Abstract
Objective
In patients with newly diagnosed atrial fibrillation (AF), do baseline risk factors and stroke prevention strategies account for the geographically diverse outcomes.
Design
Global Anticoagulant Registry in the FIELD-Atrial Fibrillation is a prospective multinational non-interventional registry of patients with newly diagnosed AF (n=52 018 patients).
Setting
Investigator sites (n=1317) were representative of the care settings/locations in each of the 35 participating countries. Treatment decisions were all determined by the local responsible clinicians.
Participants
The patients (18 years and over) with newly diagnosed AF had at least 1 investigator-determined stroke risk factor and patients were not required to meet specific thresholds of risk score for anticoagulant treatment.
Main outcomes and measures
Observed 1-year event rates and risk-standardised rates were derived.
Results
Rates of death, non-haemorrhagic stroke/systemic embolism and major bleeding varied more than three-to-four fold across countries even after adjustment for baseline factors and antithrombotic treatments. Rates of anticoagulation and antithrombotic treatment varied widely. Patients from countries with the highest rates of cardiovascular mortality and stroke were among the least likely to receive oral anticoagulants. Beyond anticoagulant treatment, variations in the treatment of comorbidities and lifestyle factors may have contributed to the variations in outcomes. Countries with the lowest healthcare Access and Quality indices (India, Ukraine, Argentina, Brazil) had the highest risk-standardised mortality.
Conclusion
The variability in outcomes across countries for patients with newly diagnosed AF is not accounted for by baseline characteristics and antithrombotic treatments. Residual mortality rates were correlated with Healthcare Access and Quality indices. The findings suggest the management of patients with AF needs to not only address guideline indicated and sustained anticoagulation, but also the treatment of comorbidities and lifestyle factors.
Trial registration number
Keywords: cardiology, anticoagulation, thromboembolism
Strengths and limitations of this study.
This is a prospective observational study where patients with newly diagnosed atrial fibrillation were identified and followed and outcomes evaluated.
All patients were managed according to local standards of care.
Remote and onsite monitoring and robust quality control methods were used.
As in any observational study the findings may have been influenced by unmeasured confounders.
Ascertainment of bleeding outcomes was according to local standards of care and thus ascertainment criteria, locally, may have influenced observed rates of bleeding.
Introduction
The 2015 Global Burden of Disease (GBD) report of 195 countries and territories suggests that atrial fibrillation (AF) prevalence is highest in Northern and Central Europe, and the USA,1 and is projected to rise globally because of ageing and population growth worldwide.2 However, whether the diverse outcomes of patients with newly diagnosed AF are accounted for by baseline risk characteristics and antithrombotic therapies is uncertain.
The gains in cardiovascular health in high-income countries are related, at least in part, to modification of cardiovascular risk factors as well as improved disease management. In the context of AF, the changes include the availability of treatment strategies for stroke prophylaxis, and/or rhythm or rate control.3–7 However, the extent to which baseline characteristics and treatment strategies account for geographical variations in outcomes is unclear.
The Global Anticoagulant Registry in the FIELD-Atrial Fibrillation (GARFIELD-AF) aimed to define geographical variations in all-cause mortality, stroke/systemic embolism (SE) and major bleeding in patients with newly diagnosed AF. The primary aim of this report was to determine whether variations in outcomes of AF are accounted for by baseline clinical risk characteristics. A secondary aim was to consider the impact of other factors including national differences in life expectancy, access to quality healthcare and stroke prevention strategies.
Methods
Design
GARFIELD-AF is the largest multinational prospective registry in AF.8 The study recruited patients from >1000 investigational sites (identified nationally as representative) in 35 countries. Patients were recruited from: Europe (Finland, Norway, Sweden, Denmark, UK, Netherlands, Belgium, Germany, Switzerland, France, Spain, Italy, Austria, Hungary, Russia, Poland, Czech Republic, Ukraine and Turkey), Asia (Singapore, China, Japan, South Korea, Thailand and India), North America (USA and Canada), Latin America (Mexico, Brazil, Argentina and Chile) and other countries including Egypt, United Arab Emirates, South Africa and Australia.
Adults ≥18 years were eligible for inclusion if they were diagnosed with non-valvular AF within 6 weeks of study entry. Patients with AF were required to have at least one risk factor for stroke, as judged by the investigator (entry to GARFIELD-AF did not require performance of a stroke risk predictor, nor a specific threshold if such a score was performed). Patients were enrolled prospectively and consecutively at sites that aimed to reflect diverse care settings (including office/outpatient practice; hospital departments including neurology, cardiology, geriatrics, internal medicine and emergency; anticoagulation clinics; and general practice).8 9
Written informed consent was obtained from all study participants. Confidentiality and anonymity of all enrolled patients was maintained.
GARFIELD-AF data were captured using an electronic case report form (eCRF). Submitted data were examined for completeness and accuracy by the coordinating centre (Thrombosis Research Institute, London, UK), and data queries were sent to study sites. An audit and quality control programme was implemented, and this included source documentation (20% of all eCRFs were monitored against source records).10 This paper adheres to the guidelines from Strengthening the Reporting of Observational Studies in Epidemiology.11
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Procedures and outcome measures
Baseline characteristics collected at study entry included: medical history, care setting, type of AF, date and method of diagnosis of AF, symptoms, antithrombotic treatment (vitamin K antagonists (VKAs), non-VKA oral anticoagulants (NOACs) and antiplatelet (AP) treatment), as well as all cardiovascular drugs. Race was classified by the investigator in agreement with the patient.8 Vascular disease included coronary artery disease with a history of acute coronary syndromes (ACS) and/or peripheral artery disease. Chronic kidney disease (CKD) was classified according to National Kidney Foundation guidelines into moderate-to-severe (stages 3–5), mild (stages 1 and 2) or none. Data on components of the CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, age 65-74, female) and HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or anemia, labile INR, age, and drugs/alcohol use) risk stratification schemes were collected and calculated retrospectively. HAS-BLED scores were calculated excluding fluctuations in international normalised ratio. In addition, the risk of death, non-haemorrhagic stroke/SE and major bleeding was evaluated with the GARFIELD-AF risk calculator.12
Patients were followed over a minimum of 24 months or until death or lost to follow-up, whichever occurred first. As reported previously, standardised definitions for clinical events, death (cardiovascular and non-cardiovascular), non-haemorrhagic stroke/SE and major bleeding) were used.8 9 Outcome events were not centrally adjudicated.
Data for this report were extracted from the study database on 30 June 2019.
Statistical analysis
Univariate data are presented as medians (first and third quartile) for continuous variables and as absolute frequencies with percentages for categorical variables.
‘Time at risk’ for each event was calculated over the first year after enrolment up to the first occurrence of an event or last follow-up or at 365 days, which ever occurred earlier. All-cause mortality, non-haemorrhagic stroke/SE and major bleeding were described as the number of events and the Kaplan-Meier event rate with 95% CIs.
In this study, national risk-standardised measures of event rates were calculated to compare the observed event rates based on case mix (ie, the clinical characteristics of patients) in each country, with the expected rates for a similar case mix. The risk-standardised event rates were calculated using the following equation:
Where the Observed event rate was the crude rate calculated for each country using the Kaplan-Meier estimator (1 minus event-free survival probability at 1 year after enrolment).
Expected event rate was calculated (using multivariable Cox regression with a series of demographic and clinical characteristics as covariates) for every patient and the national average computed.
Global (and regional) event rates were the crude rate calculated with the Kaplan-Meier rate across all countries in GARFIELD-AF without exclusion.
When the observed and expected rates were the same, the risk-standardised rate equalled the global event rates. However, when the observed event rate was greater or less than the expected rate, then the country had more or less events than expected, based on its case mix. Hence, the observed to expected ratio was greater or less than 1.0, making the risk-standardised rate higher or lower than the global rate.
Patients’ characteristics included in the initial Cox model were: age, gender, type of AF, history of hypertension, blood pressure (systolic and diastolic) and pulse rate (at enrolment), hypercholesterolaemia, smoking status (never/ex/current) and heavy alcohol consumption, diabetes mellitus (type 1 or 2), ACS, coronary artery bypass graft (CABG), vascular disease, carotid occlusive disease, venous thromboembolism, history of stroke/transient ischaemic attack/SE, history of bleeding, heart failure, moderate-to-severe CKD and cirrhosis. Fine-Gray modelling was applied to the outcomes of non-haemorrhagic stroke/SE and major bleeding with death as the competing risk. CIs for the risk-standardised measures were computed using estimates extracted from 1000 bootstrap samples. Patients with missing values were not removed from the study; single imputation was applied.
Both baseline risk factors and antithrombotic regimens (with oral AC and/or AP) at the time of diagnosis of AF (baseline) were included in the Cox model.
The observed rates in a contemporary US registry, the ORBIT-AF II, were derived to assess the representability of the US patients in GARFIELD-AF.
All analyses were performed with SAS V.9.4 (SAS Institute).
Results
Baseline demographics and clinical characteristics
Baseline characteristics were analysed for the 52 018 patients with newly diagnosed AF, enrolled consecutively into GARFIELD-AF between March 2010 and August 2016, in 35 countries. The largest cohort was recruited from Europe (57.4%), followed by Asia (26.6%), Latin America (8.2%), ‘other’ countries (4.7%) (including South Africa, Egypt, United Arab Emirates and Australia) and North America (3.1%). The rate of missing data was below <3%, with the exception of lifestyle information, body mass index (BMI) and some vital signs. Lost to follow-up was about 1% for all world regions except Asia (4.3%).
The observed variability in patients’ baseline characteristics among regions in GARFIELD-AF is reported in table 1. Patients from Asia compared with Europe tended to be younger, had a lower BMI, a lower prevalence of hypertension, hypercholesterolaemia, vascular disease and CKD. By contrast, patients from North America in GARFIELD-AF had the highest proportion of patients aged ≥75, together with the highest prevalence of diabetes, hypercholesterolaemia and prior/current smokers from any region (except ‘other Region’ where the highest prevalence of diabetes was observed). The prevalence of heart failure was consistent and approximately one in five of patients in every region. Approximately 70% of patients overall (and 91.6% of patients in North America) were categorised at having paroxysmal or unclassified AF at enrolment in this study (table 1).
Table 1.
Baseline characteristics distribution by region of enrolment
| Variable | Region | P value* | ||||
| Europe (N=29 876) |
Asia (N=13 821) |
Latin America (N=4247) |
North America (N=1619) |
Other countries (N=2455) |
||
| Sex, n (%) | ||||||
| Male | 16 313 (54.6) | 8199 (59.3) | 2231 (52.5) | 885 (54.7) | 1403 (57.2) | <0.001 |
| Female | 13 563 (45.4) | 5622 (40.7) | 2016 (47.5) | 734 (45.3) | 1051 (42.8) | |
| Age, median (Q1; Q3), years | 72.0 (64.0; 79.0) | 69.0 (60.0; 76.0) | 71.0 (63.0; 79.0) | 72.0 (64.0; 80.0) | 67.0 (59.0; 75.0) | <0.001 |
| Age, n (%), years | ||||||
| <65 | 8016 (26.8) | 4980 (36.0) | 1258 (29.6) | 441 (27.2) | 996 (40.6) | <0.001 |
| 65–69 | 4578 (15.3) | 2165 (15.7) | 628 (14.8) | 237 (14.6) | 407 (16.6) | |
| 70–74 | 5183 (17.3) | 2399 (17.4) | 708 (16.7) | 257 (15.9) | 384 (15.6) | |
| ≥75 | 12 099 (40.5) | 4277 (30.9) | 1653 (38.9) | 684 (42.2) | 668 (27.2) | |
| Race/ethnicity, n (%) | ||||||
| Caucasian | 27 934 (96.9) | 13 (0.1) | 957 (23.1) | 1421 (90.5) | 1672 (70.3) | <0.001 |
| Hispanic/Latino | 344 (1.2) | 0 (0.0) | 3000 (72.5) | 35 (2.2) | 14 (0.6) | |
| Asian | 160 (0.6) | 13 789 (99.8) | 11 (0.3) | 11 (0.7) | 305 (12.8) | |
| Black/mixed/other | 394 (1.4) | 16 (0.1) | 172 (4.2) | 103 (6.6) | 386 (16.2) | |
| BMI, median (Q1; Q3), kg/m² | 28.0 (25.1; 31.8) | 24.2 (22.0; 26.6) | 27.9 (24.8; 31.6) | 29.4 (25.4; 34.0) | 29.8 (26.0; 34.3) | <0.001 |
| Systolic blood pressure, median (Q1; Q3), mm Hg | 135.0 (120.0; 147.0) | 130.0 (118.0; 140.0) | 130.0 (120.0; 141.0) | 130.0 (118.0; 143.0) | 132.5 (120.0; 148.0) | <0.001 |
| Diastolic blood pressure, median (Q1; Q3), mm Hg | 80.0 (71.0; 90.0) | 78.0 (70.0; 86.0) | 80.0 (70.0; 86.0) | 78.0 (68.0; 86.0) | 80.0 (70.0; 90.0) | <0.001 |
| Pulse, median (Q1; Q3), bpm | 85.0 (70.0;108.0) | 82.0 (70.0; 98.0) | 80.0 (70.0; 102.0) | 89.0 (72.0; 117.0) | 98.0 (80.0; 122.0) | <0.001 |
| Type of atrial fibrillation, n (%) | ||||||
| Permanent | 4587 (15.4) | 1108 (8.0) | 666 (15.7) | 35 (2.2) | 234 (9.5) | <0.001 |
| Persistent | 4313 (14.4) | 2505 (18.1) | 625 (14.7) | 100 (6.2) | 210 (8.6) | |
| Paroxysmal | 7375 (24.7) | 5165 (37.4) | 1086 (25.6) | 345 (21.3) | 333 (13.6) | |
| New onset (unclassified) | 13 598 (45.5) | 5042 (36.5) | 1870 (44.0) | 1137 (70.3) | 1678 (68.4) | |
| Care setting specialty at diagnosis, n (%) | ||||||
| Internal medicine/neurology/geriatrics | 7077 (23.7) | 1807 (13.1) | 654 (15.4) | 345 (21.3) | 560 (22.8) | <0.001 |
| Cardiology | 16 824 (56.3) | 11 571 (83.7) | 3184 (75.0) | 968 (59.9) | 1626 (66.2) | |
| Primary care/general practice | 5972 (20.0) | 442 (3.2) | 409 (9.6) | 304 (18.8) | 269 (11.0) | |
| Care setting location at diagnosis, n (%) | ||||||
| Hospital | 16 647 (55.7) | 10 112 (73.2) | 1792 (42.2) | 615 (38.1) | 1169 (47.6) | <0.001 |
| Office/anticoagulation clinic/thrombosis centre | 9804 (32.8) | 3366 (24.4) | 1404 (33.1) | 387 (23.9) | 957 (39.0) | |
| Emergency room | 3422 (11.5) | 342 (2.5) | 1051 (24.7) | 614 (38.0) | 329 (13.4) | |
| Medical history, n (%) | ||||||
| Heart failure | 6841 (22.9) | 3072 (22.2) | 951 (22.4) | 312 (19.3) | 563 (22.9) | 0.012 |
| Acute coronary syndromes | 3262 (11.0) | 1160 (8.4) | 433 (10.2) | 209 (13.0) | 469 (19.2) | <0.001 |
| Vascular disease | 8220 (27.7) | 2629 (19.2) | 791 (18.8) | 438 (27.4) | 737 (30.2) | <0.001 |
| Carotid occlusive disease | 1071 (3.6) | 251 (1.8) | 109 (2.6) | 56 (3.5) | 51 (2.1) | <0.001 |
| VTE | 995 (3.3) | 81 (0.6) | 102 (2.4) | 73 (4.6) | 104 (4.3) | <0.001 |
| Prior stroke/TIA/SE | 3445 (11.6) | 1400 (10.2) | 492 (11.7) | 165 (10.4) | 337 (13.9) | <0.001 |
| History of bleeding | 764 (2.6) | 222 (1.6) | 173 (4.1) | 76 (4.7) | 80 (3.3) | <0.001 |
| Hypertension | 23 740 (79.7) | 9353 (67.9) | 3420 (80.8) | 1229 (76.4) | 1862 (76.2) | <0.001 |
| Hypercholesterolaemia | 13 368 (46.3) | 3743 (27.7) | 1550 (38.6) | 940 (59.3) | 1354 (56.8) | <0.001 |
| Diabetes | 6359 (21.3) | 2976 (21.5) | 1041 (24.5) | 422 (26.1) | 744 (30.3) | <0.001 |
| Cirrhosis | 148 (0.5) | 96 (0.7) | 15 (0.4) | 14 (0.9) | 20 (0.8) | 0.003 |
| Moderate to severe CKD | 3606 (12.4) | 1052 (7.8) | 282 (7.2) | 142 (9.5) | 272 (11.3) | <0.001 |
| Dementia | 381 (1.3) | 246 (1.8) | 47 (1.1) | 34 (2.1) | 56 (2.3) | <0.001 |
| Heavy alcohol use, n (%) | 486 (1.9) | 365 (3.2) | 72 (1.8) | 36 (2.7) | 69 (3.1) | <0.001 |
| Current smoker, n (%) | 2786 (10.2) | 1595 (13.0) | 348 (8.5) | 180 (12.1) | 293 (12.5) | <0.001 |
| Treatment, n (%) | ||||||
| NOAC±AP | 8240 (28.1) | 3532 (25.7) | 900 (21.5) | 715 (44.7) | 725 (29.9) | <0.001 |
| VKA±AP | 13 042 (44.4) | 4119 (30.0) | 1666 (39.9) | 361 (22.6) | 995 (41.0) | |
| AP only | 5148 (17.5) | 3807 (27.7) | 1004 (24.0) | 302 (18.9) | 500 (20.6) | |
| None | 2922 (10.0) | 2282 (16.6) | 610 (14.6) | 220 (13.8) | 206 (8.5) | |
| AP treatment, n (%) | 9074 (30.9) | 5522 (40.2) | 1666 (39.9) | 706 (44.2) | 1135 (46.8) | <0.001 |
| CHA2DS2-VASc score, median (Q1; Q3) | 3.0 (2.0; 4.0) | 3.0 (2.0; 4.0) | 3.0 (2.0; 4.0) | 3.0 (2.0; 4.0) | 3.0 (2.0; 4.0) | <0.001 |
| HAS-BLED score, median (Q1; Q3)† | 1.0 (1.0; 2.0) | 1.0 (1.0; 2.0) | 1.0 (1.0; 2.0) | 2.0 (1.0; 2.0) | 1.0 (1.0; 2.0) | <0.001 |
| GARFIELD death score, median (Q1; Q3)‡ | 5.3 (3.1; 9.4) | 3.1 (1.8; 6.0) | 6.0 (3.5; 10.9) | 5.8 (3.1; 10.9) | 4.3 (2.5; 8.5) | <0.001 |
| GARFIELD stroke score, median (Q1; Q3)§ | 1.6 (1.1; 2.4) | 1.5 (1.0; 2.3) | 1.6 (1.1; 2.4) | 1.6 (1.1; 2.4) | 1.4 (0.9; 2.3) | <0.001 |
| GARFIELD bleeding score, median (Q1; Q3)¶ | 1.7 (1.1; 2.6) | 1.3 (0.9; 2.0) | 1.6 (1.0; 2.4) | 1.6 (1.0; 2.6) | 1.6 (1.0; 2.4) | <0.001 |
*P values for categorical variables obtained from χ2 or Fisher’s exact test, as appropriate. P value for continuous variables obtained from one-way ANOVA or Kruskal-Wallis test, as appropriate.
†The risk factor ‘Labile INRs’ is not included in the HAS-BLED score as it is not collected at baseline. As a result, the maximum HAS-BLED score at baseline is 8 points (not 9).
‡Represent the risk of mortality within 2 years.
§Represent the risk of non-haemorrhagic stroke/SE within 2 years.
¶Represent the risk of major bleeding within 2 years.
ANOVA, analysis of variance; AP, antiplatelet; BMI, body mass index; CHA2DS2-VASc, Congestive Heart Failure, Hypertension, Age ≥75 [Doubled], Diabetes Mellitus, Prior Stroke or Transient Ischemic Attack [Doubled], Vascular Disease, Age 65–74, Female; CKD, chronic kidney disease; GARFIELD, Global Anticoagulant Registry in the FIELD; HAS-BLED, Hypertension, abnormal renal/liver function, stroke, bleeding history or anemia, Labile INR, age, and drugs/alcohol use.; INR, international normalized ratio; NOAC, non-vitamin K antagonist oral anticoagulant; SE, systemic embolism; TIA, transient ischaemic attack; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Standard risk assessment scores (including the GARFIELD-AF risk score) found that the calculated risks of stroke or major bleeding were similar across regions (median CHA2DS2-VASc score 3.0 in all regions). However, the GARFIELD-AF risk model for death revealed regional differences, with a lower expected rate of death in patients from Asia and highest in those from Latin America (table 1).
Treatment setting
In Asia and Latin America, patients were predominantly diagnosed and managed by cardiologists (83.7% and 75.0%, respectively), while in Europe and North America, the role of managing patients with AF was shared between cardiologists (in approximately 60% of cases), internists (~20%) and primary care (~20%). The likelihood of being diagnosed and treated in the emergency care setting was highest in North America (38.0% of patients) followed by Latin America (24.7%), ‘other’ countries (13.4%), Europe (11.5%) and Asia (2.5%).
Observed global and regional outcomes
In GARFIELD-AF, the lowest observed rate of death at 1 year was recorded in Asia (2.8; 95% CI 2.6 to 3.1) with rates less than half of those observed in ‘other’ countries (6.0; 95% CI 5.1 to 7.0) (namely, South Africa, Egypt, United Arab Emirates and Australia). Non-haemorrhagic stroke/SE rates showed less regional variability, but once again, the lowest observed rates were reported in Asia (1.0; 95% CI 0.9 to 1.2). For major bleeding, the highest observed rates were recorded in North America (2.9; 95% CI 2.2 to 3.8) and the lowest in Asia (0.9; 95% CI 0.7 to 1.0). Reflecting the high proportion of patients from Europe, the global rates across all countries in GARFIELD-AF were similar to European event rates for mortality, non-haemorrhagic stroke/SE and major bleeding (table 2).
Table 2.
Observed 1-year rates and corresponding 95% CIs for all-cause mortality, non-haemorrhagic stroke/SE and major bleeding by region and in all 35 countries in GARFIELD-AF
| Region | Outcome | ||
| Mortality | Non-haemorrhagic stroke/SE | Major bleeding | |
| Europe | 4.4 (4.2 to 4.6) | 1.2 (1.1 to 1.3) | 1.3 (1.2 to 1.4) |
| Asia | 2.8 (2.6 to 3.1) | 1.0 (0.9 to 1.2) | 0.9 (0.7 to 1.0) |
| Latin America | 5.5 (4.8 to 6.2) | 1.4 (1.1 to 1.8) | 1.3 (1.0 to 1.7) |
| North America | 5.9 (4.8 to 7.2) | 1.0 (0.6 to 1.6) | 2.9 (2.2 to 3.8) |
| Other countries | 6.0 (5.1 to 7.0) | 1.8 (1.3 to 2.4) | 1.3 (0.9 to 1.9) |
| All countries | 4.2 (4.0 to 4.4) | 1.2 (1.1 to 1.3) | 1.2 (1.1 to 1.3) |
GARFIELD-AF, Global Anticoagulant Registry in the FIELD-Atrial Fibrillation; SE, systemic embolism.
Observed and risk-standardised outcomes by country
Figures 1–3 depict the observed and risk-standardised rates of mortality, non-haemorrhagic stroke/SE and major bleeding for countries that enrolled more than 90% of the patients into GARFIELD-AF, that is, omitting countries with potentially unrepresentative findings due to low enrolment. Full details of the observed rates from all countries, including those omitted from the figures, that is, South Africa (n=639), Denmark (n=532), Egypt (n=527), Austria (n=460), United Arab Emirates (n=397), Finland (n=359), Singapore (n=306), Norway (n=270) and Switzerland (n=89), are reported in online supplemental table S1–S3.
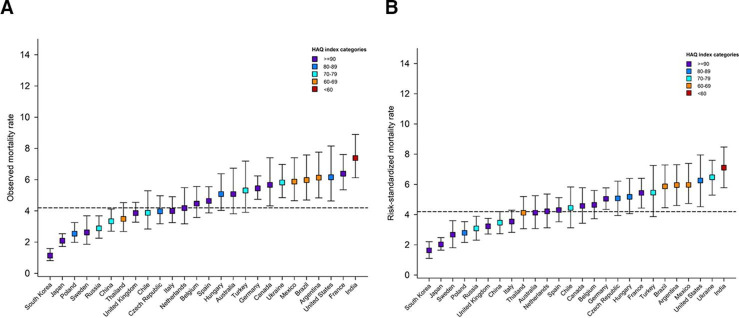
Figure 1.
Observed (A) and risk-standardised1 (B) mortality rates by country.1 Computed as the ratio of observed and predicted rate for each country, multiplied by the global observed rate. The predicted rate estimated from a Cox model with the following covariates: age, sex, type of AF, systolic and diastolic blood pressure, pulse, BMI, hypertension, diabetes, heart failure, history of stroke/TIA/SE, history of bleeding, vascular disease, acute coronary syndromes, moderate to severe CKD, hypercholesterolaemia, cirrhosis, carotid occlusive disease, VTE, dementia, smoking status, heavy alcohol consumption. CIs for risk-standardised rates are calculated through bootstrap sampling with 1000 replications. Showing only countries with more than 700 patients enrolled. Horizontal dashed line represents overall observed global rate. AF, atrial fibrillation; BMI, body mass index; CKD, chronic kidney disease; SE, systemic embolism; TIA, transient ischaemic attack; VTE, venous thromboembolism.
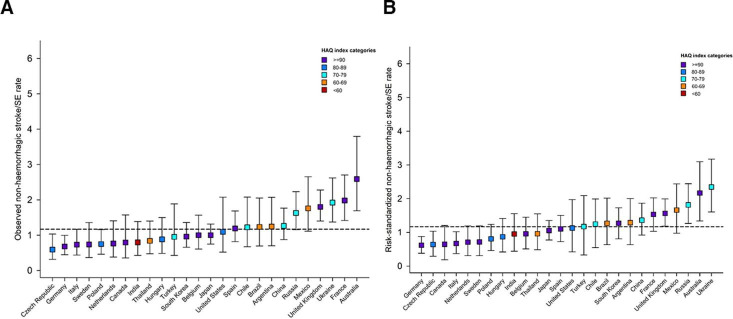
Figure 2.
Observed (A) and risk-standardised1 (B) non-haemorrhagic stroke/systemic embolism (SE) rates by country.1Computed as the ratio of observed and predicted rate for each country, multiplied by the global observed rate. The predicted rate estimated from a Fine-Gray model with the following covariates: age, sex, type of AF, systolic and diastolic blood pressure, pulse, BMI, hypertension, diabetes, heart failure, history of stroke/TIA/SE, history of bleeding, vascular disease, acute coronary syndromes, moderate to severe CKD, hypercholesterolaemia, cirrhosis, carotid occlusive disease, VTE, dementia, smoking status, heavy alcohol consumption. CIs for risk-standardised rates are calculated through bootstrap sampling with 1000 replications. Showing only countries with more than 700 patients enrolled. Horizontal dashed line represents overall observed global rate. AF, atrial fibrillation; BMI, body mass index; CKD, chronic kidney disease; HAQ, Healthcare Access and Quality; TIA, transient ischaemic attack; VTE, venous thromboembolism.
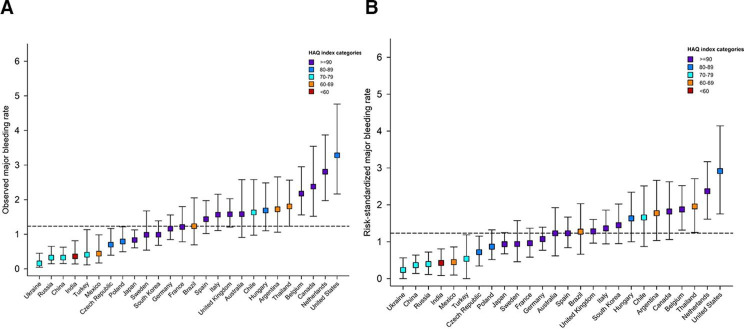
Figure 3.
Observed (A) and risk-standardised1 (B) major bleeding rates by country.1 Computed as the ratio of observed and predicted rate for each country, multiplied by the global observed rate. The predicted rate estimated from a Fine-Gray model with the following covariates: age, sex, type of AF, systolic and diastolic blood pressure, pulse, BMI, hypertension, diabetes, heart failure, history of stroke/TIA/SE, history of bleeding, vascular disease, acute coronary syndromes, moderate to severe CKD, hypercholesterolaemia, cirrhosis, carotid occlusive disease, VTE, dementia, smoking status, heavy alcohol consumption. Cis for risk-standardised rates are calculated through bootstrap sampling with 1000 replications. Showing only countries with more than 700 patients enrolled. Horizontal dashed line represents overall observed global rate. AF, atrial fibrillation; BMI, body mass index; CKD, chronic kidney disease; HAQ, Healthcare Access and Quality; SE, systemic embolism; TIA, transient ischaemic attack; VTE, venous thromboembolism.
bmjopen-2021-049933supp001.pdf (212.6KB, pdf)
Figures 1–3 show the marked variations in observed event rates by country. This variability persisted even after adjusting for all 22 baseline factors (demographics, modifiable cardiovascular risk factors and comorbidities).
India and Ukraine experienced the highest risk-standardised mortality rates, primarily driven by cardiovascular events. Marked differences were also observed for the USA, where the rate of non-cardiovascular mortality was more than threefold higher compared with cardiovascular mortality. Within most other countries the rates of cardiovascular and non-cardiovascular mortality were similar (online supplemental table S1).
To display the relation between healthcare access and outcomes in more detail, we colour-coded each country according to the Healthcare Access and Quality (HAQ) Index (overall score on a scale of 0–100) from the GBD Study 2016.13 The results show that some of the countries with highest risk-standardised mortality rates (ie, India, Mexico, Argentina and Brazil) had some of the lowest HAQ indices (HAQ: <70); only Thailand had a similarly low HAQ and a mortality rate. Conversely, the three countries with the lowest risk-standardised mortality rate (South Korea, Japan and Sweden) all obtained a high HAQ score (HAQ:≥90).
The observed mortality rate from the US study, ORBIT-AF II, was similar to the GARFIELD-AF global rate (4.3 (95% CI 3.7 to 4.9) vs 4.2 (95% CI 4.0 to 4.4) respectively) and below the global rate for non-haemorrhagic stroke/SE (ORBIT-AF-II 0.8 (95% CI 0.6 to 1.1) vs GARFIELD-AF 1.2 (95% CI 1.1 to 1.3)). Nevertheless, both GARFIELD-AF and ORBIT-AF II reported high rates of major bleeding in the US: 3.4 (95% CI 2.3 to 5.0) (GARFIELD-AF) and 3.3 (95% CI 2.8 to 3.8) (ORBIT-AF II) relative to the global rate of 1.2 (95% CI 1.1 to 1.3) in GARFIELD-AF.
The rates of each type of outcome differed by country. For instance, the lowest risk-standardised mortality rates were observed for South Korea, Japan and Sweden, while the lowest risk-standardised rates of non-haemorrhagic stroke/SE were observed in Germany, Czech Republic and Canada. The highest risk-standardised rates non-haemorrhagic stroke/SE were reported in Ukraine and Australia, and the highest risk-standardised rates of major bleeding in the Netherlands and the USA.
Antithrombotic regimen for stroke prevention at baseline
GARFIELD-AF recorded substantial differences in the overall rate of anticoagulation by region (from 73% in Europe to 56% in Asia, online supplemental figure S1a), as well as large variations within countries (online supplemental figure S1b). At the time of diagnosis of AF, the highest proportion of patients receiving NOACs was in North America (44.8%). This included 14.4% of patients who received NOAC in combination with APs. VKAs were most commonly prescribed in Europe, Latin America and ‘Other’ countries (in 44.4%, 39.8% and 41.1% of patients, respectively) (online supplemental figure S1a).
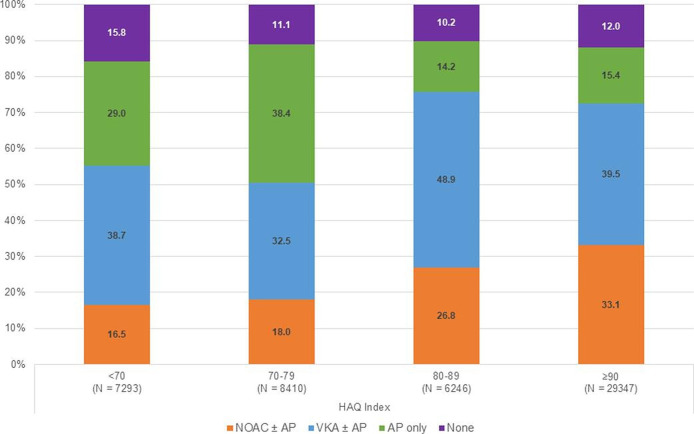
Even though CHA2DS2-VASc scores were similar across countries (online supplemental table S2), anticoagulant treatment varied threefold among countries (30%–90%) (online supplemental figure S1b). The highest rate of anticoagulation was in the Netherlands and Switzerland (90%) and lowest in China (30%), India (35%) and Ukraine (48%) (online supplemental figure S1b). More than 40% of newly diagnosed patients with AF in China and India received AP therapy only and a further 20%, approximately, received no antithrombotic therapy. Across all countries, we found a significant (p<0.001) association with the choice of antithrombotic regimen and HAQ index, that is, with a greater likelihood of AC and NOAC prescribing (and lower likelihood of AP therapy alone) with increasing HAQ score (figure 4).
Figure 4.
Baseline antithrombotic treatment distribution by Healthcare Access and Quality (HAQ) index.1 1 As HAQ index is a country measure, all patients enrolled within a specific country are assigned the same HAQ index. HAQ index of OAC+AP or AP only: <70=46.7%; 70–79=52.5%; 80–89=30.1%; ≥90=28.6%. NOAC, AP, antiplatelet; NOAC, non-vitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist.
ACs (with or without AP therapy) were prescribed to more than 70% of patients in 18 of 35 countries.
The choice of stroke prevention strategy by region and country was analysed and included in the Cox model. Even after adjustment for baseline risk factors and antithrombotic regimen (AC and/or AP treatment), substantial inter-country differences remained in the rate of non-haemorrhagic stroke/SE (online supplemental table S4).
Discussion
Our analysis revealed a wide variability in standardised rates of all-cause mortality, non-haemorrhagic stroke/SE and major bleeding across regions and countries. It also showed a wide variability in baseline characteristics and treatment patterns across regions and countries. Asians had a lower risk profile than patients of any other regions, with lower mean age, BMI, systolic and diastolic blood pressure, and pulse rate. They had lower rates of comorbidities, particularly history of ACS, vascular disease, stroke/SE, hypertension, high blood cholesterol, moderate to severe CKD, and much lower risk of death according to the GARFIELD-AF risk score. With few exceptions, patients of non-Asian regions had substantially higher rates of any of these variables and higher risk of death according to GARFIELD-AF mortality risk score, though median CHA2DS2-VASc score and GARFIELD-AF stroke risk score were similar across regions.
In addition, there was a wide variability in treatment patterns as regards stroke prevention that was not accounted for by conventional measures of stroke risk, namely CHA2DS2-VASc score.14 Such findings are consistent with other observational studies, including Practice Innovation and Clinical Excellence,15 EUR Observational Research Programme-Atrial Fibrillation16 and Global Registry on Long-Term Antithrombotic Treatments in Patients with Atrial Fibrillation.17 In our population, there were also wide variations across countries in antithrombotic therapy prescription. The rate of prescription of OAC w/wo AP agent was in the range of 70% in Europe, North America and Other Countries but approximately 60% in Latin America, and 56% in Asia. In China, India, South Korea, Singapore, Russia, United Arab Emirates, Mexico, Ukraine patients had substantially higher than global rates of AP therapy (without anticoagulation) (over 30%), and substantially lower than global average rates of OAC prescription (range 22%–58%), and a higher proportion of patients with no antithrombotic at all.
After adjusting for the baseline demographics and clinical characteristics (including modifiable cardiovascular risk factors and comorbidities), the variability in all three major outcomes among countries persisted, though attenuated. Even after including antithrombotic regimen as a model covariate, substantial differences in the expected rates of events across countries remained. OAC treatment was shown to be associated with 30% and 28% lower risks of death and stroke/SE in a previous report.18 However, type and quality of OAC matter. NOAC instead of VKA, appropriateness of NOAC dosing and quality of VKA monitoring had significant impact on outcomes,19 20 as well as adherence to treatment.21 This was not accounted for in this analysis and may explain that the differences in outcomes were only partly attenuated after adjustment.
In GARFIELD-AF there were geographic disparities, not only in antithrombotic regimens for AF, but also in other cardiovascular management measures. Indeed, AF is no longer considered as an isolated arrhythmia as it is associated with comorbidities that all need a specific therapeutic approach in other words a comprehensive management is now recommended. There may be wide geographic variations in the management of comorbidities such as CHF, diabetes, hypertension, high total and low-density lipoprotein (LDL) cholesterol, as well as other non-cardiovascular comorbidities such as respiratory failure, sepsis and malignancy. Non-cardiovascular death accounts for at least 50% of all cause death. In some regions more comprehensive treatment of comorbidities in patients with AF may have influenced cardiovascular and non-cardiovascular outcomes and may have accounted, at least in part, for the residual geographic variation in outcomes. The demonstrated clear relation of outcomes with indices of healthcare access (HAQ indices) supports this concept.
The observed differences in stroke rates, by country and by region, are not explained by the risk predictors within commonly used stroke prediction tools. These findings highlight the importance of identifying factors beyond those collected in conventional risk prediction tools to estimate outcomes in patients with AF. Such factors may include practice patterns (eg, anticoagulation quality and adherence to treatment, statin use, LDL cholesterol management, diabetes control), access to quality healthcare, and environmental, lifestyle and epigenetic characteristics. The sum impact may account for the substantial differences in risk-standardised event rates among countries.22 Achieving population-wide control of modifiable risk factors (including tobacco use, diet, physical inactivity, plasma glucose and hypertension) could abrogate a substantial part of the global stroke burden, irrespective of age, gender or ethnicity.23 24 Even small changes in the distribution of these risk factors could lead to clinically relevant reductions in the risks of cardiovascular disease, stroke, and mortality.25–27 The findings from GARFIELD-AF and other recently published global and regional studies7 28–32 suggest that high rates of potentially modifiable metabolic disorders and smoking persist. Thus, there remains considerable scope to improve the outcomes of patients with newly diagnosed AF, even in high-income and middle-income countries.
Across countries huge variations in outcomes may also be influenced by factors beyond baseline characteristics, stroke prevention and management. Outcomes may depend on access to good quality care and may reflect standardised mortality rates per country. In GARFIELD-AF, the proportion of anticoagulated patients was highly correlated with the average HAQ index (derived from national data). And it was not surprising to observe that both these measures were found to be high in most countries with low risk-standardised mortality rates. Countries with some of the lowest HAQ indices in GARFIELD-AF (India, Ukraine, Argentina and Brazil) had the highest risk-standardised mortality rates. Conversely, the lowest observed rates of mortality in Japan and South Korea persisted even after risk adjustment. Not all countries fit in this frame though. High observed mortality rates (relative to the global average) were found in countries with high HAQ indices such as USA, France and Germany, which remained greater than average even after risk adjustment.
The risk-standardised mortality rates in GARFIELD-AF appeared to be a reflection of average national life expectancy, with the lowest mortality rates in this population with newly diagnosed AF in countries with life expectancies (in years) of 82.2, 83.8, 82.6, 78.2 and 81.6, whereas countries with the highest mortalities in this AF population have life expectancies (in years) of 68.3, 71.2, 76.3, 78.7 and 74.7.33
Patients from participating centres with the highest rates of mortality and non-haemorrhagic stroke/SE were among the least likely to receive OAC for stroke prevention over the 5 years of recruitment into GARFIELD-AF. This is consistent with the observed higher rates of cardiovascular (vs non-cardiovascular) mortality in such countries and where AP therapy or no antithrombotic therapy for AF is most prevalent. However, higher rates of major bleeding were observed in the Netherlands (GARFIELD-AF) and the USA (GARFIELD-AF and ORBIT-AF II). These findings may reflect prescribing practice as in the USA where combination therapy, OAC +AP was more often used (28%) than in other countries. In the Netherlands the rate of OAC prescription is very high, in the range of 90%, chiefly with VKA (78%) and far less with NOAC (28%). These factors may account for the higher-than-expected rates of major bleeding in these two countries.
Clinical implications
Implications are twofold: first, that cardiovascular secondary prevention measures, including lifestyle measures need to be systematically addressed and anticoagulation measures applied and maintained. Second, that additional factors, beyond those in commonly used risk prediction tools (like CHADS2VASc) need to be evaluated, including renal dysfunction, smoking status and the extent of vascular disease. Such comorbidities require additional management.
Conclusions
Antithrombotic regimens varied substantially across countries as well as the observed rates of death, stroke/SE and bleeding. Differences in the event rates persisted even after adjustment for baseline characteristics and antithrombotic treatments. Other factors, including variations in clinical practice and access to quality healthcare, as well as unobserved patient-related factors, may be responsible for the substantial differences in the rates of mortality, stroke/SE and major bleeding across countries. The comprehensive management of patients with AF extends beyond anticoagulation.
Supplementary Material
Acknowledgments
We thank the physicians, nurses and patients involved in the GARFIELDAF registry. Programming support was provided by Madhusudana Rao (TRI, London, UK). Editorial support was provided by Rae Hobbs and Dr Surekha Damineni (TRI, London, UK).
Footnotes
Contributors: KAAF, J-PB, JC, SGot, SGol, SH, GK, FM, JPP, AGGT, FV and AKK contributed to the study design. YK, SO, AP, JPSS and JS contributed to the data collection. KAAF was responsible for the overall content of the manuscript. SV analysed the data. All authors supervised the data analysis, provided the interpretation of results and contributed to the drafting and critical review of the manuscript. All authors approved the final draft.
Funding: The work is supported by Kantor Charitable Foundation for the KantorKakkar Global Centre for Thrombosis Science.
Disclaimer: The funding sources had no involvement in the data collection, data analysis or data interpretation
Competing interests: KAAF has received grants and personal fees from Bayer/Janssen and AstraZeneca and personal fees from Sanofi/Regeneron and Verseon. AJC: Institutional grants and personal fees from Bayer, Boehringer Ingelheim, BMS/Pfizer and Daichi Sankyo; SG has received Personal fees from Thrombosis Research Institute and the American Heart Association, grants from Sanofi, Pfizer, Ono, Bristol Myer Squibb, the Vehicle Racing Commemorative Foundation and Nakatani Foundation for Advancement of Measuring Technologies in Biomedical Engineering. SZG has received research support from Boehringer-Ingelheim, BMS, BTG EKOS, Daiichi, Janssen, NHLBI, and the Thrombosis Research Institute; has served as a consultant for Agile, Bayer, Boehringer-Ingelheim, BMS, Daiichi, Janssen and Zafgen. SH has received personal fees from Aspen, Bayer Healthcare, BMS/Pfizer, Daiichi-Sankyo, and Sanofi. YK: Research grant from Daiichi Sankyo and Boeringer Ingelheim. Personal fees from: Daiichi Sankyo, Boehringer Ingelheim, Bayer, Bristol Meyers and Pfizer; FM is a former employee of Bayer AG. SO: consultant/advisory board payments from Bayer Pharma AG, Bristol-Myers Squibb Korea, Boehringer-Ingelheim Korea, Pfizer Korea, Sanofi-Aventis, and St Jude Medical. JPSS: Personal fee from Pfizer, Astra Zeneca, Novartis, Sanofi & BMS; JPP: Reported grants for clinical research from Abbott, American Heart Association, Boston Scientific, Gilead, Janssen Pharmaceuticals, NHLBI, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, Johnson & Johnson, LivaNova, Medtronic, Milestone, Oliver Wyman Health, Sanofi, Philips, and Up-to-Date. JS: Research grants from Bayer; personal fees from Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, BMS/Pfizer, Novartis, Sanofi, Servier; Expert witness for Boehringer Ingelheim; AGGT has received personal fees from Bayer Healthcare, Janssen Pharmaceutical Research & Development, and Portola. FV has received grants from Bayer Healthcare; personal fees from Bayer Healthcare, BMS/Pfizer, DaiichiSankyo, and Boehringer-Ingelheim. AKK has received research support from Bayer AG and Sanofi; personal fees from Bayer AG, Pfizer, Janssen, Sanofi, Verseon and Anthos Therapeutics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: * A complete list of investigators is given in the supplementary materials
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The data underlying this article will be shared on reasonable request from Karen S Pieper (KPieper@tri-london.ac.uk).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by Independent ethics committee and hospital-based institutional review board approvals were obtained, as necessary, for the registry protocol. Additional approvals were obtained from individual study sites. The registry is being conducted in accordance with the principles of the Declaration of Helsinki, local regulatory requirements, and the International Conference on Harmonisation Good Pharmacoepidemiological and Clinical Practice Guidelines.
References
- 1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke Statistics-2018 update: a report from the American heart association. Circulation 2018;137:e67–492. 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 2. Patel NJ, Atti V, Mitrani RD, et al. Global rising trends of atrial fibrillation: a major public health concern. Heart 2018;104:1989–90. 10.1136/heartjnl-2018-313350 [DOI] [PubMed] [Google Scholar]
- 3. Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med 2007;356:2388–98. 10.1056/NEJMsa053935 [DOI] [PubMed] [Google Scholar]
- 4. Koopman C, Vaartjes I, van Dis I, et al. Explaining the decline in coronary heart disease mortality in the Netherlands between 1997 and 2007. PLoS One 2016;11:e0166139. 10.1371/journal.pone.0166139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bandosz P, O'Flaherty M, Drygas W, et al. Decline in mortality from coronary heart disease in Poland after socioeconomic transformation: modelling study. BMJ 2012;344:d8136. 10.1136/bmj.d8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palmieri L, Bennett K, Giampaoli S, et al. Explaining the decrease in coronary heart disease mortality in Italy between 1980 and 2000. Am J Public Health 2010;100:684–92. 10.2105/AJPH.2008.147173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA 2014;311:183–92. 10.1001/jama.2013.284692 [DOI] [PubMed] [Google Scholar]
- 8. Kakkar AK, Mueller I, Bassand J-P, et al. International longitudinal registry of patients with atrial fibrillation at risk of stroke: global anticoagulant registry in the field (Garfield). Am Heart J 2012;163:13–19. 10.1016/j.ahj.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 9. Kakkar AK, Mueller I, Bassand J-P, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the International, observational, prospective Garfield registry. PLoS One 2013;8:e63479. 10.1371/journal.pone.0063479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. KAA F, Gersh BJ, Traore S. Evolving quality standards for large-scale registries: the GARFIELD-AF experience. European heart journal Quality of care & clinical outcomes 2017;3:114–22. [DOI] [PubMed] [Google Scholar]
- 11. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297. 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fox KAA, Lucas JE, Pieper KS, et al. Improved risk stratification of patients with atrial fibrillation: an integrated GARFIELD-AF tool for the prediction of mortality, stroke and bleed in patients with and without anticoagulation. BMJ Open 2017;7:e017157. 10.1136/bmjopen-2017-017157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fullman N, Yearwood J, Abay SM, et al. Measuring performance on the healthcare access and quality index for 195 countries and territories and selected subnational locations: a systematic analysis from the global burden of disease study 2016. Lancet 2018;391:2236–71. 10.1016/S0140-6736(18)30994-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Camm AJ, Accetta G, Ambrosio G, et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart 2017;103:307–14. 10.1136/heartjnl-2016-309832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan PS, Maddox TM, Tang F, et al. Practice-level variation in warfarin use among outpatients with atrial fibrillation (from the NCDR pinnacle program). Am J Cardiol 2011;108:1136–40. 10.1016/j.amjcard.2011.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lip GYH, Laroche C, Boriani G, et al. Regional differences in presentation and treatment of patients with atrial fibrillation in Europe: a report from the EURObservational research programme atrial fibrillation (EORP-AF) pilot General registry. Europace 2015;17:194–206. 10.1093/europace/euu201 [DOI] [PubMed] [Google Scholar]
- 17. McIntyre WF, Conen D, Olshansky B, et al. Stroke-prevention strategies in North American patients with atrial fibrillation: the GLORIA-AF registry program. Clin Cardiol 2018;41:744–51. 10.1002/clc.22936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bassand J-P, Accetta G, Al Mahmeed W, et al. Risk factors for death, stroke, and bleeding in 28,628 patients from the GARFIELD-AF registry: rationale for comprehensive management of atrial fibrillation. PLoS One 2018;13:e0191592. 10.1371/journal.pone.0191592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Camm AJ, Fox KAA, Virdone S. Comparative effectiveness of oral anticoagulants in everyday practice. Heart 2021. 10.1136/heartjnl-2020-318420. [Epub ahead of print: 16 Feb 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haas S, Ten Cate H, Accetta G, et al. Quality of vitamin K antagonist control and 1-year outcomes in patients with atrial fibrillation: a global perspective from the GARFIELD-AF registry. PLoS One 2016;11:e0164076. 10.1371/journal.pone.0164076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cools F, Johnson D, Camm AJ, et al. Risks associated with discontinuation of oral anticoagulation in newly diagnosed patients with atrial fibrillation: results from the GARFIELD-AF registry. J Thromb Haemost 2021;19:2322–34. 10.1111/jth.15415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khambhati J, Allard-Ratick M, Dhindsa D, et al. The art of cardiovascular risk assessment. Clin Cardiol 2018;41:677–84. 10.1002/clc.22930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 2016;388:761–75. 10.1016/S0140-6736(16)30506-2 [DOI] [PubMed] [Google Scholar]
- 24. Feigin VL, Norrving B, George MG, et al. Prevention of stroke: a strategic global imperative. Nat Rev Neurol 2016;12:501–12. 10.1038/nrneurol.2016.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fang N, Jiang M, Fan Y. Ideal cardiovascular health metrics and risk of cardiovascular disease or mortality: a meta-analysis. Int J Cardiol 2016;214:279–83. 10.1016/j.ijcard.2016.03.210 [DOI] [PubMed] [Google Scholar]
- 26. Guo L, Zhang S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: a meta-analysis of prospective studies. Clin Cardiol 2017;40:1339–46. 10.1002/clc.22836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Colpani V, Baena CP, Jaspers L, et al. Lifestyle factors, cardiovascular disease and all-cause mortality in middle-aged and elderly women: a systematic review and meta-analysis. Eur J Epidemiol 2018;33:831–45. 10.1007/s10654-018-0374-z [DOI] [PubMed] [Google Scholar]
- 28. NCD Risk Factor Collaboration (NCD-RisC) . Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387:1377–96. 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guthold R, Stevens GA, Riley LM, et al. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health 2018;6:e1077–86. 10.1016/S2214-109X(18)30357-7 [DOI] [PubMed] [Google Scholar]
- 30. Zhou B, Bentham J, Di Cesare M, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 2017;389:37–55. 10.1016/S0140-6736(16)31919-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011;378:31–40. 10.1016/S0140-6736(11)60679-X [DOI] [PubMed] [Google Scholar]
- 32. Kotseva K, De Bacquer D, De Backer G, et al. Lifestyle and risk factor management in people at high risk of cardiovascular disease. A report from the European Society of cardiology European action on secondary and primary prevention by intervention to reduce events (EUROASPIRE) IV cross-sectional survey in 14 European regions. Eur J Prev Cardiol 2016;23:2007–18. 10.1177/2047487316667784 [DOI] [PubMed] [Google Scholar]
- 33. World Economic Forum . Life expectancy. Available: http://reportsweforumorg/global-competitiveness-index-2017-2018/competitiveness-rankings/#series=LIFEEXPECT [Accessed Dec 2018].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-049933supp001.pdf (212.6KB, pdf)
Data Availability Statement
Data are available on reasonable request. The data underlying this article will be shared on reasonable request from Karen S Pieper (KPieper@tri-london.ac.uk).