Abstract
Selenium is a fascinating element that has a long history, most of which documents it as a deleterious element to health. In more recent years, selenium has been found to be an essential element in the diet of humans, all other mammals, and many other life forms. It has many health benefits that include, for example, roles in preventing heart disease and certain forms of cancer, slowing AIDS progression in HIV patients, supporting male reproduction, inhibiting viral expression, and boosting the immune system, and it also plays essential roles in mammalian development. Elucidating the molecular biology of selenium over the past 40 years generated an entirely new field of science which encompassed the many novel features of selenium. These features were (1) how this element makes its way into protein as the 21st amino acid in the genetic code, selenocysteine (Sec); (2) the vast amount of machinery dedicated to synthesizing Sec uniquely on its tRNA; (3) the incorporation of Sec into protein; and (4) the roles of the resulting Sec-containing proteins (selenoproteins) in health and development. One of the research areas receiving the most attention regarding selenium in health has been its role in cancer prevention, but further research has also exposed the role of this element as a facilitator of various maladies, including cancer.
Keywords: cancer, health, mouse models, selenium, selenocysteine (Sec), tRNA[Ser]Sec, Sec-tRNA[Ser]Sec, selenoproteins
1. Introduction
The element selenium was discovered in 1817 by the Swedish chemist, Jöns Jacob Berzelius [1]. He named selenium after the Greek goddess of the moon, Selene. This fascinating element has a long and unsavory history of use as a dietary component. Its first description as being deleterious for animals to ingest was reported by Marco Polo in the late 13th century [2]. In his travels in Western China, Marco Polo wrote about an illness that his “beasts of burden” acquired wherein their hooves became brittle and fell off after eating certain plants. These plants most likely were seleniferous plants, which absorb large quantities of selenium from the soil and store the selenium in their tissues. Such diseases as Polo described have been found in the 20th century in horses and cattle that grazed on the plains of the Dakota and Nebraska territories of the United States. For example, T.C. Madison, an army physician stationed at Fort Randall in Northern Nebraska in the mid-1850s, described a necrotic hoof disorder that, in addition, involved losses of hair in the mane and tail among the army horses that grazed on the plants around the fort [3].
Subsequently, Franke reported that this malady, which was found to be prevalent in the livestock living in these Great Plains states, resulted from these animals eating seleniferous plants that were rich in selenium absorbed from high levels of this element in the soil [4]. Interestingly, and to further document the harmful effects of selenium on the animals ingesting plants rich in this element, selenium poisoning in horses was thought to have played a role in General George Custer’s defeat at the Little Bighorn [5]. The military horses under Custer’s command had grazed freely on the plants surrounding the area where he and his men had waited prior to the battle; these plants were later found to be seleniferous plants. At the Battle of the Little Bighorn on 25 June 1876, Custer’s horses were reported to have had laminitis, which caused them to be lame, and led to Custer’s defeat [5].
Selenium’s role as a deleterious dietary element took a major turn for the good in 1957, when Schwarz and Foltz unexpectedly found that it prevented liver necrosis in rats [6]. The Schwartz and Foltz finding was followed by another interesting observation that selenium had an important role in anaerobic growth in Escherichia coli when this organism was grown on glucose [7]. It soon became obvious that selenium was an essential element in the diet of mammals and many other life forms when ingested in low levels, but harmful when ingested in high levels (see several chapters in [8] for an in-depth summary on these findings). The window between too little and too much selenium in the diet is rather narrow for most organisms.
Subsequently, the livestock industry found that the inclusion of selenium in the diet of livestock had many health benefits. These included enhanced fertility in male sheep and cattle, and importantly also the alleviation of numerous disorders such as white muscle disease and ill thrift in lambs and calves, pancreatic degeneration and exudative diathesis in fowl, and hepatosis dietetica in swine [9]. In many regions of the world where livestock are prevalent, the addition of selenium in the diets of livestock has saved this industry hundreds of millions of dollars [10]. With regard to humans, in certain regions in rural China, where the soil is deficient in selenium and hence the selenium status of the individuals living therein is suboptimal, maladies such as Keshan disease, a cardiomyopathy primarily in children, were found [11]. Similarly, Kashin–Beck disease, a chronic, endemic osteochondropathy, was found primarily in southwestern to northeastern China [12]. Keshan disease has been virtually eradicated in China by supplementing the diets of the populations residing in specific rural areas where the soil is deficient in selenium [13]. In the USA, the recommended daily amount of selenium is set forth by the Food and Nutrition Board of the National Academies of Medicine, and is 55 micrograms per day for men and women above 14 years of age. Women who are pregnant or lactating require 60 or 70 micrograms per day [14,15].
In addition to having roles in preventing heart disease and other muscle disorders, as well as enhancing male fertility, selenium was found to have roles as a chemopreventive agent in certain cancers [16,17,18,19], roles in boosting immune function [18,20], in suppressing viral expression [21], in slowing the development of AIDS in HIV positive patients [22] and in Simian Acquired Immunodeficiency Virus (SAIDS)-infected monkeys [23], and possibly in slowing the aging process [24].
Low molecular weight selenium-containing compounds (LMW selenocompounds) also have highly significant roles in providing benefits to mammals. The research carried out in this area constitutes a subfield within the selenium field. There are several excellent reviews on the benefits of LMW selenocompounds in health and numerous other aspects of these selenocompounds [25,26,27]. This topic will, therefore, not be further discussed herein.
Several seminal studies in the selenium field in the 1970s and 1980s provided the foundation for elucidating the molecular biology of selenium and established it as a separate and highly significant field in science. Initially, selenium was found to be an essential component of glutathione peroxidase 1 (GPX1) in 1973 [28,29], which was subsequently identified in clostridial glycine reductase as selenocysteine (Sec) [30]. Bovine GPX1 was sequenced in 1984, and the position of the Sec moiety was therefore established within the protein [31]. The gene sequences of mammalian Gpx1 [32] and bacterial glycine formate dehydrogenase [33] were determined, and the Sec residue in the corresponding proteins shown to be encoded by TGA in both genes.
Additional studies that played major roles in defining the molecular biology of selenium as a separate field of science rapidly developed, and encompassed how selenium made its way into protein as the 21st proteinogenic amino acid in the genetic code—the vast machinery dedicated to synthesizing Sec and incorporating it into protein—and the roles of the resulting Sec-containing proteins (selenoproteins) in health and development. One of the research areas receiving much attention has been the role of selenium in cancer prevention, but this finding has also exposed the potential role of this element as a facilitator of various maladies, including cancer. These aspects of the molecular biology of selenium are discussed herein.
2. Selenocysteine (Sec) tRNA[Ser]Sec
This Section describes various aspects of Sec tRNA[Ser]Sec (i.e., the transcription of tRNA[Ser]Sec, primary sequences of the two isoforms, their distributions, the synthesis of Sec on tRNA[Ser]Sec, and the incorporation of Sec into selenoproteins as the 21st amino acid in the genetic code). The reason Sec tRNA is designated tRNA[Ser]Sec is that it is initially aminoacylated with serine (Ser) by Ser-tRNA synthetase (SARS), and the Ser moiety is then uniquely converted to Sec on the tRNA (see Section 2.4 below).
2.1. Transcription of the tRNA[Ser]Sec Gene (Trsp)
Trsp is a single-copy gene in most genomes, but several organisms, including zebrafish, have more than one copy [34,35]. Transfer RNA[Ser]Sec is transcribed, like all canonical tRNAs, by RNA polymerase (Pol) III, except in Trypanosoma brucei which was reported to be transcribed by Pol II [36]. However, the promoter structure and TATA-box-binding protein utilization of tRNA[Ser]Sec are distinct from those of other tRNA genes [37,38]. While the transcription of canonical tRNA genes is dependent on the internal promoters, so called A- and B-boxes, the upstream promoters including TATA-boxes govern the transcription of tRNA[Ser]Sec and other TATA-less Pol III genes such as snU6 and 7SK. Trsp transcription is initiated at the first nucleotide within the gene, whereas all other tRNAs are transcribed with a leader sequence that must be removed by processing from the resulting transcript [39]. The tRNA[Ser]Sec transcript has a trailer sequence, and like all other tRNAs, the trailer must be processed to yield the primary sequence, wherein the ubiquitous CCA terminus is then added to prepare the completed transcript which is now ready for modification.
2.2. Primary Sequence of Sec tRNA[Ser]Sec
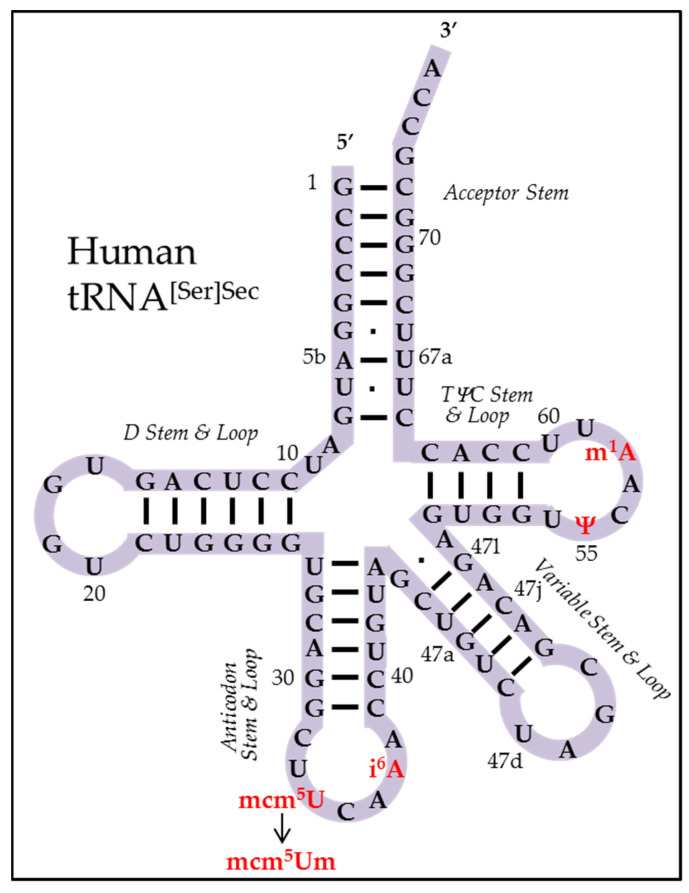
The primary sequence of Sec tRNA[Ser]Sec, which is the longest tRNA described to date, contains 90 or more nucleotides, depending on the species that encodes Trsp. In higher animals (e.g., Xenopus, birds, and mammals), four bases are modified on the 90 nucleotide primary transcript, and a portion of the Sec tRNA population is modified on the 2′-O-ribosyl moiety forming the only nucleoside modification (reviewed in [40]). The four base modifications are 5-methoxycarbonylmethyluracil (mcm5U) at position 34 (the wobble position in the anticodon), isopentenyladenosine (i6A) at position 37 (the base immediately 5′ to the anticodon), pseudouridine (Ψ) at position 55, and N1-methyladenosine (m1A) at position 58 (wherein the last two modifications occur within the TΨC loop). The single nucleoside modification occurs when a portion of the mcm5U isoform is converted to 5-methoxycarbonylmethyluracil-2′-O-methylribose (mcm5Um). The methylation of mcm5U to mcm5Um requires other modifications such as i6A and m1A, and the correct tertiary structure [41]. Interestingly, methylation of mcm5U is influenced by the selenium levels in the cell [41]. The primary structures of tRNA[Ser]Secmm5U and tRNA[Ser]Secmm5Um are shown in a cloverleaf form in Figure 1.
Figure 1.
Cloverleaf model of human tRNA[Ser]Sec. The image shows the 90 bases in human tRNA[Ser]Sec. The paired 5′ and 3′ terminal bases constitute the acceptor stem, and on the left portion of the tRNA, the D stem and loop constitute six paired and four unpaired bases. On the lower portion of the tRNA, the anticodon stem and loop constitute six paired and seven unpaired bases, and the variable stem and loop constitute five paired and four unpaired bases. On the right portion of the tRNA, the TΨC stem and loop constitute four paired and seven unpaired bases. Human tRNA[Ser]Sec contains base modifications at the following positions: 34 (mcm5U), 37 (i6A), 55 (Ψ), and 58 (m1A). The two isoforms differ from one another at position 34 by a single methyl group on the 2′-O-ribosyl moiety.
2.3. The Sec-tRNA[Ser]Sec Population
The Sec-tRNA[Ser]Sec populations in bacteria and archaea consist of a single tRNA that is aminoacylated with Ser by seryl-tRNA synthetase (SARS). The tRNA[Ser]Sec populations in mammals, birds, and Xenopus consist of two isoforms: tRNA[Ser]Secmcm5U and tRNA[Ser]Secmm5Um, both of which are aminoacylated with Ser by their corresponding SARS [18]. The levels of the two isoforms are not limiting; however, reducing the tRNA[Ser]Sec population by about half or increasing it several-fold does not generally appear to affect overall selenoprotein expression in various mammalian cells and mouse tissues [42]—albeit some minor differences in selenoprotein expression have been observed [43].
The levels of mcm5U are enriched and those of mcm5Um diminished under conditions of selenium deficiency in mammalian cells and organs, while the reverse is true under conditions of selenium sufficiency, i.e., the levels of mcm5Um are enriched, and the levels of mcm5U diminished. Interestingly, the two Sec-tRNA[Ser]Sec isoforms are involved in the synthesis of different classes of selenoproteins. Housekeeping selenoproteins, such as GPX4 and thioredoxin reductase 1 (TXNRD1), are essential to cellular function and are expressed even during selenium-deficient conditions. Housekeeping selenoproteins are expressed by the Sec-tRNA[Ser]Secmcm5U isoform. Stress-related selenoproteins, such as GPX1, are expressed in higher amounts in response to enriched selenium levels. This class of selenoproteins is expressed by the Sec-tRNA[Ser]Secmcm5Um isoform [43,44].
Detailed examinations of Ser-tRNA[Ser]Secmcm5U and Ser-tRNA[Ser]Secmcm5Um levels were carried out in various mammalian cell lines by growing human leukemia (HL-60) cells, Chinese hamster ovary (CHO) cells, and rat mammary tumor (RMT) cells in media with or without selenium supplementation (Table 1), and in various mammalian organs by subjecting mice to diets with or without selenium supplementation (Table 2). The respective tRNA populations were isolated from each cell line and from each mouse organ, labeled with 3H-serine, and the distributions of the two Sec isoforms were determined. The total amount of the two Ser-tRNA[Ser]Sec] isoforms varied considerably in the different cell lines and mammalian organs (Table 1 and Table 2), respectively. The consistent observation was that in every case the tRNA[Ser]Secmcm5U isoform was enriched in selenium-deficient conditions and the Ser-tRNA[Ser]Secmcm5Um isoform was enriched in selenium-sufficient conditions.
Table 1.
Sec-tRNA[Ser]Sec isoforms in cultured mammalian cells.
| Sec tRNA[Ser]Sec | |||||||
|---|---|---|---|---|---|---|---|
| mcm5U | mcm5Um | ||||||
| Cell Line | Selenium Supplementation a |
% of Total b | % | % of Total c | % | % of Total d | mcm5Um/ mcm5U e |
| HL-60 | +(chem. defined media) | 9.6 | 38.5 | 3.70 | 61.5 | 5.90 | 1.60 |
| −(chem. defined media) | 7.5 | 61.3 | 4.60 | 38.7 | 2.90 | 0.63 | |
| HL-60 | +(FBS) | 9.4 | 55.3 | 5.20 | 44.7 | 4.20 | 0.81 |
| −(FBS) | 7.4 | 77.0 | 5.70 | 23.0 | 1.70 | 0.30 | |
| CHO | +(FBS) | 1.01 | 45.1 | 0.46 | 54.9 | 0.55 | 1.22 |
| −(FBS) | 0.86 | 56.2 | 0.48 | 43.8 | 0.38 | 0.78 | |
| RMT | +(chem. defined media) | 1.7 | 11.8 | 0.20 | 88.2 | 1.50 | 7.47 |
| −(chem. defined media) | 1.4 | 35.7 | 0.50 | 64.3 | 0.90 | 1.80 | |
a FBS: fetal bovine serum; b percentage of tRNA[Ser]Sec population within total Ser-tRNA population; c percentages of mcm5U and mcm5Um isoforms within total tRNA[Ser]Sec population; d percentages of mcm5U or mcm5Um isoforms within total Ser-tRNA population; e amount of mcm5Um/amount of mcm5U isoforms.
Table 2.
Sec-tRNA[Ser]Sec isoforms in murine tissues.
| Sec tRNA[Ser]Sec | |||||||
|---|---|---|---|---|---|---|---|
| mcm5U | mcm5Um | ||||||
| Organ | Dietary Selenium Supplementation |
% of Total a | % | % of Total b | % | % of Total c | mcm5Um/ mcm5U d |
| Heart | + | 4.3 | 38.1 | 1.64 | 61.9 | 2.66 | 1.62 |
| − | 3.2 | 66.4 | 2.12 | 33.6 | 1.08 | 0.51 | |
| Kidney | + | 7.5 | 33.7 | 2.52 | 66.3 | 4.97 | 1.97 |
| − | 3.7 | 59.2 | 2.19 | 40.8 | 1.51 | 0.69 | |
| Liver | + | 4.5 | 33.3 | 1.50 | 66.7 | 3.00 | 2.00 |
| − | 2.8 | 57.7 | 1.62 | 42.3 | 1.18 | 0.73 | |
| Muscle | + | 1.9 | 38.6 | 0.73 | 61.4 | 1.17 | 1.59 |
| − | 1.5 | 73.3 | 1.10 | 26.7 | 0.40 | 0.35 | |
a Percentage of tRNA[Ser]Sec population within total Ser-tRNA population; b percentages of mcm5U and mcm5Um isoforms within total tRNA[Ser]Sec population; c percentages of mcm5U or mcm5Um isoforms within total Ser-tRNA population; d amount of mcm5Um/amount of mcm5U isoforms.
2.4. Biosynthesis of Sec on Sec tRNA[Ser]Sec
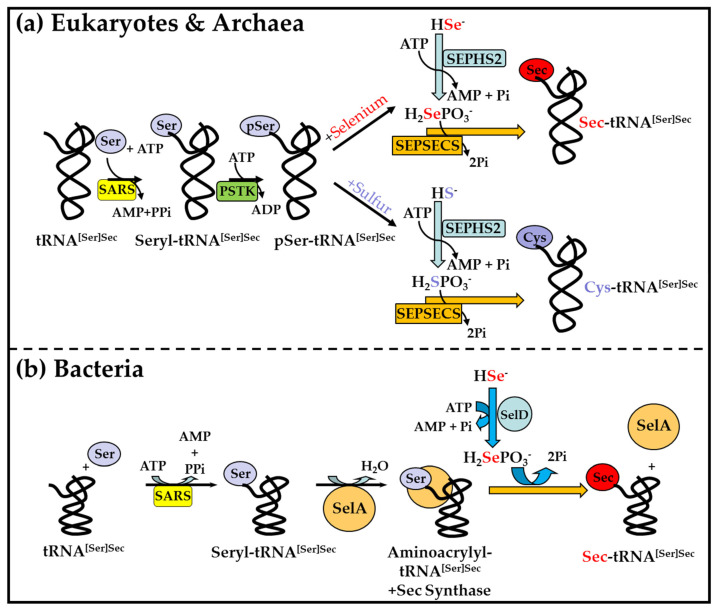
The incorporation of selenium into protein occurs as the amino acid Sec. This amino acid is biosynthesized in a unique manner on its tRNA, named Sec-tRNA[Ser]Sec [45,46]. The pathway of Sec biosynthesis is different in archaea and eukaryotes (Figure 2a), and in bacteria (Figure 2b). The unacylated tRNA[Ser]Sec is initially aminoacylated with Ser by SARS to form Ser-tRNA[Ser]Sec in all three groups. The Ser moiety on Ser-tRNA[Ser]Sec in archaea and eukaryotes (Figure 2a) is transferred to an intermediate, phosphorseryl-tRNA[Ser]Sec, by phosphoseryl-tRNA kinase (PSTK). The intermediate is then converted to Sec-tRNA[Ser]Sec in the presence of selenophosphate 2 (SEPHS2) (see [18] and references therein). On the other hand, bacteria use an enzyme, Sec synthetase (SecS or SepSecS), to convert Ser-tRNA[Ser]Sec to Sec-tRNA[Ser]Sec (Figure 2b). There is an abundance of complex machinery dedicated to incorporating Sec into protein in response to the UGA Sec codon in selenoprotein mRNA. This topic has been reviewed elsewhere in this Special Issue by Copeland and Howard [47], and, therefore, will not be further discussed herein. The insertion of Sec into protein to generate selenoproteins occurs in organisms within all three of the taxonomic domains, eukaryotes, archaea, and bacteria. Selenoproteins are found in only about 15% of archaea, about 25% of bacteria, and about half of eukaryotes [48].
Figure 2.
Pathways of Selenocysteine (Sec) biosynthesis. The biosynthetic pathways of Sec in: (a) eukaryotes and archaea; (b) bacteria. Abbreviations are: Pi—inorganic phosphate; PPi—inorganic pyrophosphate; SARS—Ser-tRNA synthetase; SelA—selenocysteine synthase; SelD—selenophosphate synthetase; H2SePO3−—selenophosphate; SEPHS2—selenophosphate synthetase 2.
2.5. Sec, the 21st Amino Acid in the Genetic Code
Sec constitutes the 21st proteinogenic amino acid in the genetic code, and Sec is encoded in selenoprotein mRNA, as noted above, by the genetic codeword UGA. UGA is a shared codon in the genetic code in organisms containing selenoproteins, and it designates either Sec or the cessation of protein synthesis depending on its location within the mRNA. A specific sequence called the Sec insertion sequence (SECIS) element, which is located immediately downstream of the Sec UGA codon in bacteria and located much further downstream of the Sec UGA codon in archaea and eukaryotes, is responsible for designating the upstream codon as Sec [49]. The classes of SECIS elements and their roles have been reviewed in detail elsewhere (see [18,45] and references therein) and will not be further considered herein.
3. Selenoproteins
3.1. Mammalian Selenoproteins
There are 25 selenoprotein genes in the human genome. They are highly conserved across mammals, with the only two known exceptions being GPX6 and SELENOV. GPX6 contains Cys instead of Sec in some species, including mice and rats, and SELENOV was lost in gorillas [50]. Some of the mammalian selenoproteins are shared with non-mammalian eukaryotes, including glutathione peroxidases (GPXs), thioredoxin reductases (TXNRDs), and selenophosphate synthetase (SEPHS), indicating an early evolutionary origin for these proteins [51].
3.1.1. Glutathione Peroxidases (GPX)
GPXs comprise a large superfamily that is widespread across all kingdoms of life [52]. They use glutathione or thiol oxidoreductases as major reductants, and their functions include detoxification of hydroperoxides, regulation of ferroptosis, and hydrogen hydroperoxide signaling, among others [51]. There are eight GPXs in mammals, five of which are selenoproteins (GPX1-4, GPX6), and three are Cys-containing homologs (GPX5, GPX7, and GPX8). GPX1 is the most abundant mammalian selenoprotein found in the cytosol of most cells. It was the first mammalian selenoprotein described, and it was instrumental in developing methods for the insertion of selenium in the form of Sec into proteins. GPX2 and GPX3 have a more localized expression. GPX2 is often termed gastrointestinal based on its initial detection in gastrointestinal tissues, and GPX3 is expressed in the kidney at very high levels and is secreted into the plasma. GPX4 is unique among the GPXs because it reduces phospholipid hydroperoxides and has a broad substrate specificity. It has received much attention recently due to its essentiality for embryonic development in mice [53,54] and its role as a master regulator of ferroptosis [55,56]. GPX6 is the most recently evolved Sec-containing GPX, present only in mammals. In mice and in a few other species, Sec was then replaced by Cys [50].
3.1.2. Thioredoxin Reductases (TXNRD)
There are three TXNRDs in mammals, all of which contain Sec, and their functions are selenium-dependent. Sec is located in the penultimate C-terminal position, as part of a characteristic GCUG motif [57]. TXNRD1 is primarily localized in the cytosol and nucleus, and uses thioredoxin 1 (TXN1) as a major substrate. Its main physiological role is the NADPH-dependent reduction of TXN1, which in turn is involved in many physiological processes. TXNRD2 is localized in the mitochondria, and it is involved in the reduction of mitochondrial thioredoxin (TXN2) and glutaredoxin 2 (GLRX2). Both TXNRD1 and TXNRD2 are present in all vertebrates and are essential in mice [58,59]. TXNRD3 contains an additional N-terminal GLRX domain, and displays glutaredoxin activity [60].
3.1.3. Iodothyronine Deiodinases (DIO)
The thyroid hormone deiodinases (DIO) consist of three selenoproteins (DIO1, DIO2, and DIO3) that are involved in the metabolism of thyroid hormones by iodothyronine deiodination [61]. Like most selenoproteins, they are thioredoxin-like proteins. Thyroid hormones regulate a variety of processes, including growth, development, and metabolic rate. Thyroxine (T4) is the main thyroid hormone in circulation, produced in the thyroid gland, and is the precursor of triiodothyronine (T3), which has a higher affinity for thyroid hormone receptors [62]. DIO1 and DIO2 catalyze the activation of T4 to T3. Conversely, DIO3, and in some conditions DIO1, can inactivate T3 by producing the inactive metabolites T2 and reverse T3 (rT3), respectively. Studies in deiodinase-deficient mice have confirmed the function of deiodinases for T3 formation [63,64,65]. Distantly related homologs have been identified in single-celled eukaryotes; however, their function is not known, but it must be different from that of mammalian deiodinases.
3.1.4. Methionine-R-Sulfoxide Reductase 1 (MSRB1)
MSRB1 is a zinc-containing selenoprotein that was initially identified as selenoprotein R [66] and selenoprotein X [67] using bioinformatic tools. It was later found to be methionine-R-sulfoxide reductase based on its repair activity on the R enantiomer of oxidized methionine residues in proteins. Methionine and cysteine are the two sulfur-containing amino acids that are the most susceptible to oxidation, which may lead to a significant alteration of their structure and the disruption of protein function. MSRB1 may protect proteins against oxidative damage by catalyzing the reduction of methionine sulfoxide back to methionine. Though structurally different to MSRA (methionine-S-sulfoxide reductase), both proteins perform complementary functions by acting in only one of the two stereoisomers. MSRA is also a selenoprotein in some unicellular eukaryotes. Two additional homologs, MSRB2 and MSRB3, which contain catalytic Cys instead of Sec, are present in mammals. MSRB2 is localized in the mitochondria, whereas MSRB3 is targeted to the endoplasmic reticulum [68].
3.1.5. Selenophosphate Synthetase 2 (SEPHS2)
As discussed above, SEPHS2 provides the active Se donor for the synthesis of Sec. Selenophosphate is synthesized from selenide and ATP [69]. SEPHS2 is a widespread protein found in all Sec-containing prokaryotes and eukaryotes. In prokaryotes, in addition to Sec, SEPHS2 also supports other forms of selenium utilization. Selenium is used in the form of selenouridine in certain tRNAs [70,71], and as a cofactor in some molybdenum-containing proteins [72,73]. In eukaryotes, Sec is the only known selenium trait.
A SEPHS2 paralog called SEPHS1 is found in some animals [74,75]. SEPHS1 is not a selenoprotein, it does not support selenophosphate and selenoprotein synthesis [76], and never carries Sec or Cys at the catalytic site. Instead, other amino acids have been observed at that position (arginine, threonine, glycine, and leucine). SEPHS1 is an essential gene in fruit flies [77] and mice [78], but its function remains unknown. The fact that SEPHS1 is present in selenoprotein-less animals [79,80] suggests that its function is not related to selenium. Nonetheless, it is believed to participate in redox homeostasis [78]. Interestingly, SEPHS1 genes in different animal lineages, e.g., insects and vertebrates, are believed to be functional homologs, but they originated independently [81].
3.1.6. Selenoprotein P (SELENOP)
SELENOP is the only selenoprotein with multiple Sec residues in mammals. It is a major selenoprotein in plasma and is synthesized primarily in the liver [82]. Its sequence contains a Sec-containing thioredoxin-like domain in its N-terminal region, and a Sec-rich domain in the C-terminus [83]. Its main function is to provide selenium to several tissues, especially the testis and brain [84,85]. SELENOP is present across metazoans, but its Sec content is highly variable. Among vertebrates, the number ranges from five in mole rats to up to 37 in amberjack fish. Human and mouse SELENOP contains 10 Sec residues. A remarkable diversity is observed in invertebrates. SELENOP was lost in most nematodes, most insects, tunicates, and Platyhelminthes, whereas in other lineages, SELENOP is particularly Sec-rich, including in annelids, ribbon worms (Nemertea), and mollusks. Topping the list is the bivalve Elliptio complanata with 133 Sec residues [86].
3.1.7. Selenoprotein N (SELENON)
SELENON (formerly SEPN1) is an endoplasmic reticulum (ER) glycoprotein that contains a calcium-binding EF-hand domain and a Sec-containing domain with a possible oxidoreductase function. The specific function of the protein remains unknown. One possible function that has been suggested is a calcium sensor through the EF-hand domain, which activates the sarcoplasmic/ER calcium ATPase 2 (SERCA2)-mediated calcium uptake into the ER in a redox-dependent manner [87]. SELENON was first identified using computational methods [67], and shortly after was associated with congenital rigid spine muscular dystrophy [88], becoming the first selenoprotein to be associated with a genetic disease. Mutations in SELENON cause a group of recessive neuromuscular disorders collectively known as SELENON-related myopathies [89]. Many mutations have been identified in homozygous or heterozygous compound patients, including mutations in the UGA codon and the SECIS element that prevent the incorporation of Sec [88,90,91].
3.1.8. Selenoprotein O (SELENOO)
SELENOO is a widespread selenoprotein present in both prokaryotes and eukaryotes. The mammalian homologs carry a Sec residue at their C-terminal penultimate position [92], while in bacteria and in many other eukaryotes, including yeast and plants, homologs have a Cys instead. Its sequence contains a protein kinase-like domain [93] but its function remained elusive [92]. A recent study [94] uncovered a novel activity for the protein kinase superfamily, wherein SELENOO transfers AMP from ATP to Ser, Thr, and Tyr residues on protein substrates, an activity termed AMPylation. The protein is localized in the mitochondria [95] and AMPylates proteins involved in redox homeostasis [94].
3.1.9. Selenoprotein I (SELENOI)
SELENOI is essential for embryonic development in mice [96]. It is a recently evolved selenoprotein only found in vertebrates [50]. Its sequence contains a CDP-alcohol phosphatidyltransferase domain, characteristic of phospholipid synthases. It was suggested that SELENOI may be an ethanolamine phosphotransferase that catalyzes the last step of the Kennedy pathway for the synthesis of phosphatidylethanolamine [97] and is localized in the Golgi apparatus [98]. However, this activity was reported for a protein truncated at Sec, so more studies are needed to establish the function of this selenoprotein. Mutations in SELENOI have been identified in patients with a form of hereditary spastic paraplegia [99,100].
3.1.10. Other Selenoproteins
Other selenoproteins have no known functions, albeit some suggested ones. SELENOW, SELENOT, SELENOH, and SELENOV belong to the redox family of selenoproteins [101]. They possess a CXXU motif within a thioredoxin-like fold domain. Based on this observation, they are proposed to be oxidoreductases of unknown functions. SELENOV is the most recently evolved selenoprotein, only present in placental mammals. It appeared by duplication of SELENOW; the two genes share the same exonic structure, but SELENOV contains a long highly variable N-terminal extension [50]. SELENOV is exclusively expressed in testis [92].
SELENOF and SELENOM are thioredoxin-like fold ER-resident selenoproteins. These proteins share ~30% of sequence identity in mammals and are distantly related homologs with a common evolutionary origin [51]. Their function, however, is not well understood. SELENOF may be involved in the regulation of protein folding, and its deletion promotes nuclear cataracts in mice [102]. Several studies examined its possible role in cancer [103,104]. Altered expression, both high and low, has been linked to a higher cancer risk in different tissues, including lung, breast, prostate, and liver [105]. Similarly, common genetic variants in SELENOF have been studied for their relationship with higher cancer risk [105]. SELENOM is expressed in the brain and shows neuroprotective properties. Altered levels of SELENOM have been linked to the early onset of Alzheimer’s disease and hepatocellular carcinoma [106]. In addition, it has also been implicated in calcium release from the ER in response to hydrogen peroxide [107]. Its deletion in mice leads to increased body weight but does not affect neuronal and cognitive function [108].
SELENOK and SELENOS share a few features that set them apart from other selenoproteins. They are ER-resident transmembrane proteins with a single transmembrane domain in the N-terminal sequence, contain a Gly-rich segment with positively charged residues, and their Sec residues are characteristically located near the C-terminus. They have been implicated in the ER-associated degradation (ERAD) of misfolded proteins [51]. SELENOK has also been proposed to link selenium levels and immunity through association with a partner enzyme, DHHC6, for protein palmitoylation [109].
3.2. Phylogenetic Distribution of Selenoproteins
Selenoproteins are present across the three domains of life: bacteria, archaea, and eukaryotes. The evidence supports that the Sec trait evolved only once: prokaryotes and eukaryotes use analogous Sec biosynthesis and insertion pathways, and some selenoprotein families are shared among bacteria, archaea, and eukaryotes. The use of Sec is not universal, however. In selenoprotein-less organisms, the functions of selenoproteins are typically replaced by Cys homologs and the Sec synthesis machinery genes are lost.
Among prokaryotes, it is estimated that 20–25% of bacteria use selenoproteins [81,110], and the proportion is even lower in archaea, with a narrow distribution of Sec-containing genomes [111]. Nonetheless, the closest relatives to eukaryotes, the archaeal Asgard lineage, was identified as the intermediate form between the prokaryotic and eukaryotic Sec insertion systems [60,112,113].
Sec is much more common among eukaryotes, and selenoproteins show a highly dynamic evolution in terms of Sec to Cys conversions and gene losses. A scattered pattern of the presence/absence of selenoproteins was already evident from the analysis of the early sequenced genomes [114,115]. Since then, thousands of genomes have been sequenced, and the use of bioinformatic tools for large-scale analyses has provided a much more detailed picture. No selenoproteins have been identified in land plants so far, although they are abundant in other Archaeplastida (plantae) lineages. Recent works have explored the diversity of selenoproteins in plantae and especially algae lineages, reporting novel eukaryotic selenoprotein families [116,117]. Fungi were traditionally thought to have lost all selenoproteins at the root of the lineage. This was recently challenged by the discovery of several Sec-containing fungi genomes, outlining multiple independent Sec- loss events, including in the yeast lineage [118]. Among animals, Sec losses have been reported in all major insect lineages [119,120], in mites [34], and in nematodes [121].
4. Mouse Models
In the early 2000s, various mouse models were developed to elucidate the role of selenoproteins in health and development [122,123,124,125]. These mouse models took advantage of the fact that selenoprotein expression is dependent on the presence of a single tRNA, Sec tRNA[Ser]Sec, and the fact that this tRNA is encoded as a single copy gene, designated Trsp [126]. Thus, by manipulating Trsp in numerous ways, several models were developed that identified the presence of two selenoprotein classes, housekeeping selenoproteins and stress-related selenoproteins, their cellular roles, and also the roles of various individual selenoproteins within these two classes.
4.1. Trsp Transgenic Mouse Models
The first mouse models that examined the role of Sec-tRNA[Ser]Sec in selenoprotein synthesis were created in 2001 and involved Trsp wild-type or mutant transgenes [92]. Three different constructs encoding either the wild-type or two different mutant transgenes were prepared. By substituting one of the bases within the anticodon loop of Trsp, the role of the mutant Sec-tRNA[Ser]Sec in selenoprotein expression could be assessed. Changing either the T at position 34 to A, or the A at position 37 to G, prevented the synthesis of the 2′-0-methyluridine at position 34 [124,127]. Therefore, these mutant mice carrying either mutant transgene permitted the evaluation of the methylribose in selenoprotein expression. Synthesis of stress-related selenoproteins was virtually abolished, while housekeeping selenoprotein expression was virtually unchanged. Stress-related selenoprotein synthesis, therefore, is dependent on the 2′-0-hydroxymethyl group, while housekeeping selenoprotein expression is carried out by Sec-tRNA[Ser]Secmcm5U. These studies did not resolve the question of whether Sec-tRNA[Ser]Secmcm5Um also supports housekeeping selenoprotein synthesis. However, it seems very plausible that this isoform can also synthesize the essential class of selenoproteins.
Various aspects of the effects of stress-related selenoprotein loss on health were also examined. Interestingly, reduced stress-related selenoprotein expression in G37 mutant mice was tissue-specific, wherein the loss was highly significant in the kidney and liver but not in the testes [124]. These mice were further studied regarding health issues and were found to be more susceptible to colon cancer [128], viral infection [129], and X-ray damage [130]. Crossing these mice with C3/TAg mice yielded offspring with accelerated rates of prostatic epithelial neoplasia, suggesting a protective role of selenoproteins in prostate cancer development [131]. Glucose intolerance was also observed in these G37 mice, which led to a diabetic-like phenotype [132].
4.2. Trsp Conditional Knockout Mouse Models
Although the total removal of Trsp in mice is embryonic lethal [122], the targeted removal of Trsp in specific tissues and organs provides an alternative model for examining the role of selenoproteins in health and development [123]. Highly significant functions of selenoproteins were elucidated in numerous organs and tissues such as skeletal muscle; heart and endothelial cells [133]; bone [134]; skin [135]; immune cells, including macrophages, hematopoietic tissues, and T cells [136,137,138]; neurons [139]; liver [82,140]; podocytes [141]; osteochondroprogenitor [134]; thyroid [142]; prostate [143]; kidney; and mammary glands [123]. For convenience to the reader, and to keep this review within the allotted length, we have summarized the major findings in each of the above in vivo Trsp conditional knockout studies in Table 3. It should also be noted that more in-depth, recent studies of endothelial cells in cell death have been carried out (see [144] in this Special Issue and references therein).
Table 3.
Trsp conditional knockout mouse models.
| Targeted Tissue or Organ 1 | Main Findings Regarding Role of Selenoproteins in Genetically-Altered Mice, Relative to Control Mice in the Study | Cre Promoter |
|---|---|---|
| Endothelial cells | Endothelial cell development/function: embryonic lethal. 14.5 d.p.c. embryos were smaller, more fragile, had poorly or under-developed vascular systems, limbs, head, and tail [133]. | TieTek2-Cre |
| Heart & Skeletal Muscle | Heart disease prevention: mice died from acute myocardial failure 12 days after birth. | MCK-Cre |
| Kidney | No increase in oxidative stress or nephropathy found in podocytes of selenoprotein-deficient mice [141]. | NPHS2-Cre |
| Liver | Liver function: severe hepatocellular degeneration—mice died between 1 and 3 months of age [82]. SELENOP and GPX3 were reduced in serum and kidney, supporting a selenium-transport role for liver-derived SELENOP [140]. Enhanced expression of phase II response genes compensated for loss of hepatic Trsp [145]. Mice used as controls to monitor selenium pools in kidney due to reduction of GPX3 imported from liver [146]. Secisbp2 gene inactivation was less detrimental than Trsp inactivation [147]. | Alb-Cre |
| Macrophages | Immune function: increased oxidative stress and expression of cytoprotective antioxidant and detoxification genes, accumulation of ROS levels, and impaired invasiveness. Altered expression of ECM and fibrosis-associated genes [148]. Balance of pro- and anti-inflammatory oxylipids during inflammation [149]. Selenoproteins protect mice from chemically-induced colitis by alleviating inflammation [150]. Role in epigenetic modulation of pro-inflammatory genes [151]. When infected with N. brasiliensis, selenium-supplemented KO mice showed a complete abrogation in M2-marker expression with a significant increase in intestinal worms and fecal eggs [152]. | LysM-Cre |
| Mammary glands | First Trsp conditional KO mouse, providing an important tool for elucidating the role of selenoproteins in health and development [123]. MMTV-Cre mice treated with DMBA had significantly more tumors, suggesting that selenoproteins protect against carcinogen-induced mammary cancer [153]. | MMTV-Cre; Wap-Cre |
| Neurons | Neuronal function: enhanced neuronal excitation followed by neurodegeneration of hippocampus. Cerebellar hypoplasia associated with degeneration of Purkinje and granule cells. Cerebellar interneurons essentially absent [139]. Selenoproteins required in post-mitotic neurons of the developing cerebellum [154]. | Tal-Cre; CamK-Cre |
| Osteo-chondroprogenitor | Kashin–Beck disease model: mice had post-natal growth retardation, chondrodysplasia, chondronecrosis, and delayed skeletal ossification characteristic of Kashin–Beck disease [134]. | Col2a1-Cre |
| Prostate | Mice developed PIN-like lesions and microinvasive carcinoma by 24 weeks, which were associated with loss of basement membrane, increased cell cycle, and apoptotic activity [143]. | PB-Cre4 |
| Skin | Role in skin and hair follicle development: runt phenotype, premature death, alopecia with flaky and fragile skin, epidermal hyperplasia with disturbed hair cycle, and an early regression of hair follicles [135]. | K14-Cre |
| T-cells | Immune function: reduction of mature T cells and a defect in T-cell-dependent antibody response. Antioxidant hyperproduction and suppression of T cell proliferation in response to T cell receptor stimulation [137]. | LCK-Cre |
| Thyroid | Mice lacking selenoproteins in thyrocytes showed increased oxidative stress in thyroid. Gross morphology remained intact for at least 6 months. Thyroid hormone levels remained normal in knockout mice; thyrotropin levels moderately elevated [142]. |
Pax8-Cre;
Tg-CreER |
1 Target organs/tissues in alphabetical order. Abbreviations: days-post-coitum (d.p.c.); 7,12-dimethylbenz[a]anthracene (DMBA); extracellular matrix (ECM); mouse mammary tumor virus (MMTV); prostatic intraepithelial neoplasia (PIN).
4.3. Trsp Knockout/Transgenic and Trsp Conditional Knockout/Transgenic Mouse Models
Models involving Trsp knockout and Trsp conditional knockout mice that were rescued with the Trsp wild-type transgene, or the G37 or A34 mutant transgenes, were constructed [125] to examine the ability of these transgenes to restore selenoprotein synthesis. As expected, the wild-type transgene completely restored selenoprotein biosynthesis, while the G37 mutant transgene restored housekeeping selenoprotein synthesis but virtually did not refurbish stress-related selenoprotein synthesis [125]. Importantly, these mice demonstrated unequivocally that stress-related selenoproteins are not essential to the livelihood of the animal, although these mice were found to be very susceptible to selenium status (see Table 4). Mice carrying the G37 mutant transgene appeared phenotypically normal, but male mice produced sperm with an abnormal morphology which accounted for their reduced fertility, while female mice produced smaller-sized litters than the corresponding wild-type mice.
Table 4.
Mouse models involving Trsp knockout (KO) or Trsp conditional KO mice rescued with wild-type (WT), G37 mutant, or A34 transgenes.
| Target Site | Model Description | Major Findings Observed in Genetically Altered Mice in Comparison to Control Mice |
|---|---|---|
| Whole Mouse | Trsp KO rescued with WT Trsp transgene | Selenoprotein synthesis was completely recovered [125]. |
| Trsp KO rescued with G37 Trsp transgene | Proper base modification in the anticodon is essential, as mutant mice synthesize stress-related selenoproteins very poorly. Male mutant mice show abnormal sperm and reduced fertility; females produced reduced litter size [43]. Trsp KO could not be rescued with A34 mutant transgene most likely due to misreading (see Text). | |
| Whole Mouse | Trsp KO rescued with promoter mutant Trsp transgene | Mice expressed tissue- and organ-specific amounts of tRNA[Ser]Sec. Lower levels of the mcm5Um isoform were observed in promoter mutant Trsp mice. Mice developed a similar neurological phenotype as SELENOP-KO mice and a reduced life span [157]. |
| Liver Alb-Cre | Trsp liver KO rescued with Trsp WT transgene | Selenoprotein synthesis was completely recovered [82]. |
| Trsp liver KO rescued with G37 mutant Trsp transgene | Housekeeping selenoprotein synthesis was recovered while stress-related selenoprotein synthesis was poorly recovered [82]. | |
| Trsp liver KO rescued with A34 mutant Trsp transgene | Housekeeping selenoprotein synthesis was recovered while stress-related selenoprotein synthesis was poorly recovered. Replacement of selenoprotein synthesis in conditional Trsp mutants resulted in normal gene expression of Phase II response enzymes [127,145]. |
The A in the wobble position of the anticodon in tRNA is converted to inosine (I) which decodes A, U, and C in the 3′ position of the corresponding codewords. Hence, this tRNA decodes UGA and the cysteine codons, UCU and UCC, and likely promotes misreading in protein synthesis, which most certainly accounts for the reason why Trsp negative mice could not be rescued with the A34 mutant transgene [155].
Additional mouse models lacking Trsp specifically in the liver and rescued with the wild-type transgene or G37 or A34 mutant transgenes were generated [82]. A mouse model involving the loss of Trsp, and rescued with a transgene carrying a deletion within the activator element, was also prepared [25]. The activator element is required for the binding of the transcription factor, STAF, to transcribe Sec tRNA[Ser]Sec [156]. The major findings of these studies are summarized in Table 4.
5. Conclusions
So much of the molecular biology of selenium has been resolved in the past 30 to 40 years (see chapters in [8]) that the selenium field has tapered off considerably. There is still much to be done, primarily in providing more detailed functions of numerous individual selenoproteins and in understanding the roles of LMW selenocompounds in health and development. Herein, we have assembled a variety of topics in the selenium field that involve the unique characteristics of transcribing Trsp, determining the primary sequences of Sec-tRNA[Ser]Secmcm5U and tRNA[Ser]Secmcm5Um isoforms and their roles in translating housekeeping and stress-related selenoproteins, discussing numerous aspects of the historical roles of selenium in health and disease, and the molecular biology of selenium and selenoproteins in health and development. We borrowed the title of the classic Italian Western “The Good, the Bad and the Ugly” to include as part of our title as it seemed to perfectly describe the historical roles of selenium in health. This element has its “good” (an essential element in the diet of mammals and many other life forms), its “bad” (the consequences of too little or too much selenium in the diet), and its “ugly” aspects (in some cases, selenium may promote cancer and other health disorders, and extreme selenium deficiency may be lethal). Likewise, selenoproteins also have their “good” (these proteins are responsible in large part for the many health benefits of selenium), their “bad” characteristics (they can promote many health disorders including cancer), and can be “ugly” (loss of several selenoproteins is lethal). It will be of considerable interest to witness the many discoveries in the selenium/selenoprotein field as they continue to unfold in the years to come.
Acknowledgments
We gratefully acknowledge Bradley A. Carlson for the many contributions he made to this review, and for his vast accomplishments in the selenium/selenoprotein research field that resulted in the more than 170 papers he has published, many of which are referenced herein.
Author Contributions
All authors contributed in all aspects of the preparation and writing of this review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Towson University’s Fisher College of Science and Mathematics and the Department of Biological Sciences, awarded to Petra Tsuji, and by grants from the National Institutes of Health, awarded to Vadim Gladyshev.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there are on conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berzelius J.J. Undersökning af en ny Mineral-kropp, funnen i de orenare sorterna af det I Falun tillverkade svafl et. Afhandlingar Fysik Kemi Och Mineral. 1818;6:42. [Google Scholar]
- 2.Marsden W. In: The travels of Marco Polo, the Venetian: The translation of Marsden Revised, with A Selection of His Notes. Wright T., editor. Franklin Classics; London, UK: 1854. [Google Scholar]
- 3.Madison T.C. Sanitary report—Fort Randall. In: Coolidge R.H., editor. Statistical Report on the Sickness and Mortality in the Army of the United States. United States, Surgeon General’s Office; Washington, DC, USA: 1856. pp. 37–41. 36th Congress Senate Executive Document. [Google Scholar]
- 4.Franke K.W. A new toxicant occurring naturally in certain samples of plant foodstuffs. J. Nutr. 1934;8:597. doi: 10.1093/jn/8.5.597. [DOI] [Google Scholar]
- 5.Hintz H.F., Thompson L.J. Custer, selenium and swainsonine. Veter Hum. Toxicol. 2000;42:242–243. [PubMed] [Google Scholar]
- 6.Schwarz K., Foltz C.M. Factor 3 Activity of Selenium Compounds. J. Biol. Chem. 1958;233:245–251. doi: 10.1016/S0021-9258(19)68065-8. [DOI] [PubMed] [Google Scholar]
- 7.Enoch H.G., Lester R.L. Effects of Molybdate, Tungstate, and Selenium Compounds on Formate Dehydrogenase and Other Enzyme Systems in Escherichia coli. J. Bacteriol. 1972;110:1032–1040. doi: 10.1128/jb.110.3.1032-1040.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatfield D.L., Schweizer U., Tsuji P.A., Gladyshev V.N., editors. Selenium Its Molecular Biology and Role in Human Health. 4th ed. Springer; New York, NY, USA: 2016. [Google Scholar]
- 9.Oldfield J.E. Selenium: A historical perspective. In: Hatfield D.L., Berry M.J., Gladyshev V.N., editors. Selenium—Its Molecular Biology and Role in Human Health. 2nd ed. Springer; New York, NY, USA: 2006. pp. 1–6. [Google Scholar]
- 10.Combs G.F., Yan L. Status of dietary selenium in cancer prevention. In: Hatfield D.L., Schweizer U., Tsuji P.A., Gladyshev V.N., editors. Selenium—Its Molecular Biology and Role in Human Health. 4th ed. Springer; New York, NY, USA: 2016. pp. 321–332. [Google Scholar]
- 11.Ge K., Xue A., Bai J., Wang S. Keshan disease-an endemic cardiomyopathy in China. Virchows Archiv A Pathol. Anat. Histopathol. 1983;401:1–15. doi: 10.1007/BF00644785. [DOI] [PubMed] [Google Scholar]
- 12.Yu F.F., Qi Z., Shang Y.-N., Ping Z.-G., Guo X. Prevention and control strategies for children Kashin-Beck disease in China: A systematic review and meta-analysis. Medicine. 2019;98:e16823. doi: 10.1097/MD.0000000000016823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H., Wang T., Li Q., Li D. Prevention of Keshan Disease by Selenium Supplementation: A Systematic Review and Meta-analysis. Biol. Trace Element Res. 2018;186:98–105. doi: 10.1007/s12011-018-1302-5. [DOI] [PubMed] [Google Scholar]
- 14.Institute of Medicine . Dietary Reference Intakes: Vitamin C, Vitamin E, Selenium, and Carotenoids. Food and Nutrition Board; Washington, DC, USA: 2000. [Google Scholar]
- 15.Peters K.M., Galinn S.E., Tsuji P.A. Selenium: Dietary Sources, Human Nutritional Requirements and Intake Across Populations. In: Hatfield D.L., Schweizer U., Tsuji P.A., Gladyshev V.N., editors. Selenium—Its Molecular Biology and Role in Human Health. 4th ed. Volume 1. Springer; New York, NY, USA: 2016. pp. 295–305. [Google Scholar]
- 16.Diwadkar-Navsariwala V., Diamond A.M. The Link between Selenium and Chemoprevention: A Case for Selenoproteins. J. Nutr. 2004;134:2899–2902. doi: 10.1093/jn/134.11.2899. [DOI] [PubMed] [Google Scholar]
- 17.Fairweather-Tait S.J., Bao Y., Broadley M., Collings R., Ford D., Hesketh J.E., Hurst R. Selenium in Human Health and Disease. Antioxid. Redox Signal. 2011;14:1337–1383. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- 18.Hatfield D.L., Tsuji P.A., Carlson B.A., Gladyshev V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014;39:112–120. doi: 10.1016/j.tibs.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rayman M.P. Proceedings of the Nutrition Society. Volume 64. CABI Publishing; Oxfordshire, UK: 2005. Selenium in cancer prevention: A review of the evidence and mechanism of action; pp. 527–542. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann P.R., Berry M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008;52:1273–1280. doi: 10.1002/mnfr.200700330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guillin O.M., Vindry C., Ohlmann T., Chavatte L. Selenium, Selenoproteins and Viral Infection. Nutrients. 2019;11:2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campa A., Shor-Posner G., Indachochea F., Zhang G., Lai H., Asthana D., Scott G.B., Baum M.K. Mortality risk in selenium-deficient HIV-positive children. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1999;20:508–513. doi: 10.1097/00042560-199904150-00015. [DOI] [PubMed] [Google Scholar]
- 23.Xu X.-M., Carlson B.A., Grimm T.A., Kutza J., Berry M.J., Arreola R., Fields K.H., Shanmugam I., Jeang K.-T., Oroszlan S., et al. Rhesus Monkey Simian Immunodeficiency Virus Infection as a Model for Assessing the Role of Selenium in AIDS. JAIDS J. Acquir. Immune Defic. Syndr. 2002;31:453–463. doi: 10.1097/00126334-200212150-00001. [DOI] [PubMed] [Google Scholar]
- 24.Cai Z., Zhang J., Li H. Selenium, aging and aging-related diseases. Aging Clin. Exp. Res. 2018;31:1035–1047. doi: 10.1007/s40520-018-1086-7. [DOI] [PubMed] [Google Scholar]
- 25.Bartolini D., Sancineto L., Fabro de Bem A., Tew K.D., Santi C., Radi R., Toquato P., Galli F. Selenocompounds in cancer therapy: An overview. Adv. Cancer Res. 2017;136:259–302. doi: 10.1016/bs.acr.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Whanger P.D. Selenocompounds in Plants and Animals and their Biological Significance. J. Am. Coll. Nutr. 2002;21:223–232. doi: 10.1080/07315724.2002.10719214. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira R.L.U., Sena-Evangelista K.C.M., de Azevedo E.P., Pinheiro F.I., Cobucci R.N., Pedrosa L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship with Diseases. Front. Nutr. 2021;8:685317. doi: 10.3389/fnut.2021.685317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flohe L., Günzler W., Schock H. Glutathione peroxidase: A selenoenzyme. FEBS Lett. 1973;32:132–134. doi: 10.1016/0014-5793(73)80755-0. [DOI] [PubMed] [Google Scholar]
- 29.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 30.Cone J.E., Del Rio R.M., Davis J.N., Stadtman T.C. Chemical characterization of the selenoprotein component of clostridial glycine reductase: Identification of selenocysteine as the organoselenium moiety. Proc. Natl. Acad. Sci. USA. 1976;73:2659–2663. doi: 10.1073/pnas.73.8.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Günzler W.A., Steffens G.J., Grossmann A., Kim S.-M.A., Ötting F., Wendel A., Flohé L. The Amino-Acid Sequence of Bovine Glutathione Peroxidase. Hoppe-Seyler’s Z Physiol. Chem. 1984;365:195–212. doi: 10.1515/bchm2.1984.365.1.195. [DOI] [PubMed] [Google Scholar]
- 32.Chambers I., Frampton J., Goldfarb P., Affara N., McBain W., Harrison P. The structure of the mouse glutathione peroxidase gene: The selenocysteine in the active site is encoded by the ‘termination’ codon, TGA. EMBO J. 1986;5:1221–1227. doi: 10.1002/j.1460-2075.1986.tb04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zinoni F., Birkmann A., Stadtman T.C., Bock A. Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc. Natl. Acad. Sci. USA. 1986;83:4650–4654. doi: 10.1073/pnas.83.13.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santesmasses D., Mariotti M., Guigó R. Computational identification of the selenocysteine tRNA (tRNASec) in genomes. PLoS Comput. Biol. 2017;13:e1005383. doi: 10.1371/journal.pcbi.1005383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X.M., Zhou X., Carlson B.A., Kim L.K., Huh T.L., Lee B.J., Hatfield D.L. The zebrafish genome contains two distinct selenocysteine tRNA[Ser]sec genes. FEBS Lett. 1999;454:16–20. doi: 10.1016/S0014-5793(99)00767-X. [DOI] [PubMed] [Google Scholar]
- 36.Aeby E., Ullu E., Yepiskoposyan H., Schimanski B., Roditi I., Mühlemann O., Schneider A. tRNASec is transcribed by RNA polymerase II in Trypanosoma brucei but not in humans. Nucleic Acids Res. 2010;38:5833–5843. doi: 10.1093/nar/gkq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J.M., Lee J.Y., Hatfield D.L., Lee B.J. Differential mode of TBP utilization in transcription of the tRNA[Ser]Sec gene and TATA-less class III genes. Gene. 1997;196:99–103. doi: 10.1016/S0378-1119(97)00211-4. [DOI] [PubMed] [Google Scholar]
- 38.Park J.M., Yang E.S., Hatfield L.L., Lee B.J. Analysis of the Selenocysteine tRNA[SER]SEC Gene Transcription in vitro Using Xenopus Oocyte Extracts. Biochem. Biophys. Res. Commun. 1996;226:231–236. doi: 10.1006/bbrc.1996.1338. [DOI] [PubMed] [Google Scholar]
- 39.Lee B.J., De-La-Pena-Cortines P., Tobian J.A., Zasloff M., Hatfield D. Unique pathway of expression of an opal suppressor phosphoserine tRNA. Proc. Natl. Acad. Sci. USA. 1987;84:6384–6388. doi: 10.1073/pnas.84.18.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlson B.A., Lee B.C., Tsuji P.A., Tobe R., Park J.M., Schweizer U., Gladyshev V.N., Hatfield D.L. Selenocysteine tRNA[Ser]Sec: From nonsense suppressor tRNA to the quintessential constituent in selenoprotein biosynthesis. In: Hatfield D.L., Schweizer U., Tsuji P.A., Gladyshev V.N., editors. Selenium—Its Molecular Biology and Role in Human Health. Springer; New York, NY, USA: 2016. [Google Scholar]
- 41.Kim L.K., Matsufuji T., Matsufuji S., Carlson B.A., Kim S.S., Hatfield D.L., Lee B.J. Methylation of the ribosyl moiety at position 34 of selenocysteine tRNA[Ser]Sec is governed by both primary and tertiary structure. RNA. 2000;6:1306–1315. doi: 10.1017/S1355838200000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chittum H.S., Baek H.J., Diamond A.M., Fernandez-Salguero P., Gonzalez F., Ohama T., Hatfield D.L., Kuehn M., Lee B.J. Selenocysteine tRNA[Ser]Sec Levels and Selenium-Dependent Glutathione Peroxidase Activity in Mouse Embryonic Stem Cells Heterozygous for a Targeted Mutation in the tRNA[Ser]Sec Gene. Biochemistry. 1997;36:8634–8639. doi: 10.1021/bi970608t. [DOI] [PubMed] [Google Scholar]
- 43.Carlson B.A., Xu X.-M., Gladyshev V.N., Hatfield D.L. Selective Rescue of Selenoprotein Expression in Mice Lacking a Highly Specialized Methyl Group in Selenocysteine tRNA. J. Biol. Chem. 2005;280:5542–5548. doi: 10.1074/jbc.M411725200. [DOI] [PubMed] [Google Scholar]
- 44.Carlson B.A. Um34 in selenocysteine tRNA is required for the expression of stress-related selenoproteins in mammals. In: Grosjean H., editor. Fine-tuning of RNA Functions by Modification and Editing. Volume 12. Topis in Current Genetics; Springer; Berlin/Heidelberg, Germany: 2005. pp. 431–438. [Google Scholar]
- 45.Xu X.-M., Carlson B.A., Mix H., Zhang Y., Saira K., Glass R.S., Berry M.J., Gladyshev V.N., Hatfield D.L. Biosynthesis of Selenocysteine on Its tRNA in Eukaryotes. PLoS Biol. 2006;5:e4. doi: 10.1371/journal.pbio.0050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan J., Palioura S., Salazar J.C., Su D., O’Donoghue P., Hohn M.J., Cardoso A., Whitman W., Söll D. RNA-dependent conversion of phosphoserine forms selenocysteine in eukaryotes and archaea. Proc. Natl. Acad. Sci. USA. 2006;103:18923–18927. doi: 10.1073/pnas.0609703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Copeland P.R., Howard M.T. Ribosome Fate during Decoding of UGA-Sec Codons. Int. J. Mol. Sci. 2021;22:13204. doi: 10.3390/ijms222413204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y., Gladyshev V.N. Comparative Genomics of Trace Element Dependence in Biology. J. Biol. Chem. 2011;286:23623–23629. doi: 10.1074/jbc.R110.172833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berry M.J., Banu L., Chen Y., Mandel S.J., Kieffer J.D., Harney J.W., Larsen P.R. Recognition of UGA as a selenocysteine codon in Type I deiodinase requires sequences in the 3′ untranslated region. Nat. Cell Biol. 1991;353:273–276. doi: 10.1038/353273a0. [DOI] [PubMed] [Google Scholar]
- 50.Mariotti M., Ridge P.G., Zhang Y., Lobanov A.V., Pringle T.H., Guigo R., Hatfield D.L., Gladyshev V.N. Composition and Evolution of the Vertebrate and Mammalian Selenoproteomes. PLoS ONE. 2012;7:e33066. doi: 10.1371/journal.pone.0033066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Labunskyy V.M., Hatfield D.L., Gladyshev V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014;94:739–777. doi: 10.1152/physrev.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toppo S., Vanin S., Bosello V., Tosatto S.C. Evolutionary and Structural Insights into the Multifaceted Glutathione Peroxidase (Gpx) Superfamily. Antioxid. Redox Signal. 2008;10:1501–1514. doi: 10.1089/ars.2008.2057. [DOI] [PubMed] [Google Scholar]
- 53.Imai H., Hirao F., Sakamoto T., Sekine K., Mizukura Y., Saito M., Kitamoto T., Hayasaka M., Hanaoka K., Nakagawa Y. Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem. Biophys. Res. Commun. 2003;305:278–286. doi: 10.1016/S0006-291X(03)00734-4. [DOI] [PubMed] [Google Scholar]
- 54.Yant L., Ran Q., Rao L., Van Remmen H., Shibatani T., Belter J.G., Motta L., Richardson A., Prolla T.A. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free. Radic. Biol. Med. 2003;34:496–502. doi: 10.1016/S0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 55.Ingold I., Berndt C., Schmitt S., Doll S., Poschmann G., Buday K., Roveri A., Peng X., Porto Freitas F.P., Seibt T., et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell. 2017;172:409–422.e21. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 56.Stockwell B.R., Angeli J.P.F., Bayir H., Bush A., Conrad M., Dixon S.J., Fulda S., Gascón S., Hatzios S.K., Kagan V.E., et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arnér E.S. Focus on mammalian thioredoxin reductases—Important selenoproteins with versatile functions. Biochim. Biophys. Acta BBA Gen. Subj. 2009;1790:495–526. doi: 10.1016/j.bbagen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 58.Conrad M., Jakupoglu C., Moreno S., Lippl S., Banjac A., Schneider M., Beck H., Hatzopoulos A.K., Just U., Sinowatz F., et al. Essential Role for Mitochondrial Thioredoxin Reductase in Hematopoiesis, Heart Development, and Heart Function. Mol. Cell. Biol. 2004;24:9414–9423. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jakupoglu C., Przemeck G.K.H., Schneider M., Moreno S., Mayr N., Hatzopoulos A.K., de Angelis M.H., Wurst W., Bornkamm G.W., Brielmeier M., et al. Cytoplasmic Thioredoxin Reductase Is Essential for Embryogenesis but Dispensable for Cardiac Development. Mol. Cell. Biol. 2005;25:1980–1988. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun Q.-A., Kirnarsky L., Sherman S., Gladyshev V.N. Selenoprotein oxidoreductase with specificity for thioredoxin and glutathione systems. Proc. Natl. Acad. Sci. USA. 2001;98:3673–3678. doi: 10.1073/pnas.051454398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bianco A.C., Salvatore D., Gereben B., Berry M.J., Larsen P.R. Biochemistry, Cellular and Molecular Biology, and Physiological Roles of the Iodothyronine Selenodeiodinases. Endocr. Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 62.Larsen P.R., Dick T.E., Markovitz B.P., Kaplan M.M., Gard T.G. Inhibition of intrapituitary thyroxine to 3.5.3′-triiodothyronine conversion prevents the acute suppression of thyrotropin release by thyroxine in hypothyroid rats. J. Clin. Investig. 1979;64:117–128. doi: 10.1172/JCI109430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez M.E., Karaczyn A., Stohn J.P., Donnelly W.T., Croteau W., Peeters R.P., Galton V.A., Forrest D., St Germain D., Hernandez A. The Type 3 Deiodinase Is a Critical Determinant of Appropriate Thyroid Hormone Action in the Developing Testis. Endocrinology. 2016;157:1276–1288. doi: 10.1210/en.2015-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schneider M.J., Fiering S.N., Pallud S.E., Parlow A.F., Germain D.L.S., Galton V.A. Targeted Disruption of the Type 2 Selenodeiodinase Gene (DIO2) Results in a Phenotype of Pituitary Resistance to T4. Mol. Endocrinol. 2001;15:2137–2148. doi: 10.1210/mend.15.12.0740. [DOI] [PubMed] [Google Scholar]
- 65.Schneider M.J., Fiering S.N., Thai B., Wu S.-Y., Germain E.S., Parlow A.F., Germain D.L.S., Galton V.A. Targeted Disruption of the Type 1 Selenodeiodinase Gene (Dio1) Results in Marked Changes in Thyroid Hormone Economy in Mice. Endocrinology. 2006;147:580–589. doi: 10.1210/en.2005-0739. [DOI] [PubMed] [Google Scholar]
- 66.Kryukov G., Kryukov V.M., Gladyshev V.N. New Mammalian Selenocysteine-containing Proteins Identified with an Algorithm That Searches for Selenocysteine Insertion Sequence Elements. J. Biol. Chem. 1999;274:33888–33897. doi: 10.1074/jbc.274.48.33888. [DOI] [PubMed] [Google Scholar]
- 67.Lescure A., Gautheret D., Carbon P., Krol A. Novel Selenoproteins Identified in Silico and in Vivo by Using a Conserved RNA Structural Motif. J. Biol. Chem. 1999;274:38147–38154. doi: 10.1074/jbc.274.53.38147. [DOI] [PubMed] [Google Scholar]
- 68.Kim H.-Y., Gladyshev V.N. Methionine Sulfoxide Reduction in Mammals: Characterization of Methionine-R-Sulfoxide Reductases. Mol. Biol. Cell. 2004;15:1055–1064. doi: 10.1091/mbc.e03-08-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Veres Z., Kim I., Scholz T., Stadtman T., Veres Z., Kim I., Scholz T., Stadtman T. Selenophosphate synthetase. Enzyme properties and catalytic reaction. J. Biol. Chem. 1994;269:10597–10603. doi: 10.1016/S0021-9258(17)34101-7. [DOI] [PubMed] [Google Scholar]
- 70.Ching W.-M., Wittwer A.J., Tsai L., Stadtman T.C. Distribution of two selenonucleosides among the selenium-containing tRNAs from Methanococcus vannielii. Proc. Natl. Acad. Sci. USA. 1984;81:57–60. doi: 10.1073/pnas.81.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Payne N.C., Geissler A., Button A., Sasuclark A.R., Schroll A.L., Ruggles E.L., Gladyshev V.N., Hondal R.J. Comparison of the redox chemistry of sulfur- and selenium-containing analogs of uracil. Free. Radic. Biol. Med. 2017;104:249–261. doi: 10.1016/j.freeradbiomed.2017.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haft D.H., Self W.T. Orphan SelD proteins and selenium-dependent molybdenum hydroxylases. Biol. Direct. 2008;3:1–6. doi: 10.1186/1745-6150-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y., Turanov A.A., Hatfield D.L., Gladyshev V.N. In silico identification of genes involved in selenium metabolism: Evidence for a third selenium utilization trait. BMC Genom. 2008;9:251. doi: 10.1186/1471-2164-9-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma C. Animal models of disease. Mod. Drug Discov. 2004;7:30–36. [Google Scholar]
- 75.Mariotti M., Santesmasses D., Guigó R. Evolution of selenophosphate synthetase. In: Hatfield D.L., Schweizer U., Tsuji P.A., Gladyshev V.N., editors. Selenium—Its Molecular Biology and Role in Human Health. 4th ed. Springer; New York, NY, USA: 2016. pp. 85–99. [Google Scholar]
- 76.Xu X.-M., Carlson B.A., Irons R., Mix H., Zhong N., Gladyshev V.N., Hatfield D.L. Selenophosphate synthetase 2 is essential for selenoprotein biosynthesis. Biochem. J. 2007;404:115–120. doi: 10.1042/BJ20070165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alsina B., Corominas M., Berry M., Baguna J., Serras F. Disruption of selenoprotein biosynthesis affects cell proliferation in the imaginal discs and brain of Drosophila melanogaster. J. Cell Sci. 1999;112:2875–2884. doi: 10.1242/jcs.112.17.2875. [DOI] [PubMed] [Google Scholar]
- 78.Na J., Jung J., Bang J., Lu Q., Carlson B.A., Guo X., Gladyshev V.N., Kim J.-H., Hatfield D.L., Lee B.J. Selenophosphate synthetase 1 and its role in redox homeostasis, defense and proliferation. Free. Radic. Biol. Med. 2018;127:190–197. doi: 10.1016/j.freeradbiomed.2018.04.577. [DOI] [PubMed] [Google Scholar]
- 79.Chapple C.E., Guigó R. Relaxation of Selective Constraints Causes Independent Selenoprotein Extinction in Insect Genomes. PLoS ONE. 2008;3:e2968. doi: 10.1371/journal.pone.0002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lobanov A.V., Hatfield D.L., Gladyshev V.N. Selenoproteinless animals: Selenophosphate synthetase SPS1 functions in a pathway unrelated to selenocysteine biosynthesis. Protein Sci. 2007;17:176–182. doi: 10.1110/ps.073261508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mariotti M., Santesmasses D., Capella-Gutierrez S., Mateo A., Arnan C., Johnson R., D’Aniello S., Yim S.H., Gladyshev V.N., Serras F., et al. Evolution of selenophosphate synthetases: Emergence and relocation of function through independent duplications and recurrent subfunctionalization. Genome Res. 2015;25:1256–1267. doi: 10.1101/gr.190538.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carlson B.A., Novoselov S.V., Kumaraswamy E., Lee B.J., Anver M.R., Gladyshev V.N., Hatfield D.L. Specific excision of the selenocysteine tRNA[Ser]Sec (Trsp) gene in mouse liver demonstrates an essential role of selenoproteins in liver function. J Biol. Chem. 2004;279:8011–8017. doi: 10.1074/jbc.M310470200. [DOI] [PubMed] [Google Scholar]
- 83.Schweizer U., Schomburg L., Köhrle J. Selenoprotein P and selenium distribution in mammals. In: Hatfield D.L., Schweizer U., Tsuji P.A., Gladyshev V.N., editors. Selenium—Its Molecular Biology and Role in Human Health. 4th ed. Springer; New York, NY, USA: 2016. pp. 261–274. [Google Scholar]
- 84.Hill K.E., Zhou J., McMahan W.J., Motley A.K., Atkins J., Gesteland R.F., Burk R.F. Deletion of Selenoprotein P Alters Distribution of Selenium in the Mouse. J. Biol. Chem. 2003;278:13640–13646. doi: 10.1074/jbc.M300755200. [DOI] [PubMed] [Google Scholar]
- 85.Motsenbocker M.A., Tappel A. A selenocysteine-containing selenium-transport protein in rat plasma. Biochim. Biophys. Acta BBA Gen. Subj. 1982;719:147–153. doi: 10.1016/0304-4165(82)90318-X. [DOI] [PubMed] [Google Scholar]
- 86.Baclaocos J., Santesmasses D., Mariotti M., Bierła K., Vetick M.B., Lynch S., McAllen R., Mackrill J.J., Loughran G., Guigó R., et al. Processive Recoding and Metazoan Evolution of Selenoprotein P: Up to 132 UGAs in Molluscs. J. Mol. Biol. 2019;431:4381–4407. doi: 10.1016/j.jmb.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chernorudskiy A., Varone E., Colombo S.F., Fumagalli S., Cagnotto A., Cattaneo A., Briens M., Baltzinger M., Kuhn L., Bachi A., et al. Selenoprotein N is an endoplasmic reticulum calcium sensor that links luminal calcium levels to a redox activity. Proc. Natl. Acad. Sci. USA. 2020;117:21288–21298. doi: 10.1073/pnas.2003847117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moghadaszadeh B., Petit N., Jaillard C., Brockington M., Roy S.Q., Merlini L., Romero N., Estournet B., Desguerre I., Chaigne D., et al. Mutations in SEPN1 cause congenital muscular dystrophy with spinal rigidity and restrictive respiratory syndrome. Nat. Genet. 2001;29:17–18. doi: 10.1038/ng713. [DOI] [PubMed] [Google Scholar]
- 89.Villar-Quiles R.N., von der Hagen M., Métay C., Gonzalez V., Donkervoort S., Bertini E., Castiglioni C., Chaigne D., Colomer J., Cuadrado M.L., et al. The clinical, histologic, and genotypic spectrum of SEPN1-related myopathy. Neurology. 2020;95:e1512–e1527. doi: 10.1212/WNL.0000000000010327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Allamand V., Richard P., Lescure A., Ledeuil C., Desjardin D., Petit N., Gartioux C., Ferreiro A., Krol A., Pellegrini N., et al. A single homozygous point mutation in a 3′untranslated region motif of selenoprotein N mRNA causes SEPN1-related myopathy. EMBO Rep. 2006;7:450–454. doi: 10.1038/sj.embor.7400648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maiti B., Arbogast S., Moyle M.W., Anderson C.B., Richard P., Guicheney P., Ferreiro A., Flanigan K., Howard M.T. A mutation in the SEPN1 selenocysteine redefinition element (SRE) reduces selenocysteine incorporation and leads toSEPN1-related myopathy. Hum. Mutat. 2009;30:411–416. doi: 10.1002/humu.20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kryukov G.V., Castellano S., Novoselov S.V., Lobanov A.V., Zehtab O., Guigó R., Gladyshev V.N. Characterization of Mammalian Selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 93.Dudkiewicz M., Szczepińska T., Grynberg M., Pawłowski K. A Novel Protein Kinase-Like Domain in a Selenoprotein, Widespread in the Tree of Life. PLoS ONE. 2012;7:e32138. doi: 10.1371/journal.pone.0032138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sreelatha A., Yee S.S., Lopez V.A., Park B.C., Kinch L.N., Pilch S., Servage K., Zhang J., Jiou J., Karasiewicz-Urbańska M., et al. Protein AMPylation by an Evolutionarily Conserved Pseudokinase. Cell. 2018;175:809–821.e19. doi: 10.1016/j.cell.2018.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Han S.-J., Lee B.C., Yim S.H., Gladyshev V.N., Lee S.-R. Characterization of Mammalian Selenoprotein O: A Redox-Active Mitochondrial Protein. PLoS ONE. 2014;9:e95518. doi: 10.1371/journal.pone.0095518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Avery J.C., Yamazaki Y., Hoffmann F.W., Folgelgren B., Hoffmann P.R. Selenoprotein I is essential for murine embryogenesis. Arch. Biochem. Biophys. 2020;689:108444. doi: 10.1016/j.abb.2020.108444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Horibata Y., Hirabayashi Y. Identification and characterization of human ethanolaminephosphotransferase. J. Lipid Res. 2007;48:503–508. doi: 10.1194/jlr.C600019-JLR200. [DOI] [PubMed] [Google Scholar]
- 98.Horibata Y., Ando H., Sugimoto H. Locations and contributions of the phosphotransferases EPT1 and CEPT1 to the biosynthesis of ethanolamine phospholipids. J. Lipid Res. 2020;61:1221–1231. doi: 10.1194/jlr.RA120000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Horibata Y., Elpeleg O., Eran A., Hirabayashi Y., Savitzki D., Tal G., Mandel H., Sugimoto H. EPT1 (selenoprotein I) is critical for the neural development and maintenance of plasmalogen in humans. J. Lipid Res. 2018;59:1015–1026. doi: 10.1194/jlr.P081620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahmed M.Y., Al-Khayat A., Al-Murshedi F., Al-Futaisi A., Chioza B.A., Fernandez-Murray J.P., Self J.E., Salter C.G., Harlalka G.V., Rawlins L.E., et al. A mutation ofEPT1 (SELENOI)underlies a new disorder of Kennedy pathway phospholipid biosynthesis. Brain. 2017;140:547–554. doi: 10.1093/brain/aww318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dikiy A., Novoselov S.V., Fomenko D.E., Sengupta A., Carlson B.A., Cerny R.L., Ginalski K., Grishin N.V., Hatfield D.L., Gladyshev V.N. SelT, SelW, SelH, and Rdx12: Genomics and Molecular Insights into the Functions of Selenoproteins of a Novel Thioredoxin-like Family. Biochemistry. 2007;46:6871–6882. doi: 10.1021/bi602462q. [DOI] [PubMed] [Google Scholar]
- 102.Kasaikina M.V., Fomenko D.E., Labunskyy V.M., Lachke S.A., Qiu W., Moncaster J.A., Zhang J., Wojnarowicz M.W., Natarajan S.K., Malinouski M., et al. Roles of the 15-kDa Selenoprotein (Sep15) in Redox Homeostasis and Cataract Development Revealed by the Analysis of Sep 15 Knockout Mice. J. Biol. Chem. 2011;286:33203–33212. doi: 10.1074/jbc.M111.259218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Canter J.A., Ernst S.E., Peters K.M., Carlson B.A., Thielman N.R.J., Grysczyk L., Udofe P., Yu Y., Cao L., Davis C.D., et al. Selenium and the 15kDa Selenoprotein Impact Colorectal Tumorigenesis by Modulating Intestinal Barrier Integrity. Int. J. Mol. Sci. 2021;22:10651. doi: 10.3390/ijms221910651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsuji P.A., Carlson B.A., Naranjo-Suarez S., Yoo M.-H., Xu X.-M., Fomenko D.E., Gladyshev V.N., Hatfield D.L., Davis C.D. Knockout of the 15 kDa Selenoprotein Protects against Chemically-Induced Aberrant Crypt Formation in Mice. PLoS ONE. 2012;7:e50574. doi: 10.1371/journal.pone.0050574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carlson B.A., Hartman J.M., Tsuji P.A. The 15 kDa Selenoprotein: Insights into Its Regulation and Function. In: Hatfield D.L., Schweizer U., Tsuji P.A., Gladyshev V.N., editors. Selenium—Its Molecular Biology and Role in Human Health. 4th ed. Springer; New York, NY, USA: 2016. pp. 235–243. [Google Scholar]
- 106.Gong T., Berry M.J., Pitts M.W. Selenoprotein M: Structure, Expression and Functional Relevance. In: Hatfield D.L., Schweizer U., Tsuji P.A., Gladyshev V.N., editors. Selenium—Its Molecular Biology and Role in Human Health. 4th ed. Springer; New York, NY, USA: 2016. pp. 253–260. [Google Scholar]
- 107.Reeves M.A., Bellinger F.P., Berry M.J. The Neuroprotective Functions of Selenoprotein M and its Role in Cytosolic Calcium Regulation. Antioxid. Redox Signal. 2010;12:809–818. doi: 10.1089/ars.2009.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pitts M.W., Reeves M.A., Hashimoto A.C., Ogawa A., Kremer P., Seale L.A., Berry M.J. Deletion of Selenoprotein M Leads to Obesity without Cognitive Deficits. J. Biol. Chem. 2013;288:26121–26134. doi: 10.1074/jbc.M113.471235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fredericks G.J., Hoffmann F.W., Hondal R.J., Rozovsky S., Urschitz J., Hoffmann P.R. Selenoprotein K Increases Efficiency of DHHC6 Catalyzed Protein Palmitoylation by Stabilizing the Acyl-DHHC6 Intermediate. Antioxidants. 2017;7:4. doi: 10.3390/antiox7010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peng T., Lin J., Xu Y.-Z., Zhang Y. Comparative genomics reveals new evolutionary and ecological patterns of selenium utilization in bacteria. ISME J. 2016;10:2048–2059. doi: 10.1038/ismej.2015.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin J., Peng T., Jiang L., Ni J.-Z., Liu Q., Chen L., Zhang Y. Comparative genomics reveals new candidate genes involved in selenium metabolism in prokaryotes. Genome Biol. Evol. 2015;7:664–676. doi: 10.1093/gbe/evv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu Y., Makarova K.S., Huang W.-C., Wolf Y.I., Nikolskaya A.N., Zhang X., Cai M., Zhang C.-J., Xu W., Luo Z., et al. Expanded diversity of Asgard archaea and their relationships with eukaryotes. Nat. Cell Biol. 2021;593:553–557. doi: 10.1038/s41586-021-03494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mariotti M., Lobanov A.V., Manta B., Santesmasses D., Bofill A., Guigó R., Gabaldón T., Gladyshev V.N. Lokiarchaeota Marks the Transition between the Archaeal and Eukaryotic Selenocysteine Encoding Systems. Mol. Biol. Evol. 2016;33:2441–2453. doi: 10.1093/molbev/msw122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lobanov A.V., Fomenko D.E., Zhang Y., Sengupta A., Hatfield D.L., Gladyshev V.N. Evolutionary dynamics of eukaryotic selenoproteomes: Large selenoproteomes may associate with aquatic life and small with terrestrial life. Genome Biol. 2007;8:1–16. doi: 10.1186/gb-2007-8-9-r198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lobanov A.V., Hatfield D.L., Gladyshev V.N. Eukaryotic selenoproteins and selenoproteomes. Biochim. Biophys. Acta BBA Gen. Subj. 2009;1790:1424–1428. doi: 10.1016/j.bbagen.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jiang L., Lu Y., Zheng L., Li G., Chen L., Zhang M., Ni J., Liu Q., Zhang Y. The algal selenoproteomes. BMC Genom. 2020;21:1–16. doi: 10.1186/s12864-020-07101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liang H., Wei T., Xu Y., Li L., Sahu S.K., Wang H., Li H., Fu X., Zhang G., Melkonian M., et al. Phylogenomics Provides New Insights into Gains and Losses of Selenoproteins among Archaeplastida. Int. J. Mol. Sci. 2019;20:3020. doi: 10.3390/ijms20123020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mariotti M., Salinas G., Gabaldón T., Gladyshev V.N. Utilization of selenocysteine in early-branching fungal phyla. Nat. Microbiol. 2019;4:759–765. doi: 10.1038/s41564-018-0354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mariotti M. Short Views on Insect Genomics and Proteomics. Volume 4. Springer; Cham, Switzerland: 2016. Selenocysteine extinction in insect; pp. 113–140. [Google Scholar]
- 120.Rispe C., Legeai F., Nabity P.D., Fernández R., Arora A.K., Baa-Puyoulet P., Banfill C.R., Bao L., Barberà M., Bouallègue M., et al. The genome sequence of the grape phylloxera provides insights into the evolution, adaptation, and invasion routes of an iconic pest. BMC Biol. 2020;18:90. doi: 10.1186/s12915-020-00820-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Otero L., Romanelli-Cedrez L., Turanov A.A., Gladyshev V.N., Miranda-Vizuete A., Salinas G. Adjustments, extinction, and remains of selenocysteine incorporation machinery in the nematode lineage. RNA. 2014;20:1023–1034. doi: 10.1261/rna.043877.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bösl M.R., Takaku K., Oshima M., Nishimura S., Taketo M.M. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp) Proc. Natl. Acad. Sci. USA. 1997;94:5531–5534. doi: 10.1073/pnas.94.11.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kumaraswamy E., Carlson B.A., Morgan F., Miyoshi K., Robinson G.W., Su D., Wang S., Southon E., Tessarollo L., Lee B.J., et al. Selective removal of the selenocysteine tRNA [Ser]Sec gene (Trsp) in mouse mammary epithelium. Mol. Cell Biol. 2003;23:1477–1488. doi: 10.1128/MCB.23.5.1477-1488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moustafa M.E., Carlson B.A., El-Saadani M.A., Kryukov G.V., Sun Q.-A., Harney J.W., Hill K.E., Combs G.F., Feigenbaum L., Mansur D.B., et al. Selective Inhibition of Selenocysteine tRNA Maturation and Selenoprotein Synthesis in Transgenic Mice Expressing Isopentenyladenosine-Deficient Selenocysteine tRNA. Mol. Cell. Biol. 2001;21:3840–3852. doi: 10.1128/MCB.21.11.3840-3852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moustafa M.E., Kumaraswamy E., Zhong N., Rao M., Carlson B.A., Hatfield D.L. Models for assessing the role of selenoproteins in health. J. Nutr. 2003;133:2494S–2496S. doi: 10.1093/jn/133.7.2494S. [DOI] [PubMed] [Google Scholar]
- 126.Hatfield D.L., Gladyshev V., Park J., Park S., Chittum H., Huh J., Carlson B., Kim M., Moustafa M., Lee B.J. Biosynthesis of selenocysteine and its incorporation into protein as the 21st amino acid. In: Barton D., Nakanishi K., Meth-Cohn O., Kelly J.W., editors. Comprehensive Natural Products Chemistry. Volume 4. Amino Acids, Peptides, Porphyrins, and Alkaloids; Pergamon; Elsevier Science; Oxford, UK: 1999. pp. 353–380. [Google Scholar]
- 127.Carlson B.A., Moustafa M., Sengupta A., Schweizer U., Shrimali R., Rao M., Zhong N., Wang S., Feigenbaum L., Lee B.J., et al. Selective Restoration of the Selenoprotein Population in a Mouse Hepatocyte Selenoproteinless Background with Different Mutant Selenocysteine tRNAs Lacking Um. J. Biol. Chem. 2007;282:32591–32602. doi: 10.1074/jbc.M707036200. [DOI] [PubMed] [Google Scholar]
- 128.Irons R., Carlson B.A., Hatfield D.L., Davis C.D. Both selenoproteins and low molecular weight selenocompounds reduce colon cancer risk in mice with genetically impaired selenoprotein expression. J. Nutr. 2006;135:1311–1317. doi: 10.1093/jn/136.5.1311. [DOI] [PubMed] [Google Scholar]
- 129.Sheridan P.A., Zhong N., Carlson B.A., Perella C.M., Hatfield D.L., Beck M.A. Decreased selenoprotein expression alters the immune response during influenzavirus infection in mice. J. Nutr. 2007;137:1466–1471. doi: 10.1093/jn/137.6.1466. [DOI] [PubMed] [Google Scholar]
- 130.Baliga M.S., Diwadkar-Navsariwala V., Koh T., Fayad R., Fantuzzi G., Diamond A.M. Selenoprotein deficiency enhances radiation-induced micronucleiformation. Mol. Nutr. Food Res. 2008;52:1300–1304. doi: 10.1002/mnfr.200800020. [DOI] [PubMed] [Google Scholar]
- 131.Diwadkar-Navsariwala V., Prins G.S., Swanson S.M., Birch L.A., Ray V.H., Hedayat S., Lantvit D.L., Diamond A.M. Selenoprotein deficiency accelerates pros-tate carcinogenesis in a transgenic model. Proc. Natl. Acad. Sci. USA. 2006;103:8179–8184. doi: 10.1073/pnas.0508218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Labunskyy V., Lee B.C., Handy D., Loscalzo J., Hatfield D.L., Gladyshev V.N. Both Maximal Expression of Selenoproteins and Selenoprotein Deficiency Can Promote Development of Type 2 Diabetes-Like Phenotype in Mice. Antioxid. Redox Signal. 2011;14:2327–2336. doi: 10.1089/ars.2010.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shrimali R.K., Weaver J.A., Miller G.F., Starost M.F., Carlson B.A., Novoselov S.V., Kumaraswamy E., Gladyshev V.N., Hatfield D.L. Selenoprotein expression is essential in endothelial cell development and cardiac muscle function. Neuromuscul. Disord. 2007;17:135–142. doi: 10.1016/j.nmd.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Downey C.M., Horton C.R., Carlson B.A., Parsons T.E., Hatfield D.L., Hallgrímsson B., Jirik F.R. Osteo-Chondroprogenitor–Specific Deletion of the Selenocysteine tRNA Gene, Trsp, Leads to Chondronecrosis and Abnormal Skeletal Development: A Putative Model for Kashin-Beck Disease. PLoS Genet. 2009;5:e1000616. doi: 10.1371/journal.pgen.1000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sengupta A., Lichti U.F., Carlson B.A., Ryscavage A.O., Gladyshev V.N., Yuspa S.H., Hatfield D.L. Selenoproteins are essential for proper keratinocyte function andskin development. PLoS ONE. 2010;5:e12249. doi: 10.1371/journal.pone.0012249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kawatani Y., Suzuki T., Shimizu R., Kelly T.K., Yamamoto M. Nrf2 and selenoproteins are essential for maintaining oxidative homeostasis in erythrocytes andprotecting against hemolytic anemia. Blood. 2011;117:986–996. doi: 10.1182/blood-2010-05-285817. [DOI] [PubMed] [Google Scholar]
- 137.Shrimali R.K., Irons R.D., Carlson B.A., Sano Y., Gladyshev V.N., Park J.M., Hatfield D.L. Selenoproteins Mediate T Cell Immunity through an Antioxidant Mechanism. J. Biol. Chem. 2008;283:20181–20185. doi: 10.1074/jbc.M802559200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Suzuki T., Kelly V.P., Motohashi H., Nakajima O., Takahashi S., Nishimura S., Yamamoto M. Deletion of the selenocysteine tRNA gene in macrophages and liver results in compensatory gene induction of cytoprotective enzymes by Nrf2. J. Biol. Chem. 2008;283:2021–2030. doi: 10.1074/jbc.M708352200. [DOI] [PubMed] [Google Scholar]
- 139.Wirth E.K., Conrad M., Winterer J., Wozny C., Carlson B.A., Roth S., Schmitz D., Bornkamm G.W., Coppola V., Tessarollo L., et al. Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. FASEB J. 2010;24:844–852. doi: 10.1096/fj.09-143974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schweizer U., Streckfuß F., Pelt P., Carlson B.A., Hatfield D.L., Köhrle J., Schomburg L. Hepatically derived selenoprotein P is a key factor for kidney but not for brain selenium supply. Biochem. J. 2005;386:221–226. doi: 10.1042/BJ20041973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Blauwkamp M.N., Yu J., Schin M.A., Burke K.A., Berry M.J., Carlson B.A., Brosius III F.C., Koenig R.J. Podocyte specific knock out of selenoproteins does not enhance nephropathy in streptozotocin diabetic C57BL/6 mice. BMC Nephrol. 2008;9:7. doi: 10.1186/1471-2369-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chiu-Ugalde J., Wirth E.K., Klein M.O., Sapin R., Fradejas-Villar N., Renko K., Schomburg L., Köhrle J., Schweizer U. Thyroid Function Is Maintained Despite Increased Oxidative Stress in Mice Lacking Selenoprotein Biosynthesis in Thyroid Epithelial Cells. Antioxid. Redox Signal. 2012;17:902–913. doi: 10.1089/ars.2011.4055. [DOI] [PubMed] [Google Scholar]
- 143.Luchman H.A., Villemaire M.L., Bismar T.A., Carlson B.A., Jirik F.R. Prostate epithelium-specific deletion of the selenocysteine tRNA gene Trsp leads to early onset intraepithelial neoplasia. Am. J. Pathol. 2014;184:871–877. doi: 10.1016/j.ajpath.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jung J., Kim Y., Na J., Qiao L., Bang J., Kwon D., Yoo T.-J., Kang D., Kim L.K., Carlson B.A., et al. Constitutive Oxidative Stress by SEPHS1 Deficiency Induces Endothelial Cell Dysfunction. Int. J. Mol. Sci. 2021;22:11646. doi: 10.3390/ijms222111646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sengupta A., Carlson B.A., Weaver J.A., Novoselov S.V., Fomenko D.E., Gladyshev V.N., Hatfield D.L. A functional link between housekeeping selenoproteins and phase II enzymes. Biochem. J. 2008;413:151–161. doi: 10.1042/BJ20080277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Malinouski M., Kehr S., Finney L., Vogt S., Carlson B.A., Seravalli J., Jin R., Handy D.E., Park T.J., Loscalzo J., et al. High-Resolution Imaging of Selenium in Kidneys: A Localized Selenium Pool Associated with Glutathione Peroxidase Antioxid. Redox Signal. 2012;16:185–192. doi: 10.1089/ars.2011.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seeher S., Atassi T., Mahdi Y., Carlson B.A., Braun D., Wirth E.K., Klein M.O., Reix N., Miniard A.C., Schomburg L., et al. Secisbp2 Is Essential for Embryonic Development and Enhances Selenoprotein Expression. Antioxid. Redox Signal. 2014;21:835–849. doi: 10.1089/ars.2013.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Carlson B.A., Yoo M.-H., Sano Y., Sengupta A., Kim J.Y., Irons R., Gladyshev V.N., Hatfield D.L., Park J.M. Selenoproteins regulate macrophage invasiveness and extracellular matrix-related gene expression. BMC Immunol. 2009;10:57. doi: 10.1186/1471-2172-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mattmiller S.A., Carlson B.A., Gandy J.C., Sordillo L.M. Reduced macrophage selenoprotein expression alters oxidized lipid metabolite biosynthesis from arachidonic and linoleic acid. J. Nutr. Biochem. 2014;25:647–654. doi: 10.1016/j.jnutbio.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 150.Kaushal N., Kudva A.K., Patterson A.D., Chiaro C., Kennett M.J., Desai D., Amin S., Carlson B.A., Cantorna M.T., Prabhu K.S. Crucial Role of Macrophage Selenoproteins in Experimental Colitis. J. Immunol. 2014;193:3683–3692. doi: 10.4049/jimmunol.1400347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Narayan V., Ravindra K.C., Liao C., Kaushal N., Carlson B.A., Prabhu K.S. Epigenetic regulation of inflammatory gene expression in macrophages by selenium. J. Nutr. Biochem. 2015;26:138–145. doi: 10.1016/j.jnutbio.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nelson S.M., Shay A.E., James J.L., Carlson B.A., Urban J., Prabhu K.S. Selenoprotein Expression in Macrophages Is Critical for Optimal Clearance of Parasitic Helminth Nippostrongylus brasiliensis. J. Biol. Chem. 2016;291:2787–2798. doi: 10.1074/jbc.M115.684738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hudson T.S., Carlson B.A., Hoeneroff M.J., Young H.A., Sordillo L., Muller W.J., Hatfield D.L., Green J.E. Selenoproteins reduce susceptibility to DMBA-induced mammary carcinogenesis. Carcinogenesis. 2012;33:1225–1230. doi: 10.1093/carcin/bgs129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wirth E.K., Bharathi B.S., Hatfield D., Conrad M., Brielmeier M., Schweizer U. Cerebellar Hypoplasia in Mice Lacking Selenoprotein Biosynthesis in Neurons. Biol. Trace Element Res. 2014;158:203–210. doi: 10.1007/s12011-014-9920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Carlson B.A. Selenocysteine tRNA[Ser]Sec mouse models for elucidating roles of selenoproteins in health and development. In: Hatfield D.L., Schweizer U., Tsuji P.A., Gladyshev V.N., editors. Selenium—Its Molecular Biology and Role in Human Health. Springer; New York, NY, USA: 2016. [Google Scholar]
- 156.Park J.M., Choi I.S., Kang S.G., Lee J.Y., Hatfield D.L., Lee B.J. Upstream promoter elements are sufficient for selenocysteine tRNA[Ser]Sec gene transcription and to determine the transcription start point. Gene. 1995;162:13–19. doi: 10.1016/0378-1119(95)00340-C. [DOI] [PubMed] [Google Scholar]
- 157.Carlson B.A., Schweizer U., Perella C., Shrimali R.K., Feigenbaum L., Shen L., Speransky S., Floss T., Jeong S.-J., Watts J., et al. The selenocysteine tRNA STAF-binding region is essential for adequate selenocysteine tRNA status, selenoprotein expression and early age survival of mice. Biochem. J. 2009;418:61–71. doi: 10.1042/BJ20081304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.