Abstract
Liver plays a pivotal role in maintaining blood glucose levels through complex processes which involve the disposal, storage, and endogenous production of this carbohydrate. Insulin is the hormone responsible for regulating hepatic glucose production and glucose storage as glycogen, thus abnormalities in its function lead to hyperglycemia in obese or diabetic patients because of higher production rates and lower capacity to store glucose. In this context, two different but complementary therapeutic approaches can be highlighted to avoid the hyperglycemia generated by the hepatic insulin resistance: 1) enhancing insulin function by inhibiting the protein tyrosine phosphatase 1B, one of the main enzymes that disrupt the insulin signal, and 2) direct regulation of key enzymes involved in hepatic glucose production and glycogen synthesis/breakdown. It is recognized that medicinal plants are a valuable source of molecules with special properties and a wide range of scaffolds that can improve hepatic glucose metabolism. Some molecules, especially phenolic compounds and terpenoids, exhibit a powerful inhibitory capacity on protein tyrosine phosphatase 1B and decrease the expression or activity of the key enzymes involved in the gluconeogenic pathway, such as phosphoenolpyruvate carboxykinase or glucose 6-phosphatase. This review shed light on the progress made in the past 7 years in medicinal plants capable of improving hepatic glucose homeostasis through the two proposed approaches. We suggest that Coreopsis tinctoria, Lithocarpus polystachyus, and Panax ginseng can be good candidates for developing herbal medicines or phytomedicines that target inhibition of hepatic glucose output as they can modulate the activity of PTP-1B, the expression of gluconeogenic enzymes, and the glycogen content.
Keywords: medicinal plants, hyperglycemia, hepatic glucose output, insulin resistance, PTP-1B inhibitors, natural products
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease characterized by high blood sugar levels (hyperglycemia), caused by insulin malfunctioning, deficient insulin secretion, or both (Liu et al., 2019). Type 2 diabetes (T2D) is the most important type of DM due to its high worldwide prevalence (American Diabetes Association, 2021). It is characterized by insulin resistance, which is defined as a poor response of insulin-sensitive tissues to normal insulin concentration (Mlinar et al., 2007). The main cause of insulin resistance has been associated to an obesogenic environment in which large amounts of free fatty acids and adipokines are responsible for impairing insulin signaling by increasing serine phosphorylation that inhibits tyrosine phosphorylation of insulin receptor (IR) and insulin receptor substrates (IRSs) (DeFronzo et al., 2015). However, it has also been reported that protein tyrosine phosphatases (PTPs) could have a more important role since they are upregulated in insulin resistant states. Insulin action is negative regulated by PTPs, particularly the PTP-1B, because they promote the dephosphorylation of tyrosine residues of IR and IRSs (Saltiel and Kahn, 2001). When insulin signaling is impaired in liver by either insulin resistance or low insulin levels, the glucose storage and production is dysregulated, increasing the hepatic glucose output rates yielding hyperglycemia in diabetic patients.
Liver represents a crucial therapeutic target for treating hyperglycemia in T2D because hepatic glucose output is the pathophysiological abnormality that contributes the most to the hyperglycemic state in fasting and postprandial state as a consequence of hepatic insulin resistance (Sharabi et al., 2015). During the overnight fast (postabsorptive state), the liver of a normal person produces glucose at a rate of approximately 1.8–2 mg/kg. min. However, this rate increases around 0.5 mg/kg min in a patient with T2D, promoting a significant rise in the basal state of glucose production (Cersosimo et al., 2018). After food ingestion and the subsequent increase in insulin levels, the suppression of glucose production is slower in a diabetic patient, promoting an evident postprandial hyperglycemia due to the excess of glucose produced in addition to that from the exogenous source (Rizza, 2010).
Medicinal plants and natural products have shown to have numerous benefits on processes involved in glucose and lipid metabolism, leading to correct homeostasis imbalances that promote metabolic diseases such as T2D (Li J. et al., 2018; Xu L. et al., 2018; Saadeldeen et al., 2020). Unlike the classic “on-target” paradigm in pharmacology, namely a drug with a specific target, the polypharmacology approach, or the binding of a drug to more than one target, could be more effective against a disease as complex as T2D due to its multiple pathophysiological abnormalities (Reddy and Zhang, 2013). In this context, extract plants and phytochemicals isolated from medicinal plants exhibit multiple mechanisms of action on assorted metabolic targets that are involved in glucose homeostasis. Therefore, efforts have been made to describe all the beneficial effects on metabolism of these extracts and molecules in recent years.
The current review summarizes the medicinal plants reported from 2015 that can potentially decrease hyperglycemia resulting from imbalance in hepatic glucose metabolism by two different approaches: improving hepatic insulin resistance by inhibiting PTP-1B and decreasing hepatic glucose output by inhibiting rate-limiting enzymes involved in the storage and production of glucose.
Methodology
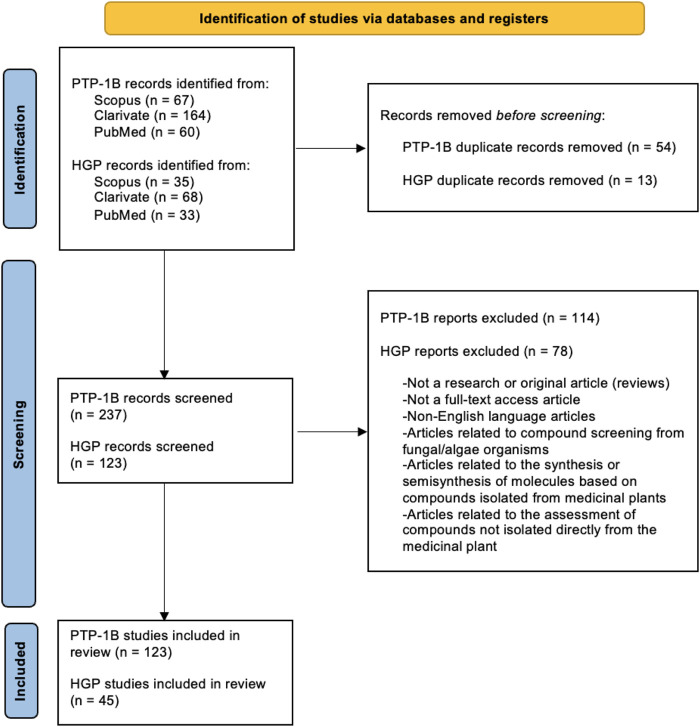
Two separate searches were performed based on the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) (Page et al., 2021) in the following databases: Scopus, Clarivate and PubMed (Figure 1). The first involved studies related to extracts or phytochemicals tested against the activity or expression of PTP-1B enzyme, while in the second, studies with extracts or phytochemicals with an effect on the glucose-producing pathways were sought. Only records related to the study of medicinal plants and their isolated compounds were considered.
FIGURE 1.
PRISMA flowchart. PTP-1B: protein tyrosine phosphatase 1B; HGP: hepatic glucose production.
Therapeutic Approaches to Reduce Hyperglycemia Resulting From Impaired Hepatic Glucose Homeostasis
Each insulin-sensitive tissue presents abnormal characteristics that contribute to hyperglycemia in an insulin-resistant state. The underlying mechanisms that give rise to insulin resistance converge on deficient insulin signalling that limits the activation of factors involved in energy metabolism. In obesity and T2D, insulin resistance has been linked mainly to defects in the signalling pathway of phosphatidylinositol 3-kinase and protein kinase B (PI3K/Akt), particularly to the Akt2 isoform (Cusi et al., 2000; Krook et al., 2000).
In normal conditions, the insulin secreted by pancreatic β cell binds to its receptor in the target cell, activating the tyrosine kinase activity, which promotes the receptor autophosphorylation and the subsequent phosphorylation of IRSs, mainly IRS-1 and IRS-2, in tyrosine residues. Afterwards, the enzyme P13K is recruited and activated by IRS to convert phosphatidylinositol 4,5-bisphosphate (PIP2) from the plasma membrane to phosphatidylinositol 3,4,5-triphosphate (PIP3), which facilitates the phosphorylation and activation of Akt at two important sites: by phosphoinositide-dependent kinase 1 (PDK1) at residue Thr308 of the catalytic domain, and by mammalian target rapamycin complex 2 (mTORC2) at residue Ser473 of the regulatory domain (Schultze et al., 2012). Specifically in liver, the activated Akt enzyme is responsible for phosphorylating different factors that are involved in the regulation of processes such as glycogen synthesis, gluconeogenesis, and glycogenolysis, which are activated or inhibited under different nutritional circumstances (Dimitriadis et al., 2021).
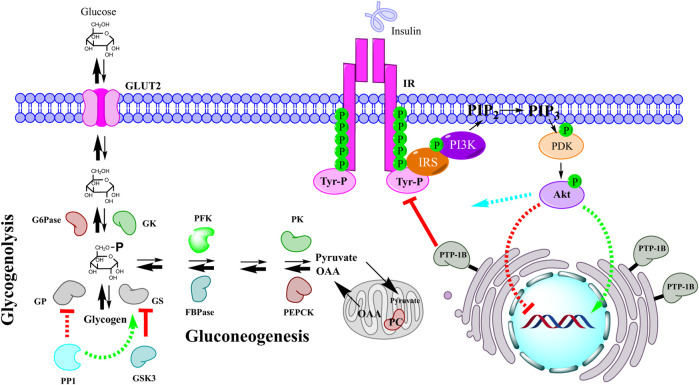
Due to hepatic insulin resistance, this hormone losses its ability to regulate glucose metabolism in liver, resulting in enhanced glucose output that contributes greatly to fasting and postprandial hyperglycemia, namely glycogen synthesis is reduced, and production of glucose is increased (Figure 2). Therefore, we proposed two approaches by which medicinal plants could ameliorated hyperglycemia through enhancing hepatic glucose metabolism: improving the function of insulin in the liver by inhibiting the enzyme PTP-1B and modulating the hepatic production/storage of glucose by regulating the enzymes involved in gluconeogenesis, glycogenolysis, and glycogenesis.
FIGURE 2.
Impaired hepatic glucose homeostasis by insulin resistance. When insulin does not work properly either due to overexpression of PTP-1B or other factors, glucose production in liver is upregulated generating a hyperglycemic state. Both gluconeogenesis and glycogenolysis are enhanced due to poor insulin signaling, namely genetic expression of gluconeogenic enzymes is not repressed and enzymes related to glycogen metabolism are not adequately regulated. Akt functions: green color indicates positive regulation, red color indicates negative regulation, and blue color represents direct or indirect regulation by phosphorylation or allosterism. IR: insulin receptor; IRS: insulin receptor substrate; PI3K: phosphoinositide 3-kinase; PIP2: phosphatidylinositol 4,5-bisphosphate; PIP3: phosphatidylinositol 3,4,5-triphosphate; PDK: phosphoinositide-dependent kinase; Akt: protein kinase B; PTP-1B: protein tyrosine phosphatase 1B; PC: pyruvate carboxylase; OAA: oxalacetate; PEPCK: phosphoenolpyruvate carboxykinase; PK: pyruvate kinase; FBPase: fructose 1,6-bisphosphatase; PFK: phosphofructokinase; GS: glycogen synthase; GP: glycogen phosphorylase; PP1: protein phosphatase 1; GSK3: glycogen synthase kinase-3; GK: glucokinase; G6Pase: glucose 6-phosphatase; GLUT2: glucose transporter 2.
Inhibition of Protein Tyrosine Phosphatase 1B
The modification of proteins through phosphorylation and dephosphorylation of tyrosine residues represents one of the main mechanisms of cell signaling regulation (Alonso et al., 2016), which is carried out by two superfamilies of enzymes: protein tyrosine kinases (PTKs), and PTPs. In this regard, the classical PTP subfamily possess a domain of 240–250 amino acids characterized by a conserved site that exhibits a catalytic mechanism based on cysteine (Denu and Dixon, 1998). Specifically, the enzyme PTP-1B is a classic intracellular PTP widely distributed in mammalian tissues that is anchored on the cytoplasmic side of the endoplasmic reticulum membrane. Despite its localization, the PTP-1B enzyme can access its substrates located on the surface of the plasma membrane during endocytosis, biosynthesis, and by the movement of the endoplasmic reticulum towards the plasma membrane in specific regions (Bakke and Haj, 2015).
Since its first isolation from the human placenta in 1988 by Tonks et al., 1988 PTP-1B has become an attractive research object due to its direct link with the etiopathogenesis of insulin resistance. In addition to the processes promoted by the obesogenic inflammatory environment, such as the serine/threonine phosphorylation of IR and IRS, and their proteasomal degradation (Mlinar et al., 2007; Ahmed et al., 2021), the dephosphorylation of these components by PTP-1B has also been implied to the termination of the insulin signal (Ahmad et al., 1995; Kenner et al., 1996; Chen et al., 1997).
Experimental data obtained from various studies have shown that the PTP-1B enzyme is one of the main negative regulators of the insulin signaling pathway. For instance, studies performed in PTP-1B knock-out mice have been shown that the absence of this enzyme produces healthy organisms that exhibit enhanced insulin sensitivity, protection against the weight gain generated by high-fat diet, and increased hepatic phosphorylation of IR and IRS after an intraperitoneal insulin injection (Elchebly et al., 1999; Klaman et al., 2000). On the other hand, it has been reported an increased PTP-1B activity in hepatic cytosolic fractions isolated from streptozotocin (STZ)-hyperglycemic rats (Meyerovitch et al., 1989), while augmented hepatic microsomal enzyme activity, content of protein, and mRNA levels have only been observed after 2 weeks of insulin treatment in these insulinopenic organisms, suggesting that elevated insulin levels are necessary to modify PTP-1B content and activity, namely hyperinsulinemia caused by insulin resistance may lead to altered PTP-1B expression and activity (Ahmad and Goldstein, 1995). Additionally, it has also been shown that insulin rises hepatic microsomal PTP-1B activity in rat hepatoma cells (Hashimoto and Goldstein, 1992). Likewise, abnormal expression and activity of PTP-1B have been reported in skeletal muscle of insulin-resistant obese people (Ahmad et al., 1997), as well as in non-obese Goto-Kakizaki rats with spontaneously generated insulin resistance (Dadke et al., 2000), and in STZ-hyperglycemic rats fed with high-fat diet (Wu et al., 2005).
Based on the aforementioned, the PTP-1B inhibition represents a good therapeutic target for the treatment of insulin resistance-related diseases, such as DM2 (Zhang et al., 2006). Hence, an arsenal of molecules with inhibitory capacity of PTP-1B activity has been generated in recent years. The methodological approaches that have been applied are the rational design of synthetic phospho-(tyrosine)-mimetic molecules to be used as competitive inhibitors, considering the structural characteristics of the protein, and the search for molecules from natural sources (Sun et al., 2018). The latter is based on the statement that nature has a great variety of structures that present diverse pharmacological effects (Atanasov et al., 2021), so natural products can be used as a starting point for the creation of powerful inhibitors.
Table 1 summarizes all medicinal plants and their identified compounds that have proved to inhibit the activity or expression of PTP-1B since 2015. It was obtained a total of 125 medicinal plants used in various traditional medicine systems around the world, mainly represented in eastern folk, such as Chinese and Vietnamese. Morus alba L. (Moraceae), a plant used in the traditional Chinese system, has been the most evaluated for this purpose. In addition to direct PTP-1B activity inhibition and molecular docking studies, some extracts and compounds were assessed to improve glucose and lipid metabolism in vivo, such as lowering blood glucose levels, improved insulin resistance and glucose intolerance, and improved lipid profile. Furthermore, their effect on glucose uptake and phosphorylation of some components of insulin signaling, such as IR, IRS, and Akt, was evaluated in cell cultures under insulin-resistant conditions.
TABLE 1.
Medicinal plants and their phytochemicals with PTP-1B inhibitory capacity.
| Medicinal plant (scientific name [Family]/Traditional medicine system or places where it is used) | Part/Extract | Isolated compounds | Experiment/Outcome | References |
|---|---|---|---|---|
| Acmella paniculata (Wall. ex DC.) R.K.Jansen [Asteraceae]/Indonesian | Aerial parts/EtOH | N-isobutyl-2E-decenamide | In vitro: PTP-1B enzyme assay/IC50 = 24 µM | Abdjul et al. (2018) |
| Agrimonia pilosa Ledeb. [Rosaceae]/Chinese | Aerial parts/EtOH | Apigenin-7-O-b-D-glucuronide-6″-methyl ester Quercetin-3-O-b-D-glycoside Kaempferol Kaempferol-3-O-a-l-rhamnoside b-sitosterol Ursolic acid Tormentic acid Methyl 2-hydroxyl tricosanoate Palmitic acid | In vitro: PTP-1B enzyme assay/IC50 = 14.35, 27.73, 42.93, 12.16, 49.78, 3.47, 0.5, 36.39, 0.1 µM | Na et al. (2016) |
| Apigenin 7-O-b-D-glucuronide Ellagic acid Agritannin | In vitro: PTP-1B enzyme assay/IC50 = 7.14, 7.73, 17.03 µM | Nguyen et al. (2017) | ||
| Akebia quinata (Thunb. ex Houtt.) Decne. [Lardizabalaceae]/Chinese | Stems/MeOH | Cyrtophyllones B Uncinatone 3-O-α-l-arabinopyranosyl olean-12-en-28-oic acid 3-O-[β-d-glucopyranosyl (1–4)-α-l-arabinopyranosyl)]olean-12-en-28-oic acid 2α,3α,23-trihydroxyoleane-12-en-28-oic acid | In vitro: PTP-1B enzyme assay/IC50 = 6.77, 5.41, 4.08, 21.8, 7.78 µM | An et al. (2016) |
| Allium cepa L. [Amaryllidaceae] | Outer skins/MeOH | Cepadial B, C Cepabifla A–C Cepadial D | In vitro: PTP-1B enzyme assay/IC50 = 22.55, 22.33, 17.01, 24.07, 14.29, 1.68 µM | Vu et al. (2020) |
| Allophylus cominia (L.) Sw. [Sapindaceae]/Cuban | Leaves/MeOH | Pheophytin A, B | In vitro: PTP-1B enzyme assay/Activity inhibition by 65 and 57% at 30 μg/ml In vitro: cell culture (L6 myotubes)/↑insulin-dependent glucose uptake (pheophytin extract) In vitro: cell culture (3T3-L1)/↓lipid accumulation on the differentiation phase, ↓lipid droplets (phaeophytin extract) | Semaan et al., 2017, 2018 |
| Angelica decursiva (Miq.) Franch. & Sav. [Apiaceae]/Korean | Whole plant/MeOH | cis-3′-Acetyl-4′-angeloylkhellactone Isorutarine | In vitro: PTP-1B enzyme assay/IC50 = 86.95, 80.09 µM | Yousof Ali et al. (2015) |
| Anoectochilus chapaensis Gagnep. [Orchidaceae]/Chinese | Whole plant/EtOH | Friedelin Sorghumol Epifriedelanol Friedelane 2a,3b-dihydroxyolean-12- en-23, 28, 30-trioic acid Quercetin Isorhamnetin Isorhamnetin-3-O-b-D-glucoside Isorhamnetin-3-O-b-d-rutinoside | In vitro: PTP-1B enzyme assay/IC50 = 6.21, 3.5, 3.75, 4.6, 2.65, 5.63, 1.75, 1.16, 1.2 µM | Cai et al. (2015) |
| Artocarpus nanchuanensis S.S.Chang S.C.Tan & Z.Y.Liu [Moraceae]/Chinese | Stems/EtOH | Hypargystilbene B, D, E | In vitro: PTP-1B enzyme assay/IC50 = 3.23, 37.31, 2.53 nM | Zhang et al. (2015) |
| Artocarpus styracifolius Pierre [Moraceae]/Chinese | Roots/EtOH | (±)-Styrastilbene A Styrastilbene B (±)-Styrastilbene C | In vitro: PTP-1B enzyme assay/IC50 = 4.52, 2.4, 8.23 µM | Li et al. (2019b) |
| Astragalus mongholicus Bunge [Fabaceae]/Chinese | Root/Aqueous | Astragaloside IV | In vitro: PTP-1B enzyme assay/IC50 = 10.34 µM In vitro: cell culture (insulin-resistant HepG2)/↑glucose consumption, ↓PTP-1B, ↑pIR, ↑pIRS1 protein levels | Zhou et al. (2021) |
| Bidens pilosa L. [Asteraceae]/Chinese | Whole plant/Aqueous In combination with Euonymus alatus (Thunb.) Siebold [Celastraceae] winged branchlet Coptis chinensis Franch. [Ranunculaceae] rhizome Cornus officinalis Siebold & Zucc. [Cornaceae] fruit Ligustrum lucidum W.T.Aiton [Oleaceae] fruit Scrophularia ningpoensis Hemsl. [Scrophulariaceae] root | Full extract | In vivo: hypertensive rats fed with HFD (2020 mg/kg b.w.)/prevention of increased body weight, ↓triglycerides, ↓LDL, ↓insulin resistance, ↑glucose tolerance, ↓ PTP-1B expression in adipose tissue | Zhu et al. (2018) |
| Bistorta officinalis Delarbre [Polygonaceae]/Chinese | Rhizome/EtOAc | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 17.43 μg/ml | Zhao et al. (2019b) |
| Boehmeria nivea (L.) Gaudich. [Urticaceae]/Chinese | Root/EtOAc | Full extract Hederagenin Pomolic acid | In vitro: PTP-1B enzyme assay/IC50 = 20.19 μg/ml, 9.53, 4.89 µM | Zhao et al. (2019b) |
| Camellia crapnelliana Tutcher [Theaceae]/Chinese | Twigs and leaves/MeOH | Camellianol B, C, E–G A1- barrigenol 22-O-angeloyl-A1-barrigenol Camelliagenin A 16-O-acetylcamelliagenin A 3β,11α,13β-trihydroxyolean-12-one α-amyrin Lupeol 3β,20-dihydroxylupane | In vitro: PTP-1B enzyme assay/IC50 = 4.87, 7.4, 20.03, 14.36, 11.08, 16.79, 2.56, 8.93, 10.16, 1.34, 19.26, 3.68, 12.44 µM | Xiong et al. (2017) |
| Cassia fistula L. [Fabaceae]/Vietnamese | Leaves/EtOAc | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 24.1 μg/ml | Trinh et al. (2017a) |
| Catharanthus roseus (L.) G.Don [Apocynaceae]/Malaysia, India, China, South Africa, and Mexico | Leaves/DCM | Vindogentianine | In vitro: PTP-1B enzyme assay/IC50 = 15.28 μg/ml In vitro: cell culture (β-TC6, C2C12)/↑glucose uptake | Tiong et al. (2015) |
| Cedrus deodara (Roxb. ex D.Don) G.Don [Pinaceae]/Aryuveda | Needles/Essential oil | Caryophyllene oxide | In vitro: PTP-1B enzyme assay/IC50 = 31.32 µM | Wang et al. (2017a) |
| Centella asiatica (L.) Urb. [Apiaceae]/Jamu | Aerial parts/Aqueous | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 13.2 μg/ml | Saifudin et al. (2016b) |
| Chaenomeles japonica (Thunb.) Lindl. ex Spach [Rosaceae]/Japanese and Chinese | Fruits/acetone | Polyphenolic extract | In vitro: cell culture (HepG2)/↓PTP-1B mRNA expression level, ↓PEPCK mRNA expression level, ↑GLUT4 mRNA expression level, ↑IRS-2 mRNA expression level, ↑pAMPK, ↑glycogen synthesis, ↓glucose production | Zakłos-Szyda and Pawlik, (2018) |
| Cinnamomum osmophloeum Kaneh. [Lauraceae]/Taiwan | Twigs and leaves/acetone | Full extract n-hexane soluble fraction ethyl acetate soluble fraction n-butanol soluble fraction water soluble fraction | In vitro: PTP-1B enzyme assay/IC50 = 1.9, 3.2, 2, 1.7, 1.9 μg/ml | Lin et al. (2016) |
| Cipadessa baccifera (Roth) Miq. [Meliaceae]/Chinese | Leaves/EtOH | Cipacinoid A | In vitro: PTP-1B enzyme assay/IC50 = 16.7 µM | Yu et al. (2016a) |
| Clausena sanki (Perr.) Molino [Rutaceae]/Chinese | Fruits/EtOH | Clausenanisines A–C, E, F Euchrestifoline Dihydromupamine Clauraila B Kurryame Clausenaline F 3-formyl-1-hydroxycarbazole Clausine Z, I Clauszoline N, M | In vitro: PTP-1B enzyme assay/IC50 = 0.58, 0.87, 28.79, 27.96, 2.47, 1.28, 15.26, 23.89, 27.93, 28.42, 4.36, 5.39, 3.96, 24.43, 26.37 µM | Liu et al. (2021) |
| Coptis chinensis Franch. [Ranunculaceae]/Chinese | Rhizome/MeOH | Berberine Epiberberine Magnoflorine Coptisine | In vitro: PTP-1B enzyme assay/IC50 = 16.43, 21.19, 28.14, 51.04 µM | Choi et al. (2015) |
| Coreopsis tinctoria Nutt. [Asteraceae]/North American and Chinese | Capitula/EtOH | Butin Taxifolin 7,3′,4′-trihydroxyflavone Quercetagitin-7-O-b-D-glucoside | In vitro: PTP-1B enzyme assay/IC50 = 20.92, 7.73, 27.93, 24.5 µM | Begmatov et al. (2020) |
| Cymbopogon nardus (L.) Rendle [Poaceae]/Jamu | Leaves/Aqueous | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 10.63 μg/ml | Saifudin et al. (2016b) |
| Dioscorea bulbifera L. [Dioscoreaceae]/Chinese | Rhizome/EtOAc | Full extract 9,10-Dihydro-2,4,6,7-phenanthrenetetrol [1,1'-Biphenanthren]-2,2',3,3',6,6',7,7'-octaol Cassigarol D | In vitro: PTP-1B enzyme assay/IC50 = 32.21 μg/ml, 23.79, 3.36, 13.16 µM | Zhao et al. (2019b) |
| Dracaena cochinchinensis (Lour.) S.C.Chen [Asparagaceae]/Chinese | Red resin/MeOH | Biflavocochin B, F, G | In vitro: PTP-1B enzyme assay/IC50 = inhibition of 75.8, 66.7, 74.9% at 10 µM | Lang et al. (2020) |
| Duranta erecta L. [Verbenaceae]/Aryuveda | Whole plant/EtOH | Full extract | In silico: Network pharmacology/phytoconstituents targeting PTP-1B In silico: molecular docking/PTP-1B binding energy: 8.9 kcal/mol (durantanin I) In vivo: diabetic rats (100, 200, and 400 mg/kg b.w.)/chronic hypoglycemic effect, ↓HbA1c, ↑glucose tolerance, ↓G6Pase and FBPase activity, ↑hexokinase activity, ↓triglycerides, LDL, VLDL, and total cholesterol, ↑HDL, ↑hepatic glycogen content, ↑glucose uptake in isolated rat hemidiaphragm | Khanal and Patil, (2020) |
| Elaeocarpus grandiflorus Sm. [Elaeocarpaceae]/Jamu | Fruits/Aqueous | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 6.9 μg/ml | Saifudin et al. (2016b) |
| Elephantopus scaber L. [Asteraceae]/Jamu | Aerial parts/Aqueous | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 2.64 μg/ml | Saifudin et al. (2016b) |
| Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. [Araliaceae]/Chinese | Stems/MeOH | (7S,8R)-3-hy- droxyl-4-methoxyl-balanophonin (7S,8R)-5-methoxyl-balanophonin Balanophonin Curcasinlignan A–C | In vitro: PTP-1B enzyme assay/IC50 = 15.2, 12.6, 16.1, 17.1, 31, 29.4 µM | Li et al. (2017) |
| Epimedium koreanum Nakai [Berberidaceae]/Chinese | Aerial parts/MeOH | Icaritin Icariside II | In vitro: PTP-1B enzyme assay/IC50 = 11.59, 9.94 µM | Kim et al. (2017a) |
| Eremophila bignoniiflora (Benth.) F. Muell. [Scrophulariaceae]/Australian | Leaves/EtOAc | 7-hydroxy-6-methyl-4-oxo-2-(3-(5-oxo-2,5- dihydrofuran-3-yl)propyl)hept-5-en-1-yl (E)-3-(3,4-dihydroxyphenyl) acrylate Galangin 3-methyl ether | In vitro: PTP-1B enzyme assay/IC50 = 52.4, 41.4 µM | Zhao et al. (2019a) |
| Eremophila lucida Chinnock [Scrophulariaceae] | Leaves/EtOAc | 5-hydroxyviscida-3,14-dien-20-oic acid | In vitro: PTP-1B enzyme assay/IC50 = 42 µM | Tahtah et al. (2016) |
| Eremophila oppositifolia R.Br. [Scrophulariaceae]/Australian | Leaves/CH₃CN | Type B dimeric fatty acids related to the branched-chain fatty acid (2E,4Z,6E)-5-(acetoxymethyl)tetradeca-2,4,6-trienoic acid (Compounds 9, 12, 13a, 13b) | In vitro: PTP-1B enzyme assay/IC50 = 24, 2.4, 12, 12 µM | Pedersen et al. (2020) |
| Eriobotrya japonica (Thunb.) Lindl. [Rosaceae]/Chinese | Leaves/EtOH | Extract of triterpenoid acids (maslinic acid, corosolic acid, oleanolic acid, and ursolic acid) | In vivo: insulin-resistant mice (200 mg/kg b.w.)/↓insulin resistance, ↑glucose tolerance, ↓triglycerides, LDL, VLDL, and total cholesterol, ↑HDL; in liver: ↑PPARg, GLUT2, and glucokinase mRNA expression levels, ↓PTP-1B mRNA expression levels | Li et al. (2020b) |
| Eucalyptus robusta Sm. [Myrtaceae]/Chinese | Leaves/EtOH | Eucarobustol A–I Macrocarpal C | In vitro: PTP-1B enzyme assay/IC50 = 1.3, 4.3, 4.3, 2.9, 4.1, 5.6, 1.8, 3.0, 1.6, 4.5 µM | Yu et al. (2016b) |
| Euphorbia hirta L. [Euphorbiaceae]/Vietnamese | Whole plant/EtOAc and n-BuOH | Full extracts | In vitro: PTP-1B enzyme assay/IC50 = 29.2, 38.3 μg/ml | Trinh et al. (2017a) |
| Ficus deltoidea Jack [Moraceae]/Malay | Leaves/EtOH | 70% EtOH extract Lupeol 3β,11β-dihydroxyolean-12-en-23-oic acid | In vitro: PTP-1B enzyme assay/IC50 = 92%, 2.88, 4.55 µM In vivo: diabetic rats (125, 250, and 500 mg/kg b.w. of 70% EtOH extract)/chronic hypoglycemic effect, ↓triglycerides, LDL, and total cholesterol, ↑HDL; in liver: ↑GLUT2 levels, ↓PEPCK, G6Pase, and PTP-1B mRNA expression levels | Abdel-Rahman et al. (2020) |
| Ficus racemosa L. [Moraceae]/Vietnamese | Fruit/EtOAc | Isoderrone Derrone Alpinumisoflavone Mucusisoflavone B | In vitro: PTP-1B enzyme assay/IC50 = 22.7, 12.6, 21.2, 2.5 µM | Trinh et al. (2017a) |
| Garcinia mangostana L. [Clusiaceae]/Southeast Asia and India | Fruits/EtOH | γ-Mangostin 8-Deoxyartanin 1,3,7-Trihydroxy-2,8-di-(3-methylbut-2-enyl)- xanthone α-Mangostin Garcinone E 9-Hydroxycalabaxanthone | In vitro: PTP-1B enzyme assay/IC50 = 0.86, 1.57, 3.28, 1.34, 0.43, 12.89 µM | Hu et al. (2021) |
| Garcinia oblongifolia Champ. ex Benth [Clusiaceae]/Vietnamese | Twigs/EtOAc | Norcowanin | In vitro: PTP-1B enzyme assay/IC50 = 14.1 µM | Trinh et al. (2017b) |
| Geranium collinum Stephan ex Willd. [Geraniaceae]/Chinese and Tajik | Root/EtOH In combination with Hypericum scabrum aerial parts (ratio: 7:3) | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 0.48 μg/ml In vitro: cell culture (L6 myotubes, in the presence of insulin)/↓PTP-1B protein, ↑IR, ↑pAkt, ↑pIRS-1, ↑pGSK3β, ↑pAMPK, ↑glucose consumption | Edirs et al. (2018) |
| Roots/EtOH | 3,3′,4,4′-Tetra-O-methylellagic acid 3,3′-Di-O-methylellagic acid Caffeic acid Quercetin Catechin Epicatechin Corilagin | In vitro: PTP-1B enzyme assay/IC50 = 21.64, 6.26, 35.81, 2.19, 0.62, 0.23, 0.87 µM | Numonov et al. (2017) | |
| Glycyrrhiza inflata Batalin [Fabaceae]/Japanese and Chinese | Roots and rhizomes/EtOAc | Licoagrochalcone A Kanzonol C Glyurallin B Gancaonin H 2′-hydroxyisolupalbigenin Gancaonin Q Glisoflavanone Glabrol Macarangaflavanone B | In vitro: PTP-1B enzyme assay/IC50 = 0.97, 0.45, 4.5, 1.48, 0.5, 0.55, 0.84, 0.31, 1.03 µM | Lin et al. (2017) |
| Glycyrrhiza uralensis Fisch. ex DC. [Fabaceae]/Chinese | Rhizomes/EtOH | Licochalcone A Licoflavone B | In vitro: PTP-1B enzyme assay/IC50 = 27.95, 15.62 µM | Guo et al. (2015) |
| Isoangustone A Angustone A | In vitro: PTP-1B enzyme assay/IC50 = 3, 0.4 µM | Ji et al. (2016) | ||
| Glyptostrobus pensilis (Staunton ex D.Don) K.Koch [Cupressaceae]/Chinese | Trunk barks/MeOH | Spiropensilisol A, B 3-epi-larixinol 3,2′-epi-larixinol Abiesinol F Larixinol (Abiesinol E) | In vitro: PTP-1B enzyme assay/IC50 = 3.3, 11.2, 17.1, 4.6, 12.9, 8.1 µM | Xiong et al. (2020) |
| Gymnema latifolium Wall. ex Wight [Apocynaceae]/Vietnamese | Aerial parts/EtOH | Gymlatinoside GL2, GL3 | In vitro: PTP-1B enzyme assay/IC50 = 22.66, 19.83 µM | Pham et al. (2020) |
| Gynostemma pentaphyllum (Thunb.) Makino [Cucurbitaceae]/Chinese | Aerial parts/EtOH | Gypenoside 2–6 | In vitro: PTP-1B enzyme assay/IC50 = 18.2, 23.5, 28.6, 8.2, 12.5 µM | Wang et al. (2017b) |
| Helicteres isora L. [Malvaceae]/Jamu | Gum/Aqueous | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 3.49 μg/ml | Saifudin et al. (2016b) |
| Houttuynia cordata Thunb. [Saururaceae]/Korea, Japan, India, and China | Aerial parts/EtOH | 3-hydroxy-1,2-dimethoxy-5-methyl-5H-dibenzoindol-4- one 4-hydroxy-1,2,3-trimethoxy- 7H-dibenzo-quinolin-7-one 7-oxodehy- droasimilobine Cepharadione B | In vitro: PTP-1B enzyme assay/IC50 = 1.254, 2.016, 2.672, 1.862 µM | Ma et al. (2017) |
| Hypericum longistylum Oliv. [Hypericaceae]/Chinese | Aerial parts/MeOH | Longistylione A–D | In vitro: PTP-1B enzyme assay/IC50 = 18.87, 16.76, 24.56, 15.96 µM | Cao et al. (2017) |
| Hypericum perforatum L. [Hypericaceae]/Chinese | Aerial parts/EtOH | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 1.08 μg/ml In vivo: insulin-resistant mice (50 and 200 mg/kg b.w.)/↓PTP-1B expression, ↑hepatic pAkt, ↑hepatic pIRS-1, ↑glucose tolerance, ↑insulin sensitivity, ↓triglycerides, improvement of lipid metabolism | Tian et al. (2015) |
| Hypericum scabrum L. [Hypericaceae]/Chinese | Aerial parts/EtOH | Quercetin | In vitro: PTP-1B enzyme assay/IC50 = 2.19 µM | Jiang et al. (2015) |
| Iris sanguinea Hornem. [Iridaceae]/Chinese | Seeds/MeOH | Kikkanol F monoacetate | In vitro: PTP-1B enzyme assay/IC50 = 7.3 µM In vitro: cell culture/↑glucose uptake (3T3-L1), ↑pAMPK (C2C12) | Yang et al. (2017) |
| Juniperus chinensis L. [Cupressaceae]/Chinese | Heartwood/MeOH | a-methyl artoflavanocoumarin | In vitro: PTP-1B enzyme assay/IC50 = 25.27 µM In vitro: cell culture (insulin-resistant HepG2)/↓PTP-1B protein, ↑pPI3K, ↑pAkt, ↑pERK1, ↑insulin-stimulated glucose uptake | Jung et al. (2017) |
| Kandelia candel (L.) Druce [Rhizophoraceae]/Vietnamese | Bark/EtOAc and n-BuOH | Full extracts | In vitro: PTP-1B enzyme assay/IC50 = 12.9, 0.02 μg/ml | Trinh et al. (2017a) |
| Lagerstroemia speciosa (L.) Pers. [Lythraceae]/Vietnamese | Leaves/n-BuOH | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 19.6 μg/ml | Trinh et al. (2017a) |
| Lantana camara L. [Verbenaceae]/Indonesian and Japanese | Aerial parts/EtOH | 24- hydroxy-lantadene B 3-hydroxy-lantadene C Icterogenin 4-epi- hederagonic acid Oleanolic acid 22b-oleanolic acid 3b-hydroxy-lantadene A 3b-hydroxy-lantadene B 22-hydroxy-oleanonic acid Lantadene B, A Oleanonic acid Lantadene D Pomonic acid Pomolic acid Lantanilic acid Camaric acid Lantanolic acid | In vitro: PTP-1B enzyme assay/IC50 = 7.3, 7.3, 11, 8.1, 2, 7.9, 7.2, 5.1, 6.9, 5.5, 5.2, 6.9, 7.9, 10.5, 10.6, 7.5, 5.1, 13 µM | Abdjul et al. (2017) |
| Lanxangia tsao-ko (Crevost & Lemarié) M.F. Newman & Škorničk [Zingiberaceae]/Asian countries | Fruits/EtOH | Tsaokoflavanol F, J, K, L, S | In vitro: PTP-1B enzyme assay/IC50 = 56.4, 75.1, 80.4, 73, 69.8 µM In vivo: db/db mice (200 and 400 mg/kg b.w.)/chronic hypoglycemic effect (full extract) | He et al. (2020) |
| Leonurus sibiricus L. [Lamiaceae]/Mongolian | Aerial parts/MeOH | Full extract | In vitro: PTP-1B enzyme assay/Activity inhibition by 40% at 10 μg/ml In vitro: cell culture (C2C12, in the presence of insulin)/↑glucose uptake | Pitschmann et al. (2016) |
| Lithocarpus polystachyus (Wall. ex A.DC.) Rehder [Fagaceae]/Chinese | Leaves/MeOH | Full extracts from five localities in China | In vitro: PTP-1B enzyme assay/IC50 = inhibition rate ranging from 84.3 to 90.3% at 1.25 mg/ml | Meng et al. (2020) |
| Litsea cubeba (Lour.) Pers. [Lauraceae]/Chinese | Twigs/EtOAc | (+)-9,9′-O-di-(E)-feruloyl-5,5′-dimethoxy secoisolariciresinol | In vitro: PTP-1B enzyme assay/IC50 = 13.5 µM | Li et al. (2019c) |
| Lonicera japonica Thunb. [Caprifoliaceae]/Chinese | Flower buds/EtOH | Lonjaponspiroside A, B | In vitro: PTP-1B enzyme assay/IC50 = 6.14, 8.42 µM | Liu et al. (2016) |
| Ludwigia octovalvis (Jacq.) P.H.Raven [Onagraceae]/Vietnamese | Aerial parts/EtOAc and n-BuOH | Full extracts | In vitro: PTP-1B enzyme assay/IC50 = 16.9, 3.3 μg/ml | Trinh et al. (2017a) |
| Macaranga denticulata (Blume) Müll.Arg. [Euphorbiaceae]/Chinese | Twings and leaves/EtOH | Macdentichalcone 1-(5,7-dihydroxy-2,2,6-trimethyl-2H-1-benzopyran-8-yl)-3-phenyl-2-propen-1-one | In vitro: PTP-1B enzyme assay/IC50 = 21, 22 µM | Lei et al. (2016) |
| Macleaya cordata (Willd.) R.Br. [Papaveraceae]/Chinese | Aerial parts/EtOH | Macleayine | In silico: molecular docking | Sai et al. (2016) |
| Maclura tricuspidata Carrière [Moraceae]/Korean, Japanese, and Chinese | Leaves/Aqueous | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 65 μg/ml In vitro: PTP-1B enzyme assay (protein chip screening method)/↓PTP-1B activity In vitro: cell culture (3T3-L1)/↓lipid droplets, ↑pIRS-1, ↑pAkt In vivo: obese mice (20 and 100 mg/kg b.w.)/↑hepatic pIRS-1, ↑hepatic pAkt ↓tryglicerides, ↓glucose tolerance | Kim et al. (2016) |
| Root barks/MeOH | Cudratricusxanthone N 1,6,7-trihydroxy-2-(1,1-dimethyl-2-propenyl)-3-methoxyxanthone Cudratricusxanthone L, A Cudraxanthone L Macluraxanthone B Cudracuspixanthone A Cudraxanthone D, M Cudraflavanone D Euchrestaflavanone C Cudraflavone C Kuwanon C | In vitro: PTP-1B enzyme assay/IC50 = 2, 3, 3, 4.3, 4.6, 3.8, 1.9, 2.8, 3.5, 5.7, 12.3, 9.4, 13.6 µM | Quang et al. (2015) | |
| Magnolia aromatica (Dandy) V.S.Kumar [Magnoliaceae]/Chinese | Twigs and leaves/EtOH | (1R,6S,7S)-1-hydroxy- cadin-4,9-dien-8-onea | In vitro: PTP-1B enzyme assay/IC50 = 83.5 µM | Wang et al. (2016b) |
| Magnolia officinalis Rehder & E.H.Wilson [Magnoliaceae]/Chinese | Root barks/MeOH | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 55.96 μg/ml In vitro: cell culture (3T3-L1 and C2C12, in the presence of insulin)/↑pIRb, ↑pERK, ↑GLUT4 translocation In vivo: db/db mice (0.5 g/kg b.w.)/chronic hypoglycemic effect | Sun et al. (2015) |
| Magnolia officinalis var. biloba Rehder & E.H.Wilson [Magnoliaceae]/Chinese | Barks/EtOH | Magterpenoid A, C | In vitro: PTP-1B enzyme assay/IC50 = 1.44, 0.81 µM | Li et al. (2018) |
| Magmenthane E, H | In vitro: PTP-1B enzyme assay/IC50 = 4.38, 3.8 µM | Li et al. (2019a) | ||
| Root and stem bark/EtOH | (±)-Mooligomers B, D, E | In vitro: PTP-1B enzyme assay/IC50 = 0.47, 2.1, 0.35, 1.22, 0.89, 0.14 µM | Li et al. (2020a) | |
| Melaleuca leucadendra (L.) L. [Myrtaceae]/Jamu | Fruit/MeOH | Betulinic acid Ursolic acid | In vitro: PTP-1B enzyme assay/IC50 = 1.5, 2.3 µM | Saifudin et al. (2016a) |
| Leaves/Aqueous | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 2.05 μg/ml | Saifudin et al. (2016b) | |
| Melicope pteleifolia (Champ. ex Benth.) T.G. Hartley [Rutaceae]/Chinese | Roots/CH2Cl2/CH3OH (1:1) | Melicoptelin B1/B2, D1/D2, E | In vitro: PTP-1B enzyme assay/IC50 = 34.4, 55.2, 66.6 µM | Xu et al. (2019b) |
| Momordica charantia L. [Cucurbitaceae] | Fruits/EtOH | 25- O-methylkaraviagein D (19R,23E)-5b,19-epox y-19,25-dimethoxycucurbita-6,23-dien-3b-ol | In vitro: PTP-1B enzyme assay/IC50 = 51.8, 54.95 µM | Yue et al. (2017) |
| Morus alba L. [Moraceae]/Chinese | Leaves/Aqueous | Full extract (mulberry leaves polysaccharide) | In vivo: insulin-resistant rats (200 mg/kg b.w.)/↑glucose tolerance, ↓insulin resistance, ↑hepatic glycogen synthesis, ↓hepatic PTP-1B expression, ↑hepatic pIRS-2, ↑hepatic pAkt-2, ↑hepatic PI3K | Ren et al. (2015) |
| Fruits/EtOH | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 11.89 μg/ml | Xiao et al. (2017b) | |
| Leave cell culture/EtOH | Morusalone A–D | In vitro: PTP-1B enzyme assay/IC50 = 2.51, 1.14, 0.35, 1.99 µM In vitro: cell culture (HepG2)/↑pIRβ and pAkt protein levels | Su et al. (2019) | |
| Morusalisin A–F | In vitro: PTP-1B enzyme assay/IC50 = 1.55, 2.24, 1.58, 1.52, 1.60, 1.14 µM | Su et al. (2020) | ||
| Leaves/EtOH | mortatarin E 3′-geranyl-3-prenyl-2′,4′,5,7- tetrahydroxyflavone | In vitro: PTP-1B enzyme assay/IC50 = 4.53, 10.53 µM In vitro: cell culture (insulin-resistant HepG2)/↑glucose uptake, ↑glycogen synthesis, ↓PTP-1B, ↑IRS1, ↑IRS2, ↓GSK3β, and ↑GLUT4 mRNA expression levels, ↓PTP-1B, ↑pIRS1, ↑PI3K, ↑pAkt, and ↑GLUT4 protein levels (mortatarin E) | Niu et al. (2020) | |
| Root bark/MeOH | Morusalfuran A–C, F Morusalnol B Morusibene A | In vitro: PTP-1B enzyme assay/IC50 = 11.02, 8.92, 7.26, 18.02, 26.56, 17.64 µM | Ha et al. (2020) | |
| Roots/EtOAc | Kuwanon L Mulberrofuran G Moracenin B (Kuwanon G) Morusinol Sanggenon G Kuwanon C Moracenin A (Kuwanon H) Kuwanon T, F, M Morusin Mulberrofuran B Cyclomorusin Sanggenofuran A | In vitro: PTP-1B enzyme assay/IC50 = 21.67, 20.03, 13.07, 30.49, 10.87, 17.48, 4.04, 9.83, 9.52, 10.71, 19.63, 4.69, 13.28, 12.72 μg/ml | Zhao et al. (2018) | |
| Morus macroura Miq. [Moraceae]/Chinese | Twigs/EtOH | Notabilisin E Taxifolin Hultenin | In vitro: PTP-1B enzyme assay/IC50 = 0.87, 5.3, 1.04 µM | Wang et al. (2015) |
| Myrtus communis L. [Myrtaceae]/Italian | Leaves/chloroform | 3β-cis-p-coumaroyloxy-2α,23-dihydroxyolean-12-en-28-oic acid 3β-trans-p-coumaroyloxy-2α,24-dihydroxy-urs-12-en-28-oic acid Maslinic acid Corosolic acid Isomyrtucommulone B 3β-O-cis-p-coumaroyl-2α-hydroxy-urs-12-en-28-oic acid Jacoumaric acid Betulinic acid Oleanolic acid Ursolic acid | In vitro: PTP-1B enzyme assay/IC50 = 15.38, 14.89, 25.73, 12.21, 8.93, 26.67, 11.93, 16.05, 8.92, 14.93 µM | Liang et al. (2020) |
| Nepenthes mirabilis (Lour.) Druce [Nepenthaceae]/Vietnamese | Whole plant/EtOAc and n-BuOH | Full extracts | In vitro: PTP-1B enzyme assay/IC50 = 1.4, 0.4 μg/ml | Trinh et al. (2017a) |
| Nigella sativa L. [Ranunculaceae]/From Turkey to India | Aerial parts/MeOH | 3-O-[α-l-Rhamnopyranosyl-(1→2)-α-l-arabinopyranpsyl]hederagenin | In vitro: PTP-1B enzyme assay/IC50 = 91.3 µM | Parveen et al. (2020) |
| Nigella sativa var. hispidula Boiss. [Ranunculaceae]/Uighur | Seeds/Petroleum ether | Nigelladine A–C | In vitro: cell culture (L6 myotubes, in the presence of insulin)/↓PTP-1B protein, ↑pAkt, ↑pIRS-1, ↑pGSK-3b, ↑pAMPK, ↑glucose consumption, ↑lactic acid production, ↑glycogen synthesis, ↑hexokinase activity | Tang et al. (2017) |
| Orthosiphon aristatus (Blume) Miq. [Lamiaceae]/Vietnamese and Indonesian | Aerial parts/MeOH | Siphonol B, D Orthosiphol B, F, G, I, N | In vitro: PTP-1B enzyme assay/IC50 = 8.18, 24.75, 9.84, 27.56, 3.82, 0.33, 1.6 µM In vitro: cell culture (3T3-L1)/↑glucose uptake | Nguyen et al. (2019) |
| Ouret lanata (L.) Kuntze [Amaranthaceae]/Ayurveda | Leaves/EtOH | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 94.66 μg/ml In vitro: cell culture (L6 myotubes)/↑adipogenesis, ↑insulin-mediated glucose uptake In vivo: diabetic rats (500 mg/kg b.w.)/antihyperglycemic effect in OSTTs by 18.44% | Riya et al. (2015) |
| Paeonia lactiflora Pall. [Paeoniaceae]/Chinese | Seeds/EtOH | Paeonilactiflorol trans-gnetin H | In vitro: PTP-1B enzyme assay/IC50 = 27.23, 27.81 µM | Zhang et al. (2019a) |
| Panax ginseng C.A.Mey. [Araliaceae]/Eastern Asia | Stems, flowers, and fruits/EtOH | 20(R)-25-methoxydammarane-3β,12β, 20-tetrol 20(R)-dammarane-3β,6α,12β, 20, 25-pentol 20(R)- protopanaxatriol 20(S)-panaxatriol 20(R)-protopanaxadiol | In vitro: PTP-1B enzyme assay/IC50 = 16.54, 10.07, 17.98, 21.02, 21.27 µM | Yang et al. (2016) |
| Panax quinquefolius L. [Araliaceae]/Chinese | Crude saponins/EtOH | 20(S)-panaxadiol (20S,24R)-dammarane-20,24-epoxy-3 β,6 α, 12 β,25- tetraol 20(R)-dammarane-3 β, 12 β,20,25-tetraol 20(S)-dammarane-3 β,6 α, 12 β,20,25-pentol 20(R)-dammarane-3 β, 12 β,20,25-tetrahydroxy-3 β-O- β- d–glucopyranoside Oleanolic acid 20(S)-protopanaxadiol | In vitro: PTP-1B enzyme assay/IC50 = 27.23, 23.63, 10.39, 6.21, 5.91, 18.99, 13.38 µM | Han et al. (2020) |
| Pandanus odorifer (Forssk.) Kuntze [Pandanaceae]/Vietnamese | Fruit/EtOAc and n-BuOH | Full extracts | In vitro: PTP-1B enzyme assay/IC50 = 20.8, 40.4 μg/ml | Trinh et al. (2017a) |
| Phyllanthus amarus Schumach. & Thonn. [Phyllanthaceae]/Vietnamese | Whole plant/EtOAc | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 74.4 μg/ml | Trinh et al. (2017a) |
| Phyllanthus niruri L. [Phyllanthaceae]/Jamu | Aerial parts/Aqueous | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 10.99 μg/ml | Saifudin et al. (2016b) |
| Phyllanthus urinaria L. [Phyllanthaceae]/Vietnamese | Whole plant/EtOAc and n-BuOH | Full extracts | In vitro: PTP-1B enzyme assay/IC50 = 14, 10.8 μg/ml | Trinh et al. (2017a) |
| Pithecellobium dulce (Roxb.) Benth. [Fabaceae]/Vietnamese | Stem/EtOAc | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 26.1 μg/ml | Trinh et al. (2017a) |
| Prunus amygdalus Batsch [Rosaceae] | Fruits/EtOH | Hexane fraction Chloroform fraction | In vitro: PTP-1B enzyme assay/IC50 = 9.66, 37.95 μg/ml | Qureshi et al. (2019) |
| Psidium guajava L. [Myrtaceae]/Worldwide | Leaves/EtOAc | Psiguadiol A–J | In vitro: PTP-1B enzyme assay/IC50 = 4.7, 11, 11.9, 10.7, 19.1, 18.9, 6.2, 9.2, 22.8, 22.8 µM | Hou et al. (2019) |
| Leaves/EtOH | Jejuguajavone A, C | In vitro: PTP-1B enzyme assay/IC50 = 10.52, 9.4 µM | Ryu et al. (2021) | |
| Psydrax subcordatus (DC.) Bridson [Rubiaceae]/African | Leaves and bark/EtOH | Subcordatanol I, III, IV | In vitro: PTP-1B enzyme assay/IC50 = 22.2, 8.9, 9.8 µM | Zhou et al. (2019) |
| Pueraria montana var. lobata (Willd.) Maesen & S.M.Almeida ex Sanjappa & Predeep [Fabaceae]/Chinese | Root/MeOH | Puerarin | In silico: molecular docking/PTP-1B binding energy: 6.4 kcal/mol | Ojo, (2021) |
| Roots/EtOH | Lupeol Lupenone | In vitro: PTP-1B enzyme assay/IC50 = 38.89, 15.11 µM | Seong et al. (2016) | |
| Full extract | In vitro: PTP-1B enzyme assay/IC50 = 0.046 mg/ml In vitro: cell culture (insulin resistant HepG2 cells)/↑glucose uptake In vivo: insulin resistant mice (0.25, 0.5, 1, and 2 g/kg b.w.)/↓AUC in OGTTs by 5.7, 10, 20, and 21% | Sun et al. (2019) | ||
| Quercus infectoria G.Olivier [Fagaceae]/Jamu | Fruits/Aqueous | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 4.68 μg/ml | Saifudin et al. (2016b) |
| Quercus wutaishanica Mayr [Fagaceae]/Chinese and Mexican | Acorn/EtOH | 3-O-galloyloleanolic acid 23- acetoxy-3-O-galloyloleanolic acid 3-acetoxy-23-O-galloyloleanolic acid Oleanolic acid 3-O-galloylursolic acid Ursolic acid | In vitro: PTP-1B enzyme assay/IC50 = 2.10, 4.17, 4.52, 17.25, 1.86, 17.37 µM | Xu et al. (2018a) |
| Leaves/EtOH | Quercetin-3-O-(2″-O-galloyl)-β-galactopyranoside Kaempferol-3-O-rutinoside Quercetin-3-O-rutinoside Quercetin Kaempferol Myricetin Dihydromyricetin 4′,5,7-trihydroxyflavanone 4′-methoxy-5′,5,7-trihydroxyflavanone Ellagic acid | In vitro: PTP-1B enzyme assay/IC50 = 5.56, 24.89, 20.56, 4.16, 3.92, 3.53, 9.58, 15.38, 20.16, 1.03 µM In vitro: cell culture (MIN6)/Protective effect on pancreatic b cells damaged by H2O2 (Quercetin-3-O-(2″-O-galloyl)-β-galactopyranoside) | Xu et al. (2018b) | |
| Reynoutria japonica Houtt. [Polygonaceae]/Japanese, South Korean, and Chinese | Roots/EtOAc | (trans)-emodin-physcion bianthrone (cis)-emodin-physcion bianthrone | In vitro: PTP-1B enzyme assay/IC50 = 2.77, 7.29 µM | Zhao et al. (2017) |
| Reynoutria multiflora (Thunb.) Moldenke [Polygonaceae]/Chinese | Roots/EtOH | Multiflorumiside H–K | In vitro: PTP-1B enzyme assay/IC50 = 1.2, 1.7, 1.5, 4.6 µM | Yang et al. (2020) |
| Rhizophora apiculata Blume [Rhizophoraceae]/India | Leaves/EtOH | Glycosin | In silico: molecular docking/PTP-1B binding energy: 6.35 kcal/mol In vivo: diabetic rats (50 mg/kg b.w.)/↓blood glucose reduction by 25% in OGTTs, ↓chronic hypoglycemic effect, ↓HbA1c, ↓triglycerides, ↓cholesterol, ↑HDL, ↑hexokinase activity, ↓G6Pase activity, ↑FBPase activity | Selvaraj et al. (2016) |
| Rhizophora mucronata Poir. [Rhizophoraceae]/Vietnamese | Bark/EtOAc and n-BuOH | Full extracts | In vitro: PTP-1B enzyme assay/IC50 = 17.2, 1.8 μg/ml | Trinh et al. (2017a) |
| Rhodiola rosea L. [Crassulaceae]/Chinese | Whole plant/MeOH | Arbutin | In vitro: PTP-1B enzyme assay/IC50 = 20.5 µM | Yuan et al. (2021) |
| Rhododendron fastigiatum Franch. [Ericaceae] | Aerial parts/EtOH | (+)-fastinoid B (-)-fastinoid B Rubiginosin A (-)-rubiginosin A Grifolinone A | In vitro: PTP-1B enzyme assay/IC50 = 47, 54.9, 40.9, 49.2, 13 µM | Huang et al. (2019) |
| Ricinus communis L. [Euphorbiaceae]/Chinese | Rhizomes/EtOH | 3α,19-dihydroxyl-ent-pimara-8 (14),15-diene | In vitro: PTP-1B enzyme assay/IC50 = 49.49% at 20 μg/ml | Zhang et al. (2019b) |
| Rubus chingii Hu [Rosaceae]/Chinese | Fruits/MeOH | Ursolic acid 2-oxopomolic acid 2α,19α-dihydroxy-3-oxo-urs- 12-en-28-oic acid | In vitro: PTP-1B enzyme assay/IC50 = 7.1, 23.7, 52.3 µM | Zhang et al. (2019c) |
| Rubus idaeus L. [Rosaceae] | Leaves/MeOH | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 3.41 μg/ml | Li et al. (2016) |
| Rubus occidentalis L. [Rosaceae] | Fruits/EtOH | Cyanidin-3-O-xylosilutinoside Cyanidin-3-O -rutinoside Quercetin-3-O-rutinoside Ellagic acid | In vitro: PTP-1B enzyme assay/IC50 = 2.58, 1.88, 2.12, 0.03 µM | Xiao et al. (2017a) |
| Salvia circinnata Cav. [Lamiaceae]/Mexican | Aerial parts/Aqueous | 6-hydroxyluteolin Pedalitin | In vitro: PTP-1B enzyme assay/IC50 = 80.1, 62 µM | Salinas-Arellano et al. (2020) |
| Salvia miltiorrhiza Bunge [Lamiaceae]/Chinese | Roots/EtOH | Cryptotanshinone Tanshinol B Tanshinonal 15,16-dihydrotanshinone I Tanshinone I Dehydrodanshenol A | In vitro: PTP-1B enzyme assay/IC50 = 5.5, 4.7, 37.6, 18.6, 27.1, 8.5 µM | Kim et al. (2017b) |
| Selected compounds of Tangzhiqing herbal formula In combination with Morus alba L. (Moraceae) Nelumbo nucifera Gaertn. (Nelumbonaceae) Crataegus pinnatifida Bunge (Rosaceae) Paeonia lactiflora Pall. (Paeoniaceae) | Nuciferine Rutin 1-Deoxynojirimycin Salvianolic acid A Salvianolic acid C Danshensu Rosmarinic acid Tanshinone IIA Cryptotanshinone Dihydrotanshinone I Quercitrin Paeoniflori | In silico: molecular docking/PTP-1B binding energy: 26.49, 86.52, 17.57, 72.09, 95.07, 49.37, 54.44, 25.66, 33.08, 22.27, 83.52, 44.46 kcal/mol | Hao et al. (2020) | |
| Selaginella rolandi-principis Alston [Selaginellaceae]/Vietnamese | Aerial parts/EtOH | Selaginolide A 2′-hydroxygenistein 6,7-dimethoxy-2′,4′-dihydroxyisoflavone | In vitro: PTP-1B enzyme assay/IC50 = 7.4, 23.02, 11.08 µM In vitro: cell culture (3T3-L1)/↑glucose uptake, ↑pIRS-1, ↑pPI3K, pAkt | Nguyen et al. (2021) |
| Selaginella tamariscina (P.Beauv.) Spring [Selaginellaceae]/Chinese | Aerial parts/MeOH | Selariscinin D Selariscinin E Amentoflavone Robustaflavone Cupressuflavone Taiwaniaflavone 3,8″-biapigenin | In vitro: PTP-1B enzyme assay/IC50 = 13.2, 9.8, 7.4, 6.2, 9.6, 5.4, 4.5 µM In vitro: cell culture (3T3-L1)/↑glucose uptake | Nguyen et al. (2015) |
| Sellaginellin U–W Sellaginellin Selariscinin A | In vitro: PTP-1B enzyme assay/IC50 = 13.8, 14.5, 14.6, 15.9, 4.8 µM | Le et al. (2017) | ||
| Selaginella uncinata (Desv.) Spring [Selaginellaceae]/Chinese | Whole plant/EtOH | Uncinatabiflavone C 7-methyl ether Robustaflavone 4′-methyl ether Robustaflavone 7- methyl ether (2R) 2, 3-dihy- droamentoflavone Amentoflavone Bilobetin (2″S) chrysocauloflavone I Delicaflavone (2S) 2,3-dihydro- 5,5″,7,7″,4′-pentahydroxy-6,6″-dimethyl-[3′-O-4‴]-biflavone | In vitro: PTP-1B enzyme assay/IC50 = 7.7, 9.2, 9.8, 16.1, 10.6, 14.6, 5.5, 6.2, 4.6 µM In vitro: cell culture (insulin-resistant HepG2)/↑glucose uptake, ↑pIRS-1, ↑pPI3K, pAkt (Uncinatabiflavone C 7-methyl ether) | Xu et al. (2019a) |
| Senna obtusifolia (L.) H.S.Irwin & Barneby [Fabaceae]/Chinese | Seeds/MeOH | Physcion Chrysophanol Emodin Alaternin Obtusin Questin Chryso-obtusin Aurantio-obtusin 2-Hydroxyemodin-1 methylether | In vitro: PTP-1B enzyme assay/IC50 = 7.28, 5.86, 3.51, 1.22, 6.44, 5.69, 14.88, 27.19, 5.22 µM In vitro: cell culture (insulin-resistant HepG2)/↑insulin-stimulated glucose uptake (alaternin and emodin) | Jung et al. (2016) |
| Silybum marianum (L.) Gaertn. [Asteraceae] | Seeds/EtOAc | Taxifolin Dihydrokaempferol Dihydroquercetin-4′-methylether Kaempferol | In vitro: PTP-1B enzyme assay/IC50 = 24.23, 27.83, 21.30, 6.79 µM | Qin et al. (2017) |
| Smilax china L. [Smilacaceae]/Thai | Leaves/EtOH | Morin Kaempferol 7-O-α-l-rhamnoside Quercetin-4′-O-β-D-glucoside 4′-methoxy-5,7-dihydroxyflavone-(3-O-7″)-4’’’,5″,7″-trihydroxyflavone Partensein 1,3,6-trihydroxyxanthone |
In vitro: PTP-1B enzyme assay/IC50 = 7.62, 10.80, 0.92, 2.68, 9.77, 24.17 µM | Zhao et al. (2016) |
| Sophora flavescens Aiton [Fabaceae] | Roots/EtOH | Sophobiflavonoid A, C | In vitro: PTP-1B enzyme assay/IC50 = 0.33, 0.35 µM | Yan et al. (2019) |
| Symplocos cochinchinensis (Lour.) S. Moore. [Symplocaceae]/Ayurveda | Bark/EtOH | Full extract | In vivo: insulin-resistant rats (250 and 500 mg/kg b.w.)/↓hepatic PTP-1B activity and expression, ↑hepatic pAkt, ↑hepatic pIRS-1, ↑glucose tolerance, ↑insulin sensitivity, ↓hepatic triglycerides, ↓expression of gluconeogenic enzymes | Antu et al. (2016) |
| Syzygium cumini (L.) Skeels [Myrtaceae]/Vietnamese, Ayurveda, Unani, and Chinese | Seeds/MeOH | Valoneic acid dilactone Rubuphenol Ellagic acid | In vitro: PTP-1B enzyme assay/IC50 = 9.37, 28.14, 25.96 µM | Sawant et al. (2015) |
| Fruit/EtOAc | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 27.5 μg/ml | Trinh et al. (2017a) | |
| Tetradium ruticarpum (A.Juss.) T.G.Hartley [Rutaceae]/East Asia | Buds/MeOH | Schinifoline Intergrifoliodiol | In vitro: PTP-1B enzyme assay/IC50 = 24.3, 47.7 µM | To et al. (2021) |
| Thonningia sanguinea Vahl [Balanophoraceae]/Angola | Rhizomes/MeOH | 2′-O- (3-O-galloyl-4,6-O-Sa-hexahydroxydiphenoyl-β-d-glucopyranosyl)-3-hydroxyphloretin 4′-O-(4,6-O-Sa-hexahydroxydiphenoyl-β-d-glucopyranosyl)- phloretin 2′-O-(3-O-galloyl-4,6-O-Sa- hexahydroxydiphenoyl-β-d-glucopyranosyl)phloretin Thonningianin B, A | In vitro: PTP-1B enzyme assay/IC50 = 24.7, 23.8, 19.3, 21.7, 4.4 µM In vitro: cell culture (HepG2)/↑insulin-stimulated IR phosphorylation (Thonningianin A) | Pompermaier et al. (2018) |
| Tinospora sagittata (Oliv.) Gagnep. [Menispermaceae]/Chinese | Rhizome/EtOAc | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 38.5 μg/ml | Zhao et al. (2019b) |
| Tradescantia spathacea Sw. [Commelinaceae]/Vietnamese | Aerial parts/MeOH | Bracteanolide A Latifolicinin C, A Oresbiusin A | In vitro: PTP-1B enzyme assay/IC50 = 7.82, 6.80, 4.55, 6.38 µM | Vo et al. (2015) |
| Ugni molinae Turcz. [Myrtaceae]/Chilean | Leaves/EtOAc | Full extract Madecassic acid Myricetin | In vitro: PTP-1B enzyme assay/IC50 = 97.2% at 2 μg/ml (full extract) In vivo: Insulin resistant mice (20, 20, and 20 mg/kg b.w.)/↓PTP-1B mRNA expression in aorta (full extract), ↑glucose tolerance (full extract and madecassic acid), ↑aortic insulin sensitivity | Arancibia-Radich et al. (2019) |
| Vaccinium myrtillus L. [Ericaceae] | Fruits/EtOH | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 6.96 μg/ml | Xiao et al. (2017b) |
| Vaccinium uliginosum L. [Ericaceae] | Fruits/EtOH | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 3.06 μg/ml | Xiao et al. (2017b) |
| Phenolic compounds Cyanidin-3-arabinoside Delphinidin-3-glucoside Cyanidin-3-galactoside Cyanidin-3-glucoside Malvidin-3-galactoside Petunidin-3-glucoside | In vitro: PTP-1B enzyme assay/IC50 = 8.91, 17.8, 19.8, 25.9, 34, 31.1 µM In vitro: cell culture (PTP-1B-overexpressed HepG2)/↑glucose consumption, ↑glycogen synthesis, ↓PTP-1B mRNA expression and protein level, ↑IRS1 and ↓GSK3β mRNA expression, ↑pIRS1, PI3K, pAkt, pAMPK, and pGSK3β protein level (cyanidin-3-arabinoside) | Tian et al. (2019) | ||
| Procyanidin B1, B2 | In vitro: PTP-1B enzyme assay/IC50 = 0.6, 4.79 µM In vitro: cell culture (HepG2)/↓PTP-1B mRNA expression and protein level | Li et al. (2021) | ||
| Viburnum macrocephalum Fortune [Viburnaceae]/Chinese | Fruits/EtOH | Viburmacrosides C Viburmacrosideand D (+)-8′-hydroxypinoresinol 4-O-b-D-glucoside (-)-olivil 4′-O-b-D-glucoside | In vitro: PTP-1B enzyme assay/IC50 = 25.8, 8.9, 28.7, 27.5 µM | Zhao et al. (2020) |
| Vigna radiata (L.) R.Wilczek [Fabaceae] | Seeds/Aqueous | Full extract | In vitro: PTP-1B enzyme assay/IC50 = 10 μg/ml In vitro: cell culture (insulin-resistant HepG2)/↑glucose uptake, ↓PEPCK and GSK3β mRNA expression level | Saeting et al. (2021) |
EtOH: ethanolic extract; MeOH: methanolic extract; EtOAc; ethyl acetate extract; PTP-1B: protein tyrosine phosphatase 1B; OSTT: oral sucrose tolerance test; OGTT: oral glucose tolerance test; IR: insulin receptor; IRS1: insulin receptor substrate 1; IRS2: insulin receptor substrate 2; GSK3β: glycogen synthase kinase 3 beta; Akt: protein kinase B; PI3K: phosphoinositide 3-kinase; ERK1: extracellular signal-regulated kinase 1; HFD: high-fat diet; HDL: high-density lipoprotein; LDL: low-density lipoprotein; VLDL: very low-density lipoprotein; PEPCK: phosphoenolpyruvate carboxykinase; FBPase: fructose 1,6-bisphosphatase; G6Pase: glucose 6-phosphatase; GLUT2: glucose transporter 2; GLUT4: glucose transporter 4; AMPK: AMP-activated protein kinase; PPARγ: peroxisome proliferator-activated receptor gamma; HbA1c: glycated hemoglobin.
Inhibition of Hepatic Glucose Output by Modulating Glucose Metabolism in Liver
The liver is a key organ that plays a crucial role in the regulation of blood glucose because it manages both storage and synthesis of glucose. The latter involves two metabolic pathways: glycogenolysis and gluconeogenesis, which constitute total hepatic glucose production (HGP) (Lee et al., 2015). Glycogenolysis consists of glycogen breakdown into glucose, being half of the basal HGP in fasting and decreasing the glycogen concentration at an almost linear rate during the first 22 h (Rothman et al., 1991; Cersosimo et al., 2018). In fasting, it is controlled by glucagon and epinephrine that activate glycogen phosphorylase (GP), the major enzyme responsible for digesting glycogen by releasing glucose 1-phosphate. In feeding condition, insulin inhibits glycogen breakdown and promotes glycogen synthesis through the activation of Akt and protein phosphatase 1 (PP1), leading the deactivation of both GP and glycogen synthase kinase-3 (GSK3), which in its active form (dephosphorylated), inactivates glycogen synthase (GS) (Han et al., 2016).
Gluconeogenesis, on the other hand, is defined as the production of glucose from a molecule that is not a carbohydrate. Its main substrates are pyruvate, glycerol, and amino acids such as alanine (Hanson and Owen, 2013). Another way to denote gluconeogenesis is as “reverse glycolysis” since both share not only substrates and final products, but also many enzymes. However, the direction of the reactions catalyzed in gluconeogenesis goes in the opposite direction, so the steps that are not shared with glycolysis can be determined as regulatory steps. These reactions are catalyzed by four rate-limiting enzymes: pyruvate carboxylase (PC), which is responsible for converting pyruvate into oxaloacetate; phosphoenolpyruvate carboxykinase (PEPCK), that converts oxaloacetate to phosphoenolpyruvate; fructose 1,6-bisphosphatase (FBPase), that dephosphorylates fructose 1,6-bisphosphate obtaining fructose 6-phosphate; and glucose 6-phosphatase (G6Pase), which is responsible for removing the phosphate group from glucose 6-phosphate, yielding novo synthesized glucose (Postic et al., 2004).
In the diabetic state, increased rates of HGP are observed as a result of an imbalance of various factors, such as the augmented availability of gluconeogenic substrates, the resistance of the liver to the action of insulin, and elevated levels of glucagon that activate HGP (Sharabi et al., 2015). Due to all these factors, the inhibition of HGP turns out to be an important therapeutic target for the reduction of hyperglycemia observed in T2D patients. In this regard, Table 2 summarizes the works made between 2015 and 2021 with extracts or natural products from 47 medicinal plants that showed to modulate hepatic glucose metabolism by inhibiting glucose production or promoting glycogen synthesis. As it can be observed, decreasing the expression of PEPCK and G6Pase is the principal mechanism related to gluconeogenesis inhibition, while phosphorylation of GSK3, promotion of GS activity, and inhibition of GP are the main mechanisms involved in glycogen breakdown and synthesis. Furthermore, although PI3K/Akt pathway stands out as a good pharmacological target to reduce insulin resistance, medicinal plants and their phytochemicals can also decrease HGP through AMP-activated protein kinase (AMPK).
TABLE 2.
Medicinal plants and their phytochemicals capable to modulate hepatic glucose metabolism.
| Medicinal plant (scientific name [Family]/Traditional medicine system or places where it is used) | Part/Extract | Isolated compounds | Experiment/Outcome | References |
|---|---|---|---|---|
| Abelmoschus esculentus (L.) Moench [Malvaceae]/Chinese | Whole plant/EtOH | Polysaccharides | In vivo: insulin-resistant mice (200 and 400 mg/kg b.w.)/↑pAkt and pGSK3β | Liao et al. (2019) |
| Ageratina petiolaris (Moc. & Sessé ex DC.) R.M.King & H.Rob. [Asteraceae]/Mexican | Aerial parts/Aqueous | Full extract | In vivo: diabetic rats (160 mg/kg b.w.)/↓Glucose production in PTTs In vitro: G6Pase inhibition assay/IC50 = 223 μg/ml | Mata-Torres et al. (2020) |
| Aloe vera (L.) Burm.f. [Asphodelaceae]/Aryuveda | Gel/EtOH | Carbohydrate fraction | In vivo: diabetic rats (27 and 54 mg/kg b.w.)/↑Liver glycogen content, ↑GS protein levels, ↓G6Pase activity | Govindarajan et al. (2021) |
| Alsophila firma (Baker) D.S.Conant [Cyatheaceae]/Mexican | Rhizome/Aqueous | Full extract | In vitro: G6Pase inhibition assay/IC50 = 341 μg/ml In vitro: FBPase inhibition assay/IC50 = 45 μg/ml | Andrade-Cetto et al. (2021b) |
| Aster spathulifolius Maxim. [Asteraceae]/Korean | Whole plant/EtOH | Full extract | In vivo: db/db mice (50, 100, and 200 mg/kg b.w.)/↑GK, ↓G6Pase and PEPCK expression | Yin et al. (2015) |
| Averrhoa bilimbi L. [Oxalidaceae]/Indian | Fruits/Aqueous | EtOAc fraction | In vivo: diabetic rats (25 mg/kg b.w.)/↓G6Pase and FBPase activity | Kurup and S, (2017) |
| Bromelia karatas L. [Bromeliaceae]/Mexican | Aerial parts/Aqueous | Full extract | In vitro: G6Pase inhibition assay/IC50 = 1,136 μg/ml | Mata-Torres et al. (2020) |
| Calea urticifolia (Mill.) DC. [Asteraceae]/Mexican | Aerial parts/Aqueous | Full extract | In vivo: diabetic rats (41 mg/kg b.w.)/↓Glucose production in PTTs In vitro: G6Pase inhibition assay/IC50 = 406 μg/ml | Andrade-Cetto et al. (2021a) |
| Calotropis procera (Aiton) W.T.Aiton [Apocynaceae]/Indian | Aerial parts/Latex | Protein fraction | In vivo: Wistar rats (5 mg/kg b.w.)/↑ pAMPK, ↓PEPCK expression, ↓blood glucose in PTTs | de Oliveira et al. (2019) |
| Caralluma fimbriata Wall. [Apocynaceae]/Indian | Stems/EtOH | Full extract | In vivo: insulin-resistant rats (200 mg/kg b.w.)/↓G6Pase and FBPase activity | Gujjala et al. (2017) |
| Caralluma quadrangula (Forssk.) N.E.Br./Apocynaceae/Saudi | Whole plant/MeOH | Russelioside B | In vivo: diabetic rats (50 mg/kg b.w.)/↑Glycogen content, ↓GP activity, ↓GS and GSK3β expression, ↓G6Pase activity and expression | Abdel-Sattar et al. (2016) |
| Chrysobalanus icaco L. [Chrysobalanaceae]/Nigerian | Leaves/Aqueous | Full extract | In vivo: diabetic rats (11.076, 22.134, and 44.268 mg/kg b.w.)/↑Liver glycogen content, ↓G6Pase activity | Ekakitie et al. (2021) |
| Cola nitida (Vent.) Schott & Endl. [Malvaceae]/African | Seeds/Aqueous | Full extract | In vivo: diabetic rats (300 mg/kg b.w.)/↓GP, G6Pase, and FBPase activities | Erukainure et al. (2019b) |
| Combretum lanceolatum Pohl ex Eichler [Combretaceae]/Brazilian | Flowers/EtOH | Full extract | In vivo: diabetic rats (500 mg/kg b.w.)/↑pAMPK and pAkt, ↓PEPCK expression | Siqueira et al. (2016) |
| Coreopsis tinctoria Nutt. [Asteraceae]/Chinese and Portuguese | Flowers/EtOAc | Marein | In vitro: cell culture (insulin-resistant HepG2)/↑Glycogen content, ↓G6Pase and PEPCK expression and protein levels | Jiang et al. (2018a) |
| Corispermum squarrosum L. [Amaranthaceae]/Mongol | Whole plant/EtOH | Oligosaccharides | In vivo: db/db mice (380 and 750 mg/kg b.w.)/In liver: ↑pIRS2, ↑pAkt, ↑IRS2, PI3K, Akt, and IR expression and protein levels | Bao et al. (2020) |
| Couroupita guianensis Aubl. [Lecythidaceae] | Leaves/Aqueous | Full extract Gold nanoparticles | In vivo: diabetic rats (100 and 2.5 mg/kg b.w.)/↑Glycogen storage, ↓G6Pase activity and expression | Manimegalai et al. (2020) |
| Edgeworthia gardneri (Wall.) Meisn. [Thymelaeaceae]/Chinese | Flowers/Aqueous | Full extract | In vitro: cell culture (insulin-resistant HepG2)/↑Glucose uptake and consumption, ↑Glycogen content, ↓Gluconeogenesis, ↑pIR, ↑pIRS1, ↑pAkt, ↑pGSK3 | Zhang et al. (2020) |
| Equisetum myriochaetum Schltdl. & Cham. [Equisetaceae]/Mexican | Aerial parts/Aqueous | Full Extract | In vivo: diabetic rats (330 mg/kg b.w.)/↓Glucose production in PTTs | Mata-Torres et al. (2020) |
| Eryngium cymosum F.Delaroche [Apiaceae]/Mexican | Aerial parts/Aqueous | Full extract | In vivo: diabetic rats (470 mg/kg b.w.)/↓ Glucose production in PTTs In vitro: G6Pase inhibition assay/IC50 = 782 μg/ml In vitro: FBPase inhibition assay/IC50 = 57.4 μg/ml | Espinoza-Hernández et al. (2021) |
| Eryngium longifolium Cav. [Apiaceae]/Mexican | Aerial parts/EtOH | Full extract | In vitro: G6Pase inhibition assay/IC50 = 780 μg/ml In vitro: FBPase inhibition assay/IC50 = 93 μg/ml | Andrade-Cetto et al. (2021b) |
| Ficus carica L. [Moraceae]/Spain | Leaves/EtOH | Full extract | In vivo: diabetic mice (2 g/kg b.w.)/↓PEPCK and G6Pase expression, ↑pAMPK In vitro: cell culture (HepG2)/↓PEPCK and G6Pase expression, ↑pAMPK | Zhang et al. (2019d) |
| Forsythia suspensa (Thunb.) Vahl [Oleaceae]/Chinese | Fruit/MeOH | Full extract | In vivo: diabetic mice (200 mg/kg b.w.)/↓PEPCK expression | Zhang et al. (2016b) |
| Graptopetalum paraguayense (N.E.Br.) E.Walther [Crassulaceae]/Taiwan | Leaves/MeOH | Full extract Partially purified fraction (HH-F3) | In vitro: cell culture (Hep3B/T2)/↓PEPCK and G6Pase expression | Jhuang et al. (2015) |
| Hyoscyamus albus L. [Solanaceae]/Mediterranean | Seeds/MeOH | Calystegine fraction | In vitro: cell culture (insulin-resistant HepG2)/↑Glucose consumption, ↓G6Pase (catalytic subunit) expression, ↑IR, IRS1/2, PI3K, Akt1/2 expression and protein levels | Kowalczuk et al. (2021) |
| Hypericum attenuatum Fisch. ex Choisy [Hypericaceae]/Chinese | Whole plant/EtOH | Full extract | In vivo: insulin-resistant mice (100, 200, and 300 mg/kg b.w.)/↓PEPCK and G6Pase expression and protein levels, ↑GS expression and protein levels, ↑pIRS, ↑PI3K, ↑pAkt, ↑GSK3 | Jin et al. (2019) |
| Iris domestica (L.) Goldblatt & Mabb. [Iridaceae]/Chinese | Leaves/EtOH | Saponins and polysaccharide fraction Flavonoid fraction | In vivo: KK-Ay-mice (200 mg/kg b.w.)/↓G6Pase and PEPCK activities, ↑Glycogen content | Guo et al. (2019) |
| Launaea acanthodes (Boiss.) Kuntze [Asteraceae]/Iran | Aerial parts/EtOH | Full extract | In vivo: diabetic rats (100, 200, and 400 mg/kg b.w.)/↑GK and GLUT2 expression, ↓PEPCK and G6Pase expression | Marvibaigi et al. (2021) |
| Lithocarpus polystachyus (Wall. ex A.DC.) Rehder [Fagaceae]/Chinese | Leaves/Aqueous | Full extract | In vivo: insulin-resistant mice (800 mg/kg b.w.)/↑Glycogen content, ↑Liver glucose influx,/↓G6Pase and PEPCK expression, ↑IR and IRS expression | Wang et al. (2016a) |
| Lupinus mutabilis Sweet [Fabaceae]/Andean | Seeds/Aqueous | Protein fraction | In vitro: cell culture (HepG2)/↓glucose production and PEPCK expression | Muñoz et al. (2018) |
| Myrianthus arboreus P.Beauv. [Urticaceae]/African | Root bark/EtOH | EtOAc fraction Isoorientin Orientin Chlorogenic acid | In vitro: cell culture (H4IIE hepatocytes)/↓G6Pase activity, ↑pAMPK | Kasangana et al. (2019) |
| Root bark/Aqueous, EtOH (EtOAc and hexane fractions), Alkaloid rich, and DCM extracts | Full extracts or fractions | In vitro: cell culture (H4IIE hepatocytes)/↓G6Pase activity, ↑pAkt, ↑pAMPK In vitro: cell culture (HepG2)/↑GS activity, ↑GSK3 | Kasangana et al. (2018) | |
| Myrica rubra (Lour.) Siebold & Zucc. [Myricaceae]/Chinese | Fruits/EtOH | Full extract | In vivo: KK-Ay mice (200 mg/kg b.w.)/↑pAMPK. ↓ PEPCK and G6Pase expression In vitro: cell culture (HepG2)/↑pAMPK, ↓PEPCK and G6Pase expression | Zhang et al. (2016a) |
| Pachylobus edulis G.Don [Burseraceae]/African and Nigerian | Leaves/EtOH | BuOH fraction | In vivo: diabetic rats (150 and 300 mg/kg b.w.)/↓GP, FBPase, and G6Pase activities | Erukainure et al. (2020) |
| Leaves/EtOAc, EtOH, and Aqueous | Full extracts | In vitro: G6Pase inhibition assay/IC50 = 0.66, 3.59, 0.05 μg/ml | Erukainure et al. (2017) | |
| Panax ginseng C.A.Mey. [Araliaceae]/Korean | Roots/EtOH | Black ginseng extract | In vivo: diabetic mice (300 and 900 mg/kg b.w.)/↓G6Pase, PEPCK and GP expression, ↑GS expression | Seo et al. (2016) |
| Plantago depressa Willd [Plantaginaceae]/Chinese | Seeds/EtOH | Plantadeprate A Plumbagine D Plantagoguanidinic acid | In vitro: cell culture (rat hepatocytes)/↓Gluconeogenesis inhibition by 8.2, 18.5, and 12.5% at 40 μM | Zheng et al. (2015) |
| Raphia hookeri G.Mann & H.Wendl. [Arecaceae] | Raffia palm wine/Concentrated in water bath | Concentrated wine | In vivo: diabetic rats (150 and 300 mg/kg b.w.)/↓GP, FBPase and G6Pase activity | Erukainure et al. (2019) |
| Rhizophora mangle L. [Rhizophoraceae]/Mexican | Bark/EtOH | Full Extract | In vivo: diabetic rats (90 mg/kg b.w.)/↓Glucose production in PTTs In vitro: G6Pase inhibition assay/IC50 = 99 μg/ml | Mata-Torres et al. (2020) |
| Rhodiola crenulata (Hook.f. & Thomson) H.Ohba [Crassulaceae]/Asian and Eastern European countries | Roots/EtOH | Full extract | In vivo: Sprague–Dawley rats (50 mg/kg b.w.)/↓PEPCK expression, ↑pAMPK In vitro: cell culture (HepG2)/↑pGSK3β and AMPK, ↓PEPCK and G6Pase expression | Lee et al. (2015) |
| Sarcopoterium spinosum (L.) Spach [Rosaceae]/Israel, Palestine, and Jordan | Root/Aqueous | Full extract | In vivo: insulin-resistant mice (35 and 100 mg/kg b.w.)/↑pIR, ↑pAkt, ↑GSK3, ↑glycogen content In vivo: KK-Ay mice (35 and 100 mg/kg b.w.)/↑pIR, ↑pAkt, ↓PEPCK expression | Rozenberg and Rosenzweig, (2018) |
| Senna alata (L.) Roxb. [Fabaceae]/Asia, Africa and South America | Leaves/EtOH | Full extract | In vivo: diabetic rats (400 mg/kg b.w.)/↑Liver glycogen content, ↑GS activity, ↓GP and FBPase activities | Mohanasundaram et al. (2021) |
| Flowers/Aqueous | Full extract EtOAc fraction n-butanol fraction Aqueous fraction | In vivo: diabetic rats (75 mg/kg b.w.)/↑Glycogen storage | Uwazie et al. (2020) | |
| Sesbania grandiflora (L.) Poir. [Fabaceae]/Aryuveda | Flowers/MeOH | Full extract | In vivo: diabetic rats (250 mg/kg b.w.)/↑Liver glycogen content, ↑GS activity, ↓GP, G6Pase, and FBPase activities | Sureka et al. (2021) |
| Shirakiopsis elliptica (Hochst.) Esser [Euphorbiaceae]/Nigerian | Leaves/EtOH | Full extract | In vivo: diabetic rats (400 and 800 mg/kg b.w.)/↑GK activity by 40.31%, ↓G6Pase activity by 37.29%, ↑Glycogen content | Ighodaro et al. (2017) |
| Smilax moranensis M.Martens & Galeotti [Smilacaceae]/Mexican | Roots/EtOH | Full Extract | In vitro: G6Pase inhibition assay/IC50 = 84 μg/ml | Mata-Torres et al. (2020) |
| Swietena humilis Zucc. [Meliaceae]/Mexican | Seeds/Aqueous | Dried aqueous extract Mexicanolide 1 Mexicanolide 2 Mexicanolide 3 | In vitro: G6Pase inhibition assay in H4IIE hepatocytes Extract: 100 μg/ml = 40.67%; 200 μg/ml = 61.11 1: 9.46 µM = 42.68% 2: 8.79 µM = 56.51% 3: 8.13 µM = 41.79% | Ovalle-Magallanes et al. (2019) |
| Tephrosia tinctoria (L.) Pers. [Fabaceae]/Aryuveda | Stems/EtOAc | EtOAc fraction | In vivo: diabetic rats (100 and 200 mg/kg b.w.)/↑Liver glycogen content, ↓G6Pase and FBPase activity | Krishnasamy and Periyasamy, (2019) |
| Terminalia catappa L. [Combretaceae]/Aryuveda | Leaves/EtOH | Full extract | In vivo: diabetic rats (300 and 500 mg/kg b.w.)/↓G6Pase and FBPase activity | Divya et al. (2019) |
| Trigonella foenum-graecum L. [Fabaceae]/Asia, Africa, and the Mediterranean region | Seeds/EtOH | Fenugreek flavonoids | In vivo: diabetic rats (0.5 g in 10 ml/kg)/↑Liver glycogen content, ↓G6Pase and FBPase activity | Jiang et al. (2018b) |
EtOH: ethanolic extract; MeOH: methanolic extract; EtOAc; ethyl acetate extract; PTT: pyruvate tolerance test; IR: insulin receptor; IRS1: insulin receptor substrate 1; IRS2: insulin receptor substrate 2; GSK3: glycogen synthase kinase 3; Akt: protein kinase B; PI3K: phosphoinositide 3-kinase; PEPCK: phosphoenolpyruvate carboxykinase; FBPase: fructose 1,6-bisphosphatase; G6Pase: glucose 6-phosphatase; GLUT4: glucose transporter 4; AMPK: AMP-activated protein kinase; GS: glycogen synthase; GP: glycogen phosphorylase.
Discussion
Insulin resistance in liver leads to the release of large amounts of glucose into the bloodstream that affects long-term homeostasis. The regulation of hepatic glucose output represents a good pharmacological target for the control of metabolic diseases such as T2D, which are characterized by the presence of this pathophysiological phenomenon. The search for new molecules capable of regulating hepatic glucose metabolism from medicinal plants has focused on screening for phytochemicals that can directly inhibit key enzymes in glucose-producing pathways. However, considering compounds with the ability to also decrease the activity of the enzymes involved in terminating the insulin signal could result in more effective glycemic control.
According to the bibliographic search, plants used in different systems of traditional medicine have shown the ability to inhibit the activity or expression of PTP-1B, which could indicate that they have a potential inhibitory effect on HGP. The determination of biological activity of full extracts and compounds isolated from medicinal plants has been approached through different perspectives. Generally, the medicinal plant is first identified using an ethnopharmacological approach. Afterwards, different types of extracts are elaborated (aqueous, ethanolic, methanolic, etc.) and then tested on the biological activity to be evaluated following several paths: 1) direct inhibition enzymatic assays, which can be complemented with structure-activity relationship (SAR) studies and molecular docking analysis to find the possible structures responsible for the bioactivity, relating them with the binding of amino acid residues present at the catalytic or regulatory sites (regarding isolated compounds); 2) the use of cell cultures to evaluate the effect of the extract or compound on the expression and protein levels of key enzymes; and 3) in vivo studies, where diabetic (hyperglycemic) animals induced with STZ or alloxan, or insulin-resistant animals generated by the consumption of high-fat diet are used.
Regarding PTP-1B, most of the studies published between 2015 and 2021 focused on conducting enzyme activity assays, and few of them had a multidisciplinary approach that encompassed enzyme assays and in vitro or in vivo studies. The main problem with the first type of studies is that, although the inhibition potency and selectivity of the molecule over the enzyme are directly evaluated, the pharmacokinetic properties of the compound are omitted. This particularity stands out since it has been reported that, despite having excellent inhibitory activity, many compounds lack adequate cellular permeability, namely they present poor absorption and low bioavailability (Zhang et al., 2017). Another aspect to highlight is that PTP-1B is almost identical to TC-PTP, another member of the PTP family with 74% identity at the catalytic site, so it is important that the identified inhibitors have a high selectivity towards PTP-1B to avoid unwanted effects (Dewang et al., 2005). Considering these facts, it would be necessary in the future to carry out more studies involving as many approaches as possible to obtain a more integrative panorama and to be able to evaluate potential inhibitors considering their pharmacokinetic properties and selectivity. Also, it is encouraged to directly evaluate the effect of medicinal plants and their compounds with reported PTP-1B inhibitory capacity on hepatic glucose metabolism.
In addition to exhibiting PTP-1B inhibitory capacity, some of the medicinal plants reported in Table 1 also improved hepatic glucose metabolism by promoting glucose consumption and glycogen synthesis, upregulating activity or expression of GS, decreasing activity or expression of key enzymes involved in glycogenolysis and gluconeogenesis such as GSK3, GP, PEPCK, FBPase, and G6Pase, and by modulating insulin signaling. The compounds isolated from these plants could have a greater modulatory capacity of hepatic glucose metabolism because they are capable of directly reducing both insulin resistance and glucose production. These species were Astragalus mongholicus (astragaloside IV), Chaenomeles japonica, Duranta erecta, Eriobotrya japonica (maslinic acid, corosolic acid, oleanolic acid, and ursolic acid), Symplocos cochinchinensis, Thonningia sanguinea (2′-O- (3-O-galloyl-4,6-O-Sa-hexahydroxydiphenoyl-β-d-glucopyranosyl)-3-hydroxyphloretin, 4′-O-(4,6-O-Sa-hexahydroxydiphenoyl-β-d-glucopyranosyl)- phloretin, 2′-O-(3-O-galloyl-4,6-O-Sa-hexahydroxydiphenoyl-β-d-glucopyranosyl)phloretin, thonningianin A, and thonningianin B), Vaccinium uliginosum (cyanidin-3-arabinoside, delphinidin-3-glucoside, cyanidin-3-galactoside, cyanidin-3-glucoside, malvidin-3-galactoside, petunidin-3-glucoside, procyanidin B1, and procyanidin B2), and Vigna radiata. On the other hand, since Coreopsis tinctoria, Lithocarpus polystachyus, and Panax ginseng were documented in both Tables 1, 2, their isolated compounds may have better glycemic control.
This work focused on summarizing the medicinal plants with the potential capacity to reduce hyperglycemia resulting from an imbalance in the hepatic metabolism of glucose, encompassing two different approaches: the inhibition of PTP-1B (improvement of hepatic insulin resistance), and the modulation of enzymes involved in gluconeogenesis and glycogenolysis/glycogenesis (decreased hepatic glucose output). In recent years, PTP-1B research has focused on the characterization of different phytochemicals from medicinal plants, such as phenolic compounds, terpenes, and alkaloids. The main methodology used was to carry out direct enzyme inhibition tests to evaluate the potency of these molecules, omitting important aspects such as selectivity or pharmacokinetics. Therefore, it is proposed to use of multidisciplinary approaches that involve in vitro studies, such as the use of cell lines or primary culture to evaluate the effect of the extracts and compounds on expression and protein levels, and in vivo studies, where the concentration of the compound in systemic circulation and its duration is determined, as well as the transformation processes involved. In this regard, not only the inhibitory activity of the compounds is evaluated, but also the impact on other pharmacological aspects that can only be observed using animal models.
On the other hand, research on medicinal plants that modulate hepatic glucose metabolism has primary focused on testing full extracts rather than compounds. However, it is worth mentioning that mixtures could have synergistic effects capable of regulating multiple targets (Caesar and Cech, 2019) and therefore compound fractions may exhibit more bioactivity than isolated molecules. Further studies are needed to identify potential multi-target phytochemicals in plants listed in Table 2. Finally, it is expected that this review will provide greater knowledge of medicinal plants and compounds for the development of drugs that improving hepatic glucose metabolism as a therapeutic target for the treatment of T2D.
We suggest that Coreopsis tinctoria, Lithocarpus polystachyus, and Panax ginseng can be good candidates for developing herbal medicines or phytomedicines that target inhibition of hepatic glucose output as they can modulate the activity of PTP-1B, the expression of gluconeogenic enzymes, and the glycogen content. However, only their full extracts are tested until now. Therefore, compounds responsible for the effects mentioned above have not been identified, and pharmacological and toxicological tests in animal models are required to assess their efficacy and safety, with the aim of moving forward to carry out clinical studies.
Acknowledgments
Authors acknowledge “Consejo Nacional de Ciencia y Tecnología (CONACyT)” for the doctoral scholarship of GM-T and FE-H.
Author Contributions
GM-T and FE-H performed the bibliographical research summarized in tables and wrote the first version of the manuscript. AA-C reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This project was partially sponsored by DGAPA PAPIIT IN226719 and IN213222.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdel-Rahman R. F., Ezzat S. M., Ogaly H. A., Abd-Elsalam R. M., Hessin A. F., Fekry M. I., et al. (2020). Ficus Deltoidea Extract Down-Regulates Protein Tyrosine Phosphatase 1B Expression in a Rat Model of Type 2 Diabetes Mellitus: a New Insight into its Antidiabetic Mechanism. J. Nutr. Sci. 9, e2. 10.1017/jns.2019.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Sattar E., El-Maraghy S. A., El-Dine R. S., Rizk S. M. (2016). Russelioside B, a Pregnane Glycoside Ameliorates Hyperglycemia in Streptozotocin Induced Diabetic Rats by Regulating Key Enzymes of Glucose Metabolism. Chem. Biol. Interact. 252, 47–53. 10.1016/j.cbi.2016.03.033 [DOI] [PubMed] [Google Scholar]
- Abdjul D. B., Yamazaki H., Maarisit W., Rotinsulu H., Wewengkang D. S., Sumilat D. A., et al. (2017). Oleanane Triterpenes with Protein Tyrosine Phosphatase 1B Inhibitory Activity from Aerial Parts of Lantana Camara Collected in Indonesia and Japan. Phytochemistry 144, 106–112. 10.1016/j.phytochem.2017.08.020 [DOI] [PubMed] [Google Scholar]
- Abdjul D. B., Yamazaki H., Maarisit W., Kirikoshi R., Takahashi O., Losung F., et al. (2018). Protein Tyrosine Phosphatase 1B Inhibitory Components and a New Unique N -alkylamide Derivative with an Endoperoxide Bridge from Aerial Parts of Indonesian Spilanthes Paniculata. Phytochemistry Lett. 24, 71–74. 10.1016/j.phytol.2018.01.013 [DOI] [Google Scholar]
- Ahmad F., Azevedo J. L., Cortright R., Dohm G. L., Goldstein B. J. (1997). Alterations in Skeletal Muscle Protein-Tyrosine Phosphatase Activity and Expression in Insulin-Resistant Human Obesity and Diabetes. J. Clin. Invest. 100, 449–458. 10.1172/JCI119552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad F., Goldstein B. J. (1995). Alterations in Specific Protein-Tyrosine Phosphatases Accompany Insulin Resistance of Streptozotocin Diabetes. Am. J. Physiol. 268, E932–E940. 10.1152/ajpendo.1995.268.5.e932 [DOI] [PubMed] [Google Scholar]
- Ahmad F., Li P. M., Meyerovitch J., Goldstein B. J. (1995). Osmotic Loading of Neutralizing Antibodies Demonstrates a Role for Protein-Tyrosine Phosphatase 1B in Negative Regulation of the Insulin Action Pathway. J. Biol. Chem. 270, 20503–20508. 10.1074/jbc.270.35.20503 [DOI] [PubMed] [Google Scholar]
- Ahmed B., Sultana R., Greene M. W. (2021). Adipose Tissue and Insulin Resistance in Obese. Biomed. Pharmacother. 137, 111315. 10.1016/j.biopha.2021.111315 [DOI] [PubMed] [Google Scholar]
- Alonso A., Nunes-Xavier C. E., Bayón Y., Pulido R. (2016). “The Extended Family of Protein Tyrosine Phosphatases,” in Methods in Molecular Biology. Editor Pulido R. (New York: Humana Press; ), 1–23. 10.1007/978-1-4939-3746-2_1 Protein Tyrosine Phosphatases [DOI] [PubMed] [Google Scholar]
- American Diabetes Association (2021). 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 44, S15–S33. 10.2337/dc21-S002 [DOI] [PubMed] [Google Scholar]
- An J. P., Ha T. K., Kim J., Cho T. O., Oh W. K. (2016). Protein Tyrosine Phosphatase 1B Inhibitors from the Stems of Akebia Quinata. Molecules 21. 10.3390/molecules21081091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Cetto A., Espinoza-Hernández F., Mata-Torres G., Escandón-Rivera S. (2021b). Hypoglycemic Effect of Two Mexican Medicinal Plants. Plants (Basel) 10, 2060. 10.3390/plants10102060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Cetto A., Espinoza-Hernández F., Mata-Torres G. (2021a). Hypoglycemic Effect of Calea Urticifolia (Mill.) DC. Evidence-Based Complement. Altern. Med. 2021, 1–10. 10.1155/2021/6625009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antu K. A., Riya M. P., Nair A., Mishra A., Srivastava A. K., Raghu K. G. (2016). Symplocos Cochinchinensis Enhances Insulin Sensitivity via the Down Regulation of Lipogenesis and Insulin Resistance in High Energy Diet Rat Model. J. Ethnopharmacol. 193, 500–509. 10.1016/j.jep.2016.09.050 [DOI] [PubMed] [Google Scholar]
- Arancibia-Radich J., González-Blázquez R., Alcalá M., Martín-Ramos M., Viana M., Arribas S., et al. (2019). Beneficial Effects of Murtilla Extract and Madecassic Acid on Insulin Sensitivity and Endothelial Function in a Model of Diet-Induced Obesity. Sci. Rep. 9, 599. 10.1038/s41598-018-36555-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasov A. G., Zotchev S., Zotchev S. B., Dirsch V. M., Supuran C. T. (2021). The International Natural Product Sciences Taskforce, and Supuran, CNatural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 20, 200–216. 10.1038/s41573-020-00114-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakke J., Haj F. G. (2015). Protein-tyrosine Phosphatase 1B Substrates and Metabolic Regulation. Semin. Cel Dev Biol 37, 58–65. 10.1016/j.semcdb.2014.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S., Wu Y. L., Wang X., Han S., Cho S., Ao W., et al. (2020). Agriophyllum Oligosaccharides Ameliorate Hepatic Injury in Type 2 Diabetic Db/db Mice Targeting INS-R/IRS-2/PI3K/AKT/PPAR-γ/Glut4 Signal Pathway. J. Ethnopharmacol. 257, 112863. 10.1016/j.jep.2020.112863 [DOI] [PubMed] [Google Scholar]
- Begmatov N., Li J., Bobakulov K., Numonov S., Aisa H. A. (2020). The Chemical Components of Coreopsis Tinctoria Nutt. And Their Antioxidant, Antidiabetic and Antibacterial Activities. Nat. Prod. Res. 34, 1772–1776. 10.1080/14786419.2018.1525377 [DOI] [PubMed] [Google Scholar]
- Caesar L. K., Cech N. B. (2019). Synergy and Antagonism in Natural Product Extracts: When 1 + 1 Does Not Equal 2. Nat. Prod. Rep. 36, 869–888. 10.1039/c9np00011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Zhao L., Tao W. (2015). Potent Protein Tyrosine Phosphatase 1B (PTP1B) Inhibiting Constituents from Anoectochilus Chapaensis and Molecular Docking Studies. Pharm. Biol. 53, 1030–1034. 10.3109/13880209.2014.957781 [DOI] [PubMed] [Google Scholar]
- Cao X., Yang X., Wang P., Liang Y., Liu F., Tuerhong M., et al. (2017). Polycyclic Phloroglucinols as PTP1B Inhibitors from Hypericum Longistylum: Structures, PTP1B Inhibitory Activities, and Interactions with PTP1B. Bioorg. Chem. 75, 139–148. 10.1016/j.bioorg.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Cersosimo E., Triplitt C., Solis-Herrera C., Mandarino L. J., DeFronzo R. A. (2018). “Pathogenesis of Type 2 Diabetes Mellitus,” in ” in Endotext . Editor Feingold K. R. (South Dartmouth: MDText.com, Inc.). . http://www.ncbi.nlm.nih.gov/pubmed/25905339 (Accessed September 1, 2021). [Google Scholar]
- Chen H., Wertheimer S. J., Lin C. H., Katz S. L., Amrein K. E., Burn P., et al. (1997). Protein-tyrosine Phosphatases PTP1B and Syp Are Modulators of Insulin-Stimulated Translocation of GLUT4 in Transfected Rat Adipose Cells. J. Biol. Chem. 272, 8026–8031. 10.1074/jbc.272.12.8026 [DOI] [PubMed] [Google Scholar]
- Choi J. S., Ali M. Y., Jung H. A., Oh S. H., Choi R. J., Kim E. J. (2015). Protein Tyrosine Phosphatase 1B Inhibitory Activity of Alkaloids from Rhizoma Coptidis and Their Molecular Docking Studies. J. Ethnopharmacol. 171, 28–36. 10.1016/j.jep.2015.05.020 [DOI] [PubMed] [Google Scholar]
- Cusi K., Maezono K., Osman A., Pendergrass M., Patti M. E., Pratipanawatr T., et al. (2000). Insulin Resistance Differentially Affects the PI 3-kinase- and MAP Kinase-Mediated Signaling in Human Muscle. J. Clin. Invest. 105, 311–320. 10.1172/JCI7535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadke S. S., Li H. C., Kusari A. B., Begum N., Kusari J. (2000). Elevated Expression and Activity of Protein-Tyrosine Phosphatase 1B in Skeletal Muscle of Insulin-Resistant Type II Diabetic Goto-Kakizaki Rats. Biochem. Biophys. Res. Commun. 274, 583–589. 10.1006/bbrc.2000.3188 [DOI] [PubMed] [Google Scholar]
- de Oliveira K. A., Moreira Gomes M. D., Vasconcelos R. P., de Abreu E. S., Fortunato R. S., Carneiro Loureiro A. C., et al. (2019). Phytomodulatory Proteins Promote Inhibition of Hepatic Glucose Production and Favor Glycemic Control via the AMPK Pathway. Biomed. Pharmacother. 109, 2342–2347. 10.1016/j.biopha.2018.11.139 [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Groop L., Henry R. R., Herman W. H., Holst J. J., et al. (2015). Type 2 Diabetes Mellitus. Nat. Rev. Dis. Primers 1, 15019–15022. 10.1038/nrdp.2015.19 [DOI] [PubMed] [Google Scholar]
- Denu J. M., Dixon J. E. (1998). Protein Tyrosine Phosphatases: Mechanisms of Catalysis and Regulation. Curr. Opin. Chem. Biol. 2, 633–641. 10.1016/S1367-5931(98)80095-1 [DOI] [PubMed] [Google Scholar]
- Dewang P. M., Hsu N. M., Peng S. Z., Li W. R. (2005). Protein Tyrosine Phosphatases and Their Inhibitors. Curr. Med. Chem. 12, 1–22. 10.2174/97816080520731090401037110.2174/0929867053363504 [DOI] [PubMed] [Google Scholar]
- Dimitriadis G. D., Maratou E., Kountouri A., Board M., Lambadiari V. (2021). Regulation of Postabsorptive and Postprandial Glucose Metabolism by Insulin-dependent and Insulin-independent Mechanisms: An Integrative Approach. Nutrients 13, 1–33. 10.3390/nu13010159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divya N., Rengarajan R. L., Radhakrishnan R., Fathi Abd Allah E., Alqarawi A. A., Hashem A., et al. (2019). Phytotherapeutic Efficacy of the Medicinal Plant Terminalia catappa L. Saudi J. Biol. Sci. 26, 985–988. 10.1016/j.sjbs.2018.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edirs S., Jiang L., Xin X., Aisa H. A. (2018). Anti-diabetic Effect and Mechanism of Kursi Wufarikun Ziyabit in L6 Rat Skeletal Muscle Cells. J. Pharmacol. Sci. 137, 212–219. 10.1016/j.jphs.2018.06.011 [DOI] [PubMed] [Google Scholar]
- Ekakitie L. I., Oyinloye B. E., Ajiboye B. O. (2021). The Ameliorative Activity of Chrysobalanus Orbicularis in Streptozotocin-Induced Type II Diabetes Mellitus Rat Model. Heliyon 7, e06596. 10.1016/j.heliyon.2021.e06596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchebly M., Payette P., Michaliszyn E., Cromlish W., Collins S., Loy A. L., et al. (1999). Increased Insulin Sensitivity and Obesity Resistance in Mice Lacking the Protein Tyrosine phosphatase-1B Gene. Science 283, 1544–1548. 10.1126/science.283.5407.1544 [DOI] [PubMed] [Google Scholar]
- Erukainure O. L., Ijomone O. M., Chukwuma C. I., Xiao X., Salau V. F., Islam M. S. (2020). Dacryodes Edulis (G. Don) H.J. Lam Modulates Glucose Metabolism, Cholinergic Activities and Nrf2 Expression, while Suppressing Oxidative Stress and Dyslipidemia in Diabetic Rats. J. Ethnopharmacol. 255, 112744. 10.1016/j.jep.2020.112744 [DOI] [PubMed] [Google Scholar]
- Erukainure O. L., Mopuri R., Oyebode O. A., Koorbanally N. A., Islam M. S. (2017). Dacryodes Edulis Enhances Antioxidant Activities, Suppresses DNA Fragmentation in Oxidative Pancreatic and Hepatic Injuries; and Inhibits Carbohydrate Digestive Enzymes Linked to Type 2 Diabetes. Biomed. Pharmacother. 96, 37–47. 10.1016/j.biopha.2017.09.106 [DOI] [PubMed] [Google Scholar]
- Erukainure O. L., Oyebode O. A., Ijomone O. M., Chukwuma C. I., Koorbanally N. A., Islam M. S. (2019a). Raffia palm (Raphia Hookeri G. Mann & H. Wendl) Wine Modulates Glucose Homeostasis by Enhancing Insulin Secretion and Inhibiting Redox Imbalance in a Rat Model of Diabetes Induced by High Fructose Diet and Streptozotocin. J. Ethnopharmacol. 237, 159–170. 10.1016/j.jep.2019.03.039 [DOI] [PubMed] [Google Scholar]
- Erukainure O. L., Sanni O., Ijomone O. M., Ibeji C. U., Chukwuma C. I., Islam M. S. (2019b). The Antidiabetic Properties of the Hot Water Extract of Kola Nut (Cola Nitida (Vent.) Schott & Endl.) in Type 2 Diabetic Rats. J. Ethnopharmacol. 242, 112033. 10.1016/j.jep.2019.112033 [DOI] [PubMed] [Google Scholar]
- Espinoza-Hernández F., Andrade-Cetto A., Escandón-Rivera S., Mata-Torres G., Mata R. (2021). Contribution of Fasting and Postprandial Glucose-Lowering Mechanisms to the Acute Hypoglycemic Effect of Traditionally Used Eryngium Cymosum F.Delaroche. J. Ethnopharmacology 279, 114339. 10.1016/j.jep.2021.114339 [DOI] [PubMed] [Google Scholar]
- Govindarajan S., Babu S. N., Vijayalakshmi M. A., Manohar P., Noor A. (2021). Aloe Vera Carbohydrates Regulate Glucose Metabolism through Improved Glycogen Synthesis and Downregulation of Hepatic Gluconeogenesis in Diabetic Rats. J. Ethnopharmacol. 281, 114556. 10.1016/j.jep.2021.114556 [DOI] [PubMed] [Google Scholar]
- Gujjala S., Putakala M., Nukala S., Bangeppagari M., Rajendran R., Desireddy S. (2017). Modulatory Effects of Caralluma Fimbriata Extract against High-Fat Diet Induced Abnormalities in Carbohydrate Metabolism in Wistar Rats. Biomed. Pharmacother. 92, 1062–1072. 10.1016/j.biopha.2017.06.016 [DOI] [PubMed] [Google Scholar]
- Guo Y., Dai R., Deng Y., Sun L., Meng S., Xin N. (2019). Hypoglycemic Activity of the Extracts of Belamcanda Chinensis Leaves (BCLE) on KK-Ay Mice. Biomed. Pharmacother. 110, 449–455. 10.1016/j.biopha.2018.11.094 [DOI] [PubMed] [Google Scholar]
- Guo Z., Niu X., Xiao T., Lu J., Li W., Zhao Y. (2015). Chemical Profile and Inhibition of α-glycosidase and Protein Tyrosine Phosphatase 1B (PTP1B) Activities by Flavonoids from Licorice (Glycyrrhiza Uralensis Fisch). J. Funct. Foods 14, 324–336. 10.1016/j.jff.2014.12.003 [DOI] [Google Scholar]
- Ha M. T., Shrestha S., Tran T. H., Kim J. A., Woo M. H., Choi J. S., et al. (2020). Inhibition of PTP1B by Farnesylated 2-arylbenzofurans Isolated from Morus alba Root Bark: Unraveling the Mechanism of Inhibition Based on In Vitro and In Silico Studies. Arch. Pharm. Res. 43, 961–975. 10.1007/s12272-020-01269-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H. S., Kang G., Kim J. S., Choi B. H., Koo S. H. (2016). Regulation of Glucose Metabolism from a Liver-Centric Perspective. Exp. Mol. Med. 48, e218–10. 10.1038/emm.2015.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.-w., Shi S.-m., Zou Y.-x., Wang Z.-c., Wang Y.-q., Shi L., et al. (2020). Chemical Constituents from Acid Hydrolyzates of Panax Quinquefolius Total Saponins and Their Inhibition Activity to α-glycosidase and Protein Tyrosine Phosphatase 1B. Chin. Herbal Medicines 12, 195–199. 10.1016/j.chmed.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. W., Owen O. E. (2013). “Gluconeogenesis,” in Metabolism Vitamins And Hormones . Elsevier, 381–386. 10.1016/B978-0-12-378630-2.00040-2 [DOI] [Google Scholar]
- Hao J., Han L., Zhang Y., Wang T. (2020). Docking Studies on Potential Mechanisms for Decreasing Insulin Resistance by the Tangzhiqing Herbal Formula. Evidence-Based Complement. Altern. Med. 2020, 1–11. 10.1155/2020/1057648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N., Goldstein B. J. (1992). Differential Regulation of mRNAs Encoding Three Protein-Tyrosine Phosphatases by Insulin and Activation of Protein Kinase C. Biochem. Biophys. Res. Commun. 188, 1305–1311. 10.1016/0006-291X(92)91373-X [DOI] [PubMed] [Google Scholar]
- He X. F., Chen J. J., Li T. Z., Zhang X. K., Guo Y. Q., Zhang X. M., et al. (2020). Nineteen New Flavanol-Fatty Alcohol Hybrids with α-Glucosidase and PTP1B Dual Inhibition: One Unusual Type of Antidiabetic Constituent from Amomum Tsao-Ko. J. Agric. Food Chem. 68, 11434–11448. 10.1021/acs.jafc.0c04615 [DOI] [PubMed] [Google Scholar]
- Hou J. Q., Fan C. L., Pei X., Zhang P. L., Deng F., Jiang W. Q., et al. (2019). Psiguadiols A-J, Rearranged Meroterpenoids as Potent PTP1B Inhibitors from Psidium Guajava. J. Nat. Prod. 82, 3267–3278. 10.1021/acs.jnatprod.9b00333 [DOI] [PubMed] [Google Scholar]
- Hu Y., Li J., Chang A. K., Li Y., Tao X., Liu W., et al. (2021). Screening and Tissue Distribution of Protein Tyrosine Phosphatase 1B Inhibitors in Mice Following Oral Administration of Garcinia Mangostana L. Ethanolic Extract. Food Chem. 357, 129759–129768. 10.1016/j.foodchem.2021.129759 [DOI] [PubMed] [Google Scholar]
- Huang G. H., Lei C., Zhu K. X., Li J. Y., Li J., Hou A. J. (2019). Enantiomeric Pairs of Meroterpenoids from Rhododendron Fastigiatum. Chin. J. Nat. Med. 17, 963–969. 10.1016/S1875-5364(19)30119-0 [DOI] [PubMed] [Google Scholar]
- Ighodaro O. M., Akinloye O. A., Ugbaja R. N., Omotainse S. O. (2017). Sapium Ellipticum (Hochst) Pax Ethanol Leaf Extract Modulates Glucokinase and Glucose-6-Phosphatase Activities in Streptozotocin Induced Diabetic Rats. Asian Pac. J. Trop. Biomed. 7, 544–548. 10.1016/j.apjtb.2017.05.009 [DOI] [Google Scholar]
- Jhuang H. J., Hsu W. H., Lin K. T., Hsu S. L., Wang F. S., Chou C. K., et al. (2015). Gluconeogenesis, Lipogenesis, and HBV Replication Are Commonly Regulated by PGC-1α-dependent Pathway. Oncotarget 6, 7788–7803. 10.18632/oncotarget.3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S., Li Z., Song W., Wang Y., Liang W., Li K., et al. (2016). Bioactive Constituents of Glycyrrhiza Uralensis (Licorice): Discovery of the Effective Components of a Traditional Herbal Medicine. J. Nat. Prod. 79, 281–292. 10.1021/acs.jnatprod.5b00877 [DOI] [PubMed] [Google Scholar]
- Jiang B.-p., Lv Q.-y., Xiang J.-m., Le L., Hu K.-p., Xu L.-j., et al. (2018a). Inhibition of Metabolic Disorders In Vivo and In Vitro by Main Constituent of Coreopsis Tinctoria. Chin. Herbal Medicines 10, 157–168. 10.1016/j.chmed.2018.03.004 [DOI] [Google Scholar]
- Jiang L., Numonov S., Bobakulov K., Qureshi M. N., Zhao H., Aisa H. A. (2015). Phytochemical Profiling and Evaluation of Pharmacological Activities of Hypericum Scabrum L. Molecules 20, 11257–11271. 10.3390/molecules200611257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Si L., Li P., Bai B., Qu J., Hou B., et al. (2018b). Serum Metabonomics Study on Antidiabetic Effects of Fenugreek Flavonoids in Streptozotocin-Induced Rats. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 1092, 466–472. 10.1016/j.jchromb.2018.06.041 [DOI] [PubMed] [Google Scholar]
- Jin D.-X., He J.-F., Luo X.-G., Zhang T.-C. (2019). Hypoglycemic Effect of Hypericum Attenuatum Choisy Extracts on Type 2 Diabetes by Regulating Glucolipid Metabolism and Modulating Gut Microbiota. J. Funct. Foods 52, 479–491. 10.1016/j.jff.2018.11.031 [DOI] [Google Scholar]
- Jung H. A., Ali M. Y., Choi J. S. (2016). Promising Inhibitory Effects of Anthraquinones, Naphthopyrone, and Naphthalene Glycosides, from Cassia Obtusifolia on α-Glucosidase and Human Protein Tyrosine Phosphatases 1B. Molecules 22, 28. 10.3390/molecules22010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H. J., Seong S. H., Ali M. Y., Min B. S., Jung H. A., Choi J. S. (2017). α-Methyl Artoflavanocoumarin from Juniperus Chinensis Exerts Anti-diabetic Effects by Inhibiting PTP1B and Activating the PI3K/Akt Signaling Pathway in Insulin-Resistant HepG2 Cells. Arch. Pharm. Res. 40, 1403–1413. 10.1007/s12272-017-0992-0 [DOI] [PubMed] [Google Scholar]
- Kasangana P. B., Eid H. M., Nachar A., Stevanovic T., Haddad P. S. (2019). Further Isolation and Identification of Anti-diabetic Principles from Root Bark of Myrianthus Arboreus P. Beauv.: The Ethyl Acetate Fraction Contains Bioactive Phenolic Compounds that Improve Liver Cell Glucose Homeostasis. J. Ethnopharmacol. 245, 112167. 10.1016/j.jep.2019.112167 [DOI] [PubMed] [Google Scholar]
- Kasangana P. B., Nachar A., Eid H. M., Stevanovic T., Haddad P. S. (2018). Root Bark Extracts of Myrianthus Arboreus P. Beauv. (Cecropiaceae) Exhibit Anti-diabetic Potential by Modulating Hepatocyte Glucose Homeostasis. J. Ethnopharmacol. 211, 117–125. 10.1016/j.jep.2017.09.017 [DOI] [PubMed] [Google Scholar]
- Kenner K. A., Anyanwu E., Olefsky J. M., Kusari J. (1996). Protein-tyrosine Phosphatase 1B Is a Negative Regulator of Insulin- and Insulin-like Growth Factor-I-Stimulated Signaling. J. Biol. Chem. 271, 19810–19816. 10.1074/jbc.271.33.19810 [DOI] [PubMed] [Google Scholar]
- Khanal P., Patil B. M. (2021). Integration of Network and Experimental Pharmacology to Decipher the Antidiabetic Action of Duranta Repens L. J. Integr. Med. 19, 66–77. 10.1016/j.joim.2020.10.003 [DOI] [PubMed] [Google Scholar]
- Kim D. H., Jung H. A., Sohn H. S., Kim J. W., Choi J. S. (2017a). Potential of Icariin Metabolites from Epimedium Koreanum Nakai as Antidiabetic Therapeutic Agents. Molecules 22. 10.3390/molecules22060986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. H., Lee S., Chung Y. W., Kim B. M., Kim H., Kim K., et al. (2016). Antiobesity and Antidiabetes Effects of a Cudrania Tricuspidata Hydrophilic Extract Presenting PTP1B Inhibitory Potential. Biomed. Res. Int. 2016, 8432759. 10.1155/2016/8432759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. H., Paudel P., Yu T., Ngo T. M., Kim J. A., Jung H. A., et al. (2017b). Characterization of the Inhibitory Activity of Natural Tanshinones from Salvia Miltiorrhiza Roots on Protein Tyrosine Phosphatase 1B. Chem. Biol. Interact. 278, 65–73. 10.1016/j.cbi.2017.10.013 [DOI] [PubMed] [Google Scholar]
- Klaman L. D., Boss O., Peroni O. D., Kim J. K., Martino J. L., Zabolotny J. M., et al. (2000). Increased Energy Expenditure, Decreased Adiposity, and Tissue-specific Insulin Sensitivity in Protein-Tyrosine Phosphatase 1B-Deficient Mice. Mol. Cel. Biol. 20, 5479–5489. 10.1128/mcb.20.15.5479-5489.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczuk A., Bourebaba N., Kornicka-Garbowska K., Turlej E., Marycz K., Bourebaba L. (2021). Hyoscyamus Albus Nortropane Alkaloids Reduce Hyperglycemia and Hyperinsulinemia Induced in HepG2 Cells through the Regulation of SIRT1/NF-kB/JNK Pathway. Cell Commun. Signal. 19, 61. 10.1186/s12964-021-00735-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnasamy R., Periyasamy S. (2019). Regulating Role of Ethyl Acetate Fraction of Tephrosia Tinctoria Pers. In Carbohydrate Metabolism and Oxidative Stress in Diabetic Rats. Biomed. Pharmacother. 114, 108842. 10.1016/j.biopha.2019.108842 [DOI] [PubMed] [Google Scholar]
- Krook A., Björnholm M., Galuska D., Jiang X. J., Fahlman R., Myers M. G., et al. (2000). Characterization of Signal Transduction and Glucose Transport in Skeletal Muscle from Type 2 Diabetic Patients. Diabetes 49, 284–292. 10.2337/diabetes.49.2.284 [DOI] [PubMed] [Google Scholar]
- Kurup S. B., Mini S. (2017). Protective Potential of Averrhoa Bilimbi Fruits in Ameliorating the Hepatic Key Enzymes in Streptozotocin-Induced Diabetic Rats. Biomed. Pharmacother. 85, 725–732. 10.1016/j.biopha.2016.11.088 [DOI] [PubMed] [Google Scholar]
- Lang G. Z., Li C. J., Gaohu T. Y., Li C., Ma J., Yang J. Z., et al. (2020). Bioactive Flavonoid Dimers from Chinese Dragon's Blood, the Red Resin of Dracaena Cochinchinensis. Bioorg. Chem. 97, 103659. 10.1016/j.bioorg.2020.103659 [DOI] [PubMed] [Google Scholar]
- Le D. D., Nguyen D. H., Zhao B. T., Seong S. H., Choi J. S., Kim S. K., et al. (2017). PTP1B Inhibitors from Selaginella Tamariscina (Beauv.) Spring and Their Kinetic Properties and Molecular Docking Simulation. Bioorg. Chem. 72, 273–281. 10.1016/j.bioorg.2017.05.001 [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Lai F. Y., Shi L. S., Chou Y. C., Yen I. C., Chang T. C. (2015). Rhodiola Crenulata Extract Suppresses Hepatic Gluconeogenesis via Activation of the AMPK Pathway. Phytomedicine 22, 477–486. 10.1016/j.phymed.2015.01.016 [DOI] [PubMed] [Google Scholar]
- Lei C., Zhang L.-B., Yang J., Gao L.-X., Li J.-Y., Li J., et al. (2016). Macdentichalcone, a Unique Polycyclic Dimeric Chalcone from Macaranga Denticulata. Tetrahedron Lett. 57, 5475–5478. 10.1016/j.tetlet.2016.10.090 [DOI] [Google Scholar]
- Li B., Fu R., Tan H., Zhang Y., Teng W., Li Z., et al. (2021). Characteristics of the Interaction Mechanisms of Procyanidin B1 and Procyanidin B2 with Protein Tyrosine phosphatase-1B: Analysis by Kinetics, Spectroscopy Methods and Molecular Docking. Spectrochimica Acta A: Mol. Biomol. Spectrosc. 259, 119910. 10.1016/j.saa.2021.119910 [DOI] [PubMed] [Google Scholar]
- Li C., Li C. J., Ma J., Chen F. Y., Li L., Wang X. L., et al. (2018a). Magterpenoids A-C, Three Polycyclic Meroterpenoids with PTP1B Inhibitory Activity from the Bark of Magnolia Officinalis Var. Biloba. Org. Lett. 20, 3682–3686. 10.1021/acs.orglett.8b01476 [DOI] [PubMed] [Google Scholar]
- Li C., Li C. J., Ma J., Huang J. W., Wang X. Y., Wang X. L., et al. (2019a). Magmenthanes A-H: Eight New Meroterpenoids from the Bark of Magnolia Officinalis Var. Biloba. Bioorg. Chem. 88, 102948. 10.1016/j.bioorg.2019.102948 [DOI] [PubMed] [Google Scholar]
- Li C., Li C. J., Xu K. L., Ma J., Huang J. W., Ye F., et al. (2020a). Novel Oligomeric Neolignans with PTP1B Inhibitory Activity from the Bark of Magnolia Officinalis Var. Biloba. Bioorg. Chem. 104, 104319. 10.1016/j.bioorg.2020.104319 [DOI] [PubMed] [Google Scholar]
- Li F., Li Y., Li Q., Shi X. (2020b). Eriobotrya Japonica Leaf Triterpenoid Acids Ameliorate Metabolic Syndrome in C57BL/6J Mice Fed with High-Fat Diet. Biomed. Pharmacother. 132, 110866. 10.1016/j.biopha.2020.110866 [DOI] [PubMed] [Google Scholar]
- Li J., Yu H., Wang S., Wang W., Chen Q., Ma Y., et al. (2018b). Natural Products, an Important Resource for Discovery of Multitarget Drugs and Functional Food for Regulation of Hepatic Glucose Metabolism. Drug Des. Devel. Ther. 12, 121–135. 10.2147/DDDT.S151860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. L., Li N., Xing S. S., Zhang N., Li B. B., Chen J. G., et al. (2017). New Neo-Lignan from Acanthopanax Senticosus with Protein Tyrosine Phosphatase 1B Inhibitory Activity. Arch. Pharm. Res. 40, 1265–1270. 10.1007/s12272-015-0659-7 [DOI] [PubMed] [Google Scholar]
- Li W., Pu Z., Yi W., Ma Q., Lin Q., Zhong G., et al. (2019b). Unusual Prenylated Stilbene Derivatives with PTP1B Inhibitory Activity from Artocarpus Styracifolius. Planta Med. 85, 1263–1274. 10.1055/a-1013-1417 [DOI] [PubMed] [Google Scholar]
- Li X., Xia H., Wang L., Xia G., Qu Y., Shang X., et al. (2019c). Lignans from the Twigs of Litsea Cubeba and Their Bioactivities. Molecules 24. 10.3390/molecules24020306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. H., Guo H., Xu W. B., Ge J., Li X., Alimu M., et al. (2016). Rapid Identification of Flavonoid Constituents Directly from PTP1B Inhibitive Extract of Raspberry (Rubus Idaeus L.) Leaves by HPLC-ESI-QTOF-MS-MS. J. Chromatogr. Sci. 54, 805–810. 10.1093/chromsci/bmw016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Staerk D., Kongstad K. T. (2020). Potential of Myrtus Communis Linn. As a Bifunctional Food: Dual High-Resolution PTP1B and α-glucosidase Inhibition Profiling Combined with HPLC-HRMS and NMR for Identification of Antidiabetic Triterpenoids and Phloroglucinol Derivatives. J. Funct. Foods 64, 103623. 10.1016/j.jff.2019.103623 [DOI] [Google Scholar]
- Liao Z., Zhang J., Liu B., Yan T., Xu F., Xiao F., et al. (2019). Polysaccharide from Okra (Abelmoschus Esculentus (L.) Moench) Improves Antioxidant Capacity via PI3K/AKT Pathways and Nrf2 Translocation in a Type 2 Diabetes Model. Molecules 24, 1906. 10.3390/molecules24101906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G. M., Chen Y. H., Yen P. L., Chang S. T. (2016). Antihyperglycemic and Antioxidant Activities of Twig Extract from Cinnamomum Osmophloeum. J. Tradit. Complement. Med. 6, 281–288. 10.1016/j.jtcme.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Kuang Y., Li K., Wang S., Song W., Qiao X., et al. (2017). Screening for Bioactive Natural Products from a 67-compound Library of Glycyrrhiza Inflata. Bioorg. Med. Chem. 25, 3706–3713. 10.1016/j.bmc.2017.05.009 [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang C., Li J., Mei Y., Liang Y. (2019). Hypoglycemic and Hypolipidemic Effects of Phellinus Linteus Mycelial Extract from Solid-State Culture in A Rat Model of Type 2 Diabetes. Nutrients 11, 296. 10.3390/nu11020296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. P., Li Y. J., Zhao Y. Y., Guo J. M., Liu Y. Y., Wang X. P., et al. (2021). Carbazole Alkaloids from the Fruits of Clausena Anisum-Olens with Potential PTP1B and α-glucosidase Inhibitory Activities. Bioorg. Chem. 110, 104775. 10.1016/j.bioorg.2021.104775 [DOI] [PubMed] [Google Scholar]
- Liu Z., Cheng Z., He Q., Lin B., Gao P., Li L., et al. (2016). Secondary Metabolites from the Flower Buds of Lonicera japonica and Their In Vitro Anti-diabetic Activities. Fitoterapia 110, 44–51. 10.1016/j.fitote.2016.02.011 [DOI] [PubMed] [Google Scholar]
- Ma Q., Wei R., Wang Z., Liu W., Sang Z., Li Y., et al. (2017). Bioactive Alkaloids from the Aerial Parts of Houttuynia Cordata. J. Ethnopharmacol. 195, 166–172. 10.1016/j.jep.2016.11.013 [DOI] [PubMed] [Google Scholar]
- Manimegalai S., Mahboob S., Al-Ghanim K. A., Al-Misned F., Govindarajan M., Anbarasu K., et al. (2020). Down-regulation of Hepatic G-6-Pase Expression in Hyperglycemic Rats: Intervention with Biogenic Gold Nanoconjugate. Saudi J. Biol. Sci. 27, 3334–3341. 10.1016/j.sjbs.2020.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvibaigi M., Hosseini S. M., Amini N. (2021). Launaea Acanthodes (Boiss) O. Kuntze Mediates Hepatic Glucose Metabolism and Ameliorates Impaired Pancreatic Function in Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 268, 113577. 10.1016/j.jep.2020.113577 [DOI] [PubMed] [Google Scholar]
- Mata-Torres G., Andrade-Cetto A., Espinoza-Hernández F. A., Cárdenas-Vázquez R. (2020). Hepatic Glucose Output Inhibition by Mexican Plants Used in the Treatment of Type 2 Diabetes. Front. Pharmacol. 11, 215–219. 10.3389/fphar.2020.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Ding L., Wang Y., Nie Q. T., Xing Y. Y., Ren Q. (2020). Phytochemical Identification of Lithocarpus Polystachyus Extracts by Ultra-high-performance Liquid Chromatography-Quadrupole Time-Of-Flight-MS and Their Protein Tyrosine Phosphatase 1B and α-glucosidase Activities. Biomed. Chromatogr. 34, e4705. 10.1002/bmc.4705 [DOI] [PubMed] [Google Scholar]
- Meyerovitch J., Backer J. M., Kahn C. R. (1989). Hepatic Phosphotyrosine Phosphatase Activity and its Alterations in Diabetic Rats. J. Clin. Invest. 84, 976–983. 10.1172/JCI114261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlinar B., Marc J., Janez A., Pfeifer M. (2007). Molecular Mechanisms of Insulin Resistance and Associated Diseases. Clin. Chim. Acta 375, 20–35. 10.1016/j.cca.2006.07.005 [DOI] [PubMed] [Google Scholar]
- Mohanasundaram S., Rangarajan N., Sampath V., Porkodi K., Pennarasi M. (2021). GC-MS and HPLC Analysis of Antiglycogenolytic and Glycogenic Compounds in Kaempferol 3-O-Gentiobioside Containing Senna alata L Leaves in Experimental Rats. Translational Metab. Syndr. Res. 4, 10–17. 10.1016/j.tmsr.2021.07.001 [DOI] [Google Scholar]
- Muñoz E. B., Luna-Vital D. A., Fornasini M., Baldeón M. E., Gonzalez de Mejia E. (2018). Gamma-conglutin Peptides from Andean Lupin Legume (Lupinus Mutabilis Sweet) Enhanced Glucose Uptake and Reduced Gluconeogenesis In Vitro . J. Funct. Foods 45, 339–347. 10.1016/j.jff.2018.04.021 [DOI] [Google Scholar]
- Na B., Nguyen P. H., Zhao B. T., Vo Q. H., Min B. S., Woo M. H. (2016). Protein Tyrosine Phosphatase 1B (PTP1B) Inhibitory Activity and Glucosidase Inhibitory Activity of Compounds Isolated from Agrimonia Pilosa. Pharm. Biol. 54, 474–480. 10.3109/13880209.2015.1048372 [DOI] [PubMed] [Google Scholar]
- Nguyen D. H., Seo U. M., Zhao B. T., Le D. D., Seong S. H., Choi J. S., et al. (2017). Ellagitannin and Flavonoid Constituents from Agrimonia Pilosa Ledeb. With Their Protein Tyrosine Phosphatase and Acetylcholinesterase Inhibitory Activities. Bioorg. Chem. 72, 293–300. 10.1016/j.bioorg.2017.04.017 [DOI] [PubMed] [Google Scholar]
- Nguyen D. T., To D. C., Tran T. T., Tran M. H., Nguyen P. H. (2021). PTP1B and α-glucosidase Inhibitors from Selaginella Rolandi-Principis and Their Glucose Uptake Stimulation. J. Nat. Med. 75, 186–193. 10.1007/s11418-020-01448-z [DOI] [PubMed] [Google Scholar]
- Nguyen P. H., Ji D. J., Han Y. R., Choi J. S., Rhyu D. Y., Min B. S., et al. (2015). Selaginellin and Biflavonoids as Protein Tyrosine Phosphatase 1B Inhibitors from Selaginella Tamariscina and Their Glucose Uptake Stimulatory Effects. Bioorg. Med. Chem. 23, 3730–3737. 10.1016/j.bmc.2015.04.007 [DOI] [PubMed] [Google Scholar]
- Nguyen P. H., Tuan H. N., Hoang D. T., Vu Q. T., Pham M. Q., Tran M. H., et al. (2019). Glucose Uptake Stimulatory and PTP1B Inhibitory Activities of Pimarane Diterpenes from Orthosiphon Stamineus Benth. Biomolecules 9, 859. 10.3390/biom9120859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu S. L., Tong Z. F., Zhang Y., Liu T. L., Tian C. L., Zhang D. X., et al. (2020). Novel Protein Tyrosine Phosphatase 1B Inhibitor-Geranylated Flavonoid from Mulberry Leaves Ameliorates Insulin Resistance. J. Agric. Food Chem. 68, 8223–8231. 10.1021/acs.jafc.0c02720 [DOI] [PubMed] [Google Scholar]
- Numonov S., Edirs S., Bobakulov K., Qureshi M. N., Bozorov K., Sharopov F., et al. (2017). Evaluation of the Antidiabetic Activity and Chemical Composition of Geranium Collinum Root Extracts-Computational and Experimental Investigations. Molecules 22. 10.3390/molecules22060983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo O. A. (2021). Puerarin as a Potential Drug Candidate for the Management of Type-2 Diabetes: Molecular Docking and Pharmacophore Modeling Studies. Biointerface Res. Appl. Chem. 11, 8751–8759. 10.33263/BRIAC112.87518759 [DOI] [Google Scholar]
- Ovalle-Magallanes B., Navarrete A., Haddad P. S., Tovar A. R., Noriega L. G., Tovar-Palacio C., et al. (2019). Multi-target Antidiabetic Mechanisms of Mexicanolides from Swietenia Humilis. Phytomedicine 58, 152891. 10.1016/j.phymed.2019.152891 [DOI] [PubMed] [Google Scholar]
- Page M. J., Moher D., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 372, n160. 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen A., Farooq M. A., Kyunn W. W. (2020). A New Oleanane Type Saponin from the Aerial Parts of Nigella Sativa with Anti-oxidant and Anti-diabetic Potential. Molecules 25, 1–13. 10.3390/molecules25092171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen H. A., Ndi C., Semple S. J., Buirchell B., Møller B. L., Staerk D. (2020). PTP1B-Inhibiting Branched-Chain Fatty Acid Dimers from Eremophila Oppositifolia Subsp. Angustifolia Identified by High-Resolution PTP1B Inhibition Profiling and HPLC-PDA-HRMS-SPE-NMR Analysis. J. Nat. Prod. 83, 1598–1610. 10.1021/acs.jnatprod.0c00070 [DOI] [PubMed] [Google Scholar]
- Pham H. T. T., Ryu B., Cho H. M., Lee B. W., Yang W. Y., Park E. J., et al. (2020). Oleanane Hemiacetal Glycosides from Gymnema Latifolium and Their Inhibitory Effects on Protein Tyrosine Phosphatase 1B. Phytochemistry 170, 112181. 10.1016/j.phytochem.2019.112181 [DOI] [PubMed] [Google Scholar]
- Pitschmann A., Zehl M., Heiss E., Purevsuren S., Urban E., Dirsch V. M., et al. (2016). Quantitation of Phenylpropanoids and Iridoids in Insulin-Sensitising Extracts of Leonurus Sibiricus L. (Lamiaceae). Phytochem. Anal. 27, 23–31. 10.1002/pca.2583 [DOI] [PubMed] [Google Scholar]
- Pompermaier L., Heiss E. H., Alilou M., Mayr F., Monizi M., Lautenschlaeger T., et al. (2018). Dihydrochalcone Glucosides from the Subaerial Parts of Thonningia Sanguinea and Their In Vitro PTP1B Inhibitory Activities. J. Nat. Prod. 81, 2091–2100. 10.1021/acs.jnatprod.8b00450 [DOI] [PubMed] [Google Scholar]
- Postic C., Dentin R., Girard J. (2004). Role of the Liver in the Control of Carbohydrate and Lipid Homeostasis. Diabetes Metab. 30, 398–408. 10.1016/S1262-3636(07)70133-7 [DOI] [PubMed] [Google Scholar]
- Qin N. B., Jia C. C., Xu J., Li D. H., Xu F. X., Bai J., et al. (2017). New Amides from Seeds of Silybum marianum with Potential Antioxidant and Antidiabetic Activities. Fitoterapia 119, 83–89. 10.1016/j.fitote.2017.04.008 [DOI] [PubMed] [Google Scholar]
- Quang T. H., Ngan N. T., Yoon C. S., Cho K. H., Kang D. G., Lee H. S., et al. (2015). Protein Tyrosine Phosphatase 1B Inhibitors from the Roots of Cudrania Tricuspidata. Molecules 20, 11173–11183. 10.3390/molecules200611173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi M. N., Numonov S., Aisa H. A. (2019). Chemical and Pharmacological Evaluation of Hulls of Prunus Dulcis Nuts. Int. J. Anal. Chem. 2019, 5861692. 10.1155/2019/5861692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A. S., Zhang S. (2013). Polypharmacology: Drug Discovery for the Future. Expert Rev. Clin. Pharmacol. 6, 41–47. 10.1586/ECP.12.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C., Zhang Y., Cui W., Lu G., Wang Y., Gao H., et al. (2015). A Polysaccharide Extract of mulberry Leaf Ameliorates Hepatic Glucose Metabolism and Insulin Signaling in Rats with Type 2 Diabetes Induced by High Fat-Diet and Streptozotocin. Int. J. Biol. Macromol. 72, 951–959. 10.1016/j.ijbiomac.2014.09.060 [DOI] [PubMed] [Google Scholar]
- Riya M. P., Antu K. A., Pal S., Chandrakanth K. C., Anilkumar K. S., Tamrakar A. K., et al. (2015). Antidiabetic Property of Aerva Lanata (L.) Juss. Ex Schult. Is Mediated by Inhibition of Alpha Glucosidase, Protein Glycation and Stimulation of Adipogenesis. J. Diabetes 7, 548–561. 10.1111/1753-0407.12216 [DOI] [PubMed] [Google Scholar]
- Rizza R. A. (2010). Pathogenesis of Fasting and Postprandial Hyperglycemia in Type 2 Diabetes: Implications for Therapy. Diabetes 59, 2697–2707. 10.2337/db10-1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman D. L., Magnusson I., Katz L. D., Shulman R. G., Shulman G. I. (1991). Quantitation of Hepatic Glycogenolysis and Gluconeogenesis in Fasting Humans with 13C NMR. Science 254, 573–576. 10.1126/science.1948033 [DOI] [PubMed] [Google Scholar]
- Rozenberg K., Rosenzweig T. (2018). Sarcopoterium Spinosum Extract Improved Insulin Sensitivity in Mice Models of Glucose Intolerance and Diabetes. PLoS One 13, e0196736. 10.1371/journal.pone.0196736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu B., Cho H. M., Zhang M., Lee B. W., Doan T. P., Park E. J., et al. (2021). Meroterpenoids from the Leaves of Psidium Guajava (Guava) Cultivated in Korea Using MS/MS-based Molecular Networking. Phytochemistry 186, 112723. 10.1016/j.phytochem.2021.112723 [DOI] [PubMed] [Google Scholar]
- Saadeldeen F. S. A., Niu Y., Wang H., Zhou L., Meng L., Chen S., et al. (2020). Natural Products: Regulating Glucose Metabolism and Improving Insulin Resistance. Food Sci. Hum. Wellness 9, 214–228. 10.1016/j.fshw.2020.04.005 [DOI] [Google Scholar]
- Saeting O., Chandarajoti K., Phongphisutthinan A., Hongsprabhas P., Sae-Tan S. (2021). Water Extract of Mungbean (Vigna Radiata L.) Inhibits Protein Tyrosine Phosphatase-1B in Insulin-Resistant HepG2 Cells. Molecules 26, 1452. 10.3390/molecules26051452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai C.-M., Qin N.-B., Jia C.-C., Li D.-H., Wang K.-B., Pei Y.-H., et al. (2016). Macleayine, a New Alkaloid from Macleaya Cordata. Chin. Chem. Lett. 27, 1717–1720. 10.1016/j.cclet.2016.06.017 [DOI] [Google Scholar]
- Saifudin A., Lallo S. A., Tezuka Y. (2016a). The Potent Inhibitors of Protein Tyrosine Phosphatase 1B from the Fruits of Melaleuca Leucadendron. Pharmacognosy Res. 8, S38–S41. 10.4103/0974-8490.178644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifudin A., Usia T., AbLallo S., Morita H., Tanaka K., Tezuka Y. (2016b). Potent Water Extracts of Indonesian Medicinal Plants against PTP1B. Asian Pac. J. Trop. Biomed. 6, 38–43. 10.1016/j.apjtb.2015.09.021 [DOI] [Google Scholar]
- Salinas-Arellano E., Pérez-Vásquez A., Rivero-cruz I., Torres-Colin R., González-Andrade M., Rangel-Grimaldo M., et al. (2020). Flavonoids and Terpenoids with PTP-1B Inhibitory Properties from the Infusion of Salvia Amarissima Ortega. Molecules 25, 1–18. 10.3390/molecules25153530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel A. R., Kahn C. R. (2001). Insulin Signalling and the Regulation of Glucose and Lipid Metabolism. Nature 414, 799–806. 10.1038/414799a [DOI] [PubMed] [Google Scholar]
- Sawant L., Singh V. K., Dethe S., Bhaskar A., Balachandran J., Mundkinajeddu D., et al. (2015). Aldose Reductase and Protein Tyrosine Phosphatase 1B Inhibitory Active Compounds from Syzygium Cumini Seeds. Pharm. Biol. 53, 1176–1182. 10.3109/13880209.2014.967784 [DOI] [PubMed] [Google Scholar]
- Schultze S. M., Hemmings B. A., Niessen M., Tschopp O. (2012). PI3K/AKT, MAPK and AMPK Signalling: Protein Kinases in Glucose Homeostasis. Expert Rev. Mol. Med. 14, e1–21. 10.1017/S1462399411002109 [DOI] [PubMed] [Google Scholar]
- Selvaraj G., Kaliamurthi S., Thirugnasambandan R. (2016). Effect of Glycosin Alkaloid from Rhizophora apiculata in Non-insulin Dependent Diabetic Rats and its Mechanism of Action: In Vivo and In Silico Studies. Phytomedicine 23, 632–640. 10.1016/j.phymed.2016.03.004 [DOI] [PubMed] [Google Scholar]
- Semaan D. G., Igoli J. O., Young L., Gray A. I., Rowan E. G., Marrero E. (2018). In Vitro anti-diabetic Effect of Flavonoids and Pheophytins from Allophylus Cominia Sw. On the Glucose Uptake Assays by HepG2, L6, 3T3-L1 and Fat Accumulation in 3T3-L1 Adipocytes. J. Ethnopharmacol. 216, 8–17. 10.1016/j.jep.2018.01.014 [DOI] [PubMed] [Google Scholar]
- Semaan D. G., Igoli J. O., Young L., Marrero E., Gray A. I., Rowan E. G. (2017). In Vitro anti-diabetic Activity of Flavonoids and Pheophytins from Allophylus Cominia Sw . On PTP1B, DPPIV, Alpha-Glucosidase and Alpha-Amylase Enzymes. J. Ethnopharmacol. 203, 39–46. 10.1016/j.jep.2017.03.023 [DOI] [PubMed] [Google Scholar]
- Seo Y. S., Shon M. Y., Kong R., Kang O. H., Zhou T., Kim D. Y., et al. (2016). Black Ginseng Extract Exerts Anti-hyperglycemic Effect via Modulation of Glucose Metabolism in Liver and Muscle. J. Ethnopharmacol. 190, 231–240. 10.1016/j.jep.2016.05.060 [DOI] [PubMed] [Google Scholar]
- Seong S. H., Roy A., Jung H. A., Jung H. J., Choi J. S. (2016). Protein Tyrosine Phosphatase 1B and α-glucosidase Inhibitory Activities of Pueraria Lobata Root and its Constituents. J. Ethnopharmacol. 194, 706–716. 10.1016/j.jep.2016.10.007 [DOI] [PubMed] [Google Scholar]
- Sharabi K., Tavares C. D., Rines A. K., Puigserver P. (2015). Molecular Pathophysiology of Hepatic Glucose Production. Mol. Aspects Med. 46, 21–33. 10.1016/j.mam.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira J. T., Batistela E., Pereira M. P., da Silva V. C., de Sousa Junior P. T., Andrade C. M., et al. (2016). Combretum Lanceolatum Flowers Ethanol Extract Inhibits Hepatic Gluconeogenesis: an In Vivo Mechanism Study. Pharm. Biol. 54, 1671–1679. 10.3109/13880209.2015.1120321 [DOI] [PubMed] [Google Scholar]
- Su C., Duan Y., Tian J., Liu J., Xie K., Chen D., et al. (2020). Morusalisins A-F, Six New Diels-Alder Type Adducts, as Potential PTP1B Inhibitors from Cell Cultures of Morus alba. Fitoterapia 146, 104682. 10.1016/j.fitote.2020.104682 [DOI] [PubMed] [Google Scholar]
- Su C., Tao X., Yin Z., Zhang X., Tian J., Chen R., et al. (2019). Morusalones A-D, Diels-Alder Adducts with 6/7/6/6/6/6 Hexacyclic Ring Systems as Potential PTP1B Inhibitors from Cell Cultures of Morus alba. Org. Lett. 21, 9463–9467. 10.1021/acs.orglett.9b03664 [DOI] [PubMed] [Google Scholar]
- Sun J., Wang Y., Fu X., Chen Y., Wang D., Li W., et al. (2015). Magnolia Officinalis Extract Contains Potent Inhibitors against PTP1B and Attenuates Hyperglycemia in Db/db Mice. Biomed. Res. Int. 2015, 139451. 10.1155/2015/139451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Qu C., Wang Y., Huang H., Zhang M., Li H., et al. (2018). PTP1B, A Potential Target of Type 2 Diabetes Mellitus. Mol. Biol. 05. 10.4172/2168-9547.1000174 [DOI] [Google Scholar]
- Sun R., Deng X., Zhang D., Xie F., Wang D., Wang J., et al. (2019). Anti-diabetic Potential of Pueraria Lobata Root Extract through Promoting Insulin Signaling by PTP1B Inhibition. Bioorg. Chem. 87, 12–15. 10.1016/j.bioorg.2019.02.046 [DOI] [PubMed] [Google Scholar]
- Sureka C., Elango V., Al-Ghamdi S., Aldossari K. K., Alsaidan M., Geddawy A., et al. (2021). Ameliorative Property of Sesbania Grandiflora on Carbohydrate Metabolic Enzymes in the Liver and Kidney of Streptozotocin-Induced Diabetic Rats. Saudi J. Biol. Sci. 28, 3669–3677. 10.1016/j.sjbs.2021.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahtah Y., Wubshet S. G., Kongstad K. T., Heskes A. M., Pateraki I., Møller B. L., et al. (2016). High-resolution PTP1B Inhibition Profiling Combined with High-Performance Liquid Chromatography-High-Resolution Mass Spectrometry-solid-phase Extraction-Nuclear Magnetic Resonance Spectroscopy: Proof-Of-Concept and Antidiabetic Constituents in Crude Extract of Eremophila Lucida. Fitoterapia 110, 52–58. 10.1016/j.fitote.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Tang D., Chen Q. B., Xin X. L., Aisa H. A. (2017). Anti-diabetic Effect of Three New Norditerpenoid Alkaloids In Vitro and Potential Mechanism via PI3K/Akt Signaling Pathway. Biomed. Pharmacother. 87, 145–152. 10.1016/j.biopha.2016.12.058 [DOI] [PubMed] [Google Scholar]
- Tian J. L., Liao X. J., Wang Y. H., Si X., Shu C., Gong E. S., et al. (2019). Identification of Cyanidin-3-Arabinoside Extracted from Blueberry as a Selective Protein Tyrosine Phosphatase 1B Inhibitor. J. Agric. Food Chem. 67, 13624–13634. 10.1021/acs.jafc.9b06155 [DOI] [PubMed] [Google Scholar]
- Tian J. Y., Tao R. Y., Zhang X. L., Liu Q., He Y. B., Su Y. L., et al. (2015). Effect of Hypericum perforatum L. Extract on Insulin Resistance and Lipid Metabolic Disorder in High-Fat-Diet Induced Obese Mice. Phytother. Res. 29, 86–92. 10.1002/ptr.5230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiong S. H., Looi C. Y., Arya A., Wong W. F., Hazni H., Mustafa M. R., et al. (2015). Vindogentianine, a Hypoglycemic Alkaloid from Catharanthus Roseus (L.) G. Don (Apocynaceae). Fitoterapia 102, 182–188. 10.1016/j.fitote.2015.01.019 [DOI] [PubMed] [Google Scholar]
- To D. C., Bui T. Q., Nhung N. T. A., Tran Q. T., Do T. T., Tran M. H., et al. (2021). On the Inhibitability of Natural Products Isolated from Tetradium Ruticarpum towards Tyrosine Phosphatase 1B (PTP1B) and α-Glucosidase (3W37): An In Vitro and In Silico Study. Molecules 26. 10.3390/molecules26123691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. (1988). Characterization of the Major Protein-Tyrosine-Phosphatases of Human Placenta. J. Biol. Chem. 263, 6731–6737. 10.1016/s0021-9258(18)68703-4 [DOI] [PubMed] [Google Scholar]
- Trinh B. T., Quach T. T., Bui D. N., Staerk D., Nguyen L. D., Jäger A. K. (2017b). Xanthones from the Twigs of Garcinia Oblongifolia and Their Antidiabetic Activity. Fitoterapia 118, 126–131. 10.1016/j.fitote.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Trinh B. T. D., Jäger A. K., Staerk D. (2017a). High-Resolution Inhibition Profiling Combined with HPLC-HRMS-SPE-NMR for Identification of PTP1B Inhibitors from Vietnamese Plants. Molecules 22. 10.3390/molecules22071228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwazie J. N., Yakubu M. T., Ashafa A. O. T., Ajiboye T. O. (2020). Identification and Characterization of Anti-diabetic Principle in Senna alata (Linn.) Flower Using Alloxan-Induced Diabetic Male Wistar Rats. J. Ethnopharmacol. 261, 112997. 10.1016/j.jep.2020.112997 [DOI] [PubMed] [Google Scholar]
- Vo Q. H., Nguyen P. H., Zhao B. T., Ali M. Y., Choi J. S., Min B. S., et al. (2015). Protein Tyrosine Phosphatase 1B (PTP1B) Inhibitory Constituents from the Aerial Parts of Tradescantia Spathacea Sw. Fitoterapia 103, 113–121. 10.1016/j.fitote.2015.03.017 [DOI] [PubMed] [Google Scholar]
- Vu N. K., Kim C. S., Ha M. T., Ngo Q. T., Park S. E., Kwon H., et al. (2020). Antioxidant and Antidiabetic Activities of Flavonoid Derivatives from the Outer Skins of Allium cepa L. J. Agric. Food Chem. 68, 8797–8811. 10.1021/acs.jafc.0c02122 [DOI] [PubMed] [Google Scholar]
- Wang H., Gu D., Wang M., Guo H., Wu H., Tian G., et al. (2017a). A Strategy Based on Gas Chromatography-Mass Spectrometry and Virtual Molecular Docking for Analysis and Prediction of Bioactive Composition in Natural Product Essential Oil. J. Chromatogr. A. 1501, 128–133. 10.1016/j.chroma.2017.04.031 [DOI] [PubMed] [Google Scholar]
- Wang J., Huang Y., Li K., Chen Y., Vanegas D., McLamore E. S., et al. (2016a). Leaf Extract from Lithocarpus Polystachyus Rehd. Promote Glycogen Synthesis in T2DM Mice. PLoS One 11, e0166557. 10.1371/journal.pone.0166557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yang J. L., Zhou P. P., Meng X. H., Shi Y. P. (2017b). Further New Gypenosides from Jiaogulan (Gynostemma Pentaphyllum). J. Agric. Food Chem. 65, 5926–5934. 10.1021/acs.jafc.7b01477 [DOI] [PubMed] [Google Scholar]
- Wang L.-J., Xiong J., Zou Y., Mei Q.-B., Wang W.-X., Hu J.-F. (2016b). Sesquiterpenoids from the Chinese Endangered Plant Manglietia Aromatica. Phytochemistry Lett. 18, 202–207. 10.1016/j.phytol.2016.10.018 [DOI] [Google Scholar]
- Wang M., Yu B. W., Yu M. H., Gao L. X., Li J. Y., Wang H. Y., et al. (2015). New Isoprenylated Phenolic Compounds from Morus Laevigata. Chem. Biodivers. 12, 937–945. 10.1002/cbdv.201400210 [DOI] [PubMed] [Google Scholar]
- Wu Y., Ouyang J. P., Wu K., Wang S. S., Wen C. Y., Xia Z. Y. (2005). Rosiglitazone Ameliorates Abnormal Expression and Activity of Protein Tyrosine Phosphatase 1B in the Skeletal Muscle of Fat-Fed, Streptozotocin-Treated Diabetic Rats. Br. J. Pharmacol. 146, 234–243. 10.1038/sj.bjp.0706306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T., Guo Z., Sun B., Zhao Y. (2017b). Identification of Anthocyanins from Four Kinds of Berries and Their Inhibition Activity to α-Glycosidase and Protein Tyrosine Phosphatase 1B by HPLC-FT-ICR MS/MS. J. Agric. Food Chem. 65, 6211–6221. 10.1021/acs.jafc.7b02550 [DOI] [PubMed] [Google Scholar]
- Xiao T., Guo Z., Bi X., Zhao Y. (2017a). Polyphenolic Profile as Well as Anti-oxidant and Anti-diabetes Effects of Extracts from Freeze-Dried Black Raspberries. J. Funct. Foods 31, 179–187. 10.1016/j.jff.2017.01.038 [DOI] [Google Scholar]
- Xiong J., Hu C. L., Wang P. P., Gao D. D., Huang F., Li J., et al. (2020). Spirobiflavonoid Stereoisomers from the Endangered conifer Glyptostrobus Pensilis and Their Protein Tyrosine Phosphatase 1B Inhibitory Activity. Bioorg. Med. Chem. Lett. 30, 126943. 10.1016/j.bmcl.2019.126943 [DOI] [PubMed] [Google Scholar]
- Xiong J., Wan J., Ding J., Wang P. P., Ma G. L., Li J., et al. (2017). Camellianols A-G, Barrigenol-like Triterpenoids with PTP1B Inhibitory Effects from the Endangered Ornamental Plant Camellia Crapnelliana. J. Nat. Prod. 80, 2874–2882. 10.1021/acs.jnatprod.7b00241 [DOI] [PubMed] [Google Scholar]
- Xu J., Wang X., Yue J., Sun Y., Zhang X., Zhao Y. (2018b). Polyphenols from Acorn Leaves (Quercus Liaotungensis) Protect Pancreatic Beta Cells and Their Inhibitory Activity against α-Glucosidase and Protein Tyrosine Phosphatase 1B. Molecules 23. 10.3390/molecules23092167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Yang L., Wang R., Zeng K., Fan B., Zhao Z. (2019a). The Biflavonoids as Protein Tyrosine Phosphatase 1B Inhibitors from Selaginella Uncinata and Their Antihyperglycemic Action. Fitoterapia 137, 104255. 10.1016/j.fitote.2019.104255 [DOI] [PubMed] [Google Scholar]
- Xu J., Cao J., Yue J., Zhang X., Zhao Y. (2018a). New Triterpenoids from Acorns of Quercus Liaotungensis and Their Inhibitory Activity against α -glucosidase, α -amylase and Protein-Tyrosine Phosphatase 1B. J. Funct. Foods 41, 232–239. 10.1016/j.jff.2017.12.054 [DOI] [Google Scholar]
- Xu L., Li Y., Dai Y., Peng J. (2018c). Natural Products for the Treatment of Type 2 Diabetes Mellitus: Pharmacology and Mechanisms. Pharmacol. Res. 130, 451–465. 10.1016/j.phrs.2018.01.015 [DOI] [PubMed] [Google Scholar]
- Xu Q. Q., Chen X. L., Xu J. F., Wang S. B., Luo J. G., Kong L. Y. (2019b). Acetophenone Derivatives from the Roots of Melicope Ptelefolia. Fitoterapia 132, 40–45. 10.1016/j.fitote.2018.11.013 [DOI] [PubMed] [Google Scholar]
- Yan H. W., Zhu H., Yuan X., Yang Y. N., Feng Z. M., Jiang J. S., et al. (2019). Eight New Biflavonoids with Lavandulyl Units from the Roots of Sophora Flavescens and Their Inhibitory Effect on PTP1B. Bioorg. Chem. 86, 679–685. 10.1016/j.bioorg.2019.01.058 [DOI] [PubMed] [Google Scholar]
- Yang J. B., Ye F., Tian J. Y., Song Y. F., Gao H. Y., Liu Y., et al. (2020). Multiflorumisides HK, Stilbene Glucosides Isolated from Polygonum Multiflorum and Their In Vitro PTP1B Inhibitory Activities. Fitoterapia 146, 104703. 10.1016/j.fitote.2020.104703 [DOI] [PubMed] [Google Scholar]
- Yang J. L., Ha T. K. Q., Lee B. W., Kim J., Oh W. K. (2017). PTP1B Inhibitors from the Seeds of Iris Sanguinea and Their Insulin Mimetic Activities via AMPK and ACC Phosphorylation. Bioorg. Med. Chem. Lett. 27, 5076–5081. 10.1016/j.bmcl.2017.09.031 [DOI] [PubMed] [Google Scholar]
- Yang N., Zhang S., Yang S., Guo Z., Zhang X., Zhao Y. (2016). The Inhibition of α-glycosidase and Protein Tyrosine Phosphatase 1B (PTP1B) Activities by Ginsenosides from Panax Ginseng C.A. Meyer and Simultaneous Determination by HPLC-ELSD. J. Funct. Foods 23, 188–197. 10.1016/j.jff.2015.12.018 [DOI] [Google Scholar]
- Yin X., Huang Y., Jung D. W., Chung H. C., Choung S. Y., Shim J. H., et al. (2015). Anti-Diabetic Effect of Aster Sphathulifolius in C57BL/KsJ-Db/db Mice. J. Med. Food 18, 987–998. 10.1089/jmf.2014.3416 [DOI] [PubMed] [Google Scholar]
- Yousof Ali M., Jung H. A., Choi J. S. (2015). Anti-diabetic and Anti-alzheimer's Disease Activities of Angelica Decursiva. Arch. Pharm. Res. 38, 2216–2227. 10.1007/s12272-015-0629-0 [DOI] [PubMed] [Google Scholar]
- Yu J. H., Liu Q. F., Sheng L., Wang G. C., Li J., Yue J. M. (2016a). Cipacinoids A-D, Four Limonoids with Spirocyclic Skeletons from Cipadessa Cinerascens. Org. Lett. 18, 444–447. 10.1021/acs.orglett.5b03487 [DOI] [PubMed] [Google Scholar]
- Yu Y., Gan L. S., Yang S. P., Sheng L., Liu Q. F., Chen S. N., et al. (2016b). Eucarobustols A-I, Conjugates of Sesquiterpenoids and Acylphloroglucinols from Eucalyptus Robusta. J. Nat. Prod. 79, 1365–1372. 10.1021/acs.jnatprod.6b00090 [DOI] [PubMed] [Google Scholar]
- Yuan Y. C., Bai X. L., Liu Y. M., Tang X. Y., Yuan H., Liao X. (2021). Ligand Fishing Based on Cell Surface Display of Enzymes for Inhibitor Screening. Anal. Chim. Acta 1156, 338359. 10.1016/j.aca.2021.338359 [DOI] [PubMed] [Google Scholar]
- Yue J., Xu J., Cao J., Zhang X., Zhao Y. (2017). Cucurbitane Triterpenoids from Momordica Charantia L. And Their Inhibitory Activity against α-glucosidase, α-amylase and Protein Tyrosine Phosphatase 1B (PTP1B). J. Funct. Foods 37, 624–631. 10.1016/j.jff.2017.07.041 [DOI] [Google Scholar]
- Zakłos-Szyda M., Pawlik N. (2018). Japanese Quince (Chaenomeles Japonica L.) Fruit Polyphenolic Extract Modulates Carbohydrate Metabolism in HepG2 Cells via AMP-Activated Protein Kinase. Acta Biochim. Pol. 65, 67–78. 10.18388/abp.2017_1604 [DOI] [PubMed] [Google Scholar]
- Zhang C. C., Geng C. A., Huang X. Y., Zhang X. M., Chen J. J. (2019a). Antidiabetic Stilbenes from Peony Seeds with PTP1B, α-Glucosidase, and DPPIV Inhibitory Activities. J. Agric. Food Chem. 67, 6765–6772. 10.1021/acs.jafc.9b01193 [DOI] [PubMed] [Google Scholar]
- Zhang P. Z., Gu J., Zhang G. L. (2015). Novel Stilbenes from Artocarpus Nanchuanensis. J. Asian Nat. Prod. Res. 17, 217–223. 10.1080/10286020.2015.1006202 [DOI] [PubMed] [Google Scholar]
- Zhang R., Yu R., Xu Q., Li X., Luo J., Jiang B., et al. (2017). Discovery and Evaluation of the Hybrid of Bromophenol and Saccharide as Potent and Selective Protein Tyrosine Phosphatase 1B Inhibitors. Eur. J. Med. Chem. 134, 24–33. 10.1016/j.ejmech.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Zhang S. N., Zeng J., Tan Y. N., Ma R. J., Zhang G. J., Wang H. S., et al. (2019b). 3α,19-Dihydroxyl-ent-pimara-8(14),15-diene, a New Diterpenoid from the Rhizomes of Ricinus communis. J. Asian Nat. Prod. Res. 21, 522–527. 10.1080/10286020.2018.1461087 [DOI] [PubMed] [Google Scholar]
- Zhang W., Hong D., Zhou Y., Zhang Y., Shen Q., Li J. Y., et al. (2006). Ursolic Acid and its Derivative Inhibit Protein Tyrosine Phosphatase 1B, Enhancing Insulin Receptor Phosphorylation and Stimulating Glucose Uptake. Biochim. Biophys. Acta 1760, 1505–1512. 10.1016/j.bbagen.2006.05.009 [DOI] [PubMed] [Google Scholar]
- Zhang X., Lv Q., Jia S., Chen Y., Sun C., Li X., et al. (2016a). Effects of flavonoid-rich Chinese bayberry (Morella rubra Sieb. et Zucc.) fruit extract on regulating glucose and lipid metabolism in diabetic KK-A(y) mice. Food Funct. 7, 3130–3140. 10.1039/c6fo00397d [DOI] [PubMed] [Google Scholar]
- Zhang X. Y., Li W., Wang J., Li N., Cheng M. S., Koike K. (2019c). Protein Tyrosine Phosphatase 1B Inhibitory Activities of Ursane-type Triterpenes from Chinese Raspberry, Fruits of Rubus Chingii. Chin. J. Nat. Med. 17, 15–21. 10.1016/S1875-5364(19)30004-4 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen J., Zeng Y., Huang D., Xu Q. (2019d). Involvement of AMPK Activation in the Inhibition of Hepatic Gluconeogenesis by Ficus Carica Leaf Extract in Diabetic Mice and HepG2 Cells. Biomed. Pharmacother. 109, 188–194. 10.1016/j.biopha.2018.10.077 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Feng F., Chen T., Li Z., Shen Q. W. (2016b). Antidiabetic and Antihyperlipidemic Activities of Forsythia Suspensa (Thunb.) Vahl (Fruit) in Streptozotocin-Induced Diabetes Mice. J. Ethnopharmacol. 192, 256–263. 10.1016/j.jep.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yan L. S., Ding Y., Cheng B. C. Y., Luo G., Kong J., et al. (2020). Edgeworthia Gardneri (Wall.) Meisn. Water Extract Ameliorates Palmitate Induced Insulin Resistance by Regulating IRS1/GSK3β/FoxO1 Signaling Pathway in Human HepG2 Hepatocytes. Front. Pharmacol. 10, 1666. 10.3389/fphar.2019.01666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B. T., Le D. D., Nguyen P. H., Ali M. Y., Choi J. S., Min B. S., et al. (2016). PTP1B, α-glucosidase, and DPP-IV Inhibitory Effects for Chromene Derivatives from the Leaves of Smilax china L. Chem. Biol. Interact. 253, 27–37. 10.1016/j.cbi.2016.04.012 [DOI] [PubMed] [Google Scholar]
- Zhao C. C., Chen J., Shao J. H., Zhang X. H., Gu W. Y., Shen J., et al. (2020). Lignan Constituents from the Fruits of Viburnum Macrocephalum F. Keteleeri and Their α-Amylase, α-Glucosidase, and Protein Tyrosine Phosphatase 1B Inhibitory Activities. J. Agric. Food Chem. 68, 11151–11160. 10.1021/acs.jafc.0c03353 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Chen M. X., Kongstad K. T., Jäger A. K., Staerk D. (2017). Potential of Polygonum Cuspidatum Root as an Antidiabetic Food: Dual High-Resolution α-Glucosidase and PTP1B Inhibition Profiling Combined with HPLC-HRMS and NMR for Identification of Antidiabetic Constituents. J. Agric. Food Chem. 65, 4421–4427. 10.1021/acs.jafc.7b01353 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Kjaerulff L., Kongstad K. T., Heskes A. M., Møller B. L., Staerk D. (2019a). 2(5H)-Furanone Sesquiterpenes from Eremophila bignoniiflora: High-Resolution Inhibition Profiling and PTP1B Inhibitory Activity. Phytochemistry 166, 112054. 10.1016/j.phytochem.2019.112054 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Kongstad K. T., Liu Y., He C., Staerk D. (2019b). Unraveling the Complexity of Complex Mixtures by Combining High-Resolution Pharmacological, Analytical and Spectroscopic Techniques: Antidiabetic Constituents in Chinese Medicinal Plants. Faraday Discuss. 218, 202–218. 10.1039/c8fd00223a [DOI] [PubMed] [Google Scholar]
- Zhao Y., Kongstad K. T., Jäger A. K., Nielsen J., Staerk D. (2018). Quadruple High-Resolution α-glucosidase/α-amylase/PTP1B/radical Scavenging Profiling Combined with High-Performance Liquid Chromatography-High-Resolution Mass Spectrometry-solid-phase Extraction-Nuclear Magnetic Resonance Spectroscopy for Identification of Antidiabetic Constituents in Crude Root Bark of Morus alba L. J. Chromatogr. A 1556, 55–63. 10.1016/j.chroma.2018.04.041 [DOI] [PubMed] [Google Scholar]
- Zheng X. M., Meng F. W., Geng F., Qi M., Luo C., Yang L., et al. (2015). Plantadeprate A, a Tricyclic Monoterpene Zwitterionic Guanidium, and Related Derivatives from the Seeds of Plantago Depressa. J. Nat. Prod. 78, 2822–2826. 10.1021/acs.jnatprod.5b00368 [DOI] [PubMed] [Google Scholar]
- Zhou J., Wu Z., Oyawaluja B. O., Coker H. A. B., Odukoya O. A., Yao G., et al. (2019). Protein Tyrosine Phosphatase 1B Inhibitory Iridoids from Psydrax Subcordata. J. Nat. Prod. 82, 2916–2924. 10.1021/acs.jnatprod.9b00770 [DOI] [PubMed] [Google Scholar]
- Zhou X., Wang L. L., Tang W. J., Tang B. (2021). Astragaloside IV Inhibits Protein Tyrosine Phosphatase 1B and Improves Insulin Resistance in Insulin-Resistant HepG2 Cells and Triglyceride Accumulation in Oleic Acid (OA)-treated HepG2 Cells. J. Ethnopharmacol. 268, 113556. 10.1016/j.jep.2020.113556 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Huang J. J., Zhang X. X., Yan Y., Yin X. W., Ping G., et al. (2018). Qing Gan Zi Shen Tang Alleviates Adipose Tissue Dysfunction with Up-Regulation of SIRT1 in Spontaneously Hypertensive Rat. Biomed. Pharmacother. 105, 246–255. 10.1016/j.biopha.2018.05.022 [DOI] [PubMed] [Google Scholar]