Abstract
Exposure of hematopoietic cells to DNA-damaging agents induces p53-independent cell cycle arrest at a G1 checkpoint. Previously, we have shown that this growth arrest can be overridden by cytokine growth factors, such as erythropoietin or interleukin-3, through activation of a phosphatidylinositol 3-kinase (PI 3-kinase)/Akt-dependent signaling pathway. Here, we show that γ-irradiated murine myeloid 32D cells arrest in G1 with active cyclin D–cyclin-dependent kinase 4 (Cdk4) but with inactive cyclin E-Cdk2 kinases. The arrest was associated with elevated levels of the Cdk inhibitors p21Cip1 and p27Kip1, yet neither was associated with Cdk2. Instead, irradiation-induced inhibition of cyclin E-Cdk2 correlated with absence of the activating threonine-160 phosphorylation on Cdk2. Cytokine treatment of irradiated cells induced Cdk2 phosphorylation and activation, and cells entered into S phase despite sustained high-level expression of p21 and p27. Notably, the PI 3-kinase inhibitor, LY294002, completely blocked cytokine-induced Cdk2 activation and cell growth in irradiated 32D cells but not in nonirradiated cells. Together, these findings demonstrate a novel mechanism underlying the DNA damage-induced G1 arrest of hematopoietic cells, that is, inhibition of Cdk2 phosphorylation and activation. These observations link PI 3-kinase signaling pathways with the regulation of Cdk2 activity.
As with other cell types, hematopoietic cells initiate apoptotic and cell cycle arrest checkpoints in response to DNA-damaging agents, such as γ irradiation (15). This has biological significance since the failure of DNA-damaged cells to die or growth arrest allows the accumulation of new mutations and may contribute to tumorigenic development (36, 45). However, both apoptotic and growth arrest responses to DNA damage in hematopoietic cells can be overridden by treatment with cytokine growth factors, such as erythropoietin (Epo) and interleukin (IL)-3. The ability of these growth factors to bypass apoptotic responses to DNA damage is likely associated with their ability to induce expression of the antiapoptotic proteins Bcl-2 and Bcl-xL (1, 40). By comparison, the mechanism by which cytokines override the growth arrest checkpoints remains undefined.
Normal progression through the mammalian cell cycle is controlled by the sequential activation of cyclin-dependent kinases (Cdks) (34). The transition from G1 to S phase is initiated by the expression of D-type cyclins and their assembly into kinase complexes with Cdk 4 (Cdk4) and Cdk6. Once activated, the cyclin D-dependent kinases phosphorylate and inactivate the tumor suppressor protein pRb (23). In early to mid-G1 phase, hypophosphorylated pRb associates with the transcription factor E2F and actively represses transcription through the recruitment of histone deacetylase. The initial phosphorylation of pRb by cyclin D-Cdk4 complexes in mid-G1 negates its ability to repress transcription and results in increased expression of cyclin E, the regulatory partner for Cdk2. Subsequently, cyclin E-Cdk2 complexes phosphorylate pRb on additional sites, causing the release of free E2F and the activated transcription of genes required for S-phase entry, including cyclin A.
Cdks are regulated by several mechanisms in addition to their association with cyclins. Activation of Cdk2 requires phosphorylation at threonine-160 by a Cdk-activating kinase (CAK), as well as the removal of inhibitory phosphorylations by Cdc25 phosphatases (34). Cdks may also be directly inhibited by association with Cdk inhibitors (CKIs), including p21Cip1 and p27Kip1 (46). Expression of p27 is induced by antiproliferative stimuli, including growth factor withdrawal, transforming growth factor beta (TGF-β), contact inhibition, and differentiation. By comparison, p21 is largely regulated by p53-dependent responses to cellular stresses, such as DNA damage.
The ability of most cells to arrest following DNA damage is dependent on the tumor suppressor p53 (29). When activated in response to a variety of cellular stresses, p53 functions as a transcription factor to induce expression of genes which elicit cell cycle arrest or programmed cell death. In so doing, p53 plays a crucial role in removing damaged cells from an organism. The importance of this response in preventing tumorigenesis is demonstrated by the frequent loss or mutation of p53 in human cancer (22). However, p53 is not commonly inactivated in leukemias (8), and DNA damage-induced cell cycle arrest checkpoints are retained in p53-deficient hematopoietic cells (40, 48). Thus, it seems likely that hematopoietic cell cycle checkpoints are mechanistically distinct.
It has previously been shown that the ability of Epo to override γ irradiation-induced growth arrest is dependent on the ability of the Epo receptor (EpoR) to activate a phosphatidylinositol 3-kinase (PI 3-kinase)/Akt signaling pathway (24, 40). For example, truncated EpoRs lacking PI 3-kinase recruitment sites retain mitogenic activity under normal culture conditions but fail to promote proliferation in irradiated myeloid cell lines. In the present study, we have examined the molecular basis of the γ irradiation-induced G1 checkpoint in hematopoietic cell lines expressing truncated EpoRs. We show that this G1 arrest correlates with induced expression of p27 and inhibits phosphorylation of Cdk2 at threonine-160. Moreover, the ability of cytokines to override this checkpoint specifically correlates with phosphorylation and activation of Cdk2 but not with altered expression of CKIs. These data define a novel DNA damage-induced checkpoint in hematopoietic cells targeting the activating phosphorylation of Cdk2.
MATERIALS AND METHODS
Cell lines and culture conditions.
32D murine myeloid cells (5) stably expressing either the wild-type (EpoR[wt]) (11)or the truncated (EpoR[H]) (33) cDNA were electroporated with 20 μg of the bcl-xL cDNA (20) in the pXM expression vector plus 2 μg of pSV2-NEO. Transfected cells were selected in 1 mg of G418/ml plus 70 pg of recombinant IL-3/ml. Clonal lines were obtained by dilution, and the constitutive expression of Bcl-xL was confirmed by Western blotting. All cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum plus either recombinant human Epo (5 U/ml) or recombinant murine IL-3 (70 pg/ml). In certain experiments, the PI 3-kinase inhibitor LY294002 (Calbiochem) was added to cell culture medium to a final concentration of 10 μM.
Flow cytometry.
Cells (n = 106) were washed with phosphate-buffered saline and were resuspended in 1 ml of propidium iodide staining solution (0.05 mg/ml propidium iodide, 01.% [wt/vol] sodium citrate, and 0.1% [wt/vol] Triton X-100) containing 2 μg of DNase-free RNase A/ml. Samples were incubated for 30 min at room temperature, and the DNA content of cells was analyzed on a FACScan (Benton Dickinson). Cell cycle distributions were determined using ModFit LT software (Verity).
Cell synchronization and γ irradiation.
Cells were synchronized in G0/G1 by culturing for 24 h in the absence of cytokine (RPMI 1640 medium supplemented with 10% fetal bovine serum). One hour prior to γ irradiation, Epo (5 U/ml) or IL-3 (1.4 ng/ml) was added to cultures as indicated. Cells were then exposed to 4 Gy of γ irradiation from a 137Cs source or were left untreated.
Immunoprecipitations.
Cells were washed twice with phosphate-buffered saline and were lysed in Tween 20 buffer (50 mM HEPES, pH 8.0, 150 mM NaCl, 1 mM EDTA, 0.1% Tween 20, 4-(2-ominoethyl)-benzenesulfonyl fluoride, pepstatin A, E-64, bestatin, leupeptin, and aprotinin) for 30 min at 4°C. Insoluble material was removed by centrifugation at 10,000 × g for 10 min. Specific proteins were precipitated with 2 μg of antiserum/ml specific for Cdk4 (Santa Cruz C-22), cyclin E (Santa Cruz M-20), or Cdk7 (Santa Cruz M-19) and were used in kinase reactions or for Western blotting.
Kinase assays.
Immune complexes were washed twice with Tween 20 buffer and twice with Cdk buffer (50 mM HEPES, pH 8.0, and 10 mM MgCl2). Glutathione S-transferase–Rb was added as a substrate for Cdk4 kinase reactions as described earlier (32). For Cdk2 kinase reactions, 10 μg of histone H1 (Roche) was added. For Cdk7 kinase reactions, 1 μg of glutathione S-transferase–Cdk2 (Santa Cruz) was used. All reactions were initiated by the addition of 0.1 pmol of ATP plus 10 μCi of [γ-32P]ATP (ICN) and proceeded at 30°C for 30 min. Reaction products were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were visualized by autoradiography or on a PhosphorImager (Molecular Dynamics).
Western blot analysis.
Total cell lysates were prepared by direct lysis in SDS-PAGE sample buffer. Lysates or immunoprecipitated proteins were resolved on SDS–12.5% PAGE gels and were transferred to nitrocellulose membrane. Membranes were probed with specific antibodies. Antibodies to Cdk2 (D-12), Cdk4 (C-22), Cdk7 (M-19), cyclin D2 (M-20), cyclin D3 (H-292), cyclin E (M-20), Kip2 p57 (M-20), Kip1 p27 (F-8), and Mat1 (FL-309) were obtained from Santa Cruz Biotechnology. Antibodies to cyclin A (Ab-3) and p21WAF1 (Ab-4) were from Oncogene Research Products. Antibodies to Rb (G3–245) were from BD Pharmingen. Cdk2 phosphorylation at tyrosine-15 was detected using antibodies raised against the conserved, phosphorylated tyrosine-15 site of Cdc2 (New England BioLabs); Cdk2 phosphorylation at threonine-160 was detected using antiserum (provided by Philipp Kaldis, NCI Frederick) raised against the phosphorylated, conserved, activating site of Cdc28p (42). Blotted proteins were visualized with Lumi-Light Blotting Reagents (Roche), as directed by the manufacturer.
RESULTS
Synchronized 32D-EpoR[H] cells arrest at a G1 checkpoint following irradiation.
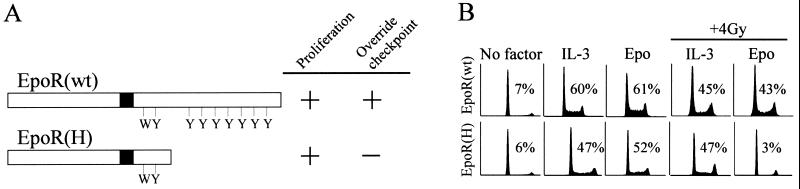
Previous studies have demonstrated that treatment of hematopoietic cells with cytokines, such as Epo or IL-3, overrides DNA damage-induced checkpoints in both G1 and G2/M phases of the cell cycle (24, 40). However, this activity is absent in cells expressing cytokine receptor mutants that are mitogenically competent but which lack a PI 3-kinase recruitment site, such as the truncated form of EpoR, EpoR[H] (Fig. 1A). In order to specifically characterize the γ irradiation-induced G1 checkpoint in hematopoietic cells, murine myeloid 32D cells expressing wild-type EpoR (32D-EpoR[wt]) or truncated EpoR (32D-EpoR[H]) were stably transfected with the antiapoptotic gene bcl-xL. Bcl-xL was used in these studies to protect against cell death during growth factor withdrawal, thereby enabling synchronization of these cells. Notably, for all assays that could be performed in the absence of synchronization, identical results were obtained using cells lacking enforced expression of Bcl-xL (data not shown). Clonal cell lines were established that could be synchronized in G0/G1 following culture for 24 h in the absence of cytokine, as indicated by the predominance of 2N DNA content in the culture (Fig. 1B). All synchronized cells could subsequently be induced to reenter the cell cycle following 24 h of treatment with Epo or IL-3. Following treatment with 4 Gy of γ irradiation, synchronized 32D-EpoR[H] cells were unable to reenter the cell cycle when cultured in Epo. The G1 arrest of this population was confirmed by a complete absence of bromodeoxyuridine labeling (not shown). By contrast, Epo efficiently induced the proliferation of irradiated 32D-EpoR[wt] cells. As a control, IL-3 induced the proliferation of all cell lines exposed to γ irradiation. Thus, synchronized 32D-EpoR[H] cells treated with Epo following γ irradiation provided a source of cells predominantly arrested at the G1 checkpoint.
FIG. 1.
Growth- and checkpoint-regulatory activities of EpoR constructs. (A) EpoR[wt] and EpoR[H] are depicted. EpoR[H] lacks the C-terminal 106 amino acids of EpoR[wt]. The positions of phosphorylable tyrosines (Y) and a conserved tryptophan (W) within the cytoplasmic domain, as well as the transmembrane domain (black box), are indicated. As previously determined (24), the ability (+) or inability (−) of each receptor to support proliferation of factor-dependent cells under normal culture conditions (Proliferation) and override DNA damage-induced cell cycle checkpoints is indicated. (B) 32D cells expressing EpoR[wt] or EpoR[H] were synchronized in G0/G1 by growth factor withdrawal (No factor). Synchronized cell cultures were supplemented with Epo or IL-3 and were either left untreated or exposed to γ irradiation (+ 4 Gy). All cultures were incubated for an additional 24 h prior to analysis by flow cytometry. Values represent the percentage of cells in S phase.
γ irradiation-induced G1 arrest of 32D cells correlates with inhibition of cyclin E-Cdk2 activity and with increased expression of p27Kip1.
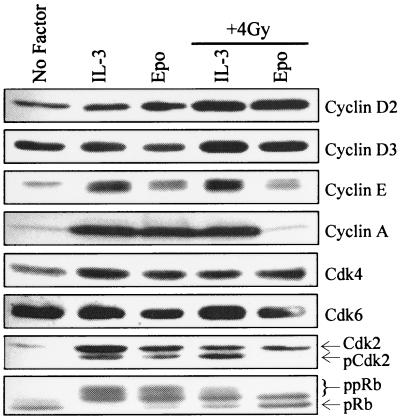
To begin characterizing the nature of the G1 cell cycle checkpoint in hematopoietic cells, the expression of common regulators of G1 cell cycle progression was evaluated. Synchronized 32D-EpoR[H] cells were restimulated with Epo or IL-3 in the presence or absence of γ irradiation, and lysates were subjected to Western blotting with specific antibodies. As shown in Fig. 2, expression of cyclins D2, D3, and E was not significantly different in Epo-treated cultures with or without γ irradiation. Cyclin D1 is not expressed in 32D cells (not shown). By contrast, irradiated cells cultured in Epo specifically lacked any detectable expression of cyclin A. Since cyclin A is first expressed in early S phase, this result is consistent with the G1 arrest of this population. Expression levels of Cdk4 and Cdk6 remained the same regardless of treatment. By contrast, two forms of Cdk2 were detected in proliferating cells, whereas only one species was expressed in growth-arrested cells. The faster-migrating form of Cdk2 (pCdk2) has been shown to result from phosphorylation on threonine-160, and it represents the active form of this kinase (21, 34). This activated form of Cdk2 was absent in cells which were arrested following irradiation (Epo, + 4 Gy), as well as in control cells which exit the cell cycle due to growth factor withdrawal (no factor).
FIG. 2.
γ irradiation-induced G1 arrest of 32D cells correlates with loss of Cdk2 threonine-160 phosphorylation and reduced pRb hyperphosphorylation. Total cell lysates were Western blotted with antibodies specific for G1 cell cycle regulators, as indicated to the right of each panel.
Cyclin A is an E2F-regulated gene whose induction depends on the sequential phosphorylation of pRb by cyclin D- and E-dependent kinases (23). To assess the status of this pathway, the phosphorylation status of pRb in each lysate was evaluated. pRb migrates as three distinct forms on denaturing gels with the fastest-migrating form representative of hypophosphorylated pRb. Two slower-migrating forms represent differentially hyperphosphorylated species (ppRb). The intermediate form of ppRb contains phosphorylations at Cdk4-specific sites, while the slowest-migrating form is phosphorylated at both Cdk4- and Cdk2-specific sites (7). As shown in Fig. 2, cells arrested by γ irradiation (Epo, + 4 Gy) contained a significant portion of hypophosphorylated pRb as well as Cdk4-phosphorylated ppRb but gave no evidence of Cdk2-phosphorylated ppRb. By comparison, irradiated cells cultured in IL-3 contained all three forms of pRb, albeit with lower levels of the Cdk2-phosphorylated form than did nonirradiated cells.
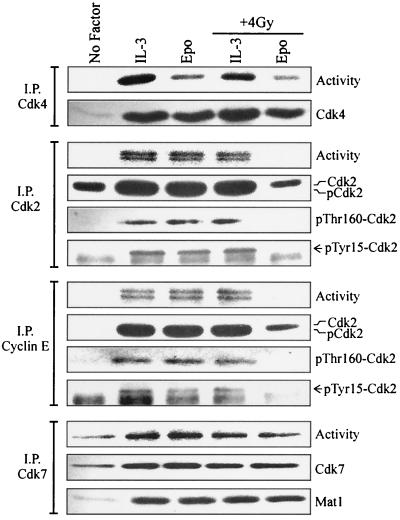
To directly assess the activity of pRb kinases in checkpoint arrested cells, Cdk4 or Cdk2 was immunoprecipitated from lysates of 32D-EpoR[H] cells and in vitro kinase reactions were performed (Fig. 3). As expected, no kinase activity was present in lysates from cells synchronized by growth factor withdrawal (no factor). Cdk4-associated kinase activity was present in lysates from arrested cells cultured in Epo following irradiation and was comparable to the activity present in lysates from cells restimulated with Epo in the absence of irradiation. Similar results were obtained when cyclin D2 complexes were assayed for kinase activity (not shown). Notably, no detectable Cdk2-associated kinase activity was present in cells arrested following irradiation (Epo, + 4 Gy). By contrast, irradiation did not inhibit IL-3-induced Cdk2 activity compared to cells restimulated with IL-3 or Epo in the absence of irradiation.
FIG. 3.
Irradiated 32D cells arrested in G1 have inactive cyclin E-Cdk2 complexes but retain active Cdk4 and Cdk7. Cell lysates from the 32D-EpoR[H] cultures represented in Fig. 1B were immunoprecipitated (I.P.) with antibodies to Cdk4, Cdk2, cyclin E, or Cdk7, and in vitro kinase assays were performed (Activity). Immunoprecipitated proteins were Western blotted with Cdk4 antibodies (Cdk4 IP), total and phosphorylation site-specific Cdk2 antibodies (Cdk2 and cyclin E IPs), or Mat1 antibodies (I.P. Cdk7).
To assess the influence of cyclin E complex formation on Cdk2 activity in irradiated cells, cyclin E-specific immunoprecipitations were Western blotted for the presence of Cdk2 (Fig. 3). Cdk2 coprecipitated with cyclin E from all cell lysates except those arrested by growth factor withdrawal. However, cyclin E immune complexes from irradiated, arrested cells (Epo, + 4 Gy) lacked any detectable kinase activity. High levels of cyclin E-associated kinase activity were obtained from all proliferating cells, including irradiated cells cultured in IL-3. Thus, γ irradiation-induced G1 arrest of 32D cells correlates with an inhibition of cyclin E-Cdk2 activity while retaining active cyclin D-Cdk4 complexes. These observations are consistent with the partially hyperphosphorylated state of pRb in these cells (Fig. 2).
Similar to the analysis of total cell lysates given for Fig. 2, anti-Cdk2 Western blotting of either Cdk2 or cyclin E immunoprecipitates appeared to detect only the slower-migrating, inactive form of Cdk2 from irradiated, arrested cells (Fig. 3; Epo, + 4 Gy), while the faster-migrating, active form (pCdk2) was present in immunoprecipitates from all proliferating cells. However, the two forms of Cdk2 were poorly resolved on SDS-PAGE gels when obtained from immunoprecipitated material. To confirm that the faster-migrating form of Cdk2 indeed corresponds to active Cdk2 phosphorylated at threonine-160, immunoprecipitated Cdk2 was Western blotted with antiserum specific for the phosphorylated, conserved activation site of Cdks. Threonine-160-phosphorylated Cdk2 was detected only in immunoprecipitates from proliferating cells, including irradiated cells cultured in IL-3. No threonine-160 phosphorylation was observed in cells arrested following irradiation (Epo, + 4 Gy). No significant differences were observed between the phosphorylated status of cyclin E-associated Cdk2 and that of the total Cdk2 population obtained from each culture.
Inhibition of Cdk2 activity may also be mediated by phosphorylation at tyrosine-15. However, no tyrosine-15 phosphorylation was detected in either Cdk2 or cyclin E immunoprecipitates from irradiated, arrested cells (Fig. 3; Epo, + 4 Gy) using phosphotyrosine-15-specific antibodies. Consistent with previous observations (21, 44), tyrosine-15 phosphorylation of Cdk2 was present in all proliferating cultures having significant S and G2 phase populations. Irradiation did not alter the amount of tyrosine-15 phosphorylation of Cdk2 or alter its distribution between total cellular versus cyclin E-associated subpopulations.
Phosphorylation of the activating threonine-160 on Cdk2 is mediated by a CAK whose identity has not been firmly established. One candidate mediator of this activity is the cyclin H-Cdk7 complex (18, 31). Cdk7-associated immune complex kinase reactions were performed from lysates of 32D-EpoR[H] cells (Fig. 3). Notably, Cdk7-associated kinase activity was present in all lysates tested, with no significant differences observed in cells arrested following irradiation compared to cycling populations. The Mat1 protein has been reported to alter the specificity of cyclin H-Cdk7 complex to favor substrates other than Cdk2 (52). As shown in Fig. 3, Mat1 was absent from active Cdk7 complexes in cells withdrawn from growth factor. However, Mat1 was associated with Cdk7 in all cytokine-treated cells, including cells arrested following irradiation and Epo treatment. Thus, there are no apparent alterations in Cdk7 activity or association with Mat1 that could account for the lack of Cdk2 phosphorylation in irradiated 32D-EpoR[H] cells cultured in Epo.
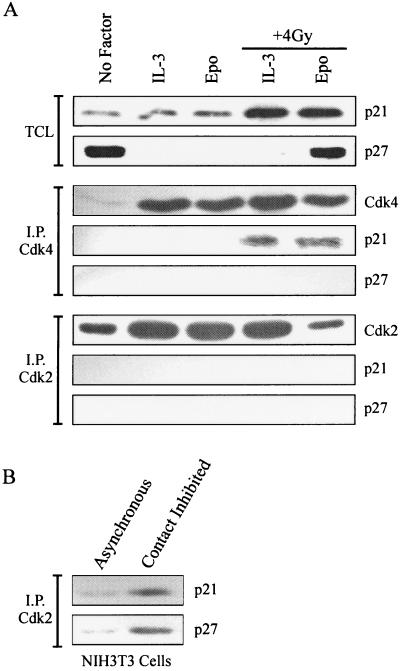
Inhibition of cyclin-Cdk activity by p21 is commonly associated with p53-dependent responses to DNA damage (29, 46). Expression of p21 and other Cip/Kip family members was evaluated by Western blot analysis of 32D-EpoR[H] cell lysates (Fig. 4). Cells synchronized in G0/G1 by growth factor withdrawal contained high levels of p27 and low levels of p21. Restimulation of cells with Epo or IL-3 significantly reduced p27 expression. Earlier studies also showed p53-dependent expression of p21 in response to DNA damage in hematopoietic cells, including 32D cells (8, 41). Indeed, p21 expression was elevated in irradiated 32D cell cultures. However, p21 expression was equivalently elevated in arrested cells (Epo, + 4 Gy) compared with proliferating cells (IL-3, + 4 Gy). By contrast, p27 expression was high in checkpoint-arrested cells (Epo, + 4 Gy) but was absent in irradiated cells cultured in IL-3. p57 expression was not detectable in any 32D cell lysates (not shown).
FIG. 4.
Expression of CKIs in irradiated 32D cells. (A) Total cell lysates (TCL) from the 32D-EpoR[H] cultures represented in Fig. 1B were Western blotted with antibodies specific for p21 or p27. Alternatively, lysates were immunoprecipitated with Cdk4 or Cdk2 antibodies, and precipitated proteins were Western blotted with antibodies specific for p21, p27, or the precipitating antibody. (B) Murine NIH 3T3 cells were grown to confluence (contact inhibited) or were collected from subconfluent cultures (asynchronous). Cell lysates were immunoprecipitated (I.P.) with Cdk2 antibodies, and precipitated proteins were Western blotted with antibodies specific for p21 or p27.
To assess the potential contribution of p21 and p27 to the inhibition of cyclin E-Cdk2 activity in checkpoint-arrested cells, Cdk-immunoprecipitated proteins were Western blotted for the presence of p21 or p27 (Fig. 4). p21 was detected in catalytically active Cdk4 immune complexes present in irradiated cells, but this inhibitor was not observed in any Cdk2 complexes analyzed. No p27 protein coprecipitated with either Cdk4 or Cdk2, regardless of treatment conditions. In keeping with these observations, we have been unable to detect p27 or p21 in cyclin E immunoprecipitations; nor was cyclin E, Cdk2, or Cdk4 present in p27 immunoprecipitates from these 32D cell lysates (not shown). To confirm that the Cdk2 antibodies and conditions used supported the precipitation of complexes with CKIs, similar immunoprecipitations were performed from lysates of murine 3T3 cells (Fig. 4B). Both p21 and p27 were readily detectable in Cdk2 immunoprecipitates from contact-inhibited 3T3 cells. As anticipated, the degree of CKI coprecipitation was significantly enhanced in contact-inhibited versus asynchronously growing cells.
Ability of cytokines to override γ irradiation-induced p27 expression and Cdk2 inactivation is dependent on a PI 3-kinase signaling pathway.
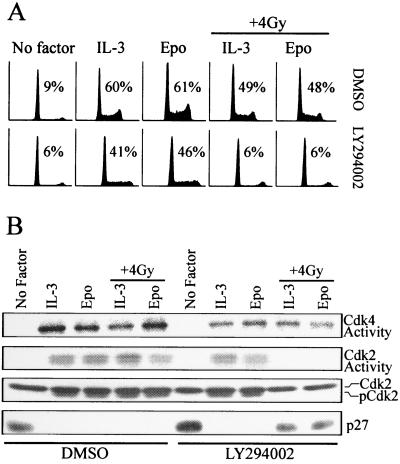
The data described above indicate that γ irradiation-induced arrest of 32D cells correlates with both inhibition of cyclin E-Cdk2 activation and elevated expression of p27 protein. In related studies, it has been recently shown that the ability of cytokines to override γ irradiation-induced cell cycle arrest requires activation of a PI 3-kinase signaling pathway (24). To determine if inhibition of this signaling pathway had similar effects on cell cycle regulators in irradiated cells, the effects of the PI 3-kinase inhibitor, LY294002, on 32D-EpoR[wt] cells were assessed. LY294002 treatment did significantly reduce the S-phase population in cytokine-treated cultures in the absence of irradiation (Fig. 5A). However, these cultures still retained greater than 40% of their population in S phase. By contrast, LY294002 completely blocked the ability of IL-3 or Epo to stimulate cell cycle progression following irradiation of synchronized cells, as indicated by the near absence of S phase. Interestingly, LY294002 treatment also abolished the ability of Epo and IL-3 to induce threonine-160 phosphorylation of Cdk2, coinciding with the inactivation of Cdk2 kinases in irradiated cells (Fig. 5B). Inhibition of PI 3-kinase in nonirradiated cells had little effect on Cdk2 activity. LY294002 also had no effect on cytokine-induced Cdk4 activity in either irradiated or nonirradiated cells. By comparison, LY294002 inhibited the Epo- and IL-3-dependent suppression of p27 expression following irradiation. Thus, the γ irradiation-induced arrest of LY294002-treated 32D-EpoR[wt] cells had a molecular phenotype identical to the arrest in 32D-EpoR[H] cells, which express receptors lacking the PI 3-kinase activation domain.
FIG. 5.
Inhibition of PI 3-kinase prevents cytokine treatment from overriding DNA damage-induced inactivation of cyclin E-Cdk2 and expression of p27. (A) 32D-EpoR[wt] cells were synchronized in G0/G1 in the absence of growth factor (No factor). Synchronized cell cultures were supplemented with LY294002 (10 μM) or vehicle control (dimethyl sulfoxide [DMSO]), plus Epo or IL-3. Parallel cultures were then left untreated or exposed to γ irradiation (+ 4 Gy). All cultures were incubated for an additional 24 h prior to analysis by flow cytometry. Values represent the percentage of cells in S phase. (B) Cell lysates prepared from the cultures represented in panel A were immunoprecipitated with Cdk4 or Cdk2 antibodies, and in vitro kinase assays were performed. Alternatively, total cell lysates were Western blotted with antibodies specific for Cdk2 or p27.
Cytokine-induced release from the G1 checkpoint correlates with phosphorylation and activation of Cdk2.
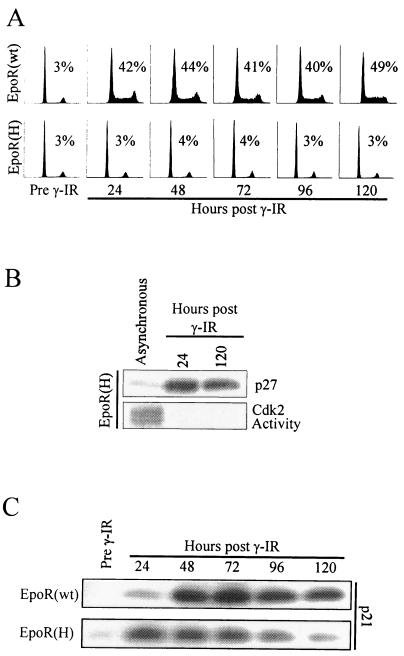
Together, the data above indicate that through activation of a PI 3-kinase signaling pathway, cytokines override DNA damage-induced expression of p27 and inactivation of Cdk2. To determine which of these activities is most closely associated with the G1 arrest in 32D cells, we examined the stability of and the kinetics of release from this checkpoint. To first assess the permanence of the cell cycle block, 32D-EpoR[H] and 32D-EpoR[wt] cells were irradiated and cultured in Epo for up to 5 days. As shown in Fig. 6A, irradiated 32D-EpoR[H] cells failed to progress out of G1 for the entire 5-day period. These cells remained viable during this time period, but by day 9 the viability of this culture rapidly declined (not shown). By contrast, irradiated 32D-EpoR[wt] cells continued to proliferate over the same time course. Cell lysates prepared from each culture at 24-h intervals were assayed for p21-, p27-, and Cdk2-associated kinase activity. Growth-arrested 32D-EpoR[H] cells showed elevated p27 expression and no Cdk2-associated kinase activity up to 5 days following irradiation (Fig. 6B), consistent with their inability to proliferate. Conversely, proliferating 32D-EpoR[wt] cells retained active Cdk2 and showed no increased p27 expression through the 5-day time course (not shown). Interestingly, irradiation-induced p21 expression displayed different kinetics in the proliferating versus arrested cell cultures (Fig. 6C). Arrested 32D-EpoR[H] cells had their highest level of p21 expression within the first 24 h following irradiation, after which p21 expression steadily diminished to background levels. In irradiated 32D-EpoR[wt] cells, p21 levels continued to increase up to 48 h following irradiation and remained high through the remainder of the time course. Irradiated 32D-EpoR[H] cells cultured in IL-3 remained asynchronous and displayed a pattern of p21 expression identical to that of 32D-EpoR[wt] cells cultured in Epo (not shown). Together, these data show that while p21 expression declined in arrested 32D-EpoR[H] cells, p27 expression remained high and Cdk2 remained inactive.
FIG. 6.
Analysis of G1 regulators in a long-term, γ irradiation-induced arrest. (A) 32D cells expressing EpoR[wt] or EpoR[H] were synchronized in G0/G1 by growth factor withdrawal (Pre γ-IR), and the cultures were then supplemented with Epo and exposed to 4 Gy of γ irradiation. Samples of each culture were assayed by flow cytometry at 24-h intervals following γ irradiation treatment. (B) Cell lysates were prepared from the 24- and 120-h post-γ irradiation cultures of 32D-EpoR[H] cells represented in panel A. Total cell lysates were Western blotted with anti-p27 antibodies. Alternatively, Cdk2 was immunoprecipitated, and in vitro kinase reactions were performed. For comparison, a lysate from asynchronously growing 32D-EpoR[H] cells was also assayed. (C) Cell lysates from the cultures represented in panel A were Western blotted with anti-p21 antibodies.
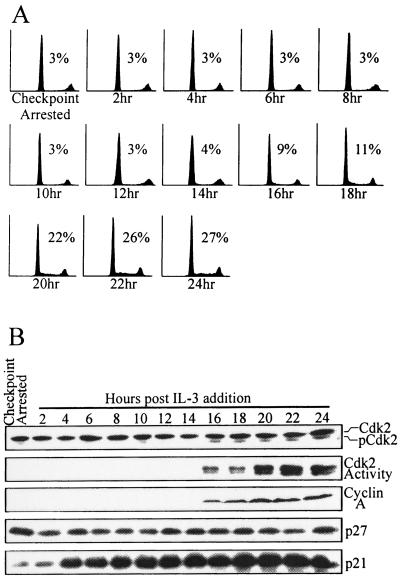
We next tested whether reversal of p27 expression or Cdk2 inactivation was more closely associated with a release from an established G1 checkpoint. Synchronized 32D-EpoR[H] cells remained uniformly arrested in G1 phase when cultured in Epo for 24 h following γ irradiation (Fig. 7A; Checkpoint Arrested). Arrested cells were then induced to override the G1 checkpoint by addition of IL-3 and began exiting G1 phase after 16 h, as indicated by the increasing percentage of S-phase cells. Lysates from cells at various time points following restimulation with IL-3 were assayed for the activities of cell cycle regulators (Fig. 7B). Cdk2 activity was undetectable up to 14 h following IL-3 restimulation, as was cyclin A expression, a marker of S-phase entry. Concurrent with the appearance of increasing S-phase populations (Fig. 7A), cells restimulated with IL-3 for 16 h contained detectable levels of Cdk2 phosphorylated at threonine-160 (pCdk2), Cdk2-associated kinase activity, and cyclin A expression. Each of these activities increased through 24 h of IL-3 restimulations in parallel with the increase in S-phase content of this culture. By comparison, expression of p27 protein showed little if any change during the 24-h period following IL-3 addition and remained elevated for up to 36 h (latter time point not shown). Consistent with earlier results (Fig. 6C), p21 protein levels were upregulated by IL-3 treatment throughout the entire time course in a manner that did not correspond to cell cycle entry.
FIG. 7.
Kinetics of cytokine-induced release from the G1 checkpoint. (A) 32D-EpoR[H] cells were synchronized in G0/G1 in the absence of growth factor. The culture was then supplemented with Epo and exposed to 4 Gy of γ irradiation. This culture was then incubated for 24 h to allow cells to arrest at the G1 checkpoint (Checkpoint Arrested) and was then supplemented with IL-3. At 2-h intervals after IL-3 addition, samples of the culture were obtained and were analyzed by flow cytometry. Values indicate the percentage of cells in S phase for each time point. (B) Cell lysates from the cultures represented in panel A were Western blotted with antibodies specific for Cdk2, cyclin A, p27, or p21. Alternatively, Cdk2 was immunoprecipitated, and in vitro kinase reactions were performed.
DISCUSSION
Normal cellular responses to DNA-damaging agents include cell cycle arrest in G1 and G2/M phases. Previously, we have shown that treatment of hematopoietic cell lines with growth-promoting cytokines can override these cell cycle checkpoints (40). Moreover, the ability of cytokines to override these checkpoints is dependent on their ability to activate a PI 3-kinase/Akt signaling pathway (24). In the present study, we report that the DNA damage-induced G1 arrest in hematopoietic cells correlates with the absence of Cdk2 phosphorylation at threonine-160 and that this appears to be a target of the cytokine-activated PI 3-kinase signaling pathway.
In many cell types, DNA damage-induced G1 arrest is associated with p53-dependent expression of p21 and with subsequent inhibition of cyclin-Cdk complexes. In murine embryonic fibroblasts, p21 expression is required for G1 arrest in response to irradiation and results in specific inhibition of cyclin E-Cdk2 but not cyclin D-Cdk4 (7). This differential inhibition of Cdk2 versus Cdk4 is similar to the irradiation-induced arrest in 32D cells. However, hematopoietic cells lacking functional p53 still retain the ability to arrest in response to DNA damage (40, 48), suggesting that other arrest mediators are functional in these cells. In fact, the results presented here show that γ irradiation does induce expression of p21 in 32D cells, but p21 levels are equally high in cells arrested at the G1 checkpoint and in those in which the arrest is overridden by cytokine treatment. Thus, p21 expression does not appear to be sufficient to induce arrest of hematopoietic cells treated with growth-promoting cytokines. Moreover, p21 expression decreased to near-background levels in cells that remained arrested 5 days after irradiation, indicating that p21 is not likely to contribute to the long-term maintenance of the G1 checkpoint.
In a separate study, exposure of hematopoietic cells to low doses of γ irradiation caused a transient slowing in G1-to-S phase progression when cells were cultured in IL-3, and this slowing could be alleviated by overexpression of Cdk4 (41). Similar to our findings, p21 was found associated with cyclin D-Cdk4 but not cyclin E-Cdk2 in irradiated cells. Notably, Cdk4 is not as effectively inhibited by p21 as is Cdk2 (46). Thus, cytokine-treated hematopoietic cells appear to maintain a sufficient pool of Cdk4 to sequester p21 and prevent inhibition of Cdk2 following irradiation. However, this apparent ability to sequester p21 is not sufficient to prevent a γ irradiation-induced arrest in the absence of PI 3-kinase activity.
Unlike p21, increased p27 expression did correlate with the establishment of the G1 arrest following γ irradiation. However, we have not been able to demonstrate the presence of p27 in any cyclin-Cdk complexes derived from irradiated or nonirradiated 32D cell lysates. Moreover, there was no apparent decrease in p27 expression as cells progressed into S phase following cytokine-induced release from the G1 checkpoint (Fig. 7B). It is not surprising that a decrease in p27 expression was not detectable prior to activation of Cdk2 in this experiment, since only a small percentage of cells begins to enter S phase 16 h following release from the checkpoint. However, by 24 h, 42% of the culture had exited G1 phase and still there was no detectable reduction in p27 expression. Thus, it does not appear that significant reduction in p27 expression is required for cytokine treatment to override the G1 checkpoint. There is also a notable difference in the pattern of p27 expression in cells that initially arrest and are subsequently released from the G1 checkpoint, compared to cells that never arrest following irradiation. Specifically, p27 levels are low in cells that do not arrest, whereas p27 levels remain high in cells released from an established G1 arrest (compare Fig. 4 and 7). The basis for this distinction remains unclear at present, but it raises the question of whether p27 plays any functional role in γ irradiation-induced arrest of hematopoietic cells. There is no prior association of p27 with established models of DNA damage response pathways, although deficiencies in p27 expression have been associated with an increased susceptibility to irradiation-induced tumor formation in mice (17). However, no significant increase in leukemias or tumors derived from hematopoietic compartments were found in p27-null mice.
The γ irradiation-induced G1 arrest of 32D cells was consistently associated with a complete lack of Cdk2 activity despite the continued presence of cyclin E-Cdk2 complexes. Recently, degradation of Cdc25A and persistent inhibitory phosphorylation of Cdk2 were shown to mediate a UV-induced, p53-independent G1 arrest of an osteosarcoma cell line (30). However, we have not observed any detectable phosphorylation of Cdk2 at inhibitory sites in irradiated, arrested 32D cells (Fig. 3). We have also not observed any detectable reduction in Cdc25A protein levels in these cells (not shown). Thus, this novel DNA damage checkpoint mechanism does not appear to be activated in γ-irradiated 32D cells.
The absence of an activating phosphorylation at threonine-160 of Cdk2 consistently correlated with establishment of the irradiation-induced G1 arrest. Moreover, Cdk2 phosphorylation and activation are the earliest events that we have observed following release from this checkpoint in response to cytokine treatment. Phosphorylation of Cdk2 is mediated by a CAK. Association of Cdks with either p21 or p27 has been reported to inhibit their phosphorylation by CAK (4, 25, 28, 47). Thus, it remains possible that Cip/Kip family members play a role in preventing access to threonine-160 of Cdk2 in irradiated 32D cells, despite our inability to detect p21 or p27 in cyclin E-Cdk2 complexes from these cells. Notably, in vitro CAK phosphorylation of Cdk4 has been found to be inhibited by stoichiometric association with p27, which could be overcome by increased concentrations of Cdk4 in the reaction (28). We have tested for a similar effect in 32D cells by enforced overexpression of Cdk2. However, 32D-EpoR[H] cells expressing up to sixfold-higher levels of Cdk2 still completely arrest in G1 phase with inactive Cdk2 following γ irradiation when cultured in Epo (A. K. Eapen, unpublished data). This implies that a more significant change in stoichiometry must be required, if indeed p27 is inhibiting Cdk2 activation in irradiated 32D cells. However, the lack of any detectable reduction in p27 expression as irradiated 32D cells reenter cycle (Fig. 7) does not support such a possibility, unless subcellular relocalization of p27 plays a significant role. Based on our observations, a more plausible explanation may be that cytokine-activated signaling pathways more directly regulate Cdk2 phosphorylation through presently undefined mechanisms.
Initially, a mammalian CAK was identified as the cyclin H-Cdk7 complex based on its ability to phosphorylate Cdk2 in vitro (18, 31). However, the Cdk7-associated kinase activity was not found to vary in a cell cycle-dependent manner in vivo. Other work suggests that addition of Mat1 to this complex can shift its specificity from Cdk2 to other substrates, including RNA polymerase II and pRB (52). In the present study, we found no alteration in Cdk7-associated kinase activity or levels of Cdk7-associated Mat1 in irradiated versus nonirradiated cells. Thus, it seems unlikely that the lack of threonine-160 phosphorylated Cdk2 in checkpoint-arrested 32D cells results from altered cyclin H-Cdk7 activity or specificity.
In yeast, CAK activity is not a function of the Cdk7 homolog Kin28. Instead, it is mediated by a monomeric kinase, Cak1 (16, 27, 51). Although a mammalian homolog of Cak1 has not been conclusively identified, a similar activity has been observed in lysates of human cells (26) and may be downregulated in a TGF-β-induced G1 arrest (35). Notably, the TGF-β-induced arrest was associated with active cyclin D-Cdk4 complexes and inactive cyclin E-Cdk2 complexes in which Cdk2 lacked phosphorylation at threonine-160. This is similar to the irradiation-induced arrest in 32D cells. Thus, it is tempting to speculate that these two growth inhibitory responses may be mediated through inactivation of the same or similar CAK activity.
Alternatively, the lack of Cdk2 phosphorylation in irradiated 32D cells could result from activation of a phosphatase. Phosphatase activity toward threonine-160 has been associated with the Cdk-associated phosphatase (37) and members of the protein phosphatase 2C family (9). However, activity for both the Cdk-associated phosphatase and protein phosphatase 2C is restricted to monomeric Cdk2 and would likely not be effective against the cyclin E-Cdk2 complexes present in G1-arrested 32D cells. Since there is no prior evidence that a CAK is inhibited or a phosphatase activated in response to DNA damage in other cell types, it is possible that this response to DNA damage is a unique property of hematopoietic cells.
Growth-promoting cytokines, such as Epo and IL-3, normally regulate progression through early G1 phase in factor-dependent hematopoietic cells. Previously, the normal growth response to cytokines has been most closely associated with induced expression of D-type cyclins and the inhibited expression of p27 (2, 3, 6). Indeed, these activities can be regulated through PI 3-kinase pathways in response to cytokines and other growth factors. PI 3-kinase activity can contribute to induced expression of D-type cyclins (19) and to increased cyclin D1 stability through Akt-dependent phosphorylation of glycogen synthase kinase 3β (13). Also, PI 3-kinase activation can effectively downregulate expression of p27, since various inhibitors of PI 3-kinase pathways have been shown to cause enhanced expression of p27 protein (6, 14, 49, 50).
Through these or other activities, PI 3-kinase activation clearly contributes to the efficiency of cytokine-induced cell cycle progression. Notably, cells expressing truncated EpoR or those treated with PI 3-kinase inhibitors (e.g., LY294002) reproducibly exhibit a reduced S-phase population compared to cells growing under control of wild-type cytokine receptors (approximately 40% S phase compared to 60% S phase, respectively). Yet, under normal culture conditions, cytokine receptors lacking the ability to directly activate PI 3-kinase do support long-term proliferation of hematopoietic cells (10, 12, 33, 38, 39, 43). By contrast, the ability of cytokines to override DNA damage-induced cell cycle checkpoints in both G1 and G2 phases is absolutely dependent on their ability to activate PI 3-kinase (24). We now find that following DNA damage, the most demonstrable effect of cytokine-induced PI 3-kinase activation is the maintenance of Cdk2 in its phosphorylated, active state. Possible effects on cyclin D stability do not appear to be significant since cyclin D-Cdk4 complexes remain active in irradiated, arrested cells. Similarly, the lack of detectable reductions in p27 expression as irradiated, arrested 32D cells reenter the cell cycle suggests that inhibited p27 expression is not a major effect of PI 3-kinase activation in this context. Instead, the ability to regulate Cdk2 phosphorylation appears to represent a novel activity for the PI 3-kinase signaling pathway. However, there remains a significant 16-h time lag between cytokine treatment and the appearance of detectable Cdk2 activity in irradiated cells. This suggests that multiple steps connect PI 3-kinase activation to the phosphorylation of Cdk2 in irradiated, hematopoietic cells.
ACKNOWLEDGMENTS
We thank Philipp Kaldis (NCI Frederick) for providing anti-threonine-160-Cdk2 antiserum. This work was performed with assistance from the Flow Cytometry Facility (University of Iowa), a 137Cs source operated by James W. Osborne (Department of Radiation Biology, University of Iowa), and core facilities of the Diabetes and Endocrinology Research Center at The University of Iowa.
D. E. Quelle was supported by a grant from the American Cancer Society (RPG-98–254-01-MGO). This work was supported by a Howard Hughes Medical Institute Biomedical Research Support Program grant and by Public Health Service grant CA-79889 from the National Cancer Institute.
REFERENCES
- 1.Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Ando K, Ajchenbaum-Cymbalista F, Griffin J D. Regulation of G1/S transition by cyclins D2 and D3 in hematopoietic cells. Proc Natl Acad Sci USA. 1993;90:9571–9575. doi: 10.1073/pnas.90.20.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ando K, Griffin J D. Cdk4 integrates growth stimulatory and inhibitory signals during G1 phase of hematopoietic cells. Oncogene. 1995;10:751–755. [PubMed] [Google Scholar]
- 4.Aprelikova O, Xiong Y, Liu E T. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J Biol Chem. 1995;270:18195–18197. doi: 10.1074/jbc.270.31.18195. [DOI] [PubMed] [Google Scholar]
- 5.Askew D S, Ashmun R A, Simmons B C, Cleveland J L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–1922. [PubMed] [Google Scholar]
- 6.Brennan P, Babbage J W, Burgering B M, Groner B, Reif K, Cantrell D A. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–689. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 7.Brugarolas J, Moberg K, Boyd S D, Taya Y, Jacks T, Lees J A. Inhibition of cyclin-dependent kinase 2 by p21 is necessary for retinoblastoma protein-mediated G1 arrest after gamma-irradiation. Proc Natl Acad Sci USA. 1999;96:1002–1007. doi: 10.1073/pnas.96.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canman C E, Gilmer T M, Coutts S B, Kastan M B. Growth factor modulation of p53-mediated growth arrest versus apoptosis. Genes Dev. 1995;9:600–611. doi: 10.1101/gad.9.5.600. [DOI] [PubMed] [Google Scholar]
- 9.Cheng A Y, Kaldis P, Solomon M J. Dephosphorylation of human cyclin-dependent kinases by protein phosphatase type 2C alpha and beta 2 isoforms. J Biol Chem. 2000;275:34744–34749. doi: 10.1074/jbc.M006210200. [DOI] [PubMed] [Google Scholar]
- 10.Damen J E, Cutler R L, Jiao H, Yi T, Krystal G. Phosphorylation of tyrosine 503 in the erythropoietin receptor (EpR) is essential for binding the P85 subunit of phosphatidylinositol (PI) 3-kinase and for EpR-associated PI 3-kinase activity. J Biol Chem. 1995;270:23402–23408. doi: 10.1074/jbc.270.40.23402. [DOI] [PubMed] [Google Scholar]
- 11.D'Andrea A D, Lodish H F, Wong G G. Expression cloning of the murine erythropoietin receptor. Cell. 1989;57:277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- 12.D'Andrea A D, Yoshimura A, Youssoufian H, Zon L I, Koo J W, Lodish H F. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth-regulatory domains. Mol Cell Biol. 1991;11:1980–1987. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diehl J A, Cheng M, Roussel M F, Sherr C J. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dijkers P F, Medema R H, Pals C, Banerji L, Thomas N S, Lam E W, Burgering B M, Raaijmakers J A, Lammers J W, Koenderman L, Coffer P J. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1) Mol Cell Biol. 2000;20:9138–9148. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 16.Espinoza F H, Farrell A, Erdjument-Bromage H, Tempst P, Morgan D O. A cyclin-dependent kinase-activating kinase (CAK) in budding yeast unrelated to vertebrate CAK. Science. 1996;273:1714–1717. doi: 10.1126/science.273.5282.1714. [DOI] [PubMed] [Google Scholar]
- 17.Fero M L, Randel E, Gurley K E, Roberts J M, Kemp C J. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher R P, Morgan D O. A novel cyclin associates with MO15/Cdk7 to form the CDK-activating kinase. Cell. 1994;78:713–724. doi: 10.1016/0092-8674(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 19.Gille H, Downward J. Multiple ras effector pathways contribute to G(1) cell cycle progression. J Biol Chem. 1999;274:22033–22040. doi: 10.1074/jbc.274.31.22033. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Garcia M, Perez-Ballestero R, Ding L, Duan L, Boise L H, Thompson C B, Nunez G. bcl-XL is the major bcl-x mRNA form expressed during murine development and its product localizes to mitochondria. Development. 1994;120:3033–3042. doi: 10.1242/dev.120.10.3033. [DOI] [PubMed] [Google Scholar]
- 21.Gu Y, Rosenblatt J, Morgan D O. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992;11:3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hainaut P, Hernandez T, Robinson A, Rodriguez-Tome P, Flores T, Hollstein M, Harris C C, Montesano R. IARC Database of p53 gene mutations in human tumors and cell lines: updated compilation, revised formats and new visualisation tools. Nucleic Acids Res. 1998;26:205–213. doi: 10.1093/nar/26.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harbour J W, Dean D C. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 2000;14:2393–2409. doi: 10.1101/gad.813200. [DOI] [PubMed] [Google Scholar]
- 24.Henry, M. K., J. T. Lynch, A. K. Eapen, and F. W. Quelle. DNA damage-induced cell cycle arrest of hematopoietic cells is overridden by activation of the PI-3 kinase/Akt signaling pathway. Blood, in press. [DOI] [PubMed]
- 25.Hitomi M, Shu J, Agarwal M, Agarwal A, Stacey D W. p21Waf1 inhibits the activity of cyclin dependent kinase 2 by preventing its activating phosphorylation. Oncogene. 1998;17:959–969. doi: 10.1038/sj.onc.1202005. [DOI] [PubMed] [Google Scholar]
- 26.Kaldis P, Solomon M J. Analysis of CAK activities from human cells. Eur J Biochem. 2000;267:4213–4221. doi: 10.1046/j.1432-1327.2000.01455.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaldis P, Sutton A, Solomon M J. The Cdk-activating kinase (CAK) from budding yeast. Cell. 1996;86:553–564. doi: 10.1016/s0092-8674(00)80129-4. [DOI] [PubMed] [Google Scholar]
- 28.Kato J Y, Matsuoka M, Polyak K, Massague J, Sherr C J. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 29.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 30.Mailand N, Falck J, Lukas C, Syljuasen R G, Welcker M, Bartek J, Lukas J. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288:1425–1429. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- 31.Makela T P, Tassan J P, Nigg E A, Frutiger S, Hughes G J, Weinberg R A. A cyclin associated with the CDK-activating kinase MO15. Nature. 1994;371:254–257. doi: 10.1038/371254a0. [DOI] [PubMed] [Google Scholar]
- 32.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J-Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura O, D'Andrea A, Kabat D, Ihle J N. Induction of tyrosine phosphorylation by the erythropoietin receptor correlates with mitogenesis. Mol Cell Biol. 1991;11:4895–4902. doi: 10.1128/mcb.11.10.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan D O. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 35.Nagahara H, Ezhevsky S A, Vocero-Akbani A M, Kaldis P, Solomon M J, Dowdy S F. Transforming growth factor beta targeted inactivation of cyclin E: cyclin-dependent kinase 2 (Cdk2) complexes by inhibition of Cdk2 activating kinase activity. Proc Natl Acad Sci USA. 1999;96:14961–14966. doi: 10.1073/pnas.96.26.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paulovich A G, Toczyski D P, Hartwell L H. When checkpoints fail. Cell. 1997;88:315–321. doi: 10.1016/s0092-8674(00)81870-x. [DOI] [PubMed] [Google Scholar]
- 37.Poon R Y, Hunter T. Dephosphorylation of Cdk2 Thr160 by the cyclin-dependent kinase-interacting phosphatase KAP in the absence of cyclin. Science. 1995;270:90–93. doi: 10.1126/science.270.5233.90. [DOI] [PubMed] [Google Scholar]
- 38.Quelle D E, Wojchowski D M. Localized cytosolic domains of the erythropoietin receptor regulate growth signaling and down-modulate responsiveness to granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci USA. 1991;88:4801–4805. doi: 10.1073/pnas.88.11.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quelle F W, Wang D, Nosaka T, Thierfelder W E, Stravopodis D, Weinstein Y, Ihle J N. Erythropoietin induces activation of Stat5 through association with specific tyrosines on the receptor that are not required for a mitogenic response. Mol Cell Biol. 1996;16:1622–1631. doi: 10.1128/mcb.16.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quelle F W, Wang J, Feng J, Wang D, Cleveland J L, Ihle J N, Zambetti G P. Cytokine rescue of p53-dependent apoptosis and cell cycle arrest is mediated by distinct Jak kinase signaling pathways. Genes Dev. 1998;12:1099–1107. doi: 10.1101/gad.12.8.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reed M F, Liu V F, Ladha M H, Ando K, Griffin J D, Weaver D T, Ewen M E. Enforced CDK4 expression in a hematopoietic cell line confers resistance to the G1 arrest induced by ionizing radiation. Oncogene. 1998;17:2961–2971. doi: 10.1038/sj.onc.1202450. [DOI] [PubMed] [Google Scholar]
- 42.Ross K E, Kaldis P, Solomon M J. Activating phosphorylation of the Saccharomyces cerevisiae cyclin-dependent kinase, cdc28p, precedes cyclin binding. Mol Biol Cell. 2000;11:1597–1609. doi: 10.1091/mbc.11.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato N, Sakamaki K, Terada N, Arai K, Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common beta subunit responsible for different signaling. EMBO J. 1993;12:4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sexl V, Diehl J A, Sherr C J, Ashmun R, Beach D, Roussel M F. A rate limiting function of cdc25A for S phase entry inversely correlates with tyrosine dephosphorylation of Cdk2. Oncogene. 1999;18:573–582. doi: 10.1038/sj.onc.1202362. [DOI] [PubMed] [Google Scholar]
- 45.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 46.Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 47.Smits V A, Klompmaker R, Vallenius T, Rijksen G, Makela T P, Medema R H. p21 inhibits Thr161 phosphorylation of Cdc2 to enforce the G2 DNA damage checkpoint. J Biol Chem. 2000;275:30638–30643. doi: 10.1074/jbc.M005437200. [DOI] [PubMed] [Google Scholar]
- 48.Strasser A, Harris A W, Jacks T, Cory S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 1994;79:329–339. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 49.Sun H, Lesche R, Li D M, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA. 1999;96:6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takuwa N, Takuwa Y. Ras activity late in G1 phase required for p27kip1 downregulation, passage through the restriction point, and entry into S phase in growth factor-stimulated NIH 3T3 fibroblasts. Mol Cell Biol. 1997;17:5348–5358. doi: 10.1128/mcb.17.9.5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thuret J Y, Valay J G, Faye G, Mann C. Civ1 (CAK in vivo), a novel Cdk-activating kinase. Cell. 1996;86:565–576. doi: 10.1016/s0092-8674(00)80130-0. [DOI] [PubMed] [Google Scholar]
- 52.Yankulov K Y, Bentley D L. Regulation of CDK7 substrate specificity by MAT1 and TFIIH. EMBO J. 1997;16:1638–1646. doi: 10.1093/emboj/16.7.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]