Abstract
As a common endocrinopathy of reproductive-aged women, polycystic ovary syndrome (PCOS) is characterized by hyperandrogenism, oligo-anovulation and polycystic ovarian morphology. It is linked with insulin resistance through preferential abdominal fat accumulation that is worsened by obesity. Over the past two millennia, menstrual irregularity, male-type habitus and sub-infertility have been described in women and confirm that these clinical features of PCOS were common in antiquity. Recent findings in normal-weight hyperandrogenic PCOS women show that exaggerated lipid accumulation by subcutaneous (SC) abdominal stem cells during development to adipocytes in vitro occurs in combination with reduced insulin sensitivity and preferential accumulation of highly-lipolytic intra-abdominal fat in vivo. This PCOS phenotype may be an evolutionary metabolic adaptation to balance energy storage with glucose availability and fatty acid oxidation for optimal energy use during reproduction. This review integrates fundamental endocrine-metabolic changes in healthy, normal-weight PCOS women with similar PCOS-like traits present in animal models in which tissue differentiation is completed during fetal life as in humans to support the evolutionary concept that PCOS has common ancestral and developmental origins.
Keywords: Polycystic ovary syndrome, Hyperandrogenism, Insulin resistance, Adipocyte, Adipose stem cells, Developmental programming, Nonhuman primates, Sheep, Body fat distribution, Metabolic adaptation
Background
Polycystic ovary syndrome (PCOS) is characterized by ovarian hyperandrogenism from altered hypothalamic-pituitary-ovarian function in combination with hyperinsulinemia from insulin resistance. With a prevalence of 6–20% in the general population, depending upon the definition of PCOS [1], its clinical manifestations of hirsutism, oligo-anovulation and polycystic ovarian morphology (PCOM) accompany glucose intolerance, dyslipidemia and preferential abdominal fat accumulation worsened by obesity [2]. These clinical manifestations of PCOS determine risks of subfertility, diabetes, metabolic syndrome and/or cardiovascular disease. Almost one-half of women with PCOS in the United States have metabolic syndrome, which is higher in prevalence than that of age-matched normal women in this country [1, 3] and of women with PCOS in other countries where obesity is less prevalent [4].
As a heritable syndrome with a polygenic origin, large genome-wide association studies (GWAS) have identified several PCOS susceptible loci in candidate genes involving insulin action, androgen biosynthesis and gonadal function [1]. These PCOS susceptible loci alone, however, have yet to explain the majority of PCOS phenotypic expression [5]. Rather, heritability of PCOS may involve one or more PCOS candidate genes interacting with environmental factors to modify target tissue phenotype through epigenetic events [6, 7], beginning before birth when an altered maternal endocrine-metabolic environment modifies fetal genetic susceptibility to PCOS, and continuing after birth into adulthood [4].
This review examines how the endocrine-metabolic characteristics of women with PCOS originally favored survival of humans in ancient times of food deprivation, but now predispose to endocrine-reproductive dysfunction in today’s obesogenic environment. It integrates clinical characteristics of PCOS women with similar PCOS-like traits present in prenatally testosterone-treated monkeys and sheep to provide evidence of developmental programming in PCOS, given that tissue differentiation in these species, as in humans, occurs during fetal life.
Clinical variables
Several variables affect the endocrine-metabolic characteristics of women with PCOS. As one such variable, different PCOS phenotypes by Rotterdam criteria vary in their degree of reproductive and metabolic dysfunction [8]. Women with National Institutes of Health (NIH)-defined PCOS (i.e., hyperandrogenism with oligo-anovulation with or without PCOM) are at greatest risk of developing menstrual irregularity, anovulatory infertility, type 2 diabetes mellitus and metabolic syndrome, as defined by increased abdominal (android) obesity, hyperglycemia, dyslipidemia and hypertension. Ovulatory women with PCOS (i.e., hyperandrogenism and PCOM) have a lower body mass index (BMI) and milder hyperinsulinemia and hyperandrogenism, which lower the risks of developing reproductive and metabolic abnormalities, while women with non-androgenic PCOS (oligo-anovulation and PCOM) have the least metabolic risk [1].
In addition, obesity coexists with abnormal insulin action in most women with PCOS [9–11]. Although not an intrinsic defect of PCOS, obesity can interact with hyperandrogenism to worsen PCOS phenotypes [1–3, 12–14] and impair insulin sensitivity [2, 15, 16]. In this regard, women with PCOS within a referral population have a more severe phenotype, including greater hyperandrogenism, higher BMI and increased risk for metabolic dysfunction, than PCOS women within an unselected background population [17, 18]. Age is another positive predictor of insulin resistance in adipose tissue [19]. These variables that adversely affect endocrine-metabolic function in PCOS were avoided in our studies by investigating healthy, normal-weight PCOS women by NIH criteria who were recruited from the general population to study a mild PCOS phenotype [17, 20] and who also were age- and BMI-balanced to controls to eliminate the effects of age and obesity on metabolic outcomes, including insulin sensitivity [15, 16, 19, 21].
In the context of metabolic function, women with NIH-defined PCOS have two distinct PCOS subtypes with different genetic heterogeneity. A reproductive endocrine subtype (23% of cases) is characterized by higher luteinizing hormone (LH) and sex hormone binding globulin (SHBG) levels with relatively low BMI and insulin levels, while a metabolic subtype (37% of cases) is characterized by higher BMI, glucose and insulin levels, with lower SHBG and LH levels. These PCOS subtypes may differ in their developmental origins [22], with their heritability variably interacting with risk-increasing environmental factors, including maternal obesity and gestational diabetes, to fully explain its prevalence. Such genetic-environmental interactions likely begin before birth, when an altered maternal-placental-fetal environment generates epigenetic modifications in fetal genetic susceptibility to PCOS that continue after birth into adulthood, with metabolic adaptations that enhance fat storage but predispose to lipotoxicity [23].
Insulin resistance
Most women with PCOS have some degree of insulin resistance due to perturbed insulin receptor/post receptor signaling, altered adipokine secretion and abnormal steroid metabolism [2] in combination with increased abdominal fat over a wide BMI range [1, 24, 25]. Clinically, insulin sensitivity (Si) and insulin resistance can be quantified by frequently sampled intravenous glucose tolerance testing (FSIVGTT) and/or Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), respectively. Healthy, normal-weight PCOS women by NIH criteria have Si and HOMA-IR values within low-normal and high-normal and ranges, respectively [26]. By total body dual-energy x-ray absorptiometry, they also exhibit preferential accumulation of abdominal fat, called android fat, which positively correlates with circulating androgen and fasting insulin levels, and remains related to serum androgen levels adjusting for serum insulin levels [24].
Intra-abdominal adipose
In women with NIH-defined PCOS, preferential abdominal fat accumulation [2, 27] promotes insulin resistance through increased intra-abdominal (visceral) fat mass, and worsens with weight gain as a risk factor for metabolic disease [28–30]. Such preferential abdominal fat accumulation in hyperandrogenic PCOS women underlies insulin resistance over a wide BMI range [25], and also occurs in healthy normal-weight PCOS women by NIH criteria in combination with adipose insulin resistance (adipose-IR), defined by the product of fasting circulating free fatty acid (FFA) and insulin levels [19, 24, 26]. In these normal-weight PCOS women, moreover, an increase in intra-abdominal fat positively correlates with serum androgen concentrations and fasting circulating levels of insulin, triglyceride (TG), as well as non-high-density lipoprotein (non-HDL) cholesterol [24].
Intra-abdominal adipose in humans is highly lipolytic and resists androgen inhibition of catecholamine-induced lipolysis (lipid breakdown) despite expressing androgen receptors [31]. Instead, intra-abdominal adipose of nonobese PCOS women shows exaggerated catecholamine-induced lipolysis despite normal insulin suppression of lipolysis [32, 33]. Consequently, an increase in intra-abdominal fat in normal-weight PCOS women likely enhances FFA delivery to the liver and muscle for energy storage, but worsens insulin resistance if increased FFA availability exceeds the capacity of these tissues to oxidize fat or convert diacylglycerols to triacylglycerols [32–34].
Subcutaneous abdominal adipose
Subcutaneous (SC) abdominal adipose normally protects against insulin resistance through a balance between lipogenesis (lipid formation) and lipolysis (lipid breakdown) in mature adipocytes combined with new adipocyte formation (i.e., adipogenesis), whereby adipose stem cells (ASCs) initially undergo commitment to preadipocytes and then differentiate into newly-formed adipocytes [35–37]. Subcutaneous adipose can thus increase its fat storage capacity through both enlargement of mature adipocytes (i.e., hypertrophy) and development of new adipocytes (i.e., hyperplasia) to buffer fatty acid influx when energy intake exceeds energy expenditure [38, 39].
Within SC adipose, androgen normally inhibits early-stage adipogenesis, diminishes insulin-stimulated glucose uptake and impairs catecholamine-stimulated lipolysis through reduced β2-adrenergic receptor and hormone-sensitive lipase (HSL) protein expression [31, 32, 35, 40]. As a result, SC abdominal adipose of PCOS women shows diminished insulin-mediated glucose uptake, reduced glucose transporter type 4 (GLUT-4) expression [41] and catecholamine lipolytic resistance from diminished protein levels of β2-adrenergic receptor, HSL and protein kinase A regulatory-IIβ component (PKA-RegIIβ) [42, 43]. As a biomarker of lipolysis, serum glycerol levels are decreased in normal-weight PCOS women with normal insulin sensitivity [42], yet increased in overweight PCOS women [44], likely because androgen-induced catecholamine lipolytic resistance in normal-weight PCOS women [43] is antagonized by impaired insulin suppression of lipolysis in overweight PCOS women [44].
Consequently, adipose-IR is often increased in healthy normal-weight women with NIH-defined PCOS compared to age- and BMI-matched normal women [26]. Moreover, adipose-IR in these women positively correlates with serum androgen and fasting TG levels, and negatively correlates with Si values [26]. These endocrine-metabolic relationships are further modified by local intracellular aldo-ketoreductase type 1C3 (AKR1C3) activity, an aldo-ketoreductase enzyme that generates testosterone (T) from androstenedione (A4) and exists predominantly in SC rather than intra-abdominal adipose [45, 46]. In overweight/obese PCOS women, increased AKR1C3-mediated T generation from A4 in SC abdominal adipose enhances lipid storage through increased lipogenesis and decreased lipolysis [47]. Similarly, in normal-weight women with NIH-defined PCOS, an increased serum total T/A4 ratio, as a marker of enhanced SC adipose AKR1C3 activity, negatively correlates with fasting serum TG levels, adjusting statistically for serum free T as a possible confounding variable [48]. This inverse relationship of serum total T/A4 ratio with fasting serum TG level in normal-weight, NIH-defined PCOS women may reduce TG turnover to counter-balance androgen inhibition of insulin-stimulated glucose uptake in SC abdominal adipocytes [40].
SC abdominal stem cells
The dynamic process of adipocyte development begins in early life [49]. Subcutaneous abdominal ASCs from normal-weight PCOS by NIH criteria compared to age−/BMI matched normal healthy women exhibit inherently altered gene expression of adipogenic/angiogenic functions involving androgen-insulin interactions through transforming growth factor (TGF)-β1 signaling [26]. When SC abdominal ASCs from normal-weight PCOS women are cultured in vitro (without exogenous androgen), exaggerated commitment of ASC to preadipocytes via zinc-finger protein 423 (ZFP423) expression negatively correlates with fasting circulating glucose levels [50]. Subsequently, accelerated lipid accumulation in newly-formed PCOS adipocytes during adipocyte maturation in vitro positively correlates with hyperandrogenemia and predicts both reduced serum FFA levels and improved systemic insulin sensitivity in vivo [50, 51]. In some SC abdominal ASCs from normal-weight PCOS, overexpression of peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT enhancer binding protein a (CEBPa) during adipocyte maturation in vitro accompanies altered dynamic chromatin remodeling, with enrichment of binding motifs for transcription factors of the activator protein-1 (AP-1) subfamily that govern adipocyte differentiation [23]. These findings suggest that triacylglycerol synthesis, lipid oxidation, free fatty acid beta-oxidation and oxidative phosphorylation may be reprogrammed in these cells to promote greater fat storage [50]. Similar studies of SC abdominal ASC gene expression and function in overweight/obese PCOS by NIH criteria have not yet been performed.
Linked with this exaggerated ZFP423-induced ASC commitment to preadipocytes in vitro is a greater formation of small SC abdominal adipocytes in normal-weight PCOS women [24, 26, 50]. A similar population of small SC abdominal adipocytes in other individuals [49, 52, 53] protects against insulin resistance through enhanced ASC commitment to preadipocyte differentiation and ZFP423 upregulation due to epigenetic changes in its promoter region [54]. An increased proportion of small SC abdominal adipocytes occurs in PCOS-like prenatally-T treated adult rhesus monkeys with increased visceral adiposity and insulin resistance [5, 55]. It also occurs in prenatally-T treated sheep with insulin resistance [56], perhaps as a compensatory adaptation to an altered intrauterine metabolic environment apart from extant androgen since enhancement of the small adipocyte population is not reversed with flutamide cotreatment [57, 58].
Finally, overexpression of PPARγ and CEBPa in some PCOS SC abdominal stem cells accompanies upregulation of AKR1C3 during adipocyte maturation in vitro. These findings correspond with an inverse relationship of serum total T/A4 ratio with serum TG level in normal-weight PCOS women [48], and suggest that reduced TG turnover in SC adipose of these individuals favors insulin sensitivity [59, 60]. AKR1C3 gene expression and activity are normally greater in preadipocytes and adipose of gluteal compared to omental fat, with gluteal fat favoring androgen activation (i.e., AKR1C3), and omental cells favoring androgen inactivation (i.e., aldo-ketoreductase type 1C2 [AKR1C2]) [46]. These differential actions of AKR1C3-mediated androgen activation by fat depot, combined with hyperandrogenemia and preferential intra-abdominal fat accumulation, likely influence body fat distribution and function in normal-weight PCOS women through a programmed mechanism to balance glucose-insulin homeostasis with fat accretion [24, 50].
Lipotoxicity
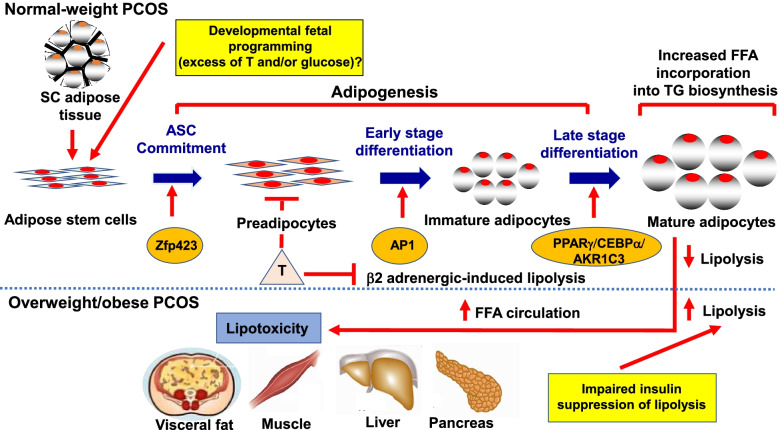
Several endocrine-metabolic characteristics of PCOS women worsen with increased adiposity. Overweight/obese compared to normal-weight women with PCOS exhibit greater preferential abdominal fat accumulation, hyperandrogenism and insulin resistance [2] accompanied by increased serum glycerol levels from impaired insulin suppression of lipolysis [44]. Their enlarged SC abdominal mature adipocytes are also more pre-disposed to a pro-inflammatory lipid depot environment than the increased number of smaller SC abdominal adipocytes present in normal-weight women with PCOS [24, 44]. Given androgen inhibition of early-stage SC abdominal adipogenesis [35], overweight/obese women with PCOS are more likely than normal-weight PCOS women to have an impaired ability to properly store fatty acid influx in SC fat as energy intake exceeds energy expenditure [38, 39], promoting ectopic lipid deposition in non-adipose tissue [1] (Fig. 1).
Fig. 1.
Altered molecular pathways of subcutaneous (SC) abdominal adipogenesis in polycystic ovary syndrome (PCOS) as a risk factor for lipotoxicity. In normal-weight PCOS women, exaggerated adipose stem cell (ASC) development to adipocytes occurs via androgen-independent mechanisms [23, 50]. Simultaneously, androgen excess inhibits early-stage adipogenesis, diminishes insulin-stimulated glucose uptake, promotes lipid storage and impairs catecholamine-stimulated lipolysis [31, 32, 35, 40, 47], favoring abdominal fat deposition and increased energy availability through hyperandrogenism and insulin resistance, respectively. These same traits are worsened in overweight/obese PCOS women who have greater preferential abdominal fat accumulation, hyperandrogenism, and systemic insulin resistance [2], along with impaired insulin suppression of lipolysis [2, 44], promoting ectopic lipid deposition and lipotoxicity [1]
Lipotoxicity refers to the ectopic lipid accumulation in non-adipose tissue where it induces oxidative/endoplasmic reticulum stress tightly linked with insulin resistance and inflammation [61]. Overweight/obese women with PCOS are at increased risk of developing lipotoxicity due to excess FFA uptake into non-adipose cells, including the muscle, liver, pancreas and ovary, which is exacerbated by increased intra-abdominal fat with high lipolytic activity [32, 33, 62–65]. Consequently, excess fatty acid influx into skeletal muscle and liver promotes diacylglycerol-induced insulin resistance, which impairs insulin signaling via increased insulin receptor serine phosphorylation, and worsens with disrupted mitochondrial oxidative phosphorylation [34, 66].
Developmental programming: dual hits from maternal-fetal endocrine-metabolic dysfunction
Through an evolutionary perspective, the high worldwide prevalence of PCOS in today’s environment and its negative impact on reproduction should have disappeared over millennia unless a beneficial effect favored both survival and reproduction [67]. There is little genetic evidence of strong selection against transmission of PCOS risk genes across multiple generations [68]. Rather, ancestral traits may have originally favored PCOS in hunter-gatherers of the late Pleistocene, or perhaps in more ancient human populations, when scarcity of food in pregnancy programmed in the fetus enhanced adipogenesis for greater fat storage to meet the metabolic demands of reproduction in later life (i.e., metabolic thrift). In this regarding, the same PCOS risk genes expressed in women with PCOS from both Chinese and European populations suggest ancient origins [68], potentially dating back before human diaspora out of sub-Saharan Africa 100,000–50,000 years ago [69, 70].
Indeed, commonality among > 20 PCOS candidate genes in women with differing PCOS phenotypes, including those diagnosed by Rotterdam or NIH criteria, or by self-report, strongly suggests shared molecular and developmental origins despite heterogeneity of PCOS phenotypic expression [71]. Moreover, rare gene variants of DENND1A involved in regulating androgen production, have been identified in ~ 50% of families with PCOS [72]. In this regard, a posttranscription form of DENND1A, DENND1A.v2, is over-expressed in some women with PCOS [73], while experimentally-induced DENND1A.v2 over-expression in human theca cells increases androgen biosynthesis and release as a fundamental PCOS trait linked to metabolic dysfunction. But without evidence of GWAS-identified, PCOS-associated, intronic gene variants of DENND1A enabling DENND1a.v2 over-expression or ovarian hyperandrogenism, investigations continue into the pathogenic mechanisms underlying this PCOS-associated gene variant [74].
A separate whole-genome sequencing study found in ~ 3% of families with PCOS, rare gene variants in anti-mullerian hormone (AMH) and its type 2 receptor (AMHR2) [75]. Both genes are involved in intra-ovarian follicle development and hypothalamic GnRH stimulation, which ultimately regulate ovarian androgen production [76]. Whether these mostly missense variants in AMH and AMHR2 induce the elevated circulating and intrafollicular levels of AMH and the ovarian hyperandrogenism of PCOS women remains unclear [75].
In addition, some PCOS candidate genes, such as thyroid adenoma associated (THADA) and insulin receptor (INSR), have been associated with metabolic syndrome and impaired glucose regulation in PCOS and type 2 diabetes [77], suggesting genetic contributions to metabolic dysfunction in women with PCOS. Moreover, in a recent Mendelian randomization study, gene variants associated with high bioavailable (unbound) circulating T levels were also linked with PCOS and type 2 diabetes [78], providing an additional causal role for female hyperandrogenism in the onset of both disorders.
Maternal-fetal endocrine-metabolic dysfunction appears to enable genetic and/or epigenetic re-programming of the female phenotype into PCOS [79]. During the second trimester of development, the human fetal ovary contains several steroidogenic enzymes, genes encoding steroid-signalling pathways, and receptors to steroids, insulin and insulin-like growth factors, when primordial ovarian follicles and abdominal fat are present [1, 80, 81]. Mid-trimester human and nonhuman primate fetal ovaries can metabolize progestins and their conjugates into androgens enabling T secretion [82, 83]. The human midgestational fetal ovary also may produce androgens in response to in utero hyperinsulinemia, particularly in a female fetus with a genetic susceptibility to PCOS. Amniotic fluid T levels are elevated in female fetuses of diabetic [84] mothers, along with theca and pancreatic beta cell hyperplasia accompanying ovarian theca-lutein cysts in hirsute female stillbirth offspring of these women [85, 86]. Amniotic fluid T levels also are elevated in female fetuses of PCOS mothers [87] during mid-gestation when regional fat depots in the human fetus develop [81]. Elongated anogenital distances, as postnatal biomarkers of mid-gestational fetal hyperandrogenism, occur in both female infants of PCOS mothers and in PCOS women [5], affirming midgestational fetal female hyperandrogenism. Interestingly, term umbilical cord T levels are elevated in only some female infants of PCOS mothers since sex differences in fetal T exposure are normally minimal at birth [4]. These findings collectively suggest that endocrine-metabolic disorders of pregnancy in mothers with PCOS can induce epigenetic modifications of fetal genetic susceptibility to PCOS after birth.
From a maternal perspective, PCOS women in pregnancy have greater serum androgen levels, higher fasting and 2-h post-prandial insulin values and elevated serum AMH levels [88–90] compared to normal mothers. Subsequently, the prevalence of gestational diabetes, glucose intolerance and type 2 diabetes in PCOS women is up to 5-fold higher than that of other women and is worsened by obesity, with about 40% of PCOS women developing gestational diabetes and other pregnancy complications [5, 8]. Moreover, PCOS women in pregnancy exhibit exaggerated dyslipidemia and elevated circulating inflammatory markers that predict gestational diabetes, hypertensive disorders and adverse obstetrical/neonatal outcomes [91, 92]. Maternal hyperandrogenemia from PCOS, however, may not directly program PCOS in offspring if placental aromatization is normal [93]. Instead, metabolic dysfunction in a PCOS mother may be transmitted through the placenta to a female fetus with a genetic susceptibility to PCOS, promoting fetal hyperinsulinemia as a cause for hyperandrogenism and altered folliculogenesis in utero [5, 87, 94–96].
Alternatively, hyperandrogenic PCOS mothers [89] with elevated serum AMH levels in pregnancy [90] may have reduced placental aromatase expression [97], providing a potential maternal androgen contribution to female fetal hyperandrogenism. Evidence for this second mechanism is that gestational exposure of mice to recombinant AMH during a critical gestational age induces maternal neuroendocrine-driven hyperandrogenism and diminishes placental aromatization, causing a PCOS-like phenotype in female offspring and their descendants over multiple generations [90, 98].
Animal models affirming developmental programming of PCOS through maternal-fetal endocrine-metabolic dysfunction
Gestational exposure of female nonhuman primates, sheep, rats, and mice to excess T, or dihydrotestosterone (DHT), induces reproductive and metabolic PCOS-like phenotypes resembling those of women with PCOS [99]. Such PCOS animal models provide unique perspectives on how hyperandrogenism and increased adiposity interact to affect PCOS phenotypic expression, given the worldwide human obesity epidemic [67].
As precocial species, in which tissue differentiation is completed during fetal life as in humans [100], prenatally T-treated monkeys and sheep provide particularly valuable mechanistic links between endocrine-metabolic dysfunction in pregnancy and its long-term metabolic-reproductive consequences in offspring. In prenatally T-treated monkeys, maternal glucose intolerance causes transient hyperinsulinemia in their female fetuses. Specifically, prenatal T-treatment in rhesus monkeys impairs maternal glucose tolerance and stimulates fetal insulin release, which then potentiates insulin action within the fetus [101]. Prenatal T-treatment in sheep also induces maternal hyperinsulinemia [102]. When these sheep are co-treated with either flutamide or rosiglitazone, juvenile insulin resistance and early adult hyperleptinemia are prevented [58].
Mid-gestational prenatal T-treatment in rhesus monkeys and sheep also programs adipose dysfunction with insulin resistance in adult offspring [94, 103]. Adult prenatally T-treated sheep with insulin resistance [58, 103, 104] also develop hypertension and hypercholesterolemia after puberty [105], although their long-term risks of developing increased adiposity and diabetes with age remain unclear. Prenatally-T treated juvenile female sheep also show increased stem cell commitment to preadipocytes and decreased preadipocyte differentiation of visceral adipocytes, with the latter prevented by dual prenatal flutamide/rosiglitazone co-treatment [57]. This increase in commitment may underlie the increased proportion of small adipocytes observed in both prenatal T-treated sheep and monkeys [55, 56].
Naturally-occurring female hyperandrogenism also occurs in some adult female macaques accompanied by PCOS-like endocrine-metabolic traits [106, 107]. A positive correlation of anogenital distance with circulating T levels in these adult monkeys suggests mid-gestational hyperandrogenic origins. Most naturally hyperandrogenic female macaques are not overweight [106], and resemble normal-weight women with PCOS [26], while those with the highest T values exhibit increased BMI, central adiposity and insulin resistance [107]. Since rhesus monkeys share > 97% DNA sequence homology with humans at protein-coding exons, evolutionary changes in the rhesus monkey exome that resemble those previously identified in human PCOS candidate genes could generate common biological phenotypes with initial selective advantages but comparable physiological consequences across primates [108].
Clinical interventions that diminish metabolic dysfunction ameliorate PCOS symptomology
Potentially programmed in part during gestation, an altered metabolic phenotype in women with PCOS could predispose them to excess weight gain in today’s obesogenic environment, emphasizing the need for appropriate clinical strategies to improve their health, reduce their risks of developing maternal-fetal complications and optimize the long-term health of their offspring [51]. Although weight loss in overweight/obese women with PCOS through lifestyle intervention, medication use and/or bariatric surgery can improve their metabolic-reproductive function [5, 109, 110], long-term effects of these clinical therapies remains uncertain, while gestational use of some medications are either contraindicated (i.e., antiandrogens) or associated with childhood adiposity and insulin resistance (i.e., metformin) [111]. A more effective strategy may be to identify young girls at risk for PCOS, perhaps by measuring facial sebum content [112], anogenital distance [113] and/or circulating AMH levels [114] in early life, and then initiate relevant interventions before puberty. Such a clinical strategy for the treatment of PCOS shifts the paradigm from disease treatment to preventive intervention, stressing early and appropriate lifestyle choices and the development of novel therapies to improve the fertility and endocrine-metabolic health of PCOS women, reduce their risks of maternal-fetal complications and optimize the long-term health of their offspring.
Conclusions
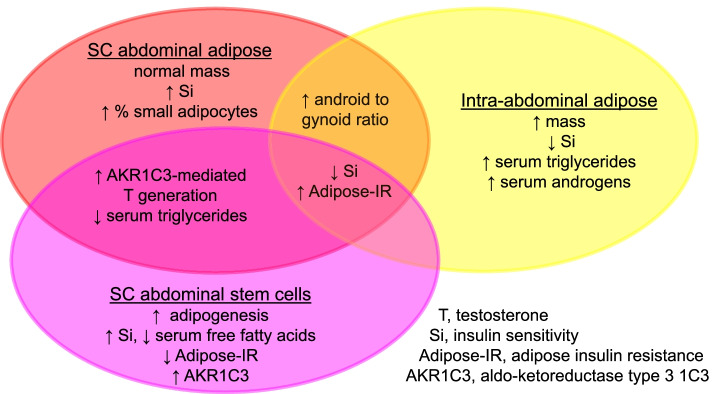
Polycystic ovary syndrome has persisted from antiquity to become the most common reproductive-metabolic disorder of reproductive-aged women. Its ancestral traits once favored abdominal fat deposition and increased energy availability through hyperandrogenism and insulin resistance, respectively, for reproduction during food deprivation. These same traits in today’s environment, however, underlie different PCOS phenotypes with variable risks for subfertility and metabolic dysfunction that are worsened by obesity. Recent studies of healthy normal-weight women with NIH-defined PCOS show enhanced AKR1C3 activity in SC abdominal adipose favoring lipid storage in combination with preferential intra-abdominal fat deposition accompanying hyperandrogenemia and low-normal insulin sensitivity. Potentially programmed as an ancestral trait by genetic inheritance and epigenetic events during early life, such a metabolic adaptation in these normal-weight PCOS women provides a balance between enhanced SC adipose TG storage and increased circulating glucose and FFA availability as energy substrate for crucial target tissues, including brain and muscle (Fig. 2). It also favors subfertility from infrequent ovulation, perhaps allowing women from antiquity sufficient time and strength for childrearing of fewer offspring who have an enhanced likelihood of childhood survival [67]. Future studies should examine how heritable PCOS characteristics are influenced by today’s obesogenic environment through an epigenetic-related metabolic adaptation that favors fat storage, but predisposes to lipotoxicity with excess weight gain and pregnancy complications. Such investigations should focus on a new perspective that PCOS may have evolutionary origins in both human and nonhuman primates.
Fig. 2.
Metabolic adaptation in polycystic ovary syndrome. Inherently accelerated adipogenesis, along with enhanced intracellular aldo-ketoreductase type 1C3-mediated testosterone generation, in subcutaneous (SC) abdominal adipose promotes lipid storage (through increased lipogenesis and decreased lipolysis) to protect against insulin resistance. Simultaneously, hyperandrogenemia accompanies preferential accumulation of highly-lipolytic intra-abdominal fat with an opposite effect. As a result, SC fat storage counterbalances increased circulating glucose and free fatty acid (FFA) availability for energy use. When energy intake exceeds fat storage capacity, excess fatty acid influx into skeletal muscle and liver promotes lipotoxicity through ectopic lipid accumulation accompanied by oxidative stress, insulin resistance and inflammation in non-adipose tissue
Acknowledgements
We thank Karla Largaespada at UCLA for subject recruitment strategies and administrative responsibilities that were crucial for the successful studies of the normal-weight PCOS and control subjects; the veterinary, pathology and animal care staff at the Wisconsin National Primate Research Center (WNPRC) and the University of Michigan, Ann Abor for outstanding care and maintenance of all animals; and Jon Levine (WNPRC) for his highly valued contributions to refining and enabling our research into the developmental origins of PCOS.
Abbreviations
- PCOS
Polycystic ovary syndrome
- PCOM
Polycystic ovarian morphology
- GWAS
Genome-wide association studies
- BMI
Body mass index
- Si
Insulin sensitivity
- FSIVGTT
Frequently sampled intravenous glucose tolerance testing
- HOMA-IR
Homeostatic Model Assessment for Insulin Resistance
- adipose-IR
Adipose insulin resistance
- TG
Triglyceride
- non-HDL
Non-high density lipoprotein
- AKR1C3
Aldo-ketoreductase type 1C3
- AKR1C2
Aldo-ketoreductase type 1C2
- T
Testosterone
- A4
Androstenedione
- SC
Subcutaneous
- ASCs
Adipose stem cells
- ZFP423
Zinc-finger protein 423
- NIH
National Institutes of Health
- PKA-RegIIβ
Protein kinase A regulatory-IIβ component
- GLUT-4
Glucose transporter type 4
- HSL
Hormone-sensitive lipase
- PPARγ
Peroxisome proliferator-activated receptor gamma
- CEBPa
CCAAT enhancer binding protein alpha
- AP-1
Activator protein-1
- FFA
Free fatty acid
- AMH
Anti-mullerian hormone
- LH
Luteinizing hormone
- SHBG
Sex hormone binding globulin
- TGF
Transforming growth factor
- AMHR2
AMH type 2 receptor
- THADA
Thyroid adenoma associated
- INSR
Insulin receptor
- DHT
Dihydrotestosterone
Authors’ contributions
D.A.D. performed clinic studies/interventions and was a major contributor in writing the manuscript. V.P. performed sheep studies and was a major contributor in writing the manuscript. G.D.C. performed all human stem cells studies and was a major contributor in writing the manuscript. D.H.A. performed nonhuman primate studies and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institutes of Health (NIH) under awards P50 HD071836 and P51 ODO11092 for the Endocrine Technologies Support Core (ETSC) through the Oregon National Primate Research Center; statistical analyses by the NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881; and the Santa Monica Bay Woman’s Club. Nonhuman primate research was supported by awards DK121559 (National Institute of Diabetes and Digestive and Kidney Diseases), R21 HD102172 (NICHD) and P51 OD011106 for the Office of Research Infrastructure Programs (ORIP) through the Wisconsin National Primate Research Center. Sheep research was supported by the Eunice Kennedy Shriver NICHD, NIH under awards R01 HD099096 and P01 HD44232. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
All studies were performed according to the Declaration of Helsinki after approval by the UCLA Institutional Review Board and signed informed consent by each subject (IRB number 12–001780). The nonhuman primate studies were reviewed and approved by the Graduate School Animal Care and Use Committee of the University of Wisconsin, Madison and were performed consistent with the USDA Animal Welfare Act and regulations and the Guide for the Care and Use of Laboratory Animals. The animal care and use program at the University of Wisconsin maintains a Public Health Services Assurance, and is fully accredited by AAALAC. All sheep studies were conducted under approved protocols of Institutional Animal Care and Use Committee of the University of Michigan, which is fully accredited by AAALAC.
Consent for publication
Not applicable.
Competing interests
D.A.D has consulted for Spruce Biosciences, Inc.; V.P., G.D.C. and D.H.A have nothing to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daniel A. Dumesic, Email: ddumesic@mednet.ucla.edu
Vasantha Padmanabhan, Email: vasantha@med.umich.edu.
Gregorio D. Chazenbalk, Email: gchazenbalk@gmail.com
David H. Abbott, Email: abbott@primate.wisc.edu
References
- 1.Chang RJ, Dumesic DA. Polycystic ovary syndrome and Hyperandrogenic states. In: Strauss JF III, Barbieri RL, editors. Yen and Jaffe’s reproductive endocrinology: physiology, pathophysiology and clinical management. 8. Philadelphia: Elsevier Saunders; 2018. pp. 520–555. [Google Scholar]
- 2.Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487–525. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Repro Update. 2010;16(4):347–363. doi: 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- 4.Dumesic DA, Hoyos LR, Chazenbalk GD, Naik R, Padmanabhan V, Abbott DH. Mechanisms of intergenerational transmission of polycystic ovary syndrome. Reproduction. 2019;159(1):R1–R13. doi: 10.1530/REP-19-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbott DH, Dumesic DA. Levine JE Hyperandrogenic origins of polycystic ovary syndrome – implications for pathophysiology and therapy. Expert Rev Endocrinol Metab. 2019;14(2):131–143. doi: 10.1080/17446651.2019.1576522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nilsson E, Benrick A, Kokosar M, Krook A, Lindgren E, Källman T, Martis MM, Højlund K, Ling C, Stener-Victorin E. Transcriptional and epigenetic changes influencing skeletal muscle metabolism in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:4465–4477. doi: 10.1210/jc.2018-00935. [DOI] [PubMed] [Google Scholar]
- 7.Vázquez-Martínez ER, Gómez-Viais YI, García-Gómez E, Reyes-Mayoral C, Reyes-Muñoz E, Camacho-Arroyo I, Cerbón M. DNA methylation in the pathogenesis of polycystic ovary syndrome. Reproduction. 2019;158(1):R27–R40. doi: 10.1530/REP-18-0449. [DOI] [PubMed] [Google Scholar]
- 8.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T. Norman RJ on behalf of the international PCOS network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33:1602–1618. doi: 10.1093/humrep/dey256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenzweig JL, Ferrannini E, Grundy SM, Haffner SM, Heine RJ, Horton ES, Kawamori R. Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3671–3689. doi: 10.1210/jc.2008-0222. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt HR. Update on treatment strategies for obesity. J Clin Endocrinol Metab. 2013;98(4):1299–1306. doi: 10.1210/jc.2012-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9(4):359–372. doi: 10.1093/humupd/dmg024. [DOI] [PubMed] [Google Scholar]
- 12.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endo Rev. 2012;33(6):981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95–109. doi: 10.1111/j.1467-789X.2012.01053.x. [DOI] [PubMed] [Google Scholar]
- 14.Yildiz BO, Knochenhauer ES, Azziz R. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(1):162–168. doi: 10.1210/jc.2007-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kakoly NS, Khomami MB, Joham AE, Corray SD, Misso ML, Norman RJ, Harrison CL, Ranasinha S, Teede HJ, Moran LJ. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update. 2018;24(4):455–467. doi: 10.1093/humupd/dmy007. [DOI] [PubMed] [Google Scholar]
- 16.Palaniappan LP, Carnethon MR, Fortmann SP. Heterogeneity in the relationship between ethnicity, BMI, and fasting insulin. Diabetes Care. 2002;25(8):1351–1357. doi: 10.2337/diacare.25.8.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mumusoglu S, Yidiz BO. Polycystic ovary syndrome phenotypes and prevalence: differential impact of diagnostic criteria and clinical versus unselected population. Curr Opin Endocr Metab Res. 2020;12:66–71. [Google Scholar]
- 18.Lizneva D, Kirubakaran R, Mykhalchenko K, Suturina L, Chernukha G, Diamond MP, Azziz R. Phenotypes and body mass in women with polycystic ovary syndrome identified in referral versus unselected populations: systematic review and meta-analysis. Fertil Steril. 2016;106(6):1510–1520.e2. doi: 10.1016/j.fertnstert.2016.07.1121. [DOI] [PubMed] [Google Scholar]
- 19.Søndergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD. How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab. 2017;102(4):1193–1199. doi: 10.1210/jc.2017-00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ezeh U, Yildiz BO, Azziz R. Referral bias in defining the phenotype and prevalence of obesity in polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98(6):E1088–E1096. doi: 10.1210/jc.2013-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hershkop K, Besor O, Santoro N, Pierpont B, Caprio S, Weiss R. Adipose insulin resistance in obese adolescents across the spectrum of glucose tolerance. J Clin Endocrinol Metab. 2016;101(6):2423–2431. doi: 10.1210/jc.2016-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dapas M, Lin FTJ, Nadkarni GN, Sisk R, Legro RS, Urbanek M, Hayes MG, Dunaif A. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: an unsupervised, phenotypic clustering analysis. PLoS Med. 2020;17(6):e1003132. doi: 10.1371/journal.pmed.1003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung KL, Sanchita S, Pham CT, Davis BA, Okhovat M, Ding X, Dumesic P, Grogan TR, Williams KJ, Morselli M, Ma F, Carbone L, Li X, Pellegrini M, Dumesic DA, Chazenbalk GD. Dynamic changes in chromatin accessibility, altered adipogenic gene expression, and total versus de novo fatty acid synthesis in subcutaneous adipose stem cells of normal-weight polycystic ovary syndrome (PCOS) women during adipogenesis: evidence of cellular programming. Clin Epigenetics. 2020;12(1):181. doi: 10.1186/s13148-020-00970-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dumesic DA, Akopians AL, Madrigal VK, Ramirez E, Margolis DJ, Sarma MK, Thomas AM, Grogan TR, Haykal R, Schooler TA, Okeya BL, Abbott DH, Chazenbalk GD. Hyperandrogenism accompanies increased intra-abdominal fat storage in Normal weight polycystic ovary syndrome women. J Clin Endocrinol Metab. 2016;101(11):4178–4188. doi: 10.1210/jc.2016-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tosi F, Di Sarra D, Kaufman JM, Bonin C, Moretta R, Bonoro E, Zanolin E, Mogetti P. Total body fat and central fat mass independently predict insulin resistance but not hyperandrogenemia in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100:661–669. doi: 10.1210/jc.2014-2786. [DOI] [PubMed] [Google Scholar]
- 26.Dumesic DA, Phan JD, Leung KL, Grogan TR, Ding X, Li X, Hoyos LR, Abbott DH, Chazenbalk GD. Adipose insulin resistance in Normal-weight polycystic ovary syndrome women. J Clin Endocrinol Metab. 2019;104(6):2171–2183. doi: 10.1210/jc.2018-02086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holte J, Bergh T, Berne C, Berglund L, Lithell H. Enhanced early insulin response to glucose in relation to insulin resistance in women with polycystic ovary syndrome and normal glucose tolerance. J Clin Endocrinol Metab. 1994;7(5):8. doi: 10.1210/jcem.78.5.8175959. [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–E1760. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN. PCOS/Troglitazone study group. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(1):48–53. doi: 10.1210/jc.2005-1329. [DOI] [PubMed] [Google Scholar]
- 30.Bjorntorp P. Metabolic implications of body fat distribution. Diabet Care. 1991;14(12):1132–1143. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- 31.Dicker A, Ryden M, Naslund E, Muehlen IE, Wiren M, Lafontan M, Arner P. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia. 2004;47:420–428. doi: 10.1007/s00125-003-1324-0. [DOI] [PubMed] [Google Scholar]
- 32.Arner P. Effects of testosterone on fat cell lipolysis. Species differences and possible role in polycystic ovarian syndrome. Biochimie. 2005;87(1):39–43. doi: 10.1016/j.biochi.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Ek I, Arner P, Rydén M, Holm C, Thörne A, Hoffstedt J, Wahrenberg H. A unique defect in the regulation of visceral fat cell lipolysis in the polycystic ovary syndrome as an early link to insulin resistance. Diabetes. 2002;51(2):484–492. doi: 10.2337/diabetes.51.2.484. [DOI] [PubMed] [Google Scholar]
- 34.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unraveling the mechanism. Lancet. 2010;375(9733):2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chazenbalk GD, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA. Androgens inhibit adipogenesis during human adipose stem cell commitment to predipocyte formation. Steroids. 2013;78(9):920–926. doi: 10.1016/j.steroids.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12(11):722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012;7(81):715–736. doi: 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- 38.Saponaro C, Gaggini M, Carli F, Gastaldelli A. The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients. 2015;7(11):9453–9474. doi: 10.3390/nu7115475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romacho T, Elsen M, Rohrborn D, Eckel J. Adipose tissue and its role in organ crosstalk. Acta Physiol (Oxf) 2014;210(4):733–753. doi: 10.1111/apha.12246. [DOI] [PubMed] [Google Scholar]
- 40.Corbould A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol. 2007;192(3):585–594. doi: 10.1677/joe.1.07070. [DOI] [PubMed] [Google Scholar]
- 41.Rosenbaum D, Harber RS, Dunaif A. Insulin resistance in polycystic ovary syndrome: decreased expression of GLUT-4 glucose transporters in adipocytes. Am J Phys. 1993;264(2 Pt 1):E197–E202. doi: 10.1152/ajpendo.1993.264.2.E197. [DOI] [PubMed] [Google Scholar]
- 42.Faulds G, Rydén M, Ek I, Wahrenberg H, Arner P. Mechanisms behind lipolytic catecholamine resistance of subcutaneous fat cells in the polycystic ovarian syndrome. J Clin Endocrinol Metab. 2003;88(5):2269–2273. doi: 10.1210/jc.2002-021573. [DOI] [PubMed] [Google Scholar]
- 43.Ek I, Arner P, Bergqvist A, Carlstrom K, Wahrenberg H. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J Clin Endocrinol Metab. 1997;82(4):1147–1153. doi: 10.1210/jcem.82.4.3899. [DOI] [PubMed] [Google Scholar]
- 44.Mannerås-Holm L, Leonhardt H, Kullberg J, Jennische E, Odén A, Holm G, Hellström M, Lönn L, Olivecrona G, Stener-Victorin E, Lönn M. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96(2):E304–E311. doi: 10.1210/jc.2010-1290. [DOI] [PubMed] [Google Scholar]
- 45.Blouin K, Veilleux A, Luu-The V, Tchernof A. Androgen metabolism in adipose tissue: recent advances. Mol Cell Endocrinol. 2009;301(1–2):97–103. doi: 10.1016/j.mce.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 46.Quinkler M, Sinha B, Tomlinson JW, Bujalska IJ, Stewart PM, Arlt W. Androgen generation in adipose tissue in women with simple obesity--a site-specific role for 17beta-hydroxysteroid dehydrogenase type 5. J Endocrinol. 2004;183(2):331–342. doi: 10.1677/joe.1.05762. [DOI] [PubMed] [Google Scholar]
- 47.O’Reilly MW, Kempegowda P, Walsh M, Taylor AE, Manolopoulos KN, Allwood JW, Semple RK, Hebenstreit D, Dunn WB, Tomlinson JW, Arlt W. AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(9):3327–3339. doi: 10.1210/jc.2017-00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dumesic DA, Tulberg A, McNamara M, Grogan TR, Abbott DH, Naik R, Lu G, Chazenbalk GD. Serum testosterone to Androstenedione ratio predicts metabolic health in Normal-weight polycystic ovary syndrome women. J Endocr Soc. 2021;5(11):bvab158. doi: 10.1210/jendso/bvab158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffsted J, Naslund E, Britton T, Concha H, Hassan M, Rydén M, Frisén J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 50.Fisch SC, Farzan Nikou A, Wright EA, Phan JD, Leung KL, Grogan TR, Abbott DH, Chazenbalk GD, Dumesic DA. Precocious subcutaneous abdominal stem cell development to adipocytes in Normal-weight polycystic ovary syndrome women. Fertil Steril. 2018;110(7):1367–1376. doi: 10.1016/j.fertnstert.2018.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dumesic DA, Tulberg A, Leung KL, Fisch SC, Grogan TR, Abbott DH, Naik R, Chazenbalk GD. Accelerated subcutaneous abdominal stem cell adipogenesis predicts insulin sensitivity in normal-weight women with polycystic ovary syndrome. Fertil Steril. 2021;116(1):232–242. doi: 10.1016/j.fertnstert.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tandon P, Wafer R, Minchin JE. Adipose morphology and metabolic disease. J Expt Biol. 2018;221(Pt Suppl 1):jeb164970. doi: 10.1242/jeb.164970. [DOI] [PubMed] [Google Scholar]
- 53.Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J, Bernard S, Arner P. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59(1):105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Longo M, Raciti GA, Zatterale F, Parrillo L, Desiderio A, Spinelli R, Hammarstedt A, Hedjazifar S, Hoffmann JM, Nigro C, Mirra P, Fiory F, Formisano P, Miele C, Smith U, Beguinot F. Epigenetic modifications of the Zfp/ZNF423 gene control murine adipogenic commitment and are dysregulated in human hypertrophic obesity. Diabetologia. 2018;61(2):369–380. doi: 10.1007/s00125-017-4471-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keller E, Chazenbalk GD, Aguilera P, Madrigal V, Grogan T, Elashoff D, Dumesic DA, Abbott DH. 2014. Impaired preadipocyte differentiation into adipocytes in subcutaneous abdominal adipose of PCOS-like female rhesus monkeys. Endocrinology. 2014;155(7):2696–2703. doi: 10.1210/en.2014-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Veiga-Lopez A, Moeller J, Patel D, Ye W, Pease A, Kinns J, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on insulin sensitivity, adiposity, and free fatty acid profile in postpubertal female sheep. Endocrinology. 2013;154(5):1731–1742. doi: 10.1210/en.2012-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puttabyatappa M, Lu C, Martin JD, Chazenbalk G, Dumesic D, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on steroidal machinery and cell differentiation markers in visceral adipocytes of female sheep. Reprod Sci. 2018;25(7):1010–1023. doi: 10.1177/1933719117746767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cardoso RC, Veiga-Lopez A, Moeller J, Beckett E, Pease A, Keller E, Madrigal V, Chazenbalk G, Dumesic D, Padmanabhan V. Developmental programming: impact of gestational steroid and metabolic milieus on adiposity and insulin sensitivity in prenatal testosterone-treated female sheep. Endocrinology. 2016;157(2):522–535. doi: 10.1210/en.2015-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nouws J, Fitch M, Mata M, Santoro N, Galuppo B, Kursawe R, Narayan D, Vash-Margita A, Pierpont B, Shulman GI, Hellerstein M, Caprio S. Altered in vivo lipid fluxes and cell dynamics in subcutaneous adipose tissues are associated with the unfavorable pattern of fat distribution in obese adolescent girls. Diabetes. 2019;68(6):1168–1177. doi: 10.2337/db18-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Umano GR, Shabanova V, Pierpont B, Mata M, Nouws J, Tricò D, Galderisi A, Santoro N, Caprio S. A low visceral fat proportion, independent of total body fat mass, protects obese adolescent girls against fatty liver and glucose dysregulation: a longitudinal study. Int J Obes. 2019;43(4):673–682. doi: 10.1038/s41366-018-0227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brennan KM, Kroener LL, Chazenbalk GD, Dumesic DA. Polycystic ovary syndrome: impact of lipotoxicity on metabolic and reproductive health. Obstet Gynecol Surv. 2019;74(4):223–231. doi: 10.1097/OGX.0000000000000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Virtue S, Vidal-Puig A. It's not how fat you are, it's what you do with it that counts. PLoS Biol. 2008;6(9):e237. doi: 10.1371/journal.pbio.0060237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801(3):209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 64.de Zegher F, Lopez-Bermejo A, Ibáñez L. Adipose tissue expandability and the early origins of PCOS. Trends Endocrinol Metab. 2009;20(9):418–423. doi: 10.1016/j.tem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Ramkissoon R, Gardner TB. Pancreatic Steatosis: an emerging clinical entity. Am J Gastroenterol. 2019;114(11):1726–1734. doi: 10.14309/ajg.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 66.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(23):1131–1141. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 67.Corbett S, Morin-Papunen L. Polycystic ovary syndrome and recent human evolution. Mol Cell Endocrinol. 2013;373(1–2):39–50. doi: 10.1016/j.mce.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 68.Dapas M, Dunaif A. The contribution of rare genetic variants to the pathogenesis of polycystic ovary syndrome. Curr Opin Endocr Metab Res. 2020;12:26–32. doi: 10.1016/j.coemr.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shriner D, Tekola-Ayele F, Adeyemo A, Rotimi CN. Ancient human migration after out-of-Africa. Sci Rep. 2016;6:26565. doi: 10.1038/srep26565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nielsen R, Akey JM, Jakobsson M, Pritchard JK, Tishkoff S, Willerslev E. Tracing the peopling of the world through genomics. Nature. 2017;541(7637):302–310. doi: 10.1038/nature21347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Day F, Karaderi T, Jones MR, Meun C, He C, Drong A, Kraft P, Lin N, Huang H, Broer L, Magi R, Saxena R, Laisk T, Urbanek M, Hayes MG, Thorleifsson G, Fernandez-Tajes J, Mahajan A, Mullin BH, Stuckey BGA, Spector TD, Wilson SG, Goodarzi MO, Davis L, Obermayer-Pietsch B, Uitterlinden AG, Anttila V, Neale BM, Jarvelin MR, Fauser B, Kowalska I, Visser JA, Andersen M, Ong K, Stener-Victorin E, Ehrmann D, Legro RS, Salumets A, McCarthy MI, Morin-Papunen L, Thorsteinsdottir U, Stefansson K, 23andMe Research Team. Styrkarsdottir U, Perry JRB, Dunaif A, Laven J, Franks S, Lindgren CM, Welt CK. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018;14(12):e1007813. doi: 10.1371/journal.pgen.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dapas M, Sisk R, Legro RS, Urbanek M, Dunaif A, Hayes MG. Family-based quantitative trait meta-analysis implicates rare noncoding variants in DENND1A in polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(9):3835–3850. doi: 10.1210/jc.2018-02496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McAllister JM, Modi B, Miller BA, Biegler J, Bruggeman R, Legro RS, Strauss JF., 3rd Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci U S A. 2014;111(15):E1519–E1527. doi: 10.1073/pnas.1400574111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tee MK, Speek M, Legeza B, Modi B, Teves ME, McAllister JM, Strauss JF, 3rd, Miller WL. Alternative splicing of DENND1A, a PCOS candidate gene, generates variant 2. Mol Cell Endocrinol. 2016;434:25–35. doi: 10.1016/j.mce.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorsic LK, Dapas M, Legro RS, Hayes MG, Urbanek M. Functional genetic variation in the anti-Müllerian hormone pathway in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(7):2855–2874. doi: 10.1210/jc.2018-02178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barbotin AL, Peigné M, Malone SA, Giacobini P. Emerging roles of anti-Müllerian hormone in hypothalamic-pituitary function. Neuroendocrinology. 2019;109(3):218–229. doi: 10.1159/000500689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tian Y, Li J, Su S, Cao Y, Wang Z, Zhao S, Zhao H. PCOS-GWAS susceptibility variants in THADA, INSR, TOX3, and DENND1A are associated with metabolic syndrome or insulin resistance in women with PCOS. Front Endocrinol (Lausanne) 2020;11:274. doi: 10.3389/fendo.2020.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, Beaumont RN, Wittemans L, Martin S, Busch AS, Erzurumluoglu AM, Hollis B, O'Mara TA; Endometrial Cancer Association Consortium, McCarthy MI, Langenberg C, Easton DF, Wareham NJ, Burgess S, Murray A, Ong KK, Frayling TM, Perry JRB Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26(2):252–258. doi: 10.1038/s41591-020-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abbott DH, Greinwald EP, Levine JE. Developmental origins of polycystic ovary syndrome: everything starts in utero. In: Diamanti-Kandarakis E, editor. Polycystic Ovary Syndrome. Elsevier Press; 2021. [Google Scholar]
- 80.Cole B, Hensinger K, Maciel GA, Chang RJ, Erickson GF. Human fetal ovary development involves the spatiotemporal expression of p450c17 protein. J Clin Endocrinol Metab. 2006;91(9):3654–3661. doi: 10.1210/jc.2006-0641. [DOI] [PubMed] [Google Scholar]
- 81.Poissonnet CM, LaVelle M, Burdi AR. Growth and development of adipose tissue. J Pediatr. 1988;113(1 Pt 1):1–9. doi: 10.1016/s0022-3476(88)80520-1. [DOI] [PubMed] [Google Scholar]
- 82.Resko JA. Androgen secretion by the fetal and neonatal rhesus monkey. Endocrinology. 1970;87(4):680–687. doi: 10.1210/endo-87-4-680. [DOI] [PubMed] [Google Scholar]
- 83.Ellinwood WE, McClellan MC, Brenner RM, Resko JA. Estradiol synthesis by fetal monkey ovaries correlates with antral follicle formation. Biol Reprod. 1983;28(2):505–516. doi: 10.1095/biolreprod28.2.505. [DOI] [PubMed] [Google Scholar]
- 84.Barbieri RL, Saltzman DH, Torday JS, Randall RW, Frigoletto FD, Ryan KJ. Elevated concentrations of the beta-subunit of human chorionic gonadotropin and testosterone in the amniotic fluid of gestations of diabetic mothers. Am J Obstet Gynecol. 1986;154(5):1039–1043. doi: 10.1016/0002-9378(86)90746-5. [DOI] [PubMed] [Google Scholar]
- 85.Driscoll SG, Benirschke K, Curtis GW. Neonatal deaths among infants of diabetic mothers. Postmortem findings in ninety-five infants. Am J Dis Child. 1960;100:818–835. doi: 10.1001/archpedi.1960.04020040820004. [DOI] [PubMed] [Google Scholar]
- 86.Hultquist GT, Olding LB. Endocrine pathology of infants of diabetic mothers. A quantitative morphological analysis including a comparison with infants of iso-immunized and of non-diabetic mothers. Acta Endocrinol Suppl (Copenh) 1981;241:1–202. [PubMed] [Google Scholar]
- 87.Palomba S, Marotta R, Di Cello A, Russo T, Falbo A, Orio F, Tolino A, Zullo F, Esposito R, La Sala GB. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clin Endocrinol. 2012;77(6):898–904. doi: 10.1111/j.1365-2265.2012.04443.x. [DOI] [PubMed] [Google Scholar]
- 88.Kent J, Dodson WC, Kunselman A, Pauli J, Stone A, Diamond MP, Coutifaris C, Schlaff WD, Alvero R, Casson P, Christman GM, Rosen RM, Hansen KR, Robinson RD, Baker V, Usadi R, Santoro N, Zhang H, Eisenberg E, Legro RS. Reproductive medicine network. Gestational weight gain in women with polycystic ovary syndrome: A controlled study. J Clin Endocrinol Metab. 2018;103(11):4315–4323. doi: 10.1210/jc.2017-02764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17(10):2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- 90.Tata B, Mimouni NEH, Barbotin AL, Malone SA, Loyens A, Pigny P, Dewailly D, Catteau-Jonard S, Sundström-Poromaa I, Piltonen TT, Dal Bello F, Medana C, Prevot V, Clasadonte J, Giacobini P. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24(6):834–846. doi: 10.1038/s41591-018-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Palomba S, Falbo A, Chiossi G, Muscogiuri G, Fornaciari E, Orio F, Tolino A, Colao A, La Sala GB, Zullo F. Lipid profile in nonobese pregnant women with polycystic ovary syndrome: a prospective controlled clinical study. Steroids. 2014;88:36–43. doi: 10.1016/j.steroids.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 92.Palomba S, Falbo A, Chiossi G, Orio F, Tolino A, Colao A, La Sala GB, Zullo F. Low-grade chronic inflammation in pregnant women with polycystic ovary syndrome: a prospective controlled clinical study. J Clin Endocrinol Metab. 2014;99(8):2942–2951. doi: 10.1210/jc.2014-1214. [DOI] [PubMed] [Google Scholar]
- 93.Hickey M, Sloboda DM, Atkinson HC, Doherty DA, Franks S, Norman RJ, Newnham JP, Hart R. The relationship between maternal and umbilical cord androgen levels and polycystic ovary syndrome in adolescence: a prospective cohort study. J Clin Endocrinol Metab. 2009;94(10):3714–3720. doi: 10.1210/jc.2009-0544. [DOI] [PubMed] [Google Scholar]
- 94.Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8(2):127–141. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dumesic DA, Goodarzi MO, Chazenbalk GD, Abbott DH. Intrauterine environment and polycystic ovary syndrome. Semin Reprod Med. 2014;32(3):159–165. doi: 10.1055/s-0034-1371087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. 2015;21(5):575–592. doi: 10.1093/humupd/dmv029. [DOI] [PubMed] [Google Scholar]
- 97.Maliqueo M, Lara HE, Sánchez F, Echiburú B, Crisosto N, Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;166(2):151–155. doi: 10.1016/j.ejogrb.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 98.Mimouni NEH, Paiva I, Barbotin AL, Timzoura FE, Plassard D, Le Gras S, Ternier G, Pigny P, Catteau-Jonard S, Simon V, Prevot V, Boutillier AL, Giacobini P. Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab. 2021;33(3):513–530.e8. doi: 10.1016/j.cmet.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stener-Victorin E, Padmanabhan P, Walters KA, Campbell RE, Benrick A, Giacobini P, Dumesic DA, Abbott DH. Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr Rev. 2020;41(4):538–576. doi: 10.1210/endrev/bnaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Padmanabhan V, Veiga-Lopez A, Abbott DH, Dumesic DA. In: Developmental programming of ovarian dysfunction. Novel concepts in ovarian endocrinology. Gonzalez-Bulnes A, editor. Kerala: Research Signpost; 2007. pp. 329–352. [Google Scholar]
- 101.Abbott DH, Bruns CR, Barnett DK, Dunaif A, Goodfriend TL, Dumesic DA, Tarantal AF. Experimentally induced gestational androgen excess disrupts glucoregulation in rhesus monkey dams and their female offspring. Am J Physiol Endocrinol Metab. 2010;299(5):E741–E751. doi: 10.1152/ajpendo.00058.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abi Salloum B, Veiga-Lopez A, Abbott DH, Burant CF, Padmanabhan V. Developmental programming: exposure to testosterone excess disrupts steroidal and metabolic environment in pregnant sheep. Endocrinology. 2015;156(6):2323–2337. doi: 10.1210/en.2014-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Padmanabhan V, Veiga-Lopez A, Abbott DH, Recabarren SE, Herkimer C. Developmental programming: impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology. 2010;151(2):595–605. doi: 10.1210/en.2009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Recabarren SE, Padmanabhan V, Codner E, Lobos A, Durán C, Vidal M, Foster DL, Sir-Petermann T. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol Endocrinol Metab. 2005;289(5):E801–E806. doi: 10.1152/ajpendo.00107.2005. [DOI] [PubMed] [Google Scholar]
- 105.King AJ, Olivier NB, Mohankumar PS, Lee JS, Padmanabhan V, Fink GD. Hypertension caused by prenatal testosterone excess in female sheep. Am J Physiol Endocrinol Metab. 2007;292(6):E1837–E1841. doi: 10.1152/ajpendo.00668.2006. [DOI] [PubMed] [Google Scholar]
- 106.Abbott DH, Rayome BH, Dumesic DA, Lewis KC, Edwards AK, Wallen K, Wilson ME, Appt SE, Levine JE. Clustering of PCOS-like traits in naturally hyperandrogenic female rhesus monkeys. Hum Reprod. 2017;32(4):923–936. doi: 10.1093/humrep/dex036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arifin E, Shively CA, Register TC, Cline JM. Polycystic ovary syndrome with endometrial hyperplasia in a cynomolgus monkey (Macaca fascicularis) Vet Pathol. 2008;45(4):512–515. doi: 10.1354/vp.45-4-512. [DOI] [PubMed] [Google Scholar]
- 108.Abbott DH, Rogers J, Dumesic DA, Levine JE. Naturally occurring and experimentally induced Rhesus macaque models for polycystic ovary syndrome: translational gateways to clinical application. Med Sci (Basel) 2019;7(12):107. doi: 10.3390/medsci7120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284. doi: 10.1038/nrendo.2018.24. [DOI] [PubMed] [Google Scholar]
- 110.Cena H, Chiovato L, Nappi RE. Obesity, polycystic ovary syndrome, and infertility: A new avenue for GLP-1 receptor agonists. J Clin Endocrinol Metab. 2020;105(8):e2695–e2709. doi: 10.1210/clinem/dgaa285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hanem LGE, Salvesen Ø, Juliusson PB, Carlsen SM, Nossum MCF, Vaage MØ, Ødegård R, Vanky E. Intrauterine metformin exposure and offspring cardiometabolic risk factors (PedMet study): a 5-10 year follow-up of the PregMet randomised controlled trial. Lancet Child Adolesc Health. 2019;3(3):166–174. doi: 10.1016/S2352-4642(18)30385-7. [DOI] [PubMed] [Google Scholar]
- 112.Homburg R, Gudi A, Shah A, Layton M, A. A novel method to demonstrate that pregnant women with polycystic ovary syndrome hyper-expose their fetus to androgens as a possible stepping stone for the developmental theory of PCOS. A pilot study. Reprod Biol Endocrinol. 2017;15(1):61. doi: 10.1186/s12958-017-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barrett ES, Hoeger KM, Sathyanarayana S, Abbott DH, Redmon JB, Nguyen RHN, Swan SH. Anogenital distance in newborn daughters of women with polycystic ovary syndrome indicates fetal testosterone exposure. J Dev Orig Health Dis. 2018;9(3):307–314. doi: 10.1017/S2040174417001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sir-Petermann T, Codner E, Maliqueo M, Echiburú B, Hitschfeld C, Crisosto N, Pérez-Bravo F, Recabarren SE, Cassorla F. Increased anti-Müllerian hormone serum concentrations in prepubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(8):3105–3109. doi: 10.1210/jc.2005-2693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.