Abstract
Background
Programmed death ligand 1 (PD-L1) contributes to tumor immunosuppression and is upregulated in aggressive meningiomas. We performed a phase II study of nivolumab, a programmed death 1 (PD-1) blocking antibody among patients with grade ≥2 meningioma that recurred after surgery and radiation therapy.
Methods
Twenty-five patients received nivolumab (240 mg biweekly) until progression, voluntary withdrawal, unacceptable toxicity, or death. Tumor mutational burden (TMB) and quantification of tumor-infiltrating lymphocytes (TIL) were evaluated as potential immunocorrelative biomarkers. Change in neurologic function was prospectively assessed using the Neurologic Assessment in Neuro-Oncology (NANO) scale.
Results
Enrolled patients had multiple recurrences including ≥3 prior surgeries and ≥2 prior courses of radiation in 60% and 72%, respectively. Nivolumab was well tolerated with no unexpected adverse events. Six-month progression-free survival (PFS-6) rate was 42.4% (95% CI: 22.8, 60.7) and the median OS was 30.9 months (95% CI: 17.6, NA). One patient achieved radiographic response (ongoing at 4.5 years). TMB was >10/Mb in 2 of 15 profiled tumors (13.3%). Baseline TIL density was low but increased posttreatment in 3 patients including both patients with elevated TMB. Most patients who achieved PFS-6 maintained neurologic function prior to progression as assessed by NANO.
Conclusion
Nivolumab was well tolerated but failed to improve PFS-6, although a subset of patients appeared to derive benefit. Low levels of TMB and TIL density were typically observed. NANO assessment of neurologic function contributed to outcome assessment. Future studies may consider rationally designed combinatorial regimens.
Keywords: immunotherapy, meningioma, PD-1, PD-L1
Key Points.
Some patients achieved durable tumor control with nivolumab monotherapy although the PFS-6 primary endpoint was not met.
Most meningiomas exhibit low tumor mutational burden and density of infiltrating T cells.
Importance of the Study.
Augmentation of anti-tumor immune responses via inhibition of the programmed death 1 (PD-1) signaling pathway has transformed therapy for many cancers. The expression level of the PD-1-binding ligand PD-L1 increases with meningioma grade and correlates with outcome. We report the first clinical trial evaluating PD-1 blockade in the treatment of patients with meningioma. In this phase II trial, nivolumab monotherapy was well tolerated and achieved durable tumor control in some patients although the primary endpoint of PFS-6 was not met. We explored tumor immunocorrelative biomarkers and noted low rates of tumor mutational burden (TMB) and tumor-infiltrating lymphocyte density in most analyzed tumors although the 2 patients with tumors demonstrating an elevated TMB are long-term survivors. In both of these patients, nivolumab induced a robust infiltration of immune effector cells. Future studies should consider combinatorial strategies to increase tumor immune infiltrate.
Meningiomas, the most common primary tumor of the central nervous system among adults, are classified by the World Health Organization (WHO) based on histopathologic features as benign (grade 1), atypical (grade 2), and anaplastic (grade 3).1 Most patients with grade 1 lesions can be followed with surveillance imaging or are effectively treated with surgery alone and have a 10-year survival rate of approximately 90%.2,3 Although surgery and radiation therapy are established therapies for grade 2 and 3 meningiomas, many of these tumors will recur; effective treatments have not been identified following progression after these modalities, resulting in a 5-year survival rate of approximately 60% and 30% for patients with grade II and III tumors, respectively.2,4,5 Systemically administered agents have been generally ineffective for recurrent meningiomas as reflected by a weighted average six-month progression-free survival (PFS-6) rate of only 26%.6
Blockade of the programmed death 1 (PD-1) signaling axis has been incorporated into the treatment paradigm of multiple malignancies, although only a subset of patients derived benefit for US Food and Drug Administration (FDA)-approved oncology indications. Nivolumab (Opdivo; Bristol-Myers Squibb), a fully human immunoglobulin subtype G4 (IgG4) monoclonal antibody targeting the PD-1 receptor is approved for the treatment of multiple cancer indications. Tumor programmed death ligand 1 (PD-L1) expression has been associated with an increased likelihood of therapeutic benefit from anti-PD-1 therapy for some cancers.7–9 Meningioma tumor cells and immune cells in the tumor microenvironment have been shown to express PD-L1,10 with expression levels correlating with meningioma grade and outcome.11–14 We therefore performed this open-label, single-arm phase II study of nivolumab monotherapy among patients with recurrent grade 2/3 meningioma. We assessed tumor mutational burden (TMB) and the density of immune cell subsets from available tumor samples. The Neurologic Assessment in Neuro-Oncology (NANO) scale15 was employed to provide an objective and quantitative measure of neurologic function among study participants.
Methods
Study Design and Patients
This single-arm, open-label phase 2 study enrolled ≥18-year-old patients with histologically confirmed grade 2 or 3 meningioma that had progressed after maximum safe resection and prior radiation therapy and had a Karnofsky score of at least 70 as well as adequate organ function. There was no limit on the number of prior progressions or treatments although patients were required to have evidence of progression at least 12 weeks after prior radiation therapy and 4 weeks or 5 half-lives (whichever was shorter) from prior systemic therapy. Exclusion criteria included patients requiring >4 mg of dexamethasone/day, any prior PD(L)-1 therapy, brachytherapy within 6 months, primarily spinal cord tumors or known autoimmune condition requiring systemic therapy within 3 months.
The study (NCT02648997) was compliant with the Declaration of Helsinki and guidelines on Good Clinical Practice. Ethics approval was obtained Dana-Farber Cancer Institute (DFCI) Institutional Review Board and all patients provided informed consent. DFCI has a proprietary and financial interest in nivolumab.
Study Procedures
Eligible patients received biweekly nivolumab (240 mg IV) until tumor progression, unacceptable toxicity, noncompliance, or withdrawal of consent. Toxicity was graded using Common Terminology Criteria for Adverse Events version 4.03. Investigator assessed response occurred every 8 weeks using clinical examination and contrast-enhanced MRI according to the Radiologic Assessment in Neuro-Oncology (RANO) criteria.16 The trial was written prior to publication of the RANO meningioma criteria,17 but a post hoc analysis of response was also performed by an independent neuro-radiologist (R.Y.H.) using these criteria. Clinically stable patients with radiologic progression were allowed to continue study therapy pending progression confirmation as per the Immunotherapy Response Assessment Criteria in Neuro-Oncology (iRANO) criteria. In such cases, the date of progression was backdated to the date of initial identification of progression.18 Patient neurologic function was assessed at baseline and at MRI assessments using the NANO scale.15
Biomarker Analyses
We used targeted next-generation exome sequencing (Oncopanel-v3) to detect mutations and copy number variations in 447 cancer genes. We processed and annotated data as previously described.19 We further filtered potential germline variants that were present at >0.1% in gnomAD version v2.120 or annotated as benign or likely benign in the ClinVar database.21 For each sample, the estimated TMB in mutations per megabase (mut/Mb) was calculated as per previous studies by dividing the number of reported (non-silent) mutations by the number of bases targeted in the corresponding version of the OncoPanel assay (1.315078 Mb in version 3).22 We validated that the estimated TMB correlated well with clinically reported TMB for 3425 CNS tumors that underwent OncoPanel analysis for which the reported TMB was available (R2 = 0.9916, P value <2.2e−16).
The protocol for immune profiling using tissue-based multiplexed cyclic immunofluorescence (t-CyCIF) of conventionally prepared formalin-fixed, paraffin-embedded (FFPE) specimens and image analysis are described elsewhere.23,24 Antibodies for t-CyCIF are listed in Supplementary Table 1.
In brief, the FFPE slides were pretreated on the Leica Bond RX (Leica Biosystems), and then incubated with 3 antibodies directly conjugated to different fluorophores. The slides were photobleached after 4-channel images were captured by using a slide-scanning microscope (CyteFinder, RareCyte). This process was repeated for 4 cycles until all antibodies listed in Supplementary Table 1 were applied. ImageJ was used for image preprocessing, registration, segmentation, and quantification, and MATLAB (version 2018a) was used for single-cell data quantification analysis, and visualization.
Semi-quantitative analysis of immune cell subsets was performed on a subset of cases for which tissue was available using immunohistochemistry (IHC) on 4-µm thick FFPE tissue sections using standard protocols with antibodies against CD4 (EP204, 1:100, Cell Marque), CD8 (C8/144B, 1:200, Dako), CD163 (10D6, 1:400, Leica Biosystems), and PD-1 (NAT105, 1:200, Cell Marque). Double staining of PD-L1 (E1L3N, 1:150, Cell Signaling) and PAX5 (24/Pax-5; 1:100, BD Biosciences) was performed as previously described.25 Positivity for each marker was scored by a board-certified neuropathologist (D.M.) who was blinded to patient outcome as an estimated percentage of all nucleated cells (tumor and immune cells) present in the stained tissue section from patients with available tumor material.
Outcomes
The primary endpoint was PFS-6 on the intent-to-treat population based on treating investigator assessment. Secondary endpoints were objective response rate (ORR), PFS, overall survival (OS), and overall safety. Time to event analyses used the Kaplan-Meier method starting from the initiation of study therapy.
Statistical Analysis
A primary endpoint of PFS-6 was selected because disease control lasting at least 6 months provides a meaningful measure of benefit in an aggressive tumor setting such as recurrent grade 2 and 3 meningioma where no effective therapy has been defined following progression after surgery and radiation therapy. A recent comprehensive RANO review, conducted by a multidisciplinary panel of neuro-oncology experts, supports the value of PFS-6 as an endpoint and defined a threshold of over 26% as worthy of further clinical investigation based on meta-analysis data from a similar target population treated with salvage therapy.6 Furthermore, clear benchmarks of radiographic response amongst these tumors have not been well established.2 With a sample size of 25 patients, this study had 90% power to detect a PFS-6 increase of 25% (26% vs 51%) using a 1-sided significance level of 0.10 (exact alpha = 0.089). For the final stage of analysis, the null hypothesis was planned to be rejected and nivolumab therapy was deemed worthy of further investigation if at least 10 patients were progression-free at the 6 months’ time point. Relationship between covariates of interest and time to event endpoints were tested using the univariate Cox Proportional Hazard model. A multivariate model was assessed for significant variables identified in the univariate analysis. Tumor volume values were highly skewed and were log-transformed.
Results
Patients and Treatment
Between March 2016 and March 2019, 25 patients were enrolled and initiated study therapy (Supplementary Figure 1). Summary demographics and baseline patient characteristics are provided in Table 1. Tumor location was primarily supratentorial (defined as those arising from convexity, falcine and parasagittal locations) in 15 patients (60%), skull-based in 8 patients (32%) and 2 patients (8%) had both supratentorial and skull-based tumors. Skull-based tumors were larger (median 1232 mm2) than those that arose in supratentorial locations (median 497 mm2). Ten patients (40%) had multifocal disease. The majority of patients (96%) had experienced multiple episodes of progression prior to enrollment, with 48% enrolling at second recurrence and 48% after 3 or more recurrences. Sixty percent of patients had undergone 3 or more prior surgeries and 72% had received at least 2 prior radiation therapy courses. Most patients (72%) had not received prior systemic therapies and 88% were not on corticosteroids at enrollment. At study initiation, 1 patient had grade 1 lymphopenia and the remaining patients all had a normal absolute lymphocyte count (ALC). All patients have discontinued study therapy, with progressive disease being the most common reason (76%). A median of 5.5 cycles of therapy was administered with a range of 1.5-25.0 cycles. Fourteen patients (56%) have died and 11 (44%) remain alive.
Table 1.
Patient Characteristics and Study Dispositiona
| Characteristic | Measure (n = 25) |
|---|---|
| Median age (yr, range) | 60 (25-88) |
| Gender, female (%) | 16 (64) |
| Grade at enrollment (%) | |
| Atypical | 18 (72) |
| Anaplastic | 7 (28) |
| KPS (%) | |
| 90-100 | 5 (20) |
| 80 | 12 (48) |
| 70 | 8 (32) |
| Multifocal (%) | 10 (40) |
| Tumor location (%) | |
| Supratentorial | 15 (60) |
| Skull base | 8 (32) |
| Supratentorial and skull base | 2 (8) |
| Median tumor volume (mm2) | 701 |
| Range | 124-3901 |
| Possibly radiation induced (%) | |
| Yes | 4 (16) |
| No | 21 (84) |
| No. of prior PD (%) | |
| 1 | 1 (4) |
| 2 | 12 (48) |
| 3 | 4 (16) |
| 4 | 3 (12) |
| ≥5 | 5 (20) |
| Resection prior to the study (%) | |
| Gross total | 0 |
| Subtotal | 3 (12) |
| Biopsy | 0 |
| None | 22 (88) |
| No. of prior resections (%) | |
| 1 | 1 (4) |
| 2 | 9 (36) |
| 3 | 7 (28) |
| ≥4 | 8 (32) |
| No. of prior radiation courses (%) | |
| 1 | 7 (28) |
| 2 | 10 (40) |
| 3 | 5 (20) |
| ≥4 | 3 (12) |
| No. of prior systemic therapies (%) | |
| 0 | 18 (72) |
| 1 | 6 (24) |
| 2 | 0 |
| 3 | 1 (4) |
| Median ALC (/mm3; range) | 1700 (740-4420) |
| Dexamethasone use at study start (%) | |
| 0 | 22 (88) |
| ≤2 mg | 1 (4) |
| >2 mg | 2 (8) |
| Mean time from initial meningioma diagnosis to enrollment in months (range) | 75.3 (105.3, 1765.9) |
| Median no. of study cycles completed | 5.5 |
| Range | 1.5-25.0 |
| Reason off-study (%) | |
| PD | 19 (76) |
| Toxicity | 1 (4) |
| Consent withdrawal | 4 (16) |
| Death | 1 (4) |
| Treatment after study discontinuation (%) | |
| Yes | 14 (56) |
| No | 4 (16) |
| Unknown | 7 (28) |
| Type of therapy after study discontinuation (%) | |
| Surgery | 8 (32) |
| External beam radiation | 4 (16) |
| Brachytherapy | 2 (8) |
| Bevacizumab | 5 (20) |
| Hydroxyurea | 2 (8) |
| Clinical trial (TORC1/TORC2 inhibitor) | 2 (8) |
| Daily temozolomide | 1 (4) |
| Current status (%) | |
| Dead | 14 (56) |
| Alive | 11 (44) |
Abbreviations: ALC, absolute lymphocyte count; GBM, glioblastoma; GS, gliosarcoma; IDH1, isocitrate dehydrogenase 1; IQR, interquartile range; KPS, Karnofsky performance score; MGMT, O6-methylguanine-DNA methyltransferase; TIL, tumor-infiltrating lymphocytes; PD, progressive disease; PD-L1, programmed death ligand 1; SD, standard deviation; TORC: target of rapamycin complex.
aPercentages are correct for denominators in each cell.
Efficacy
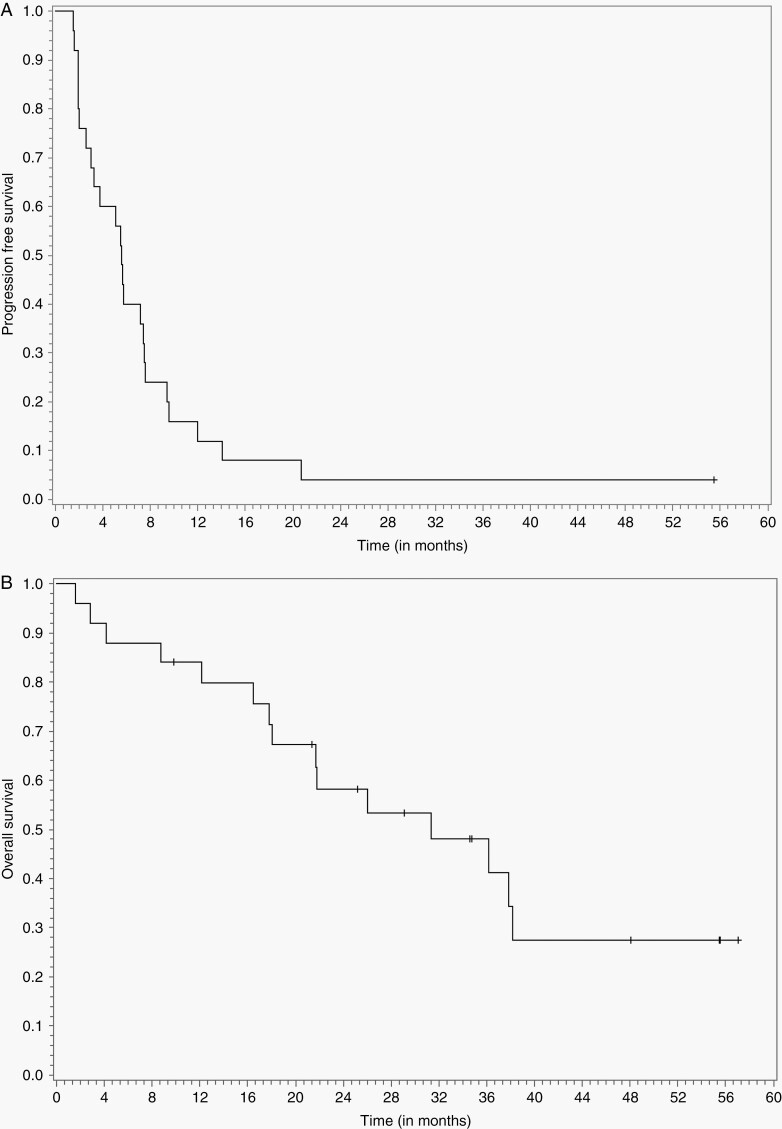
The median follow-up was 21.47 months. The median PFS and PFS-6 were 5.56 months (95% CI: 3.16, 7.40) and 42.4% (95% CI: 22.8, 60.7), respectively (Figure 1A). Median OS and overall survival at 12 months (OS-12) were 30.93 months (95% CI: 17.56, NA) and 80% (95% CI: 58, 91), respectively (Figure 1B). Per patient PFS and OS are depicted in Figure 2 and include delineation of meningioma grade at enrollment. The following variables were assessed for association with OS: age (≤60 years vs >60 years); KPS (≥80 vs <80); grade (2 vs 3); multifocality; tumor volume and logarithmically transformed tumor volume; possible radiation-induced (defined as meningiomas that developed after prior radiation for unrelated condition); number of prior recurrences (≤2 vs >2); location (supratentorial vs skull-based); number of prior surgeries (≤2 vs >2); number of prior courses of radiation therapy (≤2 vs >2); and prior exposure to systemic agents. As described in Supplementary Table 2, none of these variables are associated with PFS on univariate analysis, while supratentorial location (P = .013) and log tumor volume (P = .014) associated with OS on univariate but not multivariate analyses.
Fig. 1.
Panel A shows the number of events and the Kaplan-Meier curve for PFS per investigator assessment in all patients treated with nivolumab. Panel B shows the number of events and the Kaplan-Meier curve for OS. Abbreviations: OS, overall survival; PFS, progression-free survival.
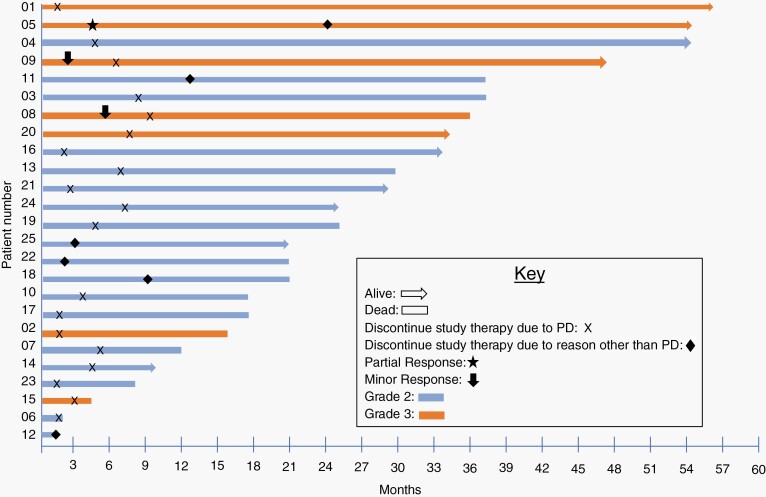
Fig. 2.
Event history plot per patient distinguished by histopathologic grade for treatment outcome.
The study was written prior to publication of the RANO meningioma criteria17 and thus utilized the RANO criteria for response assessment.16 By RANO, 1 patient (4%) achieved a near-complete radiographic response following a robust pseudoprogression that initially occurred after 2 doses of nivolumab therapy (participant 05). She underwent a subtotal resection at that time with pathologic findings revealing no evidence of active tumor and the presence of a substantial immune infiltrate as previously reported.26 Residual enhancing disease on postoperative imaging continued to gradually improve over several months resulting in classification of this patient as partial response (PR). Stable disease was the best response among 15 (60%) patients. An independent neuro-radiologist (R.Y.H.) centrally reviewed all imaging post hoc and classified response using the RANO meningioma criteria.17 Based on this analysis, 1 patient achieved a PR and 2 achieved a minor response, 1 was deemed non-evaluable, 12 patients had a best response of stable disease, and the remainder had PD. Of note, both patients who achieved a minor response had anaplastic tumors that had progressed twice prior to study enrollment.
Safety
In general, nivolumab was well tolerated and most treatment-related adverse events were not higher than grade 2 (Table 2). There were no unexpected side effects. Study therapy was interrupted in 5 patients due to adverse events that were at least possibly related to study therapy. Three of these patients successfully resumed nivolumab therapy including individual patients with grade 1 colitis, grade 2 infusion reaction, and grade 2 uveitis, respectively. The remaining 2 patients developed progressive disease before study therapy could be resumed, including 1 patient with grade 2 encephalopathy and 1 patient with grade 4 optic neuritis. There were no deaths related to study therapy, however, 1 patient with a large, skull-based atypical meningioma died from aspiration pneumonia.
Table 2.
Adverse Events ≥ Grade 2, Possibly, Probably, or Definitely Related
| Grade 2 | Grade 3 | Grade 4 | Total | |
|---|---|---|---|---|
| Endocrine | ||||
| Endocrine disorders—other | 1 | 1 | ||
| Hypothyroidism | 1 | 1 | ||
| Ophthalmic | ||||
| Optic nerve disorder | 1 | 1 | ||
| Uveitis | 1 | 1 | ||
| Gastrointestinal | ||||
| Diarrhea | 1 | 1 | ||
| Duodenal ulcer | 1a | 1 | ||
| Nausea | 1 | 1 | ||
| Pancreatitis | 1a | 1 | ||
| Vomiting | 1 | 1 | ||
| Constitutional and infusion reactions | ||||
| Fatigue | 6 | 1 | 7 | |
| Infusion-related reaction | 1 | 1 | ||
| Laboratory | ||||
| Lipase increased | 1 | 2 | 1a | 4 |
| Serum amylase increased | 1 | 1a | 2 | |
| Metabolism and nutrition | ||||
| Hyperglycemia | 2 | 2 | ||
| Nervous system | ||||
| Edema cerebral | 1 | 1 | ||
| Encephalopathy | 1 | 1 | ||
| Headache | 2 | 1 | 3 | |
| Respiratory | ||||
| Pneumonitis | 1 | 1a | 2 | |
| Total | 22 | 7 | 3 | 32 |
aOccurred during 30 days’ follow-up after study therapy discontinuation.
Neurologic Assessment Using NANO
Baseline NANO assessment at study enrollment was performed on 24 patients (96%). Among all patients, NANO was completed at 84 of 109 planned assessment time points providing an overall compliance rate of 77.1%. Among 9 patients who achieved PFS-6, 7 completed at least 70% of planned NANO assessments including baseline and off-study time points. Among these 7 patients, neurologic function remained stable by NANO measurement throughout the period of radiographic stability for 5 patients (71%). Two patients had NANO progression prior to radiographic progression, including 1 patient with NANO progression approximately 2 months prior to radiographic PD. The second patient with NANO progression without radiographic progression had strength decline to grade 3 from grade 2 at 1 time point but subsequently recovered back to grade 2 at the next evaluation time point. This patient has subsequently remained progression-free by both NANO and RANO for over 4.5 years.
An analysis of change in NANO relative to KPS at the time of progression was performed but limited by the study sample size. Among patients assessed for both KPS and NANO at progression, 1 patient had a worsened NANO score but stable KPS, while NANO and KPS were stable at progression in the remainder.
Tumor Immune Biomarker Analyses
We assessed TMB among 15 patients (60%) with adequate tumor material. Archival tumor material was used for this analysis because tumor material collected at study enrollment was not available. We found that samples from 2 patients (participants 05 and 01) exhibited an elevated TMB while the remaining 13 cases had a TMB of less than 10/Mb (median 5.32/MB; range 2.28, 9.89).
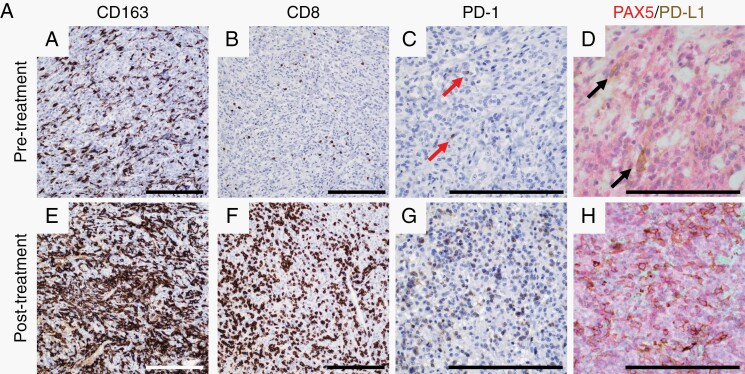
We performed immune profiling of the tumor microenvironment using IHC to quantify the percentage of immune cell subsets relative to all nucleated cells from all patients with adequate available tumor samples (n = 6; patients 03, 04, 10, 19, 22, and 24). All tumor samples used for these analyses were obtained after prior radiation therapy. Four of these patients (04, 10, 19, and 22) also had tumor samples obtained after progression on study therapy thereby providing paired tumor samples from before and after study therapy (Table 3). Among evaluated baseline tumor samples, the percentage of monocyte/macrophage cells (CD163+) was higher than CD4+ or CD8+ T cells, which in general existed at a low percentage. Most tumors also exhibited low levels of PD-1 or PD-L1 expression. For 3 patients with paired samples, there was no apparent difference in cell subsets or marker expression when comparing before and after nivolumab tumor samples. One patient (participant 19) who had a TMB of 4.8, had a marked increase in myeloid cells, CD8+ T cells, as well as expression of PD-1 and PD-L1 after anti-PD-1 therapy suggesting a possible immune activation phenomenon associated with nivolumab therapy (Figure 3A). Nonetheless, evaluation of an association between outcome and intratumoral immune cell subset levels at baseline or change after nivolumab therapy was not feasible due to the small sample size.
Table 3.
Percentage of Immune Cell Marker Positive Cell Populations
| Patient Study No. | CD163 (%) | CD8 (%) | CD4 (%) | PD-1 (%) | PAX5 (%) | PD-L1 Tumor (%) | PD-L1 Macrophage (%) | Radiographic Response | PFS (Months) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 03 | Pre | 25 | 2 | 1 | 0 | 1 | 0 | 0 | No | 7.3 |
| 03 | Post | NE | NE | NE | NE | NE | NE | NE | ||
| 24 | Pre | 10 | 5 | 2 | 1 | 1 | <1 | <1 | No | 7.4 |
| 24 | Post | NE | NE | NE | NE | NE | NE | NE | ||
| 04 | Pre | 5 | 1 | 1 | 0 | <1 | 0 | 15 | No | 5.6 |
| 04 | Post | 5 | 2 | 3 | 1 | 1 | 2 | 1 | ||
| 10 | Pre | 15 | 2 | 1 | <1 | <1 | 1 | 1 | No | 3.7 |
| 10 | Post | 15 | 5 | 1 | 0 | 1 | 0 | 1 | ||
| 19 | Pre | 15 | 2 | 1 | <1 | <1 | 2 | 1 | No | 5.4 |
| 19 | Post | 40 | 40 | 3 | 20 | 3 | 5 | 30 | ||
| 22 | Pre | 5 | 1 | <1 | <1 | 0 | 0 | 0 | No | 2.5a |
| 22 | Post | 5 | 1 | <1 | 0 | 0 | 0 | 0 |
Abbreviations: CD, cluster designation; NE, not evaluated; PAX5, paired box 5; PD-1, programmed death 1; PD-L1, programmed death ligand 1; pre, tumor sample obtained before anti-PD-1 therapy; post, tumor sample obtained after anti-PD-1 therapy.
aThis patient was censored at 2.5 months due to elective discontinuation of study therapy and continued to receive anti-PD-1 therapy on a compassionate use basis close to home without progression for 18 additional months.
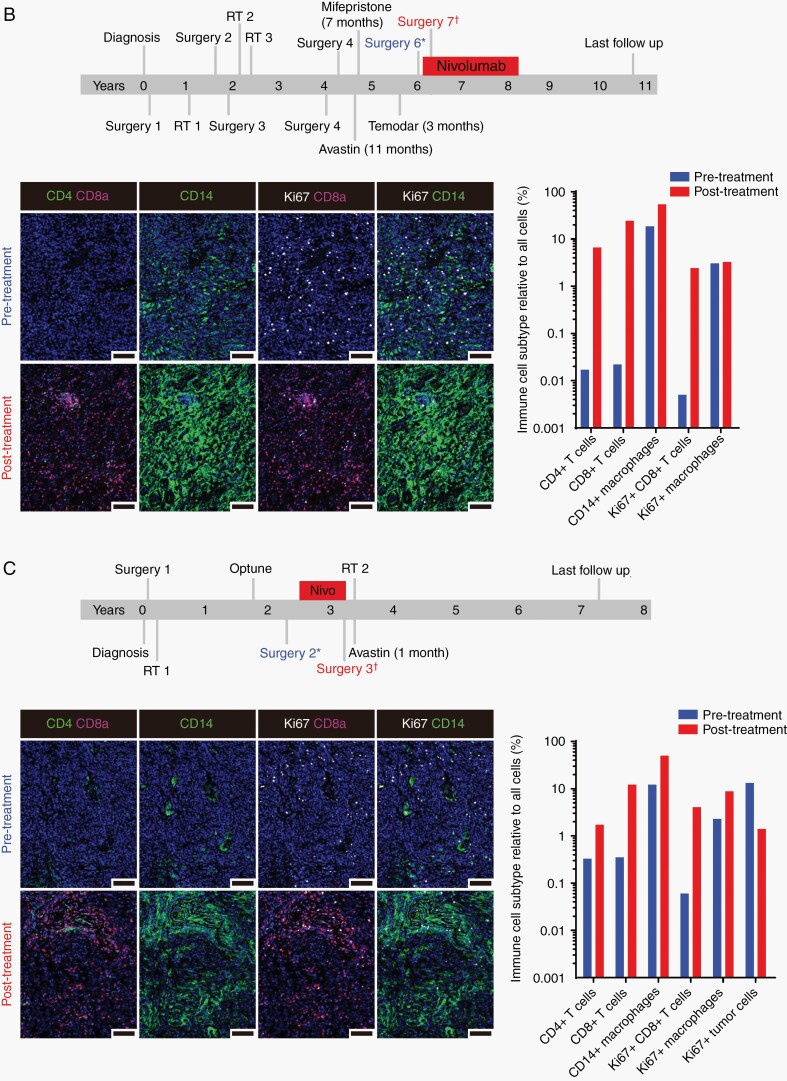
Fig. 3.
(A) Representative imaging of immune infiltrates by immunohistochemistry in paired pre- and post-study treatment samples from participant 19. The pretreated tumor sample contains moderate numbers of macrophages (a) and infrequent CD8+ T-lymphocytes (b). Rare PD-1+ lymphocytes are present (c, arrows) as are PD-L1-expressing macrophages (d, arrows). In the paired posttreatment sample, all markers are robustly increased (e–h). Scale bars = 100 µm. Treatment timeline and analysis of tissue-based multiplexed cyclic immunofluorescence (t-CyCIF) imaging from the 2 study participants with an elevated TMB including participants 05 (B) and 01 (C). Comparison of the pre- and posttreatment images showed a marked increase in CD4+ and CD8+ T cells as well as macrophages (CD14+) after nivolumab therapy. Proliferative tumor cells (CD8-, CD14-Ki67+ cells) decreased following treatment, but the proliferation of immune cell populations (CD8+ Ki67+ and CD14+ Ki67+) cells increased substantially posttreatment. Bar graphs of immune cell subtypes from before (blue bars) and after (red bars) nivolumab therapy relative to all cells (immune and nonimmune). The analysis was performed on 10 representative views (tiles). The antibodies used for this characterization are detailed in Supplementary Table 1 and analysis values are presented in Supplementary Tables 3 and 4. Scale bars = 100 µm. Abbreviations: PD-1, programmed death 1; PD-L1, programmed death ligand 1; TMB, tumor mutational burden.
We sought to better understand the tumor microenvironment among the 2 patients with high TMB (participants 05 and 01). We performed multiplexed tissue imaging (t-CyCIF) to provide a quantitative multi-marker assessment of the effects of immune checkpoint blockade on the tumor microenvironment of the paired pre- and posttreatment samples (Figure 3B and C). Of note, neither of these patients had sufficient tumor for the IHC immune analysis described above. We used a 12-plex assay that included a DNA marker (Hoechst) and 11 antibodies that mark T-cell subsets (cytotoxic, helper, and regulatory T cells), B cells, macrophages, checkpoint markers, and a marker of cell proliferation (Ki-67). We segmented the multiplexed images and measured the signal for each marker on a per cell basis. We annotated these data for cell states and quantified known immune cell subsets (Supplementary Tables 3 and 4). As previously reported, participant 05 had a heavily pretreated, recurrent grade 3 meningioma which exhibited striking elevation of TMB (38.0/Mb) that was linked with mismatch repair (MMR) deficiency from a homozygous deletion of exons 2-5 of MSH2. Of note, this patient achieved a sustained response that has been maintained for over 4.5 years after electively discontinuing study therapy following completion of 25 cycles (Figure 3B).26 Participant 01 had a recurrent grade 3 meningioma with a TMB of 19.01/Mb that exhibited multiple chromosomal abnormalities consistent with aggressive behavior including single-copy loss of chromosomes 1p, 10, 14, 18, and 22 as well as 2 copy loss of the CDKN2A/B locus on chromosome 9p (Figure 3C). Following initial resection, she developed progressive disease and underwent conventional, fractionated radiotherapy prior to developing further progressive disease. She underwent an additional surgery that confirmed recurrent tumor and enrolled in the study. She received 8 months of study nivolumab therapy before undergoing surgery due to worsened MRI changes and expressive aphasia. Histopathology of the tumor following nivolumab treatment revealed both recurrent tumor and a dense immune infiltrate. DNA sequencing did not reveal aberrations in the MLH1, MSH2, MSH6, or PMS2 genes. Postoperatively, she received additional radiotherapy followed by 3 doses of bevacizumab to treat cerebral edema and has not received any further therapy. She remains alive 57+ months from study enrollment.
Analysis of the pre- and posttreatment multiplexed t-CyCIF images from participants 05 and 01 showed a marked increase in CD4+ and CD8+ T cells as well as macrophages (CD14+) following nivolumab treatment (Figure 3B and C; Supplementary Tables 3 and 4). In the residual tumor present in the posttreatment resection from participant 01, the fraction of tumor cells that expressed the Ki67 proliferation marker decreased (16.9% before treatment to 5.8% after treatment). However, consistent with activation of an anti-tumor response, the proliferation of immune cell populations (CD8+ Ki67+ cytotoxic T cells and CD14+ Ki67+ macrophages) increased substantially posttreatment. Comparison of hematoxylin and eosin-stained tissue sections showed findings that were consistent with a pathologic complete response in participant 05 and a mixed response for participant 01 (Supplementary Figure 2). Analysis of additional t-CyCIF markers revealed an increase in B lymphocytes (CD20+), regulatory T cells (FOXP3+) as well as cells positive for PD-1 and PD-L1 expression after nivolumab therapy in posttreatment samples from both patients (Supplementary Figure 2; Supplementary Tables 3 and 4).
Discussion
We report the first clinical trial evaluating PD-1 blockade for patients with meningioma. In this open-label phase II study among patients with recurrent grade 2/3 meningioma, nivolumab monotherapy was associated with a PFS-6 rate of 42%. Although this result was higher than the 26% benchmark established by a recent meta-analysis of systemic agents,6 it did not surpass the prespecified significance threshold of 51% defined by the statistical assumptions for our sample size. Only 1 patient achieved a radiographic response, but 2 patients achieved a minor response according to the RANO meningioma criteria.17 The radiographic response occurred in a heavily pretreated patient who achieved a dramatic near complete response (CR) following initial pseudoprogression which is ongoing for over 4.5 years, as previously reported.26 Her tumor was hypermutant in association with MSH2/MSH6 loss. She electively discontinued nivolumab after 25 months and has remained progression-free for over 2 years. MMR gene/related gene deficiency as was detected in this case is rare having been detected in only 0.6% of 1080 analyzed meningiomas.26 Our responding patient suggests that the improved rate of therapeutic benefit that is the basis of FDA approval of anti-PD-1 therapy for MMR-deficient tumors27 also applies to meningioma patients with MMR-deficient tumors.
We assessed several demographic and prior treatment characteristics for association with outcome. Although this analysis was limited by our sample size, larger tumor size and skull-based location were associated with poorer outcome. Skull-based meningiomas are associated with a lower gross total resection rate compared to supratentorial tumors.28,29 In this study, we demonstrated that anti-PD-1 therapy was overall well tolerated and was not associated with unanticipated or severe adverse events.
Emerging data identify increased TMB to be associated with an improved prognosis across cancers in general30 and TMB also predicts a higher likelihood of therapeutic benefit following immune checkpoint blockade for some oncology patients.31,32 Our group and others have demonstrated that the frequency of non-synonymous mutations is increased among high-grade meningiomas compared to grade 1 meningiomas, but there is a wide range of such events across high-grade meningiomas.26,33,34 In our study, TMB was assessed among 64% (16 of 25) of tumors. TMB was low overall and was less than 10/Mb in 14 of 16 (87.5%) assessed tumor samples which is consistent with the previously published series.26,34 There was no correlation between TMB and either PFS or OS, although both patients with an elevated TMB in our study were long-term survivors and demonstrated robust infiltration by effector T cells and macrophages after anti-PD-1 therapy. In addition, proliferative tumor cells decreased while the proliferation of immune cells increased substantially following study therapy in these 2 patients.
Tumor-infiltrating lymphocytes (TIL) occur at low density among most grade 2-3 meningiomas,12,34 although a recent epigenetic analysis of 565 meningioma tumors classified 38% to an immune-enriched subset.35 In the current study, our IHC analysis of TIL density was limited by a small sample size (n = 6). Available tumor samples for this analysis were archival and thus may have not reflected the tumor microenvironment at study entry. Nonetheless, a low prevalence of CD4+ and CD8+ T cells and an elevated monocyte/macrophage infiltrate was observed in these patients which is consistent with our previously reported analysis of WHO grade 3 meningiomas.12 Among 4 patients with paired tumor samples for this analysis, one showed appreciable changes in the tumor immune microenvironment after anti-PD-1 therapy that included increases in myeloid cells and CD8+ T cells, as well as increased PD-1 and PD-L1 expression. Immune cell subset findings did not differ between responders and nonresponders, but this analysis was limited by a low number of assessed patients and markers assayed.
Our study is the first to report incorporation of the NANO scale to assess neurologic function in a clinical trial for meningioma patients. The NANO scale provides an objective, user-friendly measure of neurologic function for CNS tumor patients based on a rapidly performed and simplified neurologic examination as developed by a multidisciplinary panel of neuro-oncology experts.15 We affirmed that NANO can be efficiently integrated into the conduct of meningioma clinical trials as demonstrated by a high compliance rate in our study. We were able to objectively track neurologic function by NANO throughout the course of study therapy and demonstrated that NANO confirmed the preservation of neurologic function among nearly all patients during prolonged disease control. Of note, 1 patient exhibited neurologic decline by NANO approximately 2 months prior to demonstrating radiographic progression suggesting that NANO can be a sensitive measure of imminent treatment failure in some patients.
Several limitations are applicable to our study including lack of a randomized control arm with a primary endpoint of PFS-6, analysis of immunocorrelative analyses on a relatively small subset of enrolled patients and utilization of archival tumor samples which may have not reflected the tumor immune microenvironment at the time of study therapy. Given our small sample size, our analysis of factors associated with outcome did not adjust for multiple-testing error.
In conclusion, PD-1 blockade with nivolumab administered as monotherapy failed to increase PFS-6 in this clinical trial for unselected patients with recurrent grade 2/3 meningioma, although a subset of patients appeared to derive benefit. Nivolumab was well tolerated. The NANO scale was effectively integrated into the conduct of the study. We noted a low TIL density and low TMB as previously reported for these tumors.12,26,33,34 Two patients demonstrated an elevated TMB and both are long-term survivors. Future studies are warranted that consider exploring elevated TMB as a potential biomarker of therapeutic benefit as well as combinatorial approaches designed to enhance intratumoral infiltration and activation of lymphocytes such as integration of immune checkpoint blockade with radiation therapy. An amendment to this study to include an additional cohort of meningioma patients who will receive irradiation (initial or repeat) with nivolumab plus ipilimumab has activated to initiate accrual (NCT02648997) based on the rationale that radiation therapy may enhance anti-tumor immune responses associated with immune checkpoint blockade.36 Two other clinical trials are also evaluating such a combinatorial approach for meningioma patients (NCT03604978 and NCT04659811).
Funding
Bristol-Myers Squibb provided nivolumab. Multiplexed immunofluorescence studies were supported by National Cancer Institute Grant No. U54-CA225088 (to S.S.).
Supplementary Material
Acknowledgments
We thank Bristol-Myers Squibb for the provision of nivolumab, all clinicians and research staff involved in the study, and most importantly, all study patients and their families.
Conflict of interest statement. None declared.
Authorship statement. Conception and design: W.L.B., I.F.D., and D.A.R. Provision of study material and patients: W.L.B., L.N., E.Q.L., R.B., M.R., R.M.-F., U.C., J.S., L.D., C.T., M.C., D.L., P.Y.W., O.A.-M., E.A.C., and D.A.R. Collection and assembly of data: W.L.B., L.N., D.M.M., C.M., S.G., A.M., S.S., and D.A.R. Neuropathologic analysis: D.M.M., K.L.L., and S.S. Radiologic analysis: T.G. and D.A.R. Data analysis and interpretation: W.L.B., L.N., D.M.M., J.D., Z.D., S.H., Y.L., C.M., A.D.C., A.M., S.S., and D.A.R. Statistical analysis: W.L.B., A.M., and D.A.R. Manuscript writing: W.L.B., L.N., D.M.M., S.S., I.F.D., and D.A.R. Final approval of manuscript: W.L.B., L.N., D.M.M., J.D., Z.D., S.H., Y.L., E.Q.L., R.B., M.R., R.M.-F., U.C., C.M., S.G., A.D.C., J.S., S.K., C.T., M.C., D.L., T.G., P.Y.W., K.L.L., O.A.-M, A.M., E.A.C., S.S., I.F.D., and D.A.R.
References
- 1. Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–e391. [DOI] [PubMed] [Google Scholar]
- 2. Rogers L, Barani I, Chamberlain M, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Islim AI, Mohan M, Moon RDC, et al. Incidental intracranial meningiomas: a systematic review and meta-analysis of prognostic factors and outcomes. J Neurooncol. 2019;142(2):211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zaher A, Abdelbari Mattar M, Zayed DH, Ellatif RA, Ashamallah SA. Atypical meningioma: a study of prognostic factors. World Neurosurg. 2013;80(5):549–553. [DOI] [PubMed] [Google Scholar]
- 5. Hanft S, Canoll P, Bruce JN. A review of malignant meningiomas: diagnosis, characteristics, and treatment. J Neurooncol. 2010;99(3):433–443. [DOI] [PubMed] [Google Scholar]
- 6. Kaley T, Barani I, Chamberlain M, et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol. 2014;16(6):829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu S, Stein JE, Rimm DL, et al. Comparison of biomarker modalities for predicting response to PD-1/PD-L1 checkpoint blockade: a systematic review and meta-analysis. JAMA Oncol. 2019;5(8):1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yeung J, Yaghoobi V, Aung TN, et al. Spatially resolved and quantitative analysis of the immunological landscape in human meningiomas. J Neuropathol Exp Neurol. 2021;80(2):150–159. [DOI] [PubMed] [Google Scholar]
- 11. Han SJ, Reis G, Kohanbash G, et al. Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. J Neurooncol. 2016;130(3):543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Du Z, Abedalthagafi M, Aizer AA, et al. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget. 2015;6(7):4704–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giles AJ, Hao S, Padget M, et al. Efficient ADCC killing of meningioma by avelumab and a high-affinity natural killer cell line, haNK. JCI Insight. 2019;4(20):e130688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson MD. PD-L1 expression in meningiomas. J Clin Neurosci. 2018;57:149–151. [DOI] [PubMed] [Google Scholar]
- 15. Nayak L, DeAngelis LM, Brandes AA, et al. The Neurologic Assessment in Neuro-Oncology (NANO) scale: a tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro Oncol. 2017;19(5):625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 17. Huang RY, Bi WL, Weller M, et al. Proposed response assessment and endpoints for meningioma clinical trials: report from the Response Assessment in Neuro-Oncology Working Group. Neuro Oncol. 2019;21(1):26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramkissoon SH, Bandopadhayay P, Hwang J, et al. Clinical targeted exome-based sequencing in combination with genome-wide copy number profiling: precision medicine analysis of 203 pediatric brain tumors. Neuro Oncol. 2017;19(7):986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karczewski KJ, Francioli LC, Tiao G, et al. ; Genome Aggregation Database Consortium . The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Landrum MJ, Lee JM, Riley GR, et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014;42(Database issue):D980–D985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vokes NI, Liu D, Ricciuti B, et al. Harmonization of tumor mutational burden quantification and association with response to immune checkpoint blockade in non-small-cell lung cancer. JCO Precis Oncol. 2019;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Du Z, Lin JR, Rashid R, et al. Qualifying antibodies for image-based immune profiling and multiplexed tissue imaging. Nat Protoc. 2019;14(10):2900–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin JR, Izar B, Wang S, et al. Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes. eLife. 2018;7:e31657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dunn IF, Du Z, Touat M, et al. Mismatch repair deficiency in high-grade meningioma: a rare but recurrent event associated with dramatic immune activation and clinical response to PD-1 blockade. JCO Precis Oncol. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site - when a biomarker defines the indication. N Engl J Med. 2017;377(15):1409–1412. [DOI] [PubMed] [Google Scholar]
- 28. Meling TR, Da Broi M, Scheie D, Helseth E. Meningiomas: skull base versus non-skull base. Neurosurg Rev. 2019;42(1):163–173. [DOI] [PubMed] [Google Scholar]
- 29. Savardekar AR, Patra DP, Bir S, et al. Differential tumor progression patterns in skull base versus non-skull base meningiomas: a critical analysis from a long-term follow-up study and review of literature. World Neurosurg. 2018;112:e74–e83. [DOI] [PubMed] [Google Scholar]
- 30. Riviere P, Goodman AM, Okamura R, et al. High tumor mutational burden correlates with longer survival in immunotherapy-naive patients with diverse cancers. Mol Cancer Ther. 2021;19(10):2139–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cristescu R, Mogg R, Ayers M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362(6411). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med. 2017;377(25):2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bi WL, Greenwald NF, Abedalthagafi M, et al. Genomic landscape of high-grade meningiomas. NPJ Genom Med. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rutland JW, Gill CM, Loewenstern J, et al. NF2 mutation status and tumor mutational burden correlate with immune cell infiltration in meningiomas. Cancer Immunol Immunother. 2021;70(1):169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Choudhury A, Magill ST, Eaton CD, et al. Meningioma epigenetic grouping reveals biologic drivers and therapeutic vulnerabilities. MedRxiv. [Google Scholar]
- 36. Buchwald ZS, Wynne J, Nasti TH, et al. Radiation, immune checkpoint blockade and the abscopal effect: a critical review on timing, dose and fractionation. Front Oncol. 2018;8:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.