Abstract
Aging is accompanied by declining lung function and increasing susceptibility to lung diseases. The role of endothelial dysfunction and vascular remodeling in these changes is supported by growing evidence, but underlying mechanisms remain elusive. In this review we summarize functional, structural, and molecular changes in the aging pulmonary vasculature and explore how interacting aging and mechanobiological cues may drive progressive vascular remodeling in the lungs.
Keywords: matrix, pulmonary, senescence, stiffness, vessel
Introduction
The aging lung is susceptible to several of the most common and debilitating pulmonary diseases, including emphysema, idiopathic pulmonary fibrosis, and group 2 pulmonary hypertension. Aging-associated endothelial dysfunction and vascular remodeling have been increasingly found to play roles in organ dysfunction in the systemic vasculature but have been less well studied in the pulmonary circulation. In this review, we discuss what is known about clinical, structural, and molecular changes in the aging pulmonary vasculature, how these interact via mechanobiological feedback, and the downstream consequences for pulmonary disease pathogenesis. We highlight the current knowledge gaps in this field and the promise of further research in this area for modifying and reversing aging-associated pulmonary disease.
Clinical Assessment and Consequences of Aging-Associated Pulmonary Vascular Remodeling
Age-related changes in the systemic arterial circulation in both large elastic arteries and the microcirculation contribute to cardiovascular diseases (1). These changes precede development of overt disease and can occur in the absence of risk factors (2), suggesting that they correlate with age rather than disease. Characteristic changes with normal aging include increasing stiffness of large arteries, progressive endothelial dysfunction [loss of nitric oxide (NO)-dependent dilation] in small and conduit arteries, and impaired angiogenesis (3). These vascular changes correlate with incident systemic hypertension (4–6), left ventricular remodeling (7–9), and microvascular end-organ damage of the brain and kidney (10–14). Given these effects, it is unsurprising that systemic arterial stiffness is a significant independent predictor of cardiovascular mortality (15–19).
Although much less examined than the systemic circulation, the pulmonary vasculature appears to be subject to many of the same aging-related phenotypes. Clinical study of the normal aging pulmonary circulation in humans has been challenging because of the morbidity of invasive measurements of the lung; multiple strategies have been used (FIGURE 1). Invasive pulmonary artery catheterization of nondiseased subjects has demonstrated an increase in systolic and mean pulmonary arterial (PA) pressure with aging (20–23), accompanied by evidence of increased vascular stiffening (24). A large, population-based study by Lam et al. (25) using noninvasive echocardiography found that PA systolic pressure showed a linear correlation with age (r = 0.31), and that the increase in pressure closely mirrored that seen in the systemic circulation. Although the absolute increase in pressure seen in the pulmonary circulation is smaller than that seen in the systemic circulation with age, the relative increase is similar (25) or larger (21–23) and is accompanied by a larger relative increase in vascular resistance (21, 22).
FIGURE 1.
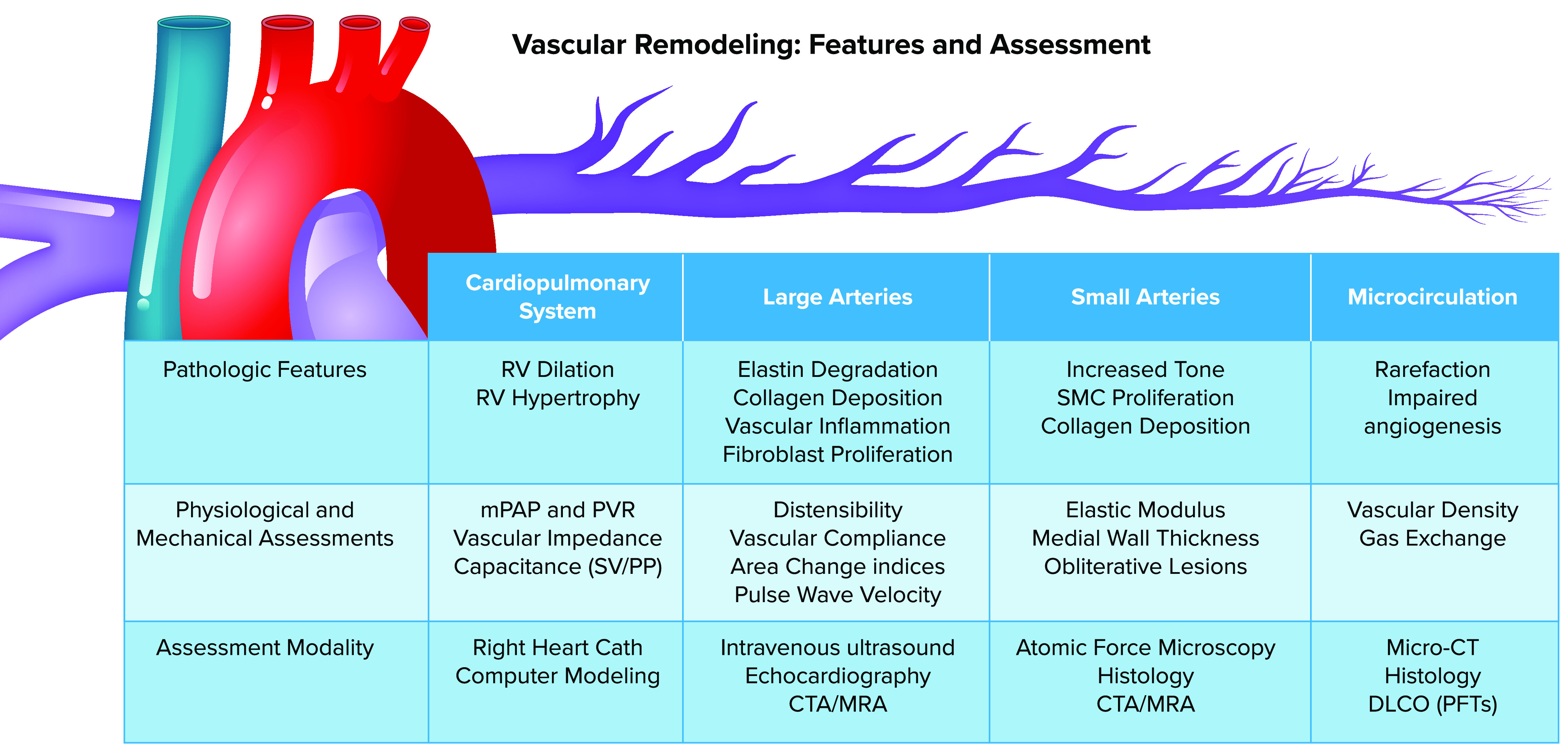
Pathophysiological features and assessment modalities for vascular remodeling in the pulmonary arterial tree This schematic of the pulmonary arterial system illustrates its branching structure, featuring large-diameter elastic arteries, smaller muscular arteries and arterioles, and extensive microcirculation. Unlike in the systemic circulation, the high compliance of the pulmonary arterial tree distributes across the entire vascular bed, contributing to impedance of the system as a whole and subsequent impact on the right ventricle (RV). The impact on the entire circulation is largely assessed invasively with flow and pressure sensors. These measurements can also be modeled based on invasive studies in humans and animals. Large arteries are most susceptible to alteration in elastin/collagen matrix and can be directly imaged with multiple modalities; reliable noninvasive physiological assessment has been technically challenging but is showing increasing feasibility with the latest technological advances. Small arteries are most susceptible to smooth muscle cell (SMC) hypertrophy and changes in tone, which can be assessed ex vivo with atomic force microscopy or examined histologically. The microcirculation suffers rarefaction and impaired angiogenesis with remodeling that can be best studied with micro-computed tomography (CT) or high-throughput, machine-assisted histology. CTA, computed tomography angiography; DlCO, diffusion capacity for carbon monoxide; mPAP, mean pulmonary artery pressure; MRA, magnetic resonance angiography; PFTs, pulmonary function tests; PP, pulse pressure; PVR, pulmonary vascular resistance; SV, stroke volume. Figure modified from Ref. 41, with permission from Frontiers in Physiology.
By increasing blood flow and oxygen demand, exercise exaggerates the hemodynamic changes seen with normal aging. Although mean PA pressure increases in all age groups with exercise, the elderly have an increase out of proportion to cardiac output and oxygen consumption compared with younger cohorts (26). Using a vascular distensibility model derived from large-animal studies, Reeves et al. (27) analyzed human exercise data and identified a decrease in vascular distensibility (1.5% vs. 2% change/mmHg) in older (age 63–81 yr) versus younger (age 14–60 yr) subjects. Age-related vascular remodeling therefore appears to require higher driving pressures in order to appropriately accommodate blood flow and recruit lung units during exercise (28).
There is strong evidence that vascular stiffening in the pulmonary circulation is associated with morbidity and mortality. In one community-based sample, elevated PA systolic pressure (PASP) was strongly associated with increased mortality, particularly for those patients with PASP > 30 mmHg or without known cardiopulmonary disease (25). Leibowitz et al. (29) showed similar findings in a separate community-based cohort of patients over the age of 85 yr. Patients with aging-related pulmonary diseases, such as chronic obstructive pulmonary disease (COPD) or interstitial lung disease, have dramatically increased mortality in the setting of pulmonary artery pressures elevated to this extent (30–33). Likewise, in cohorts of patients meeting criteria for pulmonary hypertension, vascular stiffness correlates with functional status and mortality (34–39). Several of these studies showed that stiffness measures were better predictors of mortality than typically measured resistance indexes (35, 38, 39), with particular prognostic utility in patients with normal vascular resistance (34, 38, 39). This evidence strongly links pulmonary vascular remodeling and stiffening to adverse clinical outcomes and has led multiple research groups to assess the therapeutic potential of targeting and reversing this process (40, 41).
Mechanobiological Changes in the Aging Pulmonary Vasculature: Linking Remodeling to Function
Extracellular Matrix Remodeling
Detailed structure-function studies of pulmonary vascular mechanics have been conducted in animal models and ex vivo human tissues (42). These studies have informed our growing understanding of the critical links between vascular structure, composition, stiffness, and function (41). As in the systemic circulation, the pulmonary vasculature transitions from large-diameter conduit arteries through a branching structure that ultimately reaches the alveoli as a delicate capillary network. The changes in pulmonary vascular mechanics that most influence right heart function and health are concentrated in the pulmonary arterial circulation; hence we focus here on these arterial vessels (FIGURE 1). Unlike in the systemic circulation, the compliance of the pulmonary arterial tree is distributed across the entire vascular bed rather than concentrated in the most proximal vessels (43); thus we explore remodeling as it manifests along the entirety of the pulmonary arterial vasculature.
Pulmonary arteries, like those of the systemic circulation, are composed of intimal, medial, and adventitial layers that together generate complex viscoelastic material properties. Mechanical testing studies on systemic and pulmonary arteries in dogs (44), sheep (45), and pigs (46) have emphasized their anisotropic (directional) nature. Much of this anisotropy is associated with the alignment of extracellular matrix (ECM) proteins, chief among them collagen I, which is a highly rigid molecule typically formed in highly aligned bundles to provide strength to mechanically loaded tissues. Interspersed with collagen bundles in the vascular wall are networks of elastin, an intrinsically disordered polymer possessing extraordinary compliance and ability to undergo reversible deformations (elasticity). Together these matrix components contribute to the nonlinear stiffening of vessels as loads initially borne by elastin are gradually taken on by the more rigid collagen fibers (47). Adding to their complexity, vessels have both passive mechanical properties that reflect their structure and composition as well as an active component largely defined by the number of actomyosin cross bridges dynamically engaged in the vascular smooth muscle cells that line the large arteries/arterioles of the pulmonary circulation. Changes in vessel mechanics can thus reflect changes in the proportion and molecular and cellular composition of vessels as well as the cellular activation state (tone) within the muscular layer (FIGURE 2).
FIGURE 2.
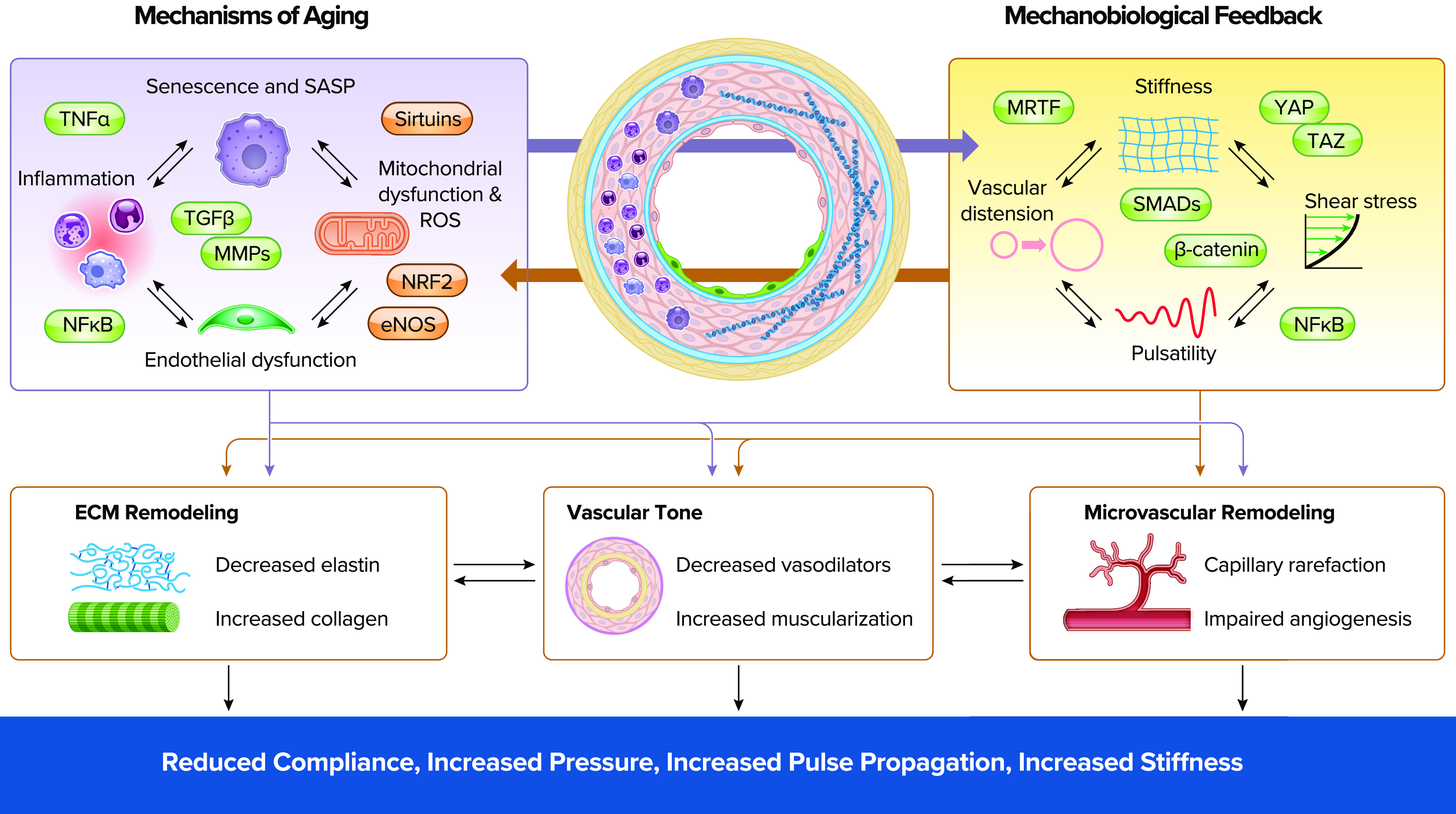
Aging and mechanobiological signals intersect to regulate vascular remodeling and pulmonary vascular mechanics The schematic cross section of a pulmonary vessel highlights known aging-related changes in inflammation, senescence, and extracellular matrix remodeling in the vessel wall. On left are shown interacting aging mechanisms of senescence, inflammation, and mitochondrial and endothelial dysfunction that together influence pulmonary vascular biology. These mechanisms engage in reciprocal interactions with the mechanobiological cellular responses to altered shear stress, stiffness, pulsatile flow, and vascular distension shown on right. Within each of these domains are shown key pathways that are increased (green) or decreased (red) in response to aging and mechanobiological cues and that link these interacting mechanisms to each other and to vascular biology. Both sets of pathways are self-reinforcing positive feedback loops containing reciprocal interactions and lacking a single initiating pathogenic mechanism. Together the interaction of aging-related and mechanobiological cues drives structural and function alterations in the pulmonary vasculature, including extracellular matrix (ECM) remodeling, increased vascular tone, and microvascular dysfunction. Over time these changes combine to alter local and systemwide pulmonary vascular mechanics and function. eNOS, nitric oxide synthase 3 or endothelial NOS; MMPs, matrix metalloproteinases; MRTF, myocardin-related transcription factor; NF-κB, nuclear factor-κB; NRF2, nuclear factor-erythroid factor 2-related factor 2; ROS, reactive oxygen species; SMADs, small Mothers Against Decapentaplegic; TAZ, transcriptional coactivator with PDZ-binding motif; TGF-β, transforming growth factor beta; TNF-α, tumor necrosis factor alpha; YAP, yes-associated protein.
Aging gradually remodels components of both the systemic and pulmonary vasculature (40, 48). The vessels of the systemic arteries exhibit gradual thickening of their walls, loss of elastin, and increased diameters of conducting arteries with age. Elastin has a remarkable half-life of ∼40 yr (49) but is mainly synthesized during development and is gradually lost with age (48, 50). Elastin-degrading metalloproteinases increase in the circulation and vessel walls with aging and may contribute to elastin loss (51, 52). Although less studied, similar aging-dependent changes, such as elastin degradation and fiber disorganization, are seen in the aging pulmonary vasculature as well (53). Similar findings are seen along with loss of pulmonary vascular compliance in pulmonary hypertension (54, 55). In this setting, absence or disorganization of elastin leads to earlier loading of collagen in the vessel wall, thereby altering wave propagation down the vascular tree and increasing right ventricular (RV) pressures and hypertrophy (56).
In contrast to elastin, collagen has a shorter half-life but is continuously produced and degraded throughout life (57). Aging increases the formation of nonenzymatic advanced glycation end products (58) that stabilize collagen, leading to loss of collagen’s elasticity and loss of vascular wall compliance (56, 59). Excess collagen deposition and accumulation are key sources of early decreases in vascular distensibility in experimental models of pulmonary hypertension (74), although hilar vessels demonstrate increased stiffness with aging without increased collagen content (53). On a molecular level, decreased nitric oxide (NO) bioavailability with aging (see below) increases the export of transglutaminase 2 to the extracellular matrix (60), where it enhances matrix stiffness by cross-linking collagen, fibronectin, fibrinogen, and laminin complexes. Alterations in matrix metalloproteinases (61), NO bioavailability (61, 62), and oxidative stress (63, 64) associated with aging have been shown to activate vascular transforming growth factor (TGF)-β signaling in both the lung and systemic circulation. TGF-β signaling, in turn, increases collagen I and collagen III deposition (65). In the systemic circulation, this gradual loss of elastin and replacement with collagen contribute directly to the decrease in aortic compliance seen starting in late middle age (50, 66).
Measures of vascular wall mechanics have traditionally emphasized quasi-static measures of deformability. Less emphasized, but likely important, are the viscoelastic (time/rate dependent) aspects of vessel mechanics. Such rate-dependent characteristics are essential to the dissipation or propagation of energy along the vascular wall that occurs with normal pulsatile loading. Changes in viscoelastic tissue mechanics with aging likely reflect altered interactions of collagen and elastin with highly charged proteoglycans distributed throughout the interstitial spaces of the vessel wall (61, 67). However, the contributions of proteoglycans (and other ECM components) to vascular viscoelasticity are less well defined than those for collagen and elastin, requiring further investigation.
Vascular Tone
Smaller muscular arteries and arterioles lack elastin and are composed of mostly smooth muscle by mass; consequently, their vessel wall mechanics are dominated by smooth muscle tone. These smaller vessels still contribute to the compliance of the pulmonary vascular tree because of the high capacitance of the pulmonary circulation. Aging-related increases in resting vascular tone have been well studied in the systemic circulation and arise primarily from impaired endothelium-dependent vasodilation (68–70). At baseline, the vascular endothelium produces NO in response to external stimuli such as acetylcholine and physiological levels of shear stress, leading to smooth muscle relaxation via NO-mediated activation of guanylate cyclase. Aging-associated pathology is felt to arise from NO scavenging by increased reactive oxygen species (ROS) that accumulate with aging (71). In animal models, inhibition of NO production can mimic aging-associated increases in arterial stiffness (72), and prolonged treatment with ROS scavengers in mice can reverse it (73). In human trials, high-dose vitamin C antioxidant infusions have similarly been shown to partially reverse age-associated declines in endothelium-mediated vasodilation (74, 75). Although less well studied, the pulmonary circulation in aged rats also shows impaired endothelium-mediated relaxation and increased oxidative stress. This was associated with high levels of ROS-generating enzymes and could be reversed through inhibition of ROS production (76).
In addition to altering NO-mediated relaxation, there is evidence that ROS can directly impact vascular smooth muscle contractility. In pulmonary arterial smooth muscle cells (PASMCs), increased ROS closely couples to increased free cytosolic Ca2+ levels, leading to increased tone and contractility. Moreover, catalase, antioxidants, and pharmacological inhibitors of potential ROS sources reduced or prevented hypoxia-induced elevation in intracellular calcium concentration ([Ca2+]i) in PASMCs (77, 78). ROS are also generated by smooth muscle cells upon exposure to vasoconstrictors such as angiotensin II or endothelin, leading to feedback loops that amplify vascular tone. ROS production and TGF-β signaling likewise mutually cross-activate (63, 64), leading to enhanced vascular stiffening and fibrosis (79). Finally, ex vivo application of cyclical stretch to mimic hypertension from arterial stiffening elevates ROS in both endothelial and smooth muscle cells (80, 81). These findings implicate high-pulsatility flow in mechanobiological remodeling of the pulmonary vasculature through endothelin, TGF-β, and NF-κB signaling (83, 84). NF-κB further promotes recruitment of inflammatory cells that amplify local oxidative stress. These multiple levels of positive feedback involving arterial stiffness, oxidative stress, contractility, and inflammatory signaling are likely critical drivers of aging-associated vascular remodeling (see FIGURE 2).
Impaired Angiogenesis and Microvascular Rarefaction
The aging vascular system tends toward rarefaction and impaired microvascular angiogenesis. Extensive mapping of the vascular and stromal networks of young versus aged organs identified marked vascular attrition in multiple organ beds including the heart, kidney, and spleen (85). These vascular changes preceded markers of accumulating ROS and cellular senescence. Endothelial signatures from organs with rarefaction compared to those without showed a strong association with increased inflammatory cytokine signaling in the endothelium as a cause or consequence of this process. Under in vitro conditions, microvascular endothelial cells derived from aged rodents exhibit impaired angiogenesis in response to VEGF and other growth factors compared with cells derived from young animals (86). Endothelial NO synthesis is involved in angiogenic responses (102), as demonstrated most clearly by significant microvascular rarefaction with long-term endothelial nitric oxide synthase (eNOS) inhibition (103) or absence of eNOS expression (104). Similarly, endothelium-specific sirtuin-1 knockout mice develop capillary rarefaction, reduced exercise-induced neovascularization, impaired endothelium-dependent vasorelaxation, and premature vascular senescence (87). Although Sirtuin1 is involved in several aspects of aging and senescence, it is well known to regulate the transcription and enzymatic activity of eNOS in the endothelium (88, 89), a likely primary driver of this phenotype.
Recent work by Caporarello et al. has extended this concept to the pulmonary vasculature. They documented that young mice recover from bleomycin lung injury with increased capillary density and repair, whereas aged mice exhibit capillary rarefaction and nonresolving lung fibrosis (90). Nos3, which codes for eNOS, was transiently upregulated only in young mice, and young mice deficient in eNOS recapitulated the capillary rarefaction and fibrotic phenotype seen in the aged rodents. This rarefaction in the vasculature may result from impaired blood flow delivery by remodeled upstream vessels, but the temporal sequence is not well understood. Whether the rarefaction itself contributes to vascular resistance or stiffening is also not a resolved question. As a low-resistance system, pulmonary vessels of small size contribute more to vascular resistance and capacitance than in the systemic circulation. Even in the systemic circulation, simulated rarefaction of distal A3 and A4 arterioles (8- to 20-µm-diameter vessels) modestly augments the global resistance of the vascular bed (91). In the lung, the consequences may be more severe given that the organ cannot reduce its perfusion and must accommodate the entirety of the cardiac output.
In summary, age-associated alterations in matrix composition in the large arteries, vascular tone in muscular arteries and arterioles, and rarefaction in the microvasculature may all contribute to vascular stiffening, increased pressures, and high-pulsatility flow in the pulmonary vasculature. These mechanical changes are sensed by pulmonary vascular cells locally through a process known as cellular mechanotransduction. We and others have studied mechanotransduction in response to vascular stiffening in the pulmonary circulation (83, 84, 92, 93, 94, 95, 97, 99–106). Increases in both flow pulsatility and matrix stiffening can activate pathways leading to matrix deposition, likely resulting in further vascular stiffening. The molecular details of these pathological feedback loops are reviewed elsewhere (41, 101, 105, 107, 108). In general, upstream mechanical cues are integrated by the cell via cell membrane- and cytoskeleton-associated signaling into a transcriptional program mediated by cytosolic-nuclear protein shuttles such as MRTF, YAP/TAZ, NF-κB, β-catenin, and Smads. Several of these pathways are also activated by growth factors, cell-cell signaling, and other nonmechanical cues, thereby integrating biological and mechanical signals. Although some mechanotransduction pathways have been found to be activated by age and stiffness in the systemic circulation (61, 109, 110), mechanobiological feedback is presently understudied in the context of lung aging. As key mediators of the cellular machinery driving vascular remodeling, such pathways may be useful targets for intervention.
Prevention and Reversal of Aging-Associated Pulmonary Vascular Remodeling
There has been great interest in reversing aging phenotypes in the vasculature, particularly given that the prominent “vascular-centric theory of aging” posits vascular dysfunction as the primary driver of aging-related pathology in the brain, kidney, cardiovascular, and endocrine systems (3, 111). Approaches to altering vascular remodeling directly through matrix modification have been pursued but have so far failed to translate into clinical practice. Elafin, an endogenously expressed elastase inhibitor, has been found to reduce elastase and metalloproteinase activity, leading to improvements in pulmonary vascular remodeling in multiple pulmonary hypertension models (112, 113). Synthetic elastase inhibitors have faced multiple challenges over several decades, with continued efforts ongoing to translate elastase inhibition into effective therapies for pulmonary diseases (114). Tissue transglutaminase inhibitors, such as cystamine, have been found to reduce vascular stiffness in aged rat aorta (115) and to delay vascular remodeling in monocrotaline-induced pulmonary hypertension (116). Similarly, BAPN, an inhibitor of the collagen cross-linking enzyme lysyl oxidase, reduces vascular remodeling in pulmonary hypertension and accelerated vascular aging models (117–120) but also leads to arterial aneurysms. These structural strategies, although aimed directly at long-term vascular changes, are likely high risk because of the consequences of long-term widespread alterations in structural protein deposition in a human compared with the impact of a few weeks of treatment in small-animal models. Improved techniques for targeting such therapies to the aging vasculature will almost certainly be required for clinical viability of this approach.
Given these challenges, many groups have pursued omics-based approaches to seek druggable targets in the aging process. Evaluation of aging using large data sets across species has yielded several common themes. Cellular and molecular changes involving oxidative metabolism, DNA damage, and telomere shortening are seen in higher mammals, whereas mechanisms like cellular senescence and inflammation are common among all species (121). Comparative single-cell RNA sequencing (RNA-seq) and proteomic analyses of lungs from young (3 mo old) and old (24 mo old) mice highlight many of the same themes (122), including increased transcriptional noise, mitochondrial oxidative phosphorylation, and proinflammatory pathway activation. These changes are seen in pulmonary vascular endothelial, mesothelial, and smooth muscle cells (123). Other groups have studied noninvasive systemic pulse-wave velocities in large human populations to gather cohorts at the extremes of arterial stiffness for age (124). Using these cohorts allows for an examination of genetic and epigenetic factors responsible for divergent age-associated stiffening (125). Data from one cohort revealed hypomethylation of a promoter region in calcium and integrin-binding protein 2, which contributes to stiffening and elevated vascular tone by increasing smooth muscle intracellular calcium concentrations (126). As in the lung, differential regulation of transcriptional programs related to oxidative stress was seen with this approach. It might be fruitful to use high-temporal-resolution magnetic resonance (MR) or other MR surrogates (127) to similarly examine cohorts with abnormal-for-age pulmonary vascular mechanics.
On the basis of these collective observations, multiple groups have explored targeting the redox state of the aging vasculature. In animal models, overexpression of antioxidant-regulating transcription factors, such as sirtuin-1 (128) and NRf2 (129) can rescue aging-associated pathology in the systemic circulation. Antioxidant drugs targeting similar pathways, such as high-dose resveratrol and 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPOL), can improve aortic stiffening and endothelium-dependent vasodilation (73, 130). Targeting of mitochondrial ROS, based on strong evidence that mitochondrial function and number decrease in arteries with age, has a large impact on vascular stiffness when genetically manipulated (131, 132). Pharmacological targeting of mitochondrial ROS using mitoQ improves endothelial dysfunction (73) and decreases pulse-wave velocity, ex vivo aortic stiffness, and aortic elastin degradation (133). In the pulmonary circulation, however, both TEMPOL and mitoQ modestly lower right ventricular pressure but do not result in improvements in pulmonary vascular remodeling in hypoxia or Sugen-hypoxia mouse pulmonary hypertension models (134, 135). This suggests either that these agents only impact age-associated remodeling or that they have decreased efficacy in the pulmonary system.
An alternative pharmacological approach is targeting aging-associated inflammation and cellular dysfunction with drugs called “senolytics.” One end result of accumulated age-associated oxidative, mitochondrial, or replicative stress is cellular senescence (136). Senescent cells exhibit a “senescence-associated secretory phenotype” (SASP) that promotes inflammation, fibrosis, and mechanobiological feedback via NF-κB, TNF-α, and TGF-β activation (137, 138). In the systemic vasculature, senescent cells accumulate with aging (139) and correlate inversely with endothelium-dependent vasodilation in vivo (140); clearance of these cells with senolytics improves vascular endothelial function in aged animals (141). Recent work (142) demonstrated that activation of senescence pathways and SASP was closely associated with irreversibility of pulmonary hypertension (PH) in a rat model, and that senolytic treatment could dramatically improve PH and PH-associated vascular remodeling even at late stages of disease. Such findings are driving interest in identifying safe and effective senolytic agents for use in aging-associated cardiovascular disease in both the systemic and pulmonary circulations (143).
Key Questions and Future Approaches
As emphasized at the outset and throughout this review, remodeling of the vasculature occurs with aging and in parallel with altered pulmonary vascular pressures and mechanics. Ultimately, such changes have been implicated, though in still relatively vague mechanistic terms, in aging-related morbidity and mortality. Key questions that emerge from these studies include: What components of remodeling are most critical to altered vascular mechanics? Can these changes be slowed or reversed? And finally, will interventions targeting pulmonary vascular remodeling ultimately result in improved function and benefit for age-related morbidity and mortality?
As a starting point for the first two questions, the complicating presence of feedback loops in vascular remodeling must be emphasized (41). Identifying fruitful points of intervention in pulmonary vascular remodeling requires a better understanding of where such processes begin and how they propagate. Notably, evidence is present for both proximal-to-distal and distal-to-proximal propagation of vascular remodeling, raising questions about the ultimate origins of aging-related mechanobiological remodeling and therefore the most beneficial target for early diagnosis and intervention (41, 108). A proximal-to-distal cascade of vascular remodeling fits with the clinical evidence that proximal vascular stiffening predicts progression of pulmonary hypertension and that reduction of pressure, flow, and shear stress protects against distal remodeling (105). However, the evidence supporting the priority of proximal stiffening in initiating remodeling is accompanied by limited abilities to measure distal vascular stiffness in living organisms. Relatively recent work employed atomic force microscopy (AFM) to invasively characterize smaller vessels in animal models of pulmonary arterial hypertension (93). This study identified increased stiffening of small vessels that preceded stiffening and thickening of larger vessels and right heart dysfunction. However, AFM was limited to measurements of intrapulmonary vessels and did not address aging-related stiffness changes.
It therefore remains unclear whether aging-related remodeling follows a distal-to-proximal or a proximal-to-distal cascade or can follow both within or across individuals. More recent efforts using AFM analysis have confirmed gradual aging-related stiffening of small intrapulmonary vessels in human lungs (144). Such measurements are only possible postmortem and therefore could not be compared to noninvasive assessments of proximal pulmonary remodeling. Resolving the point of initiation of adverse aging-related pulmonary vascular stiffening will thus require advances in methodologies in order to simultaneously assess large- and small-vessel mechanics efficiently and noninvasively. Although not possible in humans, it is notable that ex vivo perfused lungs can be used to disaggregate distal (steady flow) from proximal (oscillatory flow) vascular stiffening (145). With such methods it should be possible to study the aging vasculature in model organisms to identify the proximal versus distal site of initial pulmonary vascular stiffening and carefully reconstruct the molecular and cellular changes in composition and architecture. Merging such approaches with ongoing efforts to map the multigenomic state of cells in the aging lung (122, 146–149) could allow additional functional and molecular insight into the pathogenesis of vascular remodeling not possible through either approach alone.
Computational modeling may serve an essential function in bridging the gap between work in model organisms, from which extensive remodeling and mechanistic data can be gathered, and extrapolation of these findings to humans, in which much more limited data will be available (150–154). In parallel with modeling, advances in clinical imaging modalities that enable less invasive and more detailed characterization of pulmonary vascular mechanics would broadly benefit efforts to study the natural history of, and interventional studies to modify, pulmonary vascular mechanics. In particular, implementation of cardiac magnetic resonance to noninvasively assess key hemodynamic measures of pulmonary vascular mechanics traditionally assessed by right heart catheterization could enable such efforts (155, 156). Similarly, longitudinal chest computed tomography (CT) imaging has shown value in documenting vascular density and pruning in COPD and bronchiectasis (157–159) and could be used in similar studies of aging. In animal models, microvascular CT has already been used to assess (160) and follow microvascular injury and rarefaction during the development of PH (161). The use of advancing technology allowing noninvasive assessment of this process in aging would greatly advance our understanding of the evolution of aging-related lung diseases in the microvasculature. Advances in dynamic imaging, such as lung MR angiography (162), may also allow noninvasive assessment of pulmonary vascular capacitance at different anatomic levels and reveal the impact of age-related remodeling on blood flow and recruitment during exercise. Combined with thorough and longitudinal clinical data, computational analysis of these imaging studies may yield disease-relevant noninvasive imaging biomarkers for early vascular remodeling.
Ultimately, selection and evaluation of therapeutic interventions will be required to understand whether aging-related vascular mechanobiology can be successfully targeted and whether such interventions translate into improved vascular and pulmonary function and reduced morbidity and mortality. Such interventional studies will benefit from guidance gained from recent successes in targeting vascular function in pulmonary hypertension (163, 164). Careful selection of current and emerging end points will be key to identifying early successes supporting the expansion of pilot studies to large, extended clinical trials likely necessary to determine long-term benefit of vascular aging-targeted therapies. Given the growing weight of evidence implicating vascular mechanobiology in aging-related pulmonary diseases and overall declining cardiopulmonary function, clinical translation of mechanobiological modifications may represent an important advance for protecting pulmonary health in aging populations.
Acknowledgments
The authors are funded by National Heart, Lung, and Blood Institute Grants R01 HL-137366 (to L.E.F. and D.J.T.) and K08 HL-143197 (to P.B.D.); American Heart Association (AHA) Grant-in-Aid 17GRNT33660449 (to L.E.F.); and AHA Grant 20POST35210650 (to A.A.).
L. E. Fredenburgh reports research funding to the institution from Bayer, outside the submitted work.
P.B.D., A.A., L.E.F., and D.J.T. prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
References
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG, Wang M, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am 93: 583–604, 2009. doi: 10.1016/j.mcna.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donato AJ, Machin DR, Lesniewski LA. mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res 123: 825–848, 2018. doi: 10.1161/CIRCRESAHA.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 308: 875–881, 2012. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension 62: 1105–1110, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 43: 1239–1245, 2004. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 7.Kelly RP, Tunin R, Kass DA. Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ Res 71: 490–502, 1992. doi: 10.1161/01.res.71.3.490. [DOI] [PubMed] [Google Scholar]
- 8.Bell V, McCabe EL, Larson MG, Rong J, Merz AA, Osypiuk E, Lehman BT, Stantchev P, Aragam J, Benjamin EJ, Hamburg NM, Vasan RS, Mitchell GF, Cheng S. Relations between aortic stiffness and left ventricular mechanical function in the community. J Am Heart Assoc 6: e004903, 2017. doi: 10.1161/JAHA.116.004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Cheng S, Aragam J, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Relations of central hemodynamics and aortic stiffness with left ventricular structure and function: the Framingham Heart Study. J Am Heart Assoc 5: e002693, 2016. doi: 10.1161/JAHA.115.002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension 58: 839–846, 2011. doi: 10.1161/HYPERTENSIONAHA.111.177469. [DOI] [PubMed] [Google Scholar]
- 11.Huang N, Foster MC, Mitchell GF, Andresdottir MB, Eiriksdottir G, Gudmundsdottir H, Harris TB, Launer LJ, Palsson R, Gudnason V, Levey AS, Inker LA. Aortic stiffness and change in glomerular filtration rate and albuminuria in older people. Nephrol Dial Transplant 32: 677–684, 2017. doi: 10.1093/ndt/gfw050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S, Mitchell GF. Aortic stiffness and the risk of incident mild cognitive impairment and dementia. Stroke 47: 2256–2261, 2016. doi: 10.1161/STROKEAHA.116.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsao CW, Himali JJ, Beiser AS, Larson MG, DeCarli C, Vasan RS, Mitchell GF, Seshadri S. Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology 86: 619–626, 2016. doi: 10.1212/WNL.0000000000002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper LL, Mitchell GF. Aortic stiffness, cerebrovascular dysfunction, and memory. Pulse (Basel) 4: 69–77, 2016. doi: 10.1159/000448176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 39: 10–15, 2002. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 16.Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, Ting CT, Najjar SS, Lakatta EG, Yin FC, Chou P, Chen CH. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension 55: 799–805, 2010. doi: 10.1161/HYPERTENSIONAHA.109.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63: 636–646, 2014. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37: 1236–1241, 2001. doi: 10.1161/01.HYP.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121: 505–511, 2010. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emirgil C, Sobol BJ, Campodonico S, Herbert WH, Mechkati R. Pulmonary circulation in the aged. J Appl Physiol 23: 631–640, 1967. doi: 10.1152/jappl.1967.23.5.631. [DOI] [PubMed] [Google Scholar]
- 21.Davidson WR Jr, Fee EC. Influence of aging on pulmonary hemodynamics in a population free of coronary artery disease. Am J Cardiol 65: 1454–1458, 1990. doi: 10.1016/0002-9149(90)91354-9. [DOI] [PubMed] [Google Scholar]
- 22.Ghali JK, Liao Y, Cooper RS, Cao G. Changes in pulmonary hemodynamics with aging in a predominantly hypertensive population. Am J Cardiol 70: 367–370, 1992. doi: 10.1016/0002-9149(92)90621-5. [DOI] [PubMed] [Google Scholar]
- 23.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 34: 888–894, 2009. doi: 10.1183/09031936.00145608. [DOI] [PubMed] [Google Scholar]
- 24.Gozna ER, Marble AE, Shaw A, Holland JG. Age-related changes in the mechanics of the aorta and pulmonary artery of man. J Appl Physiol 36: 407–411, 1974. doi: 10.1152/jappl.1974.36.4.407. [DOI] [PubMed] [Google Scholar]
- 25.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 119: 2663–2670, 2009. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor BJ, Johnson BD. The pulmonary circulation and exercise responses in the elderly. Semin Respir Crit Care Med 31: 528–538, 2010. doi: 10.1055/s-0030-1265894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reeves JT, Linehan JH, Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol 288: L419–L425, 2005. doi: 10.1152/ajplung.00162.2004. [DOI] [PubMed] [Google Scholar]
- 28.Coffman KE, Curry TB, Dietz NM, Chase SC, Carlson AR, Ziegler BL, Johnson BD. The influence of pulmonary vascular pressures on lung diffusing capacity during incremental exercise in healthy aging. Physiol Rep 6: e13565, 2018. doi: 10.14814/phy2.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leibowitz D, Gilon D, Jacobs JM, Stessman-Lande I, Stessman J. Pulmonary artery systolic pressure and mortality in the oldest old. Cardiology 129: 111–116, 2014. doi: 10.1159/000365137. [DOI] [PubMed] [Google Scholar]
- 30.Hurdman J, Condliffe R, Elliot CA, Davies C, Hill C, Wild JM, Capener D, Sephton P, Hamilton N, Armstrong IJ, Billings C, Lawrie A, Sabroe I, Akil M, O’Toole L, Kiely DG. ASPIRE registry: assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J 39: 945–955, 2012. doi: 10.1183/09031936.00078411. [DOI] [PubMed] [Google Scholar]
- 31.Hurdman J, Condliffe R, Elliot CA, Swift A, Rajaram S, Davies C, Hill C, Hamilton N, Armstrong IJ, Billings C, Pollard L, Wild JM, Lawrie A, Lawson R, Sabroe I, Kiely DG. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J 41: 1292–1301, 2013. doi: 10.1183/09031936.00079512. [DOI] [PubMed] [Google Scholar]
- 32.Gall H, Felix JF, Schneck FK, Milger K, Sommer N, Voswinckel R, Franco OH, Hofman A, Schermuly RT, Weissmann N, Grimminger F, Seeger W, Ghofrani HA. The Giessen Pulmonary Hypertension Registry: survival in pulmonary hypertension subgroups. J Heart Lung Transplant 36: 957–967, 2017. doi: 10.1016/j.healun.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Oswald-Mammosser M, Weitzenblum E, Quoix E, Moser G, Chaouat A, Charpentier C, Kessler R. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest 107: 1193–1198, 1995. doi: 10.1378/chest.107.5.1193. [DOI] [PubMed] [Google Scholar]
- 34.Ploegstra MJ, Brokelman JG, Roos-Hesselink JW, Douwes JM, van Osch-Gevers LM, Hoendermis ES, van den Bosch AE, Witsenburg M, Bartelds B, Hillege HL, Berger RM. Pulmonary arterial stiffness indices assessed by intravascular ultrasound in children with early pulmonary vascular disease: prediction of advanced disease and mortality during 20-year follow-up. Eur Heart J Cardiovasc Imaging 19: 216–224, 2018. doi: 10.1093/ehjci/jex015. [DOI] [PubMed] [Google Scholar]
- 35.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol 47: 799–803, 2006. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 36.Gan CT, Lankhaar JW, Westerhof N, Marcus JT, Becker A, Twisk JW, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 132: 1906–1912, 2007. doi: 10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- 37.Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, Housten T, Champion HC, Lechtzin N, Wigley FM, Girgis RE, Hassoun PM. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med 182: 252–260, 2010. doi: 10.1164/rccm.200912-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pellegrini P, Rossi A, Pasotti M, Raineri C, Cicoira M, Bonapace S, Dini FL, Temporelli PL, Vassanelli C, Vanderpool R, Naeije R, Ghio S. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest 145: 1064–1070, 2014. doi: 10.1378/chest.13-1510. [DOI] [PubMed] [Google Scholar]
- 39.Tampakakis E, Shah SJ, Borlaug BA, Leary PJ, Patel HH, Miller WL, Kelemen BW, Houston BA, Kolb TM, Damico R, Mathai SC, Kasper EK, Hassoun PM, Kass DA, Tedford RJ. Pulmonary effective arterial elastance as a measure of right ventricular afterload and its prognostic value in pulmonary hypertension due to left heart disease. Circ Heart Fail 11: e004436, 2018. doi: 10.1161/CIRCHEARTFAILURE.117.004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohn JC, Lampi MC, Reinhart-King CA. Age-related vascular stiffening: causes and consequences. Front Genet 6: 112, 2015. doi: 10.3389/fgene.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dieffenbach PB, Maracle M, Tschumperlin DJ, Fredenburgh LE. Mechanobiological feedback in pulmonary vascular disease. Front Physiol 9: 951, 2018. doi: 10.3389/fphys.2018.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunter KS, Lammers SR, Shandas R. Pulmonary vascular stiffness: measurement, modeling, and implications in normal and hypertensive pulmonary circulations. Compr Physiol 1: 1413–1435, 2011. doi: 10.1002/cphy.c100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saouti N, Westerhof N, Postmus PE, Vonk-Noordegraaf A. The arterial load in pulmonary hypertension. Eur Respir Rev 19: 197–203, 2010. doi: 10.1183/09059180.00002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Debes JC, Fung YC. Biaxial mechanics of excised canine pulmonary arteries. Am J Physiol Heart Circ Physiol 269: H433–H442, 1995. doi: 10.1152/ajpheart.1995.269.2.H433. [DOI] [PubMed] [Google Scholar]
- 45.Fata B, Carruthers CA, Gibson G, Watkins SC, Gottlieb D, Mayer JE, Sacks MS. Regional structural and biomechanical alterations of the ovine main pulmonary artery during postnatal growth. J Biomech Eng 135: 021022, 2013. doi: 10.1115/1.4023389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fata B, Galdi E, Sacks MS. A comparative study of the main pulmonary artery and ascending aorta biomechanical behavior. Summer Bioengineering Conference American Society of Mechanical Engineers, 2011, p. 811–812. doi: 10.1115/SBC2011-53932. [DOI] [Google Scholar]
- 47.Roach MR, Burton AC. The reason for the shape of the distensibility curves of arteries. Can J Biochem Physiol 35: 681–690, 1957. [PubMed] [Google Scholar]
- 48.Thijssen DH, Carter SE, Green DJ. Arterial structure and function in vascular ageing: are you as old as your arteries? J Physiol 594: 2275–2284, 2016. doi: 10.1113/JP270597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arribas SM, Hinek A, González MC. Elastic fibres and vascular structure in hypertension. Pharmacol Ther 111: 771–791, 2006. doi: 10.1016/j.pharmthera.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol 39: 13–20, 1977. doi: 10.1016/s0002-9149(77)80004-0. [DOI] [PubMed] [Google Scholar]
- 51.Bonnema DD, Webb CS, Pennington WR, Stroud RE, Leonardi AE, Clark LL, McClure CD, Finklea L, Spinale FG, Zile MR. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs). J Card Fail 13: 530–540, 2007. doi: 10.1016/j.cardfail.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yasmin, Wallace S, McEniery CM, Dakham Z, Pulsalkar P, Maki-Petaja K, Ashby MJ, Cockcroft JR, Wilkinson IB. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol 25: 372, 2005. doi: 10.1161/01.ATV.0000151373.33830.41. [DOI] [PubMed] [Google Scholar]
- 53.Mackay EH, Banks J, Sykes B, Lee G. Structural basis for the changing physical properties of human pulmonary vessels with age. Thorax 33: 335–344, 1978. doi: 10.1136/thx.33.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang KY. The changing landscape of pulmonary arterial hypertension in 21st century. Acta Cardiol Sin 33: 510–513, 2017. doi: 10.6515/acs20170810a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tojais NF, Cao A, Lai YJ, Wang L, Chen PI, Alcazar MAA, de Jesus Perez VA, Hopper RK, Rhodes CJ, Bill MA, Sakai LY, Rabinovitch M. Codependence of bone morphogenetic protein receptor 2 and transforming growth factor-β in elastic fiber assembly and its perturbation in pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 37: 1559–1569, 2017. doi: 10.1161/ATVBAHA.117.309696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shifren A, Durmowicz AG, Knutsen RH, Faury G, Mecham RP. Elastin insufficiency predisposes to elevated pulmonary circulatory pressures through changes in elastic artery structure. J Appl Physiol (1985) 105: 1610–1619, 2008. doi: 10.1152/japplphysiol.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Humphrey J, Epstein M. Cardiovascular solid mechanics: cells, tissues, and organs. Appl Mech Rev 55: B103–B104, 2002. doi: 10.1115/1.1497492. [DOI] [Google Scholar]
- 58.Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation—a mini-review. Gerontology 58: 227–237, 2012. doi: 10.1159/000334668. [DOI] [PubMed] [Google Scholar]
- 59.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 21: 3–12, 2003. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Telci D, Collighan RJ, Basaga H, Griffin M. Increased TG2 expression can result in induction of transforming growth factor beta1, causing increased synthesis and deposition of matrix proteins, which can be regulated by nitric oxide. J Biol Chem 284: 29547–29558, 2009. doi: 10.1074/jbc.M109.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lacolley P, Regnault V, Avolio AP. Smooth muscle cell and arterial aging: basic and clinical aspects. Cardiovasc Res 114: 513–528, 2018. doi: 10.1093/cvr/cvy009. [DOI] [PubMed] [Google Scholar]
- 62.Steppan J, Bergman Y, Viegas K, Armstrong D, Tan S, Wang H, Melucci S, Hori D, Park SY, Barreto SF, Isak A, Jandu S, Flavahan N, Butlin M, An SS, Avolio A, Berkowitz DE, Halushka MK, Santhanam L. Tissue transglutaminase modulates vascular stiffness and function through crosslinking-dependent and crosslinking-independent functions. J Am Heart Assoc 6: e004161, 2017. doi: 10.1161/JAHA.116.004161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thannickal VJ, Day RM, Klinz SG, Bastien MC, Larios JM, Fanburg BL. Ras‐dependent and‐independent regulation of reactive oxygen species by mitogenic growth factors and TGF‐β1. FASEB J 14: 1741–1748, 2000. doi: 10.1096/fj.99-0878com. [DOI] [PubMed] [Google Scholar]
- 64.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor β1. J Biol Chem 270: 30334–30338, 1995. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 65.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol 588: 3971–3982, 2010. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greenberg SR. The association of medial collagenous tissue with atheroma formation in the aging human aorta as revealed by a special technique. Histol Histopathol 1: 323–326, 1986. [PubMed] [Google Scholar]
- 67.Wang Z, Lakes RS, Golob M, Eickhoff JC, Chesler NC. Changes in large pulmonary arterial viscoelasticity in chronic pulmonary hypertension. PLoS One 8: e78569, 2013. doi: 10.1371/journal.pone.0078569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.El Assar M, Angulo J, Rodríguez-Mañas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 65: 380–401, 2013. doi: 10.1016/j.freeradbiomed.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 69.Matz RL, Andriantsitohaina R. Age-related endothelial dysfunction: potential implications for pharmacotherapy. Drugs Aging 20: 527–550, 2003. doi: 10.2165/00002512-200320070-00005. [DOI] [PubMed] [Google Scholar]
- 70.Toda N. Age-related changes in endothelial function and blood flow regulation. Pharmacol Ther 133: 159–176, 2012. doi: 10.1016/j.pharmthera.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Wolin MS. Reactive oxygen species and the control of vascular function. Am J Physiol Heart Circ Physiol 296: H539–H549, 2009. doi: 10.1152/ajpheart.01167.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol (1985) 101: 1751–1759, 2006. doi: 10.1152/japplphysiol.00138.2006. [DOI] [PubMed] [Google Scholar]
- 73.Fleenor BS, Eng JS, Sindler AL, Pham BT, Kloor JD, Seals DR. Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell 13: 576–578, 2014. doi: 10.1111/acel.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension 45: 1107–1112, 2005. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- 76.Podlutsky A, Ballabh P, Csiszar A. Oxidative stress and endothelial dysfunction in pulmonary arteries of aged rats. Am J Physiol Heart Circ Physiol 298: H346–H351, 2010. doi: 10.1152/ajpheart.00972.2009. [DOI] [PubMed] [Google Scholar]
- 77.Lin MJ, Yang XR, Cao YN, Sham JS. Hydrogen peroxide-induced Ca2+ mobilization in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292: L1598–L1608, 2007. doi: 10.1152/ajplung.00323.2006. [DOI] [PubMed] [Google Scholar]
- 78.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res 91: 719–726, 2002. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- 79.Huang D, Wang Y, Wang L, Zhang F, Deng S, Wang R, Zhang Y, Huang K. Poly(ADP-ribose) polymerase 1 is indispensable for transforming growth factor-β induced Smad3 activation in vascular smooth muscle cell. PLoS One 6: e27123, 2011. doi: 10.1371/journal.pone.0027123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ali MH, Pearlstein DP, Mathieu CE, Schumacker PT. Mitochondrial requirement for endothelial responses to cyclic strain: implications for mechanotransduction. Am J Physiol Lung Cell Mol Physiol 287: L486–L496, 2004. doi: 10.1152/ajplung.00389.2003. [DOI] [PubMed] [Google Scholar]
- 81.Ding Z, Liu S, Wang X, Deng X, Fan Y, Sun C, Wang Y, Mehta JL. Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxid Redox Signal 22: 760–771, 2015. doi: 10.1089/ars.2014.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li M, Scott DE, Shandas R, Stenmark KR, Tan W. High pulsatility flow induces adhesion molecule and cytokine mRNA expression in distal pulmonary artery endothelial cells. Ann Biomed Eng 37: 1082–1092, 2009. doi: 10.1007/s10439-009-9684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scott D, Tan Y, Shandas R, Stenmark KR, Tan W. High pulsatility flow stimulates smooth muscle cell hypertrophy and contractile protein expression. Am J Physiol Lung Cell Mol Physiol 304: L70–L81, 2013. doi: 10.1152/ajplung.00342.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Chen J, Sivan U, Tan SL, Lippo L, De Angelis J, Labella R, Singh A, Chatzis A, Cheuk S, Medhghalchi M, Gil J, Hollander G, Marsden BD, Williams R, Ramasamy SK, Kusumbe AP. High-resolution 3D imaging uncovers organ-specific vascular control of tissue aging. Sci Adv 7: eabd7819, 2021. doi: 10.1126/sciadv.abd7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Murfee WL, Pacher P, Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol 15: 555–565, 2018. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Treviño-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA. Impairment of an endothelial NAD+-H2S signaling network is a reversible cause of vascular aging. Cell 173: 74–89.e20, 2018. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Förstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 106: 1652–1658, 2002. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 89.Xia N, Daiber A, Förstermann U, Li H. Antioxidant effects of resveratrol in the cardiovascular system. Br J Pharmacol 174: 1633–1646, 2017. doi: 10.1111/bph.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caporarello N, Meridew JA, Aravamudhan A, Jones DL, Austin SA, Pham TX, Haak AJ, Moo Choi K, Tan Q, Haresi A, Huang SK, Katusic ZS, Tschumperlin DJ, Ligresti G. Vascular dysfunction in aged mice contributes to persistent lung fibrosis. Aging Cell 19: e13196, 2020. doi: 10.1111/acel.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Greene AS, Tonellato PJ, Lui J, Lombard JH, Cowley AW Jr.. Microvascular rarefaction and tissue vascular resistance in hypertension. Am J Physiol Heart Circ Physiol 256: H126–H131, 1989. doi: 10.1152/ajpheart.1989.256.1.H126. [DOI] [PubMed] [Google Scholar]
- 92.Dieffenbach PB, Haeger CM, Coronata AM, Choi KM, Varelas X, Tschumperlin DJ, Fredenburgh LE. Arterial stiffness induces remodeling phenotypes in pulmonary artery smooth muscle cells via YAP/TAZ-mediated repression of cyclooxygenase-2. Am J Physiol Lung Cell Mol Physiol 313: L628–L647, 2017. doi: 10.1152/ajplung.00173.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu F, Haeger CM, Dieffenbach PB, Sicard D, Chrobak I, Coronata AM, Suárez Velandia MM, Vitali S, Colas RA, Norris PC, Marinković A, Liu X, Ma J, Rose CD, Lee SJ, Comhair SA, Erzurum SC, McDonald JD, Serhan CN, Walsh SR, Tschumperlin DJ, Fredenburgh LE. Distal vessel stiffening is an early and pivotal mechanobiological regulator of vascular remodeling and pulmonary hypertension. JCI Insight 1: e86987, 2016. doi: 10.1172/jci.insight.86987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hunter KS, Albietz JA, Lee PF, Lanning CJ, Lammers SR, Hofmeister SH, Kao PH, Qi HJ, Stenmark KR, Shandas R. In vivo measurement of proximal pulmonary artery elastic modulus in the neonatal calf model of pulmonary hypertension: development and ex vivo validation. J Appl Physiol (1985) 108: 968–975, 2010. doi: 10.1152/japplphysiol.01173.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lammers SR, Kao PH, Qi HJ, Hunter K, Lanning C, Albietz J, Hofmeister S, Mecham R, Stenmark KR, Shandas R. Changes in the structure-function relationship of elastin and its impact on the proximal pulmonary arterial mechanics of hypertensive calves. Am J Physiol Heart Circ Physiol 295: H1451–H1459, 2008. doi: 10.1152/ajpheart.00127.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li M, Tan Y, Stenmark KR, Tan W. High pulsatility flow induces acute endothelial inflammation through overpolarizing cells to activate NF-kappaB. Cardiovasc Eng Technol 4: 26–38, 2013. doi: 10.1007/s13239-012-0115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tian L, Lammers SR, Kao PH, Albietz JA, Stenmark KR, Qi HJ, Shandas R, Hunter KS. Impact of residual stretch and remodeling on collagen engagement in healthy and pulmonary hypertensive calf pulmonary arteries at physiological pressures. Ann Biomed Eng 40: 1419–1433, 2012. doi: 10.1007/s10439-012-0509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bertero T, Cottrill KA, Annis S, Bhat B, Gochuico BR, Osorio JC, Rosas I, Haley KJ, Corey KE, Chung RT, Nelson Chau B, Chan SY. A YAP/TAZ-miR-130/301 molecular circuit exerts systems-level control of fibrosis in a network of human diseases and physiologic conditions. Sci Rep 5: 18277, 2015. doi: 10.1038/srep18277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bloodworth NC, West JD, Merryman WD. Microvessel mechanobiology in pulmonary arterial hypertension: cause and effect. Hypertension 65: 483–489, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kobs RW, Muvarak NE, Eickhoff JC, Chesler NC. Linked mechanical and biological aspects of remodeling in mouse pulmonary arteries with hypoxia-induced hypertension. Am J Physiol Heart Circ Physiol 288: H1209–H1217, 2005. doi: 10.1152/ajpheart.01129.2003. [DOI] [PubMed] [Google Scholar]
- 103.Ooi CY, Wang Z, Tabima DM, Eickhoff JC, Chesler NC. The role of collagen in extralobar pulmonary artery stiffening in response to hypoxia-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 299: H1823–H1831, 2010. doi: 10.1152/ajpheart.00493.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, Hattori T, Nakashima Y, Kaibuchi K, Sueishi K, Takeshit A. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res 94: 385–393, 2004. doi: 10.1161/01.RES.0000111804.34509.94. [DOI] [PubMed] [Google Scholar]
- 105.Abe K, Shinoda M, Tanaka M, Kuwabara Y, Yoshida K, Hirooka Y, McMurtry IF, Oka M, Sunagawa K. Haemodynamic unloading reverses occlusive vascular lesions in severe pulmonary hypertension. Cardiovasc Res 111: 16–25, 2016. doi: 10.1093/cvr/cvw070. [DOI] [PubMed] [Google Scholar]
- 106.Abe K, Tawara S, Oi K, Hizume T, Uwatoku T, Fukumoto Y, Kaibuchi K, Shimokawa H. Long-term inhibition of Rho-kinase ameliorates hypoxia-induced pulmonary hypertension in mice. J Cardiovasc Pharmacol 48: 280–285, 2006. doi: 10.1097/01.fjc.0000248244.64430.4a. [DOI] [PubMed] [Google Scholar]
- 107.Schäfer M, Myers C, Brown RD, Frid MG, Tan W, Hunter K, Stenmark KR. Pulmonary arterial stiffness: toward a new paradigm in pulmonary arterial hypertension pathophysiology and assessment. Curr Hypertens Rep 18: 4, 2016. doi: 10.1007/s11906-015-0609-2. [DOI] [PubMed] [Google Scholar]
- 108.Tan W, Madhavan K, Hunter KS, Park D, Stenmark KR. Vascular stiffening in pulmonary hypertension: cause or consequence? (2013 Grover Conference series). Pulm Circ 4: 560–580, 2014. doi: 10.1086/677370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev 97: 1555–1617, 2017. doi: 10.1152/physrev.00003.2017. [DOI] [PubMed] [Google Scholar]
- 110.Huveneers S, Daemen MJ, Hordijk PL. Between Rho(k) and a hard place: the relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease. Circ Res 116: 895–908, 2015. doi: 10.1161/CIRCRESAHA.116.305720. [DOI] [PubMed] [Google Scholar]
- 111.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res 123: 849–867, 2018. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Todorovich-Hunter L, Dodo H, Ye C, McCready L, Keeley FW, Rabinovitch M. Increased pulmonary artery elastolytic activity in adult rats with monocrotaline-induced progressive hypertensive pulmonary vascular disease compared with infant rats with nonprogressive disease? Am Rev Respir Dis 146: 213–223, 1992. doi: 10.1164/ajrccm/146.1.213. [DOI] [PubMed] [Google Scholar]
- 113.Zaidi SH, You XM, Ciura S, Husain M, Rabinovitch M. Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation 105: 516–521, 2002. doi: 10.1161/hc0402.102866. [DOI] [PubMed] [Google Scholar]
- 114.von Nussbaum F, Li VM. Neutrophil elastase inhibitors for the treatment of (cardio)pulmonary diseases: Into clinical testing with pre-adaptive pharmacophores. Bioorg Med Chem Lett 25: 4370–4381, 2015. doi: 10.1016/j.bmcl.2015.08.049. [DOI] [PubMed] [Google Scholar]
- 115.Santhanam L, Tuday EC, Webb AK, Dowzicky P, Kim JH, Oh YJ, Sikka G, Kuo M, Halushka MK, Macgregor AM, Dunn J, Gutbrod S, Yin D, Shoukas A, Nyhan D, Flavahan NA, Belkin AM, Berkowitz DE. Novelty and significance. Circ Res 107: 117–125, 2010. doi: 10.1161/CIRCRESAHA.109.215228. [DOI] [PubMed] [Google Scholar]
- 116.Wang HM, Liu WZ, Tang FT, Sui HJ, Zhan XJ, Wang HX. Cystamine slows but not inverses the progression of monocrotaline-induced pulmonary arterial hypertension in rats. Can J Physiol Pharmacol 96: 783–789, 2018. doi: 10.1139/cjpp-2017-0720. [DOI] [PubMed] [Google Scholar]
- 117.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest 126: 3313–3335, 2016. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.von Kleeck R, Roberts E, Castagnino P, Bruun K, Brankovic SA, Hawthorne EA, Xu T, Tobias JW, Assoian RK. Arterial stiffness and cardiac dysfunction in Hutchinson-Gilford Progeria Syndrome corrected by inhibition of lysyl oxidase. Life Sci Alliance 4: e202000997, 2021. doi: 10.26508/lsa.202000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kerr JS, Riley DJ, Frank MM, Trelstad RL, Frankel HM. Reduction of chronic hypoxic pulmonary hypertension in the rat by beta-aminopropionitrile. J Appl Physiol Respir Environ Exerc Physiol 57: 1760–1766, 1984. doi: 10.1152/jappl.1984.57.6.1760. [DOI] [PubMed] [Google Scholar]
- 120.Nave AH, Mizikova I, Niess G, Steenbock H, Reichenberger F, Talavera ML, Veit F, Herold S, Mayer K, Vadasz I, Weissmann N, Seeger W, Brinckmann J, Morty RE. Lysyl oxidases play a causal role in vascular remodeling in clinical and experimental pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 34: 1446–1458, 2014. doi: 10.1161/ATVBAHA.114.303534. [DOI] [PubMed] [Google Scholar]
- 121.Kim SK. Common aging pathways in worms, flies, mice and humans. J Exp Biol 210: 1607–1612, 2007. doi: 10.1242/jeb.004887. [DOI] [PubMed] [Google Scholar]
- 122.Angelidis I, Simon LM, Fernandez IE, Strunz M, Mayr CH, Greiffo FR, Tsitsiridis G, Ansari M, Graf E, Strom TM, Nagendran M, Desai T, Eickelberg O, Mann M, Theis FJ, Schiller HB. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat Commun 10: 963, 2019. doi: 10.1038/s41467-019-08831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294: H2121–H2128, 2008. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- 124.Bruno RM, Nilsson PM, Engström G, Wadström BN, Empana JP, Boutouyrie P, Laurent S. Early and supernormal vascular aging: clinical characteristics and association with incident cardiovascular events. Hypertension 76: 1616–1624, 2020. doi: 10.1161/HYPERTENSIONAHA.120.14971. [DOI] [PubMed] [Google Scholar]
- 125.Laurent S, Boutouyrie P, Cunha PG, Lacolley P, Nilsson PM. Concept of extremes in vascular aging. Hypertension 74: 218–228, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12655. [DOI] [PubMed] [Google Scholar]
- 126.Mangino M, Cecelja M, Menni C, Tsai PC, Yuan W, Small K, Bell J, Mitchell GF, AortaGen Consortium, Chowienczyk P, Spector TD. Integrated multiomics approach identifies calcium and integrin-binding protein-2 as a novel gene for pulse wave velocity. J Hypertens 34: 79–87, 2016. doi: 10.1097/HJH.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gupta A, Sharifov OF, Lloyd SG, Tallaj JA, Aban I, Dell’italia LJ, Denney TS Jr, Gupta H. Novel noninvasive assessment of pulmonary arterial stiffness using velocity transfer function. J Am Heart Assoc 7: e009459, 2018. doi: 10.1161/JAHA.118.009459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wan YZ, Gao P, Zhou S, Zhang ZQ, Hao DL, Lian LS, Li YJ, Chen HZ, Liu DP. SIRT1-mediated epigenetic downregulation of plasminogen activator inhibitor-1 prevents vascular endothelial replicative senescence. Aging Cell 13: 890–899, 2014. doi: 10.1111/acel.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grimes KM, Reddy AK, Lindsey ML, Buffenstein R. And the beat goes on: maintained cardiovascular function during aging in the longest-lived rodent, the naked mole-rat. Am J Physiol Heart Circ Physiol 307: H284–H291, 2014. doi: 10.1152/ajpheart.00305.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157–168, 2008. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Park SH, Kwon OS, Park SY, Weavil JC, Andtbacka RH, Hyngstrom JR, Reese V, Richardson RS. Vascular mitochondrial respiratory function: the impact of advancing age. Am J Physiol Heart Circ Physiol 315: H1660–H1669, 2018. doi: 10.1152/ajpheart.00324.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Foote K, Reinhold J, Yu EP, Figg NL, Finigan A, Murphy MP, Bennett MR. Restoring mitochondrial DNA copy number preserves mitochondrial function and delays vascular aging in mice. Aging Cell 17: e12773, 2018. doi: 10.1111/acel.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol (1985) 124: 1194–1202, 2018. doi: 10.1152/japplphysiol.00670.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jernigan NL, Naik JS, Weise-Cross L, Detweiler ND, Herbert LM, Yellowhair TR, Resta TC. Contribution of reactive oxygen species to the pathogenesis of pulmonary arterial hypertension. PLoS One 12: e0180455, 2017. doi: 10.1371/journal.pone.0180455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pak O, Scheibe S, Esfandiary A, Gierhardt M, Sydykov A, Logan A, Fysikopoulos A, Veit F, Hecker M, Kroschel F, Quanz K, Erb A, Schäfer K, Fassbinder M, Alebrahimdehkordi N, Ghofrani HA, Schermuly RT, Brandes RP, Seeger W, Murphy MP, Weissmann N, Sommer N. Impact of the mitochondria-targeted antioxidant MitoQ on hypoxia-induced pulmonary hypertension. Eur Respir J 2018: 1701024, 2018. doi: 10.1183/13993003.01024-2017. [DOI] [PubMed] [Google Scholar]
- 136.Blokland KE, Pouwels SD, Schuliga M, Knight DA, Burgess JK. Regulation of cellular senescence by extracellular matrix during chronic fibrotic diseases. Clin Sci (Lond) 134: 2681–2706, 2020. doi: 10.1042/CS20190893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.MacNee W, Rabinovich RA, Choudhury G. Ageing and the border between health and disease. Eur Respir J 44: 1332–1352, 2014. doi: 10.1183/09031936.00134014. [DOI] [PubMed] [Google Scholar]
- 138.van der Feen DE, Berger RM, Bartelds B. Converging paths of pulmonary arterial hypertension and cellular senescence. Am J Respir Cell Mol Biol 61: 11–20, 2019. doi: 10.1165/rcmb.2018-0329TR. [DOI] [PubMed] [Google Scholar]
- 139.Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RH, Noyes RD, Richardson RS, Donato AJ. Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am J Physiol Heart Circ Physiol 305: H251–H258, 2013. doi: 10.1152/ajpheart.00197.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, Seals DR, Donato AJ. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol Heart Circ Physiol 313: H890–H895, 2017. doi: 10.1152/ajpheart.00416.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15: 973–977, 2016. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van der Feen DE, Bossers GP, Hagdorn QA, Moonen JR, Kurakula K, Szulcek R, Chappell J, Vallania F, Donato M, Kok K, Kohli JS, Petersen AH, van Leusden T, Demaria M, Goumans MT, De Boer RA, Khatri P, Rabinovitch M, Berger RM, Bartelds B. Cellular senescence impairs the reversibility of pulmonary arterial hypertension. Sci Transl Med 12: eaaw4974, 2020. doi: 10.1126/scitranslmed.aaw4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dookun E, Passos JF, Arthur HM, Richardson GD. Therapeutic potential of senolytics in cardiovascular disease. Cardiovasc Drugs Ther. In press. doi: 10.1007/s10557-020-07075-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sicard D, Haak AJ, Choi KM, Craig AR, Fredenburgh LE, Tschumperlin DJ. Aging and anatomical variations in lung tissue stiffness. Am J Physiol Lung Cell Mol Physiol 314: L946–L955, 2018. doi: 10.1152/ajplung.00415.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang Z, Chesler NC. Pulmonary vascular mechanics: important contributors to the increased right ventricular afterload of pulmonary hypertension. Exp Physiol 98: 1267–1273, 2013. doi: 10.1113/expphysiol.2012.069096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jambusaria A, Hong Z, Zhang L, Srivastava S, Jana A, Toth PT, Dai Y, Malik AB, Rehman J. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. Elife 9: e51413, 2020. doi: 10.7554/eLife.51413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Paik DT, Tian L, Williams IM, Rhee S, Zhang H, Liu C, Mishra R, Wu SM, Red-Horse K, Wu JC. Single-cell RNA sequencing unveils unique transcriptomic signatures of organ-specific endothelial cells. Circulation 142: 1848–1862, 2020. doi: 10.1161/CIRCULATIONAHA.119.041433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Feng W, Chen L, Nguyen PK, Wu SM, Li G. Single cell analysis of endothelial cells identified organ-specific molecular signatures and heart-specific cell populations and molecular features. Front Cardiovasc Med 6: 165, 2019. doi: 10.3389/fcvm.2019.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schupp JC, Adams TS, Cosme C, Raredon MS, Omote N, De Frias SP, Rose KA, Manning E, Sauler M, DeIuliis G, Ahangari F, Neumark N, Yuan Y, Habermann AC, Gutierrez AJ, Bui LT, Meyer KB, Nawijn MC, Teichmann SA, Banovich NE, Kropski JA, Niklason LE, Pe’er D, Yan X, Homer R, Rosas IO, Kaminski N. Integrated single cell atlas of endothelial cells of the human lung (Preprint). bioRxiv 10.21.347914, 2020. doi: 10.1101/2020.10.21.347914. [DOI] [PMC free article] [PubMed]
- 150.Burrowes KS, Hunter PJ, Tawhai MH. Anatomically based finite element models of the human pulmonary arterial and venous trees including supernumerary vessels. J Appl Physiol (1985) 99: 731–738, 2005. doi: 10.1152/japplphysiol.01033.2004. [DOI] [PubMed] [Google Scholar]
- 151.Tawhai M, Clark A, Donovan G, Burrowes K. Computational modeling of airway and pulmonary vascular structure and function: development of a “lung physiome”. Crit Rev Biomed Eng 39: 319–336, 2011. doi: 10.1615/critrevbiomedeng.v39.i4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tawhai MH, Bates JH. Multi-scale lung modeling. J Appl Physiol (1985) 110: 1466–1472, 2011. doi: 10.1152/japplphysiol.01289.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tawhai MH, Clark AR, Chase JG. The Lung physiome and virtual patient models: from morphometry to clinical translation. Morphologie 103: 131–138, 2019. doi: 10.1016/j.morpho.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 154.Tawhai MH, Hoffman EA, Lin CL. The lung physiome: merging imaging-based measures with predictive computational models. Wiley Interdiscip Rev Syst Biol Med 1: 61–72, 2009. doi: 10.1002/wsbm.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sanz J, Kariisa M, Dellegrottaglie S, Prat-González S, Garcia MJ, Fuster V, Rajagopalan S. Evaluation of pulmonary artery stiffness in pulmonary hypertension with cardiac magnetic resonance. JACC Cardiovasc Imaging 2: 286–295, 2009. doi: 10.1016/j.jcmg.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 156.Horvat D, Zlibut A, Orzan RI, Cionca C, Muresan ID, Mocan T, Revnic R, Agoston-Coldea L. Aging influences pulmonary artery flow and stiffness in healthy individuals: non-invasive assessment using cardiac MRI. Clin Radiol 76: 161.e19–161.e28, 2021. doi: 10.1016/j.crad.2020.09.021. [DOI] [PubMed] [Google Scholar]
- 157.Diaz AA, Maselli DJ, Rahaghi F, Come CE, Yen A, Maclean ES, Okajima Y, Martinez CH, Yamashiro T, Lynch DA, Wang W, Kinney GL, Washko GR, San José Estépar R. Pulmonary vascular pruning in smokers with bronchiectasis. ERJ Open Res 4: 00044-2018, 2018. doi: 10.1183/23120541.00044-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rahaghi FN, Argemi G, Nardelli P, Dominguez-Fandos D, Arguis P, Peinado VI, Ross JC, Ash SY, de La Bruere I, Come CE, Diaz AA, Sanchez M, Washko GR, Barbera JA, San José Estépar R. Pulmonary vascular density: comparison of findings on computed tomography imaging with histology. Eur Respir J 54: 1900370, 2019. doi: 10.1183/13993003.00370-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Washko GR, Nardelli P, Ash SY, Vegas Sanchez-Ferrero G, Rahaghi FN, Come CE, Dransfield MT, Kalhan R, Han MK, Bhatt SP, Wells JM, Aaron CP, Diaz AA, Ross JC, Cuttica MJ, Labaki WW, Querejeta Roca G, Shah AM, Young K, Kinney GL, Hokanson JE, Agustí A, San José Estépar R. Arterial vascular pruning, right ventricular size, and clinical outcomes in chronic obstructive pulmonary disease. a longitudinal observational study. Am J Respir Crit Care Med 200: 454–461, 2019. doi: 10.1164/rccm.201811-2063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Deng Y, Rowe KJ, Chaudhary KR, Yang A, Mei SH, Stewart DJ. Optimizing imaging of the rat pulmonary microvasculature by micro-computed tomography. Pulm Circ 9: 2045894019883613, 2019. doi: 10.1177/2045894019883613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Deng Y, Chaudhary K, Godoy RS, Yang A, Rowe K, Stewart DJ. Restoration of lung microcirculation after unilateral pulmonary artery banding in a rat model of severe pulmonary arterial hypertension reveals a remarkable capacity for lung microvascular repair and regeneration (Abstract). Pulmonary Vascular Research Institute Annual Conference, 2020. [Google Scholar]
- 162.Johns CS, Swift AJ, Hughes PJ, Ohno Y, Schiebler M, Wild JM. Pulmonary MR angiography and perfusion imaging—a review of methods and applications. Eur J Radiol 86: 361–370, 2017. doi: 10.1016/j.ejrad.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 163.Waxman A, Restrepo-Jaramillo R, Thenappan T, Ravichandran A, Engel P, Bajwa A, Allen R, Feldman J, Argula R, Smith P, Rollins K, Deng C, Peterson L, Bell H, Tapson V, Nathan SD. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med 384: 325–334, 2021. doi: 10.1056/NEJMoa2008470. [DOI] [PubMed] [Google Scholar]
- 164.Humbert M, McLaughlin V, Gibbs JS, Gomberg-Maitland M, Hoeper MM, Preston IR, Souza R, Waxman A, Escribano Subias P, Feldman J, Meyer G, Montani D, Olsson KM, Manimaran S, Barnes J, Linde PG, de Oliveira Pena J, Badesch DB; PULSAR Trial Investigators. Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med 384: 1204–1215, 2021. doi: 10.1056/NEJMoa2024277. [DOI] [PubMed] [Google Scholar]


