Abstract
Background
Communication is one of the most important predictors of social reintegration after stroke. Approximately 15–42% of stroke survivors experience post-stroke aphasia. Helping people recover from aphasia is one of the research priorities after a stroke. Our aim is to develop and validate a new therapy integrating dubbing techniques to improve functional communication.
Methods
The research project is structured as three work packages (WP). WP1: development of the dubbed language cinema-based therapy: Two research assistants (a speech therapist and a dubbing actor) will select the clips, mute specific words/sentences in progressive speech difficulty, and guide patients to dub them across sessions. Words to be dubbed will be those considered to be functionally meaningful by a representative sample of aphasic patients and relatives through an online survey. WP2: a randomized, crossover, interventional pilot study with the inclusion of 54 patients with post-stroke non-fluent aphasia. Patients will be treated individually in 40-min sessions twice per week for 8 weeks. Primary outcomes will be significant pre/post differences in scores in the Communicative Activity Log (CAL) questionnaire and Boston Diagnostic Aphasia Examination (BDAE) administered by a psychologist blinded to the patients’ clinical characteristics. Secondary outcomes: General Health Questionnaire (GHQ)-12, Stroke Aphasia Quality of Life Scale (SAQOL-39), Western Aphasia Battery Revised (WAB-R), and the Stroke Aphasic Depression Questionnaire (SADQ10). WP3: educational activities and dissemination of results. WP3 includes educational activities to improve public knowledge of aphasia and dissemination of the results, with the participation of the Spanish patients’ association Afasia Activa.
Discussion
This pilot clinical trial will explore the efficacy of a new therapeutic tool based on dubbing techniques and computer technology to improve functional communication of patients suffering from post-stroke aphasia with the use of standardized test assessment.
Trial registration
ClinicalTrials.govNCT04289493. Registered on 28 February 2020.
Keywords: Stroke, Aphasia, Randomized clinical trial, Protocol
Background
Stroke is a leading cause of disability, and the absolute number of people who develop stroke each year is growing worryingly, as are the number of survivors and the overall burden of stroke [1]. Communication is a vital aspect of daily functioning, being the most important predictor of social reintegration after a stroke [2]. It is estimated that approximately 15–42% of stroke survivors experience post-stroke aphasia, affecting some of the language modalities, including the production and understanding of speech, reading, and writing. Aphasia is associated not only with a greater risk of mortality after stroke, but also with long-term disability, greatly impacting the quality of life [3]. Hence, helping people recover from aphasia has been identified by consensus among survivors, caregivers, and health professionals as one of the top 10 research priorities relating to life after stroke [4]. Current international guidelines recommend speech and language therapy (SLT) for stroke survivors [5, 6]. However, according to a Cochrane meta-analysis, available SLT differ in therapy regimens (intensity, dosage, duration), delivery models (group, one-to-one, volunteer, computed-facilitated), approach, and outcome measurements [7]. Thus, there is no universally accepted SLT for post-stroke aphasia. Moreover, several SLTs use child-like materials, therefore not being generalizable for a daily adult life. This is one of the main challenges of intensive SLT since it produces high rates of early dropouts, up to 30% [7], as patients do not feel that their preferences are being covered.
Computer-based technology such as mobile apps, video games, and virtual reality is increasingly being used with success in post-stroke motor rehabilitation [8]. However, SLT has not embraced the same path [9]. To our knowledge, the first attempt at using audiovisual material for SLT purposes dates from the 60's. Researchers from the University of Chicago Speech and Language Clinic used film strips with various topics for homebound and outpatient aphasic patients. Patients showed higher engagement with therapy and increased their spontaneous self-expression [10]. Recent studies used videos in SLT for instructional or speech-triggering purposes [11, 12]. Results were positive, with an improvement in patients’ knowledge about aphasia and language skills; however, the scripts used were very limited and the patients reported their wish to have a wider and more varied range of words to practice. Besides, the use of word and sentence imitation in a computer-based intensive therapy improved the narrative production of 19 patients in a before and after trial [13]. Although a randomized clinical trial was designed to assess the effect of intensive imitation therapy (NCT00713050) [14], to our knowledge, no results other than non-randomized case series have been reported from that study [13].
We conducted a PubMed search with the terms (((computer-based) AND (therapy)) AND (stroke)) AND (aphasia), retrieving a total of 25 articles (last search conducted on October 30, 2021). Of these, only five presented the results of randomized clinical trials [15–19], and an additional completed clinical trial was found on the ClinicalTrials.gov website [20] (Table 1). Clearly, we need to change our therapeutic approach in post-stroke aphasia and take advantage of existing technology.
Table 1.
Summary of completed computer-based clinical trials on post-stroke aphasia
| Reference | Trial ID | Design | Patients | Interventions | Main results |
|---|---|---|---|---|---|
|
Kesav P et al. J Neurol Sci 2017; 380:137–141 [15] |
Clinical Trials registry India 2016/08/0120121 | Prospective open randomized, controlled trial with blinded endpoint evaluation. | 20 |
• Group A: less intensive (12 therapy sessions of conventional professional-based SLT) • Group B: more intensive (12 therapy sessions of conventional professional-based SLT + 12 h of computer-based SLT) |
• Less intensive worked better, but authors still recommend using computer-based |
| Grechuta K et al. Stroke 2019; 50:1270–1274 [16] | NCT02928822 | Randomized, controlled, parallel-group trial | 17 |
• Control group (N=8): standard treatment • Experimental group (N=9): augmented embodied therapy with the Rehabilitation Gaming System for aphasia. |
• Both groups significantly improved on the BDAE and on the lexical access-vocabulary test. • Only the Rehabilitation Gaming System for aphasia group improved on the CAL and showed therapy-induced improvements in language and communication at 16 weeks of follow-up. |
|
Palmer R et al. Lancet Neurol. 2019; 18: 821–33 [17] |
ISRCTN68798818 | Pragmatic, superiority, three-arm, individually randomized, single-blind, parallel-group trial. | 278 |
• Control group: 6 months of usual care (usual care group) • CSLT Group: Daily self-managed CSLT plus usual care • Attention control plus usual care: paper-based puzzle book activities (e.g., sudoku, spot the difference, word searches, or coloring) on a daily basis. |
• CSLT plus usual care resulted in a clinically significant improvement in personally relevant word finding but did not result in an improvement in conversation. |
|
Cherney LR et al. Clin Rehabil 2021; 35: 976–987 [18] |
NCT04413136 | Single-blind, randomized placebo-controlled trial | 32 |
• Experimental treatment (N=19): Web ORLA (Oral Reading for Language in Aphasia) • Control group (N=13): a commercially available computer game. Both groups were instructed to practice 90 min/day, 6 days/week for 6 weeks. |
• No significant difference in the gain from pre-treatment to post-treatment between groups. • The Web ORLA group showed significantly greater gains at the 6-week follow-up than the control group. |
|
Spaccavento S et al. J Communication Disorders 2021:106158 [19] |
NA | Pilot randomized non-inferiority study | 22 |
• Experimental group: computer-based • Control group: therapist-mediated aphasia treatment Both groups received one 50-min session for 5 days per week over a period of 8 weeks. |
• Participants in both groups improved in language skills, functional communication, and quality-of-life measures from pre- to post-treatment • No significant differences between groups. |
|
Elhakeem ES et al. The Egyptian J Otolaryngology 2021; 37:77 [20] |
NCT04717180 | Randomized controlled trial with blinded endpoint evaluation | 50 |
• Group I: 48 sessions using the Arabic software program • Group II: 48 sessions of conventional Therapy |
• Significant improvement from the baseline in both groups. • No significant difference in post-therapy results between groups except for some secondary items, whereas group I showed more significant improvement (phrase length, melodic line, word-finding relative to fluency, paraphasia, repetition, responsive naming, Boston naming test) |
BDAE Boston Diagnostic Aphasia Examination, CAL Communicative Activity Log, CSLT Computer-based Speech and Language Therapy, NA not available, SLT Speech and Language Therapy
Sources: https://clinicaltrials.gov [search terms (Post-stroke aphasia) AND (computer); filters: completed] and PubMED [(computer-based) AND (therapy) AND (stroke) AND (aphasia)]. Last search conducted on October 30, 2021
Factors underlying language recovery, such as visual cue integration, self-performance assessment, motor coordination, intonation, emotion, and motivation, are supported by the latest discoveries in neuroscience as separate components [21–24]. We think that including dubbing in post-stroke aphasia rehabilitation provision can link technology with language factor integration. Dubbing actors need to articulate words within a given emotional context by paying attention to an audiovisual input. In a preliminary work, we developed a single-patient open trial with dubbing techniques in a 71-year-old woman with 15 months of chronic global aphasia after having undergone classical speech therapy (i.e., mirror repetition) with two different speech therapists. Prior to the dubbing therapy, the patient could only pronounce some Spanish filler words and some short words if actively shaped and helped. After participating in only two dubbing sessions, she spontaneously incorporated the dubbed words in the proper contexts and increased the time of sustained attention as compared with the time of attention paid in previous SLT sessions [25]. In that single-case study, we excluded potential natural recovery because the acquisition of the targeted words occurred 2 years after the stroke, when the linguistic impairment was already chronic, and the patient had not incorporated other words different from those trained for during the pilot. Therefore, the linguistic improvement appears to be attributed to the experimental intervention.
Finally, trials on post-stroke aphasia can benefit from collaboration between patients, clinicians, researchers, and the use of standardized outcome measures [7]. Our research project counts on the collaboration of post-stroke aphasic patients, who are actively consulted to choose the most useful training material, and we use the standardized tests to assess our results.
Methods
Our aim is to develop and validate a new therapy integrating dubbing techniques to improve functional communication. This research project is structured as three work packages (WP). WP1: development of the dubbed language cinema-based therapy; WP2: a randomized, crossover, interventional pilot study; WP3: educational activities to improve public knowledge of aphasia and dissemination of the results. Here we present the protocol corresponding to the WP2 (clinical trial).
Design
A randomized, crossover, interventional pilot study, following the CONSORT guidelines on randomized pilot and feasibility studies [26] and the SPIRIT 2013 Recommendations for Interventional Trials [27].
Patient population
The Departments of Neurology and Rehabilitation (Speech Therapy Unit) at La Paz University Hospital and “Afasia Activa”, a non-profit patients’ association, will help with recruitment.
- Inclusion criteria:
- Non-fluent aphasia due to ischaemic stroke in the left hemisphere without neuroimaging evidence of lesions in the right hemisphere;
- Standard program of conventional speech therapy previously completed;
- Severely restricted language; poor repetition even for single words and moderately preserved language comprehension (i.e., not exceeding the 70th percentile in Repetition Scale plus exceeding the 15th percentile in Listening Comprehension Scale as an average score in word comprehension and command subscales and complex ideational material) in the Boston Diagnostic Aphasia Examination (BDAE);
- Signed informed consent. All the patients, or their guardian or legal representative, will be asked to sign a written informed consent form after a detailed explanation of the nature and purpose of this study and before undergoing any of the procedures related to the clinical trial. An aphasia-friendly information sheet containing large text and simplified language will be provided.
- Exclusion criteria:
- Any clinical condition or other characteristics that precluded appropriate follow-up;
- Simultaneous participation in any therapeutic trial assessing post-stroke recovery.
Randomization
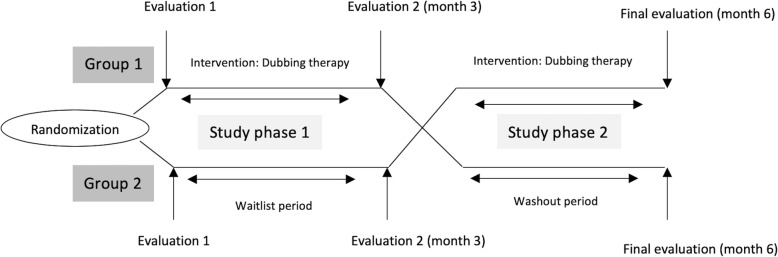
Upon signature of informed consent, the included patients will be randomly allocated (1:1) to one of the following groups (Fig. 1):
Group 1 (N=27): therapy starts within the first 3 months of their inclusion in the study, followed by a subsequent period of 3 months without therapy (washout period), thus serving as controls for the second phase of the study.
Group 2 (N=27): therapy starts between 3 and 6 months after their inclusion in the study. They do not receive speech therapy treatment during the first 3 months, thus serving as controls for the first phase of the study (waitlist controls) and as the active intervention group in the second phase.
Fig. 1.
Study design
A computer-generated random list of numbers provided by an independent statistician will be used for study allocation. The randomization sequence will be created using SAS V.9.4 statistical software (procedure “PROC PLAN”) with a 1:1 allocation. No randomization seed will be specified. The randomization seed will be generated taking the hour of the computer when the program is executed. Randomization will be performed centrally through an Interactive Web Response System in order to conceal the sequence until interventions are assigned (REDCap 8.7.4 - 2021 Vanderbilt University).
Intervention
Baseline session: first, evaluation of the baseline patient’s communication skills through BDAE and CAL. Second, to individualize the therapy, the patients and their relatives will select 48 words according to their needs from a list that was constructed by members of stroke and aphasia patients’ associations through an online survey available at https://goo.gl/forms/sxsDH9sicKaFlvas2. Each word has a corresponding 5- to 15-s clip that will be presented during the therapy following the principle of incremental learning (less complex first) using ad hoc designed software very similar to free audio editing software. Finally, the patient will receive training in the basic points of dubbing.
Sixteen 40-minute individualized dubbing sessions: one clip is played several times with sound. The dubbing actor explains the context and the speech therapist teaches the patient how to imitate the oral movements of the actor. Then, the target word is muted and the patient is encouraged to dub the gap, regardless of the synchrony, although some indicators may signal when the word should be pronounced. After each recording, the patient is rewarded by watching the dubbed fragment as many times as he/she wants to. We will score the following components: repetition, motor coordination, intonation, and synchrony. They will be evaluated at the end of the therapy considering the following ad hoc criteria for each targeted word: repetition (not achieved, achieved with difficulty, achieved); motor coordination (no impairment, mild impairment, moderate impairment, severe impairment); intonation (appropriate, not appropriate); and synchrony (appropriate, not appropriate).
Primary outcomes
A psychologist blinded to the patients’ clinical characteristics and his/her allocated group will administer the CAL [28] questionnaire and the BDAE [29] three times per patient. In brief, group 1 will be evaluated after the study group allocation, after the completion of the therapy, and after a washout period of 3 months, whereas group 2 will be evaluated after the study group allocation, after the waitlist period/prior the start of the therapy and after its completion.
Secondary outcomes
We will include additional tests following the ROMA consensus statement [30] for aphasia treatment research: General Health Questionnaire (GHQ)-12, Stroke Aphasia Quality of Life Scale (SAQOL-39), and the Western Aphasia Battery Revised (WAB-R). In addition, we will include the Stroke Aphasic Depression Questionnaire (SADQ10) to detect depressed mood and we will analyze the number of dropouts. An amendment to the protocol (protocol version 2.0; dated 7 October 2020), which was approved by the ethics committee prior the start of the recruitment, included a substudy to evaluate idiosyncratic and generalization effects with an ancillary multiple-baseline design [31]. We will randomly select 10 participants from the larger group of participants. The single-subject analysis will be comprised of three phases: baseline, treatment, and post-test. The assessor will be blind to the phase status of the participants. Generalization will be assessed by presenting 10-trial blocks of untrained words, untrained words in context, and trained words in context. In the untrained-word trials, the examiner will present a word that had not been used during treatment and will ask the participant to repeat it (e.g., please, repeat “lights”). In the untrained-word-in-context trials, the examiner will present a fill-in-the-blank phrase for target words that had not been used during treatment. Lastly, in the trained-word-in-context trials, the examiner will present a fill-in-the-blank phrase for target words previously used during treatment. We will establish the effect of the intervention for the three types of words (i.e., untrained, untrained in context, trained in context) using the Hedges-Pustejovsky-Shadish model [32]. Effect sizes will be calculated for all two-term comparisons (i.e., baseline vs. treatment, baseline vs. post-test, and treatment vs. post-test). To obtain inter-observer agreement, we will record the audio of 20% of the baseline, treatment, and post-test sessions. An agreement is defined as two independent observers recording the same trial outcome (e.g., correct, incorrect, no response). Interobserver agreement will be computed as the number of trials with agreement divided by the total number of trials multiplied by 100.
Data management and monitoring body
Data will be prospectively included in a study-specific database developed with REDcap software (REDCap 8.7.4 - 2021 Vanderbilt University). All data management will follow the principles of the European regulations for biomedical research ensuring confidentiality. In compliance with European regulations/International Conference of Harmonization Good Clinical Practice Guidelines, the investigator and the institution are required to permit direct access to authorized representatives of the Ethics Committee to review the subject’s original medical records for verification of study-related procedures and data. Monitoring will be conducted by dedicated personnel at the Clinical Trial Unit at La Paz University Hospital.
Sample size estimates
A formal sample size calculation is not possible given that this is a pilot study on a new therapy. However, based on a previous feasibility clinical trial on a different SLT in patients with post-stroke aphasia developed by our group [33], we estimated that we would need a sample size of 27 patients in each arm for an 80% power and a 0.050 two-sided significance level to detect a significant effect on the CAL evaluation [28]. Following the CONSORT guidelines for randomized pilot and feasibility trials, [26] upon the completion of this pilot trial we will estimate the sample size calculation for a definitive trial to evaluate the efficacy of this new therapy.
To achieve adequate participant enrolment to reach the target sample size, the following strategies will be implemented: stroke patients admitted to the Stroke Unit of La Paz University Hospital with post-stroke aphasia will be followed up at least 3 months after their stroke and, after the completion of the standard SLT, will be invited to participate in this study. Moreover, we will retrospectively review the clinical charts of patients discharged from the Speech Therapy Unit at La Paz University Hospital during the year before the start of the study and invite them also to participate. Finally, we will provide leaflets with the relevant study information to stroke patients’ associations. The cross-over design allows this experimental therapy to be offered to all the patients who participate in the trial, therefore reducing the possible rate of refusal to be enrolled in the control group. To ensure the follow-up, we will adapt the schedules of the dubbing sessions to the needs of the patients and their relatives.
Statistical analyses
We will use R software [34]. To evaluate the benefit of DULCINEA therapy through the CAL and the BDAE questionnaires, we will use mixed effects linear regression models, a statistical model particularly useful for longitudinal studies with repeated measures. Because of their advantage in dealing with missing values, mixed effects models are usually preferred over other approaches, allowing for an adjustment of the treatment effects by the baseline values and the period effect in crossover trials. Given this study has a crossover design with two treatment sequences (treatment-washout/waitlist-treatment) and two phases (phase 1 and phase 2) with a baseline evaluation of all the patients, we will consider treatment and phase as principal fixed effects, the treatment*phase as interaction effect and the patient nested in the treatment sequence as random effect. For pairwise post hoc comparisons, we will use the Bonferroni test. Analysis will also look for significant differences within test scores and variables scored for each dubbing session.
Discussion
Nowadays, one of the major challenges in SLT is to find a standardized, rapid, and low-cost therapy due to the increasing prevalence of stroke survivors; it is estimated that approximately 15–42% of these survivors experience post-stroke aphasia [3]. Although it has been clearly shown that SLT is beneficial for post-stroke aphasia in terms of improved functional communication, reading, writing and expressive language compared with no therapy, the current provision of SLT is heterogeneous with disparities related to the therapy regimen, delivery models, and the treatment setting [7].
In this challenging scenario, the development of an innovative therapy aimed at improving recovery from stroke as well as quality of life of stroke survivors and their relatives would represent an important advance in this field and would help to improve citizens’ health and wellbeing. In addition, computer programmes and apps to continue therapy at home, shown to improve speech output and narrative production in non-controlled studies [12, 13], could save precious time and money for society, patients, and relatives.
Using audiovisual content showing lip movements, on which dubbing techniques are based, and encouraging both healthy controls and post-stroke aphasic patients to imitate them is proven to activate a network of cortical areas involved in planning and executing speech production as demonstrated in functional MRI studies [35–37]. Therefore, there is a well-known neurobiological basis to support the effects on brain connectivity that DULCINEA therapy may produce. According to our preliminary results [25], this novel approach also helps to integrate various language requirements (the simultaneous practice of coordination of oral muscles, rhythm, and emotional intonation); to increase sustained attention and motivation, thus reducing dropouts and to improve functional language (as words are practiced within realistic frameworks, namely, the film scene). Compared with other trials on computer-based SLT, the DULCINEA trial allows the patient and their relatives to choose the target words to be dubbed, according to their needs and preferences. However, given that it is a novel therapeutic approach to post-stroke aphasia, it is unknown whether the potential improvement in functional communication is long-lasting or how long the effects last. This was one of the reasons for the choice of a cross-over design, to evaluate whether the effects last at least 3 months after the intervention. We chose this time point because it was a feasible washout period in our prior feasibility trial on aphasia treatment and it is a longer period than in other trials on aphasia, which lasted only 6 weeks [38, 39]. A 3-month period will give us a better approach to establishing the stability of the potential improvements.
Finally, it has been reported that the relevance and translation of research findings may be increased by considering research outcomes which are important to people living with aphasia [40]. In this sense, we incorporated from the very beginning the voice of patients with aphasia and their relatives, who contribute to the study design, mainly in the selection of words to be dubbed, and in the educational and dissemination activities scheduled in the DULCINEA project.
Conclusions
This pilot clinical trial exemplifies the collaboration between hospitals, universities, and patients in the development of a new therapeutic tool based on dubbing techniques in our aim to improve the recovery and quality of life of aphasic patients based on functional communication, computer-based technology, and standardized test assessment.
Trial status
Recruitment started in December 2020, and it is planned to end by March 2023. Protocol version 1.0, date 15 July 2019; Protocol version 2.0, dated October 7, 2020).
Acknowledgements
The patients’ association “Afasia Activa” is a partner of the research consortium. They have contributed to the design of the research project; in providing advice on the call application draft; in delivering a list of target words (together with two other Spanish stroke patients’ associations, “Asociación Ictus Madrid” and “Freno al Ictus”) and in organizing dissemination activities. We greatly appreciate the support of Morote Traducciones for editing assistance.
Abbreviations
- BDAE
Boston Diagnostic Aphasia Examination
- CAL
Communicative Activity Log questionnaire
- DULCINEA
DUbbing Language-therapy CINEma-based in Aphasia post-Stroke
- GHQ-12
General Health Questionnaire
- SADQ10
Stroke Aphasic Depression Questionnaire
- SAQOL-39
Stroke Aphasia Quality of Life Scale
- SLT
Speech and language therapy
- WAB-R
Western Aphasia Battery Revised
- WP
Work package
Authors’ contributions
BF: Study concept and design, manuscript draft. NB-G: Study concept and design, manuscript draft. LF-G: Study concept and design, revision for intellectual content. CS-I: Study concept and design, revision for intellectual content. CD-F: study design, revision for intellectual content; AT-R: study design, revision for intellectual content; MAL: Advice on the clinical trial design and revision for intellectual content. EdC-R: Advice on the clinical trial design and revision for intellectual content. Registration on the ClinicalTrials.gov website. RG-Z: Advice on the clinical trial design and revision for intellectual content. JL-T: Advice on the clinical trial design and revision for intellectual content. MM-A: Advice on the clinical trial design and revision for intellectual content. SP-Y: Advice on the clinical trial design and revision for intellectual content. RR-B: Advice on the clinical trial design and revision for intellectual content. GR-A: Advice on the clinical trial design and revision for intellectual content. JR-P: Advice on the clinical trial design and revision for intellectual content. JV-O: study design, revision for intellectual content; AM-B: Statistical and data monitoring plan. PB: Study concept and design, educational and dissemination strategy. All authors read and approved the final manuscript.
Funding
This study is promoted by Blanca Fuentes and the Research Foundation of La Paz University Hospital, which hosts a research consortium joined by the Department of Neurology at La Paz University Hospital, the Department of Psychology at Comillas Pontifical University, and the patients’ association Afasia Activa. This project has received funding from “la Caixa” Banking Foundation under the project code HR18-00026. Funder is not involved in any of the following processes: design of the trial, data collection, analysis, or interpretation of data nor than in writing the manuscript.
Availability of data and materials
Trial results will be published in Open Access journals. Raw data will be available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All the versions of this protocol (version 1.0, dated July 15, 2019, and version 2.0, dated October 7, 2020) have been approved by La Paz University Hospital Ethics Committee for Clinical Research. It has been registered with the ClinicalTrials.gov register: NCT04289493. All the patients, or their guardian or legal representative, will be asked to sign a written informed consent form after a detailed explanation of the nature and purpose of this study and before undergoing any of the procedures related to the clinical trial. An aphasia-friendly information sheet containing large text and simplified language will be provided.
Competing interests
The authors declare that they have no competing interests. We acknowledge the non-profit collaboration of RTVE, the Spanish Public TV corporation, that has provided us with the selected scenes to be dubbed for the patients.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Blanca Fuentes and Nereida Bueno-Guerra share the principal investigator’s responsibilities.
Contributor Information
Blanca Fuentes, Email: blanca.fuentes@salud.madrid.org.
Nereida Bueno-Guerra, Email: nbguerra@comillas.edu.
References
- 1.Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. The Lancet. Global Health. 2013;1:e259–e281. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gialanella B, Bertolinelli M, Lissi M, Prometti P. Predicting outcome after stroke: The role of aphasia. Disabil Rehabil. 2011;33:122–129. doi: 10.3109/09638288.2010.488712. [DOI] [PubMed] [Google Scholar]
- 3.Flowers HL, Skoretz SA, Silver FL, Rochon E, Fang J, Flamand-Roze C, et al. Poststroke Aphasia Frequency, Recovery, and Outcomes: A Systematic Review and Meta-Analysis. Arch Phys Med Rehabil. 2016;97:2188–2201. doi: 10.1016/j.apmr.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Pollock A, St George B, Fenton M, Firkins L. Top 10 research priorities relating to life after stroke - consensus from stroke survivors, caregivers, and health professionals. Int J Stroke. 2014;9:313–320. doi: 10.1111/j.1747-4949.2012.00942.x. [DOI] [PubMed] [Google Scholar]
- 5.Hebert D, Lindsay MP, McIntyre A, Kirton A, Rumney PG, Bagg S, et al. Canadian stroke best practice recommendations: Stroke rehabilitation practice guidelines, update 2015. Int J Stroke. 2016;11:459–484. doi: 10.1177/1747493016643553. [DOI] [PubMed] [Google Scholar]
- 6.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. AHA / ASA Guideline Guidelines for Adult Stroke Rehabilitation and Recovery. Stroke. 2016;47:e98–169. doi: 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 7.Brady M, Kelly H, Godwin J, Enderby P, Campbell P. Speech and language therapy for aphasia following stroke. Cochrane Database of Systematic Reviews 2016:Art No: CD000425. 10.1002/14651858.CD000425.pub4.www.cochranelibrary.com. [DOI] [PMC free article] [PubMed]
- 8.Lange B, Flynn SM, Rizzo AA. Game-based telerehabilitation. Eur J Phys Rehabil Med. 2009;45:143–151. [PubMed] [Google Scholar]
- 9.Wertz RT, Katz RC. Outcomes of computer-provided treatment for aphasia. Aphasiology. 2004;18:229–244. doi: 10.1080/02687030444000048. [DOI] [Google Scholar]
- 10.Wepman JM, Morency A. Filmstrips as an Adjunct to Language Therapy for Aphasia. J Speech Hear Disord. 1963;28:191–194. doi: 10.1044/jshd.2802.191. [DOI] [PubMed] [Google Scholar]
- 11.Bilda K. Video-based conversational script training for aphasia: A therapy study. Aphasiology. 2011;25:191–201. doi: 10.1080/02687031003798254. [DOI] [Google Scholar]
- 12.Fridriksson J, Hubbard HI, Hudspeth SG, Holland AL, Bonilha L, Fromm D, et al. Speech entrainment enables patients with Broca’s aphasia to produce fluent speech. Brain. 2012;135:3815–3829. doi: 10.1093/brain/aws301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan ES, Small SL. Imitation-based aphasia therapy increases narrative content: A case series. Clin Rehabil. 2017;31:1500–1507. doi: 10.1177/0269215517703765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Fowler R, Rodney D, Cherney L, Small SL. IMITATE: An intensive computer-based treatment for aphasia based on action observation and imitation. Aphasiology. 2010;24:449–465. doi: 10.1080/02687030802714157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kesav P, Vrinda SL, Sukumaran S, Sarma PS, Sylaja PN. Effectiveness of speech language therapy either alone or with add-on computer-based language therapy software (Malayalam version) for early post stroke aphasia: A feasibility study. J Neurol Sci. 2017;380:137–141. doi: 10.1016/j.jns.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Grechuta K, Rubio Ballester B, Espín Munne R, Usabiaga Bernal T, Molina Hervás B, Mohr B, et al. Augmented Dyadic Therapy Boosts Recovery of Language Function in Patients With Nonfluent Aphasia. Stroke. 2019;50:1270–1274. doi: 10.1161/STROKEAHA.118.023729. [DOI] [PubMed] [Google Scholar]
- 17.Palmer R, Dimairo M, Cooper C, Enderby P, Brady M, Bowen A, et al. Self-managed, computerised speech and language therapy for patients with chronic aphasia post-stroke compared with usual care or attention control (Big CACTUS): a multicentre, single-blinded, randomised controlled trial. Lancet Neurology. 2019;18:821–833. doi: 10.1016/S1474-4422(19)30192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherney LR, Lee JB, Kim KYA, van Vuuren S. Web-based Oral Reading for Language in Aphasia (Web ORLA®): A pilot randomized control trial. Clin Rehabil. 2021;35:976–987. doi: 10.1177/0269215520988475. [DOI] [PubMed] [Google Scholar]
- 19.Spaccavento S, Falcone R, Cellamare F, Picciola E, Glueckauf RL. Effects of computer-based therapy versus therapist-mediated therapy in stroke-related aphasia: Pilot non-inferiority study. J Commun Disord. 2021;94:106158. doi: 10.1016/j.jcomdis.2021.106158. [DOI] [PubMed] [Google Scholar]
- 20.Elhakeem ES, Saeed SSGM, Elsalakawy RNA-E, Elmaghraby RM, Ashmawy GAHO. Post-stroke aphasia rehabilitation using computer-based Arabic software program: a randomized controlled trial. The Egyptian. J Otolaryngol. 2021;37 10.1186/s43163-021-00144-3.
- 21.Jang SH. Diffusion Tensor Imaging Studies on Arcuate Fasciculus in Stroke Patients: A Review. Front Hum Neurosci. 2013;7 10.3389/fnhum.2013.00749. [DOI] [PMC free article] [PubMed]
- 22.Berthier ML, Lambon Ralph MA. Dissecting the function of networks underpinning language repetition. Front Hum Neurosci. 2014;8:2013–2015. doi: 10.3389/fnhum.2014.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dick AS, Bernal B, Tremblay P. The language connectome: New pathways, new concepts. Neuroscientist. 2014;20:453–467. doi: 10.1177/1073858413513502. [DOI] [PubMed] [Google Scholar]
- 24.Hope TMH, Prejawa S, Parker Jones Ō, Oberhuber M, Seghier ML, Green DW, et al. Dissecting the functional anatomy of auditory word repetition. Front Hum Neurosci. 2014;8:1–17 10.3389/fnhum.2014.00246. [DOI] [PMC free article] [PubMed]
- 25.Bueno-Guerra N. La importancia de los gustos del paciente en la rehabilitación logopédica y neuropsicológica del ictus. XXI Congreso Internacional AELFA-IF 2018. https//www.researchgate.net/publication/324280627_The_importance_of_the_patient’s_preferences_in_logopedic_and_neuropsychological_rehabilitation_of_stroke_La_importancia_de_los_gustos_del_paciente_en_la_rehabilitacion_logopedica_y_neuropsicologica_de ().
- 26.Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ (Online) 2016;355:1–32. doi: 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan A, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulvermuller F, Neininger B, Elbert T, Mohr B, Rockstroh B, Koebbel P, et al. Constraint-Induced Therapy of Chronic Aphasia After Stroke. Stroke 2001;32:1621–1626. 10.1161/01.STR.32.7.1621. [DOI] [PubMed]
- 29.García-Albea J, Sánchez Bernardos M, del Viso-Pabon S. Test de Boston para el diagnóstico de la afasia: Adaptación española. In: Goodglass H, Kaplan E, editors. La evaluación de la afasia y de trastornos relacionados. 2. Madrid: Editorial Médica Panamericana; 1986. pp. 129–198. [Google Scholar]
- 30.Wallace SJ, Worrall L, Rose T, Le Dorze G, Breitenstein C, Hilari K, et al. A core outcome set for aphasia treatment research: The ROMA consensus statement. Int J Stroke. 2018;0:174749301880620. doi: 10.1177/1747493018806200. [DOI] [PubMed] [Google Scholar]
- 31.Bailey JS, Burch MR. Research Methods in Applied Behavior Analysis. 2. New York: Routledge; 2017. [Google Scholar]
- 32.Hedges LV, Pustejovsky JE, Shadish WR. A standardized mean difference effect size for single case designs. Research Synthesis. Methods. 2012;3 10.1002/jrsm.1052. [DOI] [PubMed]
- 33.Haro-Martínez AM, Lubrini G, Madero-Jarabo R, Díez-Tejedor E, Fuentes B. Melodic intonation therapy in post-stroke nonfluent aphasia: a randomized pilot trial. Clin Rehabil. 2019;33:44–53. doi: 10.1177/0269215518791004. [DOI] [PubMed] [Google Scholar]
- 34.R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing 2018. https://www.r-project.org/.
- 35.Skipper JI, van Wassenhove V, Nusbaum HC, Small SL. Hearing Lips and Seeing Voices: How Cortical Areas Supporting Speech Production Mediate Audiovisual Speech Perception. Cereb Cortex. 2007;17:2387–2399. doi: 10.1093/cercor/bhl147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santhanam P, Duncan ES, Small SL. Therapy-Induced Plasticity in Chronic Aphasia Is Associated with Behavioral Improvement and Time since Stroke. Brain Connect. 2018;8:179–188. doi: 10.1089/brain.2017.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mashal N, Solodkin A, Dick AS, Elinor Chen E, Small SL. A network model of observation and imitation of speech. Front Psychol. 2012;3:1–12. doi: 10.3389/fpsyg.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Meulen I, van de Sandt-Koenderman MWME, Heijenbrok MH, Visch-Brink E, Ribber GM. Melodic intonation therapy in chronic aphasia: Evidence from a pilot randomized controlled trial. Front Hum Neurosci. 2016;10:1–9. doi: 10.3389/fnhum.2016.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Meulen I, van de Sandt-Koenderman WME, Heijenbrok-Kal MH, Visch-Brink EG, Ribbers GM. The efficacy and timing of melodic intonation therapy in subacute aphasia. Neurorehabil Neural Repair. 2014;28:536–544. doi: 10.1177/1545968313517753. [DOI] [PubMed] [Google Scholar]
- 40.Wallace SJ, Worrall L, Rose T, Le Dorze G, Cruice M, Isaksen J, et al. Which outcomes are most important to people with aphasia and their families? an international nominal group technique study framed within the ICF. Disabil Rehabil. 2017;39:1364–1379. doi: 10.1080/09638288.2016.1194899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Trial results will be published in Open Access journals. Raw data will be available from the corresponding author upon reasonable request.