Abstract
In the plant meristem, tissue-wide maturation gradients are coordinated with specialized cell networks to establish various developmental phases required for indeterminate growth. Here, we used single-cell transcriptomics to reconstruct the protophloem developmental trajectory from birth of cell progenitors to terminal differentiation in the Arabidopsis root. PHLOEM EARLY DNA-BINDING-WITH-ONE-FINGER (PEAR) transcription factors mediate lineage bifurcation by activating GTPase signaling and prime a transcriptional differentiation program. This program is initially repressed by a meristem-wide gradient of PLETHORA transcription factors. Only the dissipation of PLETHORA gradient permits activation of the differentiation program that involves mutual inhibition of early vs. late meristem regulators. Thus, for phloem development, broad maturation gradients interface with cell-type specific transcriptional regulators to stage cellular differentiation.
One-Sentence Summary:
Single-cell analysis shows how global signals in the root meristem interact with the cell type specific factors to determine distinct phases of phloem development.
Roots consist of several concentric layers of functionally distinct cell files, which initially bifurcate and establish distinct identities around the quiescent center and its surrounding stem cells. Cells within each file mature through the distinct zones of cell proliferation and differentiation (1). For example, in Arabidopsis, the development of the protophloem sieve elements involves a transient period of cell proliferation, during which, in addition to amplification of cells within the file, two lineage-bifurcating events take place (Fig. 1A) (2). Soon after the cell proliferation ceases, cells of the protophloem sieve element lineage initiate a differentiation process which culminates in enucleation, an irreversible process that gives rise to the mature conductive cells (3). Because of specific modulation of the graded distribution of the key phytohormonal cue auxin, the differentiation of protophloem sieve elements occurs faster than that of the other cell files (4). Therefore, protophloem sieve element development offers a tractable scheme to understand how the two processes of cell specialization and maturation interact.
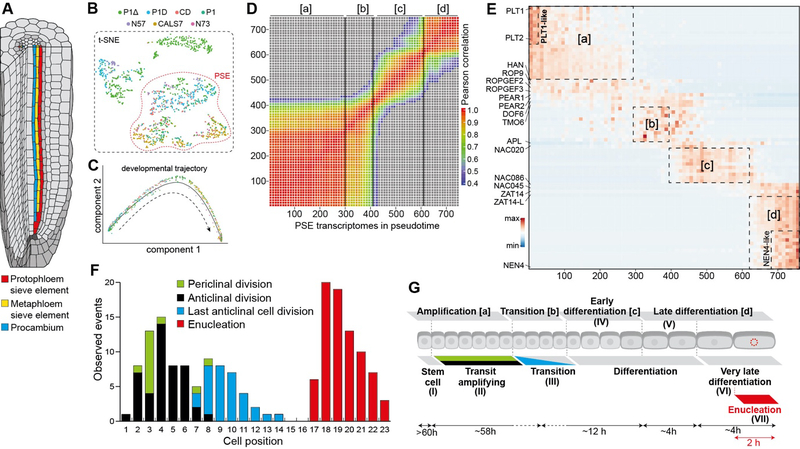
Figure 1. Phloem development at single-cell resolution.
(A) Schematic of the Arabidopsis root tip depicting position of protophloem sieve element, metaphloem sieve element and procambium cell lineages originating from a single phloem stem cell. (B) t-SNE plot of 1242 transcriptomes of cells sorted with P1Δ, P1D, CD, P1, N57, CALS7 and N73 reporter lines specific to different domains of the developing phloem. Indicated protophloem sieve element cells were used for the pseudotime trajectory analysis (Fig. S2, Supplementary Material). (C) protophloem sieve element transcriptomes ordered along developmental trajectory using Monocle 2. (D) Heatmap of Pearson correlation along the pseudotime trajectory. Vertical lines indicate 3 strongest correlation drops and separate four groups of transcriptomes with higher similarity [a], [b], [c] and [d]. (E) Gene expression heatmap of protophloem sieve element regulators and 10 most specific genes from the 4 groups defined in D) and the nested PLT1 (“PLT1-like”) or NEN4 (“NEN4-like”) expression domains in pseudotime-ordered protophloem sieve element transcriptomes. (F) Histogram of cell behavior based on long-term live imaging. (G) Seven domains and the time cells spend in each position of the developing protophloem sieve element as determined by the transcriptomics (above) and live imaging (below): (I) “stem cell”, position 1 [a], t>60h; (II) “transit amplifying”, position 2–9 [a], t=58h, SD+8.1h, (III) “transitioning”, position 8–11 [b]; (IV) “early differentiating”, position 10–15 [c], t=12h; (V) “late differentiating”, position 16–17 [d], t=4h; (VI) “very late differentiating - NEN4-like”, position 18–19 [d], t=4h; VII “enucleating”, position 19 [d], t= 2h (Movie S1, S2).
Phloem developmental trajectory at single-cell resolution.
In order to understand the process of protophloem sieve element development at a high resolution, we took a combination of approaches based on time-lapse confocal imaging (5) and single cell transcriptomics (6). Using phloem-specific marker (pPEAR1::H2B-YFP pCALS7::H2B-YFP) we precisely mapped cellular behavior of the on average of 19 cells that constitute the protophloem sieve element developmental trajectory until enucleation, which takes place every 2 hours in the final cell position. The passage of the cell from its “birth” at the stem cell until its enucleation took a minimum of 79 hours (Fig. S1, movies S1, S2). To dissect the genetic control underlying this temporal progression, we opted for deep profiling of the 19 cells that represent the developmental trajectory of protophloem sieve element, using cell sorting and well-based single cell sequencing over higher throughput but shallower droplet-based profiling (6–12). We used fluorescent reporter lines whose expression represent various spatio-temporal domains within the developmental trajectory of protophloem sieve element (Fig. S2A, B). The single-cell profiles allowed us to cluster cells together with known protophloem sieve element markers to identify 758 cells that densely sampled the 19 cell positions and captured the span of protophloem sieve element maturation (Fig. 1B, Fig. S2C–G).
We sought to use the high-resolution profile of the protophloem sieve element lineage to ask how cell passage through stable signaling gradients in the meristem controls the stages of cellular specialization. In particular, while a number of regulators of either phloem cell identity or meristem zonation have been described (13, 14), little is known about how these two regulatory processes interact to control organogenesis. Using Monocle 2 (15, 16), we projected the 758 protophloem sieve element lineage cells into a pseudo-temporal order and investigated transcriptional transitions along the developmental trajectory (Fig. 1B–D). Rather than gradual changes, we observed four transcriptomic domains separated by three narrow transition zones (Fig. 1D, E; Table S1). Based on the alignment with the temporal expression patterns of selected genes, we were able to determine that these domains correspond approximately to cells at positions 1–7 [a], 8–11 [b], 12–15 [c] and 16–19 [d], respectively (Fig. S3). To further understand which aspects of protophloem sieve element maturation these various positions represent, we extended time-lapse confocal imaging with more temporally specific marker lines pNAC86::H2B-YFP and pNEN4::H2B-YFP, active at later developmental stages (3). We found that the differentiation time, measured from the last cell division to enucleation takes around 20 hours with some variation up to the final stage defined by expression of NAC45/86-DEPENDENT EXONUCLEASE-DOMAIN PROTEIN 4 (NEN4) (active in positions 18–19), (Fig. 1E, Fig. S1D, H, I, Movies S1–S12). In summary, based on the high congruence of the single-cell transcriptome and live imaging data, we were able to assign seven distinct developmental phases along the protophloem sieve element trajectory: (I) “stem cell”, position 1; (II) “transit amplifying”, position 2–9; (III) “transitioning”, position 8–11; (IV) “early differentiating”, position 10–15; (V) “late differentiating”, position 16–17; (VI) “very late differentiating”, position 18–19; (VII) “enucleating”, position 19 (Fig. 1F, G, Fig. S1, Table S2).
PEARs promote lineage bifurcation via GTPase signaling.
Proximal to the stem cell (I) developmental phase, the first distinctive feature of the protophloem sieve element lineage is the bifurcation of the procambial and metaphloem cell files from the progenitor protophloem sieve element lineage through a pair of subsequent periclinal (asymmetric) cell divisions in the domain of transit amplifying cells (II). Using the single-cell lineage and imaging analysis, we sought to precisely map these divisions (Fig. 2A). We observed that the first periclinal division followed exclusively a rare event of phloem stem cell division (Movie S13, Fig. S4A). The second, more frequent, periclinal division was observed predominantly at position 3 (Fig. 1F). We have recently shown that the PEAR transcription factors (transcribed in domains I-IV) mediate early asymmetric divisions in the phloem lineage and laterally adjacent procambial cells in a cell autonomous and cell non-autonomous manner, respectively (17). In order to identify potential downstream effector genes for this PEAR function, we focused on the genes enriched in the expression domain of pPEAR1Δ::erVenus marker line (Methods) capturing the bifurcation events and the resulting protophloem, metaphloem and procambium cell lineages (Fig. 2B, Fig. S4B).
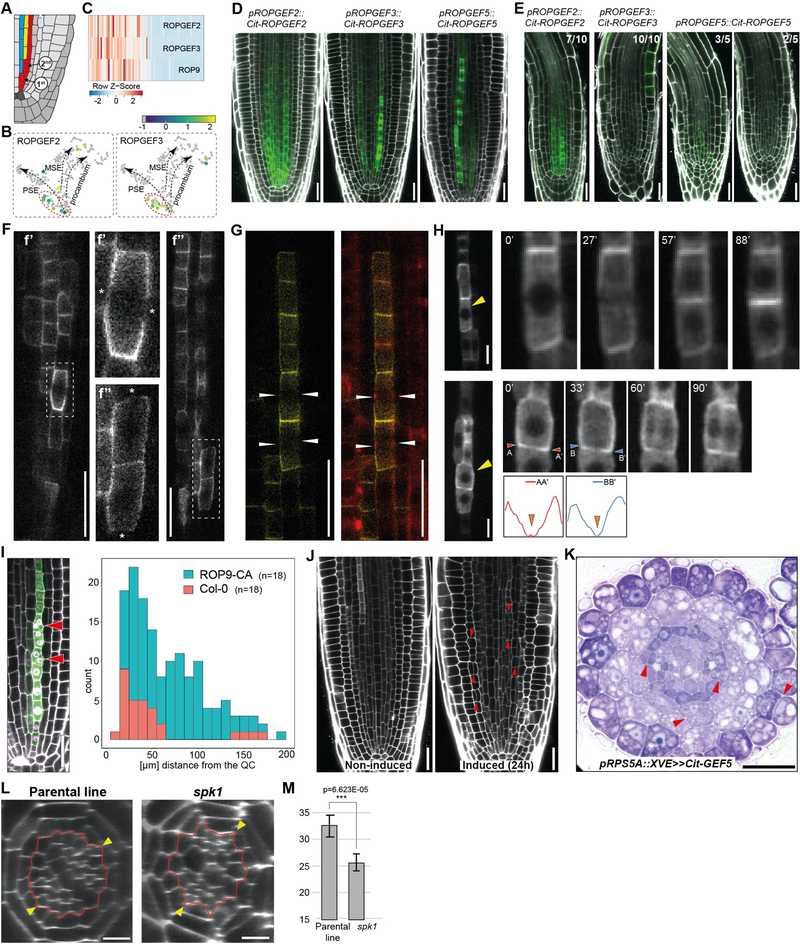
Figure 2. PEARs control asymmetric divisions by promoting ROP signaling in the phloem pole.
(A) Schematic indicating position of the two periclinal divisions in the phloem cell lineage. (B) Expression of ROPGEF2 and ROPGEF3 at the time of phloem lineage bifurcation. (C) Peak expression of ROPGEF2, 3 and ROP9 in the early phloem cells as detected in the pseudotime-ordered single cell protophloem sieve element transcriptome data. (D) Expression pattern of phloem enriched ROPGEFs. ROPGEF3 and 5 share similar expression domain – enriched in protophloem sieve element and adjacent vascular cell files; ROPGEF2 is expressed in protophloem sieve element but also in other outer procambial cells and pericycle (Fig. S4D). Scale bars: 25 μm. (E) Expression of ROPGEF2, 3 and 5 in the pear sextuple mutant background. Scale bars: 25 μm. (F) Protein localization of pROPGEF5::Cit-ROPGEF5 during anticlinal (f’) and periclinal (f”) cell division. Gaps in ROPGEF5 signal are indicated with an asterisk. Scale bars: 25 μm. (G) Depletion of Cit-ROPGEF5 membrane signal at the cortical division zone (CDZ) during cell division. CDZ is marked by accumulating cortical microtubules (mCherry-TUA5) forming pre-prophase band (white arrowheads). Scale bars: 25 μm. (H) Time course analysis of the dynamic pattern of active ROP signaling in the dividing phloem cells. Depletion of pPEAR1::mScarlet-I-MIDD1ΔN signal at the CDZ in the anticlinally (upper row) and periclinally (lower row) dividing cells (yellow arrowheads). Quantification of fluorescent signal intensity in the periclinally dividing cells. Scale bars: 10 μm. (I) Quantification of asymmetric cell divisions (red arrowheads) in the protophloem sieve element cell lineage after expression of constitutively active ROP9 (Q64L) (pPEAR1::XVE>>ROP9CA). Scale bars: 25 μm. (J) Ectopic asymmetric cell divisions (red arrowheads) 24h after induction of ectopic Cit-GEF5 expression (pRPS5A::XVE>>Cit-GEF5). Scale bars: 25 μm. (K) Toluidine blue stanning of resin sections of Cit-GEF5 overexpressing line (pRPS5A::XVE>>Cit-GEF5) 24h after induction. Red arrowheads indicate ectopic periclinal cell divisions in epidermis, endodermis and pericycle. Scale bars: 25 μm. (L) Identification of spk1 allele in the mutant screen of pRPS5A::PEAR1-GR parental line. Presented are images from non-induced plants. Scale bars: 10 μm. (M) Quantification of vascular cell files in the spk1 mutant and its parental line pRPS5A::PEAR1-GR. Both lines were not induced.
Among the sieve element enriched genes that were highly expressed in single cell profiles preceding and during the bifurcation (domain II), we identified and validated the protophloem sieve element abundant expression of Rho-related GTPase, Rho of plants 9 (ROP9) (18) as well as several genes encoding PRONE-type ROP guanine nucleotide exchange factors (ROPGEF) (Fig. 2B, C, D, Fig. S4B, C, F) (19). ROP GTPase signaling controls polarity of the multiple cell types during cell differentiation (20–22) and specific cell division events (23–25). Subsequently, we determined that ROPGEF3 and ROPGEF5 expression in the protophloem sieve element lineage is dependent upon PEAR factors, based on the spatio-temporal correlation as well as the analysis of transcriptional reporters in the pear sextuple mutant background (Fig. 2E). In addition, functional analysis of the PEAR binding sites previously indicated by the DAPseq technique (26) in the promoter region of ROPGEF genes affected their expression level (Fig. S4D) (17), suggesting a direct interaction.
In the dividing cells, ROPGEFs accumulate broadly at the cell membrane but were depleted from the expected position of cortical division zone, which demarcates the future division plane (Fig. 2F) (25). Indeed, observed gaps in ROPGEF localization coincided with the position of microtubule array called the preprophase band, the earliest marker of cell division plane in plants (Fig. 2G, Fig. S4E) (25). ROPGEFs catalyze disassociation of GDP from inactive ROP-GDP complex that enables quick binding of free cytosolic GTP and thus activates ROP signaling. In the active state, ROP-GTP interacts with a number of different effector proteins to mediate downstream signaling (27). In order to detect cellular position of the active ROP signaling in relation to the periclinal and anticlinal cell division planes in phloem, we utilized molecular biosensor of ROP signaling that consist of fluorescently tagged, ROP-GTP binding domain from MICROTUBULE DEPLETION DOMAIN1 (MIDD1ΔN) effector protein (28). Similarly to the localization of ROPGEFs, subcellular localization of active ROP signaling was detected on the cell membrane and was absent in the cortical division zone of protophloem sieve element cells during mitosis (Fig. 2H).
In order to test whether ROP signaling plays a decisive role in the selection of cell division plane, we generated an inducible line expressing the constitutively active form of ROP9 (ROP9CA) (Methods) and lines ectopically expressing phloem enriched ROPGEFs. Accumulation of ROP9CA-3xYFP on the radial walls of the protophloem sieve element lineage correlated with cell expansion to the radial direction and reorientation of the cell division plane (Fig. 2I, Fig. S4F). Ectopic expression of ROPGEFs resulted in ectopic periclinal cell divisions in the outer root layers and pericycle, which rarely undergo such division (Fig. 2J, K, Fig. S4G, H). Members of PRONE-type ROPGEF gene family in Arabidopsis have been previously proposed to act redundantly in number of processes in which they activate ROP signaling (29). On the other hand, loss of SPIKE1 (SPK1), encoding a single copy ROP interacting DOCK family GEF causes phenotypes mimicking the combinatorial rop mutants (30–32). Therefore, we focused on the loss-of-function alleles of SPK1, one of which we identified in the genetic screen for factors promoting formative (periclinal) cell divisions (Supplementary Materials). In the spk1 loss-of-function mutant, we detected a significant reduction in periclinal divisions in several tissues, including protophloem sieve element cell lineage (Fig. 2L, M, Fig. S4I, J, K). We conclude that, in the transit amplifying cells (domain II, position 2–9), PEAR function promotes the bifurcation involving the emergence of the protophloem sieve element cell lineage by switching the orientation of the cell divisions at least partially through the activation of ROPGEF-ROP signaling module.
PLETHORAs stage APL expression and phloem differentiation.
Another distinct feature of the early protophloem sieve element developmental trajectory is the transition from cell division to cell differentiation (II-III-IV). This transition mapped closely to the first major change in the protophloem sieve element transcriptome. In the first transcriptomic domain (I-II), we detected transcripts of the PLETHORA gene family (Fig. 1E), whose relatively persistent proteins are known to spread shootward through cell-to-cell movement. This movement, together with a mitotic dilution effect, contributes to the formation of the shootward protein gradient. (14). Prior work has shown that PLETHORA transcription factors broadly regulate meristem development, promoting cell division at moderate concentrations, and then permitting elongation and differentiation as levels drop (14, 33, 34). However, it is not clear how individual cell files interpret the meristem-wide PLETHORA gradient for their own specialized differentiation.
We hypothesized that the PLETHORA gradient might mediate the first transcriptional shift (i.e. domain II to III) towards protophloem sieve element differentiation by permitting a new set of transcripts to be expressed (Fig. 3A). We tested this hypothesis by driving PLETHORA2 (PLT2) under several promoters that extended its expression in the protophloem sieve element in later maturation stages than its native domain (Fig. 3B, Fig. S5A). When using the pNAC86::XVE inducible promoter, active in domains V-VII (3, 35), ectopic PLT2 delayed protophloem sieve element enucleation (Fig. 3B, Fig. S5A). Transcriptional profiling of phloem cells expressing the construct showed an upregulation of genes (Table S3) that mapped to early stages of the protophloem sieve element single-cell trajectory (from domains I-II) - the known PLT2 protein gradient (Fig. 3C). These results suggest that extending the PLT2 gradient is sufficient to prolong the early stages of meristem maturation within the protophloem sieve element lineage, providing a connection between the maturation of a specific cell file and a meristem-wide protein gradient. In addition, in the pseudo-time ordered single cells, we could detect complementary oscillatory patterns of the putative S-phase and G2-M-phase genes that were upregulated PLETHORA targets, apparently corresponding to regular progressions through the cell-cycle (Fig. 3C, Fig. S5B). Furthermore, ALTERED PHLOEM DEVELOPMENT (APL), NAC45/86 and NEN4, known key regulators of the protophloem sieve element enucleation pathway (3), were among the PLT2-downregulated genes (Fig S5C, Table S3). This is consistent with the presence of APL in the large set of genes downregulated by PLETHORA overexpression (33). We validated the downregulation of APL and NEN4 by ectopic PLT2 expression with in situ hybridization (Fig. 3D, Fig. S5D). We also monitored a shootward shift of APL expression domain in the roots after conditional ectopic induction of PLT2 expression. The induction of PLT2 in the phloem cells beyond its native domain confirmed that activation of APL-dependent genetic program requires dissipation of the PLETHORA gradient (Fig. 3E). In order to test the role of PLETHORAs in controlling the transition between transit amplification and differentiation in phloem, we used an inducible, tissue specific CRISPR/Cas9 approach to mutate PLT2 specifically in protophloem sieve element cell file (36). We observed an acceleration of the protophloem sieve element differentiation as well as the expression of pAPL::erTurq reporter towards the QC without affecting the broader meristem size or root growth, showing that loss of PLETHORA function in its native domain allows precocious expression of mid- to late-stage protophloem sieve element differentiation regulators (Fig. 3F, Fig. S5F–H).
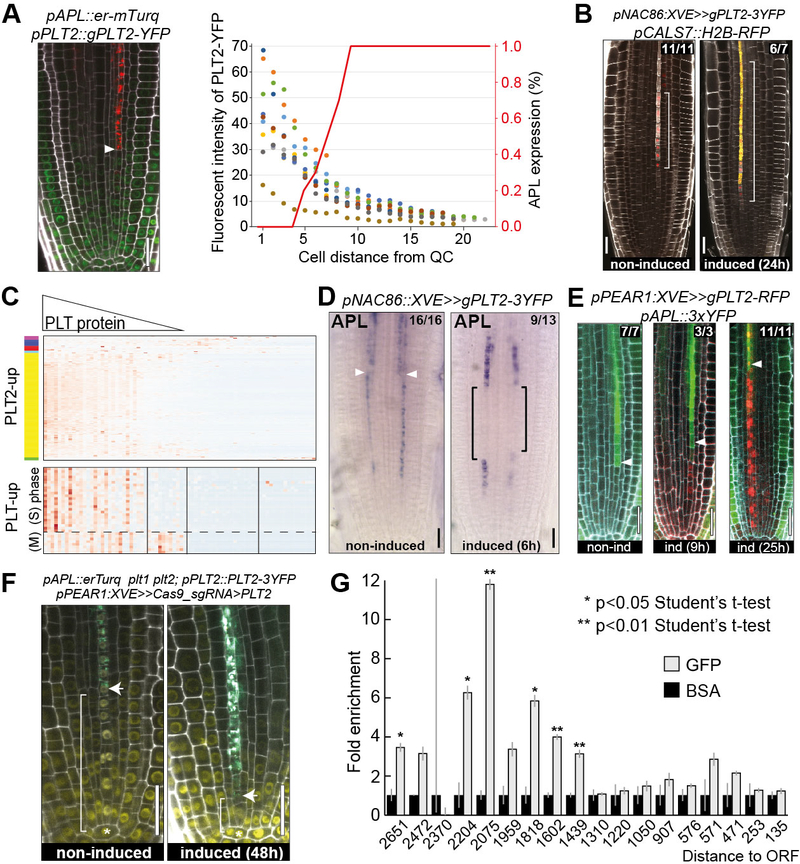
Figure 3. PLT2 inhibits phloem differentiation by directly repressing APL expression.
(A) Quantification of fluorescent intensity of PLT2-YFP in protophloem sieve element cells of 9 roots indicated with dots of different colours. Percentage of roots expressing APL in a given protophloem sieve element cell is indicated as a red line (n=9). Onset of APL expression coincides with diminishing level of PLT2 protein. Arrowhead indicates onset of APL expression in protophloem sieve element. (B) Ectopic expression of PLT2 under pNAC86::XVE promoter delays protophloem sieve element enucleation. Square brackets indicate extended expression domain of pCALS7::H2B-RFP, a reporter used for monitoring enucleation. (C) Native expression profile of PLT2 targets in protophloem sieve element cells ordered in pseudotime. Genes upregulated after 6 hours of induction of the line shown in B) are plotted. Upper panel shows gradually diminishing expression of target genes which reflects the PLT2 protein gradient. Lower panel shows PLT2 upregulated cell cycle genes with oscillatory expression pattern. (D) In situ hybridization of APL before and 6h after ectopic expression of PLT2–3xYFP. Arrowheads indicate position of protophloem sieve element enucleation beyond which point APL is expressed in phloem pole pericycle, companion cells and metaphloem sieve element (Fig. S5E). Brackets indicate pNAC086 activity domain. (E) Time course of transcriptional repression of APL in cells ectopically expressing PLT2-RFP under inducible pPEAR1::XVE promoter. (F) Early activation of APL expression 48h after phloem specific knock-out of PLT2. (G) ChIP-qPCR of PLT2–3xYFP on APL promoter revealed PLETHORA binding region −2204 to −1439 bp upstream of APL ORF. All scale bars, 25 μm.
We sought to further test whether PLT2 directly regulates the protophloem sieve element-specific differentiation program, as we found AP2 (a member of the PLETHORA family) family binding sites in the APL promoter region, as defined by the DAPseq technique (26). Indeed, we confirmed the direct binding of PLT2 to several regions of the APL promoter by ChIP-qPCR (Fig. 3G). Furthermore, along with AP2 sites, the APL promoter is also enriched for binding sites of HANABA TANARU (HAN), a GATA transcription factor. In turn, HAN is a PLETHORA target (33) and accordingly, upon ectopic PLT2 expression we detect HAN transcripts expressed in late protophloem sieve element development (Fig. S5C, I). Ectopic HAN expression under pNAC86:XVE led to a delay in enucleation (Fig. S5J), similar to PLT2 overexpression in the same domain. We conclude that the PLETHORA gradient directly (and possibly in a feedforward manner with HAN) orchestrates protophloem sieve element differentiation by cell autonomously repressing transcription of the phloem regulator APL. Overall, the results show how the PLETHORA gradient first promotes cell proliferation in the protophloem sieve element lineage and then helps to time the later stages of cellular maturation.
PEARs promote APL to orchestrate phloem differentiation.
Given the results above, we reasoned that an early phloem-specific transcription factor must activate APL expression. In order to identify genes that could fill that role, we first generated a list of sieve element genes enriched in our bulk-sorted cells from that tissue compared to published data profiling other tissue types of the root meristem (37, Fig. S6A, Table S4). We further narrowed the list by intersecting it with sieve element enriched genes identified in the cluster analysis of single-cell RNAseq profiles of the pPEAR1Δ::erVenus reporter line (Table S5; Fig. 4A, B, Fig. S6B–H). From this analysis, we identified 542 sieve element enriched genes (Table S6) and corroborated their specificity in the published whole-root scRNAseq atlas (Table S7) (12). We modeled gene regulation using a machine learning approach on the pseudotime-ordered 758 single-cell profiles and 4924 highly variable genes. Among 208 TFs in this dataset, the majority of known protophloem sieve element transcription factors (such as APL, NAC045 and NAC086) were among the top 20 regulators (Table S8). We validated the model by comparing predicted targets with genes induced by in vivo ectopic expression of the same TFs, confirming a significant overlap of targets in 3 out of 5 cases (Table S8). Among the top 20 regulators we also identified four related genes that encode early sieve element abundant PEAR transcription factors (PEAR1, PEAR2, DNA BINDING WITH ONE FINGER6, TARGET OF MONOPTEROS6) (Fig. 4C). We recently showed that simultaneous loss of six PEAR genes results in defects in protophloem sieve element differentiation (17). We subsequently profiled the transcriptomes of wildtype and pear sextuple mutant (Fig. 4D) root meristems and identified 203 downregulated genes overlapping with our protophloem sieve element specific gene list (Table S9). The expression of APL as well as its downstream targets – NAC045, NAC086 and NEN4 was lost in protophloem tissue of pear sextuple mutant (Fig. 4E, F Fig. S7A). Subsequently, expression of APL and NAC086 reporter lines was restored in the pear sextuple mutant upon induction of PEAR1, corroborating that transcriptional activation of APL in the protophloem sieve element is dependent on activity of PEAR factors (Fig. 3F).
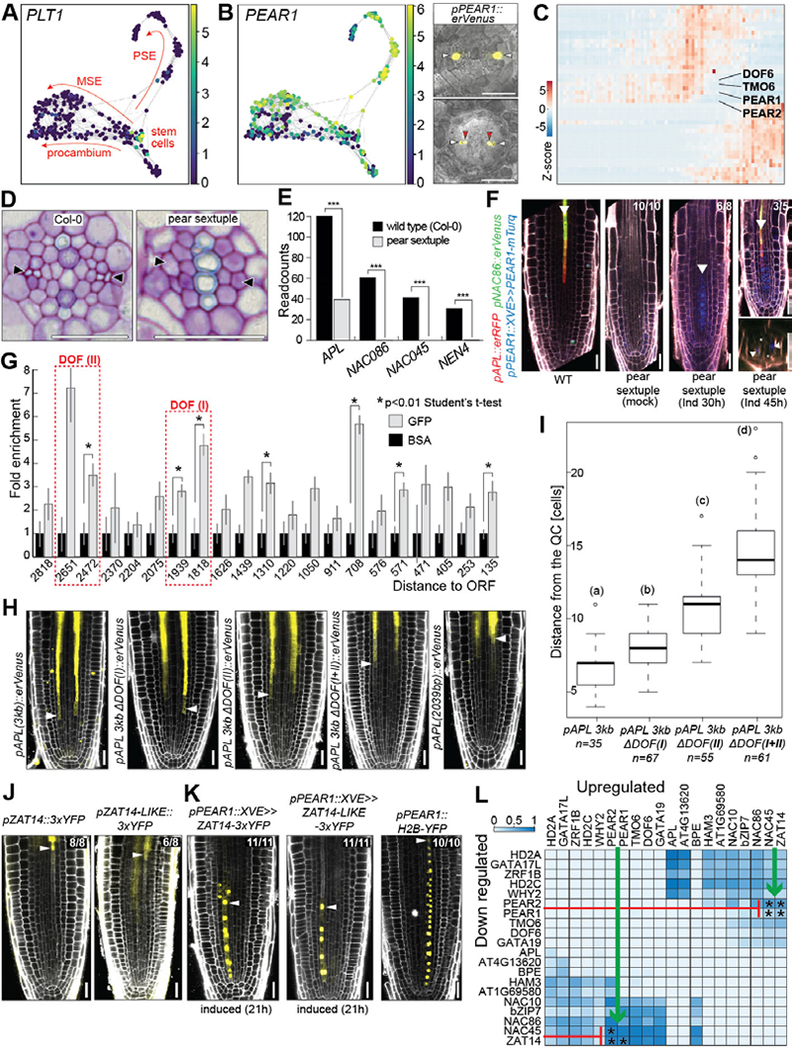
Figure 4. PEARs orchestrate phloem differentiation.
(A) Force-directed clustering of 272 single-cell transcriptomes obtained using the pPEAR1Δ::erVenus reporter. Plotted is expression of stem cell abundant PLT1. Arrows: cellular trajectories inferred from known gene expression patterns (Fig. S6). (B) Strong enrichment of PEAR1 expression in protophloem sieve element and metaphloem sieve element trajectories confirmed by pPEAR1::erVenus reporter line. White arrowheads: protophloem sieve element, red arrowheads: metaphloem sieve element. (C) Expression heatmap: PEAR genes among the earliest phloem specific transcription factors. (D) Lack of protophloem sieve element differentiation in the mature part of the pear sextuple mutant root. Arrowheads: protophloem sieve element position. (E) Lack of APL pathway activation in the roots of pear sextuple mutant based on RNASeq analysis. (F) Inducible expression of PEAR1-mTurq is sufficient to activate transcription of APL and NAC86 reporters in pear sextuple mutant background. (G) ChIP-qPCR of PEAR1-YFP shows direct interaction of PEAR1 with APL promoter at multiple positions. Two prominent PEAR1 binding sites are indicated with red dashed rectangles. (H) Expression patterns of modified pAPL reporter lines. Length of “3kb” promoter equals 2962 bp. DOF(I) and DOF(II) correspond to two enhancer elements indicated in panel G. Details of modification are provided in Fig. S7C. (I) Quantification of the onset of pAPL expression after modification of DOF binding motives. Statistically significant differences between groups were tested using Tukey’s HSD test P < 0.05. Different letters indicate significant difference at P < 0.05. (J) Expression of ZAT14 and ZAT14L during late differentiation of protophloem sieve element. Arrowheads: last cell before enucleation. (K) Ectopic expression of ZAT14 and ZAT14L under pPEAR1::XVE results in cell elongation and inhibition of cell division. Arrowheads: last cell before enucleation. pPEAR1::H2B-YFP line shows regular number of protophloem sieve element cells. (L) Heatmap shows significantly overlapping and oppositely regulated target sets of the 20 most important TFs from the GRN model. Color intensity shows a fraction of overlapping target sets. The colormap represents significantly overlapping sets (Fisher Exact Test, if p<0.05, val=1) multiplied by the fraction of overlap. Asterisk indicates experimental validation of up and downregulated sets from TF OE in vivo (Tables S15, S16). All scale bars, 25 μm.
To test whether PEAR1 can directly regulate expression of APL in its endogenous expression domain (cells 1–14), we performed chromatin immunoprecipitation (ChIP) followed by quantitative PCR (qPCR) using pPEAR1::PEAR1-GFP protein fusion and identified multiple PEAR1 binding sites within APL promoter (pAPL) (Fig. 4G). Truncation analysis of pAPL indicated presence of an enhancer element, responsible for expression of APL in the cells transitioning from cell division to cell differentiation, within 2039 bp to 2962 bp region upstream of APL open reading frame (ORF) (Fig. 4H). Our ChIP analysis detected a single strong PEAR1-GFP peak in the promoter sequence beyond 2039 bp distance from the ORF and another strong peak at the upstream end of the 2 kb region, both of which were also detected in the publicly available DAP-Seq data (Fig. 4G, Fig. S7C) (26). Furthermore, within the detected regions (−2672 to −2512 and −1946 to −1844) we identified multiple clusters of DOF binding motifs (AAAG) (26) that constitute an enhancer element required for the transcriptional activation of APL in the phloem transition zone (domain III) (Fig. 4H, I, Fig. S7C). Although the expression of APL in the protophloem sieve element is dependent on PEARs (Fig. 4F), APL expression domain extends beyond PEAR domain (cells 15–19; Fig. 1E, Fig. S3A). It is possible that either the PEAR proteins and/or APL mRNA persist this period of some 10 hours before enucleation. Alternatively, there may be intermediate factors acting downstream of PEARs to promote APL expression during late stages of phloem development. Collectively, the data supports a role for PEARs controlling the onset of APL expression to regulate a transition in phloem differentiation. The transition is controlled by the PLETHORAs, whose role in promoting division ultimately dissipates its own gradient. When PLETHORA levels decline sufficiently, PEARs can then effectively upregulate APL. The opposing regulation of APL by positively regulating PEARS and inhibitory PLETHORAs illustrates how antagonistic mechanisms – one forming a morphogen-like gradient across the meristem – orchestrate developmental timing within a cell file.
Sequential mutual inhibition directs developmental transitioning.
The final major transcriptional transition in the phloem lineage occurs between the domains IV-V. To explore this transition, we ectopically expressed NEN4 and PLT2 at various developmental stages. When expressed in early ectopic domains, NEN4 expression causes cell death, while PLT2 expression forces cells back into the cell cycle. However, later expression of these two transcription factors, have little or no visible effect on cells, showing the developmental program of domain V appears resilient to these perturbations (Fig. 3B, Fig. S5A, Fig. S9). This indicates that the high number of protophloem sieve element specific genes during the final 8 hours of differentiation remodel the cellular behavior in an irreversible manner. We next sought to explore how widely the PEARs control transcriptional programs related to this final stage of sieve element development. We combined a gene regulatory analysis in the pear mutant with systematic overexpression and modelling approaches (Fig. S7A, B, Fig. S8). Our analysis revealed that - in addition to known phloem regulators APL, NAC045, NAC086 and NAC028 - 10 out of 13 newly validated phloem enriched transcription factors are dependent on PEARs (Fig. S7A, B, Fig. 4F). Overexpression of two of these, ZAT14 (AT5G03510), which was also the 3rd most important TF in the machine learning model, and its close homolog ZAT14L (AT5G04390) led to arrest of cell cycle and premature cell elongation (Fig. 4J, K). Transcriptional profiling provided further evidence for a putative dual role in timing cell division and cell expansion (that occurs largely after enucleation in this cell lineage) (Tables S10–S14). In addition, the gene regulatory network model predicted a pattern of sequential mutual inhibition in the target sets of high-scoring transcriptional regulators (Table S15); for example, genes repressed by ZAT14 significantly overlap with genes activated by the earlier expressed PEARs and vice versa (Fig. 4L). Overexpression analysis confirmed a significant over-representation in the overlap between genes up-regulated by PEARs and down-regulated by ZAT14 (Table S16) (17).
By combining single-cell transcriptomics with live imaging, here we have mapped the cellular events from the birth of the phloem cell to its terminal differentiation into phloem sieve element cells spanning a timeframe of 79 hours. In the early part of the developmental trajectory, where cells are proliferating, the PEAR factors promote the asymmetric periclinal divisions that result in lineage bifurcation. We pinpoint the ROPGEF-ROP regulatory module as an effector of early PEAR function in promoting the periclinal cell divisions central to vascular development. In addition, the PEARs activate the final 20-hour terminal differentiation program, which highlights them as central integrators that connect early and late phloem development. Our high-resolution phloem developmental trajectory reveals three abrupt transitions in the gene expression program. The late, PEAR-regulated protophloem sieve element program is directly and antagonistically controlled by the broad PLETHORA gradient, which connects this morphogen-like gradient to cellular maturation. We propose that mutual inhibition of target genes by sequentially expressed transcription factors represents a “seesaw” mechanism (Fig. S10) that allows rapid transitions and prevent gene expression programs with conflicting effects on cellular physiology (e.g., division vs. enucleation). Similar models have been implicated in so-called attractor states in cell fate decisions in animals (38). In the future it will be interesting to determine how conserved these principles of sieve element differentiation are in an evolutionary context, as well as how extensively they apply to other differentiation trajectories in plants.
Methods summary
Single-cell transcriptomic data described in the manuscript were generated from the protophloem/metaphloem sieve element and procambial cells sorted with a use of tissue specific fluorescent reporter lines. Root tips of 5 days old Arabidopsis plants were used as a tissue material for protoplasting. RNA sequencing of the sorted cells was performed following well-based Smart-seq protocol. Obtained transcriptomes, corresponding to the cells from protophloem cell lineage, were ordered in pseudotime using Monocle2 package which generated a single linear protophloem developmental trajectory. Expression profiles and pseudotime coordinates of the known phloem-expressed genes were further confirmed with in situ and reporter lines analysis.
Gene regulatory network was modelled using a random forest machine learning approach. Selected interactions, representing mutual inhibition (the “seesaw” model), were confirmed by the transcriptome analysis of lines overexpressing a candidate gene or profiling of the loss-of-function lines.
To understand cell behaviour at different developmental phases, confocal long-term live imaging was performed with the protophloem sieve element specific and nuclear localised reporter line. Up to 5-days long movies were recorded and cell behaviour, including number and position of cell divisions, enucleation as well as the time of these events were recorded.
All the details of methods including those summarized above are provided in the supplementary materials.
Supplementary Material
Table S1: List of temporal specific genes. These were identified by comparing the transcriptomes in this domain to those of all others in pairwise comparisons. The highest qval of all these comparisons was then used for further selection (only genes with qval <0.05 are listed). Genes specific to the nested PLT1-like and NEN4-like domains can also be part of domain [a] or domain [d], respectively.
Table S2: List of developmental phases of protophloem sieve element
Table S3: List of PLT2 targets after ectopic expression in late protophloem sieve element cells. The expression in each replicate (RPKM), logarithm of the fold change (logFC, between EST/induced and DMSO/non-induced), p values (PValue), false discovery rates (FDR) are given. Upregulated genes that have GO term annotations as “cell cycle” are indicated as well as here detected specificity to M or S phase of those genes.
Table S4: List of 925 phloem enriched genes based on Shannon entropy analysis of bulk sorted cells. The gene IDs, names and description are given. RPKM values for all used replicates, including the published data run through the same bioinformatics pipeline are given (Columns D-BK). Statistics of the Shannon entropy calculations can be found in Columns BL-BS.
Table S5: List of 1192 SE enriched genes based on cluster comparisons of 272 pPEAR1Δ::erVenus single cells from the protophloem sieve element, metaphloem sieve element and procambial lineage. For further description of the applied comparisons, see M&M.
Table S6: List of the protophloem sieve element enriched genes that form the overlap between Table S4 and Table S5.
Table S7: Average expression per cluster of 467 out of 542 phloem enriched genes (Table S6) that were detected in published root expression atlas (Wendrich et al., submitted). These genes are not broadly expressed throughout the tissue types. While many genes show either protophloem sieve element or phloem specific expression, some show specific expression in other cell types. Several genes confirmed to be expressed during late protophloem sieve element cells such as NAC010, NAC73, ZAT14, RMT2 and NEN4 appear not to be expressed in the sieve element cluster. This could potentially indicate the underrepresentation of late protophloem sieve element cells within the sieve element cluster (<30 cells).
Table S8: Core predictions of the random forest GRN. The top10 most important transcription factors for each gene were selected, resulting in 49240 edges in the model (ranked by importance). The gene ID for transcription factor (TF) and the predicted target (Target), the importance of this edge (Importance), the correlation (Correlation) and the regulation based on the correlation is given (Correlation sign). These core edges of the model were in turn used to determine the most important transcription factors by the number of their target genes. The gene ID for each transcription factor (gene), number of target genes (target #), combined importance of these edges (target importance) and a short annotation for each transcription factor (annotation) is given. The model was tested for enrichment of targets determined in available overexpression or mutant data sets. The results of this enrichment test also given.
Table S9: List of differentially expressed genes in the pear sextuple root meristem. Read counts, log2 fold change, pval and padj are given. Only significantly differentially expressed genes (padj<0.05) are shown.
Table S10: List of differentially expressed genes after 10h induction of pWOL::XVE>>ZAT14. Gene IDs, mean read counts of 3 replicates for 10h induction and DMSO control (Z10 and Z_DMSO), log2foldchange, pvalue, padjust, gene loci and gene names are given for all significant differentially expressed genes (padj<0.05).
Table S11: List of differentially expressed genes after 14h induction of pWOL::XVE>>ZAT14. Gene IDs, mean read counts of 3 replicates for 14h induction and DMSO control (Z14 and Z_DMSO), log2foldchange, pvalue, padjust, gene loci and gene names are given for all significant differentially expressed genes (padj<0.05).
Table S12: List of differentially expressed genes after 10h induction of pWOL::XVE>>ZAT14L. Gene IDs, mean read counts of 3 replicates for 10h induction and DMSO control (L10 and L_DMSO), log2foldchange, pvalue, padjust, gene loci and gene names are given for all significant differentially expressed genes (padj<0.05)
Table S13: List of differentially expressed genes after 14h induction of pWOL::XVE>>ZAT14L. Gene IDs, mean read counts of 3 replicates for 10h induction and DMSO control (L14 and L_DMSO), log2foldchange, pvalue, padjust, gene loci and gene names are given for all significant differentially expressed genes (padj<0.05)
Table S14: PANTHER Gene ontology analysis of ZAT14 and/or ZAT14L targets (14h induction). Details such as test type and correction are given alongside the expected and detected number of genes for each GO category, the fold enrichment and p value.
Table S15: Significant overlap of up and down regulated, predicted targets for the Top20 transcription factors in the model, respectively.
Table S16: Summary of statistical tests for overlapping gene sets of downstream targets in over-expression data sets. Overrepresentation ratio and pval (Fisher test) are given as well as genes that are both up and down regulated by early or late transcription factors. PLT2 and ZAT14L were included as those are also important regulators in this study with very specific early or late expression and overexpression target data was available.
Table S17: List of single cell transcriptomes used in this study. Cell identifiers (ID), reporter line used to isolate the cell (sample), number of total reads, unmapped reads, reads mapped to a single locus and reads mapped to multiple loci, percent of reads mapped to single locus, reads mapped to multiple loci and generally mapped to A. thaliana genome, average library size (lib_size_avr), Cluster number when all cells passing quality thresholds were clustered (Cluster_all_cells), cluster number when only 758 protophloem sieve element were clustered (Cluster_PSE) and assigned pseudotime for protophloem sieve element cells are given (Pseudotime_PSE).
Table S18: List of fluorescence reporters and mutant lines of A. thaliana analyzed in this publication. Genetic background, introduced transgenic construct, abbreviation and origin are given.
Table S19: List of oligonucleotide sequences used in this study. Primer names, including gene names and positions and amplified fragment sizes are given together with the sequence.
Table S20: Live imaging quantification data. The time between onset of expression and enucleation in pNAC86::H2B-YFP and pNEN4::H2B-YFP line and between enucleation was quantified. For the expression length, the root and corresponding movie, the observed phloem pole (pole), movie frame with onset and enucleation and the difference (frame gene ON, frame enucleation, frames gene expression, respectively), the time of expression before enucleation in hours (time cell enucleation [h]) and the corresponding movies are given. The enucleation quantification is based only on movie S1 and movie S2 and their analysis in Fig S1A, E. Time between consecutive enucleation events and the corresponding movie are given.
Movie S1: Live-imaging of pPEAR1::H2B-YFP pCALS7::H2B-YFP pWOX5-mCherry; quantification in Fig S1A.
Movie S2: Live-imaging of pPEAR1::H2B-YFP pCALS7::H2B-YFP pWOX5-mCherry; quantification in Fig S1E.
Movie S13: Live-imaging of pPEAR1::H2B-YFP pCALS7::H2B-YFP pWOX5-mCherry; quantification in Fig S4A.
Acknowledgements:
We thank Yuki Kondo, Philip Benfey and Ottoline Leyser for providing seeds of pNAC57::2GFP, pSHR::erGFP and pPIN4::PIN4-GFP reporters, respectively and the staff of the Flow Cytometry Core Facility at CIMR for their technical support with cell sorting. We thank Satoshi Fujita (National Institute of Genetics, Japan) for providing pDONRP2rP3_MIDD1ΔNcds_stop vector. We are thankful to Raymond Wightman and Gareth Evans for technical support with microscopy experiments.
This research was funded in whole, or in part, by the Wellcome Trust [203151/Z/16/Z] and the UKRI Medical Research Council [MC_PC_17230]. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
This author manuscript is distributed under the terms of the Creative Commons Attribution license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding: This work was supported by
Finnish CoE in Molecular Biology of Primary Producers (Academy of Finland CoE program 2014–2019) decision #271832 (YH)
Gatsby Foundation GAT3395/PR3 (YH)
University of Helsinki award 799992091 (YH)
ERC Advanced Investigator Grant SYMDEV No. 323052 (YH)
NSF-BBSRC MCSB grant 1517058 (RS, YH)
NSF CAREER MCB grant 1453130 (RS)
NIH grant GM078279 (KDB)
NFS IOS grant 1934388 (KDB, DS)
NIH grant R35GM136362 (KDB)
MRC Clinical Research Infrastructure award MR/M008975/1 (BG)
Core funding from the Wellcome and MRC to the Cambridge Stem Cell Institute (BG)
Academy of Finland grants 266431 and 307335 (APM, XW)
NWO Horizon grant 050–71-054 (RH)
ERC Starting Grant TORPEDO; 714055 (BDR)
Research Foundation - Flanders (FWO; Odysseus II G0D0515N) (BDR)
Gatsby Foundation CDF grant (SEA)
BOF postdoctoral fellowship from Ghent University (JRW)
Wallenberg Academy Fellowship KAW 2016.0274 (CWM)
Wellcome Strategic Award 105031/D/14/Z (FH)
JSPS Research Fellowship for Young Scientists (KT)
JSPS KAKENHI grant JP16J00131 (KT)
JSPS Overseas Research Fellowship (YS)
MEXT KAKENHI grant 16H06280, 19H05677 (YO)
The Finnish Academy of Science (JH)
Footnotes
Competing interests: Authors declare that they have no competing interests.
Data and materials availability: All data are available in the supplementary material and raw RNA sequencing data for pooled (bulk sorted) protophloem sieve element cells, single protophloem sieve element cells and overexpression and mutant profiling is deposited at GEO-NCBI (GEO submissions GSE142259, GSE140778, GSE140977, respectively).
References
- 1.Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B, Cellular organisation of the Arabidopsis thaliana root. Development. 119, 71–84 (1993). [DOI] [PubMed] [Google Scholar]
- 2.Mähönen AP, Bonke M, Kauppinen L, Riikonen M, Benfey PN, Helariutta Y, A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes Dev. 14, 2938–2943 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuta KM, Yadav SR, Lehesranta S, Belevich I, Miyashima S, Heo J, Vatén A, Lindgren O, Rybel BD, Isterdael GV, Somervuo P, Lichtenberger R, Rocha R, Thitamadee S, Tähtiharju S, Auvinen P, Beeckman T, Jokitalo E, Helariutta Y, Arabidopsis NAC45/86 direct sieve element morphogenesis culminating in enucleation. Science. 345, 933–937 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Marhava P, Bassukas AEL, Zourelidou M, Kolb M, Moret B, Fastner A, Schulze WX, Cattaneo P, Hammes UZ, Schwechheimer C, Hardtke CS, A molecular rheostat adjusts auxin flux to promote root protophloem differentiation. Nature. 558, 297–300 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Rahni R, Birnbaum KD, Week-long imaging of cell divisions in the Arabidopsis root meristem. Plant Methods. 15, 30 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Efroni I, Mello A, Nawy T, Ip P-L, Rahni R, DelRose N, Powers A, Satija R, Birnbaum KD, Root Regeneration Triggers an Embryo-like Sequence Guided by Hormonal Interactions. Cell. 165, 1721–1733 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denyer T, Ma X, Klesen S, Scacchi E, Nieselt K, Timmermans MCP, Spatiotemporal Developmental Trajectories in the Arabidopsis Root Revealed Using High-Throughput Single-Cell RNA Sequencing. Dev. Cell. 48, 840–852.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Shulse CN, Cole BJ, Ciobanu D, Lin J, Yoshinaga Y, Gouran M, Turco GM, Zhu Y, O’Malley RC, Brady SM, Dickel DE, High-Throughput Single-Cell Transcriptome Profiling of Plant Cell Types. Cell Reports. 27, 2241–2247.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jean-Baptiste K, McFaline-Figueroa JL, Alexandre CM, Dorrity MW, Saunders L, Bubb KL, Trapnell C, Fields S, Queitsch C, Cuperus JT, Dynamics of Gene Expression in Single Root Cells of Arabidopsis thaliana. Plant Cell. 31, 993–1011 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang T-Q, Xu Z-G, Shang G-D, Wang J-W, A Single-Cell RNA Sequencing Profiles the Developmental Landscape of Arabidopsis Root. Mol Plant. 12, 648–660 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Ryu KH, Huang L, Kang HM, Schiefelbein J, Single-Cell RNA Sequencing Resolves Molecular Relationships Among Individual Plant Cells. Plant Physiol. 179, 1444–1456 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wendrich JR, Yang B-J, Vandamme N, Verstaen K, Smet W, Van de Velde C, Minne M, Wybouw B, Mor E, Arents HE, Nolf J, Van Duyse J, Van Isterdael G, Maere S, Saeys Y, De Rybel B, Vascular transcription factors guide plant epidermal responses to limiting phosphate conditions. Science. 370, 6518 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonke M, Thitamadee S, Mähönen AP, Hauser M-T, Helariutta Y, APL regulates vascular tissue identity in Arabidopsis. Nature. 426, 181–186 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Mähönen AP, Ten Tusscher K, Siligato R, Smetana O, Díaz-Triviño S, Salojärvi J, Wachsman G, Prasad K, Heidstra R, Scheres B, PLETHORA gradient formation mechanism separates auxin responses. Nature. 515, 125–129 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, Lennon NJ, Livak KJ, Mikkelsen TS, Rinn JL, The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nature Biotechnology. 32, 381–386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, Trapnell C, Reversed graph embedding resolves complex single-cell trajectories. Nature Methods. 14, 979–982 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyashima S, Roszak P, Sevilem I, Toyokura K, Blob B, Heo J, Mellor N, Help-Rinta-Rahko H, Otero S, Smet W, Boekschoten M, Hooiveld G, Hashimoto K, Smetana O, Siligato R, Wallner E-S, Mähönen AP, Kondo Y, Melnyk CW, Greb T, Nakajima K, Sozzani R, Bishopp A, Rybel BD, Helariutta Y, Mobile PEAR transcription factors integrate positional cues to prime cambial growth. Nature. 565, 490–494 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winge P, Brembu T, Kristensen R, Bones AM, Genetic structure and evolution of RAC-GTPases in Arabidopsis thaliana. Genetics. 156, 1959–1971 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berken A, Thomas C, Wittinghofer A, A new family of RhoGEFs activates the Rop molecular switch in plants. Nature. 436, 1176–1180 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z, Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell. 120, 687–700 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Molendijk AJ, Bischoff F, Rajendrakumar CS, Friml J, Braun M, Gilroy S, Palme K, Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 20, 2779–2788 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oda Y, Fukuda H, Initiation of cell wall pattern by a Rho- and microtubule-driven symmetry breaking. Science. 337, 1333–1336 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Humphries JA, Vejlupkova Z, Luo A, Meeley RB, Sylvester AW, Fowler JE, Smith LG, ROP GTPases act with the receptor-like protein PAN1 to polarize asymmetric cell division in maize. Plant Cell. 6, 2273–2284 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi P, Goshima G, Rho of Plants GTPases and cytoskeletal elements control nuclear positioning and asymmetric cell division during Physcomitrella patens branching. Current Biology. 30, 2860–2868 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Stockle D, Herrmann A, Lipka E, Lauster T, Gavidia R, Zimmermann S, Müller S, Putative RopGAPs impact division plane selection and interact with kinesin-12 POK1. Nature Plants. 2, 16120 (2016). [DOI] [PubMed] [Google Scholar]
- 26.O’Malley RC, Huang SC, Song L, Lewsey MG, Bartlett A, Nery JR, Galli M, Gallavotti A, Ecker JR, Cistrome and Epicistrome Features Shape the Regulatory DNA Landscape. Cell. 165, 1280–1292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feiguelman G, Fu Y, Yalovsky S, ROP GTPases structure-function and signaling pathways. Plant Physiology. 176, 57–79 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugiyama Y, Wakazaki M, Toyooka K, Fukuda H, Oda Y, A Novel Plasma Membrane-Anchored Protein Regulates Xylem Cell-Wall Deposition through Microtubule- Dependent Lateral Inhibition of Rho GTPase Domains. Current Biology. 27, 2522–2528 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Denninger P, Reichelt A, Schmidt VAF, Mehlhorn DG, Asseck LY, Stanley CE, Keinath NF, Evers JF, Grefen C, Distinct G RopGEFs successively drive polarization and outgrowth of root hairs. Current Biology. 29, 1854–1865 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Qiu JL, Jilk R, Marks MD, Szymanski DB, The Arabidopsis SPIKE1 gene is required for normal cell shape control and tissue development. The Plant Cell. 14, 101–118 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basu D, Le J, Zakharova T, Mallery EL, Szymanski DB, A SPIKE1 signaling complex controls actin-dependent cell morphogenesis through the heteromeric WAVE and ARP2/3 complexes. Proc. Natl. Acad. Sci. U.S.A. 105, 4044–4049 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren H, Dang X, Yang Y, Huang D, Liu M, Gao X, Lin D, SPIKE1 activates ROP GTPase to modulate petal growth and shape. Plant Physiology. 172, 358–371 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B, PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature. 449, 1053–1057 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Santuari L, Sanchez-Perez GF, Luijten M, Rutjens B, Terpstra I, Berke L, Gorte M, Prasad K, Bao D, Timmermans-Hereijgers JLPM, Maeo K, Nakamura K, Shimotohno A, Pencik A, Novak O, Ljung K, van Heesch S, de Bruijn E, Cuppen E, Willemsen V, Mähönen AP, Lukowitz W, Snel B, de Ridder D, Scheres B, Heidstra R, The PLETHORA Gene Regulatory Network Guides Growth and Cell Differentiation in Arabidopsis Roots. The Plant Cell. 28, 2937–2951 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siligato R, Wang X, Yadav SR, Lehesranta S, Ma G, Ursache R, Sevilem I, Zhang J, Gorte M, Prasad K, Wrzaczek M, Heidstra R, Murphy A, Scheres B, Mähönen AP, MultiSite Gateway-Compatible Cell Type-Specific Gene-Inducible System for Plants. Plant Physiology. 170, 627–641 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Ye L, Lyu M, Ursache R, Löytynoja A, Mähönen AP, An inducible genome editing system for plants. Nat. Plants 6, 766–772 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li S, Yamada M, Han X, Ohler U, Benfey PN, High resolution expression map of the Arabidopsis root reveals alternative splicing and lincRNA regulation. Dev Cell. 39, 508–522 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shu J, Deng H, Lineage Specifiers: New Players in the Induction of Pluripotency. Genomics, Proteomics & Bioinformatics. 11, 259–263 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lampropoulos A, Sutikovic Z, Wenzl C, Maegele I, Lohmann JU, Forner J, GreenGate - a novel, versatile, and efficient cloning system for plant transgenesis. PLoS One. 8, e83043 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Decaestecker W, Buono RA, Pfeiffer ML, Vangheluwe N, Jourquin J, Karimi M, Van Isterdael G, Beeckman T, Nowack MK, Jacobs TB, CRISPR-TSKO: A Technique for Efficient Mutagenesis in Specific Cell Types, Tissues, or Organs in Arabidopsis. Plant Cell. 31, 2868–2887 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark NM, Fisher AP, Sozzani R, “Identifying Differentially Expressed Genes Using Fluorescence-Activated Cell Sorting (FACS) and RNA Sequencing from Low Input Samples” in: Computational Cell Biology: Methods and Protocols, von Stechow L, Santos Delgado A, Eds. (Springer; New York, 2018) pp. 139–151. [DOI] [PubMed] [Google Scholar]
- 42.Zambelli F, Mastropasqua F, Picardi E, D’Erchia AM, Pesole G, Pavesi G, RNentropy: an entropy-based tool for the detection of significant variation of gene expression across multiple RNA-Seq experiments. Nucleic Acids Res. 46, e46–e46 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Picelli S, Faridani OR, Björklund ÅK, Winberg G, Sagasser S, Sandberg R, Full-length RNA-seq from single cells using Smart-seq2. Nature Protocols. 9, 171–181 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Dahlin JS, Hamey FK, Pijuan-Sala B, Shepherd M, Lau WWY, Nestorowa S, Weinreb C, Wolock S, Hannah R, Diamanti E, Kent DG, Göttgens B, Wilson NK, A single-cell hematopoietic landscape resolves 8 lineage trajectories and defects in Kit mutant mice. Blood. 131, e1–e11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf FA, Angerer P, Theis FJ, SCANPY: large-scale single-cell gene expression data analysis. Genome Biology. 19, 15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim D, Langmead B, Salzberg SL, HISAT: a fast spliced aligner with low memory requirements. Nature Methods. 12, 357–360 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup, The Sequence Alignment/Map format and SAMtools. Bioinformatics. 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L, Transcript assembly and abundance estimation from RNA-Seq reveals thousands of new transcripts and switching among isoforms. Nat Biotechnol. 28, 511–515 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandez A, Drozdzecki A, Hoogewijs K, Nguyen A, Beeckman T, Madder A, Hilson P, Transcriptional and Functional Classification of the GOLVEN/ROOT GROWTH FACTOR/CLE-Like Signaling Peptides Reveals Their Role in Lateral Root and Hair Formation. Plant Physiology. 161, 954–970 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blondel VD, Guillaume J-L, Lambiotte R, Lefebvre E, Fast unfolding of communities in large networks. J. Stat. Mech P10008 (2008). [Google Scholar]
- 51.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, Fiji: an open-source platform for biological-image analysis. Nature Methods. 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoon EK, Dhar S, Lee M-H, Song JH, Lee SA, Kim G, Jang S, Choi JW, Choe J-E, Kim JH, Lee MM, Lim J, Conservation and Diversification of the SHR-SCR-SCL23 Regulatory Network in the Development of the Functional Endodermis in Arabidopsis Shoots. Mol Plant. 9, 1197–1209 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Yang C-H, Kuo W-T, Chuang Y-T, Chen C-Y, Lin C-C, Cyclin B1 Destruction Box-Mediated Protein Instability: The Enhanced Sensitivity of Fluorescent-Protein-Based Reporter Gene System. BioMed Research International. 2013 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: List of temporal specific genes. These were identified by comparing the transcriptomes in this domain to those of all others in pairwise comparisons. The highest qval of all these comparisons was then used for further selection (only genes with qval <0.05 are listed). Genes specific to the nested PLT1-like and NEN4-like domains can also be part of domain [a] or domain [d], respectively.
Table S2: List of developmental phases of protophloem sieve element
Table S3: List of PLT2 targets after ectopic expression in late protophloem sieve element cells. The expression in each replicate (RPKM), logarithm of the fold change (logFC, between EST/induced and DMSO/non-induced), p values (PValue), false discovery rates (FDR) are given. Upregulated genes that have GO term annotations as “cell cycle” are indicated as well as here detected specificity to M or S phase of those genes.
Table S4: List of 925 phloem enriched genes based on Shannon entropy analysis of bulk sorted cells. The gene IDs, names and description are given. RPKM values for all used replicates, including the published data run through the same bioinformatics pipeline are given (Columns D-BK). Statistics of the Shannon entropy calculations can be found in Columns BL-BS.
Table S5: List of 1192 SE enriched genes based on cluster comparisons of 272 pPEAR1Δ::erVenus single cells from the protophloem sieve element, metaphloem sieve element and procambial lineage. For further description of the applied comparisons, see M&M.
Table S6: List of the protophloem sieve element enriched genes that form the overlap between Table S4 and Table S5.
Table S7: Average expression per cluster of 467 out of 542 phloem enriched genes (Table S6) that were detected in published root expression atlas (Wendrich et al., submitted). These genes are not broadly expressed throughout the tissue types. While many genes show either protophloem sieve element or phloem specific expression, some show specific expression in other cell types. Several genes confirmed to be expressed during late protophloem sieve element cells such as NAC010, NAC73, ZAT14, RMT2 and NEN4 appear not to be expressed in the sieve element cluster. This could potentially indicate the underrepresentation of late protophloem sieve element cells within the sieve element cluster (<30 cells).
Table S8: Core predictions of the random forest GRN. The top10 most important transcription factors for each gene were selected, resulting in 49240 edges in the model (ranked by importance). The gene ID for transcription factor (TF) and the predicted target (Target), the importance of this edge (Importance), the correlation (Correlation) and the regulation based on the correlation is given (Correlation sign). These core edges of the model were in turn used to determine the most important transcription factors by the number of their target genes. The gene ID for each transcription factor (gene), number of target genes (target #), combined importance of these edges (target importance) and a short annotation for each transcription factor (annotation) is given. The model was tested for enrichment of targets determined in available overexpression or mutant data sets. The results of this enrichment test also given.
Table S9: List of differentially expressed genes in the pear sextuple root meristem. Read counts, log2 fold change, pval and padj are given. Only significantly differentially expressed genes (padj<0.05) are shown.
Table S10: List of differentially expressed genes after 10h induction of pWOL::XVE>>ZAT14. Gene IDs, mean read counts of 3 replicates for 10h induction and DMSO control (Z10 and Z_DMSO), log2foldchange, pvalue, padjust, gene loci and gene names are given for all significant differentially expressed genes (padj<0.05).
Table S11: List of differentially expressed genes after 14h induction of pWOL::XVE>>ZAT14. Gene IDs, mean read counts of 3 replicates for 14h induction and DMSO control (Z14 and Z_DMSO), log2foldchange, pvalue, padjust, gene loci and gene names are given for all significant differentially expressed genes (padj<0.05).
Table S12: List of differentially expressed genes after 10h induction of pWOL::XVE>>ZAT14L. Gene IDs, mean read counts of 3 replicates for 10h induction and DMSO control (L10 and L_DMSO), log2foldchange, pvalue, padjust, gene loci and gene names are given for all significant differentially expressed genes (padj<0.05)
Table S13: List of differentially expressed genes after 14h induction of pWOL::XVE>>ZAT14L. Gene IDs, mean read counts of 3 replicates for 10h induction and DMSO control (L14 and L_DMSO), log2foldchange, pvalue, padjust, gene loci and gene names are given for all significant differentially expressed genes (padj<0.05)
Table S14: PANTHER Gene ontology analysis of ZAT14 and/or ZAT14L targets (14h induction). Details such as test type and correction are given alongside the expected and detected number of genes for each GO category, the fold enrichment and p value.
Table S15: Significant overlap of up and down regulated, predicted targets for the Top20 transcription factors in the model, respectively.
Table S16: Summary of statistical tests for overlapping gene sets of downstream targets in over-expression data sets. Overrepresentation ratio and pval (Fisher test) are given as well as genes that are both up and down regulated by early or late transcription factors. PLT2 and ZAT14L were included as those are also important regulators in this study with very specific early or late expression and overexpression target data was available.
Table S17: List of single cell transcriptomes used in this study. Cell identifiers (ID), reporter line used to isolate the cell (sample), number of total reads, unmapped reads, reads mapped to a single locus and reads mapped to multiple loci, percent of reads mapped to single locus, reads mapped to multiple loci and generally mapped to A. thaliana genome, average library size (lib_size_avr), Cluster number when all cells passing quality thresholds were clustered (Cluster_all_cells), cluster number when only 758 protophloem sieve element were clustered (Cluster_PSE) and assigned pseudotime for protophloem sieve element cells are given (Pseudotime_PSE).
Table S18: List of fluorescence reporters and mutant lines of A. thaliana analyzed in this publication. Genetic background, introduced transgenic construct, abbreviation and origin are given.
Table S19: List of oligonucleotide sequences used in this study. Primer names, including gene names and positions and amplified fragment sizes are given together with the sequence.
Table S20: Live imaging quantification data. The time between onset of expression and enucleation in pNAC86::H2B-YFP and pNEN4::H2B-YFP line and between enucleation was quantified. For the expression length, the root and corresponding movie, the observed phloem pole (pole), movie frame with onset and enucleation and the difference (frame gene ON, frame enucleation, frames gene expression, respectively), the time of expression before enucleation in hours (time cell enucleation [h]) and the corresponding movies are given. The enucleation quantification is based only on movie S1 and movie S2 and their analysis in Fig S1A, E. Time between consecutive enucleation events and the corresponding movie are given.
Movie S1: Live-imaging of pPEAR1::H2B-YFP pCALS7::H2B-YFP pWOX5-mCherry; quantification in Fig S1A.
Movie S2: Live-imaging of pPEAR1::H2B-YFP pCALS7::H2B-YFP pWOX5-mCherry; quantification in Fig S1E.
Movie S13: Live-imaging of pPEAR1::H2B-YFP pCALS7::H2B-YFP pWOX5-mCherry; quantification in Fig S4A.