Abstract
Meaningfully improved mental and behavioral health treatment is an unrealized dream. Across three factorial experiments, inferential tests in prior studies showed a pattern of negative interactions suggesting that better clinical outcomes are obtained when participants receive fewer rather than more intervention components. Further, relatively few significant main effects were found in these experiments. Modeling suggested that negative interactions amongst components may account for these patterns. This paper evaluates factors that may contribute to such declining benefit: increased attentional or effort burden; components that produce their effects via the same capacity limited mechanisms, making their effects subadditive; and a tipping point phenomenon in which those near a hypothesized “tipping point” for change will benefit markedly from weak intervention while those far from the tipping point will benefit little from even strong intervention. New research should explore factors that cause negative interactions amongst components and constrain the development of more effective treatments.
Researchers have explored multiple routes to improve the effectiveness of clinical interventions. They have developed different types of interventions and have explored increasing the intensity or duration of interventions. With regard to smoking treatment, the 2008 PHS Clinical Practice Guideline Treating Tobacco Use and Dependence (Fiore et al., 2008), reported several meta-analyses showing evidence of a dose-response relation between treatment intensity and outcome. There is also evidence that combining different types of therapies can be beneficial. For instance, adding smoking cessation counseling to pharmacotherapy increases abstinence rates (Fiore et al., 2008).
Yet, the use of multiple intervention components (e.g., >2), often yields relatively little additional benefit in comparison to less intensive treatment. Not only might more intensive or multicomponent treatment be less scalable due to its costs and complexity (Fairburn & Wilson, 2013; Glasgow & Estabrooks, 2018; Insel, 2009) but its effects may be disappointing as well. Indeed, there is a pattern of more intense or multifaceted smoking treatments yielding disappointing returns: e.g., in the case of higher than standard nicotine patch doses (Dale et al., 1995; Hughes et al., 1999; Killen, Fortmann, Davis, Strausberg, & Varady, 1999), counseling duration beyond an hour or so (Fiore et al., 2008; Lancaster & Stead, 2005, 2017), or adding relapse prevention to standard cessation counseling (Hajek et al., 2013). Sometimes more intense treatment does add significant benefit (conjoint use of the nicotine patch + gum: Fiore et al., 2008) but such instances are rare. This pattern of diminishing returns is consistent with the speculation of Brittain & Wittes (1989) who 30+ years ago suggested that main effects of combined components would be blunted by negative component interactions, many of which may fall below the threshold of statistical significance.
There are clear reasons that more intense or multicomponent treatment might be significantly more beneficial than less intensive treatments (Blankers, 2020; Carroll et al, 2020; Driessen et al, 2019; Duffy et al., 2020; Lotfizadeh et al., 2020; Smits et al., 2020). In keeping with this notion, researchers have evaluated such adjuvants as relapse prevention training, social support interventions, mindfulness content, motivational interviewing content, cognitive game interventions, physical exercise interventions, distraction via computer games, ‘resistance training,’ and medication adherence interventions (e.g., Ciccolo et al., 2014; Fiore et al., 2008; Gruder et al., 1993; Loughead et al., 2016; Schlam et al., 2020).
The belief that multicomponent treatment will be especially effective can certainly be supported by cogent argument. For instance, such treatment might address the different needs of members of a treated population: e.g., needs for social support, improved coping skills, or reduced withdrawal. Each ‘need’ could be addressed by a different treatment component. Or, multiple intervention components might yield synergies since one treatment might allow a patient to take better advantage of another intervention. For instance, an intervention designed to promote medication adherence might boost the effectiveness of smoking medications, which in turn might allow a patient to acquire coping skills better since he or she is not distracted by strong urges to smoke.
It is also possible that combining intervention components could decrease their individual contributions. For example, the combined components might all produce their effects via the same or similar mechanisms even though, in theory, they are intended to exert different, additive effects. This might occur, for instance, to the extent that different counseling interventions all produce their effects via a single nonspecific mechanism such as therapeutic alliance (Laska, Gurman, & Wampold, 2014; Martin, Garske, & Davis, 2000; Wampold & Imel, 2015). Or, diminishing returns of added components might occur because each component adds burden, perhaps creating fatigue, resentment, or cognitive overload that negates their benefits.
Our research group has recently conducted a series of factorial experiments in which smokers were randomly assigned to one of two levels of multiple factors (Baker et al., 2017; Cook et al., 2016; Fraser et al., 2014; Piper et al., 2016; Schlam et al., 2016: see Table 1). These studies were conducted as part of the Multiphase Optimization Strategy (MOST) treatment development strategy (Baker et al., 2017; Collins et al., 2011). These studies were screening experiments designed to identify intervention components that would form an especially effective integrated, multicomponent treatment that could later be evaluated in subsequent research (Collins et al., 2011). In these experiments, randomization to an ON level of a given factor causes the participant to receive an ‘active’ or more intense intervention component; randomization to an OFF level causes the participant to receive no intervention component related to that factor or to receive a weaker one. Factorial experiments permit the evaluation of multiple, randomly assigned intervention components so that the main and interaction effects of the components on outcomes can be determined. This provides a unique opportunity to determine how systematic increases in number of components affect treatment outcome.
Table 1.
Characteristics of Three Smoking Cessation Studies Using Fully Crossed Factorial Designs
| Maintenance Intervention Screening Experiment |
Motivation Screening Experiment | Population Based Study | |
|---|---|---|---|
| Study Reference | Schlam et al., 2016 | Cook et al., 2016 | Fraser et al., 2014 |
| ClinicalTrials.gov | NCT01120704 | NCT01122238 | Exempt |
| Participants | N=513 adult daily smokers recruited in 11 primary care clinics | N=517 adult daily smokers recruited in 11 primary care clinics | N=1034 adult daily smokers recruited over the internet |
| Study Design |
|
|
|
| Assessments |
|
|
|
| Outcomes | Point-prevalence abstinence at week 52 | Quit attempts, smoking reduction, and point-prevalence abstinence at 6, 12, and 26 weeks depending on the outcome | Point-prevalence abstinence at 1, 3, and 7 months follow-up. |
| Factors |
Extended Medication: ON: 26 weeks of nicotine patches plus nicotine gum OFF: 8 weeks of nicotine patches plus nicotine gum Medication Adherence Counseling: ON: Two 10-minute in-person sessions (Weeks -1 and 1) that provided information tailored to correct misconceptions regarding cessation OFF: No counseling sessions Automated Adherence Calls: ON: 7-11 brief automated medication reminder and motivation calls OFF: No automated adherence calls Electronic Medication Monitoring: ON: A “Helping Hand” (HH) gum dispenser electronically recorded gum use plus printouts of daily gum use and 5-9 adherence counseling sessions OFF: Participants carried the HH to record gum use; no gum use printouts or associated counseling Maintenance Counseling: ON: Eight 5-10 min calls OFF: No maintenance counseling |
Nicotine Patch: ON: 6 weeks of daily 14-mg nicotine patches OFF: No nicotine patches Nicotine Gum: ON: 6 weeks of 2-mg nicotine gum OFF: No nicotine gum Behavioral Reduction (BR): ON: One initial 20-minute in-person BR counseling session followed by six weekly 10-minute counseling calls designed to reduce smoking OFF: No BR counseling Motivational Interviewing (MI): ON: One initial 20-minute in-person MI counseling session followed by three biweekly, 10-minute counseling calls over the 6-week intervention period designed to enhance intrinsic motivation to reduce smoking and quit OFF: No MI counseling |
Web Site Intervention: ON: National Cancer Institute (NCI) Smokefree.gov website (step-by-step quit guide, motivational content, skill training, and interactive features) OFF: A brief, ‘lite’ version of the website; no skill-training content Quitline Counseling: ON: Five scheduled 15-30-minute proactive counseling calls from the NCI Information Service Quitline OFF: No calls Cessation Brochure: ON: The NCI Clearing the Air brochure OFF: A brief or ‘lite’ 12-page booklet; no active behavior change content Text Messaging: ON: E-mailed messages were sent for a total of 12 weeks; messages offered quitting tips, motivational content, and stressed use of treatment resources OFF: No messages Cessation Medication: ON: Mailed, 2-week “starter kit” of nicotine mini-lozenges OFF: No pharmacotherapy |
Note. All three experiments were conducted in accordance with the provisions of the World Medical Association Declaration of Helsinki; the Maintenance and Motivation studies were approved by an institutional review board; the Population study was exempt from review.
We have found a consistent pattern of effects across multiple factorial experiments, namely multiple negative interactions and few positive or synergistic interactions. That is, we find that combining intervention components tends to result in decreases in the effects of the components. In an experimental evaluation of population-based smoking cessation intervention components (the ‘Population-Based Study’: Fraser et al., 2014; also see Table 1 and Supplemental Note 1 for more information on this study), smokers were randomized to one of two levels of five factors, with each factor having an ON and OFF level: i.e., 1) website access vs. none, 2) motivational email messages for 3 months vs. none, 3) quitline counseling vs. none, 4) a lengthy smoking cessation brochure (vs. a short control brochure), and 5) nicotine mini-lozenges vs. none. Intratreatment outcomes were assessed at 1 and 3 months. Nicotine mini-lozenges produced a main effect at 1 and 3 months. There was also a 2-way interaction occurring across months 1 and 3 months; individuals who received both the website and the motivational emails had significantly lower abstinence rates than did those receiving only the website.
A second factorial experiment, the ‘Motivation Study’ (Cook et al., 2016; see Table 1 and Supplemental Note 2 for more information on this study), comprised smokers who were not willing to try to quit smoking. These individuals were randomized to 4 factors that each comprised an intervention component that was intended to reduce smoking, increase quit attempts, and ultimately, increase abstinence. Each factor had 2 levels: ON and OFF. The four factors were: (1) nicotine patch versus none; (2) nicotine gum versus none; (3) motivational interviewing (MI) versus none, and (4) behavioral reduction (BR) counseling versus none. Treatment ended at either 6 weeks or 12 weeks depending on whether a participant chose to receive a second 6-weeks of treatment. Principal outcomes were smoking abstinence, smoking reduction (cigarettes smoked/day), and whether the participant made a quit attempt.
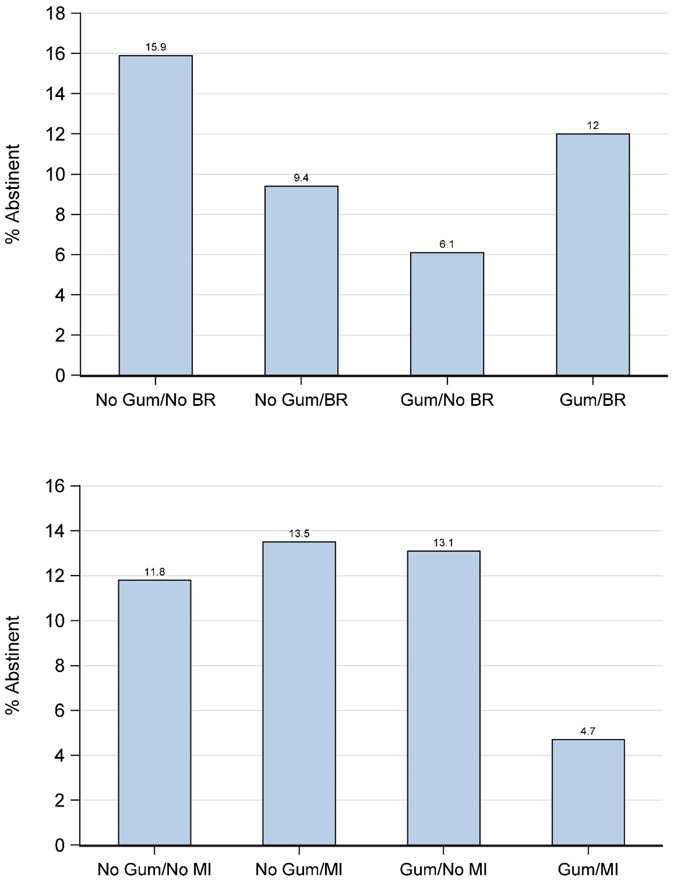
There were no main effects in the Motivation Study but there were numerous interactions, all of them negative. For instance, there were two significant 2-way interactions for abstinence at 26 weeks post treatment, which are shown in Figure 1a, b: i.e., interactions between Nicotine Gum and BR and between Nicotine Gum and MI. In Figure 1a, the condition yielding the best result is one in which neither component was ON and in Figure 1b, the condition with the worst outcome was one with both components ON. There were also two significant 4-way interactions involving smoking reduction at 12 and 26 weeks (Cook et al., 2016); in both cases the greatest smoking reduction occurred with 1- and 2-component treatments whereas 3- and 4-component combinations consistently produced less smoking reduction (data not shown). Finally, two significant 2-way negative interactions were found for the quit attempt variable (binary) at both at the 6- and 12-week marks (not reported in Cook et al., 2016). Nicotine Patch and BR both produced relatively high quit attempt rates when used alone but produced the lowest rates when used together (Supplemental Figure 1).
Figure 1.
a,b. Unpackaging Two-way Interactions Amongst Nicotine Gum, Behavioral Reduction (BR) counseling, and Motivational Interviewing (MI) on 26-week abstinence in the Motivation Study (Cook et al. 2016).
Thus, the two fully-crossed factorial studies reviewed above produced evidence of negative interactions amongst intervention components, only a single main effect, and no positive interactions. The current paper seeks to: 1) examine additional evidence that combinations of smoking treatment intervention components tend to produce negative interactions; 2) use more informative analytic methods that yield more accurate estimates of the cumulative effects of such interactions; and 3) explore mechanisms that may account for such interactions. To accomplish this, this paper will first determine whether a 3rd factorial experiment (the ‘Maintenance Study’) produces the same pattern of negative interactions using a modeling approach that more directly evaluates the presence of such effects across all components and component interactions. This analysis will then be applied in the Population-Based and the Motivation Studies as a form of replication. In a final section, data from the Maintenance Study will be used to explore three potential mechanisms that might lead to negative interactions.
Three Hypotheses Concerning the Noncomplementary Effects of Intervention Components
Our analyses show a trend for intervention components to produce reduced effects when combined. This has also been reported in other studies (e.g., McClure et al., 2014; Tombor et al., 2018). These studies, like ours (Cook et al., 2016; Fraser et al., 2014; Schlam et al., 2016) also report a relative dearth of significant main effects, which we attribute to the estimation of main effects of each component (when estimated using effect coding) when other components are simultaneously being implemented.
The current paper evaluates three different potential contributors to the occurrence of noncomplementary effects of intervention components as evaluated in factorial experiments: burden, mechanistic nonadditivity, and a tipping point phenomenon. Analyses related to such potential contributors are offered for their heuristic value: i.e., to suggest phenomena to explore in future research. These contributors are not necessarily exhaustive nor mutually exclusive and due to limitations in the data available, conclusions about their causal roles must be tentative. We first explore these using the Maintenance experiment. This experiment was chosen for further exploration because it involved a large number of factors (5) and had a rich set of assessments that permitted evaluation of the hypothesized contributors. Identifying contributors to noncomplementary component effects might suggest new strategies for enhancing treatment development and assessment.
Burden.
Intense treatment could impose burdens that affect both the patient and clinical staff, which could then affect treatment adherence, attrition, and delivery fidelity. For instance, a recent study showed that 24-weeks of nicotine patch treatment produced higher 24-week point-prevalence abstinence rates than did standard (8-week) patch treatment, while 52-week patch treatment produced weaker effects along with less adherence with medication visit attendance (the latter was just a trend: Schnoll et al., 2015). It may be that 52-weeks of medication use was so burdensome that it led to disuse of important treatment elements.
As conceptualized here, burden may occur for reasons other than sheer amount of effort or time involved. For instance, it might involve competing attentional demands or incompatible cognitive or behavioral requirements. This is illustrated in the Population-Based experiment, which showed that assignment to a second ON intervention (a short message service) caused participants to reduce their use of another component (a website); the two components interacted negatively, causing significantly lower abstinence rates. There is other evidence that use of one type of intervention can reduce use of an adjuvant intervention (Nash, Vickerman, Kellogg, & Zbikowski, 2015; Swan et al., 2010).
Mechanism overlap and capacity limitation.
Another possible explanation for non-commensurate or diminishing returns of more intense treatment involves treatment mechanisms that are overlapping and capacity limited. Thus, more intense single component treatment might yield diminishing returns because of intrinsic limits on change mechanisms. And, combining multiple components might yield disappointing results because the different components activate overlapping mechanisms, which, again, are capacity limited.
There are numerous examples of capacity limited biological and cognitive processing mechanisms: e.g., attentional and working memory processes (Cowan, 2010), and drug distribution and clearance processes (Jusko, 1989). For instance, in pharmacologic research one typically sees a clear quadratic relation between dose or number of agents on the one hand and clinical benefit on the other hand. Such nonlinear pharmacokinetic relations are due to capacity limited mechanisms with regard to drug absorption, distribution, biotransformation, receptor occupancy, and so on (Jusko, 1989; Ludden, 1991; van Ginneken, van Rossum, & Fleuren, 1974). Of course, an intensity related decline in effectiveness could reflect factors other than capacity limitation: e.g., dose-related medication side effects (i.e., another type of burden).
There are dose-related effects in smoking treatment that at least superficially fit with a capacity limitation hypothesis. For instance, the effectiveness of the nicotine patch increases up to a dose of 14-26 mg/day, but larger doses add little benefit (Dale et al., 1995; Hughes et al., 1999; Killen, Fortmann, Davis, Strausberg, & Varady, 1999). This may occur because the change mechanism, occupation of key nicotinic receptors, has achieved its’ peak; i.e., critical nicotinic receptors are saturated and desensitized (Benowitz, 2010). Similarly, there is evidence that greatly increasing counseling intensity (duration) over modest levels yields relatively little added benefit (Lancaster & Stead, 2005, 2017).
A model invoking overlapping and capacity limited change mechanisms could also account for diminished benefit when different types of interventions are combined. For instance, if different counseling approaches yield benefit due to therapeutic alliance (or another type of ‘common factor’: Laska, Gurman, & Wampold, 2014; Martin, Garske, & Davis, 2000; Wampold & Imel, 2015), and if this is capacity-limited, the different approaches would stop yielding benefit when the mechanism asymptote is reached. This could explain why it has been difficult to obtain consistent additive effects by combining different types of counseling. For instance, once smokers have received cessation counseling, there is scant evidence that adding relapse prevention content improves outcomes (Hajek et al., 2013). Of course, it is possible that some interventions do affect different change mechanisms and thereby can yield additive or synergistic benefit (e.g., combining ad lib nicotine gum or lozenges and nicotine patches: Piper et al., 2009; Smith et al., 2009).
Tipping point proximity.
It may be that “changeable” participants (i.e., those who can respond to treatment) are somewhat rare within many patient populations. Thus, the size of a treated population might create an illusion regarding the magnitude of change that is possible (a misleading ceiling effect). It would be easy to imagine that within a large population of patients, there would be many who would be helped by a more intense treatment or by combining different treatments. This would make it seem likely that adding treatments would progressively aid more patients in behavior change: e.g., each of 4 interventions might increase clinical success by 10%. However, it may be that only a relatively small proportion has a net status with regard to strengths and weaknesses that renders them likely to change. For instance, the only patients who are likely to benefit from treatment are those whose balance of strengths versus weaknesses exceeds or borders a “tipping point” for change. An important corollary of this hypothesis is that for the group of individuals near such a hypothetical tipping point for change, a wide range of aids can produce a tipping point transition (e.g., transition from smoking to abstinence). As an example, a golfer who is highly motivated to improve and has the time and energy to practice, combined with better than average coordination, might begin to shoot par regularly if aided by a wide variety of resources: e.g., obtaining better clubs, taking lessons, more practice. However, a golfer with a low tolerance for frustration and marginal coordination may as a result, be unlikely to break par even with multiple added resources. Thus, the golfer with a favorable mix of strengths and weaknesses can take advantage of a wide variety of resources. Intervention may do more for such persons, but need to do less, to create change. There are examples of tipping point phenomena in areas such as the genetic influence on disease risk (see Supplemental Note 3).
Note that our discussion of the tipping point hypothesis has involved a binary outcome. It is possible that this hypothesis is most relevant to such outcomes. However, it is also possible that it applies to polytomous or continuous outcomes as well as suggested by the range of phenomena and outcomes that have shown this rich get richer’ pattern. For instance, it has been reported with regard to both categorical and continuous measures of treatment outcome (e.g., symptom counts) (e.g., Beneciuk et al., 2017; Chambless et al., 2017; Elkins, Gallo, Pincus, & Comer, 2016; Halldorsdottir & Ollendick, 2016; Robinson et al., 2015) and with phenomena outside the treatment context (the ‘Matthew hypothesis’: Damian et al., 2015): e.g., with paired associate verbal learning (Mak & Twitchell, 2020), acquisition of social capital (Castillo, 2019; Cheng et al., 2019), vocabulary formation (James et al., 2017), foraging success of brown pelicans (Geary et al., 2019), and work success (Judge & Hurst, 2008).
Of course, this ‘rich get richer’ pattern is not uniformly found (Berndt et al., 2014; Gladstone, Forbes, Diehl, & Beardslee, 2015; Romeo et al., 2018). This might reflect the fact that in some cases intuitively appealing notions of “advantage” (key assets and fewer risks), do not, in fact, accurately reflect tipping point proximity.
The model suggests that modest treatment may especially benefit those near the tipping point. However, more intense treatment may actually result in reduced benefit; i.e., the benefit of multiple intervention components when they are combined will be less than when they are used alone. This could be because the “costs” of treatment (e.g., burden) erode some of the benefit when multiple intervention components are delivered. Also, treatment that is unnecessarily intense may actually interfere with naturally occurring change processes and create iatrogenic attributions for any success (i.e., attributing success to the treatment rather than to the individual’s own efforts and strengths). There is evidence that persons who attribute their changed behavior to their own resources and efforts will be more likely to maintain it (Deci & Ryan, 1985; Kopel & Arkowitz, 1975). In fact, there is evidence that more intensive smoking treatments can foster external attributions that are associated with a heightened risk of relapse (Harackiewicz, Sansone, Blair, Epstein, & Manderlink, 1987; see also Barefoot & Girodo, 1972; Davison & Valins, 1969).
In considering a “tipping point” hypothesis, we are sensitive to the fact that when thinking about the effects of intervention components in the presence of individual differences (e.g., related to the likelihood of successful quitting absent any intervention), we expect nonlinear change in the probability of a positive outcome even in the absence of interactions. With a binary outcome, this is due to the fact that a smoker close to the tipping point will show a greater increase in probability of success than will a smoker more distant from the tipping point, despite a constant effect of an intervention component. This will occur because logistic regression analyses index the effects of components on the logit metric (see Supplemental Note 4 for a more detailed explanation). But, as noted above, a ‘richer get richer’ effect may affect outcome beyond these nonlinear effects. In other words, being close to the tipping point would seem to have an added effect (e.g., smokers are more able or willing to take advantage of the intervention components to which they are exposed). We believe that the latter assumption makes the tipping point relevant to instances where outcomes are nonbinary; i.e., favorable status with regard to promoters and obstacles to change will enhance treatment effects even with continuous treatment outcomes (Beneciuk et al., 2017; Chambless et al., 2017; Elkins et al., 2016; Newman et al., 2019; Robinson et al., 2015). Also, it means that better use of treatment itself may serve as a measure of this latent variable.
The current research is important for several reasons. 1) It may yield new information about the effects of treatment intensity or complexity. 2) It may yield insights into novel mechanisms that affect treatment effectiveness, mechanisms that provide greater understanding of why some treatment elements produce antagonistic or subadditive effects. These insights may be relevant to the development of a broad range of psychosocial treatments. And, 3) this research may spur new models and approaches to exploring how treatments work. Also, identifying complementary intervention components is a primary goal of new treatment development methods such as the Multiphase Optimization Strategy (Baker et al., 2017; Collins et al., 2018). This research may identify factors that will influence the success of that strategy.
The Maintenance Study: Methods and Analysis of Variance Findings
Methods.
In this study and in the other studies reviewed (the Population-Based and the Motivation Studies), a considerable effort was expended to achieve high treatment fidelity across the various intervention components delivered. The interventions were either delivered via computer (e.g., Adherence Calls in the Maintenance Study), or were delivered by highly trained computer guided research staff (except for quitline counseling in the Population-Based Study, which was delivered by actual quitline counselors).
Participants in this Maintenance Intervention experiment (Schlam et al., 2016) were 513 adult smokers recruited in 11 primary care clinics from two southern Wisconsin healthcare systems from 2010 to 2013 for a smoking cessation study (See Table 1 for more study details). This was a 25 fully crossed factorial experiment with each factor having an ON and OFF level. The five factors contrasted the two factor levels on the basis of abstinence from smoking at 26 and 52 weeks post-TQD (target quit date). Half of the participants were randomized independently across factors to a level of each factor; i.e., to a more intense (“ON”) level or to an absent (“OFF”) level. The five factors were: (1) Extended Medication (26-week) versus (8-week) medication, (2) Medication Adherence Counseling (MAC) versus none, (3) Automated Adherence Calls versus none, (4) Electronic Medication Monitoring & Feedback (“Helping Hand” or HH) versus Monitoring without counseling, and (5) Maintenance Counseling versus none. (See Table 1) All participants received a base cessation medication treatment (8 weeks of combination nicotine replacement therapy [NRT: nicotine patch plus nicotine gum]), and a total of 50 minutes of counseling
Assessments included baseline smoking history questionnaires, automated medication adherence data, and daily smoking status (Robinson, Sobell, Sobell, & Leo, 2014) at study visits (Weeks 1, 4, 8) and in follow-up calls (Weeks 16, 26, 39, 52). In the analyses presented below, we report results for 52-week point-prevalence abstinence outcomes.
Main and interaction effects from the Maintenance Study.
The Maintenance experiment (Schlam et al., 2016) used logistic regression with effect coding (as in the Population Based and Motivation Studies) to examine abstinence at 52 weeks post-treatment initiation. In effect coding, targeted effects are tested with respect to the averaged effects of the other components. These analyses showed that extended (26 weeks) medication significantly increased abstinence rates versus 8-week medication (i.e., 34% vs. 27%, respectively: B = 0.34, p<.01). This was the sole main effect in the experiment.
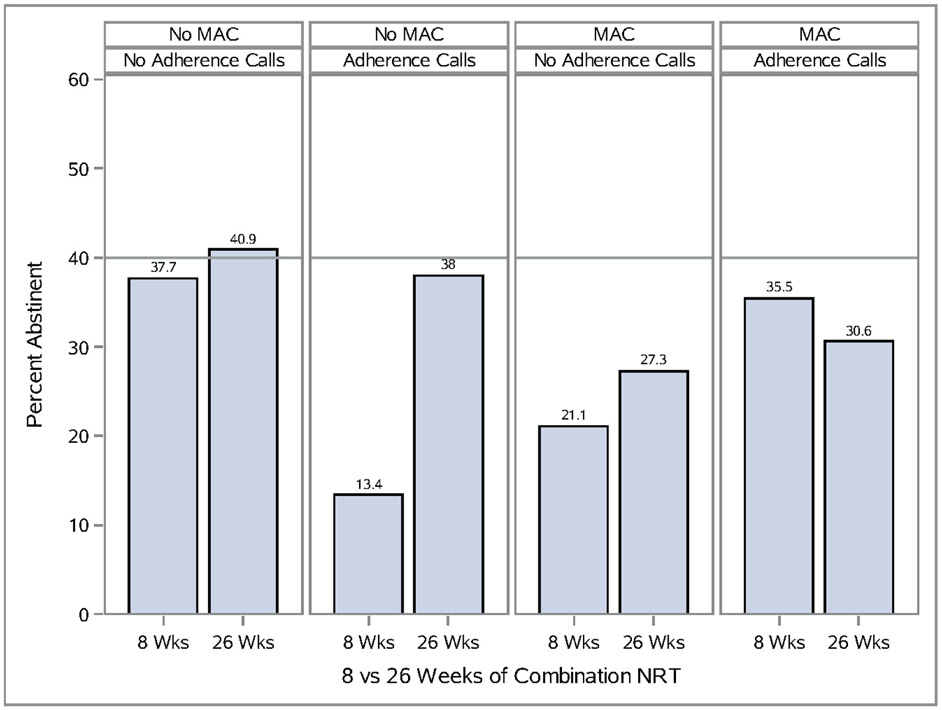
There were two statistically significant three-way interactions at week 52 (p’s<.05). An Extended Medication×MAC×Adherence Calls interaction (Figure 2) revealed that the highest abstinence rates occurred when treatment included neither adherence calls nor MAC. A Maintenance Counseling×MAC×HH counseling interaction at 52 weeks (see Supplemental Figure 2) showed that two components (e.g., maintenance counseling and helping hand counseling) produced very positive estimated effects on abstinence when each was used alone, but adding any component(s) to them produced a decidedly weaker effect.
Figure 2.
Unpackaging an Interaction Amongst Medication Adherence Counseling (MAC), Automated Adherence Calls, and Medication Duration on 52 Week Abstinence In the Maintenance Study (Schlam et al 2016).
Note that the subgroup abstinence rate data presented in the figures (Figure 2 and Supplemental Figure 1) reflect actual abstinence rates, not model based estimates. Thus, because effect coding was used, they reflect the influences of treatment factors that were not involved in the interaction. For instance, some of the participants receiving no ON intervention components in Figure 2 (i.e., neither Extended Medication, MAC, nor Adherence Calls) did receive one or more of the other intervention components (i.e., CAM, HH counseling). While assignment to these other components was random, their effects are hard to gauge. For instance, the subgroup of individuals receiving neither Extended Medication, MAC, nor Adherence Calls (i.e., receiving OFF levels of each) may have shown relatively high abstinence rates because they benefitted from the other intervention components. However, receiving these other components should also have helped those in the ON conditions for Extended Medication, MAC, and Adherence Calls, unless, as argued here, there is a diminishing return for combining a larger number of components versus a smaller number.
There were also three two-factor interactions. For instance, in an Extended Medication X MAC interaction, those who received 26 weeks of medication had higher abstinence rates when they did not receive MAC than when they did (39.4% versus 29.0%, respectively: Supplemental Figure 3). The two other significant two-way interactions in the Maintenance Study were both negative or antagonistic (i.e., the effects of two components when combined were less than would be expected based on their summed main effects [when the other component is OFF]: see Schlam et al., 2016). In both cases, the participants getting neither of the active intervention components performed better than conditions where one or more components were ON. These lower level interactions are presented not to synthesize the most important component interactions in the experiment; rather they are intended to illustrate the tendency for many of the components to interact negatively. And, as noted, these involve only the significant interactions; others may certainly fail to attain statistical significance but still function to diminish the estimated main effects of individual components as elucidated by Brittain & Wittes (1989).
Methods
Estimating Cumulative Interaction Effects
In the current study, the subadditive effects of more complex or intense treatments are demonstrated through a second analytic approach in which the outcomes for a study are determined in relation to the number of factors that are ON. This ‘Mean Abstinence Rate’ analysis approach can yield mean abstinence rates for every size treatment combination (e.g., 0 to 5 ON components). We follow this with logistic regression analyses (‘Model Based’ analyses) that illustrate the magnitude of the effects of intervention component packages that differ in the number of active components. Packages comprising multiple intervention components would be less effective to the extent that their components interact negatively. Despite recommendations for the use of effect coded factors (−1=OFF, +1=ON) in most analyses of factorial screening experiments (Collins, 2018), these analyses used dummy coded factors (0=OFF,1=ON) for clarity of illustration (see Supplemental Note 5 for a rationale for the use of dummy coding).
For each study’s respective outcome, for Model Based analyses we entered into the regression model both (a) dummy-coded predictors for each of the factors, as well as (b) additional dummy-coded predictors that account for conditions in which more than one intervention component is set to the ON condition. Note that because of the factorial design, for (‘a,’ above) we enter as many dummy-coded factor predictors as we have factors. The use of dummy coding results in a coefficient for each factor (intervention component ON vs. OFF) that reflects the simple effect of that factor when all other factors are OFF. In this paper we follow the terminology of Kugler, Dziak and Trail (2018) in referring to these as the first-order effects of each factor, to be distinguished from the main effect of a factor that reflects a component’s effect averaged across the settings of all other factors as is captured by effect coding. The second type of dummy-coded predictors entered in the model reflects the average incremental effects of component combinations for each combination with the same number of components ON. These predictors capture the degree to which multiple components (on average) enhance or detract from the sum of the first-order effects of the ON components in the package. Specifically, this second type of predictor includes separate predictors indicating whether two or more components are ON (2+), three or more components are ON (3+), and so on, up to the largest possible number of factors being ON. The coefficients for the second type of predictors provide an indication of the extent to which the combined effects of factors tend to diminish, when implemented together, relative to what is expected by adding the relevant first-order effects. Note that this model estimates an “average” effect across interactions of a common order versus evaluating an individual interaction. We thus interpret negative coefficients for the predictors in (‘b’ above) to imply the presence of negative interactions, as they indicate that the combined action of multiple factors is less than the sum of their individual first-order effects. These analyses should reveal whether a larger number of intervention components tend to commensurately increase, or decrease, effectiveness.
Results
Mean Abstinence Rates for Different Numbers of ON Components: Maintenance Study
The following analysis determined the percentages of participants in the Maintenance Study who achieved abstinence after exposure to different numbers of ON components (0-5), regardless of whether or not the component participated in a statistically detectable interaction. As noted earlier, this approach is blind to type of component; i.e., the abstinence rate associated with exposure to three components reflects the average of abstinence rates across all possible 3-component groupings. Also, although the “0” condition suggests that some participants received no active components, in fact, even participants getting no ON components did receive 8-weeks of combination NRT and four counseling sessions (totaling 50 minutes) in the Maintenance Study.
The Maintenance Study had a fully-crossed 5-factor factorial design, so the number of ON factors ranged from 0 to 5; the highest concentration of subjects being in the middle (2 or 3 conditions ON). Mean abstinence rates for the different numbers of components were, at 52 weeks (and SEs & n’s at each level of conditions ‘On”) were 0-On = 22% (SE=10%: n=18), 1-On = 32% (SE=5%: n=81), 2-On = 26% (SE=3%: n=174), 3-On = 33% (SE=4%: n=175), 4-On = 35% (SE=5%: n=83), and 5-On = 38% (SE=13%: n=13). Thus, the primary increase in abstinence rates appear to occur when moving from 0 conditions turned ON to having 1 condition turned ON (from 22% to 32%). Abstinence rates show little additional increase after having >1 condition being ON. (Note the small n’s at the ‘0’ and ‘5’ levels.)
Model-Based Analyses for All First-Order Effects (Univariable) and Combined Component Effects Accounting for the Number of ON Components: Maintenance Study
As noted earlier, in Model-Based analyses we entered dummy-coded predictors (0=OFF, 1=ON) associated with each treatment factor; in addition, we entered dummy-coded predictors indicating whether the total number of components turned on was 2 or greater (2+), 3 or greater (3+), 4 or greater (4+), or 5 (5). Thus, the variable that codes for 2 or more components reflects subjects who received 2-5 components. This approach allows for an evaluation of the average consequences of adding progressively more ON components to the treatment package. The dummy coded variables show whether treatments with multiple components turned ON yield, on average, additive or synergistic versus negative effects relative to what is expected based on the addition of their first-order effects.
This analysis allows us to interpret coefficients in terms of first-order effects of each component. As shown in Table 2, these first-order effect estimates are consistently positive for each individual component. In addition, the observation of consistently negative estimates for component combinations (2+, 3+, 4+ and 5) implies steadily diminishing returns (on average) as more components are turned ON. For example, if we consider the anticipated outcome when turning on two components (e.g., Medication Duration & Maintenance Counseling are both turned ON), the anticipated outcome would be the combination of first-order effects for these two components (.751 and .630 within logistic regression). However, the negative coefficient of the 2+ indicator (−.825), suggests that adding a second component would more than eliminate the benefit of the second component. Note, that the ‘cost’ of added components reflects an average or pooled cost effect that might not apply fully to the particular component pairing noted. It should also be noted that the effects of these number-of-components predictors are cumulative as more components are turned ON. In other words, the coefficients on 3+, 4+ and 5 component groupings, respectively, reflect the added change in effects as the number of ON components successively increases. As can be seen in Table 2 and corresponding analyses for the later studies, the lack of orthogonality (which can also be viewed as reflecting correlated predictors in our regression models) frequently leads to somewhat large standard errors in our estimated coefficients while still providing conceptually relevant data. The presence of correlated predictors and the fact that n’s are not distributed equally across number-of-component groupings compromise the ability to mount well-powered tests of component combination significance and overall prediction.
Table 2.
Model Generated Coefficients for First-order Effects and for Different Numbers of Intervention Components as Related to 52-Week Abstinence in the Maintenance Study (N=513)a
| Intervention Component Main Effects |
B | SE | OR | p-value | 95% CI for OR |
|---|---|---|---|---|---|
| Constant | −1.25 | .567 | .286 | .027 | - |
| Duration (8 vs. 26 wk) | .751 | .636 | 2.12 | .238 | (.609, 7.370) |
| Maintenance Counseling | .630 | .637 | 1.88 | .322 | (.539, 6.544) |
| Medication Adherence Counseling (MAC) | .168 | .639 | 1.18 | .792 | (.338,4.139) |
| Automated Adherence Calls | .254 | .641 | 1.29 | .692 | (.367, 4.525) |
| Helping Hand Counseling | .677 | .639 | 1.97 | .289 | (.563, 6.879) |
| 2 Components ON | −.825 | .763 | .438 | .279 | (.098, 1.954) |
| 3 Components ON | −.143 | .660 | .867 | .829 | (.238, 3.164) |
| 4 Components ON | −.382 | .317 | .683 | .573 | (.181, 2.577) |
| 5 Components ON | −.348 | .871 | .706 | .689 | (.128,3.887) |
Likelihood Ratio (LR) χ2=11.60, df = 9, p=.237; Nagelkerke R2 = .03
In the context of the current analysis, the costs associated with turning all five components ON would be the sum of the coefficient estimates for 2+, 3+, 4+ and 5. Thus, based on the estimated coefficients for these effects, the diminishing or negative returns are observed all the way through to the maximum number of ON intervention components. However, it should be recognized that particular groupings of components may still function in additive ways.
Mean and Model-Based Analyses Across the Population-Based and Motivation Studies
The patterns of averaged abstinence rates and Model-Based coefficients were computed in the Population-Based and Motivation Studies (see Table 1 for study details). The percentage of subjects abstinent as a function of the number of conditions turned ON in the Population-Based Study at 1 month follow-up reveals a substantial increase when moving from the 0-ON to the 1-ON component conditions but little meaningful increase thereafter (component number groups with SEs and n’s): 0-On = 9% (SE=5%: n=35), 1-On =25% (SE=3%: n=173), 2-On = 20% (SE=2%: n=328), 3-On =28 % (SE=3%: n=310), 4-On =24% (SE=3%: n =160), 5-On=32% (SE=9%: n=28).
The Population-Based experiment produced the same pattern of diminishing returns when abstinence rates were averaged for like-numbered component combinations (0-5). This pattern is clearly manifested in the model-derived estimates seen in Table 3. Logistic model-based coefficients for the 1-month time point show that each component by itself yielded a positive estimated effect and, with dummy coding, the effect was significant for nicotine lozenges and the website. However, each of the number-of-components predictors (2+, 3+, +4, +5) is again negative and significantly so for the 2- and 4-component combinations. Data from the 3- and 7-month follow-up time points were consistent with this pattern.
Table 3.
Model Generated Coefficients for the First-order Effects and for Different Numbers of Intervention Components as Related to 1 Month Abstinence in the Population-Based Study (N=1034)a
| Intervention Component Main Effects |
B | SE | OR | p-value | 95% CI for OR |
|---|---|---|---|---|---|
| Constant | −2.367 | .604 | .094 | <.001 | - |
| CIS Counseling | 1.321 | .643 | 3.745 | .040 | (1.063,13.200) |
| Lozenges | 1.559 | .641 | 4.754 | .015 | (1.353,16.699) |
| Cessation Booklet | 1.135 | .642 | 3.110 | .077 | (.883,10.950) |
| SmokeFree Website | 1.314 | .642 | 3.720 | .041 | (1.057,13.088) |
| Motivational Emails | 1.082 | .643 | 2.951 | .093 | (.836,10.412) |
| 2 Components ON | −1.562 | .711 | .209 | .028 | (.052,.844) |
| 3 Components ON | −.840 | .656 | .432 | .201 | (.119,1.562) |
| 4 Components ON | −1.536 | .668 | .215 | .021 | (.058,.797) |
| 5 Components ON | −.850 | .771 | .427 | .270 | (.094,1.935) |
Likelihood Ratio (LR) χ2=18.89, df = 9, p=.028; Nagelkerke R2 = .03
Data from the Motivation Study also were consistent with the diminishing returns effect. This is illustrated with an additional type of outcome variable: quit attempts (see Table 1 and Supplemental Note 2 for study details). Relevant results from this study are presented for the 6- and 12-week time points. The averaged quit attempt rates for the component groupings were: for 6-weeks the percentages (SEs & n’s) for the 5 levels of components-On were: O-On = 31% (SE=9%: n=26), 1-On = 40% (SE=5%: n=106), 2 = 44% (SE=4%: n=171), 3-On = 40% (SE=5%: n=107), 4-On = 32% (SE=10%: n=22); for 12 weeks: O-On = 13% (SE=7%: n=23), 1-On = 21% (SE=4%: n=92), 2-On = 22% (SE=4%: n-134), 3-On = 23% (SE=4%: n=99), 4-On = 30% (SE=10%: n = 20). The Model-Based estimates for quit attempts at the 12-week mark are depicted in Table 4; Supplemental Table 1 presents these estimates for the 6-week mark. At both time-points, the coefficients attached to the component first-order effects are all positive (6- and 12-weeks). However, each of the multi-component predictors (2+, 3+, 4) is again negative.
Table 4.
Model Generated Coefficients for the First-order Effects and for Different Numbers of Intervention Components as Related to Quit Attempts at Week 12 in the Motivation Study (N=517)a
| Intervention Component Main Effects |
B | SE | OR | P | 95% CI for OR |
|---|---|---|---|---|---|
| Constant | −.811 | .425 | .444 | .056 | - |
| Nicotine Patch | .352 | .497 | 1.42 | .479 | (.537,3.765) |
| Nicotine Gum | .384 | .498 | 1.47 | .441 | (.553,3.898) |
| Behavioral Intervention | .323 | .498 | 1.38 | .516 | (.521,3.663) |
| Motivational Interviewing | .516 | .500 | 1.68 | .302 | (.629,4.459) |
| 2 Components ON | −.199 | .602 | .820 | .742 | (.252,2.668) |
| 3 Components ON | −.565 | .532 | .569 | .288 | (.200,1.613) |
| 4 Components ON | −.763 | .685 | .466 | .265 | (.122,1.784) |
Likelihood Ratio (LR) χ2=3.46, df = 7, p=.839; Nagelkerke R2 = .01
Not All Component Combinations Are Created Equal: Variation in Relations of Component Combinations with Abstinence
The model-based estimates yield averaged effects of different number-of-component groupings and thus particular combinations may differ from one another in terms of their relations with abstinence. We do, in fact, see some statistically detectable variability in abstinence relations for a single one of the like-numbered component combinations examined. Thus, in comparing all possible packages with three ON components in the Maintenance experiment, we see detectable variability in the 52-week abstinence outcome across packages (Pearson χ2 exact test =17.790, df=9, p=.035). At this follow-up time point, the package with Maintenance Counseling, MAC and Helping Hand Counseling all ON, returned an abstinence rate of 0% (0/17), while the package with Counseling, MAC and Automated Adherence Calls all ON returned an abstinence rate of 50% (8/16).
In summary, all three studies showed that there was little benefit of adding components to a two-component combination. The model-based analyses similarly showed that coefficients for first-order effects associated with individual components were consistently positive while the added effects of treatment combinations were consistently negative for larger component combinations. This pattern of subadditive effects of components is consistent with the numerous statistically significant negative interactions found with regard to specific component combinations (e.g., Figures 1 & 2).
Exploring the Causes of Noncomplementary Effects with Maintenance Study Data Pursuing the Burden Hypothesis
Burden Analysis.
We explore a burden hypothesis by attending to measures of adherence and participant dropout, with an expectation that, as burden increases, adherence will decline, and participant drop-out will increase. To investigate the burden hypothesis, we examined the effects of adding intervention components to two intervention components that we thought imposed especially high levels of burden: Automated Adherence Phone Calls and Maintenance Counseling (see Table 5). Each required multiple contacts and therefore, in theory, constituted a significant burden that might render the participant sensitive to additional burden. These components also provided a sensitive, quasi-continuous index by which to show burden effects, i.e., percentage of prescribed treatment contacts completed. The Maintenance Counseling required eight 15-minute contacts for up to 22 weeks post-TQD. The Automated Adherence Phone Calls involved accepting either 7 or 11 automated phone calls depending upon the participant’s assignment to the Medication Duration factor.
Table 5.
The Relations Between Different Numbers of Intervention Components and Adherence to Components (Treatment Contacts) in the Maintenance Study (N=513)
| Intervention Components to Which Additional Intervention Components are Added |
Percentage of Treatment Contacts Completed by Participants as a Function of Number of Active Interventions (1-5) |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Automated Adherence Calls Intervention | Mean | 1.00 | 0.82 | 0.72 | 0.55 | 0.55 |
| (SD) | (0.00) | (0.34) | (0.38) | (0.40) | (0.34) | |
| N | 15 | 68 | 107 | 69 | 13 | |
| Maintenance Counseling Intervention | Mean | 0.53 | 0.42 | 0.46 | 0.43 | 0.40 |
| (SD) | (0.34) | (0.35) | (0.37) | (0.34) | (0.35) | |
| N | 16 | 70 | 103 | 61 | 13 | |
| Combination of Adherence Calls and Maintenance Counseling | Mean | 0.73 | 0.62 | 0.55 | 0.47 | |
| (SD) | N/A | (0.18) | (0.27) | (0.34) | (0.31) | |
| N | 16 | 52 | 47 | 13 | ||
The measure used to index adherence to the two components was percentage of treatment contacts completed by participants. The analytic method involved determining adherence to the Automated Adherence Phone Calls intervention or the Maintenance Counseling intervention when each intervention was used by itself and then examining the effects of adding additional intervention components via the use of dummy-coded variables, with component packages involving up to 5 components. Finally, we determined the effects of adding different numbers of interventions to a package that already comprised both Adherence Phone Calls and Maintenance Counseling interventions.
Burden Results.
Table 5 shows a fairly large and linear decrease in mean percentage of treatment contacts completed as 1-4 additional components were added to the Adherence Call intervention. With regard to the Maintenance Counseling component, the highest treatment contact attendance occurred when Maintenance Counseling was used as a sole intervention component; this decreased when a single additional component was added but did not change meaningfully with combinations of 2-5 components.
We then examined the effects of adding additional components to a 2-component package comprising both Automated Adherence Phone Calls and Maintenance Counseling Calls (Table 5). This produced a fairly large magnitude, linear decrease in visit attendance when components were added to this 2-component base.
The burden hypothesis is based in part on the assumption that nonattendance at, or nonexposure to, intervention contacts should reduce abstinence rates (and therefore account for subadditive effects on outcome variables). Therefore, we analyzed the relation between completion of Automated Adherence Phone Calls or Maintenance Calls and abstinence at 8 and 16 weeks when either of those two intervention components was used in combination with one or more additional components. In these analyses, adherence reflected the proportion of calls completed. The relation between this adherence variable and biochemically confirmed abstinence was tested in logistic regression analyses. Results showed that attendance was highly related to abstinence at both time points. For Maintenance Calls, proportion of contacts significantly predicted abstinence at 8 weeks (B=2.454, SE=.416, Wald χ2(1) = 34.74, p<.001, OR =11.63; N=247) and at 16 weeks (B=3.021, SE=.447, Wald χ2(1) = 45.63, p<.001, OR=20.51; N=247). For Adherence Calls, proportion of contacts likewise significantly predicted abstinence at 8 weeks (B=.795, SE=.340, Wald χ2(1) = 5.47, p=.019, OR=2.22; N=257) and at 16 weeks (B=.811, SE=.352, Wald χ2(1) = 5.32, p=.021, OR=2.25; N=257).
In the analyses conducted above, participants missing from follow-up were assumed to be smoking. This may have inflated the obtained association: a pattern of nonattendance across both the treatment sessions and outcome would have perforce created a strong association. Therefore, we examined the association between attendance and abstinence amongst only those who attended the 8- and 16-week abstinence assessment sessions (the criterion). This conservative analysis revealed that non-completion of calls was again related to decreased abstinence, although the magnitude of the relation was reduced. For abstinence status at 8 weeks and 16 weeks, respectively, results were: for Maintenance Calls, (n=227; B=1.83; SE=.43; OR=6.2; p<.001, and n=198; B=2.41; SE=.48; OR=11.12; p<.001); and for Automated Adherence Phone Calls (n=232; B=.26; SE=.39; OR=1.3; p=.51, and n=202; B=41; SE=.41; OR=1.50; p=.32).
Pursuing the Mechanism Overlap and Capacity Limitation Hypothesis
Mechanism Overlap Analyses.
The nonadditive mechanism hypothesis is examined in part by comparing the diminishing returns on putative treatment mechanisms that occur when active intervention components are combined. We examined this hypothesis in two ways. First, we examined whether adding especially dissimilar components affected the magnitude of the effect of adding components. We assumed that highly dissimilar components would be more likely to activate different change mechanisms, which, in theory, should produce less redundancy. Specifically, we analyzed whether adding a medication component to counseling components would produce meaningfully less reduction in effect size than when counseling components were added to one another. This derives support from evidence that counseling tends to affect mechanisms such as spending less time in the proximity of cigarettes and stress and urge coping but has little effect on withdrawal symptoms (McCarthy et al., 2010). On the other hand, medication seems to especially affect craving, which then affects cessation outcomes (e.g., Bolt, Piper, Theobald, & Baker, 2012; Piper et al., 2008). This effect was tested via an interaction between a medication factor vs. a counseling factor when each was added to a counseling component. The Maintenance Study offered the opportunity to examine the effects of a medication component (Medication Duration) when it was added to combinations of 1-4 counseling components.
We computed a logistic regression model predicting 52-week abstinence as a function of the randomly assigned treatment components. The model used dummy coding of predictors to allow the estimated coefficients to reflect the incremental contributions provided by components above a reference condition in which all components are in the “OFF” condition. We entered three sets of predictors: (1) five predictors reflecting the OFF/ON state of each of the individual components, (2) four predictors indicating the change in cumulative effect associated with having two or more, three or more, four or more, or five components ON (irrespective of which components are ON), and (3) three interactions between the Medication Duration predictor and the latter change in cumulative effect predictors (ignoring the interaction with the five components ON predictor due to redundancy). Importantly, the inclusion of the first set of predictors allows us to accommodate the likely occurrence that the five different components will vary in their first-order effects (i.e., that the individual effects of turning each component ON in isolation from the others will be different). The second set of predictors evaluates the subadditivity of turning more components ON, but now under conditions when Medication Duration is not involved (due to the presence of the third set of predictors). It is ultimately the third set of predictors that characterizes how the effects associated with turning more components ON changes when Medication Duration is ON.
Mechanism Overlap Results.
Table 6 shows the results of this analysis. Consistent with our prior analyses, we observe positive coefficients associated with each individual component variable when it is ON and the other components are OFF. However, the estimates associated with our second set of predictors indicate that turning more than one component ON, on average, results in a reduction of the effects otherwise implied by the first set of predictors. The next set of predictors is most relevant to the Mechanism Overlap hypothesis, which if correct, should manifest as positive coefficients when Medication is the added component (less reduction in the combined effects). While adding Medication Duration produced modestly positive coefficients for the 2 and 3 component combinations, it produced a relatively large negative coefficient when added to 4 counseling components. Thus, there may be less “cost” of adding a medication component to counseling when the overall number of ON components is smaller. However, the presence of the medication component seems even more prone to yield a reduction of effect in the presence of a large number of counseling components. Finally, Medication Duration had the largest impact on abstinence as a sole ON component; thus, the overall effectiveness of Medication Duration may have affected its competitiveness with other intervention components.
Table 6.
The Effects of Adding Different Numbers of Intervention Components as Related to 52-Week Abstinence in the Maintenance Study (N=513) Focusing on Interactions with and Without Medication Duration
| Intervention Effect | B | S.E | Wald | p-value |
|---|---|---|---|---|
| Constant | −.693 | .548 | 1.602 | .206 |
| Medication Duration (26 versus 8 weeks) | .944 | .744 | 1.610 | .204 |
| Maintenance Counseling (On versus Off) | .696 | .639 | 1.189 | .276 |
| Medication Adherence Counseling (On versus Off) | .411 | .636 | .418 | .518 |
| Automated Adherence Calls (On versus Off) | .792 | .643 | 1.516 | .218 |
| Electronic Medication Monitoring (With versus Without Printouts/Associated Counseling) | .643 | .642 | 1.005 | .316 |
| Two or More Intervention Components | −1.094 | .821 | 1.775 | .183 |
| Three or More Intervention Components | −.406 | .714 | .324 | .569 |
| Four or More Intervention Components | −.195 | .878 | .049 | .824 |
| Five Intervention Components | −.791 | .527 | .729 | .393 |
| Medication Duration with 2 or More Intervention Components | .093 | .682 | .019 | .892 |
| Medication Duration with 3 or More Intervention Components | .226 | .506 | .200 | .655 |
| Medication Duration with 4 or More Intervention Components | −.627 | .717 | .764 | .382 |
Likelihood Ratio (LR) χ2=15.37, df=12, p=.222 ; Nagelkerke R2 =.04
The second approach to examining the mechanism overlap hypothesis was to determine whether adding intervention components produces evidence of nonadditivity with regard to the putative mechanisms of therapeutic effectiveness. If combining different intervention components does not add cumulative benefit with regard to mechanisms of change, then this could cause subadditive returns on clinical outcomes. In addition, if there is evidence of redundancy with regard to mechanisms that are indexed as continuous variables, then these variables might serve as proxies for continuous outcome variables, suggesting that the occurrence of diminished returns is not restricted to binary outcomes.
Although the ability to formally test the relations between numbers of active components and the putative mediators is subject to the same limitations as for outcomes (i.e., relatively small numbers of subjects with few (0,1) or many (4,5) active components), the results of this analytic approach are generally consistent with the hypothesis that the tested intervention components yield redundant effects on putative mechanisms. We evaluated how adding components affected putative mediators, ones often affected by smoking treatment and that often predict smoking abstinence: i.e., measures of withdrawal (e.g., PANAS Distressed, PANAS Irritable, PANAS Upset, the PANAS Negative Affect composite, and WSWS Craving; Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Bolt et al., 2012; Etcheverry et al., 2016; Piper et al., 2008; Watson, Clark, & Tellegen, 1988), as well as motivational measures of cessation fatigue, and self-efficacy (Gwaltney, Shiffman, Balabanis, & Paty, 2005; McCarthy et al., 2010). These variables were assessed in three contacts that occurred in the first two weeks after the target quit day.
Table 7 shows the mean ratings on the various mechanism measures in relation to the different numbers of active components. In effect, these data provide an initial look at the “A” path in a mediational pathway relating treatment to the putative mediators. In general, across all mechanisms, except for self-efficacy, the pattern is that these scores are highest (at their worst) when no active components are on. These scores tend to improve when one component is added but remain stable thereafter (as more components are added), albeit there is a trend for craving to be especially low when 4 and 5 components are used. It must be remembered though, that only a very small number of participants received all 5 components, so the mean for this cell might be imprecise. With regard to self-efficacy, higher scores reflect treatment benefit. Results show essentially no evidence that adding intervention components increased self-efficacy. Thus, in no case did a greater number of intervention components improve status on the putative mediators, a finding confirmed with model based analysis (data not shown): single components produced increased benefit but additional components yielded diminishing or negative effects.
Table 7.
Means/Standard Deviations of Putative Mediators as a Function of Number of Intervention Components a Participant Received in the Maintenance Study (N=513)
| Putative Mediator Mean (Standard Deviation) Across Visits 2-4 | |||||||
|---|---|---|---|---|---|---|---|
| Number of Active Intervention Components |
PANAS Distressed |
PANAS Irritable |
PANAS Upset |
Negative PANAS |
Craving | Cessation Fatigue |
Self- Efficacy |
| 0 | 2.433 (1.374) |
2.622 (1.214) |
2.200 (1.187) |
7.256 (3.580) |
2.139 (1.344) |
2.889 (1.412) |
6.433 (.811) |
| 1 | 2.133 (1.059) |
2.406 (1.132) |
2.051 (1.018) |
6.589 (2.972) |
2.119 (1.046) |
2.500 (1.359) |
6.164 (1.126) |
| 2 | 2.247 (1.092) |
2.552 (1.071) |
2.092 (1.097) |
6.892 (3.001) |
2.075 (1.116) |
2.261 (1.325) |
6.431 (.819) |
| 3 | 2.292 (1.108) |
2.522 (1.150) |
2.207 (1.166) |
7.020 (3.103) |
2.153 (1.059) |
2.328 (1.379) |
6.277 (.900) |
| 4 | 2.078 (.938) |
2.493 (1.079) |
1.998 (.944) |
6.576 (2.699) |
1.983 (.912) |
2.241 (1.129) |
6.475 (.736) |
| 5 | 2.258 (1.042) |
2.258 (.976) |
2.076 (.758) |
6.591 (2.736) |
1.697 (.875) |
3.091 (1.892) |
6.348 (.634) |
The mechanism overlap hypothesis is based on the notion that treatment mechanisms affect outcome (abstinence) but because added components do not yield additive effects on mechanisms they do not yield additive effects on outcomes. To determine whether the mechanism measures predict outcomes, we related the mechanism measures (averaged over the first two weeks post-quit) with 52-week abstinence via univariate logistic regression. The relations were examined for only three of the mechanism measures; ones that were selected a priori based upon their addressing somewhat different conceptual domains and ones that showed strong patterns of subadditivity in relation to added components: 1) Craving, b=−.773, se=.111, Wald = 48.080 (df=1), p<.001, exp(b)=.462; Cessation fatigue, b=−.281, se=.079, Wald = 12.590 (df=1), p<.001, exp(b)=.755; and PANAS-negative affect, b=−.229, se=.040, Wald = 33.515 (df=1), p<.001, exp(b)=.795. For all three measures, a negative b coefficient reflects a negative effect on abstinence.
There is one other caveat regarding the analysis results shown in Table 7; several of the intervention components in this study are of long duration (e.g., Maintenance Counseling, Adherence Phone Calls, Extended Medication). Thus, the full differential effects of these intervention components would not have been detected when the mediators were assessed.
Pursuing the Tipping Point Hypothesis
Tipping Point Analysis.
The tipping point notion holds that for individuals near a tipping point, their status on promoters and obstacles allows them to make greater gains in response to modest treatment and gain can be conferred by a variety of interventions. Individuals who are distant from the tipping point may benefit from increased numbers of intervention components but the gains will be modest given their distance from the tipping point.
Statistically, this hypothesis is best explored from a perspective of treatment effect heterogeneity, whereby the effects of manipulated treatment factors vary across participants within a studied population when studied on a logit metric. We suspect that such heterogeneity may correspond to stronger positive factor effects for participants with a higher baseline likelihood of abstinence who are exposed to a small number of treatment component factors. At the other extreme, we suspect the presence of a subpopulation of participants for whom both (1) the likelihood of a successful outcome is low when exposed to a small number of intervention components (or to a low intensity intervention), and (2) adding components (or treatment intensity) will boost benefit only modestly.
To evaluate the tipping point hypothesis, we developed a change index to determine proximity to the tipping point. This index was developed in a separate MOST screening experiment that has not been included in the analyses presented thus far. This experiment was a 6-factor fractional factorial experiment of smoking intervention components designed to prepare smokers for cessation and help them quit (N=637; Piper et al., 2016). Multiple variables were examined to identify those that efficiently predicted smoking status at 26-week follow-up in that experiment. Thus, this approach used baseline and early post-quit predictors of ultimate clinical success to index capacity to quit successfully (tipping point proximity). Candidate variables included demographic variables (e.g., race, age, gender), nicotine dependence measures (e.g., the Fagerstrom Test of Cigarette Dependence; Fagerstrom, Russ, Yu, Yunis, & Foulds, 2012; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), the Wisconsin Inventory of Smoking Dependence Motives (Piper et al., 2004; Smith et al., 2010), contextual factors (e.g., presence of other smokers in the home, a home smoking ban), and early reaction to abstinence (smoking, withdrawal). There is considerable evidence that such factors affect likelihood of success in quitting smoking (e.g., Bolt et al., 2009).
Tipping Point Results.
We used the SAS logistic regression procedure PROC HPLOGISTIC to identify the best-fitting predictors of 26-week abstinence with selection based on Schwarz’s Bayesian criterion (SBC), which is intended to reduce multicollinearity. This procedure yielded a best-fitting model comprising gender, successful prior abstinence from smoking (> 1 month), and number of abstinent days in the first 2 weeks (a ROC analysis yielded an optimal cut-score =12 days). All three variables significantly predicted 26-week abstinence (p’s<007). When summed, this index of binary risk variables yielded scores = 0-3 (n’s: 0=100, 1=257, 2=201, 3=79). This change, risk index successfully predicted abstinence in the experimental sample in which it was derived; 26-week abstinence rates for scores 0-3 were 46%, 33.5%, 17.9%, and 12.7%, respectively. A binary version of this change index was formed by combining 0 & 1 (Low Risk) and 2 & 3 (High Risk). This yielded abstinence rates of 37% and 16.4% (χ2 with continuity correction = 31.9, p<.0001).
This change index was then used to predict 26-week abstinence in the Maintenance experiment (Schlam et al., 2016). Table 8 presents the abstinence rates of those randomized to various numbers of intervention components. Those getting 4 and 5 components were merged because of the small n’s. Results are therefore presented for participants randomized to 0-2 components, 3 components, 4-5 components, and 3-5 components (see Table 8). Comparing the last category with those randomized to 0-2 components shows that Low Risk participants showed virtually no improvement due to increased (3-5) components (OR=1.07; 95% CI: 0.65-1.76). High Risk participants on the other hand showed relatively little success with 0-2 components and only modest, statistically nonsignificant increases in success with added components (OR=1.67; 95% CI: 0.98-2.84).
Table 8.
Abstinence and Smoking Rates for Participants in the Maintenance Study (N=513) Who Were Categorized on the Basis of a Change Index Intended to Measure Likelihood of Change
| Abstinence Rates | Smoking Rates | |||
|---|---|---|---|---|
| Number of Intervention Components |
Low Risk, High Change Likelihood |
High Risk, Low Change Likelihood |
Low Risk, High Change Likelihood |
High Risk, Low Change Likelihood |
| 0-2 | 49.2% (58/118) |
24% (33/137) |
50.8% (60/118) |
75.9% (104/137) |
| 3 | 54.5% (42/77) |
32.6% (30/92) |
45.5% (35/77) |
67.4% (62/92) |
| 4-5 | 45.1% (23/51) |
39.5% (15/38) |
54.9% (28/51) |
60.5% (23/38) |
| 3-5 | 50.8% (65/128) |
34.6% (45/130) |
49.2% (63/128) |
65.4% (85/130) |
Note: The change index used comprised gender (female = 1), successful prior abstinence from smoking (> 1 month), and the number of abstinence days in the first 2 weeks. The change index score was dichotomized to low vs. high based on a receiver operating characteristic curve (ROC) analysis, which yielded a cut-off score = 12.
An assumption of the tipping point theory is that even modest intervention is enough to help low-risk individuals become abstinent. Therefore, one should see some increase in abstinence when such individuals receive 1 or 2 intervention components versus none. Unfortunately, there are too few individuals in the “no intervention” condition to afford much confidence in the pattern obtained. However, abstinence rates (and “n’s” for the number of participants in each component grouping) for the 0, 1, and 2 component groupings are, respectively: 29.4% (17), 42.5% (73), and 33.3% (165). Thus, there was evidence of some increase in abstinence due to assignment to one component to low-risk individuals, but a decrease from that level amongst those assigned to two components.
Just to ensure that the pattern of results obtained was not due to use of a particular change index, we derived a new change index using data from the Maintenance experiment itself. The use of this index produced the same pattern (data not shown) as obtained with the first index as reported above. Low-risk individuals did benefit from a small number of intervention components (0-2) with no meaningful increase from a greater number. High-risk individuals benefitted modestly from a greater number of components (3+).
One assumption of the tipping point hypothesis is that low-risk individuals will generally benefit from any of the active intervention components. This implies that some individuals respond similarly to the different intervention components, which is also consistent with components being redundant with one another. To test this, we examined correlations between adherence to one component and adherence to another (attendance/adherence scores were indexed by proportions of visits or component use episodes completed: Supplemental Note 6 and Table) for participants who were randomized to all possible pairs of intervention components. This analysis showed generally strong associations in attendance or adherence scores across pairs of components. Moreover, the associations are of roughly the same magnitude, suggesting the likely presence of a general adherence factor, a factor that may well also be positively associated with individuals close to the tipping point. This suggests a certain interchangeability of intervention components, but it does not necessarily indicate a high correlation between components with regard to their effects on abstinence.
General Discussion
Discussion of Evidence of Subadditive Effects
The relatively frequent occurrence of negative interactions amongst factors seen in inferential tests (e.g., Figures 1 & 2) caused us to examine systematically the effects of combining components. In three factorial experiments, we obtained Model Based estimates of the effects of adding up to four or five intervention components with a base treatment or no treatment. Across three studies, these analyses showed that adding components, on average, produced reductions in component effects relative to their effects if used individually. Across the three studies there were 18 component coefficients for the first-order effects and all were positive; there were also 14 coefficients for the component combinations and all were negative (including quit attempts at both 6 and 12 weeks in the Motivation Study). Modeling analyses suggest that the cumulative effects of negative interactions, including many not individually statistically significant, were cumulative up through the highest number of components, such that larger component packages continued to result in diminished contributions from added components.
Along with finding multiple negative interactions in our experiments (Cook et al., 2016; Fraser et al., 2014; Schlam et al., 2016), we found few significant positive main effects (in the original effect coding analyses or the dummy coding analyses with a single component ON). Similarly, McClure and her colleagues (McClure, et al., 2014) conducted a factorial experiment analyzing 4 factors comprising different design features of on-line smoking interventions (e.g., message tone, navigational autonomy, email reminders, and tailored testimonials). Despite considerable power (N=1865), this study yielded no main effects for any of the factors (McClure, et al., 2014). A factorial study by Tombor et al. (2018) showed no significant main effects (N=565) for five intervention modules designed to increase digital treatment engagement amongst pregnant smokers.
It should be noted that these findings were obtained in studies that differed with regard to type of outcome (quit attempts, smoking reduction, abstinence), population (those wanting to quit and those not willing to do so), types of interventions (pharmacotherapy, type of counseling, duration of treatment), and time period of assessment (4-52 weeks). Thus, at least with regard to smoking treatment, the phenomenon of subadditive returns seems fairly robust.
Finally, our results showed some variation across particular component combinations. This suggests that intensive research might be aimed at identifying such especially effective combinations. However, such combinations may be quite rare. After all, we see little evidence of synergistic interactions in the original effect coding analyses (e.g., Cook et al., 2016) and we found significant variability in component effectiveness for only one component combination in the present analyses (in the Maintenance Study) and this may be fortuitous.
The current analyses suggest that the lack of main effects in the reports of the above experiments occurs because the main effect estimates are based heavily on conditions in which other studied factors are also turned ON. In the presence of negative interactions (many of which may fall below the threshold of statistical significance), the main effect estimates may underestimate the effect of turning ON only the studied factor while keeping all other components OFF. This is consistent with prior modeling that shows that even modest negative interaction effects have the potential to bias and considerably diminish power to detect main effects (Brittain & Wittes, 1989).
While factorial experiments are powerful tools for exploring how well intervention components work together (Baker et al., 2016; Baker et al., 2017; Collins, 2018; Collins et al., 2014), it may be challenging to develop more effective psychosocial treatments by combining multiple intervention components that all exert significant main effects or positive interactions (see Chakraborty, Collins, Strecher, & Murphy, 2009; Collins et al., 2014) since they may be both rarely found and often underestimated. Collins (2018) has noted that the use of factorial designs in MOST arises in part from their successful use in engineering. However, the manipulated variables in engineering (e.g., speed, temperature) possess an independence that cannot be matched by counseling approaches that share elements in common. Moreover, cognitive processing may integrate the effects of different treatments so that they are correlated across treatments. Finally, manipulated variables in engineering research may simply be more potent.
It is important to note that it is certainly possible that synergistic or positive interactions may occur (e.g., Graham et al., 2020). Such interactions are more likely to be found where the intervention components do not yield a pattern of negative interactions (whether significant or not). In the Graham study just cited, the components were structural features of a digital intervention that involved separate smart message service functionalities. Such features may resemble those used in engineering studies where factorial experiments have been so valuable: i.e., where factors are more dissimilar and yield more divergent effects on mediators. Perhaps an evaluation of such properties should guide the selection of intervention components in behavioral research.
It is possible that we analyzed components that were too weak to produce numerous main effects or synergies. However, the components evaluated were selected based on prior research support and had strong substantive bases. For instance, the three analyzed studies explored extended medication, electronic medication tracking with counseling, extended counseling support, medication use with those unwilling to quit, and so on. All of which have considerable research support based on RCTs (e.g., Hall et al., 2009; Mooney et al., 2007; Moore et al., 2009; Schlam et al., 2018; Schnoll et al., 2015; Schnoll et al., 2010). These intervention components also seem substantively appropriate. For instance, extended medication was intended to allow participants to recover from lapses (Ferguson, Gitchell, & Shiffman, 2012), reduce late onset withdrawal exacerbations (Piasecki, Jorenby, Smith, Fiore, & Baker, 2003), and mitigate the effects of premature medication discontinuation (Medioni, Berlin, & Mallet, 2005). Extended counseling was intended to provide the social support and self-efficacy, which have been associated with positive clinical outcomes (Graham et al., 2015; Hendricks, Delucchi, & Hall, 2010; Schuck, Otten, Kleinjan, Bricker, & Engels, 2014). Similar evidence can be marshalled for the other intervention components used in the analyzed studies.
It is important to recognize that the subadditive effects we demonstrate above extend to non-factorial experimental designs; they are potentially relevant to any multicomponent treatment. Negative interactions amongst components may occur even if the experimental design does not permit their analysis. Thus, our results suggest that, in general, multicomponent treatments are likely to have their effectiveness constrained by negative interactions. Since such treatments are common in the mental and behavioral health fields, this phenomenon might have pervasive effects.
Discussion of Possible Mechanisms
Before discussing the particular mechanisms evaluated in this research, it is important to note that the finding of noncomplementary effects was not pre-ordained. It is quite reasonable to assume that, for instance, to the extent that a person has their smoking withdrawal symptoms mitigated by cessation medication, they will be better able to benefit from counseling. However, additive or synergistic effects were generally not observed.
The burden hypothesis.
The burden hypothesis holds that administering multiple intervention components concurrently can produce aversive effects such as fatigue or distraction, either of which might cause nonadherence or reduced participation in a treatment, which could in turn suppress intervention effectiveness. We evaluated the burden hypothesis in the Maintenance experiment by examining whether adherence to, or participation in, intervention components was affected by experimental assignment to additional components. We specifically addressed whether adherence to two fairly intensive components (Maintenance Counseling and Adherence Phone Calls) was eroded when increasing numbers of components were added to them.
A decline in attendance was evident for Adherence Phone Calls, for Maintenance Counseling, and for the combination of the two when other components were added to them. The decline was greater for Adherence Phone Calls, perhaps because this component involved no person-to-person interaction, making it easier to not attend.
While receiving many components was related to relatively poor adherence, it is unclear that this difference accounts for the component subaddivity. Adherence was meaningfully related to abstinence outcome but this relation may reflect bidirectional effects as some individuals might miss treatment because they were relapsing. Clearly, more research is needed to determine how burden might constrain multicomponent treatment effectiveness.
In sum, this research showed that: 1) an increasing number of co-occurring intervention components was generally associated with reduced attendance or adherence across the set of intervention components, and 2) burden-related nonadherence was associated with declines in the abstinence rates that occurred with added components (as per Fraser et al., 2014).
The mechanism overlap and capacity limitation hypothesis.
There was evidence that the second hypothesized mechanism, mechanism overlap and capacity limitation, may have significantly affected the impact of added components. Redundant effects on mechanisms were indexed by the extent to which adding components produced diminished improvement with regard to status on likely mechanisms.
An initial step was to assemble an a priori set of relevant putative component mechanisms based on previous research (i.e., relevant to the types of intervention components involved: Bolt et al., 2012; Gwaltney et al., 2005; McCarthy et al., 2010; Piper et al., 2008). The results showed that receipt of a single component reliably enhanced status with regard to virtually all mechanisms but adding two or more components did little to enhance such status. Thus, the findings of subadditive effects amongst components in terms of abstinence were echoed with regard to their effects on mechanisms and therefore serve as another indication of the robustness of the ‘diminishing returns’ effect. Subadditive returns were somewhat greater when the added components are ones thought to operate via similar versus dissimilar mechanisms (i.e., when counseling components are added to one another versus counseling added to a pharmacotherapy component). These results show that the various intervention components may exert similar effects in the “a” path of a mediational model. This pattern of effects on putative mechanisms could explain the subadditive effects pattern found in abstinence if the mechanism measures themselves are related to abstinence. Our analyses show that they significantly predict 52-week abstinence.
It is important to note that the intervention components when presented individually did, in fact, affect mechanisms positively, suggesting that the mechanisms were relevant to the nature of the interventions. However, the effects of the intervention components on the putative mechanisms were not additive when the components were combined. This may speak to the limited capacity mechanism thought to be at work; i.e., certain natural constraints, perhaps even homeostatic mechanisms (e.g., Fletcher, 1940; Solomon & Corbit, 1974), place a ceiling on change as components are combined.
Multiple reasons could be offered to account for the noncomplementary effects of components on mechanisms; e.g., different intervention components might exert effects via a single route such as self-efficacy or therapeutic alliance (e.g., Laska et al., 2014; Wampold & Imel, 2015). However, such accounts would not appear to explain the increasing subadditivity that occurs with each added component in Model-Based analyses. Thus, diminishing returns of added components may reflect one factor that lessens component effects (e.g., limited capacity change mechanisms) and another that antagonizes the effects of other components (e.g., burden).
The tipping point hypothesis.
The tipping point hypothesis holds that only a portion of participants in a trial or experiment can benefit from treatment; i.e., their status on risk factors and assets makes success (e.g., attaining abstinence) relatively likely or feasible. Thus, a sample’s status on a risk index may impose a relatively low ‘ceiling’ on change. This hypothesis also holds that participants near the tipping point will benefit from a variety of individual intervention components (a similarity in response to intervention components is suggested by correlations in adherence or contact rates across components: Supplemental Note 6).
To test this, we developed a change index that was designed to assess obstacles to successful quitting and that predicted abstinence status in a derivation sample. The risk index comprised variables similar to those that have predicted relapse risk in past research: i.e., ones reflecting contextual risk, prior quitting success, and early success (e.g., Bolt et al., 2009; Kenford et al. 1994). The inclusion of a variable that reflects early response to treatment reflects the assumption that tipping point proximity has consequences for better treatment utilization. When used in the Maintenance Study we found that participants who were at low risk for cessation failure according to the change index appeared to benefit from receiving one intervention component versus none, but there was no evidence that they achieved higher abstinence rates due to the receipt of 2 or more components. In contrast, those at high risk required a greater number of components to achieve even modest benefit. These results are in general agreement with the tipping point hypothesis. The interchangeability of intervention components for low-risk individuals (i.e., almost any sort of single component will tend to be effective) may increase the likelihood of negative interactions when multiple components are used (Brittain & Wittes, 1989; Green, Liu, & O'Sullivan, 2002), thereby eroding the benefits of added components for this group. Relatedly, the capacity limits of mechanisms might also reduce the benefit of added components if 1-2 components achieve strong effects in the low-risk group.
There are weaknesses in our analysis of the tipping point hypothesis. For instance, the change index was primarily focused on weaknesses or risks, not assets. Also, it is important to recognize that the risk index is, no doubt, an imperfect approximation to the latent dimension reflecting tipping point distance. Thus, for instance, some individuals designated as low risk were, no doubt, far from the tipping point, and therefore, difficult to change. It is also important to remember that high risk individuals would need more intervention components (than do low-risk individuals) to be moved closer to the logit inflection point assuming at least some additive benefit of some components (e.g., if 1-2 components did not ‘saturate’ change mechanisms as they did for low-risk individuals). This is because of the smaller impact of change in abstinence likelihood despite equivalent change in the logit metric amongst those distant from the tipping point.
The tipping point model seems especially relevant to instances where there is a binary outcome (abstinent vs. smoking). However, it can be examined with continuous outcomes as well, using methods similar to those modeled in this paper.
Limitations
There were few participants in key experimental conditions (e.g., those getting zero components), which limits the power or informativeness of some analyses. Also, the analyses of putative mediators were limited to general symptomatic or motivational dimensions; it is quite possible that there would have been less evidence of component overlap had measures of more specific mechanisms been available. Another major limitation is that the possible causes of such subadditivity were explored using data from a single experiment. In addition, long-term abstinence self-reports were not biochemically verified. Finally, this research involved limited ranges of participants, outcomes, and experimental designs. Thus, it is unknown if different types of intervention strategies (e.g., adaptive designs, chronic care, tailored approaches) would demonstrate subadditive effects to the same degree. For instance, there is evidence that nontailored interventions may be more susceptible to subadditive effects than are tailored or personalized interventions (Ray et al., 2014). Therefore, the generalizability of the subadditivity across different populations, change goals, and intervention types is an important target for future research. Also, the reviewed studies manipulated intensity via number of intervention components; future research should explore whether the limiting mechanisms examined in this paper constrain the effects of more intense versions of single intervention components. The general strategies that we have used to explore the causes of subadditivity might reveal why such a pattern is found with some intervention approaches and not others.
Implications
This research shows that across multiple factorial experiments, we observed a pattern such that when components were combined they yielded less benefit than the sum of their individual effects. This pattern was attributed to negative interactions amongst components that occurred across multiple experiments, change measures, and intervention types. Moreover, the magnitude of this effect and its consequences are highly meaningful; on average, such ‘costs’ grew with each component added and the resulting effect was often sufficient to eliminate any benefit of an added component(s). Moreover, these negative interactions may suppress estimates of component main effects when multiple components are used together.
Hypothesis driven analyses suggested that several phenomena contribute to the observed a pattern of negative interactions. First, there was evidence that adding intervention components may in some cases increase burden, broadly defined, such that individuals are less likely to attend or adhere to added interventions. Second, there was evidence that intervention components exerted overlapping effects on putative change mechanisms such that intervention components tended to affect the same mechanisms, and did so in a subadditive manner. Thus, a functional redundancy amongst intervention components may have reduced complementary or additive effects amongst them. Finally, we found evidence that supported a tipping point hypothesis; that is, individuals with few risks of failure appeared to be near a hypothetical tipping point of success as manifested by relatively high success rates resulting from exposure to just one or two intervention components. Conversely, ‘high risk’ individuals benefited little from few intervention components and showed only modest benefit from several.
This research cannot conclusively confirm or disconfirm any of the hypothesized causes of subadditivity and multiple processes may operate simultaneously. Therefore, the chief contributions of this research may be: demonstration of this novel constraint on treatment improvement; its heuristic value in advancing hypotheses about this constraint; and its demonstration of methods to evaluate mechanisms that might produce it.
Finally, this research has implications for treatment development. If intervention components do frequently interact negatively, then future efforts to develop more effective treatments should carefully evaluate every intervention component that might be included in a treatment package via screening experiments (Collins, 2018) to examine their interaction potential and their effects on key mechanisms. In particular, the assessment of intensity related burden (e.g., attentional demand, decision making requirements, information load, disruptions to daily life, time/contacts required) warrants additional research attention. Investigators might also wish to reduce the number of intervention components included in factorial designs in order to reduce the likelihood of negative interactions if they wish to determine intervention main effects without bias. This might reduce the efficiency of the design, but may enhance interpretability (Brittain & Wittes, 1989; Green, Liu, & O'Sullivan, 2002). Also, these results support the suggestion that promising intervention components can be identified via effect size estimates rather than by statistical significance (Collins et al., 2018); this will not reduce negative interactions but will shift focus from p-values to relative benefit. Further, existing treatment packages that contain numerous intervention components should have their constituent elements tested in factorial experiments insofar as some components might especially detract from treatment effectiveness due to especially large negative interactions with other components. Moreover, some of the analytic approaches used in the present research could be used to understand why more intensive delivery of single intervention components (e.g., longer duration counseling) does not yield commensurate benefit. If the tipping point hypothesis has merit, the development of improved risk indices might provide a useful basis for a treatment algorithm, precision medicine, or adaptive treatment, by identifying those who do, and those who do not, require more intensive intervention. Last, data consistent with the tipping point hypothesis encourage the strategy of cost-effectively increasing the reach of fairly non-intensive interventions targeted to low risk individuals likely to benefit from such interventions.
Supplementary Material
Acknowledgements.
This work was supported by National Cancer Institute grant P01 CA180945. The authors thank Wendy Theobald for her invaluable assistance with manuscript preparation.
References
- Baker TB, Collins LM, Mermelstein R, Piper ME, Schlam TR, Cook JW, … Fiore MC (2016). Enhancing the effectiveness of smoking treatment research: conceptual bases and progress. Addiction, 111(1), 107–116. doi: 10.1111/add.13154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, & Fiore MC (2004). Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev, 111(1), 33–51. doi: 10.1037/0033-295X.111.1.33 [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, Schlam TR, Cook JW, Smith SS, Loh WY, & Bolt D Are tobacco dependence and withdrawal related amongst heavy smokers? Relevance to conceptualizations of dependence. J Abnorm Psychol. 2012;121(4):909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Smith SS, Bolt DM, Loh WY, Mermelstein R, Fiore MC, … Collins LM (2017). Implementing clinical research using factorial designs: a primer. Behav Ther, 48(4), 567–580. doi: 10.1016/j.beth.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefoot JC, & Girodo M (1972). The misattribution of smoking cessation symptoms. Can J Behav Sci, 4, 358–363. [Google Scholar]
- Beneciuk JM, Hill JC, Campbell P, Afolabi E, George SZ, Dunn KM, & Foster NE (2017). Identifying treatment effect modifiers in the STarT Back Trial: a secondary analysis. J Pain, 18(1), 54–65. doi: 10.1016/j.jpain.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL (2010). Nicotine addiction. N Engl J Med, 362(24), 2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt N, Bolman C, Froelicher ES, Mudde A, Candel M, de Vries H, & Lechner L (2014). Effectiveness of a telephone delivered and a face-to-face delivered counseling intervention for smoking cessation in patients with coronary heart disease: a 6-month follow-up. J Behav Med, 37(4), 709–724. doi: 10.1007/s10865-013-9522-9 [DOI] [PubMed] [Google Scholar]
- Blankers M (2020). Commentary on Sundström et al. (2019): Digital interventions for alcohol problems—time for more research on blended therapy. Addiction, 115(5), 875–876. 10.1111/add.14962 [DOI] [PubMed] [Google Scholar]
- Bolt DM, Piper ME, McCarthy DE, Japuntich SJ, Fiore MC, Smith SS, & Baker TB (2009). The Wisconsin Predicting Patients' Relapse questionnaire. Nicotine Tob Res, 11(5), 481–492. doi: 10.1093/ntr/ntp030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt DM, Piper ME, Theobald WE, & Baker TB (2012). Why two smoking cessation agents work better than one: role of craving suppression. J Consult Clin Psychol, 80(1), 54–65. doi: 10.1037/a0026366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain E, & Wittes J (1989). Factorial designs in clinical trials: the effects of non-compliance and subadditivity. Stat Med, 8(2), 161–171. doi: 10.1002/sim.4780080204 [DOI] [PubMed] [Google Scholar]
- Carroll AJ, Mathew AR, Leone FT, Wileyto EP, Miele A, Schnoll RA, & Hitsman B (2020). Extended nicotine patch treatment among smokers with and without comorbid psychopathology. Nicotine Tob Res, 22(1), 24–31. https://doi:0.1093/ntr/nty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo RP (2019). Exploring the differential effects of social and individualistic gameplay motivations on bridging social capital for users of a massively multiplayer online game. Comput Human Behav, 91, 263–270. 10.1016/j.chb.2018.10.016 [DOI] [Google Scholar]
- Chakraborty B, Collins LM, Strecher VJ, & Murphy SA (2009). Developing multicomponent interventions using fractional factorial designs. Stat Med, 28(21), 2687–2708. doi: 10.1002/sim.3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambless DL, Milrod B, Porter E, Gallop R, McCarthy KS, Graf E, … Barber JP (2017). Prediction and moderation of improvement in cognitive-behavioral and psychodynamic psychotherapy for panic disorder. J Consult Clin Psychol, 85(8), 803–813. doi: 10.1037/ccp0000224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Wang H-y., Sigerson L, & Chau C-l. (2019). Do the socially rich get richer? A nuanced perspective on social network site use and online social capital accrual. Psychol Bull, 145(7), 734–764. 10.1037/bul0000198 [DOI] [PubMed] [Google Scholar]
- Ciccolo JT; Williams DM; Dunsiger SI; Whitworth JW; McCullough AK; Bock BB; Marcus BH; Myerson M Efficacy of Resistance Training as an Aid to Smoking Cessation: Rationale and Design of the Strength To Quit Study. Mental Health Phys. Act 2014, 7, 95–103. 10.1016/j.mhpa.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM (2018). Optimization of behavioral, biobehavioral, and biomedical interventions: the Multiphase Optimization Strategy (MOST). New York, NY: Springer. [Google Scholar]
- Collins LM, Trail JB, Kugler KC, Baker TB, Piper ME, & Mermelstein RJ (2014). Evaluating individual intervention components: making decisions based on the results of a factorial screening experiment. Trans Behav Med, 4(3), 238–251. doi: 10.1007/s13142-013-0239-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Baker TB, Mermelstein RJ, Piper ME, Jorenby DE, Smith SS, … Fiore MC (2011). The Multiphase Optimization Strategy for engineering effective tobacco use interventions. Ann Behav Med, 41(2), 208–226. doi: 10.1007/s12160-010-9253-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Dziak JJ, & Li R (2009). Design of experiments with multiple independent variables: a resource management perspective on complete and reduced factorial designs. Psychol Methods, 14(3), 202–224. doi: 10.1037/a0015826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Collins LM, Fiore MC, Smith SS, Fraser D, Bolt DM, … Mermelstein R (2016). Comparative effectiveness of motivation phase intervention components for use with smokers unwilling to quit: a factorial screening experiment. Addiction, 111(1), 117–128. doi: 10.1111/add.13161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N (2010). The magical mystery four: how is working memory capacity limited, and why? Curr Dir Psychol Sci, 19(1), 51–57. doi: 10.1177/0963721409359277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LC, Hurt RD, Offord KP, Lawson GM, Croghan IT, & Schroeder DR (1995). High-dose nicotine patch therapy. Percentage of replacement and smoking cessation. JAMA, 274(17), 1353–1358. [PubMed] [Google Scholar]
- Damian RI, Su R, Shanahan M, Trautwein U, & Roberts BW (2015). Can personality traits and intelligence compensate for background disadvantage? Predicting status attainment in adulthood. J Pers Soc Psychol, 109(3), 473–489. 10.1037/pspp0000024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison GC, & Valins S (1969). Maintenance of self-attributed and drug-attributed behavior change. J Pers Soc Psychol, 11(1), 25–33. doi: 10.1037/h0027055 [DOI] [PubMed] [Google Scholar]
- Deci EL, & Ryan RM (1985). Intrinsic motivation and self-determination in human behavior. New York: Plenum. [Google Scholar]
- Driessen M, Schulz P, Jander S, Ribbert H, Gerhards S, Neuner F, & Koch-Stoecker S (2019). Effectiveness of inpatient versus outpatient complex treatment programs in depressive disorders: a quasi-experimental study under naturalistic conditions. BMC Psychiatry, 19, 380 (2019). 10.1186/s12888-019-2371-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy D, Enrique A, Connell S, Connolly C, & Richards D, Internet-delivered cognitive behavior therapy as a prequel to face-to-face therapy for depression and anxiety: A naturalistic observation, Front Psychiatry, 2020, 1–15. 10.3389/fpsyt.2019.00902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins RM, Gallo KP, Pincus DB, & Comer JS (2016). Moderators of intensive CBT for adolescent panic disorder: the of fear and avoidance. Child Adolesc Ment Health, 21(1), 30–36. doi: 10.1111/camh.12122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etcheverry PE, Waters AJ, Lam C, Correa-Fernandez V, Vidrine JI, Cinciripini PM, & Wetter DW (2016). Attentional bias to negative affect moderates negative affect's relationship with smoking abstinence. Health Psychol, 35(8), 881–890. doi: 10.1037/hea0000338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom K, Russ C, Yu CR, Yunis C, & Foulds J (2012). The Fagerstrom Test for Nicotine Dependence as a predictor of smoking abstinence: A pooled analysis of varenicline clinical trial data. Nicotine Tob Res. doi: 10.1093/ntr/nts018 [DOI] [PubMed] [Google Scholar]
- Fairburn CG, & Wilson GT (2013). The dissemination and implementation of psychological treatments: problems and solutions. Int J Eating Disord, 46(5), 516–521. doi: 10.1002/eat.22110 [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Gitchell JG, & Shiffman S (2012). Continuing to wear nicotine patches after smoking lapses promotes recovery of abstinence. Addiction, 107(7), 1349–1353. doi: 10.1111/j.1360-0443.2012.03801.x [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, … Wewers ME (2008). Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service. [Google Scholar]
- Fletcher H (1940). Auditory patterns. Rev Modern Physics, 12(1), 47–65. doi: 10.1103/RevModPhys.12. [DOI] [Google Scholar]
- Fraser D, Kobinsky K, Smith SS, Kramer J, Theobald WE, & Baker TB (2014). Five population-based interventions for smoking cessation: a MOST trial. Trans Behav Med, 4(4), 382–390. doi: 10.1007/s13142-014-0278-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary B, Walter ST, Leberg PL, & Karubian J (2019). Condition-dependent foraging strategies in a coastal seabird: Evidence for the rich get richer hypothesis. Behav Ecol, 30(2), 356–363. 10.1093/beheco/ary173 [DOI] [Google Scholar]
- Gladstone TR, Forbes PW, Diehl A, & Beardslee WR (2015). Increasing understanding in children of depressed parents: predictors and moderators of intervention response. Depress Res Treat, 2015, 347971. doi: 10.1155/2015/347971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow RE, & Estabrooks PE (2018). Pragmatic applications of RE-AIM for health care initiatives in community and clinical settings. Prev Chronic Dis, 15, E02. doi: 10.5888/pcd15.170271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Papandonatos GD, Cobb CO, Cobb NK, Niaura RS, Abrams DB, & Tinkelman DG (2015). Internet and telephone treatment for smoking cessation: mediators and moderators of short-term abstinence. Nicotine Tob Res, 17(3), 299–308. doi: 10.1093/ntr/ntu144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Papandonatos GD, Jacobs MA, Amato MS, Cha S, Cohn AM, … Whittaker R (2020). Optimizing text messages to promote engagement with internet smoking cessation treatment: results from a factorial screening experiment. J Med Internet Res, 22(4), e17734. doi) doi: 10.2196/17734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S, Liu PY, & O'Sullivan J (2002). Factorial design considerations. J Clin Oncol, 20(16), 3424–3430. doi: 10.1200/JCO.2002.03.003 [DOI] [PubMed] [Google Scholar]
- Gruder CL, Mermelstein RJ, Kirkendol S, Hedeker D, Wong SC, Schreckengost J, Warnecke RB, Burzette R, & Miller TQ (1993). Effects of social support and relapse prevention training as adjuncts to a televised smoking-cessation intervention. J Consult Clin Psychol, 61(1), 113–120. 10.1037/0022-006X.61.1.113 [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman S, Balabanis MH, & Paty JA (2005). Dynamic self-efficacy and outcome expectancies: prediction of smoking lapse and relapse. J Abnorm Psychol, 114(4), 661–675. doi: 10.1037/0021-843X.114.4.661 [DOI] [PubMed] [Google Scholar]
- Hajek P, Stead LF, West R, Jarvis M, Hartmann-Boyce J, & Lancaster T (2013). Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev, 8, CD003999. doi: 10.1002/14651858.CD003999.pub4 [DOI] [PubMed] [Google Scholar]
- Hall SM, Humfleet GL, Munoz RF, Reus VI, Robbins JA, & Prochaska JJ (2009). Extended treatment of older cigarette smokers. Addiction, 104(6), 1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldorsdottir T, & Ollendick TH (2016). Long-term outcomes of brief, intensive CBT for specific phobias: The negative impact of ADHD symptoms. J Consult Clin Psychol, 84(5), 465–471. doi: 10.1037/ccp0000088 [DOI] [PubMed] [Google Scholar]
- Harackiewicz JM, Sansone C, Blair LW, Epstein JA, & Manderlink G (1987). Attributional processes in behavior change and maintenance: smoking cessation and continued abstinence. J Consult Clin Psychol, 55(3), 372–378. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom KO (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Delucchi KL, & Hall SM (2010). Mechanisms of change in extended cognitive behavioral treatment for tobacco dependence. Drug Alcohol Depend, 109(1-3), 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Lesmes GR, Hatsukami DK, Richmond RL, Lichtenstein E, Jorenby DE, … Heatley SA (1999). Are higher doses of nicotine replacement more effective for smoking cessation? Nicotine Tob Res, 1(2), 169–174. [DOI] [PubMed] [Google Scholar]
- Insel TR (2009). Translating scientific opportunity into public health impact: a strategic plan for research on mental illness. Arch Gen Psychiatry, 66(2), 128–133. doi: 10.1001/archgenpsychiatry.2008.540 [DOI] [PubMed] [Google Scholar]
- James E, Gaskell M Gareth WA, & Henderson L (2017). Consolidation of vocabulary during sleep: The rich get richer? Neurosci Biobehav Rev, 77, 1–13. 10.1016/j.neubiorev.2017.01.054 [DOI] [PubMed] [Google Scholar]
- Judge T,A, & Hurst C (2008). How the rich (and happy) get richer (and happier): Relationship of core self-evaluations to trajectories in attaining work success. J Appl Psychol, 93(4), 849–863. 10.1037/0021-9010.93.4.849 [DOI] [PubMed] [Google Scholar]
- Jusko WJ (1989). Pharmacokinetics of capacity-limited systems. J Clin Pharmacol, 29(6), 488–493. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA. 1994;271(8):589–594. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Davis L, Strausberg L, & Varady A (1999). Do heavy smokers benefit from higher dose nicotine patch therapy? Exp Clinl Psychopharmacol, 7(3), 226–233. [DOI] [PubMed] [Google Scholar]
- Kopel S, & Arkowitz H (1975). The role of attribution and self-perception in behavior change: implications for behavior therapy. Genet Psychol Monogr, 92(Second Half), 175–212. [PubMed] [Google Scholar]
- Kugler KC, Dziak JJ, & Trail J (2018). Coding and interpretation of effects in analysis of data from a factorial experiment. In Collins LM and Kugler KC (Eds.) Optimization of beahvioral, biobehavioral, and biomedical interventions (pp. 175–206). Springer: New York. [Google Scholar]
- Lancaster T, & Stead LF (2005). Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev (2), CD001292. doi: 10.1002/14651858.CD001292.pub2 [DOI] [PubMed] [Google Scholar]
- Lancaster T, & Stead LF (2017). Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev (3), CD001292. doi: 10.1002/14651858.CD001292.pub3 [DOI] [PubMed] [Google Scholar]
- Laska KM, Gurman AS, & Wampold BE (2014). Expanding the lens of evidence-based practice in psychotherapy: A common factors perspective. Psychotherapy, 51(4), 467–481. 10.1037/a0034332 [DOI] [PubMed] [Google Scholar]
- Lotfizadeh AD, Kazemi E, Pompa-Craven P, & Eldevik S (2020). Moderate effects of low-intensity behavioral intervention. Behav Modif, 44(1), 92–113. doi: 10.1177/0145445518796204 [DOI] [PubMed] [Google Scholar]
- Loughead J, Falcone M, Wileyto EP, Albelda B, Audrain-McGovern J, Cao W, Kurtz MM, Gur RC, & Lerman C (2016). Can brain games help smokers quit?: Results of a randomized clinical trial. Drug Alcohol Depend,168, 112–118. 10.1016/j.drugalcdep.2016.08.621 [DOI] [PubMed] [Google Scholar]
- Ludden TM (1991). Nonlinear pharmacokinetics: clinical Implications. Clinl Pharmacokinet, 20(6), 429–446. doi: 10.2165/00003088-199120060-00001 [DOI] [PubMed] [Google Scholar]
- Mak MHC, Twitchell H (2020). Evidence for preferential attachment: Words that are more well connected in semantic networks are better at acquiring new links in paired-associate learning. Psychonomic Bull Rev. 10.3758/s13423-020-01773-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DJ, Garske JP, & Davis MK (2000). Relation of the therapeutic alliance with outcome and other variables: A meta-analytic review. J Consult Clin Psychol, 68(3), 438–450. 10.1037/0022-006X.68.3.438 [DOI] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Jorenby DE, Lawrence DL, Shiffman S, & Baker TB (2010). A multi-level analysis of non-significant counseling effects in a randomized smoking cessation trial. Addiction, 105(12), 2195–2208. doi: 10.1111/j.1360-0443.2010.03089.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure JB, Peterson D, Derry H, Riggs K, Saint-Johnson J, Nair V, … Shortreed SM (2014). Exploring the "active ingredients" of an online smoking intervention: a randomized factorial trial. Nicotine Tob Res, 16(8), 1129–1139. doi: 10.1093/ntr/ntu057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medioni J, Berlin I, & Mallet A (2005). Increased risk of relapse after stopping nicotine replacement therapies: a mathematical modelling approach. Addiction, 100(2), 247–254. doi: 10.1111/j.1360-0443.2004.00961.x [DOI] [PubMed] [Google Scholar]
- Miller WR, & Rollnick S (2002). Motivational interviewing: Preparing people for change (2nd ed.). New York: Guilford Press. [Google Scholar]
- Mooney ME, Sayre SL, Hokanson PS, Stotts AL, & Schmitz JM (2007). Adding MEMS feedback to behavioral smoking cessation therapy increases compliance with bupropion: A replication and extension study. Addict Behav, 32, 875–880. doi: 10.1016/j.addbeh.2006.06.022 [DOI] [PubMed] [Google Scholar]
- Moore D, Aveyard P, Connock M, Wang D, Fry-Smith A, Barton P Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ 2009; 338: b1024. doi: 10.1136/bmj.b1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash MC, Vickerman AK, Kellogg SE, & Zbikowski MS (2015). Utilization of a web-based vs. integrated phone/web cessation program among 140,000 tobacco users: An evaluation across 10 free state quitlines. J Med Internet Res, 17(2), e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MG, Shin KE, & Lanza ST (2019). Time-varying moderation of treatment outcomes by illness duration and comorbid depression in generalized anxiety disorder. J Consult Clin Psychol, 87(3), 282–293. doi: 10.1037/ccp0000385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, & Baker TB (2003). Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. J Abnorm Psychol, 112(1), 14–27. [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Collins LM, … Baker TB (2018). A randomized controlled trial of an optimized smoking treatment delivered in primary care. Ann Behav Med. 2018 Sep 13;52(10):854–864. doi: 10.1093/abm/kax059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, & Baker TB (2008). Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. J Abnorm Psychol, 117(1), 94–105. doi:doi: 10.1037/0021-843X.117.1.94 [DOI] [PubMed] [Google Scholar]
- Piper ME, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, … Baker TB (2016). Identifying effective intervention components for smoking cessation: a factorial screening experiment. Addiction, 111(1), 129–141. doi: 10.1111/add.13162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, & Baker TB (2004). A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68). J Consult Clin Psychol, 72(2), 139–154. doi: 10.1037/0022-006X.72.2.139 [DOI] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, & Baker TB (2009). A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry, 66(11), 1253–1262. doi: 10.1001/archgenpsychiatry.2009.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray AE, Kim S-Y, White HR, Larimer ME, Mun E-Y, Clarke N, … Huh D (2014). When less is more and more is mess in brief motivational interventions: Characteristics of intervention content and their associations with drinking outcomes. Psychol Addict Behav, 15(4), 1026–1040. doi: 10.1037/a0036593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, & Leo GI (2014). Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav, 25(1), 154–162. doi: 10.1037/a0030992 [DOI] [PubMed] [Google Scholar]
- Robinson BA, Winiarski DA, Brennan PA, Foster SL, Cunningham PB, & Whitmore EA (2015). Social context, parental monitoring, and multisystemic therapy outcomes. Psychotherapy (Chic), 52(1), 103–110. doi: 10.1037/a0037948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollnick S, Mason P, & Butler C (1999). Health behavior change: A guide for practitioners. Edinburgh: Churchill Livingstone. [Google Scholar]
- Romeo RR, Christodoulou JA, Halverson KK, Murtagh J, Cyr AB, Schimmel C, … Gabrieli JDE (2018). Socioeconomic status and reading disability: neuroanatomy and plasticity in response tointervention. Cereb Cortex, 28(7), 2297–2312. doi: 10.1093/cercor/bhx131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlam TR, & Baker TB (2020). Playing around with quitting smoking: A randomized pilot trial of mobile games as a craving response strategy. Games Health J. 9(1):64–70. 10.1089/g4h.2019.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlam TR, Cook JW, Baker TB, Hayes-Birchler T, Bolt DM, Smith SS, … Piper ME (2018). Can we increase smokers' adherence to nicotine replacement therapy and does this help them quit? Psychopharmacology (Berl), 235(7), 2065–2075. doi: 10.1007/s00213-018-4903-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlam TR, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, … Baker TB (2016). Comparative effectiveness of intervention components for producing long-term abstinence from smoking: a factorial screening experiment. Addiction, 111(1), 142–155. doi: 10.1111/add.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, Leone FT, … Hitsman B (2015). Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med, 175(4), 504–511. doi: 10.1001/jamainternmed.2014.8313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, & Lerman C (2010). Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med, 152(3), 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck K, Otten R, Kleinjan M, Bricker JB, & Engels RC (2014). Predictors of cessation treatment outcome and treatment moderators among smoking parents receiving quitline counselling or self-help material. Prev Med, 69, 126–131. doi: 10.1016/j.ypmed.2014.09.014 [DOI] [PubMed] [Google Scholar]
- Smith SS, McCarthy DE, Japuntich SJ, Christiansen B, Piper ME, Jorenby DE, … Jackson TC (2009). Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med, 169(22), 2148–2155. doi: 10.1001/archinternmed.2009.426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Piper ME, Bolt DM, Fiore MC, Wetter DW, Cinciripini PM, & Baker TB (2010). Development of the Brief Wisconsin Inventory of Smoking Dependence Motives. Nicotine Tob Res, 12(5), 489–499. doi: 10.1093/ntr/ntq032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits M, Feenstra D, Bales D, Blankers M, Dekker J, Lucas Z, Kamphuis JH, Busschbach JJV, Verheul R, & Luyten P (2020). Day hospital versus intensive outpatient mentalization-based treatment: 3-year follow-up of patients treated for borderline personality disorder in a multicentre randomized clinical trial. Psychol Med, 1–11. 10.1017/S0033291720002123 [DOI] [PubMed] [Google Scholar]
- Solomon RL, & Corbit JD (1974). An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev, 81(2), 119–145. doi: 10.1037/h0036128 [DOI] [PubMed] [Google Scholar]
- Swan GE, McClure JB, Jack LM, Zbikowski SM, Javitz HS, Catz SL, … McAfee TA (2010). Behavioral counseling and varenicline treatment for smoking cessation. Am J Prev Med, 38(5), 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombor I, Beard E, Brown J, Shahab L, Michie S, & West R (2018). Randomized factorial experiment of components of the SmokeFree Baby smartphone application to aid smoking cessation in pregnancy. Trans Behav Med, 9(4):583–593. doi: 10.1093/tbm/iby073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginneken CA, van Rossum JM, & Fleuren HL (1974). Linear and nonlinear kinetics of drug elimination. I. Kinetics on the basis of a single capacity-limited pathway of elimination with or without simultaneous supply-limited elimination. J Pharmacokinet Biopharm, 2(5), 395–415. [DOI] [PubMed] [Google Scholar]
- Wampold BE, & Imel ZE (2015). The great psychotherapy debate: the evidence for what makes psychotherapy work (2nd ed.). New York, NY: Routledge/Taylor & Francis Group. [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol, 54(6), 1063–1070. doi: 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.