Abstract
Introduction:
Wildfire smoke (WFS) increases the risk of respiratory hospitalizations. We evaluated the association between WFS and asthma health care utilization (AHCU) during the 2013 wildfire season in Oregon.
Methods:
WFS particulate matter ≤2.5 microns in diameter (PM2.5) was estimated using a blended model of in-situ monitoring, chemical transport models, and satellite-based data. Asthma claims and place of service were identified from Oregon All Payer All Claims data from 2013-05-01 to 2013-09-30. The association with WFS PM2.5 was evaluated using time-stratified case-crossover designs.
Results:
The maximum WFS PM2.5 concentration during the study period was 172 μg/m3. A 10 μg/m3 increase in WFS increased risk in asthma diagnosis at emergency departments (odds ratio [OR]: 1.089, 95% Confidence Interval [CI]: 1.043–1.136), office visit (OR: 1.050, 95%CI: 1.038–1.063), and outpatient visits (OR: 1.065, 95%CI: 1.029–1.103); an association was observed with asthma rescue inhaler medication fills (OR: 1.077, 95%CI: 1.065–1.088).
Discussion:
WFS increased the risk for asthma morbidity during the 2013 wildfire season in Oregon. Communities impacted by WFS could see increases in AHCU for tertiary, secondary, and primary care.
Keywords: epidemiology, population based studies, exposure modeling
INTRODUCTION
Wildfires deteriorate air quality in the western United States (U.S.) and can impact air quality over large regions (1–3). In the Western U.S., wildfire smoke (WFS) is a significant contributor to particulate matter (PM) with aerodynamic diameters less than or equal to 2.5 microns (PM2.5), contributing to ~12% of the total daily PM2.5 (4). On days where PM2.5 concentrations exceed the daily National Ambient Air Quality Standard (NAAQS) of 35 μg/m3, WFS contributes > 70% of PM2.5 in the Western U.S. (4). Currently, wildfires result in observable increases in PM2.5 in the Pacific northwest (5). As wildfire frequency is expected to increase due to climate change (6–9). WFS is expected to be the primary source of PM in the Western U.S. by 2020 (10).
To date, population-based WFS epidemiologic studies primarily use readily-available medical claims records (11). These studies have shown that exposure to WFS increases the risk for respiratory morbidity both in the U.S. and internationally (11–14). Exposure to WFS has been associated with an increase in the risk of asthma emergency department (ED) visits (15,16) and asthma hospitalizations (17,18). WFS has been associated with increases respiratory rescue medication fills following wildfires in Canada (19) and Australia (20). These findings suggest that WFS increases the risk for asthma morbidity sufficiently to warrant public health interventions.
There are a limited number of population-based epidemiologic studies that have evaluated the impact of WFS exposure on a broad range of respiratory endpoints. In a population of school-age children, the prevalence of self-reported symptoms (e.g. wheezing, sore throat, itchy/watery eyes, dry cough at night) were higher among participants living in communities that reported smelling smoke than among participants living in communities that reported not smelling smoke following wildfires in Southern California in 2003 (21,22). In two studies of school-age children living in the Brazilian Amazon, WFS was associated with decrease in lung function (23,24). In a population of children and adults with asthma living in Australia, WFS was associated with increases in asthma symptoms, oral steroid medication use, and the use of inhaler rescue medication (20). These findings suggest that WFS is a burden on asthma morbidity and has a much broader impact on asthma beyond acute medical care.
While the effects of air pollutants like PM on hospitalizations and ED visits are costly, other markers of respiratory and asthma related morbidity impact thousands of more people (25). Few studies to date have quantified WFS on a broader range of asthma health endpoints, including office visits, urgent care visits, and medication prescription fills. These health dispositions may be less severe but more prevalent in areas vulnerable to WFS. However, health data to study the relationship between WFS and these dispositions are not always available at the state or national level. For this analysis, we selected Oregon, a state in the Western U.S. that has been affected by numerous large fires and for which we had access to relevant health data.
We evaluated health effects for the 2013 Douglas Complex and Big Windy Complex fires that occurred in southwestern Oregon, which produced a large quantity of WFS that impacted the Medford Metropolitan Statistical Area (MSA). The Douglas Complex fire and Big Windy Complex fire started due to lightning strikes from a thunderstorm that passed through the area on 2013-07-26 and burned >48,000 acres and >19,000 acres, respectively, before both fires were contained by 2013-08-21 (26). The primary aim of this study was to evaluate the associations between WFS and a broader spectrum of asthma health care utilization (AHCU) during the 2013 wildfire season across all of Oregon. A secondary aim focused on evaluating the impact of smoke from the two fires in the southwest region of the state on the metropolitan area of Medford.
METHODS
1. Estimation of WFS PM2.5 and Temperature
We estimated gridded daily PM2.5 in μg/m3 from 2013-05-01 to 2013-09-30 for Oregon using the blended method (also referred to as data fusion) detailed in Lassman et al. (27). Briefly, this method uses geographically weighted ridge regression (GWR) to estimate the expected daily PM2.5 for a given grid cell based on the following predictor variables: PM2.5 estimates from kriged surface site monitors, satellite-based aerosol optical depth (AOD), and modeled PM2.5 from the Weather Research and Forecasting with Chemistry (WRF-Chem) chemical transport model. For kriged PM2.5 estimates, we use EPA Air Quality System (AQS) PM2.5 measurements from sites in Oregon and surrounding states. We use measurements from a combination of Federal Reference Method and Federal Equivalent Method instruments in order to provide the best spatial coverage. We do not account for method or instrument bias or error explicitly in our model. This is a potential limitation and could lead to errors in our model estimates. Satellite (AOD) is from the MODerate Resolution Imaging Spectroradiometer (MODIS) instrument onboard the Terra and Aqua satellites; in particular, we are using the Level 2 AOD Dark-Target Collection 6 retrieval product at 10 km spatial resolution (28). Our modeled simulations of WRF-Chem PM2.5 (29) were focused on the Douglas Complex Fire and used similar model configurations as in Lassman et al. (27). This blended method has been shown to be more accurate at predicting surface site concentrations across a spatial area compared to any one estimation method alone (27)(Supplemental Figure S1 and S2).
To estimate the concentration of daily PM2.5 that may be attributed to WFS, we first estimated background PM2.5 concentrations during the wildfire season (i.e. baseline concentrations in absence of wildfires) by identifying the days when concentrated WFS in the atmospheric column was unlikely. We determined this by verifying smoke plumes from the National Environmental Satellite, Data, and Information Service (NESDIS) Hazard Mapping System (HMS) did not overlap a location (30,31). We found the median value from the GWR-estimated PM2.5 on days with no WFS overhead for each grid cell and then subtracted the median PM2.5 when no WFS was present from the blended GWR daily estimates of PM2.5 to estimate WFS PM2.5. We then population-weighted the daily gridded estimates of WFS PM2.5 to the county level and ZIP Code level using 2010 gridded population density estimates from the Socioeconomic Data and Applications Center (32). We also estimated average daily temperature using WRF-Chem and then population-weighted to the ZIP Code level. Our estimates of ZIP code-level population-weighted WFS PM2.5 and temperature were linked to the AHCU data and evaluated using methods detailed in Section 3.
2. Health Outcomes Records
We used the Oregon All Payer All Claims Database (APAC) from 2013 for our health outcome records. The APAC contains billing data on International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) medical diagnosis codes and National Drug Codes (NDC) pharmacy fills for persons insured in the state of Oregon. Additional patient-level data included place of service code, date of admission, admission type, age, sex, reported ZIP code of residence, and a unique de-identified patient identifier. We restricted our health outcomes to records of asthma admissions or short-acting beta-2-agonists (SABA) pharmacy fills, which are broadly used to treat symptoms of inflammatory lung conditions and the preferred class of reliever medication prescribed to almost all persons with asthma for on demand use (33). We further restricted the time period to records occurring between 2013-05-01 to 2013-09-30, which spanned two months before and two months after the Douglas Complex and Big Windy fires that took place in late July through August.
We assessed the primary diagnosis ICD-9-CM codes of 493 as a proxy measure for an asthma event; ICD-9-CM of 493 can only be assigned to confirmed cases of asthma for inpatient and emergency department places of service. For asthma inhaler rescue medication, we referenced The Healthcare Effectiveness Data and Information Set (HEDIS) Asthma Controller, and Reliever Medications table AMR-A for 2014 to identify national drug codes (NDC) codes classified as SABA with inhalation as the route of exposure (201 NDC codes met this definition) (34).
3. Time-Stratified Case-Crossover: Association between WFS PM2.5 and AHCU
Our first series of analyses evaluated the association between WFS PM2.5 and AHCU by evaluating records for a primary diagnosis of asthma for any person billed with a primary residence in Oregon at the following service places: ambulance, ED, office (defined as ambulatory care provided by a medical professional not in a hospital), inpatient hospital, outpatient hospital, SABA pharmacy fills, and urgent care facilities. We used a time-stratified case-crossover study design; this is a case only study design that controls for within-subject variability and controls for some time-varying factors such as day of the week (35,36). Asthma events are defined as a single asthma-related visit to a place of service or a SABA fill. We further restricted these records to the first observation of a unique patient to reduce the potential bias of counting the same person multiple times. For each specific asthma event, we selected referent observations on the same day of the week from 2013-05-01 to 2013-09-30. We then linked population-weighted PM2.5 and mean daily temperature values by patient-reported ZIP code and date. The associations for each place of service asthma event and WFS PM2.5 were analyzed using separate conditional logistic regressions, adjusting for temperature. Subsequent strata-specific analyses of AHCU by sex, and age categories of <15, 15–65, and >65 years were performed. Age categories were decided based on our previous work evaluating hospital admissions and WFS in Washington 2012 (17). We considered an association to be statistically significant if the 95% confidence interval (95% CI) did not contain an odds ratio (OR) of one.
4. Description of Time Series of WFS PM2.5 and AHCU by Metropolitan Statistical Area
Daily counts of AHCU for all Oregon MSAs of Bend, Corvallis, Eugene, Medford, Portland, Salem combined, and only the Medford MSA were calculated by summing AHCU events for all Oregon MSAs and for Medford; this count of AHCU events included multiple events for the same patient during the study period. The AHCU event count was then divided by 2013 U.S. Census estimates of summed Oregon MSA populations and Medford-specific populations (37) and multiplied by 100,000 to estimate the daily count per 100,000 persons from 2013-05-01 to 2013-09-30. We plotted the time series of WFS PM2.5 by MSA and the observed daily counts of AHCU for all Oregon MSAs overlayed and Medford to compare the WFS PM2.5 time series, Oregon AHCU time series, and Medford time series. Expected AHCU counts were estimated from the observed counts using natural splines with 3 degree of freedom; expected SABA fills and office visits also accounted for weekday/weekend differences using an interaction term based on observed differences in weekend/weekday trends.
5. Time Series: Association between WFS Exposure and ACHU in Medford
The descriptive plots of the asthma office visit and SABA fill counts in Medford showed a noticeable increase and deviation from the expected counts of these two markers of ACHU during the same time period there was a dramatic increase WFS PM2.5 in Medford. e investigated if AHCU time series were associated with the time series of WFS using a time series analysis of daily asthma care counts and a binary classifier of smoke of >15 μg/m3 WFS PM2.5 in the Medford MSA. A binary classifier with a strict cutoff was used to increase the specificity of capturing a day that was impacted by WFS. Separate quasi-Poisson models were fit for each service place and SABA fills, where counts were regressed on the binary classifier of smoke, average daily temperature, a natural spline with three degrees of freedom to account for seasonal trends, indicator for weekend, and a log population offset. For each outcome, we calculated relative risk ratio (RR) and 95% confidence interval (CI), percent of risk attributable to smoke exposure: (RR-1)/RR*100.
6. Software
We used R software for all data processing, statistical analyses, and visualization (38). The “tidyverse” package was used for data processing, “ggplot2” package for mapping and plotting (39) and the “survival” package for conditional logistic regression (40).
7. Institutional Review Board
This study used secondary claims data and did not require participant consent. Study design, data security and privacy using secondary claims data was reviewed and approved by the Research Integrity and Compliance Review Office at Colorado State University and The Oregon Health Authority.
RESULTS
1. Description of Wildfire Smoke
Shown in Figure 1 are fire locations and the number of smoke-impacted days where daily WFS PM2.5 > 15 μg/m3 in Oregon counties from 2013-05-01 to 2013-09-30. Oregon had several fires during the 2013 wildfire season, with the largest being the Douglas Complex and Big Windy Complex Fire that took place in the southwest corner of Oregon (Figure 1). The southwest region of Oregon had the highest number of smoke-impacted days during the study period, particularly Josephine and Jackson Counties, which includes Medford (Figure 1).
Figure 1:
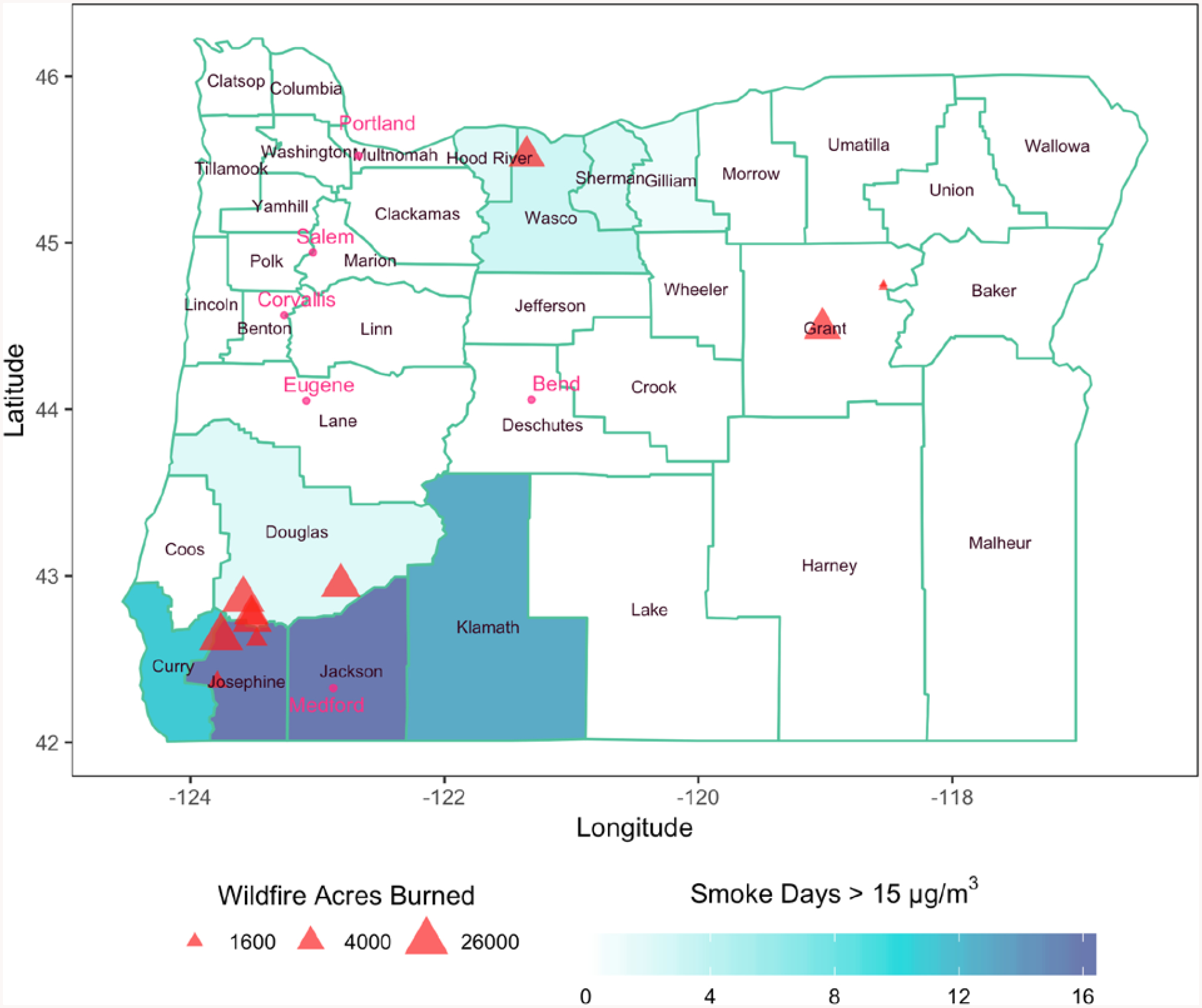
Number of smoke-impacted days where WFS PM2.5 > 15 μg/m3 in Oregon State counties from 2013-05-01 to 2013-09-30. Fire locations are represented by triangles and are proportional to acres burned by the fire. Metropolitan areas are represented by circles.
2. Descriptions of AHCU
The APAC dataset represents all insured persons who receive medical care or fill a drug prescription in Oregon. There were 54,177 asthma medical claims (ambulance n=31, ED n=1,913; inpatient hospital n=1,209; office n=33,346; outpatient hospital n=3,762; urgent care 620) and 76,025 SABA fill records for 59,175 unique persons billed for a medical expense or pharmacy fill in the state of Oregon from 2013-05-01 to 2013-09-01. The number of the first-observation-per-patient AHCU events are presented in Table 1. Office visits and SABA fills made up the majority of AHCU during the study period (Table 1).
Table 1:
Number of AHCU events by place of service from 2013-05-01 to 2013-08-30 in the state of Oregon. Age category and sex category are also included. Percentage for strata-specific ambulance place of service not presented due to small numbers in strata categories (–).
| Outcome | Cases (n) | Age Category | Sex | |||
|---|---|---|---|---|---|---|
| < 15 (%) | 15 to 65 (%) | > 65 (%) | Female (%) | Male (%) | ||
| Ambulance | 31 | -- | -- | -- | -- | -- |
| Emergency Department | 1904 | 34.1 | 57.3 | 8.6 | 58.1 | 41.9 |
| Inpatient Hospital | 586 | 29.2 | 45.7 | 25.0 | 62.4 | 37.6 |
| Outpatient Hospital | 3762 | 31.6 | 54.2 | 14.2 | 58.5 | 41.5 |
| Office | 28616 | 29.3 | 58.1 | 12.6 | 56.9 | 43.1 |
| SABA Fill | 24217 | 29.5 | 58.1 | 12.4 | 58.5 | 41.5 |
| Urgent Care Facility | 590 | 18.1 | 76.4 | 5.5 | 56.6 | 43.4 |
| -- Data were suppressed due to small numbers.(Suppression criteria n < 10) | ||||||
3. Time-Stratified Case-Crossover: Association between WFS PM2.5 and AHCU
The same-day association between WFS PM2.5 and AHCU by strata from the time-stratified case-crossover design is presented in Figure 2. For all strata combined, we observed significant associations between a 10 μg/m3 increase in WFS PM2.5 and asthma-related medical visits/admissions for the following places of service: ED admissions (OR: 1.089, 95%CI: 1.043–1.136), SABA pharmacy fills (OR: 1.077, 95%CI: 1.065–1.088), office visits (OR: 1.050, 95%CI: 1.038–1.063), and outpatient hospital admissions (OR: 1.065, 95%CI: 1.029–1.103); inpatient hospitalizations (OR: 1.072, 95%CI: 0.995–1.154). No association was observed for urgent care visits or ambulance use. ORs and 95% CIs for ‘all strata’ presented in Supplemental Table S1.
Figure 2:

Same-day association between a 10 μg/m3 increase in WFS PM2.5 and risk for AHCU event by strata, adjusting for temperature. Stratum-specific estimates for ambulance admissions not presented due to small numbers; age > 65 years stratum-specific estimate for urgent care admission not presented due to an unstable model.
Sex specific associations between a 10 μg/m3 increase in WFS PM2.5 and AHCU are presented in Figure 2. For males, increases in WFS were associated with outpatient hospital (OR: 1.079, 95%CI: 1.022–1.140), office visits (OR: 1.031, 95%CI: 1.010–1.052), and SABA pharmacy fills (OR: 1.059, 95%CI: 1.039–1.079). Elevated, but not statistically significant associations were observed for ED admission (OR: 1.078, 95%CI: 0.995–1.168) and inpatient hospital admissions (OR: 1.113, 95%CI: 0.955–1.297) (Figure 2). No association was observed for urgent care visits (OR: 0.716, 95%CI: 0.345 – 1.487) (Figure 2). Ambulance use was not analyzed due to small sample size. For females, associations were similar for ED admissions, outpatient, and inpatient hospital admissions in females (Figure 2). Notable differences in sex specific associations were observed for office visits, where females had stronger associations (OR: 1.061, 95%CI: 1.046–1.077) compared to males (OR: 1.031, 95%CI: 1.010–1.052), and for SABA fills, where females had stronger associations (OR: 1.087, 95%CI: 1.073–1.101) compared to males (OR: 1.059, 95%CI: 1.039–1.079) for SABA fills (Figure 2). Sex specific strata ORs are presented in Supplemental Table S2.
The association between WFS and age categories are presented in Figure 2. A 10 μg/m3 increase in WFS PM2.5 was associated with emergency department admissions in age 15–65 years (OR: 1.098, 95%CI: 1.043–1.156), and suggestive of an association in the age < 15 years group (OR: 1.050, 95%CI: 0.946–1.166) and > 65 years age group (OR: 1.096, 95%CI: 0.977–1.229). WFS was associated with outpatient hospital admission in the age 15–65 years strata (OR: 1.076, 95%CI: 1.030–1.123), and trended in the same direction for age < 15 years (OR: 1.045, 95%CI: 0.959–1.139) and age > 65 years (OR: 1.049, 95%CI: 0.969–1.140). We observed no association between inpatient hospital admissions and WFS when stratified by age (Figure 2). We observed associations between WFS and office visits across all age strata: age < 15 years (OR: 1.032, 95%CI: 1.006–1.058), age 15–65 years (OR:1.062, 95%CI: 1.046–1.078), and > 65 years (OR:1.036, 95%CI: 1.005–1.068). In addition, we observed associations between WFS and SABA pharmacy fills for all age strata: age < 15 years (OR: 1.056, 95%CI: 1.031–1.081), age 15–65 years (OR:1.088, 95%CI: 1.074–1.103), and age > 65 years (OR: 1.056, 95%CI: 1.024–1.090). No statistically significant association was observed with urgent care. ORs and 95%CIs for age category-specific strata are presented in Supplemental Table S3.
Our decision to evaluate the first AHCU event in the time-stratified case-crossover design was made to avoid the scenario where a second event falls on a referent day of the first event and to ensure independence of events within each subject. However, this decision could have introduced bias, as it does not account for factors that could influence multiple events over the study period. We performed sensitivity analyses to assess this bias using all events for AHCU observed during the study period. The sensitivity results are presented in Supplemental Figure S3 and mostly led to similar conclusions. For rare outcomes such as ED or urgent care admissions where a subject is unlikely to use a service frequently during the five-month window, the results were similar when we used all events and just the first event (Figure S3). For more frequent outcomes such as a SABA fill or office visit, limiting to the first event could have resulted in an overestimation of the association (Figure S3). The biggest difference observed was with inpatient events, where there was a marginally significant association when limited to the first event, but no association when considering all events (Figure S3).
4. Description of Time Series of WFS PM2.5 and AHCU by Metropolitan Statistical Area
The Douglas Complex and Big Windy Complex fires in southern Oregon produced a large quantity of WFS that impacted the Medford MSA. Figure 3A shows the average of daily ZIP-code population-weighted WFS PM2.5 concentrations in μg/m3 in each Oregon MSA from 2013-05-01 to 2013-09-30. Figure 3A shows that the Medford MSA had elevated concentrations of WFS PM2.5 in late July 2013 through August 2013, with concentrations exceeding 50 μg/m3 for multiple days. The Bend MSA may have experienced some elevated concentrations of WFS PM2.5 during late July 2013 through early August 2013 (Figure 3A).
Figure 3:
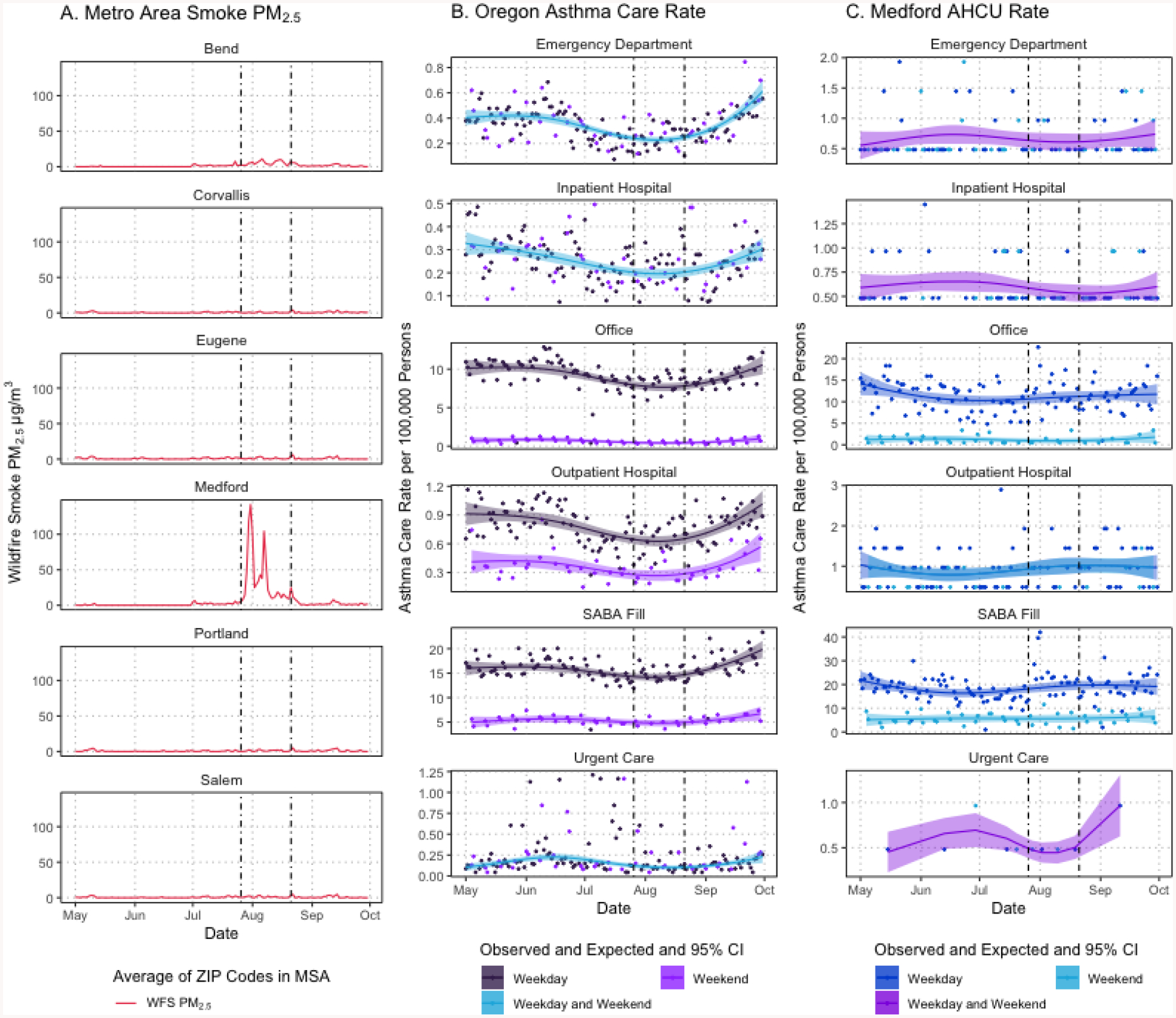
(A) Averaged daily wildfire smoke PM2.5 by MSA, (B), weekday and weekend observed and expected with 95% CI daily AHCU counts per 100,000 persons for all Oregon MSAs, (C) and weekday and weekend observed and expected with 95%CI daily AHCU counts per 100,000 persons for Medford MSA from 2013-05-01 to 2013-09-30. Points represent observed daily AHCU counts and lines and ribbons represent expected count per 100,000 persons and 95%CI. Weekend expected counts only estimated for outpatient hospital, SABA fill, and office visits; weekday expected counts for ED, inpatient hospital, and urgent care includes weekend. Dashed vertical lines represent period of interest when wildfire smoke impacted the Medford MSA.
Daily counts of AHCU per 100,000 persons by place of service and SABA fill in all Oregon MSAs over the 2013 wildfire season are shown in Figure 3B. The last week of July and first two weeks of August had the highest counts of SABA fills overall, and an elevated count of office visits relative to the earlier part of July for Medford (Figure 3C), which correspond to elevations in WFS PM2.5 (Figure 3A). Trends in the daily counts for the other places of service in Medford were not as clear due to fewer events (Figure 3C). We did not plot the counts of ambulance use during the wildfire season due to small number of events (Table 1).
5. Time Series: Association between WFS Exposure and ACHU in Medford
WFS PM2.5 >15 μg/m3 was associated with an increase in the daily counts of SABA fills (RR: 1.425, 95% CI: 1.203–1.687) and asthma-related office visits (RR: 1.366, 95% CI: 1.107–1.687) in the Medford MSA during the period of 2013-05-01 to 2013-09-30 (Figure 4). We estimated that 29.8% (95% CI: 16.9%−40.7%) of SABA fills and 26.8% (95% CI: 9.7%−40.7%) of asthma-related office visits could be attributed to WFS exposure on a given day in the Medford MSA during the study period. A marginally significant association was observed for urgent care admissions (RR: 1.602, 95% CI: 0.996–2.576). We estimate that 37.6% (95% CI: −0.4%−61.2%) of asthma-related urgent care admissions could be attributed to WFS exposure on a given day in the Medford MSA during the study period. No significant associations were observed between WFS PM2.5 >15 μg/m3 and daily counts of ED admissions, inpatient hospital admissions, and outpatient hospital admissions. Sensitivity analyses for alternative binary cutoffs of WFS PM2.5 >10 μg/m3 and >5 μg/m3 are presented in Supplemental Table S4. Results for WFS PM2.5 >10 μg/m3 and >15 μg/m3 were similar. However, the effects were not as strong across all outcomes for WFS PM2.5 >5 μg/m3 when compared to the higher cutoff values (Supplemental Table S4).
Figure 4:
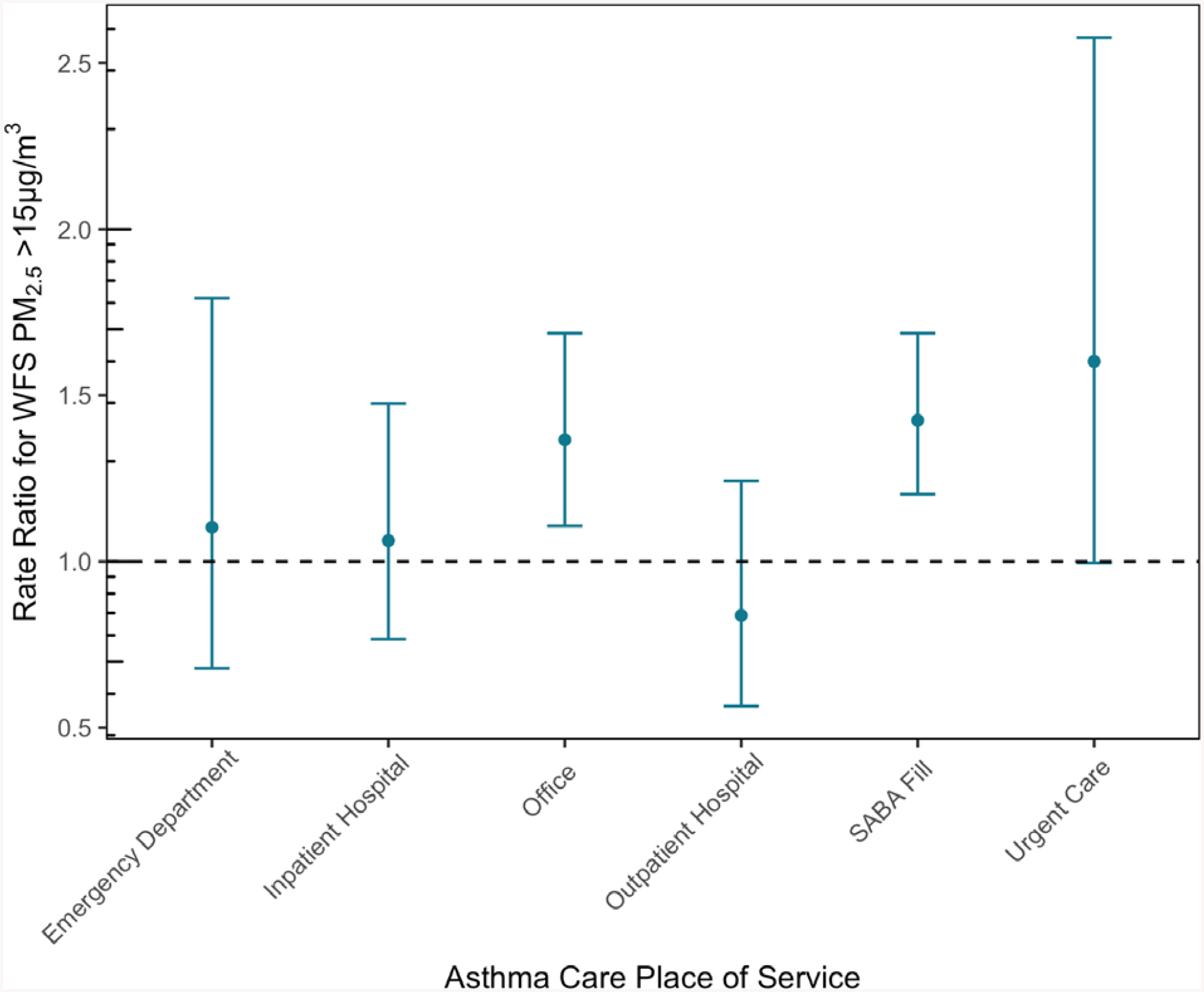
Same-day association between WFS PM2.5 >15 μg/m3 and rate ratio for asthma care utilization for Medford MSA, adjusting for temperature, seasonality, and weekend.
DISCUSSION
Results from the time-stratified case-crossover design showed WFS increases risk for AHCU, a proxy for asthma-related morbidity. The effect of WFS on asthma morbidity extends beyond more severe health endpoints like admission to an ED, to include office visits and refills of inhaler rescue medication. In our statewide evaluation, our analysis demonstrates that the risk for asthma ED admissions, office visits, outpatient admissions, and SABA fills are significantly associated with increases in WFS PM2.5 in Oregon during the study period. Effect estimates for inpatient and ambulance use were in agreement with WFS PM2.5 increasing risk for AHCU even though the 95% CIs for these estimates overlapped one for the time-stratified case-crossover design (Figure 2).
No significant association was observed between WFS and ambulance use due to the small number of events, but the effect estimate was of similar magnitude and direction to other places of service. Null associations were observed with urgent care visits in the time-stratified case-crossover design, which could be due to the availability of urgent care places of service in a given area or preference to access ED facilities over urgent care. The effect of WFS on SABA fills and asthma office visits may have been stronger in females compared to males, and in the age 15–65 age group compared to <15 and >65. It is possible that there might be some difference in the way these groups access care, however the confidence intervals overlap for the effect estimates making it difficult to draw any definitive conclusions regarding effect modification.
In our evaluation of Medford MSA, office visits and SABA fills made up the largest proportion of AHCU in this MSA. Furthermore, WFS from the Douglas and Big Windy fires increased the daily counts for both asthma-related office visits and SABA fills, where up to 26% of office visits and 29% of SABA fills on a given smoky day may be attributed to WFS during this time period. WFS also led to marginally significant increases in the urgent care admission counts. However, we did not observe significant associations with counts of ED or outpatient admissions in our evaluation of Medford, but did observe significant associations with these two outcomes in our statewide evaluation using the time-stratified case-crossover design. Differences that could explain results discrepancies could be fewer events and therefore higher variance around point estimates in Medford and differences in transforming our continuous measure of WFS to a binary cutoff, or residual confounding in either the time-series or time-stratified case-crossover design.
Although we observed some differences in our results between the state-wide time-stratified case-crossover design and our Medford time series design, our use of the time series design allowed us to calculate the percent of events attributed to WFS from the Douglas-Complex and Big Windy Complex fires for the community of Medford. This would not have been a feasible metric to calculate using a time-stratified case-crossover design as it is a case-only design.
Our sensitivity analyses of the first AHCU event compared to using all events suggested WFS increased the risk of AHCU, regardless of approach. One notable exception was with inpatient admissions, where the association trended towards significance when using single events (Figure 1) and no association when using all events (Figure S3). We believe this is likely due to duplicate counting as we were unable to determine if a person had a single event and had to stay for a multiple day or had multiple events over a couple days, and that analysis limited to the first event is likely to be less biased than the all events analysis.
In our estimation of WFS, we repeated the methods outlined in Lassman et al. (27). As in Lassman et al. (27), we found that using GWR with satellite AOD, surface monitor data, and model output outperformed any single data source. However, our estimates were not as robust as for the Washington fires of 2012 (for example, R2=0.60 compared to 0.66 and a slope of 0.58 compared to 0.78 using leave-one-out cross validation; results shown in Supplemental Figure S1 and S2). This is likely due to 1) fewer surface air quality sites in Oregon compared to Washington, 2) more localized smoke in Oregon, and 3) shorter time period of elevated PM2.5 in Oregon 2013 compared to Washington 2012. All of these factors likely resulted in more measurement error bias in this study compared to our previous study.
Measurement error in our exposure estimates could also explain our differences in results statewide vs. Medford. Our estimates of WFS are likely more accurate in Medford compared to areas in Oregon due to more surface sites located in Medford. Surface PM2.5 sites are the best predictor in our model (Figure S1 and S2), and Lassman et al. (27) demonstrated that the GWR model generally performs better in regions with more surface sites compared to regions where the GWR model relies more on the WRF-Chem estimates and satellite AOD. Furthermore, our subtraction of seasonal background PM2.5 from GWR PM2.5 does not entirely separate out PM2.5 from WFS vs. anthropogenic sources, and it is possible that some of our associations between WFS PM2.5 and AHCU could be driven by effects of anthropogenic air pollution. This imperfect identification of anthropogenic influence on our WFS PM2.5 estimate influenced our decision to use a binary classifier of WFS in our Medford analyses, where our cutoff of WFS PM2.5 >15 μg/m3 increased the specificity of capturing an area impacted by WFS on a given day. Improving the accuracy of estimating WFS PM2.5 and source apportionment of PM2.5 from WFS remains a challenge and presents opportunities for research in future studies.
The associations between WFS and asthma morbidity observed in this study are supported by findings from our previous work that evaluated cardiopulmonary hospitalizations following the 2012 Wenatchee-Complex fires that took place in Central Washington (17), where we observed an increased risk in asthma inpatient hospitalizations admitted through ED and urgent care visits. Results for the two states were comparable: in Washington, we observed a 7.6% increase in the risk of an asthma inpatient hospitalization per 10 μg/m3 increase in WFS (OR: 1.076, 95%CI: 1.019–1.136) when using a nearly identical study design for 1,456 asthma inpatient events (17). In Oregon, we found a 7.2% increase per 10 μg/m3 increase in WFS (OR: 1.072, 95%CI: 0.995–1.154) on 586 asthma inpatient events. Some differences that may explain the wider 95%CIs in this study compared to our Washington study could be attributed to the larger number of asthma inpatient events in Washington, and that WFS from the Wenatchee-Complex fire impacted a larger population (17). Another explanation that could explain the wider 95%CIs could be that we used a less specific outcome definition of inpatient visit in this study that did not contain the admitting source of ED or urgent care.
Multiple large-scale claims-based WFS epidemiologic studies have linked exposure to WFS and asthma, with a focus ED admissions (15,16) or inpatient hospital admissions (17,18) as separate outcomes. Our study provides additional evidence that WFS increases the risk of AHCU, including asthma rescue inhaler medication fills, which has also been observed in a population in British Columbia, Canada (19). To our knowledge, this study is the first to replicate these findings in a U.S. population and find an association with asthma-related office and outpatient hospital visits. We also show that the greatest burden of asthma care could be in office visits and SABA fills following a WFS event.
APAC data present an opportunity for environmental epidemiology research, as these types of data can offer insight on the broader utilization of medical care compared to just inpatient or ED records alone. APAC data also allow linking of multiple medical events on the same person over time, which would allow for assembly of retrospective cohort studies and allow environmental epidemiologists to address questions of repeat or prolonged exposure. However, use of linked medical records presents challenges in defining the cohort and population at risk, and handling right-censoring of a subject (e.g. whether due to another medical event, mortality, or loss to follow-up due to moving out of state).
Use of medical billing data and ICD-9-CM codes presents its own challenges that could result in the misclassification of proxy healthcare outcome, including translating paper records to electronic records, facility quality control, coder training and experience, unintentional coder errors, upcoding (i.e. where procedures that can be billed at a higher rate are used) (41). Despite these challenges in using medical records and APAC data, we believe that identifying novel methods, best-practices, and workflows when using linked medical records data in environmental epidemiology studies are important areas of future research.
In this study, we show that WFS increased AHCU throughout the state of Oregon. Furthermore, our analyses focused on WFS from the Douglas Complex and Big Windy fires that impacted Medford show that even in cities or areas with smaller populations WFS exposure can have a significant effect on care utilization. This study suggests that WFS may have a broad effect on persons with asthma, increasing the need for medical attention or inhaler rescue medication and office visits. Our results suggest that WFS has a larger burden on the healthcare system beyond the tertiary and secondary care of inpatient hospitalizations and emergency department admissions to include primary care office visits. Public health practitioners, healthcare providers, and pharmacies should be aware of the possible increase in care utilization in persons with asthma and other sensitive groups that may result from smoke following a wildfire.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this study was provided by the National Aeronautics and Space Administration grant number NNX15AF35G and the A.J. Kauvar Foundation.
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Colarco PR, Schoeberl MR, Doddridge BG, Marufu LT, Torres O, Welton EJ. Transport of smoke from Canadian forest fires to the surface near Washington, D.C.: Injection height, entrainment, and optical properties: SMOKE FROM CANADIAN FOREST FIRES. J Geophys Res Atmospheres. 2004. Mar 27;109(D6):n/a–n/a. [Google Scholar]
- 2.DeBell LJ. A major regional air pollution event in the northeastern United States caused by extensive forest fires in Quebec, Canada. J Geophys Res [Internet]. 2004. [cited 2017 Aug 4];109(D19). Available from: http://doi.wiley.com/10.1029/2004JD004840 [Google Scholar]
- 3.Val Martin M, Heald CL, Lamarque J-F, Tilmes S, Emmons LK, Schichtel BA. How emissions, climate, and land use change will impact mid-century air quality over the United States: a focus on effects at national parks. Atmospheric Chem Phys. 2015. Mar 10;15(5):2805–23. [Google Scholar]
- 4.Liu JC, Mickley LJ, Sulprizio MP, Dominici F, Yue X, Ebisu K, et al. Particulate air pollution from wildfires in the Western US under climate change. Clim Change. 2016. Oct;138(3–4):655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McClure CD, Jaffe DA. US particulate matter air quality improves except in wildfire-prone areas. Proc Natl Acad Sci. 2018. Jul 31;115(31):7901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford B, Val Martin M, Zelasky SE, Fischer EV, Anenberg SC, Heald CL, et al. Future Fire Impacts on Smoke Concentrations, Visibility, and Health in the Contiguous United States. GeoHealth [Internet]. 2018. Aug 3 [cited 2018 Sep 11]; Available from: http://doi.wiley.com/10.1029/2018GH000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langmann B, Duncan B, Textor C, Trentmann J, van der Werf GR. Vegetation fire emissions and their impact on air pollution and climate. Atmos Environ. 2009. Jan;43(1):107–16. [Google Scholar]
- 8.Turetsky MR, Kane ES, Harden JW, Ottmar RD, Manies KL, Hoy E, et al. Recent acceleration of biomass burning and carbon losses in Alaskan forests and peatlands. Nat Geosci. 2011. Jan;4(1):27–31. [Google Scholar]
- 9.Westerling AL, Hidalgo HG, Cayan DR, Swetnam TW. Warming and earlier spring increase western U.S. forest wildfire activity. Science. 2006. Aug 18;313(5789):940–3. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Environmental Protection Agency. The 2014 National Emissions Inventory. 2014. [Google Scholar]
- 11.Liu JC, Pereira G, Uhl SA, Bravo MA, Bell ML. A systematic review of the physical health impacts from non-occupational exposure to wildfire smoke. Environ Res. 2015. Jan;136:120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu JC, Wilson A, Mickley LJ, Dominici F, Ebisu K, Wang Y, et al. Wildfire-specific Fine Particulate Matter and Risk of Hospital Admissions in Urban and Rural Counties: Epidemiology. 2017. Jan;28(1):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu JC, Wilson A, Mickley LJ, Ebisu K, Sulprizio MP, Wang Y, et al. Who among the elderly is most vulnerable to exposure and health risks of PM2.5 from wildfire smoke? Am J Epidemiol [Internet]. 2017. May 19 [cited 2017 Jul 27]; Available from: https://academic.oup.com/aje/article-lookup/doi/10.1093/aje/kwx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid CE, Brauer M, Johnston FH, Jerrett M, Balmes JR, Elliott CT. Critical Review of Health Impacts of Wildfire Smoke Exposure. Environ Health Perspect [Internet]. 2016. Apr 15 [cited 2016 Nov 30];124(9). Available from: http://ehp.niehs.nih.gov/14-09277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston FH, Kavanagh AM, Bowman DMJS, Scott RK. Exposure to bushfire smoke and asthma: an ecological study. Med J Aust. 2002. Jun 3;176(11):535–8. [DOI] [PubMed] [Google Scholar]
- 16.Rappold AG, Cascio WE, Kilaru VJ, Stone SL, Neas LM, Devlin RB, et al. Cardio-respiratory outcomes associated with exposure to wildfire smoke are modified by measures of community health. Environ Health [Internet]. 2012. Dec [cited 2018 Jun 5];11(1). Available from: http://ehjournal.biomedcentral.com/articles/10.1186/1476-069X-11-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gan RW, Ford B, Lassman W, Pfister G, Vaidyanathan A, Fischer E, et al. Comparison of wildfire smoke estimation methods and associations with cardiopulmonary-related hospital admissions: Estimates of Smoke and Health Outcomes. GeoHealth. 2017. May;1(3):122–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin KL, Hanigan IC, Morgan GG, Henderson SB, Johnston FH. Air pollution from bushfires and their association with hospital admissions in Sydney, Newcastle and Wollongong, Australia 1994–2007. Aust N Z J Public Health. 2013. Jun;37(3):238–43. [DOI] [PubMed] [Google Scholar]
- 19.Elliott CT, Henderson SB, Wan V. Time series analysis of fine particulate matter and asthma reliever dispensations in populations affected by forest fires. Environ Health [Internet]. 2013. Dec [cited 2017 Jul 27];12(1). Available from: http://ehjournal.biomedcentral.com/articles/10.1186/1476-069X-12-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston FH, Webby RJ, Pilotto LS, Bailie RS, Parry DL, Halpin SJ. Vegetation fires, particulate air pollution and asthma: A panel study in the Australian monsoon tropics. Int J Environ Health Res. 2006. Dec;16(6):391–404. [DOI] [PubMed] [Google Scholar]
- 21.Künzli N, Avol E, Wu J, Gauderman WJ, Rappaport E, Millstein J, et al. Health effects of the 2003 Southern California wildfires on children. Am J Respir Crit Care Med. 2006. Dec 1;174(11):1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirabelli MC, Künzli N, Avol E, Gilliland FD, Gauderman WJ, McConnell R, et al. Respiratory Symptoms Following Wildfire Smoke Exposure: Airway Size as a Susceptibility Factor. Epidemiology. 2009. May;20(3):451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson L da SV, Hacon S de S, Castro HA de, Ignotti E, Artaxo P, Ponce de Leon ACM. Association between fine particulate matter and the peak expiratory flow of schoolchildren in the Brazilian subequatorial Amazon: A panel study. Environ Res. 2012. Aug;117:27–35. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson L da SV, Hacon S de S, Castro HA de, Ignotti E, Artaxo P, Saldiva PHN, et al. Acute Effects of Particulate Matter and Black Carbon from Seasonal Fires on Peak Expiratory Flow of Schoolchildren in the Brazilian Amazon. Sun Q editor. PLoS ONE. 2014. Aug 13;9(8):e104177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.U.S. Environmental Protection Agency. Benefits Mapping and Analysis Program (BenMAP) [Internet]. 2018. Available from: https://www.epa.gov/benmap/how-benmap-ce-estimates-health-and-economic-effects-air-pollution
- 26.Oregon Department of Forestry Southwest Oregon District [Internet]. 2013. [cited 2018 Jun 5]. Available from: http://www.swofire.com/2013_08_18_archive.html
- 27.Lassman W, Ford B, Gan RW, Pfister G, Magzamen S, Fischer EV, et al. Spatial and Temporal Estimates of Population Exposure to Wildfire Smoke during the Washington State 2012 Wildfire Season Using Blended Model, Satellite, and In-Situ Data: Mult-method estimates of smoke exposure. GeoHealth [Internet]. 2017. [cited 2017 Mar 31]; Available from: http://doi.wiley.com/10.1002/2017GH000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayer AM, Munchak LA, Hsu NC, Levy RC, Bettenhausen C, Jeong M-J. MODIS Collection 6 aerosol products: Comparison between Aqua’s e-Deep Blue, Dark Target, and “merged” data sets, and usage recommendations. J Geophys Res Atmospheres. 2014. Dec 27;119(24):13,965–13,989. [Google Scholar]
- 29.Grell GA, Peckham SE, Schmitz R, McKeen SA, Frost G, Skamarock WC, et al. Fully coupled “online” chemistry within the WRF model. Atmos Environ. 2005. Dec;39(37):6957–75. [Google Scholar]
- 30.Rolph GD, Draxler RR, Stein AF, Taylor A, Ruminski MG, Kondragunta S, et al. Description and Verification of the NOAA Smoke Forecasting System: The 2007 Fire Season. Weather Forecast. 2009. Apr;24(2):361–78. [Google Scholar]
- 31.Ruminski MG, Kondragunta S, Draxler RR, Zeng J. Recent changes to the Hazard Mapping System. 2016. Jun; [Google Scholar]
- 32.Center for International Earth Science Information Network - CIESIN - Columbia University. Gridded Population of the World, Version 4 (GPWv4): Population Density, Revision 10 [Internet]. NASA Socioeconomic Data and Applications Center (SEDAC); 2017. Available from: 10.7927/H4DZ068D [DOI]
- 33.Lougheed MD, Lemiere C, Ducharme FM, Licskai C, Dell SD, Rowe BH, et al. Canadian Thoracic Society 2012 Guideline Update: Diagnosis and Management of Asthma in Preschoolers, Children and Adults. Can Respir J. 2012;19(2):127–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Committee for Quality Assurance. HEDIS 2014 Final NDC Lists [Internet]. 2014. Available from: http://www.ncqa.org/hedis-quality-measurement/hedis-measures/hedis-2014/hedis-2014-final-ndc-lists
- 35.Janes H, Sheppard L, Lumley T. Case-Crossover Analyses of Air Pollution Exposure Data: Referent Selection Strategies and Their Implications for Bias. Epidemiology. 2005. Nov;16(6):717–26. [DOI] [PubMed] [Google Scholar]
- 36.Janes H, Sheppard L, Lumley T. Overlap bias in the case-crossover design, with application to air pollution exposures. Stat Med. 2005. Jan 30;24(2):285–300. [DOI] [PubMed] [Google Scholar]
- 37.U.S. Census Bureau. Metropolitan and Micropolitan Statistical Areas Totals: 2010–2016 [Internet]. 2016. Available from: https://www.census.gov/data/tables/2016/demo/popest/total-metro-and-micro-statistical-areas.html
- 38.R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2016. Available from: http://www.R-project.org/ [Google Scholar]
- 39.Wickham H tidyverse: Easily Install and Load the “Tidyverse”. [Internet]. 2017. Available from: https://CRAN.R-project.org/package=tidyverse
- 40.Therneau Terry M.. A package for survival analysis in S. [Internet]. 2015. Available from: https://CRAN.R-project.org/package=survival
- 41.O’Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring Diagnoses: ICD Code Accuracy. Health Serv Res. 2005. Oct;40(5p2):1620–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


