Abstract
Background
The ideal objective of treating a person with epilepsy is to induce remission (free of seizures for some time) using antiepileptic drugs (AEDs) and withdraw the AEDs without causing seizure recurrence. Prolonged usage of AEDs may have long‐term adverse effects. Hence, when a person with epilepsy is in remission, it is logical to attempt to discontinue the medication. The timing of withdrawal and the mode of withdrawal arise while contemplating withdrawal of AEDs. This review examines the evidence for the rate of withdrawal of AEDs (whether rapid or slow tapering) and its effect on seizure recurrence.
This is an updated version of the Cochrane Review previously published in 2020.
Objectives
To quantify risk of seizure recurrence after rapid (tapering period of three months or less) or slow (tapering period of more than three months) discontinuation of antiepileptic drugs in adults and children with epilepsy who are in remission, and to assess which variables modify the risk of seizure recurrence.
Search methods
For the latest update, on 8 November 2021, we searched: Cochrane Register of Studies (CRS Web), MEDLINE (Ovid), and SCOPUS. There were no language restrictions. CRS Web includes randomized or quasi‐randomized, controlled trials from PubMed, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP), CENTRAL, and the Specialized Registers of Cochrane Review Groups including Epilepsy.
Selection criteria
Randomized controlled trials that evaluated withdrawal of AEDs in a rapid or slow tapering after varying periods of seizure control in people with epilepsy.
Data collection and analysis
Two review authors independently assessed the trials for inclusion and extracted the data. The outcomes assessed included seizure freedom after one, two, or five years of AED withdrawal; time to recurrence of seizure following withdrawal; occurrence of status epilepticus; mortality; morbidity due to seizure, such as injuries, fractures, and aspiration pneumonia; and quality of life (assessed by validated scale).
Main results
There are two included studies in this review.
One study randomized 57 children with epilepsy with seizure freedom for at least two years to taper down the AED over one or six months. The study was not blinded and there were no details of randomization. Over the period of 54 months of follow‐up, 20/30 participants in the one‐month group remained seizure‐free compared to 15/27 participants in the six‐month group (no evidence of a difference). There was no information on time of seizure recurrence for each group to allow a comparison.
The other study involved 149 children. There was a non‐significant trend towards a lower risk of seizure recurrence after one year of AED withdrawal in participants allocated to slow tapering (risk ratio (RR) 0.76, 95% confidence interval (CI) 0.58 to 1.01; P = 0.06; very low‐certainty evidence). At the end of two years, 30 participants were seizure free in the rapid‐tapering group and 29 participants in the slow‐tapering group (RR 0.87, 95% CI 0.58 to 1.29; P = 0.48; very low‐certainty evidence). At the end of five years, 10 participants were seizure free in the rapid‐tapering group and six participants in the slow‐tapering group (RR 1.40, 95% CI 0.54 to 3.65; P = 0.49; very low‐certainty evidence). There were no data for the other outcomes.
Due to the methodological heterogeneity and the difference in the duration of tapering, we did not perform a quantitative synthesis of these studies.
Currently, one Italian trial is ongoing that is investigating if a slow or a rapid withdrawal schedule of AEDs influences return of seizures (relapse) in adults with focal or generalized epilepsy who have been seizure free for at least two years (no preliminary results available).
Authors' conclusions
In view of methodological deficiencies, and small sample size of the two included studies, we cannot draw any reliable conclusions regarding the optimal rate of tapering of AEDs. Using GRADE, we assessed the certainty of the evidence as very low for outcomes for which data were available. We judged both studies to be at an overall high risk of bias.
Further studies are needed in adults and children to investigate the optimal rate of withdrawal of AEDs and to study the effects of variables such as seizure types, aetiology, intellectual disability, electroencephalography abnormalities, presence of neurological deficits, and other comorbidities on the rate of tapering.
Plain language summary
Rapid versus slow withdrawal of antiepileptic medicines
Background
Epilepsy is a disorder where recurrent seizures (fits) are caused by abnormal electrical discharges of the brain. Antiepileptic medicines are used to prevent these seizures. Regular intake of antiepileptic medicines may have long‐term side effects. When in remission (free of seizures for some time), it is logical to attempt to stop the medicines. Two important issues are how and when to stop them.
Aim of the review
This review analyzed studies for evidence regarding rapidity of withdrawal of antiepileptic medicines. We included randomized controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) evaluating the rapid or slow withdrawal (tapering down) of these medicines after varying periods of seizure control in people with epilepsy.
Results
We included only two small studies conducted in 206 children with epilepsy. The included studies found no difference in the proportion of participants remaining seizure‐free between the rapid‐ and the slow‐tapering groups at different time points. There were no data for other measures such as status epilepticus (a long seizure), death, illness relating to seizures, and quality of life. We found no completed trials investigating antiepileptic medicine withdrawal in adults.
Currently, one Italian trial is ongoing that is investigating if a slow or a rapid withdrawal schedule of antiepileptic medicine influences return of seizures (relapse) in adults with epilepsy who have been seizure free for at least two years (no preliminary results available).
Reliability of the evidence
Evidence from the two included studies was of very low reliability. Both studies were conducted in a small number of participants and there were not enough data to detect a difference between the groups. Furthermore, they included only children, hence the results cannot be generalized to adults. Therefore, no reliable evidence is currently available on the optimal rate of tapering of antiepileptic medicines.
The evidence is current to November 2021.
Summary of findings
Summary of findings 1. Rapid versus slow withdrawal of antiepileptic drugs.
| Rapid versus slow withdrawal of antiepileptic drugs | ||||||
| Patient or population: people with epilepsy Setting: outpatients Intervention: rapid AED withdrawal Comparison: slow AED withdrawal | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with slow AED withdrawal | Risk with rapid AED withdrawal | |||||
| Seizure freedom after 1 year of AED withdrawal | Study population | RR 0.76 (0.58 to 1.01) | 149 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | Non‐significant trend towards a lower risk of seizure recurrence after 1 year of AED withdrawal in participants allocated to slow tapering. | |
| 647 per 1000 | 492 per 1000 (330 to 654) | |||||
| Seizure freedom after 2 years of AED withdrawal | Study population | RR 0.87 (0.58 to 1.29) | 149 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | No evidence of a difference, but the CI was wide and equivalence could not be inferred. | |
| 426 per 1000 | 371 per 1000 (235 to 533) | |||||
| Seizure freedom after 5 years of AED withdrawal | Study population | RR 1.40 (0.54 to 3.65) | 149 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | No evidence of a difference, but the CI was wide and equivalence could not be inferred. | |
| 88 per 1000 | 124 per 1000 (46 to 290) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AED: antiepileptic drug; CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level; information from one study with high risk of performance bias; plausible bias likely to seriously alter the results. bDowngraded one level; information from one study. cDowngraded one level; small number of participants included in this study; no statistical power was calculated.
Background
This review is an update of a previously published review in 2020 (Ayuga Loro 2020); there are no new studies, and thus, the conclusions are unchanged.
Description of the condition
Epilepsy is a chronic disorder of the brain characterized by an enduring predisposition to epileptic seizures (Fisher 2014). It is estimated that approximately one third of people with epilepsy achieve seizure freedom with one or more antiepileptic drugs (AED) (Brodie 2012). Furthermore, epilepsy can be defined as 'resolved' for people who either had an age‐dependent epilepsy syndrome (e.g. childhood absence epilepsy) but are now past the applicable age or for people who "have remained seizure‐free for the last 10 years and off anti‐seizure medicines for at least the last five years" (Fisher 2014).
Description of the intervention
The ideal objective of treating a person with epilepsy is to induce remission with AEDs and withdraw the AEDs without causing seizure recurrence. Some people with epilepsy may prefer to continue taking AEDs lifelong rather than risk a seizure recurrence. However, prolonged use of AEDs may have long‐term adverse effects and may potentially affect prognosis or quality of life. The adverse effects of AEDs on cognition and behaviour and the social stigma associated with epilepsy are increasingly recognized. When a person with epilepsy is in remission (free of seizures for some time), then it is logical to attempt to discontinue the medication.
How the intervention might work
The rate of recurrence of seizures after withdrawal of AEDs among people with epilepsy who are in remission is estimated to be between 12% and 67% (Berg 1994; MRC 1991; MRC 1993; Shih 2009). Thus, the decision to discontinue AEDs depends on the chance of remaining seizure free after drug withdrawal, the presence of factors predictive of higher risks of seizure recurrence, and the medical and social consequence of drug withdrawal compared with continuation of treatment. There are several factors that contribute to the risk of relapse. They are age at onset of seizure; age at treatment withdrawal; family history of epilepsy, recognized aetiology of epilepsy; electroencephalogram (EEG) abnormalities; number of seizures preceding remission; duration of seizure‐free period on treatment; and number of drugs needed to control the epilepsy.
Why it is important to do this review
The physician contemplating withdrawal of AEDs is faced with two important considerations: timing of withdrawal and mode of withdrawal. The timing of withdrawal (duration of seizure freedom before attempting to withdraw an AED) has not been clearly defined. The guidelines of the National Institute of Health and Care Excellence (NICE) of the United Kingdom (NICE 2012) and the guidelines of the Italian League Against Epilepsy on the withdrawal of antiepileptic drugs (Beghi 2013) recommended a minimum period of two years of seizure freedom before considering withdrawing the drugs. One study from the UK required the seizure‐free period to be at least two years (MRC 1991; MRC 1993). One systematic review found that the pooled risk ratio (RR) for seizure relapse in early (less than two years) versus late (more than two years) AED withdrawal was 1.34 (95% confidence interval (CI) 1.13 to 1.59; number needed to treat for an additional harmful effect 8), indicating that AED withdrawal in people who are not seizure free for at least two years is associated with a higher recurrence rate (Strozzi 2015).
There is no consensus on the mode of withdrawal. Tapering of AEDs ranges from rapid withdrawal to slow withdrawal spread over a period of two years (Bouma 1987; Duncan 1987; Oller‐Daurella 1975; Overweg 1981; Shinnar 1994). In other studies, the tapering period has come down to three to six months, reflecting a different clinical approach (Arts 1988; Callaghan 1988; Emerson 1981; Juul‐Jensen 1964; MRC 1991; Tennison 1994), but as yet there is no consensus in this tapering regimen. Risk of seizure relapse due to rapidity of drug discontinuation is not well defined.
We arbitrarily defined rapid tapering as when the AED(s) was/were tapered and discontinued within three months and slow tapering as when the AED(s) was/were tapered and discontinued over more than three months.
In this systematic review, we examined whether the rapidity of withdrawal of AEDs among people with epilepsy who are in remission influences the recurrence of seizures and ultimate prognosis or quality of life. We also examined the effect of variables such as age of seizure onset, seizure types, presence of neurological deficits, mental subnormality, aetiology of epilepsy, EEG findings, or duration of seizure freedom on the risk of recurrence of seizures with the two tapering regimens.
Objectives
To quantify risk of seizure recurrence after rapid (tapering period of three months or less) or slow (tapering period of more than three months) discontinuation of antiepileptic drugs in adults and children with epilepsy who are in remission, and to assess which variables modify the risk of seizure recurrence.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials that evaluated rapid or slow AED withdrawal after varying periods of seizure control (remission) in people with epilepsy.
The studies could be double blind, single blind, or unblinded.
We excluded studies involving highly specific patient samples such as neonates.
Types of participants
Men, women, and children (excluding neonates) of any age, with a diagnosis of epilepsy and who had been seizure free for a described time.
Participants may have had onset of epilepsy at any age and the seizure types could have been either focal or generalized.
Types of interventions
Studies involving rapid or slow withdrawal of AEDs. The studies may have also compared rapid or slow withdrawal with continued treatment.
Types of outcome measures
Primary outcomes
Seizure freedom after one year of AED withdrawal.
Seizure freedom after two years of AED withdrawal.
Seizure freedom after five years of AED withdrawal.
Secondary outcomes
Time to recurrence of seizure following withdrawal.
Occurrence of status epilepticus.
Mortality.
Morbidity due to seizure such as injuries, fractures, aspiration pneumonia, etc.
Quality of life (assessed by validated scales).
Search methods for identification of studies
Electronic searches
We ran searches for the original review in 2005 and subsequent searches in April 2019. For the latest update, we searched the following databases on 8 November 2021.
Cochrane Register of Studies (CRS Web), using the search strategy set out in Appendix 1.
MEDLINE (Ovid, 1946 to 5 November 2021) using the strategy outlined in Appendix 2.
SCOPUS (from 1823) citation search using the strategy outlined in Appendix 3.
CRS Web includes randomized, or quasi‐randomized, controlled trials from PubMed, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP), the Cochrane Central Register of Controlled Trials (CENTRAL), and the Specialized Registers of Cochrane Review Groups including Epilepsy. In MEDLINE (Ovid) the coverage end date always lags a few days behind the search date.
Searching other resources
We checked cross‐references from identified studies and from previous systematic reviews on the topic of AED withdrawal. We contacted colleagues and experts in the field regarding any studies that we had missed (by e‐mail on 2 July 2019).
There were no language restrictions on our searching activities.
Data collection and analysis
We performed analyses using Review Manager 5 (Review Manager 2014).
Selection of studies
Two review authors (FAL and EGT) independently screened titles and abstracts of the electronic search results, and assessed the potentially relevant trials for inclusion, with disagreements resolved by discussion.
Data extraction and management
Two review authors (FAL and EGT) independently extracted the following data and resolved differences of opinion by discussion.
-
Study methods
Design (e.g. parallel or crossover design).
Randomization method (allocation concealment and list generation).
Blinding method.
-
Participants
Number (total/per group).
Age and sex distribution.
Seizure type and epilepsy syndrome.
Duration of epilepsy.
Aetiology of epilepsy.
Neurological deficits.
Mental subnormality.
Duration of seizure freedom.
EEG abnormality before tapering.
Loss to follow‐up.
Rapidity of AED withdrawal.
Duration of follow‐up.
Outcome data as described above including the certainty of seizure recurrence.
We contacted authors of identified studies to request any missing data. If studies were only in abstract form or published without any clear description of the methodology employed, we contacted the original trialists for clarification.
Assessment of risk of bias in included studies
Two review authors (FAL and FB) independently assessed the risk of bias associated with included studies using the Cochrane risk of bias tool, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The Cochrane risk of bias tool comprises seven specific parameters: 1. random sequence generation, 2. allocation concealment, 3. blinding of participants and personnel, 4. blinding of outcome assessors, 5. incomplete outcome data, 6. selective outcome reporting, and 7. other bias. For each entry, review authors made the judgement ('low' risk of bias, 'high' risk of bias, or 'unclear' risk of bias) and provided support for the decision by an agreed review author comment or by a quote taken from the corresponding publication. We resolved any disagreements by discussion.
Measures of treatment effect
We reported dichotomous outcomes (proportion of participants who remained seizure free) as Mantel‐Haenszel RR with 95% CIs.
We reported time to event outcomes (e.g. time to seizure recurrence) as hazard ratios with 95% CIs.
We planned to report time to seizure recurrence as risk difference with 95% CIs.
Unit of analysis issues
We planned to deal with any unit of analysis issues according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
Primary analysis was by intention to treat, in which all participants were included in the treatment groups to which they were allocated, irrespective of the treatment or policy they received. Due to the small number of included studies, we performed no best‐case or worst‐case analyses.
Assessment of heterogeneity
We assessed clinical heterogeneity by evaluating similarities and differences in the methodologies and outcomes measured in the included studies and by visually inspecting forest plots. We planned to assess statistical heterogeneity using the Chi² test and I² statistic as follows (Higgins 2011): 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% may indicate considerable heterogeneity. Our intention was to use a fixed‐effect model if we did not find statistically significant heterogeneity between the included studies. If statistical heterogeneity had been present, we would have used a random‐effects model. However, despite our primary intention, due to insufficient information on outcomes and too high methodological heterogeneity, we were unable to perform any meta‐analyses.
Assessment of reporting biases
We sought all study protocols to identify any discrepancies between protocol and trial methodology.
Data synthesis
Provided we thought it clinically appropriate, and there was no important heterogeneity, we planned to summarize results in a meta‐analysis. However, because of the high methodological heterogeneity with different duration of tapering periods, we could not perform a meta‐analysis. Further research could allow results to be pooled, leading to a quantitative rather than a qualitative summary of results.
Subgroup analysis and investigation of heterogeneity
We did not carry out any subgroup analysis or formal investigation of heterogeneity.
Sensitivity analysis
We did not carry out any sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
The primary outcomes are presented in a summary of findings table. We used GRADEpro GDT software and GRADE quality assessment criteria to evaluate the certainty of evidence derived from the studies included in this review (Guyatt 2008). To assess the overall certainty of evidence for each outcome, we downgraded the evidence from 'high certainty' by one level for serious (or by two for very serious) risk of bias, indirectness of evidence, inconsistency, imprecision of effect estimates, or potential publication bias. See Table 1.
Results
Description of studies
Results of the search
Figure 1 summarizes the results of the searches and the process of screening and selecting studies for inclusion in this updated version of the review. The database searches (carried out in 2019 and 2021) yielded 119 results. After removing 48 duplicates and 26 irrelevant items, we assessed 45 full‐text articles for eligibility. Four studies were initially considered for possible inclusion (Aidaros 2010; He 2016; Gasparini 2016; Serra 2005); however, two were eventually excluded (Aidaros 2010; He 2016), as neither of them adopted a randomized controlled design (see Characteristics of excluded studies table); one study was ongoing and had no results (Gasparini 2016).
1.
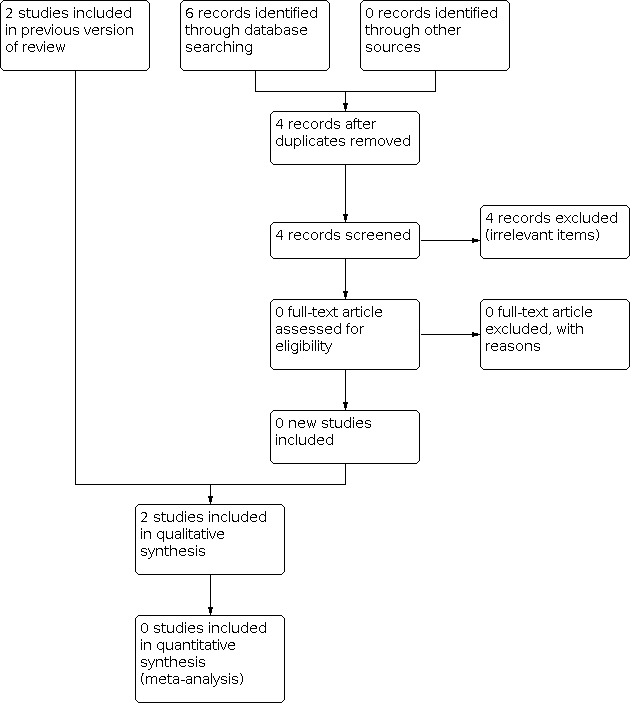
Flow diagram of this updated version of the review
Included studies
See Characteristics of included studies table.
Tennison 1994
Tennison 1994 was included in the previous version of this systematic review. The study recruited 149 children with a mean age of seizure onset of four years, mean age of 11 years at the time of starting the tapering. They were seizure free for at least 18 months. The eligible children were randomly assigned to two groups by tosses of a coin. The first toss determined the rate of tapering (six weeks or nine months), and the second toss determined whether the tapering period would begin after a seizure freedom of two or four years. EEG obtained just before the tapering was normal in 40% of participants. The six‐week tapering group (rapid‐tapering group) had 81 participants out of whom 11 were lost to follow‐up before the tapering began. The nine‐month tapering group (slow‐tapering group) had 68 participants, five of whom were lost to follow‐up before the tapering began. Thus, the rapid‐tapering group had 70 participants (41 boys and 29 girls) and the slow‐tapering group had 63 participants (38 boys and 25 girls). Sixty‐one participants had more than 10 seizures and 84 had focal seizures. The tapering period was divided into three equal parts (two weeks each in the six‐week tapering group and three months each in the nine‐month tapering group). The dose was reduced by 25% during each interval. The drugs were tapered sequentially if the participant was receiving more than one drug. Barbiturate was tapered last. Follow‐up began at the start of drug tapering. All participants were followed up for at least 11 months. Cerebral palsy was present in 43/133 participants, 22 in the rapid‐tapering group and 21 in the slow‐tapering group. Medications used included barbiturate, phenytoin, carbamazepine, and valproic acid. Twenty‐four participants in the rapid‐tapering group and 25 participants in the slow‐tapering group were receiving two or more drugs. The mean duration of follow‐up was 39 months (range 11 to 105 months).
Serra 2005
Serra 2005 was a randomized controlled study conducted in 57 children (24 girls, 33 boys; aged two to 16 years; mean 9.45 years) with a diagnosis of epilepsy controlled by an AED for at least two years. People with infantile spasms and neonatal seizures were excluded. Groups were similar at baseline regarding age, sex, type of epileptic syndrome, EEG abnormality, and AED. Thirty participants had idiopathic, 13 cryptogenic, and 14 symptomatic epilepsy. The aetiology of symptomatic seizures was hypoxic‐ischaemic injury in four participants (28.5%); neurocysticercosis in three participants (21.4%); meningoencephalitis in four participants (28.5%); and tuberous sclerosis complex, hydrocephalus, and congenital toxoplasmosis in one participant each. The EEG was normal in 41 participants and abnormal in 15 participants (there was no information for one participant). All participants were receiving monotherapy: 35 (61.4%) received carbamazepine, 12 (21%) phenytoin, six (10.5%) valproic acid, and four (7%) phenobarbital. Participants were randomized to taper the AED during one or six months. In the one‐month AED withdrawal group, the dose was reduced by 25% every 10 days; and in the six‐month group, the dose was reduced by 25% every two months. Authors of this study analyzed the frequency of seizure recurrence according to the time of AED withdrawal over 54 months of follow‐up.
Excluded studies
See Characteristics of excluded studies table.
Two studies were initially considered for possible inclusion (Aidaros 2010; He 2016); however, both were eventually excluded, as neither of them adopted a randomized controlled design.
The previous version of this review excluded nine studies (Aidaros 2010; Braathen 1996; Gebremariam 1999; Gherpelli 1992; He 2016; MRC 1991; Peters 1998; Todt 1984; Verrotti 2000).
Ongoing studies
Gasparini 2016
See Characteristics of ongoing studies table.
Gasparini 2016 is an Italian multicentre, prospective, randomized controlled trial which is ongoing. It aims to investigate if a slow or a rapid withdrawal schedule of AED monotherapy influences relapse rate in adults with focal or generalized epilepsy who have been seizure free for at least two years. Participants will be randomized to a slow (160 days) or a rapid (60 days) schedule, with follow‐up of one year after randomization (expected sample size: 350 participants). The primary outcome is the time to seizure relapse; secondary outcomes are compliance to the assigned schedule; and occurrence of status epilepticus, seizure‐related injuries, and mortality. No preliminary results are published.
Risk of bias in included studies
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Tennison 1994 was a randomized study, with random sequence generation based on coin tossing (low risk of selection bias); in this study, the clinical characteristics of the participants were similar across study groups. Serra 2005 provided no information on the methods used to generate random sequence and allocate participants (unclear risk of selection bias); however, the clinical characteristics of the participants were similar across study groups.
Blinding
Neither study was blinded (high risk of performance and detection bias) (Serra 2005; Tennison 1994).
Incomplete outcome data
Tennison 1994 had no participants lost to follow‐up after the allocation of participants to the two groups (low risk of attrition bias due to no missing outcome data). Serra 2005 provided no information on participants lost to follow‐up after randomization (unclear risk of attrition bias).
Selective reporting
Although we did not find published protocols for either study, all outcomes outlined in the methods section of each paper were analyzed and reported in the results section of both studies, so we classified them at low risk of reporting bias.
Other potential sources of bias
Tennison 1994 did not reported whether the epilepsy types (idiopathic, cryptogenic, or symptomatic) were distributed equally among groups at baseline; at baseline, there was no difference between the two groups for this variable in Serra 2005.
When more than one drug was withdrawn sequentially in Tennison 1994, the tapering exceeded the six‐week interval; the dose of barbiturates was tapered last. Of the 81 participants in the six‐week tapering group, 63 completed the protocol and of the 68 in the nine‐month tapering group, 48 completed the protocol correctly. Three participants continued therapy in the rapid‐tapering group and nine continued in the slow‐tapering group without tapering. Eleven participants in the rapid‐tapering group and five in the slow‐tapering group were lost to follow‐up before start of tapering period.
Tennison 1994 provided no information on funding. Serra 2005 received public funding from Fundação de Amparo à Pesquisa do Estado de São Paulo (São Paulo Research Foundation), maintained by endowments by the Brazilian State Government.
Neither study provided details on conflicts of interest.
Effects of interventions
See: Table 1
Because of the methodological heterogeneity and the difference in the duration of tapering, we did not perform a quantitative synthesis of the two included studies.
Primary outcomes
Seizure freedom after one year of antiepileptic drug withdrawal
One study provided information on seizure freedom after one year of AED withdrawal (Tennison 1994). At the end of one year, 40/81 participants in the rapid‐tapering group were seizure free at the end of one‐year follow‐up, compared to 44/68 in the slow‐tapering group. There was a non‐significant trend towards a lower risk of seizure recurrence after one year of AED withdrawal in participants allocated to slow tapering (RR 0.76, 95% CI 0.58 to 1.01; P = 0.06; Analysis 1.1). We downgraded the certainty of evidence from high to very low for serious risk of performance bias and for imprecision of effect estimates.
1.1. Analysis.

Comparison 1: Rapid versus slow antiepileptic drug (AED) withdrawal, Outcome 1: Seizure freedom after 1 year of AED withdrawal
Seizure freedom after two years of antiepileptic drug withdrawal
One study provided information on seizure freedom after two years of AED withdrawal (Tennison 1994). At the end of two years, 30/81 participants in the rapid‐tapering group and 29/68 in the slow‐tapering group were free of seizures (RR 0.87, 95% CI 0.58 to 1.29; P = 0.48; Analysis 1.2). We downgraded the certainty of evidence from high to very low for serious risk of performance bias and for imprecision of effect estimates.
1.2. Analysis.

Comparison 1: Rapid versus slow antiepileptic drug (AED) withdrawal, Outcome 2: Seizure freedom after 2 years of AED withdrawal
Seizure freedom after five years of antiepileptic drug withdrawal
One study provided information on seizure freedom after five years of AED withdrawal (Tennison 1994). At the end of five years of AED withdrawal, 10/81 participants in the rapid‐tapering group and 6/68 in the slow‐tapering group remained seizure free (RR 1.40, 95% CI 0.54 to 3.65; P = 0.49; Analysis 1.3). We downgraded the certainty of evidence from high to very low for serious risk of performance bias and for imprecision of effect estimates.
1.3. Analysis.

Comparison 1: Rapid versus slow antiepileptic drug (AED) withdrawal, Outcome 3: Seizure freedom after 5 years of AED withdrawal
Seizure freedom at other time points
In Tennison 1994, after three years of follow‐up, 24/81 participants in the rapid‐tapering group and 14/68 in the slow‐tapering group remained seizure free (RR 1.44, 95% CI 0.81 to 2.56; no difference). At the end of the fourth year of follow‐up, 18/81 participants in the rapid‐tapering group and 8/68 in the slow‐tapering group remained seizure free (RR 1.89, 95% CI 0.88 to 4.07; no difference).
In Serra 2005, over the period of 54 months of follow‐up, 20/30 participants in the one‐month group compared to 15/27 participants in the six‐month group remained seizure‐free (no difference).
Factors associated with seizure recurrence
In Tennison 1994, two factors were associated with higher risk of seizure recurrence: presence of intellectual disability (RR 3.1, 95% CI 1.5 to 6.2) and spikes in the EEG at the start of the tapering period (RR 1.9, 95% CI 1.0 to 3.4). The following factors were not associated with higher risk of seizure recurrence: abnormal EEG at start of the tapering period (RR 1.7, 95% CI 0.9 to 3.0); presence of cerebral palsy (RR 1.1, 95% CI 0.5 to 2.1); barbiturate tapered (RR 1.4, 95% CI 0.8 to 20.4); family history of seizures (RR 0.6, 95% CI 0.4 to 1.1); history of status epilepticus (RR 0.7, 95% CI 0.3 to 2.0); history of febrile seizures (RR 0.9, 95% CI 0.4 to 2.3); history of generalized seizures (RR 1.2, 95% CI 0.7 to 2.3); history of more than 10 seizures (RR 1.2, 95% CI 0.7 to 2.1); age at onset of seizure (RR 1.0, 95% CI 0.9 to 1.1); and age at start of tapering period (RR 1.1, 95% CI 1.0 to 1.1).
Serra 2005 did not provide data on risk factors for seizure recurrence during and after tapering of AEDs.
Secondary outcomes
Time to recurrence of seizure following withdrawal
One study provided information on time to recurrence of seizure following withdrawal, which ranged from 10 days to four years and five months (mean 13.2 months) (Serra 2005). There was no information on this outcome for each group to allow a comparison.
Occurrence of status epilepticus
No study provided information on occurrence of status epilepticus.
Mortality
No study provided information on mortality.
Morbidity due to seizures
No study provided information on morbidity due to seizures.
Quality of life
No study provided information on quality of life.
Discussion
Since the last version of this review (Ayuga Loro 2020), we found no new studies.
Summary of main results
See Table 1.
The included studies found no evidence of a difference in the proportion of participants remaining seizure‐free between the rapid‐ and the slow‐tapering groups at different time points, although in Tennison 1994, there was a non‐significant trend towards a lower risk of seizure recurrence after one year of AED withdrawal in participants allocated to slow tapering. However, these studies were probably not powered to detect a significant difference between the rapid and slow‐tapering groups because of the small sample size.
There is very limited information on factors influencing the risk of seizure recurrence during and after tapering of AEDs. In Tennison 1994, the presence of intellectual disability or spikes in the EEG obtained just before the start of the tapering period were identified as risk factors for seizure recurrence.
One study provided information on time to recurrence of seizure following withdrawal; however, there was no information on this outcome for each group to allow a comparison (Serra 2005).
There were no data for the other outcomes such as status epilepticus, mortality, morbidity, and quality of life.
Overall completeness and applicability of evidence
Both included studies were conducted in children, hence their generalizability to adults is limited. We found no completed trials investigating AED withdrawal in adults.
Currently, one Italian multicentre, prospective, randomized controlled trial is ongoing with the aim of investigating if a slow or a rapid withdrawal schedule of AED monotherapy influences relapse rate in adults with focal or generalized epilepsy who have been seizure free for at least two years (Gasparini 2016; Characteristics of ongoing studies table). Participants will be randomized to a slow (160 days) or a rapid (60 days) schedule, with follow‐up of one year after randomization (expected sample size: 350 participants). The primary outcome is the time to seizure relapse; secondary outcomes are compliance to the assigned schedule; and occurrence of status epilepticus, seizure‐related injuries, and mortality. The study is currently ongoing, and no preliminary results have been published so far. According to investigators, such a study "should contribute to better define the best withdrawal period for AED treatment in adult patients with epilepsy" (Gasparini 2016).
Certainty of the evidence
Tennison 1994 was small and not blinded. Although the procedure of randomization was adequate, protocol violation and loss to follow‐up further reduced the reliability of results. Furthermore, when participants were receiving more than one drug, the tapering period exceeded the six‐week/nine‐month tapering. Serra 2005 provided no details on the procedure of randomization, and was unblinded. In both studies, clinical characteristics of the participants were similar across study groups, suggesting that the randomization was effective in minimizing the risk of covariate imbalance.
The lack of blinding of participants appears to be a methodological limitation that is difficult to overcome in controlled trials where AED withdrawal is performed in different durations of tapering. Similarly, the blinding of healthcare providers can be difficult to establish at the start of a trial and to maintain throughout it, particularly if the study adopts a pragmatic design. Although the lack of blinding is unlikely to influence 'hard' outcomes such as seizure recurrence, it may influence subjective outcomes (e.g. quality of life), and increase the risk of performance and detection bias.
Both included studies were conducted in a small number of participants, without calculation of statistical power. Hence, it is possible that these studies were underpowered to detect a statistically significant and clinically relevant difference between the groups; in this case, the lack of a significant difference could represent a false‐negative result due to statistical error type II.
Because of these limitations, we were unable to form any firm conclusions from these two studies.
Quality of the evidence
For outcomes included in the Table 1, we found the certainty of evidence to be of very low certainty. Using GRADE, we assessed the certainty of the evidence as very low for outcomes for which data were available; we downgraded the evidence by two levels since data on seizure freedom were obtained from only one study judged at unclear or high risk of bias (Tennison 1994). Hence, we judged the certainty of the evidence for this outcome as very low (data did not provide a reliable indication of the likely effect; and the true effect is likely to be substantially different from the estimate of effect).
Potential biases in the review process
There were no major potential biases in the review process.
Agreements and disagreements with other studies or reviews
We found no other systematic review evaluating the rate of withdrawal of AEDs (whether rapid or slow tapering) and its effect on recurrence of seizure.
Authors' conclusions
Implications for practice.
The conclusions remain the same as the previous update (Ayuga Loro 2020). In view of methodological deficiencies and small sample size of the two included studies, we are unable to derive any firm conclusions regarding the optimal rate of tapering of antiepileptic drugs (AEDs). Further trials are needed to assess the optimal rate of tapering of AEDs in adults and children whose seizures are well controlled with medication.
Implications for research.
Further randomized controlled trials are needed to determine the optimal rate of tapering of AEDs in people in remission. The following questions need to be investigated.
What is the effect of structural lesions (neuroimaging abnormalities) of the brain on the rate of tapering?
What is the effect of intellectual disability on the rate of tapering?
What is the effect of electroencephalogram abnormalities on the rate of tapering?
Do different AEDs (such as benzodiazepines, barbiturates) need to be tapered at different rates?
What should be the sequence of tapering for people receiving more than one AED?
What is the optimal rate of withdrawal of AEDs in children and adults?
What is the effect of rapid and slow AED withdrawal on time to recurrence of seizures, occurrence of status epilepticus, mortality, morbidity, and quality of life?
What's new
| Date | Event | Description |
|---|---|---|
| 8 November 2021 | New citation required but conclusions have not changed | Conclusions are unchanged. |
| 8 November 2021 | New search has been performed | Searches updated 8 November 2021; no new relevant studies were identified. |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 9 April 2019 | New citation required but conclusions have not changed | Conclusions are unchanged. |
| 9 April 2019 | New search has been performed | Searches updated 9 April 2019; one new study has been included (Serra 2005). |
| 19 January 2013 | New search has been performed | Searches updated December 2012. Two additional references added to Classification pending references, January 2013. |
| 11 August 2009 | Amended | Contact details updated. |
| 3 September 2008 | Amended | Converted to new review format. |
Acknowledgements
We wish to acknowledge the valuable work that went into the original version of the review by Lakshmi Narasimhan Ranganathan and Sridharan Ramaratnam. We are indebted to the following epilepsy experts, who we contacted for information about unpublished or ongoing studies: Edoardo Ferlazzo, Vincenzo Belcastro, Stefano Sartori, Ronit Pressler, and Hans Hartmann.
This review update was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health and Social Care.
Appendices
Appendix 1. CRS Web search strategy
1. MeSH DESCRIPTOR Epilepsy Explode All WITH QUALIFIER DT AND CENTRAL:TARGET
2. MESH DESCRIPTOR Seizures EXPLODE ALL WITH QUALIFIER DT AND CENTRAL:TARGET
3. MeSH DESCRIPTOR Anticonvulsants Explode All AND CENTRAL:TARGET
4. (antiepilep* or anti‐epilep* or anticonvulsant* or anti‐convulsant* or AED or AEDs):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
5. #1 OR #2 OR #3 OR #4 AND CENTRAL:TARGET
6. MeSH DESCRIPTOR Midazolam Explode All AND CENTRAL:TARGET
7. (Dalam OR Dormicum OR Dormire OR Epistatus OR Fulsed OR Garen OR Hypnovel OR Ipnovel OR Midazolam* OR Nocturna OR Setam OR Terap OR Versed):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
8. #6 OR #7 AND CENTRAL:TARGET
9. MeSH DESCRIPTOR Methazolamide Explode All AND CENTRAL:TARGET
10. (Methazolamid* OR Methylacetazolamide OR Neptazane):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
11. #9 OR #10 AND CENTRAL:TARGET
12. MeSH DESCRIPTOR Propofol Explode All AND CENTRAL:TARGET
13. (Anepol OR Diprivan OR Disoprivan OR Disoprofol OR Fresofol OR Hypro OR Lipuro OR Plofed OR Profol OR Propofil OR Propofol* OR Propolipid OR Propovan OR Propoven OR Provive OR Recofol):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
14. #12 OR #13 AND CENTRAL:TARGET
15. MeSH DESCRIPTOR Temazepam Explode All AND CENTRAL:TARGET
16. (Dasuen OR Euhypnos OR Hydroxydiazepam OR Levanxol OR Methyloxazepam OR Nocturne OR Norkotral OR Normison OR Normitab OR Nortem OR Oxydiazepam OR Planum OR Pronervon OR Remestan OR Restoril OR Signopam OR Temaze OR Temazep* OR Temtabs OR Tenox):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
17. #15 OR #16 AND CENTRAL:TARGET
18. MeSH DESCRIPTOR Thiopental Explode All AND CENTRAL:TARGET
19. (Bomathal OR Farmotal OR Nesdonal OR Penthiobarbit* OR Pentothal OR Sodipental OR Thiomebumal OR Thionembutal OR Thiopent* OR Tiobarbital OR Tiopental* OR Trapanal):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
20. #18 OR #19 AND CENTRAL:TARGET
21. #5 OR #8 OR #11 OR #14 OR #17 OR #20 AND CENTRAL:TARGET
22. (Acemit OR Acetamide OR Acetazolamid* OR Avva OR Azm OR Azol OR Diacarb OR Diamox OR Diazomid OR Diluran OR Edemox OR Glaupax):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
23. (Barbexaclon*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
24. (Beclamid* OR Chloracon OR Hibicon OR Posedrine OR Nydrane OR Seclar):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
25. (Brivaracetam*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
26. (Bromide*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
27. (Carbamazepin* OR Carbamazepen* OR Carbamezepin* OR CBZ OR SPD417 OR "Apo‐Carbamazepine" OR Atretol OR Biston OR Calepsin OR Carbagen OR Carbatrol OR Carbazepin* OR Carbelan OR Epitol OR Equetro OR Finlepsin OR Karbamazepin OR Lexin OR Neurotop OR "Novo‐Carbamaz" OR "Nu‐Carbamazepine" OR Sirtal OR Stazepin* OR "Taro‐Carbamazepine" OR Tegretal OR Tegretol OR Telesmin OR Teril OR Timonil):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
28. (Carisbamat* OR Comfyde OR "RWJ‐333369" OR "YKP 509"):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
29. (Cenobamat* OR Xcopri OR YKP3089):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
30. (Chlormethiazol* OR Distraneurin):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
31. (Aedon OR Anxirloc OR Castilium OR Chlorepin OR Clarmyl OR Clobam OR Clobamax OR Clobator OR Clobazam* OR Clofritis OR Clopax OR Clorepin OR Frisium OR Grifoclobam OR Karidium OR Lucium OR Mystan OR Noiafren OR Onfi OR Sederlona OR Sentil OR Urbadan OR Urbanil OR Urbanol OR Urbanyl):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
32. (Antelepsin OR Antilepsin OR Chlonazepam OR Cloazepam OR Clonazepam* OR Clonex OR Clonopin OR Iktorivil OR Klonopin OR Kriadex OR Landsen OR Paxam OR Petril OR Ravotril OR Rivatril OR Rivotril OR “ro 5‐4023” OR “ro 54023”):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
33. (Calner OR Clorazepat* OR Justum OR Mendon OR "Novo‐Clopate" OR Tranxene OR Tranxilium):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
34. (Diapam OR Diastat OR Diazemuls OR Diazepam* OR Nervium OR Relanium OR Valium):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
35. (Dimethadion* OR Dimethyloxazolidinedione):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
36. (Eslicarbazepin* OR Exalief OR Stedesa OR Zebinix):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
37. (Esilgan OR Estazolam* OR Eurodin OR Nuctalon OR Prosom OR Tasedan):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
38. (Ethadion*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
39. (Aethosuximid* OR Emeside OR Ethosucci* OR Ethosuxide OR Ethosuximid* OR Etosuximid* OR Zarontin):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
40. (Ethotoin* OR Peganone):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
41. (Felbamat* OR Felbatol OR Felbamyl OR Taloxa):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
42. (Flunarizin* OR Sibelium):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
43. (Cerebyx OR Fosphenytoin* OR Prodilantin):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
44. (Gabapentin* OR Aclonium OR Fanatrex OR Gabapetin OR Gabarone OR GBP OR Gralise OR Neogab OR Neurontin OR "Novo‐Gabapentin" OR Nupentin):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
45. ("CCD‐1042" OR Ganaxolon*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
46. (Erlosamide OR Harkoseride OR Lacosamid* OR Vimpat):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
47. (Lamotrigin* OR Elmendos OR Epilepax OR "GW 273293" OR Lamictal OR Lamictin OR Lamitor OR Lamitrin OR Lamogine OR Lamotrine OR LTG):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
48. (Levetiracetam* OR Keppra OR LEV OR Levitiracetam):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
49. (Ativan OR Intensl OR Loraz OR Lorazepam* OR Lormetazepam* OR Temesta):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
50. (Losigamon*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
51. ("Magnesium sulfat*" OR "Magnesium sulphat*"):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
52. (Medazepam* OR Nobrium OR Rudotel OR Rusedal):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
53. (Mephenytoin* OR Mesantoin):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
54. (Dapaz OR Equanil OR Meprobamat* OR Meprospan OR Miltown OR Tranmep OR Visano):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
55. (Celontin OR Mesuximid* OR Methsuximide OR Petinutin):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
56. (Mephobarbit* OR Mebaral OR Mephyltaletten OR Methylphenobarbit* OR Metilfenobarbital OR Phemiton OR Prominal):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
57. (Erimin OR Nimetazepam*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
58. (Alodorm OR Arem OR Insoma OR Mogadon OR Nitrados OR Nitrazadon OR Nitrazepam* OR Ormodon OR Paxadorm OR Remnos OR Somnite OR Pacisyn):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
59. (Oxcarbazepin* OR Actinium OR Barzepin OR Carbox OR Deprectal OR "GP 47680" OR Lonazet OR OCBZ OR Oxalepsy OR OXC OR Oxcarbamazepine OR Oxetol OR Oxpin OR Oxrate OR Oxtellar OR Oxypine OR Pharozepine OR Prolepsi OR Timox OR Trexapin OR Trileptal OR Trileptin):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
60. (Paraldehyd*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
61. (Paramethadion*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
62. (E2007 OR Fycompa OR Perampanel*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
63. (Phenacemid*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
64. (Ethylphenacemid* OR Pheneturid*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
65. (Adonal OR Aephenal OR Agrypnal OR Amylofene OR Aphenylbarbit OR Aphenyletten OR Barbenyl OR Barbinal OR Barbiphen* OR Barbipil OR Barbita OR Barbivis OR Barbonal OR Barbophen OR Bardorm OR Bartol OR Bialminal OR "Blu‐Phen" OR Cabronal OR Calmetten OR Calminal OR Cardenal OR Chinoin OR Codibarbita OR Coronaletta OR Cratecil OR Damoral OR Dezibarbitur OR Dormina OR Dormiral OR Dormital OR Doscalun OR Duneryl OR Ensobarb OR Ensodorm OR Epanal OR Epidorm OR Epilol OR Episedal OR Epsylone OR Eskabarb OR Etilfen OR Euneryl OR Fenbital OR Fenemal OR Fenobarbital OR Fenosed OR Fenylettae OR Gardenal OR Gardepanyl OR Glysoletten OR Haplopan OR Haplos OR Helional OR Hennoletten OR Henotal OR Hypnaletten OR Hypnette OR "Hypno‐Tablinetten" OR Hypnogen OR Hypnolone OR Hypnoltol OR Hysteps OR Lefebar OR Leonal OR Lephebar OR Lepinal OR Lepinaletten OR Linasen OR Liquital OR Lixophen OR Lubergal OR Lubrokal OR Lumen OR Lumesettes OR Lumesyn OR Luminal OR Lumofridetten OR Luphenil OR Luramin OR Molinal OR Neurobarb OR Nirvonal OR Noptil OR "Nova‐Pheno" OR Nunol OR Parkotal OR PB OR Pharmetten OR "Phen‐Bar" OR Phenaemal OR Phenemal* OR Phenobal OR Phenobarbit* OR Phenobarbyl OR Phenoluric OR Phenolurio OR Phenomet OR Phenonyl OR Phenoturic OR Phenylethylbarbit* OR Phenylethylmalonylurea OR Phenyletten OR Phenyral OR Phob OR Polcominal OR Prominal OR Promptonal OR "Seda‐Tablinen" OR Sedabar OR Sedicat OR Sedizorin OR Sedlyn OR Sedofen OR Sedonal OR Sedonettes OR Sevenal OR Sinoratox OR Solfoton OR "Solu‐Barb" OR Sombutol OR Somnolens OR Somnoletten OR Somnosan OR Somonal OR Spasepilin OR Starifen OR Starilettae OR Stental OR Talpheno OR Teolaxin OR Teoloxin OR Thenobarbital OR Theoloxin OR Triabarb OR Tridezibarbitur OR Triphenatol OR Versomnal OR Zadoletten OR Zadonal):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
66. (Phensuximid*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
67. (Aleviatin OR Antisacer OR Auranile OR Causoin OR Citrullamon OR Citrulliamon OR Comital OR Comitoina OR Convul OR Danten OR Dantinal OR Dantoin* OR Denyl OR "Di‐Hydan" OR "Di‐Lan" OR "Di‐Phetine" OR Didan OR Difenilhidantoin* OR Difenin OR Difetoin OR Difhydan OR Dihycon OR Dihydantoin OR Dilabid OR Dilantin* OR Dillantin OR Dintoin* OR Diphantoin OR Diphedal OR Diphedan OR Diphenat OR Diphenin* OR Diphentoin OR Diphentyn OR Diphenylan OR Diphenylhydantoin* OR Diphenylhydatanoin OR Ditoinate OR Ekko OR Elepsindon OR Enkelfel OR Epamin OR Epanutin OR Epasmir OR Epdantoin* OR Epelin OR Epifenyl OR Epihydan OR Epilan OR Epilantin OR Epinat OR Epised OR Eptal OR Eptoin OR Fenantoin OR Fenidantoin OR Fenitoin* OR Fentoin OR Fenylepsin OR Fenytoin* OR "Gerot‐epilan‐D" OR Hidan OR Hidant* OR Hindatal OR Hydant* OR Ictalis OR Idantoi* OR Iphenylhydantoin OR Kessodanten OR Labopal OR Lehydan OR Lepitoin OR Lepsin OR Mesantoin OR Minetoin OR "Neos‐Hidantoina" OR Neosidantoina OR Novantoina OR Novophenytoin OR "Om‐hidantoina" OR "Om‐Hydantoine" OR Oxylan OR Phanantin* OR Phenatine OR Phenatoine OR Phenhydan* OR Phenitoin OR Phentoin OR Phentytoin OR Phenytek OR Phenytex OR Phenytoin* OR PHT OR Ritmenal OR Saceril OR Sanepil OR Silantin OR Sinergina OR Sodanthon OR Sodanto* OR Solantin OR Solantoin OR Solantyl OR Sylantoic OR Tacosal OR Thilophenyl OR TOIN OR Zentronal OR Zentropil):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
68. (Lyrica OR Pregabalin*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
69. (Mysoline OR Primidon* OR Sertan):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
70. (Gabrene OR Garene OR Halogabide OR Halogenide OR Progabid*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
71. (Ecovia OR Remacemid*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
72. ("D‐23129" OR "D23129" OR EZG OR Ezogabin* OR Retigabin* OR RTG OR Trobalt OR Potiga):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
73. (Rilutek OR Riluzol* OR Trifluoromethoxybenzothiazol*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
74. (Inovelon OR Rufinamid* OR Xilep):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
75. (Seletracetam*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
76. (Diacomit OR Stiripentol*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
77. (Sulthiam* OR Sultiam* OR Ospolot):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
78. (Talampanel*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
79. (Tiagabin* OR Gabitril):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
80. (Tiletamin*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
81. (Topiramat* OR Qudexy OR Tipiramate OR Topamax OR "Topiramic acid" OR TPM):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
82. (Tridione OR Trimethadion*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
83. (Valnoctamid*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
84. (Avugane OR Baceca OR Convulex OR Delepsine OR Depacon OR Depakene OR Depakine OR Depakote OR Deproic OR Divalprax OR Divalproex* OR DPA OR Encorate OR Epiject OR Epilex OR Epilim OR Episenta OR Epival OR Ergenyl OR Mylproin OR Orfiril OR Orlept OR Selenica OR Stavzor OR Valance OR Valcote OR Valparin OR Valpro* OR VPA OR Zalkote):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
85. (Depamide OR Valpromid*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
86. (GVG OR Sabril OR Vigabatrin*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
87. (Zonisamid* OR Exceglan OR Excegram OR Excegran OR ZNS OR Zonegran):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
88. #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86 OR #87
89. ((duration or month or months or period or periods or rapid or slow) ADJ5 (discontinu* or withdraw* or taper*)):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
90. ((discontinu* or withdraw* or taper*) ADJ5 (duration or month or months or period or periods or rapid or slow)):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
91. #89 OR #90
92. #88 AND #91
93. MESH DESCRIPTOR Epilepsy EXPLODE ALL AND CENTRAL:TARGET
94. MESH DESCRIPTOR Seizures EXPLODE ALL AND CENTRAL:TARGET
95. (epilep* OR seizure* OR convuls*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
96. #93 OR #94 OR #95
97. eclampsia:TI AND CENTRAL:TARGET
98. #96 NOT #97
99. (relaps* or recur* or reoccur* or remission or prognos*):AB,TI AND CENTRAL:TARGET
100. MESH DESCRIPTOR Remission Induction EXPLODE ALL AND CENTRAL:TARGET
101. MESH DESCRIPTOR Recurrence EXPLODE ALL AND CENTRAL:TARGET
102. MESH DESCRIPTOR Prognosis AND CENTRAL:TARGET
103. MESH DESCRIPTOR Secondary Prevention EXPLODE ALL AND CENTRAL:TARGET
104. #99 OR #100 OR #101 OR #102 OR #103
105. #98 AND #104
106. #92 AND #105
107. MESH DESCRIPTOR Diet Therapy EXPLODE ALL AND CENTRAL:TARGET
108. (diet therapy):EH,EMT,MH AND CENTRAL:TARGET
109. ketogenic:TI AND CENTRAL:TARGET
110. #107 OR #108 OR #109
111. #106 NOT #110
Appendix 2. MEDLINE search strategy
This strategy includes a modification of the Cochrane Highly Sensitive Search Strategy for identifying randomized trials published in Lefebvre 2021.
1. exp *Epilepsy/dt [Drug Therapy]
2. exp Seizures/dt [Drug Therapy]
3. exp Anticonvulsants/
4. (antiepilep$ or anti‐epilep$ or anticonvulsant$ or anti‐convulsant$ or AED or AEDs).mp.
5. exp Midazolam/
6. (Dalam or Dormicum or Dormire or Epistatus or Fulsed or Garen or Hypnovel or Ipnovel or Midazolam* or Nocturna or Setam or Terap or Versed).mp.
7. exp Methazolamide/
8. (Methazolamid* or Methylacetazolamide or Neptazane).mp.
9. exp Propofol/
10. (Anepol or Diprivan or Disoprivan or Disoprofol or Fresofol or Hypro or Lipuro or Plofed or Profol or Propofil or Propofol* or Propolipid or Propovan or Propoven or Provive or Recofol).mp.
11. exp Temazepam/
12. (Dasuen or Euhypnos or Hydroxydiazepam or Levanxol or Methyloxazepam or Nocturne or Norkotral or Normison or Normitab or Nortem or Oxydiazepam or Planum or Pronervon or Remestan or Restoril or Signopam or Temaze or Temazep* or Temtabs or Tenox).mp.
13. exp Thiopental/
14. (Bomathal or Farmotal or Nesdonal or Penthiobarbit* or Pentothal or Sodipental or Thiomebumal or Thionembutal or Thiopent* or Tiobarbital or Tiopental* or Trapanal).mp.
15. (Acemit or Acetamide or Acetazolamid* or Avva or Azm or Azol or Diacarb or Diamox or Diazomid or Diluran or Edemox or Glaupax).mp.
16. Barbexaclon*.mp.
17. (Beclamid* or Chloracon or Hibicon or Posedrine or Nydrane or Seclar).mp.
18. Brivaracetam*.mp.
19. Bromide*.mp.
20. (Carbamazepin* or Carbamazepen* or Carbamezepin* or CBZ or SPD417 or "Apo‐Carbamazepine" or Atretol or Biston or Calepsin or Carbagen or Carbatrol or Carbazepin* or Carbelan or Epitol or Equetro or Finlepsin or Karbamazepin or Lexin or Neurotop or "Novo‐Carbamaz" or "Nu‐Carbamazepine" or Sirtal or Stazepin* or "Taro‐Carbamazepine" or Tegretal or Tegretol or Telesmin or Teril or Timonil).mp.
21. (Carisbamat* or Comfyde or "RWJ‐333369" or "YKP 509").mp.
22. (cenobamat* or Xcopri or YKP3089).mp.
23. (Chlormethiazol* or Distraneurin).mp.
24. (Aedon or Anxirloc or Castilium or Chlorepin or Clarmyl or Clobam or Clobamax or Clobator or Clobazam* or Clofritis or Clopax or Clorepin or Frisium or Grifoclobam or Karidium or Lucium or Mystan or Noiafren or Onfi or Sederlona or Sentil or Urbadan or Urbanil or Urbanol or Urbanyl).mp.
25. (Antelepsin or Antilepsin or Chlonazepam or Cloazepam or Clonazepam* or Clonex or Clonopin or Iktorivil or Klonopin or Kriadex or Landsen or Paxam or Petril or Ravotril or Rivatril or Rivotril or "ro 5‐4023" or "ro 54023").mp.
26. (Calner or Clorazepat* or Justum or Mendon or "Novo‐Clopate" or Tranxene or Tranxilium).mp.
27. (Diapam or Diastat or Diazemuls or Diazepam* or Nervium or Relanium or Valium).mp.
28. (Dimethadion* or Dimethyloxazolidinedione).mp.
29. (Eslicarbazepin* or Exalief or Stedesa or Zebinix).mp.
30. (Esilgan or Estazolam* or Eurodin or Nuctalon or Prosom or Tasedan).mp.
31. Ethadion*.mp.
32. (Aethosuximid* or Emeside or Ethosucci* or Ethosuxide or Ethosuximid* or Etosuximid* or Zarontin).mp.
33. (Ethotoin* or Peganone).mp.
34. (Felbamat* or Felbatol or Felbamyl or Taloxa).mp.
35. (Flunarizin* or Sibelium).mp.
36. (Cerebyx or Fosphenytoin* or Prodilantin).mp.
37. (Gabapentin* or Aclonium or Fanatrex or Gabapetin or Gabarone or GBP or Gralise or Neogab or Neurontin or "Novo‐Gabapentin" or Nupentin).mp.
38. ("CCD‐1042" or Ganaxolon*).mp.
39. (Erlosamide or Harkoseride or Lacosamid* or Vimpat).mp.
40. (Lamotrigin* or Elmendos or Epilepax or "GW 273293" or Lamictal or Lamictin or Lamitor or Lamitrin or Lamogine or Lamotrine or LTG).mp.
41. (Levetiracetam* or Keppra or LEV or Levitiracetam).mp.
42. (Ativan or Intensl or Loraz or Lorazepam* or Lormetazepam* or Temesta).mp.
43. Losigamon*.mp.
44. ("Magnesium sulfat*" or "Magnesium sulphat*").mp.
45. (Medazepam* or Nobrium or Rudotel or Rusedal).mp.
46. (Mephenytoin* or Mesantoin).mp.
47. (Dapaz or Equanil or Meprobamat* or Meprospan or Miltown or Tranmep or Visano).mp.
48. (Celontin or Mesuximid* or Methsuximide or Petinutin).mp.
49. (Mephobarbit* or Mebaral or Mephyltaletten or Methylphenobarbit* or Metilfenobarbital or Phemiton or Prominal).mp.
50. (Erimin or Nimetazepam*).mp.
51. (Alodorm or Arem or Insoma or Mogadon or Nitrados or Nitrazadon or Nitrazepam* or Ormodon or Paxadorm or Remnos or Somnite or Pacisyn).mp.
52. (Oxcarbazepin* or Actinium or Barzepin or Carbox or Deprectal or "GP 47680" or Lonazet or OCBZ or Oxalepsy or OXC or Oxcarbamazepine or Oxetol or Oxpin or Oxrate or Oxtellar or Oxypine or Pharozepine or Prolepsi or Timox or Trexapin or Trileptal or Trileptin).mp.
53. Paraldehyd*.mp.
54. Paramethadion*.mp.
55. (E2007 or Fycompa or Perampanel*).mp.
56. Phenacemid*.mp.
57. (Ethylphenacemid* or Pheneturid*).mp.
58. (Adonal or Aephenal or Agrypnal or Amylofene or Aphenylbarbit or Aphenyletten or Barbenyl or Barbinal or Barbiphen* or Barbipil or Barbita or Barbivis or Barbonal or Barbophen or Bardorm or Bartol or Bialminal or "Blu‐Phen" or Cabronal or Calmetten or Calminal or Cardenal or Chinoin or Codibarbita or Coronaletta or Cratecil or Damoral or Dezibarbitur or Dormina or Dormiral or Dormital or Doscalun or Duneryl or Ensobarb or Ensodorm or Epanal or Epidorm or Epilol or Episedal or Epsylone or Eskabarb or Etilfen or Euneryl or Fenbital or Fenemal or Fenobarbital or Fenosed or Fenylettae or Gardenal or Gardepanyl or Glysoletten or Haplopan or Haplos or Helional or Hennoletten or Henotal or Hypnaletten or Hypnette or "Hypno‐Tablinetten" or Hypnogen or Hypnolone or Hypnoltol or Hysteps or Lefebar or Leonal or Lephebar or Lepinal or Lepinaletten or Linasen or Liquital or Lixophen or Lubergal or Lubrokal or Lumen or Lumesettes or Lumesyn or Luminal or Lumofridetten or Luphenil or Luramin or Molinal or Neurobarb or Nirvonal or Noptil or "Nova‐Pheno" or Nunol or Parkotal or PB or Pharmetten or "Phen‐Bar" or Phenaemal or Phenemal* or Phenobal or Phenobarbit* or Phenobarbyl or Phenoluric or Phenolurio or Phenomet or Phenonyl or Phenoturic or Phenylethylbarbit* or Phenylethylmalonylurea or Phenyletten or Phenyral or Phob or Polcominal or Prominal or Promptonal or "Seda‐Tablinen" or Sedabar or Sedicat or Sedizorin or Sedlyn or Sedofen or Sedonal or Sedonettes or Sevenal or Sinoratox or Solfoton or "Solu‐Barb" or Sombutol or Somnolens or Somnoletten or Somnosan or Somonal or Spasepilin or Starifen or Starilettae or Stental or Talpheno or Teolaxin or Teoloxin or Thenobarbital or Theoloxin or Triabarb or Tridezibarbitur or Triphenatol or Versomnal or Zadoletten or Zadonal).mp.
59. Phensuximid*.mp.
60. (Aleviatin or Antisacer or Auranile or Causoin or Citrullamon or Citrulliamon or Comital or Comitoina or Convul or Danten or Dantinal or Dantoin* or Denyl or "Di‐Hydan" or "Di‐Lan" or "Di‐Phetine" or Didan or Difenilhidantoin* or Difenin or Difetoin or Difhydan or Dihycon or Dihydantoin or Dilabid or Dilantin* or Dillantin or Dintoin* or Diphantoin or Diphedal or Diphedan or Diphenat or Diphenin* or Diphentoin or Diphentyn or Diphenylan or Diphenylhydantoin* or Diphenylhydatanoin or Ditoinate or Ekko or Elepsindon or Enkelfel or Epamin or Epanutin or Epasmir or Epdantoin* or Epelin or Epifenyl or Epihydan or Epilan or Epilantin or Epinat or Epised or Eptal or Eptoin or Fenantoin or Fenidantoin or Fenitoin* or Fentoin or Fenylepsin or Fenytoin* or "Gerot‐epilan‐D" or Hidan or Hidant* or Hindatal or Hydant* or Ictalis or Idantoi* or Iphenylhydantoin or Kessodanten or Labopal or Lehydan or Lepitoin or Lepsin or Mesantoin or Minetoin or "Neos‐Hidantoina" or Neosidantoina or Novantoina or Novophenytoin or "Om‐hidantoina" or "Om‐Hydantoine" or Oxylan or Phanantin* or Phenatine or Phenatoine or Phenhydan* or Phenitoin or Phentoin or Phentytoin or Phenytek or Phenytex or Phenytoin* or PHT or Ritmenal or Saceril or Sanepil or Silantin or Sinergina or Sodanthon or Sodanto* or Solantin or Solantoin or Solantyl or Sylantoic or Tacosal or Thilophenyl or TOIN or Zentronal or Zentropil).mp.
61. (Lyrica or Pregabalin*).mp.
62. (Mysoline or Primidon* or Sertan).mp.
63. (Gabrene or Garene or Halogabide or Halogenide or Progabid*).mp.
64. (Ecovia or Remacemid*).mp.
65. ("D‐23129" or "D23129" or EZG or Ezogabin* or Retigabin* or RTG or Trobalt or Potiga).mp.
66. (Rilutek or Riluzol* or Trifluoromethoxybenzothiazol*).mp.
67. (Inovelon or Rufinamid* or Xilep).mp.
68. Seletracetam*.mp.
69. (Diacomit or Stiripentol*).mp.
70. (Sulthiam* or Sultiam* or Ospolot).mp.
71. Talampanel*.mp.
72. (Tiagabin* or Gabitril).mp.
73. Tiletamin*.mp.
74. (Topiramat* or Qudexy or Tipiramate or Topamax or "Topiramic acid" or TPM).mp.
75. (Tridione or Trimethadion*).mp.
76. Valnoctamid*.mp.
77. (Avugane or Baceca or Convulex or Delepsine or Depacon or Depakene or Depakine or Depakote or Deproic or Divalprax or Divalproex$ or DPA or Encorate or Epiject or Epilex or Epilim or Episenta or Epival or Ergenyl or Mylproin or Orfiril or Orlept or Selenica or Stavzor or Valance or Valcote or Valparin or Valpro$ or VPA or Zalkote).mp.
78. (Depamide or Valpromid*).mp.
79. (GVG or Sabril or Vigabatrin*).mp.
80. (Zonisamid* or Exceglan or Excegram or Excegran or ZNS or Zonegran).mp.
81. or/1‐80
82. ((duration or month? or period? or rapid or slow) adj5 (discontinu$ or withdraw$ or taper$)).mp.
83. 81 and 82
84. exp Epilepsy/
85. exp Seizures/
86. (epilep$ or seizure$ or convuls$).mp.
87. 84 or 85 or 86
88. exp *Pre‐Eclampsia/ or exp *Eclampsia/
89. 87 not 88
90. (relaps$ or recur$ or reoccur$ or remission or prognos$).ti,ab.
91. exp REMISSION INDUCTION/ or exp RECURRENCE/ or PROGNOSIS/ or exp Secondary Prevention/ [PROGNOSIS deliberately not expanded]
92. 90 or 91
93. 89 and 92
94. exp controlled clinical trial/ or (randomi?ed or placebo or randomly).ab.
95. clinical trials as topic.sh.
96. trial.ti.
97. 94 or 95 or 96
98. exp animals/ not humans.sh.
99. 97 not 98
100. 83 and 93 and 99
101. exp *Diet Therapy/ or diet therapy.fs. or ketogenic.ti.
102. 100 not 101
103. remove duplicates from 102
Appendix 3. SCOPUS search strategy
Searched for randomized controlled trials that cited Tennison M, Greenwood R, Lewis D, Thorn M. Discontinuing antiepileptic drugs in children with epilepsy. A comparison of a six‐week and a nine‐month taper period. New England Journal of Medicine 1994;330(20):1407‐10.
List of citations refined to: ((TITLE‐ABS‐KEY((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") W/4 (analy* OR design OR evaluat* OR investigat* OR method OR procedure OR study OR studies OR trial))))
Data and analyses
Comparison 1. Rapid versus slow antiepileptic drug (AED) withdrawal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Seizure freedom after 1 year of AED withdrawal | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.58, 1.01] |
| 1.2 Seizure freedom after 2 years of AED withdrawal | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.58, 1.29] |
| 1.3 Seizure freedom after 5 years of AED withdrawal | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.54, 3.65] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Serra 2005.
| Study characteristics | ||
| Methods | Randomized, unblinded, prospective study Inclusion criteria: diagnosis of epilepsy and seizure control by an AED for ≥ 2 years 2 study arms: 1‐month AED withdrawal group (rapid tapering) or 6‐month AED withdrawal group (slow tapering) Type of randomization not specified Mean duration of follow‐up: 54 months (range: 6 months to 4 years and 3 months) |
|
| Participants | Number of participants: 57 33 boys and 24 girls Rapid‐tapering group: 30 participants (17 boys and 13 girls) Slow‐tapering group: 27 participants (16 boys and 11 girls) EEG was normal in 41 participants and abnormal in 15 participants. In 1 participant, EEG information was not available. All participants received 1 AED: 35 (61.4%) received carbamazepine, 12 (21%) phenytoin, 6 (10.5%) valproic acid, and 4 (7%) phenobarbital No difference between the 2 groups according to age, gender, family history of epilepsy, type of epileptic syndrome, EEG abnormality, and AED (P > 0.05). |
|
| Interventions | Drug tapering: rapid vs slow Rapid tapering in 1 month (30 participants); dosage reduced by 25% every 10 days Slow tapering in 6 months (27 participants); dosage reduced by 25% every 2 months EEG was routinely performed prior to randomization. |
|
| Outcomes | Proportion of participants remaining seizure free after AED withdrawal Seizure recurrence Time for seizure recurrence |
|
| Notes | Total enrolled: not specified Loss to follow‐up: not specified No flowchart Clinical characteristics of participants were similar across study groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Funding source | Low risk | Reported (sponsored by a public Foundation). |
| Conflicts of interest | Unclear risk | Not specified. |
Tennison 1994.
| Study characteristics | ||
| Methods | Randomized, unblinded, prospective study. 2 study arms: 6‐week AED withdrawal group (rapid tapering) or 9‐month AED withdrawal group (slow‐tapering group). Randomization by 2 coin tosses. The 1st toss for rate of tapering. The 2nd toss for deciding when the withdrawal of AEDs would begin, after 2 or 4 years. Mean duration of follow‐up: 39 months (range: 11–105 months). |
|
| Participants | Total number enrolled: 149 Mean age: 11 years Loss to follow‐up: 16 Number actually participated: 133; 79 boys and 54 girls Rapid‐tapering group: 70 participants (41 boys and 29 girls) Slow‐tapering group: 63 participants (38 boys and 25 girls) Total number of seizures > 10 events in 51%. 54% were free of seizure for 2 years and 46% for 4 years 63% had focal seizures Mean age of onset of seizure: 4 years in rapid‐tapering group, 5 years in slow‐tapering group Approximately 40% had normal EEG at the start of tapering period 24 participants in rapid‐tapering group and 25 participants in slow‐tapering group received ≥ 2 AEDs |
|
| Interventions | Drug tapering: rapid vs slow Rapid tapering in 6 weeks (70 participants); tapering divided into 3 equal periods of 2 weeks each; dose reduction 25% during each period Slow tapering in 9 months (63 participants); tapering divided into 3 equal periods of 3 months each; dose reduction 25% during each period When participant had > 1 AED, each drug was tapered sequentially, barbiturates were the last to be tapered. EEG was performed in all participants before tapering began. |
|
| Outcomes | Proportion of participants remaining seizure free after AED withdrawal Seizure recurrence |
|
| Notes | 16 participants lost to follow‐up; 11 in rapid‐tapering group, 5 in slow‐tapering group 63/81 in rapid‐tapering group, 48/68 in slow‐tapering group completed the protocol correctly Clinical characteristics of participants were similar across study groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate. Quote: "The eligible children were randomly assigned by two coin tosses." |
| Allocation concealment (selection bias) | Low risk | Investigators and participants were unable to predict to which group each of the participants was allocated. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Funding source | Unclear risk | Funding not reported. |
| Conflicts of interest | Unclear risk | Not specified. |
AED: antiepileptic drug; EEG: electroencephalography.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aidaros 2010 | Compared rapid vs slow withdrawal of AEDs but was a non‐randomized trial. Included 106 children with epilepsy in remission: 61 children underwent AED withdrawal over 6 months and 45 children underwent AED withdrawal over 3 months. |
| Braathen 1996 | RCT in children, in whom AEDs were withdrawn after 1–3 years of seizure freedom. The drugs were tapered over 3 months. Study did not compare rapid vs slow withdrawal. |
| Gebremariam 1999 | RCT on the withdrawal of AEDs in children after 18 or 24 months of seizure freedom. AEDs tapered sequentially if > 1 AED. Each drug tapered by 1/4 dose every 2 weeks until completely stopped. Hence, taper period was variable for each child. Study did not compare rapid vs slow withdrawal. |
| Gherpelli 1992 | Cohort study not specifically assessing drug titration. Predictive model related to other variables. |
| He 2016 | Cohort study addressing rapid vs slow withdrawal of AEDs. |
| MRC 1991 | Large RCT investigating the effects of gradual AED withdrawal compared to continuation of AED in people who had been seizure free for 2 years. Study did not compare rapid vs slow withdrawal. |
| Peters 1998 | RCT investigated withdrawal of AEDs in children who were seizure free for 6 or 12 months. Withdrawal was over 4 weeks. Study did not address the rapid vs slow withdrawal. |
| Todt 1984 | RCT of AED withdrawal after 1, 2, 3, and 4 years. Study did not compare rapid vs slow withdrawal. |
| Verrotti 2000 | RCT of AED withdrawal in children after 1 or 2 years of seizure freedom. Study did not compare rapid vs slow withdrawal. |
AED: antiepileptic drug; RCT: randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Gasparini 2016.
| Study name | Rapid versus slow withdrawal of antiepileptic monotherapy in 2‐year seizure‐free adult patients with epilepsy (RASLOW) study |
| Methods | Multicentre, prospective, randomized controlled study |
| Participants | Adults with focal or generalized epilepsy, who are seizure free on monotherapy for ≥ 2 years |
| Interventions | Slow (160 days) vs rapid (60 days) withdrawal schedule |
| Outcomes | Primary outcome: time to seizure relapse Secondary outcomes: compliance to the assigned schedule; and occurrence of status epilepticus, seizure‐related injuries, and mortality |
| Starting date | Not reported |
| Contact information | Professor Umberto Aguglia, u.aguglia@tin.it |
| Notes |
Differences between protocol and review
For the 2020 update, we distinguished between primary and secondary outcomes. Among primary outcomes, we added 'Seizure freedom after AED withdrawal assessed at one, two, and five years'. We performed a more comprehensive assessment of bias, focusing on the following methodological issues and risk of bias: random sequence generation (selection bias); allocation concealment (selection bias); blinding (performance bias and detection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); and selective reporting (reporting bias). We edited the review according to Cochrane requirements (Higgins 2011).
Contributions of authors
All review authors participated in study selection, data extraction, and analysis.
All review authors contributed to the overall writing up of the review.
Sources of support
Internal sources
No sources of support provided
External sources
National Institute for Health Research (NIHR), UK
Declarations of interest
FAL: none known. EGT: none known. FB: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Serra 2005 {published data only}
- Serra JG, Montenegro MA, Guerreiro MM. Antiepileptic drug withdrawal in childhood: does the duration of tapering off matter for seizure recurrence? Journal of Child Neurology 2005;20(7):624-6. [DOI: 10.1177/08830738050200071901] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tennison 1994 {published data only}
- Tennison M, Greenwood R, Lewis D, Thorn M. Discontinuing antiepileptic drugs in children with epilepsy: a comparison of a six-week and a nine-month taper period. New England Journal of Medicine 1994;330(20):1407-10. [DOI: 10.1056/NEJM199405193302002] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aidaros 2010 {published data only}
- Aidaros MA, Siam AG. Effect of the duration of withdrawal of antiepileptic drugs on the risk of seizure recurrence in childhood epilepsy. Egyptian Journal of Neurology, Psychiatry and Neurosurgery 2010;47(4):593-8. [Google Scholar]
Braathen 1996 {published data only}
- Braathen G, Andersson T, Gylje H, Melander H, Naglo AS, Noren L, et al. Comparison between one and three years of treatment in uncomplicated childhood epilepsy: a prospective study. I. Outcome in different seizure types. Epilepsia 1996;37(9):822-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gebremariam 1999 {published data only}
- Gebremariam A, Mengesha W, Enqusilassie F. Discontinuing anti-epileptic medication(s) in epileptic children: 18 versus 24 months. Annals of Tropical Paediatrics 1999;19(1):93-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gherpelli 1992 {published data only}
- Gherpelli JL, Kok F, dal Forno S, Elkis LC, Lefevre BH, Diament AJ. Discontinuing medication in epileptic children: a study of risk factors related to recurrence. Epilepsia 1992;33(4):681-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
He 2016 {published data only}
- He RQ, Zeng QY, Zhu P, Bao YX, Zheng RY, Xu HQ. Risk of seizure relapse after antiepileptic drug withdrawal in adult patients with focal epilepsy. Epilepsy & Behavior 2016;64(Pt A):233-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
MRC 1991 {published data only}
- Medical Research Council Antiepileptic Drug Withdrawal Study Group. Randomised study of antiepileptic drug withdrawal in patients in remission. Lancet 1991;337(8751):1175-80. [PMID: ] [PubMed] [Google Scholar]
Peters 1998 {published data only}
- Peters AC, Brouwer OF, Geerts AT, Arts WF, Stroink H, Donselaar CA. Randomized prospective study of early discontinuation of antiepileptic drugs in children with epilepsy. Neurology 1998;50(3):724-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Todt 1984 {published data only}
- Todt H. The late prognosis of epilepsy in childhood: results of a prospective follow-up study. Epilepsia 1984;25(2):137-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Verrotti 2000 {published data only}
- Verrotti A, Morresi S, Basciani F, Cutarella R, Morgese G, Chiarelli F. Discontinuation of anticonvulsant therapy in children with partial epilepsy. Neurology 2000;55(9):1393-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Gasparini 2016 {published data only}
- Gasparini S, Ferlazzo E, Giussani G, Italiano D, Cianci V, Sueri C, et al. Rapid versus slow withdrawal of antiepileptic monotherapy in 2-year seizure-free adult patients with epilepsy (RASLOW) study: a pragmatic multicentre, prospective, randomized, controlled study. Neurological Sciences 2016;37(4):579-83. [DOI: 10.1007/s10072-016-2483-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Additional references
Arts 1988
- Arts WF, Visser LH, Loonen MC, Tjiam AT, Stroink H, Stuurman PM, et al. Follow-up of 146 children with epilepsy after withdrawal of antiepileptic therapy. Epilepsia 1988;29(3):244-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Beghi 2013
- Beghi E, Giussani G, Grosso S, Iudice A, La Neve A, Pisani F, Specchio LM, Verrotti A, Capovilla G, Michelucci R, Zaccara G. Withdrawal of antiepileptic drugs: guidelines of the Italian League Against Epilepsy. Epilepsia 2013;Suppl 7:2-12. [DOI] [PubMed] [Google Scholar]
Berg 1994
- Berg AT, Shinnar S. Relapse following discontinuation of antiepileptic drugs: a meta-analysis. Neurology 1994;44(4):601-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bouma 1987
- Bouma PA, Peters AC, Arts RJ, Stijnen T, Rossum J. Discontinuation of antiepileptic therapy: a prospective study in children. Journal of Neurology, Neurosurgery, and Psychiatry 1987;50(12):1579-83. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brodie 2012
- Brodie MJ, Barry SJ, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology 2012;78(20):1548-54. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Callaghan 1988
- Callaghan N, Garrett A, Goggin T. Withdrawal of anticonvulsant drugs in patients free of seizures for two years: a prospective study. New England Journal of Medicine 1988;318(15):942-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Duncan 1987
- Duncan JS, Shorvon SD. Rates of antiepileptic drug reduction in active epilepsy – current practice. Epilepsy Research 1987;1(6):357-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Emerson 1981
- Emerson R, D'Souza BJ, Vining EP, Holden KR, Mellits ED, Freeman JM. Stopping medication in children with epilepsy: predictors of outcome. New England Journal of Medicine 1981;304(19):1125-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fisher 2014
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55(4):475-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 16 July 2019. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI: 10.1136/bmj.39489.470347.AD] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Juul‐Jensen 1964
- Juul-Jensen P. Frequency of recurrence after discontinuance of anti-convulsant therapy in patients with epileptic seizures. Epilepsia 1964;5:352-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical supplement to chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al. editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
MRC 1993
- Medical Research Council Antiepileptic Drug Withdrawal Study Group. Prognostic index for recurrence of seizures after remission of epilepsy. BMJ 1993;306(6889):1374-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2012
- NICE, The National Institute for Health and Care Excellence. NICE Clinical Guidelines, No. 137. London: National Institute for Health and Care Excellence (NICE), 2021. [Google Scholar]
Oller‐Daurella 1975
- Oller-Daurella L, Pamies R, Oller LF. Reduction or discontinuance of antiepileptic drugs in patients seizure-free for more than 5 years. In: Janz D, editors(s). Epileptology: Proceedings of the Seventh International Symposium on Epilepsy. Berlin: Georg Thieme Verlag, 1975:218-27. [Google Scholar]
Overweg 1981
- Overweg J, Rowan AJ, Binnie CD, Oosting J, Nagelkerke NJ. Prediction of seizure recurrence after withdrawal of antiepileptic drugs. In: Dam M, Gram L, Penry JK, editors(s). Advances in Epileptology: the XIIth Epilepsy International Symposium. New York: Raven Press, 1981:503-8. [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shih 2009
- Shih JJ, Ochoa JG. A Systematic Review of Antiepileptic Drug Initiation and Withdrawal. Neurologist 2009;15(3):122-131. [DOI] [PubMed] [Google Scholar]
Shinnar 1994
- Shinnar S, Berg AT, Moshe SL, Kang H, O'Dell C, Alemany M, et al. Discontinuing antiepileptic drugs in children with epilepsy: a prospective study. Annals of Neurology 1994;35(5):534-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Strozzi 2015
- Strozzi I, Nolan SJ, Sperling MR, Wingerchuk DM, Sirven J. Early versus late antiepileptic drug withdrawal for people with epilepsy in remission. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD001902. [DOI: 10.1002/14651858.CD001902.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ayuga Loro 2020
- Ayuga Loro F, Gisbert Tijeras E, Brigo F. Rapid versus slow withdrawal of antiepileptic drugs. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD005003. [DOI: 10.1002/14651858.CD005003.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranganathan 2004
- Ranganathan LN, Ramaratnam S. Rapid versus slow withdrawal of antiepileptic drugs. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No: CD005003. [DOI: 10.1002/14651858.CD005003] [DOI] [PubMed] [Google Scholar]
Ranganathan 2006
- Ranganathan LN, Ramaratnam S. Rapid versus slow withdrawal of antiepileptic drugs. Cochrane Database of Systematic Reviews 2006, Issue 2. Art. No: CD005003. [DOI: 10.1002/14651858.CD005003.pub2] [DOI] [PubMed] [Google Scholar]


