Abstract
Although murine γδ T cells are largely considered innate immune cells, they have recently been reported to form long-lived memory populations. Much remains unknown about the biology and specificity of memory γδ T cells. Here, we interrogated intestinal memory Vγ4Vδ1 T cells generated after foodborne Listeria monocytogenes (Lm) infection to uncover an unanticipated complexity in the specificity of these cells. Deep TCR sequencing revealed that a subset of non-canonical Vδ1 clones are selected by Lm infection, consistent with antigen-specific clonal expansion. Ex vivo stimulations and in vivo heterologous challenge infections with diverse pathogenic bacteria revealed that Lm-elicited memory Vγ4Vδ1 T cells are broadly reactive. The Vγ4Vδ1 T cell recall response to Lm, Salmonella enterica serovar Typhimurium (STm) and Citrobacter rodentium was largely mediated by the γδTCR as internalizing the γδTCR prevented T cell expansion. Both broadly-reactive canonical and pathogen-selected non-canonical Vδ1 clones contributed to memory responses to Lm and STm. Interestingly, some non-canonical γδ T cell clones selected by Lm infection also responded after STm infection, suggesting some level of cross-reactivity. These findings underscore the promiscuous nature of memory γδ T cells and suggest that pathogen-elicited memory γδ T cells are potential targets for broad-spectrum anti-infective vaccines.
Keywords: γδ T cells, resident memory, mucosal immunity, Listeria monocytogenes, enteric infection
INTRODUCTION
Mucosal tissues are critical interfaces with the environment for the maintenance of the body’s integrity. These barriers are constantly exposed to a wide range of stresses that need local immune monitoring to promote health. As such, mucosa resident leukocytes are crucial patrolling sentinels that must eliminate invading pathogens while maintaining a beneficial relationship with harmless commensal microorganisms and other innocuous antigens.1‘ 2 γδ T cells are unconventional lymphocytes ideally located within these barrier tissues where they provide a multitude of functions.3 In mice, most mucosal resident γδ T cells harbor invariant or semi-invariant (pairing of an invariant and a variable chain) T cell receptors (TCR)4 and are thought to respond in a TCR independent, pre-programmed fashion to cytokines or pathogen products in the periphery.5, 6 In humans, the thymus supports the development of semi-invariant type 1 and type 3 γδ T cell subsets with innate-like transcriptomic signatures that persist into adulthood.7 Because of these features, these γδ T cell subsets are commonly seen as innate-like cells.
Although immunological memory has largely been defined in the context of conventional antigen-specific αβ T cells and B cells, the recent identification of unconventional memory responses has expanded this view.8 Several studies have demonstrated that murine γδ T cells can mount long-lasting memory responses, extending the observations made more than a decade ago in non-human primate infection models.9 Using a mouse-adapted Listeria monocytogenes (Lm) strain,10 we previously showed that foodborne infection of mice generates a multifunctional resident memory γδ T cell population in the intestines and draining mesenteric lymph nodes (MLN) that is maintained in the absence of antigenic stimulation.11 This population is characterized by a CD44hi CD27neg phenotype and the expression of a semi-invariant Vγ4Vδ1 TCR (nomenclature12), consisting of an invariant, canonical Vγ4 chain associated with a more diverse Vγ1 chain. Upon secondary exposure, memory γδ T cells collaborate with conventional T cells to provide optimal protection by orchestrating the formation of organized granulomatous structures that restrain Lm growth in the MLN through IL-17A production.11, 13 Memory γδ T cell responses were subsequently reported in response to other bacterial infections14–16 and imiquimod-induced skin inflammation.17, 18 Surprisingly, several of these memory responses mobilized innate-like γδ T cells expressing semi-invariant TCR, suggesting that the role of these cells is more complex than originally proposed.
γδ T cell specificity remains mostly elusive. Nevertheless, these lymphocytes respond to a large variety of host-derived and microbial antigens that are by nature distinct from αβ T cells.19 The antigen reactivity of the different memory γδ T cell subsets reported so far remains unknown. The ligand of the canonical Vγ4Vδ1 TCR is expressed or presented by peritoneal macrophages and upregulated after intraperitoneal (i.p.) Lm infection, providing evidence of a conserved host-derived antigen consistent with the semi-restricted TCR repertoire of this γδ T cell subset.20 However, we previously showed that Lm-elicited Vγ4Vδ1 memory T cells do not respond to foodborne Salmonella enterica serovar Typhimurium (STm) infection, indicating contextual specificity to Lm.11 Similarly, Bordetella pertussis (Bp)-induced Vγ2 memory T cells demonstrated some level of specificity to Bp,15 suggesting that the ligands recognized by these two memory γδ T cell subsets may be of bacterial origin. These observations suggest that memory γδ T cells could be selected by both host derived and bacterial antigens. In line with this, the Vγ4Vδ1 T cell population elicited by foodborne Lm infection comprises both canonical and non-canonical subpopulations that may have distinct specificities and could be differentially selected by host-derived and microbial antigens upon during infection. However, it remains undefined whether exposure to Lm induces the clonal expansion and selection of Vγ4Vδ1 T cells that is commonly associated with antigen recognition and indicative of T cell specificity.
The tight association of Lm-elicited memory γδ T cells with the gastrointestinal system and their semi-invariant TCR suggest a broad role in immunosurveillance at this major entry point for pathogens. Thus, the reactivity of these adaptive γδ T cells was evaluated in response to the foodborne pathogens Lm, Yersinia pseudotuberculosis (Yp) and Citrobacter rodentium (Cr) and the murine model of STm-induced colitis. These pathogens represent a broad group of bacteria with diverse replicative life cycles. Instead of a tightly restricted specificity, our data revealed a broad TCR-mediated reactivity of Lm-elicited memory Vγ4Vδ1 T cells against pathogenic bacteria that is driven by a combination of clones expressing canonical and non-canonical Vδ1 TCRs. Although the majority of the response was carried out by cells expressing canonical TCRs, a substantial proportion of non-canonical clones were expanded upon Lm or STm infection, indicating that some form of selection occurs after infection. Interestingly, a very small number of non-canonical clones appeared to cross-react against both Lm and STm-induced colitis infections, contributing to both responses. Collectively, these findings reveal degrees of adaptive γδ T cell specificity and broad-spectrum reactivity that may provide new cellular targets for anti-infective vaccines.
RESULTS
Involvement of the TCR in γδ T cell responses to foodborne Lm infection.
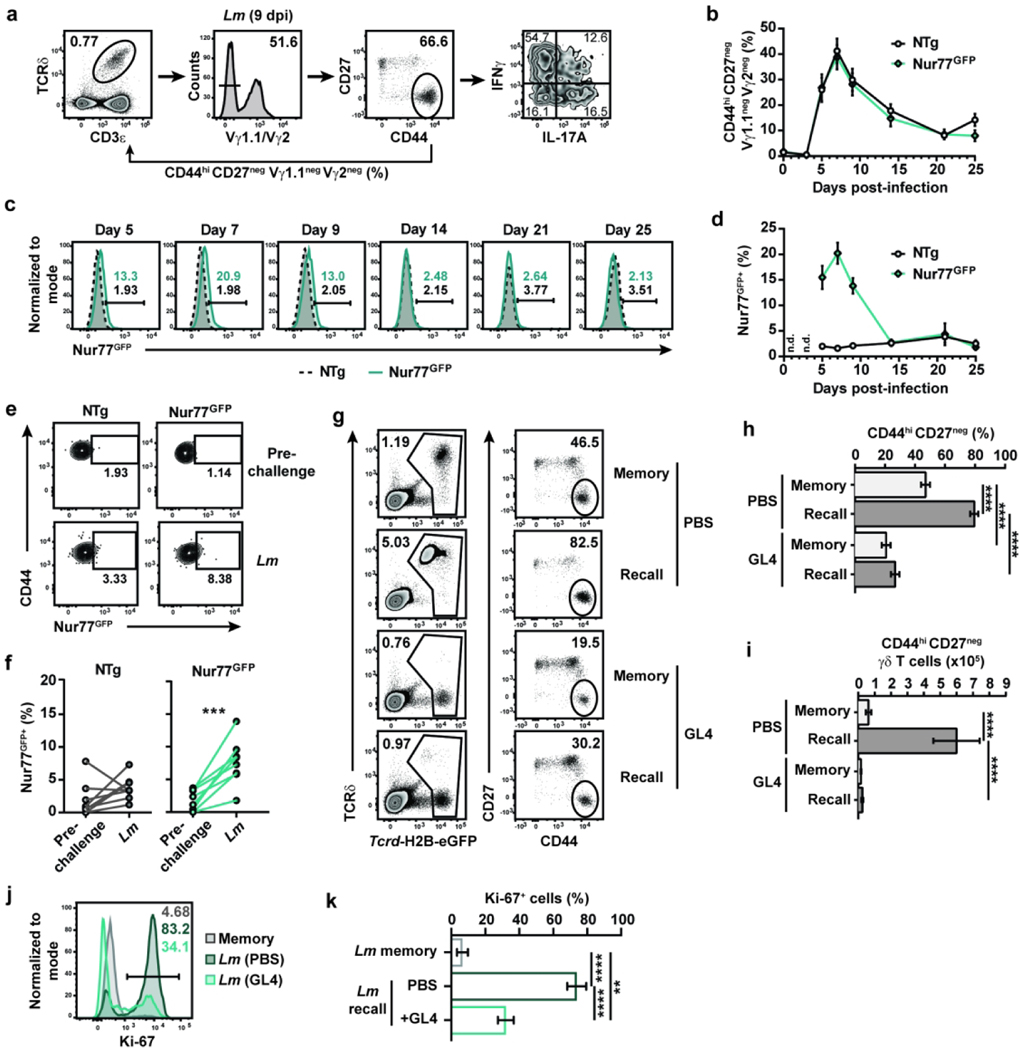
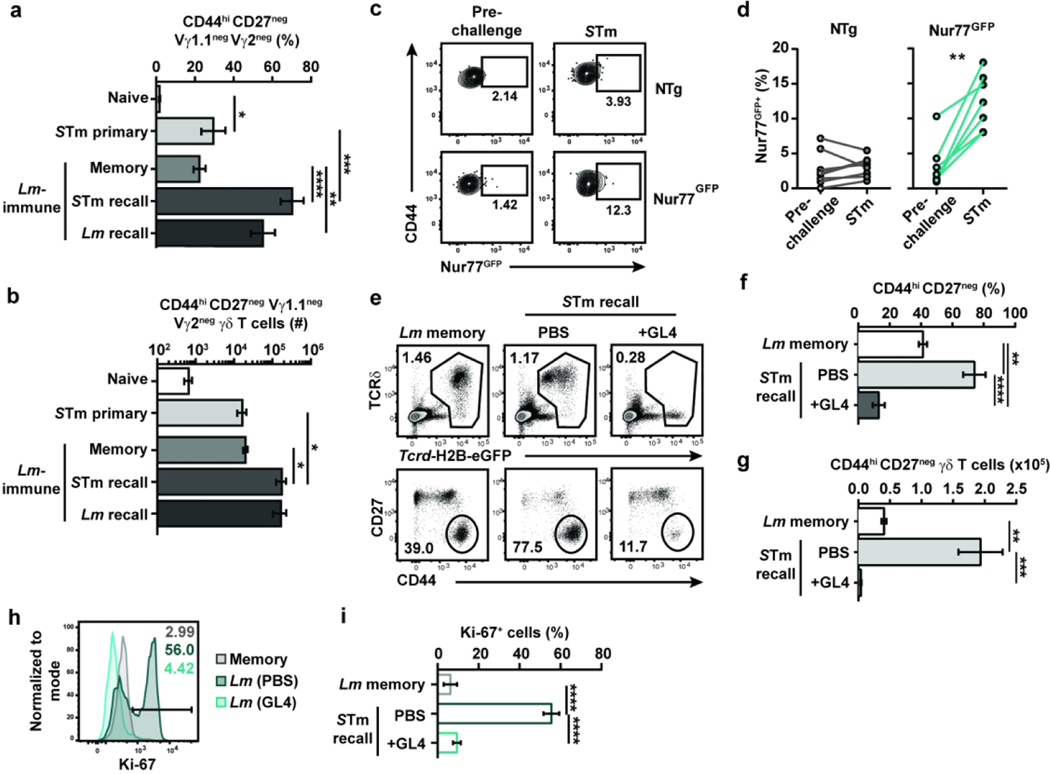
The protection provided by memory γδ T cells upon secondary Lm exposure relies on surface expression of the γδTCR,11 suggesting that TCR signaling is required for the recall of memory γδ T cells. To assess whether TCR signaling was elicited during the immune response to foodborne Lm infection, longitudinal analysis of circulating γδ T cells was performed in Nur77GFP and control non-transgenic (NTg) mice. Nur77GFP reporter mice are commonly used to assess TCR signaling in T cells as Nur77 is an orphan nuclear receptor that is upregulated upon TCR-mediated activation.21 Of note, Lm-elicited γδ T cells are generated and similarly multifunctional in both Balb/c and C57Bl/6 mice after foodborne Lm infection (11 and unpublished data). Consistent with our previous observations, Lm-elicited γδ T cells (identified as CD44hi CD27neg Vγ1.1neg Vγ2neg, see Fig. 1a) were mobilized into the blood as early as 5 days after infection, peaked around day 7 and contracted thereafter (Fig. 1b). A significant fraction of cells was GFP+ from 5 to 9 days post-infection and GFP expression rapidly vanished during contraction (Fig. 1c and d). Lm challenge infection of Lm-immune Nur77GFP mice also resulted in an increase of GFP+ cell frequency in the blood 4 days after secondary infection (Fig. 1e and f). To assess TCR mediated induction of Nur77GFP expression, we stimulated MLN single cell suspensions from Lm-immune Nur77GFP and NTg mice with anti-CD3ε and anti-CD28 or anti-TCRδ (clone GL4) antibodies and compared the expression of Nur77GFP in conventional and unconventional T cells. Nur77GFP expression was robustly upregulated in activated CD44hi CD4+ and CD8+ T cells (Fig. S1a to c). TCR engagement on CD44hi CD27neg γδ T cells led to TCR internalization and Nur77GFP expression, albeit to a lesser degree than conventional T cells (Fig. S1d to g). Hence, TCR signaling was engaged in Lm-elicited γδ T cells during both effector phases of the immune response to Lm.
Figure 1. TCR signaling shapes the Vγ4Vδ1 T cell response to foodborne Lm infection.
(a) Gating strategy for identifying CD44hi CD27neg Vγ4 T cells. The percentage of CD44hi CD27neg Vγ1.1neg Vγ2neg cells reported in the figures is among total γδ T cells and is the compound of the percentage of cells with the gates shown in the Vγ1.1/Vγ2 and CD44 vs CD27 flow plots. (b-d) CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cell frequency among total γδ T cells (b) and GFP expression among CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells (c and d) in the blood of Nur77GFP and non-transgenic (NTg) C57BL/6NJ mice at the indicated times after foodborne infection with Lm. (c) Concatenated histograms of GFP expression among CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells are shown. (d) Graph shows the mean ± SEM and is representative of 2 independent experiments with 7 to 14 mice/group. n.d.: not determined. (e and f) Longitudinal analysis of GFP expression among circulating CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells pre-challenge (25 to 31 days post-infection) and 4 days after Lm challenge infection of Lm-immune Nur77GFP and NTg mice. (e) Representative flow plots of GFP expression are shown. (f) Cumulative data from 2 independent experiments (n=2–5 mice/group/experiment) are shown. Each line represents paired data from the same mouse pre- and post-challenge. (g-i) Lm-immune Tcrd-H2B-eGFP (Balb/cJ background) mice were treated with either PBS or GL4 mAb and either challenged with Lm (“Recall”) or given PBS (“Memory”). γδ T cells (identified as TCRβneg CD8αneg GFP+ single live lymphocytes) were analyzed 5 days later in the MLN. (g) Representative flow plots are shown. The graphs depict the cumulative data of 2 independent experiments as the mean ± SEM (n=3–4 mice/group/experiment) of CD44hi CD27neg γδ T cell frequency among total γδ T cells (h) and absolute number (i). (j and k) PBS- and GL4-treated Lm-immune Tcrcf-H2B-eGFP mice were challenged with Lm, and CD44hi CD27neg γδ T cells were analyzed for Ki-67 expression 4 days later. (j) Representative expression of Ki-67 is shown. (k) Graph shows the cumulative data of 2 independent experiments as the mean ± SEM (n=3 mice/group/experiment) of Ki-67+ cell frequency. **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001
We next evaluated whether expression of the γδTCR regulates the recall response to foodborne Lm infection in γδ T cell reporter Tcrd-H2B-eGFP mice.22 γδ T cells in these mice constitutively express GFP and are identifiable in absence of surface expression of the γδTCR (Fig. 1g). Treatment of Lm-immune Tcrd-H2B-eGFP mice with the anti-TCRδ antibody clone GL4 induces the internalization of the TCR rather than depleting γδ T cells (Fig. 1g to i;11), although a significant decrease in the frequency of CD44hi CD27neg γδ T cells was noted (Fig. 1h). GL4 treatment abrogated CD44hi CD27neg γδ T cell expansion upon Lm challenge (Fig. 1h and i) and significantly inhibited their proliferation, as indicated by the substantial reduction in Ki-67 expression (Fig. 1j and k). Similar results were obtained in mice treated with the UC7–13D5 antibody, which also triggers the internalization of the γδTCR (Fig. S2a;22), although the effect was less robust (Fig. S2b to d). Consistent with these observations, TCR internalization did not overtly activate CD44hi CD27neg γδ T cells as IFNγ and IL-17A were not induced after GL4 treatment (Fig. S2e and f). Importantly, TCR internalization did not elicit a nonresponsive state as γδ T cell function was largely intact in response to TCR-independent stimulation with PMA and ionomycin (Fig. S2g to j). A subtle but significant decrease in IL-17A production was observed with or without stimulation in the GL4-treated group. As Lm burden is comparable between infected GL4- and PBS-treated Lm-immune mice,11 this suggests that surface expression of the TCR provides critical signals that mediate anamnestic γδ T cell responses.
Canonical and non-canonical Vδ1 clones participate in anamnestic responses to Lm.
The TCR dependency described above suggests that memory γδ T cells respond to antigens that are expressed or upregulated during infection. Effector γδ T cells elicited by primary Lm infection revealed an almost exclusively canonical Vγ4 chain but showed some diversity in the Vδ1 TCR repertoire,11 suggesting that variations in the CDR3δ sequence might influence the cell’s specificity and reveal some TCR-mediated selection. However, this previous study relied on traditional approaches that lacked sequencing depth as only 30 Vδ1 TCR sequences were assessed. Therefore, deep sequencing approaches were used to analyze the repertoire of sort-purified Lm-elicited Vγ4Vδ1 T cells. Since no antibody specific for the Vγ4 TCR chain was available until very recently, an indirect strategy to identify Vγ4Vδ1 T cells for cell sorting and flow cytometry analysis was used (Fig. S3a). Analysis of CDR3γ sequences confirmed that the vast majority of sorted cells expressed TRGV6 transcripts (which encodes Vγ4), of which >96% were canonical and consistent with our published observations.11 Although some of the transcripts recovered represented other TRGV gene segments, particularly TRGV2 (which encodes Vγ1.2; Fig. S3b), the presence of non-Vγ4+ cells in our sorting strategy was minimal as assessed by flow cytometric analysis using the Vγ4-specific 1C10–1F7 antibody.23 Indeed, virtually all the cells in the sorted gate were positively stained with the 1C10–1F7, but not the Vγ1.1/Vγ1.2 cross-reactive 4B2.9 antibody (Fig. S3c to e). Thus, this strategy faithfully identified Vγ4 T cells.
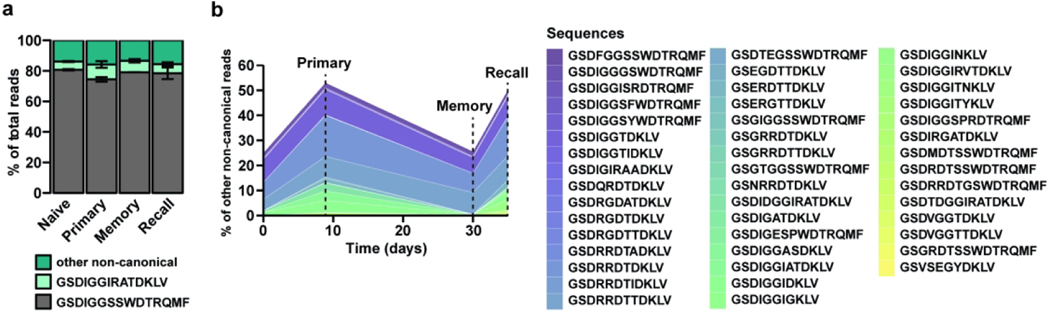
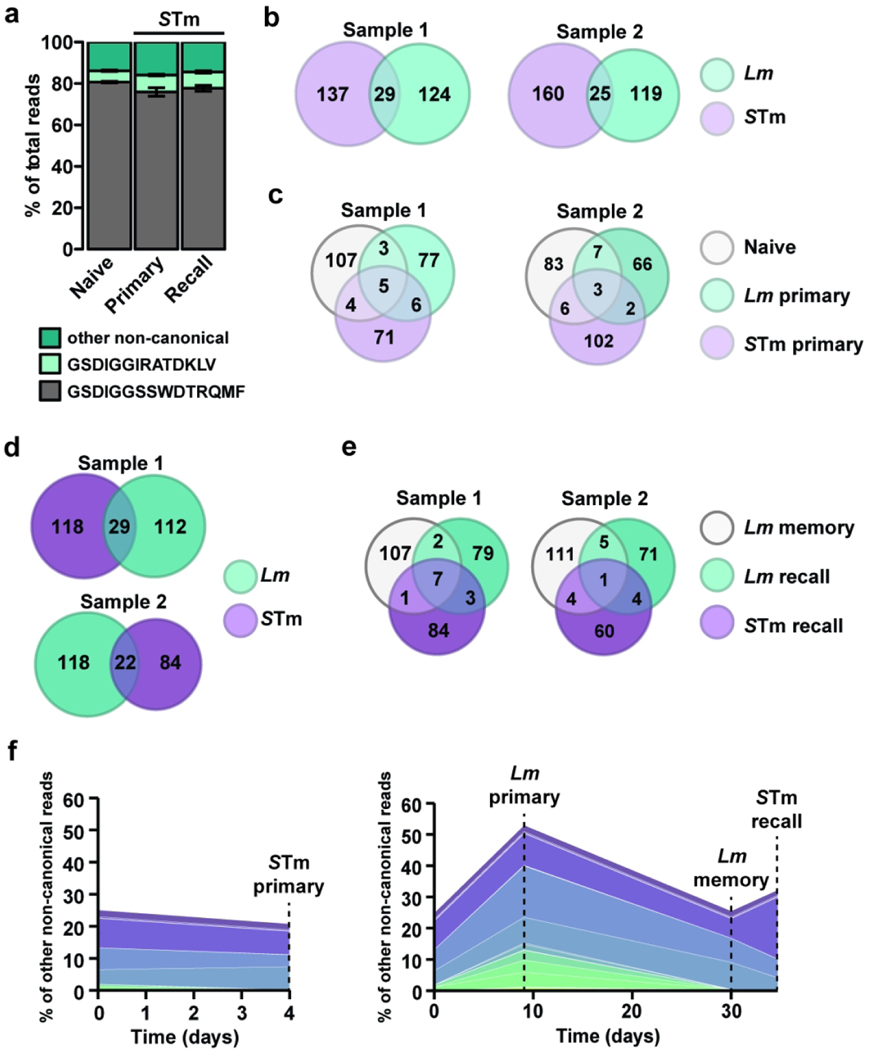
To assess whether Lm infection leads to the selection of specific Vδ1 clones, CDR3δ analysis was performed on two independent biological replicates throughout the different phases of the immune response (naïve and Lm primary, memory and secondary responses). As expected, the invariant canonical GSDIGGSSWDTRQMF sequence represented most of the repertoire throughout the immune response, followed by the non-canonical public (a rearranged TCR shared among most individuals) GSDIGGIRATDKLV sequence (Fig. 2a). However, a sizeable population did not express either conserved TCRs and will be referred to as “non-canonical” hereafter. Interestingly, a subset of non-canonical clones expanded upon primary infection, contracted at memory and expanded after recall challenge (Fig. 2b), a behavior reminiscent of antigen-specific conventional memory T cells. Similar to the heterogeneity of a polyclonal T cell response, the non-canonical population consisted mostly of infrequent clones but also included abundant clones (<0.5% or between1.5 and 17% of the non-canonical reads, respectively). These data show that while Lm infection expands the canonical and public Vγ4Vδ1 TCR, it also selects for specific non-canonical Vγ4Vδ1 TCR clones, suggesting that the TCR can impart some degree of specificity to adaptive γδ T cell responses.
Figure 2. Canonical and non-canonical clones contribute to the Vδ1 T cell recall response to Lm challenge infection.
CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells were sort purified from the MLN of mice undergoing a primary, memory or recall response to foodborne Lm infection. CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells were sort purified from the MLN, spleen and pLN from naïve mice. CDR3δ transcripts were sequenced and Vδ1 sequences selected for further analyses. (a) Percent of total reads mapping to the canonical (gray), public non-canonical (light green), or other non-canonical sequences (dark green), in the Naive, Primary, Memory and Recall samples averaged across two biological samples. (b) Percent of non-canonical reads from two combined biological samples mapping to sequences that are expanded in Primary vs Naïve and Recall vs Memory conditions. Each color represents an individual clone to aid visualization.
Microbiota is not necessary for CD44hi CD27neg γδ T cell response to Lm infection.
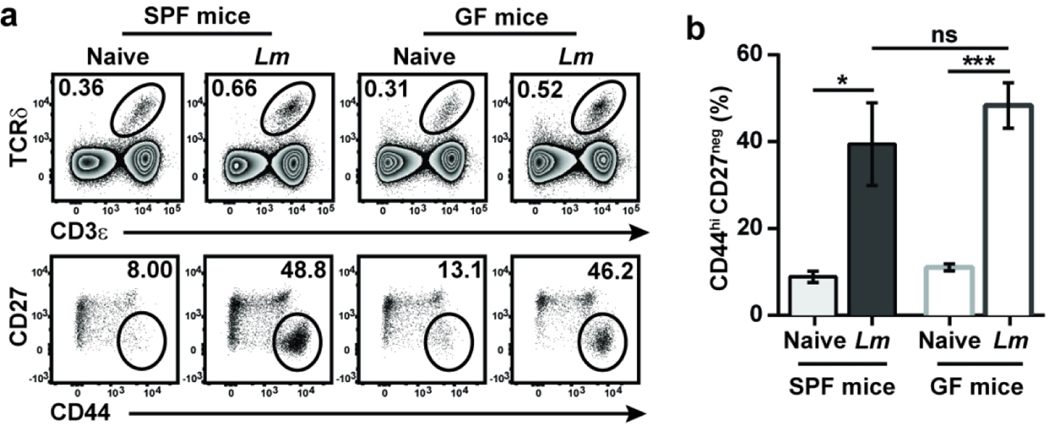
Since the majority of cells responding to Lm expressed the canonical TCR and because the ligand for the canonical Vγ4Vδ1 TCR appears to be a conserved, host-derived molecule whose expression on macrophages is regulated by infection,20 we hypothesized that some of the elicited γδ T cells might respond to other bacteria. Acute enteric infections can transiently disrupt intestinal barrier integrity, allowing commensals to disseminate extra-intestinally and induce pathogen- as well as commensal-specific conventional T cells.24Several reports have shown that subsets of γδ T cells can also be differentially modulated by the microbiota, including in the intestines.9, 25 Because Lm-elicited adaptive γδ T cells share several phenotypic and functional features with commensal-induced γδ T cells, we evaluated whether CD44hi CD27neg γδ T cells may be responding to disseminating commensals after oral Lm infection. The absence of a microbiota did not appear to impact the development of this subset at steady state as CD44hi CD27neg γδ T cells in the MLN were present at comparably low frequencies in naïve specific pathogen-free (SPF) and germ-free (GF) mice (Fig. 3a). After oral Lm infection, a similar proportion of CD44hi CD27neg γδ T cells were elicited in the MLN of SPF and GF mice (Fig. 3b), demonstrating that the microbiota is not necessary for the induction of a primary adaptive γδ T cell response after oral Lm infection.
Figure 3. Bacterial infection can elicit adaptive γδ T cell responses independent of the microbiota.
SPF and GF mice were left uninfected or foodborne infected with Lm, and γδ T cells were analyzed in the MLN at 9 dpi. (a) Representative dot plots are shown. (b) Cumulative data of the frequency of CD44hi CD27neg γδ T cells among total γδ T cells from two independent experiments are shown as mean ± SEM (n=3–6 mice/group/experiment). *, p ≤ 0.05; ***, p ≤ 0.001
Multiple pathogenic bacteria elicit a multifunctional response from memory Vγ4Vδ1 T cells ex vivo.
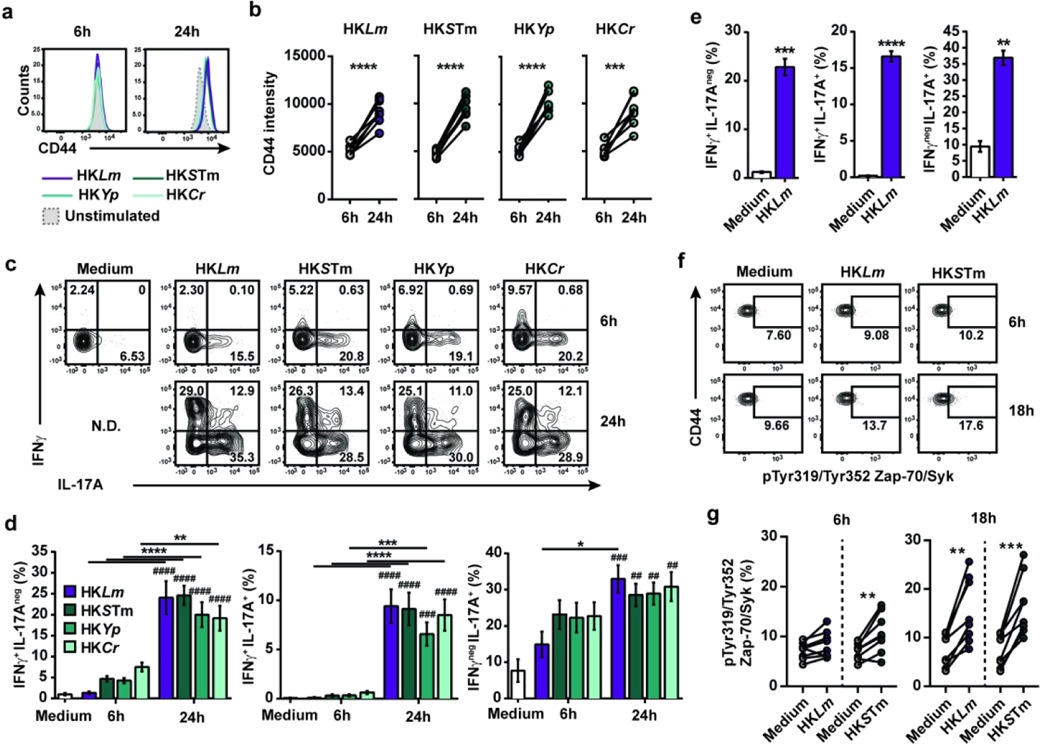
To further assess specificity, the adaptive Vγ4Vδ1 T cell response to multiple pathogens was evaluated. To this end, bulk MLN cells from Lm-immunized mice were stimulated with diverse heat-killed (HK) mouse or human pathogens including Lm, STm, Yp and Cr. CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells showed a significant increase in CD44 expression with all pathogens between 6 and 24 hours of culture (Fig. 4a and b), which was accompanied by a robust and multifunctional effector response involving IFNγ+ producers and IFNγ+ IL-17A+ co-producers at 24 but not 6 hours (Fig. 4c and d). IL-17A+ single producers were readily detectable at 6 hours and further increased at 24 hours. These multifunctional responses were not observed at 24 hours in the absence of stimulation (Fig. 4e). Interestingly, Vγ2+ T cells also produced IL-17A after 24 hours (Fig. S4a and b), but only memory Vγ4Vδ1 T cells produced IFNγ under these conditions despite the presence of IFNγ-biased Vγ1.1+ and CD27+ Vγ4 T cells (Fig. S4c to f). These results demonstrate that Lm-elicited memory Vγ4Vδ1 T cells have the capacity to respond to diverse bacteria.
Figure 4. Multifunctional response of Lm-elicited memory γδ T cells to heterologous enteropathogenic bacteria ex vivo.
Single cell suspensions of MLN from Lm-immune mice were stimulated for 6, 18 or 24 hours with the indicated heat-killed (HK) bacteria (MOI 10) or left unstimulated (medium), and memory CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells were analyzed. (a) Representative histograms of CD44 expression are shown. (b) Scatter plots show the mean ± SEM of CD44 MFI. (c) Representative flow plots of IFNγ and IL-17A production are shown. (d) Bar graphs show the mean ± SEM percentage of cytokine-producing cells. Significant differences with the unstimulated sample are depicted by # symbols. Differences between stimulated samples are represented by * symbols. All graphs depict the cumulative data from 2 independent experiments (n=4 biological replicates/condition/experiment). (e) Bar graphs show the mean ± SEM (n=4–5 samples/condition/experiment) percentage of cytokine-producing memory CD44hi CD27neg Vγ1. 1neg Vγ2neg γδ T cells after 24 hour-stimulation with HKLm and are representative of 2 independent experiments. (f) Representative flow plots of pTyr319/Tyr352 Zap-70/Syk expression by memory CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells after stimulation with the indicated HK bacteria for 6 or 18 hours. Gates were based on individual FMO controls. (g) Scatter plots depict the cumulative data of 2 independent experiments as the mean ± SEM percentage of pTyr319/Tyr352 Zap-70/Syk+ cells among memory CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells (n=4 samples/condition/experiment). A paired analysis was performed to determine significant differences between conditions. *, p ≤ 0.05; ** and ##, p ≤ 0.01; *** and ###, p ≤ 0.001; **** and ####, p ≤ 0.0001
Given the involvement of the TCR in Lm-elicited Vγ4Vδ1 T cell responses to Lm in vivo (Fig. 1), we assessed whether TCR signaling was elicited in cultures stimulated with HKLm and HKSTm. Phosphorylation of the TCR proximal tyrosine kinases Zap-70 and Syk was used to identify TCR signaling. Consistent with the minimal cytokine production observed in 6-hour cultures, only a slight increase in the phosphorylation of Zap-70/Syk was observed with HKSTm but not HKLm (Fig. 4f and g). However, a significant increase in pZap-70/Syk was detected in both cultures at 18 hours, demonstrating that TCR signaling occurred in CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells in response to HK bacteria. Collectively, these data show that Lm-elicited memory Vγ4Vδ1 T cell contact with diverse enteric bacteria induces TCR signals and results in a delayed multifunctional response.
Adaptive γδ T cells respond to diverse bacterial infections in vivo.
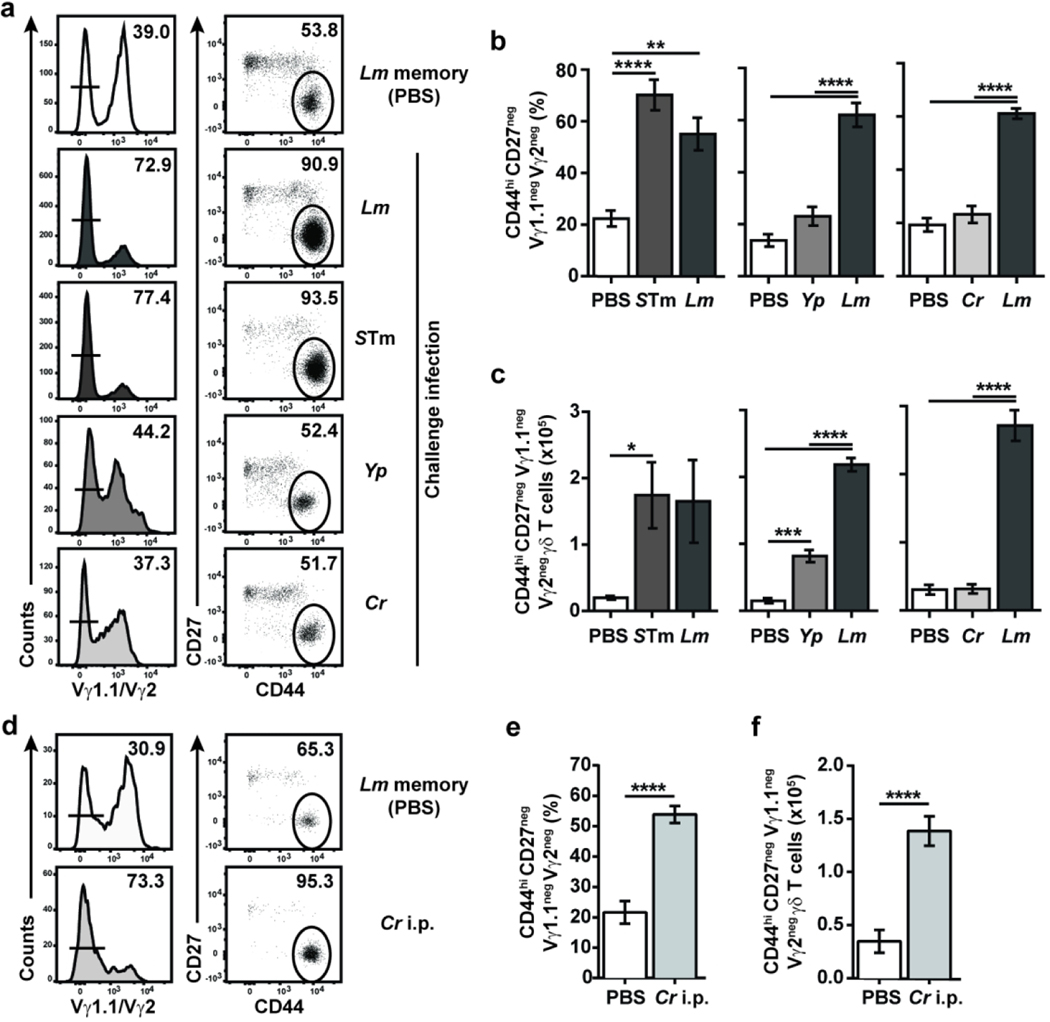
As Lm-elicited memory Vγ4Vδ1 T cells responded to diverse bacteria ex vivo, the response of Lm-elicited memory Vγ4Vδ1 T cells to distinct foodborne pathogens was evaluated in vivo. First, a murine model of STm infection-induced colitis, which closely recapitulates human STm-induced gastroenteritis,26, 27 was used in Lm-immune mice.28, 29 A significant and robust expansion of CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells was observed that was remarkably similar to the recall response induced by Lm challenge (Fig. 5a to c). Foodborne infection of Lm-immune mice with the zoonotic pathogen Yp, which has a different life cycle and colonizes intestinal and extra-intestinal sites in both humans and mice,30, 31 induced a modest increase in the numbers of CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells (Fig. 5c). In contrast, foodborne infection of Lm-immune mice with Cr, a murine pathogen widely used to model human enteropathogenic and enterohaemorrhagic E. coli infection,32 failed to induce a response of CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells in the MLN at 5 days post infection (dpi) (Fig. 5b and c). In contrast to the previously used pathogens which efficiently disseminate to the MLN, Cr is primarily confined to the lumen in immunocompetent hosts.32, 33 Since this spatial constraint may contribute to Cr’s inability to expand memory Vγ4Vδ1 T cells in vivo, Lm-immune mice were i.p. infected with Cr to bypass the gut epithelium. This led to the early colonization of the MLN and spleen (Fig. S5) and a robust response of CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells in the MLN at 5 dpi (Fig. 5d to f). Similar response patterns were observed after primary infection of naïve mice, as STm infection-induced colitis, i.p. Cr infection and, to a lesser extent, Yp infection elicited a CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cell population, whereas foodborne Cr infection failed to do so (Fig. S6). Although the level of response varied between pathogens, these data demonstrate that intestinal adaptive Vγ4Vδ1 T cells can respond to diverse bacterial pathogens with distinct replicative life cycles and suggest that the adaptive Vγ4Vδ1 T cell response is a common feature of several bacterial pathogens. Taken together, these findings indicate the broadly reactive nature of adaptive Vγ4Vδ1 T cells.
Figure 5. Differential response of memory γδ T cells to heterologous bacterial challenges in vivo.
(a-c) Lm-immune mice were left unchallenged (PBS) or challenged with the indicated bacteria. γδ T cells were analyzed in the MLN at 4 days post-recall (dpr) for STm and 5 dpr for Yp- and Cr-challenged mice. Lm-challenged mice were analyzed at the same time as the experimental group. (a) Representative flow plots are shown. Data represent the mean ± SEM (n=3–6 mice/group) of the percentage of CD44hi CD27neg Vγ1.1neg Vγ2neg T cells among total γδ T cells (b) and their absolute numbers (c) and are representative of 2 independent experiments. (d-f) Lm-immune mice received PBS (Memory) or were i.p challenged with Cr. After 5 days, γδ T cells were analyzed in the MLN. (d) Representative flow plots are shown. Data depict the mean ± SEM (n=3–5 mice/group/experiment) of the percentage of CD44hi CD27neg Vγ1.1neg Vγ2neg T cells among total γδ T cells (e) and their absolute numbers (f) from the pool of 2 independent experiments. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001
Lm-elicited γδ T cell recall responses to STm and i.p. Cr infections are mediated by the γδTCR.
A key feature of memory T cells is their ability to respond efficiently upon antigen reencounter. Therefore, a side-by-side comparison of the CD44hi CD27neg Vγ4 T cell response to STm infection in naïve and Lm-immune mice was performed. STm infection of Lm-immune mice elicited a robust response that was qualitatively similar to Lm recall infection (Fig. 6a and b). Additionally, STm infection of Lm-immune mice led to a significantly greater percentage and number of CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells compared to STm infection of naïve mice (Fig. 6a and b). These data suggest that STm infection induces a recall response of Lm-elicited memory γδ T cells.
Figure 6. The γδTCR critically regulates Lm-elicited γδ T cell recall-like response to STm infection.
(a and b) Lm-immune mice received PBS (Memory) or were challenged with either STm (STm recall) or Lm (Lm recall). Naïve mice were either given PBS (Naïve) or infected with STm (STm primary). After 4 days, γδ T cells were analyzed in the MLN. Data show the mean ± SEM (n=3–6 mice/group) of the percentage of CD44hi CD27neg Vγ1.1neg Vγ2neg T cells among total γδ T cells (a) and their absolute numbers (b) and are representative of 2 independent experiments. (c and d) GFP expression among circulating CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells pre-challenge (25 to 31 days post-infection) and 4 days after STm challenge infection of Lm-immune Nur77GFP or non-transgenic (NTg) C57BL/6NJ mice. (c) Representative flow plots of GFP expression among CD44hi CD27neg Vγ1. 1neg Vγ2neg γδ T cells are shown. (d) Cumulative data from 2 independent experiments (n=2–5 mice/group/experiment) are shown. Each line represents paired data from the same mouse pre- and post-challenge. (e-i) Lm-immune Tcrd-H2B-eGFP mice were treated with PBS or GL4 mAb and challenged with STm. γδ T cells (CD4neg CD8αneg or TCRβneg CD8αneg GFP+ single live lymphocytes) were analyzed in the MLN at 4 dpr. (e) Representative flow plots are shown. Cumulative data show the mean ± SEM (n=3–4 mice/group/experiment) of the frequency of CD44hi CD27neg γδ T cells among total γδ T cells (f) and their absolute number (g) and depict 2 independent experiments. (h) Representative histograms of Ki-67 expression by CD44hi CD27neg γδ T cells are shown. (i) Graph shows the cumulative data of 2 independent experiments as the mean ± SEM (n=3 mice/group/experiment) of Ki-67+ cell frequency. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001
Given the critical role of the γδTCR in mediating Lm-elicited memory γδ T cell recall response to Lm (Fig. 1), the importance of TCR signaling was assessed. Longitudinal analysis of circulating cells revealed a significant increase in the frequency of Nur77GFP-expressing CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells 4 days after STm challenge infection (Fig. 6c and d). Furthermore, internalization of the γδTCR in GL4-treated mice prevented CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cell expansion (Fig. 6e to g and S7a) and substantially reduced their proliferation (Fig. 6h and i) following STm challenge infection. However, GL4 treatment did not affect weight loss, fecal shedding, or MLN STm burden (Fig. S7b to d). We previously showed that Lm-elicited γδ T cells provide protection against secondary Lm infection in concert with conventional T cells and that GL4 treatment alone does not affect Lm burden.11 To determine whether TCR signaling contributes to the control of STm infection, we evaluated the effect of GL4 treatment on the ability of CD4- and CD8-depleted Lm-immune mice to control STm burden (Fig. S7e). Although STm burden in the MLN was similar between GL4- and PBS-treated mice, GL4 treatment resulted in a significant increase in STm burden in the spleen (Fig. S7f). These results reveal that the γδTCR contributes to the optimal control of STm and suggest that STm-selected and cross-reactive γδ T cells can restrict STm dissemination to the spleen or mediate local anti-bacterial effects.
GL4 treatment also abrogated CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cell response to i.p. Cr infection without affecting weight loss (Fig. S8). Altogether, these data suggest that the γδTCR is integral to the recall response of Lm-elicited memory Vγ4Vδ1 T cells to heterologous infection and suggest that cells responding to STm in Lm-immune mice arise from the Lm-elicited memory pool.
γδ T cell responses to Lm and STm infections involve cross-reactive and non-overlapping pathogen-selected non-canonical clones.
Recognition of cognate antigen elicits robust T cell clonal expansion that leads to changes in clone abundance and alterations in TCR repertoire. To determine whether Lm-elicited memory Vγ4Vδ1 T cell clones respond to STm, the TCRδ repertoire of purified CD44hi CD27neg Vγ4 T cells elicited by STm infection in naïve (STm primary) and Lm-immune mice (STm recall) were analyzed and compared to sorted Lm-elicited cells from age-matched mice (Fig. 2). The canonical and the public Vδ1 TCRs represented the majority of (~85%) the repertoire of STm primary and STm recall samples, regardless of prior exposure to Lm (Fig. 7a). Interestingly, STm and Lm primary infections selected for largely non-overlapping non-canonical clones (Fig. 7b). Only 29 and 25 overlapping clones were detected. The two infections also differentially affected the prevalence of overlapping non-canonical clones as the majority of the clonotypes analyzed showed a selective enrichment (>25% difference in read frequency) after either primary infection (Fig. 7c). Similar observations were made in Lm-immune mice infected with Lm or STm (Fig. 7d and e), suggesting that prior exposure to Lm does not impede the generation of a subsequent non-canonical response to an unrelated pathogen. Overall, these results suggest that the non-canonical Vγ4Vδ1 repertoire is differentially shaped by unrelated infections, consistent with clonal selection mediated by distinct antigens.
Figure 7. Lm and STm-colitis infections select for shared cross-reactive and distinct pathogen-specific non-canonical Vδ1 T clones in vivo.
CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells were sort-purified from the MLN during primary, memory and recall responses to Lm infection, during primary and recall responses to STm infection, or from naïve Balb/c mice (n=6–22 mice/group). CDR3δ transcripts were sequenced and Vδ1 sequences selected for further analyses. (a) Percent of total reads mapping to the canonical (gray), public non-canonical (light green), or other non-canonical sequences (dark green), in the Naive, STm Primary, and STm Recall samples averaged across two biological samples. (b) Venn diagrams show the number of expanded non-canonical clonotypes overlapping between primary STm and primary Lm. (c) Venn diagram shows the read frequency similarity of non-canonical clones present in Naïve, STm primary and Lm primary. Clonotypes with less than 25% difference between conditions are considered similar and shown as shared. (d and e) Similar analyses as in (b) and (c) were performed with Lm memory, STm-colitis challenge of Lm-immune mice and Lm recall samples. (f) Clonotypes demonstrating a conventional memory response pattern to Lm (as identified in Fig. 2b) were analyzed for their primary and recall response to STm. Percent of non-canonical reads from two combined biological samples mapping to the selected sequences in Naïve, STm Primary, Lm Primary, Lm Memory and STm Recall samples.
Although these data suggest that the vast majority of infection-elicited non-canonical Vγ4Vδ1 T cell clones are specific to a given pathogen, the presence of conserved TCR sequences that expand after Lm and STm infections raised the question as to whether some clones could respond to both insults. To tackle this question, the response of the clones identified as Lm-selected clones in Fig. 2b was assessed after primary and recall STm infections. Out of 46 total sequences, 16 clones including one abundant clonotype (>4% of reads) expanded upon primary STm infection and 13 infrequent clones and 1 highly frequent clone (<0.5% and >6% of the reads, respectively) increased upon STm challenge of Lm-immune mice (Fig. 7f and tables S1 and S2). Among these clones, 5 infrequent clones expanded in response to both primary STm infection of naïve mice and challenge STm infection of Lm-immune mice. As such, approximately 9.2% of the non-canonical clones appeared cross-reactive and responded to both Lm and STm infections. Together, our findings reveal a complex heterogeneity of the memory Vγ4Vδ1 T cell population and demonstrate that the broad bacterial reactivity of adaptive Vγ4Vδ1 T cells results from a mixture of broadly reactive canonical and public TCRs, cross-reactive non-canonical TCRs, and pathogen-selected non-canonical TCRs.
DISCUSSION
Here, a broad reactivity of the adaptive CD44hi CD27neg Vγ4Vδ1 T cell subset to gut disseminating pathogens was uncovered. This subset behaved like traditional adaptive T cells in their multifunctional response, timing of accumulation in draining LN, and selection of non-canonical TCR elements. Unlike conventional memory cells, memory Vγ4Vδ1 T cells were broadly reactive to a wide array of pathogenic bacteria capable of extra-intestinal dissemination. TCR sequencing revealed that broad bacterial reactivity was largely due to expansion of the canonical and public TCRs, with some contribution from cross-reactive non-canonical TCRs. Although Vγ4Vδ1 T cells are generally classified as innate-like,34 TCR signaling was activated in adaptive γδ T cells upon exposure to different pathogenic bacteria and their responses were abrogated by anti-TCRδ treatment, providing strong evidence of the crucial role of the γδTCR in regulating the recall response of memory Vγ4Vδ1 T cells. This suggests that antigen recognition or other TCR ligands are essential for their broad pathogen elicited response. While the Vγ4Vδ1 T cell response was broadly reactive to diverse pathogens at the population level, TCR sequencing revealed some level of pathogen specificity among a large subset of non-canonical TCRs. Thus, adaptive Vγ4Vδ1 T cells form a heterogeneous unconventional population that encompasses broadly reactive canonical and non-canonical clones, and pathogen-selected non-canonical clones which may function as gatekeepers against disseminating intestinal infections in the MLN.
Despite a uniform functional response of memory Vγ4Vδ1 T cells to all bacteria used in this study ex vivo, adaptive Vγ4Vδ1 T cells showed distinct response patterns to each pathogen in vivo. Both oral STm and foodborne Yp infection elicited an adaptive Vγ4Vδ1 T cell response, whereas foodborne Cr infection did not. A notable difference between these pathogens is their ability to efficiently disseminate extra-intestinally: while STm and Yp infection results in the colonization of the MLN,28, 31, 35 Cr is less efficient at disseminating to the MLN after foodborne infection.32 Bypassing the intestines by i.p. infection to allow Cr to colonize the MLN restored its ability to trigger a CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cell response. Although continuous sampling of the intestinal lumen by migratory DC is known to permit the dissemination of invasion defective bacteria to deeper tissues,36 our observations suggest that factors besides colonization and DC migration are necessary to elicit an optimal proliferative response from adaptive Vγ4Vδ1 T cells. Because the γδTCR mediates Vγ4Vδ1 T cell expansion and the ligand for the canonical Vγ4Vδ1 TCR is upregulated by infection,20 high pathogen burden or inflammation may be necessary to efficiently modulate the expression of canonical and non-canonical Vγ4Vδ1 T cells. Indeed, intestinal sampling only allows the uptake of a small number of bacteria.36 Furthermore, we previously demonstrated that adaptive Vγ4Vδ1 T cells do not expand in response to invasive salmonellosis, a systemic typhoid-like disease in mice, which results in lower organ burden than the STm-induced colitis model used in this study.11, 35 The inflammatory milieu may also regulate adaptive Vγ4Vδ1 T cell responses. Indeed, this study demonstrated that Lm-elicited memory CD44hi CD27neg Vγ1.1neg Vγ2neg γδ T cells expand in response to STm-induced colitis, whereas they fail to respond to invasive salmonellosis. Although these two models utilize the same pathogen, they elicit substantially different inflammatory responses.28, 29, 35 While the typhoid model is characterized by a diffuse enteritis and mononuclear immune infiltrate,35 the STm infection-induced colitis leads to a massive infiltration of neutrophils and inflammatory monocytes.28, 29 Thus, the respective contribution of inflammatory mediators and bacterial burden in the induction of adaptive Vγ4Vδ1 T cell responses needs further investigation.
In addition to regulating the generation and expansion of adaptive Vγ4Vδ1 T cells in vivo, all bacteria elicited a multifunctional response from Lm-elicited memory Vγ4Vδ1 T cells characterized by co-production of IL-17A and IFNγ ex vivo. As we previously showed that MHC-II+ cells contribute to Vγ4Vδ1 T cell memory and recall effector functions,11 bacterial pathogens may indirectly regulate γδ T cell function through DC activation and processes like antigen presentation or secretion of pro-inflammatory cytokines. Alternatively, adaptive Vγ4Vδ1 T cell responses may also be regulated by pattern recognition receptors (PRR). Indeed, γδ T cells themselves express PRR like NOD1, which has been associated with IFNγ production in IL-17 producing γδ T cells,37 and TLRs which can also mediate their functions even in the absence of TCR signaling.5; 6 These different but not mutually exclusive types of broad sensing modalities may contribute to the broad bacterial reactivity of memory Vγ4Vδ1 T cells.
Although Vγ4Vδ1 T cells are usually considered innate-like cells, TCR signaling was elicited after Lm and STm infection, and the γδTCR was critical for the recall response of Lm-elicited γδ T cells to Lm, STm and Cr infection. Analysis of the CDR3δ sequences suggests that cross-reactive and STm-selected non-canonical Vδ1 clones, in addition to broadly reactive canonical and public clones, account for the recall-like response to STm infection. This complexity is reminiscent of primate Vδ2+ T cells, which can be divided into semi-invariant, innate-like Vγ9+ T cells that sense conserved antigens38, 39 and respond to both Lm and STm,40, 41 and adaptive Vγ9- T cells that have a diverse TCR repertoire and clonally expand upon viral infection.42 The TCR of the semi-invariant human circulating Vγ9Vδ2 and intestinal Vγ4 T cell subsets can function as both an innate receptor that recognizes conserved molecules via its germline-encoded regions and a somatically rearranged receptor that confers restricted antigen specificity.43, 44 Our findings suggest that the adaptive Vγ4Vδ1 T cell response described here may also be mediated by two distinct antigen recognition modalities, with canonical and public Vδ1 clones recognizing conserved, host-derived antigens20 while non-canonical clones are selected by infection-specific ligands.
An outstanding question raised by our observations relate to the respective contributions of canonical and non-canonical clones to the overall intestinal memory Vγ4Vδ1 T cell response. Innate-like Vγ4Vδ1 T cells are important producers of IL-17A in many contexts,45 and contribute to IL-17A production after i.p. Lm infection.46 Similarly, memory Vγ4Vδ1 T cells are the main source of IL-17A early after secondary exposure to Lm.13 This suggests that canonical clones may be poised to rapidly produce large amounts of IL-17A. In contrast, the adaptive response pattern revealed by non-canonical clones and their TCR diversity suggests that these cells may be similar to conventional T cells and undergo analogous activation and differentiation steps. Although most IL-17A-producing γδ T cells develop before birth, several studies demonstrated that de novo generation can occur in the periphery of adult mice upon inflammation.47–49 Peripherally-induced γδ T cells showed some functional plasticity and were capable of co-producing IL-17A and IFNγ in culture.49 These observations suggest these cells could potentially tailor their effector functions to the pathogen encountered and the inflammatory environment they were activated in.
Collectively, the findings reported here reveal a striking heterogeneity of pathogen-reactive CD44hi CD27neg Vγ4Vδ1 memory T cells, which mount robust TCR-mediated adaptive responses to disseminating bacterial pathogens owing to the concurrent involvement of broadly-reactive canonical and public TCRs and pathogen-selected non-canonical TCRs. These responses are reminiscent of the multifunctional adaptive-like V52+ T cell response reported in macaques immunized with an attenuated form of Lm.40, 50, 51 As such, murine adaptive Vγ4Vδ1 T cells share important features with primate Vδ2+ T cells. Interestingly, Lm-elicited Vδ2+ T cells were shown to provide protection to immunized macaques against heterologous infection,40, 50, 51 which could have broad therapeutic implications. As our current understanding of how primate adaptive-like γδ T cells provide protection is limited, the mouse model of foodborne Lm infection provides an opportunity to gain insights into the mechanisms through which memory Vγ4Vδ1 T cells mediate broad-spectrum immunity and to identify means to harness these unconventional cells for anti-infective therapy.
Methods
Mice.
All experiments were performed with female mice, with the exception of experiments performed in Nur77GFP, C57BL/6NJ (NTg) mice and Tcrd-H2B-eGFP mice, which involved both males and females. Balb/c, Nur77GFP and C57BL/6NJ mice were purchased from the Jackson Laboratory (Bar Harbor) and maintained under specific pathogen-free conditions. Tcrd-H2B-eGFP mice were kindly provided by Drs. Bernard Malissen and Immo Prinz, fully backcrossed onto a Balb/c background, and bred at Stony Brook University. Age-matched 9- to 12-week-old mice were used for all experiments. Nur77GFP and C57BL/6NJ mice were infected between 6 and 9 weeks old. Animals were randomly assigned to experimental groups. Males and females were equally distributed between groups to the best extent possible. All animal experiments were conducted in accordance with the Stony Brook University Institutional Animal Care and Use Committee and National Institutes of Health guidelines. Germ-free Balb/c mice were bred and maintained at the animal facility of Pohang University of Science and Technology (Pohang, Republic of Korea). All animal experiments were performed in accordance with institutional guidelines.
Mouse infections and treatments.
Unless otherwise specified, all mouse infections with Lm, Yp and Cr were performed with foodborne infection as previously described.11, 13 Bacterial strains and infection doses are summarized in Table S3. GF and SPF control mice were infected by oral gavage with 1×108 CFU InlAM Lm, strain 10403s due to the increased susceptibility of GF mice to foodborne Lm infection. For STm infections, naïve mice were left untreated or orally treated with 20 mg streptomycin solution in PBS one day prior to intragastric inoculation of STm. Systemic infection with Cr was performed through a single i.p. injection. When indicated, mice were given i.p. injections of 100 μg anti-Cδ TCR (GL4 or UC7–13D5, Bio X Cell), 200 μg anti-CD4 (GK1.5, Bio X Cell) or 500 μg anti-CD8α (2.43, Bio X Cell) antibody, alone or in combination, or PBS on days −3, −1 and +1 relative to recall infection.
Isolation of leukocytes.
MLN were harvested and mechanically dissociated into single-cell suspensions. Viable cells from single cell suspensions were enumerated with the use of a Vi-CELL Viability Analyzer (Beckman Coulter). Cells were then directly stained or used for ex vivo stimulations.
Antibodies and Flow Cytometry.
For surface staining, cells were stained with the antibodies listed in Table S4, live/dead dye (Thermo Fisher Scientific) and anti-CD16/CD32 (Bio X Cell). For detection of Vγ4+ cells, cells were first stained with 20 μg of 1C10–1F7 antibody prior to secondary staining with a polyclonal rat anti-mouse IgG (Invitrogen). Cells were then stained with other antibodies. Intracellular cytokine staining was performed using BD Cytofix/Cytoperm Fixation/Permeabilization kit (BD Biosciences) according to the manufacturer’s instructions. Ki-67 detection was performed using eBioscience Foxp3/Transcription factor staining buffer set according to the manufacturer’s instructions (Invitrogen). For the detection of pTyr319/Tyr352 Zap-70/Syk, cells were sequentially stained with Live/Dead dye, anti-CD3ε and anti-TCRδ antibodies, fixed with 4% paraformaldehyde containing 1.5% methanol, permeabilized using 100% ice-cold methanol and finally stained with the remaining antibodies. All samples were acquired on a LSRFortessa (BD Biosciences). Data were analyzed with FlowJo software (TreeStar).
Heat-killed bacteria preparation.
Heat-killed bacteria were produced by incubating log phase growing bacteria resuspended in PBS at 56°C for 4 hours. Killing efficiency was assessed by plating undiluted bacteria on appropriate agar plates. Absence of colony was confirmed 24–48 hours later.
Ex vivo stimulations.
Single MLN cell suspensions from Lm-immune mice were adjusted to a concentration of 5×106 cells/ml in IMDM media containing 10% FBS, 10mM HEPES, 1mM sodium pyruvate, 2mM GlutaMAX™ supplement and 1X MEM non-essential amino acids solution, all from Thermo Fisher Scientific. Cells were cultured at 37°C, 5% CO2 for the incubation period indicated in the figures. All HK bacteria were added at a MOI of 10. Anti-CD3ε was used at 10 μg/ml, and plates were coated in PBS overnight the day prior to the stimulation. Anti-CD28 antibody was used at 2 μg/ml and added to the culture. GL4 antibody was either used at 1 or 10 μg/ml as indicated, and plates were coated in PBS overnight the day prior to the stimulation. Antibody-mediated stimulations were performed for 6 hours. Intracellular protein transport was blocked by the addition of BD GolgiPlug™ (BD Biosciences) 5 to 6 hours prior to the end of the stimulation.
CDR3δ and CDR3γ sequencing.
Single cell suspensions from MLN (n=6–22 mice/group) were generated as described before from Lm-infected mice at 9 (primary) and 37 (memory) dpi and 5 days after Lm recall infection. Harvest was performed 4 days post-infection for STm infected mice (primary infection and recall infection of Lm-immune mice). Naïve cells were isolated from MLN, spleen and pLN (inguinal, brachial, axillary and cervical). γδ T cells were enriched by negative selection using B220-, CD8α- and CD4-biotin antibodies and MagniSort Streptavidin Negative Selection Beads (Invitrogen). The enriched fraction was then used to sort single live CD44hi CD27neg Vγ4 T cells on a FACSAria Illu (BD) (sorting strategy showed in Fig. S3). RNA was extracted using RNeasy Mini Kit (Qiagen) according to manufacturer’s instructions. Library amplification and sequencing using Illumina MiSeq (CDR3δ) and Illumina MiSeq Nano (CDR3γ) were performed by iRepertoire Inc. (Huntsville).
CDR3δ sequencing post-analysis.
CDR3 clonotypes expressing mTRDV4 in the V region were selected for analysis. mTRDV4 sequences represented 96.04 to 99.89% of the total TCRδ reads analyzed. The canonical GSDIGGSSWDTRQMF and public GSDIGGIRATDKLV sequences were excluded from the detailed analysis, except in Fig. 2a and 7a. Non-canonical clonotypes in Fig. 2b were selected by determining which clonotypes with non-zero reads in the naive sample had a greater number of reads upon primary infection with Lm, and a greater number of reads at Lm recall relative to memory. Frequency of the selected CDR3 clonotypes were subsequently analyzed in STm primary and STm recall samples as shown in Fig. 7f. Venn diagrams in Fig. 7b and 7d show the repertoire overlap between the non-canonical sequences expanded in Lm (read counts Lm primary>Naïve) and STm primary (read counts STm-colitis>Naïve) or Lm and STm recall vs memory samples, respectively. Venn-diagrams based on read frequency similarity (Fig. 7c and 7e) use a 25% threshold calculated using the absolute difference of the percent of total non-canonical reads per compared sample. Clonotypes with percent of non-canonical reads less than 0.01% were excluded to avoid comparing non-existent sequences.
CDR3γ sequencing post-analysis.
Basic analysis of the CDR3γ clonotype representation in each sample was provided by iRepertoire Inc. (Huntsville). Segment usage was represented using GraphPad Prism 6 software (La Jolla, CA).
STm fecal shedding and organ burden.
Fecal pellets were collected, weighed and diluted in PBS. MLN were collected at day 4 and mechanically dissociated through a 70 μm filter. Serial dilutions were prepared in DPBS and plated onto lysogeny agar plates supplemented with 50 μg/ml nalixidic acid.
Statistics.
Statistical analyses were performed in GraphPad Prism software (La Jolla, CA). Ordinary one-way ANOVA with the Tukey multiple comparison test was used for comparison of more than two unpaired groups and two-way ANOVA with the Tukey multiple comparison test for longitudinal group comparisons. Comparison of Nur77GFP expression in the blood between memory and recall time points were performed using Paired Student t test. Differences in pZap-70/Syk expression were determined using paired one-way ANOVA with the Tukey multiple comparison test. Unpaired Student t test was used otherwise. Differences are represented as follow: *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported by NIH grants R01-AI076457 (BSS), R01-AI099222 (JBB), R01-AI101221 (AWMV), K12-GM102778 (ZQ), T32-AI007539 (JNI and THC), and P01-AI056172 (LP), a Mathers Charitable Foundation grant MF-1901-00210 (BSS), project IBS-R005-D1 (KKS) from the Institute for Basic Science, National Research Foundation, Korean Ministry of Science, Information/Communication Technology and Future Planning, and funds provided by The Research Foundation of New York and Stony Brook University. We thank Dr. Pablo Romagnoli for advice and discussion on the manuscript.
Footnotes
DISCLOSURE
The authors declare no conflicts of interest.
DATA AVAILABILITY
The datasets generated during this study are available at the SRA database, under the BioProject accession number PRJNA684361. The codes generated during this study are available upon request.
REFERENCES
- 1.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell 2010; 140(6): 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol 2010; 10(3): 159–169. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen MM, Witherden DA, Havran WL. gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol 2017; 17(12): 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havran WL, Chien YH, Allison JP. Recognition of self antigens by skin-derived T cells with invariant gamma delta antigen receptors. Science 1991; 252(5011): 1430–1432. [DOI] [PubMed] [Google Scholar]
- 5.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009; 31(2): 331–341. [DOI] [PubMed] [Google Scholar]
- 6.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 2009; 31(2): 321–330. [DOI] [PubMed] [Google Scholar]
- 7.Tan L, Fichtner AS, Bruni E, Odak I, Sandrock I, Bubke A et al. A fetal wave of human type 3 effector gammadelta cells with restricted TCR diversity persists into adulthood. Sci Immunol 2021; 6(58). [DOI] [PubMed] [Google Scholar]
- 8.Netea MG, Latz E, Mills KH, O’Neill LA. Innate immune memory: a paradigm shift in understanding host defense. Nat Immunol 2015; 16(7): 675–679. [DOI] [PubMed] [Google Scholar]
- 9.Khairallah C, Chu TH, Sheridan BS. Tissue Adaptations of Memory and Tissue-Resident Gamma Delta T Cells. Front Immunol 2018; 9: 2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollert T, Pasche B, Rochon M, Deppenmeier S, van den Heuvel J, Gruber AD et al. Extending the host range of Listeria monocytogenes by rational protein design. Cell 2007; 129(5): 891–902. [DOI] [PubMed] [Google Scholar]
- 11.Sheridan BS, Romagnoli PA, Pham QM, Fu HH, Alonzo F 3rd, Schubert WD et al. gammadelta T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity 2013; 39(1): 184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garman RD, Doherty PJ, Raulet DH. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell 1986; 45(5): 733–742. [DOI] [PubMed] [Google Scholar]
- 13.Romagnoli PA, Sheridan BS, Pham QM, Lefrancois L, Khanna KM. IL-17A-producing resident memory gammadelta T cells orchestrate the innate immune response to secondary oral Listeria monocytogenes infection. Proc Natl Acad Sci U S A 2016; 113(30): 8502–8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy AG, O’Keeffe KM, Lalor SJ, Maher BM, Mills KH, McLoughlin RM. Staphylococcus aureus infection of mice expands a population of memory gammadelta T cells that are protective against subsequent infection. J Immunol 2014; 192(8): 3697–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misiak A, Wilk MM, Raverdeau M, Mills KH. IL-17-Producing Innate and Pathogen-Specific Tissue Resident Memory gammadelta T Cells Expand in the Lungs of Bordetella pertussis-infected Mice. J Immunol 2017; 198(1): 363–374. [DOI] [PubMed] [Google Scholar]
- 16.Dillen CA, Pinsker BL, Marusina AI, Merleev AA, Farber ON, Liu H et al. Clonally expanded gammadelta T cells protect against Staphylococcus aureus skin reinfection. J Clin Invest 2018; 128(3): 1026–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartwig T, Pantelyushin S, Croxford AL, Kulig P, Becher B. Dermal IL-17-producing gammadelta T cells establish long-lived memory in the skin. Eur J Immunol 2015; 45(11): 3022–3033. [DOI] [PubMed] [Google Scholar]
- 18.Ramirez-Valle F, Gray EE, Cyster JG. Inflammation induces dermal Vgamma4+ gammadeltaT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc Natl Acad Sci U S A 2015; 112(26): 8046–8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chien YH, Meyer C, Bonneville M. gammadelta T cells: first line of defense and beyond. Annu Rev Immunol 2014; 32: 121–155. [DOI] [PubMed] [Google Scholar]
- 20.Aydintug MK, Roark CL, Chain JL, Born WK, O’Brien RL. Macrophages express multiple ligands for gammadelta TCRs. Mol Immunol 2008; 45(11): 3253–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 2011; 208(6): 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koenecke C, Chennupati V, Schmitz S, Malissen B, Forster R, Prinz I. In vivo application of mAb directed against the gammadelta TCR does not deplete but generates “invisible” gammadelta T cells. Eur J Immunol 2009; 39(2): 372–379. [DOI] [PubMed] [Google Scholar]
- 23.Hatano S, Tun X, Noguchi N, Yue D, Yamada H, Sun X et al. Development of a new monoclonal antibody specific to mouse Vgamma6 chain. Life Sci Alliance 2019; 2(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hand TW, Dos Santos LM, Bouladoux N, Molloy MJ, Pagan AJ, Pepper M et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science 2012; 337(6101): 1553–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan J, Chung H, Troy E, Kasper DL. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe 2010; 7(2): 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang S, Kingsley RA, Santos RL, Andrews-Polymenis H, Raffatellu M, Figueiredo J et al. Molecular pathogenesis of Salmonella enterica serotype typhimurium-induced diarrhea. Infect Immun 2003; 71(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabsch W, Tschape H, Baumler AJ. Non-typhoidal salmonellosis: emerging problems. Microbes Infect 2001; 3(3): 237–247. [DOI] [PubMed] [Google Scholar]
- 28.Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun 2003; 71(5): 2839–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin PA, Bettke JA, Tam JW, Leeds J, Bliska JB, Butler BP et al. Inflammatory monocytes provide a niche for Salmonella expansion in the lumen of the inflamed intestine. PLoS Pathog 2019; 15(7): e1007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis KM. All Yersinia Are Not Created Equal: Phenotypic Adaptation to Distinct Niches Within Mammalian Tissues. Front Cell Infect Microbiol 2018; 8: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Khairallah C, Sheridan BS, van der Velden AWM, Bliska JB. CCR2(+) Inflammatory Monocytes Are Recruited to Yersinia pseudotuberculosis Pyogranulomas and Dictate Adaptive Responses at the Expense of Innate Immunity during Oral Infection. Infect Immun 2018; 86(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins JW, Keeney KM, Crepin VF, Rathinam VA, Fitzgerald KA, Finlay BB et al. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol 2014; 12(9): 612–623. [DOI] [PubMed] [Google Scholar]
- 33.Vallance BA, Deng W, Jacobson K, Finlay BB. Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect Immun 2003; 71(6): 3443–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chien YH, Zeng X, Prinz I. The natural and the inducible: interleukin (IL)-17-producing gammadelta T cells. Trends Immunol 2013; 34(4): 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos RL, Zhang S, Tsolis RM, Kingsley RA, Adams LG, Baumler AJ. Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect 2001; 3(14–15): 1335–1344. [DOI] [PubMed] [Google Scholar]
- 36.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2001; 2(4): 361–367. [DOI] [PubMed] [Google Scholar]
- 37.Schmolka N, Papotto PH, Romero PV, Amado T, Enguita FJ, Amorim A et al. MicroRNA-146a controls functional plasticity in gammadelta T cells by targeting NOD1. Sci Immunol 2018; 3(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vantourout P, Laing A, Woodward MJ, Zlatareva I, Apolonia L, Jones AW et al. Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing gammadelta T cell biology. Proc Natl Acad Sci U S A 2018; 115(5): 1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rigau M, Ostrouska S, Fulford TS, Johnson DN, Woods K, Ruan Z et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by gammadelta T cells. Science 2020; 367(6478). [DOI] [PubMed] [Google Scholar]
- 40.Ryan-Payseur B, Frencher J, Shen L, Chen CY, Huang D, Chen ZW. Multieffector-functional immune responses of HMBPP-specific Vgamma2Vdelta2 T cells in nonhuman primates inoculated with Listeria monocytogenes DeltaactA prfA*. J Immunol 2012; 189(3): 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hara T, Mizuno Y, Takaki K, Takada H, Akeda H, Aoki T et al. Predominant activation and expansion of V gamma 9-bearing gamma delta T cells in vivo as well as in vitro in Salmonella infection. J Clin Invest 1992; 90(1): 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davey MS, Willcox CR, Hunter S, Kasatskaya SA, Remmerswaal EBM, Salim M et al. The human Vdelta2(+) T-cell compartment comprises distinct innate-like Vgamma9(+) and adaptive Vgamma9(−) subsets. Nat Commun 2018; 9(1): 1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melandri D, Zlatareva I, Chaleil RAG, Dart RJ, Chancellor A, Nussbaumer O et al. The gammadelta TCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat Immunol 2018; 19(12): 1352–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willcox CR, Vantourout P, Salim M, Zlatareva I, Melandri D, Zanardo L et al. Butyrophilin-like 3 Directly Binds a Human Vgamma4(+) T Cell Receptor Using a Modality Distinct from Clonally-Restricted Antigen. Immunity 2019; 51(5): 813–825 e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKenzie DR, Comerford I, Silva-Santos B, McColl SR. The Emerging Complexity of gammadeltaT17 Cells. Front Immunol 2018; 9: 796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD et al. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol 2008; 181(5): 3456–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng X, Wei YL, Huang J, Newell EW, Yu H, Kidd BA et al. gammadelta T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity 2012; 37(3): 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papotto PH, Goncalves-Sousa N, Schmolka N, Iseppon A, Mensurado S, Stockinger B et al. IL-23 drives differentiation of peripheral gammadelta17 T cells from adult bone marrow-derived precursors. EMBO Rep 2017; 18(11): 1957–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muschaweckh A, Petermann F, Korn T. IL-1beta and IL-23 Promote Extrathymic Commitment of CD27+CD122- gammadelta T Cells to gammadeltaT17 Cells. J Immunol 2017. [DOI] [PMC free article] [PubMed]
- 50.Frencher JT, Shen H, Yan L, Wilson JO, Freitag NE, Rizzo AN et al. HMBPP-deficient Listeria mutant immunization alters pulmonary/systemic responses, effector functions, and memory polarization of Vgamma2Vdelta2 T cells. J Leukoc Biol 2014; 96(6): 957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen L, Frencher J, Huang D, Wang W, Yang E, Chen CY et al. Immunization of Vgamma2Vdelta2 T cells programs sustained effector memory responses that control tuberculosis in nonhuman primates. Proc Natl Acad Sci U S A 2019; 116(13): 6371–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.