Abstract
Per- and poly-fluoroalkyl substances (PFAS) are widespread environmental contaminants frequently detected in drinking water supplies worldwide that have been linked to a variety of adverse reproductive health outcomes in women. Compared to men, reproductive health effects in women are generally understudied while global trends in female reproduction rates are declining. Many factors may contribute to the observed decline in female reproduction, one of which is environmental contaminant exposure. PFAS have been used in home, food storage, personal care and industrial products for decades. Despite the phase-out of some legacy PFAS due to their environmental persistence and adverse health effects, alternative, short-chain and legacy PFAS mixtures will continue to pollute water and air and adversely influence women’s health. Studies have shown that both long- and short-chain PFAS disrupt normal reproductive function in women through altering hormone secretion, menstrual cyclicity, and fertility. Here, we summarize the role of a variety of PFAS and PFAS mixtures in female reproductive tract dysfunction and disease. Since these chemicals may affect reproductive tissues directly or indirectly through endocrine disruption, the role of PFAS in breast, thyroid, and hypothalamic-pituitary-gonadal axis function are also discussed as the interplay between these tissues may be critical in understanding the long-term reproductive health effects of PFAS in women. A major research gap is the need for mechanism of action data – the targets for PFAS in the female reproductive and endocrine systems are not evident, but the effects are many. Given the global decline in female fecundity and the ability of PFAS to negatively impact female reproductive health, further studies are needed to examine effects on endocrine target tissues involved in the onset of reproductive disorders of women.
Keywords: PFAS, female reproduction, environmental contaminants, endocrine disruption
1. Introduction
Female reproductive disorders are generally understudied in the scientific community. Additionally, the role of environmental exposures in the development of reproductive disorders in women warrants further attention.(Boyles et al. 2020) In 1996, a comprehensive review on male reproductive health and environmental xenoestrogens was published.(Toppari et al. 1996) A similar focused evaluation on the association between xenoestrogen exposure and female reproductive health has yet to be conducted.(Crain et al. 2008; Gore et al. 2015) Environmental contaminants have been shown to modify short-term events like birth outcomes(Blake and Fenton 2020; Chen et al. 2021; Eick et al. 2021; Eick et al. 2020; Olsen et al. 2009a) and fertilization or implantation(Ma et al. 2021). Exposure to environmental contaminants can also modify susceptibility to long-term diseases such as endometriosis(Campbell et al. 2016; Wang et al. 2017), polycystic ovarian syndrome (PCOS)(Heffernan et al. 2018; Palioura and Diamanti-Kandarakis 2015; Vagi et al. 2014; Wang et al. 2019a), and cancer(Fenton et al. 2021; Hurley et al. 2018; Tsai et al. 2020), highlighting the importance of understanding the impact of chemical exposures on female reproductive disorders.
Studies suggest that trends in global female reproduction have been on the decline over the past few decades.(Crain et al. 2008; Hamilton and Ventura 2006) Notably, from 1960 to 2002, the general fertility rate (number of live births per 1,000 women aged 15–44 years) and total fertility rate (sums of birth rates by 5-year age groups multiplied by 5) per 1,000 women have declined by at least 44%.(Hamilton and Ventura 2006) Such trends likely result from cultural changes, such as oral contraceptive (OC) use or the trend of women starting their families at older ages; however, the role of environmental influences may contribute to the observed decline.(Crain et al. 2008) While information on global trends is limited, hormone-related disorders in women, such as precocious pubertal timing, endometriosis, and PCOS have become more common and have been attributed to environmental exposures.(Crain et al. 2008; Gore et al. 2015) Effects have also been noted by pharmaceuticals; it is well known that diethylstilbestrol (DES), a synthetic estrogenic compound, increases the risk of genital tract and breast cancers, and decreases fertility in women exposed in utero.(Crain et al. 2008; Goldberg and Falcone 1999; Hatch et al. 2006; Lagiou 2006)
Many environmental contaminants to which women are routinely exposed are labeled as endocrine-disrupting compounds (EDCs). EDCs number in the hundreds, and include persistent chemicals like diphenyldichloroethene (DDE), a breakdown product of the insecticide dichlorodiphenyltrichloroethane (DDT), and non-persistent chemicals like phthalates, which are a class of chemicals found in detergents, food packaging, medical grade plastics, and personal care products.(Hunt et al. 2016) Studies examining the relationship between EDCs and female reproductive disorders found that increased exposure to EDCs were associated with fibroids and endometriosis in women in the European Union.(Hunt et al. 2016) A number of other EDCs have been linked to reproductive disorders in women, including genistein, a phytoestrogen found in soy products; bisphenol A, a compound commonly used in plastics and epoxy resins, and dioxins, a group of chemicals produced from incinerating waste.(Caserta et al. 2008; Crain et al. 2008; Gore et al. 2015) Studies have suggested an association between these compounds and decreased fertility and fecundity, endometriosis, altered pubertal timing, as well as breast and endometrial cancers.(Caserta et al. 2008; Gore et al. 2015)
Although there are some EDCs with very strong correlations to female reproductive disease, much remains unknown about the contribution of other prevalent environmental contaminants, including per- and poly-fluoroalkyl substances (PFAS), which frequently contaminate drinking water supplies worldwide. One particular PFAS, perfluorooctanoic acid (PFOA) has been reported as an endocrine disruptor for its inhibitory effects on placental and birth outcomes(Blake and Fenton 2020; Szilagyi et al. 2020a), the thyroid(Ballesteros et al. 2017), and pubertal breast development and lactation in rodent models(Macon and Fenton 2013), but other female reproductive targets of PFAS have not been reviewed. As a result of the prevalence of EDCs and other daily environmental exposures to women, understanding the contributions of PFAS to the onset of reproductive disorders in women is critical in understanding trends in female reproduction and related diseases.
2. What are PFAS and How are Women Exposed?
The latest definition by the Organization for Economic Co-operation and Development (OECD) states that PFAS are fluorinated substances containing at least one fully fluorinated methyl or methylene carbon atom, although some exceptions exist.(OECD 2021) These chemicals are environmentally-persistent, man-made compounds that have a variety of different biological, structural, chemical, and physical properties.(Buck et al. 2011; Calafat et al. 2019; Williams et al. 2017) The ability of PFAS to persist in the environment is mainly due to the presence of a perfluoroalkyl moiety (CnF2n+1−) which exhibits chemical stability, thermal stability, hydrophobicity and lipophilicity, enabling these compounds to resist degradation.(Blake et al. 2018; Buck et al. 2011; Fu et al. 2016; Karsa 1995; Kissa and Kissa 2001; Olsen et al. 2007) PFAS are typically categorized into polymers and non-polymers and then further subdivided based on chemical properties. For example, the term “non-polymer PFAS” encompasses both perfluoroalkyl substances and polyfluoroalkyl substances.(Buck et al. 2011) It is important to consider that many of these chemicals are referred to by a variety of abbreviated names. For clarity, Table 1 summarizes numerous common PFAS based on the Environmental Protection Agency’s (EPA) CompTox Dashboard(Williams et al. 2017) official name, along with their respective CAS numbers and commonly used abbreviations or aliases in the literature.
Table 1.
Classifications of Common PFAS and their Implications in Women’s Reproductive Health.
| Compound Name By Class (CAS #) | Main Abbreviation (Other Aliases) | Carbon Number | Reported Positive, Negative, or Null Associations |
|---|---|---|---|
| Perfluoroalkyl Carboxylates | |||
| Perfluorobutanoic acid (375-22-4) | PFBA | 4 |
|
| Perfluorohexanoic acid (307-24-4) | PFHxA | 6 |
|
| Perfluoroheptanoic acid (375-85-9) | PFHpA | 7 |
|
| Perfluorooctanoic acid (335-67-1) | PFOA | 8 |
|
| Perfluorononanoic acid (375-95-1) | PFNA | 9 |
|
| Perfluorodecanoic acid (335-76-2) | PFDA (PFDeA) | 10 |
|
| Perfluoroundecanoic acid (2058-94-8) | PFUdA (PFUnA, PFUnDA PFUA) | 11 |
|
| Perfluorododecanoic acid (307-55-1) | PFDOA (PFDoDA, PFDDA) | 12 |
|
| Perfluoroalkyl Sulfonates | |||
| Perfluorobutane sulfonate (45187-15-3) | PFBS | 4 |
|
| Perfluorohexane sulfonate (108427-53-8) | PFHxS | 6 |
|
| Perfluoroheptane sulfonate (146689-46-5) | PFHpS | 7 |
|
| Perfluorooctane sulfonate (1763-23-1) | PFOS | 8 |
|
| Perfluorooctane Sulfonamido Acetic Acids | |||
| 2-(N-methyl-perfluorooctane sulfonamido) acetic acid (235531–9) | Me-PFOSA-AcOH (N-Me-FOSAA, NMePFOSA-AcOH, NMeFOSAA, Me-FOSAA) | 11 |
|
| 2-(N-ethylperfluorooct ane sulfonamido) acetic acid (2991-50-6) | Et-PFOSA-AcOH (NEtPFOSA-AcOH, NEtFOSAA, Et-FOSAA) | 12 |
|
| Perfluorooctane Sulfonamides | |||
| Perfluorooctanes ulfonamide (754-91-6) | PFOSA | 8 |
|
| Perfluoroalkyl Substance Alternatives | |||
| Ammonium perfluoro(2-methyl-3-oxahexanoate) (62037-80-3) | HFPO-DA (GenX) | 6 |
|
| Ammonium 4,8-dioxa-3H-perfluorononanoate (958445-44-8) | ADONA (PMPP) | 7 |
|
Note: Names used for each chemical is considered valid by the EPA’s CompTox Dashboard. Other names cited below that are those used in papers referenced throughout this review.
In general, PFAS are often referred to as short-chain (generally containing ≤ 7 carbons) and long-chain (containing ≥ 8 carbons), and often as legacy compounds, based on the length of time the chemicals have been evaluated. Long-chain, legacy PFAS include chemicals like perfluorooctane sulfonate (PFOS) and PFOA, which are two of the most well-studied PFAS. These compounds have long half-lives and, therefore, tend to persist in both the environment and in the human body. Although these compounds were used in a wide variety of applications for decades, production of certain PFAS, such as PFOA and PFOS, was voluntarily phased-out in the United States during the 2000s as their negative health effects were increasingly reported.(Calafat et al. 2019; DeWitt 2015; Substances and Registry 2018) This phase-out occurred in two waves: 1) the voluntary phase-out of PFOS and PFOA in 2000 and 2002 by 3M, and 2) the EPA’s PFOA Stewardship Program, which aimed to phase out 95% of PFOA and related compounds production and use by 2010, striving for their elimination by 2015.(Buck et al. 2011; EPA 2000, 2006) As a result, short-chain or structurally modified PFAS, referred to as PFAS alternatives, which include newer substances like ammonium perfluoro(2-methyl-3-oxahexanoate), or HFPO-DA, and ammonium 4,8-dioxa-3H-perfluorononanoate, or ADONA, have replaced some of the legacy PFAS.(Calafat et al. 2019; Sunderland et al. 2019; Wang et al. 2019b) These replacement compounds are proposed to decrease both the environmental and physiological burden (the amount circulating in the blood) of PFAS due to increased urinary excretion and shorter half-lives compared to long-chain PFAS.(Calafat et al. 2019; Gannon et al. 2016; Gannon et al. 2011; Nilsson et al. 2010; Olsen et al. 2009b) Although these PFAS alternatives are proposed to decrease risk for environmental persistence, many of these chemicals are unregulated and untested with respect to health risks, especially regarding reproductive tissues.(Calafat et al. 2019; Pan et al. 2020; Sunderland et al. 2019; Wang et al. 2019b) While this remains true for PFAS alternatives, there is a lack of information on other PFAS as well, notably the short-chain perfluoroalkyl carboxylic acids and perfluorooctane sulfonamides found in contemporary mixtures. The lack of reproductive effect studies in the literature for certain short-chain PFAS and PFAS alternatives compared to long-chain perfluoroalkyl carboxylic acids and perfluoroalkyl sulfonates is illustrated in Table 1.
Legacy PFAS have been used in a wide variety of commercial and industrial products since the 1950s.(Buck et al. 2011; Kissa and Kissa 2001) Some common products include textile coatings, non-stick cookware coatings, food containers, personal care products, ‘wrinkle-free’ and ‘water-proof’ products, and Class B fire-fighting foams.(Buck et al. 2011; Calafat et al. 2019; DeWitt 2015; Substances and Registry 2018) Exposure to PFAS can occur through a variety of routes, and usually as a mixture containing multiple compounds, with one of the most prevalent being contaminated drinking water and another being through dietary choices.(Blake et al. 2018; Gebbink et al. 2017; Heydebreck et al. 2015; Kaboré et al. 2018; Pan et al. 2017; Sun et al. 2016; Wei et al. 2018) Water levels of legacy PFAS like PFOS and PFOA can be measured relatively easily in comparison to short-chain and alternative PFAS, which cannot be measured accurately due to limitations in current laboratory testing and availability of standards.(DEQ 2019) In certain geographical areas, notably Michigan, Pennsylvania, New Jersey, and North Carolina, PFAS are under scrutiny due to their widespread contamination of ground, surface, and drinking water.(Park et al. 2019) PFAS are absorbed by plants grown in polluted water sources, and livestock may also accumulate PFAS in their body via consumption of contaminated water and their plant-based diet. Likewise, women may unknowingly make choices that elevate their PFAS physiologic burden including the use of cosmetics and other personal care products, non-stick cookware, PFAS-containing food storage containers, and upholstered furniture, mattresses, and stain-resistant carpeting. Numerous studies around the world have measured high serum levels of PFAS in the residents of communities with contaminated drinking water, further indicating the prevalence of PFAS contamination in the environment.(Barton et al. 2019; Group 2021; Worley et al. 2017)
Even with the phase-out of legacy PFAS (EPA 2000, 2006), exposure to PFOS and PFOA remains a problem in the United States. The Centers for Disease Control and Prevention (CDC) reported that exposure to long-chain PFAS, including PFOS and PFOA, was prevalent in the United States and that more than 95% of the population had detectable levels of PFOA, PFOS, perfluorononanoic acid (PFNA) and perfluorohexanesulfonic acid (PFHxS).(Calafat et al. 2019; CDC 2012) An evaluation of serum PFAS concentrations in participants of the United States National Health and Nutrition Examination Survey (NHANES) found that several PFAS were detected in the serum of nearly all of the U.S. general population, and that phased-out, long-chain PFAS were detected even in young adults born after the decline in legacy PFAS production and use occurred.(Calafat et al. 2019; CDC 2012; Ye et al. 2018) On the other hand, short-chain PFAS were rarely detected in these samples, suggesting that these compounds may not contribute to PFAS body burdens as much as long-chain PFAS due to their shorter half-lives and elimination through urine.(Calafat et al. 2019; CDC 2012; Ye et al. 2018)
This review specifically focuses on the effects of a subset of non-polymer PFAS on female endocrine and reproductive targets and highlights research gaps, mechanisms of interest, and the potential for reverse causality due to normal biology. The summary and perspective provided here emphasizes the need to investigate the molecular target(s) of PFAS in female reproductive tissues to elucidate the mechanistic underpinnings of PFAS-related reproductive toxicity and develop interventions to improve reproductive health.
2.1. Demographic Factors Associated with PFAS Exposure
As previously mentioned, contaminated drinking water and modifiable factors such as choice of diet and product use may contribute to an individual’s PFAS exposure. Exposure can also be exacerbated by various demographic factors such as geography, race/ethnicity, age, sex, and occupation. Understanding the impacts of these factors on PFAS exposure is crucial for identifying the racial, ethnic, or under-represented groups that may be at highest risk of experiencing the adverse health impacts of PFAS exposures.
The use of PFAS-containing aqueous film forming foams (AFFFs) at airports, military sites, and firefighting training sites along with PFAS-containing municipal and industrial waste have contributed to the contamination of ground and surface water, leaving certain geographic locations at a higher risk for increased PFAS exposures in their drinking water systems.(Anderson et al. 2016; Barton et al. 2019; Hu et al. 2016; Park et al. 2019; Weiss et al. 2012) In 2007, the EPA received a notice from a PFAS manufacturer in Alabama stating that it had discharged waste contaminated with PFAS into a wastewater treatment plant, posing serious concern for both the surrounding environment and community.(Worley et al. 2017) Serum analysis on residents of the contaminated community in 2010 revealed that mean serum concentrations of PFOA and PFOS were higher than that of the U.S. general population when compared to 2009–2010 NHANES 95th percentile values. Serum analysis of the same participants in 2016 revealed that PFOS, PFHxS and PFOA were higher than the 2013–2014 NHANES 95th percentile values but lower than serum concentrations of PFAS in residents living in areas across the United States with known PFAS exposure. Compared to 2010 serum analysis data, the authors report decreased mean serum concentrations of PFOA, PFOS, PFNA, perfluorodecanoic acid (PFDA), and 2-(N-methyl-perfluorooctane sulfonamido) acetic acid (Me-PFOSA-AcOH) in 2016, and suggest that the observed decrease may be due to reduced concentrations of PFAS in the Decatur area resulting from the EPA’s phase-out of long-chain PFAS.(Worley et al. 2017)
In another study examining the association between geographic location and PFAS exposure, Park et al.(Park et al. 2019) measured PFAS levels in women from five different sites in the Study of Women’s Health Across the Nation (SWAN, Southeast Michigan, Pittsburgh, Boston, Oakland, and Los Angeles). Analysis showed that linear PFOA, PFNA, PFHxS, and branched and linear PFOS were found in the serum of more than 97% of women, while longer chain PFAS such as PFDA, perfluoroundecanoic acid (PFUdA), perfluorododecanoic acid (PFDOA), and branched PFOA were not present as often. Additionally, study site was significantly associated with PFAS exposure. The lowest concentrations of PFAS were found in participants in the Oakland site, while participants in the Southeast Michigan site had the highest serum concentrations of PFOS. Serum concentrations of PFOS and PFHxS in participants from the Michigan and Pittsburgh sites were elevated when compared to NHANES 1999–2000 values. Geographic differences in PFAS exposure that were observed in this study may be explained by a variety of reasons, one being water contamination, since two known sites of PFAS-contaminated drinking water (Michigan and Pittsburgh sites) were included in the study. The association between PFAS exposure and geographical location has been supported by other studies, as exposure to various PFAS differed across geographic regions of the United States, Greece, Italy, Korea, the Netherlands, and Spain.(Cho et al. 2015; Ericson et al. 2007; Hu et al. 2016; Pitter et al. 2020; Vassiliadou et al. 2010; Zafeiraki et al. 2015) Additionally, a study examining serum PFOS concentrations across the United States, Poland, Korea, Belgium, Malaysia, Brazil, Italy, Colombia, and India found that PFOS concentrations were highest in residents of the United States and Poland while concentrations were lowest in India.(Kannan et al. 2004) Understanding the association between geographic location and PFAS exposure is critical because it can help identify populations at risk for developing PFAS-related health effects. For example, in Mid-Ohio Valley residents, another known site of environmental PFAS contamination, modest associations were found between PFOA exposure and preeclampsia and birth defects, while preeclampsia and low birth weights were mildly associated with PFOS exposure.(Stein et al. 2009) These findings suggest that residents of communities with elevated PFAS contamination levels are at a greater risk for PFAS-related health effects and warrants further investigation.
A subset of PFAS were detected in El Paso County, Colorado in both public and private water sources, with the likely source as the nearby use of AFFF.(Barton et al. 2019) PFHxS, PFOA, PFOS, and PFNA were detected in 99.5% of serum samples collected from participants, while perfluoroheptane sulfonate (PFHpS) was detected in 82.6% of the collected serum samples. The chemical found at the highest serum concentration across the study population was PFHxS, which had median and 90th percentile serum concentration values that were 12 and 15 times higher than the U.S. median and 90th percentile, respectively, based on 2015–2016 NHANES values. In addition to elevated PFHxS serum concentrations, PFOS and PFOA median and 90th percentile values were higher than the U.S. NHANES values. One variable that was linked to PFAS exposure in this community was race, as serum concentrations of PFHxS, PFOA, and PFHpS were significantly higher in the non-Hispanic White participants compared to Hispanic and non-Hispanic non-White participants. Similarly, another study reported that PFAS levels were lower among Hispanic participants compared to their non-Hispanic counterparts.(Eick et al. 2021) Race was also an important determinant of PFAS exposure in the study by Park et al.(Park et al. 2019), which reported that the highest serum concentrations of linear and branched PFOS, linear PFOA, and PFNA were detected in Black> White> Japanese, and Chinese women. In accordance with these findings, the latest CDC data on NHANES PFAS values across varying populations shows that PFOS, PFOA, PFNA, PFHxS, PFHpS, and PFDA levels in Mexican Americans and Hispanics were lower than those of their non-Hispanic White, non-Hispanic Black, and Asian counterparts.(CDC 2021)
Lifestyle factors such as water source, cosmetic use, and food consumption choices may play key roles in PFAS exposure levels and may explain race-related and sex-related differences. In the study by Barton et al.(Barton et al. 2019), participants who reported tap water as their primary drinking water source had significantly higher serum PFHxS and PFOA concentrations compared to participants who reported bottled water as their primary drinking water source. Drinking water source was also reported to be significantly associated with serum PFAS concentrations in pregnant African American women living in the Atlanta area, along with cosmetic use.(Chang et al. 2020) Other studies have found that food consumption choices can impact PFAS exposure, as PFAS levels were increased in African American women who reported frequent consumption of food in coated cardboard containers.(Boronow et al. 2019) Data from these studies support the hypothesis that lifestyle factors impact PFAS exposure in women.
Examining the relationship between PFAS serum concentrations and sex/age, Góralczyk et al.(Góralczyk et al. 2015) analyzed serum samples of men and women in Central Poland for 7 PFAS (PFHxS, PFOS, PFOA, PFNA, PFDA, PFUdA, PFDOA), and found that the chemical with the highest average serum concentration in both men and women was PFOS, measuring 18.49 ng/mL and 8.42 ng/mL, respectively. Generally, higher average PFAS serum concentrations were observed in men compared to women, except for PFHxS and PFDOA. Other studies have reported similar findings, specifically regarding higher PFOS and PFOA concentrations in men compared to women. Studies have also reported higher serum concentrations of PFHxS in men compared to women, which contradicts these data, and may suggest a male-specific exposure source and/or a sex-dependent difference in PFAS elimination related to female reproductive biology and menstruation.(Ericson et al. 2007; Ingelido et al. 2010; Midasch et al. 2006; Vassiliadou et al. 2010) In order to examine the relationship between age and serum PFAS concentrations, participants of this study were broken down into 4 age groups (≤25 years, 26–29 years, 30–35 years, ≥36 years), and analysis showed no statistically significant differences for any of the tested PFAS after differentiating the results by age and sex.(Góralczyk et al. 2015) Barton et al.(Barton et al. 2019) reported a positive association between PFOS and PFHxS exposure and age, while Park et al.(Park et al. 2019) noted increased PFOS and PFOA serum concentrations associated with younger age groups compared to older age groups of women. In these examples of adult exposures, there is some disagreement in the relation of age and PFAS body burden, and this could be due to lack of stratification by sex and other biological variables specific to women (parity, reproductive status). For children ages 3–11, Ye et al.(Ye et al. 2018) reported that serum levels of linear PFOS, branched PFOS, linear PFOA, PFHxS, and PFNA were similar and often higher than their corresponding levels in adults.
Occupational exposure is an important factor contributing to PFAS body burden in humans. According to the NIOSH(NIOSH 2021), workers can be exposed to PFAS through touching products containing concentrated amounts of PFAS or breathing in PFAS-contaminated air. Workers at risk for high occupational PFAS exposure include those in chemical manufacturing, chrome plating, firefighting, and ski waxing.(NIOSH 2021) In a study examining PFAS exposure in workers of a textile manufacturing plant in China, Heydebreck et al.(Heydebreck et al. 2016) reported that inhalation of fluorotelomer alcohols, which are precursors to PFAS, was 5 orders of magnitude higher than that of the general western population. In another study examining firefighters exposed to AFFFs, Rotander et al.(Rotander et al. 2015) found that serum levels of PFOS and PFHxS were elevated compared to the general population. Leary et al.(Leary et al. 2020) reported similar findings that firefighters had elevated serum PFAS levels compared to the general population. In addition to chemical manufacturing and firefighting, NIOSH lists ski waxing as an occupation with potential for high exposure to PFAS.(NIOSH 2021) Nilsson et al.(Nilsson et al. 2013) examined PFAS exposure in professional ski waxers and found that ski waxers were exposed to PFAS at levels exceeding those of the general population and above the occupational exposure standards. Overall, understanding various sociodemographic factors such as age, sex, occupation, geography, and race/ethnicity that impact PFAS exposure can help to identify high-risk exposure groups and potential health impacts of PFAS.
2.2. Reproductive and Pregnancy-Related Determinants of PFAS Physiologic Burden
Of course, exposure is only one part of the picture when understanding the human body burden of PFAS. Some long-chain PFAS are reported to be poorly eliminated from the body, with elimination half-lives on the order of years (summarized in Fenton et al. 2021). They bind to proteins in the blood and other tissues.(Forsthuber et al. 2020; Pérez et al. 2013) For example, studies have shown that albumin is a critical carrier of PFOS, PFOA, PFHxS, PFNA, and PFDA in human blood.(Forsthuber et al. 2020) Other studies examining PFAS tissue distribution have found that perfluorobutanoic acid (PFBA) accumulates at the highest concentrations in the kidney and lung, while perfluorohexanoic acid (PFHxA) accumulated at the highest concentrations in the liver and brain. PFOS and PFOA tended to accumulate more in the liver and bone but the highest overall concentrations of PFAS were observed in lung tissue.(Pérez et al. 2013) In addition to tissue distribution and accumulation, elimination of PFAS is complicated and may vary by sex, as, reproductive factors such as pregnancy, breastfeeding, and menstruation can act as important determinants of PFAS physiologic burden following exposures in women.
Pregnancy plays a key role in PFAS physiologic burden since associations between lower serum PFAS concentrations and higher parity have been reported(Berg et al. 2015; Brantsæter et al. 2013; Kato et al. 2014). In a study measuring PFAS exposure during early pregnancy in women from the Boston-area Project Viva cohort, PFOS, PFOA, PFHxS, and PFNA concentrations were reported to be higher in nulliparous women compared to parous women. The authors suggest that this finding may be due to placental transfer of PFAS and increased PFAS concentrations in breast milk.(Sagiv et al. 2015) Colles et al.(Colles et al. 2020) further examined serum levels of PFAS in Belgian mother-newborn pairs, adolescents, and adults and compared them to the Human Biomonitoring-I (HBM-I) value, which is the level of exposure to a chemical that is not expected to pose any adverse health risks over a lifetime(Umweltbundesamt 2018), much like the U.S. EPA’s Health Advisory Level.(EPA 2016) Data showed that plasma PFOS and PFOA concentrations in female participants aged 20–40 years exceeded the HBM-I value in 85% of participants, and that mothers with three or more children had lower serum and/or cord plasma levels of some PFAS compared to their age-matched counterparts. Maternal and cord blood comparison studies have reported that a mother may transfer substantial (40% or more) amounts of her body burden of PFAS to the infant through placental transfer, and this occurs with each subsequent pregnancy.(Aylward et al. 2014; Verner et al. 2016) These data suggest that pregnancy is an important determinant of PFAS physiologic burden in women and may be related to blood loss, placental transfer, and changed excretion patterns that occur during pregnancy.
In addition to transplacental transfer, breastfeeding significantly contributes to elevated PFAS measures in infants compared to their mothers.(Verner et al 2016) Breastfeeding can be an important excretion route for PFAS in post-parturient women as well, as studies have shown that PFAS levels, notably PFOS and PFOA, are higher in women who have never breastfed.(Sagiv et al. 2015) Supporting this, Colles et al.(Colles et al. 2020) reported that mothers with a history of breastfeeding or longer lactation duration tended to have lower serum and/or cord plasma levels of some PFAS compared to their counterparts. Lower serum PFAS levels were also reported in women from Cincinnati with a history of breastfeeding(Kato et al. 2014) and in Norwegian women with longer durations of breastfeeding.(Brantsæter et al. 2013) In infants and children who were breastfed, increased serum PFAS levels, with the exception of PFHxS, have been reported compared to children who were formula-fed, including those in areas of known PFAS contamination.(Herrick et al. 2017; Kato et al. 2014; Mogensen et al. 2015) Since it has been reported that lactation is a proven route of elimination for PFAS(Barbarossa et al. 2013; Fromme et al. 2010; Mogensen et al. 2015; Mondal et al. 2014; Verner et al. 2016), these findings support breastfeeding as another potential female-specific determinant of PFAS physiologic burden. Although the mechanisms are not clear, it is possible that PFAS bind to proteins transferred into milk(Fromme et al. 2009; Serrano et al. 2021), or they may mimic long-chain fatty acids(Shabalina et al. 2016) normally found in breast milk.
In addition to pregnancy and breastfeeding, menstruation appears to be a crucial route of elimination for many PFAS, potentially explaining about 30% of the discrepancy between male and female PFAS serum levels.(Wong et al. 2014; Zhou et al. 2017) Since 90–99% of PFAS in the blood are bound to serum albumin, menstrual bleeding may prove an important elimination pathway for these persistent substances.(Ding et al. 2020b; Han et al. 2003; Ylinen and Auriola 1990) For example, Park et al.(Park et al. 2019) demonstrated that for most of the PFAS studied, serum PFAS concentrations decreased in women who had experienced recent menstrual bleeding compared to those who did not report any bleeding. Another study found that menstruating women had lower serum concentrations of linear PFOA, PFNA, and branched PFOS compared to women who were not menstruating.(Ding et al. 2020a) In women who had already undergone menopause, increased PFHxS serum levels were reported compared to women who had not undergone menopause, with similar trends observed for PFOS, PFOA, and PFNA.(Colles et al. 2020) Overall, these studies suggest that reproductive factors such as pregnancy, breastfeeding, and menstruation can be important determinants of PFAS physiologic burden, and that pregnancy, nursing, and menstruation (blood loss) are primary routes of PFAS elimination in women.
Since menstruation may serve as an elimination route for PFAS, OC use may impact PFAS exposure levels in women. Rush et al.(Rush et al. 2018) found that concentrations of various PFAS, including PFOA, PFNA, PFDA, PFHxS, PFHpS, and PFOS, were increased by as much as 20.1% in women with self-reported OC use in the 12 months preceding the baseline interview compared to their counterparts. Their data also suggested that recency of OC use may impact PFAS exposure; recent OC users displayed 12.9–35.7% higher PFAS plasma concentrations compared to women who had never used OCs. In addition to recency, duration of OC use appeared to be another important determinant of PFAS exposure, as women who reported using OCs for 10 or more years had higher serum concentrations of PFOA, PFNA, PFHxS, PFHpS, and PFOS than women who reported never using OCs. One potential explanation for the increased PFAS levels observed in OC users compared to never users may be that OCs are associated with reductions in menstrual flow in both women with normal bleeding and women who experience heavy or prolonged menstrual bleeding, further supporting menstruation as a route of elimination for PFAS.(Fraser et al. 2011b; Larsson et al. 1992; Rush et al. 2018)
3. PFAS as Endocrine Disruptors
PFAS are known to have effects on metabolic, endocrine, and reproductive function, suggesting their role as endocrine disruptors.(Rashtian et al. 2019) The term “endocrine disruptor” encompasses a broad range of chemicals which can impact various organ systems in the body through altering hormone regulation or normal function. One of the ways that EDCs are unique is in their ability to produce non-monotonic dose-response curves(Vandenberg 2013), a characteristic which certain PFAS have had reported(Hines et al. 2009; Lee et al. 2021). Regarding the reproductive system, endocrine disruptors, more specifically some PFAS, have the potential to impair endogenous hormone metabolism and steroid hormone production or secretion, including that of estradiol (E2), progesterone, testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH).(Barrett et al. 2015; Behr et al. 2018; Feng et al. 2015; Nian et al. 2020; Rashtian et al. 2019; Shi et al. 2009) PFAS have also been shown to alter normal thyroid function through dysregulating thyroid-stimulating hormone (TSH), triiodothyronine (T3), and/or thyroxine (T4) levels.(Aimuzi et al. 2020; Blake et al. 2018; Chang et al. 2008; Fenton et al. 2021; Inoue et al. 2019; Jain 2013; Lau et al. 2003; Lebeaux et al. 2020; Lewis et al. 2015; Luebker et al. 2005b; Wang et al. 2014; Webster et al. 2016; Webster et al. 2014) Breast and placental tissue may be impacted by PFAS exposure, since PFAS have been shown to alter prolactin and human chorionic gonadotropin (hCG) levels, respectively.(Zhang et al. 2015; Zhang et al. 2018) These findings are critical, as thyroid hormone, prolactin, and hCG are all important targets of the reproductive system. It is important to consider that endocrine effects of PFAS may occur through directly or indirectly affecting reproductive tissues. In the mammary gland and breast, studies have shown that PFAS affect breast cells in culture and accumulate in mammary gland tissue of exposed mice, indicative of direct impact on the tissue.(Fenton et al. 2009; Pierozan and Karlsson 2018; White et al. 2009) This is also observed in the placenta, as studies have shown that PFAS exposure alters placental cell function in culture and in mice.(Bangma et al. 2020b; Blake et al. 2020; Szilagyi et al. 2020b). PFAS-induced effects on reproductive tissues can also occur indirectly through altering normal HPGA or associated endocrine tissue function.(Blake and Fenton 2020) For reproductive tissues that are less well-studied in terms of PFAS-associated outcome, it is important to consider both direct and indirect effects of these compounds. In the following sections, the various endocrine disrupting effects of PFAS, specifically as they pertain to the breast, thyroid, and female reproductive tract, will be discussed.
3.1. PFAS and the Breast
In women and other mammals, normal growth and functioning of the mammary glands involve HPGA endocrine signaling as well as signaling through the release of hormones and growth factors.(Macon and Fenton 2013) There are many different endogenous factors that can modulate growth and function of the mammary gland, including prolactin, thyroxine, and estrogens.(Macon and Fenton 2013) In this way, a number of endocrine systems within the body interact with breast tissue to ensure normal growth and function throughout a woman’s life, as displayed in Figure 1. Importantly, exogenous compounds like PFAS are known to affect many of the endocrine paths leading to the breast outlined in Figure 1, which enables them to alter normal mammary gland function or development through direct or indirect mechanisms.
Figure 1.
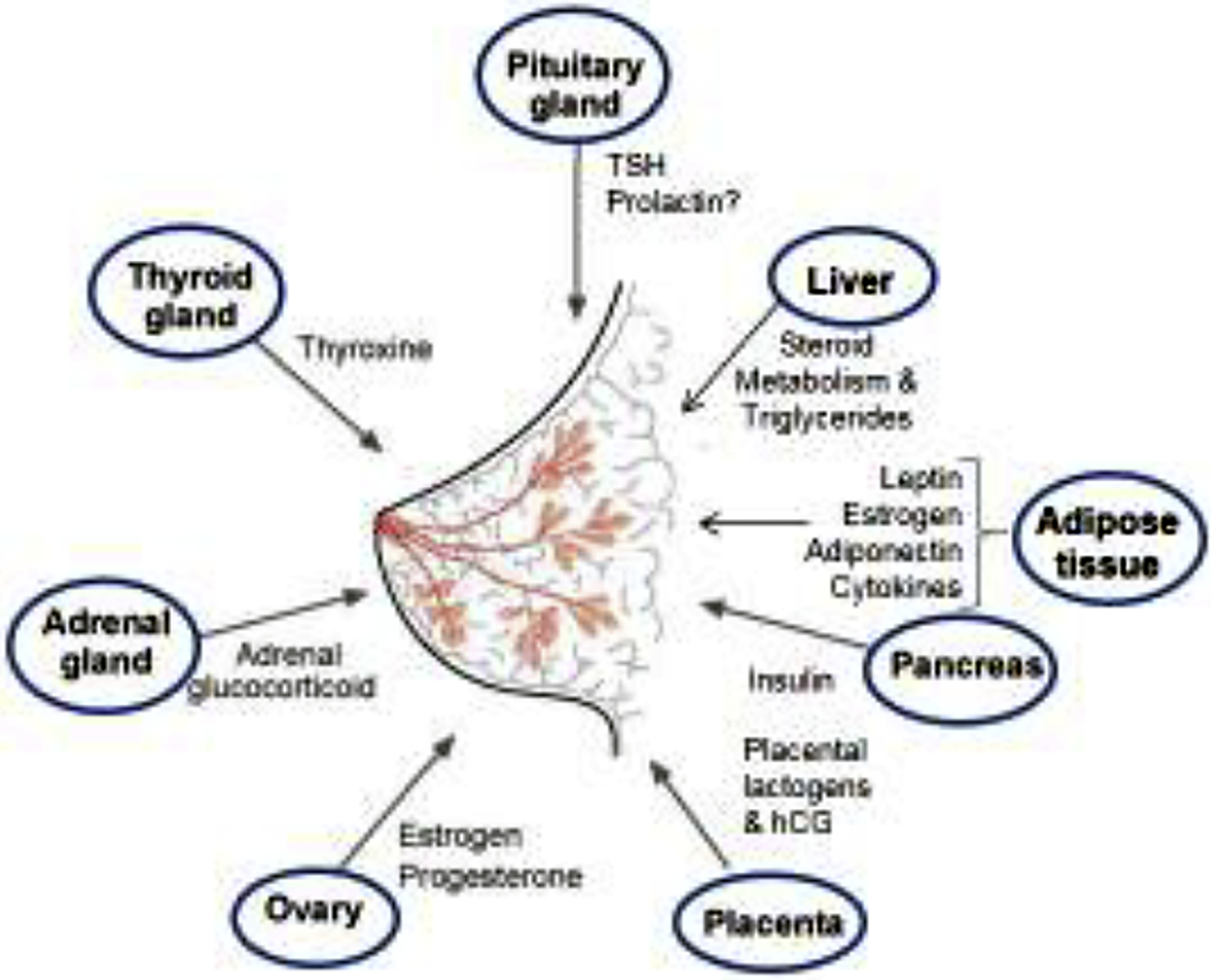
Legacy PFAS endocrine-sensitive tissue targets. PFAS affect numerous systems (blue circles) that mediate endocrine system changes in hormone output or feedback. These endocrine changes (shown) are all known to modify breast development and lactational ability. Mechanisms of action of PFAS in the breast are not known but may delay breast development and inhibit nursing duration. Adapted from Macon & Fenton, 2013. J. Mammary Gland Biol. Neoplasia 18: 43–61.
Various studies have demonstrated that one legacy PFAS, PFOA, can affect breast tissue development in both mouse models and humans.(Fenton et al. 2021), Tucker et al.(Tucker et al. 2015) found that when pregnant mice were exposed to PFOA at concentrations similar to human exposure in contaminated communities, female offspring experienced delays in mammary gland development in a dose-dependent manner. Although this delay began during puberty for female offspring, the delay persisted through young-adulthood.(Tucker et al. 2015) Other studies examining the effects of PFOA on the mammary gland of pregnant mice found that PFOA exposure can decrease mammary epithelial and terminal end bud growth in the female offspring, and that exposures during mammary bud development (latter part of gestation) was adequate to recapitulate full gestation exposures.(Macon et al. 2011) A study examining the effect of gestational PFOA exposure on three generations (P0, F1, and F2) of mice confirmed a previous report(White et al. 2007) that gestational PFOA exposure diminished lactational function and mammary gland development in the P0 and F1, respectively.(White et al. 2011) When mice in the F1 and F2 generations were exposed to low-dose PFOA through drinking water at concentrations similar to contaminated human drinking water supplies, F1 mice displayed diminished lactational morphology while those of the F2 generation had slowed mammary epithelial outgrowth at weaning. These studies defined pregnancy as a critical period for effects of PFOA on offspring and mother’s mammary tissue.
In addition to the above studies showing that breast tissue development can be altered after PFAS exposure in mice, human breast development (thelarche), which occurs in females prior to menarche may be impacted by neonatal PFAS exposure, since a main route of early PFAS exposure is through breast milk. In ethnically diverse girls aged 6–8 from the New York City, Greater Cincinnati, and San Francisco Bay Areas, later onset of breast development was observed in girls who were mixed-fed (formula and breastfeeding) or predominantly breastfed after birth.(Kale et al. 2015) The duration of breastfeeding was inversely associated with early onset of breast development in these cohorts as well and was positively associated with higher serum PFOA levels in both the Greater Cincinnati and San Francisco Bay Areas.(Kale et al. 2015; Pinney et al. 2014) Follow-up studies have reported that these 6–8-year-old girls in the Greater Cincinnati area had serum PFOA concentrations above the NHANES 95th percentile for 12–19-year-old children, while those in the San Francisco Bay Area had median serum PFOA concentrations above the U.S. median for 12–19-year-old children.(Pinney et al. 2014; Pinney et al. 2019) The relationship between PFAS exposure and thelarche timing warrants further examination.
While PFAS may affect normal mammary gland development, a main function of the breast that can be altered by PFAS exposure is lactation. One of the first papers describing the health effects of PFOA in developmentally exposed mice reported lack of weight gain and increased mortality in a dose-dependent trend(Lau et al. 2006), and follow-up studies demonstrated it was due to lack of mammary gland lactational development.(White et al. 2007) Other studies examining the effects of PFOS on prolactin-family hormone concentrations found that PFOS decreased mRNA and serum levels of prolactin-family hormones in a dose-dependent manner.(Lee et al. 2015) Prolactin-family hormone levels can be measured in women as well as markers of lactational function after PFAS exposure.(Timmermann et al. 2021) In addition to rodent models, human lactation has been reported to be altered by PFAS exposure.(Romano et al. 2016; Timmermann et al. 2017) In a study examining the association between PFAS exposure and the ability of new mothers to breastfeed, PFAS serum levels were measured throughout pregnancy and standardized breastfeeding surveys were completed until the end of breastfeeding.(Romano et al. 2016) In this cohort of women, after controlling for prior breastfeeding, serum PFOA concentrations were inversely related to the duration of breastfeeding suggesting that PFOA exposure shortens breastfeeding duration. Similarly, Timmerman et al.(Timmermann et al. 2017) found that doubling of maternal serum PFAS levels was associated with a decreased duration of breastfeeding in two Faroese birth cohorts. Doubling of serum PFOS levels in these cohorts was associated with a 1.4-month reduction in total breastfeeding duration.(Timmermann et al. 2017) These findings were confirmed in another recent study by Timmermann et al.(Timmermann et al. 2021) examining the association between serum PFAS levels during pregnancy and breastfeeding termination in the Odense Child Cohort, further suggesting an association between PFAS exposure and altered breast function (lactation duration). The ability of PFAS to affect the lipid composition of breast milk has also been examined, and a recent study reported that pregnant women with high exposure to PFAS had altered phospholipid composition of breast milk.(Lamichhane et al. 2021) This alteration marked an increase in size of milk fat globules, which were associated with slowed growth and increased markers of intestinal inflammation in infants These findings suggest that PFAS exposure can impact the fundamental quality of breast milk and adversely affect infant health as a result.(Lamichhane et al. 2021) Based on results from studies in mice and humans, PFAS exposure can delay mammary gland development, breast differentiation during late pregnancy and early lactation, and alter normal prolactin-family hormone secretion which may be involved in the observed reduction in breastfeeding duration after PFAS exposure. In the studies examining the association between PFAS exposure and duration of breastfeeding in humans, it is important to note that any switches from breastfeeding to formula feeding by mothers were likely due to hormonal triggers. Mothers enrolled in these studies were not aware that PFAS exposure was being examined at the time of enrollment, so a conscious decision to stop nursing and switch to formula feeding based on a theorized exposure was unlikely. The hormonal cues responsible for the decreased lactation and duration of breastfeeding are not well characterized and warrant further investigation.
In addition to altering mammary gland development and lactation, exposure to PFAS may increase breast cancer risk. Using NHANES data, Omoike et al.(Omoike et al. 2020) found that increased levels of PFOA, PFOS, PFNA, PFHxS, and PFDA were associated with increased odds of breast cancer. In a study examining PFAS exposure and breast cancer risk in <50-year-old Taiwanese women, a positive association was reported between PFHxS and PFOS exposure and risk of estrogen receptor-positive breast tumors.(Tsai et al. 2020) Associations between PFOS or PFOA levels and estrogen receptor-positive breast tumors, progesterone receptor-positive breast tumors, and receptor-negative breast tumors have also been reported by Mancini et al.(Mancini et al. 2020). Evaluating in utero exposure to PFAS, Cohn et al.(Cohn et al. 2020) found that high maternal 2-(N-ethylperfluorooctane sulfonamido) acetic acid (Et-PFOSA-AcOH) levels in mothers with high total cholesterol was predictive of increased breast cancer risk in their daughters while maternal PFOS levels were negatively associated with breast cancer risk. Altogether, these studies highlight the endocrine-disrupting capabilities of PFAS as they relate to breast tissue through suggesting associations between increased exposure and altered mammary gland development, impaired lactation ability, and even breast cancer risk.
3.2. PFAS and the Thyroid
An unexplored, but feasible pathway through which PFAS may affect breast tissue is through the thyroid and thyroid hormone regulation.(Neville et al. 2002) Under normal conditions, when thyroid hormone levels are low, the pituitary gland releases TSH to stimulate production of T3 and T4 by thyroid peroxidase. In healthy individuals, thyroid peroxidase antibodies (TPO-Abs) should be undetectable in the blood, since detectable levels of TPO-Abs in the blood are generally associated with autoimmune thyroid diseases.(Fröhlich and Wahl 2017) Understanding PFAS-induced alterations in thyroid function is critical because proper regulation of thyroid hormone levels is needed for normal functioning of the reproductive system, and beyond the thyroid axis’s normal role in lactation, alterations in thyroid hormone levels can lead to maladaptive changes in other reproductive hormones, menstrual irregularity, and even infertility in women.(Saran et al. 2016)
Recent studies have shown that long- and short-chain PFAS may impact thyroid function in different ways. In a study examining the effects of long- and short-chain PFAS on Fisher rat thyroid line-5 cells, PFOA, PFOS, perfluorobutane sulfonate (PFBS), PFBA, PFPA, and pentafluoropropionic anhydride had no effect on cell viability, except for PFOS at 100μM, and did not interfere with TSH-dependent cyclic adenosine monophosphate (cAMP) production.(Croce et al. 2019) Another study found that PFOA and HFPO-DA both decreased cell viability and proliferation rate in Fisher rat thyroid line-5 cells and primary normal human thyroid cells.(Zhang et al. 2021) These latter findings are similar to those of Coperchini et al.(Coperchini et al. 2020), who reported that HFPO-DA decreased cell viability in Fisher rat thyroid line-5 cells in a time- and dose-dependent manner while decreasing proliferative capabilities.
Certain PFAS have been linked to thyroid disruption in humans as well. In residents of a community with known PFAS exposure, increases in serum PFOS levels were positively associated with TSH levels and increased serum PFNA levels were positively associated with total T4 levels in a repeated sampling analysis that took into account factors such as age, sex, and education.(Blake et al. 2018) Supporting this relationship, Lin et al.(Lin et al. 2013) found that serum PFNA levels were positively associated with serum T4 levels in a cohort of Taiwanese adolescents and young adults. When stratifying by sex, Blake et al.(Blake et al. 2018) also found that increased serum PFNA levels were positively associated with T4 levels in women while serum PFHxS levels were negatively associated with serum TSH levels in women. Sex-specific associations between serum PFHxS and T4 levels have been reported by others as well.(Jain 2013; Wen et al. 2013) The observed relationship between PFAS levels and serum T4 levels may be explained through multiple mechanisms including the binding of PFAS to transthyretin(Behnisch et al. 2021), a thyroid hormone transport protein, increased T4 glucuronidation in the liver and/or increased conversion of T4 to T3 via type I deiodinase in the liver.(Blake et al. 2018; Webster et al. 2014; Weiss et al. 2009; Yu et al. 2009) In addition to associations between PFAS and T4 levels, other studies exploring the effects of PFAS on thyroid hormones have suggested positive associations between PFOA and total T3 and TSH as well.(Heffernan et al. 2018; Jain 2013; Lewis et al. 2015; Webster et al. 2016; Wen et al. 2013)
The relationship between PFAS exposure and maternal and neonatal thyroid hormone levels has also been explored in both animal models and humans. Proper thyroid hormone transfer during pregnancy is critical for embryonic development, so disruption of normal thyroid hormone transfer resulting from PFAS exposure may lead to adverse birth outcomes. In rats, studies have shown that PFOS exposure decreases maternal serum T3 and T4 concentrations during pregnancy and total T4 levels in pups.(Chang et al. 2008; Lau et al. 2003; Luebker et al. 2005b; Thibodeaux et al. 2003) In humans, certain PFAS have been shown to alter normal maternal and neonatal thyroid hormone levels as well.(Coperchini et al. 2021) In a study examining PFAS exposure and thyroid hormone levels measured in blood of pregnant women in the Shanghai Birth Cohort, several associations between PFAS levels and thyroid hormone levels were reported, including PFOA and PFHxS with increased free T4, PFNA and PFHxS with increased free T3, and PFHxS with decreased TSH.(Aimuzi et al. 2020) Similarly, when examining prenatal PFAS exposure and thyroid hormone levels in cord blood, Liang et al.(Liang et al. 2020) found positive associations between PFAS mixture concentrations and T3 or free T3 levels. The same study also reported that increased PFDA, PFUdA, and PFOA exposure were associated with increased T3 levels while upper quartiles of PFNA and PFOA were associated with decreased TSH compared to lower quartiles of exposure. While these studies suggest an association between PFAS exposure and thyroid hormone levels during pregnancy, Inoue et al.(Inoue et al. 2019) reported that this association was dependent on gestational week (GW). In pregnant women in the Danish National Birth Cohort, exposure in the upper quartile of PFOS, PFOA, PFHxS, and PFHpS were associated with higher TSH levels from GW5 to GW10 compared to lower quartiles of exposure.(Inoue et al. 2019) An association was also reported between PFDA exposure and increased free T4 levels prior to GW10.(Inoue et al. 2019) Although the studies described above report positive associations between serum PFAS levels and thyroid hormone levels in pregnant women, other studies have found that high concentrations of PFNA, PFUdA, and PFDOA were associated with lower T4 levels(Wang et al. 2014), indicating that PFAS may impact thyroid function in diverse ways depending on the amount and/or length of exposure.
In pregnant women, TPO-Ab positivity is commonly observed, and is frequently associated with adverse pregnancy outcomes.(Meena et al. 2016) Studies examining associations between PFAS exposure and thyroid function in pregnant women examined how TPO-Ab positivity affected PFAS-induced effects on thyroid function.(Coperchini et al. 2021) Aimuzi et al.(Aimuzi et al. 2020) reported that in women with TPO-Ab positivity, serum levels of long-chain PFAS were associated with increased free T3/T4 and decreased TSH. Further examining the impact of TPO-Ab status on PFAS exposure and thyroid hormone levels, Itoh et al.(Itoh et al. 2019) found that PFHxS and PFNA levels were associated with increased maternal free T3 levels in TPOAb-negative and TPO-Ab-positive women, respectively. This study also reported that PFOA levels were inversely associated with maternal TPO-Ab levels and that maternal PFAS levels were positively associated with TPO-Ab status in girls born to TPO-Ab-positive mothers. Maternal PFAS levels were inversely associated with TPO-Ab status in boys born to TPO-Ab-negative mothers. Maternal TPO-Ab status seemingly affected neonatal thyroid levels in a sex-dependent manner, as serum concentrations of certain PFAS were associated with decreased TSH or increased free T3 in girls with TPO-Ab-negative mothers. In girls with TPO-Ab-positive mothers, PFDOA was associated with lower free T4 levels.(Itoh et al. 2019) Although not reported as sex-dependent, Lebeaux et al.(Lebeaux et al. 2020) also reported that maternal TPO-Ab status influenced neonatal thyroid hormone levels. This study found that increased maternal PFOS levels were associated with increased cord serum TSH levels while increased maternal PFOA, PFOS, and PFHxS levels were associated with decreased cord serum free T4 levels in children with TPO-Ab-positive mothers. Results from these studies suggest complicated sex- and TPO-Ab-dependent associations between maternal PFAS exposure and maternal and neonatal thyroid hormones, warranting further investigation.(Itoh et al. 2019)
Since PFAS are usually found in the environment as mixtures, the effect of PFAS mixtures on maternal and neonatal thyroid hormone levels has been examined. In pregnant women and neonates from the Boston Project Viva Cohort, higher concentrations of a PFAS mixture, which included PFOA, PFOS, PFNA, PFHxS, Et-PFOSA-AcOH, and Me-PFOSA-AcOH, were associated with decreased maternal and fetal free T4 indices.(Preston et al. 2020) Analyses revealed that PFOA, PFHxS, Me-PFOSA-AcOH, and 2-(N-ethylperfluorooctane sulfonamido) acetic acid (Et-PFOSA-AcOH) were the main contributors to the observed decrease in free T4 indices. No association was reported between the PFAS mixture and maternal T4 or TSH levels, but increased PFOS was associated with increased maternal T4 levels, increased PFOA was associated with a lower maternal free T4 index, and increased PFHxS was non-linearly associated with maternal TSH levels. Increased PFHxS levels were associated with decreased T4 levels in neonates as well.(Preston et al. 2020) More details on the effects of PFAS on the newborn thyroid can be found in a recent review by Coperchini et al.(Coperchini et al. 2021) Altogether, these studies suggest the ability of certain PFAS to disrupt normal thyroid function and thyroid hormone levels, including during pregnancy, further demonstrate the endocrine-disrupting capabilities of PFAS. Alterations in thyroid hormones after PFAS exposure generally occur at relatively high doses but support for associations between PFAS and thyroid disease or thyroid hormone changes remains insufficient, especially in pregnant and lactating women.(Schrenk et al. 2020) Since the thyroid and female reproductive tract are closely linked, PFAS-induced thyroid disruption warrants further investigation, as it may contribute to adverse reproductive effects and even birth outcomes in women.
3.3. PFAS and Neuroendocrine Feedback of the Ovary
In addition to interacting directly with endocrine organs, PFAS can alter normal functioning of the HPGA and disrupt hormone regulation throughout the body. The neuroendocrine system, specifically the HPGA, plays a crucial role in maintaining normal endocrine functions, such as homeostasis and reproduction, hormone secretion, and menstrual cycles in women. HPGA regulation of the estrous cycle in rodents involves unimpaired cyclical secretion of hormones such as estrogen, progesterone, LH, GnRH, and FSH, which may be altered by PFAS exposure. Since hypothalamic regulation of hormones is critical for reproductive processes, it is important to note that in a study examining rats exposed to PFOS, those in the high-dose group displayed increased PFOS accumulation in the hypothalamus compared to other brain regions.(Austin et al. 2003) Increased numbers of atretic follicles in the ovary have also been reported in PFOS-treated mice, suggesting the ability of these chemicals upon chronic exposure to impact normal ovarian processes, such as follicle maturation and ovulation.(Feng et al. 2015) Both of these studies support the premise that PFAS, specifically PFOS, may impact neuroendocrine regulation and normal reproductive processes.
Studies examining the effects of PFAS on reproductive hormone regulation display contradictory results and warrant further examination. In a study by Barrett et al.(Barrett et al. 2015), inverse associations were observed between follicular E2 levels and 10 different PFAS concentrations in women. When stratifying the data based on parity, PFOS and perfluorooctane sulfonamide (PFOSA) serum levels were inversely related to follicular E2 and luteal progesterone levels in nulliparous women, but not parous women.(Barrett et al. 2015) Nian et al.(Nian et al. 2020) also showed that PFAS exposure may be linked to alterations in fetal gonadotropins. 10 PFAS, including the short-chain PFBS and perfluoroheptanoic acid (PFHpA), were quantified in maternal blood plasma of women in the Shanghai Birth Cohort during early pregnancy while cord blood was used to quantify various fetal sex hormones. Results showed that increases in PFBS were associated with decreases in FSH, LH, and free androgen index (FAI). PFHpA levels were inversely associated with LH and FAI, suggesting the ability of increased PFAS exposure to lower fetal sex hormone concentrations.(Nian et al. 2020)
PFAS exposure and hormone levels have been examined in vivo and in vitro, although contradictory results remain problematic. In vitro studies have demonstrated that PFOA and PFOS have both estrogenic and anti-estrogenic effects(Henry and Fair 2013) and that PFOS can alter steroidogenesis(Kraugerud et al. 2011). Other studies have corroborated that PFOA and PFOS have estrogenic effects(Bonefeld-Jorgensen et al. 2011), while some have shown that PFOS is inversely related to E2 levels.(Knox et al. 2011) Assays looking at steroidogenesis in H295R cells found that at the highest concentration tested of 10μM, PFOA and PFOS were associated with an increase in estrone secretion while only PFOA was associated with increased progesterone secretion.(Behr et al. 2018) Similar associations between high PFOA and PFOS concentrations and hormone levels were found by others using H295R cells as well(Behr et al. 2018; Du et al. 2013a; Du et al. 2013b); however, null associations between PFOA exposure and sex hormone levels have been reported.(Olsen et al. 1998) In human endometrial cells, studies have shown that PFOA, which can directly interact with progesterone, has an antagonistic effect on progesterone-regulated genes involved in embryo implantation and endometrial proliferation, suggesting a potential mechanism by which PFAS contribute to fecundity issues, discussed in Section 3.6.(Di Nisio et al. 2020) The association between PFAS exposure and reproductive hormones levels has also been examined in mice, and results showed that PFOS exposure reduced concentrations of E2, progesterone, FSH, LH, and gonadotropin-releasing hormone (GnRH).(Feng et al. 2015) The impact of PFAS levels on E2 is critical because it regulates GnRH and LH release.(Feng et al. 2015; Maeda et al. 2007) It is important to note here that differences in reported findings may be due to dose-dependent effects of PFAS tested. At concentrations below a certain threshold, these chemicals may not impact hormone production and secretion; however, above a certain threshold they may play a key role in the disruption of hormonal feedback loops.
Regarding PFAS and their effects on reproductive hormone receptors, Behr et al.(Behr et al. 2018) found that PFAS did not directly activate estrogen receptor-α (ERα) or estrogen receptor-β (ERβ) in breast carcinoma, adrenocortical carcinoma, and prostate adenocarcinoma cell lines. The authors reported that PFOA, PFOS, and HFPO-DA increased 17β-estradiol-stimulated estrogen receptor-β (Erβ) activity while PFOS, HFPO-DA, PFHxA, and PFBA increased dihydrotestosterone-stimulated androgen receptor activity. These findings have been both supported and contradicted by other studies(Du et al. 2013a; Du et al. 2013b; Kang et al. 2016; Kjeldsen and Bonefeld-Jørgensen 2013; Yao et al. 2014) Since feedback loops are based on the number of circulating hormones, activation of receptors may not be critical in understanding the effects of PFAS on the female reproductive tract. Studies examining PFAS and their association with reproductive hormones have mainly examined receptor activation or gene expression levels, so the effect of PFAS on circulating hormone levels in conjunction with receptor activity (or potentially local aromatase activity) warrants further investigation.
3.4. PFAS and Menstrual Cyclicity
There is evidence that PFAS exposures pose a threat to normal menstrual cyclicity in both animal models and in women. In animal models, only certain PFAS have been examined for disruption of estrous cyclicity. Austin et al.(Austin et al. 2003) found that intraperitoneal PFOS administration led to altered estrous cyclicity in Sprague-Dawley rats while another group reported that PFOS administered via oral gavage did not affect estrous cyclicity(Luebker et al. 2005a). Supporting the notion that PFOS alters estrous cyclicity, Feng et al.(Feng et al. 2015) found that after 3 months of PFOS exposure, estrous cycles of mice were prolonged, leading to an overall decrease in the number of estrous cycles per month. Other studies utilizing Sprague-Dawley rats have reported that daily exposure to PFDOA followed by a 14-day recovery period with no PFAS administration led to continuous diestrus.(Kato et al. 2015) Null associations between PFAS exposure and estrous cyclicity have been reported for PFHxA, PFHxS, or PFDOA.(Butenhoff et al. 2009; Loveless et al. 2009; Shi et al. 2009)
Although data are limited, studies have shown the ability of PFAS to impact reproductive hormone regulation and disrupt menstrual cyclicity through either shortening or lengthening cycle length.(Di Nisio et al. 2020; Knox et al. 2011; Kristensen et al. 2013; Lopez-Espinosa et al. 2011; Lum et al. 2017; Zhou et al. 2017) Since menstruation has been viewed as a proxy of female fecundity(Buck Louis et al. 2011) and a dysregulated menstrual cycle has been linked to lower fecundity, infertility(Harlow and Ephross 1995; Jensen et al. 1999), and female reproductive diseases such as PCOS, it is important to understanding how PFAS may impact cyclicity in women of all ages.
Examining the effects of PFAS on menstrual cycle characteristics in women planning to become pregnant, Zhou et al.(Zhou et al. 2017) measured the plasma levels of 10 different PFAS and recorded questionnaire answers regarding menstruation of the study participants. Most PFAS were positively associated with an irregular menstrual cycle, defined as variations of ≥ 7d between cycles.(Fraser et al. 2011a) Regarding menstrual cycle length, significant positive associations were reported between PFOS, PFOA, PFNA, and PFHxS and self-reported long menstrual cycles, with PFHxS having the strongest association.(Zhou et al. 2017) Decreased self-reported menorrhagia, which is the presence of heavy or prolonged menstrual periods, was commonly observed in women with increased levels of PFOA, PFOS, PFNA, and PFHxS exposure while increased self-reported hypomenorrhea, which is the presence of short or light menstrual periods, was commonly observed in women with increased levels of PFOA, PFNA, and PFHxS. One potential explanation, suggested by Zhou et al.(Zhou et al. 2017), for the relationship between increased serum PFAS levels and menstrual cycle irregularities such as light menstrual flow or irregular cycle length might be decreased menstruation as a result of higher PFAS exposure. We suggest that an alternative potential explanation for this finding is increased serum PFAS levels may result due to reduced excretion via menstrual bleeding. Further exploring the impacts of PFAS exposure on menstrual cycle irregularities, Singer et al.(Singer et al. 2018) examined blood samples and menstrual cycle characteristics of women from the Norwegian Mother and Child Cohort study and found that decreased plasma PFAS concentrations of PFOA, PFNA, PFDA, and PFUdA were associated with irregular periods but PFAS levels were not associated with cycle length.(Singer et al. 2018) An association between PFOA levels and frequency of irregular periods in girls has also been reported by Di Nisio et al.(Di Nisio et al. 2020) Factors such as parity and OC use may affect the associations between PFAS exposure and menstrual characteristics. Specifically, an association between short cycles and decreased PFHpS and PFOS levels was found in parous women while an association between increased cycle length and increased PFNA and PFUdA levels was found in recent OC users, supporting the finding that OC use may increase serum PFAS levels as a result of decreased menstruation.(Singer et al. 2018)
In another study examining PFAS and their association with menstrual cycle length, Lum et al.(Lum et al. 2017) recruited couples who had discontinued contraception and followed them until pregnancy or 12 months of attempting to achieve pregnancy. Higher serum PFOA levels were detected in women with a mean menstrual cycle length of 25–31 days compared to women with menstrual cycle lengths of ≥32 days. Some PFAS were positively associated with cycle length while others were negatively associated. Increased cycle length was observed when comparing women in the second tertile of PFDA exposure to women in the first tertile while decreased cycle length was observed when comparing women in the highest tertile of PFOA exposure to the lowest tertile.(Lum et al. 2017) Similarly, Lyngsø et al.(Lyngsø et al. 2014) reported that menstrual cycles of ≥32 days were observed in women in the highest tertile of PFOA exposure compared to the lowest tertile while cycle irregularity was reported in women in the third tertile of PFOS exposure compared to women in the first tertile. Supporting this, other studies have found that women with PFOA and PFOS exposure above the first quartile more commonly reported menstrual cycle irregularities.(Fei et al. 2009) Taken together, findings from these studies suggest an association of some long-chain PFAS with elongated menstrual cycle length, which biologically would decrease PFAS clearance, suggesting a negative feedback loop leading to higher accumulated PFAS in women with lower blood loss through menstruation. It is important to note that some of these studies were prospective(Lum et al. 2017; Singer et al. 2018; Zhou et al. 2017), while others were cross-sectional(Lyngsø et al. 2014) or retrospective(Fei et al. 2009). Due to study design differences, and potentially geographical exposure differences, it is difficult to interpret whether increased PFAS levels contribute to irregular menstruation or whether irregular menstruation leads to increased serum PFAS levels given that menstruation is thought to be a critical route of PFAS elimination in women.
3.5. PFAS and Menarche and Menopause
Although studies have shown the ability of PFAS to impact the menstrual cycle, which is highly controlled by ovarian hormones, evidence that PFAS directly alter these hormone levels is controversial. Since circulating hormone levels dictate many reproductive processes such as menarche, menstruation, and menopause, if PFAS exposure alters hormone levels in females, then it may determine the timing of these processes as well.
Studies exploring the relationship between PFAS exposure and pubertal timing in vivo have largely been performed in rodents. Examining legacy PFAS, Tucker et al.(Tucker et al. 2015) found that prenatal PFOA exposure did not alter timing of first estrus in female mouse offspring. Examining short-chain PFAS, Feng et al.(Feng et al. 2017) reported that in pregnant ICR mice, PFBS exposure delayed vaginal opening and first estrus in female offspring. Pubertal timing effects of PFAS appear to be compound-dependent, as another study found that PFHxS exposure did not affect vaginal opening, an indicator of pubertal development, in female offspring of pregnant CD-1 mice.(Chang et al. 2018) Other groups have reported similar findings, showing that PFHxA, PFHxS, and PFDOA exposure did not alter first estrus timing in rodents.(Shi et al. 2009)
Understanding the sex-specific effects of PFAS on reproductive hormones in humans is critical since EDCs can alter pubertal timing in males and females.(Maranghi and Mantovani 2012) In a study by Tsai et al.(Tsai et al. 2015), an inverse relationship between serum PFOA, PFOS and PFUdA levels and serum sex hormone-binding globulin (SHBG), FSH, and testosterone levels, mainly in females aged 12–17, was reported. In an attempt to explain these sex-specific effects, Tsai et al.(Tsai et al. 2015) suggested that female reproductive hormones may be more sensitive to PFAS exposure due to an earlier onset of puberty compared to males.
Further exploring how PFAS exposure can affect age at menarche, Di Nisio et al.(Di Nisio et al. 2020) found that increased PFOA exposure was related to increased age at menarche, suggesting that PFOA may impact female reproduction and ovarian function. These results are comparable to other studies, showing that PFOA and PFOS serum levels are associated with delayed menarche.(Kristensen et al. 2013; Lopez-Espinosa et al. 2011) Lopez-Espinosa et al.(Lopez-Espinosa et al. 2011) reported that, in girls aged 8–18, those within the highest quartile of PFOA and PFOS serum concentrations displayed delayed menarche by 130 and 138 days, respectively, compared to girls with serum PFAS concentrations in the lowest quartile. Kristensen et al.(Kristensen et al. 2013) also found that exposure to higher concentrations of PFOA in utero delayed menarche by 5.3 months compared to women with low exposure to PFOA in utero. While these studies suggest an association between PFAS levels and menarche timing, others have found null associations between PFOSA, Et-PFOSA-AcOH, Me-PFOSA-AcOH, PFOS, PFHxS, PFOA, and PFNA and menarche.(Christensen et al. 2011) One study reported associations between prenatal PFAS exposure, specifically PFOS, PFHxS, PFHpS, PFNA, and PFDA, and precocious puberty in girls.(Ernst et al. 2019)
In addition to being associated with altered menarche timing, Ding et al.(Ding et al. 2020b) demonstrated that certain PFAS, including linear PFOS, branched PFOS, linear PFOA, and PFNA, as well as PFAS mixtures were associated with earlier age at natural menopause; however, no association was found with PFHxS. When participants were grouped by PFAS exposure (low, low-medium, medium-high, high serum measures), participants in the high PFAS exposure group had earlier onset of menopause, taking place at around 50.8 years, compared to other groups which ranged from 51.8–52.8 years. Although this study found an association between PFOA and menopause timing, Dhingra et al.(Dhingra et al. 2016) found no association. Other studies support the notion that PFAS exposure is related to timing of menopause, as Knox et al.(Knox et al. 2011) demonstrated that in the oldest group of women (>51 years to ≤65 years), increasing quintiles of PFOS exposure were associated with increased odds of having already experienced menopause compared to the lowest quintile. Although hormones are a critical factor in menopause, no association was reported between serum PFOA concentrations and serum E2 levels in any age group in this study and a negative association was discovered between serum PFOS concentrations and serum E2 in all groups.
In women with a previous hysterectomy, PFOA and PFOS levels were significantly higher compared to women with no history of a hysterectomy.(Knox et al. 2011) Similarly, in a study by Taylor et al.(Taylor et al. 2014), women who had a previous history of a hysterectomy had the highest median levels of PFAS serum concentrations compared to women who were premenopausal. Additionally, strong, positive dose-response relationships for PFHxS, PFOA, PFNA, and PFOS were observed in the post-hysterectomy group along with evidence of increasing serum concentrations of each of 4 PFAS with each year since menopause occurred.(Taylor et al. 2014) Altogether, these data demonstrate an inverse association between high serum PFAS concentrations and age at menopause.(Knox et al. 2011) This is important to note because premature or early menopause is associated with increased cardiovascular risk and all-cause mortality.(Archer 2009; de Kleijn et al. 2002; Shuster et al. 2010) Additionally, menopause is thought to mark a decline in ovarian function, so early onset menopause may also be correlated with declining fertility, as well as reduced bone density.(Knox et al. 2011; Nikolaou and Templeton 2004) Decreased bone density is often observed in women with primary ovarian insufficiency as a result of estrogen deficiency and is frequently observed in post-menopausal women.(Szeliga et al. 2018) In a study by Khalil et al.(Khalil et al. 2016), PFOS and PFNA levels in post-menopausal women were associated with lower bone mineral density. The prevalence of osteoporosis in women was also significantly higher in the highest quartile of exposure to PFOA, PFHxS, and PFNA compared to women in the lowest quartile. Although these findings suggest an association between increased PFAS concentrations and early onset menopause in women, it is important to consider reverse causation in this scenario. In this case, reverse causation refers to the idea that early menopause (potentially triggered by PFAS exposures) leads to decreased menstrual bleeding, and since menstruation is thought to be a route of elimination for PFAS in women, high serum levels of PFAS in women with early onset menopause may be due to the lack of menstruation.(Knox et al. 2011) To examine the reverse causation hypothesis, Dhingra et al.(Dhingra et al. 2017) evaluated PFOA levels in relation to self-reported menopause based on previous cross-sectional studies and found that, in those same studies, earlier menopause was the cause of increased serum PFOA rather than a result of increased serum PFOA levels. Supporting this, a study by Ruark et al.(Ruark et al. 2017) also reported that in menopausal women, serum PFAS concentrations were increased due to the reduction in menstruation. The studies presented in this section suggest that regardless of potential reverse causation, certain PFAS are associated with late onset of menarche and early menopause along with altered hormone levels in specific populations of women.
3.6. PFAS and Fecundity and Birth Outcomes
As endocrine disruptors with the ability to affect hormone regulation and menarche, breast development, and menopause timing, PFAS may increase risk for adverse reproductive outcomes and infertility.(Rashtian et al. 2019) In Australian women, a study measured 32 PFAS concentrations in follicular fluid and reported that PFAS were present in all samples.(Kim et al. 2020) Levels of certain PFAS were also associated with infertility issues including endometriosis, PCOS, and genital tract infections although they were not significantly associated with fertilization rate.(Kim et al. 2020) Another study found that in women from China, ten legacy PFAS compounds along with 15 emerging PFAS were identified in over 50% of follicular fluid samples.(Kang et al. 2020) Although data is limited on emerging PFAS, studies have reported associations between these chemicals and adverse reproductive effects as well. For example, Kang et al.(Kang et al. 2020) found that the blood-follicle transfer efficiencies (ratio of PFAS concentrations in follicular fluid samples to those of paired serum samples) of perfluoroalkyl carboxylic acids decreased with increasing chain length. Blood-follicle transfer efficiencies of emerging PFAS, including chlorine-substituted perfluoroalkyl ether sulfonates, were significantly higher than that of PFOS and PFOA. In the context of in vitro fertilization (IVF), increased maternal plasma concentrations of PFOA have been associated with decreased numbers of retrieved oocytes, mature oocytes, two-pronuclei zygotes, and good-quality embryos, suggesting the ability of PFOA to impact IVF outcomes.(Ma et al. 2021) These studies suggest that PFAS in follicular fluid may contribute to fertility issues in women and human oocyte exposure or development, but further studies to confirm this theory are complex.
Examining the association between PFAS exposure and time to pregnancy (TTP) or infertility in pregnant women enrolled during antenatal care from Greenland, Poland, and Ukraine, Jørgensen et al.(Jørgensen et al. 2014) found that increased serum PFNA levels were associated with longer TTPs and increased infertility odds ratios in the pooled international sample of women and in women from Greenland. Other studies have shown that the probability of pregnancy decreased in women with higher serum levels of PFOA and PFNA compared to women with lower levels.(Lum et al. 2017) Similarly, Velez et al.(Vélez et al. 2015) found that exposure to PFOA and PFHxS, when adjusted for maternal age and body mass index (BMI) reduced fecundity by 11% and 9%, respectively, as measured by increased TTP (>12 months). Additional studies have supported the association between elevated PFOA and PFOS levels and longer TTPs.(Fei et al. 2009) Fei et al.(Fei et al. 2009) also reported that in each of the higher exposure categories for PFOS and PFOA, infertility odds increased by 70–134% and 60–154%, respectively, compared to the lowest quartile of exposure. Similar results were found in a study by Whitworth et al.(Whitworth et al. 2012), where women in the Norwegian Mother and Child Cohort with elevated PFOA and PFOS levels were at increased risk for subfecundity, defined by a TTP of >12 months. Reverse causation can be considered in this scenario, since women with longer TTPs also have longer interpregnancy intervals, which can lead to increased exposure to and accumulation of PFAS during this window.(Olsen et al. 2009a; Vélez et al. 2015) It is possible that women with longer interpregnancy intervals have more menstrual cycles, meaning they would also have more opportunities to excrete PFAS. A follow-up analysis by Whitworth et al.(Whitworth et al. 2012) found that when stratifying by parity, the odds of subfecundity were increased among parous women only, supporting the reverse causation hypothesis.(Vélez et al. 2015)
Not only are PFAS implicated in fecundity and TTP, but serum concentrations of these chemicals have also been linked to adverse birth outcomes. In rodents, PFOS and PFOA have been shown to alter birth outcomes. Lau et al.(Lau et al. 2006) reported that exposure to PFOA increased litter resorptions, fetal mortality, and fetal growth deficits while decreasing fetal birth weights. In a separate study, Lau et al.(Lau et al. 2003) found that PFOS exposure led to fetal mortality and growth deficits. Aside from PFOS and PFOA, animal studies supporting a link between PFAS exposure and birth outcomes are limited. Studies have reported links between PFHxS and PFUdA exposure and lower fetal birth weights.(Chang et al. 2018; Ramhøj et al. 2018; Takahashi et al. 2014) No effect on fetal birth weight was observed after PFNA exposure, but the number of live pups at birth was decreased along with offspring survival to weaning.(Das et al. 2015; Wolf et al. 2010)
In humans, although there is conflicting evidence between PFAS exposure and miscarriage, there is a clear association between increased umbilical cord concentrations of PFOS and preterm birth.(Arbuckle et al. 2013; Chen et al. 2021; Green et al. 2021) Additionally, some studies have shown an association between PFOA exposure and low birth weight(Green et al. 2021; Johnson et al. 2014), while others have found that most PFAS were not associated with adverse birth outcomes, specifically gestational age and birth weight, in pregnant women from San Francisco.(Eick et al. 2021) It may be that the level of PFAS contamination or what is contained in the PFAS mixture in the various communities in which these study participants were from influenced the associations with birth outcomes.
As mentioned earlier, transplacental transfer to the fetus reduces the maternal PFAS burden. Several studies have now reported PFAS in placental tissues of women.(Bangma et al. 2020a; Chen et al. 2017; Mamsen et al. 2019; Mamsen et al. 2017) Bangma et al.(Bangma et al. 2020a) examined the association between placental PFAS levels in pregnant women in North Carolina and various demographic and pregnancy-related factors and found that maternal race/ethnicity was significantly associated with differences in PFUdA levels. A near significant association between PFUdA levels and hypertensive complications of pregnancy was also reported, while no associations were observed with PFHxS, PFHpS, or PFOS. Previous literature examining PFAS exposure and pre-eclampsia diagnoses are not in complete agreement; one study reported no association(Starling et al 2014), while more recent studies report associations of PFAS exposures and hypertensive disorders of pregnancy for at least one PFAS, including PFOS, PFOA, PFNA, and PFBS.(Bangma et al. 2020a; Huang et al. 2019; Starling et al. 2014; Wikström et al. 2019) Whether these differences are due to analytical aspects of the PFAS measurement, level of contamination in the cohort, or study design have not been examined.
Examining PFAS exposure on birth outcomes, a study involving pregnant women in the Shanghai Birth Cohort found that concentrations of PFOS, PFNA, PFDA, PFUdA, and PFDOA were negatively associated with birth length only in female fetuses.(Chen et al. 2021) While the concentrations of most PFAS declined during pregnancy, concentrations of PFHxS remained consistent and perfluorobutane sulfonate (PFBS) increased.(Chen et al. 2021) Waterfield et al.(Waterfield et al. 2020) analyzed birth outcomes in an East Minneapolis-St. Paul-based cohort, where high levels of PFAS contaminated the drinking water supply, and found that average birth weight and gestational age were significantly lower in the PFAS-exposed cohort compared to controls. In addition to increased odds of low birth weight in this population, odds of pre-term birth also increased while the general fertility rate was significantly lower.(Waterfield et al. 2020) Overall, these findings suggest an association between serum PFAS levels and fecundity, TTP, and birth outcomes. These disruptions may be resultant from PFAS interfering with the HPGA.(Fei et al. 2009; Kahn et al. 2021) The thyroid may play a role in the observed effects of PFAS on fecundity and birth outcomes as well, since PFAS can disrupt thyroid hormone levels, which are critical for reproductive processes.(Aimuzi et al. 2020; Blake et al. 2018; Liang et al. 2020; Lin et al. 2013; Wang et al. 2014) The role that PFAS play in thyroid hormone disruption as it pertains to the female reproductive tract is not completely understood, since studies that have reported associations between thyroid hormones and PFAS levels have found no associations between PFAS levels and ovarian reserve or fecundability.(Crawford et al. 2017) While no association was reported, there were reported limitations including narrow study population, both in size and based on sociodemographic factors, and a limited follow-up period on fecundability that may have limited detectability of effects. Since the thyroid is known to play a critical role in fertility and the reproductive system in general, thyroid disruption may contribute to the observed effects of PFAS exposure on fecundity and birth outcomes and warrants further investigation.
3.7. PFAS and Diseases and Dysfunctions of the Female Reproductive Tract
Hormonal regulation is critical for many normal bodily processes such as puberty and altered hormone regulation can contribute to the onset of disease. PCOS is common in women of reproductive age and is one of the main causes of infertility among women(Balen et al. 2016; Wang et al. 2019a); however, the etiology of the disease is not well understood and may involve a combination of genetic and environmental factors.(Escobar-Morreale 2018; Palioura and Diamanti-Kandarakis 2015; Wang et al. 2019a). Although studies are limited, increased serum PFOA and PFOS levels are associated with PCOS in American women.(Vagi et al. 2014; Wang et al. 2019a) While positive associations between PFAS exposures and PCOS were observed by Vagi et al.(Vagi et al. 2014), Wang et al.(Wang et al. 2019a) found a dose-dependent association between PFDOA exposure and PCOS-related infertility risk in an analysis adjusted for sociodemographic or lifestyle-related variables such as age and BMI. The authors also reported an inverse association between PFUdA levels and PCOS-related infertility, but no associations were present for PFBS, PFHpA, PFHxS, PFOA, PFOS, PFNA, or PFDA. In another study, patients with PCOS had higher serum concentrations of PFOS compared to controls. (Heffernan et al. 2018) High serum PFOS concentrations were also associated with menstrual irregularities in both PCOS and control patients. These studies suggest the ability of PFAS to increase risk of PCOS as well as PCOS-related complications in women of reproductive age, potentially due to their endocrine-disrupting capabilities.
In addition to PCOS, PFAS exposure may be related to an increased risk of other reproductive diseases, including endometriosis and ovarian cancer; however, studies on these associations are limited. With regard to endometriosis, data from women aged 20–50 from NHANES 2003–2006 showed that mean levels of PFNA, PFOA, and PFOS were higher in women who reported having endometriosis compared to women who did not.(Campbell et al. 2016) Furthermore, a study by Wang et al.(Wang et al. 2017) found that certain PFAS may increase risk of endometriosis-related infertility. In a population of Chinese women of reproductive age with surgically confirmed endometriosis, increased plasma concentrations of PFBS were associated with an increased risk of endometriosis-related infertility, even in women with no previous pregnancies or other gynecologic pathology.(Wang et al. 2017) Although limited, these studies suggest an association between certain PFAS and risk of endometriosis or endometriosis-related infertility and should be further evaluated. Importantly, associations between PFAS exposure and endometrial cancer risk in humans have never been examined. In the context of ovarian cancer, the association between PFAS exposure in contaminated communities and ovarian cancer risk has been examined in two different studies.(Barry et al. 2013; Vieira et al. 2013) Barry et al.(Barry et al. 2013) did not observe an association between PFOA and ovarian cancer risk while Vieira et al.(Vieira et al. 2013) reported an association between very high PFOA levels and increased ovarian cancer risk. It is important to note that data on PFAS exposure and ovarian cancer risk is limited and the number of ovarian cancer cases in each of these studies was relatively small, warranting further investigation on this topic. Overall, the studies summarized in Sections 3.3–3.7 suggest a deleterious role of PFAS exposure in altered reproductive processes and disease onset in females and are illustrated for clarity in Figure 2. Brief summaries of those studies and other endocrine and developmental effects of PFAS on female reproductive endpoints are noted in Table 1.
Figure 2.
Female reproductive tract targets of PFAS. PFAS can disrupt normal reproductive function in females both directly through targeting the ovary, uterus, or developing embryo or indirectly through targeting other endocrine tissues or the HPGA. Various effects of PFAS on the HPGA, uterus, ovary, and developing embryo are shown as well as the specific compounds that have reported positive (red), negative (blue), or mixed (purple) associations.
4. Conclusion
Over the past few decades, global female reproduction trends have been declining and while cultural changes contribute to the observed decline, environmental contaminants, like PFAS may also play a critical role. PFAS are widespread environmental contaminants that are frequently detected in drinking water supplies and human serum worldwide. Studies have demonstrated the ability of these chemicals to target the reproductive tract in women, both through directly targeting reproductive tissues and through altering normal breast, thyroid, and HPGA function. It is important to note that the studies described in this review examining the same or similar endpoints are not always consistent; whether this is due to the levels or length of exposure time for certain populations and not others should be the topic of future systematic reviews. Many of the topics discussed in this review warrant further investigation, as study findings are limited and/or inconsistent. Studies within the same subsection reporting different findings for the same biological outcome may be due to inconsistent levels of exposure being examined and compared across studies or may be due to lack of consideration of important variables such as age, parity, or race. There are some studies reporting inconsistent results at similar exposure levels, but this is not always the case and should be noted.
It is critical for future epidemiologic studies examining the adverse reproductive effects of PFAS exposure on females to consider all the endpoints listed in this review. For example, the impacts of PFAS exposure on menstrual cyclicity, pubertal timing, and menopause timing have not been examined as routinely or as extensively as other reproductive endpoints. Combining studies would contribute significant insight into how PFAS are affecting female reproductive outcomes, since many studies are not well powered. This review highlights the findings of some (not all) studies that have examined this relationship; however, further studies are needed to understand the direct and indirect mechanistic targets of legacy, short-chain, and alternative PFAS exposure on these outcomes in women. Toxicological studies including relevant reproductive and endocrine endpoints following PFAS exposure could provide important insight on characteristics that PFAS influence and help to better understand related human health outcomes in affected populations. More generally, future work examining PFAS exposure on female reproductive end points in animals should be conducted at doses relevant to humans and blinded to treatment groups, so that in-life characteristics can be collected without bias. Using outbred strains of rodents for PFAS exposure studies would also help increase human relevance. Using inbred strains to explore the associations between PFAS exposure and health outcomes limits variability across mice. Although inbred mice would reduce the variability in response, this is not as representative of the human population, since two people in the same community can be exposed to different concentrations of PFAS and respond differently as a result. Future studies should focus on choosing sensitive animal models for the outcomes of interest. Currently, non-human primate models would have to be used to evaluate endpoints such as PFAS effects on menstrual cycle and possibly endocrine effects (due to differences between rodents and humans in reproductive cyclicity and senescence), but rodent models are suitable to evaluate effects on birth outcomes, lactation, and reproductive diseases like PCOS, endometriosis, and breast disease. In vivo exposure to PFAS was identified as an inconsistency across the studies mentioned in the above sections. While some health effects are observed in animals after exposure to PFAS at human relevant exposure levels, this is not always the case and should be addressed in future in vivo studies. For most health outcomes in women, knowledge of potential PFAS exposure sources over time (housing history, water sources) and details of parity, breast-feeding duration, and menstrual history would enhance interpretation. PFAS affects early life reproductive endpoints, such as lactation and breastfeeding duration, pubertal endpoints, fertility and possibly fecundity, and effects like these have been reported in rodent studies. Limiting PFAS in the environment based on these early life endpoints may help reduce the number of later life diseases attributed to PFAS.
Despite the findings discussed in this review, there are current literature gaps regarding the combined effects of PFAS mixtures on endocrine and reproductive targets, the mechanisms by which PFAS disrupt the endocrine system, and how interplay between PFAS endocrine target tissues leads to long-term reproductive health effects. Future studies should focus on mechanistic underpinnings of PFAS-induced reproductive outcomes in women. Based on data summarized here on the role of sociodemographic and/or reproductive factors in PFAS exposure and elimination, we suggest not only that animal or human studies include effects on reproductive tissues, but also stratification by age, race/ethnicity, and reproductive characteristics (cycling history, thyroid status, parity) is necessary to fully understand the reproductive implications of PFAS exposure across various populations of women. Additionally, the issue of reverse causality for PFAS and outcomes related to cyclicity (and potentially disease status) deserves further attention and consideration in study interpretations.
Acknowledgements:
The authors would like to thank Paige Bommarito, Rebecca Fry, and Victoria Bae-Jump for reviewing this manuscript and providing feedback.
Funding:
This work was supported by a pre-doctoral traineeship (National Research Service Award T32 ES007126) from the National Institute of Environmental Health Sciences, National Institutes of Health to BPR and National Institute of Environmental Health Sciences ZIA ES102785-11 to SEF.
Abbreviations:
- ADONA
Ammonium 4,8-dioxa-3H-perfluorononanoate
- AFFF
Aqueous film forming foam
- BMI
Body mass index
- CDC
Centers for Disease Control and Prevention
- DDE
Diphenyldichloroethene
- DDT
Dichlorodiphenyltrichloroethane
- DES
Diethylstilbestrol
- E2
Estradiol
- EDC
Endocrine-disrupting compound
- EPA
Environmental Protection Agency
- ERα
Estrogen receptor-α
- ERβ
Estrogen receptor-β
- Et-PFOSA-AcOH
2-(N-ethylperfluorooctane sulfonamido) acetic acid
- FAI
Free androgen index
- FSH
Follicle-stimulating hormone
- HFPO-DA
Ammonium perfluoro(2-methyl-3-oxahexanoate)
- GnRH
Gonadotropin-releasing hormone
- HBM-I
Human Biomonitoring-I
- HPGA
Hypothalamic-pituitary-gonadal axis
- LH
Luteinizing hormone
- Me-PFOSA-AcOH
2-(N-methyl-perfluorooctane sulfonamido) acetic acid
- NHANES
United States National Health and Nutrition Examination Survey
- NIOSH
National Institute for Occupational Safety and Health
- OC
Oral contraceptive
- PCOS
Polycystic ovarian syndrome
- PFAS
Per- and poly-fluoroalkyl substances
- PFBA
Perfluorobutanoic acid
- PFBS
Perfluorobutane sulfonate
- PFDA
Perfluorodecanoic acid
- PFDOA
Perfluorododecanoic acid
- PFHpA
Perfluoroheptanoic acid
- PFHpS
Perfluoroheptane sulfonate
- PFHxA
Perfluorohexanoic acid
- PFHxS
Perfluorohexane sulfonate
- PFNA
Perfluorononanoic acid
- PFOA
Perfluorooctanoic acid
- PFOS
Perfluorooctane sulfonate
- PFOSA
Perfluorooctane sulfonamide
- PFUdA
Perfluoroundecanoic acid
- SHBG
Sex hormone-binding globulin
- SWAN
Study of Women’s Health Across the Nation
- T3
Triiodothyronine
- T4
Thyroxine
- TPO-Ab
Thyroid peroxidase antibody
- TSH
Thyroid-stimulating hormone
- TTP
Time to pregnancy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest: The authors have no conflicts of interest to report.
Reference
- Aimuzi R, Luo K, Huang R, Huo X, Nian M, Ouyang F, Du Y, Feng L, Wang W and Zhang J 2020. Perfluoroalkyl and polyfluroalkyl substances and maternal thyroid hormones in early pregnancy. Environ Pollut 264, 114557. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Long GC, Porter RC and Anderson JK 2016. Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere 150, 678–685. [DOI] [PubMed] [Google Scholar]
- Arbuckle TE, Kubwabo C, Walker M, Davis K, Lalonde K, Kosarac I, Wen SW and Arnold DL 2013. Umbilical cord blood levels of perfluoroalkyl acids and polybrominated flame retardants. International Journal of Hygiene and Environmental Health 216, 184–194. [DOI] [PubMed] [Google Scholar]
- Archer DF 2009. Premature menopause increases cardiovascular risk. Climacteric 12 Suppl 1, 26–31. [DOI] [PubMed] [Google Scholar]
- Austin ME, Kasturi BS, Barber M, Kannan K, MohanKumar PS and MohanKumar SMJ 2003. Neuroendocrine effects of perfluorooctane sulfonate in rats. Environmental Health Perspectives 111, 1485–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward LL, M. HS, Kirman CR, Marchitti SA, Kenneke JF, English C, Mattison DR and Becker RA 2014. Relationships of chemical concentrations in maternal and cord blood: a review of available data. J Toxicol Environ Health B Crit Rev., 17(13):175–203. [DOI] [PubMed] [Google Scholar]
- Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, Stener-Victorin E, Fauser BCJM, Norman RJ and Teede H 2016. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Human Reproduction Update 22, 687–708. [DOI] [PubMed] [Google Scholar]
- Ballesteros V, Costa O, Iñiguez C, Fletcher T, Ballester F and Lopez-Espinosa MJ 2017. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: A systematic review of epidemiologic studies. Environ Int 99, 15–28. [DOI] [PubMed] [Google Scholar]
- Bangma J, Eaves LA, Oldenburg K, Reiner JL, Manuck T and Fry RC 2020a. Identifying Risk Factors for Levels of Per- and Polyfluoroalkyl Substances (PFAS) in the Placenta in a High-Risk Pregnancy Cohort in North Carolina. Environmental Science & Technology 54, 8158–8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangma J, Szilagyi J, Blake BE, Plazas C, Kepper S, Fenton SE and R CF 2020b. An assessment of serum-dependent impacts on intracellular accumulation and genomic response of per- and polyfluoroalkyl substances in a placental trophoblast model. Environ Toxicol 35, 1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarossa A, R. M, Gazzotti T, Zama D, Astolfi A, Veyrand B, Pession A and Pagliuca G 2013. Perfluoroalkyl substances in human milk: a first survey in Italy. Environ Int, 51:27–30. [DOI] [PubMed] [Google Scholar]
- Barrett ES, Chen C, Thurston SW, Haug LS, Sabaredzovic A, Fjeldheim FN, Frydenberg H, Lipson SF, Ellison PT and Thune I 2015. Perfluoroalkyl substances and ovarian hormone concentrations in naturally cycling women. Fertility and Sterility 103, 1261–1270.e1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry V, Winquist A and Steenland K 2013. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect 121, 1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton KE, Starling AP, Higgins CP, McDonough CA, Calafat AM and Adgate JL 2019. Sociodemographic and behavioral determinants of serum concentrations of per- and polyfluoroalkyl substances in a community highly exposed to aqueous film-forming foam contaminants in drinking water. nt J Hyg Environ Health, 223(221):256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnisch PA, Besselink H, Weber R, Willand W, Huang J and Brouwer A 2021. Developing potency factors for thyroid hormone disruption by PFASs using TTR-TRβ CALUX® bioassay and assessment of PFASs mixtures in technical products. [DOI] [PubMed]
- Behr AC, Lichtenstein D, Braeuning A, Lampen A and Buhrke T 2018. Perfluoroalkylated substances (PFAS) affect neither estrogen and androgen receptor activity nor steroidogenesis in human cells in vitro. Toxicol Lett, 291:251–260. [DOI] [PubMed] [Google Scholar]
- Berg V, Nøst TH, Hansen S, Elverland A, Veyhe A-S, Jorde R, Odland JØ and Sandanger TM 2015. Assessing the relationship between perfluoroalkyl substances, thyroid hormones and binding proteins in pregnant women; a longitudinal mixed effects approach. Environment international 77, 63–69. [DOI] [PubMed] [Google Scholar]
- Blake BE, Cope HA, Hall SM, Keys RD, Mahler BW, McCord J, Scott B, Stapleton HM, Strynar MJ, Elmore SA and Fenton SE 2020. Evaluation of Maternal, Embryo, and Placental Effects in CD-1 Mice following Gestational Exposure to Perfluorooctanoic Acid (PFOA) or Hexafluoropropylene Oxide Dimer Acid (HFPO-DA or GenX). Environmental Health Perspectives 128, 027006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake BE and Fenton SE 2020. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology 443, 152565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake BE, Pinney SM, Hines EP, Fenton SE and Ferguson KK 2018. Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, kidney function, and body mass index in the Fernald Community Cohort. Environmental pollution (Barking, Essex : 1987) 242, 894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonefeld-Jorgensen EC, Long M, Bossi R, Ayotte P, Asmund G, Krüger T, Ghisari M, Mulvad G, Kern P, Nzulumiki P and Dewailly E 2011. Perfluorinated compounds are related to breast cancer risk in Greenlandic Inuit: a case control study. Environ Health 10, 88–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronow KE, Brody JG, Schaider LA, Peaslee GF, Havas L and Cohn BA 2019. Serum concentrations of PFASs and exposure-related behaviors in African American and non-Hispanic white women. Journal of Exposure Science & Environmental Epidemiology 29, 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles AL, Beverly BE, Fenton SE, Jackson CL, Jukic AMZ, Sutherland VL, Baird DD, Collman GW, Dixon D, Ferguson KK, Hall JE, Martin EM, Schug TT, White AJ and Chandler KJ 2020. Environmental Factors Involved in Maternal Morbidity and Mortality. Journal of Women’s Health 30, 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantsæter AL, K.W. W, Ydersbond TA, Haug LS, Haugen M, Knutsen HK, Thomsen C, Meltzer HM, Becher G, Sabaredzovic A, Hoppin JA, Eggesbø M and Longnecker MP 2013. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ Int, 54:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Rios LI, McLain A, Cooney MA, Kostyniak PJ and Sundaram R 2011. Persistent organochlorine pollutants and menstrual cycle characteristics. Chemosphere 85, 1742–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA and van Leeuwen SPJ 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integrated environmental assessment and management 7, 513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenhoff JL, Chang S-C, Ehresman DJ and York RG 2009. Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in Sprague Dawley rats. Reproductive Toxicology 27, 331–341. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kato K, Hubbard K, Jia T, Botelho JC and Wong L-Y 2019. Legacy and alternative per- and polyfluoroalkyl substances in the U.S. general population: Paired serum-urine data from the 2013–2014 National Health and Nutrition Examination Survey. Environment international 131, 105048–105048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Raza M and Pollack AZ 2016. Perfluoroalkyl substances and endometriosis in US women in NHANES 2003–2006. Reprod Toxicol 65, 230–235. [DOI] [PubMed] [Google Scholar]
- Caserta D, Maranghi L, Mantovani A, Marci R, Maranghi F and Moscarini M 2008. Impact of endocrine disruptor chemicals in gynaecology. Human Reproduction Update 14, 59–72. [DOI] [PubMed] [Google Scholar]
- CDC. 2012. Fourth national report on human exposure to environmental chemicals: updated tables. Atlanta, GA: Department of Health and Human Services. [Google Scholar]
- CDC. 2021. Early Release: Per- and Polyfluorinated Substances (PFAS) Tables, NHANES 2011–2018. p. National Report on Human Exposure to Environmental Chemicals.
- Chang CJ, Ryan PB, Smarr MM, Kannan K, Panuwet P, Dunlop AL, Corwin EJ and Barr DB 2020. Serum per- and polyfluoroalkyl substance (PFAS) concentrations and predictors of exposure among pregnant African American women in the Atlanta area, Georgia. Environ Res, 110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Butenhoff JL, Parker GA, Coder PS, Zitzow JD, Krisko RM, Bjork JA, Wallace KB and Seed JG 2018. Reproductive and developmental toxicity of potassium perfluorohexanesulfonate in CD-1 mice. [DOI] [PubMed]
- Chang SC, Thibodeaux JR, Eastvold ML, Ehresman DJ, Bjork JA, Froehlich JW, Lau C, Singh RJ, Wallace KB and Butenhoff JL 2008. Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS). Toxicology 243, 330–339. [DOI] [PubMed] [Google Scholar]
- Chen FA-O, Yin SA-O, Kelly BA-O and Liu WA-OX 2017. Chlorinated Polyfluoroalkyl Ether Sulfonic Acids in Matched Maternal, Cord, and Placenta Samples: A Study of Transplacental Transfer. Environ Sci Technol, 51(11):6387–6394. [DOI] [PubMed] [Google Scholar]
- Chen L, Tong C, Huo X, Zhang J and Tian Y 2021. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and birth outcomes: A longitudinal cohort with repeated measurements. Chemosphere 267, 128899. [DOI] [PubMed] [Google Scholar]
- Cho CR, Lam NH, Cho BM, Kannan K and Cho HS 2015. Concentration and correlations of perfluoroalkyl substances in whole blood among subjects from three different geographical areas in Korea. Science of The Total Environment 512–513, 397–405. [DOI] [PubMed] [Google Scholar]
- Christensen KY, Maisonet M, Rubin C, Holmes A, Calafat AM, Kato K, Flanders WD, Heron J, McGeehin MA and Marcus M 2011. Exposure to polyfluoroalkyl chemicals during pregnancy is not associated with offspring age at menarche in a contemporary British cohort. Environ Int 37, 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn BA, La Merrill MA, Krigbaum NY, Wang M, Park JS, Petreas M, Yeh G, Hovey RC, Zimmermann L and Cirillo PM 2020. In utero exposure to poly- and perfluoroalkyl substances (PFASs) and subsequent breast cancer. Reprod Toxicol 92, 112–119. [DOI] [PubMed] [Google Scholar]
- Colles A, Bruckers L, Den Hond E, Govarts E, Morrens B, Schettgen T, Buekers J, Coertjens D, Nawrot T, Loots I, Nelen V, De Henauw S, Schoeters G, Baeyens W and van Larebeke N 2020. Perfluorinated substances in the Flemish population (Belgium): Levels and determinants of variability in exposure. Chemosphere, 242:125250. [DOI] [PubMed] [Google Scholar]
- Coperchini F, Croce L, Denegri M, Pignatti P, Agozzino M, Netti GS, Imbriani M, Rotondi M and Chiovato L 2020. Adverse effects of in vitro GenX exposure on rat thyroid cell viability, DNA integrity and thyroid-related genes expression. [DOI] [PubMed]
- Coperchini F, Croce L, Ricci G, Magri F, Rotondi M, Imbriani M and Chiovato L 2021. Thyroid Disrupting Effects of Old and New Generation PFAS. Front Endocrinol (Lausanne) 11, 612320–612320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC and Guillette LJ Jr. 2008. Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril 90, 911–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford NM, Fenton SE, Strynar M, Hines EP, Pritchard DA and Steiner AZ 2017. Effects of perfluorinated chemicals on thyroid function, markers of ovarian reserve, and natural fertility. Reprod Toxicol 69, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce L, Coperchini F, Tonacchera M, Imbriani M, Rotondi M and Chiovato L 2019. Effect of long- and short-chain perfluorinated compounds on cultured thyroid cells viability and response to TSH. Journal of Endocrinological Investigation 42, 1329–1335. [DOI] [PubMed] [Google Scholar]
- Das KP, Grey BE, Rosen MB, Wood CR, Tatum-Gibbs KR, Zehr RD, Strynar MJ, Lindstrom AB and Lau C 2015. Developmental toxicity of perfluorononanoic acid in mice. [DOI] [PubMed]
- de Kleijn MJ, van der Schouw YT, Verbeek AL, Peeters PH, Banga JD and van der Graaf Y 2002. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol 155, 339–345. [DOI] [PubMed] [Google Scholar]
- DEQ N 2019. GenX Investigation.
- DeWitt JC 2015. Toxicological effects of perfluoroalkyl and polyfluoroalkyl substances, Springer, Basel. [Google Scholar]
- Dhingra R, Darrow LA, Klein M, Winquist A and Steenland K 2016. Perfluorooctanoic acid exposure and natural menopause: A longitudinal study in a community cohort. Environmental research 146, 323–330. [DOI] [PubMed] [Google Scholar]
- Dhingra R, Winquist A, Darrow LA, Klein M and Steenland K 2017. A Study of Reverse Causation: Examining the Associations of Perfluorooctanoic Acid Serum Levels with Two Outcomes. Environ Health Perspect 125, 416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nisio A, Rocca MS, Sabovic I, De Rocco Ponce M, Corsini C, Guidolin D, Zanon C, Acquasaliente L, Carosso AR, De Toni L and Foresta C 2020. Perfluorooctanoic acid alters progesterone activity in human endometrial cells and induces reproductive alterations in young women. Chemosphere, 242:125208. [DOI] [PubMed] [Google Scholar]
- Ding N, Harlow SD, Batterman S, Mukherjee B and Park SK 2020a. Longitudinal trends in perfluoroalkyl and polyfluoroalkyl substances among multiethnic midlife women from 1999 to 2011: The Study of Women’s Health Across the Nation. Environ Int, 135:105381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Harlow SD, Randolph JF, Calafat AM, Mukherjee B, Batterman S, Gold EB and Park SK 2020b. Associations of Perfluoroalkyl Substances with Incident Natural Menopause: the Study of Women’s Health Across the Nation. J Clin Endocrinol Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G, Hu J, Huang H, Qin Y, Han X, Wu D, Song L, Xia Y and Wang X 2013a. Perfluorooctane sulfonate (PFOS) affects hormone receptor activity, steroidogenesis, and expression of endocrine-related genes in vitro and in vivo. Environ Toxicol Chem 32, 353–360. [DOI] [PubMed] [Google Scholar]
- Du G, Huang H, Hu J, Qin Y, Wu D, Song L, Xia Y and Wang X 2013b. Endocrine-related effects of perfluorooctanoic acid (PFOA) in zebrafish, H295R steroidogenesis and receptor reporter gene assays. Chemosphere 91, 1099–1106. [DOI] [PubMed] [Google Scholar]
- Eick SM, Enright EA, Geiger SD, Dzwilewski KLC, DeMicco E, Smith S, Park J-S, Aguiar A, Woodruff TJ, Morello-Frosch R and Schantz SL 2021. Associations of Maternal Stress, Prenatal Exposure to Per- and Polyfluoroalkyl Substances (PFAS), and Demographic Risk Factors with Birth Outcomes and Offspring Neurodevelopment: An Overview of the ECHO.CA.IL Prospective Birth Cohorts. International Journal of Environmental Research and Public Health 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick SM, Hom Thepaksorn EK, Izano MA, Cushing LJ, Wang Y, Smith SC, Gao S, Park J-S, Padula AM, DeMicco E, Valeri L, Woodruff TJ and Morello-Frosch R 2020. Associations between prenatal maternal exposure to per- and polyfluoroalkyl substances (PFAS) and polybrominated diphenyl ethers (PBDEs) and birth outcomes among pregnant women in San Francisco. Environmental Health 19, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. 2000. EPA and 3M ANNOUNCE PHASE OUT OF PFOS. United States Environmental Protection Agency. [Google Scholar]
- EPA. 2006. PFOA Stewardship Program. United States Environmental Protection Agency. [Google Scholar]
- EPA. 2016. Drinking Water Health Advisories for PFOA and PFOS. [DOI] [PubMed]
- Ericson I, M. G, Nadal M, van Bavel B, Lindström G and Domingo JL 2007. Perfluorinated chemicals in blood of residents in Catalonia (Spain) in relation to age and gender: a pilot study. Environ Int, 3(5):616–623. [DOI] [PubMed] [Google Scholar]
- Ernst A, Brix N, Lauridsen LLB, Olsen J, Parner ET, Liew Z, Olsen LH and Ramlau-Hansen CH 2019. Exposure to Perfluoroalkyl Substances during Fetal Life and Pubertal Development in Boys and Girls from the Danish National Birth Cohort. Environ Health Perspect 127, 17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Morreale HF 2018. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nature Reviews Endocrinology 14, 270–284. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L and Olsen J 2009. Maternal levels of perfluorinated chemicals and subfecundity. Human Reproduction 24, 1200–1205. [DOI] [PubMed] [Google Scholar]
- Feng X, Cao X, Zhao S, Wang X, Hua X, Chen L and Chen L 2017. Exposure of Pregnant Mice to Perfluorobutanesulfonate Causes Hypothyroxinemia and Developmental Abnormalities in Female Offspring. [DOI] [PubMed]
- Feng X, Wang X, Cao X, Xia Y, Zhou R and Chen L 2015. Chronic Exposure of Female Mice to an Environmental Level of Perfluorooctane Sulfonate Suppresses Estrogen Synthesis Through Reduced Histone H3K14 Acetylation of the StAR Promoter Leading to Deficits in Follicular Development and Ovulation. Toxicological Sciences 148, 368–379. [DOI] [PubMed] [Google Scholar]
- Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, Smith JS and Roberts SM 2021. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environmental Toxicology and Chemistry 40, 606–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton SE, Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Hines EP, White SS, Lindstrom AB, Strynar MJ and Petropoulou S-SE 2009. Analysis of PFOA in dosed CD-1 mice. Part 2. Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod Toxicol 27, 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsthuber M, Kaiser AM, Granitzer S, Hassl I, Hengstschläger M, Stangl H and Gundacker C 2020. Albumin is the major carrier protein for PFOS, PFOA, PFHxS, PFNA and PFDA in human plasma. Environment International 137, 105324. [DOI] [PubMed] [Google Scholar]
- Fraser IS, O. CH, Broder M and Munro MG 2011a. The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. [DOI] [PubMed]
- Fraser IS, Römer T, Parke S, Zeun S, Mellinger U, Machlitt A and Jensen JT 2011b. Effective treatment of heavy and/or prolonged menstrual bleeding with an oral contraceptive containing estradiol valerate and dienogest: a randomized, double-blind Phase III trial. Hum Reprod 26, 2698–2708. [DOI] [PubMed] [Google Scholar]
- Fröhlich E and Wahl R 2017. Thyroid Autoimmunity: Role of Anti-thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Frontiers in immunology 8, 521–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, C. M, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, Faber F, Hannibal I, Genzel-Boroviczény O, Koletzko B and Völkel W 2010. Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environ. Sci. Technol, 44 (18), 7123–7129. [DOI] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Völkel W, Wilhelm M and Twardella D 2009. Perfluorinated compounds--exposure assessment for the general population in Western countries. Int J Hyg Environ Health 212, 239–270. [DOI] [PubMed] [Google Scholar]
- Fu J, Gao Y, Cui L, Wang T, Liang Y, Qu G, Yuan B, Wang Y, Zhang A and Jiang G 2016. Occurrence, temporal trends, and half-lives of perfluoroalkyl acids (PFAAs) in occupational workers in China. Scientific reports 6, 38039–38039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon SA, Fasano WJ, Mawn MP, Nabb DL, Buck RC, Buxton LW, Jepson GW and Frame SR 2016. Absorption, distribution, metabolism, excretion, and kinetics of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid ammonium salt following a single dose in rat, mouse, and cynomolgus monkey. Toxicology 340, 1–9. [DOI] [PubMed] [Google Scholar]
- Gannon SA, Johnson T, Nabb DL, Serex TL, Buck RC and Loveless SE 2011. Absorption, distribution, metabolism, and excretion of [1-¹⁴C]-perfluorohexanoate ([¹⁴C]-PFHx) in rats and mice. Toxicology 283, 55–62. [DOI] [PubMed] [Google Scholar]
- Gebbink WA, van Asseldonk L and van Leeuwen SPJ 2017. Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands. Environmental Science & Technology 51, 11057–11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM and Falcone T 1999. Effect of diethylstilbestrol on reproductive function. Fertil Steril 72, 1–7. [DOI] [PubMed] [Google Scholar]
- Góralczyk K, Pachocki KA, Hernik A, Struciński P, Czaja K, Lindh CH, Jönsson BA, Lenters V, Korcz W, Minorczyk M, Matuszak M and Ludwicki JK 2015. Perfluorinated chemicals in blood serum of inhabitants in central Poland in relation to gender and age. Sci Total Environ, 532:548–555. [DOI] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J and Zoeller RT 2015. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 36, E1–e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MP, Harvey AJ, Finger BJ and Tarulli GA 2021. Endocrine disrupting chemicals: Impacts on human fertility and fecundity during the peri-conception period. Environmental Research 194, 110694. [DOI] [PubMed] [Google Scholar]
- Group EW 2021. PFAS Contamination in the U.S.
- Hamilton BE and Ventura SJ 2006. Fertility and abortion rates in the United States, 1960–2002. Int J Androl 29, 34–45. [DOI] [PubMed] [Google Scholar]
- Han X, Snow TA, Kemper RA and Jepson GW 2003. Binding of Perfluorooctanoic Acid to Rat and Human Plasma Proteins. Chemical Research in Toxicology 16, 775–781. [DOI] [PubMed] [Google Scholar]
- Harlow SD and Ephross SA 1995. Epidemiology of menstruation and its relevance to women’s health. Epidemiol Rev, 17(12):265–286. [DOI] [PubMed] [Google Scholar]
- Hatch EE, Troisi R, Wise LA, Hyer M, Palmer JR, Titus-Ernstoff L, Strohsnitter W, Kaufman R, Adam E, Noller KL, Herbst AL, Robboy S, Hartge P and Hoover RN 2006. Age at natural menopause in women exposed to diethylstilbestrol in utero. Am J Epidemiol 164, 682–688. [DOI] [PubMed] [Google Scholar]
- Heffernan AL, Cunningham TK, Drage DS, Aylward LL, Thompson K, Vijayasarathy S, Mueller JF, Atkin SL and Sathyapalan T 2018. Perfluorinated alkyl acids in the serum and follicular fluid of UK women with and without polycystic ovarian syndrome undergoing fertility treatment and associations with hormonal and metabolic parameters. Int J Hyg Environ Health 221, 1068–1075. [DOI] [PubMed] [Google Scholar]
- Henry ND and Fair PA 2013. Comparison of in vitro cytotoxicity, estrogenicity and anti-estrogenicity of triclosan, perfluorooctane sulfonate and perfluorooctanoic acid. Journal of Applied Toxicology 33, 265–272. [DOI] [PubMed] [Google Scholar]
- Herrick RL, Buckholz J, Biro FM, Calafat AM, Ye X, Xie C and Pinney SM 2017. Polyfluoroalkyl substance exposure in the Mid-Ohio River Valley, 1991–2012. Environmental Pollution 228, 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydebreck F, Tang J, Xie Z and Ebinghaus R 2015. Alternative and Legacy Perfluoroalkyl Substances: Differences between European and Chinese River/Estuary Systems. Environmental Science & Technology 49, 8386–8395. [DOI] [PubMed] [Google Scholar]
- Heydebreck F, Tang J, Xie Z and Ebinghaus R 2016. Emissions of Per- and Polyfluoroalkyl Substances in a Textile Manufacturing Plant in China and Their Relevance for Workers’ Exposure. [DOI] [PubMed]
- Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. 2009. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol. 304, 97. [DOI] [PubMed] [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, Lohmann R, Carignan CC, Blum A, Balan SA, Higgins CP and Sunderland EM 2016. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environmental Science & Technology Letters 3, 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Chen Q, Zhang L, Luo K, Chen L, Zhao S, Feng L and Zhang J 2019. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and the risk of hypertensive disorders of pregnancy. Environmental Health 18, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PA, Sathyanarayana S, Fowler PA and Trasande L 2016. Female Reproductive Disorders, Diseases, and Costs of Exposure to Endocrine Disrupting Chemicals in the European Union. J Clin Endocrinol Metab 101, 1562–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley S, Goldberg D, Wang M, Park JS, Petreas M, Bernstein L, Anton-Culver H, Nelson DO and Reynolds P 2018. Breast cancer risk and serum levels of per- and poly-fluoroalkyl substances: a case-control study nested in the California Teachers Study. Environ Health 17, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelido AM, Marra V, Abballe A, Valentini S, Iacovella N, Barbieri P, Porpora MG, Porpora MG, Domenico A.d. and De Felip E 2010. Perfluorooctanesulfonate and perfluorooctanoic acid exposures of the Italian general population. Chemosphere 80, 1125–1130. [DOI] [PubMed] [Google Scholar]
- Inoue K, Ritz B, Andersen Stine L, Ramlau-Hansen Cecilia H, Høyer Birgit B, Bech Bodil H, Henriksen Tine B, Bonefeld-Jørgensen Eva C, Olsen J and Liew Z 2019. Perfluoroalkyl Substances and Maternal Thyroid Hormones in Early Pregnancy; Findings in the Danish National Birth Cohort. Environmental Health Perspectives 127, 117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S, Araki A, Miyashita C, Yamazaki K, Goudarzi H, Minatoya M, Ait Bamai Y, Kobayashi S, Okada E, Kashino I, Yuasa M, Baba T and Kishi R 2019. Association between perfluoroalkyl substance exposure and thyroid hormone/thyroid antibody levels in maternal and cord blood: The Hokkaido Study. Environment International 133, 105139. [DOI] [PubMed] [Google Scholar]
- Jain RB 2013. Association between thyroid profile and perfluoroalkyl acids: data from NHNAES 2007–2008. Environ Res 126, 51–59. [DOI] [PubMed] [Google Scholar]
- Jensen TK, T. S, Keiding N, Schaumburg I and Grandjean P 1999. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology, 10(14):422–428. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Sutton P, Atchley DS, Koustas E, Lam J, Sen S, Robinson KA, Axelrad DA and Woodruff TJ 2014. The Navigation Guide - evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect 122, 1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen KT, Specht IO, Lenters V, Bach CC, Rylander L, Jönsson BAG, Lindh CH, Giwercman A, Heederik D, Toft G and Bonde JP 2014. Perfluoroalkyl substances and time to pregnancy in couples from Greenland, Poland and Ukraine. Environmental Health 13, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaboré HA, Vo Duy S, Munoz G, Méité L, Desrosiers M, Liu J, Sory TK and Sauvé S 2018. Worldwide drinking water occurrence and levels of newly-identified perfluoroalkyl and polyfluoroalkyl substances. The Science of the total environment 616–617, 1089–1100. [DOI] [PubMed] [Google Scholar]
- Kahn LG, Harley KG, Siegel EL, Zhu Y, Factor-Litvak P, Porucznik CA, Klein-Fedyshin M, Hipwell AE and program collaborators for Environmental Influences on Child Health Outcomes, P. 2021. Persistent organic pollutants and couple fecundability: a systematic review. Human Reproduction Update 27, 339–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale A, Deardorff J, Lahiff M, Laurent C, Greenspan LC, Hiatt RA, Windham G, Galvez MP, Biro FM, Pinney SM, Teitelbaum SL, Wolff MS, Barlow J, Mirabedi A, Lasater M and Kushi LH 2015. Breastfeeding Versus Formula-Feeding and Girls’ Pubertal Development. Maternal and Child Health Journal 19, 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Choi J-S and Park J-W 2016. Transcriptional changes in steroidogenesis by perfluoroalkyl acids (PFOA and PFOS) regulate the synthesis of sex hormones in H295R cells. Chemosphere 155, 436–443. [DOI] [PubMed] [Google Scholar]
- Kang Q, Gao F, Zhang X, Wang L, Liu J, Fu M, Zhang S, Wan Y, Shen H and Hu J 2020. Nontargeted identification of per- and polyfluoroalkyl substances in human follicular fluid and their blood-follicle transfer. Environment International 139, 105686. [DOI] [PubMed] [Google Scholar]
- Kannan K, S. C, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, Mohd MA, Olivero J, Van Wouwe N, Yang JH and Aldoust KM 2004. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ. Sci. Technol, 38 (17), 4489–4495. [DOI] [PubMed] [Google Scholar]
- Karsa DR 1995. Fluorinated surfactants: synthesis properties applications, by Erik Kissa. Marcel Dekker Inc, New York, 1994, pp. vii + 469, ISBN 0-8247-9011-1. Polymer International 36, 101–101. [Google Scholar]
- Kato H, Fujii S, Takahashi M, Matsumoto M, Hirata-Koizumi M, Ono A and Hirose A 2015. Repeated dose and reproductive/developmental toxicity of perfluorododecanoic acid in rats. Environmental Toxicology 30, 1244–1263. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong L, Chen A, Dunbar C, Webster GM, Lanphear BP and Calafat AM 2014. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003–2006. Environ. Sci. Technol, 48(16):9600–9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N, Chen A, Lee M, Czerwinski SA, Ebert JR, DeWitt JC and Kannan K 2016. Association of Perfluoroalkyl Substances, Bone Mineral Density, and Osteoporosis in the U.S. Population in NHANES 2009–2010. Environ Health Perspect 124, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YR, White N, Bräunig J, Vijayasarathy S, Mueller JF, Knox CL, Harden FA, Pacella R and Toms L-ML 2020. Per- and poly-fluoroalkyl substances (PFASs) in follicular fluid from women experiencing infertility in Australia. Environmental Research 190, 109963. [DOI] [PubMed] [Google Scholar]
- Kissa E and Kissa E 2001. Fluorinated surfactants and repellents. Science, 1–607. [Google Scholar]
- Kjeldsen LS and Bonefeld-Jørgensen EC 2013. Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res Int 20, 8031–8044. [DOI] [PubMed] [Google Scholar]
- Knox SS, Jackson T, Javins B, Frisbee SJ, Shankar A and Ducatman AM 2011. Implications of Early Menopause in Women Exposed to Perfluorocarbons. The Journal of Clinical Endocrinology & Metabolism 96, 1747–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraugerud M, Zimmer KE, Ropstad E and Verhaegen S 2011. Perfluorinated compounds differentially affect steroidogenesis and viability in the human adrenocortical carcinoma (H295R) in vitro cell assay. Toxicol Lett 205, 62–68. [DOI] [PubMed] [Google Scholar]
- Kristensen SL, Ramlau-Hansen CH, Ernst E, Olsen SF, Bonde JP, Vested A, Halldorsson TI, Becher G, Haug LS and Toft G 2013. Long-term effects of prenatal exposure to perfluoroalkyl substances on female reproduction. Human Reproduction 28, 3337–3348. [DOI] [PubMed] [Google Scholar]
- Lagiou P 2006. Intrauterine exposures, pregnancy estrogens and breast cancer risk: where do we currently stand? Breast Cancer Res 8, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane S, Siljander H, Duberg D, Honkanen J, Virtanen SM, Orešič M, Knip M and Hyötyläinen T 2021. Exposure to per- and polyfluoroalkyl substances associates with an altered lipid composition of breast milk. [DOI] [PubMed]
- Larsson G, Milsom I, Lindstedt G and Rybo G 1992. The influence of a low-dose combined oral contraceptive on menstrual blood loss and iron status. Contraception 46, 327–334. [DOI] [PubMed] [Google Scholar]
- Lau C, R. TJ, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB and Strynar MJ 2006. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol. Sci, 90(92):510–518. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL and Stevenson LA 2003. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol Sci 74, 382–392. [DOI] [PubMed] [Google Scholar]
- Leary DB, Takazawa M Fau - Kannan K, Kannan K Fau - Khalil N and Khalil N 2020. Perfluoroalkyl Substances and Metabolic Syndrome in Firefighters: A Pilot Study. [DOI] [PubMed]
- Lebeaux RM, Doherty BT, Gallagher LG, Zoeller RT, Hoofnagle AN, Calafat AM, Karagas MR, Yolton K, Chen A, Lanphear BP, Braun JM and Romano ME 2020. Maternal serum perfluoroalkyl substance mixtures and thyroid hormone concentrations in maternal and cord sera: The HOME Study. Environmental Research 185, 109395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Kang SG, Lee JT, Lee SW, Kim JH, Kim DH, Son BC, Kim KH, Suh CH, Kim SY and Park YB 2015. Effects of perfluorooctane sulfuric acid on placental PRL-family hormone production and fetal growth retardation in mice. [DOI] [PubMed]
- Lee YJ, Jung HW, Kim HY, Choi Y-J and Lee YA 2021. Early-Life Exposure to Per- and Poly-Fluorinated Alkyl Substances and Growth, Adiposity, and Puberty in Children: A Systematic Review. Front Endocrinol (Lausanne) 12, 1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RC, Johns LE and Meeker JD 2015. Serum Biomarkers of Exposure to Perfluoroalkyl Substances in Relation to Serum Testosterone and Measures of Thyroid Function among Adults and Adolescents from NHANES 2011–2012. Int J Environ Res Public Health 12, 6098–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Wang Z, Miao M, Tian Y, Zhou Y, Wen S, Chen Y, Sun X and Yuan W 2020. Prenatal exposure to perfluoroalkyl substances and thyroid hormone concentrations in cord plasma in a Chinese birth cohort. Environmental Health 19, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Wen LL, Lin LY, Wen TW, Lien GW, Hsu SH, Chien KL, Liao CC, Sung FC, Chen PC and Su TC 2013. The associations between serum perfluorinated chemicals and thyroid function in adolescents and young adults. J Hazard Mater 244–245, 637–644. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa M-J, Fletcher T, Armstrong B, Genser B, Dhatariya K, Mondal D, Ducatman A and Leonardi G 2011. Association of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) with Age of Puberty among Children Living near a Chemical Plant. Environmental Science & Technology 45, 8160–8166. [DOI] [PubMed] [Google Scholar]
- Loveless SE, Slezak B, Serex T, Lewis J, Mukerji P, O’Connor JC, Donner EM, Frame SR, Korzeniowski SH and Buck RC 2009. Toxicological evaluation of sodium perfluorohexanoate. Toxicology 264, 32–44. [DOI] [PubMed] [Google Scholar]
- Luebker DJ, Case MT, York RG, Moore JA, Hansen KJ and Butenhoff JL 2005a. Two-generation reproduction and cross-foster studies of perfluorooctanesulfonate (PFOS) in rats. Toxicology 215, 126–148. [DOI] [PubMed] [Google Scholar]
- Luebker DJ, York RG, Hansen KJ, Moore JA and Butenhoff JL 2005b. Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in Sprague-Dawley rats: dose-response, and biochemical and pharamacokinetic parameters. Toxicology 215, 149–169. [DOI] [PubMed] [Google Scholar]
- Lum KJ, R. S, Barr DB, Louis TA and Buck Louis GM 2017. Perfluoroalkyl Chemicals, Menstrual Cycle Length, and Fecundity: Findings from a Prospective Pregnancy Study. Epidemiology, 28(21):90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyngsø J, H. R-HC, Høyer BB, Støvring H, Bonde JP, Jönsson BAG, Lindh CH, Pedersen HS, Ludwicki JK, Zviezdai V and Toft G 2014. Menstrual cycle characteristics in fertile women from Greenland, Poland and Ukraine exposed to perfluorinated chemicals: a cross-sectional study. Hum Reprod, 29(22):359–367. [DOI] [PubMed] [Google Scholar]
- Ma X, Cui L, Chen L, Zhang J, Zhang X, Kang Q, Jin F and Ye Y 2021. Parental plasma concentrations of perfluoroalkyl substances and In Vitro fertilization outcomes. Environmental Pollution 269, 116159. [DOI] [PubMed] [Google Scholar]
- Macon MB and Fenton SE 2013. Endocrine disruptors and the breast: early life effects and later life disease. J Mammary Gland Biol Neoplasia 18, 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macon MB, Villanueva LR, Tatum-Gibbs K, Zehr RD, Strynar MJ, Stanko JP, White SS, Helfant L and Fenton SE 2011. Prenatal perfluorooctanoic acid exposure in CD-1 mice: low-dose developmental effects and internal dosimetry. Toxicol Sci 122, 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Adachi S, Inoue K, Ohkura S and Tsukamura H 2007. Metastin/kisspeptin and control of estrous cycle in rats. Rev Endocr Metab Disord 8, 21–29. [DOI] [PubMed] [Google Scholar]
- Mamsen LS, Björvang RD, Mucs D, Vinnars MT, Papadogiannakis N, Lindh CH, Andersen CY and Damdimopoulou P 2019. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ Int, 124:482–492. [DOI] [PubMed] [Google Scholar]
- Mamsen LS, Jönsson BAG, Lindh CH, Olesen RH, Larsen A, Ernst E, Kelsey TW and Andersen CY 2017. Concentration of perfluorinated compounds and cotinine in human foetal organs, placenta, and maternal plasma. Sci Total Environ, 596–597:597–105. [DOI] [PubMed] [Google Scholar]
- Mancini FR, Cano-Sancho G, Gambaretti J, Marchand P, Boutron-Ruault MC, Severi G, Arveux P, Antignac JP and Kvaskoff M 2020. Perfluorinated alkylated substances serum concentration and breast cancer risk: Evidence from a nested case-control study in the French E3N cohort. Int J Cancer 146, 917–928. [DOI] [PubMed] [Google Scholar]
- Maranghi F and Mantovani A 2012. Targeted toxicological testing to investigate the role of endocrine disrupters in puberty disorders. Reproductive Toxicology 33, 290–296. [DOI] [PubMed] [Google Scholar]
- Meena M, Chopra S, Jain V and Aggarwal N 2016. The Effect of Anti-Thyroid Peroxidase Antibodies on Pregnancy Outcomes in Euthyroid Women. J Clin Diagn Res 10, QC04–QC07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midasch O, Schettgen T and Angerer J 2006. Pilot study on the perfluorooctanesulfonate and perfluorooctanoate exposure of the German general population. International journal of hygiene and environmental health 209, 489–496. [DOI] [PubMed] [Google Scholar]
- Mogensen UB, Grandjean P, Nielsen F, Weihe P and Budtz-Jørgensen E 2015. Breastfeeding as an Exposure Pathway for Perfluorinated Alkylates. Environ Sci Technol 49, 10466–10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal D, Weldon RH, Armstrong BG, Gibson LJ, Lopez-Espinosa M-J, Shin H-M and Fletcher T 2014. Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environmental health perspectives 122, 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville MC, McFadden Tb Fau - Forsyth I and Forsyth I 2002. Hormonal regulation of mammary differentiation and milk secretion. [DOI] [PubMed]
- Nian M, Luo K, Luo F, Aimuzi R, Huo X, Chen Q, Tian Y and Zhang J 2020. Association between Prenatal Exposure to PFAS and Fetal Sex Hormones: Are the Short-Chain PFAS Safer? Environmental Science & Technology 54, 8291–8299. [DOI] [PubMed] [Google Scholar]
- Nikolaou D and Templeton A 2004. Early ovarian ageing. European journal of obstetrics, gynecology, and reproductive biology 113, 126–133. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Kärrman A Fau - Rotander A, Rotander A Fau - van Bavel B, van Bavel B Fau - Lindström G, Lindström G Fau - Westberg H and Westberg H 2013. Professional ski waxers’ exposure to PFAS and aerosol concentrations in gas phase and different particle size fractions. [DOI] [PubMed]
- Nilsson H, Kärrman A, Westberg H, Rotander A, van Bavel B and Lindström G 2010. A time trend study of significantly elevated perfluorocarboxylate levels in humans after using fluorinated ski wax. Environmental science & technology 44, 2150–2155. [DOI] [PubMed] [Google Scholar]
- NIOSH. 2021. Per- and polyfluoroalkyl substances (PFAS).
- OECD. 2021. Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances: Recommendations and Practical Guidance.
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL and Zobel LR 2007. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environmental health perspectives 115, 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Butenhoff JL and Zobel LR 2009a. Perfluoroalkyl chemicals and human fetal development: an epidemiologic review with clinical and toxicological perspectives. Reprod Toxicol, 27(23–24):212–230. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Chang S-C, Noker PE, Gorman GS, Ehresman DJ, Lieder PH and Butenhoff JL 2009b. A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicology 256, 65–74. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Gilliland FD, Burlew MM, Burris JM, Mandel JS and Mandel JH 1998. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. J Occup Environ Med 40, 614–622. [DOI] [PubMed] [Google Scholar]
- Omoike OE, Pack RP, Mamudu HM, Liu Y and Wang L 2020. A cross-sectional study of the association between perfluorinated chemical exposure and cancers related to deregulation of estrogen receptors. Environ Res, 110329. [DOI] [PubMed] [Google Scholar]
- Palioura E and Diamanti-Kandarakis E 2015. Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs). Reviews in Endocrine and Metabolic Disorders 16, 365–371. [DOI] [PubMed] [Google Scholar]
- Pan Y, Wang J, Yeung LWY, Wei S and Dai J 2020. Analysis of emerging per- and polyfluoroalkyl substances: Progress and current issues. TrAC Trends in Analytical Chemistry 124, 115481–115481. [Google Scholar]
- Pan Y, Zhang H, Cui Q, Sheng N, Yeung LWY, Guo Y, Sun Y and Dai J 2017. First Report on the Occurrence and Bioaccumulation of Hexafluoropropylene Oxide Trimer Acid: An Emerging Concern. Environmental Science & Technology 51, 9553–9560. [DOI] [PubMed] [Google Scholar]
- Park SK, Peng Q, Ding N, Mukherjee B and Harlow SD 2019. Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: Evidence of racial/ethnic and geographic differences in PFAS exposure. Environ Res 175, 186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez F, Nadal M, Navarro-Ortega A, Fàbrega F, Domingo JL, Barceló D and Farré M 2013. Accumulation of perfluoroalkyl substances in human tissues. Environ Int 59, 354–362. [DOI] [PubMed] [Google Scholar]
- Pierozan P and Karlsson O 2018. PFOS induces proliferation, cell-cycle progression, and malignant phenotype in human breast epithelial cells. Archives of toxicology 92, 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney SM, Biro FM, Windham GC, Herrick RL, Yaghjyan L, Calafat AM, Succop P, Sucharew H, Ball KM, Kato K, Kushi LH and Bornschein R 2014. Serum biomarkers of polyfluoroalkyl compound exposure in young girls in Greater Cincinnati and the San Francisco Bay Area, USA. Environmental Pollution 184, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney SM, Windham GC, Xie C, Herrick RL, Calafat AM, McWhorter K, Fassler CS, Hiatt RA, Kushi LH and Biro FM 2019. Perfluorooctanoate and changes in anthropometric parameters with age in young girls in the Greater Cincinnati and San Francisco Bay Area. International Journal of Hygiene and Environmental Health 222, 1038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitter G, Da Re F, Canova C, Barbieri G, Zare Jeddi M, Daprà F, Manea F, Zolin R, Bettega AM, Stopazzolo G, Vittorii S, Zambelli L, Martuzzi M, Mantoan D and Russo F 2020. Serum Levels of Perfluoroalkyl Substances (PFAS) in Adolescents and Young Adults Exposed to Contaminated Drinking Water in the Veneto Region, Italy: A Cross-Sectional Study Based on a Health Surveillance Program. Environ Health Perspect 128, 27007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston EV, Webster TF, Claus Henn B, McClean MD, Gennings C, Oken E, Rifas-Shiman SL, Pearce EN, Calafat AM, Fleisch AF and Sagiv SK 2020. Prenatal exposure to per- and polyfluoroalkyl substances and maternal and neonatal thyroid function in the Project Viva Cohort: A mixtures approach. Environment International 139, 105728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramhøj L, Hass U, Boberg J, Scholze M, Christiansen S, Nielsen F and Axelstad M 2018. Perfluorohexane Sulfonate (PFHxS) and a Mixture of Endocrine Disrupters Reduce Thyroxine Levels and Cause Antiandrogenic Effects in Rats. [DOI] [PubMed]
- Rashtian J, Chavkin DE and Merhi Z 2019. Water and soil pollution as determinant of water and food quality/contamination and its impact on female fertility. Reprod Biol Endocrinol, 17(11):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, Xu Y, Calafat AM, Yolton K, Chen A, Webster GM, Eliot MN, Howard CR, Lanphear BP and Braun JM 2016. Maternal serum perfluoroalkyl substances during pregnancy and duration of breastfeeding. Environ Res 149, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotander A, Toms LM, Aylward L, Kay M and Mueller JF 2015. Elevated levels of PFOS and PFHxS in firefighters exposed to aqueous film forming foam (AFFF). [DOI] [PubMed]
- Ruark CD, Song G, Yoon M, Verner MA, Andersen ME, Clewell HJ 3rd and Longnecker MP 2017. Quantitative bias analysis for epidemiological associations of perfluoroalkyl substance serum concentrations and early onset of menopause. Environ Int, 99:245–254. [DOI] [PubMed] [Google Scholar]
- Rush EL, Singer AB, Longnecker MP, Haug LS, Sabaredzovic A, Symanski E and Whitworth KW 2018. Oral contraceptive use as a determinant of plasma concentrations of perfluoroalkyl substances among women in the Norwegian Mother and Child Cohort (MoBa) study. Environ Int 112, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Rifas-Shiman SL, Webster TF, Mora AM, Harris MH, Calafat AM, Ye X, Gillman MW and Oken E 2015. Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environ. Sci. Technol, 49(19):11849–11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saran S, Gupta BS, Philip R, Singh KS, Bende SA, Agroiya P and Agrawal P 2016. Effect of hypothyroidism on female reproductive hormones. Indian J Endocrinol Metab 20, 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrenk D, Bignami M, Bodin L, Chipman JK, Del Mazo J, Grasl-Kraupp B, Hogstrand C, Hoogenboom LR, Leblanc J-C, Nebbia CS, Nielsen E, Ntzani E, Petersen A, Sand S, Vleminckx C, Wallace H, Barregård L, Ceccatelli S, Cravedi J-P, Halldorsson TI, Haug LS, Johansson N, Knutsen HK, Rose M, Roudot A-C, Van Loveren H, Vollmer G, Mackay K, Riolo F and Schwerdtle T 2020. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J 18, e06223–e06223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano L, Iribarne-Durán LM, Suárez B, Artacho-Cordón F, Vela-Soria F, Peña-Caballero M, Hurtado JA, Olea N, Fernández MF and Freire C 2021. Concentrations of perfluoroalkyl substances in donor breast milk in Southern Spain and their potential determinants. International Journal of Hygiene and Environmental Health 236, 113796. [DOI] [PubMed] [Google Scholar]
- Shabalina IG, Kalinovich AV, Cannon B and Nedergaard J 2016. Metabolically inert perfluorinated fatty acids directly activate uncoupling protein 1 in brown-fat mitochondria. Arch Toxicol 90, 1117–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Zhang H, Ding L, Feng Y, Xu M and Dai J 2009. The effect of perfluorododecanonic acid on endocrine status, sex hormones and expression of steroidogenic genes in pubertal female rats. Reprod Toxicol 27, 352–359. [DOI] [PubMed] [Google Scholar]
- Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR and Rocca WA 2010. Premature menopause or early menopause: long-term health consequences. Maturitas 65, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AB, Whitworth KW, Haug LS, Sabaredzovic A, Impinen A, Papadopoulou E and Longnecker MP 2018. Menstrual cycle characteristics as determinants of plasma concentrations of perfluoroalkyl substances (PFASs) in the Norwegian Mother and Child Cohort (MoBa study). Environ Res, 166:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling AP, Engel SM, Richardson DB, Baird DD, Haug LS, Stuebe AM, Klungsøyr K, Harmon Q, Becher G, Thomsen C, Sabaredzovic A, Eggesbø M, Hoppin JA, Travlos GS, Wilson RE, Trogstad LI, Magnus P and Longnecker MP 2014. Perfluoroalkyl Substances During Pregnancy and Validated Preeclampsia Among Nulliparous Women in the Norwegian Mother and Child Cohort Study. American Journal of Epidemiology 179, 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA and Dougan M 2009. Serum Levels of Perfluorooctanoic Acid and Perfluorooctane Sulfonate and Pregnancy Outcome. American Journal of Epidemiology 170, 837–846. [DOI] [PubMed] [Google Scholar]
- Substances A.f.T. and Registry D 2018. Toxicological Profile for Perfluoroalkyls.(Draft for Public Comment). US Department of Health and Human Services, Public Health Service; Atlanta, GA. [Google Scholar]
- Sun M, Arevalo E, Strynar M, Lindstrom A, Richardson M, Kearns B, Pickett A, Smith C and Knappe DRU 2016. Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environmental Science & Technology Letters 3, 415–419. [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC and Allen JG 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. Journal of Exposure Science & Environmental Epidemiology 29, 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeliga A, Maciejewska-Jeske M and Męczekalski B 2018. Bone health and evaluation of bone mineral density in patients with premature ovarian insufficiency. Prz Menopauzalny 17, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilagyi JT, Avula V and Fry RC 2020a. Perfluoroalkyl Substances (PFAS) and Their Effects on the Placenta, Pregnancy, and Child Development: a Potential Mechanistic Role for Placental Peroxisome Proliferator-Activated Receptors (PPARs). Curr Environ Health Rep 7, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilagyi JT, Freedman AN, Kepper SL, Keshava AM, Bangma JT and Fry RC 2020b. Per- and Polyfluoroalkyl Substances Differentially Inhibit Placental Trophoblast Migration and Invasion In Vitro. [DOI] [PMC free article] [PubMed]
- Takahashi M, Ishida S Fau - Hirata-Koizumi M, Hirata-Koizumi M Fau - Ono A, Ono A Fau - Hirose A and Hirose A 2014. Repeated dose and reproductive/developmental toxicity of perfluoroundecanoic acid in rats. [DOI] [PubMed]
- Taylor KW, Hoffman K, Thayer KA and Daniels JL 2014. Polyfluoroalkyl Chemicals and Menopause among Women 20–65 Years of Age (NHANES). Environmental Health Perspectives 122, 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, Butenhoff JL, Stevenson LA and Lau C 2003. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicological sciences : an official journal of the Society of Toxicology 74, 369–381. [DOI] [PubMed] [Google Scholar]
- Timmermann CAG, Andersen MS, Budtz-Jørgensen E, Boye H, Nielsen F, Jensen RC, Bruun S, Husby S, Grandjean P and Jensen TK 2021. Pregnancy exposure to perfluoroalkyl substances, prolactin concentrations and breastfeeding in the Odense Child Cohort. The Journal of Clinical Endocrinology & Metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann CAG, Budtz-Jørgensen E, Petersen MS, Weihe P, Steuerwald U, Nielsen F, Jensen TK and Grandjean P 2017. Shorter duration of breastfeeding at elevated exposures to perfluoroalkyl substances. Reprod Toxicol 68, 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ Jr., Jégou B, Jensen TK, Jouannet P, Keiding N, Leffers H, McLachlan JA, Meyer O, Müller J, Rajpert-De Meyts E, Scheike T, Sharpe R, Sumpter J and Skakkebaek NE 1996. Male reproductive health and environmental xenoestrogens. Environ Health Perspect 104 Suppl 4, 741–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M. s., Chang S-H, Kuo W-H, Kuo C-H, Li S-Y, Wang M-Y, Chang D-Y, Lu Y-S, Huang C-S, Cheng A-L, Lin C-H and Chen P-C 2020. A case-control study of perfluoroalkyl substances and the risk of breast cancer in Taiwanese women. Environment International 142, 105850. [DOI] [PubMed] [Google Scholar]
- Tsai M-S, Lin C-Y, Lin C-C, Chen M-H, Hsu SHJ, Chien K-L, Sung F-C, Chen P-C and Su T-C 2015. Association between perfluoroalkyl substances and reproductive hormones in adolescents and young adults. International Journal of Hygiene and Environmental Health 218, 437–443. [DOI] [PubMed] [Google Scholar]
- Tucker DK, Macon MB, Strynar MJ, Dagnino S, Andersen E and Fenton SE 2015. The mammary gland is a sensitive pubertal target in CD-1 and C57Bl/6 mice following perinatal perfluorooctanoic acid (PFOA) exposure. Reprod Toxicol 54, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umweltbundesamt. 2018. Ableitung von HBM-I-Werten für Perfluoroktansäure (PFOA) und Perfluoroktansulfonsäure (PFOS) – Stellungnahme der Kommission “Humanbiomonitoring” des Umweltbundesamts. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz 61, 474–487. [DOI] [PubMed] [Google Scholar]
- Vagi SJ, Azziz-Baumgartner E, Sjödin A, Calafat AM, Dumesic D, Gonzalez L, Kato K, Silva MJ, Ye X and Azziz R 2014. Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol A in polycystic ovary syndrome: a case-control study. BMC Endocr Disord 14, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN 2013. Non-monotonic dose responses in studies of endocrine disrupting chemicals: bisphenol a as a case study. Dose Response 12, 259–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassiliadou I, Costopoulou D, Ferderigou A and Leondiadis L 2010. Levels of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) in blood samples from different groups of adults living in Greece. Chemosphere 80, 1199–1206. [DOI] [PubMed] [Google Scholar]
- Vélez MP, Arbuckle TE and Fraser WD 2015. Maternal exposure to perfluorinated chemicals and reduced fecundity: the MIREC study. Human Reproduction 30, 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner MA, Ngueta G, Jensen ET, Fromme H, Völkel W, Nygaard UC, Granum B and Longnecker MP 2016. A Simple Pharmacokinetic Model of Prenatal and Postnatal Exposure to Perfluoroalkyl Substances (PFASs). Environ. Sci. Technol, 50(52):978–986. [DOI] [PubMed] [Google Scholar]
- Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF and Fletcher T 2013. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect 121, 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhang R, Jin F, Lou H, Mao Y, Zhu W, Zhou W, Zhang P and Zhang J 2017. Perfluoroalkyl substances and endometriosis-related infertility in Chinese women. Environ Int 102, 207–212. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhou W, Wu S, Liang F, Li Y, Zhang J, Cui L, Feng Y and Wang Y 2019a. Perfluoroalkyl substances exposure and risk of polycystic ovarian syndrome related infertility in Chinese women. Environ Pollut 247, 824–831. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chang W, Wang L, Zhang Y, Zhang Y, Wang M, Wang Y and Li P 2019b. A review of sources, multimedia distribution and health risks of novel fluorinated alternatives. Ecotoxicology and Environmental Safety 182, 109402–109402. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen PC, Lien GW, Chen HY, Tseng YC, Longnecker MP and Wang SL 2014. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environ Health Perspect 122, 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield G, Rogers M, Grandjean P, Auffhammer M and Sunding D 2020. Reducing exposure to high levels of perfluorinated compounds in drinking water improves reproductive outcomes: evidence from an intervention in Minnesota. Environmental Health 19, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Rauch SA, Marie NS, Mattman A, Lanphear BP and Venners SA 2016. Cross-Sectional Associations of Serum Perfluoroalkyl Acids and Thyroid Hormones in U.S. Adults: Variation According to TPOAb and Iodine Status (NHANES 2007–2008). Environ Health Perspect 124, 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Venners SA, Mattman A and Martin JW 2014. Associations between perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: a population-based cohort study. Environ Res 133, 338–347. [DOI] [PubMed] [Google Scholar]
- Wei C, Wang Q, Song X, Chen X, Fan R, Ding D and Liu Y 2018. Distribution, source identification and health risk assessment of PFASs and two PFOS alternatives in groundwater from non-industrial areas. Ecotoxicology and environmental safety 152, 141–150. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Andersson PL, Lamoree MH, Leonards PE, van Leeuwen SP and Hamers T 2009. Competitive binding of poly- and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicol Sci 109, 206–216. [DOI] [PubMed] [Google Scholar]
- Weiss O, Wiesmüller GA, Bunte A, Göen T, Schmidt CK, Wilhelm M and Hölzer J 2012. Perfluorinated compounds in the vicinity of a fire training area--human biomonitoring among 10 persons drinking water from contaminated private wells in Cologne, Germany. International journal of hygiene and environmental health 215, 212–215. [DOI] [PubMed] [Google Scholar]
- Wen LL, Lin LY, Su TC, Chen PC and Lin CY 2013. Association between serum perfluorinated chemicals and thyroid function in U.S. adults: the National Health and Nutrition Examination Survey 2007–2010. J Clin Endocrinol Metab 98, E1456–1464. [DOI] [PubMed] [Google Scholar]
- White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, Strynar MJ, Lindstrom AB, Thibodeaux JR, Wood C and Fenton SE 2007. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci 96, 133–144. [DOI] [PubMed] [Google Scholar]
- White SS, Kato K, Jia LT, Basden BJ, Calafat AM, Hines EP, Stanko JP, Wolf CJ, Abbott BD and Fenton SE 2009. Effects of perfluorooctanoic acid on mouse mammary gland development and differentiation resulting from cross-foster and restricted gestational exposures. Reprod Toxicol 27, 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SS, Stanko JP, Kato K, Calafat AM, Hines EP and Fenton SE 2011. Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice. Environ Health Perspect 119, 1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth KW, S. HL, Baird DD, Becher G, Hoppin JA, Skjaerven R, Thomsen C, Eggesbo M, Travlos G, Wilson R and Longnecker MP 2012. Perfluorinated compounds and subfecundity in pregnant women. Epidemiology, 23(22):257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström S, Lindh CH, Shu H and Bornehag C-G 2019. Early pregnancy serum levels of perfluoroalkyl substances and risk of preeclampsia in Swedish women. Scientific Reports 9, 9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AJ, Grulke CM, Edwards J, McEachran AD, Mansouri K, Baker NC, Patlewicz G, Shah I, Wambaugh JF, Judson RS and Richard AM 2017. The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. Journal of Cheminformatics 9, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf CJ, Zehr Rd Fau - Schmid JE, Schmid Je Fau - Lau C, Lau C Fau - Abbott BD and Abbott BD 2010. Developmental effects of perfluorononanoic Acid in the mouse are dependent on peroxisome proliferator-activated receptor-alpha. LID - 282896 [pii] LID - 10.1155/2010/282896 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, MacLeod M, Mueller JF and Cousins IT 2014. Enhanced Elimination of Perfluorooctane Sulfonic Acid by Menstruating Women: Evidence from Population-Based Pharmacokinetic Modeling. Environmental Science & Technology 48, 8807–8814. [DOI] [PubMed] [Google Scholar]
- Worley RR, Moore SM, Tierney BC, Ye X, Calafat AM, Campbell S, Woudneh MB and Fisher J 2017. Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ Int, 106:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao P-L, Ehresman DJ, Rae JMC, Chang S-C, Frame SR, Butenhoff JL, Kennedy GL and Peters JM 2014. Comparative in vivo and in vitro analysis of possible estrogenic effects of perfluorooctanoic acid. Toxicology 326, 62–73. [DOI] [PubMed] [Google Scholar]
- Ye X, Kato K, Wong L-Y, Jia T, Kalathil A, Latremouille J and Calafat AM 2018. Per- and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the National Health and Nutrition Examination Survey 2013–2014. International journal of hygiene and environmental health 221, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen M and Auriola S 1990. Tissue Distribution and Elimination of Perfluorodecanoic Acid in the Rat after Single Intraperitoneal Administration. Pharmacology & Toxicology 66, 45–48. [DOI] [PubMed] [Google Scholar]
- Yu WG, Liu W and Jin YH 2009. Effects of perfluorooctane sulfonate on rat thyroid hormone biosynthesis and metabolism. Environ Toxicol Chem 28, 990–996. [DOI] [PubMed] [Google Scholar]
- Zafeiraki E, Costopoulou D, Vassiliadou I, Leondiadis L, Dassenakis E, Traag W, Hoogenboom RL and van Leeuwen SP 2015. Determination of perfluoroalkylated substances (PFASs) in drinking water from the Netherlands and Greece. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 32, 2048–2057. [DOI] [PubMed] [Google Scholar]
- Zhang N, Wang WS, Li WJ, Liu C, Wang Y and Sun K 2015. Reduction of progesterone, estradiol and hCG secretion by perfluorooctane sulfonate via induction of apoptosis in human placental syncytiotrophoblasts. Placenta 36, 575–580. [DOI] [PubMed] [Google Scholar]
- Zhang S, Chen K, Li W, Chai Y, Zhu J, Chu B, Li N, Yan J, Zhang S and Yang Y 2021. Varied thyroid disrupting effects of perfluorooctanoic acid (PFOA) and its novel alternatives hexafluoropropylene-oxide-dimer-acid (GenX) and ammonium 4,8-dioxa-3H-perfluorononanoate (ADONA) in vitro. Environment International 156, 106745. [DOI] [PubMed] [Google Scholar]
- Zhang S, Tan R, Pan R, Xiong J, Tian Y, Wu J and Chen L 2018. Association of Perfluoroalkyl and Polyfluoroalkyl Substances With Premature Ovarian Insufficiency in Chinese Women. J Clin Endocrinol Metab 103, 2543–2551. [DOI] [PubMed] [Google Scholar]
- Zhou W, Zhang L, Tong C, Fang F, Zhao S, Tian Y, Tao Y and Zhang J 2017. Plasma Perfluoroalkyl and Polyfluoroalkyl Substances Concentration and Menstrual Cycle Characteristics in Preconception Women. Environ Health Perspect, 125(126):06701. [DOI] [PMC free article] [PubMed] [Google Scholar]


