Abstract
Polypropylene mesh is frequently used in urogynecology procedures; however, pain and mesh exposure into the vagina occur in ~10% of cases. Mesh-induced pain, which occurs with or without exposure, persists after removal in 50% of cases. Chronic pain history predicts poor response to mesh removal but only a fraction have this diagnosis. We hypothesize that mesh induced pain is correlated with fibrosis and failure to improve with a heightened inflammatory and fibrotic host response. Women undergoing mesh removal were offered participation in a mesh biorepository. Standardized questionnaires including visual analog scale (VAS) pelvic pain scores were completed at enrollment and 6 months after removal. Responders were considered those with ≥13 mm VAS improvement. 30 mesh-tissue explants were randomly selected for analysis. Samples were labeled for CD8, CD4 (Th) and FoxP3 (Tregs). Peri-fiber collagen deposition (fibrosis) was measured using a customized semi-quantitative assay. Concentrations of TGF-b1, bFGF, MCP-1, PDGF-BB, and IGFBP-1 in tissue were determined by immunoassay and compared to vaginal control biopsies with pathway analysis. VAS pain scores were correlated with degree of histologic fibrosis. Responders had more Tregs (7.8 vs 0.3 per mm2, p = 0.036) and patients were 1.6 times as likely to be a responder for every additional Treg/mm2 (p = 0.05). Pro-fibrotic TGF-β1 was doubled in nonresponders (p = 0.032). On pathway analysis, decreased bFGF and increased PDGF-BB provide a possible mechanism for upregulation of TGF-β1. In conclusion, fibrosis is a plausible mechanism of pain complications and the adaptive immune response likely contributes to mitigation/prevention of complications and recovery in affected patients.
Keywords: Polypropylene mesh, T regulatory cells, TGF-β1, Fibrosis, Prolapse
1. Introduction
Pelvic organ prolapse (POP) and stress urinary incontinence (SUI) are associated with severe impairments in female health, including sexual dysfunction, reduced activity, and reduced socialization [1,2]. Up to 20% of women will undergo surgery for POP or SUI in their lifetime [3,4]. Polypropylene mesh is widely used in prolapse surgery to overcome the high failure rate of suture repairs and in SUI to minimize the invasiveness of the procedure; however, even when the implanted mesh has optimal porosity and stiffness, women are still affected by mesh-induced pain and mesh exposure into the vagina in ~10% of cases [5]. Mesh-induced pain is difficult to treat and persists in 50% of cases, despite medication, physical therapy and multiple surgeries for mesh removal [6–9]. Mesh-induced pain can occur with or without exposure of the mesh through the vaginal epithelium. Although mesh-induced pain and exposure are sometimes considered two distinct complication phenotypes, there is likely pathophysiologic and symptomatic overlap between them that is poorly characterized. Understanding the pathophysiology of mesh complications and characterizing who will respond to removal has the potential to reduce poor outcomes related to mesh implants and better treat those who have already experienced these complications.
Fibrosis, driven by increased TGF-β1, appears to be an important element present in explants from women with pain and exposure mesh complications [10]. In mesh complications, pore collapse can result in crowding of mesh fibers with an overlapping foreign body response, bridging fibrosis, encapsulation and myofibroblast-induced contracture [11,12]. Indeed, both pain and exposure complications demonstrate increased collagen surrounding mesh fibers that suggests collagen over-production by fibroblasts. Fibrosis and contracture have also been implicated in adverse consequences for many other medical implants including artificial heart valves, breast implants, and joint replacements, supporting their likely importance in mesh complications [13,14].
Soluble factors such as growth factors, cytokines and chemokines play a critical role in normal wound healing and are often altered in states of chronic inflammation, chronic or heightened foreign body responses and dysregulated wound healing [15,16]. For example, platelet-derived growth factor BB (PDGF-BB) is a profibrotic growth factor that stimulates fibroblast proliferation and increases TGF-β1 activity, which in turn stimulates fibroblast activation. Fibroblast growth factor (bFGF) antagonizes TGF-β1 signaling and promotes angiogenesis during wound healing [17,18]. Monocyte chemoattractant protein (MCP-1) is a chemokine that recruits monocytic phagocytes and prolonged expression in chronic wounds causes an extended inflammatory response. Similarly, insulin-like growth factor binding protein-1 (IGFBP-1) binds anti-fibrotic insulin-like growth factor (IGF), preventing IGF from activating its receptor. High levels of IGFBP-1 are associated with severe pulmonary and liver fibrosis [19,20].
In addition to fibrosis, a strong pro-inflammatory reaction is observed in mesh complications, including a powerful M1 response, which has been shown to influence fibrosis surrounding biomaterials; a localized but robust T cell response; and a predominance of pro-inflammatory cytokines [8,21–23] ]. This may contribute directly to mesh pain through macrophage-stimulated release of pro-inflammatory factors, such as tumor necrosis factor α (TNF-α) and interleukin 1-β (IL1-β), which stimulate nociceptor neurons, and through cytotoxic T-cells, result in neuropathic pain [24–26]. We observed that Treg concentrations were inversely related to the amount of fibrosis in mesh-tissue complexes excised from women with complications, suggesting the immune system and normal wound repair mechanisms must synchronize to prevent complications after mesh implantation [10].
Despite these important laboratory findings, it is unknown whether histologic measures of fibrosis, the host immune response and/or levels of soluble biofactors observed in mesh-tissue explants removed for complications correlate with patient-reported measures such as pain. Pelvic pain attributed to mesh complications is subjective and may be influenced by factors other than the host response to the biomaterial such as pain syndromes or symptoms, expectations, fear or other motivation such as a plan to pursue litigation [27,28]. In addition, it is not known if persistent symptoms following removal is due to factors such as chronic neuroinflammation and scarring or if it is because the pain is multifactorial or not attributable to the mesh. The overall goal of this study was to define biologic factors involved in fibrogenesis following mesh implantation and correlate them with patient-reported pain outcomes and response to removal. We hypothesized that key regulators of wound healing, namely TGF-β1, PDGF-BB, bFGF, MCP-1 and IGFBP-1, would be elevated in mesh complications compared to controls. In addition, we hypothesized that patients with greater concentrations of Tregs and lower levels of fibrosis would be more likely to have improvement in pain after mesh removal than those with a profibrotic profile, more CD8+ cells and fewer T regulatory cells.
2. Methods
2.1. Patient selection and study design
After approval by the Institutional Review Board at the University of Pittsburgh Medical Center (9/11/2017), patients who underwent vaginal mesh removal for the primary indications of pain or exposure were invited to participate in the Magee Mesh Biorepository. Exclusion criteria included active infection as determined by their surgeon, chronic immunosuppression or an autoimmune disorder. Patients were consented for collection of the excised mesh-tissue complexes and an optional adjacent full thickness vaginal biopsy at the time of mesh excision. Because the vaginal biopsy was considered optional for study enrollment, additional control biopsies were obtained from women undergoing surgery for pelvic organ prolapse or stress urinary incontinence without prior mesh placement. Demographic data including age, BMI, gravity, parity, smoking status, menopausal status, use and type of hormone replacement therapy, and comorbidities as well as variables describing the initial mesh placement and removal, and complications such as tenderness and mesh deformation were collected at the time of enrollment using a standardized form developed a priori. Questionnaires on pain and symptoms including visual analog scale (VAS) pelvic pain scores and PFD-20 questionnaires were completed at enrollment and 6 months after removal (supplementary Fig. 1). Patients were considered responders (i.e. responded positively to their mesh removal) if they had at least 13 mm of improvement on the VAS scale, previously demonstrated to be the minimally important clinical difference [29]. They were considered nonresponders if they had < 13 mm of improvement or worsening of pelvic pain on the VAS scale.
30 total samples removed for pain in the absence of exposure (n = 15) or exposure with or without pain (n = 15) were randomly selected for analysis from samples in the Mesh Biorepository that had been removed for pain or exposure. When mesh-tissue complexes are obtained from women, the amount of tissue obtained is limited, especially when a portion of the specimen is needed for litigation. For analysis, specimens were divided for histology and biochemistry with the former prioritized. In order to maintain random sampling, specimens were of varied quantity which precluded our ability to perform all assays on all of the specimens. To overcome this limitation, the sample size was increased until the appropriate assays could be performed. Thus, of the 30 samples, 29 had tissue available for histology and 21 had sufficient tissue for biochemistry and one sample was added to the biochemistry group that did not have histology available (Fig. 1). The data presented in the current study represents a secondary analysis of collagen quantification, immune cell type characterization and TGF-β1 quantification previously characterized in the 29 patients with tissue available for histology (the 30th patient was included only in the biochemical analysis) [10]. As part of the current analysis, these data were correlated with patient-centered outcomes. Additionally, concentrations of TGF-β1, bFGF, MCP-1, PDGF-BB, and IGFBP-1 were determined in 21 specimens with tissue sufficient for biochemistry to further characterize the host response as shown in Fig. 1.
Fig. 1.
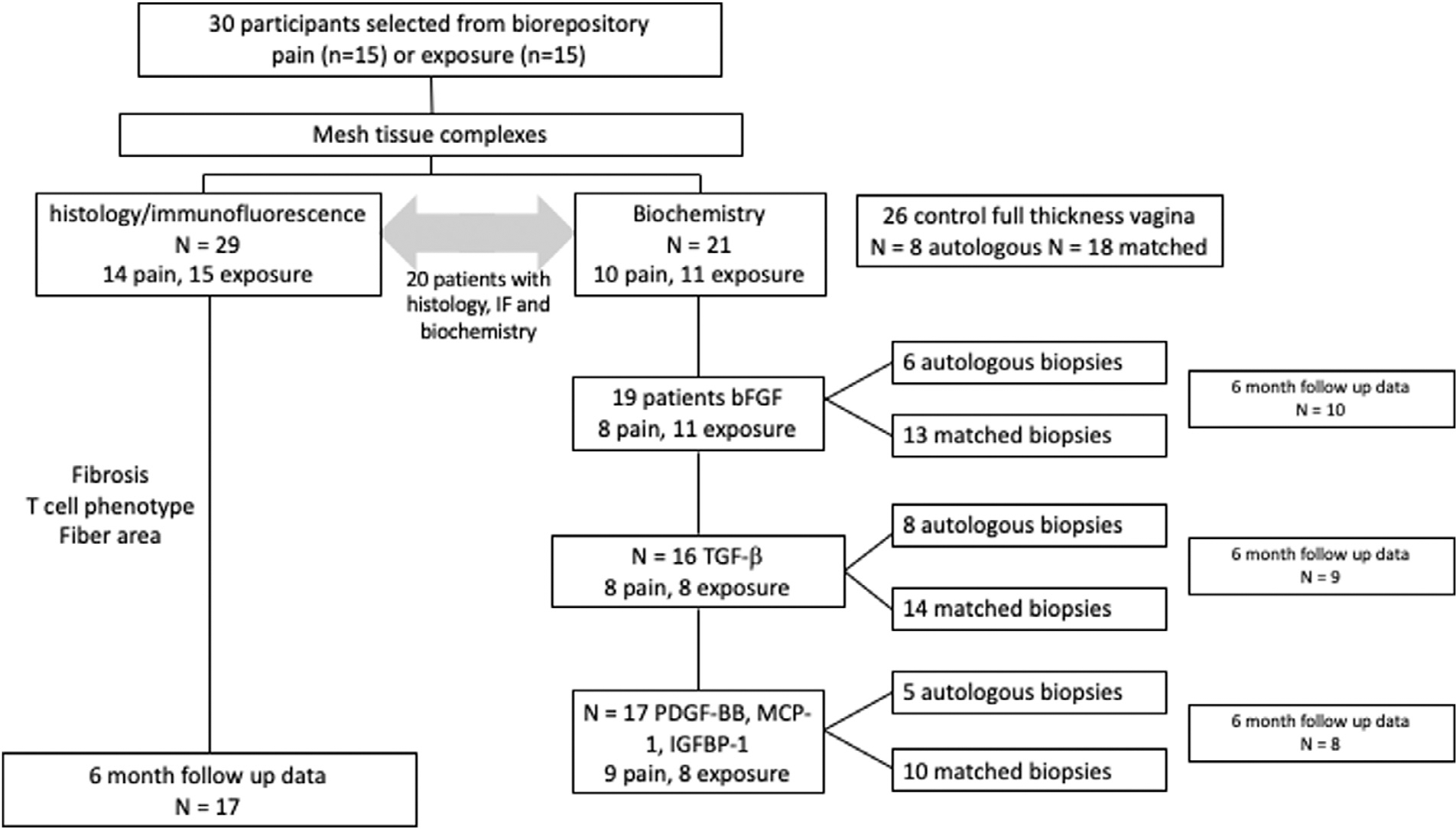
Flow chart demonstrating subgroups of patients in each analysis. Due to limited tissue for each sample and variable concentrations required for each assay, not every assay could be performed on each patient in this cohort.
2.2. Histologic preparation
Mesh tissue complexes were collected in a sterile manner, placed on ice and then imbedded in O.C.T. compound (Tissue-Tek; Sakura Finetek USA Inc, Torrance, CA), flash frozen in liquid nitrogen, sectioned at 7 μm and preserved at −80 °C.
2.3. Immunofluorescent labeling
As previously described [10], tissue sections were incubated with primary antibodies to CD4 (CD4 anti-goat 1:50, Santa Cruz) and FoxP3 (FoxP3 anti-mouse 1:150, Abcam) followed by secondary antibody incubation (Biotin anti-Goat and HRP anti-mouse at 1:500) and amplification with Alexa Flour 647 streptavidin and Alexa Flour 594 tyramide HRP conjugate. Aqueous mounting medium containing DAPI (Vectashield with DAPI, Vector Laboratories, Burlingame, CA) was applied. Separate slides were triple labeled for CD8+, FoxP3+, and DAPI to assess Treg/cytotoxic T cell ratios. Whole tissue section images were taken and analyzed using a Nikon ECLIPSE 90i upright microscope and Nikon NIS Elements Imaging Software (Nikon USA, Melville, NY). Fiber areas were measured. Samples were compared to negative controls to ensure specific labeling and were run in duplicate or triplicate. CD4+ and FoxP3+ cells per 0.25 mm2 were obtained as concentrations and total CD4+ and CD8+ cell counts were obtained per tissue section and normalized by fiber area. Tregs per 100 CD8+ cells and Tregs per 100 CD8+ cells per cm2 fiber area were calculated. To correlate the location of CD4+ and FoxP3+ cells to fibrotic response, we used a previously described customized quantification system [10], also described in Section 2.4 below. Representative IHC images can be found in Artsen et al, Acta Biomater 2019 [10].
2.4. Trichrome staining and fibrosis quantification
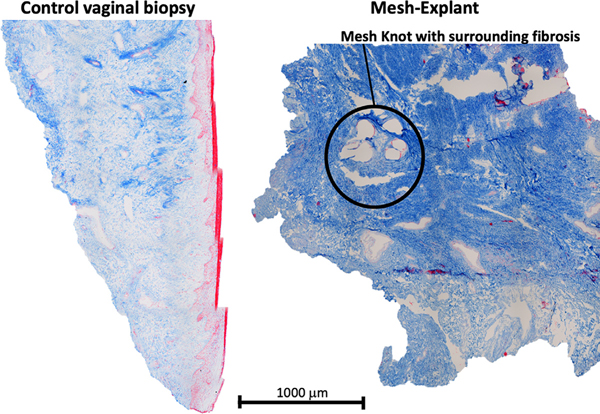
As previously described [10], a standardized Masson’s trichrome staining protocol omitting hematoxylin cell nucleus staining was applied and samples imaged using the Nikon ECLIPSE 90i upright microscope. A digital grid composed of 0.25 mm2 squares was laid over the trichrome images. In each grid, the intensity of collagen fibrosis was quantified using a digital intensity profile. Intensity in each grid was then averaged and the grids were classified as mild, moderate, and high or pathologic fibrosis, which was more than 2.5 standard deviations higher than controls (Fig. 2).
Fig. 2.
Representative trichrome images of vaginal control biopsy and mesh-explant. Pathologic fibrosis was more than 2.5 standard deviations higher than controls.
2.5. Cytokine quantification
21 of the 30 patients with mesh complications in this cohort had sufficient tissue available for biochemistry. The number of patients and autologous biopsies analyzed for each assay depended on the concentration of protein needed for each assay as detailed in Fig. 1. For TGF-β1 analysis, 16 patients with mesh complications had sufficient tissue for analysis with optional autologous control biopsies available in 8 patients (Fig. 1). 14 matched control biopsies were matched based on age (< age 50, 50–75, > 75), BMI (by NIH obesity class) and presence or absence of prolapse (≥stage 2) or stress incontinence. Protein concentrations were determined using a DC protein assay (Bio-Rad, Hercules, CA). TGF-β1 was quantified using a commercially available enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). Samples were run in duplicate with 50 μg protein.
A commercially available custom Luminex (R&D, Minneapolis, MN) kit was then used to determine concentrations of MCP-1, PDGF-BB and IGFBP-1. These chemokines and growth factors had previously been shown to demonstrate a difference between mesh and controls in preliminary studies employing a Luminex platform of proinflammatory and wound healing markers. 17 patients with mesh complications had sufficient tissue for comparison to 5 available autologous control biopsies and 10 matched controls. Those without appropriate controls were excluded from the paired analysis. Samples were run in duplicate with 80 μg protein.
The concentration of bFGF in the mesh-tissue complexes was found to be relatively high in preliminary studies. Therefore, bFGF analysis required a much smaller concentration of tissue, and bFGF was determined using a commercially available enzyme-linked immunosorbent assay kit (R&D) in 19 patients with biochemistry tissue, 6 autologous controls and 13 matched biopsies. Samples were run in duplicate with 15 μg protein.
2.6. Pathway analysis
Pathway analysis was performed using Ingenuity Pathway Analysis Software (Qiagen, Hilden, Germany) to define interactions between bFGF, TGF-β1 and PDGF-BB and references were reviewed by the primary author for relevance.
2.7. Statistical analysis
For cytokine analysis, sample size calculation suggested 11 mesh samples and 11 pooled autologous and matched controls were needed to detect an expected 45% difference at a significance level of < 0.05 and power of 80%. Sample size for histologic samples was determined by the sample size calculation for the primary analysis, namely that 14 samples were needed with a power of 80% and the standard deviation of 0.00014 seen in preliminary data to detect an expected difference in T cell populations in areas of high versus low fibrosis using a Mann–Whitney U test.
Mann–Whitney U tests and regression models were used to compare groups and determine correlation with pain score, fibrosis, or response to removal. Signed rank tests were used to compare cytokines near mesh complications with autologous and matched control biopsies. Hypothesis testing was considered significant at the p < 0.05 level. All statistical tests were completed in STATA software (version 15.1; StataCorp, College Station, Texas) or SPSS (IMB, Armonk, NY).
3. Results
3.1. Patient demographics
Women enrolled in the study were middle aged (median 56 years), overweight (29.0 kg/m2), and parous (median parity 3). Pain samples included 10 mid-urethral slings and 5 prolapse meshes. Exposure samples included 7 mid-urethral slings and 8 prolapse meshes. Median duration of implantation was 49.9 months. There were no differences in age, BMI, parity, duration of implantation, tobacco use, rate of diabetes, menopausal status, hormone use, or prior hysterectomy based on removal indication (Table 1, p > 0.05). As expected due to the study design, there were no differences in women with mesh complications and their matched controls.
Table 1.
Demographics of women with mesh complications by exposure or pain in the absence of exposure.
| Pain (n = 15) | Exposure (n = 15) | P value | |
|---|---|---|---|
|
| |||
| Age (y) | 56 (42–59) | 56 (54–68) | 0.23 |
| BMI (kg/m2) | 28.0 (25.1–32.2) | 30.0 (24.7–32.4) | 0.80 |
| Median parity (range) | 3 (2–5) | 3 (1–5) | 0.90 |
| Midurethral sling | 10 (66.7%) | 7 (46.7%) | 0.46 |
| Prolapse mesh | 5 (33.3%) | 8 (53.3%) | 0.46 |
| Duration implantation (mo) | 36.6 (15–56) | 58.5 (22–73) | 0.57 |
| Postmenopausal | 11 (73.3%) | 13 (86.7%) | 0.65 |
| Hormone therapy use* | 8 (53.3%) | 8 (53.3%) | 0.99 |
| Prior hysterectomy | 11 (73.3%) | 9 (60.0%) | 0.70 |
| Current or smoking history^ | 8 (53.3%) | 8 (53.3%) | 0.99 |
| Diabetes | 3 (20.0%) | 1 (6.7%) | 0.60 |
| Chronic steroid use | 0 (0%) | 1 (6.7%) | 0.99 |
| Baseline VAS Pelvic Pain | 30.5 (23–47) | 73 (45–95) | 0.046 |
| TGF-β1 (pg/μg protein)° | 11.8 (8.2–14.4) | 9.1 (6.9–13.3) | 0.83 |
| bFGF (pg/μg protein)+ | 6.5 (4.6–7.6) | 6.5 (4.0–10.4) | 0.99 |
| PDGF-BB (pg/μg protein)‡ | 0.4 (0.3–0.5) | 0.6 (0.4–0.7) | 0.18 |
| MCP-1 (pg/μg protein)‡ | 2.8 (1.6–3.0) | 2.1 (1.7–2.5) | 0.63 |
| IGFBP-1 (pg/μg protein)‡ | 14.2 (11.9–38.6) | 24.6 (20.2–31.6) | 0.29 |
Median (interquartile range) is listed for continuous variables, frequencies (%) for categorical variables. P values represent Mann–Whitney U for continuous variables and or Fishers Exact for categorical variables. VAS indicates visual analog scale.
includes vaginal estrogen.
smoking information missing on one patient.
TGF-β1 samples include 8 patients with pain and 8 with exposure.
FGF analysis included 7 patients with pain and 11 with exposure.
Luminex analysis included 9 patients with pain and 8 with exposure complications (see Fig. 1).
3.2. Baseline pain scores correlated linearly with degree of fibrosis
Overall median baseline VAS score was 45 (IQR 24–76) and 78.6% of patients answered “yes” to “Do you have pelvic pain?” demonstrating that many women with exposure also have pain. Median bother was 4 (scale 1–4). Women with pain in the absence of exposure had higher baseline pelvic pain scores than women with mesh removed for exposure [73 (45–95) vs 30.5 (23–47), p = 0.46]. At baseline, VAS score was associated with presence of pain and bother on the PFD-20 questionnaire (p < 0.002) and VAS scores increased linearly with amount of fibrosis (p = 0.013). Baseline VAS scores were not associated with Treg concentration or TGF-β1, PDGF-BB, MCP-1, IGFBP-1 or bFGF concentrations.
3.3. T regulatory cell concentration predicted response to removal
Six month follow up data was available for 17 of 29 patients with histology data. Of these 17 patients, there were 9 were responders with at least 13 mm improvement on the VAS pelvic pain score and 8 were nonresponders.
Responders were equally likely to have prolapse or sling mesh (5 patients vs 4 patients, p > 0.05) and equally likely to have pain or exposure complications (4 patients vs 5 patients, p > 0.05). The average age and BMI of responders and nonresponders were similar and reflected the overall demographics of the group. There was no difference in the amount of fibrosis between responders and nonresponders. However, there was a trend toward lower duration of implantation in responders (22.0 vs 55.5 months, p = 0.17).
Responders had more Tregs (7.8 vs 0.3 per mm2, p = 0.036) and a trend toward higher Treg/CD8 ratios (2.7 vs 1.1 Treg/100 CD8 cells, p = 0.07, Table 2). On logistic regression modeling, the cell density of Tregs predicted responder status. Patients were 1.6 times as likely to be a responder for every additional Treg/mm2 of tissue, (p = 0.05).
Table 2.
Characteristics of Responders and Non-responders to Mesh Removal.
| Responders (n = 9) | Non-responders (n = 8) | P value | |
|---|---|---|---|
|
| |||
| Age (y) | 57 (52.5–65.0) | 55.5 (48.8–68.0) | 0.82 |
| BMI (kg/m2) | 28.2 (26.2–31.6) | 29.6 (23.5–32.4) | 0.96 |
| Duration implantation (months) | 22.0 (10.5–61.3) | 55.5(35.0–58.9) | 0.17 |
| Baseline VAS Pelvic Pain | 45.0 (33–76) | 45.5(16–84.5) | 0.53 |
| Fibrosis level (intensity) | 54.6 (38.5–72.1) | 66.2 (35.8–82.2) | 0.89 |
| Treg concentration (cells/mm2) | 7.8 (1.3–10.2) | 0.3 (0.5–3.4) | 0.036 |
| CD8 (cells/sample) | 562.0 (289.5–829.0) | 291.0 (100.8–544.8) | 0.17 |
| Treg/CD8 ratio (Treg /100 CD8) | 2.7 (1.1–7.3) | 1.1 (0.1–1.8) | 0.07 |
| TGF-β1 (pg/μg protein)* | 6.6 (5.7–10.2) | 12.8 (10.0–13.3) | 0.032 |
| bFGF (pg/μg protein)* | 5.0 (3.81–8.37) | 7.3 (6.03–10.43) | 0.29 |
| PDGF-BB (pg/μg protein)* | 0.3 (0.3–0.6) | 0.4 (0.4–0.7) | 0.46 |
| MCP-1 (pg/μg protein)* | 2.3 (1.5–3.0) | 2.5 (1.8–2.8) | 0.88 |
| IGFBP-1 (pg/μg protein)* | 38.6 (23.6–72.3) | 17.3 (14.2–23.1) | 0.05 |
Data is presented as median (interquartile range). P values represent Mann–Whitney U tests.
Tissue for TGF-β1 quantification was available for 4 responders and 5 non-responders; for FGF 4 responders and 6 non-responders, for PDGF-BB, MCP-1 and IGFBP-1 included 3 responders and 5 nonresponders.
3.4. TGF-β1 was doubled in nonresponders
Six month follow up data was available for 9 out of 16 patients with TGF-β1 data. Nonresponders had nearly twice the level of TGF-β1 [12.8 (12.3–13.2) vs 6.6 (6.1–9.0) pg/μg protein] compared to responders (p = 0.032). bFGF, PDGF-BB, MCP-1 and IGFBP-1 concentrations were similar between responders and nonresponders (Table 2).
3.5. bFGF was decreased in mesh tissue complexes compared to controls
The median concentration of bFGF was nearly 50% decreased in mesh tissue complexes vs all controls combined (Figs. 3A and 4A, p = 0.0 0 03). This was statistically significant when mesh complication levels were compared to autologous controls 7.0 (6.5–7.8) vs 20.2 (15.4–26.4) pg/μg protein, n = 6, p = 0.028, and matched controls, [6.0 (4.4–7.6) vs 10.7 (9.7–12.0) pg/μg protein, n = 13, p = 0.0046]. bFGF was similar in pain and exposure groups and did not correlate with collagen content or baseline VAS.
Fig. 3.
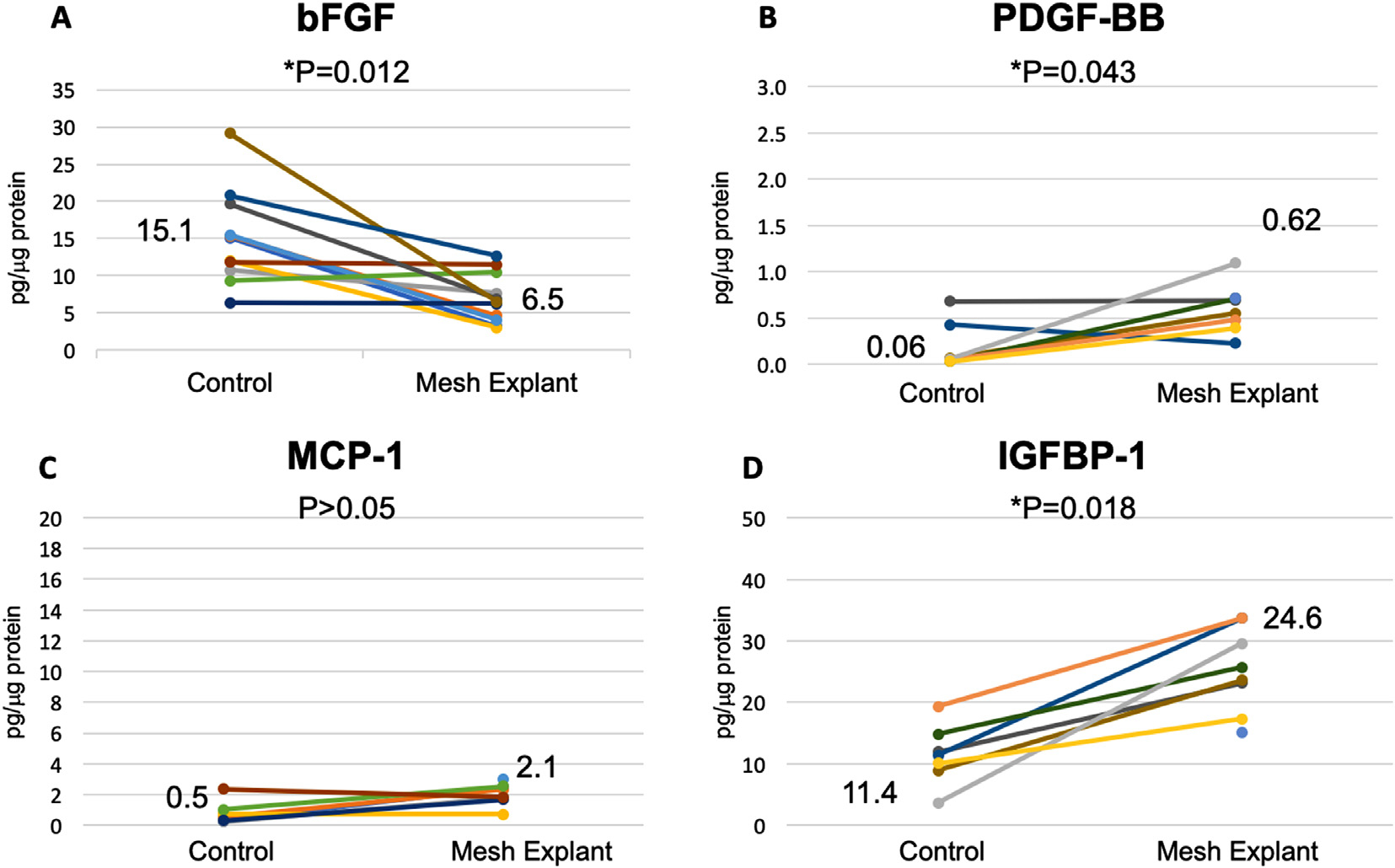
Profibrotic cytokines in mesh-tissue complexes removed for exposure compared to vaginal control biopsies. Results are given in pg/μg protein and include both autologous and matched controls. Median values are shown. P values represent Wilcoxon signed ranks given the wide variation.
Fig. 4.
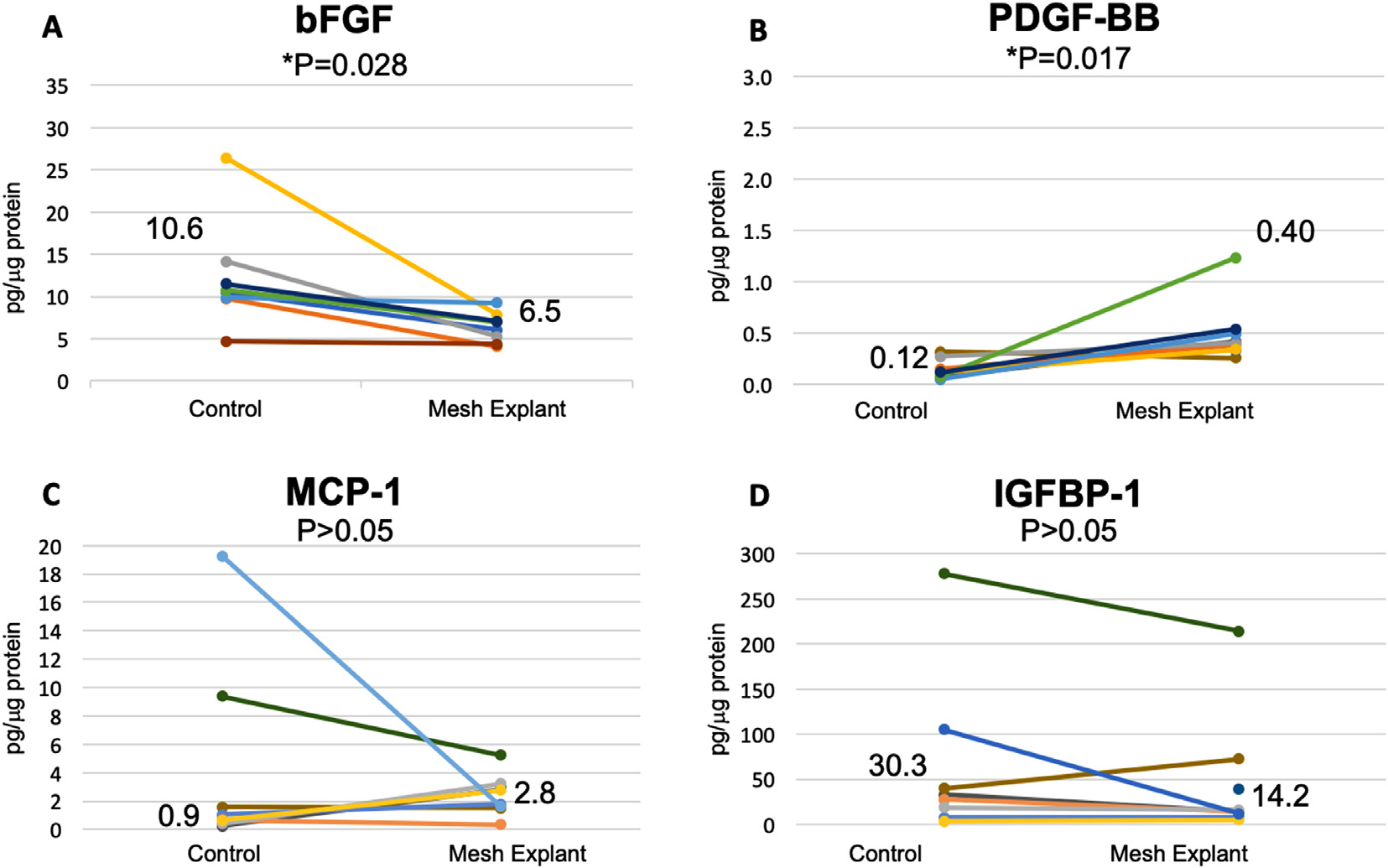
Profibrotic cytokines in mesh-tissue complexes removed for pain compared to vaginal control biopsies. Results are given in pg/μg protein and include both autologous and matched controls. Median values are shown. P values represent Wilcoxon signed ranks given the wide variation.
3.6. PDGF-BB was higher in mesh tissue complexes than controls
PDGF-BB was increased more than 5 times in mesh tissue complexes at 0.5 (0.4–0.7) pg/μg protein compared to 0.08 (0.05–0.27) pg/μg protein in control biopsies (n = 15, Figs. 3B and 4B, p = 0.002). This was seen for both autologous control biopsies [0.4 (0.4–0.5) vs 0.05 (0.03–0.2), n = 5, p = 0.0]) and matched biopsies [0.5 (0.3–0.7) vs 0.10 (0.7–0.3) n = 10, p = 0.02].
3.7. IGFBP-1 is increased in exposure complications but not pain complications
IGFBP-1 was statistically higher in tissue explanted for exposure at 24.6 (10.2–31.6) pg/μg protein compared to controls, 11.4 (8.9–14.8) pg/μg protein (p = 0.018) but not in mesh explanted for pain without exposure (Figs. 3D and 4D). MCP-1 and was similar between mesh-tissue complexes and controls (p > 0.05, Figs. 3C and 4C). There was no difference between pain and exposure groups and these analytes did not correlate with collagen content or baseline VAS.
3.8. TGF-β1 is regulated by bFGF on Pathway Analysis
Pathway analysis revealed direct regulation of TGF-β1 by bFGF and indirect regulation via PDGF-BB (Fig. 5). bFGF demonstrated inhibition of TGF-β1 dependent differentiation of fibroblasts into myofibroblasts [17]. bFGF’s dose-dependent inhibition of PDGF-BB was demonstrated in pharmacologic binding studies and PDGF-BB was seen to upregulate TGF-β1 [30,31].
Fig. 5.
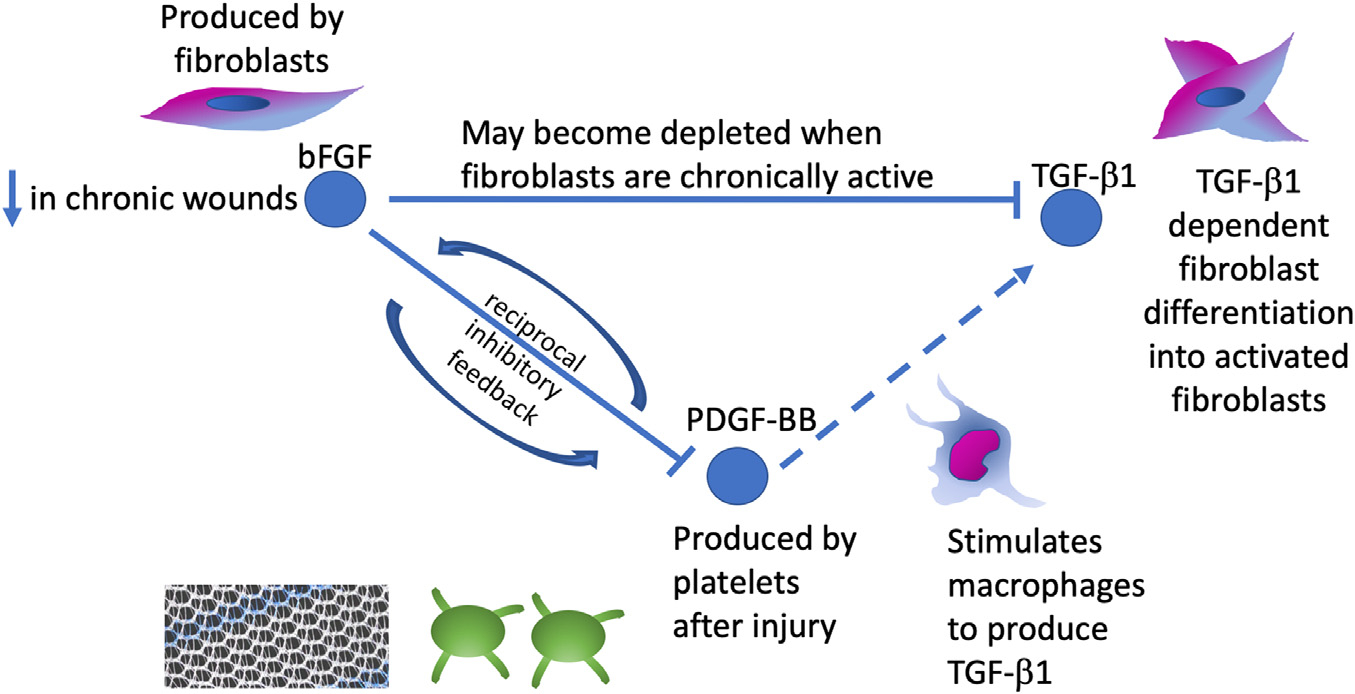
Pathway analysis of bFGF with factors TGF-β1 and PDGF-BB, which we have shown to be increased in urogynecologic mesh complications. Pathway analysis revealed bFGF regulation of TGF-β1 directly and via PDGF-BB.
4. Discussion
Polypropylene mesh complications have limited the use of transvaginal mesh for prolapse and stress urinary incontinence despite improvement in long-term success of these repairs over suture alone. A strong pro-inflammatory response has been demonstrated in mesh complications, but data increasingly also suggests a pro-fibrotic mechanism of pain resulting from poor tissue integration into mesh [8,10,22]. The results of this study support this mechanism and our hypothesis with the following important findings:1) patient-reported outcomes, specifically, pain scores, were positively correlated with degree of fibrosis; 2) increasing number of Tregs/mm2 predicted likelihood of improvement in pain after mesh removal; and 3) TGF-β1, a pro-fibrotic cytokine, was doubled in nonresponders. Additionally, decreased bFGF and increased PDGF-BB were shown in a pathway analysis to be a possible mechanism for the upregulation of TGF-β1.
4.1. Patient reported pain scores were correlated with degree of fibrosis
The difficulty of studying pelvic pain is well documented, particularly because the differential is broad and the degree of over-lap between pelvic pain symptoms is high [27,32]. However, many clinicians have documented a tender, painful band of mesh on examination [33] resulting from pore collapse, mesh contraction and bridging fibrosis seen in biomechanical and histologic analyses [11,12,34,35]. Our findings are in line with these studies by providing one of the first empiric data linking patient-perceived pain to histologic fibrosis in mesh pain complications.
4.2. Increasing Treg concentration predicted likelihood of improvement in pain after mesh removal
Improvement in pain and dyspareunia after mesh removal is reported to be as low as 57%, with only the presence of other chronic pain syndromes predicting a lower likelihood of improvement [6,28]. Here we saw a similar percentage responding to removal at 53% with similar improvements regardless of complication type and mesh type. Notably, increasing Treg concentrations predicted a positive response to mesh removal, regardless of fibrosis severity. We have previously shown that Tregs are inversely related to fibrosis in mesh-tissue explants and Tregs are well known for decreasing inflammation through suppressing T helper cells [36]. Our findings suggest that even in the case of severe fibrosis, the adaptive immune response may be responsible for prevention of complication and recovery through reducing fibrosis and inflammation; and that the altered wound healing that occurs in mesh complications could be modified by altering the adaptive immune response. In addition, pelvic pain appeared more likely to improve when mesh removal was performed after a shorter duration of implantation. This observation deserves further study, as it could be that early referral to a specialist to evaluate for removal may improve outcomes.
4.3. TGF-β1 was doubled in nonresponders and a pro-fibrotic growth factor profile seen in mesh-tissue explants may explain increased TGF-β1
We have previously shown that TGF-β1, which has been shown to facilitate myofibroblast contraction of the collagen matrix, is increased in women with mesh complications and here we demonstrate high levels in women who don’t respond to mesh removal [10,37]. The importance of TGF-β1 has been documented in the foreign body response [38] and our results further support the role of TGF-β1 and fibrosis in mesh complications and ongoing pain after mesh removal.
Decreased bFGF in mesh-tissue explants and increased PDGF-BB may account for the increased TGF-β1 observed in mesh tissue complexes from women with complications (Fig. 5). Our pathway analysis revealed that bFGF interacts directly and indirectly with TGF-β1 through PDGF-BB. bFGF is produced by fibroblasts and facilitates reepithelization and tissue remodeling and can inhibit TGF-β1 dependent differentiation of fibroblasts into myofibroblasts [18,33]. Normally, myofibroblasts undergo apoptosis with resolution of wound healing, but their persistence is a key feature in fibrosis [39]. Thus, bFGF may become depleted in counteracting TGF-β1 in this state of dysregulated wound healing.
PDGF-BB is released by platelets after injury and stimulates macrophages to produce TGF-β1, increases the proliferation and activation of fibroblasts, thus increasing ECM production and contraction [18,30]. The increase in PDGF-BB is supportive of repetitive cycles of injury [18] and repair which have been suggested as the stiffer polypropylene mesh moves against the softer vagina in a so called “brillo pad effect”. bFGF and PDGF-BB participate in reciprocal inhibitory feedback [40,41]. This negative feedback may result in an unchecked pro-fibrotic state, with elevated PDGF-BB directly contributing to decreased bFGF and resulting in increased TGF-β1 levels. Alternatively, as bFGF and PDGF-BB are both critical in neovascularization in wound healing, lower bFGF and higher PDGF-BB may also indicate dysregulated angiogenesis in mesh complications [42].
Finally, IGFBP-1 was also elevated in mesh explants for exposure but not for pain. This may suggest worsened dysregulated wound healing in exposure complications, with a pro-fibrotic, low T regulatory state preventing appropriate mesh integration and wound healing.
4.4. Strengths, limitations and future directions
This is a multidimensional study correlating patient-reported pain with histologic, immunologic and cytokine analysis of polypropylene mesh complications. By using validated pain scores, we were able to link objective observations to meaningful patient reported outcomes. Immunologic assessment included a ratio of Tregs to CD8+ effector cells, allowing for a comprehensive look at adaptive immune activation and tolerance. In addition, we supported histologic analysis with assessment of profibrotic cytokines and provided a possible mechanism for our TGF-β1 findings using pathway analysis.
Limitations include lack of a true control group, as mesh is rarely removed from women without complications. The use of matched biopsies here from women with prolapse or stress urinary incontinence provides a control group that most closely mimics women who would have mesh placed. In addition, the identification of responders and nonresponders provides a clinically meaningful comparison. Animal models also provide supporting control data, and stronger pro-inflammatory responses are seen in animals implanted with stiffer mesh materials with smaller pores, consistent with pro-inflammatory and pro-fibrotic responses seen here [21]. Although meshes removed here could not be easily grouped by biomechanical properties due to the variety of mesh removed, categorizing into sling and prolapse mesh takes into account their different mesh burden and stiffness. This is a problem inherent in human studies as so many different mesh types have been on the market. Another limitation is the and the overall small sample size and the small amount of tissue for some samples which limited the numbers for each analysis. Luminex was used in this study to help overcome this, but due to different concentration and bead region requirements, TGF-β1 and bFGF could not be performed concurrently with the other analytes through the Luminex platform. However, even with small numbers, we were able to see meaningful differences due to large effect sizes. Further studies into dysregulated wound healing in mesh complications will help confirm these findings. Finally, it is possible that patients may falsely elevate pain scores in order to receive mesh removal or for litigation purposes. However, the VAS score has been widely validated and additional measures to assess pain such as the validated PFDI-20 questions on the presence of pelvic pain and bother were reassuringly consistent.
Future studies should explore the role of myofibroblasts, which may be responsible for pathologic collagen deposition, as well as nerve fiber disruption which may contribute to response to removal. This study also suggests a potential therapeutic role of T cells which are in early clinical trials for other diseases [38,43]. These findings should inform future studies of new biomaterials and provide a potential for therapeutic manipulation of the immune response after implantation. Further studies are needed to determine if nonresponders have low Treg levels prior to mesh implantation. Indeed, phenotyping nonresponders is an important pathway moving forward so that these women can be identified ahead of time and counseled and treated appropriately.
4.5. Conclusions
Fibrosis was correlated with patient-reported pain, and higher Treg concentrations and lower levels of TGF-β1 were associated with improvement after mesh removal. Increased PDGF-BB and decreased bFGF may drive an increase in TGF-β1 seen in mesh complications. This improved understanding of the mechanism by which mesh can lead to pain moves us closer to the ultimate goal of preventing mesh complications.
Supplementary Material
Statement of Significance.
Polypropylene mesh improves anatomical outcomes in urogynecologic procedures, but is associated with complications, including pain and exposure through the vaginal epithelium. Mesh-induced pain is difficult to treat, and it is unclear why only half of women experience pain improvement after mesh removal. In this study, patient pain correlated with the presence of fibrosis and women with more T regulatory cells and lower TGF-β1 were more likely to have pain improvement following mesh removal. These findings implicate fibrosis as a mechanism of pain complications and suggest that the adaptive immune response may be responsible for prevention of complication and recovery. This improved understanding of how mesh can lead to pain moves us closer to the ultimate goal of preventing mesh complications.
Funding
This work was supported by the Pennsylvania Department of Health Tobacco Grant [grant number 6220] and the Eunice Kennedy Shriver NICHD NIH R01 HD083383–01.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.actbio.2020.07.051.
References
- [1].Lopes LG, Vasconcelos CTM, Neto JAV, Oriá MOB, Saboia DM, Gomes MLS, de Menezes PR, de Moraes Lopes MHB, A systematic review of the prevalence, risk factors, and impact of pelvic floor dysfunctions in nurses, Neurourol. Urodyn. 38 (2019) 1492–1503, doi: 10.1002/nau.24042. [DOI] [PubMed] [Google Scholar]
- [2].Verbeek M, Hayward L, Pelvic floor dysfunction and its effect on quality of sexual life, Sex. Med. Rev. 7 (2019) 559–564, doi: 10.1016/j.sxmr.2019.05.007. [DOI] [PubMed] [Google Scholar]
- [3].Barber MD, Pelvic organ prolapse, BMJ 354 (2016) i3853, doi: 10.1136/bmj.i3853. [DOI] [PubMed] [Google Scholar]
- [4].Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M, Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery, Obstet. Gynecol. 123 (2014) 1201–1206, doi: 10.1097/AOG.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maher C, Feiner B, Baessler K, Schmid C, Surgical management of pelvic organ prolapse in women, Cochrane Database Syst. Rev. (2013) CD004014, doi: 10.1002/14651858.CD004014.pub5. [DOI] [PubMed] [Google Scholar]
- [6].Hammett J, Peters A, Trowbridge E, Hullfish K, Short-term surgical outcomes and characteristics of patients with mesh complications from pelvic organ prolapse and stress urinary incontinence surgery, Int. Urogynecol. J. 25 (2014) 465–470, doi: 10.1007/s00192-013-2227-3. [DOI] [PubMed] [Google Scholar]
- [7].Cardenas-Trowers OO, Malekzadeh P, Nix DE, Hatch KD, Vaginal mesh removal outcomes: eight years of experience at an academic hospital, Female Pelvic Med. Reconstr. Surg. 23 (2017) 382–386, doi: 10.1097/SPV.0000000000000419. [DOI] [PubMed] [Google Scholar]
- [8].Nolfi AL, Brown BN, Liang R, Palcsey SL, Bonidie MJ, Abramowitch SD, Moalli PA, Host response to synthetic mesh in women with mesh complications, Am. J. Obstet. Gynecol. 215 (2016) 206.e1–8, doi: 10.1016/j.ajog.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dunn GE, Hansen BL, Egger MJ, Nygaard I, Sanchez-Birkhead AC, Hsu Y, Clark L, Changed women: the long-term impact of vaginal mesh complications, Female Pelvic Med. Reconstr. Surg. 20 (2014) 131–136, doi: 10.1097/SPV.0000000000000083. [DOI] [PubMed] [Google Scholar]
- [10].Artsen AM, Rytel M, Liang R, King GE, Meyn L, Abramowitch SD, Moalli PA, Mesh induced fibrosis: the protective role of T regulatory cells, Acta Biomater. 96 (2019) 203–210, doi: 10.1016/j.actbio.2019.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Orenstein SB, Saberski ER, Kreutzer DL, Novitsky YW, Comparative analysis of histopathologic effects of synthetic meshes based on material, weight, and pore size in mice., J. Surg. Res. 176 (2012) 423–429, doi: 10.1016/j.jss.2011.09.031. [DOI] [PubMed] [Google Scholar]
- [12].Klinge U, Klosterhalfen B, Birkenhauer V, Junge K, Conze J, Schumpelick V, Impact of polymer pore size on the interface scar formation in a rat model., J. Surg. Res. 103 (2002) 208–214, doi: 10.1006/jsre.2002.6358. [DOI] [PubMed] [Google Scholar]
- [13].Adams WP, Haydon MS, Raniere J, Trott S, Marques M, Feliciano M, Robinson JB, Tang L, Brown SA, A rabbit model for capsular contracture: development and clinical implications, Plast. Reconstr. Surg. 117 (2006) 1214–1219 discussion 1220., doi: 10.1097/01.prs.0000208306.79104.18. [DOI] [PubMed] [Google Scholar]
- [14].Vieira VJ, D’Acampora A, Neves FS, Mendes PR, Vasconcellos ZAD, Neves RD, Figueiredo CP, Capsular contracture in silicone breast implants: insights from rat models, An. Acad. Bras. Cienc. 88 (2016) 1459–1470, doi: 10.1590/0001-3765201620150874. [DOI] [PubMed] [Google Scholar]
- [15].Krzystek-Korpacka M, Kędzior K, Masłowski L, Mierzchała M, Bednarz-Misa I, Bronowicka-Szydełko A, Kubiak J, Gacka M, Płaczkowska S, Gamian A, Impact of chronic wounds of various etiology on systemic profiles of key inflammatory cytokines, chemokines, and growth factors and their interplay, Adv. Clin. Exp. Med. (2019), doi: 10.17219/acem/103845. [DOI] [PubMed] [Google Scholar]
- [16].Wynn TA, Cellular and molecular mechanisms of fibrosis., J. Pathol. 214 (2008) 199–210, doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liguori TTA, Liguori GR, Moreira LFP, Harmsen MC, Fibroblast growth factor-2, but not the adipose tissue-derived stromal cells secretome, inhibits TGF-β1-induced differentiation of human cardiac fibroblasts into myofibroblasts, Sci. Rep. 8 (2018) 16633, doi: 10.1038/s41598-018-34747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M, Growth factors and cytokines in wound healing, Wound Repair Regen. 16 (2008) 585–601, doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- [19].Guiot J, Bondue B, Henket M, Corhay JL, Louis R, Raised serum levels of IGFBP-1 and IGFBP-2 in idiopathic pulmonary fibrosis, BMC Pulm. Med. 16 (2016) 86, doi: 10.1186/s12890-016-0249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hagström H, Stål P, Hultcrantz R, Brismar K, Ansurudeen I, IGFBP-1 and IGF-I as markers for advanced fibrosis in NAFLD – a pilot study, Scand. J. Gastroenterol. 52 (2017) 1427–1434, doi: 10.1080/00365521.2017.1379556. [DOI] [PubMed] [Google Scholar]
- [21].Brown BN, Mani D, Nolfi AL, Liang R, Abramowitch SD, Moalli PA, Characterization of the host inflammatory response following implantation of prolapse mesh in rhesus macaque, Am. J. Obstet. Gynecol. 213 (2015) 668.e1–10, doi: 10.1016/j.ajog.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tennyson L, Rytel M, Palcsey S, Meyn L, Liang R, Moalli P, Characterization of the T-cell response to polypropylene mesh in women with complications, Am. J. Obstet. Gynecol. 220 (2019) 187.e1–187.e8, doi: 10.1016/j.ajog.2018.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Witherel CE, Abebayehu D, Barker TH, Spiller KL, Macrophage and fibroblast interactions in biomaterial-mediated fibrosis, Adv. Healthc. Mater. 8 (2019) e1801451, doi: 10.1002/adhm.201801451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ji R-R, Chamessian A, Zhang Y-Q, Pain regulation by non-neuronal cells and inflammation, Science 354 (2016) 572–577, doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu X-J, Zhang Y, Liu T, Xu Z-Z, Park C-K, Berta T, Jiang D, Ji R-R, Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter, Cell Res. 24 (2014) 1374–1377, doi: 10.1038/cr.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ji R-R, Xu Z-Z, Gao Y-J, Emerging targets in neuroinflammation-driven chronic pain, Nat. Rev. Drug Discov. 13 (2014) 533–548, doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee D, Chang J, Zimmern PE, Iatrogenic pelvic pain: surgical and mesh complications, Phys. Med. Rehabil. Clin. N. Am. 28 (2017) 603–619., doi: 10.1016/j.pmr.2017.03.010. [DOI] [PubMed] [Google Scholar]
- [28].Danford JM, Osborn DJ, Reynolds WS, Biller DH, Dmochowski RR, Postoperative pain outcomes after transvaginal mesh revision, Int. Urogynecol. J. 26 (2015) 65–69, doi: 10.1007/s00192-014-2455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Todd KH, Funk KG, Funk JP, Bonacci R, Clinical significance of reported changes in pain severity, Ann. Emerg. Med. 27 (1996) 485–489, doi: 10.1016/s0196-0644(96)70238-x. [DOI] [PubMed] [Google Scholar]
- [30].Fraser D, Wakefield L, Phillips A, Independent regulation of transforming growth factor-beta1 transcription and translation by glucose and platelet-derived growth factor, Am. J. Pathol. 161 (2002) 1039–1049, doi: 10.1016/s0002-9440(10)64265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Risinger GM, Updike DL, Bullen EC, Tomasek JJ, Howard EW, TGF-beta suppresses the upregulation of MMP-2 by vascular smooth muscle cells in response to PDGF-BB, Am. J. Physiol. Cell Physiol. 298 (2010) C191–201, doi: 10.1152/ajpcell.00417.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Speer LM, Mushkbar S, Erbele T, Chronic pelvic pain in women, Am. Fam. Phys. 93 (2016) 380–387. [PubMed] [Google Scholar]
- [33].Cholhan HJ, Hutchings TB, Rooney KE, Dyspareunia associated with paraurethral banding in the transobturator sling, Am. J. Obstet. Gynecol. 202 (2010) 4 81.e1–4 81.e5, doi: 10.1016/j.ajog.2010.01.061. [DOI] [PubMed] [Google Scholar]
- [34].Barone WR, Moalli PA, Abramowitch SD, Textile properties of synthetic prolapse mesh in response to uniaxial loading, Am. J. Obstet. Gynecol. 215 (2016) 326.e1–326.e9, doi: 10.1016/j.ajog.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Barone WR, Knight KM, Moalli PA, Abramowitch SD, Deformation of transvaginal mesh in response to multiaxial loading, J. Biomech. Eng. (2019) 141, doi: 10.1115/1.4041743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Corthay A, How do regulatory T cells work? Scand. J. Immunol. 70 (2009) 326–336, doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Meckmongkol TT, Harmon R, McKeown-Longo P, Van De Water L, The fibronectin synergy site modulates TGF-beta-dependent fibroblast contraction, Biochem. Biophys. Res. Commun. 360 (2007) 709–714, doi: 10.1016/j.bbrc.2007.06.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Anderson JM, Rodriguez A, Chang DT, Foreign body reaction to biomaterials, Semin. Immunol. 20 (2008) 86–100, doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Frangogiannis NG, The extracellular matrix in myocardial injury, repair, and remodeling, J. Clin. Investig. 127 (2017) 1600–1612, doi: 10.1172/JCI87491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lysiak JJ, Hussaini IM, Webb DJ, Glass WF, Allietta M, Gonias SL, Alpha 2-macroglobulin functions as a cytokine carrier to induce nitric oxide synthesis and cause nitric oxide-dependent cytotoxicity in the RAW 264.7 macrophage cell line., J. Biol. Chem. 270 (1995) 21919–21927, doi: 10.1074/jbc.270.37.21919. [DOI] [PubMed] [Google Scholar]
- [41].Russo K, Ragone R, Facchiano AM, Capogrossi MC, Facchiano A, Platelet-derived growth factor-BB and basic fibroblast growth factor directly interact in vitro with high affinity., J. Biol. Chem. 277 (2002) 1284–1291, doi: 10.1074/jbc.M108858200. [DOI] [PubMed] [Google Scholar]
- [42].Chen P-Y, Simons M, Friesel R, FRS2 via fibroblast growth factor receptor 1 is required for platelet-derived growth factor receptor beta-mediated regulation of vascular smooth muscle marker gene expression., J. Biol. Chem. 284 (2009) 15980–15992, doi: 10.1074/jbc.M809399200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Robson MC, Phillips LG, Lawrence WT, Bishop JB, Youngerman JS, Hayward PG, Broemeling LD, Heggers JP, The safety and effect of topically applied recombinant basic fibroblast growth factor on the healing of chronic pressure sores, Ann. Surg 216 (1992) 401–406 discussion 406, doi: 10.1097/00000658-199210000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.