Abstract
Introduction
Behavioral and psychological symptoms of dementia (BPSD) is the most prominent and distressing manifestation for older persons with dementia (PWD) and caregivers. Aromatherapy has demonstrated its effectiveness in managing BPSD in various studies. However, previous studies and systematic reviews have obtained inconsistent findings, and a review of qualitative studies is yet to be conducted.
Method
A mixed-methods systematic review with a convergent segregated approach was performed to evaluate the effectiveness of aromatherapy in improving the BPSD and quality of life (QoL) of PWD and in relieving the distress and burden of caregivers, as well as its safety for PWD. Both published and unpublished quantitative and qualitative studies written in English and Chinese between January 1996 and December 2020 were retrieved from 28 databases, including MEDLINE, EMBASE, and Web of Science, based on the prespecified criteria. The methodological quality was assessed by using critical appraisal tools from the Joanna Briggs Institute. Quantitative synthesis, qualitative synthesis, and integration of quantitative and qualitative evidence were performed.
Results
A total of 12 randomized controlled trials, 10 quasi-experimental studies, and 2 qualitative studies were included in the review. Some inconsistent findings regarding the effectiveness of aromatherapy in reducing the severity of BPSD were observed. Some studies reported that aromatherapy significantly improved the QoL of PWD and relieved the distress and burden of caregivers, promoted a positive experience among caregivers, and had very low adverse effects on PWD (with aromatherapy inhalation reporting no adverse effects).
Conclusion
Aromatherapy, especially in the inhalation approach, could be a potentially safe and effective strategy for managing BPSD. However, more structuralized and comparable studies with sufficient sample size, adherence monitoring, and sound theoretical basis could be conducted to obtain conclusive findings.
Keywords: Aromatherapy, Behavioral and psychological symptoms of dementia, BPSD, Dementia, Essential oil
Introduction
Dementia is an acquired brain syndrome marked by a considerable cognitive decline from a previous level of performance in cognitive domains that cannot be attributed to any other mental disorder; it affects the independence of individuals in their daily activities [1, 2]. According to the World Health Organization, about 5%–8% (>55 million) of the elderly aged 60 years and above are living with dementia, and dementia has emerged as one of the major causes of disability and dependency among the elderly worldwide [3, 4]. Behavioral and psychological symptoms of dementia (BPSD) is a term coined by the International Psychogeriatric Association (IPA) in 1996 [5] to refer to a diverse range of symptoms of “disturbed perception, thought content, mood, or behavior that frequently occur in patients with dementia” [6]. According to previous studies, over 90% of older persons with dementia (PWD) exhibit at least one BPSD symptom at any stage of their disease [7, 8, 9, 10, 11]. Behavioral symptoms of BPSD include aggression, agitation, and sleep disorders, whereas psychological symptoms include anxiety, depression, and apathy [5, 12].
BPSD is the most prominent and distressing manifestation of dementia for PWD and caregivers [13, 14, 15]. For PWD, BPSD is associated with worsening cognitive functions and progression of dementia [16] that may reduce their social functions and quality of life (QoL) [17], increase their susceptibility to abuse and neglect [18], and expose them to physical harm [19] and secondary complications, such as falls and fractures [20], which would ultimately lead to their hospitalization or institutionalization [21]. Meanwhile, formal and informal caregivers need to deal with both the cognitive deterioration of PWD and their BPSD [13]. Unlike the downward trends in cognitive and functional status in dementia, BPSD may fluctuate episodically during the progression of the disease, which introduces challenges in caring PWD and increases the burden and distress faced by caregivers [22, 23, 24] that harm their physical, psychological, and emotional well-being [25]. Studies on formal and informal caregivers reveal that the BPSD severity has a significant positive correlation with the degree of caregiver distress and burden [25, 26, 27, 28]. Therefore, effective management of BPSD should be prioritized by healthcare professionals and caregivers to reduce its negative impacts on PWD, alleviate the level of distress and burden among caregivers, and preserve the well-being of both PWD and caregivers [29].
With their high safety and low association with adverse events, nonpharmacological strategies have received much attention as safe methods for BPSD management [30] and have been recommended as a first-line approach in various studies and dementia guidelines [30, 31, 32, 33, 34]. Aromatherapy, as a nonpharmacological strategy that relies on sensory stimulation [35], has evolved from herbal and botanical medicine with at least 6,000 years of history [36]. Contemporarily, aromatherapy is defined as a natural treatment through a variety of approaches (e.g., inhalation, topical application, and massage), with the use of essential oils extracted from aromatic plants, to balance, harmonize, and promote the health of the body, mind, and spirit [37, 38, 39]. Analyses of systematic reviews and clinical guidelines on nonpharmacological strategies for managing BPSD highly recommend aromatherapy as the best strategy due to its good-quality patient-oriented evidence [40]. Aromatherapy acts on the body through olfactory stimulation or percutaneous absorption of the chemicals in essential oils [36, 41, 42, 43] and has been clinically used in BPSD management for over 20 years. Clinical studies show that aromatherapy can improve BPSD symptoms, such as sleep disturbance [44, 45], agitation [46, 47], depression [47, 48], and aggression [49, 50], but the theoretical basis for the implementation of this treatment has not been described. A systematic review of 11 randomized controlled trials (RCTs) that evaluated the effects of aromatherapy on dementia revealed that aromatherapy, except for oral intake, could effectively and safely reduce the frequencies of BPSD among PWD and could be considered a potentially effective strategy for BPSD management [51].
However, a Cochrane Review involving 13 RCTs revealed that the included trials produced inconclusive evidence regarding the effects of aromatherapy on the BPSD and QoL of PWD, and the distress and burden of caregivers; moreover, the safety of aromatherapy for PWD was not evaluated in the studies [15]. In addition, previous reviews only focused on published RCTs and ignored other quantitative, qualitative, and unpublished studies, which may lead to publication bias and generate incomplete evidence. Therefore, a mixed-methods systematic review (MMSR) with a convergent segregated approach following the Joanna Briggs Institute (JBI) methodology [52] for published and unpublished quantitative and qualitative studies was conducted to comprehensively evaluate the effectiveness and safety of aromatherapy.
Aim and Objectives
This review aims to systematically identify and review the present evidence on the effectiveness of aromatherapy in BPSD management for both PWD and caregivers, and its safety for PWD. The objectives of this review are to evaluate the effectiveness of aromatherapy in improving the BPSD severity and QoL of PWD, and the distress and burden of caregivers, as well as the safety of this treatment among PWD.
Methods
Search Strategy
A systematic literature search was conducted in December 2020 to identify both published and unpublished quantitative and qualitative studies written in English and Chinese, between January 1996 and December 2020 (after the term BPSD was introduced by IPA). The Population, Intervention, Comparison, and Outcome (PICO) search model was used to identify the search terms (Table 1). A total of 14 English databases (Academic Search Ultimate, AgeLine, AMED, APA PsycINFO, British Nursing Index, CINAHL Complete, EMBASE, Health & Medical Collection, MEDLINE, Ovid Emcare, PubMed, Research Library: Health & Medicine, Scopus, and Web of Science) and 6 Chinese databases (Airiti Library-CEPS Journals, CJN, CMCC, HyRead, NCL Periodical Information Center, and WanFang Data-Journal) were included in the search for published studies, and 8 databases (Airiti Library-CETD Theses, Dissertations & Theses @ Chinese University of Hong Kong, Google Scholar, Grey Literature Report, Networked Digital Library of Theses and Dissertations Global ETD Search, PQDT OPEN, ProQuest Dissertations & Theses, and WanFang Data-Chinese Dissertations Database) were included in the search for unpublished studies. The reference lists and bibliographies of the included studies and literature reviews were screened to identify additional relevant studies. The review was registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration no. CRD42021239188).
Table 1.
PICO model for identification of English and Chinese search terms
| PICO model | Search terms |
||
|---|---|---|---|
| English MeSH | English keywords | Chinese search terms | |
| P (Population) − older persons with dementia | “Dementia,” “frontotemporal dementia,” “dementia, vascular,” “dementia, multi-infarct,” “neurocognitive disorders,” “cognition disorders,” “Alzheimer disease,” “cognitive dysfunction” | “dement*,” “Alzheimer* disease*,” “cognit* impairment*,” “major neurocognitive disorder*,” “neurocognitive disorder*” |
|
|
| |||
| I (Intervention) − aromatherapy | “Aromatherapy,” “plant oils,” “oils, volatile,” “odorants” | “aromatherap*,” “aromacare,” “essential oil*,” “aroma therap*,” “aroma-therap*,” “aroma*,” “plant* oil*” |
|
|
| |||
| C (Comparison) − no active treatment/usual care | − | − | − |
|
| |||
| O (Outcome) − BPSD | “Behavioral symptoms,” “depression,” “depressive disorder,” “mental disorders,” “psychomotor agitation,” “anxiety,” “anxiety disorders,” “psychotic disorders,” “aggression,” “apathy,” “movement disorders,” “sleep wake disorders,” “appetite,” “feeding and eating disorders” | “anxi*,” “behave*,” “BPSD,” “Behave* and psych* symptom* of dement*,” “behave* adj3 symptom*,” “psych* adj3 symptom*,” “neuropsych* adj3 symptom*,” “aggress* behave*,” “aggress*,” “agitat*,” “depress*,” “mental disorders,” “psychosis,” “apathy,” “disinhibit*,” “motor disturb*,” “night-time behave*,” “appetite,” “eat* adj3 problem*” |
|
MeSH, Medical Subject Headings; PICO, Population, Intervention, Comparison, and Outcome.
Study Selection
After the literature search, duplicates, articles published before 1996, and articles not written in English or Chinese were removed from the results. The titles and abstracts of the remaining articles were screened by 2 reviewers based on the inclusion and exclusion criteria described in Table 2, and the full text of screened articles was retrieved for further assessment by 2 reviewers. Discrepancies were resolved through discussion.
Table 2.
Inclusion and exclusion criteria for quantitative and qualitative studies
| Inclusion criteria | Quantitative studies | Qualitative studies |
|---|---|---|
| Participants | For PWD | |
| ≥60 years old Diagnosis of dementia of any type and severity Presented with at least one symptom of BPSD For caregivers Both formal and informal caregivers Provided care to PWD in hospitals, residential care facilities, day-care facilities, or homes |
||
| Intervention | Aromatherapy: an intervention with the use of single essential oil or essential oil blends, in any dosage, frequencies, and approaches | N/A |
| Comparator Outcomes | Placebo treatment, no active treatment, or usual care | N/A |
| Primary outcome | N/A | |
| Change in PWD's severity of BPSD symptoms Secondary outcomes Change in PWD's QoL Change in caregivers' distress Change in caregivers' burden |
||
| Phenomenon of interest Context |
N/A | Explored caregivers' experience and perception on the effectiveness and safety of aromatherapy for PWD with BPSD and caregivers Taken place either in institutions (e.g., hospitals, residential care facilities) or in community (e.g., day-care facilities, PWD's homes) settings Any dosage, frequencies, and administration approaches of aromatherapy were considered All qualitative study design Qualitative data and results of mixed-methods studies |
| N/A | ||
| Types of studies | All quantitative study design Quantitative data and results of mixed-methods studies Written in English or Chinese (both traditional and simplified Chinese) Both published and unpublished studies From January 1996 to December 2020 |
|
| Exclusion criteria | ||
| Participants | Not all PWD in the study with dementia PWD with other mental disorders Other active pharmacological and/or nonpharmacological interventions Published as a conference abstract or a study protocol Not an original research study Full text could not be retrieved |
|
| Comparator Types of studies | N/A |
BPSD, behavioral and psychological symptoms of dementia; N/A, not applicable; PWD, older persons with dementia; QoL, quality of life.
Methodological Quality Assessment
The methodological quality of the included studies was independently appraised by 2 reviewers using the JBI critical appraisal tools [53]. Discrepancies were resolved through discussion.
Data Synthesis and Integration
This review followed the convergent segregated approach to synthesis and integration following the JBI methodology for MMSR [52]. Quantitative and qualitative syntheses were separately conducted followed by the integration of the resultant evidence.
The quantitative data were analyzed according to the study design, characteristics of the participants, interventions applied, and outcome measurements. For those studies with RCT or a pretest-posttest control (PPC) design and whose findings were described as means and standard deviations (SD), the effect sizes dppc2were calculated using the formula proposed by Morris [54]. An effect size of 0.2 was interpreted as small, 0.5 was interpreted as moderate, and 0.8 was interpreted as large [55]. The quantitative data from studies with similar designs, interventions, and outcome measurements, where possible, were pooled in a statistical meta-analysis using Review Manager 5.4 [56]. The mean change from the baseline to postintervention and the SD of the mean change were used in the meta-analysis. If the outcome was measured using different instruments, the standardized mean difference and 95% confidence interval (CI) were calculated. Otherwise, the weighted mean difference and 95% CI were calculated. The statistical heterogeneity was assessed by Higgins I2 [57], where I2 ≥50% indicates substantial heterogeneity [57], and a random-effects model was used for the statistical pooling. Otherwise, a fixed-effects model was used.
The qualitative data were analyzed according to the study design, characteristics of the participants, and implementation of aromatherapy. The qualitative data, where possible, were analyzed using the meta-aggregation approach [58], which involved the aggregation or synthesis of findings to generate a set of statements that represent such aggregation. The findings were assembled and categorized based on the similarities in their meanings, and these categories were then synthesized to produce a comprehensive set of synthesized findings.
The findings of the quantitative and qualitative syntheses were then configured. These findings were juxtaposed to check if the findings from the quantitative studies were supported or contradicted by those from the qualitative studies.
Results
Study Selection
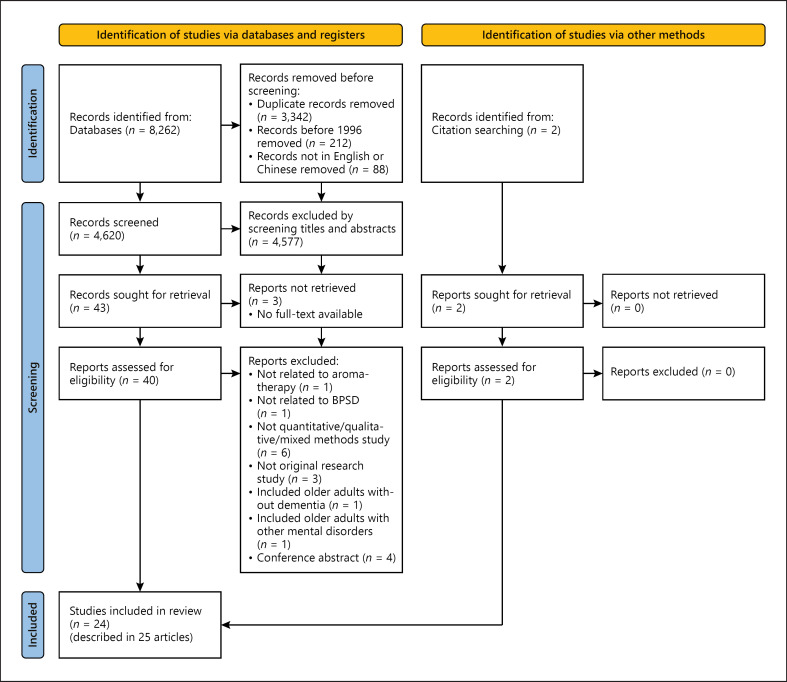
A total of 8,262 articles were identified from the databases, and 2 additional relevant articles were sourced from reference lists and bibliographies. After the screening, 45 potential articles were identified. The full texts of these articles were retrieved and reviewed, and 20 articles were eventually excluded from the review, in which 3 conference abstracts being excluded were describing interventional studies using aromatherapy on PWD with BPSD, and all of them reported positive effect in managing BPSD symptoms such as agitation, disturbed behavior, and sleep disturbance [59, 60, 61]. Finally, a total of 24 studies, as described in 25 English articles (in which one study was described in 2 articles), were included in the review, among which 22 were quantitative studies (12 RCTs and 10 quasi-experimental studies) and 2 were qualitative studies. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of the study retrieval and selection process is presented in Figure 1.
Fig. 1.
PRISMA flowchart of study retrieval and selection process. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Methodological Quality
The methodological quality of 12 RCTs was appraised using the JBI Critical Appraisal Checklist for RCTs [62] (Table 3). All RCTs satisfied more than half (≥7) of the criteria in the checklist. However, among the criteria, only half of the studies (n = 6) achieved true randomization in assigning participant and treatment groups of similar baselines. Moreover, in the treatment assignment, only 2 studies (16.7%) were able to blind their participants, and 4 studies (33.3%) were able to blind the treatment deliverers. The 10 quasi-experimental studies were appraised using the JBI Critical Appraisal Checklist for Quasi-Experimental Studies [63] (Table 4). Eight studies (80%) satisfied more than half (≥5) of the criteria in the checklist, and 2 studies (20%) satisfied only 4 criteria. Among the criteria, only 2 studies (20%) had control groups, and 3 studies (30%) had multiple measurements of the outcome both at the pre- and postintervention. The 2 qualitative studies were appraised using the JBI Critical Appraisal Checklist for Qualitative Research [64] (Table 5), and both of them satisfied more than half of the criteria (≥6). However, both studies did not state their philosophical or theoretical premises, did not locate the researchers culturally or theoretically, and did not address the influence of the researcher and vice versa.
Table 3.
Methodological quality of RCTs
| Ballard et al. [80] | Cameron et al. [65] | Fu et al. [69] | Fujii et al. [68] | Lin et al. [67] | Mascherona et al. [75] | O'Connor et al. [77] | Smallwood et al. [71] | Turten Kaymaz and Ozdemir [83] | Yang et al. [47] | Yang et al. [48]; Yang et al. [78] | Yoshiyama et al. [82] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Was true randomization used for assignment of participants to treatment groups? | Y | Y | Y | U | U | Y | Y | U | N | Y | N | U |
|
| ||||||||||||
| Was allocation to treatment groups concealed? | Y | Y | Y | U | U | Y | Y | Y | U | Y | Y | U |
|
| ||||||||||||
| Were the treatment groups similar at the baseline? | N | N | Y | Y | Y | N | U | Y | Y | Y | N | U |
|
| ||||||||||||
| Were the participants blind to treatment assignment? | Y | Y | U | u | u | U | N | u | U | U | N | U |
|
| ||||||||||||
| Were those delivering treatment blind to treatment assignment? | Y | Y | U | u | N | U | Y | Y | u | U | U | U |
|
| ||||||||||||
| Were the outcome assessors blind to treatment assignment? | Y | Y | Y | Y | N | Y | Y | Y | u | Y | Y | U |
|
| ||||||||||||
| Were treatment groups treated identically other than the intervention of interest? | N | N | N | N | Y | Y | Y | Y | Y | Y | Y | Y |
|
| ||||||||||||
| Was the follow-up complete and if not, were differences between groups in terms of their follow-up adequately described and analyzed? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
|
| ||||||||||||
| Were the participants analyzed in the groups to which they were randomized? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
|
| ||||||||||||
| Were the outcomes measured in the same way for treatment groups? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
|
| ||||||||||||
| Were outcomes measured in a reliable way? | Y | U | Y | Y | U | Y | Y | Y | Y | Y | Y | Y |
|
| ||||||||||||
| Was the appropriate statistical analysis used? | Y | U | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
|
| ||||||||||||
| Was the trial design appropriate, and any deviations from the standard RCT design (individual randomization, parallel groups) accounted for in the conduct and analysis of the trial? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
Y, yes; N, no; U, unclear; NA, not applicable; RCT, randomized controlled trial [62].
Table 4.
Methodological quality of quasi-experimental studies
| Beshara and Giddings [76] | Gray and Clair [74] | Holmes et al. [70] | Lee and Lee [81] | Moorman Li et al. [72] | Ogun-Semore [73] | Snow et al. [66] | Takeda et al. [45] | Ukwuoma [88] | Zalomonson et al. [79] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Is it clear in the study what is the “cause” and what is the “effect” (i.e., there is no confusion about which variable comes first)? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
|
| ||||||||||
| Were the participants included in any comparisons similar? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
|
| ||||||||||
| Were the participants included in any comparisons receiving similar treatment/care, other than the exposure or intervention of interest? | N | Y | Y | Y | Y | Y | Y | Y | Y | Y |
|
| ||||||||||
| Was there a control group? | N | N | N | Y | N | N | N | N | N | Y |
|
| ||||||||||
| Were there multiple measurements of the outcome both pre and post the intervention/exposure? | N | N | N | Y | U | U | Y | Y | N | N |
|
| ||||||||||
| Was the follow-up complete and if not, were differences between groups in terms of their follow-up adequately described and analyzed? | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
|
| ||||||||||
| Were the outcomes of participants included in any comparisons measured in the same way? | Y | Y | Y | Y | Y | U | Y | Y | Y | Y |
|
| ||||||||||
| Were the outcomes measured in a reliable way? | U | Y | Y | Y | Y | U | U | Y | N | Y |
|
| ||||||||||
| Was the appropriate statistical analysis used? | N | Y | Y | Y | Y | Y | Y | Y | Y | Y |
Y, yes; N, no; U, unclear; NA, not applicable [63].
Table 5.
Methodological quality of qualitative studies
| Johannessen [90] | Kilstoff and Chenoweth [91] | |
|---|---|---|
| Is there congruity between the stated philosophical perspective and the research methodology? | U | U |
| Is there congruity between the research methodology and the research question or objectives? | Y | Y |
| Is there congruity between the research methodology and the methods used to collect data? | Y | Y |
| Is there congruity between the research methodology and the representation and analysis of data? | Y | Y |
| Is there congruity between the research methodology and the interpretation of results? | Y | Y |
| Is there a statement locating the researcher culturally or theoretically? | N | N |
| Is the influence of the researcher on the research, and vice- versa, addressed? | N | N |
| Are participants, and their voices, adequately represented? | Y | Y |
| Is the research ethical according to current criteria for recent studies, and is there evidence of ethical | Y | U |
| approval by an appropriate body? | ||
| Do the conclusions drawn in the research report flow from the analysis, or interpretation, of the data? | Y | Y |
Y, yes; N, no; U, unclear; NA, not applicable [64].
Synthesis of Quantitative Data
Description of Studies
Among the 22 quantitative studies, 12 were RCTs, of which 9 had a 2-armed design and 3 had a 3-armed design, 8 used a parallel design, and 4 used a crossover design. Ten studies were quasi-experimental, of which 4 used a 1-group pretest-posttest design, 4 used a within-subjects design, 1 used a nonequivalent control group design, and 1 used a nonequivalent control group crossover design. The summaries of the RCTs and quasi-experimental studies are listed in Table 6. These studies were conducted in the USA (n = 6), UK (n = 4), Japan (n = 3), Australia (n = 2), Taiwan (n = 2), Switzerland (n = 1), Turkey (n = 1), Israel (n = 1), Korea (n = 1), and Hong Kong (n = 1). Most of these studies (55%, n = 12) were conducted in long-term residential care facility settings (e.g., nursing homes and care and attention homes), 6 in hospital inpatient settings (e.g., psychogeriatric wards and inpatient units), 2 in daycare settings (e.g., daycare centers and outpatient day programs), 1 in home-based settings, and 1 in both hospital and nursing homes.
Table 6.
Summary table of included quantitative studies
| Study | Design duration of study | Country/city setting | Symptom of BPSD sample | Intervention | Control | Effectiveness | Safety | Others |
|---|---|---|---|---|---|---|---|---|
| A. RCTs Ballard et al. [80] Cameron et al. [65] Fu et al. [69] Fujii et al. [68] |
2-Armed Parallel 4 weeks 2-Armed Crossover 3 weeks' treatment A +1-week washout +3 weeks' treatment B 3-Armed Parallel 6 weeks +6 weeks' follow-up 2-Armed Parallel 4 weeks |
UK Nursing homes UK Inpatient unit Australia Nursing homes Japan Long-term care hospital |
Agitation n = 71 Subgroup sample: 35/36 Severe dementia Mean age 78.5 years (SD 8.1) Female 59.7% General symptoms of BPSD n= 15 Subgroup sample: 6/9 Moderate-to-severe dementia Disruptive behavior n = 67 Subgroup sample: 22/23/22 Mild-to-severe dementia Mean age 84 years (SD 6.36) Female 59% General symptoms of BPSD n = 28 Subgroup sample: 14/14 Moderate-to-severe dementia Mean age 78 years (SD 10) Female 67.9% |
Aromatherapy topical application By a care assistant 2 times a day 1–2 min per time 10% Melissa essential oil Aromatherapy topical application By nursing staff 2 times a day 1 min per time <2% Melissa essential oil Aromatherapy inhalation and massage By a researcher and trained research assistants 2 times a day 3 sprays of mist to chest +5 min of massage 3% lavender mist Aromatherapy inhalation 2 times a day 3 sprays of mist to chest 3% lavender mist Aromatherapy inhalation 3 times a day 2 h per time 100% lavender essential oil 2 drops into the collar |
Placebo topical application By a care assistant 2 times a day 1–2 min per time 10% sunflower oil Placebo topical application By nursing staff 2 times a day 1 min per time <1% geranium and 0.5% lemon oil Placebo spray By researcher and trained research assistants 2 times a day Water spray to chest No active treatment |
CMAI Significant improvement dppc20.979 NPI Irritability score and aberrant motor behavior score improved significantly in intervention than control QoL parameters Significant reduction in the % of time spent socially withdrawn and significant increase in the % of time engaged in constructive activities CMAI No significant difference between intervention and control Significant increase in the scores during the washout period NPI No significant difference between intervention and control Significant increase in the scores during the washout period PAS No significant difference between intervention and control Significant increase in the scores during the washout period CMAI Mean scores reduced, but not significant No significant improvement with either intervention NPI Significant improvement dppc2 0.703 |
1 patient receiving aromatherapy topical application experienced 2 days diarrhea Possible adverse effect not described No adverse event was reported No adverse event was reported |
Adherence: weighing the bottles weekly, result not described Theoretical basis: not described Adherence: not described Theoretical basis: not described Adherence: not described Theoretical basis: not described Adherence: not described Theoretical basis: not described |
|
| ||||||||
| Lin et al. [67] | 2-Armed Crossover 3 weeks' treatment A +2 weeks' washout +3 weeks' treatment B |
Hong Kong Care and attention homes |
Agitation n = 70 Subgroup sample: 35/35 Mean age 78.29 years (SD 4.06) Female 58.6% |
Aromatherapy inhalation By care and attention homes staff 1 time a day 1 h per time 100% lavender essential oil 2 drops in a diffuser, and 2 diffusers were placed one at each side of the pillow |
Placebo inhalation By care and attention homes staff 1 time a day 1 h per times Sunflower oil 2 drops in a diffuser, and 2 diffusers were placed one at each side of the pillow |
CCMAI Significant improvement dppc20.244 No significant period and sequential effect CNPI Significant improvement dppc20.674 No significant period effect and sequential effect |
No adverse event was reported | Adherence: not described Theoretical basis: not described |
|
| ||||||||
| Mascherona et al. [75] | 2-Armed Parallel 8–14 days | Switzerland Geriatrics department |
General symptoms of BPSD n = 32 Subgroup sample: 16/16 Mean age 86.245 years (SD 6.522) Female 68.7% |
Aromatherapy inhalation and standard pharmacological treatment By nurses 4 times a day 60 min per time 6 drops 100% sweet orange essential oil into mist diffuser at 07:00, 12:00, and 15:00 6 drops 100% lavender essential oil into diffuser at 20:00 Standard pharmacological treatment |
Standard pharmacological treatment | NPI-NH Significant improvement dppc20.68 Distress scores among caregivers delivering aromatherapy inhalation decreased significantly |
No adverse event was reported | Adherence: not described Theoretical basis: not described |
|
| ||||||||
| O'Connor et al. [77] | 2-Armed Crossover 3 days' treatment A +4 days' washout +3 days treatment B + 4 days' washout |
Australia Nursing homes | Agitation n = 64 Subgroup sample: 37/27 Markedly cognitively impaired Mean age 77.6 years (SD 9.4) Female 59% |
Aromatherapy topical application By nursing staff Whenever an agitated behavior was reported 2 min per time 30% lavender essential oil 2 mL per time |
Placebo topical application By nursing staff Whenever an agitated behavior was reported 2 min per time Jojoba oil 2 mL per time | Behavioral count Agitated behavior counts were lower with intervention than with control, approaching statistical significance (p = 0.057) No significant treatment-time interactions Philadelphia Geriatric Center Affect Rating Scale Counts of positive effect |
No adverse event was reported | Adherence: not described Theoretical basis: not described |
|
| ||||||||
| Smallwood et al. [71] | 3-Armed Parallel 4 weeks | UK Hospital |
Disordered behavior n = 20 Subgroup sample: 7/7/7 (1 dropout not described in which group) Severe dementia Mean age 66.8 years (SD 11.5) Female 57.1% |
Aromatherapy massage By aromatherapist 2 times per week 1 h per time Lavender essential oil massage Conversation and aromatherapy inhalation By an aromatherapist 2 times per week 1 h per time Conversation and lavender essential oil by diffuser |
Placebo massage By aromatherapist 2 times per week 1 h per time Plain oil massage | were higher in all intervention than control periods but not to significantly different degrees Video recorded behavior No overall group differences |
Negligible side effects | Adherence: not described Theoretical basis: not described |
|
| ||||||||
| Turten Kaymaz and Ozdemir [83] | 2-Armed Parallel 4 weeks | Turkey Patients' homes |
Agitation 28 patients +28 primary caregivers Subgroup sample: 14/14 patient-caregiver pairs Mode rate-to-severe dementia Patients mean age 78.11 years (SD 7.45) Patient female 64.3% |
Aromatherapy massage and inhalation Aromatherapy massage by an aromatherapist 3 times per week 5 min per time Mixture of lemongrass and eucalyptus essential oil Aromatherapy inhalation by primary caregivers 1 time a day 1 h per time 6 drops 100% lavender essential oil to the diffuser located in the patient's room 1 m from the bed |
No intervention | CMAI Significant improvement in intervention than control NPI Significant improvement in intervention than control Significant improvement for caregiver distress in intervention than control ZBI Significant improvement in intervention than control |
Negligible side effects | Adherence: Monitored by massage and inhalation monitoring form, fully compliance was reported Theoretical basis: not described |
|
| ||||||||
| Yang et al. [47] | 2-Armed Parallel 8 weeks |
Taiwan Long-term care facilities |
Agitation, depression n = 56 Subgroup sample: 27/29 Mean age 92 years (SD 7) Female 61% |
Aromatherapy massage By trained research assistants 1 time per week 30 min per time 6% essential oil blend Regular activities |
Delay intervention Regular activities Aromatherapy massage was provided after completion of study | CCMAI No significant difference between groups regarding overall agitation CSDD-C Significant improvement dppc20.87 |
2 participants in the intervention group withdrew due to discomfort after the first aromatherapy massage session | Adherence: not described Theoretical basis: not described |
|
| ||||||||
| Yang et al. [48]; Yang et al. [78] | 3-Armed Parallel 4 weeks' +3 weeks' follow-up |
Taiwan Retirement homes for veterans and long-term care facilities |
Agitation, depression n = 186 Subgroup sample: 56/73/57 Mean age 84.08 years (SD 5.67) Female 26.3% |
Aromatherapy acupressure 5 days per week, 1 time per day No longer than 15 min per time 2.5% lavender essential oil Aromatherapy topical application 5 days per week, 1 time per day No longer than 15 min per time 2.5% lavender essential oil |
No intervention | CMAI Both interventions have significant improvement Acupressure dppc2 0.491 Topical application dppc2 0.728 Posttest score lower than post-3-week score CSDD Both interventions have significant improvement Acupressure dppc2 0.967 Topical application dppc2 1.583 Posttest score lower than post-3-week score HRV Aromatherapy acupressure was better able to inhibit the sympathetic nervous system and increase the activity of the parasympathetic nervous system compared with aromatherapy topical application |
Negligible side effects | Adherence: not described Theoretical basis: not described |
|
| ||||||||
| Yoshiyama et al. [82] | 2-Armed Crossover 4 weeks' treatment A +4 weeks' washout + 4 weeks' treatment B +4 weeks' follow-up |
Japan Nursing homes |
General symptoms of BPSD n = 12 Subgroup sample: 7/5 Mean age 82.8 years (SD 9.503) Female 100% |
Aromatherapy massage By a single researcher and an aromatherapist 3 times per week 10 min per time 1–2% essential oil blend |
Placebo massage By a single researcher and an aromatherapist 3 times per week 10 min per time Jojoba oil | CMAI No significant change for either therapy NPI-Q No significant change for either therapy CSDD The symptoms related to depression improved, but not statistically significantly, after the trials No significant change for either therapy |
Negligible side effects | Adherence: not described Theoretical basis: not described |
|
| ||||||||
| Quasi-experimental studies | ||||||||
|
| ||||||||
| Beshara and Giddings [76] |
One-group pretest-posttest 6 months |
USA Long-term care facility |
Agitation n = 10 Female 50% | Aromatherapy inhalation 9:00 a.m. and 5:00 p.m. daily Monday through Saturday with Sundays having no essential oil treatments 1 drop essential oil blend in diffuser |
− | MDS Effect of clinical aromatherapy in dementia populations is widely variable |
Possible adverse effect not described | Adherence: not described Theoretical basis: not described |
|
| ||||||||
| Gray and Clair [74] | Within-subjects 16 days | USA Residential-care facilities |
Difficult-to-manage behaviors n = 13 Female 46.2% |
Aromatherapy/ Placebo Inhalation Participants took their morning medications under 4 randomly ordered conditions: lavender, sweet orange, tea tree, or no aroma (placebo control) Each of the conditions was repeated 4 times for a total of 16 administrations 20 min before medications were dispensed, a cotton ball containing an essential oil or without oil (the control condition) was taped to the lapel of each participant |
Videotape No statistically significant differences in frequencies of resistive behaviors across the four conditions |
Possible adverse effect not described | Adherence: not described Theoretical basis: not described | |
|
| ||||||||
| Holmes et al. [70] | Within-subjects 2 weeks | UK Long-stay psychogeriatric ward |
Agitation n = 15 Severe dementia Mean age 79.0 years (SD 6.3) Female 60% |
Aromatherapy/placebo inhalation 1 time per day 2 h per time 2% lavender essential oil (intervention) or water (control) by diffuser, on alternate days A total of 5 aromatherapy and 5 placebo trials were carried out for each patient |
PAS Significant improvement in intervention than control |
Possible adverse effect not described | Adherence: not described Theoretical basis: not described | |
|
| ||||||||
| Lee and Lee [81] | Nonequivalent control group 2 weeks | Korea Hospitals and nursing homes |
Anxiety, aggressive behavior, and wandering behavior n = 43 Subgroup sample: 21/22 Mean age 77.7 years Female 62.8% |
Aromatherapy massage By the researcher and trained mental health nurses with aromatherapist certificates 1 time per day, 5 times a week 20 min per time 2% essential oil blend |
Delay intervention After completing data collection, intervention was administered to the control group | RAID Significant improvement in intervention than control dppc2 0.761 Ryden aggression scale Significant improvement in intervention than control dppc2 0.42 Algase wandering scale V2 Significant improvement in intervention than control dppc21.37 |
Possible adverse effect not described | Adherence: not described Theoretical basis: not described |
|
| ||||||||
| Moorman Li et al. [72] | One-group pretest-posttest 2 months |
USA Day-care center |
Behavioral issues (restlessness/wandering, agitation, anger, and anxiety) n = 23 Mean age 83 years Female 65.3% |
Aromatherapy inhalation 2 times per day 20 min per time Lavender essential oil in diffuser |
− | Behavior/intervention monthly flow record No. of behavioral issues was lower in the postintervention period, but did not reach statistical significance In the analysis of individual behavioral issues, there was a statistically significant difference found for the frequency of agitation |
No adverse effect reported | Adherence: not described Theoretical basis: not described |
|
| ||||||||
| Ogun-Semore [73] | One-group pretest-posttest 8 weeks |
USA. Long-term care setting |
Agitation n = 19 Mean age 72 years(SD 8.30) Female 26.3% |
Aromatherapy inhalation 4 times a week Cotton napkins with lavender essential oil placed on the table in-between each of the participants |
− | Agitation with unknown instrument Statistically significant difference between the pre- and postintervention average ratings of the level of agitation |
Possible adverse effect not described | Adherence: not described Theoretical basis: not described |
|
| ||||||||
| Snow et al. [66] | Within-subjects 4 weeks' baseline measurement + 2 weeks for each of the 5 treatment conditions (total 10 weeks) +2 weeks' postintervention measurement |
USA. Long-term care facility |
Agitation n = 7 Advanced dementia |
Aromatherapy/placebo inhalation 3 times per day Treatment conditions order: ABCBA (A = lavender essential oil, B = thyme essential oil, C = unscented grapeseed oil); all participants received all aromas in the same order A 2 × 2 inch absorbent fabric sachet was securely pinned to the front of each participant's shirt near the collarbone 2 drops 100% essential oil or unscented grapeseed oil |
Modified CMAI No treatment effect | Possible adverse effect not described | Adherence: not described Theoretical basis: not described | |
|
| ||||||||
| Takeda et al. [45] | Within-subjects 20-day control +20-day intervention | Japan Residential-care facilities |
Sleep disturbance n = 19 Mean age 80.7 years (SD 9.1) Female 47.4% |
Aromatherapy/placebo inhalation By researcher 3 types of essential oils were used for aromatherapy: (1) lavender, (2) lavender and sweet orange oil blend, and (3) Japanese cypress, Virginian cedarwood, cypress, and pine oil blend Essential oil used was selected by participants Researcher visited each participant in his or her room between 18:00 and 20:00 every day and wrapped a towel with essential oil/nothing on it around the subject's pillow before the participant went to bed |
NPI No significant difference between intervention and control Vibrometer SL: no significant differences TST: significant improvement seen with the main effect of the control and intervention periods, but no significant difference was seen with the main effect of time or period × time SE: no significant difference DLSSP: Significant improvement with the main effect of the control and intervention periods, but no significant difference was seen with the main effect of time or period × time WTASO: no significant difference EMA: a significant difference between the control and intervention periods |
Possible adverse effect not described | Adherence: not described Theoretical basis: not described | |
|
| ||||||||
| Ukwuoma [88] |
One-group pretest-posttest 4 weeks |
USA Outpatient adult day program setting |
Anxiety n = 15 Mild dementia Age 65–84 Female 60% |
Aromatherapy massage By a researcher 3 times a week 10 min per time 0.5% lavender essential oil 20 mL |
− | HAM-A No significant changes between pretest and posttest on 8 items 4 items showed an increase between pre and post: anxious mood, intellectual, GI symptoms, and behavior at intervention The increase in GI symptoms was statistically significant Two items showed a decrease between pre and post: autonomic symptoms and depressed mood The decrease in depressive mood was statistically significant Overall, there was no statistically significant difference |
Possible adverse effect not described | Adherence: not described Theoretical basis: not described |
|
| ||||||||
| Zalomonson et al. [79] | Nonequivalent control group Crossover 4 months: month 1 oil A on face→ month 2 oil A on foot → month 3 oil B on foot → month 4 oil B on face | Israel Psychogeriatric long-term care departments |
General symptoms of BPSD n = 42 Subgroup sample: 22/20 Mean age 76.1 years (SD 11.2) Female 57.2% |
Aromatherapy topical application By nurses 3 times a day 1–2 min per time 2 drops lavender essential oil |
Placebo topical application By nurses 3 times a day 1–2 min per time 2 drops sunflower seed oil | NPI No significant difference between intervention and control Use of psychotropic drugs No significant difference between intervention and control |
1 patient with exacerbation of chronic pulmonary disease while on placebo, there were 20 reported adverse events during intervention period (3 nausea, 13 sleepiness, 1 gait instability, 2 skin irritation, 1 fall) | Adherence: adherence rate was 96.4%±7.9% Theoretical basis: not described |
RCTs, randomized controlled trials; PWD, persons with dementia; BPSD, behavioral and psychological symptoms of dementia; NPI, Neuropsychiatric Inventory; SD, standard deviation; CMAI, Cohen-Mansfield Agitation Inventory; PAS, Pittsburgh Agitation Scale; CSDD, Cornell Scale for Depression in Dementia; ZBI, Zarit Burden Interview.
Participants
A total of 855 PWD with mild-to-severe dementia were included in these studies, while 1 study included 28 caregivers. The PWD sample size ranged from 7 to 186, and 59% (n = 13) of the included studies had a sample size of <30. The mean age of PWD ranged from 66.8 to 86.2 years (no data from Cameron et al. [65]), with a mean of 81.2 years (SD = 9.0). About 52% of these PWD were female (no data from Cameron et al. [65] and Snow et al. [66]).
Interventions
All studies delivered aromatherapy interventions, but their theoretical bases were not described. These interventions varied substantially in terms of their approaches, essential oils, frequencies, dosages, durations, deliverers, and settings.
Inhalation
Twelve studies delivered aromatherapy through inhalation. Among these studies, 7 used lavender (Lavandula angustifolia) essential oil alone in 100% [67, 68], 3% [69], 2% [70], and unspecified [71, 72, 73] concentrations. Two studies used different essential oils across various stages of the intervention [66, 74]. One study used 100% sweet orange (Citrus sinensis) essential oil for daytime inhalation and 100% lavender essential oil for nighttime inhalation [75]. One study used an essential oil blend with an unspecified concentration [76]. The remaining one study allowed the participants to select between 100% lavender essential oil and other 100% essential oil blends [45]. Aromatherapy inhalation was delivered using different methods. In all, six studies used diffuser [67, 70, 71, 72, 75, 76], 3 studies placed a cotton ball or fabric sachet with essential oil or dropped the essential oil on or near the collar of the participants [66, 68, 74], 1 study placed a cotton napkin with essential oil on tables [73], 1 study wrapped a towel with essential oil around the pillow of the participants [45], and 1 study sprayed essential oil mist on the chest of the participants [69]. The frequencies ranged from twice a week to 4 times a day, with each inhalation administered with 1–6 drops of essential oil and lasted from 20 min to overnight. The intervention duration lasted from 8 days to 6 months, and 2 studies involved 2 or 6 weeks of follow-up. Two studies were delivered in long-term residential care facilities by trained formal caregivers [67] or the researcher [45], 2 were delivered in hospital inpatient settings by a nurse [75] or aromatherapist [71], and others were conducted in long-term residential care facilities (n = 4), hospitals (n = 2), or daycare centers (n = 1) with unspecified deliverers.
Topical Application
Five studies delivered aromatherapy through topical application. Among these studies, 3 used lavender essential oil with 30% [77], 2.5% [48, 78], and unspecified [79] concentrations; and 2 used Melissa (Melissa officinalis) essential oil with 10% [80] or <2% [65] concentration. The frequencies ranged from once to 3 times a day, and 1 study delivered aromatherapy whenever an agitated behavior was reported. Each session lasted from 1 to 15 min. The intervention duration lasted from 3 days to 4 months, and only one study involved 3 weeks of follow-up. Two studies were delivered in long-term residential care facilities by trained care assistants [80] or nurses [77], 2 studies were delivered in hospital inpatient settings by nurses [65, 79], and 1 study was conducted in long-term residential care facilities [48, 78] with an unspecified deliverer.
Massage
Four studies delivered aromatherapy through massage. Among these studies, 1 used lavender essential oil with an unspecified concentration [71], and 3 used different essential oil blends with concentrations ranging from 1% to 6% [47, 81, 82]. The frequencies ranged from once a week to once a day, with each session lasting from 10 min to 1 h. The intervention duration lasted from 2 to 8 weeks. Two studies were delivered in long-term residential care facilities by trained research assistants [47] or the researcher and the aromatherapist [82], 1 was delivered in hospital inpatient settings by aromatherapists [71], and 1 was delivered in both hospitals and long-term residential care facilities by the researcher and nurses with aromatherapist certificates [81].
Other Approaches
Three studies delivered aromatherapy using other approaches. One study delivered aromatherapy twice a day for 6 weeks during which 3% lavender essential oil was delivered through mist spray and a 5-min hand massage by the researcher and trained research assistants in long-term residential care facilities [69]. One study delivered aromatherapy 3 times a day for 4 weeks through a combination of 3 times per week massage with essential oil blends by an aromatherapist and a once-a-day inhalation of 6 drops of 100% lavender essential oil through a diffuser by family caregivers at homes [83]. One study (described in 2 articles) involved a daily delivery of 2-min aromatherapy acupressure for 4 weeks using 2.5% lavender essential oil in long-term residential care facilities [48, 78]. Two of these studies had 3 or 6 weeks of follow-up [48, 69, 78].
Outcomes
Change in BPSD Severity for PWD
Nine studies used the Neuropsychiatric Inventory (NPI) [84] to measure neuropsychiatric symptoms of BPSD. After receiving aromatherapy, NPI decreased significantly in 5 studies compared with the control conditions, in which the aromatherapy interventions were delivered by the inhalation of 100% lavender or sweet orange essential oil with moderate effect (n = 3, dppc2= 0.674–0.763) [67, 68, 75], by the topical application of 10% Melissa essential oil with large effect (n = 1, dppc2= 0.979) [80], and by massaging an essential oil blend and inhaling 100% lavender essential oil (n = 1) [83]. Four studies reported no significant difference in NPI after delivering aromatherapy through massage, inhalation, or topical application with essential oil blends or Melissa essential oil compared with the control conditions [45, 65, 79, 82]. In those studies adopting a crossover design, no significant period and sequential effects were observed [67], and a significant increase in NPI was noted during the washout period [65].
Nine studies used the Cohen-Mansfield Agitation Inventory (CMAI) [85] to evaluate agitation. Four studies found that aromatherapy significantly relieved agitation compared with the control conditions, in which aromatherapy interventions were delivered by the inhalation of 100% lavender essential oil with small effect (n = 1, dppc2= 0.244) [67], by the topical application of 10% Melissa or 2.5% lavender essential oil with moderate to large effect (n = 2, dppc2= 0.728–0.979) [78, 80], by acupressure with 2.5% lavender essential with near moderate effect (n = 1, dppc2= 0.491) [78], and by massaging essential oil blends and inhaling 100% lavender essential oil (n = 1) [83]. Three studies that delivered aromatherapy through massage, inhalation, or topical application of 1%–6% lavender or essential oil blends revealed a decrease in agitation in the aromatherapy group; however, such change was not significant compared with the control conditions [47, 69, 82]. Two studies showed that the inhalation of 100% lavender essential oil [66] or topical application of <2% Melissa essential oil [65] had no significant treatment effect compared with the control condition. In those studies adopting a crossover design, no significant period and sequential effects were reported [67], and a significant increase in CMAI was noted during the washout period [65]. For the study with a 3-week follow-up, the follow-up CMAI score was higher than the postintervention score [78].
Three studies used the Cornell Scale for Depression in Dementia (CSDD) [86] to evaluate depression. Two studies reported a significant decrease in depression among those PWD receiving aromatherapy through acupressure, topical application, or massage with 2.5%–6% lavender essential oil with large effect (dppc2= 0.87–1.583) [47, 48]. One study that delivered aromatherapy by massage with a 1% to 2% essential oil blend reported improvement in depression, but such improvement was not significant compared with the control condition [82]. For the study with a follow-up, the follow-up CSDD score was higher than the postintervention score [78].
Two studies used the Pittsburgh Agitation Scale (PAS) [87] to evaluate agitation. One study showed a significant improvement in agitation after the inhalation of 2% lavender essential oil compared with the control condition [70]. One crossover study showed no significant difference in agitation after the topical application of <2% Melissa essential oil compared with the control condition, and the PAS score significantly increased during the washout period [65].
Ten studies used other instruments to evaluate BPSD severity. Five studies [45, 72, 73, 77, 81] showed significant improvements in BPSD after a topical application of 30% lavender essential oil, inhalation of lavender essential oil or essential oil blends with unspecified concentrations, or massage with 2% essential oil blends. One study found that the effect of essential oil blend inhalation on BPSD management widely varied, with 60% of the participants reporting a significant decrease in their disturbance behavior and the rest reporting no changes or an increase in such behavior [76]. For the other 4 studies, no significant difference was reported in anxiety, psychotropic drugs used, and behaviors related to BPSD compared with the control or preintervention condition [71, 74, 79, 88].
Change in QoL for PWD
The effects of aromatherapy on the QoL of PWD were examined in one study by using the QoL parameters from Dementia Care Mapping. The aromatherapy group receiving a 4-week topical application of 10% Melissa essential oil reported significant improvements in their QoL, with a significant reduction in the time they spend in social withdrawal behaviors, and a significant increase in the time they spend doing constructive activities [80].
Change in Caregiver Distress
The scores for caregiver distress in NPI were reported in 2 studies, both of which observed a significant reduction in caregiver distress after the PWD inhaled 100% lavender or sweet orange essential oil delivered by caregivers and/or massaged with essential oil blends by an aromatherapist [75, 83].
Change in Caregiver Burden
One study used the Zarit Burden Interview [89] to measure the change in caregiver burden and reported a significant decrease in caregiver burden after the PWD were massaged with essential oil blends by an aromatherapist and inhaled 100% lavender essential oil delivered by family caregivers [83].
Safety of Administering Aromatherapy among PWD
The possible adverse effects of aromatherapy were reported in 3 studies. One participant who received a topical application of 10% Melissa essential oil experienced diarrhea for 2 days [80], whereas 2 participants withdrew from the study due to an unspecified discomfort after being massaged with 6% essential oil blend [47]. Sleepiness, nausea, skin irritation, gait instability, and falls were reported by those participants who received a topical application of lavender essential oil [79]. Ten studies [48, 67, 68, 69, 71, 72, 75, 77, 78, 82, 83] reported negligible or no adverse events among the participants who received aromatherapy, and the remaining 9 studies [45, 65, 66, 70, 73, 74, 76, 81, 88] have not assessed the safety of aromatherapy on PWD.
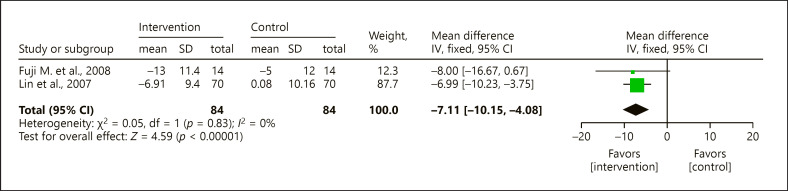
Meta-Analysis
Given inadequate reporting or the high heterogeneity among the approaches and contents of aromatherapy, only 2 RCTs had similar designs and sufficient data for meta-analysis. These studies used aromatherapy inhalation with 100% lavender essential oil as their intervention and NPI for their outcome measurement [67, 68]. The meta-analysis revealed the significant positive treatment effect of aromatherapy inhalation of 100% lavender essential oil (MD = −7.11, 95% CI = −10.15 to −4.08, p < 0.0001) and a low degree of heterogeneity (χ2 = 0.05, p = 0.83, I2 = 0%) (Fig. 2).
Fig. 2.
Forest plot of meta-analysis to 2 RCTs with aromatherapy inhalation of 100% lavender essential oil using the results of NPI as outcome measure. RCTs, randomized controlled trials; NPI, Neuropsychiatric Inventory; CI, confidence interval; SD, standard deviation.
Synthesis of Qualitative Data
Description of Studies
Two qualitative studies were included in the review, both of which were action research studies that investigated the experiences and perceptions of caregivers toward the use of aromatherapy among PWD with BPSD. One of these studies was performed in nursing homes in Norway [90], whereas the other was performed in a multicultural dementia day-care center in Australia [91]. Table 7 summarizes these studies.
Table 7.
Summary table of included qualitative studies
| Study | Design Country/city Setting | Participants | Methodology | Phenomenon of interest | Aromatherapy procedure and implementation | Conclusion | Safety | Notes |
|---|---|---|---|---|---|---|---|---|
| Johannessen [90] |
Action research Norway Nursing homes |
Residents n = 24 Nurses n = 12 | Data collection Night and daily resident reports Field notes Focus on groups and individual interview Data analysis Analysis of resident reports was made by the researcher and the responsible nurses Field notes and interviews were analyzed by the researcher alone with systematic content analysis |
Experience and perception of incorporating aromatherapy in the care of residents with dementia who suffer from anxiety and disturbed sleep patterns | By nurses 1 time per day Overnight per time 12–15 drops lavender essential into a diffuser | Nurses experienced an aromatherapy diffuser with lavender a simple and effective caring modality for residents with dementia suffering from anxiety and disturbed sleep patterns As a natural remedy, lavender was viewed as an effective tool in the care of PWD There is a need for nurses to learn more about using aromatherapy and CAM in care |
Possible adverse effect not described | Adherence: The staff were monitored by the researcher and any issues associated with the use of the specific oils were addressed and discussed Theoretical basis: not described |
|
| ||||||||
| Kilstoff and Chenoweth [91] |
Action research Australia Multicultural dementia day-care center |
Clients n= 16 Family caregivers n = 16 Day-care staff n = 7 |
Data collection Focus group discussions with memos Field notes In-depth focused interview Client observation logbooks REPDS Data analysis Content analysis of recorded focus memos, interview data, and field notes Data arising from the observation logbooks were scored and tabulated Data from REPDS were collated and analyzed numerically and then compared with perceptions of clients' dementia symptoms |
Experience and perception of a community-initiated aromatherapy hand treatment for the multicultural dementia day-care clients | By family caregivers and day-care staff 10–15 min per time Essential oil blend in pure oil base for hand massage | The aromatherapy hand massage was effective in improving clients' behaviors, emotions, and physical functioning Involvement in the hand massage program has a positive impact to all participants Family caregivers generally gained a new purpose in their caring role and became less stressed by it Clients become more alert and less agitated, function more independently, and were seen to initiate meaningful conversation with caregivers |
Possible adverse effect not described | Adherence: not described Theoretical basis: not described |
PWD, persons with dementia. CAM, complementary and alternative medicines.
Participants
The participants included 24 PWD residents and 12 nurses from 4 nursing homes [90] as well as 16 PWD clients, 16 family caregivers, and 7 staff from one dementia day-care center [91].
Aromatherapy Implementation
In these 2 studies, the caregivers were involved in the design, implementation, and evaluation of aromatherapy programs for PWD to manage their BPSD. In Johannessen [90], the nurses from nursing homes delivered overnight aromatherapy inhalation to the residents by placing 12–15 drops of lavender essential oil into a diffuser. In Kilstoff and Chenoweth [91], the family caregivers and day-care staff delivered hand massage to PWD with essential oil blends for 10–15 min. Both studies did not describe the safety issues or adverse effects of aromatherapy on PWD.
Meta-Aggregation
After analyzing the full texts of these 2 qualitative studies, 46 findings related to the effectiveness of aromatherapy in managing BPSD were identified. These findings were aggregated into 6 categories and further aggregated into 2 synthesized findings (Table 8).
Table 8.
Synthesized findings of meta-aggregation
| Findings | Categories | Synthesized findings |
|---|---|---|
| Unclear effect (C) Slight increase in BPSD symptoms (U) Reduce withdrawal (U) Reduce wandering (U) Reduce restlessness (C) Reduce repeated questioning (C) Reduce challenging behaviors (U) Reduce anxiety (U) Reduce agitation (U) No effect (C) Increase irritation (C) Improve sleep (U) Effect is short term (U) |
1.1. Effectiveness in BPSD management | 1. Effectiveness for PWD |
|
| ||
| One feel distasteful (C) More relaxed (C) More calm (C) Look forward to the treatment (C) Increase social activities (U) Increase interest in surroundings (U) Increase functional abilities (U) Increase contentment (C) Increase cheerfulness (C) Increase alertness (U) Improve mood (C) Improve communication (C) Enjoyable (U) Accept aromatherapy (C) |
1.2. Effectiveness in other aspects | |
|
| ||
| Relaxing (U) Reflect on negative attitudes (C) More focus on client (U) More effective in dealing with difficult behaviors (C) More calm (C) Less distress (C) Initiate increased difficulties in caregiving, but the beneficial effect will override it (C) Increase in understanding of the nature of dementia symptoms (C) Increase in joy and pleasure (U) Greater sense of control (C) Greater emphasis on sense of well-being (C) Facilitate better understanding of client's need (U) Develop greater coping mechanisms (C) Accept a caregiving role in a more positive way (C) |
2.1. Effectiveness in the emotion and caregiving aspects | 2. Effectiveness for caregivers |
|
| ||
| Vehicle to reconnect (C) Improve personal relationship (U) |
2.2. Effectiveness in improving relationships between caregivers and PWD | |
|
| ||
| Interest in using essential oils after the project is completed (U) Interest in using essential oil in other ways (U) Interest in learning more (U) |
2.3. Effectiveness in promoting interest in aromatherapy | |
U, unequivocal (findings accompanied by an illustration, i.e., beyond a reasonable doubt and therefore not open to challenge); C, credible (findings accompanied by an illustration lacking clear association with it and therefore open to challenge); PWD, persons with dementia; BPSD, behavioral and psychological symptoms of dementia.
Synthesized Finding 1: Effectiveness for PWD
Aromatherapy has an overall positive effect on PWD in terms of BPSD management and other aspects.
Category 1.1. Effectiveness in BPSD Management
Most of the caregivers in both studies [90, 91] claimed that BPSD, such as sleep disturbance, anxiety, agitation, and restlessness, significantly decreased among those PWD who received aromatherapy. However, some caregivers also reported either a slight increase in aggression, anger, sulking, and irritation, unclear effects, or no effects at all. Overall, aromatherapy may positively contribute to reducing BPSD among most of the PWD in these studies.
Category 1.2. Effectiveness in Other Aspects
Most of the family caregivers perceived that the PWD looked forward to and enjoyed aromatherapy. They became more alert, relaxed, calm, cheerful, interested in their surroundings and social activities, and showed improvements in their communication and functional abilities after receiving aromatherapy [91].
Synthesized Finding 2: Effectiveness for Caregivers
The caregivers claimed that they benefited from aromatherapy, that their relationships with PWD were improved, and that they were interested to learn more about the treatment.
Category 2.1. Effectiveness in the Emotion and Caregiving Aspects
The caregivers reported that aromatherapy helped them focus on their own well-being, develop greater coping mechanisms and a sense of control, and calm themselves down. However, some of them reported initial difficulties in caregiving when the PWD became more alert and inquisitive after receiving aromatherapy, but the perceived benefits of aromatherapy for these PWD overrode such initial negative effect [91]. In sum, aromatherapy generates an overall positive effect on the emotion and caregiving for caregivers.
Category 2.2. Effectiveness in Improving Relationships between Caregivers and PWD
The family caregivers reported improvements in their relationships with their PWD relatives after delivering aromatherapy [91]. They perceived aromatherapy as a vehicle for them to reconnect with their PWD relatives and improve their personal relationships.
Category 2.3. Effectiveness in Promoting Interest in Aromatherapy
The nurses reported that their participation in the aromatherapy program and the effectiveness of aromatherapy for the PWD raised their interest in essential oils and inspired them to continue using aromatherapy after the completion of the program [90].
Integration of Quantitative and Qualitative Evidence
More than half of the quantitative studies (68%, n = 15) reported improvements in BPSD severity after receiving aromatherapy compared with the control conditions, with 12 studies reporting a statistically significant difference between the aromatherapy and control conditions. However, 7 quantitative studies reported no improvements. The findings from the quantitative studies were supported by qualitative findings given that most of the caregivers in the qualitative studies reported the positive effects of aromatherapy in reducing BPSD, including sleep disturbance, agitation, and anxiety, even if some caregivers reported a slight increase in irritation, aggression, anger, and sulking. Apart from BPSD management, most caregivers from the qualitative studies reported other positive effects of aromatherapy on PWD, such as improvements in their functional abilities, alertness, communication, interest in their surroundings, and feelings of enjoyment and relaxation, all of which contribute to their QoL as reflected in a quantitative study that measured the QoL of PWD [80].
The quantitative studies reported a significant reduction in distress and burden among those caregivers who administered aromatherapy. These findings were supported by those of qualitative studies, wherein caregivers described their experiences and perceptions toward how they could benefit from aromatherapy. They generally described aromatherapy as a soothing and pleasant experience. Specifically, by delivering aromatherapy to PWD, they improved their understanding about the needs of PWD, learned to develop better coping mechanisms, and improved their relationship with the PWD. Moreover, by actively participating in the aromatherapy program and experiencing the positive effects of aromatherapy on PWD, these caregivers became more interested in knowing how to use aromatherapy in other ways, such as in reducing their own distress and burden.
While 3 quantitative studies reported that aromatherapy may have adverse effects [47, 79, 80], 10 reported negligible or no adverse events [48, 67, 68, 69, 71, 72, 75, 77, 78, 82, 83] after completing the aromatherapy program. However, these safety issues were not described in the qualitative studies.
Adherence Monitoring
Only 4 studies described the methods they used to monitor the adherence or the adherence rate of the delivered aromatherapy interventions. These methods included weighing the bottles with aromatherapy products [80], using massage and inhalation monitoring form [83], or monitoring by the researcher [90]. The adherence rates were reported as either full adherence [83] or 96.4% ± 7.9% adherence [79].
Discussion
Effectiveness and Safety of Aromatherapy
Consistent with the results of the recent Cochrane Review [15], the studies included in this review failed to reach a consensus with regard to the effectiveness of aromatherapy in reducing BPSD severity.
More than half of the included studies (68%, 15 quantitative and 2 qualitative studies) reported that aromatherapy positively contributed to reducing BPSD severity, with 12 studies reporting a statistical significance with effect sizes ranging from small to large (dppc20.244–1.583). Meanwhile, 7 quantitative studies reported no significant differences between the aromatherapy and control conditions, which may be due to the high diversity in their intervention approaches, essential oils, dosages, frequencies and duration of aromatherapy interventions, and methodologies and instruments for outcome measurement. Meanwhile, those studies that did not report positive findings had relatively small sample sizes (5–22 participants in each intervention/control condition) compared with other studies (7–73 participants in each condition), thereby increasing their chances of producing false nonsignificant results for intervention with a smaller effect [92, 93]. Furthermore, most of these studies did not monitor adherence to the treatment. Poor adherence and nonadherence may reduce the effects of aromatherapy and increase the risk of producing false nonsignificant results for the intervention.
Only one quantitative study measured the change in QoL after receiving aromatherapy [80] and reported that such treatment reduced social withdrawal and promoted constructive activities among the PWD. The positive effects of aromatherapy on the functional abilities, communication, social activities, and mood of the PWD as described in qualitative studies [90, 91] may contribute to such changes in QoL.
Two quantitative studies measured the changes in distress and/or burden among those caregivers who administered the aromatherapy interventions, and both of these studies reported a significant decrease in caregiver distress and/or burden after the delivery of the intervention [75, 83]. In the qualitative studies, the caregivers reported positive experiences and perceptions toward the program and reported that aromatherapy benefited them in both the emotion and caregiving aspects and improved their relationships with the PWD [90, 91], all of which may contribute to reducing their distress and burden. These improvements may also be attributed to how BPSD severity of PWD is reduced by aromatherapy, and the olfactory stimulation or percutaneous absorption of essential oils into the bodies of caregivers during their delivery of the intervention [36, 41, 42, 43]. Therefore, these findings suggest that delivering aromatherapy could decrease the burden or distress of caregiving.
Only 3 studies reported the possible adverse effects of the topical application or massage of essential oils, but most of these effects were only mild and temporary (e.g., sleepiness, nausea, and skin irritation). Meanwhile, those studies that delivered inhalation reported no adverse effects of aromatherapy. Aromatherapy has short-term effects as reflected in the absence of period and sequential effects or increases in BPSD severity during the washout period in crossover studies [65, 67] and during the postintervention follow-up assessments [48, 78]. These findings agreed well with those of human and animal laboratory studies that focused on the pharmacokinetics of essential oils, wherein essential oil chemicals were completely removed from the body in <4 h [94, 95, 96, 97]. Therefore, aromatherapy, especially inhalation, is a safe intervention given its very low risk of producing prolonged adverse effects on the health of the receiver.
Implications for Research and Practice
The most frequently used aromatherapy approach in the reviewed studies was inhalation (62.5%, n = 15), among which 73.3% (n = 11) reported the positive effect of aromatherapy on BPSD and 46.7% (n = 7) reported a statistical significance between the aromatherapy and control conditions. None of these studies reported any adverse effects related to aromatherapy inhalation. With the above evidence, aromatherapy inhalation is considered the safest intervention for reducing BPSD severity. However, the delivery methods, essential oils, dosages, frequencies, and duration of aromatherapy inhalation in these studies show high heterogeneity. Therefore, the most effective components of aromatherapy inhalation warrant further examination.
However, evidence on the effectiveness of aromatherapy in reducing BPSD severity among PWD was generally inconsistent, while evidence on its effects on the QoL of PWD and the distress and burden of caregivers was limited. More structuralized and comparable studies could then be conducted with sufficient sample size and adherence monitoring to obtain conclusive findings regarding the effectiveness of aromatherapy in managing BPSD.
The included studies and previous laboratory studies revealed that aromatherapy only has short-term effects, but none of the included studies reported the theoretical basis for such intervention. Theory is “a set of concepts, definitions, and propositions that explain or predict events or situations by illustrating the relationships between variables” [98] and is recommended in the development and implementation of health-related interventions [99, 100]. Theory can identify the constructs and facilitate the specification of potential active ingredients in order to improve the effects of an intervention, facilitate the accumulation of evidence, and improve one's understanding of the mechanisms behind changes [101, 102]. Future studies may develop interventions based on appropriate theories and empirical evidence to maintain the positive long-term effects of aromatherapy.
Strengths and Limitations
This systematic review was conducted by extensively searching for published and unpublished literature in academic databases to maximize the possibility of identifying and including studies related to the use of aromatherapy in BPSD management. By synthesizing both quantitative and qualitative findings, this article presents a highly comprehensive review of the current evidence related to the provision of aromatherapy to PWD experiencing BPSD.
However, this review has several limitations. First, this review only considered those studies written in English or Chinese. Therefore, some related studies written in other languages were excluded from the search. Second, this review was limited by the poor methodological quality of some studies due to their lack of relevant information. However, this limitation of the included studies was acknowledged and made explicit through their methodological quality scores. Third, given the wide variety in the approaches and components used in aromatherapy and the outcome measures used in the included studies, performing a meta-analysis of most of the included quantitative studies was impossible. Fourth, this review also considered unpublished studies that did not undergo peer review. Therefore, the validity of findings from these studies cannot be guaranteed. Fifth, despite receiving help from a librarian in sending requests to both local and oversea libraries, the full texts of several articles that might be relevant to the review were not retrieved for further assessment.
Conclusion
This MMSR aims to synthesize evidence from both published and unpublished quantitative and qualitative studies by using a convergent segregated approach [52] to evaluate the effectiveness and safety of aromatherapy in BPSD management. Evidence regarding the effectiveness of aromatherapy in reducing BPSD severity was inconsistent due to the high heterogeneity in the delivery approaches, outcome assessment methods, small sample sizes, and lack of adherence monitoring in the included studies. Some of these studies reported significant improvements in the QoL of PWD and the distress and burden of caregivers, positive experiences from the caregivers who delivered aromatherapy, and low adverse effects (with aromatherapy inhalation having no adverse effect at all) of the treatment.
Therefore, aromatherapy, especially in the inhalation approach, is a safe and effective strategy for BPSD management. However, structuralized and comparable studies with sufficient sample size, adherence monitoring, and a sound theoretical basis could be conducted to obtain conclusive findings regarding the effectiveness of aromatherapy in BPSD management.
Statement of Ethics
As this is a systematic review, ethical approval and consent to participate were not applicable. This review has been submitted for registration on PROSPERO (registration no. CRD42021239188).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This review has received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contributions
Under the supervision of C.W.H.C and I.K.Y.W., B.S.Y.L. designed the study, analyzed the data, and wrote the initial draft of the manuscript, and B.S.Y.L. and Y.H.U.Y. conducted literature searching, methodological quality appraisal, and data extraction. All the authors participated in revising and final approval of the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge the Chinese University of Hong Kong for supporting the postgraduate study undertaken by the first, third, and fifth authors; Dr. Kai Chow Choi for providing statistical consultation; and university librarians' suggestions in database searching and assistance in retrieving articles for this review.
References
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 2.World Health Organization ICD-11 for mortality and morbidity statistics. 2020 [Google Scholar]
- 3.World Health Organization Ageing and health. 2018 [Google Scholar]
- 4.World Health Organization Dementia. 2020 [Google Scholar]
- 5.IPA . The IPA complete guides to BPSD: specialists guide [Internet] Milwaukee: International Psychogeriatric Association; 2012. Available from: http://www.bsa.ualberta.ca/sites/default/files/____IPA_BPSD_Specialists_Guide_Online.pdf. [Google Scholar]
- 6.Finkel SI, Costa e Silva J, Cohen G, Miller S, Sartorius N. Behavioral and psychological signs and symptoms of dementia: a consensus statement on current knowledge and implications for research and treatment. Int J Geriatr Psychiatry. 1997;12:1060–1. doi: 10.1017/s1041610297003943. [DOI] [PubMed] [Google Scholar]
- 7.Haibo X, Shifu X, Pin NT, Chao C, Guorong M, Xuejue L, et al. Prevalence and severity of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Chinese: findings from the Shanghai three districts study. Aging Ment Health. 2013;17((6)):748–52. doi: 10.1080/13607863.2013.781116. [DOI] [PubMed] [Google Scholar]
- 8.Huang S-S, Wang W-F, Liao Y-C. Severity and prevalence of behavioral and psychological symptoms among patients of different dementia stages in Taiwan. Arch Clin Psychiatry. 2017;44((4)):89–93. [Google Scholar]
- 9.Mukherjee A, Biswas A, Roy A, Biswas S, Gangopadhyay G, Das SK. Behavioural and psychological symptoms of dementia: correlates and impact on caregiver distress. Dement Geriatr Cogn Dis Extra. 2017;7((3)):354–65. doi: 10.1159/000481568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaingankar J, Chong S, Abdin E, Picco L, Jeyagurunathan A, Seow E, et al. Behavioral and psychological symptoms of dementia: prevalence, symptom groups and their correlates in community-based older adults with dementia in Singapore. Int Psychogeriatr. 2017;29((8)):1363–76. doi: 10.1017/S1041610217000564. [DOI] [PubMed] [Google Scholar]
- 11.Makimoto K, Kang Y, Kobayashi S, Liao XY, Panuthai S, Sung HC, et al. Prevalence of behavioural and psychological symptoms of dementia in cognitively impaired elderly residents of long-term care facilities in East Asia: a cross-sectional study. Psychogeriatr. 2019;19((2)):171–80. doi: 10.1111/psyg.12380. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . The global dementia observatory reference guide (version 1.1) [Internet] Geneva: World Health Organization; 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/272669/WHO-MSD-MER-18.1-eng.pdf?ua=1. [Google Scholar]
- 13.Baharudin AD, Din NC, Subramaniam P, Razali R. The associations between behavioral-psychological symptoms of dementia (BPSD) and coping strategy, burden of care and personality style among low-income caregivers of patients with dementia. BMC Public Health. 2019;19((4)):447. doi: 10.1186/s12889-019-6868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin B, Liu H. Comparative efficacy and safety of therapy for the behavioral and psychological symptoms of dementia: a systemic review and Bayesian network meta-analysis. J Neurol. 2019;266((10)):2363–75. doi: 10.1007/s00415-019-09200-8. [DOI] [PubMed] [Google Scholar]
- 15.Ball EL, Owen-Booth B, Gray A, Shenkin SD, Hewitt J, McCleery J. Aromatherapy for dementia. Cochrane Database Syst Rev. 2020;8:CD003150. doi: 10.1002/14651858.CD003150.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canevelli M, Adali N, Cantet C, Andrieu S, Bruno G, Cesari M, et al. Impact of behavioral subsyndromes on cognitive decline in Alzheimer's disease: data from the ICTUS study. J Neurol. 2013;260((7)):1859–65. doi: 10.1007/s00415-013-6893-3. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Wang Y, Wang Z. The effectiveness of massage and touch on behavioural and psychological symptoms of dementia: a quantitative systematic review and meta-analysis. J Adv Nurs. 2017;73((10)):2283–95. doi: 10.1111/jan.13311. [DOI] [PubMed] [Google Scholar]
- 18.Cooper C, Livingston G. Mental health/psychiatric issues in elder abuse and neglect. Clin Geriatr Med. 2014;30((4)):839–50. doi: 10.1016/j.cger.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Feil DG, MacLean C, Sultzer D. Quality indicators for the care of dementia in vulnerable elders. J Am Geriatr Soc. 2007;55((Suppl 2)):S293–301. doi: 10.1111/j.1532-5415.2007.01335.x. [DOI] [PubMed] [Google Scholar]
- 20.Nourhashémi F, Andrieu S, Sastres N, Ducassé JL, Lauque D, Sinclair AJ, et al. Descriptive analysis of emergency hospital admissions of patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2001;15((1)):21–5. doi: 10.1097/00002093-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Beeri MS, Werner P, Davidson M, Noy S. The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer's disease patients. Int J Geriatr Psychiatry. 2002;17((5)):403–8. doi: 10.1002/gps.490. [DOI] [PubMed] [Google Scholar]
- 22.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu SL, Jin Y, Shi ZH, Huo YR, Guan YL, Liu MY, et al. The effects of behavioral and psychological symptoms on caregiver burden in frontotemporal dementia, Lewy body dementia, and Alzheimer's disease: clinical experience in China. Aging Ment Health. 2017;21((6)):651–7. doi: 10.1080/13607863.2016.1146871. [DOI] [PubMed] [Google Scholar]
- 24.Hiyoshi-Taniguchi K, Becker CB, Kinoshita A. What behavioral and psychological symptoms of dementia affect caregiver burnout? Clin Gerontol. 2018;41((3)):249–54. doi: 10.1080/07317115.2017.1398797. [DOI] [PubMed] [Google Scholar]
- 25.Lethin C, Leino-Kilpi H, Bleijlevens MH, Stephan A, Martin MS, Nilsson K, et al. Predicting caregiver burden in informal caregivers caring for persons with dementia living at home: a follow-up cohort study. Dementia. 2020 Apr;19((3)):640–60. doi: 10.1177/1471301218782502. [DOI] [PubMed] [Google Scholar]
- 26.Zwijsen SA, Kabboord A, Eefsting JA, Hertogh CMPM, Pot AM, Gerritsen DL, et al. Nurses in distress? An explorative study into the relation between distress and individual neuropsychiatric symptoms of people with dementia in nursing homes. Int J Geriatr Psychiatry. 2014 Apr;29((4)):384–91. doi: 10.1002/gps.4014. [DOI] [PubMed] [Google Scholar]
- 27.Kamiya M, Sakurai T, Ogama N, Maki Y, Toba K. Factors associated with increased caregivers' burden in several cognitive stages of Alzheimer's disease. Geriatr Gerontol Int. 2014 Apr;14((Suppl 2)):45–55. doi: 10.1111/ggi.12260. [DOI] [PubMed] [Google Scholar]
- 28.Song J-A, Oh Y. The association between the burden on formal caregivers and behavioral and psychological symptoms of dementia (BPSD) in Korean elderly in nursing homes. Arch Psychiatr Nurs. 2015;29((5)):346–54. doi: 10.1016/j.apnu.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Feast A, Moniz-Cook E, Stoner C, Charlesworth G, Orrell M. A systematic review of the relationship between behavioral and psychological symptoms (BPSD) and caregiver well-being. Int Psychogeriatr. 2016;28((11)):1761–74. doi: 10.1017/S1041610216000922. [DOI] [PubMed] [Google Scholar]
- 30.Dyer SM, Harrison SL, Laver K, Whitehead C, Crotty M. An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioral and psychological symptoms of dementia. Int Psychogeriatr. 2018;30((3)):295–309. doi: 10.1017/S1041610217002344. [DOI] [PubMed] [Google Scholar]
- 31.Azermai M, Petrovic M, Elseviers MM, Bourgeois J, Van Bortel LM, Vander Stichele RH. Systematic appraisal of dementia guidelines for the management of behavioural and psychological symptoms. Ageing Res Rev. 2012;11((1)):78–86. doi: 10.1016/j.arr.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Guideline Adaptation Committee . Clinical practice guidelines and principles of care for people with dementia [Internet] Australia: Guideline Adaptation Committee; 2016. Available from: https://cdpc.sydney.edu.au/wp-content/uploads/2019/06/CDPC-Dementia-Guidelines_WEB.pdf. [Google Scholar]
- 33.Tible OP, Riese F, Savaskan E, von Gunten A. Best practice in the management of behavioural and psychological symptoms of dementia. Ther Adv Neurol Disord. 2017;10((8)):297–309. doi: 10.1177/1756285617712979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee GE, Kim JY, Jung JH, Kang HW, Jung IC. Non-pharmacological interventions for patients with dementia: a protocol for a systematic review and meta-analysis. Medicine. 2019;98((38)):e17279. doi: 10.1097/MD.0000000000017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strom BS, Ytrehus S, Grov EK. Sensory stimulation for persons with dementia: a review of the literature. J Clin Nurs. 2016;25((13–14)):1805–34. doi: 10.1111/jocn.13169. [DOI] [PubMed] [Google Scholar]
- 36.Halcon LL, Buckle J. Aromatherapy. In: Snyder M, Lindquist R, editors. Complementary/ alternative therapies in nursing. New York: Springer Publishing Company; 2006. [Google Scholar]
- 37.Kusmerik J. Aromatherapy for the family. London: Institute of Classical Aromatherapy, Wigmore Publications; 1992. [Google Scholar]
- 38.Price S, Price L. Aromatherapy for health professionals. 2 ed. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- 39.International Federation of Aromatherapists. Explore aromatherapy. n.d.
- 40.Raetz J. A nondrug approach to dementia. J Fam Pract. 2013;62((10)):548. [PubMed] [Google Scholar]
- 41.Kayne SB. Aromatherapy. In: Kayne SB, editor. Complementary and alternative medicine. ProQuest Ebook Central; 2008. [Google Scholar]
- 42.Pifferi S, Menini A. The olfactory system: from odorant molecules to perception. In: Bagetta G, Cosentino M, Sakurada T, editors. Aromatherapy: basic mechanisms and evidence-based clinical use Clinical pharmacognosy series. Boca Raton: CRC Press; 2016. [Google Scholar]
- 43.Waxman SG. Clinical neuroanatomy. 29 ed. New York: McGraw-Hill; 2020. [Google Scholar]
- 44.Wolfe N, Herzberg J. Letter to the editor. Can aromatherapy oils promote sleep in severely demented patients? Int J Geriat Psychiatry. 1996;11((10)):926–7. [Google Scholar]
- 45.Takeda A, Watanuki E, Koyama S. Effects of inhalation aromatherapy on symptoms of sleep disturbance in the elderly with dementia. Evid Based Complement Alternat Med. 2017;2017:1902807–7. doi: 10.1155/2017/1902807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forrester LT, Maayan N, Orrell M, Spector AE, Buchan LD, Soares-Weiser K. Aromatherapy for dementia. Cochrane Database Syst Rev. 2014;((2)):CD003150. doi: 10.1002/14651858.CD003150.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Yang YP, Wang CJ, Wang JJ. Effect of aromatherapy massage on agitation and depressive mood in individuals with dementia. J Gerontol Nurs. 2016;42((9)):38–46. doi: 10.3928/00989134-20160615-03. [DOI] [PubMed] [Google Scholar]
- 48.Yang MH, Lin LC, Wu SC, Chiu JH, Lin JG. The use of aroma-acupoint therapy and aromatherapy to treat depression in dementia patients with severe cognitive impairment. Medicinal Aromat Plants. 2017;6((2)) [Google Scholar]
- 49.Lee SY. [The effect of lavender aromatherapy on cognitive function, emotion, and aggressive behavior of elderly with dementia] Taehan Kanho Hakhoe Chi. 2005;35((2)):303–12. doi: 10.4040/jkan.2005.35.2.303. [DOI] [PubMed] [Google Scholar]
- 50.Alzheimer's Society Aromatherapy, massage and dementia. 2020. Available from: https://www.alzheimers.org.uk/about-dementia/treatments/alternative-therapies/aromatherapy-massage.
- 51.Fung JK, Tsang HW, Chung RC. A systematic review of the use of aromatherapy in treatment of behavioral problems in dementia. Geriatr Gerontol Int. 2012;12((3)):372–82. doi: 10.1111/j.1447-0594.2012.00849.x. [DOI] [PubMed] [Google Scholar]
- 52.Lizarondo L, Stern C, Carrier J, Godfrey C, Rieger K, Salmond S, et al. Chapter 8: mixed methods systematic reviews. In: Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. JBI; 2020. [DOI] [PubMed] [Google Scholar]
- 53.Joanna Briggs Institute. Critical appraisal tools. n.d.
- 54.Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Methods. 2008;11((2)):364–86. [Google Scholar]
- 55.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 56.The Cochrane Collaboration . Review manager 5.4 (RevMan 5.4) 5.4 ed. Copenhagen: The Nordic Cochrane Centre; 2020. [Google Scholar]
- 57.Deeks JJ, Higgins JPT, Altman DG. Chapter 10: analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane handbook for systematic reviews of interventions version 60. London: Cochrane; 2019. [Google Scholar]
- 58.Lockwood C, Porrit K, Munn Z, Rittenmeyer L, Salmond S, Bjerrum M, et al. Chapter 2: systematic reviews of qualitative evidence. In: Aromataris E, Munn Z, editors. BI manual for evidence synthesis. JBI; 2020. [Google Scholar]
- 59.Holmes C, Hopkins V, Ronenvinge H, Wilkinson D, MacLaughlin V, Hensford C. The effects of aromatherapy on symptoms of agitation in patients with severe dementing illness. Neurobiol Aging. 2002;23((1)):S123. [Google Scholar]
- 60.Guendling A, Guendling PW. Aroma therapy in dementia. Eur J Integr Med. 2010;2((4)):227. [Google Scholar]
- 61.Hanson L, Cagan A, Rinehimer K, Flottemesch T, Clairmont J, Sackett-Lundeen L, et al. Lavender essential oil improves sleep in residents of a memory care assisted living facility. Alzheimer's Dementia. 2013;9((4)):655–6. [Google Scholar]
- 62.Joanna Briggs Institute JBI critical appraisal checklist for randomized control trials. 2020 [Google Scholar]
- 63.Joanna Briggs Institute JBI critical appraisal checklist for quasi-experimental studies. 2020 [Google Scholar]
- 64.Joanna Briggs Institute JBI critical appraisal checklist for qualitative research. 2020 [Google Scholar]
- 65.Cameron H, du Toit S, Richard G, Bearns L. Using lemon balm oil to reduce aggression and agitation in dementia: results of a pilot study. J Dement Care. 2011;19((5)):36–8. [Google Scholar]
- 66.Snow LA, Hovanec L, Brandt J. A controlled trial of aromatherapy for agitation in nursing home patients with dementia. J Altern Complement Med. 2004;10((3)):431–7. doi: 10.1089/1075553041323696. [DOI] [PubMed] [Google Scholar]
- 67.Lin PW, Chan WC, Ng BF, Lam LC. Efficacy of aromatherapy (Lavandula angustifolia) as an intervention for agitated behaviours in Chinese older persons with dementia: a cross-over randomized trial. Int J Geriatr Psychiatry. 2007;22((5)):405–10. doi: 10.1002/gps.1688. [DOI] [PubMed] [Google Scholar]
- 68.Fujii M, Hatakeyama R, Fukuoka Y, Yamamoto T, Sasaki R, Moriya M, et al. Lavender aroma therapy for behavioral and psychological symptoms in dementia patients. Geriatr Gerontol Int. 2008;8((2)):136–8. doi: 10.1111/j.1447-0594.2008.00461.x. [DOI] [PubMed] [Google Scholar]
- 69.Fu CY, Moyle W, Cooke M. A randomised controlled trial of the use of aromatherapy and hand massage to reduce disruptive behaviour in people with dementia. BMC Complement Altern Med. 2013;13((1)):165–73. doi: 10.1186/1472-6882-13-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holmes C, Hopkins V, Hensford C, MacLaughlin V, Wilkinson D, Rosenvinge H. Lavender oil as a treatment for agitated behaviour in severe dementia: a placebo controlled study. Int J Geriatr Psychiatry. 2002;17((4)):305–8. doi: 10.1002/gps.593. [DOI] [PubMed] [Google Scholar]
- 71.Smallwood J, Brown R, Coulter F, Irvine E, Copland C. Aromatherapy and behaviour disturbances in dementia: a randomized controlled trial. Int J Geriatr Psychiatry. 2001;16((10)):1010–3. doi: 10.1002/gps.473. [DOI] [PubMed] [Google Scholar]
- 72.Moorman Li R, Gilbert B, Orman A, Aldridge P, Leger-Krall S, Anderson C, et al. Evaluating the effects of diffused lavender in an adult day care center for patients with dementia in an effort to decrease behavioral issues: a pilot study. J Drug Assess. 2017;6((1)):1–5. doi: 10.1080/21556660.2016.1278545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogun-Semore OY. Lavender aroma therapy for agitation in elderly with dementia. Ann Arbor: Brandman University; 2019. p. p. 51. [Google Scholar]
- 74.Gray SG, Clair AA. Influence of aromatherapy on medication administration to residential-care residents with dementia and behavioral challenges. Am J Alzheimers Dis Other Demen. 2002;17((3)):169–74. doi: 10.1177/153331750201700305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mascherona I, Ferretti M, Soldini E, Biggiogero M, Maggioli C, Fontana PE. Essential oil therapy for the short-term treatment of behavioral and psychological symptoms of dementia: a monocentric randomized pilot study. Aging Clin Exp Res. 2021 Aug;33((8)):2251–9. doi: 10.1007/s40520-020-01754-2. [DOI] [PubMed] [Google Scholar]
- 76.Beshara M, Giddings D. Use of plant essential oils in treating agitation in a dementia unit 10 case studies. Int J Aromather. 2002;12((4)):207–12. [Google Scholar]
- 77.O'Connor DW, Eppingstall B, Taffe J, van der Ploeg ES. A randomized, controlled cross-over trial of dermally-applied lavender (Lavandula angustifolia) oil as a treatment of agitated behaviour in dementia. BMC Complement Altern Med. 2013;13((1)):315–21. doi: 10.1186/1472-6882-13-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang MH, Lin LC, Wu SC, Chiu JH, Wang PN, Lin JG. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. 2015;15((1)):93. doi: 10.1186/s12906-015-0612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zalomonson S, Freud T, Punchik B, Samson T, Lebedinsky S, Press Y. The results of a crossover placebo-controlled study of the effect of lavender oil on behavioral and psychological symptoms of dementia. Rejuvenation Res. 2019;22((3)):246–53. doi: 10.1089/rej.2018.2123. [DOI] [PubMed] [Google Scholar]
- 80.Ballard CG, O'Brien JT, Reichelt K, Perry EK. Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: the results of a double-blind, placebo-controlled trial with Melissa. J Clin Psychiatry. 2002;63((7)):553–8. doi: 10.4088/jcp.v63n0703. [DOI] [PubMed] [Google Scholar]
- 81.Lee S-Y, Lee J-S. The effects on aromatherapy and foot reflex massage on the cognition, anxiety, aggressive behavior and wandering behavior of elderly with dementia. J Digit Policy Management. 2013;11((12)):495–505. [Google Scholar]
- 82.Yoshiyama K, Arita H, Suzuki J. The effect of aroma hand massage therapy for people with dementia. J Altern Complement Med. 2015;21((12)):759–65. doi: 10.1089/acm.2015.0158. [DOI] [PubMed] [Google Scholar]
- 83.Turten Kaymaz T, Ozdemir L. Effects of aromatherapy on agitation and related caregiver burden in patients with moderate to severe dementia: a pilot study. Geriatr Nurs. 2017;38((3)):231–7. doi: 10.1016/j.gerinurse.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 84.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44((12)):2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 85.Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol. 1989;44((3)):M77–84. doi: 10.1093/geronj/44.3.m77. [DOI] [PubMed] [Google Scholar]
- 86.Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biol Psychiatry. 1988;23((3)):271–84. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 87.Rosen J, Burgio L, Kollar M, Cain M, Allison M, Fogleman M, et al. The Pittsburgh agitation scale: a user-friendly instrument for rating agitation in dementia patients. Am J Geriatr Psychiatry. 1994;2((1)):52–9. doi: 10.1097/00019442-199400210-00008. [DOI] [PubMed] [Google Scholar]
- 88.Ukwuoma N. Aromatherapy hand massage using lavender oil to reduce anxiety in older adults who attend adult day program. Ann Arbor: Brandman University; 2019. p. p. 45. [Google Scholar]
- 89.Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20((6)):649–55. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]
- 90.Johannessen B. Nurses experience of aromatherapy use with dementia patients experiencing disturbed sleep patterns. An action research project. Complement Ther Clin Pract. 2013;19((4)):209–13. doi: 10.1016/j.ctcp.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 91.Kilstoff K, Chenoweth L. New approaches to health and well-being for dementia day-care clients, family carers and day-care staff. Int J Nurs Pract. 1998;4((2)):70–83. doi: 10.1046/j.1440-172x.1998.00059.x. [DOI] [PubMed] [Google Scholar]
- 92.Hackshaw A. Small studies: strengths and limitations. Eur Respir J. 2008;32((5)):1141–3. doi: 10.1183/09031936.00136408. [DOI] [PubMed] [Google Scholar]
- 93.Faber J, Fonseca LM. How sample size influences research outcomes. Dent Press J Orthod. 2014;19((4)):27–9. doi: 10.1590/2176-9451.19.4.027-029.ebo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kohlert C, van Rensen I, März R, Schindler G, Graefe EU, Veit M. Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Med. 2000;66((6)):495–505. doi: 10.1055/s-2000-8616. [DOI] [PubMed] [Google Scholar]
- 95.Kohlert C, Schindler G, März RW, Abel G, Brinkhaus B, Derendorf H, et al. Systemic availability and pharmacokinetics of thymol in humans. J Clin Pharmacol. 2002;42:731–7. doi: 10.1177/009127002401102678. [DOI] [PubMed] [Google Scholar]
- 96.Li W, Hong B, Li Z, Li Q, Bi K. GC-MS method for determination and pharmacokinetic study of seven volatile constituents in rat plasma after oral administration of the essential oil of Rhizoma Curcumae. J Pharm Biomed Anal. 2018;149:577–85. doi: 10.1016/j.jpba.2017.11.058. [DOI] [PubMed] [Google Scholar]
- 97.Pavan B, Dalpiaz A, Marani L, Beggiato S, Ferraro L, Canistro D, et al. Geraniol pharmacokinetics, bioavailability and its multiple effects on the liver antioxidant and xenobiotic-metabolizing enzymes. Front Pharmacol. 2018;9:18. doi: 10.3389/fphar.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.National Cancer Institute . Theory at a glance: a gudie for health promotion practice. 2 ed. Washington, DC: Department of Health and Human Services; 2005. [Google Scholar]
- 99.Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ. 2000 Sep 16;321((7262)):694–6. doi: 10.1136/bmj.321.7262.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008 Sep 29;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ. 2000 Sep 16;321((7262)):694–6. doi: 10.1136/bmj.321.7262.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Michie S, Prestwich A. Are interventions theory-based? Development of a theory coding scheme. Health Psychol. 2010 Jan;29((1)):1–8. doi: 10.1037/a0016939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.