Abstract
Background:
Central nervous system (CNS)-active medication use is an important modifiable risk factor for falls in older adults. A fall-related injury should prompt providers to evaluate and reduce CNS-active medications to prevent recurrent falls. We evaluated change in CNS-active medications up to 12 months following a fall-related injury in community-dwelling older adults compared to a matched cohort without fall-related injury.
Methods:
Participants were from the Adult Changes in Thought study conducted at Kaiser Permanente Washington. Fall-related injury codes between 1994–2014 defined index encounters in participants with no evidence of such injuries in the preceding year. We matched each fall-related injury index encounter with up to 5 randomly selected clinical encounters from participants without injury. Using automated pharmacy data, we estimated average change in CNS-active medication use at 3, 6, and 12 months post-index according to presence or absence of CNS-active medication use prior to index.
Results:
1,516 participants with fall-related injury index encounters (449 CNS-active users, 1,067 nonusers) were matched to 7,014 index encounters from people without fall-related injuries (1,751 users, 5,236 nonusers). Among CNS-active users at the index encounter, those with fall-related injury had an average decrease in standard daily doses (SDDs) at 12 months (−0.43; 95%CI: −0.63 to −0.23), and those without injury had a greater (p=0.047) average decrease (−0.66; 95%CI: −0.78 to −0.55). Among non-users at index, those with fall-related injury had a smaller increase compared to those without injury (+0.17, 95%CI: +0.13 to +0.21, vs. +0.24, 95%CI: +0.20 to +0.28, p=0.005).
Conclusions:
The differences in CNS-active medication use change over 12 months between those with and without fall-related injury were small and unlikely to be clinically significant. These results suggest that fall risk-increasing drug use is not reduced following a fall-related injury, thus opportunities exist to reduce CNS-active medications, a potentially modifiable risk factor for falls.
Keywords: fall-related injury, medications, older adults
Introduction
Falls are the leading cause of fatal and non-fatal injury among adults aged ≥65 years in the United States.1 One in four older adults falls each year, resulting in approximately 3 million emergency department (ED) visits and 800,000 hospitalizations annually.2 ED visits and hospitalizations for fall-related injury are rising,3, 4 and it is projected that the direct medical costs of treating falls in the United States will increase to over $101 billion by 2030.5 Falls are a significant public health concern with substantial economic consequences.
Medications are a key modifiable risk factor for falls6, 7 and a central target of the Centers for Disease Control and Prevention’s (CDC) Stopping Elderly Accidents, Deaths and Injuries (STEADI) fall prevention initiative.8 Medications associated with higher fall risk have been termed fall risk-increasing drugs (FRIDs). There are several definitions of FRIDs, but central to all are medications that act on the central nervous system (CNS), termed CNS-active medications (e.g., benzodiazepines, antidepressants).9–12 CNS-active medications confer increased fall risk,13–15 with even greater risk among individuals using multiple CNS-active medications.9, 16–18 The CDC and American Geriatrics Society have provided recommendations to avoid use of CNS-active medications in older adults with a history of falls or fractures, except in the absence of safer alternatives.8–10, 12
One of the strongest risk factors for an injurious fall is a prior fall.19 Thus, a fall-related injury should serve as a “call to action” for clinicians to address modifiable risks, such as conducting a medication review, re-evaluating need for CNS-active medication(s), and reducing such medications when possible to prevent recurrent falls.8–10, 12 Furthermore, it is important for prescribers to consider fall history when initiating CNS-active medications. Evidence from epidemiologic studies suggests FRID use is not reduced following a fall-related injury.20–30 However, four important limitations of this body of literature exist.11 First, no studies included a control group without fall-related injury to account for temporal or age-related changes in FRID use. Second, most studies did not assess FRID use at clinically meaningful time points following the injury (e.g., they compared medication use at hospital admission versus discharge). Some medications need to be tapered over weeks to months, and this, along with other changes to chronic medications, would likely occur during follow up with a primary care provider, rather than at hospital discharge. Third, several studies using electronic pharmacy data to determine medication use following an injury reported use as a dichotomous variable (use or non-use) over a long time period. As a result, use is reported over a period of time (e.g., 12-month period) instead of assessing use at a specific time (e.g., at 12 months), and this imprecise measure provides no information about timing or quantity of medication fills (e.g., single versus multiple fills) over the time period. To accurately capture discontinuation or dose reduction, it is necessary to examine medication use at discrete time points following an injury. Fourth, most studies focused on discontinuation and not dose reduction. Dose reduction of CNS-active medications is an important goal of fall risk reduction,12 as it may not be realistic to completely discontinue a medication, particularly if indicated for treatment of a serious underlying condition. Thus, important gaps in knowledge remain regarding patterns of CNS-active medication use among older adults following a fall-related injury.
Data from the Adult Changes in Thought (ACT) study, conducted in community-dwelling older adults, presented an opportunity to examine whether a fall-related injury triggered medication adjustment that could address the aforementioned limitations of prior studies. The objectives of this study were to: 1) evaluate change in CNS-active medication use at 3, 6, and 12 months following fall-related injury, stratified by presence or absence of use at time of injury (index); and 2) compare this to change in use observed in a matched sample without fall-related injury at index. As compared to the matched sample, we hypothesized that individuals with fall-related injury using CNS-active medications at index would have greater reductions in dose over follow-up, and those not using at index would have lower uptake/increases in dose.
Methods
Data Source, Study Design, and Sample
This study used data from Adult Changes in Thought (ACT), an ongoing prospective cohort study in which adults aged ≥65 years and older without dementia have been randomly sampled from Kaiser Permanente Washington (KPWA), an integrated health care delivery system in Washington State.31 Participants were enrolled during three waves: original cohort between 1994–1996 (n=2,581), expansion cohort between 2000–2003 (n=811), and continuous enrollment beginning in 2004. Total enrollment across all waves at time of the data freeze (April 30, 2014) evaluated in this paper was 4947 participants. ACT participants are interviewed at follow-up study visits every two years, at which time a variety of health characteristics are assessed. In addition, cognitive function is assessed using the Cognitive Abilities Screening Instrument (CASI), a global cognitive test with scores ranging from 0–100 with higher scores indicating better cognition.32 Participants with a CASI score ≤85 undergo further in-depth clinical and neuropsychological evaluations. These data are reviewed at consensus conferences to identify dementia diagnoses using standard research definitions.33, 34 Information, including dates and diagnostic codes, from clinical encounters and healthcare utilization is available on all ACT participants from KPWA health records.
Among participants in ACT, we identified all outpatient visits, hospitalizations and ED visits following ACT enrollment for a fall-related injury, ascertained via International Classification of Diseases, Ninth Revision (ICD-9) E codes (using a definition modified from Tinetti et al.35) (Supplementary Table S1). Clinical encounters with these codes were defined as index encounters if the ACT participant had been enrolled in KPWA for ≥1 year prior to the encounter with no evidence of fall-related injuries during that prior year. These criteria were applied to ensure the index encounter captured an incident event rather than follow-up care for a prior fall. Participants could contribute multiple index encounters (i.e., multiple incident fall-related injuries) during follow-up. We excluded any index encounters occurring after an ACT participant was diagnosed with dementia, as ACT data collection ceases at this point and covariates would not be available.
We matched each fall-related injury index encounter with up to 5 randomly selected clinical encounters of other ACT participants that occurred for reasons other than fall-related injury during a comparable time period (i.e., +/− 1 calendar year). We excluded encounters that listed psychiatric diagnoses as the primary diagnosis given this would likely influence CNS-active medication use. In addition to calendar time, factors used to construct this matched cohort of non-injury index encounters included: age (+/− five years), sex, ACT study cohort (original, expansion, replacement), use of CNS-active medications at index, and type of clinical encounter (outpatient visit, ED visit, or hospitalization). This matched cohort was subject to the same inclusion criteria as above (i.e., free of dementia, enrolled in KPWA for 1+ years prior to the encounter, and no evidence of fall-related injury in prior year). Note, while we only required 1+ years of prior enrollment, all but 11 in our included sample (8,530 index encounters in both cohorts) had >2 years.
Outcome
We focused on CNS-active medications given the strong evidence base regarding falls risk and their classification as potentially inappropriate in older adults with history of falls.9 We evaluated use of anticholinergics, antidepressants, antipsychotics, benzodiazepines, other sedative hypnotics, opioids, and skeletal muscle relaxants (Supplementary Table S2). Medication name, strength, route, dispensing date, and amount dispensed were ascertained from KPWA automated pharmacy data, which includes outpatient prescription fills at both KPWA and non-KPWA pharmacies.
The primary outcome was change from index encounter in a composite measure of overall CNS-active medication burden at three time points (3, 6 and 12 months) after the index encounter. The composite measure was calculated using the standardized daily dose (SDD) approach. The SDD is associated with injurious falls,13, 15, 36 however, a clinically meaningful change score has not been established. SDD is calculated by dividing the total daily dose of each medication by the minimum effective geriatric dose for that medication (determined via a standard reference)37 and summing standardized doses of all CNS-active medications. To measure change in CNS-active medication SDD, we assessed use at index (immediately before the index encounter), and at 3, 6 and 12 months following the index encounter. At index and each follow-up time point, CNS-active medication use was defined as having any prescription fill coverage overlapping with the period 30 days before each time point. This measure takes into account adherence and early refills (Figure 1).38
Figure 1.
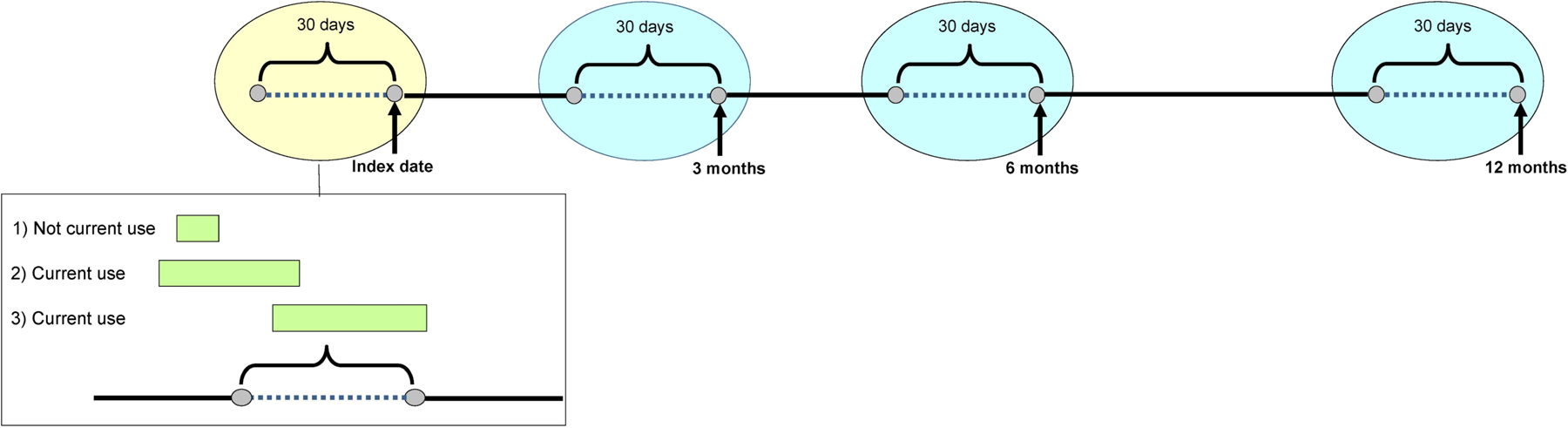
At index (yellow oval) and each follow-up time point (blue ovals), central nervous system (CNS)-active medication use was defined as having any prescription fill coverage overlapping with the period 30 days prior to each time point (dotted line in figure). This measure takes into account adherence and early refills. The green bars in the box below show three different example scenarios of prescription fills: 1) the medication fill is completed prior to the 30 day window which is considered not current use; 2) the medication fill overlaps the 30 day window which is considered current use; and 3) the medication fill starts within the 30 day window which is considered current use.
Covariates
In addition to the matching factors (age, calendar time, ACT study cohort, sex, CNS-active medication use at index, and index encounter type), we assessed numerous demographic and health-related covariates. Demographic characteristics included race (self-reported non-Hispanic white vs. other) and education level (education beyond high school vs. not). Health characteristics gathered from the ACT study visit most proximal to index date were self-rated health (fair/poor vs. good/very good/excellent), osteoarthritis, coronary artery disease (myocardial infarction, angina, coronary artery bypass graft, or angioplasty), prior stroke, frailty (performance-based gait speed <0.6 m/second or inability to complete the timed walk) and cognition (i.e., CASI score). Treatment for hypertension and treatment for diabetes were ascertained via ≥2 prescription fills from computerized pharmacy data for disease-related medications (antihypertensive medications; insulin or oral hypoglycemics) within 2 years before index.13, 36 We used ICD-9 codes to define anxiety, insomnia/sleep problems, Parkinson’s disease, urinary incontinence, and depression within 2 years before index (Supplementary Table S3). We also recorded prior fall-related injury (measured in the 1–2 years before index).
Analysis
We summarized the distribution of demographic characteristics and comorbidities separately for individuals with and without fall-related injury at index and according to CNS-active medication use at index. We then estimated average change in CNS-active medication SDD at 3, 6, and 12 months following index for both cohorts using linear regression. Models were adjusted for cohort matching factors39 and the covariates listed earlier (see Covariates). Models included indicator variables for follow-up time and group-by-time interactions to allow for testing of whether changes in dose at the three follow-up times differed between groups. Separate models were estimated according to presence or absence of CNS-active medication at index. We estimated model parameters using generalized estimating equations with an independence working correlation matrix and estimated robust standard errors using the sandwich estimator.40 We conducted sensitivity analyses: 1) excluding opioids from measures of total SDD, as opioids may be prescribed to manage pain following a fall-related injury, and 2) dividing the cohort into two time periods according to index date (1994–2003 and 2004–2014) to examine impact of potential temporal differences in prescribing. We also performed secondary analyses for the three most commonly used medication classes among people with a fall-related injury (i.e., antidepressants, benzodiazepines, opioids) in which we estimated average change in SDD among users of those respective classes prior to index. Analyses were conducted using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Study population
We identified 1,582 fall-related injury index encounters meeting study inclusion criteria; we excluded 66 of these because of incomplete covariate data. For the remaining sample of 1,516 fall-related injuries (among 1,136 ACT participants), we obtained a matched cohort of 7,014 index encounters without fall-related injury (among 2,759 ACT participants). Distributions of characteristics at index encounter in these groups are provided in Table 1 stratified by CNS-active medication use at index. Due to matching, distributions were similar with respect to age and sex, and distributions of other demographic characteristics and comorbidities were also comparable between those with and without fall-related injury. Among individuals using a CNS-active medication at index, the mean (standard deviation) SDD was 2.4 (3.0) and 2.2 (2.4) for people with and without a fall-related injury, respectively. Among those with a fall-related injury using a CNS-active medication at index, the most common classes used were antidepressants (56%), opioids (31%), and benzodiazepines and other sedative hypnotics (17%) (Table 2).
Table 1.
Participant characteristics at the index encounter according to CNS-active medication usea
| CNS-active use at index | No CNS-active use at index | |||
|---|---|---|---|---|
| Characteristic | Fall-Related Injury (N = 449) |
No Fall-Related Injury (N = 1,751) |
Fall-Related Injury (N = 1,067) |
No Fall-Related Injury (N = 5,263) |
| Age, median (25th, 75th) | 81 (77, 86) | 80 (76, 84) | 81 (76, 86) | 80 (75, 85) |
| Female | 358 (80) | 1,417 (81) | 736 (69) | 3,633 (69) |
| Non-Hispanic white | 422 (94) | 1,611 (92) | 980 (92) | 4,700 (89) |
| Some education beyond high school | 288 (64) | 1,121 (64) | 731 (69) | 3,531 (67) |
| Original ACT cohort | 296 (66) | 1,237 (71) | 749 (70) | 3,732 (71) |
| Comorbidities, | ||||
| Treatment for hypertension | 337 (75) | 1,244 (71) | 641 (60) | 3,359 (64) |
| Treatment for diabetes | 41 (9) | 198 (11) | 79 (7) | 550 (10) |
| Coronary artery disease | 132 (29) | 477 (27) | 230 (22) | 1320 (25) |
| Stroke | 38 (8) | 96 (5) | 46 (4) | 215 (4) |
| Osteoarthritis | 316 (70) | 1,293 (74) | 693 (65) | 3373 (64) |
| Urinary incontinence | 79 (18) | 268 (15) | 77 (7) | 415 (8) |
| Depression | 163 (36) | 551 (31) | 80 (8) | 422 (8) |
| Anxiety | 88 (20) | 269 (15) | 62 (6) | 316 (6) |
| Insomnia or sleep problems | 75 (17) | 305 (17) | 64 (6) | 410 (8) |
| Parkinson’s disease | 8 (2) | 18 (1) | 14 (1) | 52 (1) |
| Fair or poor self-rated health | 109 (24) | 418 (24) | 132 (12) | 857 (16) |
| Low gait speedb (<0.6m/s) | 119 (27) | 372 (21) | 136 (13) | 710 (13) |
| CASI score ≤86 | 39 (9) | 100 (6) | 52 (5) | 300 (6) |
| Prior fallsc | 67 (15) | 109 (6) | 110 (10) | 335 (6) |
Abbreviations: ACT = Adult Changes in Thought; CASI = Cognitive Abilities Screening Instrument; CNS = central nervous system
Data expressed as frequency (%) unless stated otherwise.
<0.6 meters/second or inability to complete the timed walk; for individuals with missing gait speed data, this measure was imputed using activities of daily living related to walking and reported difficulty walking a half-mile.
Prior falls were measured in the period 1–2 years prior to index.
Table 2.
Type of CNS-active medication use at index for those with and without a fall-related injury
| Fall-Related Injury (N = 449) |
No Fall-Related Injury (N = 1,751) |
|||
|---|---|---|---|---|
| N | % | N | % | |
| Specific Classes | ||||
| Antidepressants | 252 | 56 | 1,021 | 58 |
| Opioids | 139 | 31 | 523 | 30 |
| Benzodiazepine/Sedative hypnotics | 74 | 17 | 214 | 12 |
| Skeletal muscle relaxants | 14 | 3 | 59 | 3 |
| Antipsychotic | 2 | 0.4 | 14 | 0.8 |
| Anticholinergicsa | ||||
| Genitourinary antispasmodic | 63 | 14 | 170 | 10 |
| Gastrointestinal antispasmodic | 22 | 5 | 126 | 7 |
| Antihistamines | 6 | 1 | 31 | 2 |
| Antivertigo/Antiemetic | 3 | 0.7 | 37 | 2 |
Some antidepressants, muscle relaxants and antipsychotics include medications with anticholinergic effects. These anticholinergic medications were included in their primary therapeutic classes and are not summarized in the anticholinergic section.
Change in CNS-active medication dose - Overall
Users at index:
Among users of CNS-active medications at index, those with a fall-related injury, on average, had a decrease in SDD in the year following index, but those without fall-related injury had significantly greater decreases at each follow-up time point (p<0.05 for comparisons between cohorts at 3, 6, and 12 months post-index) (Table 3). CNS-active medication users with fall-related injury had average decreases of 0.38 (95% CI: −0.52 to −0.23) in SDD at 3 months, 0.39 (95% CI: −0.56 to −0.22) at 6 months, and 0.43 (95% CI: −0.63 to −0.23) in SDD at 12 months post-index. In comparison, the corresponding average decreases in SDD among users without fall-related injury were 0.66 (95% CI: −0.75 to −0.58), 0.64 (95% CI: −0.73 to −0.56), and 0.66 (95% CI: −0.78 to −0.55). The average change in SDD at 12 months from index was significantly greater in those without fall-related injury compared to those with an injury (p=0.047).
Table 3.
Average change in SDD from index of CNS-active medications according to use at index
| CNS use at index | Fall-related injury at index | SDD at index | Average change in SDD from indexb | ||
|---|---|---|---|---|---|
| 3 months | 6 months | 12 months | |||
| Primary Analysis | |||||
| Users at index | Yes | 2.4 (3.0) | −0.38 (−0.52, −0.23) | −0.39 (−0.56, −0.22) | −0.43 (−0.63, −0.23) |
| No | 2.2 (2.4) | −0.66 (−0.75, −0.58) | −0.64 (−0.73, −0.56) | −0.66 (−0.78, −0.55) | |
| p-valuea | 0.001 | 0.008 | 0.047 | ||
| Non-users at index | Yes | 0.18 (0.12, 0.24) | 0.18 (0.14, 0.22) | 0.17 (0.13, 0.21) | |
| No | 0.21 (0.18, 0.24) | 0.22 (0.19, 0.25) | 0.24 (0.20, 0.28) | ||
| p-valuea | 0.446 | 0.129 | 0.005 | ||
| Sensitivity Analysis (without opioids) | |||||
| Users at index | Yes | −0.21 (−0.38, −0.05) | −0.29 (−0.49, −0.09) | −0.31 (−0.54, −0.09) | |
| No | −0.51 (−0.62, −0.40) | −0.49 (−0.59, −0.38) | −0.53 (−0.67, −0.40) | ||
| p-valuea | 0.001 | 0.062 | 0.099 | ||
| Non-users at index | Yes | 0.13 (0.10, 0.17) | 0.15 (0.11, 0.19) | 0.15 (0.11, 0.18) | |
| No | 0.18 (0.16, 0.21) | 0.19 (0.16, 0.22) | 0.22 (0.18, 0.25) | ||
| p-valuea | 0.014 | 0.041 | 0.002 | ||
Abbreviations: ACT = Adult Changes in Thought; CNS = central nervous system; SDD = standardized daily dose
p-value corresponding to test of whether average change in SDD from index differs between those with fall-related injury and those without
Estimates for average change in SDD at each time point (relative to index) are based on adjusted regression models and standardized to a common distribution of covariates. Regression models were adjusted for the following covariates (as of index): age (spline 2df), calendar time (spline 2df), class(es) of CNS-active medications used, clinical encounter type at index, ACT cohort, gender, race/ ethnicity, education, self-rated health, frail (per gait speed), cognition, treatment for hypertension, treatment for diabetes, osteoarthritis, coronary artery disease, stroke, anxiety, depression, urinary incontinence, Parkinson’s disease, insomnia, and prior falls.
Non-users at index:
Among non-users of CNS-active medications at index, the SDD was higher in the year following index date among those with a fall-related injury and those without (Table 3). The average increase in SDD from index was lower among those with an injury compared with those without an injury during the follow-up and reached statistical significance at 12-months only (p=0.005)
Sensitivity analyses:
Similar trends as the primary analyses were observed for those who used or did not use CNS-active medications at index in the sensitivity analysis excluding opioids from the composite CNS-active measure, though inference for some of the comparisons across time points were affected (Table 3). Overall, the results from the analysis examining potential time trends confirmed our main findings. Of note, among users of CNS-active medications at index, the later cohort had a lower average SDD at index and differences in the decline in SDD between the injury and non-injury groups were of smaller magnitude numerically compared with the earlier cohort (Supplementary Tables S4 and S5).
Change in CNS-active medication dose - Class-specific
Secondary analyses examining change in SDD in the year following the index encounter for participants using antidepressants, opioids, or sedative-hypnotics at index are provided in Table 4. While the SDD of each medication class decreased over the year following index in participants with and without a fall-related injury, differences in change in SDD did not significantly differ between groups at most follow-up time points. The only exceptions were for antidepressants at 3 months (p=0.011) and for sedative-hypnotics at 6 months (p=0.023), and in both these instances, the group without fall-related injury had greater decreases.
Table 4.
Average change in SDD from index of specific CNS-active medications according to use at index
| Fall-related injury at index | Average change in SDD from indexb | |||
|---|---|---|---|---|
| 3 months | 6 months | 12 months | ||
| Antidepressants | Yes | −0.36 (−0.57, −0.15) | −0.56 (−0.82, −0.29) | −0.50 (−0.80, −0.20) |
| No | −0.62 (−0.75, −0.50) | −0.60 (−0.73, −0.46) | −0.70 (−0.87, −0.53) | |
| p-valuea | 0.011 | 0.770 | 0.216 | |
| Opioids | Yes | −0.53 (−0.70, −0.36) | −0.49 (−0.67, −0.32) | −0.63 (−0.80, −0.46) |
| No | −0.64 (−0.76, −0.52) | −0.64 (−0.74, −0.53) | −0.62 (−0.73, −0.51) | |
| p-valuea | 0.267 | 0.137 | 0.883 | |
| Benzodiazepines and other sedative hypnotics | Yes | −0.41 (−0.58, −0.24) | −0.41 (−0.62, −0.21) | −0.52 (−0.71, −0.33) |
| No | −0.62 (−0.81, −0.43) | −0.68 (−0.88, −0.48) | −0.67 (−0.87, −0.46) | |
| p-valuea | 0.054 | 0.023 | 0.158 | |
Abbreviations: ACT = Adult Changes in Thought; CNS = central nervous system; SDD = standardized daily dose
p-value corresponding to test of whether average change in SDD from index differs between those with fall-related injury and those without
Estimates for average change in SDD at each time point (relative to index) are based on adjusted regression models and standardized to a common distribution of covariates. Regression models were adjusted for the following covariates (as of index): age (spline 2df), calendar time (spline 2df), class(es) of CNS-active medications used, clinical encounter type at index, ACT cohort, gender, race/ ethnicity, education, self-rated health, frail (per gait speed), cognition, treatment for hypertension, treatment for diabetes, osteoarthritis, coronary artery disease, stroke, anxiety, depression, urinary incontinence, Parkinson’s disease, insomnia, and prior falls.
Discussion
In this cohort study of community-dwelling older adults without dementia, we found the differences in CNS-active medication use change over 12 months between those with and without fall-related injury were small and unlikely to be clinically significant. These findings suggest that FRID use is not reduced following a fall-related injury, which was unexpected. Among users of CNS-active medications at index, those who experienced a fall-related injury had, on average, a statistically significant decrease in SDD of these medications over the following year, but those without a fall-related injury had an even greater decrease. Sensitivity analyses suggested, however, that differences in SDD declines between these two groups were perhaps less pronounced in the later period (2004–2014) than in the earlier period (1994–2003). Among non-users of CNS-active medications at index, people without a fall-related injury had higher SDDs over the year following the index encounter than people with a fall-related injury. These patterns remained when we removed opioids from the SDD calculation, suggesting that results were not influenced by acute opioid use following an injury. To put our results into clinical perspective, a change of 0.5 SDD translates into a reduction by half of the minimum effective geriatric dose. For example, a 10 mg daily dose of zolpidem (minimum effective geriatric daily dose = 5 mg) is 2 SDD, and a change of −0.5 SDD translates into reduction in dose from 10 mg to 7.5 mg.
To our knowledge, this is the first study to examine use of FRIDs, specifically CNS-active medications, at repeated time points up to 12 months following a fall-related injury compared with a matched cohort without an injury. Although several observational studies have examined FRID use before and after a fall-related injury, methodological differences make direct comparison of our results difficult.11
Clinical guidelines recommend that older persons who present for medical attention because of a fall should have a multifactorial fall risk assessment, including a medication review.10 If the medication review identifies FRIDs, re-evaluation of continued need and substitution of a safer alternative or dose reduction is warranted.8, 9 Furthermore, caution should be exercised in initiating new CNS-active medications among people with a history of falling. Overall, conducting a multifactorial fall risk assessment takes time and longitudinal monitoring, especially if medication changes are made. Our results highlight that efforts are needed to enhance implementation of such services.
While we did find declines in SDDs of CNS-active medications over the 12 months following fall-related injury, we found these declines to be smaller among those with a fall-related injury compared to those without such injury. The reason for this unanticipated finding, converse to what we hypothesized, is unclear. One potential reason is that the benefit of medication use outweighs the potential risk (e.g., falls) for some individuals or safer alternatives are not available.41, 42 Another potential reason is the group with fall-related injury had more severe depression or anxiety which precluded deprescribing. A randomized multicenter trial conducted with older adults who visited an ED because of a fall and used one or more FRIDs found that FRID deprescribing was not necessary or possible for 40% of all FRIDs.42
Antidepressants may pose unique challenges for deprescribing as depression itself is a risk factor for falls,43, 44 and risks versus benefits must be carefully weighed when initiating, discontinuing, or reducing the dose of antidepressants. Given limited evidence for depression treatment duration in older adults, there is no standard for stopping maintenance treatment. Expert consensus guidelines recommend considering discontinuing antidepressants a year after remission is achieved in older patients with a single episode of depression.45 However, evidence suggests that long-term antidepressant use (>2 years) has increased over time, with this trend more prevalent in older adults (along with middle-aged adults) compared to younger adults, and that this may represent inappropriate or unnecessary use.46–48 Another barrier to deprescribing antidepressants is fear of withdrawal symptoms or disease relapse/recurrence,49 along with the complexity of deprescribing these medications, which requires gradual tapering and clinical monitoring throughout the process. In addition, some patients may require long-term treatment with antidepressants at therapeutic doses to prevent recurrence of depressive symptoms.50 Further information is needed about feasibility and safety of withdrawing antidepressants in appropriate candidates.
This study has multiple strengths. Our design addressed limitations of prior research on this topic. We included a matched cohort without fall-related injury; without this, we would have reported a reduction in FRIDs after the injury, suggesting a positive outcome. However, because the matched cohort showed a greater reduction in FRIDs, we reached a different conclusion. We evaluated new initiation of CNS-active medications among non-users of CNS-active medications at index which has not been addressed in prior research. Lastly, ACT study visit data allowed adjustment for several important covariates to minimize confounding that is not possible using administrative claims data alone.
Several limitations exist. Reliance on automated pharmacy data to ascertain CNS-active medication use means that misclassification is a possibility (i.e., an individual filled a prescription for a medication but never actually used it). We were unable to capture over-the counter antihistamines, such as sleep aids or differentiate between as-needed versus scheduled medication use. Our pre-specified plan to measure at 3, 6 and 12 months could miss medication discontinuation or reduction that occurred early after index if the medication was re-prescribed to manage adverse drug withdrawal events. The age of our dataset may not reflect current prescribing practices. A clinically meaningful change score for the SDD has not been established so it is difficult to determine how the change in SDD would translate to clinical outcomes. As with any observational study, there is also the possibility of unmeasured or residual confounding. Lastly, generalizability of our results is limited because our study sample predominately consisted of non-Hispanic white older adults without dementia enrolled in an integrated health care system.
The difference in CNS-active medication use change over 12 months between those with and without fall-related injury was small and unlikely to be clinically significant. In CNS-active medication users, the dose decreased over the year following the fall-related injury, but not as much as in people without such injury, which may be concerning. However, among participants not using CNS-active medications at the index date, the dose increased across the study period for both groups, and the change was significantly higher in those without an injury; this finding is more encouraging. These results suggest that FRID use is not reduced following a fall-related injury, thus opportunities exist to reduce CNS-active medications, a potentially modifiable risk factor for falls.
Supplementary Material
Supplementary files
Supplementary Table S1: ICD-9 and E codes used to identify participants with a fall-related injury
Supplementary Table S2: Classification of medications and minimum effective dose
Supplementary Table S3: ICD-9 codes used for covariates
Supplementary Table S4: Average change in SDD from index of CNS-active medications according to use at index (restricted to index during 1994–2003)
Supplementary Table S5: Average change in SDD from index of CNS-active medications according to use at index (restricted to index during 2004–2014)
Key Points:
Central nervous system (CNS)-active medication use is an important modifiable risk factor for falls.
Differences in CNS-active medication use change over 12 months between subjects with and without fall-related injury were small and of unclear clinical significance.
These results suggest that fall risk-increasing drug use is not reduced following a fall-related injury, thus opportunities exist to reduce CNS-active medications, a potentially modifiable risk factor for falls.
Why does this paper matter?
Our findings emphasize the importance of efforts to improve care of older adults with fall-related injury to reduce CNS-active active medication use.
Acknowledgements:
Funding Sources:
This work was supported by National Institute on Aging Grant U01AG006781 to Dr. Larson and the University of Washington School of Pharmacy Elmer M. Plein Endowed Research Fund.
Footnotes
Conflicts of Interest: No conflicts of interest to report.
References:
- 1.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA psychiatry. 2015;72(2):136–142. [DOI] [PubMed] [Google Scholar]
- 2.Bergen G, Stevens MR, Burns ER. Falls and Fall Injuries Among Adults Aged >/=65 Years - United States, 2014. MMWR Morbidity and mortality weekly report. 2016;65(37):993–998. [DOI] [PubMed] [Google Scholar]
- 3.Shankar KN, Liu SW, Ganz DA. Trends and characteristics of emergency department visits for fall-related injuries in older adults, 2003–2010. West J Emerg Med. 2017;18(5):785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orces CH. Emergency department visits for fall-related fractures among older adults in the USA: a retrospective cross-sectional analysis of the National Electronic Injury Surveillance System All Injury Program, 2001–2008. BMJ open. 2013;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houry D, Florence C, Baldwin G, et al. The CDC Injury Center’s response to the growing public health problem of falls among older adults. Am J Lifestyle Med. 2016;10(1):74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pit SW, Byles JE, Henry DA, et al. A Quality Use of Medicines program for general practitioners and older people: a cluster randomised controlled trial. Med J Aust. 2007;187(1):23–30. [DOI] [PubMed] [Google Scholar]
- 7.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012(9):CD007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. STEADI-Older adult falls prevention. Available at: https://www.cdc.gov/steadi/index.html. Updated October 16, 2020. Accessed December 1, 2020.
- 9.American Geriatrics Society 2019 Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674–694. [DOI] [PubMed] [Google Scholar]
- 10.Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49(5):664–672. [PubMed] [Google Scholar]
- 11.Hart LA, Phelan EA, Yi JY, Marcum ZA, Gray SL. Use of fall risk-increasing drugs around a fall-related injury in older adults: a systematic review. J Am Geriatr Soc. 2020;68(6):1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panel on Prevention of Falls in Older Persons AGS, British Geriatrics S. Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148–57. [DOI] [PubMed] [Google Scholar]
- 13.Gray SL, Marcum ZA, Dublin S, et al. Association between medications acting on the central nervous system and fall-related injuries in community-dwelling older adults: a new user cohort study. J Gerontol A Biol Sci Med Sci. 2020;75(5):1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seppala LJ, Wermelink A, de Vries M, et al. Fall-risk-increasing drugs: a systematic review and meta-analysis: II. Psychotropics. J Am Med Dir Assoc. 2018;19(4):371 e311–371 e317. [DOI] [PubMed] [Google Scholar]
- 15.Hanlon JT, Zhao X, Naples JG, et al. Central nervous system medication burden and serious falls in older nursing home residents. J Am Geriatr Soc. 2017;65(6):1183–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanlon JT, Boudreau RM, Roumani YF, et al. Number and dosage of central nervous system medications on recurrent falls in community elders: the Health, Aging and Body Composition study. J Gerontol A Biol Sci Med Sci. 2009;64(4):492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcum ZA, Wirtz HS, Pettinger M, et al. Anticholinergic medication use and falls in postmenopausal women: findings from the women’s health initiative cohort study. BMC Geriatr. 2016;16:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pratt NL, Ramsay EN, Kalisch Ellett LM, et al. Association between use of multiple psychoactive medicines and hospitalization for falls: retrospective analysis of a large healthcare claim database. Drug Saf. 2014;37(7):529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phelan EA, Rillamas-Sun E, Johnson L, et al. Determinants, circumstances and consequences of injurious falls among older women living in the community. Inj Prev. 2021;27(1):34–41. [DOI] [PubMed] [Google Scholar]
- 20.Beunza-Sola M, Hidalgo-Ovejero AM, Marti-Ayerdi J, Sanchez-Hernandez JG, Menendez-Garcia M, Garcia-Mata S. Study of fall risk-increasing drugs in elderly patients before and after a bone fracture. Postgrad M J. 2018;94(1108):76–80. [DOI] [PubMed] [Google Scholar]
- 21.Bennett A, Gnjidic D, Gillett M, et al. Prevalence and impact of fall-risk-increasing drugs, polypharmacy, and drug-drug interactions in robust versus frail hospitalised falls patients: a prospective cohort study. Drugs & aging. 2014;31(3):225–32. [DOI] [PubMed] [Google Scholar]
- 22.Sjoberg C, Bladh L, Klintberg L, Mellstrom D, Ohlsson C, Wallerstedt SM. Treatment with fall-risk-increasing and fracture-preventing drugs before and after a hip fracture: an observational study. Drugs & aging. 2010;27(8):653–61. [DOI] [PubMed] [Google Scholar]
- 23.Francis E, Dyks D, Kanji S. Influence of admission to a tertiary care hospital after a fall on use of potentially inappropriate medications among older patients. The Can J Hosp Pharm. 2014;67(6):429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill-Taylor B, Sketris IS, Gardner DM, et al. Concordance with a STOPP (Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions) criterion in Nova Scotia, Canada: benzodiazepine and zoplicone prescription claims by older adults with fall-related hospitalizaions. J Popul Ther Clin Pharmacol. 2016;23(1):e1–12. [PubMed] [Google Scholar]
- 25.Kragh A, Elmstahl S, Atroshi I. Older adults’ medication use 6 months before and after hip fracture: a population-based cohort study. J Am Geriatr Soc. 2011;59(5):863–868. [DOI] [PubMed] [Google Scholar]
- 26.Marvin V, Ward E, Poots AJ, et al. Deprescribing medicines in the acute setting to reduce the risk of falls. Eur J Hosp Pharm. 2017;24(1):10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMahon CG, Cahir CA, Kenny RA, et al. Inappropriate prescribing in older fallers presenting to an Irish emergency department. Age and ageing. 2014;43(1):44–50. [DOI] [PubMed] [Google Scholar]
- 28.Trenaman SC, Hill-Taylor BJ, Matheson KJ, et al. Antipsychotic drug dispensations in older adults, including continuation after a fall-related hospitalization: identifying adherence to Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions criteria using the Nova Scotia Seniors’ Pharmacare Program and Canadian Institute for Health’s Discharge Databases. Curr Ther Res Clin Exp. 2018;89:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh ME, Boland F, Moriarty F, et al. Modification of Potentially Inappropriate Prescribing Following Fall-Related Hospitalizations in Older Adults. Drugs & aging. 2019;36(5):461–470. [DOI] [PubMed] [Google Scholar]
- 30.Munson JC, Bynum JP, Bell JE, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737–46. [DOI] [PubMed] [Google Scholar]
- 32.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6(1):45–58; discussion 62. [DOI] [PubMed] [Google Scholar]
- 33.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS—ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 2011;77(4):333–333 [DOI] [PubMed] [Google Scholar]
- 34.Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 35.Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA internal medicine. 2014;174(4):588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hart LA, Marcum ZA, Gray SL, Walker RL, Crane PK, Larson EB. The Association between central nervous system-active medication use and fall-related injury in community-dwelling older adults with dementia. Pharmacotherapy. 2019;39(5):530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semla TP, Beizer JL, Higbee MD. Geriatric Dosage Handbook. 15th ed. Hudson, OH: Lexicomp. [Google Scholar]
- 38.Boudreau DM, Yu O, Chubak J, et al. Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer. Breast Cancer Res Treat. 2014;144(2):405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sjölander A, Greenland S. Ignoring the matching variables in cohort studies - when is it valid and why? Stat Med. 2013;32(27):4696–708. [DOI] [PubMed] [Google Scholar]
- 40.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. March 1986;42(1):121–30. [PubMed] [Google Scholar]
- 41.Tamblyn R, Eguale T, Buckeridge DL, et al. The effectiveness of a new generation of computerized drug alerts in reducing the risk of injury from drug side effects: a cluster randomized trial. J Am Med Inform Assoc. 2012;19(4):635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boye ND, van der Velde N, de Vries OJ, et al. Effectiveness of medication withdrawal in older fallers: results from the Improving Medication Prescribing to reduce Risk Of FALLs (IMPROveFALL) trial. Age Ageing. 2017;46(1):142–146. [DOI] [PubMed] [Google Scholar]
- 43.Biderman A, Cwikel J, Fried AV, Galinsky D. Depression and falls among community dwelling elderly people: a search for common risk factors. J Epidemiol Community Health. 2002;56(8):631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kvelde T, Lord SR, Close JC, et al. Depressive symptoms increase fall risk in older people, independent of antidepressant use, and reduced executive and physical functioning. Arch Gerontol Geriatr. 2015;60(1):190–5. [DOI] [PubMed] [Google Scholar]
- 45.Kok RM, Reynolds CF. Management of depression in older adults: a review. JAMA. 2017;317(20):2114–2122. [DOI] [PubMed] [Google Scholar]
- 46.Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. National Health and Nutrition Examination Survey. J Clin Psychiatry. 2014;75(2):169–77. [DOI] [PubMed] [Google Scholar]
- 47.Mangin D, Lawson J, Cuppage J, et al. Legacy drug-prescribing patterns in primary care. Ann Fam Med. 2018;16(6):515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Leeuwen E, van Driel ML, Horowitz MA, et al. Approaches for discontinuation versus continuation of long-term antidepressant use for depressive and anxiety disorders in adults. Cochrane Database Syst Rev. 2021;4(4):Cd013495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maund E, Dewar-Haggart R, Williams S, et al. Barriers and facilitators to discontinuing antidepressant use: A systematic review and thematic synthesis. J Affect Disord. 2019;245:38–62. [DOI] [PubMed] [Google Scholar]
- 50.The Management of Major Depressive Disorder Working Group. (2016). VA/DoD clinical practice guideline for the management of major depressive disorder. Version 3.0. Washington, DC: Veterans Health Administration and Department of Defense. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary files
Supplementary Table S1: ICD-9 and E codes used to identify participants with a fall-related injury
Supplementary Table S2: Classification of medications and minimum effective dose
Supplementary Table S3: ICD-9 codes used for covariates
Supplementary Table S4: Average change in SDD from index of CNS-active medications according to use at index (restricted to index during 1994–2003)
Supplementary Table S5: Average change in SDD from index of CNS-active medications according to use at index (restricted to index during 2004–2014)


