Abstract
Objective
To assess the influence of the emergence of severe acute respiratory syndrome coronavirus 2 and the implementation of public health measures on the seasonality of outpatient antibiotic use and their possible association with the incidence of influenza.
Methods
We performed a time-series ecological study in 1516 primary care centres of Andalusia, Spain, comparing the coronavirus disease 2019 period (April 2020 to March 2021) with the 6 previous years. We assessed the number of packs and defined daily doses per 1000 inhabitants of antibacterials and key antibiotics commonly used for acute respiratory tract infections and the number of influenza-positive cases per 100 000 inhabitants. We calculated the correlation between variables and analyzed the seasonal patterns and differences in quarterly antibiotic use.
Results
For all quarters, a significant correlation was observed between influenza activity and antibiotic use (Spearman's r = 0.94; p < 0.001). Before the pandemic period, both variables presented similar seasonal patterns. After the start of the pandemic, influenza activity was suppressed and the pattern of antibiotic use flattened into a straight line (R2 = 0.96; p = 0.022) with a quarterly change of 3.9% (p = 0.007). Total antibiotic use and antibiotics used for treating acute respiratory tract infections showed significant reductions in all quarters compared to the previous year (p < 0.01).
Discussion
The coronavirus disease 2019 pandemic has strongly influenced the seasonality of antibiotic use in primary care. The decline in respiratory viruses, among which the influenza virus is a major player that may act as a proxy for general prevalence, is proposed as a reason for the flattening of the seasonal fluctuations of outpatient antibiotic use in our region.
Keywords: Antibiotics, Primary care, Influenza, COVID-19, Seasonality, Prescribing, Outpatient
Introduction
Antibiotics are some of the most frequently prescribed drugs in both high-income and low- and middle-income countries in the outpatient setting [[1], [2], [3]]. Seasonality in antibiotic prescribing in primary care has been described around the world, in particular for countries with high annual levels of antibiotic use [1,4,5]. Seasonality refers to variations that occur with a frequency of less than a year; for antibiotic use, it is typically defined by peak values in winter and valley values in summer, mainly driven by penicillins, macrolides, and fluoroquinolones. The seasonal pattern of outpatient antibiotic use is similar to the seasonal fluctuations in the incidence of acute bronchitis, acute upper respiratory tract infections, and acute tonsillitis and is associated with the change in influenza virus activity in the community [6]. Antibiotic overuse in the community, mainly in treating acute respiratory tract infections (ARTIs), is proposed as one of the main causes of this phenomenon despite most ARTIs being caused by viruses, for which antibiotics have no role in treatment [7].
Since the introduction of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into the northern hemisphere in January 2020, coinciding with the 2019/2020 respiratory virus season, a decrease in influenza activity has been reported. Interventions designed to limit the spread of SARS-CoV-2, including lockdowns and mandatory use of face masks, are strongly proposed as causes of this beneficial collateral effect [[8], [9], [10], [11], [12]].
Antibiotic use across different healthcare settings has also been significantly influenced by the coronavirus disease 2019 (COVID-19) pandemic, with an effect on antimicrobial resistance that remains unclear. In contrast to the usage data from hospitals, where antibiotic prescriptions initially increased despite relatively low detected rates of bacterial coinfections in patients with COVID-19 [13,14], antibiotic use in primary care dropped. The magnitude of this reduction has ranged between 30% and 40% and has been notably greater for antibiotics primarily used for ARTIs [[15], [16], [17], [18]].
Based on our previously reported sharp decline in antibiotic prescribing in the community setting in our region in the second quarter of 2020, coinciding with the onset of the first wave of the COVID-19 pandemic and national lockdown [15], the objective of the present study was to assess the influence of the emergence of SARS-CoV-2 over time on the seasonal pattern of antibiotic use and its possible association with influenza virus activity in the outpatient setting of Andalusia, the most populated region in Spain.
Methods
Study design and setting
We designed an ecological study with time-series analysis in all 1516 primary care centres of 26 primary healthcare districts of the Andalusian Public Health System, with 8069 general practitioners (GPs) and paediatricians serving 8.4 million people and where an institutionally supported antimicrobial stewardship programme (ASP) for primary care centres and hospitals, the PIRASOA programme (pirasoa.iavante.es), has been in force without interruption since 2014 [5,19].
Time periods
From 14 March to 21 June 2020, a lockdown was in force in Andalusia to contain the transmission of SARS-CoV-2. Other measures implemented were isolation and testing for symptomatic individuals, segregated attention for nonrespiratory and respiratory patients in health care institutions, epidemiological investigations and quarantine of contacts, enforced public education on hand hygiene and use of masks, social distancing, travel restrictions, reduced population mobility, and promotion of telephone consultations. In this study, the COVID-19 period (from April 2020 to March 2021) was compared with the previous years.
Study variables and data sources
Quarterly seasonal fluctuations in total outpatient antibiotic use and influenza virus activity between 2014 and 2021 in Andalusia were evaluated. Influenza virus activity was measured as the number of positive cases per 100 000 inhabitants obtained from the Andalusian epidemiological sentinel surveillance network [20]. We recorded the number of packs (medication containers) of antibacterials for systemic use (ATC group J01) and the number of five key antibiotics commonly used for ARTIs in primary care (amoxicillin, amoxicillin-clavulanate, cefuroxime, azithromycin, and levofloxacin) to avoid trend biases due to the WHO's ATC/defined daily doses alterations in 2019 for certain antibiotics [21]. Fosfomycin-trometamol use was also evaluated as a comparator because it is only prescribed for urinary tract infections. We also assessed in detail the quarterly use from April 2019 to March 2021 of antibacterials for systemic use and four antibiotic groups: penicillins (J01C), cephalosporins (J01D), macrolides (J01F), and quinolones (J01M), specifically for the key antibiotics commonly used for ARTIs, measured as defined daily doses per 1000 inhabitants.
In our region, electronic prescription was established in 2007. Data on antibiotic use were recorded through the computerized pharmacy record database of reimbursed and dispensed drugs hosted by the Pharmacy Service of the Andalusian Health Service, representing 98% of the total population. In addition, we evaluated the number of GP and paediatrician consultations (face-to-face or by telephone), globally and per inhabitant, during the COVID-19 period and for the same period in the previous year.
Statistical analysis
Changes in seasonality were analyzed using the SEASON command to estimate seasonal factors for time series with a specified periodicity using IBM SPSS Statistics software version 23.0 (IBM, Armonk, NY, USA), as well as the packages TSstudio and ggplot2 with R version 4.0.5 (R Core Team, Vienna, Austria). The correlation between patterns of antibiotic prescribing in primary care and influenza activity was calculated using the two-tailed Spearman coefficient (r). To examine whether the antibiotic use pattern during the COVID-19 period fit a straight line, we performed a linear regression analysis with R, and we characterized the quarterly percentage of change of the trend, accounting for autocorrelation between quarters, with Joinpoint Regression software v4.8.0.1 (National Cancer Institute, Bethesda, MD, USA).
We analyzed the differences in antibiotic use between quarters and the number of consultations using the paired Student t-test or Wilcoxon signed-rank test, as appropriate after checking for normality, with SPSS. To assess the robustness of the findings from the paired tests for antibiotic use, we also performed a first-order autoregressive interrupted time-series analysis for each variable, accounting for the previous trend since the inception of the regional ASP in 2014, according to the Cochrane Effective Practice and Organization Care method [22]. Statistical significance was set at p < 0.05.
Ethics
The data presented in this study are part of a continuing public health monitoring of influenza activity and antibiotic use in our region. Study approval was granted by the ethics committee of the University Hospitals Virgen Macarena-Virgen del Rocio (19122014–03/2014) and valid for the Autonomous Community of Andalusia, Spain.
Results
Seasonality
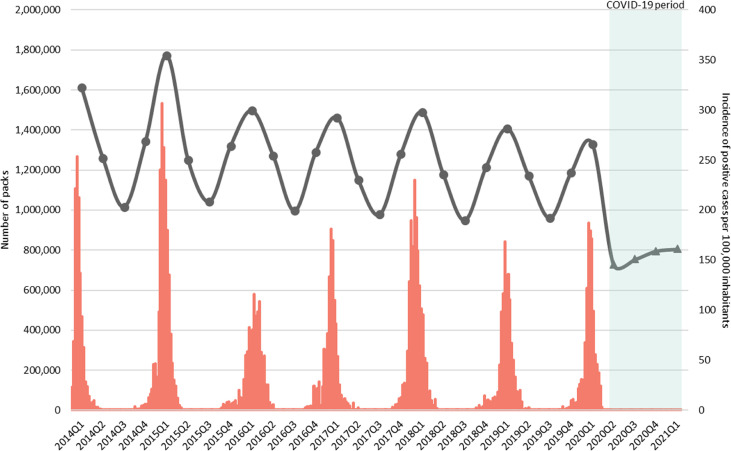
Fig. 1 represents the quarterly seasonal fluctuations of total outpatient antibiotic use and influenza virus activity between 2014 and 2021 in Andalusia. For all 29 quarters, a significant correlation was observed between outpatient antibiotic use and influenza virus activity (Spearman's r = 0.94; p < 0.001).
Fig. 1.
Quarterly seasonal fluctuations in total outpatient antibiotic use and influenza virus activity between 2014 and 2021. The black line represents antibiotic use measured as number of packs of antibacterials for systemic use prescribed in primary care during the pre-pandemic period (circles) and the coronavirus disease 2019 pandemic period (triangles), and the red stacked boxes represent the number of influenza-positive cases per 100 000 inhabitants in Andalusia, Spain. Q, quarter.
Preceding the onset of the COVID-19 pandemic, antibiotic use and influenza virus activity presented a markedly seasonal pattern characterized by higher antibiotic use and incidence of influenza cases in the first quarter of each year (winter), with a seasonal factor of 20% above the quarter's average level for antibiotic use and 241% above the quarter's average level for influenza, and the absence of virus circulation during the third quarter (summer), coinciding with the lowest rate of antibiotic use (–21% seasonal factor). In our time series, the highest values for both variables were reached in 2015 with more than 1.7 million antibiotic packs during the first quarter of 2015 and 307 influenza cases per 100 000 inhabitants in the last week of January 2015. After the start of the COVID-19 pandemic, the number of influenza-positive cases during the 2019/2020 influenza seasonal epidemic dropped rapidly, with no influenza cases reported since the end of March 2020 in our region. The number of samples collected by the sentinel surveillance network and the percentage of influenza-positive cases was 108 (0% of positive cases) in the 2020/2021 epidemic season, compared to 746 (54% of positive cases) and 778 (62% of positive cases) in 2019/2020 and 2018/2019, respectively.
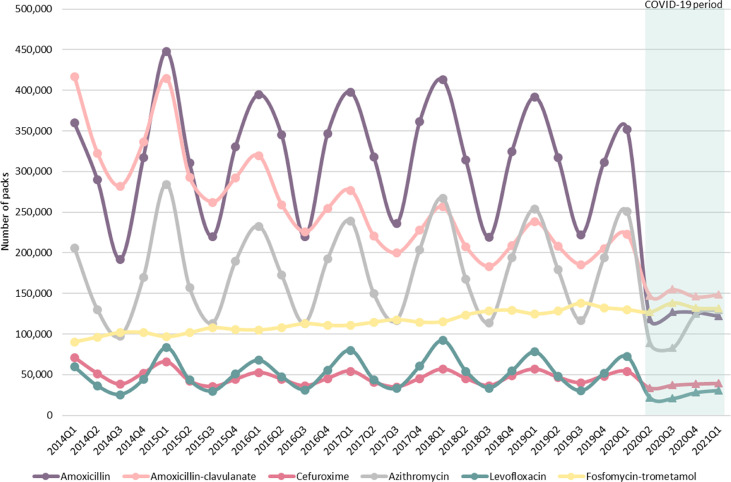
The antibiotic use pattern levelled off in the second quarter of 2020, and seasonality was no longer evident (seasonal factors: summer 2020 = –2%; winter 2021 = 5%), turning into a straight line (R2 = 0.96; p = 0.022) with an average change per quarter of 3.9% (quarterly percentage of change 95% confidence interval, 2.4–5.3; p = 0.007). Additional analyses of changes in seasonality are shown in Figs. S1 and S2. The seasonal pattern for individual antibiotics mainly used for treating ARTIs is shown in Fig. 2 . Amoxicillin, amoxicillin-clavulanate, cefuroxime, azithromycin, and levofloxacin use followed a similar oscillatory pattern related to influenza virus detection. Seasonality in the use of all of these antibiotics was removed after the emergence of the COVID-19 pandemic in the second quarter of 2020, except for fosfomycin-trometamol.
Fig. 2.
Quarterly seasonal fluctuations in amoxicillin, amoxicillin-clavulanate, cefuroxime, azithromycin, levofloxacinm and fosfomycin-trometamol use between 2014 and 2021. Lines represent antibiotic use measured as number of packs prescribed in primary care during the pre-pandemic period (circles) and the coronavirus disease 2019 pandemic period (triangles) in Andalusia, Spain. Q, quarter.
Antibiotic use
The overall use of antibiotics in the community declined during all quarters of the COVID-19 period compared with the corresponding quarters of the previous year, with reductions ranging between –21.4% and –36.8%. Since the start of the COVID-19 pandemic, all groups of antibiotics under study and the key individual antibiotics mainly used for treating ARTIs showed a dramatic reduction in all quarters with respect to the same quarters in the previous year (Table 1 ). In contrast, fosfomycin-trometamol use increased significantly in all quarters under study, except for the first quarter of the COVID-19 period, in which it remained unchanged. These findings were confirmed in the interrupted time-series analysis (Table S1 and Figs. S3–S5), which showed an immediate decrease in total antibiotic use and those antibiotics used for treating ARTIs after the start of the COVID-19 pandemic and a difference between the expected value and the observed one that remained 1 year after the start of the pandemic period.
Table 1.
Differences in antibiotic use in primary care, Andalusia, Spain 2019/2021
| Antibiotic | Quarter 2 (Apr–Jun), n = 26 |
Quarter 3 (Jul–Sept), n = 26 |
||||||
|---|---|---|---|---|---|---|---|---|
| 2019 (DID) | 2020 (DID) | Difference (%) | p-value | 2019 (DID) | 2020 (DID) | Difference (%) | p-value | |
| Total antibiotics (J01) | 12.80 | 8.10 | –36.8 | <0.0001 | 10.46 | 8.22 | –21.4 | <0.0001 |
| Penicillins | 7.74 | 4.54 | –41.3 | <0.0001 | 6.37 | 4.67 | –26.7 | <0.0001 |
| Amoxicillin | 3.88 | 1.72 | –55.6 | <0.0001 | 2.91 | 1.77 | –39.3 | <0.0001 |
| Amoxicillin-clavulanate | 3.49 | 2.55 | –27.0 | <0.0001 | 3.10 | 2.63 | –15.1 | <0.0001 |
| Cephalosporins | 1.10 | 0.83 | –24.6 | <0.0001 | 0.93 | 0.88 | –4.9 | 0.006 |
| Cefuroxime | 0.82 | 0.58 | –29.1 | <0.0001 | 0.69 | 0.63 | –8.6 | <0.0001 |
| Macrolides | 1.62 | 0.83 | –48.6 | <0.0001 | 1.13 | 0.81 | –28.4 | <0.0001 |
| Azithromycin | 1.14 | 0.58 | –49.4 | <0.0001 | 0.73 | 0.53 | –27.9 | <0.0001 |
| Quinolones | 1.25 | 0.87 | –30.5 | <0.0001 | 1.02 | 0.87 | –14.3 | <0.0001 |
| Levofloxacin | 0.56 | 0.26 | –53.4 | <0.0001 | 0.36 | 0.26 | –28.6 | <0.0001 |
| Fosfomycin-trometamol |
0.38 |
0.38 |
2.2 |
0.54 |
0.40 |
0.40 |
2.2 |
0.008 |
| Antibiotic |
Quarter 4 (Oct–Dec), n = 26 |
Quarter 1 (Jan–Mar), n = 26 |
||||||
| 2019 (DID) |
2020 (DID) |
Difference (%) |
p-value |
2020 (DID) |
2021 (DID) |
Difference (%) |
p-value |
|
| Total antibiotics (J01) | 12.92 | 8.74 | –32.4 | <0.0001 | 14.58 | 9.25 | –36.6 | <0.0001 |
| Penicillins | 7.64 | 4.55 | –40.4 | <0.0001 | 8.45 | 4.70 | –44.3 | <0.0001 |
| Amoxicillin | 3.85 | 1.81 | –53.1 | <0.0001 | 4.38 | 1.82 | –58.4 | <0.0001 |
| Amoxicillin-clavulanate | 3.43 | 2.51 | –26.9 | <0.0001 | 3.74 | 2.64 | –29.4 | <0.0001 |
| Cephalosporins | 1.13 | 0.96 | –15.0 | <0.0001 | 1.27 | 0.99 | –21.9 | <0.0001 |
| Cefuroxime | 0.84 | 0.66 | –20.7 | <0.0001 | 0.93 | 0.69 | –26.0 | <0.0001 |
| Macrolides | 1.71 | 1.12 | –34.5 | <0.0001 | 2.11 | 1.20 | –43.3 | <0.0001 |
| Azithromycin | 1.23 | 0.80 | –34.6 | <0.0001 | 1.61 | 0.86 | –46.3 | <0.0001 |
| Quinolones | 1.28 | 0.95 | –26.0 | <0.0001 | 1.54 | 1.01 | –34.6 | <0.0001 |
| Levofloxacin | 0.60 | 0.34 | –43.5 | <0.0001 | 0.83 | 0.38 | –54.2 | <0.0001 |
| Fosfomycin-trometamol | 0.38 | 0.39 | 2.6 | 0.014 | 0.38 | 0.41 | 7.3 | <0.0001 |
DID, defined daily doses per 1000 inhabitants; n, number of primary healthcare districts. Penicillins: antiinfectives classified in the group J01C, including amoxicillin and amoxicillin-clavulanate; cephalosporins: antiinfectives classified in the group J01D, including cefuroxime; macrolides: antiinfectives classified in the group J01F, including azithromycin; quinolones: antiinfectives classified in the group J01M, including levofloxacin.
Primary care consultations
There were nearly 45 million consultations in primary care during the pandemic period compared to 42 million in the previous year, resulting in 5.3 versus 5.0 consultations per inhabitant, respectively. The total number of consultations in primary care did not change, with an average of 3.5 ± 0.4 million monthly consultations in the COVID-19 period and 3.7 ± 0.5 million in the previous year (p = 0.124). However, the rate of telephone consultations increased from 4.4% in the previous year to 64.2%, exceeding the number of face-to-face consultations during the COVID-19 period (p = 0.002). Details are shown in Fig. S6.
Discussion
The results of this study show a flattening of the characteristic seasonal pattern of outpatient antibiotic use with a maintained reduction in antibiotic pressure, specifically for the antibiotics most commonly used in ARTIs, and a complete suppression of influenza virus activity since the inception of the COVID-19 pandemic in our region. The demand for primary care appointments remained stable during this period but showed a dramatic shift from face-to-face to telephone consultations.
The strong association between outpatient antibiotic use and influenza virus activity found in this work is in line with the available published evidence that shows a significant positive correlation between the variables. This correlation has been also reported for antibiotic use and the incidence of ARTIs [6].
The antimicrobial use profile was similar to that reported previously for our region [5,19]. The decreasing trend for amoxicillin-clavulanate use and the increase in the use of fosfomycin-trometamol are aligned with the educational activities of the PIRASOA programme and recommendations of the ASP guidelines [23].
Previous studies from different countries and with short follow-up periods have reported an interruption of influenza virus transmission during the 2019/2020 epidemic season or a decrease in the rates of antibiotic prescription in primary care soon after the beginning of the COVID-19 pandemic [[8], [9], [10], [11], [12],[15], [16], [17], [18],[24], [25], [26]]. Recently a pre–post study performed on an academic health system in the south of Wisconsin analyzed the incidence of respiratory virus detection and antibiotic prescription for the last 4 years, including the 2020/2021 epidemic season, in more than 80 primary care centres. Consistent with the outcomes that we found in our setting with more than 1500 centres, rates of prescription and influenza and non-influenza virus detection decreased during the pandemic period. Nevertheless, non-influenza virus detection presented the strongest correlation with antibiotic use for ARTIs in that study [27].
The emergence of COVID-19 in Spain disrupted the existing sentinel surveillance networks, which could explain why the number of samples collected and analyzed in the 2020/2021 epidemic season was lower than in the previous influenza seasons. However, no influenza-positive cases were detected, which supports the absence of circulation of influenza in our community in the 2020/2021 epidemic season.
The effect of telephone consultations on GPs' diagnostic and prescribing behaviour or patients' worries is still uncertain [15]. A recent systematic review found inconsistent evidence for the effect of remote consulting on antibiotic prescriptions in primary care, with four studies reporting higher prescribing rates, five studies showing lower rates, and three reporting no difference in remote consultations compared with face-to-face consultations [28]. In our study, one point against attributing the reduction in antimicrobial prescribing to the increase in telephone consultations is that all primary care GPs and paediatricians in the Andalusian Public Health System use the electronic prescription, so the patient is not required to be present to have antibiotics prescribed. Further studies are needed to establish the consequences of telemedicine in this setting.
The prolonged period of analysis, the population covered, and the great amount of data collected from 2014 are some of the strengths of this study. A possible explanation for our results may be that public health measures aimed to mitigate COVID-19 transmission contributed to containing other respiratory viruses such as influenza and reducing outpatient antibiotic use.
This study also has several limitations that provide scope for future research. First, observed associations are not exempt from bias, and correlations on an individual level can differ from those on a population level from ecological studies. Specifically, antibiotic reimbursement data cannot accurately reflect individual use of antibiotics, hindering ecological studies from determining what motivates patients to take or not take the antibiotics they are prescribed. Second, the use of sentinel surveillance data for influenza virus activity could have underestimated the influenza virus circulation and prevented the inclusion of non-influenza virus or other infectious diseases such as bacterial pneumonia. Third, the lack of sufficient data did not allow us to investigate possible changes in the reasons for primary care consultations or to explore the association between decreased antibiotic use and antibiotic resistance. Further studies are needed to determine if our findings led to favourable ecological outcomes.
Conclusions
The emergence of SARS-CoV-2 and the implementation of public health measures have strongly influenced the seasonality of antibiotic use. The decline in respiratory viruses, among which the influenza virus is a major player that may act as a proxy for general prevalence, is proposed as a reason for the flattening of the seasonal fluctuations in outpatient antibiotic use in our region. This information may be valuable for policymakers in promoting future strategies to reduce patient morbidity and mortality and health care costs.
Transparency declaration
ABG-G receives financial support from the Subprograma Río Hortega, Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Ciencia, Innovación y Universidades, Spain (CM19/00029). GP has a grant from the Ministerio de Economía y Competitividad, Instituto de Salud Carlos III, cofinanced by the European Development Regional Fund “A Way to Achieve Europe” and by the Spanish Network for Research in Infectious Diseases (grant REIPI RD16/0009). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have no conflicts of interest to declare.
Author contributions
JMC, ABG-G, and GP were responsible for the conception and design of the study. ABG-G and GP did the analysis and interpretation of data, drafting of the article, and final approval of the version to be submitted. RSB, RV-P, and RV made substantial contributions to the acquisition of data. ABG-G, GP, RSB, RV-P, MVG-N, and RV made critical revisions and approval of the manuscript. JMC critically revised the manuscript for important intellectual content and approved the final version to be submitted.
Acknowledgements
We acknowledge the invaluable contribution of all members of the PIRASOA programme; the regional Institutional Programme for the Prevention and Control of Healthcare-Associated Infections and Antimicrobial Stewardship for hospitals and primary care settings in Andalusia, Spain; and the doctors, clinical microbiologists, pharmacists, nurses, and other professionals from all of the health care centres who have made the implementation and continuity of the programme possible. We thank the Andalusian Health Service of the Regional Ministry of Health of Andalusia for supporting the antimicrobial stewardship programme.
Editor: M. Paul
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.12.022.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Goossens H., Ferech M., Vander Stichele R., Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 2.Versporten A., Bolokhovets G., Ghazaryan L., Abilova V., Pyshnik G., Spasojevic T., et al. Antibiotic use in eastern Europe: a cross-national database study in coordination with the WHO Regional Office for Europe. Lancet Infect Dis. 2014;14:381–387. doi: 10.1016/S1473-3099(14)70071-4. [DOI] [PubMed] [Google Scholar]
- 3.Klein E.Y., Van Boeckel T.P., Martinez E.M., Pant S., Gandra S., Levin S.A., et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115:E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suda K.J., Hicks L.A., Roberts R.M., Hunkler R.J., Taylor T.H. Trends and seasonal variation in outpatient antibiotic prescription rates in the United States, 2006 to 2010. Antimicrob Agents Chemother. 2014;58:2763–2766. doi: 10.1128/AAC.02239-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peñalva G., Fernández-Urrusuno R., Turmo J.M., Hernández-Soto R., Pajares I., Carrión L., et al. Long-term impact of an educational antimicrobial stewardship programme in primary care on infections caused by extended-spectrum β-lactamase-producing Escherichia coli in the community: an interrupted time-series analysis. Lancet Infect Dis. 2020;20:199–207. doi: 10.1016/S1473-3099(19)30573-0. [DOI] [PubMed] [Google Scholar]
- 6.Ryu S., Kim S., Kim B.I., Klein E.Y., Yoon Y.K., Chun B.C. Temporal relationship between antibiotic use and respiratory virus activities in the Republic of Korea: a time-series analysis. Antimicrob Resist Infect Control. 2018;7:56. doi: 10.1186/s13756-018-0347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Havers F.P., Hicks L.A., Chung J.R., Gaglani M., Murthy K., Zimmerman R.K., et al. Outpatient Antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherman A.C., Babiker A., Sieben A.J., Pyden A., Steinberg J., Kraft C.S., et al. The effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mitigation strategies on seasonal respiratory viruses: a tale of 2 large metropolitan centers in the United States. Clin Infect Dis. 2021;72:e154–e157. doi: 10.1093/cid/ciaa1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng W., Yu Z., Liu S., Sun W., Ling F., Pan J., et al. Successful interruption of seasonal influenza transmission under the COVID-19 rapid response in Zhejiang Province, China. Public Health. 2020;189:123–125. doi: 10.1016/j.puhe.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poole S., Brendish N.J., Clark T.W. SARS-CoV-2 has displaced other seasonal respiratory viruses: results from a prospective cohort study. J Infect. 2020;81:966–972. doi: 10.1016/j.jinf.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H., Lee H., Song K.H., Kim E.S., Park J.S., Jung J., et al. Impact of public health interventions on seasonal influenza activity during the SARS-CoV-2 outbreak in Korea. Clin Infect Dis. 2020;73:e132–e140. doi: 10.1093/cid/ciaa672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuin M., Rigatelli G., Zuliani G., Roncon L. COVID-19 restrictive measures are changing the flu season in Italy. Minerva Med. 2021 doi: 10.23736/S0026-4806.21.07359-6. [DOI] [PubMed] [Google Scholar]
- 13.Vaughn V.M., Gandhi T.N., Petty L.A., Patel P.K., Prescott H.C., Malani A.N., et al. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (COVID-19): a multi-hospital cohort study. Clin Infect Dis. 2021;72:e533–e541. doi: 10.1093/cid/ciaa1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abelenda-Alonso G., Padulles A., Rombauts A., Gudiol C., Pujol M., Alvarez-Pouso C., et al. Antibiotic prescription during the COVID-19 pandemic: a biphasic pattern. Infect Control Hosp Epidemiol. 2020;41:1371–1372. doi: 10.1017/ice.2020.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peñalva G., Benavente R.S., Pérez-Moreno M.A., Pérez-Pacheco M.D., Pérez-Milena A., Murcia J., et al. Effect of the coronavirus disease 2019 pandemic on antibiotic use in primary care. Clin Microbiol Infect. 2021;S1198–743X:48–53. doi: 10.1016/j.cmi.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malcolm W., Seaton R.A., Haddock G., Baxter L., Thirlwell S., Russell P., et al. Impact of the COVID-19 pandemic on community antibiotic prescribing in Scotland. JAC-antimicrobial Resist. 2020;2 doi: 10.1093/jacamr/dlaa105. dlaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King L.M., Lovegrove M.C., Shehab N., Tsay S., Budnitz D.S., Geller A.I., et al. Trends in U.S. outpatient antibiotic prescriptions during the COVID-19 pandemic. Clin Infect Dis. 2020;29:ciaa1896. doi: 10.1093/cid/ciaa1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain A.Z., Paudyal V., Hadi M.A. Impact of the COVID-19 pandemic on the prescribing patterns of first-line antibiotics in English primary care: a longitudinal analysis of national prescribing dataset. Antibiotics (Basel) 2021;10:591. doi: 10.3390/antibiotics10050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodríguez-Baño J., Pérez-Moreno M.A., Peñalva G., Garnacho-Montero J., Pinto C., Salcedo I., et al. Outcomes of the PIRASOA programme, an antimicrobial stewardship programme implemented in hospitals of the Public Health System of Andalusia, Spain: an ecologic study of time-trend analysis. Clin Microbiol Infect. 2020;26:358–365. doi: 10.1016/j.cmi.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Consejería de Salud y Familias. Junta de Andalucía Sistema centinela de Vigilancia de la Gripe. https://juntadeandalucia.es/organismos/saludyfamilias/areas/salud-vida/vigilancia/paginas/vigilancia-transmisibles.html
- 21.WHO . 2020. Collaborating center for drug statistics methodology. DDD alterations from 2005–2020.https://www.whocc.no/atc_ddd_alterations__cumulative/ddd_alterations/ [Google Scholar]
- 22.Cochrane Effective Practice and Organisation of Care (EPOC) EPOC Resources for review authors; 2017. Interrupted time series (ITS) analyses.https://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/analysis_in_epoc_reviews.pdf [Google Scholar]
- 23.Fernández-Urrusuno R. 3rd ed. 2018. Guía de terapéutica antimicrobiana del área Aljarafe.http://www.juntadeandalucia.es/servicioandaluzdesalud/guiaterapeuticaaljarafe/guiaTerapeuticaAljarafe/guia/guia.asp [Google Scholar]
- 24.Zhu N., Aylin P., Rawson T., Gilchrist M., Majeed A., Holmes A. Investigating the impact of COVID-19 on primary care antibiotic prescribing in North West London across two epidemic waves. Clin Microbiol Infect. 2021;27:762–768. doi: 10.1016/j.cmi.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Pol A.C., Boeijen J.A., Venekamp R.P., Platteel T., Damoiseaux R.A.M.J., Kortekaas M.F., et al. Impact of the COVID-19 pandemic on antibiotic prescribing for common infections in The Netherlands: a primary care-based observational cohort study. Antibiotics (Basel) 2021;10:196. doi: 10.3390/antibiotics10020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Partridge E., McCleery E., Cheema R., Nakra N., Lakshminrusimha S., Tancredi D.J., et al. Evaluation of seasonal respiratory virus activity before and after the statewide COVID-19 shelter-in-place order in Northern California. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.35281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lepak A.J., Taylor L.N., Stone C.A., Schulz L.T., Anderson M.C., Fox B.C., et al. Association of changes in seasonal respiratory virus activity and ambulatory antibiotic prescriptions with the COVID-19 pandemic. JAMA Intern Med. 2021 doi: 10.1001/jamainternmed.2021.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han S.M., Greenfield G., Majeed A., Hayhoe B. Impact of remote consultations on antibiotic prescribing in primary health care: systematic review. J Med Internet Res. 2020;22 doi: 10.2196/23482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.