Abstract
Water contamination is an environmental burden for the next generations, calling for advanced methods such as adsorption to remove pollutants. For instance, unwanted biowaste and invasive plants can be converted into biosorbents for environmental remediation. This would partly solve the negative effects of invasive plants, estimated at 120 billion dollars in the USA. Here we review the distribution, impact, and use of invasive plants for water treatment, with emphasis on the preparation of biosorbents and removal of pollutants such as cadmium, lead, copper, zinc, nickel, mercury, chromate, synthetic dyes, and fossil fuels. Those biosorbents can remove 90–99% heavy metals from aqueous solutions. High adsorption capacities of 476.190 mg/g for synthetic dyes and 211 g/g for diesel oils have been observed. We also discuss the regeneration of these biosorbents.
Keywords: Invasive plants, Biosorbents, Water treatment, Heavy metal ions, Synthetic dyes, Oil removal
Introduction
Industrialization and technological advancements play a considerable role in the civilization of mankind, but their repercussions are the water quality deterioration. Several publications indicated that about one billion people did not have access to clean water worldwide and this figure would incline in tune with human development (Tauqeer et al. 2021). Besides, water contamination could enhance the growth of aquatic pathogens such as harmful microbes, especially coronavirus that causes the coronavirus 2019 (COVID-19) pandemic (Sharma et al. 2020). The major culprit for such phenomenon stems from the inappropriate disposal of a wide spectrum of organic and inorganic compounds including textile dyes, heavy metals, pharmaceuticals, and hydrocarbons (Islam et al. 2021). Among the contaminants, water pollutions caused by heavy metals are alarming because just low concentrations of them could trigger the reactive oxygen species that lead to cytotoxicity. For example, long-term excess exposure to arsenic, which means overconsuming 0.05 mg/L, could express acute symptoms such as chronic respiratory disorders, sensory loss, skin discoloration, and finally skin cancer (Sodhi et al. 2019). Likewise, with the development of dye production industries, over 10,000 dyes have been expelled, the concentration of color effluents in wastewater has increased greatly (Islam et al. 2021). These contaminants not only diminish the aesthetic value of the water bodies but also interfere with the penetration of light into the water, thereby leading to disturbances in the aquatic ecosystem. Particularly, recalcitrant dyes are mostly constituted by complex aromatic structures that make them carcinogenic and obstruct their biodegradation (Nguyen et al. 2021a). Therefore, discovering practical treatments to discard such toxic pollutants is considered the need-of-the-hour commission of humans.
Disparate methods have been exploited for water purification such as coagulation, oxidative-reductive degradation, Fenton process, membrane separation, ultrafiltration, reverse osmosis, and photocatalysis (Janani et al. 2022). These processes are costly, complicated, time and energy consuming, skilled personnel, and especially create amine residues in the sludge. In the last decade, adsorption is evaluated as a promising alternative because it is the most efficient and thus most applied in water treatment (Wang et al. 2021a). However, it is the nature of the adsorbent that affect directly to the removal efficiency of pollutants. In fact, thousands of adsorbents from available sources have been recommended in the literature to remove contaminants, for instance, clay minerals, biomass, materials from agricultural wastes, and by-products (Akpomie and Conradie 2020). Biomass-based adsorbents have gained considerable attention thanks to their availability in huge quantities in most places worldwide, environmental friendliness, and demanding simple pretreatment and preparation (Mpatani et al. 2021). In particular, the use of waste biomass from invasive species as adsorbent materials for the removal of pollutants from aqueous streams could decrease their threat, in harmony with the circular economy (Ahmed et al. 2020; Lian et al. 2020).
Plant biological invasion is considered the second alarming peril to biodiversity overtaken by habitat alteration. After invading areas outside their original natural regions, either deliberately or unintentionally, invasive species damage the function of bioecosystems, the structure, and also the dynamics of other populations. The strong reproduction, fast propagation, significant tolerance, and high adaptability of exotic plants support such ecological change (Paz-Kagan et al. 2019). For instance, the aquatic weed Eichhornia crassipes, commonly called water hyacinth, could flourish over 200 kg per ha each day (Huang et al. 2021), and 125 tons per surface ha in half of a year (Istirokhatun et al. 2015). Globally, its living form covered over 4 million hectares of wetlands, consequently, threatening the aquatic survival of other organisms and causing serious human issues such as mosquito-borne malaria, and the disturbance of the water transport network (Nguyen et al. 2021e). Moreover, invasive plants are also associated with human well-being, including jeopardizing the integrity of agricultural systems, the balance of the economy, and public health. Take the USA as an example, it was estimated an economic loss of 120 billion dollars yearly driven by such exotic plants (Duenas et al. 2018). In Africa, an invasive cactus, Opuntia stricta, was recorded to make a loss of 500 to 1000 US dollars per household annually by damaging the cultivated crops (Shackleton et al. 2017; Sileshi et al. 2019). Lantana camara, a shrub with attractive flowers and had been utilized as ornamental, invaded almost all Indian pasture lands with a total area of 13.2 million ha. To control this aggressive weed, a cost of 70 US dollars for each ha was needed (Negi et al. 2019). Another highly noxious plant, Ambrosia artemisiifolia, was documented that induced pollen-borne allergy for about 13 million European and imposed economic costs of around 7.5 billion Euro each year (Schaffner et al. 2020). Because of their serious impacts on ecology and the economy, there is an urge for finding effective approaches to manage the invasive plants and utilize them as beneficial biomass sources.
Currently, the common disposal methods of exotic weed are usually manual eradication and chemical removal. Such processes produce an abundance of biomass waste, consequently, pose an increasing burden to the ecosystem. In fact, several researches have taken advantage of their great tolerance and stronger growth traits for phytoremediation (Prabakaran et al. 2019; Rezania et al. 2019; Mustafa and Hayder 2021). However, this method requires great human and financial resources, performance may be seasonal, limits to several pollutants, and generates secondary wastes after treatment (Odoh et al. 2019). Thus, there is a shift in attention to the conversion of invasive plants to biosorbents for the decontamination of pollutants. Recently, Feng et al. (2021) have summarized the potential of exotic plants for biochar productions and their added value. Several other adsorbents for pollutants removal from invasive species were well reported such as activated carbons (Bouhamidi et al. 2017; Al-Musawi et al. 2021), nanoparticles (Durairaj et al. 2019; Davarnejad et al. 2020), composites (Wang et al. 2020a; Ren et al. 2021a), and aerogels (Yang et al. 2018; Cui et al. 2021). Although these materials are quite efficient, they necessitate so further preparations, chemicals, time, and energy that they are relatively not suitable for large-scale applications. Conversely, raw biomass-waste materials are gained attention due to their simple preparation, low cost, eco-friendly, and biodegradable characteristics (Shooto 2020; Acosta-Rodríguez et al. 2021).
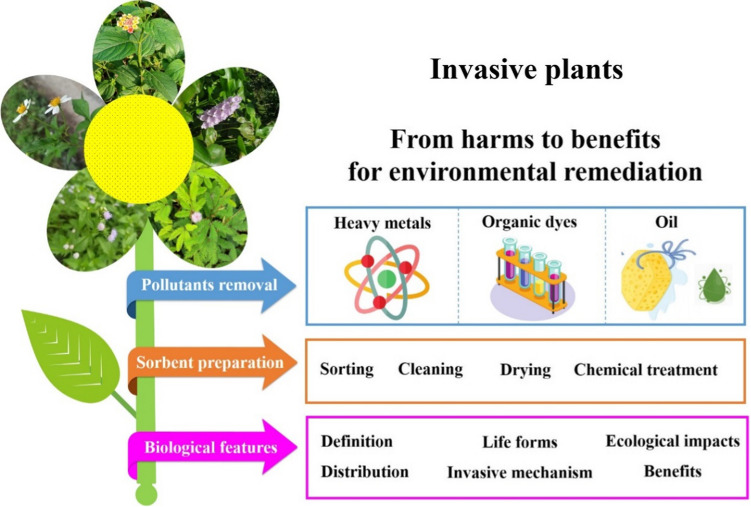
Regardless of several publications on the utilization of invasive plants biomass for medicines, phytoremediation, and biochar production, it lacks a comprehensive review that mentions the use of invasive plants as biosorbents for environmental remediation. To the best of our knowledge, this is the first review covering all aspects from the distribution, harmful properties, the biological traits, invasive mechanisms to potential applications of invasive plants in water treatment. This study spotlights the viability of such biosorbents for the treatment of many kinds of pollutants such as heavy metal, organic dyes, and oils. Therefore, the present review is systematically organized in three key sections: (i) biological traits and harms of invasive plants; (ii) insights into the preparation of biosorbents from invasive species; (iii) utilizations of invasive plant-based biosorbents for removal of heavy metals, organic dyes, and oils (Fig. 1). We briefly discuss the knowledge gaps and future directions of invasive plant-based adsorbents to orientate further researches, and their practical large-scale applications.
Fig. 1.
Conversion of biomass from harmful invasive plants into beneficial biosorbents for environmental remediation. The biosorbent preparation experiences some stages such as collecting, cleaning, drying, and treatment. Biosorbents derived from invasive plants can be used to remove heavy metals, organic dyes, and oils. This development remedies wastewaters and contributes to the prevention of powerful invasion of exotic plants
Biological characteristics of invasive plants
Anthropocentric definition
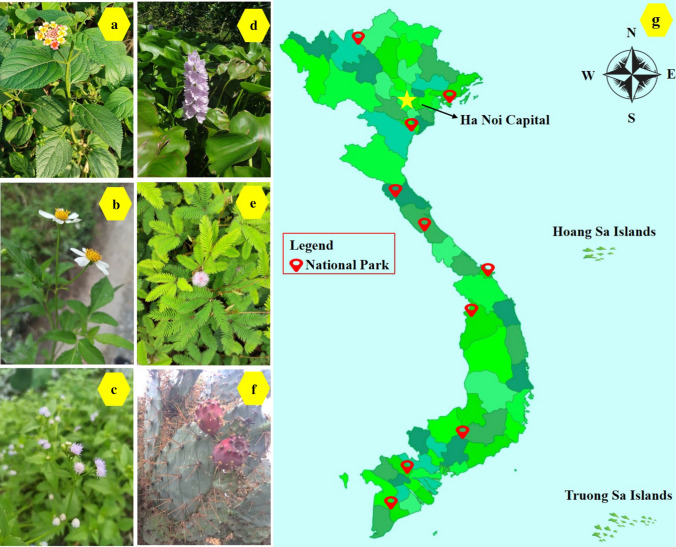
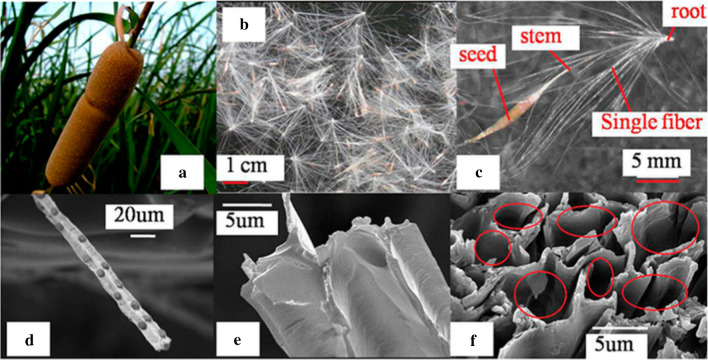
Hinging on the demography, the influence, and especially the genesis of species to definite what the invasive plant is. However, there are more common usages of the term “invasive” for the one which is both non-native and harmful for the economy and environment or even on human health (Russell and Blackburn 2017). For example, Opuntia stricta was originally grown as ornamental and fencing function in various places. After escaping cultivation and invading conserved regions, it causes many problems such as mitigating fodder production, jeopardizing public health, hindering human transportation, and influencing livestock (Witt et al. 2020). Pyšek et al. (2012) estimated there were 167 invasive species that resided in 49 plant families associated with the feedback of the resident biomes. These authors concluded that it lacked the universal measurement of influence and significantly depended on the observed context. On the other hand, Shackleton et al. (2019) overviewed relevant researches focusing on 66 invasive species. The data showed that 48% of species were attributed to having both advantages and disadvantages on local livelihoods including Lantana camara, while nearly 37% of them created major costs such as Opuntia stricta and Chromolaena odorata. Other representatives of invasive plants are illustrated in Fig. 2 based on the result of Tan et al. (2012) conducted in the national parks of Vietnam.
Fig. 2.
Invasive plants including Lantana camara (a), Bidens pilosa (b), Chromolaena odorata (c), Eichhornia crassipes (d), Mimosa pigra (e), Opuntia stricta (f), and their geographical distribution in national conservation parks in Vietnam (g)
Classification and habitat distribution of invasive plants
Invasive species commonly flourish in unusual habitats rich in resources, and thus, they have high biodiversity and wide distribution. Their extraordinary multitude of species, from aquatic weeds (e.g., Eichhornia crassipes, Pistia stratiotes) to grasses, small (e.g., Poe annua, Cynodon dactylon) and large (e.g., Phragmites australis, Sorghum halepense), forbs and herbs (e.g., Lythrum salicaria, Centaurea solstitialis), vines (e.g., Celastrus orbiculatus, Rosa multiflora), shrubs (e.g., Mimosa pigra, Lantana camara) and trees (e.g., Pinus ponderosa, Castilla elastic) (Albrecht et al. 2021). Mallick et al. (2019) reported that there were 132 genera in 165 taxa of invasive plants in the City of Odisha, India. Among, herbs were prevalent than other life forms, which accounted for 69% of the total recorded plant species. The proportion for trees, shrubs, and vines was 14, 13, and 3%, respectively. This could be explained that herbaceous plants not only possess more tolerant capacity to harsh conditions but also have tremendous viability in any environment. Weidlich et al. (2020) gathered information on exotic plant species in bio-ecological restoration from 372 articles in the first twenty years of the twenty-first century and analyzed their distribution. This systematic review showed that there were 10 researched biomes (Fig. 3). Regardless of what kind of biome, the major groups managed in restoration programs were relatively identical in terms of ratio. Nearly half of the species were exotic grasses that almost belong to Poaceae family, indicating that this invasive life form became a global issue. Forbs were recorded as the second most ubiquitous, followed by shrubs, trees, and lianas.
Fig. 3.
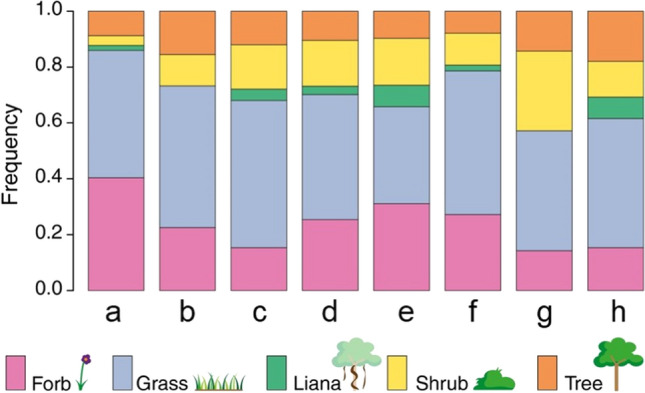
Proportion of exotic plants by growth forms—forb, grass, liana, shrub, and tree—that are controlled in restoration sites including a Chaparral, b Desert, c Savanna, d Temperature coniferous forest, e temperature deciduous forest, f Temperature grassland, g Tropical dry forest, and h Tropical rain forest. Accordingly, the distribution of major exotic species in various restoration sites was insignificantly different. Mean ± standard deviation of five biomes could be shown: 46.0 ± 5.8% grass, 24.1 ± 9.0% forb, 14.6 ± 7.1% shrub, 12.1 ± 3.5% tree, and 3.2 ± 3.1% liana. Reproduced with the permission of Wiley Online Library from reference (Weidlich et al. 2020)
Mechanism of plant invasion
Thousands of exotic species have been sent to native ecosystems over the world, either deliberately or unintentionally. The successful invasive reasons are complicated and need to be studied in the specific context of each species. Overall, such success comprises some main features related to wide bio-ecological demands and tolerances, r-selected life histories, broad geographical scopes, evolution from high diverse regions as well as anthropogenic or disturbed native environments (Jose et al. 2013). In terms of ecological process, allelopathy is fundamentally one that active secondary compounds play a critical role in biotic interference. This approach helps invasive species modify the invaded regions to improve their competitiveness (Pinzone et al. 2018). For instance, essential oil from the rhizomes of Hedychium coronarium was demonstrated to have phytotoxic effect on the germination of resident plants (Costa et al. 2019). Zheng et al. (2015) hypothesized that Chromolaena odorata could secrete a phenolic compound called odoratin. Such allelochemical joined in the defense of soil-borne pathogens and other enemy resistance of this invasive weed, thus paving the way for its colonization. Allelopathy was demonstrated as one of the most ubiquitous invasion mechanisms throughout the vascular plant phylogeny which accounted for roughly 51% of non-native species in the surveyed database. Thus, allelopathy is considered to significantly influence the native ecosystems worldwide and deserves more attention (Kalisz et al. 2021).
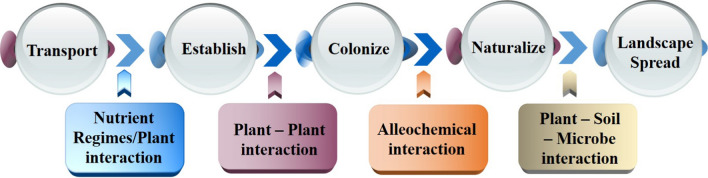
In addition, efficient resource uptake and use is another approach for plant invasion. For example, Bromus tectorum could defeat other native plants thanks to its capacity to take advantage of an increase in nitrogen in soil (Morris et al. 2016). Figure 4 illustrates the common mechanism of biological invasion of plants in a new habitat. This process comprises five stages, from transporting to spreading in the landscape, which depends on the patterns of disturbance, biomes composition, and the interactions with environmental factors (Paz-Kagan et al. 2019). By understanding such progress, people could generate effective strategies to either counter or limit the expansion of these exotic species.
Fig. 4.
Mechanism of biological invasion of plants in new habitats. Biological invasion disrupts interactions of indigenous species. (i) Nutrient regime is the first indication of new invasive transportation. (ii) Invasive plants tend to proliferate well in their regions, directly affect local species interactions. (iii) To consolidate colonization, they secrete allelochemicals into ambient environment, thereby exerting detrimental physiological effects on the growth and reproduction of native species. (iv) Naturalization signs a profound change in the distribution of plant, soil, and microbe. (v) With many strong functional traits, invasive plants spread various landscapes to complete a biological invasion and initiate a new cycle
Impacts of invasive plants
Biological invasion of the plant is a well-perceived stimulator of ecological alternation globally. Such change relates to the function of bioecosystems, the structure, and also the dynamics of other populations. For example, the strong invasion of a shrub called Rhododendron ponticum facilitates the safety of a nocturnal rodent from the threat of its major predator—the tawny owl. However, the vigorous growth of this invasive weed restrains the development of native food plants, and hence lowers local food availability (Malo et al. 2013). There is a shift to negative impacts of biological invasion since it generates catastrophe on genes, populations, native communities, and thus loss of biodiversity. Munoz and Cavieres (2019) uncovered that Taraxacum officinale, an invasive weed, was attributed to change plant–pollinator networks when co-existing with native species. In terms of the impact on native flora communities, the exotic herb Leucanthemum vulgare was found to decrease the species richness severely at larger temporal scales (Ahmad et al. 2019). Another non-native weed, Oenothera drummondii was discovered to affect more negatively in inland areas in comparison with the foredune areas (Gallego-Fernandez et al. 2019). Therefore, the ecological impacts of biological invasion fluctuate from the standard of each native species to whole biosphere patterns and processes.
Invasive plants are also associated with human well-being, including jeopardizing the integrity of agricultural systems, the balance of the economy, and public health. Indeed, Ambrosia trifida, normally known as giant ragweed, has been recognized as not only a harmful factor for reducing crop yields but relating to serious allergic symptoms for people (Park et al. 2012). Another highly noxious ragweed, Ambrosia artemisiifolia, was documented that induced pollen-borne allergy for about 13 million European and imposed economic costs of around 7.5 billion Euro each year (Schaffner et al. 2020). Moreover, this exotic herb caused a loss of 30% to sunflower and maize production yields and had the potential to lower the soybean biomass up to 70% (Savić et al. 2021). In Africa, an invasive cactus, Opuntia stricta, was estimated to create a loss of 500 to 1000 US dollars per household annually by damaging the cultivated crops. An economic loss of about one billion US dollars in the agricultural field was caused by total non-indigenous invaders in the African continent (Shackleton et al. 2017; Sileshi et al. 2019). Also, several invasive plants impose an impact on humans via the alteration of water quality. Pejchar and Mooney (2009) reported that the invasion of genus Tamarix leads to a tremendous loss of water up to 3 billion cubic meters equivalent to around 70 million US dollars. Other invasive plants influence on public health by elevating the growth of pathogen populations. For example, Virginia deer takes advantage of invaded regions by non-native shrub Lonicera maackii as a food source. However, this animal is reported to carry northeastern water tick that causes tick-borne ehrlichiosis for humans. Other cases that benefited from invasive vascular plants are recorded as the vectors of pathogens, such as scrub typhus, Lyme disease, Hantavirus, malaria, trypanosomiasis, and spotted fever (Stewart et al. 2021).
On the other hand, there are some added potential facets of invasive plants, such as food sources, ornamental benefits, cosmetics, utilizations in vector bone control, medicine, bioenergy, and green synthesis of materials (Nguyen et al. 2021d). For instance, the extraction of flowering plant Lantana camara could be applied as mosquito repellent (Sharma et al. 2021). Recently, this invasive shrub has been proved to have manifold medicinal properties, including fungicidal, insecticidal, and strongly antibacterial against E. coli, P. aeruginosa, Bacillus subtilis (Arunkumar et al. 2019). Apart from the original use as a garden ornamental, Lantana camara biomass has been used to produce biopesticides, fertilizers, pulps, paper fibers, bioenergies, fuelwoods, honey plants, and cosmetics (Negi et al. 2019). A representative of aquatic weed, water hyacinth (Eichhornia crassipes) has been applied for the production of ethanol, biodiesel, biohydrogen and developing supercapacitors. Interestingly, there are more and more studies laid emphasis on this species as adsorbents including phytoremediation for removal of heavy metals, organic compounds, and other pollutants (Guna et al. 2017). In brief, the economic benefits and positive contribution of invasive plants could outweigh their disadvantages; thus, it requires more researches on cost–benefit examinations tailoring solutions for its effective management.
Conversion of invasive plants into biosorbents
Preparation procedure
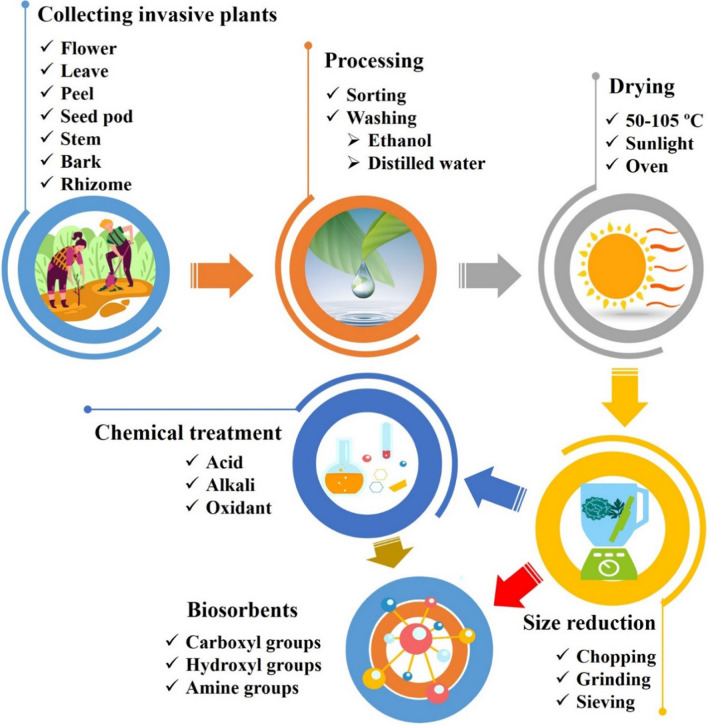
Some invasive plants can contain many toxic compounds that trigger poisonous effects on humans such as skin inflammation and fester. Hence, the procedure of detoxification is required before they can be next proceeded. Attaining the invasive plant-based raw biomass, there are overall four main steps as illustrated in Fig. 5. Initially, the invasive plants are collected from their landscapes, and they could be the aboveground biomass like leaves, stems, flowers, bark or underground biomass like rhizomes or the whole plant. Then, the explants are sorted and washed to remove dirties. Next, they are commonly desiccated under solar light or by oven at temperatures from 50–105 °C to remove the moisture content. The dried materials are subjected to size reduction by chopping or grinding before using a sieve to obtain small size particles. Most of the previous publications ended at this step and the adsorbents were stored or used directly for further studies. However, more studies applied additional steps to enhance the adsorption capacity of the materials by chemical pretreatment. The detailed procedure of each technique will be discussed in the next sections.
Fig. 5.
Preparation of biosorbents from invasive plants. Many invasive plant parts can be collected, including flowers, leaves, roots, etc., for the next processing stage. Drying procedure of invasive biomass can be carried out under solar light to remove moisture. To enhance surface area and surface chemistry, the dried samples are ground and treated by chemical agents such as acid, or alkali
Cleaning the biomass
Washing biomass not only removes the impurities, pathogen borne agents but also discards some endogenic inorganics that affect the adsorption process. Johnson et al. (2019) identified fourteen species of insects including ant, fly, wasp, bee, and beetle that were considered the visitors of exotic weed Fallopia japonica. Many of them were proved to be vectors transmitting pathogens during plant–insect interactions. Indeed, it was documented that fungal pathogens including Bipolaris species causing foliar blight in an aggressive invasive grass, Microstegium vimineum (Flory et al. 2011). On the other hand, apart from the structural carbohydrates and lignin, invasive plant biomass also comprises extraneous ingredients including inorganics. These molecules exist in various forms, such as alkali metals, alkaline earth metals, transition metals, and non-metals (Dahou et al. 2021). While such elements only account for negligible proportion, they could be dissolved in the polluted solutions and thus lower the biosorption capacity of raw biomass (Amrhar et al. 2021). Regardless of the little concern or even lack in the material preparation method, the explants could be generally washed by distilled water at ambient temperature before drying (Bansal et al. 2021). In brief, making the materials cleaner seems to be a straightforward step but plays an important role in the fabrication of biosorbents.
Grinding the biomass
Size reduction in biomass contributes significantly to the efficiency of pollutants removal of biosorbents. This step is approached by using a hammer mill (Rigueto et al. 2020) or a domestic mixer (Tounsadi et al. 2015) with initial chopping. Then, the sieves are applied to categorize the material by appropriate size for either further investigation or utilization. The underlying aim of reducing feedstock size is to enhance the surface areas of the adsorbents and shorten the diffusion path of them, in order words, increase the interactions of materials and pollutants (Nguyen et al. 2021b). For example, Hossain et al. (2012) investigated the effect of three different particle sizes of garden grass raw biomass on copper removal. The authors concluded that the adsorption capacity rose from approximately 6 to 11.17 mg/g when diminishing the particle size more than a half from 150 μm. Similarly, Khamparia and Jaspal (2016) found that the removal efficiency of Rhodamine B by Argemone mexicana seed-based adsorbent inclined significantly in tune with the decrease in the particles size from 710 to 300 μm. Regardless of the fact that size reduction demands a noticeable amount of resources and cost for scale-up investigation, this step is essential for an effective discard of contaminants by adsorption.
Chemical pretreatment
Invasive plant biomass has recalcitrant traits due to its intrinsic complicated structures. Indeed, most biomass tissues built up by cellulose, hemicellulose, and lignin possess numerous complex bonds and functional groups (Wang et al. 2021b). Chemical pretreatments contribute relatively to the disruption of the lignocellulose biomass, thus helping the treated materials increase surface area and porosity. These improvements then facilitate the adsorption capacity of biosorbents for pollutants removal. Frequently, acids, bases, and oxidizing agents are utilized as chemicals for such purposes (Sheng et al. 2021). Raw biomass would experience hydrolysis reaction during the treatment with dilute acid, e.g., oxalic acid, hydrochloric acid (HCl), or alkali, typically sodium hydroxide (NaOH). The acid could break down interchain bonds of hemicellulose while the base solution helps to decrease lignin proportion (Mallesh et al. 2020). To sum up, the chemical modifiers benefit biomass by reducing soluble organic compounds and improving the pollutants elimination efficiency.
Several publications reported the investigation of chemical treatments on invasive plant biomass. For example, Shooto (2020) examined the effects of HCl and NaOH on the adsorption of Cr(VI) and Pb(II) heavy metal ions of biosorbent derived from Acorus calamus rhizome. The authors found that such treatment helps increase the surface area of raw biomass from 5.78 to 9.98 cm2/g by acid treatment and nearly twofold by base treatment. Although both biosorbents have the adsorption capacity for Pb(II) higher than Cr(VI), the overall trend illustrated that NaOH acted the best modifier, followed by HCl. In particular, the maximum removal of adsorbent for Cr(VI) and Pb(II) treated by alkaline solution inclined over 10 mg/g compared with pristine biomass. Similarly, Ye et al. (2010) used NaOH to treat rice husk and the treated adsorbent showed significantly higher elimination of Cd(II) than natural one. However, chemical pretreatments have some disadvantages related to costs of chemicals, facilities, and demanding further drying processes (Jönsson and Martín 2016). Thus, evaluation of the efficiency of such modifications on various exotic plants should be more concerned.
Removal of heavy metal ions by invasive plants
Occurrence of heavy metal ions
The term heavy metal implies a metallic element toxic that cause a poisonous level at low concentrations (Gemeda et al. 2021; Lisak 2021). Their occurrence in nature can be detected through metal corrosion, soil erosion, or releasing heavy metals from ore mining (Malik et al. 2019). Over the past decades, the presence of heavy metal ions in aqueous media such as groundwater, surface water, and seawater has been alarmingly serious (Lim et al. 2018; Liu et al. 2019a; Karaouzas et al. 2021). For example, Chowdhury et al. (2016) reported the co-exposure of a large amount of lead, arsenic, cadmium, copper, etc., in the drinking water from taps and plumbing pipes inside the building. Sibal and Espino (2018) investigated the occurrence and analytical determination of heavy metals in many lakes in Asian countries. Palansooriya et al. (2020) overviewed the recent occurrence of many kinds of contaminants including heavy metals co-existing in drinking water sources. These contaminations are mainly due to human activities since the discharge of heavy metal ions is occurred during the production of batteries, fertilizers, metallurgical industries, and electronic devices without pretreatment (Rathi et al. 2021).
Literatures reports adverse effects of heavy metal ions as carcinogenic substrates, or toxic agents against neurons (Fu and Xi 2020; Qasem et al. 2021). Many metals, such as lead, mercury and cadmium, cause cardiovascular diseases and cancer mortalities which result in adverse effects on human health as well as aquatic creatures (Vareda et al. 2019; Ajiboye et al. 2021). As a result, the removal of heavy metal ions is an urgent task. Nowadays, a number of techniques involving chemical precipitation, ionic exchange, membrane filtration and ultrafiltration have been adopted to treat the pollution of heavy metal ions (Carolin et al. 2017; Vasseghian et al. 2021). However, the main disadvantages of these techniques are high cost, time consuming procedures, and complex operations (Qin et al. 2020). Adsorption technique using biosorbents is highly recommended because it offers a wide range of advantages such as eco-friendliness, high performance, and simple procedure (Karaouzas et al. 2021). The use of invasive plants as biosorbents for the removal of heavy metal ions may meet these critical requirements.
Plausible adsorption mechanism of heavy metal ions
The use of biosorbents from invasive plants is very beneficial because they exhibit the efficient and eco-friendly sequestration of cadmium (Ayuba et al. 2019). With the constitution of many chemical groups such as hydroxyl, amine, and carboxyl, these adsorbents can have high affinity to heavy metal cations (Pyrzynska 2019). According to Table 1, many works pointed out that some of the underlying mechanisms of metal cations adsorption are ionic exchange, and electrostatic attraction. The former relies on the formation of chelation and sequestration between divalent, trivalent metal cations with adjacent carboxyl, hydroxyl, and amine groups on the biosorbent structure (Fig. 6). Such affinity leads to the stable coordination complexation of metallic compounds to release the protons (H+) or cations (e.g., Na+, K+) into the solutions (Wong et al. 2014). The latter mechanism relies on the formation of electrostatic attraction between positively charged metal cations and negatively charged surface of the biosorbent (Saleh and Ali 2018; Verma et al. 2020; Peng et al. 2021). This may enhance the immobilization and sequestration of heavy metal ions in the structure of the biosorbent.
Table 1.
Adsorption performance of biosorbents from invasive plants for the treatment of heavy metal ions
| Plant | Part | Biosorbent features | Target metals | pH | Adsorption results | Adsorption mechanism | Refs. |
|---|---|---|---|---|---|---|---|
| Arundo donax L | Leaf | Particle size: 750 μm | Cd(II) | 5.5 | 27.9 mg/g | Electrostatic attraction, chemical reaction | (Ammari 2014) |
| Cyperus papyrus | Stem | Treated with H2O2 and NaOH | Cd(II) | 3.5 | 0.028–5.97 mg/g at 24 °C | Monolayer chemisorption | (Bakyayita et al. 2015) |
| Eichhornia crassipes | Whole | Treated with NaOH, particle size: 2000 μm | Cd(II) | 5 | 12.4 mg/g at 25 °C | Ionic exchange, electrostatic interaction, complexation | (Saraswat and Rai 2010) |
| Eichhornia crassipes | Whole | Not reported | Cd(II) | 4.84 | 12.60 mg/g | Ionic exchange | (Mahamadi and Nharingo 2010) |
| Glebionis coronaria | Stem | Particle size: < 160 μm, surface area: 1.741 m2/g, point of zero charge: 6.5 | Cd(II) | 6.5 | 18.31 mg/g at 25 °C | Electrostatic interaction, ionic sorbent–sorbate interaction | (Tounsadi et al. 2015) |
| Diplotaxis harra | Stem | Particle size: < 160 μm, surface area: 1.612 m2/g, point of zero charge: 6.29 | Cd(II) | 7.5 | 25.24 mg/g at 25 °C | Electrostatic interaction, ionic sorbent–sorbate interaction | (Tounsadi et al. 2015) |
| Undaria pinnatifida (brown algae invasive species) | Whole | Treated with CaCl2, particle size: 10–16 mesh; surface area: 0.3 m2/g | Cd(II) | 3 | 121.4 mg/g at 20 °C | Ionic exchange, complexation, coordination, and micro-precipitation | (Cazón et al. 2013) |
| Acorus calamus | Rhizome | Treated by HCl and NaOH, particle size: 800–1000 μm, surface area: 9.98 m2/g for biosorbent treated with HCl, and 11.65 m2/g for biosorbent treated with NaOH | Pb(II) | 7–8.5 | 76.12 mg/g for biosorbent treated with HCl, and 88.08 mg/g for biosorbent treated with NaOH | Electrostatic interaction, reduction, and anionic adsorption | (Shooto 2020) |
| Cyperus papyrus | Stem | Treated with H2O2 and NaOH | Pb(II) | 4.5 | 0.732–1.231 mg/g at 24 °C | Monolayer chemisorption | (Bakyayita et al. 2015) |
| Eichhornia crassipes | Whole | Treated with HNO3, particle size: 2.5 mm | Pb(II) | 4.84 | 26.32 mg/g for Pb(II) | Ionic exchange | (Mahamadi and Nharingo 2010) |
| Eupatorium adenophorum | Whole | Particle size: 40–60 mesh | Pb(II) | 5.0 | 2.236 mg/g at 26 °C | Chemisorption or chemical adsorption | (Guo et al. 2009) |
| Prosopis juliflora | Seedpod | Particle size: 100 mesh | Pb(II) | 6.0 | 40.322 mg/g at 28 °C | Chemisorption, ionic exchange | (Jayaram and Prasad 2009) |
| Prosopis juliflora | Seedpod | Particle size: 75–150 μm | Pb(II) | 6.0 | 1.4 mg/g at 27 °C | Monolayer chemisorption | (Gautam et al. 2020) |
| Moringa oleifera | Seed | Particle size: 75–150 μm | Pb(II) | 6.0 | 5.6 mg/g at 25 °C | Monolayer chemisorption | (Gautam et al. 2020) |
| Sargassum muticum Seaweed | Whole | Particle size: 500 μm | Pb(II) | 5.0 | 76 mg/g at 25 °C | Chelation | (Hannachi and Hafidh 2020) |
| Alternanthera philoxeroides (Mart.) Griseb | Whole | Particle size: < 125 μm | Cr(VI) | 2.0 | 17.71 mg/g, at 20 °C | Monolayer chemisorption, ion exchange, chelation | (Wang and Qin 2006) |
| Acorus calamus | Rhizome | Treated by HCl and NaOH, particle size: 800–1000 μm, surface area: 9.98 m2/g for biosorbent treated with HCl, and 11.65 m2/g for biosorbent treated with NaOH | Cr(VI) | 1.0 | Acorus calamus treated by acid: 21.93 mg/g, Acorus calamus treated by base: 24.48 mg/g | Electrostatic interaction, reduction and anionic adsorption (fraction of Cr(VI) is reduced to Cr(III) by electrons donating functional groups on the biomaterial surface) | (Shooto 2020) |
| Eichhornia crassipes | Whole | Treated with NaOH, particle size: 2000 μm | Cr(VI) | 2.0 | 5.6 mg/g at 25 °C | Ion exchange, electrostatic interaction, complexation | (Saraswat and Rai 2010) |
| Eupatorium adenophorum | Stem | Particle size: 60 mesh | Cr(VI) | 1.0 | 89.22 mg/g at 308 K, 99.9% of Cr(VI) removal | (i) Electrostatic interaction (via binding of Cr(VI) with positively charged biomass surface), (ii) reduction in Cr(VI) to Cr(III); (iii) electronic repulsion or complexation of Cr(III) to release Cr(III) ions | (Song et al. 2016) |
| Phragmites australis | Whole | Particle size: < 250 μm | Cr(VI) | 5.0 | 21.32 mg/g at 25 °C | Electrostatic interactions, reduction and chelation or complexation with the functional groups of adsorbents | (Mahmoud et al. 2021) |
| Ziziphus spina-christi | Whole | Particle size: < 250 μm | Cr(VI) | 4.0 | 15.5 mg/g at 25 °C | Electrostatic interactions, reduction and chelation or complexation | (Mahmoud et al. 2021) |
| Melaleuca diosmifolia | Leaf | Particle size: 500 μm; surface area: 0.99 m2/g | Cr(VI) | 7.0 | 62.5 mg/g at 24 °C | Adsorption-coupled reduction | (Kuppusamy et al. 2016a) |
| Sargassum muticum Seaweed | Whole | Particle size: 125–250 μm | Cr(VI) | 2.0 | 196.1 mg/g at 20 °C | Ionic exchange, surface complexation and electrostatic attraction | (Bermúdez et al. 2012) |
| Alternanthera philoxeroides | Whole | Particle size: < 125 μm | Zn(II) | 4.0 | 18.57 mg/g at 20 °C | Monolayer chemisorption, ion exchange, chelation | (Wang and Qin 2006) |
| Cyperus Rotundus | Whole | Surface area: 1.027 m2/g, pore volume: 9.035 cm3/g, point of zero charge: 5.0, and particle size: 150–330 μm | Zn(II) | 8.0 | 208 mg/g at 30 °C | Electrostatic attraction | (Ramesh et al. 2013) |
| Centaurea nicaeensis | Flower | Surface area: 0.76 m2/g, and particle size: 400 μm | Zn(II) | 2.0 | 13.86 mg/g at 25 °C | Electrostatic interactions | (Dhouibi et al. 2020) |
| Eichhornia crassipes | Whole | Treated with NaOH, and particle size: 2000 μm | Zn(II) | 6.0 | 9.3 mg/g at 25 °C | Ion exchange, electrostatic interaction, complexation | (Saraswat and Rai 2010) |
| Undaria pinnatifida (brown algae invasive species) | Whole | Treated with CaCl2 Size: 10–16 mesh, surface area: 0.3 m2/g | Zn(II) | 4.0 | 100.0 mg/g at 20 °C | Ionic exchange, complexation, coordination, and micro-precipitation | (Cazón et al. 2013) |
| Cyperus Rotundus | Whole | Particle size: 150–330 μm; surface area: 1.027 m2/g, pore volume: 9.035 cm3/g, point of zero charge: 5.0 | Cu(II) | 6.0 | 500 mg/g at 30 °C | Electrostatic attraction | (Ramesh et al. 2013) |
| Centaurea nicaeensis | Flower | Particle size: 400 μm, surface area: 0.76 m2/g | Cu(II) | 4.0 | 24.53 mg/g at room temperature | Electrostatic interactions | (Dhouibi et al. 2020) |
| Imperata cylindrica | Leaf | Treated with NaOH, particle size: 180–355 μm, point of zero charge: 7.34 | Cu(II) | 5.0 | 11.64 mg/g at 310 K | Monolayer chemisorption, film diffusion | (Hanafiah et al. 2009) |
| Eichhornia crassipes | Whole | Treated with 10% EDTA | Co(II) | 5.0 | 20.0 mg/g at 28 °C, 100% of Co(II) was removed the industrial samples | Electrostatic interactions caused by carboxyl, hydroxyl, amino, or sulfonic groups on biosorbent | (Acosta-Rodríguez et al. 2021) |
| Glebionis coronaria | Stem | Particle size: < 160 μm, surface area: 1.741 m2/g, point of zero charge: 6.5 | Co(II) | 6.5 | 24.52 mg/g at 25 °C | Electrostatic interaction, ionic sorbent–sorbate interaction | (Tounsadi et al. 2015) |
| Diplotaxis harra | Stem | Particle size: < 160 μm, surface area: 1.612 m2/g, point of zero charge: 6.29 | Co(II) | 7.5 | 33.02 mg/g at 25 °C | Electrostatic interaction, ionic sorbent–sorbate interaction | (Tounsadi et al. 2015) |
| Alternanthera philoxeroides (Mart.) Griseb | Whole | Particle size: < 125 μm | Ni(II) | 4.0 | 9.73 mg/g at 20 °C | Monolayer chemisorption, ion exchange, chelation | (Wang and Qin 2006) |
| Cyperus laevigatus | Leaf | Particle size: 40–50 mesh | Se(IV) | 2.0 | 110.5 mg/g at 25 °C, optimized by response surface methodology | Not reported | (Badr et al. 2020) |
| Undaria pinnatifida Seaweed | Whole | Treated with CaCl2, particle size: 10–16 mesh | Hg(II) | 7.0 | 161.2 mmol/g at 20 °C | Electrostatic attraction | (Plaza et al. 2011) |
| Undaria pinnatifida (brown algae invasive species) | Whole | Particle size: 5000–1000 μm | Cs(I) | 146.19 mg/g | Ionic exchange through carboxyl, sulfate, amine groups were involved in the adsorption process | (Hu et al. 2020) | |
| Undaria pinnatifida | Whole | Particle size: 5000–1000 μm | Sr(II) | 190.13 mg/g | Ionic exchange | (Hu et al. 2020) |
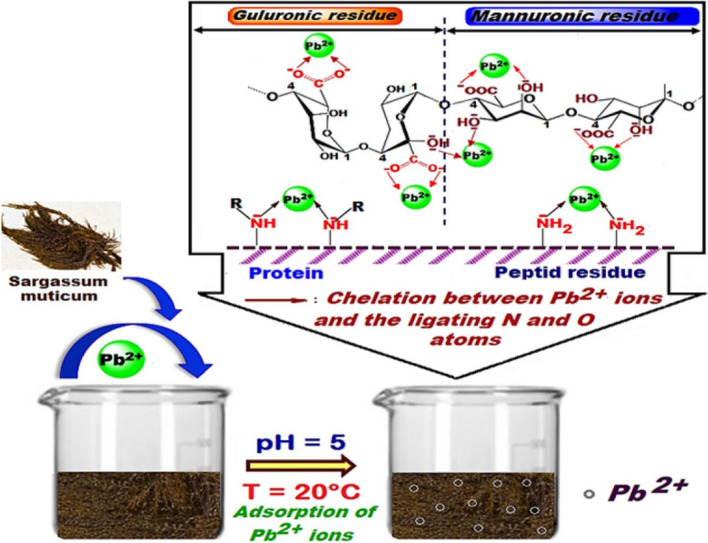
Fig. 6.
Proposed mechanism for the adsorption of lead heavy metal ions over Sargassum muticum (Japanese wireweed) biosorbent. Here, several functional groups, e.g., amine, carboxyl, and hydroxyl, on the surface of the biosorbent are attributable to the protein or peptide residues of invasive plants. They may play a vital role in the chelation of heavy metals with N and O atoms on surface functional groups. The sequestration of heavy metals over biosorbents can also be induced by electrostatic interactions. Reprinted with the permission of Elsevier from the reference (Hannachi and Hafidh 2020)
Cadmium
Cadmium (Cd) presents as one of the most toxic non-biodegradable heavy metals in the aqueous media and soils (Peng et al. 2021). Cadmium is often detected in many kinds of industrial effluents, pigment production, and metal refining activities (Zhang et al. 2021). This element is also responsible for detrimental effects such as kidney damage, lung insufficiency, cancer diseases, and deficiency of bones and blood (Luo et al. 2018). According to the World Health Organization, the permissible concentration of cadmium in drinking water is extremely low, at 5 × 10–3 mg/L (Pyrzynska 2019). Because of the high toxicity, and environmental accumulation, the removal of cadmium from the aqueous matrixes has been received great attention (Brião et al. 2020; Khan et al. 2020; Feng et al. 2022).
Many works reported the adsorption performance of biosorbents from invasive plants for the removal of cadmium ions (Table 1). Indeed, Tounsadi et al. (2015) discovered that the biosorbents from Diplotaxis harra and Glebionis coronaria plant stems can remove Cd(II) ions efficiently. In this study, pH points between 6.5 and 7.5 were found to acquire the maximum adsorption of 18.31 mg/g at 25 °C. Among inorganic ions, Al3+ showed the strongest inhibition due to its high affinity to Cd(II) ions. They indicated that functional groups on the surface of the Diplotaxis harra and Glebionis coronaria might participate in Cd(II) biosorption. Ammari (2014) reported the use of Arundo donax reed leaves for the treatment of Cd(II) in the aqueous solutions. They optimized the condition for the highest Cd(II) removal efficiency of 97% and monolayer adsorption capacity of 27.9 mg/g under the dosage of 3.5 g/L, at pH 5.5.
To enhance the removal efficiency and adsorption capacity, the chemical modification of biosorbents from invasive plants was suggested (Bakyayita et al. 2015). The chemicals are often used such as NaOH, KOH, CaCl2, K2CO3, HCl, and so forth. As treated with these chemicals, the surface of biosorbents can be modified with many functional groups which link to the improvement of adsorption (Saraswat and Rai 2010). For example, Cazón et al. (2013) used Undaria pinnatifida—a brown alga invasive species for the preparation of biosorbent. They demonstrated that the biomass treated with CaCl2 gave very high adsorption capacity (121.4 mg/g) to Cd(II) at pH 3. This outcome is explained due to the participation of many possible mechanisms such as ionic exchange, complexation, coordination, and micro-precipitation. At the same trend, Bakyayita et al. (2015) found the enormous improvement of adsorption capacity of Cyperus papyrus stem biosorbent from 0.028 mg/g to 5.97 mg/g after the modification with NaOH. However, the use of chemicals for biomass modification should be limited to ensure critical green strategies.
Lead
As similar to cadmium, lead (Pb) also presents as toxic non-biodegradable heavy metal (Ajiboye et al. 2021). The previous works listed many detrimental impacts of lead exposure on human health, involving serious damages to the nervous system, liver, and reproductive organisms (Ghorbani et al. 2020; Qasem et al. 2021; Vasseghian et al. 2021). Lead also links to several adverse effects on the intelligence quotient degrees and physical growths in children (Kuang et al. 2020; Naranjo et al. 2020). Over the decades, lead can be released from the environment through human activities such as transportation, agriculture, ceramic, and battery productions (Ramos-Guivar et al. 2021). The presence of lead in wastewater is impactful because it can cause long-term accumulation and enter the human body through the food chains and contaminated water (O’Connor et al. 2020; Jarvis and Fawell 2021). As a result, the treatment of lead from the aqueous solution is an urgent requirement.
Eupatorium adenophorum is a highly growing species that invades wide tropical and subtropical areas in Asia and Africa (Zhu et al. 2021). It strongly hampers the germination and growth of native plants, causing severe ecological damages (Liu et al. 2021). Some works utilized the biomass of this species for environmental remediation. For example, Guo et al. (2009) employed the adsorption of lead ions by the biosorbents based on Eupatorium adenophorum stems. In this study, the authors found the optimum pH for Pb(II) adsorption at 5.0 to reach the high removal efficiencies (84–90%). From the thermodynamic parameters such as Gibbs free energy and enthalpy, the authors made a deduction about the nature of spontaneous and endothermic biosorption of Pb(II) over Eupatorium adenophorum stems. Many invasive plants such as Prosopis juliflora and Moringa oleifera are locally prevalent in Uttar Pradesh, India. They are rapidly grown, drought-resistant, strongly invasive, and harmful to local plants. Taking advantage of available biomass sources, many studies have used them as green adsorbents for the removal of lead. Indeed, Gautam et al. (2020) reported the good outcomes of lead adsorption with capacity (1.4–5.6 mg/g) and efficiency (72–86%) at pH 6.0 using the above biosorbents. However, their adsorption performance was generally low, and the reusable property was not reported yet. Many kinds of invasive biomass treated with HNO3, CaCl2, HCl or NaOH gave clear improvements (26.32–76.12 mg/g), but there needs to be more investigated in further studies (Mahamadi and Nharingo 2010; Shooto 2020).
Chromium
Chromium is one of the potentially toxic and carcinogenic heavy metals prevalently present in industrial wastewater because it is related to anthropogenic activities such as metal cleaning, plating, mining industries (Peng and Guo 2020). According to World Health Organization, the permissible concentration of total chromium in drinking water is recommended at 0.05 mg/L (Vaiopoulou and Gikas 2020). Although trivalent Cr(III) is the most prevalent chromium present in water, Cr(VI) offers a higher degree of carcinogenicity, mobility, and toxicity (Jobby et al. 2018; Tumolo et al. 2020). Cr(VI) exposure possibly results in many serious health hazards such as skin irritations, kidney dysfunction, or lung carcinoma (Bakshi and Panigrahi 2018; Vareda et al. 2019; Coetzee et al. 2020). As a result, the practical treatment of Cr(VI) pollution is of greater interest than Cr(III).
In general, the pH condition strongly affects the adsorption process of hexavalent chromium anions (Mitra et al. 2017). It was suggested that the removal of Cr(VI) should often be employed under the acidic solution conditions (pH 1–4). This finding may be the role of several interactions that influence the mechanism of chromium anions over the biosorbents. Indeed, Song et al. (2016) pointed out three stages of proposed mechanisms including (i) electrostatic interaction via binding of Cr(VI) anions with positively charged biomass surface, (ii) reduction in Cr(VI) to Cr(III), and (iii) electronic repulsion or complexation of Cr(III) to release Cr(III) ions. Mahmoud et al. (2021) also agreed with this hypothesis since they demonstrated the main role of electrostatic interactions, reduction and chelation or complexation between Cr(VI), and the functional groups of the biosorbent.
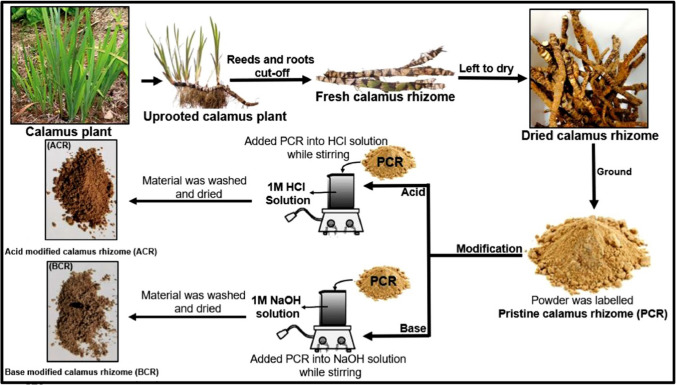
There are many works that demonstrated the potential of invasive plants as biosorbents to remove Cr(VI) from water. For example, Kuppusamy et al. (2016a) used the dried Melaleuca diosmifolia leaves to remove Cr(VI) at 24 °C with high uptake capacity (62.5 mg/g). Song et al. (2016) investigated the performance of Eupatorium adenophorum stems for the adsorption of Cr(VI) at very acidic solution (pH 1). As a result, 99% of Cr(VI) could be removed by this biosorbent. Recently, Shooto (2020) treated Acorus calamus rhizomes through chemical modification with HCl or NaOH (Fig. 7) to obtain a higher surface area (9.98–11.65 m2/g). The diversity of surface functional groups such as carboxyl groups (–COOH), hydroxyl groups (–OH) belonging to lignocellulose, and carbonyl groups (–C = O) on the treated biosorbents was detected using Fourier transform infrared spectroscopy. As a result, the authors found that the modification with alkaline agent gave a better adsorption performance than the cases modified with acidic agent or without any modification. More interestingly, the Acorus calamus rhizome biosorbents could be recycled at least three times without any significant decrease in the final cycle.
Fig. 7.
Synthesis of Acorus calamus rhizome biosorbents. Here, the rhizomes were firstly washed, pretreated, dried, and ground. Subsequently, HCl or NaOH solution was immersed to treat the surface of rhizomes powder. Reprinted with the permission of Elsevier from the reference (Shooto 2020)
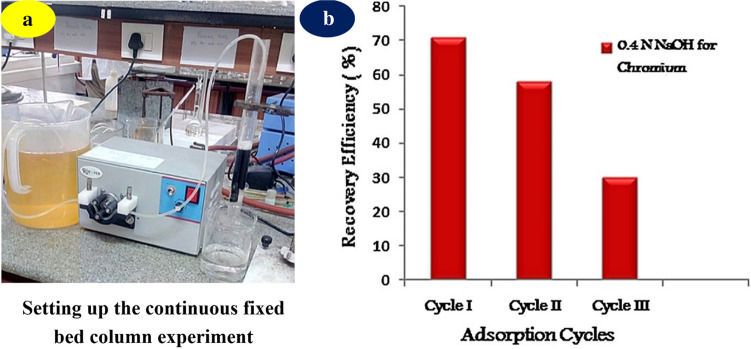
Considering the continuous adsorption mode, the fixed bed column is more appropriate in the scale-up industrial treatment for chromium anions. Inspired by this fact, Nithya et al. (2020) reported the removal of Cr(VI) anions using chemically modified Lantana camara biosorbent (Fig. 8a). In their study, H2SO4 with the ratio of 1:1 by weight was used to modify the surface of adsorbent. The fixed bed study was optimized with three parameters including Cr(VI) concentration, flow rate of the Cr(VI) solution, and bed height. The findings revealed that the fixed bed adsorption capacity was highly reached, at 362.8 mg/g. More importantly, the biosorbent could be easily desorbed by 0.4 N NaOH solution at the flow rate of 4 mL/min. The regeneration efficiency of the reused fixed column was reported with three cycles (Fig. 8b). To sum up, these results indicated the great potential of invasive plant biosorbents for the treatment of hexavalent chromium ions.
Fig. 8.
Setting up the continuous fixed bed column (a), and the regeneration experiments of H2SO4 modified Lantana camara biosorbent for the removal of Cr(VI) anions (b). Here, 0.4 N NaOH was used as an eluent solvent for desorbing Cr(VI) at the rate of 4 mL/min. The high regeneration efficiency could be still obtained after three cycles, and the final cycle gave an efficiency of about 30%. Reprinted with the permission of Elsevier from the reference (Nithya et al. 2020)
Zinc
Zinc (Zn) is an essential micronutrient element that engages in the biochemical and physiological processes of living tissues (Gombart et al. 2020). The fundamental role of zinc has been admitted in boosting the immune system to reduce the risk of infection against pathogens such as the coronavirus disease 2019 (COVID-19) pandemic (Gasmi et al. 2020; Gorji and Khaleghi Ghadiri 2021). Among the heavy metals, zinc also exhibits as one of the most benign metal elements (Wang et al. 2018). According to World Health Organization, the permissible concentrations of zinc in surface water, groundwater, and drinking water are recommended as 0.01, 0.05, and 3 mg/L, respectively (Ngabura et al. 2018). However, the presence of zinc and its inorganic compounds is increasingly prevalent in industrial effluents (Hołtra and Zamorska-Wojdyła 2020). Many fabric, wood, metal mining, and batteries production industries often discharge a large amount of zinc into the aqueous matrixes (Chen et al. 2019). The health issues including skin inflammations, fevers, irritability, and vomiting have been reported due to zinc exposure (Wei et al. 2021). Considering as a renewable biomass resource, the invasive plant may, therefore, be a good choice to handle the contamination of zine from wastewater bodies (Turkmen Koc et al. 2021).
Zinc adsorption was often studied in competition with other heavy metals such as cadmium and chromium. Saraswat and Rai (2010) studied the use of Eichhornia crassipes dead biosorbent for the simultaneous removal of Zn(II), Cd(II), and Cr(VI) ions. It was pointed out that maximum adsorption capacity at 25 °C followed by Cd(II) > Zn(II) > Cr(VI). They suggested that the main mechanism of ion exchange and electrostatic interaction controlled this simultaneous adsorption. Apart from batch mode, the flow mode of heavy metals adsorption was also investigated since it exhibits more applicability. Cazón et al. (2013) reported the biosorption on Undaria pinnatifida plant for simultaneous removal of zinc and cadmium ions. They found that there was not nearly adsorption competition in the bicomponent system. By using Fourier transform infrared spectroscopy analysis, carboxylic groups containing nitrogen and sulfur were found to contribute significantly to Zn(II) and Cd(II) adsorption.
Copper
Over the centuries, copper (Cu) has been widely used in ordinary activities as well as advanced applications such as photovoltaic cells and phytotherapies (Barsova et al. 2019). This element belongs to a group of essential micronutrients for multiple activities such as photosynthesis, metabolism, and reproduction processes in living organisms (Rehman et al. 2019). However, the exceeding discharge of copper and its compounds into the water and soil environments by anthropogenic activities has resulted in many imperative risks to human health and aquatic animals (Tang et al. 2019; Vardhan et al. 2019). The pollution of copper should be addressed by several green approaches including biosorption (Al-Saydeh et al. 2017; Krstić et al. 2018). Using invasive plants as bioremediators for the removal of copper may be one of the efficient solutions.
Ramesh et al. (2013) reported the very high maximum copper adsorption capacity (up to 500 mg/g at 30 °C) obtained by Cyperus rotundus biosorbent. In this study, they found the optimum dose of biosorbent and equilibrium time was 1.0 g/L, and 30 min, respectively. Considering waste reuse as a critical green approach, Dhouibi et al. (2020) have successfully optimized the simultaneous adsorption of copper and zinc over Centaurea nicaeensis residue after hydrodistillation process. The authors found the best fittings (R2 ≥ 0.99) of experimental data with kinetic and isotherm models were pseudo-first-order and Sips equations, respectively. Moreover, the optimal conditions of operating parameters such as pH, temperature, and biosorbent dose were undertaken through response surface methodology. By using Minitab statistical software, the highest percentages of copper and zinc removal efficiency were obtained, at 92% and 82%, respectively. This result suggested the good adoption of response surface methodology as a powerful tool for the effective optimization of metal adsorptive removal.
Cobalt
Cobalt (Co) and its alloys are widely used in the production of electronic devices, ceramic products, and ferromagnetic materials (Taka et al. 2018; Abbas et al. 2021). It has a common radioisotope (60Co), which is frequently applied for radiotherapy and many industrial applications, such as leveling devices and thickness gauges (Nayl et al. 2020). Although cobalt is a micronutrient essential for animal and plant growth, its exposure at high concentrations can lead to many serious influences on human health, involving vomiting, pneumonia, heart failure, and so forth (Zhuang et al. 2018). The occurrence of cobalt pollution is mainly originated from many human activities, especially ore mining, electroplating, and battery manufacturing (Liu et al. 2019b). According to World Health Organization, the permissible concentration of Co(II) ions in drinking water is very low, at 0.05 mg/L (Islam et al. 2018). Because cobalt reflects high toxicity and bio-accumulative nature, the treatment of cobalt pollution in water is very necessary.
With many advantages of low-cost production and local availability, invasive plants can be great biosorbents for the elimination of cobalt from wastewater. Indeed, Tounsadi et al. (2015) undertook the simple and rapid cobalt removal procedure by using Glebionis coronaria and Diplotaxis harra stems. After 120 min of adsorption, about 60% of Co(II) was removed from the solutions at a dose of 10 g/L at room temperature (25 °C). Very recently, Acosta-Rodríguez et al. (2021) enhanced the cobalt removal efficiency up to 100% in the practical industrial samples using water hyacinth (Eichhornia crassipes) as a robust biosorbent. To obtain such promising results, water hyacinth biomass was treated with 10% ethylenediaminetetraacetic acid. In vivo experiment, the authors reported a lower removal of 17.3% after four weeks of incubation through the phytoremediation pathway. Consequently, invasive plants can become high-value, but zero-cost biosorbents for the removal of cobalt. At present, more studies should focus on the treatment of heavy metals in such ways.
Other heavy metals
Apart from the treatment of above mentioned heavy metal ions, the biosorbents from the invasive plants can remove a range of toxic metals such as nickel, selenium, mercury, cesium, and strontium ions (Table 1). Undaria pinnatifida is known as seaweed which is present in the sea environment. After being treated with CaCl2, the biosorbents from this species could remove Hg(II) from water (Plaza et al. 2011). In addition, Hu et al. (2020) reported the potential of a marine brown alga invasive species, namely Undaria pinnatifida for the treatment of cesium (Cs+) and strontium (Sr2+) ions. They also applied the molecular dynamics simulation for the prediction of interaction between alginate and Sr2+ cations. The results of isotherm titration calorimetry exhibited a high competition between Sr2+ and alginate on marine algae. The maximum adsorption capacity values were reached at 146.2–190.1 mg/g, which may be attributable to the ionic exchange between metal ions with functional groups of marine algae. By the optimization using response surface methodology with 24 factorial experimental design, Badr et al. (2020) obtained the maximum selenium uptake value of 110.5 mg/g at 25 °C. Accordingly, the authors investigated the effect of process parameters on the uptake of Se(IV) over Cyperus laevigatus biomass and found their optimized points at initial concentration (400 mg/L), contact time (8 h), biosorbents dose (1 g/L), and pH 2. From the encouraging adsorption results, the biosorbents from invasive plants can be good materials for the treatment of heavy metal ions.
Removal of synthetic dyes by invasive plants
Occurrence of synthetic dyes
One of the first discovered synthetic dyes was mauveine by William Perkin in 1865 (Tkaczyk et al. 2020). Over the past centuries, the revolution of synthetic dyes has globally extended to many fields such as textile, food, beverage, printing, paper, and so forth (Bulgariu et al. 2019; Benkhaya et al. 2020). Up to now, over 100,000 synthetic organic dyes have been produced and available commercially worldwide (Tkaczyk et al. 2020). The global value of dyes and pigments market in 2021 is estimated at 36.4 billion dollars by Grand View Research Inc., United States. Although some are highly safe to be used in the color food, beverage, cosmetic, and pharmaceutical industries, a major number of organic dyes are underestimated as emergent contaminants in the aquatic media (Hassan and Carr 2018). This is attributable to their highly stable and non-biodegradable structure, which is not easy for treatment using chemical, physical and biological methods (Tran et al. 2019). Moreover, due to the highly visible and accumulative ability, the existence of synthetic dyes can lead to the blockage of solar light into water (Saya et al. 2021). The photosynthetic performance of aquatic plants and organisms can be therefore lessened. Many works have also indicated that the organic dyes are potentially teratogenetic, carcinogenic, and mutagenic compounds, posing serious threats to human health as well as to marine life (Jun et al. 2020; Tkaczyk et al. 2020; Saya et al. 2021). To solve these problems, the proper treatments of synthetic organic dyes are required. Among the current techniques, adsorption offers high performance, eco-friendliness, and effectiveness for the removal of emergent pollutants including synthetic organic dyes (Tran et al. 2020a, c; Dang et al. 2021). This section will discuss some plausible dye adsorption mechanisms as well as the adsorptive treatment using invasive plants as low-cost biosorbents.
Plausible adsorption mechanism of synthetic dyes
In general, chemisorption is the common mechanism based on the interaction of functional groups on the synthetic dyes and the surface of biosorbents (Ali 2018; Khadir et al. 2020). It is found that biosorbents from invasive plants can possess many biosubstrates such as cellulose, hemicellulose, polysaccharides, and so forth (Feng et al. 2021). These macro-compounds supply many hydroxyl, ketone, and amine groups that can interact with the synthetic dye molecules during the chemisorption (Stavrinou et al. 2018). Specifically, the presence of functional groups such as amine, carboxyl, and hydroxyl on the surface of biosorbent may take main responsibility for the hydrogen bonding, electrostatic, and n–π interactions with organic dye molecules (Fig. 9). A large number of relevant studies have proposed the main mechanisms take responsibility for the sequestration of the synthetic dyes onto biosorbents (Table 2). To have more insights into the removal of heavy metal ions over biosorbents from invasive plants, the following section will discuss their adsorption performance and controlling mechanisms.
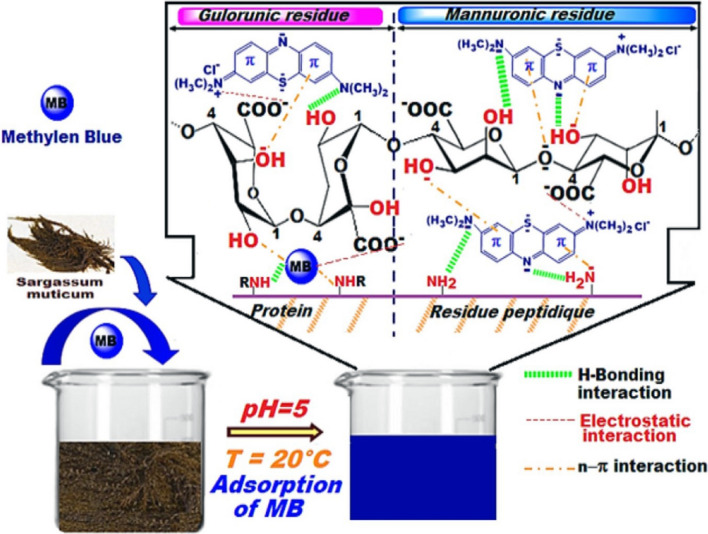
Fig. 9.
Proposed mechanism for the adsorption of organic dyes over the biosorbent. Here, the presence of functional groups, e.g., amine, carboxyl, and hydroxyl, on the surface of biosorbent may take main responsibility for the hydrogen bonding, electrostatic, and n–π interactions with organic dye molecules. Reprinted with the permission of Elsevier from the reference (Hannachi and Hafidh 2020)
Table 2.
Adsorption performance of biosorbents from invasive plants for the treatment of organic dyes
| Plant | Part | Biosorbent features | Target dyes | pH | Uptake capacity | Adsorption mechanism | Refs. |
|---|---|---|---|---|---|---|---|
| Sargassum muticum seaweed | Whole | Particle size: 100–500 μm, point of zero charge: 5.45 | Methylene blue | 1–11 | 142.87 mg/g at 25 °C | Electrostatic interaction, hydrogen bonding, hydrophobic–hydrophobic interaction | (Atouani et al. 2019) |
| Sargassum muticum seaweed | Whole | Particle size: 500 μm | Methylene blue | 5.0 | 92 mg/g at 25 °C | Electrostatic attractions, hydrogen bonding, n − π electron donor–acceptor interactions | (Hannachi and Hafidh 2020) |
| Lantana camara | Stem | Treated with oxalic acid, particle size: 250–350 μm | Methylene blue | 4.84 | Raw and modified biosorbents: 23.25–111.12 mg/g, respectively, at 30 °C | Film diffusion, ionic exchange, chemical interaction | (Banerjee et al. 2016) |
| Ailanthus Excelsa | Leaf | Particle size: 150–600 μm, point of zero charge: 7.6 | Methylene blue | 10.0 | 18.867 mg/g at 27 °C | Electrostatic interaction | (Bansal et al. 2021) |
| Calotropis gigantea | Seedpod | Particle size: 250–400 μm | Methylene blue | 6.0 | 8.36 mg/g at 21 °C | Electrostatic interaction | (Ammar et al. 2021) |
| Juncus effusus | Whole | Not reported | Methylene blue | 11.0 | 117.93 mg/g at 35 °C | Monolayer chemisorption, π–π stacking interactions, mesopore-filling, intra-particle diffusion | (Liu et al. 2016) |
| Imperata cylindrica | Whole | Treated with boiling distilled water, particle size: 125–150 μm | Methylene blue | 8.46 | 27.40 mg/g at 26 °C, optimized by response surface methodology | Monolayer chemisorption | (Su et al. 2014) |
| Glebionis coronaria | Stem | Particle size: 120 μm, surface area: 1.741 m2/g, point of zero charge: 6.5 | Methylene blue | 11.0 | 258.76 mg/g at 25 °C | Not reported | (Tounsadi et al. 2016) |
| Diplotaxis harra | Stem | Particle size: 120 μm, surface area: 1.612 m2/g, point of zero charge: 6.29 | Methylene blue | 11.0 | 185.59 mg/g at 25 °C | Not reported | (Tounsadi et al. 2016) |
| Cortaderia selloana | Flower spike | Not reported | Methylene blue | Not reported | 34.48 mg/g at 25 °C | Monolayer chemisorption | (Jia et al. 2017) |
| Melaleuca diosmifolia | Leaf | Particle size: 500 μm, surface area: 0.99 m2/g | Methylene blue | 10.0 | 119.05 mg/g at 24 °C | Electrostatic interaction | (Kuppusamy et al. 2016b) |
| Carpobrotus edulis | Whole | Particle size: < 250 μm | Crystal violet | 2–11 | 17.70 mg/g at 23 °C | Not reported | (Dabagh et al. 2021) |
| Acacia mearnsii | Bark | Treated with acetone and sulfuric acid and removed tannins, surface area: 4.867 m2/g | Crystal violet | 10.0 | 280 mg/g at 30 °C | Not reported | (Silva et al. 2018) |
| Cyperus Rotundus | Whole | Particle size: 150–300 μm, point of zero charge: 5.6 | Crystal violet | 8.0 | 84.13 mg/g at 20 °C | Electrostatic interaction | (Suyamboo and Srikrishnaperumal 2014) |
| Centaurea nicaeensis | Stem | Point of zero charge: 9 | Crystal violet | 10.1 | 476.190 mg/g at 25 °C, regeneration: eleven cycles, first: 98.3% and final > 90%, ethanol as eluent solvent | hydrogen bonding | (Naderi et al. 2018) |
| Glebionis coronaria | Stem | Particle size: 120 μm, surface area: 1.741 m2/g, point of zero charge: 6.5 | Malachite green | 11.0 | 117.32 mg/g at 25 °C | Not reported | (Tounsadi et al. 2016) |
| Diplotaxis harra | Stem | Particle size: 120 μm, surface area: 1.612 m2/g, point of zero charge: 6.29 | Malachite green | 11.0 | 64.37 mg/g at 25 °C | Not reported | (Tounsadi et al. 2016) |
| Typha angustifolia | Leaf | Particle size: 1250–2000 μm | Malachite green | 8.0 | 75.27 mg/g at 35 °C | Chemical interactions | (Guechi and Hamdaoui 2013) |
| Melaleuca diosmifolia | Leaf | Particle size: 500 μm, surface area: 0.99 m2/g | Malachite green | 2.0 | 116.28 mg/g at 24 °C | Electrostatic interaction | (Kuppusamy et al. 2016b) |
| Lantana camara | Stem | Treated with oxalic acid, particle size: 250–350 μm | Rhodamine B | 4.84 | 34.24 mg/g at 30 °C | Film diffusion, ionic exchange, chemical interaction | (Banerjee et al. 2016) |
| Trapa natans | Peel | Particle size: 85–75 mesh, point of zero charge: 5.8 | Rhodamine B | 7.0 | 3.0 mg/g at 30 °C | Liquid-film, intra-particle diffusions | (Khan et al. 2013) |
| Trapa natans | Chestnut shell | Treated with 0.2 M NaOH, surface area: 1.127 m2/g, pore volume: 0.0067 cm3/g, pore radius: 1.09 nm | Rhodamine B | 8.0 | 136.46 mg/g, and 90.36% removal, regeneration study: five consecutive cycle, methanol as a green eluent | Electrostatic attraction, van der Waals force, H-bonding, and π–π stacking | (Qaiyum et al. 2021) |
| Lantana camara | Whole | Particle size: 44 mesh, surface area: 123 m2/g, pore volume: 0.07 cm3/g, point of zero charge: 6.2 | Alizarin red S | 2.0 | 0.507 mg/g, sixth regeneration cycle, the adsorption remained at 70.2%, desorption by 0.1 M NaOH | Electrostatic attraction | (Gautam et al. 2014) |
| Cyperus Rotundus | Whole | Treated with HCl, particle size: 1000–2000 μm | Acid orange 7 | 7.0 | 35.69 mg/g at 20 °C | Not reported | (Azarpira and Balarak 2016) |
| Eichhornia crassipes | Root | Particle size: 2–20 mesh, surface area: 8.07 m2/g | Red reactive dye | 2.0 | 43.28 mg/g at 30 °C, 95% of dye removal for 110 min | Monolayer chemisorption | (Rigueto et al. 2020) |
| Melaleuca diosmifolia | Leaf | Particle size: 500 μm, surface area: 0.99 m2/g | Acridine orange | 2.0 | 126.58 mg/g at 24 °C | Electrostatic interaction | (Kuppusamy et al. 2016b) |
| Melaleuca diosmifolia | Leaf | Particle size: 500 μm, surface area: 0.99 m2/g | Eriochrome black T | 2.0 | 94.34 mg/g at 24 °C | Electrostatic interaction | (Kuppusamy et al. 2016b) |
Methylene blue
Methylene blue (C16H18N3SCl) is a cationic and thiazine dye (Bayomie et al. 2020). It was first synthesized in 1876 by Heinrich Caro and was widely used as a synthetic dye for the textile industry (Mashkoor and Nasar 2020). Some physicochemical properties of methylene blue include solid crystals and maximum light absorption at around 665 nm (Tran et al. 2020d; Nguyen et al. 2021c). Methylene blue has been thoroughly studied in both positive and negative effects. Acting as a synthetic textile dye, it exhibits as one of the most common colorings and staining agents (Ahmad et al. 2020). Consequently, a large amount of this textile dye is yearly discharged in the environment without the proper treatment (Santoso et al. 2020). Exposure to methylene blue can cause many inharmonious impacts on human health and the aquatic environment (Din et al. 2021). Indeed, Din et al. (2021) revealed that methylene blue is considered a potentially carcinogenic pollutant, and toxic agent with many general symptoms such as high blood pressure, skin irritation, vomiting, headache, and fever. The removal of methylene blue dye using low-cost biosorbents is therefore highly recommended.
Su et al. (2014) optimized the removal process of methylene blue by Imperata cylindrica using the response surface methodology. They obtained very good removal performance (99.09%) under the optimum conditions such as time of 40 min, pH 9, and dose of 1.0 g/L. Moreover, equilibrium isotherm adhered best to Langmuir model, which exhibited the monolayer behavior of adsorption process. Banerjee et al. (2016) reported the potential of Lantana camara stems treated by oxalic acid for the removal of methylene blue dye in both batch and continuous flow modes. In this research, the modified Lantana camara gave maximum adsorption capacity value at 111.12 mg/g, which was approximately fourfolds higher than that of raw biosorbent. With the fixed bed depth of modified Lantana camara at 15 cm, the highest removal efficiency for methylene blue was obtained at 94%. Sulfuric acid was also found as an efficient desorbing solvent for this fixed bed. Atouani et al. (2019) found that Sargassum muticum seaweed can adsorb methylene blue in the pH-independent condition. More importantly, the dye uptake capacity was very high, 142.87 mg/g at 25 °C. The authors assumed the main role of electrostatic, hydrogen bonding, hydrophobic–hydrophobic interactions in the enhancement of adsorption process.
Crystal violet
Crystal violet (C25N3H30Cl) is known as hexamethyl pararosaniline chloride and a compound of triarylmethane (Putri et al. 2020). It is commonly used as a histological stain as well as in the textile industry (Sacco et al. 2018). In general, exposure to crystal violet can cause many severe environmental problems such as the reduction in water quality (Tran et al. 2020b). Crystal violet can cause eye burn that leads to permanent eye damage (Zhou et al. 2014). It was reported that the concentration of crystal violet at 1 μg/L can be harmful and possibly mutagenic to human bodies (Fabryanty et al. 2017). Therefore, the removal of crystal violet from the aquatic matrix has been of enormous interest.
Cyperus rotundus is known as purple nutsedge mostly distributed in Africa. This species notably diminishes crop yields due to its superior competition with local plants (Samra et al. 2021). However, it can be utilized to convert effortlessly into effective biosorbents. Suyamboo and Srikrishnaperumal (2014) studied the crystal violet biosorption potential of Cyperus rotundus invasive plant. Through the batch experimental setup, they obtained an adsorption capacity of 84.13 mg/g at 20 °C. Moreover, it was proved that the biosorption was thermodynamically a spontaneous and exothermic process. Naderi et al. (2018) applied the powerful and efficient statistical tools based on artificial neural network and simulated annealing for predicting and optimizing the biosorption of crystal violet dye. Under the optimized conditions, the dye adsorption capacity was extremely high, 476.190 mg/g at room temperature. More interestingly, the Centaurea nicaeensis stem-based biosorbent could remove 98.3% of crystal violet from water. The regeneration study could also endure up to eleven cycles without any significant decrease in the removal efficiency at the final cycle (> 90%). It can be consequently concluded that Centaurea nicaeensis invasive plant is an ideal biosorbent for the treatment of synthetic dyes from the wastewater.
Malachite green
Malachite green (C23H25ClN2) is a triphenylmethane dye used globally for therapeutic treatment in aquaculture against fungal and protozoan infections in fish (Zhou et al. 2019). Although it is no longer authorized for medicinal usage in food-producing animals in many European Union countries, the illegal consumption of malachite green still extends worldwide (Ma et al. 2020). This may be attributable to its low production cost and high efficacy against parasites and fungal diseases. However, several works reported that the bioaccumulation of malachite green its primary metabolites can be potentially hazardous to the health of humans and other organisms (Zhou et al. 2019; Ma et al. 2020; Ren et al. 2021b). Indeed, exposure to malachite green dye brings many adverse effects such as carcinogenic and genotoxic risks (Tewari et al. 2018; Teymori et al. 2019). Because a large amount of the residue exists in the aquaculture activities, it is important to remove the malachite green dye from the aquatic water.
Typha angustifolia is a perennial macrophyte aquatic herb with the characteristics of fast growth and high productivity (Xiong et al. 2021). This aggressive invasive plant often found in the wetlands, sedge meadows, and river banks. Guechi and Hamdaoui (2013) used the cattail leaves of Typha angustifolia as a biosorbent for the malachite green treatment with the uptake capacity of 75.27 mg/g at 35 °C. Tounsadi et al. (2016) reported the usage of stems from Glebionis coronaria and Diplotaxis harra for the removal of malachite green from wastewater. They found that the biosorption was best favorable at pH 11 and 25 °C. Under these conditions, the adsorption capacity values were measured at between 64.37 mg/g and 117.32 mg/g. Equilibrium data were fitted best with nonlinear Redlich–Peterson equation. However, the plausible mechanisms of malachite green adsorption over Glebionis coronaria and Diplotaxis harra have not been reported yet.
Rhodamine B
Rhodamine B (C28H31ClN2O3) is a xanthene dye, which is used as a water tracer fluorescent for the direction of flow and transport (Wang et al. 2020b). This dye stands an extensive value in the textile industries due to the low production cost, high stability, and non-biodegradability (Worathitanon et al. 2019). It is still controversial whether Rhodamine B is a carcinogenic and neurotoxic agent to human health (Al-Buriahi et al. 2022). Although many countries ban the use of Rhodamine B as textile colorant, it is prevalently present in most water sources because of the illegal utilization (Al-Gheethi et al. 2022). As a result, Rhodamine B seriously affects the water quality and photosynthesis of aquatic creatures (Bhat et al. 2020). Taking advantage of invasive plants as low-cost biosorbents for the removal of Rhodamine B dye is very beneficial for environmental remediation.
Trapa natans is a typical floating-leaved aquatic plant that strongly competes for light, nutrients, and spaces with other aquatic species (Dodd et al. 2021). It also reduces the quality of local water and threatens the ecological balance of the habitat (Yuan et al. 2021). In an effort to mitigate the adverse effects of this species, Khan et al. (2013) used Trapa natans as biosorbent to treat Rhodamine B dye. The adsorption could be performed under mild conditions such as pH 7.0 and contact time of 20–120 min. However, the maximum adsorption capacity measured from Langmuir isotherm model was relatively low, only 3.0 mg/g at 30 °C. Very recently, Qaiyum et al. (2021) significantly improved the adsorption efficiency of Trapa natans chestnut shells by modifying with 0.2 M NaOH. The excellent adsorption capacity and removal efficiency were obtained at 136.46 mg/g, and 90.36%, respectively. Considering the better adsorption results than those obtained by Khan et al. (2013), the authors explained that alkaline treatment considerably lessens the amount of lignocellulosic inherent in the Trapa natans shells. Therefore, the NaOH activation enhanced the stability of biosorbent compared with the raw material. Moreover, electrostatic attraction, van der Waals force, H-bonding, and π–π stacking were proposed as the main mechanism for the adsorption of Rhodamine B dye over the Trapa natans biomass.
Other synthetic dyes
Some synthetic dyes including alizarin red, acid orange, red reactive dye, acridine orange, and eriochrome black can be efficiently treated by biosorbents from invasive plants (Table 2). For example, Gautam et al. (2014) used Lantana camara for removing alizarin red with the capacity of 0.507 mg/g. The biosorbent exhibited high recyclability up to six cycles using 0.1 M NaOH as desorbing solvent. Kuppusamy et al. (2016b) demonstrated the multifunctional application of Melaleuca diosmifolia leaves for the treatment of both acid orange and red reactive dye. They performed the adsorption process at pH 2 and obtained high capacity, at 94.34–126.58 mg/g. Electrostatic interaction was ascribed to controlling the adsorption of these dyes onto Melaleuca diosmifolia-based adsorbent. Moreover, some food dyes such as tartrazine can be easily treated by biosorbent and the activated carbon derived from Moringa oleifera seeds (Reck et al. 2018).
The biosorbents derived from invasive plants have demonstrated their potential for the remediation of heavy metal ions, synthetic organic dyes, and oil recovery (Rathi et al. 2021; Saheed et al. 2021; Saravanan et al. 2021). However, there are very few studies that investigated these biosorbents for the treatment of many emergent pollutants such as pharmaceutical drugs, pesticides, and other organic compounds. It is expected that future researches will extend the potential of invasive plants to address these aspects.
Removal of oils by invasive plants
Nowadays, oil spillage phenomena have posed a considerable threat to not only aquatic ecosystems but also the whole biosphere. Adsorbents from biomass receive more concerns to resolve such problems thanks to their biodegradability and cost-effectiveness. Apart from agricultural waste that is pretty well documented, invasive plants appear to be a promising source of natural fibers for the oil recovery system (Lv et al. 2018). For example, fibers isolated from fruit of cattail (Typha), a genus of dominated noxious weed, were noticed for their possibility of oil removal (Fig. 10). Each gram of this bulk material could uptake 13.4 g of motor oil and 14.6 g of vegetable fats. It was the exclusive bamboo-shaped framework of cattail fiber providing vacancy and thus high surface area enabling the oil adsorption process (Cui et al. 2014). Moreover, such structure was outstanding for oil retention to prevent the leak of treated oil during the recovery. Truly, Cao et al. (2016) estimated that nearly 90% of adsorbed oil was retained even after a day of dripping. The other contributors to this effectiveness were low density (617.8 kg m−3) and the wax covering the surface of cattail fiber that accounted for 11.5% of Soxhlet extraction (Wu et al. 2021). Consequently, cattail natural fibers are expected as environmentally friendly and effective biomass for the fabrication of oil biosorbents.
Fig. 10.
Photographs of a Mature cattail stalk, and b Cattail tufts. Scanning electron microscope microphotographical images of c Cattail tuft, d Longitudinal section of cattail fiber, e Cross section of cattail fiber, and f Cross section of the cattail fiber. Cattail fibers have a down-like structure, including root, stem, seed, and several fibers. They possess four-dimensional open spaces with an average length of 7.9 ± 1.2 mm. With exceptionally hydrophobic and oleophilic features, cattail fibers could adsorb up to 12–26 g oils (engine, vegetable and used oils) per gram of fibers. Reproduced with the permission of Taylor & Francis from reference (Cao et al. 2016)
There are other sources of fiber based oil adsorbents derived from invasive plants. For example, the ones derived from the stem of Arundo donax were so microstructural that could uptake the amount of commercial oil up to fivefold their weight. In particular, 300–2500 μm long fibers showed porous orthotropic morphology but distinct adsorption capacity to different pollutant viscosity. The result showed that the smaller these fibers were, the better oil uptake in high viscosity liquid that contained one kind of oil, such as kerosene, pump oil, virgin naphtha, and crude oil (Fiore et al. 2019). The milkweed (Asclepias syriaca) floss had a superhydrophobic trait and hollow structure occupying 90% of its total volume. Such characteristics along with high wax content were beneficial to the oil adsorption capacity of these fibers which was over 100 g/g of engine oil (Panahi et al. 2020). Table 3 illustrates typical natural fibers derived from invasive plants, their characteristics in terms of the average diameter, length, the percentage of wax content, and their adsorption capacity for several types of oils. Briefly, the conversion of noxious biomass into oil biosorbents could be a great potential for sustainable development and environmental protection.
Table 3.
Adsorption performance of biosorbents from invasive plants for the treatment of oils
| Plant | Part | Material features | Oil type | Adsorption capacity (g/g) | Refs. | ||
|---|---|---|---|---|---|---|---|
| Diameter (μm) | Length (μm) | Wax Content (%) | |||||
| Arundo donax | Stem | 100–800 | 300–2500 | Not reported | Kerosene, | 46.1 | (Fiore et al. 2019) |
| Arundo donax | Stem | 100–800 | 300–2500 | Not reported | Virgin naphtha | 50.5 | (Fiore et al. 2019) |
| Arundo donax | Stem | 100–800 | 300–2500 | Not reported | Pump oil, | 61.0 | (Fiore et al. 2019) |
| Arundo donax | Stem | 100–800 | 300–2500 | Not reported | Crude oil | 51.7 | (Fiore et al. 2019) |
| Calotropis gigantea | Seed | 30.4 | Not reported | 1.8–3 | Rapeseed oil | 41.7 | (Zheng et al. 2016) |
| Calotropis gigantea | Seed | 30.4 | Not reported | 1.8–3 | Paraffin oil | 37.6 | (Zheng et al. 2016) |
| Typha | Fruit | 10–17 | 7900 | 11.5 | Engine oil | 13.4 | (Cao et al. 2016) |
| Typha | Fruit | 10–17 | 7900 | 11.5 | Vegetable oil | 14.6 | (Cao et al. 2016) |
| Urtica dioica | Stem | 3.94 | 6500 | 0.52 | Engine oil | 19.29 | (Viju and Thilagavathi 2019) |
| Urtica dioica | Stem | 3.94 | 6500 | 0.52 | Crude oil | 22.39 | (Viju and Thilagavathi 2019) |
| Asclepias syriaca | Seed | 24 | 2700 | 2–3 | Engine oil | 126 | (Panahi et al. 2020) |
| Populus nigra | Seed | 3–12 | 4000 | 4–9 | Diesel | 211 | (Likon et al. 2013) |
Perspective
Although plant biological invasion is considered an alarming peril to biodiversity and the ecosystem, it can be seen that they are potential sources for various applications. In fact, invasive plants are widely distributed, have strong tolerance and aggressive development. Although there are some detrimental effects on the ecology, economy, and human health of such species, they are exploited as animal feed, food source, ornamental, and traditional medicine (Feng et al. 2021). Because of their abundant biomass, several exotic species have exhibited added values for paper production (Starešinič et al. 2021) and cosmetics (Nguyen et al. 2021d). In particular, they are great sources for producing energies such as bio-oils, biodiesels, and biogases (Van Meerbeek et al. 2015). In terms of generating materials, this biomass could be converted into biochars (Feng et al. 2021), activated carbons (Bouhamidi et al. 2017; Al-Musawi et al. 2021), nanoparticles (Durairaj et al. 2019; Davarnejad et al. 2020), composites (Wang et al. 2020a; Ren et al. 2021a), and aerogels (Yang et al. 2018; Cui et al. 2021).
The use of waste biomass from invasive species as adsorbent materials for the removal of pollutants from aqueous streams could decrease their threat, in harmony with the circular economy. It is imperative that the distribution and total biomass of exotic weeds requires more updated and precise data worldwide. The collection and conservation of such sources need more concerns to secure the quality of raw biomass. Moreover, most studies about such biosorbents have been investigated in synthetic aqueous solutions. In fact, the actual treated environment is considered more complicated than that in the simulated laboratories. Therefore, it requires more in situ investigations to examine the actual influence of biosorbents on the habit including microbial community and other aquatic organisms. Such endeavors could facilitate invasive plant-based adsorbents to large-scale applications, especially for industrial ones. The following areas should be more involved to enhance this purpose for environmental remediation:
As invasive plants are widely distributed and have strong development, the clear statistic data of the classification, biological features, and their biomass should be continuously updated. The comparison of the exotic plants for phytoremediation and conversion to biosorbents needs to be gained more interest to find the best method managing and utilizing them.
Future studies are needed to increase the extent of invasive plant biomass on carbon, capture, and sequestration. The long-term influences on soil and aquatic environment of such materials require more patterns and evaluations.
Mathematic models including the adsorption factors on simultaneous or several pollutants uptake should be concentrated to elucidate the kinetics and competitive mechanisms. Besides, the application of several advanced optimization software such as response surface methodology should be exploited to shorten the experimental progress. These facilitate the applications of biosorbents in large-scale expected more effective results.
The experiments mentioned in this work have been mostly conducted in batch systems that are different from the actual environment. Therefore, more investigation on invasive plant-based biosorbents for pollutants removal in continuous systems are required. Also, the evaluation of economic aspects should be considered apart from technical ones to acknowledge the ultimate cost.
Attention should be paid to the reuse of invasive plant-based adsorbents over various cycles to avoid solid wastes. The further utilization of recycled materials should be tested after regeneration and desorption studies for sustainable treatment.
The modification of invasive plant-based adsorbents such as physical, chemical, and composite fabrication should be further investigated to improve the removal of contaminants. Besides, eco-friendly modified chemicals should be exploited for such modifications to limit secondary contamination.
Conclusion
The review presented the distribution as well as harmful impacts of invasive plants and implicated their potential as low-cost and biodegradable biosorbents. The biosorbents from invasive plants possess many functional groups that make them ideal materials for removing heavy metals, organic dyes, and oil pollutants. The invasive biomass pretreatment and chemical modification were also assessed to improve the adsorption performance. Other characteristics of particle size, surface areas, and pore volume of biosorbents are significantly connected to their performance in the adsorption process. Several plausible mechanisms for pollutants treatment were proposed to have insight into the role of chemisorption, electrostatic interaction, ionic exchange, chelation, hydrophobic, and π–π stacking interactions. Besides, the desorption and regeneration processes have been also mentioned to elucidate the practical value of biosorbents from invasive plants. Several prospects of invasive plant-based adsorbents for environmental remediation, and knowledge gaps are pinpointed for further studies.
Acknowledgements
The authors would love to appreciate the effort of researchers all over the world in the fight against the COVID-19 pandemic.
Authors’ contributions
DTCN was involved in conceptualization; data curation; investigation; methodology; writing—original draft; and writing—review and editing. TVT was involved in conceptualization; data curation; investigation; methodology; writing—original draft; and writing—review and editing. PSK was involved in writing—review and editing; data curation; and supervision. ATMD was involved in writing—review and editing; data curation; and supervision. AAJ was involved in writing—review and editing; data curation; and supervision. D-VNV was involved in writing—review and editing; data curation; supervision; and project administration.
Funding
There was no external funding for this study.
Data availability
The authors declare that all data and materials support their published claims and comply with field standards.
Code availability
The authors declare that software application or custom code supports their published claims and comply with field standards.
Declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Human and animal rights
Research does not involve any human participants and/or animals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Duyen Thi Cam Nguyen and Thuan Van Tran authors are contributed equally to this work.
References
- Abbas MN, Al-Tameemi IM, Hasan MB, Al-Madhhachi A-ST. Chemical removal of cobalt and lithium in contaminated soils using promoted white eggshells with different catalysts. South African J Chem Eng. 2021;35:23–32. doi: 10.1016/j.sajce.2020.11.002. [DOI] [Google Scholar]
- Acosta-Rodríguez I, Rodríguez-Pérez A, Pacheco-Castillo NC, et al. Removal of cobalt (II) from waters contaminated by the biomass of Eichhornia crassipes. Water. 2021;13:1725. doi: 10.3390/w13131725. [DOI] [Google Scholar]
- Ahmad A, Khan N, Giri BS, et al. Removal of methylene blue dye using rice husk, cow dung and sludge biochar: Characterization, application, and kinetic studies. Bioresour Technol. 2020;306:123202. doi: 10.1016/j.biortech.2020.123202. [DOI] [PubMed] [Google Scholar]
- Ahmad R, Khuroo AA, Hamid M, et al. Scale and season determine the magnitude of invasion impacts on plant communities. Flora. 2019;260:151481. doi: 10.1016/j.flora.2019.151481. [DOI] [Google Scholar]
- Ahmed A, Bakar MSA, Hamdani R, et al. Valorization of underutilized waste biomass from invasive species to produce biochar for energy and other value-added applications. Environ Res. 2020;186:109596. doi: 10.1016/j.envres.2020.109596. [DOI] [PubMed] [Google Scholar]
- Ajiboye TO, Oyewo OA, Onwudiwe DC. Simultaneous removal of organics and heavy metals from industrial wastewater: a review. Chemosphere. 2021;262:128379. doi: 10.1016/j.chemosphere.2020.128379. [DOI] [PubMed] [Google Scholar]
- Akpomie KG, Conradie J. Banana peel as a biosorbent for the decontamination of water pollutants a review. Environ Chem Lett. 2020;18:1085–1112. doi: 10.1007/s10311-020-00995-x. [DOI] [Google Scholar]
- Al-Buriahi AK, Al-Gheethi AA, Senthil Kumar P, et al. Elimination of rhodamine B from textile wastewater using nanoparticle photocatalysts: a review for sustainable approaches. Chemosphere. 2022;287:132162. doi: 10.1016/j.chemosphere.2021.132162. [DOI] [PubMed] [Google Scholar]
- Al-Gheethi AA, Azhar QM, Senthil Kumar P, et al. Sustainable approaches for removing Rhodamine B dye using agricultural waste adsorbents: a review. Chemosphere. 2022;287:132080. doi: 10.1016/j.chemosphere.2021.132080. [DOI] [PubMed] [Google Scholar]
- Al-Musawi TJ, Mengelizadeh N, Taghavi M, et al. Activated carbon derived from Azolla filiculoides fern: a high-adsorption-capacity adsorbent for residual ampicillin in pharmaceutical wastewater. Biomass Convers Biorefinery. 2021 doi: 10.1007/s13399-021-01962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saydeh SA, El-Naas MH, Zaidi SJ. Copper removal from industrial wastewater: a comprehensive review. J Ind Eng Chem. 2017;56:35–44. doi: 10.1016/j.jiec.2017.07.026. [DOI] [Google Scholar]
- Albrecht MA, Dell ND, Engelhardt MJ, et al. Recovery of herb-layer vegetation and soil properties after pile burning in a Midwestern oak woodland. Restor Ecol. 2021 doi: 10.1111/rec.13547. [DOI] [Google Scholar]
- Ali SM. Fabrication of a nanocomposite from an agricultural waste and its application as a biosorbent for organic pollutants. Int J Environ Sci Technol. 2018;15:1169–1178. doi: 10.1007/s13762-017-1477-x. [DOI] [Google Scholar]
- Ammar C, El-Ghoul Y, Jabli M. Characterization and valuable use of Calotropis gigantea seedpods as a biosorbent of methylene blue. Int J Phytoremediation. 2021 doi: 10.1080/15226514.2021.1876629. [DOI] [PubMed] [Google Scholar]
- Ammari TG. Utilization of a natural ecosystem bio-waste; leaves of Arundo donax reed, as a raw material of low-cost eco-biosorbent for cadmium removal from aqueous phase. Ecol Eng. 2014;71:466–473. doi: 10.1016/j.ecoleng.2014.07.067. [DOI] [Google Scholar]
- Amrhar O, El Gana L, Mobarak M. Calculation of adsorption isotherms by statistical physics models: a review. Environ Chem Lett. 2021;19:4519–4547. doi: 10.1007/s10311-021-01279-8. [DOI] [Google Scholar]
- Atouani ES, Belattmania Z, Reani A (2019) Brown seaweed Sargassum muticum as low-cost biosorbent of methylene blue. Int J Environ Res 13:131–142. 10.1007/s41742-018-0161-4
- Arunkumar B, Jeyakumar SJ, Jothibas M. A sol-gel approach to the synthesis of CuO nanoparticles using Lantana camara leaf extract and their photo catalytic activity. Optik (stuttg) 2019;183:698–705. doi: 10.1016/j.ijleo.2019.02.046. [DOI] [Google Scholar]
- Ayuba S, Mohammadib AA, Yousefic M, Changanic F. Performance evaluation of agro-based adsorbents for the removal of cadmium from wastewater. Desalin Water Treat. 2019;142:293–299. doi: 10.5004/dwt.2019.23455. [DOI] [Google Scholar]
- Azarpira H, Balarak D. Biosorption of acid orang 7 using dried cyperus rotundus: Isotherm studies and error functions. Int J ChemTech Res. 2016;9:543–549. [Google Scholar]
- Badr NBE, Al-Qahtani KM, Mahmoud AED. Factorial experimental design for optimizing selenium sorption on Cyperus laevigatus biomass and green-synthesized nano-silver. Alexandria Eng J. 2020;59:5219–5229. doi: 10.1016/j.aej.2020.09.051. [DOI] [Google Scholar]
- Bakshi A, Panigrahi AK. A comprehensive review on chromium induced alterations in fresh water fishes. Toxicol Reports. 2018;5:440–447. doi: 10.1016/j.toxrep.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakyayita GK, Norrström A-C, Kulabako RN. Competitive and noncompetitive batch sorption studies of aqueous Cd (II) and Pb (II) uptake onto Coffea canephora husks, Cyperus papyrus stems, and Musa spp. peels. J Chem. 2015 doi: 10.1155/2015/696098. [DOI] [Google Scholar]
- Banerjee S, Gautam RK, Rai P, Chattopadhyaya MC. Adsorptive removal of toxic dyes from aqueous phase using notorious weed Lantana camara (Linn.) as biosorbent. Res Chem Intermed. 2016;42:5677–5708. doi: 10.1007/s11164-015-2397-3. [DOI] [Google Scholar]
- Bansal S, Pandey PK, Upadhayay S. Methylene blue dye removal from wastewater using ailanthus excelsa roxb as adsorbent. Water Conserv Sci Eng. 2021;6:1–9. doi: 10.1007/s41101-020-00097-3. [DOI] [Google Scholar]
- Barsova N, Yakimenko O, Tolpeshta I, Motuzova G. Current state and dynamics of heavy metal soil pollution in Russian federation—a review. Environ Pollut. 2019;249:200–207. doi: 10.1016/j.envpol.2019.03.020. [DOI] [PubMed] [Google Scholar]
- Bayomie OS, Kandeel H, Shoeib T, et al. Novel approach for effective removal of methylene blue dye from water using fava bean peel waste. Sci Rep. 2020;10:7824. doi: 10.1038/s41598-020-64727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkhaya S, M’ rabet S, El Harfi A. A review on classifications, recent synthesis and applications of textile dyes. Inorg Chem Commun. 2020;115:107891. doi: 10.1016/j.inoche.2020.107891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermúdez YG, Rico ILR, Guibal E, et al. Biosorption of hexavalent chromium from aqueous solution by Sargassum muticum brown alga. application of statistical design for process optimization. Chem Eng J. 2012;183:68–76. doi: 10.1016/j.cej.2011.12.022. [DOI] [Google Scholar]
- Bhat SA, Rashid N, Rather MA, et al. Highly efficient catalytic reductive degradation of Rhodamine-B over Palladium-reduced graphene oxide nanocomposite. Chem Phys Lett. 2020;754:137724. doi: 10.1016/j.cplett.2020.137724. [DOI] [Google Scholar]
- Bouhamidi Y, Kaouah F, Nouri L, et al. Adsorption of diethyl and dibutyl phthalates onto activated carbon produced from Albizia julibrissin pods: kinetics and isotherms. Int J Environ Sci Technol. 2017;14:271–284. doi: 10.1007/s13762-016-1141-x. [DOI] [Google Scholar]
- Brião de Vargasde Andrade GJR, da Silva MGC, Vieira MGA. Removal of toxic metals from water using chitosan-based magnetic adsorbents. Rev Environ Chem Lett. 2020;18:1145–1168. doi: 10.1007/s10311-020-01003-y. [DOI] [Google Scholar]
- Bulgariu L, Escudero LB, Bello OS, et al. The utilization of leaf-based adsorbents for dyes removal: a review. J Mol Liq. 2019;276:728–747. doi: 10.1016/j.molliq.2018.12.001. [DOI] [Google Scholar]
- Cao S, Dong T, Xu G, Wang F. Study on structure and wetting characteristic of cattail fibers as natural materials for oil sorption. Environ Technol. 2016;37:3193–3199. doi: 10.1080/09593330.2016.1181111. [DOI] [PubMed] [Google Scholar]
- Carolin CF, Kumar PS, Saravanan A, et al. Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J Environ Chem Eng. 2017;5:2782–2799. doi: 10.1016/j.jece.2017.05.029. [DOI] [Google Scholar]
- Cazón JP, Viera M, Donati E, Guibal E. Zinc and cadmium removal by biosorption on Undaria pinnatifida in batch and continuous processes. J Environ Manage. 2013;129:423–434. doi: 10.1016/j.jenvman.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Chen M, Wang D, Ding S, et al. Zinc pollution in zones dominated by algae and submerged macrophytes in Lake Taihu. Sci Total Environ. 2019;670:361–368. doi: 10.1016/j.scitotenv.2019.03.167. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Mazumder MAJ, Al-Attas O, Husain T. Heavy metals in drinking water: occurrences, implications, and future needs in developing countries. Sci Total Environ. 2016;569:476–488. doi: 10.1016/j.scitotenv.2016.06.166. [DOI] [PubMed] [Google Scholar]
- Coetzee JJ, Bansal N, Chirwa EMN. Chromium in environment, its toxic effect from chromite-mining and ferrochrome industries, and its possible bioremediation. Expo Heal. 2020;12:51–62. doi: 10.1007/s12403-018-0284-z. [DOI] [Google Scholar]
- Costa RO, Jose CM, Grombone-Guaratini MT, Matos DMS. Chemical characterization and phytotoxicity of the essential oil from the invasive Hedychium coronarium on seeds of Brazilian riparian trees. Flora. 2019;257:151411. doi: 10.1016/j.flora.2019.05.010. [DOI] [Google Scholar]
- Cui F, Li H, Chen C, et al. Cattail fibers as source of cellulose to prepare a novel type of composite aerogel adsorbent for the removal of enrofloxacin in wastewater. Int J Biol Macromol. 2021;191:171–181. doi: 10.1016/j.ijbiomac.2021.09.022. [DOI] [PubMed] [Google Scholar]
- Cui Y, Xu G, Liu Y. Oil sorption mechanism and capability of cattail fiber assembly. J Ind Text. 2014;43:330–337. doi: 10.1177/1528083712452902. [DOI] [Google Scholar]
- da Silva JS, da Rosa MP, Beck PH, et al. Preparation of an alternative adsorbent from Acacia Mearnsii wastes through acetosolv method and its application for dye removal. J Clean Prod. 2018;180:386–394. doi: 10.1016/j.jclepro.2018.01.201. [DOI] [Google Scholar]
- Dabagh A, Bagui A, Abali M, et al. Adsorption of Crystal Violet from aqueous solution onto eco-friendly native Carpobrotus edulis plant. Mater Today Proc. 2021;37:3980–3986. doi: 10.1016/j.matpr.2020.10.349. [DOI] [Google Scholar]
- Dahou T, Defoort F, Khiari B, et al. Role of inorganics on the biomass char gasification reactivity: a review involving reaction mechanisms and kinetics models. Renew Sustain Energy Rev. 2021;135:110136. doi: 10.1016/j.rser.2020.110136. [DOI] [Google Scholar]
- Dang HH, Nguyen DTC, Nguyen TT, et al. Zeolitic-imidazolate framework-derived N-self-doped porous carbons with ultrahigh theoretical adsorption capacities for tetracycline and ciprofloxacin. J Environ Chem Eng. 2021;9:104938. doi: 10.1016/j.jece.2020.104938. [DOI] [Google Scholar]
- Davarnejad R, Azizi A, Mohammadi M, Mansoori S. A green technique for synthesising iron oxide nanoparticles by extract of centaurea cyanus plant: an optimised adsorption process for methylene blue. Int J Environ Anal Chem. 2020 doi: 10.1080/03067319.2020.1756273. [DOI] [Google Scholar]
- Dhouibi N, Binous H, Dhaouadi H, Dridi-Dhaouadi S. Hydrodistillation residues of Centaurea nicaeensis plant for copper and zinc ions removal: Novel concept for waste re-use. J Clean Prod. 2020;261:121106. doi: 10.1016/j.jclepro.2020.121106. [DOI] [Google Scholar]
- Din MI, Khalid R, Najeeb J, Hussain Z. Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies- a critical review. J Clean Prod. 2021;298:126567. doi: 10.1016/j.jclepro.2021.126567. [DOI] [Google Scholar]
- Dodd LL, Harms NE, Schad AN. Reciprocal competitive effects of congeneric invaders, Trapa natans L. and Trapa bispinosa Roxb. Var. iinumai Nakano, in established freshwater plant cultures. Aquat Bot. 2021;174:103419. doi: 10.1016/j.aquabot.2021.103419. [DOI] [Google Scholar]
- Duenas M-A, Ruffhead HJ, Wakefield NH, et al. The role played by invasive species in interactions with endangered and threatened species in the United States: a systematic review. Biodivers Conserv. 2018;27:3171–3183. doi: 10.1007/s10531-018-1595-x. [DOI] [Google Scholar]
- Durairaj K, Senthilkumar P, Velmurugan P, et al. Sol-gel mediated synthesis of silica nanoparticle from Bambusa vulgaris leaves and its environmental applications: kinetics and isotherms studies. J Sol-Gel Sci Technol. 2019;90:653–664. doi: 10.1007/s10971-019-04922-7. [DOI] [Google Scholar]
- Fabryanty R, Valencia C, Soetaredjo FE, et al. Removal of crystal violet dye by adsorption using bentonite – alginate composite. J Environ Chem Eng. 2017;5:5677–5687. doi: 10.1016/j.jece.2017.10.057. [DOI] [Google Scholar]
- Feng Q, Wang B, Chen M, et al. Invasive plants as potential sustainable feedstocks for biochar production and multiple applications: a review. Resour Conserv Recycl. 2021;164:105204. doi: 10.1016/j.resconrec.2020.105204. [DOI] [Google Scholar]
- Feng W, Fan D, Li K, et al. Removal of cadmium from rice grains by acid soaking and quality evaluation of decontaminated rice. Food Chem. 2022;371:131099. doi: 10.1016/j.foodchem.2021.131099. [DOI] [PubMed] [Google Scholar]
- Fiore V, Piperopoulos E, Calabrese L. Assessment of Arundo donax fibers for oil spill recovery applications. Fibers. 2019;7:75. doi: 10.3390/fib7090075. [DOI] [Google Scholar]
- Flory SL, Kleczewski N, Clay K. Ecological consequences of pathogen accumulation on an invasive grass. Ecosphere. 2011;2:1–12. doi: 10.1890/ES11-00191.1. [DOI] [Google Scholar]
- Fu Z, Xi S. The effects of heavy metals on human metabolism. Toxicol Mech Methods. 2020;30:167–176. doi: 10.1080/15376516.2019.1701594. [DOI] [PubMed] [Google Scholar]
- Gallego-Fernandez JB, Martínez ML, Garcia-Franco JG, Zunzunegui M. The impact on plant communities of an invasive alien herb, Oenothera drummondii, varies along the beach-coastal dune gradient. Flora. 2019;260:151466. doi: 10.1016/j.flora.2019.151466. [DOI] [Google Scholar]
- Gasmi A, Tippairote T, Mujawdiya PK, et al. Micronutrients as immunomodulatory tools for COVID-19 management. Clin Immunol. 2020;220:108545. doi: 10.1016/j.clim.2020.108545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam AK, Singh NB, Shukla SP, Mohan D. Lead removal efficiency of various natural adsorbents (Moringa oleifera, Prosopis juliflora, peanut shell) from textile wastewater. SN Appl Sci. 2020;2:1–11. doi: 10.1007/s42452-020-2065-0. [DOI] [Google Scholar]
- Gautam RK, Gautam PK, Chattopadhyaya MC, Pandey JD. Adsorption of Alizarin red S onto biosorbent of Lantana camara: kinetic, equilibrium modeling and thermodynamic studies. Proc Natl Acad Sci India Sect A Phys Sci. 2014;84:495–504. doi: 10.1007/s40010-014-0154-4. [DOI] [Google Scholar]
- Gemeda FT, Guta DD, Wakjira FS, Gebresenbet G. Occurrence of heavy metal in water, soil, and plants in fields irrigated with industrial wastewater in Sabata town, Ethiopia. Environ Sci Pollut Res. 2021;28:12382–12396. doi: 10.1007/s11356-020-10621-6. [DOI] [PubMed] [Google Scholar]
- Ghorbani M, Seyedin O, Aghamohammadhassan M. Adsorptive removal of lead (II) ion from water and wastewater media using carbon-based nanomaterials as unique sorbents: a review. J Environ Manage. 2020;254:109814. doi: 10.1016/j.jenvman.2019.109814. [DOI] [PubMed] [Google Scholar]
- Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020;12:236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorji A, Khaleghi Ghadiri M. Potential roles of micronutrient deficiency and immune system dysfunction in the coronavirus disease 2019 (COVID-19) pandemic. Nutrition. 2021;82:111047. doi: 10.1016/j.nut.2020.111047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guechi E-K, Hamdaoui O. Cattail leaves as a novel biosorbent for the removal of malachite green from liquid phase: data analysis by non-linear technique. Desalin Water Treat. 2013;51:3371–3380. doi: 10.1080/19443994.2012.749191. [DOI] [Google Scholar]
- Guna V, Ilangovan M, Anantha Prasad MG, Reddy N. Water hyacinth: a unique source for sustainable materials and products. ACS Sustain Chem Eng. 2017;5:4478–4490. doi: 10.1021/acssuschemeng.7b00051. [DOI] [Google Scholar]
- Guo S, Li W, Zhang L, et al. Kinetics and equilibrium adsorption study of lead (II) onto the low cost adsorbent—Eupatorium adenophorum spreng. Process Saf Environ Prot. 2009;87:343–351. doi: 10.1016/j.psep.2009.06.003. [DOI] [Google Scholar]
- Hanafiah M, Zakaria H, Ngah WSW. Preparation, characterization, and adsorption behavior of Cu (II) ions onto alkali-treated weed (Imperata cylindrica) leaf powder. Water Air Soil Pollut. 2009;201:43–53. doi: 10.1007/s11270-008-9926-2. [DOI] [Google Scholar]
- Hannachi Y, Hafidh A. Biosorption potential of Sargassum muticum algal biomass for methylene blue and lead removal from aqueous medium. Int J Environ Sci Technol. 2020;17:3875–3890. doi: 10.1007/s13762-020-02742-9. [DOI] [Google Scholar]
- Hassan MM, Carr CM. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere. 2018;209:201–219. doi: 10.1016/j.chemosphere.2018.06.043. [DOI] [PubMed] [Google Scholar]
- Hołtra A, Zamorska-Wojdyła D. The pollution indices of trace elements in soils and plants close to the copper and zinc smelting works in Poland’s Lower Silesia. Environ Sci Pollut Res. 2020;27:16086–16099. doi: 10.1007/s11356-020-08072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Ngo HH, Guo WS, Setiadi T. Adsorption and desorption of copper (II) ions onto garden grass. Bioresour Technol. 2012;121:386–395. doi: 10.1016/j.biortech.2012.06.119. [DOI] [PubMed] [Google Scholar]
- Hu Y, Guo X, Wang J. Biosorption of Sr2+ and Cs+ onto Undaria pinnatifida: Isothermal titration calorimetry and molecular dynamics simulation. J Mol Liq. 2020;319:114146. doi: 10.1016/j.molliq.2020.114146. [DOI] [Google Scholar]
- Huang H, Liu J, Chen L, et al. Multiple drivers, interaction effects, and trade-offs of efficient and cleaner combustion of torrefied water hyacinth. Sci Total Environ. 2021;786:147278. doi: 10.1016/j.scitotenv.2021.147278. [DOI] [PubMed] [Google Scholar]
- Islam A, Teo SH, Taufiq-Yap YH, et al. Step towards the sustainable toxic dyes and heavy metals removal and recycling from aqueous solution-A comprehensive review. Resour Conserv Recycl. 2021;175:105849. doi: 10.1016/j.resconrec.2021.105849. [DOI] [Google Scholar]
- Islam MA, Morton DW, Johnson BB, et al. Opportunities and constraints of using the innovative adsorbents for the removal of cobalt(II) from wastewater: a review environ nanotechnology. Monit Manag. 2018;10:435–456. doi: 10.1016/j.enmm.2018.10.003. [DOI] [Google Scholar]
- Istirokhatun T, Rokhati N, Rachmawaty R, et al. Cellulose isolation from tropical water hyacinth for membrane preparation. Procedia Environ Sci. 2015;23:274–281. doi: 10.1016/j.proenv.2015.01.041. [DOI] [Google Scholar]
- Janani R, Gurunathan B, Sivakumar K, et al. Advancements in heavy metals removal from effluents employing nano-adsorbents: way towards cleaner production. Environ Res. 2022;203:111815. doi: 10.1016/j.envres.2021.111815. [DOI] [PubMed] [Google Scholar]
- Jarvis P, Fawell J. Lead in drinking water – an ongoing public health concern? Curr Opin Environ Sci Heal. 2021;20:100239. doi: 10.1016/j.coesh.2021.100239. [DOI] [Google Scholar]
- Jayaram K, Prasad MNV. Removal of Pb (II) from aqueous solution by seed powder of Prosopis juliflora DC. J Hazard Mater. 2009;169:991–997. doi: 10.1016/j.jhazmat.2009.04.048. [DOI] [PubMed] [Google Scholar]
- Jia Z, Li Z, Ni T, Li S. Adsorption of low-cost absorption materials based on biomass (Cortaderia selloana flower spikes) for dye removal: kinetics, isotherms and thermodynamic studies. J Mol Liq. 2017;229:285–292. doi: 10.1016/j.molliq.2016.12.059. [DOI] [Google Scholar]
- Jobby R, Jha P, Yadav AK, Desai N. Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: a comprehensive review. Chemosphere. 2018;207:255–266. doi: 10.1016/j.chemosphere.2018.05.050. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Breger B, Drummond F. Novel plant–insect interactions in an urban environment: enemies, protectors, and pollinators of invasive knotweeds. Ecosphere. 2019;10:e02885. doi: 10.1002/ecs2.2885. [DOI] [Google Scholar]
- Jönsson LJ, Martín C. Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol. 2016;199:103–112. doi: 10.1016/j.biortech.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Jose S, Singh HP, Batish DR, Kohli RK. Invasive plant ecology. FL: CRC Press Boca Raton; 2013. [Google Scholar]
- Jun B-M, Heo J, Taheri-Qazvini N, et al. Adsorption of selected dyes on Ti3C2Tx MXene and Al-based metal-organic framework. Ceram Int. 2020;46:2960–2968. doi: 10.1016/j.ceramint.2019.09.293. [DOI] [Google Scholar]
- Kalisz S, Kivlin SN, Bialic-Murphy L. Allelopathy is pervasive in invasive plants. Biol Invasions. 2021;23:367–371. doi: 10.1007/s10530-020-02383-6. [DOI] [Google Scholar]
- Karaouzas I, Kapetanaki N, Mentzafou A, et al. Heavy metal contamination status in Greek surface waters: a review with application and evaluation of pollution indices. Chemosphere. 2021;263:128192. doi: 10.1016/j.chemosphere.2020.128192. [DOI] [PubMed] [Google Scholar]
- Khadir A, Negarestani M, Ghiasinejad H. Low-cost sisal fibers/polypyrrole/polyaniline biosorbent for sequestration of reactive orange 5 from aqueous solutions. J Environ Chem Eng. 2020;8:103956. doi: 10.1016/j.jece.2020.103956. [DOI] [Google Scholar]
- Khamparia S, Jaspal D. Investigation of adsorption of Rhodamine B onto a natural adsorbent Argemone mexicana. J Environ Manage. 2016;183:786–793. doi: 10.1016/j.jenvman.2016.09.036. [DOI] [PubMed] [Google Scholar]
- Khan FSA, Mubarak NM, Khalid M, et al. Magnetic nanoadsorbents’ potential route for heavy metals removal—a review. Environ Sci Pollut Res. 2020;27:24342–24356. doi: 10.1007/s11356-020-08711-6. [DOI] [PubMed] [Google Scholar]
- Khan TA, Nazir M, Khan EA. Adsorptive removal of rhodamine B from textile wastewater using water chestnut (Trapa natans L.) peel: adsorption dynamics and kinetic studies. Toxicol Environ Chem. 2013;95:919–931. doi: 10.1080/02772248.2013.840369. [DOI] [Google Scholar]
- Krstić V, Urošević T, Pešovski B. A review on adsorbents for treatment of water and wastewaters containing copper ions. Chem Eng Sci. 2018;192:273–287. doi: 10.1016/j.ces.2018.07.022. [DOI] [Google Scholar]
- Kuang W, Chen Z, Shi K, et al. Adverse health effects of lead exposure on physical growth, erythrocyte parameters and school performances for school-aged children in eastern China. Environ Int. 2020;145:106130. doi: 10.1016/j.envint.2020.106130. [DOI] [PubMed] [Google Scholar]
- Kuppusamy S, Thavamani P, Megharaj M, et al. Potential of Melaleuca diosmifolia leaf as a low-cost adsorbent for hexavalent chromium removal from contaminated water bodies. Process Saf Environ Prot. 2016;100:173–182. doi: 10.1016/j.psep.2016.01.009. [DOI] [Google Scholar]
- Kuppusamy S, Thavamani P, Megharaj M, et al. Potential of Melaleuca diosmifolia as a novel, non-conventional and low-cost coagulating adsorbent for removing both cationic and anionic dyes. J Ind Eng Chem. 2016;37:198–207. doi: 10.1016/j.jiec.2016.03.021. [DOI] [Google Scholar]
- Lian W, Yang L, Joseph S, et al. Utilization of biochar produced from invasive plant species to efficiently adsorb Cd (II) and Pb (II) Bioresour Technol. 2020;317:124011. doi: 10.1016/j.biortech.2020.124011. [DOI] [PubMed] [Google Scholar]
- Likon M, Remškar M, Ducman V, Švegl F. Populus seed fibers as a natural source for production of oil super absorbents. J Environ Manage. 2013;114:158–167. doi: 10.1016/j.jenvman.2012.03.047. [DOI] [PubMed] [Google Scholar]
- Lim JY, Mubarak NM, Abdullah EC, et al. Recent trends in the synthesis of graphene and graphene oxide based nanomaterials for removal of heavy metals — a review. J Ind Eng Chem. 2018;66:29–44. doi: 10.1016/j.jiec.2018.05.028. [DOI] [Google Scholar]
- Lisak G. Reliable environmental trace heavy metal analysis with potentiometric ion sensors - reality or a distant dream. Environ Pollut. 2021;289:117882. doi: 10.1016/j.envpol.2021.117882. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu G, Yuan Z, et al. Occurrence, potential health risk of heavy metals in aquatic organisms from Laizhou Bay, China. Mar Pollut Bull. 2019;140:388–394. doi: 10.1016/j.marpolbul.2019.01.067. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang B, Su Z. Enhanced adsorptive removal of methylene blue from aqueous solution by soft rush (Juncus effusus) Desalin Water Treat. 2016;57:1671–1683. doi: 10.1080/19443994.2014.975284. [DOI] [Google Scholar]
- Liu X, Wu J, Wang J. Electro-enhanced removal of cobalt ions from aqueous solution by capacitive deionization. Sci Total Environ. 2019;697:134144. doi: 10.1016/j.scitotenv.2019.134144. [DOI] [PubMed] [Google Scholar]
- Liu Y, Luo S-H, Hua J, et al. Characterization of defensive cadinenes and a novel sesquiterpene synthase responsible for their biosynthesis from the invasive Eupatorium adenophorum. New Phytol. 2021;229:1740–1754. doi: 10.1111/nph.16925. [DOI] [PubMed] [Google Scholar]
- Luo M, Lin H, Li B, et al. A novel modification of lignin on corncob-based biochar to enhance removal of cadmium from water. Bioresour Technol. 2018;259:312–318. doi: 10.1016/j.biortech.2018.03.075. [DOI] [PubMed] [Google Scholar]
- Lv N, Wang X, Peng S, et al. Superhydrophobic/superoleophilic cotton-oil absorbent: preparation and its application in oil/water separation. RSC Adv. 2018;8:30257–30264. doi: 10.1039/C8RA05420G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Fang W, Zhang H, et al. Transcriptome analysis of zebra fish (Danio rerio) eggs following treatment with malachite green. Aquaculture. 2020;514:734500. doi: 10.1016/j.aquaculture.2019.734500. [DOI] [Google Scholar]
- Mahamadi C, Nharingo T. Utilization of water hyacinth weed (Eichhornia crassipes) for the removal of Pb (II), Cd (II) and Zn (II) from aquatic environments: an adsorption isotherm study. Environ Technol. 2010;31:1221–1228. doi: 10.1080/09593331003646604. [DOI] [PubMed] [Google Scholar]
- Mahmoud AED, Fawzy M, Hosny G, Obaid A. Equilibrium, kinetic, and diffusion models of chromium (VI) removal using Phragmites australis and Ziziphus spina-christi biomass. Int J Environ Sci Technol. 2021;18:2125–2136. doi: 10.1007/s13762-020-02968-7. [DOI] [Google Scholar]
- Malik LA, Bashir A, Qureashi A, Pandith AH. Detection and removal of heavy metal ions: a review. Environ Chem Lett. 2019;17:1495–1521. doi: 10.1007/s10311-019-00891-z. [DOI] [Google Scholar]
- Mallesh D, Anbarasan J, Kumar PM, et al. Synthesis, characterization of carbon adsorbents derived from waste biomass and its application to CO2 capture. Appl Surf Sci. 2020;530:147226. doi: 10.1016/j.apsusc.2020.147226. [DOI] [Google Scholar]
- Mallick SN, Ekka NX, Kumar S, Sahu SC. Invasive alien flora in and around an urban area of India. diversity and ecology of invasive plants. Boston: IntechOpen; 2019. [Google Scholar]
- Malo AF, Godsall B, Prebble C, et al. Positive effects of an invasive shrub on aggregation and abundance of a native small rodent. Behav Ecol. 2013;24:759–767. doi: 10.1093/beheco/ars202. [DOI] [Google Scholar]
- Mashkoor F, Nasar A. Magsorbents: Potential candidates in wastewater treatment technology–a review on the removal of methylene blue dye. J Magn Magn Mater. 2020;500:166408. doi: 10.1016/j.jmmm.2020.166408. [DOI] [Google Scholar]
- Mitra S, Sarkar A, Sen S. Removal of chromium from industrial effluents using nanotechnology: a review. Nanotechnol Environ Eng. 2017;2:11. doi: 10.1007/s41204-017-0022-y. [DOI] [Google Scholar]
- Morris KA, Stark JM, Bugbee B, Norton JM. The invasive annual cheatgrass releases more nitrogen than crested wheatgrass through root exudation and senescence. Oecologia. 2016;181:971–983. doi: 10.1007/s00442-015-3544-7. [DOI] [PubMed] [Google Scholar]
- Mpatani FM, Han R, Aryee AA, et al. Adsorption performance of modified agricultural waste materials for removal of emerging micro-contaminant bisphenol A: a comprehensive review. Sci Total Environ. 2021 doi: 10.1016/j.scitotenv.2021.146629. [DOI] [PubMed] [Google Scholar]
- Munoz AA, Cavieres LA. Sharing of pollinators between the invasive Taraxacum officinale and co-flowering natives is not related to floral similarity in the high-Andes. Flora. 2019;261:151491. doi: 10.1016/j.flora.2019.151491. [DOI] [Google Scholar]
- Mustafa HM, Hayder G. Recent studies on applications of aquatic weed plants in phytoremediation of wastewater: a review article. Ain Shams Eng J. 2021;12:355–365. doi: 10.1016/j.asej.2020.05.009. [DOI] [Google Scholar]
- Naderi P, Shirani M, Semnani A, Goli A. Efficient removal of crystal violet from aqueous solutions with Centaurea stem as a novel biodegradable bioadsorbent using response surface methodology and simulated annealing: Kinetic, isotherm and thermodynamic studies. Ecotoxicol Environ Saf. 2018;163:372–381. doi: 10.1016/j.ecoenv.2018.07.091. [DOI] [PubMed] [Google Scholar]
- Naranjo VI, Hendricks M, Jones KS. Lead toxicity in children: an unremitting public health problem. Pediatr Neurol. 2020;113:51–55. doi: 10.1016/j.pediatrneurol.2020.08.005. [DOI] [PubMed] [Google Scholar]
- Nayl AA, Ahmed IM, Abd-Elhamid AI, et al. Selective sorption of 134Cs and 60Co radioisotopes using synthetic nanocopper ferrocyanide-SiO2 materials. Sep Purif Technol. 2020;234:116060. doi: 10.1016/j.seppur.2019.116060. [DOI] [Google Scholar]
- Negi GCS, Sharma S, Vishvakarma SCR, et al. Ecology and use of Lantana camara in India. Bot Rev. 2019;85:109–130. doi: 10.1007/s12229-019-09209-8. [DOI] [Google Scholar]
- Ngabura M, Hussain SA, Ghani WAWA, et al. Utilization of renewable durian peels for biosorption of zinc from wastewater. J Environ Chem Eng. 2018;6:2528–2539. doi: 10.1016/j.jece.2018.03.052. [DOI] [Google Scholar]
- Nguyen DTC, Dang HH, Vo D-VN, et al. Biogenic synthesis of MgO nanoparticles from different extracts (flower, bark, leaf) of Tecoma stans (L.) and their utilization in selected organic dyes treatment. J Hazard Mater. 2021;404:124146. doi: 10.1016/j.jhazmat.2020.124146. [DOI] [PubMed] [Google Scholar]
- Nguyen DTC, Le HTN, Nguyen TT, et al. Engineering conversion of Asteraceae plants into biochars for exploring potential applications: a review. Sci Total Environ. 2021;797:149195. doi: 10.1016/j.scitotenv.2021.149195. [DOI] [PubMed] [Google Scholar]
- Nguyen DTC, Le HTN, Nguyen TT, et al. Multifunctional ZnO nanoparticles bio-fabricated from Canna indica L. flowers for seed germination, adsorption, and photocatalytic degradation of organic dyes. J Hazard Mater. 2021;420:126586. doi: 10.1016/j.jhazmat.2021.126586. [DOI] [PubMed] [Google Scholar]
- Nguyen DTC, Nguyen TT, Le HTN, et al. The sunflower plant family for bioenergy, environmental remediation, nanotechnology, medicine, food and agriculture a review. Environ Chem Lett. 2021;19:3701–3726. doi: 10.1007/s10311-021-01266-z. [DOI] [Google Scholar]
- Nguyen TTV, Tri N, Tran BA, et al. Synthesis, characteristics, oil adsorption, and thermal insulation performance of cellulosic aerogel derived from water hyacinth. ACS Omega. 2021;6:26130–26139. doi: 10.1021/acsomega.1c03137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithya K, Sathish A, Kumar PS. Packed bed column optimization and modeling studies for removal of chromium ions using chemically modified Lantana camara adsorbent. J Water Process Eng. 2020;33:101069. doi: 10.1016/j.jwpe.2019.101069. [DOI] [Google Scholar]
- O’Connor D, Hou D, Ok YS, Lanphear BP. The effects of iniquitous lead exposure on health. Nat Sustain. 2020;3:77–79. doi: 10.1038/s41893-020-0475-z. [DOI] [Google Scholar]
- Odoh CK, Zabbey N, Sam K, Eze CN. Status, progress and challenges of phytoremediation-An African scenario. J Environ Manage. 2019;237:365–378. doi: 10.1016/j.jenvman.2019.02.090. [DOI] [PubMed] [Google Scholar]
- Palansooriya KN, Yang Y, Tsang YF, et al. Occurrence of contaminants in drinking water sources and the potential of biochar for water quality improvement: a review. Crit Rev Environ Sci Technol. 2020;50:549–611. doi: 10.1080/10643389.2019.1629803. [DOI] [Google Scholar]
- Panahi S, Moghaddam MK, Moezzi M. Assessment of milkweed floss as a natural hollow oleophilic fibrous sorbent for oil spill cleanup. J Environ Manage. 2020;268:110688. doi: 10.1016/j.jenvman.2020.110688. [DOI] [PubMed] [Google Scholar]
- Park MJ, Park JH, Hong SH, Shin HD. First Asian report of leaf spot of Ambrosia trifida caused by Septoria epambrosiae. Plant Dis. 2012;96:289. doi: 10.1094/PDIS-10-11-0845. [DOI] [PubMed] [Google Scholar]
- Paz-Kagan T, Silver M, Panov N, Karnieli A. Multispectral approach for identifying invasive plant species based on flowering phenology characteristics. Remote Sens. 2019;11:953. doi: 10.3390/rs11080953. [DOI] [Google Scholar]
- Pejchar L, Mooney HA. Invasive species, ecosystem services and human well-being. Trends Ecol Evol. 2009;24:497–504. doi: 10.1016/j.tree.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Peng H, Guo J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: a review. Environ Chem Lett. 2020;18:2055–2068. doi: 10.1007/s10311-020-01058-x. [DOI] [Google Scholar]
- Peng Z, Lin X, Zhang Y, et al. Removal of cadmium from wastewater by magnetic zeolite synthesized from natural, low-grade molybdenum. Sci Total Environ. 2021;772:145355. doi: 10.1016/j.scitotenv.2021.145355. [DOI] [PubMed] [Google Scholar]
- Pinzone P, Potts D, Pettibone G, Warren R. Do novel weapons that degrade mycorrhizal mutualisms promote species invasion? Plant Ecol. 2018;219:539–548. doi: 10.1007/s11258-018-0816-4. [DOI] [Google Scholar]
- Plaza J, Viera M, Donati E, Guibal E. Biosorption of mercury by Macrocystis pyrifera and Undaria pinnatifida: Influence of zinc, cadmium and nickel. J Environ Sci. 2011;23:1778–1786. doi: 10.1016/S1001-0742(10)60650-X. [DOI] [PubMed] [Google Scholar]
- Prabakaran K, Li J, Anandkumar A, et al. Managing environmental contamination through phytoremediation by invasive plants: a review. Ecol Eng. 2019;138:28–37. doi: 10.1016/j.ecoleng.2019.07.002. [DOI] [Google Scholar]
- Putri KNA, Keereerak A, Chinpa W. Novel cellulose-based biosorbent from lemongrass leaf combined with cellulose acetate for adsorption of crystal violet. Int J Biol Macromol. 2020;156:762–772. doi: 10.1016/j.ijbiomac.2020.04.100. [DOI] [PubMed] [Google Scholar]
- Pyrzynska K. Removal of cadmium from wastewaters with low-cost adsorbents. J Environ Chem Eng. 2019;7:102795. doi: 10.1016/j.jece.2018.11.040. [DOI] [Google Scholar]
- Pyšek P, Jarošík V, Hulme PE, et al. A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Chang Biol. 2012;18:1725–1737. doi: 10.1111/j.1365-2486.2011.02636.x. [DOI] [Google Scholar]
- Qaiyum MA, Mohanta J, Kumari R, et al. Alkali treated water chestnut (Trapa natans L.) shells as a promising phytosorbent for malachite green removal from water. Int J Phytoremediation. 2021;6:1–9. doi: 10.1080/15226514.2021.1977912. [DOI] [PubMed] [Google Scholar]
- Qasem NAA, Mohammed RH, Lawal DU. Removal of heavy metal ions from wastewater: a comprehensive and critical review. NPJ Clean Water. 2021;4:36. doi: 10.1038/s41545-021-00127-0. [DOI] [Google Scholar]
- Qin H, Hu T, Zhai Y, et al. The improved methods of heavy metals removal by biosorbents: a review. Environ Pollut. 2020;258:113777. doi: 10.1016/j.envpol.2019.113777. [DOI] [PubMed] [Google Scholar]
- Ramesh ST, Gandhimathi R, Hamoneth Joesun J, Nidheesh PV. Novel agricultural waste adsorbent, Cyperus rotundus, for removal of heavy metal mixtures from aqueous solutions. Environ Eng Sci. 2013;30:74–81. doi: 10.1089/ees.2012.0192. [DOI] [Google Scholar]
- Ramos-Guivar JA, Flores-Cano DA, Caetano Passamani E. Differentiating nanomaghemite and nanomagnetite and discussing their importance in arsenic and lead removal from contaminated effluents: a critical review. Nanomaterials. 2021;11:2310. doi: 10.3390/nano11092310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathi BS, Kumar PS, Vo D-VN. Critical review on hazardous pollutants in water environment: occurrence, monitoring, fate, removal technologies and risk assessment. Sci Total Environ. 2021;797:149134. doi: 10.1016/j.scitotenv.2021.149134. [DOI] [PubMed] [Google Scholar]
- Reck IM, Paixão RM, Bergamasco R, et al. Removal of tartrazine from aqueous solutions using adsorbents based on activated carbon and Moringa oleifera seeds. J Clean Prod. 2018;171:85–97. doi: 10.1016/j.jclepro.2017.09.237. [DOI] [Google Scholar]
- Rehman M, Liu L, Wang Q, et al. Copper environmental toxicology, recent advances, and future outlook: a review. Environ Sci Pollut Res. 2019;26:18003–18016. doi: 10.1007/s11356-019-05073-6. [DOI] [PubMed] [Google Scholar]
- Ren L, Zhao G, Pan L, et al. Efficient removal of dye from wastewater without selectivity using activated carbon-juncus effusus porous fibril composites. ACS Appl Mater Interfaces. 2021;13:19176–19186. doi: 10.1021/acsami.0c22104. [DOI] [PubMed] [Google Scholar]
- Ren Q, Kong C, Chen Z, et al. Ultrasonic assisted electrochemical degradation of malachite green in wastewater. Microchem J. 2021;164:106059. doi: 10.1016/j.microc.2021.106059. [DOI] [Google Scholar]
- Rezania S, Park J, Rupani PF, et al. Phytoremediation potential and control of Phragmites australis as a green phytomass: an overview. Environ Sci Pollut Res. 2019;26:7428–7441. doi: 10.1007/s11356-019-04300-4. [DOI] [PubMed] [Google Scholar]
- Rigueto CVT, Piccin JS, Dettmer A, et al. Water hyacinth (Eichhornia crassipes) roots, an amazon natural waste, as an alternative biosorbent to uptake a reactive textile dye from aqueous solutions. Ecol Eng. 2020;150:105817. doi: 10.1016/j.ecoleng.2020.105817. [DOI] [Google Scholar]
- Russell JC, Blackburn TM. The rise of invasive species denialism. Trends Ecol Evol. 2017;32:3–6. doi: 10.1016/j.tree.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Sacco O, Matarangolo M, Vaiano V, et al. Crystal violet and toxicity removal by adsorption and simultaneous photocatalysis in a continuous flow micro-reactor. Sci Total Environ. 2018;644:430–438. doi: 10.1016/j.scitotenv.2018.06.388. [DOI] [PubMed] [Google Scholar]
- Saheed IO, Da OhW, Suah FBM. Chitosan modifications for adsorption of pollutants – a review. J Hazard Mater. 2021;408:124889. doi: 10.1016/j.jhazmat.2020.124889. [DOI] [PubMed] [Google Scholar]
- Saleh TA, Ali I. Synthesis of polyamide grafted carbon microspheres for removal of rhodamine B dye and heavy metals. J Environ Chem Eng. 2018;6:5361–5368. doi: 10.1016/j.jece.2018.08.033. [DOI] [Google Scholar]
- Samra RM, Soliman AF, Zaki AA, et al. Bioassay-guided isolation of a new cytotoxic ceramide from Cyperus rotundus L. South African J Bot. 2021;139:210–216. doi: 10.1016/j.sajb.2021.02.007. [DOI] [Google Scholar]
- Santoso E, Ediati R, Kusumawati Y, et al. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater Today Chem. 2020;16:100233. doi: 10.1016/j.mtchem.2019.100233. [DOI] [Google Scholar]
- Saraswat S, Rai JPN. Heavy metal adsorption from aqueous solution using Eichhornia crassipes dead biomass. Int J Miner Process. 2010;94:203–206. doi: 10.1016/j.minpro.2010.02.006. [DOI] [Google Scholar]
- Saravanan A, Kumar PS, Karishma S, et al. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere. 2021;264:128580. doi: 10.1016/j.chemosphere.2020.128580. [DOI] [PubMed] [Google Scholar]
- Savić A, Oveisi M, Božić D, et al. Competition between ambrosia artemisiifolia and ambrosia trifida: is there a threat of a stronger competitor? Weed Res. 2021;61:298–306. doi: 10.1111/wre.12479. [DOI] [Google Scholar]
- Saya L, Gautam D, Malik V, et al. Natural polysaccharide based graphene oxide nanocomposites for removal of dyes from wastewater: a review. J Chem Eng Data. 2021;66:11–37. doi: 10.1021/acs.jced.0c00743. [DOI] [Google Scholar]
- Schaffner U, Steinbach S, Sun Y, et al. Biological weed control to relieve millions from Ambrosia allergies in Europe. Nat Commun. 2020;11:1–7. doi: 10.1038/s41467-020-15586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton RT, Shackleton CM, Kull CA. The role of invasive alien species in shaping local livelihoods and human well-being: a review. J Environ Manage. 2019;229:145–157. doi: 10.1016/j.jenvman.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Shackleton RT, Witt ABR, Piroris FM, van Wilgen BW. Distribution and socio-ecological impacts of the invasive alien cactus Opuntia stricta in eastern Africa. Biol Invasions. 2017;19:2427–2441. doi: 10.1007/s10530-017-1453-x. [DOI] [Google Scholar]
- Sharma M, Alexander A, Saraf S, et al. Mosquito repellent and larvicidal perspectives of weeds Lantana camara L. and Ocimum gratissimum L. found in central India. Biocatal Agric Biotechnol. 2021;34:102040. doi: 10.1016/j.bcab.2021.102040. [DOI] [Google Scholar]
- Sharma VK, Jinadatha C, Lichtfouse E. Environmental chemistry is most relevant to study coronavirus pandemics. Environ Chem Lett. 2020;18:993–996. doi: 10.1007/s10311-020-01017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Y, Tan X, Gu Y, et al. Effect of ascorbic acid assisted dilute acid pretreatment on lignin removal and enzyme digestibility of agricultural residues. Renew Energy. 2021;163:732–739. doi: 10.1016/j.renene.2020.08.135. [DOI] [Google Scholar]
- Shooto ND. Removal of toxic hexavalent chromium (Cr (VI)) and divalent lead (Pb (II)) ions from aqueous solution by modified rhizomes of Acorus calamus. Surfaces and Interfaces. 2020;20:100624. doi: 10.1016/j.surfin.2020.100624. [DOI] [Google Scholar]
- Sibal LN, Espino MPB. Heavy metals in lake water: a review on occurrence and analytical determination. Int J Environ Anal Chem. 2018;98:536–554. doi: 10.1080/03067319.2018.1481212. [DOI] [Google Scholar]
- Sileshi GW, Gebeyehu S, Mafongoya PL. The threat of alien invasive insect and mite species to food security in Africa and the need for a continent-wide response. Food Secur. 2019;11:763–775. doi: 10.1007/s12571-019-00930-1. [DOI] [Google Scholar]
- Sodhi KK, Kumar M, Agrawal PK, Singh DK. Perspectives on arsenic toxicity, carcinogenicity and its systemic remediation strategies. Environ Technol Innov. 2019;16:100462. doi: 10.1016/j.eti.2019.100462. [DOI] [Google Scholar]
- Song D, Pan K, Tariq A, et al. Adsorptive removal of toxic chromium from waste-Water using wheat straw and Eupatorium adenophorum. PLoS ONE. 2016;11:e0167037. doi: 10.1371/journal.pone.0167037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starešinič M, Boh Podgornik B, Javoršek D, et al. Fibers obtained from invasivei alien plant species as a base material for paper production. Forests. 2021;12:527. doi: 10.3390/f12050527. [DOI] [Google Scholar]
- Stavrinou A, Aggelopoulos CA, Tsakiroglou CD. Exploring the adsorption mechanisms of cationic and anionic dyes onto agricultural waste peels of banana, cucumber and potato: adsorption kinetics and equilibrium isotherms as a tool. J Environ Chem Eng. 2018;6:6958–6970. doi: 10.1016/j.jece.2018.10.063. [DOI] [Google Scholar]
- Stewart PS, Hill RA, Stephens PA, et al. Impacts of invasive plants on animal behaviour. Ecol Lett. 2021;24:891–907. doi: 10.1111/ele.13687. [DOI] [PubMed] [Google Scholar]
- Su CX-H, Teng TT, Alkarkhi AFM, Low LW. Imperata Cylindrica (Cogongrass) as an adsorbent for methylene blue dye removal: Process optimization. Water, Air, Soil Pollut. 2014;225:1–12. doi: 10.1007/s11270-014-1941-x. [DOI] [Google Scholar]
- Suyamboo BK, Srikrishnaperumal R. Biosorption of crystal violet onto cyperus rotundus in batch system: kinetic and equilibrium modeling. Desalin Water Treat. 2014;52:3535–3546. doi: 10.1080/19443994.2013.803315. [DOI] [Google Scholar]
- Taka AL, Fosso-Kankeu E, Pillay K, Mbianda XY. Removal of cobalt and lead ions from wastewater samples using an insoluble nanosponge biopolymer composite: adsorption isotherm, kinetic, thermodynamic, and regeneration studies. Environ Sci Pollut Res. 2018;25:21752–21767. doi: 10.1007/s11356-018-2055-6. [DOI] [PubMed] [Google Scholar]
- Tan DT, Thu PQ, Dell B. Invasive plant species in the national parks of Vietnam. Forests. 2012;3:997–1016. doi: 10.3390/f3040997. [DOI] [Google Scholar]
- Tang J, Zhang J, Ren L, et al. Diagnosis of soil contamination using microbiological indices: a review on heavy metal pollution. J Environ Manage. 2019;242:121–130. doi: 10.1016/j.jenvman.2019.04.061. [DOI] [PubMed] [Google Scholar]
- Tauqeer M, Khan ME, Ji RS, et al. Metal and metal oxide nanomaterials for wastewater decontamination metal metal-oxides and metal-organic frameworks for environmental remediation. New York: Springer; 2021. [Google Scholar]
- Tewari K, Singhal G, Arya RK. Adsorption removal of malachite green dye from aqueous solution. Rev Chem Eng. 2018;34:427–453. doi: 10.1515/revce-2016-0041. [DOI] [Google Scholar]
- Teymori M, Khorsandi H, Aghapour AA, et al. Electro-Fenton method for the removal of Malachite Green: effect of operational parameters. Appl Water Sci. 2019;10:39. doi: 10.1007/s13201-019-1123-5. [DOI] [Google Scholar]
- Tkaczyk A, Mitrowska K, Posyniak A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: a review. Sci Total Environ. 2020;717:137222. doi: 10.1016/j.scitotenv.2020.137222. [DOI] [PubMed] [Google Scholar]
- Tounsadi H, Khalidi A, Abdennouri M, Barka N. Biosorption potential of Diplotaxis harra and Glebionis coronaria L. biomasses for the removal of Cd (II) and Co (II) from aqueous solutions. J Environ Chem Eng. 2015;3:822–830. doi: 10.1016/j.jece.2015.03.022. [DOI] [Google Scholar]
- Tounsadi H, Khalidi A, Abdennouri M, Barka N. Potential capability of natural biosorbents: Diplotaxis harra and Glebionis coronaria L. on the removal efficiency of dyes from aqueous solutions. Desalin Water Treat. 2016;57:16633–16642. doi: 10.1080/19443994.2015.1079739. [DOI] [Google Scholar]
- Tran TV, Nguyen DTC, Le HTN, et al. Facile synthesis of manganese oxide-embedded mesoporous carbons and their adsorbability towards methylene blue. Chemosphere. 2019;227:455–461. doi: 10.1016/j.chemosphere.2019.04.079. [DOI] [PubMed] [Google Scholar]
- Tran TV, Nguyen H-TT, Dang HH, et al. Central composite design for optimizing the organic dyes remediation utilizing novel graphene oxide@CoFe2O4 nanocomposite. Surfaces and Interfaces. 2020;21:100687. doi: 10.1016/j.surfin.2020.100687. [DOI] [Google Scholar]
- Tran TV, Nguyen H, Le PHA, et al. Microwave-assisted solvothermal fabrication of hybrid zeolitic–imidazolate framework (ZIF-8) for optimizing dyes adsorption efficiency using response surface methodology. J Environ Chem Eng. 2020;8:104189. doi: 10.1016/j.jece.2020.104189. [DOI] [Google Scholar]
- Tran TV, Nguyen VH, Nong LX, et al. Hexagonal Fe-based MIL-88B nanocrystals with NH2 functional groups accelerating oxytetracycline capture via hydrogen bonding. Surfaces and Interfaces. 2020;20:100605. doi: 10.1016/j.surfin.2020.100605. [DOI] [Google Scholar]
- Tran TV, Phan T-QT, Nguyen DTC, et al. Recyclable Fe3O4@ C nanocomposite as potential adsorbent for a wide range of organic dyes and simulated hospital effluents. Environ Technol Innov. 2020;20:101122. doi: 10.1016/j.eti.2020.101122. [DOI] [Google Scholar]
- Tumolo M, Ancona V, De Paola D, et al. Chromium pollution in European water, sources, health risk, and remediation strategies: an overview. Int J Environ Res Public Health. 2020;17:5438. doi: 10.3390/ijerph17155438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkmen Koc SN, Kipcak AS, Moroydor Derun E, Tugrul N. Removal of zinc from wastewater using orange, pineapple and pomegranate peels. Int J Environ Sci Technol. 2021;18:2781–2792. doi: 10.1007/s13762-020-03025-z. [DOI] [Google Scholar]
- Vaiopoulou E, Gikas P. Regulations for chromium emissions to the aquatic environment in Europe and elsewhere. Chemosphere. 2020;254:126876. doi: 10.1016/j.chemosphere.2020.126876. [DOI] [PubMed] [Google Scholar]
- Van Meerbeek K, Appels L, Dewil R, et al. Biomass of invasive plant species as a potential feedstock for bioenergy production. Biofuels, Bioprod Biorefin. 2015;9:273–282. doi: 10.1002/bbb.1539. [DOI] [Google Scholar]
- Vardhan KH, Kumar PS, Panda RC. A review on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. J Mol Liq. 2019;290:111197. doi: 10.1016/j.molliq.2019.111197. [DOI] [Google Scholar]
- Vareda JP, Valente AJM, Durães L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: a review. J Environ Manage. 2019;246:101–118. doi: 10.1016/j.jenvman.2019.05.126. [DOI] [PubMed] [Google Scholar]
- Vasseghian Y, Dragoi E-N, Almomani F, et al. Graphene-based membrane techniques for heavy metal removal: a critical review. Environ Technol Innov. 2021;24:101863. doi: 10.1016/j.eti.2021.101863. [DOI] [Google Scholar]
- Verma M, Kumar A, Singh KP, et al. Graphene oxide-manganese ferrite (GO-MnFe2O4) nanocomposite: One-pot hydrothermal synthesis and its use for adsorptive removal of Pb2+ ions from aqueous medium. J Mol Liq. 2020;315:113769. doi: 10.1016/j.molliq.2020.113769. [DOI] [Google Scholar]
- Viju S, Thilagavathi G. Oil spill cleanup by bonded nettle fibrous mat. J Inst Eng Ser E. 2019;100:93–100. doi: 10.1007/s40034-018-0131-6. [DOI] [Google Scholar]
- Wang B, Li F, Wang L. Enhanced hexavalent chromium (Cr (VI)) removal from aqueous solution by Fe–Mn oxide-modified cattail biochar: adsorption characteristics and mechanism. Chem Ecol. 2020;36:138–154. doi: 10.1080/02757540.2019.1699537. [DOI] [Google Scholar]
- Wang C, Xiong C, He Y, et al. Facile preparation of magnetic Zr-MOF for adsorption of Pb (II) and Cr (VI) from water: Adsorption characteristics and mechanisms. Chem Eng J. 2021;415:128923. doi: 10.1016/j.cej.2021.128923. [DOI] [Google Scholar]
- Wang J, Um P, Dickerman B, Liu J. Zinc, magnesium, selenium and depression: a review of the evidence potential mechanisms and implications. Nutrients. 2018;10:584. doi: 10.3390/nu10050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zou C, Yang H, et al. Effects of cellulose, hemicellulose, and lignin on the combustion behaviours of biomass under various oxygen concentrations. Bioresour Technol. 2021;320:124375. doi: 10.1016/j.biortech.2020.124375. [DOI] [PubMed] [Google Scholar]
- Wang T, Liu S, Mao W, et al. Novel Bi2WO6 loaded N-biochar composites with enhanced photocatalytic degradation of rhodamine B and Cr(VI) J Hazard Mater. 2020;389:121827. doi: 10.1016/j.jhazmat.2019.121827. [DOI] [PubMed] [Google Scholar]
- Wang X-S, Qin Y. Removal of Ni (II), Zn (II) and Cr (VI) from aqueous solution by Alternanthera philoxeroides biomass. J Hazard Mater. 2006;138:582–588. doi: 10.1016/j.jhazmat.2006.05.091. [DOI] [PubMed] [Google Scholar]
- Wei Z, Gu H, Van Le Q, et al. Perspectives on phytoremediation of zinc pollution in air, water and soil. Sustain Chem Pharm. 2021;24:100550. doi: 10.1016/j.scp.2021.100550. [DOI] [Google Scholar]
- Weidlich EWA, Flórido FG, Sorrini TB, Brancalion PHS. Controlling invasive plant species in ecological restoration: a global review. J Appl Ecol. 2020;57:1806–1817. doi: 10.1111/1365-2664.13656. [DOI] [Google Scholar]
- Witt ABR, Nunda W, Makale F, Reynolds K. A preliminary analysis of the costs and benefits of the biological control agent Dactylopius opuntiae on Opuntia stricta in Laikipia County, Kenya. Biocontrol. 2020;65:515–523. doi: 10.1007/s10526-020-10018-x. [DOI] [Google Scholar]
- Wong C-W, Barford JP, Chen G, McKay G. Kinetics and equilibrium studies for the removal of cadmium ions by ion exchange resin. J Environ Chem Eng. 2014;2:698–707. doi: 10.1016/j.jece.2013.11.010. [DOI] [Google Scholar]
- Worathitanon C, Jangyubol K, Ruengrung P, et al. High performance visible-light responsive Chl-Cu/ZnO catalysts for photodegradation of rhodamine B. Appl Catal B Environ. 2019;241:359–366. doi: 10.1016/j.apcatb.2018.09.048. [DOI] [Google Scholar]
- Wu S, Zhang J, Li C, et al. Characterization of potential cellulose fiber from cattail fiber: a study on micro/nano structure and other properties. Int J Biol Macromol. 2021 doi: 10.1016/j.ijbiomac.2021.10.088. [DOI] [PubMed] [Google Scholar]
- Xiong J-Q, Cui P, Ru S, et al. Unravelling metabolism and microbial community of a phytobed co-planted with Typha angustifolia and Ipomoea aquatica for biodegradation of doxylamine from wastewater. J Hazard Mater. 2021;401:123404. doi: 10.1016/j.jhazmat.2020.123404. [DOI] [PubMed] [Google Scholar]
- Yang J, Xu P, Xia Y, Chen B. Multifunctional carbon aerogels from typha orientalis for oil/water separation and simultaneous removal of oil-soluble pollutants. Cellulose. 2018;25:5863–5875. doi: 10.1007/s10570-018-1994-x. [DOI] [Google Scholar]
- Ye H, Zhu Q, Du D. Adsorptive removal of Cd (II) from aqueous solution using natural and modified rice husk. Bioresour Technol. 2010;101:5175–5179. doi: 10.1016/j.biortech.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Yuan C, Bai X, Zhu T, et al. Long-term effects of the harvesting of trapa natans on local water quality and aquatic macrophyte community in Lake Erhai. China Front Environ Sci. 2021;9:246. doi: 10.3389/fenvs.2021.706746. [DOI] [Google Scholar]
- Zhang Y, Zhang Y, Akakuru OU, et al. Research progress and mechanism of nanomaterials-mediated in-situ remediation of cadmium-contaminated soil: a critical review. J Environ Sci. 2021;104:351–364. doi: 10.1016/j.jes.2020.12.021. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Feng Y, Zhang L, et al. Integrating novel chemical weapons and evolutionarily increased competitive ability in success of a tropical invader. New Phytol. 2015;205:1350–1359. doi: 10.1111/nph.13135. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Zhu Y, Wang A, Hu H. Potential of Calotropis gigantea fiber as an absorbent for removal of oil from water. Ind Crops Prod. 2016;83:387–390. doi: 10.1016/j.indcrop.2016.01.009. [DOI] [Google Scholar]
- Zhou X, Zhang J, Pan Z, Li D. Review of methods for the detection and determination of malachite green and leuco-malachite green in aquaculture. Crit Rev Anal Chem. 2019;49:1–20. doi: 10.1080/10408347.2018.1456314. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhang M, Wang X, et al. Removal of crystal violet by a novel cellulose-based adsorbent: comparison with native cellulose. Ind Eng Chem Res. 2014;53:5498–5506. doi: 10.1021/ie404135y. [DOI] [Google Scholar]
- Zhu X, Yi Y, Huang L, et al. Metabolomics eeveals the allelopathic potential of the invasive plant Eupatorium adenophorum. Plants. 2021;10:1473. doi: 10.3390/plants10071473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ST, Yin YN, Wang JL. Simultaneous detection and removal of cobalt ions from aqueous solution by modified chitosan beads. Int J Environ Sci Technol. 2018;15:385–394. doi: 10.1007/s13762-017-1388-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that all data and materials support their published claims and comply with field standards.
The authors declare that software application or custom code supports their published claims and comply with field standards.