Abstract
Background
Targeted therapies directed at specific driver oncogenes have improved outcomes for individuals with advanced non‐small cell lung cancer (NSCLC). Approximately 5% of lung adenocarcinomas, the most common histologic subtype of NSCLC, harbour rearrangements in the anaplastic lymphoma kinase (ALK) gene leading to constitutive activity of the ALK kinase. Crizotinib was the first tyrosine kinase inhibitor (TKI) demonstrated to be effective in advanced NSCLC. Next‐generation ALK TKIs have since been developed including ceritinib, alectinib, brigatinib, ensartinib, and lorlatinib, and have been compared with crizotinib or chemotherapy in randomised controlled trials (RCTs). These ALK‐targeted therapies are currently used in clinical practice and are endorsed in multiple clinical oncology guidelines.
Objectives
To evaluate the safety and efficacy of ALK inhibitors given as monotherapy to treat advanced ALK‐rearranged NSCLC.
Search methods
We conducted electronic searches in the Cochrane Lung Cancer Group Specialised Register, Cochrane Central Register of Controlled Trials, MEDLINE, and Embase. We also searched conference proceedings from the American Society for Clinical Oncology (ASCO), European Society of Medical Oncology (ESMO), and International Association for the Study of Lung Cancer (IASLC) World Conference on Lung Cancer, as well as the reference lists of retrieved articles. All searches were conducted from 2007 until 7 January 2021.
Selection criteria
We included RCTs comparing ALK inhibitors with cytotoxic chemotherapy or another ALK inhibitor in individuals with incurable locally advanced or metastatic pathologically confirmed ALK‐rearranged NSCLC.
Data collection and analysis
Two review authors independently assessed studies for eligibility, extracted study characteristics and outcome data, and assessed risk of bias using the Cochrane risk of bias tool for each included study. We assessed the certainty of evidence using GRADE. Primary outcomes were progression‐free survival (PFS) and adverse events (AE); secondary outcomes were overall survival (OS), OS at one year, overall response rate (ORR) by RECIST (Response Evaluation Criteria in Solid Tumours) criteria, and health‐related quality of life (HRQoL). We performed a meta‐analysis for all outcomes, where appropriate, using the fixed‐effect model. We reported hazard ratios (HR) for PFS, OS, and a composite HRQoL of life outcome (time to deterioration), and risk ratios (RR) for AE, ORR, and one‐year OS. We presented 95% confidence intervals (95% CIs) and used the I² statistic to investigate heterogeneity. We planned comparisons of 'ALK inhibitor versus chemotherapy' and 'next‐generation ALK inhibitor versus crizotinib’ with subgroup analysis by type of ALK inhibitor, line of treatment, and baseline central nervous system involvement.
Main results
Eleven studies (2874 participants) met our inclusion criteria: six studies compared an ALK inhibitor (crizotinib, ceritinib, and alectinib) to chemotherapy, and five studies compared a next‐generation ALK inhibitor (alectinib, brigatinib, and lorlatinib) to crizotinib. We assessed the evidence for most outcomes as of moderate to high certainty. Most studies were at low risk for selection, attrition, and reporting bias; however, no RCTs were blinded, resulting in a high risk of performance and detection bias for outcomes reliant on subjective measurement.
ALK inhibitor versus chemotherapy
Treatment with ALK inhibitors resulted in a large increase in PFS compared to chemotherapy (HR 0.45, 95% CI 0.40 to 0.52, 6 RCTs, 1611 participants, high‐certainty evidence). This was found regardless of line of treatment.
ALK inhibitors may result in no difference in overall AE rate when compared to chemotherapy (RR 1.01, 95% CI 1.00 to 1.03, 5 RCTs, 1404 participants, low‐certainty evidence).
ALK inhibitors slightly improved OS (HR 0.84, 95% CI 0.72 to 0.97, 6 RCTs, 1611 participants, high‐certainty evidence), despite most included studies having a significant number of participants crossing over from chemotherapy to receive an ALK inhibitor after the study period.
ALK inhibitors likely increase ORR (RR 2.43, 95% CI 2.16 to 2.75, 6 RCTs, 1611 participants, moderate‐certainty evidence) including in measurable baseline brain metastases (RR 4.88, 95% CI 2.18 to 10.95, 3 RCTs, 108 participants) when compared to chemotherapy. ALK inhibitors result in a large increase in the HRQoL measure, time to deterioration (HR 0.52, 95% CI 0.44 to 0.60, 5 RCTs, 1504 participants, high‐certainty evidence) when compared to chemotherapy.
Next‐generation ALK inhibitor versus crizotinib
Next‐generation ALK inhibitors resulted in a large increase in PFS (HR 0.39, 95% CI 0.33 to 0.46, 5 RCTs, 1263 participants, high‐certainty evidence), particularly in participants with baseline brain metastases.
Next‐generation ALK inhibitors likely result in no difference in overall AE (RR 1.00, 95% CI 0.98 to 1.01, 5 RCTs, 1263 participants, moderate‐certainty evidence) when compared to crizotinib.
Next‐generation ALK inhibitors likely increase OS (HR 0.71, 95% CI 0.56 to 0.90, 5 RCTs, 1263 participants, moderate‐certainty evidence) and slightly increase ORR (RR 1.18, 95% CI 1.10 to 1.25, 5 RCTs, 1229 participants, moderate‐certainty evidence) including a response in measurable brain metastases (RR 2.45, 95% CI 1.7 to 3.54, 4 RCTs, 138 participants) when compared to crizotinib.
Studies comparing ALK inhibitors were conducted exclusively or partly in the first‐line setting.
Authors' conclusions
Next‐generation ALK inhibitors including alectinib, brigatinib, and lorlatinib are the preferred first systemic treatment for individuals with advanced ALK‐rearranged NSCLC. Further trials are ongoing including investigation of first‐line ensartinib. Next‐generation inhibitors have not been compared to each other, and it is unknown which should be used first and what subsequent treatment sequence is optimal.
Keywords: Humans; Anaplastic Lymphoma Kinase; Anaplastic Lymphoma Kinase/genetics; Carcinoma, Non-Small-Cell Lung; Carcinoma, Non-Small-Cell Lung/drug therapy; Carcinoma, Non-Small-Cell Lung/genetics; Lung Neoplasms; Lung Neoplasms/drug therapy; Lung Neoplasms/genetics; Progression-Free Survival; Protein Kinase Inhibitors; Protein Kinase Inhibitors/adverse effects
Plain language summary
Targeted treatment of non‐small cell lung cancer with an anaplastic lymphoma kinase (ALK) gene mutation
Background
The most common type of lung cancer is non‐small cell lung cancer (NSCLC). About 5% of NSCLC will be driven by a gene mutation known as anaplastic lymphoma kinase (ALK). Targeted treatments for those with advanced (not curable) ALK‐mutated NSCLC cancer have been developed and found to be more effective than chemotherapy. The first ALK inhibitor to be developed was crizotinib. Newer ALK‐targeted drugs have also been developed and include ceritinib, alectinib, brigatinib, ensartinib, and lorlatinib. In this review we looked at treatments that target ALK‐mutated NSCLC to find out how well they work.
Objectives
The primary objective of this review was to find out whether people with ALK‐mutated NSCLC given treatments targeted towards ALK live longer without recurrence and have fewer side effects than those treated with chemotherapy. We also planned to evaluate whether newer ALK‐targeted drugs achieve this better than crizotinib.
Study characteristics
We searched the main medical databases and records of conferences up to 7 January 2021. We found 11 studies (2874 participants): six studies compared an ALK‐targeted drug to chemotherapy, and five studies compared a newer ALK‐targeted drug to crizotinib. The studies were conducted in people with advanced ALK‐mutated NSCLC using these drugs as their first or later treatment. A total of five different ALK inhibitors were used across studies: alectinib, brigatinib, ceritinib, crizotinib, and lorlatinib.
Results
People treated with ALK‐targeted drugs lived longer without their cancer growing than those on chemotherapy. These improvements were also seen in people with cancer that had spread to the brain. People receiving ALK‐targeted drugs lived longer overall, even when some had received chemotherapy first. ALK‐targeted drugs cause a similar number of side effects as chemotherapy. ALK‐targeted drugs caused more tumours to reduce in size and resulted in a longer time until worsening of symptoms when compared to chemotherapy.
People treated with newer ALK‐targeted drugs lived longer without their cancer growing than those receiving crizotinib, including in people with cancer involving the brain. People treated with newer ALK‐targeted drugs as first treatment were likely to live longer overall with a similar number of overall side effects. Newer ALK‐targeted drugs caused more tumours to reduce in size when compared to crizotinib.
The evidence for most reported measures was of moderate or high certainty.
Conclusions
The best first treatment for people with incurable ALK‐mutated lung cancer is a newer ALK inhibitor such as alectinib, brigatinib, ceritinib, or lorlatinib. More studies are needed to determine which of these options is best and what treatment should be used when the cancer grows after these medicines have been given.
Summary of findings
Summary of findings 1. ALK inhibitor compared to any cytotoxic chemotherapy for advanced anaplastic lymphoma kinase (ALK)‐rearranged non‐small cell lung cancer.
| ALK inhibitor compared to any cytotoxic chemotherapy for advanced anaplastic lymphoma kinase (ALK)‐rearranged non‐small cell lung cancer | |||||
| Patient or population: advanced anaplastic lymphoma kinase (ALK)‐rearranged non‐small cell lung cancer Intervention: ALK inhibitor Comparison: chemotherapy | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with any cytotoxic chemotherapy | Risk with ALK inhibitor | ||||
| Progression‐free survival: all participants | Study population | HR 0.45 (0.40 to 0.52) | 1611 (6 RCTs) | ⊕⊕⊕⊕ HIGH | |
| 0 per 1000 | 0 per 1000 | ||||
| Progression‐free survival in people with central nervous system disease | Study population | HR 0.51 (0.41 to 0.62) | 581 (6 RCTs) | ⊕⊕⊕⊕ HIGH | |
| 0 per 1000 | 0 per 1000 | ||||
| Overall adverse events | Study population | RR 1.01 (1.00 to 1.03) | 1404 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | |
| 964 per 1000 | 974 per 1000 (954 to 993) | ||||
| Grade 5 adverse events (excluding progressive disease) | Study population | RR 2.03 (0.89 to 4.66) | 1611 (6 RCTs) | ⊕⊕⊝⊝ LOW 3 | |
| 8 per 1000 | 15 per 1000 (7 to 36) | ||||
| Overall survival | Study population | HR 0.84 (0.72 to 0.97) | 1611 (6 RCTs) | ⊕⊕⊕⊕ HIGH | |
| 0 per 1000 | 0 per 1000 | ||||
| Overall response rate | Study population | RR 2.43 (2.16 to 2.75) | 1611 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 4 | |
| 275 per 1000 | 671 per 1000 (597 to 756) | ||||
| Health‐related quality of life: time to deterioration in composite endpoint (cough, dyspnoea, and chest pain) | Study population | HR 0.52 (0.44 to 0.60) | 1504 (5 RCTs) | ⊕⊕⊕⊕ HIGH | |
| 0 per 1000 | 0 per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for risk of bias, as trials were unblinded, and adverse event is a subjective outcome. 2Downgraded one level for imprecision, as summary statistic includes both no difference in adverse events and potential reduction in adverse events for chemotherapy. 3Downgraded two levels for imprecision, as there were fewer than 100 events, and the summary statistic includes both gain and no effect. 4Downgraded one level for inconsistency, as I² greater than 75% (85%).
Summary of findings 2. Next‐generation ALK inhibitor compared to crizotinib for advanced anaplastic lymphoma kinase (ALK)‐rearranged non‐small cell lung cancer.
| Next‐generation ALK inhibitor compared to crizotinib for advanced anaplastic lymphoma kinase (ALK)‐rearranged non‐small cell lung cancer | |||||
| Patient or population: advanced anaplastic lymphoma kinase (ALK)‐rearranged non‐small cell lung cancer Intervention: next‐generation ALK inhibitor Comparison: crizotinib | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with crizotinib | Risk with next‐generation ALK inhibitor | ||||
| Progression‐free survival: overall population | Study population | HR 0.39 (0.32 to 0.46) | 1263 (5 RCTs) | ⊕⊕⊕⊕ HIGH | |
| 0 per 1000 | 0 per 1000 | ||||
| Progression‐free survival in people with central nervous system disease | Study population | HR 0.25 (0.19 to 0.34) | 406 (5 RCTs) | ⊕⊕⊕⊕ HIGH | |
| 0 per 1000 | 0 per 1000 | ||||
| Overall adverse events | Study population | RR 1.00 (0.98 to 1.01) | 1263 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| 990 per 1000 | 990 per 1000 (970 to 1000) | ||||
| Grade 5 adverse events (excluding progressive disease) | Study population | RR 0.85 (0.49 to 1.47) | 1263 (5 RCTs) | ⊕⊕⊝⊝ LOW 2 | |
| 42 per 1000 | 36 per 1000 (21 to 62) | ||||
| Overall survival | Study population | HR 0.71 (0.56 to 0.90) | 1263 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | |
| 0 per 1000 | 0 per 1000 | ||||
| Overall response rate | Study population | RR 1.18 (1.10 to 1.25) | 1229 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| 691 per 1000 | 816 per 1000 (760 to 864) | ||||
| Health‐related quality of life: time to deterioration in composite endpoint (cough, dyspnoea, and chest pain) | Study population | HR 1.10 (0.72 to 1.68) | 303 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 5 | |
| 0 per 1000 | 0 per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for risk of bias, as assessors for adverse events were not blinded to treatment allocation, which might have affected this outcome. 2Downgraded two levels for imprecision, as there were fewer than 100 events, and the summary statistic includes both gain and no effect. 3Downgraded one level for inconsistency, as trials found both a positive effect and no effect. 4Downgraded one level for imprecision, as there were fewer than 400 participants (303). 5Downgraded one level for indirectness, as result was based on population from only one trial.
Background
Description of the condition
Lung cancer leads to the most cancer deaths worldwide of any tumour type, causing approximately one‐quarter of all cancer deaths annually (Siegel 2019).
Non‐small cell lung cancer (NSCLC) comprises approximately 85% of lung cancers overall (Novello 2016), and is further divided into squamous and non‐squamous histological subtypes, including adenocarcinoma. Typically, NSCLC is diagnosed at an advanced stage, with five‐year survival for stage IV cancer estimated at 3% to 6% (Chansky 2017). Platinum doublet chemotherapies have been the standard first‐line chemotherapy and achieve modest improvements, with median overall survival (OS) between 7.9 months found in Schiller 2002 and 11.8 months in Scagliotti 2008.
Evolution in understanding lung cancer at the molecular level has led to new and tailored therapies showing significant efficacy in defined subgroups of lung cancer patients (Hirsch 2016). Potentially targetable mutations have been found in up to 64% of lung adenocarcinomas (Kris 2014); however, not all of these mutations have clinically validated treatments.
Non‐squamous NSCLC has been further classified according to the presence of driver gene mutations, and current international molecular testing guidelines recommend universal testing of patients with advanced non‐squamous NSCLC for mutations in the epidermal growth factor receptor (EGFR) and rearrangements of the anaplastic lymphoma kinase (ALK), ROS proto‐oncogene 1, receptor tyrosine kinase (ROS1), and BRAF genes (Kalemkerian 2018). This is regardless of clinical characteristics. Furthermore, more extended panels are recommended in some patients to include detection of KRAS proto‐oncogene, GTPase (KRAS), erb‐b2 receptor tyrosine kinase 2 (ERBB2), MET proto‐oncogene, receptor tyrosine kinase (MET), and ret proto‐oncogene (RET) gene alterations. These driver mutations are considered mutually exclusive, to the extent that sequential testing strategies can be used. Targeted therapies are only effective against a tumour where the specific target (or gene mutation) has been found on molecular testing of biopsy material or circulating tumour DNA.
Advanced NSCLC driven by EGFR mutations were the first molecularly defined subgroup where an impact on survival was demonstrated using a targeted therapy approach. First‐line tyrosine kinase inhibitors (TKI), including erlotinib and gefitinib, have demonstrated efficacy superior to chemotherapy, but only when tailored to treat cancers driven by sensitising mutations in exons 18 to 21 of the EGFR gene (Fukuoka 2011).
In 2007, ALK gene rearrangements were discovered to occur in NSCLC (Rikova 2007; Soda 2007). Patients with ALK‐rearranged advanced NSCLC have subsequently been identified as the next subgroup of lung cancer patients to gain survival benefit from targeted therapy. Approximately 5% of NSCLC is driven by the ALK oncogene (Barlesi 2016; Solomon 2009), with patients typically being younger, light or never‐smokers with adenocarcinoma histology (Shaw 2009).
Independent of the discovery that driver mutations respond to targeted therapy, immunotherapy has recently demonstrated efficacy in NSCLC. Checkpoint inhibitors, including PD‐1 and PD‐L1 targeting antibodies such as pembrolizumab, atezolizumab, and nivolumab, have changed the landscape of advanced NSCLC treatment with improved OS and durable disease response when compared to chemotherapy (Kim 2018; Reck 2021).
Subgroup analysis of second‐line immunotherapy trials have demonstrated that patients with NSCLC driven by EGFR or ALK mutations do not gain the same benefits from immunotherapy when given as a single agent (Gainor 2016). Most subsequent trials have excluded patients with ALK‐rearranged NSCLC, and immunotherapy appears to be less effective in this group (Mazieres 2019). More recently, trials are under way to investigate the combination of targeted therapy (including ALK inhibitors) with immunotherapy (Moya‐Horno 2018). This strategy is not current standard practice, and in fact one trial revealed unacceptable toxicity with the combination of gefitinib and durvalumab immunotherapy (Creelan 2019).
Although first‐line EGFR‐targeted therapy combined with chemotherapy has been shown to be effective (Seike 2018), this has not been demonstrated in the ALK setting. International guidelines currently recommend that ALK inhibitors be used as monotherapy.
This review focuses on the role of targeted therapy, specifically ALK inhibitors, to treat patients with NSCLC driven by the ALK gene rearrangement.
Description of the intervention
Over the last decade, multiple TKIs have been developed to target the ALK fusion kinase. These ALK inhibitors are medications taken orally up to twice a day and continuously to maintain effect.
Crizotinib (formerly known as PF02341066) was the first‐in‐class ALK inhibitor and is a TKI with activity against ALK, c‐MET, and ROS1 kinases. Crizotinib has been compared to chemotherapy in the treatment of ALK‐rearranged advanced NSCLC in phase III clinical trials. In both the first‐ and second‐line setting (Shaw 2013; Solomon 2014), crizotinib was the first drug shown to have significantly higher overall response rates (ORR) and progression‐free survival (PFS) than chemotherapy in this subgroup of lung cancer patients. Although these trials were not of cross‐over design, participants were allowed to receive crizotinib after progression on chemotherapy which affected the OS outcomes. The impact of cross‐over on OS is also apparent in subsequent ALK inhibitor trials. Longer follow‐up of the initial phase III first‐line crizotinib studies demonstrates a four‐year OS of 57%. The median OS is not yet reached at 46 months follow‐up (Solomon 2018). This is a significant and meaningful outcome when considering the five‐year survival more broadly for stage IV lung adenocarcinoma is estimated at 2% (Cetin 2011).
Despite the efficacy of crizotinib in treating ALK‐rearranged NSCLC, acquired resistance to crizotinib inevitably results in disease progression whilst on treatment. The pattern and mechanism of disease progression can guide the most appropriate next step in treatment (Lin 2017). A high rate of central nervous system (CNS) metastases occurs in this setting, Costa 2015, due to lower levels of crizotinib reaching the brain (Costa 2011), which may represent a pharmacokinetic failure of the TKI. At a molecular level, acquired resistance mechanisms can include employment of other oncogenic pathways to bypass the inhibited ALK fusion protein or alteration of the ALK target through the gain of additional mutations (Camidge 2012).
This acquired resistance was the trigger to develop more potent, CNS‐penetrant, and specific next‐generation ALK inhibitors including ceritinib, alectinib, brigatinib, ensartinib, and lorlatinib. Each TKI has a different potency (Gainor 2016a), with some also able to inhibit kinases other than the ALK fusion kinase, including ROS1, RET, and NTRK. This review is limited to efficacy against the ALK fusion kinase. Next‐generation ALK inhibitors were initially tested in crizotinib‐resistant patients and have been found to be effective, including for the treatment of CNS metastases (Gainor 2015). Evidence has rapidly evolved to demonstrate efficacy in the first‐line setting compared to crizotinib in Shaw 2017 and chemotherapy in Soria 2017. Current guidelines give an option of four different ALK inhibitors (ASCO guideline), all supported by phase III trial data. With an approach of sequential ALK inhibitors, survival outcomes achieved in ALK‐positive patients are unprecedented in the advanced NSCLC setting. Some real‐world publications now estimate a median OS of nearly seven years (Pacheco 2019), and five‐year OS of 60% (Pacheco 2019a), which is comparable to resected stage II lung cancer (Goldstraw 2016).
How the intervention might work
The chromosomal rearrangement of the ALK gene results in a constitutively active ALK fusion kinase protein. This kinase was demonstrated in preclinical studies to be an oncogenic driver, Soda 2008, that was sensitive to inhibition by a TKI (McDermott 2008). This finding was rapidly followed by early‐phase studies including patients with lung cancer, confirming clinical efficacy and safety of crizotinib. International guidelines responding to results of phase III clinical trials recommend first‐line targeted therapy in NSCLC patients with ALK gene rearrangements rather than chemotherapy or immunotherapy (Planchard 2018).
Why it is important to do this review
ALK inhibitors have already been established as the standard of care in treating advanced ALK‐positive lung cancer. The necessary development of multiple potent next‐generation ALK inhibitors contributes to a rapidly evolving treatment paradigm. Our initial literature search found a number of expert opinion publications (Cameron 2015), but only one systematic review assessing efficacy (Barrows 2019), which included publications up until July 2017. Further relevant phase III trials have been published since. A systematic review and meta‐analysis of ALK inhibitor toxicities has been completed (Costa 2018), including studies up to July 2017.
With multiple next‐generation drugs being tested in first and subsequent lines of treatment, determining the ideal sequence becomes more complex. Sequencing strategies have been proposed based on potential resistance mechanisms (Gainor 2016a), but with a rapidly evolving evidence base, a robust assessment of high‐level clinical trial outcomes is needed to inform and update the optimal treatment algorithm for this specific group of lung cancer patients.
An issue confronting clinicians now is which ALK inhibitor should be used first and in what sequence to gain the optimal outcomes for ALK‐rearranged NSCLC patients (Recondo 2018).
Objectives
To evaluate the safety and efficacy of ALK inhibitors given as monotherapy to treat advanced ALK‐rearranged NSCLC.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that were open‐label, single‐blind, or double‐blind studies. We did not apply language restrictions and included abstracts where sufficient data were provided.
Types of participants
We included studies involving participants with advanced (stage III or IV) NSCLC harbouring ALK gene rearrangements diagnosed histologically or cytologically using immunohistochemistry or fluorescence in situ hybridisation (FISH) analysis. There were no limitations by age, gender, or demographics. We did not include people with ROS1 mutations.
Types of interventions
We considered any administration of therapies targeting the oncogenic ALK fusion kinase (ALK inhibitors) versus chemotherapy or other ALK inhibitors, when used as a first (naive to systemic therapy) or subsequent (previously treated) line of treatment.
ALK inhibitors included but were not limited to crizotinib, ceritinib, alectinib, entrectinib, lorlatinib, brigatinib, and ensartinib. We excluded studies that involved ALK inhibitors in combination with other systemic treatments. EGFR and ALK‐driven lung cancers can be considered different pathologies, and as such, we did not include EGFR‐targeted therapies in this review.
We completed the following comparisons.
Any ALK inhibitor versus any cytotoxic chemotherapy
One ALK inhibitor versus another ALK inhibitor
The only studies found for comparison 2 were next‐generation ALK inhibitors compared to crizotinib.
Types of outcome measures
Primary outcomes
Progression‐free survival (PFS): defined as the time from date of randomisation to date of objective disease progression by Response Evaluation Criteria in Solid Tumours (RECIST 1.1; Eisenhauer 2009) or death from any cause, whichever occurred first.
Adverse events (AE) as reported by the included trials individually. We presented the incidence of overall, grade 3 and 4, grade 5 AEs and by AE type (gastrointestinal, haematological, hepatic, and general). AE grade (1 to 5) was defined by common terminology criteria for AEs (CTCAE v4). We also presented treatment reductions, which includes possible dose reduction, interruption, and treatment discontinuation as a measure of tolerability.
Secondary outcomes
Overall survival (OS): defined as time from date of randomisation to date of death from any cause, or study end date if the participant was alive.
OS at one year: measured as a percentage alive one year after randomisation.
Overall response rate (ORR) by RECIST 1.1 criteria (Eisenhauer 2009). We also reported partial response and complete response rates.
Health‐related quality of life (HRQoL): as measured on a validated generic or disease‐specific scale.
Search methods for identification of studies
Electronic searches
We searched the following databases from 2007 to 7 January 2021.
Cochrane Lung Cancer Group Specialised Register
Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 1)
MEDLINE (Appendix 2)
Embase (Appendix 3)
The Cochrane Lung Cancer Group Information Specialists developed the search strategies for the three main databases: CENTRAL (Appendix 1), MEDLINE (Appendix 2), and Embase (Appendix 3). The search string for MEDLINE was developed according to the Cochrane Highly Sensitive Search Strategy, sensitivity maximising version (2008 version) as referenced in Section 6.4.11.1 and detailed in box 6.4.b of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We also conducted a search of ClinicalTrials.gov (clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/).
We commenced searches from 2007, which is the year that ALK rearrangements were discovered in NSCLC. We placed no restrictions on language or publication type, including abstract format.
Searching other resources
We also searched proceedings from the following conferences from 2017 to 7 January 2021:
American Society for Clinical Oncology (ASCO);
European Society of Medical Oncology (ESMO);
International Association for the Study of Lung Cancer (IASLC) World Conference on Lung Cancer.
We also searched the reference lists of included studies and narrative reviews.
Data collection and analysis
We used standard Cochrane methodologies for data collection (Higgins 2011).
Selection of studies
Four review authors used the Covidence tool for screening studies identified by the search (Covidence). Two review authors independently screened the title/abstract of each record to assess eligibility. We obtained the full texts of all studies deemed potentially relevant, and two review authors independently assessed these for eligibility according to the inclusion criteria for the review.
Any discordant evaluations were reviewed by a fifth review author to reach consensus. We documented the reasons for exclusion of studies excluded at full‐text stage in the report and recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009). In the case of multiple publications for the same study, we included the most mature outcome data.
Data extraction and management
Two review authors independently extracted the following data from each included study.
Study details: study citation and name if applicable, study sponsor and location, study design and period, exclusion and inclusion criteria, year of publication, number of participants randomised , participant discontinuation rate, and duration of study follow‐up.
Participant baseline characteristics: age, gender, ethnicity , Eastern Cooperative Oncology Group (ECOG) performance status, smoking status, stage of NSCLC, histological subtype, CNS metastases at recruitment, previous treatment.
Intervention: dose, route, frequency, and duration of targeted therapy and comparator intervention.
Outcomes: PFS, AE, OS, one‐year OS, ORR, HRQoL measures, median OS, median PFS, and reported cross‐over rate.
The comparisons are outlined in the Types of interventions section.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the risk of bias tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were discussed with a third review author (LC or VJ) to reach consensus. We evaluated risk of bias according to the following domains.
Selection bias: random sequence generation
Selection bias: allocation concealment
Performance bias: blinding of participants and personnel, evaluated for each outcome measure
Detection bias: blinding of outcome assessors, evaluated for each outcome measure
Attrition bias: incomplete outcome data, evaluated for each outcome measure
Reporting bias: selective outcome reporting
Other bias
We assessed each risk of bias domain as low, high, or unclear risk of bias. We anticipated that a number of studies would not be blinded due to the different methods of drug administration. For each included study, we reviewed the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We judged studies as at low risk of performance bias if they were blinded, or if the lack of blinding could not have affected the outcome, such as in the case of mortality. We assessed blinding and incomplete outcome data separately for different outcomes or classes of outcomes. We also assessed blinding of outcome assessors who used RECIST criteria of tumour measurement to assess response. Where publications stated independent central review of images, this was considered to be blinded, as the assessor would have no knowledge of participant allocation. In contrast, investigators would be aware of the allocated treatment and open to bias. For example, an investigator‐assessed PFS was considered high risk, and an independent central review of PFS was considered low risk of detection bias.
Measures of treatment effect
We reported hazard ratios (HRs) and their standard errors (SEs) for time‐to‐event outcomes: PFS, OS, and time to deterioration (TTD). If data had been analysed using a Cox proportional hazards model, we used this to produce a direct estimate of the HR and its SE. If HRs were not supplied, but survival curves were given, we used these to estimate the HRs. If an HR had been supplied with a measure of error, which is not an SE (such as a confidence interval (CI) or P value), we used the inbuilt calculator in Review Manager 5 to convert these to an SE (Review Manager 2020).
We anticipated that participant cross‐over in the included studies would result in OS differences being less apparent when presented as HRs. Hence, for OS and PFS we also presented median length of survival time with CIs. In addition, we presented the study reported cross‐over rate (%) from control group to comparator TKI after leaving the study.
For the continuous outcome, HRQoL, we planned to report the results as mean differences (MDs), and calculate them with 95% CIs for studies that used the same scale. If quality of life had been measured using different scales in the different studies, we would calculate standardised mean differences (SMDs) with 95% CIs. Upon extracting all available HRQoL scales, we identified EORTC QLQ‐C30, Aaronson 1993, and EORTC QLQ‐LC13, Koller 2017, as the most consistently used. The threshold for clinically meaningful difference on these scales was consistently reported as 10 points in the studies included and elsewhere (Anota 2015). From these scales, we selected TTD as a composite endpoint (cough, dyspnoea, and chest pain) as the most clinically applicable measure. This endpoint was presented as a HR in the included studies.
For the dichotomous outcomes, ORR, AE, and one‐year OS, we presented results as risk ratios (RRs) with 95% CIs. If the number of events and the total number of participants were not presented in the paper, we used percentages to back calculate the number of events. If only a summary RR was presented, we used generic inverse variance to permit the calculation of an overall summary statistic.
Unit of analysis issues
The unit of analysis was the individual participant. We excluded studies with a cross‐over design. For any studies with multiple intervention groups, we planned to divide the analysis into pairwise comparisons (e.g. A versus placebo, B versus placebo, A versus B) to ensure that a group of participants was not included twice in the same meta‐analysis. When A and B had to be analysed together, we would halve the placebo group to avoid double‐counting. If outcomes were presented as a summary and as components, we did not report a summary statistic to avoid double‐counting.
Dealing with missing data
We presented data as reported in the included studies. Where available, we used intention‐to‐treat (ITT) data. We made no assumptions for participants missing to follow‐up. We needed to contact one author to clarify randomisation method and allocated bias accordingly. We did not impute any data.
Assessment of heterogeneity
We presented the included studies on forest plot graphs and visually inspected the graphs for heterogeneity. We explored potential causes of heterogeneity. For pooled analyses, we used Review Manager 5 to calculate the I² statistic (Review Manager 2020). We defined unexplained heterogeneity as an I² statistic greater than 75%. If overall heterogeneity was greater than 90% in a meta‐analysis, we did not produce a summary statistic.
Assessment of reporting biases
We planned that if 10 or more studies were included in a meta‐analysis, we would create a funnel plot to assess possible publication bias or small‐study effects. We did not limit the search to English studies in order to avoid language bias.
Data synthesis
We analysed data using Review Manager 5 software (Review Manager 2020). Where multiple publications referred to the same study, we included the most mature data set for outcomes. If appropriate, we performed meta‐analyses and used forest plots for illustration and synthesis. We expected studies to be clinically similar and so predetermined that a fixed‐effect model would be used.
For the primary outcome of PFS and for OS, we calculated an overall HR using the generic inverse variance method to combine the summary data from each of the studies.
The definition of baseline CNS metastases as a site of disease varied across studies. When reporting PFS, we elected to use a definition of 'any' baseline CNS involvement. When reporting intracranial ORR, we elected to use a definition of 'measurable' baseline CNS involvement, and where prior radiotherapy was described, we presented ORR in those participants who had not received prior radiotherapy.
We reported summarised HRs as a clinically relevant HRQoL outcome rather than the intended MD or SMD.
For dichotomous data supplied for AEs, ORR, and one‐year OS, we combined data to produce an overall RR using the Mantel‐Haenszel method with CIs.
Subgroup analysis and investigation of heterogeneity
Planned subgroup analysis included line of treatment, type of ALK inhibitor, and baseline CNS involvement. Line of treatment was categorised as the ALK inhibitor being given as first treatment or as a subsequent line of therapy. The types of ALK inhibitor identified were crizotinib, ceritinib, alectinib, lorlatinib and brigatinib. Our search did not find any studies that exclusively recruited patients with baseline CNS involvement. Some studies presented outcomes for participants who had baseline CNS involvement but did not present outcomes for non‐CNS participants. We presented PFS and intracranial ORR for those studies that reported these selected outcomes for participants with baseline CNS involvement rather than performing a formal subgroup analysis.
Sensitivity analysis
We conducted sensitivity analyses to confirm the robustness of analysis results when appropriate. If sufficient studies were eligible for the analysis, we would select studies with low risk of bias for the majority of risk of bias domains. We also performed sensitivity analysis on the primary outcomes by utilising a random‐effects model and comparing results to our default fixed‐effect model.
Summary of findings and assessment of the certainty of the evidence
We created two summary of findings tables to present the results for each comparison evaluated in the review (see Table 1 and Table 2). The outcomes in the tables included PFS, overall AEs, OS, one‐year OS, ORR, and HRQoL. We used the GRADE approach to rate the certainty of evidence for the meta‐analysis and presented the results in the summary of findings table (GRADEpro GDT). We assessed the quality of a body of evidence using the five GRADE considerations: study limitations, consistency of effect, imprecision of results, indirectness, and publication bias (Guyatt 2008). We justified decisions to down‐ or upgrade the certainty of the evidence using footnotes, and added comments and footnotes to the summary of findings tables to aid readers' understanding of the results.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies.
Results of the search
The goal of this systematic review was to include all relevant literature. A total of 2428 records were retrieved from searching the databases electronically up to 7 January 2021. After removal of duplicate references, 2153 records remained. Four review authors (EC, LC, NH, and TM) excluded 1996 of these records based on screening of titles and abstracts. We selected 157 potentially relevant records for full‐text screening. We excluded 36 records representing 15 studies (Blackhall 2017; Cho 2017; Chow 2019; EUCTR2012‐003474‐36‐BE; Felip 2016; Gao 2016; JPRN‐JapicCTI‐184073; Kim 2016; Lenderking 2017; Liang 2019; NCT02134912; Park 2020; Reckamp 2019; Wolf 2015; Zhao 2015). One study Zhao 2015 was initially identified as randomised, but on careful translation from the publication in Mandarin, we confirmed an alternative allocation method of treatment assignment, which is not randomisation (Schulz 2002), and decided to exclude the small study from analysis due to wrong study design. The remaining 121 records represented 17 studies, of which 11 were included in the review (ALESIA 2019; ALEX 2017; ALTA‐1L 2019; ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; CROWN 2020; J‐ALEX 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018), and 6 were assessed as ongoing studies (eXalt3 2020; NCT03737994; NCT04009317; NCT04318938; NCT04632758; Popat 2019). A PRISMA study flow diagram is presented in Figure 1.
1.
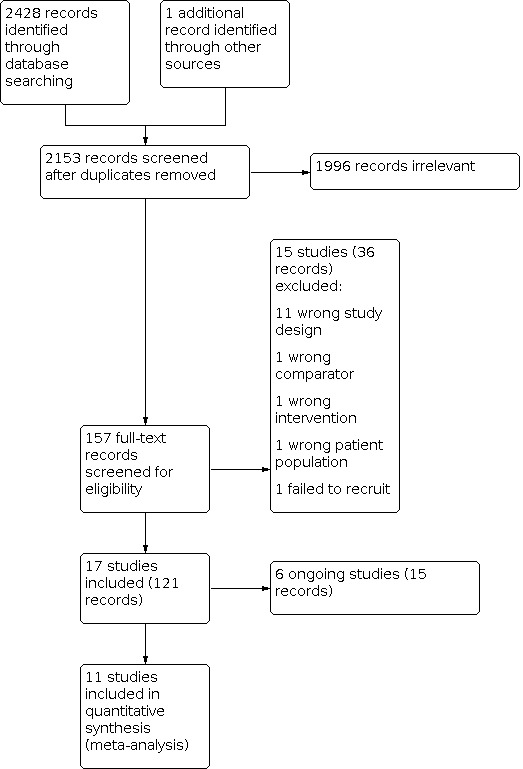
Study flow diagram.
Included studies
The 11 included studies were RCTs published in English between 2013 and 2020. Three studies enrolled participants from Asian countries only (ALESIA 2019; J‐ALEX 2017; PROFILE 1029 2018), and eight studies enrolled participants internationally from multiple continents (ALEX 2017; ALTA‐1L 2019; ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; CROWN 2020; PROFILE 1007 2013; PROFILE 1014 2014). All 11 studies were sponsored by the pharmaceutical industry. The included studies randomised participants into two intervention groups, with a total of 2874 participants recruited. Chemotherapy was given intravenously, and ALK inhibitors were administered orally. All of the included studies comparing two ALK inhibitors allocated crizotinib as a control which was compared to a next‐generation ALK inhibitor. All studies used the RECIST v1.1 for radiology assessment.
We divided the included studies into our predetermined comparisons by intervention strategies:
ALK inhibitor versus cytotoxic chemotherapy;
next‐generation ALK inhibitor versus crizotinib.
Six studies compared ALK inhibitors versus cytotoxic chemotherapy (ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018), and five studies compared a next‐generation ALK inhibitor to crizotinib (ALESIA 2019; ALEX 2017; ALTA‐1L 2019; CROWN 2020; J‐ALEX 2017).
ALK inhibitor versus chemotherapy
We identified six studies comparing ALK inhibitor to cytotoxic chemotherapy for the treatment of ALK‐positive NSCLC (ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018). All six studies were phase III, open‐label RCTs.
Population
The inclusion criteria across the studies were similar: participants were adults age 18 or over with histologically or cytologically confirmed locally advanced, recurrent, or metastatic NSCLC and an ECOG performance score of 0 to 1. Three studies included patients who had all received prior treatment (second line) (ALUR 2018; ASCEND‐5 2017; PROFILE 1007 2013), and three studies included patients with no prior treatment exposure (first line) (ASCEND‐4 2017; PROFILE 1014 2014; PROFILE 1029 2018). All studies included people with NSCLC stage IV, IIIB, or locally advanced and not amenable to radical chemoradiotherapy.
The number of randomised participants varied from 107, ALUR 2018, to 376, ASCEND‐4 2017. A total of 1611 participants were recruited in the six studies.
Participant characteristics were well‐balanced across the majority of studies. In two studies there were no differences in baseline characteristics between intervention and control arms (PROFILE 1007 2013; PROFILE 1014 2014). All studies reported a mean participant age of between 48 and 59. ALUR 2018 had a lower percentage of Asian participants than other studies. PROFILE 1029 2018 included only Asian participants. Predominant histology in all studies was adenocarcinoma, and most studies had stage IV disease participants (87.5% to 99%). In PROFILE 1029 2018, the proportions of stage IV disease participants (87.5% versus 93.2%), female gender (51.9% versus 58.3%), and those with baseline brain metastases (20.2% versus 31.1%) were lower in the crizotinib group compared to the chemotherapy group.
In ASCEND‐4 2017, the proportions of females (54% versus 61%), Asians (40% versus 44%), and never‐smokers (57% versus 65%) were lower in the ceritinib group compared to the chemotherapy group. In ASCEND‐5 2017, the proportions of females (59% versus 53%) and never‐smokers (62% versus 53%) were higher in the ceritinib group compared to the chemotherapy group. ALUR 2018 had a higher proportion of never‐smokers (48.6% versus 45.7%) and lower proportions of females (43.1% versus 51.4%), Asians (6.9% versus 20%), and those with baseline brain metastases (65.3% versus 74.3%) in the alectinib group compared to the chemotherapy group.
Setting
All six studies were multinational studies enrolling participants from centres in North America, South and Central America, Europe, Asia, and Australasia.
Intervention
All six studies compared an ALK inhibitor with chemotherapy. First‐line studies used doublet chemotherapy combining pemetrexed and carboplatin or cisplatin (ASCEND‐4 2017; PROFILE 1014 2014; PROFILE 1029 2018). Second‐line studies compared an ALK inhibitor to single‐agent three‐weekly pemetrexed or docetaxel chemotherapy (ALUR 2018; ASCEND‐5 2017; PROFILE 1007 2013). ALK inhibitor varied by study, with ALUR 2018 using alectinib 600 mg twice daily, PROFILE 1007 2013, PROFILE 1014 2014, and PROFILE 1029 2018 using crizotinib 250 mg twice daily, and ASCEND‐4 2017 and ASCEND‐5 2017 using ceritinib 750 mg once daily. Cross‐over was allowed in all studies (cross‐over rates are presented in Table 3).
1. ALK inhibitor versus chemotherapy: median progression‐free survival and overall survival .
| Study | Median progression‐free survival | Median overall survival | Median follow‐up | Median overall survival | Median follow‐up | % Cross‐over | |
|
ALK inhibitor months (95% CI) |
Chemotherapy months (95% CI) |
ALK inhibitor months (95% CI) |
ALK inhibitor months |
Chemotherapy months (95% CI) |
Chemotherapy months |
||
| ALUR 2018 | 7.1 (6.3 to 10.8) | 1.6 (1.3 to 4.1) | 12.6 (9.7 to NR) | NS | NR (NR to NR) | NS | 70.6 |
| PROFILE 1007 2013 | 7.7 (6.0 to 8.8) | 3 (2.6 to 4.3) | 20.3 (18.1 to NR) | 51 | 22.8 (18.6 to NR) | 53.1 | 89 |
| PROFILE 1014 2014 | 10.9 (8.3 to 13.9) | 7 (6.8 to 8.2) | NR (45.8 to NR) | 46 | 47.5 (32.2 to NR) | 46 | 74.9 |
| PROFILE 1029 2018 | 11.1 (8.3 to 12.6) | 6.8 (5.7 to 7.0) | 28.5 (26.4 to NR) | 22.5 | 27.7 (23.9 to NR) | 21.6 | 80.6 |
| ASCEND‐4 2017 | 16.6 (12.6 to 27.2) | 8.1 (5.8 to 11.1) | NR (29.3 to NE) | 19.7 | 26.2 (22.8 to NE) | NS | 42.7 |
| ASCEND‐5 2017 | 5.4 (4.1 to 6.9) | 1.6 (1.4 to 2.8) | 18.1 (13.4 to 23.9) | 16.6 | 20.1 (11.9 to 25.1) | 16.4 | 64.7 |
CI: confidence interval NE: not estimable NR: not reached NS: not stated
Study and treatment duration
Median study duration was 23 months. Median treatment duration ranged from 20.1 weeks, ALUR 2018, to 66.4 weeks, ASCEND‐4 2017, in the ALK inhibitor groups, and from 6 weeks, ALUR 2018, to 26.9 weeks, ASCEND‐4 2017, in the chemotherapy groups.
Outcomes
Primary outcomes
PFS was the primary outcome in all six studies (ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018). Despite initially utilising independent review committee (IRC) review, the 2019 ALUR 2018 update and 2020 ALEX 2017 update did not do so. ASCEND‐4 2017, ASCEND‐5 2017, PROFILE 1014 2014, and PROFILE 1029 2018 used PFS assessed by an IRC as a primary outcome.
PFS outcomes were analysed and presented by subgroup, as follows.
ALUR 2018: by age, gender, ethnicity, baseline CNS disease, prior radiotherapy, and ECOG status.
ASCEND‐4 2017: by investigator and blinded IRC as well as age, gender, ethnicity, baseline CNS disease, prior adjuvant treatment, smoking and performance status.
ASCEND‐5 2017: by age, gender, ethnicity, baseline CNS disease, disease burden, smoking and performance status.
PROFILE 1014 2014 and PROFILE 1029 2018: by age, gender, ethnicity, performance and smoking status, baseline CNS disease, histology, time since diagnosis, and extent of disease.
PROFILE 1007 2013: by age, gender, ethnicity, smoking and performance status, baseline CNS disease, histology, and prior EGFR TKI.
Secondary outcomes
ORR as per RECIST (v1.1) criteria, and AEs as per Common Terminology Criteria for Adverse Events (CTCAE v4.0) were secondary outcomes in all six studies (CTCAE v4). Four studies assessed CNS response as a secondary outcome (ALUR 2018; ASCEND‐5 2017; PROFILE 1014 2014; PROFILE 1029 2018). Six studies assessed HRQoL as a secondary outcome (ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018). All studies used EORTC QLQ‐C30 and EORTC QLQ‐LC13 questionnaires to measure HRQoL outcomes.
Next‐generation ALK inhibitor versus crizotinib
We identified five studies that compared a next‐generation ALK inhibitor to crizotinib (ALESIA 2019; ALEX 2017; ALTA‐1L 2019; CROWN 2020; J‐ALEX 2017). All five studies were phase III RCTs with an open‐label trial design.
Population
The inclusion criteria were similar across studies. Participants were adults age 18 or over with histologically or cytologically confirmed stage IIIB or IV NSCLC ALK‐altered NSCLC with ECOG performance score 0 to 2. All five studies included a proportion of participants with baseline brain metastases. Three studies included patients who had not received any prior systemic treatments (ALESIA 2019; ALEX 2017; CROWN 2020). Two studies permitted but did not require participants to have received one line of previous chemotherapy (ALTA‐1L 2019; J‐ALEX 2017).
The number of participants varied from 187, ALESIA 2019, to 303, ALEX 2017. A total of 1263 participants were recruited in the five studies.
In ALESIA 2019, the proportion of females was higher and the proportions of never‐smokers and those with adenocarcinoma were lower in the alectinib group compared to the chemotherapy group. In ALEX 2017, the proportions of females and adenocarcinoma patients were lower, and the median age, proportions of never‐smokers, and participants with baseline brain metastases were higher in the alectinib group compared to the crizotinib group. In J‐ALEX 2017, the proportions of never‐smokers and those with baseline brain metastases were lower in the alectinib group compared to the crizotinib group. All participants in the J‐ALEX 2017 and ALESIA 2019 studies were of Asian ethnicity.
In the ALTA‐1L 2019 study, the proportions of females and adenocarcinoma patients were lower, and the proportions of Asians, never‐smokers, and those with stage IV disease were higher in the brigatinib group compared to the crizotinib group. In CROWN 2020, the proportions of never‐smokers and females were lower, and the median age was higher in the lorlatinib group compared to the crizotinib group.
Setting
Three studies were multicentre, international trials with recruitment centres in North and South America, Europe, Asia, and Australasia (ALEX 2017; ALTA‐1L 2019; CROWN 2020). ALESIA 2019 was an international study in Asian countries only. J‐ALEX 2017 recruited only in Japan.
Intervention
All studies tested ALK inhibitors. All studies compared a next‐generation ALK inhibitor (alectinib, brigatinib, or lorlatinib) to crizotinib. All trials used crizotinib at 250 mg twice daily. ALEX 2017 and ALESIA 2019 used alectinib 600 mg twice daily as the next‐generation ALK inhibitor, whereas J‐ALEX 2017 used alectinib 300 mg twice daily. ALTA‐1L 2019 administered brigatinib 90 mg once daily for seven days which was then escalated to 180 mg once daily. CROWN 2020 used lorlatinib 100 mg once daily. Only ALTA‐1L 2019 permitted cross‐over.
ALESIA 2019, ALEX 2017, and CROWN 2020 were first‐line studies, whereas J‐ALEX 2017 and ALTA‐1L 2019 included a mix of treatment‐naive and pre‐treated patients.
Study and treatment duration
Median study duration was 19 months. CROWN 2020 and J‐ALEX 2017 did not report median treatment duration. In the other three studies (ALESIA 2019; ALEX 2017; ALTA‐1L 2019), median treatment duration ranged from 9.2 months, ALTA‐1L 2019, to 17.9 months, ALEX 2017, in the next‐generation ALK inhibitor group, and from 7.4 months, ALTA‐1L 2019, to 12.6 months, ALESIA 2019, in the crizotinib group.
Outcomes
Primary outcomes
PFS was the primary outcome in all five studies (ALESIA 2019; ALEX 2017; ALTA‐1L 2019. CROWN 2020; J‐ALEX 2017). All five studies used an independent blinded outcome assessor. ALTA‐1L 2019, CROWN 2020, and J‐ALEX 2017 used PFS as assessed by a blinded IRC as a primary outcome. ALESIA 2019 and ALEX 2017 used investigator‐assessed PFS as a primary outcome. All studies except CROWN 2020 performed subgroup analysis for PFS. ALESIA 2019 analysed by baseline CNS disease with or without previous radiotherapy, age, gender, smoking and ECOG status. In ALTA‐1L 2019, PFS outcomes were subgrouped by prior treatment, baseline CNS disease, age, gender, ethnicity, ECOG status, as well as independent and blinded independent review outcomes. In ALEX 2017, PFS outcomes were subgrouped by baseline CNS disease with or without prior radiotherapy, age, gender, ethnicity, smoking and ECOG status. In J‐ALEX 2017, PFS was subgrouped by prior treatment line, baseline CNS disease, age, gender, smoking status, and disease stage.
Secondary outcomes
All five studies included OS, ORR as per RECIST 1.1 criteria, AEs as per CTCAE (CTCAE v4), CNS response or time to CNS progression, and patient‐reported outcome measures for HRQoL as secondary outcomes (ALESIA 2019; ALEX 2017; ALTA‐1L 2019; CROWN 2020; J‐ALEX 2017). All five studies used EORTC QLQ‐C30 and EORTC QLQ‐LC13 questionnaires to measure HRQoL outcomes.
Excluded studies
We excluded 15 studies for the reasons described below (see Characteristics of excluded studies) (Blackhall 2017; Cho 2017; Chow 2019; EUCTR2012‐003474‐36‐BE; Felip 2016; Gao 2016; JPRN‐JapicCTI‐184073; Kim 2016; Lenderking 2017; Liang 2019; NCT02134912; Park 2020; Reckamp 2019; Wolf 2015; Zhao 2015).
Wrong study design: 11 studies (Blackhall 2017; Chow 2019; EUCTR2012‐003474‐36‐BE; Felip 2016; Gao 2016; Kim 2016; Lenderking 2017; Liang 2019; Reckamp 2019; Wolf 2015; Zhao 2015).
Wrong comparator: one study (Cho 2017).
Wrong intervention: one study (Park 2020).
Wrong patient population: one study (JPRN‐JapicCTI‐184073).
Failed to recruit: one study (NCT02134912).
Risk of bias in included studies
Study designs and methods were generally well reported. Judgements regarding risk of bias across all included studies and for each individual domain in the included studies are presented in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Generation of the randomised sequence was adequate in nine studies (ALESIA 2019; ALEX 2017; ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; CROWN 2020; J‐ALEX 2017; PROFILE 1007 2013; PROFILE 1014 2014). Two studies did not report the methods used to generate random allocation (ALTA‐1L 2019; PROFILE 1029 2018). Nine studies described satisfactory methods for allocation concealment (ALESIA 2019; ALEX 2017; ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; CROWN 2020; J‐ALEX 2017; PROFILE 1007 2013; PROFILE 1014 2014). Two studies did not report the method of allocation concealment (ALTA‐1L 2019; PROFILE 1029 2018).
Blinding
We assessed risk of bias for the blinding of participants, personnel, and assessors for survival outcomes as low if the outcome could not be affected by knowledge of treatment arms and if the studies included independent central review. We assessed risk of bias as high if outcomes were subjective and could be affected by knowledge of treatment arm, such as for HRQoL and AEs, and if the study did not include independent review of survival outcomes.
Blinding of participants and personnel
All 11 included studies had an open‐label design with a potential risk of performance bias (ALESIA 2019; ALEX 2017; ALTA‐1L 2019; ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; CROWN 2020; J‐ALEX 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018).
We assessed 8 of the 11 studies as at low risk of bias for survival outcomes (ALTA‐1L 2019; ASCEND‐4 2017; ASCEND‐5 2017; CROWN 2020; J‐ALEX 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018), as the studies utlilised independent central review of PFS. Three of the 11 studies were assessed at high risk of bias because PFS was assessed by investigators; not by independent central review (ALESIA 2019; ALEX 2017; ALUR 2018). In all 11 studies, OS would not have been influenced by the lack of blinding of participants or personnel.
All 11 included studies were at high risk of bias for blinding of participants and personnel for subjective outcomes such as HRQoL and AEs, given the open‐label nature of the studies (ALESIA 2019; ALEX 2017; ALTA‐1L 2019; ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; CROWN 2020; J‐ALEX 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018).
Blinding of outcome assessors
Eight of the 11 included studies were at low risk of bias for objective survival outcomes because PFS was assessed by independent central review (ALTA‐1L 2019; ASCEND‐4 2017; ASCEND‐5 2017; CROWN 2020; J‐ALEX 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018). Three of the 11 studies were at high risk of bias for objective survival outcomes because PFS was assessed by investigators; not by independent central review (ALESIA 2019; ALEX 2017; ALUR 2018). In all 11 studies, OS would not have been influenced by the lack of blinding of outcome assessors.
All 11 studies were at high risk of bias for subjective outcomes given the open‐label design of the studies (ALESIA 2019; ALEX 2017; ALTA‐1L 2019; ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; CROWN 2020; J‐ALEX 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018).
There was a high risk of bias across the included studies for blinding of participants, personnel, and outcome assessors for subjective outcomes, given the open‐label design of the studies. There was an overall low risk of bias for objective survival outcomes, as the majority of the studies included independent central review of survival outcomes.
Incomplete outcome data
All 11 studies were at low risk of attrition bias (ALESIA 2019; ALEX 2017; ALTA‐1L 2019; ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; CROWN 2020; J‐ALEX 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018), as there were no significant differences between treatment arms in participants lost to follow‐up, and the total number of participants included in each outcome was reported.
Selective reporting
All 11 studies were at low risk of reporting bias given that studies were preregistered with all stated outcomes having been reported (ALESIA 2019; ALEX 2017; ALTA‐1L 2019; ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; CROWN 2020; J‐ALEX 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018).
Other potential sources of bias
One study was at high risk of other sources of bias. Baseline patient characteristics were unbalanced with regard to ethnicity and smoking status in the ASCEND‐5 2017 study.
Effects of interventions
Comparison 1: ALK inhibitor versus chemotherapy
See Table 1.
1. Primary outcome: progression‐free survival
Six studies provided information on PFS (ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018). The studies compared ALK inhibitors (crizotinib, ceritinib, and alectinib) to chemotherapy.
ALK inhibitors resulted in a large increase in PFS when compared to chemotherapy (hazard ratio (HR) 0.45, 95% confidence interval (CI) 0.40 to 0.52, P = 0.02, I² = 62%, 6 RCTs, 1611 participants, high‐certainty evidence) (Figure 4, Analysis 1.1).
4.

Forest plot of comparison: 1 ALK inhibitor versus chemotherapy, outcome: 1.1 Progression‐free survival subgrouped by line of treatment.
1.1. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 1: Progression‐free survival subgrouped by line of treatment
Subgroup analysis
Line of treatment
Three studies recruited treatment‐naive participants (first‐line) (ASCEND‐4 2017; PROFILE 1014 2014; PROFILE 1029 2018), and three studies recruited participants who had previously received chemotherapy, PROFILE 1007 2013, or sequential chemotherapy and crizotinib (ALUR 2018; ASCEND‐5 2017). Hazard ratios were similar for first‐line (HR 0.47) and second‐line (HR 0.43) subgroups, and the test for subgroup differences showed no difference (test for subgroup differences: Chi² = 0.50, df = 1 (P = 0.48), I² = 0%).
Type of ALK inhibitor
1.2. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 2: Progression‐free survival subgrouped by type of ALK inhibitor
Chemotherapy was compared to crizotinib in three studies (PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018), ceritinib in two studies (ASCEND‐4 2017; ASCEND‐5 2017), and alectinib in one study (ALUR 2018). All hazard ratios favoured ALK inhibitors. The test for subgroup differences indicated a difference by type of ALK inhibitor (test for subgroup differences: Chi² = 11.97, df = 2 (P = 0.003), I² = 83.3%), which on visual inspection of the plot is explained by the alectinib subgroup having a lower HR of 0.2 reported in a single study (ALUR 2018).
Baseline CNS involvement
1.3. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 3: Progression‐free survival in people with CNS disease
All six studies reported PFS according to baseline CNS involvement. PFS was not defined according to brain progression only. The baseline rate of CNS involvement ranged from 20.2%, PROFILE 1029 2018, to 65.3%, ALUR 2018, in the ALK inhibitor arm, and from 27%, PROFILE 1014 2014, to 74.3%, ALUR 2018, in the chemotherapy arm. Baseline CNS involvement was defined as 'any' in all six studies.
ALK inhibitors resulted in a large increase in PFS when compared to chemotherapy for participants with baseline CNS disease (HR 0.51, 95% CI 0.41 to 0.62, P = 0.009, I² = 68%, 6 RCTs, 581 participants, high‐certainty evidence).
Sensitivity analysis
Analysis of PFS undertaken using a random‐effects model did not significantly alter the results presented using the default fixed‐effect model (random‐effects model: HR 0.43, 95% CI 0.35 to 0.54).
2. Primary outcome: adverse events
Overall AE rates
Five studies reported overall AE rates. PROFILE 1029 2018 reported by AE type and grade, but did not report overall rates and was therefore not included. The threshold for reporting grade 1 and 2 AE ranged from > 5%, ALUR 2018, to > 20%, PROFILE 1007 2013, frequency.
ALK inhibitors may result in no difference in overall AE rate when compared to chemotherapy (risk ratio (RR) 1.01, 95% CI 1.00 to 1.03, P = 0.18, I² = 36%, 5 RCTs, 1404 participants, low‐certainty evidence) (Figure 5, Analysis 1.4).
5.

Forest plot of comparison: 1 ALK inhibitor versus chemotherapy, outcome: 1.4 Overall adverse events subgrouped by line of treatment.
1.4. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 4: Overall adverse events subgrouped by line of treatment
Subgroup analysis
Line of treatment
Two studies were in the first‐line setting (ASCEND‐4 2017; PROFILE 1014 2014), and three were in the second or subsequent line of treatment setting (ALUR 2018; ASCEND‐5 2017; PROFILE 1007 2013). Subgroup analysis of overall AEs by line of treatment revealed no difference (test for subgroup differences: Chi² = 0.37, df = 1 (P = 0.55), I² = 0%).
Type of ALK inhibitor
1.5. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 5: Overall adverse events subgrouped by type of ALK inhibitor
Chemotherapy was compared to crizotinib in two studies (PROFILE 1007 2013; PROFILE 1014 2014), ceritinib in two studies (ASCEND‐4 2017; ASCEND‐5 2017), and alectinib in one study (ALUR 2018). Subgroup analysis of overall AEs by type of ALK inhibitor revealed no difference (test for subgroup differences: Chi² = 3.92, df = 2 (P = 0.14), I² = 49%).
Sensitivity analysis
Analysis of overall AE rates undertaken using a random‐effects model did not significantly alter the results presented using the default fixed‐effect model (random‐effects model: HR 1.01, 95% CI 1.00 to 1.03).
Grade 3 to 4 AE rates
Five studies reported grade 3 and 4 adverse event rates (ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; PROFILE 1007 2013; PROFILE 1014 2014).
There are slightly reduced grade 3 and 4 AEs in favour of chemotherapy compared to ALK inhibitors (RR 1.09, 95% CI 1.0 to 1.19, P = 0.09, I² = 50%, 5 RCTs, 1404 participants) (Analysis 1.6).
1.6. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 6: Grade 3/4 adverse events subgrouped by line of treatment
Subgroup analysis
Line of treatment
Two studies were in the first‐line setting (ASCEND‐4 2017; PROFILE 1014 2014), and three were in the second or subsequent line of treatment setting (ALUR 2018; ASCEND‐5 2017; PROFILE 1007 2013). Subgroup analysis of grade 3 and 4 AEs by line of treatment revealed no difference (test for subgroup differences: Chi² = 0.44, df = 1 (P = 0.51), I² = 0%).
Type of ALK inhibitor
1.7. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 7: Grade 3/4 adverse events subgrouped by type of ALK inhibitor
Chemotherapy was compared to crizotinib in two studies (PROFILE 1007 2013; PROFILE 1014 2014), ceritinib in two studies (ASCEND‐4 2017; ASCEND‐5 2017), and alectinib in one study (ALUR 2018). Subgroup analysis of grade 3 and 4 AEs by type of ALK inhibitor revealed a difference (test for subgroup differences: Chi² = 6.23, df = 2 (P = 0.04), I² = 67.9%). Visual inspection of the plot indicated more grade 3 and 4 AEs with ceritinib than with chemotherapy (HR 1.2).
Grade 5 AE rates
Six studies reported grade 5 AE rates (toxic deaths). Three studies included deaths due to progressive disease in the grade 5 definition (ASCEND‐4 2017; PROFILE 1007 2013; PROFILE 1014 2014), and three studies reported deaths due to disease progression separately (ALUR 2018; ASCEND‐5 2017; PROFILE 1029 2018). We analysed total presented grade 5 AEs (Analysis 1.8) and grade 5 AEs excluding disease progression (Analysis 1.9) for all six studies.
1.8. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 8: Total Grade 5 adverse events subgrouped by line of treatment
1.9. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 9: Grade 5 adverse events (excluding progressive disease) subgrouped by line of treatment
The evidence suggests an increase in total reported grade 5 AEs in participants who received ALK inhibitors compared to those who received chemotherapy (RR 3.33, 95% CI 2.02 to 5.48, P = 0.25, I² = 25%, 6 RCTs, 1611 participants, low‐certainty evidence) (Analysis 1.8).
The evidence suggests there was little to no increase in grade 5 AEs (excluding disease progression) in participants who received ALK inhibitors compared to those who received chemotherapy (RR 2.03, 95% CI 0.89 to 4.66, P = 0.57, I² = 0%, 6 RCTs, 1611 participants, low‐certainty evidence) (Figure 6, Analysis 1.9). Most deaths were not treatment related across the six studies. When reported for chemotherapy arms, two were listed as treatment related but unclear cause, one due to sepsis, and two due to pneumonitis. When reported for ALK inhibitors, one was due to ventricular arrhythmia, three to pneumonitis, one to interstitial lung disease, one to cardiac failure, one to respiratory failure and pneumonia, and in one participant the cause of death was unknown. Of note, there was variable reporting of treatment‐related deaths and small numbers, hence firm conclusions cannot be drawn from this information.
6.

Forest plot of comparison: 1 ALK inhibitor versus chemotherapy, outcome: 1.9 Grade 5 adverse events (excluding progressive disease) subgrouped by line of treatment.
Subgroup analysis
Line of treatment
Two studies were in the first‐line setting (ASCEND‐4 2017; PROFILE 1014 2014), and three were in the second or subsequent line of treatment setting (ALUR 2018; ASCEND‐5 2017; PROFILE 1007 2013). Subgroup analysis of grade 5 AEs (excluding disease progression) by line of treatment revealed no difference (test for subgroup differences: Chi² = 0.15, df = 1 (P = 0.7), I² = 0%).
Type of ALK inhibitor
1.10. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 10: Grade 5 adverse events (excluding progressive disease) subgrouped by ALK inhibitor
Chemotherapy was compared to crizotinib in three studies (PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018), ceritinib in two studies (ASCEND‐4 2017; ASCEND‐5 2017), and alectinib in one study (ALUR 2018). Subgroup analysis of grade 5 AEs excluding disease progression by type of ALK inhibitor revealed no difference (test for subgroup differences: Chi² = 3.11, df = 2 (P = 0.2), I² = 35.7%).
Treatment reductions
We presented dose reduction, treatment interruption, and treatment discontinuation due to AEs. ALUR 2018 was the only study to report dose reduction and treatment interruption, finding no evidence of a difference between ALK inhibitors and chemotherapy. Five studies reported treatment discontinuation rates (ALUR 2018; ASCEND‐5 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018). There is likely no difference between ALK inhibitors and chemotherapy in treatment discontinuation rates (RR 1.03, 95% CI 0.73 to 1.45, P = 0.02, I² = 65%, 5 RCTs, 1235 participants) (Analysis 1.11).
1.11. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 11: Dose intensity
Types of AEs
We presented types of AEs where comparable data were available and categorised them into gastrointestinal (Analysis 1.12), haematological (Analysis 1.13), hepatic (Analysis 1.14), and general (Analysis 1.15). Due to a wide range in terminology used across studies, we did not present cardiac, visual, and respiratory AEs. We could not compare rare and drug‐specific AEs across multiple studies. We were not able to calculate summary statistics for Analysis 1.12 and Analysis 1.14 due to risk of a unit of analysis error. For example, double‐counting would occur if we summarised 'any' and 'grade 3 to 4' diarrhoea. The included AE types for Analysis 1.15 were diverse, and a summary statistic would not be clinically useful.
1.12. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 12: Gastrointestinal adverse events
1.13. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 13: Haematological adverse events
1.14. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 14: Hepatic adverse events
1.15. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 15: General any grade adverse events
The evidence suggests a decrease in haematological AEs in participants who received ALK inhibitors compared to those who received chemotherapy (Analysis 1.13).
Grade 3 to 4 anaemia: RR 0.2, 95% CI 0.12 to 0.40, P = 0.67, I² = 0%, 5 RCTs, 1380 participants.
Grade 3 to 4 neutropenia: RR 0.52, 95% CI 0.40 to 0.68, P = 0.002, I² = 73%, 6 RCTs, 1611 participants.
Grade 3 to 4 thrombocytopenia: RR 0.13, 95% CI 0.04 to 0.43, P = 0.54, I² = 0%, 3 RCTs, 781 participants.
3. Secondary outcome: overall survival
Six studies reported OS (ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018).
ALK inhibitors result in a slight increase in OS when compared to chemotherapy (HR 0.84, 95% CI 0.72 to 0.97, P = 0.88, I² = 0%, 6 RCTs, 1611 participants, high‐certainty evidence) (Figure 7, Analysis 1.16).
7.

Forest plot of comparison: 1 ALK inhibitor versus chemotherapy, outcome: 1.16 Overall survival subgrouped by line of treatment.
1.16. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 16: Overall survival subgrouped by line of treatment
Subgroup analysis
Line of treatment
Three studies were in the first‐line setting (ASCEND‐4 2017; PROFILE 1014 2014; PROFILE 1029 2018), and three were in the second or subsequent lines of treatment setting (ALUR 2018; ASCEND‐5 2017; PROFILE 1007 2013). Subgroup analysis of OS by line of treatment revealed no difference (test for subgroup differences: Chi² = 0.83, df = 1 (P = 0.36), I² = 0%), although the first‐line summary statistic (HR 0.78) did not cross line of neutral, whilst the second‐line summary statistic (HR 0.89) did.
Type of ALK inhibitor
1.17. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 17: Overall survival subgrouped by type of ALK inhibitor
Chemotherapy was compared to crizotinib in three studies (PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018), ceritinib in two studies (ASCEND‐4 2017; ASCEND‐5 2017), and alectinib in one study (ALUR 2018). Subgroup analysis of OS by type of ALK inhibitor revealed no difference (test for subgroup differences: Chi² = 0.09, df = 2 (P = 0.95), I² = 0%).
4. Secondary outcome: overall survival at one year
Two studies reported OS at one year (PROFILE 1029 2018; PROFILE 1014 2014). Risk ratios visually favoured ALK inhibitors, but data could not be pooled due to the presence of heterogeneity (I² = 92%) (Analysis 1.18).
1.18. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 18: Overall survival at 1 year
Further survival data
All six studies reported median PFS and OS. These values (measured in months) are presented in Table 3, including reported confidence intervals and the cross‐over rate measured as the percentage of participants in the chemotherapy group who received the intervention ALK inhibitor after discontinuing the study. All studies had a significant rate of cross‐over. Median OS had not been reached at the time of publication in three studies (ALUR 2018; ASCEND‐4 2017; PROFILE 1014 2014), with median lengths of follow‐up ranging from 16.4 to 53.1 months across all studies.
5. Secondary outcome: overall response rate
All six studies reported ORR. There was unexplained heterogeneity (I² = 85%) for the pooled result, which we explored in subgroup analysis.
ALK inhibitors likely result in a higher ORR than chemotherapy (RR 2.43, 95% CI 2.16 to 2.75, P < 0.001, I² = 85%, 6 RCTs, 1611 participants, moderate‐certainty evidence) (Analysis 1.19).
1.19. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 19: Overall response rate by line of treatment
Subgroup analysis
Line of treatment
Three studies were in the first‐line setting (ASCEND‐4 2017; PROFILE 1014 2014; PROFILE 1029 2018), and three were in the second or subsequent lines of treatment setting (ALUR 2018; ASCEND‐5 2017; PROFILE 1007 2013). Subgroup analysis of ORR by line of treatment revealed a difference (test for subgroup differences: Chi² = 18.09, df = 1 (P < 0.001), I² = 94.5%). Visual inspection of the plot confirmed that all studies favoured ALK inhibitors, but more strongly in the second‐line.
Type of ALK inhibitor
1.20. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 20: Overall response rate subgrouped by type of ALK inhibitor
Chemotherapy was compared to crizotinib in three studies (PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018), ceritinib in two studies (ASCEND‐4 2017; ASCEND‐5 2017), and alectinib in one study (ALUR 2018). Subgroup analysis of ORR by type of ALK inhibitor revealed a difference (test for subgroup differences: Chi² = 10.97, df = 2 (P = 0.004), I² = 81.8%). Visual inspection of the forest plot indicated that a single study of alectinib, ALUR 2018, had a larger ORR improvement (RR 13.1) than was seen with crizotinib or ceritinib.
Partial and complete response
Five studies reported partial and complete response rate (ALUR 2018; ASCEND‐4 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018).
ALK inhibitors likely result in a higher partial response rate than chemotherapy (RR 2.30, 95% CI 2.03 to 2.59, P <0.0001, I² = 83%, 5 RCTs, 1380 participants). ALK inhibitors likely result in a higher complete response rate than chemotherapy (RR 2.70, 95% CI 0.80 to 9.14, P = 0.84, I² = 0%, 5 RCTs, 1380 participants) (Analysis 1.21).
1.21. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 21: Partial and complete response rate
There was heterogeneity in the partial response analysis. This is to be expected, as partial response is the largest component of the ORR outcome, which is discussed above.
CNS involvement
Three studies specifically reported the ORR in baseline measurable CNS metastases (Analysis 1.22) (ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017).
1.22. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 22: Overall and complete response rate in people with measurable baseline CNS disease
ALK inhibitors likely result in a higher ORR in baseline CNS metastases than chemotherapy (RR 4.88, 95% CI 2.18 to 10.95, P = 0.17, I² = 44%, 3 RCTs, 108 participants). ALK inhibitors may result in little to no effect on complete response rate in baseline CNS metastases (RR 1.56, 95% CI 0.32 to 7.57, P = 0.83, I² = 0%, 2 RCTs, 71 participants).
6. Secondary outcome: health‐related quality of life
Five studies reported HRQoL (ASCEND‐4 2017; ASCEND‐5 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018); the remaining study has not yet published HRQoL outcomes (ALUR 2018). The five studies reporting HRQoL all used the EORTC QLQ‐C30 and EORTC QLQ‐LC13 questionnaires (Aaronson 1993; Koller 2017). All five studies reported time to deterioration in a composite endpoint (cough, dyspnoea, and chest pain) (ASCEND‐4 2017; ASCEND‐5 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018).
Participants who received ALK inhibitors had a larger increase in the time to deterioration of the composite endpoint (cough, dyspnoea, and chest pain) than those who received chemotherapy (HR 0.52, 95% CI 0.44 to 0.60, P = 0.43, I² = 0%, 5 RCTs, 1504 participants, high‐certainty evidence) (Analysis 1.23).
1.23. Analysis.

Comparison 1: ALK inhibitor versus chemotherapy, Outcome 23: Quality of life: time to deterioration in composite endpoint (cough, dyspnoea, and chest pain)
Comparison 2: next‐generation ALK inhibitor versus crizotinib
See Table 2.
Three studies recruited treatment‐naive participants (first‐line) (ALESIA 2019; ALEX 2017; CROWN 2020), and two studies recruited participants who were permitted to have had one line of previous chemotherapy (ALTA‐1L 2019; J‐ALEX 2017). In ALTA‐1L 2019 and J‐ALEX 2017, 26% and 36% of participants, respectively, received prior chemotherapy, with neither study reporting outcomes according to prior treatment. No studies exclusively recruited participants who had received a prior line of therapy (second‐line), therefore we have not presented subgroup analysis by line of treatment.
1. Primary outcome: progression‐free survival
All five studies provided information on PFS (ALESIA 2019; ALEX 2017; ALTA‐1L 2019; CROWN 2020; J‐ALEX 2017). The studies compared next‐generation ALK inhibitors (alectinib, brigatinib, and lorlatinib) to crizotinib.
Next‐generation ALK inhibitors resulted in a large increase in PFS when compared to crizotinib (HR 0.39, 95% CI 0.32 to 0.46, P = 0.32, I² = 14%, 5 RCTs, 1263 participants, high‐certainty evidence) (Figure 8, Analysis 2.1).
8.

Forest plot of comparison: 2 Next‐generation ALK inhibitor versus crizotinib, outcome: 2.1 Progression‐free survival subgrouped by type of ALK inhibitor.
2.1. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 1: Progression‐free survival subgrouped by type of ALK inhibitor
Subgroup analysis
Type of ALK inhibitor
Crizotinib was compared to alectinib in three studies (ALESIA 2019; ALEX 2017; J‐ALEX 2017), brigatinib in one study (ALTA‐1L 2019), and lorlatinib in one study (CROWN 2020). All hazard ratios favoured next‐generation ALK inhibitors. Subgroup analysis by type of next‐generation ALK inhibitor revealed no difference (test for subgroup differences: Chi² = 4.16, df = 2 (P = 0.12), I² = 52%).
Baseline CNS site of disease
2.2. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 2: Progression‐free survival in people with CNS disease
All five studies reported PFS according to any baseline CNS involvement (Analysis 2.2). PFS was not defined according to brain progression only. The baseline rate of CNS involvement ranged from 13.6%, J‐ALEX 2017, to 42%, ALEX 2017, in the next‐generation ALK inhibitor arm and from 27%, CROWN 2020, to 38%, ALEX 2017, in the crizotinib arm.
Next‐generation ALK inhibitors resulted in a large increase in PFS when compared to crizotinib for participants with baseline CNS disease (HR 0.25, 95% CI 0.19 to 0.34, P = 0.05, I² = 58%, 5 RCTs, 406 participants, high‐certainty evidence).
Sensitivity analysis
Analysis of PFS undertaken using a random‐effects model did not significantly alter the results presented using the default fixed‐effect model (random‐effects model: 0.39, 95% CI 0.32 to 0.46).
2. Primary outcome: adverse events
Overall AE rates
All five studies reported overall AE rates. The threshold for reporting grade 1 and 2 AEs ranged from > 10%, ALESIA 2019; ALEX 2017; CROWN 2020, to > 20%, ALTA‐1L 2019; J‐ALEX 2017, frequency (Figure 9, Analysis 2.3).
9.

Forest plot of comparison: 2 Next‐generation ALK inhibitor versus crizotinib, outcome: 2.3 Overall adverse events subgrouped by type of ALK inhibitor.
2.3. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 3: Overall adverse events subgrouped by type of ALK inhibitor
Next‐generation ALK inhibitors likely result in no difference in overall AE rate when compared to crizotinib (RR 1.00, 95% CI 0.98 to 1.01, P = 0.52, I² = 0%, 5 RCTs, 1263 participants, moderate‐certainty evidence).
Subgroup analysis
Type of ALK inhibitor
Crizotinib was compared to alectinib in three studies (ALESIA 2019; ALEX 2017; J‐ALEX 2017), brigatinib in one study (ALTA‐1L 2019), and lorlatinib in one study (CROWN 2020). Subgroup analysis of overall AEs by type of next‐generation ALK inhibitor revealed no difference (test for subgroup differences: Chi² = 2.61, df = 2 (P = 0.27), I² = 23.3%).
Sensitivity analysis
Analysis of overall AE rates undertaken using a random‐effects model did not significantly alter the results presented using the default fixed‐effect model (random‐effects model: HR 1.00, 95% CI 0.99 to 1.01).
Grade 3 to 4 AE rates
All five studies reported grade 3 and 4 adverse event rates (Analysis 2.4).
2.4. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 4: Grade 3/4 adverse events subgrouped by type of ALK inhibitor
There is likely no difference in grade 3 and 4 AEs between next‐generation ALK inhibitors and crizotinib (RR 0.98, 95% CI 0.88 to 1.08, P < 0.001, I² = 88%, 5 RCTs, 1263 participants). There was high heterogeneity, which we explored further with subgroup analysis by type of next‐generation ALK inhibitor.
Crizotinib was compared to alectinib in three studies (ALESIA 2019; ALEX 2017; J‐ALEX 2017), brigatinib in one study (ALTA‐1L 2019), and lorlatinib in one study (CROWN 2020). Subgroup analysis by next‐generation ALK inhibitor type revealed a difference (test for subgroup difference: Chi² = 27.23, df = 2 (P < 0.001), I² = 92.7%). Visual inspection of the plot favoured alectinib (RR 0.73), but not brigatinib (RR 1.20) or lorlatinib (RR 1.30) in comparison to crizotinib.
Grade 5 AE rates
All five studies reported grade 5 AE rates (toxic deaths) (Figure 10, Analysis 2.5).
10.

Forest plot of comparison: 2 Next‐generation ALK inhibitor versus crizotinib, outcome: 2.5 Grade 5 adverse events (excluding progressive disease) subgrouped by type of ALK inhibitor.
2.5. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 5: Grade 5 adverse events (excluding progressive disease) subgrouped by type of ALK inhibitor
Only one study presented grade 5 AE rates with and without progressive disease included in the definition (CROWN 2020). We analysed grade 5 AEs excluding disease progression for all five studies (Analysis 2.5).
The evidence suggests there is no difference in grade 5 AEs (excluding disease progression) between participants who received next‐generation ALK inhibitors and those who received crizotinib (RR 0.85, 95% CI 0.49 to 1.47, P = 0.62, I² = 0%, 5 RCTs, 1263 participants, low‐certainty evidence).
Crizotinib was compared to alectinib in three studies (ALESIA 2019; ALEX 2017; J‐ALEX 2017), brigatinib in one study (ALTA‐1L 2019), and lorlatinib in one study (CROWN 2020). Subgroup analysis of grade 5 AEs (excluding progressive disease) by next‐generation ALK inhibitor type revealed no difference (test for subgroup difference: Chi² = 0.98, df = 2 (P = 0.61), I² = 0%).
Treatment reductions
Four studies reported dose reduction rates due to AEs (ALESIA 2019; ALEX 2017; ALTA‐1L 2019; CROWN 2020). Next‐generation ALK inhibitors likely result in little to no difference in dose reduction rates when compared to crizotinib (RR 1.27, 95% CI 1.02 to 1.59, P = 0.51, I² = 0%, 4 RCTs, 1056 participants) (Analysis 2.6).
2.6. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 6: Dose intensity
Four studies reported treatment interruption rates due to AEs (ALESIA 2019; ALEX 2017; CROWN 2020; J‐ALEX 2017). Next‐generation ALK inhibitors likely result in little to no difference in treatment interruption rates when compared to crizotinib (RR 0.83, 95% CI 0.71 to 0.97, P = 0.002, I² = 79%, 4 RCTs, 988 participants) (Analysis 2.6).
All five studies reported treatment discontinuation rates due to AEs. There is likely no difference in discontinuation rates between next‐generation ALK inhibitors and crizotinib (RR 0.86, 95% CI 0.63 to 1.16, P = 0.22, I² = 30%, 5 RCTs, 1263 participants) (Analysis 2.6).
Types of AEs
We presented types of AEs where comparable data were available and categorised them into gastrointestinal (Analysis 2.7), haematological (Analysis 2.8), hepatic (Analysis 2.9), and general (Analysis 2.10). We were not able to calculate summary statistics for categories of AEs due to the presence of significant heterogeneity (I² > 75%).
2.7. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 7: Gastrointestinal adverse events
2.8. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 8: Haematological adverse events
2.9. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 9: Hepatic adverse events
2.10. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 10: General adverse events
3. Secondary outcome: overall survival
All five studies reported OS comparing next‐generation ALK inhibitors (alectinib, brigatinib, and lorlatinib) to crizotinib.
Next‐generation ALK inhibitors likely result in an increase in OS when compared to crizotinib. (HR 0.71, 95% CI 0.56 to 0.90, P = 0.19, I² = 34%, 5 RCTs, 1263 participants, moderate‐certainty evidence) (Figure 11, Analysis 2.11).
11.

Forest plot of comparison: 2 Next‐generation ALK inhibitor versus crizotinib, outcome: 2.11 Overall survival subgrouped by type of ALK inhibitor.
2.11. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 11: Overall survival subgrouped by type of ALK inhibitor
Crizotinib was compared to alectinib in three studies (ALESIA 2019; ALEX 2017; J‐ALEX 2017), brigatinib in one study (ALTA‐1L 2019), and lorlatinib in one study (CROWN 2020). Subgroup analysis of OS by next‐generation ALK inhibitor type revealed no difference (test for subgroup difference: Chi² = 1.93, df = 2 (P = 0.38), I² = 0%).
4. Secondary outcome: overall survival at one year
Two studies reported OS at one year (ALEX 2017; ALTA‐1L 2019). The evidence suggests that next‐generation ALK inhibitors result in no difference in OS at one year when compared to crizotinib (RR 0.98, 95% CI 0.67 to 1.43, P = 0.71, I² = 0%, 2 RCTs, 578 participants) (Analysis 2.12).
2.12. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 12: Overall survival at 1 year subgrouped by type of ALK inhibitor
Further survival data
All five studies reported median PFS and OS. These values are presented measured in months in Table 4, including reported confidence intervals and the cross‐over rate measured as the percentage of participants in the crizotinib group who received the next‐generation ALK inhibitor after discontinuing the study. Median PFS for crizotinib consistently approximated 10 months in all 5 studies, and 34 months in 2 studies (ALEX 2017; J‐ALEX 2017), but is not yet reached in 3 studies (ALESIA 2019; ALTA‐1L 2019; CROWN 2020). Median OS for crizotinib was reported in two studies at 57.4, ALEX 2017, and 43.7 months, J‐ALEX 2017, but had not been reached in the other three studies. Median OS for next‐generation ALK inhibitors had not been reached at the time of publication in any of the five studies. Median lengths of follow‐up ranged from 14.8 to 48.2 months, as presented in Table 4. In contrast to comparison 1, only one study allowed cross‐over.
2. Next‐generation ALK versus crizotinib: median progression‐free survival and overall survival.
| Study | Median progression‐free survival | Median overall survival | Median follow‐up | Median overall survival | Median follow‐up | % Cross‐over | |
|
Next‐generation ALK inhibitor months (95% CI) |
Crizotinib months (95% CI) |
Next‐generation ALK inhibitor months (95% CI) |
Next‐generation ALK inhibitor months |
Crizotinib months (95% CI) |
Crizotinib | ||
| ALEX 2017 | 34.8 (17.7 to NE) | 10.9 (9.1 to 12.9) | NR (NR to NR) | 48.2 | 57.4 (34.6 to NR) | 23.3 | 0 |
| J‐ALEX 2017 | 34.1 (22.1 to NE) | 10.2 (8.3 to 12) | NE (NE to NE) | 42.4 | 43.7 (41.5 to NE) | 42.2 | 0 |
| ALTA‐1L 2019 | NR (NR to NR) | 9.8 (9.0 to 12.9) | NR (NR to NR) | 24.9 | NR (NR to NR) | 15.2 | 25.4 |
| ALESIA 2019 | NE (16.7 to NE) | 10.7 (7.4 to NE) | NR (NR to NR) | 27.8 | NR (NR to NR) | 22.8 | 0 |
| CROWN 2020 | NE (NR to NR) | 9.3 (7.6 to 11.1) | NR (NR to NR) | 18.3 | NR (NR to NR) | 14.8 | 0 |
CI: confidence interval NE: not estimable NR: not reached
5. Secondary outcome: overall response rate
All five studies reported overall, partial, and complete response rates.
Participants who received next‐generation ALK inhibitors likely had a slightly higher ORR (RR 1.18, 95% CI 1.10 to 1.25, P = 0.69, I² = 0%, 5 RCTs, 1229 participants, moderate‐certainty evidence) (Analysis 2.13).
2.13. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 13: Overall response rate subgrouped by type of ALK inhibitor
Subgroup analysis of ORR by type of next‐generation ALK inhibitor revealed no difference (test for subgroup difference: Chi² = 1.57, df = 2 (P = 0.46), I² = 0%).
Participants who received next‐generation ALK inhibitors likely had a slightly higher partial response rate (RR 1.15, 95% CI 1.07 to 1.24, P = 0.70, I² = 0%, 5 RCTs, 1229 participants) and complete response rate (RR 1.87, 95% CI 1.11 to 3.16, P = 0.80, I² = 0%, 5 RCTs, 1229 participants) than those who received crizotinib (Analysis 2.14).
2.14. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 14: Partial and complete response rate
ORR in baseline CNS disease
Four studies specifically reported response rate (overall and complete) in baseline measurable CNS metastases (Analysis 2.15) (ALESIA 2019; ALEX 2017; ALTA‐1L 2019; CROWN 2020).
2.15. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 15: Overall and complete response rate in people with measurable baseline CNS disease
Next‐generation ALK inhibitors likely result in an increased ORR in baseline measurable CNS lesions when compared with crizotinib (RR 2.45, 95% CI 1.7 to 3.54, P = 0.25, I² = 27%, 4 RCTs, 138 participants).
Next‐generation ALK inhibitors may result in a large increase in complete response rate in baseline CNS lesions when compared with crizotinib (RR 8.85, 95% CI 2.88 to 27.20, P = 0.98, I² = 0%, 4 RCTs, 138 participants) (Analysis 2.15).
6. Secondary outcome: health‐related quality of life
Two studies reported HRQoL (ALEX 2017; ALTA‐1L 2019), and three have not yet published HRQoL outcomes (ALESIA 2019; CROWN 2020; J‐ALEX 2017). Both studies reporting HRQoL used the EORTC QLQ‐C30 and EORTC QLQ‐LC13 questionnaires (Aaronson 1993; Koller 2017). Only ALEX 2017 reported time to deterioration in a composite endpoint (cough, dyspnoea, and chest pain), and we were not able to perform a meta‐analysis (Analysis 2.16).
2.16. Analysis.

Comparison 2: Next‐generation ALK inhibitor versus crizotinib, Outcome 16: Quality of life: time to deterioration in composite endpoint (cough, dyspnoea, and chest pain)
Discussion
Summary of main results
We obtained data for 2874 participants from 11 studies investigating ALK inhibitors as monotherapy for the treatment of advanced ALK‐rearranged NSCLC. Six studies (1611 participants) compared an ALK inhibitor to chemotherapy (ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018), and five studies (1263 participants) compared next‐generation ALK inhibitors to crizotinib (ALESIA 2019; ALEX 2017; ALTA‐1L 2019; CROWN 2020; J‐ALEX 2017). Our primary outcomes were PFS and AEs. Secondary outcomes were OS, OS at one year, ORR, and HRQoL. Data were available for all of our predetermined primary and secondary outcome measures in the majority of studies.
ALK inhibitor versus chemotherapy
In the six studies identified for this comparison, crizotinib, ceritinib, and alectinib were compared to chemotherapy in the first‐line (three studies) or second/subsequent line (three studies) setting.
Treatment with ALK inhibitors results in a large improvement in PFS compared to chemotherapy, which was the primary outcome measure in all included studies. This was the case regardless of the line of treatment and for participants with baseline CNS involvement. Subgroup analysis by type of ALK inhibitor and line of treatment detected a difference, although all studies favoured ALK inhibitors, and the apparent larger increase in PFS with alectinib was based on a single study (ALUR 2018). ALUR 2018 was a small study comparing alectinib to chemotherapy in the second‐line setting and included a high rate of patients with baseline CNS involvement. Targeted therapy is known to penetrate the brain more effectively than chemotherapy, which may explain the findings in ALUR 2018. We assessed the certainty of the evidence for the outcome of PFS as high.
None of the included studies were blinded, and as such, AE reporting was open to performance and detection bias, which resulted in an assessment of low‐certainty evidence. There may be no difference in overall AE rate between ALK inhibitor and chemotherapy, regardless of line of treatment or type of ALK inhibitor. As expected with different classes of medicine, there was a wide range of AEs, some of which were only specific to one drug type; as a result, not all could be compared in our selected categories. To avoid a unit of analysis error and given the variety of AE types, the only category permitting the calculation of a summary statistic was haematological toxicity, which favoured ALK inhibitors. Reported grade 5 AE (toxic death) rates appeared to be higher with ALK inhibitors, but when this outcome was analysed as excluding disease progression as the stated cause of death, little to no difference between groups was demonstrated.
The duration of time on treatment was higher for ALK inhibitor than for chemotherapy. This is an important consideration when assessing the frequency of AEs, including death, which may be overestimated in the ALK inhibitor arms due to longer exposure time to the drug.
ALK inhibitors result in a slight increase in OS compared to chemotherapy regardless of line of treatment or type of ALK inhibitor. We assessed the certainty of the evidence for the outcome of OS as high. The magnitude of benefit, in trials where cross‐over was allowed, was much less than was seen with PFS. As presented in a table summarising median PFS and OS (Table 3), a significant number of participants randomised to chemotherapy later crossed over to receive an ALK inhibitor. For the outcome measure of survival at one year, there was a high level of unexplained heterogeneity which prevented pooled analysis. Of the two included studies, one study showed a large benefit for crizotinib, and the other failed to show a discernible difference.
ALK inhibitors likely result in a higher ORR and complete response rate compared to chemotherapy. This effect appeared to be stronger in the second or subsequent line setting and with alectinib. There was heterogeneity for ORR, which on inspection of the forest plot appeared to be largely contributed by the ALUR 2018 study. In participants who had baseline measurable CNS metastases, ALK inhibitors likely result in a higher ORR. We assessed the certainty of the evidence for the outcome of ORR as moderate.
Five studies reported HRQoL measures, all of which showed an improvement in the time to deterioration composite endpoint (cough, dyspnoea, and chest pain) for ALK inhibitors compared to chemotherapy. We assessed the certainty of the evidence for this outcome as high.
Next‐generation ALK inhibitor versus crizotinib
The planned comparison of one ALK inhibitor versus another ALK inhibitor was tailored to the five included studies, whereby next‐generation ALK inhibitors (alectinib, lorlatinib, and brigatinib) were compared to crizotinib as the control group. All included studies allowed or mandated treatment in the first‐line setting, hence subgroup analysis by line of treatment was not possible.
Next‐generation ALK inhibitors result in a large improvement in PFS compared to crizotinib, regardless of type of inhibitor and including those participants with baseline CNS involvement. We assessed the certainty of the evidence for this outcome as high.
Next‐generation ALK inhibitors likely result in no difference in overall and grade 3 or 4 AEs rates compared to crizotinib, although subgroup analysis of grade 3 or 4 AEs favoured alectinib over crizotinib, but not lorlatinib or brigatinib. We did not undertake summary analysis for our categorised types of AEs, and some AEs specific to drugs were not reported consistently across studies to permit inclusion in the review. We assessed the certainty of the evidence as moderate.
There may be no difference in grade 5 AEs excluding disease progression between next‐generation ALK inhibitors and crizotinib, including in subgroup analysis by type of next‐generation ALK inhibitor. We assessed the certainty of the evidence as low.
Next‐generation ALK inhibitors likely result in an increase in OS compared to crizotinib, regardless of type of inhibitor. The effect measure (HR 0.71) favoured next‐generation ALK inhibitors over crizotinib more strongly than ALK inhibitors versus chemotherapy (HR 0.84), which may be because four of the five studies did not allow cross‐over. Median OS was not yet reached in the next‐generation ALK inhibitor arms of the five included studies. We assessed the certainty of the evidence as moderate.
Next‐generation ALK inhibitors likely had a slightly increased overall, partial, and complete response rate compared to crizotinib regardless of the type of inhibitor. We assessed the certainty of the evidence as moderate. In participants with baseline measurable CNS disease, next‐generation ALK inhibitors likely result in an increased ORR compared to crizotinib.
Two studies reported HRQoL outcomes, but not with comparable endpoints. As a result, we could not perform a meta‐analysis and comparison of effects on HRQoL for next‐generation ALK inhibitors versus crizotinib. We assessed the certainty of the evidence for the outcome of HRQoL as very low.
Overall completeness and applicability of evidence
We nominated PFS as our primary efficacy outcome, which was found to be the primary endpoint by design in all of the included trials. Median OS for people with advanced ALK‐rearranged NSCLC has been estimated beyond six years in observational studies (Pacheco 2019), which is consistent with most of the studies included in this review not yet reaching median OS values. Cross‐over to the control arm after the trial limited interpretation of the magnitude of benefit in OS for ALK inhibitors compared to chemotherapy, whereas in studies comparing next‐generation ALK inhibitors to crizotinib there was limited cross‐over, thereby giving a more accurate representation of effects on median OS.
The following limitations may affect the strength of the conclusions of this review.
There was no access to individual patient data for analysis, therefore we could not interpret the influence of individual patient characteristics such as gender, age, ethnicity, or smoking status on outcomes in this review.
There was some limitation in interpretation of CNS outcomes given the variety of definitions of baseline CNS involvement and variability in the use of CNS imaging. Some studies presented measurable and non‐measurable baseline brain metastases, whereas others included all baseline CNS disease for subgroup analysis. In addition, not all studies clearly specified those who had or had not received previous brain radiotherapy. In restricting our subgroup analysis to studies with the same baseline definition of CNS involvement, fewer were included in the ORR analysis.
Different ALK inhibitors have various unique side effects. Not all studies assessed or reported these uniformly, with AEs such as pulmonary toxicity or visual changes not defined in such a way to allow subgroup analysis across studies, therefore this review does not demonstrate the variety of specific AEs caused by different ALK inhibitors.
We found no studies that were performed exclusively in the second‐line setting for the comparison crizotinib versus next‐generation ALK inhibitors, hence subgroup analysis was not possible by line of treatment for this comparison. Furthermore, we were not able to subgroup by type of previous treatment as intended.
In selecting a single most commonly reported measure of HRQoL, the number of trials included in analyses of next‐generation ALK inhibitors compared to crizotinib was insufficient to allow us to draw a meaningful conclusion. HRQoL measures are important outcomes given that these medications are often taken for years until progression. This may be better analysed in the future with further publication of HRQoL outcomes in the more recent trials.
We are aware of some ongoing studies for new next‐generation ALK inhibitors (eXalt3 2020; NCT03737994; NCT04009317; NCT04318938; NCT04632758; Popat 2019). In this rapidly evolving field, this review will need to be updated in the future. Newer ALK‐targeted drugs such as the macrocyclic molecule TPX‐0131, Murray 2021; NCT04849273, are in development in response to resistance to next‐generation ALK inhibitors.
Quality of the evidence
All 11 included studies were parallel RCTs, with the majority being multicentre international studies. One included study was conducted in one country, Japan (J‐ALEX 2017). In three studies all participants were Asian (ALESIA 2019; J‐ALEX 2017; PROFILE 1029 2018).
Seven trials permitted cross‐over (ALTA‐1L 2019; ALUR 2018; ASCEND‐4 2017; ASCEND‐5 2017; PROFILE 1007 2013; PROFILE 1014 2014; PROFILE 1029 2018), which led to an underestimation of the HRs for OS in studies comparing ALK inhibitors and chemotherapy.
With regard to risk of bias assessment, a consistent evaluation across studies was an increased risk of performance and detection bias for outcomes reliant on subjective measurement such as HRQoL and AEs. This was a consequence of all included studies being open‐label. We considered this as low risk of bias for objective assessments such as death and if an independent review committee was used in assessing radiological response. Blinding would have been possible in designing trials comparing ALK inhibitors due to tablet form, but would have been less practical when comparing intravenous chemotherapy to targeted therapy in tablet form. It was not possible to investigate the risk of publication bias and complete a funnel plot given the insufficient number of included studies.
Using the GRADE approach (Table 1; Table 2), we considered the certainty of evidence to be moderate to high for most outcomes. For the comparison of ALK inhibitors versus chemotherapy, we assessed the certainty of the evidence as high for all PFS outcomes, OS, and HRQoL. For this same comparison, we downgraded the certainty of the evidence to moderate for ORR due to inconsistency of results and high heterogeneity. We downgraded the certainty of the evidence to low for AEs due to lack of blinding and imprecision of summary statistics with both gains and no effect. For the comparison of next‐generation ALK inhibitors versus crizotinib, we considered the certainty of the evidence as high for PFS outcomes, but downgraded to moderate for AEs due to lack of blinding and for OS due to inconsistency of trial findings. We downgraded the certainty of the evidence to low for grade 5 AEs due to imprecision with reports of gains and no effect, and very low for HRQoL given that limited reported results precluded meta‐analysis.
The overall number of participants across studies (2874) was adequate to give power to support the conclusions made for our primary outcomes.
Potential biases in the review process
We implemented a wide search that included ongoing trial registry databases and conference proceedings. We performed an updated search during the review process to include the most up‐to‐date literature. We did not apply language or publication status restrictions, and are confident that all relevant trials were included in the review or listed as ongoing studies. We prespecified all the review outcomes and subgroups prior to completion of the analysis. Subsequent comparison to currently published reviews revealed no publications that we had missed, which gave us further confidence in our search strategy. By limiting our included studies to RCTs, we pursued the highest level of evidence; however, in doing so we did not include several earlier‐phase studies that have been instrumental in drug registration and clinical use including description of AEs (Kim 2016; Wolf 2015)
Agreements and disagreements with other studies or reviews
Our findings support the use of first‐line next‐generation ALK inhibitors and align with international clinical guidelines (ASCO guideline; ESMO guideline), although these guidelines predate the evidence for lorlatinib.
We are aware of systematic reviews and meta‐analyses published in the last two years on this topic, and have compared our findings to the seven most relevant reviews (Barrows 2019; Breadner 2020; Chuang 2021; Costa 2018; Elliott 2020; Kassem 2019; Khan 2019)
Chuang 2021 published the most recent relevant review of which we are aware. They limited their review to first‐line therapy with the aim of using indirect comparison and network meta‐analysis to rank first‐line ALK inhibitors. Chuang 2021 included six studies, five of which were included in our second comparison first‐line subgroup analysis, and included an ensartinib trial, eXalt3 2020, which has only been published as conference proceedings and so was allocated as an ongoing study in our January search. The Chuang 2021 authors concluded that all next‐generation ALK inhibitors improved PFS when compared to crizotinib (HR 0.41). They undertook subgroup analysis for baseline CNS involvement, concluding that next‐generation ALK inhibitors also resulted in improved PFS when compared to crizotinib in these participants. Our conclusions were similar. They concluded that lorlatinib had the best PFS using surface under the cumulative ranking curve (SUCRA) analysis, whereby our PFS subgroup analysis by type of next‐generation ALK inhibitor did not demonstrate a significant difference.
To our knowledge, ours is the first review to demonstrate a significant increase in OS when comparing next‐generation ALK inhibitors to crizotinib.
Breadner 2020 undertook a recent review, which is the most comparable systematic review and meta‐analysis of which we are aware. They included only RCTs and undertook the same comparisons (ALK inhibitor versus chemotherapy and first‐generation versus second‐generation ALK inhibitors). We were pleased to find that the 10 trials included in Breadner 2020 were all found in our search. They were the first to find a significant benefit in OS for ALK inhibitors compared to chemotherapy (HR 0.84), and we concluded the exact same estimate. For the second comparison we included an additional next‐generation ALK inhibitor trial (lorlatinib, CROWN 2020), which had not yet been published at the time of their search in September 2019. This indicates how rapidly new evidence is arising in this field, with our search (January 2021) conducted only 16 months later. With this additional publication and updated data included, we found a likely significant improvement in OS for next‐generation ALK inhibitors compared to crizotinib, whereas the Breadner 2020 authors reported a trend only. In contrast to our review, Breadner 2020 designated OS as a primary outcome and did not undertake subgroup analysis by line of treatment.
Elliott 2020 also recently published a review of similar design to our study and limited study inclusion to RCTs. Two extra studies were included in the Elliott 2020 review due to their inclusion criteria permitting comparison of different doses of an ALK inhibitor and cross‐over design. The primary outcome for Elliot and colleagues was treatment‐related death; however, PFS was presented with similar conclusions to our review: all ALK inhibitors improved PFS relative to chemotherapy. After meta‐analysis, OS was only slightly increased with ALK inhibitors compared to chemotherapy; the Elliott 2020 authors also cautioned that interpretation of OS outcomes may be influenced by cross‐over rates.
Barrows 2019 undertook a systematic review of ALK inhibitors for advanced ALK‐positive NSCLC with a larger number of studies found due to inclusion of early‐phase clinical trials and observational studies in the search strategy. Barrows 2019 also presented survival outcomes and was better able to draw conclusions on sequencing of ALK inhibitors, albeit from study designs of lower‐quality evidence. Reported median OS from observational studies including sequential ALK inhibitors were up to 89.6 months from diagnosis, which may explain why the median OS has not been reached in the majority of RCTs included in our review. This review aimed to identify optimal sequencing of ALK inhibitors, but found no direct comparisons of different ALK inhibitor sequences.
Khan 2019 undertook a review of ALK inhibitors in the same context as our review, assessing similar comparisons and with PFS as a primary outcome. Nine of our 11 studies were also found in their search strategy conducted in 2018, but they also included retrospective studies. They reported similar findings for PFS, ORR, and intracranial response but found no significant statistical difference in OS when comparing ALK inhibitors to chemotherapy. Due to the inclusion of updated data, we found a significant difference favouring ALK inhibitors. In both Khan 2019 and our review, cross‐over is discussed as a potential confounder to the OS outcome. Khan and colleagues present more detailed HRQoL outcome measures than our review, which continued to favour ALK inhibitors over chemotherapy.
Costa 2018 compared AE rates and the AE profiles for crizotinib, ceritinib, alectinib, and brigatinib from phase I to III clinical trials and concluded that ALK inhibitors to have an acceptable safety profile and low rate of treatment‐related deaths, but did not compare ALK inhibitors to chemotherapy.
Kassem 2019 presented AE profiles for the same four ALK inhibitors as Costa 2018 and could describe the AE profiles of each drug in detail. With the inclusion of earlier‐phase studies, most conclusions relied on cross‐trial comparisons, and rates were not compared to chemotherapy. They also found a low treatment‐related death rate (0% to 1%).
Authors' conclusions
Implications for practice.
ALK inhibitors result in improved progression‐free survival and overall survival, overall response rate, and health‐related quality of life in comparison to chemotherapy, with similar overall adverse event rates. Next‐generation ALK inhibitors including alectinib, brigatinib, and lorlatinib achieve superior progression‐free survival and overall survival compared to crizotinib as the first‐line treatment for patients with advanced ALK‐rearranged non‐small cell lung cancer (NSCLC) and represent the standard of care in this setting.
We were unable to draw any conclusions on the optimal sequence of ALK inhibitors, as the design of included trials did not compare sequential treatment strategies.
Implications for research.
Future first‐line randomised controlled trials (RCTs) in advanced ALK‐rearranged NSCLC should no longer use crizotinib as the control arm.
Given that patients are on ALK inhibitors for long durations, it is important that future RCTs continue to assess health‐related quality of life outcomes.
Further studies are required to inform which next‐generation ALK inhibitor is the best initial treatment for advanced ALK‐rearranged NSCLC. Some reviews have used statistical methods to indirectly compare first‐line next‐generation ALK inhibitors (Chuang 2021), and although we identified one prospective study comparing alectinib and brigatinib in the second‐line setting (Popat 2019), we are not aware of a first‐line RCT designed to answer this question.
Future trials will require a design that can compare different ALK inhibitor sequences. We identified an ongoing study with a randomised design with multiple second‐line ALK inhibitors arms being allocated according to detected ALK resistance mutations (NCT03737994). As reflected in this design, an approach involving repeat genetic testing at progression may be required to allocate sequence of therapies based on resistance mechanism rather than one formula for all patients.
Ideal sequencing of ALK inhibitors may be addressed by well‐designed large prospective databases of real‐world data rather than the RCTs included in this review.
What's new
| Date | Event | Description |
|---|---|---|
| 21 January 2022 | Amended | Correcting minor formatting error. |
History
Protocol first published: Issue 10, 2019 Review first published: Issue 1, 2022
Acknowledgements
Leiyang Vivian Sun for contribution to the design and publication of the protocol.
Members of the Lung Cancer Group who peer reviewed the protocol and review: Fergus Macbeth, Nichole Taske, Sophie Paget‐Bailly, Michael Duruisseaux, Noelle O'Rourke, Corynne Marchal, Marta Roque i Figuls.
Information Specialists: François Calais and Giorgio Maria Agazzi.
Sign‐off Editor: Virginie Westeel.
Appendices
Appendix 1. CENTRAL search strategy
#1. MeSH descriptor: [Carcinoma, Non‐Small‐Cell Lung] explode all trees #2. nsclc #3. "lung cancer*" #4. "lung carcinom*" #5. "lung neoplasm*" #6. "lung tum*" #7. "non small cell*" #8. "nonsmall cell*" #9. (#3 or #4 or #5 or #6) and (#7 or #8) #10. #1 #2 or #9 #11. "anaplastic lymphoma kinase" #12. alk #13. "anaplastic lymphoma kinase" #14. "anaplastic lymphoma receptor tyrosine kinase" #15. "CD 246" #16. MeSH descriptor: [Molecular Targeted Therapy] explode all trees #17. crizotinib #18. xalkori #19. ceritinib #20. zykadia #21. LDK378 #22. alectinib #23. entrectinib #24. brigatinib #25. AP26113 #26. lorlatinib #27. X396 OR Ensartinib #28. "X 396" #29. MeSH descriptor: [Receptor Protein‐Tyrosine Kinases] explode all trees #30. MeSH descriptor: [Protein Kinase Inhibitors] explode all trees #31. #13 or #14 or #15 #32. #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 #33. #10 and #31 and #32
Appendix 2. MEDLINE search strategy
| 1 | Carcinoma, Non‐Small‐Cell Lung[MeSH Terms] |
| 2 | nsclc[Title/Abstract] |
| 3 | lung cancer*[Title/Abstract] |
| 4 | lung carcinoma[Title/Abstract] |
| 5 | lung neoplasm*[Title/Abstract] |
| 6 | lung tumor*[Title/Abstract] |
| 7 | lung tumour*[Title/Abstract] |
| 8 | non small cell*[Title/Abstract] |
| 9 | nonsmall cell*[Title/Abstract] |
| 10 | (#3 OR #4 OR #5 OR #6 OR #7) AND (#8 OR #9) |
| 11 | #1 OR #2 OR #10 |
| 12 | anaplastic lymphoma kinase[Supplementary Concept] |
| 13 | alk[Title/Abstract] |
| 14 | anaplastic lymphoma kinase[Title/Abstract] |
| 15 | anaplastic lymphoma receptor tyrosine kinase[Title/Abstract] |
| 16 | CD246[Title/Abstract] |
| 17 | CD 246[Title/Abstract] |
| 18 | molecular targeted therapy[MeSH Terms] |
| 19 | target*[Title/Abstract] |
| 20 | crizotinib[Supplementary Concept] |
| 21 | crizotinib[Title/Abstract] |
| 22 | PF 02341066 |
| 23 | PF02341066 |
| 24 | xalkori[Title/Abstract] |
| 25 | ceritinib[Supplementary Concept] |
| 26 | ceritinib[Title/Abstract] |
| 27 | zykadia[Title/Abstract] |
| 28 | LDK378 |
| 29 | CH5424802[Supplementary Concept] |
| 30 | alectinib[Title/Abstract] |
| 31 | RO5424802 |
| 32 | entrectinib[Supplementary Concept] |
| 33 | entrectinib[Title/Abstract] |
| 34 | RXDX‐101 |
| 35 | NMS‐E628 |
| 36 | brigatinib[Title/Abstract] |
| 37 | AP26113 |
| 38 | 7‐amino‐12‐fluoro‐2,10,16‐trimethyl‐15‐oxo‐10,15,16,17‐tetrahydro‐2H‐8,4‐(metheno)pyrazolo(4,3‐h)(2,5,11)benzoxadiazacyclotetradecine‐3‐carbonitrile |
| 39 | lorlatinib[Title/Abstract] |
| 40 | PF06463922 |
| 41 | PF‐06463922 |
| 42 | ensartinib[Title/Abstract] |
| 43 | X396 |
| 44 | receptor protein‐tyrosine kinases[MeSH Terms] |
| 45 | protein kinase inhibitors[MeSH Terms] |
| 54 | (((AP26113[Supplementary Concept]) OR AP26113) OR brigatinib[Title/Abstract]) OR Alunbrig[Title/Abstract] |
| 58 | ((((entrectinib[Supplementary Concept]) OR entrectinib[Title/Abstract]) OR RXDX‐101) OR NMS‐E628) OR N‐(5‐(3,5‐difluorobenzyl)‐1H‐indazol‐3‐yl)‐4‐(4‐methyl‐1‐piperazinyl)‐2‐(tetrahydro‐2H‐pyran‐4‐ylamino)benzamide |
| 60 | #12 OR #13 OR #14 OR #15 OR #16 OR #17 |
| 61 | #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #54 OR #58 |
| 62 | #11 AND #60 AND #61 |
| 63 | randomized controlled trial[Publication Type] |
| 64 | controlled clinical trial[Publication Type] |
| 65 | randomized[Title/Abstract] |
| 66 | placebo[Title/Abstract] |
| 67 | drug therapy[MeSH Subheading] |
| 68 | randomly[Title/Abstract] |
| 69 | trial[Title/Abstract] |
| 70 | groups[Title/Abstract] |
| 71 | #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 |
| 72 | animals [MeSH Terms] NOT humans [MeSH Terms] |
| 73 | #71 NOT #72 |
| 74 | #62 AND #73 |
Appendix 3. Embase search strategy
| #50 | #11 AND #48 AND #49 | |
| #49 | 'crossover procedure'/exp OR 'double‐blind procedure'/exp OR 'randomized controlled trial'/exp OR 'single‐blind procedure'/exp OR random* OR factorial* OR crossover* OR (cross NEXT/1 over*) OR placebo* OR (doubl* NEAR/1 blind*) OR (singl* NEAR/1 blind*) OR assign* OR allocat* OR volunteer* | |
| #48 | #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 | |
| #47 | 'x 396':ti,ab | |
| #46 | 'nms e628':ti,ab | |
| #45 | 'rxdx 101':ti,ab | |
| #44 | 'pf 02341066':ti,ab | |
| #43 | 'pf 06463922':ti,ab | |
| #42 | ro5424802:ti,ab | |
| #41 | xalkori:ti,ab | |
| #40 | alunbrig:ti,ab | |
| #39 | lorbrena:ti,ab | |
| #38 | zykadia:ti,ab | |
| #37 | alecensa:ti,ab | |
| #36 | x396:ti,ab | |
| #35 | pf06463922:ti,ab | |
| #34 | ap26113:ti,ab | |
| #33 | rxdx101:ti,ab | |
| #32 | ch5424802:ti,ab | |
| #31 | ldk378:ti,ab | |
| #30 | pf02341066:ti,ab | |
| #29 | ensartinib:ti,ab | |
| #28 | 'lorlatinib':ti,ab | |
| #27 | 'lorlatinib'/exp | |
| #26 | 'brigatinib':ti,ab | |
| #25 | 'brigatinib'/exp | |
| #24 | 'entrectinib':ti,ab | |
| #23 | 'entrectinib'/exp | |
| #22 | 'alectinib':ti,ab | |
| #21 | 'alectinib'/exp | |
| #20 | 'ceritinib':ti,ab | |
| #19 | 'ceritinib'/exp | |
| #18 | 'crizotinib':ti,ab | |
| #17 | 'crizotinib'/exp | |
| #16 | 'protein tyrosine kinase inhibitor'/exp | |
| #15 | 'tyrosine kinase inhib*':ti,ab | |
| #14 | 'anaplastic lymphoma kinase':ti,ab | |
| #13 | 'anaplastic lymphoma kinase inhibitor'/exp | |
| #12 | alk:ti,ab | |
| #11 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 | |
| #10 | 'non‐small cell*':ab,ti | |
| #9 | 'nonsmall cell*':ab,ti | |
| #8 | 'lung tumour*':ab,ti | |
| #7 | 'lung tumor*':ab,ti | |
| #6 | 'lung neoplasm*':ab,ti | |
| #5 | 'lung cancer*':ab,ti | |
| #4 | 'lung carcinom*':ab,ti | |
| #3 | 'nsclc':ab,ti | |
| #2 | 'lung tumor'/exp | |
| #1 | 'non small cell lung cancer'/exp |
Data and analyses
Comparison 1. ALK inhibitor versus chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Progression‐free survival subgrouped by line of treatment | 6 | Hazard Ratio (IV, Fixed, 95% CI) | 0.45 [0.40, 0.52] | |
| 1.1.1 1st line | 3 | Hazard Ratio (IV, Fixed, 95% CI) | 0.47 [0.40, 0.56] | |
| 1.1.2 2nd or subsequent line | 3 | Hazard Ratio (IV, Fixed, 95% CI) | 0.43 [0.36, 0.52] | |
| 1.2 Progression‐free survival subgrouped by type of ALK inhibitor | 6 | Hazard Ratio (IV, Fixed, 95% CI) | 0.45 [0.40, 0.52] | |
| 1.2.1 Alectinib | 1 | Hazard Ratio (IV, Fixed, 95% CI) | 0.20 [0.12, 0.33] | |
| 1.2.2 Crizotinib | 3 | Hazard Ratio (IV, Fixed, 95% CI) | 0.45 [0.38, 0.53] | |
| 1.2.3 Ceritinib | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 0.52 [0.43, 0.64] | |
| 1.3 Progression‐free survival in people with CNS disease | 6 | 581 | Hazard Ratio (IV, Fixed, 95% CI) | 0.51 [0.41, 0.62] |
| 1.4 Overall adverse events subgrouped by line of treatment | 5 | 1404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [1.00, 1.03] |
| 1.4.1 1st line | 2 | 719 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [1.00, 1.03] |
| 1.4.2 2nd or subsequent line | 3 | 685 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.98, 1.04] |
| 1.5 Overall adverse events subgrouped by type of ALK inhibitor | 5 | 1404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [1.00, 1.03] |
| 1.5.1 Alectinib | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.09] |
| 1.5.2 Crizotinib | 2 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.99, 1.02] |
| 1.5.3 Ceritinib | 2 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [1.01, 1.05] |
| 1.6 Grade 3/4 adverse events subgrouped by line of treatment | 5 | 1404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.00, 1.19] |
| 1.6.1 1st line | 2 | 719 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.00, 1.26] |
| 1.6.2 2nd or subsequent line | 3 | 685 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.94, 1.20] |
| 1.7 Grade 3/4 adverse events subgrouped by type of ALK inhibitor | 5 | 1404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.00, 1.19] |
| 1.7.1 Alectinib | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.42, 1.32] |
| 1.7.2 Crizotinib | 2 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.85, 1.16] |
| 1.7.3 Ceritinib | 2 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.09, 1.31] |
| 1.8 Total Grade 5 adverse events subgrouped by line of treatment | 6 | 1611 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [2.02, 5.48] |
| 1.8.1 1st line | 3 | 926 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.60 [1.78, 7.29] |
| 1.8.2 2nd or subsequent line | 3 | 685 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [1.50, 6.18] |
| 1.9 Grade 5 adverse events (excluding progressive disease) subgrouped by line of treatment | 6 | 1611 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.89, 4.66] |
| 1.9.1 1st line | 3 | 926 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.77, 6.93] |
| 1.9.2 2nd or subsequent line | 3 | 685 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.47, 5.98] |
| 1.10 Grade 5 adverse events (excluding progressive disease) subgrouped by ALK inhibitor | 6 | 1611 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.89, 4.66] |
| 1.10.1 Alectinib | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.94] |
| 1.10.2 Crizotinib | 3 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.69, 6.41] |
| 1.10.3 Ceritinib | 2 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.32 [0.74, 25.29] |
| 1.11 Dose intensity | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.11.1 Treatment reductions | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.09, 1.54] |
| 1.11.2 Treatment interruption | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.64, 6.91] |
| 1.11.3 Treatment discontinuation | 5 | 1235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.73, 1.45] |
| 1.12 Gastrointestinal adverse events | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.12.1 Nausea any | 5 | 1504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.18, 1.44] |
| 1.12.2 Nausea Grade 3 to 4 | 5 | 1504 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.19, 5.47] |
| 1.12.3 Vomiting any | 5 | 1504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.66, 2.16] |
| 1.12.4 Vomiting Grade 3 to 4 | 5 | 1504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.73, 2.63] |
| 1.12.5 Diarrhoea any | 6 | 1611 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.64 [3.12, 4.25] |
| 1.12.6 Diarrhoea Grade 3 to 4 | 6 | 1611 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.89 [1.45, 5.77] |
| 1.12.7 Constipation any | 6 | 1610 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.16, 1.60] |
| 1.12.8 Constipation Grade 3 to 4 | 6 | 1610 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.67, 8.95] |
| 1.13 Haematological adverse events | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.13.1 Anaemia Grade 3 to 4 | 5 | 1380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.12, 0.40] |
| 1.13.2 Neutropenia Grade 3 to 4 | 6 | 1611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.40, 0.68] |
| 1.13.3 Thrombocytopenia Grade 3 to 4 | 3 | 781 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.04, 0.43] |
| 1.14 Hepatic adverse events | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.14.1 Increased alanine aminotransferase Grade 3 to 4 | 3 | 954 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.52 [5.19, 17.46] |
| 1.14.2 Increased aspartate aminotransferase Grade 3 to 4 | 3 | 954 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.12 [3.75, 17.60] |
| 1.14.3 Increased bilirubin any grade | 2 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [0.35, 16.21] |
| 1.14.4 Increased bilirubin Grade 3 to 4 | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.15 General any grade adverse events | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.15.1 Fatigue | 6 | 1611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.69, 0.95] |
| 1.15.2 Loss of appetite | 5 | 1504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.96, 1.31] |
| 1.15.3 Oedema | 3 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.14 [2.39, 4.13] |
| 1.15.4 Rash any grade | 3 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.57, 1.15] |
| 1.16 Overall survival subgrouped by line of treatment | 6 | 1611 | Hazard Ratio (IV, Fixed, 95% CI) | 0.84 [0.72, 0.97] |
| 1.16.1 1st line | 3 | 926 | Hazard Ratio (IV, Fixed, 95% CI) | 0.78 [0.62, 0.97] |
| 1.16.2 2nd or subsequent line | 3 | 685 | Hazard Ratio (IV, Fixed, 95% CI) | 0.89 [0.73, 1.09] |
| 1.17 Overall survival subgrouped by type of ALK inhibitor | 6 | Hazard Ratio (IV, Fixed, 95% CI) | 0.84 [0.72, 0.97] | |
| 1.17.1 Alectinib | 1 | Hazard Ratio (IV, Fixed, 95% CI) | 0.91 [0.49, 1.69] | |
| 1.17.2 Crizotinib | 3 | Hazard Ratio (IV, Fixed, 95% CI) | 0.83 [0.69, 0.99] | |
| 1.17.3 Ceritinib | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 0.85 [0.64, 1.11] | |
| 1.18 Overall survival at 1 year | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.19 Overall response rate by line of treatment | 6 | 1611 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [2.16, 2.75] |
| 1.19.1 1st line | 3 | 926 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.79, 2.30] |
| 1.19.2 2nd or subsequent line | 3 | 685 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.08 [3.03, 5.48] |
| 1.20 Overall response rate subgrouped by type of ALK inhibitor | 6 | 1611 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [2.16, 2.75] |
| 1.20.1 Alectinib | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.12 [1.86, 92.68] |
| 1.20.2 Crizotinib | 3 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [1.83, 2.40] |
| 1.20.3 Ceritinib | 2 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [2.45, 3.97] |
| 1.21 Partial and complete response rate | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.21.1 Partial response | 5 | 1380 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [2.03, 2.59] |
| 1.21.2 Complete response | 5 | 1380 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [0.80, 9.14] |
| 1.22 Overall and complete response rate in people with measurable baseline CNS disease | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.22.1 Overall response rate | 3 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.88 [2.18, 10.95] |
| 1.22.2 Complete response rate | 2 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.32, 7.57] |
| 1.23 Quality of life: time to deterioration in composite endpoint (cough, dyspnoea, and chest pain) | 5 | 1504 | Hazard Ratio (IV, Fixed, 95% CI) | 0.52 [0.44, 0.60] |
| 1.23.1 Crizotinib | 3 | 897 | Hazard Ratio (IV, Fixed, 95% CI) | 0.52 [0.43, 0.61] |
| 1.23.2 Ceritinib | 2 | 607 | Hazard Ratio (IV, Fixed, 95% CI) | 0.51 [0.38, 0.70] |
Comparison 2. Next‐generation ALK inhibitor versus crizotinib.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Progression‐free survival subgrouped by type of ALK inhibitor | 5 | 1263 | Hazard Ratio (IV, Random, 95% CI) | 0.39 [0.32, 0.46] |
| 2.1.1 Alectinib | 3 | 697 | Hazard Ratio (IV, Random, 95% CI) | 0.40 [0.32, 0.49] |
| 2.1.2 Brigatinib | 1 | 275 | Hazard Ratio (IV, Random, 95% CI) | 0.49 [0.33, 0.73] |
| 2.1.3 Lorlatinib | 1 | 291 | Hazard Ratio (IV, Random, 95% CI) | 0.28 [0.19, 0.41] |
| 2.2 Progression‐free survival in people with CNS disease | 5 | 800 | Hazard Ratio (IV, Fixed, 95% CI) | 0.25 [0.19, 0.34] |
| 2.3 Overall adverse events subgrouped by type of ALK inhibitor | 5 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.98, 1.01] |
| 2.3.1 Alectinib | 3 | 697 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.97, 1.01] |
| 2.3.2 Brigatinib | 1 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.97, 1.01] |
| 2.3.3 Lorlatinib | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.99, 1.04] |
| 2.4 Grade 3/4 adverse events subgrouped by type of ALK inhibitor | 5 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.88, 1.08] |
| 2.4.1 Alectinib | 3 | 697 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.62, 0.86] |
| 2.4.2 Brigatinib | 1 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.01, 1.42] |
| 2.4.3 Lorlatinib | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.09, 1.56] |
| 2.5 Grade 5 adverse events (excluding progressive disease) subgrouped by type of ALK inhibitor | 5 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.49, 1.47] |
| 2.5.1 Alectinib | 3 | 697 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.27, 1.62] |
| 2.5.2 Brigatinib | 1 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.35, 1.93] |
| 2.5.3 Lorlatinib | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.41, 4.96] |
| 2.6 Dose intensity | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.6.1 Dose reduction | 4 | 1056 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.02, 1.59] |
| 2.6.2 Treatment interruption | 4 | 988 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.71, 0.97] |
| 2.6.3 Treatment discontinuation | 5 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.63, 1.16] |
| 2.7 Gastrointestinal adverse events | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.7.1 Nausea Any Grade | 5 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.26, 0.38] |
| 2.7.2 Vomiting Any Grade | 5 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.22, 0.35] |
| 2.7.3 Diarrhoea Any Grade | 5 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.37, 0.50] |
| 2.7.4 Constipation Any Grade | 5 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.61, 0.84] |
| 2.7.5 Nausea Grade 3 to 4 | 5 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.13, 0.81] |
| 2.7.6 Vomiting Grade 3 to 4 | 5 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.64] |
| 2.7.7 Diarrhoea Grade 3 to 4 | 5 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.23, 1.63] |
| 2.7.8 Constipation Grade 3 to 4 | 4 | 960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.08, 4.40] |
| 2.8 Haematological adverse events | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.8.1 Anaemia Grade 3 to 4 | 3 | 801 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.98, 6.77] |
| 2.8.2 Neutropenia Grade 3 to 4 | 5 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.03, 0.19] |
| 2.9 Hepatic adverse events | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.9.1 Increased Alanine Aminotransferase Grade 3 to 4 | 5 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.18, 0.49] |
| 2.9.2 Increased Aspartate Aminotransferase Grade 3 to 4 | 4 | 1076 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.27, 0.82] |
| 2.9.3 Increased Bilirubin Any Grade | 3 | 697 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.29 [5.48, 32.24] |
| 2.9.4 Increased Bilirubin Grade 3 to 4 | 3 | 697 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.52 [0.68, 18.37] |
| 2.10 General adverse events | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.10.1 Fatigue Any Grade | 4 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.99] |
| 2.10.2 Loss of Appetite Any Grade | 4 | 960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.13, 0.31] |
| 2.10.3 Oedema Any Grade | 4 | 1076 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.57, 0.83] |
| 2.10.4 Rash Any Grade | 5 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.33, 2.47] |
| 2.11 Overall survival subgrouped by type of ALK inhibitor | 5 | 1263 | Hazard Ratio (IV, Fixed, 95% CI) | 0.71 [0.56, 0.90] |
| 2.11.1 Alectinib | 3 | 697 | Hazard Ratio (IV, Fixed, 95% CI) | 0.62 [0.45, 0.85] |
| 2.11.2 Brigatinib | 1 | 275 | Hazard Ratio (IV, Fixed, 95% CI) | 0.92 [0.59, 1.43] |
| 2.11.3 Lorlatinib | 1 | 291 | Hazard Ratio (IV, Fixed, 95% CI) | 0.72 [0.41, 1.26] |
| 2.12 Overall survival at 1 year subgrouped by type of ALK inhibitor | 2 | 578 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.67, 1.43] |
| 2.12.1 Alectinib | 1 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.55, 1.52] |
| 2.12.2 Brigatinib | 1 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.60, 1.86] |
| 2.13 Overall response rate subgrouped by type of ALK inhibitor | 5 | 1229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.10, 1.25] |
| 2.13.1 Alectinib | 3 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.05, 1.22] |
| 2.13.2 Brigatinib | 1 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.01, 1.41] |
| 2.13.3 Lorlatinib | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.08, 1.49] |
| 2.14 Partial and complete response rate | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.14.1 Partial Response | 5 | 1229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.07, 1.24] |
| 2.14.2 Complete Response | 5 | 1229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.11, 3.16] |
| 2.15 Overall and complete response rate in people with measurable baseline CNS disease | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.15.1 Overall response rate | 4 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [1.70, 3.54] |
| 2.15.2 Complete Response Rate | 4 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.85 [2.88, 27.20] |
| 2.16 Quality of life: time to deterioration in composite endpoint (cough, dyspnoea, and chest pain) | 1 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ALESIA 2019.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial (2:1) Study grouping: parallel group |
|
| Participants |
Baseline characteristics Alectinib 600 mg orally, twice daily, continuously
Crizotinib 250 mg orally, twice daily, continuously
Inclusion criteria Histologically or cytologically confirmed diagnosis of advanced or recurrent (stage IIIB not amenable for multimodality treatment) or metastatic (stage IV) NSCLC that is ALK‐positive as assessed by the Ventana immunohistochemistry (IHC) test. Sufficient tumour tissue available to perform ALK IHC is required. Ventana IHC testing will be performed at the designated central laboratory. Life expectancy of at least 12 weeks. ECOG PS of 0 to 2. No history of receiving systemic treatment for advanced, recurrent (stage IIIB not amenable for multimodality treatment) or metastatic (stage IV) NSCLC. Adequate haematologic function: platelet count >= 100 × 10^9/L; absolute neutrophil count (ANC) >= 1500 cells per microlitre; haemoglobin >= 9.0 g/dL. Adequate renal function: an estimated glomerular filtration rate (eGFR) calculated using the Modification of Diet in Renal Disease (MDRD) formula of >= 45 millilitres per minute per 1.73 square metre. Participants must have recovered from effects of any major surgery or significant traumatic injury at least 28 days before receiving the first dose of study treatment. Measurable disease (by Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1)) before administration of study treatment. Previous brain or leptomeningeal metastases are allowed if the patient is asymptomatic (e.g. diagnosed incidentally at study baseline). Asymptomatic central nervous system (CNS) lesions may be treated at the discretion of the investigator as per local clinical practice. If patient has neurological symptoms or signs because of CNS metastasis, the patient must complete whole‐brain radiation or gamma knife irradiation treatment. In all cases, radiation treatment must be completed >= 14 days before enrolment, and disease must be clinically stable. For all females of childbearing potential, a negative serum pregnancy test result must be obtained within 3 days prior to starting study treatment. For women who are not postmenopausal (>= 12 months of non‐therapy‐induced amenorrhoea) or surgically sterile (absence of ovaries and/or uterus), agreement to remain abstinent or to use single or combined contraceptive methods that result in a failure rate of < 1% per year during the treatment period and for at least 3 months after the last dose of study drug. Abstinence is acceptable only if it is in line with the preferred and usual lifestyle of the participant. Periodic abstinence (e.g. calendar, ovulation, symptothermal, or postovulation methods) and withdrawal are not acceptable methods of contraception. Examples of contraceptive methods with a failure rate of < 1% per year include tubal ligation, male sterilisation, hormonal implants, established, proper use of combined oral or injected hormonal contraceptives, and certain intrauterine devices. Alternatively, 2 methods (e.g. 2 barrier methods such as a condom and a cervical cap) may be combined to achieve a failure rate of < 1% per year. Barrier methods must always be supplemented with the use of a spermicide. For men, agreement to remain abstinent or to use a condom plus an additional contraceptive method that together result in a failure rate of < 1% per year during the treatment period and for at least 3 months after the last dose of study drug. Abstinence is acceptable only if it is in line with the preferred and usual lifestyle of the participant. Periodic abstinence (e.g. calendar, ovulation, symptothermal, or postovulation methods) and withdrawal are not acceptable methods of contraception. Exclusion criteria A malignancy within the previous 3 years (other than curatively treated basal cell carcinoma of the skin, early gastrointestinal (GI) cancer by endoscopic resection, in situ carcinoma of the cervix, or any cured cancer that is considered to have no impact in progression‐free survival (PFS) or overall survival (OS) for the current NSCLC). Any GI disorder that may affect absorption of oral medications, such as malabsorption syndrome or status post‐major bowel resection. Liver disease characterised by: alanine aminotransferase (ALT) or aspartate aminotransferase (AST) greater than 3 × the upper limit of normal (ULN) (>= 5 × ULN for participants with concurrent liver metastases) confirmed on 2 consecutive measurements; or impaired excretory function (e.g. hyperbilirubinaemia), synthetic function, or other conditions of decompensated liver disease such as coagulopathy, hepatic encephalopathy, hypoalbuminaemia, ascites, and bleeding from oesophageal varices; or acute viral or active autoimmune, alcoholic, or other types of hepatitis. National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 Grade 3 or higher toxicities because of any previous therapy (e.g. radiotherapy) (excluding alopecia) which have not shown improvement and are strictly considered to interfere with current study medication. History of organ transplant. Co‐administration of anticancer therapies other than those administered in this study. Baseline QTc > 470 ms or symptomatic bradycardia. Administration of strong/potent cytochrome P4503A inhibitors or inducers within 14 days prior to the receiving the first dose of study treatment and during treatment with alectinib or crizotinib. Administration of agents with potential QT interval prolonging effects within 14 days prior to receiving the first dose of study drug. History of hypersensitivity to any of the additives in the alectinib or crizotinib drug formulation. Pregnant or lactating. Known HIV‐positivity or AIDS‐related illness. Any clinically significant concomitant disease or condition that could interfere with, or for which the treatment might interfere with, the conduct of the study or the absorption of oral medications or that would, in the opinion of the Principal Investigator, pose an unacceptable risk to the participant in this study. Any psychological, familial, sociological, or geographical condition that potentially hampers compliance with the study protocol requirements or follow‐up procedures; those conditions should be discussed with the participant before study entry. Number enrolled: 187 Number in control group: 67 (crizotinib) Number in treatment group: 125 (alectinib) Number of withdrawal (treatment/control): 1/1 |
|
| Interventions |
Intervention characteristics Alectinib 600 mg orally, twice daily, continuously
Crizotinib 250 mg orally, twice daily, continuously
|
|
| Outcomes | Progression‐free survival (PFS) as determined by investigator using Response Evaluation Criteria in Solid Tumour (RECIST) v1.1 [ Time Frame: from the date of randomisation to the date of the first documented disease progression or death, whichever occurred first (up to overall period of approximately 40 months) ] PFS as determined by Independent Review Committee (IRC) using RECIST v1.1 [ Time Frame: baseline, Week 8, thereafter every 8 weeks until disease progression, death, or withdrawal from the study and 4 weeks after permanent discontinuation (up to overall period of approximately 40 months) ] Percentage of participants with objective response of complete response (CR) or partial response (PR) as determined by investigator using RECIST v1.1 [ Time Frame: baseline, Week 8, thereafter every 8 weeks until disease progression, death, or withdrawal from the study and 4 weeks after permanent discontinuation (up to overall period of approximately 40 months) ] Time to progression of disease in the CNS as determined by IRC using RECIST v1.1 [ Time Frame: baseline, Week 8, thereafter every 8 weeks until disease progression, death, or withdrawal from the study and 4 weeks after permanent discontinuation (up to overall period of approximately 40 months) ] Time to progression of disease in the CNS as determined by IRC using Response Assessment in Neuro‐Oncology (RANO) [ Time Frame: baseline, Week 8, thereafter every 8 weeks until disease progression, death, or withdrawal from the study and 4 weeks after permanent discontinuation (up to overall period of approximately 40 months) ] Duration of response (DOR) assessed by investigator using RECIST v1.1 [ Time Frame: baseline, Week 8, thereafter every 8 weeks until disease progression, death, or withdrawal from the study and 4 weeks after permanent discontinuation (up to overall period of approximately 40 months) ] Overall survival time [ Time Frame: baseline, until death (up to overall period of approximately 40 months) ] Percentage of participants with non‐serious adverse events and serious adverse events [ Time Frame: up to overall period of approximately 40 months ] Time to deterioration assessed using EORTC QLQ‐C30 score [ Time Frame: baseline, Week 4, thereafter every 4 weeks until disease progression, death, or withdrawal from the study and 4 weeks after permanent discontinuation (up to overall period of approximately 40 months) ] Time to deterioration assessed using EORTC QLQ‐LC13 score [ Time Frame: baseline, Week 4, thereafter every 4 weeks until disease progression, death, or withdrawal from the study and 4 weeks after permanent discontinuation (up to overall period of approximately 40 months) ] Area under the plasma concentration‐time curve (AUC) of alectinib and its metabolite [ Time Frame: baseline and Week 4 predose (within 2 hours before administration of study drug) ] Maximum plasma concentration observed (Cmax) of alectinib and its metabolite [ Time Frame: baseline and Week 4 predose (within 2 hours before administration of study drug) ] Time to Cmax (Tmax) of alectinib and its metabolite [ Time Frame: baseline and Week 4 predose (within 2 hours before administration of study drug) ] |
|
| Identification |
Trial name: ALESIA Sponsorship source: F. Hoffmann‐La Roche Country: China, South Korea, Thailand |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was done centrally via an interactive voice or web response system, with stratification by ECOG PS (0 or 1 vs 2) and baseline CNS metastases (yes vs no)." |
| Allocation concealment (selection bias) | Low risk | Randomisation was done centrally via an interactive voice or web response. |
| Blinding of participants and personnel for objective outcomes | High risk | Primary endpoint was investigator‐assessed PFS rather than independent PFS. Comment: this was judged as high risk of bias |
| Blinding of participants and personnel for subjective outcomes | High risk | Open‐label study |
| Blinding of outcome assessment for objective outcomes | High risk | Primary endpoint was investigator‐assessed PFS rather than independent PFS. Comment: this was judged as high risk of bias Included IRC‐assessed PFS |
| Blinding of outcome assessors for subjective outcomes | High risk | Open‐label study, outcomes such as patient‐reported outcome measures and quality of life influenced by risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Intention‐to‐Treat Population"; participants accounted for as per CONSORT diagram |
| Selective reporting (reporting bias) | Low risk | All outcomes from registration reported and listed July 2016. |
| Other bias | Low risk | Groups well balanced, up front 2:1 randomisation set |
ALEX 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Alectinib 600 mg orally, twice daily, continuously
Crizotinib 250 mg orally, twice daily, continuously
Inclusion criteria Histologically or cytologically confirmed diagnosis of advanced or recurrent (stage IIIB not amenable for multimodality treatment) or metastatic (stage IV) NSCLC that is ALK‐positive as assessed by the Ventana immunohistochemistry (IHC) test. Life expectancy of at least 12 weeks. ECOG PS of 0 to 2. Patients with no prior systemic treatment for advanced or recurrent (stage IIIB not amenable for multimodality treatment) or metastatic (stage IV) NSCLC. Adequate renal and haematologic function. Participants must have recovered from effects of any major surgery or significant traumatic injury at least 28 days before the first dose of study treatment. Measurable disease by Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 (v1.1) prior to the administration of study treatment. Prior brain or leptomeningeal metastases allowed if asymptomatic (e.g. diagnosed incidentally at study baseline). Negative pregnancy test for all females of childbearing potential. Use of highly effective contraception as defined by the study protocol Exclusion criteria Patients with a previous malignancy within the past 3 years. Any gastrointestinal (GI) disorder or liver disease. National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) (version 4.0) Grade 3 or higher toxicities due to any prior therapy (e.g. radiotherapy) (excluding alopecia). History of organ transplant. Co‐administration of anticancer therapies other than those administered in this study. Patients with baseline QTc > 470 ms or symptomatic bradycardia. Recipient of strong/potent cytochrome P4503A inhibitors or inducers within 14 days prior to the first dose until the end of study treatment. Recipient of any drug with potential QT interval‐prolonging effects within 14 days prior to the first dose for all participants and whilst on treatment through the end of the study for crizotinib‐treated participants only. History of hypersensitivity to any of the additives in the alectinib and crizotinib drug formulation. Pregnancy or lactation. Any clinically significant disease or condition (or history of) that could interfere with, or for which the treatment might interfere with, the conduct of the study or the absorption of oral medications, or that would, in the opinion of the Principal Investigator, pose an unacceptable risk to the participant in this study. Any psychological, familial, sociological, or geographical condition potentially hampering compliance with the study protocol requirements and/or follow‐up procedures; those conditions should be discussed with the participant before trial entry Number eligible: 354 Number enrolled: 303 Number in treatment group: alectinib 152 Number in control group: crizotinib 151 Number withdrawal from trial (treatment/control): 68/105 Number completing trial (treatment/control): 84/46 |
|
| Interventions |
Intervention characteristics Alectinib 600 mg orally, twice daily, continuously
Crizotinib 250 mg orally, twice daily, continuously
|
|
| Outcomes | Progression‐free survival (PFS) by investigator assessment [ Time Frame: randomisation to first documented disease progression or death, whichever occurs first (assessed every 8 weeks up to 33 months) ] PFS Independent Review Committee (IRC)‐assessed [ Time Frame: randomisation to first documented disease progression or death, whichever occurs first (assessed every 8 weeks up to 33 months) ] Percentage of participants with central nervous system (CNS) progression as determined by IRC using RECIST V1.1 criteria [ Time Frame: randomisation to CNS progressive disease as first occurrence of disease progression (assessed every 8 weeks up to 33 months) ] Percentage of participants with CNS progression as determined by IRC using Revised Assessment in Neuro Oncology (RANO) Criteria [ Time Frame: randomisation to the first occurrence of disease progression in the CNS (assessed every 8 weeks up to 33 months) ] Percentage of participants with objective response rate (ORR) of complete response (CR) or partial response (PR) as determined by the investigators according to RECIST V1.1 criteria [ Time Frame: randomisation to first documented disease progression or death, whichever occurs first (assessed every 8 weeks up to 33 months) ] Duration of response (DOR) according to RECIST V1.1 criteria as assessed by the investigators [ Time Frame: first occurrence of objective response to first documented disease progression or death, whichever occurs first (assessed every 8 weeks up to 33 months) ] Overall survival (OS) [ Time Frame: from randomisation until death (up to 43 months) ] Percentage of participants with adverse events [ Time Frame: baseline up to 28 months in the crizotinib arm and up to 30 months in the alectinib arm ] Area under the concentration‐time curve (AUC) of alectinib Maximum concentration (Cmax) of alectinib Time to reach Cmax (Tmax) of alectinib AUC of alectinib metabolite Cmax of alectinib metabolite Tmax of alectinib metabolite Time to deterioration by EORTC QLQ‐C30 [ Time Frame: baseline, every 4 weeks until disease progression (up to 33 months) ] |
|
| Identification |
Trial name: ALEX Sponsorship source: Hoffmann‐La Roche Country: multinational (North/South America, Europe, Asia) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "block‐stratified randomization procedure with the use of an interactive or Web‐based response system)" |
| Allocation concealment (selection bias) | Low risk | ixRS system in protocol |
| Blinding of participants and personnel for objective outcomes | High risk | Quote: "The primary end point was investigator‐ assessed progression‐free survival." Comment: this was judged as high risk of bias |
| Blinding of participants and personnel for subjective outcomes | High risk | Open‐label study, outcomes influenced by risk of bias |
| Blinding of outcome assessment for objective outcomes | High risk | Quote: "The primary end point was investigator‐ assessed progression‐free survival." Comment: this was judged as high risk of bias, as there was no independent central review for the primary outcome Study included IRC‐assessed PFS as secondary outcome. |
| Blinding of outcome assessors for subjective outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for, intention‐to‐treat analysis used. |
| Selective reporting (reporting bias) | Low risk | All outcomes from registration reported in the results. |
| Other bias | Low risk | Balanced baseline characteristics |
ALTA‐1L 2019.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Brigatinib 90 mg orally, daily for 7 days, then 180 mg daily, continuously
Crizotinib 250 mg orally, daily, continuously
Inclusion criteria Have histologically or cytologically confirmed stage IIIB (and not a candidate for definitive multimodality therapy) or stage IV NSCLC. Must have documented ALK‐rearrangement. Have sufficient tumour tissue available for central analysis. Have at least 1 measurable (i.e. target) lesion per Response Evaluation Criteria in Solid Tumours (RECIST) v1.1. Recovered from toxicities related to prior anticancer therapy to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) (NCI CTCAE v 4.0) grade <= 1. Male or female participants >= 18 years old. Have adequate organ function, as defined by the study protocol. Have ECOG PS <= 2. Have normal QT interval on screening electrocardiogram evaluation, defined as QT interval corrected (Fridericia) (QTcF) of <= 450 ms in males or <= 470 ms in females. For female participants of childbearing potential, have a negative pregnancy test documented prior to randomisation. For female and male participants who are fertile, agree to use a highly effective form of contraception, as defined by the study protocol. Provide signed and dated informed consent indicating that the participant has been informed of all pertinent aspects of the study, including the potential risks, and is willingly participating. Have the willingness and ability to comply with scheduled visit and study procedures. Exclusion criteria Previously received an investigational antineoplastic agent for NSCLC. Previously received any prior TKI, including ALK‐targeted TKIs. Previously received more than 1 regimen of systemic anticancer therapy for locally advanced or metastatic disease. Received chemotherapy or radiation within 14 days of first dose of study drug, except stereotactic radiosurgery (SRS) or stereotactic body radiation therapy (SBRT). Received antineoplastic monoclonal antibodies within 30 days of the first dose of study drug. Had major surgery within 30 days of the first dose of study drug; minor surgical procedures such as catheter placement or minimally invasive biopsies are allowed. Have been diagnosed with another primary malignancy other than NSCLC, except for adequately treated non‐melanoma skin cancer or cervical cancer in situ; definitively treated non‐metastatic prostate cancer; or patients with another primary malignancy who are definitively relapse‐free with at least 3 years elapsed since the diagnosis of the other primary malignancy. Have symptomatic central nervous system metastases (parenchymal or leptomeningeal) at screening or asymptomatic disease requiring an increasing dose of corticosteroids to control symptoms within 7 days prior to randomisation. Have current spinal cord compression (symptomatic or asymptomatic and detected by radiographic imaging). Patients with leptomeningeal disease and without cord compression are permitted. Be pregnant, planning a pregnancy, or breastfeeding. Have significant, uncontrolled, or active cardiovascular disease, as defined by the study protocol. Have uncontrolled hypertension. Have a history or the presence at baseline of pulmonary interstitial disease, drug‐related pneumonitis, or radiation pneumonitis. Have an ongoing or active infection. Have a known history of HIV infection. Have a known or suspected hypersensitivity to brigatinib or its excipients and/or crizotinib or its excipients. Have malabsorption syndrome or other gastrointestinal (GI) illness or condition. Have any condition or illness that, in the opinion of the investigator, would compromise the participant's safety or interfere with evaluation of the study drug. Number eligible: 311 Number enrolled: 275 Number in treatment group: 137 Number in control group: 138 Number of withdrawals (treatment/control): 5/8 |
|
| Interventions | Brigatinib 90 mg orally, daily for 7 days, then 180 mg daily, continuously
Crizotinib 250 mg orally, daily, continuously
|
|
| Outcomes | Progression‐free survival (PFS) [ Time Frame: baseline up to approximately 36 months ] Objective response rate (ORR) [ Time Frame: baseline up to approximately 36 months ] Intracranial ORR [ Time Frame: baseline up to approximately 36 months ] Intracranial PFS [ Time Frame: baseline up to approximately 36 months ] Overall survival (OS) [ Time Frame: baseline up to approximately 36 months ] Duration of response [ Time Frame: baseline up to approximately 36 months ] Time to response (TTR) [ Time Frame: baseline up to approximately 36 months ] Disease control rate (DCR) [ Time Frame: baseline up to approximately 36 months ] Health‐related quality of life (HRQoL) [ Time Frame: baseline until 30 days after the last dose of study treatment (approximately 3 years) ] Percentage of participants with adverse events [ Time Frame: baseline until 30 days after the last dose of study treatment (approximately 3 years) ] |
|
| Identification |
Trial name: ALTA‐1L Sponsorship source: Ariad Pharmaceuticals Country: multinational (Europe, Asia, North America) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel for objective outcomes | Low risk | Objective overall survival would not be influenced by blinding. Independent review for RECIST PFS + OS assessed by blinded independent central review |
| Blinding of participants and personnel for subjective outcomes | High risk | Open‐label study |
| Blinding of outcome assessment for objective outcomes | Low risk | Overall survival would not be influenced by blinding. Independent review for RECIST PFS assessed by blinded independent central review Comment: this was judged as low risk of bias |
| Blinding of outcome assessors for subjective outcomes | High risk | High risk of bias for subjective outcomes, as open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants (1 per group) did not receive treatment but were included in the intention‐to‐treat analysis. See Figure 1 CONSORT diagram. |
| Selective reporting (reporting bias) | Low risk | Registered April 2016. All outcomes reported (quality of life was reported in separate publication). |
| Other bias | Low risk | Balanced baseline characteristics |
ALUR 2018.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Alectinib 600 mg orally, daily, continuously
Docetaxel 75 mg/m² intravenously, every 3 weeks or pemetrexed 500 mg/m² intravenously, every 3 weeks continuously
Inclusion criteria Histologically or cytologically confirmed diagnosis of advanced or recurrent (stage IIIB not amenable for multimodality treatment) or metastatic (stage IV) NSCLC that is ALK‐positive. ALK‐positivity must have been determined by a validated fluorescence in situ hybridisation (FISH) test (recommended probe, Vysis ALK Break‐Apart Probe) or a validated immunohistochemistry (IHC) test (recommended antibody, clone D5F3). Participant had received 2 prior systemic lines of therapy, which must have included 1 line of platinum‐based chemotherapy and 1 line of crizotinib. Prior CNS or leptomeningeal metastases allowed if asymptomatic. Participants with symptomatic CNS metastases for whom radiotherapy is not an option will be allowed to participate in this study. Measurable disease by Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 prior to the administration of study treatment. ECOG PS of 0 to 2. For all females of childbearing potential, a negative pregnancy test must be obtained within 3 days before starting study treatment. Exclusion criteria Patients with a previous malignancy within the past 3 years are excluded (other than curatively treated basal cell carcinoma of the skin, early gastrointestinal (GI) cancer by endoscopic resection, or in situ carcinoma of the cervix). Patients who have received any previous ALK inhibitor other than crizotinib. Any GI disorder that may affect absorption of oral medications. Pretreatment: higher proportion of ECOG 2, brain metastases, female gender, and Asian ethnicity in chemotherapy group Number enrolled: 107 Number in control group: chemotherapy 35 (docetaxel 25, pemetrexed 9) Number in treatment group: 72 alectinib Number of withdrawal (treatment/control): 20/10 |
|
| Interventions |
Intervention characteristics Alectinib 600 mg orally, daily, continuously
Docetaxel 75 mg/m² intravenously, every 3 weeks or pemetrexed 500 mg/m² intravenously, every 3 weeks
|
|
| Outcomes | Progression‐free survival (PFS) using RECIST version 1.1 as assessed by investigator [ Time Frame: randomisation to first documented disease progression, death from any cause, or study end (up to 33 months) ] Percentage of participants with CNS objective response rate (ORR) with measurable CNS metastases at baseline using RECIST version 1.1 as assessed by Independent Review Committee (IRC) [ Time Frame: baseline through study end (up to 33 months) ] PFS using RECIST version 1.1 as assessed by IRC [ Time Frame: approximately 15 months (tumour assessments at baseline, every 6 weeks until progressive disease (PD), death, or withdrawal from study prior to PD) ] Percentage of participants with objective response of complete response (CR) or partial response (PR) using RECIST version 1.1 as assessed by investigator and IRC [ Time Frame: approximately 15 months (tumour assessments at baseline, every 6 weeks until PD, death, or withdrawal from study prior to PD) ] Percentage of participants with disease control using RECIST version 1.1 as assessed by investigator and IRC [ Time Frame: approximately 15 months (tumour assessments at baseline, every 6 weeks until PD, death, or withdrawal from study prior to PD) ] Duration of response (DOR) using RECIST version 1.1 as assessed by investigator and IRC [ Time Frame: from the first documented CR or PR to the first documented disease progression, death, or study end (up to 33 months) ] PFS in the intention‐to‐treat population with CNS metastases at baseline (C‐ITT) using RECIST version 1.1 as assessed by investigator and IRC [ Time Frame: approximately 15 months (tumour assessments at baseline, every 6 weeks until PD, death, or withdrawal from study prior to PD) ] Time to CNS progression in C‐ITT population using RECIST version 1.1 as assessed by IRC [ Time Frame: approximately 15 months (tumour assessments at baseline, every 6 weeks until PD, death, or withdrawal from study prior to PD) ] Percentage of participants with disease control in C‐ITT population using RECIST version 1.1 as assessed by IRC [ Time Frame: from first documented CR, PR, or stable disease (SD) lasting at least 5 weeks through study end (up to 33 months) ] Percentage of participants with ORR in C‐ITT population using RECIST version 1.1 as assessed by IRC [ Time Frame: baseline through study end (up to 33 months) ] Duration of response for lesions in the CNS (C‐DOR) using RECIST version 1.1 as assessed by IRC [ Time Frame: from the first documented CR or PR to the first documented disease progression, death, or study end (up to 33 months) ] Overall survival (OS) [ Time Frame: randomisation to death from any cause, through study end (up to 33 months) ] Plasma concentration of alectinib Compliance of EORTC QLQ‐C30 over time [ Time Frame: baseline through Week 138 ] Percentage of participants with adverse events (AEs) [ Time Frame: baseline through study end (up to 33 months) ] |
|
| Identification |
Trial name: ALUR Sponsorship source: Hoffmann‐La Roche Country: multinational (Europe, Asia) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Central randomization was performed via a block‐stratified randomization procedure (block size 6) using an interactive voice or web‐based response system" |
| Allocation concealment (selection bias) | Low risk | Quote: "The following steps were taken to keep the study team blinded: no aggregate review of patients indicating the treatment allocation was performed" |
| Blinding of participants and personnel for objective outcomes | High risk | Investigator‐assessed PFS, therefore outcome could have been influenced by blinding Comment: this was judged to be high risk |
| Blinding of participants and personnel for subjective outcomes | High risk | Open‐label |
| Blinding of outcome assessment for objective outcomes | High risk | OS not influenced by blinding. Investigator‐assessed PFS, therefore outcome could have been influenced by blinding Comment: this was judged to be high risk |
| Blinding of outcome assessors for subjective outcomes | High risk | Open‐label study, outcomes could have been influenced by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The ITT population comprised all patients randomized. The safety population comprised all patients who received 1 dose of assigned study medication." |
| Selective reporting (reporting bias) | Low risk | Trial date listed prior and all outcomes reported, and all prespecified outcomes reported from trial. |
| Other bias | Low risk | Baseline characteristics well balanced. |
ASCEND‐4 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Ceritinib 750 mg orally, daily, continuously
Cisplatin 75 mg/m²/pemetrexed 500 mg/m² or carboplatin area under the curve (AUC) 5‐6/pemetrexed 500 mg/m² x 4 intravenously every 3 weeks followed by maintenance pemetrexed 500 mg/m² every 3 weeks
Inclusion criteria Patient has a histologically or cytologically confirmed diagnosis of NSCLC that is ALK‐positive as assessed by the Ventana immunohistochemistry (IHC) test. The test will be performed at Novartis designated central laboratories. Patient has newly diagnosed stage IIIB (who is not a candidate for definitive multimodality therapy) or stage IV NSCLC or relapsed locally advanced or metastatic NSCLC not previously treated with any systemic anticancer therapy (e.g. cytotoxic drugs, monoclonal antibody therapy, crizotinib or other ALK inhibitors, or other targeted therapies, either experimental or not), with the exception of neo‐adjuvant or adjuvant therapy. Patient has at least 1 measurable lesion as defined by Response Evaluation Criteria in Solid Tumours (RECIST) 1.1. Exclusion criteria Patient with known hypersensitivity to any of the excipients of LDK378 (microcrystalline cellulose, mannitol, crospovidone, colloidal silicon dioxide, and magnesium stearate). Patient with a history of severe hypersensitivity reaction to platinum containing drugs, pemetrexed, or any known excipients of these drugs. Patient with symptomatic CNS metastases who is neurologically unstable or has required increasing doses of steroids within the 2 weeks prior to screening to manage CNS symptoms. Number eligible: 425 Number enrolled: 376 Number in treatment group: 189 ceritinib Number in control group: 187 chemotherapy Number of withdrawals (treatment/control): 94/145 Number completing trial (treatment/control): 95/30 |
|
| Interventions |
Intervention characteristics Ceritinib 750 mg orally, daily, continuously
Cisplatin 75 mg/m²/pemetrexed 500 mg/m² or carboplatin AUC 5‐6/pemetrexed 500 mg/m² x 4 intravenously, every 3 weeks, followed by maintenance pemetrexed 500 mg/m² every 3 weeks, continuously
|
|
| Outcomes | Progression‐free survival by blinded independent review committee Overall survival Overall response rate Duration of response Disease control rate Time to response Patient‐reported outcomes with time to deterioration for quality of life Adverse events |
|
| Identification |
Trial name: ASCEND‐4 Sponsorship source: Novartis Country: multinational (Europe, Asia, South/Central America) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was done via interactive response technology" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was done via interactive response technology" |
| Blinding of participants and personnel for objective outcomes | Low risk | Quote: "Intracranial response was assessed based on images collected for the blinded independent review committee, by an independent central neuroradiologist" Overall survival would not be influenced by blinding. |
| Blinding of participants and personnel for subjective outcomes | High risk | Open‐label |
| Blinding of outcome assessment for objective outcomes | Low risk | Quote: "Intracranial response was assessed based on images collected for the blinded independent review committee, by an independent central neuroradiologist" Overall survival would not be influenced by blinding. |
| Blinding of outcome assessors for subjective outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Any withdrawals were stated in text. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | High risk | Quote: "overall intracranial response rate; intracranial disease control rate; duration of intracranial response; intracranial clinical benefit rate (this was added post hoc)" Comment: post hoc analysis for intracranial outcomes; this was deemed as high risk |
ASCEND‐5 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Ceritinib 750 mg orally, daily, continuously
Pemetrexed 500 mg/m² or docetaxel 75 mg/m² intravenously, every 3 weeks, continuously
Inclusion criteria Histologically or cytologically confirmed diagnosis of NSCLC carrying an ALK rearrangement as assessed by the US Food and Drug Administration (FDA)‐approved fluorescence in situ hybridisation (FISH) test using Vysis ALK Break Apart FISH Probe (Abbott Molecular Inc) test and scoring algorithm (including positivity criteria). Stage IIIB or IV NSCLC. Age 18 years or older at the time of informed consent. Life expectancy ≥ 12 weeks World Health Organization performance status of 0 to 2. Patients who had received previous treatment with crizotinib for the treatment of locally advanced or metastatic NSCLC. A minimum of 21 days of treatment with crizotinib was required to qualify as 1 prior course of crizotinib (unless crizotinib was discontinued due to progressive disease (PD) after a shorter treatment course). Greater than 1 prior course of crizotinib was allowed. Patients might have had discontinued crizotinib therapy for disease progression, intolerance, or other reason. No particular sequence of prior crizotinib and chemotherapy was required for enrolment, and either could comprise the last treatment received by the patient. Patients who had received 1 or 2 prior regimens (including platinum‐based doublet) of cytotoxic chemotherapy to treat their locally advanced or metastatic NSCLC: prior therapy with bevacizumab was allowed if it was a component of the previous platinum‐based regimen. Prior maintenance therapy (e.g. bevacizumab, pemetrexed) was allowed if it was a component of the previous platinum‐based regimen. For chemotherapy regimens given every 21 or 28 days, a minimum of 2 cycles was required to qualify as a prior chemotherapy regimen (unless chemotherapy was discontinued due to PD after 1 cycle). If chemotherapy was discontinued for a reason other than PD after only 1 cycle, then this regimen did not count as a prior line of chemotherapy. Prior cytotoxic chemotherapy was to include a platinum‐based doublet. If patient received 2 prior chemotherapy regimens, patient must not have received both pemetrexed and docetaxel. No particular sequence of prior crizotinib and chemotherapy was required for enrolment, and either could comprise the last treatment received by the patient. Patients who had documented disease progression at study enrolment. At least 1 measurable lesion as defined by Response Evaluation Criteria in Solid Tumours (RECIST) 1.1. A previously irradiated site lesion was counted as a target lesion only if there was clear sign of progression since the irradiation. Patients had to have recovered from all toxicities related to prior anticancer therapies to grade ≤ 1 (Common Terminology Criteria for Adverse Events (CTCAE) v 4.03). However, patients with any grade of alopecia were allowed to enter the study. Patients with the following laboratory values at the screening visit:
Patients with the following laboratory values within the laboratory normal limits or corrected to within normal limits with supplements during screening:
Written informed consent for the main study was obtained prior to any screening procedures. If consent could not be expressed in writing, it was formally documented and witnessed, ideally via an independent trusted witness. Willingness and ability to comply with scheduled visits, treatment plans, laboratory tests, and other study procedures. Exclusion criteria Patients with known hypersensitivity to any of the excipients of ceritinib (microcrystalline cellulose, mannitol, crospovidone, colloidal silicon dioxide, and magnesium stearate). Patients with a history of severe hypersensitivity reaction to pemetrexed, if the investigator’s choice for chemotherapy was pemetrexed. Patients with a history of severe hypersensitivity reaction to docetaxel or to other drugs formulated with polysorbate 80, if the investigator’s choice for reference therapy was docetaxel. Patients with symptomatic CNS metastases who were neurologically unstable or required increasing doses of steroids within the 2 weeks prior to study entry to manage CNS symptoms. History of carcinomatous meningitis. History of interstitial lung disease or interstitial pneumonitis, including clinically significant radiation pneumonitis (i.e. affecting activities of daily living or requiring therapeutic intervention). Prior therapy with other ALK inhibitor investigational or approved agents with the exception of crizotinib. Prior systemic anticancer (including investigational) therapy aside from crizotinib and 1 to 2 regimens of previous cytotoxic chemotherapy for locally advanced or metastatic NSCLC. Patients who had thoracic radiotherapy to lung fields ≤ 4 weeks prior to starting the study treatment or patients who had not recovered from radiotherapy‐related toxicities. For all other anatomic sites (including radiotherapy to thoracic vertebrae and ribs), radiotherapy ≤ 2 weeks prior to starting the study treatment or patients who had not recovered from side effects of such procedure. Palliative radiotherapy for bone lesions ≤ 2 weeks prior to starting study treatment was allowed. Patients who had major surgery (e.g. intrathoracic, intra‐abdominal, or intrapelvic) within 4 weeks prior (2 weeks for resection of brain metastases) to starting study treatment or who had not recovered from side effects of such procedures. Video‐assisted thoracic surgery (VATS) and mediastinoscopy was not counted as major surgery, and patients could be enrolled in the study ≥ 2 weeks after the procedure. Presence or history of a malignant disease other than NSCLC that was diagnosed and/or required therapy within the past 3 years. Exceptions to this exclusion included completely resected basal cell and squamous cell skin cancers, and completely resected carcinoma in situ of any type. Clinically significant, uncontrolled heart disease and/or recent cardiac event (within 6 months), such as:
Impairment of gastrointestinal (GI) function or GI disease that might significantly alter the absorption of ceritinib (e.g. ulcerative diseases, uncontrolled nausea, vomiting, diarrhoea, or malabsorption syndrome). Patients treated with medications that met 1 of the following criteria and that could not be discontinued at least 1 week prior to the start of treatment with ceritinib and for the duration of the study:
Patients treated with warfarin sodium (Coumadin) or any other coumarin‐derivative anticoagulants. Patients who received unstable or increasing doses of corticosteroids. If patients were on corticosteroids for endocrine deficiencies or tumour‐associated symptoms (non‐CNS), dose was to be stabilised (or decreased) for at least 5 days before first dose of study treatment. Patients treated with any enzyme‐inducing anticonvulsant that could not be discontinued at least 1 week before first dose of study treatment, and for the duration of the study. Patients on non‐enzyme‐inducing anticonvulsants were eligible. Pregnant or nursing (lactating) women. Women of childbearing potential, defined as all women physiologically capable of becoming pregnant, unless they were using highly effective methods of contraception during dosing and for 3 months after stopping study medication. Sexually active males had to use a condom during intercourse whilst taking the drug and for 3 months after stopping ceritinib treatment and were not to father a child during this period. Male patients randomised to chemotherapy (docetaxel or pemetrexed) were not to father a child for at least 6 months after the last dose of treatment or as per the locally approved package label. A condom was also required to be used by vasectomised men in order to prevent delivery of the drug via seminal fluid. Other severe, acute, or chronic medical (including uncontrolled diabetes mellitus) or psychiatric conditions or laboratory abnormalities that in the opinion of the investigator might increase the risk associated with study participation, or that could interfere with the interpretation of study results. History of pancreatitis or a history of increased amylase or lipase that was due to pancreatic disease Number eligible: 231 Number enrolled: 231 Number in control group: 116 Number in treatment group: 115 Number of withdrawal (treatment/control): 82/105 |
|
| Interventions |
Intervention characteristics Ceritinib 750 mg orally, daily, continuously
Pemetrexed 500 mg/m² or docetaxel 75 mg/m², intravenously, every 3 weeks, continuously
|
|
| Outcomes |
Primary outcome Progression‐free survival (PFS) per blinded independent review committee (BIRC) [ Time Frame: from the date of randomisation to the date of first radiologically documented disease progression or death due to any cause up to approximately 24 months ] Secondary outcomes Overall survival (OS) [ Time Frame: Month 18 ] Overall response rate (ORR) [ Time Frame: Month 18 ] Duration of response (DOR) [ Time Frame: Month 18 ] Disease control rate (DCR) [ Time Frame: Month 18 ] Time to response (TTR) [ Time Frame: Month 18 ] Patient‐reported outcomes (PRO) [ Time Frame: screening, followed by every 6 weeks until Month 18, after Month 18 every 9 weeks ] Time to definitive deterioration [ Time Frame: from the date of randomisation to the date of event for disease‐related symptoms ] Overall intracranial response rate (OIRR) [ Time Frame: screening, followed by every 6 weeks until Month 18, after Month 18 every 9 weeks ] Intracranial disease control rate (IDCR) [ Time Frame: screening, followed by every 6 weeks until Month 18, after Month 18 every 9 weeks ] Duration of intracranial response (DOIR) [ Time Frame: screening, followed by every 6 weeks until Month 18, after Month 18 every 9 weeks ] |
|
| Identification |
Trial name: ASCEND‐5 Sponsorship source: Novartis Country: multinational (USA, Italy, Japan, Canada, Spain, Switzerland) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomly allocated eligible patients using interactive response technology (IRT) in a 1:1 ratio (block randomisation with a block size of four used) to receive either ceritinib or chemotherapy" |
| Allocation concealment (selection bias) | Low risk | Quote: "patient randomisation list was produced by the IRT provider using a validated, automated system. Each patient was uniquely identified in the study by a patient number that was assigned when the patient was first identified for screening, which was retained as the primary identifier for the patient throughout their entire participation in the study. A separate medication randomisation list was produced under the responsibility of Novartis using a validated system that automated the random assignment of medication numbers to medication packs containing ceritinib. Chemotherapy was locally sourced and the medication number was provided by the IRT system." |
| Blinding of participants and personnel for objective outcomes | Low risk | Quote: "The primary endpoint was progression‐free survival (the time from randomisation to the first radiologically documented disease progression [according to RECIST 1.1 and assessed by the masked IRC] or death from any cause)." |
| Blinding of participants and personnel for subjective outcomes | High risk | Open‐label study |
| Blinding of outcome assessment for objective outcomes | Low risk | Quote: "The primary endpoint was progression‐free survival (the time from randomisation to the first radiologically documented disease progression [according to RECIST 1.1 and assessed by the masked IRC] or death from any cause). The key secondary efficacy endpoint was overall survival" |
| Blinding of outcome assessors for subjective outcomes | High risk | Open‐label study, outcomes influenced by risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for as per CONSORT diagram. |
| Selective reporting (reporting bias) | Low risk | All outcomes from registration reported. |
| Other bias | Low risk | Small differences between study groups with regard to baseline characteristics: smoker/never smoker (10% difference), Asian race (7% difference), ECOG PS 1/2 (4% in ECOG 2) |
CROWN 2020.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Lorlatinib 100 mg orally, daily, continuously
Crizotinib 250 mg orally, twice daily, continuously
Inclusion criteria Histologically or cytologically confirmed diagnosis of locally advanced or metastatic ALK‐positive NSCLC; at least 1 extracranial measurable target lesion not previously irradiated. CNS metastases allowed if asymptomatic and not currently requiring corticosteroid treatment. Availability of an archival formalin fixed paraffin embedded (FFPE) tissue specimen. No prior systemic NSCLC treatment. ECOG PS 0, 1, or 2. Age ≥ 18 years. Adequate bone marrow, liver, renal, pancreatic function. Negative pregnancy test for females of childbearing potential Exclusion criteria Spinal cord compression unless good pain control attained. Major surgery within 4 weeks prior to randomisation. Radiation therapy within 2 weeks prior to randomisation, including stereotactic or partial brain irradiation. Whole brain irradiation within 4 weeks prior to randomisation. Active bacterial, fungal, or viral infection. Clinically significant cardiovascular disease, active or within 3 months prior to enrolment. Ongoing cardiac dysrhythmias, uncontrolled atrial fibrillation, bradycardia, or congenital long QT syndrome. Predisposing characteristics for acute pancreatitis in the last month prior to randomisation. History of extensive, disseminated, bilateral or presence of Grade 3 or 4 interstitial fibrosis or interstitial lung disease. Active malignancy (other than NSCLC, non‐melanoma skin cancer, in situ cervical cancer, papillary thyroid cancer, lobular carcinoma in situ (LCIS)/ductal carcinoma in situ (DCIS) of the breast, or localised prostate cancer) within the last 3 years prior to randomisation. Concurrent use of any of the following food or drugs within 12 days prior to the first dose of lorlatinib or crizotinib: known strong CYP3A inhibitors, known strong CYP3A inducers, known P gp substrates with a narrow therapeutic index. Concurrent use of CYP3A substrates with narrow therapeutic indices within 12 days prior to the first dose of lorlatinib or crizotinib. Other severe acute or chronic medical or psychiatric condition, including recent or active suicidal ideation or behaviour, or laboratory abnormality that may increase the risk associated with study participation or interfere with the interpretation of study results. Investigational site staff members directly involved in the conduct of the study and their family members, or Pfizer employees, including their family members, directly involved in the conduct of the study. Participation in other studies involving investigational drug(s) within 2 weeks prior to study entry and/or during study participation Pretreatment: nil significant Number enrolled: 296 Number in control group: 147 Number in treatment group: 142 Number of withdrawal (treatment/control): 4/18 |
|
| Interventions |
Intervention characteristics Lorlatinib 100 mg orally, daily, continuously
Crizotinib 250 mg orally, twice daily, continuously
|
|
| Outcomes | Progression‐free survival (PFS) based on blinded independent central review (BICR) assessment [ Time Frame: from time of study start up to 33 months ] Overall survival (OS) [ Time Frame: from time of study start up to 125 months ] PFS based on investigator's assessment [ Time Frame: from time of study start up to 33 months ] Objective response (OR) based on BICR and on investigator's assessment [ Time Frame: from time of study start up to 33 months ] Intracranial objective response (IC‐OR) based on BICR assessment [ Time Frame: from time of study start up to 33 months ] Intracranial time to progression (IC‐TTP) based on BIRC assessment [ Time Frame: from time of study start up to 33 months ] Duration of response (DR) based on BIRC assessment [ Time Frame: from time of study start up to 33 months ] Intracranial duration of response (IC‐DR) based on BICR assessment [ Time Frame: from time of study start up to 33 months ] Time to tumour response (TTR) based on BIRC assessment [ Time Frame: from time of study start up to 33 months ] Intracranial time to tumour response (IC‐TTR) based on BICR assessment [ Time Frame: from time of study start up to 33 months ] Second progression‐free survival (PFS2) based on investigator's assessment [ Time Frame: from time of study start up to 45 months ] Adverse event as graded by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v 4.03 [ Time Frame: from time of study start up to 33 months ] Laboratory abnormalities as graded by NCI CTCAE v 4.03 [ Time Frame: from time of study start up to 33 months ] Vital signs (blood pressure, pulse rate) and body weight [ Time Frame: from time of study start up to 33 months ] Electrocardiograms (ECG) [ Time Frame: from time of study start up to 33 months ] Echocardiograms or multigated acquisition scan (MUGA) [ Time Frame: from time of study start up to 33 months ] Ophthalmology [ Time Frame: from time of study start up to 33 months ] Patient reported outcomes (PRO) as assessed by EORTC QLQ‐C30, EORTC QLQ‐LC13, and EQ‐5D‐5L [ Time Frame: from time of study start up to 33 months ] Tumour tissue biomarkers [ Time Frame: from time of study start up to 33 months ] Peripheral blood cfDNA (circulating free DNA) biomarkers [ Time Frame: from time of study start up to 33 months ] |
|
| Identification |
Sponsorship source: Pfizer Country: USA, Spain, Japan, Canada, France, Korea, Hong Kong, Australia Comments: CROWN |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of interactive response technology (IRT) for assignment and randomisation number (stated in protocol) |
| Allocation concealment (selection bias) | Low risk | IRT system stores allocation generation |
| Blinding of participants and personnel for objective outcomes | Low risk | Blinded independent review committee |
| Blinding of participants and personnel for subjective outcomes | High risk | Open‐label study |
| Blinding of outcome assessment for objective outcomes | Low risk | Blinded independent review committee |
| Blinding of outcome assessors for subjective outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in CONSORT diagram. |
| Selective reporting (reporting bias) | Low risk | Trial listed prior to starting, and all planned outcomes were reported on. |
| Other bias | Low risk | Characteristics well balanced in study, slightly higher mean age in lorlatinib group (5 years) |
J‐ALEX 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Alectinib 300 mg orally, twice daily, continuously
Crizotinib 250 mg orally, daily, continuously
Inclusion criteria Histologically or cytologically confirmed ALK‐positive by immunohistochemistry and fluorescence in situ hybridisation (FISH) or reverse transcription‐polymerase chain reaction (RT‐PCR). Stage IIIB NSCLC not amenable to curative radiotherapy, stage IV NSCLC. Chemotherapy‐naive or has received 1 regimen of chemotherapy for NSCLC (continuous maintenance is included in the initial therapy). Note that neoadjuvant or postoperative adjuvant chemotherapy will only be counted as 1 regimen if the cancer recurs within 6 months after finishing therapy. Response Evaluation Criteria in Solid Tumours (RECIST) (v1.1) measurable disease. Exclusion criteria Treated with an ALK inhibitor in the past. Meningeal metastases, or brain metastases that are symptomatic or require treatment. Pleural effusion, ascites, or pericardial effusion requiring drainage. Concurrent or prior radiographically evident interstitial lung disease. HIV antibody‐positive, hepatitis B surface or hepatitis C virus (HCV) antigen‐positive, or HCV antibody‐positive. Another primary cancer with a disease‐free interval of less than 5 years Number eligible: 234 Number enrolled: 207 Number in control group: 104 Number in treatment group: 103 Number of withdrawal (treatment/control): 24/61 |
|
| Interventions |
Intervention characteristics Alectinib 300 mg orally, twice daily, continuously
Crizotinib 250 mg orally, twice daily, continuously
|
|
| Outcomes |
Primary outcome Progression‐free survival as assessed by the Independent Review Facility Secondary outcomes Investigator‐assessed progression‐free survival Overall survival Proportion of participants who achieved an objective response Duration of response Time to response Time to progression of brain metastatic lesions in participants who had them at baseline Time to onset of brain metastatic lesions in participants who did not have them at baseline Health‐related quality of life Safety Pharmacokinetics |
|
| Identification |
Trial name: J‐ALEX Sponsorship source: Chugai Pharmaceutical Co, Ltd. Country: Japan |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned in a 1:1 ratio, using a stratified permuted‐block method" |
| Allocation concealment (selection bias) | Low risk | Quote: "an interactive web response system (IxRS; EPS Corporation, Tokyo, Japan) using a randomisation code generated independently by the IxRS vendor." |
| Blinding of participants and personnel for objective outcomes | Low risk | Quote: "Members of the Independent Review Facility (IRF) who assessed the primary endpoint were masked to treatment and to the investigators’ assessment for each patient." |
| Blinding of participants and personnel for subjective outcomes | High risk | Open‐label |
| Blinding of outcome assessment for objective outcomes | Low risk | Quote: "Members of the Independent Review Facility (IRF) who assessed the primary endpoint were masked to treatment and to the investigators’ assessment for each patient." |
| Blinding of outcome assessors for subjective outcomes | High risk | Quote: "open‐label design" ; "Secondary endpoints were investigator‐assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals stated in text; see CONSORT diagram in figure. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | Balanced baseline characteristics |
PROFILE 1007 2013.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants | Crizotinib 250 mg orally, twice daily, continuously
Pemetrexed 500 mg/m² or docetaxel 75 mg/m², intravenously, every 3 weeks, continuously
Inclusion criteria Eligible patients had locally advanced or metastatic NSCLC that was positive for ALK rearrangements as determined by fluorescence in situ hybridisation (FISH), age of at least 18 years, progressive disease after 1 prior platinum‐based chemotherapy regimen, measurable disease as assessed with the use of Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1, and an ECOG PS of 0, 1, or 2. Patients with stable brain metastases that had been treated previously or were untreated and asymptomatic. Adequate organ function, written informed consent Exclusion criteria Prior ALK therapy; concurrent treatment within another clinical trial; spinal cord compression, carcinomatosis meningitis, or leptomeningeal disease; acute cardiac or cerebrovascular event in preceding 3 months, ongoing cardiac dysrhythmia, uncontrolled atrial fibrillation, QTc > 470 ms. Prior treatment with crizotinib; predominant squamous cell carcinoma if assigned to arm B pemetrexed (chemo arm); pre‐existing > Grade 2 peripheral neuropathy or hypersensitivity to polysorbate 80 if assigned to arm B docetaxel; pregnancy or breastfeeding; concurrent potent CYP3A4 inhibitor, inducers or substrates; prior malignancy within 3 years (other than non‐melanoma skin cancer, localised cervical cancer, or localised and presumed cured prostate cancer); known interstitial fibrosis or interstitial lung disease; other acute or chronic medical or psychiatric conditions, or laboratory abnormalities which would make patient inappropriate for entry in investigator's opinion Number eligible: 588 Number enrolled: 347 Number in control group: 174 Number in treatment group: 173 Number of withdrawal (treatment/control): 87/143 |
|
| Interventions |
Intervention characteristics Crizotinib 250 mg orally, twice daily, continuously
Pemetrexed 500 mg/m² or docetaxel 75 mg/m², intravenously, every 3 weeks, continously
|
|
| Outcomes |
Primary outcome Progression‐free survival (PFS) [ Time Frame: randomisation until progressive disease (PD) or initiation of antitumour therapy in the absence of PD or death, assessed every 6 weeks (up to 112 weeks) ] Secondary outcomes Overall survival (OS) [ Time Frame: randomisation until death (up to 4.5 years) ] Overall survival probability at Months 6 and 12 [ Time Frame: Month 6, 12 ] Percentage of participants with objective response (OR) [ Time Frame: randomisation until PD or initiation of antitumour therapy in the absence of PD or death, assessed every 6 weeks (up to 112 weeks) ] Percentage of participants with disease control at Week 6 [ Time Frame: Week 6 ] Percentage of participants with disease control at Week 12 [ Time Frame: Week 12 ] Duration of response (DR) [ Time Frame: randomisation until PD or initiation of antitumour therapy in the absence of PD or death, assessed every 6 weeks (up to 112 weeks) ] Time to tumour response (TTR) [ Time Frame: randomisation until PD or initiation of antitumour therapy in the absence of PD or death, assessed every 6 weeks (up to 112 weeks) ] Plasma concentration of crizotinib [ Time Frame: predose on Cycle 1 Day 1, Cycle 1 Day 15, and Day 1 of Cycles 2, 3, 5 ] Number of participants with categorical maximum QTcF for crizotinib [ Time Frame: predose on Day 1 of Cycle 1, 2 to 6 hours postdose on Day 1 of Cycle 1, 2 ] Plasma concentration of soluble c‐Met ectodomain and hepatocyte growth factor scatter proteins [ Time Frame: predose on Day 1 of Cycle 1, 2 to 6 hours postdose on Day 1 of Cycle 2, end of treatment (up to 112 weeks) ] Time to deterioration (TTD) in participant‐reported pain, dyspnoea, and cough [ Time Frame: Baseline up to end of treatment (up to 112 weeks) ] EORTC QLQ‐C30 [ Time Frame: baseline, Day 1 of each cycle until disease progression, end of treatment (up to 112 weeks) ] EORTC QLQ‐LC13 [ Time Frame: baseline, Day 1 of each cycle until disease progression, end of treatment (up to 112 weeks) ] EQ‐5D Visual Analogue Scale (VAS) [ Time Frame: baseline, Day 1 of each cycle until disease progression, end of treatment (up to 112 weeks) ] |
|
| Identification |
Trial name: PROFILE 1007 Sponsorship source: Pfizer Country: international (the UK, South Korea, Japan, Italy, France, Australia, China, Germany, Canada) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients will be randomized in a 1:1 ratio based on a randompermuted block design using a centralized Interactive Voice Response System(IVRS)/websit" |
| Allocation concealment (selection bias) | Low risk | Quote: "randompermuted block design using a centralized Interactive Voice Response System(IVRS)/websit" |
| Blinding of participants and personnel for objective outcomes | Low risk | Quote: "all scans were subject to central review by independent radiologists who were unaware of the group assignments." |
| Blinding of participants and personnel for subjective outcomes | High risk | Open‐label study |
| Blinding of outcome assessment for objective outcomes | Low risk | Quote: "all scans were subject to central review by independent radiologists who were unaware of the group assignments." |
| Blinding of outcome assessors for subjective outcomes | High risk | Open‐label study, outcomes influenced by risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Efficacy end points were analyzed mainly in the intention‐to‐treat population." |
| Selective reporting (reporting bias) | Low risk | All clinical outcomes reported as outlined on ClinicalTrials.gov. |
| Other bias | Low risk | Baseline characteristics well balanced. |
PROFILE 1014 2014.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Crizotinib 250 mg orally, twice daily, continuously
Cisplatin 75 mg/m²/pemetrexed 500 mg/m² or carboplatin area under the curve (AUC) 5 or 6/pemetrexed 500 mg/m², intravenously, every 3 weeks, continuously
Inclusion criteria Proven diagnosis of locally advanced not suitable for local treatment, recurrent, and metastatic non‐squamous cell carcinoma of the lung. Positive for translocation or inversion events involving the ALK gene locus. No prior systemic treatment for locally advanced or metastatic disease; patients with brain metastases only if treated and neurologically stable with no ongoing requirement for corticosteroids. Evidence of a personally signed and dated informed consent document and willingness and ability to comply with scheduled visits, treatment plans, laboratory tests, and other study procedures including completion of patient‐reported outcome measures. 18 years of age or older, with the exception of India, which has an upper age limit of 65 years old Exclusion criteria Current treatment on another therapeutic clinical trial. Prior therapy directly targeting ALK. Any of the following within the 3 months prior to starting study treatment: myocardial infarction, severe/unstable angina, coronary/peripheral artery bypass graft, congestive heart failure, or cerebrovascular accident including transient ischaemic attack; appropriate treatment with anticoagulants is permitted. Ongoing cardiac dysrhythmias of National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) Grade >=2, uncontrolled atrial fibrillation of any grade, or QTc interval > 470 ms. Pregnancy or breastfeeding. Use of drugs or foods that are known potent CYP3A4 inducers/inhibitors. Concurrent use of drugs that are CYP3A4 substrates with narrow therapeutic indices. Known HIV infection. Known interstitial lung disease or interstitial fibrosis. Other severe acute or chronic medical conditions (including severe gastrointestinal conditions such as diarrhoea or ulcer) or psychiatric conditions, or laboratory abnormalities that would impart, in the judgment of the investigator and/or sponsor, excess risk associated with study participation or study drug administration, and which would make the patient inappropriate for entry into this study Number enrolled: 343 Number in treatment group: 172 crizotinib Number in control group: 171 chemotherapy Number withdrawal from trial (treatment/control): 92/61 |
|
| Interventions |
Intervention characteristics Crizotinib 250 mg orally, twice daily, continuously
Cisplatin 75 mg/m²/pemetrexed 500 mg/m² or carboplatin AUC 5 or 6/pemetrexed 500 mg/m² intravenously, every 3 weeks
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Identification |
Trial name: PROFILE 1014 Sponsorship source: Pfizer Country: multinational (North/South America, Europe, Asia) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The centralised random permuted block design will be used to balance treatment assignments across the strata. |
| Allocation concealment (selection bias) | Low risk | Correspondence with authors confirmed that the method of randomisation included interactive voice response systems (IVRS). |
| Blinding of participants and personnel for objective outcomes | Low risk | Quote: "All scans were submitted for central independent radiologic review by radiologists who were unaware of the group assignments." |
| Blinding of participants and personnel for subjective outcomes | High risk | Open‐label study, outcomes such as patient‐reported outcome measures and quality of life influenced by risk of bias |
| Blinding of outcome assessment for objective outcomes | Low risk | Quote: "All scans were submitted for central independent radiologic review by radiologists who were unaware of the group assignments." |
| Blinding of outcome assessors for subjective outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for, intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes from registration reported. |
| Other bias | Low risk | Balanced baseline characteristics |
PROFILE 1029 2018.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Crizotinib 250 mg orally, twice daily, continously
Pemetrexed 500 mg/m² + cisplatin 75 mg/m² intravenously, every 3 weeks, maximum 6 cycles or pemetrexed 500 mg/m² + carboplatin AUC 5/6 intravenously, every 3 weeks, maximum 6 cycles
Inclusion criteria Histologically or cytologically proven diagnosis of locally advanced not suitable for local treatment, recurrent and metastatic non‐squamous cell carcinoma of the lung. Positive for translocation or inversion events involving the ALK gene locus. No prior systemic treatment for locally advanced or metastatic disease. Patients with brain metastases only if treated and neurologically stable for at least 2 weeks and not taking any medications contraindicated in the exclusion criteria. Evidence of a personally signed and dated informed consent document and willingness and ability to comply with scheduled visits, treatment plans, laboratory tests, and other study procedures including completion of patient‐reported outcome measures. Exclusion criteria Current treatment in another therapeutic clinical trial. Prior therapy directly targeting ALK. Any of the following within the 3 months prior to starting study treatment: myocardial infarction, severe/unstable angina, coronary/peripheral artery bypass graft, congestive heart failure, or cerebrovascular accident including transient ischaemic attack. Appropriate treatment with anticoagulants is permitted. Ongoing cardiac dysrhythmias of National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) Grade >= 2, uncontrolled atrial fibrillation of any grade, or QTc interval > 470 ms. Pregnancy or breastfeeding. Use of drugs or foods that are known potent CYP3A inducers/inhibitors. Concurrent use of drugs that are CYP3A substrates with narrow therapeutic indices. Known HIV infection. History of extensive disseminated/bilateral or known presence of Grade 3 or 4 interstitial fibrosis or interstitial lung disease, including a history of pneumonitis, hypersensitivity pneumonitis, interstitial pneumonia, interstitial lung disease, obliterative bronchiolitis, and pulmonary fibrosis, but not history of prior radiation pneumonitis. Other severe acute or chronic medical conditions (including severe gastrointestinal conditions such as diarrhoea or ulcer) or psychiatric conditions, or endstage renal disease on haemodialysis, or laboratory abnormalities that would impart, in the judgement of the investigator and/or sponsor, excess risk associated with study participation or study drug administration, and which would make the patient inappropriate for entry into this study Pretreatment: higher proportion of brain metastases in chemotherapy group Number completing trial (treatment/control: 43/76 Number enrolled: 207 Number in control group: 103 chemotherapy Number in treatment group: 104 crizotinib Number of withdrawal (treatment/control): 59/101 (discontinued) |
|
| Interventions |
Intervention characteristics Crizotinib 250 mg orally, twice daily, continously
Pemetrexed 500 mg/m² + cisplatin 75 mg/m² intravenously, every 3 weeks, maximum 6 cycles or pemetrexed 500 mg/m² + carboplatin AUC 5/6 intravenously, every 3 weeks, maximum 6 cycles
|
|
| Outcomes | Progression‐free survival (PFS) based on independent radiology review (IRR) by treatment arm [ Time Frame: randomisation to objective progression, death, or last tumour assessment without progression before any additional anticancer therapy (whichever occurred first, assessed up to 33 months) ] Objective response rate (ORR) ‐ percentage of participants with objective response based on IRR [ Time Frame: randomisation to objective progression, death, or last tumour assessment without progression before any additional anticancer therapy (assessed up to 33 months) ] Overall survival (OS) [ Time Frame: from randomisation to death or last date known alive for those not known to have died (whichever occurred first, assessed up to 33 months) ] Percentage of participants with disease control at 12 weeks based on IRR [ Time Frame: from randomisation to Week 12 ] Estimate of the percentage of participants surviving at 1 year and at 18 months [ Time Frame: from randomisation to 18 months ] Duration of response (DR) based on IRR [ Time Frame: from objective response to date of progression, death, or last tumour assessment without progression before any additional anticancer therapy (whichever occurred first, assessed up to 33 months) ] Time to tumour response (TTR) based on IRR [ Time Frame: randomisation to first documentation of objective tumour response (assessed up to 33 months) ] Intracranial time to progression (IC‐TTP) based on IRR [ Time Frame: randomisation to objective intracranial progression or last tumour assessment without progression before any additional anticancer therapy (whichever occurred first, assessed up to 33 months) ] Extracranial time to progression (EC‐TTP) based on IRR [ Time Frame: randomisation to objective extracranial progression or last tumour assessment without progression before any additional anticancer therapy (whichever occurred first, assessed up to 33 months) ] Time to deterioration (TTD) in participant‐reported pain, dyspnoea, or cough [ Time Frame: from baseline to deterioration whilst on study treatment. For participants with no deterioration, data were censored at the last date when QLQ‐LC13 assessment for pain, dyspnoea, or cough was completed (assessed up to 33 months) ] Change from baseline in functioning and global quality of life as assessed by the EORTC QLQ‐C30 [ Time Frame: Cycle 1 Day 1 to end of treatment or withdrawal, no later than 4 weeks (+/− 1 week) from last dose of study medication or when decision was made to withdraw from the study (whichever was sooner, assessed up to 33 months) ] Change from baseline scores in QLQ‐C30 symptoms as assessed by the EORTC QLQ‐C30 [ Time Frame: Cycle 1 Day 1 to end of treatment or withdrawal, no later than 4 weeks (+/− 1 week) from last dose of study medication or when decision was made to withdraw from the study (whichever was sooner, assessed up to 33 months) ] Change from baseline in lung cancer symptom scores as assessed by the EORTC QLQ‐ LC13 [ Time Frame: Cycle 1 Day 1 to end of treatment or withdrawal, no later than 4 weeks (+/− 1 week) from last dose of study medication or when decision was made to withdraw from the study (whichever was sooner, assessed up to 33 months) ] Change from baseline in general health status as assessed by EQ‐5D Visual Analogue Scale (VAS) [ Time Frame: Cycle 1 Day 1 to end of treatment or withdrawal, no later than 4 weeks (+/− 1 week) from last dose of study medication or when decision was made to withdraw from the study (whichever was sooner, assessed up to 33 months) ] Change from baseline in general health status as assessed by EQ‐5D‐ Index [ Time Frame: Cycle 1 Day 1 to end of treatment or withdrawal, no later than 4 weeks (+/− 1 week) from last dose of study medication or when decision was made to withdraw from the study (whichever was sooner, assessed up to 33 months) ] Percentage of participants with visual disturbance as assessed by Visual Symptom Assessment Questionnaire (VSAQ‐ALK) [ Time Frame: Cycle 1 Day 1 to end of treatment or withdrawal, no later than 4 weeks (+/− 1 week) from last dose of study medication or when decision was made to withdraw from the study (whichever was sooner, assessed up to 33 months) ] Agreement between central laboratory ALK fluorescence in situ hybridisation (FISH) and ALK immunohistochemistry (IHC) test results ‐ molecular profiling evaluable [ Time Frame: screening, less than or equal to 28 days prior to dosing ] Percentage of participants with treatment‐emergent adverse events (AEs; all causalities) [ Time Frame: from the first dose of study medication until 28 days after the last dose of study medication. However, all AEs entered in the database from the treatment start were included in AE analyses (assessed up to 33 months). ] Percentage of participants with treatment‐emergent AEs (treatment related) [ Time Frame: from the first dose of study medication until 28 days after the last dose of study medication. However, all AEs entered in the database from the treatment start were included in AE analyses (assessed up to 33 months). ] |
|
| Identification |
Trial name: PROFILE 1029 Sponsorship source: Pfizer Country: multinational (Asia) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel for objective outcomes | Low risk | Quote: "RECIST‐ defined progression, as assessed by IRR." |
| Blinding of participants and personnel for subjective outcomes | High risk | Open‐label |
| Blinding of outcome assessment for objective outcomes | Low risk | Quote: "RECIST‐ defined progression, as assessed by IRR." |
| Blinding of outcome assessors for subjective outcomes | High risk | Open‐label study, outcomes such as patient‐reported outcome measures and quality of life influenced by risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Intention‐to‐Treat Population" ; participants accounted for as per CONSORT diagram |
| Selective reporting (reporting bias) | Low risk | All outcomes from registration reported. |
| Other bias | Low risk | Baseline characteristics balanced except for brain metastases (20.2% in ceritinib group vs 31.1% in chemotherapy group). |
CNS: central nervous system ECOG PS: Eastern Cooperative Oncology Group Performance Status EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core EORTC QLQ‐LC13: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Lung Cancer Module NSCLC: non‐small cell lung cancer RECIST: Response Evaluation Criteria in Solid Tumours TKI: tyrosine kinase inhibitor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blackhall 2017 | Wrong study design |
| Cho 2017 | Wrong comparator |
| Chow 2019 | Wrong study design |
| EUCTR2012‐003474‐36‐BE | Wrong study design |
| Felip 2016 | Wrong study design |
| Gao 2016 | Wrong study design |
| JPRN‐JapicCTI‐184073 | Wrong patient population |
| Kim 2016 | Wrong study design |
| Lenderking 2017 | Wrong study design |
| Liang 2019 | Wrong study design |
| NCT02134912 | Failed to recruit |
| Park 2020 | Wrong intervention |
| Reckamp 2019 | Wrong study design |
| Wolf 2015 | Wrong study design |
| Zhao 2015 | Wrong study design |
Characteristics of ongoing studies [ordered by study ID]
eXalt3 2020.
| Study name | Study comparing X‐396 (ensartinib) to crizotinib in ALK positive non‐small cell lung cancer (NSCLC) patients |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
| Participants |
Baseline characteristics Oral X‐396 (ensartinib) at 225 mg orally, daily, continously
Oral crizotinib at 250 mg orally, twice daily, continously
Inclusion criteria
Adequate organ system function, defined as follows:
Note that the following pertains to patients enrolled in France.
Exclusion criteria
Patients receiving the following:
Clinically significant cardiovascular disease including:
Inability or unwillingness to comply with study and/or follow‐up procedures outlined in the protocol. Note that the following pertains to patients enrolled in France.
|
| Interventions | Ensartinib 22 mg orally, daily, continously
Crizotinib 250 mg orally, twice daily, continously
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes
|
| Starting date | May 2016 |
| Contact information | |
| Notes | clinicaltrials.gov/ct2/show/NCT02767804 |
NCT03737994.
| Study name | Targeted treatment for ALK positive patients who have previously been treated for non‐squamous non‐small cell lung cancer |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
| Participants |
Baseline characteristics ALK L1198F mutation (alone or in combination with another ALK mutation) receive crizotinib orally, daily, continously. Cycles repeat every 21 days.
Cy1156Y mutation receive either lorlatinib orally, daily, alectinib orally, twice daily, or brigatinib orally, daily, continuously. Cycles repeat every 21 days.
Compound mutation receive lorlatinib orally, daily, continuously. Cycles repeat every 21 days.
F1174 receive either lorlatinib orally, daily, alectinib orally, twice daily, or brigatinib orally, daily, continuously. Cycles repeat every 21 days.
G1202 (including G1202del and G1202R) receive either lorlatinib orally, daily or brigatinib orally, daily, continuously. Cycles repeat every 21 days.
I1171 mutation receive either lorlatinib orally, daily, ceritinib orally, daily, or brigatinib orally, daily. Cycles repeat every 21 days.
L1196 (including L1196M) mutation receive either lorlatinib orally, daily, ceritinib orally, daily, alectinib orally, twice daily, brigatinib orally, daily, or ensartinib orally, daily, continuously. Cycles repeat every 21 days.
MET amplification receive crizotinib orally, daily, continuously. Cycles repeat every 21 days.
No ALK‐resistant mutations receive lorlatinib orally, daily, ceritinib orally, daily, alectinib orally, twice daily, brigatinib orally, daily, ensartinib orally, daily, or pemetrexed +/− carboplatin. ALK inhibitor cycles repeat every 21 days. Chemotherapy cycles repeat every 21 days, up to 6 cycles.
Overall
Inclusion criteria PRIOR TO STEP 1 REGISTRATION. Patients must have histologically or cytologically confirmed stage IV ALK‐positive non‐squamous non‐small cell lung carcinoma (NSCLC) (includes M1a, M1b, M1c stage disease, American Joint Committee on Cancer (AJCC) 8th edition). ALK rearrangement must have been demonstrated by a US Food and Drug Administration (FDA)‐approved assay (Vysis fluorescence in situ hybridisation (FISH) or Ventana immunohistochemistry (IHC)) or by next‐generation sequencing (NGS). Patient must be willing and able to undergo a fresh biopsy, or if patient has a biopsy after progression on current TKI within 3 months of study enrolment (and has continued TKI for clinical benefit per treating physician), this tissue may be used. Must have sufficient tissue. Patient must have progressive disease as defined by Response Evaluation Criteria in Solid Tumours (RECIST) 1.1 after 1 second‐generation ALK inhibitor, including LDK378 (ceritinib), alectinib, ensartinib, and brigatinib (may not have received more than 1 second‐generation ALK inhibitor). Patient may have received prior crizotinib; however, the second‐generation ALK inhibitor received must be the last treatment given prior to study enrolment. Prior lorlatinib (third‐generation ALK inhibitor) is not allowed. Prior chemotherapy is not allowed except for 1 prior cycle received at the time of original diagnosis of metastatic NSCLC with no evidence of disease progression following the cycle. NOTE: prior adjuvant or neoadjuvant chemotherapy is allowed if last dose was received more than 12 months prior to enrolment. The patient or a legally authorised representative must provide study‐specific informed consent prior to step 1 registration. PRIOR TO STEP 2 REGISTRATION. Absolute neutrophil count (ANC) >= 1500 cells/mm^3 (within 28 days prior to step 2 registration). Platelets >= 100,000 cells/mm^3 (within 28 days prior to step 2 registration). Estimated creatinine clearance >= 60 mL/min by the Cockcroft Gault formula (within 28 days prior to step 2 registration). Total bilirubin =< 1.5 x upper limit of normal (ULN) (except for patients with documented Gilbert's syndrome) (within 28 days prior to step 2 registration). Aspartate aminotransferase (AST) =< 2.5 x ULN, =< 5 x ULN if liver metastases are present (within 28 days prior to step 2 registration). Alanine aminotransferase (ALT) =< 2.5 x ULN, =< 5 x ULN if liver metastases are present (within 28 days prior to step 2 registration). Patients with asymptomatic treated or untreated brain metastases are eligible. Treated brain metastases are eligible as long as patients have measurable disease outside the brain according to RECIST 1.1. Patients must be on a stable or decreasing dose of steroids for at least 7 days prior to step 2 registration. Anticonvulsants are allowed as long as the patient is neurologically stable and not deteriorating. Patients enrolled with asymptomatic brain metastases (mets) must have at least 1 measurable target extracranial lesion according to RECIST 1.1. ECOG PS 0 to 2/acute effects of any prior therapy resolved to baseline severity or to Common Terminology Criteria for Adverse Events (CTCAE) grade =< 1 (except for alopecia, hearing loss). Not taking any medications that may interact with selected study medication based on stratification. Patients must be able to take oral medications (i.e. swallow whole tablets/capsules). All females of childbearing potential must have a blood test or urine study within 14 days prior to step 2 registration to rule out pregnancy. A female of childbearing potential is any woman, regardless of sexual orientation or whether they have undergone tubal ligation, who meets the following criteria: has not undergone a hysterectomy or bilateral oophorectomy; or has not been naturally postmenopausal for at least 24 consecutive months (i.e. has had menses at any time in the preceding 24 consecutive months). Women must not be pregnant or breastfeeding due to potential harm to the foetus or infant from ALK inhibitors and the unknown risk. Women of childbearing potential and sexually active males must agree to use an accepted and effective method of contraception or to abstain from sexual intercourse for the duration of their participation in the study. Exclusion criteria PRIOR TO STEP 2 REGISTRATION: Major surgery within 2 weeks of study entry. Minor surgical procedures (e.g. port insertion, pleurex catheter placement) are allowed, and all wounds must not show signs of infection or draining. PRIOR TO STEP 2 REGISTRATION: Palliative radiation therapy (RT) (< 10 fractions) must have been completed at least 48 hours prior to study entry. Stereotactic or small field brain irradiation must have been completed at least 1 week prior to study entry. Whole brain RT or other palliative RT must have been completed at least 2 weeks prior to study entry. PRIOR TO STEP 2 REGISTRATION: Prior dose of next‐generation ALK inhibitor (LDK378 (ceritinib), alectinib, ensartinib, lorlatinib) within 5 days prior to step 2 registration. Prior dose of brigatinib within 7 days prior to step 2 registration. PRIOR TO STEP 2 REGISTRATION: History of interstitial lung disease or interstitial fibrosis, including a history of pneumonitis, obliterative bronchiolitis, pulmonary fibrosis. Patients with a history of prior radiation pneumonitis are not excluded. PRIOR TO STEP 2 REGISTRATION: Active inflammatory gastrointestinal disease (such as Crohn's, ulcerative colitis), chronic diarrhoea, symptomatic diverticular disease, or any gastrointestinal disease that would affect the absorption of oral medications or increase the risk of toxicity. PRIOR TO STEP 2 REGISTRATION: Clinically significant cardiovascular abnormalities, as determined by the treating/registering physician, such as uncontrolled hypertension, congestive heart failure New York Heart Association classification of 3, unstable angina, or poorly controlled arrhythmia, or myocardial infarction within 6 months. PRIOR TO STEP 2 REGISTRATION: Active and clinically significant bacterial, fungal, or viral infection. PRIOR TO STEP 2 REGISTRATION: Patients with active or chronic pancreatitis based on lipase elevation, symptoms, and radiographic findings. PRIOR TO STEP 2 REGISTRATION: Other concomitant serious illness or organ system dysfunction that in the opinion of the investigator would either compromise patient safety or interfere with the evaluation of the safety of the study drug. PRIOR TO STEP 2 REGISTRATION: Patients must not plan to receive any other investigational agents during the course of therapy. PRIOR TO STEP 2 REGISTRATION: Patients with active malignancy other than ALK‐positive non‐squamous NSCLC within the last 2 years are excluded (note: adequately treated basal cell or squamous cell skin cancer, in situ cervical cancer, papillary thyroid cancer treated with curative intent, adequately treated stage I or II cancer from which the patient is currently in complete remission, or any other cancer from which the patient has been disease‐free for 2 years are eligible). PRIOR TO STEP 2 REGISTRATION: No chemotherapy and/or immunotherapy allowed after step 1 registration. |
| Interventions |
Intervention characteristics ALK L1198F mutation (alone or in combination with another ALK mutation) receive crizotinib orally, daily, continously. Cycles repeat every 21 days.
Cy1156Y mutation receive either lorlatinib orally, daily, alectinib orally, twice daily, or brigatinib orally, daily, continously. Cycles repeat every 21 days.
Compound mutation receive lorlatinib orally, daily, continuously. Cycles repeat every 21 days.
F1174 receive either lorlatinib orally, daily, alectinib orally, twice daily, or brigatinib orally, daily, continuously. Cycles repeat every 21 days.
G1202 (including G1202del and G1202R) receive either lorlatinib orally, daily or brigatinib orally, daily, continuously. Cycles repeat every 21 days.
I1171 mutation receive either lorlatinib orally, daily, ceritinib orally, daily, or brigatinib orally, daily, continuously. Cycles repeat every 21 days.
L1196 (including L1196M) mutation receive either lorlatinib orally, daily, ceritinib orally, daily, alectinib orally, twice daily, brigatinib orally, daily, or ensartinib orally, daily, continuously. Cycles repeat every 21 days.
MET amplification receive crizotinib orally, daily, continously. Cycles repeat every 21 days.
No ALK‐resistant mutations receive lorlatinib orally, daily, ceritinib orally, daily, alectinib orally, twice daily, brigatinib orally, daily, ensartinib orally, daily, continuously, or pemetrexed +/− carboplatin, intravenously, every 3 weeks. ALK inhibitor cycles repeat every 21 days. Chemotherapy cycles repeat every 21 days, up to 6 cycles.
|
| Outcomes | Objective response rate (ORR) [ Time Frame: up to 24 weeks ] Progression‐free survival (PFS) [ Time Frame: from second step registration to the date of the first recorded occurrence of disease progression or death from any cause (whichever occurs first), assessed up to 7 years ] Duration of response [ Time Frame: from the first occurrence of a documented best overall response (BOR) of complete response (CR) or partial response (PR) to the first date of recorded disease progression or death from any cause (whichever occurs first), assessed up to 7 years ] Overall survival (OS) [ Time Frame: from second step registration to the date of death from any cause, assessed up to 7 years ] Intracranial objective response rate [ Time Frame: up to 7 years] Incidence of adverse events [ Time Frame: up to 30 days post‐treatment ] Biopsy mutation and circulating free DNA (cfDNA) mutation results [ Time Frame: up to 30 days post‐treatment ] |
| Starting date | April 2019 |
| Contact information | |
| Notes | clinicaltrials.gov/ct2/show/NCT03737994 |
NCT04009317.
| Study name | Study of TQ‐B3139 versus crizotinib in the first line treatment of subjects with anaplastic lymphoma kinase (ALK) positive non‐small cell lung cancer (NSCLC) |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
| Participants |
Baseline characteristics TQ‐B3139 tablet 600 mg administered orally, twice daily in 28‐day cycle.
Crizotinib tablet 250 mg administered orally, twice daily in 28‐day cycle.
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics TQ‐B3139 tablet 600 mg administered orally, twice daily, continuously in 28‐day cycle.
Crizotinib tablet 250 mg administered orally, twice daily, continuously in 28‐day cycle.
|
| Outcomes | Progression‐free survival (PFS) [ Time Frame: up to 36 months ] Objective response rate (ORR) [ Time Frame: up to 36 months ] Disease control rate (DCR) [ Time Frame: up to 36 months ] Overall survival (OS) [ Time Frame: up to 36 months ] |
| Starting date | August 2019 |
| Contact information | Li Zhang, Doctor; zhangli@sysucc.org.cn |
| Notes | clinicaltrials.gov/ct2/show/NCT04009317 |
NCT04318938.
| Study name | Advancing brigatinib properties in ALK+ NSCLC patients by deep phenotyping |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
| Participants |
Baseline characteristics 1L: Any approved second‐generation ALK TKI (investigator's choice) 2L: Any ALK TKI including brigatinib (investigator's choice)
1L: Brigatinib 90 mg orally, daily for 7/7 then 180 mg orally, daily, continuously 2L: Any ALK TKI (investigator's choice)
Inclusion criteria Fully informed written consent and any locally required authorisation (EU Data Privacy Directive) given by the patient. Male or female ≥ 18 years of age. NOTE: There are no data that indicate special gender distribution, therefore patients will be enrolled in the study gender‐independently. Histologically confirmed locally advanced (stage III) and not suitable for curative treatment (i.e. R0 operation or definitive chemo‐/radiation, or metastatic (stage IV) ALK+ NSCLC). NOTE: Documentation of ALK rearrangement by a positive result of any ALK assay approved in Germany (i.e. positivity for at least 1 of the 3: immunohistochemistry (IHC), next‐generation sequencing, fluorescence in situ hybridisation (FISH)) must be available at baseline. Treatment can already be started based on a local ALK+ test result, but subsequent central testing of the baseline biopsy for molecular profiling, including determination of ALK variant and TP53 status, should be made possible for all patients. No prior therapy for metastatic ALK+ NSCLC including therapy with ALK inhibitors. However, 1 or 2 cycles of chemotherapy as well as cerebral irradiation before inclusion in the study will be allowed. At least 1 measurable (i.e. target) lesion per RECIST v1.1 ECOG PS ≤ 2. Have adequate organ function, as determined by the following:
Willingness and ability to comply with scheduled visit and study procedures. Patient willing to participate in accompanying research programme. Collection of current biopsy during screening must be feasible. NOTE: For each patient a formalin‐fixed, paraffin‐embedded (FFPE) tumour tissue block must be available for biomarker evaluation. Excisional, incisional, or core needle biopsies are appropriate, whilst fine‐needle aspirations are insufficient. Women of childbearing potential must have a negative pregnancy test within 7 days prior to randomisation. Women must not be breastfeeding. Female patients who: are postmenopausal for at least 1 year before the screening visit, OR are surgically sterile, OR if they are of childbearing potential, agree to practice highly effective non‐hormonal contraception from the time of signing the informed consent through at least 4 months after the last dose of study drug, or agree to completely abstain from heterosexual intercourse. Male patients, even if surgically sterilised (i.e. status postvasectomy), who: agree to practice effective barrier contraception during the entire study treatment period and through at least 4 months after the last dose of study drug, OR agree to completely abstain from heterosexual intercourse Exclusion criteria History or presence at baseline of pulmonary interstitial disease, drug‐related pneumonitis, or radiation pneumonitis. Uncontrolled hypertension, defined as hypertension treated* with antihypertensive drugs AND blood pressure ≥ 160 mmHg (systolic) or ≥ 100 mmHg (diastolic) in repeated measurements. Untreated elevated blood pressure is not an exclusion criterion and should receive adequate antihypertensive adjustment. *NOTE: In case of treatment, at least 3 antihypertensive drugs should have been used with the intention to control hypertensive disease. Systemic treatment with strong cytochrome P‐450 (CYP) 3A inhibitors, strong CYP3A inducers, or moderate CYP3A inducers or treatment with any investigational systemic anticancer agents, chemotherapy, or radiation therapy (except for stereotactic radiosurgery or stereotactic body radiation therapy) within 14 days of randomisation. Treatment with antineoplastic monoclonal antibodies within 30 days of randomisation. Major surgery within 30 days of randomisation. Minor surgical procedures, such as catheter placement or minimally invasive biopsies, are allowed. Current spinal cord compression (symptomatic or asymptomatic) as detected by radiographic imaging. Patients with leptomeningeal disease without cord compression are allowed. Significant or uncontrolled cardiovascular disease, defined as to the following: if an acute myocardial infarction has ensued in the past 6 months, successful reperfusion has to be documented, and the patient has to be free of symptoms. New York Heart Association class III or IV heart failure (i.e. marked limitation in activity due to symptoms, even during less‐than‐ordinary activity, e.g. walking short distances (20 to 100 m); comfortable only at rest) within 6 months prior to randomisation. Any history of clinically significant ventricular arrhythmia, defined as ventricular tachycardia (VT), ventricular fibrillation (VF), or cardiac arrest. Cerebrovascular accident or transient ischaemic attack within 6 months prior to first dose of study drug. Malabsorption syndrome or other gastrointestinal illness or condition that could affect oral absorption of the study drug. Active severe or uncontrolled chronic infection, including, but not limited to, the requirement for intravenous antibiotics for longer than 2 weeks. History of HIV infection. Testing is not required in the absence of history. Chronic hepatitis B (surface antigen‐positive) or chronic active hepatitis C infection. Testing is not required in the absence of history. Any serious medical condition or psychiatric illness that could, in the investigator's opinion, potentially compromise patient safety or interfere with the completion of treatment according to this protocol. Known or suspected hypersensitivity to brigatinib or other TKI or their excipients. Life‐threatening illness unrelated to cancer. Involvement in the planning and/or conduct of the study (applies to both Takeda staff and/or staff of sponsor and study site). Patient who might be dependent on the sponsor, site, or the investigator. Patient who has been incarcerated or involuntarily institutionalised by court order or by the authorities (according to national Medicinal Products Act (Arzneimittelgesetz, AMG)). Patients who are unable to consent because they do not understand the nature, significance, and implications of the clinical trial and therefore cannot form a rational intention in the light of the facts (according to national AMG). Legal incapacity or limited legal capacity. Females who are pregnant or breastfeeding. Patients who have symptomatic CNS metastases (parenchymal or leptomeningeal) at screening or asymptomatic disease requiring an increasing dose of corticosteroids to control symptoms within 7 days prior to randomisation. NOTE: If a patient has worsening neurological symptoms or signs due to CNS metastasis, the patient needs to complete local therapy and be neurologically stable (with no requirement for an increasing dose of corticosteroids or use of anticonvulsants) for 7 days prior to randomisation. Rare hereditary galactose intolerance, total lactase deficiency, or glucose‐galactose malabsorption |
| Interventions |
Intervention characteristics 1L: Any approved second‐generation ALK TKI (investigator's choice) 2L: Any ALK TKI including brigatinib (investigator's choice)
1L: Brigatinib 90 mg orally, daily for 7/7 then 180 mg orally, daily, continuously 2L: Any ALK TKI (investigator's choice)
|
| Outcomes | Progression‐free survival (PFS) of first‐line treatment according to RECIST v1.1 [ Time Frame: 68 months ] PFS of second‐line treatment according to RECIST v1.1 [ Time Frame: 68 months TNT first line (TNT1, i.e. time‐to‐next treatment for the first line) [ Time Frame: 68 months ] TNT second line (TNT2, i.e. time‐to‐next treatment for the second line [ Time Frame: 68 months ] TNT1/2 (time‐to‐next treatment for the first and second line together) [ Time Frame: 68 months ] Overall survival (OS) [ Time Frame: 68 months] Intracranial overall response rate (iORR) [ Time Frame: 68 months ] Intracranial duration of response (iDOR) according to RECIST v1.1 [ Time Frame: 68 months ] Time to intracranial progression (TTiP) [ Time Frame: 68 months ] Safety (rate of adverse events) [ Time Frame: 68 months ] Quality of life assessed by 12‐item Short Form Health Survey (SF‐12) [ Time Frame: 68 months ] Quality of life assessed by EORTC QLQ‐BN20 [ Time Frame: 68 months ] |
| Starting date | March 2020 |
| Contact information | Michael Thomas, Prof: Michael.Thomas@med.uni‐heidelberg.de Regina Eickhoff, Dr; eickhoff.regina@ikf‐khnw.de |
| Notes | clinicaltrials.gov/ct2/show/NCT04318938 |
NCT04632758.
| Study name | Study comparing WX‐0593 to crizotinib in ALK positive non‐small cell lung cancer (NSCLC) patients |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
| Participants |
Included criteria: ≥ 18 years female or male patient has an ECOG PS ≤ 2. Life expectancy of at least 12 weeks. At least 1 measurable lesion (according to RECIST v1.1). Histologically or cytologically confirmed diagnosis of advanced or recurrent or metastatic NSCLC that is ALK‐positive by an Abbott FISH assay in the central lab. Randomisation will occur after ALK‐positive confirmation is received from the central lab or local test using a method including Abbott FISH, reverse transcription‐polymerase chain reaction (RT‐PCR), or Ventana IHC. No brain metastasis, or asymptomatic brain metastasis, or symptomatic brain metastasis but stable for more than 4 weeks after treatment, and has stopped systemic hormone treatment (prednisone of > 10 mg/day or equivalent hormone) for more than 2 weeks. Patients must have normal function defined as follows:
Any surgery or prior radiation (expect for palliative radiation)/operations must have been completed at least 4 weeks prior to first dosing. Palliative radiation must have been completed at least 48 hours prior to first dosing. Patients must be able to understand and volunteer to sign the informed consent. Exclusion criteria Patients that have previously received cancer therapy (i.e. other targeted therapies, chemotherapy, immunotherapy, biologic therapy, hormonal therapy). Patients with tumour meningeal metastasis. Clinically significant cardiovascular disease within 6 months prior to first dosing. 2 consecutive corrected QT interval (QTc) > 480 ms through electrocardiogram (ECG) examination during screening, ≥2 arrhythmias, ≥ 2 heart failure (according to Common Terminology Criteria for Adverse Events (CTCAE) 4.03), atrial fibrillation and ventricular fibrillation of any grade, or clinically significant supraventricular or ventricular arrhythmia requiring treatment or intervention. Patients needing medications that may prolong QT interval or induce torsades de pointes within 14 days prior to the first dosing or during the study. Continuous use of corticosteroids for more than 30 days, or require chronic use of corticosteroids or other immunosuppressants. Past history of a large area of diffuse/interstitial pulmonary fibrosis, or known history of Grade 3 or 4 interstitial pulmonary fibrosis or interstitial lung disease. Patients with Grade > 1 nausea, vomiting, or diarrhoea (CTCAE 4.03), other gastrointestinal (GI) dysfunction or GI disease that may potentially affect drug absorption. Patients at risk for GI perforation or intestinal obstruction. Patient has received other investigational drug within 1 month prior to first dosing. Patient received other clinical trial treatment within 1 month prior to the first dose of the investigational drug. Patients who are hepatitis B surface antigen (HBsAg)‐positive and/or hepatitis B core antibody (HBcAB)‐positive and hepatitis B virus (HBV) DNA > 10^3 copies/mL, or hepatitis C virus (HCV) antibody‐positive, or syphilis antibody‐positive or known HIV infected. Patients who cannot suspend the use of a strong CYP3A4 inducer or inhibitor at least 1 week prior to this study and during the study. Patients who cannot suspend the use of a CYP3A4 substrate at least 1 week prior to this study and during the study, and the therapeutic index is low. Females who are pregnant or breastfeeding. Pregnant or lactating female patients or a positive pregnancy test at baseline for females of childbearing potential. Female patients who are unwilling to use effective contraceptive measures during the entire course of the study and within 6 months after the end of the study, or male patients who plan to have children. Concurrent diseases that may seriously affect patient safety or impact patient completion of the study as determined by the investigator (such as clinically uncontrolled hypertension (blood pressure > 160/110 mmHg), severe diabetes, or thyroid disease). Drug abusers and alcoholics. Drug or alcohol abuse. Alcohol abuse refers to drinking 14 units of alcohol per week: 1 unit = 285 mL of beer, or 25 mL of spirits, or 100 mL of wine; history of definitive neurological or mental disorder, including epilepsy or dementia. Patients with other malignant tumours within 5 years prior to screening (except for cured basal cell carcinoma of the skin, cervical carcinoma in situ, thyroid carcinoma in situ, and papillary thyroid carcinoma). Patients with added risks associated with the study or may interfere with the interpretation of study results as determined by the investigator, or deemed unsuitable by the investigator and/or sponsor |
| Interventions | WX‐0593 60 mg orally, daily for 7 days, then 180 mg orally, daily 28‐day cycle continuously Type: TKI Crizotinib 250 mg orally, twice daily, continuously Type: TKI |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | June 2019 |
| Contact information | Shunjiang Yu, CMO: shunjiang.yu@qilu‐pharma.com Yuankai Shi, MD; syuankaipumc@126.com |
| Notes | Qilu Pharmaceutical Co., Ltd clinicaltrials.gov/ct2/show/NCT04632758 |
Popat 2019.
| Study name | Brigatinib versus crizotinib in ALK‐positive non‐small‐cell lung cancer |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
| Participants |
Baseline characteristics Brigatinib 7‐day lead in 90 mg orally, daily, then 180 mg orally, daily, continuously
Alectinib 600 mg orally, twice daily, continuously
Inclusion criteria Have ECOG PS of 0 to 2. Have histologically or cytologically confirmed stage IIIB (locally advanced or recurrent) or stage IV NSCLC. Must meet 1 of the following criteria: have documentation of ALK rearrangement by a positive result from the Vysis ALK Break‐Apart fluorescence in situ hybridisation (FISH) Probe Kit or the Ventana ALK (D5F3) CDx Assay or Foundation Medicine's FoundationOne CDx. Have documented ALK rearrangement by a different test and be able to provide tumour sample to the central laboratory. (NOTE: central laboratory ALK rearrangement testing results are not required to be obtained before randomisation). Had progressive disease whilst on crizotinib, as assessed by the investigator or treating physician. (NOTE: crizotinib does not need to be the last therapy a participant received. The participant may have received chemotherapy as his/her last therapy). Treatment with crizotinib for at least 4 weeks before progression. Have had no other ALK inhibitor other than crizotinib. Have had no more than 2 prior regimens of systemic anticancer therapy (other than crizotinib) in the locally advanced or metastatic setting. NOTE: a systemic anticancer therapy regimen will be counted if it is administered for at least 1 complete cycle. A new anticancer agent used as maintenance therapy will be counted as a new regimen. Neoadjuvant or adjuvant systemic anticancer therapy will be counted as a prior regimen if disease progression/recurrence occurred within 12 months upon completion of this neoadjuvant or adjuvant therapy. (Systemic therapy followed by maintenance therapy will be considered as 1 regimen if the maintenance therapy consists of a drug or drugs that were used in the regimen that immediately preceded maintenance.) Have at least 1 measurable (i.e. target) lesion per RECIST v1.1. Have recovered from toxicities related to prior anticancer therapy to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v4.03 Grade <= 1. (NOTE: treatment‐related alopecia or peripheral neuropathy that are Grade > 1 are allowed, if deemed irreversible). Have adequate organ function, as determined by the following:
Suitable venous access for study‐required blood sampling (i.e. including pharmacokinetic and laboratory safety tests) Exclusion criteria Had participated in the control (crizotinib) arm of Study AP26113‐13‐301 (ALTA 1L). Had received crizotinib within 7 days of randomisation. Have a history or presence at baseline of pulmonary interstitial disease, drug‐related pneumonitis, or radiation pneumonitis. Have uncontrolled hypertension. Patients with hypertension should be under treatment for control of blood pressure upon study entry. Had received systemic treatment with strong cytochrome P‐450 (CYP) 3A inhibitors, moderate CYP3A inhibitors, strong CYP3A inducers, or moderate CYP3A inducers within 14 days before randomisation. Treatment with any investigational systemic anticancer agents within 14 days or 5 half‐lives, whichever is longer, before randomisation. Had received chemotherapy or radiation therapy within 14 days of randomisation except for stereotactic radiosurgery (SRS) or stereotactic body radiation therapy. Had received antineoplastic monoclonal antibodies within 30 days of randomisation. Had major surgery within 30 days of randomisation. Minor surgical procedures, such as catheter placement or minimally invasive biopsies, are allowed. Have symptomatic CNS metastases (parenchymal or leptomeningeal) at screening (patients with asymptomatic brain metastases or those who have stable symptoms and did not require an increased dose of corticosteroids to control symptoms within 7 days before randomisation will be enrolled). NOTE: If a patient has worsening neurological symptoms or signs due to CNS metastasis, the patient needs to complete local therapy and be neurologically stable (with no requirement for an increasing dose of corticosteroids or use of anticonvulsants) for 7 days before randomisation. Have current spinal cord compression (symptomatic or asymptomatic and detected by radiographic imaging). Patients with leptomeningeal disease and without cord compression are allowed. Have significant, uncontrolled, or active cardiovascular disease, specifically including, but not restricted to, the following: myocardial infarction within 6 months before randomisation. Unstable angina within 6 months before randomisation. New York Heart Association class III or IV heart failure within 6 months before randomisation. History of clinically significant atrial arrhythmia (including clinically significant bradyarrhythmia), as determined by the treating physician. Any history of clinically significant ventricular arrhythmia. Had cerebrovascular accident or transient ischaemic attack within 6 months before first dose of study drug. Have malabsorption syndrome or other gastrointestinal illness or condition that could affect oral absorption of the study drug. Have an ongoing or active infection, including, but not limited to, the requirement for intravenous antibiotics. Have a known history of HIV infection. Testing is not required in the absence of history. Known hepatitis B surface antigen‐positive, or known or suspected active hepatitis C infection. Have a known or suspected hypersensitivity to brigatinib or alectinib or their excipients. Life‐threatening illness unrelated to cancer |
| Interventions |
Intervention Characteristics Brigatinib 7‐day lead in 90 mg orally, daily, then 180 mg orally, daily, continuously
Alectinib 600 mg orally, twice daily, continuously
|
| Outcomes | Progression‐free survival (PFS) as assessed by blinded independent review committee (BIRC) per RECIST v1.1 [ Time Frame: up to 5 years ] Time to intracranial progression (iPD) as assessed by BIRC per modified RECIST v1.1 [ Time Frame: up to 5 years ] Overall survival (OS) [ Time Frame: up to 5 years ] Objective response rate (ORR) as assessed by investigator and BIRC per RECIST v1.1 [ Time Frame: up to 5 years ] Time to response as assessed by investigator and BIRC [ Time Frame: up to 5 years ] Duration of response (DOR) as assessed by BIRC [ Time Frame: up to 5 years ] Intracranial objective response rate (iORR) as assessed by BIRC per modified RECIST v1.1 [ Time Frame: up to 5 years ] Intracranial duration of response (iDOR) as assessed by the BIRC per modified RECIST v1.1 [ Time Frame: up to 5 years] Health‐related quality of life (HRQoL) from EORTC QLQ‐C30 v3.0 score [ Time Frame: first dose of study drug up to 30 days after last dose (approximately 9 years) ] |
| Starting date | May 2016 |
| Contact information | |
| Notes | pubmed.ncbi.nlm.nih.gov/30280657/ |
CNS: central nervous system ECOG PS: Eastern Cooperative Oncology Group Performance Status EORTC QLQ‐BN20: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Brain Cancer Module EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core EORTC QLQ‐LC13: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Lung Cancer Module NSCLC: non‐small cell lung cancer RECIST: Response Evaluation Criteria in Solid Tumours TKI: tyrosine kinase inhibitor
Differences between protocol and review
We simplified comparisons based on the studies found in the search strategy.
We presented lines of treatment as subgroup analysis; however, this was not possible for the second comparison.
We found no data to perform subgroup analysis on the type of previous treatment.
We considered baseline central nervous system involvement to be the most relevant site of disease to investigate with subgroup analysis.
We undertook a sensitivity analysis using a random‐effects model for the primary outcomes only.
Contributions of authors
LC, NH, BS, RM, and VJ designed the review strategy.
LC, NH, EC, and TM screened titles and abstracts for potential relevance using the Covidence tool, and assessed the full‐text reports according to the defined criteria for inclusion in the review.
Disagreements resolved by VJ.
NH and EC extracted data.
EC, NH, and TM assessed risk of bias.
All authors have been involved in the analysis, interpretation of data, and writing of the review.
All authors have reviewed and approved the final draft of this review.
Sources of support
Internal sources
-
New Source of support, Other
No sources of support provided
External sources
-
New Source of support, Other
No sources of support provided
Declarations of interest
Laird B Cameron: none known. LC is a member of the Thoracic Oncology Group Australasia (formerly Australasian Lung Cancer Trials Group) and chairperson of the New Zealand Lung Oncology Special Interest Group.
Nadia Hitchen: none known.
Elias Chandran: none known.
Tessa Morris: none known
Renée Manser: none known.
Benjamin J Solomon is an author on phase III clinical studies of crizotinib, ceritinib, and lorlatinib. He has served on advisory boards for Pfizer, Novartis, Roche‐Genentech, AstraZeneca, Merck, Bristol Myers Squibb, Gritstone Oncology, and Loxo Oncology, and he has spoken at meetings where costs were sponsored by industry.
Vanessa Jordan: none known.
Edited (no change to conclusions)
References
References to studies included in this review
ALESIA 2019 {published data only}
- NCT02838420.A study to evaluate and compare the efficacy and safety of alectinib versus crizotinib and to evaluate the pharmacokinetics of alectinib in Asian participants with treatment-naive anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC). clinicaltrials.gov/ct2/show/NCT02838420.
- Zhou C, Kim SW, Reungwetwattana T, Zhou J, Zhang Y, He J, et al.Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet 2019;7(5):437‐46. [DOI: 10.1016/S2213-2600(19)30053-0] [DOI] [PubMed] [Google Scholar]
- Zhou C, Lu Y, Kim S-W, Reungwetwattana T, Zhou J, Zhang Y, et al.Primary results of ALESIA: a randomised, phase III, open-label study of alectinib vs crizotinib in Asian patients with treatment-naive ALK1 advanced NSCLC. Annals of Oncology 2018;29:viii740. [DOI: 10.1093/annonc/mdy424.062] [DOI] [Google Scholar]
- Zhou C, Lu Y, Kim S-W, Reungwetwattana T, Zhou J, Zhang Y, et al.Primary results of ALESIA: phase III, randomised open label study of alectinib (ALC) vs crizotinib (CRZ) in Asian patients (pts) with treatment-naive ALK+ advanced non-small-cell lung cancer (NSCLC). Annals of Oncology 2018;29(9):IX 174. [DOI: 10.1093/annonc/mdy483.001] [DOI] [Google Scholar]
ALEX 2017 {published data only}
- Camidge DR, Dziadziuszko R, Peters S, Mok T, Noe J, Nowicka M, et al.Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non–small cell lung cancer in the global phase III ALEX study. Journal of Thoracic Oncology 2019;14(7):1233-43. [DOI: 10.1016/j.jtho.2019.03.007] [DOI] [PubMed] [Google Scholar]
- Camidge DR, Peters S, Mok T, Gadgeel SM, Cheema PK, Pavlakis N, et al.Updated efficacy and safety data from the global phase III ALEX study of alectinib (ALC) vs crizotinib (CZ) in untreated advanced ALK+ NSCLC. Journal of Clinical Oncology 2018;36(15 Suppl):9043. [DOI: 10.1200/JCO.2018.36.15-suppl.9043] [DOI] [Google Scholar]
- Dziadziuszko R, Mok TS, Camidge DR, Shaw AT, Noe J, Nowicka M, et al.Impact of the EML4-ALK variant on the efficacy of alectinib (ALC) in untreated ALK1 advanced NSCLC (aNSCLC) in the global phase III ALEX study. Annals of Oncology 2018;29:viii494‐5. [DOI: 10.1093/annonc/mdy292.002] [DOI] [Google Scholar]
- Dziadziuszko R, Peters S, Mok T S K, Camidge D R, Noé J, Nowicka M, et al.Circulating free DNA as a prognostic biomarker in patients with advanced ALK+ NSCLC treated with alectinib from the global phase III ALEX trial. Journal of Clinical Oncology 2019;37(15 Suppl):9053. [Google Scholar]
- EUCTR2013-004133-33-PL.Alectinib versus crizotinib in previously untreated patients with ALK-positive non-small cell lung cancer. trialsearch.who.int/Trial2.aspx?TrialID=NCT02075840 27/02/2014.
- Gadgeel S, Peters S, Mok T, Shaw AT, Kim DW, Ou SI, et al.Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Annals of Oncology 2018;29(11):2214‐22. [DOI: 10.1093/annonc/mdy405] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffknecht P, Mok T, Shaw AT, Camidge DR, Gadgeel S, Rosell R, et al.Final progression-free survival (PFS), updated overall survival (OS), and safety data from the global, randomized, phase 3 ALEX study of alectinib (Alc) versus crizotinib (CRZ) in untreated advanced alk+ non-small cell lung cancer (NSCLC). Oncology Research and Treatment 2020;43:236. [DOI: 10.1159/000506491] [DOI] [Google Scholar]
- Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim DW, et al.Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Annals of Oncology 2020;31(8):1056-64. [DOI: 10.1016/j.annonc.2020.04.478] [DOI] [PubMed] [Google Scholar]
- Mok T, Peters S, Camidge D R, Gadgeel S, Ignatius Ou S, Kim D, et al.Patients with alk IHC-positive/fish-negative NSCLC benefit from ALK TKI treatment: response data from the global ALEX trial. Journal of Thoracic Oncology 2017;12(11):S1826. [Google Scholar]
- Mok T, Peters S, Ross Camidge D, Gadgeel S Ou SI, Kim D, et al.Patients with ALK IHC-positive/FISH-negative NSCLC benefit from ALK TKI treatment: response data from the global ALEX trial. Journal of Thoracic Oncology 2017;12(11):S1739‐40. [Google Scholar]
- Mok T, P Solange, Camidge D R, Noé J, Gadgeel S, Ou Sai-Hong I, et al.Outcomes according to ALK status determined by central immunohistochemistry or fluorescence In situ hybridization in patients with ALK-positive NSCLC enrolled in the phase 3 ALEX study. Journal of Thoracic Oncology 2021;16(2):259-68. [DOI] [PubMed] [Google Scholar]
- Mok TSK, Peters S, Camidge DR, Ou S-HI, Ahn JS, Tan EH, et al.Alectinib (ALC) vs crizotinib (CRZ) in treatment-näive ALK+ non-small-cell lung cancer (NSCLC): Asian vs non-Asian subgroup analysis of the ALEX study. Annals of Oncology 2017;28:x191. [DOI: 10.1093/annonc/mdx729.009] [DOI] [Google Scholar]
- Mok TSK, Shaw AT, Camidge RD, Gadgeel SM, Rosell R, Dziadziuszko R, et al.Final PFS, updated OS and safety data from the randomised, phase III ALEX study of alectinib (ALC) versus crizotinib (CRZ) in untreated advanced ALK1 NSCLC. Annals of Oncology 2019;30:v607. [DOI: 10.1093/annonc/mdz260.006] [DOI] [Google Scholar]
- NCT02075840.A study comparing alectinib with crizotinib in treatment-naive anaplastic lymphoma kinase-positive advanced non-small cell lung cancer participants. clinicaltrials.gov/ct2/show/NCT02075840.
- PER-064-14.Randomized, multicenter, phase iii, open-label study of alectinib versus crizotinib in treatment-naïve anaplastic lymphoma kinase−positive advanced non−small cell lung cancer. trialsearch.who.int/Trial2.aspx?TrialID=PER-064-14.
- Perol M, Pavlakis N, Levchenko E, Platania M, Oliveira J, Novello S, et al.Patient-reported outcomes from the randomized phase III ALEX study of alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer. Lung Cancer 2019;138:79‐87. [DOI: 10.1016/j.lungcan.2019.10.002] [DOI] [PubMed] [Google Scholar]
- Perol M, Peters S, Pavlakis N, Levchenko E, Platania M, Oliveira J, et al.Patient-reported outcomes (PROs) in ALEX: a phase III study of alectinib (ALEC) vs crizotinib (CRIZ) in non-small-cell lung cancer (NSCLC). Journal of Thoracic Oncology 2018;13(4):S80‐1. [Google Scholar]
- Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al.Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. New England Journal of Medicine 2017;377(9):829‐38. [DOI: 10.1056/NEJMoa1704795] [DOI] [PubMed] [Google Scholar]
- Peters S, Mok TSK, Gadgeel SM, Rosell R, Dziadziuszko R, Kim DW, et al.Updated overall survival (OS) and safety data from the randomized, phase III ALEX study of alectinib (ALC) versus crizotinib (CRZ) in untreated advanced ALK + NSCLC. Journal of Clinical Oncology 2020;38(15 Suppl):9518. [Google Scholar]
- Shaw AT, Peters S, Mok T, Gadgeel SM, Ahn JS, Ou SH, et al.Alectinib versus crizotinib in treatment-naive advanced ALK positive non-small cell lung cancer (NSCLC): primary results of the global phase III ALEX study. In: Journal of Clinical Oncology. Vol. 35. 2017.
- Thomas M, Camidge DR, Peters S, Mok T, Gadgeel SM, Cheema P, et al.ENCORE: updated efficacy and safety data from the global phase III ALEX study of alectinib (ALC) vs. crizotinib (CZ) in untreated advanced ALK+ NSCLC. In: Pneumologie. Vol. 73. 2019. [DOI: 10.1055/s-0039-1678027] [DOI]
ALTA‐1L 2019 {published data only}
- Ahn MJ, Kim HR, Yang JCH, Han JY, Lee JS, Hochmair MJ, et al.Brigatinib (BRG) versus crizotinib (CRZ) in Asian versus non-Asian patients (pts) in the phase III ALTA-1L trial. Journal of Clinical Oncology 2019;37(15 Suppl):9026. [DOI: 10.1200/JCO.2019.37.15_suppl.9026] [DOI] [Google Scholar]
- Ahn M J, Kim H R, Yang J C H, Han J Y, Li J Y C, Hochmair MJ, et al.Brigatinib (BRG) versus crizotinib (CRZ) in Asian vs non-Asian patients (pts): update from ALTA-1L. Annals of Oncology 2020;31:S843-4. [Google Scholar]
- Califano R, Camidge D, Kim HR, Ahn MJ, Yang J, Han J, et al.Brigatinib versus crizotinib in patients with ALK inhibitor naive advanced ALK+ NSCLC: results from the phase 3 ALTA-1L trial. Lung Cancer 2020;139:S59‐60. [DOI: 10.1016/S0169-5002(20)30164-1] [DOI] [Google Scholar]
- Califano R, Hochmair MJ, Gridelli C, Delmonte A, Garcia Campelo MR, Bearz A, et al.Brigatinib (BRG) vs crizotinib (CRZ) in the phase III ALTA-1L trial. Annals of Oncology 2019;30:ii41. [DOI: 10.1093/annonc/mdz063.004] [DOI] [Google Scholar]
- Camidge D R, Kim HR, Ahn MJ, Yang JCH, HanJY, Hochmair MJ, et al.Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: second interim analysis of the phase III ALTA-1L trial. Journal of Clinical Oncology 2020;38(31):3592-603. [DOI: 10.1200/JCO.20.00505] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camidge DR, Kim HR, Ahn MJ, Yang, JC, Han JY, Lee JS, et al.Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. New England Journal of Medicine 2018;379(21):2027‐39. [DOI: 10.1056/NEJMoa1810171] [DOI] [PubMed] [Google Scholar]
- Camidge R, Kim HR, Ahn M, Yang JC, Han J, Lee J, et al .Brigatinib vs crizotinib in patients with ALK inhibitor-naive advanced ALK+ NSCLC: first report of a phase 3 trial (ALTA-1L). Journal of Thoracic Oncology 2018;13(10):S184‐S185. [DOI: 10.1016/j.jtho.2018.08.011] [Google Scholar]
- Camidge R, Kim HR, Ahn MJ, Yang JCH, HanJY, Hochmair MJ, et al.Brigatinib vs crizotinib in patients with ALK inhibitor-naive advanced ALK+ NSCLC: updated results from the phase III ALTA-1L trial. Annals of Oncology 2019;30(Suppl 9):ix195‐6. [DOI: 10.1093/annonc/mdz446] [DOI] [Google Scholar]
- Campelo RG, Lin H, Perol M, Jahanzeb M, Popat S, Zhu Y, et al.P1.01-124 Health-related quality of life (HRQoL) data in a phase 3 study of first-line brigatinib versus crizotinib in NSCLC (ALTA-1L). Journal of Thoracic Oncology 2019;14(10):S411‐2. [DOI: 10.1016/j.jtho.2019.08.839] [DOI] [Google Scholar]
- Campelo RG, Lin HM, Perol M, Jahanzeb M, Popat S, Zhu Y, et al.Health-related quality of life (HRQoL) results from ALTA-1L: phase 3 study of brigatinib vs crizotinib as first-line (1L) ALK therapy in advanced ALK+ nonsmall cell lung cancer (NSCLC). Journal of Clinical Oncology 2019;37(15 Suppl):9084. [DOI: 10.1200/JCO.2019.37.15_suppl.9084] [DOI] [Google Scholar]
- Cranmer H, Liu Y, Kay S, Shen J.PCN23 Overall survival estimates adjusting for treatment crossover in patients previously untreated with an ALK inhibitor with ALK+ NSCLC using data from the Alta-1L clinical trial. Value in Health 2020;23:S424-5. [DOI: 10.1016/j.jval.2020.08.160] [DOI] [Google Scholar]
- EUCTR2015-003447-19-NL.A phase 3 multicenter open-label study of brigatinib versus crizotinib in ALK-positive advanced lung cancer patients. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2015-003447-19-GB.
- Griesinger F, Camidge DR, Kim HR, Ahn MJ, Yang JCH, Han JY, et al.Updated results from the phase 3 ALTA-1L trial of brigatinib (BRG) vs. crizotinib (CRZ) in ALK inhibitor-naive advanced ALK + NSCLC. Oncology Research and Treatment 2020;43:232. [DOI: 10.1159/000506491] [DOI] [Google Scholar]
- NCT02737501.ALTA-1L study: a phase 3 study of brigatinib versus crizotinib in anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC) participants. clinicaltrials.gov/ct2/show/NCT02737501.
- Ng TL, Narasimhan N, Gupta N, Venkatakrishnan K, Kerstein D, Camidge DR, et al.Early-onset pulmonary events associated with brigatinib use in advanced NSCLC. Journal of Thoracic Oncology 2020;15(7):1190-9. [DOI: 10.1016/j.jtho.2020.02.011] [DOI] [PubMed] [Google Scholar]
- Popat S, Kim HR, Ahn MJ, Yang JCH, Han JY, Hochmair MJ, et al.Intracranial efficacy of brigatinib (BRG) versus crizotinib (CRZ): updated results from the ALTA-1L trial. Annals of Oncology 2020;31:S840-1. [DOI: 10.1016/j.annonc.2020.08.1614] [DOI] [Google Scholar]
- Popat S, Kim HR, Ahn MJ, Yang JCH, Han JY, Hochmair MJ, et al.Intracranial efficacy of brigatinib (BRG) versus crizotinib (CRZ) in the phase III ALTA-1L trial. Annals of Oncology 2018;29(Suppl 8):viii746. [DOI: 10.1093/annonc/mdy424.070] [DOI] [Google Scholar]
- Popat S, Tiseo M, Gettinger S, Peters S, Haney J, Kerstein D, et al.ALTA-1L (ALK in lung cancer trial of brigatinib in 1st line): a randomized, phase 3 trial of brigatinib (BRG) versus crizotinib (CRZ) in tyrosine kinase inhibitor (TKI)-naive, advanced anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer. Annals of Oncology 2016;27:vi448. [DOI: 10.1093/annonc/mdw383.89] [DOI] [Google Scholar]
- Tiseo M, Popat S, Gettinger SN, Peters S, Haney J, Kerstein D, et al.Design of ALTA-1L (ALK in lung cancer trial of brigatinib in first-line), a randomized phase 3 trial of brigatinib (BRG) versus crizotinib (CRZ) in tyrosine kinase inhibitor (TKI)-naive patients (pts) with advanced anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC). Journal of Clinical Oncology 2017;35(15 Suppl):TPS9098. [Google Scholar]
- Yang JCH, Kim HR, Ahn MJ, Han JY, Hochmair MJ, Lee KH, et al.Brigatinib (BRG) versus crizotinib (CRZ) in patients (Pts) with ALK inhibitor-naive advanced ALK1 NSCLC from ALTA-1L. Annals of Oncology 2019;30:vi83. [DOI: 10.1093/annonc/mdz339.006] [DOI] [Google Scholar]
ALUR 2018 {published data only}
- De Castro J, Novello S, Mazieres J, Oh IJ, Migliorino MR, Helland A, et al.CNS efficacy results from the phase III ALUR study of alectinib versus chemotherapy in previously treated ALK1 NSCLC. Annals of Oncology 2017;28(Suppl 5):v481. [DOI: 10.1093/annonc/mdx380.048] [DOI] [Google Scholar]
- EUCTR2015-000634-29-SK.A study of alectinib versus pemetrexed or docetaxel in patients with anaplastic lymphoma kinase-positive advanced non small cell lung cancer. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2015-000634-29-PT.
- Mazieres J, Novello S, De Castro J, Migliorino MR, Helland Å, Dziadziuszko R, et al.P1.01-013 Patient-reported outcomes and safety from the phase III ALUR study of alectinib versus chemotherapy in pre-treated ALK+ NSCLC. Journal of Thoracic Oncology 2017;12(11):S1897. [DOI: 10.1016/j.jtho.2017.09.667] [DOI] [Google Scholar]
- Mazieres J, Novello S, De Castro J, Migliorino MR, Helland A, Dziadziuszko R, et al.Patient-reported outcomes and safety from the phase III ALUR study of alectinib versus chemotherapy in pre-treated ALK+ NSCLC. Journal of Thoracic Oncology 2017;12(11):S1897. [Google Scholar]
- NCT02604342.Alectinib versus pemetrexed or docetaxel in anaplastic lymphoma kinase (ALK)-Positive advanced non-small cell lung cancer (NSCLC) participants previously treated with platinum-based chemotherapy and crizotinib. clinicaltrials.gov/ct2/show/NCT02604342.
- Novello S, Mazières J, Oh IJ, Castro J, Migliorino MR, Helland Å, et al.Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Annals of Oncology 2018;29(6):1409‐16. [DOI: 10.1093/annonc/mdy121] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novello S, Mazieres J, Oh IJ, De Castro J, Migliorino MR, Helland Å, et al.Primary results from the phase III ALUR study of alectinib versus chemotherapy in previously treated ALK+ non-small-cell lung cancer (NSCLC). Annals of Oncology 2017;28:v639. [DOI: 10.1093/annonc/mdx440.058] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J, Helland A, Oh IJ, Migliorino MR, Dziadziuszko R, De Castro Carpeno J, et al.OA02.07 Phase 3 ALUR study of alectinib in pretreated ALK+ NSCLC: final efficacy, safety and targeted genomic sequencing analyses. Journal of Thoracic Oncology 2019;14(10):S210. [DOI: 10.1016/j.jtho.2019.08.416] [DOI] [Google Scholar]
- Wolf J, Oh IJ, Mazieres J, De Castro J, Revil C, Koth A, et al.ALUR: a phase 3 study of alectinib versus chemotherapy in previously treated ALK+ non-small cell lung cancer (NSCLC). Annals of Oncology 2016;27(Suppl 6):VI449. [DOI: 10.1093/annonc/mdw383.90] [DOI] [Google Scholar]
ASCEND‐4 2017 {published data only}
- De Castro G, Shao-Weng Tan D, Crino L, Wu YL, Paz-Ares L, Wolf J, et al.First-line ceritinib versus chemotherapy in patients with ALK-rearranged (ALK+) NSCLC: a randomized, phase 3 study (ASCEND-4). Journal of Thoracic Oncology 2017;12(1):S7. [Google Scholar]
- Erratum: department of Error (The Lancet (2017) 389(10072) (917-929) (S014067361730123X) (10.1016/S0140-6736(17)30123-X)). Lancet 2017;389(10072):917-29. [DOI: 10.1016/S0140-6736(17)30123-X] [DOI]
- NCT01828099.LDK378 versus chemotherapy in previously untreated patients with ALK rearranged non-small cell lung cancer. clinicaltrials.gov/ct2/show/NCT01828099.
- Soria JC, Tan DSW, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al.First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389(10072):917-29. [DOI: 10.1016/S0140-6736%2817%2930123-X] [DOI] [PubMed] [Google Scholar]
- Tan DS, Geater S, Yu CJ, Tsai CM, Hsia TC, Zhou J, et al.First-line ceritinib versus chemotherapy in patients (pts) with advanced ALK rearranged (ALK1) non-small cell lung cancer (NSCLC): ASCEND-4 Asian subgroup analysis. Annals of Oncology 2019;30:v599‐600. [DOI: 10.1093/annonc/mdz259.016] [DOI] [Google Scholar]
- Tan DSW, Soria JC, De Castro G, Wu YL, Paz-Ares L, Wolf J, et al.Pros with ceritinib versus chemotherapy in patients with previously untreated ALK-rearranged nonsquamous NSCLC (ASCEND-4). Journal of Thoracic Oncology 2017;12(1):S1176‐7. [Google Scholar]
ASCEND‐5 2017 {published data only}
- Ceritinib outperforms chemo as second-line treatment. Cancer Discovery 2016;6(12):OF5. [DOI: 10.1158/2159-8290.CD-NB2016-135] [DOI] [PubMed]
- Kiura K, Imamura F, Kagamu H, Matsumoto S, Hida T, Nakagawa K, et al.Phase 3 study of ceritinib versus chemotherapy in ALK-rearranged NSCLC patients previously treated with chemotherapy and crizotinib (ASCEND-5): Japanese subset. Japanese Journal of Clinical Oncology 2018;48(4):367‐75. [DOI: 10.1093/jjco/hyy016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall H.Ceritinib versus chemotherapy in ALK-positive lung cancer. Lancet Respiratory Medicine 2016;4(12):952-3. [DOI: 10.1016/S2213-2600(16)30377-0] [DOI] [PubMed] [Google Scholar]
- Mok TSK, Scagliotti G, Kim TM, Crino L, Liu G, Gridelli C, et al.Patient-reported outcomes (PROs) in ASCEND-5: a randomized, phase 3 study of ceritinib versus chemotherapy (CT) in patients (pts) with advanced anaplastic lymphoma kinase rearranged (ALK+) NSCLC previously treated with CT and crizotinib (CRZ). Annals of Oncology 2016;27:ix142‐3. [DOI: 10.1093/annonc/mdw594.008] [DOI] [Google Scholar]
- NCT01828112.LDK378 versus chemotherapy in ALK rearranged (ALK positive) patients previously treated with chemotherapy (platinum doublet) and crizotinib. clinicaltrials.gov/ct2/show/NCT01828112.
- Scagliotti G, Kim TM, Crino L, Liu G, Gridelli C, Novello S, et al.PR Ceritinib vs chemotherapy (CT) in patients (pts) with advanced anaplastic lymphoma kinase (ALK)-rearranged (ALK+) non-small cell lung cancer (NSCLC) previously treated with CT and crizotinib (CRZ): results from the confirmatory phase 3 ASCEND-5 study. Annals of Oncology 2016;27(Suppl 6):VI579. [DOI: 10.1093/annonc/mdw435.41] [DOI] [Google Scholar]
- Shaw AT, Kim TM, Crinò L, Gridelli C, Kiura K, Liu G, et al.Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncology 2017;18(7):874‐86. [DOI: 10.1016/S1470-2045(17)30339-X] [DOI] [PubMed] [Google Scholar]
CROWN 2020 {published data only}
- CTRI/2018/01/011325.Study with investigational drug PF-06463922 and comparator crizotinib in patients with a specific type of advanced lung cancer. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=22321&EncHid=&modid=&compid=%27,%2722321det%27.
- EUCTR2016-003315-35-PL.Study with investigational drug PF-06463922 and comparator crizotinib in patients with a specific type of advanced lung cancer. clinicaltrialsregister.eu/ctr-search/search?query=2016-003315-35.
- Lorlatinib outperforms crizotinib in NSCLC. Cancer Discovery 2021;11(1):OF5. [DOI: 10.1158/2159-8290.CD-NB2020-110] [DOI] [PubMed]
- NCT03052608.A study of lorlatinib versus crizotinib in first line treatment of patients with ALK-positive NSCLC. clinicaltrials.gov/ct2/show/NCT03052608.
- Shaw A, Bauer T, Takahashi T, Baik C, Goto Y, Polli A, et al.First-line lorlatinib versus crizotinib for advanced anaplastic lymphoma kinase-positive (ALK+) non-small cell lung cancer. Journal of Thoracic Oncology 2018;13(10):S584. [DOI: 10.1016/j.jtho.2018.08.863] [DOI] [Google Scholar]
- Shaw At, Bauer TM, Marinis F, Felip E, Goto Y, Liu G, et al.First-line lorlatinib or crizotinib in advanced alk-positive lung cancer. New England Journal of Medicine 2020;383(21):2018-29. [DOI: 10.1056/NEJMoa2027187] [DOI] [PubMed] [Google Scholar]
- Shaw AT, Bauer TM, Takahashi T, Baik CS, Polli A, Carpentieri M, et al.A randomized, open-label comparison of lorlatinib versus crizotinib as first-line treatment for advanced anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer. Annals of Oncology 2017;28:v493. [DOI: 10.1093/annonc/mdx380.081] [DOI] [Google Scholar]
- Solomon B, Bauer TM, De Marinis F, Felip E, Goto Y, Lie G, et al.Lorlatinib versus crizotinib in the first-line treatment of patients (pts) with advanced ALK-positive non-small cell lung cancer (NSCLC): results of the phase III CROWN study. Annals of Oncology 2020;31(Suppl 4):S1180-1. [DOI: 10.1016/j.annonc.2020.08.2282] [DOI] [Google Scholar]
J‐ALEX 2017 {published data only}
- Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, et al.Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390(10089):29‐39. [DOI: 10.1016/S0140-6736(17)30565-2] [DOI] [PubMed] [Google Scholar]
- Hsu JC, Jaminion F, Guerini E, Tanaka T, Golding S, Balas B, et al.Population pharmacokinetic and exposure-efficacy/safety analyses for bridging J-ALEX to global population with alectinib 600 mg BID dose regimen. Journal of Clinical Oncology 2017;35(15 Suppl):e20616. [DOI: 10.1007/s10928-017-9536-y] [DOI] [Google Scholar]
- JPRN-JapicCTI-132316.Open-label randomized phase III study of the efficacy and safety of CH5424802(AF802) in ALK-positive advanced or recurrent non-small cell lung cancer with crizotinib control. www.clinicaltrials.jp/user/showCteDetailE.jsp?japicId=JapicCTI-132316.
- Kim Y, Hida T, Nokihara H, Kondo M, Azuma K, Seto T, et al.Alectinib (ALC) versus crizotinib (CRZ) in ALK-positive non-small cell lung cancer (ALK+ NSCLC): primary results from phase III study (J-ALEX). Journal of Thoracic Oncology 2017;12(1):S378‐9. [Google Scholar]
- Nakagawa K, Hida T, Nokihara H, Morise M, Azuma K, Kim YH, et al.Final progression-free survival results from the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer 2020;139:195-9. [DOI] [PubMed] [Google Scholar]
- Nishio M, Nakagawa K, Mitsudomi T, Yamamoto N, Tanaka T, Kuriki H, et al.Analysis of central nervous system efficacy in the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer 2018;121:37-40. [DOI: 10.1016/j.lungcan.2018.04.015] [DOI] [PubMed] [Google Scholar]
- Nokihara H, Hida T, Kondo M, Hak Kim Y, Azuma K, Seto T, et al.Alectinib (ALC) versus crizotinib (CRZ) in ALK-inhibitor naive ALK-positive non-small cell lung cancer (ALK+ NSCLC): primary results from the J-ALEX study. Journal of Clinical Oncology 2016;34(15 Suppl):9008. [Google Scholar]
- Seto T, Nishio M, Hida T, Nokihara H, Morise M, Hak Kim Y, et al.Final PFS analysis and safety data from the phase III J-ALEX study of alectinib (ALC) vs. crizotinib (CRZ) in ALK-inhibitor naive ALK-positive non-small cell lung cancer (ALK+ NSCLC). Journal of Clinical Oncology 2019;37(15 Suppl):9092. [DOI: 10.1200/JCO.2019.37.15_suppl.9092] [DOI] [Google Scholar]
- Takiguchi Y, Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, et al.Updated efficacy and safety of the J-ALEX study comparing alectinib (ALC) with crizotinib (CRZ) in ALK-inhibitor naive ALK fusion positive non-small cell lung cancer (ALK+ NSCLC). Journal of Clinical Oncology 2017;35(15 Suppl):9064. [Google Scholar]
PROFILE 1007 2013 {published data only}
- Blackhall F, Hirsh V, Kim DW, Besse B, Nokihara H, Han JY, et al .Impact of crizotinib on patient-reported general health status compared with single-agent chemotherapy in a phase III study of advanced ALK-positive non-small cell lung cancer (NSCLC). European Journal of Cancer 2013;49(Suppl 2):S799‐800. [DOI: 10.1016/S0959-8049%2813%2970065-0] [DOI] [Google Scholar]
- Blackhall F, Kim DW, Besse B, Nokihara H, Han JY, Wilner KD, et al.Patient-reported outcomes and quality of life in PROFILE 1007: a randomized trial of crizotinib compared with chemotherapy in previously treated patients with ALK-positive advanced non-small-cell lung cancer. Journal of Thoracic Oncology 2014;9(11):1625‐33. [DOI: 10.1097/JTO.0000000000000318] [DOI] [PubMed] [Google Scholar]
- Erratum: patient-reported outcomes and quality of life in PROFILE 1007: a randomized trial of crizotinib compared with chemotherapy in previously treated patients with ALK-positive advanced non-small-cell lung cancer. Journal of Thoracic Oncology 2015;10(11):1657. [DOI: 10.1097/JTO.0000000000000702] [DOI] [PubMed]
- EUCTR2009-012595-27-BG.Phase 3, randomized, open-label study of the efficacy and safety of pf 02341066 versus standard of care chemotherapy (pemetrexed or docetaxel) in patients with advanced non-small cell lung cancer (Nsclc) harboring a translocation or inversion event involving the anaplastic lymphoma kinase (Alk) gene locus. trialsearch.who.int/Trial2.aspx?TrialID=NCT00932893 (first received 30 June 2009).
- NCT00932893.An investigational drug, PF-02341066 is being studied versus standard of care in patients with advanced non-small cell lung cancer with a specific gene profile involving the anaplastic lymphoma kinase (ALK) gene. clinicaltrials.gov/ct2/show/NCT00932893.
- Nishio M, Kim DW, Wu YL, Nakagawa K, Solomon BJ, Shaw AT, et al.Crizotinib versus chemotherapy in Asian patients with ALK-positive advanced non-small cell lung cancer. Cancer Research and Treatment 2018;50(3):691‐700. [DOI: 10.4143/crt.2017.280] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AT, Janne PA, Besse B, Solomon BJ, Blackhall FH, Camidge DR, et al.Crizotinib versus chemotherapy in ALK+ advanced non-small cell lung cancer (NSCLC): final survival results from PROFILE 1007. Journal of Clinical Oncology 2016;34(15 Suppl):9066. [Google Scholar]
- Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, et al.Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. New England Journal of Medicine 2013;368(25):2385‐94. [DOI: 10.1056/NEJMoa1214886] [DOI] [PubMed] [Google Scholar]
PROFILE 1014 2014 {published data only}
- EUCTR2010-021336-33-PT.Cancer of the lung with special characteristics (nonsquamous histology and ALK fusion gene event). trialsearch.who.int/Trial2.aspx?TrialID=NCT01154140.
- Felip E, Blackhall FH, Mok T, Cappuzzo F, Wilner KD, Reisman A, et al.Impact of crizotinib on patient-reported general health status compared with chemotherapy in patients with no prior systemic treatment for advanced non-squamous ALK-positive non-small cell lung cancer (NSCLC). Journal of Clinical Oncology 2015;33(15 Suppl):8101. [Google Scholar]
- Lu Y, Zhou J, Chung CH, Masters E, Wilner K, Selaru P, et al.Patient-reported symptoms and quality of life (QOL) in East Asian patients with ALK+ NSCLC treated with crizotinib versus chemotherapy. Journal of Thoracic Oncology 2017;12(1):S1167. [Google Scholar]
- Mok TSK, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, et al.Overall survival (OS) for first-line crizotinib versus chemotherapy in ALK1 lung cancer: updated results from PROFILE 1014. Annals of Oncology 2017;28(Suppl 5):v605-49. [DOI: 10.1093/annonc/mdx440.053] [DOI] [Google Scholar]
- Nakagawa K, Kim DW, Wu YL, Solomon BJ, Mekhail T, Felip E, et al.First-line crizotinib versus pemetrexed + cisplatin/carboplatin in Asian patients with advanced ALK+ NSCLC in PROFILE 1014. Annals of Oncology 2014;25(Suppl 5):v2. [DOI: 10.1093/annonc/mdu395.1] [DOI] [Google Scholar]
- NCT01154140.A clinical trial testing the efficacy of crizotinib versus standard chemotherapy pemetrexed plus cisplatin or carboplatin in patients with ALK positive non squamous cancer of the lung. trialsearch.who.int/Trial2.aspx?TrialID=NCT01154140.
- Solomon BJ, Cappuzzo F, Felip E, Blackhall FH, Costa DB, Kim DW, et al.Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK-positive non-small-cell lung cancer: results from PROFILE 1014. Journal of Clinical Oncology 2016;34(24):2858‐65. [DOI: 10.1200/JCO.2015.63.5888] [DOI] [PubMed] [Google Scholar]
- Solomon BJ, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, et al.Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non-small-cell lung cancer. Journal of Clinical Oncology 2018;36(22):2251‐8. [DOI: 10.1200/JCO.2017.77.4794] [DOI] [PubMed] [Google Scholar]
- Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al.First-line crizotinib versus chemotherapy in ALK-positive lung cancer. New England Journal of Medicine 2014;371(23):2167‐77. [DOI: 10.1056/NEJMoa1408440] [DOI] [PubMed] [Google Scholar]
- Wilner K, Usari T, Polli A, Kim E.Comparison of cardiovascular effects of crizotinib and chemotherapy in patients (pts) with ALK-positive (+) advanced non-small cell lung cancer (NSCLC). Advanced NSCLC 2016;11(4 Suppl 1):S133. [DOI: 10.1016/S1556-0864%2816%2930284-2] [DOI] [Google Scholar]
- Wilner KD, Usari T, Polli A, Kim EE.Comparison of cardiovascular effects of crizotinib and chemotherapy in ALK-positive advanced non-small-cell lung cancer. Future Oncology 2019;15(10):1097‐103. [DOI: 10.2217/fon-2018-0869] [DOI] [PubMed] [Google Scholar]
PROFILE 1029 2018 {published data only}
- NCT01639001.A study of crizotinib versus chemotherapy in previously untreated ALK positive East Asian non-small cell lung cancer patients. clinicaltrials.gov/ct2/show/NCT01639001.
- Wu YL, Lu S, Lu Y, Zhou J, Shi YK, Sriuranpong V, et al.Results of PROFILE 1029, a phase III comparison of first-line crizotinib versus chemotherapy in East Asian patients with ALK-positive advanced non–small cell lung cancer. Journal of Thoracic Oncology 2018;13(10):1539‐48. [DOI: 10.1016/j.jtho.2018.06.012] [DOI] [PubMed] [Google Scholar]
- Zhou J, Lu Y, Chung CH, Masters E, Wilner K, Tang Y, Wu YL.Patient reported general health status in a study of crizotinib versus chemotherapy in patients with non-small cell lung cancer (NSCLC). Journal of Thoracic Oncology 2017;12(1):S1169. [Google Scholar]
References to studies excluded from this review
Blackhall 2017 {published data only}
- Blackhall F, Ross Camidge D, Shaw AT, Soria JC, Solomon BJ, et al.Final results of the large-scale multinational trial PROFILE 1005: efficacy and safety of crizotinib in previously treated patients with advanced/metastatic ALK-positive non-small-cell lung cancer. ESMO Open 2017;2(3):e000219. [DOI: 10.1136/esmoopen-2017-000219] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cho 2017 {published data only}
- Cho BC, Kim DW, Bearz A, Laurie SA, McKeage M, Borra G, et al.ASCEND-8: a randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)-rearranged metastatic non-small cell lung cancer (NSCLC). Journal of Thoracic Oncology 2017;12(9):1357‐67. [DOI: 10.1016/j.jtho.2017.07.005] [DOI] [PubMed] [Google Scholar]
- Cho BC, Obermannova R, Bearz A, Kim D, Orlov S, Borra G, et al.Efficacy and updated safety of ceritinib (450 mg or 600 mg) with low-fat meal vs 750 mg fasted in ALK+ metastatic NSCLC. Journal of Thoracic Oncology 2017;12(11):S1757. [Google Scholar]
- Cho BC, Obermannova R, Bearz A, McKeage M, Kim DW, Batra U, et al.Efficacy and safety of ceritinib (450 mg/d or 600 mg/d) with food versus 750-mg/d fasted in patients with ALK receptor tyrosine kinase (ALK)–positive NSCLC: primary efficacy results from the ASCEND-8 study. Journal of Thoracic Oncology 2019;14(7):1255-65. [DOI: 10.1016/j.jtho.2019.03.002] [DOI] [PubMed] [Google Scholar]
Chow 2019 {published data only}
- Chow LQ, Barlesi F, Bertino EM, den Bent MJ, Wakelee H, Wen PY, et al.Results of the ASCEND-7 phase II study evaluating ALK inhibitor (ALKi) ceritinib in patients (pts) with ALK+ non-small cell lung cancer (NSCLC) metastatic to the brain. Annals of Oncology 2019;30(Suppl 5):v602‐3. [Google Scholar]
EUCTR2012‐003474‐36‐BE {published data only}
- EUCTR2012-003474-36-BE.A clinical study of oral LDK378 in adult patients with ALK-activated non-small cell lung cancer and who have not been previously treated with crizotinib. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2012-003474-36 (first received 25 October 2012).
Felip 2016 {published data only}
- Felip E, Orlov S, Park K, Yu C, Tsai CM, Nishio M, et al.Phase 2 study of ceritinib in ALKi-naive patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC): whole body responses in the overall pt group and in pts with baseline brain metastases (BM). In: Annals of Oncology. Vol. 27. 2016. [DOI: 10.1093/annonc/mdw383.3] [DOI]
Gao 2016 {published data only}
- Gao L, Zhang Y, Hu B, Liu J, Kong P, Lou S, et al.Phase II multicenter, randomized, double-blind controlled study of efficacy and safety of umbilical cord–derived mesenchymal stromal cells in the prophylaxis of chronic graft-versus-host disease after HLA-haploidentical stem-cell transplantation. Journal of Clinical Oncology 2016;34(24):2843-50. [DOI] [PubMed] [Google Scholar]
JPRN‐JapicCTI‐184073 {published data only}
- JPRN-JapicCTI-184073.A study comparing adjuvant alectinib versus adjuvant platinum-based chemotherapy in patients with ALK positive non-small cell lung cancer. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-JapicCTI-184073.
Kim 2016 {published data only}
- Ahn M, Camidge DR, Tiseo M, Reckamp K, Hansen K, Kim S, et al.Brigatinib in crizotinib-refractory ALK+ NSCLC: updated efficacy and safety results from ALTA, a randomized phase 2 trial. Journal of Thoracic Oncology 2017;12(11):S1755‐6. [Google Scholar]
- Ahn MJ, Camidge DR, Tiseo M, Reckamp KL, Hansen KH, Kim SW, et al.Brigatinib (BRG) in crizotinib (CRZ)-refractory ALK+ non-small cell lung cancer (NSCLC): updates from ALTA, a pivotal randomized phase 2 trial. Journal of Clinical Oncology 2017;35(15 Suppl):e20503. [Google Scholar]
- Camidge DR, Kim DW, Tiseo M, Langer CJ, Ahn MJ, Shaw AT, et al.Exploratory analysis of brigatinib activity in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer and brain metastases in two clinical trials. Journal of Clinical Oncology 2018;36(26):2693‐701. [DOI: 10.1200/JCO.2017.77.5841] [DOI] [PubMed] [Google Scholar]
- Camidge DR, Tiseo M, Ahn M, Reckamp K, Hansen K, Kim S, et al.Depth of target lesion response to brigatinib and its association with outcomes in patients with ALK+ NSCLC in the ALTA trial. Journal of Thoracic Oncology 2017;12(11):S1892. [Google Scholar]
- Camidge DR, Tiseo M, Ahn MJ, Reckamp K, Hansen K, Kim SW, et al.Brigatinib in crizotinib-refractory ALK+ NSCLC: central assessment and updates from ALTA, a pivotal randomized phase 2 trial. Journal of Thoracic Oncology 2017;12(1):S1167‐9. [Google Scholar]
- Hochmair M, Ahn MJ, Camidge DR, Tiseo M, Reckamp K, Holmskov Hansen K, et a.Updated efficacy and safety results from ALTA, a randomized phase 2 trial of brigatinib in crizotinib-refractory ALK+ NSCLC. Oncology Research and Treatment 2018;41:271. [DOI: 10.1159/000492737] [DOI] [Google Scholar]
- Hochmair MJ, Tiseo M, Reckamp KL, West HL, Groen HJ, Langer CJ, et al.Brigatinib in crizotinib-refractory ALK1 NSCLC: updates from the pivotal randomized phase 2 trial (ALTA). Annals of Oncology 2017;28:iii35‐6. [DOI: 10.1093/annonc/mdx085] [DOI] [Google Scholar]
- Huber RM, Hansen KH, Paz-Ares Rodriguez L, West HL, Reckamp KL, Leighl NB, et al.Brigatinib in crizotinib-refractory ALK+ non-small cell lung cancer: 2-year follow-up on systemic and intracranial outcomes in the phase 2 ALTA trial. Journal of Thoracic Oncology 2020;15(3):P404-15. [DOI: 10.1016/j.jtho.2019.11.004] [DOI] [PubMed] [Google Scholar]
- Huber RM, Kim DW, Ahn MJ, Langer CJ, Tiseo M, West H, et al.Brigatinib (BRG) in crizotinib (CRZ)-refractory ALK+ non-small cell lung cancer (NSCLC): efficacy updates and exploratory analysis of CNS ORR and overall ORR by baseline (BL) brain lesion status. Journal of Clinical Oncology 2018;36(15 Suppl):9061. [DOI: 10.1200/JCO.2018.36.15-suppl.9061] [DOI] [Google Scholar]
- Kim DW, Tiseo M, Ahn MJ, Reckamp KL, Hansen KH, Kim SW, et al.Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. Journal of Clinical Oncology 2017;35(22):2490‐8. [DOI: 10.1200/JCO.2016.71.5904] [DOI] [PubMed] [Google Scholar]
- Kim DW, Tiseo M, Ahn MJ, Reckamp KL, Hansen KH, Kim SW, et al.Brigatinib (BRG) in patients (pts) with crizotinib (CRZ)-refractory ALK+ non-small cell lung cancer (NSCLC): first report of efficacy and safety from a pivotal randomized phase (ph) 2 trial (ALTA). Journal of Clinical Oncology 2016;34(15 Suppl):9007. [Google Scholar]
- Langer C, Huang H, Reichmann W, Lustgarten S, Kerstein D, Camidge DR.Overall survival (OS) after disease progression (PD) on brigatinib in patients with crizotinib-refractory ALK+NSCLC in ALTA. Journal of Thoracic Oncology 2017;12(11):S1893‐4. [Google Scholar]
- Langer CJ, Huang H, Huang J, Kerstein D, Reichmann W, Speck RM, et al.Patient-reported outcomes and quality of life in ALTA: the randomized phase 2 study of brigatinib (BRG) in advanced ALK+ non-small cell lung cancer (NSCLC). Journal of Clinical Oncology 2017;35(15 Suppl):9066. [Google Scholar]
- Lee DH, Kim DW, Camidge DR, Langer CJ, Huber RM, Tiseo M, et al.Brigatinib (BRG) in Asian versus non-Asian patients (PTS) with crizotinib (CRZ)-refractory ALK1 NSCLC in the phase II ALTA trial. Annals of Oncology 2019;30:v634‐5. [DOI: 10.1093/annonc/mdz260.065] [DOI] [Google Scholar]
- Lenderking WR, Lin H, Speck RM, Zhu Y, Huang H, Huang J, et al.Patient-reported outcomes and quality of life in advanced ALK+ non-small-cell lung cancer trial of brigatinib (ALTA). Future Oncology 2019;15(24):2841‐55. [DOI: 10.2217/fon-2019-0185] [DOI] [PubMed] [Google Scholar]
- NCT02094573.A study to evaluate the efficacy of brigatinib (AP26113) in participants with anaplastic lymphoma kinase (ALK)-positive, non-small cell lung cancer (NSCLC) previously treated with crizotinib. clinicaltrials.gov/ct2/show/NCT02094573.
- Ou SHI, Tiseo M, Camidge DR, Ahn MJ, Huber RM, Hochmair MJ, et al.Brigatinib (BRG) in patients (pts) with crizotinib (CRZ)-refractory ALK+ non-small cell lung cancer (NSCLC) and brain metastases in the pivotal randomized phase 2 ALTA trial. Journal of Clinical Oncology 2017;35(15 Suppl):e20502. [Google Scholar]
- Ou SHI, Tiseo M, Camidge R, Ahn MJ, Huber RM, Hochmair MJ, et al.Intracranial efficacy of brigatinib (BRG) in patients (Pts) with crizotinib (CRZ)-refractory anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC) and baseline CNS metastases. Annals of Oncology 2017;28:v480‐1. [DOI: 10.1093/annonc/mdx380.047] [DOI] [Google Scholar]
Lenderking 2017 {published data only}
- Lenderking WR, Speck RM, Huang JT, Huang H, Kerstein D, Reichmann W, et al.Evaluating clinically meaningful change of the EORTC QLQ-C30 in patients with NSCLC. Value in Health 2017;20(5):A120. [Google Scholar]
Liang 2019 {published data only}
- Liang F, Shen D.EP1.14-02 comparative efficacy of first-line ceritinib at a dose of 450mg with food and alectinib in advanced ALK+ NSCLC. Journal of Thoracic Oncology 2019;14(10):S1032. [DOI: 10.1016/j.jtho.2019.08.2287] [DOI] [Google Scholar]
NCT02134912 {published data only}
- NCT02134912.S1300: Pemetrexed Disodium With or Without Crizotinib in Treating Patients With Stage IV Non-Small Cell Lung Cancer That Has Progressed After Crizotinib. clinicaltrials.gov/ct2/show/NCT02134912.
Park 2020 {published data only}
- Park S, Lee YG, Park JH, Lee GW, Kang EJ, Choi YJ, et al.A phase III, open-label, randomized study of atezolizumab in combination with carboplatin + paclitaxel + bevacizumab compared with pemetrexed + cisplatin or carboplatin with stage IV non-squamous non-small cell lung cancer (NSCLC) with activating EGFR mutation or ALK translocation (ATLAS Trial). Journal of Clinical Oncology 2020;38(15 Suppl):TPS9636. [Google Scholar]
Reckamp 2019 {published data only}
- Reckamp K, Lin HM, Huang J, Proskorovsky I, Reichmann W, Krotneva S, et al.Comparative efficacy of brigatinib versus ceritinib and alectinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small cell lung cancer. Current Medicine Respiratory Opinion 2019;35(4):569-76. [DOI: 10.1080/03007995.2018.1520696] [DOI] [PubMed] [Google Scholar]
Wolf 2015 {published data only}
- Wolf J, Schneider CP, Potzner M, Cazorla Arratia P, Shen J, Branle F, et al.The phase II ASCEND-7 (CLDK378A2205) trial: ceritinib in patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) metastatic to the brain and/or leptomeninges. Oncology Research and Treatment 2015;38:138. [DOI: 10.1159/000439070] [DOI] [Google Scholar]
Zhao 2015 {published data only}
- Zhao J, Zhang K, Zhang L, Wang H.Clinical efficacy of crizotinib in advanced ALK positive non-small cell lung cancer. Zhongguo fei ai za zhi [Chinese Journal of Lung Cancer] 2015;18(10):616‐20. [DOI: 10.3779/j.issn.1009-3419.2015.10.03] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
eXalt3 2020 {published data only}
- EUCTR2015-004147-40-NL.A clinical trial comparing ensartinib (study drug) to crizotinib in lung cancer patients. trialsearch.who.int/Trial2.aspx?TrialID=NCT02767804.
- Horn L, Wu Y, Reck M, Wakelee H, Liang C, Harrow K, et al.eXalt3: phase 3 randomized study comparing ensartinib to crizotinib in anaplastic lymphoma kinase positive non-small cell lung cancer patients. Journal of Thoracic Oncology 2018;13(10):S582. [DOI: 10.1016/j.jtho.2018.08.858] [DOI] [Google Scholar]
- Horn L, Wu YL, Reck M, Liang C, Tan F, Harrow K, et al.EXalt3: a phase III study of ensartinib (X-396) in anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC). Journal of Clinical Oncology 2017;35(15):TPS8578. [Google Scholar]
- Horn L, Wu YL, Reck M, Liang C, Tan F, Harrow K, et al.EXalt3: phase 3 randomized study comparing ensartinib to crizotinib in anaplastic lymphoma kinase (ALK) positive non-small cell lung cancer (NSCLC) patients. Journal of Thoracic Oncology 2018;13(4):S116. [Google Scholar]
- Horn L, Wu YL, Reck M, Liang C, Tan F, Harrow K et al.EXalt3: a phase III study of ensartinib (X-396) in anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC). Annals of Oncology 2017;28:iii51. [DOI: 10.1093/annonc/mdx085] [DOI] [Google Scholar]
- Horn L, Wu YL, Reck M, Wakelee H, Liang C, Harrow K, et al.eXalt3: phase III randomized study comparing ensartinib to crizotinib in anaplastic lymphoma kinase positive non-small cell lung cancer patients. Annals of Oncology 2018;29(Suppl 8):viii543. [DOI: 10.1093/annonc/mdy292.121] [DOI] [Google Scholar]
- Horn L, Wu YL, Reck M, Wakelee HA, Liang C, Tan F, et al.EXalt3: phase 3 randomized study comparing ensartinib to crizotinib in anaplastic lymphoma kinase (ALK) positive non-small cell lung cancer (NSCLC) patients. Journal of Clinical Oncology 2018;36(15 Suppl):TPS9115. [DOI: 10.1200/JCO.2018.36.15-suppl.TPS9115] [DOI] [Google Scholar]
- NCT02767804.eXalt3: study comparing X-396 (ensartinib) to crizotinib in ALK positive non-small cell lung cancer (NSCLC) patients. clinicaltrials.gov/ct2/show/NCT02767804.
- Selvaggi G, Wakelee HA, Mok T, Wu Y-L, Reck M, Chiappori A, et al.ID:1882 Phase III randomized study of ensartinib vs crizotinib in anaplastic lymphoma kinase (ALK) positive NSCLC patients: eXalt3. IASLC 2020 Virtual Presidential Symposium 2020;15(10 Suppl):e41-2.
- Wu Y, Mok T, Reck M, Wakelee H, Liang C, Tan F, et al.First-line ensartinib (X396) versus crizotinib in advanced alk-rearranged NSCLC (eXalt3): a randomized, open-label, phase 3 study. Journal of Thoracic Oncology 2017;12(11):S2402. [Google Scholar]
NCT03737994 {published data only}
- NCT03737994.Biomarker/ALK inhibitor combinations in treating patients with stage IV ALK positive non-small cell lung cancer (The NCI-NRG ALK master protocol). clinicaltrials.gov/ct2/show/NCT03737994.
NCT04009317 {published data only}
- NCT04009317.Study of TQ-B3139 versus crizotinib in the first line treatment of subjects with anaplastic lymphoma kinase (ALK) positive non-small cell lung cancer (NSCLC). clinicaltrials.gov/ct2/show/NCT04009317.
NCT04318938 {published data only}
- NCT04318938.Advancing brigatinib properties in ALK+ NSCLC patients by deep phenotyping. clinicaltrials.gov/ct2/show/NCT04318938.
NCT04632758 {published data only}
- NCT04632758.Study comparing WX-0593 to crizotinib in ALK positive non-small cell lung cancer (NSCLC) patients. clinicaltrials.gov/ct2/show/NCT04632758.
Popat 2019 {published data only}
- NCT03596866.An efficacy study comparing brigatinib versus alectinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer participants who have progressed on crizotinib. clinicaltrials.gov/ct2/show/NCT03596866.
- Popat S, Liu G, Lu S, Song G, Samnotra V, Yang JCH.Phase III ALTA-3 study of brigatinib (BRG) versus alectinib (ALC) in patients (pts) with advanced anaplastic lymphoma kinase (ALK)−positive non–small cell lung cancer (NSCLC) that progressed on crizotinib (CRZ). Annals of Oncology 2019;30:v653-4. [DOI: 10.1093/annonc/mdz260.108] [DOI] [Google Scholar]
Additional references
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al.The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993;85:365-76. [DOI] [PubMed] [Google Scholar]
Anota 2015
- Anota A, Hamidou Z, Paget-Bailly S, Chibaudel B, Bascoul-Mollevi C, Auquier P, et al.Time to health-related quality of life score deterioration as a modality of longitudinal analysis for health-related quality of life studies in oncology: do we need RECIST for quality of life to achieve standardization? Quality Life Research 2015;24(1):5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
ASCO guideline
- Hanna NH, Robinson AG, Temin S, Baker S Jr, Brahmer JR, Ellis PM, et al.Therapy for stage IV non–small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. Journal of Clinical Oncology 2021;39(9):1040-91. [DOI] [PubMed] [Google Scholar]
Barlesi 2016
- Barlesi F, Mazieres J, Merlio JP, Debieuvre D, Mosser J, Lena H, et al.Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French cooperative thoracic intergroup (IFCT). Lancet 2016;387(10026):1415-26. [DOI] [PubMed] [Google Scholar]
Barrows 2019
- Barrows SM, Wright K, Copley-Merriman C, Kaye JA, Chioda M, Wiltshire R, et al.Systematic review of sequencing of ALK inhibitors in ALK-positive non-small-cell lung cancer. Lung Cancer (Auckland) 2019;10:11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Breadner 2020
- Breadner D, Blanchette P, Shanmuganathan S, Boldt RG, Raphael J.Efficacy and safety of ALK inhibitors in ALK-rearranged non-small cell lung cancer: a systematic review and meta-analysis. Lung Cancer 2020;144:57-63. [DOI] [PubMed] [Google Scholar]
Cameron 2015
Camidge 2012
- Camidge DR, Doebele RC.Treating ALK-positive lung cancer - early successes and future challenges. Nature Reviews Clinical Oncology 2012;9(5):268-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cetin 2011
- Cetin K, Ettinger DS, Hei YJ, O'Malley CD.Survival by histologic subtype in stage IV nonsmall cell lung cancer based on data from the surveillance, epidemiology and end results program. Clinical Epidemiology 2011;3:139-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chansky 2017
- Chansky K, Detterbeck FC, Nicholson AG, Rusch VW, Vallieres E, Groome P, et al.The IASLC lung cancer staging project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. Journal of Thoracic Oncology 2017;12(7):1109-21. [DOI] [PubMed] [Google Scholar]
Chuang 2021
- Chuang C-H, Chen L, Chang H-M, Tsai Y-C, Wu K-L, Chen I-H, et al.Systematic review and network meta-analysis of anaplastic lymphoma kinase (ALK) inhibitors for treatment-naïve ALK-positive lung cancer. Cancers (Basel) 2021;13(8):1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Costa 2011
- Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, et al.CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. Journal of Clinical Oncology 2011;29(15):e443-5. [DOI] [PubMed] [Google Scholar]
Costa 2015
- Costa DB, Shaw AT, Ou SH, Solomon BJ, Riely GJ, Ahn MJ, et al.Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. Journal of Clinical Oncology 2015;33(17):1881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Costa 2018
- Costa RB, Costa RL, Talamantes SM, Kaplan JB, Bhave MA, Rademaker A, et al.Systematic review and meta-analysis of selected toxicities of approved ALK inhibitors in metastatic non-small cell lung cancer. Oncotarget 2018;9(31):22137-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence.Version accessed 20 September 2017. Melbourne, Australia: Veritas Health Innovation, 2014. Available at covidence.org.
Creelan 2019
- Creelan BC, Yeh T, Kim S-W, Nogami N, Kim D-W, Chow LQ, et al.Phase I study of gefitinib (G) + durvalumab (D) for locally advanced/metastatic non-small cell lung cancer (NSCLC) harbouring epidermal growth factor receptor (EGFR) sensitising mutations. Annals of Oncology 2019;30(Suppl 2):ii31-7. [Google Scholar]
CTCAE v4
- US Department of Health and Human Services, National Institutes of Health, National Cancer Institute.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published: May 28, 2009 (v4.03: June 14, 2010). www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
Eisenhauer 2009
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al.New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45(2):228-47. [DOI] [PubMed] [Google Scholar]
Elliott 2020
- Elliott J, Bai Z, Hsieh SC, Kelly SE, Chen L, Skidmore B, et al.ALK inhibitors for non-small cell lung cancer: a systematic review and network meta-analysis. PLoS ONE 2020;15(2):e0229179. [DOI] [PMC free article] [PubMed] [Google Scholar]
ESMO guideline
- Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al.Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2019;30(5):863-70. [DOI: 10.1093/annonc/mdy474] [DOI] [PubMed] [Google Scholar]
Fukuoka 2011
- Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al.Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). Journal of Clinical Oncology 2011;29(21):2866-74. [DOI] [PubMed] [Google Scholar]
Gainor 2015
- Gainor JF, Sherman CA, Willoughby K, Logan J, Kennedy E, Brastianos PK, et al.Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. Journal of Thoracic Oncology 2015;10(2):232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gainor 2016
- Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al.EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clinical Cancer Research 2016;22(18):4585-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gainor 2016a
- Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, et al.Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discovery 2016;6(10):1118-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldstraw 2016
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al.The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. Journal of Thoracic Oncology 2016;11(1):39-51. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT: GRADEpro Guideline Development Tool.Version accessed 20 September 2017. McMaster University and Evidence Prime, 2021. Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al.GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/. [1119964792]
Hirsch 2016
- Hirsch FR, Suda K, Wiens J, Bunn PA Jr.New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 2016;388(10048):1012-24. [DOI] [PubMed] [Google Scholar]
Kalemkerian 2018
- Kalemkerian GP, Narula N, Kennedy EB, Biermann WA, Donington J, Leighl NB, et al.Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. Journal of Clinical Oncology 2018;36(9):911. [DOI] [PubMed] [Google Scholar]
Kassem 2019
- Kassem L, Shohdy KS, Lasheen S, Abdel-Rahman O, Ali A, Abdel-Malek RR.Safety issues with the ALK inhibitors in the treatment of NSCLC: a systematic review. Critical Review Oncology Hematology 2019;134:56-64. [DOI] [PubMed] [Google Scholar]
Khan 2019
- Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al.ALK inhibitors in the treatment of ALK positive NSCLC. Frontiers in Oncology 2019;8:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2018
- Kim J, Cho J, Lee MH, Lim JH.Relative efficacy of checkpoint inhibitors for advanced NSCLC according to programmed death-ligand-1 expression: a systematic review and network meta-analysis. Scientific Reports 2018;8(1):11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koller 2017
- Koller M, Hjermstad MJ, Tomaszewski KA, Tomaszewska IM, Hornslien K, Harle A, et al on behalf of the EORTC .Quality of Life Group, EORTC Lung Cancer Group, and European Society of Thoracic Surgeons. An international study to revise the EORTC questionnaire for assessing quality of life in lung cancer patients. Annals of Oncology 2017;28(11):2874-81. [DOI: 10.1093/annonc/mdx453.] [DOI] [PubMed] [Google Scholar]
Kris 2014
- Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, et al.Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311(19):1998-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2017
- Lin JJ, Riely GJ, Shaw AT.Targeting ALK: precision medicine takes on drug resistance. Cancer Discovery 2017;7(2):137-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazieres 2019
- Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al.Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Annals of Oncology 2019;30(8):1321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDermott 2008
- McDermott U, Iafrate AJ, Gray NS, Shioda T, Classon M, Maheswaran S, et al.Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Research 2008;68(9):3389-95. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moya‐Horno 2018
- Moya-Horno I, Viteri S, Karachaliou N, Rosell R.Combination of immunotherapy with targeted therapies in advanced non-small cell lung cancer (NSCLC). Therapeutic Advances in Medical Oncology 2018;10:1758834017745012. [DOI: 10.1177/1758834017745012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2021
- Murray BW, Zhai D, Deng W, Zhang X, Ung J, Nguyen V, et al.TPX-0131, a potent CNS-penetrant, next-generation inhibitor of wild-type ALK and ALK-resistant mutations. Molecular Cancer Therapeutics 2021;20(9):1499-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04849273
- NCT04849273.A Study of TPX-0131, a Novel Oral ALK Tyrosine Kinase Inhibitor, in Patients With ALK+ Advanced or Metastatic NSCLC. clinicaltrials.gov/ct2/show/NCT04849273.
Novello 2016
Pacheco 2019
- Pacheco JM, Gao D, Smith D, Purcell T, Hancock M, Bunn P, et al.Natural history and factors associated with overall survival in stage IV ALK-rearranged non-small cell lung cancer. Journal of Thoracic Oncology 2019;14(4):691-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pacheco 2019a
- Pacheco JM, Camidge DR.Is long-term survival possible for patients with stage IV ALK+ non-small cell lung cancer? Expert Review of Respiratory Medicine 2019;13(5):399-401. [DOI] [PubMed] [Google Scholar]
Planchard 2018
- Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al.Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2018;29(Suppl 4):iv192-237. [DOI] [PubMed] [Google Scholar]
Reck 2021
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al.Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%. Journal of Clinical Oncology 2021;39(21):2339-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Recondo 2018
- Recondo G, Facchinetti F, Olaussen KA, Besse B, Friboulet L.Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? National Review of Clinical Oncology 2018;15(11):694-708. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5).Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rikova 2007
Scagliotti 2008
- Scagliotti GV, Parikh P, Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al.Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. Journal of Clinical Oncology 2008;26(21):3543-51. [1527-7755: (Electronic)] [DOI] [PubMed]
Schiller 2002
Schulz 2002
- Schulz KF, Grimes DA.Generation of allocation sequences in randomised trials: chance, not choice. Lancet 2002;359(9305):515-9. [DOI] [PubMed] [Google Scholar]
Seike 2018
- Seike M, Inoue A, Sugawara S, Morita S, Hosomi Y, Ikeda S, et al.Phase III study of gefitinib (G) versus gefitinib+carboplatin+pemetrexed (GCP) as first-line treatment for patients (pts) with advanced non-small cell lung cancer (NSCLC) with EGFR mutations (NEJ009). Annals of Oncology 2018;29(Suppl 8):viii496. [DOI: 10.1093/annonc/mdy292.005] [DOI] [Google Scholar]
Shaw 2009
- Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al.Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. Journal of Clinical Oncology 2009;27(26):4247-53. [1527-7755: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaw 2013
Shaw 2017
- Shaw AT, Peters S, Mok T, Gadgeel SM, Ahn JS, Ou S-HI, et al.Alectinib versus crizotinib in treatment-naive advanced ALK-positive non-small cell lung cancer (NSCLC): primary results of the global phase III ALEX study. ASCO Annual Meeting 2017. [0732-183X]
Siegel 2019
- Siegel RL, Miller KD, Jemal A.Cancer statistics, 2019. CA: a Cancer Journal for Clinicians 2019;69(1):7-34. [DOI] [PubMed] [Google Scholar]
Soda 2007
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al.Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448(7153):561-6. [DOI] [PubMed] [Google Scholar]
Soda 2008
- Soda M, Takada S, Takeuchi K, Choi YL, Enomoto M, Ueno T, et al.A mouse model for EML4-ALK-positive lung cancer. Proceedings of the National Academy of Science of the United States of America 2008;105(50):19893-7. [1091-6490: (Electronic)] [DOI] [PMC free article] [PubMed]
Solomon 2009
- Solomon B, Varella-Garcia M, Camidge DR.ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non-small cell lung cancer. Journal of Thoracic Oncology 2009;4(12):1450-4. [1556-1380: (Electronic)] [DOI] [PubMed] [Google Scholar]
Solomon 2014
Solomon 2018
- Solomon BJ, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, et al.Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non-small-cell lung cancer. Journal of Clinical Oncology 2018;36(22):2251-8. [DOI] [PubMed] [Google Scholar]


