Abstract
We have developed a model system of human fibrosarcoma cell lines that do or do not possess and express an oncogenic mutant allele of N-ras. HT1080 cells contain an endogenous mutant allele of N-ras, whereas the derivative MCH603 cell line contains only wild-type N-ras. In an earlier study (S. Gupta et al., Mol. Cell. Biol. 20:9294–9306, 2000), we had shown that HT1080 cells produce rapidly growing, aggressive tumors in athymic nude mice, whereas MCH603 cells produced more slowly growing tumors and was termed weakly tumorigenic. An extensive analysis of the Ras signaling pathways (Raf, Rac1, and RhoA) provided evidence for a potential novel pathway that was critical for the aggressive tumorigenic phenotype and could be activated by elevated levels of constitutively active MEK. In this study we examined the role of phosphoinositide 3-kinase (PI 3-kinase) in the regulation of the transformed and aggressive tumorigenic phenotypes expressed in HT1080 cells. Both HT1080 (mutant N-ras) and MCH603 (wild-type N-ras) have similar levels of constitutively active Akt, a downstream target of activated PI 3-kinase. We find that both cell lines constitutively express platelet-derived growth factor (PDGF) and PDGF receptors. Transfection with tumor suppressor PTEN cDNA into HT1080 and constitutively active PI 3-kinase–CAAX cDNA into MCH603 cells, respectively, resulted in several interesting and novel observations. Activation of the PI 3-kinase/Akt pathway, including NF-κB, is not required for the aggressive tumorigenic phenotype in HT1080 cells. Activation of NF-κB is complex: in MCH603 cells it is mediated by Akt, whereas in HT1080 cells activation also involves other pathway(s) that are activated by mutant Ras. A threshold level of activation of PI 3-kinase is required in MCH603 cells before stimulatory cross talk to the RhoA, Rac1, and Raf pathways occurs, without a corresponding activation of Ras. The increased levels of activation seen were similar to those observed in HT1080 cells, except for Raf and MEK, which were more active than HT1080 levels. This cross talk results in conversion to the aggressive tumorigenic phenotype. This latter observation is consistent with our previous observation that overstimulation of the activity of endogenous members of Ras signaling pathways, activated MEK in particular, is a prerequisite for aggressive tumorigenic growth.
Members of the Ras superfamily are small GTP-binding proteins that function as activating transducers of signaling pathways, whose members are commonly kinases or transcription factors (3, 5). Three members of this family, namely, H-ras, K-ras, and N-ras, have been implicated in human cancers. Mutations in ras alleles have been found in more than 30% of human cancers. The mutations invariably result in chronic GTP binding to the Ras molecule and its consequent chronic activation. This state results in constitutive activation of Ras-dependent signaling pathways. Among these are the Raf, Rac1, RhoA, and phosphoinositide (PI) 3-kinase signal transduction cascades (29). These pathways have been shown to regulate mitogenesis signals, motility and invasiveness, actin cytoskeletal architecture, and cell survival, respectively (10, 21, 38, 39). Derangement of the normal regulation of these cellular processes, as occurs when mutant Ras proteins are expressed, is deleterious for the normal behavior of the cells in question and contributes to the progression to a cancerous state.
A variety of experimental procedures, usually utilizing rodent cells, have shown that downstream members of each of the signaling pathways identified above, when mutated, function as transforming oncogenes (23). Among these genes are PI 3-kinase and its downstream target Akt, also known as protein kinase B (2, 41). PI 3-kinase activates Akt, a serine threonine kinase (25), which in turn phosphorylates a number of substrates, including Bad, caspase 9, Forkhead transcription factors, and IKKα (6, 9, 13, 33). Phosphorylation of Bad, procaspase 9, and Forkhead transcription factors inactivates these proapoptotic molecules, whereas phosphorylation of IKKα activates this kinase, leading eventually to activation of the antiapoptotic NF-κB transcription factor. Each of these substrates is implicated in cell survival. One of the major cell survival factors is NF-κB, whose activation status is dependent upon binding to the IκB protein. The IκB protein complexes with NF-κB and sequesters it in the cytoplasm, thereby preventing it from entering the nucleus. Degradation of IκB, following phosphorylation by IKK, releases NF-κB, which then enters the nucleus and activates its target genes (22, 40, 48). Activation of NF-κB is associated with increased cell survival and cell proliferation (4, 49, 50). One proposed mechanism for the activation of IKK is phosphorylation mediated by Akt (33, 42). However, other mechanisms also exist that do not involve the degradation of IκB (27, 44).
In addition to being activated by Ras-GTP, PI 3-kinase may also be activated directly by contact with activated growth factor receptors, including platelet-derived growth factor (PDGF) (20, 46). Dysregulated PI 3-kinase activity is likely to play an important role in cancer progression. One indication of this has been the identification of the PTEN tumor suppressor gene (26, 45). PTEN is a common target of inactivating mutations in a variety of sporadic human cancers. In addition, germ line mutations in the PTEN gene are associated with Cowden's disease, an inherited hamartoma syndrome that includes an elevated risk of breast and thyroid cancers (31). The PTEN protein functions as both a protein and a lipid phosphatase. It is the lipid phosphatase activity that is critical for its tumor-suppressing function (30). PTEN lipid phosphatase catalyzes the dephosphorylation of the 3 position of PI 3,4,5-triphosphate (PIP3) and PI 3,4,-biphosphate (PIP2), both of which are the lipid byproducts of the lipid kinase activity of PI 3-kinase. The Akt molecule binds to PIP3 via its pleckstrin homology (PH) domain. In this complex with PIP3, Akt is then phosphorylated and activated by the PI-dependent kinase, PDK-1 (1, 8). Thus, normal cells integrate the activities of PI 3-kinase and PTEN to facilitate homeostasis with respect to PI 3-kinase-mediated signal transduction and cell cycle control. Overactivation of PI 3-kinase or loss of PTEN function is likely to cause dysregulation of this finely balanced control. An illustration of this is that expression of wild-type PTEN transfected into PTEN-null cancer cells results in induction of G1 arrest and/or apoptosis (12, 16). Conversely, this arrest can be overridden by a constitutively active form of Akt (52, 55).
We have developed an experimental model system comprising the human fibrosarcoma cell line HT1080, which possesses one mutant N-ras allele, and its derivative, MCH603, which has deleted the mutant allele and possesses only wild-type N-ras (35). Examination of these cells has shown that HT1080 has a typical transformed phenotype in culture, including disorganized actin stress fibers and the ability to grow in soft agar, plus an aggressive tumorigenic phenotype in vivo in immunodeficient mice. By contrast, MCH603 cells have “reversed” their transformed phenotype; they have restored a well-organized actin stress fiber distribution in the cytoplasm and are no longer able to grow in soft agar. When implanted into immunodeficient mice they continue to form tumors but with much slower kinetics. We have described these cells as having a weak tumorigenic phenotype (35).
When we examined the activation of a number of Ras signaling pathways, namely, the Raf, Rac1, and RhoA pathways, we found that all members were constitutively active in HT1080 but had basal activity in MCH603 cells (36). However, we noted that Akt was constitutively active in both cell lines. Since this was not due to oncogenic Ras expression in MCH603 cells, we looked for another explanation. In this study we found that both cell lines constitutively synthesize and secrete PDGF and contain cell surface PDGF receptor (PDGFR). Thus, this provides a mechanism for constitutive activation of PI 3-kinase, resulting in the activation of Akt.
Although HT1080 and MCH603 cells have different transformed and tumorigenic phenotypes and yet both have constitutively active Akt, it is formally possible that there may be quantitative and qualitative differences in the activation of PI 3-kinase and/or Akt and their downstream substrates in the two cell lines that play a role in the expression of these phenotypes. In order to determine this, we have modulated the activation of PI 3-kinase and Akt by stable transfection of HT1080 and MCH603 cells with PTEN and an activated mutant of PI 3-kinase (hereafter termed PI3Kact), respectively. Examination of the biochemical and biological properties of the parental and transfectant cells has revealed several unexpected and novel findings with respect to both signal transduction pathways and biological behavior.
MATERIALS AND METHODS
Molecular constructs.
The expression plasmids used in this study were as follows: PI3Kact-pCMV(hyg)P110CAAX5′myc is derived from pSG5P110CAAX5′myc (51) and encodes the catalytic domain of PI 3-kinase. The constitutively active protein product, PI3Kact, is permanently plasma membrane associated. The construct pCDNA3PTEN(wt) (Neo) encodes a full-length wild-type PTEN cDNA (52), whose expression is driven from a heterologous cytomegalovirus promoter.
Cell culture and stable transfection.
The HT1080 cell line has one mutant and one wild-type N-ras allele (28, 35). MCH603 is a variant of HT1080 and contains only wild-type N-ras (35). The cell lines were maintained in Dulbecco minimal essential medium (DMEM) supplemented with 10% fetal calf serum (FCS; Life Technologies). The HT1080 and MCH603 cell lines were transfected with the PTEN(wt) and PI3Kact plasmids, respectively. Clones from each transfection were selected and maintained in medium containing the relevant selective antibiotic (either 800 μg of Geneticin [Gibco-BRL] or 36 U of hygromycin B [Calbiochem] per ml for the HT1080 and MCH603 transfectants, respectively). Subconfluent (70%) 100-mm dishes of MCH603 cells or HT1080 cells were transfected with 5 μg of linearized DNA or vector control DNA, using 30 μl of Lipofectin (Gibco-BRL) in Optimem medium (Gibco-BRL).
Growth in soft agar.
Logarithmically growing cells (104 or 106) were plated in single-cell suspension in a 0.3% top agar overlay in DMEM supplemented with 10% FCS, above a 0.5% bottom agar layer (in DMEM–10% FCS) in 60-mm dishes as previously described (35). Plates were fed periodically with 1 ml of DMEM–10% FCS. Colonies (>0.1 mm) were inspected under the microscope and counted after 3 weeks.
Actin cytoskeleton staining and morphology.
Cells grown on glass slides (Nunc) were washed with phosphate-buffered saline (PBS) and fixed with 3.7% paraformaldehyde in PBS for 10 min. After a wash with PBS, cells were permeabilized with PBS containing 0.1% Triton X-100 for 5 min. The slides were then washed, and the actin stress fibers were visualized by staining the cells with fluorescein-conjugated phalloidin (0.005 U/μl; Molecular Probes) for 20 min at room temperature and mounted in ProLong Fade antifade (Molecular Probes).
Immunoblot analyses.
Subconfluent cells were serum starved for 18 h, and the cells were then lysed in lysis buffer comprised of 1% sodium dodecyl sulfate (SDS) in 20 mM Tris (pH 7.4), 1 mM CaCl2, 1 mM phenylmethylsulfonyl fluoride, and 0.2 mM sodium orthovanadate. Total cell lysates, each containing 60 μg of protein, were electrophoresed by SDS–7.5% polyacrylamide gel electrophoresis (PAGE) and transferred to Immobilon-P membranes (Millipore). The membranes were then probed with the relevant antibodies. These included PDGFR-α and PDGFR-β (Santa Cruz Biotechnology), Akt/PKB, Phospho-Akt/PKB (Ser473), total Bad, Phospho-Bad, total IκBα, and Phospho-IκBα (New England Biolabs). Following incubation with horseradish peroxidase-conjugated secondary antibody, bound proteins were detected by incubation with a chemiluminescent detection system (Pierce) as previously described (7). In order to test for secreted PDGF in the conditioned medium, subconfluent HT1080 and MCH603 cells were exposed to serum-free medium for 18 h. The conditioned medium was then concentrated in the Centricon (Millipore) apparatus, followed by PAGE under reducing or nonreducing conditions and immunoblotting, using PDGF-A (E-10) and PDGF-B (P-20) antibodies (Santa Cruz Biotechnology).
Activated Ras, Rac1, and RhoA assays.
Subconfluent cells were serum starved for 18 h and then lysed with 1 × Mg2+ lysis buffer (Ras and Rac Activation Assay Kits; Upstate Biotechnology). Each cell lysate (500 μg) was affinity precipitated with 10 μl of Raf-1 RBD, PAK-1 PBD agarose, or glutathione S-transferase (GST)-C21–Sepharose conjugate (43) at 4°C overnight for the Ras, Rac-Cdc42, or RhoA activation assays, respectively. The beads were collected, washed, and resuspended in 6× Laemmli sample buffer. Western blot analysis was performed as described elsewhere (7), using 1 μg of mouse monoclonal anti-Ras, anti-Rac1 (Upstate Biotechnology), and anti-RhoA (Santa Cruz Biotechnology) antibodies per ml. Horseradish peroxidase-conjugated anti-mouse immunoglobulin G (Santa Cruz Biotechnology) was used as the secondary antibody. A chemiluminescence detection system (Pierce) was used for detection of the relevant proteins. To determine the total Ras, Rac1, or RhoA levels, immunoblots were performed using N-Ras(F155), Rac1(C-14), or RhoA(26C4) antibodies (Santa Cruz Biotechnology) that recognize total protein.
Kinase assays.
MEK, ERK, JNK, and Akt kinase assays were performed according to the manufacturer's protocols (New England Biolabs), using subconfluent cultures that had been serum starved (0.25% FCS) for 18 h, and have been described elsewhere (18). Briefly, cells were washed twice with PBS, scraped into 500 μl of lysis buffer, and incubated on ice for 20 min. After centrifugation at 14,000 × g for 20 min, the supernatants were incubated with the relevant antibodies. The resulting immunoprecipitates were employed in kinase assays. The activated MEK assay was carried out by incubating immunoprecipitated phospho-MEK with ERK protein and cold ATP (New England Biolabs MEK1/2Kinase Assay Kit). The activated ERK assay was carried out by incubating immunoprecipitated phospho-ERK with Elk-1 fusion protein and cold ATP (New England Biolabs p44/p42 ERK Assay Kit). The JNK assays were carried out by incubating the JNK–c-Jun fusion protein complex with cold ATP (New England Biolabs JNK/SAPK Assay Kit). The Akt-P assay was carried out by incubating the immunoprecipitated total Akt with GSK3 protein and cold ATP (New England Biolabs Akt Assay Kit). For MEK, ERK, and JNK assays, the relevant gel was transferred onto an Immobilon membrane, and Western blot analysis was performed. The blots were performed using phospho-ERK (Thr202/Tyr204) monoclonal antibody for the MEK assay, phospho-Elk-1 (Ser383) polyclonal antibody for the ERK assay, phospho–c-Jun (Ser63) polyclonal antibody for the JNK assay, and phospho-GSK3 α/β (Ser219) rabbit polyclonal antibody for the Akt assay. The Raf-1 assay was performed as described by Graham et al. (17). For the Raf-1 assay, the γ-32P-labeled mitogen-activated protein (MAP) Kinase (ERK) proteins in the gel were visualized by autoradiography. To determine the total Raf, MEK, ERK, JNK, and Akt levels, immunoblots were performed using the respective antibodies that recognize total protein.
Elk-1 and NF-κB luciferase reporter assays.
To measure Elk-1 activation, a dual luciferase reporter assay kit (Promega) was used as previously described (18). For NF-κB assays, approximately 2 × 105 parental HT1080 and MCH603 cells and the MCH603/PI3Kact or HT1080/PTEN stable transfectant cells were cotransfected in six-well plates with the pUC13-based Δ56FosdE-luc plasmid (measures basal expression but is not Ras responsive) and the NF-κB reporter, (HIV-kB)3-luc (54). The latter plasmid has three tandem copies of the two adjacent NF-κB sites from the human immunodeficiency virus enhancer (six total tandem NF-κB sites) inserted just upstream of the minimal Fos promoter present in Δ56FosdE-luc. The Effectene kit (Qiagen) was used for these transient transfections. Following transfection, the cells were kept in serum-starved medium for 24 h. Tumor necrosis factor alpha (TNF-α; 10 ng/ml) was then added to the culture medium, and both treated and untreated control cultures were incubated for a further 4-h period. The luciferase activity of each sample was measured with the dual luciferase assay kit (Promega) and normalized with an internal control Renilla luciferase.
Tumorigenicity assays.
Cells were trypsinized and resuspended in 0.2 ml of DMEM, and then 107 cells were injected subcutaneously into the flanks of 4- to 6-week-old nude athymic mice. Tumors were measured in three dimensions with linear calipers at weekly intervals.
RESULTS
We have shown previously that HT1080 (mutant N-ras) cells have constitutively active Raf-dependent (Raf/MEK/ERK/Elk-1), Rac1 (Rac1/Cdc42/JNK), and RhoA signaling pathways (18). Conversely, MCH603 (wild-type N-ras) cells have basal levels of activity of these signal transduction proteins (18). Interestingly, both HT1080 and MCH603 cells have significant levels of constitutively active Akt. The fact that MCH603 does not possess a mutant ras allele and yet has constitutively active levels of Akt, approximating those found in HT1080 cells, infers an alternative mechanism of chronic activation.
HT1080 and MCH603 constitutively secrete PDGF.
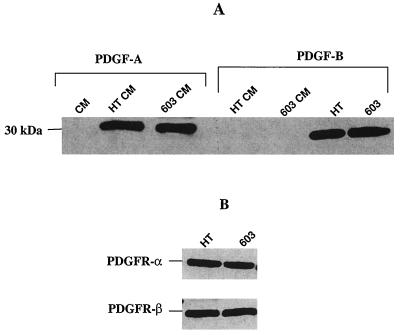
Concentrated conditioned media and cell lysates from both HT1080 and MCH603 serum-starved cell cultures were electrophoresed and immunoblotted with antibodies to PDGF-A and PDGF-B. Both forms of PDGF were expressed at similar levels by both cell types. However, whereas PDGF-A is secreted into the medium, PDGF-B remains associated with the cells (Fig. 1A). Analyses of PDGF dimers under nonreducing conditions indicated that the predominant secreted form is PDGF-AA (data not shown). Immunoblotting of cell lysates showed that both the α and β forms of the PDGFR are expressed (Fig. 1B). Constitutive secretion of PDGF-A and subsequent binding to and activation of its cognate receptor is, therefore, the probable mechanism for downstream activation of PI 3-kinase and Akt. It is known that the catalytic subunit of PI 3-kinase associates with, and is activated by, the autophosphorylated PDGFR (20, 34, 46).
FIG. 1.
Western blot analysis performed on HT1080 and MCH603 cell lysates or their respective conditioned media to determine the levels of secreted PDGF-A and PDGF-B (A) or surface membrane-bound PDGFR-α and PDGFR-β (B). HT, HT1080; 603, MCH603; CM, conditioned medium.
Modulation of PI 3-kinase and Akt/PKB activity. (i) HT1080 cells.
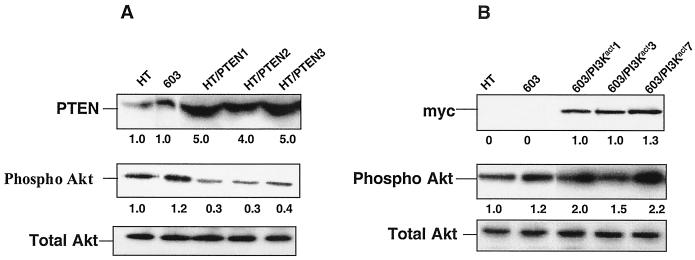
We wished to downregulate constitutive activity of PI 3-kinase and/or Akt in HT1080 cells. Initially, we attempted downregulation of PI 3-kinase activity via stable transfection with PI 3-kinase dominant-negative cDNAs. Unfortunately, none of the constructs tested (24) had the desired effect (data not shown). Thus, we resorted to expressing the tumor suppressor protein PTEN in these cells. PTEN is a dual-specificity phosphatase that catalyzes the dephosphorylation of PIP3, thereby inhibiting the activation of Akt (30). As shown in Fig. 2A, severalfold-higher levels of expression of PTEN were observed in the HT1080/PTEN stable transfectants, compared to parental HT1080 cells. Correspondingly, there was a decline in the level of expression of activated phospho-Akt. This decline in activity was confirmed in Akt assays (Fig. 3A).
FIG. 2.
Western blot analysis of the cell lysates from HT1080/PTEN transfectants (A) and MCH603/PI3Kact transfectants (B) to determine the levels of PTEN (A), myc-tagged PI 3-kinase (B), phospho-Akt, and total Akt. Three independent HT1080/PTEN and MCH603/PI3Kact clones were analyzed. The fold level of the individual proteins (PTEN and phospho-Akt) is relative to 1.0 for HT1080 control cells. HT, HT1080; 603, MCH603.
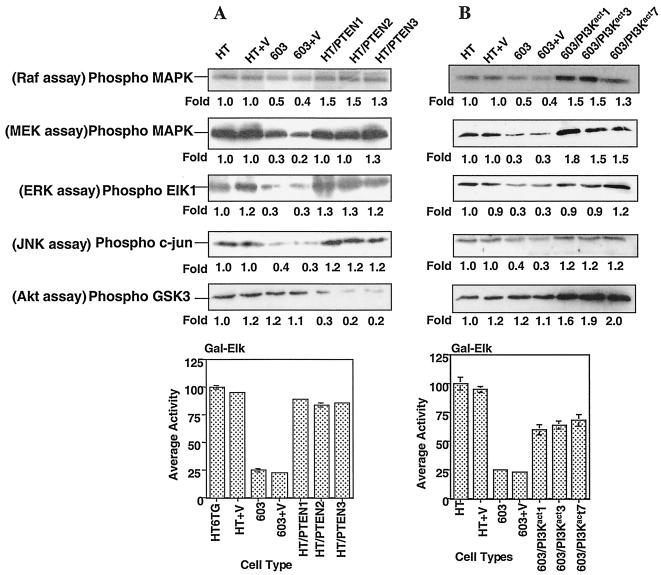
FIG. 3.
In vitro Raf, MEK, ERK, and JNK kinase assays and Elk-1 activation assays performed on HT1080/PTEN (A) and MCH603/PI3Kact (B) transfectants. For the kinase assays the fold level is relative to 1.0 for HT1080 control cells, and for the Elk-1 luciferase reporter the activities are expressed as the percent relative to 100% for HT1080. Three independent HT1080/PTEN clones and MCH603/PI3Kact clones were analyzed. HT, HT1080; 603, MCH603; V, vector only (control). The error bars indicate the standard deviations.
(ii) MCH603 cells.
Although these cells already express significant levels of constitutively active Akt, and presumably PI 3-kinase, we wanted to elevate the activity levels even further in order to determine if this may have an effect on in vitro transformed phenotypic traits and in vivo tumorigenicity. To accomplish this, MCH603 cells were stably transfected with a constitutively activated PI 3-kinase–CAAX expression vector (PI3Kact) that contains a myc epitope tag. As shown in Fig. 2B, the transfectants express high levels of the myc epitope tag and, correspondingly, higher levels of activated Akt.
Effects on other Ras-dependent signaling pathways. (i) HT1080/PTEN cells.
The lipid phosphatase activity of PTEN dephosphorylates phosphoinositides and would be expected to have inhibitory effects on PI 3-kinase-mediated activation of RhoA-, Rac1-, and Raf-dependent signaling pathways. The protein phosphatase acivity of this dual-specificity phosphatase may also have PIP3-independent effects on signal transduction. In the case of the HT1080/PTEN transfectants, however, levels of constitutively active RhoA, Rac1, and JNK and members of the Raf-dependent pathway (Raf/MEK/ERK/Elk-1) remained high, approximating the levels found in parental HT1080 cells (Fig. 3A and 4). These levels of constitutive activity are presumably mediated by the mutant N-Ras protein (see Fig. 3A) in a PI 3-kinase-independent manner.
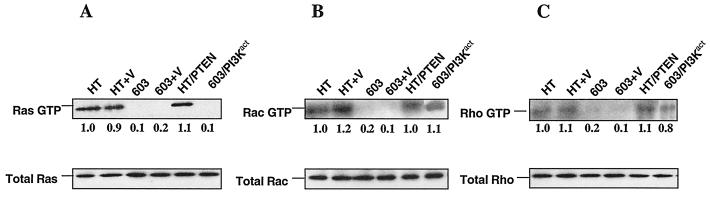
FIG. 4.
Pull-down assays of activated Ras, Rac, and Rho. The GTP-bound forms of Ras, Rac, and Rho were pulled down with GST fusion proteins, corresponding to the Ras-binding domain of Raf-1 (Raf-1 RBD), the p21-binding domain (PBD) of human PAK-1, and the C21 binding domain of Rho, respectively, conjugated to agarose beads. The Ras-GTP, Rac-GTP, and Rho-GTP proteins bound to the beads were identified using anti-Ras (A), anti-Rac (B), and anti-Rho (C) antibodies, respectively, in a Western immunoblot. Immunoblot analysis of total cell lysates identified the levels of total protein.
(ii) MCH603/PI3Kact cells.
Clear evidence of activation of multiple signaling pathways was found in these cells (Fig. 3B and 4). Persistent activation of RhoA, Rac1, and JNK and members of the Raf-dependent pathway (Raf/MEK/ERK/Elk-1) were observed. However, no activation of Ras was seen (Fig. 4A). Thus, the activation of these pathways was independent of Ras activation and was due either to direct signaling from activated PI 3-kinase or via cross talk between members of the distinct pathways. Quantitation of the levels of activity of the various members of the signaling pathways examined revealed approximately twofold-higher levels of Akt activity in the transfectants, as expected. Levels of activated RhoA and Rac1 approximated that seen in HT1080 cells. A modest but reproducible increase in levels of activated Raf-1 (approximately 1.5-fold) and MEK (1.5- to 1.8-fold) over that seen in HT1080 was observed. The levels of activated ERK and Elk-1 were approximately the same as seen in the HT1080 cells. All levels of constitutive activity were markedly higher than those found in the parental MCH603 cells.
Effects on NF-κB activation.
Akt activation, either mediated by PI 3-kinase or other signal transduction pathways, has been shown to be an antiapoptotic survival factor via activation of NF-κB and/or Bad (2, 27, 42). This property may well contribute to the tumor-forming properties of cancer cells. Thus, we wished to determine if activation or downregulation of the activities of these factors affected the tumorigenic phenotypes of HT1080 and MCH603 cells. Akt activation has been reported to activate NF-κB via IκB degradation (33, 42), although other mechanisms of activation have been reported (27, 44). In our studies we examined the status of IκB-α and NF-κB in the parental and transfectant cells.
(i) IκB-α phosphorylation.
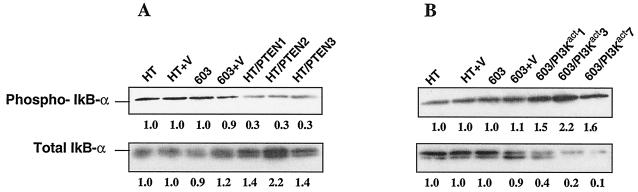
Degradation of IκB subunits is facilitated by their phosphorylation by IKK (22, 40, 48). Thus, the level of phosphorylated IκB-α, relative to the levels of total IκB-α protein, is indicative of the degradative process. In Fig. 5A we see that HT1080 and MCH603 have comparable levels of phospho-IκB-α relative to the total IκB protein levels. In contrast, the HT1080/PTEN transfectants clones have reduced levels of phospho-IκB-α. Interestingly, the levels of total IκB-α protein increased in these transfectants. Presumably, this is due to the increased stability of the unphosphorylated IκB-α. Thus, lowered Akt activity, mediated by the PTEN lipid phosphatase, results in decreased degradation of IκB-α.
FIG. 5.
Western blot analysis performed on HT1080/PTEN transfectants (A) and MCH603/PI3Kact (B) transfectants to determine the levels of phospho-IkB and total IkB in these cells.
The MCH603/PI3Kact transfectants exhibit the opposite characteristics. Increased levels of phospho-IκB-α were seen (Fig. 5B) with correspondingly greatly reduced levels of total IκB-α protein. This is consistent with the increased levels of constitutive Akt activity in these transfectants (Fig. 1B) and is indicative of an increased rate of degradation of IκB-α protein, presumably resulting in a release of IκB-bound NF-κB.
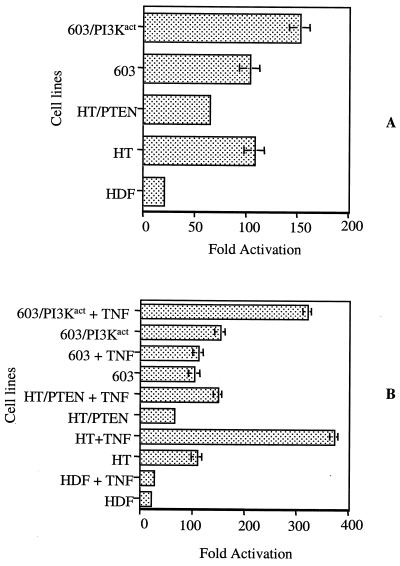
(ii) NF-κB activation. The levels of NF-κB activity in the parental and transfectant cells were assayed, using an NF-κB reporter assay (54). The relative fold activity was determined using an internal control, the Δ56FosdE-luc expression vector.
Consistent with their essentially equal levels of constitutive Akt activity, HT1080 and MCH603 cells had approximately the same fold NF-κB activities. In the HT1080/PTEN transfectants the level of activated NF-κB decreased but did not decline to the level seen in normal human diploid fibroblast (HDF) cells (Fig. 6A). Since the RhoA, Rac1, and Raf signaling pathways remain constitutively active in HT1080 cells, we interpret this to indicate that activation of NF-κB occurs via Akt-dependent and -independent pathways in these cells. In an attempt to clarify this further, we treated the various cell lines with TNF-α, a cytokine that stimulates NF-κB activation via multiple pathways (15, 22). Both HT1080 and HT1080/PTEN NF-κB activity levels were elevated by TNF-α, whereas the level of NF-κB activity in MCH603 cells was unaffected by TNF-α (Fig. 6B). However, the level of NF-κB activity in the MCH603/PI3Kact transfectants was substantially increased in the presence of TNF-α (Fig. 6B). It should be noted here that the MCH603/PI3Kact cells possess constitutively active RhoA, Rac1, and Raf pathways but not constitutively active Ras (Fig. 3 and 4). Taken together, these data suggest that NF-κB activation in MCH603 cells is Akt dependent, whereas in HT1080 and MCH603/PI3Kact cells activation is mediated by both Akt-dependent and independent pathways.
FIG. 6.
In vitro luciferase reporter assays were performed to determine NF-κB activities in HT1080, HT1080/PTEN, MCH603, and MCH603/PI3Kact cells with (B) or without (A) TNF-α (TNF) treatment. Normal HDFs were used as a control for basal level activity. The NF-κB luciferase reporter activities are presented as the average fold activation. HT, HT1080; 603, MCH603.
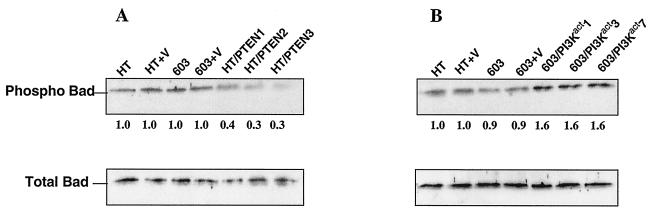
Effects on Bad.
Another mechanism whereby activated Akt may function as a survival factor is by phosphorylating the proapoptotic protein Bad, thereby inactivating it and inhibiting the Bad-mediated apoptotic pathway (13). This is, indeed, what was observed: the levels of phosphorylated Bad decreased in the HT1080/PTEN transfectants and increased in the MCH603/PI3Kact transfectants, relative to their respective parental cells (Fig. 7).
FIG. 7.
Western blot analysis performed on HT1080/PTEN transfectants (A) and 603/PI3Kact transfectants (B) to determine the level of Phospho-Bad and total Bad in these cells relative to the levels in the parental HT1080 and MCH603 cells.
Biological effects of modulating PI 3-kinase and Akt activity.
Activation of PI 3-kinase has been shown to have dramatic effects on the biological behavior of cells, including the transformation of rodent cells (24). We therefore examined a number of phenotypic traits expressed in culture that are associated with neoplastic transformation, plus tumorigenic growth in vivo.
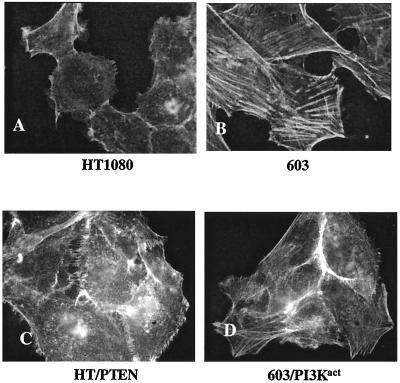
(i) Actin stress fibers.
We had earlier shown (18), as is illustrated in Fig. 8A and B, that HT1080 cells have disorganized actin, whereas MCH603 cells have restored an extensive cytoskeleton of actin stress fibers. There was no restoration of actin stress fibers in the HT1080/PTEN transfectants (Fig. 8C). Thus, it appears that phosphoinositide-mediated activation of the Akt pathway is not the determining factor with respect to regulation of actin stress fiber formation. However, as seen in Fig. 8D, increased activation of Akt in the MCH603/PI3Kact transfectants is associated with a dramatic loss of actin stress fibers. It should be noted that these cells have also constitutively activated RhoA, Rac1, and Raf/MEK/ERK/Elk-1 signaling pathways (Fig. 3 and 4) and, therefore, more closely resemble HT1080 cells in this regard.
FIG. 8.
Actin stress fiber organization in HT1080, MCH603, HT1080/PTEN, and MCH603/PI3Kact cells. The cells were stained with fluorescein-conjugated phalloidin. Magnification, ×160.
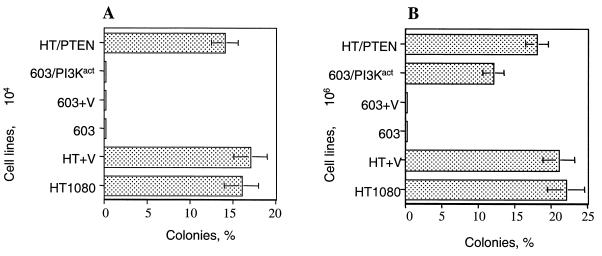
(ii) Anchorage-independent growth. Our earlier studies had shown that HT1080 cells grow well in soft agar, whereas MCH603 cells are incapable of forming colonies in this medium (18). Downregulation of constitutive Akt activity in the HT1080/PTEN transfectants had no effect on this ability to form colonies in soft agar (Fig. 9), whereas MCH603/PI3Kact transfectants had a partially restored ability to grow. Colonies were able to form when cells were plated at high density (106 cells per dish) but not when plated at low density (104 cells per dish). The HT1080 cells form colonies at both plating densities. It should again be noted that the expression of PI3Kact in the transfectants activates the RhoA, Rac1, and Raf/MEK/ERK/Elk-1 signaling pathways (Fig. 3 and 4). These same pathways remain constitutively active in the HT1080/PTEN transfectants. Thus, the partial restoration of anchorage-independent growth is not dependent on the constitutive activity of Akt per se but is associated with activation of other Ras-associated signaling pathways.
FIG. 9.
Anchorage-independent assays. Totals of 104 cells (A) or 106 cells (B) were plated per 60-mm petri dish in soft agar. Colonies (>0.1 mm) were counted after incubation for 3 weeks at 37°C, with periodic refeeding with fresh growth medium. The error bars indicate standard deviations.
(iii) Tumor formation.
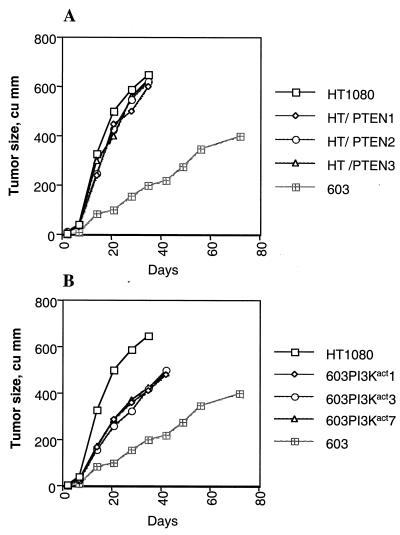
HT1080 and MCH603 cells both form tumors in immune-deficient mice. However, the kinetics of tumor formation differ dramatically. HT1080 cells form aggressively growing tumors that reach a large size within 3 weeks, whereas MCH603 cells form tumors much more slowly. We have termed these phenotypes as aggressive and weak tumorigenic phenotypes, respectively (18, 35). Stable elevated levels of expression of the tumor suppressor protein PTEN in HT1080/PTEN transfectants had no effect on the aggressive tumorigenic phenotype (Fig. 10A). Conversely, elevated levels of activated PI 3-kinase protein in the MCH603/PI3Kact transfectants resulted in a conversion from a weak to an aggressive tumorigenic phenotype, albeit not one as aggressive as that seen with HT1080 and HT1080/PTEN cells (Fig. 10B). As with the other biological phenotypes examined, the aggressive and weak tumorigenic phenotypes cannot be a direct consequence of PI 3-kinase or Akt activity. Thus, the antiapoptotic function of NF-κB and inactivation of the proapoptotic factor, Bad, do not seem to influence the aggressive and weak tumorigenic phenotypes of HT1080 and MCH603, respectively. As discussed in more detail below, the activation of MEK in the MCH603/PI3Kact transfectants is a likely candidate for orchestrating the conversion from weak to aggressive tumor-forming ability.
FIG. 10.
Tumorigenicity assays of HT1080, MCH603, HT1080/PTEN, and 603/PI3Kact cells. Each point is the average size of the tumor sizes of all sites inoculated (total of 6 for the parental cells and 18 for the transfectants, combining three independent clones).
DISCUSSION
We have developed an experimental model system that utilizes the HT1080 human fibrosarcoma cell line, possessing a mutant N-ras allele, and its derivative, MCH603, in which the mutant N-ras allele has been deleted (35). In the HT1080 cells all Ras-dependent pathways examined, namely, the Raf, Rac1, RhoA, and PI 3-kinase/Akt pathways, were constitutively active, presumably as a consequence of the permanent activated status of the mutant N-Ras protein (18). In contrast, the derivative MCH603 cells exhibit only basal levels of activity of these pathways, with the singular exception of Akt and p38 MAP kinase. We show here that this is probably due to constitutive expression of PDGF and the activation of its cognate PDGFR.
Elevated levels of expression of the lipid phosphatase PTEN protein in HT1080 cells resulted in a significant decrease in activity of Akt. This indicates that the constitutive activation of Akt is mediated via PI 3-kinase generated PIP3, rather than some other pathway, for example Ca2+-calmodulin-dependent protein kinase II (53). Although Akt activity was significantly decreased, the levels of constitutive activity of the RhoA-, Rac1-, and Raf-dependent pathways remained high. Presumably, this is due to the continued stimulation by the endogenous mutant N-Ras protein, whose constitutive activity was unaffected by PTEN.
It is interesting that elevated levels of expression of the PTEN protein did not affect the proliferation of the HT1080/PTEN transfectants, since others have reported that overexpression of PTEN induces G1 arrest and/or apoptosis (12, 16). However, most of these studies employed transient-transfection methodologies. Also, the cell lines examined were null for PTEN activity (37). Stable transfections of endogenous wild-type PTEN glioma cells with wild-type PTEN cDNA and its subsequent overexpression did not noticeably affect the proliferation of the cells in culture (16). We experienced a similar lack of effect on the growth of HT1080 cells, which are PTEN wild type (data not shown), even though the HT1080/PTEN transfectants express severalfold-higher levels of PTEN protein than the endogenous levels of wild-type PTEN expressed in HT1080. However, the increased levels of PTEN protein did correspond with a decrease in Akt activity. This suggests that the physiological level of endogenous wild-type PTEN in both HT1080 and MCH603 cells did not influence the PIP3-mediated constitutive activation of Akt and further suggests that a threshold level of PTEN protein is required for its inhibitory effect.
Elevating the level of activity of PI 3-kinase in the MCH603/PI3Kact transfectants had dramatic effects on the constitutive activities of other putative Ras-dependent pathways examined. The RhoA-, Rac1-, and Raf-dependent pathways were all activated, presumably in an activated PI 3-kinase-dependent fashion involving positive cross talk (47, 51). Interestingly, endogenous Ras was not activated. There has been some debate as to whether low or high levels of activated PI 3-kinase stimulate the activation of Ras (51). In these cells there is clearly no activation of endogenous Ras: thus, PI 3-kinase-mediated activation of these “Ras-dependent” pathways occurs downstream of Ras. It is noteworthy that activation of members of the Raf pathway, in particular MEK, exceeded the levels seen in HT1080 cells even though Ras itself was not activated.
The fact that MCH603 cells have significant levels of Akt activity, which is PI 3-kinase mediated, and yet do not exhibit activation of the RhoA-, Rac1-, and Raf-dependent pathways, suggests that a threshold level of activation is required to initiate the cross talk activation of multiple pathways. Whether this reflects an on/off switch to the activated state, as posited by Ferrell (14), will require further experimentation to determine.
PI 3-kinase-mediated activation of Akt and its subsequent upregulation of the activity of the transcription factor, NF-κB, have been shown to be important modulators of antiapoptotic cell survival (33, 42). Additionally, both Akt and PI 3-kinase, in their activated form, have been shown to have transforming activity in experimental rodent and avian cell systems (2, 11, 24).
Examination of NF-κB activity in the HT1080 and MCH603 parental and HT1080/PTEN and MCH603/PI3Kact transfectant cells revealed evidence of complex, multiple pathways of regulation. The complexity of NF-κB activation has been addressed by many investigators. Activation may be effected by oncogenic Ras through Raf-dependent and Raf-independent MAP kinase signaling pathways (15, 19, 32). The Raf-independent pathway appears to signal via Rac and p38 or a closely related kinase. Raf-dependent activation also converges with Raf-independent activation at the level of p38 activation. Furthermore, activation may be effected by PI 3-kinase, either as a consequence of activation of PI 3-kinase by oncogenic Ras or independently of Ras (27, 44).
In the case of the parental HT1080 and MCH603 cells, the basal levels of NF-κB activity were similar and significantly higher than those of normal HDFs. The fact that HT1080 and MCH603 cells have similar levels of constitutive activity of NF-κB under conditions of serum starvation is interesting, given that HT1080 has the capacity to stimulate activity via oncogenic Ras-dependent signaling, as well as PDGF-mediated PI 3-kinase and Akt signaling, whereas MCH603 cells possess only the latter mechanism. This presumably reflects an upper threshold level of activity under this physiological condition. In both HT1080 and MCH603 cells, activation of NF-κB appears to be associated with, and presumably dependent upon, IκB degradation and the release of NF-κB sequestered in the cytosol. The parental HT1080 and MCH603 cells, however, differ dramatically in their responsiveness to TNF-α stimulation of NF-κB activity. Whereas the activity in HT1080 cells is amplified manyfold, the activity in MCH603 cells is unaltered, as is the case with HDF cells. Thus, it would seem that TNF-α stimulatory effects are mediated only through oncogenic Ras or one or more downstream signaling partners, independently of PI 3-kinase-mediated Akt activation.
Support for this notion is given by the fact that elevated PTEN expression in HT1080/PTEN transfectants reduces the level of constitutive NF-κB activity below that seen in MCH603 cells but not to the level seen in HDFs. This indicates that the constitutive activation of NF-κB in HT1080 is dependent on both PI 3-kinase/Akt and oncogenic Ras signaling. Also, overexpression of activated PI 3-kinase in MCH603/PI3Kact transfectants results in levels of constitutive NF-κB activity that are somewhat higher than those seen in MCH603 or HT1080 cells. In these cells exposure to TNF-α does result in an amplification of NF-κB activity to levels approximating those seen in TNF-α-stimulated HT1080 cells. This result is consistent with the observation that the RhoA, Rac1 and Raf signaling pathways all become constitutively activated in these transfectants. It also indicates that Ras-GTP per se is not directly required for TNF-α-mediated stimulation.
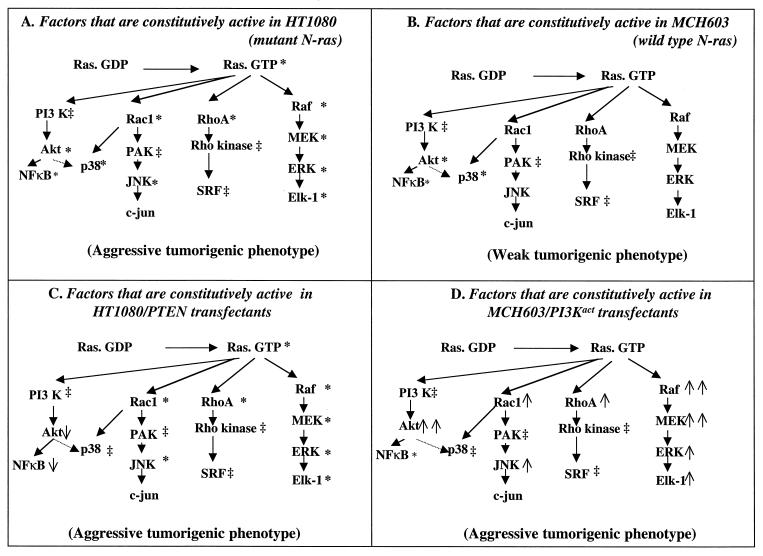
A major goal of this study was to determine whether constitutive activation of PI 3-kinase and Akt contributed to the aggressive tumorigenic phenotype of HT1080 fibrosarcoma cells. Our data, which are summarized in Fig. 11, clearly demonstrate that downregulation of this antiapoptotic survival pathway does not demonstrably affect the aggressive tumorigenic phenotype in HT1080/PTEN transfectants. The fact that the HT1080/PTEN transfectants retain the oncogenic Ras-dependent constitutive activation of the RhoA, Rac1, and Raf signaling pathways seems the most likely mechanism for retaining the aggressive tumorigenic phenotype. Consistent with this notion is the observation that overexpression of activated PI 3-kinase in the MCH603/PI3Kact transfectants results in constitutive activation of the RhoA-, Rac1-, and Raf-dependent signaling pathways, accompanied by a conversion from the weak to the aggressive tumorigenic phenotype (Fig. 11).
FIG. 11.
Summary of levels of constitutive activity of members of the PI 3-kinase, RhoA, Rac1, and Raf signaling pathways and of the tumorigenic phenotypes in parental HT1080 and MCH603 cells and their respective transfectants, HT1080/PTEN and MCH603/PI3Kact. ∗, Proteins constitutively active in HT1080 or MCH603; ↓ or ↑, decrease or increase, respectively, in the constitutive activity of individual factors tested in HT1080/PTEN and MCH603/PI3Kact relative to their respective parental cells; ↑↑, activity higher than that seen in HT1080 cells; ‡, not tested.
In earlier studies we have shown that, in the absence of mutant N-Ras in the MCH603 cells, overexpression of activated MEK results in the conversion to an aggressive tumorigenic phenotype (18). This overexpression, coupled with a lack of effect when activated Raf or Rac1 were expressed, led us to speculate that the overexpression of activated MEK in these cells stimulated the activation of a possibly novel pathway that is critical for the conversion to an aggressive tumorigenic phenotype. Consistent with this notion is the observation in this study that the levels of endogenous activated MEK in MCH603/PI3Kact transfectants are higher than that seen in HT1080 cells. Thus, the same putative novel pathway may be activated in these cells. Further experimentation is required to test this hypothesis.
The generality of the phenomena described here with respect to other human cancers and cell lines must await further examination. If a novel pathway is confirmed and found to be general for human cancers that express mutant Ras proteins, this may provide an important target for cancer therapy.
ACKNOWLEDGMENTS
We thank Julian Downward and Craig Hauser for the gifts of plasmids and Albert Baldwin, Jr., for critical reading of the manuscript.
These studies were supported by NCI grants CA69515 (E.J.S.) and CA85772 (Y.E.W.) and DOD grant DAMD17-00-1-0037.
REFERENCES
- 1.Alessi D, Deak M, Casamayor A, Caudwell F, Morrice N, Norman D, Gaffney P. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 2.Aoki M, Osvaldo B, Bellacosa A, Tsichlis P, Vogt P K. The Akt kinase: molecular determinants of oncogenicity. Proc Natl Acad Sci USA. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar-Sagi D, Hall A. Ras and Rho GTPases: a family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 4.Beg A A, Baltimore D. An essential role of NF-kappaB in preventing TNF-alpha induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 5.Boettner B, Van Aelst L. Rac and Cdc42 effectors. Prog Mol Subcell Biol. 1999;22:135–158. doi: 10.1007/978-3-642-58591-3_7. [DOI] [PubMed] [Google Scholar]
- 6.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 7.Burnett W N. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 8.Cantley L C, Neel B G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E J, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter C L, Cantley L C. Phosphoinositide kinases. Curr Opin Cell Biol. 1996;8:153–158. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]
- 11.Chang H W, Aoki M, Fruman D, Auger K R, Bellacosa A, Tsichlis P N, Cantley L C, Roberts T M, Vogt P K. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 12.Davies M A, Koul D, Dhesi H, Berman R, McDonnell T J, McConkey D, Yung W K, Steck P A. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res. 1999;59:2551–2556. [PubMed] [Google Scholar]
- 13.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 14.Ferrell J E., Jr How responses get more switch-like as you move down a protein kinase cascade. Trends Biochem Sci. 1997;22:288–289. doi: 10.1016/s0968-0004(97)82217-7. [DOI] [PubMed] [Google Scholar]
- 15.Frost J A, Swantek J L, Stippec S, Yin M J, Gaynor R, Cobb M H. Stimulation of NFkappa B activity by multiple signaling pathways requires PAK1. J Biol Chem. 2000;275:19693–19699. doi: 10.1074/jbc.M909860199. [DOI] [PubMed] [Google Scholar]
- 16.Furnari F B, Lin H, Huang H J S, Cavenee W K. Growth suppression of glioma cells by PTEN requires a functional phosphatase catalytic domain. Proc Natl Acad Sci USA. 1997;94:12479–12484. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham A, McCleese A, Malarkey K, Gould G W, Plevin R. Role of receptor desensitization, phosphatase induction and intracellular cyclic AMP in the termination of mitogen-activated protein kinase activity in UTP-stimulated Eahy 9266 endothelial cells. Biochem J. 1996;315:563–569. doi: 10.1042/bj3150563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Plattner R, Der C J, Stanbridge E J. Dissection of ras-dependent signaling pathways controlling aggressive tumor growth of human fibrosarcoma cells: evidence for a potential novel pathway. Mol Cell Biol. 2000;20:9294–9306. doi: 10.1128/mcb.20.24.9294-9306.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jefferies C A, O'Neill L A. Rac1 regulates interleukin 1-induced nuclear factor kappaB activation in an inhibitory protein kappaBalpha-independent manner by enhancing the ability of the p65 subunit to transactivate gene expression. J Biol Chem. 2000;275:3114–3120. doi: 10.1074/jbc.275.5.3114. [DOI] [PubMed] [Google Scholar]
- 20.Jones S M, Klinghoffer R, Prestwich G D, Toker A, Kazlauskas A. PDGF induces an early and a late wave of PI 3-kinase activity, and only the late wave is required for progression through G1. Curr Biol. 1999;9:512–521. doi: 10.1016/s0960-9822(99)80235-8. [DOI] [PubMed] [Google Scholar]
- 21.Joneson T, Bar-Sagi D. Ras effectors and their role in mitogenesis and oncogenesis. J Mol Med. 1997;75:587–593. doi: 10.1007/s001090050143. [DOI] [PubMed] [Google Scholar]
- 22.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 23.Khosravi-Far R, Solski P A, Clark G J, Kinch M S, Der C J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klippel A, Escobedo M A, Wachowicz M S, Apell G, Brown T W, Giedlin M A, Kavanaugh W M, Williams L T. Activation of phosphatidylinositol 3-kinase is sufficient for cell cycle entry and promotes cellular changes characteristic of oncogenic transformation. Mol Cell Biol. 1998;18:5699–5711. doi: 10.1128/mcb.18.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klippel A, Kavanaugh W, Pot D, Williams L T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–345. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner S H, Giovanella B C, Ittmann M, Tycko B, Hibshoosh H, Wigler M H, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 27.Madrid L V, Wang C Y, Guttridge D C, Schottelius A J G, Baldwin A S, Jr, Mayo M W. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-κB. Mol Cell Biol. 2000;20:1626–1638. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall C J, Hall A, Weiss R A. A transforming gene present in human sarcoma cell lines. Nature. 1982;299:171–173. doi: 10.1038/299171a0. [DOI] [PubMed] [Google Scholar]
- 29.McCormick F. Activators and effectors of ras p21 proteins. Curr Opin Genet Dev. 1994;4:71–76. doi: 10.1016/0959-437x(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 30.Myers M P, Pass I, Batty I H, Van der Kaay J, Stolarov J P, Hemmings B A, Wigler M H, Downes C P, Tonks N K. The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc Natl Acad Sci USA. 1998;95:13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelen M R, van Staveren W C, Peeters E A, Hassel M B, Gorlin R J, Hamm H, Lindboe C F, Fryns J P, Sijmons R H, Woods D G, Mariman E C, Padberg G W, Kremer H. Germline mutations in the PTEN/MMAC1 gene in patients with Cowden disease. Hum Mol Genet. 1997;6:1383–1387. doi: 10.1093/hmg/6.8.1383. [DOI] [PubMed] [Google Scholar]
- 32.Norris J L, Baldwin A S., Jr Oncogenic Ras enhances NF-κB transcriptional activity through Raf-dependent and Raf-independent mitogen-activated protein kinase signaling pathways. J Biol Chem. 1999;274:13841–13846. doi: 10.1074/jbc.274.20.13841. [DOI] [PubMed] [Google Scholar]
- 33.Ozes O N, Mayo L D, Gustin J A, Pfeffer S R, Pfeffer L M, Donner D B. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 34.Palmer H J, Maher V M, McCormick J. Effect of retinoids on growth factor-induced anchorage independent growth of human fibroblasts. J In Vitro Cell Dev Biol. 1989;25:1009–1015. doi: 10.1007/BF02624134. [DOI] [PubMed] [Google Scholar]
- 35.Plattner R, Anderson M J, Sato K Y, Fasching C L, Der C J, Stanbridge E J. Loss of oncogenic ras expression does not correlate with loss of tumorigenicity in human cells. Proc Natl Acad Sci USA. 1996;93:6665–6670. doi: 10.1073/pnas.93.13.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plattner R, Gupta S, Khosravi-Far R, Sato K Y, Perucho M, Der C J, Stanbridge E J. Contribution of the ERK and JNK mitogen-activated protein kinase cascades to Ras transformation of HT1080 fibrosarcoma and DLD-1 colon carcinoma cells. Oncogene. 1999;18:1807–1817. doi: 10.1038/sj.onc.1202482. [DOI] [PubMed] [Google Scholar]
- 37.Ramaswamy S, Nakamura N, Vazquez F, Batt D B, Perera S, Roberts T M, Sellers W R. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1999;96:2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridley A J, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–99. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 39.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez M S, Wright J, Thompson J, Thomas D, Baleux F, Virelizier J L, Hay R T, Arenzana-Seisdedos F. Identification of lysine residues required for signal-induced ubiquitination and degradation of I kappa B-alpha in vivo. Oncogene. 1996;12:2425–2435. [PubMed] [Google Scholar]
- 41.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 42.Romashkova J A, Makarov S S. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 43.Sander E E, Van Delft S, Ten Klooster J P, Reid T, Van der Kammen R A, Michiels F, Collard J G. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol. 1998;143:1385–1398. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sizemore N, Leung S, Stark G R. Activationof phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-κB p65/RelA subunit. Mol Cell Biol. 2000;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steck P A, Pershouse M A, Jasser S A, Yung W K, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng D H, Tavtigian S V. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 46.Susa M, Keeler M, Varticovski L. Platelet-derived growth factor activates membrane-associated phosphatidylinositol 3-kinase and mediates its translocation from the cytosol. Detection of enzyme activity in detergent-solubilized cell extracts. J Biol Chem. 1992;267:22951–22956. [PubMed] [Google Scholar]
- 47.Tang Y, Yu J, Field J. Signals from the Ras, Rac, and Rho GTPases converge on the Pak protein kinase in Rat-1 fibroblasts. Mol Cell Biol. 1999;19:1881–1891. doi: 10.1128/mcb.19.3.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Traenckner E B, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-alpha-induced apoptosis by NF-kappa-B. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 50.Wang C Y, Mayo M W, Baldwin A S., Jr TNF-alpha and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 51.Wennstrom S, Downward J. Role of phosphoinositide 3-kinase in activation of ras and mitogen-activated protein kinase by epidermal growth factor. Mol Cell Biol. 1999;6:4279–4288. doi: 10.1128/mcb.19.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu X, Senechal K, Neshat M S, Whang Y E, Sawyers C L. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1998;95:15587–15591. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yano S, Tokumitsu H, Soderling T R. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;39:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 54.Ye X, Mehlen P, Rabizadeh S, VanArsdale T, Zhang H, Shin H, Wang J J, Leo E, Zapata J, Hauser C A, Reed J C, Bredesen D E. TRAF family proteins interact with the common neurotrophin receptor and modulate apoptosis induction. J Biol Chem. 1999;274:30202–30208. doi: 10.1074/jbc.274.42.30202. [DOI] [PubMed] [Google Scholar]
- 55.Zhang P, Steinberg B M. Overexpression of PTEN/MMAC1 and decreased activation of Akt in human papillomavirus-infected laryngeal papillomas. Cancer Res. 2000;60:1457–1462. [PubMed] [Google Scholar]