Abstract
Early diagnosis of congenital toxoplasmosis is rendered difficult when specific immunoglobulin M (IgM) and/or IgA antibodies are absent in the blood of the newborn infant. Since maternal IgG antibodies can cross the placenta, determination of IgG antibodies in newborn infants has hitherto not been used routinely for the diagnosis of congenital infection. The aim of this study was to assess the diagnostic usefulness of an immunoblot assay which compares the early IgG profiles between the mother and her child (comparative IgG profile between mother and child; CGMC test) directed against a total cell lysate of Toxoplasma gondii tachyzoites. Serum samples from 97 newborn infants at risk of toxoplasma infection were obtained from umbilical cord blood at birth or postnatally until 3 months of life and were directly compared with serum samples from the respective mothers. Congenital toxoplasmosis was diagnosed only when IgG-reactive protein bands that were present in any newborn serum samples were absent in the corresponding maternal serum sample. Congenital infection was defined by conventional serological assays when IgM and/or IgA antibodies were present in newborn infant blood or when IgG titers rose within the first 12 months or were persistently stable for more than 8 months. Using these criteria, congenital infection was definitely confirmed in 11 cases. Three additional cases were diagnosed based on indicative data. The CGMC test, which was performed without knowledge of the results of conventional serologal assays, had sensitivity and specificity of 82.4 and 93.0%, respectively, and positive and negative predictive values of 73.7 and 95.7%, respectively. When true positives and true negatives were considered, the comparative IgG profile had a ratio of 90.9% true results. The CGMC test thus is useful as an additional assay for the rapid diagnosis of congenital toxoplasmosis when paired serum samples from mother and child are available.
Whereas Toxoplasma gondii infection in the immunocompetent adult usually causes no serious clinical symptoms, congenital infection with this protozoan parasite can lead to abortion or severe disease in the newborn infant (17). However, only 10 to 15% of congenitally infected infants have clinical symptoms at birth (4, 5). More than 80% of infected infants who are clinically asymptomatic at birth may later develop sequelae (10, 24), which most often affect the eyes and which may be prevented when treatment is started early and continued during the first year of life (11). Therefore, early diagnosis of congenital toxoplasmosis is urgently demanded.
Diagnosis of prenatally acquired T. gondii infection is based mainly on serological tests. On one hand, the presence of specific immunoglobulin M (IgM), IgA and/or IgE antibodies in fetal blood is highly predictive of congenital toxoplasmosis (14, 16), although misinterpretation due to possible contamination by maternal blood during the first few days after birth has to be taken into consideration (10). On the other hand, the absence of specific IgM and/or IgA antibodies has been described for up to 33% (3, 12; G. Enders, unpublished data) of prenatally infected infants. This situation might especially occur (i) when the fetus has been infected early during pregnancy (1); (ii) when the immune system of the newborn is immature due to short-term pregnancy; or (iii) when maternal IgG, which is able to cross the placental barrier, suppresses the IgM response in the fetus (22). In asymptomatic newborns of mothers with serological evidence of acute infection during pregnancy, decision about treatment usually is made immediately, even though congenital infection has not been proven. In infants who are at high risk of congenital toxoplasmosis but who have no detectable IgM and/or IgA antibodies at birth, diagnosis must rely either on direct detection of the parasite in cord blood, cerebrospinal fluid, or body tissues by using culture techniques or on PCR (6, 8). Finally, a rise in IgG titers within the first 12 months of life or persistently positive IgG titers beyond the first 12 months of life define congenital toxoplasmosis (10). However, such determinations are time-consuming and not only imperil the infant's health but also increase parental anxiety.
We therefore analyzed in this study whether a comparison of specific IgG antibody profiles of serum samples from the newborn infant and its mother, as determined by the immunoblot technique, might be useful for the rapid diagnosis of congenital toxoplasmosis. We postulated that IgG antibodies which are present in an infant's serum sample(s) but absent in the mother's serum sample are generated by the newborn infant as a result of prenatally acquired infection. Since nonspecific so-called natural antibodies against T. gondii have been described to occur beyond 3 months of life (15), only serum samples drawn from infants before this time point were included in this study.
MATERIALS AND METHODS
We analyzed serum samples from 97 newborn infants who were delivered from mothers with a serological indication of recently acquired T. gondii infection (seroconversion, increase in IgG antibodies, and/or presence of IgM antibodies). Serum samples were obtained from umbilical cord blood or from newborn blood that was drawn after birth or at different times during the first 3 months of life. In addition, serum samples from the respective mothers were analyzed. Maternal sera were obtained during pregnancy, starting from week 12 of pregnancy; when a definite diagnosis could not be achieved at an early time, sera were obtained up to 2 months postpartum. For the CGMC test (comparative IgG profile between mother and child), 39 (40.2%) of the corresponding mother-infant serum samples were drawn on the same day, most of them (n = 31) on the day of birth. The time interval between drawing of the maternal sample and drawing of the infant sample for the other 58 paired samples spanned 3 to 47 weeks. Serological analysis for toxoplasmosis was carried out with well-established conventional serological methods and confirmed infection during the first trimester of pregnancy in 40 cases and during the third trimester in 28 cases. Serum samples obtained at birth or within 14 days after birth were available for 64 and 11 newborns, respectively. Since screening for toxoplasmosis is not mandatory in Germany, the initial serum samples from the remaining children were obtained between the ages of 1 and 3 months (n = 22).
In maternal serum samples, IgG antibodies were detected by a specific enzyme-linked immunosorbent assay (ELISA) (Cobas Core; Hoffmann-La Roche, Basel, Switzerland) (21) and/or a specific immunofluorescence assay (bioMérieux, Marcy l'Etoile, France) (23). For IgM antibody detection, an immunosorbent agglutination assay with a slight in-house modification (ISAGA; bioMérieux) (16) and a specific ELISA (Cobas Core or an ELISA from Sorin, Saluggia, Italy) were used. IgA antibodies were determined with an IgA-specific ELISA (Sorin). The avidity of IgG antibodies was assessed by an ELISA from Labsystems, Helsinki, Finland. Most tests were performed as suggested by the manufacturers.
In serum samples from newborns and infants, IgG antibodies were measured by the above-mentioned specific immunofluorescence assay and/or by the Cobas Core ELISA. IgM antibodies were determined by ISAGA, and IgA antibodies were determined by the ELISA from Sorin, using serum dilutions of 1:16 and 1:20, respectively. In all instances, initial and follow-up sera were tested simultaneously.
Congenital toxoplasmosis was defined as suggested by Lebech et al. (10) with a slight modification: identification of specific IgM and/or IgA antibodies in the newborn within the first 6 months of life and a rise in or persistence at high levels of specific IgG antibody titers during follow-up investigations. We classified the cases into three categories: (i) confirmed cases, where follow-up controls were available for more than 8 months after birth and where the CGMC test gave unambigious results when samples were repeatedly tested (n = 64) (group a), (ii) cases in which diagnosis was indicative but follow-up controls were available only for less than 8 months (n = 24) (group b), and (iii) cases in which the CGMC test gave inconsistent results when samples were repeatedly tested and thus normally would have been excluded from the interpretation for routine diagnosis (n = 9) (group c).
Serum samples from the newborn infants and their respective mothers were retrospectively analyzed in parallel by using a modification of a previously described immunoblot technique for the diagnosis of toxoplasma infection (7). Briefly, a total cell lysate of 5 × 105 T. gondii tachyzoites of parasite strain BK (25) per μl was separated by sodium dodecyl sulfate–11% polyacrylamide gel electrophoresis and transferred electrophoretically to 0.45-μm-pore-size nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Serum samples from the infants and their mothers were incubated overnight at a dilution of 1:100 with antigen-loaded nitrocellulose strips (equivalent to 2 × 106 parasites per strip), followed by 90 min of incubation with alkaline phosphatase-conjugated antihuman IgG antiserum (Dianova, Hamburg, Germany) at a dilution of 1:5,000. Finally, IgG-reactive protein bands were visualized by using 5-bromo-4-chloro-3-indolylphosphate incorporating nitroblue tetrazolium as the substrate. It was important to incubate serum samples from the infants and their mothers with neighboring nitrocellulose strips from identical immunoblots in order to directly compare reactive protein bands. Congenital toxoplasmosis was diagnosed only when at least one IgG-reactive protein band was present in any of the newborn serum samples but absent in the corresponding maternal serum samples, irrespective of the molecular mass of the reactive antigen. All immunoblotting reactions were performed at least twice to prove reproducibility, and immunoblots were read with no knowledge of the results of conventional toxoplasma serological analyses by at least two independent scientists.
RESULTS
The IgG profiles for a total cell lysate of T. gondii were directly compared between serum samples from 97 newborn infants at risk of congenital toxoplasmosis and serum samples from their corresponding mothers by using the immunoblot technique. Conventional serological analysis demonstrated congenital infection in 14 cases of group a, in 3 cases of group b, and in 2 cases of group c.
The absence of toxoplasma infection was diagnosed by conventional serological analysis for 50 cases of group a, for 21 cases of group b, and for 7 cases of group c (Table 1). IgG-reactive antigens that were present in serum samples from infants but absent in corresponding maternal serum samples could be identified correctly in 11 cases in which conventional serological analysis definitely confirmed congenital toxoplasmosis. Similarly, all three cases of congenital toxoplasmosis in group b could be diagnosed correctly by the CGMC test. In total, eight cases showed discordant results between conventional serological analysis and the CGMC test. In all cases, the newborn sample was drawn on the day of birth. Five of these cases demonstrated an IgG profile indicative of congenital infection but for which conventional serological analysis failed to diagnose diaplacental transmission of the parasite. Three of these paired mother-infant serum samples (60%) were drawn at birth. In the other two cases, maternal samples were drawn at week 21 or 28 of pregnancy. In three cases, the CGMC test failed to correctly diagnose congenital toxoplasmosis compared to conventional serological analysis. One of the corresponding maternal serum samples was drawn at week 34 of pregnancy; in the other two cases, maternal serum samples were obtained at the time of delivery.
TABLE 1.
Comparison of conventional serological analysis and comparative IgG profiles for mother and child
| IgG profile of congenital toxoplasmosis for: | No. of cases with the following result for serological analysis of congenital toxoplasmosis:
|
|
|---|---|---|
| Positive | Negative | |
| All cases (n = 88) | ||
| Positive | 14 | 5 |
| Negative | 3 | 66 |
| Group a (confirmed cases) (n = 64) | ||
| Positive | 11 | 2 |
| Negative | 3 | 48 |
| Group b (indicative cases) (n = 24) | ||
| Positive | 3 | 3 |
| Negative | 0 | 18 |
With conventional serological analysis as a standard, statistical analysis of the CGMC test revealed sensitivity and specificity of the comparative IgG profile of 82.4 and 93.0%, respectively, and positive and negative predictive values of 73.7 and 95.7%, respectively. When true positives and true negatives were considered, the comparative IgG profile had a ratio of 90.9% true results.
Since the definition of positivity was based on the presence of at least one IgG-reactive antigen present in the sample from the child and absent in the corresponding sample from the mother, interpretation of the CGMC test was simple and resulted in 100% agreement between different scientists who interpreted the test. However, a total of nine cases gave inconsistent results when repeatedly analyzed in the CGMC test (group c) and thus normally would not have been included from the interpretation for routine diagnosis; two and seven cases with inconsistent CGMC test results were found positive and negative for congenital toxoplasmosis by conventional serologic testing, respectively.
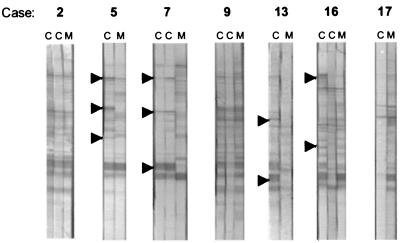
No preference of IgG antibodies that were generated by the child could be identified for the immunodominant toxoplasma antigen, SAG1/P30. In nearly all cases of congenital infection, the child developed IgG antibodies against at least two antigens of T. gondii. Although these antibodies were directed against antigens that varied in size, all of the IgG-reactive antigens were consistently larger than 30 kDa (Fig. 1).
FIG. 1.
Representative examples of comparative IgG profiles of mothers (M) and children (C). Arrowheads mark IgG-reactive antigens that were present in the child but absent in the mother.
Four of the 14 individuals with a confirmed diagnosis of congenital toxoplasmosis (group a) had symptoms such as retinochoroiditis or hydrocephalus at birth (Table 2). Eight infants with confirmed congenital toxoplasmosis were IgA and/or IgM positive at birth. With the CGMC test, four of the remaining individuals in whom IgA and IgM were negative would have been diagnosed well before the age of 3 months (Table 2). Otherwise, these cases would have been diagnosed later than 8 months after birth by the persistence of IgG antibodies. It is important to note that three of these individuals were asymptomatic at birth.
TABLE 2.
Characterization of cases with a confirmed diagnosis of congenital toxoplasmosis (n= 14)
| Case | Resulta for:
|
Clinical symptoms at birth | |||
|---|---|---|---|---|---|
| IgM | IgA | IgG persistence | CGMC test | ||
| 1 | + | + | NT | + | Retinochoroiditis |
| 2 | + | + | NT | + | Hydrocephalus |
| 3 | + | + | NT | + | Hydrocephalus |
| 4 | + | + | NT | + | None |
| 5 | + | + | NT | + | None |
| 6 | + | + | NT | + | None |
| 7 | + | + | NT | + | None |
| 8 | + | + | NT | − | None |
| 9 | − | − | + | + | Retinochoroiditis |
| 10 | − | − | + | + | None |
| 11 | − | − | + | + | None |
| 12 | − | − | + | + | None |
| 13 | − | − | + | − | None |
| 14 | − | − | + | − | None |
+, antibody positive; −, antibody negative; NT, not tested.
In contrast to the eight cases (57.1%) in which the standard early tests for infection (detection of IgM and/or IgA antibodies) correctly diagnosed congenital toxoplasmosis, the additional performance of the CGMC test largely improved the sensitivity of early detection of infants with congenital toxoplasmosis (85.7%). However, the combination of the IgM-IgA test with the CGMC test failed to diagnose congenital infection well before the age of 3 months in 2 of the 14 confirmed cases of congenital toxoplasmosis (Table 2). These two cases, however, became positive in the CGMC test when serum samples that were drawn at 3 months (case 14) or 8 months (case 13) of life were tested.
DISCUSSION
Diagnosis of congenital toxoplasmosis is problematic when specific IgM and/or IgA antibodies in serum samples from newborn infants delivered by mothers who have seroconverted are lacking (2). In those cases, diagnosis must rely on follow-up controls in order to identify rising or persistently high IgG antibody titers (10). This long follow-up time unnecessarily delays a definite diagnosis and leaves the parents in a state of uncertainty. Thus, it would be advantageous to develop a test that allows early confirmation of congenital infection; such a test would aid in decision making for therapy as well.
Since IgG antibodies can be transmitted via the placenta to the fetus, this antibody class was thought not to be useful for diagnosing congenital infections (20). Indeed, with well-established serological assays, such as, for example, the ELISA, differentiation between maternal IgG antibodies and IgG antibodies generated by the fetus or newborn infant had been impossible so far. A first approach to distinguishing between maternal IgG antibodies and those that are generated by the child was introduced by the use of an enzyme-linked immunofiltration assay (13). This test had a sensitivity of 80.5% when serum samples that were drawn at 1 month after birth were compared with maternal serum samples (13).
The immunoblot technique not only allows a direct comparison of IgG profiles between the mother and her child but also identifies antigens that are differentially recognized by the humoral immune responses of the mother and her child. In nearly all cases in which the CGMC test was positive, the child developed IgG antibodies that were directed against at least two different antigens of T. gondii that varied in size and that were consistently larger than 30 kDa. This fact is important, because it has been reported that serum samples from uninfected control individuals react with antigens of between 14 and 21 kDa (18). In this latter study, which was performed more than a decade ago, the sensitivity for diagnosing congenital toxoplasmosis by identification of IgG-reactive antibodies that were present in serum samples from newborn infants but absent in those from mothers was only 37.5% (18). Therefore, this approach was not investigated more intensively at that time. The reason for the significant difference in sensitivities between these two studies is unclear. However, antigen preparations, gel conditions, dilutions of patient sera, and sources of secondary antibodies were different. Finally, a similar approach in which vitreous fluid was compared with serum has also been successfully applied for the determination of intraocular synthesis of antibodies in ocular toxoplasmosis (19).
The CGMC test in combination with a determination of IgA and/or IgM antibodies at or early after birth resulted in a sensitivity of 85.7%, making this test useful as an additional assay for confirming congenital infection as early as possible. Timing of the maternal sample versus the infant sample might be critical for the CGMC test. It could be argued that if the maternal sample were obtained early in pregnancy, the immunoblot result might not reflect the entire spectrum of antigen reactivities that could develop and be transferred to the infant later in the pregnancy. This situation could result in false-positive CGMC results. Conversely, maternal blood obtained late after delivery might have new reactivities not present at the time of delivery and thus could result in false-negative CGMC results. However, a precise analysis of the eight cases in which the CGMC test had either a false-positive result or a false-negative result showed that most of the respective mother-infant samples were drawn on the day of delivery. In the three remaining cases, the maternal sample was obtained during week 21, 28, or 34 of pregnancy, making it unlikely that the timing of obtaining maternal blood has a greater influence on the CGMC test result.
The sensitivity of this combined test system could be increased by inclusion of serum samples which were drawn later than 3 months of life. However, since natural antibodies have been reported to occur after 3 months of life (15) and since early diagnosis is needed in order to initiate therapy, only CGMC test results that were obtained from umbilical cord blood or serum samples that were drawn between 1 and 3 months of life were included in this study.
It should be noted that in nine cases, contrary results were obtained when the CGMC test was repeatedly performed. Such inconsistent CGMC test results usually would have to be excluded from routine diagnosis, and such cases still would have to be diagnosed by methods based on conventional serological analysis. It is thus important to note that a control sample drawn from an infant 4 to 6 weeks later than the first sample always has to be investigated in order to confirm the diagnosis. Nevertheless, our results indicate that the CGMC test is a method that is easy to perform and that helps to shorten the period until a definite diagnosis can be obtained. Most importantly, the combination of the CGMC test with the standard early tests for infection (detection of IgM and/or IgA antibodies) seems to be useful for improving the sensitivity of the early diagnosis of congenital toxoplasmosis.
ACKNOWLEDGMENTS
We thank Jack S. Remington (Stanford University, Stanford, Calif.) and Eskild Petersen (Statens Serum Institute, Copenhagen, Denmark) for critically reading the manuscript and for helpful suggestions.
REFERENCES
- 1.Decoster A. Detection of IgA anti-P30 (SAG1) antibodies in acquired and congenital toxoplasmosis. Curr Top Microbiol Immunol. 1996;219:199–207. doi: 10.1007/978-3-642-51014-4_18. [DOI] [PubMed] [Google Scholar]
- 2.Decoster A, Caron A, Darcy F, Capron A. IgA antibodies against P30 as markers of congenital and acute toxoplasmosis. Lancet. 1988;i:1104–1107. doi: 10.1016/s0140-6736(88)90523-5. [DOI] [PubMed] [Google Scholar]
- 3.Decoster A, Darcy F, Caron A, Vinatier D, Houze-de-L'Aulnoit D, Vittu G, Niel G, Heyer F, Lecolier B, Delcroix M, et al. Anti-P30 antibodies as prenatal markers of congenital toxoplasma infection. Clin Exp Immunol. 1992;87:310–315. doi: 10.1111/j.1365-2249.1992.tb02993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desmonts G, Couvreur J. Congenital toxoplasmosis. A prospective study of 378 pregnancies. N Engl J Med. 1974;290:1110–1116. doi: 10.1056/NEJM197405162902003. [DOI] [PubMed] [Google Scholar]
- 5.Desmonts G, Couvreur J. Toxoplasmosis in pregnancy and its transmission to the fetus. Bull NY Acad Med. 1974;50:146–159. [PMC free article] [PubMed] [Google Scholar]
- 6.Groß U, Roggenkamp A, Janitschke K, Heesemann J. Improved sensitivity of the polymerase chain reaction for detection of Toxoplasma gondii in biological and human clinical specimens. Eur J Clin Microbiol Infect Dis. 1992;11:33–39. doi: 10.1007/BF01971268. [DOI] [PubMed] [Google Scholar]
- 7.Groß U, Roos T, Appoldt D, Heesemann J. Improved serological diagnosis of Toxoplasma gondii infection by detection of immunoglobulin A (IgA) and IgM antibodies against P30 by using the immunoblot technique. J Clin Microbiol. 1992;30:1436–1441. doi: 10.1128/jcm.30.6.1436-1441.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hohlfeld P, Daffos F, Costa J M, Thulliez P, Forestier F, Vidaud M. Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain-reaction test on amniotic fluid. N Engl J Med. 1994;331:695–699. doi: 10.1056/NEJM199409153311102. [DOI] [PubMed] [Google Scholar]
- 9.Koppe J G, Loewer-Sieger D H, de Roever-Bonnet H. Results of 20-year follow-up of congenital toxoplasmosis. Lancet. 1986;i:254–256. doi: 10.1016/s0140-6736(86)90785-3. [DOI] [PubMed] [Google Scholar]
- 10.Lebech M, Joynson D H M, Seitz H M, Thulliez P, Gilbert R E, Dutton G N, Ovlisen B, Petersen E. Classification system and case definitions of Toxoplasma gondii infection in immunocompetent pregnant women and their congenitally infected offspring. Eur J Clin Microbiol Infect Dis. 1996;15:799–804. doi: 10.1007/BF01701522. [DOI] [PubMed] [Google Scholar]
- 11.McAuley J, Boyer K M, Patel D, Mets M, Swisher C, Roizen N, Wolters C, Stein L, Stein M, Schey W, et al. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago collaborative treatment trial. Clin Infect Dis. 1994;18:38–72. doi: 10.1093/clinids/18.1.38. [DOI] [PubMed] [Google Scholar]
- 12.Naot Y, Desmonts G, Remington J S. IgM enzyme-linked immunosorbent assay test for the diagnosis of congenital Toxoplasma infection. J Pediatr. 1981;98:32–36. doi: 10.1016/s0022-3476(81)80528-8. [DOI] [PubMed] [Google Scholar]
- 13.Pinon J M, Chemla C, Villena I, Foudrinier F, Aubert D, Puygauthier-Toubas D, Leroux B, Dupouy D, Quereux C, Talmud M, Trenque T, Potron G, Pluot M, Remy G, Bonhomme A. Early neonatal diagnosis of congenital toxoplasmosis: value of comparative enzyme-linked immunofiltration assay immunological profiles and anti-Toxoplasma gondii immunoglobulin M (IgM) or IgA immunocapture and implications for postnatal therapeutic strategies. J Clin Microbiol. 1996;34:579–583. doi: 10.1128/jcm.34.3.579-583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinon J M, Toubas D, Marx C, Mougeot G, Bonnin A, Bonhomme A, Villaume M, Foudrinier F, Lepan H. Detection of specific immunoglobulin E in patients with toxoplasmosis. J Clin Microbiol. 1990;28:1739–1743. doi: 10.1128/jcm.28.8.1739-1743.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potasman I, Araujo F G, Remington J S. Toxoplasma antigens recognized by naturally occurring human antibodies. J Clin Microbiol. 1986;24:1050–1054. doi: 10.1128/jcm.24.6.1050-1054.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pratlong F, Boulot P, Villena I, Issert E, Tamby I, Cazenave J, Dedet J P. Antenatal diagnosis of congenital toxoplasmosis: evaluation of the biological parameters in a cohort of 286 patients. Br J Obstet Gynaecol. 1996;103:552–557. doi: 10.1111/j.1471-0528.1996.tb09805.x. [DOI] [PubMed] [Google Scholar]
- 17.Remington J S, Desmonts G. Toxoplasmosis. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. Philadelphia, Pa: The W. B. Saunders Co.; 1990. pp. 89–195. [Google Scholar]
- 18.Remington J S, Araujo F G, Desmonts G. Recognition of different Toxoplasma antigens by IgM and IgG antibodies in mothers and their congenitally infected newborns. J Infect Dis. 1985;155:1020–1024. doi: 10.1093/infdis/152.5.1020. [DOI] [PubMed] [Google Scholar]
- 19.Riss J M, Carboni M E, Franck J Y, Mary C J, Dumon H, Ridings B. Toxoplasmose oculaire: interet de l'immunoblot pour la mise en evidence d'une synthese intra-oculaire d'anticorps. Pathol Biol (Paris) 1995;43:772–778. [PubMed] [Google Scholar]
- 20.Roos T, Martius J, Groß U, Schrod L. Systematic serologic screening for toxoplasmosis in pregnancy. Obstet Gynecol. 1993;81:243–250. [PubMed] [Google Scholar]
- 21.Santaro F, Afchain D, Pierce R, Cesbron J Y, Ovlaque G, Capron A. Serodiagnosis of Toxoplasma infection using a purified parasite protein (P30) Clin Exp Immunol. 1985;62:262–269. [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki Y, Kobayashi A. Induction of tolerance to Toxoplasma gondii in newborn mice by maternal antibody. Parasitol Res. 1990;76:424–427. doi: 10.1007/BF00933551. [DOI] [PubMed] [Google Scholar]
- 23.Walton B C, Benchoff B M, Brooks W H. Comparison of indirect fluorescent antibody test and methylene blue dye test for detection of antibodies to Toxoplasma gondii. Am J Trop Med Hyg. 1966;15:149–152. doi: 10.4269/ajtmh.1966.15.149. [DOI] [PubMed] [Google Scholar]
- 24.Wilson C B, Remington J S, Stagno S, Reynolds D W. Development of adverse sequelae in children born with subclinical congenital Toxoplasma infection. Pediatrics. 1980;66:767–774. [PubMed] [Google Scholar]
- 25.Winser J, Verlinde J D, van Thiel H, Davel J, van der Elst P. Isolation of Toxoplasma from cerebrospinal fluid of a living infant in Holland. Proc Soc Exp Biol Med. 1948;67:292–294. doi: 10.3181/00379727-67-16279. [DOI] [PubMed] [Google Scholar]