Introduction
Impressive clinical responses observed in cancer patients treated with checkpoint-specific monoclonal antibodies (mAbs) have restored immunologists’ confidence in the ability of a patient’s immune system to recognize malignant cells and to eliminate them (1,2). More importantly, these clinical findings have convinced clinical oncologists, even the most skeptical ones, that T cell-based immunotherapy is an effective strategy to treat malignant diseases. As a result, this type of immune therapy is widely used either alone or in combination with chemotherapy, radiotherapy and/or various immune modulators for the treatment of patients with various malignant diseases. However, many cancer patients fail to respond to therapy with checkpoint-specific mAbs (3). While the mechanisms of this resistance to immune therapies are not understood, there is a growing awareness that the escape mechanisms cancer cells use to avoid recognition and destruction by the host immune system represent a major obstacle to successful immunotherapy. Multiple escape mechanisms used by tumors have been identified and characterized in the course of recent years. They include: i) defective or lack of recognition of tumor cells by the host immune system because of abnormalities in the expression and/or function of HLA class I antigen processing machinery. The latter plays a crucial role in the synthesis and expression of HLA class I molecule and tumor antigen-derived peptide complexes, which mediate the interactions between cancer cells and cognate cytotoxic T cells (4,5); ii) immunosuppressive agents, whether cellular or soluble, in the tumor microenvironment (TME), which inhibit the development of a tumor antigen-specific immune responses and/or the anti-tumor activity of tumor antigen-specific cytotoxic T cells. The immunoinhibitory characteristics of the TME have been well documented in the literature (6,7); iii) the presence in cancer patients of dysfunctional or exhausted effector T cells, which are sensitive to apoptosis and are defective or completely unable to exercise anti-tumor activities (8); iv) enrichment in lymphoid- or myeloid-derived regulatory cells (Tregs and myeloid-derived suppressor cells (MDSC), respectively) in the TME and in the periphery of patients with cancer (9,10); v) up-regulated expression of various inhibitory receptors on immune cells and of complementary immunosuppressive ligands on tumor cells (e.g., PD1/PD-L1), favoring checkpoint inhibition of immune responses (11); and vi) presence of metabolic checkpoints in tumor cells, such as glucose depravation, essential amino acid depravation or hypoxia, which interfere with functions of immune cells in the TME (12).
Emerging evidence indicates that tumor cell-derived exosomes, a subpopulation of small extracellular vesicles (EVs), are an additional key player in the escape of tumor cells from immune surveillance (13). Exosomes are produced by both normal and malignant cells, but tumor cells produce them in excess (13). Exosomes circulate freely in body fluids, contain bioactive cargoes including proteins, lipids and nucleic acids and deliver their cargos to distant or near recipient cells. In this paper, we will describe: (i) the characteristics of melanoma cell-derived exosomes (MTEX) separated by immunoaffinity capture from exosomes produced by non-malignant cells (nonMTEX); (ii) the characteristics of the tumor antigen, chondroitin sulphate proteoglycan 4 (CSPG4) (14,15), which we use as a target to separate MTEX from nonMTEX; and (iii) the evidence supporting the role MTEX play in immune suppression in melanoma.
Characteristics of melanoma cell-derived exosomes (MTEX)
Like other types of cancer cells, melanoma cells produce and release into body fluids various types of EVs. Exosomes are small EVs ranging in size from 30–150nm which differ from other EVs by a unique biogenesis (16). Exosomes are formed in the endocytic compartment of parent cells by a vesiculation process involving reverse invagination of the multivesicular body (MVB) membrane and resulting in the formation of numerous intraluminal vesicles. When MVBs filled with intraluminal vesicles fuse with the surface membrane of the parent cell, the vesicles (i.e., exosomes) are released into the tissue space and disseminate throughout all body fluids (17). Melanoma cells produce more exosomes than normal melanocytes, and melanoma patients’ plasma contains increased levels of exosomes carrying a wide variety of cellular components, including proteins, lipids and nucleic acids (18). The molecular content of exosomes mimics that of their parent cell (19), and circulating exosomes are emerging as faithful tumor cell surrogates potentially useful as a liquid biopsy (19). Additionally, tumor cell-derived exosomes (TEX) carry a variety of biologically active molecules, including enzymes, growth factors, oncogenes and signaling immunoregulatory proteins (13). Upon interaction with recipient cells, exosomes deliver their cargo to recipient cells and alter their functions (20). In the TME, exosomes serve as a communication system between the tumor and the immune system (21). We and others have shown that exosomes in melanoma patients’ plasma contain an excess of immunosuppressive proteins (e.g., FasL, TGF-β, TRAIL, PD-L1) and inhibit functions of primary human immune cells in vitro and in vivo (21–24). Further, exosomes isolated from supernatants of melanoma cell lines, and containing only melanoma cell-derived exosomes, are highly enriched in inhibitory proteins and mediate strong immunosuppression (23).
Melanoma patients’ plasma contains exosomes produced by malignant and non- malignant cells. To analyze the phenotypic and functional characteristics of melanoma cell-derived exosomes (MTEX), we have developed an immunoaffinity-based method to separate them from nonMTEX. In this method, first the total exosome population is isolated from plasma using size exclusion chromatography (SEC), as described (25). Exosomes recovered in Fractions #3,4 are separated using immune capture with the CSPG4-specific mAb into MTEX and nonMTEX, as also previously described (26) and as shown in Fig. 1. The immune-captured MTEX are tested by on-bead flow cytometry for the expression of CSPG4 using a CSPG4-specific labeled mAb, which recognizes an epitope distinct and spatially-distant from that recognized by the CSPG4-specific mAb used to capture MTEX. The CSPG4 antigen is expressed on MTEX, but is not detected on nonMTEX. Representative results generated by experiments performed with the CSPG4-specific mAb TP41.1 for capture and mAb TP61.1 for detection, which recognize distinct and spatially distant CSPG4 epitopes, are shown in Fig. 2. The data show that MTEX are positive (99%) and nonMTEX are negative for CSPG4. The protein cargo of successfully separated MTEX can now be further examined by on-bead flow cytometry using labeled mAbs which recognize melanoma associated antigens (MAAs) or other proteins of interest in the MTEX cargo (24). The MTEX and nonMTEX fractions can also be used for RNA or DNA extraction or can be co-incubated with various immune or non-immune cell types to determine their abilities to alter functions of recipient cells.
Figure 1.
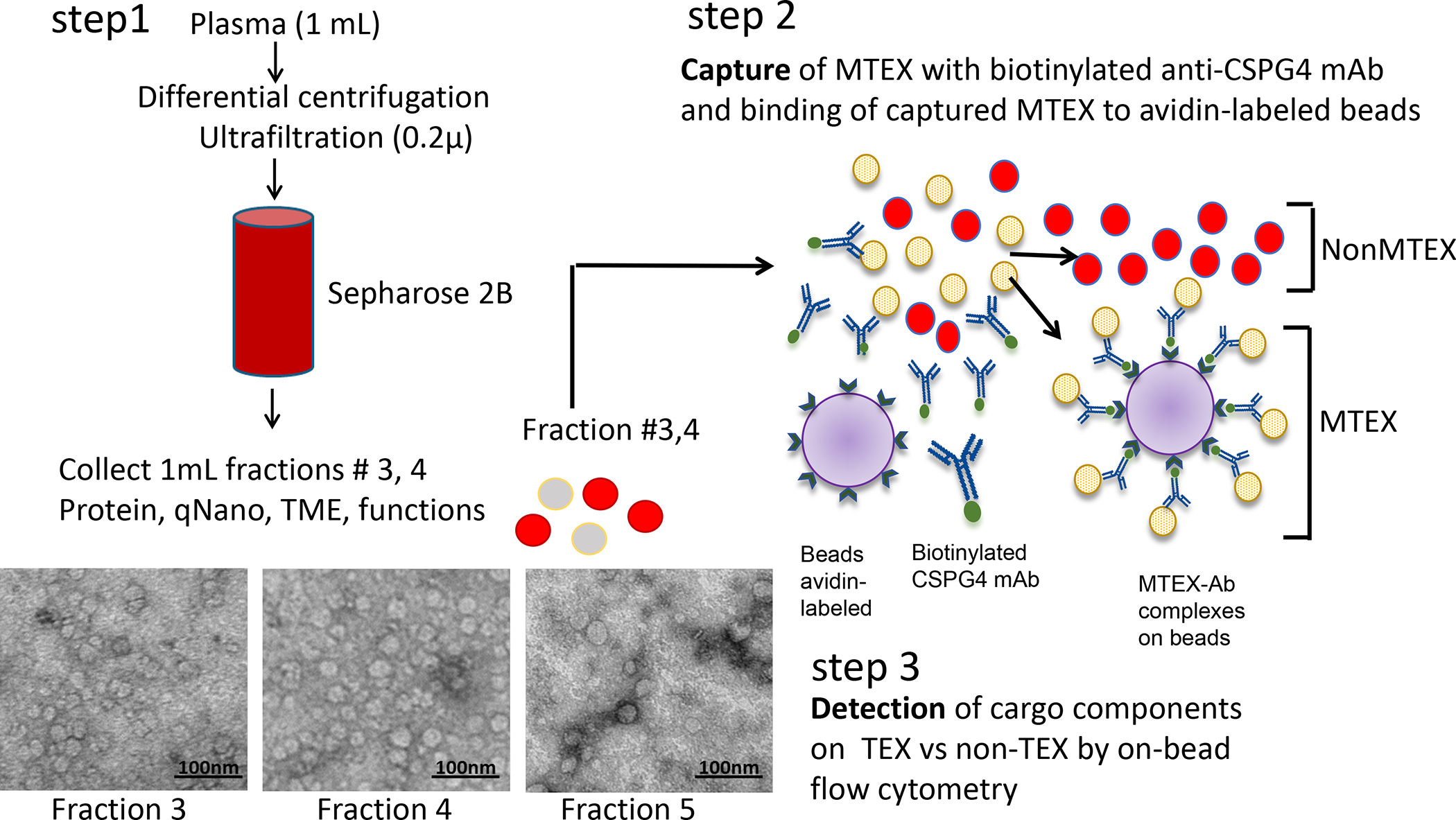
Methods for isolation of exosomes from plasma of patients with melanomaby size exclusion chromatography (SEC) and separation of total exosomes recovered in fractions # 3 and 4 into MTEX and nonMTEX by immune capture with biotinylated anti-CSPG4 mAb. Detection of CSSPG4 antigen on MTEX and non-MTEX was performed by on-bead flow cytometry as described (26).
Figure 2.
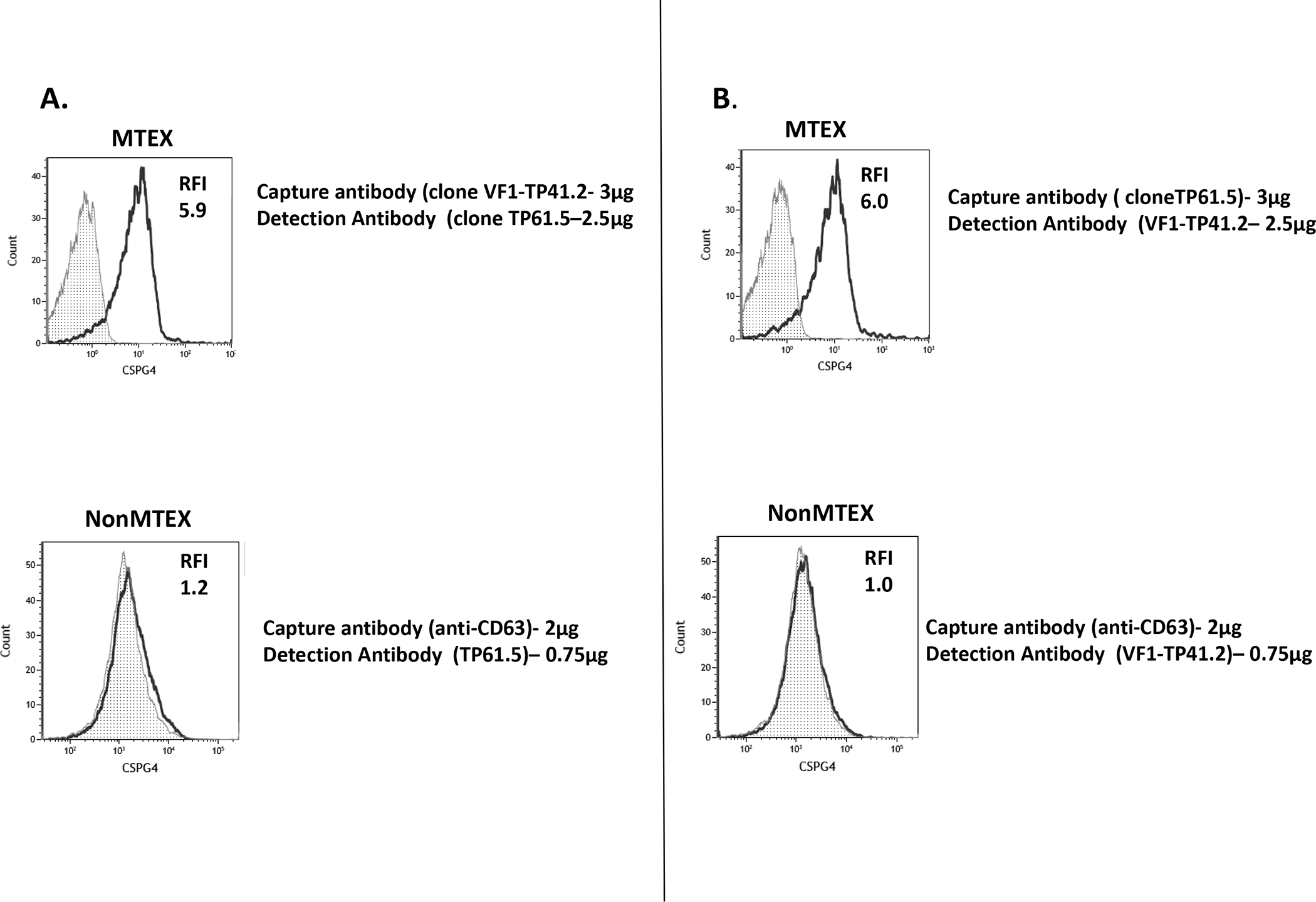
Specificity of anti-CSPG4 mAb clones TP4 and TP6, which recognize distinct epitopes of CSPG4 antigen, for detection of this antigen on MTEX and nonMTEX. In A, nearly all MTEX immunocaptured with clone TP4 mAb and detected with clone TP6 mAb were CSPG4(+). NonMTEX were negative for CSPG4. In B, nearly all MTEX immunocaptured with clone TP6 and detected with clone TP4 mAb were CSPG4(+). NonMTEX were negative for CSPG4. In A and B, the CSPG4-specific capture mAbs were used at the protein concentration of 3ug, while anti-CD63 mAb for capture of nonMTEX was 2ug. Note that dilutions of capture and detection mAbs are critical for the success of capture as well as detection of CSPG4+ exosomes.
Characteristics of chondroitin sulphate proteoglycan 4 (CSPG4) as a target for isolation of melanoma cell-derived exosomes from melanoma patients’ plasma.
The rationale for selection of CSPG4 as a target antigen for immune capture of MTEX from melanoma patients’ plasma is based on extensive evaluation of its expression on melanoma and normal human tissues. Like CD44, CSPG4 is a member of the CSPG family of cancer-associated proteins. CSPG4 is also known as a high molecular weight- melanoma-associated antigen (HMW-MAA), or neuron-glial antigen 2 (NG2) (14, 15). The CSPG family members are key bioactive molecules that play a major role in tumor growth, migration and neo- angiogenesis.
CSPG4 is highly expressed on melanoma cells in about 80% of primary and metastatic tumors with limited inter- and intra-lesional heterogeneity (14,15). It is expressed not only on differentiated melanoma cells, but also on malignant melanoma initiating cells (MMICs). The latter are defined as cells which can form spheres in vitro and are highly tumorigenic in immunodeficient mice. These cells express high levels of aldehyde dehydrogenase and are stained by the ABCB5-specific mAb RK1 we have developed (unpublished results). In addition, as shown in Fig. 3, exosomes isolated from the spent medium of cultured melanoma cells by sequential differential centrifugation, filtration through a 0.2u filter and size exclusion chromatography are stained by CSPG4-specific mAbs with high intensity.
Figure 3.
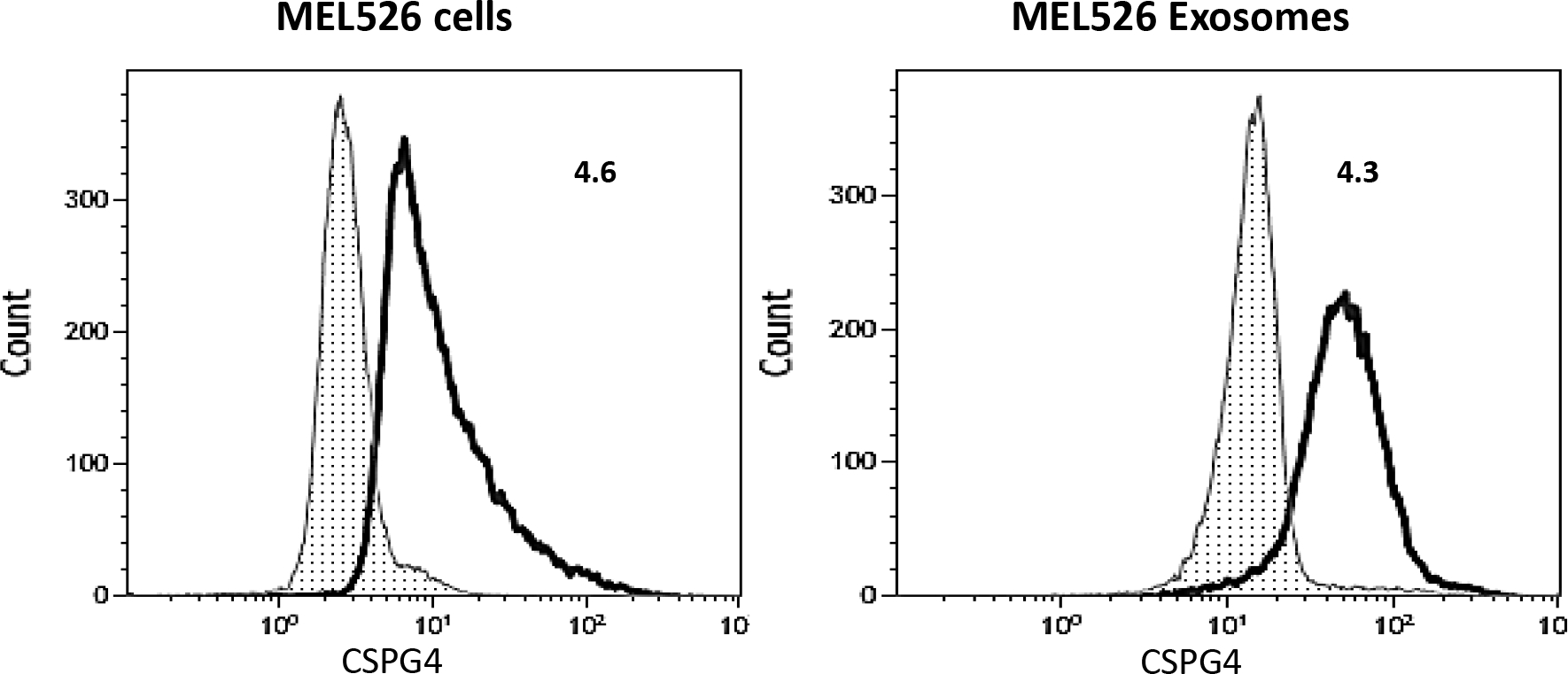
CSPG4 expression levels on Mel526 cells and on exosomes produced by MEL526 cells. Exosomes were captured with biotinylated anti-63 mAb from supernatants of MEL526 cells, and on bead-cytometry was used for detection of CSPG4 on exosomes as described (26). Mel526 cells express high levels of surface CSPG4, and the exosomes these cells produce also carry high levels of CSPG4. Numbers indicate Relative Fluorescence Index (RFI) values, which are nearly the same for cells and exosomes. Figure 3 is reproduced with permission from ref. 26.
Data about the expression of CSPG4 in normal tissues are conflicting. In our own experience (14,15,27), immunohistochemical staining with mAbs which recognize distinct epitopes of CSPG4 has not detected expression of this antigen in any normal tissue with the exception of activated pericytes in the TME (28,29). Similar results have been reported by Morgan and his collaborators (30, 31) using different techniques. This conclusion has been corroborated by several lines of evidence. First, by analyzing 94 normal tissues from different organs with a reverse protein assay, we could detect CSPG4 expression above the threshold level in only 2 out of the 4 small bowel samples tested. Second, no toxicity was detected: (i) in mice injected with large amounts of CSPG4-specific mAb cross-reacting with the mouse CSPG4 homologue [32] and ii) in patients and dogs with melanoma as well as in rats with a chemically induced chondrosarcoma [33–35] who developed CSPG4-specific antibodies following immunizations with CSPG4 mimics. Lastly, CSPG4-specific CAR+T cells did not lyse various types of normal cells which are not stained by CSPG4-specific mAbs [36]. In contrast to our results summarized above, the data reported in the Protein Atlas, which have been obtained utilizing commercially available anti-CSPG4 antibodies have indicated that CSPG4 has a broad distribution in normal tissues. This conflicting evidence is likely caused by the lack of specificity of some of the commercial antibodies used to generate the data presented in the Protein Atlas. For instance, the rabbit antiserum provided by Sigma does not appear to be specific for CSPG4, since it recognizes a molecule with a molecular weight different from that of CSPG4 in Western blotting. Furthermore, the same Ab reacts with cells in which CSPG4 has been knocked out by CRISPR. The conflict that exists between our data and recommendations published in the Protein Atlas has led to confusion among investigators using commercial anti-CSPG4 mAbs for immune capture. We wish to emphasize the specificity of our anti-CSPG4 mAbs for epitopes overexpressed on melanoma (or other tumor) cells and the lack of their reactivity with normal human tissues. Clearly, such specificity is the key to successful immune capture of CSPG4+ cells or exosomes. Some of the commercially-available mAbs targeting CSPG4 fail to meet similar standards for tumor cell specificity and thus cannot be reliably used for immune capture of MTEX.
Conclusion
The high expression levels of CSPG4 on melanoma in combination with the lack of its detection in normal tissues (14,15,27), except for activated pericytes in the TME (28,29), has provided the rationale for its use as a target antigen for separation of MTEX from nonMTEX fractions of exosomes in plasma of melanoma patients. The ability to perform this separation by immunoaffinity capture has allowed us for the first time to measure the ratios of MTEX/nonMTEX in plasma of patients with metastatic melanoma. We have found that this ratio may vary from 20–60% (24). In addition, we have been able to compare the phenotype and functional properties of MTEX and nonMTEX (24). This comparison has shown that MTEX carry an abundance of immunosuppressive proteins and inhibit numerous functions of human primary T cells and NK cells ex vivo, as also described elsewhere (23). As a result, MTEX may promote tumor immune escape and tumor progression. In contrast, nonMTEX which are enriched in co-stimulatory proteins, might stimulate immune cell activity (24, 26).
It is noteworthy that CSPG4 is also expressed on cancer cells in glioma, head and neck cancer, mesothelioma, triple negative breast cancer, ovarian cancer and malignancies of orthopedic interest (14,15). Therefore, the methodology we have developed for isolation of melanoma cell-derived exosomes may be applicable to other human malignancies. However, the CSPG4 expression level on cancer cells in the above listed cancers is in general lower than that on melanoma cells. Therefore, the methodology we have developed may require some adjustments in order to increase its sensitivity. Among them is the use of a cocktail of mAbs which recognize distinct and spatially distant epitopes of CSPG4 instead of a single epitope. The development of this approach is facilitated by the availability of a large panel of mAbs which recognize distinct and spatially distant CSPG4 epitopes (14) that we have developed and make available to the scientific community.
References
- 1.Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science. 2018 Mar 23;359(6382):1350–1355. doi: 10.1126/science.aar4060. Epub 2018 Mar 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss SA, Wolchok JD, Sznol M (2019) Immunotherapy of Melanoma: Facts and Hopes. Clin Cancer Res. 2019 Sep 1;25(17):5191–5201. doi: 10.1158/1078-0432.CCR-18-1550. Epub 2019 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pires da Silva I, Lo S, Quek C, Gonzalez M, Carlino MS, Long GV, Menzies AM. Site-specific response patterns, pseudoprogression, and acquired resistance in patients with melanoma treated with ipilimumab combined with anti-PD-1 therapy. Cancer. 2019. Oct 4. doi: 10.1002/cncr.32522. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. (2000) Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. [DOI] [PubMed] [Google Scholar]
- 5.Cai L, Michelakos T, Yamada T, Fan S, Wang X, Schwab JH, Ferrone CR, Ferrone S (2018) Defective HLA class I antigen processing machinery in cancer. Cancer Immunol Immunother. 2018 Jun;67(6):999–1009. doi: 10.1007/s00262-018-2131-2. Epub 2018 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munn DH, Mellor AL (2016) IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. 2016 Mar;37(3):193–207. doi: 10.1016/j.it.2016.01.002. Epub 2016 Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohta A (2016) A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment. Front Immunol 2016 Mar 29;7:109. doi: 10.3389/fimmu.2016.00109. eCollection 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann TK, Dworacki G, Tsukihiro T, Meidenbauer N, Gooding W, Johnson JT, Whiteside TL. (2002) Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002 Aug;8(8):2553–62. [PubMed] [Google Scholar]
- 9.Whiteside TL (2015) The role of regulatory T cells in cancer immunology. Immunotargets Ther. 2015 Aug 5;4:159–71. doi: 10.2147/ITT.S55415. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostrand-Rosenberg S (2018) Myeloid derived-suppressor cells: their role in cancer and obesity. Curr Opin Immunol. 2018 Apr;51:68–75. doi: 10.1016/j.coi.2018.03.007. Epub 2018 Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Chen W, Xu ZP, Gu W (2019) PD-L1 Distribution and Perspective for Cancer Immunotherapy-Blockade, Knockdown, or Inhibition. Front Immunol. 2019 Aug 27;10:2022. doi: 10.3389/fimmu.2019.02022. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damgaci S, Ibrahim-Hashim A, Enriquez-Navas PM, Pilon-Thomas S, Guvenis A, Gillies RJ (2018) Hypoxia and acidosis: immune suppressors and therapeutic targets. Immunology. 2018 Jul;154(3):354–362. doi: 10.1111/imm.12917. Epub 2018 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiteside TL (2017) Exosomes carrying immunoinhibitory proteins and their role in cancer. Clin Exp Immunol. 2017 Sep;189(3):259–267. doi: 10.1111/cei.12974. Epub 2017 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campoli MR, Chang CC, Kageshita T, Wang X, McCarthy JB, Ferrone S (2004) Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol, 24:267–296, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Campoli M, Ferrone S, Wang X (2010) Functional and clinical relevance of chondroitin sulfate proteoglycan 4. Adv Can Res, 109:73–121, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Ruivo CF, Adem B, Silva M, Melo SA (2017) The Biology of Cancer Exosomes: Insights and New Perspectives. Cancer Res. 2017 Dec 1;77(23):6480–6488. doi: 10.1158/0008-5472.CAN-17-0994. Epub 2017 Nov 21. [DOI] [PubMed] [Google Scholar]
- 17.Hessvik NP, Llorente A (2018) Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018 Jan;75(2):193–208. doi: 10.1007/s00018-017-2595-9. Epub 2017 Jul 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012 Jun;18(6):883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiteside TL (2017) The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol. 2017 Dec;13(28):2583–2592. doi: 10.2217/fon-2017-0343. Epub 2017 Dec 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulcahy LA, Pink RC, Carter DR (2014) Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014 Aug 4;3. doi: 10.3402/jev.v3.24641. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurywchak P, Tavormina J, Kalluri R (2018) The emerging roles of exosomes in the modulation of immune responses in cancer. Genome Med. 2018 Mar 26;10(1):23. doi: 10.1186/s13073-018-0535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H, Man Q, Zhong W, Antelo LF, Wu B, Xiong X, Liu X, Guan L, Li T, Liu S, Yang R, Lu Y, Dong L, McGettigan S, Somasundaram R, Radhakrishnan R, Mills G, Lu Y, Kim J, Chen YH, Dong H, Zhao Y, Karakousis GC, Mitchell TC, Schuchter LM, Herlyn M, Wherry EJ, Xu X, Guo W (2018) Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018 Aug;560(7718):382–386. doi: 10.1038/s41586-018-0392-8. Epub 2018 Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL (2009) Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009 Sep 15;183(6):3720–30. doi: 10.4049/jimmunol.0900970. Epub 2009 Aug 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma P, Diergaarde B, Ferrone S, Kirkwood JM, Whiteside TL. Melanoma cell-derived exosomes in plasma of melanoma patients mediate suppression of immune effector cells. Sci Reports, In Press, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong CS, Funk S, Muller L, Boyiadzis M, Whiteside TL (2016) Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell Vesicles. 2016 Mar 24;5:29289. doi: 10.3402/jev.v5.29289. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma P, Ludwig S, Muller L, Hong CS, Kirkwood JM, Ferrone S, Whiteside TL (2017) Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J Extracell Vesicles. 2018; 15;7(1):1435138. doi: 10.1080/20013078.2018.1435138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Katayama A, Wang Y, Yu L, Favoino E, Sakakura K, Favole A, Tsuchikawa T, Silver S, Watkins SC, Kageshita T, Ferrone S (2011) Functional characterization of an scFV antibody that immunotherapeutically targets the common cancer cell surface proteoglycan CSPG4. Cancer Res, 71:7410–7422, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlingemann RO, Rietveld FJ, de Waal RM, Ferrone S, Ruiter DJ (1990) Expression of the high molecular weight melanoma associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am J Pathol, 136:1393–1405, 1990 [PMC free article] [PubMed] [Google Scholar]
- 29.Maciag PC, Seavy MM, Pan ZK, Ferrone S, Paterson Y (2008) Cancer immunotherapy targeting the high molecular weight melanoma-associated antigen protein results in a broad antitumor response and reduction of pericytes in the tumor vasculature. Cancer Res, 68:8066–8075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beard RE, Zheng Z, Lagisetty KH, Burns WR, Tran E, Hewitt SM, Abate-Daga D, Rosati SF, Fine HA, Ferrone S, Rosenberg SA, Morgan RA (2014) Multiple chimeric antigen receptors successfully target chondroitin sulfate proteoglycan 4 in several different cancer histologies and cancer stem cells. J Immunother Cancer. 2:25, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beard RE, Abate-Daga D, Rosati SF, Zheng Z, Wunderlich JR, Rosenberg SA, Morgan RA (2013) Gene expression profiling using nanostring digital RNA counting to identify potential target antigens for melanoma immunotherapy. Clin Cancer Res. 2013 Sep 15;19(18):4941–50. doi: 10.1158/1078-0432.CCR-13-1253. Epub 2013 Sep 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Osada T, Wang Y, Yu L, Sakakura K, Katayama A, McCarthy J, Brufsky A, Chivukula M, Khoury T, Hsu D, Lyerly H, Clay T, Ferrone S (2010) CSPG4 as a new target for antibody-based immunotherapy of triple negative breast cancer. J Natl Cancer Inst, 102:1496–1512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittelman A, Chen ZJ, Yang H, Wong GY, Ferrone S (1992) Human high molecular weight melanoma-associated antigen (HMW-MAA) mimicry by mouse anti-idiotypic monoclonal antibody MK2–23: induction of humoral anti-HMW-MAA immunity and prolongation of survival in patients with stage IV melanoma. Proc Natl Acad Sci USA, 89:466–470, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riccardo F, Iussich S, Maniscalco L, Mayayo SL, Rosa GL, Arigoni M, Maria RD, Gattino F, Lanzardo S, Lardone E, Martano M, Morello E, Prestigio S, Fiore A, Quaglino E, Zabarino S, Ferrone S, Buracco P, Cavallo F (2014) CSPG4-specific immunity and survival prolongation in dogs with oral malignant melanoma immunized with human CSPG4 DNA. Clin Cancer Res. 20:3753–62, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Léger O, Johnson-Léger C, Jackson E, Coles B, Dean C (1994) The chondroitin sulfate proteoglycan NG2 is a tumour-specific antigen on the chemically induced rat chondrosarcoma HSN. Int J Cancer. 1994 Sep 1;58(5):700–5. [DOI] [PubMed] [Google Scholar]
- 36.Geldres C, Savoldo B, Hoyos V, Caruana I, Zhang M, Yvon E, Del Vecchio M, Creighton CJ, Ittmann MM, Ferrone S, Dotti G (2014) T lymphocytes redirected against the chondroitin sulfate proteoglycan-4 control the growth of multiple solid tumors both in vitro and in vivo. Clin Cancer Res. 20:962–71, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]


