ABSTRACT.
Antibiotics are recommended by the WHO as part of the management of uncomplicated severe acute malnutrition in children. We evaluated whether azithromycin, an antibiotic with antimalarial properties, improved malarial parasitemia outcomes in children with severe acute malnutrition compared with amoxicillin, an antibiotic commonly used for severe acute malnutrition that does not have antimalarial properties. Total of 301 children were randomized (1:1) to a single oral dose of azithromycin or a 7-day course of amoxicillin and followed for 8 weeks. We found no significant evidence that children receiving azithromycin had improved parasitemia outcomes relative to amoxicillin. Although azithromycin may have advantages over amoxicillin in terms of dosing and administration for uncomplicated severe acute malnutrition, it may not yield additional benefit for malaria outcomes.
INTRODUCTION
WHO guidelines for uncomplicated severe acute malnutrition include treatment with a broad-spectrum antibiotic due to the potential for asymptomatic infection when the immune system is suppressed.1,2 In many settings, the most commonly-used antibiotic is a 5- to 7-day course of amoxicillin. The evidence base for the use of antibiotics in uncomplicated severe acute malnutrition is mixed, with one study in Niger demonstrating no significant benefit of amoxicillin and another in Malawi showing significantly improved outcomes in children receiving amoxicillin versus placebo.3,4 Routine antibiotics for uncomplicated severe acute malnutrition may be beneficial in treating asymptomatic infection, however may unnecessarily contribute to selection for antibiotic resistance if used at large scale in children without established infection. Other classes of antibiotics may have additional benefits in children with severe acute malnutrition. Azithromycin has been previously shown to have some activity against the apicoplast in Plasmodium falciparum in vitro.5,6 In population-based studies, a reduction in malaria parasitemia and mortality has been observed in communities receiving mass azithromycin distribution compared with placebo.7,8 Although azithromycin is not adequate as monotherapy for treatment of malaria, it may provide some protection against malaria infection during recovery from severe acute malnutrition.
Here, we present results of a prespecified secondary analysis of a trial of azithromycin versus amoxicillin for severe acute malnutrition, comparing malarial parasitemia 8 weeks following admission to an outpatient nutritional program in Burkina Faso. We then evaluated whether children with malarial parasitemia at baseline had a differential response to azithromycin versus amoxicillin treatment in anthropometric outcomes over their 8-week treatment course. We hypothesized that malarial parasitemia would be lower in children receiving azithromycin compared with amoxicillin, and that children with malarial parasitemia at baseline would have greater response to treatment if they received azithromycin compared with amoxicillin.
MATERIALS AND METHODS
Complete methods for the trial have been previously reported.9 In brief, we conducted a 1:1 randomized controlled trial of single dose oral azithromycin (20 mg/kg) compared a 7-day course of oral amoxicillin (standard of care) among children aged 6–59 months with uncomplicated severe acute malnutrition. This study was conducted in six Centers de Santé et de Promotion Sociale in Boromo District, Burkina Faso, which are primary healthcare facilities that offer basic preventative and curative care, including an outpatient nutritional program. The study was reviewed and approved by the Institutional Review Boards at the University of California, San Francisco and the Comité Institutionnel d’Ethique at the Center de Recherche en Santé de Nouna. Written informed consent was obtained from the caregiver of each child.
The study was conducted from June through December 2020, which coincides with the rainy season and high malnutrition season.10 Seasonal malaria chemoprevention (SMC) is distributed monthly from July through October to all children aged 3–59 months, and children in this study were eligible to receive SMC routinely. Participants were eligible if they were aged 6–59 months, had mid-upper arm circumference (MUAC) < 11.5 cm and/or weight-for-height Z-score (WHZ) < 3, and did not have any clinical complications requiring inpatient treatment. Participants were recruited via malnutrition screening days at the health facilities and community-based screening by community health workers. At baseline, caregivers completed a demographic, socioeconomic, and dietary survey.
Anthropometric measurements including weight (Seca 874dr scale, Seca, Germany), height (ShorrBoard Infant/Child Measuring Board, Weight and Measure, LLC, Olney, MD), and MUAC (Shorr Child MUAC tape, Weight and Measure, LLC) were collect from each child at enrollment, weekly until nutritional recovery, and at 8 weeks for all children. Anthropometric measurements were collected in triplicate and the median was used for analyses. All children were tested for malaria using a malaria rapid diagnostic test (RDT; SD Bioline Malaria Ag P.f/Pan, Abbott, Abbott Park, IL) and had a tympanic temperature measurement at enrollment and 8 weeks. The RDT was considered positive if the HRP2 (Plasmodium falciparum) and/or pLDH (Plasmodium species) bands were positive. Children with a positive RDT were referred for care. Hemoglobin was measured in a subset of children in four of the six healthcare facilities due to supply shortages at baseline and 8 weeks (HemoCue, Ängelholm, Sweden). Children with fever at each routine time point for the nutritional program received an RDT and appropriate treatment if the RDT was positive outside of the study. At each study time point, we recorded whether the child had received medical care for malaria since their last study visit.
The sample size for the trial was based on the trial’s primary outcome, weight gain in g/kg/day.9 For malaria outcomes, assuming a 10% prevalence of malarial parasitemia at baseline and 150 children per arm, we estimated that we would have at least 80% power to detect an absolute difference of 7.8% in malarial parasitemia positivity in children receiving azithromycin compared with amoxicillin. We used an unadjusted logistic regression model to estimate odds ratios in children randomized to azithromycin compared with amoxicillin for malarial parasitemia positivity by RDT, RDT positivity plus fever (tympanic temperature ≥ 37.5°C), and anemia (hemoglobin < 11 g/dL). We used a linear regression model to evaluate mean differences in hemoglobin as a continuous variable in children randomized to azithromycin compared with amoxicillin. As a sensitivity analysis because RDTs can remain positive for many weeks and because children with a positive RDT received antimalarials (artemether lumefantrine),11 we restricted analyses only to those with a negative baseline RDT. To evaluate whether children with malaria and without malaria at baseline had differential change in weight over the 8-week study period in g/kg/day, we constructed a linear regression model with an interaction term for randomized treatment group by baseline malarial parasitemia status. All analyses were conducted in R v3.6.2 (The R Foundation for Statistical Computing).
RESULTS
Of 301 children enrolled, 161 were randomized to azithromycin and 140 were randomized to amoxicillin (Supplemental Figure 1). Baseline RDT positivity was 12% (amoxicillin: 12.1%; azithromycin: 11.8%; Supplemental Table 1). At 8 weeks, RDT positivity was 11.2% in the amoxicillin group versus 8.7% in the azithromycin group (odds ratio [OR] = 0.75 azithromycin versus amoxicillin, 95% confidence interval [CI] = 0.34–1.63, P = 0.46). There was no evidence of a difference in fever, positive RDT plus fever by arm, hemoglobin, anemia, or clinic visits (Table 1). Sensitivity analyses restricting only to children with a negative baseline RDT did not qualitatively affect results (Supplemental Table 2).
Table 1.
Malaria outcomes at 8 weeks by study arm (N = 284)
| Azithromycin (N = 150) | Amoxicillin (N = 133) | Odds Ratio or Mean Difference (95% CI) | P value | |
|---|---|---|---|---|
| Positive RDT for malaria | 13 (8.7%) | 15 (11.2%) | 0.75 (0.34 to 1.63) | 0.46 |
| Fever* | 7 (4.7%) | 5 (3.7%) | 1.26 (0.39 to 4.36) | 0.70 |
| Positive RDT plus fever* | 6 (4.0%) | 4 (3.0%) | 1.35 (0.38 to 5.40) | 0.64 |
| Hemoglobin, g/dL2 | 9.2 (1.2) | 9.3 (1.5) | −0.07 (−0.44 to 0.30) | 0.70 |
| Anemia (< 11.0 g/dL)† | 94 (92.2%) | 81 (86.2%) | 1.93 (0.77 to 5.09) | 0.17 |
| Received medical care since last study visit‡ | ||||
| Week 1 | 5 (3.3%) | 7 (5.2%) | 0.62 (0.18 to 1.98) | 0.42 |
| Week 2 | 6 (3.9%) | 7 (5.1%) | 0.75 (0.24 to 2.32) | 0.62 |
| Week 3 | 8 (5.5%) | 5 (3.7%) | 1.50 (0.49 to 5.06) | 0.49 |
| Week 4 | 6 (4.1%) | 5 (3.8%) | 1.08 (0.32 to 3.83) | 0.90 |
| Week 8 | 9 (6.3%) | 9 (7.5%) | 0.83 (0.32 to 2.21) | 0.71 |
CI = confidence interval; RDT = rapid diagnostic test.
Fever defined as tympanic temperature > 37.5°C.
In subset with hemoglobin measurements at 8 weeks (N = 196).
Received care at a primary healthcare facility per caregiver report.
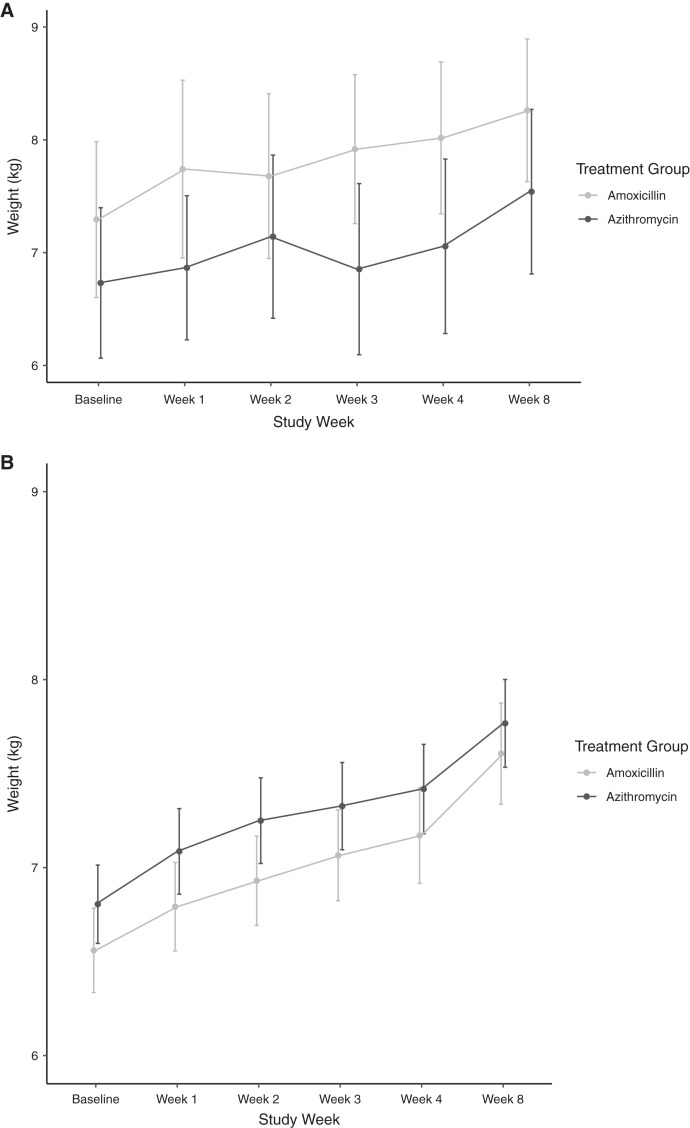
We found no evidence of a difference in weight gain among children receiving azithromycin compared with amoxicillin over the 8-week study period who were RDT positive (N = 36) versus negative (N = 265) at baseline (P for interaction = 0.79). Mean difference in weight gain in children receiving azithromycin compared with amoxicillin who were RDT positive at baseline was 0.05 g/kg/day (95% CI = 1.2–3.3 g/kg/day, P = 0.94) and in those who were RDT negative was −0.13 g/kg/day (95% CI = 0.6–0.3 g/kg/day, P = 0.58). Figure 1 shows mean weight by study arm at each study time point. There was no evidence of a difference in weight over time in children randomized to azithromycin compared with amoxicillin by malaria positivity.
Figure 1.
Mean weight at each study time point by randomized treatment group and among children with (A) and without (B) malaria infection at baseline. Grey lines indicate measurements in children randomized to amoxicillin and black lines to azithromycin. Points indicate the mean weight measurement at each time point and bars indicate the 95% confidence interval.
DISCUSSION
We found no evidence of a difference in malarial parasitemia as detected by RDT 8 weeks after enrollment in children with severe acute malnutrition receiving azithromycin compared with amoxicillin. The proportion of children with malarial parasitemia at baseline and over the follow-up period was lower than has been previously reported in studies of severe acute malnutrition in the Sahel.12 The Sahel experiences seasonal fluctuations in malnutrition, with the malnutrition season occurring during the rainy season leading up to the annual harvest.13 The rainy season coincides with the malaria season, during which malaria transmission and mortality is highest.10,12,14 Although the relationship between malaria infection and acute malnutrition is unclear,15 malnutrition may predispose children to infection or make it more difficult to recover, and malaria infection may predispose children to acute weight loss. Administration of an antibiotic with antimalarial properties could hypothetically be useful if it improved both nutritional and malaria outcomes. A larger study that could detect smaller but still meaningful differences in malaria outcomes—such as the 25% relative reduction estimated in this study—would improve understanding of azithromycin’s effect on malaria infection in children with uncomplicated severe acute malnutrition.
Because malaria infection is thought to contribute to weight loss in children, we hypothesized that children randomized to an antibiotic with antimalarial properties may have better outcomes if they also had malaria at baseline compared with those receiving an antibiotic without antimalarial properties. Although numbers for subgroup analyses were small, we did not find evidence of a difference in response to azithromycin compared with amoxicillin among those with and without malarial parasitemia at baseline. A previous study evaluating the relationship between malaria and nutritional status in Niger found no evidence of effect modification of the relationship between communities receiving biannual mass azithromycin distribution to children compared with placebo.16 Although azithromycin has antimalarial properties in vivo5 and has been shown to reduce parasitemia when administered to entire communities of children,7 it is a weak antimalarial and may only have limited antimalarial effects when administered at the individual level. The primary analysis did not find evidence of a difference in weight gain between children receiving azithromycin and amoxicillin,17 and this result does not appear to be moderated by malarial parasitemia.
This study has several limitations. The parent study was designed as a pilot study for a full-scale randomized controlled trial9 and thus confidence intervals were wide for most outcomes. Subgroup analyses were especially underpowered, given the relatively few malaria cases at baseline. The study used a single point measurement of malarial parasitemia using an RDT. The use of a single-point measurement is a poor surrogate for incidence or prevalence of infection over the course of the study, as some children may have been infected during the course of the study and subsequently recovered and were no longer antigenemic. HRP2-based RDTs can have imperfect specificity and sensitivity due to persistent antigenemia after treatment of malaria and among lower parasite density infections.18 Further, febrile children were screened for malaria and received treatment if positive by RDT routinely outside of the study. We did not record specific diagnoses, but overall receipt of medical care was infrequent and nondifferential by arm, suggesting that overall results were likely not affected by diagnosis of malaria and subsequent receipt of antimalarials during the study period. Malarial parasitemia prevalence was lower in this study than anticipated. This may have been due in part to SMC distributions or other malaria interventions co-occurring at the time of the study. We did not collect data as to whether children enrolled in the study received SMC, although all were eligible for up to four rounds of SMC and distributions were ongoing in the study area during our enrollment period. We did not collect data on other malaria intervention activities such as bed net use during the study.
We found no evidence that azithromycin significantly improved malarial parasitemia 8 weeks after administration in children with uncomplicated severe acute malnutrition. If azithromycin were to be considered as an alternative antibiotic to amoxicillin for routine treatment of severe acute malnutrition, it may not be sufficient to prevent or treat malaria infection in these children.
Supplemental tables
Note: Supplemental tables and figure appear at www.ajtmh.org.
REFERENCES
- 1. World Health Organization , 2010. Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 2.Bourke CD, Berkley JA, Prendergast AJ, 2016. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol 37: 386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isanaka S. et al. , 2016. Routine amoxicillin for uncomplicated severe acute malnutrition in children. N Engl J Med 374: 444–453. [DOI] [PubMed] [Google Scholar]
- 4.Trehan I. et al. , 2013. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med 368: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahl EL, Rosenthal PJ, 2007. Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrob Agents Chemother 51: 3485–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sidhu ABS, Sun Q, Nkrumah LJ, Dunne MW, Sacchettini JC, Fidock DA, 2007. In vitro efficacy, resistance selection, and structural modeling studies implicate the malarial parasite apicoplast as the target of azithromycin. J Biol Chem 282: 2494–2504. [DOI] [PubMed] [Google Scholar]
- 7.Arzika AM. et al. , 2019. Biannual mass azithromycin distributions and malaria parasitemia in pre-school children in Niger: a cluster-randomized, placebo-controlled trial. PLoS Med 16: e1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keenan JD. et al. , 2020. Cause-specific mortality of children younger than 5 years in communities receiving biannual mass azithromycin treatment in Niger: verbal autopsy results from a cluster-randomised controlled trial. Lancet Glob Health 8: 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien K. et al. , 2021. Azithromycin for uncomplicated severe acute malnutrition: study protocol for a pilot randomized controlled trial. Pilot Feasibility Stud 7: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burki TK, 2013. Malaria and malnutrition: Niger’s twin crises. Lancet 382: 587–588. [DOI] [PubMed] [Google Scholar]
- 11.Dalrymple U, Arambepola R, Gething PW, Cameron E, 2018. How long do rapid diagnostic tests remain positive after anti-malarial treatment? Malar J 17: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oldenburg CE, Guerin PJ, Berthé F, Grais RF, Isanaka S, 2018. Malaria and nutritional status among children with severe acute malnutrition in Niger: a prospective cohort study. Clin Infect Dis 67: 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belesova K, Gasparrini A, Sie A, Sauerborn R, Wilkinson P, 2018. Annual crop yield variation, child survival, and nutrition among subsistence farmers in Burkina Faso. Am J Epidemiol 187: 242–250. [DOI] [PubMed] [Google Scholar]
- 14.im Kampe EO, Muller O, Sie A, Becher H, 2015. Seasonal and temporal trends in all-cause and malaria mortality in rural Burkina Faso, 1998–2007. Malar J 14: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das D. et al. , 2018. Complex interactions between malaria and malnutrition: a systematic literature review. BMC Med 16: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arzika AM. et al. , 2020. Malaria parasitemia and nutritional status during the low transmission season in the presence of azithromycin distribution among preschool children in Niger. Am J Trop Med Hyg 103: 1315–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien KS. et al. , 2021. Comparing azithromycin to amoxicillin in the management of uncomplicated severe acute malnutrition in Burkina Faso: a pilot randomized trial. medRxiv. Available at: 10.1101/2021.06.15.21258967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acquah FK. et al. , 2021. Diagnostic performance of an ultrasensitive HRP2-based malaria rapid diagnostic test kit used in surveys of afebrile people living in southern Ghana. Malar J 20: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.