Abstract
Although many regional multiple sclerosis (MS) databases existed in the United States and Canada, there was no single clinician-derived registry that examined this disease as a group across the North American continent. This distinction is important because information that results from such a database can potentially give perspectives about MS that cannot be derived from any single regional registry. A partnership was forged between the pharmaceutical industry and the Consortium of Multiple Sclerosis Centers (CMSC) to create a registry of patients with MS from Canada and the United States, including Puerto Rico. Case report forms were created to collect physician-derived information, and the Patient-Reported Outcomes Measurement Information System (PROMIS) was selected to capture patient-reported outcomes. As of November 2021, 754 of 1000 patients have been enrolled. Completion of recruitment is expected by the end of 2021. Twenty-five centers are participating, with an expected total of 30, including five centers from Canada. Clinical status, health economic outcomes, magnetic resonance images, and, soon, biomarkers relevant to understanding relapses and progression are collected. The short-term goal is to understand and better treat MS disease progression, and the long-term goal is its prevention. The North American Registry for Care and Research in Multiple Sclerosis (NARCRMS) is one of few clinician/patient-generated registries that examines MS across North America, including Puerto Rico. Information derived from the natural history studies should help physicians, the pharmaceutical industry, and regulatory bodies understand MS better and improve quality of life for patients with MS worldwide.
Keywords: Biomarkers, Epidemiology, Disease-modifying therapies (DMTs), Health economics, Magnetic resonance imaging (MRI), Multiple sclerosis (MS), Registry
There was consensus among multiple sclerosis (MS) thought leaders that a large registry of physician/clinician-based patient data from the United States and Canada would be helpful in understanding the natural history of MS in the era of disease-modifying therapies (DMTs). It was recognized that such an endeavor would be expensive and beyond the scope of any single entity. Accordingly, it was believed that a partnership could be formed of stakeholders in the understanding of the disease and that, together, these stakeholders could develop a viable entity to examine the natural history of MS across Canada and the United States, including Puerto Rico. The model developed by the North American Registry for Care and Research in Multiple Sclerosis (NARCRMS) paralleled that of the Alzheimer’s Disease Neuroimaging Initiative (ADNI),1 a historic study of brain aging aiming to expedite discovery to prevent, treat, and some day cure Alzheimer disease. The ADNI is a partnership of the National Institute on Aging, pharmaceutical and neuroimaging industries with an interest in Alzheimer disease, and Alzheimer disease centers across the United States. Based on the model of the ADNI, a white paper was created and a business model developed. Six pharmaceutical companies (see the Funding/Support section), all with products for MS, came together with the Consortium of Multiple Sclerosis Centers (CMSC), and NARCRMS was formed. A Clinical Advisory Board, formerly known as the Steering Committee, composed of thought leaders in the field (Appendix S1, which is published in the online version of this article at ijmsc.org), was convened, and the process of developing NARCRMS commenced.
Rationale for Development of NARCRMS
The goals of NARCRMS, among others, are twofold: 1) The short-term goal is to define factors leading to disease progression so that preventable aspects of disease progression can be addressed early to reduce or prevent long-term disability. 2) The long-term goal is to understand the early events in the lives of patients with MS that lead to occurrence of the disease so that the disease can be prevented in future generations.
The past decade has seen substantial expansion of the armamentarium of DMTs for MS. Despite these advances in treatment, fundamental questions about the natural history of MS with the use of DMTs remain largely unknown. Broadly, these unknowns include, but are not limited to, the following: 1) Best choice of initial therapy. This is an area of ongoing concern for patients, and registries are ideally suited to provide answers as to how a group of patients using one class of therapy fares compared with others in a phase 4 clinical setting, with the inherent limitations of confounding factors that influence choice of therapy. 2) The long-term impact of current treatments. Do patients taking one class of therapy experience long-term adverse effects not evident in the clinical trial? For example, acute promyelocytic leukemia as a complication of mitoxantrone was not apparent until many years after the trial, and such information could readily become available through a longitudinal registry.2 3) Impact of relapses on disease progression. There is considerable controversy regarding whether progressive MS is an independent variable unrelated to relapses or occurs as a consequence of relapses because a variety of studies have questioned the long-term impact of relapses on disease progression.3 4) Predictive value of early no evidence of disease activity on long-term disability. There is no consensus that no evidence of disease activity is a predictor of secondary progression, and this area can be addressed by natural history longitudinal registries.4 5) Early markers of disease progression. What is the impact of early signs in their predictive values for progression? Among others, what is the impact of race, ethnicity, early relapse history, and early markers on magnetic resonance imaging (MRI)? Do biomarkers such as neurofilament light chains in blood reflect long-term disease state?5 6) Impact of regional environmental factors on disease progression. This is an area that cannot be examined by a single regional registry and, instead, requires the uniform collection of data from multiple registries across the United States and Canada. Regional differences can be expected because multiple confounding factors, such as the gut microbiome, sun exposure, diet, and vitamin D, vary greatly among different parts of the country6 and influence the final expression of the disease. 7) The impact of genetics on MS phenotype and disease course. This will be examined in conjunction with the International Multiple Sclerosis Genetics Consortium (IMSGC).7 8) The long-term impact of MS on patients, families, and society. The socioeconomic toll of MS on individuals, families, and society are understudied, and the real impact of DMTs can be gauged only by the improvement in quality of life rendered by the various treatments. Health economics and outcomes research (HEOR) measures this only in part. The true impact can be assessed only from patients, and the collection of patient-reported outcomes (PROs) on the global impact of this disease will be an important aspect of NARCRMS.
Until now, glimpses of insight into these questions in the North American population have been obtained through clinical trials and several regional and national center-based registries. The North American Research Committee on Multiple Sclerosis (NARCOMS) is detailed in an accompanying article in this issue of the Journal.8 The New York State Multiple Sclerosis Consortium linked selective centers in the state and collected longitudinal information.9 MS COSTAR (COmputer STored Study Ambulatory Record) was an early attempt at developing a computerized national database in MS, but the effort could not be sustained except in a handful of centers.10 The University of California at San Francisco–based Expression, Proteomics, Imaging, Clinical (EPIC) study systematically studied patients at that center.4 Comprehensive Longitudinal Investigation of Multiple Sclerosis (CLIMB) is a registry developed at Brigham and Women’s Hospital.11 EPIC and CLIMB have joined efforts to create the Serially Unified Multicenter Multiple Sclerosis Investigation (SUMMIT).12 However, limitations of study duration and type of data collected (clinical, PROs, biospecimens, MRIs, and catchment area) have rendered an incomplete picture of the MS landscape.13,14 To develop a more complete understanding of MS natural history across its many dimensions, the CMSC supported the creation of NARCRMS. Around the same time, the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS) registry was launched and sponsored by Biogen to capture digital information from electronic medical records of patients with MS from selected participating centers in the United States and Europe at their regular clinic visits.15
Structure of NARCRMS
The development and administration of NARCRMS is overseen by the Board of Governors of the CMSC. The standard rules of operating procedures of NARCRMS are approved by the CMSC Board of Governors. The organizational chart of the registry is shown in Figure S1. The day-to-day operations and activities of the registry are conducted by the NARCRMS Leadership, which includes the project director, Dr Kottil Rammohan, University of Miami; the associate director, Dr David Li, University of British Columbia (previously, Dr David Jones, University of Virginia); and the CMSC chief executive officer, Ms June Halper. The Leadership implements the goals set by the Clinical Advisory Board composed of principal investigators from the various sites, thought leaders in MS, and representatives from the National Multiple Sclerosis Society and the Multiple Sclerosis Association of America. Additional input is derived from the NARCRMS Industry Advisory Board and Patient Advisory Board. The Patient Advisory Board in particular is paramount to ensuring that NARCRMS is meeting patients’ needs and incorporating patient voices regarding the usefulness and importance of the collected data. These perspectives are captured through quarterly meetings of the advisory boards with representatives from the Leadership.
Registry Design
NARCRMS is a prospective longitudinal registry involving the participation of approximately 30 CMSC-affiliated MS centers in the United States and Canada (Figure 1). The NARCRMS Leadership has established an organizational structure consisting of five main cores, each of which focuses on a particular area of research. The goal of the study is to systematically collect information considered relevant to the pathogenesis and progression of MS from early childhood events to the present and to continually assess the course of individual patients as they progress through their disease. No attempts will be made to intervene, and any change in therapy will be initiated by the treating neurologist independent of NARCRMS. Such changes in therapy will be documented for further analysis.
Figure 1.
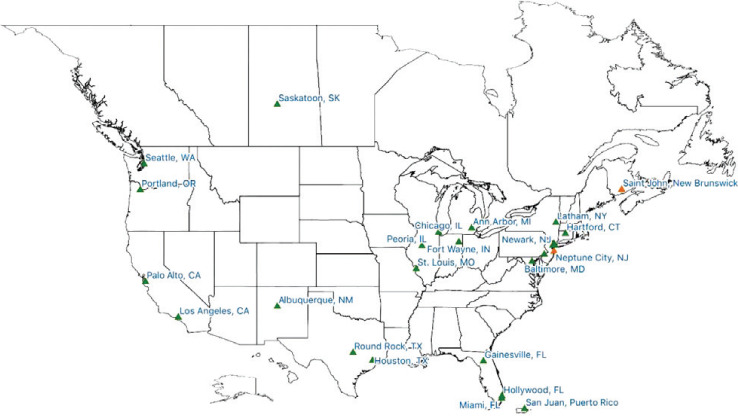
Geographic distribution of currently participating sites (green) and sites pending official enrollment (orange) as of January 2021
Operations Center with Information Technology Services and Data Management Core
Social & Scientific Systems, a DLH Holdings Company, was selected as the operations center, with information technology and data management services also provided. Social & Scientific Systems facilitates the collection and sharing of NARCRMS data and provides support at both the site level, for the collection of physician-generated data, and the patient level, to ensure uniform collection of PROs.
The registry is structured on the OpenClinica (OC) platform (https://www.openclinica.com/), which is a CFR 21 Part 11–compliant cloud-based system for electronic data capture and data management. The system allows for complete validation of documentation and audits and has security certifications that could be used for clinical trials. Although all participating centers use OC Enterprise for data entry, access to the data is provided through “Insight,” the reporting portal of OC. Every NARCRMS stakeholder, including site staff, advisory board members, and Leadership, has access to the entire database, including uploaded MRIs.
Clinical Core
The patients recruited into the registry are the heart of the operation of NARCRMS. The clinical core is expected to enroll 1000 patients from approximately 30 clinical centers across Canada and the United States, including Puerto Rico. The first participant was recruited into NARCRMS in December 2016, and as of mid-April 2021, a total of 26 sites have been recruited, including two centers from Canada, with 849 patients in the study as of this writing. Accrual of patients into the registry can be observed in real time at http://www.NARCRMS.org. The electronic case report forms were carefully prepared by an ad hoc committee of investigators from existing MS registries. Every database approached generously shared their case report forms, which enabled the committee to choose the best for incorporation into NARCRMS. The design of the data capture was to examine all aspects of the course of MS from the “cradle to the grave,” a concept that will provide information on progression as well as prevention, the two tenets of this registry. Four types of visits are captured. The enrollment visit captures all information that preceded the diagnosis of MS, including status at study entry, comorbidities, and information from early childhood and adulthood that could have potential effects on pathogenesis. Annual follow-up visits capture performance of various metrics that examine the physical and cognitive status of patients. Patients visits also occur after documented relapses and every time a change in DMT occurs. Sites receive remuneration for each participant visit to support their efforts with recruiting and retaining participants. The exact remuneration depends on whether a site submits MRIs as part of their participation in NARCRMS.
An important part of the collection of data includes PROs. After consultations with Cindy Nowinski, MD, PhD, Northwestern University Feinberg School of Medicine, and Amber Salter, PhD, Washington University, St Louis, measures from the Patient-Reported Outcomes Measurement Information System (PROMIS)16,17 were chosen to be collected as PROs. These measures, now available in English and Spanish, were also selected after close consultation with the NARCRMS Patient Advisory Board to ensure that they captured outcomes most meaningful to patients.
Neuroimaging Core
Brain MRIs have proved to be an important bio-marker for disease activity and progression, and collecting quality brain MRIs remains a priority for studying MS in NARCRMS. Regional brain volumetric analysis may hold the key to better understanding disease progression as patients transition from the earliest to the latest stages of MS. The University of British Columbia was selected as the neuroimaging core for the management of brain MRIs for NARCRMS. The neuroimaging core is responsible for ensuring collection, quality control, and storage of MRI data in accordance with CMSC guidelines.18 Originally, NARCRMS planned to collect MRIs acquired as part of routine clinical care. However, after initial data collection it became evident that many of the clinically acquired images did not satisfy the CMSC recommendations for standardized MRIs, and there was substantial variability in MRI acquisition parameters across sites as well as between scans for the same individual over time. This inconsistency made meaningful comparison of the MRIs longitudinally over time impossible. Therefore, the difficult decision was made to limit collection of MRIs to a select subset of sites. These sites successfully passed quality checks to provide sample images from approved scanners (3 T or 1.5 T) using standardized sequences as recommended by the CMSC as optimal studies for MS diagnosis and follow-up. Although we recognize that significant spinal cord disease can influence progression, standardization of spinal cord imaging across sites poses an even greater challenge than collection of brain MRIs, and the decision was made to collect only brain MRIs.
Biomarkers Core
This planned core will collect and store biomaterials, including cerebrospinal fluid when available, peripheral blood mononuclear cells from selected sites, and serum. This core will also share biomaterials for analysis of DNA with the IMSGC from consenting participants.7 A separate genetics core is, therefore, not planned for NARCRMS.
Finally, if appropriate funding is available, study of the gut microbiome will also be undertaken, especially because regional differences in MS can best be correlated to changes in the gut microbiome. Regional differences in the gut microbiome have already been identified across the largely diverse continental North America and islands of Puerto Rico.6
HEOR Core
Because this disorder predominantly affects young people in the prime of their lives, the toll of lost productivity from MS is truly incalculable. More incalculable is the toll that MS takes on direct and indirect aspects of mental health, disruptions of personal connections, and social isolations, and particularly regarding depression and suicide. Much of this “doom and gloom” scenario has changed in recent years due to the advent of DMTs. An individual with MS today can focus on leading a normal or nearly normal life with all of the fulfillments of any other individual without the disorder but with some inconveniences of having to use a DMT in their day-to-day life. How does one calculate the impact of the use of a DMT? Although an incomplete measure of success, the NARCRMS HEOR core seeks to understand at least the financial impact of saved resources for patients using DMTs. Started as an ad hoc advisory group of the Industry Advisory Board, the HEOR was established as a standing core, actively examining the impact of the various DMTs on savings of lost wages and utilization of health care resources in individuals taking the various DMTs.
Enrollment
The enrollment phase (2016–2021) consists of the recruitment of 1000 patients with MS across 30 participating MS centers, including five centers anticipated from Canada. Patients with a definitive diagnosis of either relapsing-remitting or progressive MS in the past 15 years are eligible for enrollment. Data collection begins with the participant’s enrollment visit at their participating MS center. At this visit, detailed information regarding individual demographics, family history, infections and immunizations, occupational history, use of hormones and diet supplementations, as well as HEOR data are collected electronically. Physician-reported data across physical and cognitive domains are also collected at this time. Physical evaluation includes assessment with the Expanded Disability Status Scale, the Nine-Hole Peg Test, and the Multiple Sclerosis Functional Composite.19 Cognitive evaluation includes testing with the Symbol Digit Modalities Test20 and the California Verbal Learning Test.21 Detailed demographic data about patients with completed enrollments as of April 2021 are given in Table 1.
Table 1.
Demographic data for the 754 participants with completed enrollments as of April 2021
| Factor | Participants, No. (%) |
|---|---|
| Gender | |
| Female | 563 (74.67) |
| Male | 189 (25.07) |
| Transgender female | 0 |
| Transgender male | 2 (0.27) |
| Ethnicity (self-report) | |
| Non-Hispanic/Latino | 574 (76.13) |
| Hispanic/Latino | 163 (21.62) |
| Unknown | 17 (2.25) |
| Race (self-report) | |
| White | 640 (86.25) |
| African American | 80 (10.78) |
| Asian/Pacific Islander | 10 (1.34) |
| Aboriginal/Native American/Alaskan Native | 1 (0.13) |
| Unknown | 23 (3.05) |
| Educational level | |
| Less than high school | 3 (0.39) |
| High school diploma | 172 (22.81) |
| Vocational certificate | 45 (5.97) |
| Undergraduate degree | 380 (50.4) |
| Graduate degree | 153 (20.29) |
| Unknown | 1 (0.13) |
| Income, USD ($) | |
| <25,000 | 99 (13.13) |
| 25,000–49,999 | 144 (19.1) |
| 50,000–99,999 | 216 (28.65) |
| 100,000–199,999 | 190 (25.2) |
| >200,000 | 68 (9.02) |
| Unknown | 37 (4.91) |
| Marital status | |
| Single (never married) | 215 (28.51) |
| Married | 415 (55.04) |
| Divorced/separated | 82 (10.88) |
| Widowed | 2 (0.27) |
| Living with partner | 39 (5.17) |
| Unknown | 1 (0.13) |
In addition, patients will begin to submit PROs through text message or e-mail at the time of their enrollment visit. The NARCRMS PROs are derived from the National Institutes of Health’s PROMIS tool16,17 and include Global Health, Fatigue Short Form (SF) 8a, Pain Interference SF 6b, Emotional Distress/Depression SF 8a, and two optional measures, Cognitive Function Abilities SF 6a, and Physical Function SF 6b.
Challenges
Developing and Sustaining the Registry
Maintaining a longitudinal registry is an expensive undertaking beyond the capabilities of any individual institution or investigator. However, every investigator recognizes the value of such a registry, and fledgling registries have been generated over time at multiple institutions. The financial burden posed by expenses for maintaining registries often result in many of these registries becoming nonviable. The financial burden of NARCRMS is supported by generous contributions from participating pharmaceutical partners. As long as NARCRMS is able to provide valuable information to industry and investigators, the enterprise will remain viable. It is imperative that NARCRMS adapt to the changing environment of health care delivery so that the natural history of MS can continue to be collected in this changing landscape of MS care. The hope of NARCRMS Leadership is that this entity will sustain itself with grants from national agencies and philanthropic organizations and foundations rather than depending on pharmaceutical support in the long term. At that time, it would be appropriate to make the information in NARCRMS available as an open-source entity for all stakeholders, including those not contributing to the collection of information; the information is presently limited to the participating sites, industry supporters, and advisory board members.
Retention of Participants
Retention of participants poses a significant challenge to the success of all longitudinal research. NARCRMS has developed several mechanisms to maximize the retention of participants. First, before enrollment, prospective participants are evaluated by local study coordinators to ensure geographic stability and commitment to the success of NARCRMS. Second, the annual follow-up visits are designed to coincide with the participant’s annual visit to their neurologist, which minimizes the travel burden for participants. Third, separate quarterly meetings of the NARCRMS leadership team with study personnel and with each advisory board reinforces the mission of NARCRMS and allows for course adjustment to ensure that study protocols best serve the needs of participants. Last, patients who are open to group participation will be encouraged to join a support group for NARCRMS enrollees.
COVID-19 Pandemic
The COVID-19 pandemic triggered a paradigm shift in the practice of medicine. Although neurology has always been a leader in the use of telemedicine, the pandemic posed unanticipated significant challenges to patient care and research.22,23 Although patient enrollment in NARCRMS was surprisingly minimally affected by the pandemic (Figure S2), physician-reported elements of the neurologic examination have been affected. Nevertheless, these challenges have presented an opportunity for innovation. We have been exploring the use of novel tools to obtain these data remotely, including wearable devices for collection of performance metrics. The future of collection of usable data on patients can change even after the pandemic is behind us.
Promotion of Registry Use
A database is only as useful as it is used. When large sets of data are generated, the ability to make sense of that information requires special tools of bioinformatics and statistics. The ability of NARCRMS data to be of value to patients is wholly dependent on its widespread use by investigators. The NARCRMS team is currently implementing several strategies to promote awareness and use of the registry. These strategies include the publication of preliminary NARCRMS data in the scientific literature and at scientific conferences (Appendix S2), advertisement at neurology and MS conferences, exhibition at CMSC annual meetings, and promotion through social media. In addition, analytics training sessions are held at participating sites to train investigators on how to best use NARCRMS data. Although data use is currently restricted to NARCRMS’ stakeholders, it is our hope to implement a more liberal data access policy to disseminate results of information derived from NARCRMS. Such data can set the standards against which change is measured so as to be helpful to regulatory agencies such as the US Food and Drug Administration or the European Medicines Agency.
Conclusions
Although much can be learned about MS from every patient with MS, some information will become evident only when large groups of patients are examined as a whole through disease-specific registries. Although much can be learned from regional registries, the true impact of environment on MS can be ascertained only when information is examined from across the continent. The impact of geography through the diversity of its populations can shed new light on the more complete understanding of this complex disorder. Unlike NARCOMS, which is a patient-only–derived registry, NARCRMS primarily focuses on physician/clinician-derived data in addition to patient-reported information in a longitudinal setting across Canada, the continental United States, and Puerto Rico. Much can be learned from such an entity, and the goal of this registry is to understand and better treat progressive MS in the short term, and in the long term to prevent the occurrence of this disorder in future generations. As the great Sylvia Lawry, founder of the National Multiple Sclerosis Society, once said, MS will someday stand not for multiple sclerosis, but for mystery solved!
PRACTICE POINTS
The North American Registry for Care and Research in Multiple Sclerosis (NARCRMS) is a physician/clinician-based registry and longitudinal database of clinical records and patient-centered outcomes. NARCRMS collects complete patient and physician data sets to track the incidence, prevalence, and longitudinal history of MS over time.
NARCRMS provides deidentified longitudinal data from patients enrolled across the United States (including Puerto Rico) and Canada to all stakeholders, including sites, industry supporters, and advisory board members.
NARCRMS provides clinicians and researchers with a greater, more integrated ability to track the incidence, prevalence, and longitudinal history of MS. Through information sharing, NARCRMS will improve the understanding of MS, facilitate care at every level, and assist with the design of clinical drug trials.
Supplementary Material
Acknowledgments
The NARCRMS Leadership appreciates the original Steering Committee that convened with them on June 11, 2014, in Crystal City, Virginia, to initiate the formal development of NARCRMS. These thought leaders include Stanley Cohan, MD; Anne Cross, MD; Gary Cutter, PhD; Corey Ford, MD, PhD; Colleen Harris, MN, NP, MSCN; Stephen Hauser, MD; Nicholas LaRocca, PhD; Robert Lisak, MD; Fred Lublin, MD; Aaron Miller, MD; Michael Racke, MD; Barbara Teter, PhD, MPH; Anthony Traboulsee, MD, FRCPC; Howard Weiner, MD; and Michael Weiner, MD.
Footnotes
Financial Disclosures: Dr Rammohan reports receiving honoraria for advisory board membership or consulting from Biogen, Novartis, Roche Genentech, Genzyme, EMD Serono, Alexion, and Viela Bio/MedImmune and grants from Biogen, Novartis, Roche Genentech, Genzyme, EMD Serono, and Alexion. Dr Li reports receiving honoraria for advisory board membership or consulting from Biogen; grants from Sanofi Genzyme, Novartis, and Roche; lectures/education grants from Academy of Health Care Learning, Biogen, Consortium of Multiple Sclerosis Centers (CMSC), and Sanofi Genzyme; and contracts from Roche and Sanofi Genzyme. Ms Halper reports receiving honoraria from EMD Serono. The other authors declare no conflicts of interest.
Funding/Support: The pharmaceutical companies that initially supported NARCRMS included Acorda, Biogen, Genentech, Genzyme, Novartis, and Teva. Current sponsors include Biogen, Celgene/Bristol Myers Squibb, EMD Serono, Genentech, Novartis, and Sanofi Genzyme.
References
- 1.Mueller SG, Weiner MW, Thal LJ et al. The Alzheimer’s disease neuroimaging initiative. Neuroimaging Clin N Am . 2005;15:869–877. xi–xii. doi: 10.1016/j.nic.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghalie RG, Mauch E, Edan G et al. A study of therapy-related acute leukaemia after mitoxantrone therapy for multiple sclerosis. Mult Scler . 2002;8:441–445. doi: 10.1191/1352458502ms836oa. [DOI] [PubMed] [Google Scholar]
- 3.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med . 2000;343:1430–1438. doi: 10.1056/NEJM200011163432001. [DOI] [PubMed] [Google Scholar]
- 4.University of California, San Francisco MS-EPIC Team. Cree BAC, Hollenbach JA, Bove R et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol . 2019;85:653–666. doi: 10.1002/ana.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Disanto G, Barro C, Benkert P et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol . 2017;81:857–870. doi: 10.1002/ana.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks AW, Priya S, Blekhman R, Bordenstein SR. Gut microbiota diversity across ethnicities in the United States. PLoS Biol . 2018;16:e2006842. doi: 10.1371/journal.pbio.2006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Multiple Sclerosis Genetics Consortium Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science . 2019;365:eaav7188. doi: 10.1126/science.aav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrie RA, Cutter GR, Fox RJ, Vollmer T, Tyry T, Salter A. NARCOMS and other registries in multiple sclerosis: issues and insights. Int J MS Care . 2021;23:276–284. doi: 10.7224/1537-2073.2020-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs LD, Wende KE, Brownscheidle CM et al. A profile of multiple sclerosis: the New York State Multiple Sclerosis Consortium. Mult Scler . 1999;5:369–376. doi: 10.1177/135245859900500511. [DOI] [PubMed] [Google Scholar]
- 10.Paty D, Studney D, Redekop K, Lublin F. MS COSTAR: a computerized patient record adapted for clinical research purposes. Ann Neurol . 1994;36(suppl):S134–S135. doi: 10.1002/ana.410360732. [DOI] [PubMed] [Google Scholar]
- 11.Gauthier SA, Glanz BI, Mandel M, Weiner HL. A model for the comprehensive investigation of a chronic autoimmune disease: the multiple sclerosis CLIMB study. Autoimmun Rev . 2006;5:532–536. doi: 10.1016/j.autrev.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Bove R, Chitnis T, Cree BA et al. SUMMIT (Serially Unified Multicenter Multiple Sclerosis Investigation): creating a repository of deeply phenotyped contemporary multiple sclerosis cohorts. Mult Scler . 2018;24:1485–1498. doi: 10.1177/1352458517726657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bebo BF, Jr, Fox RJ, Lee K, Utz U, Thompson AJ. Landscape of MS patient cohorts and registries: recommendations for maximizing impact. Mult Scler . 2018;24:579–586. doi: 10.1177/1352458517698250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurwitz BJ. Registry studies of long-term multiple sclerosis outcomes: description of key registries. Neurology . 2011;76(suppl 1):S3–S6. doi: 10.1212/WNL.0b013e3182050225. [DOI] [PubMed] [Google Scholar]
- 15.Mowry EM, Bermel RA, Williams JR et al. Harnessing real-world data to inform decision-making: Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS) Front Neurol . 2020;11:632. doi: 10.3389/fneur.2020.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook KF, Jensen SE, Schalet BD et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol . 2016;73:89–102. doi: 10.1016/j.jclinepi.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senders A, Hanes D, Bourdette D, Whitham R, Shinto L. Reducing survey burden: feasibility and validity of PROMIS measures in multiple sclerosis. Mult Scler . 2014;20:1102–1111. doi: 10.1177/1352458513517279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Traboulsee A, Simon JH, Stone L et al. Revised recommendations of the Consortium of MS Centers Task Force for a standardized MRI protocol and clinical guidelines for the diagnosis and follow-up of multiple sclerosis. AJNR Am J Neuroradiol . 2016;37:394–401. doi: 10.3174/ajnr.A4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer JS, Rudick RA, Cutter GR, Reingold SC. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler . 1999;5:244–250. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 20.Strober L, DeLuca J, Benedict RH et al. Symbol Digit Modalities Test: a valid clinical trial endpoint for measuring cognition in multiple sclerosis. Mult Scler . 2019;25:1781–1790. doi: 10.1177/1352458518808204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elwood RW. The California Verbal Learning Test: psychometric characteristics and clinical application. Neuropsychol Rev . 1995;5:173–201. doi: 10.1007/BF02214761. [DOI] [PubMed] [Google Scholar]
- 22.Vogel AC, Schmidt H, Loud S, McBurney R, Mateen FJ. Impact of the COVID-19 pandemic on the health care of >1,000 people living with multiple sclerosis: a cross-sectional study. Mult Scler Relat Disord . 2020;46:102512. doi: 10.1016/j.msard.2020.102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manacorda T, Bandiera P, Terzuoli F et al. Impact of the COVID-19 pandemic on persons with multiple sclerosis: early findings from a survey on disruptions in care and self-reported outcomes. J Health Serv Res Policy . 2021;26:189–197. doi: 10.1177/1355819620975069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


