Abstract
Background/purpose
Ridge resorption after tooth extraction may result in inadequate bone volume and unfavorable ridge architecture for ideal implant placement. The use of bone substitutes has been advocated to fill extraction sites and to enhance primary implant stability. This study was made to evaluate the clinical efficacy of novel 3D printed nano-porous hydroxyapatite (3DP HA, test group) in comparison to nano-crystalline bone graft (NanoBone®, control group) in alveolar ridge preservation prior to implant placement.
Materials and methods
Thirty patients were randomized into two groups following tooth extraction. All extracted sockets were filled with 3DP HA or NanoBone® and covered with a non-resorbable membrane. After four months, cone-beam computed tomography (CBCT) and intraoral scanner were used to measure dimensional changes of bone and soft tissue surface. Bone core specimens were harvested for histological analysis during implant osteotomy. Implant stability was assessed using a modified damping capacity analysis.
Results
At four months postoperatively, dimensional changes in soft tissue surface resorption were less in the test group than in the control group; however, alveolar bone resorption was the same in both groups. Histological analysis revealed new bone formation, residual graft and fibrous connective tissue in both groups. The average primary implant stability (IST) value for both groups was approximately 70. There was no statistically significant difference in all parameters between two groups (p > 0.05).
Conclusion
3DP HA could potentially be used as an alternative bone graft material for alveolar ridge preservation.
Keywords: 3D printing, Alveolar ridge preservation, Bone graft, Dimensional analysis, Hydroxyapatite
Introduction
Implant therapy has gained increasing popularity in recent decades, with reliable long-term outcomes. Local conditions of the implant site have an important role, especially in the quality and quantity of the available bone.1 After extraction, significant resorption of the alveolar ridge in both horizontal and vertical dimensions is expected.2 Most studies have reported that alveolar ridge volume loss after extraction is an inevitable and irreversible process.3,4 Rapid resorption rate following tooth removal is found in the first six months5,6 and continues at a mean 0.5–1% per year for life.7 To overcome this resorption, a method of alveolar ridge preservation (ARP) has been published in many studies.8, 9, 10, 11, 12, 13 In order to preserve alveolar bone and avoid the need for ridge augmentation, the use of several socket grafting materials including allograft, xenograft and alloplastic biomaterials has been reported.9,10,14, 15, 16, 17, 18, 19 Alloplastic bone substitute such as hydroxyapatite (HA) has been frequently used due to its osteoconductive property, practically unlimited quantity, and the fact that there is no risk of disease transmission.20 This HA is the least soluble form of the naturally occurring calcium phosphate salts and provides an osteoconductive scaffolding function, being highly resistant to physiologic resorption.21
Recently, a combination of 3-dimensional powder printing process and low-temperature phase transformation could produce a novel low-crystalline nano-hydroxyapatite structure.22 This low-temperature technique allows for production of bone graft material with both osteoconductivity and osteoclastic resorbability in vivo due to their low crystallinity in comparison with the typical high-temperature sintering route.22, 23, 24, 25, 26
The aim of this study was to evaluate the clinical efficacy of this novel 3D printed nano-porous hydroxyapatite bone substitute in comparison with commercial bone graft (NanoBone®) in alveolar ridge preservation prior to implant placement.
Materials and methods
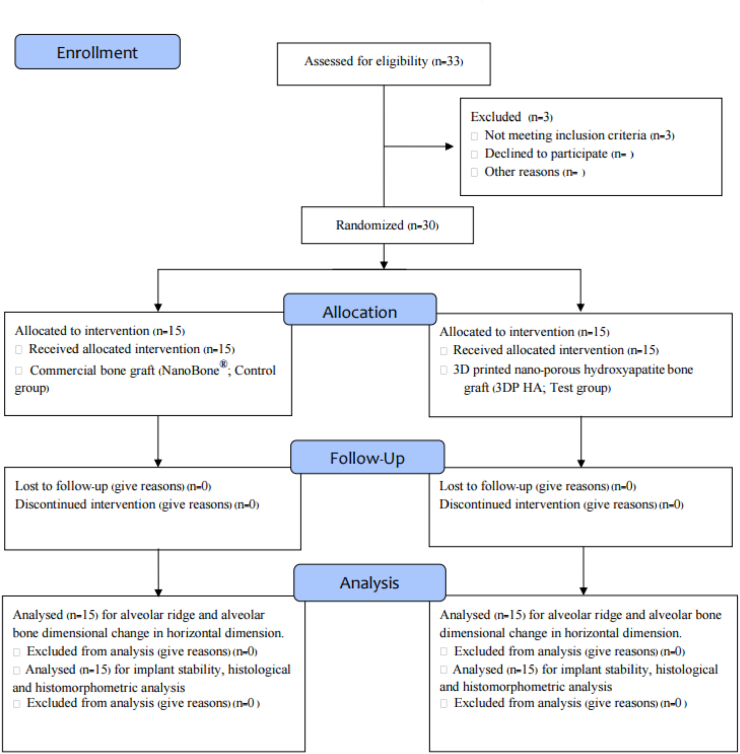
This study was a single-blinded, prospective, randomized, controlled, clinical trial with 30 patients who needed tooth extraction and implant replacement during the period of September 2018 to January 2020. The study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki and the International Conference on Harmonization (ICH) for Good Clinical Practice (GCP). The protocol was registered in the Thai Clinical Trials Registry (Study ID: TCTR20181026001) and approved by the research ethics committee of Thammasat University (Approval No. 011/2561). The flowchart of the study design and timeline according to CONSORT guidelines is shown in Fig. 1. All patients were informed of the details and purpose of the study and signed an informed consent before being enrolled in the study. All surgical treatments and measurements were performed by one surgeon (PK).
Figure 1.
Flowchart of the study design and timeline.
Based on previous reports,27 the sample size was calculated considering differences of at least 1 mm in dimensional changes between the two groups and assuming a standard deviation of 1.25. It was defined that 12 teeth per group were necessary to provide an 80% power with an α of 0.05. Considering an attrition rate of about 20%, at least 13 teeth should be included in each treatment group. Assuming a 15% loss to follow-up, hence the number of samples was 15 teeth per group (test and control).
Inclusion criteria
-
•
Good general health (ASA 1, 2)
-
•
Controlled periodontitis
-
•
Age >18 years old or with parental approval
-
•
Cigarette smoking habit of less than ten cigarettes per day
-
•
Single-tooth extraction in the anterior/premolar areas and only teeth with an intact buccal bone plate (more than 50%) were included in the study population.
-
•
All extraction sites presented a minimum width of 2 mm of keratinized gingival tissue, with signed informed consent by the patients.
Exclusion criteria
-
•
Pregnancy or lactation in females
-
•
Smoking habit of more than ten cigarettes/day, but subjects smoking less than ten cigarettes per day were requested to stop smoking for two weeks before and after surgery.
-
•
Bone disease or the use of medicines that interfere with bone metabolism
-
•
A history of head and neck radiotherapy or the presence of dehiscence or fenestration on the bone wall of the socket
Surgical procedures
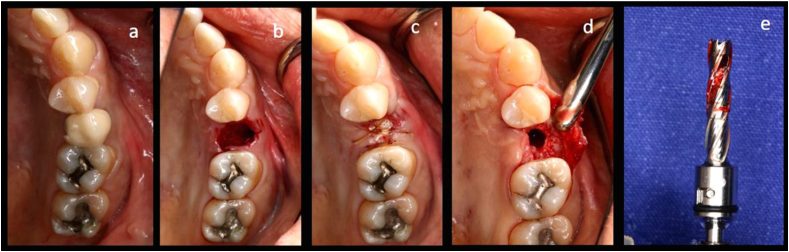
All surgical procedures were performed by one surgeon using local anesthesia. After atraumatic extraction, the walls of the extraction socket were evaluated. An intraoral scanner (CEREC AC Omnicam 1.0, Dentsply Sirona, Hessen, Germany) and CBCT (DentiiScan 1.1, NSTDA, Khlong Luang, Thailand) were used for baseline data. The extracted sockets were randomized into two groups, with the test group sockets being filled with 3DP HA (granule size 1 mm, MTEC, Khlong Luang, Thailand), prepared as described previously,22 while the control group sockets were filled with NanoBone® (granule size 0.6 mm, Artoss, Rostock, Germany). All filled sockets were covered with non-resorbable membrane (CytoplastTM TXT-200, Osteogenics, TX, USA). All patients were recalled at one, two, four and sixteen weeks postoperatively to monitor clinical response after ARP. After four months, the intraoral scan and CBCT were taken again before the implant placement. A bone core biopsy was harvested during implant osteotomy for histological and histomorphometric analysis (Fig. 2). Implant primary stability at the time of implant placement and three months later prior to prosthesis insertion were measured by using a modified damping capacity analysis device (AnyCheck IMT-100, Neobiotech Co. Ltd., Wonju-si, Republic of Korea).
Figure 2.
Process diagram of clinical procedure; a) Cracked tooth #25; b) #25 Atraumatic extraction; c) Placement of HA hydroxyapatite granules in alveolar socket after atraumatic extraction, with closing of the socket by Cytoplast™ and suturing; d, e) After four months, harvesting of the grafted area with a trephine bur in formalin solution.
Data analysis
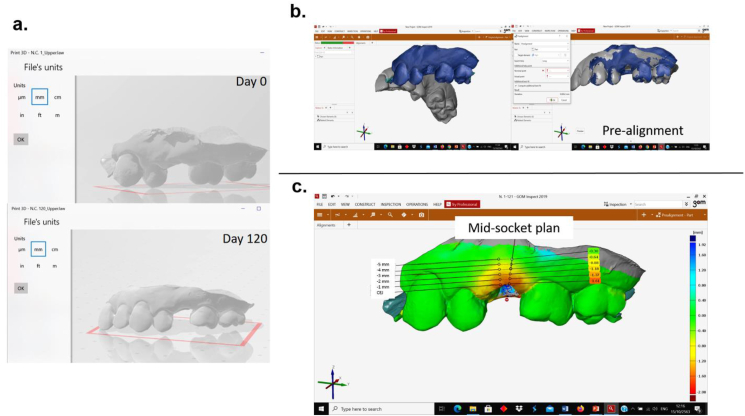
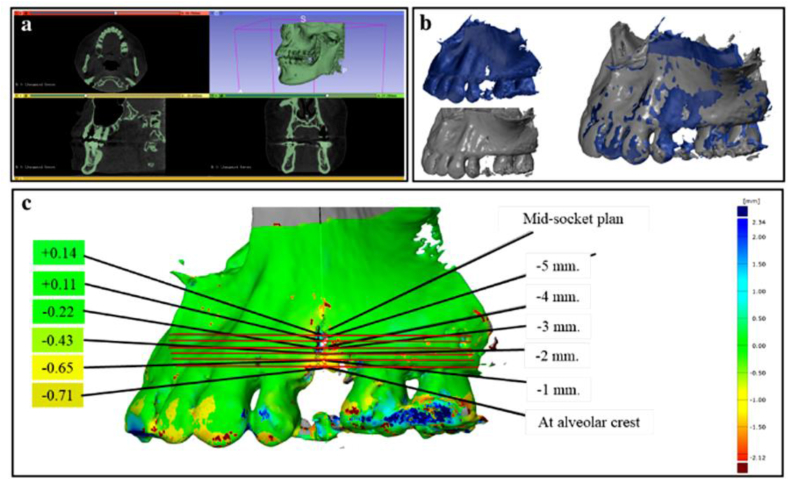
Stereolithography (STL) image file of soft tissue surface at the time of extraction and four months after ARP were exported into the 3D metrology software (GOM Inspect 2019, GOM GmbH, Braunschweig, Germany) for linear measurement analysis (Fig. 3). To measure the alveolar bone changes, two DICOM files acquired from CBCT from two time points were segmented and reconstructed into 3D STL image files by using 3D slicer software (3D Slicer version 4.10.1, http://www.slicer.org).28 These 3D models were aligned and analyzed using GOM inspect software (Fig. 4).
Figure 3.
Process for measuring soft tissue surface dimensional changes of alveolar ridge; a) Created 3D image file into 3D STL image files from intraoral scanner; b) Two STL image files at the extraction time (D0) and four months (D120) later were imported into the 3D metrology software and pre-aligned; c) After superimposition of two STL files, the horizontal dimensional changes of alveolar ridge were analyzed using GOM Inspect software.
Figure 4.
Process for measuring the alveolar bone changes; a) Created 3D image file and reconstructed files from CBCT into 3D STL images by using 3D slicer software; b) Two STL image files from CBCT immediately after extraction and four months later were pre-aligned; c) After superimposition of two STL files before measuring the alveolar bone changes, the horizontal dimensional changes of alveolar bone were analyzed using GOM Inspect software.
Histologic/histomorphometric analysis
Following fixation in buffered formalin solution, the trephined specimens were decalcified and the sections from the middle part of each specimen were stained with haematoxylin and eosin (H&E). At least eight fields of view per sample were examined and photographed with light microscope (Nikon DS-U3, Nikon Instruments Inc., NY, USA) equipped with camera. Histological observation was evaluated in each section, and histomorphometric analysis was calculated for the percentage of new bone formation, connective tissue, and residual graft particles.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 8.0 (GraphPad, California, USA). Shapiro–Wilks test was used to validate the normal distribution of the data, and all results were analyzed by Manne–Whitney test, which are nonparametric tests. Regression analysis was used to investigate the influence of variables on the clinical outcomes. The level of statistical significance was set at p < 0.05.
Results
All extracted sites had four walls of socket with more than 50% of buccal and palatal/lingual bone plate. There were 15 patients with 3DP HA and 15 patients with NanoBone® who underwent the ridge preservation procedure. The demographic data of the patients in each group are shown in Table 1.
Table 1.
Demographic data of the participants in the control groups and the test groups.
| Characteristic | NanoBone® (Control group, n = 15) | 3DP HA (Test group, n = 15) | p-value | ||
|---|---|---|---|---|---|
| Age, mean ± SD, (years) | 60.13 ± 2.31 years | 60.33 ± 1.54 years | 0.825 | ||
| Gender n (%) | Male, n (%) | 2 (13.33%) | 3 (20%) | – | |
| Female, n (%) | 13 (86.67%) | 12 (80%) | – | ||
| Periodontal parameters, mean ± SD, (mm) | Keratinized tissue | 4.67 ± 1.82 mm | 4.19 ± 1.41 mm | 0.432 | |
| PPD | 3.59 ± 0.94 mm | 3.99 ± 1.21 mm | 0.320 | ||
| Tooth types (%) | Anterior, n (%) | 1 (6.67%) | 2 (13.33%) | – | |
| Premolar, n (%) | 14 (93.33%) | 13 (86.67%) | – | ||
| Location | Maxillary, n (%) | 8 (53.33%) | 9 (60%) | ||
| Mandible, n (%) | 7 (46.67%) | 6 (40%) | |||
| Causes of extraction | Endodontic failure, n (%) | 6 (40.0%) | 7 (46.66%) | – | |
| Periodontitis, n (%) | 5 (33.33%) | 4 (26.67%) | – | ||
| Fractures, n (%) | 4 (26.67%) | 4 (26.67%) | – | ||
SD, standard deviation; n, number; mm, millimeter; 3DP HA, 3D printed nano-porous hydroxyapatite; PPD, periodontal probing depth.
Soft tissue surface dimensional changes of alveolar ridge
Table 2 shows that the changes in dimension of alveolar ridge resorption in the control group was greater than those of the test group. However, no significant difference in the measured dimensional changes at all locations between the two groups was observed (p > 0.05).
Table 2.
Soft tissue surface dimensional changes of alveolar ridge (intraoral scanner STL data) and horizontal dimensional changes of alveolar bone (CBCT data) immediately after tooth extraction and four months after preservation in each group (Mean ± SD).
| Location | Soft tissue surface dimensional changes of alveolar ridge |
Horizontal dimensional changes of alveolar bone |
||||
|---|---|---|---|---|---|---|
| NanoBone® (Control group; n = 15) | 3DP HA (Test group; n = 15) | p-value | NanoBone® (Control group; n = 15) | 3DP HA (Test group; n = 15) | p-value | |
| At gingival margin | −2.45 ± 0.67 | −2.40 ± 0.59 | 0.664 | −0.97 ± 0.14 | −0.92 ± 0.28 | 0.691 |
| 1 mm | −2.33 ± 0.66 | −2.27 ± 0.57 | 0.693 | −0.86 ± 0.11 | −0.84 ± 0.29 | 0.644 |
| 2 mm | −2.28 ± 0.72 | −2.07 ± 0.56 | 0.892 | −0.63 ± 0.16 | −0.65 ± 0.19 | 0.953 |
| 3 mm | −1.90 ± 0.77 | −1.92 ± 0.60 | 0.984 | −0.44 ± 0.13 | −0.46 ± 0.18 | 0.983 |
| 4 mm | −1.74 ± 0.80 | −1.72 ± 0.58 | 0.995 | −0.31 ± 0.10 | −0.37 ± 0.10 | 0.920 |
| 5 mm | −1.56 ± 0.84 | −1.51 ± 0.58 | 0.963 | −0.27 ± 0.10 | −0.27 ± 0.22 | 0.892 |
| Total difference | −2.03 ± 0.79 | −1.98 ± 0.64 | 0.219 | −0.58 ± 0.29 | −0.59 ± 0.32 | 0.265 |
n, number; mm, millimeter; 3DP HA, 3D printed nano-porous hydroxyapatite.
Dimensional changes of alveolar bone
The alveolar bone changes at the coronal part were greater than the apical part (Table 2). At all levels of measurement, no significant difference in bone preservation between two groups was observed (p > 0.05).
Insertion torque and implant stability
All implants were placed at a mean insertion torque >35 Ncm in both groups. There was good primary implant stability with an average IST value of 69–70 in all preserved sites. After three months, an increase in implant stability value was observed in all implants. There was no statistical difference in IST value between the two groups (p > 0.05) (Table 3).
Table 3.
Implant stability test (IST) at the time of implant placement (IST1) and three months later before inserting the prosthesis (IST2) in each group (Mean ± SD).
| Implant stability | NanoBone® (Control group, n = 15) | 3DP HA (Test group, n = 15) | p-value |
|---|---|---|---|
| IST1 | 70.6 ± 2.77 | 69.2 ± 1.93 | 0.198 |
| IST2 | 74.8 ± 2.92 | 73.8 ± 2.87 | 0.977 |
IST, implant stability test; n, number; 3DP HA, 3D printed nano-porous hydroxyapatite.
Histological and histomorphometric analysis
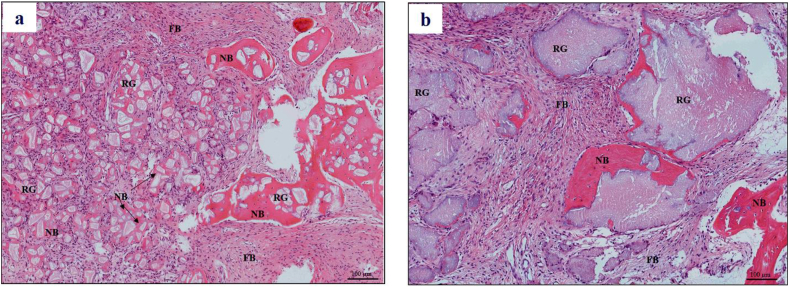
New bone formation and residual graft materials were seen in all harvested samples (Fig. 5). The percentage of new bone formation, residual graft and connective tissue were shown in Table 4. No statistically significant difference between the two groups was observed (p > 0.05).
Figure 5.
Histological observation four months after surgery. H&E staining demonstrated new bone (NB), fibrous tissue (FB) and residual hydroxyapatite granules (RG) in: a) 3DP HA (test group) and b) NanoBone® (control group) (magnification × 200).
Table 4.
Histomorphometric data of the NanoBone® group and the 3DP HA group four months after preservation (Mean ± SD).
| Measurement | NanoBone® (Control group, n = 15) | 3DP HA (Test group, n = 15) | p-value |
|---|---|---|---|
| New bone formation (%) | 31.35 ± 4.82 | 33.20 ± 6.73 | 0.385 |
| Residual bone graft (%) | 31.69 ± 6.42 | 27.04 ± 7.91 | 0.090 |
| Connective tissue (%) | 36.96 ± 8.56 | 39.76 ± 4.03 | 0.264 |
n, number; 3DP HA, 3D printed nano-porous hydroxyapatite.
Discussion
Post extraction resorption was unavoidable, but with the aid of membrane and bone graft materials the resorption can be minimized. Several studies reported significant reduction in resorption when bone graft was filled in extracted sockets in comparison with spontaneous healing by only blood clotting.21 This was also observed in our study, using 3DP HA and NanoBone® as socket-filling bone substitute materials.
In this study, linear measurement by measuring the distance between two surface points at interested level makes it possible to represent the dimensional changes with high sensitivity and accuracy.4,19,27 Using stereolithography (STL) data of soft tissue surface, the mean horizontal ridge changes were −1.51 to −2.40 mm in the test group and −1.56 to −2.45 mm in the control group. These results were comparable to the range as reported in the systematic review and meta-analysis by Avila-Ortiz et al.29 which concluded that dimensional change in ridge preservation was typically −1.1 to −3.5 mm. Our results also agree with other studies which showed that the use of bone grafts for ridge preservation was effective in preventing ridge resorption after tooth extraction.11,19,30, 31, 32, 33
The mean horizontal dimensional changes of alveolar bone at four months post extraction were −0.27 to −0.92 mm in the test group and −0.27 to −0.97 mm in the control group, while previous studies found −3.8 mm34, -3.6 mm35 and -3.06 mm36 six months after no ridge preservation. Also, ridge preservation demonstrated better efficacy in the horizontal dimension.13 The total mean of soft tissue surface dimensional changes of alveolar ridge differences was −2.03 mm in the control group and −1.98 mm in the test group, while the total mean of horizontal dimensional changes of alveolar bone differences was −0.58 mm in the control group and −0.59 mm in the test group. This study showed that soft tissue surface changes more than bone changes.
Histological study revealed that all the samples in the grafted area were occupied by connective tissue, new bone and residual bone graft granules. Newly formed bone in both groups was in direct contact with bone graft granules, indicating their comparable good bioactivity and osteoconductivity. New bone was found mainly at the apical part of the socket in the NanoBone® group, while both the apical and coronal parts of the socket were filled with new bone in 3DP HA group (Fig. 5). The histomorphometric measurements showed residual graft of 27.04% in the test group and 31.69% in the control group. Typically, hydroxyapatite was reported to be stable and displayed limited resorption. The low residual content of 3DP HA observed in this in vivo situation further confirmed that the resorbable nature of 3DP HA resulted from the use of a low-temperature process which was previously seen in an in vitro resorption test.26 Although the residual content was still greater than the 10–13% of residual deproteinized bovine bone, as reported by Consistent with Felice et al. (2009)37 from a clinical implant placement perspective, such a difference did not present any handling difficulty during the placement of the trephining cores and the implant.
In this study, four months after ridge preservation, using test and control bone graft materials minimized ridge resorption and provided good integration in all 30 cases. An average IST value of 70 was seen in the control group and 69 in the test group at the time of implant placement, which indicates good stability for implant osseointegration.38 An average IST value of 74 in the control group and 73 in the test group before insertion of the crown showed that good stability can be reached in prosthesis insertion, that the grafting procedure did not impair osseointegration,39 and that implants placed in bone regenerated using 3DP HA were able to sustain loading and provide similar long-term results to those placed in pristine bone.40
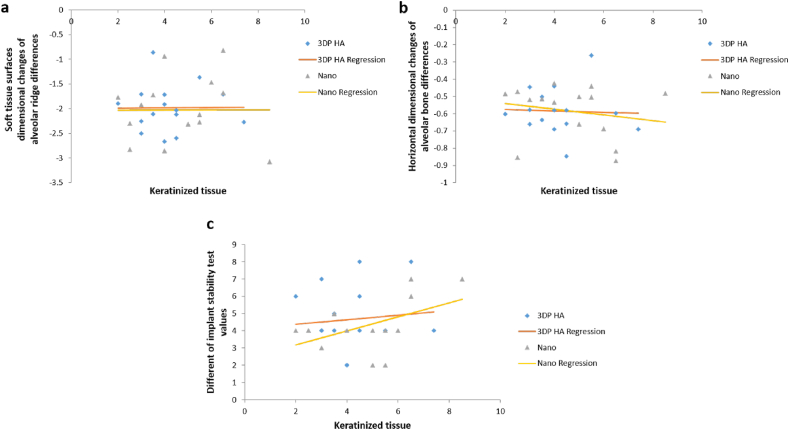
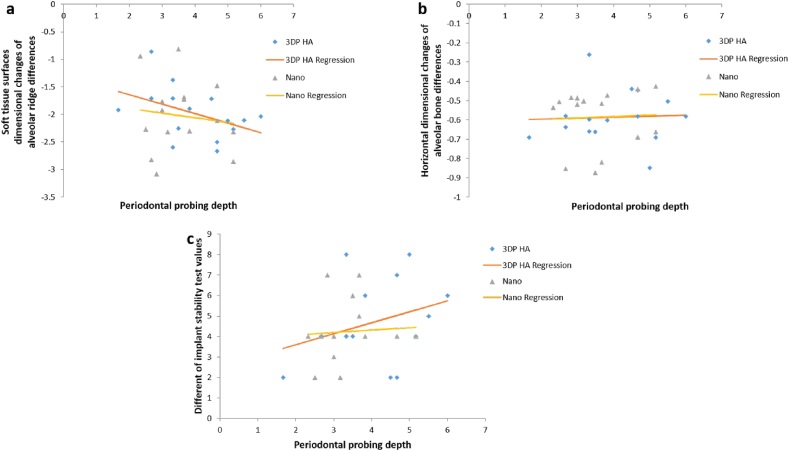
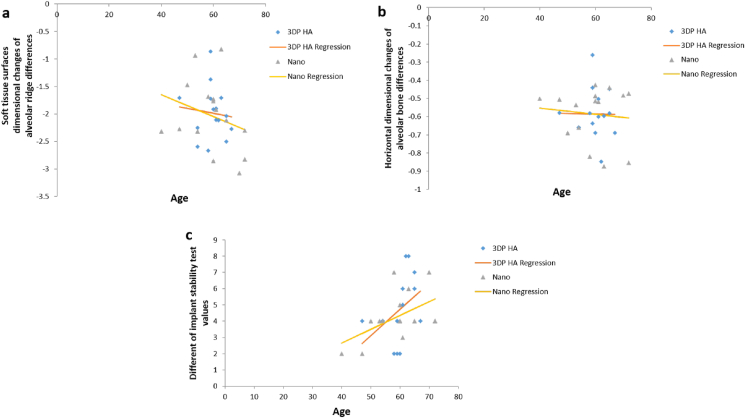
The regression analysis in Table 5 identified the influence of contributing factors such as keratinized tissue, periodontal probing depth (PPD), and age did not affect clinical outcomes after ridge preservation in dimensional changes of soft tissue surface, dimensional changes of alveolar bone, and IST in both groups. Because the subjects were randomly selected based on their need for tooth extraction with ridge preservation, the two groups (control, test) did not differ with respect to age, PPD, keratinized tissue and favorable bone configuration in extracted socket. Therefore, the graph in Figure 6, Figure 7, Figure 8 show the same outcome when comparing the two groups. However, the gender, tooth type, tooth location and causes of extracted teeth were not interpreted in the regression analysis because of the limited number of the cases.
Table 5.
Regression analysis with dimensional changes and IST as dependent variable
| Variables | Group | p-value | Regression coefficient | Standard error | 95% Confidence Interval |
|---|---|---|---|---|---|
| Keratinized tissue VS Soft tissue surface dimensional changes of alveolar ridge differences | NanoBone® | 0.997 | 0.001 | 0.099 | −0.215 to 0.216 |
| 3DP HA | 0.976 | 0.003 | 0.094 | −0.199 to 0.205 | |
| Keratinized tissue VS Horizontal dimensional changes of alveolar bone differences | NanoBone® | 0.479 | −0.017 | 0.023 | −0.066 to 0.033 |
| 3DP HA | 0.876 | −0.004 | 0.027 | −0.062 to 0.053 | |
| Keratinized tissue VS IST differences | NanoBone® | 0.060 | 0.406 | 0.196 | −0.018 to 0.831 |
| 3DP HA | 0.747 | 0.128 | 0.390 | −0.714 to 0.971 | |
| PPD VS Soft tissue surface dimensional changes of alveolar ridge differences | NanoBone® | 0.665 | −0.084 | 0.191 | −0.496 to 0.327 |
| 3DP HA | 0.103 | −0.173 | 0.098 | −0.385 to 0.040 | |
| PPD VS Horizontal dimensional changes of alveolar bone differences | NanoBone® | 0.859 | 0.008 | 0.045 | −0.089 to 0.106 |
| 3DP HA | 0.882 | 0.005 | 0.031 | −0.063 to 0.072 | |
| PPD VS IST differences | NanoBone® | 0.791 | 0.118 | 0.435 | −0.823 to 1.059 |
| 3DP HA | 0.237 | 0.536 | 0.432 | −0.398 to 1.470 | |
| Age VS Soft tissue surface dimensional changes of alveolar ridge differences | NanoBone® | 0.514 | −0.019 | 0.019 | −0.061 to 0.021 |
| 3DP HA | 0.733 | −0.009 | 0.026 | −0.065 to 0.047 | |
| Age VS Horizontal dimensional changes of alveolar bone differences | NanoBone® | 0.713 | −0.002 | 0.005 | −0.012 to 0.008 |
| 3DP HA | 0.961 | −0.001 | 0.007 | −0.016 to 0.016 | |
| Age VS IST differences | NanoBone® | 0.060 | 0.085 | 0.039 | 0.002 to 0.168 |
| 3DP HA | 0.128 | 0.161 | 0.099 | −0.053 to 0.376 |
IST, implant stability test; PPD, periodontal pocket depth; 3DP HA, 3D printed nano-porous hydroxyapatite; VS, versus.
Figure 6.
Scatterplot illustrating the effect of keratinized tissue to dimensional changes of soft tissue surfaces, alveolar bone and IST values in Nanobone® group VS 3DP HA group by regression analysis.
Figure 7.
Scatterplot illustrating the effect of PPD to dimensional changes of soft tissue surfaces, alveolar bone and IST values in Nanobone® group VS 3DP HA group by regression analysis.
Figure 8.
Scatterplot illustrating the effect of age to dimensional changes of soft tissue surfaces, alveolar bone and IST values in Nanobone® group VS 3DP HA groups by regression analysis.
The limitations of this study were the absence of a negative control group of unassisted socket healing which might not allow for complete evaluation of the overall effectiveness of the two bone grafts, the small number of participants, and the relatively short follow-up times.
Within all parameters of this study, no statistically significant differences were found in alveolar ridge resorption after alveolar ridge preservation with 3DP HA bone graft material or commercial bone graft material (NanoBone®) in terms of 3-dimensional changes of soft tissue and bony tissue, IST value after implantation, and histomorphometric analysis. Overall results indicated that 3DP HA bone graft material can potentially be used as bone grafting material for alveolar ridge preservation.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was supported by Thammasat University Research Unit in Tissue Engineering and Implant Dentistry. This project was funded by National Research Council of Thailand.
References
- 1.Turkyilmaz I., Tözüm T.F., Tumer C. Bone density assessments of oral implant sites using computerized tomography. J Oral Rehabil. 2007;34:267–272. doi: 10.1111/j.1365-2842.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- 2.Hansson S., Halldin A. Alveolar ridge resorption after tooth extraction: a consequence of a fundamental principle of bone physiology. J Dent Biomech. 2012;3 doi: 10.1177/1758736012456543. 1758736012456543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo M.G., Lindhe J. Ridge alterations following tooth extraction with and without flap elevation: an experimental study in the dog. Clin Oral Implants Res. 2009;20:545–549. doi: 10.1111/j.1600-0501.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 4.Schropp L., Wenzel A., Kostopoulos L., Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restor Dent. 2003;23:313–323. [PubMed] [Google Scholar]
- 5.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pietrokovski J., Massler M. Alveolar ridge resorption following tooth extraction. J Prosthet Dent. 1967;17:21–27. doi: 10.1016/0022-3913(67)90046-7. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson G.E., Persson G. Morphologic changes of the mandible after extraction and wearing of dentures. A longitudinal, clinical, and x-ray cephalometric study covering five years. Odontol Revy. 1967;18:27–54. [PubMed] [Google Scholar]
- 8.Araujo M., Linder E., Lindhe J. Effect of a xenograft on early bone formation in extraction sockets: an experimental study in the dog. Clin Oral Implants Res. 2009;20:1–6. doi: 10.1111/j.1600-0501.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 9.Araújo M.G., Lindhe J. Ridge preservation with the use of Bio-Oss collagen: a six-month study in the dog. Clin Oral Implants Res. 2009;20:433–440. doi: 10.1111/j.1600-0501.2009.01705.x. [DOI] [PubMed] [Google Scholar]
- 10.Brkovic B., Prasad H., Rohrer M., et al. Beta-tricalcium phosphate/type I collagen cones with or without a barrier membrane in human extraction socket healing: clinical, histologic, histomorphometric, and immunohistochemical evaluation. Clin oral investig. 2012;16:581–590. doi: 10.1007/s00784-011-0531-1. [DOI] [PubMed] [Google Scholar]
- 11.Carmagnola D., Adriaens P., Berglundh T. Healing of human extraction sockets filled with Bio-Oss. Clin Oral Implants Res. 2003;14:137–143. doi: 10.1034/j.1600-0501.2003.140201.x. [DOI] [PubMed] [Google Scholar]
- 12.Fickl S., Zuhr O., Wachtel H., Bolz W., Huerzeler M.B. Hard tissue alterations after socket preservation: an experimental study in the beagle dog. Clin Oral Implants Res. 2008;19:1111–1118. doi: 10.1111/j.1600-0501.2008.01575.x. [DOI] [PubMed] [Google Scholar]
- 13.Fickl S., Zuhr O., Wachtel H., Kebschull M., Hürzeler M.B. Hard tissue alterations after socket preservation with additional buccal overbuilding: a study in the beagle dog. J Clin Periodontol. 2009;36:898–904. doi: 10.1111/j.1600-051X.2009.01463.x. [DOI] [PubMed] [Google Scholar]
- 14.Calasans-Maia M.D., Ascoli F.O., Novellino A.T.N., Rossi A.M., Granjeiro J. Comparative histological evaluation of tibil bone repair in rabbits treated with xenografts. Acta Ortopédica Bras. 2008;17:340–343. [Google Scholar]
- 15.Fernandes P.G., Novaes A.B., Jr., de Queiroz A.C., et al. Ridge preservation with acellular dermal matrix and anorganic bone matrix cell-binding peptide P-15 after tooth extraction in humans. J Periodontol. 2011;82:72–79. doi: 10.1902/jop.2010.100241. [DOI] [PubMed] [Google Scholar]
- 16.Festa V.M., Addabbo F., Laino L., Femiano F., Rullo R. Porcine-derived xenograft combined with a soft cortical membrane versus extraction alone for implant site development: a clinical study in humans. Clin Implant Dent Relat Res. 2013;15:707–713. doi: 10.1111/j.1708-8208.2011.00398.x. [DOI] [PubMed] [Google Scholar]
- 17.Gonshor A., McAllister B.S., Wallace S.S., Prasad H. Histologic and histomorphometric evaluation of an allograft stem cell-based matrix sinus augmentation procedure. Int J Oral Maxillofac Implants. 2011;26:123–131. [PubMed] [Google Scholar]
- 18.Spinato S., Agnini A., Chiesi M., Agnini A.M., Wang H.L. Comparison between graft and no-graft in an immediate placed and immediate nonfunctional loaded implant. Implant Dent. 2012;21:97–103. doi: 10.1097/ID.0b013e318248866c. [DOI] [PubMed] [Google Scholar]
- 19.Vanhoutte V., Rompen E., Lecloux G., Rues S., Schmitter M., Lambert F. A methodological approach to assessing alveolar ridge preservation procedures in humans: soft tissue profile. Clin Oral Implants Res. 2014;25:304–309. doi: 10.1111/clr.12144. [DOI] [PubMed] [Google Scholar]
- 20.Darby I. Periodontal materials. Aust Dent J. 2011;56(Suppl 1):107–118. doi: 10.1111/j.1834-7819.2010.01301.x. [DOI] [PubMed] [Google Scholar]
- 21.Govindaraj S., Costantino P.D., Friedman C.D. Current use of bone substitutes in maxillofacial surgery. Facial Plast Surg. 1999;15:73–81. doi: 10.1055/s-2008-1064302. [DOI] [PubMed] [Google Scholar]
- 22.Suwanprateeb J., Suvannapruk W., Wasoontararat K. Low-temperature preparation of calcium phosphate structure via phosphorization of 3D-printed calcium sulfate hemihydrate based material. J Mater Sci Mater Med. 2010;21:419–429. doi: 10.1007/s10856-009-3883-1. [DOI] [PubMed] [Google Scholar]
- 23.Suwanprateeb J., Thammarakcharoen F., Phanphiriya P., Chokevivat W., Suvannapruk W., Chernchujit B. Preparation and characterizations of antibiotic impregnated microporous nano-hydroxyapatite for osteomyelitis treatment. Biomed Eng Appl Basis Commun. 2014;26:1450041. [Google Scholar]
- 24.Suwanprateeb J., Thammarakcharoen F., Wasoontararat K., Chokevivat W., Phanphiriya P. Preparation and characterization of nanosized silver phosphate loaded hydroxyapatite by single step co-conversion process. Mater Sci Eng C. 2012;32:2122–2128. doi: 10.1016/j.msec.2012.05.051. [DOI] [PubMed] [Google Scholar]
- 25.Suwanprateeb J., Thammarakcharoen F., Wasoontararat K., Chokevivat W., Phanphiriya P. Single step preparation of nanosilver-loaded calcium phosphate by low-temperature co-conversion process. J Mater Sci Mater Med. 2012;23:2091–2100. doi: 10.1007/s10856-012-4690-7. [DOI] [PubMed] [Google Scholar]
- 26.Thammarakcharoen F., Palanuruksa P., Suwanprateeb J. In vitro resorbability of three different processed hydroxyapatite. Key Eng Mater. 2015;659:3–7. [Google Scholar]
- 27.Jung R.E., Philipp A., Annen B.M., et al. Radiographic evaluation of different techniques for ridge preservation after tooth extraction: a randomized controlled clinical trial. J Clin Periodontol. 2013;40:90–98. doi: 10.1111/jcpe.12027. [DOI] [PubMed] [Google Scholar]
- 28.Fedorov A., Beichel R., Kalpathy-Cramer J., et al. 3D Slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging. 2012;30:1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avila-Ortiz G., Elangovan S., Kramer K.W., Blanchette D., Dawson D.V. Effect of alveolar ridge preservation after tooth extraction: a systematic review and meta-analysis. J Dent Res. 2014;93:950–958. doi: 10.1177/0022034514541127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Artzi Z., Tal H., Dayan D. Porous bovine bone mineral in healing of human extraction sockets. Part 1: histomorphometric evaluations at nine months. J Periodontol. 2000;71:1015–1023. doi: 10.1902/jop.2000.71.6.1015. [DOI] [PubMed] [Google Scholar]
- 31.Artzi Z., Tal H., Dayan D. Porous bovine bone mineral in healing of human extraction sockets: 2. Histochemical observations at nine months. J Periodontol. 2001;72:152–159. doi: 10.1902/jop.2001.72.2.152. [DOI] [PubMed] [Google Scholar]
- 32.Buser D., Chappuis V., Bornstein M.M., Wittneben J.G., Frei M., Belser U.C. Long-term stability of contour augmentation with early implant placement following single-tooth extraction in the esthetic zone: a prospective, cross-sectional study in 41 patients with a five-to-nine-year follow-up. J Periodontol. 2013;84:1517–1527. doi: 10.1902/jop.2013.120635. [DOI] [PubMed] [Google Scholar]
- 33.Serino G., Rao W., Iezzi G., Piattelli A. Polylactide and polyglycolide sponge used in human extraction sockets: bone formation following three months after its application. Clin Oral Implants Res. 2008;19:26–31. doi: 10.1111/j.1600-0501.2007.01311.x. [DOI] [PubMed] [Google Scholar]
- 34.Hammerle C.H., Araujo M.G., Simion M. Evidence-based knowledge on the biology and treatment of extraction sockets. Clin Oral Implants Res. 2012;23(Suppl 5):80–82. doi: 10.1111/j.1600-0501.2011.02370.x. [DOI] [PubMed] [Google Scholar]
- 35.Barone A., Ricci M., Tonelli P., Santini S., Covani U. Tissue changes of extraction sockets in humans: a comparison of spontaneous healing vs. ridge preservation with secondary soft tissue healing. Clin Oral Implants Res. 2013;24:1231–1237. doi: 10.1111/j.1600-0501.2012.02535.x. [DOI] [PubMed] [Google Scholar]
- 36.Camargo P.M., Lekovic V., Weinlaender M., et al. Influence of bioactive glass on changes in alveolar process dimensions after exodontia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90 doi: 10.1067/moe.2000.110035. 581–6. [DOI] [PubMed] [Google Scholar]
- 37.Felice P., Marchetti C., Iezzi G., et al. Vertical ridge augmentation of the atrophic posterior mandible with interpositional bloc grafts: bone from the iliac crest vs. bovine anorganic bone. Clinical and histological results up to one year after loading from a randomized-controlled clinical trial. Clin Oral Implants Res. 2009;20:1386–1393. doi: 10.1111/j.1600-0501.2009.01765.x. [DOI] [PubMed] [Google Scholar]
- 38.Bornstein M.M., Hart C.N., Halbritter S.A., Morton D., Buser D. Early loading of nonsubmerged titanium implants with a chemically modified sand-blasted and acid-etched surface: six-month results of a prospective case series study in the posterior mandible focusing on peri-implant crestal bone changes and implant stability quotient (ISQ) values. Clin Implant Dent Relat Res. 2009;11:338–347. doi: 10.1111/j.1708-8208.2009.00148.x. [DOI] [PubMed] [Google Scholar]
- 39.Molly L., Vandromme H., Quirynen M., Schepers E., Adams J.L., van Steenberghe D. Bone formation following implantation of bone biomaterials into extraction sites. J Periodontol. 2008;79:1108–1115. doi: 10.1902/jop.2008.070476. [DOI] [PubMed] [Google Scholar]
- 40.Fiorellini J.P., Nevins M.L. Localized ridge augmentation/preservation. A systematic review. Ann Periodontol. 2003;8:321–327. doi: 10.1902/annals.2003.8.1.321. [DOI] [PubMed] [Google Scholar]