Abstract
Background
Self‐management interventions help people with chronic obstructive pulmonary disease (COPD) to acquire and practise the skills they need to carry out disease‐specific medical regimens, guide changes in health behaviour and provide emotional support to enable them to control their disease. Since the 2014 update of this review, several studies have been published.
Objectives
Primary objectives
To evaluate the effectiveness of COPD self‐management interventions compared to usual care in terms of health‐related quality of life (HRQoL) and respiratory‐related hospital admissions. To evaluate the safety of COPD self‐management interventions compared to usual care in terms of respiratory‐related mortality and all‐cause mortality.
Secondary objectives
To evaluate the effectiveness of COPD self‐management interventions compared to usual care in terms of other health outcomes and healthcare utilisation. To evaluate effective characteristics of COPD self‐management interventions.
Search methods
We searched the Cochrane Airways Trials Register, CENTRAL, MEDLINE, EMBASE, trials registries and the reference lists of included studies up until January 2020.
Selection criteria
Randomised controlled trials (RCTs) and cluster‐randomised trials (CRTs) published since 1995. To be eligible for inclusion, self‐management interventions had to include at least two intervention components and include an iterative process between participant and healthcare provider(s) in which goals were formulated and feedback was given on self‐management actions by the participant.
Data collection and analysis
Two review authors independently selected studies for inclusion, assessed trial quality and extracted data. We resolved disagreements by reaching consensus or by involving a third review author. We contacted study authors to obtain additional information and missing outcome data where possible. Primary outcomes were health‐related quality of life (HRQoL), number of respiratory‐related hospital admissions, respiratory‐related mortality, and all‐cause mortality. When appropriate, we pooled study results using random‐effects modelling meta‐analyses.
Main results
We included 27 studies involving 6008 participants with COPD. The follow‐up time ranged from two‐and‐a‐half to 24 months and the content of the interventions was diverse. Participants' mean age ranged from 57 to 74 years, and the proportion of male participants ranged from 33% to 98%. The post‐bronchodilator forced expiratory volume in one second (FEV1) to forced vital capacity (FVC) ratio of participants ranged from 33.6% to 57.0%. The FEV1/FVC ratio is a measure used to diagnose COPD and to determine the severity of the disease. Studies were conducted on four different continents (Europe (n = 15), North America (n = 8), Asia (n = 1), and Oceania (n = 4); with one study conducted in both Europe and Oceania).
Self‐management interventions likely improve HRQoL, as measured by the St. George’s Respiratory Questionnaire (SGRQ) total score (lower score represents better HRQoL) with a mean difference (MD) from usual care of ‐2.86 points (95% confidence interval (CI) ‐4.87 to ‐0.85; 14 studies, 2778 participants; low‐quality evidence). The pooled MD of ‐2.86 did not reach the SGRQ minimal clinically important difference (MCID) of four points. Self‐management intervention participants were also at a slightly lower risk for at least one respiratory‐related hospital admission (odds ratio (OR) 0.75, 95% CI 0.57 to 0.98; 15 studies, 3263 participants; very low‐quality evidence). The number needed to treat to prevent one respiratory‐related hospital admission over a mean of 9.75 months' follow‐up was 15 (95% CI 8 to 399) for participants with high baseline risk and 26 (95% CI 15 to 677) for participants with low baseline risk. No differences were observed in respiratory‐related mortality (risk difference (RD) 0.01, 95% CI ‐0.02 to 0.04; 8 studies, 1572 participants ; low‐quality evidence) and all‐cause mortality (RD ‐0.01, 95% CI ‐0.03 to 0.01; 24 studies, 5719 participants; low‐quality evidence).
We graded the evidence to be of ‘moderate’ to ‘very low’ quality according to GRADE. All studies had a substantial risk of bias, because of lack of blinding of participants and personnel to the interventions, which is inherently impossible in a self‐management intervention. In addition, risk of bias was noticeably increased because of insufficient information regarding a) non‐protocol interventions, and b) analyses to estimate the effect of adhering to interventions. Consequently, the highest GRADE evidence score that could be obtained by studies was ‘moderate’.
Authors' conclusions
Self‐management interventions for people with COPD are associated with improvements in HRQoL, as measured with the SGRQ, and a lower probability of respiratory‐related hospital admissions. No excess respiratory‐related and all‐cause mortality risks were observed, which strengthens the view that COPD self‐management interventions are unlikely to cause harm. By using stricter inclusion criteria, we decreased heterogeneity in studies, but also reduced the number of included studies and therefore our capacity to conduct subgroup analyses. Data were therefore still insufficient to reach clear conclusions about effective (intervention) characteristics of COPD self‐management interventions. As tailoring of COPD self‐management interventions to individuals is desirable, heterogeneity is and will likely remain present in self‐management interventions.
For future studies, we would urge using only COPD self‐management interventions that include iterative interactions between participants and healthcare professionals who are competent using behavioural change techniques (BCTs) to elicit participants' motivation, confidence and competence to positively adapt their health behaviour(s) and develop skills to better manage their disease. In addition, to inform further subgroup and meta‐regression analyses and to provide stronger conclusions regarding effective COPD self‐management interventions, there is a need for more homogeneity in outcome measures. More attention should be paid to behavioural outcome measures and to providing more detailed, uniform and transparently reported data on self‐management intervention components and BCTs. Assessment of outcomes over the long term is also recommended to capture changes in people's behaviour. Finally, information regarding non‐protocol interventions as well as analyses to estimate the effect of adhering to interventions should be included to increase the quality of evidence.
Plain language summary
Self‐management for people with chronic obstructive pulmonary disease
Review question We looked at the current evidence on the effects of self‐management interventions for people with chronic obstructive pulmonary disease (COPD). In particular, we assessed their effectiveness on health‐related quality of life (HRQoL) and hospital admissions related to COPD. We also wanted to assess whether self‐management interventions are safe by evaluating the number of deaths.
Background COPD is a common and long‐term lung condition that slowly worsens over the years, and causes symptoms such as breathlessness, coughing, wheezing and increased sputum (mucus) production. This leads to loss of well‐being (also known as reduction in HRQoL) in people with COPD. Self‐management interventions encourage people to develop the skills and behaviours they need to successfully manage their disease, and the emotional and practical issues that may go along with it. In this update, we reviewed the current evidence on the effects of self‐management on HRQoL, hospital admissions related to COPD, deaths from any cause and related to COPD, as well as other health outcomes.
Search date We searched for studies up until January 2020.
Study characteristics We included 27 studies, involving 6008 participants, that evaluated the effectiveness and safety of COPD self‐management interventions. The average age of the participants ranged between 57 and 74 years. Between 33% to 98% of the participants in the studies were male. Studies were conducted on four different continents (15 in Europe, eight in North America, one in Asia, and four in Oceania; with one study conducted in both Europe and Oceania). All studies had control groups of participants who received usual care – that is, care typical for people with COPD. The studies lasted between two‐and‐a‐half to 24 months.
Key results Self‐management interventions improved HRQoL in people with COPD compared to usual care, but this did not reach a clinically meaningful improvement. The number of participants with at least one hospital admission related to COPD was reduced amongst those who participated in a self‐management intervention. We found no difference in number of deaths between self‐management and usual care groups, which strengthens the view that COPD self‐management interventions are unlikely to cause harm. We have been strict about only including studies that met our definition of a COPD self‐management intervention. Despite this, the studies were still quite different from one another in terms of the intervention components used, duration of the self‐management intervention and the study populations. It should be noted, that heterogeneity in future interventions will be inevitable as individual tailoring of self‐management interventions is desirable; it will never be a 'one size fits all' intervention.
Quality of the evidence Our confidence in the evidence for the main findings in this review ranged from ‘very low’ to ‘moderate’, due to the nature of the COPD self‐management intervention – none of the studies prevented participants and personnel from knowing what treatment the participants were getting. Additionally, none of the studies provided detailed information about the extent to which participants adhere to the self‐management intervention or whether any further treatments were given during the course of the study. Consequently, study evidence could not be graded higher than ‘moderate’ in any of the studies.
Summary of findings
Summary of findings 1. Self‐management interventions compared to usual care for people with chronic obstructive pulmonary disease.
| Self‐management interventions compared to usual care for people with chronic obstructive pulmonary disease | |||||||
| Patients or population: people with chronic obstructive pulmonary disease (COPD) Settings: hospital, outpatient clinic, primary care, home‐based Intervention: COPD self‐management interventions Comparison: usual care | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Risk with usual care | Risk with self‐management interventions | Difference | |||||
| HRQoL Assessed with: St. George’s Respiratory Questionnaire adjusted total score Scale from: 0 to 100 Note: lower scores indicate better HRQoL Follow‐up range: 3 to 12 months |
The mean HRQoL ranged from 30.9 to 71.1 points | ‐ | 2.86 points lower (4.87 lower to 0.85 lower) | ‐ | 2778 (15 comparisons of studies) |
⊕⊕⊝⊝ Lowa | ‐ |
| Respiratory‐related hospital admissions Assessed with: number of participants with at least one respiratory‐related hospital admission Follow‐up range: 3 to 12 months |
317 per 1000 | 258 per 1000 (209 to 312) |
‐ | OR 0.75 (0.57 to 0.98) | 3263 (16 comparisons of studies) |
⊕⊝⊝⊝ Very lowb | ‐ |
| Respiratory‐related mortality Assessed with: number of respiratory‐related deaths Follow‐up range: 3 to 24 months |
4.2%# | 7.0% (3.8 to 12.5)# |
2.7% more participants (0.4 fewer to 8.3 more)# |
OR 1.70 (0.89 to 3.26)# | 1572 (8 comparisons of studies) |
⊕⊕⊝⊝ Lowc | Pooled risk difference of 0.01 (95% CI ‐0.02 to 0.04)# |
| All‐cause mortality Assessed with: number of all‐cause deaths Follow‐up range: 3 to 24 months |
8.4%# | 7.3% (5.1 to 10.4)# |
1.1% fewer participants (3.3 fewer to 2.0 more)# |
OR 0.86 (0.59 to 1.26)# | 5719 (25 comparisons of studies) |
⊕⊕⊝⊝ Lowd | Pooled risk difference of ‐0.01 (95% CI ‐0.03 to 0.01)# |
| All‐cause hospital admissions Assessed with: number of participants with at least one all‐cause hospital admission Follow‐up range: 3 to 12 months |
397 per 1000 | 367 per 1000 (318 to 415) |
‐ | OR 0.88 (0.71 to 1.08) | 2633 (11 comparisons of studies) |
⊕⊕⊕⊝ Moderatee | ‐ |
| Health status ‐ Dyspnoea Assessed with mMRC Dyspnoea Scale total score Scale from: 0 to 4 Note: lower scores indicate less dyspnoea Follow‐up range: 3 to 12 months |
The mean dyspnoea score ranged from 2.1 to 3.1 | ‐ | 0.31 lower (1.23 lower to 0.6 higher) | ‐ | 356 (3 comparisons of studies) |
⊕⊕⊝⊝ Lowf | ‐ |
| ED visits Assessed with: mean number of visits Follow‐up range: 12 to 24 months |
The mean number of ED visits ranged from 0.7 to 3.1 | ‐ | 0.52 lower (0.89 lower to 0.15 lower) | ‐ | 1939 (6 comparisons of studies) |
⊕⊕⊝⊝ Lowg | ‐ |
| Health status ‐ Anxiety and depression Assessed with HADS total score Scale from: 0 to 21 Note: higher scores indicate more active symptoms of anxiety and depression Follow‐up range: 3 to 24 months |
The mean anxiety score ranged from 4.7 to 10.2 The mean depression score ranged from 3.8 to 9.1 |
‐ | Anxiety: 0.57 lower (1.01 lower to 0.13 lower) Depression: 0.45 lower (0.80 lower to 0.10 lower) |
‐ | Anxiety: 1647 Depression: 1653 (9 comparisons of studies) |
⊕⊕⊕⊝ Moderateh | ‐ |
| COPD exacerbations Assessed with: number of COPD exacerbations per participant (regardless of definition) Follow‐up range: 12 to 24 months |
The mean number of COPD exacerbations ranged from 1.2 to 2.8 | ‐ | 0.06 lower (0.26 lower to 0.15 higher) | ‐ | 1401 (7 comparisons of studies) |
⊕⊕⊕⊝ Moderatei | ‐ |
|
*The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). #The absolute and relative effects do not include comparisons of studies that reported zero events (respiratory‐related mortality: two studies with no deaths and thus excluded; all‐cause mortality: three studies with no deaths and thus excluded). The reported effects are in this case overestimated and should be interpreted with caution. As a result, the pooled risk difference that includes all study data is more accurate. CI: confidence interval; COPD: chronic obstructive pulmonary disease; ED: emergency department; GRADE: Grading of Recommendation, Assessment, Development and Evaluation; HADS: Hospital Anxiety and Depression Scale; HRQoL: health‐related quality of life; mMRC: modified Medical Research Council; OR: odds ratio. | |||||||
|
GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | |||||||
aThe majority of the studies had high risk of bias. Heterogeneity was moderate (I2 = 60%) (risk of bias ‐1, inconsistency ‐1). bThe majority of the studies had high risk of bias. Heterogeneity was moderate (I2 = 49%). The 95% CI was wide (risk of bias ‐1, inconsistency ‐1, imprecision ‐1). cThe majority of the studies had high risk of bias. Heterogeneity was substantial (I2 = 63%) (risk of bias ‐1, inconsistency ‐1). dThe majority of the studies had high risk of bias. Heterogeneity was substantial (I2 = 63%) (risk of bias ‐1, inconsistency ‐1). eThe majority of the studies had high risk of bias (risk of bias ‐1). fThe majority of the studies had high risk of bias. Only three studies were included in this meta‐analysis (risk of bias ‐1, imprecision ‐1). gThe majority of the studies had high risk of bias. Heterogeneity was considerable (I2 = 96%) (risk of bias ‐1, inconsistency ‐1). hThe majority of the studies had high risk of bias (risk of bias ‐1). iThe majority of the studies had high risk of bias (risk of bias ‐1).
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a chronic progressive lung condition characterised by exacerbations — acute distressing of symptoms, such as increased dyspnoea, cough and wheeze, and increased and altered sputum production (Anthonisen 1987; Rodriguez‐Roisin 2000) — that cause impaired health‐related quality of life (HRQoL), increased hospitalisations and mortality (GOLD 2021). COPD is both preventable and, although not fully reversible, treatable (GOLD 2021). In 2019, COPD ranked third in the leading causes of death globally (WHO 2020). It is predicted that by 2060, there will be over 5.4 million deaths annually from COPD and related conditions (WHO 2018). Apart from personal distress, COPD confers a substantial and increasing economic and social burden on society (GOLD 2021), with its exacerbations accounting for most direct costs (Toy 2010). The high and growing prevalence of COPD makes it a major problem of chronic morbidity and mortality in health care worldwide.
Description of the intervention
Management of COPD is complex and can be difficult for people with COPD due to heterogeneous disease progression, high symptom burden and fluctuation of symptoms (Agusti 2010; Donaldson 2005; Kessler 2011). Self‐management interventions have been described as an essential part of COPD disease management. They aim to help people develop skills to manage the disease more effectively, and have the goal to empower the individual during all stages of the disease (Bourbeau 2009; Effing 2012). This is important for people with chronic disease, such as COPD, who are responsible for their day‐to‐day care over the duration of the illness (Lorig 2003).
Lorig and Holman were amongst the first to suggest that a successful self‐management intervention should include the following essential skills and attributes: problem‐solving, decision‐making, resource utilisation, the formation of a partnership between participant and healthcare professional, action‐planning and self‐tailoring (Lorig 2003). Skills mastery, modelling, interpretation of symptoms and social persuasion are believed to contribute to enhanced self‐efficacy in people with COPD (Lorig 2003). Self‐efficacy is defined as having the confidence to effectively manage one's health, and has been recognised as a powerful factor in inducing new health behaviours in individuals, such as smoking cessation, regular exercise or physical activity, diet habits and coping with breathlessness (Bourbeau 2004; Effing 2012). The debate on the definition and most effective content of self‐management was ongoing during the previous update of this review in 2014 (Zwerink 2014).
In 2016, an international expert group reached consensus regarding a definition of a COPD self‐management intervention (Effing 2016): "A COPD self‐management intervention is structured but personalised and often multi‐component, with goals of motivating, engaging, and supporting the patients to positively adapt their health behaviour(s) and develop skills to better manage their disease.
The ultimate goals of self‐management are: a) optimising and preserving physical health; b) reducing symptoms and functional impairments in daily life and increasing emotional well‐being, social well‐being, and quality of life; and c) establishing effective alliances with healthcare professionals, family, friends and community.
The process requires iterative interactions between patients and healthcare professionals who are competent in delivering self‐management interventions. These patient‐centred interactions focus on: 1) identifying needs, health beliefs, and enhancing intrinsic motivations; 2) eliciting personalised goals; 3) formulating appropriate strategies (e.g. exacerbation management) to achieve these goals; and if required 4) evaluating and re‐adjusting strategies. Behaviour change techniques are used to elicit patient motivation, confidence and competence. Literacy sensitive approaches are used to enhance comprehensibility.”
We developed our review inclusion criteria in line with the definition above.
Different frameworks have been developed to characterise the underlying mechanisms of changing the behaviour of an individual (Michie 2011). Behavioural change techniques (BCTs) are defined as “an observable, replicable, and irreducible component of an intervention designed to alter or redirect causal processes that regulate behaviour” (Michie 2013). These techniques are proposed to be an ‘active ingredient’ (e.g. feedback, self‐monitoring and reinforcement) and can be used alone or in combination, and in a variety of intervention forms (e.g. face‐to‐face, written or digital) (Michie 2013). BCTs are perceived as imperative to elicit motivation, confidence and competence of participants in COPD self‐management interventions (Effing 2016). Previous COPD self‐management intervention studies conclude that participant activation and long‐term behaviour change are crucial characteristics to achieve improvement of health status (Benzo 2012; Disler 2012; Effing 2012; Nici 2012; Nici 2014).
How the intervention might work
Self‐management interventions are directed towards behavioural change and include a variety of components, such as self‐treatment of exacerbations, symptom management, smoking cessation, physical activity and dietary intake. Due to a significant heterogeneity of content within self‐management interventions, the reported effects are diverse. This heterogeneity complicates the formulation of clear conclusions regarding effective intervention components and implementation in clinical practice. However, the current vision to personalise treatment based on participant characteristics will lead to more participant‐tailored treatment approaches, and heterogeneity will therefore in the future also be inevitable (Agusti 2014; Singh 2017; Trappenburg 2013). To be successful, a self‐management intervention has to lead to positive behaviour change in the individual behaviours targeted by the intervention (Bourbeau 2015). Primary health behaviour targets for COPD self‐management interventions are: adequate medication intake (e.g. adherence, inhalation technique), smoking cessation, increasing levels of physical activity and exercise, managing breathlessness, using energy conservation techniques, avoiding aggravating factors (e.g. smoke, pollution), and using stress management strategies (Bourbeau 2015). It is important to note that even when people with COPD are aware of the benefits of self‐management, this does not mean they will be motivated to positively adapt their health behaviour (Bourbeau 2015). However, if individuals are able to perceive the risks associated with a given condition, they may be more likely to seek health‐improving behaviours in order to prevent the condition or reduce its progression (Hayden 2009; Rosenstock 1974).
COPD self‐management interventions are associated with a reduced number of exacerbations days and hospitalisations and decreased healthcare costs, as well as improved HRQoL (Effing 2009; Lenferink 2017; Zwerink 2014). A 2017 Cochrane Review evaluated the effects of COPD self‐management interventions, including action plans for COPD exacerbations, compared with usual care (Lenferink 2017). In line with other COPD self‐management reviews, it concluded that COPD exacerbation action plans are associated with improvements in HRQoL and a lower probability of respiratory‐related hospital admissions (Lenferink 2017). Although no excessive all‐cause mortality risk was observed, results showed a small but significantly higher respiratory‐related mortality rate for self‐management (including an action plan for COPD exacerbations) compared to usual care (Lenferink 2017). Another systematic review published in 2017 found that COPD self‐management interventions generally improved HRQoL and, in addition, reduced emergency visits (Newham 2017). Furthermore, Newham and colleagues found that BCTs addressing mental health showed increased improvements in those outcomes (Newham 2017). Jonkman 2016 aimed to identify components of self‐management interventions for people with chronic conditions (chronic heart failure, COPD, type 2 diabetes mellitus) that affect improvements in HRQoL. They concluded that the duration of the intervention involving ongoing healthcare professional support showed positive associations with all‐cause hospital admissions. This conclusion reminds us that self‐management is not a time‐limited intervention, but an ongoing process of reviewing, problem‐solving, and collaboration between the healthcare professional and chronically ill person, which needs a whole systems approach for effective implementation (Jonkman 2016).
Why it is important to do this review
The original Cochrane Review regarding COPD self‐management interventions was published in 2003 (Monninkhof 2002; Monninkhof 2003). The first update of the review, published in 2007, concluded that self‐management interventions were associated with improved HRQoL and reduced hospital admissions with no indication of detrimental effects on the other health outcomes (Effing 2007). The second update of the review, published in 2014, strengthened the evidence for associations between the intervention and improved quality of life, reduced respiratory‐related hospitalisations and improved dyspnoea (Zwerink 2014). In addition, this update concluded that self‐management interventions were associated with reduced all‐cause hospitalisations (Zwerink 2014). However, because of heterogeneity amongst interventions, study populations, follow‐up time and outcome measures, it was not possible to formulate clear conclusions regarding effective components and characteristics of self‐management interventions (Zwerink 2014). The latest update of the review included studies until August 2011. Since then, multiple studies have been published and new opinions have been formed regarding the limitations and contents of self‐management interventions for people with COPD.
Previous systematic reviews regarding the effectiveness of COPD self‐management interventions recommended that further research should focus on: 1) identifying effective components of interventions and identifying participant‐specific factors that may modify these; and 2) characterisation of behavioural change theories and strategies that underpin COPD self‐management interventions (Jolly 2016; Jonkman 2016; Lenferink 2017; Newham 2017; Zwerink 2014). Therefore, in the current review, we intended to assess not only the effectiveness and safety of COPD self‐management interventions, but also tried to identify effective self‐management intervention characteristics (e.g. integration of various self‐management intervention components and behavioural change techniques).
Objectives
Primary objectives
To evaluate the effectiveness of COPD self‐management interventions compared to usual care in terms of HRQoL and respiratory‐related hospital admissions.
To evaluate the safety of COPD self‐management interventions compared to usual care in terms of respiratory‐related mortality and all‐cause mortality.
Secondary objectives
To evaluate the effectiveness of COPD self‐management interventions compared to usual care in terms of other health outcomes and healthcare utilisation.
To evaluate effective characteristics of COPD self‐management interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and cluster‐randomised trials (CRTs) assessing the effectiveness of self‐management interventions for people with COPD. For CRTs, we performed meta‐analyses only if they had been adjusted to account for clustering (or could be adjusted by ourselves). In line with the previous update, we excluded studies published before 1995, as we believe that the primary focus of self‐management interventions before 1995 consisted of improving knowledge through education rather than initiating and enabling sustained behavioural change (Zwerink 2014).
Types of participants
All included participants were required to have a diagnosis of COPD according to the GOLD classification criteria (that is, a post‐bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio < 0.70)(GOLD 2021), a measure used to diagnose COPD and to determine the severity of the disease, as shown by baseline FEV1/FVC ratio spirometry, or in case of uncertainty, confirmed by study authors. Therefore, inclusion using only e.g. International Classification of Diseases (ICD) codes (WHO 2019) was insufficient. We excluded participants with a primary diagnosis of asthma.
Types of interventions
To be included, self‐management interventions had to be defined as structured interventions for participants with COPD aimed at improvement of self‐health behaviours and self‐management skills using an iterative process in at least two of its intervention components (i.e. smoking cessation, self‐recognition of exacerbations, use of an exacerbation action plan, home‐based exercise or physical activity, diet, medication intake (e.g. adherence, inhalation technique), or coping with breathlessness). An iterative process was defined as an interaction between participants and healthcare professional(s), including at least two contact moments, in which goals were formulated and feedback was given to develop participants’ self‐management skills. Interventions needed to include techniques directed at achieving behavioural change. We included interventions only if they incorporated at least the two following BCT clusters, defined according to Michie 2013: ‘goals and planning’ and ‘feedback and monitoring’.
We excluded interventions classified as pulmonary rehabilitation or exercise classes offered in a hospital, at a rehabilitation centre or in a community‐based setting. We included interventions that incorporated unsupervised home‐based exercise programmes if they met all the other study criteria.
We included only studies with usual care as the comparator, defined as de facto routine clinical care.
Types of outcome measures
Primary outcomes
Health‐related quality of life (HRQoL)
Respiratory‐related hospital admissions
Respiratory‐related mortality
All‐cause mortality
Secondary outcomes
All‐cause hospital admissions
Use of (other) healthcare facilities (e.g. number of emergency department visits, number of all‐cause and respiratory‐related hospital admission days in total and per participant, general practitioner, number of nurse and specialist visits)
-
Number of COPD exacerbations, based on:
COPD symptom scores (e.g. symptom diary)
Courses of oral corticosteroids or antibiotics, or both
Health status (e.g. dyspnoea, impact of COPD on life, anxiety and/or depression)
Self‐efficacy
Days lost from work
Exercise capacity and physical activity
Self‐management behaviour
Patient activation
Health literacy
Search methods for identification of studies
Electronic searches
The previously published version of this review included searches up to August 2011 (Zwerink 2014). We re‐assessed all previously included studies for inclusion in this update. The search period for this update is 2011 to January 2020. Studies were identified from searches of the following databases and trials registries.
Cochrane Airways Register, through the Cochrane Register of Studies (CRS).
Cochrane Central Register of Controlled Trials (CENTRAL), through the CRS.
MEDLINE (Ovid) ALL.
EMBASE (Ovid).
ClinicalTrials.gov (www.ClinicalTrials.gov).
World Health Organization International Clinical Trials Registry Platform (ICTRP).
We searched all sources from 2011 up to 23 January 2020, with no restrictions on language or publication type. See Appendix 1 for details of the search strategies. We performed an updated database search from January 2020 to March 2021. We added potentially eligible studies from this search to 'Studies awaiting classification', and we will incorporate these into the review at the next update, if inclusion criteria are met.
Searching other resources
We checked reference lists of all primary studies, reviewed articles for additional references, and re‐evaluated the included studies from the previous version of this review against the updated inclusion and exclusion criteria.
Data collection and analysis
Selection of studies
Because of the large number of studies found, we used Cochrane's 'Screen4Me' workflow to help assess the results of our search for RCTs. Screen4Me includes three components: 1) known assessments: a service that matches records in the search results to records that have already been screened in Cochrane Crowd and have been labelled as 'RCT' or as 'not an RCT'; 2) the RCT classifier: a machine‐learning model that distinguishes RCTs from non‐RCTs; and if appropriate, 3) Cochrane Crowd: Cochrane's citizen science platform where 'the crowd' helps to identify and describe health evidence.
Following use of the Screen4Me workflow, any two of the team of review authors (JS, TE, AL, MB, JP, MZ or PV) independently assessed titles and abstracts of all references retrieved using Covidence software (Covidence 2016). Subsequently, two review authors (of JS, TE, AL, MB, JP or MZ) independently reviewed full‐text versions of potentially relevant reports to determine eligibility for inclusion based on the criteria stated above, using Covidence.
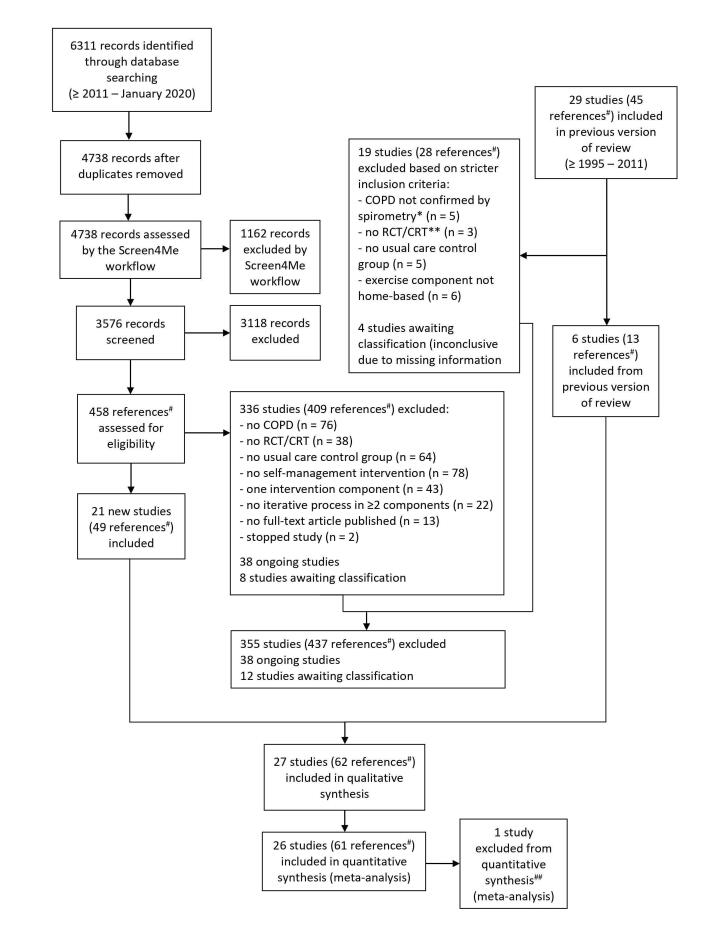
At the start of screening, we conducted calibration exercises to enhance the validity of the screening process. Therefore, all review authors independently assessed 50 titles and abstracts, and 10 full‐text articles. Subsequently, we compared screening results and discussed differences between review authors' judgements. We then updated a detailed worksheet to clarify the decision process. During the subsequent review process, any disagreements were resolved through discussion between the two review authors concerned. If consensus could not be reached, we consulted a third review author. Where necessary, we contacted authors of potentially eligible studies to ask for further information regarding inclusion criteria. Detailed information regarding this process can be found in the PRISMA flow diagram (Figure 1) and ‘Excluded studies’ section (Moher 2009).
1.
*FEV1/FVC ratio <0.7
**The previous version of this review included both RCTs and non‐RCTs
#References included full‐text articles, trial register abstracts, conference abstracts, and sub‐studies
##One study could not be included in any meta‐analyses because of insufficient available data
RCT: Randomised controlled trial; CRT: Cluster‐randomised controlled trial
Data extraction and management
Two review authors (of JS, TE, AL, MB, JP and MZ) independently extracted the following data from included studies using Covidence (Covidence 2016): relevant outcome measures, sample size, demographics of participants, disease severity, setting, duration and contents of the intervention. We used standard data extraction forms and spreadsheets for study characteristics and outcome data. At the start of the data extraction, review authors independently extracted data from five studies. We compared results, and discussed any differences between review authors. We then optimised the data extraction form for study characteristics and outcome data.
One review author (JS) transferred data into the Review Manager Web (RevMan Web) file(RevMan Web 2021). We double‐checked the accuracy of data entry for newly included studies by comparing data presented in the RevMan Web file with the data‐extraction forms (one of TE, AL, MB or JP).
Assessment of risk of bias in included studies
Two review authors (of JS, TE, AL, MB, JP and MZ) independently assessed risk of bias in the included studies using the Cochrane tool known as the 'risk of bias 2'(RoB 2) tool (Sterne 2019), as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019, hereafter referred to as the Cochrane Handbook), for the following five domains.
Bias arising from the randomisation process.
Bias due to deviations from intended interventions.
Bias due to missing outcome data.
Bias in measurement of the outcome.
Bias in selection of the reported result.
For the findings of each included study, two review authors (of JS, TE, AL, MB, JP and MZ) independently answered signalling questions to reach a risk of bias judgement related to each domain using a predefined algorithm. Subsequently, the overall risk of bias was assessed for each included study as 'high risk', 'low risk' or 'some concerns', using criteria detailed in the Cochrane Handbook (Higgins 2019). Again, we conducted calibration exercises at the start of the risk of bias assessments. Therefore, review authors independently assessed risk of bias in five studies. We compared results and discussed any differences between review authors. We resolved disagreements through discussion, and if necessary, involved a third review author (JS, TE, AL, MB, JP or MZ).
We report the grade of each potential bias per outcome of the included studies, together with a justification for our judgement, in the ‘Included studies’ section. In case of a CRT, we used a special variant of the RoB 2 tool (Sterne 2019), that focuses mainly on groups of participants from the clusters.
Assessment of bias in conducting the systematic review
We conducted this review according to our prespecified protocol. We detail deviations from the protocol in the ‘Differences between protocol and review’ section.
Measures of treatment effect
We synthesised study results using random‐effects modelling (REM) in RevMan Web (RevMan Web 2021), and displayed these in forest plots. For continuous outcomes, we reported mean differences (MDs) or the standardised mean differences (SMDs) with the 95% confidence intervals (CIs). We used final scores in our meta‐analyses if available, but if unavailable, we included the change from baseline scores. For dichotomous outcomes, we reported odds ratios (ORs) with corresponding 95% CIs or, in case of outcomes with few events, risk differences (RDs) with corresponding 95% CIs.
We determined the clinical relevance of treatment effects by using the minimal clinically important difference (MCID), when available. We calculated numbers needed to treat for an additional beneficial outcome (NNTB) for respiratory‐related hospitalisations, all‐cause hospitalisations, respiratory‐related mortality and all‐cause mortality, using pooled ORs and control group data from individual studies within the meta‐analysis with Visual Rx 4 (Visual Rx 2016). The calculation of NNTBs was performed in four steps: 1) we calculated the mean control event risks over the mean follow‐up duration of the studies with the highest and lowest baseline risks; 2) we calculated the usual care event risks per study (proportion (%) of participants who had at least one respiratory‐related hospital admission divided by the total number of usual care); 3) we made two equal groups, one including the studies with the highest baseline risks and one including the lowest baseline risks; and 4) we calculated the mean usual care event risk per group (using the same procedure as for calculating the risk per study).
Unit of analysis issues
The unit of analysis in the included RCTs was the participant. In case the unit of analysis was a cluster, we adjusted for this by inflating the standard errors, as outlined in Section 16.3.6 of the Cochrane Handbook (Higgins 2019). This method requires an intra‐cluster correlation coefficient (ICC). We ran sensitivity analyses for primary outcomes using adjustments of clustering assuming ICCs of 0.02 and 0.04. Furthermore, we included studies that compared more than two intervention groups in a meta‐analysis by making multiple pair‐wise comparisons. To avoid double‐counting of usual care group participants, we divided the usual care group number by two, to have two entries for the study in the meta‐analysis.
Dealing with missing data
In case of missing or incomplete data, we contacted study authors to request missing data. If study authors did not respond, we made a second ‐ and when necessary ‐ a third attempt to request missing data. If study authors did not respond after our third attempt, we analysed the available data and reported that data were missing. If we thought the missing data presented major bias, we took this into consideration in the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) rating for affected outcomes (Guyatt 2011). We listed the study authors who have provided us with data for this and previous versions of the review in the ‘Acknowledgements’.
Assessment of heterogeneity
We explored variability among studies using the I2 statistic (Higgins 2019). When substantial heterogeneity (I2 > 50%) was detected, we discussed possible explanations and critically reconsidered the appropriateness of a meta‐analysis. Furthermore, in the meta‐analyses, we used a REM (estimated mean of a distribution of effects), rather than a fixed‐effect model (FEM), to account for heterogeneity.
Assessment of reporting biases
We explored possible reporting bias by assessing asymmetry in funnel plots to determine whether studies selectively reported as indicated in the paragraph, ‘Assessment of risk of bias in included studies’. We conducted a funnel plot when at least ten studies had been included.
Data synthesis
After exploring whether pooling of study outcomes was possible, we calculated a summary statistic for each study, to describe the observed intervention effect in the same way for every study. If appropriate, we performed a meta‐analysis using RevMan Web (RevMan Web 2021). We considered a meta‐analysis when at least three studies reported sufficient data for the outcome. Because of the nature of the intervention analysed in this review, we expected clinical heterogeneity between the studies. We planned to perform meta‐analyses using a REM if pooling was possible, but considered a FEM if the included interventions were very similar.
For primary outcomes, we performed primary and secondary analyses. The primary analysis included the final study endpoint outcome scores, regardless of length of follow‐up. The secondary analyses included short‐term (≤ 6 months), medium‐term (> 6 to ≤ 12 months), and long‐term (> 12 months) follow‐up. For the short‐ and medium‐term follow‐up, follow‐up scores closest to 6 and 12 months, respectively, were included. For the long‐term follow‐up, final scores were included if the follow‐up was longer than 12 months. For secondary outcomes, we only performed meta‐analyses including the final study end point outcome scores, regardless of length of follow‐up.
Subgroup analysis and investigation of heterogeneity
We performed preplanned subgroup analyses when at least three studies could be included in each subgroup. We defined the following subgroup analyses a priori to explain possible heterogeneity between study results.
Duration of the intervention (< 8 weeks versus ≥ 8 weeks). No information is available regarding the most effective self‐management intervention duration. Effects of interventions with a shorter duration may well differ from those of longer duration.
Inclusion of participants in the acute phase (having an acute exacerbation of COPD) versus stable state (at least four weeks post exacerbation and six weeks post hospitalisation). Acute exacerbations may hamper self‐management improvements. Awareness of the clinical sequelae of acute exacerbations of COPD enables approaches such as early post‐exacerbation rehabilitation to mitigate its negative effects (Goldstein 2014).
COPD self‐management interventions delivered in different income countries (low‐ and middle‐income countries versus high‐income countries). We classified countries according to the World Bank list of economies (World Bank 2021). We expected a priori more room for improvement after the implementation of a self‐management intervention in low‐ and middle‐income countries compared to high‐income countries as we expected that some elements of self‐management interventions may already have been included as part of usual care in high‐income countries but not in low‐ and middle‐income countries. The latter also face challenges with COPD diagnosis and management, including poorly‐resourced primary care systems and lack of trained workforces (Mills 2014).
COPD self‐management interventions delivered in different care settings: primary care versus secondary and tertiary care. Self‐management interventions delivered in primary care may appear to be less effective (Jolly 2018). This may be driven by large heterogeneity in COPD populations, interventions and outcomes. We therefore decided that it is important to look at the effects in different healthcare settings separately.
-
Inclusion of the following self‐management intervention components:
COPD exacerbation action plan component (inclusion of a COPD exacerbation action plan component versus no COPD exacerbation action plan component in the self‐management intervention). An exacerbation action plan is defined as a guideline (a hard copy or via audiovisual media) for participants with COPD describing when and how to act in case of worsening COPD‐related symptoms, indicating (the onset of) an exacerbation. Inclusion of COPD exacerbation action plans may result in improved HRQoL and lower probability of respiratory‐related hospital admissions (Lenferink 2017).
Home‐based exercise or physical activity component (inclusion of a home‐based exercise or physical activity component in the self‐management intervention versus no exercise component in the self‐management intervention). Increased exercise capacity may result in better HRQoL and potentially fewer hospital admissions (McCarthy 2015).
Smoking cessation component (inclusion of a smoking cessation component in the self‐management intervention versus no smoking cessation component in the self‐management intervention). Smoking cessation may result in improved HRQoL (Cheruvu 2016; Van Eerd 2016).
Diet component (inclusion of a diet component in the self‐management intervention versus no diet component in the self‐management intervention) ‐ for example, evaluation and optimisation of participants' diet and nutritional intake.
COPD medication component (inclusion of a medication component in the self‐management intervention versus no medication component in the self‐management intervention) ‐ for example, advice about medication intake, adherence and inhalation technique.
Coping with breathlessness component (inclusion of a coping with breathlessness component in the self‐management intervention versus no coping with breathlessness component in the self‐management intervention).
Self‐recognition of COPD exacerbations component (inclusion of a self‐recognition of COPD exacerbations component in the self‐management intervention versus no self‐recognition of COPD exacerbations component in the self‐management intervention).
The effects of COPD self‐management interventions with and without use of digital technology. We expected that COPD self‐management interventions with use of digital technology may have an added positive impact on HRQoL, hospital admissions and exercise capacity (McLean 2011; McCabe 2017).
-
The integration of behavioural change techniques (BCTs) in COPD self‐management interventions. The BCT taxonomy (version 1) is a hierarchically structured, cross‐domain list of 93 distinct BCTs described in 16 different clusters: 1) Goals and planning, 2) Feedback and monitoring, 3) Social support, 4) Shaping of knowledge, 5) Natural consequences, 6) Comparison of behaviours, 7) Associations, 8) Repetition and substitution, 9) Comparison of outcomes, 10) Reward and threat, 11) Regulation, 12) Antecedents, 13) Identity, 14) Scheduled consequences, 15) Self‐belief, and 16) Covert learning (Michie 2013). BCTs applied in self‐management interventions were extracted by using the mobile BCT taxonomy application (BCT Taxonomy; Michie 2013). We only extracted data that were explicitly reported in published articles of included studies. We performed the following two subgroup analyses.
COPD self‐management interventions by integration of two BCTs versus less than two BCTs in the intervention (Michie 2013).
The number of BCT taxonomy clusters in COPD self‐management interventions: ‘lower or equal’ versus ‘higher’ than the median of BCT clusters found in all included interventions (Michie 2013).
We used the formal test for subgroup interactions in RevMan Web (RevMan Web 2021).
Sensitivity analysis
We planned the following sensitivity analyses, which we conducted using different assumptions to investigate the robustness of effect sizes found in this review.
Assumption of small‐study effects: to identify whether review findings were dependent on study characteristics (e.g. studies with low and high numbers of included participants), by using REM versus FEM.
Assumption of influencing outliers: to explore whether review findings were dependent on variation in results, by excluding those studies with outlying results from the analysis.
Summary of findings and assessment of the certainty of the evidence
Using the criteria outlined in the Cochrane Handbook (Higgins 2019), we created a Table 1 (SOF table), including key information concerning the quality of evidence, the magnitude of effect of the self‐management intervention and the sum of available data for the main outcomes. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it related to studies that contributed data to the meta‐analyses for prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook (Higgins 2019), by using GRADEpro GDT software. In the SOF table footnotes and comments, we included justifications for decisions to downgrade the quality of studies, to aid the reader’s understanding of the review.
Results
Description of studies
See Characteristics of included studies.
Results of the search
Searches over the period January 2011 until January 2020 identified 6311 titles and abstracts (Figure 1). After de‐duplication and pre‐screening by Screen4Me workflow, 3576 records remained. We identified 458 potentially eligible articles, from which 21 studies were included. In addition, six of 29 studies included in our previous update (1995 to 2011) met the stricter inclusion criteria of this update (Figure 1). Therefore, a total of 27 studies (62 references) have been included in this review. One of these 27 studies could not be included in any quantitative syntheses (meta‐analyses) because of insufficient data (Emery 1998). Another study included two intervention groups versus one usual care group (Coultas 2005); all three study groups were included in meta‐analyses.
An update search in March 2021 identified 1280 titles and abstracts. After de‐duplication and prescreening by Screen4Me workflow, 640 records remained. We identified 55 potentially eligible articles. From these, 22 studies were excluded; six studies were classified as ongoing; 26 studies await classification; and one study ‐ Ozturk 2020 ‐ will be fully incorporated in a future update of this review, if the study criteria of this future update remain unchanged.
Included studies
We tabulated details of the 27 included studies (Benzo 2016; Bischoff 2012; Bösch 2007; Bourbeau 2003; Bringsvor 2018; Bucknall 2012; Coultas 2005; Emery 1998; Fan 2012; Ferrone 2019; Gallefoss 1999; Hernández 2015; Johnson‐Warrington 2016; Jolly 2018; Jonsdottir 2015; Kessler 2018; Lenferink 2019; Liang 2019; Martin 2004; Mitchell 2014; Rice 2010; Rose 2018; Sanchez‐Nieto 2016; Tabak 2014; Titova 2015; Walters 2013; Wang 2019). Participant characteristics can be found in Table 2, and the intervention and follow‐up details in Table 3. Coultas 2005 used two intervention groups and one usual care group. Two of the 27 included studies were CRTs (Liang 2019; Walters 2013); the others were RCTs. Because Walters 2013 did not adjust their reported outcomes for clustering, we manually adjusted the data using a calculated average cluster size of 5.8710 participants (i.e. 182 participants across 31 practices) and an ICC of 0.05, resulting in a design effect of 1.24.
1. Characteristics of participants in included studies.
| Study |
Randomised COPD participants (number) |
Lost to follow‐up (%) |
Age (years; mean (SD)) |
Gender (% male) |
Current smokers (%) |
FEV1/FVC ratio (SD) |
FEV1% predicted (SD) |
|||||||
| Self‐management | Usual care | Self‐management | Usual care | Self‐management | Usual care | Self‐management | Usual care | Self‐management | Usual care | Self‐management | Usual care | Self‐management | Usual care | |
| Benzo 2016 | 108 | 107 | 14.8 | 0.9 | 67.9 (9.8) | 68.1 (9.2) | 43 | 48 | NR | NR | 48.2 (13.8) | 47.7 (13.8) | 40.5 (17.1) | 40.3 (17.2) |
| Bischoff 2012 | 55 | 55 | 10.9* | 20* | 65.5 (11.5) | 63.5 (10.3) | 67 | 51 | 29 | 33 | 43 (78)** | 38 (69)** | 66.3 (16.5) | 67.0 (18.0) |
| Bösch 2007 | 38 | 12 | 21.1* | 8.3* | 63.8 (8.4) | 64.6 (6.8) | NR | NR | 13.3 | 27.3 | NR | NR | 45.9 (17.5) | 47.8 (16.9) |
| Bourbeau 2003 | 96 | 95 | 10.4 | 16.8 | 69.4 (6.5) | 69.6 (7.4) | 52 | 59 | 25 | 26 | 46 | 45 | NR | NR |
| Bringsvor 2018 | 92 | 90 | 31.5* | 22.2 | 68.5 (8.16) | 69.3 (9.02) | 59 | 63 | NR | NR | 45.2 (12.4) | 45.1 (12.7) | 45.2 (14.4) | 44.8 (16.2) |
| Bucknall 2012 | 232 | 232 | 16.8 | 21.1 | 70.0 (9.3) | 68.3 (9.2) | 38 | 35 | 39 | 39 | 46.4 (0.12) | 45.4 (0.12) | 41.2 (13.4) | 39.8 (13.8) |
| Coultas 2005#,a | 72 | 73 | 31.9 | 30.1 | 68.3 (6.6) | 68.8 (10.4) | 42.9 | 53.8 | 28.6 | 27.5 | 48.08 (12.35) | 52.05 (12.99) | NR | NR |
| Coultas 2005#,b | 72 | 73 | 29.2 | 30.1 | 70.1 (7.0) | 68.8 (10.4) | 32.7 | 53.8 | 23.5 | 27.5 | 49.85 (11.18) | 52.05 (12.99) | NR | NR |
| Emery 1998 | 25 | 25 | 8.0* | 0.0* | 67.4 (5.9) | 67.4 (5.9) | 40 | 48 | 14 | 20 | 45 (11) | 43 (12) | 43 (18) | 43 (18) |
| Fan 2012 | 209 | 217 | 17.2 | 9.2 | 66.2 (8.4) | 65.8 (8.2) | 97.6 | 96.3 | 28.2 | 27.2 | 47 (12) | 47 (12) | 38.2 (14.3) | 37.8 (14.5) |
| Ferrone 2019 | 84 | 84 | 14.3 | 11.9 | 68.6 (9.6) | 67.9 (9.8) | 40.5 | 52.4 | 39.3 | 57.1 | 55.6 (11.8) | 53.6 (10.4) | 55.5 (14.5) | 53.2 (14.7) |
| Gallefoss 1999 | 31 | 31 | 16.1 | 12.9 | 57 (9) | 58 (10) | 48 | 52 | 39 | 39 | 55 (9) | 52 (10) | 59 (9) | 56 (11) |
| Hernández 2015 | 76 | 84 | 22.4 | 34.5 | 73 (8) | 75 (9) | 83 | 86 | 13 | 14 | 47 (13) | 47 (15) | 41 (19) | 44 (20) |
| Johnson‐Warrington 2016 | 38 | 39 | 10.5 | 7.7 | 67.6 (8.5) | 68.3 (7.7) | 38.4 | 33.3 | 35.9 | 46.2 | 47.1 (14.0) | 42.8 (10.5) | 40.5 (15.7) | 42.5 (11.7) |
| Jolly 2018 | 289 | 288 | 14.5 | 2.4 | 70.7 (8.8) | 70.2 (7.8) | 63 | 64 | 26 | 19 | NR | NR | 71.2 (18.9) | 72.1 (18.7) |
| Jonsdottir 2015 | 60 | 59 | 20.0 | 11.9 | 59.4 (4.7) | 58.7 (4.4) | 39.6 | 51.9 | 50.0 | 69.2 | NR | NR | 54.0 (17.6) | 60.9 (17.3) |
| Kessler 2018 | 172 | 173 | 20.3 | 26.0 | 67.3 (8.9) | 66.6 (9.6) | 69.4 | 69.8 | 21.7 | 21.0 | 45.7 (11.3) | 43.7 (11.3) | 37.8 (12.4) | 36.4 (12.3) |
| Lenferink 2019 | 102 | 99 | 16.7 | 15.2 | 68.8 (9.0) | 68.2 (8.9) | 64.7 | 63.6 | 19.6 | 20.2 | 49.3 (14.3) | 48.5 (12.2) | 53.4 (16.1) | 50.7 (14.3) |
| Liang 2019 | 157 | 115 | 28.0 | 33.0 | 66.6 (10.8) | 61.7 (10.1) | 60.5 | 62.6 | 53.5 | 71.3 | 57 (13) | 57 (10) | 69.0 (20.5) | 70.8 (19.3) |
| Martin 2004 | 44 | 49 | 20.5 | 8.2 | 71.1 (68.7, 73.5)## | 69.1 (63.5, 74.7)## | 34.1 | 65.3 | NR | NR | NR | NR | 35.4 (31.6, 39.2)## | 34.3 (31.2, 37.4)## |
| Mitchell 2014 | 89 | 89 | 27.0 | 18.0 | 69 (8) | 69 (10.1) | 60.7 | 49.5 | 20.2 | 22.1 | 49.8 (13.4) | 50.5 (11.1) | 56.0 (16.8) | 60.0 (17.4) |
| Rice 2010 | 372 | 371 | 9.7 | 12.9 | 69.1 (9.4) | 70.7 (9.7) | 97.6 | 98.4 | 21.6 | 23.0 | 53.0 (14.5) | 54.4 (14.3) | 36.1 (14.5) | 38.2 (14.4) |
| Rose 2018 | 237 | 238 | 12.7 | 19.7 | 71 (9.2) | 71 (9.7) | 50 | 44 | 23 | 26 | 50 (12.6) | 52 (13.0) | 43 (17.0) | 45 (17.8) |
| Sanchez‐Nieto 2016 | 51 | 45 | 7.8$ | 15.6$ | 68.2 (7.2) | 67.1 (6.8) | 92.2 | 88.9 | 37.3 | 35.6 | 53 (17) | 55 (10) | 47.3 (14.4) | 44.3 (11.9) |
| Tabak 2014 | 15 | 14 | 33.3$ | 71.4$ | 64.1 (9.0) | 62.8 (7.4) | 50.0 | 50.0 | 36.4 | 33.3 | 36.5 (29.5‐51.0)$$ | 33.5 (26.0‐52.0)$$ | 50.0 (33.3‐61.5)$$ | 36.0 (26.0‐53.5)$$ |
| Titova 2015^ | 91 | 81 | 44.0 | 39.5 | 73.6 (9.2) | 72.2 (9.4) | 42.9 | 42.5 | 33.0 | 40.0 | NR | NR | 33.6 (9.9) | 33.4 (9.4) |
| Walters 2013 | 90 | 92 | 17.8 | 13.0 | 68.2 (7.9) | 67.3 (7.6) | 54 | 51 | 48 | 36 | 56 (12) | 50 (11) | 54.0 (13.4) | 56.4 (13.2) |
| Wang 2019 | 77 | 77 | 6.5 | 7.8 | 68.7 (6.2) | 69.2 (6.1) | 76.6 | 80.5 | 44.2 | 41.6 | 55.2 (18.2) | 56.7 (16.9) | 58.4 (17.3) | 59.2 (18.2) |
COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; NR: not reported; SD: standard deviation.
*No deaths reported and included in these data; **Post‐bronchodilator FEV1/FVC < lower limit of normal; #Study with one usual care group and two intervention groups, number of participants in usual care group halved in meta‐analyses; ##mean (95% confidence interval); $unclear whether the deaths were included in these data (self‐management: 0; usual care: 2); $$median (interquartile range); ^Different baseline data reported in 2015 and 2017 articles, data of 2017 article included in this Table; anurse‐assisted medical management intervention group; bnurse‐assisted collaborative management intervention group
2. Characteristics of interventions in included studies.
| Study |
Follow‐up (months) |
Setting; provision intervention | Time period intervention (months); duration of sessions | Included components with iterative process |
| Benzo 2016 | 12 | Outpatient clinic | 12; 2 FTF individual sessions (first visit 120 min, second visit not reported) and 6 phone calls (mean duration 28.6 min (SD 10.0). |
Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan Home‐based exercise or physical activity component Coping with breathlessness |
| Bischoff 2012 | 24 | General practice | 24; 2‐4 FTF individual sessions (60 min each) scheduled in 4 to 6 consecutive weeks, 6 phone calls |
Smoking cessation (optional) Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan: Home‐based exercise or physical activity component (optional) Diet COPD medication intake (i.e. adherence, inhalation technique) Coping with breathlessness Managing anxiety and stress |
| Bösch 2007 | 12 | Outpatient clinic; (University) hospital |
12; 4 FTF group sessions (120 min each) and final session scheduled 6 weeks later |
Smoking cessation (optional) Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan Home‐based exercise or physical activity component Diet COPD medication intake (i.e. adherence, inhalation technique) Coping with breathlessness Leisure activities and travelling |
| Bourbeau 2003 | 12 (24*) | (University) hospital | 12; 7 FTF individual sessions (60 min each) scheduled in 7 to 8 consecutive weeks, 18 phone calls |
Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan Home‐based exercise or physical activity component (optional) Diet COPD medication intake (i.e. adherence, inhalation technique) Coping with breathlessness Leisure activities and travelling Energy conservation during day‐by‐day activities Relaxation exercises Adopting a healthy lifestyle Long‐term oxygen (optional) |
| Bringsvor 2018 | 3 | Meeting locations in the participants’ municipalities. | 2.5; 11 FTF group sessions (120 min) scheduled weekly |
Smoking cessation (optional) Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan Home‐based exercise or physical activity component Diet COPD medication intake (i.e. adherence, inhalation technique) Coping with breathlessness Psychological issues Information about the healthcare system, including local, regional and national “offers” for persons with COPD |
| Bucknall 2012 | 12 | Home‐based | 12; 4 FTF individual sessions (40 min each) in 2 months, at least 6 subsequent home visits, 828 phone calls intervention group |
Smoking cessation Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan Diet (optional) COPD medication intake (i.e. adherence, inhalation technique) Coping with breathlessness |
| Coultas 2005a | 6 | Home‐based | 6; 1 FTF individual session (mean duration 64 min (SD 23.1) and mean 6 (SD 1.8) phone calls (mean duration 10 min (SD 5.4) |
Smoking cessation (optional) Self‐recognition of COPD exacerbations (optional) Use of a COPD exacerbation action plan (optional) COPD medication intake (i.e. adherence, inhalation technique) (optional) |
| Coultas 2005b | 6 | Home‐based | 6; 1 FTF individual session (mean duration 64 min (SD 23.1) and mean 6 (SD 1.8) phone calls (mean duration 10 min (SD 5.4) |
Smoking cessation (optional) Self‐recognition of COPD exacerbations (optional) Use of a COPD exacerbation action plan (optional) COPD medication intake (i.e. adherence, inhalation technique) (optional) |
| Emery 1998 | 2.5 | Rehabilitation centre; (University) hospital |
2.5; 26 FTF group sessions (16 lectures of 60 min and 10 management sessions of 60 min) |
Self‐recognition of COPD exacerbations COPD medication intake (i.e. adherence, inhalation technique) Coping with breathlessness Relaxation exercises Coping skills training |
| Fan 2012 | 12# | Outpatient clinic | 12; 4 FTF individual sessions (90 min each) scheduled weekly, 1 FTF group session, 6 phone calls (duration not specified) |
Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan COPD medication intake (i.e. adherence, inhalation technique) |
| Ferrone 2019 | 12 | General practice | 9; 2 FTF individual sessions (first visit 60 min (baseline evaluation) and 5 to 7 min (encounter with physician) and second visit after 3 months of 45 min) and either a phone call or FTF visit at 6 and 9 months (15 to 30 min each) |
Smoking cessation (optional) Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan Home‐based exercise or physical activity component COPD medication intake (i.e. adherence, inhalation technique) Coping with breathlessness |
| Gallefoss 1999 | 12 | Outpatient clinic | 1‐2; 1 or 2 FTF individual sessions by a nurse and 1 or 2 by physiotherapist (40 min each), 2 FTF group sessions (120 min each) |
Smoking cessation (optional) Self‐recognition of COPD exacerbations COPD medication intake (i.e. adherence, inhalation technique) Coping with breathlessness |
| Hernández 2015 | 12 (84$) | Outpatient clinic; (University) hospital |
12; Participants with no mobility problems: 1 FTF individual session (40 min) at home by primary care team, 3 FTF group sessions at outpatient clinic (2 x 90 min, 1 x 120 min) Participants with mobility problems: 4 FTF individual sessions (15 min each), 1 FTF individual session (120 min) or 1 FTF group session (40 min), all at home by primary care team All participants: Web based calls at least once per month (15 min each) |
Smoking cessation (optional) Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan Home‐based exercise or physical activity component Diet COPD medication intake (i.e. adherence, inhalation technique) Coping with breathlessness Comorbid condition (no further explanation regarding the content) |
| Johnson‐Warrington 2016 | 3 | (University) hospital; Home‐based |
3; 1 FTF individual session (30 to 45 min) and 6 phone calls (5 to 20 min each) |
Smoking cessation (optional) Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan Home‐based exercise or physical activity component COPD medication intake (i.e. adherence, inhalation technique) Coping with breathlessness |
| Jolly 2018 | 12 | General practice; Home‐based |
5.5; 4 individual phone calls (first call 35 to 60 min, other calls 15 to 20 min) scheduled at 3, 7 and 11 weeks |
Smoking cessation (optional) Self‐recognition of COPD exacerbations (optional) Use of a COPD exacerbation action plan (optional) Home‐based exercise or physical activity component COPD medication intake (i.e. adherence, inhalation technique) |
| Jonsdottir 2015 | 12 | Clinical research centre located on a university‐hospital | 1‐2; 1 FTF group session (120 min), 3 to 4 FTF individual sessions (30 to 45 min), and 4 phone calls (5 to 10 min each) |
Smoking cessation (optional) Self‐recognition of COPD exacerbations Home‐based exercise or physical activity component Diet COPD medication intake (i.e. adherence, inhalation technique) Coping with breathlessness Utilization of health care Prevent further decline of disease within the aim of enhancing health of patient and family Coping with feelings of shame and guilt |
| Kessler 2018 | 12 | Outpatient clinic Home‐based |
12; 1 FTF group session (90 to 120 min), 4 FTF individual sessions (60 to 90 min), and multiple phone calls (duration not specified) |
Smoking cessation (optional) Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan Home‐based exercise or physical activity component (optional) Diet COPD medication intake (i.e. adherence, inhalation technique) Coping with breathlessness |
| Lenferink 2019 | 12 | (University) hospital | 12; 2‐3 FTF group sessions (120 to 240 min), 2 FTF individual sessions (60 min), and 3 phone calls (10 to 15 min each) |
Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan COPD medication intake (i.e. adherence, inhalation technique) Coping with breathlessness Self‐recognition of increase in in comorbid symptoms and use of an action plan for these comorbidities (CHF, IHD, anxiety and depression) |
| Liang 2019 | 12 | General practice | 2; 3 FTF individual sessions (duration not specified), and 9 phone calls (duration not specified) |
Smoking cessation (optional) Home‐based exercise or physical activity component COPD medication intake (i.e. adherence, inhalation technique) |
| Martin 2004 | 12 | General practice; (University) hospital; Home‐based; Ambulance service |
12 4 FTF individual sessions and respiratory nurse visits at 3, 6 and 12 months |
Use of a COPD exacerbation action plan COPD medication intake (i.e. adherence, inhalation technique) Guidance regarding treatment for coexisting conditions (e.g. when/how to use oxygen therapy, and when to use diuretics) |
| Mitchell 2014 | 6 | General practice; Home‐based |
1; 1 FTF individual session (30 to 45 min) by a physiotherapist and 2 phone calls (duration not specified) |
Smoking cessation (optional) Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan Home‐based exercise or physical activity component Management of psychological consequences (e.g. dealing with anger, depression, disease acceptance) |
| Rice 2010 | 12 | Outpatient clinic | 12; 1 FTF group session (60 to 90 min) by a respiratory therapist case manager, 12 monthly phone calls (10 to 15 min each) |
Smoking cessation (optional) Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan COPD medication intake (i.e. adherence, inhalation technique) |
| Rose 2018 | 12 | Outpatient clinic | 12; 1 FTF individual session (40 min), 21 phone calls (duration not specified) |
Smoking cessation (optional) Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan COPD medication intake (i.e. adherence, inhalation technique) Advance care planning |
| Sanchez‐Nieto 2016 | 12 | (University) hospital | 3; 1 FTF group session (40 min), and 3 FTF individual sessions (20 min each) |
Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan Home‐based exercise or physical activity component COPD medication intake (i.e. adherence, inhalation technique) |
| Tabak 2014 | 9 | Outpatient clinic; Primary care physiotherapy practices |
9; 2 FTF group sessions (90 min each) by a nurse practitioner, 1 FTF individual session and 1 x intake by the physiotherapist, additional meetings after 1, 3, 6 and 9 months |
Smoking cessation (optional) Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan Home‐based exercise or physical activity component |
| Titova 2015 | 24 | Home‐based | 24; 6 FTF individual sessions (1 x at discharge, 5 x home visits at 3 and 14 days, and at 6, 12, 24 months) by the specialist nurse, 1 e‐learning programme (15 min), at least 24 phone calls |
Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan Coping with breathlessness |
| Walters 2013 | 12 | Home‐based | 12; 16 individual phone calls (30 min each) |
Smoking cessation (optional) Self‐recognition of COPD exacerbations Use of a COPD exacerbation action plan Home‐based exercise or physical activity component (optional) Diet (optional) COPD medication intake (i.e. adherence, inhalation technique) (optional) Alcohol (optional) Psychosocial (optional) |
| Wang 2019 | 12 | (University) hospital | 3; 5 to 6 FTF individual sessions (45 min each), 3 home visits (45 to 60 min each), and weekly phone calls scheduled over 3 months (10 to 15 min each) |
Smoking cessation (optional) Home‐based exercise or physical activity component COPD medication intake (i.e. adherence, inhalation technique) Coping with breathlessness Respiratory muscle training (pursed lip breathing and abdominal breathing) Coughing techniques Long‐term home oxygen therapy (optional) |
COPD: chronic obstructive pulmonary disease; FTF: face‐to‐face; min: minute(s)
*Second year data based on provincial health insurance and hospitalisation database records; #study was terminated early after a mean follow‐up time of 250 days; $two groups were passively followed up for 6 additional years; anurse‐assisted medical management intervention group; bnurse‐assisted collaborative management intervention group
Participants and recruitment
A total of 6008 participants (self‐management intervention n = 3074; usual care n = 2934) were assessed in the 27 included studies (Table 2). Dropout rates in the studies ranged from 0% to 71.4%, and a total of 5125 (85%) participants completed the study follow‐up.
Interventions
The content of self‐management interventions in the 27 included studies was diverse (Table 3). The follow‐up duration was three months or less in three (11%) studies, six months in two (7%), nine months in one (4%), 12 months in 18 studies (67%), and 24 months in three studies (11%). Self‐management interventions were delivered individually in 15 (56%) studies (Benzo 2016; Bischoff 2012; Bourbeau 2003; Bucknall 2012; Coultas 2005; Ferrone 2019; Johnson‐Warrington 2016; Jolly 2018; Liang 2019; Martin 2004; Mitchell 2014; Rose 2018; Titova 2015; Walters 2013; Wang 2019), in small groups in three studies (11%) (Bösch 2007; Bringsvor 2018; Emery 1998), and included both individual and group sessions in nine (33%) studies (Fan 2012; Gallefoss 1999; Hernández 2015; Jonsdottir 2015; Kessler 2018; Lenferink 2019; Rice 2010; Sanchez‐Nieto 2016; Tabak 2014). The median duration of the intervention, including self‐management reinforcement, was nine months (interquartile range (IQR) 3.0 to 12.0). The intervention duration was three months or less in nine (33%) studies (Bringsvor 2018; Emery 1998; Gallefoss 1999; Johnson‐Warrington 2016; Jonsdottir 2015; Liang 2019; Mitchell 2014; Sanchez‐Nieto 2016; Wang 2019), over three months and up to six months in two (7%) studies (Coultas 2005; Jolly 2018), nine months in two (7%) studies (Ferrone 2019; Tabak 2014), 12 months in 12 (44%) studies (Benzo 2016; Bösch 2007; Bourbeau 2003; Bucknall 2012; Fan 2012; Hernández 2015; Kessler 2018; Lenferink 2019; Martin 2004; Rice 2010; Rose 2018; Walters 2013), and 24 months in two (7%) studies (Bischoff 2012; Titova 2015).
A ‘COPD exacerbation action plan’ was part of the self‐management intervention in 23 (85%) studies; both ‘self‐recognition of COPD exacerbations’ and ‘a medication component’ were each part of 22 study interventions (81%); ‘home‐based exercise or physical activity component’ was part of 17 study interventions (63%); a ‘coping with breathlessness component’ was present in 16 study interventions (59%); a ‘smoking cessation component’ was part of 15 study interventions (56%); and a diet component was present in nine study interventions (33%).
A median of 4.0 (IQR 3.0 to 7.0) BCT clusters was detected per study intervention, with a minimum of two BCT clusters (Bischoff 2012; Bösch 2007; Bringsvor 2018; Coultas 2005) and a maximum 11 BCT clusters (Johnson‐Warrington 2016; Mitchell 2014). The BCT clusters that were integrated in COPD self‐management interventions groups to promote the uptake and optimal use of COPD self‐management behaviour patterns were: goals and planning (n = 28, all intervention groups of 27 studies, one study with two intervention groups); feedback and monitoring (n = 28, all intervention groups of 27 studies, one study with two intervention groups); shaping knowledge (n = 19, all but nine comparisons (Bischoff 2012; Bösch 2007; Bringsvor 2018; Coultas 2005; Emery 1998; Liang 2019; Rose 2018; Titova 2015)); social support (n = 16, all but twelve comparisons (Bischoff 2012; Bösch 2007; Bourbeau 2003; Bringsvor 2018; Coultas 2005; Emery 1998; Ferrone 2019; Gallefoss 1999; Kessler 2018; Martin 2004; Sanchez‐Nieto 2016; Tabak 2014)); natural consequences (n = 9 (Bucknall 2012; Fan 2012; Gallefoss 1999; Johnson‐Warrington 2016; Jolly 2018; Jonsdottir 2015; Liang 2019; Mitchell 2014; Wang 2019)); repetition and substitution (n = 9 (Benzo 2016; Bourbeau 2003; Bucknall 2012; Fan 2012; Johnson‐Warrington 2016; Jonsdottir 2015; Lenferink 2019; Mitchell 2014; Titova 2015)); regulation (n = 7 (Emery 1998; Fan 2012; Gallefoss 1999; Johnson‐Warrington 2016; Jolly 2018; Jonsdottir 2015; Mitchell 2014)); comparison of behaviour (n = 6 (Bourbeau 2003; Bucknall 2012; Hernández 2015; Johnson‐Warrington 2016; Jonsdottir 2015; Mitchell 2014)); associations (n = 6 (Bourbeau 2003; Fan 2012; Gallefoss 1999; Hernández 2015; Johnson‐Warrington 2016; Mitchell 2014)); antecedents (n = 6 (Bourbeau 2003; Emery 1998; Gallefoss 1999; Johnson‐Warrington 2016; Jolly 2018; Mitchell 2014)); identity (n = 3 (Johnson‐Warrington 2016; Jonsdottir 2015; Mitchell 2014)); self‐belief (n = 3 (Bucknall 2012; Jonsdottir 2015; Walters 2013)); comparison of outcomes (n = 1 (Jolly 2018)); and reward and threat (n = 1 (Liang 2019)). There were no scheduled consequences or covert learning reported in any of the self‐management interventions.
Adherence
Half of the studies (n = 13) reported details regarding participants’ adherence to the self‐management intervention. Of these, nine studies reported adherence as the number or percentage of sessions attended by participants. In Emery 1998, the self‐management group attended approximately 88% of both the education and stress management sessions. In Gallefoss 1999, they used a per‐protocol analysis and withdrew intervention group participants who did not attend the individual or group sessions (n = 5, 16%). In Bischoff 2012, the total number of sessions that were offered to participants depended on participants' needs, with a minimum of two. Participants in Bischoff 2012 received a mean of 3.4 (SD 1.5) sessions; 13% did not attend any sessions or telephone calls. Fan 2012 reported that, during the entire follow‐up period, eight of 209 participants in the self‐management intervention group and 10 of 217 participants in the usual care group either did not attend any scheduled visits or formally withdrew from the study. The study authors also reported that in the self‐management intervention group, 87% completed all four individual educational visits and 57% completed the scheduled group visit (Fan 2012). Early termination after the intervention was enforced by the Data and Safety Monitoring Committee and the apparently low attendance rate of the group visit may well be a consequence (Fan 2012).
Tabak 2014 reported that the self‐management module on the web portal, including the self‐treatment of COPD exacerbations, was used on 86% of treatment days per participant. Benzo 2016 reported that 85% of the participants in the self‐management intervention group received a complete intervention, defined as at least 70% of 21 phone calls completed. In Kessler 2018, 100% of the participants in the self‐management group completed all four initial individual home coaching sessions; 66.7% achieved at least 80% of their phone and group coaching; and 89% achieved at least 80% for weekly phone health status transmission, which demonstrated that most participants adhered to the intervention. Liang 2019 reported that only 31% of the participants completed the full self‐management intervention; 26% partially completed the intervention; and 43% did not receive the intervention. Rose 2018 reported that 29% of the participants were 100% compliant with all 12 weekly phone calls, and 31% of the participants were 100% compliant with all nine subsequent monthly phone calls.
Jolly 2018 reported adherence regarding medication. Participants in the self‐management intervention improved medication adherence in six months compared to baseline, with higher proportions having: an inhaler check (86% versus 55%); an agreed care plan with a healthcare provider (44% versus 30%); written advice about what to do if symptoms worsened (23% versus 17%); and an antibiotic rescue pack (37% versus 29%).
Comparisons
As per our inclusion criteria, self‐management interventions that included an iterative process for at least two of its intervention components were compared with usual care in 27 studies. Coultas 2005 used two intervention groups and one usual care group. In meta‐analyses, both intervention groups were compared with the same usual care group, resulting in one extra comparison.
Outcomes
See Additional Table 4 for details on the number of included studies reporting outcomes of interest.
3. Number of studies reporting outcomes of interest.
| Studies | |
| Primary outcomes | |
| Health‐related quality of life scores | 23 |
| Respiratory‐related hospital admissions | 20 |
| Respiratory‐related mortality | 8 |
| All‐cause mortality | 24 |
| Secondary outcomes | |
| All‐cause hospital admissions | 18 |
| Respiratory‐related hospitalisation days | 5 |
| All‐cause hospitalisation days | 8 |
| Emergency department visits | 13 |
| General practitioner visits | 7 |
| Specialist visits | 5 |
| COPD exacerbations | 8 |
| Use of courses of oral steroids and antibiotics | 11 |
| Health status | 6 |
| Anxiety or depression, or both | 13 |
| Self‐efficacy | 9 |
| Days lost from work | 1 |
| Exercise capacity and physical activity | 7 |
| Self‐management behaviour | 2 |
| Patient activation | 1 |
| Health literacy | 0 |
COPD: chronic obstructive pulmonary disease.
Missing data
We have listed the authors from whom we received responses to requests for additional data in the ‘Acknowledgements’. However, not all study authors were able to provide the requested additional information. If the requested data were not provided, we described the data that were available.
Excluded studies
For the period 2011 to January 2020, we excluded 355 studies (437 references) following the assessment of the full‐text articles (Figure 1). The most frequent exclusion reason was that studies could not be classified as a COPD self‐management intervention (n = 78, 22.0%). For the period 1995 to 2011, we excluded 19 of the previously included studies (Zwerink 2014), because of stricter inclusion criteria for the population target (i.e. COPD diagnosis) and the self‐management intervention.
Studies awaiting classification
Twelve studies await classification because we could not reach the study authors to verify whether the studies met our eligibility criteria (Abdulsalim 2017; Aboumatar 2017; Alharbey 2019; Efraimsson 2008; Ghanem 2010; Heidari 2018; Hill 2010; Jiang 2012; Khdour 2009; Li 2014; Li Z 2015; Liu 2013).
Ongoing studies
We identified 38 ongoing studies (Boer 2011; Bourne 2017; Cecere Feemster 2013; Chen 2018; ChiCTR1800018197; ChiCTR‐TRC‐12002559; Chien 2016; Costa 2015; Dewan 2011a; Ding 2019; Doheny 2013; Duran 2017; Ergan 2018; Fleehart 2015; Gonzalez 2015; Hernandez 2016; Imanalieva 2016; IRCT201504149014N61; IRCT2017030432764N2; James 2012; Ko 2015; Moreno 2017; NCT02258646; NCT02924870; NCT03012256; NCT03084874; NCT03216603; NCT03721315; NL3827 (NTR4009); Padilla‐Zarate 2013; Paquin 2014; Reguera 2017; Sano 2016; Siddharthan 2018; Sirichana 2014; Thomas 2019; NL5277 (NTR5558); Zanaboni 2016).
Risk of bias in included studies
We present an overview of our risk of bias assessment per outcome in Figure 2. We performed assessments based on the content of the study articles, with no extra information requested from study authors. Further details and the rationale for judgements for primary outcomes, per outcome and per study, can be found in the Risk of bias (tables) section.
2.

Risk of bias overview for each outcome according to authors' judgements
None of the included studies reported blinding of participants and personnel, and none provided sufficient information regarding the balance of non‐protocol interventions over the study groups. Also, none of the included studies provided sufficient information regarding analyses to estimate the effect of adhering to interventions. These limitations resulted in high‐risk scores for all studies in domain 2 – ‘deviations from the intended interventions’ – of the risk of bias assessment form. As a result, we had to consider the overall risk of bias in each assessed study as ‘high’.
Other potential sources of bias
We explored possible reporting bias by assessing asymmetry in funnel plots. The St. George’s Respiratory Questionnaire (SGRQ) and respiratory‐related hospital admissions funnel plots seem to show a gap on both the lower left and right side of the graphs (funnel plots not shown). This could indicate that smaller studies with effects in favour of both the self‐management intervention and usual care group are published less frequently. By contrast, the funnel plot of all‐cause mortality seems to show a gap on the left side of the graph, indicating that smaller studies and studies of moderate size with effects in favour of self‐management interventions are published less frequently. For the latter, the same could be suggested by the funnel plot of all‐cause hospital admissions (funnel plot not shown). We could not rule out the contribution of other study factors to funnel plot asymmetry.
Effects of interventions
See: Table 1
We included a ‘Table 1’ (SOF table) of the 27 included studies. This table reflects the endpoints related to HRQoL, hospital admissions, mortality, dyspnoea, emergency department visits, anxiety and depression, and COPD exacerbations.
Health‐related quality of life
St. George's Respiratory Questionnaire (SGRQ)
COPD‐specific HRQoL was measured by the SGRQ in 16 studies (Bourbeau 2003; Bucknall 2012; Coultas 2005; Fan 2012; Gallefoss 1999; Hernández 2015; Jolly 2018; Jonsdottir 2015; Kessler 2018; Liang 2019; Martin 2004; Rice 2010; Rose 2018; Titova 2015; Walters 2013; Wang 2019). For primary analysis, mean adjusted SGRQ total scores, regardless of length of follow‐up, of 14 studies with 2778 participants could be included in the meta‐analysis (Bourbeau 2003; Bucknall 2012; Coultas 2005; Fan 2012; Gallefoss 1999; Hernández 2015; Jolly 2018; Jonsdottir 2015; Kessler 2018; Liang 2019; Rice 2010; Titova 2015; Walters 2013; Wang 2019), in which 15 comparisons between self‐management interventions versus usual care could be made, as two intervention groups from Coultas 2005 were included. The meta‐analysis showed lower mean SGRQ total scores (MD ‐2.86, 95% CI ‐4.87 to ‐0.85; low‐quality evidence; Analysis 1.1; Figure 3), indicating likely better HRQoL in the intervention group compared to the usual care group, with substantial heterogeneity (I2 = 60%). The pooled MD of ‐2.86 did not reach the MCID of four points (Jones 2005). Five individual studies reached the MCID of four points for the SGRQ score (Bucknall 2012; Hernández 2015; Rice 2010; Titova 2015; Wang 2019). Sensitivity analyses using FEM resulted in a similar effect size (MD ‐3.03, 95% CI ‐4.18 to ‐1.88) compared to REM. Liang 2019 showed a discrepancy with regard to SGRQ results. Whereas the authors described better HRQoL for the self‐management intervention group after follow‐up, they presented SGRQ scores that were higher (meaning worse). Because our contact attempts (in which we asked for clarification) remained unanswered, we included the presented data (worse HRQoL) in our primary analysis and performed a sensitivity analysis on SGRQ adjusted total score without the Liang 2019 study, resulting in a higher MD of ‐3.25 (95% CI ‐5.27 to ‐1.23) with lower heterogeneity (I2 = 58%) compared to the primary analysis. Sensitivity analyses using ICCs of 0.02 and 0.04 for the CRT of Walters 2013 resulted in similar effect sizes (MD ‐2.86, 95% CI ‐4.87 to ‐0.85).
1.1. Analysis.

Comparison 1: Self‐management versus usual care (primary outcomes), Outcome 1: Health‐related quality of life (HRQoL): adjusted SGRQ total score (primary analysis)
3.

Forest plot of comparison: self‐management versus usual care, outcome: 1.1 HRQoL: adjusted SGRQ total score (primary analysis)
For the meta‐analysis of short‐term (≤ 6 months' follow‐up) effects, seven studies could be included (including the Coultas study with two intervention groups)(Bourbeau 2003; Coultas 2005; Jolly 2018; Liang 2019; Titova 2015; Walters 2013; Wang 2019). No difference in SGRQ total score between self‐management interventions and usual care was detected (MD ‐2.36, 95% CI ‐4.92 to 0.20; I2 = 54%; Analysis 1.2; Figure 4). For medium‐term (> 6 to ≤ 12 months' follow‐up) effects, 13 studies could be included in the meta‐analysis (Bourbeau 2003; Bucknall 2012; Fan 2012; Gallefoss 1999; Hernández 2015; Jolly 2018; Jonsdottir 2015; Kessler 2018; Liang 2019; Rice 2010; Titova 2015; Walters 2013; Wang 2019), which showed probably lower SGRQ total scores for self‐management interventions compared to usual care (MD ‐2.65, 95% CI ‐4.78 to ‐0.52; I2 = 65%; Analysis 1.2; Figure 4). Analysis for long‐term (> 12 months' follow‐up) effects could not be performed, because of an insufficient number of studies (n < 3).
1.2. Analysis.

Comparison 1: Self‐management versus usual care (primary outcomes), Outcome 2: Health‐related quality of life (HRQoL): adjusted SGRQ total score (secondary analysis)
4.

Forest plot of comparison: Self‐management versus usual care, outcome: 1.2 HRQoL: adjusted SGRQ total score (secondary analysis)
Two studies reported insufficient data for inclusion in the SGRQ meta‐analyses (Martin 2004; Rose 2018). Rose 2018 could not be included due to lack of per‐group participant numbers at different time points for SGRQ total scores. This study reported no change in SGRQ scores at six and 12 months' follow‐up. Martin 2004 also found no difference in SGRQ total score after 12 months' follow‐up.
Chronic Respiratory Questionnaire (CRQ)
Five studies measured COPD‐specific HRQoL with the Chronic Respiratory Questionnaire (CRQ) (Benzo 2016; Bischoff 2012; Johnson‐Warrington 2016; Lenferink 2019; Mitchell 2014). The CRQ consists of four domain scores: dyspnoea, fatigue, emotional function and mastery (sense of control over the disease) (Guyatt 1987). A higher CRQ domain score indicates better HRQoL and the MCID is reflected by a change in a CRQ domain score of at least 0.5 on a 7‐point scale (Jaeschke 1989; Redelmeier 1996).
For primary analyses, mean domain end point scores of five studies, with a total of 738 participants, could be included in the meta‐analyses. Whereas these studies showed higher mean domain CRQ scores in the self‐management intervention compared to usual care, the evidence suggests that self‐management interventions do not improve CRQ domain scores, with MDs of 0.13 (95% CI ‐0.10 to 0.35), 0.12 (95% CI ‐0.09 to 0.33), 0.23 (95% CI –0.01 to 0.47) and 0.20 (95% CI ‐0.06 to 0.46) for dyspnoea, mastery, fatigue and emotional function, respectively (Analysis 1.3).
1.3. Analysis.

Comparison 1: Self‐management versus usual care (primary outcomes), Outcome 3: Health‐related quality of life (HRQoL): CRQ domain scores (primary analysis)
For the meta‐analysis of short‐term (≤ 6 months' follow‐up) effects, three studies could be included (Johnson‐Warrington 2016; Lenferink 2019; Mitchell 2014). Domain scores showed no difference between self‐management interventions and usual care, with MDs of 0.23 (95% CI ‐0.15 to 0.61), 0.12 (95% CI ‐0.33 to 0.57), 0.11 (95% CI ‐0.55 to 0.77) and 0.22 (95% CI ‐0.37 to 0.82) for dyspnoea, mastery, fatigue and emotional function, respectively (Analysis 1.4). For medium‐term (> 6 to ≤ 12 months' follow‐up) effects, again, three studies could be included in the meta‐analysis (Benzo 2016; Lenferink 2019; Mitchell 2014). Domain scores showed no difference between self‐management interventions and usual care, with MDs of 0.13 (95% CI ‐0.9 to 0.34), 0.17 (95% CI ‐0.07 to 0.41), 0.22 (95% CI ‐0.15 to 0.59) and 0.12 (95% CI ‐0.27 to 0.51) for dyspnoea, mastery, fatigue and emotional function, respectively (Analysis 1.4). Analysis for long‐term (> 12 months' follow‐up) effects could not be performed, because of an insufficient number of studies (n < 3).
1.4. Analysis.

Comparison 1: Self‐management versus usual care (primary outcomes), Outcome 4: Health‐related quality of life (HRQoL): CRQ domain scores (secondary analyses)
Clinical COPD Questionnaire (CCQ)
In two studies with a total of 170 participants (Ferrone 2019; Tabak 2014), COPD‐specific HRQoL was measured with the Clinical COPD Questionnaire (CCQ). No meta‐analysis could be performed. A lower score indicates better HRQoL and the MCID of the CCQ total score is reflected by a change in score of 0.4 or more on a 6‐point scale (Kocks 2006). Ferrone 2019 reported clinically relevant lower CCQ total scores in the COPD self‐management intervention group (mean 1.89; SD 1.07) compared to the usual care group (CCQ total mean 2.79; SD 1.28). Tabak 2014 reported no differences between the small study groups after three months' follow‐up (self‐management intervention (n = 12): mean 1.8, SE 0.24; usual care (n = 12): mean 2.3, SE 0.26).
Other HRQoL measures
Coultas 2005 and Walters 2013 used the Short Form‐36 (SF‐36) to measure generic HRQoL. Both studies reported no effects on SF‐36 domain scores for the intervention group compared to usual care. Fan 2012 used the SF‐12, a reduced version of the SF‐36, and reported no improvement on SF‐12 domain scores for participants in the self‐management intervention group compared to the usual care group who completed 12 months of study visits.
Bucknall 2012 and Tabak 2014 reported generic HRQoL using EuroQol‐5 Dimensions (EQ‐5D). Both Bucknall 2012 and Tabak 2014 reported no differences in the EQ‐5D areas under the curve between the groups after follow‐up.
In Tabak 2014, the individual participants' HRQoL state was also reported using a vertical visual analogue scale (VAS): self‐management intervention (72.3; SE 3.1) and usual care (62.4; SE 3.5). No statistical test was performed.
Generic HRQoL was measured using the Illness Intrusiveness Rating Scale (IIRS) in Coultas 2005 and Jonsdottir 2015, the Short Form‐12 (SF‐12) in Fan 2012, and the Sickness Impact Profile (SIP) in Emery 1998. Both Coultas 2005 and Jonsdottir 2015 reported beneficial effects of the self‐management intervention on the total IIRS. Coultas 2005 found lower participant‐reported illness intrusiveness in the nurse‐assisted collaborative management group compared to usual care at six months' follow‐up (mean change ‐7.0, 95% CI ‐15.0 to ‐0.5). Jonsdottir 2015 found less perceived intrusiveness of COPD and its treatment for participants in the self‐management intervention, demonstrated by IIRS total score (self‐management intervention: mean 31.57, SD 17.31; usual care: mean 27.84, SD 14.5; P = 0.014) at 12 months' follow‐up. Finally, Emery 1998 found improvement in total function in the control group as measured by the SIP (baseline: mean 14.2, SD 8.6; after a 10‐week intervention: mean 10.4, SD 7.8; P < 0.001), whereas the self‐management intervention group showed no change.
Respiratory‐related hospital admissions
Respiratory‐related hospital admissions were reported in 19 studies (Benzo 2016; Bösch 2007; Bourbeau 2003; Bucknall 2012; Coultas 2005; Fan 2012; Gallefoss 1999; Hernández 2015; Johnson‐Warrington 2016; Jolly 2018; Lenferink 2019; Martin 2004; Mitchell 2014; Rice 2010; Sanchez‐Nieto 2016; Tabak 2014; Titova 2015; Walters 2013; Wang 2019). Coultas 2005 had two intervention groups included in the meta‐analysis.
For primary analyses, regardless of length of follow‐up, 15 studies including 3263 participants, could be included in a meta‐analysis (Benzo 2016; Bourbeau 2003; Bucknall 2012; Coultas 2005; Fan 2012; Gallefoss 1999; Hernández 2015; Johnson‐Warrington 2016; Lenferink 2019; Mitchell 2014; Rice 2010; Sanchez‐Nieto 2016; Tabak 2014; Titova 2015; Walters 2013). A lower probability of at least one respiratory‐related hospital admission was noted amongst participants receiving the self‐management intervention compared with those who received usual care (OR 0.75, 95% CI 0.57 to 0.98; very low‐quality evidence; Analysis 1.5; Figure 5). Pooled study results showed moderate heterogeneity (I2 = 48%). Sensitivity analyses using FEM resulted in a similar effect size (OR 0.74, 95% CI 0.63 to 0.88) compared to REM. Sensitivity analyses using ICCs of 0.02 and 0.04 for the Walters 2013 CRT resulted in similar effect sizes (OR 0.75, 95% CI 0.57 to 0.98).
1.5. Analysis.

Comparison 1: Self‐management versus usual care (primary outcomes), Outcome 5: Healthcare utilisation: respiratory‐related hospital admissions (number of participants with at least one admission) (primary analysis)
5.

Forest plot of comparison: self‐management versus usual care, outcome: 1.4 Healthcare utilisation: respiratory‐related hospital admissions (number of participants with at least one admission)
The meta‐analysis of short‐term (≤ 6 months' follow‐up) effects included four studies (Coultas 2005; Johnson‐Warrington 2016; Mitchell 2014; Tabak 2014), and showed no difference between self‐management interventions and usual care (OR 0.84, 95% CI 0.45 to 1.55; Analysis 1.6). For medium‐term (> 6 to ≤ 12 months' follow‐up) effects, 11 studies were included (Benzo 2016; Bourbeau 2003; Bucknall 2012; Fan 2012; Gallefoss 1999; Hernández 2015; Lenferink 2019; Rice 2010; Sanchez‐Nieto 2016; Titova 2015; Walters 2013). No difference between self‐management and usual care was detected (OR 0.74, 95% CI 0.53 to 1.03; Analysis 1.6). Analysis for long‐term (> 12 months' follow‐up) effects could not be performed due to an insufficient number of studies (n < 3).
1.6. Analysis.

Comparison 1: Self‐management versus usual care (primary outcomes), Outcome 6: Healthcare utilisation: respiratory‐related hospital admissions (number of participants with at least one admission) (secondary analyses)
The Martin 2004 study could not be included in the meta‐analysis due to lack of SDs. In this study, more respiratory‐related hospitalisations were found in the intervention group (1.1 per patient per year) compared to usual care (0.7 per patient per year).
The study‐specific NNTBs for respiratory‐related hospital admissions ranged from 15 (95% CI 8 to 399) to 26 (95% CI 15 to 677). To calculate NNTB, the pooled effect on respiratory‐related hospital admissions (OR 0.75, 95% CI 0.57 to 0.98) was used, and this was applied to the mean usual care event risks over the mean follow‐up duration of the study comparisons with the highest and lowest baseline risks. The eight comparisons with the highest baseline risks for respiratory‐related hospital admissions had a mean control event risk (mean observed risk of the respiratory‐related hospital admissions in the usual care group) of 48.6 (Figure 6) (Benzo 2016; Bourbeau 2003; Bucknall 2012; Johnson‐Warrington 2016; Lenferink 2019; Sanchez‐Nieto 2016; Tabak 2014; Titova 2015). Over a mean of 9.75 months' follow‐up, 15 participants (95% CI 8 to 399) with high baseline risk of respiratory‐related hospital admissions needed to be treated to prevent one person with at least one respiratory‐related hospital admission. The eight comparisons with the lowest baseline risks for respiratory‐related hospital admissions had a mean usual care event risk of 17.6 (Figure 7) (Coultas 2005 (with two intervention groups); Fan 2012; Gallefoss 1999; Hernández 2015; Mitchell 2014; Rice 2010; Walters 2013). Over a mean of 9.75 months' follow‐up, 26 participants (95% CI 15 to 677) with low baseline risk of respiratory‐related hospital admissions needed to be treated to prevent one person with at least one respiratory‐related hospital admission.
6.
Cates plot of participants with COPD with high baseline risk of respiratory‐related hospital admissions. In the usual care group, 49 of 100 participants had at least one respiratory‐related hospital admission over a mean of 9.75 months, compared to 42 (95% CI 35 to 49) of 100 participants in the self‐management intervention group.
7.
Cates plot of participants with COPD with low baseline risk of respiratory‐related hospital admissions. In the usual care group, 18 of 100 participants had at least one respiratory‐related hospital admission over a mean of 9.75 months, compared to 14 (95% CI 11 to 18) of 100 participants in the self‐management intervention group.
Seven studies with 1572 participants were included in a meta‐analysis on the mean number of respiratory‐related hospital admissions (Bösch 2007; Bucknall 2012; Jolly 2018; Lenferink 2019; Tabak 2014; Titova 2015; Wang 2019). For primary analyses, regardless of length of follow‐up, no difference between self‐management interventions and usual care was found (MD ‐0.29, 95% CI ‐0.60 to 0.01; Analysis 1.7). Using FEM in the sensitivity analysis produced similar effects (MD ‐0.00, 95% CI ‐0.02 to 0.01).
1.7. Analysis.

Comparison 1: Self‐management versus usual care (primary outcomes), Outcome 7: Healthcare utilisation: respiratory‐related hospital admissions (mean number per participant) (primary analysis)
The meta‐analysis of short‐term (≤ 6 months' follow‐up) effects included three studies (Jolly 2018; Tabak 2014; Wang 2019), and showed no difference (MD ‐0.01, 95% CI ‐0.04 to 0.02; Analysis 1.8). For medium‐term (> 6 to ≤ 12 months' follow‐up) effects, six studies were included (Bösch 2007; Bucknall 2012; Jolly 2018; Lenferink 2019; Titova 2015; Wang 2019). No difference was detected (MD ‐0.33, 95% CI ‐0.68 to 0.01; Analysis 1.8). Analysis for long‐term (> 12 months' follow‐up) effects could not be performed, due to an insufficient number of studies (n < 3).
1.8. Analysis.

Comparison 1: Self‐management versus usual care (primary outcomes), Outcome 8: Healthcare utilisation: respiratory‐related hospital admissions (mean number per participant) (secondary analyses)
Mortality
Mortality was reported as an outcome measure in nine studies (Bucknall 2012; Fan 2012; Johnson‐Warrington 2016; Kessler 2018; Lenferink 2019; Rice 2010; Rose 2018; Sanchez‐Nieto 2016; Titova 2015). In addition to formal mortality data, we extracted mortality data from sections describing the participant flow and reasons for losses to follow‐up from 15 studies (Benzo 2016; Bourbeau 2003; Bringsvor 2018; Coultas 2005; Ferrone 2019; Gallefoss 1999; Hernández 2015; Jolly 2018; Jonsdottir 2015; Liang 2019; Martin 2004; Mitchell 2014; Tabak 2014; Walters 2013; Wang 2019). Three studies provided no information on mortality (Bischoff 2012, 110 participants; Bösch 2007, 50 participants; Emery 1998, 49 participants), and could not be included in the meta‐analyses.
Respiratory‐related mortality
We included data from eight studies in the meta‐analysis of respiratory‐related mortality (Bucknall 2012; Fan 2012; Gallefoss 1999; Johnson‐Warrington 2016; Lenferink 2019; Tabak 2014; Titova 2015; Wang 2019). No difference in mortality risk was found between self‐management intervention and usual care groups (risk difference (RD) 0.01, 95% CI ‐0.02 to 0.04; I2 = 63%; 1572 participants ; low‐quality evidence; Analysis 1.9; Figure 8). Three studies reported no deaths in the self‐management and usual care groups after 12 months' follow‐up (Gallefoss 1999; Wang 2019), and after three months' follow‐up (Tabak 2014). Sensitivity analysis using a FEM produced similar results on respiratory‐related mortality (RD 0.03, 95% CI 0.00 to 0.05). It was not possible to calculate the NNTB for respiratory‐related mortality because the 95% CI of the pooled RD for respiratory‐related mortality included the possibilities of both benefit and harm.
1.9. Analysis.

Comparison 1: Self‐management versus usual care (primary outcomes), Outcome 9: Mortality: respiratory‐related mortality (primary analysis)
8.

Forest plot of comparison: self‐management versus usual care, outcome: 1.6 Mortality: respiratory‐related mortality)
For the meta‐analysis of short‐term (≤ 6 months' follow‐up) effects, four studies could be included (Gallefoss 1999; Johnson‐Warrington 2016; Tabak 2014; Wang 2019). No difference was found between self‐management interventions and usual care (RD ‐0.01, 95% CI ‐0.05 to 0.03; Analysis 1.10). For medium‐term (> 6 to ≤ 12 months' follow‐up) effects, seven studies could be included in the meta‐analysis (Bucknall 2012; Fan 2012; Gallefoss 1999; Kessler 2018; Lenferink 2019; Tabak 2014; Wang 2019). No difference between self‐management interventions and usual care was detected (RD 0.00, 95% CI ‐0.01 to 0.02; Analysis 1.10). Analysis for long‐term (> 12 months' follow‐up) effects could not be performed, due to an insufficient number of studies (n < 3).
1.10. Analysis.

Comparison 1: Self‐management versus usual care (primary outcomes), Outcome 10: Mortality: respiratory‐related mortality (secondary analyses)
All‐cause mortality
We included data from 24 studies with 5719 participants in the meta‐analysis for all‐cause mortality (Coultas 2005 had two intervention groups) (Benzo 2016; Bourbeau 2003; Bringsvor 2018; Bucknall 2012; Coultas 2005; Fan 2012; Ferrone 2019; Gallefoss 1999; Hernández 2015; Johnson‐Warrington 2016; Jolly 2018; Jonsdottir 2015; Kessler 2018; Lenferink 2019; Liang 2019; Martin 2004; Mitchell 2014; Rice 2010; Rose 2018; Sanchez‐Nieto 2016; Tabak 2014; Titova 2015; Walters 2013; Wang 2019). No difference in mortality risk was found between self‐management intervention and usual care group participants (RD ‐0.01, 95% CI ‐0.03 to 0.01; I2 = 63%; low‐quality evidence; Analysis 1.11; Figure 9). Three studies reported no deaths in the self‐management and usual care groups (Gallefoss 1999; Tabak 2014; Wang 2019). Sensitivity analysis using a FEM resulted in a similar result on all‐cause mortality (RD ‐0.01, 95% CI ‐0.02 to 0.01). It was not possible to calculate the NNTB for all‐cause mortality, because the 95% CI of the pooled RD for all‐cause mortality included the possibilities of both benefit and harm. Sensitivity analyses using ICCs of 0.02 and 0.04 for the Walters 2013 CRT resulted in similar effect sizes (RD ‐0.01, 95% CI ‐0.03 to 0.01).
1.11. Analysis.

Comparison 1: Self‐management versus usual care (primary outcomes), Outcome 11: Mortality: all‐cause mortality (primary analysis)
9.

Forest plot of comparison: self‐management versus usual care, outcome: 1.7 Mortality: all‐cause mortality
For the meta‐analysis of short‐term (≤ 6 months' follow‐up) effects, nine studies could be included (Coultas 2005; Gallefoss 1999; Johnson‐Warrington 2016; Jolly 2018; Jonsdottir 2015; Liang 2019; Mitchell 2014; Tabak 2014; Wang 2019). No difference between self‐management interventions and usual care was detected (RD ‐0.00, 95% CI ‐0.01 to 0.01; Analysis 1.12). For medium‐term (> 6 to ≤ 12 months' follow‐up) effects, 21 studies could be included in the meta‐analyses (Benzo 2016; Bourbeau 2003; Bringsvor 2018; Bucknall 2012; Fan 2012; Ferrone 2019; Gallefoss 1999; Hernández 2015; Jolly 2018; Jonsdottir 2015; Kessler 2018; Lenferink 2019; Liang 2019; Martin 2004; Rice 2010; Rose 2018; Sanchez‐Nieto 2016; Tabak 2014; Titova 2015; Walters 2013; Wang 2019). Again, no difference between self‐management interventions and usual care was found (RD ‐0.01, 95% CI ‐0.02 to 0.01; Analysis 1.12). Analysis for long‐term (> 12 months' follow‐up) effects could not be performed, because an insufficient number of studies including outcomes of interest (n < 3) was available.
1.12. Analysis.

Comparison 1: Self‐management versus usual care (primary outcomes), Outcome 12: Mortality: all‐cause mortality (secondary analyses)
All‐cause hospital admissions
Ten studies, with a total of 2633 participants, were included in a meta‐analysis for number of participants with at least one all‐cause hospital admission (Benzo 2016; Bucknall 2012; Coultas 2005; Fan 2012; Hernández 2015; Johnson‐Warrington 2016; Lenferink 2019; Mitchell 2014; Rice 2010; Tabak 2014; the Coultas 2005 study had two intervention groups). There was no between‐group difference in all‐cause hospital admissions (OR 0.88, 95% CI 0.71 to 1.08; moderate‐quality evidence; Analysis 2.1). Heterogeneity was low (I2 = 20%). Sensitivity analysis using FEM showed similar results (OR 0.85, 95% CI 0.72 to 1.01).
2.1. Analysis.

Comparison 2: Self‐management versus usual care (secondary outcomes), Outcome 1: Healthcare utilisation: all‐cause hospital admissions (number of participants with at least one admission)
Four studies could not be included in this meta‐analysis due to lack of required information (Bösch 2007; Kessler 2018; Martin 2004; Titova 2015). Two of these studies suggested a reduction in total number of all‐cause hospital admissions, favouring the self‐management intervention compared to usual care (Bösch 2007; Titova 2015). The other two studies reported no difference in all‐cause hospital admissions compared to usual care (Kessler 2018; Martin 2004). It was not possible to calculate the NNTB for all‐cause hospital admissions because the 95% CI of the pooled OR for at least one all‐cause hospital admission included the possibilities of both benefit and harm.
Seven studies reported the mean number of all‐cause hospital admissions (Bucknall 2012; Ferrone 2019; Jolly 2018; Lenferink 2019; Martin 2004; Rose 2018; Tabak 2014). No difference in this mean number was found (MD ‐0.01, 95% CI ‐0.06 to 0.04; Analysis 2.2). Sensitivity analysis using FEM showed similar results (MD ‐0.01, 95% CI ‐0.06 to 0.04). The Jolly 2018 study dominated the overall effect with a weight of 87% due to extremely small SEs and CIs. However, sensitivity analysis excluding Jolly 2018 showed similar results on all‐cause hospital admissions (MD ‐0.05, 95% CI ‐0.19 to 0.08). The Bourbeau 2003 study could not be included in the meta‐analysis because SDs were missing.
2.2. Analysis.

Comparison 2: Self‐management versus usual care (secondary outcomes), Outcome 2: Healthcare utilisation: all‐cause hospital admissions (mean number per participant)
Healthcare utilisation
Respiratory‐related hospitalisation days
Five studies assessed the number of respiratory‐related hospitalisation days per participant (Benzo 2016; Johnson‐Warrington 2016; Kessler 2018; Lenferink 2019; Sanchez‐Nieto 2016). Four of these, including 819 participants, could be included in the meta‐analysis (Benzo 2016; Kessler 2018; Lenferink 2019; Sanchez‐Nieto 2016). No differences were found between the self‐management intervention and usual care groups (MD ‐0.62, 95% CI ‐2.27 to 1.03; Analysis 2.3). Heterogeneity was substantial (I2 = 68%). Sensitivity analyses using a FEM showed similar results (MD ‐0.57, 95% CI ‐1.18 to 0.05). The Johnson‐Warrington 2016 study could not be included in the meta‐analysis because insufficient data were reported. The difference in the median number of respiratory‐related hospitalisation days in this study was not different between the self‐management intervention and usual care group (median 12.0, IQR 9.0 to 33.8 versus median 15.0, IQR 3.5 to 32.0).
2.3. Analysis.

Comparison 2: Self‐management versus usual care (secondary outcomes), Outcome 3: Healthcare utilisation: respiratory‐related hospitalisation days (per participant)
All‐cause hospitalisation days
Eight studies assessed the number of all‐cause hospitalisation days per participant (Bourbeau 2003; Bucknall 2012; Hernández 2015; Johnson‐Warrington 2016; Kessler 2018; Lenferink 2019; Rice 2010; Rose 2018). Six studies with 2073 participants could be included in the meta‐analysis (Bourbeau 2003; Bucknall 2012; Hernández 2015; Kessler 2018; Lenferink 2019; Rice 2010). No between‐group differences were found (MD ‐0.51, 95% CI ‐1.85 to 0.84; Analysis 2.4). Heterogeneity was moderate (I2 = 43%). Sensitivity analysis using a FEM showed similar results (MD ‐0.59, 95% CI ‐1.29 to 0.12). Two studies could not be included in the meta‐analysis, because insufficient data were reported (Johnson‐Warrington 2016; Rose 2018). The Johnson‐Warrington 2016 study reported lower median number of all‐cause bed days for readmission in the self‐management group (9.0, IQR 1.0 to 30.0) compared to the usual care group (16.5, IQR 3.8 to 39.8); however, the CI includes no difference. The Rose 2018 study reported lower median number of all‐cause bed days in the intervention group (8.0, IQR 4.0 to 22.0) compared to the usual care group (11.0, IQR 4.0 to 15.0), with a difference in hospitalisation day risk ratio of 0.84 (95% CI 0.78 to 0.90), favouring self‐management for those at risk.
2.4. Analysis.

Comparison 2: Self‐management versus usual care (secondary outcomes), Outcome 4: Healthcare utilisation: all‐cause hospitalisation days (per participant)
Emergency department (ED) visits
Thirteen studies reported ED visits (Bourbeau 2003; Bucknall 2012; Coultas 2005; Fan 2012; Ferrone 2019; Hernández 2015; Jolly 2018; Mitchell 2014; Rice 2010; Rose 2018; Sanchez‐Nieto 2016; Tabak 2014; Wang 2019). Five studies with 865 participants were included in a meta‐analysis for number of participants with at least one ED visit (Fan 2012; Ferrone 2019; Mitchell 2014; Sanchez‐Nieto 2016; Tabak 2014). Self‐management intervention participants may have a slightly lower probability of at least one ED visit compared to usual care participants (OR 0.53, 95% CI 0.32 to 0.87; low‐quality evidence; Analysis 2.5), with a low heterogeneity (I2 = 34%). Sensitivity analysis using a FEM produced a similar result (OR 0.59, 95% CI 0.43 to 0.81).
2.5. Analysis.

Comparison 2: Self‐management versus usual care (secondary outcomes), Outcome 5: Healthcare utilisation: emergency department visits (number of participants with at least one visit)
Six studies with 1939 participants could be included in a meta‐analysis for the mean number of ED visits per participant (Bourbeau 2003; Bucknall 2012; Ferrone 2019; Jolly 2018; Rose 2018; Wang 2019). The meta‐analysis showed a slightly lower risk of ED visits for self‐management intervention participants compared to usual care participants (MD 0.52, 95% CI 0.89 to 0.15; Analysis 2.6), with considerable heterogeneity (I2 = 96%). Sensitivity analysis using a FEM showed a similar result (MD ‐0.32, 95% CI ‐0.38 to ‐0.25). The outlier in this meta‐analysis was the study by Wang 2019, showing a very strong effect size favouring self‐management (MD ‐1.80, 95% CI ‐2.18 to ‐1.42). Sensitivity analysis without the Wang 2019 study showed no difference in risk of ED visits (MD ‐0.28, 95% CI ‐0.60 to 0.03).
2.6. Analysis.

Comparison 2: Self‐management versus usual care (secondary outcomes), Outcome 6: Healthcare utilisation: emergency department visits (mean number per participant)
Three studies could not be included in the meta‐analysis, because different methods were used to report the outcome (Coultas 2005; Hernández 2015; Rice 2010). Coultas 2005 reported respiratory‐related ED visits and reported no differences between groups in COPD‐related visits after six months' follow‐up. Hernández 2015 reported a lower mean number of respiratory‐related ED visits for self‐management intervention participants (mean 10, SD 12.11) compared to usual care participants (mean 23, SD 27.4). These data could not be included in a meta‐analysis because there were different approaches for co‐ordination of hospital admissions between study groups. Eighty percent of the admissions in the self‐management intervention group were co‐ordinated between primary care and the hospital team, and therefore bypassing the ED (Hernández 2015). All admissions in the usual care group were processed as unplanned through the ED (Hernández 2015). Rice 2010 found fewer visits in the self‐management intervention group compared to the usual care group (67.0 versus 91.2 per 100 person‐years, P = 0.02).
General practitioner (GP) visits
Seven studies reported GP visits (Bischoff 2012; Bourbeau 2003; Bucknall 2012; Gallefoss 1999; Jolly 2018; Martin 2004; Mitchell 2014). Four studies with 1113 participants were included in a meta‐analysis (Bucknall 2012; Gallefoss 1999; Jolly 2018; Martin 2004). No difference between the self‐management intervention and usual care groups was found (MD ‐0.21, 95% CI ‐0.86 to 0.25; Analysis 2.7). Sensitivity analyses using a FEM resulted in similar results (MD ‐0.11, 95% CI ‐0.25 to 0.02). Three studies could not be included in the meta‐analysis either because different methods were used to report the outcome (Bischoff 2012; Mitchell 2014), or because of missing SDs (Bourbeau 2003). Bourbeau 2003 reported fewer unscheduled GP visits for the self‐management intervention group compared to the usual care group. Nevertheless, the scheduled GP visits were comparable between the groups. CIs of group differences in the Bischoff 2012 study (self‐management intervention: n = 20, usual care: n = 18; OR 1.09, 95% CI 0.42 to 2.81) and the Mitchell 2014 study (self‐management intervention: n = 78, usual care: n = 73; OR 1.15, 95% CI 0.75 to 1.76) did not find a difference.
2.7. Analysis.

Comparison 2: Self‐management versus usual care (secondary outcomes), Outcome 7: Healthcare utilisation: GP visits (mean number per participant)
Specialist visits
Five studies reported data on specialist visits (Bourbeau 2003; Coultas 2005; Ferrone 2019; Martin 2004; Mitchell 2014). No meta‐analysis could be performed because different methods and definitions were used to report these visits. Bourbeau 2003 reported comparable unscheduled (self‐management intervention n = 24, usual care n = 26) and scheduled specialist visits (self‐management intervention n = 347, usual care n = 316) in both groups. Coultas 2005 reported no change in between‐group difference from baseline to six months' follow‐up (nurse‐assisted medical management versus usual care: mean change 1.3, 95% CI ‐1.5 to 4.1; nurse‐assisted collaborative management versus usual care: mean change 0.4, 95% CI ‐2.2 to 3.4).
Ferrone 2019 reported a lower number of urgent physician visits for the self‐management group compared to the usual care group after 12 months' follow‐up (between‐group difference: 1.46, 95% CI 0.90 to 2.02, P < 0.001). Martin 2004 reported a non‐significant higher number of all‐cause doctor and nurse visits in the self‐management intervention group compared to the usual care group (mean 15.6, SD 12.68 versus mean 11.6, SD 8.02). Mitchell 2014 observed a reduction in the number of nurse specialist home visits for respiratory reasons in the self‐management intervention compared to usual care group (OR 0.42, 95% CI 0.19 to 0.91).
Number of COPD exacerbations
A meta‐analysis, including 1401 participants from seven studies, of the mean number of exacerbations per participant (regardless of definition used) (Benzo 2016; Bischoff 2012; Bösch 2007; Fan 2012; Jonsdottir 2015; Kessler 2018; Lenferink 2019), resulted in lower mean exacerbations per participant for the self‐management intervention, but the CI includes no difference (MD ‐0.06, 95% CI ‐0.26 to 0.15; Analysis 2.8). Sensitivity analysis using a FEM produced similar results (MD ‐0.06, 95% CI ‐0.26 to 0.15).
2.8. Analysis.

Comparison 2: Self‐management versus usual care (secondary outcomes), Outcome 8: COPD exacerbations (mean number per participant)
Four studies reported COPD exacerbations based on symptoms (Bischoff 2012; Fan 2012; Kessler 2018; Lenferink 2019). Data from these studies were included in a meta‐analysis, with 1047 participants, resulting in no difference in mean COPD exacerbations between self‐management intervention and usual care (MD 0.05, 95% CI ‐0.22 to 0.31; Analysis 2.8), with low heterogeneity (I2 = 0%).
Five studies reported the total number of exacerbations (Bischoff 2012; Bourbeau 2003; Fan 2012; Lenferink 2019; Tabak 2014). Bischoff 2012 reported 280 exacerbations in the self‐management intervention group (n = 55) and 235 in the usual care group (n = 55), with no between‐group difference (first year follow‐up rate ratio 1.10, 95% CI 0.86 to 1.40; second year follow‐up rate ratio 1.16, 95% CI 0.81 to 1.67). Bourbeau 2003 reported a difference in exacerbation rates between both groups, with 299 exacerbations in the self‐management intervention group (n = 96) and 362 exacerbations in the usual care group (n = 95) after 12 months' follow‐up (P = 0.06). Fan 2012 reported 600 self‐reported exacerbations in the self‐management intervention group (n = 209) and 610 in the usual care group (n = 217), with no between‐group difference during the first 12 months' follow‐up (rate ratio 1.03, 95% CI 0.97 to 1.10). Tabak 2014 reported 33 (median 2.0, IQR 1.0 to 3.0) exacerbations in the self‐management intervention group (n = 12); exacerbation data for the usual care group was not available. Lenferink 2019 reported 216 exacerbations in the self‐management intervention group (n = 102) and 230 exacerbations in the usual care group (n = 99), extracted from diary data, after 12 months' follow‐up. Whereas no difference in COPD exacerbation rates was found between both groups (rate per 100 person‐years 0.91, 95% CI 0.65 to 1.26), a shorter duration per COPD exacerbation was observed by Lenferink 2019 for the self‐management intervention group (median days 8.1, IQR 4.8 to 10.1) compared to the usual care group (median days 9.5, IQR 7.0 to 15.1) (P = 0.021).
Use of oral steroids and antibiotics
The majority of the studies (n = 16) did not report any data on the use of oral steroids or antibiotics and thus could not be included in a meta‐analysis (Bourbeau 2003; Bringsvor 2018; Bucknall 2012; Coultas 2005; Emery 1998; Hernández 2015; Johnson‐Warrington 2016; Jolly 2018; Jonsdottir 2015; Kessler 2018; Liang 2019; Rose 2018; Tabak 2014; Titova 2015; Walters 2013; Wang 2019). Two studies reported data on combined use of oral steroids and antibiotics (Benzo 2016; Bischoff 2012). Bischoff 2012 reported a similar number of participants who started prednisolone, antibiotics or both, to manage exacerbations in the self‐management intervention group (n = 16, 11%) compared to the usual care group (n = 13, 10%) in the first year of follow‐up. In the second year of follow‐up, a higher number of exacerbations in the self‐management intervention group were managed by starting prednisolone, antibiotics or both (OR 3.98, 95% CI 1.10 to 15.58). Benzo 2016 reported no difference between groups in the use of antibiotic‐prednisone combination at three, six and nine months after discharge. There was greater use of the written action plan from nine to 12 months, and therefore a greater use of antibiotic‐prednisone combination in the self‐management intervention group (n = 54, 65.9%) compared to the usual care group (n = 34, 43.0%) (P = 0.004).
Courses of oral steroids
Six studies reported the use of oral steroids (Fan 2012; Gallefoss 1999; Lenferink 2019; Martin 2004; Rice 2010; Sanchez‐Nieto 2016). The numbers of participants who used at least one course of oral steroids were available for three studies (Gallefoss 1999; Rice 2010; Sanchez‐Nieto 2016). Data from these studies were included in a meta‐analysis, with 881 participants, resulting in no difference in oral steroid use (OR 4.19, 95% CI 0.35 to 50.65; Analysis 2.9), with considerable heterogeneity (I2 = 96%). Sensitivity analysis using a FEM resulted in a higher probability of using at least one course of oral steroids for self‐management interventions with a smaller CI (OR 8.98, 95% CI 5.95 to 13.56; I2 = 96%), likely due to less beneficial small‐study effects. The Rice 2010 study included a very large population compared to Gallefoss 1999 and Sanchez‐Nieto 2016. The proportion of participants who received at least one course of oral steroids in the self‐management intervention group was relatively high (97.6%) compared to other studies (Gallefoss 1999 = 69.2%; Sanchez‐Nieto 2016 = 38.3%). The OR in Rice 2010 was 32.7 which is probably an overestimation of the risk ratio due to the fact that the event is common. An additional sensitivity analysis without the Rice 2010 study was not possible because of the limited number of studies. This meta‐analysis should therefore be interpreted with caution.
2.9. Analysis.

Comparison 2: Self‐management versus usual care (secondary outcomes), Outcome 9: Courses of oral steriods (number of participants who used at least one course)
Fan 2012 reported a higher mean of 2.5 exacerbations per patient‐year treated with prednisolone in the self‐management intervention group compared with 2.1 in the usual care group (rate ratio 1.25, 95% CI 1.05 to 1.48). In Martin 2004, no difference between both study groups was detected (self‐management intervention: 2.3 courses, 95% CI 1.4 to 3.2; usual care: 1.3 courses, 95% CI 0.8 to 1.8). Lenferink 2019 reported that a higher number of self‐management intervention participants (n = 34, 51.5%) than usual care participants (n = 23, 32.9%) with a COPD exacerbation initiated a course of oral prednisolone within two days from the COPD exacerbation start in at least 75% of the exacerbations.
Courses of antibiotics
Seven studies reported the use of antibiotics (Bösch 2007; Fan 2012; Lenferink 2019; Martin 2004; Mitchell 2014; Rice 2010; Sanchez‐Nieto 2016). Data regarding participants who used at least one course of antibiotics were available in three studies (Mitchell 2014; Rice 2010; Sanchez‐Nieto 2016). All three studies, with a total of 1012 participants, could be included in a meta‐analysis. Results show higher use of antibiotics in the self‐management group compared to usual care (OR 3.95, 95% CI 1.37 to 11.43; Analysis 2.10), with considerable heterogeneity (I2 = 85%). Sensitivity analysis using a FEM also resulted in a higher probability of participants in the self‐management intervention group using at least one course of antibiotics for the self‐management interventions (OR 5.88, 95% CI 4.19 to 8.25) with similar considerable heterogeneity (I2 = 85%). As with oral steroids, Rice 2010 reported much higher rates of antibiotic use in the self‐management intervention group compared to usual care.
2.10. Analysis.

Comparison 2: Self‐management versus usual care (secondary outcomes), Outcome 10: Courses of antibiotics (number of participants who used at least one course)
Bösch 2007 reported a reduction in the mean number of exacerbations that were treated with antibiotics in the self‐management intervention group (mean exacerbations 2.0, SD 1.4 to mean exacerbations 1.4; SD 1.6), with no changes observed in the usual care group. Fan 2012 reported a slightly higher mean of 2.7 exacerbations per patient‐year treated with an antibiotic in the self‐management intervention group compared with a mean of 2.5 in the usual care group, but the CI indicates no difference (rate ratio 1.11; 95% CI 0.97 to 1.27). In Martin 2004, there was no difference in the use of antibiotics between the groups after 12 months' follow‐up (self‐management intervention: 3.6, 95% CI 2.5 to 4.7, versus usual care: 2.5, 95% CI 1.7 to 3.3). Lenferink 2019 reported no difference in the number of patient‐reported antibiotics between the self‐management intervention group (rate per 100 person‐years: 1.3, n = 102) and usual care group (rate per 100 person‐years: 1.2, n = 99) (incidence rate ratio 1.08, 95% CI 0.70 to 1.67).
Health status
COPD assessment test (CAT)
Two studies reported data on the impact of COPD on a person’s life, measured with the CAT (Ferrone 2019; Lenferink 2019). Ferrone 2019 found an improvement in CAT score for the self‐management intervention group compared to usual care group after 12 months' follow‐up (adjusted difference: 9.3, 95% CI 7.8 to 10.8). Lenferink 2019 reported no between‐group differences in CAT score for self‐management interventions compared to usual care.
Dyspnoea
Five studies assessed the effect of self‐management interventions on dyspnoea as measured by the modified Medical Research Council questionnaire (mMRC) (Bösch 2007; Hernández 2015; Jolly 2018; Lenferink 2019; Liang 2019). Three studies, representing 356 participants, were included in a meta‐analysis (Bösch 2007; Hernández 2015; Lenferink 2019). No difference in dyspnoea scores was found (MD ‐0.31, 95% CI ‐1.23 to 0.60; Analysis 2.11). Sensitivity analyses using a FEM resulted in a lower effect on dyspnoea score for self‐management interventions, but the CI includes no difference (MD ‐0.04, 95% CI ‐0.25 to 0.17). Two studies could not be included in a meta‐analysis, because different methods were used to report the outcome (Jolly 2018; Liang 2019). Jolly 2018 reported no differences in the level of breathlessness for self‐management interventions compared to usual care (OR 1.1, 95% CI 0.7 to 1.5). Liang 2019 reported non‐significant median change from baseline differences between groups in mMRC grades (self‐management intervention: median 1 (IQR 1 to 2); usual care: median 1 (IQR 0 to 2); P = 0.74).
2.11. Analysis.

Comparison 2: Self‐management versus usual care (secondary outcomes), Outcome 11: Health status: modified Medical Research Council Dyspnoea Scale (mMRC)
Anxiety and depression
Thirteen studies assessed the effect of self‐management interventions on anxiety and depression, as measured by the Hospital Anxiety and Depression Scale (HADS)(Zigmond 1983), a 21‐unit scale in which higher scores indicate more severe symptoms (Bucknall 2012; Emery 1998; Hernández 2015; Johnson‐Warrington 2016; Jolly 2018; Jonsdottir 2015; Kessler 2018; Lenferink 2019; Liang 2019; Mitchell 2014; Rose 2018; Titova 2015; Walters 2013). Nine studies could be included in a meta‐analysis, with 1647 participants for the HADS–anxiety score, and 1653 participants for the HADS–depression score (Bucknall 2012; Hernández 2015; Johnson‐Warrington 2016; Jolly 2018; Jonsdottir 2015; Lenferink 2019; Mitchell 2014; Titova 2015; Walters 2013). The meta‐analyses showed probably better mean HADS–anxiety scores (MD ‐0.57, 95% CI ‐1.01 to ‐0.13; moderate‐quality evidence) and probably better mean HADS–depression scores (MD ‐0.45, 95% CI ‐0.80 to ‐0.10; moderate‐quality evidence) for the self‐management intervention compared to usual care (Analysis 2.12); heterogeneity was low for both HADS analyses (I2 = 21% and 6%, respectively). Sensitivity analyses using a FEM produced similar results (HADS–anxiety score MD ‐0.57, 95% CI ‐0.94 to ‐0.20; HADS–depression score MD ‐0.45, 95% CI ‐0.79 to ‐0.12).
2.12. Analysis.

Comparison 2: Self‐management versus usual care (secondary outcomes), Outcome 12: Health status: Hospital Anxiety and Depression Scale (HADS)
Four studies could not be included in meta‐analyses on anxiety and depression because insufficient data were reported by authors or they did not use the HADS to assess the outcome (Emery 1998; Kessler 2018; Liang 2019; Rose 2018). Kessler 2018 and Liang 2019 reported no difference for HADS scores between the self‐management intervention and usual care groups (Kessler 2018 adjusted total score MD 0.2, 95% CI ‐0.3 to 0.7; Liang 2019 self‐management intervention: anxiety median 0 (IQR 0 to 4) and depression median 1 (IQR 0 to 3.25); usual care: anxiety median 0 (IQR 0 to 3) and depression median 0 (IQR 0 to 1.25). Rose 2018 could not be included in the meta‐analysis due to lack of per‐group participant numbers for both HADS anxiety and depression scores. They found no evidence that the self‐management intervention changed HADS scores at 6 and 12 months' follow‐up (no effects reported). Emery 1998 used different units of measurement to assess anxiety and depression. Anxiety was assessed by the anxiety subscales of the State‐Trait Anxiety Inventory (STAI) and the Hopkins Symptom Checklist. Depression was assessed by the Center for Epidemiological Studies‐Depression inventory (CES‐D), the depression subscale of the Hopkins Symptom Checklist and the Bradburn Affect‐Balance Scale. No differences in anxiety and depression were found between education and stress management (ESM) and waiting list groups (usual care) after 10 weeks' follow‐up.
Self‐efficacy
Nine studies reported data on self‐efficacy (Bischoff 2012; Bringsvor 2018; Bucknall 2012; Fan 2012; Johnson‐Warrington 2016; Jolly 2018; Lenferink 2019; Mitchell 2014; Walters 2013), but no meta‐analysis could be performed because of insufficient data and the use of different outcome measures. Three of these studies measured self‐efficacy by using the COPD Self‐Efficacy Scale (CSES). Bischoff 2012 reported differences in participants’ self‐efficacy between the intervention and control groups according to the CSES total (MD ‐0.17, 95% CI ‐0.64 to 0.30) and domain scores after 24 months' follow‐up. Bucknall 2012 also reported lower CSES total scores in the self‐management intervention group, but the CI indicates no difference (MD 2.65, 95% CI ‐5.85 to 11.14). Lenferink 2019 reported a reduction in the behavioural risk factors domain of the CSES (MD ‐0.26, 95% CI ‐0.52 to ‐0.01). Bringsvor 2018 measured self‐efficacy by using the General Self‐Efficacy Scale (GSE), but reported no mean change differences (P = 0.18). Fan 2012 measured participants’ self‐efficacy by a self‐developed 8‐item questionnaire and reported an improvement in the self‐management intervention group after 12 months' follow‐up (MD 0.65, 95% CI 0.02 to 1.29). In Johnson‐Warrington 2016 and Mitchell 2014, self‐efficacy was assessed with the Pulmonary Rehabilitation Adapted Index of Self‐Efficacy (PRAISE). Johnson‐Warrington 2016 found no difference between change in self‐efficacy in groups (self‐management intervention: mean change 0.54, SD 9.48; usual care: mean change 2.34, SD 8.73). Mitchell 2014 also reported no between‐group difference in PRAISE score (MD 1.47, 95% CI ‐0.65 to 3.60; P = 0.21). Jolly 2018 measured self‐efficacy with the Stanford self‐efficacy score, but found no difference between the self‐management intervention group compared to the usual care group after 12 months' follow‐up (MD 0.1, 95% CI ‐0.1 to 0.4). Walters 2013 assessed self‐efficacy with the Self‐Efficacy for Managing Chronic Disease (SE MCD); no improvement in self‐efficacy was reported (self‐management intervention versus usual care: β coefficient 0.41, 95% CI ‐0.56 to 1.37).
Days lost from work
Only Gallefoss 1999 reported days lost from work. No differences between groups were observed. Almost 50% of the participants with COPD in this study were employed. Only three of 14 (21%) participants in the self‐management intervention group and two of 13 (15%) in the usual care group reported absence from work.
Exercise capacity and physical activity
Six studies, with 772 participants, measured exercise capacity using the six‐minute walking test (6MWT) and could be included in the meta‐analysis (Bösch 2007; Bourbeau 2003; Hernández 2015; Kessler 2018; Tabak 2014; Wang 2019). Compared to usual care, self‐management interventions may result in an improvement in exercise capacity, with an MD of 45.14 meters (95% CI 9.16 to 81.13; Analysis 2.13). Heterogeneity was considerable (I2 = 86%). Sensitivity analysis using a FEM resulted in similar effects (MD 46.57, 95% CI 33.73 to 59.41; I2 = 86%). The pooled MD of 45.14 meters reached the MCID of 25 meters and therefore is considered clinically relevant (Holland 2010). Two studies – Tabak 2014 and Wang 2019 – found a very high mean distance walked for the self‐management intervention group compared to usual care (Tabak 2014: MD 99.66, 95% CI 66.45 to 132.75; Wang 2019: MD 89.00, 95% CI 63.36 to 114.64). The large variation in effect sizes seems to be the main contributor to the considerable heterogeneity in this analysis.
2.13. Analysis.

Comparison 2: Self‐management versus usual care (secondary outcomes), Outcome 13: Exercise capacity: six‐minute walk test (6MWT)
Two studies assessed exercise capacity with both the Incremental Shuttle Walk Test (ISWT) and Endurance Shuttle Walk Test (ESWT) (Johnson‐Warrington 2016; Mitchell 2014). In Johnson‐Warrington 2016, no differences were found between the self‐management intervention and usual care groups for both ISWT (self‐management intervention: mean change (metres (m)) 45, 95% CI 0 to 70; usual care: mean change (m) 30, 95% CI 0 to 95) and ESWT (self‐management intervention: mean change (seconds) 178.5, 95% CI ‐3.75 to 443.50; usual care: mean change (seconds) 155, 95% CI 21 to 618.50). Mitchell 2014 reported a between‐group difference in the change in distance walked on the ISWT at six weeks' follow‐up, but there were no differences reported at six months' follow‐up. Furthermore, the ESWT time improved in the self‐management intervention group compared to the usual care group at six weeks and was maintained between six weeks and six months.
Jonsdottir 2015 assessed self‐reported physical activity of different intensities (walking, moderate intensity, and vigorous intensity) using the International Physical Activity Questionnaire short version (IPAQ). Jonsdottir 2015 reported a higher score on the subscale ‘vigorous’ for the self‐management intervention group after 12 months' follow‐up (P = 0.02). Furthermore, they reported a lower score on the subscale ‘walking’ with time in both groups (P = 0.02).
Self‐management behaviour
Three studies reported data on self‐management behaviour (Bringsvor 2018; Lenferink 2019; Walters 2013). However, a meta‐analysis was not possible, because different outcome measures were used. Two of these studies measured self‐management behaviour and knowledge using the Partners in Health scale (PIH) (Lenferink 2019; Walters 2013). Walters 2013 reported an interaction of treatment group by time for the overall PIH score (β coefficient 0.15, 95% CI 0.03 to 0.29) and for the PIH knowledge domain (β coefficient 0.25, 95% CI 0.00 to 0.50). Furthermore, an increase over time in both groups for the PIH coping domain was observed (β coefficient 0.15, 95% CI 0.04 to 0.26). Lenferink 2019 reported no between‐group differences for the overall PIH score and PIH domain scores between self‐management intervention and usual care groups (between‐group difference: 0.28, 95% CI ‐2.43 to 3.00). Bringsvor 2018 used the 'Health education impact Questionnaire' (HeiQ 2) to measure eight self‐management domains: 1) Positive and active engagement in life; 2) Health‐directed activities; 3) Skill and technique acquisition; 4) Constructive attitudes and approaches; 5) Self‐monitoring and insight; 6) Health service navigation; 7) Social integration and support; and 8) Emotional distress. Positive changes were observed in intention‐to‐treat (ITT) analyses for the 'Constructive attitudes and approaches' domain (MD in change 0.14, 95% CI 0.00 to 0.27; P < 0.01) and 'Skill and technique acquisition' domain (MD in change 0.06, 95% CI ‐0.06 to 0.19; P = 0.04).
Patient activation
One study – Titova 2015 – measured patient activation using the Patient Activation Measure (PAM) and reported no differences between self‐management intervention and usual care in the mean values of the PAM scores at 6 months (MD in change 0.5, 95% CI ‐6.2 to 7.3), 12 months (MD in change 0.5, 95% CI ‐5.8 to 6.7) and 24 months' follow‐up (MD in change 3.1, 95% CI ‐4.5 to 10.8).
Health literacy
No studies reported data on health literacy.
Subgroup analyses
We performed 28 of the a priori 42 defined subgroup analyses for three outcomes: HRQoL, respiratory‐related hospital admissions, and all‐cause mortality (see Table 5). Fourteen subgroup analyses on outcomes of interest could not be performed due to an inadequate number of studies (n ≤ 2) in one of the two subgroups, and were therefore not presented.
4. Subgroup analyses.
| HRQoL | Respiratory‐related hospital admissions | All‐cause mortality | ||||
| Group description | Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 |
| Duration of intervention: 1. Short (< 8 weeks) versus 2. Long (≥ 8 weeks) |
2* | 12* | 2* | 13* | 3 | 21 |
| COPD stability at inclusion: 1. Acute; versus 2. Stable |
2* | 7* | 3 | 8 | 4 | 14 |
| Income country: 1. Low/medium; versus 2. High |
1* | 13* | 0* | 15* | 1* | 23* |
| Care setting intervention: 1. Primary; versus 2. Secondary/tertiary |
7 | 6 | 5 | 9 | 10 | 13 |
| COPD exacerbation action plan component: 1. Yes; versus 2. No |
11 | 3 | 15* | 0* | 21 | 3 |
| Home‐based exercise/physical activity component: 1. Yes; versus 2. No |
8 | 6 | 8 | 7 | 15 | 9 |
| Smoking cessation component: 1. Yes; versus 2. No |
9 | 5 | 6 | 9 | 13 | 11 |
| Diet component: 1. Yes; versus 2. No |
6 | 8 | 4 | 11 | 7 | 17 |
| Medication component: 1. Yes; versus 2. No |
13* | 1* | 10 | 5 | 19 | 5 |
| Coping with breathlessness component: 1. Yes; versus 2. No |
9 | 5 | 8 | 7 | 13 | 11 |
| Self‐recognition of COPD exacerbations component: 1. Yes; versus 2. No |
12* | 2* | 13* | 2* | 19 | 5 |
| Use of digital technology: 1. Yes; versus 2. No |
2* | 12* | 3 | 12 | 3 | 21 |
| Integration of BCT clusters: 1. 2 BCTs; versus 2. > 2 BCTs |
1* | 14* | 1* | 15* | 2* | 23* |
| Integration of BCT clusters: 1. ≤ median of 4; versus 2. > median of 4 |
4 | 10 | 5 | 10 | 10 | 14 |
BCT: behaviour change technique; COPD: chronic obstructive pulmonary disease; HRQoL: health‐related quality of life
*Subgroup analysis could not be performed because of an insufficient number of studies in one of the subgroups.
Duration of intervention: short (< 8 weeks) versus longer (≥ 8 weeks)
No difference was found in all‐cause mortality between studies with short intervention duration (n = 3; RD ‐0.00, 95% CI ‐0.02 to 0.02) or longer intervention duration (n = 21; RD ‐0.02, 95% CI ‐0.04 to 0.01) (test for subgroup differences: Chi2 = 0.93, degrees of freedom (df) = 1 (P = 0.33), I2 = 0%) (forest plots not shown).
COPD stability at time of inclusion: acute phase versus stable phase
No difference was found in respiratory‐related hospital admissions between studies that included participants in acute phase (n = 3; OR 0.59, 95% CI 0.32 to 1.08) or stable phase (n = 8; OR 0.74, 95% CI 0.48 to 1.15) (test for subgroup differences: Chi2 = 0.35, df = 1 (P = 0.55), I2 = 0%) (forest plots not shown).
Also, no difference was found in all‐cause mortality between studies that included participants in acute phase (n = 4; RD ‐0.00, 95% CI ‐0.07 to 0.06) or stable phase (n = 14; RD 0.00, 95% CI ‐0.02 to 0.02) (test for subgroup differences: Chi2 = 0.02, df = 1 (P = 0.89), I2 = 0%) (forest plots not shown).
Country of intervention: low‐ and medium‐income versus high‐income
We classified included studies into a low‐, medium‐ or high‐income country according to the World Bank list of economies (World Bank 2021). Only one study was conducted in a middle‐income country (Wang 2019), while the other included studies were all conducted in high‐income countries. We were therefore not able to create subgroups of sufficient size to permit meta‐analyses on low‐ and middle‐income countries versus high‐income countries.
Care setting of intervention: primary care versus secondary and tertiary care
No difference for the effects on HRQoL was detected between studies that were conducted in a primary care setting (n = 7; MD ‐3.53, 95% CI ‐5.15 to ‐1.92) compared to a secondary and tertiary care setting (n = 6; MD ‐2.48, 95% CI ‐6.69 to 1.73) (test for subgroup differences: Chi2 = 0.21, df = 1 (P = 0.65), I2 = 0%) (forest plots not shown).
No difference was also found in respiratory‐related hospital admissions between studies conducted in a primary care setting (n = 5; OR 0.87, 95% CI 0.46 to 1.63) versus a secondary and tertiary care setting (n = 9; OR 0.64, 95% CI 0.52 to 0.79) (test for subgroup differences: Chi2 = 0.82, df = 1 (P = 0.36), I2 = 0%) (forest plots not shown).
No difference in the effects on all‐cause mortality was found for studies conducted in a primary care setting (n = 10; RD 0.01, 95% CI ‐0.01 to 0.02) versus a secondary and tertiary care setting (n = 13; RD ‐0.02, 95% CI ‐0.06 to 0.01) (test for subgroup differences: Chi2 = 2.34, df = 1 (P = 0.13), I2 = 57.2%) (forest plots not shown).
Use of self‐management intervention components:
Inclusion of a ‘COPD exacerbation action plan component’
We found no difference in HRQoL for studies with a COPD exacerbation action plan as a self‐management intervention component (n = 11; MD ‐2.57, 95% CI ‐4.11 to ‐1.04) versus studies without a plan (n = 3; MD ‐3.13, 95% CI ‐13.29 to 7.02) (test for subgroup differences: Chi2 = 0.01, df = 1 (P = 0.92), I2 = 0%) (forest plots not shown).
No difference for all‐cause mortality was found for studies with a COPD exacerbation action plan as a self‐management intervention component (n = 21; RD ‐0.01, 95% CI ‐0.03 to 0.01) versus studies without a plan (n = 3; RD 0.01, 95% CI ‐0.01 to 0.03) (test for subgroup differences: Chi2 = 1.86, df = 1 (P = 0.17), I2 = 46.2%) (forest plots not shown).
Inclusion of a ‘home‐based exercise programme or physical activity component’
For HRQoL, we found no difference between the subgroups (studies with a home‐based exercise or physical activity component: n = 8 (MD ‐2.51, 95% CI ‐5.78 to 0.77) versus studies without a home‐based exercise or physical activity component: n = 6 (MD ‐3.18, 95% CI ‐5.53 to ‐0.83) (test for subgroup differences: Chi2 = 0.11, df = 1 (P = 0.74), I2 = 0%) (forest plots not shown)).
For respiratory‐related hospital admissions, no difference was found between the inclusion of a home‐based exercise or physical activity component (n = 8; OR 0.82, 95% CI 0.50 to 1.33) versus the absence of a home‐based exercise or physical activity component (n = 7; OR 0.72, 95% CI 0.51 to 1.02) (test for subgroup differences: Chi2 = 0.18, df = 1 (P = 0.67), I2 = 0%) (forest plots not shown).
For all‐cause mortality, again, no difference was found between the inclusion of a home‐based exercise or physical activity component (n = 15; RD ‐0.02, 95% CI ‐0.04 to 0.00) versus the absence of a home‐based exercise or physical activity component (n = 9; RD 0.01, 95% CI ‐0.03 to 0.05) (test for subgroup differences: Chi2 = 1.74, df = 1 (P = 0.19), I2 = 42.6%) (forest plots not shown).
Inclusion of a ‘smoking cessation component’
No effect was observed in a subgroup analysis on HRQoL (studies including a smoking cessation component: n= 9 (MD ‐3.37, 95% CI ‐5.95, ‐0.79) versus studies without a smoking cessation component: n = 5 (MD 0.99, 95% CI ‐3.16 to 1.18) (test for subgroup differences: Chi2 = 1.91, df = 1, (P = 0.17), I2 = 47.8%) (forest plots not shown)).
A subgroup analysis on respiratory‐related hospital admissions showed a between‐group difference of studies with a smoking cessation component (n = 6; OR 1.10, 95% CI 0.68 to 1.79) versus studies without a smoking cessation component (n = 9; OR 0.60, 95% CI 0.44 to 0.82) (test for subgroup differences: Chi2 = 4.20, df = 1 (P = 0.04)), including a substantial variability in effect estimates from the different subgroups (I2 = 76.2%) (Analysis 3.1; Figure 10).
3.1. Analysis.

Comparison 3: Subgroup analyses: self‐management versus usual care, Outcome 1: Healthcare utilisation: respiratory‐related hospital admissions (subgroup by smoking cessation component)
10.

Forest plot of comparison: self‐management versus usual care, outcome: 3.1 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by smoking cessation component)
For all‐cause mortality, no difference was found between the group of studies that included a smoking cessation component (n = 13; RD ‐0.02, 95% CI ‐0.04 to 0.01) versus the group of studies that did not include a smoking cessation component (n = 11; RD 0.01, 95% CI ‐0.03 to 0.04) (test for subgroup differences: Chi2 = 1.23, df = 1 (P = 0.27), I2 = 18.4%) (forest plots not shown).
Inclusion of a ‘diet component’
For HRQoL, no effect was observed between the subgroups (including diet component: n = 6 (MD ‐1.84, 95% CI ‐3.94 to 0.25); no diet component: n = 8 (MD ‐3.55, 95% CI ‐6.54 to ‐0.57) (test for subgroup differences: Chi2 = 0.84, df = 1 (P = 0.36), I2 = 0%) (forest plots not shown)).
We found no subgroup differences in respiratory‐related hospital admissions between studies with a diet component (n = 4; OR 1.22, 95% CI 0.57 to 2.58) and without a diet component (n = 11; OR 0.65, 95% CI 0.52 to 0.80) (test for subgroup differences: Chi2 = 2.49, df = 1 (P = 0.11), I2 = 59.8%) (forest plots not shown).
For all‐cause mortality, no difference was observed between studies including a diet component (n = 7; RD ‐0.03, 95% CI ‐0.07 to 0.02) versus without a diet component (n = 17; RD ‐0.00, 95% CI ‐0.02 to 0.01) (test for subgroup differences: Chi2 = 0.88, df = 1 (P = 0.35), I2 = 0%) (forest plots not shown).
Inclusion of a ‘medication component’
For respiratory‐related hospital admissions, no difference was observed between the subgroups (studies including a medication component: n = 10 (OR 0.82, 95% CI 0.59 to 1.16) versus studies with no medication component: n = 5 (MD 0.60; 95% CI 0.40 to 0.91) (test for subgroup differences: Chi2 = 1.29, df = 1 (P = 0.26), I2 = 22.8%) (forest plots not shown)).
We also did not find a difference for all‐cause mortality for studies with a medication component (n = 19; RD ‐0.01, 95% CI ‐0.03 to 0.01) versus without a medication component (n = 5; RD ‐0.01, 95% CI ‐0.07 to 0.05) (test for subgroup differences: Chi2 = 0.00, df = 1 (P = 0.97), I2 = 0%) (forest plots not shown).
Inclusion of a ‘coping with breathlessness component’
No effect was found in a subgroup analysis on HRQoL for studies with a 'coping with breathlessness' component (n = 9; MD ‐3.73, 95% CI ‐6.93 to ‐0.52) versus studies without such a component (n = 5; MD ‐1.89, 95% CI ‐4.41 to 0.63) (test for subgroup differences: Chi2 = 0.78, df = 1 (P = 0.38), I2 = 0%) (forest plots not shown).
No effect was observed in a subgroup analysis on respiratory‐related hospital admissions for studies with a 'coping with breathlessness' component (n = 8; OR 0.77, 95% CI 0.48 to 1.24) versus studies without such a component (n = 7; OR 0.71, 95% CI 0.56 to 0.91) (test for subgroup differences: Chi2 = 0.08, df = 1 (P = 0.77), I2 = 0%) (forest plots not shown).
No difference was found in effects on all‐cause mortality between studies with a 'coping with breathlessness' component (n = 13; RD ‐0.01, 95% CI ‐0.04 to 0.01) versus studies without such a component (n = 11; RD ‐0.00, 95% CI ‐0.03 to 0.02) (test for subgroup differences: Chi2 = 0.40, df = 1 (P = 0.53), I2 = 0%) (forest plots not shown).
Inclusion of a ‘self‐recognition of COPD exacerbations component’
For all‐cause mortality, we found no difference between studies with a 'self‐recognition of COPD exacerbations' component (n = 19; RD ‐0.01, 95% CI ‐0.03 to 0.01) and studies without such a component (n = 5; RD ‐0.00, 95% CI ‐0.03 to 0.02) (test for subgroup differences: Chi2 = 0.27, df = 1 (P = 0.60), I2 = 0%) (forest plots not shown).
Use of digital technology
No difference was observed for the probability of respiratory‐related hospital admissions between studies that incorporated digital technology (n = 3; OR 0.85, 95% CI 0.17 to 4.15) versus studies without digital technology (n = 12; OR 0.74, 95% CI 0.58 to 0.95) (test for subgroup differences: Chi2 = 0.03, df = 1 (P = 0.87), I2 = 0%) (forest plots not shown).
There was also no difference observed in the risk of all‐cause mortality for studies with digital technology (n = 3; RD ‐0.00, 95% CI ‐0.15 to 0.15) versus without digital technology (n = 21; RD ‐0.01, 95% CI ‐0.03 to 0.01) (test for subgroup differences: Chi2 = 0.01, df = 1 (P = 0.92), I2 = 0%) (forest plots not shown).
Integration of behavioural change technique (BCT) clusters: high number of BCT clusters (> median of 4) versus low number of BCT clusters (≤ median of 4)
We observed no difference for the effects on HRQoL amongst studies with a high number of BCT clusters (n = 10; MD ‐2.62, 95% CI ‐5.37 to 0.13) versus a low number of BCT clusters (n = 4; MD ‐3.79, 95% CI ‐6.02 to ‐1.56) (test for subgroup differences: Chi2 = 0.42, df = 1 (P = 0.52), I2 = 0%) (forest plots not shown).
Subgroup analyses on BCT clusters integrated in the self‐management intervention also showed no differences in respiratory‐related hospital admissions in studies with a high number of BCT clusters (n = 10; OR 0.86, 95% CI 0.60 to 1.22) compared to studies with a low number of BCT clusters (n = 5; OR 0.57, 95% CI 0.43 to 0.76) (test for subgroup differences: Chi2 = 3.08, df = 1 (P = 0.08)), including a substantial variability in effect estimates from different subgroups (I2 = 67.6%) (forest plots not shown).
No difference between subgroups on all‐cause mortality was found amongst studies with a low number of BCT clusters (n = 10; RD ‐0.03, 95% CI ‐0.06 to 0.01) and studies with a high number of BCT clusters (n = 14; RD 0.00, 95% CI ‐0.01 to 0.02) (test for subgroup differences: Chi2 = 2.78, df = 1 (P = 0.10)), including a substantial variability in effect estimates from different subgroups (I2 = 64.1%) (forest plots not shown).
Recently published studies
The updated search from January 2020 to March 2021 identified one study (Ozturk 2020), that met all the current inclusion criteria. This study included several COPD self‐management intervention components; namely, smoking cessation, exercise and physical activity, coping with breathlessness, energy‐saving techniques, psychological assessment and nutritional training. They observed beneficial effects in the self‐management intervention compared to usual care in HRQoL measured by the total SGRQ score (P = 0.02), health status measured by the CAT score (P < 0.001), anxiety and depression symptoms measured by the HADS score (anxiety: P = 0.01; depression: P = 0.01) (Ozturk 2020).
Discussion
Summary of main results
This is an update of a review previously published in 2014 (Zwerink 2014). We systematically evaluated 25 RCTs and two CRTs (described in 38 articles) on the effectiveness of COPD self‐management interventions compared to usual care. Compared to the previous review update, we included 21 new self‐management studies. We had to exclude 19 of 29 previously included studies, due to the application of stricter inclusion criteria regarding COPD self‐management interventions and COPD diagnosis (Effing 2016), and because some of the previously included studies were not RCTs. Positive effects of COPD self‐management interventions on HRQoL and respiratory‐related hospitalisations were detected (Effing 2007; Monninkhof 2002; Monninkhof 2003; Zwerink 2014). The lack of observed effects regarding respiratory‐related and all‐cause mortality has strengthened the view that COPD self‐management interventions are unlikely to cause harm. Applying stricter inclusion criteria has led to less heterogeneity in interventions, and has also resulted in fewer studies being eligible for review. Most predefined subgroup analyses did not show differences; the small numbers of studies in many of these subgroup analyses may have contributed to this. As a result, we have been unsuccessful in identifying potentially effective (intervention) characteristics that can be linked to COPD self‐management intervention outcomes.
We observed a beneficial effect for COPD self‐management interventions on HRQoL, measured by the SGRQ adjusted total score which did not reach the MCID of four points (Jones 2005). We determined a priori that, in addition to analysing the final outcome points, if possible, we would also analyse short‐term (≤ 6 months' follow‐up), medium‐term (> 6 to ≤ 12 months' follow‐up), and long‐term (> 12 months' follow‐up) effects of the primary outcomes in this review. For the SGRQ, these analyses could be performed for short‐ and medium‐term effects. Only the analysis for the medium‐term effects showed a pooled beneficial effect favouring self‐management. This could suggest that HRQoL may further improve when people with COPD develop their self‐management skills over time. However, due to the highly overlapping CIs of short‐ and medium‐term SGRQ effects, and the impossibility of performing a subgroup difference test, this interpretation should be treated with caution, and no conclusions can be drawn from our data regarding the influence of time on HRQoL improvements.
A beneficial self‐management effect was observed for respiratory‐related hospital admissions. Participants in self‐management intervention study arms were at a lower risk for at least one respiratory‐related hospital admission compared to participants who received usual care. Fifteen participants with high baseline risk and 14 participants with low baseline risk needed to be treated to prevent one respiratory‐related hospital admission over a mean follow‐up of 9.75 months.
We observed no difference between self‐management interventions and usual care for the risk of all‐cause mortality.
No effect was found on respiratory‐related mortality (RD 0.01, 95% CI ‐0.02 to 0.04). In a review regarding the effect of COPD self‐management interventions, including exacerbation action plans, published in 2017 (Lenferink 2017), a very small, but higher respiratory‐related mortality rate was found in the self‐management intervention group compared to the usual care group (RD 0.03, 95% CI 0.05 to 0.05). The two studies that dominated this negative effect were also included in the current analysis (Bucknall 2012; Fan 2012), but with more studies and participants included (1572 versus 1219 participants), no detrimental effect was detected.
In the current update, we did not find a difference in the probability of all‐cause hospital admissions in the self‐management intervention group compared to the usual care group. The previous 2014 update reported that the probability of having one or more all‐cause hospital admissions was higher in the self‐management group (Zwerink 2014). The included studies in our meta‐analysis (n = 10) included four of the six previously included studies. Six newly included studies showed heterogeneity of effect sizes. The lack of effect on all‐cause hospitalisations in our review may be explained by the fact that most COPD self‐management intervention studies are still predominantly directed towards COPD and do not include treatment components directed towards frequently existing comorbidities (Zwerink 2014).
A beneficial difference was observed for self‐management interventions on ED visits, both for the number of participants with at least one visit and the mean number of visits per participant. However, the lack of clear definitions of ED visits in most studies and considerable heterogeneity in the mean number of ED visits per participant meaning that these results should be interpreted with caution.
A higher use of oral corticosteroids and antibiotics was reported in the self‐management groups. The higher probability of using at least one course of oral steroids was found after sensitivity analysis and should be interpreted with caution. Only three studies were included in this sensitivity analysis (Gallefoss 1999; Rice 2010; Sanchez‐Nieto 2016), with the Rice 2010 study having a large population and high proportion of events. No sensitivity analysis excluding Rice 2010 could be performed because of the limited number of studies. Higher use of both corticosteroids and antibiotics in the self‐management groups may have been triggered by the use of exacerbation action plans encouraging earlier initiation of self‐treatment with corticosteroids, and when necessary, antibiotics. However, the differences in medication use could as likely be caused by actual undertreatment of exacerbations in the usual care group.
Anxiety and depression, measured by the HADS, were both reduced in participants assigned to the self‐management intervention. Whereas the presence of a mental health component in most of the interventions would have been a very plausible explanation, this was not the case, as only two of the nine studies included in the meta‐analyses had a mental health component (Jonsdottir 2015; Walters 2013). However, six of the nine studies included a ‘coping with breathlessness’ component (Bucknall 2012; Hernández 2015; Jonsdottir 2015; Lenferink 2019; Titova 2015; Walters 2013), which may have contributed to the positive effects on anxiety and depression. Respiratory health and especially breathlessness in COPD can trigger anxiety symptoms (Heslop‐Marshall 2014). Importantly, the baseline levels of anxiety and depression in most of the included studies were quite high (mean total HADS scores > 11 in both study groups) (Spinhoven 1997; Zigmond 1983), resulting in room for improvement for this parameter.
The MD of 45 meters between self‐management intervention and usual care groups in the 6MWT was clinically relevant, favouring self‐management. As only two of the six studies in this meta‐analysis included a ‘home‐based exercise component’ in their self‐management intervention, it is not plausible that this component was the only contributor to the improvement in walking distance. The ‘COPD exacerbation action plan component’ – included in five of the six studies – may also have played a role in this improvement as it encourages prompt treatment of exacerbations and therefore may have led to less severe exacerbations, a faster recovery, and possibly a better physical condition.
Subgroup analyses
The total number of included studies provided the opportunity to perform several subgroup analyses to try to gain greater insight into the “black box” of COPD self‐management interventions. However, only one subgroup analysis showed a difference between effects in the subgroups. A limited number of studies in the majority of subgroup analyses may have contributed to the lack of effects.
Studies without a smoking cessation component showed a lower probability on respiratory‐related hospital admissions favouring self‐management compared to studies with a smoking cessation component. This is the opposite effect of what was expected, as smoking cessation is associated with a reduction in hospitalisations (Godtfredsen 2002). A possible explanation may be that participants in both the self‐management and usual care groups of studies without a smoking cessation component may have already quit smoking before entering the study, and may therefore have already achieved the beneficial effect on respiratory‐related hospital admission before study entry, leading to less room for improvement by the self‐management intervention. The numbers of current smokers in study groups included in this subgroup analysis were however fairly comparable at baseline (range of current smokers in self‐management intervention groups: 13.0% to 53.5%; range of current smokers in usual care groups: 14.0% to 71.3%). Unfortunately, no data were reported regarding the participants that actually stopped smoking during the intervention, and we cannot rule out differences in other variables between the two groups that may explain this effect. A meta‐regression analysis would have provided us with the possibility to adjust for potential effect modifiers (Higgins 2019). Unfortunately, due to an insufficient number of studies in our review, we could not use this very promising statistical technique, as at least 10 studies for each characteristic modelled are needed. However, the technique should be considered if future reviews include significantly more studies.
Overall completeness and applicability of evidence
Our review showed beneficial effects on HRQoL and respiratory‐related hospital admissions. Additionally, beneficial effects were detected for ED visits, anxiety, depression and exercise capacity. Also, no increase in mortality was detected in the COPD self‐management interventions, which strengthens the view that these interventions are unlikely to cause harm. Lastly, our results showed higher use of antibiotic courses in the self‐management group.
This review included 6008 participants with COPD having a post‐bronchodilator FEV1 to FVC ratio of less than 0.7. We included studies conducted in 13 different countries on four different continents (15 in Europe, eight in North America, one in Asia, and four in Oceania; with one study conducted in both Europe and Oceania), suggesting that our findings can be generalised across various high‐income healthcare settings. Ideally, more studies from Asia would have been included, but we encountered problems with gaining required inclusion information from six potentially eligible Asian studies (Abdulsalim 2017; Alharbey 2019; Ghanem 2010; Li Z 2015; Liu 2013; Lou 2015). Having a better distribution of included studies over all continents would certainly increase the generalisability of the review results. Our searches were current up to January 2020.
There are some limitations to the generalisability of our results. We had difficulties collecting essential information regarding 12 studies (Abdulsalim 2017; Aboumatar 2017; Alharbey 2019; Efraimsson 2008; Ghanem 2010; Heidari 2018; Hill 2010; Jiang 2012; Khdour 2009; Li 2014; Liu 2013; Lou 2015). Based on the information provided in the publications, we were not able to check whether the studies included only participants that met our COPD diagnosis criteria, and whether they had at least two self‐management intervention components provided to all included participants using an iterative process. We made at least three attempts to request information from the authors of these studies. Unfortunately, we received no response from the authors.
Three of the included studies (11%) had follow‐up periods of three months or less (Bringsvor 2018; Emery 1998; Johnson‐Warrington 2016). Depending on the time of participant enrolment (e.g. during summer), seasonal variation may have influenced the outcomes in these studies (e.g. the number of exacerbations). This may have resulted in an under‐ or overestimation of the actual effect. The study by Fan 2012 was prematurely stopped with a mean follow‐up of 250 days, because of a higher number of deaths in the intervention group compared with the control group that could not be explained satisfactorily by the study authors. It is therefore uncertain if a true effect was observed. The results of this study need to be interpreted with caution.
Moreover, we were not able to perform a meta‐analysis on physical activity outcomes, because of limited studies including this component. Many studies that incorporated a sole physical activity component were excluded from this review, as inclusion required at least two components with an iterative process.
Over the span of 25 years, views about what is required for COPD self‐management interventions have changed. Nowadays, it is agreed that self‐management interventions should incorporate BCTs and encourage activation of participants. In addition, social support and digital technology have more frequently been integrated. Whereas usual care is diverse across countries, it is likely that usual care has been optimised over the years, and that self‐management approaches are increasingly embedded in usual care. This leads to the expectation that the observed benefits of self‐management interventions compared to usual care will be diminishing.
Quality of the evidence
All 27 included studies in our review were judged as having an overall high risk of bias for several reasons. Due to the nature of COPD self‐management interventions, it is not possible to blind participants and personnel during RCTs and CRTs. In addition, none of the studies provided detailed information regarding the distribution of non‐protocol interventions to the study groups during the follow‐up period of the study. Finally, not a single study reported whether an appropriate analysis was used to estimate the effect of adhering to interventions. As a result, all studies scored ‘high risk’ in the same domain of the risk of bias 2 (ROB 2) tool (i.e. domain 2 – ‘deviations from the intended interventions’)(Sterne 2019), and consequently scored ‘high’ on overall risk of bias. This directly affected the GRADE score (Guyatt 2011); outcomes were downgraded from high‐ to moderate‐quality evidence, from moderate‐ to low‐quality evidence, and from low‐ to very low‐quality evidence.
With the assigned overall high risk of bias score and moderate heterogeneity, the quality of evidence for HRQoL was graded as low. The improvement in HRQoL, measured by the adjusted SGRQ total score, did not reach the MCID. Therefore, we need to consider this carefully as the positive effects may only have been clinically relevant for part of the population. Furthermore, the overall high risk of bias score, moderate heterogeneity of included studies and a wide 95% CI resulted in very low‐quality evidence for respiratory‐related hospital admissions. We graded the quality of evidence for all‐cause and respiratory‐related mortality as low because of the high risk of bias in all included studies, and substantial heterogeneity resulting in inconsistency in both mortality outcomes. Finally, we graded the quality of evidence for all other secondary outcomes as moderate to low; assessments were based on fewer studies or smaller sample sizes, or both.
Because of the nature of the self‐management intervention, blinding of personnel and participants to group assignment is complicated and will be very unlikely in future studies. Improvement in the risk of bias due to deviations from intended interventions may be achieved if studies: a) provide better descriptions of non‐protocol interventions used in study groups (e.g. by using online repositories); and b) detail intervention implementation failures and non‐adherence to the intervention; or c) apply and describe appropriate analyses to estimate the effect of adhering to interventions. By doing this, the overall risk of bias will be reduced, and consequently, the overall quality of the evidence will improve.
Serious inconsistency in effect sizes affected the quality of evidence of more than half of the outcomes. Heterogeneity in intervention content may have contributed to this inconsistency. In this review, we have tried to decrease this heterogeneity by applying stricter inclusion criteria in line with the most recent published definition of COPD self‐management interventions (Effing 2016). However, because of the nature of COPD self‐management interventions and because individual tailoring is desirable, heterogeneity in future interventions will be inevitable; it will never be a 'one size fits all' intervention.
Potential biases in the review process
We observed heterogeneity in clinical diversity (e.g. care setting, intervention components, BCTs, intensity and duration), (primary) outcome measures, and statistical diversity (e.g. variability in intervention effects). As inclusion of studies in this review was not based on reported outcome measures, we observed a broad spectrum of outcome measures with various methods for assessment (e.g. different questionnaires for the same outcome measure) and various calculations (e.g. mean number versus the percentage of participants). We could therefore not perform all predefined meta‐analyses due to insufficient (< 3 studies) similarly‐reported outcome data.
Studies were only eligible if self‐management interventions had at least two intervention components that were offered to all included participants. Several self‐management intervention studies that incorporated intervention components and characteristics tailored to the individual (e.g. personalised care plans), could not confirm that at least two specific components were offered to each included participant, and therefore could not be included in this review.
Furthermore, studies were only included when data or authors confirmed that all included participants met COPD spirometry FEV1/FVC criteria (GOLD 2021). However, crucial information on COPD diagnosis remained missing, even after several contact attempts with authors. We have therefore been unable to include all potentially eligible studies.
In this review update, we classified the Titova 2015 study as an RCT. However, we acknowledge that this classification is tentative, as it remains unclear whether random sequence allocation was performed at the participant or health centre level. If random sequence allocation was performed solely at health centre level, the study should have been classified as a CRT.
A priori, we expected to see heterogeneity amongst studies due to the nature of the self‐management intervention. We therefore decided to use a REM for the meta‐analyses. This model weighs by study, rather than number of participants, when heterogeneity is present. However, when only a few large studies and many small studies are included, this may result in bias introduced by small‐study effects. We therefore performed several sensitivity analyses using FEM meta‐analysis. However, bias introduced by small‐study effects was unlikely, as the observed effect sizes in FEM and REM were comparable, except for the meta‐analysis on the use of oral corticosteroids where a non‐significant REM resulted in a significant FEM analysis.
Self‐management interventions were not always described in sufficient detail to allow coding of all applied BCTs. It is possible that some BCTs were present in interventions but were not adequately described by study authors. For standardisation purposes, we decided to use only data that were explicitly reported in published articles of included studies, and we coded BCTs by using the mobile BCT Taxonomy application (BCT Taxonomy). So, we have not used any extra information that was provided by authors for determining BCTs (e.g. unpublished protocols). Our approach has almost certainly led to an underestimation of the number (and variety) of BCTs and may therefore have contributed to the lack of found effects in subgroup analyses regarding BCTs. There is a significant need for providing more detailed, uniformly and transparently reported data on BCTs used in self‐management interventions in future studies – for example, by using online journal repositories – to increase the meaningfulness of the BCT subgroup analysis.
Agreements and disagreements with other studies or reviews
A previous Cochrane Review by Lenferink and colleagues on COPD self‐management interventions that include action plans for exacerbations of COPD (Lenferink 2017), reported similar beneficial effects on HRQoL, measured by SGRQ (MD ‐2.69, 95% CI ‐4.49 to ‐0.90), and respiratory‐related hospital admissions (OR 0.69, 95% CI 0.51 to 0.94). However, they observed a small negative effect on respiratory‐related mortality (RD 0.03, 95% CI 0.05 to 0.05), which was not observed in the present review. Their review highlighted that self‐management interventions including exacerbation action plans with a smoking cessation programme contributed to significant improvements in HRQoL (Lenferink 2017). However, this could not be confirmed by subgroup analyses in our review.
A review by Jonkman 2016 aimed to quantify the diversity in components of self‐management interventions and aimed to identify intervention components that improve HRQoL, measured by SGRQ, in chronically ill participants (i.e. COPD, chronic heart failure, diabetes), by conducting individual patient data analysis. They found that self‐management interventions improve HRQoL in participants with COPD at 12 months (SMD 0.08, 95% CI 0.00 to 0.16), but not at 6 months (SMD 0.05, 95% CI ‐0.05 to 0.15). This finding could not be confirmed in our review. Their subgroup analyses did not identify any intervention components that were associated with the intervention effects (Jonkman 2016). Furthermore, a risk reduction was found at 12 months in respiratory‐related hospital admissions (RR 0.77, 95% CI 0.64 to 0.93) and all‐cause hospital admissions (RR 0.84, 95% CI 0.73 to 0.96). It was also observed that a longer duration of self‐management interventions conferred a reduction in respiratory‐related hospital admissions (hazard ratio 0.79, 95% CI 0.66 to 0.94) and all‐cause hospitalisations (hazard ratio 0.80, 95% CI 0.69 to 0.92). These results strengthen the finding that self‐management interventions with longer follow‐up duration should be recommended for clinical practice, rather than interventions with short‐term follow‐up. Whether the observed lower risks are clinically relevant is unclear, because there is no MCID for hospitalisations.
Jordan 2015 conducted a review, including ten RCTs, on the effectiveness of supported self‐management interventions that were delivered to participants with COPD who had recently been discharged from hospital. As in the current review, a beneficial effect on HRQoL, measured by SGRQ, was reported (MD 3.48, 95% CI 1.29 to 6.40), but the Jordan 2015 review included studies that showed high rates of loss to follow‐up. Furthermore, no distinct beneficial effects were found on mortality, anxiety and depression, and exercise capacity.
Another review on COPD self‐management interventions by Newham 2017 observed that self‐management interventions were significantly more effective than usual care. In line with the current review, they found significant improvements in HRQoL, measured by SGRQ, and a reduced number of ED visits. In addition, self‐management interventions that tackle mental health concerns were considered to be more effective than those that focused on symptom management alone. As in the current review, they used an established taxonomy – Michie 2013 – to assess the integration of BCTs into self‐management interventions of included studies. Also in line with our review, they observed no significant association between number of BCTs and improvement in HRQoL (Newham 2017).
Furthermore, a review by Jolly 2018 on the effectiveness of community‐based self‐management interventions reported no effects on HRQoL (measured by SGRQ) or anxiety and depression. This review only included studies conducted in primary care, and therefore only included participants with mild or moderate COPD. In our review, we performed a subgroup analysis on HRQoL in studies delivered in primary care settings (n = 7) versus secondary and tertiary care settings (n = 6) and found no significant between‐group differences in effects. Jolly 2018 argued that people with COPD in primary care may still benefit from self‐management support, although it may be ineffective in its current form. Further research in this primary care COPD population is recommended to identify suitable and effective self‐management interventions for the less severe primary care population.
Finally, a recent review by Song 2021 aimed to evaluate 'blended' (i.e. eHealth combined with individual face‐to‐face) self‐management interventions compared to: 1) eHealth interventions with and without usual care; 2) face‐to‐face interventions with or without usual care; and 3) usual care only, in participants with COPD and asthma. In participants with COPD, they revealed beneficial effects of the blended self‐management intervention on HRQoL (measured by SGRQ, CAT and CRQ), exercise capacity and hospital admissions. However, the comparator was unclear.
Authors' conclusions
Implications for practice.
Self‐management interventions in people with COPD are associated with improvement in HRQoL, as measured by the SGRQ; a reduction in both respiratory‐related admissions and ED visits; a likely improvement in both anxiety and depression symptoms, and exercise capacity; and probably more use of antibiotics. No differences were found in other outcome parameters. In addition, the lack of observed effects regarding respiratory‐related and all‐cause mortality strengthens the view that COPD self‐management interventions are unlikely to cause harm. By using stricter inclusion criteria, we have decreased the heterogeneity amongst studies, but also reduced the number of studies that could be included in this review and therefore our capacity to do subgroup analyses. Consequently, the data are insufficient to permit clear conclusions about effective (intervention) characteristics of COPD self‐management interventions (e.g. duration of the intervention, intervention components). Because tailoring of self‐management interventions to individuals is desirable, heterogeneity is and will in all likelihood remain present in self‐management interventions.
Future clinical practice may focus on the following strategy:
Ensuring that the self‐management interventions meet the criteria of the definition of COPD self‐management interventions (Effing 2016) (e.g. include multiple intervention components with an iterative process between healthcare provider(s) and participants, directed towards behaviour change).
Implications for research.
Future studies and systematic reviews of studies should focus on the following points and improvements:
Providing more detailed, uniformly and transparently reported data on the self‐management intervention components and the BCTs used.
Achieving greater homogeneity in outcome measures, with greater attention to behavioural outcome measures.
Assessing outcomes over the long term (> 12 months' follow‐up), as COPD self‐management interventions are directed towards behavioural change, which is often not achieved in a short period of time and if this is the case, it would be of interest to know whether this change is maintained.
Providing more detailed information regarding deviations from intended interventions to allow for higher certainty of evidence of outcomes (i.e. information regarding distribution of non‐protocol interventions, as well as analyses used to estimate the effect of adhering to intervention).
What's new
| Date | Event | Description |
|---|---|---|
| 13 March 2023 | Amended | Correction to typo in the Results section (subgroup analyses, page 32) |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 1, 2003
| Date | Event | Description |
|---|---|---|
| 14 January 2022 | Amended | Title changed |
| 27 January 2020 | New citation required and conclusions have changed | Complete rewrite of the review conducted. 21 new studies added. New risk of bias assessment completed for all included studies. References in background updated. Literature search run in March 2021 and not fully incorporated see Studies awaiting classification. |
| 27 January 2020 | New search has been performed | New literature search run. |
| 7 July 2014 | Amended | We amended the data for all‐cause hospitalisations. This outcome now favours the self‐management group (OR 0.60 95%CI 0.45 to 0.89) and the review was revised accordingly. |
| 31 August 2011 | New search has been performed | New literature search run |
| 31 August 2011 | New citation required and conclusions have changed | Complete rewrite of the review conducted. Summary of findings table added. 14 new studies added. New risk of bias assessment completed for all included studies. References in background updated Change of title—'education' removed |
| 25 March 2008 | Amended | Converted to new review format. |
| 21 August 2007 | New citation required and conclusions have changed | New studies: N = 7 (Bourbeau 2003; Boxall 2005; Coultas 2005a; Coutas 2005b; Martin 2004; Monninkhof 2003; Rea 2004) What these studies have added: Data on health related quality of life; exacerbations (hospitalisations, requirement for oral steroids); lung function (FEV1). Quality of life scores and respiratory‐related hospital admission now show significant benefits. Lung function parameters do not show a significant difference. Steroid‐treated exacerbations were not significantly different. How this has changed the review: The review now demonstrates that from the self‐management interventions assessed in the studies assembled in the review, patients were less likely to require hospital admissions when treated with this type of intervention. There was a small improvement in total quality of life scores measured by the St George's Respiratory Questionnaire. There were no indications of detrimental effects in other outcome parameters. The effects of different components of self‐management interventions and their requisite intensity requires more research. |
Risk of bias
Risk of bias for analysis 1.1 Health‐related quality of life (HRQoL): adjusted SGRQ total score (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Gallefoss 1999 | Low risk of bias | Comment: At inclusion they signed a written consent and were then randomised to an intervention group or a control group. | High risk of bias |
Effect of assignment:
Comment: No information reported about blinding, but it is almost impossible to blind these patients because of the nature of the intervention. Furthermore, no information was reported whether deviations arose from the intended intervention because of the trial context. Effect of adhering: "Forty‐six percent of those educated (12 of 26) received a standard treatment plan. Non‐standard treatment plans, incorporating the use of oral steroids as the first line of action in the yellow zone, were followed in some cases, for example, if the patient already used high dosages of inhaled steroids as maintenance therapy, or could report that a double or triple increase in inhaled steroids previously had shown marginal effect on the course of attacks/exacerbations. Among those 14 patients receiving non‐standard treatment plans, eight patients did not want to or were not able to use peak flow monitoring as a basis for change in medication. For those patients, symptom‐only based treatment plans were issued." Gallefoss 2002, p. 425. "In the intervention group, four patients failed to complete the educational program (social problems (n = 1), unannounced emigration (n = 1), failure to meet at educational group sessions for unknown reasons (n = 1) and serious myocardial infarction (n = 1))." Gallefoss 2002, p. 427. Comment: No information reported about adherence to the programme of the other patients (who completed the programme). |
High risk of bias | “In the intervention group, four patients failed to complete the educational programme (social problems (n = 1), unannounced emigration (n = 1), failure to meet at educational group sessions for unknown reasons (n = 1) and serious myocardial infarction (n = 1)). Another patient was withdrawn from the study during the follow‐up due to lymphoma (n = 1). This left us with 26 patients (81%) for a 1‐year follow‐up. The patients who were withdrawn from the intervention group did not, to our knowledge, have any serious deterioration in their obstructive lung disease, and none were hospitalised. In the control group four patients were withdrawn (lack of co‐operation (n = 2), diagnosis of rectal cancer (n = 1) and emigration (n = 1)). Two of the withdrawn control group patients were hospitalised for exacerbations of their COPD. This left us with 27 patients (84%) for the 1‐year follow‐up” Gallefoss 2002, p. 427. Comment: The number of dropouts was relatively low, and reasons for dropout were comparable over groups |
High risk of bias | "Prebronchodilator spirometry was performed before randomization and at 12 mo follow‐up by standard methods using a Jaeger MasterLab Body Box (Würzburg, Germany). The technical staff did not know whether the patients belonged to the control or intervention groups." Gallefoss 1999, p. 2003. Comment: No information reported regarding the assessors of the primary outcome. Number of prednisolone courses was retrospectively self‐reported at 12 months of follow‐up. |
Some concerns | "We defined a priori the patient as compliant when dispensed regular medication was greater than 75% of prescribed regular medication during the study period." Gallefoss 1999, p. 2002. | High risk of bias | Unpredictable direction of the outcome. |
| Bourbeau 2003 | Low risk of bias | “… central computergenerated list of random numbers. Randomization was stratified per center and in blocks of 6, and patients were assigned to the self‐management program (intervention group) or to usual care.” p. 586. “The blocking factor was not known by the investigators or their staff in each participating center." p. 586. “The use of respiratory medications was similar between study groups, except that oral steroids were used less commonly in the intervention group (7%) than in the usual care group (13%). None of the disease severity characteristics were otherwise different between the study groups.” p. 586. Comment: Random sequence allocation was adequately performed. |
High risk of bias |
Effect of assignment:
"Since a double‐blind design was impossible..." p. 586.
Comment: Participants and personnel were not blinded. ITT analysis was performed. Effect of adhering: "Since a double‐blind design was impossible..." p. 586. Comment: Participants and personnel were not blinded. Comment: There might be some imbalance in non‐protocol interventions such as exercise evaluation (not mandatory). However, no information available. |
Low risk of bias | ‐ | Low risk of bias | "...an independent evaluator unaware of the patient assignment was responsible for the evaluation process in each center. The evaluator was cautioned not to ask about the workbook modules and types of contact.” p. 586. Comment: Outcome assessment was blinded. |
Some concerns | Comment: No protocol or prespecified analysis plan available. | High risk of bias | Unpredictable direction of the outcome. |
| Coultas 2005 | Low risk of bias | “Patients were randomly assigned to one of three intervention groups (usual care (UC), nurse‐assisted medical management (MM), or nurse‐assisted collaborative management (CM)) using a computer‐generated random list.” p. 2018. “Although the distribution of most of the baseline characteristics of the three groups was similar (Tables 2 and 3), only the distribution of gender and the tangible domain of social support were significantly different among the intervention groups.” p. 2020. Comment: It is unclear how allocation concealment was guaranteed. Observed imbalances are probably compatible with chance. Random sequence generation was adequately performed. |
High risk of bias |
Effect of assignment:
“Of the 217 patients enrolled in the study, 151 (69.6%) completed the 6‐month intervention and follow‐up data collection.” p. 2020.
“The reasons for the failure to complete the study were patient was unavailable for follow‐up (26.7%) and death (3.7%) [Fig 1]. The frequency of patients being unavailable for follow‐up was equally distributed among the three intervention groups (Fig 1). Overall, the demographic characteristics of the patients who dropped out of the study were similar to those who completed the study (data not shown). However, patients who dropped out of the study had more severe airflow obstruction, higher levels of distress, and lower quality of life, as measured with the SGRQ, compared with the patients who had completed the study (data not shown).” p. 2020.
Comment: Not reported whether deviations arose because of the trial context. Only patients who completed the 6‐month intervention and follow‐up data collection were analysed (per‐protocol analyses). Effect of adhering: Comment: Patients and personnnel were probably aware of assignment to the intervention during the trial. |
High risk of bias | “The reasons for the failure to complete the study were patient was unavailable for follow‐up (26.7%) and death (3.7%).” p. 2020. “However, patients who dropped out of the study had more severe airflow obstruction, higher levels of distress, and lower quality of life, as measured with the SGRQ, compared with the patients who had completed the study (data not shown).” p. 2020. Comment: Only patients who completed the 6‐month intervention and follow‐up data collection were analysed. |
Low risk of bias | “To limit interviewer bias, each interviewer who obtained the baseline and 6‐month outcome data was blinded to the patient’s treatment group.” p. 2023. "Although self‐reported health‐care utilization may be subject to recall bias, it has been shown to be an acceptable method of measurement and may actually result in an underestimation of utilization with more frequent use.” p. 2023. |
Some concerns | Comment: No protocol available. Measures and analysis intentions were only reported in the method section of the paper. | High risk of bias | Unpredictable direction of the outcome. |
| Coultas 2005 | Low risk of bias | “Patients were randomly assigned to one of three intervention groups (usual care (UC), nurse‐assisted medical management (MM), or nurse‐assisted collaborative management (CM)) using a computer‐generated random list.” p. 2018. “Although the distribution of most of the baseline characteristics of the three groups was similar (Tables 2 and 3), only the distribution of gender and the tangible domain of social support were significantly different among the intervention groups.” p. 2020. Comment: It is unclear how allocation concealment was guaranteed. Observed imbalances are probably compatible with chance. Random sequence generation was adequately performed. |
High risk of bias |
Effect of assignment:
“Of the 217 patients enrolled in the study, 151 (69.6%) completed the 6‐month intervention and follow‐up data collection.” p. 2020.
“The reasons for the failure to complete the study were patient was unavailable for follow‐up (26.7%) and death (3.7%) [Fig 1]. The frequency of patients being unavailable for follow‐up was equally distributed among the three intervention groups (Fig 1). Overall, the demographic characteristics of the patients who dropped out of the study were similar to those who completed the study (data not shown). However, patients who dropped out of the study had more severe airflow obstruction, higher levels of distress, and lower quality of life, as measured with the SGRQ, compared with the patients who had completed the study (data not shown).” p. 2020.
Comment: Not reported whether deviations arose because of the trial context. Only patients who completed the 6‐month intervention and follow‐up data collection were analysed (per‐protocol analyses). Effect of adhering: Comment: Patients and personnnel were probably aware of assignment to the intervention during the trial. |
High risk of bias | “The reasons for the failure to complete the study were patient was unavailable for follow‐up (26.7%) and death (3.7%).” p. 2020. “However, patients who dropped out of the study had more severe airflow obstruction, higher levels of distress, and lower quality of life, as measured with the SGRQ, compared with the patients who had completed the study (data not shown).” p. 2020. Comment: Only patients who completed the 6‐month intervention and follow‐up data collection were analysed. |
Low risk of bias | “To limit interviewer bias, each interviewer who obtained the baseline and 6‐month outcome data was blinded to the patient’s treatment group.” p. 2023. "Although self‐reported health‐care utilization may be subject to recall bias, it has been shown to be an acceptable method of measurement and may actually result in an underestimation of utilization with more frequent use.” p. 2023. |
Some concerns | Comment: No protocol available. Measures and analysis intentions were only reported in the method section of the paper. | High risk of bias | Unpredictable direction of the outcome. |
| Rice 2010 | Some concerns | "We assigned subjects in equal proportions to each of the two treatment arms by permuted‐block randomisation." Appendix 1, p. 3. "The baseline characteristics of the two treatment groups were generally well matched, with differences in age, lung function, and ED visits (not significant)." p. 891. Comment: Information on the method of allocation concealment was not reported. |
High risk of bias |
Effect of assignment:
"We performed a randomized, adjudicator‐blinded, controlled, 1‐year trial." p. 890.
"The status of all 743 patients was determined after 1 year. Electronic medical records were available for all hospitalizations and ED visits that occurred within the VA medical system. The success rate for obtaining discharge summaries and ED records for self‐reported events that occurred outside the VA was 99% in the usual care group and 98% in the disease management group." p. 891. Effect of adhering: Comment: 336 out of 372 (90.3%) patients completed disease management 323 out of 371 (87%) completed usual care. |
Low risk of bias | "55% of patients in the usual care group and 60% of patients in the disease management group returned a completed the Saint George’s Respiratory Questionnaire in response to a single mailing at the end of the study. low response rates on SGRQ leading to a high risk of bias. However, data on healthcare utilisation seem complete with no risk of bias." p. 892. | Low risk of bias | "Blinded pulmonologists independently reviewed all discharge summaries and ED reports and assigned a primary cause for each." p. 891. | High risk of bias | Comment: Primary outcome in the paper is reported as a combined outcome measure, whereas it was planned to analyse the two outcomes separately. | High risk of bias | Unpredictable direction of the outcome. |
| Bucknall 2012 | Low risk of bias | “We used a minimisation technique to stratify randomisation of participants by demographic factors (deprivation category of area of residence,11 age and sex, FEV1 per cent predicted at the time of randomisation, smoking status, participation in pulmonary rehabilitation within two years, and number of previous admissions) to control for key aspects of disease severity and predictors of readmission. We constructed a computer generated sequence by using the method of randomised permuted blocks of length four, with two allocations being made at random and two by minimisation. Treatment group allocations were obtained by telephone, after baseline assessments had been made.” p 2. Comment: Random sequence allocation was adequately performed. |
High risk of bias |
Effect of assignment:
Comment: No blinding of patients and personnel. Data for the primary outcome (time to first acute COPD hospital admission or death) were available for all patients and analysed with a Cox proportional hazards model adjusting for the stratification variables. Effect of adhering: Comment: No blinding of patients and personnel. Seventy‐five (42%) of 180 intervention group participants who completed 12 months of follow‐up were judged to have become successful self managers. |
High risk of bias | Comment: For secondary outcome measures (e.g. SGRQ, HADS, CSES) not all data were available for all patients randomised (Table 2). | Low risk of bias | ‐ | Some concerns | ‐ | High risk of bias | Unpredictable direction of the outcome. |
| Fan 2012 | Low risk of bias | “Randomization lists were generated on the basis of random, permuted blocks of variable size to ensure approximate balance over time.” p. 674. “The CSP Coordinating Center in Boston, Massachusetts, randomly assigned eligible patients in equal numbers to 2 groups, stratifying patients per site to allow for possible regional differences in patient characteristics and clinical practice patterns.” p. 674. Comment: Random sequence generation was adequately performed. The allocation was adequately concealed. Authors didn’t report whether there were any significant differences. Most characteristics are equally balanced, but there is a bit larger difference in the following characteristics (>5%):
|
High risk of bias |
Effect of assignment:
“The 2 groups differed on the basis of a complex behavioral intervention that made blinding impossible.” p. 674.
Comment: No blinding of participants and personnel.
"For the primary intention‐to‐treat analysis, the null hypothesis was that the COPD hospitalization hazard rate for intervention did not differ from that for standardized care."p. 675.
"Also, with respect to time to first COPD hospitalization, we conducted a futility analysis to assess what might have resulted if the trial had continued." p. 676.
"When the study was stopped, the 1‐year cumulative incidence of COPD‐related hospitalization was 27% in the intervention group and 24% in the usual care group (hazard ratio, 1.13 [95% CI, 0.70 to 1.80]; P 0.62)." p. 677. Effect of adhering: "During the entire follow‐up period extending to September 2009, a total of 8 patients in the intervention group and 10 in the usual care group either did not attend scheduled study visits or formally withdrew from the study. Among patients in the intervention group, 87% completed all 4 individual educational visits and 57% completed the group visit. A review of the recorded sessions found that case managers covered 77% to 89% of the educational items in each session. The ranges of average scores across sites that provided audio recordings for each session were 18.3 to 31 for session 1 (13 sites), 12 to 19 for session 2 (12 sites), 12.7 to 23 for session 3 (10 sites), and 6 to 18 for session 4 (10 sites)." p. 677. |
High risk of bias | “This multisite, randomized, controlled trial of an educational and acute care management program was stopped early when a safety monitoring board noted more deaths in the intervention group.” p. 674 Comment: There is incomplete outcome data due to early termination of the study. |
Low risk of bias | “Telephone‐based ascertainment of study outcomes (COPD hospitalizations and exacerbations) was performed by centralized research staff blinded to assignment. All outcomes were collected by centralized staff blinded to study group, and all hospitalizations were adjudicated by a committee that was also blinded to study group.” p. 674. Comment: Research staff blinded to study group contacted patients every 2 months to determine whether they developed symptoms of a COPD exacerbation, along with details of treatment and health care use. Deaths were ascertained by staff at the study sites, by review of VA electronic medical and administrative records and by a search of the Social Security Death Index. Extensive multivariate analyses failed to identify any factors alone or combined that could plausibly explain the difference in mortality. Two of the authors who were blinded to study group reviewed all available medical information and did not find anything in the deaths that would not be expected in frail patients with COPD. |
Some concerns | Comment: The primary and secondary outcomes were reported, only healthcare costs as (secondary objective) were not reported. | High risk of bias | Predicted directon: Favours comparator, because more deaths in the intervention group were observed. There is still a chance that this was because of chance. |
| Walters 2013 | High risk of bias |
Randomisation process:
"After recruitment, practices were randomised using a code generated by investigators from a random numbers table stratified in blocks of four by Rural, Remote and Metropolitan Areas (RRMA) classifications in Tasmania. Allocation occurred independently using sequentially numbered, opaque and sealed envelopes." p. 2. Timing of identification or recruitment of participants: GPs identified patients with COPD aged over 45 years seen within the previous 12 months through database searches, based on a diagnostic code for COPD or prescription of tiotropium. Comment: Most variables are equally distributed, but more smokers (not statistically significant, p=0.102) and more previous admissions in the intervention group (statistically significant, p=0.028), so potentially more room for improvement. Although, this small number of differences identified as ‘statistically significant’ at the conventional 0.05 threshold should usually be considered to be compatible with chance. |
High risk of bias |
Effect of assignment:
‐ Effect of adhering: "The limitations of the study were the high rate of misclassification of COPD in general practices, which impacted on enrolment, and higher than expected withdrawal rates, especially in the HM group, which reduced the intended statistical power." p. 6. Comment: Nine patients discontinued and 8 patients withdrew in the intervention group. |
Low risk of bias | Comment: Figure 1: 9 intervention patients discontinued, but they attended follow‐up. In Table 2, no patient numbers are mentioned. | Low risk of bias | Comment: Blinding of participants or research officers was not possible given the nature of the study. | Low risk of bias | Comment: Data that produced this result analysed in accordance with a pre‐specified analysis plan. | High risk of bias | Unpredictable direction of the outcome. |
| Hernández 2015 | Low risk of bias | "A computer‐generated list of random numbers with no restrictions and administered by personnel who were not involved in the study ensured blinded randomisation." p. 2. "(…) and administered by personnel who were not involved in the study." p. 2. Comment: Random sequence generation was adequately performed. No blinding of participants or personnel. The patients of the two groups showed similar characteristics, except that influenza and pneumococcal vaccination were more prevalent in the IC group (P<0.01). |
High risk of bias |
Effect of assignment:
Comment: It was not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analysis performed. Effect of adhering: "As a sensitivity analysis, we repeated all analyses after excluding patients who had a previous contact with any IC programme (n = 15)." p. 2. "25 patients (22%) died and 16 (14%) were lost during the 1‐year follow‐up, and the remaining 114 patients (IC, n = 59 and UC, n = 55) completed the 12‐month assessment visit. Patients lost to follow‐up exhibited a higher affectation of activities of daily living and health‐related quality of life at baseline than those who were followed up, without differences in other sociodemographic, clinical, functional and medical care‐related variables." p. 3. Comment: It is not mentioned in which way these patients were distributed over the two groups, nor are the results of the sensitivity analysis reported. Although the approach was not adequately adopted at the community level and thus the intervention was not continued after the 1 year of follow‐up, this was not a failure of implementing the intervention, since the intervention stopped at 1 year according to the protocol. |
High risk of bias | Comment: Refusal of n=5 in the intervention group. Lost to follow‐up 12 in intervention group and 29 in usual care group. So in total 22% died and 14% were lost to follow‐up. Patients lost to follow‐up exhibited a higher affectation of activities of daily living and health‐related quality of life at baseline than those who were followed up, (no differences in other sociodemographic, clinical, functional and medical care‐related variables) this could have biased the results between the intervention group and usual care group. | Low risk of bias | "A blind evaluation of the study group carried out before randomisation and after the 12‐month follow‐up consisted of a patient interview and analysis of medical records, self‐administered questionnaires and lung function testing." p. 2. | Some concerns | "Although the study was officially registered as part of the EU Grant (CIP‐ICT‐PSP‐2007.225025), the RCT was not included in the clinicaltrials.gov registry because at that time it was not compulsory." p. 5. Comment: No protocol available. |
High risk of bias | Direction: favours experimental. Comment: More patients were lost to follow‐up in the usual care group and patients lost to follow‐up exhibited a higher affectation of activities of daily living and health‐related quality of life at baseline than those who were followed up. |
| Jonsdottir 2015 | Low risk of bias | "After accepting the invitation, prior to entering the research centre, the research nurses randomized the patients to experimental and control groups in accordance with CONSORT requirements using a computer‐generated list of random numbers." p. 2638. | High risk of bias |
Effect of assignment:
Comment: Not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analysis was not carried out as stated in the article, but in table 3 all patients that completed the follow‐up seem to be included. Effect of adhering: "To minimize treatment bias the data were concealed from the nurses providing the treatment and the treatment notes were concealed from the nurses conducting data collection." p. 2640. "Both groups followed standardized protocols and had regular meetings to maintain consistency. Few family members participated. This actually changed the nature of the study from being a family‐centered intervention as planned, into becoming focused on patients, with family involvement when possible." p. 2640. "Attendance at treatment sessions in the experimental group was more than 80% in the patient/family conversations (43/48 attended three or four out of the four possible meetings) and the group meeting (40/48). Half of the patients in the experimental group smoked (24/48). Of those, 58% (14/24) participated in the smoking cessation treatment." p. 2641. |
High risk of bias | "Three patients died in the experimental group... The dropout rate was 20% (n = 12) in the experimental group and 12% (n = 7) in the control group. Also additional missing data on outcome variables as displayed in table 3. The dropout rate for family members was 31% (n = 5) and 50% (n = 7) in the experimental and control groups respectively." p. 2640. Comment: No info on dropout reasons or characteristics of dropouts. |
Low risk of bias | "To minimize treatment bias the data were concealed from the nurses providing the treatment and the treatment notes were concealed from the nurses conducting data collection." p. 2640. | High risk of bias | No information, not registered in a trial register. | High risk of bias | Unpredictable direction of the outcome. |
| Titova 2015 | High risk of bias |
Randomisation process:
“They were randomly allocated to either integrated care (IC) or usual care (UC) based on their address of permanent residence. In order to create two pairs of districts with approximately equal numbers of citizens, a pair‐wise matching of districts was carried out. It was decided by lottery that participants from District Pair 1 were assigned to the UC group, and participants from District Pair 2 were assigned to the IC group.” Titova 2015, p. 2.
“There were no significant differences in baseline characteristics between the IC and the UC groups, but when comparing those who died during follow‐up with those who were still alive after two years, those who died had a significantly higher PaCO2 and lower BMI at baseline (Table 1). Those who died during follow‐up also had significantly more HA and HD during the year prior to study start.” Titova 2015, p. 4.
Comment: No information provided about the allocation concealment. Timing of identification or recruitment of participants: "They were randomly allocated to either IC or UC group based on their address of permanent residence. Patient data were registered at discharge and 6, 12, and 24 months after discharge from the TUH." Titova 2017, p 3. Comment: If allocation was known before study inclusion, suitable patients could be selected for the intervention. |
High risk of bias |
Effect of assignment:
“The study was a prospective, open, single‐centre intervention study.” Titova 2015, p. 2.
"The principal comparisons of changes were performed on an intention‐to treat basis." Titova 2017, p. 4.
Comment: No blinding of participants and personnel. Effect of adhering: ‐ |
High risk of bias | “172 patients were randomly allocated to the IC group (n = 91) or the UC group (n = 81), respectively. After two years of follow‐up, a total of 100 patients (58% of the included patients), 51 patients in the IC group and 49 patients in the UC group, were available for evaluation (Figure 1).” Titova 2017, p. 5. "Those who died had a significantly higher PaCO2 and lower BMI at baseline (Table 1). Those who died during follow‐up also had significantly more HA and HD during the year prior to study start." Titova 2015, p. 4. Comment: No mortality reported; however, Figure 1 shows higher mortality for the IC group, n=35 (38.4%), compared to the UC group, n=21 (25.9%). |
High risk of bias | “Information concerning the number and duration of the COPD exacerbations, as well as the time from onset of symptoms until the start of self‐initiated treatment is insufficient due to many incomplete registrations in “My COPD book.” Titova 2015, p. 9. "“Data concerning HA (hospital admissions) and HD (hospital days) were collected from the hospital registry database’s medical charts.” Titova 2015, p. 3. Comment: Unclear who was the outcome assessor. |
Some concerns | Trial register reported number of hospitalisations as the primary outcome. Secondary outcomes were quality of life, lung function, use of medication, cost‐effectiveness. However, the article reported not all secondary outcomes (e.g. cost‐effectiveness). | High risk of bias | Unpredictable direction of the outcome. |
| Jolly 2018 | High risk of bias | "When a patient is identified as eligible for the study, and has given written, informed consent to take part, the researcher randomises the patient using a web‐based programme hosted by the Primary Care Clinical Trials Unit (PC‐CTU), University of Birmingham. Centre specific randomisation lists were produced by a statistician at the trials unit. Participants are individually randomised, stratified by centre. The four recruitment centres are Birmingham and West‐Midlands South; Greater Manchester; North West Midlands and Oxfordshire/Gloucestershire. Only the PC‐CTU have access to the allocation sequence." Sidhu 2015 (protocol paper), p. 4. "The study groups were generally well balanced in terms of patient characteristics, although there was a higher proportion of current smokers in the telephone health coaching group. The usual care group reported a higher level of self reported moderate and vigorous physical activity, but this was not reflected in the accelerometry data at baseline." Jolly 2018 (results paper), p. 5. |
High risk of bias |
Effect of assignment:
Comment: Patients were informed of their allocation at the end of the recruitment appointment; they were not masked to treatment allocation. All data were analysed by intention to treat. Effect of adhering: "We achieved good fidelity of delivery of the intervention, with 75% of intervention participants receiving all four calls and only four patients receiving none." p. 11. "The dose and coverage of intervention delivery were high: 86.4% (999/1156) of the scheduled calls were delivered and 75.4% (218/289) of participants received all four calls." No information regarding pulmonary rehabilitation, oxygen use. |
High risk of bias | "There was imbalance in the follow‐up rates between telephone health coaching (82.7%; 37 withdrawals) and usual care (96.2%; 7 withdrawals)." p. 4. "Participants who did not provide data at 12 months were more likely to be in GOLD stage 3, to be smokers, had lower levels of self‐reported physical activity, and to live alone than responders. " p. 5. Comment: For health‐related quality of life, data were available for 75% of intervention patients (217 of 289), although some sensitivity analyses were performed. |
Some concerns | "Patients were informed of their allocation at the end of the recruitment appointment; they were not masked to treatment allocation. Data were entered into the study database by researchers at the University of Birmingham who were masked to the treatment allocation." p. 3. Comment: No information about outcome assessors. |
Low risk of bias | ‐ | High risk of bias | Unpredictable direction of the outcome. |
| Kessler 2018 | Low risk of bias | "Patients were allocated to groups in a 1:1 fashion according to a pre‐specified randomisation list generated before the study by a partial‐minimisation computer algorithm under supervision of the study sponsor. Patients were assigned a randomisation number by study staff at each centre in sequential numerical order through a telephone‐based interactive voice response system. Randomisation was stratified by smoking status (current or former), need for respiratory assistance (none, or on LTOT and/or HMV), and centre." p. 3. | High risk of bias |
Effect of assignment:
"For practical reasons, the study was open; neither the patients nor the investigators were blinded to the COPD management strategy." p. 3.
"The primary analysis of the primary outcome was performed in the intention‐to‐treat (ITT) population (all patients who entered the follow‐up period). The number of hospitalisation days fitting the established criteria was normalised on a yearly basis for each patient and compared between both groups using the non‐parametric Wilcoxon rank‐sum test." p. 4.
"The open design of the study, though necessary owing to the extensive differences between the two disease management programmes, may have led to some bias because doctors could have had a tendency to discharge patients in the disease management arm earlier than those in the usual management arm." p. 12. Effect of adhering: Comment: 15 patients (<10%) received <25% self‐management training. |
Low risk of bias | "…leaving 157 (91%) and 162 (94%) patients in the ITT population, 137 (80%) and 128 (74%) of whom completed at least 12 months of follow‐up." p. 5. | Low risk of bias | "Because hospitalisation decisions might have been subject to local practices, social considerations, bed availability, and so on, all hospitalisations were reviewed by an independent, three‐member end‐point validation committee (EVC) to assess the reason and appropriateness of each hospitalisation, determine whether the hospitalisation fulfilled the protocol definition, and determine the extent to which it was to be considered in the efficacy assessments (fully, partially, not at all)." p. 3. | Low risk of bias | "During the study, the protocol was amended to accelerate recruitment and allow study completion (supplementary material)." p. 3. Comment: Inclusion criteria were expanded from COPD patients receiving LTOT to patients with severe COPD, with or without LTOT; the minimum number of patients was reduced from 520 to 306 by retaining the initial assumptions for sample size and removing a multiple comparisons adjustment; the follow‐up period was reduced from 2 years to 1 year; and the contact frequency between DM patients and CMs was increased to improve patient training (either group or phone session, once per month). Prior to these changes, 72 patients on LTOT were enrolled in the study. | High risk of bias | Unpredictable direction of the outcome. |
| Wang 2019 | Low risk of bias | "The allocation sequence was generated and released to the interventionist on a case‐by‐case basis by an independent department that specialised in supplying randomly generated sequences for research. The randomisation sequence was recorded." p. 151. "Participants were randomly assigned to groups after consent was obtained and baseline data were collected." p. 151. "There were no significant differences in demographic and clinical data between the two groups at baseline (Table 1)." p. 151. |
High risk of bias |
Effect of assignment:
Comment: It is not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analysis was used. Effect of adhering: "154 participants were enrolled, among whom, 143 completed the 12‐month study program. The attrition rate was 7.14%." p. 151. "...adherence to the self‐management intervention was reported by the participants but not monitored by the nurses." p. 155. Comment: No info on reasons of adherence by patients reported in the current publication. |
Low risk of bias | Comment: Relatively low attrition rate. | Low risk of bias | "To minimize researcher bias, the interventionists who provided care were blinded to the participants’ baseline and allocation sequence. The statistician was blinded to the participants’ results during the trial." p. 151. Comment: Not possible to blind the treatment for patients and people delivering the treatment. |
Some concerns | Comment: No pre‐specified analysis plan published which can be checked. However they mention: The statistician was blinded to the participants’ results during the trial. | High risk of bias | Unpredictable direction of the outcome. |
| Liang 2019 | High risk of bias | "Group or solo GP clinics with ≥1000 patients on their databases were approached directly or with assistance from primary care organisations. After obtaining signed agreement, clinics were block randomised (block sizes of four and six) to usual care or intervention arms using an externally managed web‐based randomisation programme. Clinics were notified of their allocation." p. 3. | High risk of bias |
Effect of assignment:
‐ Effect of adhering: "67 participants (43%) did not receive any part of the intervention." p. 7. "A key limitation is the low uptake of the intervention components." p. 10. |
High risk of bias | Comment: Data were not available for all patients randomised. | Low risk of bias | "The effective analysis was according to an intention‐to‐treat principle." p. 4. | Low risk of bias | Results were analysed according to the published design paper. | High risk of bias | Predicted direction: Favours comparator, because general practices could exclude patients from selection to participate in the intervention due to the cluster randomisation approach. Adherence to the intervention was low. |
Risk of bias for analysis 1.3 Health‐related quality of life (HRQoL): CRQ domain scores (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.3.1 HRQoL: CRQ ‐ dyspnoea | ||||||||||||
| Bischoff 2012 | Some concerns | "We randomised participants by using a computer generated two block randomisation procedure with stratification on severity of COPD (mild or moderate v severe airflow obstruction), smoking status (current v former smoker), and frequency of exacerbations in the previous 24 months (<2 v ≥ 2 exacerbations)". p. 2.
"Table 1 shows that at inclusion in the study, patients’ characteristics were well balanced between the three study groups, except for sex.". p. 7. Comment: Random sequence generation was adequately performed. No information on who performed the allocation. There is another paper published, with different patient numbers (Uijen, British Journal of General Practice, June 2012), this paper shows a higher number of patients in the intervention group (n= 64) compared to the Bischoff’s paper (n= 55), but a lower number of patients in the usual care group (n= 49) compared to the Bischoff’s paper (n= 55). This raised some concerns about the randomisation process. Clarification could not be given by the authors. |
High risk of bias |
Effect of assignment:
"Our primary analysis was based on the intention to treat principle and included all available data for all participants. We compared self management with usual care and routine monitoring with usual care. We did not impute any missing data." p. 3.
Comment: It is not possible to blind patients / carers /people delivering the intervention due to the nature of this intervention. Effect of adhering: "“We prevented potential treatment contamination caused by provision of self management, routine monitoring, and usual care within the same." p. 2. "Seven (13%) patients did not receive any session or telephone contact, and 48 (87%) received two or more sessions." p. 4. "Six (11%) patients did not receive any consultation, and 42 (76%) patients received two or more consultations." p. 4. Comment: There was some non‐adherence in intervention group (at least 10%), this may all have led to some effect in the usual care group and a smaller difference between the intervention and usual care group. |
Some concerns | Comment: Almost 16% of the participants dropped out during follow‐up (intervention 11%; usual care 20%). However, baseline characteristics did not differ between dropouts and participants who finished follow‐up. Exclusion is well described in flow chart. Intention‐to‐treat analyses were used. | Low risk of bias | Comment: The outcome assessor was blinded (p. 2). Outcome assessment was performed with standardised questionnaires and a telephonic exacerbation assessment system (TEXAS). | Some concerns | Comments: The trial register includes outcomes that haven’t been reported in this paper, but the outcomes that are reported in this paper have been mentioned in the trial register. | High risk of bias | Unpredictable direction of the outcome. |
| Mitchell 2014 | Low risk of bias | “Patients were assigned to either usual care or SPACE FOR COPD via a web‐based, concealed allocation programme, using simple randomisation codes prepared by the trial statistician (J. Bankart).” p. 1539.
"Randomisation was conducted by the trial investigator responsible for administering the intervention (K.E. Mitchell).” p. 1539. Comment: Random sequence allocation was adequately performed. The method of allocation concealment was not reported, but they say it is concealed. The only significant baseline difference between treatment groups was the CRQ‐SR dyspnoea score; therefore, this was corrected for in subsequent analyses. |
High risk of bias |
Effect of assignment:
“Lack of participant blinding may have increased motivation when receiving the treatment and attempts to satisfy the researchers might have increased the observed treatment effects in the intervention arm. We cannot, therefore, rule out the possible impact of attention.” p. 1546.
Comment: No blinding of participants. Analysis was carried out on an intention‐to‐treat basis. Effect of adhering: Comment: Withdrawal around intervention group: 27% and UC: 17%. The adherence of using the manual in by the intervention patients was not reported. |
Low risk of bias | Comment: Data were not available for all randomised patients (withdrawal intervention: 27%, usual care: 17%). However, they found no significant differences in demographics or baseline variables between those who completed and those who did not complete the study. | Low risk of bias | “The assessments at week 6 and 6 months were conducted by a member of the research team who was blind to randomisation allocation (V. Johnson‐Warrington)." p. 1540 Comment: The outcome assessment was blinded. |
Low risk of bias | Comment: No signs for selective outcome reporting. The primary outcome measure and most of the secondary outcome measures were reported. | High risk of bias | Unpredictable direction of the outcome. |
| Johnson‐Warrington 2016 | Low risk of bias | "Participants were randomized to receive usual care or SPACE for COPD via a web‐based, concealed allocation program (www.sealedenvelope.com) using simple random permuted block 1:1 randomization by VJ‐W. Randomization was performed after the participants completed the baseline assessment, with treatment allocation prior to hospital discharge." p. 1162. | Some concerns |
Effect of assignment:
"Analysis was conducted on an intention‐to‐treat basis." p. 1163. Effect of adhering: Patients and personnel were not blinded. No information regarding adherence reported. |
High risk of bias | Some patients were missing: n= 78 were randomized (n= 39 to each arm). Included in the analysis: Intervention: n= 35 and usual care: n= 36. | Low risk of bias | "All outcomes were measured at baseline (during admission but as close to discharge as possible) and 3 months after randomization, by a clinician blinded to treatment allocation." p. 1163. | Some concerns | No protocol including a pre‐specified analysis plan was available. | High risk of bias | Unpredictable direction of the outcome. |
| Benzo 2016 | Low risk of bias | Comment: Patients were randomly assigned, using an online, computer‐generated, simple binomial randomization program to one of the two groups, stratified by center. | High risk of bias |
Effect of assignment:
"85% of the individuals in the intervention group received the intervention as intended, defined as 15 (.70%) of 21 calls completed. Reasons for not completing the intervention were death during the study period (n = 3), unable to contact (n = 6), and refusal to complete the scheduled calls (n = 7)." p. 674.
Comment: It was possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analysis was used. Effect of adhering: "Attendance at pulmonary rehabilitation visits anytime in the first 12 months after discharge tended to be higher in the intervention group (53% vs. 43%; P = 0.056)." p. 675. "In the intervention group, 85% of the individuals received a complete intervention, defined as 15 (.70%) of 21 calls completed." p. 674. |
Low risk of bias | ‐ | Low risk of bias | Comment: Outcome assessors were blinded. | High risk of bias | Comment: No analysis plan available in the trial register. Furthermore, the trial register mentions that the outcomes will be assessed in the 12 months time frame, but not mentioned how and when they were assessed. Although 12 months analyses show no differences, the additional time points that were reported (not prespecified) are in favour of experimental. | High risk of bias | Predicted direction: Favours experimental. |
| Lenferink 2019 | Low risk of bias | Comment: Allocation was stratified on potential confounders. Only a baseline difference in 6MWD was reported. | High risk of bias |
Effect of assignment:
Comment: It was not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analyses were performed. Effect of adhering: "The 12‐month follow‐up was completed by 83.3% of the individuals in the self‐management group and 84.8% in the UC group (figure 1). Patients completed 81.3% (self‐management: 82.3%; UC: 80.2%) of the diary data." p. 6. Comment: The effect of adherence to the intervention was not analysed. However, it was observed that a high number of patients in completed follow‐up and their symptom diaries. Adherence to action plans was measured, but not yet reported in the current publication. |
Low risk of bias | Comment: Relatively low number of drop‐outs. The imputated data analyses showed similar results as the observed data analyses. | Low risk of bias | "Wherever possible, though, assessors of outcomes were blinded to the treatment group." p. 3. | Low risk of bias | Comment: Some small changes in final analyses compared to analysis plan that was published with the study protocol. | High risk of bias |
Additional bias:
"Based on the exacerbation rates/patient/day from the COPE‐II study (intervention: 0.116; control: 0.176, both with an estimated standard deviation of 0.17) and allowing for overdispersion and an attrition rate of 10%, 105 patients per group provided 80% power to detect an effect of this size, with a two‐tailed p<0.05." p. 4.
Comment: The study included less patients (intervention: n= 102; usual care: n= 99) than aimed by power calculation (both groups n= 105). Unpredicted direction of the outcome. |
| Subgroup 1.3.2 HRQoL: CRQ ‐ mastery | ||||||||||||
| Bischoff 2012 | Some concerns | "We randomised participants by using a computer generated two block randomisation procedure with stratification on severity of COPD (mild or moderate v severe airflow obstruction), smoking status (current v former smoker), and frequency of exacerbations in the previous 24 months (<2 v ≥ 2 exacerbations)". p. 2.
"Table 1 shows that at inclusion in the study, patients’ characteristics were well balanced between the three study groups, except for sex.". p. 7. Comment: Random sequence generation was adequately performed. No information on who performed the allocation. There is another paper published, with different patient numbers (Uijen, British Journal of General Practice, June 2012), this paper shows a higher number of patients in the intervention group (n= 64) compared to the Bischoff’s paper (n= 55), but a lower number of patients in the usual care group (n= 49) compared to the Bischoff’s paper (n= 55). This raised some concerns about the randomisation process. Clarification could not be given by the authors. |
High risk of bias |
Effect of assignment:
"Our primary analysis was based on the intention to treat principle and included all available data for all participants. We compared self management with usual care and routine monitoring with usual care. We did not impute any missing data." p. 3.
Comment: It is not possible to blind patients / carers /people delivering the intervention due to the nature of this intervention. Effect of adhering: "“We prevented potential treatment contamination caused by provision of self management, routine monitoring, and usual care within the same." p. 2. "Seven (13%) patients did not receive any session or telephone contact, and 48 (87%) received two or more sessions." p. 4. "Six (11%) patients did not receive any consultation, and 42 (76%) patients received two or more consultations." p. 4. Comment: There was some non‐adherence in intervention group (at least 10%), this may all have led to some effect in the usual care group and a smaller difference between the intervention and usual care group. |
Some concerns | Comment: Almost 16% of the participants dropped out during follow‐up (intervention 11%; usual care 20%). However, baseline characteristics did not differ between dropouts and participants who finished follow‐up. Exclusion is well described in flow chart. Intention‐to‐treat analyses were used. | Low risk of bias | Comment: The outcome assessor was blinded (p. 2). Outcome assessment was performed with standardised questionnaires and a telephonic exacerbation assessment system (TEXAS). | Some concerns | Comments: The trial register includes outcomes that haven’t been reported in this paper, but the outcomes that are reported in this paper have been mentioned in the trial register. | High risk of bias | Unpredictable direction of the outcome. |
| Mitchell 2014 | Low risk of bias | “Patients were assigned to either usual care or SPACE FOR COPD via a web‐based, concealed allocation programme, using simple randomisation codes prepared by the trial statistician (J. Bankart).” p. 1539.
"Randomisation was conducted by the trial investigator responsible for administering the intervention (K.E. Mitchell).” p. 1539. Comment: Random sequence allocation was adequately performed. The method of allocation concealment was not reported, but they say it is concealed. The only significant baseline difference between treatment groups was the CRQ‐SR dyspnoea score; therefore, this was corrected for in subsequent analyses. |
High risk of bias |
Effect of assignment:
“Lack of participant blinding may have increased motivation when receiving the treatment and attempts to satisfy the researchers might have increased the observed treatment effects in the intervention arm. We cannot, therefore, rule out the possible impact of attention.” p. 1546.
Comment: No blinding of participants. Analysis was carried out on an intention‐to‐treat basis. Effect of adhering: Comment: Withdrawal around intervention group: 27% and UC: 17%. The adherence of using the manual in by the intervention patients was not reported. |
Low risk of bias | Comment: Data were not available for all randomised patients (withdrawal intervention: 27%, usual care: 17%). However, they found no significant differences in demographics or baseline variables between those who completed and those who did not complete the study. | Low risk of bias | “The assessments at week 6 and 6 months were conducted by a member of the research team who was blind to randomisation allocation (V. Johnson‐Warrington)." p. 1540 Comment: The outcome assessment was blinded. |
Low risk of bias | Comment: No signs for selective outcome reporting. The primary outcome measure and most of the secondary outcome measures were reported. | High risk of bias | Unpredictable direction of the outcome. |
| Benzo 2016 | Low risk of bias | Comment: Patients were randomly assigned, using an online, computer‐generated, simple binomial randomization program to one of the two groups, stratified by center. | High risk of bias |
Effect of assignment:
"85% of the individuals in the intervention group received the intervention as intended, defined as 15 (.70%) of 21 calls completed. Reasons for not completing the intervention were death during the study period (n = 3), unable to contact (n = 6), and refusal to complete the scheduled calls (n = 7)." p. 674.
Comment: It was possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analysis was used. Effect of adhering: "Attendance at pulmonary rehabilitation visits anytime in the first 12 months after discharge tended to be higher in the intervention group (53% vs. 43%; P = 0.056)." p. 675. "In the intervention group, 85% of the individuals received a complete intervention, defined as 15 (.70%) of 21 calls completed." p. 674. |
Low risk of bias | ‐ | Low risk of bias | Comment: Outcome assessors were blinded. | High risk of bias | Comment: No analysis plan available in the trial register. Furthermore, the trial register mentions that the outcomes will be assessed in the 12 months time frame, but not mentioned how and when they were assessed. Although 12 months analyses show no differences, the additional time points that were reported (not prespecified) are in favour of experimental. | High risk of bias | Predicted direction: Favours experimental. |
| Johnson‐Warrington 2016 | Low risk of bias | "Participants were randomized to receive usual care or SPACE for COPD via a web‐based, concealed allocation program (www.sealedenvelope.com) using simple random permuted block 1:1 randomization by VJ‐W. Randomization was performed after the participants completed the baseline assessment, with treatment allocation prior to hospital discharge." p. 1162. | Some concerns |
Effect of assignment:
"Analysis was conducted on an intention‐to‐treat basis." p. 1163. Effect of adhering: Patients and personnel were not blinded. No information regarding adherence reported. |
High risk of bias | Some patients were missing: n= 78 were randomized (n= 39 to each arm). Included in the analysis: Intervention: n= 35 and usual care: n= 36. | Low risk of bias | "All outcomes were measured at baseline (during admission but as close to discharge as possible) and 3 months after randomization, by a clinician blinded to treatment allocation." p. 1163. | Some concerns | No protocol including a pre‐specified analysis plan was available. | High risk of bias | Unpredictable direction of the outcome. |
| Lenferink 2019 | Low risk of bias | Comment: Allocation was stratified on potential confounders. Only a baseline difference in 6MWD was reported. | High risk of bias |
Effect of assignment:
Comment: It was not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analyses were performed. Effect of adhering: "The 12‐month follow‐up was completed by 83.3% of the individuals in the self‐management group and 84.8% in the UC group (figure 1). Patients completed 81.3% (self‐management: 82.3%; UC: 80.2%) of the diary data." p. 6. Comment: The effect of adherence to the intervention was not analysed. However, it was observed that a high number of patients in completed follow‐up and their symptom diaries. Adherence to action plans was measured, but not yet reported in the current publication. |
Low risk of bias | Comment: Relatively low number of drop‐outs. The imputated data analyses showed similar results as the observed data analyses. | Low risk of bias | "Wherever possible, though, assessors of outcomes were blinded to the treatment group." p. 3. | Low risk of bias | Comment: Some small changes in final analyses compared to analysis plan that was published with the study protocol. | High risk of bias |
Additional bias:
"Based on the exacerbation rates/patient/day from the COPE‐II study (intervention: 0.116; control: 0.176, both with an estimated standard deviation of 0.17) and allowing for overdispersion and an attrition rate of 10%, 105 patients per group provided 80% power to detect an effect of this size, with a two‐tailed p<0.05." p. 4.
Comment: The study included less patients (intervention: n= 102; usual care: n= 99) than aimed by power calculation (both groups n= 105). Unpredicted direction of the outcome. |
| Subgroup 1.3.3 HRQoL: CRQ ‐ fatigue | ||||||||||||
| Bischoff 2012 | Some concerns | "We randomised participants by using a computer generated two block randomisation procedure with stratification on severity of COPD (mild or moderate v severe airflow obstruction), smoking status (current v former smoker), and frequency of exacerbations in the previous 24 months (<2 v ≥ 2 exacerbations)". p. 2.
"Table 1 shows that at inclusion in the study, patients’ characteristics were well balanced between the three study groups, except for sex.". p. 7. Comment: Random sequence generation was adequately performed. No information on who performed the allocation. There is another paper published, with different patient numbers (Uijen, British Journal of General Practice, June 2012), this paper shows a higher number of patients in the intervention group (n= 64) compared to the Bischoff’s paper (n= 55), but a lower number of patients in the usual care group (n= 49) compared to the Bischoff’s paper (n= 55). This raised some concerns about the randomisation process. Clarification could not be given by the authors. |
High risk of bias |
Effect of assignment:
"Our primary analysis was based on the intention to treat principle and included all available data for all participants. We compared self management with usual care and routine monitoring with usual care. We did not impute any missing data." p. 3.
Comment: It is not possible to blind patients / carers /people delivering the intervention due to the nature of this intervention. Effect of adhering: "“We prevented potential treatment contamination caused by provision of self management, routine monitoring, and usual care within the same." p. 2. "Seven (13%) patients did not receive any session or telephone contact, and 48 (87%) received two or more sessions." p. 4. "Six (11%) patients did not receive any consultation, and 42 (76%) patients received two or more consultations." p. 4. Comment: There was some non‐adherence in intervention group (at least 10%), this may all have led to some effect in the usual care group and a smaller difference between the intervention and usual care group. |
Some concerns | Comment: Almost 16% of the participants dropped out during follow‐up (intervention 11%; usual care 20%). However, baseline characteristics did not differ between dropouts and participants who finished follow‐up. Exclusion is well described in flow chart. Intention‐to‐treat analyses were used. | Low risk of bias | Comment: The outcome assessor was blinded (p. 2). Outcome assessment was performed with standardised questionnaires and a telephonic exacerbation assessment system (TEXAS). | Some concerns | Comments: The trial register includes outcomes that haven’t been reported in this paper, but the outcomes that are reported in this paper have been mentioned in the trial register. | High risk of bias | Unpredictable direction of the outcome. |
| Mitchell 2014 | Low risk of bias | “Patients were assigned to either usual care or SPACE FOR COPD via a web‐based, concealed allocation programme, using simple randomisation codes prepared by the trial statistician (J. Bankart).” p. 1539.
"Randomisation was conducted by the trial investigator responsible for administering the intervention (K.E. Mitchell).” p. 1539. Comment: Random sequence allocation was adequately performed. The method of allocation concealment was not reported, but they say it is concealed. The only significant baseline difference between treatment groups was the CRQ‐SR dyspnoea score; therefore, this was corrected for in subsequent analyses. |
High risk of bias |
Effect of assignment:
“Lack of participant blinding may have increased motivation when receiving the treatment and attempts to satisfy the researchers might have increased the observed treatment effects in the intervention arm. We cannot, therefore, rule out the possible impact of attention.” p. 1546.
Comment: No blinding of participants. Analysis was carried out on an intention‐to‐treat basis. Effect of adhering: Comment: Withdrawal around intervention group: 27% and UC: 17%. The adherence of using the manual in by the intervention patients was not reported. |
Low risk of bias | Comment: Data were not available for all randomised patients (withdrawal intervention: 27%, usual care: 17%). However, they found no significant differences in demographics or baseline variables between those who completed and those who did not complete the study. | Low risk of bias | “The assessments at week 6 and 6 months were conducted by a member of the research team who was blind to randomisation allocation (V. Johnson‐Warrington)." p. 1540 Comment: The outcome assessment was blinded. |
Low risk of bias | Comment: No signs for selective outcome reporting. The primary outcome measure and most of the secondary outcome measures were reported. | High risk of bias | Unpredictable direction of the outcome. |
| Benzo 2016 | Low risk of bias | Comment: Patients were randomly assigned, using an online, computer‐generated, simple binomial randomization program to one of the two groups, stratified by center. | High risk of bias |
Effect of assignment:
"85% of the individuals in the intervention group received the intervention as intended, defined as 15 (.70%) of 21 calls completed. Reasons for not completing the intervention were death during the study period (n = 3), unable to contact (n = 6), and refusal to complete the scheduled calls (n = 7)." p. 674.
Comment: It was possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analysis was used. Effect of adhering: "Attendance at pulmonary rehabilitation visits anytime in the first 12 months after discharge tended to be higher in the intervention group (53% vs. 43%; P = 0.056)." p. 675. "In the intervention group, 85% of the individuals received a complete intervention, defined as 15 (.70%) of 21 calls completed." p. 674. |
Low risk of bias | ‐ | Low risk of bias | Comment: Outcome assessors were blinded. | High risk of bias | Comment: No analysis plan available in the trial register. Furthermore, the trial register mentions that the outcomes will be assessed in the 12 months time frame, but not mentioned how and when they were assessed. Although 12 months analyses show no differences, the additional time points that were reported (not prespecified) are in favour of experimental. | High risk of bias | Predicted direction: Favours experimental. |
| Johnson‐Warrington 2016 | Low risk of bias | "Participants were randomized to receive usual care or SPACE for COPD via a web‐based, concealed allocation program (www.sealedenvelope.com) using simple random permuted block 1:1 randomization by VJ‐W. Randomization was performed after the participants completed the baseline assessment, with treatment allocation prior to hospital discharge." p. 1162. | Some concerns |
Effect of assignment:
"Analysis was conducted on an intention‐to‐treat basis." p. 1163. Effect of adhering: Patients and personnel were not blinded. No information regarding adherence reported. |
High risk of bias | Some patients were missing: n= 78 were randomized (n= 39 to each arm). Included in the analysis: Intervention: n= 35 and usual care: n= 36. | Low risk of bias | "All outcomes were measured at baseline (during admission but as close to discharge as possible) and 3 months after randomization, by a clinician blinded to treatment allocation." p. 1163. | Some concerns | No protocol including a pre‐specified analysis plan was available. | High risk of bias | Unpredictable direction of the outcome. |
| Lenferink 2019 | Low risk of bias | Comment: Allocation was stratified on potential confounders. Only a baseline difference in 6MWD was reported. | High risk of bias |
Effect of assignment:
Comment: It was not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analyses were performed. Effect of adhering: "The 12‐month follow‐up was completed by 83.3% of the individuals in the self‐management group and 84.8% in the UC group (figure 1). Patients completed 81.3% (self‐management: 82.3%; UC: 80.2%) of the diary data." p. 6. Comment: The effect of adherence to the intervention was not analysed. However, it was observed that a high number of patients in completed follow‐up and their symptom diaries. Adherence to action plans was measured, but not yet reported in the current publication. |
Low risk of bias | Comment: Relatively low number of drop‐outs. The imputated data analyses showed similar results as the observed data analyses. | Low risk of bias | "Wherever possible, though, assessors of outcomes were blinded to the treatment group." p. 3. | Low risk of bias | Comment: Some small changes in final analyses compared to analysis plan that was published with the study protocol. | High risk of bias |
Additional bias:
"Based on the exacerbation rates/patient/day from the COPE‐II study (intervention: 0.116; control: 0.176, both with an estimated standard deviation of 0.17) and allowing for overdispersion and an attrition rate of 10%, 105 patients per group provided 80% power to detect an effect of this size, with a two‐tailed p<0.05." p. 4.
Comment: The study included less patients (intervention: n= 102; usual care: n= 99) than aimed by power calculation (both groups n= 105). Unpredicted direction of the outcome. |
| Subgroup 1.3.4 HRQoL: CRQ ‐ emotional function | ||||||||||||
| Bischoff 2012 | Some concerns | "We randomised participants by using a computer generated two block randomisation procedure with stratification on severity of COPD (mild or moderate v severe airflow obstruction), smoking status (current v former smoker), and frequency of exacerbations in the previous 24 months (<2 v ≥ 2 exacerbations)". p. 2.
"Table 1 shows that at inclusion in the study, patients’ characteristics were well balanced between the three study groups, except for sex.". p. 7. Comment: Random sequence generation was adequately performed. No information on who performed the allocation. There is another paper published, with different patient numbers (Uijen, British Journal of General Practice, June 2012), this paper shows a higher number of patients in the intervention group (n= 64) compared to the Bischoff’s paper (n= 55), but a lower number of patients in the usual care group (n= 49) compared to the Bischoff’s paper (n= 55). This raised some concerns about the randomisation process. Clarification could not be given by the authors. |
High risk of bias |
Effect of assignment:
"Our primary analysis was based on the intention to treat principle and included all available data for all participants. We compared self management with usual care and routine monitoring with usual care. We did not impute any missing data." p. 3.
Comment: It is not possible to blind patients / carers /people delivering the intervention due to the nature of this intervention. Effect of adhering: "“We prevented potential treatment contamination caused by provision of self management, routine monitoring, and usual care within the same." p. 2. "Seven (13%) patients did not receive any session or telephone contact, and 48 (87%) received two or more sessions." p. 4. "Six (11%) patients did not receive any consultation, and 42 (76%) patients received two or more consultations." p. 4. Comment: There was some non‐adherence in intervention group (at least 10%), this may all have led to some effect in the usual care group and a smaller difference between the intervention and usual care group. |
Some concerns | Comment: Almost 16% of the participants dropped out during follow‐up (intervention 11%; usual care 20%). However, baseline characteristics did not differ between dropouts and participants who finished follow‐up. Exclusion is well described in flow chart. Intention‐to‐treat analyses were used. | Low risk of bias | Comment: The outcome assessor was blinded (p. 2). Outcome assessment was performed with standardised questionnaires and a telephonic exacerbation assessment system (TEXAS). | Some concerns | Comments: The trial register includes outcomes that haven’t been reported in this paper, but the outcomes that are reported in this paper have been mentioned in the trial register. | High risk of bias | Unpredictable direction of the outcome. |
| Mitchell 2014 | Low risk of bias | “Patients were assigned to either usual care or SPACE FOR COPD via a web‐based, concealed allocation programme, using simple randomisation codes prepared by the trial statistician (J. Bankart).” p. 1539.
"Randomisation was conducted by the trial investigator responsible for administering the intervention (K.E. Mitchell).” p. 1539. Comment: Random sequence allocation was adequately performed. The method of allocation concealment was not reported, but they say it is concealed. The only significant baseline difference between treatment groups was the CRQ‐SR dyspnoea score; therefore, this was corrected for in subsequent analyses. |
High risk of bias |
Effect of assignment:
“Lack of participant blinding may have increased motivation when receiving the treatment and attempts to satisfy the researchers might have increased the observed treatment effects in the intervention arm. We cannot, therefore, rule out the possible impact of attention.” p. 1546.
Comment: No blinding of participants. Analysis was carried out on an intention‐to‐treat basis. Effect of adhering: Comment: Withdrawal around intervention group: 27% and UC: 17%. The adherence of using the manual in by the intervention patients was not reported. |
Low risk of bias | Comment: Data were not available for all randomised patients (withdrawal intervention: 27%, usual care: 17%). However, they found no significant differences in demographics or baseline variables between those who completed and those who did not complete the study. | Low risk of bias | “The assessments at week 6 and 6 months were conducted by a member of the research team who was blind to randomisation allocation (V. Johnson‐Warrington)." p. 1540 Comment: The outcome assessment was blinded. |
Low risk of bias | Comment: No signs for selective outcome reporting. The primary outcome measure and most of the secondary outcome measures were reported. | High risk of bias | Unpredictable direction of the outcome. |
| Benzo 2016 | Low risk of bias | Comment: Patients were randomly assigned, using an online, computer‐generated, simple binomial randomization program to one of the two groups, stratified by center. | High risk of bias |
Effect of assignment:
"85% of the individuals in the intervention group received the intervention as intended, defined as 15 (.70%) of 21 calls completed. Reasons for not completing the intervention were death during the study period (n = 3), unable to contact (n = 6), and refusal to complete the scheduled calls (n = 7)." p. 674.
Comment: It was possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analysis was used. Effect of adhering: "Attendance at pulmonary rehabilitation visits anytime in the first 12 months after discharge tended to be higher in the intervention group (53% vs. 43%; P = 0.056)." p. 675. "In the intervention group, 85% of the individuals received a complete intervention, defined as 15 (.70%) of 21 calls completed." p. 674. |
Low risk of bias | ‐ | Low risk of bias | Comment: Outcome assessors were blinded. | High risk of bias | Comment: No analysis plan available in the trial register. Furthermore, the trial register mentions that the outcomes will be assessed in the 12 months time frame, but not mentioned how and when they were assessed. Although 12 months analyses show no differences, the additional time points that were reported (not prespecified) are in favour of experimental. | High risk of bias | Predicted direction: Favours experimental. |
| Johnson‐Warrington 2016 | Low risk of bias | "Participants were randomized to receive usual care or SPACE for COPD via a web‐based, concealed allocation program (www.sealedenvelope.com) using simple random permuted block 1:1 randomization by VJ‐W. Randomization was performed after the participants completed the baseline assessment, with treatment allocation prior to hospital discharge." p. 1162. | Some concerns |
Effect of assignment:
"Analysis was conducted on an intention‐to‐treat basis." p. 1163. Effect of adhering: Patients and personnel were not blinded. No information regarding adherence reported. |
High risk of bias | Some patients were missing: n= 78 were randomized (n= 39 to each arm). Included in the analysis: Intervention: n= 35 and usual care: n= 36. | Low risk of bias | "All outcomes were measured at baseline (during admission but as close to discharge as possible) and 3 months after randomization, by a clinician blinded to treatment allocation." p. 1163. | Some concerns | No protocol including a pre‐specified analysis plan was available. | High risk of bias | Unpredictable direction of the outcome. |
| Lenferink 2019 | Low risk of bias | Comment: Allocation was stratified on potential confounders. Only a baseline difference in 6MWD was reported. | High risk of bias |
Effect of assignment:
Comment: It was not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analyses were performed. Effect of adhering: "The 12‐month follow‐up was completed by 83.3% of the individuals in the self‐management group and 84.8% in the UC group (figure 1). Patients completed 81.3% (self‐management: 82.3%; UC: 80.2%) of the diary data." p. 6. Comment: The effect of adherence to the intervention was not analysed. However, it was observed that a high number of patients in completed follow‐up and their symptom diaries. Adherence to action plans was measured, but not yet reported in the current publication. |
Low risk of bias | Comment: Relatively low number of drop‐outs. The imputated data analyses showed similar results as the observed data analyses. | Low risk of bias | "Wherever possible, though, assessors of outcomes were blinded to the treatment group." p. 3. | Low risk of bias | Comment: Some small changes in final analyses compared to analysis plan that was published with the study protocol. | High risk of bias |
Additional bias:
"Based on the exacerbation rates/patient/day from the COPE‐II study (intervention: 0.116; control: 0.176, both with an estimated standard deviation of 0.17) and allowing for overdispersion and an attrition rate of 10%, 105 patients per group provided 80% power to detect an effect of this size, with a two‐tailed p<0.05." p. 4.
Comment: The study included less patients (intervention: n= 102; usual care: n= 99) than aimed by power calculation (both groups n= 105). Unpredicted direction of the outcome. |
Risk of bias for analysis 1.5 Healthcare utilisation: respiratory‐related hospital admissions (number of participants with at least one admission) (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Gallefoss 1999 | Low risk of bias | Comment: At inclusion they signed a written consent and were then randomised to an intervention group or a control group. | High risk of bias |
Effect of assignment:
Comment: No information reported about blinding, but it is almost impossible to blind these patients because of the nature of the intervention. Furthermore, no information was reported whether deviations arose from the intended intervention because of the trial context. Effect of adhering: "Forty‐six percent of those educated (12 of 26) received a standard treatment plan. Non‐standard treatment plans, incorporating the use of oral steroids as the first line of action in the yellow zone, were followed in some cases, for example, if the patient already used high dosages of inhaled steroids as maintenance therapy, or could report that a double or triple increase in inhaled steroids previously had shown marginal effect on the course of attacks/exacerbations. Among those 14 patients receiving non‐standard treatment plans, eight patients did not want to or were not able to use peak flow monitoring as a basis for change in medication. For those patients, symptom‐only based treatment plans were issued." Gallefoss 2002, p. 425. "In the intervention group, four patients failed to complete the educational program (social problems (n = 1), unannounced emigration (n = 1), failure to meet at educational group sessions for unknown reasons (n = 1) and serious myocardial infarction (n = 1))." Gallefoss 2002, p. 427. Comment: No information reported about adherence to the programme of the other patients (who completed the programme). |
High risk of bias | “In the intervention group, four patients failed to complete the educational programme (social problems (n = 1), unannounced emigration (n = 1), failure to meet at educational group sessions for unknown reasons (n = 1) and serious myocardial infarction (n = 1)). Another patient was withdrawn from the study during the follow‐up due to lymphoma (n = 1). This left us with 26 patients (81%) for a 1‐year follow‐up. The patients who were withdrawn from the intervention group did not, to our knowledge, have any serious deterioration in their obstructive lung disease, and none were hospitalised. In the control group four patients were withdrawn (lack of co‐operation (n = 2), diagnosis of rectal cancer (n = 1) and emigration (n = 1)). Two of the withdrawn control group patients were hospitalised for exacerbations of their COPD. This left us with 27 patients (84%) for the 1‐year follow‐up” Gallefoss 2002, p. 427. Comment: The number of dropouts was relatively low, and reasons for dropout were comparable over groups |
High risk of bias | "Prebronchodilator spirometry was performed before randomization and at 12 mo follow‐up by standard methods using a Jaeger MasterLab Body Box (Würzburg, Germany). The technical staff did not know whether the patients belonged to the control or intervention groups." Gallefoss 1999, p. 2003. Comment: No information reported regarding the assessors of the primary outcome. Number of prednisolone courses was retrospectively self‐reported at 12 months of follow‐up. |
Some concerns | "We defined a priori the patient as compliant when dispensed regular medication was greater than 75% of prescribed regular medication during the study period." Gallefoss 1999, p. 2002. | High risk of bias | Unpredictable direction of the outcome. |
| Bourbeau 2003 | Low risk of bias | “… central computergenerated list of random numbers. Randomization was stratified per center and in blocks of 6, and patients were assigned to the self‐management program (intervention group) or to usual care.” p. 586. “The blocking factor was not known by the investigators or their staff in each participating center." p. 586. “The use of respiratory medications was similar between study groups, except that oral steroids were used less commonly in the intervention group (7%) than in the usual care group (13%). None of the disease severity characteristics were otherwise different between the study groups.” p. 586. Comment: Random sequence allocation was adequately performed. |
High risk of bias |
Effect of assignment:
"Since a double‐blind design was impossible..." p. 586.
Comment: Participants and personnel were not blinded. ITT analysis was performed. Effect of adhering: "Since a double‐blind design was impossible..." p. 586. Comment: Participants and personnel were not blinded. Comment: There might be some imbalance in non‐protocol interventions such as exercise evaluation (not mandatory). However, no information available. |
Low risk of bias | ‐ | Low risk of bias | "...an independent evaluator unaware of the patient assignment was responsible for the evaluation process in each center. The evaluator was cautioned not to ask about the workbook modules and types of contact.” p. 586. Comment: Outcome assessment was blinded. |
Some concerns | Comment: No protocol or prespecified analysis plan available. | High risk of bias | Unpredictable direction of the outcome. |
| Coultas 2005 | Low risk of bias | “Patients were randomly assigned to one of three intervention groups (usual care (UC), nurse‐assisted medical management (MM), or nurse‐assisted collaborative management (CM)) using a computer‐generated random list.” p. 2018. “Although the distribution of most of the baseline characteristics of the three groups was similar (Tables 2 and 3), only the distribution of gender and the tangible domain of social support were significantly different among the intervention groups.” p. 2020. Comment: It is unclear how allocation concealment was guaranteed. Observed imbalances are probably compatible with chance. Random sequence generation was adequately performed. |
High risk of bias |
Effect of assignment:
“Of the 217 patients enrolled in the study, 151 (69.6%) completed the 6‐month intervention and follow‐up data collection.” p. 2020.
“The reasons for the failure to complete the study were patient was unavailable for follow‐up (26.7%) and death (3.7%) [Fig 1]. The frequency of patients being unavailable for follow‐up was equally distributed among the three intervention groups (Fig 1). Overall, the demographic characteristics of the patients who dropped out of the study were similar to those who completed the study (data not shown). However, patients who dropped out of the study had more severe airflow obstruction, higher levels of distress, and lower quality of life, as measured with the SGRQ, compared with the patients who had completed the study (data not shown).” p. 2020.
Comment: Not reported whether deviations arose because of the trial context. Only patients who completed the 6‐month intervention and follow‐up data collection were analysed (per‐protocol analyses). Effect of adhering: Comment: Patients and personnnel were probably aware of assignment to the intervention during the trial. |
High risk of bias | “The reasons for the failure to complete the study were patient was unavailable for follow‐up (26.7%) and death (3.7%).” p. 2020. “However, patients who dropped out of the study had more severe airflow obstruction, higher levels of distress, and lower quality of life, as measured with the SGRQ, compared with the patients who had completed the study (data not shown).” p. 2020. Comment: Only patients who completed the 6‐month intervention and follow‐up data collection were analysed. |
Low risk of bias | “To limit interviewer bias, each interviewer who obtained the baseline and 6‐month outcome data was blinded to the patient’s treatment group.” p. 2023. "Although self‐reported health‐care utilization may be subject to recall bias, it has been shown to be an acceptable method of measurement and may actually result in an underestimation of utilization with more frequent use.” p. 2023. |
Some concerns | Comment: No protocol available. Measures and analysis intentions were only reported in the method section of the paper. | High risk of bias | Unpredictable direction of the outcome. |
| Coultas 2005 | Low risk of bias | “Patients were randomly assigned to one of three intervention groups (usual care (UC), nurse‐assisted medical management (MM), or nurse‐assisted collaborative management (CM)) using a computer‐generated random list.” p. 2018. “Although the distribution of most of the baseline characteristics of the three groups was similar (Tables 2 and 3), only the distribution of gender and the tangible domain of social support were significantly different among the intervention groups.” p. 2020. Comment: It is unclear how allocation concealment was guaranteed. Observed imbalances are probably compatible with chance. Random sequence generation was adequately performed. |
High risk of bias |
Effect of assignment:
“Of the 217 patients enrolled in the study, 151 (69.6%) completed the 6‐month intervention and follow‐up data collection.” p. 2020.
“The reasons for the failure to complete the study were patient was unavailable for follow‐up (26.7%) and death (3.7%) [Fig 1]. The frequency of patients being unavailable for follow‐up was equally distributed among the three intervention groups (Fig 1). Overall, the demographic characteristics of the patients who dropped out of the study were similar to those who completed the study (data not shown). However, patients who dropped out of the study had more severe airflow obstruction, higher levels of distress, and lower quality of life, as measured with the SGRQ, compared with the patients who had completed the study (data not shown).” p. 2020.
Comment: Not reported whether deviations arose because of the trial context. Only patients who completed the 6‐month intervention and follow‐up data collection were analysed (per‐protocol analyses). Effect of adhering: Comment: Patients and personnnel were probably aware of assignment to the intervention during the trial. |
High risk of bias | “The reasons for the failure to complete the study were patient was unavailable for follow‐up (26.7%) and death (3.7%).” p. 2020. “However, patients who dropped out of the study had more severe airflow obstruction, higher levels of distress, and lower quality of life, as measured with the SGRQ, compared with the patients who had completed the study (data not shown).” p. 2020. Comment: Only patients who completed the 6‐month intervention and follow‐up data collection were analysed. |
Low risk of bias | “To limit interviewer bias, each interviewer who obtained the baseline and 6‐month outcome data was blinded to the patient’s treatment group.” p. 2023. "Although self‐reported health‐care utilization may be subject to recall bias, it has been shown to be an acceptable method of measurement and may actually result in an underestimation of utilization with more frequent use.” p. 2023. |
Some concerns | Comment: No protocol available. Measures and analysis intentions were only reported in the method section of the paper. | High risk of bias | Unpredictable direction of the outcome. |
| Rice 2010 | Some concerns | "We assigned subjects in equal proportions to each of the two treatment arms by permuted‐block randomisation." Appendix 1, p. 3. "The baseline characteristics of the two treatment groups were generally well matched, with differences in age, lung function, and ED visits (not significant)." p. 891. Comment: Information on the method of allocation concealment was not reported. |
High risk of bias |
Effect of assignment:
"We performed a randomized, adjudicator‐blinded, controlled, 1‐year trial." p. 890.
"The status of all 743 patients was determined after 1 year. Electronic medical records were available for all hospitalizations and ED visits that occurred within the VA medical system. The success rate for obtaining discharge summaries and ED records for self‐reported events that occurred outside the VA was 99% in the usual care group and 98% in the disease management group." p. 891. Effect of adhering: Comment: 336 out of 372 (90.3%) patients completed disease management 323 out of 371 (87%) completed usual care. |
Low risk of bias | "55% of patients in the usual care group and 60% of patients in the disease management group returned a completed the Saint George’s Respiratory Questionnaire in response to a single mailing at the end of the study. low response rates on SGRQ leading to a high risk of bias. However, data on healthcare utilisation seem complete with no risk of bias." p. 892. | Low risk of bias | "Blinded pulmonologists independently reviewed all discharge summaries and ED reports and assigned a primary cause for each." p. 891. | High risk of bias | Comment: Primary outcome in the paper is reported as a combined outcome measure, whereas it was planned to analyse the two outcomes separately. | High risk of bias | Unpredictable direction of the outcome. |
| Bucknall 2012 | Low risk of bias | “We used a minimisation technique to stratify randomisation of participants by demographic factors (deprivation category of area of residence,11 age and sex, FEV1 per cent predicted at the time of randomisation, smoking status, participation in pulmonary rehabilitation within two years, and number of previous admissions) to control for key aspects of disease severity and predictors of readmission. We constructed a computer generated sequence by using the method of randomised permuted blocks of length four, with two allocations being made at random and two by minimisation. Treatment group allocations were obtained by telephone, after baseline assessments had been made.” p 2. Comment: Random sequence allocation was adequately performed. |
High risk of bias |
Effect of assignment:
Comment: No blinding of patients and personnel. Data for the primary outcome (time to first acute COPD hospital admission or death) were available for all patients and analysed with a Cox proportional hazards model adjusting for the stratification variables. Effect of adhering: Comment: No blinding of patients and personnel. Seventy‐five (42%) of 180 intervention group participants who completed 12 months of follow‐up were judged to have become successful self managers. |
Low risk of bias | Comment: Only for secondary outcome measures (e.g. SGRQ, HADS, CSES) not all data were available for all patients randomised (Table 3). | Low risk of bias | ‐ | Some concerns | ‐ | High risk of bias | Unpredictable direction of the outcome. |
| Fan 2012 | Low risk of bias | “Randomization lists were generated on the basis of random, permuted blocks of variable size to ensure approximate balance over time.” p. 674. “The CSP Coordinating Center in Boston, Massachusetts, randomly assigned eligible patients in equal numbers to 2 groups, stratifying patients per site to allow for possible regional differences in patient characteristics and clinical practice patterns.” p. 674. Comment: Random sequence generation was adequately performed. The allocation was adequately concealed. Authors didn’t report whether there were any significant differences. Most characteristics are equally balanced, but there is a bit larger difference in the following characteristics (>5%):
|
High risk of bias |
Effect of assignment:
“The 2 groups differed on the basis of a complex behavioral intervention that made blinding impossible.” p. 674.
Comment: No blinding of participants and personnel.
"For the primary intention‐to‐treat analysis, the null hypothesis was that the COPD hospitalization hazard rate for intervention did not differ from that for standardized care."p. 675.
"Also, with respect to time to first COPD hospitalization, we conducted a futility analysis to assess what might have resulted if the trial had continued." p. 676.
"When the study was stopped, the 1‐year cumulative incidence of COPD‐related hospitalization was 27% in the intervention group and 24% in the usual care group (hazard ratio, 1.13 [95% CI, 0.70 to 1.80]; P 0.62)." p. 677. Effect of adhering: "During the entire follow‐up period extending to September 2009, a total of 8 patients in the intervention group and 10 in the usual care group either did not attend scheduled study visits or formally withdrew from the study. Among patients in the intervention group, 87% completed all 4 individual educational visits and 57% completed the group visit. A review of the recorded sessions found that case managers covered 77% to 89% of the educational items in each session. The ranges of average scores across sites that provided audio recordings for each session were 18.3 to 31 for session 1 (13 sites), 12 to 19 for session 2 (12 sites), 12.7 to 23 for session 3 (10 sites), and 6 to 18 for session 4 (10 sites)." p. 677. |
High risk of bias | “This multisite, randomized, controlled trial of an educational and acute care management program was stopped early when a safety monitoring board noted more deaths in the intervention group.” p. 674 Comment: There is incomplete outcome data due to early termination of the study. |
Low risk of bias | “Telephone‐based ascertainment of study outcomes (COPD hospitalizations and exacerbations) was performed by centralized research staff blinded to assignment. All outcomes were collected by centralized staff blinded to study group, and all hospitalizations were adjudicated by a committee that was also blinded to study group.” p. 674. Comment: Research staff blinded to study group contacted patients every 2 months to determine whether they developed symptoms of a COPD exacerbation, along with details of treatment and health care use. Deaths were ascertained by staff at the study sites, by review of VA electronic medical and administrative records and by a search of the Social Security Death Index. Extensive multivariate analyses failed to identify any factors alone or combined that could plausibly explain the difference in mortality. Two of the authors who were blinded to study group reviewed all available medical information and did not find anything in the deaths that would not be expected in frail patients with COPD. |
Some concerns | Comment: The primary and secondary outcomes were reported, only healthcare costs as (secondary objective) were not reported. | High risk of bias | Predicted directon: Favours comparator, because more deaths in the intervention group were observed. There is still a chance that this was because of chance. |
| Walters 2013 | High risk of bias |
Randomisation process:
"After recruitment, practices were randomised using a code generated by investigators from a random numbers table stratified in blocks of four by Rural, Remote and Metropolitan Areas (RRMA) classifications in Tasmania. Allocation occurred independently using sequentially numbered, opaque and sealed envelopes." p. 2. Timing of identification or recruitment of participants: GPs identified patients with COPD aged over 45 years seen within the previous 12 months through database searches, based on a diagnostic code for COPD or prescription of tiotropium. Comment: Most variables are equally distributed, but more smokers (not statistically significant, p=0.102) and more previous admissions in the intervention group (statistically significant, p=0.028), so potentially more room for improvement. Although, this small number of differences identified as ‘statistically significant’ at the conventional 0.05 threshold should usually be considered to be compatible with chance. |
High risk of bias |
Effect of assignment:
‐ Effect of adhering: "The limitations of the study were the high rate of misclassification of COPD in general practices, which impacted on enrolment, and higher than expected withdrawal rates, especially in the HM group, which reduced the intended statistical power." p. 6. Comment: Nine patients discontinued and 8 patients withdrew in the intervention group. |
Low risk of bias | Comment: Figure 1: 9 intervention patients discontinued, but they attended follow‐up. In Table 3, no patient numbers are mentioned. | Low risk of bias | Comment: Blinding of participants or research officers was not possible given the nature of the study. | Low risk of bias | Comment: Data that produced this result analysed in accordance with a pre‐specified analysis plan. | High risk of bias | Unpredictable direction of the outcome. |
| Mitchell 2014 | Low risk of bias | “Patients were assigned to either usual care or SPACE FOR COPD via a web‐based, concealed allocation programme, using simple randomisation codes prepared by the trial statistician (J. Bankart).” p. 1539.
"Randomisation was conducted by the trial investigator responsible for administering the intervention (K.E. Mitchell).” p. 1539. Comment: Random sequence allocation was adequately performed. The method of allocation concealment was not reported, but they say it is concealed. The only significant baseline difference between treatment groups was the CRQ‐SR dyspnoea score; therefore, this was corrected for in subsequent analyses. |
High risk of bias |
Effect of assignment:
“Lack of participant blinding may have increased motivation when receiving the treatment and attempts to satisfy the researchers might have increased the observed treatment effects in the intervention arm. We cannot, therefore, rule out the possible impact of attention.” p. 1546.
Comment: No blinding of participants. Analysis was carried out on an intention‐to‐treat basis. Effect of adhering: Comment: Withdrawal around intervention group: 27% and UC: 17%. The adherence of using the manual in by the intervention patients was not reported. |
Low risk of bias | Comment: Data were not available for all randomised patients (withdrawal intervention: 27%, usual care: 17%). However, they found no significant differences in demographics or baseline variables between those who completed and those who did not complete the study. | Low risk of bias | “The assessments at week 6 and 6 months were conducted by a member of the research team who was blind to randomisation allocation (V. Johnson‐Warrington)." p. 1540 Comment: The outcome assessment was blinded. |
Low risk of bias | Comment: No signs for selective outcome reporting. The primary outcome measure and most of the secondary outcome measures were reported. | High risk of bias | Unpredictable direction of the outcome. |
| Tabak 2014 | Low risk of bias | "Patients were randomized using a computer‐generated randomization list (Blocked Stratified Randomization version 5; Steven Piantadosi), where randomization was applied in blocks of two and four.” p. 936. “Participants were allocated by a data manager in order of inclusion following the randomization list, placed in a sealed envelope.” p. 936. Comment: There was a significant difference in dyspnea (MRC scale) but this seems by chance. All other parameters seem evenly distributed. |
Some concerns |
Effect of assignment:
"To present the outcome measures over time in both groups, a mixed‐model analysis for repeated measures was performed (intention to treat)." p. 938.
Comment: Patients and personnel were not blinded. No information was reported regarding deviations from the intervention that arose because of the trial context. Effect of adhering: "Participants in the control group received only usual care. Patients in the control group were allowed to attend regular physiotherapy sessions if this was prescribed as part of usual care." p. 937. “Although some patients in the intervention group quit the physiotherapy modules ( exercising and activity coach) due to weak (n=l, < T l ) or unstable condition (n=l, <T2) and personal circumstances (n=2, <T4), they persisted in using the web portal and triage diary til! T4.” p. 939. Comment: Most outcome measures are reported for 3 months follow‐up, whereas there was a total of 9 months follow‐up. There was a high number of withdrawals for the 9 months follow‐up. However, there were more drop‐outs in the usual care control group than in the intervention group. |
High risk of bias | “A large number of patients were not able or willing to continue study participation: 33% in the intervention group and 86% in the control group.” p. 939. Comment: Most outcome measures are reported for 3 months follow‐up, whereas there was a total of 9 months follow‐up. There was a high number of withdrawals for the 9 months follow‐up (more dropouts in the control group). Reasons reported for patients from the control group: too much effort (n=2), exacerbation (n‐1), hospitalisation (n‐2), kidney problems (n‐1), exacerbation, and cancer (n‐1). |
Low risk of bias | ‐ | Low risk of bias | Comment: Not all outcome measures were reported for the 9 months follow‐up. Exacerbations (duration) were not reported. Also, no information or results provided for the use of diaries in the usual care control group. | High risk of bias | Unpredictable direction of the outcome. |
| Hernández 2015 | Low risk of bias | "A computer‐generated list of random numbers with no restrictions and administered by personnel who were not involved in the study ensured blinded randomisation." p. 2. "(…) and administered by personnel who were not involved in the study." p. 2. Comment: Random sequence generation was adequately performed. No blinding of participants or personnel. The patients of the two groups showed similar characteristics, except that influenza and pneumococcal vaccination were more prevalent in the IC group (P<0.01). |
High risk of bias |
Effect of assignment:
Comment: It was not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analysis performed. Effect of adhering: "As a sensitivity analysis, we repeated all analyses after excluding patients who had a previous contact with any IC programme (n = 15)." p. 2. "25 patients (22%) died and 16 (14%) were lost during the 1‐year follow‐up, and the remaining 114 patients (IC, n = 59 and UC, n = 55) completed the 12‐month assessment visit. Patients lost to follow‐up exhibited a higher affectation of activities of daily living and health‐related quality of life at baseline than those who were followed up, without differences in other sociodemographic, clinical, functional and medical care‐related variables." p. 3. Comment: It is not mentioned in which way these patients were distributed over the two groups, nor are the results of the sensitivity analysis reported. Although the approach was not adequately adopted at the community level and thus the intervention was not continued after the 1 year of follow‐up, this was not a failure of implementing the intervention, since the intervention stopped at 1 year according to the protocol. |
Low risk of bias | ‐ | Low risk of bias | "A blind evaluation of the study group carried out before randomisation and after the 12‐month follow‐up consisted of a patient interview and analysis of medical records, self‐administered questionnaires and lung function testing." p. 2. | Some concerns | "Although the study was officially registered as part of the EU Grant (CIP‐ICT‐PSP‐2007.225025), the RCT was not included in the clinicaltrials.gov registry because at that time it was not compulsory." p. 5. Comment: No protocol available. |
High risk of bias | Unpredicted direction of outcome. |
| Titova 2015 | High risk of bias |
Randomisation process:
“They were randomly allocated to either integrated care (IC) or usual care (UC) based on their address of permanent residence. In order to create two pairs of districts with approximately equal numbers of citizens, a pair‐wise matching of districts was carried out. It was decided by lottery that participants from District Pair 1 were assigned to the UC group, and participants from District Pair 2 were assigned to the IC group.” Titova 2015, p. 2.
“There were no significant differences in baseline characteristics between the IC and the UC groups, but when comparing those who died during follow‐up with those who were still alive after two years, those who died had a significantly higher PaCO2 and lower BMI at baseline (Table 1). Those who died during follow‐up also had significantly more HA and HD during the year prior to study start.” Titova 2015, p. 4.
Comment: No information provided about the allocation concealment. Timing of identification or recruitment of participants: "They were randomly allocated to either IC or UC group based on their address of permanent residence. Patient data were registered at discharge and 6, 12, and 24 months after discharge from the TUH." Titova 2017, p 3. Comment: If allocation was known before study inclusion, suitable patients could be selected for the intervention. |
High risk of bias |
Effect of assignment:
“The study was a prospective, open, single‐centre intervention study.” Titova 2015, p. 2.
"The principal comparisons of changes were performed on an intention‐to treat basis." Titova 2017, p. 4.
Comment: No blinding of participants and personnel. Effect of adhering: ‐ |
High risk of bias | “172 patients were randomly allocated to the IC group (n = 91) or the UC group (n = 81), respectively. After two years of follow‐up, a total of 100 patients (58% of the included patients), 51 patients in the IC group and 49 patients in the UC group, were available for evaluation (Figure 1).” Titova 2017, p. 5. "Those who died had a significantly higher PaCO2 and lower BMI at baseline (Table 2). Those who died during follow‐up also had significantly more HA and HD during the year prior to study start." Titova 2015, p. 4. Comment: No mortality reported; however, Figure 1 shows higher mortality for the IC group, n=35 (38.4%), compared to the UC group, n=21 (25.9%). |
High risk of bias | “Information concerning the number and duration of the COPD exacerbations, as well as the time from onset of symptoms until the start of self‐initiated treatment is insufficient due to many incomplete registrations in “My COPD book.” Titova 2015, p. 9. "“Data concerning HA (hospital admissions) and HD (hospital days) were collected from the hospital registry database’s medical charts.” Titova 2015, p. 3. Comment: Unclear who was the outcome assessor. |
Some concerns | Trial register reported number of hospitalisations as the primary outcome. Secondary outcomes were quality of life, lung function, use of medication, cost‐effectiveness. However, the article reported not all secondary outcomes (e.g. cost‐effectiveness). | High risk of bias | Unpredictable direction of the outcome. |
| Sanchez‐Nieto 2016 | Some concerns | "Controlled, randomized, parallel‐group, single‐blind study with follow‐up of 1 year. After consenting to take part in the study, simple randomization was carried out separately at each site by means of a list of computer‐generated random numbers, assigning the patients to two groups." p. 1940. Comment: No information reported regarding concealed allocation sequence. |
High risk of bias |
Effect of assignment:
Comment: Patients and personnel were not blinded. No proper intention‐to‐treat analysis used, as the baseline characteristics of the people that have dropped out haven’t been included in the analyses. Patients lost to follow‐up were evaluated until the time of the last contact with them by the research staff. Effect of adhering: Comment: No information reported regarding adherence. |
High risk of bias | "In the end, 85 patients (88.5%) completed the study: 38 patients in the CG (18 (47.4%) in area VI and 20 (52.6%) in area I); and 47 patients in the IG (25 (53.2%) in area VI and 22 (46.8%) in area I; P=0.593). In the CG, seven patients were lost to follow‐up (one protocol violation and six dropouts), and in the IG, four were lost (one protocol violation and three dropouts)." p. 1942. Comment: Data were not available for all randomised patients. |
Low risk of bias | "Because double‐blinding was not possible, an independent evaluator, who did not know the patients’ group assignments, was responsible for evaluating the outcome variables." p. 1940. | High risk of bias | Comment: No trial register number and study protocol were available. | High risk of bias | Unpredictable direction of the outcome. |
| Benzo 2016 | Low risk of bias | Comment: Patients were randomly assigned, using an online, computer‐generated, simple binomial randomization program to one of the two groups, stratified by center. | High risk of bias |
Effect of assignment:
"85% of the individuals in the intervention group received the intervention as intended, defined as 15 (.70%) of 21 calls completed. Reasons for not completing the intervention were death during the study period (n = 3), unable to contact (n = 6), and refusal to complete the scheduled calls (n = 7)." p. 674.
Comment: It was possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analysis was used. Effect of adhering: "Attendance at pulmonary rehabilitation visits anytime in the first 12 months after discharge tended to be higher in the intervention group (53% vs. 43%; P = 0.056)." p. 675. "In the intervention group, 85% of the individuals received a complete intervention, defined as 15 (.70%) of 21 calls completed." p. 674. |
Low risk of bias | ‐ | Low risk of bias | Comment: Outcome assessors were blinded. | High risk of bias | Comment: No analysis plan available in the trial register. Furthermore, the trial register mentions that the outcomes will be assessed in the 12 months time frame, but not mentioned how and when they were assessed. Although 12 months analyses show no differences, the additional time points that were reported (not prespecified) are in favour of experimental. | High risk of bias | Predicted direction: Favours experimental. |
| Johnson‐Warrington 2016 | Low risk of bias | "Participants were randomized to receive usual care or SPACE for COPD via a web‐based, concealed allocation program (www.sealedenvelope.com) using simple random permuted block 1:1 randomization by VJ‐W. Randomization was performed after the participants completed the baseline assessment, with treatment allocation prior to hospital discharge." p. 1162. | Some concerns |
Effect of assignment:
"Analysis was conducted on an intention‐to‐treat basis." p. 1163. Effect of adhering: Patients and personnel were not blinded. No information regarding adherence reported. |
High risk of bias | Some patients were missing: n= 78 were randomized (n= 39 to each arm). Included in the analysis: Intervention: n= 35 and usual care: n= 36. | Low risk of bias | "All outcomes were measured at baseline (during admission but as close to discharge as possible) and 3 months after randomization, by a clinician blinded to treatment allocation." p. 1163. | Some concerns | No protocol including a pre‐specified analysis plan was available. | High risk of bias | Unpredictable direction of the outcome. |
| Lenferink 2019 | Low risk of bias | Comment: Allocation was stratified on potential confounders. Only a baseline difference in 6MWD was reported. | High risk of bias |
Effect of assignment:
Comment: It was not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analyses were performed. Effect of adhering: "The 12‐month follow‐up was completed by 83.3% of the individuals in the self‐management group and 84.8% in the UC group (figure 1). Patients completed 81.3% (self‐management: 82.3%; UC: 80.2%) of the diary data." p. 6. Comment: The effect of adherence to the intervention was not analysed. However, it was observed that a high number of patients in completed follow‐up and their symptom diaries. Adherence to action plans was measured, but not yet reported in the current publication. |
Low risk of bias | Comment: Relatively low number of drop‐outs. The imputated data analyses showed similar results as the observed data analyses. | Low risk of bias | "Wherever possible, though, assessors of outcomes were blinded to the treatment group." p. 3. | Low risk of bias | Comment: Some small changes in final analyses compared to analysis plan that was published with the study protocol. | High risk of bias |
Additional bias:
"Based on the exacerbation rates/patient/day from the COPE‐II study (intervention: 0.116; control: 0.176, both with an estimated standard deviation of 0.17) and allowing for overdispersion and an attrition rate of 10%, 105 patients per group provided 80% power to detect an effect of this size, with a two‐tailed p<0.05." p. 4.
Comment: The study included less patients (intervention: n= 102; usual care: n= 99) than aimed by power calculation (both groups n= 105). Unpredicted direction of the outcome. |
Risk of bias for analysis 1.7 Healthcare utilisation: respiratory‐related hospital admissions (mean number per participant) (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Bösch 2007 | Some concerns | Information from the author regarding allocation concealment: “Pick of envelope. Enrolment and selection were right before the start of the study‐ a selection bias cannot be fully excluded". Comment: The method used to generate the random sequence generation was not clearly reported. The information received from the author regarding allocation concealment is too concise to assess risk of bias. |
High risk of bias |
Effect of assignment:
Comment: Blinding of participants and personnel was not reported. No information reported whether patients were analysed in the wrong intervention group or were excluded from the analysis. No intention‐to‐treat analyses were performed. Effect of adhering: Comment: Eight (21%) participants in the intervention group and one (8%) participant in the control group dropped out, without clearly reported reasons. |
Some concerns | Comment: Only the patients who completed the study follow‐up were included in the baseline characteristics and analysis (per protocol analysis). Eight (21%) participants in the intervention group and one (8%) participant in the control group dropped out. Reasons for drop‐out were not clearly reported. Furthermore, the trial report provides no information about the extent of missing outcome data. | Some concerns | Comment: There was no protocol available. Some of the outcomes were self‐reported outcomes (e.g. frequency of avoidance of physical exertion, frequency of sport activities, frequency of impairment of lifestyle), which could have been influenced by knowledge of the received intervention. Blinding of outcome assessment was not reported. | Some concerns | Comment: No signs for selective outcome reporting, results were reported extensively; however, no protocol was available. | High risk of bias | Unpredictable direction of the outcome. |
| Bucknall 2012 | Low risk of bias | “We used a minimisation technique to stratify randomisation of participants by demographic factors (deprivation category of area of residence,11 age and sex, FEV1 per cent predicted at the time of randomisation, smoking status, participation in pulmonary rehabilitation within two years, and number of previous admissions) to control for key aspects of disease severity and predictors of readmission. We constructed a computer generated sequence by using the method of randomised permuted blocks of length four, with two allocations being made at random and two by minimisation. Treatment group allocations were obtained by telephone, after baseline assessments had been made.” p 2. Comment: Random sequence allocation was adequately performed. |
High risk of bias |
Effect of assignment:
Comment: No blinding of patients and personnel. Data for the primary outcome (time to first acute COPD hospital admission or death) were available for all patients and analysed with a Cox proportional hazards model adjusting for the stratification variables. Effect of adhering: Comment: No blinding of patients and personnel. Seventy‐five (42%) of 180 intervention group participants who completed 12 months of follow‐up were judged to have become successful self managers. |
Low risk of bias | Comment: Only for secondary outcome measures (e.g. SGRQ, HADS, CSES) not all data were available for all patients randomised (Table 3). | Low risk of bias | ‐ | Some concerns | ‐ | High risk of bias | Unpredictable direction of the outcome. |
| Tabak 2014 | Low risk of bias | "Patients were randomized using a computer‐generated randomization list (Blocked Stratified Randomization version 5; Steven Piantadosi), where randomization was applied in blocks of two and four.” p. 936. “Participants were allocated by a data manager in order of inclusion following the randomization list, placed in a sealed envelope.” p. 936. Comment: There was a significant difference in dyspnea (MRC scale) but this seems by chance. All other parameters seem evenly distributed. |
Some concerns |
Effect of assignment:
"To present the outcome measures over time in both groups, a mixed‐model analysis for repeated measures was performed (intention to treat)." p. 938.
Comment: Patients and personnel were not blinded. No information was reported regarding deviations from the intervention that arose because of the trial context. Effect of adhering: "Participants in the control group received only usual care. Patients in the control group were allowed to attend regular physiotherapy sessions if this was prescribed as part of usual care." p. 937. “Although some patients in the intervention group quit the physiotherapy modules ( exercising and activity coach) due to weak (n=l, < T l ) or unstable condition (n=l, <T2) and personal circumstances (n=2, <T4), they persisted in using the web portal and triage diary til! T4.” p. 939. Comment: Most outcome measures are reported for 3 months follow‐up, whereas there was a total of 9 months follow‐up. There was a high number of withdrawals for the 9 months follow‐up. However, there were more drop‐outs in the usual care control group than in the intervention group. |
High risk of bias | “A large number of patients were not able or willing to continue study participation: 33% in the intervention group and 86% in the control group.” p. 939. Comment: Most outcome measures are reported for 3 months follow‐up, whereas there was a total of 9 months follow‐up. There was a high number of withdrawals for the 9 months follow‐up (more dropouts in the control group). Reasons reported for patients from the control group: too much effort (n=2), exacerbation (n‐1), hospitalisation (n‐2), kidney problems (n‐1), exacerbation, and cancer (n‐1). |
Low risk of bias | ‐ | Low risk of bias | Comment: Not all outcome measures were reported for the 9 months follow‐up. Exacerbations (duration) was not reported. Also, no information or results provided for the use of diaries in the usual care control group. | High risk of bias | Unpredictable direction of the outcome. |
| Titova 2015 | High risk of bias |
Randomisation process:
“They were randomly allocated to either integrated care (IC) or usual care (UC) based on their address of permanent residence. In order to create two pairs of districts with approximately equal numbers of citizens, a pair‐wise matching of districts was carried out. It was decided by lottery that participants from District Pair 1 were assigned to the UC group, and participants from District Pair 2 were assigned to the IC group.” Titova 2015, p. 2.
“There were no significant differences in baseline characteristics between the IC and the UC groups, but when comparing those who died during follow‐up with those who were still alive after two years, those who died had a significantly higher PaCO2 and lower BMI at baseline (Table 1). Those who died during follow‐up also had significantly more HA and HD during the year prior to study start.” Titova 2015, p. 4.
Comment: No information provided about the allocation concealment. Timing of identification or recruitment of participants: "They were randomly allocated to either IC or UC group based on their address of permanent residence. Patient data were registered at discharge and 6, 12, and 24 months after discharge from the TUH." Titova 2017, p 3. Comment: If allocation was known before study inclusion, suitable patients could be selected for the intervention. |
High risk of bias |
Effect of assignment:
“The study was a prospective, open, single‐centre intervention study.” Titova 2015, p. 2.
"The principal comparisons of changes were performed on an intention‐to treat basis." Titova 2017, p. 4.
Comment: No blinding of participants and personnel. Effect of adhering: ‐ |
High risk of bias | “172 patients were randomly allocated to the IC group (n = 91) or the UC group (n = 81), respectively. After two years of follow‐up, a total of 100 patients (58% of the included patients), 51 patients in the IC group and 49 patients in the UC group, were available for evaluation (Figure 1).” Titova 2017, p. 5. "Those who died had a significantly higher PaCO2 and lower BMI at baseline (Table 2). Those who died during follow‐up also had significantly more HA and HD during the year prior to study start." Titova 2015, p. 4. Comment: No mortality reported; however, Figure 1 shows higher mortality for the IC group, n=35 (38.4%), compared to the UC group, n=21 (25.9%). |
High risk of bias | “Information concerning the number and duration of the COPD exacerbations, as well as the time from onset of symptoms until the start of self‐initiated treatment is insufficient due to many incomplete registrations in “My COPD book.” Titova 2015, p. 9. "“Data concerning HA (hospital admissions) and HD (hospital days) were collected from the hospital registry database’s medical charts.” Titova 2015, p. 3. Comment: Unclear who was the outcome assessor. |
Some concerns | Trial register reported number of hospitalisations as the primary outcome. Secondary outcomes were quality of life, lung function, use of medication, cost‐effectiveness. However, the article reported not all secondary outcomes (e.g. cost‐effectiveness). | High risk of bias | Unpredictable direction of the outcome. |
| Jolly 2018 | High risk of bias | "When a patient is identified as eligible for the study, and has given written, informed consent to take part, the researcher randomises the patient using a web‐based programme hosted by the Primary Care Clinical Trials Unit (PC‐CTU), University of Birmingham. Centre specific randomisation lists were produced by a statistician at the trials unit. Participants are individually randomised, stratified by centre. The four recruitment centres are Birmingham and West‐Midlands South; Greater Manchester; North West Midlands and Oxfordshire/Gloucestershire. Only the PC‐CTU have access to the allocation sequence." Sidhu 2015 (protocol paper), p. 4. "The study groups were generally well balanced in terms of patient characteristics, although there was a higher proportion of current smokers in the telephone health coaching group. The usual care group reported a higher level of self reported moderate and vigorous physical activity, but this was not reflected in the accelerometry data at baseline." Jolly 2018 (results paper), p. 5. |
High risk of bias |
Effect of assignment:
Comment: Patients were informed of their allocation at the end of the recruitment appointment; they were not masked to treatment allocation. All data were analysed by intention to treat. Effect of adhering: "We achieved good fidelity of delivery of the intervention, with 75% of intervention participants receiving all four calls and only four patients receiving none." p. 11. "The dose and coverage of intervention delivery were high: 86.4% (999/1156) of the scheduled calls were delivered and 75.4% (218/289) of participants received all four calls." No information regarding pulmonary rehabilitation, oxygen use. |
High risk of bias | "There was imbalance in the follow‐up rates between telephone health coaching (82.7%; 37 withdrawals) and usual care (96.2%; 7 withdrawals)." p. 4. "Participants who did not provide data at 12 months were more likely to be in GOLD stage 3, to be smokers, had lower levels of self‐reported physical activity, and to live alone than responders. " p. 5. |
Some concerns | "Patients were informed of their allocation at the end of the recruitment appointment; they were not masked to treatment allocation. Data were entered into the study database by researchers at the University of Birmingham who were masked to the treatment allocation." p. 3. Comment: No information about outcome assessors. |
Low risk of bias | ‐ | High risk of bias | Unpredictable direction of the outcome. |
| Lenferink 2019 | Low risk of bias | Comment: Allocation was stratified on potential confounders. Only a baseline difference in 6MWD was reported. | High risk of bias |
Effect of assignment:
Comment: It was not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analyses were performed. Effect of adhering: "The 12‐month follow‐up was completed by 83.3% of the individuals in the self‐management group and 84.8% in the UC group (figure 1). Patients completed 81.3% (self‐management: 82.3%; UC: 80.2%) of the diary data." p. 6. Comment: The effect of adherence to the intervention was not analysed. However, it was observed that a high number of patients in completed follow‐up and their symptom diaries. Adherence to action plans was measured, but not yet reported in the current publication. |
Low risk of bias | Comment: Relatively low number of drop‐outs. The imputated data analyses showed similar results as the observed data analyses. | Low risk of bias | "Wherever possible, though, assessors of outcomes were blinded to the treatment group." p. 3. | Low risk of bias | Comment: Some small changes in final analyses compared to analysis plan that was published with the study protocol. | High risk of bias |
Additional bias:
"Based on the exacerbation rates/patient/day from the COPE‐II study (intervention: 0.116; control: 0.176, both with an estimated standard deviation of 0.17) and allowing for overdispersion and an attrition rate of 10%, 105 patients per group provided 80% power to detect an effect of this size, with a two‐tailed p<0.05." p. 4.
Comment: The study included less patients (intervention: n= 102; usual care: n= 99) than aimed by power calculation (both groups n= 105). Unpredicted direction of the outcome. |
| Wang 2019 | Low risk of bias | "The allocation sequence was generated and released to the interventionist on a case‐by‐case basis by an independent department that specialised in supplying randomly generated sequences for research. The randomisation sequence was recorded." p. 151. "Participants were randomly assigned to groups after consent was obtained and baseline data were collected." p. 151. "There were no significant differences in demographic and clinical data between the two groups at baseline (Table 1)." p. 151. |
High risk of bias |
Effect of assignment:
Comment: It is not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analysis was used. Effect of adhering: "154 participants were enrolled, among whom, 143 completed the 12‐month study program. The attrition rate was 7.14%." p. 151. "...adherence to the self‐management intervention was reported by the participants but not monitored by the nurses." p. 155. Comment: No info on reasons of adherence by patients reported in the current publication. |
Low risk of bias | Comment: Relatively low attrition rate. | Low risk of bias | "To minimize researcher bias, the interventionists who provided care were blinded to the participants’ baseline and allocation sequence. The statistician was blinded to the participants’ results during the trial." p. 151. Comment: Not possible to blind the treatment for patients and people delivering the treatment. |
Some concerns | Comment: No pre‐specified analysis plan published which can be checked. However they mention: The statistician was blinded to the participants’ results during the trial. | High risk of bias | Unpredictable direction of the outcome. |
Risk of bias for analysis 1.9 Mortality: respiratory‐related mortality (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Gallefoss 1999 | Low risk of bias | Comment: At inclusion they signed a written consent and were then randomised to an intervention group or a control group. | High risk of bias |
Effect of assignment:
Comment: No information reported about blinding, but it is almost impossible to blind these patients because of the nature of the intervention. Furthermore, no information was reported whether deviations arose from the intended intervention because of the trial context. Effect of adhering: "Forty‐six percent of those educated (12 of 26) received a standard treatment plan. Non‐standard treatment plans, incorporating the use of oral steroids as the first line of action in the yellow zone, were followed in some cases, for example, if the patient already used high dosages of inhaled steroids as maintenance therapy, or could report that a double or triple increase in inhaled steroids previously had shown marginal effect on the course of attacks/exacerbations. Among those 14 patients receiving non‐standard treatment plans, eight patients did not want to or were not able to use peak flow monitoring as a basis for change in medication. For those patients, symptom‐only based treatment plans were issued." Gallefoss 2002, p. 425. "In the intervention group, four patients failed to complete the educational program (social problems (n = 1), unannounced emigration (n = 1), failure to meet at educational group sessions for unknown reasons (n = 1) and serious myocardial infarction (n = 1))." Gallefoss 2002, p. 427. Comment: No information reported about adherence to the programme of the other patients (who completed the programme). |
High risk of bias | “In the intervention group, four patients failed to complete the educational programme (social problems (n = 1), unannounced emigration (n = 1), failure to meet at educational group sessions for unknown reasons (n = 1) and serious myocardial infarction (n = 1)). Another patient was withdrawn from the study during the follow‐up due to lymphoma (n = 1). This left us with 26 patients (81%) for a 1‐year follow‐up. The patients who were withdrawn from the intervention group did not, to our knowledge, have any serious deterioration in their obstructive lung disease, and none were hospitalised. In the control group four patients were withdrawn (lack of co‐operation (n = 2), diagnosis of rectal cancer (n = 1) and emigration (n = 1)). Two of the withdrawn control group patients were hospitalised for exacerbations of their COPD. This left us with 27 patients (84%) for the 1‐year follow‐up” Gallefoss 2002, p. 427. Comment: The number of dropouts was relatively low, and reasons for dropout were comparable over groups |
High risk of bias | "Prebronchodilator spirometry was performed before randomization and at 12 mo follow‐up by standard methods using a Jaeger MasterLab Body Box (Würzburg, Germany). The technical staff did not know whether the patients belonged to the control or intervention groups." Gallefoss 1999, p. 2003. Comment: No information reported regarding the assessors of the primary outcome. Number of prednisolone courses was retrospectively self‐reported at 12 months of follow‐up. |
Some concerns | "We defined a priori the patient as compliant when dispensed regular medication was greater than 75% of prescribed regular medication during the study period." Gallefoss 1999, p. 2002. | High risk of bias | Unpredictable direction of the outcome. |
| Fan 2012 | Low risk of bias | “Randomization lists were generated on the basis of random, permuted blocks of variable size to ensure approximate balance over time.” p. 674. “The CSP Coordinating Center in Boston, Massachusetts, randomly assigned eligible patients in equal numbers to 2 groups, stratifying patients per site to allow for possible regional differences in patient characteristics and clinical practice patterns.” p. 674. Comment: Random sequence generation was adequately performed. The allocation was adequately concealed. Authors didn’t report whether there were any significant differences. Most characteristics are equally balanced, but there is a bit larger difference in the following characteristics (>5%):
|
High risk of bias |
Effect of assignment:
“The 2 groups differed on the basis of a complex behavioral intervention that made blinding impossible.” p. 674.
Comment: No blinding of participants and personnel.
"For the primary intention‐to‐treat analysis, the null hypothesis was that the COPD hospitalization hazard rate for intervention did not differ from that for standardized care."p. 675.
"Also, with respect to time to first COPD hospitalization, we conducted a futility analysis to assess what might have resulted if the trial had continued." p. 676.
"When the study was stopped, the 1‐year cumulative incidence of COPD‐related hospitalization was 27% in the intervention group and 24% in the usual care group (hazard ratio, 1.13 [95% CI, 0.70 to 1.80]; P 0.62)." p. 677. Effect of adhering: "During the entire follow‐up period extending to September 2009, a total of 8 patients in the intervention group and 10 in the usual care group either did not attend scheduled study visits or formally withdrew from the study. Among patients in the intervention group, 87% completed all 4 individual educational visits and 57% completed the group visit. A review of the recorded sessions found that case managers covered 77% to 89% of the educational items in each session. The ranges of average scores across sites that provided audio recordings for each session were 18.3 to 31 for session 1 (13 sites), 12 to 19 for session 2 (12 sites), 12.7 to 23 for session 3 (10 sites), and 6 to 18 for session 4 (10 sites)." p. 677. |
High risk of bias | “This multisite, randomized, controlled trial of an educational and acute care management program was stopped early when a safety monitoring board noted more deaths in the intervention group.” p. 674 Comment: There is incomplete outcome data due to early termination of the study. |
Low risk of bias | “Telephone‐based ascertainment of study outcomes (COPD hospitalizations and exacerbations) was performed by centralized research staff blinded to assignment. All outcomes were collected by centralized staff blinded to study group, and all hospitalizations were adjudicated by a committee that was also blinded to study group.” p. 674. Comment: Research staff blinded to study group contacted patients every 2 months to determine whether they developed symptoms of a COPD exacerbation, along with details of treatment and health care use. Deaths were ascertained by staff at the study sites, by review of VA electronic medical and administrative records and by a search of the Social Security Death Index. Extensive multivariate analyses failed to identify any factors alone or combined that could plausibly explain the difference in mortality. Two of the authors who were blinded to study group reviewed all available medical information and did not find anything in the deaths that would not be expected in frail patients with COPD. |
Some concerns | Comment: The primary and secondary outcomes were reported, only healthcare costs as (secondary objective) were not reported. | High risk of bias | Predicted directon: Favours comparator, because more deaths in the intervention group were observed. There is still a chance that this was because of chance. |
| Bucknall 2012 | Low risk of bias | “We used a minimisation technique to stratify randomisation of participants by demographic factors (deprivation category of area of residence,11 age and sex, FEV1 per cent predicted at the time of randomisation, smoking status, participation in pulmonary rehabilitation within two years, and number of previous admissions) to control for key aspects of disease severity and predictors of readmission. We constructed a computer generated sequence by using the method of randomised permuted blocks of length four, with two allocations being made at random and two by minimisation. Treatment group allocations were obtained by telephone, after baseline assessments had been made.” p 2. Comment: Random sequence allocation was adequately performed. |
High risk of bias |
Effect of assignment:
Comment: No blinding of patients and personnel. Data for the primary outcome (time to first acute COPD hospital admission or death) were available for all patients and analysed with a Cox proportional hazards model adjusting for the stratification variables. Effect of adhering: Comment: No blinding of patients and personnel. Seventy‐five (42%) of 180 intervention group participants who completed 12 months of follow‐up were judged to have become successful self managers. |
Low risk of bias | Comment: Only for secondary outcome measures (e.g. SGRQ, HADS, CSES) not all data were available for all patients randomised (Table 3). | Low risk of bias | ‐ | Some concerns | ‐ | High risk of bias | Unpredictable direction of the outcome. |
| Tabak 2014 | Low risk of bias | "Patients were randomized using a computer‐generated randomization list (Blocked Stratified Randomization version 5; Steven Piantadosi), where randomization was applied in blocks of two and four.” p. 936. “Participants were allocated by a data manager in order of inclusion following the randomization list, placed in a sealed envelope.” p. 936. Comment: There was a significant difference in dyspnea (MRC scale) but this seems by chance. All other parameters seem evenly distributed. |
Some concerns |
Effect of assignment:
"To present the outcome measures over time in both groups, a mixed‐model analysis for repeated measures was performed (intention to treat)." p. 938.
Comment: Patients and personnel were not blinded. No information was reported regarding deviations from the intervention that arose because of the trial context. Effect of adhering: "Participants in the control group received only usual care. Patients in the control group were allowed to attend regular physiotherapy sessions if this was prescribed as part of usual care." p. 937. “Although some patients in the intervention group quit the physiotherapy modules ( exercising and activity coach) due to weak (n=l, < T l ) or unstable condition (n=l, <T2) and personal circumstances (n=2, <T4), they persisted in using the web portal and triage diary til! T4.” p. 939. Comment: Most outcome measures are reported for 3 months follow‐up, whereas there was a total of 9 months follow‐up. There was a high number of withdrawals for the 9 months follow‐up. However, there were more drop‐outs in the usual care control group than in the intervention group. |
High risk of bias | “A large number of patients were not able or willing to continue study participation: 33% in the intervention group and 86% in the control group.” p. 939. Comment: Most outcome measures are reported for 3 months follow‐up, whereas there was a total of 9 months follow‐up. There was a high number of withdrawals for the 9 months follow‐up (more dropouts in the control group). Reasons reported for patients from the control group: too much effort (n=2), exacerbation (n‐1), hospitalisation (n‐2), kidney problems (n‐1), exacerbation, and cancer (n‐1). |
Low risk of bias | ‐ | Low risk of bias | Comment: Not all outcome measures were reported for the 9 months follow‐up. Exacerbations (duration) was not reported. Also, no information or results provided for the use of diaries in the usual care control group. | High risk of bias | Unpredictable direction of the outcome. |
| Titova 2015 | High risk of bias |
Randomisation process:
“They were randomly allocated to either integrated care (IC) or usual care (UC) based on their address of permanent residence. In order to create two pairs of districts with approximately equal numbers of citizens, a pair‐wise matching of districts was carried out. It was decided by lottery that participants from District Pair 1 were assigned to the UC group, and participants from District Pair 2 were assigned to the IC group.” Titova 2015, p. 2.
“There were no significant differences in baseline characteristics between the IC and the UC groups, but when comparing those who died during follow‐up with those who were still alive after two years, those who died had a significantly higher PaCO2 and lower BMI at baseline (Table 1). Those who died during follow‐up also had significantly more HA and HD during the year prior to study start.” Titova 2015, p. 4.
Comment: No information provided about the allocation concealment. Timing of identification or recruitment of participants: "They were randomly allocated to either IC or UC group based on their address of permanent residence. Patient data were registered at discharge and 6, 12, and 24 months after discharge from the TUH." Titova 2017, p 3. Comment: If allocation was known before study inclusion, suitable patients could be selected for the intervention. |
High risk of bias |
Effect of assignment:
“The study was a prospective, open, single‐centre intervention study.” Titova 2015, p. 2.
"The principal comparisons of changes were performed on an intention‐to treat basis." Titova 2017, p. 4.
Comment: No blinding of participants and personnel. Effect of adhering: ‐ |
High risk of bias | “172 patients were randomly allocated to the IC group (n = 91) or the UC group (n = 81), respectively. After two years of follow‐up, a total of 100 patients (58% of the included patients), 51 patients in the IC group and 49 patients in the UC group, were available for evaluation (Figure 1).” Titova 2017, p. 5. "Those who died had a significantly higher PaCO2 and lower BMI at baseline (Table 2). Those who died during follow‐up also had significantly more HA and HD during the year prior to study start." Titova 2015, p. 4. Comment: No mortality reported; however, Figure 1 shows higher mortality for the IC group, n=35 (38.4%), compared to the UC group, n=21 (25.9%). |
High risk of bias | “Information concerning the number and duration of the COPD exacerbations, as well as the time from onset of symptoms until the start of self‐initiated treatment is insufficient due to many incomplete registrations in “My COPD book.” Titova 2015, p. 9. "“Data concerning HA (hospital admissions) and HD (hospital days) were collected from the hospital registry database’s medical charts.” Titova 2015, p. 3. Comment: Unclear who was the outcome assessor. |
Some concerns | Trial register reported number of hospitalisations as the primary outcome. Secondary outcomes were quality of life, lung function, use of medication, cost‐effectiveness. However, the article reported not all secondary outcomes (e.g. cost‐effectiveness). | High risk of bias | Unpredictable direction of the outcome. |
| Johnson‐Warrington 2016 | Low risk of bias | "Participants were randomized to receive usual care or SPACE for COPD via a web‐based, concealed allocation program (www.sealedenvelope.com) using simple random permuted block 1:1 randomization by VJ‐W. Randomization was performed after the participants completed the baseline assessment, with treatment allocation prior to hospital discharge." p. 1162. | Some concerns |
Effect of assignment:
"Analysis was conducted on an intention‐to‐treat basis." p. 1163. Effect of adhering: Patients and personnel were not blinded. No information regarding adherence reported. |
High risk of bias | Some patients were missing: n= 78 were randomized (n= 39 to each arm). Included in the analysis: Intervention: n= 35 and usual care: n= 36. | Low risk of bias | "All outcomes were measured at baseline (during admission but as close to discharge as possible) and 3 months after randomization, by a clinician blinded to treatment allocation." p. 1163. | Some concerns | No protocol including a pre‐specified analysis plan was available. | High risk of bias | Unpredictable direction of the outcome. |
| Lenferink 2019 | Low risk of bias | Comment: Allocation was stratified on potential confounders. Only a baseline difference in 6MWD was reported. | High risk of bias |
Effect of assignment:
Comment: It was not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analyses were performed. Effect of adhering: "The 12‐month follow‐up was completed by 83.3% of the individuals in the self‐management group and 84.8% in the UC group (figure 1). Patients completed 81.3% (self‐management: 82.3%; UC: 80.2%) of the diary data." p. 6. Comment: The effect of adherence to the intervention was not analysed. However, it was observed that a high number of patients in completed follow‐up and their symptom diaries. Adherence to action plans was measured, but not yet reported in the current publication. |
Low risk of bias | Comment: Relatively low number of drop‐outs. The imputated data analyses showed similar results as the observed data analyses. | Low risk of bias | "Wherever possible, though, assessors of outcomes were blinded to the treatment group." p. 3. | Low risk of bias | Comment: Some small changes in final analyses compared to analysis plan that was published with the study protocol. | High risk of bias |
Additional bias:
"Based on the exacerbation rates/patient/day from the COPE‐II study (intervention: 0.116; control: 0.176, both with an estimated standard deviation of 0.17) and allowing for overdispersion and an attrition rate of 10%, 105 patients per group provided 80% power to detect an effect of this size, with a two‐tailed p<0.05." p. 4.
Comment: The study included less patients (intervention: n= 102; usual care: n= 99) than aimed by power calculation (both groups n= 105). Unpredicted direction of the outcome. |
| Wang 2019 | Low risk of bias | "The allocation sequence was generated and released to the interventionist on a case‐by‐case basis by an independent department that specialised in supplying randomly generated sequences for research. The randomisation sequence was recorded." p. 151. "Participants were randomly assigned to groups after consent was obtained and baseline data were collected." p. 151. "There were no significant differences in demographic and clinical data between the two groups at baseline (Table 1)." p. 151. |
Some concerns |
Effect of assignment:
Comment: It is not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analysis was used. Effect of adhering: "154 participants were enrolled, among whom, 143 completed the 12‐month study program. The attrition rate was 7.14%." p. 151. "...adherence to the self‐management intervention was reported by the participants but not monitored by the nurses." p. 155. Comment: No info on reasons of adherence by patients reported in the current publication. |
Low risk of bias | Comment: Relatively low attrition rate. | Low risk of bias | "To minimize researcher bias, the interventionists who provided care were blinded to the participants’ baseline and allocation sequence. The statistician was blinded to the participants’ results during the trial." p. 151. Comment: Not possible to blind the treatment for patients and people delivering the treatment. |
Some concerns | Comment: No pre‐specified analysis plan published which can be checked. However they mention: The statistician was blinded to the participants’ results during the trial. | Some concerns | Unpredictable direction of the outcome. |
Risk of bias for analysis 1.11 Mortality: all‐cause mortality (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Gallefoss 1999 | Low risk of bias | Comment: At inclusion they signed a written consent and were then randomised to an intervention group or a control group. | High risk of bias |
Effect of assignment:
Comment: No information reported about blinding, but it is almost impossible to blind these patients because of the nature of the intervention. Furthermore, no information was reported whether deviations arose from the intended intervention because of the trial context. Effect of adhering: "Forty‐six percent of those educated (12 of 26) received a standard treatment plan. Non‐standard treatment plans, incorporating the use of oral steroids as the first line of action in the yellow zone, were followed in some cases, for example, if the patient already used high dosages of inhaled steroids as maintenance therapy, or could report that a double or triple increase in inhaled steroids previously had shown marginal effect on the course of attacks/exacerbations. Among those 14 patients receiving non‐standard treatment plans, eight patients did not want to or were not able to use peak flow monitoring as a basis for change in medication. For those patients, symptom‐only based treatment plans were issued." Gallefoss 2002, p. 425. "In the intervention group, four patients failed to complete the educational program (social problems (n = 1), unannounced emigration (n = 1), failure to meet at educational group sessions for unknown reasons (n = 1) and serious myocardial infarction (n = 1))." Gallefoss 2002, p. 427. Comment: No information reported about adherence to the programme of the other patients (who completed the programme). |
High risk of bias | “In the intervention group, four patients failed to complete the educational programme (social problems (n = 1), unannounced emigration (n = 1), failure to meet at educational group sessions for unknown reasons (n = 1) and serious myocardial infarction (n = 1)). Another patient was withdrawn from the study during the follow‐up due to lymphoma (n = 1). This left us with 26 patients (81%) for a 1‐year follow‐up. The patients who were withdrawn from the intervention group did not, to our knowledge, have any serious deterioration in their obstructive lung disease, and none were hospitalised. In the control group four patients were withdrawn (lack of co‐operation (n = 2), diagnosis of rectal cancer (n = 1) and emigration (n = 1)). Two of the withdrawn control group patients were hospitalised for exacerbations of their COPD. This left us with 27 patients (84%) for the 1‐year follow‐up” Gallefoss 2002, p. 427. Comment: The number of dropouts was relatively low, and reasons for dropout were comparable over groups |
High risk of bias | "Prebronchodilator spirometry was performed before randomization and at 12 mo follow‐up by standard methods using a Jaeger MasterLab Body Box (Würzburg, Germany). The technical staff did not know whether the patients belonged to the control or intervention groups." Gallefoss 1999, p. 2003. Comment: No information reported regarding the assessors of the primary outcome. Number of prednisolone courses was retrospectively self‐reported at 12 months of follow‐up. |
Some concerns | "We defined a priori the patient as compliant when dispensed regular medication was greater than 75% of prescribed regular medication during the study period." Gallefoss 1999, p. 2002. | High risk of bias | Unpredictable direction of the outcome. |
| Bourbeau 2003 | Low risk of bias | “… central computergenerated list of random numbers. Randomization was stratified per center and in blocks of 6, and patients were assigned to the self‐management program (intervention group) or to usual care.” p. 586. “The blocking factor was not known by the investigators or their staff in each participating center." p. 586. “The use of respiratory medications was similar between study groups, except that oral steroids were used less commonly in the intervention group (7%) than in the usual care group (13%). None of the disease severity characteristics were otherwise different between the study groups.” p. 586. Comment: Random sequence allocation was adequately performed. |
High risk of bias |
Effect of assignment:
"Since a double‐blind design was impossible..." p. 586.
Comment: Participants and personnel were not blinded. ITT analysis was performed. Effect of adhering: "Since a double‐blind design was impossible..." p. 586. Comment: Participants and personnel were not blinded. Comment: There might be some imbalance in non‐protocol interventions such as exercise evaluation (not mandatory). However, no information available. |
Low risk of bias | ‐ | Low risk of bias | "...an independent evaluator unaware of the patient assignment was responsible for the evaluation process in each center. The evaluator was cautioned not to ask about the workbook modules and types of contact.” p. 586. Comment: Outcome assessment was blinded. |
Some concerns | Comment: No protocol or prespecified analysis plan available. | High risk of bias | Unpredictable direction of the outcome. |
| Martin 2004 | High risk of bias | "Patients were randomly assigned to the intervention (care plan) or control (usual care) groups." p. 192. Comment: The method of random sequence allocation was not reported. The method of allocation concealment was not reported. Significant differences in gender and cigarette consumption (the latter can be a predictor of worse outcomes). No information reported about smokers – no‐smokers. |
High risk of bias |
Effect of assignment:
Comment: Blinding of patients and personnel was not reported. The intervention group patients did however receive a care plan with specific instructions for the patient and/or carer. No intention‐to‐treat analysis was used. Effect of adhering: Comment: No information regarding adherence was reported. |
High risk of bias | "Three subsequently withdrew for personal reasons. A further 13 died during the follow‐up period (...) (nine in the intervention group and four in the control group (NS)" p. 192. Comments: The number of withdrawals was higher in the intervention group compared to the usual care control group. However, no information was provided regarding the differences in drop‐out rates. |
Some concerns | “Quality of life was measured by the St George’s Respiratory Questionnaire (SGRQ). The questionnaire was administered by the research nurse (DMcN) at each visit.” p. 192. Comment: The blinding of outcome assessment was not reported. However, it seems that the research nurse was aware of the intervention received. |
Some concerns | Comment: No signs of selective reporting, however, no protocol available. | High risk of bias | Unpredictable direction of the outcome. |
| Coultas 2005 | Low risk of bias | “Patients were randomly assigned to one of three intervention groups (usual care (UC), nurse‐assisted medical management (MM), or nurse‐assisted collaborative management (CM)) using a computer‐generated random list.” p. 2018. “Although the distribution of most of the baseline characteristics of the three groups was similar (Tables 2 and 3), only the distribution of gender and the tangible domain of social support were significantly different among the intervention groups.” p. 2020. Comment: It is unclear how allocation concealment was guaranteed. Observed imbalances are probably compatible with chance. Random sequence generation was adequately performed. |
High risk of bias |
Effect of assignment:
“Of the 217 patients enrolled in the study, 151 (69.6%) completed the 6‐month intervention and follow‐up data collection.” p. 2020.
“The reasons for the failure to complete the study were patient was unavailable for follow‐up (26.7%) and death (3.7%) [Fig 1]. The frequency of patients being unavailable for follow‐up was equally distributed among the three intervention groups (Fig 1). Overall, the demographic characteristics of the patients who dropped out of the study were similar to those who completed the study (data not shown). However, patients who dropped out of the study had more severe airflow obstruction, higher levels of distress, and lower quality of life, as measured with the SGRQ, compared with the patients who had completed the study (data not shown).” p. 2020.
Comment: Not reported whether deviations arose because of the trial context. Only patients who completed the 6‐month intervention and follow‐up data collection were analysed (per‐protocol analyses). Effect of adhering: Comment: Patients and personnnel were probably aware of assignment to the intervention during the trial. |
High risk of bias | “The reasons for the failure to complete the study were patient was unavailable for follow‐up (26.7%) and death (3.7%).” p. 2020. “However, patients who dropped out of the study had more severe airflow obstruction, higher levels of distress, and lower quality of life, as measured with the SGRQ, compared with the patients who had completed the study (data not shown).” p. 2020. Comment: Only patients who completed the 6‐month intervention and follow‐up data collection were analysed. |
Low risk of bias | “To limit interviewer bias, each interviewer who obtained the baseline and 6‐month outcome data was blinded to the patient’s treatment group.” p. 2023. "Although self‐reported health‐care utilization may be subject to recall bias, it has been shown to be an acceptable method of measurement and may actually result in an underestimation of utilization with more frequent use.” p. 2023. |
Some concerns | Comment: No protocol available. Measures and analysis intentions were only reported in the method section of the paper. | High risk of bias | Unpredictable direction of the outcome. |
| Coultas 2005 | Low risk of bias | “Patients were randomly assigned to one of three intervention groups (usual care (UC), nurse‐assisted medical management (MM), or nurse‐assisted collaborative management (CM)) using a computer‐generated random list.” p. 2018. “Although the distribution of most of the baseline characteristics of the three groups was similar (Tables 2 and 3), only the distribution of gender and the tangible domain of social support were significantly different among the intervention groups.” p. 2020. Comment: It is unclear how allocation concealment was guaranteed. Observed imbalances are probably compatible with chance. Random sequence generation was adequately performed. |
High risk of bias |
Effect of assignment:
“Of the 217 patients enrolled in the study, 151 (69.6%) completed the 6‐month intervention and follow‐up data collection.” p. 2020.
“The reasons for the failure to complete the study were patient was unavailable for follow‐up (26.7%) and death (3.7%) [Fig 1]. The frequency of patients being unavailable for follow‐up was equally distributed among the three intervention groups (Fig 1). Overall, the demographic characteristics of the patients who dropped out of the study were similar to those who completed the study (data not shown). However, patients who dropped out of the study had more severe airflow obstruction, higher levels of distress, and lower quality of life, as measured with the SGRQ, compared with the patients who had completed the study (data not shown).” p. 2020.
Comment: Not reported whether deviations arose because of the trial context. Only patients who completed the 6‐month intervention and follow‐up data collection were analysed (per‐protocol analyses). Effect of adhering: Comment: Patients and personnnel were probably aware of assignment to the intervention during the trial. |
High risk of bias | “The reasons for the failure to complete the study were patient was unavailable for follow‐up (26.7%) and death (3.7%).” p. 2020. “However, patients who dropped out of the study had more severe airflow obstruction, higher levels of distress, and lower quality of life, as measured with the SGRQ, compared with the patients who had completed the study (data not shown).” p. 2020. Comment: Only patients who completed the 6‐month intervention and follow‐up data collection were analysed. |
Low risk of bias | “To limit interviewer bias, each interviewer who obtained the baseline and 6‐month outcome data was blinded to the patient’s treatment group.” p. 2023. "Although self‐reported health‐care utilization may be subject to recall bias, it has been shown to be an acceptable method of measurement and may actually result in an underestimation of utilization with more frequent use.” p. 2023. |
Some concerns | Comment: No protocol available. Measures and analysis intentions were only reported in the method section of the paper. | High risk of bias | Unpredictable direction of the outcome. |
| Rice 2010 | Some concerns | "We assigned subjects in equal proportions to each of the two treatment arms by permuted‐block randomisation." Appendix 1, p. 3. "The baseline characteristics of the two treatment groups were generally well matched, with differences in age, lung function, and ED visits (not significant)." p. 891. Comment: Information on the method of allocation concealment was not reported. |
High risk of bias |
Effect of assignment:
"We performed a randomized, adjudicator‐blinded, controlled, 1‐year trial." p. 890.
"The status of all 743 patients was determined after 1 year. Electronic medical records were available for all hospitalizations and ED visits that occurred within the VA medical system. The success rate for obtaining discharge summaries and ED records for self‐reported events that occurred outside the VA was 99% in the usual care group and 98% in the disease management group." p. 891. Effect of adhering: Comment: 336 out of 372 (90.3%) patients completed disease management 323 out of 371 (87%) completed usual care. |
Low risk of bias | "55% of patients in the usual care group and 60% of patients in the disease management group returned a completed the Saint George’s Respiratory Questionnaire in response to a single mailing at the end of the study. low response rates on SGRQ leading to a high risk of bias. However, data on healthcare utilisation seem complete with no risk of bias." p. 892. | Low risk of bias | "Blinded pulmonologists independently reviewed all discharge summaries and ED reports and assigned a primary cause for each." p. 891. | High risk of bias | Comment: Primary outcome in the paper is reported as a combined outcome measure, whereas it was planned to analyse the two outcomes separately. | High risk of bias | Unpredictable direction of the outcome. |
| Bucknall 2012 | Low risk of bias | “We used a minimisation technique to stratify randomisation of participants by demographic factors (deprivation category of area of residence,11 age and sex, FEV1 per cent predicted at the time of randomisation, smoking status, participation in pulmonary rehabilitation within two years, and number of previous admissions) to control for key aspects of disease severity and predictors of readmission. We constructed a computer generated sequence by using the method of randomised permuted blocks of length four, with two allocations being made at random and two by minimisation. Treatment group allocations were obtained by telephone, after baseline assessments had been made.” p 2. Comment: Random sequence allocation was adequately performed. |
High risk of bias |
Effect of assignment:
Comment: No blinding of patients and personnel. Data for the primary outcome (time to first acute COPD hospital admission or death) were available for all patients and analysed with a Cox proportional hazards model adjusting for the stratification variables. Effect of adhering: Comment: No blinding of patients and personnel. Seventy‐five (42%) of 180 intervention group participants who completed 12 months of follow‐up were judged to have become successful self managers. |
Low risk of bias | Comment: Only for secondary outcome measures (e.g. SGRQ, HADS, CSES) not all data were available for all patients randomised (Table 2). | Low risk of bias | ‐ | Some concerns | ‐ | High risk of bias | Unpredictable direction of the outcome. |
| Fan 2012 | Low risk of bias | “Randomization lists were generated on the basis of random, permuted blocks of variable size to ensure approximate balance over time.” p. 674. “The CSP Coordinating Center in Boston, Massachusetts, randomly assigned eligible patients in equal numbers to 2 groups, stratifying patients per site to allow for possible regional differences in patient characteristics and clinical practice patterns.” p. 674. Comment: Random sequence generation was adequately performed. The allocation was adequately concealed. Authors didn’t report whether there were any significant differences. Most characteristics are equally balanced, but there is a bit larger difference in the following characteristics (>5%):
|
High risk of bias |
Effect of assignment:
“The 2 groups differed on the basis of a complex behavioral intervention that made blinding impossible.” p. 674.
Comment: No blinding of participants and personnel.
"For the primary intention‐to‐treat analysis, the null hypothesis was that the COPD hospitalization hazard rate for intervention did not differ from that for standardized care."p. 675.
"Also, with respect to time to first COPD hospitalization, we conducted a futility analysis to assess what might have resulted if the trial had continued." p. 676.
"When the study was stopped, the 1‐year cumulative incidence of COPD‐related hospitalization was 27% in the intervention group and 24% in the usual care group (hazard ratio, 1.13 [95% CI, 0.70 to 1.80]; P 0.62)." p. 677. Effect of adhering: "During the entire follow‐up period extending to September 2009, a total of 8 patients in the intervention group and 10 in the usual care group either did not attend scheduled study visits or formally withdrew from the study. Among patients in the intervention group, 87% completed all 4 individual educational visits and 57% completed the group visit. A review of the recorded sessions found that case managers covered 77% to 89% of the educational items in each session. The ranges of average scores across sites that provided audio recordings for each session were 18.3 to 31 for session 1 (13 sites), 12 to 19 for session 2 (12 sites), 12.7 to 23 for session 3 (10 sites), and 6 to 18 for session 4 (10 sites)." p. 677. |
High risk of bias | “This multisite, randomized, controlled trial of an educational and acute care management program was stopped early when a safety monitoring board noted more deaths in the intervention group.” p. 674 Comment: There is incomplete outcome data due to early termination of the study. |
Low risk of bias | “Telephone‐based ascertainment of study outcomes (COPD hospitalizations and exacerbations) was performed by centralized research staff blinded to assignment. All outcomes were collected by centralized staff blinded to study group, and all hospitalizations were adjudicated by a committee that was also blinded to study group.” p. 674. Comment: Research staff blinded to study group contacted patients every 2 months to determine whether they developed symptoms of a COPD exacerbation, along with details of treatment and health care use. Deaths were ascertained by staff at the study sites, by review of VA electronic medical and administrative records and by a search of the Social Security Death Index. Extensive multivariate analyses failed to identify any factors alone or combined that could plausibly explain the difference in mortality. Two of the authors who were blinded to study group reviewed all available medical information and did not find anything in the deaths that would not be expected in frail patients with COPD. |
Some concerns | Comment: The primary and secondary outcomes were reported, only healthcare costs as (secondary objective) were not reported. | High risk of bias | Predicted directon: Favours comparator, because more deaths in the intervention group were observed. There is still a chance that this was because of chance. |
| Walters 2013 | High risk of bias |
Randomisation process:
"After recruitment, practices were randomised using a code generated by investigators from a random numbers table stratified in blocks of four by Rural, Remote and Metropolitan Areas (RRMA) classifications in Tasmania. Allocation occurred independently using sequentially numbered, opaque and sealed envelopes." p. 2. Timing of identification or recruitment of participants: GPs identified patients with COPD aged over 45 years seen within the previous 12 months through database searches, based on a diagnostic code for COPD or prescription of tiotropium. Comment: Most variables are equally distributed, but more smokers (not statistically significant, p=0.102) and more previous admissions in the intervention group (statistically significant, p=0.028), so potentially more room for improvement. Although, this small number of differences identified as ‘statistically significant’ at the conventional 0.05 threshold should usually be considered to be compatible with chance. |
High risk of bias |
Effect of assignment:
‐ Effect of adhering: "The limitations of the study were the high rate of misclassification of COPD in general practices, which impacted on enrolment, and higher than expected withdrawal rates, especially in the HM group, which reduced the intended statistical power." p. 6. Comment: Nine patients discontinued and 8 patients withdrew in the intervention group. |
Low risk of bias | Comment: Figure 1: 9 intervention patients discontinued, but they attended follow‐up. In Table 3, no patient numbers are mentioned. | Low risk of bias | Comment: Blinding of participants or research officers was not possible given the nature of the study. | Low risk of bias | Comment: Data that produced this result analysed in accordance with a pre‐specified analysis plan. | High risk of bias | Unpredictable direction of the outcome. |
| Mitchell 2014 | Low risk of bias | “Patients were assigned to either usual care or SPACE FOR COPD via a web‐based, concealed allocation programme, using simple randomisation codes prepared by the trial statistician (J. Bankart).” p. 1539.
"Randomisation was conducted by the trial investigator responsible for administering the intervention (K.E. Mitchell).” p. 1539. Comment: Random sequence allocation was adequately performed. The method of allocation concealment was not reported, but they say it is concealed. The only significant baseline difference between treatment groups was the CRQ‐SR dyspnoea score; therefore, this was corrected for in subsequent analyses. |
High risk of bias |
Effect of assignment:
“Lack of participant blinding may have increased motivation when receiving the treatment and attempts to satisfy the researchers might have increased the observed treatment effects in the intervention arm. We cannot, therefore, rule out the possible impact of attention.” p. 1546.
Comment: No blinding of participants. Analysis was carried out on an intention‐to‐treat basis. Effect of adhering: Comment: Withdrawal around intervention group: 27% and UC: 17%. The adherence of using the manual in by the intervention patients was not reported. |
Low risk of bias | Comment: Data were not available for all randomised patients (withdrawal intervention: 27%, usual care: 17%). However, they found no significant differences in demographics or baseline variables between those who completed and those who did not complete the study. | Low risk of bias | “The assessments at week 6 and 6 months were conducted by a member of the research team who was blind to randomisation allocation (V. Johnson‐Warrington)." p. 1540 Comment: The outcome assessment was blinded. |
Low risk of bias | Comment: No signs for selective outcome reporting. The primary outcome measure and most of the secondary outcome measures were reported. | High risk of bias | Unpredictable direction of the outcome. |
| Tabak 2014 | Low risk of bias | "Patients were randomized using a computer‐generated randomization list (Blocked Stratified Randomization version 5; Steven Piantadosi), where randomization was applied in blocks of two and four.” p. 936. “Participants were allocated by a data manager in order of inclusion following the randomization list, placed in a sealed envelope.” p. 936. Comment: There was a significant difference in dyspnea (MRC scale) but this seems by chance. All other parameters seem evenly distributed. |
Some concerns |
Effect of assignment:
"To present the outcome measures over time in both groups, a mixed‐model analysis for repeated measures was performed (intention to treat)." p. 938.
Comment: Patients and personnel were not blinded. No information was reported regarding deviations from the intervention that arose because of the trial context. Effect of adhering: "Participants in the control group received only usual care. Patients in the control group were allowed to attend regular physiotherapy sessions if this was prescribed as part of usual care." p. 937. “Although some patients in the intervention group quit the physiotherapy modules ( exercising and activity coach) due to weak (n=l, < T l ) or unstable condition (n=l, <T2) and personal circumstances (n=2, <T4), they persisted in using the web portal and triage diary til! T4.” p. 939. Comment: Most outcome measures are reported for 3 months follow‐up, whereas there was a total of 9 months follow‐up. There was a high number of withdrawals for the 9 months follow‐up. However, there were more drop‐outs in the usual care control group than in the intervention group. |
High risk of bias | “A large number of patients were not able or willing to continue study participation: 33% in the intervention group and 86% in the control group.” p. 939. Comment: Most outcome measures are reported for 3 months follow‐up, whereas there was a total of 9 months follow‐up. There was a high number of withdrawals for the 9 months follow‐up (more dropouts in the control group). Reasons reported for patients from the control group: too much effort (n=2), exacerbation (n‐1), hospitalisation (n‐2), kidney problems (n‐1), exacerbation, and cancer (n‐1). |
Low risk of bias | ‐ | Low risk of bias | Comment: Not all outcome measures were reported for the 9 months follow‐up. Exacerbations (duration) was not reported. Also, no information or results provided for the use of diaries in the usual care control group. | High risk of bias | Unpredictable direction of the outcome. |
| Hernández 2015 | Low risk of bias | "A computer‐generated list of random numbers with no restrictions and administered by personnel who were not involved in the study ensured blinded randomisation." p. 2. "(…) and administered by personnel who were not involved in the study." p. 2. Comment: Random sequence generation was adequately performed. No blinding of participants or personnel. The patients of the two groups showed similar characteristics, except that influenza and pneumococcal vaccination were more prevalent in the IC group (P<0.01). |
High risk of bias |
Effect of assignment:
Comment: It was not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analysis performed. Effect of adhering: "As a sensitivity analysis, we repeated all analyses after excluding patients who had a previous contact with any IC programme (n = 15)." p. 2. "25 patients (22%) died and 16 (14%) were lost during the 1‐year follow‐up, and the remaining 114 patients (IC, n = 59 and UC, n = 55) completed the 12‐month assessment visit. Patients lost to follow‐up exhibited a higher affectation of activities of daily living and health‐related quality of life at baseline than those who were followed up, without differences in other sociodemographic, clinical, functional and medical care‐related variables." p. 3. Comment: It is not mentioned in which way these patients were distributed over the two groups, nor are the results of the sensitivity analysis reported. Although the approach was not adequately adopted at the community level and thus the intervention was not continued after the 1 year of follow‐up, this was not a failure of implementing the intervention, since the intervention stopped at 1 year according to the protocol. |
Low risk of bias | ‐ | Low risk of bias | "A blind evaluation of the study group carried out before randomisation and after the 12‐month follow‐up consisted of a patient interview and analysis of medical records, self‐administered questionnaires and lung function testing." p. 2. | Some concerns | "Although the study was officially registered as part of the EU Grant (CIP‐ICT‐PSP‐2007.225025), the RCT was not included in the clinicaltrials.gov registry because at that time it was not compulsory." p. 5. Comment: No protocol available. |
High risk of bias | Unpredictable direction of outcome. |
| Jonsdottir 2015 | Low risk of bias | "After accepting the invitation, prior to entering the research centre, the research nurses randomized the patients to experimental and control groups in accordance with CONSORT requirements using a computer‐generated list of random numbers." p. 2638. | High risk of bias |
Effect of assignment:
Comment: Not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analysis was not carried out as stated in the article, but in table 3 all patients that completed the follow‐up seem to be included. Effect of adhering: "To minimize treatment bias the data were concealed from the nurses providing the treatment and the treatment notes were concealed from the nurses conducting data collection." p. 2640. "Both groups followed standardized protocols and had regular meetings to maintain consistency. Few family members participated. This actually changed the nature of the study from being a family‐centered intervention as planned, into becoming focused on patients, with family involvement when possible." p. 2640. "Attendance at treatment sessions in the experimental group was more than 80% in the patient/family conversations (43/48 attended three or four out of the four possible meetings) and the group meeting (40/48). Half of the patients in the experimental group smoked (24/48). Of those, 58% (14/24) participated in the smoking cessation treatment." p. 2641. |
High risk of bias | "Three patients died in the experimental group... The dropout rate was 20% (n = 12) in the experimental group and 12% (n = 7) in the control group. Also additional missing data on outcome variables as displayed in table 3. The dropout rate for family members was 31% (n = 5) and 50% (n = 7) in the experimental and control groups respectively." p. 2640. Comment: No info on dropout reasons or characteristics of dropouts. |
Low risk of bias | "To minimize treatment bias the data were concealed from the nurses providing the treatment and the treatment notes were concealed from the nurses conducting data collection." p. 2640. | High risk of bias | No information, not registered in a trial register. | High risk of bias | Unpredictable direction of the outcome. |
| Titova 2015 | High risk of bias |
Randomisation process:
“They were randomly allocated to either integrated care (IC) or usual care (UC) based on their address of permanent residence. In order to create two pairs of districts with approximately equal numbers of citizens, a pair‐wise matching of districts was carried out. It was decided by lottery that participants from District Pair 1 were assigned to the UC group, and participants from District Pair 2 were assigned to the IC group.” Titova 2015, p. 2.
“There were no significant differences in baseline characteristics between the IC and the UC groups, but when comparing those who died during follow‐up with those who were still alive after two years, those who died had a significantly higher PaCO2 and lower BMI at baseline (Table 1). Those who died during follow‐up also had significantly more HA and HD during the year prior to study start.” Titova 2015, p. 4.
Comment: No information provided about the allocation concealment. Timing of identification or recruitment of participants: "They were randomly allocated to either IC or UC group based on their address of permanent residence. Patient data were registered at discharge and 6, 12, and 24 months after discharge from the TUH." Titova 2017, p 3. Comment: If allocation was known before study inclusion, suitable patients could be selected for the intervention. |
High risk of bias |
Effect of assignment:
“The study was a prospective, open, single‐centre intervention study.” Titova 2015, p. 2.
"The principal comparisons of changes were performed on an intention‐to treat basis." Titova 2017, p. 4.
Comment: No blinding of participants and personnel. Effect of adhering: ‐ |
High risk of bias | “172 patients were randomly allocated to the IC group (n = 91) or the UC group (n = 81), respectively. After two years of follow‐up, a total of 100 patients (58% of the included patients), 51 patients in the IC group and 49 patients in the UC group, were available for evaluation (Figure 1).” Titova 2017, p. 5. "Those who died had a significantly higher PaCO2 and lower BMI at baseline (Table 1). Those who died during follow‐up also had significantly more HA and HD during the year prior to study start." Titova 2015, p. 4. Comment: No mortality reported; however, Figure 1 shows higher mortality for the IC group, n=35 (38.4%), compared to the UC group, n=21 (25.9%). |
High risk of bias | “Information concerning the number and duration of the COPD exacerbations, as well as the time from onset of symptoms until the start of self‐initiated treatment is insufficient due to many incomplete registrations in “My COPD book.” Titova 2015, p. 9. "“Data concerning HA (hospital admissions) and HD (hospital days) were collected from the hospital registry database’s medical charts.” Titova 2015, p. 3. Comment: Unclear who was the outcome assessor. |
Some concerns | Trial register reported number of hospitalisations as the primary outcome. Secondary outcomes were quality of life, lung function, use of medication, cost‐effectiveness. However, the article reported not all secondary outcomes (e.g. cost‐effectiveness). | High risk of bias | Unpredictable direction of the outcome. |
| Sanchez‐Nieto 2016 | Some concerns | "Controlled, randomized, parallel‐group, single‐blind study with follow‐up of 1 year. After consenting to take part in the study, simple randomization was carried out separately at each site by means of a list of computer‐generated random numbers, assigning the patients to two groups." p. 1940. Comment: No information reported regarding concealed allocation sequence. |
High risk of bias |
Effect of assignment:
Comment: Patients and personnel were not blinded. No proper intention‐to‐treat analysis used, as the baseline characteristics of the people that have dropped out haven’t been included in the analyses. Patients lost to follow‐up were evaluated until the time of the last contact with them by the research staff. Effect of adhering: Comment: No information reported regarding adherence. |
High risk of bias | "In the end, 85 patients (88.5%) completed the study: 38 patients in the CG (18 (47.4%) in area VI and 20 (52.6%) in area I); and 47 patients in the IG (25 (53.2%) in area VI and 22 (46.8%) in area I; P=0.593). In the CG, seven patients were lost to follow‐up (one protocol violation and six dropouts), and in the IG, four were lost (one protocol violation and three dropouts)." p. 1942. Comment: Data were not available for all randomised patients. |
Low risk of bias | "Because double‐blinding was not possible, an independent evaluator, who did not know the patients’ group assignments, was responsible for evaluating the outcome variables." p. 1940. | High risk of bias | Comment: No trial register number and study protocol were available. | High risk of bias | Unpredictable direction of the outcome. |
| Benzo 2016 | Low risk of bias | Comment: Patients were randomly assigned, using an online, computer‐generated, simple binomial randomization program to one of the two groups, stratified by center. | High risk of bias |
Effect of assignment:
"85% of the individuals in the intervention group received the intervention as intended, defined as 15 (.70%) of 21 calls completed. Reasons for not completing the intervention were death during the study period (n = 3), unable to contact (n = 6), and refusal to complete the scheduled calls (n = 7)." p. 674.
Comment: It was possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analysis was used. Effect of adhering: "Attendance at pulmonary rehabilitation visits anytime in the first 12 months after discharge tended to be higher in the intervention group (53% vs. 43%; P = 0.056)." p. 675. "In the intervention group, 85% of the individuals received a complete intervention, defined as 15 (.70%) of 21 calls completed." p. 674. |
Low risk of bias | ‐ | Low risk of bias | Comment: Outcome assessors were blinded. | High risk of bias | Comment: No analysis plan available in the trial register. Furthermore, the trial register mentions that the outcomes will be assessed in the 12 months time frame, but not mentioned how and when they were assessed. Although 12 months analyses show no differences, the additional time points that were reported (not prespecified) are in favour of experimental. | High risk of bias | Predicted direction: Favours experimental. |
| Johnson‐Warrington 2016 | Low risk of bias | "Participants were randomized to receive usual care or SPACE for COPD via a web‐based, concealed allocation program (www.sealedenvelope.com) using simple random permuted block 1:1 randomization by VJ‐W. Randomization was performed after the participants completed the baseline assessment, with treatment allocation prior to hospital discharge." p. 1162. | Some concerns |
Effect of assignment:
"Analysis was conducted on an intention‐to‐treat basis." p. 1163. Effect of adhering: Patients and personnel were not blinded. No information regarding adherence reported. |
High risk of bias | Some patients were missing: n= 78 were randomized (n= 39 to each arm). Included in the analysis: Intervention: n= 35 and usual care: n= 36. | Low risk of bias | "All outcomes were measured at baseline (during admission but as close to discharge as possible) and 3 months after randomization, by a clinician blinded to treatment allocation." p. 1163. | Some concerns | No protocol including a pre‐specified analysis plan was available. | High risk of bias | Unpredictable direction of the outcome. |
| Rose 2018 | Low risk of bias | "Randomisation was performed according to a centralised, computer generated 1:1 randomisation schedule stratified by study site. Because of the nature of the intervention and co‐location of research staff within the respiratory clinics, healthcare providers, patients and outcome assessors were not blinded, though treating respirologists were not informed of study allocation." p. 3. Comment: Some differences between intervention and control group were reported in home‐oxygen therapy (around 6% between groups). |
High risk of bias |
Effect of assignment:
Comment: Due to the nature of the intervention it was not possible to blind patients and personnel. Treating respirologists were not informed of study allocation. Effect of adhering: "Of the 221 participants with case manager contact for all 12 weekly calls, 29% were 100% compliant, 62% 50–99% compliant, and 22% <50% compliant i.e., completed call activities. Of the 203 participants with case manager contact for all nine monthly calls, 31% were 100% compliant, 47% 50–99% compliant, and 22% <50% compliant. Most (65%) participants did not refuse any calls; 20% refused two, 5% refused three, and 1% refused ⩾4 calls. Most common reason for refusal was being too busy (69% of refused calls), other reasons were too fatigued or ill (20%), or not interested in talking when called (6%)." p. 5. |
Low risk of bias | Comment: Only a lot of missing data for questionnaire data. Even a lot of baseline data are missing. | Low risk of bias | Comment: Intention‐to‐treat analysis was used. | Low risk of bias | No signs for selective outcome reporting. | High risk of bias | Unpredictable direction of the outcome. |
| Bringsvor 2018 | Low risk of bias | “The allocation ratio was 1:1. Included participants were given a running number (based on the alphabetical order of last names), and then the numbers were randomized by a statistician (using MATLAB) with no access to the participant list.” p. 3678. “(...) an attrition of 20% was expected, this indicates that at a minimum of six participants were to be allocated to each group. In municipalities with fewer than 12 registered participants, it was therefore not possible to establish two groups, and we decided to allocate one group to either intervention or control. (...) participants from municipalities with only one group were randomized to either intervention or control at the municipality level.” p. 3678‐3679. |
High risk of bias |
Effect of assignment:
“All models were estimated using intention‐to‐treat (ITT) analysis and per‐protocol analysis (PPA). The ITT analysis was conducted for all available data, while inclusion in the PPA required participants to follow the protocol sufficiently; ie, we included in the PPA all participants who had participated in more than half of the group conversations.” p. 3680.
Comment: Patients and personnel were not blinded. Effect of adhering: “The attrition rate was 22.2% in the control group and 31.5% in the intervention group (ITT).” p. 3681. "37 patients did not receive allocated intervention (participated <6 sessions).” p. 3681. Comment: Not reported whether there were used analyses with regard to the effect of adhering to intervention. |
High risk of bias | “One study limitation is that the follow‐up measurements were limited because of a clerical error, where 22 intervention group participants did not receive follow‐up questionnaires (Figure 1) after leaving the groups. This introduced unnecessary missing data in the ITT analysis. Unfortunately, we lack sufficient information to determine whether this affected the results, although there were no significant differences between these 22 and other participants at baseline on any of the self‐management‐related domains.” p. 3685. | Low risk of bias | “Data were blinded during the analyses, ie, the groups were labeled with non‐identifying terms.”p. 3680. Comment: Not reported whether outcome assessors were aware of the intervention during the assessments. |
Some concerns | Comment: In the Trial register the following pre‐defined primary outcomes were reported: Change in Health promoting competence, HeiQ (Time Frame: Before, after, and 3,6 and 12 months after comlepting the intervention (self‐management program)). However, measurements were only performed at baseline and after 12 months follow‐up. No detailed protocol was available. | High risk of bias | Unpredictable direction of the outcome. |
| Jolly 2018 | High risk of bias | "When a patient is identified as eligible for the study, and has given written, informed consent to take part, the researcher randomises the patient using a web‐based programme hosted by the Primary Care Clinical Trials Unit (PC‐CTU), University of Birmingham. Centre specific randomisation lists were produced by a statistician at the trials unit. Participants are individually randomised, stratified by centre. The four recruitment centres are Birmingham and West‐Midlands South; Greater Manchester; North West Midlands and Oxfordshire/Gloucestershire. Only the PC‐CTU have access to the allocation sequence." Sidhu 2015 (protocol paper), p. 4. "The study groups were generally well balanced in terms of patient characteristics, although there was a higher proportion of current smokers in the telephone health coaching group. The usual care group reported a higher level of self reported moderate and vigorous physical activity, but this was not reflected in the accelerometry data at baseline." Jolly 2018 (results paper), p. 5. |
High risk of bias |
Effect of assignment:
Comment: Patients were informed of their allocation at the end of the recruitment appointment; they were not masked to treatment allocation. All data were analysed by intention to treat. Effect of adhering: "We achieved good fidelity of delivery of the intervention, with 75% of intervention participants receiving all four calls and only four patients receiving none." p. 11. "The dose and coverage of intervention delivery were high: 86.4% (999/1156) of the scheduled calls were delivered and 75.4% (218/289) of participants received all four calls." No information regarding pulmonary rehabilitation, oxygen use. |
High risk of bias | "There was imbalance in the follow‐up rates between telephone health coaching (82.7%; 37 withdrawals) and usual care (96.2%; 7 withdrawals)." p. 4. "Participants who did not provide data at 12 months were more likely to be in GOLD stage 3, to be smokers, had lower levels of self‐reported physical activity, and to live alone than responders. " p. 5. |
Some concerns | "Patients were informed of their allocation at the end of the recruitment appointment; they were not masked to treatment allocation. Data were entered into the study database by researchers at the University of Birmingham who were masked to the treatment allocation." p. 3. Comment: No information about outcome assessors. |
Low risk of bias | ‐ | High risk of bias | Unpredictable direction of the outcome. |
| Kessler 2018 | Low risk of bias | "Patients were allocated to groups in a 1:1 fashion according to a pre‐specified randomisation list generated before the study by a partial‐minimisation computer algorithm under supervision of the study sponsor. Patients were assigned a randomisation number by study staff at each centre in sequential numerical order through a telephone‐based interactive voice response system. Randomisation was stratified by smoking status (current or former), need for respiratory assistance (none, or on LTOT and/or HMV), and centre." p. 3. | High risk of bias |
Effect of assignment:
"For practical reasons, the study was open; neither the patients nor the investigators were blinded to the COPD management strategy." p. 3.
"The primary analysis of the primary outcome was performed in the intention‐to‐treat (ITT) population (all patients who entered the follow‐up period). The number of hospitalisation days fitting the established criteria was normalised on a yearly basis for each patient and compared between both groups using the non‐parametric Wilcoxon rank‐sum test." p. 4.
"The open design of the study, though necessary owing to the extensive differences between the two disease management programmes, may have led to some bias because doctors could have had a tendency to discharge patients in the disease management arm earlier than those in the usual management arm." p. 12. Effect of adhering: Comment: 15 patients (<10%) received <25% self‐management training. |
Low risk of bias | "…leaving 157 (91%) and 162 (94%) patients in the ITT population, 137 (80%) and 128 (74%) of whom completed at least 12 months of follow‐up." p. 5. | Low risk of bias | "Because hospitalisation decisions might have been subject to local practices, social considerations, bed availability, and so on, all hospitalisations were reviewed by an independent, three‐member end‐point validation committee (EVC) to assess the reason and appropriateness of each hospitalisation, determine whether the hospitalisation fulfilled the protocol definition, and determine the extent to which it was to be considered in the efficacy assessments (fully, partially, not at all)." p. 3. | Low risk of bias | "During the study, the protocol was amended to accelerate recruitment and allow study completion (supplementary material)." p. 3. Comment: Inclusion criteria were expanded from COPD patients receiving LTOT to patients with severe COPD, with or without LTOT; the minimum number of patients was reduced from 520 to 306 by retaining the initial assumptions for sample size and removing a multiple comparisons adjustment; the follow‐up period was reduced from 2 years to 1 year; and the contact frequency between DM patients and CMs was increased to improve patient training (either group or phone session, once per month). Prior to these changes, 72 patients on LTOT were enrolled in the study. | High risk of bias | Unpredictable direction of the outcome. |
| Wang 2019 | Low risk of bias | "The allocation sequence was generated and released to the interventionist on a case‐by‐case basis by an independent department that specialised in supplying randomly generated sequences for research. The randomisation sequence was recorded." p. 151. "Participants were randomly assigned to groups after consent was obtained and baseline data were collected." p. 151. "There were no significant differences in demographic and clinical data between the two groups at baseline (Table 1)." p. 151. |
High risk of bias |
Effect of assignment:
Comment: It is not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analysis was used. Effect of adhering: "154 participants were enrolled, among whom, 143 completed the 12‐month study program. The attrition rate was 7.14%." p. 151. "...adherence to the self‐management intervention was reported by the participants but not monitored by the nurses." p. 155. Comment: No information on reasons of adherence by patients reported in the current publication. |
Low risk of bias | Comment: Relatively low attrition rate. | Low risk of bias | "To minimize researcher bias, the interventionists who provided care were blinded to the participants’ baseline and allocation sequence. The statistician was blinded to the participants’ results during the trial." p. 151. Comment: Not possible to blind the treatment for patients and people delivering the treatment. |
Some concerns | Comment: No pre‐specified analysis plan published which can be checked. However they mention: The statistician was blinded to the participants’ results during the trial. | High risk of bias | Unpredictable direction of the outcome. |
| Ferrone 2019 | Some concerns | "After obtaining informed consent, subjects were randomly assigned by opaque sealed envelopes in blocks of four, stratified by site, in a ratio of 1:1 to IDM or usual care. The randomization sequence was generated by our biostatistician. Study personnel were blinded to the sequence and blocking factor." p. 8. "In the IDM group, there were numerically more women and more GOLD D patients. The usual care arm had a numerically lower baseline CAT score (better QoL) and more smokers." p. 2. |
High risk of bias |
Effect of assignment:
"Data were analyzed using a complete case analysis approach." p. 8.
Comment: Patients and personnel were not blinded. No intention‐to‐treat analysis was uses. 12 patients that withdrew directly after randomisation have not been included in the analyses. Effect of adhering: Comment: No information regarding adherence reported. |
Low risk of bias | "A constrained longitudinal data analysis was conducted on the CAT score as a sensitivity analysis to evaluate the impact of missing data from patients lost to follow up." p. 7. "When, as a sensitivity analysis, a constrained longitudinal model was used to analyze CAT scores, the between‐group difference was found to be significant (p < 0.001)." p. 2. Comment: 87% of the randomised patients were analysed. However, 12 patients did not show up after randomisation. | Low risk of bias | ‐ | Low risk of bias | No signs for selective outcome reporting; however, no protocol available. | High risk of bias | Unpredictable direction of the outcome. |
| Lenferink 2019 | Low risk of bias | Comment: Allocation was stratified on potential confounders. Only a baseline difference in 6MWD was reported. | High risk of bias |
Effect of assignment:
Comment: It was not possible to blind the treatment for patients and people delivering the treatment. Intention‐to‐treat analyses were performed. Effect of adhering: "The 12‐month follow‐up was completed by 83.3% of the individuals in the self‐management group and 84.8% in the UC group (figure 1). Patients completed 81.3% (self‐management: 82.3%; UC: 80.2%) of the diary data." p. 6. Comment: The effect of adherence to the intervention was not analysed. However, it was observed that a high number of patients in completed follow‐up and their symptom diaries. Adherence to action plans was measured, but not yet reported in the current publication. |
Low risk of bias | Comment: Relatively low number of drop‐outs. The imputated data analyses showed similar results as the observed data analyses. | Low risk of bias | "Wherever possible, though, assessors of outcomes were blinded to the treatment group." p. 3. | Low risk of bias | Comment: Some small changes in final analyses compared to analysis plan that was published with the study protocol. | High risk of bias |
Additional bias:
"Based on the exacerbation rates/patient/day from the COPE‐II study (intervention: 0.116; control: 0.176, both with an estimated standard deviation of 0.17) and allowing for overdispersion and an attrition rate of 10%, 105 patients per group provided 80% power to detect an effect of this size, with a two‐tailed p<0.05." p. 4.
Comment: The study included less patients (intervention: n= 102; usual care: n= 99) than aimed by power calculation (both groups n= 105). Unpredicted direction of the outcome. |
| Liang 2019 | High risk of bias | "Group or solo GP clinics with ≥1000 patients on their databases were approached directly or with assistance from primary care organisations. After obtaining signed agreement, clinics were block randomised (block sizes of four and six) to usual care or intervention arms using an externally managed web‐based randomisation programme. Clinics were notified of their allocation." p. 3. | High risk of bias |
Effect of assignment:
‐ Effect of adhering: "67 participants (43%) did not receive any part of the intervention." p. 7. "A key limitation is the low uptake of the intervention components." p. 10. |
High risk of bias | Comment: Data were not available for all patients randomised. | Low risk of bias | "The effective analysis was according to an intention‐to‐treat principle." p. 4. | Low risk of bias | Results were analysed according to the published design paper. | High risk of bias | Predicted direction: Favours comparator, because general practices could exclude patients from selection to participate in the intervention due to the cluster randomisation approach. Adherence to the intervention was low. |
Acknowledgements
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways. We thank the Cochrane Editorial Team, consisting of Rebecca Fortescue (Co‐ordinating Editor), Liz Stovold (Information Specialist), Emma Dennett (Managing Editor), and Emma Jackson (Assistant Managing Editor), who commented critically on the review. We would also like to thank Lucy Goldsmith and Rebecca Fortescue for their critical comments and helpful discussions regarding statistical issues.
The authors and Cochrane Airways’ Editorial Team are grateful to peer reviewers Ana Isabel Gonzalez‐Gonzalez (Spain) and Karen Royals (Australia) for their time and comments on this review.
We would like to thank the following study authors for their assistance: R Andújar‐Espinosa (Sanchez‐Nieto 2016), R Benzo (Benzo 2016), E Bischoff (Bischoff 2012), J Bourbeau (Bourbeau 2003; Kessler 2018), D Bösch (Bösch 2007), H Bringsvor (Bringsvor 2018), C Bucknall and A McConnachie (Bucknall 2012), H Cameron‐Tucker (Cameron‐Tucker 2016), S Cheng (Cheng 2017), A Collinsworth (Collinsworth 2018), D Coultas (Coultas 2005), C Emery (Emery 1998), V Fan (Fan 2012), A Hussey and C Licskai (Ferrone 2019), J Garcia‐Aymerich (Garcia‐Aymerich 2007), J George (Liang 2019), R Goldstein (Hill 2010), C Hernández (Hernández 2015), K Heslop‐Marshall (Heslop‐Marshall 2015), V Johnson‐Warrington (Johnson‐Warrington 2016), K Jolly (Jolly 2018), H Jonsdottir (Jonsdottir 2015), A Lenferink (Lenferink 2019), F Ko (Ko 2016), K Mitchell (Mitchell 2014), I Orts (Folch‐Ayora 2019), K Rayce (withdrawn study, no reference), K Rice (Rice 2010), L Rose (Rose 2018), J Stevens (Bohingamu 2018), M Tabak (Tabak 2014), R Taylor (Martin 2004), S Taylor (Taylor 2012b), E Titova (Titova 2015), J Walters (Walters 2013), and A Zakrisson (Zakrisson 2019).
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health and Social Care.
Appendices
Appendix 1. Search strategies
| Source | Search strategy |
|
Airways Register (CRS) (Date of most recent search: 23 January 2020) |
1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All 2 MeSH DESCRIPTOR Bronchitis, Chronic 3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*) 4 COPD:MISC1 5 (COPD OR COAD OR COBD):TI,AB,KW 6 #1 OR #2 OR #3 OR #4 OR #5 7 MeSH DESCRIPTOR Self Care Explode All 8 MeSH DESCRIPTOR Education 9 MeSH DESCRIPTOR Patient Education as Topic 10 educat* 11 self‐manag* 12 self manag* 13 self‐car* or "self car*" 14 train* or instruct* 15 patient cent* or patient‐cent* 16 patient‐focus* or "patient focus*" 17 patient‐education or "patient education" 18 management plan or management‐plan 19 management* NEAR1 program* 20 behavior* or behaviour* 21 disease* NEAR2 management* 22 self‐efficac* 23 empower* 24 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 25 #6 and #24 |
|
CENTRAL (CRS) (Date of most recent search: 23 January 2020) |
1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All 2 MeSH DESCRIPTOR Bronchitis, Chronic 3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*) 4 COPD:MISC1 5 (COPD OR COAD OR COBD):TI,AB,KW 6 #1 OR #2 OR #3 OR #4 OR #5 7 MeSH DESCRIPTOR Self Care Explode All 8 MeSH DESCRIPTOR Education 9 MeSH DESCRIPTOR Patient Education as Topic 10 educat* 11 self‐manag* 12 self manag* 13 self‐car* or "self car*" 14 train* or instruct* 15 patient cent* or patient‐cent* 16 patient‐focus* or "patient focus*" 17 patient‐education or "patient education" 18 management plan or management‐plan 19 management* NEAR1 program* 20 behavior* or behaviour* 21 disease* NEAR2 management* 22 self‐efficac* 23 empower* 24 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 25 #6 and #24 |
|
MEDLINE (Ovid) (Date of most recent search: 23 January 2020) |
1. exp Pulmonary Disease, Chronic Obstructive/ 2. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).tw. 3. (COPD or AECOPD or AECB).tw. 4. or/1‐3 5. Self Care/ 6. Self‐Management/ 7. Education/ 8. Patient Education as Topic/ 9. educat$.ti,ab. 10. (self‐manag$ or self manage$).tw. 11. (self‐car$ or self car$).tw. 12. (patient cent$ or patient‐cent$).tw. 13. (patient‐focus$ or patient focus$).tw. 14. (management adj2 (plan$ or program$)).tw. 15. (behavior$ or behaviour$).tw. 16. (disease* adj2 management$).tw. 17. (self‐efficac$ or self efficac$).tw. 18. empower$.tw. 19. or/5‐18 20. 4 and 19 21. (controlled clinical trial or randomized controlled trial).pt. 22. (randomized or randomised).ab,ti. 23. placebo.ab,ti. 24. randomly.ab,ti. 25. trial.ab,ti. 26. groups.ab,ti. 27. or/21‐26 28. Animals/ 29. Humans/ 30. 28 not (28 and 29) 31. 27 not 30 32. 20 and 31 33. limit 32 to yr="2011 ‐Current" |
|
EMBASE (Ovid) (Date of most recent search: 23 January 2020) |
1. chronic obstructive lung disease/ 2. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).tw. 3. (COPD or AECOPD or AECB).tw. 4. or/1‐3 5. exp self care/ 6. education/ 7. patient education/ 8. educat$.ti,ab. 9. (self‐manag$ or self manage$).tw. 10. (self‐car$ or self car$).tw. 11. (patient cent$ or patient‐cent$).tw. 12. (patient‐focus$ or patient focus$).tw. 13. (management adj2 (plan$ or program$)).tw. 14. (behavior$ or behaviour$).tw. 15. (disease* adj2 management$).tw. 16. (self‐efficac$ or self efficac$).tw. 17. empower$.tw. 18. or/5‐17 19. 4 and 18 20. Randomized Controlled Trial/ 21. randomization/ 22. controlled clinical trial/ 23. Double Blind Procedure/ 24. Single Blind Procedure/ 25. Crossover Procedure/ 26. (clinica$ adj3 trial$).tw. 27. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 28. exp Placebo/ 29. placebo$.ti,ab. 30. random$.ti,ab. 31. ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 32. (crossover$ or cross‐over$).ti,ab. 33. or/20‐32 34. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 35. human/ or normal human/ or human cell/ 36. 34 and 35 37. 34 not 36 38. 33 not 37 39. 19 and 38 40. limit 39 to yr="2011 ‐Current" |
|
Clinicaltrial.gov (Date of most recent search: 23 January 2020) |
Study type: Interventional Condition: copd Intervention: self‐management |
|
WHO trials registry (Date of most recent search: 23 January 2020) |
Condition: copd Intervention: self‐management |
Data and analyses
Comparison 1. Self‐management versus usual care (primary outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Health‐related quality of life (HRQoL): adjusted SGRQ total score (primary analysis) | 14 | 2778 | Mean Difference (IV, Random, 95% CI) | ‐2.86 [‐4.87, ‐0.85] |
| 1.2 Health‐related quality of life (HRQoL): adjusted SGRQ total score (secondary analysis) | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 HRQoL: adjusted SGRQ total short term follow‐up (≤ 6 months) | 7 | 1461 | Mean Difference (IV, Random, 95% CI) | ‐2.36 [‐4.92, 0.20] |
| 1.2.2 HRQoL: adjusted SGRQ total medium term follow‐up (>6‐≤12 months) | 13 | 2651 | Mean Difference (IV, Random, 95% CI) | ‐2.65 [‐4.78, ‐0.52] |
| 1.3 Health‐related quality of life (HRQoL): CRQ domain scores (primary analysis) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 HRQoL: CRQ ‐ dyspnoea | 5 | 738 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.10, 0.35] |
| 1.3.2 HRQoL: CRQ ‐ mastery | 5 | 738 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.09, 0.33] |
| 1.3.3 HRQoL: CRQ ‐ fatigue | 5 | 738 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.01, 0.47] |
| 1.3.4 HRQoL: CRQ ‐ emotional function | 5 | 738 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.06, 0.46] |
| 1.4 Health‐related quality of life (HRQoL): CRQ domain scores (secondary analyses) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 HRQoL: CRQ ‐ dyspnoea (≤ 6 months) | 3 | 386 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.15, 0.61] |
| 1.4.2 HRQoL: CRQ ‐ mastery (≤6 months) | 3 | 386 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.33, 0.57] |
| 1.4.3 HRQoL: CRQ ‐ fatigue (≤6 months) | 3 | 386 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.55, 0.77] |
| 1.4.4 HRQoL: CRQ ‐ emotional function (≤6 months) | 3 | 386 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.37, 0.82] |
| 1.4.5 HRQoL: CRQ ‐ dyspnoea (>6‐≤12 months) | 3 | 557 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.09, 0.34] |
| 1.4.6 HRQoL: CRQ ‐ mastery (>6‐≤12 months) | 3 | 557 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.07, 0.41] |
| 1.4.7 HRQoL: CRQ ‐ fatigue (>6‐≤12 months) | 3 | 557 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.15, 0.59] |
| 1.4.8 HRQoL: CRQ ‐ emotional function (>6‐≤12 months) | 3 | 557 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.27, 0.51] |
| 1.5 Healthcare utilisation: respiratory‐related hospital admissions (number of participants with at least one admission) (primary analysis) | 15 | 3263 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.57, 0.98] |
| 1.6 Healthcare utilisation: respiratory‐related hospital admissions (number of participants with at least one admission) (secondary analyses) | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 Healthcare utilisation: respiratory‐related hospital admissions ≤ 6 months (number of participants with at least one admission) | 4 | 437 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.45, 1.55] |
| 1.6.2 Healthcare utilisation: respiratory‐related hospital admissions > 6 to ≤ 12 months (number of participants with at least one admission) | 11 | 2826 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.53, 1.03] |
| 1.7 Healthcare utilisation: respiratory‐related hospital admissions (mean number per participant) (primary analysis) | 7 | 1572 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.60, 0.01] |
| 1.8 Healthcare utilisation: respiratory‐related hospital admissions (mean number per participant) (secondary analyses) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.8.1 Healthcare utilisation: respiratory‐related hospital admissions ≤ 6 months (mean number per participant) | 3 | 709 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 1.8.2 Healthcare utilisation: respiratory‐related hospital admissions >6 ‐ ≤12 months (mean number per participant) | 6 | 1548 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.68, 0.01] |
| 1.9 Mortality: respiratory‐related mortality (primary analysis) | 8 | 1572 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 1.10 Mortality: respiratory‐related mortality (secondary analyses) | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 1.10.1 Respiratory‐related ≤ 6 months mortality | 4 | 309 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 1.10.2 Respiratory‐related > 6 to ≤ 12 months mortality | 6 | 1322 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 1.11 Mortality: all‐cause mortality (primary analysis) | 24 | 5719 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 1.12 Mortality: all‐cause mortality (secondary analyses) | 24 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 1.12.1 All‐cause ≤ 6 months mortality | 9 | 1678 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 1.12.2 All‐cause > 6 to ≤ 12 months mortality | 21 | 5240 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
Comparison 2. Self‐management versus usual care (secondary outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Healthcare utilisation: all‐cause hospital admissions (number of participants with at least one admission) | 10 | 2633 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.71, 1.08] |
| 2.2 Healthcare utilisation: all‐cause hospital admissions (mean number per participant) | 7 | 1914 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 2.3 Healthcare utilisation: respiratory‐related hospitalisation days (per participant) | 4 | 819 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐2.27, 1.03] |
| 2.4 Healthcare utilisation: all‐cause hospitalisation days (per participant) | 6 | 2073 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.85, 0.84] |
| 2.5 Healthcare utilisation: emergency department visits (number of participants with at least one visit) | 5 | 865 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.32, 0.87] |
| 2.6 Healthcare utilisation: emergency department visits (mean number per participant) | 6 | 1939 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.89, ‐0.15] |
| 2.7 Healthcare utilisation: GP visits (mean number per participant) | 4 | 1113 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.68, 0.25] |
| 2.8 COPD exacerbations (mean number per participant) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.8.1 COPD exacerbations (regardless of definition) | 7 | 1401 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.26, 0.15] |
| 2.8.2 COPD exacerbations (symptom based) | 4 | 1047 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.22, 0.31] |
| 2.9 Courses of oral steriods (number of participants who used at least one course) | 3 | 881 | Odds Ratio (M‐H, Random, 95% CI) | 4.19 [0.35, 50.65] |
| 2.10 Courses of antibiotics (number of participants who used at least one course) | 3 | 1012 | Odds Ratio (M‐H, Random, 95% CI) | 3.95 [1.37, 11.43] |
| 2.11 Health status: modified Medical Research Council Dyspnoea Scale (mMRC) | 3 | 356 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐1.23, 0.60] |
| 2.12 Health status: Hospital Anxiety and Depression Scale (HADS) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.12.1 Health status: Hospital Anxiety and Depression Scale (HADS) ‐ anxiety | 9 | 1647 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.01, ‐0.13] |
| 2.12.2 Health status: Hospital Anxiety and Depression Scale (HADS) ‐ depression | 9 | 1653 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.80, ‐0.10] |
| 2.13 Exercise capacity: six‐minute walk test (6MWT) | 6 | 772 | Mean Difference (IV, Random, 95% CI) | 45.14 [9.16, 81.13] |
Comparison 3. Subgroup analyses: self‐management versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by smoking cessation component) | 15 | 3263 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.57, 0.98] |
| 3.1.1 Smoking cessation component | 6 | 1662 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.68, 1.79] |
| 3.1.2 No smoking cessation component | 9 | 1601 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.44, 0.82] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Benzo 2016.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: hospital (inpatient) Assessed for eligibility: 969 Randomly assigned: SM: 108; UC: 107 Completed: SM: 92; UC: 106 Mean age: SM: 67.9 (SD 9.8) years; UC: 68.1 (SD 9.2) years Gender (% male): SM: 43; UC: 48 COPD diagnosis: GOLD criteria, confirmed with spirometry (FEV1/FVC ratio < 0.7) Inclusion of participants in acute phase: yes, during hospitalisation Major inclusion criteria: admission for a COPD exacerbation Major exclusion criteria: medical conditions that would impair their ability to participate in the study or to provide informed consent; receiving hospice care |
| Interventions |
Mode: individual sessions at hospital outpatient clinics, telephone calls, educational booklet Duration: two face‐to‐face individual sessions (first visit 120 min, second visit not reported) and 6 phone calls (mean duration 28.6 min (SD 10.0) Professional: (respiratory) nurse, respiratory therapist Assignment of case manager: yes, accessible to participant during the complete follow‐up period Self‐management components: self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, home based exercise or physical activity component, coping with breathlessness Self‐management topics: (maintenance) medication Behavioural change techniques: 5 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, repetition and substitution |
| Outcomes | 1. Rate of COPD hospitalisation 2. Quality of life 3. Physical activity 4. Number of COPD exacerbations, based on emergency department visits, nurse triage, or urgent care clinics |
| Notes | Source of funding: supported by NHLBI grant R01 HL09468 (RB, principal investigator) from the National Institutes of Health Conflict of interest: none declared |
Bischoff 2012.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 24 months Control group: usual care |
| Participants |
Recruitment: general practice Assessed for eligibility: 748 Randomly assigned: SM: 55; UC: 55 Completed: SM: 49; UC: 44 Mean age: SM: 65.5 (SD 11.5) years; UC: 63.5 (SD 10.3) years Gender (% male): SM: 67; UC: 51 COPD diagnosis: GOLD criteria, confirmed with spirometry (FEV1/FVC ratio < 0.7) Inclusion of participants in the acute phase: not reported Major inclusion criteria: aged at least 35 years, post‐bronchodilator ratio of FEV1/FVC < 0.70 Major exclusion criteria: post‐bronchodilator FEV1 < 30% predicted, treatment by a respiratory physician, severe comorbid conditions with a reduced life expectancy, inability to communicate in the Dutch language, and objections to one or more of the modes of disease management used in the study |
| Interventions |
Mode: individual sessions at the general practice, paper modules "Living well with COPD", telephone calls Duration: 2 to 4 individual face‐to‐face sessions of one hour each, scheduled over 4 to 6 consecutive weeks; 6 telephone calls to reinforce self‐management skills Professional: practice nurse of each participating practice Assignment of case managers: yes, accessible to participants during the complete follow‐up period Self‐management components: smoking cessation, self‐recognition of COPD exacerbations, COPD exacerbation action plan, exercise / physical activity component (optional), diet, medication, coping with breathlessness, managing anxiety and stress Self‐management topics: keeping a healthy and fulfilling lifestyle Behavioural change techniques: 2 clusters: goals and planning, feedback and monitoring |
| Outcomes | 1. Change from baseline in health‐related quality of life (CRQ) 2. Change in CRQ domain scores 3. Exacerbation frequency and management, based on symptoms 4. Total and five domain scores for self‐efficacy (CSES) |
| Notes | A third group of participants (n = 55) were assigned to routine monitoring through scheduled periodic monitoring visits as an adjunct to usual care. However, this group did not include an action plan. Source of funding: this study was funded by the Netherlands Organisation for Health Research and Development (ZonMw) and Partners in Care Solutions for COPD (PICASSO). Conflict of interest: no authors received any support from any company for the submitted work; no authors have any relationship with any company that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. |
Bösch 2007.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: outpatient clinic Assessed for eligibility: not reported Randomly assigned: SM: 38; UC: 12 Completed: SM: 30; UC: 11 Mean age: SM: 63.8 (SD 8.4) years; UC: 64.6 (SD 6.8) years Gender (% male): 63% of 41 participants who completed the study; the distribution of males per group is not reported COPD diagnosis: GOLD criteria (FEV1/FVC ratio < 0.7), confirmed by authors Inclusion of participants in the acute phase: not reported Major inclusion criteria: diagnosis of COPD with obstruction proven by spirometry and a FEV1/FVC < 70% Major exclusion criteria: comorbidities which significantly influence symptoms, capacity or spirometry (symptomatic cardiopulmonary disease) |
| Interventions |
Mode: group sessions (six to eight participants) at the participants' homes Duration: four face‐to‐face group sessions of two hours each with the final session scheduled six weeks later Professional: respiratory nurse under supervision of a respiratory specialist Assignment of case managers: yes, accessible to participants during the complete follow‐up period Self‐management components: smoking cessation (optional), self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, home‐based exercise or physical activity component, diet, COPD medication intake (i.e. adherence, inhalation technique), coping with breathlessnes, leisure activities and travelling Self‐management topics: not reported Behavioural change techniques: 2 clusters: goals and planning, feedback and monitoring |
| Outcomes | 1. mMRC 2. Courses of antibiotics 3. FEV1 (L) 4. Hospital admissions 5. 6MWT 6. COPD exacerbations, based on treatment with antibiotics |
| Notes | Sources of funding: not reported Conflict of interest: none declared |
Bourbeau 2003.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 and 24 months Control group: usual care |
| Participants |
Recruitment: hospital (outpatient) Assessed for eligibility: not reported Randomly assigned: SM: 96; UC: 95 Completed: SM: 86; UC: 79 Mean age: SM: 69.4 (SD 6.5) years; UC: 69.6 (SD 7.4) years Gender (% male): SM: 52; UC: 59 COPD diagnosis: FEV₁ after the use of a bronchodilator between 25% and 70% of the predicted normal value and FEV₁/FVC ratio less than 70% Inclusion of participants in the acute phase: no Major inclusion criteria: hospitalised at least once in the preceding year for an exacerbation; stable COPD (respiratory symptoms and medication unchanged for at least 4 weeks before enrolment); at least 50 years of age; current or previous smoker (at least 10 pack‐years); FEV₁ after the use of a bronchodilator between 25% and 70% of the predicted normal value 14 and FEV₁/FVC ratio < 70%; no previous diagnosis of asthma, left congestive heart failure, terminal disease, dementia, or uncontrolled psychiatric illness; no participation in a respiratory rehabilitation programme in the past year; and no long‐term‐care facility stays Major exclusion criteria: participants with asthma as a primary diagnosis and those with major comorbidities (documented left ventricular failure and any terminal disease), dementia or uncontrolled psychiatric illness |
| Interventions |
Mode: individual sessions at the participant's home, "Living well with COPD" programme with patient workbook, telephone calls Duration: seven face‐to‐face individual sessions of one hour each, scheduled in seven to eight consecutive weeks, 18 telephone calls (weekly calls for the eight weeks' educational period; after eight weeks, monthly phone calls for 12 months) Professional: experienced health professionals (nurses, respiratory therapists, a physiotherapist) who acted as case managers with the supervision and collaboration of the treating physician Assignment of case managers: "The programme was supervised by experienced and trained health professionals..." (Bourbeau 2006, p. 586) “Half‐day training sessions were dedicated to interactive lecturing sessions on each aspect of COPD given by different members of the multidisciplinary team. The rest of the training days included workshops oriented toward how to assess patient needs and the acquisition of motivational and teaching skills using group discussion, demonstration and practice of techniques, case scenarios, and role modeling" (Bourbeau 2006, p. 1705). The case‐manager was accessible to participants during the complete follow‐up period. Self‐management components: self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, home‐based exercise or physical activity component (optional), diet, COPD medication intake (i.e. adherence, inhalation technique), coping with breathlessness, leisure activities and travelling, energy conservation during day‐by‐day activities, relaxation exercises, adopting a healthy lifestyle, long‐term oxygen (optional) Self‐management topics: smoking cessation, exercise Behavioural change techniques: 7 clusters: goals and planning, feedback and monitoring, shaping knowledge, comparison of behaviour, associations, repetition and substitution, antecedents |
| Outcomes | 1. Hospital admissions 2. Scheduled and unscheduled physician visits 3. Emergency department visits 4. Health‐related quality of life (SGRQ) 5. Pulmonary function 6. Functional exercise capacity 7. COPD exacerbations, based on symptoms |
| Notes | Completed first year of follow‐up: N = 165 (based on hospital registry database) Completed second year of follow‐up: N = 175 (based on provincial health insurance and hospitalisation database records) Source of funding: this study was funded by an unrestricted grant from Boehringer Ingelheim Canada, Burlington, Ontario, in partnership with the Fonds de la Recherche en Santé du Québec (FRSQ), Montreal, Quebec. Conflict of interest: none declared |
Bringsvor 2018.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 3 months Control group: usual care |
| Participants |
Recruitment: hospital (outpatient) Assessed for eligibility: 2309 Randomly assigned: SM: 92; UC: 90 Completed: SM: 55; UC: 70 Mean age: SM: 68.5 (SD 8.16) years; UC: 69.3 (SD 9.02) years Gender (% male): SM: 59; UC: 63 COPD diagnosis: GOLD criteria, confirmed with spirometry (FEV1/FVC ratio <0.7) Inclusion of participants in acute phase: no Major inclusion criteria: registered ICD‐10 code J44.0, 1, 8, or 9 after 1 January 2010; age ≥ 18 years; confirmed COPD grade II to IV, according to the GOLD; and the ability to read and speak Norwegian Major exclusion criteria: substantial cognitive impairment reported in a medical journal (e.g. severe dementia, severe Alzheimer’s disease), substantial alcohol or drug abuse, or both, or a life expectancy < 12 months due to comorbidity |
| Interventions |
Mode: group sessions at meeting locations in the participants' home municipalities Duration: 11 face‐to‐face group sessions (120 min) scheduled weekly Professional: (respiratory) nurse, physiotherapist (co‐moderator) Assignment of case managers: yes, accessible to participants during the complete follow‐up period Self‐management components: smoking cessation (optional), self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, home‐based exercise or physical activity component, diet, COPD medication intake (i.e. adherence, inhalation technique), coping with breathlessness, psychological issues, information about the healthcare system, including local, regional and national “offers” for persons with COPD Self‐management topics: not reported Behavioural change techniques: 2 clusters: goals and planning, feedback and monitoring |
| Outcomes | 1. Lung function 2. Dyspnoea (mMRC) 3. CAT 4. Self‐management (HeiQ version 2) 5. GSE 6. SOC‐13 |
| Notes | Source of funding: this work was supported by the Western Norway Regional Health Authority [grant number 2013/911836] and the Norwegian Extra Foundation for Health and Rehabilitation [grant number 2015/RB13639] Conflict of interest: none declared |
Bucknall 2012.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: hospital (inpatient) Assessed for eligibility: 1405 Randomly assigned: SM: 232; UC: 232 Completed: SM: 211; UC: 200 Mean age: SM: 70.0 (SD 9.3) years; UC: 68.3 (SD 9.2) years Gender (% male): SM: 38; UC: 35 COPD diagnosis: chronic irreversible airflow limitation with FEV₁ < 70% predicted and a FEV₁ /FVC ratio of < 70% Inclusion of participants in the acute phase: not reported Major inclusion criteria: admitted to hospital with an acute exacerbation of COPD Major exclusion criteria: a history of asthma or left ventricular failure, evidence of active malignant disease or any evidence of confusion/poor memory, assessed with the abbreviated mental test (scores of 9/10 or 10/10 required) |
| Interventions |
Mode: individual sessions at the participant's home, adapted "Living well with COPD" booklets, telephone calls Duration: four face‐to‐face individual sessions of 40 minutes each, scheduled fortnightly, over a two‐month period. There were also 828 phone calls to the intervention group participants (mean 4.6 phone calls per intervention participant). There were at least 6 subsequent home visits (but more frequently on request) thereafter for a total of 12 months Professional: study nurse Assignment of case managers: "Study nurses’ training was based on self regulation theory" (Bucknall 2012, p. 2). "Nurses were trained to deliver a structured self‐management programme in four fortnightly home visits (…). Nurses without previous respiratory training completed three half day training sessions" (p. 3). Case managers were accessible to participants during the complete follow‐up period. Self‐management components: smoking cessation, self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, diet (optional), COPD medication intake (i.e. adherence, inhalation technique), coping with breathlessness Self‐management topics: exercise Behavioural change techniques: 8 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, repetition and substitution, self‐belief |
| Outcomes | 1. Time to first acute hospital admission with a COPD exacerbation 2. Death due to COPD within 12 months of randomisation 3. Morbidity (change from baseline at 6 and 12 months in SGRQ) 4. Likelihood of anxiety or depression (HADS) 5. Sense of self‐efficacy (CSES) 6. Quality of life (EuroQol 5D) |
| Notes | Self‐management materials based on the "Living Well with COPD" programme and previously adapted for the UK population and healthcare setting by an iterative process, were used (p. 2). Extra information from author: "We used adapted “Living with COPD” booklets and daily diary cards (Stockley et al. – originally developed for use in Bronchiecistasis, piloted these and adapted them for this study, to include a line for recording steroid and antibiotic usage." Source of funding: in addition to funding from the Chief Scientist Office, Scottish Health Department (CZH/4/246), this study was supported by educational grants from Boehringer Ingelheim, GlaxoSmithKline, and Astra Zeneca. Conflict of interest: in addition to the Chief Scientist Office grant (CZH/4/246), CEB’s institution received financial support for the employment of a research fellow from Boehringer Ingelheim, GlaxoSmithKline, and Astra Zeneca, and JC holds other grants; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. |
Coultas 2005.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 6 months Intervention 1: nurse‐assisted medical management (MM) Intervention 2: nurse‐assisted collaborative management (CM) Control group: usual care |
| Participants |
Recruitment: primary care clinics Assessed for eligibility: 217 Randomly assigned: MM: 72; CM: 72; UC: 73 Completed: MM: 49; CM: 51; UC: 51 Mean age: MM: 68.3 (SD 6.6) years; CM: 70.1 (SD 7.0) years; UC 68.8 (SD 10.4) years Gender (% male): MM: 42.9%; CM: 32.7%; UC: 53.8% COPD diagnosis: COPD‐related diagnosis code (International Classification of Diseases, Ninth Revision: codes 491, 492, 496), FEV1 < 80%; FEV1/FVC < 70%, confirmed by authors Inclusion of participants in acute phase: no Major inclusion criteria: current or former smoker with at least a 20‐pack‐year smoking history, at least one respiratory symptom (e.g. cough, shortness of breath or wheeze), airflow obstruction (i.e. FEV1/FVC ratio, 70%; and FEV1, 80% predicted) during the past 12 months Major exclusion criteria: not reported |
| Interventions |
Mode: MM: enhance participant knowledge. CM: enhance participant knowledge and facilitating the adoption of healthy behaviour including lifestyle and self‐management skills Duration: 1 face‐to‐face individual session (mean 64 min ± 23.1), mean 6.0 ± 1.8 telephone calls (10.0 min ± 5.4) Professional: nurse Assignment of case manager: no Self‐management components: smoking cessation (optional), self‐recognition of COPD exacerbations (optional), use of a COPD exacerbation action plan (optional), COPD medication intake (i.e. adherence, inhalation technique) (optional) Self‐management topics: coping with breathlessness, review of symptoms and medications, education about COPD symptoms and medications Behavioural change techniques: MM: 2 clusters: goals and planning, feedback and monitoring. CM: 3 clusters: goals and planning, feedback and monitoring, social support |
| Outcomes | 1. Health status 2. SGRQ 3. SF‐36 4. Perceived illness intrusiveness 5. Doctor visits 6. ER visits 7. Hospital admissions |
| Notes |
Note 1: baseline characteristics are given only for the group of participants who completed the six‐month follow‐up period.
Note 2: dropout percentages are high: MM: 32.0%; UC: 30.1%.
Note 3: participants who dropped out of the study had more severe airflow obstruction, higher levels of distress and lower quality of life compared with participants who completed the study.
Note 4: content of the interventions is not described properly, whereas the training of the nurses providing the intervention was described in detail.
Note 5: outcome measures of self‐efficacy and social support and BSI‐18 and CES‐D scores were measured but not reported in the article. Source of funding: a grant from Robert Wood Johnson Foundation Conflict of interest: not reported |
Emery 1998.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 2.5 months Control group: usual care |
| Participants |
Recruitment: announcements, word of mouth, advertisements in weekly newspapers for older adults and physician referral Eligible: 92 Randomly assigned: SM: 25; UC: 25 Completed: SM: 23; UC: 25 Mean age: SM: 67.4 (SD 5.9) years; UC: 67.4 (SD 7.1) years Gender (% male): SM: 40; UC: 48 COPD diagnosis: airflow obstruction demonstrated on spirometry (i.e. the FEV1/FVC) Inclusion of participants in acute phase: yes, during hospitalisation Major inclusion criteria: stable COPD; > 50 years; FEV1/VC < 70; > six months of clinical symptoms of COPD Major exclusion criteria: significant cardiac disease; other diseases affecting exercise tolerance or learning skills last three months; asthma without fixed obstruction |
| Interventions |
Mode: group education sessions Duration: 26 face‐to‐face group sessions (16 lectures of 60 min and 10 management sessions of 60 min) Professional: clinical psychologist Assignment of case managers: not reported Self‐management components: self‐recognition of COPD exacerbations, COPD medication intake (i.e. adherence, inhalation technique), coping with breathlessness, relaxation exercises, coping skills training Self‐management topics: not reported Behavioural change techniques: 3 clusters: goals and planning, feedback and monitoring, regulation and substitution |
| Outcomes | 1. Health status 2. SIP 3. HRQoL‐MHLC 4. Health knowledge test 5. FEV₁ % predicted |
| Notes | We disregarded the third arm because it was focused on pulmonary rehabilitation. Source of funding: this work was supported by grants from the National Heart, Lung and Blood Institute (HL45290) and the National Institute on Aging (AG00029). Conflict of interest: not reported |
Fan 2012.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 months Control group: guideline‐based usual care |
| Participants |
Recruitment: outpatient clinic Assessed for eligibility: 467 Randomly assigned: SM: 209; UC: 217 Completed: SM: 193; UC: 203 Mean age: SM: 66.2 (SD 8.4) years; UC: 65.8 (SD 8.2) years Gender (% male): SM: 97.6; UC: 96.3 COPD diagnosis: GOLD, a post‐bronchodilator ratio of FEV₁/FVC < 0.70 with an FEV₁ < 80% predicted. At baseline and 1‐year study visits, post‐bronchodilator spirometry performed according to ATS criteria. Inclusion of participants in the acute phase: no Major inclusion criteria: hospitalised for COPD in the 12 months before enrolment, post‐bronchodilator ratio of FEV₁ to FVC < 0.70 with an FEV₁ < 80% predicted, older than 40 years, current or past history of cigarette smoking (> 10 pack‐years), at least 1 visit in the past year to either a primary care or pulmonary clinic at a Veterans Affairs medical centre, no COPD exacerbation in the past 4 weeks, ability to speak English, and access to a telephone Major exclusion criteria: primary diagnosis of asthma or any medical conditions that would impair ability to participate in the study or to provide informed consent |
| Interventions |
Mode: individual and group sessions at hospital outpatient clinics, telephone calls, educational booklet Duration: four face‐to‐face individual sessions of 90 minutes each, scheduled weekly. The individual lessons were reinforced during a group session and by six phone calls, one per month for three months and every three months thereafter. Professional: case manager (various health‐related professionals). Assignment of case managers: before starting the study, all case managers received a three‐day training course with workshops covering detailed aspects of the self‐management programme, and all were supervised by the site investigator. Case managers were accessible to participants during the complete follow‐up period. Self‐management components: self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, COPD medication intake (i.e. adherence, inhalation technique) Self‐management topics: not reported Behavioural change techniques: 9 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, associations, repetition and substitution, regulation, antecedents |
| Outcomes | 1. Time from randomisation to first COPD hospitalisation 2. All‐cause mortality 3. Number of COPD exacerbations, based on symptoms 4. Health‐related quality of life 5. Patient satisfaction 6. Medication adherence 7. COPD‐related knowledge, skill acquisition and self‐efficacy |
| Notes | This multi site RCT of an educational and acute care management programme was stopped early when a safety monitoring board noted excess mortality in the intervention group. The mean follow‐up time was 250 days. Source of funding: Veterans Affairs Cooperative Study Program Conflict of interest: none declared |
Ferrone 2019.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: general practice Assessed for eligibility: 1186 Randomly assigned: SM: 84; UC: 84 Completed: SM: 72; UC: 74 Mean age: SM: 68.6 (SD 9.6) years; UC: 67.9 (SD 9.8) years Gender (% male): SM: 40.5; UC: 52.4 COPD diagnosis: GOLD criteria, post‐bronchodilator FEV1 of ≤ 70% after four puffs of salbutamol and FEV1/FVC ratio < 0.7 Inclusion of participants in acute phase: no Major inclusion criteria: ≥ 40 years of age, current or ex‐smokers with a minimum 10 pack‐year smoking history, a post‐bronchodilator FEV1 of ≤ 70% after four puffs of salbutamol and FEV1/FVC ratio < 0.7, a history of at least 2 exacerbations in the past 3 years or 1 exacerbation in the past year Major exclusion criteria: COPD exacerbation in the past 4 weeks, diagnosis of asthma prior to the age of 40 years, use of long‐term supplemental oxygen, comorbid illness that would interfere with study participation, scheduled for COPD rehabilitation, terminal illness |
| Interventions |
Mode: individual sessions at general practice, phone calls Duration: 2 face‐to‐face individual sessions (first visit 60 min (baseline evaluation) and 5 to7 min (encounter with physician) and second visit of 45 min after 3 months) and either a phone call or face‐to‐face visit at 6 and 9 months (15 to 30 min each) Professional: respiratory specialist, CRE Assignment of case managers: yes, accessible to participants during the complete follow‐up period Self‐management components: smoking cessation (optional), self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, home‐based exercise or physical activity component, COPD medication intake (i.e. adherence, inhalation technique), coping with breathlessness Self‐management topics: diet, energy conservation, advanced care/end‐of‐life planning, travel planning, COPD pathophysiology Behavioural change techniques: 6 clusters: goals and planning, feedback and monitoring, shaping knowledge, natural consequences, associations, regulation |
| Outcomes | 1. COPD‐related quality of life (CAT and CCQ) 2. Knowledge (Bristol Knowledge Questionnaire) 3. Predicted FEV1 and FEV1/FVC ratio 4. COPD exacerbations (required prednisolone or antibiotics, or both) 5. COPD‐related health service utilisation (including unscheduled physician and ED visits, and hospitalisation) |
| Notes | Source of funding: this study was funded by Asthma Research Group Windsor Essex Inc. through unrestricted project grants by GlaxoSmithKline and Pfizer Canada Ltd. Conflict of interest: MF and ZR reported grants from Pfizer and GlaxoSmithKline during the conduct of the study; outside the current work. CJL reported grants and personal fees from AstraZeneca, Boehringer Ingelheim, and Novartis; grants from Pfizer and Bayer; and personal fees from GlaxoSmithKline, outside the current work. The remaining authors declare no competing interests. |
Gallefoss 1999.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: hospital (outpatient) Assessed for eligibility: not reported Randomly assigned: SM: 31; UC: 31 Completed: SM: 26; UC: 27 Mean age: SM: 57 (SD 9) years; UC: 58 (SD 10) years Gender (% male): SM: 48; UC: 52 COPD diagnosis: FEV₁ equal to or higher than 40% and lower than 80% of predicted Inclusion of participants in the acute phase: not reported Major inclusion criteria: participants with COPD, < 70 years of age, a FEV₁ equal to or higher than 40% and lower than 80% of predicted Major exclusion criteria: not suffering from any serious disease such as unstable coronary heart disease, heart failure, serious hypertension, diabetes mellitus, kidney or liver failure |
| Interventions |
Mode: individual and group sessions at an outpatient clinic Duration: 1 or 2 face‐to‐face individual sessions with a nurse and 1 or 2 face‐to‐face individual sessions with a physiotherapist of 40 minutes each. Two 2‐hour group education sessions (five to eight persons) were scheduled on two separate days. Professional: nurse, physiotherapist, pharmacist, medical doctor Assignment of case managers: specially trained nurse, accessible to participants during the complete follow‐up period Self‐management components: smoking cessation (optional), self‐recognition of COPD exacerbations, COPD medication intake (i.e. adherence, inhalation technique), coping with breathlessness Self‐management topics: exercise, diet Behavioural change techniques: 6 clusters: goals and planning, feedback and monitoring, shaping knowledge, natural consequences, associations, regulation |
| Outcomes | 1. Health‐related quality of life (SGRQ and four simple questions) 2. Hospital admissions 3. Days lost from work 4. GP consultation 5. FEV₁ % predicted |
| Notes | Source of funding: Norwegian Medical Associations Fund for Quality Improvement Conflict of interest: not reported |
Hernández 2015.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 (and 72 months passive follow‐up thereafter) Control group: usual care |
| Participants |
Recruitment: hospital (outpatient) Assessed for eligibility: 860 Randomly assigned: SM: 71; UC: 84 Completed: SM: 54; UC: 55 Mean age: SM: 73 (SD 8) years; UC: 75 (SD 9) years Gender (% male): SM: 83; UC: 86 COPD diagnosis: a person not involved in the study identified the cases with COPD (ICD9‐CM 491, 492, 493 or 496) as the primary diagnosis for admission. However, lung function testing was also assessed before randomisation. COPD confirmed with spirometry (FEV1/FVC < 70%). Inclusion of participants in the acute phase: no Major inclusion criteria: clinically stable COPD participants with a history of at least two hospital admissions owing to severe respiratory exacerbations during two consecutive years. "We considered a broad spectrum of COPD diagnostic terms that include chronic obstructive inflammatory diseases; namely, emphysema, asthma, tuberculosis, chronic bronchitis and COPD, aged above 45 years and living at home within the healthcare area of the hospital (Barcelona‐Esquerra)" (p. 2). Major exclusion criteria: nursing home or not living in the area, participants in another randomised controlled trial, died prior to contact |
| Interventions |
Mode: individual and group sessions at an outpatient clinic and at participants' homes Duration: at least one face‐to‐face individual session of 40 minutes at the participant's home within 72 hours after entry into the study by the primary care team (participants without mobility problems), four face‐to‐face individual sessions of 15 minutes education each at the participant's home by the primary care team (participants with mobility problems), one two‐hour individual or group educational programme of 40 minutes. Three group sessions for participants without mobility problems (two comprehensive assessments of 90 minutes each at the outpatient clinic and one 2‐hour educational programme) and for participants with mobility problems, the programme was done at home. In all visits, the nurses dedicated 15 minutes for education. Professional: specialised respiratory nurse, primary care team (physician, nurse and social worker) Assignment of case managers: the community care teams received training: a 2‐hour face‐to‐face educational training and 1‐day stay at the hospital ward, aiming at enhancing home‐based management of frail COPD participants. Case managers were accessible to participants during the complete follow‐up period. Self‐management components: smoking cessation (optional), self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, home‐based exercise or physical activity component, diet, COPD medication intake (i.e. adherence, inhalation technique), coping with breathlessness, comorbid condition (no further explanation regarding content) Self‐management topics: vaccination Behavioural change techniques: 7 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, comparison of behaviour, associations, antecedents |
| Outcomes | 1. Mental status 2. Activities of daily living (Lawton index) 3. Anxiety and depression (HADS) 4. Health‐related quality of life (SGRQ) 5. Sleepiness (Epworth sleepiness scale) 6. 6MWT 7. Nocturnal pulse oximetry and body mass distribution 8. Exacerbations |
| Notes | Source of funding: this study was funded by NEXES (Supporting Healthier and Independent Living for Chronic Patients and Elderly). Conflict of interest: none declared |
Johnson‐Warrington 2016.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 3 months Control group: usual care |
| Participants |
Recruitment: hospital (inpatient) Assessed for eligibility: 464 Randomly assigned: SM: 38; UC: 39 Completed: SM: 35; UC: 36 Mean age: SM: 67.6 (SD 8.5) years; UC: 68.3 (SD 7.7) years Gender (% male): SM: 38.4; UC: 33.3 COPD diagnosis: COPD confirmed with spirometry (FEV1/FVC ratio <0.7) Inclusion of patients in acute phase: yes, during hospitalisation Major inclusion criteria: established diagnosis of COPD and grade 2–5 dyspnoea according to the Medical Research Council Major exclusion criteria: reason for admission was not an acute exacerbation of COPD, unable to safely participate in unsupervised exercise (i.e. due to psychiatric, locomotive, cardiac or neurological impairments), involved in other research, unable to read English, had previously received SPACE (Self‐management Program of Activity Coping and Education) for COPD or completed pulmonary rehabilitation within the previous 6 months, had four or more admissions in the previous 12 months |
| Interventions |
Mode: individual session at the hospital, written educational information Duration: 1 face‐to‐face individual session (30 to 45 min) and 6 phone calls (5 to 20 min each) Professional: physiotherapist Assignment of case managers: yes, accessible to participants during the complete follow‐up period Self‐management components: smoking cessation (optional), self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, home‐based exercise, COPD medication intake, coping with breathlessness Self‐management topics: diet, correct device use Behavioural change techniques: 11 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, associations, repetition and substitution, regulation, antecedents, identity |
| Outcomes | 1. Respiratory‐related hospital readmission at 3 months 2. Quality of life (CRQ‐SR) 3. Anxiety and depression (HADS) 4. Bristol COPD Knowledge Questionnaire 5. ISWT 6. ESWT 7. Pulmonary Rehabilitation Adapted Index of Self‐Efficacy 8. Ready for home survey |
| Notes | Source of funding: SJS and KR were supported by the Collaboration for Leadership in Applied Health Research and Care, East and West Midlands, respectively, and the NIHR Leicester Respiratory Biomedical Research Unit (BRU). Conflict of interest: none declared |
Jolly 2018.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: general practice Assessed for eligibility: 1146 Randomly assigned: SM: 289; UC: 288 Completed: SM: 247; UC: 281 Mean age: SM: 70.7 (SD 8.8) years; UC: 70.2 (SD 7.8) years Gender (% male): SM: 63; UC: 64 COPD diagnosis: according to UK guidelines (FEV1/FVC ratio <0.7), confirmed by authors Inclusion of participants in acute phase: no Major inclusion criteria: on the practice COPD register, mild dyspnoea (MRC grades 1 (only breathless on strenuous exercise) or 2 (only get short of breath when hurrying on level ground or up a slight hill)), FEV1/FVC < 0.7 after post‐bronchodilator spirometry, aged 18 years or over Major exclusion criteria: level of dyspnoea of MRC grade 3 or greater, terminal disease or severe psychiatric disorder (confirmed by their GP) |
| Interventions |
Mode: individually tailored written supportive materials (i.e. information leaflet, standard written information), followed by telephone calls Duration: 4 individual phone calls (first call 35 to 60 min, other calls 15 to 20 min) scheduled at 3, 7 and 11 weeks Professional: nurse Assignment of case managers: yes, accessible to participants during the complete follow‐up period Self‐management components: smoking cessation (optional), self‐recognition of COPD exacerbations (optional), use of a COPD exacerbation action plan (optional), physical activity, COPD medication intake (i.e. adherence, inhalation technique) Self‐management topics: coping with breathlessness Behavioural change techniques: 8 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of outcomes, regulation, antecedents |
| Outcomes | 1. SGRQ‐C 2. MRC dyspnoea scale 3. Self‐reported physical activity 4. Psychological morbidity 5. Self‐efficacy (Stanford self‐efficacy scale) 6. Health state utility (EuroQoL 5 Dimensions 5 Levels) |
| Notes | ‐ |
Jonsdottir 2015.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: 291 Assessed for eligibility: 291 Randomly assigned: SM: 60; UC: 59 Completed: SM: 52; UC: 48 Mean age: SM: 59.4 (SD 4.7) years; UC: 58.7 (SD 4.4) years Gender (% male): SM: 39.6; UC: 51.9 COPD diagnosis: GOLD criteria (FEV1/FVC ratio < 0.7), confirmed by authors Inclusion of participants in acute phase: no Major inclusion criteria: aged 45 to 65 with mild and moderate COPD (grade II and III) as the primary disease Major exclusion criteria: another major disease (among them, individuals with asthma who had more than 200 mL or 12% increase in FEV1 after inhalation of 200 µg albuterol in the postbronchodilator spirometry), non‐Icelandic speaking, not capable of travelling to the treatment site, participated in a structured rehabilitation programme for people with COPD 6 months prior to the screening |
| Interventions |
Mode: group and individual sessions at a clinical research centre located on a university‐hospital campus, followed by telephone calls Duration: 1 face‐to‐face group session (120 min), 3 to 4 face‐to‐face individual sessions (30 to 45 min), and 4 phone calls (5 to 10 min each) Professional: (respiratory) nurse, peer led, research team Assignment of case managers: no Self‐management components: smoking cessation (optional), self‐recognition of COPD exacerbations, physical exercise, diet, COPD medication intake (i.e. adherence, inhalation technique), coping with breathlessness, utilisation of health care, prevention of further decline of disease with the aim of enhancing health of participant and family, and coping with feelings of shame and guilt Self‐management topics: skills in managing treatment and consequences in daily life, knowledge about and skills in maintaining safe environment (pollution, cold/hot weather, smoke‐free environment, infections), skills in communication with family, relatives and health professionals Behavioural change techniques: 10 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, repetition and substitution, regulation, identity, self‐belief |
| Outcomes | 1. SGRQ‐C 2. IIRS 3. IPAQ short version 4. COPD exacerbations (self‐reported), measured by the question: ‘How often during the previous 6 months have you had a serious exacerbation of the lungs?’ |
| Notes | Source of funding: this research was funded by the Icelandic Research Fund, University of Iceland’s Research Fund, Landspitali‐University Hospital’s Research Fund, Icelandic Nurses’ Association’s Research Fund, and the Oddur Olafsson Fund. Conflict of interest: none declared |
Kessler 2018.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: hospital (outpatient) Assessed for eligibility: not reported Randomly assigned: SM: 172; UC: 173 Completed: SM: 137; UC: 128 Mean age: SM: 67.3 (SD 8.9) years; UC: 66.6 (SD 9.6) years Gender (% male): SM: 69.4; UC: 69.8 COPD diagnosis: GOLD criteria, confirmed with spirometry (FEV1/FVC ratio < 0.7) Inclusion of participants in acute phase: not reported Major inclusion criteria: COPD patients (a post‐bronchodilator FEV1/FVC ratio ≤ 70%; an FEV1 < 50% of the predicted value), aged ≥ 35 years, a ≥ 10 pack‐year smoking history, at least one severe exacerbation in the previous year, could receive all relevant COPD treatments including long‐term oxygen therapy and home mechanical ventilation Major exclusion criteria: not expected to survive longer than 6 months, unable to read or speak the country language or had cognitive/psychiatric disease, on continuous treatment of > 10 mg per day prednisone or equivalent for more than 6 weeks, living in a nursing home |
| Interventions |
Mode: 'Living Well with COPD programme', group and individual sessions, phone calls Duration: 1 face‐to‐face group session (90 to 120 min), 4 face‐to‐face individual sessions (60 to 90 min), and multiple phone calls (duration not specified) Professional: respiratory specialist, case manager Assignment of case managers: yes, accessible to participants during the complete follow‐up period Self‐management components: smoking cessation (optional), self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, exercise programme (optional), diet, COPD medication intake, coping with breathlessness Self‐management topics: not reported Behavioural change techniques: 3 clusters: goals and planning, feedback and monitoring, shaping knowledge |
| Outcomes | 1. Unplanned all‐cause hospitalisation days 2. Number of COPD exacerbations, based on symptoms 3. 6MWD 4. BODE index 5. Anxiety and depression (HADS) 6. Health status (SGRQ‐C) 7. Safety (adverse events, serious adverse events and deaths) |
| Notes | Source of funding: Air Liquide Healthcare. Conflict of interest: JB, IDZ, PC, DK, ST, JLV, RWDN, and RK were investigators in the COMET trial and have received honoraria from Air Liquide Healthcare, sponsors of the COMET trial. DG was an employee of Air Liquide Healthcare at the time when the study was conducted. SR is a director of HEVA HEOR, which received consulting fees from Air Liquide Healthcare to perform a health economic analysis. The authors report no other conflicts of interest in this work. |
Lenferink 2019.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: hospital (outpatient) Assessed for eligibility: 1586 Randomly assigned: SM: 102; UC: 99 Completed: SM: 85; UC: 84 Mean age: SM: 68.8 (SD 9.0) years; UC: 68.2 (SD 8.9) years Gender (% male): SM: 64.7; UC: 63.6 COPD diagnosis: GOLD criteria, confirmed with spirometry (FEV1/FVC ratio < 0.7) Inclusion of participants in acute phase: no. Major inclusion criteria: a clinical diagnosis of COPD according to the GOLD criteria (FEV1 80% of the predicted value and FEV1/FVC < 0.70); 1 or more diagnostic comorbidities (ischaemic heart disease, history of myocardial infarction, angina pectoris, heart failure (defined according to the ESC guidelines), diabetes (steroid‐induced or stable diabetes type 1 or 2)); active symptoms of anxiety or depression, or both (using a cut‐off score of ≥ 11 from the HADS and/or having symptoms that are currently being treated); 3 or more COPD exacerbations, defined as respiratory problems that required a course of oral corticosteroids/antibiotics in the two years preceding study entry; and/or 1 or more hospitalisations for respiratory problems in the two years preceding study entry; ≥ 40 years of age; stable at the time of inclusion (at least 4 weeks post‐exacerbation, 6 weeks post‐hospitalisation or post‐rehabilitation); able to understand and read English or Dutch Major exclusion criteria: terminal cancer, end stage of COPD or another serious disease with low survival rate (expected survival < 12 months), other serious lung disease (e.g. α1‐antitrypsin deficiency; interstitial lung diseases), people with cognitive impairment (MMSE < 24), people who are currently enrolled in other randomised controlled trials or intensive case management programmes |
| Interventions |
Mode: group and individual sessions at the hospital, written symptom diary and action plan, telephone calls Duration: 2 to 3 face‐to‐face group sessions (120 to 240 min), 2 face‐to‐face individual sessions (60 min), and 3 phone calls (10 to 15 min each) Professional: respiratory nurse and cardiac, mental health and/or diabetes nurses Assignment of case managers: yes, accessible to participants during the complete follow‐up period. Self‐management components: self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, COPD medication intake (i.e. adherence, inhalation technique), coping with breathlessness, self‐recognition of increase in comorbid symptoms and use of an action plan for these comorbidities (CHF, IHD, anxiety and depression) Self‐management topics: exercise, diet, knowledge regarding COPD and comorbidities, immunisations, physical fitness and relaxation exercises Behavioural change techniques: 5 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, repetition and substitution |
| Outcomes | 1. COPD exacerbation days, based on symptoms 2. Number of COPD exacerbations, based on symptoms, per participant 3. Duration of COPD exacerbations, based on symptoms, per participant per year |
| Notes | Source of funding: this study was supported by the Lung Foundation Netherlands (grant number 3.4.11.061), Lung Foundation Australia (Australian Lung Foundation Boehringer Ingelheim COPD Research Fellowship 2010), Repat Foundation, GlaxoSmithKline (unrestricted grant) and Stichting Astma Bestrijding. Funding information for this article has been deposited with the Crossref Funder Registry. Conflict of interest: A Lenferink reports grants from Stichting Astmabestrijding and GlaxoSmithKline (unrestricted grant), during the conduct of the study. J van der Palen reports grants from Netherlands Lung Foundation, during the conduct of the study. PDLPM van der Valk has nothing to disclose. P Cafarella has nothing to disclose. A van Veen has nothing to disclose. S Quinn has nothing to disclose. CGM Groothuis‐Oudshoorn has nothing to disclose. MG Burt has nothing to disclose. M Young has nothing to disclose. PA Frith has nothing to disclose. TW Effing reports grants from The Repat Foundation, Australian Lung Foundation and Dutch Asthma Foundation, during the conduct of the study. |
Liang 2019.
| Study characteristics | |
| Methods | Design: CRT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: general practice Assessed for eligibility: 1050 Randomly assigned: SM: 157; UC: 115 Completed: SM: 138; UC: 100 Mean age: SM: 66.6 (SD 10.8) years; UC: 61.7 (SD 10.1) years Gender (% male): SM: 60.5; UC: 62.6 COPD diagnosis: GOLD criteria, confirmed with spirometry (FEV1/FVC ratio < 0.7) Inclusion of participants in acute phase: no Major inclusion criteria: ≥ 40 years old, ≥ 2 clinic visits during the previous year, self‐reported being a current/ex‐smoker (≥ 10 pack‐year smoking history), documented diagnosis of COPD on clinic records or were being treated with COPD‐specific medications Major exclusion criteria: terminal illness (anticipated survival < 12 months), unable to provide informed consent (e.g. cognitive impairment), pre‐existing interstitial lung disease, unstable cardiovascular status, comorbidities preventing participation in an exercise training programme, contraindications to spirometry, completed pulmonary rehabilitation in the previous 24 months |
| Interventions |
Mode: individual sessions at the general practice; phone calls Duration: 3 face‐to‐face individual sessions (duration not specified), and 9 phone calls (duration not specified) Professional: physiotherapist, research assistants, pharmacist Assignment of case managers: no Self‐management components: smoking cessation (optional), home based exercise, COPD medication intake (i.e. adherence, inhalation technique) Self‐management topics: not reported Behavioural change techniques: 5 clusters: goals and planning, feedback and monitoring, social support, natural consequences, reward and threat |
| Outcomes | 1. HRQoL (SGRQ) 2. CAT 3. Dyspnoea (mMRC) 4. Lung function (FEV1 % predicted) 5. Anxiety and depression (HADS) 6. HSI 7. Smoking abstinence |
| Notes | Source of funding: this study was supported by Boehringer Ingelheim, Eastern Melbourne Primary Health Network, Lung Foundation Australia and National Health and Medical Research Council. Conflict of interest: MJ Abramson reports grants from Boehringer Ingelheim, during the conduct of the study; grants from Pfizer, assistance with conference attendance and personal fees for consultancy from Sanofi, outside the submitted work. G Russell has nothing to disclose. AE Holland is a current member of the Lung Foundation Australia COPD‐X: Concise Guide for Primary Care Advisory Committee. NA Zwar is a current member of the Lung Foundation Australia COPD Guidelines Committee. B Bonevski has nothing to disclose. A Mahal has nothing to disclose. P Eustace has nothing to disclose. E Paul has nothing to disclose. K Phillips is the Lung Foundation Australia General Manager of Consumer Programs. The Lung Foundation Australia works in collaboration and receives funding from pharmaceutical companies outlined in the foundation’s annual reports (available at lungfoundation.com.au/about‐us/annual‐reports/). NS Cox has nothing to disclose. S Wilson has nothing to disclose. J George reports grants from Boehringer Ingelheim, during the conduct of the study; grants from Pfizer, and personal fees for consultancy from GlaxoSmithKline, outside the submitted work; and is a current member of the Lung Foundation Australia COPD Guidelines Committee. J Liang has nothing to disclose. |
Martin 2004.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: general practice Assessed for eligibility: not reported Randomly assigned: SM: 44; UC: 49 Completed: SM: 35; UC: 45 Mean age: SM: 71.1 (95% CI 68.7 to 73.5) years; UC: 69.1 (95% CI 63.5 to 74.7) years Gender (% male): SM: 34.1; UC: 65.3 COPD diagnosis: GOLD criteria (FEV1/FVC ratio < 0.7), confirmed by authors Inclusion of participants in acute phase: no (use of the plan was commenced at a time when each participant was in a stable condition) Major inclusion criteria: diagnosis of COPD, aged 55 or over, at least one hospital admission or two acute exacerbations of COPD requiring GP care during the previous 12 months, an MMSE score > 22 Major exclusion criteria: terminally ill, coexisting lung cancer, admission to hospital with cardiac disease within previous 12 months, receiving home oxygen therapy |
| Interventions |
Mode: individual sessions at a general practice, hospital, ambulance service, emergency department or home‐based Duration: 4 face‐to‐face individual sessions and respiratory nurse visits at 3, 6 and 12 months Professional: respiratory physician, respiratory nurse, GP, ED consultant, medical staff Assignment of case managers: no Self‐management components: use of a COPD exacerbation action plan, COPD medication intake, guidance regarding treatment for coexisting conditions (e.g. when/how to use oxygen therapy, and when to use diuretics) Self‐management topics: smoking cessation, coping with breathlessness/breathing techniques, self‐recognition of exacerbations Behavioural change techniques: 3 clusters: goals and planning, feedback and monitoring, shaping knowledge |
| Outcomes | 1. Health care utilisation (GP visits, hospital admissions, ambulance calls) 2. Quality of life (SGRQ) 3. Medication use (courses of oral steroids and antibiotics) |
| Notes | Three participants subsequently withdrew for personal reasons. However, it was not reported from which group(s) they withdrew. A further 13 people died during the follow‐up period (nine in the intervention group and four in the control group). Source of funding: this study was supported by South Link Health Inc., a nonprofit consortium of general practitioners. Conflict of interest: none declared |
Mitchell 2014.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 6 months Control group: usual care |
| Participants |
Recruitment: general practice Assessed for eligibility: 326 Randomly assigned: SM: 89; UC: 95 Completed: SM: 65; UC: 79 Mean age: SM: 69 (SD 8 years); UC: 69 (SD 10.1) years Gender (% male): SM: 60.7; UC: 49.5 COPD diagnosis: COPD confirmed by spirometry, with a FEV₁/FVC ratio < 0.7 Inclusion of participants in the acute phase: no Major inclusion criteria: have a diagnosis of COPD confirmed by spirometry, with a FEV₁/FVC ratio < 0.7, grade 2‐5 MRC dyspnoea scale, clinically stable for 4 weeks Major exclusion criteria: unable to undertake an exercise regime due to neurological, musculoskeletal or cognitive comorbidities, unable to read English to the reading age of an 8‐year‐old, completed pulmonary rehabilitation within the previous 12 months |
| Interventions |
Mode: individual sessions at a GP's office or home‐based, telephone calls, workbook Duration: one face‐to‐face individual session for 30 to 45 minutes by a physiotherapist and two telephone calls at two and four weeks into the programme to reinforce skills and provide encouragement to progress Professional: physiotherapist, trainee health psychologist Assignment of case managers: yes, but after a second phone call, no access to the case manager Self‐management components: smoking cessation (optional), self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, home‐based exercise, management of psychological consequences (e.g. dealing with anger, depression, disease acceptance) Self‐management topics: diet, (maintenance) medication, coping with breathlessness Behavioural change techniques: 11 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, associations, repetition and substitution, regulation, antecedents, identity |
| Outcomes | 1. Health status (CRQ dyspnoea domain) 2. Fatigue, emotion and mastery domains of the CRQ 3. Disease knowledge (Bristol COPD Knowledge Questionnaire) 4. Anxiety and depression (HADS) 5. Exercise capacity (ISWT, ESWT) 6. Self‐efficacy (Pulmonary Rehabilitation Adapted Index of Self‐Efficacy) 7. Healthcare utilisation (admissions, GP visits, ED visits, nurse home visits) 8. Medication use (courses of antibiotics) 8. Self‐reported smoking status |
| Notes | Source of funding: National Institute for Health Research (NIHR) Conflict of interest: none declared |
Rice 2010.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: hospital (Veterans Affairs medical centres) Assessed for eligibility: 1739 Randomly assigned: SM: 372; UC: 371 Completed: SM: 336; UC: 323 Mean age: SM: 69.1 (SD 9.4) years; UC: 70.7 (SD 9.7) years Gender (% male): SM: 97.6%; UC: 98.4% COPD diagnosis: clinical diagnosis of COPD with post‐bronchodilator spirometry showing a FEV₁ < 70% predicted and a FEV₁/FVC < 0.70 Inclusion of participants in the acute phase: not reported Major inclusion criteria: a diagnosis of COPD at high risk of hospitalisation as predicted by one or more of the following during the previous year: hospital admission or ED visit for COPD, chronic home oxygen use or course of systemic corticosteroids for COPD Major exclusion criteria: inability to have access to a home telephone line or sign a consent form, any condition that would preclude effective participation in the study or likely to reduce life expectancy to less than a year |
| Interventions |
Mode: group sessions at an outpatient clinic, one‐page handout summary and number for help line, telephone calls Duration: one face‐to‐face group session (60 to 90 min) by a respiratory therapist case manager, 12 monthly phone calls (10 to 15 minutes each) Professional: respiratory therapist case manager Assignment of case managers: "case managers were respiratory therapists who had completed a one‐day training session.” Appendix 1, p. 2. The case manager was accessible to participants during the complete follow‐up period. Self‐management components: smoking cessation (optional), self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, COPD medication intake Self‐management topics: exercise, oximetry, recommendation concerning influenza and pneumococcal vaccinations, instruction in hand hygiene Behavioural change techniques: 4 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge |
| Outcomes | 1. Hospital admissions and ED visits for COPD 2. All‐cause hospitalisations and all‐cause ED visits 3. Hospital and intensive care unit lengths of stay 4. Respiratory medication use 5. Change in respiratory quality of life (SGRQ) 6. All‐cause mortality |
| Notes | Source of funding: this study was supported by an unrestricted grant from the Veterans Integrated Service Network 23 Primary Care and Research Services and by the Center for Chronic Disease Outcomes Research, a Veterans Affairs Health Services Research and Development Center of Excellence Conflict of interest: several study authors (i.e. K.L.R., M.C., D.E.N.) reported that they or family members received financial benefits from a commercial entity. The other study authors (i.e. H.E.B., J.G., T.M.S., D.B.N., S.K., M.T., L.J.G., C.B., do not have financial relationships with a commercial entity that has an interest in the subject of this manuscript |
Rose 2018.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: hospital (inpatient) Assessed for eligibility: 8696 (2100 documented COPD diagnosis) Randomly assigned: SM: 237; UC: 238 Completed: SM: 207; UC: 191 Mean age: SM: 71 (SD 9.2) years; UC: 71 (SD 9.7) years Gender (% male): SM: 50; UC: 44 COPD diagnosis: GOLD criteria, confirmed with spirometry (FEV1/FVC ratio < 0.7) Inclusion of participants in acute phase: yes, on emergency department presentation and/or hospital admission for COPD exacerbation, or during attendance at respirology outpatient clinic Major inclusion criteria: COPD diagnosis according to GOLD criteria and published Canadian reference values confirmed by a respirologist or internist, ≥ 50 years of age, 1 or more emergency department visits or hospital admissions for COPD exacerbation in previous 12 months, and ≥ 2 prognostically‐important COPD‐associated comorbidities (as defined by GOLD and Canadian Thoracic Society Guidelines) identified via medical record screening Major exclusion criteria: primary diagnosis of asthma (action plans differ substantially), terminal diagnosis, dementia, uncontrolled psychiatric illness, inability to understand English, no telephone access, inability to attend follow‐up, resident in a long‐term care facility, enrolled in the provincial tele‐home monitoring programme, and no family physician |
| Interventions |
Mode: individual session (standardised education session based on 'Living Well with COPD') at an outpatient clinic; telephone calls Duration: 1 face‐to‐face individual session (40 min), 21 phone calls (duration not specified). Professional: Case manager (nurse practitioner or respiratory therapist, both trained as COPD educators) Assignment of case managers: yes, accessible to participants during the complete follow‐up period Self‐management components: smoking cessation (optional), self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, COPD medication intake (i.e. adherence, inhalation technique), advance care planning Self‐management topics: exercise Behavioural change techniques: 3 clusters: goals and planning, feedback and monitoring, social support |
| Outcomes | 1. Number of ED visits 2. Number of hospital admissions and hospitalised days 3. Mortality 4. Time to first ED presentation 5. BODE index 6. Quality of life (EQ‐5D‐3L, SGRQ) 7. Anxiety and depression (HADS) 8. Self‐efficacy (CSES) 9. Satisfaction (CSQ8) 10. Caregiver impact |
| Notes | Source of funding: this trial was funded through the Building Bridges to Integrate Care (BRIDGES) program led by the University of Toronto’s Departments of Medicine and Family and Community Medicine and funded through the Ministry of Health and Long Term Care. L. Rose holds a CIHR New Investigator Award. Funding information for this article has been deposited with the Crossref Funder Registry. Conflict of interest: none declared |
Sanchez‐Nieto 2016.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: hospital (outpatient) Assessed for eligibility: 250 Randomly assigned: SM: 51; UC: 45 Completed: SM: 47; UC: 38 Mean age: SM: 68.2 (SD 7.2) years; UC: 67.1 (SD 6.8) years Gender (% male): SM: 92.2; UC: 88.9 COPD diagnosis: post‐bronchodilator FEV1/FVC < 70% Inclusion of participants in acute phase: no Major inclusion criteria: clinical stability (at least in the 3 months prior to randomisation, with no change in medication or usual symptoms); active smoker or prior history of smoking of at least 10 pack‐years; post‐bronchodilator FEV1/FVC < 70%; normal cognitive status (assessed by the intersecting pentagons test) to read and understand written texts, and receive training in inhalation techniques or self‐care education sessions; physical status that allows for regular walking or exercise; no diagnoses of asthma, advanced heart failure, unstable ischaemic heart disease, terminal disease, dementia or uncontrolled psychiatric disorders; ability to read texts; no participation in any pulmonary rehabilitation program in the previous year Major exclusion criteria: not reported |
| Interventions |
Mode: group and individual sessions at the hospital; written material with treatment instructions Duration: 1 face‐to‐face group session (40 min), and 3 face‐to‐face individual sessions (20 min each) Professional: respiratory specialist, nurse, physiotherapist Assignment of case managers: yes (telephone assistance to intervention participants), accessible to participants during the complete follow‐up period Self‐management components: self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, home‐based physical exercise, COPD medication intake Self‐management topics: main characteristics of the disease Behavioural change techniques: 3 clusters: goals and planning, feedback and monitoring, shaping knowledge |
| Outcomes | 1. Combined number of hospital admissions, and A&E department visits for COPD exacerbations 2. Hospitalisatons for COPD exacerbations 3. A&E visits for COPD exacerbations 4. Lengths of stay 5. Antibiotic or glucocorticoid treatment 6. All‐cause mortality |
| Notes | Source of funding: Gas Medi SA, Boehringer Ingelheim, Chiesi, Menarini Conflict of interest: none declared |
Tabak 2014.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 9 months Control group: usual care |
| Participants |
Recruitment: hospital, primary care physiotherapy practices Assessed for eligibility: not reported (101 participants eligible) Randomly assigned: SM: 15; UC: 14 Completed: SM: 10; UC: 2 Mean age: SM: 64.1 (SD 9.0) years; UC: 62.8 (SD 7.4) years Gender (% male): SM: 50.0; UC: 50.0 COPD diagnosis: GOLD II‐IV, a clinical diagnosis of COPD according to the GOLD criteria, confirmed with spirometry (FEV1/FVC ratio < 0.7) Inclusion of participants in the acute phase: no Major inclusion criteria: fulfill COPE‐II study (effects of self‐treatment and an exercise programme within a self‐management programme in outpatients with COPD) criteria: no exacerbation in the month prior to enrolment, three or more exacerbations or one hospitalisation for respiratory problems in the 2 years preceding study entry, a computer with Internet access at home Major exclusion criteria: other serious disease with a low survival rate, other diseases influencing bronchial symptoms and/or lung function, severe psychiatric illness, uncontrolled diabetes mellitus or a hospitalisation for diabetes mellitus in the 2 years preceding the study, need for regular oxygen therapy, maintenance therapy with antibiotics, known Alpha‐1 antitrypsin deficiency, disorders or progressive disease seriously influencing walking ability |
| Interventions |
Mode: individual and group sessions at the outpatient clinic, primary care physiotherapy practices and at the participant's home, web‐based teleconsultation module Duration: at least 1 face‐to‐face individual session by the primary care physiotherapist (no protocol for education, offered as blended care, depending on physiotherapist and participant) and a teleconsultation module. For research purposes, there was one intake by a physiotherapist for baseline measure activity coach and explanations. Furthermore, there were additional meetings after 1, 3, 6 and 9 months. Before the start of the programme, participants had to attend 2 group sessions of 90 minutes each by a nurse practitioner. Professional: respiratory nurse practitioner, respiratory physiotherapist Assignment of case managers: no Self‐management components: smoking cessation (optional), self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, home‐based exercise (web‐based) Self‐management topics: diet, (maintenance) medication, coping with breathlessness/breathing techniques Behavioural change techniques: 3 clusters: goals and planning, feedback and monitoring, shaping knowledge |
| Outcomes | 1. Use of application 2. Adherence (online diary, exercise scheme) 3. Satisfaction (Client Satisfaction Questionnaire) 4. Hospitalisations (number and length of stay) 5. Emergency department visits 6. COPD exacerbations, based on symptoms 7. Level of activity (activity coach, accelerometer) 8. Self‐perceived activity levels (Baecke Phsycial Activity Questionnaire) 9. Exercise tolerance (6MWT) 10. Fatigue (Multidimensional Fatigue Inventory 20) 11. Health status (CCQ) 12. Dyspnoea (MRC) 13. Quality of life (EuroQol‐5D) |
| Notes | Source of funding: NL Agency, a division of the Dutch Ministry of Economic Affairs (grant CALLOP9089) Conflict of interest: none declared |
Titova 2015.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 24 months Control group: usual care |
| Participants |
Recruitment: hospital (inpatient) Assessed for eligibility: 199 Randomly assigned: SM: 91; UC: 81 Completed: SM: 51; UC: 49 Mean age: SM: 74.1 (SD 9.26) years; UC: 72.6 (SD 9.33) years Gender (% male): SM: 42.9; UC: 43.2 COPD diagnosis: GOLD criteria (FEV1/FVC ratio <0.7), confirmed by authors Inclusion of participants in the acute phase: yes, during hospitalisation Major inclusion criteria: admission due to AECOPD, COPD (GOLD stage III or IV, 2007), living in the Trondheim municipality, ability to communicate in Norwegian, ability to sign the informed consent form Major exclusion criteria: any serious diseases that might cause a very short lifespan (expected survival time less than six months) |
| Interventions |
Mode: individual sessions at the participant’s home, telephone calls, e‐learning programme, “My COPD book” Duration: six face‐to‐face individual sessions (one at discharge, five joint visits at home at approximately 3 days, 14 days, 6 months, 12 months, and 24 months post‐ discharge) by the specialist nurse, one interactive 15‐minute e‐learning programme, at least 24 telephone calls (routine phone calls at least once a month and during COPD exacerbations) Professional: specialist nurse Assignment of case managers: not reported Self‐management components: self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, coping with breathlessness Self‐management topics: smoking cessation, (maintenance) medication Behavioural change techniques: 4 clusters: goals and planning, feedback and monitoring, social support, repetition and substitution |
| Outcomes | 1. Hospital utilisation (admissions caused by AECOPD, in‐hospital days due to AECOPD) 2. Mortality 3. Inhaled medication use (long‐acting bronchodilators) |
| Notes | Source of funding: Central Norway Regional Health Authority and the Research Council of Norway Conflict of interest: none declared |
Walters 2013.
| Study characteristics | |
| Methods | Design: CRT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: general practice Assessed for eligibility: 1207 Randomly assigned: SM: 90; UC: 92 Completed: SM: 74; UC: 80 Mean age: SM: 68.2 (SD 7.9) years; UC: 67.3 (SD 7.6) years Gender (% male): SM: 54; UC: 51 COPD diagnosis: postbronchodilator FEV1/FVC < 0.7, FEV1 30‐80% Inclusion of participants in acute phase: not reported Major inclusion criteria: smoking history > 10 pack‐years, postbronchodilator FEV1/FVC < 0.7, FEV1 30% to 80%; able to complete procedures and provide informed consent Major exclusion criteria: unable to participate in self‐care activities due to mental or physical incapacity, end‐stage cancer, poor English language skills and nursing home resident |
| Interventions |
Mode: mentor telephone call sessions Duration: 16 individual phone calls (30 min each) Professional: community health nurses Assignment of case managers: yes, accessible to participants during the complete follow‐up period Self‐management components: smoking cessation (optional), self‐recognition of COPD exacerbations, use of a COPD exacerbation action plan, physical activity (optional), diet (optional), COPD medication intake (optional), alcohol (optional), psychosocial (optional) Self‐management topics: not reported Behavioural change techniques: 5 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, self‐belief |
| Outcomes | 1. Quality of life (SF‐36 and SGRQ) 2. Patients' self‐management behaviour and knowledge (PIH scale) 3. Self‐efficacy (SEMCD) 4. Anxiety and depression (HADS, CES‐D and PCL‐C) 5. Well‐being (SWLS) 6. Hospital admissions |
| Notes | Source of funding: this work was supported by the National Health and Medical Research Council (NHMRC) project grant ID490028, a Royal Hobart Hospital Research Foundation grant and a University of Tasmania Institutional Research Grant. Conflict of interest: Lung Foundation Australia/Boehringer Ingelheim chronic obstructive pulmonary disease (COPD) Research Fellowship for JW |
Wang 2019.
| Study characteristics | |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: hospital (inpatient) Assessed for eligibility: 479 Randomly assigned: SM: 77; UC: 77 Completed: SM: 72; UC: 71 Mean age: SM: 68.7 (SD 6.2) years; UC: 69.2 (SD 6.1) years Gender (% male): SM: 76.6; UC: 80.5 COPD diagnosis: GOLD criteria, confirmed with spirometry (FEV1/FVC ratio < 0.7) Inclusion of participants in acute phase: yes, during hospitalisation Major inclusion criteria: aged 40 years or older; diagnosis of Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage II, III or IV; COPD documented by pulmonary function testing; participants hospitalised for acute exacerbation of COPD; willing to sign an informed consent form Major exclusion criteria: severe sensory or cognitive impairment or symptomatic ischaemic heart disease; a coexisting respiratory condition (e.g. asthma or lung cancer); inability to be contacted by phone/mobile phone; participation in another research program or inability to provide informed consent |
| Interventions |
Mode: individual sessions at the hospital and at home; booklet; telephone calls Duration: 5 to 6 face‐to‐face individual sessions (45 min each), 3 home visits (45 to 60 min each), and weekly phone calls scheduled over 3 months (10 to 15 min each) Professional: nurse Assignment of case managers: not reported Self‐management components: smoking cessation (optional), home‐based exercise or physical activity, COPD medication intake (i.e. adherence, inhalation technique), coping with breathlessness, respiratory muscle training (pursed lip breathing and abdominal breathing), coughing techniques, long‐term home oxygen therapy (optional) Self‐management topics: not reported Behavioural change techniques: 5 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequeces |
| Outcomes | 1. COPD‐related hospital admissons 2. Emergency department visits 3. Exercise tolerance (6MWT) 4. Health‐related quality of life (SGRQ) 5. Participant satisfaction (CTCPSQ) |
| Notes | Source of funding: the work described in this paper was supported by a grant from the Education Department of Guizhou Province, China. Conflict of interest: none declared |
AECOPD: acute exacerbations of COPD; ATS: American Thoracic Society; A&E: accident and emergency; BODE: Body‐mass index, airflow Obstruction, Dyspnea, and Exercise; BSI‐18: Brief Symtom Inventory 18; CAT: COPD Assessment Test; CCQ: Clinical COPD Questionnaire; CES‐D: Centers for Epidemiologic Studies – Depression; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; CM: collaborative management; CRE: certified respiratory educator; CRQ: Chronic Respiratory (Disease) Questionnaire; CSES: COPD Self‐Efficacy Scale; CSQ: Client Satisfaction Questionnaire; CTCPSQ: COPD Transitional Care Patient Satisfaction Questionnaire; ED: emergency department; ER: emergency room; ESC: European Society of Cardiology; ESWT: Endurance‐Shuttle Walk Test; EuroQol 5D: European Quality of Life Five Dimension; FEV1/FEV1: forced expiratory volume in one second (measured in litres (L)); FVC: forced vital capacity; GOLD: Global initiative for chronic Obstructive Lung Disease; GSE: General Self‐Efficacy Scale; GP: general practitioner; HADS: Hospital Anxiety and Depression Scale; HeiQ: Health education impact Questionnaire; HRQoL: health‐related quality of life; HSI: Heaviness of Smoking Index; ICD: International Classification of Diseases; IHD: ischaemic heart disease; IIRS: Illness Intrusiveness Rating Scale; IPAQ: International Physical Activity Questionnaire; ISWT: Incremental Shuttle Walking Test; MHLC: Multidimensional Health Locus of Control; min: minute(s); MM: medical management; MMSE: Mini Mental State Examination; (m)MRC: (modified) Medical Research Council; PCL‐C: Post‐traumatic Stress Disorder Checklist; PIH: Partners in Health Scale; RCT: randomised controlled trial; SD: standard deviation; SEMCD: Self‐Efficacy for Managing Chronic Disease; SF‐36: 36‐Item Short Form Health Survey; SGRQ: St. George's Respiratory Questionnaire; SGRQ‐C: St. George's Respiratory Questionnaire ‐ COPD‐Specific Version; SIP: Sickness Impact Profile; SM: self‐management; SOC‐13: Sense of Coherence Scale – 13; SR: self‐reported; SWLS: Satisfaction With Life Scale; UC: usual care; 6MWD: six‐minute walk distance
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aboumatar 2019 | No verification of COPD |
| ACTRN12616001039471 | One intervention component |
| Akinci 2011 | Included in previous review update; non‐RCT / CRT |
| Ali 2018 | No stratification on COPD |
| Altenburg 2015 | No iterative process |
| Anonymous 2012 | No self‐management / home‐based exercise programme |
| Ansari 2017 | Non‐RCT / CRT |
| Apps 2013 | Non‐RCT / CRT |
| Arbillaga‐Etxarri 2018 | One intervention component |
| Barberan‐Garcia 2014 | Non‐RCT / CRT |
| Barnestein‐Fonseca 2011 | Not all participants have had their COPD diagnosis verified (with spirometry) |
| Baron 2011 | No usual care / control group |
| Barradell 2017 | No usual care / control group |
| Barradell 2018 | No usual care / control group |
| Basri 2017 | No self‐management / home‐based exercise programme |
| Bausewein 2012 | No stratification on COPD |
| Bavarsad 2015 | One intervention component |
| Beekman 2014 | No self‐management / home‐based exercise programme |
| Bentley 2014 | One intervention component |
| Benzo 2017 | No verification of COPD |
| Benzo 2019 | No usual care / control group |
| Berkhof 2014 | No self‐management / home‐based exercise programme |
| Berkhof 2015 | No self‐management / home‐based exercise programme |
| Bhadhuri 2019 | Non‐RCT / CRT |
| Bi 2021 | No usual care / control group |
| Billington 2015 | No usual care / control group |
| Bischoff 2011 | Non‐RCT / CRT |
| Blackstock 2016 | No usual care / control group |
| Bohingamu 2018 | No self‐management / home‐based exercise programme |
| Boland 2014 | No iterative process |
| Bosma 2011 | No self‐management / home‐based exercise programme |
| Bove 2016 | One intervention component |
| Bower 2012 | No stratification on COPD |
| Browne 2013 | No usual care / control group |
| Buckingham 2015 | No verification of COPD |
| Bunker 2012 | No verification of COPD |
| Cameron‐Tucker 2011 | Abstract of pilot study; no published results available |
| Cameron‐Tucker 2016 | No usual care / control group |
| Carcereny 2016 | One intervention component |
| Casanas 2019 | No stratification on COPD |
| Casas 2006 | Included in previous review update; not all participants meet COPD spirometry criteria |
| Casey 2012 | No self‐management / home‐based exercise programme |
| Cecere 2012 | No self‐management / home‐based exercise programme |
| Chan 2011 | One intervention component |
| Chan 2016 | No usual care / control group |
| Chang 2019 | No usual care / control group |
| Chatwin 2014 | No usual care / control group; no self‐management / home‐based exercise programme |
| Chavannes 2009 | Included in previous review update; non‐RCT / CRT |
| Chen 2011 | One intervention component |
| Cheng 2017 | One intervention component |
| Christenhusz 2012 | No usual care / control group |
| Chuang 2011 | Included in previous review update; non‐RCT / CRT |
| Collinsworth 2018 | Not all participants have had their COPD diagnosis verified by spirometry |
| Cordova 2016 | One intervention component |
| Coultas 2013 | No usual care / control group |
| Coultas 2014 | No usual care / control group |
| Coultas 2016 | No usual care / control group |
| Coventry 2019 | Non‐RCT / CRT |
| Csikesz 2016 | No abstract or published results available |
| Cully 2012 | No verification of COPD; no stratification on COPD |
| Cully 2017 | No verification of COPD; no stratification on COPD |
| Dabrowska 2017 | One intervention component |
| Davis 2016 | Study was stopped due to participant recruitment issues |
| De Jongh 2013 | No self‐management / home‐based exercise programme |
| Demeyer 2017a | One intervention component |
| Demeyer 2017b | No verification of COPD |
| Deng 2013 | Non‐RCT / CRT |
| De Roos 2018 | No self‐management / home‐based exercise programme |
| De San Miguel 2013 | No self‐management / home‐based exercise programme |
| Dewan 2011b | No stratification on COPD |
| Dimitri 2012 | No usual care / control group |
| Dogan 2017 | Non‐RCT / CRT |
| Donesky 2012 | No usual care / control group |
| Doward 2017 | Non‐RCT / CRT |
| Drennan 2014 | No self‐management / home‐based exercise programme |
| DRKS00006021 | One intervention component |
| Due 2014 | Non‐RCT / CRT |
| Durheim 2014 | No usual care / control group |
| Durheim 2015 | No usual care / control group |
| Dwinger 2013 | No stratification on COPD |
| Effing 2009 | Included in previous review update; no usual care / control group |
| Effing 2011 | Included in previous review update; no usual care / control group |
| Emme 2014 | No self‐management / home‐based exercise programme |
| Etxarri 2017 | One intervention component |
| EUCTR2013‐002671‐18‐AT | No usual care / control group |
| Fairbrother 2011 | Non‐RCT / CRT |
| Fairbrother 2013 | Non‐RCT / CRT |
| Farmer 2014 | No usual care / control group |
| Faulkner 2010 | Included in previous review update; no home‐based exercise component |
| Ferreira 2016 | No self‐management / home‐based exercise programme |
| Fish 2012 | No stratification on COPD |
| Fitzsimmons 2011 | No self‐management / home‐based exercise programme |
| Fitzsimmons 2016 | No self‐management / home‐based exercise programme |
| Flink 2017 | No stratification on COPD |
| Folch‐Ayora 2019 | Not all participants have had their COPD diagnosis verified with spirometry |
| Foot 2017 | No self‐management / home‐based exercise programme |
| Fors 2018 | No iterative process |
| Fortin 2013 | No stratification on COPD |
| Freund 2016 | No verification of COPD; no stratification on COPD |
| Frith 2017a | No usual care / control group |
| Frith 2017b | No usual care / control group |
| Gaeckle 2016 | No self‐management / home‐based exercise programme |
| Garcia‐Aymerich 2007 | No verification of COPD |
| Gellis 2012 | No stratification on COPD |
| Goossens 2014 | No self‐management / home‐based exercise programme |
| Goris 2013 | One intervention component |
| Grabenhorst 2013 | No self‐management / home‐based exercise programme |
| Granados‐Santiago 2019 | No self‐management / home‐based exercise programme |
| Gurgun 2011 | No self‐management / home‐based exercise programme |
| Hæsum 2017 | No iterative process |
| He 2015 | Abstract only; no full‐text article available |
| Heaton 2019 | No iterative process |
| Hegelund 2019 | No iterative process |
| Heslop‐Marshall 2018 | No iterative process |
| Hilberink 2011 | No verification of COPD |
| Ho 2016 | No self‐management / home‐based exercise programme |
| Houben 2014 | No self‐management / home‐based exercise programme |
| Houben 2019 | No self‐management / home‐based exercise programme |
| Howard 2014 | No usual care / control group |
| Huang 2017 | Not all participants have had their COPD diagnosis verified with spirometry |
| ISRCTN30110012 | No usual care / control group |
| ISRCTN32281812 | Study was stopped due to participant recruitment issues |
| ISRCTN77785397 | No usual care / control group |
| Janaudis‐Ferreira 2018 | No self‐management / home‐based exercise programme |
| Jarab 2012 | No iterative process |
| Jennings 2015 | One intervention component |
| Ji 2019 | One intervention component |
| Jokar 2012 | No verification of COPD |
| Jonkers 2012 | No verification of COPD |
| Kalter‐Leibovici 2018 | No usual care / control group |
| Kanabar 2015 | No self‐management / home‐based exercise programme |
| Kara 2004 | Included in previous review update; no home‐based exercise programme. |
| Kato 2017 | No self‐management / home‐based exercise programme |
| Kenealy 2015 | No self‐management / home‐based exercise programme |
| Kennedy 2013 | No stratification on COPD |
| Khan 2019 | No self‐management / home‐based exercise programme |
| Kheirabadi 2008 | Included in previous review update; no verification of COPD |
| Khoshkesht 2015 | No verification of COPD |
| Kiser 2012 | No verification of COPD |
| Ko 2016 | No self‐management / home‐based exercise programme |
| Koff 2009 | Included in previous review update; no home‐based exercise programme |
| Korsbakke 2016 | No self‐management / home‐based exercise programme |
| Kruis 2011 | No iterative process |
| Kruis 2014a | No iterative process |
| Kruis 2014b | No iterative process |
| Labrecque 2011 | Non‐RCT / CRT |
| Lahham 2018 | No usual care / control group |
| Lainscak 2013 | No self‐management / home‐based exercise programme |
| Lam 2011 | Non‐RCT / CRT |
| Larson 2017a | No usual care / control group |
| Larson 2017b | No usual care / control group |
| La Torre 2018 | No stratification on COPD |
| Lavesen 2012 | No self‐management / home‐based exercise programme |
| Lavesen 2016 | No self‐management / home‐based exercise programme |
| Lee 2015 | No iterative process |
| Leiva‐Fernandez 2011 | One intervention component |
| Leiva‐Fernandez 2012 | One intervention component |
| Leiva‐Fernandez 2014 | Not all participants have had their COPD diagnosis verified with spirometry |
| Li J 2015 | Abstract only; no full‐text article available |
| Lilholt 2015 | No self‐management / home‐based exercise programme |
| Lilholt 2016 | No self‐management / home‐based exercise programme |
| Li P 2015 | No iterative process |
| Li Z 2015 | No published documents or information available |
| Lopez‐Lopez 2019 | No self‐management / home‐based exercise programme |
| Luhr 2018 | One intervention component |
| Marchioro 2011 | No self‐management / home‐based exercise programme |
| Maricoto 2019 | No stratification on COPD |
| Martinez 2014 | Non‐RCT / CRT |
| Martinez 2019 | One intervention component |
| McDonald 2011 | Non‐RCT / CRT |
| Moayeri 2019 | No verification of COPD |
| Monninkhof 2003 | Included in previous review update; no home‐based exercise component |
| Morganroth 2011 | No verification of COPD (based on ICD codes) |
| Morganroth 2016 | No verification of COPD (based on ICD codes) |
| Moriyama 2015 | Non‐RCT / CRT |
| Moullec 2008 | Included in previous review update; non‐RCT / CRT |
| Moy 2015a | No verification of COPD (based on ICD codes) |
| Moy 2015b | No verification of COPD (based on ICD codes) |
| Moy 2016 | No verification of COPD (based on ICD codes) |
| Mozaffari 2018 | No verification of COPD |
| Murphy 2011 | No self‐management / home‐based exercise programme |
| NCT01543217 | No usual care / control group |
| NCT01867970 | No stratification on COPD |
| NCT01871025 | One intervention component |
| NCT01897298 | One intervention component |
| NCT01921556 | No self‐management / home‐based exercise programme |
| NCT01985529 | No usual care / control group |
| NCT02035566 | No published documents or other information available |
| NCT02078622 | No verification of COPD |
| NCT02085161 | No usual care / control group |
| NCT02567474 | No iterative process |
| NCT02742597 | No verification of COPD; no stratification on COPD |
| NCT02754232 | Non‐RCT / CRT |
| NCT03387735 | No stratification on COPD |
| NCT03654092 | One intervention component |
| Ng 2017 | No iterative process |
| Nguyen 2008 | Included in previous review update; no usual care / control group |
| Nguyen 2009 | Included in previous review update; no usual care / control group |
| Nguyen 2011 | No usual care / control group |
| Nguyen 2012 | No usual care / control group |
| Nguyen 2013 | No usual care / control group |
| Nguyen 2016 | One intervention component |
| Nguyen 2018 | One intervention component |
| Nikoletou 2016 | No usual care / control group |
| Ninot 2011 | Included in previous review update; no home‐based exercise programme |
| NTR3945 | One intervention component |
| Nyberg 2017 | Non‐RCT / CRT |
| Nyberg 2019 | Non‐RCT / CRT |
| O'Donnel 2018 | Non‐RCT / CRT |
| O'Dwyer 2016 | No verification of COPD; no stratification on COPD |
| Orme 2016 | One intervention component |
| Orme 2018 | No verification of COPD |
| Özkaptan 2016 | Non‐RCT / CRT |
| Paneroni 2016 | No self‐management / home‐based exercise programme |
| Papp 2017 | No stratification on COPD |
| Pascual 2011 | No stratification on COPD |
| Peian 2013 | No verification of COPD |
| Perkins‐Porras 2018 | No verification of COPD (physician diagnosis) |
| Phan 2015 | No self‐management / home‐based exercise programme |
| Pinnock 2012 | Non‐RCT / CRT |
| Pinnock 2013 | No usual care / control group |
| Pommer 2012 | No verification of COPD; no stratification on COPD |
| Pothirat 2015 | Non‐RCT / CRT |
| Poureslami 2016 | No usual care / control group |
| Pradella 2015 | No self‐management / home‐based exercise programme |
| Rea 2004 | Included in previous review update; no verification of COPD (by spirometry) |
| Renn 2018 | No verification of COPD (based on ICD codes) |
| Rice 2011 | Non‐RCT / CRT |
| Ritchie 2012 | No verification of COPD; no stratification on COPD |
| Ritchie 2016 | No verification of COPD |
| Rixon 2017 | No verification of COPD |
| Roberts 2011 | No usual care / control group |
| Robinson 2019 | No usual care / control group |
| Rojas‐Gomez 2014 | No iterative process |
| Russo 2015 | No usual care / control group |
| Saini 2018 | Non‐RCT / CRT |
| Sanchez 2018 | No self‐management / home‐based exercise programme |
| Sanders 2012 | Non‐RCT / CRT |
| Sassi‐Dambron 1995 | Included in previous review update; no usual care / control group |
| Scalvini 2016 | No self‐management / home‐based exercise programme |
| Schmidt 2018 | No usual care / control group |
| Schou 2012 | No self‐management / home‐based exercise programme |
| Schuz 2015 | Non‐RCT / CRT |
| Scuffham 2018 | No stratification on COPD |
| Seyedi 2018 | No self‐management / home‐based exercise programme |
| Siddique 2012 | No iterative process |
| Silva 2018 | No usual care / control group |
| Silver 2017 | No iterative process |
| Sinclair 2017 | No stratification on COPD |
| Sink 2018 | No self‐management / home‐based exercise programme |
| Slok 2014 | No self‐management / home‐based exercise programme |
| Slok 2016a | No self‐management / home‐based exercise programme |
| Slok 2016b | No self‐management / home‐based exercise programme |
| Smidth 2014 | No usual care / control group |
| Sohanpal 2012 | No usual care / control group |
| Song 2014 | Not all participants have had their COPD diagnosis verified with spirometry |
| Sorensen 2015 | No verification of COPD; no iterative process |
| Soriano 2018 | No self‐management / home‐based exercise programme |
| Stamenova 2019 | No verification of COPD (by spirometry) |
| Steinhauser 2017 | No verification of COPD; no stratification on COPD |
| Stenlund 2019 | No verification of COPD |
| Steurer‐Stey 2014 | No self‐management / home‐based exercise programme |
| Stolz 2018 | No self‐management / home‐based exercise programme |
| Stoop 2015 | No verification of COPD |
| Stulbarg 2002 | Included in previous review update; no usual care / control group |
| Talboom‐Kamp 2017a | No RCT / CRT |
| Talboom‐Kamp 2017b | Non‐RCT / CRT |
| Tang 2012 | No verification of COPD |
| Tashkin 2012 | One intervention component |
| Taylor 2012 | No iterative process |
| Theander 2015 | No stratification on COPD |
| Thom 2018 | Not all participants have had their COPD diagnosis verified with spirometry |
| Thoonsen 2011 | No verification of COPD; no stratification on COPD |
| Titova 2016 | Non‐RCT / CRT |
| To 2019 | One intervention component |
| Tommelein 2014 | No verification of COPD |
| Tong 2012 | No self‐management / home‐based exercise programme |
| Torres‐Sanchez 2018 | No self‐management / home‐based exercise programme |
| Touchette 2012 | No verification of COPD; no stratification on COPD |
| Trappenburg 2011 | No usual care / control group |
| Troosters 2011 | No usual care / control group |
| Tsai 2016 | One intervention component |
| Udsen 2014 | Study protocol; no iterative process |
| Ulrik 2013 | Non‐RCT / CRT |
| Valderramas 2018 | No self‐management / home‐based exercise programme |
| Valenza 2018 | No self‐management / home‐based exercise programme |
| Van der Weegen 2015 | No stratification on COPD |
| Van Wetering 2009 | Included in previous review update; no home‐based exercise programme |
| Vasilopoulou 2017 | No self‐management / home‐based exercise programme |
| Vayisoglu 2019 | No self‐management / home‐based exercise programme |
| Velardo 2017 | No usual care / control group |
| Verwey 2014 | No stratification on COPD |
| Vianello 2016 | No self‐management / home‐based exercise programme |
| Vivodtzev 2012 | One intervention component |
| Voncken‐Brewster 2015 | No stratification on COPD |
| Vorrink 2016 | One intervention component |
| Vorrink 2017 | One intervention component |
| Wadell 2013 | No self‐management / home‐based exercise programme |
| Wakabayashi 2011 | Included in previous review update; no usual care / control group |
| Walker 2018 | No self‐management / home‐based exercise programme |
| Walters 2012 | Non‐RCT / CRT |
| Wan 2017 | No usual care / control group |
| Wan 2019 | No verification of COPD |
| Wang 2012 | No self‐management / home‐based exercise programme |
| Wang 2018 | No self‐management / home‐based exercise programme |
| Wang CH 2014 | No usual care / control group |
| Wang H 2017 | No self‐management / home‐based exercise programme |
| Wang J‐X 2017 | No self‐management / home‐based exercise programme |
| Wang K 2017 | No self‐management / home‐based exercise programme |
| Wang Y 2014 | No iterative process |
| Wei 2014 | One intervention component |
| Weldam 2016 | No usual care / control group |
| Weldam 2017 | No usual care / control group |
| Whelan 2019 | No usual care / control group |
| White 2019 | Non‐RCT / CRT |
| Wilson 2011 | No usual care / control group |
| Wilson 2015 | No usual care / control group |
| Windisch 2018 | No usual care / control group |
| Wood‐Baker 2012 | Non‐RCT / CRT |
| Wootton 2014 | No self‐management / home‐based exercise programme |
| Wootton 2017 | One intervention component |
| Wu M 2018 | One intervention component |
| Wu W 2017 | One intervention component |
| Wu W 2018 | No self‐management / home‐based exercise programme |
| Wu X 2016 | One intervention component |
| Xi 2015 | No published documents or information available |
| Xin 2016 | No iterative process |
| Yamaguti 2012 | No self‐management / home‐based exercise programme |
| Yan 2017 | No published documents or information available |
| Yan 2018 | No self‐management / home‐based exercise programme |
| Yan 2019 | No usual care / control group |
| Yan J 2016 | No usual care / control group |
| Yan XN 2016 | No usual care / control group |
| Yazdani 2018 | No self‐management / home‐based exercise programme |
| Yilmaz 2017 | One intervention component |
| Ying 2013 | One intervention component |
| Yu 2013 | No usual care / control group |
| Yu 2014 | Non‐RCT / CRT |
| Yuan 2015 | No stratification on COPD |
| Zakrisson 2019 | No verification of COPD (based on ICD codes) |
| Zambom 2011 | No self‐management / home‐based exercise programme |
| Zhai 2016 | No published documents or information available |
| Zhang 2012 | No published documents or information available |
| Zhang 2013 | No self‐management / home‐based exercise programme |
| Zhang 2014 | No published documents or information available |
| Zhang H 2016 | One intervention component |
| Zhang M 2016 | No self‐management / home‐based exercise programme |
| Zhao 2017 | No published documents or information available |
| Zheng 2019 | One intervention component |
| Zhou 2016 | No usual care / control group |
| Zhu 2018 | No self‐management / home‐based exercise programme |
| Zuo 2015 | No published documents or information available |
| Zwar 2012 | No verification of COPD |
| Zwar 2016 | No verification of COPD |
| Zwerink 2013 | No usual care / control group |
Characteristics of studies awaiting classification [ordered by study ID]
Abdulsalim 2017.
| Methods | Design: RCT Follow‐up: 24 months Control group: usual care |
| Participants |
Recruitment: not reported. Assessed for eligibility: 328. Randomly assigned: SM: 130; UC: 130. Completed: SM: 104; UC: 98. Mean age: SM: 60.6 (SD 7.9) years; UC: 61.1 (SD 8.4) years. Gender (% male): SM: 96.9; UC: 94.4. COPD diagnosis: GOLD criteria, no spirometry (FEV1/FVC ratio <0.7) reported. Inclusion of participants in acute phase: not reported. Major inclusion criteria: confirmed diagnosis of COPD as per GOLD guidelines. Major exclusion criteria: not reported. |
| Interventions |
Mode: 6 monthly counselling sessions, monthly phone calls, information leaflets. Duration: counselling sessions 15‐20 min each. Professional: pharmacist. Assignment of case manager: unclear. Self‐management components: unclear whether each included participant received at least two intervention components including an iterative process. Self‐management topics: unclear. Behavioural change techniques: at least goals and planning, feedback and monitoring, other unclear. |
| Outcomes | 1. MAQ |
| Notes | More information regarding COPD spirometry, intervention components and iterative process needed. |
Aboumatar 2017.
| Methods | Design: RCT Follow‐up: 6 months Control group: usual care |
| Participants |
Recruitment: hospital (inpatient). Assessed for eligibility: 969. Randomly assigned: not reported. Completed: not reported. Mean age: not reported per group. Gender (% male): not reported per group. COPD diagnosis: COPD diagnosis based on ICD9 codes 491.x , 492.x, 493.2, and 496. Inclusion of participants in acute phase: yes, during hospitalisation. Major inclusion criteria: admitted with a diagnosis of an acute COPD exacerbation; or, had a previous COPD diagnosis (ICD9 codes 491.x , 492.x, 493.2, and 496) and are receiving additional treatment to control COPD symptoms – (e.g. nebulizer treatments, steroids) in the current hospitalization. Major exclusion criteria: terminal illness with less than 6 months life expectancy. |
| Interventions |
Mode: unclear. Duration: unclear. Professional: (respiratory) nurse. Assignment of case manager: unclear. Self‐management components: Tailored Transition Support,Individualized COPD selfmanagement education and support, Facilitated access to services. Self‐management topics: unclear. Behavioural change techniques: at least goals and planning, feedback and monitoring, other unclear. |
| Outcomes | 1. combined number of COPD‐related hospitalizations and ED visits per participant at 6 months post discharge 2. quality of life (SGRQ) |
| Notes | More information regarding COPD spirometry, intervention components and iterative process needed. |
Alharbey 2019.
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: unclear. Assessed for eligibility: not reported. Randomly assigned: not reported. Completed: not reported. Mean age: not reported. Gender (% male): not reported. COPD diagnosis: not reported. Inclusion of participants in acute phase: not reported. Major inclusion criteria: not reported. Major exclusion criteria: not reported. |
| Interventions |
Mode: unclear. Duration: unclear. Professional: (respiratory) nurse. Assignment of case manager: unclear. Self‐management components: unclear. Self‐management topics: unclear. Behavioural change techniques: unclear. |
| Outcomes | 1. perceived awareness (UCOPD) 2. self‐efficacy (10 measurement items from a validated and reliable COPD self‐efficacy scale by Wigal) 3. perceived severity (HBM instrument by Champion) 4. behavioral intention (1‐item scale: “I intended to engage in the COPD recommended behavior.”) |
| Notes | More information regarding COPD spirometry, intervention components and iterative process needed. |
Efraimsson 2008.
| Methods | Design: RCT Follow‐up: 3 to 5 months Control group: usual care |
| Participants |
Recruitment: nurse‐led primary healthcare clinic. Assessed for eligibility: 110. Randomly assigned: SM: 26, UC: 26. Completed: SM: 26, UC: 26. Mean age: SM: 66 (SD 9.4) years; UC: 67 (SD 10.4) years. Gender (% male): SM: 50.0, UC: 50.0. COPD diagnosis: mild, moderate, severe or very severe COPD based on spirometry, lung capacity after bronchodilator use, based on GOLD criteria. Inclusion of participants in the acute phase: not reported. Major inclusion criteria: diagnosed with mild, moderate, severe or very severe COPD based on spirometry, lung capacity after bronchodilator use, based on GOLD criteria. Major exclusion criteria: diagnosed severe mental disorders such as schizophrenia, dementia or alcohol or drug abuse. |
| Interventions |
Mode: individual sessions at the outpatient and nurse‐led primary healthcare clinic Duration: two face‐to‐face individual sessions for self‐care education during 3‐5 months for one hour each by the nurse Professional: COPD nurse, physician, if needed: dietician, medical social worker, physical therapist, occupational therapist Training of case managers: not reported Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD, smoking cessation, exercise or physical activity component Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques, other: instructions on the coughing technique to prevent infections and exacerbations, measurement on oxygen saturation before and after exertion, psycho‐social counselling and support, counselling on infection prevention Exercise programme: yes (optional), dialogue on physical activity and exercise. When needed, a dietician, a medical social worker, a physical therapist and an occupational therapist were consulted. Smoking cessation programme: yes (optional), motivational dialogue on smoking cessation based on Prochaska and DiClementes’ transtheoretical model of the stages of change. The model is based on open questions to help participants reflect on their smoking habits and empower patients to quit smoking. Behavioural change techniques: ten clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, associations, repetition and substitution, comparison of outcomes, reward and threat, regulation, antecedents, identity, scheduled consequences, self‐belief, covert learning. Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, contact healthcare providers for support |
| Outcomes | 1. health‐related quality of life (SGRQ) 2. smoking 3. COPD knowledge |
| Notes | Included in previous review update; more information regarding intervention components and iterative process needed |
Ghanem 2010.
| Methods | Design: RCT Follow‐up: 2 months Control group: usual care |
| Participants |
Recruitment: hospital (inpatient) Assessed for eligibility: not reported. Randomly assigned: 39. Completed: 39. Mean age: SM: 56.96 (SD 11.59) years; UC: 56.43 (SD 9.03) years. Gender (% male): not reported. COPD diagnosis: moderate to severe COPD according to GOLD. Inclusion of participants in acute phase: yes, during hospitalisation. Major inclusion criteria: admission for a COPD exacerbation. Major exclusion criteria: unable to read or write, locomotor problems, cognitive impairment, ischaemic heart disease, aortic valve disease, cancer or lung disease other than COPD. |
| Interventions |
Mode: individual sessions, face‐to‐face, booklet, home‐based. Duration: two face‐to‐face individual sessions (first visit 120 min, second visit not reported) and 6 phone calls (mean duration 28.6 min (SD 10.0). Professional: respiratory nurse, respiratory specialist. Assignment of case manager: yes, accessible for participant during the complete follow‐up period. Self‐management components: education regarding the disease, exercise programme, advice about nutrition, advice about medication. Iterative process unclear. Self‐management topics: unclear. Behavioural change techniques: unclear. |
| Outcomes | 1. CRQ 2. SF‐36 3. FEV1 (L and % of predicted) 4. FEV1/FVC 5. 6MWT |
| Notes | Included in previous review update; more information regarding COPD spirometry, intervention components and iterative process needed. |
Heidari 2018.
| Methods | Design: RCT Follow‐up: 3 months Control group: usual care |
| Participants |
Recruitment: participants referred to the clinic. Assessed for eligibility: 85. Randomly assigned: SM: 25; UC: 25. Completed: SM: 22; UC: 29. Mean age: SM: 59.54 (SD 7.43) years; UC: 60.05 (SD 5.17) years. Gender (% male): SM: 86.4; UC: 84.2. COPD diagnosis: GOLD criteria, confirmed with spirometry (FEV1/FVC ratio < 0.7). Inclusion of participants in acute phase: not reported. Major inclusion criteria: certified diagnosis of moderate or severe COPD by a pulmonologist according to the GOLD criteria, aged 45–70 years, a BMI of <30, being literate, having a strong understanding of the Persian language, having a constant prescription drug regime, and not suffering from another serious and restrictive disease (such as a major psychological disorder, neural disease, musculoskeletal disease, cancer, or cardiac or angina attack in the last month). Major exclusion criteria: hospitalization during the study, requiring use of oxygen or spray during the 6‐minute walking test, dealing with serious stress, failure to attend any personal or group education sessions, and noncompliance with a practical program that was determined at monthly visits (for intervention group). |
| Interventions |
Mode: self‐management plan based on the 5A model. Duration: unclear. Professional: physician, nurse. Assignment of case manager: unclear. Self‐management components: unclear whether each included participant received at least two intervention components including an iterative process. Self‐management topics: unclear. Behavioural change techniques: at least goals and planning, feedback and monitoring, other unclear. |
| Outcomes | 1. spirometry 2. 6MWT 3. Borg scale |
| Notes | More information regarding COPD spirometry, intervention components and iterative process needed. |
Hill 2010.
| Methods | Design: RCT Follow‐up: 3 months Control group: usual care |
| Participants |
Recruitment: primary care setting. Assessed for eligibility: 131. Randomly assigned: 110. Completed: 93. Mean age: SM: 63.4 (SD 9.6) years; UC: 65.7 (SD 9.9) years. Gender (% male): SM: 44.0; UC: 46.5. COPD diagnosis: postbronchodilator ratio of FEV1/FVC < 0.7 and FEV1 < 80% predicted. Inclusion of participants in acute phase: not reported. Major inclusion criteria: confirmed diagnosis of COPD as per GOLD guidelines. Major exclusion criteria: unable to perform spirometry for a medical reason; unable to communicate in written or spoken English. |
| Interventions |
Mode: individual sessions, face‐to‐face, written teaching manual, primary care practice. Duration: two individual sessions of one hour. Professional: certified COPD educator. Assignment of case manager: unclear. Self‐management components: unclear whether each included participant received at least two intervention components including an iterative process. Self‐management topics: unclear. Behavioural change techniques: unclear. |
| Outcomes | 1. Bristol COPD Knowledge Questionnaire |
| Notes | Included in previous review update; more information regarding intervention components and iterative process needed |
Jiang 2012.
| Methods | Design: RCT Follow‐up: 10 months Control group: usual care |
| Participants |
Recruitment: hospital (outpatient). Assessed for eligibility: 295. Randomly assigned: SM: 50; UC: 50. Completed: SM: 49; UC: 47. Mean age: SM: 65.2 (SD 8.96) years; UC: 64.7 (SD 8.05) years. Gender (% male): SM: 71.4; UC: 68.1. COPD diagnosis: moderate or severe COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD). Inclusion of participants in acute phase: not reported. Major inclusion criteria: moderate or severe COPD according to the GOLD, disease duration < 2 years since COPD diagnosis, at least one COPD exacerbation (defined as a complex of respiratory events/symptoms (increase or new onset) with a duration of 3 days requiring a change in treatment). Major exclusion criteria: unable to communicate clearly and give informed consent, concurrent oncologic or psychiatric diseases, drug or alcohol abuse history. |
| Interventions |
Mode: weekly phone calls, audio CD, the self‐help manual, instruction booklet. Duration: 35 min per phone call. Professional: nurse. Assignment of case manager: unclear. Self‐management components: unclear whether each included participant received at least two intervention components including an iterative process. Self‐management topics: unclear. Behavioural change techniques: at least goals and planning, feedback and monitoring, other unclear. |
| Outcomes | 1. uncertainty level (Mishel’s Uncertainty in Illness Scale‐Adult) 2. CSQ 3. anxiety (State‐Trait Anxiety Inventory scale) 4. depression (HADS‐depression) 5. quality of life (SF‐36) |
| Notes | More information regarding COPD spirometry, intervention components and iterative process needed. |
Khdour 2009.
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants |
Recruitment: hospital (outpatient clinic). Assessed for eligibility: not reported. Randomly assigned: 173. Completed: 143. Mean age: SM: 65.6 (SD 10.1) years; UC: 67.3 (SD 9.2) years Gender (% male): SM: 43.7; UC: 44.2. COPD diagnosis: confirmed diagnosis of COPD (by the hospital consultant) for at least 1 year, having a FEV of 30‐80% of the predicted normal value Inclusion of participants in acute phase: no. Major inclusion criteria: confirmed diagnosis of COPD for at least 1 year, having a FEV of 30‐80% of the predicted normal value and > 45 years old. Major exclusion criteria: congestive heart failure; moderate to severe learning difficulties (as judged by hospital consultant); attended a pulmonary rehabilitation programme in the last six months; severe mobility problems or terminal illness. |
| Interventions |
Mode: individual sessions, face‐to‐face, telephone, hospital (outpatient clinic). Duration: one session of one hour, reinforcement at each outpatient visit every six months, two telephone calls at three and nine months. Professional: clinical pharmacist. Accessibility of case managers: not reported. Self‐management components: unclear. Self‐management topics: unclear. Behavioural change techniques: at least 2 clusters: goals and planning, feedback and monitoring, other unclear. |
| Outcomes | 1. SGRQ 2. FEV1 3. Hospital admissions for acute exacerbations 4. ED visits for acute exacerbations 5. GP visits, scheduled and unscheduled 6. COPD knowledge questionnaire 7. Adherence to prescribed medication |
| Notes | Included in previous review update; more information from authors needed on COPD diagnosis, intervention components including an iterative process. |
Li 2014.
| Methods | Design: RCT Follow‐up: 3 months Control group: usual care |
| Participants |
Recruitment: hospital (inpatient). Assessed for eligibility: 114. Randomly assigned: SM: 57; UC: 57. Completed: SM: 56; UC: 56. Mean age: SM: 70.91 (SD 9.17) years; UC: 72.18 (SD 8.53) years. Gender (% male): SM: 87.5; UC: 91.1. COPD diagnosis: COPD characterized by inhaled bronchodilator FEV1/FVC < 70%, FEV 1% predicted percentage < 80%. Inclusion of participants in acute phase: yes, during hospitalisation. Major inclusion criteria: a diagnosis of COPD characterized by inhaled bronchodilator FEV1/FVC < 70%, FEV 1% predicted percentage < 80%; ability to care for themselves during stable periods; and willingness to sign an informed consent form. Major exclusion criteria: a co‐existent medical problem (e.g, bronchial asthma, suspected malignancy, cardiac failure); cognitive impairment or lack of social support; or limb movement disorder. |
| Interventions |
Mode: home‐based rehabilitation programme, phone calls, home visits. Duration: phone calls at 3, 5, 7, and 9 weeks after discharge, and home visits at 72 hours and 3 months post‐discharge. Professional: respiratory nurse, community nurse. Assignment of case manager: unclear. Self‐management components: unclear whether each included participant received at least two intervention components including an iterative process. Self‐management topics: unclear. Behavioural change techniques: at least goals and planning, feedback and monitoring, other unclear. |
| Outcomes | 1. quality of life (SGRQ) 2. GHQ‐12 3. BMI |
| Notes | More information needed from authors on COPD diagnosis, intervention delivery, intervention components and an iterative process. |
Liu 2013.
| Methods | Design: RCT Follow‐up: 4 months Control group: usual care |
| Participants |
Recruitment: not reported. Assessed for eligibility: not reported. Randomly assigned: SM: 29; UC: 28. Completed: not reported. Mean age: SM: 69.4 (SD 3.3) years; UC: 68.8 (SD 1.4) years. Gender (% male): SM: 72.4; UC: 82.1. COPD diagnosis: COPD according to the 2007 guidelines of the Chinese Society of Respiratory Disease. Inclusion of participants in acute phase: not reported. Major inclusion criteria: COPD according to the 2007 guidelines of the Chinese Society of Respiratory Disease,17 their clinical condition was stable at the time of inclusion, there was no history of bronchial asthma, a test for bronchiectasis was negative, no oral glucocorticoid treatment had been taken within the previous three months, and a computer with Internet access was available in the home. Major exclusion criteria: not reported. |
| Interventions |
Mode: 6 monthly counselling sessions, monthly phone calls, information leaflets. Duration: counselling sessions 15‐20 min each. Professional: pharmacist. Assignment of case manager: unclear. Self‐management components: pursed‐lip breathing, deep inspiration‐slow blowing – making a fist, deep inhale‐holdingslow exhale, global exercise. Unclear whether each included participant received at least two intervention components including an iterative process. Self‐management topics: unclear. Behavioural change techniques: unclear. |
| Outcomes | 1. pulmonary function tests (FEV1/FVC ratio) 2. exercise capacity (6MWT) 3. quality of life (SGRQ) |
| Notes | More information regarding COPD spirometry, intervention components and iterative process needed. |
Lou 2015.
| Methods | Design: RCT Follow‐up: 48 months Control group: usual care |
| Participants |
Recruitment: healthcare units/centres in rural areas. Assessed for eligibility: 8,217. Randomly assigned: Self‐management (SM): 4,197; Usual care (UC): 4,020. Completed: SM: 3,418; UC: 2,803. Mean age: SM: 71.2 ± 7.4 years; UC: 71.5 ± 7.8 years. Gender (% male): SM: 47.8; UC: 47.9. COPD diagnosis: the subjects had to have a diagnosis of COPD according to the criteria proposed by the GOLD . Inclusion of participants in acute phase: no. Major inclusion criteria: at baseline, the subjects had to have a diagnosis of COPD according to the criteria proposed by GOLD . Major exclusion criteria: presence of fever, active tuberculosis, changes in radiographic images or medication in the 4 weeks immediately preceding recruitment, primary diagnosis of asthma or obvious bronchiectasis, cystic fibrosis, interstitial lung disease, previous lung‐volume‐reduction surgery, lung transplantation, pneumonectomy, uncontrolled or serious conditions that could potentially affect spirometry tests, and refusal to fill out psychological questionnaires. |
| Interventions |
Mode: group and individual face‐to‐face sessions. Duration: 104 group sessions of 40‐60 minutes lecture each every 2 weeks, 104 individual follow‐up sessions at least once every two weeks. Every 2 months, the professionals examined the subjects collectively at the health‐care units. Professional: respiratory specialist, nurse psychologist, (respiratory) physiotherapist, peer led dietician, GPs, psychiatrists, rehabilitation specialists, other experts. Assignment of case manager: unclear. Self‐management components: education regarding COPD, smoking cessation, exercise or physical activity component, other: psychological counselling, review and adjustment of outpatient COPD medication. Unclear whether each included participant received at least two intervention components including an iterative process. Self‐management topics: unclear. Behavioural change techniques: at least goals and planning, feedback and monitoring, other unclear. |
| Outcomes | 1. health status (BODE index) 2. changes in COPD knowledge, awareness and risk factors (survey) 3. changes in anxiety and depression symptoms (HADS) 4. changes in hospital admissions and ED visits 5. changes in medication regimens |
| Notes | More information regarding COPD spirometry, intervention components and iterative process needed. |
Ozturk 2020.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Potential eligible study screened from updated database search (January 2020 to March 2021). This study will be incorporated into the review at the next update, if inclusion criteria are met. No data extraction has been performed. |
COPD: Chronic Obstructive Pulmonary Disease; CRQ: Chronic Respiratory (Disease) Questionnaire; CSQ: Client Satisfaction Questionnaire; ED: emergency department; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; GHQ: General Health Questionnaire; GOLD: Global Initiative for Chronic Obstructive Lung Disease; GP: General Practitioner; HADS: Hospital Anxiety and Depression Scale; HMB: Health Belief Model; ICD: International Classification of Diseases; MAQ: Multidimensional Anxiety Questionnaire; RCT: Randomised Controlled Trial; SD: Standard deviation; SF‐36: 36‐Item Short Form Health Survey; SGRQ: St. George's Respiratory Questionnaire; SM: Self‐management; UC: Usual care; UCOPD: understanding COPD questionnaire; 6MWD: six‐minute walk distance
Characteristics of ongoing studies [ordered by study ID]
Boer 2011.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Bourne 2017.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Cecere Feemster 2013.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Chen 2018.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
ChiCTR1800018197.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
ChiCTR‐TRC‐12002559.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Chien 2016.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Costa 2015.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Dewan 2011a.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Ding 2019.
| Study name | MH‐COPD |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Doheny 2013.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Duran 2017.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Ergan 2018.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Fleehart 2015.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Gonzalez 2015.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Hernandez 2016.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Imanalieva 2016.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
IRCT201504149014N61.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
IRCT2017030432764N2.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
James 2012.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Ko 2015.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Moreno 2017.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
NCT02258646.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
NCT02924870.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
NCT03012256.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
NCT03084874.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
NCT03216603.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
NCT03721315.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
NL3827 (NTR4009).
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
NL5277 (NTR5558).
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Padilla‐Zarate 2013.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Paquin 2014.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Reguera 2017.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Sano 2016.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Siddharthan 2018.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Sirichana 2014.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Thomas 2019.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Zanaboni 2016.
| Study name | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | More information needed from authors about intervention components, including whether the intervention involves an iterative process. |
Differences between protocol and review
We changed the title to 'Self‐management interventions for people with chronic obstructive pulmonary disease', in line with recent self‐management studies.
We redrafted the Background section.
We expanded the Objectives to include the outcomes of interest.
For inclusion in this review, participants with COPD in included studies needed to have a diagnosis of COPD in line with the GOLD classification criteria (GOLD 2021), meaning a post‐bronchodilator FEV1 to FVC ratio of less than 0.70 (confirmed by (provided) spirometry data or confirmed in writing by authors).
We included only randomised controlled trials and cluster‐randomised trials that compared intervention to usual care in this update.
We included COPD self‐management intervention studies only if they incorporated at least two intervention components with an iterative process between healthcare provider and participant, in line with the most recent definition of COPD self‐management interventions (Effing 2016).
We also removed reference to modes of delivering the intervention and reframed as requiring interventions to be aimed at behaviour change.
For primary outcomes, we performed primary (using end point scores) and secondary analyses (using scores from different follow‐up times).
For dichotomous outcomes with few events, we reported risk differences with corresponding 95% confidence intervals.
We added mortality as a primary outcome. Hospitalisations were defined as respiratory‐related (primary outcome) or all‐cause (secondary outcome). We defined dyspnoea, impact of COPD on life, anxiety and/or depression as sub‐outcomes of health status. We also defined the use of other healthcare facilities, and added self‐management behaviours, patient activation and health literacy as secondary outcomes.
We used the Cochrane risk of bias 2 tool for the 2021 update.
We removed ‘Courses of steroids’ and ‘Exercise’ from the summary of findings table, and added 'Respiratory‐related mortality' and 'COPD exacerbations'.
We calculated the number needed to treat for an additional beneficial outcome (NNTB) for primary outcomes.
We performed sensitivity analysis on studies with contradictory results to investigate the robustness of the effect size, by excluding those studies from a meta‐analysis.
We performed sensitivity analyses on cluster‐randomised controlled trials using intra‐cluster correlation coefficients of 0.02 and 0.04, in case authors did not adjust their data for clustering and did not report such a coefficient.
We added the following extra subgroup analyses.
The duration of the intervention (< 8 weeks versus ≥ 8 weeks).
Inclusion of participants in the acute phase (having an acute exacerbation of COPD) versus stable state (at least four weeks post exacerbation and six weeks post hospitalisation).
COPD self‐management interventions delivered in different income countries (self‐management interventions in low‐ and middle‐income countries versus high‐income countries).
COPD self‐management interventions delivered in different care settings: primary care versus secondary and tertiary care.
Smoking cessation component (inclusion of a smoking cessation component in the self‐management intervention versus no smoking cessation component in the self‐management intervention).
Diet component (e.g. evaluation and optimisation of participants' diet and nutritional intake) (inclusion of a diet component in the self‐management intervention versus no diet component in the self‐management intervention).
COPD medication component (e.g. advice about medication intake, adherence, inhalation technique) (inclusion of a medication component in the self‐management intervention versus no medication component in the self‐management intervention).
Coping with breathlessness component (inclusion of a coping with breathlessness component in the self‐management intervention versus no coping with breathlessness component in the self‐management intervention).
Self‐recognition of COPD exacerbations component (inclusion of a self‐recognition of COPD exacerbations component in the self‐management intervention versus no self‐recognition of COPD exacerbations component in the self‐management intervention).
The effects of COPD self‐management interventions with and without use of digital technology.
The integration of behavioural change techniques in COPD self‐management interventions.
Contributions of authors
Jade Schrijver coordinated the review, independently assessed the eligibility of titles, abstracts and full‐text versions of potentially relevant reports, independently extracted data from the included studies, assessed risk of bias for included studies, managed and analysed the data, generated the summary of findings table and wrote the review update.
Anke Lenferink independently assessed the eligibility of titles, abstracts and full‐text versions of potentially relevant reports, independently extracted data from the included studies, assessed risk of bias for included studies, double‐checked data entry, supported data management and data analysis, provided a methodological perspective, and critically revised the review update.
Marjolein Brusse‐Keizer independently assessed the eligibility of titles, abstracts and full‐text versions of potentially relevant reports, independently extracted data from the included studies, assessed risk of bias for included studies, double‐checked data entry, provided a methodological perspective, and critically revised the review update.
Marlies Zwerink independently assessed the eligibility of titles, abstracts and full‐text versions of potentially relevant reports, independently extracted data from the included studies, assessed risk of bias, and critically revised the review update.
Paul van der Valk independently assessed the eligibility of titles and abstracts, provided a clinical perspective, and critically revised the review update.
Job van der Palen independently assessed the eligibility of titles, abstracts and full‐text versions of potentially relevant reports, independently extracted data from the included studies, assessed risk of bias for included studies, double‐checked data entry, provided a methodological perspective, and critically revised the review update.
Tanja Effing independently assessed the eligibility of titles, abstracts and full‐text versions of potentially relevant reports, independently extracted data from the included studies, assessed risk of bias for included studies, double‐checked data entry, supported data management and data analysis, provided a methodological perspective, helped write the review, and critically revised the review update.
Contributions of editorial team
Rebecca Fortescue (Co‐ordinating Editor) edited the review; advised on methodology; approved the review prior to publication.
Emma Dennett (Managing Editor): co‐ordinated the editorial process; advised on interpretation and content; edited the review.
Emma Jackson (Assistant Managing Editor): conducted peer review; edited reference sections and other sections generally in the protocol and the review.
Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; edited the search methods section.
Lucy Goldsmith: checked the data entry prior to the full write‐up of the review.
Sources of support
Internal sources
-
All authors, Other
The authors declare that no such funding was received for this systematic review
External sources
-
All authors, Netherlands
Dutch Foundation for Asthma Prevention
Declarations of interest
JS: is a researcher, employed by Medisch Spectrum Twente, Enschede, the Netherlands. She received funding to complete work on this review from the Dutch Foundation for Asthma Prevention, that was in no way able to influence the results of the review.
AL: is an epidemiologist and an assistant professor at the Health Technology and Services Research section, University of Twente, the Netherlands. She is also a researcher at Medisch Spectrum Twente, Enschede, the Netherlands. She coordinated the Lenferink 2019* study, included in this review. She has no conflict of interest with regard to the current review.
MB‐K: is an epidemiologist, employed by Medisch Spectrum Twente, Enschede, the Netherlands. She is also a researcher at the Health Technology and Services Research section, University of Twente, the Netherlands. She was involved in the study of Tabak 2014**, included in this review. She has no conflict of interest with regard to the current review.
MZ: is a data‐analyst and an epidemiologist, employed by Medisch Spectrum Twente, Enschede, the Netherlands. She coordinated the previous update of this review (Zwerink 2014).
PvdV: is a medical doctor, pulmonologist and respiratory researcher at the Department of Pulmonary Medicine, Medisch Spectrum Twente, Enschede, the Netherlands. He was involved in the Lenferink 2019* and Tabak 2014** studies, both included in this review. He has no conflict of interest with regard to the current review.
JvdP: is Professor of Evaluation and Assessment in Health Research at University of Twente, Enschede, the Netherlands. He is also an epidemiologist and research coordinator at Medisch Spectrum Twente, Enschede, the Netherlands. He was involved in the Lenferink 2019* and Tabak 2014** studies, both included in this review. He has no conflict of interest with regard to the current review.
TE: is an epidemiologist affiliated with Flinders University and University of Adelaide, Adelaide, Australia. She was involved in the Lenferink 2019* study, included in this review. She has no conflict of interest with regard to the current review.
* The Lenferink 2019 study was supported by the Lung Foundation Netherlands (grant number 3.4.11.061), Lung Foundation Australia (Australian Lung Foundation Boehringer Ingelheim COPD Research Fellowship 2010), Repat Foundation, GlaxoSmithKline (unrestricted grant) and Dutch Foundation for Asthma Prevention. ** The Tabak 2014 study was supported by the NL Agency, a division of the Dutch Ministry of Economic Affairs (grant CALLOP9089).
Edited (no change to conclusions)
References
References to studies included in this review
Benzo 2016 {published and unpublished data}
- Benzo R, McEvoy C. Effect of health coaching delivered by a respiratory therapist or nurse on self-management abilities in severe COPD: analysis of a large randomized study. Respiratory Care 2019;64(9):1065-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzo R, Vickers K, Ernst D, Tucker S, McEvoy C, Lorig K. Development and feasibility of a self-management intervention for chronic obstructive pulmonary disease delivered with motivational interviewing strategies. Journal of Cardiopulmonary Rehabilitation and Prevention 2013;33(2):113-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzo R, Vickers K, Novotny PJ, Tucker S, Hoult J, Neuenfeldt P, et al. Health coaching and chronic obstructive pulmonary disease rehospitalization: a randomized study. American Journal of Respiratory and Critical Care Medicine 2016;194(6):672-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bischoff 2012 {published and unpublished data}
- Bischoff E, Akkermans R, Bourbeau J, Vercoulen J, Van Weel C, Schermer T. Comprehensive self management and routine monitoring in chronic obstructive pulmonary disease patients in general practice: randomised controlled trial. European Respiratory Journal 2013;42:1084s [P5104]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff EW, Akkermans R, Bourbeau J, Van Weel C, Vercoulen JH, Schermer TR. Comprehensive self management and routine monitoring in chronic obstructive pulmonary disease patients in general practice: randomised controlled trial. BMJ 2012;345(e7642):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bösch 2007 {published and unpublished data}
- Bösch D, Feierabend M, Becker A. COPD outpatient education programme (ATEM) and BODE index [Ambulante COPD–patientenschulung (ATEM) und BODE–index]. Pneumologie 2007;61(10):629-35. [DOI] [PubMed] [Google Scholar]
Bourbeau 2003 {published and unpublished data}
- Bourbeau J, Collet JP, Schwartzman K, Ducruet T, Nault D, Bradley C. Economic benefits of self-management education in COPD. Chest 2006;130(6):1704-11. [DOI] [PubMed] [Google Scholar]
- Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupré A, Bégin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Archives of Internal Medicine 2003;163(5):585-91. [DOI] [PubMed] [Google Scholar]
- Gadoury MA, Schwartzman K, Rouleau M, Maltais F, Julien M, Beaupré A, et al. Self-management reduces both short- and long-term hospitalisation in COPD. European Respiratory Journal 2005;26(5):853–7. [DOI] [PubMed] [Google Scholar]
- Sedeno MF, Nault D, Hamd DH, Bourbeau J. A self-management education program including an action plan for acute COPD exacerbations. Journal of Chronic Obstructive Pulmonary Disease 2009;6(5):352-8. [DOI] [PubMed] [Google Scholar]
Bringsvor 2018 {published and unpublished data}
- Bringsvor HB, Langeland E, Oftedal BF, Skaug K, Assmus J, Bentsen SB. Effects of a COPD self-management support intervention: a randomized controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2018;13:3677-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bucknall 2012 {published and unpublished data}
- Bucknall CE, Miller G, Lloyd SM, Cleland J, McCluskey S, Cotton M, et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ 2012;344(e1060):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coultas 2005 {published and unpublished data}
- Coultas D, Frederick J, Barnett B, Singh G, Wludyka P. A randomized trial of two types of nurse-assisted home care for patients with COPD. Chest 2005;128(4):2017-24. [DOI] [PubMed] [Google Scholar]
Emery 1998 {published and unpublished data}
- Emery CF, Schein RL, Hauck ER, MacIntyre NR. Psychological and cognitive outcomes of a randomized trial of exercise among patients with chronic obstructive pulmonary disease. Health Psychology 1998;17(3):232-40. [DOI] [PubMed] [Google Scholar]
Fan 2012 {published data only (unpublished sought but not used)}
- Fan VS, Gaziano JM, Lew R, Bourbeau J, Adams SG, Leatherman S, et al. A comprehensive care management program to prevent Chronic Obstructive Pulmonary Disease hospitalizations: a randomized, controlled trial. Annals of Internal Medicine 2012;156(10):673-83. [DOI] [PubMed] [Google Scholar]
- Fan VS, Niewoehner DE, Lew R. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations. Annals of Internal Medicine 2012;157(7):530-1. [DOI] [PubMed] [Google Scholar]
Ferrone 2019 {published and unpublished data}
- Ferrone M, Masciantonio MG, Malus N, Stitt L, O'Callahan T, Roberts Z, et al. The impact of integrated disease management in high-risk COPD patients in primary care. NPJ Primary Care Respiratory Medicine 2019;29:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02343055. COPE with COPD trial [Collaborative self-management patient education will improve health outcomes in COPD]. clinicaltrials.gov/show/NCT02343055 (first received 21 January 2015).
Gallefoss 1999 {published and unpublished data}
- Gallefoss F, Bakke PS, Kjaersgaard P. Quality of life assessment after patient education in a randomized controlled study on asthma and chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1999;159(3):812-7. [DOI] [PubMed] [Google Scholar]
- Gallefoss F, Bakke PS. Cost-benefit and cost-effectiveness analysis of self-management in patients with COPD: a 1-year follow-up randomized, controlled trial. Respiratory Medicine 2002;96(6):424-31. [DOI] [PubMed] [Google Scholar]
- Gallefoss F, Bakke PS. How does patient education and self-management among asthmatics and patients with chronic obstructive pulmonary disease affect medication? American Journal of Respiratory and Critical Care Medicine 1999;160(6):2000-5. [DOI] [PubMed] [Google Scholar]
- Gallefoss F, Bakke PS. Impact of patient education and self management on morbidity in asthmatics and patients with chronic obstructive pulmonary disease. Respiratory Medicine 2000;94(3):279-87. [DOI] [PubMed] [Google Scholar]
- Gallefoss F. The effects of patient education in COPD in a 1-year follow-up randomised, controlled trial. Patient Education and Counseling 2004;52(3):259-66. [DOI] [PubMed] [Google Scholar]
Hernández 2015 {published and unpublished data}
- Hernández C, Alonso A, Garcia-Aymerich J, Serra I, Marti D, Rodriguez-Roisin R, et al. Effectiveness of community-based integrated care in frail COPD patients: a randomised controlled trial. Primary Care Respiratory Medicine 2015;25:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson‐Warrington 2016 {published data only}
- ISRCTN84599369. A study for the delivery of standardised self management (SPACE Self management Programme of Activity, Coping and Education) at the time of discharge after an acute exacerbation of COPD - is it effective? ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN84599369 (first received 26 March 2013).
- Johnson-Warrington V, Rees K, Gelder C, Morgan MD, Singh SJ. Can a supported self-management program for COPD upon hospital discharge reduce readmissions? A randomized controlled trial. International Journal of Chronic Obstructive Pulmonary disease 2016;11(1):1161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jolly 2018 {published and unpublished data}
- Jolly K, Sidhu M, Hewitt C, Daley A, Jordan R, Coventry P, et al. Telephone health coaching in primary care patients with MRC I/II COPD: randomised controlled trial. European Respiratory Journal 2017;50(Suppl 61):OA2914. [Google Scholar]
- Jolly K, Sidhu MS, Hewitt CA, Coventry PA, Daley A, Jordan R, et al. Self management of patients with mild COPD in primary care: randomised controlled trial. BMJ 2018;361:k2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu MS, Daley A, Jordan R, Coventry PA, Heneghan C, Jowett S, et al. Patient self-management in primary care patients with mild COPD - protocol of a randomised controlled trial of telephone health coaching. BMC Pulmonary Medicine 2015;15(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jonsdottir 2015 {published and unpublished data}
- Jonsdottir H, Amundadottir OR, Gudmundsson G, Halldorsdottir BS, Hrafnkelsson B, Ingadottir TS. Effectiveness of a partnership-based self-management programme for patients with mild and moderate chronic obstructive pulmonary disease: a pragmatic randomized controlled trial. Journal of Advanced Nursing 2015;71(11):2634-49. [DOI] [PubMed] [Google Scholar]
- Jonsdottir H, Gunnarsdottir A, Halldorsdottir B, Gudmundsson G, Stefansdottir I, Jonsson JS, et al. Effectiveness of a partnership based self-management program for individuals with mild to moderate COPD and their families. European Respiratory Journal 2013;42(Suppl 57):416s [P2083]. [Google Scholar]
Kessler 2018 {published and unpublished data}
- Bourbeau J, Casan P, Tognella S, Haidl P, Texereau JB, Kessler R. An international randomized study of a home-based self-management program for severe COPD: the COMET. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:1447-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbeau J, Granados D, Roze S, Durand-Zaleski I, Casan P, Kohler D, et al. Cost-effectiveness of the COPD Patient Management European Trial home-based disease management program. International Journal of Chronic Obstructive Pulmonary Disease 2019;14:645-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbeau J, Kessler R, Casan P, Koehler D, Tognella S, JL Viejo, et al. An international randomised study of a home-based self-management program for severe COPD: the COPD patient management European trial. Canadian Journal of Respiratory Critical Care and Sleep Medicine 2017;1(2):98. [Google Scholar]
- Kessler R, Casan-Clara P, Koehler D, Tognella S, Viejo JL, Dal Negro RW, et al. COMET: a multicomponent home-based disease-management programme versus routine care in severe COPD. European Respiratory Journal 2018;51(1):1701612. [DOI] [PubMed] [Google Scholar]
- Kessler R, Casan P, Koehler D, Tognella S, Luis Viejo J, Dal Negro R, et al. A home-centered disease management program in severe chronic obstructive pulmonary disease (Results of the COPD patient Management European Trial-COMET). European Respiratory Journal 2016;48(Suppl 60):OA4806. [Google Scholar]
Lenferink 2019 {published and unpublished data}
- Lenferink A, Frith P, Van der Valk P, Buckman J, Sladek R, Cafarella P, et al. A self-management approach using self-initiated action plans for symptoms with ongoing nurse support in patients with chronic obstructive pulmonary disease (COPD) and comorbidities: the COPE-III study protocol. Contemporary Clinical Trials 2013;36(1):81-9. [DOI] [PubMed] [Google Scholar]
- Lenferink A, Van der Palen J, Van der Valk P, Cafarella P, Van Veen A, Quinn S, et al. Effects of self-management action plans for COPD patients with comorbidities on health status and self-efficacy. European Respiratory Journal 2017;50(Suppl 61):PA3456. [Google Scholar]
- Lenferink A, Van Der Palen J, Van Der Valk P, Cafarella P, Van Veen A, Quinn S, et al. Self-management action plans for COPD patients with comorbidities reduce exacerbation duration and respiratory-related hospitalizations - the COPE-III study. American Journal of Respiratory and Critical Care Medicine 2017;195:A7003. [Google Scholar]
- Lenferink A, Van Der Palen J, Van Der Valk P, Cafarella P, Van Veen A, Quinn S, et al. Self-management action plans for patients with chronic obstructive pulmonary disease and comorbidities reduce exacerbation duration and respiratory-related hospitalisations - the COPE-III study. American Journal of Respiratory and Critical Care Medicine 2017;195:A7003. [Google Scholar]
- Lenferink A, Van der Palen J, Van der Valk PD, Cafarella P, Van Veen A, Quinn S, et al. Exacerbation action plans for patients with COPD and comorbidities: a randomised controlled trial. European Respiratory Journal 2019;54(5):1802134. [DOI: 10.1183/13993003.02134-2018] [DOI] [PubMed] [Google Scholar]
Liang 2019 {published and unpublished data}
- Liang J, Abramson MJ, Russell G, Holland AE, Zwar NA, Bonevski B, et al. Interdisciplinary COPD intervention in primary care: a cluster randomised controlled trial. European Respiratory Journal 2019;53(4):1801530. [DOI] [PubMed] [Google Scholar]
Martin 2004 {published data only}
- Martin IR, McNamara D, Sutherland FR, Tilyard MW, Taylor DR. Care plans for acutely deteriorating COPD: a randomized controlled trial. Chronic Respiratory Disease 2004;1(4):191-5. [DOI] [PubMed] [Google Scholar]
Mitchell 2014 {published and unpublished data}
- Apps L, Harrison S, Williams J, Steiner M, Morgan M, Singh S. A self-management programme of activity, coping and education (SPACE) for COPD: patients' perspective [Abstract]. European Respiratory Journal 2012;40(Suppl 56):266s [P1471]. [Google Scholar]
- Dritsaki M, Johnson-Warrington V, Mitchell K, Singh S, Rees K. An economic evaluation of a self-management programme of activity, coping and education for patients with chronic obstructive pulmonary disease. Chronic Respiratory Disease 2016;13(1):48-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KE, Johnson-Warrington V, Apps LD, Bankart J, Sewell L, Williams JE, et al. A self-management programme for COPD: a randomised controlled trial. European Respiratory Journal 2014;44(6):1538-47. [DOI] [PubMed] [Google Scholar]
- Mitchell KE, Warrington V, Sewell L, Bankart J, Williams JE, Steiner M, et al. A randomised controlled trial of a self-management programme of activity coping and education - SPACE FOR COPD: impact on physical activity at 6 weeks. American Journal of Respiratory and Critical Care Medicine 2013;187:A5952. [Google Scholar]
- Mitchell-Wagg K, Warrington V, Apps L, Sewell L, Bankart J, Steiner M, et al. A self-management programme of activity coping and education (SPACE) for COPD: results from a randomised controlled trial [Abstract]. Thorax 2012;67(Suppl 2):A25 [S49]. [Google Scholar]
- Wagg K, Warrington V, Apps L, Sewell L, Bankart J, Steiner M, et al. A self-management programme of activity coping and education (SPACE) for COPD: 6 week results from a randomised controlled trial [Abstract]. European Respiratory Journal 2012;40(Suppl 56):548s [3092]. [Google Scholar]
Rice 2010 {published and unpublished data}
- Rice KL, Dewan N, Bloomfield HE, Grill J, Schult TM, Nelson DB, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2010;182(7):890-6. [DOI] [PubMed] [Google Scholar]
Rose 2018 {published and unpublished data}
- Rose L, Istanboulian L, Carriere L, Price A, Lee L, Rezaie S, et al. Program of integrated care for patients with chronic obstructive pulmonary disease and multiple comorbidities (pic COPD+): a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2017;195:A6739. [DOI] [PubMed] [Google Scholar]
- Rose L, Istanboulian L, Carriere L, Thomas A, Lee H-B, Rezaie S, et al. Program of integrated care for patients with chronic obstructive pulmonary disease and multiple comorbidities (PIC COPD +): a randomised controlled trial. European Respiratory Journal 2018;51(1):1701567. [DOI] [PubMed] [Google Scholar]
Sanchez‐Nieto 2016 {published and unpublished data}
- Sanchez-Nieto JM, Andujar-Espinosa R, Bernabeu-Mora R, Hu C, Galvez-Martinez B, Carrillo-Alcaraz A, et al. Efficacy of a self-management plan in exacerbations for patients with advanced COPD. International Journal of Chronic Obstructive Pulmonary Disease 2016;11(1):1939-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tabak 2014 {published and unpublished data}
- NTR3072. CoCo in COPD treatment: evaluation of use, satisfaction and clinical effects. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=NTR3072 (first received 19 September 2011).
- Tabak M, Brusse-Keizer M, Van der Valk P, Hermens H, Vollenbroek-Hutten M. A telehealth program for self-management of COPD exacerbations and promotion of an active lifestyle: a pilot randomized controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2014;9:935-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak M, Brusse-Keizer M, Van Ommeren C, Kotte H, Weltevreden P, Hermens H, et al. A telecare programme for self-management of COPD exacerbations and promotion of an active lifestyle. European Respiratory Journal 2013;42(Suppl 57):1041s [P4911]. [Google Scholar]
Titova 2015 {published and unpublished data}
- Titova E, Salvesen Ø, Bentsen SB, Sunde S, Steinshamn S, Henriksen AH. Does an integrated care intervention for COPD patients have long-term effects on quality of life and patient activation? A prospective, open, controlled single-center intervention study. PLoS One 2017;12(1):e0167887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titova E, Steinshamn S, Henriksen A. An integrated care management program (ICMP) for patients with severe COPD; mortality, hazards and causes. European Respiratory Journal 2015;46(Suppl 59):PA3072. [Google Scholar]
- Titova E, Steinshamn S, Indredavik B, Henriksen AH. Long term effects of an integrated care intervention on hospital utilization in patients with severe COPD: a single centre controlled study. Respiratory Research 2015;16(8):1-10. [DOI: 10.1186/s12931-015-0170-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2013 {published data only}
- Licskai C, Ferrone M, Malus N, Stitt L, O'Callahan T, Roberts Z, et al. COPD collaborative self-management in primary care: a randomized controlled trial. In: European Respiratory Journal. Vol. 48. 2016:OA1994.
- Walters J, Cameron-Tucker H, Wills K, Schüz N, Scott J, Robinson A, et al. Effects of telephone health mentoring in community-recruited chronic obstructive pulmonary disease on self-management capacity, quality of life and psychological morbidity: a randomised controlled trial. BMJ Open 2013;3(9):e003097. [CENTRAL: 871543] [EMBASE: 2013640708] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2019 {published and unpublished data}
- Wang LH, Zhao Y, Chen LY, Zhang L, Zhang YM. The effect of a nurse-led self-management program on outcomes of patients with chronic obstructive pulmonary disease. Clinical Respiratory Journal 2019;14(2):148-57. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aboumatar 2019 {published data only}
- Aboumatar H, Naqibuddin M, Chung S, Chaudhry H, Kim SW, Saunders J, et al. Effect of a program combining transitional care and long-term self-management support on outcomes of hospitalized patients with chronic obstructive pulmonary disease: a randomized clinical trial. JAMA 2018;320(22):2335-43. Retraction in: JAMA 2019; 322(14):1417-8. [DOI] [PubMed] [Google Scholar]
ACTRN12616001039471 {published data only}
- ACTRN12616001039471. Efficacy of cognitive behavioural therapy and/or simulation-based learning resources on the mental health of chronic obstructive pulmonary disease patients. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371204 (first received 1 August 2016).
Akinci 2011 {published data only}
- Akinci A, Olgun N. The effectiveness of nurse-led, home-based pulmonary rehabilitation in patients with COPD in Turkey. Rehabilitation Nursing 2011;36(4):159–65. [DOI] [PubMed] [Google Scholar]
Ali 2018 {published data only}
- Ali L, AF Fors, Ekman I. Belief in medication among people with chronic obstructive pulmonary disease and/or chronic heart failure. European Journal of Cardiovascular Nursing 2018;17(1 Suppl 1):21-2. [Google Scholar]
Altenburg 2015 {published data only}
- Altenburg WA, Ten Hacken NH, Bossenbroek L, Kerstjens HA, Greef MH, Wempe JB. Short- and long-term effects of a physical activity counselling programme in COPD: a randomized controlled trial. Respiratory Medicine 2015;109(1):112-21. [DOI] [PubMed] [Google Scholar]
Anonymous 2012 {published data only}
- Anonymous. Summaries for patients: vitamin D treatment of chronic obstructive pulmonary disease. Annals of Internal Medicine 2012;156(2):I26-I26. [DOI] [PubMed] [Google Scholar]
Ansari 2017 {published data only}
- Ansari S, Hosseinzadeh H, Dennis S, Zwar N. Empowering primary care patients with COPD in the context of multi-morbidity through the pilot APCOM self-management program. European Respiratory Journal 2017;50(Suppl 61):PA1604. [Google Scholar]
Apps 2013 {published data only}
- Apps LD, Mitchell KE, Harrison SL, Sewell L, Williams JE, Young HM, et al. The development and pilot testing of the Self-management Programme of Activity, Coping and Education for Chronic Obstructive Pulmonary Disease (SPACE for COPD). International Journal of COPD 2013;8:317-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arbillaga‐Etxarri 2018 {published data only}
- Arbillaga-Etxarri A, Gimeno-Santos E, Barberan-Garcia A, Balcells E, Benet M, Borrell E, et al. Long-term efficacy and effectiveness of a behavioural and community-based exercise intervention (Urban Training) to increase physical activity in patients with COPD: a randomised controlled trial. European Respiratory Journal 2018;52(4):1800063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barberan‐Garcia 2014 {published data only}
- Barberan-Garcia A, Vogiatzis I, Solberg HS, Vilaro J, Rodriguez DA, Garasen HM, et al. Effects and barriers to deployment of telehealth wellness programs for chronic patients across 3 European countries. Respiratory Medicine 2014;108(4):628-37. [DOI] [PubMed] [Google Scholar]
Barnestein‐Fonseca 2011 {published data only}
- Barnestein-Fonseca P, Leiva-Fernandez J, Vidal-Espana F, Garcia-Ruiz A, Prados-Torres D, Leiva-Fernandez F. Efficacy and safety of a multifactor intervention to improve therapeutic adherence in patients with chronic obstructive pulmonary disease (COPD): protocol for the ICEPOC study. Trials 2011;12:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baron 2011 {published data only}
- Baron K. COPD intervention investigation: comparing the effect of an outpatient counseling session after discharge to an educational counseling session on admission day 2 on hospitalization rates in patients with COPD. In: 50th Annual Assembly of the New York State Council of Health-System Pharmacists; Apr 29-May 1; Verona (NY). 2011.
Barradell 2017 {published data only}
- Barradell A, Sohanpal R, Pinnock H, Taylor S. Patient and Public Involvement (PPI) in TANDEM: a Tailored intervention for anxiety and depression management in COPD. European Respiratory Journal 2017;50(Suppl 61):PA942-PA942. [Google Scholar]
Barradell 2018 {published data only}
- Barradell A, Sohanpal R, Mammoliti K-M, Moore A, Pinnock H, Taylor SJ. Participant experiences in TANDEM feasibility pilot. European Respiratory Journal 2018;52(Suppl 62):PA924. [Google Scholar]
Basri 2017 {published data only}
- Basri R, Tahir M, Naseem M. Short-term effects of chest physiotherapy in acute exacerbation of chronic obstructive pulmonary disease. Journal of Medical Sciences 2017;25(3):323-7. [Google Scholar]
Bausewein 2012 {published data only}
- Bausewein C, Jolley C, Reilly C, Lobo P, Kelly J, Bellas H, et al. Development, effectiveness and cost-effectiveness of a new out-patient Breathlessness Support Service: study protocol of a phase III fast-track randomised controlled trial. BMC Pulmonary Medicine 2012;12(58):[10 p.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bavarsad 2015 {published data only}
- Bavarsad MB, Shariati A, Eidani E, Latifi M. The effect of home-based inspiratory muscle training on exercise capacity, exertional dyspnea and pulmonary function in COPD patients. Iranian journal of Nursing and Midwifery Research 2015;20(5):613-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beekman 2014 {published data only}
- Beekman E, Mesters I, Hendriks EJ, Muris JW, Wesseling G, Evers SM, et al. Exacerbations in patients with chronic obstructive pulmonary disease receiving physical therapy: a cohort-nested randomised controlled trial. BMC Pulmonary Medicine 2014;14(1):[10 p.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bentley 2014 {published data only}
- Bentley CL, Mountain GA, Thompson J, Fitzsimmons DA, Lowrie K, Parker SG, et al. A pilot randomised controlled trial of a Telehealth intervention in patients with chronic obstructive pulmonary disease: challenges of clinician-led data collection. Trials 2014;15(1):[12 p.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benzo 2017 {published data only}
- Benzo R, Novotny P, McEvoy C. Health coaching improves self management in COPD after a hospitalization. American Journal of Respiratory and Critical Care Medicine 2017;195(Meeting Abstracts):A6738. [Google Scholar]
Benzo 2019 {published data only}
- Benzo R, Hoult JP, Thomas BE, Lam N, Seifert S, Kramer K. Effect of home pulmonary rehab plus health coaching on COPD self-management: a randomized study. American Journal of Respiratory and Critical Care Medicine 2019;199(9):A5729. [Google Scholar]
Berkhof 2014 {published data only}
- Berkhof FF, Hesselink AM, Vaessen DL, Uil SM, Kerstjens HA, Van den Berg JW. The effect of an outpatient care on-demand-system on health status and costs in patients with COPD. A randomized trial. Respiratory Medicine 2014;108(8):1163-70. [DOI] [PubMed] [Google Scholar]
Berkhof 2015 {published data only}
- Berkhof FF, Van den Berg JW, Uil SM, Kerstjens HA. Telemedicine, the effect of nurse-initiated telephone follow up, on health status and health-care utilization in COPD patients: a randomized trial. Respirology 2015;20(2):279-85. [DOI] [PubMed] [Google Scholar]
Bhadhuri 2019 {published data only}
- Bhadhuri A, Al-Janabi H, Jowett S, Jolly K. Incorporating household spillovers in cost utility analysis: a case study using behavior change in COPD. International Journal of Technology Assessment in Health Care 2019;35:212-20. [DOI] [PubMed] [Google Scholar]
Bi 2021 {published data only}
- Bi J, Yang W, Hao P, Zhao Y, Wei D, Sun Y, et al. WeChat as a platform for baduanjin intervention in patients with stable chronic obstructive pulmonary disease in China: retrospective randomized controlled trial. MIR mHealth and uHealth 2021;9(2):e23548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Billington 2015 {published data only}
- Billington J, Coster S, Murrells T, Norman I. Evaluation of a nurse-led educational telephone intervention to support self-management of patients with chronic obstructive pulmonary disease: a randomized feasibility study. COPD 2015;12(4):395-403. [DOI] [PubMed] [Google Scholar]
Bischoff 2011 {published data only}
- Bischoff EW, Hamd DH, Sedeno M, Benedetti A, Schermer TR, Bernard S, et al. Effects of written action plan adherence on COPD exacerbation recovery. Thorax 2011;66(1):26-31. [DOI] [PubMed] [Google Scholar]
Blackstock 2016 {published data only}
- FC Blackstock, KE Webster, CF McDonald, Hill CJ. Emotional state associated with self-management behaviour in people with COPD. American Journal of Respiratory and Critical Care Medicine 2016;193:A3991. [Google Scholar]
Bohingamu 2018 {published data only}
- Bohingamu Mudiyanselage S, Stevens J, Watts JJ, Toscano J, Kotowicz MA, Steinfort CL, et al. Personalised telehealth intervention for chronic disease management: a pilot randomised controlled trial. Journal of Telemedicine and Telecare 2018;25(6):343-52. [DOI] [PubMed] [Google Scholar]
Boland 2014 {published data only}
- Boland M, Kruis A, Tsiachristas A, Assendelft W, Gusselkoo J, Blom C, et al. Cost-effectiveness of an integrated care program for COPD: the RECODE cluster randomized trial. European Respiratory Journal 2014;44(Suppl 58):1980. [Google Scholar]
- Boland M, Tsiachristas A, Chavannes NM, Rutten-van Molken M. Does registration of performance indicators improve health outcomes in COPD? European Respiratory Journal 2015;46:OA3286. [Google Scholar]
- Boland MR, Kruis A, Tsiachristas A, Assendelft W, Gussekloo J, Blom C, et al. Cost-effectiveness of a COPD disease management program in primary care: the RECODE cluster randomized trial. Value in Health 2014;17(7):A595. [DOI] [PubMed] [Google Scholar]
- Boland MR, Kruis AL, Tsiachristas A, Assendelft WJ, Gussekloo J, Blom CM, et al. Cost-effectiveness of integrated COPD care: the RECODE cluster randomised trial. BMJ Open 2015;5(10):e007284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bosma 2011 {published data only}
- Bosma H, Lamers F, Jonkers CC, Van Eijk JT. Disparities by education level in outcomes of a self-management intervention: the DELTA trial in the Netherlands. Psychiatric Services 2011;62(7):793-5. [DOI] [PubMed] [Google Scholar]
Bove 2016 {published data only}
- Bove DG, Lomborg K, Jensen AK, Overgaard D, Lindhardt BØ, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention in patients with advanced COPD: a randomised controlled trial. Thorax 2016;121:109-16. [DOI] [PubMed] [Google Scholar]
- Bove DG, Overgaard D, Lomborg K, Lindhardt BO, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention versus usual care for managing anxiety and dyspnoea in patients with severe chronic obstructive pulmonary disease: a randomised controlled trial protocol. BMJ Open 2015;5(7):e008031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DG Bove, Midtgaard J, Kaldan G, Overgaard D, Lomborg K. Home-based COPD psychoeducation: a qualitative study of the patients' experiences. Journal of Psychosomatic Research 2017;98:71-7. [DOI] [PubMed] [Google Scholar]
Bower 2012 {published data only}
- Bower P, Kennedy A, Reeves D, Rogers A, Blakeman T, Chew-Graham Carolyn, et al. A cluster randomised controlled trial of the clinical and cost-effectiveness of a 'whole systems' model of self-management support for the management of long- term conditions in primary care: trial protocol. Implementation Science 2012;7(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Browne 2013 {published data only}
- Browne P, Olive S, Staunton L, Clark A, Wilson E, Galey P, et al. The effects of maintenance schedules following pulmonary rehabilitation in patients with chronic obstructive pulmonary disease [Abstract]. In: British Thoracic Society Winter Meeting; 2013 Dec 4-6; London (UK). 2013.
Buckingham 2015 {published data only}
- Buckingham S, Kendall M, Ferguson S, Macnee W, Sheikh A, White P, et al. HELPing older people with very severe chronic obstructive pulmonary disease (HELP-COPD): mixed-method feasibility pilot randomised controlled trial of a novel intervention. npj Primary Care Respiratory Medicine 2015;25:15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bunker 2012 {published data only}
- Bunker JM, Reddel HK, Dennis SM, Middleton S, Van Schayck C, Crockett AJ, et al. A pragmatic cluster randomized controlled trial of early intervention for chronic obstructive pulmonary disease by practice nurse-general practitioner teams: study protocol. Implementation Science 2012;7:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cameron‐Tucker 2011 {published data only}
- Cameron-Tucker H, Joseph L, Edwards B, Wood-Baker RC. Telephone health-mentoring, a walking action plan and rehabilitation [Abstract]. Respirology 2011;16(Suppl 1):P33. [Google Scholar]
Cameron‐Tucker 2016 {published data only}
- Cameron-Tucker HL, Wood-Baker R, Joseph L, Walters JA, Schuz N, Walters EH. A randomized controlled trial of telephone-mentoring with home-based walking preceding rehabilitation in COPD. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:1991-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carcereny 2016 {published data only}
- Carcereny CH, Seijas N, Folch A, Roman M, Orts I, Aibar J, et al. Impact of therapeutic education program for COPD patients on preventing readmissions (APRENDEEPOC). European Respiratory Journal 2016;48(Suppl 60):PA1614. [Google Scholar]
Casanas 2019 {published data only}
- Casanas R, Martin Royo J, Fernandez-San-Martin MI, Raya Tena A, Mendioroz J, Sauch Valmana G, et al. Effectiveness of a psychoeducation group intervention conducted by primary healthcare nurses in patients with depression and physical comorbidity: study protocol for a randomized, controlled trial. BMC Health Services Research 2019;19(1):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Casas 2006 {published and unpublished data}
- Casas A, Troosters T, Garcia-Aymerich, Roca J, Hernández, Pozo F, et al. Integrated care prevents hospitalisations for exacerbations in COPD. European Respiratory Journal 2006;28(1):123-30. [DOI] [PubMed] [Google Scholar]
- Garcia-Aymerich J, Hernandez C, Alonso A, Casas A, Rodriguez-Roisin R, Anto J, et al. Effects of an integrated care intervention on risk factors of COPD readmission. Respiratory Medicine 2007;101:1462-9. [DOI] [PubMed] [Google Scholar]
Casey 2012 {published data only}
- Casey D, Murphy K, Cooney A, Devane D, McCarthy B, Kirwan C, et al. A cluster randomised controlled trial evaluating the effectiveness of a structured pulmonary rehabilitation education programme for improving the health status of people with chronic obstructive pulmonary disease (COPD): the PRINCE study. American Journal of Respiratory and Critical Care Medicine 2012;185:A2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey D, Murphy K, Declan D, Cooney A, McCarthy B, Mee L, et al. A cluster randomised controlled trial evaluating the effectiveness of a structured pulmonary rehabilitation education programme in a primary care setting for people with chronic obstructive pulmonary disease (COPD): the PRINCE study. European Respiratory Journal 2013;42(Suppl 57):396s. [Google Scholar]
Cecere 2012 {published data only}
- Cecere LM, Slatore CG, Uman JE, Evans LE, Udris EM, Bryson CL, et al. Adherence to long-acting inhaled therapies among patients with chronic obstructive pulmonary disease (COPD). COPD: Journal of Chronic Obstructive Pulmonary Disease 2012;9(3):251-8. [DOI] [PubMed] [Google Scholar]
Chan 2011 {published data only}
- Chan AW, Lee A, Suen LK, Tam WW. Tai chi Qigong improves lung functions and activity tolerance in COPD clients: a single blind, randomized controlled trial. Complementary Therapies in Medicine 2011;19(1):3-11. [DOI] [PubMed] [Google Scholar]
Chan 2016 {published data only}
- Chan H-Y, Dai Y-T, Hou I-C. Evaluation of a tablet-based instruction of breathing technique in patients with COPD. International Journal of Medical Informatics 2016;94:263-70. [DOI] [PubMed] [Google Scholar]
Chang 2019 {published data only}
- Chang YY, Dai YT. The efficacy of a flipping education program on improving self-management in patients with chronic obstructive pulmonary disease: a randomized controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2019;14:1239-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chatwin 2014 {published data only}
- Chatwin M, Hawkins G, Panicchia L, Woods A, Hanak A, Lucas R, et al. Randomised crossover trial of telemonitoring in chronic respiratory patients (TeleCRAFT trial). Thorax 2016;71(4):305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatwin M, Hawkins G, Paniccia L, Woods A, Lucas R, Hanak A, et al. Randomised crossover trial of telemonitoring in chronic respiratory patients (TeleCRAFT trial*): No impact on hospital admissions and quality of life (QOL). European Respiratory Journal 2014;44(Suppl 58):4830. [Google Scholar]
Chavannes 2009 {published data only}
- Chavannes NH, Grijsen M, den Akker M, Schepers H, Nijdam M, Tiep B, et al. Integrated disease management improves one-year quality of life in primary care COPD patients: a controlled clinical trial. Primary Care Respiratory Journal 2009;18(3):171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2011 {published data only}
- Chen J, Chen PC. The individualized smoking counselling for smoking cessation in COPD patients and general smokers. Respirology 2011;16(Suppl 2):116 [983]. [Google Scholar]
Cheng 2017 {published data only}
- Cheng SW, Alison J, Dennis S, Stamatakis E, Spencer L, McNamara R, et al. A behaviour change intervention to reduce sedentary time in people with chronic obstructive pulmonary disease: protocol for a randomised controlled trial. Journal of Physiotherapy 2017;63(3):182. [DOI] [PubMed] [Google Scholar]
Christenhusz 2012 {published data only}
- Christenhusz Lieke CA, Prenger R, Pieterse ME, Seydel ER, Van der Palen J. Cost-effectiveness of an intensive smoking cessation intervention for COPD outpatients. Nicotine and Tobacco Research 2012;14(6):657-63. [DOI] [PubMed] [Google Scholar]
Chuang 2011 {published and unpublished data}
- Chuang C, Levine SH, Rich J. Enhancing cost-effective care with a patient-centric chronic obstructive pulmonary disease program. Population Health Management 2011;14(3):133-6. [DOI] [PubMed] [Google Scholar]
Collinsworth 2018 {published and unpublished data}
- Collinsworth AW, Brown RM, James CS, Stanford RH, Alemayehu D, Priest EL. The impact of patient education and shared decision making on hospital readmissions for COPD. International Journal of Chronic Obstructive Pulmonary Disease 2018;13:1325-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cordova 2016 {published data only}
- Cordova FC, Ciccolella D, Grabianowski C, Gaughan J, Brennan K, Goldstein F, et al. A telemedicine-based intervention reduces the frequency and severity of COPD exacerbation symptoms: a randomized, controlled trial. Telemedicine Journal and E-Health 2016;22(2):114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coultas 2013 {published data only}
- Coultas D, Jackson B, Uhm M, Singh K, Bae S. Risk factors for self-reported hospitalizations among stable outpatients with COPD. European Respiratory Journal 2013;42:P5108. [Google Scholar]
Coultas 2014 {published data only}
- Coultas D, Russo R, Peoples J, Ashmore J, Sloan J, Jackson B, et al. Improvements in self-efficacy and readiness to engage in physical activity are associated with improved health outcomes among patients with COPD. European Respiratory Journal 2014;44(Suppl 58):3489. [Google Scholar]
Coultas 2016 {published data only}
- Ashmore J, Russo R, Peoples J, Sloan J, Jackson BE, Bae S, et al. Chronic obstructive pulmonary disease self-management activation research trial (COPD-SMART): design and methods. Contemporary Clinical Trials 2013;35(2):77-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BE Jackson, Coultas D, Russo R, Ashmore J, Sloan J, Uhm M, et al. Benefits of a lifestyle physical activity intervention for COPD are limited to patients with moderate impairment. American Journal of Respiratory and Critical Care Medicine 2015;191:A4448. [Google Scholar]
- Coultas D, Jackson B, Russo R, Peoples J, Ashmore J, Sloan J, et al. A lifestyle physical activity intervention for COPD improved mental health-related quality of life among severely impaired patients. American Journal of Respiratory and Critical Care Medicine 2017;195:A6733. [Google Scholar]
- Coultas D, Jackson B, Singh K, Bae S. Longitudinal change in self-reported physical activity (SRPA) is useful among patients with COPD. In: European Respiratory Journal. Vol. 50. 2017:PA3454.
- Coultas DB, Jackson BE, Russo R, Peoples J, Singh KP, Sloan J, et al. Home-based physical activity coaching, physical activity, and health care utilization in chronic obstructive pulmonary disease. Chronic obstructive pulmonary disease self-management activation research trial secondary outcomes. In: Annals of the American Thoracic Society. Vol. 15. 2018:470-8. [DOI] [PMC free article] [PubMed]
- Coultas DB, Jackson BE, Russo R, Peoples J, Sloan J, Singh KP, et al. A lifestyle physical activity intervention for patients with COPD: a randomized controlled trial. In: Annals of the American Thoracic Society. Vol. 13. 2016:617-26. [DOI] [PMC free article] [PubMed]
- Coultas DB, Jackson BE, Russo R, Peoples J, Sloan J, Singh KP, et al. A lifestyle physical activity intervention for patients with COPD: a randomized controlled trial. In: European Respiratory Journal. Vol. 13. 2016:617-26. [DOI] [PMC free article] [PubMed]
- Jackson BE, Coultas DB, Ashmore J, Russo R, Peoples J, Uhm M, et al. Domain-specific self-efficacy is associated with measures of functional capacity and quality of life among patients with moderate to severe chronic obstructive pulmonary disease. In: Annals of the American Thoracic Society. Vol. 11. 2014:310-15. [DOI] [PMC free article] [PubMed]
Coventry 2019 {published data only}
- Coventry PA, Blakemore A, Baker E, Sidhu M, Fitzmaurice D, Jolly K. The push and pull of self-managing mild COPD: an evaluation of participant experiences of a nurse-led telephone health coaching intervention. Qualitative Health Research 2019;29(5):658-71. [DOI] [PubMed] [Google Scholar]
Csikesz 2016 {published data only}
- Csikesz N, Nici LCC. A physician-led intervention to prevent COPD re-admissions. American Journal of Respiratory and Critical Care Medicine 2016;193:A3994. [Google Scholar]
Cully 2012 {published data only}
- Cully JA, Armento ME, Mott J, Nadorff MR, Naik AD, Stanley MA, et al. Brief cognitive behavioral therapy in primary care: a hybrid type 2 patient-randomized effectiveness-implementation design. Implementation Science 2012;7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cully 2017 {published data only}
- Cully JA, Stanley MA, Petersen NJ, Hundt NE, Kauth MR, Naik AD, et al. Delivery of brief cognitive behavioral therapy for medically ill patients in primary care: a pragmatic randomized clinical trial. Journal of General Internal Medicine 2017;32(9):1014-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dabrowska 2017 {published data only}
- Dabrowska M, Luczak K, Miszczuk M, Domagata I, Lubanski W, Leszczynski A, et al. Impact of short training of inhalation technique on the course of asthma and COPD. European Respiratory Journal 2017;50(Suppl 61):PA536. [Google Scholar]
Davis 2016 {published data only}
- Davis E, Marra C, Gamble J-M, Farrell J, Lockyer J, FitzGerald JM, et al. Effectiveness of a pharmacist-driven intervention in COPD (EPIC): study protocol for a randomized controlled trial. Trials 2016;17(1):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Jongh 2013 {published data only}
- De Jong C, Kocks J, Kerstjens H, Van der Molen T. Health status based treatment of COPD patients in primary care. A randomized controlled pilot study. European Respiratory Journal 2013;42(Suppl 57):26s [268]. [Google Scholar]
Demeyer 2017a {published data only}
- Demeyer H, Waschki B, Polkey M, Furlanetto K, Donaire-Gonzalez D, Anto JM, et al. The survival effect of physical activity in patients with COPD: every step counts. European Respiratory Journal 2017;50(Suppl 61):OA512. [Google Scholar]
Demeyer 2017b {published data only}
- Demeyer H, Louvaris Z, Frei A, Rabinovich RA, Jong C, Gimeno-Santos E, et al. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax 2017;72(5):415-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deng 2013 {published data only}
- Deng GJ, Liu FR, Zhong QL, Chen J, Yang MF, He HG. The effect of non-pharmacological staged interventions on fatigue and dyspnoea in patients with chronic obstructive pulmonary disease: a randomized controlled trial. International Journal of Nursing Practice 2013;19(6):636-43. [DOI] [PubMed] [Google Scholar]
De Roos 2018 {published data only}
- De Roos P, Lucas C, Strijbos JH, Van Trijffel E. Effectiveness of a combined exercise training and home-based walking programme on physical activity compared with standard medical care in moderate COPD: a randomised controlled trial. Physiotherapy 2018;104(1):116-21. [DOI] [PubMed] [Google Scholar]
De San Miguel 2013 {published data only}
- De San Miguel K, Smith J, Lewin G. Telehealth remote monitoring for community-dwelling older adults with chronic obstructive pulmonary disease. Telemedicine Journal and E-health 2013;19(9):652-7. [DOI] [PubMed] [Google Scholar]
Dewan 2011b {published data only}
- Dewan NA, Rice KL, Caldwell M, Hilleman DE. Economic evaluation of a disease management program for chronic obstructive pulmonary disease. Chest 2011;8(3):153-9. [DOI] [PubMed] [Google Scholar]
Dimitri 2012 {published data only}
- Dimitri A, Livermore N, Sharpe L, Gandevia SC, Mckenzie MK, Butler JE. CBT reduces ratings of breathing difficulty in response to external resistive loads in people with COPD. Respirology 2012;17(Suppl 1):32. [Google Scholar]
Dogan 2017 {published data only}
- Dogan U, Ovayolu N. The effects of health education given by nurses to COPD patients on the daily oxygen concentrator usage time. Advances in Respiratory Medicine 2017;85(1):15-21. [DOI] [PubMed] [Google Scholar]
Donesky 2012 {published data only}
- Donesky DM, Nguyen HQ, Benditt J, Carrieri-Kohlman V. Effects of a dyspnea self-management program on hospitalizations and urgent care in COPD. American Journal of Respiratory and Critical Care Medicine 2012;185:A4866. [Google Scholar]
Doward 2017 {published data only}
- Doward L, Svedsater H, Whalley D, Crawford R, Leather D, Lay-Flurrie J, et al. Salford Lung Study in chronic obstructive pulmonary disease (SLS COPD): follow-up interviews on patient-centred outcomes. npj Primary Care Respiratory Medicine 2017;27(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drennan 2014 {published data only}
- Drennan IR, Dainty KN, Hoogeveen P, Atzema CL, Barrette N, Hawker G, et al. Expanding paramedicine in the community (EPIC): study protocol for a randomized controlled trial. Trials 2014;15(1):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
DRKS00006021 {published data only}
- DRKS00006021. Influence of a controlled inspiratory muscle training on the course of COPD after acute exacerbation (AECOPD): a controlled study. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00006021 (first received 9 May 2014).
Due 2014 {published data only}
- Due TD, Thorsen T, Kousgaard MB, Siersma VD, Waldorff FB. The effectiveness of a semi-tailored facilitator-based intervention to optimise chronic care management in general practice: a stepped-wedge randomised controlled trial. BMC Family Practice 2014;15(1):1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Durheim 2014 {published data only}
- Durheim MT, Smith PJ, Babyak MA, Mabe SK, Emery CF, Blumenthal JA, et al. Physical function as measured by 6-minute walk distance or accelerometry predicts clinical outcomes in COPD patients independent of GOLD 2011. American Journal of Respiratory and Critical Care Medicine 2014;189:A6679. [Google Scholar]
Durheim 2015 {published data only}
- Durheim MT, Smith PJ, Babyak MA, Mabe SK, Martinu T, Welty-Wolf KE, et al. Six-minute-walk distance and accelerometry predict outcomes in chronic obstructive pulmonary disease independent of Global Initiative for Chronic Obstructive Lung Disease 2011 Group. Annals of the American Thoracic Society 2015;12(3):349-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwinger 2013 {published data only}
- Dwinger S, Dirmaier J, Herbarth L, Konig H-H, Eckardt M, Kriston L, et al. Telephone-based health coaching for chronically ill patients: study protocol for a randomized controlled trial. Trials 2013;14(1):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Effing 2009 {published data only}
- Effing T, Kerstjens H, Valk P, Zielhuis G, Palen J. (Cost)-effectiveness of self-treatment of exacerbations on the severity of exacerbations in patients with COPD: the COPE II study. Thorax 2009;64(11):956-62. [DOI] [PubMed] [Google Scholar]
Effing 2011 {published and unpublished data}
- Effing T, Zielhuis G, Kerstjens H, Van der Valk P, Van der Palen P. Community-based physiotherapeutic exercise in COPD self-management: a randomised controlled trial. Respiratory Medicine 2011;105(3):418-26. [DOI] [PubMed] [Google Scholar]
Emme 2014 {published data only}
- Emme C, Mortensen EL, Rydahl-Hansen S, Ostergaard B, Jakobsen AS, Schou L, et al. The impact of virtual admission on self-efficacy in patients with chronic obstructive pulmonary disease - a randomised clinical trial. Journal of Clinical Nursing 2014;23(21-22):3124-37. [DOI] [PubMed] [Google Scholar]
Etxarri 2017 {published data only}
- Etxarri AA, Gimeno-Santos E, Barberan-Garcia A, Rodriguez GA, Balcells E, Simonet P, et al. Effectiveness of an intervention of Urban Training in patients with COPD: a randomized controlled trial. European Respiratory Journal 2017;50(Suppl 61):OA513. [Google Scholar]
EUCTR2013‐002671‐18‐AT {published data only}
- EUCTR2013-002671-18-AT. To evaluate the effect of inhaled medication together with exercise and activity training on exercise capacity and daily activites in patients with chronic lung disease with obstruction of airways [An explorarory, 12 week, randomised, partially double-blinded, placebo-controlled parallel group trial to explore the effects of once daily treatments of orally inhaled tiotropium + olodaterol fixed dose combination or tiotropium (both delivered by Respimat® inhaler), supervised exercise training and behavior modification on exercise capacity and physical activity in patients with Chronic Obstructive Pulmonary Disease (COPD)]. www.clinicaltrialsregister.eu/ctr-search/trial/2013-002671-18/PT (first received 7 April 2014).
Fairbrother 2011 {published data only}
- Fairbrother P, Pinnock H, Hanley J, McCloughan L, Todd A, McKinstry B. Perspectives of patients and healthcare professionals on the impact of telemedicine on hospital admissions for chronic obstructive pulmonary disease (COPD): a nested qualitative study. European Respiratory Journal 2011;38:p4986. [Google Scholar]
- Fairbrother P, Pinnock H, Hanley J, McCloughan L, Todd A, McKinstry BC. Perspectives of patients and healthcare professionals on the impact of telemedicine on hospital admissions for chronic obstructive pulmonary disease (COPD): a nested qualitative study [Abstract]. In: 6th IPCRG World Conference; 2012 April 25-28; Edinburgh, Scotland. 2012:Abstract 209.
Fairbrother 2013 {published data only}
- Fairbrother P, Pinnock H, Hanley J, McCloughan L, Sheikh A, Pagliari C, et al. Exploring telemonitoring and self-management by patients with chronic obstructive pulmonary disease: a qualitative study embedded in a randomized controlled trial. Patient Education and Counseling 2013;93(3):403-10. [DOI] [PubMed] [Google Scholar]
Farmer 2014 {published data only}
- Farmer A, Toms C, Hardinge M, Williams V, Rutter H, Tarassenko L. Self-management support using an Internet-linked tablet computer (the EDGE platform)-based intervention in chronic obstructive pulmonary disease: protocol for the EDGE-COPD randomised controlled trial. BMJ Open 2014;4(1):e004437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer A, Williams V, Velardo C, Shah SA, Yu LM, Rutter H, et al. Self-management support using a digital health system compared with usual care for chronic obstructive pulmonary disease: randomized controlled trial. Journal of Medical Internet Research 2017;19(5):e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer A, Williams V, Velardo C, Shah SA, Yu L-M, Rutter H, et al. Self-management support using an internet-linked tablet computer based intervention in chronic obstructive pulmonary disease (EDGE): randomised controlled trial. npj Primary Care Respiratory Medicine 2016;26(16022):10-CR024. [Google Scholar]
Faulkner 2010 {published and unpublished data}
- Faulkner J, Walshaw E, Campbell J, Jones R, Taylor R, Price D, et al. The feasibility of recruiting patients with early COPD to a pilot trial assessing the effects of a physical activity intervention. Primary Care Respiratory Journal 2010;19(2):124-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferreira 2016 {published data only}
- Ferreira TJ, Harrison S, Carr J, Gershon A, Carr S, Fishbein D, et al. Can patients with COPD assimilate disease specific information at a time of being acutely unwell due to an exacerbation of their disease? European Respiratory Journal 2016;48(Suppl 60):OA4814. [Google Scholar]
Fish 2012 {published data only}
- Fish LJ, Gierisch JM, Stechuchak KM, Grambow SC, Rohrer LD, Bastian LA. Correlates of expected positive and negative support for smoking cessation among a sample of chronically ill veterans. Addictive Behaviors 2012;37(1):135. [DOI] [PubMed] [Google Scholar]
Fitzsimmons 2011 {published data only}
- Fitzsimmons DA, Thompson J, Hawley M, Mountain GA. Preventative tele-health supported services for early stage chronic obstructive pulmonary disease: a protocol for a pragmatic randomized controlled trial pilot. BMC Health Services Research 2011;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzsimmons 2016 {published data only}
- Fitzsimmons DA, Thompson J, Bentley CL, Mountain GA. Comparison of patient perceptions of Telehealth-supported and specialist nursing interventions for early stage COPD: a qualitative study. Trials 2016;16(1):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flink 2017 {published data only}
- Flink M, Lindblad M, Frykholm O, Kneck A, Nilsen P, Arestedt K, et al. The Supporting Patient Activation in Transition to Home (sPATH) intervention: a study protocol of a randomised controlled trial using motivational interviewing to decrease re-hospitalisation for patients with COPD or heart failure. BMJ Open 2017;7(7):e014178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Folch‐Ayora 2019 {published data only}
- Folch-Ayora A, Orts-Cortes MI, Macia-Soler L, Andreu-Guillamon MV, Moncho J. Patient education during hospital admission due to exacerbation of chronic obstructive pulmonary disease: effects on quality of life - controlled and randomized experimental study. Patient Education and Counseling 2019;102(3):51119. [DOI] [PubMed] [Google Scholar]
Foot 2017 {published data only}
- Foot H, Freeman C, Hemming K, Scott I, Coombes ID, Williams ID, et al. Reducing medical admissions into hospital through optimising medicines (REMAIN HOME) study: protocol for a stepped-wedge, cluster-randomised trial. BMJ Open 2017;7:e015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fors 2018 {published data only}
- Fors A, Blanck E, Ali L, Ekberg-Jansson A, Fu M, Lindstrom KI, et al. Effects of a person-centred telephone-support in patients with chronic obstructive pulmonary disease and/or chronic heart failure: a randomized controlled trial. PLOS One 2018;13(8):e0203031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fors A, Blanck E, Ali L, Swedberg K, Ekman I. Person-centred telephone-support is effective in patients with chronic obstructive pulmonary disease and/or chronic heart failure. Six-month follow-up of a randomized controlled trial. European Journal of Heart Failure 2018;20(Suppl 1):194. [Google Scholar]
Fortin 2013 {published data only}
- Fortin M, Chouinard MC, Bouhali T, Dubois MF, Gagnon C, Belanger M. Evaluating the integration of chronic disease prevention and management services into primary health care. BMC Health Services Research 2013;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freund 2016 {published data only}
- Freund T, Peters-Klimm F, Boyd CM, Mahler C, Gensichen J, Erler A, et al. Medical assistant-based care management for high-risk patients in small primary care practices: a cluster randomized clinical trial. Annals of Internal Medicine 2016;164(5):323-30. [DOI] [PubMed] [Google Scholar]
Frith 2017a {published data only}
- Frith P, Troosters T, Lavoie KL, Leidy N, Maltais F, Sedeno M, et al. Bronchodilator therapy and exercise added to self-management behaviour-modification: effects on physical activity in COPD. Respirology 2017;22(Suppl 2):84-5. [Google Scholar]
Frith 2017b {published data only}
- Frith P, Troosters T, Bourbeau J, Maltais F, Leidy N, Erzen D, et al. Effect of tiotropium and olodaterol, alone and with exercise training, on exercise endurance in COPD. Respirology 2017;22(Suppl 2):41-2. [Google Scholar]
Gaeckle 2016 {published data only}
- Gaeckle N, Ciccolella D, Criner A, Criner GC. Participation in a telemedicine program for chronic obstructive pulmonary disease improves daily symptoms. American Journal of Respiratory and Critical Care Medicine 2016;193:A1688. [Google Scholar]
Garcia‐Aymerich 2007 {published and unpublished data}
- Garcia-Aymerich J, Hernandez C, Alonso A, Casas A, Rodriguez-Roisin R, Anto JM, et al. Effects of an integrated care intervention on risk factors of COPD readmission. Respiratory Medicine 2007;101(7):1462-9. [DOI] [PubMed] [Google Scholar]
Gellis 2012 {published data only}
- Gellis ZD, Kenaley B, McGinty J, Bardelli E, Davitt J, Have TT. Outcomes of a telehealth intervention for homebound older adults with heart or chronic respiratory failure: a randomized controlled trial. Gerontologist 2012;52(4):541-52. [DOI] [PubMed] [Google Scholar]
Goossens 2014 {published data only}
- Goossens LM, Utens CM, Smeenk FW, Donkers B, Van Schayck OC, Rutten-van Molken MP. Should I stay or should I go home? A latent class analysis of a discrete choice experiment on hospital-at-home. Value in Health 2014;17(5):588-96. [DOI] [PubMed] [Google Scholar]
Goris 2013 {published data only}
- Goris S, Tasci S, Elmali F. The effects of training on inhaler technique and quality of life in patients with COPD. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2013;26(6):336-44. [DOI] [PubMed] [Google Scholar]
Grabenhorst 2013 {published data only}
- Grabenhorst M, Jehn M, Maldener N, Liebers U, Kohler F, Witt C. Telemedicine in patients with COPD: feasibility and benefit of regular exercise testing via remote patient monitoring [Abstract]. Pneumologie 2013;67:P377. [Google Scholar]
Granados‐Santiago 2019 {published data only}
- Granados-Santiago M, Valenza MC, Lopez-Lopez L, Prados-Roman E, Rodriguez-Torres J, Cabrera-Martos I. Shared decision-making and patient engagement program during acute exacerbation of COPD hospitalization: a randomized control trial. Patient Education and Counseling 2019;103(4):702-8. [DOI] [PubMed] [Google Scholar]
Gurgun 2011 {published data only}
- Gurgun A, Deniz S, Argin M, Karapolat HC. The effects of nutritional supplementation added to pulmonary rehabilitation in muscle wasted chronic obstructive pulmonary disease: a randomised, controlled, prospective study. European Respiratory Journal 2011;183:A3972. [DOI] [PubMed] [Google Scholar]
Hæsum 2017 {published data only}
- Hæsum LK, Ehlers LH, Hejlesen OK. The long-term effects of using telehomecare technology on functional health literacy: results from a randomized trial. Public Health 2017;150:43-50. [DOI] [PubMed] [Google Scholar]
He 2015 {published data only}
- He S, Niu M, Ni J. Application of health education path in health education of patients with chronic obstructive pulmonary disease. Chinese Nursing Research 2015;29(2B):552-5. [Google Scholar]
Heaton 2019 {published data only}
- Heaton PC, Frede S, Kordahi A, Lowery L, Moorhead B, Kirby J, et al. Improving care transitions through medication therapy management: a community partnership to reduce readmissions in multiple health-systems. Journal of the American Pharmacists Association 2019;59(3):319-28. [DOI] [PubMed] [Google Scholar]
Hegelund 2019 {published data only}
- Hegelund A, Andersen IC, Andersen MN, Bodtger U. The impact of a personalised action plan delivered at discharge to patients with COPD on readmissions: a pilot study. Scandinavian Journal of Caring Sciences 2020;34(4):909-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heslop‐Marshall 2018 {published data only}
- Heslop K, Newton J, Baker C, Burns G, Carrick-Sen D, De Soyza A. Effectiveness of cognitive behavioural therapy (CBT) interventions for anxiety in patients with chronic obstructive pulmonary disease (COPD) undertaken by respiratory nurses: the COPD CBT CARE study: (ISRCTN55206395). BMC Pulmonary Medicine 2013;13(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop K, Stenton C, Jambon M, Newton J, Carrick-Sen D, Baker C, et al. Recruitment for psychological therapy in chronic obstructive pulmonary disease (COPD). What factors influence participation? European Respiratory Journal 2013;42(Suppl 57):P3655. [Google Scholar]
- Heslop K, Stenton C, Newton J, Carrick-Sen D, Baker C, Graham B, et al. A randomised controlled trial of cognitive behavioural therapy (CBT) delivered by respiratory nurses to reduce anxiety in chronic obstructive pulmonary disease (COPD). European Respiratory Journal 2016;Suppl 60:OA289. [Google Scholar]
- Heslop-Marshall K, Baker C, Carrick-Sen D, Newton J, Echevarria C, Stenton C, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Research 2018;4:00094-2018. [DOI: 10.1183/23120541.00094-2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop-Marshall K, Baker C, Carrick-Sen D, Newton J, Stenton C, Burns G, et al. A randomised controlled trial (RCT) of cognitive behavioural therapy (CBT) for patients with chronic obstructive pulmonary disease. Thorax 2017;72(Suppl 3):A6. [Google Scholar]
- Heslop-Marshall K, Baker C, Carrick-Sen D, Stenton SC, Newton J, Burns GP, et al. Prevalence of anxiety and patient characteristics from a randomised controlled trial (RCT) to identify if cognitive behavioural therapy (CBT) by respiratory nurses reduces anxiety in COPD. Thorax 2015;70:A237. [Google Scholar]
Hilberink 2011 {published data only}
- Hilberink SR, Jacobs JE, Breteler MH, De Vries H, Grol RP. General practice counseling for patients with chronic obstructive pulmonary disease to quit smoking: impact after 1 year of two complex interventions. Patient Education and Counseling 2011;83(1):120-4. [DOI] [PubMed] [Google Scholar]
Ho 2016 {published data only}
- Ho TW, Huang CT, Chiu HC, Ruan SY, Tsai YJ, Yu CJ, et al. Effectiveness of telemonitoring in patients with chronic obstructive pulmonary disease in Taiwan - a randomized controlled trial. Scientific Reports 2016;6:23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Houben 2014 {published data only}
- Houben CH, Spruit MA, Wouters EF, Janssen DJ. A randomised controlled trial on the efficacy of advance care planning on the quality of end-of-life care and communication in patients with COPD: the research protocol. BMJ Open 2014;4(1):e004465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Houben 2019 {published data only}
- Houben CH, Spruit MA, Luyten H, Pennings HJ, Van den Boogaart VE, Creemers JP, et al. Cluster-randomised trial of a nurse-led advance care planning session in patients with COPD and their loved ones. Thorax 2019;74(4):328-36. [DOI] [PubMed] [Google Scholar]
Howard 2014 {published data only}
- Howard C, Dupont S. 'The COPD breathlessness manual': a randomised controlled trial to test a cognitive-behavioural manual versus information booklets on health service use, mood and health status, in patients with chronic obstructive pulmonary disease. npj Primary Care Respiratory Medicine 2014;24:14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2017 {published data only}
- Huang B, Willard-Grace R, De Vore D, Wolf J, Chirinos C, Tsao S, et al. Correction to: health coaching to improve self-management and quality of life for low income patients with chronic obstructive pulmonary disease (COPD): protocol for a randomized controlled trial. BMC Pulmonary Medicine 2019;19(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Willard-Grace R, De Vore D, Wolf J, Chirinos C, Tsao S, et al. Health coaching to improve self-management and quality of life for low income patients with chronic obstructive pulmonary disease (COPD): protocol for a randomized controlled trial. BMC Pulmonary Medicine 2017;17(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN30110012 {published data only}
- ISRCTN30110012. Evaluating a group-based maintenance self-management intervention for patients with COPD. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN30110012 (first received 12 August 2019).
ISRCTN32281812 {published data only}
- ISRCTN32281812. Effectiveness of a pharmacist intervention in patients with lung disease. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN32281812 (first received 17 May 2017).
ISRCTN77785397 {published data only}
- ISRCTN77785397. Using the internet to help individuals stay healthy and prevent further reductions in health from existing chronic diseases [Maximizing the effects of self-management interventions on chronic disease outcomes: the development of a chronic obstructive pulmonary disease (COPD) web-based patient portal]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN77785397 (first received 8 November 2011).
Janaudis‐Ferreira 2018 {published data only}
- Janaudis-Ferreira T, Carr SJ, Harrison SL, Gershon AS, Milner SC, Carr S, et al. Can patients with COPD assimilate disease-specific information during an acute exacerbation? Results of a pilot randomized controlled trial. Chest 2018;3:588-96. [DOI] [PubMed] [Google Scholar]
Jarab 2012 {published data only}
- Jarab AS, Alqudah SG, Khdour M, Shamssain M, Mukattash TL. Impact of pharmaceutical care on health outcomes in patients with COPD. International Journal of Clinical Pharmacy 2012;34(1):53-62. [DOI] [PubMed] [Google Scholar]
Jennings 2015 {published data only}
- Jennings JH, Thavarajah K, Mendez MP, Eichenhorn M, Kvale P, Yessayan L. Predischarge bundle for patients with acute exacerbations of COPD to reduce readmissions and ED visits: a randomized controlled trial. Chest 2015;147(5):1227-34. [DOI] [PubMed] [Google Scholar]
Ji 2019 {published data only}
- Ji M, Wang A-H, Ye J, Shen Y-H, Chen C-M, Yu C, et al. Effects of the health belief model following acute exacerbation of chronic obstructive pulmonary disease in a hospital in China. Journal of Thoracic Disease 2019;11(8):3593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jokar 2012 {published data only}
- Jokar Z, Mohammadi F, Khankeh H, Fallah Tafti S. Effect of home-based pulmonary rehabilitation on fatigue in patients with COPD. HAYAT 2012;18(5):64-72. [Google Scholar]
Jonkers 2012 {published data only}
- Jonkers CC, Lamers F, Bosma H, Metsemakers JF, Van Eijk JT. The effectiveness of a minimal psychological intervention on self-management beliefs and behaviors in depressed chronically ill elderly persons: a randomized trial. International Psychogeriatrics 2012;24(2):288-97. [DOI] [PubMed] [Google Scholar]
Kalter‐Leibovici 2018 {published data only}
- Kalter-Leibovici O, Benderly M, Freedman LS, Kaufman G, Molcho Falkenberg Luft T, Murad H, et al. Disease management plus recommended care versus recommended care alone for ambulatory COPD patients. China NLM 2018;197(12):1565-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanabar 2015 {published data only}
- Kanabar P, Warrington V, Houchen-Wolloff L, Singh S. Investigating the profile of physical activity in COPD patients 7 days post discharge from a respiratory-related admission. Does brief advice have an effect? American Journal of Respiratory and Critical Care Medicine 2015;70:A146. [Google Scholar]
Kara 2004 {published data only}
- Kara M, Asti T. Effects of education on self-efficacy of Turkish patients with chronic obstructive pulmonary disease. Patient Education and Counseling 2004;55(1):114-20. [DOI] [PubMed] [Google Scholar]
Kato 2017 {published data only}
- Kato D, Dobashi K, Fueki M, Tomioka S, Yamada H, Fueki N. Short-term and long-term effects of a self-managed physical activity program using a pedometer for chronic respiratory disease: a randomized controlled trial. Journal of Physical Therapy Science 2017;29:807-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kenealy 2015 {published data only}
- Kenealy TW, Parsons MJ, Rouse AP, Doughty RN, Sheridan NF, Harre Hindmarsh JK, et al. Telecare for diabetes, CHF or COPD: effect on quality of life, hospital use and costs. A randomised controlled trial and qualitative evaluation. PLOS One 2015;10(3):e0116188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kennedy 2013 {published data only}
- Kennedy A, Bower P, Reeves D, Blakeman T, Bowen R, Chew-Graham C, et al. Implementation of self management support for long term conditions in routine primary care settings: cluster randomised controlled trial. BMJ 2013;346(7913):f2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2019 {published data only}
- Khan MA, Khan N, Walley JD, Hicks J, Ahmed M, Sheikh FI, et al. Effectiveness of delivering integrated COPD care at public healthcare facilities: a cluster randomised trial in Pakistan. BJGP Open 2019;3(1):[11 p.]. [DOI: 10.3399/bjgpopen18X101634] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kheirabadi 2008 {published data only}
- Kheirabadi GR, Keypour M, Attaran N, Bagherian R, Maracy MR. Effect of add-on "self-management and behaviour modification" education on severity of COPD. Tanaffos 2008;7(3):23-30. [Google Scholar]
Khoshkesht 2015 {published data only}
- IRCT138905064443N2. The effect of pulmonary rehabilitation program on self-efficacy and severity of symptoms among patients with chronic obstructive pulmonary disease. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=IRCT138905064443N2 (first received 14 May 2011).
- Khoshkesht S, Zakerimoghadam M, Ghiyasvandian S, Kazemnejad A, Hashemian M. The effect of home-based pulmonary rehabilitation on self-efficacy in chronic obstructive pulmonary disease patients. Journal of the Pakistan Medical Association 2015;65(10):1041-6. [PubMed] [Google Scholar]
- Moghadam MZ, Ghiyasvandian S, Kazemnejad A, Hashemian M, Khoshkesht S. Effect of pulmonary rehabilitation program on the intensity of fatigue and dyspnea of patients with chronic obstructive pulmonary disease. Iranian Journal of Allergy, Asthma, and Immunology 2013;12(9S):S139. [Google Scholar]
Kiser 2012 {published data only}
- Kiser K, Jonas D, Warner Z, Scanlon K, Bryant SB, DeWalt DA. A randomized controlled trial of a literacy-sensitive self-management intervention for chronic obstructive pulmonary disease patients. Journal of General Internal Medicine 2012;27(2):190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ko 2016 {published data only}
- Ko FW, Cheung NK, Rainer TH, Lum C, Wong I, Hui DS. Comprehensive care programme for patients with chronic obstructive pulmonary disease: a randomised controlled trial. Thorax 2016;72(2):122-8. [DOI] [PubMed] [Google Scholar]
Koff 2009 {published and unpublished data}
- Koff PB, Jones RH, Cashman JM, Voelkel NF, Vandivier RW. Proactive integrated care improves quality of life in patients with COPD. European Respiratory Journal 2009;33(5):1031-8. [DOI] [PubMed] [Google Scholar]
Korsbakke 2016 {published data only}
- Korsbakke LK, Ehlers L, Hejlesen OK. Interaction between functional health literacy and telehomecare: short-term effects from a randomized trial. Nursing and Health Sciences 2016;18(3):328-33. [DOI] [PubMed] [Google Scholar]
Kruis 2011 {published data only}
- Kruis A, Boland M, Assendelft P, Gussekloo J, Tsiachristas A, Rutten M, et al. RECODE: RCT on effectiveness of integrated COPD management in primary care. European Respiratory Journal 2011;38(55):912s [P4990]. [Google Scholar]
Kruis 2014a {published data only}
- Kruis A, Boland M, Assendelft W, Gussekloo J, Tsiachristas A, Stijnen T, et al. Is integrated disease management of COPD effective? Results of the RECODE cluster randomized controlled trial in real world patients. European Respiratory Journal 2014;44(Suppl 58):4413. [Google Scholar]
- Kruis AL, Boland M, Assendelft WJ, Gussekloo J, Tsiachristas A, Stijnen T. Is integrated disease management of COPD effective? Results of the RECODE cluster randomized controlled trial in real world patients. In: 7th International Primary Care Respiratory Group (IPCRG) World Conference; 2014 May 21 - 24 May; Athens, Greece. 2014.
Kruis 2014b {published data only}
- Kruis AL, Boland MR, Assendelft WJ, Gussekloo J, Tsiachristas A, Stijnen T, et al. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: results of cluster randomised trial. BMJ 2014;349(7976):g5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruis AL, Boland MR, Assendelft WJ, Gussekloo J, Tsiachristas A, Stijnen T, et al. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: results of cluster randomised trial. Nederlands Tijdschrift voor Geneeskunde 2015;159(13):A8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Labrecque 2011 {published data only}
- Labrecque M, Rabhi K, Laurin C, Favreau H, Moullec G, Lavoie K, et al. Can a self-management education program for patients with chronic obstructive pulmonary disease improve quality of life? Canadian Respiratory Journal 2011;18(5):e77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moullec G, Favreau H, Lavoie KL, Labrecque M. Does a self-management education program have the same impact on emotional and functional dimensions of HRQoL? Journal of Chronic Obstructive Pulmonary Disease 2012;9(1):36-45. [DOI] [PubMed] [Google Scholar]
Lahham 2018 {published data only}
- Lahham A, McDonald CF, Mahal A, Lee AL, Hill CJ, Burge AT, et al. Home-based pulmonary rehabilitation for people with COPD: a qualitative study reporting the patient perspective. Chronic Respiratory Disease 2018;15(2):123-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lainscak 2013 {published data only}
- Lainscak M, Kadivec S, Kosnik M, Benedik B, Bratkovic M, Jakhel T, et al. Discharge coordinator intervention prevents hospitalizations in patients with COPD: a randomized controlled trial. Journal of the American Medical Directors Association 2013;14(6):450.e1-e6. [DOI] [PubMed] [Google Scholar]
Lam 2011 {published data only}
- Lam A. Practice innovations: delivering medication therapy management services via Videoconference interviews. Consultant Pharmacist 2011;26(10):764-74. [DOI] [PubMed] [Google Scholar]
Larson 2017a {published data only}
- Larson J, Tucker S, Buckery D. Increasing light physical activity and decreasing sedentary time in people with chronic obstructive pulmonary disease - a preliminary study. Respirology 2017;22(Suppl 3):80. [Google Scholar]
Larson 2017b {published data only}
- Larson JL, Han MK, McAuley E. Active for life with COPD: preliminary study. American Journal of Respiratory and Critical Care Medicine 2017;195:A4270. [Google Scholar]
La Torre 2018 {published data only}
- La Torre G, Cocchiara RA, Sordo EL, Chiarini M, Siliquini R, Firenze A, et al. Counseling intervention to improve quality of life in patients with pre-existing acute myocardial infarction (AMI) or chronic obstructive pulmonary disease (COPD): a pilot study. Journal of Preventive Medicine and Hygiene 2018;59(2):E153-8. [PMC free article] [PubMed] [Google Scholar]
Lavesen 2012 {published data only}
- Lavesen M, Overgaard R, Mazurek S, Just A, Overgaard D. Do telephone interventions of patients with COPD prevent readmission? European Respiratory Journal 2012;40(Suppl 56):220s [P1203]. [Google Scholar]
Lavesen 2016 {published data only}
- Lavesen M, Ladelund S, Frederiksen AJ, Lindhardt BO, Overgaard D. Nurse-initiated telephone follow-up on patients with chronic obstructive pulmonary disease improves patient empowerment, but cannot prevent readmissions. Danish Medical Journal 2016;63(10):A5276. [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee H, Yoon JY, Lim Y, Jung H, Kim S, Yoo Y, et al. The effect of nurse-led problem-solving therapy on coping, self-efficacy and depressive symptoms for patients with chronic obstructive pulmonary disease: a randomised controlled trial. Age and Ageing 2015;44(3):397-403. [DOI] [PubMed] [Google Scholar]
Leiva‐Fernandez 2011 {published data only}
- Leiva-Fernandez F, Barnestein-Fonseca P, Leiva-Fernandez J, Vidal-Espana F, Garcia-Ruiz A, Prados-Torres D. Effectiveness of a multifactorial intervention to improve adherence in patients with chronic obstructive pulmonary disease (COPD) icepoc study. Value in Health 2011;14(7):A487-8. [Google Scholar]
Leiva‐Fernandez 2012 {published data only}
- Leiva-Fernandez F, Leiva-Fernandez J, Zubeldia-Santoyo F, Garcia-Ruiz A, Prados-Torres D, Barnestein-Fonseca P. Efficacy of two educational interventions about inhalation techniques in patients with chronic obstructive pulmonary disease (COPD). TECEPOC: study protocol for a partially randomized controlled trial (preference trial). Trials 2012;13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leiva‐Fernandez 2014 {published data only}
- Leiva-Fernandez J, Leiva-Fernandez F, Garcia-Ruiz A, Prados-Torres D, Barnestein-Fonseca P. Efficacy of a multifactorial intervention on therapeutic adherence in patients with chronic obstructive pulmonary disease (COPD): a randomized controlled trial. BMC Pulmonary Medicine 2014;14(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li J 2015 {published data only}
- Li J. Study on TCM nursing on the improvement of pulmonary function of patients with COPD in stable stage. Chinese Medicine Modern Distance Education of China 2015;13(14):120-1. [Google Scholar]
Lilholt 2015 {published data only}
- PH Lilholt, LK Hæsum, OK Hejlesen. Exploring user experience of a telehealth system for the Danish TeleCare North Trial. Studies in Health Technology and Informatics 2015;210:301-5. [PubMed] [Google Scholar]
Lilholt 2016 {published data only}
- Lilholt PH, Hæsum LK, Ehlers LH, Hejlesen OK. Specific technological communication skills and functional health literacy have no influence on self-reported benefits from enrollment in the TeleCare North trial. International Journal of Medical Informatics 2016;91:60-6. [DOI] [PubMed] [Google Scholar]
Li P 2015 {published data only}
- Li P, Gong Y, Zeng G, Ruan L, Li G. A new mode of community continuing care service for COPD patients in China: Participation of respiratory nurse specialists. International Journal of Clinical and Experimental Medicine 2015;8(9):15878-88. [PMC free article] [PubMed] [Google Scholar]
Li Z 2015 {published data only}
- Li Z, Zeng K, He X. Study on influence of cognitive-behavioral intervention on quality of life of COPD patients with chronic obstructive pulmonary disease. Chinese Medicine Modern Distance Education of China 2015;29(8A):2714-16. [Google Scholar]
Lopez‐Lopez 2019 {published data only}
- Lopez-Lopez L, Valenza MC, Rodriguez-Torres J, Torres-Sanchez I, Granados-Santiago M, Valenza-Demet G. Results on health-related quality of life and functionality of a patient-centered self-management program in hospitalized COPD: a randomized control trial. Disability and Rehabilitation 2019;42(25):3687-95. [DOI] [PubMed] [Google Scholar]
Luhr 2018 {published data only}
- Luhr K, Eldh AC, Theander K, Holmefur M. Effects of a self-management programme on patient participation in patients with chronic heart failure or chronic obstructive pulmonary disease: a randomized controlled trial. European Journal of Cardiovascular Nursing 2018;18(3):185-93. [DOI] [PubMed] [Google Scholar]
Marchioro 2011 {published data only}
- Marchioro JC, Belmonte G, Pradela C, Maia MN, Nascimento OA, Jardim JR. Effects of home-based pulmonary rehabilitation in COPD patients - adaptation to patients' real life. American Journal of Respiratory and Critical Care Medicine 2011;183(1 MeetingAbstracts):A6438. [Google Scholar]
Maricoto 2019 {published data only}
- Maricoto T, Correia-de-Sousa J, Taborda-Barata L. Inhaler technique education in elderly patients with asthma or COPD: impact on disease exacerbations - a protocol for a single-blinded randomised controlled trial. BMJ Open 2019;9(1):e022685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 2014 {published data only}
- Martinez CH, Moy ML, Nguyen HQ, Cohen MD, Kadri R, Roman P, et al. Internet-mediated recruitment of rural veterans in a randomized controlled trial of a walking program for COPD (abstract). American Journal of Respiratory and Critical Care Medicine 2014;189:A5362. [Google Scholar]
Martinez 2019 {published data only}
- Martinez IM, Alonso PC, Sanchez RL, Arce RA, Diaz PP, Arboleya AL. Effectiveness of a brief educational intervention relating to the correct use of inhalers on the prevention of exacerbation in patients suffering from chronic obstructive pulmonary disease. SEMERGEN 2019;45(1):15-22. [DOI] [PubMed] [Google Scholar]
McDonald 2011 {published data only}
- McDonald VM, Higgins I, LG Wood, Gibson PG. Multidimensional assessment and individualised management (MDAIM) of obstructive airway diseases (OAD) in older adults - a pilot clinical trial. Respirology 2011;16(Suppl 1):P13 [TO 019]. [Google Scholar]
Moayeri 2019 {published data only}
- Moayeri F, Dunt D, Hsueh Y-SA, Doyle C. Cost-utility analysis of telephone-based cognitive behavior therapy in chronic obstructive pulmonary disease (COPD) patients with anxiety and depression comorbidities: an application for willingness to accept concept. Rehabilitation Nursing 2019;19(3):331-40. [DOI] [PubMed] [Google Scholar]
Monninkhof 2003 {published and unpublished data}
- Monninkhof E, Van der Valk P, Schermer T, Van der Palen J, Van Herwaarden C, Zielhuis G. Economic evaluation of a comprehensive self-management programme in patients with moderate to severe chronic obstructive pulmonary disease. Chronic Respiratory Disease 2004;1(1):7-16. [DOI] [PubMed] [Google Scholar]
- Monninkhof E, Van der Valk P, Van der Palen J, Van Herwaarden C, Zielhuis G. Effects of a comprehensive self-management programme in patients with chronic obstructive pulmonary disease. European Respiratory Journal 2003;22(5):815-20. [DOI] [PubMed] [Google Scholar]
Morganroth 2011 {published data only}
- Morganroth ML, Johnson T, Pape G, Rozenfeld Y, Siemienczuk J, Heffner JE. An integrated pulmonary and primary care COPD disease management program: impact on clinical outcomes. American Journal of Respiratory and Critical Care Medicine 2011;183:A2264. [Google Scholar]
Morganroth 2016 {published data only}
- Morganroth M, Pape G, Rozenfeld Y, Heffner JE. Multidisciplinary COPD disease management program: impact on clinical outcomes. Postgraduate Medicine 2016;128(2):239-49. [DOI] [PubMed] [Google Scholar]
Moriyama 2015 {published data only}
- Moriyama M, Takeshita Y, Haruta Y, Hattori N, Ezenwaka CE. Effects of a 6-month nurse-led self-management program on comprehensive pulmonary rehabilitation for patients with COPD receiving home oxygen therapy. Rehabilitation Nursing 2015;40(1):40-51. [DOI] [PubMed] [Google Scholar]
Moullec 2008 {published and unpublished data}
- Moullec G, Ninot G, Varray A, Desplan J, Hayot M, Prefaut C. An innovative maintenance follow-up program after a first inpatient pulmonary rehabilitation. Respiratory Medicine 2008;102(4):556-66. [DOI] [PubMed] [Google Scholar]
- Moullec G, Ninot G. An integrated programme after pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: effect on emotional and functional dimensions of quality of life. Clinical Rehabilitation 2010;24(2):122-36. [DOI] [PubMed] [Google Scholar]
Moy 2015a {published data only}
- Moy ML, Martinez CH, Kadri R, Roman P, Holleman RG, Kim HM, et al. Long-term effects of an internet-mediated pedometer-based walking program in COPD: a randomized controlled trial. Journal of Medical Internet Research 2015;191(Meeting Abstracts):A2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moy 2015b {published data only}
- Moy ML, Collins RJ, Martinez CH, Kadri R, Roman P, Holleman RG, et al. An internet-mediated pedometer-based program improves health-related quality-of-life domains and daily step counts in COPD: a randomized controlled trial. Chest 2015;148(1):128-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moy 2016 {published data only}
- Moy ML, Martinez CH, Kadri R, Roman P, Holleman RG, Kim HM, et al. Long-term effects of an Internet-mediated pedometer-based walking program for chronic obstructive pulmonary disease: randomized controlled trial. Journal of Medical Internet Research 2016;18(8):e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mozaffari 2018 {published data only}
- Mozaffari M, Azami S, Naderi M. Determining the effect of implementing an educational package on quality of life among patients with chronic obstructive pulmonary disease referring to teaching hospitals affiliated with Ilam University of Medical Sciences in 2016. Journal of Family Medicine and Primary Care 2018;7(3):606-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2011 {published data only}
- Murphy K, Casey D, Devane D, Cooney A, McCarthy B, Mee L, et al. A cluster randomised controlled trial evaluating the effectiveness of a structured pulmonary rehabilitation education programme for improving the health status of people with chronic obstructive pulmonary disease (COPD): the PRINCE Study protocol. BMC Pulmonary Medicine 2011;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Casey D, Devane D, Cooney A, McCarthy B, Mee L, et al. The effectiveness of a structured education pulmonary rehabilitation programme for improving the health status of people with Chronic Obstructive Pulmonary Disease (COPD): the PRINCE study. Irish Journal of Medical Science 2011;180(Suppl 12):S457. [Google Scholar]
NCT01543217 {published data only}
- NCT01543217. Use of a respiratory care practitioner disease management (RCP-DM) program for patients hospitalized with COPD. clinicaltrials.gov/ct2/show/NCT01543217 (first received 12 March 2012).
NCT01867970 {published data only}
- NCT01867970. Interactive tool to support self-management through lifestyle feedback, aimed at physical activity of COPD/DM patients (RCTIt'sLiFe!) [RCT It's LiFe! to evaluate the effectiveness of the monitoring and feedback tool and the corresponding counseling protocol (self-management support program) to be executed by practice nurses in primary care]. clinicaltrials.gov/show/NCT01867970 (first received 4 June 2013).
NCT01871025 {published data only}
- NCT01871025. Early incentive and mobilization during COPD exacerbation (TIME). clinicaltrials.gov/show/nct01871025 (first received 6 June 2013).
NCT01897298 {published data only}
- NCT01897298. Urban training for COPD patients [Effectiveness of an intervention of urban training in patients with chronic obstructive pulmonary disease (COPD): a randomised controlled trial]. clinicaltrials.gov/show/NCT01897298 (first received 11 July 2013).
NCT01921556 {published data only}
- NCT01921556. The improving care in chronic obstructive lung disease study a cluster randomized trial (CAROL) [The improving care in chronic obstructive lung disease study: CAROL improving processes of care and quality of life of COPD patients in primary care: a cluster randomized trial]. clinicaltrials.gov/show/NCT01921556 (first received 13 August 2013).
NCT01985529 {published data only}
- NCT01985529. A controlled study of community-based exercise training in patients with moderate COPD. clinicaltrials.gov/show/NCT01985529 (first received 15 November 2013).
NCT02035566 {published data only}
- NCT02035566. Telehome monitoring for chronic disease management [Effectiveness of telehome monitoring on quality of life and health resources utilization among people with chronic disease residing in rural Maryland]. clinicaltrials.gov/ct2/show/NCT02035566 (first received 14 January 2014).
NCT02078622 {published data only}
- NCT02078622. Use of respiratory therapists (RTs) to improve outcomes and quality of life in patients with COPD (RTQOL) [A prospective randomized controlled trial to evaluate the use of respiratory therapists (RTs) to improve outcomes and quality of life in patients diagnosed with COPD]. clinicaltrials.gov/show/nct02078622 (first received 5 March 2014).
NCT02085161 {published data only}
- NCT02085161. To evaluate the effect of inhaled medication together with exercise and activity training on exercise capacity and daily activities in patients with chronic lung disease with obstruction of airways. clinicaltrials.gov/show/nct02085161 (first received 12 March 2014).
NCT02567474 {published data only}
- NCT02567474. Effect of self management program on clinical status of COPD patients [Effect of self management program based on 5A model on clinical status of chronic obstructive pulmonary disease]. clinicaltrials.gov/show/NCT02567474 (first received 5 October 2015).
NCT02742597 {published data only}
- NCT02742597. Patient-centred innovations for persons with multimorbidity - Ontario (PACEinMM-ON). clinicaltrials.gov/show/nct02742597 (first received 19 April 2016).
NCT02754232 {published data only}
- NCT02754232. Telemedical training for chronically ill COPD patients: a cross sectoral study. clinicaltrials.gov/show/nct02754232 2016.
NCT03387735 {published data only}
- NCT03387735. Multiple chronic conditions for older adults [Heart-related multiple chronic conditions in primary care: behavioral technology]. clinicaltrials.gov/show/nct03387735 (first received 2 January 2017).
NCT03654092 {published data only}
- NCT03654092. Home-based exercise training for COPD patients (HOMEX-2) [Effects of a long-term home-based exercise training program using minimal equipment vs usual care in COPD patients: a multicenter randomized controlled trial]. clinicaltrials.gov/show/nct03654092 (first received 31 August 2018).
Ng 2017 {published data only}
- Ng WI, Smith GD. Effects of a self-management education program on self-efficacy in patients with COPD: a mixed-methods sequential explanatory designed study. International Journal of COPD 2017;12:2129-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2008 {published data only}
- Nguyen HQ, Donesky-Cuenco D, Wolpin S, Reinke LF, Benditt JO, Paul SM, et al. Randomized controlled trial of an internet-based versus face-to-face dyspnea self-management program for patients with chronic obstructive pulmonary disease: pilot study. Journal of Medical Internet Research 2008;10(2):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2009 {published data only}
- Nguyen HQ, Gill DP, Wolpin S, Steele BG, Benditt JO. Pilot study of a cell phone-based exercise persistence intervention post-rehabilitation for COPD. International Journal of COPD 2009;4:301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2011 {published and unpublished data}
- Nguyen HQ, Donesky-Cuenco D, Wolpin S, Benditt JO, Paul S, Carrieri-Kohlman V. A randomized controlled trial of an internet-based dyspnea self-management program in patients with COPD. In: American Journal of Respiratory and Critical Care Medicine. Vol. 183. 2011:A5818.
Nguyen 2012 {published data only}
- Nguyen HQ, Donesky-Cuenco DM, Benditt J, Paul S, Carrieri-Kohlman V. Moderators of improvement in self-efficacy for managing dyspnea in patients with COPD participating in a dyspnea self-management program. American Journal of Respiratory and Critical Care Medicine 2012;185:A4867. [Google Scholar]
Nguyen 2013 {published data only}
- Nguyen HQ, Donesky D, Reinke LF, Wolpin S, Chyall L, Benditt JO, et al. Internet-based dyspnea self-management support for patients with chronic obstructive pulmonary disease. Journal of Pain and Symptom Management 2013;46(1):43-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2016 {published data only}
- Nguyen HQ, Bailey A, Coleman KJ, Desai S, Fan VS, Gould MK, et al. Patient-centered physical activity coaching in COPD (Walk On!): a study protocol for a pragmatic randomized controlled trial. Contemporary Clinical Trials 2016;46:18-29. [DOI] [PubMed] [Google Scholar]
Nguyen 2018 {published data only}
- Nguyen HQ, Moy LM, Fan VS, Gould KM, Xiang A, Bailey A, et al. Applying the pragmatic-explanatory continuum indicator summary to the implementation of a physical activity coaching trial in chronic obstructive pulmonary disease. Nursing Outlook 2018;66(5):455-63. [DOI] [PubMed] [Google Scholar]
Nikoletou 2016 {published data only}
- Nikoletou D, Man WD-C, Mustfa N, Moore J, Rafferty G, Grant RL, et al. Evaluation of the effectiveness of a home-based inspiratory muscle training programme in patients with chronic obstructive pulmonary disease using multiple inspiratory muscle tests. Disability and Rehabilitation 2016;38(3):250-9. [DOI] [PubMed] [Google Scholar]
Ninot 2011 {published data only}
- Ninot G, Moullec G, Picot MC, Jaussent A, Hayot M, Desplan M, et al. Cost-saving effect of supervised exercise associated to COPD self-management education program. Respiratory Medicine 2011;105(3):377-85. [DOI] [PubMed] [Google Scholar]
NTR3945 {published data only}
- NTR3945. COPD-GRIP study. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=NTR3945 (first received 9 April 2013).
Nyberg 2017 {published data only}
- Nyberg A, Tistad M, Wadell K. Effects of an internet based tool for self-management in patients with COPD - a controlled pragmatic pilot trial. European Respiratory Journal 2017;50(Suppl 61):OA515. [Google Scholar]
Nyberg 2019 {published data only}
- Nyberg A, Tistad M, Wadell K. Can the COPD web be used to promote self-management in patients with COPD in Swedish primary care: a controlled pragmatic pilot trial with 3-month and 12-month follow-up. Scandinavian Journal of Primary Health Care 2019;37(1):69-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Donnel 2018 {published data only}
- O'Donnell DE. Increasing physical activity in chronic obstructive pulmonary disease one step at a time. American Journal of Respiratory and Critical Care Medicine 2018;198(8):977-8. [DOI] [PubMed] [Google Scholar]
O'Dwyer 2016 {published data only}
- O'Dwyer SM, MacHale E, Sulaiman I, Holmes M, Hughes C, D'Arcy S, et al. The effect of providing feedback on inhaler technique and adherence from an electronic audio recording device, INCA®, in a community pharmacy setting: study protocol for a randomised controlled trial. Trials 2016;17(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orme 2016 {published data only}
- Anonymous. Correction: study protocol for Chronic Obstructive Pulmonary Disease-Sitting and ExacerbAtions Trial (COPD-SEAT): a randomised controlled feasibility trial of a home-based self-monitoring sedentary behaviour intervention. BMJ Open 2016;6 (e013014)(11):no pages. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme M, Weedon A, Esliger D, Saukko P, Morgan M, Steiner M, et al. Study protocol for Chronic Obstructive Pulmonary Disease-Sitting and ExacerbAtions Trial (COPD-SEAT): a randomised controlled feasibility trial of a home-based self-monitoring sedentary behaviour intervention. BMJ Open 2016;6(10):e013014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orme 2018 {published data only}
- Orme MW, Weedon AE, Saukko PM, Esliger DW, Morgan MD, Steiner MC, et al. Findings of the chronic obstructive pulmonary disease-sitting and exacerbations trial (COPD-SEAT) in reducing sedentary time using wearable and mobile technologies with educational support: randomized controlled feasibility trial. JMIR Mhealth Uhealth 2018;6(4):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Özkaptan 2016 {published data only}
- Bal Ozkaptan B, Kapucu S. Home nursing care with the self-care model improves self-efficacy of patients with chronic obstructive pulmonary disease. Japan Journal of Nursing Science 2016;13(3):365-77. [DOI] [PubMed] [Google Scholar]
Paneroni 2016 {published data only}
- Paneroni M, Scalvini S, Bernocchi P, Galli T, Baratti D, La Rovere MT, et al. Home telerehabilitation maintenance program for patients affected by COPD and CHF. European Respiratory Journal 2016;48(Suppl 60):OA268. [Google Scholar]
Papp 2017 {published data only}
- Papp ME, Wandell PE, Lindfors P, Nygren-Bonnier M. Effects of yogic exercises on functional capacity, lung function and quality of life in participants with obstructive pulmonary disease: a randomized controlled study. European Journal of Physical and Rehabilitation Medicine 2017;53(3):447-61. [DOI] [PubMed] [Google Scholar]
Pascual 2011 {published data only}
- Pascual CR, Galan EP, Guerrero JL, Colino RM, Soler PA, Calvo MH, et al. Rationale and methods of the multicenter randomised trial of a heart failure management programme among geriatric patients (HF-Geriatrics). BMC Public Health 2011;11:[8 p.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peian 2013 {published data only}
- Peian L, Yanan Z, Peipei C, Pan Z, Jiaxi Y, Ning Z, et al. Supporting smoking cessation in chronic obstructive pulmonary disease with behavioral intervention: a randomized controlled trial. BMC Family Practice 2013;14(1):91-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perkins‐Porras 2018 {published data only}
- Perkins-Porras L, Riaz M, Okekunle A, Zhelezna S, Chakravorty I, Ussher M. Feasibility study to assess the effect of a brief mindfulness intervention for patients with chronic obstructive pulmonary disease: a randomized controlled trial. Chronic Respiratory Disease 2018;15(4):400-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phan 2015 {published data only}
- Phan T, Carter O, Waterer G, Chung LP, Hawkins M, Rudd C, et al. Investigating the efficacy of cognitive behavioural therapy (CBT) on the mental health of chronic obstructive pulmonary disease (COPD) patients: a randomized control trial. Respirology 2015;20(Suppl 3):33. [Google Scholar]
Pinnock 2012 {published data only}
- Pinnock H, Fairbrother P, Hanley J, McCloughlan L, Todd A, McKinstry BC. Perspectives of patient and professional participants on telehealthcare and the impact on self-management: qualitative study nested in the TELESCOT COPD trial. Thorax 2012;67(Suppl 2):A145 [P186]. [Google Scholar]
Pinnock 2013 {published data only}
- Pinnock H, Hanley J, McCloughan L, Todd A, Krishan A, Lewis S, et al. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: researcher blind, multicentre, randomised controlled trial. BMJ 2013;347(7933):[16 p.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pommer 2012 {published data only}
- Pommer AM, Pouwer F, Denollet J, Pop VJ. Managing co-morbid depression and anxiety in primary care patients with asthma and/or chronic obstructive pulmonary disease: study protocol for a randomized controlled trial. Trials 2012;13(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pothirat 2015 {published data only}
- Pothirat C, Chaiwong W, Phetsuk N, Liwsrisakun C, Bumroongkit C, Deesomchok A, et al. Long-term efficacy of intensive cycle ergometer exercise training program for advanced COPD patients. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:133-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poureslami 2016 {published data only}
- Poureslami I, Kwan S, Lam S, Khan NA, Fitzgerald JM. Assessing the effect of culturally specific audiovisual educational interventions on attaining self-management skills for chronic obstructive pulmonary disease in Mandarin- and Cantonese-speaking patients: a randomized controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:1811-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pradella 2015 {published data only}
- Pradella CO, Belmonte GM, Maia MN, Delgado CS, Luise AP, Nascimento OA, et al. Home-based pulmonary rehabilitation for subjects with COPD: a randomized study. Respiratory Care 2015;60(4):526-32. [DOI] [PubMed] [Google Scholar]
Rea 2004 {published and unpublished data}
- Rea H, McAuley S, Stewart A, Lamont C, Roseman P, Didsbury P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Internal Medicine Journal 2004;34(11):608-14. [DOI] [PubMed] [Google Scholar]
Renn 2018 {published data only}
- Renn BN, Hundt NE, Sansgiry S, Petersen NJ, Kauth MR, Kunik ME, et al. Integrated brief cognitive behavioral therapy improves illness intrusiveness in veterans with chronic obstructive pulmonary disease. Annals of Behavioral Medicine 2018;52(8):686-96. [DOI] [PubMed] [Google Scholar]
Rice 2011 {published data only}
- Rice KL. A COPD disease management program reduced a composite of hospitalizations or emergency department visits. Annals of Internal Medicine 2011;154(6):JC3-5. [DOI] [PubMed] [Google Scholar]
Ritchie 2012 {published data only}
- Ritchie C, Richman J, Sobko H, Bodner E, Phillips B, Houston T. The E-coach transition support computer telephony implementation study: protocol of a randomized trial. Contemporary Clinical Trials 2012;33(6):1172-9. [DOI] [PubMed] [Google Scholar]
Ritchie 2016 {published data only}
- Ritchie CS, Houston TK, Richman JS, Sobko HJ, Berner ES, Taylor BB, et al. The E-Coach technology-assisted care transition system: a pragmatic randomized trial. Translational Behavioral Medicine 2016;6(3):428-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rixon 2017 {published data only}
- Rixon L, Hirani SP, Cartwright M, Beynon M, Doll H, Steventon A, et al. A RCT of telehealth for COPD patients' quality of life: the whole system demonstrator evaluation. Clinical Respiratory Journal 2017;11(4):459-69. [DOI] [PubMed] [Google Scholar]
Roberts 2011 {published data only}
- Roberts M, Robinson TC. Telemed: bringing technology to the homes of patients with chronic obstructive pulmonary disease - lessons learnt. Respirology 2011;16:P9 [TO 001]. [Google Scholar]
Robinson 2019 {published data only}
- Robinson SA, Wan JS, Kantorowski A, Moy ML. A web-based physical activity intervention benefits persons with COPD and low self-efficacy: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2019;199(9):A5722. [Google Scholar]
Rojas‐Gomez 2014 {published data only}
- Rojas-Gomez J, Nystrom P, Gauder R, Sampsel D, Wetzel S, Bloch K, al et. Pilot study in the use of human patient simulator (HPS) as a novel approach to COPD self-management. Chest 2014;146(4 suppl 2):23A. [Google Scholar]
Russo 2015 {published data only}
- Russo R, Coultas D, Ashmore J, Peoples J, Sloan J, Jackson BE, et al. Chronic obstructive pulmonary disease self-management activation research trial (COPD-SMART): results of recruitment and baseline patient characteristics. Contemporary Clinical Trials 2015;41C:192-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saini 2018 {published data only}
- Saini P, Dekker M, Van Genugten L, Priori R, Klee M. Online coaching for physical activity in COPD patients: user engagement and determinants. European Respiratory Journal 2018;52(Suppl 62):PA3644. [Google Scholar]
Sanchez 2018 {published data only}
- Sanchez IT, Granados-Santiago M, Lopez-Lopez L, Lucena-Aguilera MD, Romero-Fernandez R, Valenza MC. Efficacy of a comprehensive education program including inhaler training and COPD management during hospitalization of AECOPD, a randomized controlled clinical. European Respiratory Journal 2018;52(Suppl 62):PA3836. [Google Scholar]
Sanders 2012 {published data only}
- Sanders C, Rogers A, Bowen R, Bower P, Hirani S, Cartwright M, et al. Exploring barriers to participation and adoption of telehealth and telecare within the Whole System Demonstrator trial: a qualitative study. BMC Health Services Research 2012;12:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sassi‐Dambron 1995 {published data only}
- Sassi-Dambron DE, Eakin EG, Ries AL, Kaplan RM. Treatment of dyspnea in COPD. A controlled clinical trial of dyspnea management strategies. Chest 1995;107(3):724-9. [DOI] [PubMed] [Google Scholar]
Scalvini 2016 {published data only}
- Scalvini S, Bernocchi P, Baratti D, Gatti T, Paneroni M, La Rovere MT, et al. Multidisciplinary telehealth program for patients affected by chronic heart failure and chronic obstructive pulmonary disease. European Journal of Heart Failure 2016;18:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2018 {published data only}
- Schmidt B, Bourbeau J, Sedeno M, Li PZ, Troosters T, Hamilton A, et al. Impact of meeting behavioral targets in a self-management behaviour modification program designed to improve physical activity in COPD patients. Pneumologie 2018;72(Suppl 1):[no pagination]. [Google Scholar]
Schou 2012 {published data only}
- Schou L, Ostergaard B, Rasmussen L, Rydahl-Hansen S, Svarre Jakobsen A, Emme C, et al. Cognitive function of patients with COPD after virtual admission: a randomized clinical trial. European Respiratory Journal 2012;40(Suppl 56):81s [P549]. [Google Scholar]
Schuz 2015 {published data only}
- Schuz N, Walters JA, Cameron-Tucker H, Scott J, Wood-Baker R, Walters EH. Patient anxiety and depression moderate the effects of increased self-management knowledge on physical activity: a secondary analysis of a randomised controlled trial on health-mentoring in COPD. COPD 2015;12(5):502-9. [DOI] [PubMed] [Google Scholar]
Scuffham 2018 {published data only}
- Scuffham PA, Byrnes JM, Pollicino C, Cross D, Goldstein S, Ng S-K. The impact of population-based disease management services on health care utilisation and costs: results of the capiche trial. Journal of General Internal Medicine 2018;1:41-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seyedi 2018 {published data only}
- Seyedi Chegeni P, Gholami M, Azargoon A, Hossein Pour AH, Birjandi M, Norollahi H. The effect of progressive muscle relaxation on the management of fatigue and quality of sleep in patients with chronic obstructive pulmonary disease: a randomized controlled clinical trial. Complementary Therapies in Clinical Practice 2018;31:64-70. [DOI] [PubMed] [Google Scholar]
Siddique 2012 {published data only}
- Siddique HH, Olson RH, Parenti CM, Rector TS, Caldwell M, Dewan NA, et al. Randomized trial of pragmatic education for low-risk COPD patients: impact on hospitalizations and emergency department visits. International Journal of Chronic Obstructive Pulmonary Disease 2012;7(1):719-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silva 2018 {published data only}
- Silva IG, Silva BS, Areire AP, Santos AP, Lima FF, Ramos D, et al. Functionality of patients with chronic obstructive pulmonary disease at 3 months follow-up after elastic resistance training: a randomized clinical trial. Pulmonology 2018;24(6):354-7. [DOI] [PubMed] [Google Scholar]
Silver 2017 {published data only}
- Silver PC, Kollef MH, Clinkscale D, Watts P, Kidder R, Eads B, et al. A respiratory therapist disease management program for subjects hospitalized with COPD. Respiratory Care 2017;62(1):1-9. [DOI] [PubMed] [Google Scholar]
Sinclair 2017 {published data only}
- Sinclair C, Auret KA, Evans SF, Williamson F, Dormer S, Wilkinson A, et al. Advance care planning uptake among patients with severe lung disease: a randomised patient preference trial of a nurse-led, facilitated advance care planning intervention. Journal of Pain and Symptom Management 2017;7(2):e013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sink 2018 {published data only}
- Sink E, Patel K, Groenendyk J, Peters R, Som A, Kim E, et al. Effectiveness of a novel, automated telephone intervention on time to hospitalisation in patients with COPD: a randomised controlled trial. Journal of Telemedicine and Telecare 2018;26(3):132-9. [DOI] [PubMed] [Google Scholar]
Slok 2014 {published data only}
- Slok AH, In 't Veen JC, Chavannes NH, Van der Molen T, Molken MP, Kerstjens HA, et al. Effectiveness of the Assessment of Burden of Chronic Obstructive Pulmonary Disease (ABC) tool: study protocol of a cluster randomised trial in primary and secondary care. BMC Pulmonary Medicine 2014;14(1):[9 p.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slok 2016a {published data only}
- Slok A, Kotz D, Van Breukelen G, Chavannes N, Rutten-Van Molken M, Kerstjens H, et al. The assessment of burden of COPD tool improves health related quality of life. European Respiratory Journal 2016;48(Suppl 60):OA1997. [Google Scholar]
Slok 2016b {published data only}
- Slok AH, Kotz D, Van Breukelen G, Chavannes NH, Rutten-Van Molken MP, Kerstjens HA, et al. Effectiveness of the Assessment of Burden of COPD (ABC) tool on health-related quality of life in patients with COPD: a cluster randomised controlled trial in primary and hospital care. BMJ Open 2016;6(7):[12 p.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smidth 2014 {published data only}
- Smidth MC. The effect of an active implementation of a disease management program for COPD [Abstract]. In: 7th International Primary Care Respiratory Group (IPCRG) World Conference; 2014 May 21-24; Athens, Greece. 2014.
Sohanpal 2012 {published data only}
- Sohanpal R, Seale C, Taylor SJ. Learning to manage COPD: a qualitative study of reasons for attending and not attending a COPD-specific self-management programme. Trials 2012;9(3):163-74. [DOI] [PubMed] [Google Scholar]
Song 2014 {published data only}
- Song HY, Yong SJ, Hur HK. Effectiveness of a brief self-care support intervention for pulmonary rehabilitation among the elderly patients with chronic obstructive pulmonary disease in Korea. Rehabilitation Nursing 2014;39(3):147-56. [DOI] [PubMed] [Google Scholar]
Sorensen 2015 {published data only}
- Sorensen SS, Pedersen KM, Ehlers L. Design of a randomized controlled trial (RCT) evaluating outcome and cost-effectiveness of a local case management intervention of patients suffering from chronic obstructive pulmonary disease (COPD) [Abstract]. Value in Health 2013;16(7):A576. [Google Scholar]
- Sorensen SS, Pedersen KM, Weinreich UM, Ehlers L. Economic evaluation of community-based case management of patients suffering from chronic obstructive pulmonary disease. Applied Health Economics and Health Policy 2016;15(3):413-24. [DOI] [PubMed] [Google Scholar]
- Sorensen SS, Pedersen KM, Weinreich UM, Ehlers LH. Design, and participant enrollment, of a randomized controlled trial evaluating effectiveness and cost-effectiveness of a community-based case management intervention, for patients suffering from COPD. Open Access Journal of Clinical Trials 2015;7:53-62. [Google Scholar]
Soriano 2018 {published data only}
- Soriano JB, Garcia-Rio F, Vazquez-Espinosa E, Conforto JI, Hernando-Sanz A, Lopez-Yepes L, et al. A multicentre, randomized controlled trial of telehealth for the management of COPD. Respiratory Medicine 2018;144:74-81. [DOI] [PubMed] [Google Scholar]
Stamenova 2019 {published data only}
- Stamenova V, Yang R, Engel K, Liang K, Van Lieshout F, Lalingo E, et al. Technology-enabled self-monitoring of chronic obstructive pulmonary disease with or without asynchronous remote monitoring: protocol for a randomized controlled trial. JMIR Research Protocols 2019;8(8):e13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steinhauser 2017 {published data only}
- Steinhauser KE, Stewart A, Olsen MK, Stechuchak KM, Zervakis J, Ammarell N, et al. Addressing patient emotional and existential needs during serious illness: results of the outlook randomized controlled trial. Journal of Pain and Symptom Management 2017;54(6):898-908. [DOI] [PubMed] [Google Scholar]
Stenlund 2019 {published data only}
- Stenlund T, Nyberg A, Lundell S, Wadell K. Web-based support for self-management strategies versus usual care for people with COPD in primary healthcare: a protocol for a randomised, 12-month, parallel-group pragmatic trial. BMJ Open 2019;9(10):e030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steurer‐Stey 2014 {published data only}
- Steurer-Stey C, Markun S, Lana KD, Frei A, Held U, Wensing M, et al. The improving care in chronic obstructive lung disease study: CAROL improving processes of care and quality of life of COPD patients in primary care: study protocol for a randomized controlled trial. Trials 2014;15(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stolz 2018 {published data only}
- Stolz D, Hirsch HH, Schilter D, Louis R, Rakic J, Boeck L, et al. Intensified therapy with inhaled corticosteroids and long-acting beta2-agonists at the onset of upper respiratory tract infection to prevent chronic obstructive pulmonary disease exacerbations. A multicenter, randomized, double-blind, placebo-controlled trial. American Journal of Respiratory and Critical Care Medicine 2018;197(9):1136-46. [DOI] [PubMed] [Google Scholar]
Stoop 2015 {published data only}
- Stoop CH, Nefs G, Pommer AM, Pop VJ, Pouwer F. Effectiveness of a stepped care intervention for anxiety and depression in people with diabetes, asthma or COPD in primary care: a randomized controlled trial. Journal of Affective Disorders 2015;184:269-76. [DOI] [PubMed] [Google Scholar]
Stulbarg 2002 {published data only}
- Carrieri-Kohlman V, Nguyen HQ, Donesky-Cuenco D, Demir-Deviren S, Neuhaus J, Stulbarg MS. Impact of brief or extended exercise training on the benefit of a dyspnea self-management program in COPD. Journal of Cardiopulmonary Rehabilitation 2005;25(5):275-84. [DOI] [PubMed] [Google Scholar]
- Davis AHT, Carrieri-Kohlman V, Janson SL, Gold WM, Stulbarg MS. Effects of treatment on two types of self-efficacy in people with chronic obstructive pulmonary disease. Journal of Pain and Symptom Management 2006;31(1):60-70. [DOI] [PubMed] [Google Scholar]
- Donesky-Cuenco DA, Janson S, Neuhaus J, Neilands TB, Carrieri-Kohlman V. Adherence to a home-walking prescription in patients with chronic obstructive pulmonary disease. Heart and Lung 2007;36(5):348-63. [DOI] [PubMed] [Google Scholar]
- Nguyen HQ, Carrieri-Kohlman V. Dyspnea self-management in patients with chronic obstructive pulmonary disease: moderating effects of depressed mood. Psychosomatics 2005;46(5):402-10. [DOI] [PubMed] [Google Scholar]
- Stulbarg MS, Carrieri-Kohlman V, Demir-Deviren S, Nguyen HQ, Adams L, Tsang AH, et al. Exercise training improves outcomes of a dyspnea self-management program. Journal of Cardiopulmonary Rehabilitation 2002;22(2):109-21. [DOI] [PubMed] [Google Scholar]
Talboom‐Kamp 2017a {published data only}
- Talboom-Kamp E, Kasteleyn M, Verdijk N, Chavannes N, Harmans L, Talboom I, et al. Stimulation of usage of self-management platforms through integration in disease management. European Respiratory Journal 2017;50(Supplement 61):PA3878. [Google Scholar]
Talboom‐Kamp 2017b {published data only}
- Talboom-Kamp E, Verdijk N, Kasteleyn M, Van Geloven N, Talboom I, Harmans L, et al. Effect on health status of integrated self-management platforms in COPD disease-management. European Respiratory Journal 2017;50(Suppl 61):OA2912. [Google Scholar]
Tang 2012 {published data only}
- Tang CY, Blackstock FC, Clarence M, Taylor NF. Early rehabilitation exercise program for inpatients during an acute exacerbation of chronic obstructive pulmonary disease: a randomized controlled trial. Journal of Cardiopulmonary Rehabilitation and Prevention 2012;32(3):163-9. [DOI] [PubMed] [Google Scholar]
Tashkin 2012 {published data only}
- Tashkin DP, Rabinoff M, Noble EP, Ritchie TL, Simmons MS, Connett J. Association of dopamine-related gene alleles, smoking behavior and decline in FEV1 in subjects with COPD: findings from the lung health study. Journal of Chronic Obstructive Pulmonary Disease 2012;9(6):620-8. [DOI] [PubMed] [Google Scholar]
Taylor 2012 {published data only}
- Taylor SJ, Sohanpal R, Bremner SA, Devine A, McDaid D, Fernandez J-L, et al. Self-management support for moderate-to-severe chronic obstructive pulmonary disease: a pilot randomised controlled trial. British Journal of General Practice 2012;62(603):e687-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Theander 2015 {published data only}
- Theander K, Arne M, Hasselgren M, Lisspers K, Stallberg B, Zakrisson A-B. Effects of a self-management program for patients with COPD or chronic heart failure (CHF) on self-efficacy related to exercise and fatigue - the SAFS study. European Respiratory Journal 2015;46(Suppl 59):PA3862. [Google Scholar]
Thom 2018 {published data only}
- Thom DH, Willard-Grace R, Tsao S, Hessler D, Huang B, DeVore D, et al. Randomized controlled trial of health coaching for vulnerable patients with chronic obstructive pulmonary disease (COPD). Annals of the American Thoracic Aociety 2018;15(10):1159-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thoonsen 2011 {published data only}
- Thoonsen B, Groot M, Engels Y, Prins J, Verhagen S, Galesloot C, et al. Early identification of and proactive palliative care for patients in general practice, incentive and methods of a randomized controlled trial. BMC Family Practice 2011;12:[7 p.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Titova 2016 {published data only}
- Titova EV, Sunde S, Steinshamn S, Henriksen AH. Integrated disease management (IDM) intervention for COPD patients has long-term effects on patient activation. European Respiratory Journal 2016;48:PA726. [Google Scholar]
To 2019 {published data only}
- To KW. The effects of an education-based adherence intervention on adherence of inhalation therapy among patients with chronic respiratory diseases. www.proquest.com/openview/7d070c859649964e7102810742442adf/1?pq-origsite=gscholar&cbl=2026366&diss=y (accessed prior to 4 July 2021).
Tommelein 2014 {published data only}
- Tommelein E, Mehuys E, Van Hees T, Adriaens E, Van Bortel L, Christiaens T, et al. Effectiveness of pharmaceutical care for patients with chronic obstructive pulmonary disease (PHARMACOP): a randomized controlled trial. British Journal of Clinical Pharmacology 2014;77(5):756-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommelein E, Mehuys E, Van Hees T, Adriaens E, Van Bortel L, Christiaens T, et al. Effectiveness of pharmaceutical care for patients with COPD: translated review of the recently published PHARMACOP trial. Journal de Pharmacie de Belgique 2014;3:4-14. [PubMed] [Google Scholar]
Tong 2012 {published data only}
- Tong C, Hart D, Corna N, Forbes-Faulkner L, Goodman M, al Masson S. Application of self-management systems evaluation trial (asset) for COPD patients in counties manukau (funded by the primary health care innovations fund). Respirology 2012;17:TP-180. [Google Scholar]
Torres‐Sanchez 2018 {published data only}
- Torres-Sanchez I, Valenza MC, Cebria I Iranzo MD, Lopez-Lopez L, Moreno-Ramirez MP, Ortiz-Rubio A. Effects of different physical therapy programs on perceived health status in acute exacerbation of chronic obstructive pulmonary disease patients: a randomized clinical trial. Disability and Rehabilitation 2018;40(17):2025-31. [DOI] [PubMed] [Google Scholar]
Touchette 2012 {published data only}
- Touchette DR, Masica AL, Dolor RJ, Schumock GT, Choi YK, Kim Y, et al. Safety-focused medication therapy management: a randomized controlled trial. Journal of the American Pharmacists Association 2012;52(5):603-12. [DOI] [PubMed] [Google Scholar]
Trappenburg 2011 {published data only}
- Trappenburg JC, Monninkhof EM, Bourbeau J, Troosters T, Schrijvers AJ, Verheij TJ, et al. Effect of an action plan with ongoing support by a case manager on exacerbation-related outcome in patients with COPD: a multicentre randomised controlled trial. Thorax 2011;66(11):977-84. [DOI] [PubMed] [Google Scholar]
Troosters 2011 {published data only}
- Troosters T, Weisman I, Dobbels F, Giardino N, Valluri SR. Assessing the impact of tiotropium on lung function and physical activity in GOLD Stage II COPD patients who are naive to maintenance respiratory therapy: a study protocol. Open Respiratory Medicine Journal 2011;5:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsai 2016 {published data only}
- Tsai L, McNamara R, Moddel C, McKenzie D, Alison J, McKeough Z. Telerehabilitation improves exercise capacity and quality of life in people with chronic obstructive pulmonary disease (COPD): a randomised controlled trial. Respirology 2016;21(Suppl 2):136. [Google Scholar]
- Tsai LL, McNamara R, Moddel C, McKenzie D, Alison J, McKeough Z. Telerehabilitation in people with chronic obstructive pulmonary disease (COPD): a randomised controlled trial. European Respiratory Journal 2016;48(Suppl 60):PA2065. [Google Scholar]
- Tsai LL, McNamara RJ, Moddel C, Alison JA, McKenzie DK, McKeough ZJ. Home-based telerehabilitation via real-time videoconferencing improves endurance exercise capacity in patients with COPD: the randomized controlled TeleR Study. Respirology 2016;22(4):699-707. [DOI] [PubMed] [Google Scholar]
Udsen 2014 {published data only}
- Udsen FW, Lilholt PH, Hejlesen O, Ehlers LH. Effectiveness and cost-effectiveness of telehealthcare for chronic obstructive pulmonary disease: study protocol for a cluster randomized controlled trial. Trials 2014;15(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ulrik 2013 {published data only}
- Ulrik, CS. No benefit and potential harm with an educational and care management programme for chronic obstructive pulmonary disease. Evidence-Based Medicine 2013;18(2):72-3. [DOI] [PubMed] [Google Scholar]
Valderramas 2018 {published data only}
- Valderramas S, Kovelis D, Mazzarin C, Biazim SK, Gomes AR. Can exercise capacity be improved after home exercise in COPD patients? European Respiratory Journal 2018;52(Suppl 62):PA3825. [Google Scholar]
Valenza 2018 {published data only}
- Valenza MC, Torres-Sanchez I, Lopez-Lopez L, Cabrera-Martos I, Ortiz-Rubio A, Valenza-Demet G. Effects of home-based neuromuscular electrical stimulation in severe chronic obstructive pulmonary disease patients: a randomized controlled clinical trial. European Journal of Physical and Rehabilitation Medicine 2018;54(3):323-32. [DOI] [PubMed] [Google Scholar]
Van der Weegen 2015 {published data only}
- Van der Weegen S, Verwey R, Spreeuwenberg M, Tange H, Van der Weijden T, Witte L. It's LiFe! Mobile and web-based monitoring and feedback tool embedded in primary care increases physical activity: a cluster randomized controlled trial. Journal of Medical Internet Research 2015;17(7):e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Wetering 2009 {published data only}
- Hoogendoorn M, Wetering CR, Schols AM, Rutten-van Mölken MP. Is INTERdisciplinary COMmunity-based COPD management (INTERCOM) cost-effective? European Respiratory Journal 2010;35(1):79-87. [DOI] [PubMed] [Google Scholar]
- Van Wetering CR, Hoogendoorn M, Mol SJ, Rutten-van Mölken MP, Schols AM. Short- and long-term efficacy of a community-based COPD management programme in less advanced COPD: a randomised controlled trial. Thorax 2010;65(1):7-13. [DOI] [PubMed] [Google Scholar]
Vasilopoulou 2017 {published data only}
- Vasilopoulou M, Papaioannou AI, Kaltsakas G, Louvaris Z, Chynkiamis N, Spetsioti S, et al. Home-based maintenance tele-rehabilitation reduces the risk for acute exacerbations of COPD, hospitalisations and emergency department visits. European Respiratory Journal 2017;49(5):OA273. [DOI] [PubMed] [Google Scholar]
Vayisoglu 2019 {published data only}
- Vayisoglu SK, Zincir H. The health action process approach-based program's effects on influenza vaccination behavior. Journal for Nurse Practitioners 2019;15(7):517-24. [Google Scholar]
Velardo 2017 {published data only}
- Velardo C, Shah SA, Gibson O, Clifford G, Heneghan C, Rutter H, et al. Digital health system for personalised COPD long-term management. BMC Medical Informatics and Decision Making 2017;17(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verwey 2014 {published data only}
- Verwey R, Van der Weegen S, Spreeuwenberg M, Tange H, Van der Weijden T, Witte L. A monitoring and feedback tool embedded in a counselling protocol to increase physical activity of patients with COPD or type 2 diabetes in primary care: study protocol of a three-arm cluster randomised controlled trial. BMC Family Practice 2014;15(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vianello 2016 {published data only}
- Vianello A, Fusello M, Gubian L, Rinaldo C, Dario C, Concas A, et al. Home telemonitoring for patients with acute exacerbation of chronic obstructive pulmonary disease: a randomized controlled trial. BMC Pulmonary Medicine 2016;16(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vivodtzev 2012 {published data only}
- Vivodtzev I, Debigare R, Gagnon P, Mainguy V, Saey D, Dube A, et al. Functional and muscular effects of neuromuscular electrical stimulation in patients with severe COPD: a randomized clinical trial. Chest 2012;141(3):716-25. [DOI] [PubMed] [Google Scholar]
Voncken‐Brewster 2015 {published data only}
- Voncken-Brewster V, Tange H, De Vries H, Nagykaldi Z, Winkens B, Van der Weijden T. A randomized controlled trial evaluating the effectiveness of a web-based, computer-tailored self-management intervention for people with or at risk for COPD. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:1061-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vorrink 2016 {published data only}
- Vorrink SN, Kort HS, Troosters T, Zanen P, Lammers JJ. Efficacy of an mHealth intervention to stimulate physical activity in COPD patients after pulmonary rehabilitation. European Respiratory Journal 2016;48(4):1019-29. [DOI] [PubMed] [Google Scholar]
Vorrink 2017 {published data only}
- Vorrink S, Huisman C, Kort H, Troosters T, Lammers JW. Perceptions of patients with chronic obstructive pulmonary disease and their physiotherapists regarding the use of an eHealth intervention. JMIR Human Factors 2017;4(3):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wadell 2013 {published data only}
- Wadell K, Webb KA, Preston ME, Amornputtisathaporn N, Samis L, Patelli J, et al. Impact of pulmonary rehabilitation on the major dimensions of dyspnea in COPD. Journal of Chronic Obstructive Pulmonary Disease 2013;10(4):425-35. [DOI] [PubMed] [Google Scholar]
Wakabayashi 2011 {published data only}
- Wakabayashi R, Motegi T, Yamada K, Ishii T, Jones RC, Hyland ME, et al. Efficient integrated education for older patients with chronic obstructive pulmonary disease using the Lung Information Needs Questionnaire. Geriatrics and Gerontology International 2011;11(4):422-30. [DOI] [PubMed] [Google Scholar]
Walker 2018 {published data only}
- Walker PP, Pompilio PP, Zanaboni P, Bergmo TS, Prikk K, Malinovschi A, et al. Telemonitoring in chronic obstructive pulmonary disease (CHROMED). A randomized clinical trial. American Journal of Respiratory and Critical Care Medicine 2018;198(5):620-8. [DOI] [PubMed] [Google Scholar]
Walters 2012 {published data only}
- Walters EH, Walters J, Wills KE, Robinson A, Wood-Baker R. Clinical diaries in COPD: compliance and utility in predicting acute exacerbations. International Journal of COPD 2012;7:427-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2017 {published data only}
- Wan ES, Kantorowski A, Homsy D, Teylan M, Kadri R, Richardson CR, et al. Promoting physical activity in COPD: insights from a randomized trial of a web-based intervention and pedometer use. Respiratory Medicine 2017;130:102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2019 {published data only}
- Wan ES, Kantorowski A, Kadri R, Richardson CR, Gagnon D, Garshick E, et al. Internet-mediated, pedometer-based physical activity intervention reduces risk of future acute exacerbations in COPD: a randomized trial. American Journal of Respiratory and Critical Care Medicine 2019;199(9):A4274. [Google Scholar]
Wang 2012 {published data only}
- Wang M, Li J, Li S, Wang H, Yu X, Zhang H. Effect of traditional Chinese medicine on outcomes in patients with mild/moderate chronic obstructive pulmonary disease: study protocol for a randomized placebo-controlled trial. Trials 2012;13:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2018 {published data only}
- Wang L, Wu K, Chen X, Liu Q. The effects of tai chi on lung function, exercise capacity and health related quality of life for patients with chronic obstructive pulmonary disease: a pilot study. Heart, Lung and Circulation 2018;8:1206-12. [DOI] [PubMed] [Google Scholar]
Wang CH 2014 {published data only}
- Wang CH, Chou PC, Joa WC, Chen LF, Sheng TF, Ho SC, et al. Mobile-phone-based home exercise training program decreases systemic inflammation in COPD: a pilot study. BMC Pulmonary Medicine 2014;14(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang H 2017 {published data only}
- Wang H, Wei Z, Li X, Li Y. Efficacy of emotion regulation for patients suffering from chronic obstructive pulmonary disease. Iranian Journal of Public Health 2017;46(1):50-4. [PMC free article] [PubMed] [Google Scholar]
Wang J‐X 2017 {published data only}
- Wang J-X. Efficacy of assisted breathing training combined with abdominal massage care in management of constipation in patients with chronic obstructive pulmonary disease. World Chinese Journal of Digestology 2017;25(22):2056-60. [Google Scholar]
Wang K 2017 {published data only}
- Wang K, Zeng G-Q, Li R, Luo Y-W, Wang M, Hu Y-H, et al. Cycle ergometer and inspiratory muscle training offer modest benefit compared with cycle ergometer alone: a comprehensive assessment in stable COPD patients. International Journal of Chronic Obstructive Pulmonary Disease 2017;12:2655-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang Y 2014 {published data only}
- Wang Y, Zang XY, Bai J, Liu SY, Zhao Y, Zhang Q. Effect of a health belief model-based nursing intervention on Chinese patients with moderate to severe chronic obstructive pulmonary disease: a randomised controlled trial. Journal of Clinical Nursing 2014;23(9-10):1342-53. [DOI] [PubMed] [Google Scholar]
Wei 2014 {published data only}
- Wei L, Yang X, Li J, Liu L, Luo H, Zheng Z, Wei Y. Effect of pharmaceutical care on medication adherence and hospital admission in patients with chronic obstructive pulmonary disease (COPD): a randomized controlled study. Journal of Thoracic Disease 2014;6(6):656-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weldam 2016 {published data only}
- Weldam S, Schuurmans M, Zanen P, Sachs A, Heijmans M, Lammers J-W. Effectiveness of a new nursing intervention for primary care COPD patients. European Respiratory Journal 2016;48(Suppl 60):OA290. [Google Scholar]
Weldam 2017 {published data only}
- Weldam SW, Schuurmans MJ, Zanen P, Heijmans MJ, Sachs AP, Lammers J-W. The effectiveness of a nurse-led illness perception intervention in COPD patients: a cluster randomised trial in primary care. ERJ Open Research 2017;3(4):00115-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whelan 2019 {published data only}
- Whelan ME, Velardo C, Rutter H, Tarassenko L, Farmer AJ. Mood monitoring over one year for people with chronic obstructive pulmonary disease using a mobile health system: retrospective analysis of a randomized controlled trial. JMIR Mhealth and Uhealth 2019;7(11):e14946. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2019 {published data only}
- White P, Gilworth G, Lewin S, Hogg L, Tuffnell R, Taylor SJ, et al. Improving uptake and completion of pulmonary rehabilitation in COPD with lay health workers: feasibility of a clinical trial. International Journal of Chronic Obstructive Pulmonary Disease 2019;14:631-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2011 {published data only}
- Wilson JS, Elborn JS, Fitzsimons D, McCrum-Gardner E. Do smokers with chronic obstructive pulmonary disease report their smoking status reliably? A comparison of self-report and bio-chemical validation. International Journal of Nursing Studies 2011;48(7):856-62. [DOI] [PubMed] [Google Scholar]
Wilson 2015 {published data only}
- Wilson AM, Browne P, Olive S, Clark A, Galey P, Dix E, et al. The effects of maintenance schedules following pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomised controlled trial. BMJ Open 2015;5(3):e005921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Windisch 2018 {published data only}
- Windisch W, Schwarz SB, Magnet FS, Dreher M, Schmoor C, Storre JH, et al. Using web-based videos to improve inhalation technique in COPD patients requiring hospitalization: a randomized controlled trial. PLOS One 2018;13(10):e0201188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood‐Baker 2012 {published data only}
- Wood-Baker R, Reid D, Robinson A, Walters EH. Clinical trial of community nurse mentoring to improve self-management in patients with chronic obstructive pulmonary disease. International Journal of COPD 2012;7:407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wootton 2014 {published data only}
- Wootton SL, Ng LW, McKeough ZJ, Jenkins S, Hill K, Eastwood PR, et al. Ground-based walking training improves quality of life and exercise capacity in COPD. European Respiratory Journal 2014;44(4):885-94. [DOI] [PubMed] [Google Scholar]
Wootton 2017 {published data only}
- Wootton SL, Hill K, Alison JA, Ng LW, Jenkins S, Eastwood PR, et al. Effects of ground-based walking training on daily physical activity in people with COPD: a randomised controlled trial. Respiratory Medicine 2017;132:139-45. [DOI] [PubMed] [Google Scholar]
Wu M 2018 {published data only}
- Wu M, Zhou LQ, Li S, Zhao S, Fan HJ, Sun JM, et al. Efficacy of patients' preferred exercise modalities in chronic obstructive pulmonary disease: a parallel-group, randomized, clinical trial. Clinical Respiratory Journal 2018;12(4):1581-90. [DOI] [PubMed] [Google Scholar]
Wu W 2017 {published data only}
- Wu W, Guan L, Zhang X, Li X, Yang Y, Guo B, et al. Effects of two types of equal-intensity inspiratory muscle training in stable patients with chronic obstructive pulmonary disease: a randomised controlled trial. Respiratory Medicine 2017;132:84-91. [DOI] [PubMed] [Google Scholar]
Wu W 2018 {published data only}
- Wu W, Liu X, Li P, Li N, Wang Z. Effect of liuzijue exercise combined with elastic band resistance exercise on patients with COPD: a randomized controlled trial. Evidence-based Complementary and Alternative Medicine 2018;2018:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Liu X, Liu J, Li P, Wang Z. Effectiveness of water-based Liuzijue exercise on respiratory muscle strength and peripheral skeletal muscle function in patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease 2018;13:1713-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu X 2016 {published data only}
- Wu XH. Clinical study on therapy of integrated medicine combined with nursing on chronic obstructive pulmonary disease. Chinese Medicine Modern Distance Education of China 2016;14(7):109-10.
Xi 2015 {published data only}
- Xi M, Qin Q, Tang C. Influence of transitional care model on pulmonary function and quality of life of discharged patients with COPD. Chinese Nursing Research 2015;29(3C):1052-4.
Xin 2016 {published data only}
- Xin C, Xia Z, Jiang C, Lin M, Li G. The impact of pharmacist-managed clinic on medication adherence and health-related quality of life in patients with COPD: a randomized controlled study. Patient Preference and Adherence 2016;10:1197-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamaguti 2012 {published data only}
- Yamaguti WP, Claudino RC, Neto AP, Chammas MC, Gomes AC, Salge JM, et al. Diaphragmatic breathing training program improves abdominal motion during natural breathing in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2012;93(4):571-7. [DOI] [PubMed] [Google Scholar]
- Yamaguti WP. Effects of a short-term diaphragmatic breathing program on respiratory mechanics and functional capacity of COPD patients: a randomized controlled trial [thesis]. Sao Paulo: Universidade de Sao Paulo, 2011. [DOI: 10.11606/T.5.2011.tde-02082011-143720] [DOI] [Google Scholar]
Yan 2017 {published data only}
- Yan X, Qinfang O. Application of cognitive behavior intervention based on concept of humanism for elderly patients with chronic ob-structive pulmonary disease. Chinese Nursing Research 2017;31(5):551-4. [Google Scholar]
Yan 2018 {published data only}
- Yan Y, Liu L, Zeng J, Zhang L. Evaluation and exploration on the effect of the management of chronic obstructive pulmonary disease in rural areas through an internet-based network consulting room. Medical Principles and Practice 2018;27(3):222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan 2019 {published data only}
- Yan J, Wang L, Liu C, He Z, Zhu S, Long L, Yang G. The effects of hospital outreach intervention on decreasing hospitalizations and medical cost of patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2019;199(9):A2379. [Google Scholar]
Yan J 2016 {published data only}
- Yan J, Wang L, Liu C, Yuan H, Wang X, Yu B, Luo Q. Effect of a hospital outreach intervention programme on decreasing hospitalisations and medical costs in patients with chronic obstructive pulmonary disease in China: protocol of a randomised controlled trial. BMJ Open 2016;6(6):e009988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan XN 2016 {published data only}
- Yan XN. Analysis on the syndrome differentiation nursing strategy for acute exacerbation chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2016;14(7):121-2. [Google Scholar]
Yazdani 2018 {published data only}
- Yazdani R, Marefati H, Shahesmaeili A, Nakhaei S, Bagheri A, Dastoorpoor M. Effect of aerobic exercises on serum levels of apolipoprotein A1 and apolipoprotein B, and their ratio in patients with chronic obstructive pulmonary disease. Tanaffos 2018;17(2):82-9. [PMC free article] [PubMed] [Google Scholar]
Yilmaz 2017 {published data only}
- Yilmaz CK, Kapucu S. The effect of progressive relaxation exercises on fatigue and sleep quality in individuals with COPD. Holistic Nursing Practice 2017;31(6):369-77. [DOI] [PubMed] [Google Scholar]
Ying 2013 {published data only}
- Ying S, Zhou X, Zhou L, Hu X, Liu Y. Effect of exercise combined with encouragement on quality of life of patients with chronic obstructive pulmonary disease. Nan Fang Yi Ke Da Xue Xue Bao 2013;33(9):1312-5. [PubMed] [Google Scholar]
Yu 2013 {published data only}
- Yu X-Q, Li J-S, Li S-Y, Xie Y, Wang M-H, Zhang H-L, et al. Functional and psychosocial effects of pulmonary Daoyin on patients with COPD in China: study protocol of a multicenter randomized controlled trial. Journal of Chinese Integrative Medicine 2013;11(2):140-6. [DOI] [PubMed] [Google Scholar]
Yu 2014 {published data only}
- Yu S-H, Guo A-M, Zhang X-J. Effects of self-management education on quality of life of patients with chronic obstructive pulmonary disease. International Journal of Nursing Sciences 2014;1(1):53-7. [Google Scholar]
Yuan 2015 {published data only}
- Yuan X, Tao Y, Zhao JP, Liu XS, Xiong WN, Xie JG, et al. Long-term efficacy of a rural community-based integrated intervention for prevention and management of chronic obstructive pulmonary disease: a cluster randomized controlled trial in China's rural areas. Brazilian Journal of Medical and Biological Research 2015;48(11):1023-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zakrisson 2019 {published data only}
- Zakrisson A-B, Theander K, Arne M, Hasselgren M, Lisspers K, Stallberg B. A complex intervention of self-management for patients with COPD or CHF in primary care improved performance and satisfaction with regard to own selected activities: a longitudinal follow-up. Journal of Advanced Nursing 2019;75(1):175-86. [DOI] [PubMed] [Google Scholar]
Zambom 2011 {published data only}
- Zambom F, Cabollero P, Hernandez M, Gorostiaga E, Ibanez J, Hueto J. Improvement in skeletal muscle dysfunction after twice-weekly exercise training in COPD patients [Abstract]. European Respiratory Journal 2011;38(55):879s [P4806]. [Google Scholar]
Zhai 2016 {published data only}
- Zhai ZG. The effect evaluation of combined traditional Chinese and western medicine for respiratory rehabilitation training to improve the ability of exercise in patients with chronic obstructive pulmonary disease. China Health Industry 2016;14(2):75-7. [Google Scholar]
Zhang 2012 {published data only}
- Zhang LH, Wu JJ, Wang ZC. Effects of 24-form tai chi with respiratory rehabilitation training on pulmonary function and quality of life of patients with COPD. Acta Universitatis traditionis Medicalis Sinensis Pharmacologiaeque Shanghai 2012;26(4):53-6. [Google Scholar]
Zhang 2013 {published data only}
- Zhang J, Song Y-L, Bai C-X. MIOTIC study: a prospective, multicenter, randomized study to evaluate the long-term efficacy of mobile phone-based Internet of Things in the management of patients with stable COPD. International Journal of Chronic Obstructive Pulmonary Disease 2013;8:433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang Y, Yang W, Li A. Influence of group work on self-management of community elderly patients with chronic obstructive pulmonary disease. Chinese Nursing Research 2014;28(12C):4501-4. [Google Scholar]
Zhang H 2016 {published data only}
- Zhang H, Guo X, Chen N. Influence of Ba Duan Jin on quality of life of patients with stable chronic obstructive pulmonary disease. Chinese Nursing Research 2016;30(6A):1953-6. [Google Scholar]
Zhang M 2016 {published data only}
- Zhang M, Xv G, Luo C, Meng DJ, Ji Yan. Qigong Yi Jinjing promotes pulmonary function, physical activity, quality of life and emotion regulation self-efficacy in patients with chronic obstructive pulmonary disease: a pilot study. Journal of Alternative and Complementary Medicine 2016;22(10):810-17. [DOI] [PubMed] [Google Scholar]
Zhao 2017 {published data only}
- Zhao DX, Chen SY, Zhou YM, Li XC, Zou WF, Chen XM, et al. Establishment and application effect appraisement of community chronic obstructive pulmonary disease integrated management system. Chinese Journal of Tuberculosis and Respiratory Diseases 2017;40(2):102-7. [DOI] [PubMed] [Google Scholar]
Zheng 2019 {published data only}
- Zheng A, Wang L. Application effect of rational emotional behavior therapy for home chronic obstructive pulmonary disease patients with depression. Chinese Nursing Research 2019;33(7):1135-40. [Google Scholar]
Zhou 2016 {published data only}
- Zhou L-Q, Li X-Y, Li Y, Guo B-P, Guan L-L, Chen X, et al. Inspiratory muscle training followed by non-invasive positive pressure ventilation in patients with severe chronic obstructive pulmonary disease: a randomized controlled trial. Journal of Southern Medical University 2016;36(8):1069-74. [PubMed] [Google Scholar]
Zhu 2018 {published data only}
- Zhu S, Shi K, Yan J, He Z, Wang Y, Yi Q, et al. A modified 6-form Tai Chi for patients with COPD. Complementary Therapies in Medicine 2018;39:36-42. [DOI] [PubMed] [Google Scholar]
Zuo 2015 {published data only}
- Zuo AW, Xiong GZ. Effect comparison of integrated traditional Chinese medicine nursing and routine nursing application to chronic obstructive pulmonary disease. Chinese Medicine Modern Distance Education of China 2015;13(4):121-2. [Google Scholar]
Zwar 2012 {published data only}
- Zwar NA, Hermiz O, Comino E, Middleton S, Vagholkar S, Xuan W, et al. Care of patients with a diagnosis of chronic obstructive pulmonary disease: a cluster randomised controlled trial. Medical Journal of Australia 2012;197(7):394-8. [DOI] [PubMed] [Google Scholar]
Zwar 2016 {published data only}
- Zwar NA, Bunker JM, Reddel HK, Dennis SM, Middleton S, Van Schayck OC, et al. Early intervention for chronic obstructive pulmonary disease by practice nurse and GP teams: a cluster randomized trial. Family Practice 2016;33(6):663-70. [DOI] [PubMed] [Google Scholar]
Zwerink 2013 {published data only}
- Zwerink M, Van der Palen J, Van der Valk P, Brusse-Keizer M, Effing T. Relationship between daily physical activity and exercise capacity in patients with COPD. Respiratory Medicine 2013;107(2):242-8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abdulsalim 2017 {published data only}
- Abdulsalim S, Unnikrishnan MK, Manu MK, Alrasheedy AA, Godman B, Morisky DE. Structured pharmacist-led intervention programme to improve medication adherence in COPD patients: a randomized controlled study. Research in Social and Administrative Pharmacy 2017;14(10):909-14. [DOI] [PubMed] [Google Scholar]
- Abdulsalim S, Unnikrishnan MK, Manu MK, Alsahali S, Alrasheedy AA, Martin AP, et al. Impact of a clinical pharmacist intervention on medicine costs in patients with chronic obstructive pulmonary disease in India. Pharmacoeconomics Open 2019;4:331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CTRI/2014/08/004848. Assessment of quality of life in chronic lung disease [Evaluation of structured individualized pharmacist intervention programme on economic, clinical, and humanistic outcomes of COPD patients in a tertiary care hospital]. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=8428&EncHid=&modid=&compid=%27,%278428det%27 (first received 19 March 2012).
- Suhaj A, Manu MK, Unnikrishnan MK, Vijayanarayana K, Mallikarjuna RC. Effectiveness of clinical pharmacist intervention on health-related quality of life in chronic obstructive pulmonary disorder patients - a randomized controlled study. Journal of Clinical Pharmacy and Therapeutics 2016;41(1):78-83. [DOI] [PubMed] [Google Scholar]
- Suhaj A, Unnikrishnan M, Mohan MK, Rao CM, Vijayanarayana K. The effectiveness of clinical pharmacist intervention on health related quality of life in chronic obstructive pulmonary disorder patients - a randomized controlled study. Respirology 2015;20:58. [DOI] [PubMed] [Google Scholar]
Aboumatar 2017 {published data only}
- Aboumatar H, Naqibuddin M, Chung S, Adebowale H, Bone L, Brown T, et al. Better Respiratory Education and Treatment Help Empower (BREATHE) study: methodology and baseline characteristics of a randomized controlled trial testing a transitional care program to improve patient-centered care delivery among chronic obstructive pulmonary disease patients. Contemporary Clinical Trials 2017;62:159-67. [DOI] [PubMed] [Google Scholar]
- Thurber EG, Aboumatar H. Do patient comorbidities impact the effectiveness of a COPD self-management program? Journal of Clinical and Translational Science 2018;2(S1):41. [Google Scholar]
Alharbey 2019 {published data only}
- Alharbey R, Chatterjee S. An mHealth assistive system "MyLung" to empower patients with chronic obstructive pulmonary disease: design science research. JMIR Formative Research 2019;3(1):e12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Efraimsson 2008 {published and unpublished data}
- Efraimsson EÖ, Hillervik C, Ehrenberg A. Effects of COPD self-care management education at a nurse-led primary health care clinic. Scandinavian Journal of Caring Sciences 2008;22(2):178-85. [DOI] [PubMed] [Google Scholar]
Ghanem 2010 {published data only}
- Ghanem M, Abd ELaal E, Mehany M, Tolba K. Home-based pulmonary rehabilitation program: effect on exercise tolerance and quality of life in chronic obstructive pulmonary disease patients. Annals of Thoracic Medicine 2010;5(1):18-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heidari 2018 {published data only}
- Heidari M, Fayazi S, Borsi SH, Latifi M, Moradbeigi K, Torghi MT, et al. Effect of the 5A model on clinical status indexes of COPD patients. Rehabilitation Nursing 2018;43(3):158-66. [DOI] [PubMed] [Google Scholar]
- IRCT2013061713694N1. The effect of self management program on indicators of clinical status in patients with chronic obstructive pulmonary disease. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=IRCT2013061713694N1 (first received 21 November 2012).
Hill 2010 {published data only}
- Hill K, Mangovski-Alzamora S, Blouin M, Guyat G, Heels-Andell D, Bragaglia P, et al. Disease-specific education in the primary care setting increases the knowledge of people with chronic obstructive pulmonary disease: a randomized controlled trial. Patient Education and Counseling 2010;81(1):14-8. [DOI] [PubMed] [Google Scholar]
Jiang 2012 {published data only}
- Jiang X, He G. Effects of an uncertainty management intervention on uncertainty, anxiety, depression, and quality of life of chronic obstructive pulmonary disease outpatients. Research in Nursing and Health 2012;35(4):409-18. [DOI] [PubMed] [Google Scholar]
Khdour 2009 {published and unpublished data}
- Khdour MR, Agus AM, Kidney JC, Smyth BM, McElnay JC, Crealey GC. Cost-utility analysis of a pharmacy-led self-management programme for patients with COPD. International Journal of Clinical Pharmacy 2011;33(4):665-73. [DOI] [PubMed] [Google Scholar]
- Khdour MR, Kidney JC, Smyth BM, McElnay JC. Clinical pharmacy-led disease and medicine management programme for patients with COPD. British Journal of Clinical Pharmacology 2009;68(4):588-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2014 {published and unpublished data}
- Li J-M, Cheng S-Z, Cai W, Zhang Z-H, Liu Q-H, Xie B-Z, et al. Transitional care for patients with chronic obstructive pulmonary disease. International Journal of Nursing Sciences 2014;1(2):157-64. [Google Scholar]
Liu 2013 {published data only}
- Liu F, Cai H, Tang Q, Zou Y, Wang H, Xu Z, et al. Effects of an animated diagram and video-based online breathing program for dyspnea in patients with stable COPD. Patient Preference and Adherence 2013;7:905-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lou 2015 {published data only}
- Lou P, Chen P, Zhang P, Yu J, Wang Y, Chen N, et al. A COPD health management program in a community-based primary care setting: a randomized controlled trial. Respiratory Care 2015;60(1):102-12. [DOI] [PubMed] [Google Scholar]
Ozturk 2020 {published data only}
- Ozturk BO, Alpaydın AO, Ozalevli S, Guler N, Cimilli C. Self-management training in chronic obstructive lung disease improves the quality of life. Turkish Thoracic Journal 2020;21(4):266-73. [DOI: 10.5152/TurkThoracJ.2019.19015] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Boer 2011 {published data only}
- Boer L, Schermer T, Koopman A, Peters J, Heijdra Y, Vercoulen J, et al. Effects of case management on hospitalisation and exacerbation rate in severe, complex COPD: a randomized controlled trial. European Respiratory Journal 2011;38(55):230s [P1248]. [Google Scholar]
Bourne 2017 {published data only}
- Bourne C, Houchen-Wolloff L, Kanabar P, Bankart M, Singh S. A self-management programme of activity, coping and education - SPACE for COPD - in primary care: a pragmatic trial. Thorax 2018;73(Suppl 4):A167-8. [Google Scholar]
- Bourne CL, Kanabar P, Mitchell K, Schreder S, Houchen-Wolloff L, Bankart MJ, et al. A self-management programme of activity, coping and education - SPACE for COPD(C) - in primary care: the protocol for a pragmatic trial. BMJ Open 2017;7(7):e014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN17942821. A self-management programme of activity coping and education - SPACE for COPD - in primary care: a pragmatic trial. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN17942821 (first received 11 February 2015).
Cecere Feemster 2013 {published data only}
- Cecere Feemster LM, Collins BF, Uman J, Au DH. Factors explaining variation in dyspnea, health-related quality of life, and medication use among veterans with COPD. American Journal of Respiratory and Critical Care Medicine 2013;187:A5699. [Google Scholar]
Chen 2018 {published data only}
- Chen K-Y, Hung M-H, Chang M-H, Kuo C, Lin C-M, Chuang L-P, et al. Four-weeks remote pulmonary rehabilitation protocol with mobile apps of real-time heart rate monitoring for gold category B/C/D-A study design. Respirology 2018;23(Suppl 2):82. [Google Scholar]
ChiCTR1800018197 {published data only}
- ChiCTR1800018197. A real world study for the effect of TCM pulmonary rehabilitation training to protect the pulmonary function of patients with mild to moderate COPD. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ChiCTR1800018197 (first received 4 April 2018).
ChiCTR‐TRC‐12002559 {published data only}
- ChiCTR-TRC-12002559. The effects of a transitional care programme on patients with chronic diseases at high risk for readmission: a randomized controlled trial. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ChiCTR-TRC-12002559 (first received 9 October 2012).
Chien 2016 {published data only}
- Chien C-L, Liu Y-F, Liu W-T, Lu C-C, Wang P-C, Chiang L-L. Impact of multidisciplinary self-management education program in patients with chronic obstructive pulmonary disease: self-efficacy, exercise tolerance, and quality of life. American Journal of Respiratory and Critical Care Medicine 2016;193:A7863. [Google Scholar]
Costa 2015 {published data only}
- Costa F, Porcu A, Balestracci S, Mignani D, Magnani F, Pianta T, et al. Cost and effectiveness of 2 years integrated care intervention in COPD. European Respiratory Journal 2015;46:PA679. [Google Scholar]
Dewan 2011a {published data only}
- Dewan N, Rice K, Morrow L, Caldwell M. Long-term outcomes of disease management in chronic obstructive lung disease: results of VISN 23 randomized controlled trial. Chest 2011;140:921A. [Google Scholar]
Ding 2019 {published data only}
- Ding H, Karunanithi M, Ireland D, McCarthy L, Hakim R, Phillips K, et al. Evaluation of an innovative mobile health programme for the self-management of chronic obstructive pulmonary disease (MH-COPD): protocol of a randomised controlled trial. BMJ Open 2019;9(4):e025381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doheny 2013 {published data only}
- Doheny S, Lynch A, Dunican K, Cabrera A, Silva M. The effectiveness of pharmacist-provided self-management education to patients with chronic obstructive pulmonary disease. Journal of the American Pharmacists Association 2013;53(2):e107. [Google Scholar]
Duran 2017 {published data only}
- Duran CA, Winnicka L, Bista A, Charles K, Joshi A, Khan SU, et al. Reducing hospital readmission among high risk patients admitted with COPD exacerbation: randomized trial of a respiratory therapist conducted home-based structured intervention. American Journal of Respiratory and Critical Care Medicine 2017;195:A7005. [Google Scholar]
Ergan 2018 {published data only}
- Ergan B, Goktalay T, Ergun P, lmaz D, Ocakli B, Gurgun A, et al. A multicenter randomized trial for the effectiveness of structured discharge and follow-up protocol on readmission rate in COPD patients receiving LTOT/NIV: one-year interim analysis. European Respiratory Journal 2018;52(Suppl 62):OA5410. [Google Scholar]
Fleehart 2015 {published data only}
- Fleehart S, Nguyen HQ, Fan VS, Hunter C, Chen Z, Reinke LF. The effect of psychosocial behavioral therapy for patients with COPD and depression. American Journal of Respiratory and Critical Care Medicine 2015;191:A6395. [Google Scholar]
Gonzalez 2015 {published data only}
- Gonzalez IC, Espinosa RA, Hu C, Martinez BG, Miranda CF, Birlanga OM, et al. A self-treatment programme in patients with COPD: effectiveness for the reduction of severe exacerbations. European Respiratory Journal 2015;46(Suppl 59):PA3705. [Google Scholar]
Hernandez 2016 {published data only}
- Hernandez C, Seijas N, Folch A, Grane C, Orts I, Esquinas C, et al. Impact of a structured therapeutic education program for COPD patients on preventing readmissions (APRENDEEPOC Program). American Journal of Respiratory and Critical Care Medicine 2016;193:A5541. [Google Scholar]
Imanalieva 2016 {published data only}
- Imanalieva A, Vinnikov D, Brimkulov N. Patient education with telephone follow-up for chronic obstructive pulmonary disease and essential hypertension. European Respiratory Journal 2016;48:PA2063. [Google Scholar]
IRCT201504149014N61 {published data only}
- IRCT201504149014N61. Effect of self-care education versus no education on self-efficacy in patients with chronic obstructive pulmonary disease. en.irct.ir/trial/9500 (first received 25 April 2015).
IRCT2017030432764N2 {published data only}
- IRCT2017030432764N2. The effect of Orem Self Care Model on patients with chronic obstructive pulmonary disease. en.irct.ir/trial/25441 (first received 19 May 2017).
James 2012 {published data only}
- James S, Patry R. Pulmonary rehabilitation provided by a pharmacist and its impact on patient care. Journal of the American Pharmacists Association 2012;52:258. [Google Scholar]
Ko 2015 {published data only}
- Ko FW, Cheung N-K, Rainer T, Lum CC, Hui D. Intergrated care programme for patients with chronic obstructive pulmonary disease (COPD) - a randomized controlled trial. Respirology 2015;20:38. [DOI] [PubMed] [Google Scholar]
Moreno 2017 {published data only}
- Moreno MP, Lopez L, Torres I, Cabrera I, Ortiz A, Valenza CM. Cardiorespiratory functionality improvements after a brief occupational therapy intervention: a pilot study. European Respiratory Journal 2017;50(Suppl 61):x. [Google Scholar]
NCT02258646 {published data only}
- NCT02258646. Long-term integrated telerehabilitation of COPD patients. A multi-center trial. clinicaltrials.gov/show/NCT02258646 (first received 7 October 2014).
NCT02924870 {published data only}
- NCT02924870. Long-term effect of an health education program on daily physical activity in patients with moderate to very severe chronic obstructive pulmonary disease (EA-EPOC). clinicaltrials.gov/show/nct02924870 (first received 5 October 2016).
NCT03012256 {published data only}
- NCT03012256. The DIVERT-CARE (Collaboration Action Research & Evaluation) Trial (DIVERT-CARE) [The DIVERT-CARE (Collaboration Action Research & Evaluation) Trial: A multi provincial pragmatic cluster randomized trial of cardio-respiratory management in home care]. clinicaltrials.gov/show/NCT03012256 (first received 6 January 2016).
NCT03084874 {published data only}
- NCT03084874. Efficacy of a coaching program to promote physical activity and reduce sedentary behavior after a COPD hospitalization [Efficacy of a coaching program to promote physical activity and reduce sedentary behavior after a COPD exacerbation that required hospitalization]. clinicaltrials.gov/show/nct03084874 (first received 21 March 2017).
NCT03216603 {published data only}
- NCT03216603. Health literacy in people with chronic obstructive pulmonary disease [Health literacy. An intervention on organized information and health care for people with chronic obstructive pulmonary disease (COPD)]. ClinicalTrials.gov/show/NCT03216603 (first received 13 July 2017).
NCT03721315 {published data only}
- NCT03721315. Health literacy activation RCT among the COPD patients and designated support dyad [A randomized controlled trial of health literacy activation among the chronic obstructive pulmonary disease patients and designated support dyad]. clinicaltrials.gov/show/nct03721315 (first received 26 October 2018).
NL3827 (NTR4009) {published data only}
- NL3827 (NTR4009). PRACTISS COPD [Pulmonary rehabilitation of COPD: a trial of sustained internet based self-management support]. www.trialregister.nl/trial/3827 (first received 1 February 2013).
NL5277 (NTR5558) {published data only}
- NL5277 (NTR5558). Efficacy of a physical activity coaching system for patients with COPD [Effectiviteitsonderzoek naar een coachingsprogramma voor het behouden van fysieke activiteit in patienten met COPD]. www.trialregister.nl/trial/5277 (first received 1 April 2015).
Padilla‐Zarate 2013 {published data only}
- Padilla-Zarate MP, Cortes-Poza D, Martinez-Soto JM, Herrera-Cenobio T, Del Carmen Vazquez-Bautista M, Garcia-Flores M, et al. Self-care and quality of life after nursing counseling in patients with chronic obstructive pulmonary disease. Revista Mexicana de Enfermeria Cardiologica 2013;21(1):15-23. [Google Scholar]
Paquin 2014 {published data only}
- Paquin S, Landry L, Nault D, Dagenais J, Lefrancois E, St-Jules D, et al. Telehome care for patients with chronic pulmonary disease: the experience of a Canadian second line respiratory specialty care service. American Journal of Respiratory and Critical Care Medicine 2014;189:A1395. [Google Scholar]
Reguera 2017 {published data only}
- Reguera BJ, Lopez EM, Martin ML, Monteagudo LJ, Gutierrez NG, Casamitjana JV, et al. Efficacy of an integrated internet community program after pulmonary rehabilitation for COPD patients: a pilot randomized control trial. European Respiratory Journal 2017;50(Suppl 61):OA514. [Google Scholar]
Sano 2016 {published data only}
- Sano E, Ueki J, Sasaki S, Kuriyama S, Muraki K, Nagashima O, et al. Self-management education using interactive application software for tablet computer to improve health status in patients with COPD: a randomized controlled trial. European Respiratory Journal 2016;85:PA3736. [Google Scholar]
Siddharthan 2018 {published data only}
- Siddharthan T, Pollard SL, Quaderi SA, Mirelman AJ, Cárdenas MK, Kirenga B, et al. Effectiveness-implementation of COPD case finding and self-management action plans in low- and middle-income countries: global excellence in COPD outcomes (GECo) study protocol. Trials 2018;19(1):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sirichana 2014 {published data only}
- Sirichana W, Patel MH, Wang X, Taylor M, Barjaktarevic I, Kleerup EC, et al. Choices of spirometry measures for remote patient monitoring in COPD. American Journal of Respiratory and Critical Care Medicine 2014;189:A2971. [Google Scholar]
Thomas 2019 {published data only}
- Thomas B, Ridgeway JL, Novotny PJ, Benzo R. Home-based rehabilitation for COPD: a qualitative analysis of a large randomized study. American Journal of Respiratory and Critical Care Medicine 2019;199(9):A5735. [Google Scholar]
Zanaboni 2016 {published data only}
- Zanaboni P, Dinesen B, Hjalmarsen A, Hoaas H, Holland AE, Oliveira CC, et al. Long-term integrated telerehabilitation of COPD patients: a multicentre randomised controlled trial (iTrain). BMC Pulmonary Medicine 2016;16(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Agusti 2010
- Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respiratory Research 2010;11(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Agusti 2014
- Agusti A. The path to personalised medicine in COPD. Thorax 2014;69(9):857-64. [DOI] [PubMed] [Google Scholar]
Anthonisen 1987
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Annals of Internal Medicine 1987;106:196-204. [DOI] [PubMed] [Google Scholar]
BCT Taxonomy [Computer program]
- University College London BCT Taxonomy. Version accessed prior to 3 December 2020. London: University College London, 2020.
Benzo 2012
- Benzo R. Collaborative self-management in chronic obstructive pulmonary disease: learning ways to promote patient motivation and behavioral change. Chronic Respiratory Disease 2012;9(4):257-8. [DOI] [PubMed] [Google Scholar]
Bourbeau 2004
- Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Education and Counseling 2004;52(3):271-7. [DOI] [PubMed] [Google Scholar]
Bourbeau 2009
- Bourbeau J, Van der Palen J. Promoting effective self-management programmes to improve COPD. European Respiratory Journal 2009;33(3):461-3. [DOI] [PubMed] [Google Scholar]
Bourbeau 2015
- Bourbeau J, Lavoie KL, Sedeno M. Comprehensive self-management strategies. Seminars in Respiratory and Critical Care Medicine 2015;36(4):630-8. [DOI: 10.1055/s-0035-1556059] [DOI] [PubMed] [Google Scholar]
Cheruvu 2016
- Cheruvu VK, Odhiambo L A, Mowls DS, Zullo MD, Gudina AT. Health-related quality of life in current smokers with COPD: factors associated with current smoking and new insights into sex differences. International Journal of COPD 2016;11(1):2211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence 2016 [Computer program]
- Veritas Health Innovation Covidence. Version accessed prior to 2 December 2021. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Disler 2012
- Disler RT, Gallagher RD, Davidson PM. Factors influencing self-management in chronic obstructive pulmonary disease: an integrative review. International Journal of Nursing Studies 2012;49(2):230-42. [DOI] [PubMed] [Google Scholar]
Donaldson 2005
- Donaldson GC, Wilkinson TM, Hurst JR, Perera WR, Wedzicha JA. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2005;171(5):446-52. [DOI] [PubMed] [Google Scholar]
Effing 2009
- Effing T, Kerstjens H, Van der Valk P, Zielhuis G, Van der Palen J. (Cost)-effectiveness of self-treatment of exacerbations on the severity of exacerbations in patients with COPD: the COPE II study. Thorax 2009;64(11):956-62. [DOI] [PubMed] [Google Scholar]
Effing 2012
- Effing TW, Bourbeau J, Vercoulen J, Apter AJ, Coultas D, Meek P, et al. Self-management programmes for COPD: moving forward. Chronic Respiratory Disease 2012;9(1):27-35. [DOI] [PubMed] [Google Scholar]
Effing 2016
- Effing TW, Vercoulen JH, Bourbeau J, Trappenburg J, Lenferink A, Cafarella P. Definition of a COPD self-management intervention: international expert group consensus. European Respiratory Journal 2016;48:46-54. [DOI] [PubMed] [Google Scholar]
Godtfredsen 2002
- Godtfredsen NS, Vestbo J, Osler M, Prescott E. Risk of hospital admission for COPD following smoking cessation and reduction: a Danish population study. Thorax 2002;57(11):967-72. [DOI: 10.1136/thorax.57.11.967] [DOI] [PMC free article] [PubMed] [Google Scholar]
GOLD 2021
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2021 report. goldcopd.org/2021-gold-reports/ (accessed prior to 31 May 2021).
Goldstein 2014
- Goldstein R, Brooks D. Pulmonary rehabilitation at the time of the COPD exacerbation. Clinics in Chest Medicine 2014;35(2):391-8. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to May 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guyatt 1987
- Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987;42(10):773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brożek J, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Hayden 2009
- Hayden JA. Introduction to Health Behavior Theory. Mississauga (ON): Jones and Bartlett Learning, 2009. [Google Scholar]
Heslop‐Marshall 2014
- Heslop-Marshall K, De Soyza A. Are we missing anxiety in people with chronic obstructive pulmonary disease (COPD)? Annals of Depression and Anxiety 2014;1(5):1023. [Google Scholar]
Higgins 2019
- Higgings JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
Hill 2010
- Hill K, Mangovski‐Alzamora S, Blouin M, Guyatt G, Heels‐Andell D, Bragaglia P, et al. Disease‐specific education in the primary care setting increases the knowledge of people with chronic obstructive pulmonary disease: a randomized controlled trial. Patient Education and Counseling 2010;81(1):14-8. [DOI] [PubMed] [Google Scholar]
Holland 2010
- Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Archives of Physical Medicine and Rehabilitation 2010;91(2):221-5. [DOI] [PubMed] [Google Scholar]
Jaeschke 1989
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials 1989;10(4):407-15. [DOI: 10.1016/0197-2456(89)90005-6] [DOI] [PubMed] [Google Scholar]
Jolly 2016
- Jolly K, Majothi S, Sitch AJ, Heneghan NR, Riley RD, Moore DJ, et al. Self-management of health care behaviors for COPD: a systematic review and meta-analysis. International Journal of COPD 2016;17(11):305-26. [DOI: 10.2147/COPD.S90812] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jolly 2018
- Jolly K, Sidhu MS, Bates E, Majothi S, Sitch A, Bayliss S, et al. Systematic review of the effectiveness of community-based self-management interventions among primary care COPD patients. npj Primary Care Respiratory Medicine 2018;28(1):44. [DOI: 10.1038/s41533-018-0111-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2005
- Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD: Journal of Chronic Obstructive Pulmonary Disease 2005;2(1):75-9. [DOI] [PubMed] [Google Scholar]
Jonkman 2016
- Jonkman NH, Schuurmans MJ, Groenwold RH, Hoes AW, Trappenburg Jaap CA. Identifying components of self-management interventions that improve health-related quality of life in chronically ill patients: systematic review and meta-regression analysis. Patient Education and Counseling 2016;99(7):1087-98. [DOI] [PubMed] [Google Scholar]
Jordan 2015
- Jordan RE, Majothi S, Heneghan NR, Blissett DB, Riley RD, Sitch AJ, et al. Supported self-management for patients with moderate to severe chronic obstructive pulmonary disease (COPD): an evidence synthesis and economic analysis. Health Technology Assessment 2015;19(36):1-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 2011
- Kessler R, Partridge MR, Miravitlles M, Cazzola M, Vogelmeiere C, Leynaud D, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. European Respiratory Journal 2011;37(2):264-72. [DOI] [PubMed] [Google Scholar]
Kocks 2006
- Kocks JW, Tuinenga MG, Uil SM, Van den Berg JW, Ståhl E, Van der Molen T. Health status measurement in COPD: the minimal clinically important difference of the clinical COPD questionnaire. Respiratory Research 2006;7(1):62. [DOI: 10.1186/1465-9921-7-62] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lenferink 2017
- Lenferink A, Brusse-Keizer M, Van der Valk PD, Frith PA, Zwerink M, Monninkhof EM, et al. Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD011682. [DOI: 10.1002/14651858.CD011682.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lorig 2003
- Lorig KR, Holman HR. Self-management education: history, definition, outcomes, and mechanisms. Annals of Behavioral Medicine 2003;26(1):1-7. [DOI] [PubMed] [Google Scholar]
McCabe 2017
- McCabe C, McCann M, Brady AM. Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD011425. [DOI: 10.1002/14651858.CD011425.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2015
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD003793. [DOI: 10.1002/14651858.CD003793.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McLean 2011
- McLean S, Nurmatov U, Liu JL, Pagliari C, Car J, Sheikh A. Telehealthcare for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 7. Art. No: CD007718. [DOI: 10.1002/14651858.CD007718.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Michie 2011
- Michie S, Van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implementation Science 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michie 2013
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of Behavioral Medicine 2013;46(1):81-95. [DOI] [PubMed] [Google Scholar]
Mills 2014
- Mills A. Health care systems in low- and middle-income countries. New England Journal of Medicine 2014;370(6):552-7. [DOI: 10.1056/NEJMra1110897] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, on behalf of the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;339:[8 p.]. [DOI: 10.1136/bmj.b2535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Newham 2017
- Newham JJ, Presseau J, Heslop-Marshall K, Russell S, Ogunbayo OJ, Netts P, et al. Features of self-management interventions for people with COPD associated with improved health-related quality of life and reduced emergency department visits: a systematic review and meta-analysis. International Journal of COPD 2017;12:1705-20. [DOI] [PMC free article] [PubMed]
Nici 2012
- Nici L, ZuWallack R. An official American Thoracic Society Workshop Report: the integrated care of the COPD patient. Proceedings of the American Thoracic Society 2012;9(1):9-18. [DOI] [PubMed] [Google Scholar]
Nici 2014
- Nici L, Bontly TD, ZuWallack R, Gross N. Self-management in chronic obstructive pulmonary disease: time for a paradigm shift? Annals of the American Thoracic Society 2014;11(1):101-7. [DOI] [PubMed] [Google Scholar]
Redelmeier 1996
- Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. Journal of Clinical Epidemiology 1996;49(11):1215-9. [DOI: 10.1016/s0895-4356(96)00206-5] [DOI] [PubMed] [Google Scholar]
RevMan Web 2021 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). Version 3.0.1. The Cochrane Collaboration, 2021. Available at revman.cochrane.org.
Rodriguez‐Roisin 2000
- Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000;117(5):398S-401S. [DOI] [PubMed] [Google Scholar]
Rosenstock 1974
- Rosenstock IM. Historical origins of the health belief model. Health Education and Behavior 1974;2(4):328-35. [DOI: 10.1177/109019817400200403] [DOI] [Google Scholar]
Singh 2017
- Singh D, Miravitlles M, Vogelmeier C. Chronic obstructive pulmonary disease individualized therapy: tailored approach to symptom management. Advances in Therapy 2017;34(2):281-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2021
- Song X, Hallensleben C, Zhang W, Jiang Z, Shen H, Gobbens RJ, et al. Blended self-management interventions to reduce disease burden in patients with chronic obstructive pulmonary disease and asthma: systematic review and meta-analysis. Journal of Medical Internet Research 2021;23(3):e24602. [DOI] [PMC free article] [PubMed]
Spinhoven 1997
- Spinhoven PH, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the hospital anxiety and depression scale (HADS) in different groups of Dutch subjects. Psychological Medicine 1997;27(2):363-70. [DOI: 10.1017/s0033291796004382] [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI: 10.1136/bmj.l4898] [DOI] [PubMed] [Google Scholar]
Toy 2010
- Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. Journal of Chronic Obstructive Pulmonary Disease 2010;7(3):214-28. [DOI] [PubMed] [Google Scholar]
Trappenburg 2013
- Trappenburg J, Jonkman N, Jaarsma T, Van Os-Medendorp H, Kort H, Wit N, et al. Self-management: one size does not fit all. Patient Education and Counseling 2013;92(1):134-7. [DOI] [PubMed] [Google Scholar]
Van Eerd 2016
- Van Eerd EA, Van der Meer RM, Van Schayck OC, Kotz D. Smoking cessation for people with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD010744. [DOI: 10.1002/14651858.CD010744.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Visual Rx 2016 [Computer program]
- Visual Rx. Version 4. Cates C, 2016. Available at www.nntonline.net/visualrx/.
WHO 2018
- World Health Organization (WHO). Updated WHO projections of mortality and causes of death, 2016 to 2060; March 2018. Available at www.who.int/healthinfo/global_burden_disease/projections_method.pdf.
WHO 2019
- World Health Organization (WHO). International Statistical Classification of Diseases and Related Health Problems (ICD)-11; September 2019. Available at https://www.who.int/classifications/classification-of-diseases.
WHO 2020
- World Health Organization (WHO). The top 10 causes of death; December 2020. Available at www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed prior to 5 July 2021).
World Bank 2021
- World Bank. New country classifications by income level: 2020-2021. datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed prior to 30 May 2021).
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67(6):361-70. [DOI: 10.1111/j.1600-0447.1983.tb09716.x] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Effing 2007
- Effing TW, Monninkhof EM, Van der Valk PD, Zielhuis GA, Van Herwaarden CL, Partridge MR, et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD002990. [DOI: 10.1002/14651858.CD002990.pub2] [DOI] [PubMed] [Google Scholar]
Monninkhof 2002
- Monninkhof EM, Van der Valk PD, Van der Palen J, Van Herwaarden CL, Partidge MR, Walters EH, et al. Self-management education for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2002, Issue 4. Art. No: CD002990. [DOI: 10.1002/14651858.CD002990] [DOI] [PubMed] [Google Scholar]
Monninkhof 2003
- Monninkhof E, Van der Valk P, Van der Palen J, Van Herwaarden C, Partridge MR, Zielhuis G. Self-management education for patients with chronic obstructive pulmonary disease: a systematic review. Thorax 2003;58(5):394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zwerink 2014
- Zwerink M, Brusse-Keizer M, Van der Valk PD, Zielhuis GA, Monninkhof EM, Van der Palen P, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD002990. [DOI: 10.1002/14651858.CD002990.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]