds on host physiology and health has to be confirmed to further develop therapeutic approaches. Additional studies are also needed to clarify the possible interactions between gut microflora and drug action, since a significant number of patients are prescribed various medications. Overall, it must be fully understood that the GI microflora plays a crucial role in the pathogenesis of CNS diseases and could be considered a new organ, signified as the second brain. Future investigations in neurotherapeutics will provide important information regarding gut microbiota as a new boundary between human health and various diseases.
ACKNOWLEDGMENTS
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We thank Jane Fowler and William T. Emery for their valuable guidance in the revision of this article.
We regret the omission of many colleagues’ research articles because of space constraints.
Biographies

Shokufeh Ghasemian Sorboni is a Ph.D. student in Microbiology specializing in infectious diseases at the Faculty of Science, Department of Microbiology, University of Tehran, Iran. Her research focuses on the human gut microbiota, using culture-independent (molecular methods) and culture-dependent (culturomics) approaches. She is currently a junior researcher at the Advanced Medical Biotechnology Laboratory at the University of Tehran.

Hanieh Shakeri Moghaddam is a Ph.D. student in Microbiology at Simon Fraser University (Canada). She got her master’s in Microbiology from Ferdowsi University of Mashhad, Iran. Her research focuses on the drinking water microbiome. Her passion for microbiome studies started when she was a master’s student and started reading more about the human microbiome and its connection with human diseases and moods. This insatiable thirst for learning more about this world led her to write this review. Following her Ph.D. studies, she hopes in the near future to become a specialist in microbiota studies.

Reza Jafarzadeh-Esfehani is currently a researcher at the Blood Borne Infections Research Center, Academic Center for Education, Culture and Research (ACECR)-Khorasan Razavi and founder/CEO of the Sepehr Salamat company in Iran, developing diagnostic assistance tools in different fields of medicine. He qualified in Medicine in 2017 from Sabzevar University of Medical Sciences and graduated with a Ph.D. degree in Medical Genetics from Mashhad University of Medical Sciences in 2021. His main focus is on developing artificial intelligence tools for diagnosis of genetic syndromes and interpretation of clinical next-generation sequencing data.

Saman Soleimanpour, Ph.D, who specializes in Medical Microbiology, has been an assistant professor of microbiology at the Faculty of Medicine, Mashhad University of Medical Sciences, since 2015. He completed his master's degree in Medical Microbiology from the Razi Vaccine and Serum Research Institute and Ph.D. in Bacteriology from Mashhad University of Medical Sciences, Iran. He is the head of Tuberculosis Reference Laboratory-Northeast of Iran. He is a molecular microbiologist whose research is focused on tuberculosis vaccines and the role of microbiota and bacteria in the treatment of cancer. Microbiota and its relationship with some diseases also represent one of Saman's main research areas. For over 16 years, his work has focused on clinical and epidemiological microbiology research, with a special emphasis on the evaluation of new diagnostics, optimized use of existing and new anti-TB vaccines, and also the human microbiome and its impacts on health.
APPENDIX
GLOSSARY
- Dopamine
A catecholaminergic neurotransmitter synthesized by the central and peripheral nervous systems that acts by binding to G-protein-coupled receptors.
- γ-Aminobutyric acid (GABA)
An inhibitory neurotransmitter in the central nervous system and a nonproteinogenic amino acid that naturally exists in different kinds of foods.
- Serotonin (5-hydroxytryptamine)
A monoamine neurotransmitter regulating almost all brain functions, including memory, mood, and sleep.
- Short-chain fatty acids (SCFAs)
Main metabolites produced by microbiota through anaerobic fermentation of undigestible foods, including fiber and starch.
- Blood-brain barrier (BBB)
A unique property of the central nervous system vasculature that provides a highly selectively permeable membrane protecting neurons against circulating pathogens and toxins.
- Germfree (GF) mouse model
A model system bred in isolators and without exposure to microorganisms allowing the study of complete absence of microbes in a living animal.
- Specific pathogen-free (SPF) mice
Mice that are totally free of a list of pathogens.
- Postsynaptic density protein-95 (PSD-95)
A member of the membrane-associated guanylate kinase family encoded by DLG4, which is an important postsynaptic scaffolding protein.
- Brain-derived neurotrophic factor (BDNF)
A growth factor from the neurotrophic family that is a key mediator of plastic neuronal changes related to memory and learning.
- Microbe-associated molecular patterns (MAMPs)
Conserved molecular signatures in whole classes of microbes that are absent from the host.
- Experimental autoimmune encephalomyelitis (EAE)
An animal model of demyelinating diseases such as MS, in which the T lymphocytes recognize the central nervous system as an exogenous immunogen, resulting in the demyelinating process.
- Lipopolysaccharide (LPS)
A large molecule consisting of polysaccharide and lipids found in the outer membrane of Gram-negative bacteria that induces an immune response.
- Antimicrobial peptides (AMPs)
Host defense peptides and parts of the innate immune response that include cationic or amphiphilic alpha-helical peptides.
- Segmented filamentous bacteria (SFB)
A group of host-adopted commensal immune modulatory organisms colonizing the mammalian gut.
- Metagenomic shotgun DNA sequencing
Sequencing the DNA of a complete community of microbes
- α-Diversity
Term referring to diversity within a specific local sample or a specific ecological community.
- β-Diversity
Term referring to differences between microbial communities from different environments.
- Leaky gut
A condition in which undigested food particles and bacteria leak into the bloodstream from a damaged intestine.
- Alpha-synuclein
A highly soluble unfolded protein found in neural tissue and the Lewy bodies seen in synucleinopathies localized in the cellular organelles of neurons.
- Bacterial 16S rRNA
A useful gene for bacterial classification.
- Human milk oligosaccharides (HMOs)
A biologically diverse group of undigestible sugars and the third most abundant solid component of milk.
- Operational taxonomic unit (OTU)
A group of similar microbial individuals based on similarity of the 16S rRNA gene (or other selected marker genes) sequences (e.g., 95 to 99%).
- Psychobiotics
Beneficial prebiotics or probiotics influencing the bacterium-brain relationship when ingested in appropriate amounts.
- Paraprobiotics
Nonviable probiotics that have lost their viability after exposure to factors disrupting their cell structures.
REFERENCES
- 1.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. 2012. Host-gut microbiota metabolic interactions. Science 336:1262–1267. 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 2.Qin Y, Wade PA. 2018. Crosstalk between the microbiome and epigenome: messages from bugs. J Biochem 163:105–112. 10.1093/jb/mvx080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapira M. 2016. Gut microbiotas and host evolution: scaling up symbiosis. Trends Ecol Evol 31:539–549. 10.1016/j.tree.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Falony G, Vandeputte D, Caenepeel C, Vieira-Silva S, Daryoush T, Vermeire S, Raes J. 2019. The human microbiome in health and disease: hype or hope. Acta Clin Belg 74:53–64. 10.1080/17843286.2019.1583782. [DOI] [PubMed] [Google Scholar]
- 5.Ding R-X, Goh W-R, Wu R-N, Yue X-Q, Luo X, Khine WWT, Wu J-R, Lee Y-K. 2019. Revisit gut microbiota and its impact on human health and disease. J Food Drug Anal 27:623–631. 10.1016/j.jfda.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tierney BT, Yang Z, Luber JM, Beaudin M, Wibowo MC, Baek C, Mehlenbacher E, Patel CJ, Kostic AD. 2019. The landscape of genetic content in the gut and oral human microbiome. Cell Host Microbe 26:283–295. 10.1016/j.chom.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park W. 2018. Gut microbiomes and their metabolites shape human and animal health. Springer, New York, NY. [DOI] [PubMed] [Google Scholar]
- 8.Moos WH, Faller DV, Harpp DN, Kanara I, Pernokas J, Powers WR, Steliou K. 2016. Microbiota and neurological disorders: a gut feeling. Biores Open Access 5:137–145. 10.1089/biores.2016.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Price J, Mahurkar A, Rahnavard G, Crabtree J, Orvis J, Hall AB, Brady A, Creasy HH, McCracken C, Giglio MG, McDonald D, Franzosa EA, Knight R, White O, Huttenhower C. 2017. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 550:61–66. 10.1038/nature23889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosca A, Leclerc M, Hugot JP. 2016. Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front Microbiol 7:455. 10.3389/fmicb.2016.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PW. 2016. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci 39:763–781. 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bain CC, Cerovic V. 2020. Interactions of the microbiota with the mucosal immune system. Clin Exp Immunol 199:9–11. 10.1111/cei.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. 2011. Human nutrition, the gut microbiome and the immune system. Nature 474:327–336. 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto J-M, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker J-D, Raes J, Hansen T, Bork P, Wang J, Ehrlich SD, Pedersen O, MetaHIT Consortium . 2013. Richness of human gut microbiome correlates with metabolic markers. Nature 500:541–546. 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 15.Ma Q, Xing C, Long W, Wang HY, Liu Q, Wang R-F. 2019. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J Neuroinflammation 16:1–14. 10.1186/s12974-019-1434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang D-W, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, Pollard EL, Roux S, Sadowsky MJ, Lipson KS, Sullivan MB, Caporaso JG, Krajmalnik-Brown R. 2017. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5:10. 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharon G, Cruz NJ, Kang D-W, Gandal MJ, Wang B, Kim Y-M, Zink EM, Casey CP, Taylor BC, Lane CJ, Bramer LM, Isern NG, Hoyt DW, Noecker C, Sweredoski MJ, Moradian A, Borenstein E, Jansson JK, Knight R, Metz TO, Lois C, Geschwind DH, Krajmalnik-Brown R, Mazmanian SK. 2019. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 177:1600–1618. 10.1016/j.cell.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, Ferrari C, Guerra UP, Paghera B, Muscio C, Bianchetti A, Volta GD, Turla M, Cotelli MS, Gennuso M, Prelle A, Zanetti O, Lussignoli G, Mirabile D, Bellandi D, Gentile S, Belotti G, Villani D, Harach T, Bolmont T, Padovani A, Boccardi M, Frisoni GB. 2017. Association of brain amyloidosis with proinflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging 49:60–68. 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Tremlett H, Fadrosh DW, Faruqi AA, Zhu F, Hart J, Roalstad S, Graves J, Lynch S, Waubant E, US Network of Pediatric MS Centers . 2016. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur J Neurol 23:1308–1321. 10.1111/ene.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A. 2011. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6:e28032. 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, Broughton E, Hagan H, Carroll C. 2014. Accumulation of α-synuclein in the bowel of patients in the preclinical phase of Parkinson’s disease. Acta Neuropathol 127:235–241. 10.1007/s00401-013-1214-6. [DOI] [PubMed] [Google Scholar]
- 22.Nemani K, Ghomi RH, McCormick B, Fan X. 2015. Schizophrenia and the gut-brain axis. Prog Neuropsychopharmacol Biol Psychiatry 56:155–160. 10.1016/j.pnpbp.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, Rey FE. 2017. Gut microbiome alterations in Alzheimer’s disease. Sci Rep 7:1–11. 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM. 2015. Colonic bacterial composition in Parkinson’s disease. Mov Disord 30:1351–1360. 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 25.Kang D-W, Park JG, Ilhan ZE, Wallstrom G, LaBaer J, Adams JB, Krajmalnik-Brown R. 2013. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One 8:e68322. 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn AB, Jordan S, Baker BJ, Carlson NS. 2017. The maternal infant microbiome: considerations for labor and birth. MCN Am J Matern Child Nurs 42:318–325. 10.1097/NMC.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stiemsma LT, Michels KB. 2018. The role of the microbiome in the developmental origins of health and disease. Pediatrics 141:e20172437. 10.1542/peds.2017-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visconti A, Le Roy CI, Rosa F, Rossi N, Martin TC, Mohney RP, Li W, de Rinaldis E, Bell JT, Venter JC, Nelson KE, Spector TD, Falchi M. 2019. Interplay between the human gut microbiome and host metabolism. Nat Commun 10:1–10. 10.1038/s41467-019-12476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carabotti M, Scirocco A, Maselli MA, Severi C. 2015. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28:203. [PMC free article] [PubMed] [Google Scholar]
- 30.Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. 2011. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 108:3047–3052. 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. 2014. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci 34:15490–15496. 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith PA. 2015. The tantalizing links between gut microbes and the brain. Nature 526:312–314. 10.1038/526312a. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt C. 2015. The microbiome may yield a new class of psychobiotics for the treatment of anxiety, depression and other mood disorders. Nature 518:S12–S12. 10.1038/518S13a. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins TA, Nguyen JC, Polglaze KE, Bertrand PP. 2016. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 8:56. 10.3390/nu8010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Vos WM, de Vos EA. 2012. Role of the intestinal microbiome in health and disease: from correlation to causation. Nutrition Rev 70:S45–S56. 10.1111/j.1753-4887.2012.00505.x. [DOI] [PubMed] [Google Scholar]
- 36.Lozupone C, Stombaugh J, Gordon J, Jansson J, Knight R. 2012. Diversity, stability and resilience of the human gut microbiota. Nature 489:220–230. 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grenham S, Clarke G, Cryan JF, Dinan TG. 2011. Brain-gut-microbe communication in health and disease. Front Physiol 2:94. 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cryan JF, O’Mahony SM. 2011. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil 23:187–192. 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 39.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. 2009. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 1:6ra14. 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H-X, Wang Y-P. 2016. Gut microbiota-brain axis. Chin Med J (Engl) 129:2373–2380. 10.4103/0366-6999.190667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vickers NJ. 2017. Animal communication: when I’m calling you, will you answer, too? Curr Biol 27:R713–R715. 10.1016/j.cub.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 42.Cryan JF, Dinan TG. 2012. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13:701–712. 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 43.Foster JA, Neufeld K-AM. 2013. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 36:305–312. 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Jiang C, Li G, Huang P, Liu Z, Zhao B. 2017. The gut microbiota and Alzheimer’s disease. J Alzheimers Dis 58:1–15. 10.3233/JAD-161141. [DOI] [PubMed] [Google Scholar]
- 45.Mancuso C, Santangelo R. 2018. Alzheimer’s disease and gut microbiota modifications: the long way between preclinical studies and clinical evidence. Pharmacol Res 129:329–336. 10.1016/j.phrs.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 46.Miyake S, Yamamura T. 2019. Gut environmental factors and multiple sclerosis. J Neuroimmunol 329:20–23. 10.1016/j.jneuroim.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 47.Qian Y, Yang X, Xu S, Wu C, Song Y, Qin N, Chen S-D, Xiao Q. 2018. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun 70:194–202. 10.1016/j.bbi.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Santocchi E, Guiducci L, Fulceri F, Billeci L, Buzzigoli E, Apicella F, Calderoni S, Grossi E, Morales MA, Muratori F. 2016. Gut to brain interaction in autism spectrum disorders: a randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry 16:183. 10.1186/s12888-016-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheperjans F, Aho V, Pereira PAB, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. 2015. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30:350–358. 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 50.Lyte M. 2013. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog 9:e1003726. 10.1371/journal.ppat.1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherwin E, Dinan TG, Cryan JF. 2018. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci 1420:5–25. 10.1111/nyas.13416. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Kasper LH. 2014. The role of microbiome in central nervous system disorders. Brain Behav Immun 38:1–12. 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarangi AN, Goel A, Singh A, Sasi A, Aggarwal R. 2017. Faecal bacterial microbiota in patients with cirrhosis and the effect of lactulose administration. BMC Gastroenterol 17:125. 10.1186/s12876-017-0683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dinan TG, Cryan JF. 2017. Brain-gut-microbiota axis and mental health. Psychosom Med 79:920–926. 10.1097/PSY.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 55.Holzer P, Farzi A. 2014. Neuropeptides and the microbiota-gut-brain axis, p 195–219. In Microbial endocrinology: the microbiota-gut-brain axis in health and disease. Springer, New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rogers G, Keating D, Young R, Wong M, Licinio J, Wesselingh S. 2016. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry 21:738–748. 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. 2010. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170:1179–1188. 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Lyte M. 2013. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog 9:e1003726. 10.1371/journal.ppat.1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lyte M. 2014. Microbial endocrinology and the microbiota-gut-brain axis, p 3–24. In Microbial endocrinology: the microbiota-gut-brain axis in health and disease. Springer, New York, NY. [DOI] [PubMed] [Google Scholar]
- 60.Badawy AA. 2017. Tryptophan availability for kynurenine pathway metabolism across the life span: control mechanisms and focus on aging, exercise, diet, and nutritional supplements. Neuropharmacology 112:248–263. 10.1016/j.neuropharm.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 61.McCarville JL, Chen GY, Cuevas VD, Troha K, Ayres JS. 2020. Microbiota metabolites in health and disease. Annu Rev Immunol 38:147–170. 10.1146/annurev-immunol-071219-125715. [DOI] [PubMed] [Google Scholar]
- 62.Kennedy PJ, Cryan JF, Dinan TG, Clarke G. 2017. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 112:399–412. 10.1016/j.neuropharm.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Fülling C, Dinan TG, Cryan JF. 2019. Gut microbe to brain signaling: what happens in vagus. Neuron 101:998–1002. 10.1016/j.neuron.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Roager HM, Licht TR. 2018. Microbial tryptophan catabolites in health and disease. Nat Commun 9:1–10. 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Collins SM, Bercik P. 2009. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 136:2003–2014. 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 66.Evrensel A, Ceylan ME. 2015. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci 13:239–244. 10.9758/cpn.2015.13.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bastiaanssen TF, Cowan CS, Claesson MJ, Dinan TG, Cryan JF. 2019. Making sense of… the microbiome in psychiatry. Int J Neuropsychopharmacol 22:37–52. 10.1093/ijnp/pyy067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silva YP, Bernardi A, Frozza RL. 2020. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 11:25. 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alhabeeb H, AlFaiz A, Kutbi E, AlShahrani D, Alsuhail A, AlRajhi S, Alotaibi N, Alotaibi K, AlAmri S, Alghamdi S, AlJohani N. 2021. Gut hormones in health and obesity: the upcoming role of short chain fatty acids. Nutrients 13:481. 10.3390/nu13020481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rea K, Dinan TG, Cryan JF. 2020. Gut microbiota: a perspective for psychiatrists. Neuropsychobiology 79:50–62. 10.1159/000504495. [DOI] [PubMed] [Google Scholar]
- 71.Morais LH, Schreiber HL, Mazmanian SK. 2021. The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol 19:241–215. 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- 72.Krautkramer KA, Fan J, Bäckhed F. 2021. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol 19:77–18. 10.1038/s41579-020-0438-4. [DOI] [PubMed] [Google Scholar]
- 73.Grochowska M, Wojnar M, Radkowski M. 2018. The gut microbiota in neuropsychiatric disorders. Acta Neurobiol Exp (Wars) 78:69–81. [PubMed] [Google Scholar]
- 74.Cussotto S, Clarke G, Dinan TG, Cryan JF. 2019. Psychotropics and the microbiome: a chamber of secrets. Psychopharmacology (Berl) 236:1411–1432. 10.1007/s00213-019-5185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Margolis KG, Cryan JF, Mayer EA. 2021. The microbiota-gut-brain axis: from motility to mood. Gastroenterology 160:1486–1501. 10.1053/j.gastro.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Więdłocha M, Marcinowicz P, Janoska-Jaździk M, Szulc A. 2021. Gut microbiota, kynurenine pathway, and mental disorders: review. Prog Neuro-Psychopharmacol Biol Psychiatry 106:110145. 10.1016/j.pnpbp.2020.110145. [DOI] [PubMed] [Google Scholar]
- 77.Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O’Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG. 2019. The microbiota-gut-brain axis. Physiol Rev 99:1877–2013. 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 78.Chrobak A, Nowakowski J, Dudek D. 2016. Interactions between the gut microbiome and the central nervous system and their role in schizophrenia, bipolar disorder and depression. Arch Psych Psych 18:5–11. 10.12740/APP/62962. [DOI] [Google Scholar]
- 79.Dash S, Clarke G, Berk M, Jacka FN. 2015. The gut microbiome and diet in psychiatry: focus on depression. Curr Opin Psychiatry 28:1–6. 10.1097/YCO.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 80.Jacka FN, O’Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, Castle D, Dash S, Mihalopoulos C, Chatterton ML, Brazionis L, Dean OM, Hodge AM, Berk M. 2017. A randomised controlled trial of dietary improvement for adults with major depression (the “SMILES” trial). BMC Med 15:1–13. 10.1186/s12916-017-0791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jacka FN. 2017. Nutritional psychiatry: where to next? EBioMedicine 17:24–29. 10.1016/j.ebiom.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. 2016. The central nervous system and the gut microbiome. Cell 167:915–932. 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. 2014. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 20:509–518. 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 84.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto J-M, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, MetaHIT Consortium, et al. 2010. A human gut microbial gene catalogue established by metagenomic sequencing. nature 464:59–65. 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tognini P. 2017. Gut microbiota: a potential regulator of neurodevelopment. Front Cell Neurosci 11:25. 10.3389/fncel.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tau GZ, Peterson BS. 2010. Normal development of brain circuits. Neuropsychopharmacology 35:147–168. 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. 2004. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 558:263–275. 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tarsa L, Goda Y. 2002. Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci USA 99:1012–1016. 10.1073/pnas.022575999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwon SE, Chapman ER. 2011. Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron 70:847–854. 10.1016/j.neuron.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.El-Husseini AE-D, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. 2000. PSD-95 involvement in maturation of excitatory synapses. Science 290:1364–1368. 10.1126/science.290.5495.1364. [DOI] [PubMed] [Google Scholar]
- 91.Béïque JC, Andrade R. 2003. PSD-95 regulates synaptic transmission and plasticity in rat cerebral cortex. J Physiol 546:859–867. 10.1113/jphysiol.2002.031369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Furness JB. 2012. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9:286–294. 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 93.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. 2011. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 108:16050–16055. 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gualtieri P, Marchetti M, Cioccoloni G, De Lorenzo A, Romano L, Cammarano A, Colica C, Condò R, Di Renzo L. 2020. Psychobiotics regulate the anxiety symptoms in carriers of allele A of IL-1β gene: a randomized, placebo-controlled clinical trial. Mediat Inflamm 2020:1–11. 10.1155/2020/2346126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. 2011. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141:599–609. 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 96.Strandwitz P. 2018. Neurotransmitter modulation by the gut microbiota. Brain Res 1693:128–133. 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de los Reyes-Gavilán CG, Salazar N. 2016. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 7:185. 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bourassa MW, Alim I, Bultman SJ, Ratan RR. 2016. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neuroscience Lett 625:56–63. 10.1016/j.neulet.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mu C, Yang Y, Zhu W. 2016. Gut microbiota: the brain peacekeeper. Front Microbiol 7:345. 10.3389/fmicb.2016.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y, Wang Z, Wang Y, Li F, Jia J, Song X, Qin S, Wang R, Jin F, Kitazato K, Wang Y. 2018. The gut-microglia connection: implications for central nervous system diseases. Front Immunol 9:2325. 10.3389/fimmu.2018.02325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abdel-Haq R, Schlachetzki JC, Glass CK, Mazmanian SK. 2019. Microbiome-microglia connections via the gut-brain axis. J Exp Med 216:41–59. 10.1084/jem.20180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermöhlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. 2015. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18:965–977. 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ferguson BJ, Dovgan K, Takahashi N, Beversdorf DQ. 2019. The relationship among gastrointestinal symptoms, problem behaviors, and internalizing symptoms in children and adolescents with autism spectrum disorder. Front Psychiatry 10. 10.3389/fpsyt.2019.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vericat A, Orden AB. 2019. Neurodevelopment in infants with moderate neonatal risk and its association with biological and environmental factors. Adv Neurodev Disord 3:297–305. 10.1007/s41252-019-00116-y. [DOI] [Google Scholar]
- 105.Nuriel-Ohayon M, Neuman H, Koren O. 2016. Microbial changes during pregnancy, birth, and infancy. Front Microbiol 7:1031. 10.3389/fmicb.2016.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gibson MK, Wang B, Ahmadi S, Burnham C-AD, Tarr PI, Warner BB, Dantas G. 2016. Developmental dynamics of the preterm infant gut microbiota and antibiotic resistome. Nat Microbiol 1:16024. 10.1038/nmicrobiol.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, Da Mesquita S, Frost EL, Gaultier A, Harris TH, Cao R, Hu S, Lukens JR, Smirnov I, Overall CC, Oliver G, Kipnis J. 2018. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci 21:1380–1391. 10.1038/s41593-018-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morimoto K, Nakajima K. 2019. Role of the immune system in the development of the central nervous system. Front Neurosci 13:916. 10.3389/fnins.2019.00916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Daneman R, Prat A. 2015. The blood-brain barrier. Cold Spring Harb Perspect Biol 7:a020412. 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kiecker C. 2018. The origins of the circumventricular organs. J Anat 232:540–553. 10.1111/joa.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lawson L, Perry V, Gordon S. 1992. Turnover of resident microglia in the normal adult mouse brain. Neuroscience 48:405–415. 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 112.Filiano AJ, Gadani SP, Kipnis J. 2015. Interactions of innate and adaptive immunity in brain development and function. Brain Res 1617:18–27. 10.1016/j.brainres.2014.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lenz KM, Nelson LH. 2018. Microglia and beyond: innate immune cells as regulators of brain development and behavioral function. Front Immunol 9:698. 10.3389/fimmu.2018.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Korin B, Ben-Shaanan TL, Schiller M, Dubovik T, Azulay-Debby H, Boshnak NT, Koren T, Rolls A. 2017. High-dimensional, single-cell characterization of the brain’s immune compartment. Nat Neurosci 20:1300–1309. 10.1038/nn.4610. [DOI] [PubMed] [Google Scholar]
- 115.Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, Ben-Yehuda H, David E, Zelada González F, Perrin P, Keren-Shaul H, Gury M, Lara-Astaiso D, Thaiss CA, Cohen M, Bahar Halpern K, Baruch K, Deczkowska A, Lorenzo-Vivas E, Itzkovitz S, Elinav E, Sieweke MH, Schwartz M, Amit I. 2016. Microglia development follows a stepwise program to regulate brain homeostasis. Science 353:aad8670. 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- 116.Rosenzweig N, Dvir-Szternfeld R, Tsitsou-Kampeli A, Keren-Shaul H, Ben-Yehuda H, Weill-Raynal P, Cahalon L, Kertser A, Baruch K, Amit I, Weiner A, Schwartz M. 2019. PD-1/PD-L1 checkpoint blockade harnesses monocyte-derived macrophages to combat cognitive impairment in a tauopathy mouse model. Nat Commun 10:1–15. 10.1038/s41467-019-08352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rojas OL, Pröbstel A-K, Porfilio EA, Wang AA, Charabati M, Sun T, Lee DSW, Galicia G, Ramaglia V, Ward LA, Leung LYT, Najafi G, Khaleghi K, Garcillán B, Li A, Besla R, Naouar I, Cao EY, Chiaranunt P, Burrows K, Robinson HG, Allanach JR, Yam J, Luck H, Campbell DJ, Allman D, Brooks DG, Tomura M, Baumann R, Zamvil SS, Bar-Or A, Horwitz MS, Winer DA, Mortha A, Mackay F, Prat A, Osborne LC, Robbins C, Baranzini SE, Gommerman JL. 2019. Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell 176:610–624. 10.1016/j.cell.2018.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, Blecher R, Ulas T, Squarzoni P, Hoeffel G, Coulpier F, Siopi E, David FS, Scholz C, Shihui F, Lum J, Amoyo AA, Larbi A, Poidinger M, Buttgereit A, Lledo P-M, Greter M, Chan JKY, Amit I, Beyer M, Schultze JL, Schlitzer A, Pettersson S, Ginhoux F, Garel S. 2018. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172:500–516. 10.1016/j.cell.2017.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Emani R, Alam C, Pekkala S, Zafar S, Emani M, Hänninen A. 2015. Peritoneal cavity is a route for gut-derived microbial signals to promote autoimmunity in non-obese diabetic mice. Scand J Immunol 81:102–109. 10.1111/sji.12253. [DOI] [PubMed] [Google Scholar]
- 120.Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, Liu C, Klotz L, Stauffer U, Baranzini SE, Kümpfel T, Hohlfeld R, Krishnamoorthy G, Wekerle H. 2017. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci USA 114:10719–10724. 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kadowaki A, Miyake S, Saga R, Chiba A, Mochizuki H, Yamamura T. 2016. Gut environment-induced intraepithelial autoreactive CD4+ T cells suppress central nervous system autoimmunity via LAG-3. Nat Commun 7:1–16. 10.1038/ncomms11639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Haghikia A, Jörg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee D-H, May C, Wilck N, Balogh A, Ostermann AI, Schebb NH, Akkad DA, Grohme DA, Kleinewietfeld M, Kempa S, Thöne J, Demir S, Müller DN, Gold R, Linker RA. 2015. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43:817–829. 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 123.Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL, Crabtree-Hartman E, Sand IK, Gacias M, Zhu Y, Casaccia P, Cree BAC, Knight R, Mazmanian SK, Baranzini SE. 2017. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci USA 114:10713–10718. 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao C-C, Patel B, Yan R, Blain M, Alvarez JI, Kébir H, Anandasabapathy N, Izquierdo G, Jung S, Obholzer N, Pochet N, Clish CB, Prinz M, Prat A, Antel J, Quintana FJ. 2016. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22:586–597. 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Möhle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, French T, Hambardzumyan D, Matzinger P, Dunay IR, Wolf SA. 2016. Ly6Chi monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep 15:1945–1956. 10.1016/j.celrep.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 126.Belkaid Y, Hand TW. 2014. Role of the microbiota in immunity and inflammation. Cell 157:121–141. 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rabot S, Jaglin M, Daugé V, Naudon L. 2016. Impact of the gut microbiota on the neuroendocrine and behavioural responses to stress in rodents. Ocl 23:D116. 10.1051/ocl/2015036. [DOI] [Google Scholar]
- 128.Kallinteris NL, Lu X, Wu S, Hu H, Li Y, Gulfo JV, Humphreys RE, Xu M. 2003. Ii-Key/MHC class II epitope hybrid peptide vaccines for HIV. Vaccine 21:4128–4132. 10.1016/s0264-410x(03)00493-6. [DOI] [PubMed] [Google Scholar]
- 129.Zhao Y, Cong L, Jaber V, Lukiw WJ. 2017. Microbiome-derived lipopolysaccharide enriched in the perinuclear region of Alzheimer’s disease brain. Front Immunol 8:1064. 10.3389/fimmu.2017.01064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schroeder BO, Bäckhed F. 2016. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med 22:1079–1089. 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 131.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. 2001. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291:881–884. 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 132.Kabat AM, Srinivasan N, Maloy KJ. 2014. Modulation of immune development and function by intestinal microbiota. Trends Immunol 35:507–517. 10.1016/j.it.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, Umesaki Y, Mathis D, Benoist C, Relman DA, Kasper DL. 2012. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 149:1578–1593. 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Littman DR, Rudensky AY. 2010. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140:845–858. 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 135.Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, Eberl G, Snel J, Kelly D, Cerf-Bensussan N. 2009. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31:677–689. 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 136.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139:485–498. 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wesemann DR, Portuguese AJ, Meyers RM, Gallagher MP, Cluff-Jones K, Magee JM, Panchakshari RA, Rodig SJ, Kepler TB, Alt FW. 2013. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature 501:112–115. 10.1038/nature12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fransen F, Zagato E, Mazzini E, Fosso B, Manzari C, El Aidy S, Chiavelli A, D’Erchia AM, Sethi MK, Pabst O, Marzano M, Moretti S, Romani L, Penna G, Pesole G, Rescigno M. 2015. BALB/c and C57BL/6 mice differ in polyreactive IgA abundance, which impacts the generation of antigen-specific IgA and microbiota diversity. Immunity 43:527–540. 10.1016/j.immuni.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 139.Antonini M, Lo Conte M, Sorini C, Falcone M. 2019. How the interplay between the commensal microbiota, gut barrier integrity and mucosal immunity regulates brain autoimmunity. Front Immunol 10:1937. 10.3389/fimmu.2019.01937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. 2019. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol 16:461–478. 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 141.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. 2013. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341:569–573. 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thorburn AN, Macia L, Mackay CR. 2014. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 40:833–842. 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 143.Maslowski KM, Mackay CR. 2011. Diet, gut microbiota and immune responses. Nat Immunol 12:5–9. 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 144.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–450. 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 145.Schiering C, Wincent E, Metidji A, Iseppon A, Li Y, Potocnik AJ, Omenetti S, Henderson CJ, Wolf CR, Nebert DW, Stockinger B. 2017. Feedback control of AHR signalling regulates intestinal immunity. Nature 542:242–245. 10.1038/nature21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hubbard TD, Murray IA, Perdew GH. 2015. Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metab Dispos 43:1522–1535. 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bekebrede AF, Keijer J, Gerrits WJ, de Boer VC. 2020. The molecular and physiological effects of protein-derived polyamines in the intestine. Nutrients 12:197. 10.3390/nu12010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kibe R, Kurihara S, Sakai Y, Suzuki H, Ooga T, Sawaki E, Muramatsu K, Nakamura A, Yamashita A, Kitada Y, Kakeyama M, Benno Y, Matsumoto M. 2014. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci Rep 4:4548. 10.1038/srep04548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Harris JR, Korolchuk VI. 2018. Biochemistry and cell biology of ageing. I Biomedical science. Springer, New York, NY. [Google Scholar]
- 150.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W. 2010. Through ageing and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5:e10667. 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, Stanton C, van Sinderen D, O’Connor M, Harnedy N, O’Connor K, Henry C, O’Mahony D, Fitzgerald AP, Shanahan F, Twomey C, Hill C, Ross RP, O’Toole PW. 2011. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA 108:4586–4591. 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Donini LM, Savina C, Cannella C. 2009. Nutrition in the elderly: role of fiber. Arch Gerontol Geriatrics 49:61–69. 10.1016/j.archger.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 153.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HMB, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald GF, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O’Toole PW. 2012. Gut microbiota composition correlates with diet and health in the elderly. Nature 488:178–184. 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 154.Yoo BB, Mazmanian SK. 2017. The enteric network: interactions between the immune and nervous systems of the gut. Immunity 46:910–926. 10.1016/j.immuni.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Paun A, Danska JS. 2016. Modulation of type 1 and type 2 diabetes risk by the intestinal microbiome. Pediatr Diabetes 17:469–477. 10.1111/pedi.12424. [DOI] [PubMed] [Google Scholar]
- 156.Saffrey MJ. 2014. Aging of the mammalian gastrointestinal tract: a complex organ system. Age (Dordr) 36:9603. 10.1007/s11357-013-9603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rolig AS, Mittge EK, Ganz J, Troll JV, Melancon E, Wiles TJ, Alligood K, Stephens WZ, Eisen JS, Guillemin K. 2017. The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol 15:e2000689. 10.1371/journal.pbio.2000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Collins SM, Surette M, Bercik P. 2012. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10:735–742. 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 159.Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, Kitzman DW, Kushugulova A, Marotta F, Yadav H. 2018. Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging 4:267–285. 10.3233/NHA-170030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cowen T, Johnson R, Soubeyre V, Santer RM. 2000. Restricted diet rescues rat enteric motor neurones from age related cell death. Gut 47:653–660. 10.1136/gut.47.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Saffrey MJ. 2013. Cellular changes in the enteric nervous system during ageing. Dev Biol 382:344–355. 10.1016/j.ydbio.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 162.Van Ginneken C, Schäfer K-H, Van Dam D, Huygelen V, De Deyn PP. 2011. Morphological changes in the enteric nervous system of aging and APP23 transgenic mice. Brain Res 1378:43–53. 10.1016/j.brainres.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 163.Gamage P, Ranson R, Patel B, Yeoman M, Saffrey M. 2013. Myenteric neuron numbers are maintained in aging mouse distal colon. Neurogastroenterol Motil 25:e495–e505. 10.1111/nmo.12114. [DOI] [PubMed] [Google Scholar]
- 164.Hanani M, Fellig Y, Udassin R, Freund HR. 2004. Age-related changes in the morphology of the myenteric plexus of the human colon. Auton Neurosci 113:71–78. 10.1016/j.autneu.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 165.Abalo R, Vera G, Rivera AJ, Martin MI. 2007. Age-related changes in the gastrointestinal tract: a functional and immunohistochemical study in guinea-pig ileum. Life Sci 80:2436–2445. 10.1016/j.lfs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 166.Phillips RJ, Kieffer EJ, Powley TL. 2003. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci 106:69–83. 10.1016/S1566-0702(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 167.Phillips RJ, Kieffer EJ, Powley TL. 2004. Loss of glia and neurons in the myenteric plexus of the aged Fischer 344 rat. Anat Embryol (Berl) 209:19–30. 10.1007/s00429-004-0426-x. [DOI] [PubMed] [Google Scholar]
- 168.Phillips RJ, Walter GC, Ringer BE, Higgs KM, Powley TL. 2009. Alpha-synuclein immunopositive aggregates in the myenteric plexus of the aging Fischer 344 rat. Exp Neurol 220:109–119. 10.1016/j.expneurol.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Calvani R, Picca A, Lo Monaco MR, Landi F, Bernabei R, Marzetti E. 2018. Of microbes and minds: a narrative review on the second brain aging. Front Med (Lausanne) 5:53. 10.3389/fmed.2018.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D. 2016. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxidat Med Cell Longevity 2016:1–18. 10.1155/2016/3565127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Leal SS, Gomes CM. 2015. Calcium dysregulation links ALS defective proteins and motor neuron selective vulnerability. Front Cell Neurosci 9:225. 10.3389/fncel.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hetz S, Acikgoez A, Moll C, Jahnke H-G, Robitzki AA, Metzger R, Metzger M. 2014. Age-related gene expression analysis in enteric ganglia of human colon after laser microdissection. Front Aging Neurosci 6:276. 10.3389/fnagi.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Marcolla CS, Alvarado CS, Willing BP. 2019. Early life microbial exposure shapes subsequent animal health. Can J Anim Sci 99:661–677. 10.1139/cjas-2019-0029. [DOI] [Google Scholar]
- 174.Smith LK, Wissel EF. 2019. Microbes and the mind: how bacteria shape affect, neurological processes, cognition, social relationships, development, and pathology. Perspect Psychol Sci 14:397–418. 10.1177/1745691618809379. [DOI] [PubMed] [Google Scholar]
- 175.Heiss CN, Olofsson LE. 2019. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J Neuroendocrinol 31:e12684. 10.1111/jne.12684. [DOI] [PubMed] [Google Scholar]
- 176.Kabouridis PS, Lasrado R, McCallum S, Chng SH, Snippert HJ, Clevers H, Pettersson S, Pachnis V. 2015. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 85:289–295. 10.1016/j.neuron.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.McVey Neufeld K, Mao Y, Bienenstock J, Foster J, Kunze W. 2013. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil 25:183–188. 10.1111/nmo.12049. [DOI] [PubMed] [Google Scholar]
- 178.Grasset E, Puel A, Charpentier J, Collet X, Christensen JE, Tercé F, Burcelin R. 2017. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric NO-dependent and gut-brain axis mechanism. Cell Metab 25:1075–1090. e5. 10.1016/j.cmet.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 179.Pierre JF, Barlow-Anacker AJ, Erickson CS, Heneghan AF, Leverson GE, Dowd SE, Epstein ML, Kudsk KA, Gosain A. 2014. Intestinal dysbiosis and bacterial enteroinvasion in a murine model of Hirschsprung’s disease. J Pediatr Surg 49:1242–1251. 10.1016/j.jpedsurg.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ward N, Pieretti A, Dowd S, Cox S, Goldstein A. 2012. Intestinal aganglionosis is associated with early and sustained disruption of the colonic microbiome. Neurogastroenterol Motil 24:874–e400. 10.1111/j.1365-2982.2012.01937.x. [DOI] [PubMed] [Google Scholar]
- 181.Hyland NP, Cryan JF. 2016. Microbe-host interactions: influence of the gut microbiota on the enteric nervous system. Dev Biol 417:182–187. 10.1016/j.ydbio.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 182.Obata Y, Pachnis V. 2016. The effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology 151:836–844. 10.1053/j.gastro.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fransen F, van Beek AA, Borghuis T, Aidy SE, Hugenholtz F, van der Gaast-de Jongh C, Savelkoul HFJ, De Jonge MI, Boekschoten MV, Smidt H, Faas MM, de Vos P. 2017. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front Immunol 8:1385. 10.3389/fimmu.2017.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.D’Amato A, Di Cesare Mannelli L, Lucarini E, Man AL, Le Gall G, Branca JJV, Ghelardini C, Amedei A, Bertelli E, Regoli M, Pacini A, Luciani G, Gallina P, Altera A, Narbad A, Gulisano M, Hoyles L, Vauzour D, Nicoletti C. 2020. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity-and neurotransmission-related proteins in young recipients. Microbiome 8:1–19. 10.1186/s40168-020-00914-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim M-S, Kim Y, Choi H, Kim W, Park S, Lee D, Kim DK, Kim HJ, Choi H, Hyun D-W, Lee J-Y, Choi EY, Lee D-S, Bae J-W, Mook-Jung I. 2020. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 69:283–294. 10.1136/gutjnl-2018-317431. [DOI] [PubMed] [Google Scholar]
- 186.Li Y, Ning L, Yin Y, Wang R, Zhang Z, Hao L, Wang B, Zhao X, Yang X, Yin L, Wu S, Guo D, Zhang C. 2020. Age-related shifts in gut microbiota contribute to cognitive decline in aged rats. Aging (Albany, NY) 12:7801–7817. 10.18632/aging.103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fattorusso A, Di Genova L, Dell’Isola GB, Mencaroni E, Esposito S. 2019. Autism spectrum disorders and the gut microbiota. Nutrients 11:521. 10.3390/nu11030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, DiRienzo M, Christensen DL, Wiggins LD, Pettygrove S, Andrews JG, Lopez M, Hudson A, Baroud T, Schwenk Y, White T, Rosenberg CR, Lee L-C, Harrington RA, Huston M, Hewitt A, Esler A, Hall-Lande J, Poynter JN, Hallas-Muchow L, Constantino JN, Fitzgerald RT, Zahorodny W, Shenouda J, Daniels JL, Warren Z, Vehorn A, Salinas A, Durkin MS, Dietz PM, EdS1 . 2020. Prevalence of autism spectrum disorder among children aged 8 years: autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill Summ 69:1–12. 10.15585/mmwr.ss6904a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li Q, Han Y, Dy ABC, Hagerman RJ. 2017. The gut microbiota and autism spectrum disorders. Front Cell Neurosci 11:120. 10.3389/fncel.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bölte S, Girdler S, Marschik PB. 2019. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci 76:1275–1297. 10.1007/s00018-018-2988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ramirez-Celis A, Becker M, Nuño M, Schauer J, Aghaeepour N, Van de Water J. 2021. Risk assessment analysis for maternal autoantibody-related autism (MAR-ASD): a subtype of autism. Mol Psychiatry 26:1551–1510. 10.1038/s41380-020-00998-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Estes ML, McAllister AK. 2015. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci 16:469–486. 10.1038/nrn3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang LW, Tancredi DJ, Thomas DW. 2011. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatrics 32:351–360. 10.1097/DBP.0b013e31821bd06a. [DOI] [PubMed] [Google Scholar]
- 194.Mazurek MO, Vasa RA, Kalb LG, Kanne SM, Rosenberg D, Keefer A, Murray DS, Freedman B, Lowery LA. 2013. Anxiety, sensory over-responsivity, and gastrointestinal problems in children with autism spectrum disorders. J Abnorm Child Psychol 41:165–176. 10.1007/s10802-012-9668-x. [DOI] [PubMed] [Google Scholar]
- 195.Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, Liu M, Molitoris DR, Green JA. 2010. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16:444–453. 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 196.Horvath K, Perman JA. 2002. Autistic disorder and gastrointestinal disease. Curr Opin Pediatr 14:583–587. 10.1097/00008480-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 197.Jenkins TP, Rathnayaka Y, Perera PK, Peachey LE, Nolan MJ, Krause L, Rajakaruna RS, Cantacessi C. 2017. Infections by human gastrointestinal helminths are associated with changes in faecal microbiota diversity and composition. PLoS One 12:e0184719. 10.1371/journal.pone.0184719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Leung JM, Graham AL, Knowles SC. 2018. Parasite-microbiota interactions with the vertebrate gut: synthesis through an ecological lens. Front Microbiol 9:843. 10.3389/fmicb.2018.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Navarro F, Liu Y, Rhoads JM. 2016. Can probiotics benefit children with autism spectrum disorders? World J Gastroenterol 22:10093–10102. 10.3748/wjg.v22.i46.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.De Angelis M, Francavilla R, Piccolo M, De Giacomo A, Gobbetti M. 2015. Autism spectrum disorders and intestinal microbiota. Gut Microbes 6:207–213. 10.1080/19490976.2015.1035855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Connolly N, Anixt J, Manning P, Ping I, Lin D, Marsolo KA, Bowers K. 2016. Maternal metabolic risk factors for autism spectrum disorder: an analysis of electronic medical records and linked birth data. Autism Res 9:829–837. 10.1002/aur.1586. [DOI] [PubMed] [Google Scholar]
- 202.Schultz ST, Klonoff-Cohen HS, Wingard DL, Akshoomoff NA, Macera CA, Ji M, Bacher C. 2006. Breastfeeding, infant formula supplementation, and autistic disorder: the results of a parent survey. Int Breastfeed J 1:16–17. 10.1186/1746-4358-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liu Y, Fatheree NY, Mangalat N, Rhoads JM. 2010. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 299:G1087–G1096. 10.1152/ajpgi.00124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jonkers D, Penders J, Masclee A, Pierik M. 2012. Probiotics in the management of inflammatory bowel disease. Drugs 72:803–823. 10.2165/11632710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 205.Zhang Y, Li L, Guo C, Mu D, Feng B, Zuo X, Li Y. 2016. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: a meta-analysis. BMC Gastroenterol 16:62. 10.1186/s12876-016-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Giannetti E, Staiano A. 2016. Probiotics for irritable bowel syndrome: clinical data in children. J Pediatr Gastroenterol Nutr 63:S25–S26. [DOI] [PubMed] [Google Scholar]
- 207.Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, Caruso D, Pearson C, Kiang S, Dahm JL, Hong X, Wang G, Wang M-C, Zuckerman B, Wang X. 2016. The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics 137:e20152206. 10.1542/peds.2015-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Finegold SM, Downes J, Summanen PH. 2012. Microbiology of regressive autism. Anaerobe 18:260–262. 10.1016/j.anaerobe.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 209.Finegold SM. 2011. Desulfovibrio species are potentially important in regressive autism. Med Hypotheses 77:270–274. 10.1016/j.mehy.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 210.Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Väisänen M-L, Nelson MN, Wexler HM. 2000. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol 15:429–435. 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- 211.Kraneveld A, Szklany K, de Theije C, Garssen J. 2016. Gut-to-brain axis in autism spectrum disorders: central role for the microbiome. Int Rev Neurobiol 131:263–287. 10.1016/bs.irn.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 212.Onore C, Careaga M, Ashwood P. 2012. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun 26:383–392. 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. 2013. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155:1451–1463. 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Doenyas C. 2018. Gut microbiota, inflammation, and probiotics on neural development in autism spectrum disorder. Neuroscience 374:271–286. 10.1016/j.neuroscience.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 215.MacFabe DF. 2015. Enteric short-chain fatty acids: microbial messengers of metabolism, mitochondria, and mind: implications in autism spectrum disorders. Microb Ecol Health Dis 26:28177. 10.3402/mehd.v26.28177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sa’ad H, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. 2010. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta 1801:1175–1183. [DOI] [PubMed] [Google Scholar]
- 217.Needham BD, Adame MD, Serena G, Rose DR, Preston GM, Conrad MC, Campbell AS, Donabedian DH, Fasano A, Ashwood P, Mazmanian SK. 2021. Plasma and fecal metabolite profiles in autism spectrum disorder. Biol Psychiatry 89:451–462. 10.1016/j.biopsych.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kang D-W, Adams JB, Vargason T, Santiago M, Hahn J, Krajmalnik-Brown R. 2020. Distinct fecal and plasma metabolites in children with autism spectrum disorders and their modulation after microbiota transfer therapy. mSphere 5:e00314-20. 10.1128/mSphere.00314-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tandon R, Keshavan MS, Nasrallah HA. 2008. Schizophrenia,“just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr Res 102:1–18. 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 220.Hoftman GD, Dienel SJ, Bazmi HH, Zhang Y, Chen K, Lewis DA. 2018. Altered gradients of glutamate and gamma-aminobutyric acid transcripts in the cortical visuospatial working memory network in schizophrenia. Biol Psychiatry 83:670–679. 10.1016/j.biopsych.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kesby J, Eyles D, McGrath J, Scott J. 2018. Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience. Transl Psychiatry 8:1–12. 10.1038/s41398-017-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rowland LM, Summerfelt A, Wijtenburg SA, Du X, Chiappelli JJ, Krishna N, West J, Muellerklein F, Kochunov P, Hong LE. 2016. Frontal glutamate and γ-aminobutyric acid levels and their associations with mismatch negativity and digit sequencing task performance in schizophrenia. JAMA Psychiatry 73:166–174. 10.1001/jamapsychiatry.2015.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kirkpatrick B, Miller B, García-Rizo C, Fernandez-Egea E. 2014. Schizophrenia: a systemic disorder. Clin Schizophr Relat Psychoses 8:73–79. 10.3371/CSRP.KIMI.031513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. 2015. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2:258–270. 10.1016/S2215-0366(14)00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Severance EG, Prandovszky E, Castiglione J, Yolken RH. 2015. Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep 17:27. 10.1007/s11920-015-0574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.De Palma G, Blennerhassett P, Lu J, Deng Y, Park AJ, Green W, Denou E, Silva MA, Santacruz A, Sanz Y, Surette MG, Verdu EF, Collins SM, Bercik P. 2015. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat Commun 6:1–13. 10.1038/ncomms8735. [DOI] [PubMed] [Google Scholar]
- 227.Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P. 2016. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 21:786–796. 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 228.Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. 2016. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 165:1762–1775. 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet M-F, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. 2016. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167:1469–1480. e12. 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, Liu Y, Cheng K, Zhou C, Wang H, Zhou X, Gui S, Perry SW, Wong M-L, Licinio J, Wei H, Xie P. 2019. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv 5:eaau8317. 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dinan T, Borre Y, Cryan J. 2014. Genomics of schizophrenia: time to consider the gut microbiome? Mol Psychiatry 19:1252–1257. 10.1038/mp.2014.93. [DOI] [PubMed] [Google Scholar]
- 232.Nguyen TT, Kosciolek T, Eyler LT, Knight R, Jeste DV. 2018. Overview and systematic review of studies of microbiome in schizophrenia and bipolar disorder. J Psychiatr Res 99:50–61. 10.1016/j.jpsychires.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM, Whittredge PB, Fu T, Worringer K, Brown HE, Wang J, Kaykas A, Karmacharya R, Goold CP, Sheridan SD, Perlis RH. 2019. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci 22:374–385. 10.1038/s41593-018-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dickerson FB, Stallings C, Origoni A, Katsafanas E, Savage CL, Schweinfurth LA, Goga J, Khushalani S, Yolken RH. 2014. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. The primary care companion for CNS disorders 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CLG, Adamos MB, Sweeney KM, Origoni AE, Khushalani S, Dickerson FB, Yolken RH. 2017. Probiotic normalization of Candida albicans in schizophrenia: a randomized, placebo-controlled, longitudinal pilot study. Brain Behav Immun 62:41–45. 10.1016/j.bbi.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Doshi A, Chataway J. 2017. Multiple sclerosis, a treatable disease. Clin Med 17:530–536. 10.7861/clinmedicine.17-6-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ochoa-Repáraz J, Kirby TO, Kasper LH. 2018. The gut microbiome and multiple sclerosis. Cold Spring Harb Perspect Med 8:a029017. 10.1101/cshperspect.a029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Harbo HF, Gold R, Tintoré M. 2013. Sex and gender issues in multiple sclerosis. Ther Adv Neurol Disord 6:237–248. 10.1177/1756285613488434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Frohman EM, Racke MK, Raine CS. 2006. Multiple sclerosis: the plaque and its pathogenesis. N Engl J Med 354:942–955. 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 240.Fletcher JM, Lalor S, Sweeney C, Tubridy N, Mills K. 2010. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol 162:1–11. 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Legroux L, Arbour N. 2015. Multiple sclerosis and T lymphocytes: an entangled story. J Neuroimmune Pharmacol 10:528–546. 10.1007/s11481-015-9614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Weissert R. 2013. The immune pathogenesis of multiple sclerosis. J Neuroimmune Pharmacol 8:857–866. 10.1007/s11481-013-9467-3. [DOI] [PubMed] [Google Scholar]
- 243.Correale J, Gaitan M. 2015. Multiple sclerosis and environmental factors: the role of vitamin D, parasites, and Epstein-Barr virus infection. Acta Neurol Scand 132:46–55. 10.1111/ane.12431. [DOI] [PubMed] [Google Scholar]
- 244.Oksenberg JR. 2013. Decoding multiple sclerosis: an update on genomics and future directions. Expert Rev Neurother 13:11–19. 10.1586/14737175.2013.865867. [DOI] [PubMed] [Google Scholar]
- 245.Schepici G, Silvestro S, Bramanti P, Mazzon E. 2019. The gut microbiota in multiple sclerosis: an overview of clinical trials. Cell Transplant 28:1507–1527. 10.1177/0963689719873890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, Clarke G, Cryan JF. 2016. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 6:e774. 10.1038/tp.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Guan NL, Kundu P, Gulyás B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S. 2014. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6:263ra158. 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Libbey J, Sanchez J, Doty D, Sim J, Cusick M, Cox J, Fischer K, Round J, Fujinami R. 2018. Variations in diet cause alterations in microbiota and metabolites that follow changes in disease severity in a multiple sclerosis model. Benef Microbes 9:495–513. 10.3920/BM2017.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rea K, Dinan TG, Cryan JF. 2016. The microbiome: a key regulator of stress and neuroinflammation. Neurobiol Stress 4:23–33. 10.1016/j.ynstr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Tankou SK, Regev K, Healy BC, Tjon E, Laghi L, Cox LM, Kivisäkk P, Pierre IV, Hrishikesh L, Gandhi R, Cook S, Glanz B, Stankiewicz J, Weiner HL. 2018. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol 83:1147–1161. 10.1002/ana.25244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Dickson DW, Fujishiro H, Orr C, DelleDonne A, Josephs KA, Frigerio R, Burnett M, Parisi JE, Klos KJ, Ahlskog JE. 2009. Neuropathology of non-motor features of Parkinson disease. Parkinsonism Related Disorders 15:S1–S5. 10.1016/S1353-8020(09)70769-2. [DOI] [PubMed] [Google Scholar]
- 252.Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag A-E, Lang AE. 2017. Parkinson disease. Nat Rev Dis Primers 3:1–21. 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 253.Braak H, de Vos RA, Bohl J, Del Tredici K. 2006. Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396:67–72. 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 254.Twelves D, Perkins KS, Counsell C. 2003. Systematic review of incidence studies of Parkinson’s disease. Mov Disord 18:19–31. 10.1002/mds.10305. [DOI] [PubMed] [Google Scholar]
- 255.Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, Grigoletto F, Amaducci L, Inzitari D. 2000. Parkinson’s disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. Neurology 55:1358–1363. 10.1212/wnl.55.9.1358. [DOI] [PubMed] [Google Scholar]
- 256.Parashar A, Udayabanu M. 2017. Gut microbiota: implications in Parkinson’s disease. Parkinsonism Relat Disord 38:1–7. 10.1016/j.parkreldis.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, Buchman AS, Larson EB, Crane PK, Kaye JA, Kramer P, Woltjer R, Trojanowski JQ, Weintraub D, Chen-Plotkin AS, Irwin DJ, Rick J, Schellenberg GD, Watson GS, Kukull W, Nelson PT, Jicha GA, Neltner JH, Galasko D, Masliah E, Quinn JF, Chung KA, Yearout D, Mata IF, Wan JY, Edwards KL, Montine TJ, Zabetian CP. 2013. APOE ε4 increases risk for dementia in pure synucleinopathies. JAMA Neurol 70:223–228. 10.1001/jamaneurol.2013.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mulak A, Bonaz B. 2015. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol 21:10609–10620. 10.3748/wjg.v21.i37.10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pfeiffer RF. 2003. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurology 2:107–116. 10.1016/S1474-4422(03)00307-7. [DOI] [PubMed] [Google Scholar]
- 260.Ternák G, Kuti D, Kovács KJ. 2020. Dysbiosis in Parkinson’s disease might be triggered by certain antibiotics. Med Hypotheses 137:109564. 10.1016/j.mehy.2020.109564. [DOI] [PubMed] [Google Scholar]
- 261.Miraglia F, Colla E. 2019. Microbiome, Parkinson’s disease, and molecular mimicry. Cells 8:222. 10.3390/cells8030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cersosimo MG, Benarroch EE. 2012. Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol Dis 46:559–564. 10.1016/j.nbd.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 263.Poewe W. 2008. Non‐motor symptoms in Parkinson’s disease. Eur J Neurol 15:14–20. 10.1111/j.1468-1331.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 264.Paillusson S, Clairembault T, Biraud M, Neunlist M, Derkinderen P. 2013. Activity‐dependent secretion of alpha‐synuclein by enteric neurons. J Neurochem 125:512–517. 10.1111/jnc.12131. [DOI] [PubMed] [Google Scholar]
- 265.Niehaus I, Lange J. 2003. Endotoxin: is it an environmental factor in the cause of Parkinson’s disease? Occup Environ Med 60:378–378. 10.1136/oem.60.5.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Forsythe P, Bienenstock J, Kunze WA. 2014. Vagal pathways for microbiome-brain-gut axis communication, p 115–133. In Microbial endocrinology: the microbiota-gut-brain axis in health and disease. Springer, New York, NY. [DOI] [PubMed] [Google Scholar]
- 267.Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A. 2011. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6:e28032. 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Tufekci KU, Genc S, Genc K. 2011. The endotoxin-induced neuroinflammation model of Parkinson’s disease. Parkinson’s Dis 2011:1–25. 10.4061/2011/487450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Dutta G, Zhang P, Liu B. 2008. The lipopolysaccharide Parkinson’s disease animal model: mechanistic studies and drug discovery. Fundam Clin Pharmacol 22:453–464. 10.1111/j.1472-8206.2008.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wallen ZD, Appah M, Dean MN, Sesler CL, Factor SA, Molho E, Zabetian CP, Standaert DG, Payami H. 2020. Characterizing dysbiosis of gut microbiome in PD: evidence for overabundance of opportunistic pathogens. bioRxiv 10.1101/2020.01.13.905166. [DOI] [PMC free article] [PubMed]
- 271.Unger MM, Möller JC, Mankel K, Eggert KM, Bohne K, Bodden M, Stiasny-Kolster K, Kann PH, Mayer G, Tebbe JJ, Oertel WH. 2011. Postprandial ghrelin response is reduced in patients with Parkinson’s disease and idiopathic REM sleep behaviour disorder: a peripheral biomarker for early Parkinson’s disease? J Neurol 258:982–990. 10.1007/s00415-010-5864-1. [DOI] [PubMed] [Google Scholar]
- 272.Ivanov II, Honda K. 2012. Intestinal commensal microbes as immune modulators. Cell Host Microbe 12:496–508. 10.1016/j.chom.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Nishio J, Honda K. 2012. Immunoregulation by the gut microbiota. Cell Mol Life Sci 69:3635–3650. 10.1007/s00018-012-0993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bested AC, Logan AC, Selhub EM. 2013. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances. I. Autointoxication revisited. Gut Pathog 5:5–16. 10.1186/1757-4749-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Caricilli AM, Castoldi A, Câmara NOS. 2014. Intestinal barrier: a gentlemen’s agreement between microbiota and immunity. World J Gastrointest Pathophysiol 5:18–32. 10.4291/wjgp.v5.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Guo S, Al-Sadi R, Said HM, Ma TY. 2013. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol 182:375–387. 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Sui Y-T, Bullock KM, Erickson MA, Zhang J, Banks W. 2014. Alpha synuclein is transported into and out of the brain by the blood-brain barrier. Peptides 62:197–202. 10.1016/j.peptides.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, Onofrj M. 2009. Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun 23:55–63. 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 279.Dobbs R, Charlett A, Purkiss A, Dobbs S, Weller C, Peterson D. 1999. Association of circulating TNF‐α and IL‐6 with ageing and parkinsonism. Acta Neurol Scand 100:34–41. 10.1111/j.1600-0404.1999.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 280.Chen H, O’Reilly EJ, Schwarzschild MA, Ascherio A. 2008. Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am J Epidemiol 167:90–95. 10.1093/aje/kwm260. [DOI] [PubMed] [Google Scholar]
- 281.Block ML, Zecca L, Hong J-S. 2007. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69. 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 282.Rite I, Machado A, Cano J, Venero JL. 2007. Blood-brain barrier disruption induces in vivo degeneration of nigral dopaminergic neurons. J Neurochem 101:1567–1582. 10.1111/j.1471-4159.2007.04567.x. [DOI] [PubMed] [Google Scholar]
- 283.Perez-Pardo P, Dodiya HB, Engen PA, Forsyth CB, Huschens AM, Shaikh M, Voigt RM, Naqib A, Green SJ, Kordower JH, Shannon KM, Garssen J, Kraneveld AD, Keshavarzian A. 2019. Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut 68:829–843. 10.1136/gutjnl-2018-316844. [DOI] [PubMed] [Google Scholar]
- 284.Nair AT, Ramachandran V, Joghee NM, Antony S, Ramalingam G. 2018. Gut microbiota dysfunction as reliable noninvasive early diagnostic biomarkers in the pathophysiology of Parkinson’s disease: a critical review. J Neurogastroenterol Motil 24:30–42. 10.5056/jnm17105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Förster C. 2008. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol 130:55–70. 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Clairembault T, Leclair-Visonneau L, Coron E, Bourreille A, Le Dily S, Vavasseur F, Heymann M-F, Neunlist M, Derkinderen P. 2015. Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol Commun 3:12. 10.1186/s40478-015-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Çamcı G, Oğuz S. 2016. Association between Parkinson’s disease and Helicobacter pylori. J Clin Neurol 12:147–150. 10.3988/jcn.2016.12.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Fasano A, Bove F, Gabrielli M, Petracca M, Zocco MA, Ragazzoni E, Barbaro F, Piano C, Fortuna S, Tortora A, Di Giacopo R, Campanale M, Gigante G, Lauritano EC, Navarra P, Marconi S, Gasbarrini A, Bentivoglio AR. 2013. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord 28:1241–1249. 10.1002/mds.25522. [DOI] [PubMed] [Google Scholar]
- 289.Tan AH, Mahadeva S, Thalha AM, Gibson PR, Kiew CK, Yeat CM, Ng SW, Ang SP, Chow SK, Tan CT, Yong HS, Marras C, Fox SH, Lim S-Y. 2014. Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism Relat Disord 20:535–540. 10.1016/j.parkreldis.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 290.Adams B, Nunes JM, Page MJ, Roberts T, Carr J, Nell TA, Kell DB, Pretorius E. 2019. Parkinson’s disease: a systemic inflammatory disease accompanied by bacterial inflammagens. Front Aging Neurosci 11:210. 10.3389/fnagi.2019.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Feng Y-K, Wu Q-L, Peng Y-W, Liang F-Y, You H-J, Feng Y-W, Li G, Li X-J, Liu S-H, Li Y-C, Zhang Y, Pei Z. 2020. Oral P. gingivalis impairs gut permeability and mediates immune responses associated with neurodegeneration in LRRK2 R1441G mice. J Neuroinflammation 17:1–12. 10.1186/s12974-020-02027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pompei A, Cordisco L, Amaretti A, Zanoni S, Matteuzzi D, Rossi M. 2007. Folate production by bifidobacteria as a potential probiotic property. Appl Environ Microbiol 73:179–185. 10.1128/AEM.01763-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Surwase SN, Jadhav JP. 2011. Bioconversion of l-tyrosine to L-DOPA by a novel bacterium Bacillus sp. Amino Acids 41:495–506. 10.1007/s00726-010-0768-z. [DOI] [PubMed] [Google Scholar]
- 294.Cassani E, Privitera G, Pezzoli G, Pusani C, Madio C, Iorio L, Barichella M. 2011. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol Dietol 57:117–121. [PubMed] [Google Scholar]
- 295.Ho L, Zhao D, Ono K, Ruan K, Mogno I, Tsuji M, Carry E, Brathwaite J, Sims S, Frolinger T, Westfall S, Mazzola P, Wu Q, Hao K, Lloyd TE, Simon JE, Faith J, Pasinetti GM. 2019. Heterogeneity in gut microbiota drive polyphenol metabolism that influences α-synuclein misfolding and toxicity. J Nutr Biochem 64:170–181. 10.1016/j.jnutbio.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M, Vandeputte D, Tito RY, Chaffron S, Rymenans L, Verspecht C, De Sutter L, Lima-Mendez G, D’hoe K, Jonckheere K, Homola D, Garcia R, Tigchelaar EF, Eeckhaudt L, Fu J, Henckaerts L, Zhernakova A, Wijmenga C, Raes J. 2016. Population-level analysis of gut microbiome variation. Science 352:560–564. 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 297.Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, Mujagic Z, Vila AV, Falony G, Vieira-Silva S, Wang J, Imhann F, Brandsma E, Jankipersadsing SA, Joossens M, Cenit MC, Deelen P, Swertz MA, Weersma RK, Feskens EJM, Netea MG, Gevers D, Jonkers D, Franke L, Aulchenko YS, Huttenhower C, Raes J, Hofker MH, Xavier RJ, Wijmenga C, Fu J, LifeLines Cohort Study . 2016. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 352:565–569. 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP, Knight R, Payami H. 2017. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32:739–749. 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kim Y-K. 2020. Method for diagnosing parkinson’s disease through bacterial metagenome analysis. Google Patents. [Google Scholar]
- 300.Hegelmaier T, Lebbing M, Duscha A, Tomaske L, Tönges L, Holm JB, Bjørn Nielsen H, Gatermann SG, Przuntek H, Haghikia A. 2020. Interventional influence of the intestinal microbiome through dietary intervention and bowel cleansing might improve motor symptoms in Parkinson’s disease. Cells 9:376. 10.3390/cells9020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Yang D, Zhao D, Ali Shah SZ, Wu W, Lai M, Zhang X, Li J, Guan Z, Zhao H, Li W, Gao H, Zhou X, Yang L. 2019. The role of the gut microbiota in the pathogenesis of Parkinson’s disease. Front Neurol 10. 10.3389/fneur.2019.01155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Haikal C, Chen Q-Q, Li J-Y. 2019. Microbiome changes: an indicator of Parkinson’s disease? Transl Neurodegener 8:1–9. 10.1186/s40035-019-0175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina AM, Winblad B, Jönsson L, Liu Z, Prince M. 2017. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 13:1–7. 10.1016/j.jalz.2016.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Alzheimer’s Association. 2019. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dementia 15:321–387. 10.1016/j.jalz.2019.01.010. [DOI] [Google Scholar]
- 305.Braak H, Braak E. 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 306.Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, Trojanowski JQ. 2010. Total and phosphorylated tau protein as biological markers of Alzheimer’s disease. Exp Gerontol 45:30–40. 10.1016/j.exger.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Reitz C. 2012. Alzheimer’s disease and the amyloid cascade hypothesis: a critical review. Int J Alzheimer’s Dis 2012:1–11. 10.1155/2012/369808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Mucke L, Selkoe DJ. 2012. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med 2:a006338. 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Bachurin SO, Gavrilova SI, Samsonova A, Barreto GE, Aliev G. 2018. Mild cognitive impairment due to Alzheimer disease: contemporary approaches to diagnostics and pharmacological intervention. Pharmacol Res 129:216–226. 10.1016/j.phrs.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 310.Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, Moir RD. 2010. The Alzheimer’s disease-associated amyloid β-protein is an antimicrobial peptide. PLoS One 5:e9505. 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sochocka M, Zwolińska K, Leszek J. 2017. The infectious etiology of Alzheimer’s disease. Curr Neuropharmacol 15:996–1009. 10.2174/1570159X15666170313122937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Cappellano G, Carecchio M, Fleetwood T, Magistrelli L, Cantello R, Dianzani U, Comi C. 2013. Immunity and inflammation in neurodegenerative diseases. Am J Neurodegener Dis 2:89–107. [PMC free article] [PubMed] [Google Scholar]
- 313.Papassotiropoulos A, Lambert J-C, Wavrant-De Vrièze F, Wollmer MA, von der Kammer H, Streffer JR, Maddalena A, Huynh K-D, Wolleb S, Lutjohann D, Schneider B, Thal DR, Grimaldi LME, Tsolaki M, Kapaki E, Ravid R, Konietzko U, Hegi T, Pasch T, Jung H, Braak H, Amouyel P, Rogaev EI, Hardy J, Hock C, Nitsch RM. 2005. Cholesterol 25-hydroxylase on chromosome 10q is a susceptibility gene for sporadic Alzheimer’s disease. Neurodegener Dis 2:233–241. 10.1159/000090362. [DOI] [PubMed] [Google Scholar]
- 314.Wozniak MA, Frost AL, Itzhaki RF. 2009. Alzheimer’s disease-specific tau phosphorylation is induced by herpes simplex virus type 1. J Alzheimers Dis 16:341–350. 10.3233/JAD-2009-0963. [DOI] [PubMed] [Google Scholar]
- 315.Readhead B, Haure-Mirande J-V, Ehrlich ME, Gandy S, Dudley JT. 2019. Clarifying the potential role of microbes in Alzheimer’s disease. Neuron 104:1036–1037. 10.1016/j.neuron.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Allen HB. 2019. The essential role of biofilms in Alzheimer’s disease. Microbiol Infect Dis 3:1–3. 10.33425/2639-9458.1060. [DOI] [Google Scholar]
- 317.Woods JJ, Skelding KA, Martin KL, Aryal R, Sontag E, Johnstone DM, Horvat JC, Hansbro PM, Milward EA. 2020. Assessment of evidence for or against contributions of Chlamydia pneumoniae infections to Alzheimer’s disease etiology. Brain Behav Immun 83:22–32. 10.1016/j.bbi.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 318.Hill JM, Lukiw WJ. 2015. Microbial-generated amyloids and Alzheimer’s disease (AD). Front Aging Neurosci 7:9. 10.3389/fnagi.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Vogt NM, Romano KA, Darst BF, Engelman CD, Johnson SC, Carlsson CM, Asthana S, Blennow K, Zetterberg H, Bendlin BB, Rey FE. 2018. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res Ther 10:124. 10.1186/s13195-018-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Harach T, Marungruang N, Duthilleul N, Cheatham V, Mc Coy KD, Frisoni G, Neher JJ, Fåk F, Jucker M, Lasser T, Bolmont T. 2017. Reduction of Aβ amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep 7:41802. 10.1038/srep41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Zhang L, Wang Y, Xiayu X, Shi C, Chen W, Song N, Fu X, Zhou R, Xu Y-F, Huang L, Zhu H, Han Y, Qin C. 2017. Altered gut microbiota in a mouse model of Alzheimer’s disease. J Alzheimers Dis 60:1241–1257. 10.3233/JAD-170020. [DOI] [PubMed] [Google Scholar]
- 322.Dodiya HB, Kuntz T, Shaik SM, Baufeld C, Leibowitz J, Zhang X, Gottel N, Zhang X, Butovsky O, Gilbert JA, Sisodia SS. 2019. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J Exp Med 216:1542–1560. 10.1084/jem.20182386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Zhuang Z-Q, Shen L-L, Li W-W, Fu X, Zeng F, Gui L, Lü Y, Cai M, Zhu C, Tan Y-L, Zheng P, Li H-Y, Zhu J, Zhou H-D, Bu X-L, Wang Y-J. 2018. Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis 63:1337–1346. 10.3233/JAD-180176. [DOI] [PubMed] [Google Scholar]
- 324.Mawanda F, Wallace R. 2013. Can infections cause Alzheimer’s disease? Epidemiol Rev 35:161–180. 10.1093/epirev/mxs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, Holsinger LJ, Arastu-Kapur S, Kaba S, Lee A, Ryder MI, Potempa B, Mydel P, Hellvard A, Adamowicz K, Hasturk H, Walker GD, Reynolds EC, Faull RLM, Curtis MA, Dragunow M, Potempa J. 2019. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv 5:eaau3333. 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Wang M, Cao J, Gong C, Amakye WK, Yao M, Ren J. 2021. Exploring the microbiota-Alzheimer’s disease linkage using short-term antibiotic treatment followed by fecal microbiota transplantation. Brain Behav Immun 96:227–238. 10.1016/j.bbi.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 327.Honarpisheh P, Reynolds CR, Blasco Conesa MP, Moruno Manchon JF, Putluri N, Bhattacharjee MB, Urayama A, McCullough LD, Ganesh BP. 2020. Dysregulated gut homeostasis observed prior to the accumulation of the brain amyloid-β in Tg2576 mice. Int J Mol Sci 21:1711. 10.3390/ijms21051711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Shukla PK, Delotterie DF, Xiao J, Pierre JF, Rao R, McDonald MP, Khan MM. 2021. Alterations in the gut-microbial-inflammasome-brain axis in a mouse model of Alzheimer’s disease. Cells 10:779. 10.3390/cells10040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, Musch MW, Liao F, Ward JF, Holtzman DM, Chang EB, Tanzi RE, Sisodia SS. 2016. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep 6:30028. 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Minter MR, Hinterleitner R, Meisel M, Zhang C, Leone V, Zhang X, Oyler-Castrillo P, Zhang X, Musch MW, Shen X, Jabri B, Chang EB, Tanzi RE, Sisodia SS. 2017. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APP SWE/PS1 ΔE9 murine model of Alzheimer’s disease. Sci Rep 7:1–18. 10.1038/s41598-017-11047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Cai Z, Hussain MD, Yan L-J. 2014. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int J Neurosci 124:307–321. 10.3109/00207454.2013.833510. [DOI] [PubMed] [Google Scholar]
- 332.Arulsamy A, Tan QY, Balasubramaniam V, O’Brien TJ, Shaikh MF. 2020. Gut microbiota and epilepsy: a systematic review on their relationship and possible therapeutics. ACS Chem Neurosci 11:3488–3498. 10.1021/acschemneuro.0c00431. [DOI] [PubMed] [Google Scholar]
- 333.Şafak B, Altunan B, Topçu B, Topkaya AE. 2020. The gut microbiome in epilepsy. Microbial Pathogenesis 139:103853. 10.1016/j.micpath.2019.103853. [DOI] [PubMed] [Google Scholar]
- 334.Jennum P, Gyllenborg J, Kjellberg J. 2011. The social and economic consequences of epilepsy: a controlled national study. Epilepsia 52:949–956. 10.1111/j.1528-1167.2010.02946.x. [DOI] [PubMed] [Google Scholar]
- 335.Jennum P, Pickering L, Christensen J, Ibsen R, Kjellberg J. 2017. Morbidity and mortality of childhood-and adolescent-onset epilepsy: a controlled national study. Epilepsy Behav 66:80–85. 10.1016/j.yebeh.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 336.Levira F, Thurman DJ, Sander JW, Hauser WA, Hesdorffer DC, Masanja H, Odermatt P, Logroscino G, Newton CR, Epilepsy ECotILA . 2017. Premature mortality of epilepsy in low‐and middle‐income countries: a systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia 58:6–16. 10.1111/epi.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Chatzikonstantinou S, Gioula G, Kimiskidis VK, McKenna J, Mavroudis I, Kazis D. 2021. The gut microbiome in drug‐resistant epilepsy. Epilepsia Open 6:28–37. 10.1002/epi4.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Round JL, Mazmanian SK. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9:313–323. 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Vezzani A. 2014. Epilepsy and inflammation in the brain: overview and pathophysiology: epilepsy and inflammation in the brain. Epilepsy Curr 14:3–7. 10.5698/1535-7511-14.s2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lindefeldt M, Eng A, Darban H, Bjerkner A, Zetterström CK, Allander T, Andersson B, Borenstein E, Dahlin M, Prast-Nielsen S. 2019. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes 5:5. 10.1038/s41522-018-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Peng A, Qiu X, Lai W, Li W, Zhang L, Zhu X, He S, Duan J, Chen L. 2018. Altered composition of the gut microbiome in patients with drug-resistant epilepsy. Epilepsy Res 147:102–107. 10.1016/j.eplepsyres.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 342.Xie G, Zhou Q, Qiu C-Z, Dai W-K, Wang H-P, Li Y-H, Liao J-X, Lu X-G, Lin S-F, Ye J-H, Ma Z-Y, Wang W-J. 2017. Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J Gastroenterol 23:6164–6171. 10.3748/wjg.v23.i33.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Kitamura S, Sugihara K, Kuwasako M, Tatsumi K. 1997. The role of mammalian intestinal bacteria in the reductive metabolism of zonisamide. J Pharm Pharmacol 49:253–256. 10.1111/j.2042-7158.1997.tb06790.x. [DOI] [PubMed] [Google Scholar]
- 344.Dahlin M, Prast-Nielsen S. 2019. The gut microbiome and epilepsy. EBioMedicine 44:741–746. 10.1016/j.ebiom.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Sewal RK, Modi M, Saikia UN, Chakrabarti A, Medhi B. 2017. Increase in seizure susceptibility in sepsis like condition explained by spiking cytokines and altered adhesion molecules level with impaired blood brain barrier integrity in experimental model of rats treated with lipopolysaccharides. Epilepsy Res 135:176–186. 10.1016/j.eplepsyres.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 346.Gómez-Eguílaz M, Ramón-Trapero J, Pérez-Martínez L, Blanco J. 2018. The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: a pilot study. Benef Microbes 9:875–881. 10.3920/BM2018.0018. [DOI] [PubMed] [Google Scholar]
- 347.Yeom JS, Park JS, Kim Y-S, Kim RB, Choi D-S, Chung J-Y, Han T-H, Seo J-H, Park ES, Lim J-Y, Woo H-O, Youn H-S, Park C-H. 2019. Neonatal seizures and white matter injury: role of rotavirus infection and probiotics. Brain and Development 41:19–28. 10.1016/j.braindev.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 348.Medel‐Matus JS, Shin D, Dorfman E, Sankar R, Mazarati A. 2018. Facilitation of kindling epileptogenesis by chronic stress may be mediated by intestinal microbiome. Epilepsia Open 3:290–294. 10.1002/epi4.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. 2018. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173:1728–1741. e13. 10.1016/j.cell.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, Dichgans M, Liesz A. 2016. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci 36:7428–7440. 10.1523/JNEUROSCI.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Houlden A, Goldrick M, Brough D, Vizi ES, Lénárt N, Martinecz B, Roberts I, Denes A. 2016. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun 57:10–20. 10.1016/j.bbi.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Tang WW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. 2013. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368:1575–1584. 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung Y-M, Wu Y, Schauer P, Smith JD, Allayee H, Tang WHW, DiDonato JA, Lusis AJ, Hazen SL. 2011. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472:57–63. 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yin J, Liao S‐X, He Y, Wang S, Xia G‐H, Liu F‐T, Zhu J‐J, You C, Chen Q, Zhou L, Pan S‐Y, Zhou H‐W. 2015. Dysbiosis of gut microbiota with reduced trimethylamine‐N‐oxide level in patients with large‐artery atherosclerotic stroke or transient ischemic attack. Jaha 4:e002699. 10.1161/JAHA.115.002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Winek K, Dirnagl U, Meisel A. 2016. The gut microbiome as therapeutic target in central nervous system diseases: implications for stroke. Neurotherapeutics 13:762–774. 10.1007/s13311-016-0475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Griffin JL, Wang X, Stanley E. 2015. Does our gut microbiome predict cardiovascular risk? A review of the evidence from metabolomics. Circ Cardiovasc Genet 8:187–191. 10.1161/CIRCGENETICS.114.000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Winek K, Engel O, Koduah P, Heimesaat MM, Fischer A, Bereswill S, Dames C, Kershaw O, Gruber AD, Curato C, Oyama N, Meisel C, Meisel A, Dirnagl U. 2016. Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke 47:1354–1363. 10.1161/STROKEAHA.115.011800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J. 2016. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med 22:516–523. 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Lee J, d’Aigle J, Atadja L, Quaicoe V, Honarpisheh P, Ganesh BP, Hassan A, Graf J, Petrosino J, Putluri N, Zhu L, Durgan DJ, Bryan RM, McCullough LD, Venna VR. 2020. Gut microbiota–derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res 127:453–465. 10.1161/CIRCRESAHA.119.316448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Sun J, Wang F, Ling Z, Yu X, Chen W, Li H, Jin J, Pang M, Zhang H, Yu J, Liu J. 2016. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res 1642:180–188. 10.1016/j.brainres.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 361.Brenner LA, Stearns-Yoder KA, Hoffberg AS, Penzenik ME, Starosta AJ, Hernandez TD, Hadidi DA, Lowry CA. 2017. Growing literature but limited evidence: a systematic review regarding prebiotic and probiotic interventions for those with traumatic brain injury and/or posttraumatic stress disorder. Brain Behav Immun 65:57–67. 10.1016/j.bbi.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 362.Kerry RG, Patra JK, Gouda S, Park Y, Shin H-S, Das G. 2018. Benefaction of probiotics for human health: a review. J Food Drug Anal 26:927–939. 10.1016/j.jfda.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Vallianou N, Stratigou T, Christodoulatos GS, Tsigalou C, Dalamaga M. 2020. Probiotics, prebiotics, synbiotics, postbiotics, and obesity: current evidence, controversies, and perspectives. Curr Obes Rep 9:179–114. 10.1007/s13679-020-00379-w. [DOI] [PubMed] [Google Scholar]
- 364.Draper K, Ley C, Parsonnet J. 2017. Probiotic guidelines and physician practice: a cross-sectional survey and overview of the literature. Benef Microbes 8:507–519. 10.3920/BM2016.0146. [DOI] [PubMed] [Google Scholar]
- 365.Whitehead TR, Price NP, Drake HL, Cotta MA. 2008. Catabolic pathway for the production of skatole and indoleacetic acid by the acetogen Clostridium drakei, Clostridium scatologenes, and swine manure. Appl Environ Microbiol 74:1950–1953. 10.1128/AEM.02458-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Kumar A, Sperandio V. 2019. Indole signaling at the host-microbiota-pathogen interface. mBio 10:e01031-19. 10.1128/mBio.01031-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Russell WR, Duncan SH, Scobbie L, Duncan G, Cantlay L, Calder AG, Anderson SE, Flint HJ. 2013. Major phenylpropanoid‐derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res 57:523–535. 10.1002/mnfr.201200594. [DOI] [PubMed] [Google Scholar]
- 368.Wlodarska M, Luo C, Kolde R, d’Hennezel E, Annand JW, Heim CE, Krastel P, Schmitt EK, Omar AS, Creasey EA, Garner AL, Mohammadi S, O’Connell DJ, Abubucker S, Arthur TD, Franzosa EA, Huttenhower C, Murphy LO, Haiser HJ, Vlamakis H, Porter JA, Xavier RJ. 2017. Indoleacrylic acid produced by commensal peptostreptococcus species suppresses inflammation. Cell Host Microbe 22:25–37. 10.1016/j.chom.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. 2009. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 106:3698–3703. 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Cervantes-Barragan L, Chai JN, Tianero MD, Di Luccia B, Ahern PP, Merriman J, Cortez VS, Caparon MG, Donia MS, Gilfillan S, Cella M, Gordon JI, Hsieh C-S, Colonna M. 2017. Lactobacillus reuteri induces gut intraepithelial CD4+ CD8αα+ T cells. Science 357:806–810. 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Obata Y, Castaño Á, Boeing S, Bon-Frauches AC, Fung C, Fallesen T, de Agüero MG, Yilmaz B, Lopes R, Huseynova A, Horswell S, Maradana MR, Boesmans W, Vanden Berghe P, Murray AJ, Stockinger B, Macpherson AJ, Pachnis V. 2020. Neuronal programming by microbiota regulates intestinal physiology. Nature 578:284–289. 10.1038/s41586-020-1975-8. [DOI] [PubMed] [Google Scholar]
- 372.Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao C-C, Ardura-Fabregat A, de Lima KA, Gutiérrez-Vázquez C, Hewson P, Staszewski O, Blain M, Healy L, Neziraj T, Borio M, Wheeler M, Dragin LL, Laplaud DA, Antel J, Alvarez JI, Prinz M, Quintana FJ. 2018. Microglial control of astrocytes in response to microbial metabolites. Nature 557:724–728. 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Rothhammer V, Quintana FJ. 2019. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol 19:184–197. 10.1038/s41577-019-0125-8. [DOI] [PubMed] [Google Scholar]
- 374.Wheeler MA, Rothhammer V, Quintana FJ. 2017. Control of immune-mediated pathology via the aryl hydrocarbon receptor. J Biol Chem 292:12383–12389. 10.1074/jbc.R116.767723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Rothhammer V, Kenison JE, Tjon E, Takenaka MC, de Lima KA, Borucki DM, Chao C-C, Wilz A, Blain M, Healy L, Antel J, Quintana FJ. 2017. Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proc Natl Acad Sci USA 114:2012–2017. 10.1073/pnas.1615413114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao C-C, Patel B, Yan R, Blain M, Alvarez JI, Kébir H, Anandasabapathy N, Izquierdo G, Jung S, Obholzer N, Pochet N, Clish CB, Prinz M, Prat A, Antel J, Quintana FJ. 2016. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22:586–597. 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Morelli L, Capurso L. 2012. FAO/WHO guidelines on probiotics: 10 years later. J Clin Gastroenterol 46:S1–S2. 10.1097/MCG.0b013e318269fdd5. [DOI] [PubMed] [Google Scholar]
- 378.Cheng L-H, Liu Y-W, Wu C-C, Wang S, Tsai Y-C. 2019. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J Food Drug Anal 27:632–648. 10.1016/j.jfda.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Arnold LE, Luna RA, Williams K, Chan J, Parker RA, Wu Q, Hollway JA, Jeffs A, Lu F, Coury DL, Hayes C, Savidge T. 2019. Probiotics for gastrointestinal symptoms and quality of life in autism: a placebo-controlled pilot trial. J Child Adolesc Psychopharmacol 29:659–669. 10.1089/cap.2018.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Nimgampalle M, Kuna Y. 2017. Anti-Alzheimer properties of probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s disease-induced albino rats. J Clin Diagn Red 11:KC01. 10.7860/JCDR/2017/26106.10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Athari Nik Azm S, Djazayeri A, Safa M, Azami K, Ahmadvand B, Sabbaghziarani F, Sharifzadeh M, Vafa M. 2018. Lactobacilli and bifidobacteria ameliorate memory and learning deficits and oxidative stress in β-amyloid (1–42) injected rats. Appl Physiol Nutr Metab 43:718–726. 10.1139/apnm-2017-0648. [DOI] [PubMed] [Google Scholar]
- 382.Asl ZR, Sepehri G, Salami M. 2019. Probiotic treatment improves the impaired spatial cognitive performance and restores synaptic plasticity in an animal model of Alzheimer’s disease. Behav Brain Res 376:112183. 10.1016/j.bbr.2019.112183. [DOI] [PubMed] [Google Scholar]
- 383.Rezaeiasl Z, Salami M, Sepehri G. 2019. The effects of probiotic Lactobacillus and Bifidobacterium strains on memory and learning behavior, long-term potentiation (ltp), and some biochemical parameters in β-amyloid-induced rat’s model of alzheimer’s disease. Prev Nutr Food Sci 24:265–273. 10.3746/pnf.2019.24.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Pärtty A, Kalliomäki M, Wacklin P, Salminen S, Isolauri E. 2015. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr Res 77:823–828. 10.1038/pr.2015.51. [DOI] [PubMed] [Google Scholar]
- 385.Castelli V, d’Angelo M, Quintiliani M, Benedetti E, Cifone MG, Cimini A. 2021. The emerging role of probiotics in neurodegenerative diseases: new hope for Parkinson’s disease? Neural Regen Res 16:628–634. 10.4103/1673-5374.295270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Hsieh T-H, Kuo C-W, Hsieh K-H, Shieh M-J, Peng C-W, Chen Y-C, Chang Y-L, Huang Y-Z, Chen C-C, Chang P-K, Chen K-Y, Chen H-Y. 2020. Probiotics alleviate the progressive deterioration of motor functions in a mouse model of Parkinson’s disease. Brain Sci 10:206. 10.3390/brainsci10040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. 2014. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 388.Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, Cryan JF. 2017. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry 82:472–487. 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 389.Ghanizadeh A, Moghimi-Sarani E. 2013. A randomized double blind placebo controlled clinical trial of N-acetylcysteine added to risperidone for treating autistic disorders. BMC Psychiatry 13:196. 10.1186/1471-244X-13-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Grimaldi R, Cela D, Swann JR, Vulevic J, Gibson GR, Tzortzis G, Costabile A. 2017. In vitro fermentation of B-GOS: impact on faecal bacterial populations and metabolic activity in autistic and non-autistic children. FEMS Microbiol Ecol 93:fiw233. 10.1093/femsec/fiw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Lee Y-S, Lai D-M, Huang H-J, Lee-Chen G-J, Chang C-H, Hsieh-Li HM, Lee G-C. 2021. Prebiotic lactulose ameliorates the cognitive deficit in Alzheimer’s disease mouse model through macroautophagy and chaperone-mediated autophagy pathways. J Agric Food Chem 69:2422–2437. 10.1021/acs.jafc.0c07327. [DOI] [PubMed] [Google Scholar]
- 392.Lorente-Picón M, Laguna A. 2021. New avenues for Parkinson’s disease therapeutics: disease-modifying strategies based on the gut microbiota. Biomolecules 11:433. 10.3390/biom11030433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Barathikannan K, Chelliah R, Rubab M, Daliri EB-M, Elahi F, Kim D-H, Agastian P, Oh S-Y, Oh DH. 2019. Gut microbiome modulation based on probiotic application for anti-obesity: a review on efficacy and validation. Microorganisms 7:456. 10.3390/microorganisms7100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Krumbeck JA, Rasmussen HE, Hutkins RW, Clarke J, Shawron K, Keshavarzian A, Walter J. 2018. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 6:1–16. 10.1186/s40168-018-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. 2019. Effect of probiotic and prebiotic versus placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin Nutr 38:522–528. 10.1016/j.clnu.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 396.Barichella M, Pacchetti C, Bolliri C, Cassani E, Iorio L, Pusani C, Pinelli G, Privitera G, Cesari I, Faierman SA, Caccialanza R, Pezzoli G, Cereda E. 2016. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology 87:1274–1280. 10.1212/WNL.0000000000003127. [DOI] [PubMed] [Google Scholar]
- 397.Sanctuary MR, Kain JN, Chen SY, Kalanetra K, Lemay DG, Rose DR, Yang HT, Tancredi DJ, German JB, Slupsky CM, Ashwood P, Mills DA, Smilowitz JT, Angkustsiri K. 2019. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS One 14:e0210064. 10.1371/journal.pone.0210064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Gocan AG, Bachg D, Schindler AE, Rohr UD. 2012. Balancing steroidal hormone cascade in treatment-resistant veteran soldiers with PTSD using a fermented soy product (FSWW08): a pilot study. Hormone Mol Biol Clin Invest 10:301–314. 10.1515/hmbci-2011-0135. [DOI] [PubMed] [Google Scholar]
- 399.Aguilar-Toalá J, Garcia-Varela R, Garcia H, Mata-Haro V, González-Córdova A, Vallejo-Cordoba B, Hernández-Mendoza A. 2018. Postbiotics: an evolving term within the functional foods field. Trends Food Sci Technol 75:105–114. 10.1016/j.tifs.2018.03.009. [DOI] [Google Scholar]
- 400.Oberg T, Steele J, Ingham S, Smeianov V, Briczinski E, Abdalla A, Broadbent J. 2018. Correction to: intrinsic and inducible resistance to hydrogen peroxide in Bifidobacterium species. J Ind Microbiol Biotechnol 45:765–765. 10.1007/s10295-018-2054-0. [DOI] [PubMed] [Google Scholar]
- 401.Lin HV, Frassetto A, Kowalik EJ, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, Marsh DJ. 2012. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One 7:e35240. 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Cuevas-González P, Liceaga A, Aguilar-Toalá J. 2020. Postbiotics and paraprobiotics: from concepts to applications. Food Res Int 136:109502. 10.1016/j.foodres.2020.109502. [DOI] [PubMed] [Google Scholar]
- 403.Sánchez B, Delgado S, Blanco‐Míguez A, Lourenço A, Gueimonde M, Margolles A. 2017. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res 61:1600240. 10.1002/mnfr.201600240. [DOI] [PubMed] [Google Scholar]
- 404.Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Võsa U, Mujagic Z, Masclee AAM, Jonkers DMAE, Oosting M, Joosten LAB, Netea MG, Franke L, Zhernakova A, Fu J, Wijmenga C, McCarthy MI. 2019. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet 51:600–605. 10.1038/s41588-019-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O’Sullivan O, Clarke G, Stanton C, Dinan TG, Cryan JF. 2018. Short‐chain fatty acids: microbial metabolites that alleviate stress‐induced brain-gut axis alterations. J Physiol 596:4923–4944. 10.1113/JP276431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Shultz SR, MacFabe DF, Ossenkopp K-P, Scratch S, Whelan J, Taylor R, Cain DP. 2008. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology 54:901–911. 10.1016/j.neuropharm.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 407.Lach G, Schellekens H, Dinan TG, Cryan JF. 2018. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics 15:36–59. 10.1007/s13311-017-0585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Nishida K, Sawada D, Kuwano Y, Tanaka H, Sugawara T, Aoki Y, Fujiwara S, Rokutan K. 2017. Daily administration of paraprobiotic Lactobacillus gasseri CP2305 ameliorates chronic stress-associated symptoms in Japanese medical students. J Functional Foods 36:112–121. 10.1016/j.jff.2017.06.031. [DOI] [Google Scholar]
- 409.Liu W-H, Chuang H-L, Huang Y-T, Wu C-C, Chou G-T, Wang S, Tsai Y-C. 2016. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav Brain Res 298:202–209. 10.1016/j.bbr.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 410.Antunes LCM, Han J, Ferreira RB, Lolić P, Borchers CH, Finlay BB. 2011. Effect of antibiotic treatment on the intestinal metabolome. Antimicrob Agents Chemother 55:1494–1503. 10.1128/AAC.01664-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, Engstrand L. 2010. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One 5:e9836. 10.1371/journal.pone.0009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, Patil KR, Bork P, Typas A. 2018. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555:623–628. 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Ticinesi A, Milani C, Lauretani F, Nouvenne A, Mancabelli L, Lugli GA, Turroni F, Duranti S, Mangifesta M, Viappiani A, Ferrario C, Maggio M, Ventura M, Meschi T. 2017. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci Rep 7:1–11. 10.1038/s41598-017-10734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Tomizawa Y, Kurokawa S, Ishii D, Miyaho K, Ishii C, Sanada K, Fukuda S, Mimura M, Kishimoto T. 2021. Effects of psychotropics on the microbiome in patients with depression and anxiety: considerations in a naturalistic clinical setting. Int J Neuropsychopharmacol 24:97–107. 10.1093/ijnp/pyaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Gunics G, Farkas S, Motohashi N, Shah A, Harsukh G, Kawase M, Molnár J. 2002. Interaction between 3,5-diacetyl-1,4-dihydropyridines and ampicillin and erythromycin on different Escherichia coli strains. Int J Antimicrob Agents 20:227–229. 10.1016/s0924-8579(02)00159-0. [DOI] [PubMed] [Google Scholar]
- 416.Gunics G, Motohashi N, Molnár J, Farkas S, Kawase M, Saito S, Shah A. 2001. Enhanced antibacterial effect of erythromycin in the presence of 3,5-dibenzoyl-1,4-dihydropyridines. Anticancer Res 21:269–273. [PubMed] [Google Scholar]
- 417.Kruszewska H, Zareba T, Tyski S. 2000. Antimicrobial activity of selected non-antibiotics: activity of methotrexate against Staphylococcus aureus strains. Acta Poloniae Pharmaceutica 57:117–119. [PubMed] [Google Scholar]
- 418.Kruszewska H, Zareba T, Tyski S. 2002. Search of antimicrobial activity of selected non-antibiotic drugs. Acta Pol Pharm 59:436–438. [PubMed] [Google Scholar]
- 419.de Meij TG, de Groot EF, Eck A, Budding AE, Kneepkens CF, Benninga MA, van Bodegraven AA, Savelkoul PH. 2016. Characterization of microbiota in children with chronic functional constipation. PLoS One 11:e0164731. 10.1371/journal.pone.0164731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Vijayvargiya P, Busciglio I, Burton D, Donato L, Lueke A, Camilleri M. 2018. Bile acid deficiency in a subgroup of patients with irritable bowel syndrome with constipation based on biomarkers in serum and fecal samples. Clin Gastroenterol Hepatol 16:522–527. 10.1016/j.cgh.2017.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Chait YA, Mottawea W, Tompkins TA, Hammami R. 2020. Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci Rep 10:1–11. 10.1038/s41598-020-74934-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Baumgart DC, Sandborn WJ. 2012. Crohn’s disease. Lancet 380:1590–1605. 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 423.Luppens S, Kara D, Bandounas L, Jonker M, Wittink F, Bruning O, Breit T, Ten Cate J, Crielaard W. 2008. Effect of Veillonella parvula on the antimicrobial resistance and gene expression of Streptococcus mutans grown in a dual‐species biofilm. Oral Microbiol Immunol 23:183–189. 10.1111/j.1399-302X.2007.00409.x. [DOI] [PubMed] [Google Scholar]
- 424.Müller RT, Pos KM. 2015. The assembly and disassembly of the AcrAB-TolC three-component multidrug efflux pump. Biol Chem 396:1083–1089. 10.1515/hsz-2015-0150. [DOI] [PubMed] [Google Scholar]
- 425.Flowers SA, Evans SJ, Ward KM, McInnis MG, Ellingrod VL. 2017. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy 37:261–267. 10.1002/phar.1890. [DOI] [PubMed] [Google Scholar]
- 426.Morgan AP, Crowley JJ, Nonneman RJ, Quackenbush CR, Miller CN, Ryan AK, Bogue MA, Paredes SH, Yourstone S, Carroll IM, Kawula TH, Bower MA, Sartor RB, Sullivan PF. 2014. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS One 9:e115225. 10.1371/journal.pone.0115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Kothari D, Patel S, Kim S-K. 2019. Probiotic supplements might not be universally effective and safe: a review. Biomed Pharmacother 111:537–547. 10.1016/j.biopha.2018.12.104. [DOI] [PubMed] [Google Scholar]
- 428.Doron S, Snydman DR. 2015. Risk and safety of probiotics. Clin Infect Dis 60:S129–S134. 10.1093/cid/civ085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Liu X, Cao S, Zhang X. 2015. Modulation of gut microbiota–brain axis by probiotics, prebiotics, and diet. J Agric Food Chem 63:7885–7895. 10.1021/acs.jafc.5b02404. [DOI] [PubMed] [Google Scholar]
- 430.Islam SU. 2016. Clinical uses of probiotics. Medicine (Baltimore, MD) 95:e2658. 10.1097/MD.0000000000002658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Frye RE, Slattery J, MacFabe DF, Allen-Vercoe E, Parker W, Rodakis J, Adams JB, Krajmalnik-Brown R, Bolte E, Kahler S, Jennings J, James J, Cerniglia CE, Midtvedt T. 2015. Approaches to studying and manipulating the enteric microbiome to improve autism symptoms. Microb Ecol Health Dis 26:26878. 10.3402/mehd.v26.26878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Vallabhaneni S, Walker TA, Lockhart SR, Ng D, Branch IDP, Chiller T, Melchreit R, Brandt ME, Smith RM. 2015. Fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement—Connecticut, 2014. MMWR Morbid Mortal Wkly Rep 64:155. [PMC free article] [PubMed] [Google Scholar]
- 433.Belizário JE, Napolitano M. 2015. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front Microbiol 6:1050. 10.3389/fmicb.2015.01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Ng SC, Chan FK, Chan PK. 2020. Screening FMT donors during the COVID-19 pandemic: a protocol for stool SARS-CoV-2 viral quantification. Lancet Gastroenterol Hepatol 5:642–643. 10.1016/S2468-1253(20)30124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Kang D-W, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, Caporaso JG, Krajmalnik-Brown R. 2019. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci Rep 9:1–9. 10.1038/s41598-019-42183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Zhao H, Gao X, Xi L, Shi Y, Peng L, Wang C, Zou L, Yang Y. 2019. Mo1667 fecal microbiota transplantation for children with autism spectrum disorder. Gastrointest Endoscopy 89:AB512–AB513. 10.1016/j.gie.2019.03.857. [DOI] [Google Scholar]
- 437.Urbonas V, Cervinskiene J. 2018. Fecal transplantation and its role in autism spectrum disorders. Wiley, Hoboken, NJ. [Google Scholar]
- 438.Liu J, Wan G-B, Huang M-S, Agyapong G, Zou T-l, Zhang X-Y, Liu Y-W, Song Y-Q, Tsai Y-C, Kong X-J. 2019. Probiotic therapy for treating behavioral and gastrointestinal symptoms in autism spectrum disorder: a systematic review of clinical trials. Curr Med Sci 39:173–184. 10.1007/s11596-019-2016-4. [DOI] [PubMed] [Google Scholar]
- 439.Shaaban SY, El Gendy YG, Mehanna NS, El-Senousy WM, El-Feki HS, Saad K, El-Asheer OM. 2018. The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr Neurosci 21:676–681. 10.1080/1028415X.2017.1347746. [DOI] [PubMed] [Google Scholar]
- 440.Slykerman RF, Kang J, Van Zuy N, Barthow C, Wickens K, Stanley T, Coomarasamy C, Purdie G, Murphy R, Crane J, Mitchell EA. 2018. Effect of early probiotic supplementation on childhood cognition, behavior, and mood: a randomized, placebo-controlled trial. Acta Paediatr 107:2172–2178. 10.1111/apa.14590. [DOI] [PubMed] [Google Scholar]
- 441.Grimaldi R, Gibson GR, Vulevic J, Giallourou N, Castro-Mejía JL, Hansen LH, Gibson EL, Nielsen DS, Costabile A. 2018. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome 6:133. 10.1186/s40168-018-0523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Xie W-R, Yang X-Y, Xia HH-X, Wu L-H, He X-X. 2019. Hair regrowth following fecal microbiota transplantation in an elderly patient with alopecia areata: a case report and review of the literature. World J Clin Cases 7:3074–3081. 10.12998/wjcc.v7.i19.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Cai T, Shi X, Yuan L-Z, Tang D, Wang F. 2019. Fecal microbiota transplantation in an elderly patient with mental depression. Int Psychogeriatr 31:1525–1526. 10.1017/S1041610219000115. [DOI] [PubMed] [Google Scholar]
- 444.Okubo R, Koga M, Katsumata N, Odamaki T, Matsuyama S, Oka M, Narita H, Hashimoto N, Kusumi I, Xiao J, Matsuoka YJ. 2019. Effect of bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: a proof-of-concept study. J Affect Disord 245:377–385. 10.1016/j.jad.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 445.Tomasik J, Yolken RH, Bahn S, Dickerson FB. 2015. Immunomodulatory effects of probiotic supplementation in schizophrenia patients: a randomized, placebo-controlled trial. Biomarker Insights 10:BMI.S22007. 10.4137/BMI.S22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Haghighat N, Rajabi S, Mohammadshahi M. 2021. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: a randomized, double-blinded, clinical trial. Nutr Neurosci 24:490–410. 10.1080/1028415X.2019.1646975. [DOI] [PubMed] [Google Scholar]
- 447.Makkawi S, Camara-Lemarroy C, Metz L. 2018. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol Neuroimmunol Neuroinflamm 5:e459. 10.1212/NXI.0000000000000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Salehipour Z, Haghmorad D, Sankian M, Rastin M, Nosratabadi R, Dallal MMS, Tabasi N, Khazaee M, Nasiraii LR, Mahmoudi M. 2017. Bifidobacterium animalis in combination with human origin of Lactobacillus plantarum ameliorate neuroinflammation in experimental model of multiple sclerosis by altering CD4+ T cell subset balance. Biomed Pharmacother 95:1535–1548. 10.1016/j.biopha.2017.08.117. [DOI] [PubMed] [Google Scholar]
- 449.Salami M, Kouchaki E, Asemi Z, Tamtaji OR. 2019. How probiotic bacteria influence the motor and mental behaviors as well as immunological and oxidative biomarkers in multiple sclerosis? A double-blind clinical trial. J Functional Foods 52:8–13. 10.1016/j.jff.2018.10.023. [DOI] [Google Scholar]
- 450.Secher T, Kassem S, Benamar M, Bernard I, Boury M, Barreau F, Oswald E, Saoudi A. 2017. Oral administration of the probiotic strain Escherichia coli Nissle 1917 reduces susceptibility to neuroinflammation and repairs experimental autoimmune encephalomyelitis-induced intestinal barrier dysfunction. Front Immunol 8:1096. 10.3389/fimmu.2017.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Huang H, Xu H, Luo Q, He J, Li M, Chen H, Tang W, Nie Y, Zhou Y. 2019. Fecal microbiota transplantation to treat Parkinson’s disease with constipation: a case report. Medicine (Baltimore, MD) 98:e16163. 10.1097/MD.0000000000016163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Sun M-F, Zhu Y-L, Zhou Z-L, Jia X-B, Xu Y-D, Yang Q, Cui C, Shen Y-Q. 2018. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun 70:48–60. 10.1016/j.bbi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 453.Zhou Z-L, Jia X-B, Sun M-F, Zhu Y-L, Qiao C-M, Zhang B-P, Zhao L-P, Yang Q, Cui C, Chen X, Shen Y-Q. 2019. Neuroprotection of fasting mimicking diet on MPTP-induced Parkinson’s disease mice via gut microbiota and metabolites. Neurotherapeutics 16:741–760. 10.1007/s13311-019-00719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Borzabadi S, Oryan S, Eidi A, Aghadavod E, Kakhaki RD, Tamtaji OR, Taghizadeh M, Asemi Z. 2018. The effects of probiotic supplementation on gene expression related to inflammation, insulin and lipid in patients with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Arch Iran Med 21:289–295. [PubMed] [Google Scholar]
- 455.Tamtaji OR, Taghizadeh M, Kakhaki RD, Kouchaki E, Bahmani F, Borzabadi S, Oryan S, Mafi A, Asemi Z. 2019. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr 38:1031–1035. 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 456.Georgescu D, Ancusa OE, Georgescu LA, Ionita I, Reisz D. 2016. Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: is there hope? Clin Interv Aging 11:1601–1608. 10.2147/CIA.S106284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Fujii Y, Nguyen TTT, Fujimura Y, Kameya N, Nakamura S, Arakawa K, Morita H. 2019. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer’s disease. Biosci Biotechnol Biochem 83:2144–2152. 10.1080/09168451.2019.1644149. [DOI] [PubMed] [Google Scholar]
- 458.Zhan G, Yang N, Li S, Huang N, Fang X, Zhang J, Zhu B, Yang L, Yang C, Luo A. 2018. Abnormal gut microbiota composition contributes to cognitive dysfunction in SAMP8 mice. Aging (Albany, NY) 10:1257–1267. 10.18632/aging.101464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Leblhuber F, Steiner K, Schuetz B, Fuchs D, Gostner JM. 2018. Probiotic supplementation in patients with Alzheimer’s dementia-an explorative intervention study. Curr Alzheimer Res 15:1106–1113. 10.2174/1389200219666180813144834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Den H, Dong X, Chen M, Zou Z. 2020. Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidative stress in adults with Alzheimer’s disease or mild cognitive impairment: a meta-analysis of randomized controlled trials. Aging (Albany, NY) 12:4010–4039. 10.18632/aging.102810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E, Tajabadi-Ebrahimi M, Asemi Z. 2019. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: a randomized, double-blind, controlled trial. Clin Nutr 38:2569–2575. 10.1016/j.clnu.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 462.Hwang Y-H, Park S, Paik J-W, Chae S-W, Kim D-H, Jeong D-G, Ha E, Kim M, Hong G, Park S-H, Jung S-J, Lee S-M, Na K-H, Kim J, Chung Y-C. 2019. Efficacy and safety of Lactobacillus plantarum C29-fermented soybean (DW2009) in individuals with mild cognitive impairment: a 12-week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients 11:305. 10.3390/nu11020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Kobayashi Y, Kinoshita T, Matsumoto A, Yoshino K, Saito I, Xiao J-Z. 2019. Bifidobacterium breve A1 supplementation improved cognitive decline in older adults with mild cognitive impairment: an open-label, single-arm study. J Prev Alzheimers Dis 6:70–75. [DOI] [PubMed] [Google Scholar]
- 464.Agahi A, Hamidi G, Salami M, Alinaghipour A, Daneshvar Kakhaki R, Soheili M. 2018. The effect of probiotic supplementations on cognitive function in patients with primary and secondary Alzheimer. J Arak Univ Med Sci 20:1–9. [Google Scholar]
- 465.He Z, Cui B-T, Zhang T, Li P, Long C-Y, Ji G-Z, Zhang F-M. 2017. Fecal microbiota transplantation cured epilepsy in a case with Crohn’s disease: the first report. World J Gastroenterol 23:3565–3568. 10.3748/wjg.v23.i19.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Bagheri S, Heydari A, Alinaghipour A, Salami M. 2019. Effect of probiotic supplementation on seizure activity and cognitive performance in PTZ-induced chemical kindling. Epilepsy Behav 95:43–50. 10.1016/j.yebeh.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 467.Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, Ajami NJ, Putluri N, Graf J, Bryan RM, McCullough LD. 2018. Age‐related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol 84:23–36. 10.1002/ana.25250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Xia G-H, You C, Gao X-X, Zeng X-L, Zhu J-J, Xu K-Y, Tan C-H, Xu R-T, Wu Q-H, Zhou H-W, He Y, Yin J. 2019. Stroke dysbiosis index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Front Neurol 10:397. 10.3389/fneur.2019.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Akhoundzadeh K, Vakili A, Shadnoush M, Sadeghzadeh J. 2018. Effects of the oral ingestion of probiotics on brain damage in a transient model of focal cerebral ischemia in mice. Iran J Med Sci 43:32. [PMC free article] [PubMed] [Google Scholar]
- 470.Gong C, Yang L, Liu K, Shen S, Zhang Q, Li H, Cheng Y. 2021. Effects of antibiotic treatment and probiotics on the gut microbiome of 40 infants delivered before term by cesarean section analysed by using 16S rRNA quantitative polymerase chain reaction sequencing. Med Sci Monit 27:e928467-1. 10.12659/MSM.928467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Rahmati H, Momenabadi S, Vafaei AA, Bandegi AR, Mazaheri Z, Vakili A. 2019. Probiotic supplementation attenuates hippocampus injury and spatial learning and memory impairments in a cerebral hypoperfusion mouse model. Mol Biol Rep 46:4985–4995. 10.1007/s11033-019-04949-7. [DOI] [PubMed] [Google Scholar]
- 472.Jahangiri S, Tadayonfar M, Rakhshani MH. 2017. The effect of synbiotics on the constipation of stroke patients admitted to ICU. J Adv Med Biomed Res 25:105–112.. [Google Scholar]
- 473.Liu S, Guo R, Liu F, Yuan Q, Yu Y, Ren F. 2020. Gut microbiota regulates depression-like behavior in rats through the neuroendocrine-immune-mitochondrial pathway. Neuropsychiatr Dis Treat 16:859–869. 10.2147/NDT.S243551. [DOI] [PMC free article] [PubMed] [Google Scholar]