Abstract
Reactive oxygen species (ROS) are rapidly eliminated and reproduced in organisms, and they always play important roles in various biological functions and abnormal pathological processes. Evaluated ROS have frequently been observed in various cancers to activate multiple pro-tumorigenic signaling pathways and induce the survival and proliferation of cancer cells. Hydrogen peroxide (H2O2) and superoxide anion (O2•−) are the most important redox signaling agents in cancer cells, the homeostasis of which is maintained by dozens of growth factors, cytokines, and antioxidant enzymes. Therefore, antioxidant enzymes tend to have higher activity levels to maintain the homeostasis of ROS in cancer cells. Effective intervention in the ROS homeostasis of cancer cells by chelating agents or metal complexes has already developed into an important anti-cancer strategy. We can inhibit the activity of antioxidant enzymes using chelators or metal complexes; on the other hand, we can also use metal complexes to directly regulate the level of ROS in cancer cells via mitochondria. In this review, metal complexes or chelators with ROS regulation capacity and with anti-cancer applications are collectively and comprehensively analyzed, which is beneficial for the development of the next generation of inorganic anti-cancer drugs based on ROS regulation. We expect that this review will provide a new perspective to develop novel inorganic reagents for killing cancer cells and, further, as candidates or clinical drugs.
Keywords: anti-cancer, reactive oxygen species, chelators, metal complexes, antioxidant enzymes, SOD1, TrxR, mitochondria
1. Introduction
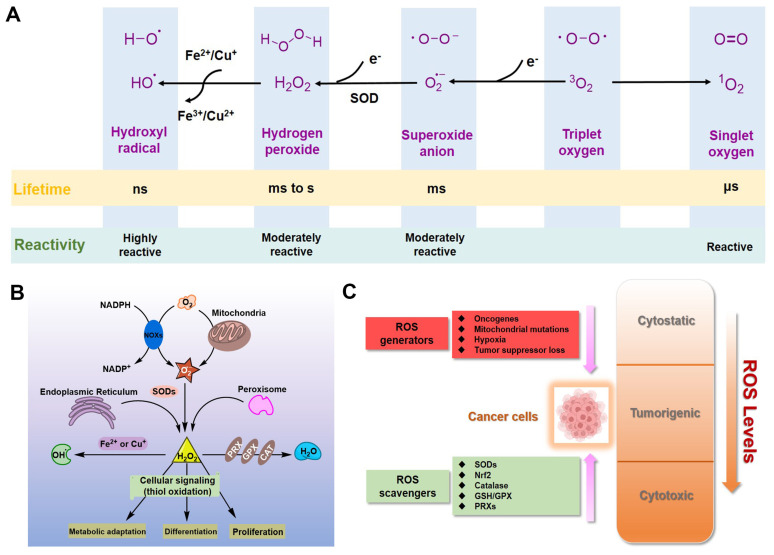
For eukaryotic cells, reactive oxygen species (ROS) encompass a group of molecules derived from oxygen, such as hydrogen peroxide (H2O2), superoxide anion (O2•−), organic hydroperoxides (ROOH), singlet molecular oxygen (1O2), hydroxyl radical (•OH), alkoxyl radical (•OR), and peroxyl radical (•OOR) [1,2,3]. ROS are mainly formed by reduction–oxidation reactions or by electronic excitation (Figure 1A) [1,2,3] and have evolved as regulators of multiple signaling pathways [4,5,6,7,8]. Two species, H2O2 and O2•−, are the most important redox signaling agents in the cells [4,5,6,7,8]. H2O2 is the major ROS in organisms, with its concentration always maintained within 1~100 nM under normal conditions [8]. For O2•−, the concentration is also maintained at about 0.01 nM, much lower than that of H2O2 [8].
Figure 1.
ROS in cancer cells. (A) Generation and chemical structures of ROS. (B) Brief metabolic process and signal regulation of intracellular ROS. (C) Balancing ROS generation and scavenging in cancer cells to remain in the tumorigenic range.
Dozens of growth factors, cytokines, and antioxidant enzymes control the homeostasis of intracellular H2O2 and O2•− (Figure 1B) [9]. O2•− is prominently generated by the mitochondrial electron transport chain (Mito-ETC), NADPH oxidase (NOX) complex, and endoplasmic reticulum (ER) system, and is rapidly converted to H2O2 by superoxide dismutases (SODs) [10,11,12]. Subsequently, H2O2 is mainly detoxified to H2O by catalase (CAT), glutathione peroxidase (GPX), and peroxiredoxin (Prx) [12]. It is worth mentioning that •OH formed by metal-catalyzed Fenton reaction is the most reactive ROS; it can oxidize biological macromolecules indiscriminately, such as DNA, proteins, and lipids [12]. Therefore, maintaining the homeostasis of intracellular ROS is essential for cell growth, proliferation, and survival [6,9,10].
Due to the well-established role of ROS in cell signaling, cancer cells always have higher levels of endogenous ROS to enhance rapid cell growth and proliferation through the mitogen-activated protein kinase (MAPK)/extracellular-regulated kinase 1/2 (ERK1/2), phosphoinositide-3-kinase (PI3K)/Akt, nuclear factor-κB (NF-κB), and hypoxia-sensitive α (HIF1α) pathways [13,14,15,16,17,18]. Indeed, higher levels of ROS have already been observed in various cancer cells [11,19]. If the intracellular ROS levels increase dramatically to toxic concentrations, oxidative stress will cause irreversible damage and may eventually lead to the death of cancer cells [11,20]. To maintain the elevated mitogenic signaling without incurring substantial oxidative damage by a proper balance of ROS, the antioxidant enzymes in cancer cells, such as Cu/Zn superoxide dismutase (SOD1), GPX, and Prx, should harbor higher levels of activity (Figure 1C) [21,22,23,24].
Elevated levels of ROS are always involved in the initiation and progression of cancer. Hence, intervening in the homeostasis of ROS in cancer cells is an effective anti-cancer strategy [25,26]. So far, a variety of chelators or metal complexes based on the regulation of ROS have been reported as anti-cancer agents [27,28,29,30,31,32,33,34]. For those antioxidant enzymes where the active center is a metal ion, chelators can be used to competitively bind to the metal ion and thus inhibit the enzymatic activity to achieve the regulation of intracellular ROS, including SOD1 inhibitors tetrathiomolybdate (ATN-224) and LD100 [32,33]. On the other hand, several metal complexes can regulate the ROS levels in cancer cells through other mechanisms to achieve anti-cancer purposes, such as TxrR inhibition and mitochondrial dysfunction [34,35,36]. Regulating the relative level of ROS in cancer cells through the mechanism of metal coordination has become an important branch with broad prospects in the field of cancer therapy.
In this review, chelators or metal complexes with ROS regulation capacity and with anti-cancer applications are collectively and comprehensively analyzed [32,33,34,35,36,37,38]. The strategy based on coordination chemistry to regulate the level of intracellular ROS has developed into an important method to kill cancer cells. We expect that further study of ROS regulation by metal coordination will provide a new perspective to develop novel reagents for killing cancer cells and, further, as candidates or clinical drugs for cancer therapy.
2. Inorganic SOD1 Inhibitors with Anti-Cancer Prospects
In mammals, the main biological function of SODs is to catalyze the dismutation of O2•− into H2O2 and O2 [39,40]. Cu/Zn superoxide dismutase (SOD1), the major SOD, mainly exists in the formation of homodimers in cells and is widely distributed in the nucleus, the cytoplasm, and the intermembrane space (IMS) of mitochondria [41]. Next, Mn superoxide dismutase (SOD2) exclusively exists in the mitochondrial matrix [42]. An extracellular form of SOD (EC-SOD), also a Cu/Zn-containing SOD, is tetrameric and exists in most mammals [42]. Besides this, SOD1 also regulates multiple redox signals to control growth and metabolic pathways, such as glucose metabolism and transcription [5,43,44,45]. Therefore, SODs, especially SOD1, are the first firewall to resist oxidative stress.
Recently, emerging evidence from researchers has indicated that SOD1 is usually overexpressed in cancer cells; its activity is essential to maintain higher ROS levels under the critical threshold during aberrant energy metabolism of cancer progression [41]. For example, SOD1 accumulations were observed not only in the cytoplasm but also in the nucleus of human primary breast and mammary cancers [46]. Besides this, prostate cancer cells (DU145) also have higher levels of activity and expression of SOD1, compared with normal prostate cells (RWPE-1) [5]. In vitro studies also showed that the fast growth of non-small cell lung cancer (NSCLC) and leukemia depends on the high activity of SOD1, which controls the oncogenic KRAS and EGFR pathways [47,48], as well as other cancer cells and xenograft tumors [49]. In general, SOD1 is recognized as a promising anti-cancer target, and several small-molecule targeting drugs for SOD1 have already entered the preclinical and clinical development stages [50].
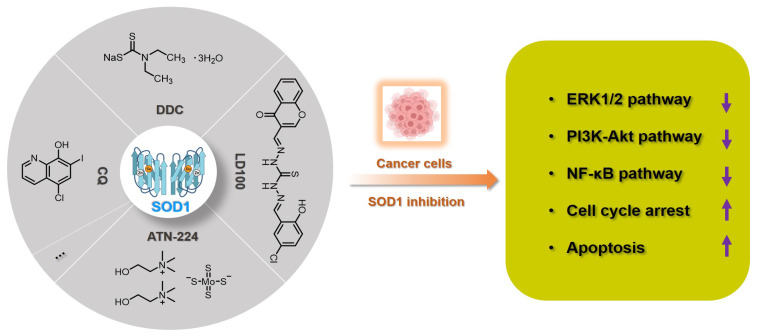
Since the activity of SOD1 mainly comes from the copper ion in the active center, a vast majority of SOD1 inhibitors are competitive chelators of copper ions. In 1975, Heikkila et al. found that diethyldithiocarbamate (DDC) can competitively bind to copper ions (Figure 2), thereby inhibiting SOD1 activity at a millimolar level [51]. After being inhibited by DDC, SOD1 cannot restore enzyme activity through dialysis, but adding CuSO4 during dialysis restores SOD1 activity [51]. In 1979, Misra systematically explored the mechanism by which DDC inhibits SOD1 activity [52]. In Phase I, one DDC molecule first coordinates with the copper(II) center in SOD1, with retention of activity. In Phase II, a second DDC displaces the copper(II) center, with a loss of activity. The shortcomings of DDC as a SOD1 inhibitor are mainly reflected in its high working concentration and poor specificity, such as its interference with the activity of cytochrome c oxidase [53,54]. Nevertheless, DDC still has a wide range of anti-cancer applications, and DDC effectively inhibits SOD1 activity to kill cancer cells [55,56,57].
Figure 2.
Summary of SOD1 inhibitors based on copper chelation. The specific activity inhibition of SOD1 selectively kills cancer cells by regulating the intracellular ROS signaling network.
In 2005, Ding et al. found that clioquinol (5-chloro-7-iodo-8-hydroxyquinoline, CQ), a metal chelator of copper/zinc/iron, is another SOD1 inhibitor (Figure 2), because the copper and zinc ions in the active sites of SOD1 are coordinated by CQ [58]. Structural characterization of the zinc(II) and copper(II) complexes with CQ indicated that the stoichiometry of ligand to metal is 2:1 [59]. Therefore, CQ can effectively inhibit SOD1 at micromolar concentrations (IC50: 6.7~43.1 μM) and induce the death of a variety of cancer cells through the caspase-3-mediated apoptosis pathway [58]. It cannot be ignored that CQ has the risk of destroying copper homeostasis during its inhibition of SOD1 activity in cells [60].
Tetrathiomolybdate is an orally available copper chelator that has been shown to have efficacy as an anti-angiogenic and anti-tumor agent in multiple cancers [61,62]. ATN-224 is the second-generation choline salt of tetrathiomolybdate with improved performance (Figure 2) and is being evaluated in several phase II trials in cancer patients [63]. Doñate et al. found that ATN-224 can selectively bind copper with high affinity, and SOD1 is the main target for the anti-angiogenic activity of this chelator [32,64]. ATN-224 also inhibits intracellular SOD1 activity at micromolar concentrations (IC50: 1.4~185 μM), but has specificity for copper binding, all of which makes it one of the most popular SOD1 inhibitors [62,64]. Every sulfur atom in tetrathiomolybdate can coordinate with copper and may then form metal clusters with copper enzymes, thereby inhibiting the activity of copper proteins, such as SOD1, cytochrome c oxidase, and ceruloplasmin [65,66]. Therefore, ATN-224 may also interfere with intracellular copper homeostasis or inhibit other copper enzymes. In cancer treatment, ATN-224-mediated SOD1 inhibition led to the downregulation of PDGF and increase of O2•−, prevented the formation of high levels of H2O2, and protected protein tyrosine phosphatases from oxidation by H2O2 [62]. Therefore, SOD1 inhibition by ATN-224 results in the down-regulation of multiple signaling pathways for cancer cell function, such as ERK1/2 and anti-apoptotic factor Mcl1 [50,62].
Considering that the known SOD1 inhibitors have various defects, such as low efficiency, weak specificity, and interference with the homeostasis of metal ions, we designed a next-generation SOD1 inhibitor (LD100) based on copper coordination chemistry and the catalytic cycle in the active site (Figure 2) [33]. LD100 was designed through the combination of thiosemicarbazone and phenol derivatives, because thiosemicarbazone contains a copper chelating moiety, -C(SH)-NH-, and the phenolic hydroxyl can further facilitate the copper coordination. Besides this, LD100 also contains a fluorescent group chromone, which not only can be used to track the entry of LD100 into cells, but also enables LD100 to better occupy the substrate channel of SOD1. Therefore, LD100 has a strong binding ability to copper ions in solution and can effectively inhibit the activity of SOD1 in vitro and in vivo (IC50 of LD100 to SOD1 in HeLa cells: 0.18 μM) [33]. Through specific inhibition of SOD1 activity, LD100 can efficiently up-regulate the intracellular concentration of O2•−, down-regulate the concentration of H2O2, down-regulate the phosphorylation of ERK1/2, and finally induce the apoptosis of cancer cells [33]. In summary, LD100 may be the most effective SOD1 inhibitor so far and has application prospects for cancer treatment. Using this inhibitor, we also systematically explored the mechanism of how SOD1 activity inhibition selectively kills cancer cells [5]. The rapid growth and proliferation of cancer cells always depend on higher SOD1 activity, so cancer cells are more sensitive to SOD1 inhibition. During SOD1 inhibition in cancer cells, LD100 could repress the ERK, PI3K-Akt, and NF-κB pathways; arrest the cell cycle; and induce mitochondria-dependent apoptosis [5].
SOD1 is indeed a recognized target for cancer treatment. At present, a variety of chelators have been used for SOD1 inhibition. LD100 may be the most effective inhibitor designed through coordination chemistry. However, the use of inorganic strategies to develop anti-cancer drugs based on SOD1 inhibition still requires further efforts. First, we need to solve the problem of compatibility between targeted and clinical deliveries. On the other hand, we also should reduce the side effects of chelating agents while ensuring the efficiency of SOD1 inhibition. The summary of SOD1 metal-chelating inhibition can provide a reference for the design of SOD1 inhibitors with anti-cancer effects in the future.
3. Anti-Cancer Metal Complexes Inhibiting Antioxidant Enzymes
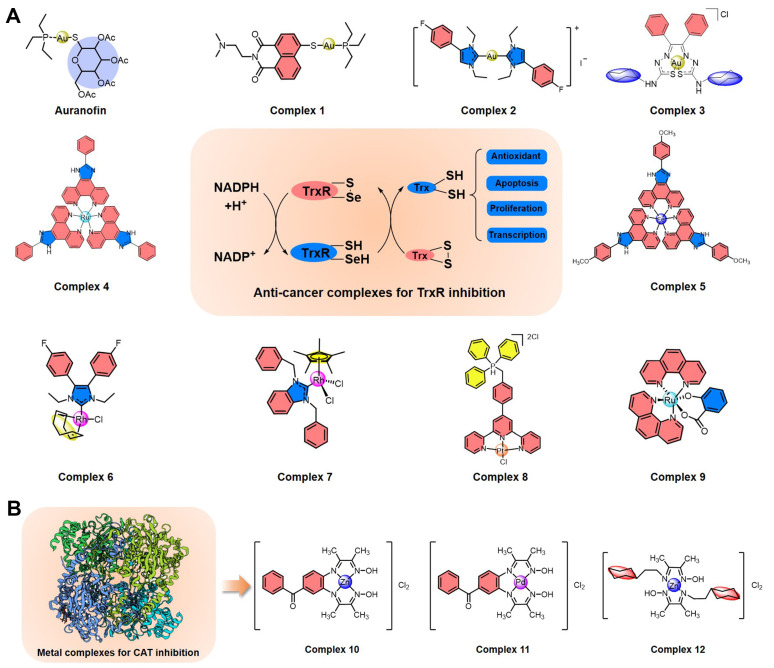
Compared to normal cells, cancer cells always harbor higher levels of ROS and antioxidant enzymes to induce uncontrolled proliferation and a high metabolic rate [67]. In addition to the aforementioned chelators used to inhibit SOD1 activity, a variety of metal complexes targeting other members of antioxidant enzyme systems have also been developed. In higher organisms, thioredoxin (Trx) and thioredoxin reductase (TrxR) are the core members of the Trx system and have also been recognized as another key modulator of cancer development [68]. The main function of TrxR is to reduce the oxidized disulfide of Trxs to the reduced dithiol form by taking electrons for NADPH, and high TrxR activity is also crucial for cancer cells (Figure 3A) [68]. According to the Hard and Soft Acids and Bases (HSAB) theory, sulfur/selenium of TrxR (soft base) should have a high affinity for gold and other noble metals (soft acid). Hence, the use of metal complexes to inhibit the activity of TrxR has also been developed as an anti-cancer strategy, by reducing the level of reduced Trx in cancer cells [68,69,70,71].
Figure 3.
Summary of metal complexes inhibiting TrxR and CAT. (A) Structures of anti-cancer metal complexes for TrxR inhibition to regulate intracellular ROS levels. (B) Structures of metal complexes for CAT inhibition.
As early as 1998, Schirmer et al. first found that auranofin (Figure 3A), an organic gold compound with anti-cancer activity, can inhibit TrxR in the nanomolar range through the formation of a covalent interaction between the gold atom and disulfide bond [72,73]. Inspired by TrxR inhibition by auranofin, Ott et al. developed another gold(I) phosphine complex (1) (Figure 3A) [74]. The ligand of 1 contains a pharmacophore of the naphthalimide class with anti-cancer activity, including a heterocyclic naphthalimide core for DNA intercalation and a side chain containing a protonable nitrogen for DNA phosphate backbone contraction. In cancer cells, 1 can effectively inhibit TrxR activity, suppress rapid proliferation, and promote mitochondrial-dependent apoptosis. This complex is also enriched in the nucleus of tumor cells, possibly exerting the anti-cancer activity of naphthalimide. In 2019, Ott et al. used another gold(I) biscarbene complex (2) (Figure 3A) with an N-heterocyclic carbene ligand to improve the inhibition efficiency of TrxR and to enhance the antiproliferative effects of cancer cells [75]. Besides this, Pizarro et al. also synthesized a gold(III) benzil bis(thiosemicarbazonate) complex (3) (Figure 3A) that can be enriched in the cytoplasm and mitochondria of MCF-7 cells and effectively inhibit TrxR activity, leading to a dramatic alteration of the cellular redox state and to the induction of cell death [76]. Of course, there are also various other gold complexes with anti-cancer prospects that can effectively inhibit the activity of TrxR.
In addition to gold complexes, a variety of other metal (platinum, ruthenium, rhodium, iridium, iron, palladium, silver) complexes also inhibit TrxR and may have anti-cancer potential. In 2014, Chen et al. developed a ruthenium polypyridyl complex (4) as an inducer of ROS-mediated apoptosis in cancer cells by targeting TrxR (Figure 3A) [37]. This complex can effectively inhibit TrxR at the micromolar level within a few minutes and can suppress cancer cell growth through cell cycle arrest and induction of apoptosis. Furthermore, an iron(II) complex (5) with a phenanthroline derivative as a ligand was also reported by Chen et al. in 2016 (Figure 3A) [77], with inhibitory efficiency for TrxR improved by at least an order of magnitude compared with that of 4. Complex 5 also kills cancer cells by ROS-mediated apoptosis by targeting TrxR, and it further improves compatibility in animal models [37]. Next, rhodium complexes are also a large class of conventional TrxR inhibitors, such as rhodium(I) complex 6 and rhodium(III) complex 7 [78,79], all of which can kill cancer cells by TrxR-inhibition-mediated ROS regulation (Figure 3A). Recently, Chen et al. also found that a novel triphenylphosphonium-modified terpyridine platinum(II) complex (8) is an inhibitor of mitochondrial TxrR with enhancement of caspase-3-independent apoptosis by increasing cellular ROS [80]. Besides this, a ruthenium(II) salicylate complex (9) with anti-cancer potential was developed by Lan-mei Chen et al., which can selectively kill cancer cells, induce apoptosis, trigger cell cycle arrest and DNA damage, and promote the accumulation of ROS by specific TrxR inhibition [81]. Overall, metal complexes based on TrxR inhibition regulate the level of reduced Trx, destroy ROS homeostasis, and then kill cancer cells by apoptosis.
In addition, a few metal complexes have been developed to target other antioxidant enzymes such as Prx and CAT. Wang et al. demonstrated that a ruthenium complex [(η6-arene)Ru(en)Cl]+ can inhibit the enzymatic activity of human peroxiredoxin I through coordination with the catalytic site Cys173 [82]. However, due to the numerous subtypes of intracellular Prxs, it is difficult to design highly efficient and targeted metal complexes. It is worth mentioning that Shahraki et al. recently developed several potential anti-cancer metal complexes with the potential to inhibit CAT (Figure 3B) [83,84,85]. For example, both zinc(II) complex 10 and palladium(II) complex 11 can bind to the CAT enzyme cavity and alter the structure and conformation of CAT [83]. Besides this, zinc(II) complex 12 with a novel bidentate Schiff base ligand can also inhibit CAT by the conformational changes from van der Waals forces and hydrogen bonds; it also has anti-cancer activity in human colon cancer cells [84].
Contributions from the Ott group, Chen group, Shahraki group, and others have greatly enriched the entry of metal complexes targeting the antioxidant enzyme system as promising anti-cancer agents [74,75,76,77,78,79,80,81,82,83,84,85]. Despite the importance of metal complexes as antioxidant enzyme inhibitors, no clinical anti-cancer drug targets TrxR, CAT, or Prx currently, which may be due to several major challenges in the field. For example, the metal complexes designed by targeting cysteine have the risk of non-specific effects. We may also need to improve the efficiency of metal complexes in inhibiting antioxidant enzymes and reduce the interference of ROS homeostasis in normal cells during drug treatment. The summary of anti-cancer metal complexes targeting the antioxidant enzyme systems in this chapter is expected to give inspiration for anti-cancer drug design, which might potentiate the clinical application of metal complexes in cancer treatment.
4. Anti-Cancer Metal Complexes Activating ROS-Mediated Signaling from Mitochondria
Functional mitochondria are essential for higher energy supply to cancer cells through oxidative phosphorylation [86], and they control various vital cellular parameters, including ATP production, oxidation–reduction status, ROS, cytosolic calcium and biosynthetic precursor levels, oncogenic signaling, innate immunity, and apoptosis through the activation of mitochondrial permeability transition pores [86,87,88]. In cancer cells, the metabolism is reprogrammed for energy supply from oxidative phosphorylation to aerobic glycolysis [89,90], and mitochondrial biogenesis and quality control are always upregulated [91,92].
ROS overproduction in the mitochondria of cancer cells promotes cancer progression by increasing genomic instability, regulating gene expression, and controlling multiple signaling pathways [91,92]. On the other hand, oxidative damage to mitochondria and mitochondrial DNA impairs the oxidative phosphorylation process and results in further ROS production, which forms a vicious cycle involving ROS, mitochondria, genomic instability, and cancer deterioration [93,94,95]. Hence, the major contributor to cancer development from mitochondria is ROS, especially from dysfunctioning or malfunctioning mitochondria [94]. So far, regulating the function of mitochondria in cancer cells to control the level of intracellular ROS has already been developed as a powerful anti-cancer method [94,95]. Compared with normal cells, the mitochondria of cancer cells always have a higher membrane potential [96], and the delivery of inorganic drugs into mitochondria based on the higher mitochondrial membrane potential allows selective targeting of cancer cell mitochondria to kill cancer cells [97,98]. Recently, anti-cancer metal complexes targeting mitochondria have drawn strong interest because of their strong anti-cancer activities, limited side effects, and versatile photophysical properties.
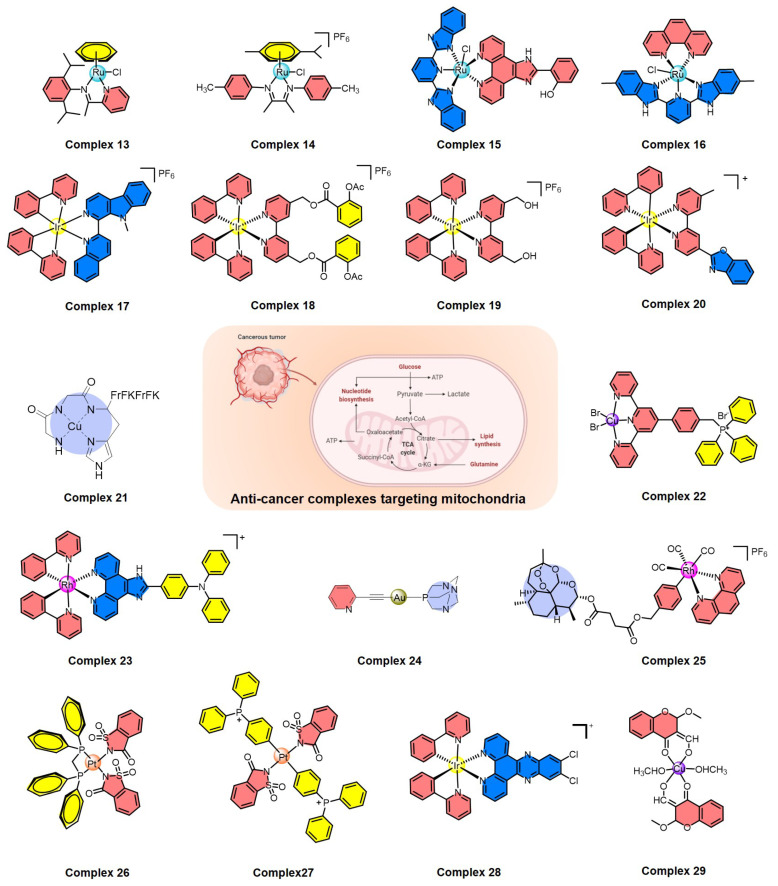
In recent years, a variety of ruthenium complexes that act on mitochondria have been developed, and these complexes can often up-regulate intracellular ROS levels and activate ROS-mediated signaling to kill cancer cells (Figure 4). In 2017, Liu et al. reported a half-sandwich ruthenium(II) complex (13) with an N^N-chelated imino-pyridyl ligand as a selective anti-cancer agent; it evaluates intracellular ROS, disrupts mitochondrial membrane potential, and then kills A549 cancer cells [99]. They also developed another mitochondria-targeted half-sandwich ruthenium(II) diimine complex (14) as an anti-cancer agent via ROS-mediated signaling; 14 can effectively locate in the mitochondria and inhibit the migration of cancer cells [100]. These half-sandwich ruthenium complexes and derivatives have great value for development as novel theranostic candidates due to their mitochondrial imaging and anti-cancer prospects. Of course, the metal complexes acting on mitochondria are not limited to the half-sandwich structure, such as ruthenium polypyridyl complexes. For example, Chen et al. synthesized a mixed-ligand ruthenium polypyridyl complex (15) with an ortho-phenolic group on the ligand; 15 can enter cancer cells through endocytosis and then translocate from lysosomes to the mitochondria, where 15 activates mitochondrial dysfunction and up-regulates the intracellular ROS level to selectively kill cancer cells [101]. Subsequently, Chen et al. also found that aquation of ruthenium complex 16 can effectively enhance its hydrophilicity and cellular uptake, thus significantly increasing its anti-cancer efficacy by mitochondrial dysfunction [102], which provides valuable information for the rational design of next-generation ruthenium polypyridyl complexes.
Figure 4.
Summary of metal complexes activating ROS-mediated signaling by mitochondrial dysfunction.
In addition, cyclometalated iridium complexes are another type of anti-cancer agent acting on functional mitochondria (Figure 4). Mao et al. reported several representative cyclometalated iridium complexes with anti-cancer activity through loss of mitochondrial membrane potential and elevation of intracellular ROS, such as iridium complexes 17 and 18 [103,104]. For 17, the ubiquitin-proteasome system (UPS) was also induced and resulted in the collapse of mitochondria and subsequent cytoplasmic vacuolation because of the rapid loss of mitochondrial functions [103]. For 18, caspase-dependent apoptosis, caspase-independent paraptosis, and metastasis were controlled, and this complex also showed tumor growth inhibition in vivo [104]. Similarly, mitochondria-targeting cyclometalated iridium complexes were also used as potent anti-glioma stem cell agents, such as complex 19 [105]. On the other hand, two iridium complexes were synthesized as necroptosis inducers in cisplatin-resistant cancer cells, such as complex 20, which can selectively accumulate in mitochondria, disrupt the mitochondrial membrane potential, and circumvent drug resistance, leading to the activation of receptor-interacting serine-threonine kinase 3 (RIPK3) and mixed lineage kinase domain-like pseudokinase (MLKL) [106]. Therefore, the development of iridium-based complexes offers the opportunity to bypass drug resistance and improve the efficiency of killing cancer cells.
Recently, many other metal complexes targeting mitochondria have also been developed to kill cancer cells by regulating the level of intracellular ROS (Figure 4), such as copper complexes 21 and 22 [107,108], rhodium complex 23 [109], gold complex 24 [110], and rhenium complex 25 [111]. Interestingly, 24 is also a necroptosis inducer and a potential option in cases of apoptosis resistance; it disrupts the normal function of mitochondria, leading to ROS elevation in colorectal adenocarcinoma cells [110]. Another thing worth mentioning is that rhenium complex 25 can induce both apoptosis and ferroptosis in cancer cells through mitochondrial dysfunction, caspase cascade, glutathione depletion, glutathione peroxidase 4 inactivation, and lipid peroxidation, which is a promising strategy to induce both apoptosis and ferroptosis at the same time [111].
A variety of metal complexes have been developed that not only act on mitochondria but also can bind to DNA (Figure 4), which can cause excessive DNA damage to kill cancer cells, including platinum complexes 26 and 27 [112,113], iridium complex 28 [114], and copper complex 29 [115]. Taking 27 as an important example, 27 can interact with DNA through groove binding and has the potential to break DNA [113]. Mechanistic studies indicated that this complex causes excessive generation of ROS and displays dual action by targeting both mitochondria and genomic DNA [113]. Therefore, complexes acting on mitochondria and genomic DNA at the same time have broad prospects in the field of designing new non-polar drugs in the future.
5. Conclusions
In this review, we systematically summarized the latest advances in developing inorganic chelators and metal complexes as anti-cancer agents through regulating intracellular ROS levels, with a particular focus on those targeting mitochondria and antioxidant enzyme systems, including SOD1, TrxR, and CAT. Metal coordination and metal complexes display a preeminent combination of biological activity, cell permeability, and stability in cancer cells. Using inorganic strategies to destroy the ROS homeostasis in cancer cells is powerful and gives significant prospects for the future application of chelators and metal complexes as anti-cancer drugs.
For the antioxidant enzyme systems, a large number of inorganic inhibitors have been developed, such as SOD1 inhibitors (DDC, CQ, ATN-224, and LD100), TrxR inhibitors (auranofin and 1–9), and CAT inhibitors (10–12). Generally, these chelators or metal complexes can induce apoptosis, arrest the cell cycle, regulate ROS signaling pathways, and kill cancer cells through antioxidant enzyme inhibition and consequent ROS elevation. Although ATN-224 has entered clinical testing, there is still a lack of inorganic anti-cancer drugs targeting antioxidant enzyme systems. The problems that need to be solved mainly involve the efficiency and specificity of antioxidant enzyme inhibition, the targeting of cancer tissues, and toxic side effects.
Multiple strategies of cancer-selective mitochondrial damage can lead to apoptosis, necroptosis, and ferroptosis by the utilization of metal complexes, such as ruthenium polypyridyl complexes, cyclometalated iridium complexes, and so on (13–29). For the high performance of anti-cancer metal complexes acting on mitochondria, several challenges still need to be overcome, including anti-cancer activity, mitochondrial targeting, and toxic side effects. Metal complexes with dual effects on functional mitochondria and genomic DNA may have better application prospects in improving anti-cancer performance. In order to further reduce the side effects, we can improve the efficiency of metal complexes targeting cancer cell mitochondria, such as by conjugation of mitochondria-targeting peptides and DNAs.
The summary of anti-cancer chelators and metal complexes targeting antioxidant enzyme systems or mitochondria in this review is expected to give inspiration for the design of next-generation inorganic anti-cancer drugs. To design novel SOD1 inhibitors, we can further optimize the type of coordination group and adjust the molecular conformation of chelators to match the active cavity of SOD1. For the rational design of anti-cancer metal complexes, we can not only adjust the combinatorial mode of ligands and metal ions, but also apply different metal ions to different systems. Besides this, the development of multifunctional metal complexes may be another direction for the design of anti-cancer drugs, such as the combination of antioxidant enzyme inhibition, mitochondrial destruction, and genomic DNA interaction. In any case, the application of cancer-targeting groups is an optional strategy to reduce off-target effects. In the future, research on the development of inorganic anti-cancer drugs is highly expected to result in clinical trials for efficient and side-effect-free cancer therapeutics.
Author Contributions
X.L. and X.D. conceived and designed the format of the paper; X.L., Y.W., M.L. and H.W. edited the article and drew the structures of the compounds; X.L. reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [21701128]; Hubei Provincial Natural Science Foundation of China [2017CFB206]; and Educational Commission of Hubei Province of China [Q20171601].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Waszczak C., Carmody M., Kangasjärvi J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018;69:209–236. doi: 10.1146/annurev-arplant-042817-040322. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg F., Ramnath N., Nagrath D. Reactive oxygen species in the tumor microenvironment: An overview. Cancers. 2019;11:1191. doi: 10.3390/cancers11081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang B., Chen Y., Shi J. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 2019;119:4881–4985. doi: 10.1021/acs.chemrev.8b00626. [DOI] [PubMed] [Google Scholar]
- 4.Marcec M.J., Gilroy S., Poovaiah B.W., Tanaka K. Mutual interplay of Ca2+ and ROS signaling in plant immune response. Plant Sci. 2019;283:343–354. doi: 10.1016/j.plantsci.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Li X., Chen Y., Zhao J., Shi J., Wang M., Qiu S., Hu Y., Xu Y., Cui Y., Liu C., et al. The specific inhibition of SOD1 selectively promotes apoptosis of cancer cells via regulation of the ROS signaling network. Oxidative Med. Cell. Longev. 2019;2019:9706792. doi: 10.1155/2019/9706792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milkovic L., Cipak Gasparovic A., Cindric M., Mouthuy P.A., Zarkovic N. Short overview of ROS as cell function regulators and their implications in therapy concepts. Cells. 2019;8:793. doi: 10.3390/cells8080793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 9.D’Autréaux B., Toledano M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 10.Reczek C.R., Chandel N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015;33:8–13. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moloney J.N., Cotter T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018;80:50–64. doi: 10.1016/j.semcdb.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Branicky R., Noë A., Hekimi S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018;217:1915–1928. doi: 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su X., Shen Z., Yang Q., Sui F., Pu J., Ma J., Ma S., Yao D., Ji M., Hou P. Vitamin C kills thyroid cancer cells through ROS-dependent inhibition of MAPK/ERK and PI3K/AKT pathways via distinct mechanisms. Theranostics. 2019;9:4461. doi: 10.7150/thno.35219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y., Liang R., Zhang X., Wang J., Shan C., Liu S., Li L., Zhang S. Copper chaperone for superoxide dismutase promotes breast cancer cell proliferation and migration via ROS-mediated MAPK/ERK signaling. Front. Pharmacol. 2019;10:356–367. doi: 10.3389/fphar.2019.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steelman L.S., Abrams S.L., Whelan J., Bertrand F.E., Ludwig D.E., Bäsecke J., Libra M., Stivala F., Milella M., Tafuri A., et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 16.Yeo D., Hwang S.J., Kim W.J., Youn H.J., Lee H.J. The aqueous extract from Artemisia capillaris inhibits acute gastric mucosal injury by inhibition of ROS and NF-κB. Biomed. Pharmacother. 2018;99:681–687. doi: 10.1016/j.biopha.2018.01.118. [DOI] [PubMed] [Google Scholar]
- 17.Park S.A., Na H.K., Kim E.H., Cha Y.N., Surh Y.J. 4-Hydroxyestradiol induces anchorage-independent growth of human mammary epithelial cells via activation of IκB kinase: Potential role of reactive oxygen species. Cancer Res. 2009;69:2416–2424. doi: 10.1158/0008-5472.CAN-08-2177. [DOI] [PubMed] [Google Scholar]
- 18.Castelli S., Ciccarone F., Tavian D., Ciriolo M.R. ROS-dependent HIF1α activation under forced lipid catabolism entails glycolysis and mitophagy as mediators of higher proliferation rate in cervical cancer cells. J. Exp. Clin. Cancer Res. 2021;40:1–18. doi: 10.1186/s13046-021-01887-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szatrowski T.P., Nathan C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 20.Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., Castoria G., Migliaccio A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y., Rosen D.G., Zhou Y., Feng L., Yang G., Liu J., Huang P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: Role in cell proliferation and response to oxidative stress. J. Biol. Chem. 2005;280:39485–39492. doi: 10.1074/jbc.M503296200. [DOI] [PubMed] [Google Scholar]
- 22.Saydam N., Kirb A., Demir Ö., Hazan E., Oto Ö., Saydam O., Güner G. Determination of glutathione, glutathione reductase, glutathione peroxidase and glutathione S-transferase levels in human lung cancer tissues. Cancer Lett. 1997;119:13–19. doi: 10.1016/S0304-3835(97)00245-0. [DOI] [PubMed] [Google Scholar]
- 23.Murawaki Y., Tsuchiya H., Kanbe T., Harada K., Yashima K., Nozaka K., Tanida O., Kohno M., Mukoyama T., Nishimuki E., et al. Aberrant expression of selenoproteins in the progression of colorectal cancer. Cancer Lett. 2008;259:218–230. doi: 10.1016/j.canlet.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Oberley T.D., Oberley L.W. Antioxidant enzyme levels in cancer. Histol. Histopathol. 1997;12:525–535. [PubMed] [Google Scholar]
- 25.De Sá Junior P.L., Câmara D.A.D., Porcacchia A.S., Fonseca P.M.M., Jorge S.D., Araldi R.P., Ferreira A.K. The roles of ROS in cancer heterogeneity and therapy. Oxidative Med. Cell. Longev. 2017;2017:2467940. doi: 10.1155/2017/2467940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chio I.I.C., Tuveson D.A. ROS in cancer: The burning question. Trends Mol. Med. 2017;23:411–429. doi: 10.1016/j.molmed.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zehra S., Cirilli I., Silvestri S., Gómez-Ruiz S., Tabassum S., Arjmand F. Structure elucidation, in vitro binding studies and ROS-dependent anti-cancer activity of Cu (II) and Zn (II) phthaloylglycinate (phen) complexes against MDA-MB-231 cells. Metallomics. 2021;13:mfab064. doi: 10.1093/mtomcs/mfab064. [DOI] [PubMed] [Google Scholar]
- 28.Guo W., Ye S., Cao N., Huang J., Gao J., Chen Q. ROS-mediated autophagy was involved in cancer cell death induced by novel copper (II) complex. Exp. Toxicol. Pathol. 2010;62:577–582. doi: 10.1016/j.etp.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Liu J., Guo W., Li J., Li X., Geng J., Chen Q., Gao J. Tumor-targeting novel manganese complex induces ROS-mediated apoptotic and autophagic cancer cell death. Int. J. Mol. Med. 2015;35:607–616. doi: 10.3892/ijmm.2015.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marloye M., Berger G., Gelbcke M., Dufrasne F. A survey of the mechanisms of action of anticancer transition metal complexes. Future Med. Chem. 2016;8:2263–2286. doi: 10.4155/fmc-2016-0153. [DOI] [PubMed] [Google Scholar]
- 31.Sîrbu A., Palamarciuc O., Babak M.V., Lim J., Ohui K., Enyedy E.A., Shova S., Darvasiová D., Rapta P., Ang W.H., et al. Copper (II) thiosemicarbazone complexes induce marked ROS accumulation and promote nrf2-mediated antioxidant response in highly resistant breast cancer cells. Dalton Trans. 2017;46:3833–3847. doi: 10.1039/C7DT00283A. [DOI] [PubMed] [Google Scholar]
- 32.Donate F., Juarez J.C., Burnett M.E., Manuia M.M., Guan X., Shaw D.E., Smith E.L.P., Timucin C., Braunstein M.J., Batuman O.A., et al. Identification of biomarkers for the antiangiogenic and antitumour activity of the superoxide dismutase 1 (SOD1) inhibitor tetrathiomolybdate (ATN-224) Br. J. Cancer. 2008;98:776–783. doi: 10.1038/sj.bjc.6604226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong X., Zhang Z., Zhao J., Lei J., Chen Y., Li X., Chen H., Tian J., Zhang D., Liu C., et al. The rational design of specific SOD1 inhibitors via copper coordination and their application in ROS signaling research. Chem. Sci. 2016;7:6251–6262. doi: 10.1039/C6SC01272H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalaivani P., Saranya S., Poornima P., Prabhakaran R., Dallemer F., Padma V.V., Natarajan K. Biological evaluation of new nickel (II) metallates: Synthesis, DNA/protein binding and mitochondrial mediated apoptosis in human lung cancer cells (A549) via ROS hypergeneration and depletion of cellular antioxidant pool. Eur. J. Med. Chem. 2014;82:584–599. doi: 10.1016/j.ejmech.2014.05.075. [DOI] [PubMed] [Google Scholar]
- 35.Zhang P., Sadler P.J. Redox-active metal complexes for anticancer therapy. Eur. J. Inorg. Chem. 2017;2017:1541–1548. doi: 10.1002/ejic.201600908. [DOI] [Google Scholar]
- 36.Imberti C., Zhang P., Huang H., Sadler P.J. New designs for phototherapeutic transition metal complexes. Angew. Chem. Int. Ed. 2020;59:61–73. doi: 10.1002/anie.201905171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo Z., Yu L., Yang F., Zhao Z., Yu B., Lai H., Wong K., Ngai S.M., Zheng W., Chen T. Ruthenium polypyridyl complexes as inducer of ROS-mediated apoptosis in cancer cells by targeting thioredoxin reductase. Metallomics. 2014;6:1480–1490. doi: 10.1039/C4MT00044G. [DOI] [PubMed] [Google Scholar]
- 38.Ng C.H., Kong S.M., Tiong Y.L., Maah M.J., Sukram N., Ahmad M., Khoo A.S.B. Selective anticancer copper (II)-mixed ligand complexes: Targeting of ROS and proteasomes. Metallomics. 2014;6:892–906. doi: 10.1039/C3MT00276D. [DOI] [PubMed] [Google Scholar]
- 39.Borgstahl G.E.O., Oberley-Deegan R.E. Superoxide dismutases (SODs) and SOD mimetics. Antioxidants. 2018;7:156. doi: 10.3390/antiox7110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinett N.G., Peterson R.L., Culotta V.C. Eukaryotic copper-only superoxide dismutases (SODs): A new class of SOD enzymes and SOD-like protein domains. J. Biol. Chem. 2018;293:4636–4643. doi: 10.1074/jbc.TM117.000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papa L., Manfredi G., Germain D. SOD1, an unexpected novel target for cancer therapy. Genes Cancer. 2014;5:15–21. doi: 10.18632/genesandcancer.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X., Zhang H., Sapio R., Yang J., Wong J., Zhang X., Guo J.Y., Pine S., Remmen H.V., Li H., et al. SOD1 regulates ribosome biogenesis in KRAS mutant non-small cell lung cancer. Nat. Commun. 2021;12:1–15. doi: 10.1038/s41467-021-22480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsang C.K., Liu Y., Thomas J., Zhang Y., Zheng X.F.S. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat. Commun. 2014;5:1–11. doi: 10.1038/ncomms4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X., Qiu S., Shi J., Wang S., Wang M., Xu Y., Nie Z., Liu C., Liu C. A new function of copper zinc superoxide dismutase: As a regulatory DNA-binding protein in gene expression in response to intracellular hydrogen peroxide. Nucleic Acids Res. 2019;10:5074–5085. doi: 10.1093/nar/gkz256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddi A.R., Culotta V.C. SOD1 integrates signals from oxygen and glucose to repress respiration. Cell. 2013;152:224–235. doi: 10.1016/j.cell.2012.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papa L., Hahn M., Marsh E.L., Evans B.S., Germain D. SOD2 to SOD1 switch in breast cancer. J. Biol. Chem. 2014;289:5412–5416. doi: 10.1074/jbc.C113.526475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glasauer A., Sena L.A., Diebold L.P., Mazar A.P., Chandel N.S. Targeting SOD1 reduces experimental non–small-cell lung cancer. J. Clin. Investig. 2014;124:117–128. doi: 10.1172/JCI71714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Somwar R., Erdjument-Bromage H., Larsson E., Shum D., Lockwood W.W. Superoxide dismutase 1 (SOD1) is a target for a small molecule identified in a screen for inhibitors of the growth of lung adenocarcinoma cell lines. Proc. Natl. Acad. Sci. USA. 2011;108:16375–16380. doi: 10.1073/pnas.1113554108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez M.L., Shah N., Kenny T.C., Jenkins Jr E.C., Germain D. SOD1 is essential for oncogene-driven mammary tumor formation but dispensable for normal development and proliferation. Oncogene. 2019;38:5751–5765. doi: 10.1038/s41388-019-0839-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Che M., Wang R., Li X., Wang H.Y., Zheng X.F.S. Expanding roles of superoxide dismutases in cell regulation and cancer. Drug Discov. Today. 2016;21:143–149. doi: 10.1016/j.drudis.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heikkila R.E., Cabbat F.S., Cohen G. In vivo inhibition of superoxide dismutase in mice by diethyldithiocarbamate. J. Biol. Chem. 1976;251:2182–2185. doi: 10.1016/S0021-9258(17)33675-X. [DOI] [PubMed] [Google Scholar]
- 52.Misra H.P. Reaction of copper-zinc superoxide dismutase with diethyldithiocarbamate. J. Biol. Chem. 1979;254:11623–11628. doi: 10.1016/S0021-9258(19)86530-4. [DOI] [PubMed] [Google Scholar]
- 53.Singh N., Savanur M.A., Srivastava S., D’Silva P., Mugesh G. A manganese oxide nanozyme prevents the oxidative damage of biomolecules without affecting the endogenous antioxidant system. Nanoscale. 2019;11:3855–3863. doi: 10.1039/C8NR09397K. [DOI] [PubMed] [Google Scholar]
- 54.Griffiths D.E., Wharton D.C. Studies of the electron transport system XXXV. Purification and properties of cytochrome oxidase. J. Biol. Chem. 1961;236:1850–1856. doi: 10.1016/S0021-9258(19)63315-6. [DOI] [PubMed] [Google Scholar]
- 55.Skrott Z., Cvek B. Diethyldithiocarbamate complex with copper: The mechanism of action in cancer cells. Mini Rev. Med. Chem. 2012;12:1184–1192. doi: 10.2174/138955712802762068. [DOI] [PubMed] [Google Scholar]
- 56.Feuser P.E., Cordeiro A.P., Silveira G.B., Borges Corrêa M.E.A., Silveira P.C.L., Sayer C., Hermes de Araújo P.H., Machado-de-Ávila R.A., Dal Bó A.G. Co-encapsulation of sodium diethyldithiocarbamate (DETC) and zinc phthalocyanine (ZnPc) in liposomes promotes increases phototoxic activity against (MDA-MB 231) human breast cancer cells. Colloids Surf. B Biointerfaces. 2021;197:111434. doi: 10.1016/j.colsurfb.2020.111434. [DOI] [PubMed] [Google Scholar]
- 57.Cho H.Y., Mavi A., Chueng S.T.D., Borges Corrêa M.E.A., Silveira P.C.L., Sayer C., Araújo P.H.H., Machado-de-Ávila R.A., Dal Bó A.G. Tumor homing reactive oxygen species nanoparticle for enhanced cancer therapy. ACS Appl. Mater. Interfaces. 2019;11:23909–23918. doi: 10.1021/acsami.9b07483. [DOI] [PubMed] [Google Scholar]
- 58.Ding W.Q., Liu B., Vaught J.L., Yamauchi H., Lind S.E. Anticancer activity of the antibiotic clioquinol. Cancer Res. 2005;65:3389–3395. doi: 10.1158/0008-5472.CAN-04-3577. [DOI] [PubMed] [Google Scholar]
- 59.Di Vaira M., Bazzicalupi C., Orioli P., Messori L., Bruni B., Zatta P. Clioquinol, a drug for Alzheimer’s disease specifically interfering with brain metal metabolism: Structural characterization of its zinc (II) and copper (II) complexes. Inorg. Chem. 2004;43:3795–3797. doi: 10.1021/ic0494051. [DOI] [PubMed] [Google Scholar]
- 60.Katsuyama M., Kimura E., Ibi M., Iwata K., Matsumoto M., Asaoka N., Yabe-Nishimura C. Clioquinol inhibits dopamine-β-hydroxylase secretion and noradrenaline synthesis by affecting the redox status of ATOX1 and copper transport in human neuroblastoma SH-SY5Y cells. Arch. Toxicol. 2021;95:135–148. doi: 10.1007/s00204-020-02894-0. [DOI] [PubMed] [Google Scholar]
- 61.Brewer G.J., Dick R.D., Grover D.K., LeClaire V., Tseng M., Wicha M., Pienta K., Redman B.G., Jahan T., Sondak V.K., et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin. Cancer Res. 2000;6:1–10. [PubMed] [Google Scholar]
- 62.Juarez J.C., Manuia M., Burnett M.E., Betancourt O., Boivin B., Shaw D.E., Tonks N.K., Mazar A.P., Donate F. Superoxide dismutase 1 (SOD1) is essential for H2O2-mediated oxidation and inactivation of phosphatases in growth factor signaling. Proc. Natl. Acad. Sci. USA. 2008;105:7147–7152. doi: 10.1073/pnas.0709451105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin J., Zahurak M., Beer T.M., Ryan C.J., Wilding G., Mathew P., Morris M., Callahan J.A., Gordon G., Reich S.D., et al. Urologic Oncology: Seminars and Original Investigations. Volume 5. Elsevier; Amsterdam, The Netherlands: 2013. A non-comparative randomized phase II study of 2 doses of ATN-224, a copper/zinc superoxide dismutase inhibitor, in patients with biochemically recurrent hormone-naïve prostate cancer; pp. 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Juarez J.C., Betancourt O., Pirie-Shepherd S.R., Guan X., Price M.L., Shaw D.E., Mazar A.P., Doñate F. Copper binding by tetrathiomolybdate attenuates angiogenesis and tumor cell proliferation through the inhibition of superoxide dismutase. Clin. Cancer Res. 2006;12:4974–4982. doi: 10.1158/1078-0432.CCR-06-0171. [DOI] [PubMed] [Google Scholar]
- 65.Maiti B.K., Moura J.J. Diverse biological roles of the tetrathiomolybdate anion. Coord. Chem. Rev. 2021;429:213635. doi: 10.1016/j.ccr.2020.213635. [DOI] [Google Scholar]
- 66.Alvarez H.M., Xue Y., Robinson C.D., Canalizo-Hernandez M.A., Marvin R.G., Kelly R.A., Mondragon A., Penner-Hahn J.E., O’Halloran T.V. Tetrathiomolybdate inhibits copper trafficking proteins through metal cluster formation. Science. 2010;327:331–334. doi: 10.1126/science.1179907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bindoli A., Rigobello M.P. Principles in redox signaling: From chemistry to functional significance. Antioxid. Redox Signal. 2013;18:1557–1593. doi: 10.1089/ars.2012.4655. [DOI] [PubMed] [Google Scholar]
- 68.Zhang J., Li X., Han X., Liu R., Fang J. Targeting the thioredoxin system for cancer therapy. Trends Pharmacol. Sci. 2017;38:794–808. doi: 10.1016/j.tips.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 69.Meng C., Qian J., Xu Z., Liu J., Shan W., Zhu P., Zhu W., Miao J., Ling C., Ling Y. Efficacy of novel methylenecyclohexenone derivatives as TrxR inhibitors in suppressing the proliferation and metastasis of human cancer cells. Bioorg. Chem. 2020;105:104360. doi: 10.1016/j.bioorg.2020.104360. [DOI] [PubMed] [Google Scholar]
- 70.Lei H., Wang G., Zhang J., Han Q. Inhibiting TrxR suppresses liver cancer by inducing apoptosis and eliciting potent antitumor immunity. Oncol. Rep. 2018;40:3447–3457. doi: 10.3892/or.2018.6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bian M., Fan R., Zhao S., Liu W. Targeting the thioredoxin system as a strategy for cancer therapy: Miniperspective. J. Med. Chem. 2019;62:7309–7321. doi: 10.1021/acs.jmedchem.8b01595. [DOI] [PubMed] [Google Scholar]
- 72.Gromer S., Arscott L.D., Williams C.H., Schirmer R.H., Becker K. Human placenta thioredoxin reductase: Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J. Biol. Chem. 1998;273:20096–20101. doi: 10.1074/jbc.273.32.20096. [DOI] [PubMed] [Google Scholar]
- 73.Barnard P.J., Berners-Price S.J. Targeting the mitochondrial cell death pathway with gold compounds. Coord. Chem. Rev. 2007;251:1889–1902. doi: 10.1016/j.ccr.2007.04.006. [DOI] [Google Scholar]
- 74.Ott I., Qian X., Xu Y., Vlecken D.H., Marques I.J., Kubutat D., Bagowski C.P. A gold (I) phosphine complex containing a naphthalimide ligand functions as a TrxR inhibiting antiproliferative agent and angiogenesis inhibitor. J. Med. Chem. 2009;52:763–770. doi: 10.1021/jm8012135. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt C., Albrecht L., Balasupramaniam S., Misgeld R., Karge B., Brönstrup M., Prokop A., Baumann K., Ingo Ott S.R. A gold (i) biscarbene complex with improved activity as a TrxR inhibitor and cytotoxic drug: Comparative studies with different gold metallodrugs. Metallomics. 2019;11:533–545. doi: 10.1039/c8mt00306h. [DOI] [PubMed] [Google Scholar]
- 76.Rodríguez-Fanjul V., López-Torres E., Mendiola M.A., Pizarro A.M. Gold (III) bis (thiosemicarbazonate) compounds in breast cancer cells: Cytotoxicity and thioredoxin reductase targeting. Eur. J. Med. Chem. 2018;148:372–383. doi: 10.1016/j.ejmech.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 77.Xie L., Luo Z., Zhao Z., Chen T. Anticancer and antiangiogenic iron (II) complexes that target thioredoxin reductase to trigger cancer cell apoptosis. J. Med. Chem. 2017;60:202–214. doi: 10.1021/acs.jmedchem.6b00917. [DOI] [PubMed] [Google Scholar]
- 78.Fan R., Bian M., Hu L., Liu W. A new rhodium (I) NHC complex inhibits TrxR: In vitro cytotoxicity and in vivo hepatocellular carcinoma suppression. Eur. J. Med. Chem. 2019;183:111721. doi: 10.1016/j.ejmech.2019.111721. [DOI] [PubMed] [Google Scholar]
- 79.Truong D., Sullivan M.P., Tong K.K.H., Steel T.R., Prause A., Lovett J.H., Andersen J.W., Jamieson S.M.F., Harris H.H., Ott I., et al. Potent inhibition of thioredoxin reductase by the Rh derivatives of anticancer M (arene/Cp*)(NHC) Cl2 complexes. Inorg. Chem. 2020;59:3281–3289. doi: 10.1021/acs.inorgchem.9b03640. [DOI] [PubMed] [Google Scholar]
- 80.Wang K., Zhu C., He Y., Zhang Z., Zhou W., Muhammad N., Guo Y., Wang X., Guo Z. Restraining Cancer Cells by Dual Metabolic Inhibition with a Mitochondrion-Targeted Platinum (II) Complex. Angew. Chem. Int. Ed. 2019;58:4638–4643. doi: 10.1002/anie.201900387. [DOI] [PubMed] [Google Scholar]
- 81.Chen J., Zhang Y., Jie X., She J., Dongye G., Zhong Y., Deng Y., Wang J., Guo B., Chen L.-M. Ruthenium (II) salicylate complexes inducing ROS-mediated apoptosis by targeting thioredoxin reductase. J. Inorg. Biochem. 2019;193:112–123. doi: 10.1016/j.jinorgbio.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 82.Lin Y., Wang J., Zheng W., Luo Q., Wu K., Du J., Zhao Y., Wang F. Organometallic ruthenium anticancer complexes inhibit human peroxiredoxin I activity by binding to and inducing oxidation of its catalytic cysteine residue. Metallomics. 2019;11:546–555. doi: 10.1039/c8mt00352a. [DOI] [PubMed] [Google Scholar]
- 83.Shahraki S., Delarami H.S., Saeidifar M. Catalase inhibition by two Schiff base derivatives. Kinetics, thermodynamic and molecular docking studies. J. Mol. Liq. 2019;287:111003. doi: 10.1016/j.molliq.2019.111003. [DOI] [Google Scholar]
- 84.Shahraki S., Saeidifar M., Delarami H.S., Kazemzadeh H. Molecular docking and inhibitory effects of a novel cytotoxic agent with bovine liver catalase. J. Mol. Struct. 2020;1205:127590. doi: 10.1016/j.molstruc.2019.127590. [DOI] [Google Scholar]
- 85.Shahraki S., Razmara Z., Shiri F. A paramagnetic oxalato-bridged binuclear copper (II) complex as an effective catalase inhibitor. Spectroscopic and molecular docking studies. J. Mol. Struct. 2020;1208:127865. doi: 10.1016/j.molstruc.2020.127865. [DOI] [Google Scholar]
- 86.Wallace D.C. Mitochondria and cancer. Nat. Rev. Cancer. 2012;12:685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pustylnikov S., Costabile F., Beghi S., Facciabene A. Targeting mitochondria in cancer: Current concepts and immunotherapy approaches. Transl. Res. 2018;202:35–51. doi: 10.1016/j.trsl.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jeena M.T., Kim S., Jin S., Ryu J.H. Recent progress in mitochondria-targeted drug and drug-free agents for cancer therapy. Cancers. 2020;12:4. doi: 10.3390/cancers12010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. doi: 10.1126/science.124.3215.269. [DOI] [PubMed] [Google Scholar]
- 90.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 91.Zong W.X., Rabinowitz J.D., White E. Mitochondria and cancer. Mol. Cell. 2016;61:667–676. doi: 10.1016/j.molcel.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vyas S., Zaganjor E., Haigis M.C. Mitochondria and cancer. Cell. 2016;166:555–566. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Idelchik M.P.S., Begley U., Begley T.J., Melendez J.A. Mitochondrial ROS control of cancer. Semin. Cancer Biol. 2017;47:57–66. doi: 10.1016/j.semcancer.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang Y., Karakhanova S., Hartwig W., D’ Haese J.G., Philippov P.P., Werner J., Bazhin A.V. Mitochondria and mitochondrial ROS in cancer: Novel targets for anticancer therapy. J. Cell. Physiol. 2016;231:2570–2581. doi: 10.1002/jcp.25349. [DOI] [PubMed] [Google Scholar]
- 95.Zou Z., Chang H., Li H., Wang S. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis. 2017;22:1321–1335. doi: 10.1007/s10495-017-1424-9. [DOI] [PubMed] [Google Scholar]
- 96.Zielonka J., Joseph J., Sikora A., Hardy M., Ouari O., Vasquez-Vivar J., Cheng G., Lopez M., Kalyanaraman B. Mitochondria-targeted triphenylphosphonium-based compounds: Syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem. Rev. 2017;117:10043–10120. doi: 10.1021/acs.chemrev.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo X., Yang N., Ji W., Zhang H., Dong X., Zhou Z., Li L., Shen H., Yao S., Huang W. Mito-Bomb: Targeting mitochondria for cancer therapy. Adv. Mater. 2021;43:2007778. doi: 10.1002/adma.202007778. [DOI] [PubMed] [Google Scholar]
- 98.Dong L., Gopalan V., Holland O., Neuzil J. Mitocans Revisited: Mitochondrial Targeting as Efficient Anti-Cancer Therapy. Int. J. Mol. Sci. 2020;21:7941. doi: 10.3390/ijms21217941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tian M., Li J., Zhang S., Guo L., He X., Kong D., Zhang H., Liu Z. Half-sandwich ruthenium (II) complexes containing N^N-chelated imino-pyridyl ligands that are selectively toxic to cancer cells. Chem. Commun. 2017;53:12810–12813. doi: 10.1039/C7CC08270C. [DOI] [PubMed] [Google Scholar]
- 100.Xu Z., Kong D., He X., Guo L., Ge X., Liu X., Zhang H., Li J., Yang Y., Liu Z. Mitochondria-targeted half-sandwich ruthenium II diimine complexes: Anticancer and antimetastasis via ROS-mediated signalling. Inorg. Chem. Front. 2018;5:2100–2105. doi: 10.1039/C8QI00476E. [DOI] [Google Scholar]
- 101.Zhao Z., Luo Z., Wu Q., Zheng W., Feng Y., Chen T. Mixed-ligand ruthenium polypyridyl complexes as apoptosis inducers in cancer cells, the cellular translocation and the important role of ROS-mediated signaling. Dalton Trans. 2014;43:17017–17028. doi: 10.1039/C4DT01392A. [DOI] [PubMed] [Google Scholar]
- 102.Li M., Lai L., Zhao Z., Chen T. Aquation is a crucial activation step for anticancer action of ruthenium (II) polypyridyl complexes to trigger cancer cell apoptosis. Chem. Asian J. 2016;11:310–320. doi: 10.1002/asia.201501048. [DOI] [PubMed] [Google Scholar]
- 103.He L., Wang K.N., Zheng Y., Cao J., Zhang M., Tan C., Ji L., Mao Z. Cyclometalated iridium (iii) complexes induce mitochondria-derived paraptotic cell death and inhibit tumor growth in vivo. Dalton Trans. 2018;47:6942–6953. doi: 10.1039/C8DT00783G. [DOI] [PubMed] [Google Scholar]
- 104.Wu X.W., Zheng Y., Wang F.X., Cao J., Zhang H., Zhang D., Tan C., Ji L., Mao Z. Anticancer IrIII-aspirin conjugates for enhanced metabolic immuno-modulation and mitochondrial lifetime imaging. Chem. Eur. J. 2019;25:7012–7022. doi: 10.1002/chem.201900851. [DOI] [PubMed] [Google Scholar]
- 105.Peng W., Hegazy A.M., Jiang N., Chen X., Qi H., Zhao X., Pu J., Ye R., Li R. Identification of two mitochondrial-targeting cyclometalated iridium (III) complexes as potent anti-glioma stem cells agents. J. Inorg. Biochem. 2020;203:110909. doi: 10.1016/j.jinorgbio.2019.110909. [DOI] [PubMed] [Google Scholar]
- 106.Guan R., Xie L., Wang L., Zhou Y., Chen Y., Ji L., Chao H. Necroptosis-inducing iridium (III) complexes as regulators of cyclin-dependent kinases. Inorg. Chem. Front. 2021;8:1788–1794. doi: 10.1039/D0QI01430C. [DOI] [Google Scholar]
- 107.Li X., Hao S., Han A., Yang Y., Fang G., Liu J., Wang S. Intracellular Fenton reaction based on mitochondria-targeted copper (ii)–peptide complex for induced apoptosis. J. Mater. Chem. B. 2019;7:4008–4016. doi: 10.1039/C9TB00569B. [DOI] [Google Scholar]
- 108.Shao J., Li M., Guo Z., Jin C., Zhang F., Ou C., Xie Y., Tan S., Wang Z., Zheng S., et al. TPP-related mitochondrial targeting copper (II) complex induces p53-dependent apoptosis in hepatoma cells through ROS-mediated activation of Drp1. Cell Commun. Signal. 2019;17:1–18. doi: 10.1186/s12964-019-0468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peng Y.B., Tao C., Tan C.P., Zhao P. Mitochondrial targeted rhodium (III) complexes: Synthesis, characterized and antitumor mechanism investigation. J. Inorg. Biochem. 2021;218:111400. doi: 10.1016/j.jinorgbio.2021.111400. [DOI] [PubMed] [Google Scholar]
- 110.Mármol I., Virumbrales-Muñoz M., Quero J., Sánchez-de-Diego C., Fernández L., Ochoa I., Cerrada E., Yoldi M.J.R. Alkynyl gold (I) complex triggers necroptosis via ROS generation in colorectal carcinoma cells. J. Inorg. Biochem. 2017;176:123–133. doi: 10.1016/j.jinorgbio.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 111.Ye R.R., Chen B.C., Lu J.J., Ma X., Li R. Phosphorescent rhenium (I) complexes conjugated with artesunate: Mitochondrial targeting and apoptosis-ferroptosis dual induction. J. Inorg. Biochem. 2021;223:111537. doi: 10.1016/j.jinorgbio.2021.111537. [DOI] [PubMed] [Google Scholar]
- 112.Icsel C., Yilmaz V.T., Aygun M., Cevatemre B., Alper P., Ulukaya E. Palladium (II) and platinum (II) saccharinate complexes with bis (diphenylphosphino) methane/ethane: Synthesis, S-phase arrest and ROS-mediated apoptosis in human colon cancer cells. Dalton Trans. 2018;47:11397–11410. doi: 10.1039/C8DT02389A. [DOI] [PubMed] [Google Scholar]
- 113.Yilmaz V.T., Icsel C., Turgut O.R., Aygun M., Erkisa M., Turkdemir M.H., Ulukaya E. Synthesis, structures and anticancer potentials of platinum (II) saccharinate complexes of tertiary phosphines with phenyl and cyclohexyl groups targeting mitochondria and DNA. Eur. J. Med. Chem. 2018;155:609–622. doi: 10.1016/j.ejmech.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 114.Zhang H., Tian L., Xiao R., Zhou Y., Zhang Y., Hao J., Liu Y., Wang J. Anticancer effect evaluation in vitro and in vivo of iridium (III) polypyridyl complexes targeting DNA and mitochondria. Bioorg. Chem. 2021;115:105290. doi: 10.1016/j.bioorg.2021.105290. [DOI] [PubMed] [Google Scholar]
- 115.Yousuf I., Arjmand F., Tabassum S., Toupet L., Khan R.A., Siddiqui M.A. Mechanistic insights into a novel chromone-appended Cu (II) anticancer drug entity: In vitro binding profile with DNA/RNA substrates and cytotoxic activity against MCF-7 and HepG2 cancer cells. Dalton Trans. 2015;44:10330–10342. doi: 10.1039/C5DT00770D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.