Abstract
Background
Fructose consumption increases risk factors for cardiometabolic disease. It is assumed that the effects of free sugars on risk factors are less potent because they contain less fructose. We compared the effects of consuming fructose, glucose or their combination, high fructose corn syrup (HFCS), on cardiometabolic risk factors.
Methods
Adults (18–40 years; BMI 18–35 kg/m2) participated in a parallel, double-blinded dietary intervention during which beverages sweetened with aspartame, glucose (25% of energy requirements (ereq)), fructose or HFCS (25% and 17.5% ereq) were consumed for two weeks. Groups were matched for sex, baseline BMI and plasma lipid/lipoprotein concentrations. 24-h serial blood samples were collected at baseline and after the intervention. Primary outcomes were 24-h triglyceride AUC, LDL-cholesterol (C), and apolipoprotein (apo)B. Interactions between fructose and glucose were assessed post hoc.
Findings
145 subjects (26.0±5.8 years; body mass index 25.0±3.7 kg/m2) completed the study. As expected, the increase of 24-h triglycerides compared with aspartame was highest during fructose consumption (25%: 6.66 mmol/Lx24h 95% CI [1.90 to 11.63], P=0.0013 versus aspartame), intermediate during HFCS consumption (25%: 4.68 mmol/Lx24h 95% CI [−0.18 to 9.55], P=0.066 versus aspartame) and lowest during glucose consumption. In contrast, the increase of LDL-C was highest during HFCS consumption (25%: 0.46 mmol/L 95% CI [0.16 to 0.77], P=0.0002 versus aspartame) and intermediate during fructose consumption (25%: 0.33 mmol/L 95% CI [0.03 to 0.63], P=0.023 versus aspartame), as was the increase of apoB (HFCS-25%: 0.108 g/L 95%CI [0.032 to 0.184], P=0.001; fructose 25%: 0.072 g/L 95%CI [−0.004 to 0.148], P=0.074 versus aspartame).
The post hoc analyses showed significant interactive effects of fructose*glucose on LDL-C and apoB (both P<0.01), but not on 24-h triglyceride (P=0.340).
Conclusion
A significant interaction between fructose and glucose contributed to increases of lipoprotein risk factors when the two monosaccharides were co-ingested as HFCS. Thus, the effects of HFCS on lipoprotein risks factors are not solely mediated by the fructose content and it cannot be assumed that glucose is a benign component of HFCS. Our findings suggest that HFCS may be as harmful as isocaloric amounts of pure fructose and provide further support for the urgency to implement strategies to limit free sugar consumption.
Keywords: Glucose, Fructose, Interaction, Diet Intervention Trial, Lipoproteins
Background
Global sugar consumption is at an all-time high 1. This causes reason for concern as sugar consumption appears to be causal or contributory in the development of metabolic diseases. Clinical trials 2,3 and literature reviews 4,5 suggest that it is the unregulated metabolism of fructose in the liver that mediates many of these undesirable health outcomes. Therefore, the French Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement, et du travail (ANSES) recommended an upper limit of 100 g total sugar/day 5, which is approximately twice the upper limit recommended by the World Health Organization 1. However, this recommendation is based on evidence suggesting that 50 g of pure fructose/day is a safe level of consumption and on the assumption that only the fructose component, and not the glucose component, contributes to the metabolic dysregulation resulting from the consumption of sucrose or high fructose corn syrup (HFCS). Therefore, since 100 g of sucrose contain 50 g of fructose, 100 g of sucrose/day would equal a safe level of consumption. However, we previously reported results that do not support this assumption that the adverse metabolic effects of sugar consumption are solely proportional to the fructose content 6. We investigated the effects of consuming 25% energy requirement (ereq) as HFCS- (HFCS-55: 55% fructose, 45% glucose)-, fructose- or glucose-sweetened beverages on risk factors for CVD in young adults. The increases of low density lipoprotein cholesterol (LDL-C), non-high density lipoprotein cholesterol (nonHDL-C) and apolipoprotein B (apoB) induced by HFCS were not at a level approximating 55% of the increases induced by isocaloric 100% fructose; instead they tended to exceed the increases induced by pure fructose. We concluded additional studies were needed to confirm this unexpected pattern 6. The objectives of the current study were to confirm this unexpected pattern in a larger number of subjects and with additional groups consuming HFCS or fructose, and to determine if interactions between glucose and fructose contributed to the adverse effects of consuming HFCS on some risk factors. Thus, we implemented post hoc statistical testing for interactions between fructose and glucose in subjects consuming beverages sweetened with fructose, glucose, HFCS or aspartame.
Methods
Study design –
Participants in this study are a subset from a 5 year National Institutes of Health (NIH)-funded project. The University of California, Davis (UCD) Institutional Review Board approved the experimental protocol. A detailed study protocol can be found in Supplement 1. This study was a double-blinded, parallel assignment, diet intervention study with three phases: 1) a 3.5-day inpatient baseline period during which subjects resided at the UCD Clinical and Translational Science Center’s Clinical Research Center (CCRC); 2) a 12-day outpatient intervention period; and 3) a 3.5-day inpatient intervention period at the CCRC. During day 2 and day 3 of the baseline and intervention inpatient periods, the subjects were provided and consumed energy-balanced meals consisting of conventional foods. Daily energy requirements (ereq) were calculated by the Mifflin equation 7 with adjustment for activity of 1.3 on the days of the 24-h serial blood collections, and adjustment of 1.5 for the other inpatient days. The baseline inpatient diet contained 55% of energy mainly as complex carbohydrate, 30% fat, and 15% protein. The meals during the intervention inpatient period were comparable to baseline meals, except for the substitution of complex carbohydrate for an isocaloric amount of sugar in the assigned drink. The timing of inpatient meals and their energy distribution were: breakfast, 09:00 (25% of daily ereq); lunch, 13:00 (35% of daily ereq); dinner, 18:00 (40% of daily ereq).
Participants –
Participants were recruited from October 2008 to February 2014 through online listings (craigslist.com) and local flyers. Eligibility was assessed through telephone and in-person interviews with medical history, complete blood count, and serum biochemistry panel. Inclusion criteria included age (18–40 y), body mass index (BMI) (18–35 kg/m2), and self-reported stable body weight during the prior 6 months. Exclusion criteria; detailed in Supplement 1; included absence of disease, atypical eating patterns, and a high level of exercise. All subjects provided informed written consent.
Group assignment –
Assignment to the groups was not randomized; the experimental groups were matched for sex, BMI, and concentrations of fasting triglyceride (TG), cholesterol, HDL-C, and insulin in plasma collected during the in-person interviews. The study included eight experimental groups with a total of 187 participants and was designed to compare the metabolic effects of consuming beverages sweetened with aspartame (Asp) (non-caloric control), glucose (25% ereq (G25)), fructose (25% (F25) and 17.5% ereq (F17.5)), HFCS (25% (HFCS25), 17.5% (HFCS17.5) and 10% ereq) and sucrose (25% ereq). We previously reported data from 16 of 28 participants from each of 3 groups (25% ereq fructose (F25), 25% ereq glucose (G25) and 25% ereq HFCS (HFCS25)) 6 and the data from 4 groups (Asp, 10% ereq HFCS, 17.5% ereq HFCS (HFCS17.5), HFCS25) 8.
Study Beverages –
All beverages were prepared by the study staff. Sugar-sweetened beverages were flavored with an unsweetened drink mix (Kool-Aid; Kraft Foods, Northfield, IL) in addition to the respective sugar. Asp-sweetened beverages contained a fruit-flavored drink mix (Market Pantry®, Target, Minneapolis, MN). The sugar-sweetened beverages contained glucose (STALEYDEX® crystalline dextrose, Tate & Lyle, Hoffman Estates, IL, USA), fructose (KRYSTAR® crystalline fructose, Tate & Lyle, Hoffman Estates, IL, USA), or HFCS (ISOSWEET® 5500, Tate & Lyle, Hoffman Estates, IL, USA). These beverages were formulated by a designated staff person as 15% sugar in water (weight/weight). The amount of beverage provided (mean ± SE for all subjects: 1081 ± 12 grams divided into three servings) was standardized among the 6 groups and based on individual energy requirements. During the outpatient phase, the subjects were instructed to drink three servings per day of the provided beverages, to consume their usual diet, and to refrain from consuming other sugar-containing beverages, including fruit juice. To monitor compliance, a biomarker (riboflavin) as added to the beverages and fluorometrically measured in urine samples 8. Subjects were informed that they were being monitored to ensure beverage consumption, but were not provided details about the method. Urinary riboflavin was assessed in urine samples collected during baseline, during the middle and end of outpatient intervention, and during inpatient intervention.
Procedures
Blood pressure was measured during the morning and evening of each inpatient day and results are reported in Table 1 in Supplement 2. Subjects were instructed to continue their normal activity during the outpatient intervention period and to not introduce new exercise or workout routines. Physical activity was not monitored objectively; subjects were asked to fill out modified versions of the Baecke Physical Activity Questionnaire 9 at the CCRC screening visit and during the first day of the intervention inpatient period. The intervention questionnaire queried the physical activity specific to the prior 2-week outpatient period. The questionnaires were analyzed to quantify physical activity (sports, workouts, exercise programs, plus biking and/or walking to work and/or classes) in hours/week. 24-h serial blood collections, consisting of 32 samples collected every 30 or 60 minutes were performed on the third day of the inpatient period at baseline and intervention. Additional blood was collected at three fasting draws (08:00, 08:30 and 09:00 h) and three late-night postprandial draws (22:00, 23:00 and 24:00 h); the plasma was pooled and multiple aliquots of the fasting pool and postprandial pool were stored. The timing of the late-night postprandial period was based on our previous studies in which TG peaked around 23:00 h 3,10.
Primary and secondary outcomes –
The primary outcomes of this study were the changes (2wk-0wk) in 24-h TG area under the curve (AUC), fasting plasma levels of nonHDL-C, apoB, and LDL-C. Secondary outcomes included the changes in 24-h uric acid AUC, postprandial levels for apoCIII, postprandial levels of LDL-C, nonHDL-C and apoB, fasting oxidized (ox)LDL, 24-h plasma glucose and insulin concentrations, amplitudes of post-meal glucose and insulin peaks, and body weight.
Plasma concentrations of triglyceride, uric acid, insulin, and glucose were measured in the 32 serial plasma samples and the 24-h AUC was calculated by the trapezoidal method. Glucose and insulin amplitudes were calculated as the difference between the post-meal peak concentrations minus the pre-meal nadirs for each meal and averaged for the 3 meals. Glucose was measured with an automated glucose analyzer (YSI, Inc., Yellow Springs, OH) and insulin with radioimmunoassay (Millipore). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from the means of the glucose and insulin concentrations in three fasting samples [fasting plasma insulin (μU/ml) x fasting plasma glucose (mmol/22.5)] and results are shown in Table 1 in Supplement 2. The concentrations of triglyceride, uric acid, cholesterol, HDL-C, LDL-C, apoB and apoCIII were measured with the Polychem Chemistry Analyzer (PolyMedCo Inc.) with reagents from MedTest DX. Fasting concentrations of oxidized LDL (oxLDL) were measured via ELISA (Mercodia, Uppsala, Sweden). The intra- and inter-assay coefficients of variation for our laboratory were as follows: triglyceride: 3.1%, 7.6% (intra-assay, inter-assay); total cholesterol: 2.3%, 4.4%; HDL cholesterol: 3.0%, 5.5%; direct LDL cholesterol: 2.4%, 4.7%; apoB: 3.5%, 6.9%; apoCIII: 2.0%, 6.5%; uric acid: 1.9%, 5.6%; oxLDL: 5.3%, 7.6%, glucose: 3.6%, 4.5%, and insulin: 6.5%, 7.6%.
Statistical analysis –
We based the initial sample size calculation on the effect sizes (mean group difference/standard deviation) obtained for fasting apolipoprotein B (apoB) and small dense LDL-C (1.23 and 0.96 respectively) in our previous study comparing the effects of consuming 25% ereq as fructose- or glucose-sweetened 3. It indicated that 25 subjects per group would be sufficient to detect differences in apoB with a significance of P < 0.05 and 80% power in a 7-group analyses. This sample size was not achieved in all groups due to funding constraints; and was exceeded in the F25, G25 and HFCS25 groups in order to pursue the aims of an NIH-funded ancillary project (1R01 HL107256).
The change (Δ: intervention minus baseline) for each outcome was analyzed by multivariable (group, sex) generalized linear model analysis of covariance (ANCOVA) (SAS 9.4). The model was adjusted for BMI and outcome at baseline. This model allowed for testing of outcomes that were significantly different from baseline concentrations as least squares means of Δ different from zero and identified significant differences between groups by Tukey-Kramer’s multiple-comparisons test. P-values <0.05 were considered significant.
Post hoc statistical analyses –
To determine the effects of fructose and glucose and their interaction, Δ of each outcome was analyzed by multivariable (fructose, glucose, sex) generalized linear model ANCOVA that included the interaction term fructose*glucose. Each beverage intervention was described in the model as its proportion of fructose and glucose as separate variables (Table 3 in Supplement 2), e.g. the 25% and 17.5% ereq fructose-SB was inputted as 100 or 70, respectively, for the fructose variable and 0 for the glucose variable, while the 25% ereq HFCS-SB was inputted as 55 for fructose and 45 for glucose. The model included adjustment for BMI and outcome at baseline. The proportion of variance explained by the covariates was calculated as follows: (type III sum of squares/corrected total sum of squares) * 100.
A multivariable regression model assessed the effects of the mean Δ of plasma glucose or insulin amplitudes on ΔnonHDL-C, ΔLDL-C, ΔapoB, Δ24-h TG AUC, Δpostprandial apoCIII, Δ24-h uric acid AUC in all groups and Δfasting oxLDL in the four groups consuming fructose-containing beverages. The model was adjusted for sex, BMI, and outcome at baseline.
The study was registered with clinicaltrials.gov, identifier NCT01103921.
Results
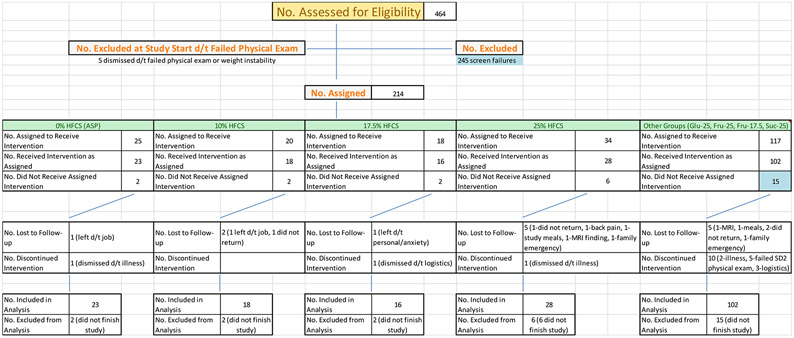
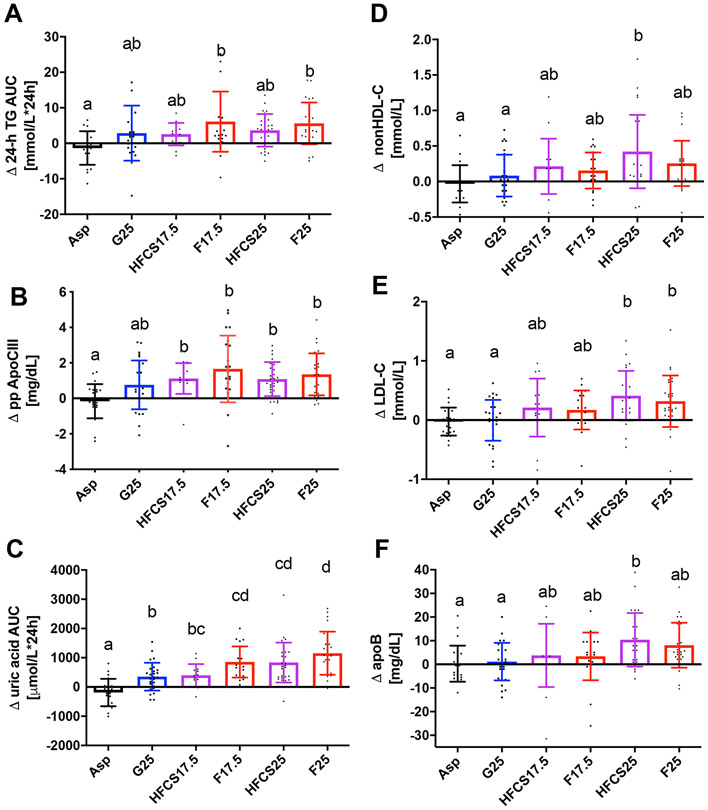
Two hundred and six healthy young adults were allocated into one of eight intervention groups during October 2008 to February 2014 (Figure 1). Here we report on one hundred and fifty-nine subjects assigned to Asp, G25, HFCS17.5, F17.5, HFCS25 and F25. Of these 159 subjects, seven ended their participation, and one was dismissed for a medical reason (kidney stone), prior to receiving intervention beverages. After receiving intervention, five subjects discontinued their participation – one had scheduling conflicts with their job (Asp), one experienced back pain (HFCS25), one was dismissed due to magnetic resonance imaging (MRI) finding (kidney stone) (HFCS25), one subject had a family emergency preventing study completion (HFCS17.5) and one subject was lost to follow-up (HFCS25). The samples from one subject were not analyzed due to sickness (vomiting) during the final 24-h serial blood collection (HFCS25). The data from one subject (HFCS17.5) were included in the fasting analyses, but not postprandial analyses, because a family emergency prevented completion of the 24-h serial blood collection during the intervention period. The current article reports the results from 145 participants consuming beverages containing Asp (n = 23), G25 (n = 28), F25 (n = 28), F17.5 (n = 22), HFCS25 (n = 28) and HFCS17.5 (n = 16). The baseline characteristics in the six experimental groups are presented in Table 1. There were no significant differences in anthropometric or metabolic parameters between the six groups of subjects. We found no differences in urinary riboflavin concentrations between the groups or between the unmonitored (outpatient) and monitored (inpatient) phases of the study (Figure 1 in Supplement 2). Subjects reported 4.6 ± 3.4(SD) hour/week of physical activity at screening and they reported 3.8 ± 3.1 hours/week physical activity on the first day of inpatient intervention. The decrease in reported activity during the 2 weeks of study compared to pre-study was significant (P = 0.005), but was not affected by group (p= 0.75). None of the main outcomes (TG, lipoproteins, uric acid) were affected by the reported pre-study level of physical activity nor by reported change in physical activity. Table 2 contains all baseline values and adjusted differences from baseline alongside the results of the multivariable ANCOVA (effects of group and sex). The intervention (effect of group) significantly affected all reported outcomes, excluding body weight and fasting apoCIII. The differences between the groups (Tukey-Kramer’s post-test) for the primary outcomes are presented in Figure 2. The changes of TG 24-h-AUC in the F25 and F17.5, groups were larger compared with Asp (Figure 2A). The changes of nonHDL-C, LDL-C, and apoB in the HFCS25 group were significantly greater than in the Asp and G25 groups (Figure 2D-F). The consumption of F25 led to larger increases of LDL-C than Asp or G25 (Figure 2E). There were no statistically significant differences between the F25 and HFCS25 or between F17.5 and HFCS17.5, however the results confirm the unexpected pattern we previously reported in a subset of subjects 6, both HFCS groups tended to have higher increases of nonHDL-C, LDL-C and apoB (Table 2) than the subjects consuming fructose at the same level (% ereq).
Figure 1. Trial profile.
LTF – lost to follow-up; Discon. – discontinued participation
Table 1.
Baseline anthropometric and metabolic parameters
| Aspartame-0% | Glucose-25% | HFCS-17.5% | Fructose-17.5% | HFCS-25% | Fructose-25% | |
|---|---|---|---|---|---|---|
| No. of participants | 23 | 28 | 16 | 22 | 28 | 28 |
| Female (%) | 12 (52.2) | 13 (46.4) | 9 (56.2) | 11 (50) | 13 (46.4) | 13 (46.4) |
| Age, y | 25 ± 6 | 26 ± 6 | 24 ± 5 | 26 ± 5 | 27 ± 7 | 27 ± 6 |
| BMI, kg/m2 | 24.8 ± 3.3 | 25.8 ± 3.4 | 24.2 ± 3.3 | 24.8 ± 4.4 | 24.9 ± 4.0 | 25.4 ± 3.7 |
| Body fat, % | 27.0 ± 9.8 | 28.9 ± 8.4 | 25.9 ± 9.6 | 27.0 ± 9.7 | 26.0 ± 9.7 | 29.0 ± 10.3 |
| Waist circumference, cm | 75.2 ± 6.4 | 79.0 ± 9.3 | 73.3 ± 7.7 | 76.3 ± 10.8 | 77.0 ± 10.1 | 78.3 ± 10.2 |
| Fst glucose, mmol/L | 5.02 ± 0.37 | 4.99 ± 0.34 | 4.98 ± 0.34 | 4.96 ± 0.34 | 5.03 ± 0.35 | 5.02 ± 0.4 |
| Fst insulin, pmol/L | 87.9 ± 37.9 | 88.8 ± 30.7 | 81.7 ± 19.3 | 93.2 ± 31 | 90.4 ± 35.8 | 102.5 ± 68.4 |
| Fst TG, mmol/L | 1.14 ± 0.59 | 1.15 ± 0.53 | 1.1 ± 0.39 | 1.39 ± 0.52 | 1.22 ± 0.57 | 1.12 ± 0.39 |
| Fst C, mmol/L | 3.86 ± 0.66 | 4.19 ± 0.8 | 4.27 ± 0.9 | 4.21 ± 0.77 | 4.08 ± 0.89 | 3.9 ± 0.64 |
| Fst HDL-C, mmol/L | 1.02 ± 0.19 | 1.18 ± 0.39 | 1.2 ± 0.24 | 1.1 ± 0.2 | 1.18 ± 0.36 | 1.15 ± 0.24 |
| Fst LDL-C, mmol/L | 2.17 ± 0.6 | 2.39 ± 0.78 | 2.42 ± 0.85 | 2.39 ± 0.64 | 2.37 ± 0.71 | 2.16 ± 0.64 |
| Fst uric acid, μmol/L | 272.1 ± 62.4 | 283.9 ± 65.5 | 261.9 ± 47.7 | 278.7 ± 54.9 | 270.6 ± 69.7 | 268.8 ± 57.5 |
Abbreviations: BMI=body mass index; fst=fasting; TG=triglycerides; C=cholesterol
Conversion factors: To convert glucose to mg/dL, divide by 0.0555; insulin to μU/mL, divide by 6.945; triglycerides to mg/dL, divide by 0.0113; cholesterol to ml/dL, divide by 0.0295 uric acid to ml/dL divide by 59.485
Values are mean ± SD
Table 2.
Body weight and plasma concentrations of risk factors at baseline and adjusted difference after consuming Aspartame or sugar-sweetened beverages for two weeks.
| Aspartame | Glucose-25% | HFCS-17.5% | Fructose-17.5% | HFCS-25% | Fructose-25% | effect of |
Total variation accounted for in % [95% CI] a |
p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Body weight, kg | |||||||||
| Baseline | 71.8 ± 10.6 | 75.5 ± 12.8 | 69.9 ± 14.3 | 72.5 ± 15 | 72.9 ± 14.5 | 75.7 ± 12.9 | |||
| Δ | −0.03 | 0.56 | 0.32 | 0.02 | 0.79 | 0.07 | sugar | 6.55% [0.00% to 12.65%] | 0.090 |
| [95% CI] | [−0.53 to 0.47] | [0.11 to 1.02] | [−0.28 to 0.92] | [−0.49 to 0.53] | [0.34 to 1.24] | [−0.38 to 0.53] | sex | 0.60% [0.00% to 5.43%] | 0.347 |
| FST non–HDL cholesterol, mmol/L | |||||||||
| Baseline | 2.84 ± 0.67 | 3.01 ± 0.8 | 3.07 ± 0.8 | 3.11 ± 0.76 | 2.9 ± 0.8 | 2.75 ± 0.62 | |||
| Δ | −0.07 | 0.06 | 0.24 | 0.19 | 0.38 | 0.22 | sugar | 14.18% [2.95% to 22.41%] | 0.000 |
| [95% CI] | [−0.22 to 0.07] | [−0.08 to 0.19] | [0.07 to 0.42] | [0.04 to 0.34] | [0.24 to 0.51] | [0.09 to 0.35] | sex | 1.82% [0.00% to 8.20%] | 0.081 |
| PP non–HDL cholesterol, mmol/L | |||||||||
| Baseline | 2.62 ± 0.68 | 2.78 ± 0.79 | 2.92 ± 0.8 | 2.98 ± 0.71 | 2.65 ± 0.71 | 2.51 ± 0.63 | |||
| Δ | −0.05 | 0.14 | 0.38 | 0.32 | 0.51 | 0.41 | sugar | 22.34% [8.96% to 31.39%] | <0.0001 |
| [95% CI] | [−0.20 to 0.09] | [0.00 to 0.27] | [0.19 to 0.56] | [0.17 to 0.47] | [0.38 to 0.64] | [0.28 to 0.55] | sex | 4.73% [0.29% to 13.02%] | 0.003 |
| FST LDL cholesterol, mmol/L | |||||||||
| Baseline | 2.17 ± 0.6 | 2.39 ± 0.78 | 2.42 ± 0.85 | 2.39 ± 0.64 | 2.37 ± 0.71 | 2.16 ± 0.64 | |||
| Δ | −0.04 | 0.00 | 0.25 | 0.19 | 0.42 | 0.29 | sugar | 16.94% [4.88% to 25.54%] | <0.0001 |
| [95% CI] | [−0.20 to 0.11] | [−0.14 to 0.14] | [0.07 to 0.43] | [0.03 to 0.34] | [0.28 to 0.56] | [0.15 to 0.43] | sex | 0.6% [0% to 6.87%] | 0.150 |
| PP LDL cholesterol, mmol/L | |||||||||
| Baseline | 80 ± 22 | 86 ± 29 | 89 ± 28 | 90 ± 22 | 86 ± 26 | 80 ± 23 | |||
| Δ | 0.00 | 0.10 | 0.31 | 0.22 | 0.50 | 0.27 | sugar | 16.58% [4.58% to 25.15%] | <0.0001 |
| [95% CI] | [−5.49 to 5.85] | [−1.24 to 9.07] | [4.72 to 18.89] | [2.51 to 14.15] | [14.35 to 24.61] | [5.15 to 15.51] | sex | 3.16% [0.00% to 10.65%] | 0.017 |
| FST apoB, mg/dL | |||||||||
| Baseline | 65 ± 17 | 73 ± 24 | 69 ± 22 | 73 ± 23 | 70 ± 19 | 64 ± 14 | |||
| Δ | −0.01 | 0.04 | 0.12 | 0.11 | 0.27 | 0.18 | sugar | 12.3% [1.9% to 20.2%] | 0.0001 |
| [95% CI] | [−4.18 to 3.52] | [−2.04 to 4.96] | [−0.10 to 9.15] | [0.32 to 8.21] | [6.98 to 13.93] | [3.35 to 10.38] | sex | 2.7% [0.0% to 9.8%] | 0.030 |
| PP apoB, mg/dL | |||||||||
| Baseline | 62 ± 17 | 69 ± 24 | 66 ± 20 | 72 ± 19 | 65 ± 18 | 61 ± 15 | |||
| Δ | −1.21 | 2.97 | 7.77 | 4.9 | 11.89 | 8.07 | sugar | 16.04% [4.20% to 24.50%] | <0.0001 |
| [95% CI] | [−5.09 to 2.67] | [−0.56 to 6.49] | [2.95 to 12.58] | [0.90 to 8.89] | [8.38 to 15.39] | [4.53 to 11.61] | sex | 4.80% [0.30% to 13.00%] | 0.004 |
| FST apoCIII, mg/dL | |||||||||
| Baseline | 7.3 ± 2.5 | 7.9 ± 2.0 | 8.1 ± 1.9 | 8.2 ± 2.1 | 8.2 ± 2.7 | 7.4 ± 1.9 | |||
| Δ | −0.05 | 0.63 | 0.53 | 0.77 | 0.63 | 0.55 | sugar | 4.33% [0.00% to 9.22%] | 0.270 |
| [95% CI] | [−0.56 to 0.45] | [0.18 to 1.09] | [−0.07 to 1.13] | [0.26 to 1.29] | [0.18 to 1.09] | [0.10 to 1.01] | sex | 4.80% [0.33% to 13.05%] | 0.008 |
| PP apoCIII. mg/dL | |||||||||
| Baseline | 6.7 ± 3.0 | 7.4 ± 2.2 | 7.5 ± 2.1 | 7.6 ± 1.9 | 7.4 ± 2.5 | 7 ± 1.9 | |||
| Δ | −0.12 | 0.7 | 1.19 | 1.67 | 1.08 | 1.32 | sugar | 16.73% [4.90% to 25.33%] | <0.0001 |
| [95% CI] | [−0.63 to 0.39] | [0.23 to 1.16] | [0.56 to 1.82] | [1.14 to 2.19] | [0.61 to 1.54] | [0.86 to 1.79] | sex | 2.13% [0.00% to 8.79%] | 0.056 |
| 24-h AUC uric acid.μmol/Lx24-h | |||||||||
| Baseline | 104 ± 24 | 108 ± 26 | 101 ± 20 | 106 ± 21 | 102 ± 27 | 102 ± 23 | |||
| Δ | −184.4 | 348.6 | 399.1 | 854.8 | 822.7 | 1142.7 | sugar | 36.84% [22.47% to 45.61%] | <0.0001 |
| [95% CI] | [−416 to 48] | [137 to 559] | [113 to 690] | [619 to 1095] | [613 to 1035] | [934 to 1356] | sex | 2.92% [0.00% to 10.17%] | 0.01 |
| 24-h AUC triglyceride mmol/Lx24-h | |||||||||
| Baseline | 29.4 ± 17.9 | 31.8 ± 16.4 | 29.7 ± 11.3 | 35.9 ± 14.5 | 32.2 ± 14.7 | 29.3 ± 11.6 | |||
| Δ | −1.11 | 2.62 | 2.96 | 5.82 | 3.57 | 5.65 | sugar | 12.40% [1.86% to 20.31%] | 0.001 |
| [95% CI] | [−3.57 to 1.36] | [0.37 to 4.86] | [−0.10 to 6.02] | [3.28 to 8.37] | [1.33 to 5.80] | [3.40 to 7.90] | sex | 0.98% [0.00% to 6.45%] | 0.196 |
Adjusted difference in means from a multivariate regression model (sugar, sex) adjusted for outcome at baseline and BMI
Data were estimated from Multivariate regression model (sugar, sex) adjusted for outcome at baseline and BMI
FST – fasting; PP – postprandial; Baseline data is presented as mean ± SD
Conversion factors: To convert triglyceride to mg/dL, divide by 0.0113; cholesterol to ml/dL, divide by 0.0295 uric acid to ml/dL divide by 59.485
Figure 2.
Changes (Δ - 2wk - 0wk) in 24-h TG AUC (A), postprandial apoCIII (B), 24-h uric acid AUC (C) and fasting nonHDL-C (D), LDL-C (E) and apoB (F) in subjects consuming Asp- (n=23), or sugar- sweetened beverages for two weeks (G25:n=28, HFCS17.5:n=16, F17.5:n=22, HFCS25:n=28 , F25:n=28). Groups without shared Post-scripts are significantly different, Tukey-Kramer’s post-test. Data shown as mean ± SD.
The changes of postprandial apoCIII in both of the fructose and HFCS groups differed significantly from that in the Asp group (Figure 2B). Compared with Asp and G25, consumption of both concentrations of fructose and HFCS25, increased uric acid 24-h AUC (Figure 2C). The mean change in subjects consuming F25 was also larger than in those consuming HFCS17.5 (Figure 2C). While there were no statistically significant differences between the F25 and HFCS25 or between F17.5 and HFCS17.5, the increases in 24-h TG AUC, postprandial apoCIII and, 24-h uric acid AUC tended to be higher in the fructose group than in the HFCS group consuming the same concentration of the sugars. Both HFCS groups tended to have larger increases of postprandial nonHDL-C, LDL-C, and apoB (Table 2) than the fructose group at the same level of consumption (%ereq) (Figure 1 in Supplement 2).
We measured fasting oxLDL in samples from subjects consuming Asp, G25, F25, and HFCS25 (Table 4 in Supplement 2). Only the subjects consuming HFCS25 exhibited a significant increase from baseline of oxLDL after two weeks (adjusted difference in means from baseline 6.17 U/L [95%CI, 4.45 to 7.89], P <0.0001). This increase was significantly larger than in the other three groups (Tukey-Kramer post-test adjusted mean difference from HFSC25: Asp −5.88 [95%CI, −9.29 to −2.47], P=0.0001, F25 −4.75 [95%CI, −8.0 to −1.5], P=0.0013, and G25 −4.84 [95%CI, −8.12 to −1.62], P=0.0009).
The change of body weight (Δbody weight) was not different between the groups after the two-week intervention (Table 2). However, as Δbody weight changed within some groups (G25 and HFCS25), we assessed the effects of Δbody weight on all outcomes in a separate model (Table 2 in Supplement 2). Δbody weight contributed significantly to the Δfasting nonHDL-C, LDL-C, and apoB. Nevertheless, the variance explained by the effect of group within the same model was approximately three times higher than that explained by Δbody weight and was only slightly reduced by the inclusion of Δbody weight as a co-variable.
Post hoc analyses testing the effect of fructose, glucose and their interaction (fructose*glucose) were conducted. Table 3 lists the variation accounted for by these factors. The fructose component significantly contributed to the changes of all measured variables, excluding oxLDL, while the glucose component only contributed to Δ24-h TG AUC and Δ24-h uric acid AUC. This analysis indicates that the interaction of fructose*glucose contributed to the increases of fasting and postprandial apoB, LDL-C, nonHDL-C, and fasting oxLDL while it did not affect Δ24-h uric acid AUC, Δ24-h TG AUC, or Δpostprandial apoCIII (Table 3).
Table 3:
Variation accounted for by fructose, glucose or their combination on measured outcomes
| Total variation accounted for in % [95% CI] |
P-value | |
|---|---|---|
| Fasting nonHDL-C | ||
| F | 4.97% [0.4% to 11.3%] | 0.002 |
| G | 0.75% [0.0% to 5.9%] | 0.209 |
| F*G | 5.86% [0.7% to 14.6%] | 0.002 |
| Postprandial nonHDL-C | ||
| F | 11.20% [10.6% to 21.3%] | <0.0001 |
| G | 1.58% [1.1% to 7.7%] | 0.062 |
| F*G | 5.39% [4.9% to 13.9%] | 0.002 |
| Fasting LDL-C | ||
| F | 5.84% [0.7% to 14.5%] | 0.001 |
| G | 0.08% [0.0% to 3.3%] | 0.661 |
| F*G | 6.17% [0.8% to 15.0%] | 0.001 |
| Postprandial LDL-C | ||
| F | 4.01% [0.1% to 11.9%] | 0.006 |
| G | 0.46% [0.0% to 5.0%] | 0.306 |
| F*G | 8.43% [1.8% to 18.0%] | 0.0002 |
| Fasting apoB | ||
| F | 4.44% [0.2% to 5.6%] | 0.004 |
| G | 0.34% [0.0% to 0.5%] | 0.396 |
| F*G | 4.51% [0.3% to 12.6%] | 0.005 |
| Postprandial apoB | ||
| F | 6.64% [1.0% to 15.6%] | 0.001 |
| G | 1.37% [0.0% to 7.3%] | 0.099 |
| F*G | 5.21% [0.4% to 13.7%] | 0.003 |
| 24-h TG AUC | ||
| F | 10.90% [3.1% to 21.0%] | <0.0001 |
| G | 2.30% [0.0% to 9.1%] | 0.049 |
| F*G | 0.42% [0.0% to 4.9%] | 0.340 |
| Postprandial apoCIII | ||
| F | 11.67% [3.6% to 21.9%] | <.0001 |
| G | 1.94% [0.0% to 8.4%] | 0.064 |
| F*G | 0.03% [0.0% to 2.5%] | 0.8099 |
| 24-h uric acid AUC | ||
| F | 32.72% [20.6% to 43.3%] | <.0001 |
| G | 4.82% [0.3% to 13.1%] | 0.001 |
| F*G | 0.00% [0.0% to 0.8%] | 0.943 |
| Fasting ox.LDLa | ||
| F | 0.57% [0.0% to 6.5%] | 0.384 |
| G | 0.45% [0.0% to 6.1%] | 0.538 |
| F*G | 15.26% [4.7% to 27.6%] | <0.0001 |
Data were estimated from Mulivariate regression model (fructose, glucose, fructose*glucose) adjusted for outcome at Baseline and sex
F- fructose, G – glucose
We performed post hoc analyses to assess if postprandial plasma glucose or insulin concentrations may have a role in the synergistic effects of fructose and glucose on nonHDL-C, LDL-C, and apoB. The analyses tested the effects of the changes of plasma glucose or insulin post-meal amplitudes on these outcomes in the four groups that consumed fructose or HFCS-sweetened beverages. Consumption of G25 and HFCS25 significantly increased the mean post-meal glucose amplitudes compared with ASP, F17.5 and F25 (Table 1 in Supplement 2). The changes in the glucose amplitudes were positively associated with the changes of nonHDL-C, LDL-C, apoB, and oxLDL but not with the changes of 24-h TG AUC, postprandial apoCIII, or 24-h uric acid AUC (Table 5 in Supplement 2). The Δmean insulin amplitudes were only associated with Δfasting oxLDL. The individual effects of insulin and glucose amplitudes on the change of oxLDL were attenuated when both were included in the same model (data not shown) indicating that the effects are likely to be mediated through a common pathway.
Discussion
In agreement with fructose being the principal driver of the metabolic dysregulation induced by sugar, we found that the increases of 24-h TG AUC, postprandial apoCIII, and 24-h uric acid AUC were largest after fructose consumption. However, relative to the aspartame control group, the increases of nonHDL-C, LDL-C, and apoB were highest in subjects consuming HFCS. Post hoc statistical analyses demonstrate that a significant interaction between the glucose and fructose in HFCS contributes to this unexpected pattern. We propose a two-step mechanism, detailed below, to explain this synergy between fructose and glucose.
The differences between fructose and glucose metabolism that explain the reported results for 24-h TG AUC, postprandial apoCIII, and 24-h uric acid AUC have been extensively reviewed 4,11,12. We therefore, present only a brief description. Most cell types, including hepatocytes, metabolize glucose through mechanisms regulated by energy need. In contrast, the liver metabolizes about 85% of consumed fructose immediately after absorption from the intestine independently of hepatic energy requirements 13. This unregulated fructose metabolism leads to ATP depletion in hepatocytes, subsequent upregulation of the purine degradation pathway and increased circulating uric acid concentrations 8,14,15. Increased levels of uric acid are strongly associated with and predictive of metabolic syndrome, fatty liver, and CVD 12,16. Interestingly, the inhibition of xanthine oxidase, a key enzyme in fructose-induced uric acid synthesis, reduced features of metabolic syndrome in fructose-fed rats 17,18. This suggests that uric acid might be a key mediator of the metabolic disturbance induced by fructose. In support of this, we have previously reported that the increase of uric acid significantly contributed to the changes of fasting and postprandial LDL-C, nonHDL-C and apoB observed in young adults consuming 0, 10, 17.5 and 25% of energy requirement as HFCS 8.
Unregulated fructose metabolism promotes apoCIII synthesis via increased expression of sterol regulatory element-binding protein (SREBP)-1c and carbohydrate-responsive element-binding protein (ChREBP) 8,11,19,20. Furthermore, fructose increases de novo lipogenesis (DNL) via activation of SREBP-1c 21,22 and via increased substrate availability for lipid synthesis 3,20,23. This leads to an increase in the assembly of very dense lipoprotein (VLDL) particles 24 and elevated circulating TG. Our interaction analysis confirms that the increases of TG, apoCIII, and uric acid are mainly driven by the fructose component and any contribution by glucose is additive at most (Table 3).
The high VLDL levels in circulation lead to downstream increases of intermediate density lipoprotein and then LDL particles 25, representing the first step of our proposed two-step mechanism (Figure 3). Subjects consuming HFCS exhibited higher post-meal glucose peaks compared with the groups consuming pure fructose (adjusted difference in means from baseline HFCS25: +0.966 mmol/L, F25: −0.179 mmol/L; difference in adjusted means between HFCS25 and F25 P<0.0001). We propose that increased glucose availability in the circulation is the second step of the proposed mechanism (Figure 3). Hyperglycemia leads to delayed lipoprotein clearance 26,27 possibly via non-enzymatic glycation of LDL particles, which additionally facilitates oxidation of LDL particles 28-30. In this study, oxLDL was increased only in the HFCS group and the interaction analysis demonstrated that only the co-ingestion of glucose and fructose contributed to this increase, while the individual monosaccharides did not. A post hoc analysis revealed that the changes of post-meal glucose amplitudes, but not post-meal insulin amplitudes, were significantly associated with the increases of apoB, nonHDL-C, LDL-C, and fasting oxLDL in the four groups of subjects consuming fructose or HFCS (Table 4 in Supplement 2). This supports the plausibility that higher post-meal glucose excursions cause delayed lipoprotein clearance which is the second step of our proposed mechanism explaining the significant interaction of fructose and glucose in subjects consuming HFCS.
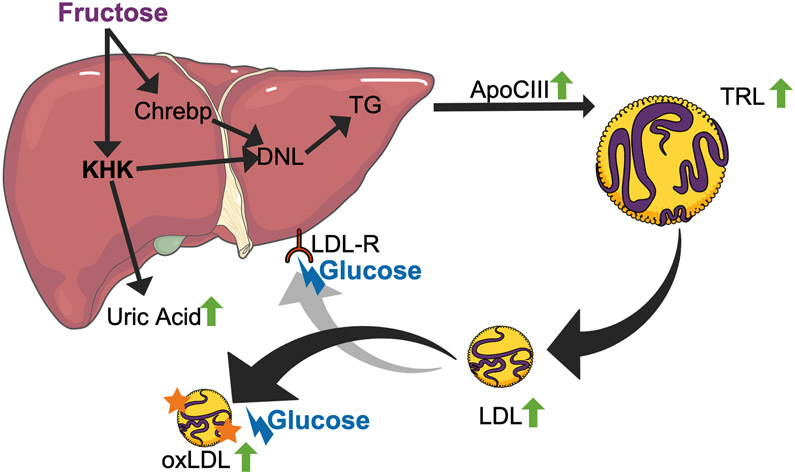
Figure 3. ‘Co-ingestion of glucose and fructose - Two-step’ theory.
‘First step’ - Fructose: Fructose influx into the liver induces immediate changes in the lipid metabolism. This causes an increase in uric acid due to the degradation AMP via the purine degradation pathway, an increase in apoCIII expression via ChREBP and an increase in de novo lipogenesis, through an increase in substrate and the action of transcription factors (ChREBP and SREBP). These processes lead to an increase in postprandial triglyceride rich lipoproteins (TRL). Hydrolysis of the triglyceride via lipoprotein lipase converts TRL into intermediate density lipoprotein and then LDL particles.
‘Second step’ - Glucose: Postprandial glucose levels lead to non-enzymatic glycation of LDL particles and interfere with the clearance of these particles. Further, plasma glucose aids in the oxidation of LDL particles with results in oxidized LDL particles.
LDL-R – LDL receptor; KHK – ketohexokinase; TG – triglycerides; TRL – TG-rich lipoprotein
In summary, we proposed a two-step mechanism wherein the fructose component of HFCS promotes VLDL synthesis and secretion, which subsequently leads to higher plasma concentrations of nonHDL-C, LDL-C, and apoB (first step). The glucose component of HFCS amplifies the post-meal glucose levels which contribute to the delay of hepatic LDL particle clearance and facilitate LDL modification (second step). More research is needed to corroborate our novel finding that an interaction of fructose and glucose contributes to increases of nonHDL-C, LDL-C, apoB and oxLDL in subjects consuming HFCS and our two-step mechanism explaining this synergy.
Our results suggest that the adverse health outcomes arising from diets high in dietary sugar cannot be attributed solely to fructose. Therefore, dietary guidelines for sugar consumption should not be based on the assumption that all of the adverse effects of dietary sugars are proportional to their fructose content 5. These results also challenge the relevance of meta-analyses 31,32 that concluded that fructose consumption has no unique effect on postprandial triglycerides, apoB, LDL-C, or nonHDL-C compared with all other dietary carbohydrates, including HFCS. Our data provide further evidence that, compared with starch and glucose, fructose does indeed have unique effects on these risk factors. In contrast, the data indicate that, compared with HFCS, fructose does not have unique effects on apoB, LDL-C, and nonHDL-C. However, this lack of unique effects does not represent evidence that fructose consumption is safe. Nevertheless Chiavaroli et al. 31 concluded that fructose consumption has no unique effect on lipids/lipoproteins compared with all other dietary carbohydrates and that this should be considered in clinical practice guidelines. Our data support the meta-analysis by Te Morenga et al. 33 comparing high sugar to low sugar diets that demonstrated that excess consumption of free sugar increases TG, cholesterol, and LDL-C independent of body weight gain.
One strength of the present study is the inclusion of two different doses of HFCS and fructose. In addition, the lipoprotein risk factors were measured in both the fasting and postprandial state. The comparable outcomes in both doses of sugar and during both the fasting and postprandial states strengthen our results. The two-days of inpatient residence prior to the start of the serial blood collections provided standardization of diet and activity levels, which helped to minimize variation. The inclusion of young individuals with a broad range of BMIs makes our data applicable to a wide population.
A limitation of our study is that the participants consumed ad libitum diets with the study beverages during the 12-day outpatient period, which prevents us from drawing conclusions regarding the effects of precise levels of sugar consumption. While 24-h food intake recalls of the pre-study and outpatient intervention eating periods were collected, they did not rectify this limitation due to poor subject compliance and the strong likelihood of under-reporting. Accurate assessment of dietary intake data from free-living subjects remains a challenge for the field of nutrition research. The intervention was delivered as sugar-sweetened beverages as they are the main source of added sugar in western diets 34. Providing a portion of the intervention sugar from solid foods would have better represented actual dietary patterns. Our intervention was short-term and a longer interventions might lead to different outcomes with regard to the interaction between glucose and fructose. In addition, when assigning the participants to the different beverage groups we ensured that the groups were matched with regard to baseline characteristics and risk factors. It is possible that the lack of randomization potentially introduced a bias in the assignment of subjects to the experimental groups. Finally, the assessment of synergy was performed post hoc and further research is required to confirm these results. Future studies should address these limitations. Optimally, these studies would include an eucaloric dietary protocol that provides all subjects with matched meals that vary only in the amount of complex carbohydrate that has been replaced by the sugar in the experimental beverages. Additional outcomes that would provide mechanistic insights like HbA1c, fructosamine, LDL particle glycation and the fractional clearance rate of LDL-C 35, should be included. Studies could also be designed to manipulate circulating glucose levels in order to specifically test the second step of our proposed 2-step mechanism. These could include dietary protocols in which consumption of fructose-sweetened beverages are followed by consumption of meals containing liquid glucose, solid glucose or starch.
The current findings provide evidence for an interaction of glucose and fructose when they are co-ingested as HFCS. This could prove to be an important mechanistic insight regarding the pathophysiology of excess sugar consumption. It is also highly relevant as it is has repeatedly been argued that the actual consumption of fructose is low and that the effects of fructose are diluted by the glucose component in dietary sugars 36. These results indicate that dietary guidelines for sugar consumption should not be based on the assumption that the adverse effects of sugar are solely induced by the fructose content 5. Our findings suggest that a commonly-consumed dietary sugar – HFCS – may be as harmful as isocaloric amounts of pure fructose. These results provide further support for the urgency to implement strategies aimed at limiting free sugar consumption.
Supplementary Material
Acknowledgements
We would like to thank the subjects who participated in the study. We thank the nursing staff at the University of California, Davis, Clinical Research Center for their dedicated nursing support. We also acknowledge and thank Janet Peerson, for expert advice on the statistical analyses, and Yanet Benyam, for her help with the data extraction from the physical activity questionnaires.
National Institutes of Health (NIH)/National Heart, Lung and Blood Institute 1R01 HL09133 (PI: Havel) and 1R01 HL107256 (PI: Havel). National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research UL1 RR024146 (PI: Berglund). Bettina Hieronimus was supported by a research fellowship from the German Research Foundation (DFG) HI 2113/1-1. Kimber Stanhope was supported by a Building Interdisciplinary Research Careers in Women’s Health award (K12 HD051958; PI: Gold) funded by the National Institute of Child Health and Human Development, Office of Research on Women's Health, Office of Dietary Supplements, and the National Institute of Aging. Nancy Keim’s research is supported by intramural USDA-ARS CRIS 2032-51530-025-00D. Havel’s research program has also received support from U24 DK092993 and a multi-campus grant from the University of California Office of the President (Award #142691).
Footnotes
Declaration of interests
The authors declare no competing interests.
Data sharing
Email the corresponding author for the paper to request the relevant data.
Disclaimer: The contents of this article represent the authors’ views and do not constitute an official position of the National Institutes of Health or the United States Government.
Clinical Trial Registration: clinicaltrials.gov Identifier: NCT01103921
Contributor Information
Dr. Bettina Hieronimus, Max Rubner-Institut, Department of Child Nutrition, Haid-und-Neu-Strasse 9, 76131 Karlsruhe, Germany; Department of Molecular Biosciences, School of Veterinary Medicine, University of California, Davis, CA
Valentina Medici, Division of Gastroenterology and Hepatology, University of California, Davis, CA
Andrew A. Bremer, Department of Pediatrics, School of Medicine, University of California, Davis, CA; Pediatric Growth and Nutrition Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD
Vivien Lee, Department of Molecular Biosciences, School of Veterinary Medicine, University of California, Davis, CA.
Marinelle V. Nunez, Department of Nutrition, University of California, Davis, CA
Desiree M. Sigala, Department of Molecular Biosciences, School of Veterinary Medicine, University of California, Davis, CA
Nancy L. Keim, Department of Nutrition, University of California, Davis, CA; United States Department of Agriculture, Western Human Nutrition Research Center, Davis, CA
Peter J. Havel, Department of Molecular Biosciences, School of Veterinary Medicine, University of California, Davis, CA; Department of Nutrition, University of California, Davis, CA
Kimber L. Stanhope, Department of Molecular Biosciences, School of Veterinary Medicine, University of California, Davis, CA
References
- 1.WHO. Sugars Intake for Adults and Children.; 2015. doi:978 92 4 154902 8 [PubMed]
- 2.Aeberli I, Hochuli M, Gerber PA, et al. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care. 2013;36(1):150–156. doi: 10.2337/dc12-0540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322–1334. doi: 10.1172/JCI37385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taskinen M-R, Packard CJ, Borén J. Dietary fructose and the metabolic syndrome. Nutrients. 2019;11(1987). doi: 10.3390/nu11091987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tappy L, Morio B, Azzout-Marniche D, et al. French recommendations for sugar intake in adults: A novel approach chosen by ANSES. Nutrients. 2018;10(8):1–16. doi: 10.3390/nu10080989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanhope KL, Bremer AA, Medici V, et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab. 2011;96(10):E1596–605. doi:jc.2011-1251 [pii] 10.1210/jc.2011-1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–247. [DOI] [PubMed] [Google Scholar]
- 8.Stanhope KL, Medici V, Bremer AA, et al. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr. 2015;101(6):1144–1154. doi: 10.3945/ajcn.114.100461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936 [DOI] [PubMed] [Google Scholar]
- 10.Stanhope K, Griffen SC, Bair BR, Swarbrick M, Keim N, Havel PJ. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr. 2008;87(5):1194–1203. doi:87/5/1194 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hieronimus B, Stanhope KL. Dietary fructose and dyslipidemia : new mechanisms involving apolipoprotein CIII. Curr Opin Lipidol. 2019;31(1):1–9. doi: 10.1097/MOL.0000000000000653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62(10):3307–3315. doi: 10.2337/db12-1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francey C, Cros J, Rosset R, et al. The extra-splanchnic fructose escape after ingestion of a fructose–glucose drink: An exploratory study in healthy humans using a dual fructose isotope method. Clin Nutr ESPEN. 2019;29:125–132. doi: 10.1016/j.clnesp.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 14.Caliceti C, Calabria D, Roda A, Cicero AFG. Fructose intake, serum uric acid, and cardiometabolic disorders: A critical review. Nutrients. 2017;9(4):1–15. doi: 10.3390/nu9040395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruun JM, Maersk M, Belza A, Astrup A, Richelsen B. Consumption of sucrose-sweetened soft drinks increases plasma levels of uric acid in overweight and obese subjects: A 6-month randomised controlled trial. Eur J Clin Nutr. 2015;69(8):949–953. doi: 10.1038/ejcn.2015.95 [DOI] [PubMed] [Google Scholar]
- 16.King C, Lanaspa MA, Jensen T, Tolan DR, Sánchez-Lozada LG, Johnson RJ. Uric Acid as a Cause of the Metabolic Syndrome. Contrib Nephrol. 2018;192:88–102. doi: 10.1159/000484283 [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Ren Physiol. 2006;290:625–631. doi: 10.1152/ajprenal.00140.2005 [DOI] [PubMed] [Google Scholar]
- 18.Sánchez-Lozada LG, Tapia E, Bautista-García P, et al. Effects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndrome. Am J Physiol - Ren Physiol. 2008;294(4):710–718. doi: 10.1152/ajprenal.00454.2007 [DOI] [PubMed] [Google Scholar]
- 19.Hieronimus B, Griffen SC, Keim NL, et al. Effects of Fructose or Glucose on Circulating ApoCIII and Triglyceride and Cholesterol Content of Lipoprotein Subfractions in Humans. J Clin Med. 2019;8(7):913. doi: 10.3390/jcm8070913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taskinen M-R, Söderlund S, Bogl LH, et al. Adverse effects of fructose on cardiometabolic risk factors and hepatic lipid metabolism in subjects with abdominal obesity. J Intern Med. 2017;140(6):874–888. doi: 10.1111/joim.12632 [DOI] [PubMed] [Google Scholar]
- 21.Kim M, Lai M, Herman MA, et al. ChREBP regulates fructose-induced glucose production independently of insulin signaling. J Clin Invest. 2016;126(11):4372–4386. doi: 10.1172/JCI81993.tor [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koo H-Y, Miyashita M, Cho BHS, Nakamura MT. Replacing dietary glucose with fructose increases ChREBP activity and SREBP-1 protein in rat liver nucleus. Biochem Biophys Res Commun. 2009;390(2):285–289. doi: 10.1016/j.bbrc.2009.09.109 [DOI] [PubMed] [Google Scholar]
- 23.Schwarz JM, Noworolski SM, Wen MJ, et al. Effect of a high-fructose weight-maintaining diet on lipogenesis and liver fat. J Clin Endocrinol Metab. 2015;100(6):2434–2442. doi: 10.1210/jc.2014-3678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adiels M, Taskinen M-R, Packard C, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49(4):755–765. doi: 10.1007/s00125-005-0125-z [DOI] [PubMed] [Google Scholar]
- 25.Sigurdsson G, Nicoll A, Lewis B. Conversion of very low density lipoprotein to low density lipoprotein. A metabolic study of apolipoprotein B kinetics in human subjects. J Clin Invest. 1975;56(6):1481–1490. doi: 10.1172/JCI108229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Bucala R, Milne R. Epitopes close to the apolipoprotein B low density lipoprotein receptor-binding site are modified by advanced glycation end products. Proc Natl Acad Sci U S A. 1998;95(13):7643–7647. doi: 10.1073/pnas.95.13.7643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bucala R, Makita Z, Vega G, et al. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci U S A. 1994;91(20):9441–9445. doi: 10.1073/pnas.91.20.9441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotani K, Yamada S, Uurtuya S, Yamada T, Taniguchi N, Sakurabayashi I. The association between blood glucose and oxidized lipoprotein(a) in healthy young women. Lipids Health Dis. 2010;9:2–5. doi: 10.1186/1476-511X-9-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen NG, Azhar S, Abbasi F, Carantoni M, Reaven GM. The relationship between plasma glucose and insulin responses to oral glucose, LDL oxidation, and soluble intercellular adhesion molecule-1 in healthy volunteers. Atherosclerosis. 2000;152(1):203–208. doi: 10.1016/S0021-9150(99)00460-8 [DOI] [PubMed] [Google Scholar]
- 30.Lamharzi N, Renard CB, Kramer F, et al. Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions: Potential role of glucose-oxidized LDL. Diabetes. 2004;53(12):3217–3225. doi: 10.2337/diabetes.53.12.3217 [DOI] [PubMed] [Google Scholar]
- 31.Chiavaroli L, de Souza RJ, Ha V, et al. Effect of fructose on established lipid targets: A systematic review and meta-analysis of controlled feeding trials. J Am Heart Assoc. 2015;4(9):1–23. doi: 10.1161/JAHA.114.001700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang DD, Sievenpiper JL, De Souza RJ, et al. Effect of fructose on postprandial triglycerides: A systematic review and meta-analysis of controlled feeding trials. Atherosclerosis. 2014;232(1):125–133. doi: 10.1016/j.atherosclerosis.2013.10.019 [DOI] [PubMed] [Google Scholar]
- 33.Te Morenga LA, Howatson AJ, Jones RM, Mann J. Dietary sugars and cardiometabolic risk: Systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr. 2014;100(1):65–79. doi: 10.3945/ajcn.113.081521 [DOI] [PubMed] [Google Scholar]
- 34.Bailey RL, Fulgoni VL, Cowan AE, Gaine PC. Sources of added sugars in young children, adolescents, and adults with low and high intakes of added sugars. Nutrients. 2018;10(1). doi: 10.3390/nu10010102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinagre CGC, Ficker ES, Finazzo C, et al. Enhanced removal from the plasma of LDL-like nanoemulsion cholesteryl ester in trained men compared with sedentary healthy men. J Appl Physiol. 2007;103(4):1166–1171. doi: 10.1152/japplphysiol.01176.2006 [DOI] [PubMed] [Google Scholar]
- 36.Tappy L Fructose-containing caloric sweeteners as a cause of obesity and metabolic disorders. J Exp Biol. 2018;221(Suppl 1):jeb164202. doi: 10.1242/jeb.164202 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.