Abstract
Quantification of contaminant concentrations in baleen whales is important for individual and population level health assessments but is difficult due to large migrations and infrequent resighings. The use of baleen allows for a multiyear retrospective analysis of contaminant concentrations without having to collect repeated samples from the same individual. Here we provide case studies of mercury analysis using cold vapor atomic absorption spectroscopy in three individual humpback whales (Megaptera novaeangliae), a 44.5-year-old female and two males aged ≥35 and 66 years, over approximately three years of baleen growth. Mercury concentrations in the female's baleen were consistently 2–3 times higher than in either male. Age did not affect mercury concentrations in baleen; the younger male had comparable levels to the older male. In the female, mercury concentrations in the baleen did not change markedly during pregnancy but mercury did spike during the first half of lactation. Stable isotope profiles suggest that diet likely drove the female's high mercury concentrations. In conclusion, variations in baleen mercury content can be highly individualistic. Future studies should compare sexes as well as different populations and species to determine how the concentrations of mercury and other contaminants vary by life history parameters and geography.
Keywords: Marine, Mammal, Mercury, Toxicology, Whale, Physiology, Baleen
Marine; Mammal; Mercury; Toxicology; Whale; Physiology; Baleen.
1. Introduction
Baleen whales are long lived and have the potential to accumulate toxic concentrations of contaminants, such as mercury, in their metabolically active tissues. Mercury is an endocrine disruptor (Iavicoli et al., 2009; Rice et al., 2014) and the release of mercury during activities that are mediated by the endocrine system (e.g., fasting and pregnancy) may interfere with normal functions. Therefore, effective conservation and management of baleen whales requires an improved understanding of their contaminant exposure. Contaminants can damage the functioning of systems throughout the body, leading to declines in health, as well as elevated mortality and reduced natality (reviewed in Wolfe et al., 1998). Unfortunately, contaminant information is lacking for many baleen whales due to the inherent difficulties of studying large underwater species with long lifespans and extensive migrations.
Baleen is a continuously growing keratinous tissue that hangs from the upper jaw of mysticete whales and functions as a filter feeding apparatus that is unique to this clade (Schell et al., 1989). Humpback whales (Megaptera novaeangliae) have approximately 600 total baleen plates (Werth et al., 2018) and each plate is triangular, and composed of long α-keratin bundles embedded in a fingernail-like calcium-salt matrix (Werth et al., 2020). Like many other mammalian external keratinous tissues (e.g., hair, nails, claws, and hooves), baleen grows slowly at its base throughout life (Fudge et al., 2009), although the rate of growth can vary by individual, age, and body condition. Because the baleen plates are continuously growing and are composed mainly of material derived from food, they can provide a temporal record of contaminant exposure, including exposure to mercury (Hobson et al., 2004). The concentration of mercury in baleen is positively correlated with mercury concentration in metabolically active tissues such as muscle, liver, and kidney (Hobson et al., 2004) and is a useful tool to examine multi-year exposure patterns. Baleen can be collected at necropsy or during harvest and many baleen plates from various whale populations are stored in museum collections, making it an ideal tissue type for retrospective contaminant analysis.
Inorganic mercury in marine systems can be converted to methylmercury by bacteria, which can then bioaccumulate in animals and biomagnify in food webs (Boudou and Ribeyre, 1997; Kidd et al., 2012; Morel et al., 1998). Methylmercury levels in phytoplankton are up to 105 times higher than in surrounding seawater (Lee and Fisher, 2016). Higher trophic level organisms (e.g., toothed whales that consume mammals) will generally have higher methylmercury body burdens than species that consume lower trophic level organisms (Haraguchi et al., 2000; Simmonds et al., 2002). Baleen whales eat relatively low on the trophic scale, with many species eating zooplankton and small schooling fish. Humpback whales eat a varied diet consisting of krill and various schooling fish (e.g., anchovy (Engraulidae), herring (Clupeidae), sand lance (Ammodytes), capelin (Mallotus villosus)) (Clapham et al., 1997; Soerensen et al., 2010). However, the long lifespan of the humpback whale (>90 years; Chittleborough, 1959) can result in high contaminant body burdens through bioaccumulation.
In addition to long lifespans, humpback whales also exhibit dramatic changes in body condition across the year; fat is gained prior to southward migration and lost during migration, overwinter fasting, and pregnancy. Body condition changes can influence body burdens of contaminants (Bengtson Nash et al., 2013; Polischuk et al., 2002), with higher concentrations of contaminants during periods of low body condition. Humpback whales are capital breeders that fast during migration and breeding, utilizing large blubber energy stores that are developed during the summer feeding season (Chittleborough, 1965; Clapham, 2018). Due to extreme fluctuations in lipid stores, periods of breeding or fasting may represent a phase of elevated risk of heightened mercury concentrations (i.e., in circulating plasma) to both individuals and nutritionally dependent young (Bengtson Nash et al., 2013). During periods of fasting or high metabolic demands, mercury can be released from fat reserves and become bioavailable and result in the redistribution of mercury across the body (Bengtson Nash et al., 2013; Debier et al., 2006). When mercury is in plasma, it can travel to tissues that are more sensitive to contaminants, such as the brain and thyroid (Aschner an Aschner, 1990; Mori et al., 2006; Wada et al., 2009; Watanabe et al., 1999). These repeated changes in body mass that occur in humpback whales during migration and pregnancy may therefore result in increased exposure to bioavailable mercury and other contaminants during particularly sensitive periods of development (e.g., pregnancy).
Mercury concentrations in baleen have the potential to serve as a useful indicator of mercury (and other contaminant) body burden during and across annual cycle stages of migration, breeding, pregnancy and lactation. Mercury exposure damages numerous life systems, including the reproductive system, further exacerbating cetacean population declines. Marine pollution, especially that of nearshore feeding habitats critical to migratory species such as humpback whales, has reached crisis levels (Selin, 2009; Strode et al., 2010). To date, there have been no studies examining mercury content over multiple years in this species. Quantifying how mercury accumulates and offloads during various life stages could be useful for understanding possible deleterious health effects of mercury in this important population. Our study provides a case study of mercury levels in baleen of three humpback whales: a 44.5-year-old female who experienced two pregnancies during the baleen growth period, and two males, a ≥35-year-old and 66-year-old, all from the North Pacific population. The female was expected to have lower concentrations of mercury than the males as she would have offloading capabilities through pregnancy and lactation. The ≥35-year-old male was expected to have lower concentrations than the 66-year-old whale, as the older whale had more time to accumulate mercury. Baleen has been shown to correlate with organ tissues (Hobson et al., 2004) and the results of this study may help guide future work on contaminant concentrations in baleen with the goal of yielding valuable multiyear analyses on pollutant exposures in large free-living cetaceans.
2. Methods
2.1. Study animals
We studied the baleen recovered during necropsy of one female (SEAK 68) and two male (SEAK 441, SEAK 1536) humpback whales (Table 1). All three were from the North Pacific population and were found dead on beaches in southeast Alaska. Lactation periods are expected to last for approximately 10 months after conception; conception was averaged to occur in January (most conceptions are likely to occur December through February). Fasting periods occur when the whales are migrating to Hawai'i and back and occur in the winter months, from approximately October until May.
Table 1.
Life history information of the three humpback whales (Megaptera novaeangliae) used In this study. Age was determined via earplug analysis and year of birth is approximate. Cause of death was determined by findings at necropsies.
| Whale ID | Sex | Age (Appx. year of birth) | Length (m) | Cause of death |
|---|---|---|---|---|
| SEAK 68 | Female | 44.5 (1956) | 13.87 | Ship strike |
| SEAK 441 | Male | 66 (1950) | 13.90 | Chronic Illness |
| SEAK 1536 | Male | ≥35 (<1983) | 11.80 | Ship strike |
SEAK 68 was an adult female that was first reported in the Alaska fluke catalog in 1975 and was seen with a calf five of the thirteen years she was spotted; her pregnancies were tracked through the fluke catalog. She was found dead in 2001 in Glacier Bay, Alaska and upon examination was determined to be pregnant (fetus length 39.2 cm), with cause of death identified as ship strike (Gulland, 2001). Earplug analysis indicates she was approximately 44.5 years old (Gabriele et al., 2010) and previous studies (Lowe et al., 2021) using stable isotopes (SI) show her baleen covers 3.5 years of growth from 1998-2001.
SEAK 441 was an adult male (66 years old) that was one of the longest sighted humpbacks in the world, with a sighting record spanning 45 years (Gabriele et al., 2021). His baleen record spans 4.5 years from 2012-2016 (Lowe et al., 2021). He was first sighted in 1972 and died in 2016 (Gabriele et al., 2021). His necropsy showed extensive external whale lice, scattered superficial erosions and ulcerations suggestive of generalized debilitation or immunosuppression, multiple tissue infections, and multiple severe caudal vertebrae abnormalities indicative of prolonged disease (Gabriele et al., 2021). Measurement of blubber during necropsy indicated poor body condition and low oil content.
SEAK 1536 was found dead in 2018 and estimated to be ≥ 35 years old based on sighting history (Savage, 2018). Extensive hemorrhaging and bone fractures suggest that he was killed by a ship strike (Savage, 2018). His baleen was grown over approximately 4 years, from 2015-2018.
2.2. Baleen cleaning and estimating date of growth
Baleen was removed from each whale during necropsy or from the beach after the whale died and, if necessary, cleaned of any remaining tissue and sent to Northern Arizona University (Flagstaff, AZ) for analysis. Baleen plates were then marked at each centimeter from the base to the tip along the posterior face of each plate, starting approximately 1 cm from the labial edge lengthwise. The newest grown baleen, at the base of the plate, was designated as the “zero cm” point and was assigned an estimated growth date of the day before the whale was found dead. Baleen for two of the whales (SEAK 68, SEAK 441) had previously been analyzed for stable isotopes of nitrogen, allowing estimation of growth rate of the baleen plate via counting the number of annual cycles in ratio of 15N/14N (δ15N) (for full methodology see Rogers et al., in preparation). Briefly, the δ15N changes seasonally as whales migrate between isotopically distinct summer and winter latitudes (Hobson et al., 2004; Hobson and Schell, 1998; Lubetkin et al., 2008) and each year was determined from δ15N peaks after smoothing from four nearest neighbors. For SEAK 1536 and SEAK 441, every 4 cm was sampled for total mercury. SEAK 68 was sampled with a 1–11 cm range due to extensive prior analysis of her baleen plate that did not allow for evenly spaced sampling.
2.3. Mercury analysis
We measured total mercury; however, mercury incorporated into baleen keratin is likely to be nearly all methylated, similar to what has been found in feathers (Furness et al., 1986; Furness, 1993). Baleen was powdered using an electric rotary grinder (Dremel Model 395 Type 5) to abrade a short (<1.0 cm) transverse groove across the posterior face of the plate, with the powder collected on a piece of weigh paper (Rogers et al., in preparation). Total number of baleen samples was n = 20 for SEAK 68, n = 15 for SEAK 441, and n = 15 for SEAK 1536.
To extract the powdered baleen for mercury, 15–93 mg of dried baleen tissue was used per sample. Each sample underwent an open acid digestion (adapted from Furness, 1993) by adding 1.5 mL hydrochloric acid (HCl; Fisher Chemical), 1 mL of 5% potassium permanganate in 0.1% HCl (KMnO4; Fisher Chemical, Waltham, MA), 100-200uL hydrogen peroxide (H2O2), and 7 mL deionized (DI) water and digesting overnight at room temperature. After 12–15 h, samples were capped and put into a hot water bath at 85ᵒC for 2 h. Samples were filtered using glass fiber syringe filters (0.45 um). Each sample was diluted to 15 mL with DI water prior to analysis. QA/QC samples, extraction blanks, and a certified reference standard (10 ppb Hg tuna fish flesh homogenate; IAEA-436) were prepared along with samples to ensure quality control and determine extraction efficiencies ( extraction efficiency 99%). Standards were made from 1000 μg/mL Hg standard stock (PerkinElmer, Waltham, MA) and serially diluted to 10 ppb, 5 ppb, and 0.5 ppb concentrations. An analysis QC sample (10 ppm Hg; Inorganic Ventures) was diluted to 1 ppb and run as an unknown sample. Prior to analysis, 100 uL hydroxylamine hydrochloride (NH2OH • HCl; Medivators) was added to each sample. Total Hg concentrations were analyzed using a FIMS-100 cold-vapor atomic absorption analyzer with an auto-sampling unit (PerkinElmer, Waltham, MA) and argon gas. Tin (II) chloride dihydrate (SnCl2; Fischer Chemical, Waltham, MA) was used as the reductant and HCl as the carrier solution.
2.4. Statistical analysis
Statistical analyses were done using R 4.0.2 (R Core Team, 2020), package ‘nlme’. To understand how mercury content changed between individuals, we used mixed linear effects (nlme package, Bates et al., 2011) to model the dependent variable (mercury concentration) as a function of individual (whale ID). As samples were obtained from a single baleen plate for each individual, we included the distance from baseline (cm) from each sample as random factors. The models were constructed as follows:
| Mercury concentration ∼ WhaleID, random = list(∼1|cm) |
3. Results
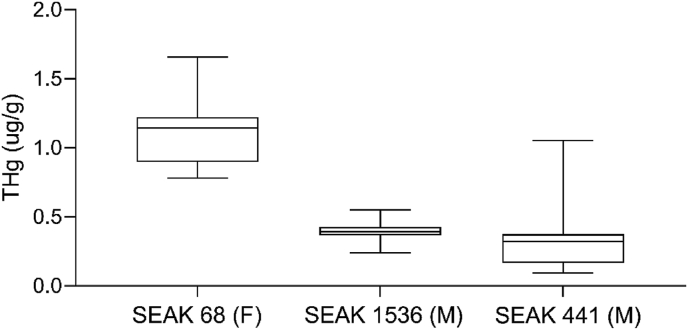
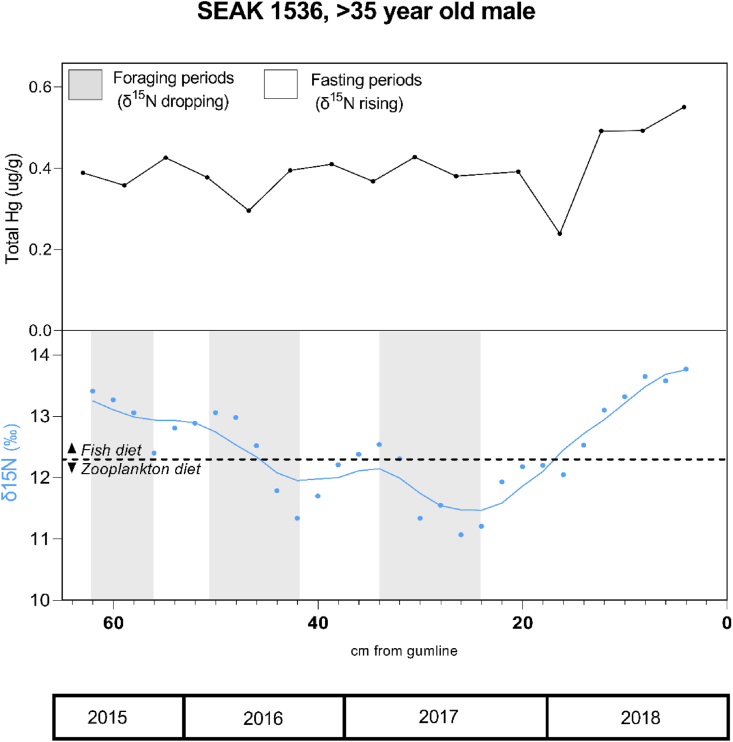
Total mercury was detectable in all samples, ranging from 0.096 – 1.65 μg/g. Total mercury concentrations varied among individuals (Table 2; Figure 1). The two male whales (Figures 3, 4) had lower, relatively stable mercury concentrations throughout the baleen growth period compared to the female (Table 2, Figure 1). The average total mercury concentration of the baleen in the 44.5-year-old female was 30% higher during lactation than at other points during her baleen growth (Table 1, Figure 2), with the two highest mercury concentrations occurring during the first half of lactation. The older male that died from a chronic illness showed fluctuations in mercury concentration during the last 6–9 months of life, with an increase in mercury at 8 cm (0.79 μg/g) and then a decrease to a low value at 6 cm (0.16 μg/g) before rising again to the highest level seen along the plate at 4 cm (1.05 μg/g, approximately four months before death) (Figure 3). The ≥35-year-old male had relatively low and stable mercury concentrations, with a dip (16 cm) during the winter/fasting season before death and the highest concentration occurring just before death (in summer, Figure 4).
Table 2.
Total mercury concentrations in the baleen of three humpback whales (Megaptera novaeangliae) in ug mercury per gram of baleen powder. Whale ID includes sex and age as determined from necropsy, sighting history and/or earplug analysis.
| Whale ID (sex, age) | Avg ±SD (ug/g) |
|---|---|
| SEAK 68 (F, 44.5) | 1.12 ± 0.22 |
| During pregnancy | 1.00 ± 0.15 |
| During lactation | 1.34 ± 0.20 |
| SEAK 1536 (M, ≥35) | 0.40 ± 0.08 |
| SEAK 441 (M, 66) | 0.35 ± 0.26 |
Figure 1.
Total mercury concentrations (ug mercury per g baleen powder) from three humpback whales (Megaptera novaeangliae). X axis indicates stranding ID along with sex (M/F).
Figure 3.
Total mercury concentrations and δ15N along a baleen plate from SEAK 441, a 66-year-old male humpback whale (Megaptera novaeangliae). Dates are extrapolated via stable isotope analysis from date of death. Top panel is total mercury concentrations in ug of mercury per gram of dried baleen powder. Lower panel shows δ15N (‰, blue) versus air with gray boxes representing areas of expected feeding periods; line is smoothed from four nearest neighbors. Stable isotope inference of prey consumption can be determined via troughs; peaks do not represent an increase in fish consumption.
Figure 4.
Total mercury concentrations and δ15N along a baleen plate from SEAK 1536, a >35-year-old male humpback whale (Megaptera novaeangliae). Dates are extrapolated via stable isotope analysis from date of death. Top panel is total mercury concentrations in ug of mercury per gram of dried baleen powder. Lower panel shows δ15N (‰, blue) versus air with gray boxes representing areas of expected feeding periods; line is smoothed from four nearest neighbors. Stable isotope inference of prey consumption can be determined via troughs; peaks do not represent an increase in fish consumption.
Figure 2.
Total mercury concentrations and δ15N along a baleen plate from SEAK 68, a 44.5-year-old female humpback whale (Megaptera novaeangliae). Dates are extrapolated via stable isotope analysis from date of death. Top panel is total mercury concentrations in ug of mercury per gram of dried baleen powder. Blue boxes represent period of expected pregnancy based on sighting history; yellow box is the period of expected lactation. Lower panel shows δ15N (‰, blue) versus air with gray boxes representing areas of expected feeding periods; line is smoothed from four nearest neighbors. Stable isotope inference of prey consumption can be determined via troughs; peaks do not represent an increase in fish consumption.
4. Discussion
Total mercury was detected in all samples along the baleen plates for the three humpback whales, demonstrating that this sample type is viable for analysis of multi-year patterns of mercury concentrations. Total mercury concentration did not vary based on fasting or feeding season. The female showed consistently higher total mercury levels than did the two males and had much larger variation along the length of the baleen. Total mercury concentrations were highest during the expected period of lactation compared to pregnancy and nonpregnancy. Individual variation in total mercury accumulation could be due to sex, age, or diet, among other factors.
During the gestation period, contaminants can be transferred to the fetus through the placenta (Chen et al., 2014; Clarkson et al., 2007) or via milk during lactation (Habran et al., 2011, 2013; Noël et al., 2016). Methylmercury is lipophilic and readily binds to proteins (Lin et al., 2012; Steele and Opella, 1997); whale milk has high fat and protein content (Lauer and Baker, 1969; Ohta et al., 1953), making it likely that methylmercury transfers to the neonate through milk. Female striped dolphins (Stenella coeruleoalba) were estimated to lose 4–9% of their organochlorine loads during gestation and 72–91% during lactation, showing that, for some species, the bulk of pollutant offloading occurs during lactation (Tanabe et al., 1982). However, we did not find differences in total mercury concentrations during either pregnancy and, rather than a decline, we observed an increase during lactation. This could be due to the excretion and offloading differences in contaminants between adipose tissues and circulating levels. It is possible that the mercury levels in humpback blubber might decline during lactation and increase in circulating concentrations, leading to an increase in mercury in the baleen. Minke whale baleen was significantly correlated with concentrations in tissues such as kidney, liver and muscle (Hobson et al., 2004), showing that baleen could be a good indicator of internal contaminants. However, how the patterns of mercury accumulation and offloading in various humpback whale sample types (i.e., organs, baleen) change during migration, fasting, pregnancy, and lactation warrants further investigation with larger sample sizes to determine how well baleen reflects each internal tissue.
Elevated total mercury concentrations in the female compared to the two males could be due to individual differences in prey consumption. Nitrogen isotopes are commonly used to assess diet and quantification of δ15N allowed for insight into the prey consumption of two of the three whales (SEAK 68 and SEAK 441; Rogers et al., in preparation). The δ15N analysis revealed that SEAK 68 was likely consuming mostly small fish whereas SEAK 441 preyed on zooplankton (Figures 1 and 2; Rogers et al., in preparation). Differences in diet could account for the higher concentration of total mercury in the baleen of SEAK 68 compared with SEAK 441 because fish are at a higher trophic level than zooplankton. SEAK 1536 fed on both zooplankton and fish, depending on year, but did not show an increase in total mercury concentration that coincided with a switch to fish consumption. Future analysis should account for individual variation in diet and its influence on contaminant uptake.
Because methylmercury bioaccumulates, we expected that total mercury concentration in baleen would increase with age in both males and females, as seen in the tissues of other marine mammals (Wagemann and Muir, 1984; Wagemann et al., 1998), including polar bear hair (Dietz et al., 2006), beluga whale teeth (Outridge et al., 2002) and minke whale baleen (Hobson et al., 2004). However, the 66-year-old male in the study had a mean concentration of mercury that was similar to that of the ≥35-year-old male, and both were approximately a third of that observed in the 44.5-year-old female, a difference that is likely due to diet (see above). It is also possible that the ≥35-year-old male was closer in age to the 66-year-old whale, explaining why they had similar mercury concentrations in their baleen; since the ≥35-year-old male is a minimum of 35, it is possible that both whales are 60 + years old. Due to the small sample size, it is unknown if males’ mercury concentrations asymptote as they age or if the individuals in these case studies represent their age classes. The relationship between age and mercury, as well as tissue tropism of mercury in whales, warrants further study with a larger sample size of humpback whales from various areas and of different sexes and age classes.
Mercury concentrations in the baleen of minke (Balaenoptera acutorostrata, Hobson et al., 2004) and bowhead whales (Eubalaena glacilias; Pomerleau et al., 2018) peaked at ∼0.58–0.59 μg/g; the female humpback whale in this study peaked at 1.66 μg/g. Minke and humpback whales feed on similar prey (flexible feeders that will eat krill, capelin (M. villosus), and herring; Nemoto, 1970; Skaug et al., 1997) but minke whales are much smaller (8–9 m) than humpbacks (∼15 m) and offloading rates of mercury are slower in larger animals (Kidd et al., 2012; Trudel and Rasmussen, 2006). Because of this, long-lived cetacean species should be examined for contaminant concentrations regardless of their prey preferences, because animals that feed low on the trophic scale may still harbor unexpectedly high concentrations of contaminants. Future studies should compare baleen mercury concentrations from a variety of species throughout the world to better understand the conservation and management implications.
The accumulation of contaminants into biologically inactive tissues such as baleen, hair, and feathers outside of the growth zone, raises the question of how much of a contaminant is offloaded from the animal when those tissues are shed. Humpback whales grow approximately 15 g of baleen per plate per year (Werth et al., 2020). Given approximately 600 plates per individual (Werth et al., 2018), the total amount of baleen grown per year is ∼9000 g. The female with high mercury concentrations would therefore offload ∼10,080 μg of mercury per year into her baleen plates. For the two males (SEAK 1536 and SEAK 441), the mean mercury concentrations were much lower, making the average mercury offload ∼3,600 and 3,150 μg per year, respectively. Mercury concentration in minke whale baleen is positively correlated with mercury concentration in metabolically active tissues, such as muscle, liver and kidney (Hobson et al., 2004). Necropsy teams should routinely collect both baleen and internal samples when possible so that this relationship can be assessed for humpback whales. Studies examining how mercury is incorporated into baleen and the affinity of mercury for keratin will be useful to determine how baleen reflects other body tissues.
Baleen is a useful tissue type for multi-year studies, but the growth rate, and thus the resolution for mercury, is too low to determine patterns over days or weeks. Additionally, baleen is opportunistically available only from deceased individuals, so baleen studies must be retrospective. Historic baleen samples go back as far as the late 19th century for some species (https://arctos.database.museum/guid/DMNS:Mamm:6700), making it possible to examine contaminant increases over timescales greater than a century. We recommend, future studies examining mercury concentrations in baleen model the effects of sex, diet, habitat use, body size, populations, and species.
5. Conclusion
Sampling the same individual whale at different ages can reveal how mercury accumulation changes as the animal migrates and fasts, and for females, as she gestates and lactates; these are important factors in understanding how certain sexes or populations might be at risk for mercury toxicity. Even though marine mammals are exposed to toxic concentrations of mercury (López-Berenguer et al., 2020; Wagemann et al., 1998), few whale studies examine accumulation across life stages, likely due to the difficulty of obtaining temporal records of mercury accumulation in free-living cetaceans. Use of baleen could produce enough data to assess population-wide levels of contamination, which is important for both ecological monitoring as well as informing groups that consume baleen whales. Mercury exposure will continue to increase with anthropogenic development, creating a need for additional biomonitoring techniques, such as the use of baleen, in order to track the impacts of pollution and of global efforts to combat it.
Declarations
Author contribution statement
Carley L. Lowe: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Renee Jordan-Ward: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kathleen E. Hunt: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Matthew C. Rogers: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Alexander J. Werth: Analyzed and interpreted the data; Wrote the paper.
Chris Gabriele, Janet Neilson, Frank A. von Hippel & C. Loren Buck: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Aschner M., Aschner J.L. Mercury neurotoxicity: mechanisms of blood-brain barrier transport. Neurosci. Biobehav. Rev. 1990;14(2):169–176. doi: 10.1016/s0149-7634(05)80217-9. [DOI] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S., Christensen R.H.B., Singmann H., et al. 2011. Package ‘lme4’. Linear Mixed-effects Models using S4 classes. R package version. 1(6) [Google Scholar]
- Bengtson Nash S.M., Waugh C.A., Schlabach M. Metabolic concentration of lipid soluble organochlorine burdens in the blubber of southern hemisphere humpback whales through migration and fasting. Environ. Sci. Technol. 2013;47(16):9404–9413. doi: 10.1021/es401441n. [DOI] [PubMed] [Google Scholar]
- Boudou A., Ribeyre F. Mercury in the food web: accumulation and transfer mechanisms. Met. Ions Biol. Syst. 1997;34:289–320. [PubMed] [Google Scholar]
- Chen Z., Myers R., Wei T., Bind E., Kassim P., Wang G., Ji Y., Hong X., Caruso D., Bartell T. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J. Expo. Sci. Environ. Epidemiol. 2014;24(5):537–544. doi: 10.1038/jes.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittleborough R.G. Determination of age in the humpback whale, Megaptera nodosa (Bonnaterre) Marine and Freshwater Res. 1959;10(2):125–143. [Google Scholar]
- Chittleborough R. Dynamics of two populations of the humpback whale, Megaptera novaeangliae (Borowski) Mar. Freshw. Res. 1965;16(1):33–128. [Google Scholar]
- Clapham P.J. Elsevier; 2018. Humpback Whale: Megaptera novaeangliae, Encyclopedia of marine Mammals; pp. 489–492. [Google Scholar]
- Clapham P.J., Leatherwood S., Szczepaniak I., Brownell R.L., Jr. Catches of humpback and other whales from shore stations at Moss Landing and Trinidad, California, 1919–1926. Mar. Mamm. Sci. 1997;13(3):368–394. [Google Scholar]
- Clarkson T.W., Vyas J.B., Ballatori N. Mechanisms of mercury disposition in the body. Am. J. Ind. Med. 2007;50(10):757–764. doi: 10.1002/ajim.20476. [DOI] [PubMed] [Google Scholar]
- Debier C., Chalon C., Le Bœuf B.J., de Tillesse T., Larondelle Y., Thomé J.-P. Mobilization of PCBs from blubber to blood in northern elephant seals (Mirounga angustirostris) during the post-weaning fast. Aquat. Toxicol. 2006;80(2):149–157. doi: 10.1016/j.aquatox.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Dietz R., Riget F., Born E.W., Sonne C., Grandjean P., Kirkegaard M., Andreasen C. Trends in mercury in hair of Greenlandic polar bears (Ursus maritimus) during 1892 – 2001. Environ. Sci. Technol. 2006;40(4):1120–1125. doi: 10.1021/es051636z. [DOI] [PubMed] [Google Scholar]
- Fudge D.S., Szewciw L.J., Schwalb A.N. Morphology and development of blue whale baleen: an annotated translation of Tycho Tullberg's classic 1883 paper. Aquat. Mamm. 2009;35(2):226. [Google Scholar]
- Furness R., Muirhead S., Woodburn M. Using bird feathers to measure mercury in the environment: relationships between mercury content and moult. Mar. Pollut. Bull. 1986;17(1):27–30. [Google Scholar]
- Furness R.W. Springer; 1993. Birds as Monitors of Pollutants, Birds as Monitors of Environmental Change; pp. 86–143. [Google Scholar]
- Gabriele C.M., Lockyer C., Straley J.M., Jurasz C.M., Kato H. Sighting history of a naturally marked humpback whale (Megaptera novaeangliae) suggests ear plug growth layer groups are deposited annually. Mar. Mamm. Sci. 2010;26(2):443–450. [Google Scholar]
- Gabriele C.M., Taylor L.F., Huntington K.B., Buck C.L., Hunt K.E., Lefebvre K.A., Lockyer C., Lowe C., Moran J.R., Murphy A., et al. National Park Service; Fort Collins, Colorado: 2021. Humpback Whale #441 (Festus): Life, Death, Necropsy, and Pathology, Natural Resource Report NPS/GLBA/NRR—2021/2250. [Google Scholar]
- Gulland F. 2001. Humpback Whale SEAK 68 Necropsy Report Glacier Bay National Park: Unpublished Report. [Google Scholar]
- Habran S., Debier C., Crocker D.E., Houser D.S., Das K. Blood dynamics of mercury and selenium in northern elephant seals during the lactation period. Environ. Pollut. 2011;159(10):2523–2529. doi: 10.1016/j.envpol.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Habran S., Pomeroy P.P., Debier C., Das K. Changes in trace elements during lactation in a marine top predator, the grey seal. Aquat. Toxicol. 2013;126:455–466. doi: 10.1016/j.aquatox.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Haraguchi K., Endo T., Sakata M., Masuda Y., Simmonds M. Contamination survey of heavy metals and organochlorine compounds in cetacean products purchased in Japan. J. Food Hyg. Soc. Jpn. 2000;41(4):287–296. [Google Scholar]
- Hobson K., Riget F., Outridge P., Dietz R., Born E. Baleen as a biomonitor of mercury content and dietary history of North Atlantic minke whales (Balaenopetra acutorostrata): combining elemental and stable isotope approaches. Sci. Total Environ. 2004;331(1-3):69–82. doi: 10.1016/j.scitotenv.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Hobson K.A., Schell D.M. Stable carbon and nitrogen isotope patterns in baleen from eastern Arctic bowhead whales (Balaena mysticetus) Can. J. Fish. Aquat. Sci. 1998;55(12):2601–2607. [Google Scholar]
- Iavicoli I., Fontana L., Bergamaschi A. The effects of metals as endocrine disruptors. J. Toxicol. Environ. Health, Part B. 2009;12(3):206–223. doi: 10.1080/10937400902902062. [DOI] [PubMed] [Google Scholar]
- Kidd K., Clayden M., Jardine T. Wiley; Hoboken, NJ: 2012. Bioaccumulation and Biomagnification of Mercury through Food Webs. [Google Scholar]
- Lauer B., Baker B. Whale milk. I. Fin whale (Balaenoptera physalus) and beluga whale (Delphinapterus leucas) milk: gross composition and fatty acid constitution. Can. J. Zool. 1969;47(1):95–97. doi: 10.1139/z69-017. [DOI] [PubMed] [Google Scholar]
- Lee C.S., Fisher N.S. Methylmercury uptake by diverse marine phytoplankton. Limnol. Oceanogr. 2016;61(5):1626–1639. doi: 10.1002/lno.10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Yee N., Barkay T. 2012. Microbial Transformations in the Mercury Cycle. [Google Scholar]
- López-Berenguer G., Peñalver J., Martínez-López E. A critical review about neurotoxic effects in marine mammals of mercury and other trace elements. Chemosphere. 2020;246:125688. doi: 10.1016/j.chemosphere.2019.125688. [DOI] [PubMed] [Google Scholar]
- Lowe C.L., Hunt K.E., Robbins J, Seton R.E., Rogers M.C., Gabriele C.M., Neilson J.L., Landry S., Teerlink S.S., Buck C.L. Patterns of cortisol and corticosterone concentrations in humpback whale (Megaptera novaeangliae) baleen are associated with different causes of death. Conserv. Physiol. 2021;9(1):coab096. doi: 10.1093/conphys/coab096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C.L., Hunt K.E., Rogers M.C., Neilson J.L., Robbins J., Gabriele C.M., Teerlink S., Seton R.E., Buck C.L. Multi-year progesterone profiles during pregnancy in baleen of humpback whales (Megaptera novaeangliae) Conserv. Physiol. 2021 doi: 10.1093/conphys/coab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubetkin S., Zeh J., Rosa C., George J. Age estimation for young bowhead whales (Balaena mysticetus) using annual baleen growth increments. Can. J. Zool. 2008;86(6):525–538. [Google Scholar]
- Morel F.M., Kraepiel A.M., Amyot M. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Systemat. 1998;29(1):543–566. [Google Scholar]
- Mori K., Yoshida K., Hoshikawa S., Ito S., Yoshida M., Satoh M., Watanabe C. Effects of perinatal exposure to low doses of cadmium or methylmercury on thyroid hormone metabolism in metallothionein-deficient mouse neonates. Toxicology. 2006;228(1):77–84. doi: 10.1016/j.tox.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Nemoto T. 1970. Feeding Pattern of Baleen Whales in the Ocean. [Google Scholar]
- Noël M., Jeffries S., Lambourn D.M., Telmer K., Macdonald R., Ross P.S. Mercury accumulation in harbour seals from the northeastern Pacific Ocean: the role of transplacental transfer, lactation, age and location. Arch. Environ. Contam. Toxicol. 2016;70(1):56–66. doi: 10.1007/s00244-015-0193-0. [DOI] [PubMed] [Google Scholar]
- Ohta K., Watarai T., Oishi T., Ueshiba Y., Hirose S., Yoshizawa T., Akikusa Y., Sato M., Okano H. Composition of fin whale milk. Proc. Jpn. Acad. 1953;29(7):392–398. [Google Scholar]
- Outridge P.M., Hobson K.A., McNeely R., Dyke A. A comparison of modern and preindustrial levels of mercury in the teeth of beluga in the Mackenzie Delta, Northwest Territories, and walrus at Igloolik, Nunavut, Canada. Arctic. 2002:123–132. [Google Scholar]
- Polischuk S., Norstrom R., Ramsay M. Body burdens and tissue concentrations of organochlorines in polar bears (Ursus maritimus) vary during seasonal fasts. Environ. Pollut. 2002;118(1):29–39. doi: 10.1016/s0269-7491(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Pomerleau C., Matthews C.J., Gobeil C., Stern G.A., Ferguson S.H., Macdonald R.W. Mercury and stable isotope cycles in baleen plates are consistent with year-round feeding in two bowhead whale (Balaena mysticetus) populations. Polar Biol. 2018;41(9):1881–1893. [Google Scholar]
- Rice K.M., Walker E.M., Jr., Wu M., Gillette C., Blough E.R. Environmental mercury and its toxic effects. J. Prevent. Med. Pub. Health. 2014;47(2):74. doi: 10.3961/jpmph.2014.47.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers. M., Moran, J., Gabriele, C., Neilson, J., Weiss, C., Masterman, A., Straley, J. (In Review) Stable isotope analysis of Northeast Pacific humpback whale baleen: Dynamic foraging habits and evidence of stravation in response to a marine heatwave. Deep Sea Research Part II: Topical Studies in Oceanography.
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- Savage K. 2018. NMFS Necropsy Report #2018046. [Google Scholar]
- Schell D., Saupe S., Haubenstock N. Springer; 1989. Natural Isotope Abundances in Bowhead Whale (Balaena mysticetus) Baleen: Markers of Aging and Habitat Usage. [Google Scholar]
- Selin N.E. Global biogeochemical cycling of mercury: a review. Annu. Rev. Environ. Resour. 2009;34:43–63. [Google Scholar]
- Simmonds M., Haraguchi K., Endo T., Cipriano F., Palumbi S., Troisi G. Human health significance of organochlorine and mercury contaminants in Japanese whale meat. J. Toxicol. Environ. Health, Part A. 2002;65(17):1211–1235. doi: 10.1080/152873902760125714. [DOI] [PubMed] [Google Scholar]
- Skaug H.J., Gjosæter H., Haug T., Nilssen K.T., Lindstrøm U. Do minke whales (Balaenoptera acutorostrata) exhibit particular prey preferences? J. Northwest Atl. Fish. Sci. 1997;22 [Google Scholar]
- Soerensen A.L., Sunderland E.M., Holmes C.D., Jacob D.J., Yantosca R.M., Skov H., Christensen J.H., Strode S.A., Mason R.P. An improved global model for air-sea exchange of mercury: high concentrations over the North Atlantic. Environ. Sci. Technol. 2010;44(22):8574–8580. doi: 10.1021/es102032g. [DOI] [PubMed] [Google Scholar]
- Steele R.A., Opella S.J. Structures of the reduced and mercury-bound forms of MerP, the periplasmic protein from the bacterial mercury detoxification system. Biochemistry. 1997;36(23):6885–6895. doi: 10.1021/bi9631632. [DOI] [PubMed] [Google Scholar]
- Strode S., Jaeglé L., Emerson S. Vertical transport of anthropogenic mercury in the ocean. Global Biogeochem. Cycles. 2010;24(4) [Google Scholar]
- Tanabe S., Tatsukawa R., Maruyama K., Miyazaki N. Transplacental transfer of PCBs and chlorinated hydrocarbon pesticides from the pregnant striped dolphin (Stenella coeruleoalba) to her fetus. Agric. Biol. Chem. 1982;46(5):1249–1254. [Google Scholar]
- Trudel M., Rasmussen J.B. Bioenergetics and mercury dynamics in fish: a modelling perspective. Can. J. Fish. Aquat. Sci. 2006;63(8):1890–1902. [Google Scholar]
- Wada H., Cristol D.A., McNabb F.A., Hopkins W.A. Suppressed adrenocortical responses and thyroid hormone levels in birds near a mercury-contaminated river. Environ. Sci. Technol. 2009;43(15):6031–6038. doi: 10.1021/es803707f. [DOI] [PubMed] [Google Scholar]
- Wagemann R., Muir D.C. Western Region, Department of Fisheries and Oceans Canada; 1984. Concentrations of Heavy Metals and Organochlorines in marine Mammals of Northern Waters: Overview and Evaluation. [Google Scholar]
- Wagemann R., Trebacz E., Boila G., Lockhart W. Methylmercury and total mercury in tissues of arctic marine mammals. Sci. Total Environ. 1998;218(1):19–31. doi: 10.1016/s0048-9697(98)00192-2. [DOI] [PubMed] [Google Scholar]
- Watanabe C., Yoshida K., Kasanuma Y., Kun Y., Satoh H. UteroMethylmercury exposure differentially affects the activities of Selenoenzymes in the fetal mouse brain. Environ. Res. 1999;80(3):208–214. doi: 10.1006/enrs.1998.3889. [DOI] [PubMed] [Google Scholar]
- Werth A.J., Rita D., Rosario M.V., Moore M.J., Sformo T.L. How do baleen whales stow their filter? A comparative biomechanical analysis of baleen bending. J. Exp. Biol. 2018;221(23):jeb189233. doi: 10.1242/jeb.189233. [DOI] [PubMed] [Google Scholar]
- Werth A.J., Sformo T.L., Lysiak N.S., Rita D., George C. Baleen turnover and gut transit in mysticete whales and its environmental implications. Polar Biol. 2020;43:707–723. [Google Scholar]
- Wolfe M.F., Schwarzbach S., Sulaiman R.A. Effects of mercury on wildlife: a comprehensive review. Environ. Toxicol. Chem.: Int. J. 1998;17(2):146–160. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.