Summary
Background
For many cardiovascular risk factors there is no lower limit to which further reduction will result in decreased disease risk; this includes values within ranges considered normal for healthy adults. This seems to be true for new emerging metabolic risk factors identified by innovative technological advances. Further, there seems to be ever evolving evidence of differential responses to lifestyle interventions by sex and body compositions in the normal range. In this secondary analysis, we had the opportunity to test these principles for newly identified molecular biomarkers of cardiometabolic risk in a young (21–50 years), normal weight healthy population undergoing calorie restriction for two years.
Methods
The Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE™) was a 24-month, multicenter, randomized controlled trial (May 2007-November 2012) in healthy, adults without obesity to evaluate the potential for calorie restriction (CR) to promote anti-aging adaptations, including those associated with disease risk. 218 participants (age 37.9 ± 7.2 years and body mass index (BMI) 25.1 ± 1.7 kg/m2, mean±SD) were randomized 2:1 to 24 months of CR (prescribed as 25% reduction from baseline calorie intake) versus ad libitum (AL). Fasting plasma from baseline, 12, and 24 months was used for assessments of lipoproteins, metabolites, and inflammatory markers using nuclear magnetic resonance spectroscopy.
Findings
Averaging 11.9% CR, the CR group had reductions at 12 and 24 months in the cardiovascular disease risk markers, apolipoprotein B and GlycA, and risks for insulin resistance and type 2 diabetes—Lipoprotein Insulin Resistance Index and Diabetes Risk Index (all PCRvsAL≤0.0009). Insulin resistance and diabetes risk improvements resulted from CR-induced alterations in lipoproteins, specifically reductions in triglyceride-rich lipoprotein particles and low-density lipoprotein particles, a shift to larger high-density lipoprotein particles (more effective cholesterol transporters), and reductions in branched chain amino acids (BCAAs) (all PCRvsAL≤0.004). These CR responses were more pronounced in overweight than normal weight participants and greater in men than women.
Interpretation
In normal to slightly overweight adults without overt risk factors or disease, 12 months of ∼12% CR improved newly identified risk markers for atherosclerotic cardiovascular disease, insulin resistance and type 2 diabetes. These markers suggest that CR improves risks by reducing inflammation and BCAAs and shifting lipoproteins from atherogenic to cholesterol transporting. Additionally, these improvements are greater for men and for those with greater BMIs indicating sex and BMI-influences merit attention in future investigations of lifestyle-mediated improvements in disease risk factors.
Funding
The CALERIE™ trial design and implementation were supported by a National Institutes of Health (NIH) U-grant provided to four institutions, the three intervention sites and a coordinating center (U01 AG022132, U01 AG020478, U01 AG020487 U01 AG020480). For this secondary analysis including sample acquisition and processing, data analysis and interpretation, additional funding was provided by the NIH to authors as follows: R01 AG054840 (MO, VBK); R33 AG070455 (KMH, DCP, MB, SBR, CKM, LMR, SKD, CFP, CJR, WEK); P30 DK072476 (CKM, LMR); and U54 GM104940 (CKM, LMR).
Keywords: Nuclear magnetic resonance spectroscopy, Cardiovascular disease, Type 2 diabetes risk, Insulin resistance
Research in context.
Evidence before this study
This is a secondary analysis of the only in-human randomized controlled intervention of medium term caloric restriction. Previous findings from this intervention showed that in young to middle aged, normal to slightly overweight adults, two years of ∼12% caloric restriction (CR) improved traditional cardiometabolic risk factors within ranges considered normal. These improvements suggested modest lifestyle interventions in mid-life have the potential to prevent later-life onset of cardiovascular disease, diabetes and obesity. However, CR responses for emerging molecular cardiometabolic biomarkers, specifically, nuclear magnetic resonance (NMR)-derived cardiometabolic risk markers, had never been performed. Additionally, CR response differences by weight status and sex were unclear.
Added value of this study
This study describes the effects of two-years of calorie restriction in humans on nuclear magnetic resonance (NMR)-derived measures of cardiometabolic risk and response differences by weight status and sex.
Implications of all the available evidence
Two years of ∼12% CR improved NMR-derived molecular biomarkers of cardiometabolic risk via reductions in inflammation and branched chain amino acids and a shift from atherogenic to cholesterol transporting lipoproteins. These CR benefits were influenced by body mass index (BMI) and sex, with greater effects for overweight versus normal weight and for men versus women.
Alt-text: Unlabelled box
Introduction
Calorie restriction (CR) improves risk factors for atherosclerotic cardiovascular disease (ASCVD) including dyslipidemia, elevated blood pressure, and diabetes mellitus as well as cardiometabolic risks comprising the metabolic syndrome, atherogenic dyslipidemia (elevated triglycerides and fasting glucose, reduced high density lipoprotein (HDL)-cholesterol), hypertension, and large waist circumference).1, 2, 3, 4, 5 These risk factors reflect the excess visceral and subcutaneous adiposity driving the dysmetabolism of prediabetes and type 2 diabetes mellitus (T2D).1 Likely CR improves the risk factors– both in animal models and in humans- by imposing negative energy balance.5, 6, 7
Cardiovascular incidence and mortality risk increase continuously for all conventional cardiometabolic risk factors even with values well below the conventional clinical disease thresholds.8 This is also true for newly emerging molecular biomarkers of cardiovascular risk. Nuclear magnetic resonance (NMR) spectroscopy-based assessments of lipoprotein particle classes, subclasses and systemic inflammation have extended and enhanced cardiovascular risk assessments beyond those traditionally reported in a standard lipid panel. Also, NMR-assessed metabolites include glucose, citrate, ketone bodies, alanine, and the branched chain amino acids (BCAAs: valine, leucine and isoleucine provide insight into associations between dysmetabolism and cardiovascular disease, especially with BCAAs having emerged as novel markers of obesity, insulin resistance, cardiovascular disease risk, and cardiovascular mortality.11, 12, 13, 14, 15, 16 Several combinations of these NMR-determined molecules serve as multi-component markers of cardiometabolic risk. For example, the Lipoprotein Insulin Resistance Index (LP-IR) uses six parameters of lipoprotein subclass and size to identify individuals with insulin resistance.9 LP-IR is associated with T2D incidence with scores validated against homeostasis model assessments of insulin resistance (HOMA-IR) and clamp-derived measures of insulin resistance.9,17 The Diabetes Risk Index (DRI) incorporates LP-IR with valine and leucine to enhance prediction of future T2D.18,19 Offering a composite measure of systemic inflammation, GlycA is an NMR signal composed of several glycosylated acute phase proteins that is associated with increased cardiometabolic risk.10,20, 21, 22 Together, these novel NMR markers of ASCVD and T2D increase awareness and knowledge of the metabolic changes leading to increased risk of ASCVD-related events.
The Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE™) study was a two-year, multicenter, randomized controlled trial designed to evaluate the potential for CR to promote anti-aging adaptations in resting metabolic rate and core body temperature.23,24 In healthy individuals without obesity, CR of ∼300 kcal/day reduced resting metabolic rate adjusted for weight change at 12 months, but exerted little influence on core body temperature.24 Also, CR decreased body weight, body mass index (BMI), blood pressure, and improved plasma cholesterol concentrations.5 CALERIE provided the unique opportunity to address two novel outstanding questions of two years of modest calorie restriction in metabolically normal individuals: whether there are effects on emerging measures of cardiometabolic risk and whether these effects were modified by sex or beginning BMI strata.
Methods
Study design and participants
CALERIE™ Phase 2 was a multicenter, randomized controlled trial (May 2007—November 2012) aimed at evaluating the time-course effect of 25% CR (a 25% reduction in calorie intake from baseline) over a two-year period in healthy, men (aged 21–50 years) and women who were premenopausal (aged 21–47 years), without obesity (BMI 22.0–27.9 kg/m²).23
The study protocol (NCT00427193) was approved by the institutional review boards at all participating clinical centers (Washington University School of Medicine, St Louis, MO; Pennington Biomedical Research Center, Baton Rouge, LA; Tufts University, Boston, MA), and the coordinating center (Duke University, Durham, NC). All study participants provided written informed consent and study oversight was provided by a Data and Safety Monitoring Board.
Randomization and interventions
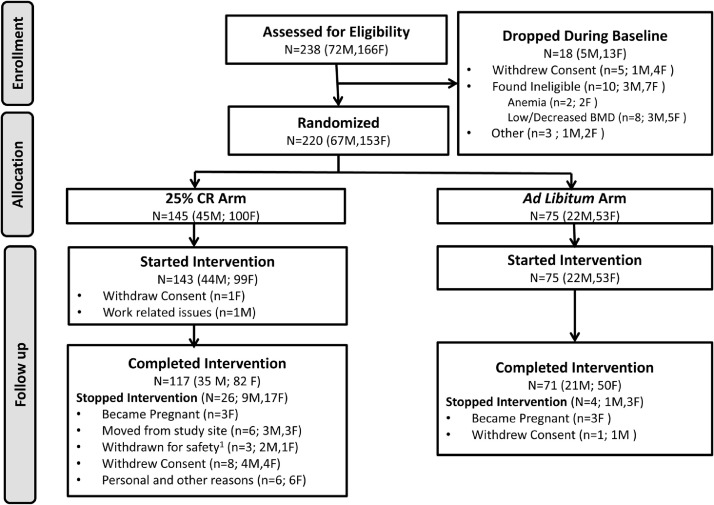
Participants were assigned randomly at a ratio of 2:1 to a 25% CR behavioral intervention or to an AL control group.24 Randomization used a permuted block technique stratified by site, sex, and BMI. Between May 2007 and Feb 2010, 238 participants began the baseline assessments, 220 were randomized to the study arms, and 218 started the assigned intervention. Of the 218 participants, 143 (66%) were assigned to the 25% CR diet group; 75 (34%) were assigned to the AL diet group. In the two study arms, 117 (82%) of the participants in the CR group and 71 (95%) of the participants in the AL group completed the study. Sample size calculations were based on a 2:1 (CR:AL) allocation and expectation of 10% attrition.23 For 225 persons enrolled and 180 persons completing the study, there was 93% and 94% power (alpha=0.05) to detect expected group differences for primary outcomes of resting metabolic rate and core temperature, respectively.23 Resting metabolic rate and core temperature expected group differences were (mean ± standard deviation) 60 ± 1107 kCal/d and 0.20 ± 0.36 °C, respectively, derived from CALERIE Phase 1 data, literature, and expert opinion.23 Figure 1 shows the previously reported CONSORT diagram.5
Figure 1.
CALERIE CONSORT diagram. Reproduced with permission.5
Extensive details of the intervention are provided in previous publications.23, 24, 25 At baseline, participants in the CR group were prescribed a 25% restriction in calorie intake based on energy requirements from two consecutive doubly labeled water measurements over a 4-week period.
For determinations of energy expenditure, urine samples were collected before and at several time points after participants ingested each dose of water containing deuterium (²H) and oxygen 18 (¹⁸O); samples were analyzed for isotopic enrichment by isotope ratio mass spectrometry. Energy expenditure (kcal/day) was calculated based on the difference in the elimination rates of the two isotopes.26 All participants underwent doubly labeled water measurements and dual-energy x-ray absorptiometry (DEXA) at 6-month intervals during the intervention. Energy intake (percent CR) was computed from doubly labeled water-derived total daily energy expenditure and DEXA-derived changes in body composition; as above, doubly labeled water and DEXA laboratory and radiographic analyses precluded the use of percent CR as a real time adherence measure.27 During the intervention, percent CR adherence and prescription modifications were based on attaining individualized weight loss trajectories. Retrospective analyses indicated these calculated weight loss trajectories permitted less weight loss than was needed to achieve 25% CR as determined by doubly labeled water and DEXAs performed during the intervention; thus, these differences contributed to the CR group attaining an average of ∼12% CR. For the first 27 days of the intervention, all participant meals and snacks were prepared in a metabolic kitchen at each clinical center to facilitate adoption of the CR diet; subsequently, meal provision was offered periodically to enhance adherence. Throughout the majority of the intervention, participants self-selected their own meals in accordance with their CR prescription and received intensive guidance from nutrition and behavioral interventionists. Also, participants attended group and individual counselling sessions throughout the intervention. A Computerized Tracking System was employed to monitor adherence and respond to challenges in real time.25,27, 28, 29, 30 Individualized counselling was tailored accord to whether weight change followed or deviated from the individualized weight loss trajectories. . Participants assigned to the AL control group were instructed to continue their habitual diets and did not receive counselling.
Study outcomes
This secondary analysis addressed NMR-determined cardiovascular risk measures that were assessed in all participants (CR and AL) at baseline, 12 months, and 24 months, Details on the primary outcome measures have been reported previously.5
NMR spectroscopy analysis
Venous blood was drawn after an overnight fast. Ethylenediaminetetraacetic acid (EDTA) plasma samples were processed and stored frozen at −80 °C until testing. NMR LipoProfile® testing31,32 is currently deployed on the high-throughput Vantera® NMR Clinical Analyzer (Morrisville, NC); a single “scan” (proton NMR spectrum) of a plasma or serum specimen provides average sizes for three lipoprotein classes,33 concentrations of 24 lipoprotein particle subclasses, several small molecule metabolites, and GlycA.10,31,32 The NMR analysis, which reported lipoprotein subclass particle concentrations and sizes as well as concentrations of several key metabolites, was performed using the LP4 deconvolution algorithm.33 Diameter ranges of the lipoprotein classes and subclasses can be found in Table 1. Of note, low density lipoprotein (LDL) particles, high density lipoprotein (HDL) particles, and the LDL and HDL subclasses generated from the LP4 algorithm have been calibrated to agree more closely to absolute concentrations of LDL and HDL particles determined by their apolipoprotein compositions. Mean triglyceride-rich lipoprotein (TRL), LDL and HDL particle sizes are weighted averages derived from the sum of the diameters of each subclass multiplied by the relative mass percentage of each. For the LP4 algorithm, linear regression of the lipoprotein subclass signal areas against serum lipid and apolipoprotein levels measured by chemical assays in a large reference range study population (n = 698) provided the conversion factors to generate NMR-derived concentrations of total cholesterol, triglycerides, the cholesterol in TRL, LDL and HDL fractions, apolipoprotein B (ApoB) and apolipoprotein A-I (ApoA-I). NMR-derived concentrations of these parameters are highly correlated with those measured by standard methods. Details of the NMR quantification of the BCAAs and ketone bodies have been reported previously.11,12 Assay development, analytical performance evaluation and clinical validation of LP-IR (0–100; least to most insulin resistant) and DRI (1–100; least to greatest risk of developing T2D) also have been reported.9,17,19
Table 1.
NMR measured lipoprotein diameter ranges.
| Lipoprotein Class | Lipoprotein Subclass | Description | Estimated Diameter Range or Median (nm) |
|---|---|---|---|
| TG-Rich Lipoprotein Particle (TRLP) Concentrations (nmol/L) | |||
| TRLP | Total TRLP | 24- 240 | |
| L-TRLP | Large TRLP | 50–89 | |
| M-TRLP | Medium TRLP | 37–49 | |
| S-TRLP | Small TRLP | 30–36 | |
| VS-TRLP | Very Small TRLP | 24–29 | |
| LDL Particle (LDLP) Concentrations (nmol/L) | |||
| LDLP | Total LDLP | 19–23 | |
| L-LDLP | Large LDLP | 21.5–23 | |
| M-LDLP | Medium LDLP | 20.5–21.4 | |
| S-LDLP | Small LDLP | 19–20.4 | |
| HDL Particle (HDLP) Concentrations (μmol/L) | |||
| HDLP | Total HDLP | 7.5–12 | |
| L-HDLP | Large HDLP | 10.3–12.0 | |
| M-HDLP | Medium HDLP | 8.7–9.5 | |
| S-HDLP | Small HDLP | 7.4–7.8 | |
| H7P | HDLP subspecies | 12.0 | |
| H6P | HDLP subspecies | 10.8 | |
| H5P | HDLP subspecies | 10.3 | |
| H4P | HDLP subspecies | 9.5 | |
| H3P | HDLP subspecies | 8.7 | |
| H2P | HDLP subspecies | 7.8 | |
| H1P | HDLP subspecies | 7.4 | |
| Mean Particle Sizes (nm) | |||
| TRLZ | — | TRL Size | 30–100 |
| LDLZ | — | LDL Size | 19–22.5 |
| HDLZ | — | HDL Size | 7.4–13 |
Statistical analysis
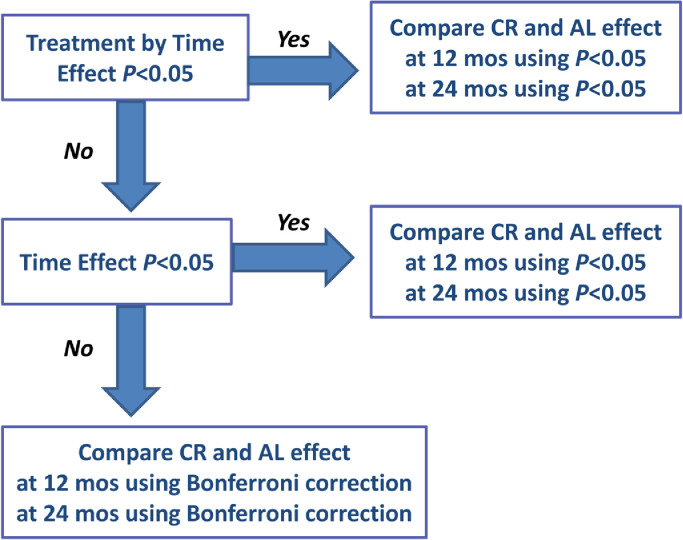
The same statistical methods used in the primary analysis of the CALERIE™ trial were applied.27,34 Intention-to-treat (ITT) analyses included all available observations; ITT analyses include participants in the groups to which they were assigned regardless of intervention adherence and are considered primary analyses for randomized controlled trials Wilcoxon and Fisher exact tests were used to evaluate baseline between-group differences. Repeated measures ANCOVA, as implemented under mixed models,35 was applied with change from baseline as the dependent variable, and treatment, time, and the treatment by time interaction as independent variables. The approximate normality of each outcome and of the change score of the outcome were confirmed by examination. Site, sex, BMI stratum (normal weight [22·0–24·9 kg/m²] and overweight [25·0–27·9 kg/m²]), and the baseline value were included as covariates to ensure statistical balance not captured by randomization, and to reduce error variance.36 To avoid arbitrary modeling assumptions with respect to linearity, time was treated as a categorical variable; similarly, an unstructured covariance structure among the repeated observations was assumed. Hypotheses of specific interest, for example between-group differences (e.g., CR versus AL) at the individual time points and within-group changes over time, were tested by defining contrasts among the regression parameters; predicted mean changes (standard errors (SEs)) are the adjusted values from this model For testing of the statistical significance of group effects, for each outcome, test of group differences at a particular time point used a hierarchical gatekeeping strategy (Figure 2).37 First, the treatment-by-time interaction term was tested. If the interaction was significant, between-group differences (CR vs. AL) at each time point were tested at α = 0.05 and reported as PCRvsAL <0.05 in the table. If the group X time interaction was non-significant, the treatment main effect was tested next. If the treatment main effect was significant, between-group differences (CR vs. AL) at each time point were tested at a value of α=0·05 and reported as PCRvsAL <0.05. If neither of the treatment-by-visit interaction or the treatment main effect were statistically significant, a Bonferroni correction was applied at each time point, with p values adjusted by multiplying the nominal p value by the number of tests (truncated at 1.0).37
Figure 2.
Decision Tree for Statistical Analyses. To control for type-I effect, assessment of each outcome used a hierarchical gatekeeping strategy as illustrated. First, the treatment-by-visit interaction term was tested; if significant, between-group differences at each time point were tested at α=0·05. If not significant, the treatment main effect was tested next; if significant, between-group differences at each time point were tested at α=0·05. If neither of the treatment-by-visit interaction or the treatment main effect were significant, Bonferroni correction was applied at each time point, with p values adjusted by multiplying the nominal p value by the number of tests (truncated at 1·0).
First, the treatment-by-time interaction term was tested. If the interaction was significant, between-group differences (CR vs. AL) at each time point were tested at α = 0.05 and reported as PCRvsAL <0.05. If the interaction was not significant, the treatment main effect was tested next. If the treatment main effect was significant, between-group differences (CR vs. AL) at each time point were tested at α = 0.05 and reported as PCRvsAL <0.05. If neither of the treatment-by-visit interaction or the treatment main effect were significant, Bonferroni correction was applied at each time point, with p values adjusted by multiplying the nominal p value by the number of tests (truncated at 1·0).27 Analyses were done using SAS, version 9.4.
Role of funding source
CALERIE™ was conducted as a National Institutes of Health (NIH) U-grant (U01 AG022132, U01 AG020478, U01 AG020487 U01 AG020480). As defined by this mechanism, the study design and conduct was a collaborative effort between internal NIH and external study investigators. Additional project funding was provided by NIH R01 AG054840 (MO, VBK); R33 AG070455 (KMH, DCP, MB, SBR, CKM, LMR, SKD, CFP, CJR, WEK); P30 DK072476 (CKM, LMR); and U54 GM104940 (CKM, LMR) and without any additional scientific contribution or other role in this report. All authors had access to the data and accept responsibility to submit for publication.
Results
Baseline characteristics of the two study groups are shown in Table 2. Information regarding changes in energy intake, body weight, body composition, BMI, and adherence to the study intervention have been published previously.5,23,24,38 Briefly, during year one, the CR group had pronounced caloric and weight reductions; in year 2, the CR group had caloric intakes and weights that were reduced from baseline yet remained stable over the duration of year two.24 Specifically, the CR group achieved an average 11.9% CR and demonstrated reductions in body mass (8.4 kg at 12 months and 7.5 kg at 24 months), fat mass (6.1 kg at 12 months and 5.3 kg at 24 months) and fat free mass (2.20 kg at 12 months and 2.17 kg at 24 months).5,24
Table 2.
Baseline characteristics of CALERIE™ study participants.
| Ad libitum group (n = 75) | Calorie restriction group (n = 143) | |
|---|---|---|
| Age, years | 37.9 (6.9) | 38.0 (7.3) |
| Sex | ||
| Women | 53 (71%) | 99 (69%) |
| Men | 22 (29%) | 44 (31%) |
| Race/Ethnicity White African American Other* |
57 (76%) 11 (15%) 7 (9%) |
111 (78%) 15 (11%) 17 (12%) |
| Height, m | 168.4 (8.3) | 168.9 (8.6) |
| Weight, kg | 71.5 (8.7) | 72.0 (9.5) |
| BMI, kg/m2 | 25.1 (1.6) | 25.2 (1.8) |
| Fat mass,% | 33.6 (6.6) | 32.9 (6.1) |
| Fat free mass, kg | 47.6 (8.6) | 48.5 (9.2) |
| Energy intake, kcal/d | 2390 (384.8) | 2467 (405.6) |
Data are n (%) or mean (SD). Abbreviations: BMI, body mass index. *Other includes Asian, Pacific Islander, and Native American. There were no significant between-group differences in baseline characteristics.
Cardiovascular and T2D risk measures
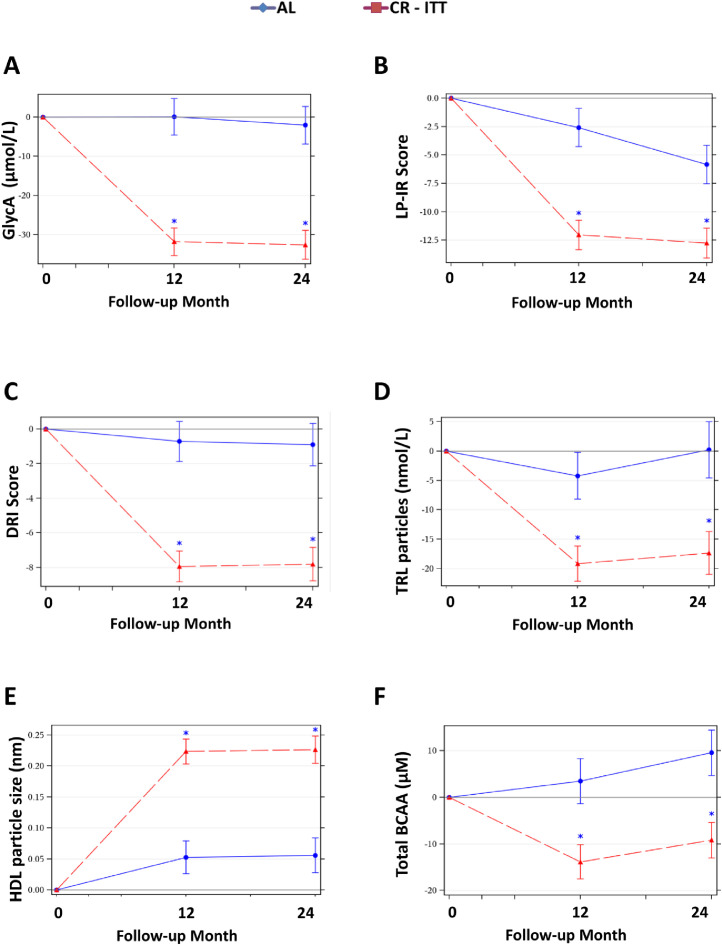
Results of NMR measures are summarized in Table 3, Table 4, Table 5. At both 12 and 24 months, as compared to AL, the CR group had reductions in emerging molecular biomarkers for T2D and cardiovascular disease; these included reductions in ApoB, a pro-atherosclerotic apolipoprotein, GlycA (Figure 3A), a composite inflammatory marker, and LP-IR and DRI (Figure 3B,C), indices reflecting insulin resistance and T2D risk, (scored 0–100 and 1–100, respectively). (Table 3; all P < 0.001 for CR versus AL (PCRvsAL)).
Table 3.
NMR-measured markers of cardiovascular and type 2 diabetes risk at baseline, and changes from baseline at 12, and 24 monthsa.
| Ad libitum group (n = 75) | Calorie restriction group (n = 143) | Between-group p-valueb | |
|---|---|---|---|
| Apolipoprotein B (mg/dL) | |||
| Baseline | 73.9 (2.4) | 70.5 (1.6) | 0.21 |
| Change at month 12 | −3.76 (1.39)* | −10.56 (1.05)*** | <0.0001 |
| Change at month 24 | −1.92 (1.46) | −9.83 (1.11)*** | <0.0001 |
| Apolipoprotein A-I (mg/dL) | |||
| Baseline | 134.7 (2.5) | 137.6 (2.2) | 0.68 |
| Change at month 12 | −1.41 (1.86) | −3.29 (1.39)* | 0.40 |
| Change at month 24 | −1.81 (2.12) | 1.30 (1.61) | 0.23 |
| GlycA (µmol/L) | |||
| Baseline | 334.1 (5.6) | 326.7 (4.8) | 0.12 |
| Change at month 12 | 0.06(4.66) | −31.79 (3.52)*** | <0.0001 |
| Change at month 24 | −2.07 (4.80) | −32.58 (3.70)*** | <0.0001 |
| LP-IR score (0–100) | |||
| Baseline | 30.8 (2.6) | 33.7 (1.9) | 0.42 |
| Change at month 12 | −2.59 (1.68) | −12.03 (1.29)*** | <0.0001 |
| Change at month 24 | −5.83 (1.70)** | −12.75 (1.32)*** | 0.0009 |
| DRI score (1–100) | |||
| Baseline | 22.1 (2.0) | 23.9 (1.4) | 0.33 |
| Change at month 12 | −0.72 (1.15) | −7.94 (0.89)*** | <0.0001 |
| Change at month 24 | −0.90 (1.23) | −7.81 (0.97)*** | <0.0001 |
Abbreviations: LP-IR, Lipoprotein Insulin Resistance Index; DRI, Diabetes Risk Index.
Baseline values are the observed mean (SE); change scores are the least-squares adjusted means (SE) from the ITT repeated measures analysis.
Between-group p-value tests for a significant between-group difference in the change score at the time point. All comparisons are controlled for baseline values. All p-values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see text).
*, **, and *** indicated within group differences at the P<0.05, 0.01, and 0.001 values, respectively. Between group significant findings p<0.0017 are bolded and italicized for emphasis.
Table 4.
NMR-measured lipid and lipoproteins levels at baseline, and changes from baseline at 12, and 24 monthsa.
| Ad libitum group (n = 75) | Calorie restriction group (n = 143) | Between-group p-valueb | |
|---|---|---|---|
| Total cholesterol (mg/dL) | |||
| Baseline | 156.5 (3.4) | 153.5 (2.4) | 0.29 |
| Change at month 12 | −4.98 (2.09)* | −14.66 (1.58)*** | 0.0002 |
| Change at month 24 | −3.03 (2.36) | −11.34 (1.81)*** | 0.004 |
| TRL cholesterol (mg/dL) | |||
| Baseline | 24.8 (1.3) | 23.7 (0.9) | 0.70 |
| Change at month 12 | −1.22 (0.79) | −5.16 (0.59)*** | <0.0001 |
| Change at month 24 | −0.72 (0.87) | −4.69 (0.66)*** | 0.0002 |
| LDL cholesterol (mg/dL) | |||
| Baseline | 78.6 (2.5) | 75.6 (1.8) | 0.30 |
| Change at month 12 | −4.20 (1.58)* | −10.80 (1.19)*** | 0.0006 |
| Change at month 24 | −2.74 (1.62) | −10.14 (1.25)*** | 0.0002 |
| HDL cholesterol (mg/dL) | |||
| Baseline | 53.1 (1.4) | 54.2 (1.2) | 0.64 |
| Change at month 12 | 0.72 (0.91) | 1.49 (0.68) | 0.47 |
| Change at month 24 | 0.47 (1.05) | 3.75 (0.80)*** | 0.010 |
| Triglycerides (mg/dL) | |||
| Baseline | 119.5 (6.6) | 118.2 (4.4) | 0.94 |
| Change at month 12 | −6.33 (3.87) | −29.42 (2.92)*** | <0.0001 |
| Change at month 24 | −5.86 (4.03) | −26.53 (3.10)*** | <0.0001 |
| TRL triglycerides (mg/dL) | |||
| Baseline | 81.7 (6.3) | 79.8 (4.2) | 0.90 |
| Change at month 12 | −5.62 (3.68) | −25.62 (2.79)*** | <0.0001 |
| Change at month 24 | −5.40 (3.80) | −24.00 (2.92)*** | <0.0001 |
| Total TRL particles (nmol/L) | |||
| Baseline | 139.2 (6.9) | 129.5 (4.2) | 0.41 |
| Change at month 12 | −4.21 (3.98) | −19.17 (2.98)*** | 0.002 |
| Change at month 24 | 0.20 (4.78) | −17.33 (3.64)*** | 0.003 |
| Large TRL particles (nmol/L) | |||
| Baseline | 2.3 (0.5) | 2.5 (0.3) | 0.42 |
| Change at month 12 | −0.51 (0.29) | −1.76 (0.22)*** | 0.0005 |
| Change at month 24 | −0.43 (0.32) | −1.69 (0.25)*** | 0.0013 |
| Medium TRL particles (nmol/L) | |||
| Baseline | 17.6 (1.4) | 17.7 (1.1) | 0.90 |
| Change at month 12 | −1.59 (1.10) | −4.54 (0.83)*** | 0.026 |
| Change at month 24 | −1.34 (1.07) | −4.53 (0.83)*** | 0.015 |
| Small TRL particles (nmol/L) | |||
| Baseline | 62.3 (4.8) | 48.6 (2.8) | 0.014 |
| Change at month 12 | −2.26 (3.42) | −7.40 (2.56)** | 0.43 |
| Change at month 24 | 4.39 (3.55) | −5.27 (2.71) | 0.053 |
| Very small TRL particles (nmol/L) | |||
| Baseline | 56.8 (6.1) | 60.6 (3.6) | 0.014 |
| Change at month 12 | 3.01 (4.11) | −5.71 (3.08) | 0.16 |
| Change at month 24 | 0.29 (4.13) | −6.71 (3.18) | 0.33 |
| TRL size (nm) | |||
| Baseline | 42.4 (0.8) | 43.5 (0.60) | 0.18 |
| Change at month 12 | −0.41 (0.59) | −2.87 (0.44)*** | 0.0007 |
| Change at month 24 | −1.97 (0.52)*** | −3.01 (0.41)*** | 0.10 |
| Total LDL particles (nmol/L) | |||
| Baseline | 1187.7 (36.2) | 1162.1 (27.6) | 0.51 |
| Change at month 12 | −58.64 (23.25)* | −170.11 (17.48)*** | <0.0001 |
| Change at month 24 | −39.89 (22.77) | −163.83 (17.47)*** | <0.0001 |
| Large LDL particles (nmol/L) | |||
| Baseline | 337.4 (22.7) | 302.5 (15.6) | 0.17 |
| Change at month 12 | −4.54 (16.81) | −22.10 (12.72) | 0.78 |
| Change at month 24 | −15.02 (15.46) | −27.34 (12.06) | 1.00 |
| Medium LDL particles (nmol/L) | |||
| Baseline | 293.0 (35.4) | 243.4 (20.9) | 0.41 |
| Change at month 12 | −20.30 (23.69) | −54.03 (17.66)** | 0.48 |
| Change at month 24 | 12.89 (24.16) | −28.84 (18.54) | 0.32 |
| Small LDL particles (nmol/L) | |||
| Baseline | 557.4 (42.1) | 616.3 (29.0) | 0.11 |
| Change at month 12 | −30.54 (26.92) | −92.79 (20.27)*** | 0.06 |
| Change at month 24 | −34.13 (26.66) | −106.53 (20.62)*** | 0.027 |
| LDL size (nm) | |||
| Baseline | 21.1 (0.06) | 21.0 (0.04) | 0.12 |
| Change at month 12 | −0.000 (0.04) | −0.002 (0.03) | 1.00 |
| Change at month 24 | −0.01 (0.04) | −0.009 (0.03) | 1.00 |
| Total HDL particles (µmol/L) | |||
| Baseline | 20.3 (0.3) | 20.7 (0.3) | 0.36 |
| Change at month 12 | −0.28 (0.26) | −1.27 (0.19)*** | 0.002 |
| Change at month 24 | −0.47 (0.28) | −0.73 (0.22)** | 0.46 |
| Large HDL particles (µmol/L) | |||
| Baseline | 2.4 (0.1) | 2.4 (0.1) | 0.73 |
| Change at month 12 | 0.10 (0.10) | 0.63 (0.08)*** | <0.0001 |
| Change at month 24 | 0.13 (0.12) | 0.69 (0.09)*** | 0.0001 |
| Medium HDL particles (µmol/L) | |||
| Baseline | 6.2 (0.3) | 6.3 (0.2) | 0.97 |
| Change at month 12 | 0.05 (0.19) | −0.53 (0.14)*** | 0.013 |
| Change at month 24 | 0.23 (0.19) | −0.08 (0.15) | 0.19 |
| Small HDL particles (µmol/L) | |||
| Baseline | 11.7 (0.3) | 12.0 (0.3) | 0.38 |
| Change at month 12 | −0.26 (0.29) | −1.25 (0.22)*** | 0.006 |
| Change at month 24 | −0.67 (0.27)* | −1.22 (0.21)*** | 0.10 |
| H7 particles (µmol/L) | |||
| Baseline | 0.46 (0.04) | 0.49 (0.03) | 0.74 |
| Change at month 12 | 0.02 (0.04) | 0.09 (0.03)** | 0.12 |
| Change at month 24 | −0.01 (0.04) | 0.10 (0.03)*** | 0.009 |
| H6 particles (µmol/L) | |||
| Baseline | 1.06 (0.11) | 1.09 (0.09) | 0.73 |
| Change at month 12 | −0.05 (0.10) | 0.30 (0.07)*** | 0.003 |
| Change at month 24 | 0.02 (0.12) | 0.44 (0.09)*** | 0.006 |
| H5 particles (µmol/L) | |||
| Baseline | 0.88 (0.07) | 0.84 (0.05) | 0.68 |
| Change at month 12 | 0.08 (0.08) | 0.17 (0.06)* | 0.65 |
| Change at month 24 | 0.07 (0.08) | 0.12 (0.06) | 1.00 |
| H4 particles (µmol/L) | |||
| Baseline | 1.52 (0.13) | 1.60 (0.07) | 0.07 |
| Change at month 12 | 0.13 (0.10) | −0.23 (0.08)** | 0.004 |
| Change at month 24 | 0.01 (0.12) | 0.05 (0.09) | 0.79 |
| H3 particles (µmol/L) | |||
| Baseline | 4.71 (0.23) | 4.66 (0.17) | 0.51 |
| Change at month 12 | −0.12 (0.18) | −0.33 (0.14)* | 0.66 |
| Change at month 24 | 0.18 (0.18) | −0.18 (0.14) | 0.19 |
| H2 particles (µmol/L) | |||
| Baseline | 9.85 (0.26) | 10.25 (0.21) | 0.34 |
| Change at month 12 | −0.28 (0.25) | −1.04 (0.19)*** | 0.012 |
| Change at month 24 | −0.58 (0.24)* | −1.06 (0.19)*** | 0.11 |
| H1 particles (µmol/L) | |||
| Baseline | 1.81 (0.18) | 1.79 (0.14) | 0.85 |
| Change at month 12 | 0.04 (0.15) | −0.19 (0.11) | .37 |
| Change at month 24 | −0.08 (0.14) | −0.13 (0.11) | 1.00 |
| HDL size (nm) | |||
| Baseline | 9.2(0.04) | 9.1 (0.04) | 0.55 |
| Change at month 12 | 0.05 (0.03) | 0.22 (0.02)*** | <0.0001 |
| Change at month 24 | 0.06 (0.03) | 0.23 (0.02)*** | <0.0001 |
Abbreviations: HDL, high density lipoprotein; LDL, low density lipoprotein; TRL, triglyceride-rich lipoprotein.
Baseline values are the observed mean (SE); change scores are the least-squares adjusted means (SE) from the ITT repeated measures analysis.
Between-group p-value tests for a significant between-group difference in the change score at the time point. All comparisons are controlled for baseline values. All p-values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see text).
*, **, and *** indicated within group differences at the p<0.05, 0.01, and 0.001 values, respectively. Between group significant findings p<0.0017 are bolded and italicized for emphasis.
Table 5.
NMR-measured small molecule metabolite levels at baseline, and changes from baseline at 12, and 24 months a.
| Ad libitum group (n = 75) | Calorie restriction group (n = 143) | Between-group p-value b | |
|---|---|---|---|
| Total BCAAs (µM) | |||
| Baseline | 310.1 (7.3) | 313.2 (4.6) | 0.42 |
| Change at month 12 | 3.47 (4.83) | −13.85 (3.67)*** | 0.003 |
| Change at month 24 | 9.55 (4.86) | −9.20 (3.79)* | 0.002 |
| Valine (µM) | |||
| Baseline | 182.5 (3.8) | 184.0 (2.6) | 0.80 |
| Change at month 12 | −0.45 (2.47) | −8.44 (1.88)*** | 0.008 |
| Change at month 24 | 3.52 (2.50) | −6.99 (1.94)*** | 0.0006 |
| Leucine (µM) | |||
| Baseline | 92.3 (3.1) | 92.5 (1.7) | 0.67 |
| Change at month 12 | 3.45 (2.31) | −3.42 (1.75) | 0.014 |
| Change at month 24 | 4.04 (2.44) | −2.55 (1.88) | 0.027 |
| Isoleucine (µM) | |||
| Baseline | 36.0 (1.5) | 36.7 (0.9) | 0.38 |
| Change at month 12 | 0.75 (1.20) | −0.78 (0.90) | 0.59 |
| Change at month 24 | 1.73 (1.11) | 0.78 (0.85) | 0.96 |
| Alanine (µM) | |||
| Baseline | 274.7 (8.9) | 275.2 (6.7) | 0.92 |
| Change at month 12 | −0.32 (7.65) | −31.48 (5.76)*** | 0.0008 |
| Change at month 24 | −0.58 (8.18) | −23.52 (6.32)*** | 0.023 |
| Fasting Glucose (mg/dL) | |||
| Baseline | 90.4(0.9) | 90.5 (0.7) | 0.92 |
| Change at month 12 | −0.64 (0.61) | −2.52 (0.46)*** | 0.011 |
| Change at month 24 | −0.44 (0.68) | −1.81 (0.53)** | 0.10 |
| Citrate (µM) | |||
| Baseline | 91.9 (2.3) | 93.1 (2.0) | 0.96 |
| Change at month 12 | −6.07 (2.13)** | −2.26 (1.60) | 0.27 |
| Change at month 24 | −2.73 (2.44) | −1.28 (1.87) | 1.0 |
| Total ketone bodies (µM) | |||
| Baseline | 196.9 (10.9) | 218.8 (11.5) | 0.87 |
| Change at month 12 | 9.04 (19.05) | 47.57 (14.09)** | 0.10 |
| Change at month 24 | −4.62 (16.71) | 34.47 (12.77)* | 0.055 |
| β-hydroxybutyrate (µM) | |||
| Baseline | 117.0 (7.1) | 128.3 (7.6) | 0.91 |
| Change at month 12 | −0.76 (11.70) | 23.23 (8.66)* | 0.09 |
| Change at month 24 | −7.90 (10.27) | 17.95 (7.86)* | 0.039 |
| Acetoacetate (µM) | |||
| Baseline | 52.2 (3.5) | 56.5 (2.8) | 0.40 |
| Change at month 12 | 10.20 (5.88) | 20.18 (4.37)*** | 0.33 |
| Change at month 24 | 6.51 (5.06) | 17.37 (3.89)*** | 0.16 |
| Acetone (µM) | |||
| Baseline | 27.7 (2.1) | 34.0 (2.6) | 0.44 |
| Change at month 12 | −2.77 (3.07) | 4.86 (2.27) | 0.043 |
| Change at month 24 | −5.39 (2.24)* | −0.33 (1.72) | 0.06 |
Abbreviations: BCAA, branched chain amino acids.
Baseline values are the observed mean (SE); change scores are the least-squares adjusted means (SE) from the ITT repeated measures analysis.
Between-group p-value tests for a significant between-group difference in the change score at the time point. All comparisons are controlled for baseline values. All p-values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see text).
*, **, and *** indicated within group differences at the P < 0.05, 0.01, and 0.001 values, respectively. Between group significant findings p < 0.0017 are bolded and italicized for emphasis.
Figure 3.
Mean (± SE) Changes in Representative NMR Parameters. The NMR parameter changes (means±SE) by treatment group are shown for (A) the composite inflammatory measure, GlycA, (B) Lipoprotein Insulin Resistance Index (LP-IR), (C) Diabetes Risk Index (DRI), (D) Triglyceride-rich lipoprotein particles (TRLP), (E) HDL particle size, and (F) total branched chain amino acids (BCAA). All comparisons are controlled for baseline values. All p-values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see text). Asterisk indicates p < 0.05 for a significant between-group difference in the change score at the time point. Included figures are meant to demonstrate the range and heterogeneity of alternative responses over time among different parameters.
Cholesterol metabolism and lipoproteins
CR improved cholesterol metabolism, a key mediator of cardiovascular disease. For lipids found in traditional clinical panels, as compared to AL, CR reduced NMR-measured total-cholesterol, and LDL-cholesterol, and triglycerides (Table 4; 12 and 24-month PCRvsAL<0.005). For HDL cholesterol, as compared to AL, CR increased concentrations but only at 24 months (Table 4; 24-month PCRvsAL = 0.01).
CR benefits on lipoprotein metabolism extended beyond those observed for traditional cholesterol panel components. (Table 4). At 12 months, the CR but not the AL group had reductions in the following molecular biomarkers of lipid metabolism: total (Figure 3D), large, and medium TRL particles, and thus, TRL size; total LDL particles; and total, medium and small HDL particles with increases in large HDL particles and HDL size (Figure 3E; all 12-month PCRvAL<0.05). At 24 months, CR-mediated responses remained superior to AL for total, large, and medium TRL particles; total LDL particle numbers; as well as large HDL particles and HDL size (all 24-month PCRvsAL<0.05). Also, at 24 months, CR-mediated reductions in small LDL particles were superior to AL (24-month PCRvsAL = 0.027).
Seven HDL subspecies (H1-H7) were analyzed for understanding effects of CR on changes in HDL subpopulations. At 12 months, the CR but not the AL group had increases in the larger HDL subtype, H6, and reductions in the smaller subtypes H4 and H2 (all 12-month PCRvsAL < 0.02). At 24 months, CR responses were superior to AL for increases in H6 as well as H7 (both 24-month PCRvsAL < 0.009). As larger HDL subpopulations more effectively transport cholesterol, these changes demonstrate a health benefit of CR.
Systemic metabolism
To inform how CR reduced ASCVD and T2D risks, evaluations were performed for CR versus AL responses in molecular biomarkers of whole body metabolism, NMR-assessed small metabolites (Table 5). At both 12 and 24 months, the CR but not the AL group had reductions in valine, leucine, total BCAAs (Figure 3F), and alanine (all 12- and 24-month PCRvsAL<0.03). As previously reported, the CR group had reductions in glucose at 12 and 24 months in CR, yet this change differed from AL at 12 months only (12-month PCRvsAL = 0.01). Consistent with an energy deficit state in the CR group, total ketone bodies, β-hydroxybutyrate, and acetoacetate were increased at 12 and 24 months; however, significant between-group differences for ketone bodies were observed only for β-hydroxybutyrate (24-month PCRvsAL = 0.038) and acetone (12-month PCRvsAL=0.043).
Heterogeneity by BMI and sex
There were significant differential effects observed by prespecified BMI and sex strata.
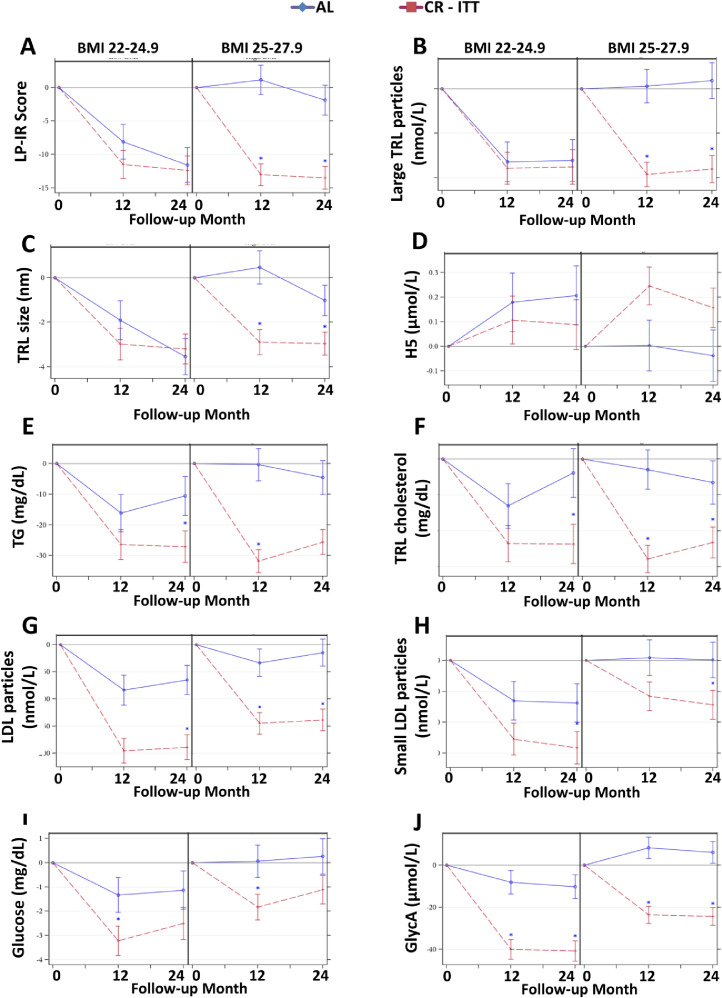
BMI effects. For multiple NMR-determined molecular markers of cardiometabolic risk, intervention responses were impacted by BMI. Participants with BMI of 25.0 to 27.9 kg/m2 had greater effects with CR compared to AL for reductions in LP-IR, large TRL particles, and TRL size with increases in H5; however, for those with a BMI 22.0 to 24.9 kg/m2, these measures responded similarly in CR and AL (Figure 4A-D; BMI by treatment interaction effect P < 0.05; for higher BMI only, 12- and 24-month PCRvsAL < 0.0001). Also, CR participants with BMI 25.0 to 27.9 kg/m2 had reductions in total triglycerides and TRL cholesterol at 12 and 24 months, while those with BMI 22.0 to 24.9 kg/m2 had reductions only after 24 months (Figure 4E-F; BMI by treatment by time interaction effect P < 0.05; for higher BMI only, 12- and 24-month PCRvsAL < 0.0001). For several NMR markers, significant BMI main effects reflect that differences in CR and AL were similar regardless of BMI, but overall reductions were greater for those with lower BMIs; these markers include total and small LDL particles, fasting glucose, and GlycA (Figure 4G-J; BMI main effect P < 0.05).
Figure 4.
BMI Effects on Mean (± SE) Changes in NMR Parameters. The NMR parameter changes (means±SE) by treatment group by BMI (22.0–24.9 versus 25.0–27.9 kg/m2) for (A) Lipoprotein Insulin Resistance Index (LP-IR), (B) Large triglyceride-rich lipoprotein (TRL) particles, (C) TRL size (D) HDL particle subtype H5, (E) triglycerides (TG), (F) TRL cholesterol, (G) LDL particles; (H) small LDL particles; (I) glucose, and (J) GlycA. All comparisons are controlled for baseline values. All p-values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see text). Asterisk indicates p < 0.05 for a significant between-group difference in the change score at the time point. Included figures are meant to demonstrate the range and heterogeneity of responses over time among different BMI strata.
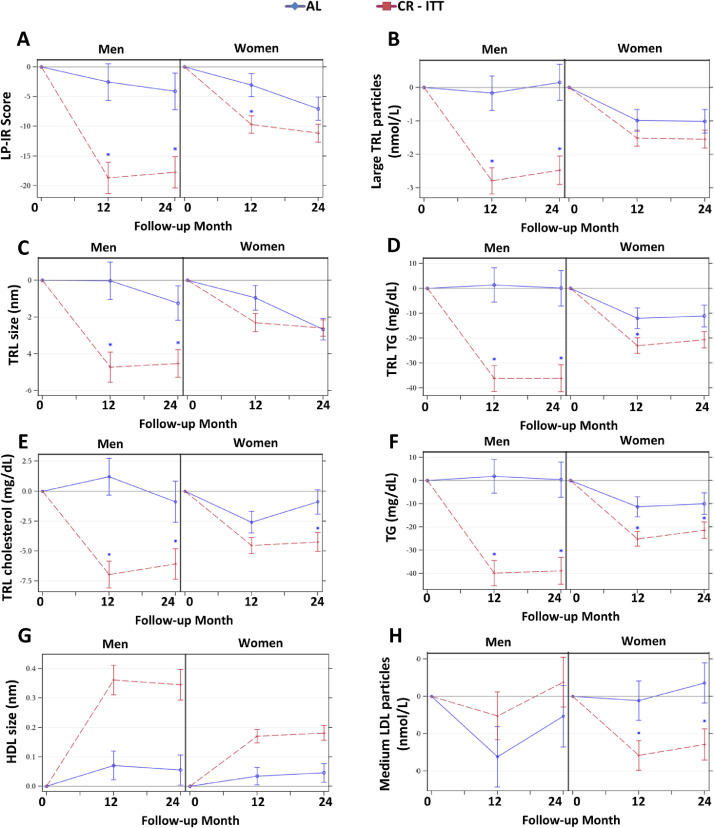
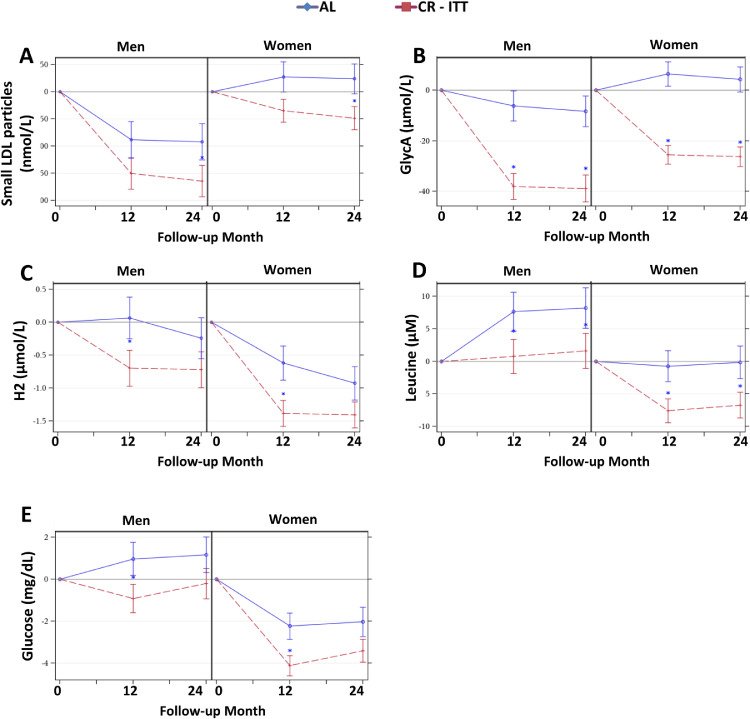
Sex effects. For several NMR -determined molecular markers of cardiometabolic risk, treatment effects differed by sex (sex by treatment interaction effects P < 0.05): compared with men in the AL group, men assigned to CR had superior responses for LP-IR, large TRL particles, TRL size, TRL triglycerides, TRL cholesterol, total triglycerides, HDL size, and medium LDL particles (Figure 5A-G; for all sex by treatment interaction P < 0.05; 12- and 24-month PCRvsAL < 0.05); however, for women, treatment group differences were less remarkable. One exception was the observation for medium LDL particles: for women at 12 and 24 months, medium LDL particles were reduced in CR; for men, there was no group difference in medium LDL particle responses (Figure 5H; sex by treatment interaction P < 0.05; for women only, 12- and 24-month PCRvsAL < 0.05). For several measures, CR responses were superior to AL similarly in both sexes, but regardless of group, the magnitude of response was greater for one sex: men had greater reductions in small LDL particles and GlycA (Figure 6A-B), while women had greater responses for the HDL subpopulation H2, leucine, and glucose (Figure 6C-E; sex main effect P < 0.05).
Figure 5.
Sex by Treatment Effects on Mean (± SE) Changes in NMR Parameters. The NMR parameter changes (means±SE) by treatment group by sex for (A) Lipoprotein Insulin Resistance Index (LP-IR), (B) Large triglyceride-rich lipoprotein (TRL) particles, (C) TRL size, (D) TRL triglycerides (TG), (E) TRL cholesterol, (F) TG, (G) HDL size, and (H) Medium LDL particles. All p-values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see text). Asterisk indicates p < 0.05 for a significant between-group difference in the change score at the time point. Included figures are meant to demonstrate the range and heterogeneity of alternative responses over time within the different sex strata.
Figure 6.
Sex Main Effects on Mean (± SE) Changes in NMR Parameters. The NMR parameter changes (means±SE) by treatment group by sex for (A) small LDL particles, (B) GlycA, (C) HDL particle subtype H2 (D) Leucine (E) Glucose. All p-values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see text). Asterisk indicates p < 0.05 for a significant between-group difference in the change score at the time point. Included figures are meant to demonstrate the range and heterogeneity of alternative responses over time within the different sex strata.
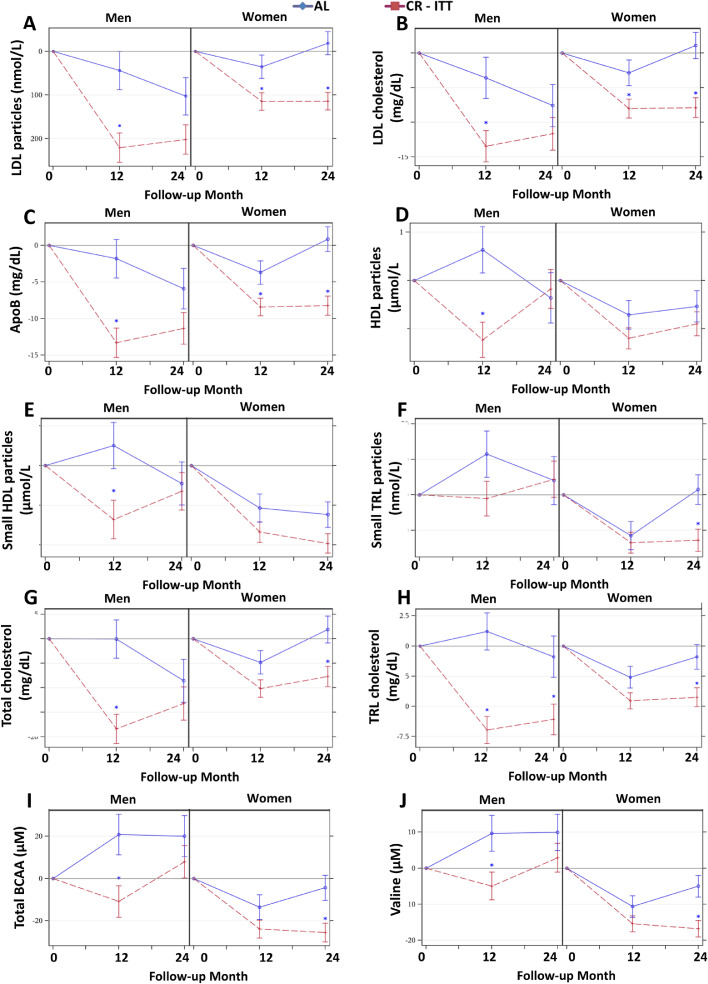
For several molecular biomarkers, there were unique responses by sex and group over time (time by sex by treatment interactions). These were most notable for differences according to sex and group in CR responses that were sustained at 24 months. While both sexes had CR-induced reductions at 12 months in total LDL particles, LDL cholesterol, and Apo B (Figure 7A-C), group differences were sustained at 24 months only for CR women (sex by treatment by time effects P < 0.05). For some measures, CR responses occurred for one sex at only one time point: CR men had reductions in total and small HDL particles at 12 months (Figure 7D-E); CR women had reductions in small TRL particles at 24 months (Figure 7F); reductions in total cholesterol, TRL cholesterol, total BCAAs, and valine occurred in CR men at 12 months and CR women at 24 months (Figure 7G-J; for all, sex by treatment by time effects P < 0.05).
Figure 7.
Sex by Treatment by Time Effects on Mean (± SE) Changes in NMR Parameters. The NMR parameter changes (means±SE) by treatment group by sex for (A) LDL particles, (B) LDL cholesterol, (C) ApoB, (D) HDL particles (E) small HDL particles (F) small TRL particles, (G) total cholesterol, (H) TRL cholesterol, (I) total branched chain amino acids (BCAA), and (J) Valine. All p-values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see text). Asterisk indicates p < 0.05 for a significant between-group difference in the change score at the time point. Included figures are meant to demonstrate the range and heterogeneity of alternative responses over time within the different sex strata.
Discussion
Over 24 months, ∼12% CR improved NMR-determined molecular markers of ASCVD, insulin resistance (LP-IR), and T2D (DRI). Remarkably, these improvements occurred in young (21–50 years), healthy adults with normal weight to moderate overweight with minimal underlying cardiometabolic risk. For most measures, CR versus AL differences were more pronounced for those overweight versus normal weight, and for men versus women. This is important, as evidence that modest calorie restriction of about 300 kg calories per day without substantive changes of macronutrients in the diet results in improvements in emerging cardiovascular risk factors even in healthy, young and middle aged individuals; further, we found the effects of calorie restriction on these cardiovascular risk markers to be more pronounced in men than women, and overweight than normal weight persons.
Additionally, these changes highlight that CR effects are pleiotropic, including multiple cardiometabolic risk components. CR improves atherogenic dyslipidemia risk not only through reductions in total cholesterol, LDL cholesterol, and triglycerides, but with contributions from reductions in ApoB and a shift towards larger HDL particles more effective for cholesterol transport. Additionally, effects on GlycA and BCAAs emphasize the impact of CR on improving systemic inflammation and altered metabolism, both important components of cardiometabolic risk.
One of the key findings from this work is that CR improved NMR-determined molecular markers for insulin resistance (LP-IR) and T2D risk (DRI). Previously reported, insulin resistance as measured by Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was reduced in the CR group and not the AL group, although the between-group difference was significant only at 24 months.5 LP-IR is a measure of insulin resistance in the liver as well as in the periphery.9 Therefore, CR appears to benefit both liver-related insulin resistance (reflected in the many lipoprotein changes) and peripheral insulin resistance (reflected in both HOMA-IR and LP-IR). In addition to insulin resistance, CR reduced the DRI at both 12 and 24 months. Greater scores for both LP-IR (0–100) and DRI (1–100) confer increasing hazards of T2D development.17,19 CR-mediated reductions in LP-IR (from ∼ 34 to ∼22) and DRI (from ∼24 to ∼16) correspond to shifts from the second lowest to the lowest quartiles, comparable to 52% and 59% age- and sex-adjusted hazard reductions for incident T2D over 7.5 years (Prevention of Renal and Vascular Endstage Disease (PREVEND) LP-IR HR=2.07, 95% CI=1.16 - 3.68; DRI HR=2.42 (1.33 - 4.4)).17,19 Thus, CR appears to reduce the risk of T2D even in apparently healthyadults without obesity. The implications for preventing longer term development of ASCVD and T2D through middle to late adult age is an open but pertinent question. It is possible that by resetting the baseline for individuals in the CR group at a younger age, as compared to their AL colleagues, those in CR may avoid the ravages of these aging-related diseases for a longer period. This could be addressed with long-term follow-up studies of participants in lifestyle trials, such as CALERIE™.
Reductions in insulin resistance and diabetes risk in response to CR were accompanied by improvements in NMR-determined lipoproteins, indicative of a reduced risk associated with atherogenic dyslipidemia. Atherogenic dyslipidemia—also known as diabetic dyslipidemia—is characterized by having an abundance of circulating triglyceride-enriched particles such as very low-density lipoprotein, smaller, cholesterol-depleted LDL particles, and cholesterol-enriched larger HDL particles.9,39 CR shifted lipoproteins to a less atherogenic profile through reductions in circulating cholesterol, triglycerides, large TRL particles, TRL size, and total and small LDL particles. These reductions were accompanied by reductions in small HDL and increases in large HDL particles; this represents a shift towards larger HDL subclasses. This was also reflected by increases in the larger HDL subpopulations H6 and H7, accompanied by decreases in the smaller subpopulation H2. Larger HDL particles, more effective at transporting cholesterol, are associated with reduced cardiovascular and T2D risks, while H2, the most abundant of the small HDL subpopulations, is increased in association with insulin resistance, large waist circumference, hypertension, visceral adiposity, and T2D.40 Our findings for changes in the HDL subpopulations were consistent with the reduction in insulin resistance and T2D risk observed with CR. The shift from small to large HDL particles without a concomitant increase in the numbers of HDL particles accounts for the lack of reduction in ApoA-I. However, both total and small LDL particles were reduced and consistent with a reduction in ApoB. The reduction in ApoB—even without an increase in ApoA-I—leads to a reduction in the ApoB/ApoA-I ratio, a well-known marker of cardiovascular risk.41,42 The magnitude of reduction in ApoB, ∼10 mg/dl at both 12 and 24 months, associates with a 23% lower risk of coronary heart disease (in43 OR = 0.77, 95% CI = 0.74–0.80). Thus, CR alterations in lipoprotein profile, including HDL particle subspecies and the ApoB/ApoA-I ratio, support reductions in atherogenic dyslipidemia-associated cardiovascular risk.
CR also resulted in favorable responses in molecular biomarkers of altered metabolism, including BCAAs and ketone bodies. Circulating concentrations of total BCAAs, isoleucine, leucine and valine are associated with states of energetic excess, such as obesity and insulin resistance:13,16,44 additionally, these metabolic intermediates may impair cardiac, skeletal muscle, and liver metabolism directly.45, 46, 47, 48 Ketone bodies, characteristic of the fasted state, are produced by the liver in response to low glucose and insulin plasma concentrations. Thus, CR-mediated reductions in amino acids and increases in ketones are indicative of a shift from a state of energetic substrate balance or excess to energetic deficit. Increases in ketone bodies along with reductions in fasting glucose were most notable after the first 12 months of CR, reflecting reductions in energy intake, weight, fat mass, and fat-free mass. Reductions in amino acids at 12 months were sustained at 24 months, during the “weight maintenance stage” of the intervention period and despite declines in adherence, indicating a persistent enhancement of energetic balance.
In addition to beneficial changes in biomarkers of altered metabolism, CR reduced GlycA concentrations, an emerging biomarker of inflammation-associated cardiometabolic risk.10 GlycA concentrations are greater in chronic inflammatory diseases, including systemic inflammatory conditions associated with greater cardiovascular risk such as rheumatoid arthritis, and conditions with low grade inflammation such as obesity and T2D20,22; thus, reductions in GlycA with CR reflect improved cardiovascular risk related to inflammation.
CALERIE™ was designed to have adequate power to address sex differences for the primary outcomes of resting metabolic rate and core body temperature.23,24 Sex-specific responses to CR were present for multiple NMR biomarkers, with many favorable responses being greater for men than women. Interestingly, for many of these, the seeming significant CR effects were due to men in AL deteriorating metabolically in a more pronounced fashion over two years. This finding may reflect the protective effects of endogenous, cycling hormones such as estrogen in women. It is also possible in the absence of an imposed diet, that women select a healthier AL diet than men or have disproportionate changes in body composition changes. Notably, for those in AL, women and men did not differ in change in energy intake, but men ate more protein. For those in CR, men versus women had greater reductions in fat mass. Weight loss may also help account for a sex-specific temporal pattern. CR responses in men were robust after 12 months while responses in women were more notable after 24 than 12 months (Figure 5, Figure 6, Figure 7). Thus, while CR responses in men may require weight loss, CR responses in women may not be as dependent upon weight loss but rather a longer duration of sustained CR and weight maintenance.
In addition to sex differences, another key design component of the parent study was to examine response differences according to baseline BMI. CR responses in NMR measures were more robust for overweight (BMI 25.0–27.9 kg/m2) than normal weight (BMI 22.0–24.9 kg/m2) individuals; these findings were notable for TRL cholesterol, TRL-related particles, and total triglycerides. In part, this derives from effects of the AL diet having a more negative effect on those with greater BMIs than those with lesser BMIs (Figure 4); thus, for those with greater BMIs, CR provides a greater benefit by preventing the deleterious effects of an AL diet. For those with BMIs in the overweight range, benefits accrued after 12 months of CR. In contrast, persons with normal BMIs may require a longer duration, as long as 24 months of CR, to derive benefits. With CR, normal weight participants accrued superior effects to overweight participants for total and small LDL particles, fasting glucose, and GlycA (Figure 4); these differences by BMI may reflect subclinical metabolic dysfunction in persons with overweight that merit additional investigation of BMI-specific responses to cardiometabolic risk reduction. On the other hand, findings here indicate that CR produces significant cardiometabolic benefits even for persons in a healthy BMI range.
There are several limitations of this analysis. Although the CR intervention was over 24 months, these measures were available only at these three windows in time; thus, it is not clear when the critical shifts in lipids and metabolites occurred. Similarly, these measures were obtained in the fasted state, and do not permit assessment of post-prandial implications; future analyses could perform similar NMR assessments in residual oral glucose tolerance samples to address shifts in these measures with a glucose load. While the original design was for 25% CR, participants in the CR group attained closer to 12% CR from baseline. Rather than a lack of participant compliance, this reflects use of real time adherence assessments that underestimated weight change needed to achieve the prescribed CR, and thus is not viewed as a limitation but as a reflection on the intervention delivery. Rather the mean attainment of 12% CR over two years is a study strength, especially since this degree and length of CR in humans has never been studied. Similarly other limitations are tempered by the strengths of an extremely stringent and consistent protocol across clinical sites for all outcome measures and time-points and use of centralized laboratories for sample analyses. As a critical example of this, samples were all obtained in the fasting state, verified by participants staying overnight in a metabolic unit prior to collection.
In summary, in young (21–50 yrs), healthy, normal to moderately overweight adults, after 12 and 24 months of 12% CR, there were improved NMR measures of insulin resistance, T2D, and ASCVD risk. These benefits accrued through alterations in multiple cardiometabolic risk domains, including the following: lipoproteins associated with atherogenic dyslipidemia; BCAAs associated with heart, muscle, and liver metabolic impairments; and systemic inflammation. While responses may be more robust for men and those with greater BMIs, CR yields significant cardiovascular benefits across atherogenic, metabolic, and inflammatory measures even in young (21–50 yrs), healthy adult without obesity. These findings may have implications for the ability of CR, started at young and middle age, to delay time to disease development (prolong health span) and perhaps even mortality with increasing age.
Contributors
Kim M. Huffman: conceptualization, funding acquisition, data verification, visualization, writing, reviewing and editing
Daniel C. Parker: conceptualization, visualization, writing, reviewing and editing
Manjushri Bhapkar: data curation, formal analysis, visualization, writing, reviewing and editing
Susan B. Racette: investigation, project administration, conceptualization, reviewing and editing
Corby Martin: investigation, project administration, conceptualization, reviewing and editing
Leanne M. Redman: investigation, project administration, conceptualization, reviewing and editing
Sai Krupa Das: investigation, project administration, conceptualization, reviewing and editing
Margery A. Connelly: conceptualization, methodology, resources, project administration, writing, reviewing and editing
Carl F. Pieper: conceptualization, funding acquisition, data curation, formal analysis, visualization, writing, reviewing and editing
Melissa Orenduff: conceptualization, methodology, project administration, writing, reviewing and editing
Leanna M. Ross: conceptualization, writing, reviewing and editing
Megan E. Ramaker: conceptualization, visualization, writing, reviewing and editing
James L. Dorling: conceptualization, visualization, writing, reviewing and editing
Clifford J Rosen: conceptualization, visualization, writing, reviewing and editing
Irina Shalaurova: methodology, resources, project administration, writing, reviewing and editing
James D. Otvos: conceptualization, methodology, resources, project administration, writing, reviewing and editing
Virginia B. Kraus: conceptualization, funding acquisition, project administration, writing, reviewing and editing
William E. Kraus: conceptualization, funding acquisition, data verification, visualization, writing, reviewing and editing
Declaration of interest
MAC, IS, and JDO are employees of Labcorp. All other authors have no conflicts interests.
Acknowledgments
Funding
The CALERIE™ trial design and implementation were supported by a National Institutes of Health (NIH) U-grant provided to four institutions, the three intervention sites and a coordinating center (U01 AG022132, U01 AG020478, U01 AG020487 U01 AG020480). For this secondary analysis including sample acquisition and processing, data analysis and interpretation, additional funding was provided by the NIH to authors as follows: R01 AG054840 (MO, VBK); R33 AG070455 (KMH, DCP, MB, SBR, CKM, LMR, SKD, CFP, CJR, WEK); P30 DK072476 (CKM, LMR); and U54 GM104940 (CKM, LMR).
Data sharing statement
The data are freely available at CALERIE.duke.edu, which contains a detailed description of the CALERIE study design, procedures, findings, forms, data dictionaries, and de-identified. Please see https://calerie.duke.edu/.
References
- 1.Alberti K.G., Zimmet P., Shaw J. Metabolic syndrome–a new world-wide definition. A consensus statement from the international diabetes federation. Diabetic Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 2.Adiels M., Olofsson S.O., Taskinen M.R., Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28(7):1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 3.Fontana L., Meyer T.E., Klein S., Holloszy J.O. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA. 2004;101(17):6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer T.E., Kovacs S.J., Ehsani A.A., Klein S., Holloszy J.O., Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47(2):398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 5.Kraus W.E., Bhapkar M., Huffman K.M., et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. The Lancet Diabetes Endocrinol. 2019;7(9):673–683. doi: 10.1016/S2213-8587(19)30151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colman R.J., Beasley T.M., Kemnitz J.W., Johnson S.C., Weindruch R., Anderson R.M. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattison J.A., Colman R.J., Beasley T.M., et al. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGarrah R.C., Haynes C., Dowdy Z.E., Shah S., Kraus W. GlycA, a novel biomarker of systemic inflammation, improves cardiovascular risk prediction in a high-risk coronary catheterization cohort. J Am Coll Cardiol. 2015;65(10S):A1606. D. [Google Scholar]
- 9.Shalaurova I., Connelly M.A., Garvey W.T., Otvos J.D. Lipoprotein insulin resistance index: a lipoprotein particle-derived measure of insulin resistance. Metab Syndr Relat Disord. 2014;12(8):422–429. doi: 10.1089/met.2014.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otvos J.D., Shalaurova I., Wolak-Dinsmore J., et al. GlycA: a composite nuclear magnetic resonance biomarker of systemic inflammation. Clin. Chem. 2015;61(5):714–723. doi: 10.1373/clinchem.2014.232918. [DOI] [PubMed] [Google Scholar]
- 11.Wolak-Dinsmore J., Gruppen E.G., Shalaurova I., et al. A novel NMR-based assay to measure circulating concentrations of branched-chain amino acids: elevation in subjects with type 2 diabetes mellitus and association with carotid intima media thickness. Clin Biochem. 2018;54:92–99. doi: 10.1016/j.clinbiochem.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Garcia E., Shalaurova I., Matyus S.P., et al. Ketone bodies are mildly elevated in subjects with type 2 diabetes mellitus and are inversely associated with insulin resistance as measured by the lipoprotein insulin resistance index. J Clin Med. 2020;9(2) doi: 10.3390/jcm9020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huffman K.M., Shah S.H., Stevens R.D., et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32(9):1678–1683. doi: 10.2337/dc08-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah S.H., Hauser E.R., Bain J.R., et al. High heritability of metabolomic profiles in families burdened with premature cardiovascular disease. Mol Syst Biol. 2009;5:258. doi: 10.1038/msb.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah S.H., Crosslin D.R., Haynes C.S., et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55(2):321–330. doi: 10.1007/s00125-011-2356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newgard C.B., An J., Bain J.R., et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flores-Guerrero J.L., Connelly M.A., Shalaurova I., et al. Lipoprotein insulin resistance index, a high-throughput measure of insulin resistance, is associated with incident type II diabetes mellitus in the prevention of renal and vascular end-stage disease study. J Clin Lipidol. 2019;13(1):129–137. doi: 10.1016/j.jacl.2018.11.009. e1. [DOI] [PubMed] [Google Scholar]
- 18.Flores-Guerrero J.L., Oste M.C.J., Kieneker L.M., et al. Plasma branched-chain amino acids and risk of incident type 2 diabetes: results from the PREVEND prospective cohort study. J Clin Med. 2018;7(12) doi: 10.3390/jcm7120513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores-Guerrero J.L., Gruppen E.G., Connelly M.A., et al. A newly developed diabetes risk index, based on lipoprotein subfractions and branched chain amino acids, is associated with incident type 2 diabetes mellitus in the PREVEND cohort. J Clin Med. 2020;9(9) doi: 10.3390/jcm9092781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connelly M.A., Gruppen E.G., Otvos J.D., Dullaart R.P. Inflammatory glycoproteins in cardiometabolic disorders, autoimmune diseases and cancer. Clin Chim Acta. 2016;459:177–186. doi: 10.1016/j.cca.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Connelly M.A., Otvos J.D., Shalaurova I., Playford M.P., Mehta N.N. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J Transl Med. 2017;15(1):219. doi: 10.1186/s12967-017-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta N.N., Dey A.K., Maddineni R., Kraus W.E., Huffman K.M. GlycA measured by NMR spectroscopy is associated with disease activity and cardiovascular disease risk in chronic inflammatory diseases. Am J Prevent Cardiol. 2020;4 doi: 10.1016/j.ajpc.2020.100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rochon J., Bales C.W., Ravussin E., et al. Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. J Gerontol Series A, Biol Sci Med Sci. 2011;66(1):97–108. doi: 10.1093/gerona/glq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravussin E., Redman L.M., Rochon J., et al. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol Series A, Biol Sci Med Sci. 2015;70(9):1097–1104. doi: 10.1093/gerona/glv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rickman A.D., Williamson D.A., Martin C.K., et al. The CALERIE study: design and methods of an innovative 25% caloric restriction intervention. Contemp Clin Trials. 2011;32(6):874–881. doi: 10.1016/j.cct.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong W.W., Roberts S.B., Racette S.B., et al. The doubly labeled water method produces highly reproducible longitudinal results in nutrition studies. J. Nutr. 2014;144(5):777–783. doi: 10.3945/jn.113.187823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Racette S.B., Das S.K., Bhapkar M., et al. Approaches for quantifying energy intake and%calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. Am J Physiol Endocrinol Metabol. 2012;302(4):E441–E448. doi: 10.1152/ajpendo.00290.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Look A.R.G., Wadden T.A., West D.S., et al. The look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14(5):737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pieper C., Redman L., Racette S., et al. Development of adherence metrics for caloric restriction interventions. Clinical trials. 2011;8(2):155–164. doi: 10.1177/1740774511398369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anton S.D., LeBlanc E., Allen H.R., et al. Use of a computerized tracking system to monitor and provide feedback on dietary goals for calorie-restricted diets: the POUNDS LOST study. J Diabetes Sci Technol. 2012;6(5):1216–1225. doi: 10.1177/193229681200600527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeyarajah E.J., Cromwell W.C., Otvos J.D. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26(4):847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Matyus S.P., Braun P.J., Wolak-Dinsmore J., et al. NMR measurement of LDL particle number using the Vantera clinical analyzer. Clin Biochem. 2014;47(16–17):203–210. doi: 10.1016/j.clinbiochem.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Makri A., Cheung A., Sinaii N., et al. Lipoprotein particles in patients with pediatric cushing disease and possible cardiovascular risks. Pediatr. Res. 2019;86(3):375–381. doi: 10.1038/s41390-019-0438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dmitrienko A., Millen B.A., Brechenmacher T., Paux G. Development of gatekeeping strategies in confirmatory clinical trials. Biom J. 2011;53(6):875–893. doi: 10.1002/bimj.201100036. [DOI] [PubMed] [Google Scholar]
- 35.Laird N.M., Ware J.H. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 36.Lewis J.A. Statistical principles for clinical trials (ICH E9): an introductory note on an international guideline. Stat Med. 1999;18(15):1903–1942. doi: 10.1002/(sici)1097-0258(19990815)18:15<1903::aid-sim188>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 37.Dmitrienko A., Tamhane A.C. Mixtures of multiple testing procedures for gatekeeping applications in clinical trials. Stat Med. 2011;30(13):1473–1488. doi: 10.1002/sim.4008. [DOI] [PubMed] [Google Scholar]
- 38.Das S.K., Roberts S.B., Bhapkar M.V., et al. Body-composition changes in the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE)-2 study: a 2-y randomized controlled trial of calorie restriction in nonobese humans. Am J Clin Nutr. 2017;105(4):913–927. doi: 10.3945/ajcn.116.137232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garvey W.T., Kwon S., Zheng D., et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52(2):453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 40.Hoeting N.A., Rohatgi A. Deep phenotyping of HDL particles: characterization of seven HDL species and their relationship to cardiometabolic phenotypes in a multi-ethnic population (Dallas Heart Study) Arterioscl Thromb Vasc Biol. 2019;38:170. C. R. [Google Scholar]
- 41.Avogaro P., Bon G.B., Cazzolato G., Quinci G.B. Are apolipoproteins better discriminators than lipids for atherosclerosis? Lancet. 1979;1(8122):901–903. doi: 10.1016/s0140-6736(79)91375-8. [DOI] [PubMed] [Google Scholar]
- 42.Raitakari O.T., Makinen V.P., McQueen M.J., et al. Computationally estimated apolipoproteins B and A1 in predicting cardiovascular risk. Atherosclerosis. 2013;226(1):245–251. doi: 10.1016/j.atherosclerosis.2012.10.049. [DOI] [PubMed] [Google Scholar]
- 43.Ference B.A., Kastelein J.J.P., Ray K.K., et al. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA. 2019;321(4):364–373. doi: 10.1001/jama.2018.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glynn E.L., Piner L.W., Huffman K.M., et al. Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia. 2015;58(10):2324–2335. doi: 10.1007/s00125-015-3705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGarrah R.W., Zhang G.F., Christopher B.A., et al. Dietary branched-chain amino acid restriction alters fuel selection and reduces triglyceride stores in hearts of Zucker fatty rats. Am J Physiol Endocrinol Metabol. 2020;318(2):E216–EE23. doi: 10.1152/ajpendo.00334.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang Y.J., Sun S.J., Cao W.X., et al. Excessive ROS production and enhanced autophagy contribute to myocardial injury induced by branched-chain amino acids: roles for the AMPK-ULK1 signaling pathway and alpha7nAChR. Biochim Biophys Acta Mol Basis Dis. 2020;1867(1) doi: 10.1016/j.bbadis.2020.165980. [DOI] [PubMed] [Google Scholar]
- 47.Zhao H., Zhang F., Sun D., et al. Branched-chain amino acids exacerbate obesity-related hepatic glucose and lipid metabolic disorders via attenuating Akt2 signaling. Diabetes. 2020;69(6):1164–1177. doi: 10.2337/db19-0920. [DOI] [PubMed] [Google Scholar]
- 48.Crossland H., Smith K., Isdir I., Phillips B.E., Atherton P.J., Dilkinson D.J. Exploring mechanistic links between extracellular BCAA & muscle insulin resistance: an in vitro approach. Am J Physiol Cell Physiol. 2020 doi: 10.1152/ajpcell.00377.2020. In press. [DOI] [PubMed] [Google Scholar]